- 1Department of Clinical Sciences, Faculty of Veterinary Medicine, Semnan University, Semnan, Iran
- 2Department of Surgery and Radiology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
- 3Department of Internal Medicine, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
Introduction: Platelet indices changes in severely ill people and in dogs with inflammation are compatible findings. This study aimed to compare platelet indices between dogs with clinical benign prostatic hyperplasia (BPH) and healthy controls. Additionally, to determine whether there is a correlation between the relative prostatic size (Srel) and the platelet indices in BPH dogs.
Methods: Thirty-five adult intact male dogs of different breeds were allocated to the experimental groups: dogs with clinical BPH (groups A; n = 24; median age of 6 years; the median weight of 8.50 kg) and healthy dogs (group B; n = 11; median age 5.50 years; the median weight of 7.00 kg) based on physical examination, clinical signs, and Srel detected by ultrasonographic findings. The individual prostatic volume (IPV) was divided by the expected prostatic volume (EPV) to determine the relative prostatic size in dogs over 4 years old. Platelet indices were compared between the two groups, and a correlation between Srel and these indices was calculated.
Results: The median Srel of dogs in group A was significantly higher (P = 0.001), and the mean plateletcrit (PCT) was significantly lower (P = 0.003) compared with those in group B. Srel showed a significant negative correlation with PLT and PCT (r = −0.388; P = 0.02 and r = −0.402; P = 0.01). Receiver operating characteristic (ROC) analysis showed PLT and PCT thresholds for estimating Srel > 1 with 75% and 87.5% sensitivity and 71.82 and 63.64% specificity.
Discussion: The findings of this study support the use of platelet indices like PLT and PCT to detect clinical BPH in dogs. However, more research is needed to confirm their utility in conjunction with other previously described diagnostic factors.
Introduction
Benign prostatic hyperplasia (BPH) is the most common prostate disease in intact male dogs (1–3). BPH is an age-related spontaneous disease that many pet owners overlook (4, 5). Although the pathogenesis of BPH is unknown, androgen derivatives, androgen-to-estrogen ratio, and inflammation caused by chemokine released from the prostatic stroma all play a role in its development (6). A study of intact male dogs older than 5 and 9 years revealed that BPH affected 80 and 95% of them, respectively (7). Periodic bloody discharge, haematuria, haematospermia, dyschezia, strangury, incontinence, constipation, tenesmus, and stilted gait are the most common clinical signs of BPH (8). History, clinical signs, ultrasonography, and digital rectal examination (DRE) are non-invasive methods of diagnosing BPH. In contrast, fine-needle aspiration (FNA) and biopsy, as the gold standard of diagnosis, are invasive methods. The DRE has low sensitivity because it only examines the caudal part of the prostate. It is not suitable for large or small breeds, so it does not apply to all dogs (9). Clinically, a prostate biopsy is the gold standard, and FNAs are not routinely used to distinguish BPH from other prostatic lesions in living animals (10).
The ultrasound examination gives information about the size, shape, contour, echogenicity, symmetry, and adjacent soft tissues. Prostate size in dogs can be determined by the relative prostatic size (Srel) (11). The ratio between the prostate volume of an individual dog and the normal prostate volume is computed. As a result, dogs with a Srel > 1 had larger prostates than expected (11). In recent studies, circulating biomarkers such as canine prostatic specific esterase (CPSE) have been studied as prostate health markers. It was supported by the serum CPSE level in BPH-affected dogs being higher than that of healthy dogs. There is no data on the concentration of a specific esterase in dogs with different prostate disorders (12–14). CPSE displays promising potential for detecting BPH. However, it is not commonly used in clinical diagnosis as the prostate-specific antigen (PSA) in humans (12).
Platelets mediate hemostasis play a significant role in host inflammation. Hence, in a resting state, platelets circulate in the blood. Several platelet indices that may serve as proxy markers for platelet activation can be determined using optical-based automated hematology analyzers (15, 16). Two factors may contribute to the increased number of large platelets in the bloodstream during inflammation. (1) increased cytokine concentrations result in thrombocytosis, which produces large immature platelets regardless of platelet concentrations; (2) after activation, platelets undergo a change in shape, resulting in the formation of small protrusions on their surface (17).
MPV increases have been linked to acute appendicitis (18), pancreatitis (19), infectious endocarditis (20), myocardial infarction (21) and malaria (22). Several studies have found that increased MPV at admission and during hospitalization predicts a poorer outcome in critically ill patients with sepsis (23). Within 24 h of diagnosis, the only platelet parameter associated with increased mortality in dogs with surgically treated septic peritonitis was MPV (24). The higher MPV indicates that activated platelets are associated with inflammation. In a canine model, MPV and PDW can be used to diagnose and monitor dogs with endotoxemia (25).
Platelets play a substantial role in inflammatory responses, hemostasis, thrombosis, and wound healing by producing and secreting inflammatory and pro-inflammatory cytokines, such as IL-8 and 6 (26). Cytokines, chemokines, and inflammatory mediators are believed to be important in the pathogenesis, symptoms, and progression of BPH (27). Alternatively, thrombosis is affected by androgenic hormones such as dihydrotestosterone and the ratio of androgen to estrogen, affecting platelet indices (28). As a result of the alteration in the androgen-to-estrogen ratio in BPH, changing platelet indices should be expected. As a result, the purpose of this study was to compare platelet indices in dogs with clinical BPH and healthy controls. In addition, to see if there is a link between relative prostatic size (Srel) and platelet indices in BPH dogs.
Materials and methods
Study design
A prospective, case-controlled observational study on dogs with naturally occurring clinical BPH was conducted at the Semnan Veterinary Academic Hospital (SVAH) between August 2018 and July 2019. The current study was approved by the Faculty of Veterinary Medicine's Ethics Committee in biological research (REC12-96). All experiments were carried out in accordance with national guidelines and applicable national laws on animal protection. The study used privately owned dogs of various breeds and enrolled them only with the owner's permission.
Study population
This study included client-owned intact male dogs over the age of four who were clinically and ultrasonographically diagnosed with BPH and were otherwise healthy. The reproductive history (fertility or previous mating), physical examination, and clinical signs such as tenesmus, dysuria, constipation, stilted gait, hemorrhagic urethral discharge, and intermittent hematuria (7), as well as Srel detected by ultrasonography, were used to make a presumptive diagnosis of BPH (29). Platelet clumps visible in the blood film, urinary turbidity, history of renal failure (protein-losing nephropathy, chronic kidney disease, and ureteral obstruction), and coagulation disorders (thrombocytopenia, increased PT, and aPTT), current antiandrogenic and anticoagulant medication, and asymmetric and heterogeneous prostate on ultrasonographic findings were all exclusion criteria.
Ultrasonographic evaluation
Two independent radiologists evaluated the prostate appearance, border, and size using a 5–7.5 MHz micro convex probe (MyLab™ClassC; Esaote Spa, Genua, Italy) based on a standardized protocol (29). The individual prostatic volume (IPV) and the expected prostatic volume (EPV) were calculated according to Kamolpatana et al. (11) and Sannamwong et al. (29) as IPV = (1/2.6) * (Length × Width × Height) + 1.8 cm3 and EPV = 0.33 × body weight (BW) (kg) + 3.28, respectively. The IPV was compared with the EPV to obtain a relative prostatic size (Srel = IPV/EPV). The dogs were divided into two groups based on Srel and clinical signs of BPH; dogs with Srel > 1 and BPH-related clinical signs (Group A; n = 27) and dogs with Srel < 1 with no clinical signs of prostate disorders (Group B; n = 11) (Supplementary material). The prostate gland was also evaluated by sonography for cysts, parenchymal homogeneity, and shape symmetry.
Sample collection and laboratory methods
Peripheral blood was collected into plain and EDTA vacutainer tubes prior to any treatment (Deltalab). Within 1 h of blood collection, CBCs and blood smears were performed. Blood smears were prepared and stained with Giemsa (Merck), and platelet aggregation was assessed immediately after blood collection. PLT, PCT, MPV, and PDW were measured using a dog-validated automatic blood cell counter (Nihon Kohden, Celltac Alpha VET MEK-6550, Japan).
Statistical analysis
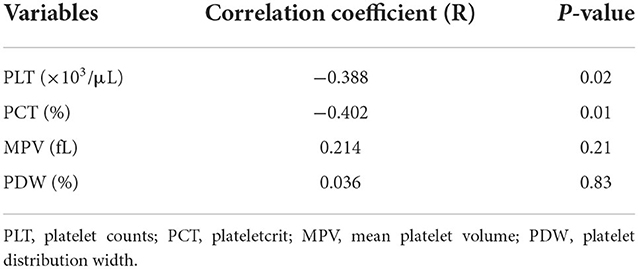
Statistical analysis was performed using MedCalc software (MedCalc, version 15.2, Statistical Software). Data was tested for normality using the Kolmogorov-Smirnov test and reported as mean ± standard deviation (SD) when normally distributed and median and interquartile range when not normally distributed. Threshold platelet indices were calculated by the receiver operating characteristic (ROC) analysis for diagnosing BPH. The correlation between Srel and platelet indices was determined using Spearman's rank correlation coefficient. Hence, the correlation coefficient (r); 0.20–0.39, 0.40–0.59, and 0.60–0.79 are weal, moderate and strong, respectively.
Results
Ultrasonographic findings of the canine prostate
There mainly were symmetrical enlargements and homogeneous parenchyma in the prostates of dogs in BPH dogs (Figure 1). The prostate of some cases (n = 3) was asymmetric and heterogeneous because of intraparenchymal cysts and nodules in the ultrasound evaluation, and these cases were excluded from the study. There were no visible changes in the prostate parenchyma in the B group. Group A included six mongrels and 18 other dogs from six defined breeds, i.e., Terrier (n = 10), Siberian Husky (n = 3), Spitz (n = 2), German Sphered (n = 1), Belgian Malinois (n = 1), and Pekingese (n = 1). The weight ranged from 3 to 55 kg, with a median of 8.30 kg (interquartile range [IQR]: 6.1–16). Age ranged from 4 to 15 years, with a median of 6.0 years (IQR: 5.25–7.75). Group B included three mongrels and eight other dogs from five defined breeds, i.e., Shih tzo (n = 3), Terrier (n = 2), German Sphered (n = 1), Pekingese (n = 1), and Pomer (n = 1). Weight ranged from 4.5 to 30 kg, with a median of 7.0 kg (IQR: 4.5–30.0). Age ranged from 4.5 to 10 years, with a median of 5.50 years (IQR: 5.0–7.5).
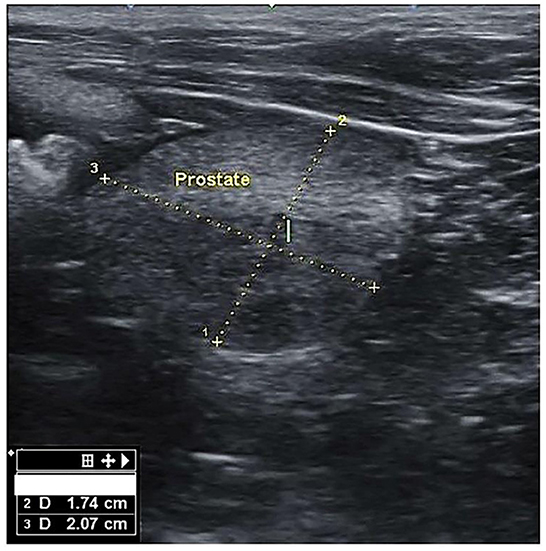
Figure 1. Transverse section of the prostate gland in dogs suffering from BPH, showing the height (1) and width (2).
Comparison of Srel and platelet indices between groups
The median Srel in group A was significantly higher than in group B (1.40; IQR: 1.12–1.96 vs. 0.82; IQR: 0.55–0.96, P = 0.001) (Figure 2). Most of the platelets of group B in blood smears were uniform and medium in size, whereas platelets of all dogs from group A showed anisocytosis. Platelet clumping was not detected in any of the blood smears. The mean PCT of dogs in group A decreased significantly (P = 0.003) compared with those in group B (13.54 ± 1.19%) and (0.20 ± 0.11%) (Figure 3). Compared to group B, a higher MPV (P = 0.969; Figure 4) and a lower mean of PLT (P = 0.233; Figure 5) and PDW (P = 0.903; Figure 6) were noted in group A.
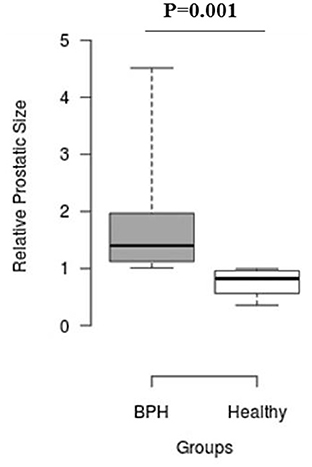
Figure 2. Box plots of relative prostatic size (Srel) in BPH and Healthy groups. Center lines show the medians; box limits indicate the 25th and 75th percentiles. n = 24, 11 sample points.
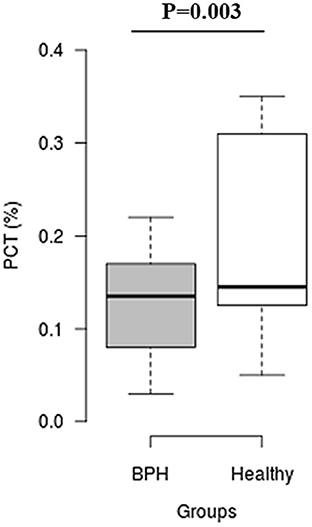
Figure 3. Box plots of plateletcrit (PCT) in BPH and Healthy groups. Center lines show the medians; box limits indicate the 25th and 75th percentiles. n = 24, 11 sample points.
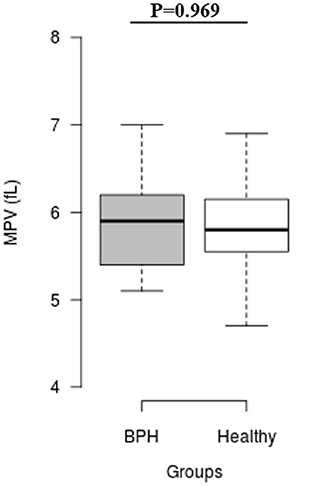
Figure 4. Box plots of mean platelet volume (MPV) in BPH and Healthy groups. Center lines show the medians; box limits indicate the 25th and 75th percentiles. n = 24, 11 sample points.
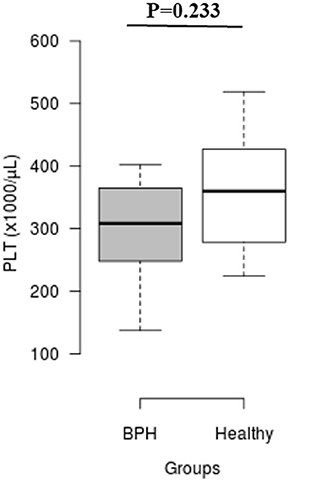
Figure 5. Box plots of platelet counts (PLT) in BPH and Healthy groups. Center lines show the medians; box limits indicate the 25th and 75th percentiles. n = 24, 11 sample points.
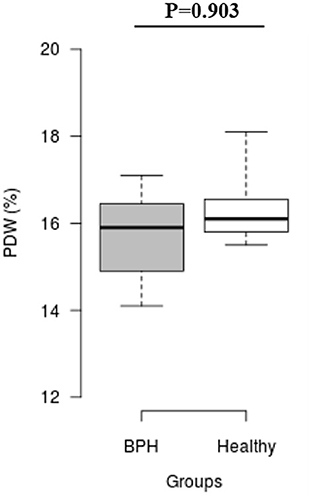
Figure 6. Box plots of platelet distribution width (PDW) in BPH and Healthy groups Center lines show the medians; box limits indicate the 25th and 75th percentiles. n = 24, 11 sample points.
Correlation between Srel and platelet indices
A significant negative correlation was found between Srel and PLT and PCT (r = −0.388; P = 0.02 and r = −0.402; P = 0.01, respectively). The correlation coefficient (Table 1) shows that MPV and PDW were weakly correlated (r = 0.214; P = 0.21 and r = 0.036; P = 0.83, respectively).
Cut-off values of platelet indices based on Srel for diagnosing clinical BPH
ROC analysis showed PLT threshold for estimating Srel > 1 with 75% sensitivity, 71.82% specificity (AUC; 0.716) was ≤ 326 × 103 (Figure 7). The PCT-based cut-off value Srel > 1 was ≤ 0.17% (AUC; 0.782, 87.5% sensitivity, 63.64% specificity; Figure 8).
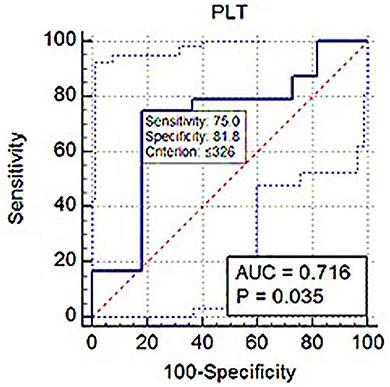
Figure 7. ROC (receiver operating characteristic) curve analysis of PLT for Srel > 1. The solid blue line between the two blue dashed lines is the corresponding cut-off point with sensitivity and specificity. The blue dashed lines, one on the upper left side and the other on the lower right side of the solid blue line, are the upper and lower 95% confidence bounds, respectively.
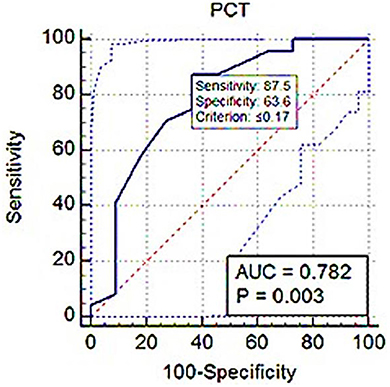
Figure 8. ROC (receiver operating characteristic) curve analysis of PCT for Srel > 1. The solid blue line between the two blue dashed lines is the corresponding cut-off point with sensitivity and specificity. The blue dashed lines, one on the upper left side and the other on the lower right side of the solid blue line, are the upper and lower 95% confidence bounds, respectively.
Discussion
The most common prostate disorder in dogs is BPH, which is followed by hormonal atrophy, squamous metaplasia, cysts, neoplasia, and inflammation (24). This study looked at the relationship between platelet indices and Srel in BPH dogs, taking into account evidence linking these indices to a variety of non-hematological illnesses in dogs (30, 31). Platelet mass is measured with the PCT and calculated using PLT and MPV (32). The studies evaluating PCT in malignant and benign tumors were limited. A higher PCT was found in benign epithelial ovarian masses than in healthy individuals, but the difference was insignificant (33). In this study, PCT was significantly lower in BPH dogs compared to healthy ones (P = 0.003). A reduction in platelet production by the bone marrow or an increase in giant platelet consumption by pro-inflammatory molecules may be associated with changes in these indices (34, 35). It has been shown that blood vessels protect themselves from tumor cells by natural killer cells, and tumor cells are protected by platelets from natural killer cells, resulting in lower platelet consumption in the early stages of tumors (36, 37). Platelets play a role in tumor angiogenesis and inflammation by producing and releasing vascular endothelial growth factor (VEGF) (38).
In terms of platelet function, large platelets are more active than small platelets (39). MPV has been studied in a variety of cancer types (40). A study showed that patients with benign goiter had significantly higher MPV levels than healthy people (41). Rifaioglu et al. (42) reported that asymptomatic prostatitis patients had a higher MPV than healthy individuals (34). However, other studies have presented conflicting results regarding the influence of prostate disorders on platelet indices (42–47). In the present study, a significant difference was not observed in MPV between the two groups.
PDW is a morphometric indicator used to describe the distribution of peripheral platelet size. There have been few studies on the relationship between various tumors and PDW, and even those that have been conducted have yielded contradictory results. PDW was significantly higher in ovarian cancer patients than in healthy people (48). However, some studies found a significant decrease in PDW levels in patients with non-small cell lung cancer, breast cancer, and malignant adnexal cancer (49–51). This study found no significant difference in PDW levels between BPH and healthy dogs.
Local hypoxia incidence in BPH exacerbates inflammation since low levels of reactive oxygen species encourage neovascularization and angiogenesis. Prostate stromal cells can also increase VEGF-2, VEGF-7, and tumor growth factor-β (TGF-β) secretion, all of which play important roles in determining prostatic growth rates in response to hypoxia (52, 53). The PLT and PCT correlate negatively with the relative prostatic size in BPH dogs. A reduction in platelet production by the bone marrow or an increase in giant platelet consumption by proinflammatory molecules may be associated with changes in these indices (34, 35). In inflammatory conditions, the relationship between PLT and MPV is inverse. As thrombopoiesis accelerates, large active circulating platelets migrate to inflamed sites where they are intensely consumed (51).
Eventually, in the ROC curve analysis, the AUC value was from 0.5 to 1; the closer the AUC value was to 1, the better the diagnostic effect. An AUC of between 0.7 and 0.9 showed high accuracy. In this study, the PLT and PCT use to distinguish BPH dogs from healthy dogs had a large AUC, increased sensitivity, and high precision.
Findings from the study by Fu et al. (44) suggest that MPV and PDW are associated with prostate cancer in patients. A specific mechanism showing a relationship between prostate cancer and MPV and PDW has not been precisely defined.
The function of platelets may differ based on sex hormones. Researchers suggest sex hormones have non-genomic effects on platelets or genomic impacts on megakaryocytes. Thromboxane A2 receptors were increased in healthy men by testosterone (T) treatment, which caused platelet activation. The arachidonate receptors increasing on blood platelets have proposed the link between T and platelet reactivity (54). However, T-stimulated endothelium also secretes nitric oxide (NO) that indirectly inhibits platelet activation (55). Platelet variable values are affected by thrombopoiesis intensity. Thrombopoiesis is affected by androgen hormones and the androgen-to-estrogen ratio (38). Based on Karolszak et al.'s (45) findings, androgen concentrations negatively correlate with various measurements of platelet morphology, such as MPV, PCT, PDW, and platelet large cell ratio (P-LCR). Therefore, changes in platelet indices should be expected because of the alteration in the androgen concentrations and androgen to estrogen ratio in BPH.
The current study discovered that: (1) PLT, MPV, and PDW measurements in dogs with BPH were not significantly different from controls; (2) PCT values in BPH dogs were significantly lower; (3) PLT and PCT were moderately negatively correlated with Srel; and (4) PLT and PCT sensitivity and specificity calculated by ROC analysis for estimating Srel can be used in conjunction with other diagnostic markers to evaluate clinical BPH in dogs. The findings of this study support the use of platelet indices like PLT and PCT to detect clinical BPH in dogs. However, more research is needed to confirm their utility in conjunction with other previously described diagnostic factors.
The current study has a limitation due to a lack of access to a valid and specific CPSE-ELISA kit. As a result, we were unable to determine whether the relationship between platelet indices and this diagnostic marker differed between BPH dogs and healthy controls. More research is needed to determine whether there are any statistically significant differences.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Iranian Society for the Prevention of Cruelty to Animals and Semnan University Research Council supervised this study under Iranian animal Ethics guidelines. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
HH: study design, data, and primary author of the manuscript. MA-h: study design, data, result analysis, and manuscript editing. MM: study design and manuscript editing. DS: data and result analysis. RN: manuscript editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the Deanship of Scientific Research, Semnan University, Semnan, Iran.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1031292/full#supplementary-material
References
1. Krawiec DR, Hefin D. Study of prostatic disease in dogs: 177 cases (1,981 ± 1,986). J Am Vet Med Assoc. (1992) 200:1119–22.
2. Foster RA. Common lesions in the male reproductive tract of cats and dogs (review). Vet Clin North Am Small Anim Pract. (2012) 42:527–45. doi: 10.1016/j.cvsm.2012.01.007
3. Levy X, Nizanski W, von Heimendahl A, Mimouni P. Diagnosis of common prostatic conditions in dogs: an update. Reprod Domest Anim. (2014) 49:50–7. doi: 10.1111/rda.12296
4. Berry SJ, Strandberg JD, Saunders WJ, Coffey DS. Development of canine benign prostatic hyperplasia with age. Prostate. (1986) 9:363–73. doi: 10.1002/pros.2990090406
5. Palmieri C, Lean FZ, Akter SH, Romussi S, Grieco V. A retrospective analysis of 111 canine prostatic samples: histopathological findings and classification. Res Vet Sci. (2014) 97:568–73. doi: 10.1016/j.rvsc.2014.11.006
6. Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, et al. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. (2004) 172:1399–403. doi: 10.1097/01.ju.0000139539.94828.29
7. Cunto M, Mariani E, Anicito EG, Ballotta G, Zambelli D. Clinical approach to prostatic diseases in the dog. Reprod Domest Anim. (2019) 54:815–22. doi: 10.1111/rda.13437
8. Polisca A, Troisi A, Fontaine E, Menchetti L, Fontbonne A. A retrospective study of canine prostatic diseases from 2002 to 2009 at the Alfort Veterinary College in France. Theriogenology. (2016) 85:835–40. doi: 10.1016/j.theriogenology.2015.10.030
9. Mukaratirwa S, Chitura T. Canine subclinical prostatic disease: Histological prevalence and validity of digital rectal examination as a screening test. J South Afr Vet Assoc. (2007) 78:66–8. doi: 10.4102/jsava.v78i2.292
10. Root Kustritz MV. Collection of tissue and culture samples from the canine reproductive tract. Theriogenology. (2006) 66:567–74. doi: 10.1016/j.theriogenology.2006.05.003
11. Kamolpatana K, Johnston GR, Johnston SD. Determination of canine prostatic volume using transabdominal ultrasonography. Vet Radiol Ultrasound. (2000) 41:73–7. doi: 10.1111/j.1740-8261.2000.tb00430.x
12. Holst BS, Holmroos E, Frilling L, Hanas S, Langborg LM, Franko Hansson K. The association between the serum concentration of canine prostate-specific esterase (CPSE) and the size of the canine prostate. Theriogenology. (2017) 93:33–39. doi: 10.1016/j.theriogenology.2017.01.032
13. Pinheiro D, Machado J, Viegas C, Baptista C, Bastos E, Magalhães J, et al. Evaluation of biomarker canine-prostate specific arginine esterase (CPSE) for the diagnosis of benign prostatic hyperplasia. BMC Vet Res. (2017) 13:76–83. doi: 10.1186/s12917-017-0996-5
14. Alonge S, Melandri M, Leoci R, Lacalandra GM, Aiudi G. Canine prostate-specific esterase (CPSE) as an useful biomarker in preventive screening program of canine prostate: CPSE threshold value assessment and its correlation with ultrasonographic prostatic abnormalities in asymptomatic dogs. Reprod Dom Anim. (2018) 53:359–64. doi: 10.1111/rda.13113
15. Macey MG, Carty E, Webb L, Chapman ES, Zelmanovic D, Okrongly D, et al. Use of mean platelet component to measure platelet activation on the ADVIA 120 haematology system. Cytometry. (1999) 38:250–5. doi: 10.1002/(SICI)1097-0320(19991015)38:5<250::AID-CYTO8>3.0.CO;2-K
16. Goddard A, Leisewitz AL, Kristensen AT, Schoeman JP. Platelet indices in dogs with babesia rossi infection. Vet Clin Pathol. (2015) 44:493–7. doi: 10.1111/vcp.12306
17. Kim HK, Kim JE, Ham CK, Lee DS, Park S, Cho HI. Prognostic value of platelet indices as determined by ADVIA 120 in patients suspected of having disseminated intravascular coagulation. Int J Lab Hematol. (2008) 30:117–23. doi: 10.1111/j.1751-553X.2007.00904.x
18. Aydogan A, Akkucuk S, Arica S, Motor S, Karakus A, Ozkan OV, et al. The analysis of mean platelet volume and platelet distribution width levels in appendicitis. Indian J Surg. (2015) 77:495–500. doi: 10.1007/s12262-013-0891-7
19. Mimidis K, Papadopoulos V, Kotsianidis J, Filippou D, Spanoudakis E, Bourikas G, et al. Alterations of platelet function, number and indexes during acute pancreatitis. Pancreatology. (2004) 4:22–7. doi: 10.1159/000077024
20. Gunebakmaz O, Kaya MG, Kaya EG, Ardic I, Yarlioglues M, Dogdu O, et al. Mean platelet volume predicts embolic complications and prognosis in infective endocarditis. Int J Infect Dis. (2010) 14:e982–5. doi: 10.1016/j.ijid.2010.05.019
21. von Ruecker A, Hufnagel P, Dickerhoff R, Murday H, Bidlingmaier F. Qualitative and quantitative changes in platelets after coronary-artery bypass surgery may help identify thrombotic complications and infections. Klin Wochenschr. (1989) 67:1042–7. doi: 10.1007/BF01727006
22. Chandra S, Chandra H. Role of haematological parameters as an indicator of acute malarial infection in uttarakhand state of India. Mediterr J Hematol Infect Dis. (2013) 5:e2013009. doi: 10.4084/mjhid.2013.009
23. Gao Y, Li Y, Yu X, Guo S, Ji X, Sun T, et al. The impact of various platelet indices as prognostic markers of septic shock. PLoS ONE. (2014) 9:e103761. doi: 10.1371/journal.pone.0103761
24. Yilmaz Z, Eralp O, Ilcol YO. Evaluation of platelet count and its association with plateletcrit, mean platelet volume, and platelet size distribution width in a canine model of endotoxemia. Vet Clin Pathol. (2008) 37:159–63. doi: 10.1111/j.1939-165X.2008.00023.x
25. Llewellyn EA, Todd JM, Sharkey LC, Rendahl A. A pilot study evaluating the prognostic utility of platelet indices in dogs with septic peritonitis. J Vet Emerg Crit Care. (2017) 27:569–78. doi: 10.1111/vec.12628
26. Mazzucco L, Borzini P, Gope R. Platelet-derived factors involved in tissue repair-from signal to function. Transf Med Rev. (2010) 24:218–34. doi: 10.1016/j.tmrv.2010.03.004
27. Castro P, Xia C, Gomez L, Lamb DJ, Ittmann M. Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate. (2004) 60:153–9. doi: 10.1002/pros.20051
28. Zhao S, Tang J, Shao S, Yan Y. The relationship between benign prostatic hyperplasia/lower urinary tract symptoms and mean platelet volume: the role of metabolic syndrome. Urol Int. (2016) 96:449–58. doi: 10.1159/000443313
29. Sannamwong N, Saengklub N, Sriphuttathachot P, Ponglowhapan S. Formula derived prostate volume determination of normal healthy intact dogs in comparison to dogs with clinical BPH. In: Proceeding of the 7th International Symposium on Canine and Feline Reproduction. Whistler, BC (2012). p. 226.
30. Schwartz D, Sharkey L, Armstrong PJ, Knudson C, Kelley J. Platelet volume and plateletcrit in dogs with presumed primary immune-mediated thrombocytopenia. J Vet Intern Med. (2014) 28:1575–9. doi: 10.1111/jvim.12405
31. Mehain SO, Haines JM, Lee PM. Platelet indices as biomarkers for characterization and determination of severity in canine chronic enteropathy. Vet J. (2019) 248:37–41. doi: 10.1016/j.tvjl.2019.04.003
32. Chandrashekar V. Plateletcrit as a screening tool for detection of platelet quantitative disorders. J Hematol. (2013) 2:22–6. doi: 10.4021/jh70w
33. Danese S, Motte C, Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. (2004) 99:938–45. doi: 10.1111/j.1572-0241.2004.04129.x
34. Kayahan H, Akarsu M, Ozcan MA, Demir S, Ates H, Unsal B, et al. Reticulated platelet levels in patients with ulcerative colitis. Int J Colorectal Dis. (2007) 22:1429–35. doi: 10.1007/s00384-007-0330-y
35. Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2017) 46:75–9. doi: 10.1016/j.intimp.2017.02.027
36. Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. (2010) 30:2362–7. doi: 10.1161/ATVBAHA.110.207514
37. Tunce T, Ozgun A, Emirzeoglu L, Celik S, Bilgi O, Karagoz B. Mean platelet volume as a prognostic marker in metastatic colorectal cancer patients treated with bevacizumab-combined chemotherapy. Asian Pac J Cancer Prev. (2014) 15:6421–3. doi: 10.7314/APJCP.2014.15.15.6421
38. Baldane S, Ipekci SH, Sozen M, Kebapcilar L. Mean platelet volume could be a possible biomarker for papillary thyroid carcinomas. Asian Pac J Cancer Prev. (2015) 16:2671–4. doi: 10.7314/APJCP.2015.16.7.2671
39. Mangalpally KK, Siqueiros-Garcia A, Vaduganathan M, Dong JF, Kleiman NS, Guthikonda S. Platelet activation patterns in platelet size sub-populations: differential responses to aspirin in vitro. J Thromb Thrombolysis. (2010) 30:251–62. doi: 10.1007/s11239-010-0489-x
40. Wu YY, Zhang X, Qin YY, Qin JQ, Lin FQ. Mean platelet volume/platelet count ratio in colorectal cancer: a retrospective clinical study. BMC Cancer. (2019) 19:1–7. doi: 10.1186/s12885-019-5504-9
41. Rifaioglu MM, Demirbas O, Gokce H, Davarci M. Mean platelet volume: a predictive factor for the diagnosis of nonsymptomatic prostatitis: results of univariate and multivariate models. Am J Men's Health. (2017) 11:35–40. doi: 10.1177/1557988315621144
42. Karaman H, Karakukcu C, Kocer D. Can mean platelet volume serve as a marker for prostatitis? Int J Med Sci. (2013) 10:1387–91. doi: 10.7150/ijms.6126
43. Smith JR, Smith KF, Brainard BM. Platelet parameters from an automated hematology analyzer in dogs with inflammatory clinical diseases. Vet J. (2014) 201:406–11. doi: 10.1016/j.tvjl.2014.07.009
44. Fu S, Zhang X, Niu Y, Wang RT. Prostate specific antigen, mean platelet volume, and platelet distribution width in combination to discriminate prostate cancer from benign prostate hyperplasia. Asian Pac J Cancer Prev. (2018) 19:699–702. doi: 10.22034/APJCP.2018.19.3.699
45. Karolczak K, Konieczna L, Kostka T, Witas PJ, Soltysik B, Baczek T, et al. Testosterone and dihydrotestosterone reduce platelet activation and reactivity in older men and women. Aging. (2018) 10:902–29. doi: 10.18632/aging.101438
46. Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer. (2019) 10:81–91. doi: 10.7150/jca.27210
47. Ozaksit G, Tokmak A, Kalkan H, Yesilyurt H. Value of the platelet to lymphocyte ratio in the diagnosis of ovarian neoplasms in adolescents. Asian Pac J Cancer Prev. (2015) 16:2037–41. doi: 10.7314/APJCP.2015.16.5.2037
48. Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. (2014) 83:97–101. doi: 10.1016/j.lungcan.2013.08.020
49. Ma X, Wang Y, Sheng H, Tian W, Qi Z, Teng F, Xue F. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J Obstet Gynaecol Res. (2014) 40:178–83. doi: 10.1111/jog.12151
50. Okuturlar Y, Gunaldi M, Tiken EE, Oztosun B, Inan YO, Ercan T, et al. Utility of peripheral blood parameters in predicting breast cancer risk. Asian Pac J Cancer Prev. (2015) 16:2409–12. doi: 10.7314/APJCP.2015.16.6.2409
51. Zareifar S, Farahmand Far MR, Golfeshan F, Cohan N. Changes in platelet count and mean platelet volume during infectious and inflammatory disease and their correlation with ESR and CRP. J Clin Lab Anal. (2014) 28:245–8. doi: 10.1002/jcla.21673
52. Briganti A, Capitanio U, Suardi N, Gallina A, Salonia A, Bianchi M, et al. Benign prostatic hyperplasia and its aetiologies. Eur Urol Suppl. (2009) 8:865–71. doi: 10.1016/j.eursup.2009.11.002
53. Wang L, Yang JR, Yang JY, Liu ZT. Chronic inflammation in benign prostatic hyperplasia: implications for therapy. Med Hypotheses. (2008) 70:1021–3. doi: 10.1016/j.mehy.2007.08.022
54. Ajayi AAL, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. (1995) 91:2742–7. doi: 10.1161/01.CIR.91.11.2742
Keywords: area under curve, dogs, BPH, platelet indices, relative prostatic size
Citation: Hosseinpour H, Ahmadi-hamedani M, Masoudifard M, Shirani D and Narenj Sani R (2022) Assessment of the utility of platelet indices to diagnose clinical benign prostatic hyperplasia in dogs. Front. Vet. Sci. 9:1031292. doi: 10.3389/fvets.2022.1031292
Received: 29 August 2022; Accepted: 23 November 2022;
Published: 08 December 2022.
Edited by:
Tanmoy Rana, West Bengal University of Animal and Fishery Sciences, IndiaReviewed by:
Yu Ueda, North Carolina State University, United StatesIbrahim Emam, Cairo University, Egypt
Copyright © 2022 Hosseinpour, Ahmadi-hamedani, Masoudifard, Shirani and Narenj Sani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmood Ahmadi-hamedani, YWhtYWRpLmhhbWVkYW5pQHNlbW5hbi5hYy5pcg==
 Hediyeh Hosseinpour
Hediyeh Hosseinpour Mahmood Ahmadi-hamedani
Mahmood Ahmadi-hamedani Majid Masoudifard2
Majid Masoudifard2