- 1Department of Veterinary Microbiology, Parasitology and Biotechnology, College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, Morogoro, Tanzania
- 2National Livestock Resources Research Institute, Kampala, Uganda
- 3Southern African Centre for Infectious Disease Surveillance (SACIDS) Foundation for One Health Sokoine University of Agriculture, Morogoro, Tanzania
- 4Department of Knowledge Management, Sokoine National Agricultural Library, Sokoine University of Agriculture, Morogoro, Tanzania
- 5Department of Microbiology and Immunology, Weill Bugando School of Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
- 6Department of Microbiology and Immunology, School of Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- 7Department of Veterinary Public Health, College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, Morogoro, Tanzania
Antimicrobial use in livestock production has been linked to antimicrobial resistance (AMR) worldwide; however, optimization of their use has been considered an important strategy in dealing with it. The aims of this study were as follows: (a) to assess the literature on antimicrobial usage (practices, frequency, class, type) in cattle and poultry production with regard to resistance in Escherichia coli (E. coli) including multidrug resistance (MDR) (b) summarize evidence for quantitative (volumes of active antimicrobial ingredients) and quality (identify and quantify active ingredient) and (c) to identify data gaps. Peer reviewed literature search was conducted by querying two online databases: PubMed and Google scholar from November 15, 2018 to February 2019. The inclusion criteria for eligibility were articles: published in English between 2008 and 2018, including poultry (chicken) or cattle or both, E. coli bacteria of choice, antimicrobial use on farms, quantitative data and quality of antimicrobial used. Microsoft Excel was used for data extraction and Rayyan software for eligibility studies. The search retrieved 1,446 probable articles including those from the reference list of significant papers, of which twenty-four articles remained on full text review with more than a third of the studies being conducted in Nigeria. Farm surveys and antimicrobial sales were identified as the main sources of data and the mean quantities of antimicrobials based on sales data were 23,234, 41,280.87, and 1,538,443 kg of the active ingredient in Nigeria, Zambia and South Africa, respectively. One study from Cameroon determined the quantities of active ingredients based on dose metrics while another study still from Cameroon mentioned the quality of antimicrobials. Tetracyclines, beta-lactams/aminoglycosides and fluoroquinolones were the most common classes of antimicrobials (antibiotics) used. Our review reveals a dearth of information in Sub- Saharan Africa on the quantity and quality of veterinary drugs and yet they play a role in the overall picture of antimicrobial resistance. This finding gives an opportunity in the area of focus for future research as far as resistance and multidrug resistance are concerned in food producing animals.
Introduction
Antimicrobial use (AMU) in livestock production, is not only for improving productivity and sustainability but also as growth enhancers (1). Its use involves different classes of antimicrobials of varying doses and their implementation methods depend on the livestock species and production system (2). Owing to the increasing demand for dietary protein intake of foods of animal origin, livestock production in developing economies has become intensive whereby AMU is inevitable (3, 4). However, there is mounting evidence over the years that the dependence of food producing animals on antimicrobials due to their indiscriminate and inappropriate usage has led to the selection, emergence, and spread of antimicrobial resistant bacterial strains in both animals and humans (5, 6). Although its magnitude is unknown, it is likely to vary depending on the type and quantity of antimicrobial used. This resistance phenomenon is of ultimate global health concern and the situation is worsened by the emergence of multiple drug resistance (MDR) in food animals. Increased levels of antimicrobial resistance (AMR) in livestock production either reduce farm productivity or increase disease treatment costs (7). Consequently, several calls have been made to optimize this usage in order to limit the growth of AMR in humans (8–10).
A previous study by O'Neill (10), predicted antimicrobial consumption in food animals to rise by 67% by 2030 globally, and nearly double in Brazil, Russia, India, China, and South Africa. This rise was probably attributed to the growth in consumer demand for livestock dietary products (eggs, meat, milk) in middle-income countries and a shift to large-scale farms where antimicrobials are used routinely (3, 11). Earlier studies by McEwen and Fedorka-Cray (12) and Moulin et al. (13) indicated that in Europe and the United States antimicrobials in livestock production represent the largest fraction (66–80%) of the total global usage.
AMU measurement in livestock production is of importance, as it addresses several issues among which include; monitoring AMU over time, setting benchmarks to promote AMU reduction, and correlating the association between AMU and AMR. However, data across studies cannot be compared due to diverse metric systems in the measurement or quantification of antimicrobials (14). This is further complicated by inadequate resources and research capacity which is typical of developing countries (8).
Although research has increased in recent years on the role of poor-quality veterinary medicine, its impact has not been incorporated into the overall picture of antimicrobial resistance by the scientific community (15). This knowledge gap in veterinary antimicrobials can be exploited in the emergence of antimicrobial resistance (16).
In the current article, we reviewed and summarized original peered-reviewed research articles on AMU in cattle and poultry production in sub-Saharan Africa. The aim of this study was to assess the literature on antimicrobial usage (practices, frequency, class, type) in cattle and poultry production with regard to resistance in E. coli including MDR, summarize evidence for quantitative (volumes of active antimicrobial ingredients) and quality (identify and quantify active ingredient) of antimicrobials from 2008 to 2018.
Materials and methods
This review covers the use of antimicrobials in cattle and poultry production, with the following research question: What is the pattern of antimicrobial use in terms of classes and purpose; what methods are used to quantify antimicrobials and their quality with regard to the occurrence of resistance in E. coli including MDR? This systematic review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines (17). It was conducted in four steps: database search, evaluation of the articles, data extraction and Library formation/summary. Search criteria were defined and verified by researchers, and also modalities on how to settle disagreements before the initiation of the study.
Data sources and search strategy
A multifaceted search was conducted by querying two online databases: PubMed and Google scholar between November 15, 2018 and February 2019 for published literature in English. Boolean operators (AND/OR) were used among keywords like antimicrobial usage, quantity, quality, livestock, poultry, chicken, cattle, dairy and beef followed by specific names of individual countries in sub-Saharan Africa for relevant articles published between 2008 and 2018. In addition, reference lists of relevant articles were searched manually for supplementary literature. This period (2008 and 2018) is justified by numerous studies conducted on antimicrobial use and resistance in cattle and poultry production. The final search string and the number of citations used in this study are shown in Supplementary Table 1.
Eligibility article assessment/evaluation
The inclusion criteria were, studies; (i) published in English (ii) focused on quality, qualitative and quantitative data on antimicrobial use in poultry or cattle or other livestock species but poultry or cattle inclusive (iii) conducted between 2008 and 2018 in any of the 46 countries in sub-Saharan Africa, (iv) original research study (v) mentioned about E. coli. Citations of included articles were downloaded and stored as Comma delimited files. The files were eventually exported to Rayyan online application software for selection eligibility by two researchers (RA and FD). The researchers independently screened the relevant articles based on their titles and abstracts against the search criteria (first screening), followed by full text reading (second screening). Contentions in article selection were resolved on consensus by the researchers.
Quality assessment
Articles were graded based on the grading approach by the GRADE Working Group (18) for human research, on full text review since we did not come across that for animal research. This approach grades an article on the basis of quality, directness, and consistency for quality of evidence. In our review, quality was given a score of two, one on evidence of statistical analysis and another on bias or design limitations. The directness score was based on whether the methods and results presented were clear and easily understood and the consistency score was on the fact that the results and conclusion presented appeared to be consistent with the methodology. When the three scoring categories are combined, each article could receive a maximum score of plus four (+4) and a minimum score of minus six (−6) (Table 1).
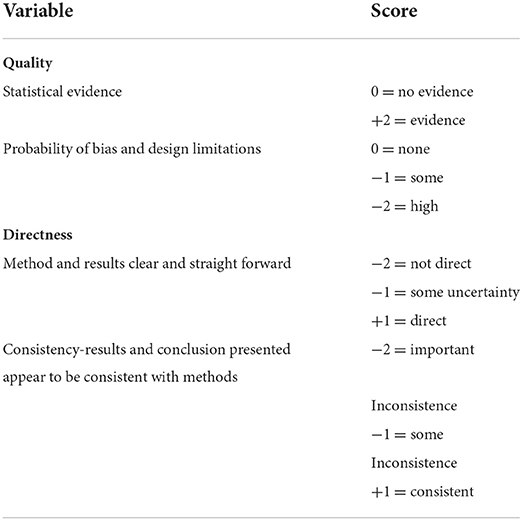
Table 1. Scoring system for generating a grade for articles on full text review based on the GRADE approach.
Data extraction and management
Data from eligible study articles were extracted and summarized onto a Microsoft® Office Excel 2007 framework sheet by RA and revised independently by FD. For each article, information was documented systematically in detail of publication (country, author, year of study, study unit, sample type, study population (cattle, poultry, goat, sheep, and pigs) and antimicrobial use (AMU), Supplementary Table 2. To minimize bias, articles were carefully scrutinized during data extraction due to variations in study execution and reporting methodologies. In circumstances where information was not clear, the onus was upon the researchers to either include or exclude it on full text review or contact the author by email for clarity.
Results
Eligible studies
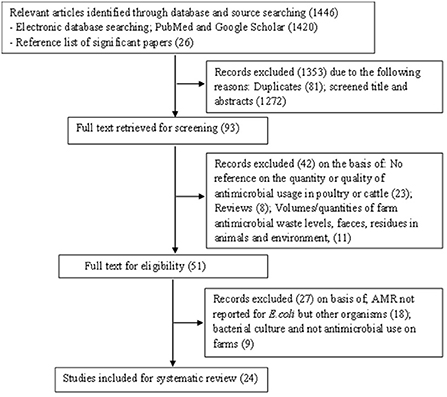
A total of 1,446 articles were retrieved from two online databases: PubMed and Google scholar as well as through a manual search of reference lists of relevant articles. On screening and duplicate removal, 93 articles remained for the initial title and abstract screening. Of these 51 articles were eligible for full text review based on inclusion criteria. However, twenty-seven articles were excluded with reason on full text review. In total 24 articles were included in this systematic review as shown in PRISMA flow diagram Figure 1.
Description of the included studies and data sources
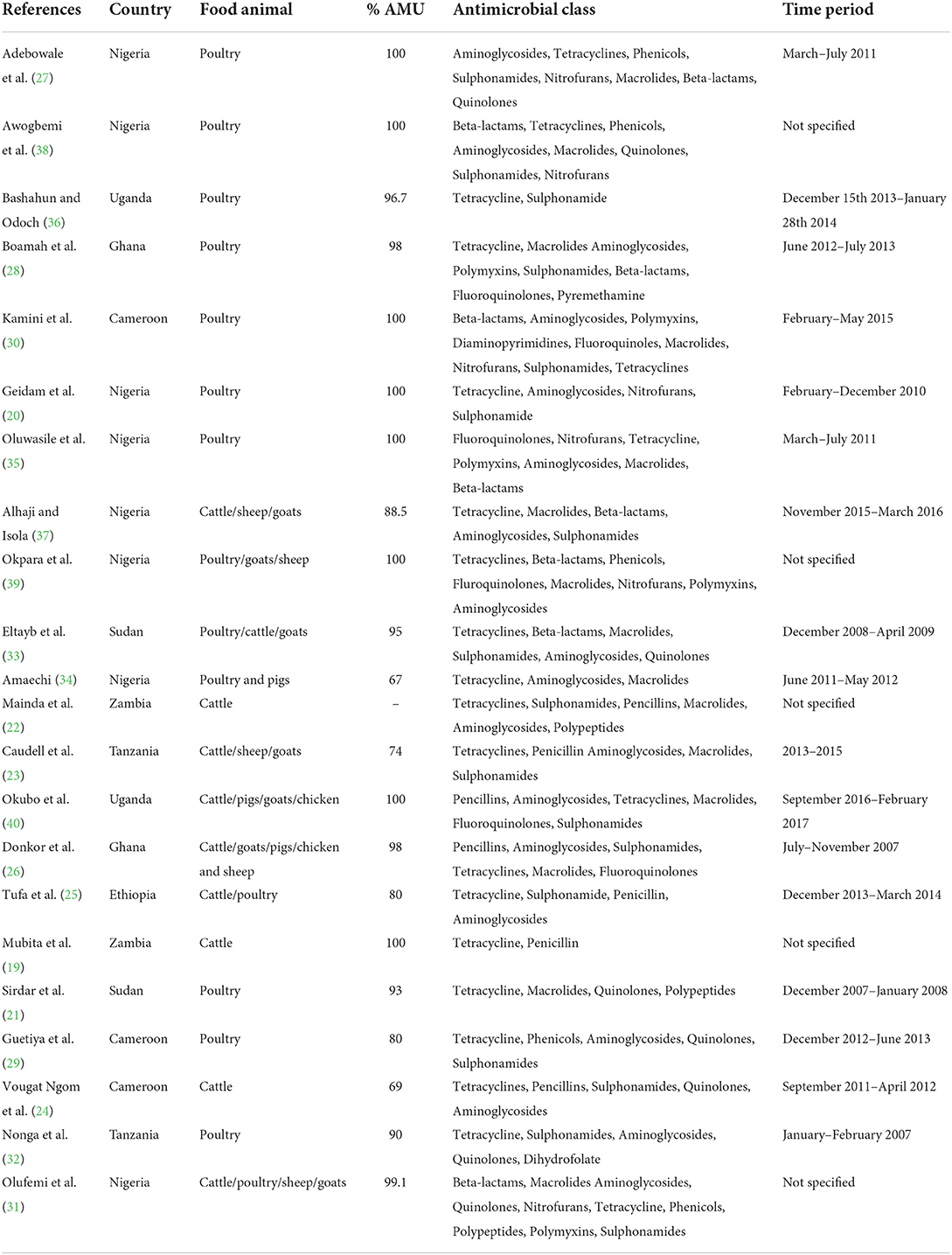
As shown in Table 2, twenty-four studies were included in the final analysis of this systematic review. Nearly a third (9/24, 38%), of the studies were conducted in Nigeria and the rest in other sub-Saharan African countries. Almost half (10/24, 42%) of the relevant articles identified were based on poultry, three on cattle (beef or dairy) and eleven on more than one animal species with either both cattle and poultry inclusive or one of them. Antimicrobial use or data (prevalence of use/antimicrobial classes, or antimicrobials sold) was mentioned in all the studies and these studies were cross-sectional in design. Two data sources were identified; farm surveys and antimicrobial sales data. Of the 24 studies, twenty collected data through farm surveys only, two compiled data from antimicrobial sales alone and two collected from both farm surveys and antimicrobial sales. Three studies estimated the quantities of antimicrobials from sales data both nationally and regionally, one study estimated the quantities based on dose metrics from farm data and only one study mentioned about the quality of antimicrobials.
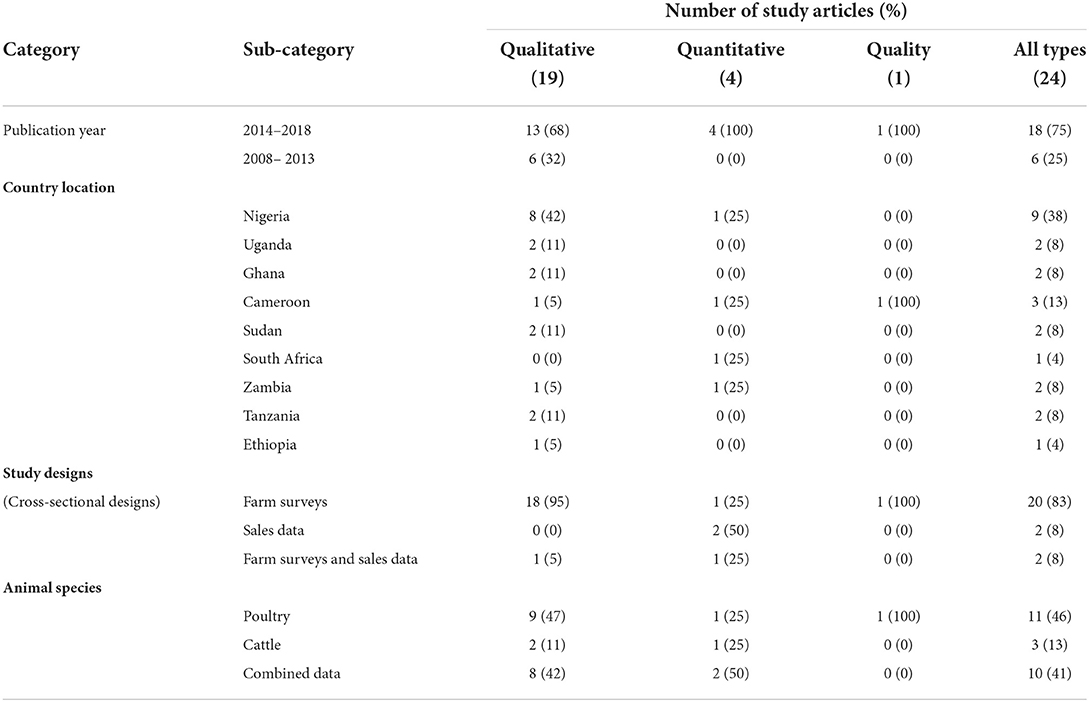
Table 2. Summary of 24 articles on antimicrobial use (AMU) stratified by study year, country location, study design, and livestock species.
Antimicrobial use in food producing animals
Antimicrobials were used in poultry and cattle production for different purposes. They were either used for therapeutic/ prophylactic purposes or as growth enhancers. However, the highest usage was observed in poultry. Seven articles indicated that antimicrobials were mostly used for therapeutic purposes (19–25), two for prophylactic (26, 27), four for both prophylactic and therapeutic (28–31), and nine for all purposes (32–40).
Antimicrobial usage percentage on the farms varied from 67% in Nigeria to 100% in Cameroon, Nigeria and Zambia. The commonly used antimicrobial classes were tetracyclines, beta-lactams/aminoglycosides and fluoroquinolones (Table 3). Of the 24 articles, four studies reported on antimicrobial sales. One of the studies estimated a mean quantity of 1,538,443 kg over a period of 3 years (41) based on national sales in South Africa, another study reported a mean quantity of 23,234 kg over a period of 3 years (42) based on the sales in South-Western region of Nigeria while the third study reported a mean quantity of 41,280.87 kg sold over a period of 1 year in Zambia (22) and the fourth study reported on the brands of antimicrobials marketed by the drug shop outlets without specifying the volumes or quantities sold in North-Eastern Nigeria (20). However, a study by Kamini et al. (30) reported on the quantitative estimates based on dose metrics (defined daily doses) of active antimicrobial ingredients. Only one study reported quality determination using High-performance liquid chromatography (HPLC) (24).
Assessment of antimicrobial resistance
Seven studies reported on different antimicrobial resistance (19, 22–29, 38–40) levels within and between countries. The proportions of AMR of E. coli isolates ranged from 6.5% in Zambia (22) to 100% in Nigeria (38). Clinical and Laboratory Standards Institute (CLSI) (43) guidelines were used for antimicrobial susceptibility testing (AST) in most of the studies and EUCAST (European Committee on Antimicrobial Susceptibility Testing) (44) in only one study (40). Overall E. coli isolates were screened with varying amounts of antibiotics ranging from 6 (22) to 14 (39) across the respective studies using disk diffusion (5/6) and broth microdilution (1/6) as the main methods of AST. Susceptibility testing was frequently performed on tetracycline, gentamicin, ampicillin, chloramphenicol, ciprofloxacin, cotrimoxazole, -augmentin, trimethoprim-sulfamethoxazole nalidixic acid, amoxicillin, kanamycin, and streptomycin.
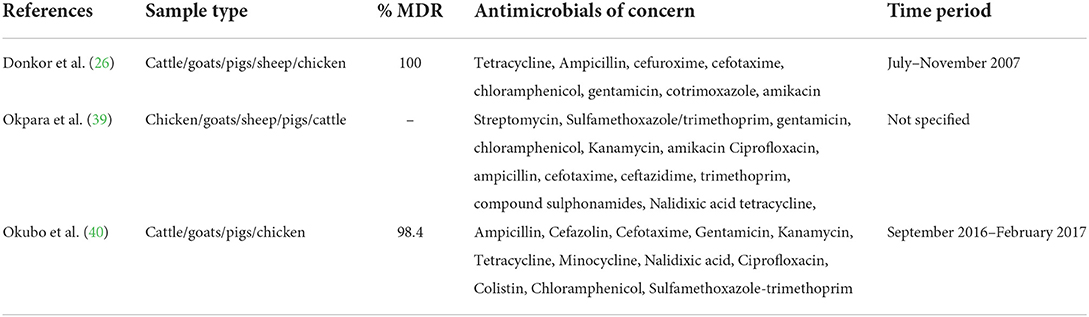
Multidrug resistance in Escherichia coli
Three studies reported on multidrug resistance. The proportion of multidrug resistance (MDR) strains among E. coli, which is an indicator organism, is shown in Table 4. These studies defined MDR as non-susceptibility to antimicrobial agents belonging to at least three or more different antimicrobial classes (26, 39, 40). The MDR E. coli proportions ranged from 98.4% in Uganda (40) to 100% in Ghana (26).
Discussion
Information on antimicrobial use in food animals is useful for several reasons, among which include raising awareness, identification of use pattern trends over time, antimicrobial resistance data integration, and evaluation of effective measures on judicious use of antimicrobials (45). Several studies on antimicrobial use have been conducted over the past decade and in this review, most of the studies were between 2008 and 2018. The majority of 9/24 of the studies were conducted in Nigeria. This implies that the public health significance of resistance to food production animals is recognized in Nigeria by the government since it provides research funding as reflected in two studies (27, 31). Although other studies in Nigeria did not indicate the source of funding.
Article type
We reviewed 24 articles on antimicrobial use in poultry and cattle production published in English since 2008. A number of articles (n = 22) reported on qualitative (proportion) usage on farms with time frames although a few did not specify. The importance of the time frame is that it simplifies the interpretation of data since usage is dependent on the observation period. Information on proportion of usage is important among other reasons; such as comparing use patterns across countries and conducting risk assessments. Interestingly, only one article from Cameroon was identified on the quality of antimicrobials (24). Although not verified, probably this reflects language bias, as it is likely that some studies were published in languages other than English, or were outside the scope of the search engine. Quality of antimicrobials is of importance as low or poor quality may play a role in infection treatment failure due to incorrect active substances. One stud estimated quantities of antimicrobials based on dose metrics (30). Quantitative data is dose dependent, and when coupled with antimicrobial resistance data may potentially help in explaining the association between antimicrobial usage and antimicrobial resistance (46). Since antimicrobial active principles/substances vary in their potency, usage of dose-based metrics results in a fairer comparison between antimicrobials. However, there is no universally accepted dose standard, as these vary by country, species, route of application, and indication (47). Even if doses are standardized, estimating the number of doses from gross amounts of active ingredients is challenging because animals (especially poultry and pigs) may increase their body size over production (48).
Data sources
In most of the studies, farm surveys and antimicrobial sales were the two main sources of data for this review. However, farm surveys were the primary source of data, since most of these countries have not yet developed a national antimicrobial use monitoring system. Farm surveys which can either be longitudinal or cross sectional, have an advantage over antimicrobial sales in that they give detailed information on the species for which the antimicrobial is being used, the purpose for the use, dosage form, treatment duration, and production type. Unreliable antimicrobial sales figures make accurate antimicrobial use data collection difficult and labor intensive. However, when comprehensive antimicrobial sales data are used in monitoring antimicrobial use trends over time, as long as the production animal population is stable. Antimicrobial use data when collated by national surveillance systems are used in determining the impact of large-scale interventions, as performed in Norway (49).
Antimicrobial use
Antimicrobial use frequency (qualitative data) from specific studies suggests a diversity of antimicrobials used for both prophylactic and therapeutic purposes, as well as growth promotion, although results are difficult to compare across studies. Tetracyclines, fluoroquinolones and beta-lactams/aminoglycosides were the common antimicrobials used on the farms regardless of the species of food animal in the various studies. This probably suggests that these antimicrobials are readily available in these countries over the counter and are inexpensive compared to third-generation antimicrobials. This finding concurs with observations by Chantziaras et al. (50), in one of their studies on antimicrobial use in livestock production in Europe. This could be due to the non-existent/lack of enforcement of regulatory measures in developing countries which has resulted in abuse of those classes of antimicrobials in food production animals unlike in developed countries where it can be attributed to the prescription tendency of veterinarians. The unregulated use of critically important antibiotics like fluoroquinolones used in human medicine in food producing animals is worrisome (51).
Antimicrobial and multidrug resistance
The resistance prevalence ranged from 6.5 to 100% and that of multidrug resistance from 33.3 to 100%. This could be due to unregulated use and administration of antimicrobials which exert selection pressure on the emergence of resistant bacterial strains. Secondly, the numerous resistance patterns also imply that livestock practices in Africa are reliant on antimicrobials (52). Regarding the species type, poultry had the highest prevalence of resistant or multidrug resistant Escherichia coli in our study. This can be exemplified by rapid growth and high financial returns and easy management by farmers in close proximity (Intensive system) where antimicrobial usage is high to curb morbidity and mortality. Our findings coincided with studies carried out in developing countries like Thailand and Vietnam (53, 54) but higher than in developed countries like Denmark which was in the range: (of 4–65%) (55–58). This is probably because of long term monitoring and surveillance, biosecurity measures, and the ban of growth promoters in food producing animals in developed countries. Such policies and measures would have an impact on the emergence, development and spread of antimicrobial resistance in food animal production in Sub-Saharan Africa.
The pathogen prevalence in poultry and cattle and the level of antimicrobial resistance and susceptibility test to different antimicrobials is enough evidence to guide antimicrobial selection and support for judicious use. However, the lack of antimicrobial use monitoring systems and research capacity limitations typical of many LMICs represent other challenges (8). Therefore, animal health workers or veterinarians rarely collect samples for bacterial identification and antimicrobial sensitivity tests. Our findings demonstrate that antimicrobial resistance in food producing animals is a problem and is associated with the unregulated administration of antimicrobials by farmers and also the non-existence of regulatory use measures. Bearing in mind that antimicrobial resistance is of worldwide concern in humans and livestock, policies based on regulatory control of antimicrobial use are necessary and farmers training on judicious antimicrobial use to reduce the risk/number of AMR pathogens transmitted to humans via direct and indirect contact with livestock and poultry.
This review has some limitations. We managed to gain full access to two online databases and so there was a possibility of not recovering key articles due to search strategy boundaries as well as search interfaces. However, we minimized this effect by referring to the reference list of significant research articles. This review covers 24 articles published in English so there is a possibility that there were similar articles published in other languages in some Sub-Saharan countries which may offer similar or different findings. Questionnaire based antimicrobial use surveys cannot detect misuse and off-label use, and as such approaches like prescription, reviews are needed. Furthermore, we included a few developing countries mostly those from Southeast Asia because those countries in addition to increased levels of animal product production and consumption where AMU is inevitable to meet the demand of the increased population are also considered to be a hotspot of infectious disease and AMR. Future studies should compare developing countries not included in this study to those developed countries not considered in terms of AMU and AMR.
Conclusion
This study has revealed a high level of antimicrobial usage, especially tetracyclines, fluoroquinolones and beta-lactams/aminoglycosides in cattle and poultry production in sub-Saharan Africa. This is likely to intensify the already high prevalence of antimicrobial resistance and multidrug resistance in the region. This, coupled with low enforcement of antimicrobial regulatory measures in most of the sub-Saharan African countries is of concern to food animals and public health. Secondly, the review has indicated a deficit of studies on the estimates of quantity and quality of antimicrobials used in food producing animals (poultry and cattle) in sub-Saharan Africa yet they play a role in the overall picture of antimicrobial resistance This therefore has given us a node of focus for future research. The study has also confirmed that antimicrobials of veterinary importance as defined in the WHO list (59) as the highest priority critically important antimicrobials in humans were still used in poultry and cattle production.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RA conceived the idea, performed a literature search, data extraction, and drafted the first version of the manuscript. FD cleaned the data and revised the first version of the manuscript. MS, MM, and SK were involved in the revision of the final manuscript before submission. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wish to thank the members of the Antimicrobial Community of Practice (CoP) at the SACIDS Foundation for One Health based at Sokoine University of Agriculture for their continuous encouragement during the review process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1000457/full#supplementary-material
References
1. Onu PN, Ude FE, Okpaniezeani PW. Effect of graded levels of dietary penicillin of the growth rate and feed conversion of broiler chicks. JASR. (2004) 4:25–32. doi: 10.4314/jasr.v4i2.2813
2. Sawant AA, Sordillo LM, Jayarao BM. A survey on antibiotic usage in dairy herds in Pennsylvania. J Dairy Sci. (2005) 88:2991–9. doi: 10.3168/jds.S0022-0302(05)72979-9
3. Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. (2015) 112:5649–54. doi: 10.1073/pnas.1503141112
4. UN FAO. The State of Food Agriculture. (2009). Available online at: http://www.fao.org/docrep/012/i0680e/i0680e.pdf (accessed February 13, 2018).
5. Aarestrup FM, Wegener HC. The effects of antibiotic usage in food animals on the development of antimicrobial resistance of importance for humans in Campylobacter and Escherichia coli. Microbes Infect. (1999) 1:639–44. doi: 10.1016/S1286-4579(99)80064-1
6. Gyssens IC. Quality measures of antimicrobial drug use. Int J Antimicrob Agents. (2001) 17:9–19. doi: 10.1016/S0924-8579(00)00208-9
7. Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. (2017) 1:e316–27. doi: 10.1016/S2542-5196(17)30141-9
8. Landers TF, Cohen B, Wittum TE, Larson EL, Faan C. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. (2012) 127:4–22. doi: 10.1177/003335491212700103
9. Woolhouse M, Ward M, van Bunnik B, Farrar J. Antimicrobial resistance in humans, livestock and the wider environment. Philos Trans R Soc Lond B Biol Sci. (2015) 370:20140083. doi: 10.1098/rstb.2014.0083
10. O'Neill J. Antimicrobials in agriculture and the environment: reducing unnecessary use and waste. In: The Review on Antimicrobial Resistance. Report by HM Government and the Wellcome Trust, London, United Kingdom. (2015). Available online at: https://amr-review.org/Publications.html (accessed January 15, 2019).
11. Mehdi Y, Létourneau-Montminy MP, Gaucher ML, Chorfi Y, Suresh G, Rouissi T, et al. Use of antibiotics in broiler production: global impacts and alternatives. Anim Nutr. (2018) 4:170–8. doi: 10.1016/j.aninu.2018.03.002
12. McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. (2002) 34 (Suppl. 3):S93–106. doi: 10.1086/340246
13. Moulin G, Cavalié P, Pellanne I, Chevance A, Laval A, Millemann Y, et al. A comparison of antimicrobial usage in human and veterinary medicine in France from 1999 to 2005. J Antimicrob Chemother. (2008) 62:617–25. doi: 10.1093/jac/dkn213
14. Collineau L, Belloc C, Stark KD, Hemonic A, Postma M, Dewulf J, et al. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health. (2017) 64:165–84. doi: 10.1111/zph.12298
15. Zaman M. Bitter Pills: The Global War on Counterfeit Drugs. Oxford: Oxford University Press (2018).
16. Clifford K, Desai D, Prazeres da Costa C, Meyer H, Klohe K, Winkler A, et al. Antimicrobial resistance in livestock and poor quality veterinary medicines. Bull World Health Organ. (2018) 96:662–4. doi: 10.2471/BLT.18.209585
17. Moher D, Liberati A, Tetzlaff J, Altman D, Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoSMed. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. Guyatt GH, Oxman AD, Scheunemann H.J, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–82. doi: 10.1016/j.jclinepi.2010.09.011
19. Mubita C, Syakalima M, Chisenga C, Munyeme M, Bwalya M, Chifumpa G, et al. Antibiograms of faecal Escherichia coli and Enterococci species isolated from pastoralist cattle in the interface areas of the Kafue basin in Zambia–Short communication. Vet Arh. (2008) 78:179–85.
20. Geidam YA, Ibrahim UI, Grema HA, Sanda KA, Suleiman A, Mohzo DL. Patterns of antibiotic sales by drug stores & usage in poultry farms: a questionnaire-based survey in Maiduguri, Northeastern Nigeria. J Anim Vet Adv. (2012) 11:2852–55. doi: 10.3923/javaa.2012.2852.2855
21. Sirdar MM, Picard J, Bisschop S, Gummow B. A questionnaire survey of poultry layer farmers in Khartoum State, Sudan, to study their antimicrobial awareness and usage patterns. Onderstepoort J Vet Res. (2012) 79:1–8. doi: 10.4102/ojvr.v79i1.361
22. Mainda G, Bessell PR, Muma JB, McAteer SP, Chase-Topping ME, Gibbons J, et al. Prevalence and patterns of antimicrobial resistance among Escherichia coli isolated from Zambian dairy cattle across different production systems. Sci Rep. (2015) 5:12439. doi: 10.1038/srep12439
23. Caudell MA, Quinlan MB, Subbiah M, Call DR, Roulette CJ, Roulette JW, et al. Antimicrobial use and veterinary care 498 among agro-pastoralists in Northern Tanzania. PLoS ONE. (2017) 12:1–18. doi: 10.1371/journal.pone.0170328
24. Vougat Ngom RRB, Tomdieu T, Ziébé R, Foyet HS, Moritz M, Vondou L, et al. Quality of veterinary pharmaceuticals and their use by pastoralists in the Far North Region of Cameroon. Pastoralism Res Policy Pract. (2017) 7:1–14. doi: 10.1186/s13570-017-0081-5
25. Tufa TB, Gurmu F, Beyi AF, Hogeveen H, Beyene TJ, Ayana D, et al. Veterinary medicinal product usage among food animal producers and its health implications in Central Ethiopia. BMC Vet Res. (2018) 14:1–7. doi: 10.1186/s12917-018-1737-0
26. Donkor ES, Newman MJ, Yeboah-Manu D. Epidemiological aspects of nonhuman antibiotic usage and resistance: implications for the control of antibiotic resistance in Ghana. Trop Med Int Heal. (2012) 17:462–8. doi: 10.1111/j.1365-3156.2012.02955.x
27. Adebowale OO, Adeyemo OK, Awoyomi O, Dada R, Adebowale O. Antibiotic use and practices in commercial poultry laying hens in Ogun state Nigeria. Rev Elev Vet Pays Trop. (2016) 69:41–5. doi: 10.19182/remvt.31170
28. Boamah VE, Agyare C, Odoi H, Dalsgaard A. Antibiotic practices and factors influencing the use of antibiotics in selected poultry farms in Ghana. J Antimicrob Agents. (2016) 2:1–8. doi: 10.4172/2472-1212.1000120
29. Guetiya WR, Zambou ENF, Anyangwe FF, Njimou JR, Coman MM, Verdenelli MC, et al. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Bri Poult Sci. (2016) 57:483–93. doi: 10.1080/00071668.2016.1180668
30. Kamini MG, Keutchatang FT, Huguette YM, Kansci Germain A, Gabriel MN. Antimicrobial usage in the chicken farming in Yaoundé, Cameroon: a cross sectional study. Int J Food Contam. (2016) 3:1–6. doi: 10.1186/s40550-016-0034-6
31. Olufemi EO, Eniola F, Ademola AM, Morenike AD. Antimicrobials in animal production: usage and practices among livestock farmers in Oyo and Kaduna States of Nigeria. Trop Anim Health Prod. (2016) 48:189–97. doi: 10.1007/s11250-015-0939-8
32. Nonga HE, Mariki M, Karimuribo ED, Mdegela RH. Antimicrobial usage and residue in Morogoro. Pakistan J Nutr. (2009) 8:203–7. doi: 10.3923/pjn.2009.203.207
33. Eltayb A, Barakat S, Marrone G, Shaddad S, Lundborg CS. Antibiotic use and resistance in animal farming: a quantitative and qualitative study on knowledge and practices among farmers in Khartoum. Sudan Zoonoses Public Health. (2012) 59:330–8. doi: 10.1111/j.1863-2378.2012.01458.x
34. Amaechi NA. Survey on antibiotic usage in pigs and poultry birds in Abia State, Nigeria. Glob J Med Res C Microbiol Pathol. (2014) 14:1–9.
35. Oluwasile BB, Agbaje M, Ojo OE, Dipeolu MA. Antibiotic usage pattern in selected poultry farms in Ogun State. Sokoto J Vet Sci. (2014) 12:45–50. doi: 10.4314/sokjvs.v12i1.7
36. Bashahun GMD, Odoch AT. Assessment of antibiotic usage in intensive poultry farms in Wakiso District, Uganda. Livest Res Rural Dev. (2015) 27:247. Available online at: http://www.lrrd.org/lrrd27/12/bash27247.html
37. Alhaji NB, Isola TO. Antimicrobial usage by pastoralists in food animals in north- Central Nigeria: the associated socio-cultural drivers for antimicrobials misuse and public health implications. One Heal. (2018) 6:41–7. doi: 10.1016/j.onehlt.2018.11.001
38. Awogbemi J, Adeyeye M, Akinkunmi EO. A survey of antimicrobial agents usage in poultry farms and antibiotic resistance in Escherichia coli and staphylococci isolates from the poultry in Ile-Ife, Nigeria. J Infect Dis Epidemiol. (2018) 4:4–11. doi: 10.23937/2474-3658/1510047
39. Okpara EO, Ojo OE, Awoyomi OJ, Dipeolu MA, Oyekunle MA, Schwarz S. Antimicrobial usage and presence of extended-spectrum β-lactamase-producing Enterobacteriaceae in animal-rearing households of selected rural and peri-urban communities. Vet Microbiol. (2018) 218:31–9. doi: 10.1016/j.vetmic.2018.03.013
40. Okubo T, Yossapol M, Maruyama F, Wampande EM, Kakooza S, Ohya K, et al. Phenotypic and genotypic analyses of antimicrobial resistant bacteria in livestock in Uganda. Transbound Emerg Dis. (2018) 66:317–26. doi: 10.1111/tbed.13024
41. Eagar H, Swan G, Van Vuuren M. A survey of antimicrobial usage in animals in South Africa with specific reference to food animals. J S Afr Vet Assoc. (2012) 83:15–23. doi: 10.4102/jsava.v83i1.16
42. Adesokan HK, Akanbi IO, Akanbi I.M, Obaweda RA. Pattern of antimicrobial usage in livestock animals in south western Nigeria: The need for alternative plans. Onderstepoort J Vet Res. (2015) 82:816. doi: 10.4102/ojvr.v82i1.816
43. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Sixth Informational Supplement. CLSI document M100-S26. Wayne, PA: Clinical and Laboratory Standards Institute (2016).
44. European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST Clinical Breakpoint Tables Version 7.1. (2017). Available online at: http://www.eucast.org/clinical_breakpoints (accessed January 28, 2019).
45. Werner N, McEwen SA., Kreienbrock L. Monitoring antimicrobial drug usage in animals: methods and applications. Microbiol Spectrum. (2018) 6:ARBA-0015-2017. doi: 10.1128/microbiolspec.ARBA-0015-2017
46. European Medicines Agency (EMA). Guidance on Collection and Provision of National Data on Antimicrobial Use by Animal Species/Categories. London: European Medicines Agency (EMA) (2018).
47. Radke BR. Towards an improved estimate of antimicrobial use in animals: adjusting the “population correction unit” calculation. Can Rech Vet. (2017) 81:235–40.
48. Boulianne M, Arsenault J, Daignault D, Archambault M, Letellier A, Dutil L. Drug use and antimicrobial resistance among Escherichia coli and Enterococcus spp. Isolates from chicken and turkey flocks slaughtered in Quebec, Canada. Rev Can Rech Vet. (2016) 80:49–59.
49. Grave K, Kaldhusdal M, Kruse H, Harr LMF, Flatlandsmo K. What has happened in Norway after the ban of avoparcin? Consumption of antimicrobials by poultry. Prev Vet Med. (2004) 62:59–72. doi: 10.1016/j.prevetmed.2003.08.009
50. Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother. (2014) 69:827–34. doi: 10.1093/jac/dkt443
51. OIE List of Antimicrobials of Veterinary Importance Criteria Used for Categorization List of Antimicrobials. (2007). Available online at: https://www.oie.int/doc/ged/D9840.PDF (accessed February 3, 2019).
52. Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP. Antibiotics in agriculture and the risk to human health: how worried should we be? Evol Appl. (2015) 8:240–5. doi: 10.1111/eva.12185
53. Boonyasiri A, Tangkoskul T, Seenama C, Saiyarin J, Tiengrim S, Thamlikitkul V. Prevalence of ABr bacteria in healthy adults, foods, food animals, and the environment in selected areas in Thailand. Pathog Global Health. (2014) 108:235–45. doi: 10.1179/2047773214Y.0000000148
54. Usui M, Ozawa S, Onozato H, Kuge R, Obata Y, Uemae T, et al. Antimicrobial susceptibility of indicator bacteria isolated from chickens in Southeast Asian countries (Vietnam, Indonesia and Thailand). J Vet Med Sci. (2014) 76:685–92. doi: 10.1292/jvms.13-0423
55. Geser N, Stephan R, Hächler H. Occurrence and characteristics of extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet Res. (2012) 8:21. doi: 10.1186/1746-6148-8-21
56. DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria From Food Animals, Food and Humans in Denmark. (2014). Available online at: www.danmap.org (accessed June 21, 2016).
57. European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA J. (2016) 14:4380. doi: 10.2903/j.efsa.2016.4380
58. NethMap-MARAN. Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2014. Lelystad, Netherland: Central Veterinary Institute of Wageningen University and Research Centre in collaboration with the Food and Consumer Product Safety Authority (NVWA), the National Institute for Public Health and the Environment (RIVM) and the Netherlands Veterinary Medicines Authority (SDa) (2015).
Keywords: antimicrobial use, cattle, poultry, quality, quantity, sub-Saharan Africa
Citation: Azabo R, Dulle F, Mshana SE, Matee M and Kimera S (2022) Antimicrobial use in cattle and poultry production on occurrence of multidrug resistant Escherichia coli. A systematic review with focus on sub-Saharan Africa. Front. Vet. Sci. 9:1000457. doi: 10.3389/fvets.2022.1000457
Received: 22 July 2022; Accepted: 30 September 2022;
Published: 24 October 2022.
Edited by:
Ioannis Magouras, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Vathsala Mohan, The New Zealand Institute for Plant and Food Research Ltd., New ZealandTakele Beyene Tufa, Addis Ababa University, Ethiopia
Madelaine Norström, Norwegian Veterinary Institute (NVI), Norway
Copyright © 2022 Azabo, Dulle, Mshana, Matee and Kimera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rogers Azabo, cm9nZXJzLmF6YWJvQHNhY2lkcy5vcmc=
 Rogers Azabo
Rogers Azabo Frankwell Dulle
Frankwell Dulle Stephen E. Mshana
Stephen E. Mshana Mecky Matee
Mecky Matee Sharadhuli Kimera
Sharadhuli Kimera