- 1Department of Parasitology, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt
- 2Department of Medical Parasitology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
- 3Department of Zoonoses, Faculty of Veterinary Medicine, Benha University, Benha, Egypt
- 4Department of Animal Medicine, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
- 5Department of Biotechnology, College of Science, Taif University, Taif, Saudi Arabia
- 6Department of Parasitology, Faculty of Medicine, Assiut University, Assiut, Egypt
- 7Animal Health Research Institute, Assiut, Egypt
- 8Department of Parasitology, Faculty of Veterinary Medicine, Sohag University, Sohag, Egypt
- 9Department of Hygiene and Zoonoses, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
- 10Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Sohag, Egypt
Cystic echinococcosis has been considered one of the major parasitic zoonoses which is associated with severe economic losses. The present study was undertaken to investigate the occurrence, organ distribution, cyst fertility, and viability of cystic echinococcosis in slaughtered camels and cattle from various abattoirs in Assiut Governorate, Egypt. The work also involved morphological, morphometric, and molecular identification of the parasite. The occurrence of hydatid cysts was investigated in total number of 100 lungs of camels and 574 liver and lungs of cattle admitted to three slaughterhouses at Assiut Governorate, Egypt. Moreover, several individual variable factors, including organ involvement, age, sex, and hydatid cyst characteristics, were studied to identify their possible association with the occurrence of the disease. Genomic DNA was extracted from the hydatid cysts, followed by molecular identification of the parasite through amplification of ribosomal DNA internal transcribed spacer (ITS) regions. Hydatid cysts were found in 6 camels (6%) out of 100 inspected camels, while 5 hydatid cysts (0.87%) were detected in a total number of 574 cattle examined. The parasite was detected exclusively in lungs of camels, while lungs were the main organ infected by the parasite in cattle and one hydatid cyst was found in the liver (0.17%). In camel, 66.7, 16.65, and 16.65%of detected cysts were fertile, sterile, and calcified, respectively, while in cattle, these percentages were 60, 20, and 20%, respectively. None of the studied variable factors were significantly associated with the occurrence of the disease in camels, with the exception that all cysts were found in the lung. Conversely, we found a significant association (P < 0.05) between the age and sex of the slaughtered cattle and the occurrence of hydatid cysts. In this respect, the rate of infection was higher in female cattle and those cattle more than 5 years (P < 0.05). The morphological, morphometric, and molecular studies confirmed the presence of the parasite. Taken together, our results concluded that camels and cattle play a potential role in maintaining the transmission cycle of this zoonotic parasite.
Introduction
Cystic echinococcosis (CE) is a parasitic zoonotic disease with a worldwide distribution, particularly in developing countries (1–4). This disease is caused by a tapeworm of the species Echinococcus granulosus (EG) complex that has several distinct genotypes (5). The adult tapeworm inhabits the intestine of the definitive hosts, i.e., carnivores, who contract the infection through the consumption of the viscera of the intermediate hosts (3). The epidemiological profile of the disease includes human hosts and a wide range of wild and domestic hosts, including cattle, sheep, goats, and camels, which serve as intermediate hosts (3, 6). Carnivores then contaminate the environment, resulting in the spread of the infection, while human and other intermediate hosts contract the infection from fecal matter via oral routes and/or indirect contact with canines (3). In humans and other intermediate hosts, the larval stage of the parasite can reside and grow in the liver and lung, but rarely in other visceral organs (7). The pathogenesis of the disease and its clinical impact depend on the severity of the infection and the organ involved and the occasional rupture of hydatid cysts might lead to sudden death resulting from anaphylaxis, hemorrhage, and metastasis (8, 9). It is therefore not surprising to state that CE might represent a life-threatening disease in humans if left untreated, as it affects around one million people worldwide (10, 11). In addition, CE has been ranked as the second most significant helminthic disease associated with serious global economic losses, besides being a cosmopolitan disease (12–15). These huge economic losses result from the waste of animal protein because the edible organs are deemed unfit for human consumption (16, 17). According to the World Health Organization (WHO), echinococcosis results in around 19,300 deaths and 871,000 disability-adjusted life-years (18).
In accordance with its distribution, several previous studies revealed the endemicity of the disease in Egypt and neighboring countries of the Mediterranean basin and Middle East (19–30). The prevalence rates varied greatly among intermediate hosts of various species because of the influence of a variety of environmental factors and hygienic conditions (25), which is potentiated by the presence of infected stray dogs that are mostly used for guarding purposes or by the ease access of these dogs to slaughter houses (25, 29).
One of the most important strategies used for controlling the disease is accurate detection. The role of morphology criteria in the differentiation of the taxa of E. granulosus has been documented repeatedly (31–34). Several trials have been performed using light microscopy and, more recently, scanning electron microscopy (SEM) in detection of the parasite (34, 35). Light microscopy was reported as a valid method for identifying E. granulosus strains in several previous studies based on its larval hook morphology (33, 35). However, other studies have considered that its morphological identification based solely on ordinary microscopy is insufficient to differentiate the various strains of E. granulosus (34, 36–38). In this respect, SEM provides many advantages over light microscopy and facilitates the visualization and measurements of the large and small larval rostellar hooks, particularly in some countries where molecular studies cannot be performed (39, 40). Polymerase chain reaction (PCR) is a widely accepted molecular tool for epidemiological studies aimed at identifying and quantifying Echinococcus spp. in various tissues and body fluids, either in reservoirs or hosts (41–44). To our knowledge, E. granulosus is an assemblage of cryptic species that differ in morphology, host specificity, pathogenicity, and mitochondrial and nuclear genomes, making the taxonomy of this parasites is challenging issue (45). Several molecular approaches that can distinguish the genotypes of E. granulosus revealed that they are associated with distinct intermediate hosts, including sheep, goats, pigs, horses, cattle, camels, and cervids (5, 46, 47). Based on the most recent molecular phylogeny of the six nuclear genes and mitochondrial genomes, E. granulosus sensu lato comprises 5 species and 8 genotypes that represent intraspecific variants (48, 49). According to these analyses, G2 genotype has a variant of G3, while G6-G7 and G8-G10 genotypes are considered as distinct species. It seems that this great diversity has led to phylogenetic differences and affinities within the same genus. Therefore, it is not surprising to find that various hypotheses regarding the origin and geographic dispersal of the causative agents of CE have been reported (45).
Providing periodical updates on the available epidemiological data of the disease through field surveys performed for surveillance purposes, together with the investigation of the genotypes of the E. granulosus strains circulating in a given endemic area, may be crucial for the development of vaccines, diagnostic tests, and control strategies targeting hydatid disease (45, 50). Given the fact that limited information is available about the real contribution of camel and cattle in transmission of the disease in Upper Egypt, the present study was undertaken to investigate the occurrence, organ distribution, cyst fertility, and viability of CE in slaughtered camel and cattle from various abattoirs in Assiut Governorate, Egypt, followed by the morphological, morphometric, and molecular identification of the parasite.
Materials and Methods
Ethical Considerations
The study protocol was approved by the local guidance of Research, Publication and Ethics Committee of the Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt, which complies with all relevant Egyptian legislations in publication and research. The ethical approval number is KFS-2015/1.
Study Area and Sample Collection and Preparation
The present study was conducted to determine the occurrence of CE in camels (N = 100) and cattle (N = 574) slaughtered during the period of October 2015 to December 2017. The animals were admitted to different abattoirs (N = 3) in Assiut Governorate (Assiut, Bani-Adi, and Dairout abattoirs). Assiut Governorate is located in Upper Egypt (latitude, 27° 10′ 48.4824″ N; and longitude 31° 11′ 21.4188 ″W). The liver and lungs of camels and cattle were examined for the detection of hydatid cysts through visual inspection, palpation, and systematic incision of each lung (51). Any hydatid cysts obtained from the lungs and liver of each species were collected during the postmortem examination; then extracted, counted, and carefully removed during evisceration by dissection; and finally placed into sterile flasks (thermo flasks) and transported to the laboratory at the Department of Medical Parasitology, Faculty of Medicine, Assiut University, for the experimental work. The methodology included the morphological and microscopic identification of the hydatid cysts, together with sending samples of parasite material to the Molecular Biology Unit, Assiut University, Assiut, Egypt, for further examination and molecular analysis. The samples used for morphological and microscopic identification were preserved in 10% formalin and stored in closed containers at 4°C, while those used for molecular identification were kept in clean sterile bottles containing 70% ethanol (52).
Morphological and Microscopic Examination of the Hydatid Cysts
The hydatid cysts were subjected to morphological examination to check their shape, size, viability, and condition according to a protocol described elsewhere (53, 54). The condition of the cysts was classified into three categories, as follows: fertile hydatid cysts, containing protoscoleces and/or daughter cysts; sterile hydatid cysts, full of fluid but without protoscoleces; and calcified hydatid cysts, with a tough thickened wall and absence of protoscoleces or fluid (53, 54). The hydatid fluid of each cyst was aspirated using 21 gauge needle. The collected fluid was centrifuged at 252 g for 5 min. The last drops of the sediment were then transferred to a slide, mounted with a glass cover slip, and observed under a microscope for the presence of protoscoleces, brood capsules, and taeniid hooks. When scolices could not be detected, the whole cyst was opened in a Petri dish, in which the fluid and germinal layer scrapings were examined for the presence of protoscoleces or brood capsules. Microscopic examination of the cyst fluid was performed to look for viable protoscoleces after dropping 0.1% eosin into the fluid. The specimens of hydatid cysts were also processed and prepared for SEM according to the protocol described elsewhere (40). Briefly, the hydatid fluid was aspirated from 3 hydatid cysts in cattle and 4 hydatid cysts in camels, then the parasite materials were prepared from the fluid by repeated centrifugations. This step was then followed by washing several times in phosphate buffer and fixation in mixture of 3% glutaraldehyde with 0.1 M phosphate buffer. The re-suspended samples were centrifuged twice at 112 g for 5 min and the resulting pellet was then resuspended in 1% osmium tetroxide prepared at room temperature. The morphometric analysis of the preparations was then done using SEM (Joel, JSM-5400LV Scanning Electron Microscope, Tokyo 1993, Japan) (40). The identified structures, including small and large hooks, were also examined using SEM for a different characteristics and aspects including length and the width of each hook and the guard angle, following to the protocols described elsewhere (39, 40, 55, 56).
Preparation of Parasite Material for the Extraction of DNA
It is important to note that camel and cattle samples were processed separately using the same protocol. The processing of the cyst samples was carried out as described elsewhere (52). Briefly, the cyst wall was opened and the hydatid fluids were collected into marked test tubes, then centrifuged. The supernatant was discarded and the sediment (parasite material), which contains free scolices and brood capsules) was collected into clean sterile bottles containing 70% ethanol and stored at −20°C until use (52). After washing the samples with nucleic-acid-free water, to remove the ethanol, total genomic DNA was extracted from parasite material using the QIAamp DNA Mini Kit (QIAGEN, Germany), according to the manufacturer's protocol and as described elsewhere (50). The DNA was then stored in sterile DNAse- and RNAse-free microtubes and kept at −20°C.
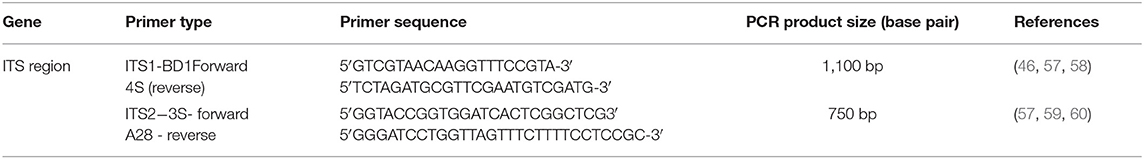
Molecular Identification (PCR)
Table 1 lists the primers used for the amplification of the DNA obtained from protoscoleces of hydatid cysts. The primers targeted the ribosomal DNA (rDNA) region spanning the internal transcribed spacers ITS-1 and ITS-2 regions which is validated for E. granulosus diagnosis (46, 57, 58). The PCR was designed for ITS1 and ITS2 regions and the amplification conditions were as per a protocol described elsewhere (5, 61), with slight modification. Briefly, the PCR mixture (25 μl) contained 12.5 μl of Master mix (Promega), 1 μl of forward primer (10 p/mol), 1 μl of reverse primer (10 p/mol), 1 μl of DNA (50 ng/μl of DNA template), and 9.5 μl of deionized distilled water. The amplification included an initial denaturation step of 5 min at 95°C; followed by 40 cycles of 1 min at 95°C, 30 s at 60°C, and 3 min at 72°C; and a final extension at 72°C for 10 min. Five microliter of the resultant amplified PCR products were analyzed in 1.6% (w/v) agarose gels in Tris-acetate-EDTA (TAE) buffer stained with ethidium bromide, transilluminated under ultraviolet light, and photographed. A known positive control comprising a reference strain was included [kindly provided by Professor Refaat Khalifa (Animal Health Research Institute, Assiut, Egypt)], while purified water was used as the negative control. Controls were processed in parallel with the experimental samples, to detect possible contamination.
Data Analysis
Data were collected, organized and then analyzed using SPSS, 23. Fisher's exact test was used to compare frequencies of presence of hydatid cyst in different organs in camels and cattle besides studying the potential explanatory individual variable factors associated with the occurrence of the disease. Furthermore, normality of quantitative parameters (length and width of hooks, handle, and blade of small hook and large hooks) were assessed using normal probability plots and the Kolmogorov-Smirnov test generated with the Proc T-test procedure of Statistical Analysis System (SAS®, version 9.2, SAS Institute, Cary, NC, USA) to study the statistical differences between cattle and camel. For all analyses, P ≤ 0.05 was defined as significant.
Results
Occurrence of Hydatid Cysts in Camel and Cattle Samples and the Potential Explanatory Individual Variable Factors
In the present study, hydatid cysts were detected in 6% of the examined lungs of camels. Table 2 summarizes the variable factors associated with the occurrence of the disease in camels. In accordance with organ distribution of hydatid cysts in camels, 6 hydatid cysts were detected in the lungs, whereas no cysts were found in the liver (P < 0.05). The remaining factors were not significantly associated with the occurrence of cysts. Regarding the age factor in camels, the rate of cysts was higher in camels older than 5 years of age than younger camels (<5 years of age). In this context, Table 2 shows that the occurrence of hydatid cysts in camels older than 5 years of age was 10% (4 out of 40 examined camels), whereas in younger camels (<5 years of age) this value was 3.33% (2 out of 60 examined camels). In accordance with sex, the occurrence of hydatid cysts in male camels was 6.7% (6 out of 90 examined camels) and no hydatid cysts were detected in females. Out of the 6 detected hydatid cysts, 4 (66.7 %) were fertile, 1 was sterile (16.65%), and another was calcified (16.65%). Furthermore, in camels, the current investigation showed that 75% (3 out of 4 examined cysts) of the fertile cysts were viable and 25% (1 out of 4 examined cysts) were non-viable. In addition, 3 hydatid cysts were found as single cysts in camels and another three animals experienced multiple hydatid cysts.
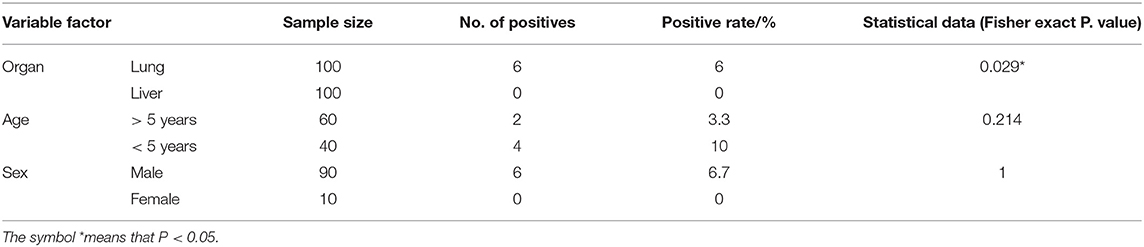
Table 2. Summarizes the data of the possible associations between the occurrence of hydatid cysts in camels and the potential explanatory individual variable factors of these detected cysts.
In accordance with their occurrence in cattle, hydatid cysts were found in 0.87% of examined cattle. The potential explanatory individual variable factors associated with the occurrence of hydatid cysts in cattle are illustrated in Table 3. As shown in the table, the majority of hydatid cysts were detected in the lungs (0.7%), while one hydatid cyst was found in the liver (0.17%). The age and sex factors were the variables that were significantly associated with the occurrence of hydatid cysts (P < 0.05). The occurrence of hydatid cysts in female cattle older than 5 years of age was 2.6% (5 out of 175 examined cattle), while no hydatid cysts were detected in younger male cattle (<5 years of age). In addition, out of the 5 detected hydatid cysts, 3 (60%) were fertile, 1 was sterile (20%), and another was calcified (20%). Regarding the viability of fertile hydatid cysts, only 33.3% (1 out of 3 examined cysts) of the fertile cysts isolated from cattle were non-viable, while 66.7% (2 out of 3 examined cysts) were viable. In accordance with the number of cysts in cattle, 4 cattle had single cyst and only one animal exhibited multiple hydatid cysts.
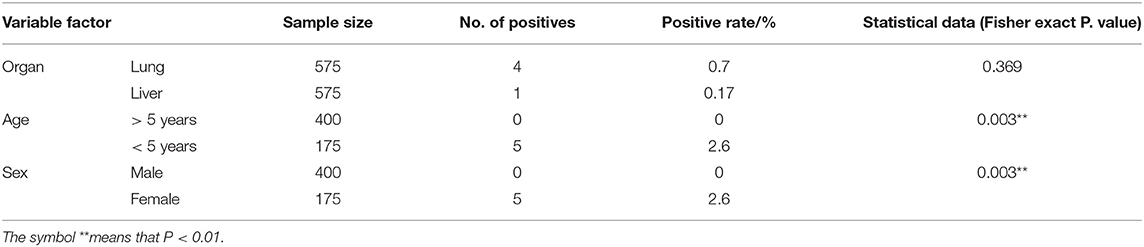
Table 3. Summarizes the data of the possible associations between the occurrence of hydatid cysts in cattle and the potential explanatory individual variable factors of these detected cysts.
Molecular, Morphological, and Morphometric Identification of Hydatid Cysts
In accordance with the molecular identification of hydatid cysts (46, 57, 58, 62), the DNA fragments amplified from the rDNA extracted from hydatid cyst protoscoleces of the organs from infected camels and cattle were approximately 1,100 base pairs (bp) and 750 bp in length for ITS1 and ITS2, respectively (Supplementary Figures 1, 2). Figure 1 illustrates the gross morphological appearance of examples of the hydatid cysts detected in camels. Several instances of multiple scolices in viable and non-viable fertile hydatid cysts, and different-shaped and -sized hooks in fertile viable hydatid cysts in the lungs of camels were also observed (Figure 1). Similar to that observed in camels, the morphological and microscopic examinations of the hydatid cysts encountered in the lungs of cattle are shown in Figure 2, several instances of multiple scolices in viable and non-viable solitary fertile hydatid cysts, as well as different-shaped and -sized hooks in fertile viable hydatid cysts, were detected in the lungs of cattle (Figure 2). On the other hand, SEM visualization of the morphometric appearance of the small and large hooks of hydatid cysts obtained from the infected lung of camel is shown in Figure 3, while that of cattle are depicted in Figures 4, 5. The morphometric characteristics and measurements of the hooks of cattle and camel are shown in Table 4. As shown in Figures 3–5, morphologically, the small hooks of cattle lungs had a flatter concavity of the handle and much thinner blade with a pointed end, while the large hooks of cattle lungs were generally smaller than those of the camel lungs. Regarding their morphometry, the blade of small hooks of cattle lungs was narrow, the concavity between the handle and the guard was shallower, and the handle was longer and narrower than those of camel lungs. Moreover, the large hooks of cattle lungs were smaller in total length, width, and handle length, with a similar blade length. Collectively, Figures 3–5 depict the presence of slight but clear differences in the morphology and morphometry of the small and large hooks of cysts obtained from the lungs of camels and cattle.
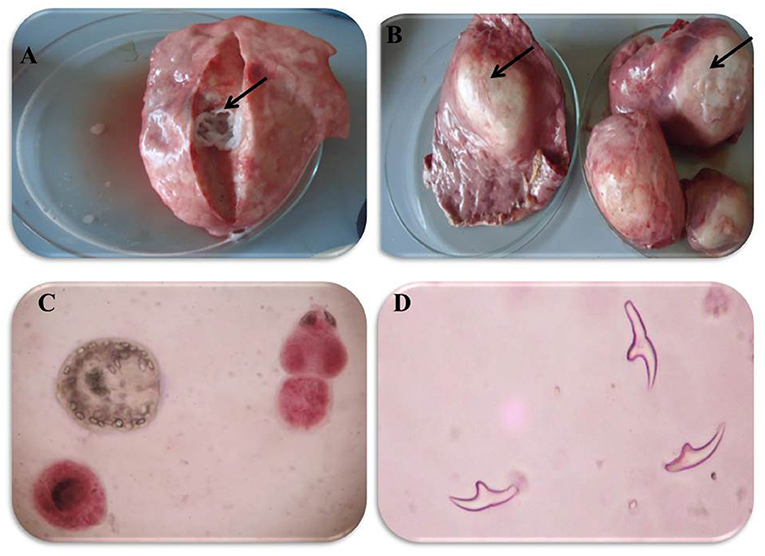
Figure 1. Morphological appearance of Hydatid cyst in the lung of camels. (A) Solitary sterile hydatid cysts. (B) Multiple hydatid cysts. (C) Multiple scolices in viable and non-viable fertile hydatid cyst, using 0.1% Eosin stain (X400) and (D) different-shaped and sized hooks in fertile viable-hydatid cysts.
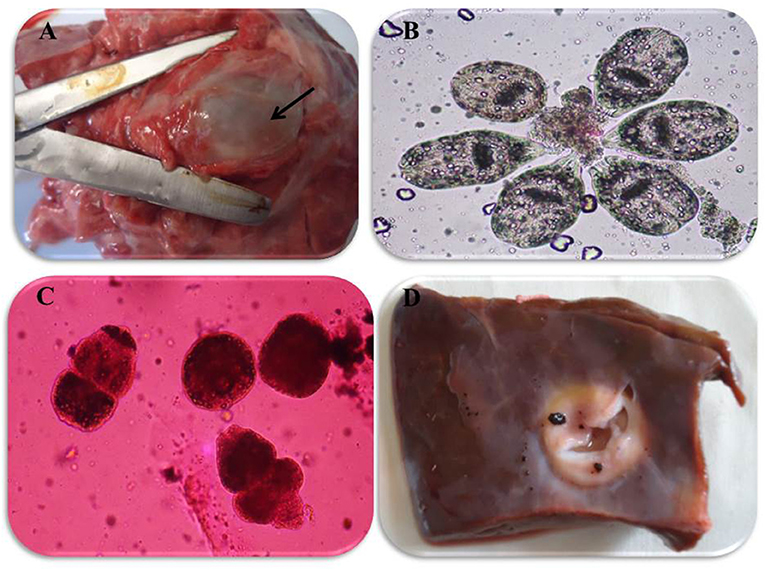
Figure 2. Morphological appearance of hydatid cyst in lung and liver of cattle. (A) Solitary viable fertile hydatid cysts in the lung of cattle. (B) Multiple scolices in viable fertile hydatid cyst in the lung of cattle using 0.1% eosin stain (X100). (C) Multiple scolices in non-viable fertile hydatid cyst in the lung of cattle using 0.1% eosin stain (X100) and (D) calcified hydatid cysts in the liver of cattle.
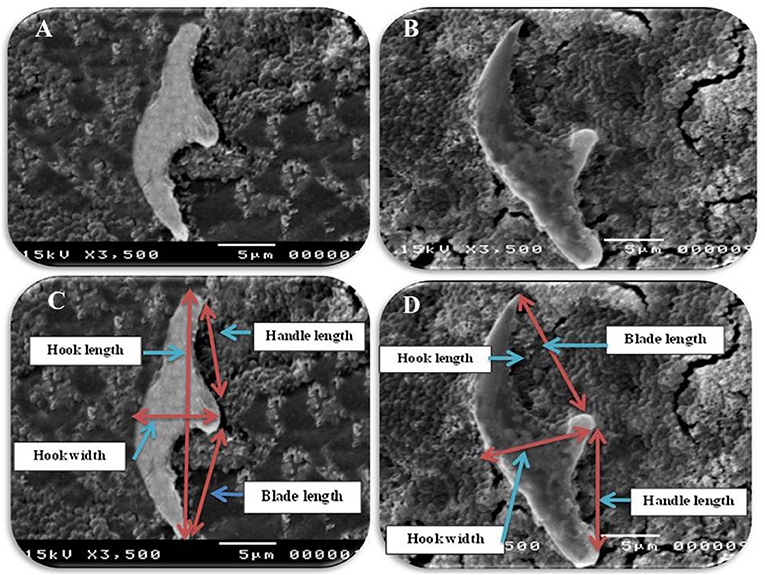
Figure 3. Morphometric appearance of small and large hook of hydatid cyst isolated from lung of camel using SEM. (A) The small hook of hydatid cyst. (B) The large hook of hydatid cyst. (C) Measurement the small hook of hydatid cyst; total hook length = 27 μm (arrow), total hook width = 9 μm (arrow), handle length = 9 μm (arrow) and blade length = 10 μm (arrow) and (D) measurement the large hook of hydatid cyst; Total hook length = 34 μm (arrow), total hook width = 12 μm (arrow), handle length = 14 μm (arrow) and blade length = 15 μm (arrow).
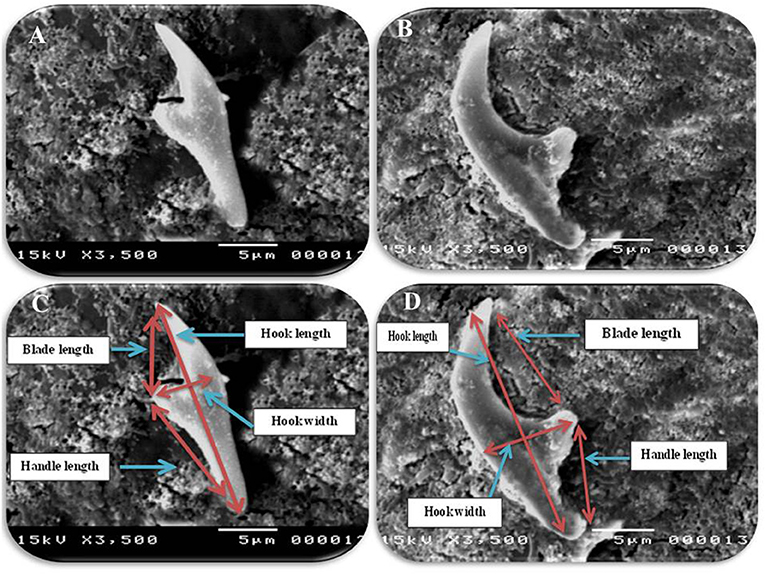
Figure 4. Morphometric appearance of small and large hook of hydatid cyst isolated from cattle lung using SEM. (A) The small hook of hydatid cyst isolated from cattle lung. (B) The large hook of hydatid cyst isolated from cattle lung. (C) Measurement the small hook of hydatid cyst isolated from cattle lung; Total hook length = 22 μm (arrow), total hook width = 6 μm (arrow), handle length = 10 μm (arrow) and blade length = 5 μm (arrow) and (D) measurement the large hook of hydatid cyst isolated from cattle lung; total hook length = 28 μm (arrow), total hook width = 8 μm (arrow), handle length = 8 μm (arrow) and blade length = 16 μm (arrow).
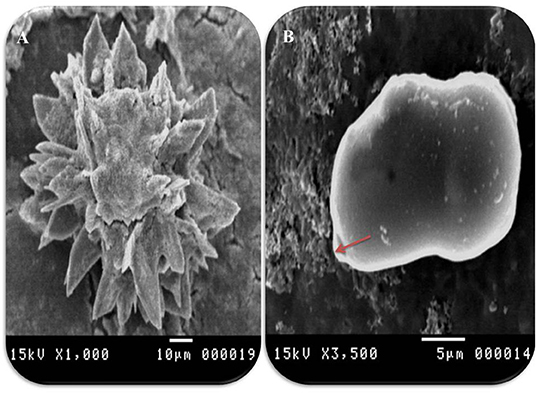
Figure 5. Morphometric appearance of hydatid cyst isolated from cattle lung using SEM. (A) SEM of rostellar hooks from protoscoleces of hydatid cyst, isolated from cattle showing alternating large and small hooks, and (B) SEM of brood capsules showing exterior outgrowth in hydatid cyst isolated from a cattle lung (arrow).
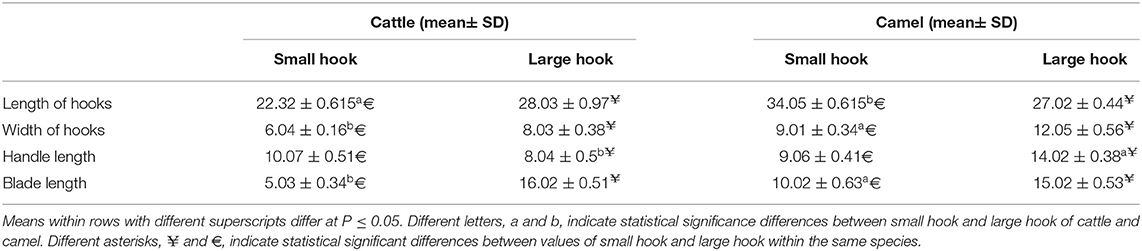
Table 4. The morphometric measurements (μm) of total length, width, handle, and blade of large and small hooks hydatid cysts retrieved from lungs of cattle and camel [Values are means ± error of the mean (SEM)].
Discussion
The present study provides interesting data related to the occurrence of CE and to several potential explanatory individual variable factors associated with the occurrence of hydatid cysts in camels and cattle from slaughterhouses in Assiut Governorate, Egypt. The confirmation of the results was performed using a set of molecular identification tools and by studying the ultrastructure of the protoscoleces and hooks in the detected cysts via SEM. Given the fact, limited information are available about CE in upper Egypt, our study provides novel contribution about the occurrence of this disease and the real contribution of camels and cattle in maintenance the epidemiological foci of this disease of zoonotic importance in this area. As shown in our work, hydatid cysts were detected in 6% of examined lungs of camels. A previous survey of hydatid disease in camels and cattle in the same studied area reported a prevalence rate of 7.67% in camels, while no infection was reported in cattle (54). Another study reported a seroprevalence rate of 6% in camels harboring hydatid cysts (63). The frequency of CE observed in camels in the present work is similar to that detected previously by Dyab et al. (64) in Assiut Governorate (64) and to that reported by Lahmar et al. (65) in Tunisia (65). In contrast, higher occurrence rates of CE, of 9 and 24.15% were reported in camels from the same region of Egypt (66, 67). Moreover, Barghash et al. (26) reported higher rates of 18.7% in camels from Cairo, Dairut, Mallawy, and Kafr-ElShikh, Egypt (26). Another study of CE in various municipals abattoirs in Cairo, Giza, and Beni-Suef governorate, Egypt, reported a higher prevalence rate of 10.82% (29). Conversely, lower values of 2.35 and 5% have been reported in camels from Beni Suef and Upper Egypt, respectively (25, 68). In the present study, CE was only reported in 0.87% of examined cattle. The present rates of CE are lower than those of a previous study performed in cattle from Egypt, which reported an occurrence rate of 3.3% (69). Another study performed in Cairo reported that 18.4% of the examined cattle were infected by CE (70). In contrast, the occurrence rate obtained in the present study was higher than those reported in cattle slaughtered in abattoirs in Assiut, Mansoura, and other provinces of Upper Egypt, where hydatid cysts were noted in 0.4, 0.068, and 0.004% of the examined animals, respectively (25, 71, 72). The discrepancy in the occurrence of CE in camels and cattle in the present study vs. those reported in several previous studies may be attributed to several factors, such as hygienic practices during slaughter, sex and age of the slaughtered animals, the method of detection, the geographic location, and various climatic conditions (25, 45, 73, 74). The unhygienic disposal of condemned carcasses and infected organs, the ease of access stray dogs to slaughter houses, and the unauthorized slaughter are also relevant factors in the transmission of CE (29, 30, 74, 75).
In accordance with the studied potential variable factors, the statistical analysis revealed that the number and type of cysts, organ involved, and fertility and viability of hydatid cysts were not significantly associated with the occurrence of the of CE in camels or cattle. The present study also showed that CE were only detected in the lungs of camels, while cysts occurred mainly in the lungs of cattle and only one cyst was reported in the liver of cattle, which is consistent with several previous reports, mainly from Egypt (69, 70, 72). However, some previous reports revealed that the parasite exhibited single-organ involvement, although the involvement of two organs has also been recorded, which is apparently associated with the specific geographic regions and strain of the parasite (25, 76, 77). Regarding the age as variable factor, our study reveals that occurrence of hydatid cysts in aged camels at rate of 10% (4 out of 40 examined camels), whereas in younger male camels, this value was 3.33% (2 out of 60 examined camels). Furthermore, the present data illustrate the occurrence of hydatid cysts in aged cattle at a rate of 2.6% (5 infected out of 175 examined animals), while no infection was detected among young cattle (<5 years of age) (N = 400). The present findings agree with several previous studies performed either at the national (Egypt) or international level (70, 78, 79). This observation could be attributed to the fact that older animals are exposed to infection more than young ones and aged animals get slaughtered more than young ages, since their production (calves/milk) and working capacity decrease (80). Immunity represent an additional factor might involve this difference since older ages have weak immune system to combat the infection (81). Furthermore, the present study showed that female cattle were infected exclusively, at rate of 2.6% (5 infected out of 175 examined animals), while no infection was detected in the examined male cattle. This observation may be explained by the fact that female animals are not slaughtered at younger ages, as the owners mostly keep them for breeding, obtaining calves and for milk production; in contrast, male cattle are slaughtered at younger ages (70, 82, 83). The management practices might also contribute to this difference since males move far away for grazing; meanwhile females are usually kept homesteads, making females are more exposed to infection than males (84).
Considering the sex as potential variable factor, all detected hydatid cysts in camels were found in males and no cysts were detected in females. Meanwhile, all detected hydatid cysts in cattle were found in females. Reviewing the available literature, a previous study reported an infection rate of 16.89% in female and 13.55% in male cattle in Northwest Iran (85). This difference might be attributed to the low number of females analyzed in our study compared with males. In the present study, single cysts were detected in 80% of positive samples and multiple cysts were present in 20% of infected cattle, while single and multiple cysts were reported at an equal percentage of 50% in camels. Our results are consistent with those reported in Southern Italy, where 78.8% of the cysts were single entities (86). Regarding hydatid cyst viability in camels, 75% of the examined cysts were viable while 25% of the cysts were non-viable. Meanwhile, our data revealed that 66.7% of hydatid cysts detected in cattle were viable, while 33.3% of the examined fertile cysts were non-viable. Similar results were recorded in camels in Addis Ababa, where 66.6% of the detected hydatid cysts were viable (87). Another previous study performed in Southeastern Iran found that 57.14% of the hydatid cysts detected in camels were viable (88). Regarding the type of cyst, the current investigation showed that 4 of hydatid cysts (66.7 %) were fertile, 1 was sterile (16.65%), and another was calcified (16.65%). In addition, 75% of the fertile cysts in camels were viable and 25% were non-viable. Moreover, 3 hydatid cysts were found as single cysts in camels and another three animals experienced multiple hydatid cysts. On the other hand, our study found that 60% of hydatid cysts in cattle were fertile, 20% were sterile, and 20% were calcified. In addition, only 33.3% of the examined fertile cysts isolated from cattle were non-viable, while 66.7% were viable. Moreover, 4 cattle had single cyst and only one animal showed multiple hydatid cysts. Similar to the present results obtained in cattle, another study performed in Eastern Ethiopia found that 80% of the cysts were fertile, 17.3% were sterile, and 2.85% were calcified (79). Another study from Southern Italy revealed that 42.7% of cysts were sterile and 57.3% were calcified/caseous, while no fertile cysts were found (86). In this context, camelids and porcine are suitable hosts that frequently contain fertile cysts (45).
In accordance with the morphological and morphometric data (Table 4, Figures 3–5), the reported data indicated slight but clear differences in the morphology and statistical significant differences morphometric measurements of the small and large hooks obtained from the lungs of camels and cattle, which is consistent with the results of several previous studies (31–34, 39, 40). However, the limitations of the use of SEM, i.e., the need for infrastructure or financial constraints, favor the performance of molecular techniques vs. purchasing an SEM instrument (89).
Among other molecular targets, PCR was used to amplify the ITS region of ribosomal DNA as a genetic marker, which provides a simple and powerful tool for the accurate identification and differentiation of Echinococcus strains (90). In the present work, the size of the DNA fragment amplified for rDNA-ITS1 and rDNA-ITS2 was 1,100 and 750 bp, respectively, in both camel and cattle samples. Similar results were reported in several previous studies (57, 59, 62). A review of the available literature showed that CE is widespread in many countries where camels are raised, including Egypt (52, 91–94). This finding implies the widespread presence of CE across the area of Upper Egypt. Among others, recent molecular data suggested that the prevalence of infection of E. canadensis G6 might be higher than previously described, and that this genotype exists as a complex of different strains that differ in a wide variety of criteria (92, 94, 95). Clearly, future genotypic characterization of the major strains circulating in the country using phylogenetic studies would provide interesting information about the genetic relatedness of the parasite, both at the regional and international levels.
Conclusions and Recommendations
In summary, the results of this study demonstrated the occurrence of CE among camels and cattle in Upper Egypt. Moreover, our findings conclude that camels and cattle play a potential role in the maintenance of the zoonotic foci and the transmission cycle of the parasite in Egypt. Our results also suggest the possible spreading of this zoonotic disease to other provinces in Egypt, together with animal movements. Further research and epidemiological studies are recommended to explore the involvement of other intermediate hosts in Egypt and identify the species of Echinococcus species that are circulating throughout the country, which is important information for combating this zoonotic disease.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AG, RK, AD, DY, and MA involved in the conception of the research idea and methodology design, supervision, and performed data analysis and interpretation. AS, SM, AT, RB, FE-G, and EE participated in methodology, sampling, and data analysis. AG and EE drafted and prepared the manuscript for publication for publication and revision. RK, AD, DY, and MA contributed their scientific advice. All authors read and approved the final manuscript.
Funding
This work was supported by the Taif University Researchers Supporting Program (Project number: TURSP-2020/269), Taif University, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors also thank the veterinarians and directors of the abattoirs for their support and help in providing data and during collection of the samples throughout the study. The authors also would like to thank Dr. Kirsty Jensen from University of Edinburgh for improving the English style of the paper.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.750640/full#supplementary-material
References
1. Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. (2006) 12:296–303. doi: 10.3201/eid1202.050499
2. Alvarez Rojas CA, Romig T, Lightowlers MW. Echinococcus granulosus sensu lato genotypes infecting humans–review of current knowledge. Int J Parasitol. (2014) 44:9–18. doi: 10.1016/j.ijpara.2013.08.008
3. Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol. (2017) 95:315–493. doi: 10.1016/bs.apar.2016.11.001
4. Khan SN, Ali R, Khan S, Norin S, Rooman M, Akbar NU, et al. Cystic echinococcosis: an emerging zoonosis in southern regions of Khyber Pakhtunkhwa, Pakistan. BMC Vet Res. (2021) 17:139. doi: 10.1186/s12917-021-02830-z
5. Nakao M, McManus DP, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. (2007) 134:713–22. doi: 10.1017/S0031182006001934
6. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. (2004) 17:107–5. doi: 10.1128/CMR.17.1.107-135.2004
7. Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. (2009) 13:125–33. doi: 10.1016/j.ijid.2008.03.037
8. Getaw A, Beyene D, Ayana D, Megersa B, Abunna F. Hydatidosis: prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia, Ethiopia. Acta Trop. (2010) 113:221–5. doi: 10.1016/j.actatropica.2009.10.019
9. Ahmadi NA, Meshkehkar M. An abattoir-based study on the prevalence and economic losses due to cystic echinococcosis in slaughtered herbivores in Ahwaz, south-western Iran. J Helminthol. (2011) 85:33–9. doi: 10.1017/S0022149X10000234
10. Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. (2007) 7:385–94. doi: 10.1016/S1473-3099(07)70134-2
11. Casulli A. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob Health. (2020) 8:e470–e1. doi: 10.1016/S2214-109X(20)30066-8
12. Torgerson PR, Deplazes P. Echinococcosis: diagnosis and diagnostic interpretation in population studies. Trends Parasitol. (2009) 25:164–70. doi: 10.1016/j.pt.2008.12.008
13. Romig T, Omer RA, Zeyhle E, Huttner M, Dinkel A, Siefert L, et al. Echinococcosis in sub-Saharan Africa: emerging complexity. Vet Parasitol. (2011) 181:43–7. doi: 10.1016/j.vetpar.2011.04.022
14. Youssefi MR, Mirshafiei S, Moshfegh Z, Soleymani N, Rahimi MT. Cystic echinococcosis is an occupational disease? J Parasit Dis. (2016) 40:586–90. doi: 10.1007/s12639-014-0543-2
15. Essa A. Worms and human disease – second edition. J Clin Pathol. (2004) 57:110–1. doi: 10.1136/jcp.57.1.110-b
16. Tembo W, Emmanue Nonga H. A survey of the causes of cattle organs and/or carcass condemnation, financial losses and magnitude of foetal wastage at an abattoir in Dodoma, Tanzania. Onderstepoort J Vet Res. (2015) 82:E1–7. doi: 10.4102/ojvr.v82i1.855
17. Jemal D, Kebede B. The study of major parasitic causes of organ condemnation and financial losses in cattle slaughtered at Hawassa Municipal Abattoir, Ethiopia. Cogent Food Agric. (2016) 2:1201183. doi: 10.1080/23311932.2016.1201183
18. World health organization (WHO). Echinococcosis fact sheet. (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/echinococcosis (accessed July 11, 2020).
19. Battelli G, Mantovani A, Seimenis A. Cystic echinococcosis and the Mediterranean Region: a long-lasting association. Parassitologia. (2002) 44:43–57.
20. Kandeel A, Ahmed ES, Helmy H, El Setouhy M, Craig PS, Ramzy RM. A retrospective hospital study of human cystic echinococcosis in Egypt. East Mediterr Health J. (2004) 10:349–57.
21. Capuano F, Rinaldi L, Maurelli MP, Perugini AG, Veneziano V, Garippa G, et al. Cystic echinococcosis in water buffaloes: epidemiological survey and molecular evidence of ovine (G1) and buffalo (G3) strains. Vet Parasitol. (2006) 137:262–8. doi: 10.1016/j.vetpar.2006.01.016
22. Cringoli G, Rinaldi L, Musella V, Veneziano V, Maurelli MP, Di Pietro F, et al. Geo-referencing livestock farms as tool for studying cystic echinococcosis epidemiology in cattle and water buffaloes from southern Italy. Geospat Health. (2007) 2:105–11. doi: 10.4081/gh.2007.259
23. Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol. (2012) 18:1425–37. doi: 10.3748/wjg.v18.i13.1425
24. Kouidri M, Benchaib-Khoudja F, Boulkaboul A, Selles M. Prevalence, fertility and viability of cystic echinococcosis in sheep and cattle of Algeria. Bulgarian J Vet Med. (2012) 15:191–7.
25. Omar M, Sultan K, Haridy M, Ali A. Prevalence of Cystic Echinococcosis in slaughtered ruminants in different abattoirs, upper Egypt. Am J Anim Vet Sci. (2013) 8:117–21. doi: 10.3844/ajavsp.2013.117.121
26. Barghash S, El-Sayed R, El-Alfy N, Abou-Elnour B, El-Kattan A, Sadek AS. Prevalence and molecular identification of Echinococcus granulosus in humans and slaughtered animals in Egypt. Europ J Biomed Pharm Sci. (2017) 4:34–42
27. Manciulli T, Mariconti M, Vola A, Lissandrin R, Brunetti E. Cystic Echinococcosis in the Mediterranean. Curr Trop Med Rep. (2017) 4:235–44. doi: 10.1007/s40475-017-0129-z
28. Scala A, Bosco A, Pipia AP, Tamponi C, Musella V, Costanzo N, et al. Cystic echinococcosis in cattle dairy farms: spatial distribution and epidemiological dynamics. Geospat Health. (2017) 12:562. doi: 10.4081/gh.2017.562
29. El-Dakhly KM, Arafa WM, El-Nahass ESN, Shokier KAM, Noaman AF. The current prevalence and diversity of cystic echinococcosis in slaughtered animals in Egypt. J Parasitic Dis. (2019) 43:711–7. doi: 10.1007/s12639-019-01151-1
30. Borhani M, Fathi S, Lahmar S, Ahmed H, Abdulhameed MF, Fasihi Harandi M. Cystic echinococcosis in the Eastern Mediterranean region: neglected and prevailing! PLoS Negl Trop Dis. (2020) 14:e0008114. doi: 10.1371/journal.pntd.0008114
31. Hobbs R, Lymbery A, Thompson R. Rostellar hook morphology of Echinococcus granulosus (Batsch, 1786) from natural and experimental Australian hosts, and its implications for strain recognition. Parasitology. (1990) 101:273–81. doi: 10.1017/S0031182000063332
32. Pednekar RP, Gatne ML, Thompson RC, Traub RJ. Molecular and morphological characterisation of Echinococcus from food producing animals in India. Vet Parasitol. (2009) 165:58–65. doi: 10.1016/j.vetpar.2009.06.021
33. Gholami S, Irshadullah M, Mobedi I. Rostellar hook morphology of larval Echinococcus granulosus isolates from the Indian buffalo and Iranian sheep, cattle and camel. J Helminthol. (2011) 85:239–45. doi: 10.1017/S0022149X10000520
34. Singh BB, Sharma JK, Tuli A, Sharma R, Bal MS, Aulakh RS, et al. Prevalence and morphological characterisation of Echinococcus granulosus from north India. J Parasit Dis. (2014) 38:36–40. doi: 10.1007/s12639-012-0189-x
35. Ahmadi NA. Using morphometry of the larval rostellar hooks to distinguish Iranian strains of Echinococcus granulosus. Ann Trop Med Parasitol. (2004) 98:211–20. doi: 10.1179/000349804225003217
36. Hussain A, Maqbool A, Tanveer A, Anees A. Studies on morphology of Echinococcus granulosus from different animal-dog origin. Punjab Univ J Zool. (2005) 20:151–7.
37. Yildiz K, Gurcan IS. The detection of Echinococcus granulosus strains using larval rostellar hook morphometry. Türkiye Parazitoloji Dergisi. (2009) 33:199–202.
38. Mustafa I, Shahbaz M, Asif S, Khan MR, Saeed U, Sadiq F, et al. Availability, cyst characteristics and hook morphology of Echinococcus granulosus isolates from livestock (Cattle, Sheep and Goats) in Central Punjab, Pakistan. Kafkas Univ Vet Fak Derg. (2015) 21:849–54. doi: 10.9775/kvfd.2015.13755
39. Antoniou M, Tselentis Y. Studies on Echinococcus granulosus using the scanning electron microscope. II. The hooks. Parasitol Res. (1993) 79:543–6. doi: 10.1007/BF00932237
40. Elmajdoub L, Rahman W, Wajidi M, Siti-Azizah M. Studies on the protoscoleces and hooks of Echinococcus granulosus from libya by scanning electron microscope. Acta Med Int. (2014) 1:74–81. doi: 10.5530/ami.2014.2.5
41. Khademvatan S, Yousefi E, Rafiei A, Rahdar M, Saki J. Molecular characterization of livestock and human isolates of Echinococcus granulosus from south-west Iran. J Helminthol. (2012) 87:1–5. doi: 10.1017/S0022149X12000296
42. Chaya D, Parija SC. Performance of polymerase chain reaction for the diagnosis of cystic echinococcosis using serum, urine, and cyst fluid samples. Trop Parasitol. (2014) 4:43–6. doi: 10.4103/2229-5070.129164
43. Moradi M, Meamar AR, Akhlaghi L, Roozbehani M, Razmjou E. Detection and genetic characterization of Echinococcus granulosus mitochondrial DNA in serum and formalin-fixed paraffin embedded cyst tissue samples of cystic echinococcosis patients. PLoS ONE. (2019) 14:e0224501. doi: 10.1371/journal.pone.0224501
44. Yousefi E, Rafiei A, Rashidi I, Khademvatan S, Foroutan M. Molecular characterization of Echinococcus granulosus in paraffin-embedded human tissues from Southwest Iran. Asian Pacific J Trop Med. (2019) 12:507. doi: 10.4103/1995-7645.271290
45. Romig T, Ebi D, Wassermann M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet Parasitol. (2015) 213:76–84. doi: 10.1016/j.vetpar.2015.07.035
46. Bowles J, McManus DP. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int J Parasitol. (1993) 23:969–72. doi: 10.1016/0020-7519(93)90065-7
47. Thompson RC. The taxonomy, phylogeny and transmission of Echinococcus. Exp Parasitol. (2008) 119:439–46. doi: 10.1016/j.exppara.2008.04.016
48. Kinkar L, Laurimae T, Sharbatkhori M, Mirhendi H, Kia EB, Ponce-Gordo F, et al. New mitogenome and nuclear evidence on the phylogeny and taxonomy of the highly zoonotic tapeworm Echinococcus granulosus sensu stricto. Infect Genet Evol. (2017) 52:52–8. doi: 10.1016/j.meegid.2017.04.023
49. Laurimae T, Kinkar L, Moks E, Romig T, Omer RA, Casulli A, et al. Molecular phylogeny based on six nuclear genes suggests that Echinococcus granulosus sensu lato genotypes G6/G7 and G8/G10 can be regarded as two distinct species. Parasitology. (2018) 145:1929–37. doi: 10.1017/S0031182018000719
50. McManus DP, Thompson RC. Molecular epidemiology of cystic echinococcosis. Parasitology. (2003) 127(Suppl):S37–51. doi: 10.1017/S0031182003003524
51. Eckert J, Gemmell MA, Meslin FO.-X, Pawlowski ZS World Health Organisation "WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Eckert J, Gemmell MA, Meslin F-X, Pawłowski ZS, editors. Paris: World Organisation for Animal Health (2001).
52. Osman A, Aradaib I, Ashmaig A-L, Gameel A. Detection and differentiation of Echinococcus granulosus-complex using a simple PCR-based assay. Int J Trop Med. (2009) 4:24–6.
53. Himonas C, Antoniadou-Sotiriadou K, Papadopoulos E. Hydatidosis of food animals in Greece: prevalence of cysts containing viable protoscoleces. J Helminthol. (1994) 68:311–3. doi: 10.1017/S0022149X00001541
54. Dyab KA, Hassanein R, Hussein AA, Metwally SE, Gaad HM. Hydatidosis among man and animals in Assiut and Aswan Governorates. J Egypt Soc Parasitol. (2005) 35:157–66.
55. Mahmoud LH, el-Garhy MF. A histochemical study on the hydatid cyst and electron microscopy of the hydatid sand of Echinococcus granulosus. J Egypt Soc Parasitol. (2002) 32:647–56.
56. Antoniou M, Tselentis Y. Studies on Echinococcus granulosus using the scanning electron microscope. I. preparations of the parasite for infection of the final host. Parasitol Res. (1993) 79:537–42. doi: 10.1007/BF00932236
57. Bowles J, Blair D, McManus DP. A molecular phylogeny of the genus Echinococcus. Parasitology. (1995) 110:317–28. doi: 10.1017/S0031182000080902
58. Hansh W, Awad A. Genotyping study of hydatid cyst by sequences of ITS1 – rDNA in Thi-Qar – Southern of Iraq. Int J Curr Microbiol Appl Sci. (2016) 5:350–61. doi: 10.20546/ijcmas.2016.508.037
59. Gasser RB, Chilton NB. Characterisation of taeniid cestode species by PCR-RFLP of ITS2 ribosomal DNA. Acta Trop. (1995) 59:31–40. doi: 10.1016/0001-706X(94)00085-F
60. Bowles J, Blair D, McManus DP. A molecular phylogeny of the human schistosomes. Mol Phylogenet Evol. (1995) 4:103–9. doi: 10.1006/mpev.1995.1011
61. White BA. PCR Protocols : Current Methods and Applications. Totowa, NJ: Humana Press (1993). doi: 10.1385/0896032442
62. Gholami S, Sosari M, Fakhar M, Sharif M, Daryani A, Hashemi M, et al. Molecular characterization of Echinococcus granulosus from hydatid cysts isolated from human and animals in golestan province, North of Iran. Iran J Parasitol. (2012) 7:8–16.
63. Ghada M, Osama H. Seroprevalence of hydatidosis in camels of Assuit Province, Egypt. Madridge J Vaccine. (2017) 1:1–4.
64. Dyab A, Mohamed G, Abdella O. Seroprevalence of hydatidosis in camels of Assuit Province, Egypt. Madridge J Vaccines. (2017) 1:5–8. doi: 10.18689/mjv-1000102
65. Lahmar S, Trifi M, Naceur S, Bouchhima T, Lahouar N, Lamouchi I, et al. Cystic echinococcosis in slaughtered domestic ruminants from Tunisia. J Helminthol. (2012) 87:1–8. doi: 10.1017/S0022149X12000430
66. Ali A. Some studies on ecto and endoparasites of camels in Assiut Governorate. Assiut: M.V.Sc. of parasitology in Assiut Universitym (2005).
67. Khalifa R, Abdel-Rahman S, Monib MS, Yones D, Saleh DAS. Characteristics of hydatid cyst of camel strain of Echinococcus granulosus in Assiut. El-Minia Med J. (2005) 16:202–14.
68. Gab-Allah HM, Saba S. Incidence of hydatid cyst in slaughtered animals and their relation to public health at Sharkia Province Egypt. J Agric Res. (2010) 88:285–90. doi: 10.21608/ejar.2010.180436
69. Mahdy O. Epidemiological and molecular characterization of antigens extracted from Hydatid cysts of camel, cattle and donkeys in Egypt. Int J Basic Appl Sci. (2014) 3:93–8. doi: 10.14419/ijbas.v3i2.2127
70. Abo-Aziza FAM, Oda SS, Aboelsoued D, Farag TK, Almuzaini AM. Variabilities of hydatidosis in domestic animals slaughtered at Cairo and Giza abattoirs, Egypt. Vet World. (2019) 12:998–1007. doi: 10.14202/vetworld.2019.998-1007
71. Ahmed S. Seroepidemiological studies on hydatid cyst in animals and man. Ph.D, Assiut, Egypt (2006).
72. Abbas I, Al kappany Y, Al-Araby M. Prevalence and molecular characterization of hydatid cyst isolates from cattle in Egypt. Asian J Anim Vet Adv. (2016) 11:794–804. doi: 10.3923/ajava.2016.794.804
73. Sanli A, Onen A, Karapolat S, Atinkaya C, Yuncu G, Eyuboglu GM, et al. Social factors associated with pulmonary hydatid cyst in Aegean, Turkey. Afr Health Sci. (2011) 11(Suppl. 1):S82–5. doi: 10.4314/ahs.v11i3.70075
74. Otero-Abad B, Torgerson P. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Neglect Trop Dis. (2013) 7:e2249. doi: 10.1371/journal.pntd.0002249
75. Oudni-M'rad M, M'rad S, Babba H. Molecular and epidemiology data on cystic echinococcosis in Tunisia. In: Current Topics in Echinococcosis. Croatia: Intech publisher (2015). p. 55–74. doi: 10.5772/60891
76. Gottstein B. Hydatid disease, major tropical syndromes by body system. Systemic infections. Cambridge: Cambridge University Press (2000) 169 p.
77. El-Majdoub L, Drah M. Light microscopy of the hooklets of protoscolices of hydatid cysts infecting sheep and camels from misurata, libya and their possible role in ‘parasite strain’recognition. Int J Infect Dis. (2008) 12:e128–9. doi: 10.1016/j.ijid.2008.05.320
78. Zewdu E, Teshome T, Makwoya A. Bovine hydatidosis in ambo municipality abattoir, West Shoa, Ethiopia. Ethiop Vet J. (2010) 14:1–14.
79. Mulatu M, Mekonnen B, Tassew H, Kumar A. Bovine hydatidosis in eastern part of Ethiopia. Momona Ethiop J Sci. (2013) 5:107–14. doi: 10.4314/mejs.v5i1.85334
80. Ibrahim K, Thomas R, Peter K, Omer RA. A molecular survey on cystic echinococcosis in Sinnar area, Blue Nile state (Sudan). Chin Med J. (2011) 124:2829–33. doi: 10.3760/cma.j.issn.0366-6999.2011.18.006
81. Himonas C, Frydas S, Antoniadol-Sotiriadou K. The fertility of hydatid cysts in food animals in Greece. In: Helminth Zoonoses. Dordrecht: Springer (1987). p. 12–21. doi: 10.1007/978-94-009-3341-5_2
82. Haleem S, Niaz S, Qureshi NA, Ullah R, Alsaid MS, Alqahtani AS, et al. Incidence, risk factors, and epidemiology of cystic echinococcosis: a complex socioecological emerging infectious disease in Khyber Pakhtunkhwa, Province of Pakistan. Biomed Res Int. (2018) 2018:5042430. doi: 10.1155/2018/5042430
83. Guduro GG, Desta AH. Cyst, viability, and economic significance of hydatidosis in Southern, Ethiopia. J Parasitol Res. (2019) 2019:2038628. doi: 10.1155/2019/2038628
84. Parija S. Medical Parasitolgy, Protozology and Helminthology. Text and Atlas 2nd Ed. India Ichennia Medical Books Publisher (2004). p. 221–9.
85. Taghavi M, Mirzaei M, Fartashvand M. An abattoir survey of liver and lung hydatidosis in Northwest Iran. J Nov Appl Sci. (2013) 2: 710–2.
86. Rinaldi L, Maurelli M, Veneziano V, Capuano F, Perugini A, Cringoli S. The role of cattle in the epidemiology of Echinococcus granulosus in an endemic area of southern Italy. Parasitol Res. (2008) 103:175–9. doi: 10.1007/s00436-008-0948-x
87. Saleh MA, Mahran OM, Al-Salahy MB. Circulating oxidative stress status in dromedary camels infested with sarcoptic mange. Vet Res Commun. (2011) 35:35–45. doi: 10.1007/s11259-010-9450-x
88. Fathi S, Dehaghi MM, Radfar MH. Occurrence of hydatidosis in camels (Camelus dromedarius) and their potential role in the epidemiology of Echinococcus granulosus in Kerman area, southeast of Iran. Compar Clin Pathol. (2012) 21:921–7. doi: 10.1007/s00580-011-1200-0
89. Owen G. Purchasing an electron microscope?–Considerations and scientific strategies to help in the decision making process. Microscopy Vancouver, BC (2018).
90. Hasan HF, Fadhil MH, Fadhil ZH. Molecular characterization of Echinococcus granulosus isolated from 703 human and domestic animals in Kirkuk, Iraq. Anim Res Int. (2016) 13:2544–7.
91. Karine B, Piarroux R, Dia M, Schneegans F, Beurdeley A, Godot V, et al. Combined eco-epidemiological and molecular biology approaches to assess Echinococcus granulosus transmission to humans in Mauritania: Occurence of the 'camel' strain and human cystic echinococcosis. Trans R Soc Trop Med Hygiene. (2002) 96:383–6. doi: 10.1016/S0035-9203(02)90369-X
92. M'Rad S, Filisetti D, Oudni-M'rad M, Mekki M, Belguith M, Nouri A, et al. Molecular evidence of ovine (G1) and camel (G6) strains of Echinococcus granulosus in Tunisia and putative role of cattle in human contamination. Vet Parasitol. (2005) 129:267–72. doi: 10.1016/j.vetpar.2005.02.006
93. Maillard S, Benchikh-Elfegoun M, Knapp J, Bart J.-M, Koskei P, et al. Taxonomic position and geographical distribution of the common sheep G1 and camel G6 strains of Echinococcus granulosus in three African countries. Parasitol Res. (2007) 100:495–503. doi: 10.1007/s00436-006-0286-9
94. Khalifa N, Khater H, Fahmy H, Radwan MEI, Jsa A. Genotyping and phylogenetic analysis of cystic echinococcosis isolated from camels and humans in Egypt. Am J Epidemiol Infect Dis. (2014) 2:74–82. doi: 10.12691/ajeid-2-3-2
Keywords: molecular, morphometric, hydatid cyst, camel, cattle, Egypt, epidemiological
Citation: Gareh A, Saleh AA, Moustafa SM, Tahoun A, Baty RS, Khalifa RMA, Dyab AK, Yones DA, Arafa MI, Abdelaziz AR, El-Gohary FA and Elmahallawy EK (2021) Epidemiological, Morphometric, and Molecular Investigation of Cystic Echinococcosis in Camel and Cattle From Upper Egypt: Current Status and Zoonotic Implications. Front. Vet. Sci. 8:750640. doi: 10.3389/fvets.2021.750640
Received: 30 July 2021; Accepted: 07 September 2021;
Published: 04 October 2021.
Edited by:
Hui Zhang, South China Agricultural University, ChinaReviewed by:
Wenchao Li, Anhui Science and Technology University, ChinaDr. Sumbal Haleem, Kohat University of Science and Technology, Pakistan
Copyright © 2021 Gareh, Saleh, Moustafa, Tahoun, Baty, Khalifa, Dyab, Yones, Arafa, Abdelaziz, El-Gohary and Elmahallawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ehab Kotb Elmahallawy, ZWVoYWFAdW5pbGVvbi5lcw==
 Ahmed Gareh1
Ahmed Gareh1 Amin Tahoun
Amin Tahoun Roua S. Baty
Roua S. Baty Amer R. Abdelaziz
Amer R. Abdelaziz Fatma A. El-Gohary
Fatma A. El-Gohary Ehab Kotb Elmahallawy
Ehab Kotb Elmahallawy