- Department of Animal Production, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia
Introduction: Consumer interest in poultry and high-quality meat products has increased. Probiotics are used in the diet to improve the quality of broiler meat. The aim of this study was to investigate the effects of multi-strain probiotics (RISCO-NUTRIFOUR®, RNF) on the quality and physicochemical properties of broiler meat.
Methods: A total of 288 broilers received six feed treatments for 1-14 days in water and 15-28 days in feed. T1-T3 received 0.4%, 0.2%, and 0.1% RNF, respectively; T4 received 0.1% Bacillus subtilis (BS; CLOSTAT®); T5 received 0.1% Saccharomyces cerevisiae (SC); and T6 received 0.0% probiotic (NC). The meat quality and physicochemical properties of the broiler breast were evaluated on day 28.
Results and discussion: RNF, especially at 0.1% RNF, significantly reduced cooking losses was more tender (required the least SF), and improved average body weight at day 28 and total numerical feed conversion ratio compared to controls. The RNF probiotic had a positive effect on the texture profile (especially 0.4% RNF), sensory properties, and body weight (especially at 0.2% RNF). In conclusion, 0.4% RNF is recommended to achieve the best texture profile, 0.2% RNF to achieve the best juiciness and overall sensory acceptability as well as the best target weight of the broilers, and 0.1% RNF to achieve the most tender texture and the lowest cooking losses at day 28 compared to the controls.
1 Introduction
Chicken is the most popular poultry species and accounts for one third of the world’s meat production for human consumption, providing both meat and eggs (1). Chicken meat is an important source of high-quality protein and is in high demand worldwide due to its nutritional value and affordability (2, 3). As consumer preference shifts toward healthier diets, meat quality has become a critical factor in poultry production (4, 5).
The widespread use of antibiotics in poultry has raised concerns about antibiotic resistance, imbalance of the intestine microbiota, and the accumulation of residues in meat products. As a result, probiotics have emerged as viable, safe, and effective alternatives to antibiotics that promote gut health, immunity, and meat quality without undesirable side effects (1, 6). Among the various dietary supplements (7), probiotics such as Bacillus subtilis and Saccharomyces cerevisiae have shown potential to improve meat quality (5, 8). Probiotics have gained considerable attention in poultry nutrition as natural growth promoters and alternatives to antibiotics that improve gut health, feed efficiency, and overall meat quality.
Meat quality is significantly influenced by the microbial and physicochemical properties of the meat. Physicochemical properties, such as pH and color, and technological meat quality, such as water retention, drip loss, cooking loss, and shear force, are the most important and noticeable indices (1, 2, 9, 10). However, the effects of probiotics on these parameters are inconsistent across studies, and conflicting results are reported regarding their effects on broiler meat characteristics (4, 11–14).
This well-designed study addresses the demand for high-quality poultry meat by testing RISCO–NUTRIFOUR, a multi-strain probiotic (Bacillus substiles, Lactobacillus Parabunchneri, Saccharomyces cerevisiae yeast, Lactobacillus harbinensis, Rhodopseudomonas palustris, Rhodopseudomonas shaeroides, and Candida ethanolic) and its effects on meat quality parameters such as pH, color, water-holding capacity, cooking loss, texture, and sensory properties. The effects of multi-strain probiotics, such as RISCO-NUTRIFOUR® (RNF) on the physicochemical and technological quality of broiler meat, especially when administered by different routes, continue to be the subject of active research. The aim of this trial was to evaluate the effects of RNF probiotic supplementation-administered in water from day 1 to 14 and in feed from day 15 to 28-on the physicochemical properties, technological meat quality parameters, texture profile analysis (TPA), and sensory attributes of broiler breast meat, carcass characteristics, and feed conversion ratio of broiler chickens.
2 Materials and methods
2.1 Housing birds and experimental design
This study was conducted in June 2022 at King Saud University (KSU) Experimental Poultry Research Unit using 288 day-old Ross 308 chicks. The experiment complied with all applicable methods and procedures approved by the KSU Scientific Research Ethics Committee under the institutional approval code KSU-SE-21-47. The chicks were separated according to feather sex, weighed individually, and randomly divided equally into 6 experimental groups. Each treated group had 8 replicates with 6 birds each (3♂ and 3♀) (48 chicks per group). Experimental groups 1–3: groups treated with RNF at the three RNF doses 1, 2, and 3 (0.4, 0.2, and 0.1% RNF, respectively). Group 4: group treated with Bacillus substiles (BS, Clostat®). Group 5: group treated with Saccharomyces cerevisiae yeast (SC). Group 6: non-treated group (negative control).
The study investigated the effects of RISCO–NUTRIFOUR probiotic supplementation using two different administration methods: Water supplementation from 1 to 14 days and feed supplementation from 15 to 28 days. Supplementation in drinking water ensures a steady intake, especially in the first two weeks when feed intake is still in progress and the digestive system is not yet mature, from the 15th onwards, as the digestive system matures and feed intake increases, nutrient absorption becomes more efficient through feed. The broilers were treated with 1 of six water–based (from 1 to 14 days) and feed–based (from 15 to 28 days) treatments: 4 L/ton (0.4%), 2 L/ton (0.2%), 1 L/ton (0.1%), 0.1% Clostat “1:128,” 0.1% SC, and negative control (NC) 0%. Al Raya Specialties Industrial Factory in Riyadh, Saudi Arabia, manufactures the RISCO-NUTRIFOUR® solution, a probiotic mixture. RNF contains Bacillus substiles (1 × 10^9 colony-forming units (CFU)/ml), Lactobacillus Parabunchneri (1 × 10^9 CFU/mL), Saccharomyces cerevisiae yeast (1 × 10^5 CFU/mL), Lactobacillus harbinensis (1 × 10^9 CFU/mL), Rhodopseudomonas palustris (1 × 10^7 CFU/mL), Rhodopseudomonas shaeroides (1 × 10^7 CFU/mL), and Candida ethanolic (1 × 10^5 CFU/mL).
The experimental starter (0–14 days) and grower (15–28 days) diets were formulated as a mash according to the nutritional requirements of the Ross 308 Management Guide recommendations (Aviagen, 2019, New York, NY, USA), as shown in Supplementary Table S1. Feed and water were provided ad libitum. Chicks were housed in electrically controlled heated battery cages, with a room temperature of 35°C on arrival and a gradual decrease (2° C every 3 days) until day 24. The average outside temperature was approximately 26.4°C, and humidity ranged from light to moderate. The light bulb program was on continuously for 24 h during the first week of life and was on 23 h and off for 1 h during the rest of the experimental period. The broilers were housed in the same cage, which was 58 cm long, 50 cm wide, and 35 cm high. The stocking density was 6 birds per 0.30 m2. All chicks were immunized against Gumboro disease, Newcastle disease, and infectious bronchitis (Fort Dodge Animal Health-USA).
2.2 Bioactive chemicals analysis of RISCO–NUTRIFOUR
The procedures for the separation of chemical mixtures by gas chromatography–mass spectrometry (GC–MS; Agilent Technologies, Palo Alto, CA, USA) was described in detail by Azzam et al. (15). The bioactive chemicals were expressed as a proportion of the extracted samples.
2.3 Carcass traits, target weight, feed conversion ratio, and meat quality evaluation
The weights of the chicks at arrival and at the end of the trial were converted to the average target weight and used to calculate the daily weight gain. Then, the feed conversion ratio (1–28 d) was calculated by dividing the feed intake by the gain throughout these periods. On day 28, the broilers were slaughtered according to standard practice in Saudi Arabia, and eight male broilers per group (n = 8) were randomly selected to evaluate meat quality and carcass traits. The slaughter of broilers according to Islamic law complies with halal standards, with an emphasis on humane and respectful treatment during the slaughter process without anesthetic. The slaughtering was done with a very sharp knife and by a qualified person to allow for a faster process while minimizing the suffering of the birds, which is critical under halal standards, with an emphasis on maintaining the cleanliness and consistency of the meat. Slaughter weight and carcass weight (excluding head, neck, feathers, shanks, abdominal fat, and eviscerated organs) were measured. The carcass yield (%) was calculated as the ratio between carcass weight and slaughter weight. Breast, leg, wing, kidney, pancreas, lymphoid organs (liver, bursa, spleen, and thymus), and offal (gizzard, proventriculus, heart, and liver without gall bladder) were separated and weighed in the same manner. The percentage yield for each portion was estimated in relation to the live weight at slaughtering (16). After cutting, samples of the pectoral muscle were taken from each carcass to determine the physicochemical parameters (pH and color) and samples of the pectoralis major muscle to determine the textural characteristics and sensory properties. The samples were stored in the refrigerator at 4°C for 24 h after slaughter to measure the ultimate physicochemical parameters and then immediately stored at −20°C to determine the meat quality parameters. The frozen samples were thawed at 4°C before being tested for water-holding capacity (WHC), cooking loss (CL), myofibrillar fragmentation index (MFI), shear force (SF), texture profile analysis (TPA), and sensory evaluation.
2.4 Physico-chemical properties (pH value, core temperature, and color measurements)
The internal temperature and pH parameters of the pectoral muscles were determined 15 min and 24 h post-mortem using a thermocouple thermometer, taking the average of three pH measurements on the inner surface of the pectoral muscles at different locations for each sample. The pH was measured by inserting electrodes into the meat samples using a Hanna Instruments pH meter with microprocessor (model pH 211, Woonsocket, RI, USA).
The color parameters (L*, a*, and b* values and their derivatives) of the breast samples were measured 24 h post-mortem, whereby the average of two color measurements on the inside of the breasts was determined for each sample. Breast muscle color measurements were taken using a Minolta Chroma-Meter (Konica Minolta, Tokyo, Japan) with a CR400 head at an illumination setting compatible with D65 illumination (17). The coordinates L*, a*, and b* were evaluated according to the CIELAB system, where L* corresponds to lightness, a* to redness (between green and red), and b* to yellowness (between blue and yellow). The measurements were made after calibrating the device with a white reference tile at Y = 86.10, x = 0.3188, and y = 0.3362.
The center of the plane is neutral, and the distance from the center axis represents the color saturation “chroma,” while the angle on the chromaticity axes refers to the hue angle (18). In order to obtain a particularly realistic assessment of how consumers imagine the color of meat, chroma, delta color change (∆E), browning index (BI), whiteness index (WI), and hue angle (h*) were derived from the color coordinates and calibration values and formulated as described by Valizadeh et al. (19) and Cázares-Gallegos et al. (20).
2.5 Meat quality indicators
To measure water-holding capacity (WHC), the compression method described by Wilhelm et al. (21) was used. Thawed samples with a wet weight of about 2 to 3 g were carefully clamped between two sheets of filter paper and pressed for 5 min with a pressure device over two acrylic plates with a force of 10 kg; the samples were weighed again. The samples were analyzed in duplicate. Finally, the percentage of WHC was determined using the following equation: WHC (%) = 100 – [((Initial weight of sample – Final weight of sample/Initial weight of sample)) × 100]. Cooking loss (CL) is a common method for evaluating the water-holding capacity of meat during cooking. It is calculated as the percentage weight loss during the cooking process as described by Hussein et al. (22). In brief, the breast meat samples were weighed raw (initial weight, W₁). Samples are placed on a standard tabletop grill preheated to a specific temperature (e.g., 170–180°C). The samples are cooked until they reach an internal temperature of 75°C, which is recommended for poultry meat. The internal temperature is measured with a thermometer inserted into the thickest part of the breast. After cooking, the samples are cooled to room temperature (~20–25°C). The cooked samples are weighed again (final weight, W₂). To calculate the cooking loss (%):
Where: W₁ = Initial raw weight (g), W₂ = Final cooked weight (g).
The MFI of muscle samples was measured as an indirect indicator of calpain activity using the method described by Suliman et al. (23). Thawed, scissor-cut samples (4 g) were homogenized in 40 mL MFI buffer (2°C) for 30 s using a blender. After washing several times with MFI buffer, the absorbance was measured at 540 nm using a spectrophotometer (HACH DR/3000, Loveland, CO, USA). The MFI was calculated by multiplying the absorbance of the resulting 0.5 mg/mL solution by 200. To calculate the shear force (SF) as an index of breast meat tenderness, five rectangular core samples of 2*1*1 cm3 in size from each chilled, cooked sample were cut longitudinally parallel to the muscle fibers using a manual corer. The greatest force (N/cm2) of the TA-HD Texture Analyzer (Stable Micro Systems Ltd., Godalming, UK) equipped with a Warner-Bratzler shear barb with a triangular opening blade, could be applied vertically to the fibers. The crosshead was configured to move at 200 mm/min. From a distance of 15 mm, the device was operated at speeds of 2, 2, and 10 mm/s during the pre-, during-, and post-tests. The SF values were calculated using the maximum point of the generated curve.
2.6 Texture profile analysis (TPA)
The TPA was performed with a TA-HD Texture Analyzer. To determine the TPA, the cooked breast muscle fibers were scored parallel to the longitudinal direction using a hand-held coring device. A cylindrical piston was used to compress the samples to 80% of their original height over two test cycles. The force-time curves of the deformation were determined using test-specific analyzes in the texturometer. The velocities used were 2, 5 and 5 mm/s in the pre-, intermediate and post-test. The hardness, springiness, cohesiveness, and chewiness of the samples were measured as described by Novaković and Tomašević (24).
2.7 Sensory evaluation
The frozen meat samples were thawed overnight at 4°C, then wrapped in aluminum foil and cooked in the oven at 200°C until a core temperature of 70°C was reached. After cooking, the samples were cut into small pieces of approximately 2 cm3 and given a random code number for identification. Twenty-four trained KSU taste panelists were asked for a sensory evaluation of the meat. The mean of all panel ratings was calculated to determine the characteristics of the sample. The evaluation was carried out according to the method described by Grunert et al. (25) using a 9-point hedonic scale, whereby the meat samples used for the sensory evaluation were divided into the following groups based on the category scaling: 9, 8, 7, 6, 5, 4, 3, 2, 1 = extremely like, very like, moderately like, somewhat like, neither like nor dislike, somewhat dislike, moderately dislike, very like dislike, extremely dislike, respectively. Water and crackers were served to remove any residual taste in the mouth from the previous samples.
2.8 Statistical analysis
The Ryan-Einot-Gabriel-Welsch and Quiot (REGWQ) test, also known as the “Ryan’s method,” is used to determine statistically significant differences (p < 0.05) between independent treatment groups in a balanced 1-way ANOVA reporting means ± standard error of the mean (SEM) based on a completely randomized design using the general linear model (GLM) of SAS (26) software (Cary, NC, USA).
The equation of the model was:
Where Yij is an individual observation, μ is the overall mean, Ti is the effect of the ith treatment, and eij is the random residual error. Before starting the statistical analysis, the Kolmogorov–Smirnov test was performed to ensure that the data were normal.
For the statistical analysis, a typical experimental design was established for this study, which included multiple treatment groups with standardized protocols for humane slaughter and random sampling, and data collection with a sufficient number of birds (8 per group) for the evaluation of carcass traits and meat quality. Six chicks per cage (8 cages per group) were used to evaluate growth performance.
3 Results
3.1 Physico-chemical traits, meat quality, texture profile analysis, and sensory evaluation of the breast
The data on the physicochemical parameters the breast meat of 28-day-old broilers treated with RNF in water from 1 to 14 days and with feed from 15 to 28 days are presented in Table 1. The treatments had no significant effect (p > 0.05) on pH 15 min, core temperature 24 h post-mortem, ultimate color components and color derivatives. However, 0.4% RNF had a significantly higher ultimate breast pH (pH24 h) compared to the other treatment groups.
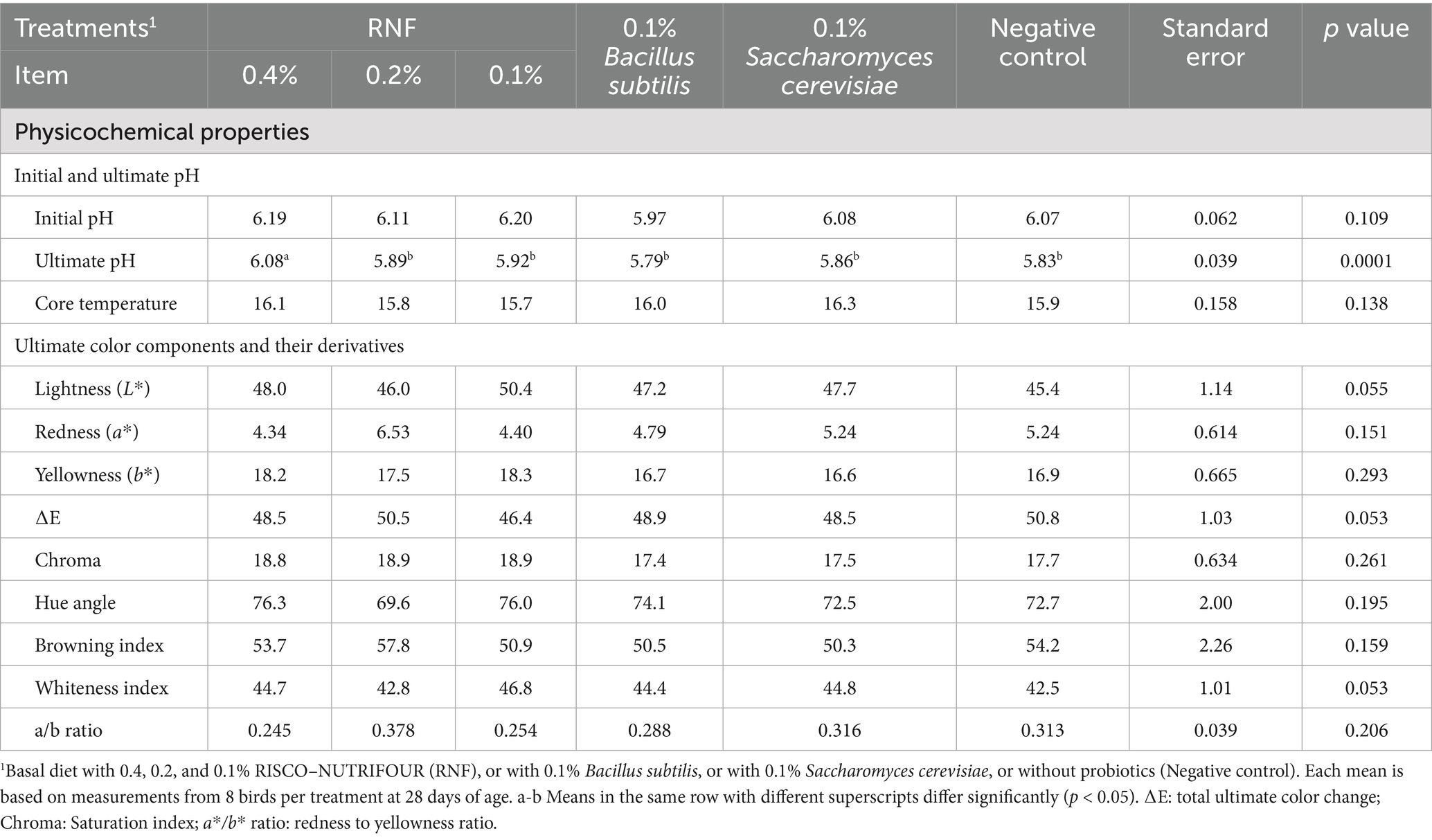
Table 1. Initial and ultimate pH, core temperature, color components and color derivatives at day 28 in breast meat of broiler treated with RISCO–NUTRIFOUR probiotics in a water and feed bases.
The meat quality characteristics for the breast samples at 28 days of age are shown in Table 2. CL% and SF of the breast samples differed (p < 0.05) between treatments. The 0.1% RNF treated group had the lowest CL% and SF values, indicating that the 0.1% RNF treated group had the most favorable CL% (8.42%) and tenderness (the lowest tenderness; 3.42). Although WHC% and MFI were similar between groups (p > 0.05), the 0.1% RNF treated group had the highest numerical (p > 0.05) from WHC and MFI, which decreased with increasing RNF dosage.
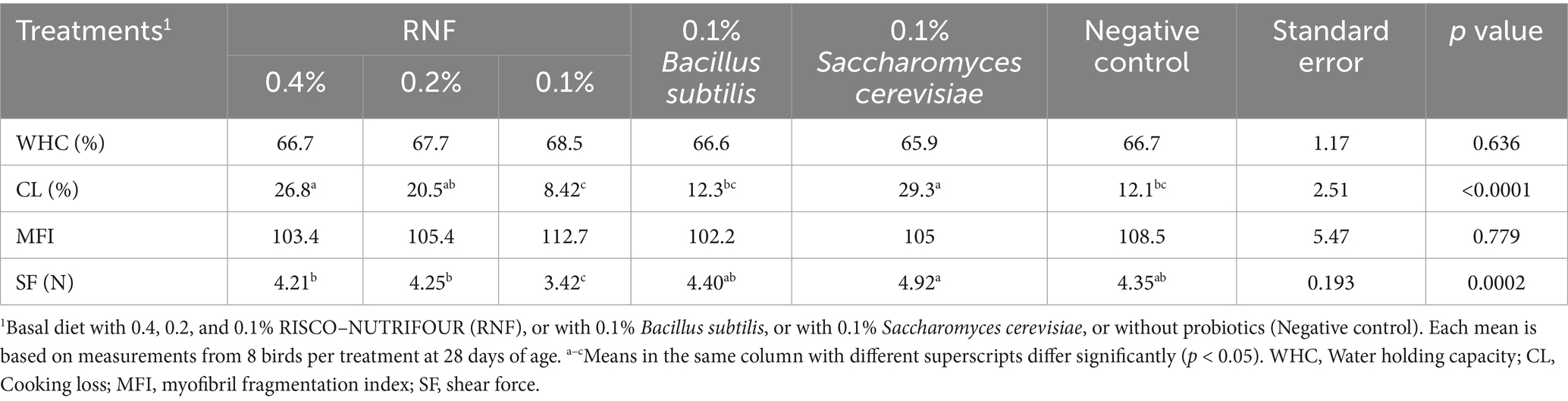
Table 2. Meat quality characteristics on day 28 of age in breast meat of broilers treated with RISCO–NUTRIFOUR probiotics in a water and feed base.
The TPA for the broiler samples on day 28 are shown in Table 3. Hardness (N), springiness, and cohesiveness of the breast meat samples differed (p < 0.05) between the treatment groups. Hardness was higher in groups treated with 0.1% BS and 0.1% SC probiotics, lower in groups treated with the RNF-probiotic mixture, and intermediate in the negative control group.
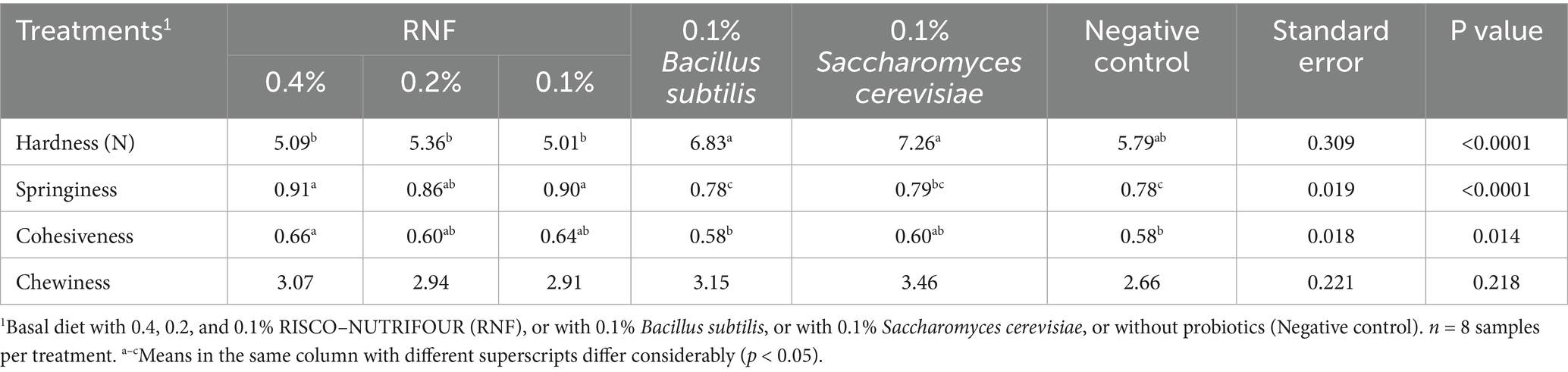
Table 3. Texture profile analysis (TPA) on day 28 of age in breast meat of broilers treated with RISCO–NUTRIFOUR probiotics in a water and feed base.
The RNF treatments, particularly at 0.4%, gave the most significant values for springiness and cohesiveness in the meat samples when compared to the negative control, with the 0.4% RNF treated group having the best values for springiness and cohesiveness. The chewiness values were similar between treatments (p > 0.05).
The sensory evaluation of the broiler samples at 28 days of age is shown in Table 4. Consumer ratings of tenderness and flavor were similar between treatments (p > 0.05), while juiciness and overall acceptability were highest in broilers treated with 0.2% RNF in water from 1 to 14 days of age and in feed from 15 to 28 days of age compared to the other groups.
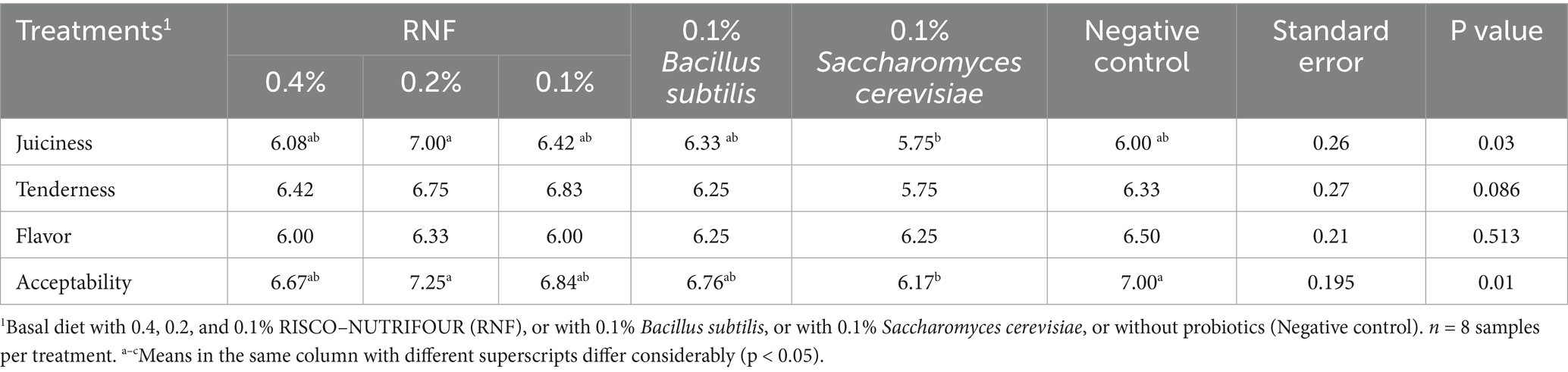
Table 4. Sensory attributes on day 28 of age in breast meat of broilers treated with RISCO–NUTRIFOUR probiotics in a water and feed base.
3.2 Carcass traits, target weight, and feed conversion ratio
The carcass characteristics (live slaughter weight (g), hot carcass weight (g), carcass yield (%), and relative carcass composition weights (% to live weight)) of 28-day-old broilers treated with RNF in water from 1 to 14 days and with feed for 15 to 28 days are shown in Table 5. The live weight, carcass weight, carcass yield, and carcass composition of the broilers did not differ (p > 0.05) between the experimental groups.
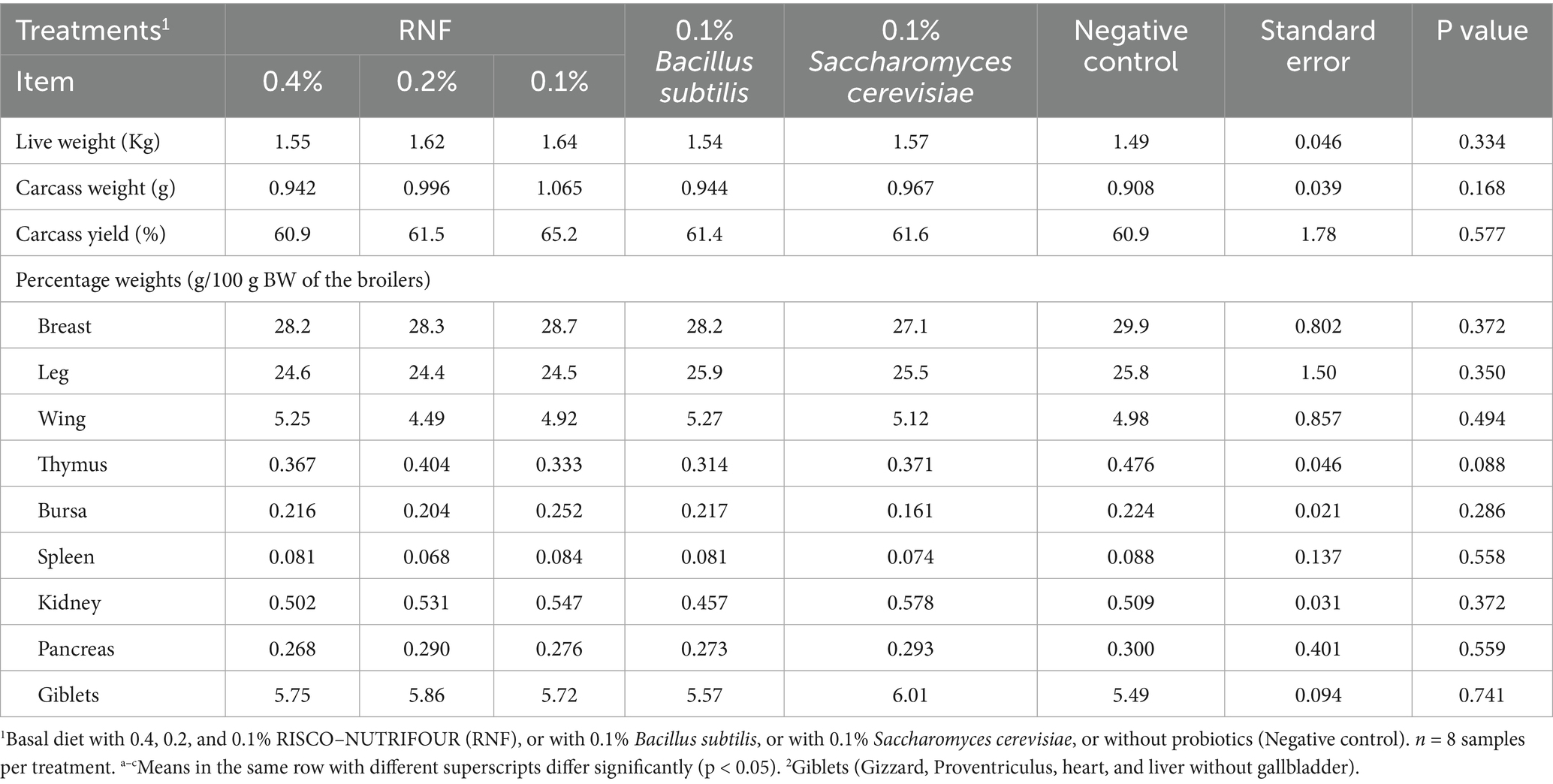
Table 5. Carcass traits measured at day 28 of broilers supplemented with RISCO–NUTRIFOUR probiotics in a water and feed bases.
Figure 1 shows the FCR of broilers treated with RNF in water for 1–14 days and with feed for 15–28 days. Water supplementation with RNF resulted in a lower (p > 0.05) FCR in broiler chicks compared to the negative control. However, FCR improved quantitatively between days 15 and 28, indicating that treatments with water supplementation may not be as effective in achieving optimal results. During the 1–14 days, 0.2% RNF treatment reduced FCR by 5.08% compared to the negative control (1.24 vs. 1.18). During 15–28 days, 0.2% RNF treatment improved (p > 0.05) FCR by 5.30% compared to negative control (1.51 vs 1.43). Thus, RNF was more effective when administered via feed rather than water compared to the control. The average body weight at day 28 and the overall FCR of broilers receiving RNF in water from 1 to 14 days and in feed from 15 to 28 days are shown in Figure 2. The 0.2% RNF treatment resulted in a significantly (p < 0.05) higher body weight (1.558 kg) on day 28 and a trend toward better feed conversion (1.40) throughout the period compared to the other treatments.
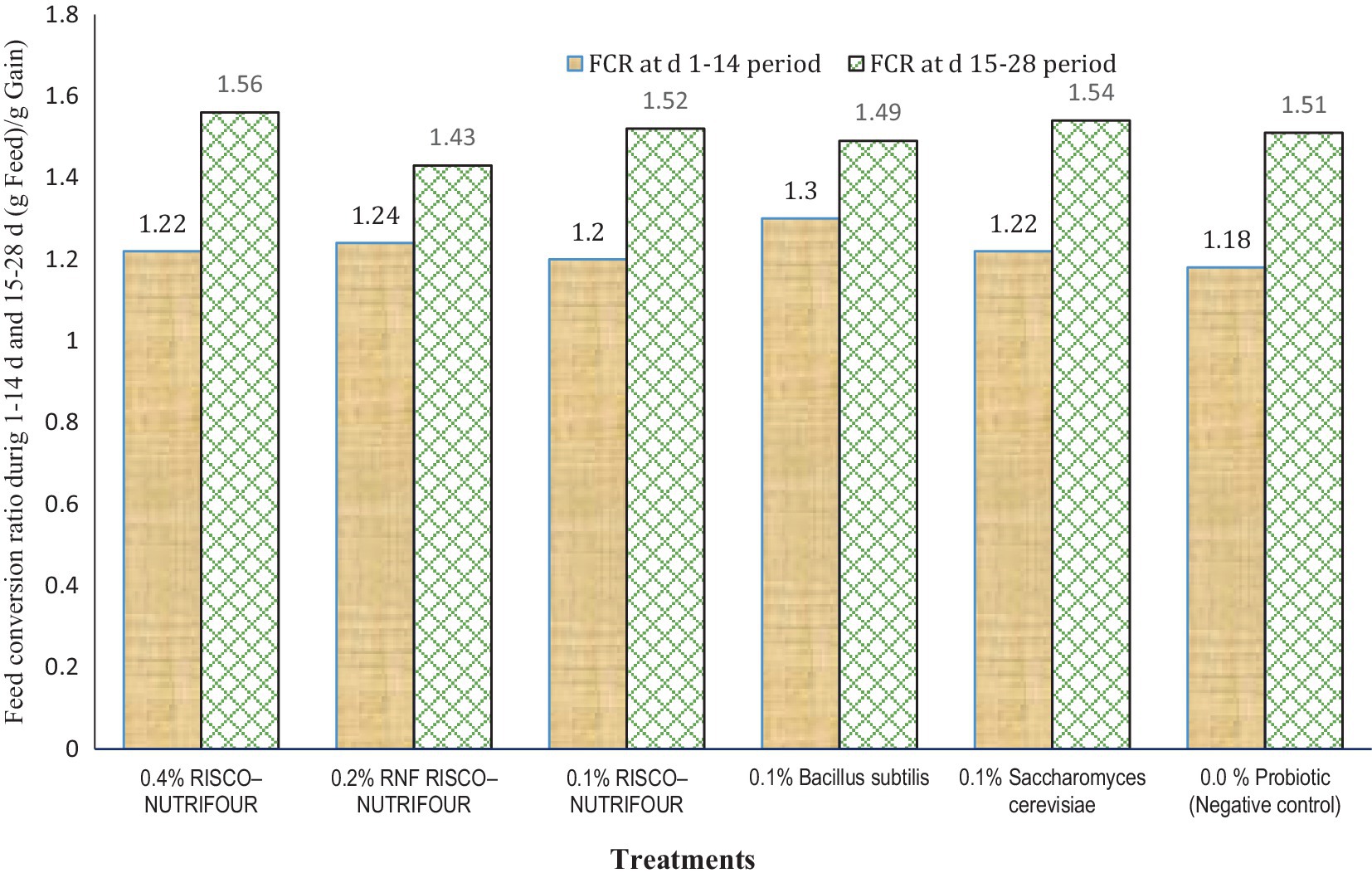
Figure 1. Feed conversion ratio (FCR) during 1–14 days’ period (p = 0.225; SEM ± 0.394); FCR during 15–28 days’ period (p = 0.394; SEM ± 0.042); n = 8 replicated cages.
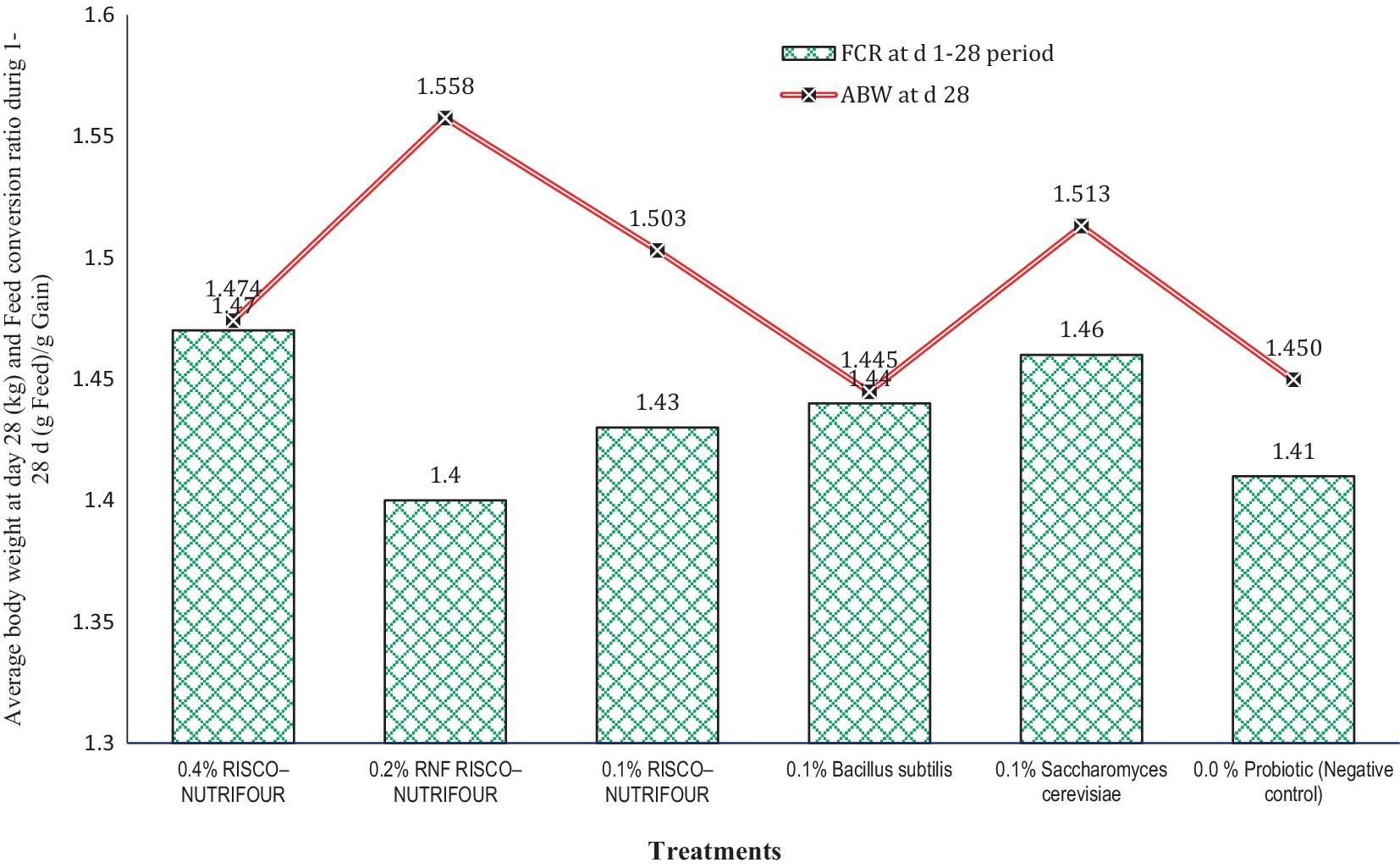
Figure 2. Average body weight at day 28 (kg; p = 0.041; SEM ± 0.027) and feed conversion ratio during 1–28 days’ periods (p = 0.041; SEM ± 0.027; FCR = feed intake (g)/gain (g)); n = 8 replicated cages.
4 Discussion
Probiotics, live non-pathogenic microorganisms added to human and animal diets, colonize the intestinal environment to promote a balanced flora consisting of species commonly found in the poultry gut (27–30). Probiotics have the ability to reduce pathogens, and improve the quality of broiler meat (30–32). The effectiveness of probiotics in exerting beneficial effects depends on their ability to colonize the intestine, which is influenced by various factors. These include the feeding program, the type, dose, and frequency of probiotic administration, the presence of prebiotics, and host-related factors such as age, health status, genetic characteristics, the pH of the intestine. In addition, external environmental conditions play a crucial role in determining probiotic colonization and functionality (30, 33–35).
In this study, the effects of probiotic supplementation on the meat quality of broilers were investigated. Meat quality parameters such as pH, lightness, redness, yellowness, cooking loss, water holding capacity, shear force, texture profile, and sensory evaluation were assessed. Analysis of REGWQ showed that the physicochemical data of the breast meat at day 28 were similar in the RNF groups, except for the ultimate pH, which was higher in the highest RNF group compared to the other groups. The pH was considered a general signal for meat quality testing, reflecting the conversion of glycogen to lactic acid in the muscle pre and post mortem (9). At that time, there was a direct relationship between pH and meat quality, including tenderness, water-holding capacity, color, juiciness, and shelf-life. Meat generally had a pH between 5.0 and 7.0 (36). Some of the studies that examined meat quality found that the use of probiotics increased redness and yellowness of breast meat and decreased lightness (5, 37), while probiotics increased all parameters of meat color in thigh meat (13, 38). In contrast, some studies found that probiotic supplementation had no effect on yellowness, redness, or lightness (5, 13, 37, 39). The addition of probiotics has been shown to consistently improve the redness and yellowness of broiler meat, which could be an indication of improved meat quality as perceived by the consumer. These differences between trials were more significant for thigh meat than breast meat, indicating that probiotics had a different impact depending on the anatomical location of the muscle.
This study showed that the use of RNF probiotics in broilers significantly improves meat quality. The 0.4% RNF improved the breast texture profile of the breast through improved springiness by 16.67% indicating better elasticity and resilience of meat after compression, and improved cohesiveness by 13.79% compared to the negative control, indicating improved structural integrity and firmness of the meat, resulting in better meat texture.
The 0.2% RNF showed a 21.7% increase in juiciness, increasing meat palatability and consumer satisfaction, and a 17.5% increase in overall acceptability, indicating a higher consumer preference for RNF-treated meat, compared to the SC group, and a 7.45% increase in average body weight at day 28 compared to the negative control, indicating improved growth performance with the 0.2% RNF supplementation. Also, the lowest (best) FCR was observed at RNF 0.2%, indicating better feed efficiency compared to the other groups. The lower FCR value indicates that the broilers fed 0.2% RNF utilized the feed more efficiently, resulting in higher weight gain per unit of feed consumed.
The 0.1% RNF showed a 71.3% reduction in cooking loss, which improved water-holding capacity, as well as a 30.5% reduction in shear force and 30.99% reduction in hardness, which improved meat tenderness compared to the SC group at day 28. Thus, the study shows that RNF supplementation significantly improves meat quality, especially in terms of water retention, tenderness, and sensory properties. Tang et al. (5) found that dietary supplementation with BS can improve meat quality and carcass characteristics of broilers, which is beneficial to consumers due to the improved fatty acid profile and amino acid composition. Other parameters studied were not significantly altered by the treatments. Previous research on probiotics in broilers has primarily focused on growth rather than meat quality, leaving a gap in the literature (40, 41). As a result, this study presents information about meat quality.
Similarly, studies on other meat quality metrics show that probiotic supplementation has no effect on pH, cooking loss, shear force, or drip loss of broiler meat (5, 13, 39). In addition, some studies found that probiotic supplementation increased pH and WHC in breast meat (42, 43) while reducing cooking loss, shear force, and drip loss in breast and thigh meat (13, 37, 38). The increase in WHC% and decrease in CL% in breast meat show that RNF probiotics, especially at the 0.1% level, can alter protein structures in muscle, improving their ability to bind moisture during cooking. This improves both the sensory properties of the meat and its nutritional value. The various research studies on the effect of adding probiotics to broilers vary widely, including the breed of chicken, the type of probiotic, the level and dosage, and the location of measurement. Therefore, a study was needed to further investigate these effects on meat quality indicators.
Several factors contribute to the heterogeneity of the study results. In some studies, measurements were taken on both the leg and the breast, while in others only the breast or the leg was examined. These differences highlight the complex relationships between probiotic supplementation and meat quality in broilers. This study provides a detailed assessment of the effects of probiotics on numerous meat quality traits in broilers. The study found that the addition of RNF probiotics, particularly at 0.1%, had a significant effect on meat texture profile, sensory characteristics, cooking loss, and shear force, all of which were significantly improved in the broiler breast meat. In addition, numerical improvements in WHC and MFI were observed in the breast meat portions of broilers receiving RNF at a low concentration (0.1%). The results have important implications for the chicken industry, particularly with regard to improving meat quality by optimizing feed formulation.
5 Conclusion
Based on the finding obtained in this study, the 0.4% RNF is recommended to achieve a 16.67% improvement in springiness and a 13.79% improvement in cohesiveness meat texture compared to the negative control. With 0.2% RNF, a 21.7% increase in juiciness and a 17.5% increase in overall acceptability compared to the SC group, and a 7.45% increase in average body weight at day 28 compared to the negative control and overall feed conversion compared to other groups is recommended. It is recommended that 0.1% RNF achieves a 71.3% reduction in cooking loss, improving water-holding capacity, a 30.5% reduction in shear force and a 30.99% reduction in hardness, improving meat tenderness compared to the SC group on day 28. The study thus shows that supplementation with RNF significantly improves meat quality, particularly in term of water retention, tenderness, and sensory properties, and points to avenues for further research and standardization in poultry production. These results also contribute to a better understanding of the role of RISCONUTRIFOUR probiotics in improving meat quality and meeting consumer demands for nutritious and high-quality poultry products.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by King Saud University (KSU-SE-21-47). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AA-a: Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. MA-G: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing. MQ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AM: Data curation, Investigation, Methodology, Writing – review & editing. MA: Investigation, Writing – review & editing. MA-B: Data curation, Software, Writing – review & editing. EH: Data curation, Validation, Writing – review & editing. GS: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Ongoing Research Funding Program (ORF-2025-690), King Saud University, Riyadh, Saudi Arabi.
Acknowledgments
The authors extend their appreciation to the Ongoing Research Funding Program (ORF-2025-690), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1517078/full#supplementary-material
References
1. Halder, N, Sunder, J, De, AK, Bhattacharya, D, and Joardar, SN. Probiotics in poultry: a comprehensive review. J Basic Appl Zool. (2024) 85:23. doi: 10.1186/s41936-024-00379-5
2. Popova, T. Effect of probiotics in poultry for improving meat quality. Curr Opin Food Sci. (2017) 14:72–7. doi: 10.1016/j.cofs.2017.01.008
3. Vlaicu, PA, Untea, AE, Varzaru, I, Saracila, M, and Oancea, AG. Designing nutrition for health—incorporating dietary by-products into poultry feeds to create functional foods with insights into health benefits, risks, bioactive compounds, food component functionality and safety regulations. Food Secur. (2023) 12:4001. doi: 10.3390/foods12214001
4. Mohammed, A, Mahmoud, M, Zaki, R, and Cheng, H. Effect of a probiotic supplement (Bacillus subtilis) on struggling behavior, immune response, and meat quality of shackled broiler chickens exposed to pre-slaughter stress. Poult Sci. (2024, 2024) 103:104051. doi: 10.1016/j.psj.2024.104051
5. Tang, X, Liu, X, and Liu, H. Effects of dietary probiotic (Bacillus subtilis) supplementation on carcass traits, meat quality, amino acid, and fatty acid profile of broiler chickens. Front Vet Sci. (2021) 8:767802. doi: 10.3389/fvets.2021.767802
6. Rashid, S, Alsayeqh, AF, Akhtar, T, Abbas, RZ, and Ashraf, R. Probiotics: alternative to antibiotics in poultry production. Int J Vet Sci. (2023) 12:45–53. doi: 10.47278/journal.ijvs/2022.175
7. Thong, HT, and Duc, HV. Potential substitutes of antibiotics for swine and poultry production In: AA Kamboh, editor. Antibiotics and probiotics in animal food-impact and regulation. London: IntechOpen (2022)
8. Abd El-Hack, ME, El-Saadony, MT, Shafi, ME, Qattan, SY, Batiha, GE, Khafaga, AF, et al. Probiotics in poultry feed: A comprehensive review. J Anim Physiol Anim Nutr. (2020) 104:1835–50. doi: 10.1111/jpn.13454
9. Mir, NA, Rafiq, A, Kumar, F, Singh, V, and Shukla, VJJo. f. s., and technology. Determinants of broiler chicken meat quality and factors affecting them: a review. J Food Sci Technol. (2017) 54:2997–3009. doi: 10.1007/s13197-017-2789-z
10. Popova, T, Petkov, E, Ignatova, M, Vlahova-Vangelova, D, Balev, D, Dragoev, S, et al. Meat quality of male layer-type chickens slaughtered at different ages. Agriculture. (2023) 13:624. doi: 10.3390/agriculture13030624
11. Kim, H, Yan, F, Hu, J, Cheng, H, and Kim, Y. Effects of probiotics feeding on meat quality of chicken breast during postmortem storage. Poult Sci. (2016) 95:1457–64. doi: 10.3382/ps/pew055
12. Macelline, WSP, Cho, HM, Awanthika, HT, Wickramasuriya, SS, Jayasena, DD, Tharangani, RH, et al. Determination of the growth performances and meat quality of broilers fed Saccharomyces cerevisiae as a probiotic in two different feeding intervals. Korean J Poultry Sci. (2017) 44:161–72. doi: 10.5536/KJPS.2017.44.3.161
13. Yu, L, Peng, Z, Dong, L, Wang, H, and Shi, S. Enterococcus faecium NCIMB 10415 supplementation improves the meat quality and antioxidant capacity of muscle of broilers. J Anim Physiol Anim Nutr. (2019) 103:1099–106. doi: 10.1111/jpn.13097
14. Zhang, Z, Zhou, T, Ao, X, and Kim, I. Effects of β-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize–soybean meal based diets. Livest Sci. (2012) 150:419–24. doi: 10.1016/j.livsci.2012.10.003
15. Azzam, MM, Qaid, MM, Al-Mufarrej, SI, Al-Garadi, MA, Albaadani, HH, and Alhidary, IA. Rumex nervosus leaves meal improves body weight gain, duodenal morphology, serum thyroid hormones, and cecal microflora of broiler chickens during the starter period. Poult Sci. (2020) 99:5572–81. doi: 10.1016/j.psj.2020.08.023
16. Suliman, GM, Hussein, EOS, Al-Owaimer, AN, Alhotan, RA, Al-Garadi, MA, Mahdi, JMH, et al. Betaine and nano-emulsified vegetable oil supplementation for improving carcass and meat quality characteristics of broiler chickens under heat stress conditions. Front Vet Sci. (2023) 10:1147020. doi: 10.3389/fvets.2023.1147020
17. Kokoszyński, D, Żochowska-Kujawska, J, Kotowicz, M, Wegner, M, Arpášová, H, Włodarczyk, K, et al. Carcass characteristics, physicochemical traits, texture and microstructure of young and spent quails meat. Poult Sci. (2024) 103:103763:103763. doi: 10.1016/j.psj.2024.103763
18. Ly, BCK, Dyer, EB, Feig, JL, Chien, AL, and Del Bino, S. Research techniques made simple: cutaneous colorimetry: a reliable technique for objective skin color measurement. J Invest Dermatol. (2020) 140:e1:115080, 115080–2.e1. doi: 10.1016/j.jid.2019.11.003
19. Valizadeh, S, Naseri, M, Babaei, S, Hosseini, SMH, and Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int J Biol Macromol. (2019) 134:604–12. doi: 10.1016/j.ijbiomac.2019.05.071
20. Cázares-Gallegos, R, Silva-Vázquez, R, Hernández-Martínez, C, Gutiérrez-Soto, J, Kawas-Garza, J, Hume, M, et al. Performance, carcass variables, and meat quality of broilers supplemented with dietary Mexican oregano oil. Brazilian J Poultry Sci. (2019) 21:801. doi: 10.1590/1806-9061-2018-0801
21. Wilhelm, AE, Maganhini, MB, Hernández-Blazquez, FJ, Ida, EI, and Shimokomaki, M. Protease activity and the ultrastructure of broiler chicken PSE (pale, soft, exudative) meat. Food Chem. (2010) 119:1201–4. doi: 10.1016/j.foodchem.2009.08.034
22. Hussein, E, Suliman, G, Alowaimer, A, Ahmed, S, Abd El-Hack, M, Taha, A, et al. Growth, carcass characteristics, and meat quality of broilers fed a low-energy diet supplemented with a multienzyme preparation. Poult Sci. (2020) 99:1988–94. doi: 10.1016/j.psj.2019.09.007
23. Suliman, GM, Al-Owaimer, AN, Hussein, EOS, Abuelfatah, K, and Othman, MB. Meat quality characteristics of the Arabian camel (Camelus dromedarius) at different ages and post-mortem ageing periods. Asian Australas J Anim Sci. (2020) 33:1332–8. doi: 10.5713/ajas.19.0589
24. Novaković, S., and Tomašević, I. (2017). A comparison between Warner-Bratzler shear force measurement and texture profile analysis of meat and meat products: a review. In "59th International Meat Industry Conference, IOP Conf Series: Earth and Environmental Science", 85, pp. 012063–011315.
25. Grunert, KG, Bredahl, L, and Brunsø, K. Consumer perception of meat quality and implications for product development in the meat sector—a review. Meat Sci. (2004) 66:259–72. doi: 10.1016/S0309-1740(03)00130-X
26. SAS (2012). SAS Institute/STAT 9.3. User's guide: Mathematical programming examples. Cary NC: SAS Inst. 2–12.
27. FAO/WHO. Guidelines for the evaluation of probiotics in food, report of a joint FAO/WHO working group on drafting guideline for the evaluation of probiotic in food. Geneva: World Health Organization (2002).
28. Getachew, T. A review on effects of probiotic supplementation in poultry performance and cholesterol levels of egg and meat. J World's Poultry Res. (2016) 6:31–6.
29. Park, YH, Hamidon, F, Rajangan, C, Soh, KP, Gan, CY, Lim, TS, et al. Application of probiotics for the production of safe and high-quality poultry meat. Korean J Food Sci Anim Resour. (2016) 36:567–76. doi: 10.5851/kosfa.2016.36.5.567
30. Yibar, A, and Uzabaci, E. Meta-analysis to predict the effects of probiotics on meat quality of broiler. Journal of animal physiology animal. Nutrition. (2024) 108:1616–23. doi: 10.1111/jpn.14006
31. Bai, K, Huang, Q, Zhang, J, He, J, Zhang, L, and Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult Sci. (2017) 96:74–82. doi: 10.3382/ps/pew246
32. Zhou, X, Wang, Y, Gu, Q, and Li, W. Effect of dietary probiotic, Bacillus coagulans, on growth performance, chemical composition, and meat quality of Guangxi yellow chicken. Poult Sci. (2010) 89:588–93. doi: 10.3382/ps.2009-00319
33. Iqbal, MZ, Qadir, MI, Hussain, T, Janbaz, KH, Khan, YH, and Ahmad, B. Probiotics and their beneficial effects against various diseases. Pakistan J Pharmaceut Sci. (2014):27.
34. Mazziotta, C, Tognon, M, Martini, F, Torreggiani, E, and Rotondo, JC. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells. (2023) 12:184. doi: 10.3390/cells12010184
35. Ohashi, Y, and Ushida, K. Health-beneficial effects of probiotics: its mode of action. Anim Sci J. (2009) 80:361–71. doi: 10.1111/j.1740-0929.2009.00645.x
36. Heymich, M-L, Srirangan, S, and Pischetsrieder, M. Stability and activity of the antimicrobial peptide Leg1 in solution and on meat and its optimized generation from chickpea storage protein. Food Secur. (2021) 10:1192. doi: 10.3390/foods10061192
37. Liu, X, Ma, A, Zhi, T, Hong, D, Chen, Z, Li, S, et al. Dietary effect of Brevibacillus laterosporus S62-9 on chicken meat quality, amino acid profile, and volatile compounds. Food Secur. (2023) 12:288. doi: 10.3390/foods12020288
38. Mohammed, AA, Zaki, R, Negm, E, Mahmoud, M, and Cheng, H. Effects of dietary supplementation of a probiotic (Bacillus subtilis) on bone mass and meat quality of broiler chickens. Poult Sci. (2021) 100:100906:100906. doi: 10.1016/j.psj.2020.11.073
39. Moon, S-G, Kothari, D, Lee, W-D, Kim, J-I, Kim, K-I, Kim, Y-G, et al. Potential probiotic acceptability of a novel strain of Paenibacillus konkukensis SK 3146 and its dietary effects on growth performance, Intestinal Microbiota, and Meat Quality in Broilers. Animals. (2022) 12:1471. doi: 10.3390/ani12111471
40. Leão, APA, Alvarenga, RR, and Zangeronimo, MG. In ovo inoculation of probiotics for broiler chickens: systematic review and meta-analysis. Anim Feed Sci Technol. (2021) 280:115080:115080. doi: 10.1016/j.anifeedsci.2021.115080
41. Uzabaci, E, and Yibar, A. Effects of probiotic supplementation on broiler growth performance: a meta-analysis of randomised controlled trials. Anim Prod Sci. (2023) 63:645–51. doi: 10.1071/AN22295
42. Park, J, and Kim, I. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult Sci. (2014) 93:2054–9. doi: 10.3382/ps.2013-03818
Keywords: broilers meat quality, feed conversion ratio, meat texture, RISCO-NUTRIFOUR probiotic, physicochemical properties, sensory attributes
Citation: Al-abdullatif AA, Al-Garadi MA, Qaid MM, Matar AM, Alobre MM, Al-Badwi MA, Hussein EO and Suliman GM (2025) Effects of water and feed based RISCO-NUTRIFOUR probiotic supplementation on the technological and physicochemical quality of broiler breast meat. Front. Vet. Sci. 12:1517078. doi: 10.3389/fvets.2025.1517078
Edited by:
Oyegunle Emmanuel Oke, Federal University of Agriculture, Abeokuta, NigeriaReviewed by:
Khalid M. Mahrose, Zagazig University, EgyptNikola Čobanović, University of Belgrade, Serbia
Copyright © 2025 Al-abdullatif, Al-Garadi, Qaid, Matar, Alobre, Al-Badwi, Hussein and Suliman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulaziz A. Al-abdullatif, YWFsYWJkdWxsYXRpZkBrc3UuZWR1LnNh; Maged A. Al-Garadi, bWFsZ2FyYWRpQGtzdS5lZHUuc2E=
 Abdulaziz A. Al-abdullatif
Abdulaziz A. Al-abdullatif Maged A. Al-Garadi
Maged A. Al-Garadi Mohammed M. Qaid
Mohammed M. Qaid Abdulkareem M. Matar
Abdulkareem M. Matar Mohsen M. Alobre
Mohsen M. Alobre Mohammed A. Al-Badwi
Mohammed A. Al-Badwi Elsayed O. Hussein
Elsayed O. Hussein Gamaleldin M. Suliman
Gamaleldin M. Suliman