- 1College of Animal Science and Technology, Northeast Agricultural University, Harbin, China
- 2College of Animal Husbandry and Veterinary Medicine, Jinzhou Medical University, Jinzhou, China
This study was conducted to investigate the effects and mechanism of quercetin on chicken quality in broilers. We selected 480 AA broilers (1 day old) and randomly allotted those to four treatments (negative control and 0.2, 0.4, or 0.6 g of quercetin per kg of diet) for 42 days. Compared with the control group, the supplementation with 0.4 g of quercetin significantly increased the pH45min and L* value of the thigh muscle and decreased the shearing force of the thigh muscle and breast muscle and drip loss of the thigh muscle (P < 0.05). The supplementation with 0.6 g/kg of quercetin significantly increased the pH45min and L* value of the thigh muscle, and pH45min of breast muscle and decreased the drip loss of the thigh muscle (P < 0.05). Sensory scores of meat color, tenderness, and juiciness also were improved with increasing quercetin concentration (P < 0.05). The inosinic acid (IMP) content of the breast and thigh muscles of broilers was significantly increased by supplementation with 0.6 g/kg of quercetin (P < 0.05). Supplementation with 0.2, 0.4, and 0.6 g of quercetin significantly reduced mRNA expression of L-FABP (P < 0.05, P < 0.05, and P < 0.05); supplementation with 0.4 and 0.6 g/kg of quercetin significantly increased mRNA expression of PKB and AMPKα1 (P < 0.05 and P < 0.05); supplementation with 0.6 g/kg of quercetin in the diet significantly reduced mRNA expression of SREBP1 and HMGR (P < 0.05 and P < 0.05) and significantly increased mRNA expression of CPT1 and PPARγ (P < 0.05 and P < 0.05); and supplementation with 0.2, 0.4, and 0.6 g/kg of quercetin significantly increased mRNA expression of PI3K, LPL, and Apo A1 and significantly reduced mRNA expression of ACC and FATP1 in the breast muscle of broilers (P > 0.05). PI3k, PKB, AMPK, SREBP1, and L-FABP were significantly and positively correlated with pH45min (P < 0.05); PPARγ was significantly and positively correlated with shear force (P < 0.05); CPT1 was significantly and positively correlated with the L* value (P < 0.05); and HMGR was significantly and positively correlated with drip loss (P < 0.05). In conclusion, quercetin improved the meat quality, protecting it against lipid oxidation and deposition by regulating the PI3K/PKB/AMPKα1 signaling pathway in the breast muscle of broilers.
Introduction
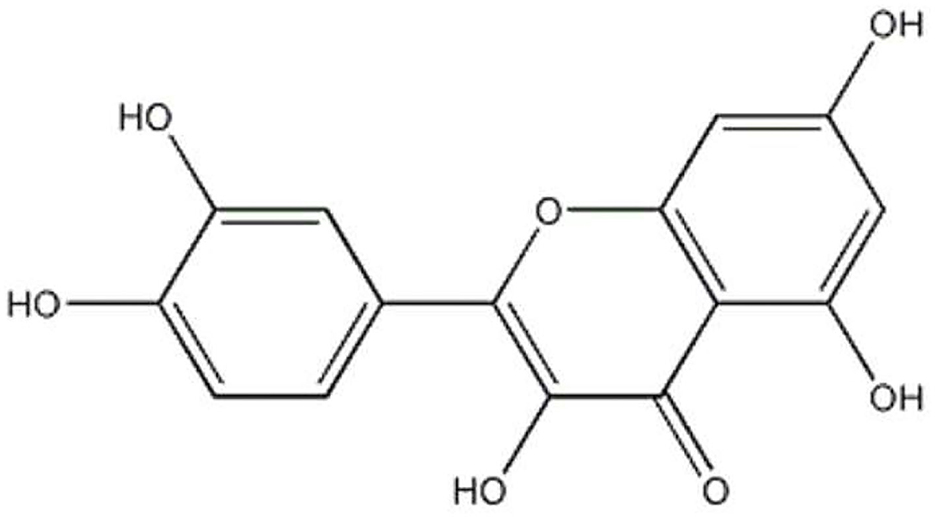
Quercetin is a natural flavonoid found in fruits and vegetables, including red onions, tea, apples, capers, broccoli, parsley, and red grapes (1). Quercetin is present in plants, and it presents many different glycosidic forms (formed by attaching to a glycosyl group), which directly reflect the bioactivity profile of this substance. The chemical structure of quercetin is characterized by the catechol (3′, 4′o-dihydroxy) group in ring B, and by the double bond in the ring C between C-2 and C-3 in conjunction with the 4-carbonyl group as well as 3-, 5-, and 7-hydroxyl groups (Figure 1). Quercetin has been revealed to mediate a multitude of physiological functions with a broad spectrum of pharmacological properties, including anti-inflammatory, anti-diabetic, lipid modulatory, and anti-oxidative capacities (2).
5′-Monophosphate-activated protein kinase (AMPK) preserves the energy homeostasis of cells, including lipid metabolism, which is regarded as a central regulator of energy homeostasis (3). The AMPK signaling pathway might be involved in the energy metabolism alterations in the skeletal muscles of broiler chickens (4). AMPK is also a principal upstream regulator of fatty acid oxidation and lipolysis (5). AMPK may regulate fatty acid metabolism by the acetyl-CoA carboxylase (ACC)/malonyl CoA-carnitine palmitoyltransferase (CPT) pathway in the muscle of broiler chickens (6).
Hence, we hypothesized that quercetin inhibited lipid oxidation and deposition in broilers. The lipid-lowering activity of quercetin was evaluated by analyzing meat quality, chemical composition of meat, and tasting test scores. The possible mechanisms of quercetin on the suppression of lipid oxidation and deposition were investigated based on the phosphatidylinositol 3-kinase (PI3K)/serine–threonine protein kinase (PKB/AKT)/AMPK signaling pathway. To the best of our knowledge, this is the first study investigating the lipid-regulatory activity and underlying mechanisms of quercetin in meat.
Materials and methods
Birds, diets, and experimental design
All study procedures were performed in accordance with the guidelines set forth by the Animal Welfare Committee of Northeast Agricultural University (Harbin, People's Republic of China). Housing, management, and care of the birds conformed to the guidelines of Agricultural Animal in Agricultural Research and Teaching of Heilongjiang Province (HEI Animal Management Certificate No. 11928).
A total of 480 AA broilers (1 day old) were obtained from a commercial facility (Yi-nong Poultry, Harbin, People's Republic of China). The birds were randomly allotted to four experimental treatments, comprising six replicates of 20 birds in each treatment. All birds were raised in stainless steel cages (316 × 400 × 400 mm) under continuous light in a controlled room for 42 days. The room temperature was maintained at 33°C for the first 3 days. Then, the temperature was reduced to 24°C until the end of the experiment.
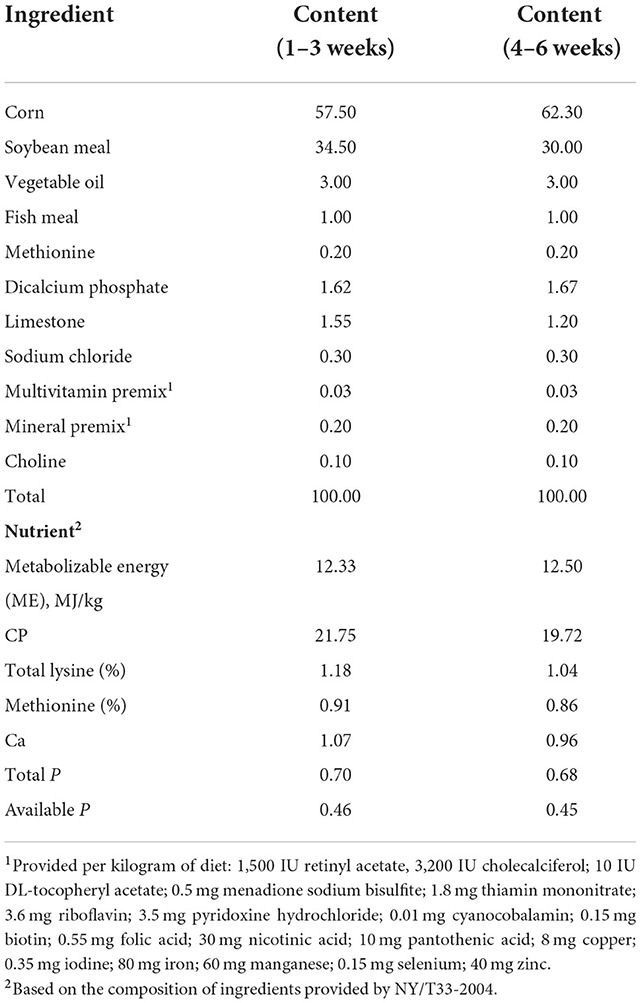
The experimental diets were based on corn and soybean meal, and quercetin was added at four concentrations: 0, 0.2, 0.4, and 0.6 g/kg of diet. Feeding was divided into two phases: the starter phase from 1 to 21 days and the grower phase from 21 to 42 days. The basal diet was formulated to meet the nutritional requirements suggested by Chinese Broiler Feeding Standards (NY/T33-2004) (Table 1). Quercetin (purity of quercetin dihydrate powder ≥97%, Sigma-Aldrich, St. Louis, MO) was mixed with the basal diet.
Methods
Carcass characteristics
At the age of 42 days, 12 chickens per treatment (six per replicate pen) were randomly selected and slaughtered for carcass analyses. Each of these birds was deprived of feed for 12 h and individually weighed prior to slaughter. The percentage of the carcass, eviscerated, semi-eviscerated, breast muscle, thigh muscle, and abdominal fat was calculated according to the weight of the carcass, eviscerated, semi-eviscerated, breast muscle, thigh muscle, and abdominal fat.
Chicken quality
pH
The pH24h and pH45min were measured by inserting a pH meter electrode (Sentron 1001 pH System, Roden, the Netherlands) into the right breast and thigh muscle for 45 min and 24 h after slaughter, which was calibrated at pH 4.0 and pH 7.0 (7).
Color
The color of the right breast and thigh muscles (three measurements per sample) that were exposed to air at room temperature for 30 min was measured using a MiniScan XE (HunterLab, Reston, VA) chromameter based on the L* (lightness), a* (redness), and b* (yellowness) values (8).
Warner–Bratzler shear force
Breast and thigh muscle samples of 2.54 cm thickness of from each chicken were stored at 4°C for 24 h until cooking. Steaks were placed in polyethylene bags and cooked until 71°C in the water bath, and the samples were removed and cooled in an ice slurry (1.0 ± 0.5°C) for 15 min (8). Then, six 1.27-cm-diameter cores were removed parallel with the muscle fiber orientation, and each core was sheared using a Warner–Bratzler shear force device attached to a texture analyzer (model TA-XT2i, Stable Micro Systems, UK) fitted with a tension/compression load cell of 30 kg and a crosshead speed of 240 mm/min (9).
Drip loss
At 24 h postmortems, the breast and thigh muscles were weighed and immediately placed in a plastic bag, hung from a hook, and stored at 2 °C for 4 days. After hanging, the samples were wiped with an absorbent paper and weighed again. The difference in weight corresponded to the drip loss and was expressed as the percentage of initial muscle weight (7).
Chemical composition
Proximate chemical analysis of the carcass subsections was performed. Each subsection was dried and ground separately to measure the content of crude protein and ether extract (fat).
Inosinic acid (IMP)
IMP concentrations in the breast and thigh muscles were determined by HPLC (4.6 × 250 mm, 5 μm particle size, Waters Corp.) (10).
Sensory evaluation
In total, 20 potential assessors between 18 and 33 years old (50% men and 50%women) were recruited from our department who have experience in meat quality analysis of at least 1 year. The evaluation of meat sensory attributes was performed in the breast and thigh muscles of six birds from each treatment group 2 days after the broilers were slaughtered. The breast and thigh muscle samples were cooked and randomly assigned to the 20 assessors. Color, odor, flavor, tenderness, and juiciness were tested. Score standards of the tasting trial: 1–3 is poor, 4–6 is good, and 7–9 is excellent (11, 12).
PI3K/PKB/AMPK signaling pathway
Real-time quantitative PCR analysis (RT-qPCR)
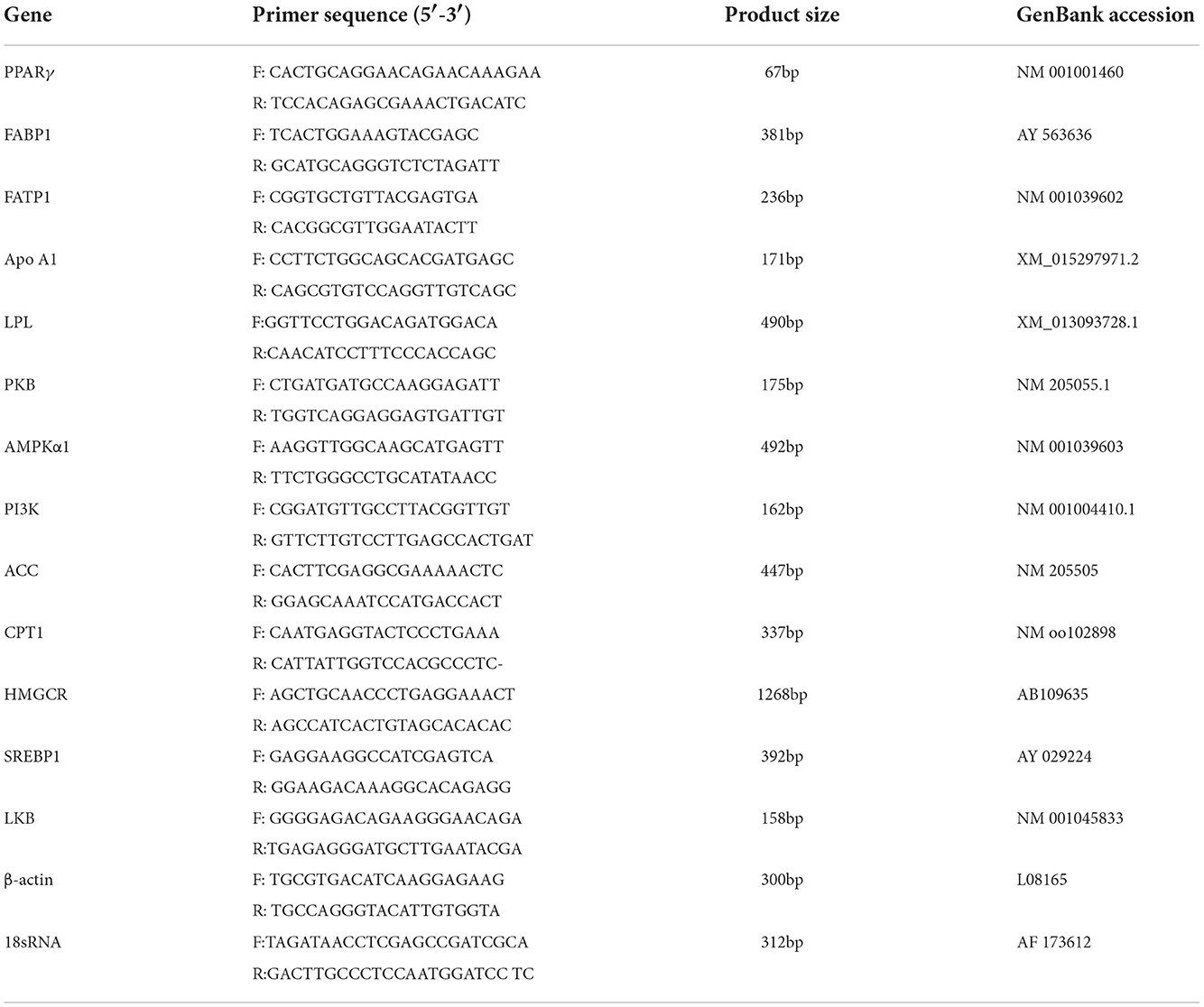
The total RNA was isolated using the TRIzol reagent. The SuperScript First-Strand Synthesis System (Life Technologies, Grand Island, NY) was adopted to synthesize first-strand cDNA from the total RNA isolated. The quantity of purified cDNAs was determined by RT-qPCR (Life Technologies, Grand Island, NY). mRNA expression levels were normalized to β-actin as reference genes. Calculations were performed using the following formulas: ΔCt (corrected sample) = mean value of target gene–mean value of internal reference gene, and ΔΔ Ct = ΔCt–mean value of the control group (Table 2).
Statistical analysis
All data were subjected to one-way ANOVA with a completely randomized design with four treatments and six replicates in each treatment. The data were subjected to ANOVA, using SPSS 20.0 software. The experimental data were presented as means ± SEM, and a P-value of <0.05 was considered statistically significant.
Results
Chicken quality
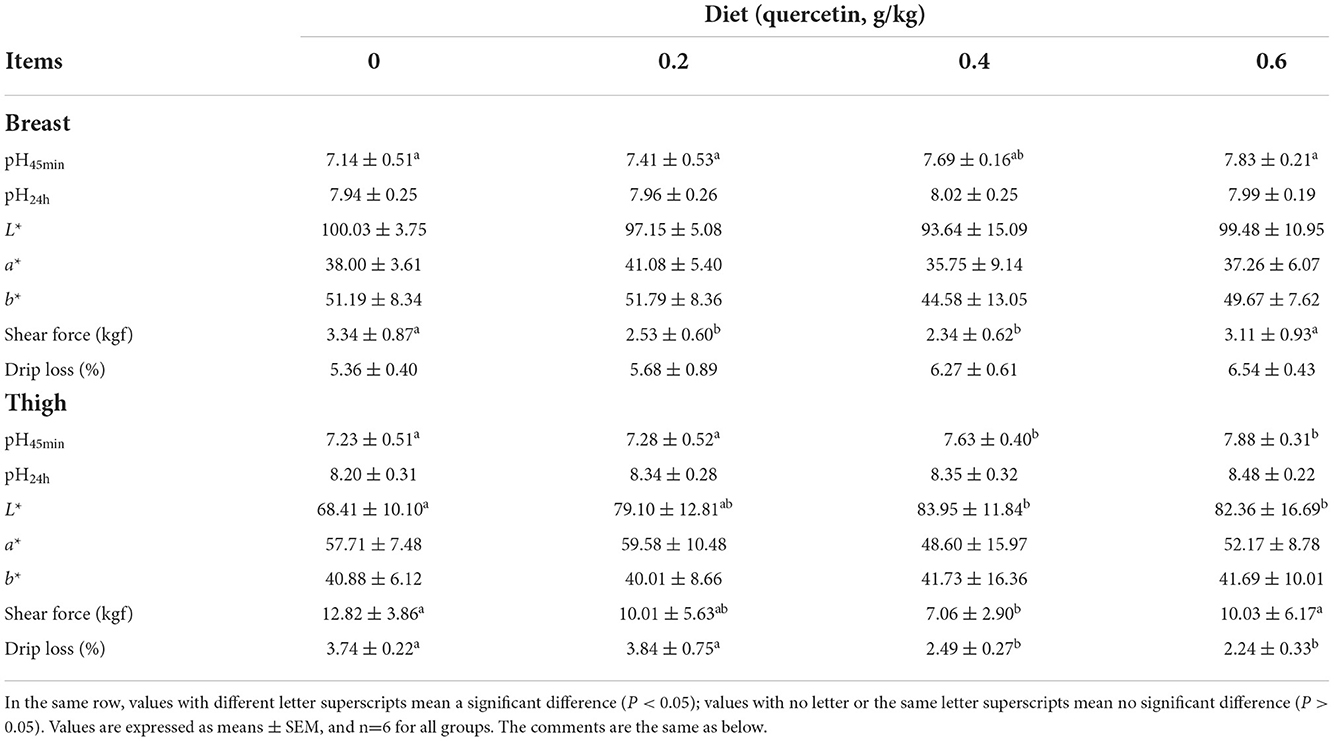
Compared with the control group, the shearing force significantly reduced in the breast muscle of broilers supplemented with 0.2 g/kg of quercetin (P < 0.05). The shearing force of the breast and thigh muscles of broilers supplemented with 0.4 g/kg of quercetin was significantly lower than that of the control group (P < 0.05). The breast muscles of broilers in the 0.6 g/kg quercetin group showed significantly increased pH45min (P < 0.01). The pH45min (P < 0.05), L* value (P < 0.05), and drip loss (P < 0.05) in thigh muscles of broilers in 0.4 and 0.6 g/kg quercetin groups were found to be significantly changed compared with those of the control group (Table 3).
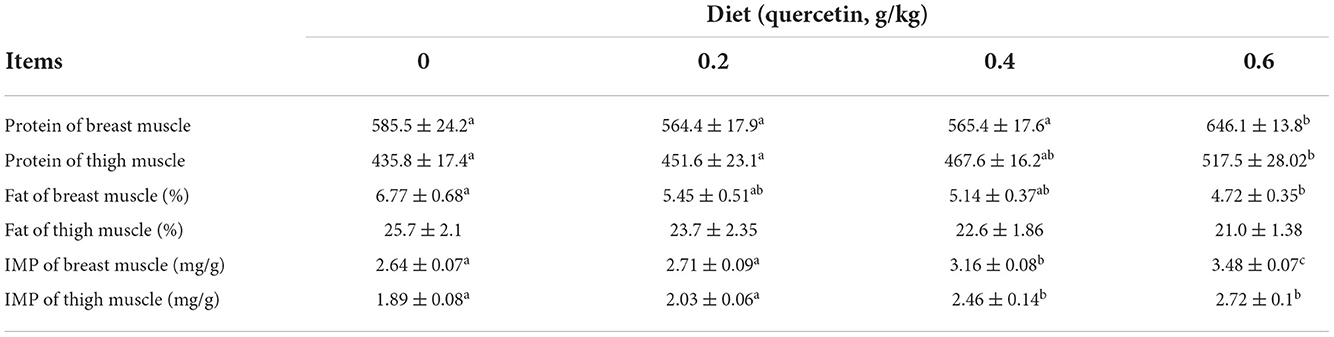
Compared with the control group, the protein content significantly increased in the breast and thigh muscles (P < 0.05), and the fat content significantly reduced in the breast muscle of broilers given 0.6 g/kg of quercetin (P < 0.05); the IMP content was significantly enhanced in the breast and thigh muscles of broilers in 0.4 and 0.6 g/kg quercetin groups (P < 0.05) (Table 4).
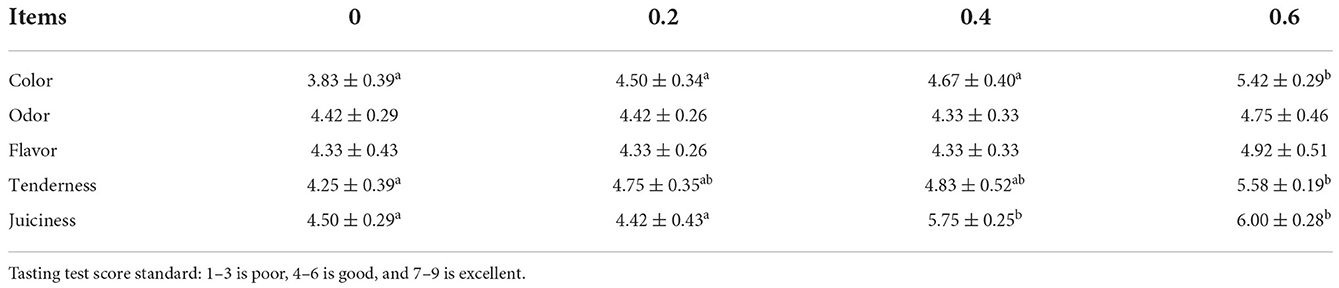
Comparing with the control group, the score of juiciness in the 0.4 g/kg quercetin group was significantly increased (P < 0.05), and scores of meat color, tenderness, and juiciness were significantly increased in the 0.6 g/kg quercetin group (P < 0.05) (Table 5).
The mechanism of quercetin ameliorating carcass characteristics and chicken quality in broilers
The mRNA expression of FABP1(L-FABP) significantly decreased in the 0.2 g/kg quercetin group compared with the control group (P < 0.05). The mRNA expression of FABP1(L-FABP) was significantly downregulated, and that of PKB and AMPKα1 was significantly upregulated in the 0.4 g/kg quercetin group compared with the control group (P < 0.05). The mRNA expression of FABP1(L-FABP), SREBP1, and HMGR was significantly downregulated, and that of PKB, AMPKα1, CPT1, and PPARγ was significantly upregulated in the 0.6 g/kg quercetin group compared with the control group (P < 0.05). The mRNA expression of PI3K, LPL, and Apo A1 was upregulated, and that of ACC and FATP1 was downregulated in the breast muscle of broilers in 0.2, 0.4, and 0.6 g/kg quercetin groups (P > 0.05) (Figure 2).
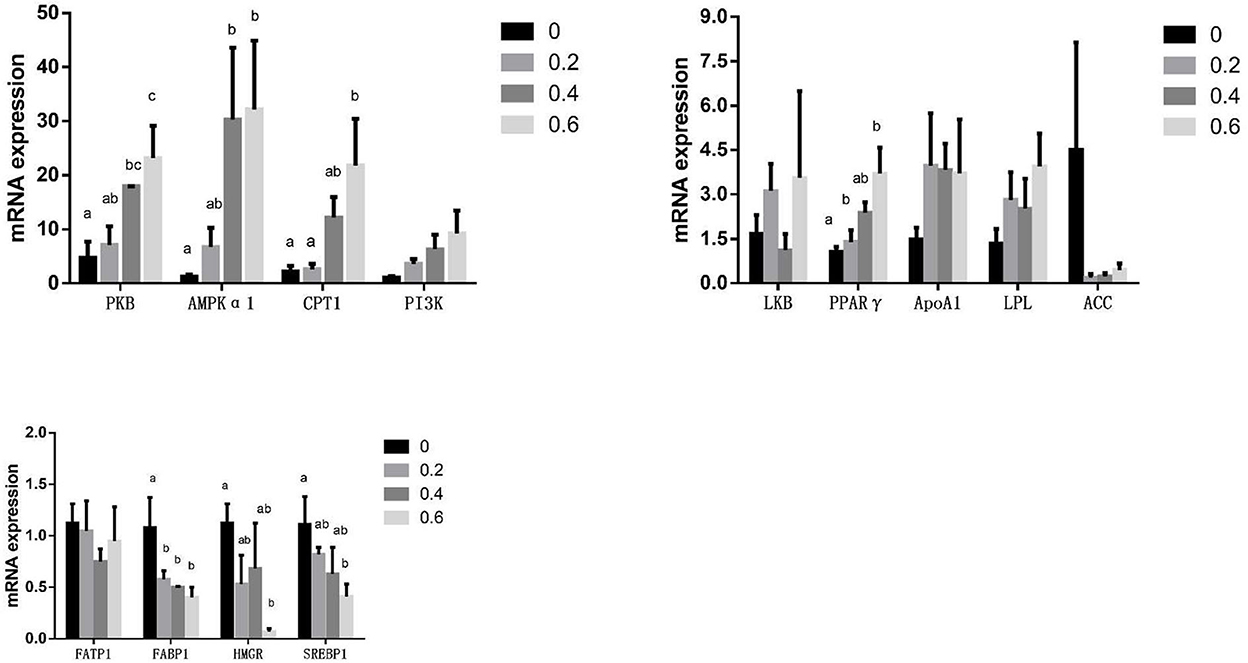
Figure 2. Effects of quercetin on mRNA expression of genes related to the PI3K/PKB/AMPK signaling pathway in the breast muscle of AA broilers.
Correlation between chicken quality and PI3K/PKB/AMPK signaling pathway
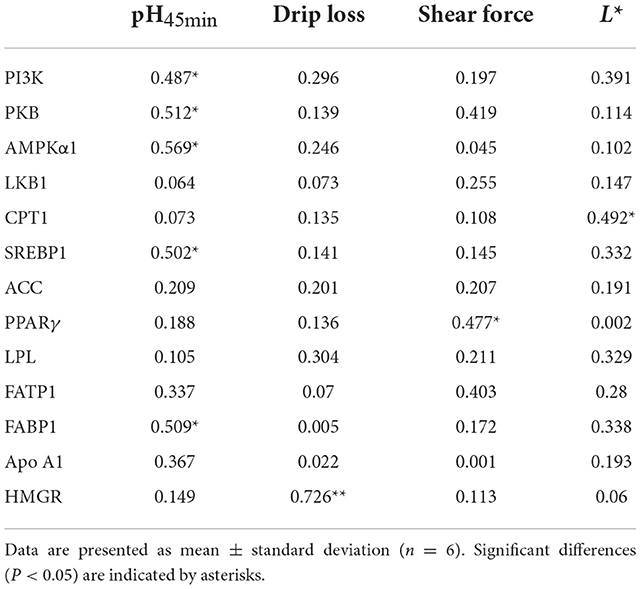
The correlation coefficient is great than 0.6, suggesting that a correlation exists between the chicken quality and PI3K/PKB/AMPK signaling pathway—the larger the value, the stronger the correlation. PI3k, PKB, AMPK, SREBP1, and L-FABP were significantly and positively correlated with pH45min (P < 0.05). PPARγ was significantly and positively correlated with shear force (P < 0.05). CPT1 was significantly and positively correlated with the L* value (P < 0.05). HMGR was significantly and positively correlated with drip loss (P < 0.05) (Table 6).
Discussion
Chicken quality
Muscle pH is an important factor affecting meat quality. pH is a potential indicator of quality characteristics of meat; a rapid postmortem decline in pH may denature protein, resulting in pale color and low water-holding capacity. Normally, muscle pH ranges from 7.35 to 7.45 and remains stable. After slaughtering and bleeding, the physiological regulation function of the body deteriorates, and lactic acid is formed by glycolysis, which rapidly decreases muscle pH (13). The rapid decrease in muscle pH inhibits the tenderization function of the Ca2+-activating enzyme system, which accelerates the formation of pale, soft, and exudation (PSE) meat (14). Therefore, an increase in pH of poultry meat after slaughter may delay the formation of PSE meat to some extent. Genistein and hesperidin supplemented with 20 mg/kg significantly increased muscle pH45min of broilers (15). This study showed that supplementation with 0.4 and 0.6 g/kg of quercetin could markedly significantly increase pH45min in the breast and thigh muscles of broilers (P < 0.05). Results from this study are supported by the study conducted by Jiang (16), who reported that supplementation of broiler diets with 0.4 g/kg of quercetin increased muscle pH45min. Hence, quercetin delayed the formation of PSE meat, and the result is confirmed by the study by Swatland (17).
The previous studies have measured the L* value to estimate the incidence of paleness or the pale, soft, and exudative condition in the breast muscle of broilers. Customarily, flesh color dominated consumer preference for chickens (18). Supplementation with 0.5 and 1 g/kg of quercetin had no significant effect on the L* value of broilers (19). However, the current study showed that the 0.4 and 0.6 g/kg quercetin group significantly increased the L* value of the thigh muscles (P < 0.05). Moreover, alfalfa flavonoids significantly increased the L* value of rabbit meat (20). This probably resulted from the difference in the myoglobin content of the muscle among breeds and difference in concentrations of quercetin (21). Therefore, the result suggested that dietary quercetin supplementation improved the flesh color of the thigh muscles in AA broilers.
Shearing force is an intuitive indicator of muscle tenderness. The lower the shear force, the better the tenderness. Quercetin supplementation significantly increased the shearing force of breast muscle in broilers (19). Our study showed that supplementation with 0.4 g/kg of quercetin significantly reduced the shearing force of the breast and thigh muscles (P < 0.05), and supplementation with 0.2 g/kg of quercetin reduced the shearing force of the breast muscle (P < 0.05) (Table 5). The difference could have resulted from the difference in breeds, age, or muscle composition of broilers (22).
The drip loss is a quantitative indicator of muscle hydration. The lower the drip loss, the stronger the muscle water-holding capacity. Muscle pH affects the muscle water-holding capacity, which was significantly negatively correlated to drip loss (23, 24). Supercritical CO2 extraction of Schisandra chinensis (SCESC) reduced lipid peroxidation and thus protected the integrity of the membrane, exerted the normal function, and reduced drip loss (25). Our results showed that supplementation with 0.4 and 0.6 g/kg of quercetin significantly decreased drip loss of the thigh muscle (P < 0.05), while supplementation with 0.6 g/kg of quercetin also significantly increased the pH45min of the breast and thigh muscles in broilers (P < 0.05), and these results corroborated the previous findings of Schafer (23) and Le Bihan-Duval (24).
SCESC significantly increased the protein content of the breast muscle (26). In this study, the protein content of the breast and thigh muscles of the broilers significantly increased in the 0.6 g/kg quercetin group (P < 0.05) and the fat content of the breast muscle significantly reduced (P < 0.05). The increased protein content of the breast muscle suggested that quercetin partly improved the nutritional value of the breast muscle. Simultaneously, IMP is an important indicator for measuring the meat flavor and juiciness (27). When the muscle samples were heated in water, fat produced obvious meat flavor. The content of IMP was also closely related to water-holding capacity; the present study showed that supplementation with 0.4 and 0.6 g/kg of quercetin significantly increased the IMP content of the breast and thigh muscles of the broilers (P < 0.05), together with drip loss, and it suggested that the decline in drip loss resulted from increasing the IMP content (26).
Color, odor, tenderness, juiciness, and flavor are mostly considered the most important sensory criterion in poultry products. Quercetin enhanced the meat quality of the thigh muscle without adverse effects on color and sensory characteristics in broilers (16). Moreover, quercetin supplementation modified the sensory quality of lamb and goat meat, respectively (27, 28). Among these degradation products, IMP has a positive and even synergistic impact on umami taste (29). Our data showed that the 0.4 g/kg quercetin group showed a significantly increased score of juiciness (P < 0.05); the 0.6 g/kg quercetin group showed significantly increased scores of meat color, tenderness, and juiciness (P < 0.05); better sensory evaluation was accompanied with increasing quercetin. The results of IMP in this experiment were supported by Khan et al. (29), who found that the content of IMP was directly associated with tenderness and juiciness.
The mechanism of quercetin ameliorates carcass characteristics and chicken quality in broilers
The previous results of transcriptome sequencing showed that the AMPK signaling pathway is one of the main signaling pathways of lipid metabolism. We previously reported 505 differentially expressed genes of the AMPK signaling pathway we found in the quercetin treatments compared with the control and is the first differentially expressed gene signaling pathway (30).
The AMPK signaling pathway regulates fatty acid synthesis and fatty acid oxidation by controlling lipid metabolism (31, 32) and acts as an energy sensor by regularly responding to cellular energy demands via sensing the balance in the AMP-to-ATP ratio (33). Liraglutide is shows to reduce visceral fat mass by inducing beige fat development in obese mice through AMPK signaling (34). In this study, supplementation with 0.4 and 0.6 g/kg of quercetin significantly increased the expression of AMPKα1 mRNA in the breast muscle of broilers (P < 0.05). The change in muscle pH is one of the most significant changes that occur during abatage and affects meat quality attributes such as texture, color, and water-holding capacity. IMP contributes to the pleasant and fresh flavor of the meat (35). Our results that quercetin affected pH and IMP through increasing the expression of AMPKα1 mRNA in the breast muscle of broilers were supported by Shen et al. (36).
These results show that the PI3K/PKB (AKT) signaling pathway participated in adipogenesis (37–39). Taken together, the results are consistent with findings of previous research showing that quercetin improved lipid metabolism and reduced abdominal fat deposition by activating the PI3K/PKB signaling pathway (40). Our results showed that the mRNA expression of PI3K and PKB significantly increased in the 0.4 and 0.6 g/kg quercetin group. Together with the results of AMPKα1 in this experiment, quercetin activated the PI3K/PKB (AKT)/AMPKα1 signaling pathway, affected fatty acid synthesis and fatty acid oxidation, and thus improved chicken quality.
Fatty acid synthesis and fatty acid oxidation are a complex transcriptional cascade, including the downstream activation of PPARγ, CPT1, HMGR, SREBP1, and L-FABP, which facilitate the activation of the transcription of genes connected to the phenotype of adipocytes (29, 41). Previous studies have shown that blue honeysuckle berry (BHBE) promoted the phosphorylation of AMPK and further reduced the expression of lipogenic transcription-related genes PPARγ and SREBP1, while AMPK inhibitor attenuated the suppressive effect of BHBE on lipogenesis (42).
The transcription factor peroxisome proliferator-activated receptor (PPARγ) plays a key role in regulating adipogenesis and is expressed in the late stages of differentiation. A previous study showed that 50% Polygonum cuspidatum ethanol extract (PEE) alleviated lipid accumulation on 3T3-L1 adipocytes and downregulated the mRNA and protein production of adipogenesis-related SREBP1 and PPARγ (43). CPT1 adjusts the β-oxidation of fatty acids by catalyzing the conversion of fatty acyl-CoA into fatty acylcarnitine in mitochondria (44). Instant fermented teas are shown to heighten energy expenditure by increasing CPT1 expression (45). Berteroin was found to significantly increase the expression of mitochondrial fatty acid oxidation-related genes CPT1 and PPARγ, and the phosphorylation of AMPK in HepG2 cells (46). Peroxisome proliferator-activated receptor (HMGR) is a rate-limiting enzyme for cholesterol synthesis. It was found that Schisandra chinensis fruit (SF) extract may decrease lipid accumulation by upregulating AMPK and downregulating HMGR (38). A study showed that salsalate reduces atherosclerosis through AMPK, which inhibits fatty acid and cholesterol synthesis through the phosphorylation of HMGCR in mice (47). Sterol regulatory element-binding protein (SREBP1) is found to particularly involve in the activation of the genes controlling fatty acid metabolism and de novo lipogenesis. A study showed that berberine may prevent lipid metabolism disorders by decreasing the expressions of SREBP1 and SREBP2 and increasing the expression of AMPKα1 (48). The effect of DLBS3733 on lipogenesis and cholesterologenesis is revealed by its activity to increase phosphorylated AMPK and repress the expressions of total SREBP and HMGCR in HepG2 cells (49). Previous studies reported that liver-type fatty acid-binding protein (FABP1/L-FABP) is an important candidate gene for traits of intramuscular and abdominal fat in poultry, and that an FABP1 polymorphism is significantly associated with the deposition of intramuscular and abdominal fat in poultry (50, 51). FABP1 has been found in the cytosol, nucleus, and mitochondria and has multiple roles. Livias reported that due to the role of FABP1 in trafficking fatty acids to the nucleus and also its ability to bind PPARs, the decrease in placentas from women with pre-gestational obesity altered pathways related to placental lipid handling and fatty acid metabolism (52). Our study showed that supplementation with 0.6 g/kg of quercetin group significantly increased the expression of CPT1 and PPARγ (P < 0.05) and decreased the expression of HMGR, SREBP1, and FABP1 (P < 0.05) by activating AMPK, and thus prevented lipid deposition and promoted lipid transport and lipid β-oxidation.
The correlation analysis showed that there was a significant positive correlation between the pH45min and mRNA expression of PI3K, PKB, AMPKα1, SREBP1, and FABP1 (P < 0.05); there was also a significant positive correlation between drip loss and mRNA expression of HMGCR (P < 0.05); shear force and mRNA expression of PPARγ (P < 0.05); and L* value and mRNA expression of CPT1 (P < 0.05).
Conclusion
This study demonstrated that quercetin ameliorates the meat quality and protected against lipid oxidation and deposition by activating the PI3K/PKB/AMPKα1 signaling pathway in the breast muscle of broilers (Figure 3). However, as a widespread flavonoid, quercetin is a safe and dietary supplement based on its broad range of biological effects in animals. Quercetin could be used as functional additive ingredients to ameliorate chicken quality. Future studies should investigate the optimal benefits of quercetin, especially in dosing regimens and adjuvants that may amplify any perceived bioactive effects of quercetin in vivo.
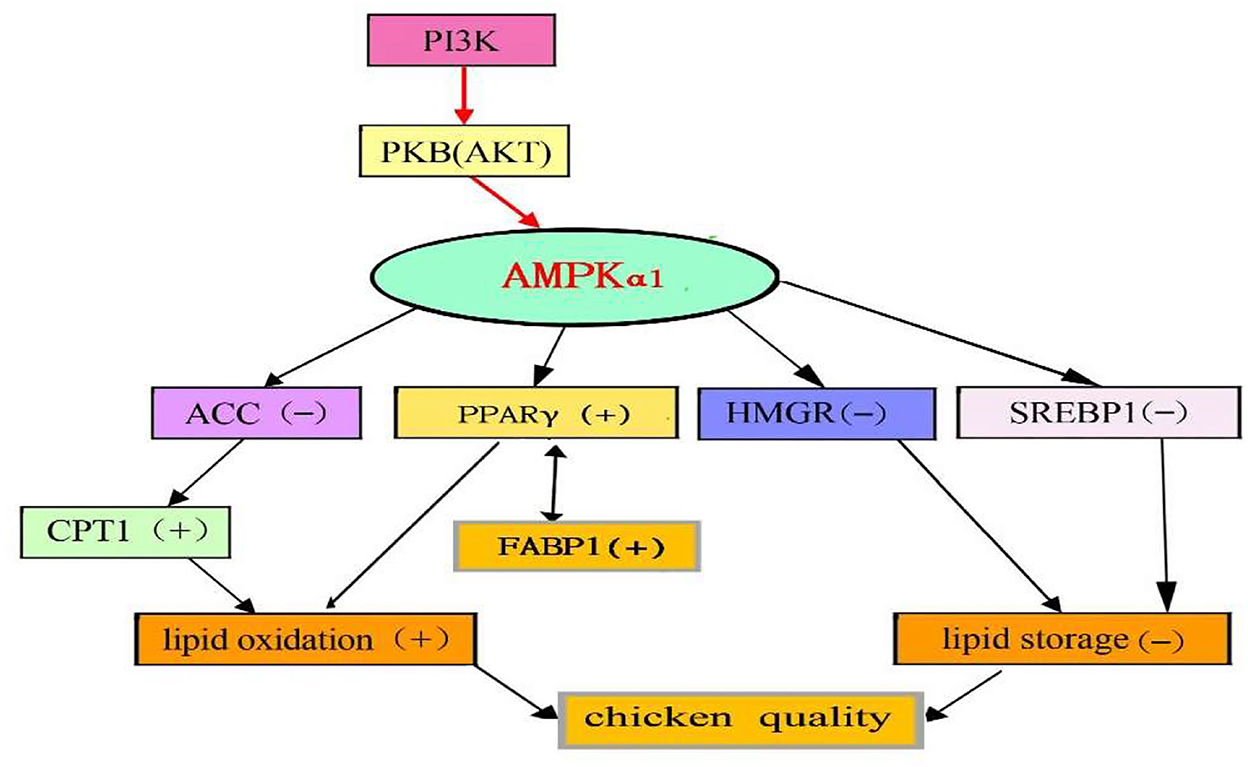
Figure 3. Proposed model of AMPK actions on gene expressions in the breast muscle of chickens supplemented with quercetin [(–), down, (+), up] change.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was reviewed and approved by HEI Animai Management Certificate No. 11928.
Author contributions
MW and YL participated in the design of the study and critically revised the first manuscript. BW, HW, and SZ provided some technical support for the experiment. JL, MD, and HL performed the experiments and participated in the statistical analysis. YL modified the manuscript and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This study were supported by the National Natural Science Foundation of China (32072749) and the Natural Science Foundation of Heilongjiang Province (YQ2019C007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xiao L, Liu L, Guo X, Zhang S, Wang J, Zhou F, et al. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: a critical role of NADPH oxidase. Food Chem Toxicol. (2017) 105:22.-33. doi: 10.1016/j.fct.2017.03.048
2. Kobori M, Takahashi Y, Sakurai M, Akimoto Y, Tsushida T, Oike H, et al. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Moler Nutr Food Rese. (2016) 60:300-12. doi: 10.1002/mnfr.201500595
3. Horman S, Browne GJ, Krause U, Patel JV, Vertommen D, Bertrand L, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. (2002) 12:1419–23. doi: 10.1016/S0960-9822(02)01077-1
4. Hu X, Cai Y, Kong L, Lin H, Song Z, Buyse J. Effects of dietary corticosterone on the central adenosine monophosphate-activated protein kinase (AMPK) signaling pathway in broiler chickens. J Anim Sci. (2020) 7:skaa202. doi: 10.1093/jas/skaa202
5. Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS, et al. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Rese. (2012) 53:2490. doi: 10.1194/jlr.R025882
6. Joubert R, Métayer Coustard S, Swennen Q, Sibut V, Crochet S, Cailleau-Audouin E, et al. The beta-adrenergic system is involved in the regulation of the expression of the expression of avian uncoupling protein in the chicken. Domest Anim Endocrin. (2010) 38:115–25. doi: 10.1016/j.domaniend.2009.08.002
7. Berri C, Besnard J, Relandeau C. Increasing dietary lysine increases final pH and decreases drip loss of broiler breast meat. Poult Sci. (2008) 87:480–4. doi: 10.3382/ps.2007-00226
8. Honikel KO. Reference methods for the assessment of physical characteristics of meat. Meat Sci. (1998) 49:447–57. doi: 10.1016/S0309-1740(98)00034-5
9. Fabre R, Dalzotto G, Perlo F, Bonato P, Teira G, Tisocco O, et al. Cooking method effect on Warner-Bratzler shear force of different beef muscles. Meat Sci. (2018) 138:10–14. doi: 10.1016/j.meatsci.2017.12.005
10. Jayasena DD, Jung S, Kim HJ, Alahakoon AU, Jo C. Effect of sex on flavor-related and functional compounds in freeze-dried broth made from korean native chicken. Korean J Food Sci An. (2014) 34:448–456. doi: 10.5851/kosfa.2014.34.4.448
11. Silva FA, Amaral DS, Guerra IC, Dalmas PS, Arcanjo NM, Bezerra TK, et al. The chemical and sensory qualities of smoked blood sausage made with the edible by-products of goat slaughter. Meat Sci. (2013) 94:34–8. doi: 10.1016/j.meatsci.2013.01.004
12. Bou R, Guardiola F, Grau A, Grimpa S, Manich A, Barroeta A, et al. Influence of dietary fat source, alpha-tocopherol, and ascorbic acid supplementation on sensory quality of dark chicken meat. Poultry Sci. (2001) 80:800–7. doi: 10.1093/ps/80.6.800
13. El Rammouz R, Babile R, Fernandez X. Effect of ultimate pH on the physicochemical and biochemical characteristics of turkey breast muscle showing normal rate of postmortem pH fall. Poult Sci. (2004) 83:1750–7. doi: 10.1093/ps/83.10.1750
14. Barbut S, Sosnicki AA, Lonergan SM, Knapp T, Ciobanu DC, Gatcliffe LJ, et al. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. (2008) 79:46–63. doi: 10.1016/j.meatsci.2007.07.031
15. Kamboh AA, Zhu WY. Individual and combined effects of genistein and hesperidin supplementation on meat quality in meat-type broiler chickens. J Sci Food Agric93. (2013) 3362–7. doi: 10.1002/jsfa.6185
16. Jiang ZY, Jiang SQ, Lin YC, Xi PB, Yu DQ, Wu TX, et al. Effects of soybean isoflavone on growth performance, meat quality, and antioxidation in male broilers. Poul Sci. (2007) 86:1356–62. doi: 10.1093/ps/86.7.1356
17. Swatland HJ. How pH causes paleness or darkness in chicken breast meat. Meat Sci. (2008) 80:396–400. doi: 10.1016/j.meatsci.2008.01.002
18. Kennedy OB, Stewart-Knox BJ, Mitchell PC, Thurnham DI. Flesh colour dominates consumer preference for chicken. Appetite. (2004) 44:181–186. doi: 10.1016/j.appet.2004.11.002
19. Goliomytis M, Tsoureki D, Simitzis PE, Charismiadou MA, Hager-Theodorides AL, Deligeorgis SG, et al. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult Sci. (2014) 93:1957–62. doi: 10.3382/ps.2013-03585
20. Dabbou S, Gasco L, Rotolo L, Pozzo L, Tong JM, Dong XF, et al. Effects of dietary alfalfa flavonoids on the performance, meat quality and lipid oxidation of growing rabbits. Asian Austral J Anim Sci. (2018) 31:270–7. doi: 10.5713/ajas.17.0284
21. Mancini RA, Hunt MC. Current research in meat color. Meat Sci. (2005) 71:100–21. doi: 10.1016/j.meatsci.2005.03.003
22. Wattanachant S, Benjakul S, Ledward DA. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult Sci. (2004) 83:123–8. doi: 10.1093/ps/83.1.123
23. Schafer A, Rosenvold K, Purslow PP, Andersen HJ, Henckel P. Physiological and structural events post mortem of importance for drip loss in pork. Meat Sci. (2002) 61:355–66. doi: 10.1016/S0309-1740(01)00205-4
24. Le Bihan-Duval E, Berri C, Baeza E, Millet N, Beaumont C. Estimation of the genetic parameters of meat characteristics and of their genetic correlations with growth and body composition in an experimental broiler line. Poult Sci. (2001) 80:839–43. doi: 10.1093/ps/80.7.839
25. Ushiroda C, Naito Y, Takagi T, Uchiyama K, Mizushima K, Higashimura Y, et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J Clin Biochem Nutr. (2019) 65:34–46. doi: 10.3164/jcbn.18-116
26. Yan J, Shan A, Wang H, Ma D, Yan C. Influence of supercritical co2 extraction of schisandra chinensis on antioxidant status, carcass and meat quality of aa broilers. J Poul Sci. (2013) 50:219–27. doi: 10.2141/jpsa.0110171
27. Andres S, Huerga L, Mateo J, Tejido ML, Bodas R, Moran L, et al. The effect of quercetin dietary supplementation on meat oxidation processes and texture of fattening lambs. Meat Sci. (2014) 96: 806–11. doi: 10.1016/j.meatsci.2013.09.020
28. Cho SK, Jo CR, Jung SME, Kim MK, Oh HM, Lee BD, et al. Effects of dietary quercetin on the feed utilization, blood parameters, and meat quality in Korean native goats. J Anim Sc Technol52. (2010) 297–304. doi: 10.5187/JAST.2010.52.4.297
29. Khan MI, Jo C, Tariq MR. Meat flavor precursors and factors influencing flavor precursors-A systematic review. Meat Sci. (2015) 110:278–84. doi: 10.1016/j.meatsci.2015.08.002
30. Wang M, Mao Y, Wang B, Wang S, Li Y. Quercetin improving lipid metabolism by regulating lipid metabolism pathway of ileum mucosa in broilers. Oxid Med Cell Longevity. (2020) 2020:1–17. doi: 10.1155/2020/8686248
31. Liou CJ, Lee YK, Ting NC, Chen YL, Shen S, Wu SC, et al. Protective effects of licochalcone A ameliorates obesity and non-fatty liver disease via promotion of the Sirt-1/AMPK pathway in mice fed a high-fat diet. Cells. (2019) 8:447. doi: 10.3390/cells8050447
32. Luo JM, Qi JM, Wang WJ, Luo ZH, Liu L, Zhang GG, et al. Antiobesity effect of flaxseed polysaccharide via inducing satiety due to leptin resistance removal and promoting lipid metabolism through the AMP-activated protein kinase (AMPK) signaling pathway. J Agri Food Chem. (2019) 67:7040–9. doi: 10.1021/acs.jafc.9b02434
33. Zhao NQ, Li XY, Wang L, Feng ZL, Li XF, Wen YF, et al. Palmitate induces fat accumulation by activating C/EBPBβ-mediated G0S2 expression in hepg2 cells. WJGO. (2017), 7705–7715. doi: 10.3748/wjg.v23.i43.7705
34. Zhou J, Poudel A, Chandramani-Shivalingappa P, Xu B, Welchko R, Li L, et al. Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRT-1-PGC1-α cell signaling pathway. Endocrine. (2019) 64:271–83. doi: 10.1007/s12020-018-1826-7
35. Howgate P. Kinetics of degradation of adenosine triphosphate in chill-stored rainbow trout (Oncorhynchus mykiss). Int J Food Sci Tech. (2005) 40:579–88. doi: 10.1111/j.1365-2621.2005.00924.x
36. Shen QW, Means WJ, Underwood KR, Thompson SA, Zhu MJ, McCormick RJ, et al. Early post-mortem AMP-activated protein kinase (AMPK) activation leads to phosphofructokinase-2 and-1 (PFK-2 and PFK-1) phosphorylation and the development of pale, soft, and exudative (PSE) conditions in porcine longissimus muscle. J Agr Food Chem. (2006) 54:5583–9. doi: 10.1021/jf060411k
37. Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. (2019) 14:1483. doi: 10.7150/ijbs.27173
38. Manning BD, Toker A. Akt/pkb signaling: navigating the network. Cell. (2017) 169:381–405. doi: 10.1016/j.cell.2017.04.001
39. Zhu D, Wang J, Sun X, Chen J, Duan Y, Pan J, et al. Septin4_i1 regulates apoptosis in hepatic stellate cells through peroxisome proliferator-activated receptor-γ/AKT/B-cell lymphoma 2 pathway. J Histochem Cytochem. (2015) 63, 163–169. doi: 10.1369/0022155414567230
40. Ying L, Chaudhry MT, Xiao F, Mao Y, Li Y. The effects and mechanism of quercetin dietary supplementation in streptozotocin-induced hyperglycemic arbor acre broilers. Oxid Med Cell Longevity. (2020) 2020:1–11. doi: 10.1155/2020/9585047
41. Lee JE, Schmidt H, Lai B, Ge K. Transcriptional and epigenomic regulation of adipogenesis. Mole Cell Biol. (2019) 39:00601–18. doi: 10.1128/MCB.00601-18
42. Liu HT, Wu CG, Wang S, Gao SM, Liu JS, Dong ZQ, et al. Extracts and lignans of Schisandra chinensis fruit alter lipid and glucose metabolism in vivo and in vitro. J Funct Foods. (2015) 19:296–307. doi: 10.1016/j.jff.2015.09.049
43. Da-Hye C, Joon-Hee H, Keun-Hyung Y, Min H, Sun-Yeop L, Ka-Hee P, et al. Antioxidant and anti-obesity activities of polygonum cuspidatum extract through alleviation of lipid accumulation on 3t3-l1 adipocytes. J Microbiol Biotechn. (2020) 30:21–30. doi: 10.4014/jmb.1910.10040
44. Zheng JL, Luo Z, Liu CX, Chen QL, Tan XY, Zhu Q, et al. Differential effects of acute and chronic zinc (Zn) exposure on hepatic lipid deposition and metabolism in yellow catfish pelteobagrus fulvidraco. Aquat Toxicol. (2013) 132–133:173–81. doi: 10.1016/j.aquatox.2013.02.002
45. Sun Y, Wang YW, Song PP, Wang HS, Xu N, Wang YJ, et al. Antiobesity effects of instant fermented teas in vitro and in mice with high-fat-diet-induced obesity. Food Funct. (2019) 10:3502–13. doi: 10.1039/C9FO00162J
46. Kim YJ, Park SY, Lee JH. Berteroin ameliorates lipid accumulation through AMPK-mediated regulation of hepatic lipid metabolism and inhibition of adipocyte differentiation. Life Sci. (2021) 282:119668. doi: 10.1016/j.lfs.2021.119668
47. Day EA, Ford RJ, Smith BK, Houde VP, Stypa S, Rehal S, et al. Salsalate reduces atherosclerosis through ampkβ1 in mice. Mol Metab. (2021) 53:101321. doi: 10.1016/j.molmet.2021.101321
48. Li YJ, Zhao XM, Feng XY, Liu XM, Deng C, Hu CH, et al. Berberine alleviates olanzapine-induced adipogenesis via the AMPK–SREBP pathway in 3t3-l1 cells. Inte J Moler Sci. (2016) 17:1865–77. doi: 10.3390/ijms17111865
49. Tandrasasmita OM, Berlian G, Tjandrawinata RR. Molecular mechanism of DLBS3733, a bioactive fraction of Lagerstroemia speciosa (L.) Pers., on ameliorating hepatic lipid accumulation in HepG2 cells. Biomed Pharmacothe. (2021) 141:111937. doi: 10.1016/j.biopha.2021.111937
50. He J, Chen J, Lu L, Yong T, Tao Z, Wang D, et al. A novel snp of liver-type fatty acid-binding protein gene in duck and its associations with the intramuscular fat. Mol Biol Rep. (2012) 39:1073–7. doi: 10.1007/s11033-011-0833-z
51. He J, Tian Y, Li J, Shen J, Tao Z, Fu Y, et al. Expression pattern of l-fabp gene in different tissues and its regulation of fat metabolism-related genes in duck. Mol Biol Rep. (2013) 40:189–95. doi: 10.1007/s11033-012-2048-3
52. Livia B, Carolina SF, Marcelle AS, Daniela BM, Antonio M, Carla L, et al. Decreased fatty acid transporter FABP1 and increased isoprostanes and neuroprostanes in the human term placenta: implications for inflammation and birth weight in maternal pre-gestational obesity. Nutr. (2021) 13:2768. doi: 10.3390/nu13082768
Keywords: quercetin, chicken quality, PI3K, PKB, AMPK
Citation: Wang M, Wang B, Zhou S, Liu J, Lu H, Wu H, Ding M and Li Y (2022) Quercetin ameliorates chicken quality by activating the PI3K/PKB/AMPK signaling pathway in broilers. Front. Vet. Sci. 9:951512. doi: 10.3389/fvets.2022.951512
Received: 12 July 2022; Accepted: 14 November 2022;
Published: 12 December 2022.
Edited by:
Tugay Ayasan, Osmaniye Korkut Ata University, TurkeyReviewed by:
Miroslav Juzl, Mendel University in Brno, CzechiaMuhammad Sohaib, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2022 Wang, Wang, Zhou, Liu, Lu, Wu, Ding and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Li, bGl5YW9sendAMTYzLmNvbQ==
 Mi Wang
Mi Wang Bo Wang1
Bo Wang1