- 1Department of Emergency and Critical Care, Angell Animal Medical Center, Boston, MA, United States
- 2Department of Pathology, Angell Animal Medical Center, Boston, MA, United States
- 3BluePearl, Tampa, FL, United States
Objective: Detection of bacteremia on peripheral blood smear (PBS) is rare and may be a poor prognostic indicator for small animal patients. This study aimed to determine the relationship between bacteremia on PBS and survival to discharge in clinically ill patients presenting through the Emergency Department (ED).
Methods: This retrospective study analyzed data from two veterinary tertiary care facilities from 2014 to 2024. Records from 16 client-owned animals presenting to the ED with PBS-detected bacteremia were reviewed. The primary outcome was survival to discharge. Secondary outcomes evaluated associations between survival in these patients with glucose level, leukocyte count, toxic change, band neutrophils, total bilirubin, blood pressure, and antibiotic use. Statistical comparisons between categorical data were made using Fisher’s exact test. A p-value of <0.05 was considered significant.
Results: In-hospital mortality of the 16 patients was 75% (12/16). Hyperglycemia was positively associated with survival (p = 0.0099). All survivors were cats. No other parameters showed statistical significance between survivors and non-survivors.
Conclusion: PBS-detected bacteremia in clinically ill small animals was associated with a high in-hospital mortality in this study. Further investigation is warranted to better understand its clinical relevance and potential diagnostic utility.
1 Introduction
The primary objective of this study was to evaluate any association between bacteremia when detected on peripheral blood smear (PBS) in clinically ill small animal patients, and prognosis to discharge. Bacteremia, characterized by the presence of bacteria in the blood, is an uncommon, but concerning finding on a PBS (1, 2). Unlike conditions such as sepsis, which involves a life-threatening infection and dysregulated or insufficient host response, bacteremia may not necessarily indicate an advanced infection on its own (3, 4). Given the lower bacterial load in the blood that can be associated with bacteremia, it may be transient and handled by the immune system without medical intervention (3, 5). However, the detection of bacteremia on PBS in clinically unwell small animals raises concern for more severe illness and poorer prognosis (1, 6). Although bacterial load was not directly measured in the present study, detection of bacteremia on PBS in the emergency setting may serve as an early marker of disease severity.
There can be many different sources of bacteremia ranging from a peripheral wound, surgical site, venous access site, blood contamination, or a source of infection throughout the body leading to hematogenous spread (4). Though a PBS does not replace the recommendation for blood culture and further diagnostics (7, 8), it can serve as a rapid diagnostic tool to guide the initial treatment plan and expedite antibiotic therapy in lieu of pending or absent blood culture results (2, 9, 10). There is currently very limited veterinary literature evaluating whether the presence of bacteremia identified on PBS correlates with prognosis to discharge (2, 9).
The objective of this study was to report in-hospital mortality associated with PBS-detected bacteremia in clinically ill small animals, regardless of underlying cause. Our primary hypothesis was that the detection of bacteremia on PBS would be associated with a poor prognosis to discharge. A secondary aim was to evaluate whether survival in this bacteremic population was associated with other clinical parameters, such as blood glucose concentration (11).
2 Materials and methods
2.1 Study design
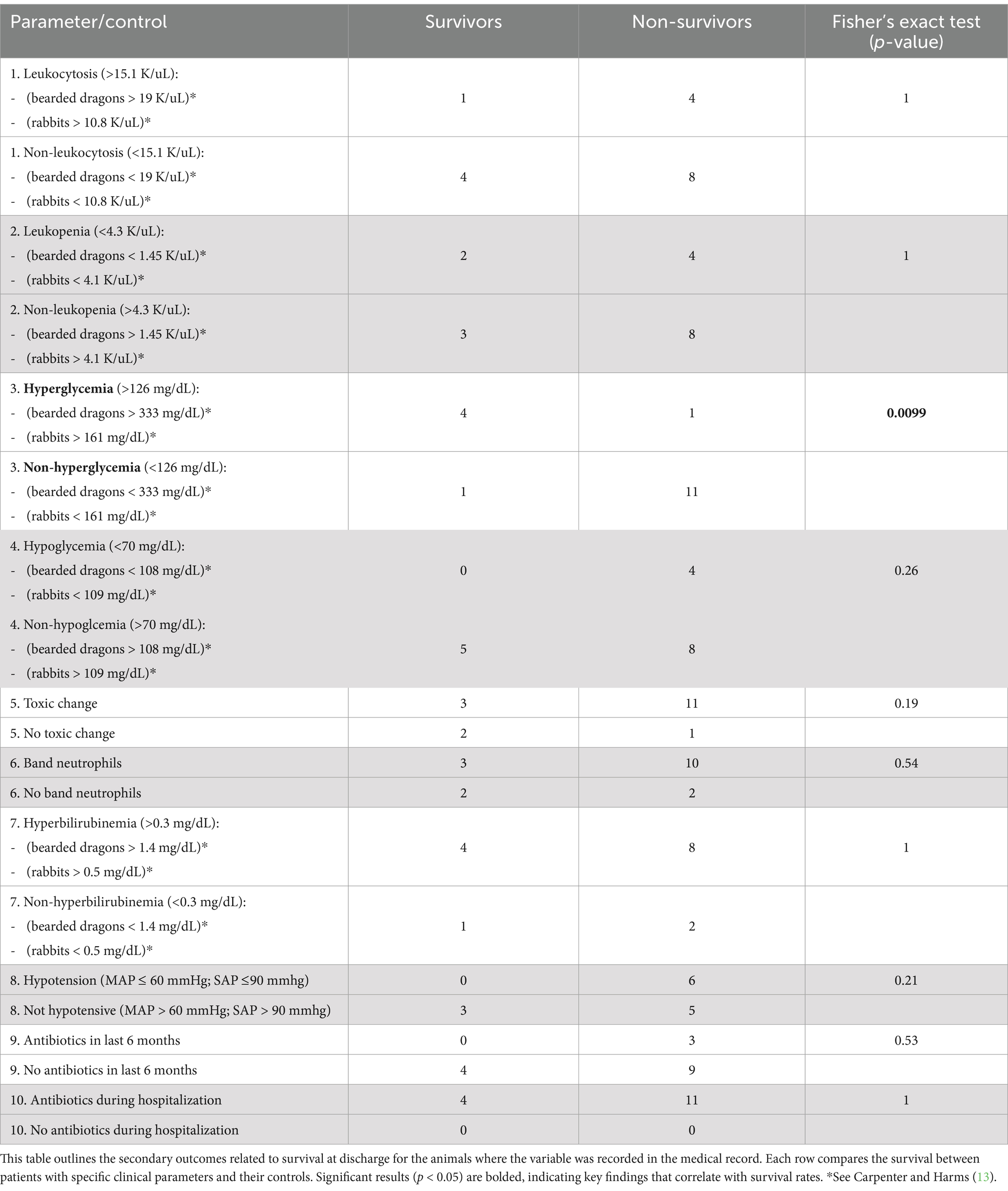
This multi-institutional, retrospective study was conducted at two veterinary tertiary care facilities. The medical record database at each facility was searched for the word “bacteremia.” Patients where bacteremia was identified on PBS by pathologist review at time of presentation or during hospitalization were considered for inclusion. Medical records spanning 2014 to 2024 were reviewed to extract relevant clinical data, including diagnostic findings and treatment outcomes. The primary outcome assessed was survival to discharge, with secondary outcomes describing the relationship between survival and various clinical parameters in this bacteremic patient population. The parameters that were evaluated included the patient’s signalment, serum glucose concentration, white blood cell count (WBC), presence of toxic change, band neutrophils, serum total bilirubin, blood pressure, and antibiotic usage while in hospital and over the 6 months prior to hospital admission.
2.2 Diagnostic techniques
Blood smears were prepared and stained using Wright-Giemsa stain. These smears were then examined by a board-certified clinical pathologist, with detailed descriptions of bacteria and cell morphology noted. At the included institutions, a pathologist review of a PBS is triggered by a technologist alert or CBC abnormality. Hypotension was defined as a systolic blood pressure of less than or equal to 90 mmHg. A mean arterial pressure of less than or equal to 60 mmHg was also included if an oscillometric blood pressure measurement was recorded in the record (12). The lowest blood pressure recorded during the hospitalization period was used. Antibiotic usage was recorded both during hospitalization and, when documented, within the 6 months prior to hospital admission (Table 1). Biochemical parameters were categorized using species-specific preset laboratory reference ranges, in combination with literature reference limits as detailed in Table 1 (12, 13). Blood glucose and total bilirubin concentrations were measured from serum samples. The biochemical results recorded were from initial blood work submitted at time of admission.
For the purpose of identifying possible sepsis in this patient population, sepsis was defined for the use in this study as a combination of a positive blood culture and available criteria that may indicate organ dysfunction (4, 8). The criteria that were considered were a leukocytosis or leukopenia, presence of band neutrophils, hypotension, and hyperbilirubinemia. These criteria were extrapolated from the human sequential organ failure assessment (SOFA) score and SIRS criteria for dogs and cats (4, 8, 14).
2.3 Statistical analysis
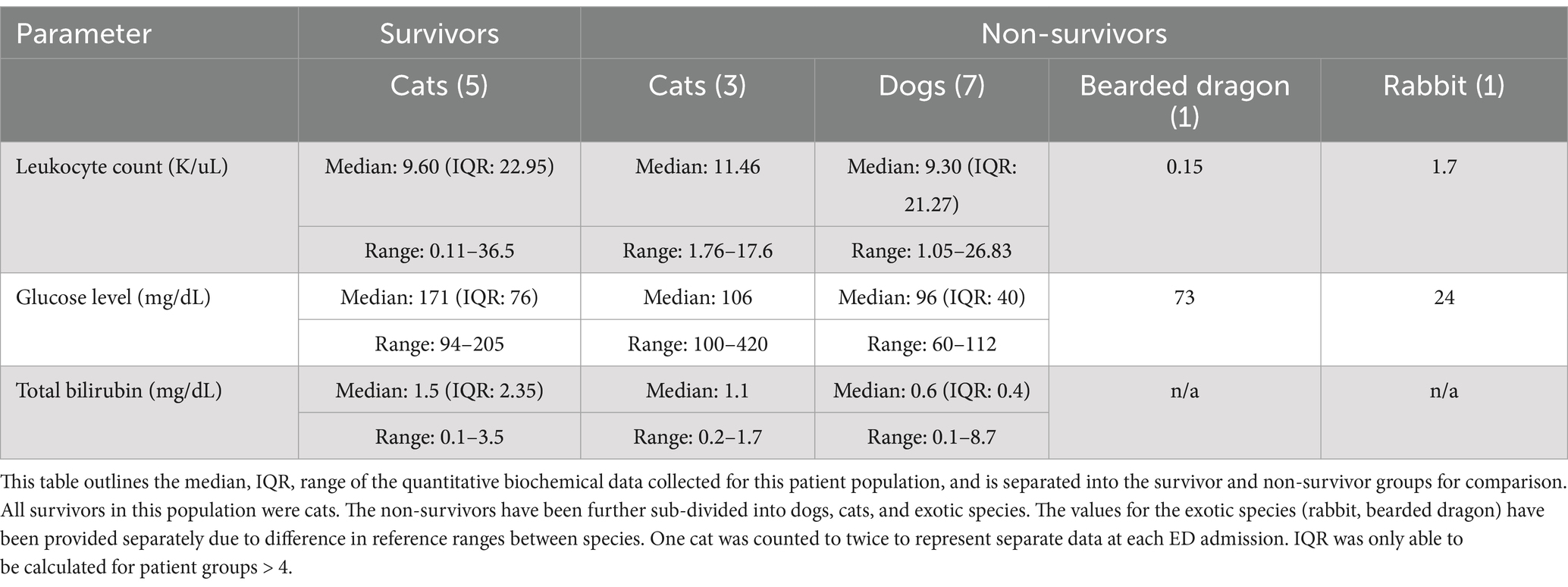
Statistical comparisons were performed using Fisher’s exact test to assess associations between categorical variables and the primary outcome being survival to discharge. Due to the small sample size, subgroup analysis by species was not statistically feasible. Descriptive statistics for each species are provided, and inferential statistics were conducted on the combined dataset. Continuous clinical variables (i.e., glucose level, WBC count, total bilirubin, and blood pressure) were converted into categorical variables based on established species-specific reference intervals and thresholds derived from veterinary literature (see Section 2.2). A p-value of <0.05 was considered statistically significant. Descriptive statistics, including medians, interquartile ranges (IQR), and full ranges, were reported for continuous variables in Table 2, with group stratification by survival status.
3 Results
Sixteen client-owned animals presenting to the ED from 2015 to 2024 were found to have bacteremia (see Supplementary Figure 1) noted on PBS at time of presentation or during hospitalization, and met the inclusion criteria for this study. The study documented high in-hospital mortality with 75% (12/16) of the animals not surviving to discharge. Euthanasia accounted for 58% (7/12) of cases that did not survive to discharge. Also of note, all surviving patients (4/16) were cats. All patients included in the study were admitted to the ED for a variety of clinical signs. Presenting complaints were often nonspecific and commonly included signs such as lethargy, vomiting, and hyporexia. None of the patients included had been previously determined to be septic, or had positive blood cultures prior to detection of bacteria on PBS (4, 14). Duration of hospitalization for non-survivors ranged from 1 to 11 days, while survivors were hospitalized for 1 to 5 days. One cat presented to the Emergency Department (ED) on two separate occasions and was found to be bacteremic on PBS at both visits. At the first presentation, the cat was treated as an outpatient and survived to discharge. However, the cat re-presented 24 h later with clinical decline, and repeated PBS again showed bacteremia. This patient was ultimately euthanized during the second hospitalization. For the purpose of clinical parameter comparison, both visits are included in the analysis (Table 1), once under “survivor” and once under “non-survivor,” to reflect the findings at each point of care. However, for primary outcome reporting (i.e., overall survival), this cat is counted only once, as a non-survivor.
The study population consisted of 7 dogs (43%), 7 cats (43%), one Holland Lop rabbit (6%), and one bearded dragon (6%). Dog breeds included German Shepherd (1), Great Pyrenees (1), Wire Fox Terrier (1), Miniature Pinscher (1), Miniature Poodle (1), Beagle (1), and an Italian Greyhound (1). Their ages ranged from 7 months to 19 years with a median age of 10.45 years. Among the cats, the Domestic Shorthair (4), Bengal (1), Siberian (1), and British Shorthair (1) were represented, with ages from 4 months to 15 years with a median age of 6.33 years. Of the patients, 71% (5/7) of the dogs and 57% (4/7) of the cats were male. Of the dogs included, 43% (3/7) were castrated males, 28.5% (2/7) were intact males, and 28.5% (2/7) were spayed females. Of the cats included, 28.5% (2/7) were castrated males, 28.5% (2/7) were intact males, and 43% (3/7) were spayed females.
Pathologist review of PBS described confirmed intracellular phagocytized bacteria within neutrophils, monocytes, or thrombocytes in 10 cases, suspected intracellular bacteria in 3 cases, and extracellular bacteria in 3 cases. Several patients exhibited both intra-and extracellular organisms. Morphological descriptions of bacteria varied (small rods, cocci, diplococci, coccobacilli), but no consistent differences were noted between survivors and non-survivors. In exotic species (rabbit and bearded dragon), heterophil rather than neutrophil toxic changes predominated.
Neither the presence of leukocytosis or leukopenia was associated with survival (p = 1). The presence of toxic change and band neutrophils was not significantly associated with survival (p = 0.19 and p = 0.54, respectively). Similarly, elevated bilirubin levels did not correlate significantly with survival outcomes (p = 1). No patients with hypotension survived (0 alive, 6 deceased), but hypotension was not associated with survival when compared with normotensive and hypertensive patients (p = 0.21). The use of antibiotics, both in the 6 months prior to presentation and during hospitalization, was not significantly associated with survival (p = 0.53 and p = 1, respectively). Of the 17 total ED presentations, 7 received antibiotics prior to PBS evaluation. Three of those had documentation of antibiotic administration within the 6 months preceding presentation. Antibiotics administered during hospitalization included ampicillin-sulbactam (15/17), enrofloxacin (10/17), metronidazole (3/17), ceftazidime (1/17), clindamycin (1/17), doxycycline (2/17), and piperacillin-tazobactam (2/17). Several patients received more than one agent. Antibiotics recorded in the 6 months prior to presentation included amoxicillin-clavulanate (3/3), chloramphenicol (1/3), enrofloxacin (1/3), doxycycline (1/3), and metronidazole (2/3). Due to the retrospective nature of this study, complete antibiotic history was not available for all patients.
Hyperglycemia was found to have a significant association with survival. Animals with high glucose levels had better survival outcomes compared to those with normoglycemia and hypoglycemia in this patient population (p = 0.0099). The results of these categorical comparisons, including leukocyte count, glucose, bilirubin, blood pressure, presence of toxic change, and antibiotic use, are summarized in Table 1. Descriptive statistics for leukocyte counts, glucose levels, and total bilirubin by survival group, and subdivided by species, are presented in Table 2.
Of the 16 patients, 6 had cultures submitted after PBS-detected bacteremia was noted. The patients with blood cultures submitted included 3 dogs and 3 cats. Three of the submitted cultures yielded bacterial growth with species and susceptibility profiles. Five of the six had received antibiotics prior to culture collection. Among the 3 patients with positive cultures, all of which were non-survivors, two met the study’s outlined sepsis criteria (Section 2.2). Two were dogs and one was a cat. All had leukocytosis (range: 17.6–26.83 K/μL; mean: 20.6 K/μL), band neutrophils, toxic change, and hyperbilirubinemia (range: 0.7–8.7 mg/dL; mean: 3.7 mg/dL). Glucose levels were within reference intervals (range: 99–106 mg/dL; mean: 102.6 mg/dL). In one dog, the blood culture was positive for Group G beta-hemolytic Streptococcus, suspected to be due to a septic joint. In a second dog, the blood culture was positive for Leclercia adecarboxylata, associated with a dialysis catheter. The cat had a blood culture that was positive for Streptococcus dysgalactiae subsp. Equisimilis, suspected to be due to urosepsis. In all three cases, the cultured organism matched the bacterial morphology noted on initial PBS review.
4 Discussion
Peripheral blood smears can be useful in detecting bacteremia in sick small animal patients and may provide diagnostic and prognostic information that can hasten the onset of treatment (15, 16). This study aimed to evaluate the significance of detecting bacteremia by PBS and its relationship with survival to discharge in a sick small animal population. No cases of bacteremia detected were found to be inconsequential in this patient population given that all were clinically ill at presentation. The findings suggest that bacteremia identified on PBS in clinically ill small animal patients is associated with a poor prognosis for survival.
All patients included in this study were clinically ill at time of presentation and PBS evaluation. However, asymptomatic animals were not intentionally excluded. All cases where bacteremia was identified on PBS during a retrospective review of electronic medical records were included. All animals identified had presented through the ED for various clinical signs. No instances of PBS-detected bacteremia were found for routine wellness visits or in other non-emergent settings. This supports the clinical impression that the presence of bacteremia found on PBS is a rare, but significant finding. However, it should be noted that the pathologists at the included institutions do not provide blood smear evaluations for all peripheral blood samples submitted to the laboratory. Instead, the pathologist review is triggered by a technologist alert, or a CBC abnormality. Because of this, it is possible that the results could be biased toward sick patients, decreasing the overall detected incidence of bacteremia identified on PBS in the population, and potentially overlooking bacteremia seen on blood smears of healthy pets.
Hyperglycemia was positively associated with survival. This finding is consistent with existing literature suggesting that hyperglycemia in septic or critically ill patients may not always be detrimental, and may reflect a metabolic response to stress (17, 18). In human and veterinary medicine, transient hyperglycemia has been observed in association with systemic inflammation, trauma, congestive heart failure, and sepsis, where it may play a protective role by supporting increased energy demands of immune cells (17, 19). Conversely, persistent or severe hyperglycemia has been associated with poorer outcomes in prolonged critical illness (19–21). The finding that hyperglycemia was associated with better outcomes in this cohort adds to the ongoing discussion about stress hyperglycemia in acute illness (17). All survivors in this study were cats, a species known to exhibit stress hyperglycemia due to adrenergic stimulation during acute illness or hospitalization (12, 22). It is possible that transient stress hyperglycemia in these patients served as a physiologic response to support increased energy demands, rather than indicating a primary derangement in glucose metabolism. In this context, hyperglycemia may be an adaptive response rather than a negative prognostic indicator.
Subgroup analysis was of low value given the small number of patients and thus was not reported. The lack of significant survival differences related to toxic change or band neutrophils is consistent with earlier research that suggests these indicators are not necessarily predictive of poor outcomes in small animals (23). However, one equine study did find the presence of band neutrophils and toxic change to be associated with decreased survival (24). To avoid overinterpretation of these findings, no single clinical or hematologic parameter should be relied upon to determine prognosis. Rather, a multifactorial approach is beneficial when evaluating animals with PBS-detected bacteremia, particularly given the small sample size and retrospective nature of this study.
Given the rarity of PBS-detected bacteremia, all eligible cases across species were included to capture the span of this clinical finding. Though bacteremia identified on PBS is a reported finding in reptile species, it remains rare in both domesticated bearded dragon and small mammal species (25). To date, literature related to bacteremia on PBS in rabbits has been limited to experimental models of sepsis and hematological studies (26). Case reports in domestic rabbits have noted bacteremia diagnosed by culture or associated histopathology, with minimal documentation of direct visualization on PBS. This highlights the novelty of PBS-detected bacteremia in rabbits. While no prognostic conclusions cannot be drawn from a single case, this finding may warrant further consideration in future studies if additional cases are identified.
In addition to being a poor prognostic indicator for survival, PBS-detected bacteremia may also serve as an early indicator of sepsis. However, interpreting bacteremia on PBS as an indicator of sepsis is complicated by the current lack of consensus on how sepsis is clinically identified in veterinary medicine (4). Additionally, due to the retrospective nature of this study, it was not possible to uniformly apply SIRS criteria, definitively identify sources of infection, or calculate APPLEfull or SOFA scores for all patients (4, 14). Given the retrospective design, small sample size, and limitations in sepsis classification, the primary objective of this study was to directly evaluate the association between PBS-detected bacteremia and survival to discharge. A future prospective study that more specifically explores the relationship between PBS-detected bacteremia and the incidence of sepsis would be a valuable contribution to the current body of literature.
This study had several limitations, primarily related to its small sample size, lack of a comparator group, and its retrospective design. While exploratory patterns were observed, the small sample size limits definitive conclusions regarding associations between specific clinical parameters and survival outcomes. Antibiotic selection was not standardized, which may limit the interpretation of its effect on survival. The study lacked a comparator group of patients without bacteremia on PBS, and as such, conclusions are limited to associations within this cohort. Furthermore, the variation in diagnostic and treatment approaches between patients could have influenced the outcomes. Another limitation of this study is the potential influence of PBS-detected bacteremia on euthanasia decisions. Though none of the cases were noted to be euthanized solely based on PBS findings from the records, it is possible that visualization of bacteria on a blood smear could have contributed to clinician perception of a poor prognosis. Because of this, we cannot definitively determine the extent to which PBS findings may have biased outcomes as retrospective records did not uniformly document rationale for euthanasia. Future studies would need a larger, multicenter cohort to strengthen the statistical power of these findings. As we get closer to defining the parameters involved in the diagnosis of sepsis, standardization of diagnostic and treatment protocols could provide clearer guidance for clinicians managing patients with bacteremia.
In conclusion, bacteremia identified on PBS in clinically ill small animal patients is a rare, but meaningful diagnostic finding that is associated with poor prognosis for survival to discharge. The presence of PBS-detected bacteremia supports the importance of timely diagnostics and consideration of early intervention, though prospective studies are needed to determine its impact on treatment decisions (9, 25). Though not all hospitals have an in-house clinical pathology laboratory, PBS can still function as a useful bedside tool for emergency clinicians who are awaiting the results of more definitive testing such as blood cultures and have a high clinical suspicion for infection. Incorporating PBS evaluation into emergency settings may facilitate earlier recognition of serious illness and support timely therapeutic interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because only retrospective analysis of existing pathology and biochemical data was collected as part of clinician-driven care provided to patients at the institution hospitals. No new interaction with animals was included. Written informed consent was not obtained from the owners for the participation of their animals in this study because only anonymized data was utilized. No client or patient identifying information is presented.
Author contributions
SF: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. MW: Conceptualization, Supervision, Writing – review & editing. PE: Data curation, Writing – review & editing. VS-S: Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors wish to thank Ian DeStefano for his efforts in data curation at the Cummings School of Veterinary Medicine. Thank you to the reviewers for their valuable feedback on the manuscript. Graphpad Software, Inc. was used for statistical analysis calculations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1550732/full#supplementary-material
Abbreviations
PBS, Peripheral blood smear; ED, Emergency Department.
References
1. Fife, A, Hill, D, Barton, C, and Burden, P. Gram negative septicaemia diagnosed on peripheral blood smear appearances. J Clin Pathol. (1994) 47:82–4. doi: 10.1136/jcp.47.1.82
2. Van der Meer, W, Theunisse, LA, and Vreeken, J. Bacteria in blood smears: overwhelming sepsis or trivial contamination? Acta Haematol. (2002) 107:220–3. doi: 10.1159/000058318
3. Smith, DA, and Nehring, SM. Bacteremia In. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023). Available from: https://www.ncbi.nlm.nih.gov/books/NBK441979/
4. Cortellini, S, Bateman, S, Chan, DL, Goggs, R, Hopper, K, Menard, JM, et al. Defining sepsis in small animals. J Vet Emerg Crit Care. (2024) 34:97–109. doi: 10.1111/vec.13359
5. Ristuccia, PA, Hoeffner, RA, Digamon-Beltran, M, and Cunha, BA. Detection of bacteremia by buffy coat smears. Scand J Infect Dis. (1987) 19:215–7. doi: 10.3109/00365548709032401
6. Brooks, GF, Pribble, AH, and Beaty, HN. Early diagnosis of bacteremia by buffy-coat examinations. Arch Intern Med. (1973) 132:673–5. doi: 10.1001/archinte.1973.03650110029006
7. Wardrop, KJ, Reine, NJ, Birkenheuer, AJ, Callan, MB, Kohn, B, Lappin, MR, et al. Update on canine and feline blood donor screening for blood-borne pathogens. J Vet Intern Med. (2016) 30:15–35. doi: 10.1111/jvim.13823
8. Long, B, and Koyfman, A. Best clinical practice: blood culture utility in the emergency department. J Emerg Med. (2016) 51:529–39. doi: 10.1016/j.jemermed.2016.07.003
9. Lehmann, LS, and Spivak, JL. Rapid and definitive diagnosis of infectious diseases using peripheral blood smears. J Intensive Care Med. (1992) 7:36–47. doi: 10.1177/088506669200700105
10. Li, T, Zhang, L, Liu, Y, Wang, E, Xi, Y, and Zhang, Y. Critical role of the peripheral blood smear for early diagnosis of bacteremia and/or fungemia: a case-based approach. Int J Lab Hematol. (2024) 46:160–4. doi: 10.1111/ijlh.14154
11. Morgan, RK, Cortes, Y, and Murphy, L. Pathophysiology and aetiology of hypoglycaemic crises. J Small Anim Pract. (2018) 59:659–69. doi: 10.1111/jsap.12911.30251421
12. Silverstein, DC, and Hopper, K. Small Animal Critical Care Medicine. 3rd ed. St. Louis (MO): Elsevier (2022).
13. Carpenter, JW, and Harms, CA. Carpenter’s exotic animal formulary. 6th ed. St. Louis (MO): Elsevier (2023).
14. Bateman, S. SIRS, sepsis, and MODS In: DC Silverstein and K Hopper, editors. Small Animal Critical Care Medicine. 3rd ed. St. Louis (MO): Elsevier (2022). 32–9.
15. Ekeng, B, Emanghe, U, Davies, A, and Oladele, R. A critical review of diagnostic methods for disseminated histoplasmosis with special focus on resource-limited settings. Curr Fungal Infect Rep. (2023) 17:1–9. doi: 10.1007/s12281-023-00454-4
16. Reik, H, and Rubin, SJ. Evaluation of the buffy-coat smear for rapid detection of bacteremia. JAMA. (1981) 245:357–9. doi: 10.1001/jama.1981.03310290025016.7451453
17. Knieriem, M, Otto, CM, and Macintire, D. Hyperglycemia in critically ill patients. Compend Contin Educ Vet. (2007) 29:360–72.
18. Ray, CC, Callahan-Clark, J, Beckel, NF, and Walters, PC. The prevalence and significance of hyperglycemia in hospitalized cats. J Vet Emerg Crit Care (San Antonio). (2009) 19:347–51. doi: 10.1111/j.1476-4431.2009.00435.x
19. van Vught, LA, Wiewel, MA, Klein Klouwenberg, PM, Hoogendijk, AJ, Scicluna, BP, Ong, DS, et al. Admission hyperglycemia in critically ill sepsis patients: association with outcome and host response. Crit Care Med. (2016) 44:1338–46. doi: 10.1097/CCM.0000000000001658.26992065
20. Brady, CA, Hughes, D, and Drobatz, KJ. Association of hyponatremia and hyperglycemia with outcome in dogs with congestive heart failure. J Vet Emerg Crit Care (San Antonio). (2004) 14:177–82. doi: 10.1111/j.1534-6935.2004.00118.x
21. Hardie, EM, Rawlings, CA, and George, JW. Plasma-glucose concentrations in dogs and cats before and after surgery: comparison of healthy animals and animals with sepsis. Am J Vet Res. (1985) 46:1700–4.4030475. doi: 10.2460/ajvr.1985.46.08.1700
22. Nelson, RW, and Reusch, CE. Animal models of disease: classification and etiology of diabetes in dogs and cats. J Endocrinol. (2014) 222:T1–9. doi: 10.1530/JOE-14-0202.24982466
23. Mare, TA, Treacher, DF, Shankar-Hari, M, Beale, R, Lewis, SM, Chambers, DJ, et al. Diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in systemic inflammation. Crit Care. (2015) 19:57. doi: 10.1186/s13054-015-0778-z
24. Lambert, JL, Fernandez, NJ, and Roy, MF. Association of band cells and toxic neutrophils with systemic inflammatory response syndrome and outcome in horses. J Vet Intern Med. (2016) 30:1284–92. doi: 10.1111/jvim.13968
25. Capobianco, CM, Bosch, SN, Stacy, NI, and Wellehan, JF. Lactococcus garvieae–associated septicemia in a central bearded dragon. J Vet Diagn Invest. (2024) 36:477–80. doi: 10.1177/10406387241239912
Keywords: bacteremia, peripheral blood smear, hyperglycemia, pathologist, emergency, sepsis
Citation: Ford SS, Whelan M, Ewing P and Sinnott-Stutzman VB (2025) Bacteremia detected on peripheral blood smear in small animal patients presenting to the Emergency Department and its association with prognosis to discharge. Front. Vet. Sci. 12:1550732. doi: 10.3389/fvets.2025.1550732
Edited by:
Gabriele Rossi, Murdoch University, AustraliaReviewed by:
Corrin John Boyd, Murdoch University, AustraliaElizabeth Anne Rozanski, Tufts University, United States
Bridget M. Lyons, Cornell University, United States
Copyright © 2025 Ford, Whelan, Ewing and Sinnott-Stutzman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Summer Scout Ford, c2ZvcmRAbXNwY2Eub3Jn
 Summer Scout Ford
Summer Scout Ford Megan Whelan
Megan Whelan Patty Ewing2
Patty Ewing2