- 1Sarasota Dolphin Research Program, Brookfield Zoo Chicago ℅ Mote Marine Laboratory, Sarasota, FL, United States
- 2Brookfield Zoo Chicago, Animal Health, Welfare and Science, Brookfield, IL, United States
- 3Department of Veterinary Clinical Medicine, College of Veterinary Medicine, University of Illinois, Urbana, IL, United States
- 4National Marine Mammal Foundation, San Diego, CA, United States
- 5New College of Florida, Sarasota, FL, United States
- 6Dolphin Quest, San Diego, CA, United States
- 7National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Office of Protected Resources, Silver Spring, MD, United States
- 8Department of Biological Sciences, Institute of Environment, Florida International University, North Miami, FL, United States
- 9Department of Clinical Sciences, Ohio State University College of Veterinary Medicine, Columbus, OH, United States
- 10Department of Comparative, Diagnostic and Population Medicine, College of Veterinary Medicine, University of Florida, Gainesville, FL, United States
A juvenile male common bottlenose dolphin (Tursiops truncatus) was examined as part of a long-term dolphin research and monitoring program in Sarasota Bay, Florida. Scars consistent with a shark bite, identified as a possible tiger shark (Galeocerdo cuvier), were observed at the proximal ventral peduncle bilaterally, involving the area at the distal genital slit. A left ventrolateral urinary tract-body wall fistula was identified at the cranial margin of the healed shark bite scar. The area was closely associated with palpable scar tissue at the base of the penis within the genital slit. Physical and ultrasonographic examination and attempts at urinary catheter placement supported findings of a urethral stricture with a urethral or vesicocutaneous fistula. Hematuria was detected on urinalysis, and mild hydronephrosis and lymphadenopathy were observed via ultrasonography. Despite experiencing substantial soft tissue trauma from the shark bite and subsequently developing a urinary tract obstruction with fistula formation, this animal has maintained good body condition since the health exam. Due to the location of the urethral obstruction and fistula, this animal may not be able to reproduce if it survives to breeding age. Long-term prognosis will likely be determined by the sequelae of potential progressive hydronephrosis. This case report documents a rare medical condition as a result of a shark bite not previously described in a free-ranging bottlenose dolphin, including unique historical and ensuing behavioral/health data, which is rarely possible when monitoring free-ranging wildlife.
Introduction
Common bottlenose dolphins (Tursiops truncatus) are widely distributed in estuarine and coastal marine ecosystems and are valuable sentinels to assess marine ecosystem health (1). Some populations have been closely monitored over multiple decades, the results of which have greatly expanded our knowledge of dolphin health, biology, and population structure and have identified environmental and anthropogenic stressors affecting their survival. The long-term resident dolphins of Sarasota Bay, Florida, United States, have been studied by the Sarasota Dolphin Research Program (SDRP) since 1970 (2). The individually identifiable residents are monitored through monthly systematic photo-identification surveys throughout the community range that extends from southern Tampa Bay to Venice, Florida (2). The community consists of approximately 170 individuals, studied over at least six generations, spanning as many as five concurrent generations within a given maternal lineage, and includes individuals up to 67 years of age (3). Periodic catch-and-release health assessments (1) supplement photo-identification survey data with background life history, morphometric, and health information (4). During a 2023 health assessment, a juvenile male bottlenose dolphin was examined and found to have a large, healed shark bite over the proximal ventral peduncle and genital slit associated with a urinary tract-body wall fistula.
Urinary tract fistulas can be congenital or acquired due to underlying pelvic disease or trauma. Lower urinary tract fistulas can occur as uroenteric, urogenital, and urocutaneous fistulas, including vesicocutaneous (VCF, bladder to skin) (5) and urethrocutaneous (urethra to skin) forms. These fistulas develop when the ureter, bladder, or urethra forms a connection or tract with an adjacent structure, allowing urine leakage from the urinary system. Congenital forms (urogenital > urorectal fistulas) (6–9) have been uncommonly reported in dogs (10–12), cats (13), cows (14), sheep (15), and horses (16). Acquired forms generally involve any inflammatory process that compromises the lower urinary tract. The most common causes include urinary calculi, infection, neoplasia, trauma, iatrogenic, and radiation therapy (5). Clinical signs are characterized by the type and location of the fistula, typically including urine leakage through single or multiple discharge sites outside the body through the skin, reproductive, or lower gastrointestinal tract. Genitourinary symptoms may include local or abdominal pain, changes in urinary frequency, dermatitis at the leakage site, recurrent cystitis, and can lead to life-threatening infections, including sepsis. Visualization of the exact location and path of a urinary system fistula often requires advanced imaging for confirmation. While nephrolithiasis (17, 18), ureteral obstruction (19), prostatitis (20), patent urachus (21), and cystitis (22, 23) have been reported in dolphins, to the authors’ knowledge, no previous reports of urinary fistulas have been reported in this taxon.
Case description
A 126.6 kg, approximately 4.4-year-old (radiographically determined age (24); see Supplementary material 1), male bottlenose dolphin (222 cm long from tip of the upper jaw to the fluke notch; maximum girth of 123 cm), from Sarasota Bay, Florida (27.29282, −82.54878), was evaluated during population health assessments on 12 May 2023. Photographic identification of the animal’s dorsal fin through systematic monthly surveys identified that this animal entered Sarasota Bay approximately 6 months prior to the health assessment (first sighting on 14 November 2022). It is unknown where the dolphin originated, as resident communities of bottlenose dolphins are found along the western coast of Florida—such as Charlotte Harbor to the south (25) and Tampa Bay to the north of Sarasota (26)—and in near-and-offshore waters in the Gulf of Mexico (48). Photo documentation from the initial sighting confirmed the presence of a large, healed scar consistent with a shark bite on the peduncle (Figure 1A). Prior to the examination, the animal had been observed across 10 survey days from 14 November 2022 to 09 May 2023, primarily in the southern portion of the Sarasota study area (Figure 2). Observations from survey vessels documented the animal in groups of 1–13 dolphins (average = 5) and engaging in normal feeding and social behavior.
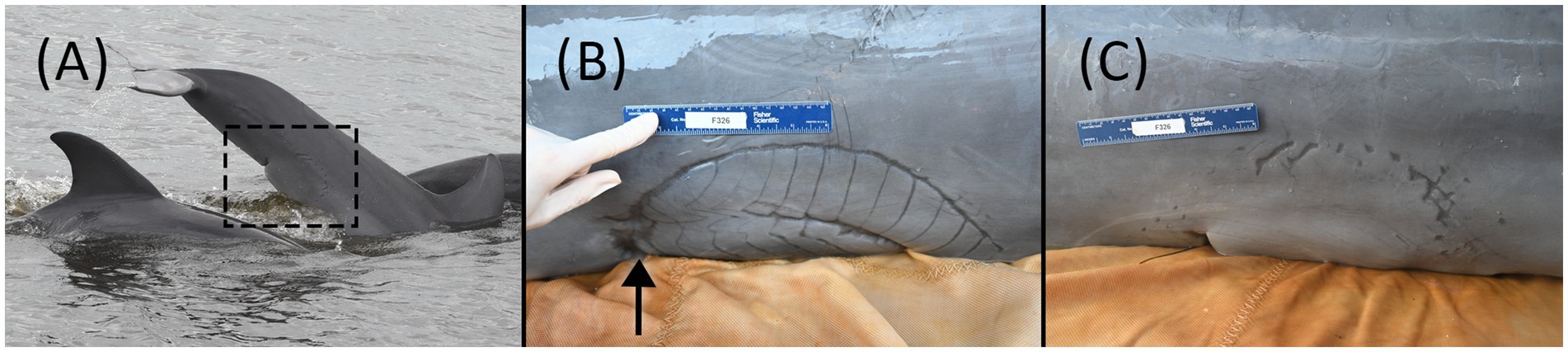
Figure 1. (A) First boat-based observation of F326 “Squirt” on 14 November 2022. The shark bite was present and healed at the time of the first sighting. (B) Shark bite wound over the left-side proximal ventral peduncle and genital slit during the health assessment examination on 12 May 2023. Urine was intermittently projected from a 1 mm pinpoint circular defect in the skin (arrow shows approximate location), consistent with a fistula, at the cranial portion of the shark bite scar. (C) Shark bite wound on the right-side proximal ventral peduncle and genital slit during the health assessment examination on 12 May 2023.
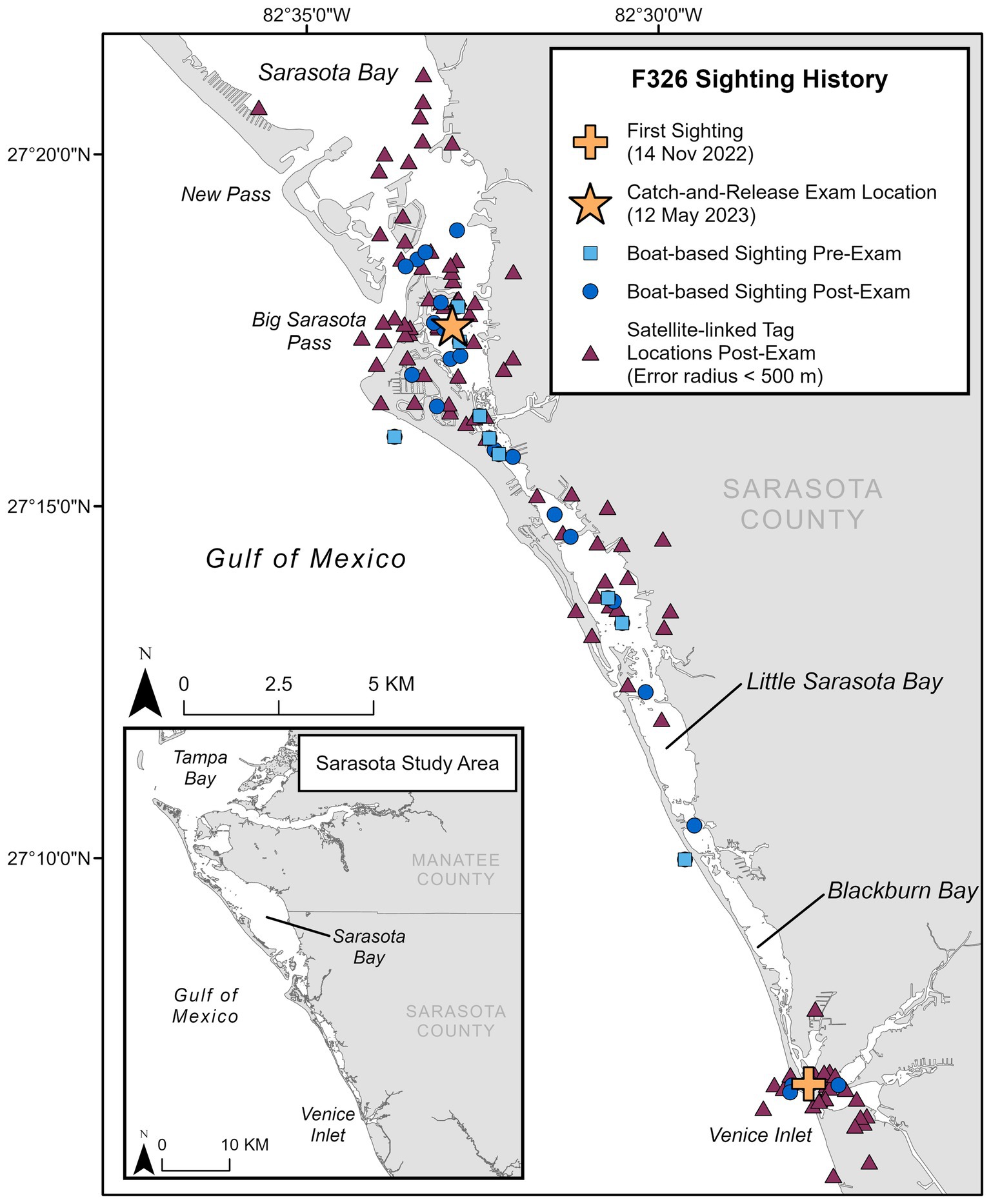
Figure 2. Sighting history for F326 “Squirt,” including first sighting location in the study area, location of catch-and-release health exam, locations during monthly photo-identification boat-based surveys pre- and post-examination (through November 2024), and satellite-linked tag locations post-examination.
The methodology described in Wells et al. (1) was used to perform a hands-on physical examination and diagnostic sampling (NOAA/NMFS Scientific Research Permit #26622). The animal was calm and amenable to handling initially in the water and subsequently on a soft, padded, and shaded mat on the veterinary examination vessel. On physical examination, the dolphin was bright, alert, and responsive, with no abnormal findings in heart rate, respiratory rate, or mucous membranes. The most significant finding was a semicircular healed scar (35 × 11 cm) on the ventral and lateral proximal peduncle, consistent with a shark bite (Figures 1B,C). Individual longitudinal scars (1.5–2.5 cm apart) were demarcated on the left side of the peduncle, with two clear semicircular scars, one inside the other. On the right side, the scar was less defined, having one semicircular scar of similar size. The cranial margin of the bite wound scar was the widest on the left cranial aspect and coursed across the distal genital slit (base of the penis) bilaterally. The caudal margin of the smaller semicircle on the left was associated with a 2 × 2 cm defect in the ventral peduncle. At the cranial portion of the semicircular scar on the left ventral body wall, there was a 1 mm pinpoint circular defect in the skin and underlying soft tissue from which yellow fluid (urine) projected intermittently in a stream from the body, consistent with a urethral or vesicocutaneous fistula based on its location (see Supplementary video 1). Off-white caseous debris was present within the genital slit, covering the distal 4 cm of the tip of the penis. On palpation within the caudal genital slit, firm scar tissue was present at the base of the penis. Attempts to pass a red rubber catheter (5 Fr Kendall, Covidien, Mansfield, MA and 8 Fr Kendall, Cardinal Health, Waukegan, IL) for routine sterile urine collection were unsuccessful, as the catheter could only be advanced 12 cm into the distal urethra, suggesting a distal urethral stricture or obstruction. Urine flow was not observed from the genital slit, penis/urethra, at any time during the examination.
A full-body in-water ultrasound was performed in sternal recumbency. Ultrasound of the urinary tract revealed bilaterally symmetrical kidneys considered to be normal in size for this species and aged animal (maximum diameter: L = 6.7 cm; R = 6.5 cm). There were focal areas of mild hydronephrosis within individual renicules that were distended with anechoic fluid (< 30 renicules affected per kidney; Figures 3B,C). The collecting ducts were mildly distended bilaterally (maximum diameter: L = 3.1 mm; R = 3.6 mm). No nephroliths were identified. Along the caudodorsal wall of the bladder, there was a hyperechoic structure bulging into the dorsal trigone of the bladder wall, creating a mass effect (Figures 3D,E). This mass-like structure (2.8 cm wide × 5.8 cm long) appeared to extend dorsolaterally through the mid-penile shaft and extended to the blubber skin layer of the lateral body wall. The forestomach was moderately distended with a mixture of whole fish and fluid of mixed echogenicity, indicating that the animal had recently foraged. The remainder of the organs examined were unremarkable.
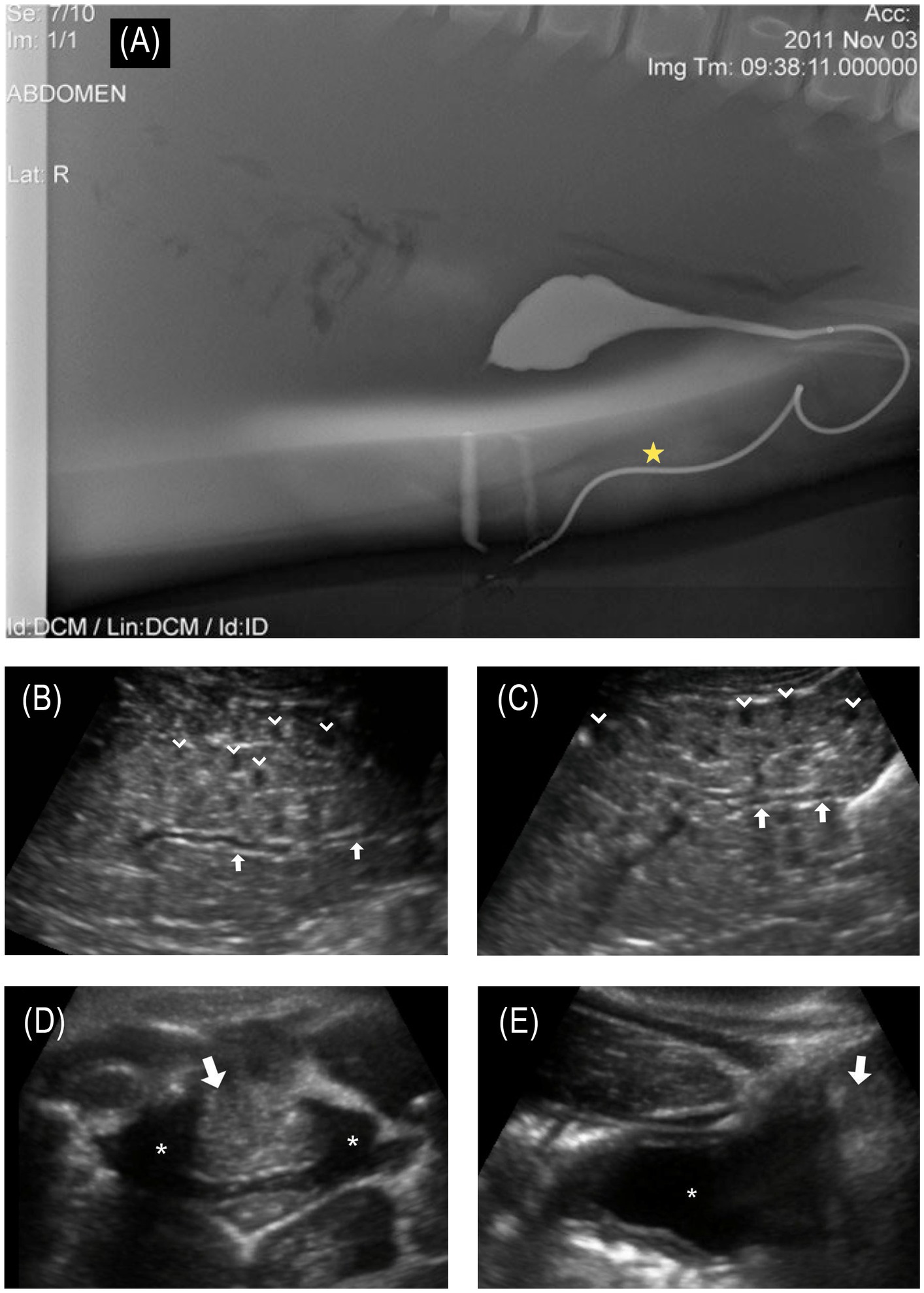
Figure 3. (A) © Sur et al. (17); Published by Mary Ann Liebert, Inc. Normal male bottlenose dolphin retrograde cystourethrogram demonstrates normal bladder and urethral anatomy; of note, the urethra has a marked curvature at the base of the sigmoid flexure. The star denotes the approximate location of the fistula that was observed in the affected animal. (B) Longitudinal ultrasound of the left kidney and (C) right kidney. Collecting ducts are slightly distended (arrows in images B,C). Multifocal reniculi with mild hydronephrosis are visible (chevrons); (D) transverse and (E) longitudinal ultrasound images centered on the urinary bladder (asterisk in images D,E). Along the caudodorsal wall of the bladder, there was a hyperechoic structure (arrows in images D,E), bulging into the trigone of the bladder wall, creating a mass effect. This mass-like structure (2.8 cm wide × 5.8 cm length) appeared to extend dorsolaterally through the mid-penile shaft and extended to the blubber skin layer of the lateral body wall.
Dorsoventral and lateral radiographs of the thorax, vertebrae, pectoral flipper, and peduncle/pelvic region were acquired on board the examination vessel. Radiographs of the thorax and skeleton were unremarkable. Irregular undulation margins of the ventral cutaneous body wall margin consistent with the bite wound/scar tissue were observed.
Point of care venous blood gas showed the animal to have mild respiratory and metabolic acidosis with appropriate systemic compensation and increased glucose (Supplementary Table 1) over the course of the examination, consistent with a stress response. Complete blood count, serum biochemical analysis, protein electrophoresis (EPH), iron panel, thyroid hormones, and cortisol were within reported reference ranges with no indication of renal compromise (Supplementary Table 1) (27–30). Testosterone was consistent with prepuberty (Supplementary Table 1) (31). The urinalysis was unremarkable except for having a moderate amount of blood on the dipstick and a large amount of amorphous debris on cytological examination (32). Urine cultures were not submitted. The cytology of blowhole, gastric, and fecal samples was unremarkable.
Prior to release, the dolphin received a freeze brand (326) for long-term identification and a Wildlife Computers SPLASH10 satellite-linked finmount tag for tracking his movements. The observation of urine intermittently squirting from the side of the body led to this dolphin being referred to as “Squirt.”
Discussion
We considered the injury to be shark-inflicted based on the crescent shape of the wound and the presence of widely spaced teeth impressions. The identification of predator species involved in non-lethal lesions on dolphins is challenging. However, the size (particularly the width) and shape of the wound can be used to identify the predator species involved (33–35). Bull sharks (Carcharhinus leucas) and tiger sharks (Galeocerdo cuvier) are common predators of the Sarasota dolphin community (36–38) and in the nearshore Gulf of Mexico coastal areas; though as previously mentioned, this bite occurred prior to the animal moving into the Sarasota study area, and it is unknown from where the animal originated. Dusky sharks (Carcharhinus obscurus) are also found in the Gulf of Mexico, as are occasional white sharks (Carcharodon carcharias), both of which are also considered dolphin predators (33, 36–39). Carcharhinid shark bites (e.g., bull and dusky sharks) are crescent-shaped and clean-cut, while white sharks are wider and parabolic (33, 34). Tiger shark bites are broad in shape and tend to leave widely spaced rake marks (34), consistent with what was observed on this animal. The longitudinal scars were measured 1.5–2.5 cm apart, larger than the interdental distance of bull or dusky sharks but within the range of tiger sharks (35). Though scar growth over time cannot be ruled out, the wound’s shape, widely spaced rake marks, and three independent expert opinions indicate the predator was most likely a tiger shark.
Free-ranging cetaceans can survive tremendous trauma and physical challenges, both natural (e.g., shark bites, harmful algal blooms, hurricanes) and anthropogenic (e.g., entanglement, boat strikes, and oil spills); however, these threats can also result in substantial morbidity and mortality. Long-term monitoring, research, and health assessments of wild dolphin populations, such as the community in Sarasota Bay, have provided critical global reference data essential for evaluating at-risk populations and investigating causes of mortality [(e.g., 40–43)]. While most common bottlenose dolphin populations are not currently considered threatened, and the vast majority of dolphins in Sarasota Bay are in good health, the loss of even a single animal may have significant effects on a population at the local level (44). The animal described in this case study survived the initial shark interaction; however, the resulting wounds and subsequent scarring have most likely prevented any possibility of this animal successfully reproducing if/when it reaches sexual maturity. Monthly photographic surveys have proven to be a valuable tool for documenting population dynamics, serving to identify new individuals or the disappearance of well-known ones within this population. These efforts identified this animal as a new addition to the local community and documented its survival from a sizable shark bite. Following the health assessment, the animal was observed on more than 20 boat-based photographic surveys, in group sizes ranging from 1 to 11 dolphins (average 4.38) and engaging in typical feeding and social behavior (at the time of this communication, November 2024, Figure 2). Although the satellite-linked tag was shed after approximately 3.5 months (as designed), as of this writing, the dolphin spends a great deal of time in inshore waters and canals where it is reported by the public, often with photo documentation from shore. Post-health assessment sightings, public reports, and satellite-linked locations confirm that the animal has stayed in the southern part of the Sarasota study area, which is consistent with his sighting history prior to catch-and-release sampling (Figure 2). The dolphin is readily identifiable by its freeze-brand and will continue to be monitored during monthly SDRP photo-identification surveys.
Thorough hands-on health evaluations of individuals in a population can detect findings that are not apparent from observations alone (such as disease prevalence, immune dysfunction, contaminant levels, and individual health), as demonstrated by the identification of a urinary fistula in this animal. While it is not possible to determine exactly when the injury occurred, it is likely that it happened several months prior to the initial sighting, based on the wound being fully healed. Had this animal not developed a fistula to allow urine passage, it likely would have succumbed to the sequelae of urinary obstruction (17–19, 45) despite recovering from the wounds associated with a shark bite. Examination findings of a urethral stricture/obstruction, along with a small-diameter urethral or vesicocutaneous fistula, would prevent the animal from fully emptying its bladder of urine, likely contributing to the mild hydronephrosis and dilated collecting ducts identified on ultrasonographic examination. Hematuria may be due to chronic trauma to the bladder, urethra, or leakage from the tissue making up the fistulous tract. In comparing the location of the fistula to cross-sectional anatomy from contrast-enhanced computed tomography (CT) and previously published cystourethrogram (Figure 3A), the location of the fistula is most likely associated with a urethral fistula but a vesicocutaneous tract cannot be ruled out.
While not possible in this case, imaging contrast studies, such as CT and magnetic resonance imaging (MRI), are the most sensitive diagnostic techniques for identifying fistulas and associated abnormalities. Additionally, a fistulogram, urethroscopy, cystoscopy, and ureteroscopy can also be helpful in diagnosing a fistula in some cases. Despite the unavailability of these advanced diagnostic modalities in this case, physical examination, diagnostic testing, and ultrasonographic imaging allowed for the diagnosis of this animal’s urethral/vesicocutaneous fistula. Abdominal and pelvic ultrasound is one of the most widely used modalities for diagnosing urinary tract disease, but it can be more difficult to identify fistulous tracks with this modality. In this case, the ultrasound detected abnormalities closely associated with the bladder and base of the penis (Figures 3D,E) at the level of the scars from the shark bite; however, the fistula itself would not have been identified without an out-of-water examination, demonstrating the value of a thorough physical evaluation as part of the complete health assessment process. The location of the fistula, the yellow color of the fluid, the expected urinalysis results, and the shrinking size of the bladder after fluid excretion from the fistula confirmed the fluid to be urine and the findings to be consistent with the animal having a urethral or vesicocutaneous fistula.
Hematological and serum biochemical parameters, as well as most urinalysis findings, were within reference intervals for bottlenose dolphins from this geographical site, supporting that the animal did not have a systemic infection or localized cystitis at the time of the examination (29). These findings support that there is unlikely to have been any ongoing ascending leakage of saltwater retrograde up the fistula. Total protein, albumin, sodium, PCV, and BUN values (Supplementary Table 1) were at the upper threshold of the range for juveniles (29), suggesting that this animal was mildly dehydrated. Additionally, there was evidence of recent consumption of a high-protein meal, which may have also contributed to these findings. Alkaline phosphatase was also within the upper threshold of values (29, 46), supporting a lack of acute illness (46, 47) and may be associated with growth.
Treatment was not possible for this individual or generally for free-ranging dolphins. Treatment and rehabilitation for ill, injured, or stranded dolphins are rare and determined through collaborative local, regional, and federal response agencies, their capabilities, and species management plans. For people, domestic species, and dolphins in managed care, CT, including a contrast urethrogram, urethroscopy, reestablishing urethral patency, and repair of the fistula, would be recommended or considered.
The prognosis for this dolphin with urethral obstruction, urethral/vesicocutaneous fistula, and early signs of post-renal urinary obstruction (mild hydronephrosis, dilated collecting ducts) is unknown. The animal survived initial recovery from a substantial shark bite from which trauma and scar tissue resulted in the findings described in this report. While this juvenile has maintained good body condition and did not have findings consistent with ascending infection or azotemia (elevated renal values), it is likely that continued chronic increased pressure in the bladder, due to the inability to urinate normally, will result in continued increased retrograde pressure through the ureters to the kidneys that will likely cause progressive renal compromise over time. As long as the animal has a fistula, the risk of ascending infection will remain increased. Additionally, reproductive fluids, including semen, will also not be able to pass, prohibiting this animal from successful reproduction.
This case highlights how attempted predation events can have enduring impacts on a local dolphin community, even when the dolphin survives the predation attempt. It also illustrates the sometimes-exceptional abilities of small cetaceans to recover from major traumatic injuries while underscoring the importance of long-term monitoring in conservation studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Mote Marine Laboratory’s Institutional Animal Care and Use Committee (IACUC). The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the participants for the publication of this case report.
Author contributions
KW: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing, Project administration. JM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Project administration, Resources. CT: Writing – review & editing, Data curation, Investigation, Project administration, Supervision. RF: Data curation, Investigation, Project administration, Supervision, Writing – review & editing. JS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. DF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JK: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. EH: Formal analysis, Resources, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. MW: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. RW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Health assessment support was obtained from Dolphin Quest, Inc., Florida Atlantic University/Harbor Branch Oceanographic Institute, Fundación Oceanogràfic, University of Southern Denmark, NOAA John H. Prescott Grants Program, College of Charleston, Clearwater Marine Aquarium, and the National Marine Mammal Foundation. Monthly photoidentification survey support was obtained from the Charles and Margery Barancik Foundation. The authors declare that this study received funding from Dolphin Quest, Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We would like to thank the staff, volunteers, students, and collaborators of the Sarasota Dolphin Research Program for helping to ensure safe and successful health assessment operations, and especially the Florida Fish and Wildlife Conservation Commission for providing our dolphin catcher, catch boat, and experienced staff. We thank Kim Bassos-Hull for photo and video coverage during the examination and Jonathan Crossman for his assistance with figure preparation. A special thanks to Demian Chapman and Jack Morris for providing their expert opinion as to which shark species could be responsible for the observed scar. This study was conducted under NOAA/NMFS Scientific Research Permit #26622 and according to protocols approved by Mote Marine Laboratory’s Institutional Animal Care and Use Committee (IACUC).
Conflict of interest
JS is an owner of Dolphin Quest, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1551129/full#supplementary-material
References
1. Wells, RS, Rhinehart, HL, Hansen, LJ, Sweeney, JC, Townsend, FI, Stone, R, et al. Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. EcoHealth. (2004) 1:246–54. doi: 10.1007/s10393-004-0094-6
2. Wells, RS. Learning from nature: bottlenose dolphin care and husbandry. Zoo Biol. (2009) 28:635–51. doi: 10.1002/zoo.20252
3. Wells, RS. The Sarasota dolphin research program in 2020: celebrating 50 years of research, conservation, and education. Aquat Mamm. (2020) 46:502–3. doi: 10.1578/AM.46.5.2020.502
4. Wells, RS. Social structure and life history of bottlenose dolphins near Sarasota Bay, Florida: insights from four decades and five generations In: J Yamagiwa and L Karczmarski, editors. Primates and cetaceans. Primatology monographs. Tokyo, Japan: Springer (2014). 149–72.
5. Vázquez Gálvez, A, Blas Reina, A, García Martínez, FE, Figueroa Sánchez, TO, and Arriaga, J. Vesicocutaneous Fistula In: R Sotelo, CF Polotti, and J Arriaga, editors. Urinary fistula. Cham, Switzerland: Springer International Publishing (2022). 71–81.
6. Suess, RP, Martin, RA, Moon, ML, and Dallman, MJ. Rectovaginal fistula with atresia ani in three kittens. Cornell Vet. (1992) 82:141–53.
7. Rahal, SC, Vicente, CS, Mortari, AC, Mamprim, MJ, and Caporalli, EHG. Rectovaginal fistula with anal atresia in 5 dogs. Can Vet J. (2007) 48:827–30. doi: 10.17221/8769-VETMED
8. Embertson, RM. Selected urogenital surgery concerns and complications. Vet Clin North Am Equine Pract. (2008) 24:643–61. doi: 10.1016/j.cveq.2008.10.007
9. Gangwar, AK. Congenital anomalies and their surgical correction in ruminants. Adv Anim Vet Sci. (2014) 2:369–76. doi: 10.14737/journal.aavs/2014/2.7.369.376
10. Goulden, B, Bergman, MM, and Wyburn, RS. Canine urethrorectal fistulae. J Small Anim Pract. (1973) 14:143–50. doi: 10.1111/j.1748-5827.1973.tb06909.x
11. Chandler, JC, and MacPhail, CM. Congenital urethrorectal fistulas. Compend Contin Educ Pract Vet. (2001) 23:995–1002.
12. Thompson, JL, Liuti, T, Albuquerque, C, and Murgia, D. Congenital Urethrovaginal fistula with blind-ending vagina in a female Pseudohermaphrodite dog with urinary incontinence. J Am Anim Hosp Assoc. (2021) 57:237–41. doi: 10.5326/JAAHA-MS-7114
13. John, MK, Cari, AO, Jody, PL, and Rodney, EO. Inherited and congenital diseases of the feline lower urinary tract. Vet Clin Small Anim Pract. (1996) 26:265–79. doi: 10.1016/S0195-5616(96)50207-X
14. Özak, A, Hö, N, Yardimci, C, and Kabak, YB. Anorectal malformation with Colovesical and Colourethral fistula in two calves. Kafkas Univ Vet Fak Derg. (2015) 21:287–9. doi: 10.9775/kvfd.2014.12166
15. Vahar, M, Hosseni, SM, Omidzahir, S, Kenari, EO, Iraee, MA, and Ziabari, AH. Report of congenital colonobladder fistula with atresia ani in a lamb and treatment by surgery. Asian Pac J Trop Dis. (2015) 5:S181–3. doi: 10.1016/S2222-1808(15)60885-4
16. Cruz, AM, Barber, SM, Kaestner, SBR, and Townsend, HGG. Urethrorectal fistula in a horse. Can Vet J. (1999) 40:122–4.
17. Sur, RL, Meegan, JM, Smith, CR, Schmitt, T, L’Esperance, J, Hendrikson, D, et al. Surgical Management of Nephrolithiasis in the bottlenose dolphin: collaborations between the urologist and veterinarian. J Endourol Case Rep. (2018) 4:62–5. doi: 10.1089/cren.2017.0143
18. Meegan, JM, and Smith, CR. Chapter 82 - dolphin nephrolithiasis In: E Miller, N Lamberski, and P Calle, editors. Fowler’s zoo and wild animal medicine current therapy, vol. 10. New Delhi: W.B. Saunders (2023). 565–72.
19. Schmitt, TL, and Sur, RL. Treatment of ureteral Calculus obstruction with laser lithotripsy in an Atlantic bottlenose dolphin (Tursiops truncatus). J Zoo Wildl Med. (2012) 43:101–9. doi: 10.1638/2011-0002.1
20. Suárez-Santana, CM, Sierra, E, Díaz-Delgado, J, Zucca, D, De Quirós, YB, Puig-Lozano, R, et al. Prostatic lesions in Odontocete cetaceans. Vet Pathol. (2018) 55:466–72. doi: 10.1177/0300985818755252
21. Larson, KM, Maretelli, PR, Khong, LF, and Fernando, NM. Multiple birth defects in a captive-born 10-day-old indo-Pacific bottlenose dolphin (Tursiops aduncus) with a likely association to a head-first presentation at parturition. (2012). Available online at: https://www.vin.com/doc/?id=6699186.
22. Reidarson, TH, and McBain, J. The combined use of Itraconazole and Flucytosine in the treatment of chronic Candida cystitis in a bottlenose dolphin (Tursiops truncatus). (1995). Available online at: https://www.vin.com/doc/?id=6696240.
23. Vargas-Castro, I, Crespo-Picazo, JL, Fayos, M, Jiménez-Martínez, MDLÁ, Torre-Fuentes, L, Álvarez, J, et al. New insights into the pathogenesis and transmission of Brucella pinnipedialis: systemic infection in two bottlenose dolphins (Tursiops truncatus). Microbiol Spectr. (2023) 11:e01997–23. doi: 10.1128/spectrum.01997-23
24. Barratclough, A, Sanz-Requena, R, Marti-Bonmati, L, Schmitt, TL, Jensen, E, and García-Párraga, D. Radiographic assessment of pectoral flipper bone maturation in bottlenose dolphins (Tursiops truncatus), as a novel technique to accurately estimate chronological age. PLoS One. (2019) 14:e0222722. doi: 10.1371/journal.pone.0222722
25. Bassos-Hull, K, Perrtree, RM, Shepard, CC, Schilling, S, Barleycorn, AA, Allen, JB, et al. Long-term site fidelity and seasonal abundance estimates of common bottlenose dolphins (Tursiops truncatus) along the southwest coast of Florida and responses to natural perturbations. J Cetacean Res Manage. (2013) 13:19–30. doi: 10.47536/jcrm.v13i1.551
26. Urian, KW, Hofmann, S, Wells, RS, and Read, AJ. Fine-scale population structure of bottlenose dolphins (Tursiops truncatus) in Tampa Bay, Florida. Mar Mamm Sci. (2009) 25:619–38. doi: 10.1111/j.1748-7692.2009.00284.x
27. St. Aubin, DJ, Ridgway, SH, Wells, RS, and Rhinehart, H. Dolphin thyroid and adrenal hormones: circulating levels in wild and semidomesticated Tursiops truncatus, and influence of sex, age and season. Mar Mamm Sci. (1996) 12:1–13. doi: 10.1111/j.1748-7692.1996.tb00301.x
28. St. Aubin, DJ. Endocrinology In: L Dierauf and FMD Gulland, editors. CRC handbook of marine mammal medicine. Boca Raton, Florida: CRC Press (2001). 165–92.
29. Schwacke, LH, Hall, AJ, Townsend, FI, Wells, RS, Hansen, LJ, Hohn, AA, et al. Hematologic and serum biochemical reference intervals for free-ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. Am J Vet Res. (2009) 70:973–85. doi: 10.2460/ajvr.70.8.973
30. Hart, LB, Wells, RS, Kellar, N, Balmer, BC, Hohn, AA, Lamb, SV, et al. Adrenal hormones in common bottlenose dolphins (Tursiops truncatus): influential factors and reference intervals. PLoS One. (2015) 10:e0127432. doi: 10.1371/journal.pone.0127432
31. Robeck, TR, O’Brien, JK, and Atkinson, S. Chapter 11 - reproduction In: FMD Gulland, LA Dierauf, and KL Whitman, editors. CRC handbook of marine mammal medicine. Boca Raton: CRC Press (2018). 169–208.
32. Deming, A, Stacy, NI, Balmer, BC, Sweeney, J, Fauquier, DA, and Wells, RS. Urinalysis in free-ranging bottlenose dolphins (Tursiops truncatus). from Sarasota Bay, Florida: (2012) https://www.vin.com/doc/?id=6699186.
33. Long, DJ, and Jones, RE. White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean In: AP Klimley and DG Ainley, editors. Great white sharks: The biology of Carcharodon carcharias. New York, NY, USA: Academic Press (1996). 293–307.
34. Heithaus, MR. Shark attacks on bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia: attack rate, bite scar frequencies and attack seasonality. Mar Mamm Sci. (2001) 17:526–39. doi: 10.1111/j.1748-7692.2001.tb01002.x
35. Lowry, D, De Castro, ALF, Mara, K, Whitenack, LB, Delius, B, Burgess, GH, et al. Determining shark size from forensic analysis of bite damage. Mar Biol. (2009) 156:2483–92. doi: 10.1007/s00227-009-1273-3
36. Irvine, B, Wells, RS, and Gilbert, PW. Conditioning an Atlantic bottle-nosed dolphin, Tursiops truncatus, to repel various species of sharks. J Mammal. (1973) 54:503–5.
38. Wilkinson, KA, Wells, RS, Pine, WE, and Borkhataria, RR. Shark bite scar frequency in resident common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar Mammal Sci. (2017) 33:678–86. doi: 10.1111/mms.12385
39. Heithaus, MR. Predator–prey and competitive interactions between sharks (order Selachii) and dolphins (suborder Odontoceti): a review. J Zool. (2001) 253:53–68. doi: 10.1017/S0952836901000061
40. Schwacke, LH, Smith, CR, Townsend, FI, Wells, RS, Hart, LB, Balmer, BC, et al. Health of common bottlenose dolphins (Tursiops truncatus) in Barataria Bay, Louisiana, following the Deepwater horizon oil spill. Environ Sci Technol. (2014) 48:93–103. doi: 10.1021/es403610f
41. Lane, SM, Smith, CR, Mitchell, J, Balmer, BC, Barry, KP, McDonald, T, et al. Reproductive outcome and survival of common bottlenose dolphins sampled in Barataria Bay, Louisiana, USA, following the Deepwater horizon oil spill. Proc R Soc B Biol Sci. (2015) 282:20151944. doi: 10.1098/rspb.2015.1944
42. Smith, CR, Rowles, TK, Hart, LB, Townsend, F, Wells, RS, Zolman, ES, et al. Slow recovery of Barataria Bay dolphin health following the Deepwater horizon oil spill (2013-2014), with evidence of persistent lung disease and impaired stress response. Endanger Species Res. (2017) 33:127–42. doi: 10.3354/esr00778
43. Barratclough, A, Wells, RS, Schwacke, LH, Rowles, TK, Gomez, FM, Fauquier, DA, et al. Health assessments of common bottlenose dolphins (Tursiops truncatus): past, present, and potential conservation applications. Front Vet Sci. (2019) 6:444. doi: 10.3389/fvets.2019.00444
44. Lacy, RC, Wells, RS, Scott, MD, Allen, JB, Barleycorn, AA, Urian, KW, et al. Assessing the viability of the Sarasota Bay Community of Bottlenose Dolphins. Front Mar Sci. (2021) 8:788086. doi: 10.3389/fmars.2021.788086
45. Venn-Watson, S, Smith, C, Johnson, S, Daniels, R, and Townsend, F. Clinical relevance of urate nephrolithiasis in bottlenose dolphins Tursiops truncatus. Dis Aquat Org. (2010) 89:167–77. doi: 10.3354/dao02187
46. Andersen, S. Physiological ranges of blood-chemical parameters in captive harbour porpoise, Phocaena phocaena (L). Nord Vet Med. (1968) 20:267–78.
47. Fothergill, MB, Schwegman, CA, Garratt, PA, Govender, A, and Robertson, WD. Serum alkaline phosphatase- changes in relation to state of health and age of dolphins. Aquat Mamm. (1991) 17:71–5.
Keywords: ultrasound, health assessment, cetacean, marine mammal, trauma, radiograph, elasmobranch, tiger shark
Citation: Wilkinson KA, Langan JN, Meegan JM, Toms CN, Faulkner Allen R, Sweeney JC, Fauquier DA, Kiszka JJ, Hostnik ET, Barratclough A, Walsh MT and Wells RS (2025) Case Report: Shark bite resulting in a urethral obstruction and urinary tract-body wall fistula in a bottlenose dolphin (Tursiops truncatus) in Sarasota Bay, Florida. Front. Vet. Sci. 12:1551129. doi: 10.3389/fvets.2025.1551129
Edited by:
Natascha Wosnick, Federal University of Paraná, BrazilReviewed by:
Michael James Murray, Monterey Bay Aquarium, United StatesKelly Melillo-Sweeting, Dolphin Communication Project, United States
Copyright © 2025 Wilkinson, Langan, Meegan, Toms, Faulkner Allen, Sweeney, Fauquier, Kiszka, Hostnik, Barratclough, Walsh and Wells. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krystan A. Wilkinson, a3J5c3RhbkBtb3RlLm9yZw==; Jennifer N. Langan, amVubmlmZXIubGFuZ2FuQGJyb29rZmllbGR6b28ub3Jn
†These authors share first authorship
 Krystan A. Wilkinson
Krystan A. Wilkinson Jennifer N. Langan2,3*†
Jennifer N. Langan2,3*† Robyn Faulkner Allen
Robyn Faulkner Allen Jeremy J. Kiszka
Jeremy J. Kiszka Eric T. Hostnik
Eric T. Hostnik Michael T. Walsh
Michael T. Walsh Randall S. Wells
Randall S. Wells