- 1National Animal Protozoa Laboratory, China Agricultural University, Beijing, China
- 2Key Laboratory of Animal Epidemiology of the Ministry of Agriculture, China Agricultural University, Beijing, China
The apicomplexan parasite Neospora caninum causes neosporosis, an illness that leads to abortion or stillbirth in cattle, causing massive economic losses to the livestock industry. Rapid and viable diagnosis is the premise of prevention and control for neosporosis. In this study, we screened a new microneme protein 26 (NcMIC26) through western blot and mass spectrometry identification from the excretory secretion antigen (ESA) of N. caninum tachyzoites. NcMIC26 is subcellularly localized to the microneme of parasites. NcMIC26 is a specific antigen of N. caninum and has no cross-reaction with Toxoplasma gondii. Therefore, NcMIC26 has the potential to be a candidate diagnostic antigen for neosporosis. To test this hypothesis, recombinant NcMIC26 (rNcMIC26) was expressed in Escherichia coli (E. coli), and an indirect ELISA for detecting anti-N. caninum antibodies in cattle was established. Compared with that of the indirect immunofluorescent antibody test (IFAT), the positive coincidence rate of the ELISA based on rNcMIC26 was 76.53% (75/98), which was higher than that of an ELISA based on rSRS2 (66.33%), and the negative coincidence rate was 84.62% (33/39). It is noteworthy that 30 positive samples confirmed by IFAT were consistent with the rNcMIC26 ELISA but were negative by the rNcSRS2 ELISA. Our research illustrated that NcMIC26 is a dependable diagnostic marker for the serodiagnosis of N. caninum infection in cattle and could be utilized as a supplementary antigen for missed detection by NcSRS2.
Introduction
Neospora caninum is an obligate intracellular apicomplexan parasite that is the etiologic agent of neosporosis for a variety of animals, for which canids are the definitive hosts (1). The disease tends to be globally distributed and is most serious in cattle (2). Abortion is the main clinical symptom of infection, and neosporosis is one of the main causes of cattle abortion worldwide (3). Reproductive loss is the main clinical outcome of neosporosis in cattle and a major reason for the economic impact on the dairy and beef cattle trade (2). In the absence of an effective treatment or vaccine against bovine neosporosis, control of the disease depends on an accurate diagnosis of neosporosis-infected cattle for timely treatment or early elimination of livestock and other farm management measures.
The recombinant antigens used or validated for indirect ELISA are based on different biological function-associated antigens, including the surface antigens NcSAG1 (4), NcSRS2 (5), NcP40 (6), NcSAG4 (7), and rNcSRS9 (8); dense granule antigens NcGRA2 (9), NcGRA6 (10), and NcGRA7 (11); microneme antigen NcMIC10 (12); and other antigens, such as Neospora profilin (13). The NcSRS2 antigen is the most widely used and shows excellent diagnostic parameters (14). However, it is hard to examine the antibody response simultaneously against different antigens to which a host is differentially exposed depending on the stage (the acute or chronic stage) of N. caninum infection by using single antigens in ELISA. Similar to most other apicomplexan parasites, the process of invasion is necessary for N. caninum to survive and replicate within the host (15), and the proteins discharged by tachyzoites, known as the excretory secretion antigens (ESAs), are the most common targets of host immune reactions; recognizable proof of ESAs included in invasion may be valuable for revealing the critical target for and the prevailing antigen of N. caninum (16). In the present study, we screened a microneme protein 26 (NcMIC26) from N. caninum ESAs. The localization of NcMIC26 is in the microneme of parasites, and it partially colocalizes with NcMIC4. Recombinant NcMIC26 was expressed in Escherichia coli; a reliable, sensitive, and specific diagnostic test based on recombinant NcMIC26 was developed; and its diagnostic potential in an ELISA was evaluated.
Materials and Methods
Parasites and Cell Cultures
N. caninum Nc-1 strain tachyzoites were propagated in African green monkey kidney (Vero) cells cultured in Dulbecco's modified Eagle's medium (DMEM) (M&C, China) containing 25 mM glucose and 4 mM glutamine and supplemented with 2% fetal bovine serum (FBS, Gibco, USA). Cells were incubated at 37°C with 5% CO2 in a humidified incubator.
Preparation of N. caninum Tachyzoite ESA and Soluble N. caninum Lysate Antigen
To find a new N. caninum diagnostic antigen, we employed mass spectrometry-based proteomics to identify proteins present in the N. caninum tachyzoite using two different approaches. The first approach was identifying the proteins present in the tachyzoite-secreted fraction (ESA). ESA were obtained according to a method involving Toxoplasma gondii that was previously described (17). Briefly, the tachyzoites were harvested from Vero cell cultures. Twenty-seven-gauge needles were used to disrupt the cells, and lysates were filtered through a 5-μm syringe filter. The purified tachyzoites were washed three times in DMEM by centrifugation at 900 × g for 10 min. The freshly purified tachyzoites were incubated (5 × 107 parasites/mL) in a serum-free medium (DMEM) at 37°C for 3 h and cooled for 10 min on ice. The supernatant separated from the parasites and containing ESA was collected by centrifugation at 20,000 × g for 10 min at 4°C. The parasites were lysed using a RIPA buffer (Beyotime, China) supplemented with a cocktail of protease inhibitors (Sigma, USA).
The second approach was to identify the secreted proteins in the culture medium of intracellular tachyzoite cultures. Nc1 tachyzoites were inoculated in Vero cells. The medium was discarded after 3 days before its egress and washed with PBS three times, and then a serum-free DMEM was cultured at 37°C for 24 h. A dialysis bag (Harveybio, China) was used to concentrate the collected secreted proteins from intracellular culture. The medium collected from the Vero cell culture served as the control.
Identification of N. caninum-Specific Antigen From Tachyzoite ESA
The polyclonal antiserum against N. caninum or T. gondii was generated in mice or rabbits using tachyzoites lysate antigen as described previously (18). Six- to eight-week-old female BALB/c mice and 2-month-old rabbits were purchased from the Academy of Military Medical Sciences Laboratory Animal Center (Beijing, China). Mice and rabbits were immunized subcutaneously every 2 weeks with 100 μg (mice) or 2 mg/kg (rabbits) tachyzoites lysate antigen in an equal volume of Freund's complete adjuvant (Sigma, USA) for the first injection and 50 μg (mice) or 1 mg/kg (rabbits) for the second and third injections. The anti-N. caninum or anti-T. gondii sera were collected 10 days after the final immunization. Animal experiments were conducted according to the institutional guidelines for animal ethics.
SDS-PAGE and western blot were used to identify the collected N. caninum proteins, which were performed as previously described (6). The N. caninum tachyzoite ESA and lysate samples were loaded into a 12% SDS-PAGE gel with equal loads. Separated protein bands were visualized in gels by silver staining according to the manufacturer's protocol for the Silver Stain Kit (Beyotime Biotechnology Co., Ltd., China) or transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA) together with a visible prestained protein marker (TransGen Biotech Co., Ltd., China) after electrophoresis (Bio-Rad). The membranes were blocked with 5% (w/v) skim milk (BD Difco, USA) in PBS for 1 h at 37°C, rinsed with a washing buffer, and incubated with a mouse polyclonal antiserum against N. caninum (1:400) or a rabbit polyclonal antiserum against T. gondii (1:400) for 1 h at 37°C. The blots were washed five times with PBST (1% Tween-20), followed by incubation with horseradish peroxidase (HRP)-labeled goat antimouse IgG (H + L) (1:5,000, Sigma, USA) or HRP-labeled goat antirabbit IgG (1:10,000, Sigma, USA). Finally, enhanced chemiluminescence reagents (CoWin Biotech Co., Ltd., China) were used to observe the reaction bands after 5 s of exposure time.
According to the western blot results, the corresponding specific bands (realized by anti-N. caninum but not anti-T. gondii antibodies) in SDS-PAGE protein strips were cut out for mass spectrometry (MS) and protein identification (Beijing Protein Innovation Co., Ltd., China) to obtain the corresponding peptide information and determine the N. caninum-specific antigen. Analysis of the DNA and protein sequences was performed using The Toxoplasma Genomics Resource (ToxoDB) website (https://toxodb.org/toxo/) and the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/). Conserved domains are available on the NCBI Conserved Domain Search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Gene Cloning, Recombinant Protein Expression, and Purification
Total RNA was extracted from purified N. caninum tachyzoites, reverse-transcribed (TransGen Biotech Co., Ltd., China), and used as a template for PCR. Primers were designed for the region 271–1,014 bp of the NcMIC26 RNA sequence (ToxoDB, NCLIV_033690), with low similarity with the homologous gene in Toxoplasma gondii. The primer sequences were as follows—forward primer: 5′-AGCAAATGGGTCGC@@UGGATCCLINE@@GTTCTGGATTTCATAGACTTGG-3′, containing a BamHI site, and reverse primer: 5′-TCGAGTGCGGCCGC@@UAAGCTTLINE@@CGAAGTCCATTCGCCCCACGTT-3′, containing a HindIII site. The truncated NcMIC26 gene was amplified with 2 × ExTaq Mix polymerase (TransGen Biotech Co., Ltd., China), and PCR was performed using the following procedure: 95°C for 5 min; followed by 35 cycles at 95°C for 30 s, 57°C for 1 min, and 72°C for 1 min; and extension at 72°C for 10 min.
The resultant PCR products were ligated into the pET-28a expression vector backbone after BamHI and HindIII double enzymatic digestion. The insert sequences were sequenced and aligned to the NcMIC26 gene sequence reported previously (19). The recombinant plasmid was named pET-28a-NcMIC26.
The recombinant plasmid was transformed into Escherichia coli Transetta (DE3). Expressed as a His tag fusion protein following induction with 0.8 mM IPTG for 6 h at 37°C, rNcMIC26 was purified by column chromatography using Ni-NTA Superflow columns and stored at −80°C until use.
NcMIC26 Subcellular Localization
NcMIC26 endogenous epitope tags were generated as described previously (20). Briefly, the plasmid pNc_Cas9CRISPR::sgNcMIC26 and the plasmid pLIC-HA-DHFR-NcMIC26 (a template of homologous repair amplicon) were constructed, and the primers are listed in Table S1. Then, the parental Nc1 strain was cotransfected with the plasmid pNc_Cas9CRISPR::sgNcMIC26, and a linearized homologous repair was completed. The transgenic parasites were grown under pyrimethamine (1 μM) selection pressure to the third generation and then screened to confirm the purity of the selected strains. The selected strain was named NcMIC26-HA.
IFAT was used for NcMIC26 subcellular localization in parasites as previously described (20). Briefly, 1 × 105 N. caninum tachyzoites were seeded onto HFFs that were already arranged on glass coverslips in 12-well-plates (Corning costar, USA). Infected cells were incubated at 37°C with 5% CO2 for 30 h, fixed for 30 min in 4% formaldehyde, permeabilized with 0.25% Triton X-100 for 15 min, and then blocked with 3% bovine serum albumin (BSA) for 30 min. Subsequently, the cells were incubated with a mouse anti-HA monoclonal antibody (1:50, Sigma-Aldrich) and a rabbit antiNcSRS2 polyclonal antibody [1:500, (20)], the primary antibodies were detected with FITC-conjugated goat-anti mouse IgG (H + L) (1:100, Sigma, USA) and Cy3-conjugated goat-anti rabbit IgG (H + L) (1:100, Sigma, USA), respectively, and the nuclear DNA was stained with DAPI (1:200, Sigma, USA). Finally, a Leica confocal microscope system (Leica, TCS SP52, Germany) was used to obtain images.
Specificity Analysis
The expression and purity of recombinant protein were analyzed by SDS-PAGE electrophoresis and visualized by Coomassie blue staining. Besides, SDS-PAGE and western blots were used to confirm the reactogenicity and antigenic specificity of N. caninum. Western blots were followed as described above; rNcMIC26 was subjected to electrophoresis and transferred electrophoretically onto a PVDF membrane. After blocking, the blots were incubated with mouse polyclonal antiserum against N. caninum (1:400), mouse N. caninum-negative serum (1:400), mouse polyclonal antiserum against T. gondii (1:400), and mouse anti-His monoclonal antibody (1:500) for 1 h at 37°C. After washing, the cells were incubated with a horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (H + L) secondary antibody (1:5,000).
Indirect ELISA
Indirect ELISA tests based on the purified recombinant protein NcMIC26 were developed as previously described (21). Optimal dilutions of the antigen and bovine sera were determined by checkerboard titration. N. caninum-positive and negative sera samples were employed to each assay. We diluted the His-fused rNcMIC26 in a coating buffer (0.05 M carbonate–bicarbonate buffer, pH 9.6) to a final concentration of 1 μg/well, added it to 96-well flat-bottom plates (Guangzhou Jet Bio-filtration Co., Ltd., China), and incubated it at 37°C for 1 h and then at 4°C overnight. After four washes with a washing buffer (PBS containing 0.1% Tween 20), the plates were blocked with a blocking buffer (PBS containing 5% horse serum) at 37°C for 1 h. The plates were washed four times, the cattle sera were diluted in a diluent solution (PBS containing 2% horse serum, 1:200), and 100 μl was added to each of the duplicate wells of the ELISA plate and incubated for 1 h at 37°C. The plates were rinsed as before and incubated with the HRP-conjugated goat anti-bovine IgG antibody (Southern Biotechnology Associates, Inc., USA) diluted in a diluent solution (1:25,000) at 100 μl/well for 0.5 h at 37°C. Finally, the plate was rinsed, and bound antibodies were detected by incubation with a 100 μl/well tetramethylbenzidine (TMB) substrate (M&C Gene Technology Co., Ltd., China) and color rendering at room temperature for 10 min. The reaction was stopped with a stop solution (2 M sulfuric acid, 50 μl/well), and the absorbance was measured at 450/630 nm in an ELISA plate reader (Bio-Rad). Every experiment was repeated three times. The cutoff point was determined as the mean OD450/630nm for N. caninum-negative sera kept in our laboratory (n = 30) plus three standard deviations. The positive antibody of N. caninum was found in samples whose OD value ≥ cutoff point, and the sample OD value < cutoff point was negative for N. caninum.
Data Analysis
The sera used in this study were as follows: gold-standard panel sera consisting of IFAT-defined negative sera (n = 39) and IFAT-defined positive sera (n = 98) from cattle. The number of positive or negative sera samples of the two diagnostic methods was counted manually. Statistical analysis of the data was performed using SPSS 22. Kappa coefficient was used to evaluate the level of agreement between ELISA methods (based on NcSRS2 or NcMIC26) and the gold standard (IFAT). The specificity and sensitivity of detecting N. caninum serum antibodies by ELISA were determined using the following formulas: sensitivity = (number of ELISA test-positive sera/number of IFAT test-positive sera) × 100%; specificity = number of ELISA test-negative sera/number of IFAT test-negative sera) × 100%). Indirect ELISA methods based on the rNcMIC26 and rNcSRS2 (5) antigens were used to detect these sera, and the relevant data were compared and analyzed.
Ethics Statement
The experiments with animals in this study were performed strictly according to the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of China and approved by the Institutional Animal Care and Use Committee of China Agricultural University (under the certificate of Beijing Laboratory Animal employee ID: CAU20161210-2).
Results
Identified NcMIC26 From ESA of N. caninum
The collected ESA proteins were separated by SDS-PAGE, and western blot was used to identify antigen reactivity and specificity (Figure 1). Three specific protein bands (50–70 kDa from Figure 1A; 14–30, 50–70 kDa from Figure 1B) of N. caninum showed no reaction with T. gondii-positive serum. The tryptic peptides were analyzed by LC-MS/MS, corresponding to a total of 121 proteins after the appropriate cutoff filters were applied to the results (Table 1). First, we excluded proteins that were inconsistent with their corresponding band mass. Then, we mainly screened for proteins related to the three major secretory organelles and, eventually, determined NcMIC26 to be a candidate antigen of N. caninum, which was present in the tachyzoite-secreted fraction ESA (50–70 kDa from Figure 1A).
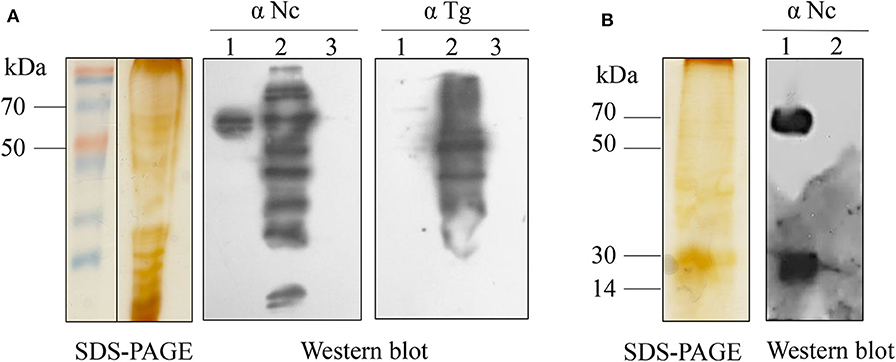
Figure 1. SDS-PAGE and western blot analyses of N. caninum ESAs. (A) SDS-PAGE and western blot analyses of N. caninum tachyzoite-secreted fraction ESAs. Lane 1, N. caninum tachyzoite-secreted fraction ESA; lane 2, N. caninum tachyzoite lysate; lane 3, DMEM control. Western blot using anti-N. caninum serum (1:400) or anti-T. gondii serum (1:400). (B) SDS-PAGE and western blot analyses of N. caninum tachyzoite-secreted protein for intracellular culture. Lane 1, N. caninum tachyzoite secreted protein from intracellular culture; lane 2, Vero cell control. Western blot using anti-N. caninum serum (1:400).
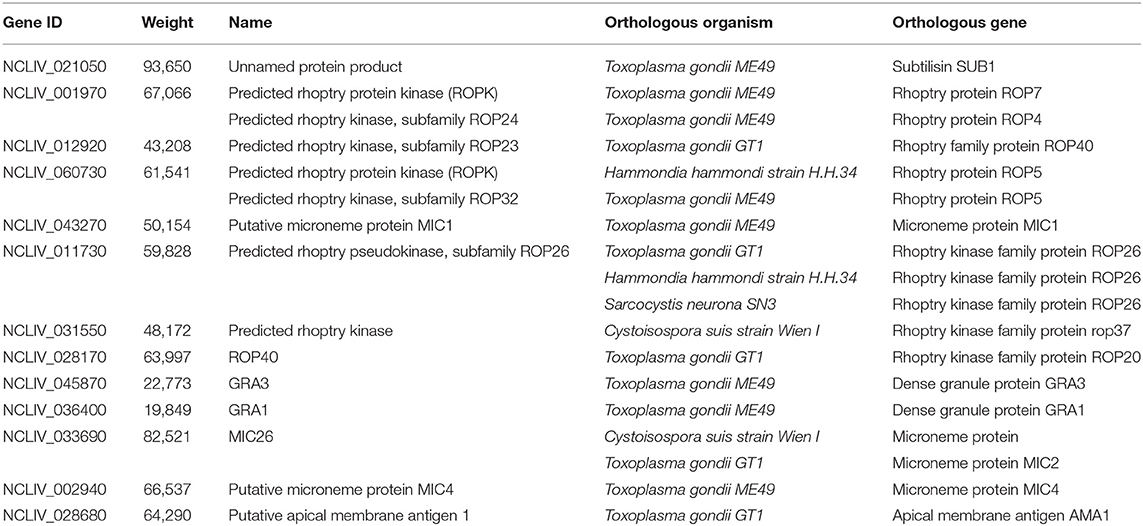
The gene sequence and the amino acid sequence of N. caninum NcMIC26 were obtained from ToxoDB (http://toxodb.org/toxo/; Gene ID: NCLIV_033690). The full-length NcMIC26 protein is composed of 756 amino acids, and a hydrophobic region at the N-terminus has characteristics of a signal peptide (1–33 amino acid). The mature protein has a predicted molecular weight of 80 kDa. Two putative transmembrane spanning helixes were found between amino acid residues 12–34 and 687–709, near the N-terminus and C-terminus of NcMIC26, respectively. In addition, the protein contains a Von Willebrand factor type A (vWA) domain (67–248) and five thrombospondin type 1 (TSP-1) repeat regions (261–325, 330–388, 394–449, 454–510, and 516–575). The NcMIC26 amino acid sequence aligned to other similar Apicomplexa proteins; the deduced NcMIC26 amino acid sequence was 45% identical to that of N. canimum MIC2 (NCLIV_022970), 44% identical to that of T. gondii MIC2 (TGGT1_201780), 59% identical to that of Cystoisospora suis microneme protein (CSUI_022748), and 52.76% identical to that of Sarcocystis neurona SO SN1 syntenic protein (SRCN_7088).
Recombinant NcMIC26 Had No Cross-Reaction With T. gondii-Positive Serum
The coding sequence of the 744 bp truncated NcMIC26 gene encoding a target protein of 248 amino acids was inserted into the bacterial expression vector pET-28a and expressed as a His fusion protein in E. coli, with a predicted molecular mass of 35 kDa. The recombinant NcMIC26 was expressed mainly as inclusion bodies (Figure 2A). The molecular mass of the purified recombinant protein was 35 kDa, as expected. Western blot analysis showed that rNcMIC26 reacted strongly with N. caninum-positive serum and had no reaction with T. gondii-positive serum (Figure 2B). This result indicates that the recombinant protein rNcMIC26 has strong reactivity and that rNcMIC26 does not react with T. gondii-positive serum, possibly making it a candidate diagnostic antigen for neosporosis.
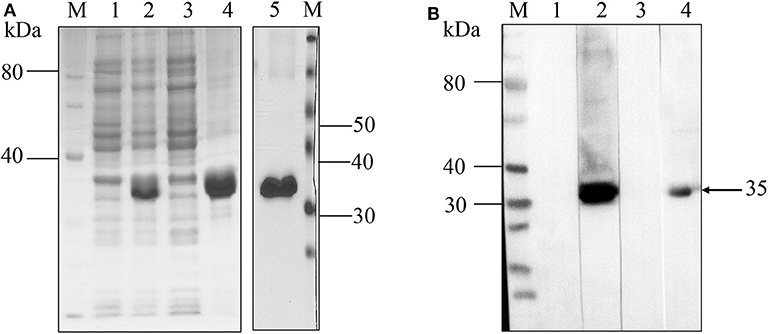
Figure 2. SDS-PAGE and western blot analyses of recombinant NcMIC26 protein. (A) Expression and purification of rNcMIC26. Recombinant protein expression patterns were analyzed by SDS-PAGE and visualized by Coomassie blue staining. M: protein marker; lane 1, uninduced protein; lane 2, induced protein; lane 3, supernatant; lane 4: inclusion bodies; lane 5, recombinant protein purified by Ni-NTA Superflow columns. (B) Western blotting was used to confirm the reactogenicity and antigenic specificity of N. caninum. M: protein marker; lanes 1–4 loading sample: purified rNcMIC26. The incubated antibodies were as follows: lane 1, non-infected mouse serum (1:400); lane 2, mouse N. caninum-positive serum (1:400); lane 3, mouse T. gondii-positive serum (1:400); lane 4, mouse anti-His monoclonal antibody (1:500); and HRP-labeled goat anti-mouse IgG (H + L) secondary antibody (1:5,000).
NcMIC26 Localized on Micronemes
To localize NcMIC26, NcMIC26 was fused with a triple hemagglutinin (3 × HA) epitope tag in the C-terminus by single homologous recombination (Figure 3A). NcMIC26 subcellular localization was visualized by immunofluorescent staining using a mouse anti-HA monoclonal antibody (1:50, Sigma-Aldrich) and rabbit anti-NcSRS2 polyclonal antibody (1:500) (20).
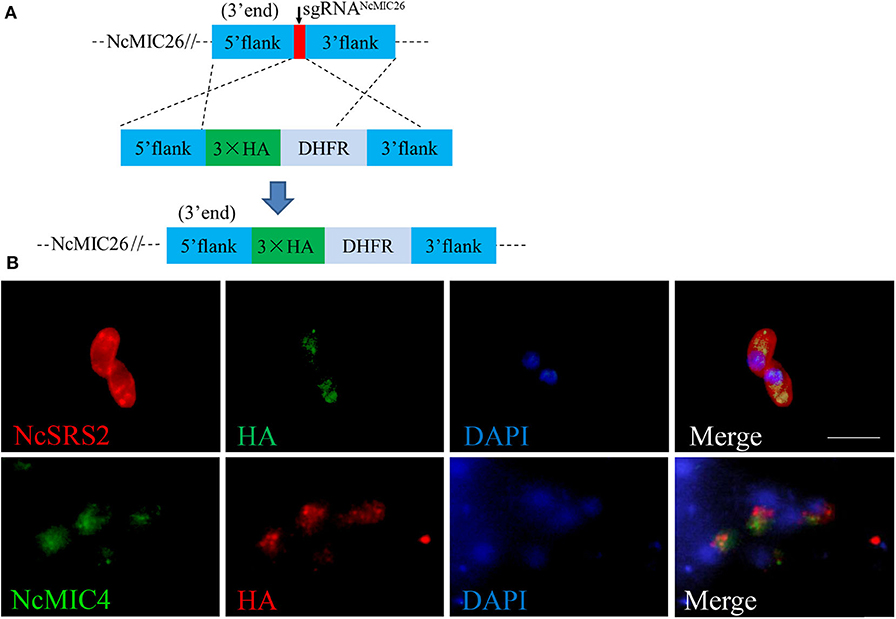
Figure 3. Characterization of NcMIC26 in N. caninum. (A) Schematic diagram showing NcMIC26 with an HA tag at its endogenous locus. (B) Localization of NcMIC26. NcMIC26, stained with mouse anti-HA antibody, was distributed in the microneme of NcMIC26-HA parasites. NcSRS2 was used as a marker to indicate the outlines of parasites; NcMIC4 was used as a marker to indicate the microneme of parasites. Visualized with Cy3-conjugated goat-anti-rabbit IgG (H + L) and FITC-conjugated goat-antimouse IgG (H + L), and nuclear DNA was stained with DAPI (blue). Scale bar, 5 μm.
The anti-HA-labeled parasites showed that NcMIC26 was distributed in the micronemes of parasites (Figure 3B) and partially colocalized with another microneme marker, NcMIC4 (22), suggesting that NcMIC26 is a microneme protein in N. caninum.
Diagnosis of N. caninum Infection in Cattle by ELISA With rNcMIC26
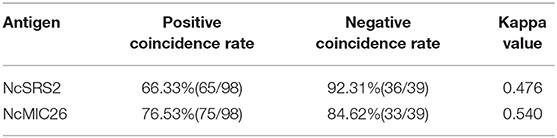
Ninety-eight samples of N. caninum-positive and 39 samples of N. caninum-negative bovine serum were defined by IFAT and evaluated by indirect ELISA based on the rNcMIC26 and rSRS2 antigens; OD values were listed in Table S2. The sensitivity and specificity of the ELISA were evaluated. The results are shown in Table 2. Compared with that of IFAT, the positive coincidence rate (sensitivity) of the ELISA based on rNcMIC26 (cutoff = 0.150) was 76.53% (75/98), and the negative coincidence rate (specificity) was 84.62% (33/39), while those of the ELISA based on rNcSRS2 (cutoff = 0.178) were 66.33% (65/98) and 92.31% (36/39), respectively. The sensitivity of the ELISA based on NcMIC26 was better than that of the ELISA based on NcSRS2. In addition, compared with NcSRS2 (kappa = 0.476), the NcMIC26-based ELISA test (kappa = 0.540) was more in agreement with the IFAT test through the calculation of the kappa value.
Afterward, a total of 137 serum samples were analyzed statistically. As shown in Table 3, the diagnosis results for bovine serum were not consistent. It is interesting to note that of the 98 N. caninum-positive bovine serum samples, 17 samples were confirmed as positive only by the ELISA based on rNcSRS2, while they were confirmed as negative by the ELISA based on rNcMIC26, and 30 samples were confirmed as positive by the ELISA based on rNcMIC26, while they were confirmed as negative by the ELISA based on rNcSRS2.
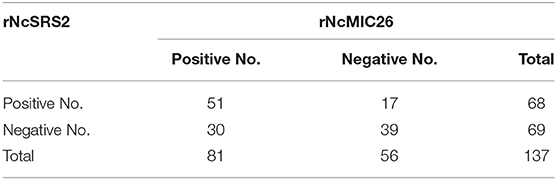
Table 3. Comparison between the ELISA based on rNcMIC26t and rSRS2 for the diagnosis of neosporosis in cattle.
Discussion
N. caninum is a recently recognized protozoan parasite. Until 1988 (23), it was misdiagnosed as T. gondii. It is structurally, antigenically, and molecularly related to T. gondii, but these organisms are biologically distinct. One of the parameters to be evaluated is diagnostic specificity, that is, the proportion of test negatives among all animals that are true negative. Cross-reactivities among N. caninum and T. gondii have been reported (24). An increasing number of proteins have been identified as cross-antigens between the two closely related parasites, for example, MIC3 and AMA1 (25), affecting the diagnostic specificity of serological tests. Therefore, it is urgent to screen new dominant antigens with improved diagnostic abilities and to establish more sensitive and specific serological diagnostic methods.
In this study, a new diagnostic antigen, NcMIC26, from N. caninum tachyzoite ESA was screened. This antigen is composed of a Von Willebrand figure type A (vWA) domain and five thrombospondin type 1 (TSP-1) repeat regions. Since its identification, the vWA domain has drawn incredible interest because of its far-reaching effects and its association in a wide assortment of vital cellular functions. In T. gondii, the VWA domain likely intervenes in the protein–protein interaction of these proteins with their binding partners, which plays a pivotal part in cell adhesion and intrusion by interceding gliding motility (26). Within the TSP repeat region, several motifs are present that have been implicated in cell binding (27). For most MICs, secretion is started in vitro before parasites initiate egress from the host cells. Sera from actually infected cattle recognized an overwhelming protein band with an atomic mass indistinguishable from that of NcMIC26 in N. caninum ESA. However, it is possible that there are some proteins from the lysis of parasites in the detected proteins. To avoid this, we have selected the proteins from the three major secretory organelles (microneme, rhoptry, and dense granule) and detected its secretion ability in the following screening process. Finally, NcMIC26, which contains signal peptide and a transmembrane region, was confirmed as a secreted protein by secretion assays (data were not shown). In addition to the affirmation that the bovine antiserum recognized rNcMIC26, the results of the current study suggest that the bovine antisera against the whole parasite recognized NcMIC26 as an immunodominant antigen.
To assess whether recombinant NcMIC26 can be an appropriate antigen for the diagnosis of N. caninum disease in cattle, purified recombinant NcMIC26 was assessed in an ELISA. IFAT and the rNcSRS2-based ELISA were used as the comparison test. IFATs are based on intaglio tachyzoites and are respected, among others, as reference serological tests (“gold-standard tests”) (28). NcSRS2 is an immunodominant surface protein that is displayed within the bradyzoites and tachyzoites of N. caninum (29), and empowers specific serological diagnosis of neosporosis. These results demonstrated that the recombinant NcMIC26 expressed in E. coli ought to be a valuable diagnostic reagent for the detection of antibodies to N. caninum in cattle. Moreover, our detection information reflects a substantial discrepancy in the overall serum assessment determined by ELISA based on NcSRS2 or NcMIC26. The sensitivity of the ELISA based on NcMIC26 was better than that of the ELISA based on NcSRS2, and a considerable portion of N. caninum-positive serum can recognize only one antigen (only NcSRS2 or only NcMIC26). Using single antigens in ELISAs is insufficient to examine simultaneously the antibody response against different antigens to which a host is differentially exposed depending on the stage of infection (i.e., the acute or chronic stage). Our data indicated that the ELISA test utilizing NcMIC26 could be used as a supplementary antigen for missed detection by NcSRS2 to improve N. caninum diagnosis, while further study should focus on using both antigens in the same ELISA to prove the advantage. On the other hand, compared with that of IFAT, the positive coincidence rate of the ELISA based on rNcSRS2 (66.33%) in our research may be lower than that in other investigations (30). Considering that our serum samples are from cattle that were naturally infected with N. caninum, the stage of infection with N. caninum was inconsistent, and the antibody titers may be diverse.
Conclusion
In this study, we screened a new microneme protein 26 (NcMIC26) from the excretory secretion antigen (ESA) of N. caninum tachyzoites. This study characterized NcMIC26 as an effective microneme protein that is recognized by the sera of N. caninum-infected animal hosts. The ELISA specific to NcMIC26 established in this study can aid in a more conclusive determination of N. caninum infection in cattle. Ensuing studies will be vital to extending the detection affectability of the assay, and more importantly, it can also be utilized as a supplementary antigen for missed discovery by SRS2. The combination of these two antigens may be considered to obtain more accurate detection information in clinical diagnosis.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
JL conceived the project. XW performed the experiments and drafted the manuscript. JL and QL participated in the design of the study and helped to draft the manuscript. XW, JY, and XS participated in the interpretation of the data. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (2017YFD0501200), the National Natural Science Foundation of China (31772730 and 31972700), and the National Key Basic Research Program (973 program) of China (2015CB150300).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.00357/full#supplementary-material
Table S1. Primers of constructed plasmids pNc_Cas9CRISPR::sgNcMIC26 and pLIC-HA-DHFR-NcMIC26.
Table S2. The data of tested samples for indirect ELISA based on the rNcMIC26 and rNcSRS2.
Abbreviations
N. caninum, Neospora caninum; T. gondii, Toxoplasma gondii; E. coli, Escherichia coli; ESA, excretory secretion antigen; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; LC-MS/MS, liquid chromatography mass spectrometer; WB, western blotting; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; IFAT, indirect immunofluorescent antibody test; IPTG, isopropyl-β-d-thiogalactoside; MIC, microneme protein; SRS, surface antigen related protein; SAG, surface antigen; GRA, dense granule protein; DHFR, dihydrofolatereductase; HRP, horseradish peroxidase; FITC, fluorescein isothiocyanate.
References
1. McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. (1998) 28:1473–8. doi: 10.1016/S0020-7519(98)00138-6
2. Dubey JP, Schares G. Neosporosis in animals–the last five years. Vet Parasitol. (2011) 180:90–108. doi: 10.1016/j.vetpar.2011.05.031
3. Waldner CL, Janzen ED, Henderson J, Haines DM. Outbreak of abortion associated with Neospora caninum infection in a beef herd. J Am Vet Med Assoc. (1999) 215:1485–90:1448–9.
4. Chahan B, Gaturaga I, Huang X, Liao M, Fukumoto S, Hirata H, et al. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet Parasitol. (2003) 118:177–85. doi: 10.1016/j.vetpar.2003.10.010
5. Liu J, Yu J, Wang M, Liu Q, Zhang W, Deng C, et al. Serodiagnosis of Neospora caninum infection in cattle using a recombinant tNcSRS2 protein-based ELISA. Vet Parasitol. (2007) 143:358–63. doi: 10.1016/j.vetpar.2006.08.034
6. He P, Li J, Gong P, Liu C, Zhang G, Yang J, et al. Neospora caninum surface antigen (p40) is a potential diagnostic marker for cattle neosporosis. Parasitol Res. (2013) 112:2117–20. doi: 10.1007/s00436-013-3309-3
7. Hu J, Ferroglio E, Trisciuoglio A. Immunoblot diagnosis of infection with Neospora caninum in cattle based on recombinant NcSAG4 Antigen. Parasitol Res. (2011) 108:1055–8. doi: 10.1007/s00436-011-2286-7
8. Jimenez-Ruiz E, Bech-Sabat G, Alvarez-Garcia G, Regidor-Cerrillo J, Hinojal-Campana L, Ortega-Mora LM. Specific antibody responses against Neospora caninum recombinant rNcGRA7, rNcSAG4, rNcBSR4 and rNcSRS9 proteins are correlated with virulence in mice. Parasitology. (2013) 140:569–79. doi: 10.1017/S0031182012002041
9. Jin C, Yu L, Wang Y, Hu S, Zhang S. Evaluation of Neospora caninum truncated dense granule protein 2 for serodiagnosis by enzyme-linked immunosorbent assay in dogs. Exp Parasitol. (2015) 157:88–91. doi: 10.1016/j.exppara.2015.07.003
10. Liddell S, Lally NC, Jenkins MC, Dubey JP. Isolation of the cDNA encoding a dense granule associated antigen (NCDG2) of Neospora caninum. Mol Biochem Parasitol. (1998) 93:153–8. doi: 10.1016/S0166-6851(98)00031-0
11. Hamidinejat H, Seifi ASM, Namavari MM, Shayan P, Kefayat M. Development of an indirect ELISA using different fragments of recombinant Ncgra7 for detection of Neospora caninum infection in cattle and water buffalo. Iran J Parasitol. (2015) 10:69–77.
12. Yin J, Qu G, Cao L, Li Q, Fetterer R, Feng X, et al. Characterization of Neospora caninum microneme protein 10 (NcMIC10) and its potential use as a diagnostic marker for neosporosis. Vet Parasitol. (2012) 187:28–35. doi: 10.1016/j.vetpar.2012.01.003
13. Hiasa J, Nishimura M, Itamoto K, Xuan X, Inokuma H, Nishikawa Y. Enzyme-linked immunosorbent assays based on Neospora caninum dense granule protein 7 and profilin for estimating the stage of neosporosis. Clin Vaccine Immunol. (2012) 19:411–7. doi: 10.1128/CVI.05669-11
14. Alvarez-Garcia G, Garcia-Culebras A, Gutierrez-Exposito D, Navarro-Lozano V, Pastor-Fernandez I, Ortega-Mora LM. Serological diagnosis of bovine neosporosis: a comparative study of commercially available ELISA tests. Vet Parasitol. (2013) 198:85–95. doi: 10.1016/j.vetpar.2013.07.033
15. Santos JM, Soldati-Favre D. Invasion factors are coupled to key signalling events leading to the establishment of infection in apicomplexan parasites. Cell Microbiol. (2011) 13:787–96. doi: 10.1111/j.1462-5822.2011.01585.x
16. Pollo-Oliveira L, Post H, Acencio ML, Lemke N, van den Toorn H, Tragante V, et al. Unravelling the Neospora caninum secretome through the secreted fraction (ESA) and quantification of the discharged tachyzoite using high-resolution mass spectrometry-based proteomics. Parasit Vectors. (2013) 6:335. doi: 10.1186/1756-3305-6-335
17. Norouzpour Deilami K, Daryani A, Ahmadpour E, Sharif M, Dadimoghaddam Y, Sarvi S, et al. Excretory-secretory antigens: a suitable candidate for immunization against ocular toxoplasmosis in a murine model. Comp Immunol Microbiol Infect Dis. (2014) 37:369–74. doi: 10.1016/j.cimid.2014.10.003
18. Yang D, Liu J, Hao P, Wang J, Lei T, Shan D, et al. MIC3, a novel cross-protective antigen expressed in Toxoplasma gondii and Neospora caninum. Parasitol Res. (2015) 114:3791–9. doi: 10.1007/s00436-015-4609-6
19. Reid AJ, Vermont SJ, Cotton JA, Harris D, Hill-Cawthorne GA, Konen-Waisman S, et al. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLos Pathog. (2012) 8:e1002567. doi: 10.1371/journal.ppat.1002567
20. Yang C, Liu J, Ma L, Zhang X, Zhang X, Zhou B, et al. NcGRA17 is an important regulator of parasitophorous vacuole morphology and pathogenicity of Neospora caninum. Vet Parasitol. (2018) 264:26–34. doi: 10.1016/j.vetpar.2018.03.018
21. Dong J, Otsuki T, Kato T, Park EY. Development of a diagnostic method for neosporosis in cattle using recombinant Neospora caninum proteins. BMC Biotechnol. (2012). 12:19. doi: 10.1186/1472-6750-12-19
22. Li W, Liu J, Wang J, Fu Y, Nan H, Liu Q. Identification and characterization of a microneme protein (NcMIC6) in Neospora caninum. Parasitol Res. (2015) 114:2893–902 doi: 10.1007/s00436-015-4490-3
23. Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. (1988) 192:1269–85.
24. Zhang H, Lee EG, Yu L, Kawano S, Huang P, Liao M, et al. Identification of the cross-reactive and species-specific antigens between Neospora caninum and Toxoplasma gondii tachyzoites by a proteomics approach. Parasitol Res. (2011) 109:899–911. doi: 10.1007/s00436-011-2332-5
25. Zhang H, Compaore MK, Lee EG, Liao M, Zhang G, Sugimoto C, et al. Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol Biochem Parasitol. (2007) 151:205–12. doi: 10.1016/j.molbiopara.2006.11.005
26. Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. (2004) 14:528–32. doi: 10.1016/j.tcb.2004.08.002
27. Lovett JL, Howe DK, Sibley LD. Molecular characterization of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol Biochem Parasitol. (2000) 107:33–43. doi: 10.1016/S0166-6851(99)00228-5
28. Conrad PA, Sverlow K, Anderson M, Rowe J, BonDurant R, Tuter G, et al. Detection of serum antibody responses in cattle with natural or experimental Neospora infections. J Vet Diagn Invest. (1993) 5:572–8. doi: 10.1177/104063879300500412
29. Fuchs N, Sonda S, Gottstein B, Hemphill A. Differential expression of cell surface- and dense granule-associated Neospora caninum proteins in tachyzoites and bradyzoites. J Parasitol. (1998) 84:753–8. doi: 10.2307/3284583
Keywords: Neospora caninum, ESA, microneme protein 26, ELISA, cattle
Citation: Wang X, Song X, Yang J, Liu Q and Liu J (2020) Characterization of Neospora Caninum Microneme Protein 26 and Its Potential Use as a Diagnostic Marker for Neosporosis in Cattle. Front. Vet. Sci. 7:357. doi: 10.3389/fvets.2020.00357
Received: 28 January 2020; Accepted: 22 May 2020;
Published: 17 July 2020.
Edited by:
Annunziata Giangaspero, University of Foggia, ItalyReviewed by:
Ezio Ferroglio, University of Turin, ItalyYoshifumi Nishikawa, Obihiro University of Agriculture and Veterinary Medicine, Japan
Andrew Hemphill, University of Bern, Switzerland
Copyright © 2020 Wang, Song, Yang, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu, bGl1amluZ3ZldEBjYXUuZWR1LmNu
 Xianmei Wang1
Xianmei Wang1 Qun Liu
Qun Liu Jing Liu
Jing Liu