Abstract
A 2-year-old, 3.8 kg spayed female Pomeranian was presented for a routine health examination with no clinical signs. Physical examination revealed no cardiac murmur. Blood tests were unremarkable and electrocardiography revealed normal sinus rhythm. Thoracic radiography showed a normal cardiac silhouette. Transthoracic echocardiography identified a left atrial size at the upper limit of the normal range (left atrium-to-aortic root ratio: 1.7) and a markedly visualized tubular structure along the posterior wall of the left atrium, presumed to be the coronary sinus. Color Doppler imaging revealed continuous flow into the right atrium without evidence of atrial septal defect. There was no evidence of pulmonary hypertension and a bubble study excluded an intracardiac right-to-left shunt. Subsequent computed tomography identified a 3.6 mm partial defect between the midportion of the coronary sinus and the left atrium, consistent with a partial unroofed coronary sinus. Despite the absence of coronary sinus dilation, contrast enhancement patterns were similar in both the coronary sinus and the left atrium, supporting the presence of a left-to-right shunt. No other congenital abnormalities were identified, and regular follow-up with echocardiography and clinical sign monitoring was recommended. Whole-exome sequencing revealed four unique missense single nucleotide variants in genes potentially implicated in cardiac development. This case highlights that unroofed coronary sinus can occur without clinical signs or associated anomalies in dogs and further presents potential genetic variants identified through whole-exome sequencing, contributing to a better understanding of this rare defect.
1 Introduction
A cardiac shunt is a congenital heart defect characterized by an abnormal passage between two cardiac chambers or between distinct circulatory pathways, causing blood to bypass its normal route (1, 2). In dogs, congenital heart diseases such as pulmonic stenosis and aortic stenosis are relatively common (3, 4), whereas unroofed coronary sinus (UCS) is exceedingly rare, with only two veterinary cases reported to date (5, 6).
UCS involves partial or complete defect of the coronary sinus roof, resulting in an abnormal communication with the left atrium (LA). First described by Raghib et al. (7) in 1965, UCS is considered a rare form of atrial septal defect, which accounts for 0.1% of all congenital heart diseases in humans (8). Most human cases of UCS are incidentally detected in asymptomatic individuals (9). When not identified during childhood, the defect is often diagnosed later in adulthood, with one study reporting cases in individuals aged 30 to 56 years (10). Larger defects can lead to right ventricular volume overload and heart failure, necessitating surgical closure (8).
In veterinary medicine, asymptomatic UCS has not been previously documented, and the genetic factors underlying UCS in dogs remain unknown. Here, we report a case of an asymptomatic UCS in a dog, discovered incidentally during a routine health examination, and diagnosed using transthoracic echocardiography and computed tomography (CT). We also present potential genetic variants identified through whole-exome sequencing (WES), contributing to our understanding of this rare anomaly in dogs.
2 Case description
A 2-year-old spayed female Pomeranian dog weighing 3.8 kg was presented for a routine health examination with no history of cardiovascular disease or clinical signs. Vital signs were unremarkable, with a systolic blood pressure of 144 mmHg and a heart rate of 80 beats per minute. No cardiac murmur was detected. Blood tests were unremarkable, and electrocardiography (ECG) revealed a normal sinus rhythm.
Thoracic radiography (DRGEM, Seoul, Republic of Korea) demonstrated a cardiac silhouette within normal limits, with a vertebral heart size of 10.8 (reference range: 10.69 ± 0.62) and a vertebral left atrial size of 2.1 (reference range: 2.17 ± 0.26) (11). Pulmonary vessels appeared normal, as the cranial lobar pulmonary vessels measured less than 1.2 times the diameter of the proximal third of the fourth rib, and the caudal lobar vessels did not exceed the ninth rib thickness (12) (Figure 1).
Figure 1
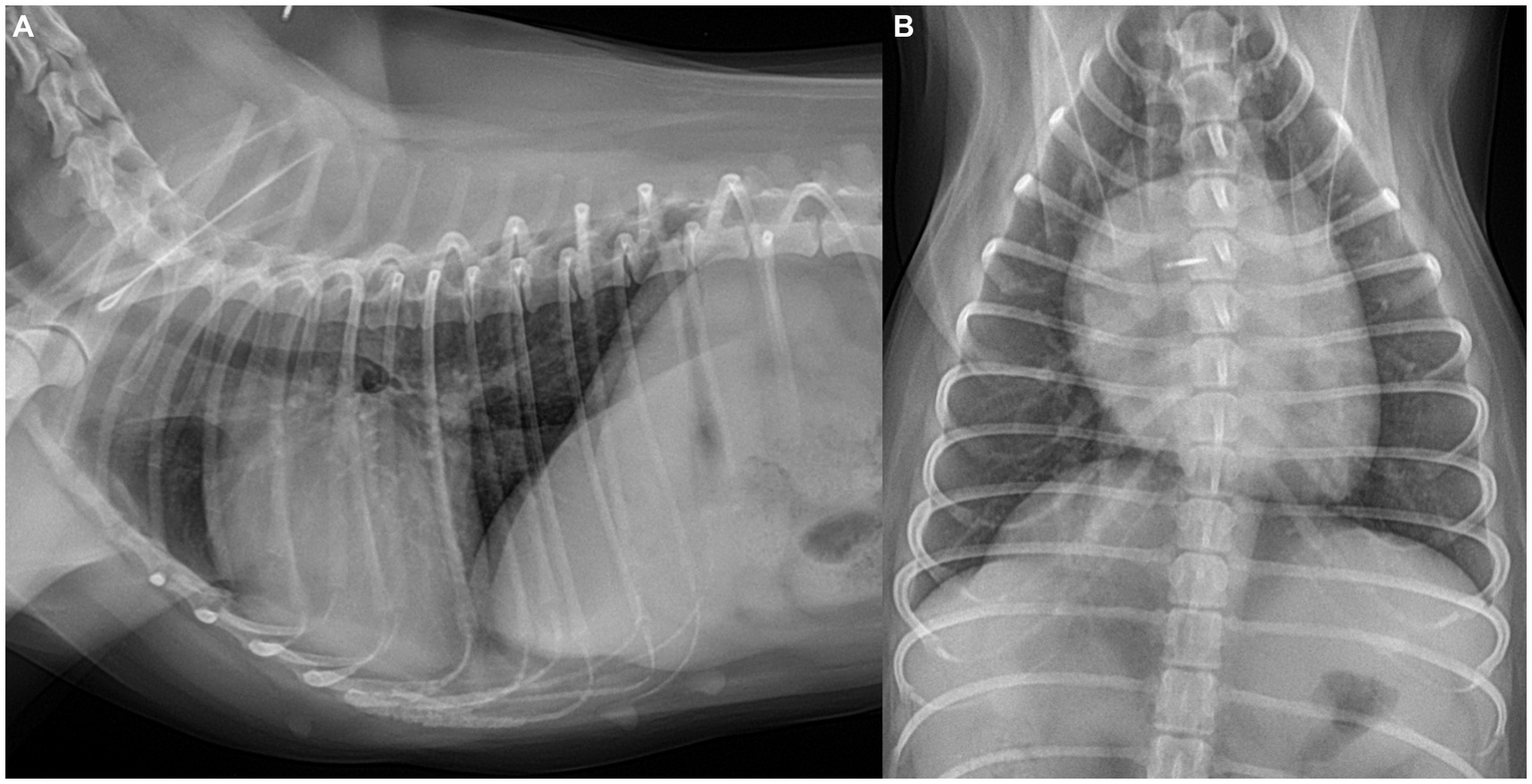
Thoracic radiographs of the dog. (A) Right lateral and (B) ventrodorsal views. Radiographs revealed a cardiac silhouette within normal limits, with the pulmonary vessels and caudal vena cava also measuring within normal limits.
Transthoracic echocardiography (GE Vivid 7, Horten, Norway) using a 5-MHz transducer identified a LA size at the upper limit of the normal range, with a left atrium-to-aortic root ratio of 1.7 (upper reference limit: 1.62–1.83) (13). The left ventricle, right atrium (RA) and right ventricle appeared normal in size. There was no evidence of pulmonary hypertension, systolic dysfunction, or diastolic dysfunction. Color Doppler imaging identified a markedly visualized tubular structure running along the posterior aspect of the LA and extending caudally to the RA near the tricuspid valve annulus, which was presumed to be the coronary sinus. Color Doppler imaging demonstrated a continuous blood flow of unclear origin into the RA, with a velocity of 0.5 m/s (Figure 2). No atrial septal defect was identified. The pulmonary-to-systemic blood flow ratio (Qp/Qs) was 1.3 (reference range: 0.71–1.29) (14). Contrast-enhanced echocardiography using hand-agitated saline confirmed the absence of an intracardiac right-to-left shunt. As the origin of the continuous blood flow into the right atrium was not clearly identified on echocardiography, additional CT (SIEMENS Healthineers, Erlangen, Germany) was performed to differentiate potential congenital cardiac anomalies that could cause continuous shunting into the right atrium, such as UCS, coronary artery fistula, or partial or total anomalous pulmonary venous connection (5, 15, 16).
Figure 2
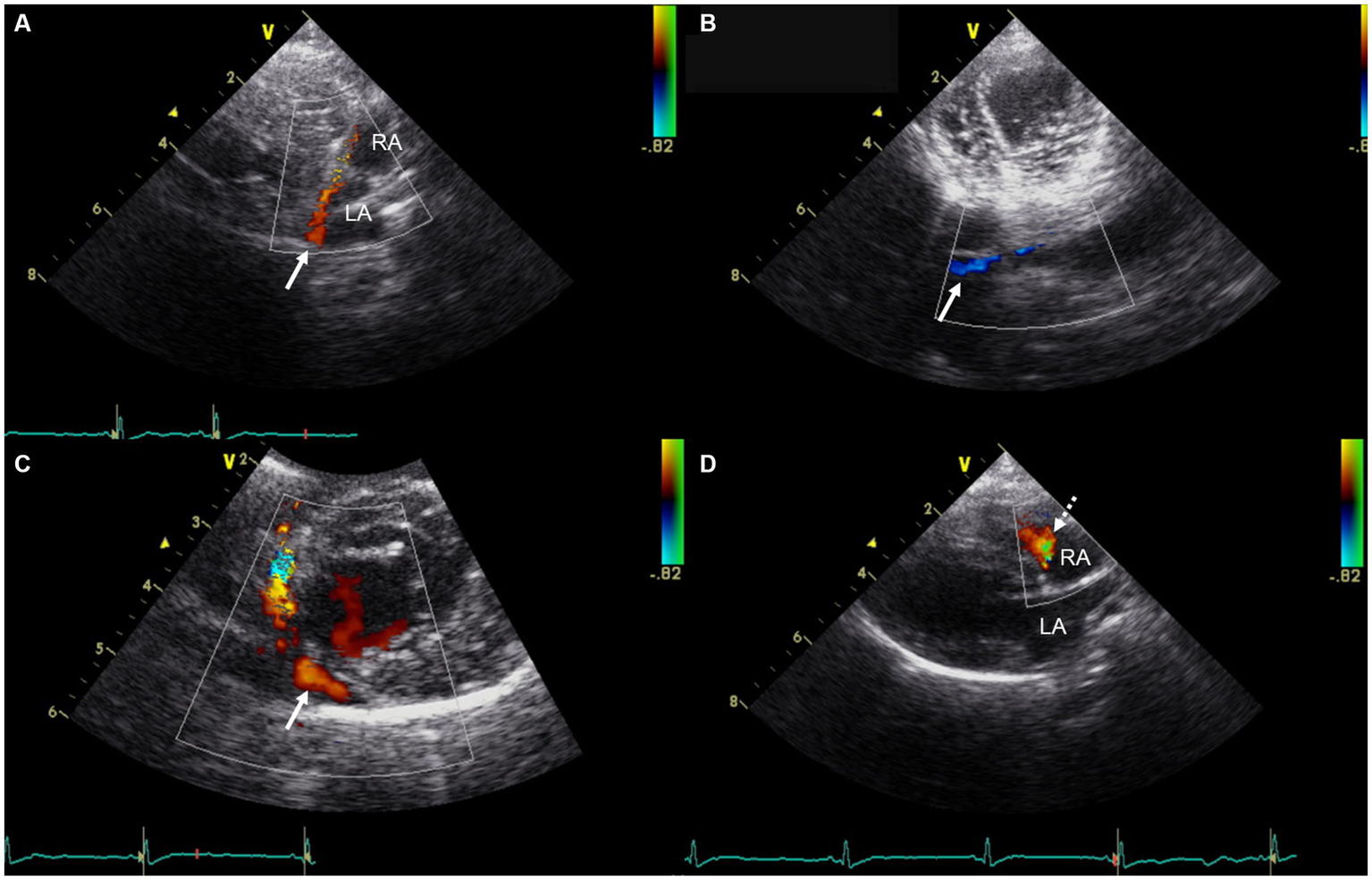
Transthoracic echocardiographic images showing a markedly visualized coronary sinus and continuous blood flow into the right atrium. (A) Modified right parasternal long-axis view. (B) Modified left apical four-chamber view at the level of the chordae tendineae. (C) Modified right parasternal short-axis view. (D) Right parasternal long-axis four-chamber view. In panels (A–C), Color Doppler imaging identified a markedly visualized tubular structure (white arrow) running along the posterior aspect of the LA and extending caudally to the RA, near the tricuspid valve annulus, which is presumed to represent the coronary sinus. In panel (D), Color Doppler imaging demonstrates continuous blood flow (white dashed arrow) of unidentified origin entering the RA. LA, left atrium; RA, right atrium.
The CT findings confirmed a partial defect (3.6 mm) between the midportion of the coronary sinus and the left atrium (Figure 3). The coronary sinus measured 3.5 mm in diameter at both the ostium and the tubular segment. Considering that the reported mean ostium diameter in dogs is 5.5 ± 1.3 mm (17, 18), no obvious dilation was identified in this case. Contrast enhancement patterns on CT were similar in both the coronary sinus and LA, supporting the presence of a left-to-right shunt from the LA to the coronary sinus (5). No other congenital anomalies were observed. Based on these findings, the dog was diagnosed with a partial UCS. Serial echocardiographic evaluations and monitoring for clinical signs suggestive of right-sided volume overload or pulmonary hypertension were recommended to assess potential progression of the condition over time.
Figure 3
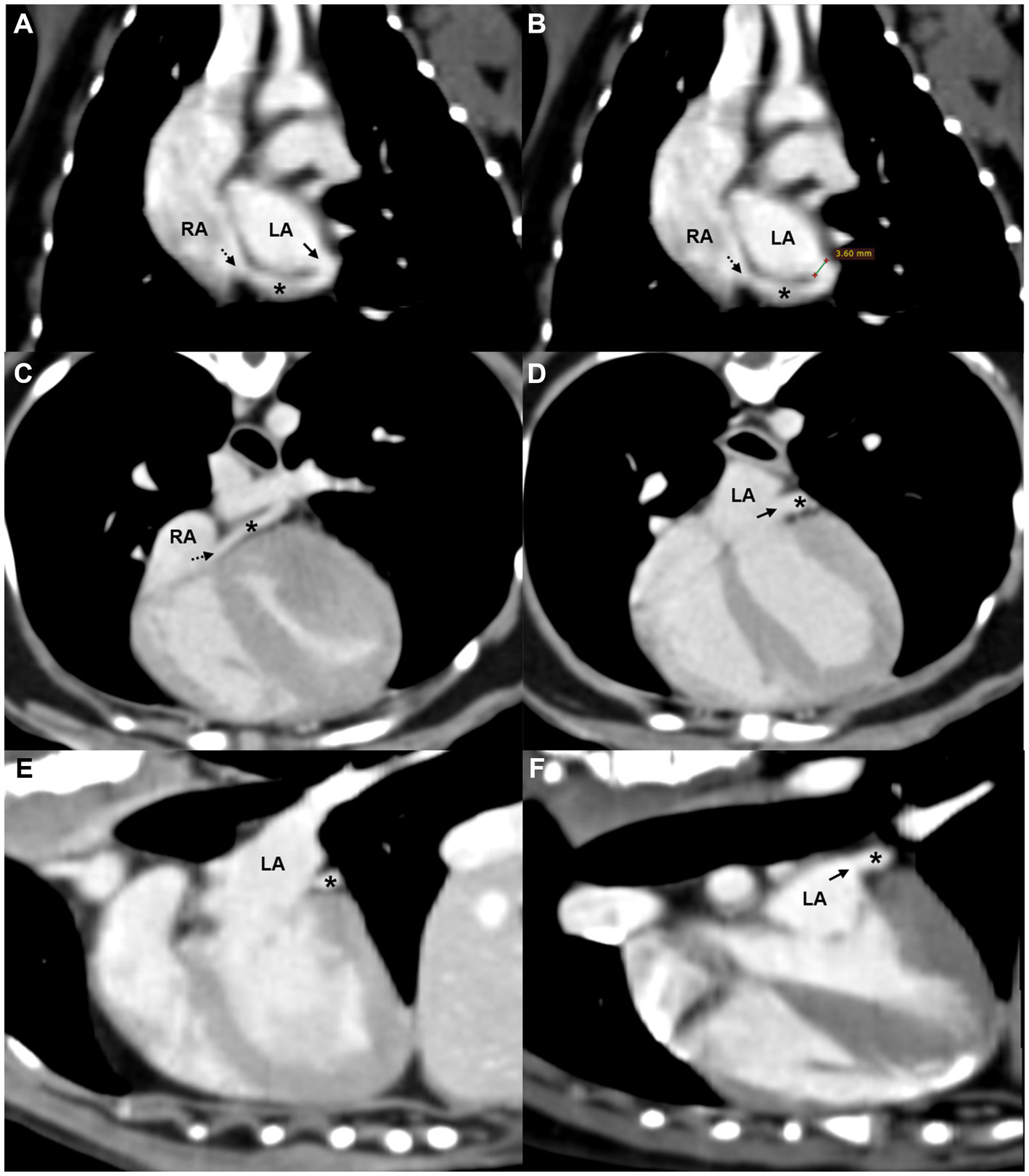
Oblique reformatted CT images of the (A,B) dorsal, (C,D) axial, and (E,F) sagittal planes showing a partial defect between the coronary sinus and the LA. A tubular structure consistent with the coronary sinus (asterisk) courses along the posterior aspect of the LA and drains into the RA via the coronary sinus ostium (black dashed arrow). A partial defect (black solid arrow) in the septum between the coronary sinus and LA is visible in panels (A,D,F), with similar contrast enhancement noted between the two chambers. Measurement of the defect is shown in panel (B). LA, left atrium; RA, right atrium; *, coronary sinus.
To investigate potential genetic factors underlying UCS in this dog, WES was conducted following previously established methods (19). WES was performed on DNA isolated from the blood of the dog by Theragen Bio (Seongnam, Republic of Korea) using the Illumina NovaSeq6000 platform (Illumina, San Diego, CA, United States). Exomes were captured using the SureSelect XT Canine All Exon V2 kit (Agilent Technologies, Santa Clara, CA, United States). Sequencing reads were aligned to the CanFam3.1 genome using the BWA-MEM (version 0.7.1). Variants were identified using SnpEff software (version 4.3). An initial set of 87,610 variants was identified, which was filtered based on annotation criteria, yielding 14,926 variants. Further filtering using public databases and literature reduced the variants to 230 (20, 21), with 13 identified as potentially pathogenic based on SIFT predictions. To evaluate the conservation and functional significance of these variants, comparisons were made with sequences from 12 reference species, including Canis lupus familiaris, C. lupus dingo, Zalophus californianus, Ursus americanus, Lynx canadensis, Suricata suricatta, Felis catus, and Homo sapiens. Cross-species analysis revealed that four candidate missense SNVs were unique to the subject and absent in other reference species.
Consequently, we identified four candidate missense SNVs (Figure 4). A heterozygous SNV was present in CEP250 at position Chr24:24092656G>A (rs852125333), leading to c.2545G>A, resulting in a p.Ala849thr change. The HHEX gene had a heterozygous SNV at position Chr28:7234149A>C (rs3349242635), leading to c.139A>C, resulting in a p.Thr47Pro alteration. A heterozygous SNV was identified in CSPG4 at position Chr30:38397649G>A (rs852344774), leading to c.5089C>T, resulting in a p.Pro1697Ser change. Lastly, CDK15 had a heterozygous SNV at Chr37:11734372A>G (rs852796654), leading to c.355A>G, resulting in a p.Thr119Ala alteration. All variants were classified as missense variants with moderate predicted impact and were assessed as deleterious based on SIFT prediction scores ranging from 0.003 to 0.021.
Figure 4
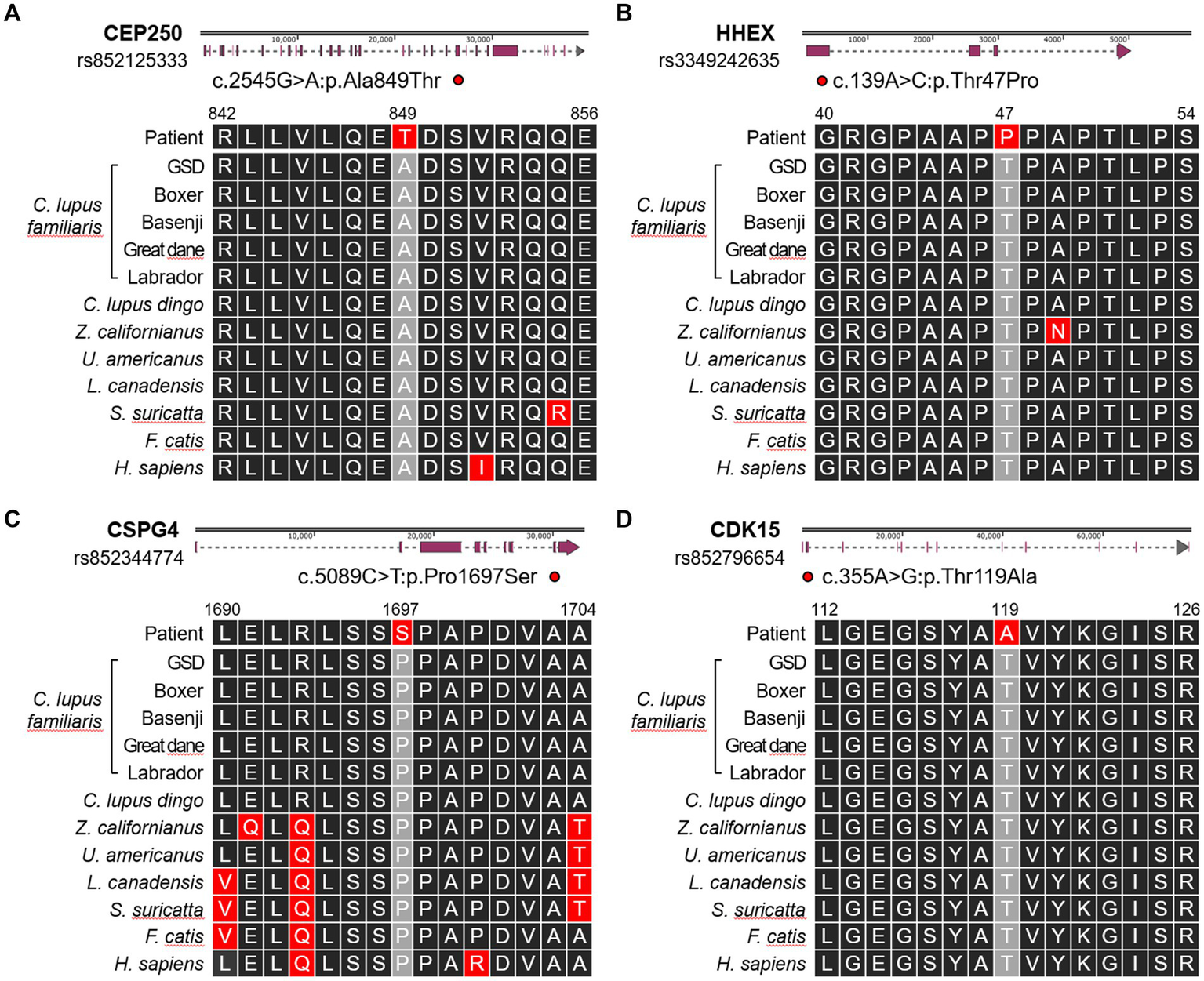
Schematic illustration showing the locations of the single-nucleotide polymorphisms present in (A) CEP250, (B) HHEX, (C) CSPG4, and (D) CDK15 genes. Altered amino acid sequences are compared with 12 references including Canis lupus familiaris (German Shepherd Dog, Boxer, Basenji, Great Dane, Labrador Retriever), Canis lupus dingo (dingo), Zalophus californianus (California sea lion), Ursus americanus (American black bear), Lynx canadensis (Canada lynx), Suricata suricatta (meerkat), Felis catis (domestic cat), and Homo sapiens (Human).
3 Discussion
UCS is a rare congenital cardiac anomaly characterized by partial or complete absence of the coronary sinus roof, leading to an abnormal communication between the coronary sinus and the LA (5, 7–9). Normally, the coronary sinus drains deoxygenated blood from the myocardium into the RA (22). However, in the presence of UCS, blood shunts from the LA into the coronary sinus and then into the RA, functionally resembling an atrial septal defect. The physiological impact varies with the size of the defect; larger UCS lesions can produce significant volume overload on the right heart (5, 8, 10). In humans, UCS often coexists with other congenital cardiac anomalies, such as persistent left superior vena cava (PLSVC), atrial septal defect and endocardial cushion defect (8).
In the present case, UCS was incidentally discovered in an asymptomatic dog. Transthoracic echocardiography revealed a markedly visualized coronary sinus on Color Doppler imaging. Additionally, although no atrial septal defect was identified, continuous blood flow of undetermined origin entering the RA was observed. No cardiac remodeling or pulmonary hypertension was detected, and a bubble study ruled out a right-to-left shunt. On subsequent CT, a partial defect between the LA and the coronary sinus was identified, consistent with a diagnosis of partial UCS. No additional congenital cardiac anomalies were detected. Although ECG-gated CT was not performed, the LA and coronary sinus exhibited similar contrast enhancement patterns on the CT angiogram, suggesting the presence of a left-to-right shunt (5). Accordingly, the continuous flow entering the right atrium was considered to result from UCS.
The absence of clinical signs at the time of diagnosis was likely attributable to the small defect size and minimal shunting. This was supported by a Qp/Qs ratio of 1.3, indicating a left-to-right shunt of hemodynamically insignificant magnitude (23). The LA/Ao ratio was 1.7, which, although within the reported normal range, was close to the upper limit (13). While slight overestimation of the LA/Ao ratio cannot be excluded, it is also possible that the calculated Qp/Qs underestimated the actual shunt volume, particularly given the small body size of the patient (3.8 kg) and the operator-dependent nature of these measurements. Despite the absence of gross coronary sinus dilation, the prominent Color Doppler signal in the coronary sinus was interpreted as a result of increased blood flow into this structure, which may precede anatomical enlargement. Therefore, periodic echocardiographic follow-up was recommended, not only to reassess shunt volume but also to monitor for additional indicators of hemodynamic progression, such as right atrial and right ventricular dilation, progressive coronary sinus enlargement, and reversal of shunt flow. In addition, it was recommended to monitor for clinical signs of right ventricular overload or pulmonary hypertension, such as dyspnea, fatigue, palpitations, cyanosis, syncope, and peripheral edema (24).
Although transthoracic echocardiography identified continuous flow into the right atrium, the UCS defect could not be directly visualized. If 2D or 3D transesophageal echocardiography had been performed, the defect might have been detected, as transesophageal echocardiography offers better visualization of posterior cardiac structures (25). Cardiac magnetic resonance imaging can also aid in UCS diagnosis by offering detailed anatomical assessment and flow quantification via phase-contrast cine magnetic resonance imaging. Additionally, ECG-gated CT may improve detection of small defects by reducing motion artifacts (26). Such detailed assessments are particularly relevant when considering surgical or interventional repair in hemodynamically significant cases. Therefore, the potential application of these modalities in veterinary cardiology warrants further investigation, especially for rare and often underdiagnosed anomalies such as UCS.
In humans, UCS is morphologically classified based on the size and location of the defect and the presence or absence of a PLSVC, which significantly influences surgical planning and clinical management (8). UCS is commonly classified into four types based on the extent and location of the defect and the presence or absence of PLSVC: Type I (completely unroofed with PLSVC), Type II (completely unroofed without PLSVC), Type III (partially unroofed in the midportion), and Type IV (partially unroofed in the terminal portion) (27, 28). In the present case, the defect was located in the midportion of the coronary sinus without associated vascular anomalies, corresponding to Type III. A modified classification by Xie et al. (8) further divides UCS into three main types—complete absence of the coronary sinus (Type I), midportion unroofing (Type II), and terminal portion unroofing (Type III)—each with “a” (with PLSVC) and “b” (without PLSVC) subtypes. According to this scheme, the present case is classified as Type IIb. Although such classification systems have not yet been established in veterinary medicine, their application could help standardize diagnostic approaches and assist in surgical decision-making, highlighting the need for further research in this field. In veterinary medicine, UCS is only rarely diagnosed. This may reflect not only the true rarity of the condition but also a significant degree of underdiagnosis. The present case suggests that, as in humans, asymptomatic UCS can occur in dogs and emphasizes that the condition should not be overlooked even in the absence of clinical signs.
To date, only two cases of UCS have been reported in the veterinary literature. A previous case reported by Zani et al. (6) described a French Bulldog with severe pulmonary stenosis, in which angiography during right ventricular catheterization revealed anomalous venous drainage from a persistent left cranial vena cava into the left atrium. A subsequent bubble study confirmed a right-to-left shunt, as evidenced by immediate opacification of the left atrium following agitated saline injection. Although the authors did not specifically mention UCS, the direct drainage of persistent left cranial vena cava into the left atrium strongly suggests the presence of UCS type I (29). However, no anatomical confirmation or advanced imaging-based diagnosis of UCS was provided. This case underscores the importance of thorough diagnostic evaluation, including complete echocardiographic assessment and cross-sectional imaging modalities such as CT and cardiac magnetic resonance imaging, to accurately identify rare congenital anomalies such as UCS, especially in asymptomatic patients. Shin et al. (5) reported a case in a Dachshund with a larger UCS defect (15 mm) that caused pulmonary overcirculation and right ventricular volume overload, necessitating surgical intervention. In contrast, the coronary sinus defect in the present case was smaller (3.6 mm), which may account for the lack of clinical signs and cardiac remodeling. Unlike previous reports, this is the first case of UCS in a dog without clinical signs or concurrent cardiac anomalies.
In an effort to shed light on the genetic basis of UCS in dogs, we investigated four candidate genes that might contribute to UCS in a dog. UCS, a rare congenital heart defect, presents significant challenges for genetic investigation due to the limited availability of genomic data (30). While genes like NF1 and SMN2 have been implicated in congenital heart defects in humans (31, 32), no equivalent findings have been reported in other animals. To address this limitation, we utilized public databases and comparative analyses across 12 reference species, identifying SNV sites conserved in all species except the subject. This finding suggests that the identified mutations are unique to this dog and may contribute to the pathogenesis of UCS. As a future diagnostic approach, large-scale genomic analyses in a cohort of dogs diagnosed with UCS will be important for identifying shared genetic mutations and clarifying the causal relationship between these mutations and UCS development.
In the subject, we have identified four candidate missense SNVs: CEP250, HHEX, CSPG4, and CDK15. Each of these genes has been implicated, either directly or indirectly, in congenital heart defect. First, CEP250, a centrosomal protein, has been linked to left-sided congenital heart lesions through GWAS and eQTL analyses (33, 34). HHEX, a Homeobox gene family member, regulates early cardiac development and cardiomyocyte formation (35). CSPG4, a transmembrane proteoglycan, is essential for vascular development and stability (36). CDK15, a cyclin-dependent kinase involved in cell cycle regulation and tissue development, plays a role in cardiovascular development (21, 37).
The limitations of this case report are as follows. First, although regular cardiac monitoring was recommended to evaluate potential disease progression, follow-up data were not available at the time of writing; therefore, the longitudinal course of the condition could not be documented. Second, the absence of ECG-gated CT imaging may have introduced motion artifacts, potentially affecting the precision of anatomical measurements (9). Lastly, genetic analysis was restricted to a single case, limiting the statistical significance of our findings.
This report presents the first documented case of UCS without clinical signs or associated congenital anomalies in a dog, emphasizing that this rare condition should not be overlooked even in asymptomatic cases. Additionally, four candidate genes potentially associated with UCS are reported, providing a foundation for further genetic investigations into this rare condition. Further research and multi-institutional cooperation are needed to clarify the genetic mechanisms of UCS and establish evidence-based guidelines for its diagnosis in veterinary medicine.
Statements
Data availability statement
The Whole Exome Sequencing (WES) raw data generated and analyzed in this study have been deposited in the NCBI Sequence Read Archive (SRA) and are publicly available. The data can be accessed using the following accession numbers: BioProject PRJNA1294427, BioSample SAMN50096542, and SRA SRR34653185 via the direct link https://www.ncbi.nlm.nih.gov/sra/SRR34653185.
Ethics statement
Ethical approval was not required for this case report, as the data were obtained during routine clinical examination of a single client-owned dog without any experimental intervention. Written informed consent was obtained from the owner for the participation of the animal and for publication of this case. The study was conducted in accordance with local legislation and institutional ethical guidelines, The case report was based solely on diagnostic procedures and clinical findings obtained during standard veterinary care, with informed consent from the owner. Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the participants for the publication of this case report.
Author contributions
YC: Writing – original draft, Writing – review & editing. WY: Writing – original draft, Writing – review & editing. B-YP: Writing – original draft, Writing – review & editing. Y-JJ: Writing – original draft, Writing – review & editing. KL: Writing – original draft, Writing – review & editing. HY: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the professors and clinicians of the Teaching Hospital of Jeonbuk National University, as well as the veterinary team at Guardian Angel Veterinary Hospital, for their assistance and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1611021/full#supplementary-material
Abbreviations
CT, Computed tomography; ECG, Electrocardiography; LA, Left atrium; PLSVC, Persistent left superior vena cava; Qp/Qs, Pulmonary-to-systemic blood flow ratio; RA, Right atrium; UCS, Unroofed coronary sinus; WES, Whole-exome sequencing.
References
1.
de MadronEChetboulVBussadoriC. Congenital cardiopathies In: Clinical echocardiography of the dog and cat. Saint Louis, MO: Elsevier (2015). 286–322.
2.
SommerRJHijaziZMRhodesJF. Pathophysiology of congenital heart disease in the adult: part I: shunt lesions. Circulation. (2008) 117:1090–9. doi: 10.1161/CIRCULATIONAHA.107.714402
3.
SchropeDP. Prevalence of congenital heart disease in 76,301 mixed-breed dogs and 57,025 mixed-breed cats. J Vet Cardiol. (2015) 17:192–202. doi: 10.1016/j.jvc.2015.06.001
4.
TidholmA. Retrospective study of congenital heart defects in 151 dogs. J Small Anim Pract. (1997) 38:94–8. doi: 10.1111/j.1748-5827.1997.tb03326.x
5.
ShinTShimamuraSHirataSKoderaCKomaiSTaniH. Surgical repair of an unroofed coronary sinus in a dog. J Vet Cardiol. (2023) 48:1–6. doi: 10.1016/j.jvc.2023.04.006
6.
ZaniABecchettiELeonardiPSinatraA. Persistent left cranial vena cava draining into the left atrium associated with pulmonary stenosis in a French Bulldog. J Vet Cardiol. (2014) 16:121–5. doi: 10.1016/j.jvc.2014.03.003
7.
RaghibGRuttenbergHDAndersonRCAmplatzKAdamsPEdwardsJE. Termination of left superior vena cava in left atrium, atrial septal defect, and absence of coronary sinus; a developmental complex. Circulation. (1965) 31:906–18. doi: 10.1161/01.CIR.31.6.906
8.
XieMXYangYLChengTOWangXFLiKRenPPet al. Coronary sinus septal defect (unroofed coronary sinus): echocardiographic diagnosis and surgical treatment. Int J Cardiol. (2013) 168:1258–63. doi: 10.1016/j.ijcard.2012.11.113
9.
Babapoor-FarrokhranSKallaABozorgniaBAmanullahA. Wide unroofed coronary sinus and cryptogenic stroke: a case report. Eur Heart J Case Rep. (2020) 4:1–6. doi: 10.1093/ehjcr/ytaa421
10.
MaJZhengYXuSTengHLvLLiYet al. The value of cardiac CT in the diagnosis of unroofed coronary sinus syndrome. BMC Cardiovasc Disord. (2022) 22:516. doi: 10.1186/s12872-022-02966-2
11.
NaHLeeYLeeSKChoiHLeeYLeeK. The breed-specific vertebral heart score and vertebral left atrial size in toy breed dogs. Thai J Vet Med. (2022) 52:753–9.
12.
SchwarzTJohnsonV. The heart and major vessels In: BSAVA manual of canine and feline thoracic imaging. Gloucester: British Small Animal Veterinary Association (2008). 99.
13.
RishniwMCaivanoDDicksonDVatneLHarrisJMatosJN. Two-dimensional echocardiographic left-atrial-to-aortic ratio in healthy adult dogs: a reexamination of reference intervals. J Vet Cardiol. (2019) 26:29–38. doi: 10.1016/j.jvc.2019.11.001
14.
SerresFChetboulVTissierRGouniVDesmyterASampedranoCCet al. Quantification of pulmonary to systemic flow ratio by a Doppler echocardiographic method in the normal dog: repeatability, reproducibility, and reference ranges. J Vet Cardiol. (2009) 11:23–9. doi: 10.1016/j.jvc.2009.04.001
15.
BodeEFLongoMBrehenyCDel-PozoJCulshawGJMartinez-PereiraY. Total anomalous pulmonary venous connection in a mature dog. J Vet Cardiol. (2019) 21:10–7. doi: 10.1016/j.jvc.2018.11.003
16.
JacobsGJCalvertCAHallDGKrausM. Diagnosis of right coronary artery to right atrial fistula in a dog using two-dimensional echocardiography. J Small Anim Pract. (1996) 37:387–90. doi: 10.1111/j.1748-5827.1996.tb02422.x
17.
PifferCRPifferMISantiFPDayoubMC. Anatomic observations of the coronary sinus in the dog (Canis familiaris). Anat Histol Embryol. (1994) 23:301–8. doi: 10.1111/j.1439-0264.1994.tb00479.x
18.
GenainMAMorletAHerrtageMMuresianHAnselmeFLatremouilleCet al. Comparative anatomy and angiography of the cardiac coronary venous system in four species: human, ovine, porcine, and canine. J Vet Cardiol. (2018) 20:33–44. doi: 10.1016/j.jvc.2017.10.004
19.
JangYJParkBYTaeHJSimJAhnD. Whole-exome sequencing analysis of patent ductus arteriosus in a Japanese macaque (Macaca fuscata). J Med Primatol. (2024) 53:e12686. doi: 10.1111/jmp.12686
20.
GaoYWuDChenBChenYZhangQZhaoP. Rare variants in novel candidate genes associated with nonsyndromic patent ductus arteriosus identified with whole-exome sequencing. Front Genet. (2022) 13:921925. doi: 10.3389/fgene.2022.921925
21.
ChenBHouAZhaoLLiuYShiXDuBet al. Next generation sequencing identify rare copy number variants in non-syndromic patent ductus arteriosus. Front Genet. (2020) 11:600787. doi: 10.3389/fgene.2020.600787
22.
HermansonJWde LahuntaA. The heart and arteries In: Miller and Evans’ anatomy of the dog. 5th ed. Saint Louis, MO: Elsevier (2018). 1038–9.
23.
BoonJA. Congenital shunts and AV valve dysplasia In: Veterinary echocardiography. 2nd ed. Hoboken, NJ: John Wiley & Sons (2011)
24.
CintezăEEFilipCDuicăGNicolaeGNicolescuAMBălgrădeanM. Unroofed coronary sinus: update on diagnosis and treatment. Rom J Morphol Embryol. (2019) 60:33–40. PMID:
25.
BansalRCMartensTPHuHRabkinDG. Unroofed coronary sinus discovered incidentally during cardiac surgery: systematic approach to diagnosis by transesophageal echocardiography. CASE. (2021) 5:384–91. doi: 10.1016/j.case.2021.07.014
26.
KimHChoeYHParkSWJunTGKangISYangJHet al. Partially unroofed coronary sinus: MDCT and MRI findings. AJR Am J Roentgenol. (2010) 195:W331–6. doi: 10.2214/AJR.09.3689
27.
JoffeDCRivoJOxornDC. Coronary sinus atrial septal defect. Anesth Analg. (2008) 107:1163–5. doi: 10.1213/ane.0b013e3181806974
28.
OotakiYYamaguchiMYoshimuraNOkaSYoshidaMHasegawaT. Unroofed coronary sinus syndrome: diagnosis, classification, and surgical treatment. J Thorac Cardiovasc Surg. (2003) 126:1655–6. doi: 10.1016/S0022-5223(03)01019-5
29.
BorgeatKWesselowskiSBessoJLoughranKConnollyDJ. Anomalous left atrial drainage of the vena cava in an adult French Bulldog. CASE (Phila). (2020) 4:146–51. doi: 10.1016/j.case.2020.03.001
30.
MuthiahR. Coronary sinus atrial septal defect (unroofed coronary sinus) with total anomalous pulmonary venous connection—a case report. Case Rep Clin Med. (2017) 6:1–18. doi: 10.4236/crcm.2017.61001
31.
BenderLPMeyerMRFRosaRFMRosaRCMTrevisanPZenPRG. Unroofed coronary sinus in a patient with neurofibromatosis type 1. Rev Paul Pediatr. (2013) 31:546–9. doi: 10.1590/S0103-05822013000400019
32.
KrupickovaSRigbyMLJicinskaHMaraisGRubensMCarvalhoJS. Total anomalous pulmonary venous connection to unroofed coronary sinus diagnosed in a fetus with spinal muscular atrophy type I. Ultrasound Obstet Gynecol. (2017) 50:657–8. doi: 10.1002/uog.17432
33.
FloriotSBelluttiLCastilleJMoisonPMessiaenSPassetBet al. CEP250 is required for maintaining centrosome cohesion in the germline and fertility in male mice. Front Cell Dev Biol. (2022) 9:754054. doi: 10.3389/fcell.2021.754054
34.
HanchardNASwaminathanSBucasasKFurthnerDFernbachSAzamianMSet al. A genome-wide association study of congenital cardiovascular left-sided lesions shows association with a locus on chromosome 20. Hum Mol Genet. (2016) 25:2331–41. doi: 10.1093/hmg/ddw071
35.
WuYJinXZhangYZhengJYangR. Genetic and epigenetic mechanisms in the development of congenital heart diseases. World J Pediatr Surg. (2021) 4:e000196. doi: 10.1136/wjps-2020-000196
36.
StallcupWB. The NG2 proteoglycan in pericyte biology In: Pericyte biology: novel concepts. Cambridge, MA: Academic Press (2018). 5–19.
37.
HuangCDuRJiaXLiuKQiaoYWuQet al. CDK15 promotes colorectal cancer progression via phosphorylating PAK4 and regulating β-catenin/MEK-ERK signaling pathway. Cell Death Differ. (2022) 29:14–27. doi: 10.1038/s41418-021-00828-6
Summary
Keywords
canine, congenital heart disease, atrial septal defect, case report, echocardiography, computed tomography, genetic variant
Citation
Choi Y, Yoon W, Park B-Y, Jang Y-J, Lee K and Yoon H (2025) Case Report: A rare case of asymptomatic unroofed coronary sinus in a dog: diagnostic imaging and genetic findings. Front. Vet. Sci. 12:1611021. doi: 10.3389/fvets.2025.1611021
Received
13 April 2025
Accepted
08 July 2025
Published
30 July 2025
Volume
12 - 2025
Edited by
Sang-Kwon Lee, Kyungpook National University, Republic of Korea
Reviewed by
Howard Dobson, Invicro, United States
Szymon Graczyk, Nicolaus Copernicus University in Toruń, Poland
Updates
Copyright
© 2025 Choi, Yoon, Park, Jang, Lee and Yoon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hakyoung Yoon, hyyoon@jbnu.ac.kr
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.