- 1Department of Orthopedics, The Second People’s Hospital of Yibin, Yibin, Sichuan, China
- 2Department of Nephrology, The Second People’s Hospital of Yibin, Yibin, Sichuan, China
Purpose: This study aims to identify the association between preoperative Modic changes and the recurrence of lumbar disc herniation (LDH) in patients who have undergone percutaneous endoscopic lumbar discectomy (PELD).
Methods: The PubMed, Web of Science, EMBASE, and CNKI databases were searched from their inception until 19 March 2025. Early recurrence was defined as herniation occurring within 6 months postoperatively, whereas late recurrence referred to herniation occurring after 12 months. Odds ratios (ORs) with 95% confidence intervals (CIs) were combined, and subgroup analyses were conducted according to the recurrence type.
Results: Twenty-seven studies involving 10,116 patients were included, with the majority of studies originating from China (25/27). The recurrence rates for patients without and with Modic changes were 7.44% and 16.41%, respectively (type I: 15.01%; type II/III: 18.14%; P < 0.001). The presence of Modic changes was associated with a significantly increased risk of recurrence (OR = 2.96, 95% CI: 2.29–3.82, P < 0.001), and subgroup analyses by the recurrence period (early or late) showed consistent findings. However, patients with Modic type II/III changes did not have a higher risk of recurrence than those with Modic type I changes (OR = 1.13, P = 0.217).
Conclusion: Preoperative Modic changes are associated with postoperative recurrence among LDH patients undergoing PELD, and the presence of Modic changes is related to a significantly higher risk of early and late recurrence.
Introduction
Lumbar disc herniation (LDH) is one of the most common degenerative spinal disorders, primarily caused by degeneration or external stress that leads to the nucleus pulposus protruding through the annulus fibrosus and compressing adjacent nerve roots (1). This condition often results in low back pain, radiculopathy, and, in some cases, motor or sensory dysfunction (2). The incidence of LDH has been increasing steadily and is considered a major contributor to reduced quality of life and work capacity among the working-age population. Epidemiological studies have shown that approximately 60%–80% of adults experience low back pain at some point in their lives, with LDH being one of the leading causes (3). Although conservative treatments—such as pharmacotherapy, physical therapy, and spinal traction—may offer symptom relief in the early stages, surgical intervention remains the most effective treatment option for patients with persistent or worsening symptoms unresponsive to conservative management (4). Common surgical techniques include open discectomy, microdiscectomy, and minimally invasive procedures such as percutaneous endoscopic lumbar discectomy (PELD). These procedures generally provide rapid symptom relief and significantly improve patients' quality of life. However, postoperative recurrence remains a clinical concern, with reported recurrence rates ranging from 5% to 15% (5). Recurrence is often associated with inadequate rehabilitation, residual disc fragments, or further degeneration of the intervertebral disc. Therefore, while surgical treatment offers favorable short-term outcomes, postoperative rehabilitation and long-term management are equally important to prevent recurrence and ensure sustained recovery.
Recent studies have suggested a potential association between Modic changes—vertebral endplate and bone marrow signal alterations detected on magnetic resonance imaging (MRI)—and postoperative recurrence following PELD (6). Modic changes, commonly classified into three types (type I: inflammatory, type II: fatty degeneration, and type III: sclerosis), are considered imaging indicators of degenerative changes at the vertebral endplate–disc interface (6). These changes are increasingly observed in patients with LDH and are thought to reflect underlying pathological processes such as endplate damage, inflammatory responses, and biomechanical alterations (7).
Although some studies have indicated that the presence of Modic changes may be associated with an increased risk of recurrent disc herniation after PELD, the existing evidence remains inconclusive and somewhat inconsistent. Therefore, we conducted a meta-analysis to further clarify the relationship between preoperative Modic changes and postoperative recurrence in patients undergoing PELD, aiming to provide a more robust evidence base for clinical decision-making and surgical risk assessment.
Materials and methods
This meta-analysis was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (8).
Literature search
The PubMed, EMBASE, CNKI, and Web of Science databases were searched from their inception until 19 March 2025. The following terms were used in the search: lumbar disc herniation, LDH, percutaneous endoscopic lumbar discectomy, percutaneous transforaminal endoscopic discectomy, PETD, PED, recurrence, and Modic. A detailed search PubMed strategy is shown in Supplementary File S1. Meanwhile, MeSH terms and free texts were applied. References for the included studies were also reviewed.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) patients were diagnosed with LDH and underwent PELD; (2) the presence or absence of Modic changes was evaluated before the surgery by MRI according to previously reported criteria (9); (3) recurrence rates for patients with and without Modic (type I, II, or III) changes were reported; (4) detailed information was provided for the calculation of odds ratios (ORs) with 95% confidence intervals (CIs) to investigate the association between Modic changes and recurrence risk; (5) the study was published in English or Chinese; (6) the full text was available.
Studies were excluded if they met any of the following criteria: (1) contained overlapping or duplicate data; or (2) were meeting abstracts, letters, animal trials, editorials, reviews, or case reports.
Data extraction
We extracted the following data from each included study: first author, publication year, country, sample size, follow-up duration, number of patients, and number of patients experiencing recurrence with non-Modic changes, type I Modic change, type II Modic change, and type III Modic change, and ORs and 95% CIs.
In this meta-analysis, early recurrence was defined as recurrence occurring within 6 months after surgery (10), and late recurrence was defined as recurrence occurring after 12 months (11).
Quality assessment
All included studies were cohort studies, and the Newcastle–Ottawa Scale (NOS) was used to assess their methodological quality (12). Studies with an NOS score ≥6 were defined as high-quality.
The literature search, study selection, data collection, and quality assessment were independently conducted by two authors (XL and HR), and any disagreements were resolved through consensus or consultation with a third reviewer (LP).
Statistical analysis
In our study, all statistical analyses were performed using STATA 17.0 software. Heterogeneity among the included studies was evaluated using the I2 statistic and the Q-test. When significant heterogeneity was detected (I2 > 50% and/or P < 0.1), a random-effects model was applied; otherwise, a fixed-effects model was used. ORs and 95% CIs were calculated to evaluate the association between Modic changes and recurrence risk. Subgroup analyses based on the recurrence period were also performed. Sensitivity analyses were performed to identify potential sources of heterogeneity and assess the stability of the pooled results. In addition, Begg's funnel plot and Egger's test were conducted to detect publication bias, with significant publication bias defined as P < 0.05 (13, 14). If significant publication bias was detected, the trim-and-fill method was applied to identify potentially unpublished studies (15).
Results
Literature search and selection process
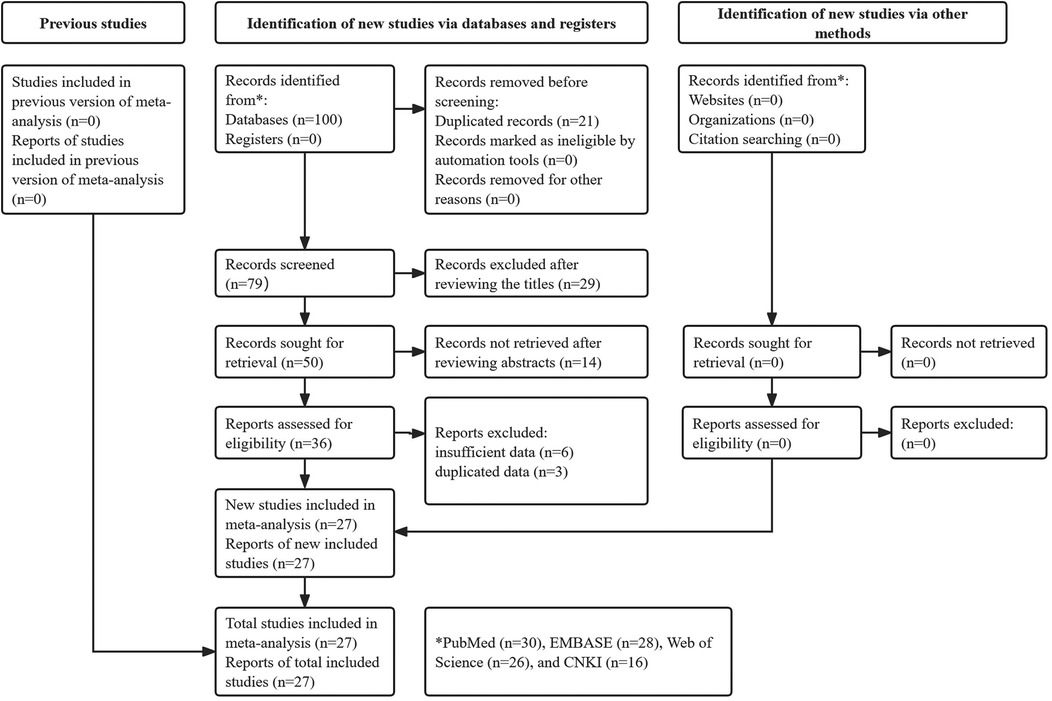
A total of 100 records were identified through searches of the four databases, and 21 duplicate records were removed. After reviewing the titles, abstracts, and full texts, 29, 14, and 9 publications were excluded, respectively. Eventually, 27 studies were included in this meta-analysis (10, 16–41) (Figure 1).
Basic characteristics of included studies
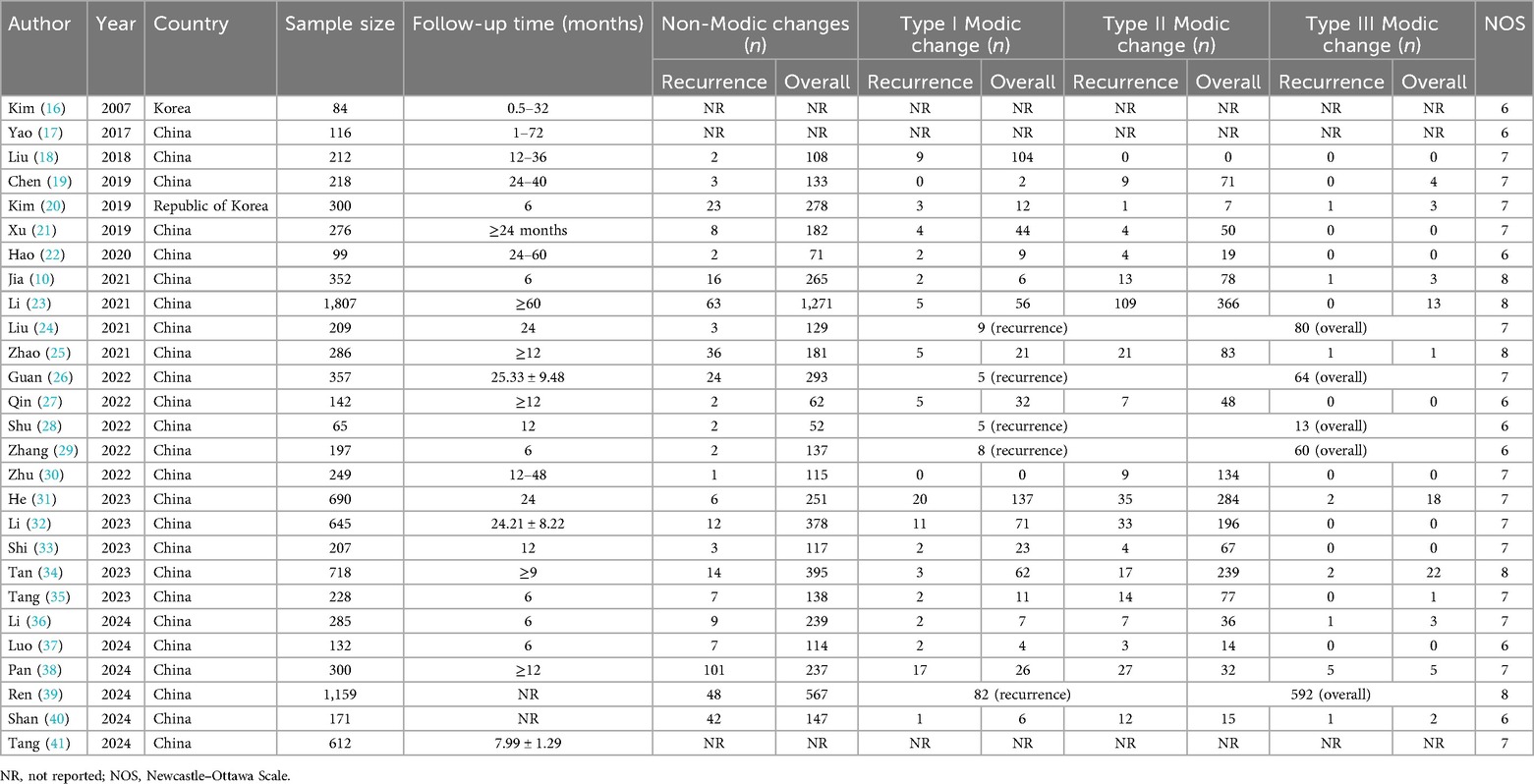
Among the 27 included studies, 10,116 patients were enrolled, with sample sizes ranging from 84 to 1,807. Most studies were conducted in China (25/27). Six studies focused on early recurrence (within 6 months), while 15 examined late recurrence (after 12 months). All of the included studies were deemed high quality. Specific data are presented in Table 1.
Recurrence rates in LDH patients after PELD
First, we calculated the recurrence rates of LDH in patients with and without Modic changes. The results showed that the recurrence rate in patients with Modic changes (16.41%, 547/3,333) was significantly higher than that in patients without Modic changes (7.44%, 436/5,424) (P < 0.001). In detail, the recurrence rates in patients with type I and type II/III Modic changes were 15.01% (95/633) and 18.14% (343/1,891), respectively (P = 0.072).
Association between preoperative Modic changes and recurrence in LDH patients receiving PELD
Based on the pooled results of the meta-analysis, the presence of Modic changes was significantly associated with an increased risk of recurrence (OR = 2.96, 95% CI: 2.29–3.82, P < 0.001; I2 = 72.7%, P < 0.001) (Figure 2). Subgroup analysis by the recurrence period showed similar findings (late: OR = 3.36, 95% CI: 2.23–5.04, P < 0.001; early: OR = 3.84, 95% CI: 2.69–5.48, P < 0.001) (Supplementary Figure S1).
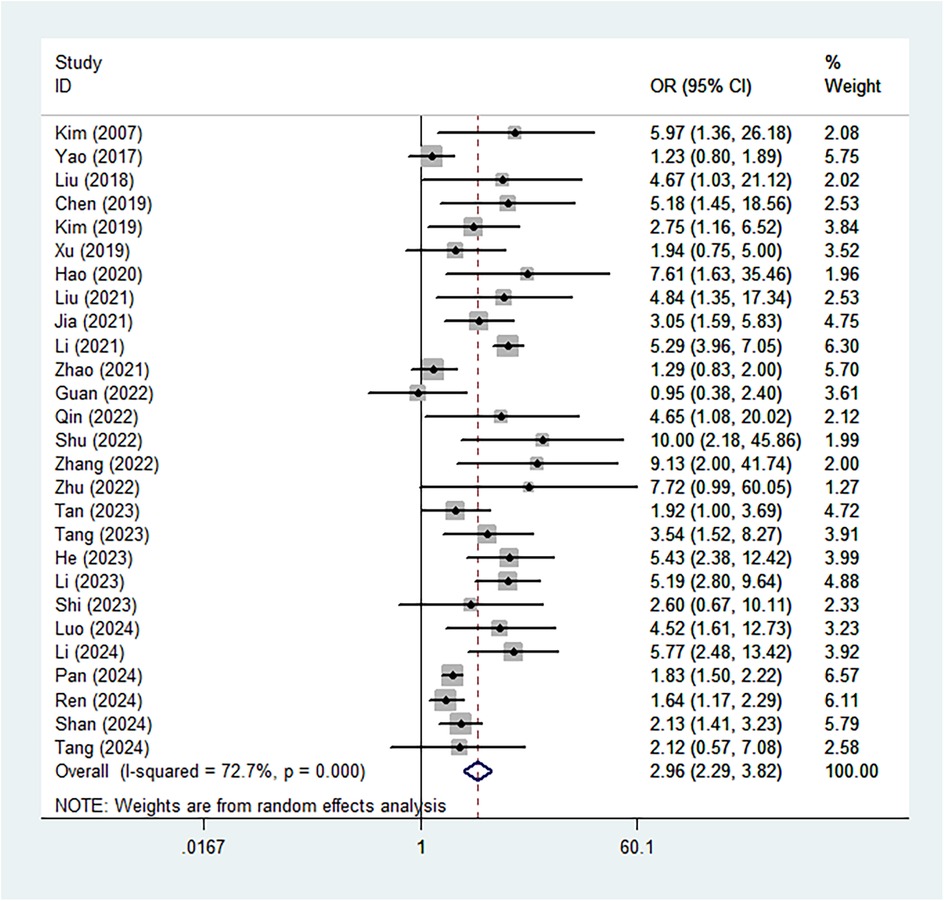
Figure 2. Association between the presence of Modic changes and postoperative recurrence in patients with lumbar disc herniation after the percutaneous endoscopic lumbar discectomy.
In addition, the association between different types of Modic changes and recurrence risk was also explored. However, the recurrence rates of LDH in patients with type I versus type II/III Modic changes were not statistically different (OR = 1.13, 95% CI: 0.93–1.38, P = 0.217) (Figure 3). Subgroup analyses based on the recurrence period yielded consistent results (late: OR = 1.17, 95% CI: 0.95–1.45, P = 0.135; early: OR = 0.65, 95% CI: 0.36–1.20, P = 0.170) (Supplementary Figure S2).
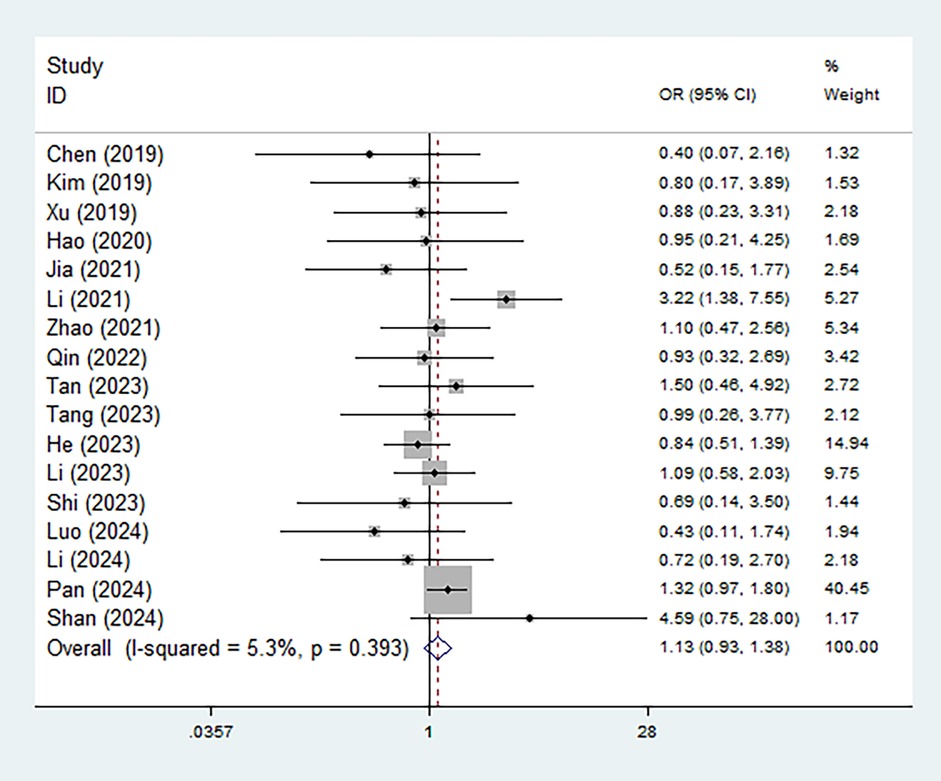
Figure 3. Association between the type of Modic changes (II/III vs. I) and postoperative recurrence in patients with lumbar disc herniation after the percutaneous endoscopic lumbar discectomy.
Sensitivity analysis
Sensitivity analysis indicated that the results were stable and reliable, and no individual study had a significant impact on the overall findings (Figure 4).
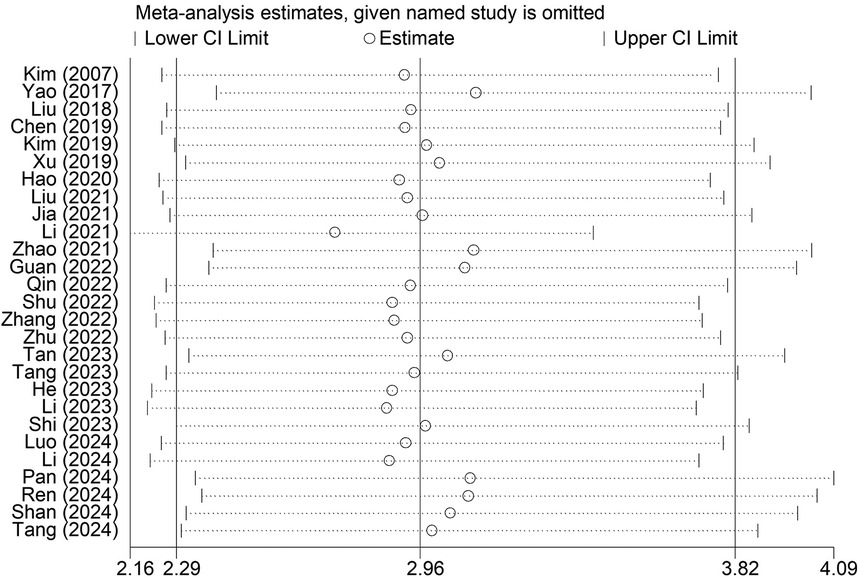
Figure 4. Sensitivity analysis for the association between the presence of Modic changes and postoperative recurrence in patients with lumbar disc herniation after the percutaneous endoscopic lumbar discectomy.
Publication bias
According to Begg's funnel plot (Figure 5) and Egger's test (P = 0.039), obvious publication bias was detected. Therefore, the trim-and-fill method was applied, revealing seven potentially unpublished studies (Figure 6). However, these seven studies did not affect the overall conclusion (random-effects filled OR = 2.49, 95% CI: 1.96–3.16, P < 0.001; fixed-effects filled OR = 2.28, 95% CI: 2.05–2.54, P < 0.001).
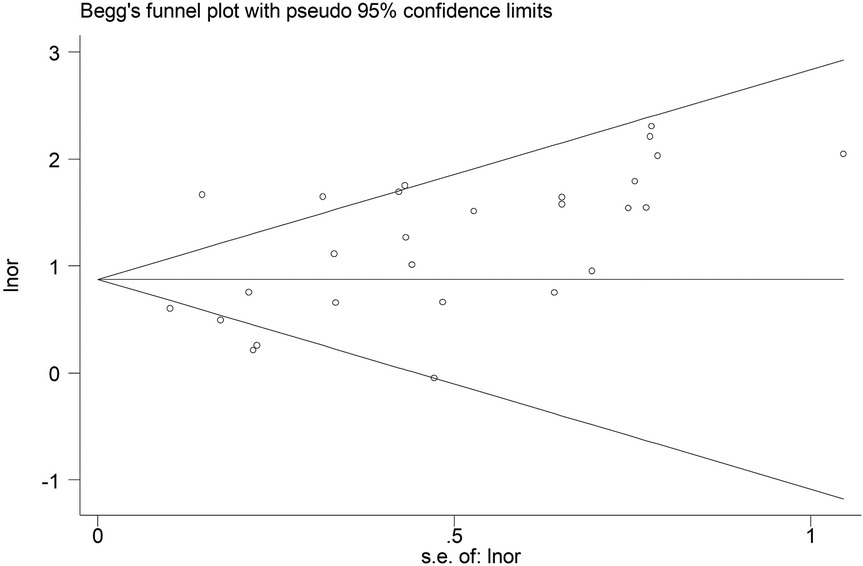
Figure 5. Begg's funnel plot for the association between the presence of Modic changes and postoperative recurrence in patients with lumbar disc herniation after the percutaneous endoscopic lumbar discectomy.
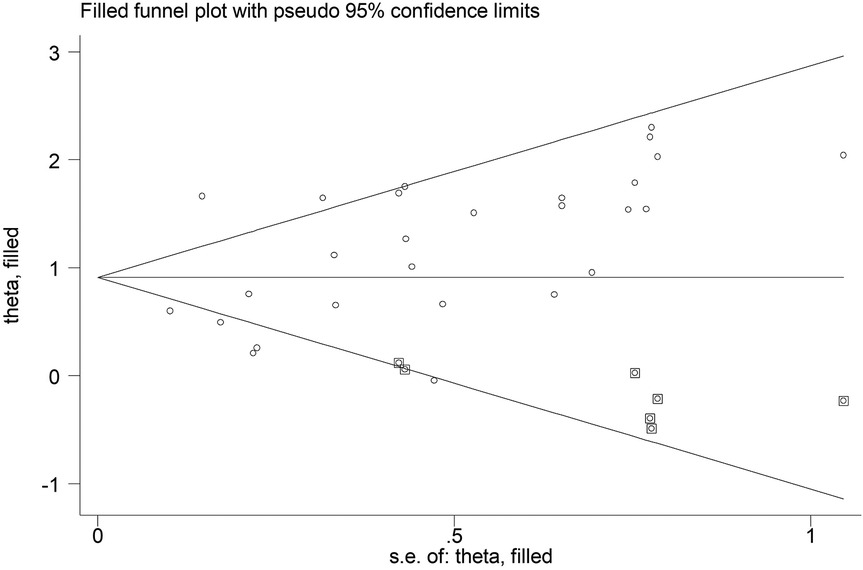
Figure 6. Filled Begg's funnel plot for the association between the presence of Modic changes and postoperative recurrence in patients with lumbar disc herniation after the percutaneous endoscopic lumbar discectomy.
Discussion
In the current meta-analysis, we included 27 studies with 10,116 patients and evaluated the association between preoperative Modic changes and postoperative recurrence of LDH among patients undergoing PELD. Our pooled results indicated that the presence of Modic changes, regardless of the type, was significantly associated with an increased risk of postoperative recurrence. Therefore, preoperative evaluation of Modic changes is essential for LDH patients. However, due to the limitations of the included studies, more prospective cohort studies or randomized trials are needed to verify our findings.
Several mechanisms have been proposed to explain why patients with LDH accompanied by Modic changes may exhibit a higher risk of postoperative recurrence following PELD. First, Modic changes often reflect structural damage to the vertebral endplates, which can lead to intervertebral segmental instability. This biomechanical alteration may expose the operated disc to increased mechanical stress, thereby accelerating disc degeneration and increasing the likelihood of reherniation (42). Second, particularly in patients with Modic type I changes, a persistent inflammatory microenvironment around the endplate and bone marrow may not resolve following surgical decompression. Such inflammation may contribute to ongoing degeneration and residual or recurrent symptoms (43). In addition, Modic changes are commonly associated with advanced disc degeneration, including reduced water content, annular fissures, and fragmentation of the nucleus pulposus. These degenerative changes can impair the disc's ability to structurally recover after surgery, making it more susceptible to recurrent herniation (44). Moreover, in patients with Modic changes, the annulus fibrosus is often more severely compromised, which may lead to incomplete repair of annular defects and residual disc fragments postoperatively—factors that have been linked to recurrence (45). Finally, Modic-related alterations in load transmission across the vertebral body may cause abnormal stress redistribution, further promoting recurrent disc protrusion either at the surgical level or at adjacent segments (46). Although these mechanisms are not fully elucidated, they highlight the potential role of Modic changes in influencing surgical outcomes and underscore the need for careful preoperative assessment and postoperative management in this patient population.
Beyond their potential association with postoperative recurrence, preoperative assessment of Modic changes may offer additional clinical value in the comprehensive management of LDH patients. First, Modic changes may serve as imaging biomarkers that reflect the degree of vertebral endplate degeneration and intervertebral disc pathology, thus aiding in surgical decision-making and risk stratification. Identifying Modic changes preoperatively could help surgeons anticipate technical challenges during discectomy and select the most appropriate surgical approach or extent of decompression (47). Second, Modic changes—especially type I—are often associated with more severe preoperative low back pain and a higher incidence of residual postoperative symptoms. Therefore, evaluating Modic status may help predict patient prognosis beyond herniation recurrence, including pain persistence and recovery of function (48, 49). In such cases, patients may benefit from tailored perioperative management strategies, such as enhanced rehabilitation programs, anti-inflammatory interventions, or adjunctive treatments targeting endplate inflammation. Moreover, the presence of Modic changes may indicate a more advanced degenerative process that could predispose patients to adjacent segment disease or long-term spinal instability (44). Consequently, integrating Modic change assessment into the preoperative evaluation may facilitate long-term treatment planning and improve patient counseling regarding expected outcomes and potential complications.
However, this meta-analysis has some limitations. First, most of the included studies were from China, which may affect the universality of our conclusion. Therefore, additional studies from other countries are needed. Second, all of the included studies were retrospective in design, which may affect the stability of the pooled findings. Third, we were unable to perform more subgroup analyses based on other confounding factors such as age and sex. Finally, only a few studies explored the association between Modic changes and symptom relief after PELD, and we did not define postoperative symptoms as one of our observation indicators.
Conclusion
Preoperative Modic changes are associated with postoperative recurrence in LDH patients undergoing PELD, and the presence of Modic changes is associated with a significantly higher risk of early and late recurrence. However, well-designed prospective cohort studies or randomized trials are required to validate this association and further clarify any causal mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XL: Conceptualization, Supervision, Visualization, Writing – original draft. HR: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. LP: Investigation, Resources, Software, Writing – original draft. JY: Data curation, Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1694557/full#supplementary-material
Supplementary File 1 | Search strategy in the PubMed database.
References
1. Zhang AS, Xu A, Ansari K, Hardacker K, Anderson G, Alsoof D, et al. Lumbar disc herniation: diagnosis and management. Am J Med. (2023) 136(7):645–51. doi: 10.1016/j.amjmed.2023.03.024
2. Amin RM, Andrade NS, Neuman BJ. Lumbar disc herniation. Curr Rev Musculoskelet Med. (2017) 10(4):507–16. doi: 10.1007/s12178-017-9441-4
3. Pojskic M, Bisson E, Oertel J, Takami T, Zygourakis C, Costa F. Lumbar disc herniation: epidemiology, clinical and radiologic diagnosis WFNS spine committee recommendations. World Neurosurg X. (2024) 22:100279. doi: 10.1016/j.wnsx.2024.100279
4. Awadalla AM, Aljulayfi AS, Alrowaili AR, Souror H, Alowid F, Mahdi AMM, et al. Management of lumbar disc herniation: a systematic review. Cureus. (2023) 15(10):e47908. doi: 10.7759/cureus.47908
5. Gadjradj PS, Harhangi BS, Amelink J, van Susante J, Kamper S, van Tulder M, et al. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: a systematic review and meta-analysis. Spine (Phila Pa 1976). (2021) 46(8):538–49. doi: 10.1097/BRS.0000000000003843
6. Zhao J, Zeng L, Zhao S, Liang G, Sha B, Fu H, et al. Associations of recurrent lumbar disc herniation after percutaneous endoscopic lumbar discectomy with age, body mass index, modic change, disc degeneration and sacral slope: a quantitative review. Exp Ther Med. (2024) 27(5):195. doi: 10.3892/etm.2024.12483
7. Verheijen EJA, van der Vlist NRE, Bartels EC, van Haagen O, Vleggeert-Lankamp CLA. The effect of a transforaminal epidural injection in patients with lumbar disc herniation is not correlated with the presence of type II modic changes. Brain Spine. (2025) 5:104222. doi: 10.1016/j.bas.2025.104222
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
9. Li L, Wang C, Zhang H, Liu Z, Lian Z, Li H, et al. New-occurrence of postoperative modic changes and its influence on the surgical prognosis after percutaneous endoscopic lumbar disc discectomy. Orthop Surg. (2025) 17(2):482–91. doi: 10.1111/os.14308
10. Jia M, Sheng Y, Chen G, Zhang W, Lin J, Lu S, et al. Development and validation of a nomogram predicting the risk of recurrent lumbar disk herniation within 6 months after percutaneous endoscopic lumbar discectomy. J Orthop Surg Res. (2021) 16(1):274. doi: 10.1186/s13018-021-02425-2
11. Parker SL, Mendenhall SK, Godil SS, Sivasubramanian P, Cahill K, Ziewacz J, et al. Incidence of low back pain after lumbar discectomy for herniated disc and its effect on patient-reported outcomes. Clin Orthop Relat Res. (2015) 473(6):1988–99. doi: 10.1007/s11999-015-4193-1
12. Wang Y, Li J, Chang S, Dong Y, Che G. Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: a systematic review and two meta-analyses based on four million cases. J Thorac Oncol. (2021) 16(11):1893–908. doi: 10.1016/j.jtho.2021.07.001
13. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50(4):1088–101. doi: 10.2307/2533446
14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
15. Wang Y, Li P, Li J, Lai Y, Zhou K, Wang X, et al. The prognostic value of pretreatment Glasgow prognostic score in patients with esophageal cancer: a meta-analysis. Cancer Manag Res. (2019) 11:8181–90. doi: 10.2147/CMAR.S203425
16. Kim JM, Lee SH, Ahn Y, Yoon DH, Lee CD, Lim ST. Recurrence after successful percutaneous endoscopic lumbar discectomy. Minim Invasive Neurosurg. (2007) 50(2):82–5. doi: 10.1055/s-2007-982504
17. Yao Y, Liu H, Zhang H, Wang H, Zhang C, Zhang Z, et al. Risk factors for recurrent herniation after percutaneous endoscopic lumbar discectomy. World Neurosurg. (2017) 100:1–6. doi: 10.1016/j.wneu.2016.12.089
18. Liu M. Percutaneous transforaminal endoscopic discectomy for lumbar disc herniation with Modic type I changes (Master's thesis). Taian Medical College, Taishan, China (2018).
19. Chen H. Analysis of factors influencing recurrence of lumbar disc herniation after endoscopic surgery (Master's thesis). Xinjiang Medical University, Xinjiang, China (2019).
20. Kim HS, You JD, Ju CI. Predictive scoring and risk factors of early recurrence after percutaneous endoscopic lumbar discectomy. Biomed Res Int. (2019) 2019:6492675. doi: 10.1155/2019/6492675
21. Xu J, Li Y, Wang B, Lv G-H, Wu P, Dai Y, et al. Percutaneous endoscopic lumbar discectomy for lumbar disc herniation with modic changes via a transforaminal approach: a retrospective study. Pain Physician. (2019) 22(6):E601–8.31775413
22. Hao L, Li S, Liu J, Shan Z, Fan S, Zhao F. Recurrent disc herniation following percutaneous endoscopic lumbar discectomy preferentially occurs when Modic changes are present. J Orthop Surg Res. (2020) 15(1):176. doi: 10.1186/s13018-020-01695-6
23. Li Y, Wang B, Li H, Chang X, Wu Y, Hu Z, et al. Adjuvant surgical decision-making system for lumbar intervertebral disc herniation after percutaneous endoscopic lumber discectomy: a retrospective nonlinear multiple logistic regression prediction model based on a large sample. Spine J. (2021) 21(12):2035–48. doi: 10.1016/j.spinee.2021.07.012
24. Liu J. Analysis of related risk factors after percutaneous endoscopic lumbar discectomy for recurrent lumbar disc herniation (Master's thesis). Xinjiang Medical University, Xinjiang, China (2021).
25. Zhao C, Zhang H, Wang Y, Xu D, Han S, Meng S, et al. Nomograms for predicting recurrent herniation in PETD with preoperative radiological factors. J Pain Res. (2021) 14:2095–109. doi: 10.2147/JPR.S312224
26. Guan Y, Wu J, Fan S, Shen X, Xu B, Shen G, et al. Clinical study on the influencing factors of recurrence of single-segment lumbar intervertebral disc herniation after percutaneous transforaminal endoscopy. Chin J Tradit Med Traumatol Orthop. (2022) 30(2):40–4.
27. Qin X, Zhai Y, Li H, Sun X, Zhang C. Effects of Modic changes on the clinical outcomes of percutaneous endoscopic transforaminal discectomy for treatment of lumbar disc herniation. J Tradit Chin Orthop Traumatol. (2022) 34(9):17–23.
28. Shu Y. A comparative study of limited discectomy and aggressive discectomy in patients with lumbar disc herniation who had underwent percutaneous endoscopic lumbar discectomy (Master's thesis). Xinjiang Medical University, Xinjiang, China (2022).
29. Zhang W, Yuan C, Yang X, Song Z, Yang H, Wu C, et al. Study on the relationship between Modic change and early re-protrusion after percutaneous endoscopic lumbar discectomy. Chin J Tradit Med Traumatol Orthop. (2022) 30(5):51–3.
30. Zhu H, Hussain Z, Zhang M, Ji F, Mao H, Li H, et al. Percutaneous endoscopic lumbar discectomy for lumbar disc herniation with type II Modic changes. World Neurosurg. (2022) 164:e143–9. doi: 10.1016/j.wneu.2022.04.056
31. He H, Ma J, Xiong C, Wei T, Tang A, Chen Y, et al. Development and validation of a nomogram to predict the risk of lumbar disk reherniation within 2 years after percutaneous endoscopic lumbar discectomy. World Neurosurg. (2023) 172:e349–56. doi: 10.1016/j.wneu.2023.01.026
32. Li X, Pan B, Cheng L, Li G, Liu J, Yuan F. Development and validation of a prognostic model for the risk of recurrent lumbar disc herniation after percutaneous endoscopic transforaminal discectomy. Pain Physician. (2023) 26(1):81–90. doi: 10.36076/ppj.2023.26.81
33. Shi Z, Li P, Wu W, Jiang Y, Wang Y. Analysis of the efficacy of percutaneous endoscopic interlaminar discectomy for lumbar disc herniation with different types/grades of modic changes. J Pain Res. (2023) 16:1927–40. doi: 10.2147/JPR.S403266
34. Tan L. Risk factors for recurrence after percutaneous endoscopic lumbar discectomy and the construction of a predictive model based on the theory of “prevention of recurrence after healing” in the treatment of untreated diseases (Master's thesis). Guangzhou University of Chinese Medicine, Guangzhou, China (2023).
35. Tang M, Zeng F, Chang X, Fang Q, He M, Yin S. Nomogram development and validation for predicting postoperative recurrence of lumbar disc herniation based on paraspinal functional muscle cross-sectional area. J Jinan Univ. (2023) 44(6):602–11.
36. Li ZP, Liu LL, Liu H, Tan JH, Li XL, Xu Z, et al. Radiologic analysis of causes of early recurrence after percutaneous endoscopic transforaminal discectomy. Global Spine J. (2024) 14(1):113–21. doi: 10.1177/21925682221096061
37. Luo J, Bao J, Qiu H, Zhang F. Risk factors and predictive efficacy analysis of early recurrence after lumbar discectomy under intervertebral foramen endoscopy. Zhejiang J Trauma Surg. (2024) 29(3):416–8, +422.
38. Pan YH, Wan D, Wang Q, Shen WJ, Yang JR, Wang ZY, et al. Association of spinal–pelvic parameters with recurrence of lumbar disc herniation after endoscopic surgery: a retrospective case–control study. Eur Spine J. (2024) 33(2):444–52. doi: 10.1007/s00586-023-08073-w
39. Ren G, Liu L, Zhang P, Xie Z, Wang P, Zhang W, et al. Machine learning predicts recurrent lumbar disc herniation following percutaneous endoscopic lumbar discectomy. Global Spine J. (2024) 14(1):146–52. doi: 10.1177/21925682221097650
40. Shan ZM, Ren XS, Shi H, Zheng SJ, Zhang C, Zhuang SY, et al. Machine learning prediction model and risk factor analysis of reoperation in recurrent lumbar disc herniation patients after percutaneous endoscopic lumbar discectomy. Global Spine J. (2024) 14(8):2240–51. doi: 10.1177/21925682231173353
41. Tang M, Wang S, Wang Y, Chen M, Chang X, He M, et al. Development and validation of a nomogram predicting postoperative recurrent lumbar disc herniation based on activity factors. Risk Manag Healthc Policy. (2024) 17:689–99. doi: 10.2147/RMHP.S453819
42. Luo M, Wang Z, Zhou B, Yang G, Shi Y, Chen J, et al. Risk factors for lumbar disc herniation recurrence after percutaneous endoscopic lumbar discectomy: a meta-analysis of 58 cohort studies. Neurosurg Rev. (2023) 46(1):159. doi: 10.1007/s10143-023-02041-0
43. Zeng Z, Qin J, Guo L, Hirai T, Gui Z, Liu T, et al. Prediction and mechanisms of spontaneous resorption in lumbar disc herniation: narrative review. Spine Surg Relat Res. (2024) 8(3):235–42. doi: 10.22603/ssrr.2023-0152
44. Brooks M, Dower A, Abdul Jalil MF, Kohan S. Radiological predictors of recurrent lumbar disc herniation: a systematic review and meta-analysis. J Neurosurg Spine. (2021) 34(3):481–91. doi: 10.3171/2020.6.SPINE20598
45. Jin Q, Chen L, Wu K, Feng Z, Yuan Y, Wang Y. Buttock pain in lumbar disc herniation: clinical characteristics, risk factors, and surgical outcomes. J Neurosurg Spine. (2025) 42(5):572–8. doi: 10.3171/2025.1.SPINE241170
46. Takahashi H, Aoki Y, Inoue M, Saito J, Nakajima A, Sonobe M, et al. Characteristics of relief and residual low back pain after discectomy in patients with lumbar disc herniation: analysis using a detailed visual analog scale. BMC Musculoskelet Disord. (2021) 22(1):167. doi: 10.1186/s12891-021-04015-z
47. Hong X, Liu L, Bao J, Shi R, Fan Y, Wu X. Characterization and risk factor analysis for reoperation after microendoscopic diskectomy. Orthopedics. (2015) 38(6):e490–6. doi: 10.3928/01477447-20150603-57
48. Shen Z, Zhong ZM, Wu Q, Zheng S, Shen X, Chen J. Predictors for poor outcomes after percutaneous endoscopic lumbar discectomy: a retrospective study of 241 patients. World Neurosurg. (2019) 126:e422–31. doi: 10.1016/j.wneu.2019.02.068
Keywords: Modic changes, lumbar disc herniation, percutaneous endoscopic lumbar discectomy, recurrence, meta-analysis
Citation: Li X, Ren H, Peng L and Yuan J (2025) Association between Modic changes and recurrence of lumbar disc herniation after percutaneous endoscopic lumbar discectomy: a meta-analysis. Front. Surg. 12:1694557. doi: 10.3389/fsurg.2025.1694557
Received: 28 August 2025; Revised: 5 November 2025;
Accepted: 11 November 2025;
Published: 27 November 2025.
Edited by:
Siying Song, MD Anderson Cancer Center, United StatesReviewed by:
Wentao Wan, Tianjin Medical University, ChinaShaocheng Liu, Capital Medical University, China
Copyright: © 2025 Li, Ren, Peng and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaqin Yuan, eWpxdHR4c0AxNjMuY29t
†These authors have contributed equally to this work
 Xianfeng Li
Xianfeng Li Honghong Ren2,†
Honghong Ren2,† Jiaqin Yuan
Jiaqin Yuan