- 1Department of Animal Biosciences, University of Guelph, Guelph, ON, Canada
- 2Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, ON, Canada
- 3Department of Animal Science, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
- 4Consultant, Spring Hill, TN, United States
- 5Department of Clinical Studies, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
Introduction: Camelina oil contains a greater concentration of omega-3 (n-3) a-linolenic acid (C18:3n-3; ALA) than omega-6 (n-6) linoleic acid (C18:2n-6; LA), in comparison to alternative fat sources commonly used to formulate canine diets. Omega-3 FAs are frequently used to support canine skin and coat health claims and reduce inflammation and oxidative stress; however, there is a lack of research investigating camelina oil supplementation and its effects on these applications in dogs. The objective of this study was to evaluate the effects of camelina oil supplementation on coat quality, skin barrier function, and circulating inflammatory and oxidative marker concentrations.
Methods: Thirty healthy [17 females; 13 males; 7.2 ± 3.1 years old; 27.4 ± 14.0 kg body weight (BW)] privately-owned dogs of various breeds were used. After a 4-week wash-in period consuming sunflower oil (n6:n3 = 1:0) and a commercial kibble, dogs were blocked by age, breed, and size, and randomly assigned to one of three treatment oils: camelina (n6:n3 = 1:1.18), canola (n6:n3 = 1:0.59), flaxseed (n6:n3 = 1:4.19) (inclusion level: 8.2 g oil/100 g of total food intake) in a randomized complete block design. Transepidermal water loss (TEWL) was measured using a VapoMeter on the pinna, paw pad, and inner leg. Fasted blood samples were collected to measure serum inflammatory and oxidative marker concentrations using enzyme-linked immunosorbent assay (ELISA) kits and spectrophotometric assays. A 5-point-Likert scale was used to assess coat characteristics. All data were collected on weeks 0, 2, 4, 10, and 16 and analyzed using PROC GLIMMIX in SAS.
Results: No significant changes occurred in TEWL, or inflammatory and oxidative marker concentrations among treatments, across weeks, or for treatment by week interactions. Softness, shine, softness uniformity, color intensity, and follicle density of the coat increased from baseline in all treatment groups (P < 0.05).
Discussion: Outcomes did not differ (P > 0.05) among treatment groups over 16-weeks, indicating that camelina oil is comparable to existing plant-based canine oil supplements, flaxseed, and canola, at supporting skin and coat health and inflammation in dogs. Future research employing an immune or exercise challenge is warranted, as the dogs in this study were not subjected to either.
Introduction
Dogs are unable to produce the omega-6 (n-6) linoleic acid (C18:2n-6; LA) and the omega-3 (n-3) α-linolenic acid (C18:3n-3; ALA), endogenously, and as such, these must be obtained in the diet (1). Omega-3 fatty acids (FAs) in particular have been linked to numerous health benefits, including a reduction in inflammation and oxidative stress, and improved skin and coat health properties, which are directly associated (2–7).
There is a competitive relationship between the n-6 and n-3 FA pathways for the use of the Δ5- and Δ6-desaturase and elongase enzymes needed to convert LA and ALA into longer chain FAs. Consequently, a balanced dietary n-6:n-3 ratio is needed to ensure sufficient conversion to longer chain FAs in both pathways. Specifically, and most notably, LA is converted into arachidonic acid (AA), and ALA is converted into eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (8). Both AA and EPA and DHA are parent compounds for the production of pro- and anti-inflammatory eicosanoids, respectively. An increase in endogenous n-6 AA results in a prothrombotic, pro-constructive, and pro-inflammatory state, whereas increased EPA and DHA give rise to resolvins, which are anti-inflammatory and pro-resolving. Greater concentrations of n-6 FAs and a higher n-6:n-3 ratio allow for greater conversion of n-6 FAs to AA and more pro-inflammatory effects. In contrast, greater concentrations of n-3 FAs and a lower n-6:n-3 ratio allow for increased production of EPA and more anti-inflammatory effects (9). As a result, excessive amounts of n-6 FAs and a high n-6:n-3 ratio promote the pathogenesis of many inflammatory, autoimmune, and dermatological disorders, whereas greater concentrations of n-3 FAs and a low n-6:n-3 ratio exert suppressive effects (10).
In order to formulate canine diets to meet the ideal n-6:n-3 ratio of between 5:1 and 10:1, n-3 rich ingredients are typically required (11). Two oils commonly used to increase n-3 inclusion in canine diets are fish oil, as a result of its high levels of EPA and DHA (180 mg EPA, 120 mg DHA/1,000 mg of oil provided in the most common fish oil capsules in the United States today, however, doses vary widely between supplements), and flaxseed oil, due to its favorable n-6:n-3 ratio of 1:4.19 (12–15). However, large-scale fish oil production required to meet the demands of the growing pet food industry is not environmentally sustainable long-term, and the high abundance of ALA in flaxseed oil makes it susceptible to oxidation, making its use in commercial diets difficult (12, 15). Additionally, flaxseed crops are sensitive to various climates, diseases, and pests, making both of these options less than desirable (12, 14, 15). Alternative animal-based (beef, 1:0.05; milk, 1:0.07; eggs, 1:0.05) and plant-based (canola, 1:0.59; corn, 1:0.01; soybean, 1:0.12; and sunflower oil, 1:0.00) lipid sources commonly used in canine diet formulations all have higher concentrations of n-6 FAs rather than n-3 FAs (15–17). This leaves room in the market for an alternative plant-based oil source that is economically and environmentally sustainable, with good shelf-stability and a favorable concentration of n-3 FAs that could contribute to achieving the ideal n-6:n-3 ratio in canine diets.
The oil seed camelina (Camelina sativa) is considered a low-input, high-yield crop due to its short growing season and resistance to various seasons, climates, and soil types (18–21). The product of this robust crop, camelina oil, provides a rich source of n-3 FAs as a result of its desirable n-6:n-3 ratio of 1:1.8 (22). Additionally, camelina oil contains high concentrations of tocopherols and polyphenols, which have been associated with improved skin and coat health due to their antioxidant properties (22). Due to camelina oil being naturally high antioxidants as well as having a slightly lower concentrations of n-3 FAs in contrast to flaxseed oil, it's shelf-stability is better by comparison (23).
Additional data from this study suggests camelina oil to be safe for canine consumption (24). The inclusion of oil supplements in canine diets is often associated with claims of maintenance or support of skin and coat health, but currently there is no data directly comparing the effects of camelina oil supplementation to the effects of other oils approved for use in pet foods on markers of skin and coat health and inflammation. The objective of this study was to compare the effects of dietary camelina oil supplementation to those of flaxseed oil and canola oil supplementation on skin and coat health and inflammatory and oxidative markers in healthy, adult dogs. Outcomes include changes in oxidative and inflammatory biomarkers and coat quality. Additionally, skin barrier function and integrity was assessed by measuring transepidermal water loss (TEWL). Authors hypothesize that camelina oil (n-3:n-6 = 1:1.8) is comparable, flaxseed (n-3:n-6 = 1:4.19) and canola oil (n-3:n-6 = 1:0.59) in terms of its effects on oxidative and inflammatory markers, coat quality, and TEWL.
Materials and methods
Animals and housing
This experiment was approved by the University of Guelph's Animal Care Committee (AUP #4365) and was carried out in accordance with national and institutional guidelines for the care and use of animals. Thirty client-owned, adult (7.2 ± 3.1 years) dogs of mixed sex (17 females: 16 spayed, one intact; 13 males: 10 neutered, three intact), weight (27.4 ± 14.0 kg) and breed participated in this study (Table 1). All dogs were deemed healthy based on their previous medical history as well as a pre-study physical examination performed by a licensed veterinarian, complete blood count (CBC), and serum biochemistry profile. During the recruitment process, dogs were excluded if they had any skin conditions, received any pro- or anti-inflammatory medications 2-months prior to baseline samples, had abnormalities on their physical examination, CBC, or serum biochemistry, or were younger than 2 years of age. Dogs were housed at their owners' homes for the duration of the study, they followed their usual daily routines. Pet owners were instructed to provide no supplements, medications, antibiotics, antifungals, antiparasitics, or topical creams without notifying the researchers. Prior to week 10, dog #10, consuming FLX, withdrew from the study due to circumstances unrelated to the research trial or treatment diet.
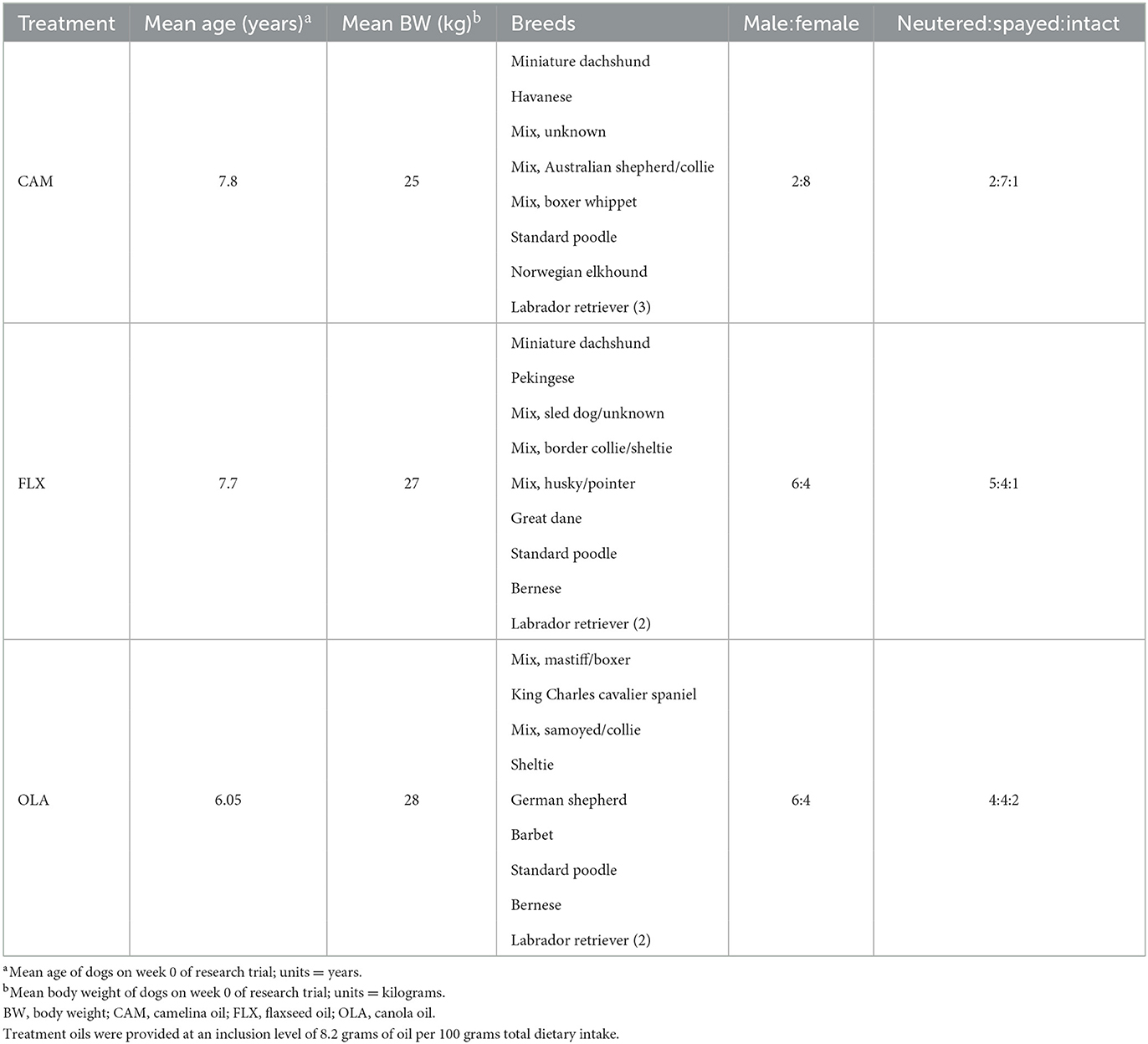
Table 1. Mean age, mean body weight, breeds, and male:female and neutered:spayed:intact ratios of 30 client-owned dogs enrolled in a research trial investigating the effects of three oil supplements (camelina, canola, flaxseed) on transepidermal water loss, inflammatory and oxidative markers, and coat quality over a 16-week period.
Dietary treatments
Over a 4-week wash-in period, all dogs were acclimated to a dry extruded commercial kibble (SUMMIT Three Meat Reduced Calorie Recipe, Petcurean, Chilliwack, BC, Canada; Table 2), sunflower oil (SA Kernel-Trade, Kuiv, Ukraine; Table 3), and beef-based treats (Beef Tendersticks, The Crump Group, Brampton, ON, Canada; proximate analysis: metabolizable energy 3039 kcal/kg; crude protein minimum 65%; crude fat minimum 5.1%; crude fiber maximum 4.0%; moisture max 9.56%). Oil was included in the diet at 8.2 grams of oil per 100 grams of total food intake, bringing the total dietary lipid content to 20% on an as-fed basis. Treats were included in the diet up to 2.5 grams per 100 grams total intake, and the remaining proportion of the diet was provided as kibble. During the wash-in period and throughout the study, daily portions of food, oil, and treats were pre-weighed by researchers and provided to the owners in 2-week intervals to be offered to dogs daily at a frequency determined by the owner. To avoid the occurrence of lipid peroxidation, owners were instructed to mix the oil with the food immediately before feeding. Any leftover kibble, oil, and/or treats were returned to researchers and subsequently weighed and recorded. Dogs were initially fed to meet their estimated maintenance energy requirements (110 kcal ME × kg BW0.75), and BW was recorded every 2 weeks starting at baseline. Each dog's food allotment was then adjusted accordingly to maintain baseline BW throughout the study. No abnormal observations were reported by owners throughout the 16-week study period in terms of diet tolerance (i.e., vomiting, stool quality, halitosis, etc.).
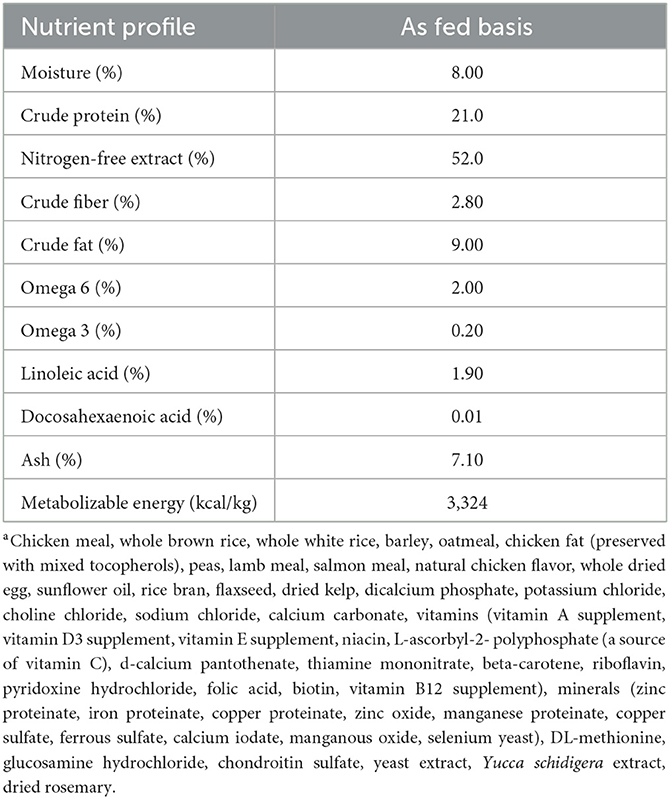
Table 2. Proximate analysis, metabolizable energy, omega-6 and omega-3, and linoleic and docosahexaenoic acid content of a commercial extruded kibblea on an as-fed basis, fed to 30 client-owned dogs during a skin and coat health trial over a 16-week period.
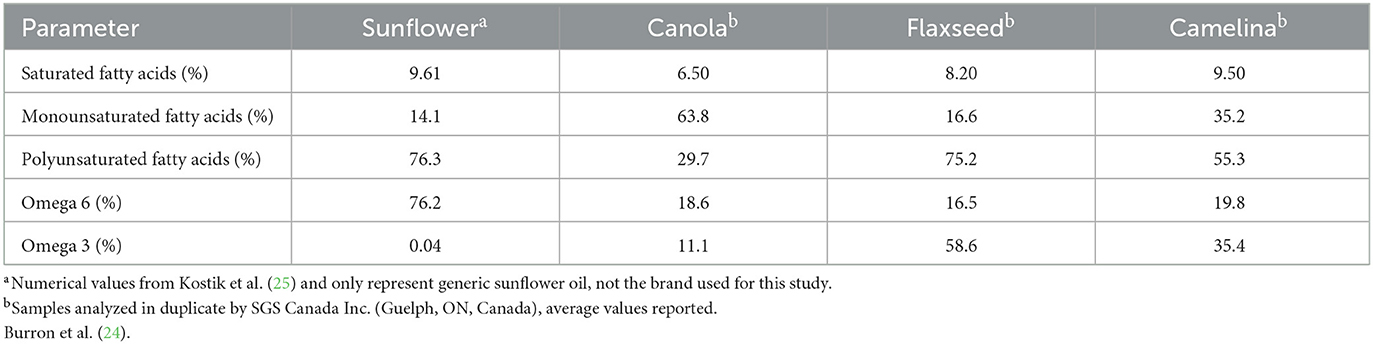
Table 3. Analyzed fatty acid profiles of camelina oil, canola oil, flax oil, and sunflower oil fed to 30 client-owned dogs top dressed on commercial kibble during a skin and coat health trial over a 16-week feeding period.
Study design
This study was conducted using a randomized complete block design (RCBD) with repeated measures. Following the 4-week wash-in period, dogs were blocked by breed, age, and BW and groups were randomly assigned to one of 3 treatment oils: camelina oil (CAM) (n = 10; eight females; two males), flaxseed oil (FLX) (n = 10; five females; five males), or canola oil (OLA) (n = 10; four females; six males). The sunflower oil used during the wash-in was replaced with either CAM, FLX, or OLA, and feeding continued as described for 16 weeks. Both OLA and FLX were chosen as control groups for this study as they are commonly used to formulate canine diets and provide a source of n-3 FAs.
Blood collection
Dogs were fasted for a minimum of 10 h overnight and blood samples were collected via cephalic venipuncture using a syringe (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Of the collected blood, 5 mL was put into a serum vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Blood was allowed to clot and was centrifuged at 7,200 × g for 15 min using an accuSpin Micro 17 centrifuge (Thermo Fisher Scientific, Waltham, MA, USA). Then, the serum aliquots were frozen at −80°C until later analysis.
Inflammatory and oxidative markers
Serum samples were analyzed for prostaglandin E2 (PGE2) (Canine Prostaglandin E2 ELISA Kit MBS013017, MyBioSource, Vancouver, BC) and junction plakoglobin (JUP) (Canine Junction Plakoglobin ELISA Kit MBS104997, MyBioSource, Vancouver, BC) using commercially available ELISA (Enzyme-linked immunosorbent assay) kits. Samples were run in duplicate according to the manufacturer's instructions. Serum glycosaminoglycan (GAG) (dimethyl methylene blue) and nitric oxide (NO) (Griess Reaction; Molecular Probes, Eugene, OR) concentrations were determined using spectrophotometric assays (26, 27). Serum NO and GAG samples were analyzed as previously described by MacNicol et al. (28).
Skin barrier function
Skin barrier function and integrity were assessed by measuring TEWL, which is defined as the amount of water that passively evaporates through skin to the external environment due to a water vapor pressure gradient on both sides of the skin barrier and is commonly used to characterize skin barrier function and integrity (29, 30). On weeks 0, 2, 4, 10, and 16, TEWL was measured using a VapoMeter® SWL-3 (Delfin Technologies Ltd, Kuopio, Finland), according to the manufacturer's instructions. Since privately-owned dogs were used, it was not feasible to shave multiple patches for TEWL measurements, and as a result, researchers chose three body sites with little hair to measure TEWL, including: the right paw pad, right pinna, and right inner thigh. Ten measurements were taken per body site and the average was used for analyses. Once the averages were calculated, any values above or below the average by 50 g/m2/h or more were considered outliers and removed. All dogs were brought to the University of Guelph by their owners on collection days to ensure environmental conditions during collections remained consistent. All measurements were carried out by a single operator, in the same order of body sites, and in a climate-controlled room to maintain consistency between samples and to avoid variation in VapoMeter® readings due to temperature and humidity fluctuations (29). Room conditions were stable at 22–23°C ambient temperature and 44–50% ambient relative humidity. The evaporation rate value is calculated in grams of water per square meter per hour (g/m2/h). All dogs were behaviorally acclimated to the use of the VapoMeter®, the researchers involved in sample collection, and the collection room, prior to the first sample day to minimize stress, thereby reducing variation in measurements. If dogs were wet due to weather upon arrival they were dried with a towel, to reduce variation further.
Coat quality
Two researchers blinded to treatment were trained to perform a subjective coat assessment on weeks 0, 2, 4, 10, and 16 using a 5-point Likert scale (under Supplementary material). A Likert scale was used to measure the softness, shedding, dander, shine, spring, softness uniformity, color, color uniformity, and follicle density of the coat. Follicle density was assessed on the center of the back of the dogs by scoring the thickness/amount of hair coming from individual follicles. To increase consistency among dogs given different management practices in each household, all dogs were bathed 2 weeks prior to each assessment and owners were instructed to keep dogs dry and to not brush or groom them during this period.
Statistical analysis
Data are presented as mean ± SD unless otherwise stated. All statistical analyses were performed using the PROC GLIMMIX of SAS Studio® software (v.9.4., SAS Institute Inc., Cary, NC, USA). Dog was the experimental unit, and treatment, TEWL site, and sex, and age were treated as fixed effects (age and sex data not presented). Week was treated as a repeated measure. An analysis of variance (ANOVA) was performed to assess the effects of treatment on inflammatory and oxidative marker concentrations, TEWL, and coat scores. When the fixed effects were significant, the means were separated using Tukey–Kramer adjustments. Significance was declared at a P ≤ 0.05. Trends were declared at P ≤ 0.10.
Results
Inflammatory and oxidative markers
Prostaglandin E2
There were no differences among treatments (P = 0.973), across weeks (P = 0.397), or for treatment by week interactions (P = 0.987) (Table 4). Additionally, no differences were observed due to sex (P = 0.937) or age (P = 0.274).
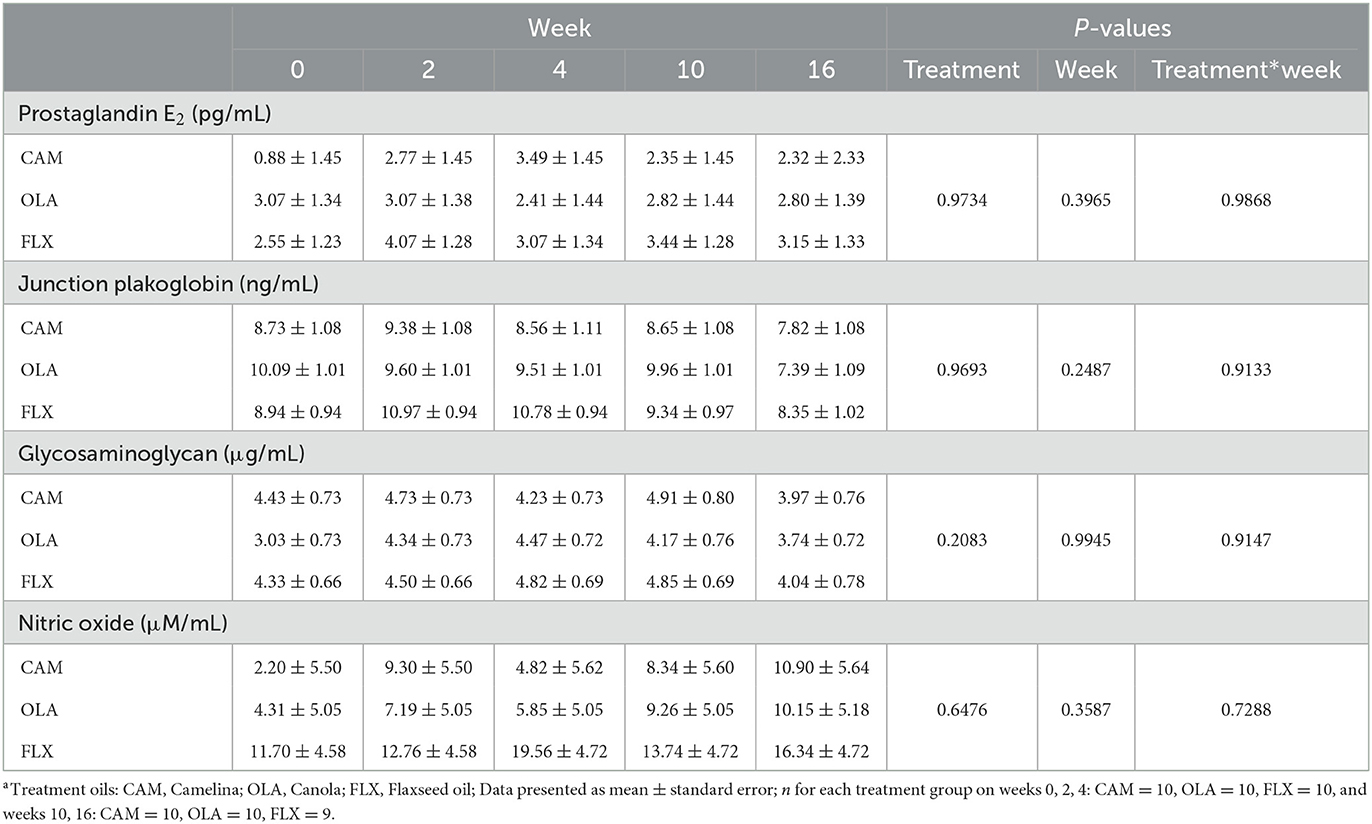
Table 4. Serum prostaglandin E2, junction plakoglobin, glycosaminoglycan, and nitric oxide concentrations of healthy adult dogs supplemented one of three treatment oilsa on weeks 0, 2, 4, 10, and 16 of a skin and coat health trial, presented as lsmeans ± standard error.
Junction plakoglobin
There were no differences among treatments (P = 0.969), across weeks (P = 0.249), or for treatment by week interactions (P = 0.913) (Table 4). No differences were observed due to sex (P = 0.914) or age (P = 0.743).
Glycosaminoglycan
There were no differences among treatments (P = 0.208), across weeks (P = 0.995), or for treatment by week interactions (P = 0.915) (Table 4). Concentrations of GAG tended to be greater in males compared to females (P = 0.078). There were no differences observed due to age (P = 0.329).
Nitric oxide
There were no differences among treatments (P = 0.648), across weeks (P = 0.359), or for treatment by week interactions (P = 0.729) (Table 4). No differences were observed due to sex (P = 0.226) or age (P = 0.424).
Transepidermal water loss
Of the 4,440 individual TEWL measurements collected throughout the study period, 18 were considered outliers and removed [D = Dog, W = Week; Paw pad: D6W2(CAM), D8W16(FLX)(2 values), D9W16(FLX), D17W4(CAM), D18W2(FLX), D18W4(FLX)(2 values), D23W10(CAM), D23W16(CAM); Inner ear: D5W4(OLA), D5W10(OLA), D12W10(OLA); Inner leg: D6W2(CAM), D6W10(CAM), D12W0(OLA), D16W0(FLX), D29W0(FLX)]. These outliers could often be attributed to changes in the environment, leading to signs of stress or excitement in the dogs (i.e., researchers entering and leaving the room, noises occurring outside of the sample room, and in the case of some outliers these samples were taken near the end of the collection period and the dogs would become impatient, no longer wanting to remain in the same spot for samples).
There were no differences among treatments (P = 0.726), across weeks (P = 0.738), or for treatment by week interactions (P = 0.996). Additionally, there were no differences for site by week (P = 0.378), or sex (P = 0.274) (Table 5). However, there were differences observed among sites (P < 0.0001), in that TEWL values for the paw pad were greater than those of the pinna or inner thigh. Additionally, there was a trend observed in age (P = 0.072), in that senior dogs (11–14 years; n = 3) tended to have lower mean TEWL values compared to young (2–4 years; n = 7), young adult (5–7 years; n = 9), and adult dogs (8–10 years; n = 9).
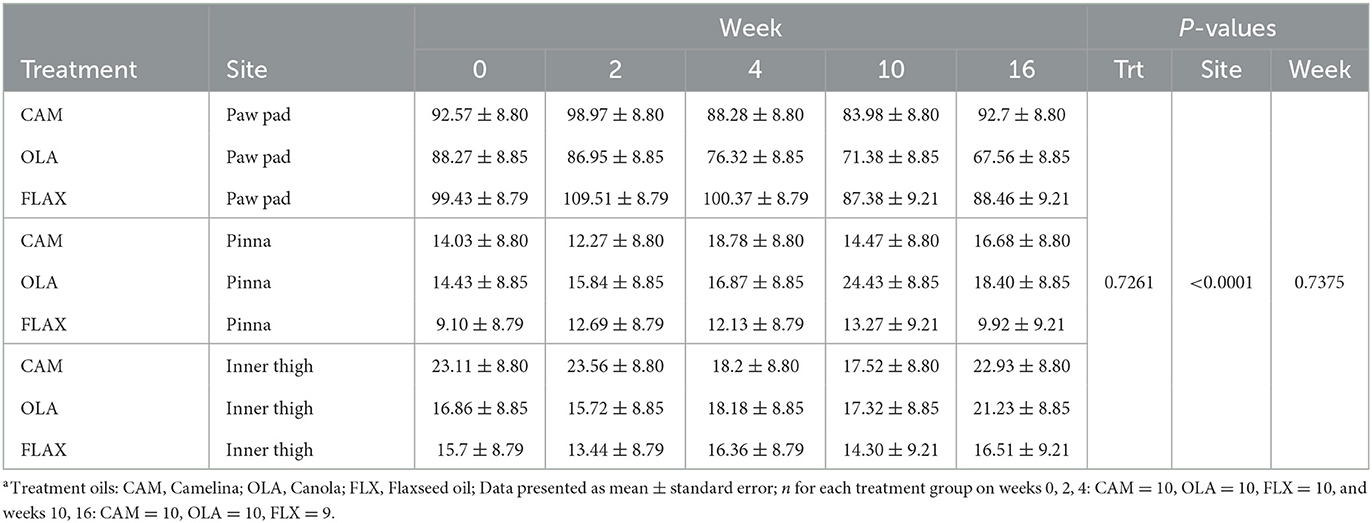
Table 5. Mean transepidermal water loss (TEWL) values (g/m2/h) of the right paw pad, right pinna, and right inner thigh of healthy adult dogs supplemented one of three treatment oilsa on weeks 0, 2, 4, 10, and 16 of a skin and coat health trial, presented as lsmeans ± standard error.
Coat quality
Softness
There were no differences among treatments (P = 0.539), for treatment by week interactions (P = 0.757), or due to age (P = 0.479), week by age (0.338) or week by sex (P = 0.738) interactions. However, there were differences observed across weeks for pooled data (P = 0.005) in that softness was greater on week 10 and 16 compared to week 0, and greater on week 10 compared to week 2. Week 4 was not different from any other time points (Figure 1). Additionally, softness was greater in females compared to males (P = 0.026).
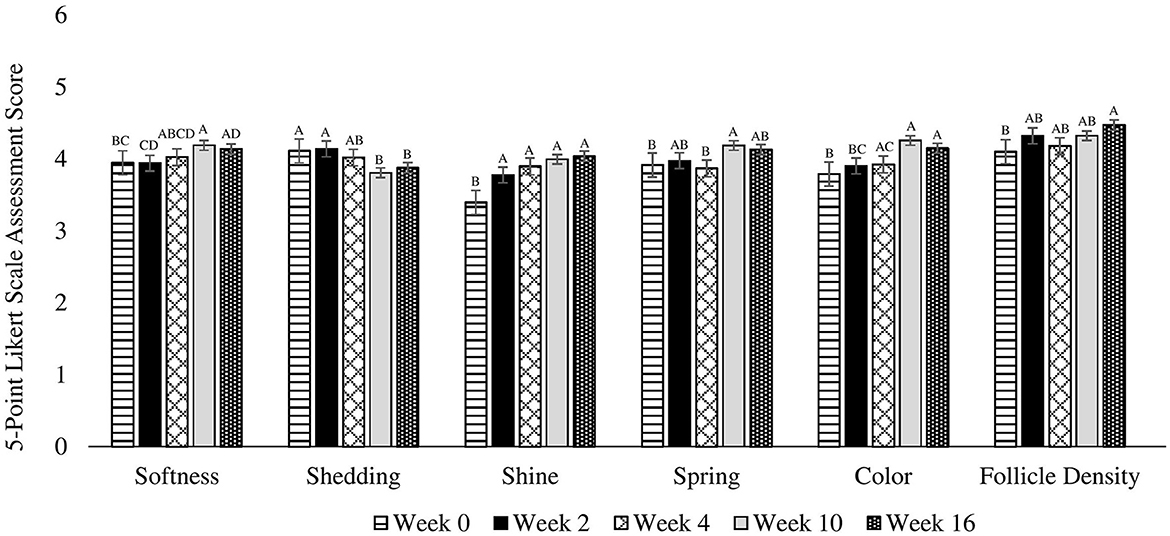
Figure 1. Mean coat quality assessment scores completed using a 5-point Likert scale on 30 client owned healthy adult dogs fed one of three treatment oils (camelina oil, canola oil, flaxseed oil) and commercial kibble. A, B, C, DBars without a common letter differ significantly (P < 0.05).
Shedding
There were no differences among treatments (P = 0.882), due to age (0.894) or sex (P = 0.760), or for treatment by week (P = 0.444), week by age (P = 0.302), or week by sex (P = 0.514) interactions. For pooled data across weeks, shedding was greater on weeks 0 and 2 compared to weeks 10 and 16 (P = 0.004). Week 4 was not different from any other time points (Figure 1).
Dander
There were no differences among treatments (P = 0.648), due to age (P = 0.114) or sex (P = 0.349), across weeks (P = 0.129), or for treatment by week (P = 0.869), week by age (P = 0.171), or week by sex (P = 0.163) interactions (Figure 1).
Shine
There were no differences among treatments (P = 0.815), due to age (P = 0.945), or sex (P = 0.191), or treatment by week (P = 0.998), week by age (0.992), or week by sex (P = 0.375) interactions. However, there were differences across weeks for pooled data (P < 0.0001) in that shine on weeks 2, 4, 10, and 16 was greater than at week 0 (Figure 1).
Spring
There were no differences among treatments (P = 0.918), due to age (P = 0.663) or sex (P = 0.401), or for treatment by week (P = 0.397), week by age (P = 0.773), or week by sex (P = 0.997) interactions. However, there were differences across weeks for pooled data (P = 0.014) in that spring was greater on week 10 compared to week 4 and 0. There were no differences on weeks 2 and 16 (Figure 1).
Softness uniformity
There were no differences among treatments (P = 0.969), due to age (P = 0.860) or sex (P = 0.132), or for treatment by week (P = 0.799), week by age (P = 0.996), or week by sex (P = 0.142) interactions. However, a trend was observed across weeks for pooled data (P = 0.065) in that softness uniformity tended to be greater on week 16 compared to week 0. Weeks 2, 4, and 10 were not different from any other time points (Figure 1).
Fur color
There were no differences among treatments (P = 0.323), due to age (P = 0.770) or sex (P = 0.546), or for treatment by week (P = 0.567), week by age (P = 0.345), or week by sex (P = 0.954) interactions. However, there were differences across weeks for pooled data (P < 0.0001) in that color was higher on weeks 4, 10, and 16 compared to week 0. Additionally, color was greater on week 10 and 16 compared to week 2. Furthermore, color tended to be higher on week 10 compared to week 4 (Figure 1).
Fur color uniformity
There were no differences among treatments (P = 0.541), due to age (P = 0.893) or sex (P = 0.911), across weeks (P = 0.362), or for treatment by week (P = 0.291), week by age (P = 0.787), or week by sex (P = 0.910) interactions (Figure 1).
Follicle density
There were no differences among treatments (P = 0.873), due to age (P = 0.795) or sex (P = 0.854), or for treatment by week (P = 0.670), week by age (P = 0.846), or week by sex (P = 0.299) interactions. However, there were differences across weeks for pooled data (P = 0.027) in that follicle density was greater on week 16 compared to week 0. Weeks 2, 4, and 10 were not different from any other time points (Figure 1).
Discussion
The purpose of this study was to assess the effects of camelina oil supplementation on skin and coat health compared to canola and flaxseed oil, two oils currently used to formulate canine diets. The results presented herein suggest no differences in TEWL, coat quality, or the inflammatory and oxidative markers assessed due to treatment over the 16-week period.
Inflammatory and oxidative markers
In the current study, concentrations of GAG tended to be higher in males compared to females. Studies in humans by (1) Larking (31) and (2) Claassen and Werner (32) found that, similar to the present study, females have lower concentrations of GAG. Claassen and Werner analyzed GAG in thyroid cartilage while Larking measured GAG excretion in the tissue. Since GAG is a marker of cartilage turnover, Claassen and Werner attribute their findings to greater cartilage turnover in males, while Larking accredits their findings to the males in their study having a greater mean height (31, 32). It is possible that the female dogs in the present experiment had a smaller average height and lower cartilage mineralization than the males, which contributed to the lower concentration of circulating GAGs observed. However, height and cartilage mineralization were not measured in the present study. Furthermore, the observation made in our study was only a tendency; this, combined with the dearth of work carried out in dogs and lack of equal distribution of male/female, intact/neutered/spayed dogs in the current study make it difficult to form any cogent conclusions. Future research should investigate this relationship further using a dog model.
No significant changes were observed in PGE2, JUP, GAG, or NO concentrations over the 16-week study period. It is possible that the stability of these concentrations across time and among treatments is attributed to the lack of exercise or immune challenge experienced by the dogs on the current study. It is well-established that both exercise and immune challenges result in a wide range of physiological and biochemical adaptations, the magnitude of which is directly related to the intensity and duration of the exercise or immune challenge encountered (33–36). This wide range of physiological and biochemical adaptations include changes in inflammatory and oxidative biomarker concentrations (28, 33).
Dogs and horses both experience increased PGE2 concentrations following exercise. In horses, NO and GAG concentrations increase following exercise and compared to baseline, but no change was observed in dogs (28, 33). Pearson et al. attribute these results, similar to previous findings, to variations in NO production depending on exercise intensity, suggesting that it is possible that the lack of changes observed in NO concentration in the current study is due to the low intensity of the exercise experienced by the dogs (33). Markers like PGE2, NO, GAG, and JUP are often upregulated during times of immune challenge/disease (37–40). A myriad of studies completed in humans suggest no effects of n-3 PUFA supplementation on inflammatory or immune markers in healthy individuals (41–43). As an example, Pot et al. found that supplementing fish oil and sunflower oil to healthy individuals had no effect on chemokine, cytokine, or cell adhesion molecule concentration compared to baseline (41). Healthy individuals, similar to the canine subjects of our study, generally have low levels of circulating inflammatory markers. Thus, the chance that low levels of inflammation are reduced even further by an intervention with oil is very small and difficult to measure. The dogs of the present study were healthy upon recruitment and on every sample period based on a veterinary examination, as well as CBC and biochemistry analysis, indicating a lack of immune response that would elicit an inflammatory response. Additionally, the dogs did not participate in any intense exercise prior to or on sample days, and thus had no known reason to elicit any exercise stress induced response impacting markers of inflammatory or oxidative stress. For safety and animal care purposes, no procedures with the potential to cause harm to the animals, like an inflammatory or immune challenge, can be carried out in client-owned dogs. Additionally, the objective of the present study was to determine how these three oils compare to one another in terms of their effects on these biomarkers to gauge their use in dog food formulations for typical pets, not to evaluate their performance following an exercise or immune challenge. Future studies should compare the effects of these three oils and their performance following exercise and immune challenge.
Transepidermal water loss
In the present study, mean TEWL values were significantly greater when measured on the paw pad compared to the inner leg and inner ear. This is likely the result of the tubular, unbranched eccrine glands that open directly onto the skin of the paw pads and noses of canines. These glands allow sweat to be released from these areas, contributing to the water-loss detected by the VapoMeter, and thereby likely contributing to greater TEWL values compared to the inner leg and pinna (44). Additionally, TEWL values were found to be lower in senior dogs compared to young, young adult, and adult dogs. Similar findings have been observed in other canine and human studies and although the exact mechanism behind these observations is unclear, there are various theories (45, 46). The thickness of the stratum corneum and flattening of corneocytes increases with age, while natural moisturizing factors, stratum corneum hydration, and epidermal lipid synthesis are reduced (47–53). Additionally, the density of dermal capillaries decreases with age, which may lower skin temperature and in turn decrease water diffusion (51, 54). All of these findings provide examples of mechanisms that increase the path length and resistance of a water molecule and subsequently contribute to lower TEWL in older individuals, and in agreement with the present study.
Coat quality
Spring and follicle density increased significantly from baseline. This is likely due, at least in part, to the growth of winter coats as the study began at the end of summer and went into the winter (September–January). Dogs have a light summer undercoat that is shed before a thick winter undercoat grows in, which could explain the increase in spring and follicle density. This further supports the observation of the present study in that shedding was greater in all dogs at the beginning of the study at weeks 0 and 2, compared to weeks 10 and 16.
Softness, shine, and color of the dogs' coats increased from baseline. This is likely a result of the dogs consuming an increased amount of n-3 FAs following baseline, which can be further metabolized into EPA and DHA, though with limited efficiency. Supplementation of fish oil, a rich source of EPA and DHA, was found to improve skin and hair coat quality in dogs from baseline based on a clinical score, with maximal improvement occurring after 8 weeks (55). The positive effects on skin and coat health are thought to be due to an increase in EPA and DHA in the erythrocyte membrane, along with increased total lipids in the hair shaft (55). The same study observed that following supplement withdrawal, skin and coat health clinical scores remained the same for 1 month and began to deteriorate following the second month (55). Although we did not take measurements on week 8, we did take measurements on week 10, and this is where we saw the largest improvement (i.e., softness, shedding, shine, spring, and color). This is most likely due to the increase in ALA, which is the parent compound of EPA and DHA, the dogs received from their treatment oil (CAM 1:1.8, FLX 1:4.19, OLA 1:0.59) in comparison to the wash-in sunflower oil (1:0). It is important to note that our study had no negative control group, since the absence of an oil supplement would alter all macronutrient intakes and our aim was to compare to existing approved oil supplements. As a result it cannot be ruled out that the observed changes in coat quality may be a result of the placebo effect. Future studies should consider employing a control group fed no oil supplement to rule out the possibility of the placebo effect impacting observations.
All dogs in the current study were considered healthy, with no known dermatological conditions or skin disorders. The coats of these dogs were in relatively good condition at baseline, and future research should investigate these oil supplements and their effects on skin and coat health in dogs with poor skin and coat quality as a result of conditions like atopic dermatitis. It is important to note that ectoparasites, particularly fleas in dogs, can negatively impact skin and coat health (56). In this study, although complete blood count and biochemistry values were assessed, and physical examinations were performed by a licensed veterinarian prior to study recruitment and throughout the entire trial, diagnostic and preventive control in terms of ectoparasites was not considered, and this is a limitation of this study. Authors recommend future studies consider using more specific techniques as inclusion criteria when recruiting participants in order to ensure the absence and prevention of parasites and their potential impact on skin and coat health.
Conclusion
In conclusion, camelina oil is comparable to canola and flaxseed oil in terms of its effects on skin barrier function, coat quality, and the circulating inflammatory and oxidative markers measured in the current study when fed to healthy adult dogs, subjected to no physical or immunological challenge, and observed for 16-weeks. Canola and flaxseed oil are commonly used in canine food formulations. Flaxseed oil specifically has the ability to support skin and coat health claims, making camelina oil a potential alternative plant-based oil source with high concentrations of ALA that could contribute to achieving the ideal n-6:n-3 ratio in canine diets, while supporting skin and coat health claims.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by University of Guelph Animal Care Committee. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
AS and WP: conceptualization and funding acquisition. AS, WP, and DM: methodology. TR, SB, KP, and CG: study conduct. TR: formal analysis and writing—original draft preparation. TR, SB, DWM, CG, KP, LT, DM, WP, and AS: writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by the Canadian Agricultural Partnership program as part of the diverse field crops clusters and funding from with Smart Earth Camelina. The kibble was provided by Petcurean, and the treats were provided by Crumps' Naturals.
Acknowledgments
Authors would like to thank the undergraduate and graduate students who assisted with this project, and all of the dogs and their owners for their commitment and cooperation during this study.
Conflict of interest
AS is the Champion Petfoods Chair in Canine and Feline Nutrition, Physiology and Metabolism and additionally consults for Champion Petfoods. AS has received various honoraria and research funding from various pet food manufacturers and ingredient suppliers and was a former employee of P&G Petcare and Mars Petcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1085890/full#supplementary-material
References
1. National Research Council Fats and Fatty Acids. Nutrient Requirements of Dogs and Cats. Washington, DC: The National Academies Press (2006). p. 81–110.
2. Billman GE, Kang JX, Leaf A. Prevention of ischemia-induced cardiac sudden death by N−3 polyunsaturated fatty acids in dogs. Lipids. (1997) 32:1161–8. doi: 10.1007/s11745-997-0149-2
3. Hock CE, Beck LD, Bodine RC, Reibel DK. Influence of dietary n-3 fatty acids on myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. (1990) 259. doi: 10.1152/ajpheart.1990.259.5.H1518
4. De Lorgeril M, Renaud S, Salen P, Monjaud I, Mamelle N, Martin J, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. (1994) 343:1454–9. doi: 10.1016/S0140-6736(94)92580-1
5. Wander RC, Hall JA, Gradin JL, Du S, Jewell DE. The ratio of dietary (N-6) to (N-3) fatty acids influences immune system function, eicosanoid metabolism, lipid peroxidation and vitamin E status in aged dogs. J Nutr. (1997) 127:1198–205. doi: 10.1093/jn/127.6.1198
6. Kirby NA, Hester SL, Rees CA, Kennis RA, Zoran DL, Bauer JE. Skin surface lipids and skin and hair coat condition in dogs fed increased total fat diets containing polyunsaturated fatty acids. J Anim Physiol Anim Nutr. (2009) 93:505–11. doi: 10.1111/j.1439-0396.2008.00832.x
7. Logas D, Kunkle GA. Double-blinded crossover study with Marine Oil supplementation containing high-dose icosapentaenoic acid for the treatment of canine pruritic skin disease. Vet Dermatol. (1994) 5:99–104. doi: 10.1111/j.1365-3164.1994.tb00020.x
8. Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP. Conversion of α-linolenic acid in humans is influenced by the absolute amounts of α-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr. (2006) 84:44–53. doi: 10.1093/ajcn/84.1.44
9. Zivkovic AM, Telis N, German JB, Hammock BD. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif Agric. (2011) 65:106–11. doi: 10.3733/ca.v065n03p106
10. Simopoulos A. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Dietet. (2003) 56:1–22. doi: 10.1159/000073788
11. Vaughn DM, Reinhart GA, Swaim SF, Lauten SD, Garner CA, Boudreaux MK, et al. Evaluation of effects of dietary N-6 to n-3 fatty acid ratios on leukotriene B synthesis in dog skin and neutrophils. Vet Dermatol. (1994) 5:163–73. doi: 10.1111/j.1365-3164.1994.tb00028.x
13. Brunner EJ, Jones PJ, Friel S, Bartley M. Fish, human health and marine ecosystem health: policies in collision. Int J Epidemiol. (2008) 38:93–100. doi: 10.1093/ije/dyn157
14. Oken E, Choi AL, Karagas MR, Mariën K, Rheinberger CM, Schoeny R, et al. Which fish should I eat? Perspectives influencing fish consumption choices. Environ Health Perspect. (2012) 120:790–8. doi: 10.1289/ehp.1104500
15. Sarker PK, Bureau DP, Hua K, Drew MD, Forster I, Were K, et al. Sustainability issues related to feeding salmonids: a Canadian perspective. Rev Aquacult. (2013) 5:199–219. doi: 10.1111/raq.12013
16. AAFCO. Official Publication. Champaign, IL: Association of American Feed Control Officials (2020).
18. Moser BR. Camelina (Camelina sativa L.) oil as a biofuels feedstock: golden opportunity or false hope? Lipid Technol. (2010) 22:270–3. doi: 10.1002/lite.201000068
19. Berti M, Gesch R, Eynck C, Anderson J, Cermak S. Camelina uses, genetics, genomics, production, and management. Ind Crops Prod. (2016) 94:690–710. doi: 10.1016/j.indcrop.2016.09.034
20. Putnam DH, Budin JT, Field LA, Breene WM. Camelina: a promising low-input oilseed. In:Janick J, Simon JE, , editors. New Crops.New York, NY: Wiley (1993). p. 314–22.
21. Vollmann J, Eynck C. Camelina as a sustainable oilseed crop: contributions of plant breeding and genetic engineering. Biotechnol J. (2015) 10:525–35. doi: 10.1002/biot.201400200
22. Zubr J, Matthäus B. Effects of growth conditions on fatty acids and tocopherols in camelina sativa oil. Ind Crops Prod. (2002) 15:155–62. doi: 10.1016/S0926-6690(01)00106-6
23. Eidhin DN, Burke J, O'Beirne D. Oxidative stability of N3-rich camelina oil and Camelina oil-based spread compared with plant and fish oils and sunflower spread. J Food Sci. (2003) 68:345–53. doi: 10.1111/j.1365-2621.2003.tb14163.x
24. Burron S, Richards T, Patterson K, Grant C, Akhtar N, Trevizan L, et al. Safety of dietary camelina oil supplementation in healthy, adult dogs. Animals. (2021) 11:2603. doi: 10.20944/preprints202107.0363.v1
25. Kostik V, Memeti S, Bauer B. Fatty acid composition of edible oils and fats. J Hyg EngDes. (2013) 4:112–6.
26. Chandrasekhar S, Esterman MA, Hoffman HA. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem. (1987) 161:103–8. doi: 10.1016/0003-2697(87)90658-0
27. Goldberg RL, Kolibas LM. An improved method for determining proteoglycans synthesized by chondrocytes in culture. Connect Tissue Res. (1990) 24:265–75. doi: 10.3109/03008209009152154
28. MacNicol JL, Lindinger MI, Pearson W. A Time-course evaluation of inflammatory and oxidative markers following high-intensity exercise in horses: A pilot study. J Appl Physiol. (2018) 124:860–5. doi: 10.1152/japplphysiol.00461.2017
29. Green M, Kashetsky N, Feschuk A, Maibach HI. Transepidermal water loss (TEWL): environment and pollution—a systematic review. Skin Health Dis. (2022) 2:e104. doi: 10.1002/ski2.104
30. Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. (1989) 30:323–33. doi: 10.1016/S0022-2275(20)38361-9
31. Larking P. Total glycosaminoglycans in the plasma of adults: effects of age and gender, and relationship to plasma lipids: a preliminary study. Biochem Med Metab Biol. (1989) 42:192–7. doi: 10.1016/0885-4505(89)90055-8
32. Claassen H, Werner J. Gender-specific distribution of glycosaminoglycans during cartilage mineralization of human thyroid cartilage. J Anat. (2004) 205:371–80. doi: 10.1111/j.0021-8782.2004.00348.x
33. Pearson W, Guazzelli Pezzali J, Antunes Donadelli R, Wagner A, Buff P. The time course of inflammatory biomarkers following a one-hour exercise bout in canines: a pilot study. Animals. (2020) 10:486. doi: 10.3390/ani10030486
34. Bessa AL, Oliveira VN G, Agostini G, Oliveira RJ, Oliveira AC, et al. Exercise intensity and recovery. J Strength Condit Res. (2016) 30:311–9. doi: 10.1519/JSC.0b013e31828f1ee9
35. Thirupathi A, Pinho RA, Ugbolue UC, He Y, Meng Y, Gu Y. Effect of running exercise on oxidative stress biomarkers: a systematic review. Front Physiol. (2021) 11:610112. doi: 10.3389/fphys.2020.610112
36. König D, Neubauer O, Nics L, Kern N, Berg A, Bisse E, et al. Biomarkers of exercise-induced myocardial stress in relation to inflammatory and oxidative stress. Exerc Immunol Rev. (2007) 13:15–36.
37. Nasrallah R, Hassouneh R, Hébert RL. PGE2, kidney disease, and cardiovascular risk: beyond hypertension and diabetes. J Am Soc Nephrol. (2015) 27:666–76. doi: 10.1681/ASN.2015050528
38. Tewari D. Role of nitric oxide in neurodegeneration: function, regulation, and inhibition. Curr Neuropharmacol. (2021) 19:114–26. doi: 10.2174/18756190MTA2pMjApy
39. Shi D, Sheng A, Chi L. Glycosaminoglycan-protein interactions and their roles in human disease. Front Mol Biosci. (2021) 8:639666. doi: 10.3389/fmolb.2021.639666
40. Fang W. Down-regulated γ-catenin expression is associated with tumor aggressiveness in esophageal cancer. World J Gastroenterol. (2014) 20:5839. doi: 10.3748/wjg.v20.i19.5839
41. Pot GK, Geelen A, Majsak-Newman G, Harvey LJ, Nagengast FM, Witteman BJ, et al. Increased consumption of fatty and lean fish reduces serum C-reactive protein concentrations but not inflammation markers in feces and in colonic biopsies. J Nutr. (2009) 140:371–6. doi: 10.3945/jn.109.113472
42. Muldoon MF, Laderian B, Kuan DC, Sereika SM, Marsland AL, Manuck SB. Fish oil supplementation does not lower C-reactive protein or interleukin-6 levels in healthy adults. J Intern Med. (2015) 279:98–109. doi: 10.1111/joim.12442
43. Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. (2012) 107:S159–70. doi: 10.1017/S0007114512001559
44. Carrier CA, Seeman JL, Hoffmann G. Hyperhidrosis in naïve purpose-bred beagle dogs (Canis familiaris). J Am Assoc Lab Anim Sci. (2011) 50:396–400.
45. Kottner J, Lichterfeld A, Blume-Peytavi U. Transepidermal water loss in young and aged healthy humans: a systematic review and meta-analysis. Arch Dermatol Res. (2013) 305:315–23. doi: 10.1007/s00403-012-1313-6
46. Watson A, Fray T, Clarke S, Yates D, Markwell P. Reliable use of the SERVOMED evaporimeter EP-2™ to assess transepidermal water loss in the canine. J Nutr. (2002) 132:136–45. doi: 10.1093/jn/132.6.1661S
47. Hadgraft J, Lane ME. Transepidermal water loss and skin site: a hypothesis. Int J Pharm. (2009) 373:1–3. doi: 10.1016/j.ijpharm.2009.02.007
48. Xiao P, Imhof RE. Two dimensional finite element modelling for dynamic water diffusion through stratum corneum. Int J Pharm. (2012) 435:88–92. doi: 10.1016/j.ijpharm.2012.01.047
49. Marks R. Measurement of biological ageing in human epidermis. Br J Dermatol. (1981) 104:627–33. doi: 10.1111/j.1365-2133.1981.tb00748.x
50. Boireau-Adamezyk E, Baillet-Guffroy A, Stamatas GN. Age-dependent changes in stratum corneum barrier function. Skin Res Technol. (2014) 20:409–15. doi: 10.1111/srt.12132
51. Alikhan A, Gorouhi F, Zargari O. Fox-Fordyce disease exacerbated by hyperhidrosis. Pediatr Dermatol. (2010) 27:162–5. doi: 10.1111/j.1525-1470.2010.01102.x
52. Rawlings AV. Recent advances in skin ‘barrier' research. J Pharm Pharmacol. (2010) 62:671–7. doi: 10.1211/jpp.62.06.0002
53. Tagami H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsic aging. Arch Dermatol Res. (2007) 300:1–6. doi: 10.1007/s00403-007-0799-9
55. Combarros D, Castilla-Castaño E, Lecru L, Pressanti C, Amalric N, Cadiergues M. A prospective, randomized, double blind, placebo-controlled evaluation of the effects of an N-3 essential fatty acids supplement (Agepi® N3) on clinical signs, and fatty acid concentrations in the erythrocyte membrane, hair shafts and skin surface of dogs with poor quality coats. Prostaglandins Leukotrienes Essential Fatty Acids. (2020) 159:102140. doi: 10.1016/j.plefa.2020.102140
Keywords: omega-3, omega-6, canine nutrition, skin and coat health, flaxseed oil, canola oil, camelina oil
Citation: Richards TL, Burron S, Ma DWL, Pearson W, Trevizan L, Minikhiem D, Grant C, Patterson K and Shoveller AK (2023) Effects of dietary camelina, flaxseed, and canola oil supplementation on inflammatory and oxidative markers, transepidermal water loss, and coat quality in healthy adult dogs. Front. Vet. Sci. 10:1085890. doi: 10.3389/fvets.2023.1085890
Received: 31 October 2022; Accepted: 10 February 2023;
Published: 09 March 2023.
Edited by:
Bangyuan Wu, China West Normal University, ChinaReviewed by:
Christopher Thomas, Cardiff University, United KingdomMarie Christine Cadiergues, Ecole Nationale Vétérinaire de Toulouse (ENVT), France
Jirayu Tanprasertsuk, NomNomNow Inc., United States
Copyright © 2023 Richards, Burron, Ma, Pearson, Trevizan, Minikhiem, Grant, Patterson and Shoveller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna K. Shoveller, YXNob3ZlbGxAdW9ndWVscGguY2E=
 Taylor L. Richards
Taylor L. Richards Scarlett Burron1
Scarlett Burron1 David W. L. Ma
David W. L. Ma Wendy Pearson
Wendy Pearson Anna K. Shoveller
Anna K. Shoveller