- Bio-Safety Research Institute and College of Veterinary Medicine, Jeonbuk National University, Iksan, Republic of Korea
Background: The African swine fever virus (ASFV), prevalent globally, causes high mortality and morbidity in domestic pigs. However, there is a lack of effective treatment or vaccines against ASFV infection despite the ongoing research in this field.
Methods: In this systematic review and meta-analysis, we conducted a quantitative evaluation of the efficacy of non-replicating vaccines against ASFV. The vaccine efficacy (VE) was analyzed based on three key disease outcomes: mortality, fever, and clinical symptoms after infection.
Results: The search of relevant electronic databases yielded 23 studies for inclusion in the review. Vaccination with subunit vaccines significantly reduced mortality risk in vaccinated pigs compared to that in controls (p = 0.02), with a relative risk (RR) of 0.90 (95% CI: 0.83–0.98), indicating a VE of 10% (95% CI: 2–17). However, subunit vaccines did not substantially reduce the risk of fever and other clinical symptoms in vaccinated pigs, with a RR of 0.97 (95% CI: 0.93–1.01) for both outcomes. Moreover, inactivated vaccines did not provide any protection against mortality (RR = 1.01, 95% CI: 0.95–1.06) or other clinical signs (RR = 1.00, 95% CI: 1.00–1.00). No significant between-study heterogeneity was detected, indicating consistent findings across different vaccination trials. Thus, currently available non-replicating vaccines fail to deliver the protection required for field applications.
Conclusion: Currently, subunit vaccines are more likely to serve as long-term options for vaccine development strategies. Further research is essential to deepen our understanding of the roles and significance of humoral and cellular immune responses against ASFV, and to identify critical viral antigens that can induce effective protective immunity.
1 Introduction
African swine fever (ASF) is a highly contagious and deadly disease that affects domestic and wild members of the Suidae family. The disease was first identified in Africa in the 1920s and subsequently spread to Europe, Asia, and the Americas in the following decades (1). It is caused by the African swine fever virus (ASFV), a large enveloped DNA virus of the Asfarviridae family, which is characterized by a complex icosahedral structure and a genome that encodes over 150 proteins (2). The virus displays considerable genotypic diversity, with 24 genotypes identified based on the nucleotide sequence diversity of the B646L gene, which encodes the major capsid protein p72 (3). ASFV exhibits pronounced phenotypic variability, driven in large part by its immune-evasion mechanisms that modulate innate and adaptive host defenses (4). ASFV spreads through direct contact with infected pigs, contaminated pork products, and biological vectors such as soft ticks of the Ornithodoros genus, which can host the virus for long durations (5, 6). Its high environmental stability further facilitates disease transmission. Since the disease was first identified in Africa, it has spread globally and has had a significant economic impact on the swine industry (1). Acute clinical forms of ASF manifest as high fever, hemorrhage, respiratory distress, and cause up to 100% mortality, whereas subacute and chronic forms present milder symptoms and have lower mortality rates depending on the viral strain (7, 8). Owing to the absence of effective vaccines or treatments, ASFV continues to pose a significant threat to swine populations worldwide, thereby emphasizing the need for ongoing research and improved control measures to mitigate its spread and impact (6).
In the pursuit of developing an effective ASFV vaccine, live attenuated vaccines (LAVs) have frequently demonstrated the most robust protection as discussed in our previous study (9). However, despite their high efficacy, the deployment of LAVs is hampered by significant safety concerns, including post-vaccination fever, clinical reactions, and lingering risks of reversion to virulence or chronic infection (10, 11). Unlike LAVs, subunit and inactivated vaccines (hereafter referred to as non-replicating vaccines) do not use live pathogens, thereby eliminating the risk of reversion to a virulent form. A notable benefit of non-replicating vaccines, particularly subunit vaccines, is their ability to achieve high expression levels, while maintaining low production costs. Additionally, these vaccines enable the differentiation of infected from vaccinated animals (DIVA) by excluding specific antigens or encoding immunogens that can serve as vaccine markers, allowing companion diagnostic tests to distinguish infected from vaccinated animals (1, 12). Over the past few decades, non-replicating vaccines have become essential for managing infectious diseases in pigs and have been highly effective in promoting animal health, lowering mortality rates, and boosting productivity in swine populations (13–15). Notably, recombinant subunit vaccines for diseases such as classical swine fever (16–18), foot-and-mouth disease (19), and porcine circovirus type 2 (20–23) have been successfully developed and approved by regulatory bodies. An inactivated vaccine targeting porcine parvovirus, a major contributor to reproductive failure and economic losses in pigs, has also undergone field testing (13, 24). These advancements highlight the critical role of non-replicating vaccines as indispensable tools for disease control and productivity improvement in modern pig farming. Given the safety concerns associated with ASF LAVs, non-replicating vaccines have been explored as alternative approaches that offer several advantages, particularly in terms of safety and stability. While subunit and inactivated vaccines offer enhanced safety, stability, and DIVA compatibility, they come with notable drawbacks. These vaccine platforms typically induce weaker immune responses compared to LAVs, especially in terms of inducing cellular immunity such as CD8+ T-cell responses (25, 26). Moreover, non-replicating vaccines often require adjuvants and multiple booster doses to achieve and maintain protection (25). Given these trade-offs, it is imperative to comprehensively assess the feasibility of non-replicating vaccines as safer means to combat ASFV in endemic and at-risk regions.
Subunit vaccines can be formulated to focus the immune response on specific components of the pathogen, such as its surface proteins, to enhance the specificity of the immune response and reduce the risk of unwanted side effects (10, 27, 28). Subunit vaccines consist of purified antigens specifically derived from pathogenic microorganisms that stimulate the host’s immune system to produce targeted antibodies (29). These vaccines contain proteins, peptides, or polysaccharides with immunogenic epitopes that trigger an immune response (12, 27, 29). Despite the genetic diversity of ASFV, several of its proteins, including p12, CD2v, p72, pp220, p54, p30, and pp62, involved in different stages of viral attachment and internalization have been reported to be immunogenic (7, 12, 30). For instance, antibodies targeting p12, p72, or p54 block viral adsorption, whereas antibodies against p30 inhibit ASFV internalization (27, 31, 32). These discoveries have intensified the focus on these proteins for developing recombinant protein-based, DNA-based, and viral-vectored vaccines against ASFV. Inactivated vaccines rely on physical or chemical inactivation of the virus and are often combined with specific adjuvants to improve immune responses (33–35).
Several reviews have examined the research on non-replicating vaccines against ASF (1, 4, 7, 10, 12, 27–29, 31, 32, 36–40) and have provided valuable insights into the strategies used for formulation and administration, the immunogenicity, and potential protective effectiveness of these vaccines in domestic pig populations. Despite these thorough reviews, there is a significant lack of quantitative assessments of the efficacy of non-replicating vaccines against ASF in domestic pigs. This lack limits our complete understanding of the effectiveness of these vaccines in ASF control, and underscores the need for additional research to address this knowledge gap. Meta-analysis serves as a valuable tool in veterinary science for evaluating the efficacy and safety of treatments aimed at enhancing animal health, productivity, and reproduction (41, 42). It combines data from multiple independent studies on a specific topic to produce stronger and more statistically powerful estimates than those obtained from individual studies (43–45). This approach offers more reliable insights into a research topic and helps identify sources of variation among the study results (44). Thus, to address the gaps in the current knowledge on the efficacy of non-replicating ASF vaccines, we conducted a systematic review and meta-analysis (SRMA) of controlled ASF vaccine trials to evaluate the effectiveness of inactivated and subunit vaccines against virulent ASFV strains in domestic pigs to offer critical insights into their potential role in ASF control.
2 Materials and methods
2.1 Research question and search strategy
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (46). The PRISMA guideline checklist for this study is provided in Supplementary material 1. The review question was structured using the “PICO” (population, intervention, comparator, and outcome) framework. The “population” included domestic pigs; “intervention” involved pigs vaccinated with inactivated or subunit vaccines against ASFV infection; the “comparator” group included unvaccinated pigs or those administered a placebo; and key disease “outcomes” included mortality rates, fever, and clinical signs associated with ASF in both vaccinated and control groups. A comprehensive literature search was performed across the international databases MEDLINE (via PubMed), Web of Science, and Scopus, along with several Korean databases including RISS, KISS, and ScienceOn, to identify relevant studies published until May 28, 2024. The following search terms were used: (ASF OR African swine fever OR ASFV OR African swine fever virus) AND (immun* OR vaccin* OR interven* OR treatment OR efficacy OR safety OR effect OR protect* OR mitigat* OR control OR antibody OR prevent* OR subunit OR DNA OR recombinant OR vector OR candidate OR antigen OR inactivated OR killed) AND (swine OR pigs OR piglets OR gilts OR sows OR weaner OR feeders OR finishers).
2.2 Inclusion and exclusion criteria
Primary studies investigating the efficacy of inactivated and/or subunit vaccines against ASF in domestic pigs of any breed in an experimental challenge model were included in the review. Studies that evaluated vaccine efficacy (VE) against virulent ASFV strains were considered eligible. The VE should have been assessed based on at least one clinical disease outcome such as mortality, fever, or ASF-related symptoms. No language restrictions were imposed in the inclusivity criteria. Studies involving animals other than domestic pigs, those using tick bites as the challenge method, review articles, and in vitro studies were excluded from the review. In this study, the VE was defined as the ability to confer protection against disease and prevent the clinical manifestations of ASFV infection. Therefore, studies focusing on humoral and/or cellular immune responses rather than clinical outcomes were excluded, as immune responses are regarded proxy indicators of protection, and the presence of antibodies does not consistently correlate with protection against ASFV infection (47, 48). Furthermore, experimental studies without control groups or full-text access were also excluded. After screening the titles and abstracts, the relevant articles were reviewed completely to confirm their eligibility.
2.3 Data extraction
Three independent reviewers (EN, MT, and FH) extracted data from the eligible studies, and any discrepancies were resolved through discussion and consensus. The extracted information included author names; publication year; country; target antigen/protein; source strain; vaccine type; vaccine dose; vaccination route; name of the adjuvant; challenge strain; challenge dose; number of animals in each experimental group; and disease outcomes such as mortality, fever, and other clinical signs. All outcomes were considered dichotomous variables, and the number of animals that experienced each outcome was recorded. Apart from fever, all reported ASF symptoms (e.g., lethargy, anorexia, recumbence, cyanosis, skin hemorrhages, joint swelling, labored breathing, coughing, ocular discharge, and digestive disorders) were collectively grouped and analyzed as the “clinical signs” outcome. Animals exhibiting at least one of the above symptoms after ASFV challenge were classified as diseased, as long as the study author attributed the symptom to ASFV infection. This approach was deemed appropriate due to the broad range of symptoms associated with ASFV infection in domestic pigs, making it unlikely for an animal to display all symptoms simultaneously. On the other hand, animals explicitly reported by the author as not developing any ASFV-attributable symptom were considered fully protected.
2.4 Risk-of-bias assessment
The internal and external validity of the included studies was assessed by three independent reviewers (EN, MT, and FH) against the Animal Research: Reporting in vivo Experiments 2.0 (ARRIVE 2.0) guidelines checklist (49). Eighteen RoB items were assessed and each was rated as either low, unclear, or high RoB. Discrepancies were resolved through discussions until a consensus was reached.
2.5 Statistical analyses of the data
The VE was assessed by examining three main disease outcomes: mortality, fever, and clinical signs after infection. As mentioned previously, all ASF clinical signs reported in the primary studies (excluding fever) were collectively grouped and analyzed under the “clinical signs” outcome. The meta-analysis was undertaken using R statistical software, version 4.1.2, employing the “meta,” “metafor,” and “dmetar” packages (50–53). The effect of vaccination was estimated using risk ratios (RR) and their corresponding 95% confidence intervals (CI). The VE was calculated as the percentage of cases preventable through vaccination, using the formula (1 - RR) * 100 (54). The 95% CIs for VE were determined using the substitution method (55). A random-effects model was used to combine effect sizes, accounting for the expected heterogeneity across studies. The Mantel–Haenszel method was employed to assign weights to each study during data pooling (56). The between-study variance of true effect sizes (τ2), was estimated using the Paule-Mandel estimator (57), whereas the Knapp-Hartung adjustment was applied to stabilize variance and calculate CIs around the pooled effect estimates (58). The presence of between-study heterogeneity was evaluated using Cochran’s statistic and the I2 test (59). The Q-statistic was used to test the null hypothesis that all studies in the analysis shared a common effect size, whereas the I2 statistic indicated the proportion of observed variance due to true effects rather than sampling error. Heterogeneity was considered significantly high if I2 was > 50% and the p-value was < 0.05. A subgroup analysis was conducted to compare effect sizes across groups and to identify moderators associated with variability in effect sizes. Publication bias was assessed by visual inspection of the funnel plot symmetry and quantitatively using Egger’s regression test (60). When publication bias was confirmed, the Duval and Tweedie trim-and-fill method was applied to estimate the unbiased effect by imputing missing studies into the funnel plot (61). The trim-and-fill method is a nonparametric, funnel-plot-based data-augmentation technique that addresses potential publication bias by evaluating asymmetry in a funnel plot. It iteratively removes the most extreme small-study effects causing the asymmetry and then fills by imputing mirror-image studies to restore symmetry (62). This strategy enables recalculation of a more balanced pooled effect estimate.
3 Results
3.1 Search results
A comprehensive search initially identified 4,983 studies from electronic databases and other sources. After removal of duplicates, 3,498 studies were subjected to title and abstract screening. Of these, 3,157 were deemed irrelevant and excluded from the review. Thus, 341 studies remained for full-text review, of which a further 318 were excluded for reasons such as the use of vaccines other than inactivated or subunit, lack of relevant clinical outcomes, unrelated data, missing full text, or study focus outside domestic pigs. Finally, 23 studies met the inclusion criteria and were incorporated into both qualitative and quantitative analyses; 19 studies were on subunit vaccines and 4 on inactivated vaccines (Figure 1). According to Rodrigues et al. (2015), vaccine designs such as virus-like particles (VLPs), DNA vaccines, vectored vaccines, and vectored VLPs are often classified as subunit vaccines because they deliver only specific antigens of the pathogen, either as proteins or genetic material (63). Consistent with this classification, the subunit vaccines evaluated in this study include protein-, DNA-, and viral vector-based vaccines.
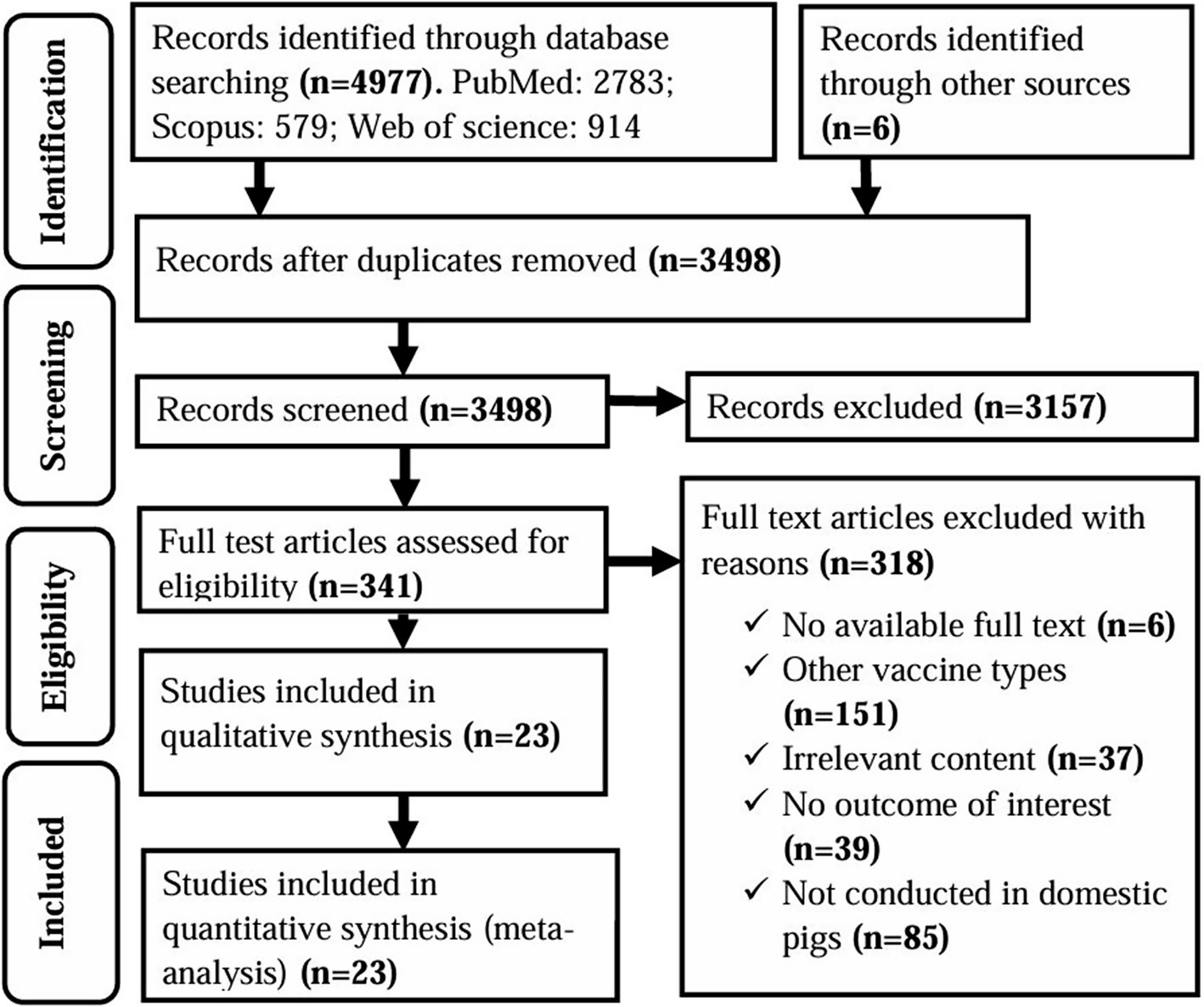
Figure 1. PRISMA flow diagram of the selection of studies for use in a systematic review and meta-analysis of the efficacy of non-replicating vaccines against ASF in domestic pigs.
3.2 Study characteristics
After a comprehensive evaluation of the identified records, 23 studies met the inclusion criteria of the SRMA. Regarding subunit vaccines, 10 studies were conducted in Spain, four in the United States (USA), two in the United Kingdom (UK), and one each in China, Serbia, and Russia. In terms of vaccine type, 10 studies investigated viral vectored vaccines (64–73), two focused on protein-based vaccines (74, 75), four focused on DNA-based vaccines (47, 76–78), and the remaining three explored combination or heterologous prime-boost vaccination strategies (Figure 2). For instance, one study examined a combined DNA-protein vaccine (79), another primed pigs with a DNA-based vaccine and provided a booster of recombinant vaccinia viruses (80), whereas a third study involved priming twice with plasmid DNA followed by a boost with BA71∆CD2 (81), a recombinant live attenuated vaccine. For vaccine delivery, 13 studies employed intramuscular immunization, two used the subcutaneous route, and four studies utilized a combination of both routes. Detailed information regarding the experimental design of studies investigating the efficacy of subunit vaccines against ASFV in domestic pigs is provided in Table 1. Regarding the inactivated vaccines, two studies were conducted in Germany (33, 34), one in Spain (26), and one in Vietnam (82). In terms of the inactivation method, three studies utilized chemical inactivation with binary ethylenimine (BEI) (26, 33, 82), whereas one study employed gamma irradiation (34). Regarding the vaccination route, three studies employed intramuscular immunization, and one used both intramuscular and intradermal routes. Table 2 depicts the main characteristics of the studies assessing the efficacy of inactivated vaccines against ASF in domestic pigs.
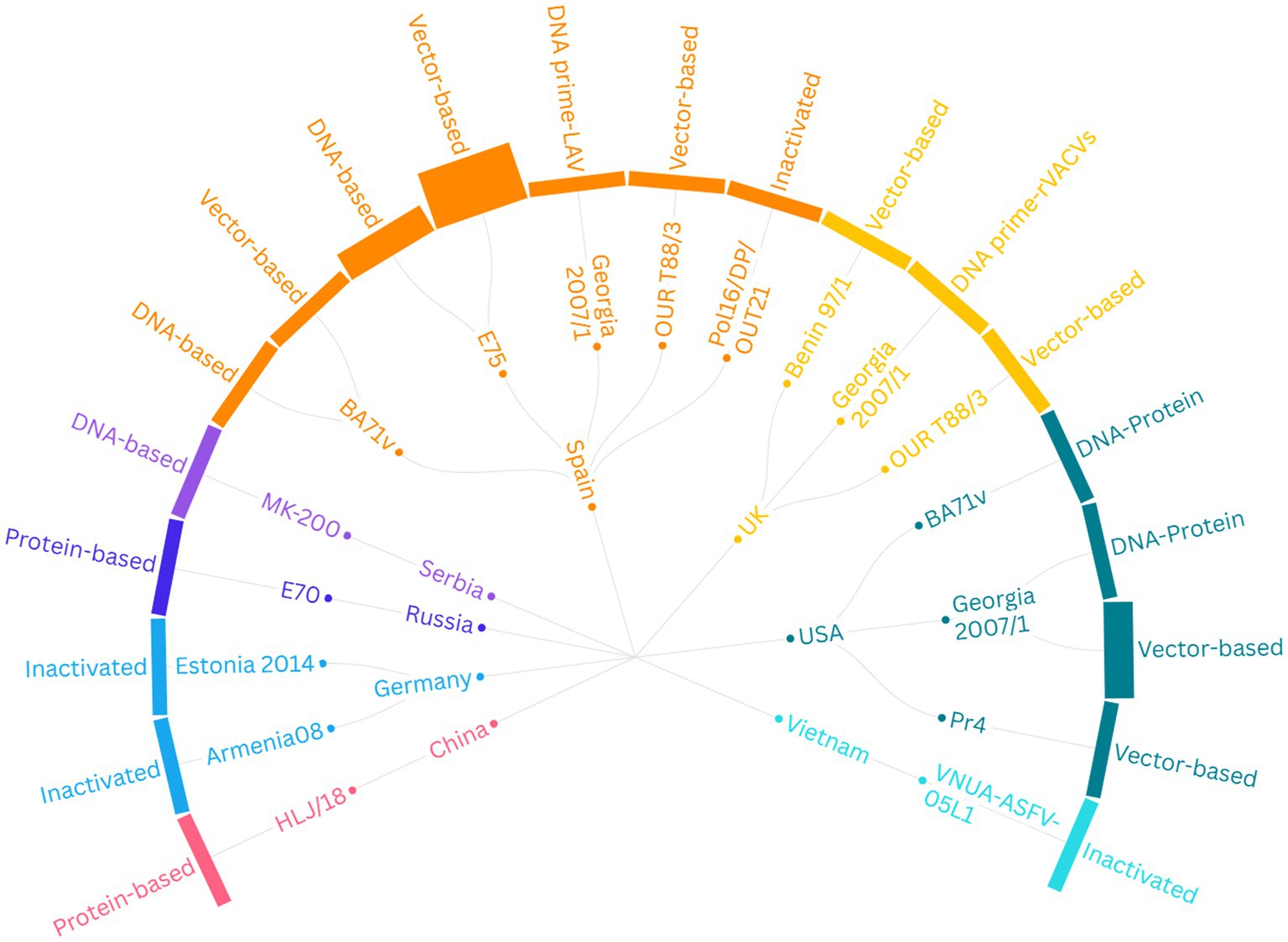
Figure 2. Summary of the experimental designs of 23 studies evaluating the efficacy of non-replicating vaccines against ASFV in domestic pigs. The graph’s structure moves from the outer layer to the center, displaying (1) the type of vaccine, (2) the viral strain from which the antigen was derived, and (3) the country where the study was conducted. LAV, Live Attenuated Vaccine (BA71ΔCD2); rVACVs, Recombinant Vaccinia Viruses; USA, United States of America; UK, United Kingdom.
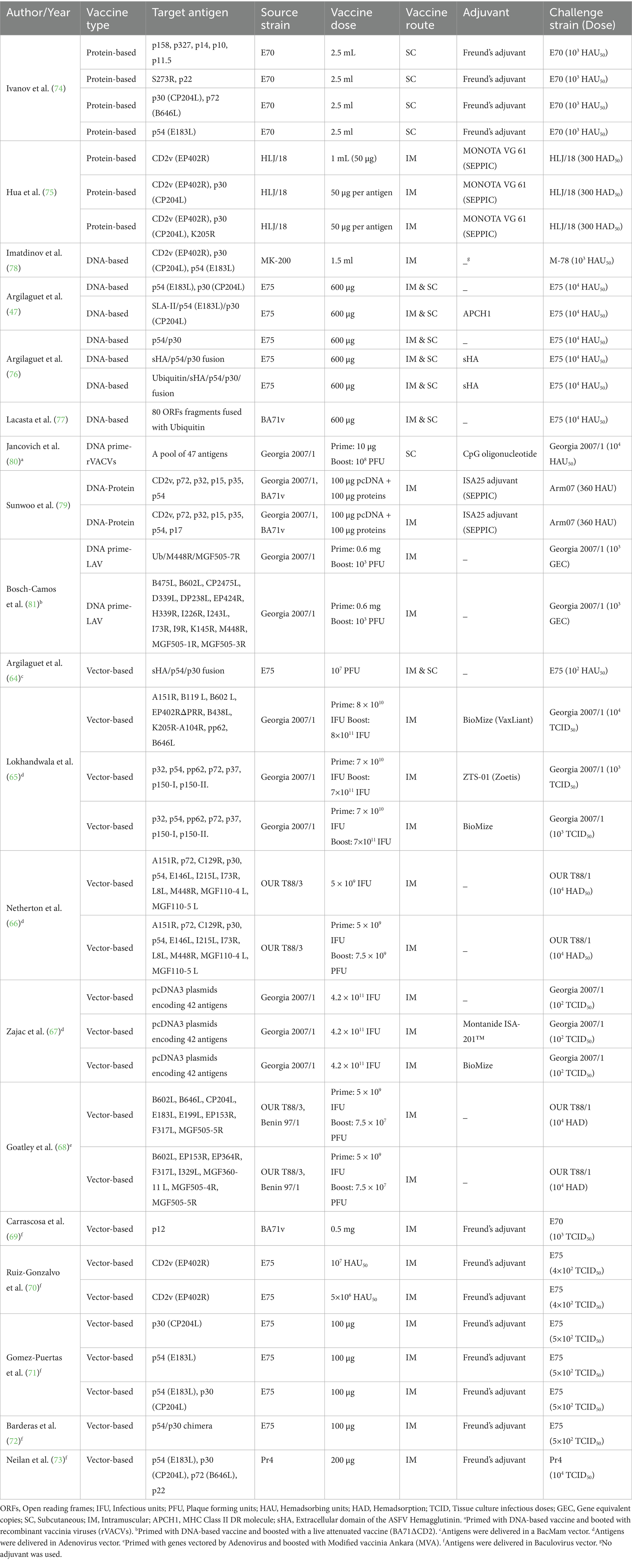
Table 1. Characteristics of 19 studies included in the systematic review and meta-analysis of the efficacy of subunit vaccines against African swine fever in domestic pigs.
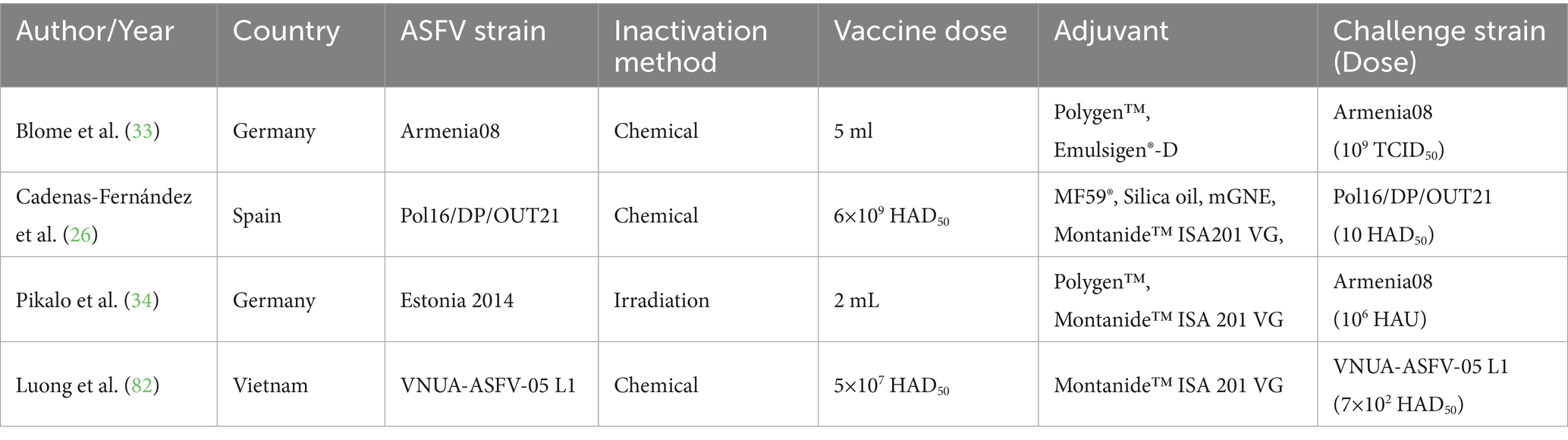
Table 2. Characteristics of 4 studies included in the meta-analysis of the efficacy of inactivated vaccines against African Swine Fever (ASF) in domestic pigs.
3.3 Quality assessment
All 23 studies included in the SRMA were evaluated for potential RoB using the ARRIVE 2.0 guidelines checklist, which assesses 18 specific items (Figure 3). Notably, no study demonstrated a consistently low RoB across all evaluated domains. All studies (100%) did not provide details on sample size determination or personnel blinding during experiments and outcome assessments, and they were rated with an unclear RoB for these items. In one study (68), some vaccinated animals showed ASF-related clinical signs after challenge and were treated with the nonsteroidal anti-inflammatory and antipyretic drug flunixin meglumine. This study was rated as high RoB for the “Study limitations and potential sources of bias” category, as the protective effect observed in the animals was likely influenced by the treatment rather than solely by the vaccine. Seven studies (30.4%) did not disclose their funding sources or the role of the funders, leading to an unclear RoB, whereas the remaining studies (69.6%) were rated as low RoB in this category. Regarding animal randomization, four studies (17.4%) described their randomization strategies and eight studies (34.8%) used antibody-negative pigs or randomly assigned pigs in experimental groups, earning these studies a low RoB rating for randomization or control of cofounders. In summary, most studies were judged to have either a low or unclear RoB across the 18 items, based on the ARRIVE 2.0 guidelines.
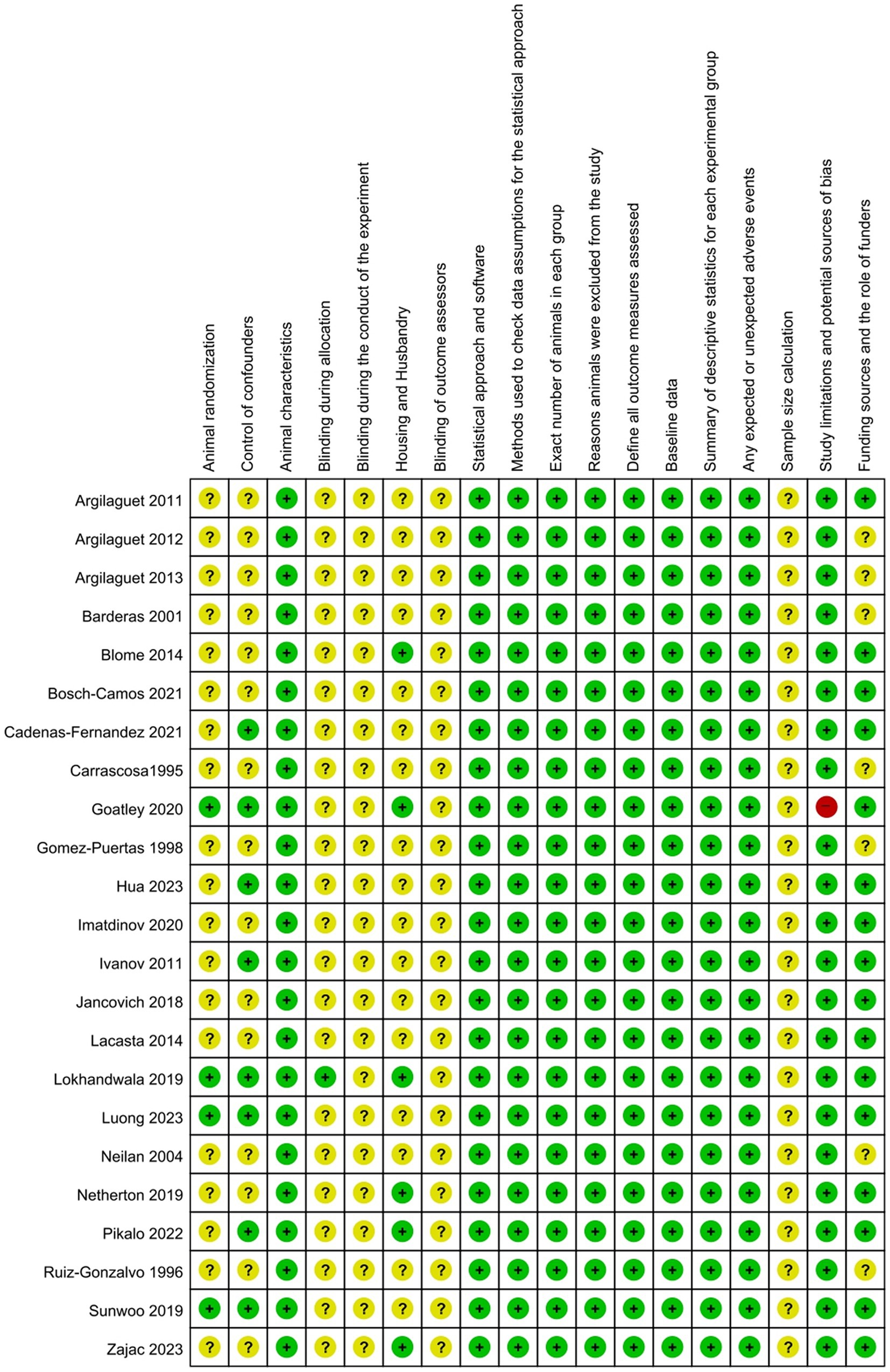
Figure 3. Risk-of-bias assessment of the eligible studies based on the ARRIVE guidelines checklist. Green color denotes a low risk of bias, red denotes a high risk of bias, and yellow indicates an unclear risk of bias.
3.4 Meta-analysis of subunit vaccines
3.4.1 Mortality outcome
Overall, vaccinated pigs had a significantly lower risk of mortality compared to the controls (p = 0.02), with a pooled RR of 0.90 (95% CI: 0.83–0.98). This effect size corresponds to a VE of 10% (95% CI: 2–17) (Figure 4). Specifically, viral-vectored vaccines exhibited the highest efficacy (RR = 0.85, 95% CI: 0.71–1), followed by DNA-based (RR = 0.89, 95% CI: 0.69–1.15), and protein-based (RR = 0.97, 95% CI: 0.55–1.72) vaccines, although the differences between these groups were not statistically significant (Figure 5). Furthermore, no significant heterogeneity was found (I2 = 16, p = 0.26), indicating consistent findings across studies.
![Forest plot showing studies comparing vaccinated and control groups. Each study displays the number of events and total participants. The plot includes risk ratio (RR), 95% confidence intervals (CI), and weight percentage for each study. The overall RR is 0.90 with a CI of [0.83; 0.98] indicating statistical significance. The plot includes a vertical line at RR = 1, a diamond representing the overall effect, and details on heterogeneity and prediction interval.](https://www.frontiersin.org/files/Articles/1614479/fvets-12-1614479-HTML-r2/image_m/fvets-12-1614479-g004.jpg)
Figure 4. Forest plot of the efficacy of subunit vaccines against ASF in domestic pigs based on mortality outcome. The pooled effect estimate is shown as the RR with its corresponding 95% confidence interval, as calculated using a random-effects model.
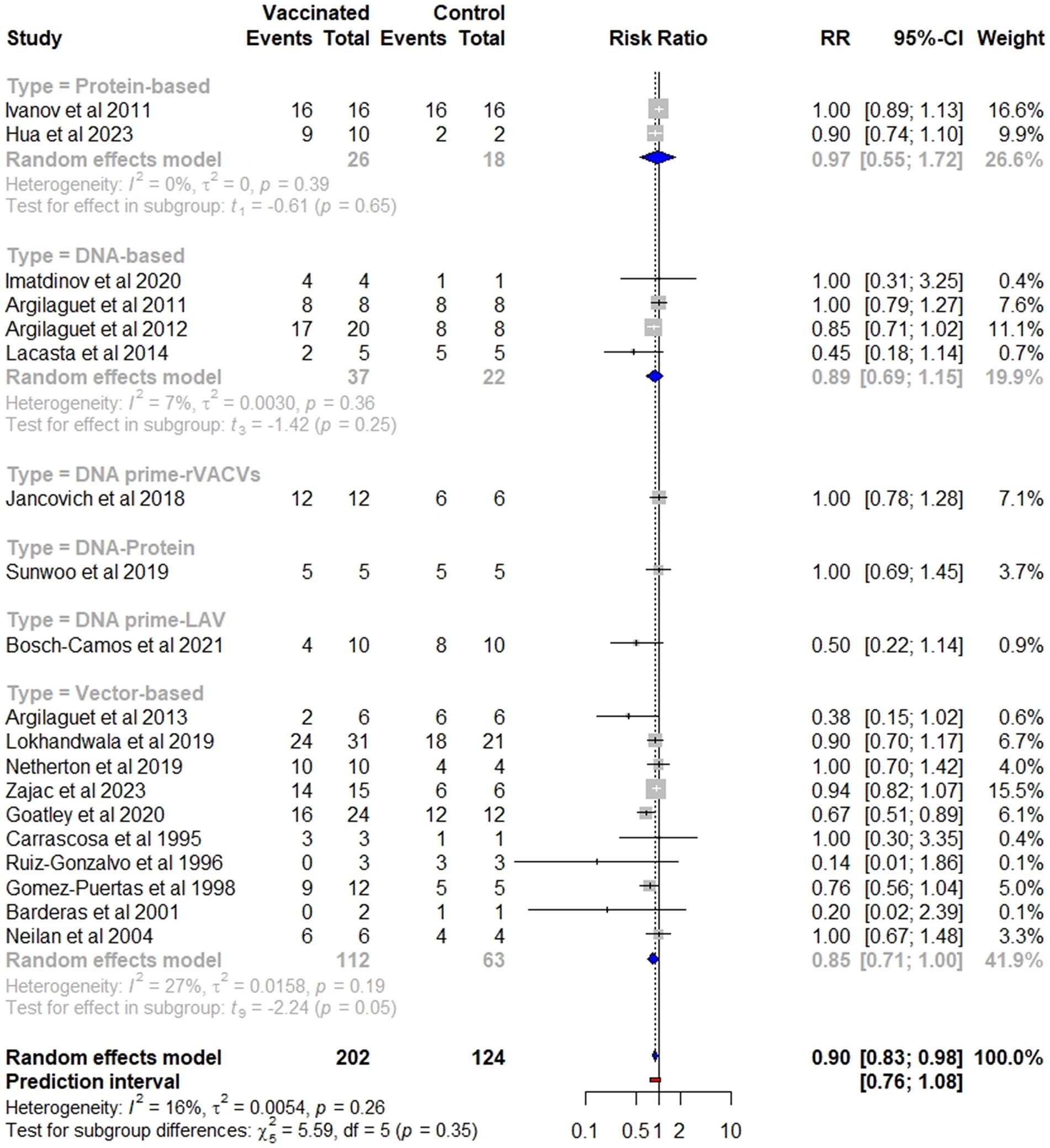
Figure 5. Subgroup analysis of the effectiveness of subunit vaccines in preventing mortality, categorized by vaccine type.
3.4.2 Fever and clinical signs outcomes
In contrast to the mortality outcome, the subunit vaccines did not significantly reduce the risk of fever (p = 0.17) or other ASF-associated clinical symptoms (p = 0.19) in vaccinated pigs compared to those in controls. The overall protection against fever among vaccinated pigs (RR = 0.97, 95% CI: 0.93–1.01) was comparable to the protection against other clinical signs (RR = 0.97, 95% CI: 0.93–1.02). These effect sizes corresponded to a VE of 3% (Figure 6).
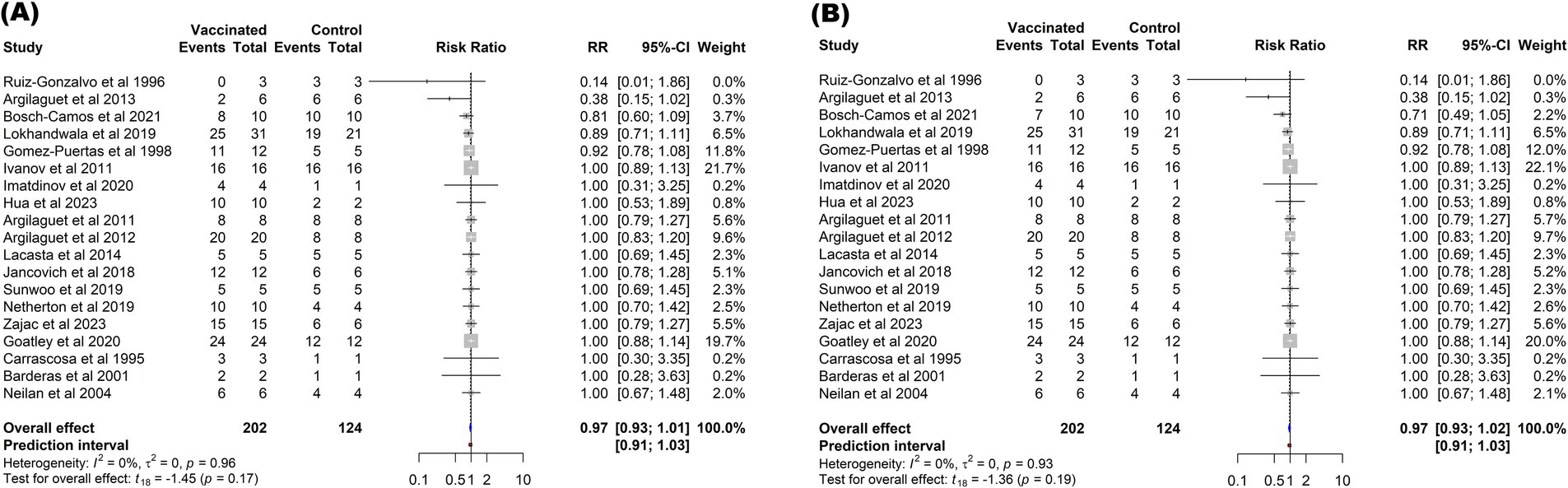
Figure 6. Forest plot of the efficacy of subunit vaccines against ASF in domestic pigs based on fever (A), and clinical signs (B) outcomes. The pooled effect estimate is shown as the RR with its corresponding 95% confidence interval, as calculated using a random-effects model.
3.5 Meta-analysis of inactivated vaccines
In contrast to the subunit vaccines, immunization of pigs with inactivated vaccines failed to confer protection (Figure 7). The risk of mortality among vaccinated pigs (RR = 1.01, 95% CI: 0.95–1.06) was comparable to that observed in non-vaccinated controls (p = 0.71). Similarly, currently developed inactivated vaccines did not provide any protection against fever (RR = 1.00, 95% CI: 1.00–1.00) or other clinical symptoms associated with ASF (RR = 1.00, 95% CI: 1.00–1.00) when compared to control groups.
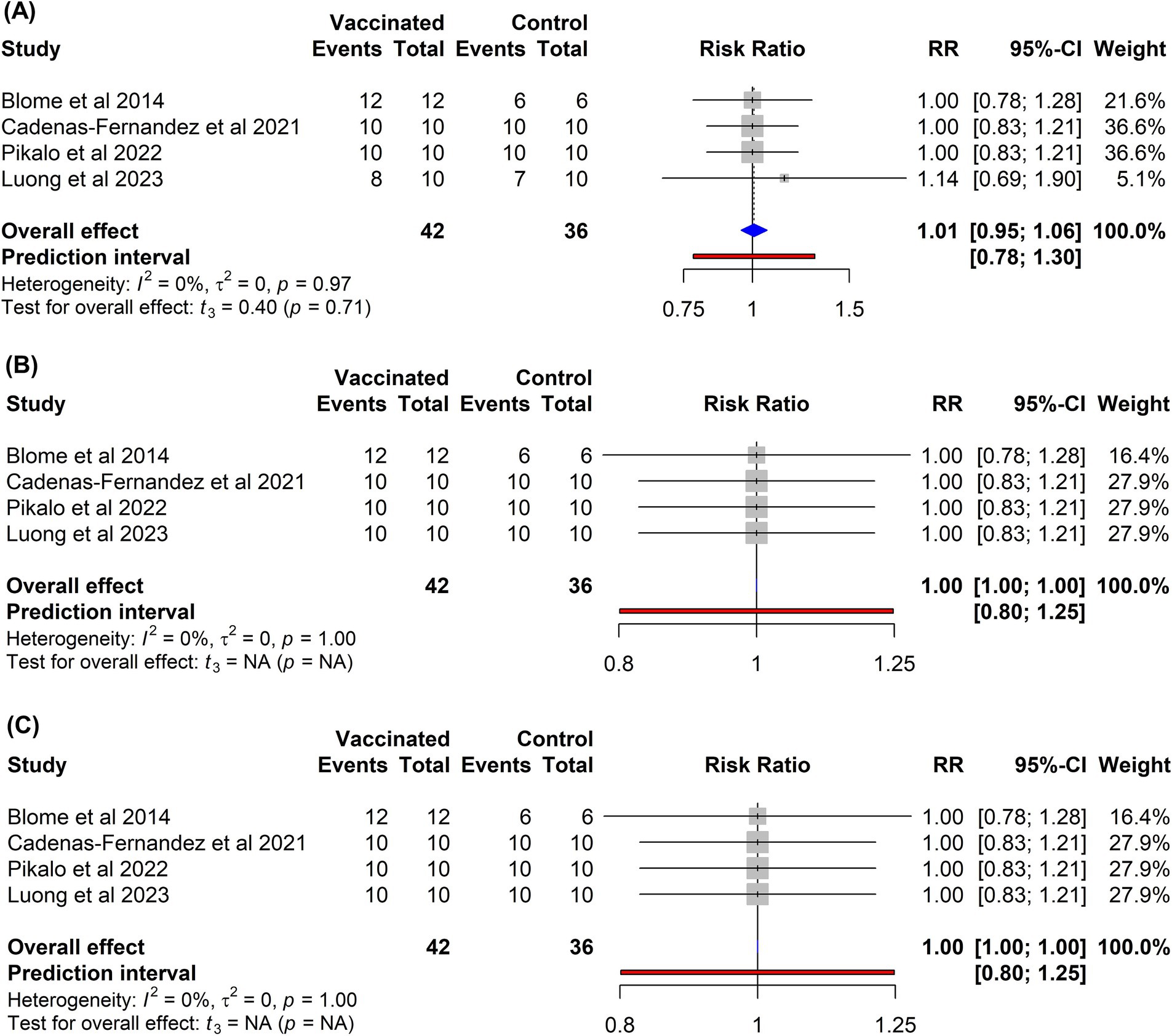
Figure 7. Forest plot of the efficacy of inactivated vaccines against ASF in domestic pigs based on mortality (A), clinical signs (B), and fever (C) outcomes. The pooled effect estimate is shown as the RR with its corresponding 95% confidence interval, as calculated using a random-effects model.
3.6 Publication bias
To evaluate the likelihood of publication bias, funnel plots were constructed with the effect size on the x-axis and standard error on the y-axis (Figure 8). Visual examination of these plots revealed an asymmetric distribution of studies for all outcomes, suggesting publication bias. Given the subjective nature of the funnel plot interpretation, Egger’s regression test was performed to further investigate the significance of funnel plot asymmetry. Egger’s test yielded statistically significant results for mortality (t = −3.015, p = 0.007), confirming the presence of publication bias for this outcome. To assess the influence of publication bias on the pooled effect size, the trim-and-fill method was used to generate a corrected estimate that accounted for potentially missing studies. The analysis identified three studies that were likely missing due to publication bias. Following the application of the trim-and-fill method, the adjusted RR was 0.89 (95% CI: 0.80–1.00). This adjusted effect size closely aligns with the initial pooled estimate (RR = 0.90, 95% CI: 0.83–0.98), indicating that the potential presence of publication bias did not affect the overall conclusions. Furthermore, the analyses of clinical signs (t = −2.109, p = 0.05) and fever (t = −1.993, p = 0.06) did not yield statistically significant results, suggesting that publication bias was unlikely to have influenced these specific disease outcomes.
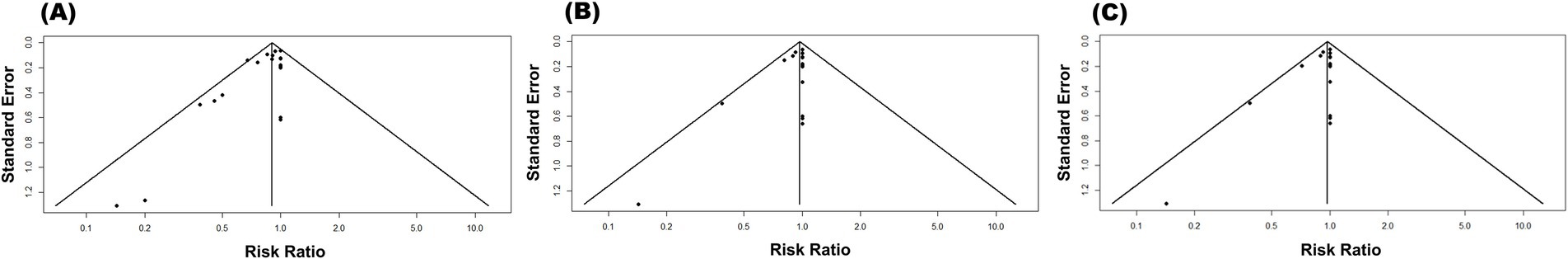
Figure 8. Funnel plot for publication bias on the efficacy of subunit vaccines in preventing ASF in domestic pigs considering the mortality (A), clinical signs (B), and fever (C) outcomes.
4 Discussion
ASFV poses a significant threat to the global swine industry because of its severe morbidity and mortality rates. The complex genome structure of the virus, intricate life cycle, and absence of an effective vaccine pose remarkable challenges in controlling this devastating disease. Various vaccine platforms have been explored to address the challenges of vaccine development; each platform has distinct advantages and limitations. Given the safety concerns associated with live-attenuated vaccines, non-replicating vaccines have been explored as safer alternatives for ASFV control. These vaccines are designed without live pathogens capable of replication; instead, they use inactivated or non-viable components of a pathogen to elicit an immune response without causing illness (83). This category also includes viral vector-based vaccines that utilize replication-deficient viral vectors (84, 85). Thus, the current manuscript provides a quantitative evaluation of the efficacy of non-replicating vaccines developed to protect domestic pigs against ASFV infection. The analysis focused on VE by examining three key disease outcomes: mortality, fever, and clinical symptoms after the infection.
The meta-analysis revealed that vaccination with subunit vaccines significantly reduced the mortality risk in vaccinated pigs compared to that in control pigs (p = 0.02), with a RR of 0.90 (95% CI: 0.83–0.98). This effect size suggests that 10% of the vaccinated pigs were protected against virulent ASFV. Notably, no significant heterogeneity was detected among the studies (I2 = 16, p = 0.26), indicating consistent results across the different trials (Figure 4). Although statistically significant results can be compelling, they should be interpreted with caution when assessing the clinical, epidemiological, and economic relevance of a vaccine. On one hand, even a vaccine with a small effect size could offer substantial value in contexts where partial protection aids in disease control, especially in cases where the disease has a devastating impact. On the other hand, a very high statistically significant effect may not always translate into a meaningful impact if the vaccine does not reduce morbidity, mortality, or transmission rates, especially for diseases with high economic and epidemiological impact like ASF. As illustrated in Figures 4, 6, while the reduction in mortality was statistically significant, the practical value of subunit vaccines remains limited owing to the minimal protection they provide against fever and other ASF-related symptoms. ASFV presents a distinctive challenge owing to its genetic complexity, boasting a sizable genome that encodes approximately 150–167 open reading frames (ORFs), depending on the strain (4, 40). This genetic intricacy limits the selection of antigenic determinants and epitopes capable of eliciting strong, prolonged immunity, thus rendering the development of an effective subunit vaccine extremely complex (27, 31).
In clinical trials, killed vaccines have consistently failed to protect pigs from ASFV, even when exposed to homologous strains (82). Research has indicated that effective protection against ASFV is closely linked to antigen-specific antibodies and CD8 + T-cell responses (6, 31). The poor efficacy of inactivated vaccines is often attributed to their inability to elicit robust humoral and cellular immunity after administration (12, 86). These vaccines cannot replicate or infect host cells, they do not trigger antigen processing via the MHC-I pathway and thus poorly prime CD8+ cytotoxic T-cell responses necessary for clearing infected macrophages (1). Moreover, although antibodies are elicited, they are typically non-neutralizing and fail to prevent ASFV replication, and in some cases may even enhance disease severity (26). Furthermore, common inactivation methods (e.g., chemical or irradiation) can impair critical conformational epitopes and reduce antigen integrity, undermining effective antibody binding (87, 88). To enhance the immunogenicity of inactivated vaccines, recent formulations have incorporated advanced adjuvants, such as Polygen and Emulsigen D. These adjuvants are designed to stimulate both humoral and cellular immune responses, including interferon gamma production, which is essential for protection against ASFV (12, 33). Despite these groundbreaking discoveries, the current meta-analysis revealed that vaccination of pigs with inactivated vaccines did not confer any protection against mortality (RR = 1.01, 95% CI: 0.95–1.06), fever (RR = 1.00, 95% CI: 1.00–1.00), or other ASF-related symptoms (RR = 1.00, 95% CI: 1.00–1.00) when compared to that in control groups (Figure 7). These findings clearly indicate that the use of inactivated or killed vaccines is not a feasible strategy and holds limited promise for preventing ASF. According to Pikalo et al. (2022), the effective generation of robust cell-mediated immunity typically requires viral replication within the host (34), which may explain the lower efficacy of inactivated and subunit vaccines in comparison to that of live attenuated vaccines. Furthermore, the complexity of virions, coupled with their intracellular and extracellular localization, makes viral neutralization difficult and often results in the creation of antibodies that provide no protection or potentially worsen the disease (26, 27, 79).
Unlike protein-based subunit vaccines, DNA and vector-based vaccines are more immunogenic (36). This is because they enable intracellular antigen expression, allowing presentation via MHC-I pathways, which is crucial for activating CD8+ T cells (4, 36). The recognition of vector-associated pathogen-associated molecular patterns (PAMPs) such as dsRNA or viral proteins by pattern recognition receptors (PRRs), including TLR3, TLR7, TLR9 in endosomes, and the cGAS–STING cytosolic DNA-sensing pathway, initiates dendritic cell (DC) maturation and triggers type I interferon (IFN-α/β) production (89). This signaling also upregulates co-stimulatory molecules and induces robust secretion of cytokines like IL-12p70, TNF-α, and IFN-γ, which collectively bridge innate sensing to adaptive immunity, leading to effective CD4+ and CD8+ T-cell responses necessary for clearance of infected macrophages (89). However, this contrasts with the findings of the subgroup analysis. The results showed no statistically significant differences in protection between pigs vaccinated with protein-based vaccines, DNA-based vaccines, viral-vectored vaccines, or their combinations (p = 0.31), as illustrated in Figure 5. This clearly indicates that the mechanisms underlying ASFV protection remain poorly understood, and the significance of antibody-mediated and cell-mediated immune responses in ASFV protection is yet to be elucidated (47). Antibody-dependent enhancement (ADE) of ASFV infection and disease progression is common, as demonstrated in multiple studies involving swine immunization with attenuated or subunit vaccines (12, 66, 79). Additionally, previous research has highlighted the pivotal role of CD8+ T cells in viral clearance, albeit the presence of IFNγ-specific T cells alone does not guarantee complete protection against ASFV infection and disease (47, 48). Further investigations are essential to determine the optimal antibody response that ensures protection while avoiding the harmful effects of excessive antibody production. From an applied perspective, this understanding is crucial for the development of effective and safe immunization strategies.
This study had a few limitations. Although the search strategy effectively retrieved the most relevant studies from electronic databases without language restrictions, the exclusive use of English-spelled search terms may have resulted in the omission of studies published in other languages. The fact that only four studies on inactivated vaccines met our inclusion criteria reflects a limited evidence base, which may reduce analytical robustness and external validity. This scarcity undermines statistical power and constrains our ability to generalize findings across diverse swine populations and real-world settings. Consequently, any conclusions regarding the effectiveness of inactivated vaccines must be considered preliminary and context-specific, rather than definitive. We also acknowledge that we did not plan to contact vaccine companies or research institutes for unpublished data that could have been eligible for inclusion. Moreover, the findings indicated a potential publication bias for the mortality outcomes, which could have affected the statistical power in pooling the effect size. However, the presence of publication bias should be interpreted with caution as it does not necessarily pose a threat to the validity of the findings. In fact, after applying the trim-and-fill method, the adjusted effect size was estimated to be 0.89 (95% CI: 0.80–1.00), which is comparable to the overall pooled effect size of 0.90 (95% CI: 0.83–0.98) as reported previously. Thus, publication bias may exist; however, it does not have a significant impact on the estimated effect size. Additionally, the variations in vaccination protocols, particularly regarding the breed and age of pigs included in the trials, along with small sample size of animals used in the vaccine experiments, pose significant limitations. Consequently, extrapolating experimental findings to natural field settings should be approached with caution to ensure accurate interpretation and applicability. Furthermore, although many trials have evaluated humoral and cellular immune responses, and viral shedding post ASF vaccination or infection, these data were excluded from the meta-analysis. This exclusion may have affected the assessment of immune response dynamics and the related findings available in the scientific literature.
5 Conclusion
This study provided a comprehensive assessment of the effectiveness of current non-replicating vaccine candidates in protecting pigs against ASFV. Compared to live-attenuated vaccines, non-replicating vaccines can deliver significant benefits, including enhanced specificity, stability, and safety, while also enabling DIVA. Overall, the findings of this study indicate that the use of inactivated vaccines represents an unsuccessful strategy and holds limited promise for preventing ASF. In contrast, subunit vaccines against ASFV provide approximately 10% protection against mortality and 3% protection against fever and other clinical symptoms in domestic pigs. From a scientific perspective, this marks a significant step forward in the pursuit of an ASF vaccine, suggesting that the development of an effective subunit vaccine is a realistic goal with continued efforts. However, from a practical standpoint, the clinical, epidemiological, and economic relevance of currently available subunit vaccines remains limited. This limitation arises because most vaccinated pigs developed viremia or a chronic form of ASF, which may pose a serious threat to animal health and complicating disease control efforts. The virus’s persistence and potential for undetected spread undermine control measures. At present, subunit vaccines are likely to serve as a long-term choice in vaccine development strategies. Further research is essential to deepen our understanding of the roles and significance of humoral and cellular immune responses against ASFV infection, as well as to identify critical viral antigens and delivery systems that can induce effective protective immunity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
EN: Writing – review & editing, Formal analysis, Data curation, Writing – original draft, Software, Investigation, Methodology, Visualization. MT: Formal analysis, Data curation, Methodology, Visualization, Writing – review & editing. FH: Methodology, Visualization, Investigation, Writing – review & editing. GW: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00347286). This subject was supported by the National Institute of Wildlife Disease Control and Prevention as a Specialized Graduate School Support Project for Wildlife Disease Specialists.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1614479/full#supplementary-material
References
1. Bosch-Camós, L, López, E, and Rodriguez, F. African swine fever vaccines: a promising work still in progress. Porcine Health Manag. (2020) 6:17. doi: 10.1186/s40813-020-00154-2
2. Wang, T, Luo, R, Sun, Y, and Qiu, HJ. Current efforts towards safe and effective live attenuated vaccines against African swine fever: challenges and prospects. Infect Dis Poverty. (2021) 10:83–9. doi: 10.1186/s40249-021-00920-6
3. Qu, H, Ge, S, Zhang, Y, Wu, X, and Wang, Z. A systematic review of genotypes and serogroups of African swine fever virus. Virus Genes. (2022) 58:77–87. doi: 10.1007/s11262-021-01879-0
4. Teklue, T, Sun, Y, Abid, M, Luo, Y, and Qiu, HJ. Current status and evolving approaches to African swine fever vaccine development. Transbound Emerg Dis. (2020) 67:529–42. doi: 10.1111/tbed.13364
5. Cheng, J, and Ward, MP. Risk factors for the spread of African swine fever in China: a systematic review of Chinese-language literature. Transbound Emerg Dis. (2022) 69:e1289–98. doi: 10.1111/tbed.14573
6. Gaudreault, NN, Madden, DW, Wilson, WC, Trujillo, JD, and Richt, JA. African swine fever virus: an emerging DNA arbovirus. Front Vet Sci. (2020) 7:215. doi: 10.3389/fvets.2020.00215
7. Li, Z, Chen, W, Qiu, Z, Li, Y, Fan, J, Wu, K, et al. African swine fever virus: a review. Life. (2022) 12:1255. doi: 10.3390/life12081255
8. Schulz, K, Staubach, C, and Blome, S. African and classical swine fever: similarities, differences and epidemiological consequences. Vet Res. (2017) 48:84. doi: 10.1186/s13567-017-0490-x
9. Ntakiyisumba, E, Tanveer, M, and Won, G. A comprehensive analysis of the current strategy for developing live attenuated vaccines against African swine fever: a systematic review and meta-analysis. Vaccine. (2025) 57:127243. doi: 10.1016/j.vaccine.2025.127243
10. Gaudreault, NN, and Richt, JA. Subunit vaccine approaches for African swine fever virus. Vaccine. (2019) 7:56. doi: 10.3390/vaccines7020056
11. King, K, Chapman, D, Argilaguet, JM, Fishbourne, E, Hutet, E, Cariolet, R, et al. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine. (2011) 29:4593–600. doi: 10.1016/j.vaccine.2011.04.052
12. Turlewicz-Podbielska, H, Kuriga, A, Niemyjski, R, Tarasiuk, G, and Pomorska-Mól, M. African swine fever virus as a difficult opponent in the fight for a vaccine-current data. Viruses. (2021) 13:1212. doi: 10.3390/v13071212
13. Foerster, T, Streck, AF, Speck, S, Selbitz, HJ, Lindner, T, Truyen, U, et al. An inactivated whole-virus porcine parvovirus vaccine protects pigs against disease but does not prevent virus shedding even after homologous virus challenge. J Gen Virol. (2016) 97:1408–13. doi: 10.1099/jgv.0.000446
14. Liu, J, Hu, G, Liu, S, Ren, G, Gao, L, Zhao, Z, et al. Evaluating passive immunity in piglets from sows vaccinated with a PEDV S protein subunit vaccine. Front Cell Infect Microbiol. (2024) 14:1498610. doi: 10.3389/fcimb.2024.1498610
15. Wu, M-C, Wu, HC, Lee, JW, Chang, WC, and Chu, CY. A protein-based subunit vaccine with biological adjuvants provides effective protection against Pasteurella multocida in pigs. Vet Res. (2023) 54:17. doi: 10.1186/s13567-023-01150-4
16. Moormann, RJM, Bouma, A, Kramps, JA, Terpstra, C, and de Smit, HJ. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet Microbiol. (2000) 73:209–19. doi: 10.1016/S0378-1135(00)00146-2
17. Blome, S, Moß, C, Reimann, I, König, P, and Beer, M. Classical swine fever vaccines—state-of-the-art. Vet Microbiol. (2017) 206:10–20. doi: 10.1016/j.vetmic.2017.01.001
18. Xia, S-L, Xiang, GT, Lei, JL, du, M, Wang, Y, Zhou, M, et al. Efficacy of the marker vaccine rAdV-SFV-E2 against classical swine fever in the presence of maternally derived antibodies to rAdV-SFV-E2 or C-strain. Vet Microbiol. (2016) 196:50–4. doi: 10.1016/j.vetmic.2016.10.001
19. Eblé, PL, Weerdmeester, K, van Hemert-Kluitenberg, F, and Dekker, A. Intradermal vaccination of pigs against FMD with 1/10 dose results in comparable vaccine efficacy as intramuscular vaccination with a full dose. Vaccine. (2009) 27:1272–8. doi: 10.1016/j.vaccine.2008.12.011
20. Poulsen Nautrup, B, Van Vlaenderen, I, and Mellencamp, MA. A chimeric vaccine against porcine circovirus type 2: Meta-analysis of comparative clinical trials. Vaccine. (2023) 11:584. doi: 10.3390/vaccines11030584
21. Da Silva, N, Carriquiry, A, O’Neill, K, Opriessnig, T, and O’Connor, AM. Mixed treatment comparison meta-analysis of porcine circovirus type 2 (PCV2) vaccines used in piglets. Prev Vet Med. (2014) 117:413–24. doi: 10.1016/j.prevetmed.2014.10.006
22. Chae, C. Commercial porcine circovirus type 2 vaccines: efficacy and clinical application. Vet J. (2012) 194:151–7. doi: 10.1016/j.tvjl.2012.06.031
23. Do, DT, Tran, KDV, Quach, AT, Lee, D, Chang, FC, Wu, CP, et al. A comparative efficacy test of 1 versus 2 doses of CIRCOQ PCV2 subunit vaccine against naturally occurring PCV2-type d in piglets with high maternally derived antibodies (MDAs) on a Vietnamese swine farm. Can J Vet Res. (2021) 85:93–100.
24. Jóźwik, A, Manteufel, J, Selbitz, HJ, and Truyen, U. Vaccination against porcine parvovirus protects against disease, but does not prevent infection and virus shedding after challenge infection with a heterologous virus strain. J Gen Virol. (2009) 90:2437–41. doi: 10.1099/vir.0.012054-0
25. Coronado, L, Perera, CL, Rios, L, Frías, MT, and Pérez, LJ. A critical review about different vaccines against classical swine fever virus and their repercussions in endemic regions. Vaccines (Basel). (2021) 9:154. doi: 10.3390/vaccines9020154
26. Cadenas-Fernández, E, Sánchez-Vizcaíno, JM, van den Born, E, Kosowska, A, van Kilsdonk, E, Fernández-Pacheco, P, et al. High doses of inactivated African swine fever virus are safe, but Do not confer protection against a virulent challenge. Vaccine. (2021) 9:242. doi: 10.3390/vaccines9030242
27. Sánchez, EG, Pérez-Núñez, D, and Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. (2019) 265:150–5. doi: 10.1016/j.virusres.2019.03.022
28. Wu, K, Liu, J, Wang, L, Fan, S, Li, Z, Li, Y, et al. Current state of global African swine fever vaccine development under the prevalence and transmission of ASF in China. Vaccines (Basel). (2020) 8:531. doi: 10.3390/vaccines8030531
29. Zhang, H, Zhao, S, Zhang, H, Qin, Z, Shan, H, Cai, X, et al. Vaccines for African swine fever: an update. Front Microbiol. (2023) 14:1139494. doi: 10.3389/fmicb.2023.1139494
30. Wang, T, Sun, Y, Huang, S, and Qiu, HJ. Multifaceted immune responses to African swine fever virus: implications for vaccine development. Vet Microbiol. (2020) 249:108832. doi: 10.1016/j.vetmic.2020.108832
31. Arias, M, de la Torre, A, Dixon, L, Gallardo, C, Jori, F, Laddomada, A, et al. Approaches and perspectives for development of African swine fever virus vaccines. Vaccine. (2017) 5:35. doi: 10.3390/vaccines5040035
32. Sang, H, Miller, G, Lokhandwala, S, Sangewar, N, Waghela, SD, Bishop, RP, et al. Progress toward development of effective and safe African swine fever virus vaccines. Front Vet Sci. (2020) 7:84. doi: 10.3389/fvets.2020.00084
33. Blome, S, Gabriel, C, and Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine. (2014) 32:3879–82. doi: 10.1016/j.vaccine.2014.05.051
34. Pikalo, J, Porfiri, L, Akimkin, V, Roszyk, H, Pannhorst, K, Kangethe, RT, et al. Vaccination with a gamma irradiation-inactivated African swine fever virus is safe but does not protect against a challenge. Front Immunol. (2022) 13:832264. doi: 10.3389/fimmu.2022.832264
35. Kamboj, A, Dumka, S, Saxena, MK, Singh, Y, Kaur, BP, da Silva, SJR, et al. A comprehensive review of our understanding and challenges of viral vaccines against swine pathogens. Viruses. (2024) 16:833. doi: 10.3390/v16060833
36. Wang, Z, Ai, Q, Huang, S, Ou, Y, Gao, Y, Tong, T, et al. Immune escape mechanism and vaccine research progress of African swine fever virus. Vaccine. (2022) 10:344. doi: 10.3390/vaccines10030344
37. Ravilov, RK, Rizvanov, AA, Mingaleev, DN, Galeeva, AG, Zakirova, EY, Shuralev, EA, et al. Viral vector vaccines against ASF: problems and prospectives. Front Vet Sci. (2022) 9:830244. doi: 10.3389/fvets.2022.830244
38. Monoldorova, S, Koltsova, G, Titov, I, Yoo, I, Gogin, A, Jeong, J, et al. African swine fever vaccine development: current status and challenges ahead. Thai J Vet Med. (2022) 52:1–12. doi: 10.56808/2985-1130.3186
39. Brake, DA. African swine fever modified live vaccine candidates: transitioning from discovery to product development through harmonized standards and guidelines. Viruses. (2022) 14:2619. doi: 10.3390/v14122619
40. Blome, S, Franzke, K, and Beer, M. African swine fever – a review of current knowledge. Virus Res. (2020) 287:198099. doi: 10.1016/j.virusres.2020.198099
41. Lean, I, Rabiee, AR, Duffield, TF, and Dohoo, IR. Invited review: use of meta-analysis in animal health and reproduction: methods and applications. J Dairy Sci. (2009) 92:3545–65. doi: 10.3168/jds.2009-2140
42. Ntakiyisumba, E, Lee, S, and Won, G. Evidence-based approaches for determining effective target antigens to develop vaccines against post-weaning Diarrhea caused by enterotoxigenic Escherichia coli in pigs: a systematic review and network Meta-analysis. Animals. (2022) 12:2136. doi: 10.3390/ani12162136
43. Paudyal, N, Pan, H, Liao, X, Zhang, X, Li, X, Fang, W, et al. A meta-analysis of major foodborne pathogens in Chinese food commodities between 2006 and 2016. Foodborne Pathog Dis. (2018) 15:187–97. doi: 10.1089/fpd.2017.2417
44. Field, AP, and Gillett, R. How to do a meta-analysis. Br J Math Stat Psychol. (2010) 63:665–94. doi: 10.1348/000711010X502733
45. Lee, S-I, Ntakiyisumba, E, and Won, G. Systematic review and network meta-analysis to compare vaccine effectiveness against porcine edema disease caused by Shiga toxin-producing Escherichia coli. Sci Rep. (2022) 12:6460. doi: 10.1038/s41598-022-10439-x
46. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021):372. doi: 10.1136/bmj.n160
47. Argilaguet, JM, Pérez-Martín, E, Gallardo, C, Salguero, FJ, Borrego, B, Lacasta, A, et al. Enhancing DNA immunization by targeting ASFV antigens to SLA-II bearing cells. Vaccine. (2011) 29:5379–85. doi: 10.1016/j.vaccine.2011.05.084
48. Revilla, Y, Pena, L, and Viñuela, E. Interferon-gamma production by African swine fever virus-specific lymphocytes. Scand J Immunol. (1992) 35:225–30. doi: 10.1111/j.1365-3083.1992.tb02854.x
49. Percie du Sert, N, Hurst, V, Ahluwalia, A, Alam, S, Avey, MT, Baker, M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. (2020) 40:1769–77. doi: 10.1177/0271678X20943823
50. Balduzzi, S, Rücker, G, and Schwarzer, G. How to perform a meta-analysis with R: a practical tutorial. BMJ Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
51. Harrer, M, Cuijpers, P, Furukawa, T, and Ebert, D. Doing meta-analysis with R: A hands-on guide. Boca Raton, FL: Chapman and Hall, CRC (2021).
52. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: R Core Team (2013).
53. Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
54. De Oliveira, MM, Pereira, CR, de Oliveira, IRC, Godfroid, J, Lage, AP, Dorneles, EMS, et al. Efficacy of Brucella abortus S19 and RB51 vaccine strains: a systematic review and meta-analysis. Transbound Emerg Dis. (2022) 69:e32–51. doi: 10.1111/tbed.14259
55. Daly, LE. Confidence limits made easy: interval estimation using a substitution method. Am J Epidemiol. (1998) 147:783–90. doi: 10.1093/oxfordjournals.aje.a009523
56. Mantel, N, and Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22:719–48.
57. Paule, RC, and Mandel, J. Consensus values and weighting factors. J Res Natl Bur Stand. (1982) 87:377–85. doi: 10.6028/jres.087.022
58. Hartung, J, and Knapp, G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. (2001) 20:3875–89. doi: 10.1002/sim.1009
59. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
60. Sabarimurugan, S, Kumarasamy, C, Baxi, S, Devi, A, and Jayaraj, R. Systematic review and meta-analysis of prognostic microRNA biomarkers for survival outcome in nasopharyngeal carcinoma. PLoS One. (2019) 14:e0209760. doi: 10.1371/journal.pone.0209760
61. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
62. Duval, S. The trim and fill method. Publication bias in meta-analysis: Prevention, assessment and adjustments. pp. 127–144. (2005).
63. Rodrigues, AF, Soares, HR, Guerreiro, MR, Alves, PM, and Coroadinha, AS. Viral vaccines and their manufacturing cell substrates: new trends and designs in modern vaccinology. Biotechnol J. (2015) 10:1329–44. doi: 10.1002/biot.201400387
64. Argilaguet, JM, Pérez-Martín, E, López, S, Goethe, M, Escribano, JM, Giesow, K, et al. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antivir Res. (2013) 98:61–5. doi: 10.1016/j.antiviral.2013.02.005
65. Lokhandwala, S, Petrovan, V, Popescu, L, Sangewar, N, Elijah, C, Stoian, A, et al. Adenovirus-vectored African swine fever virus antigen cocktails are immunogenic but not protective against intranasal challenge with Georgia 2007/1 isolate. Vet Microbiol. (2019) 235:10–20. doi: 10.1016/j.vetmic.2019.06.006
66. Netherton, CL, Goatley, LC, Reis, AL, Portugal, R, Nash, RH, Morgan, SB, et al. Identification and immunogenicity of African swine fever virus antigens. Front Immunol. (2019) 10:1318. doi: 10.3389/fimmu.2019.01318
67. Zajac, MD, Trujillo, JD, Yao, J, Kumar, R, Sangewar, N, Lokhandwala, S, et al. Immunization of pigs with replication-incompetent adenovirus-vectored African swine fever virus multi-antigens induced humoral immune responses but no protection following contact challenge. Front Vet Sci. (2023) 10:1208275. doi: 10.3389/fvets.2023.1208275
68. Goatley, LC, Reis, AL, Portugal, R, Goldswain, H, Shimmon, GL, Hargreaves, Z, et al. A Pool of eight virally vectored African swine fever antigens protect pigs against fatal disease. Vaccines (Basel). (2020) 8:234. doi: 10.3390/vaccines8020234
69. Carrascosa, AL, Sastre, I, and Viñuela, E. Production and purification of recombinant African swine fever virus attachment protein p12. J Biotechnol. (1995) 40:73–86. doi: 10.1016/0168-1656(95)00035-O
70. Ruiz-Gonzalvo, F, Rodríguez, F, and Escribano, JM. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology. (1996) 218:285–9. doi: 10.1006/viro.1996.0193
71. Gómez-Puertas, P, Rodríguez, F, Oviedo, JM, Brun, A, Alonso, C, Escribano, JM, et al. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology. (1998) 243:461–71. doi: 10.1006/viro.1998.9068
72. Barderas, MG, Rodríguez, F, Gómez-Puertas, P, Avilés, M, Beitia, F, Alonso, C, et al. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch Virol. (2001) 146:1681–91. doi: 10.1007/s007050170056
73. Neilan, JG, Zsak, L, Lu, Z, Burrage, TG, Kutish, GF, Rock, DL, et al. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology. (2004) 319:337–42. doi: 10.1016/j.virol.2003.11.011
74. Ivanov, V, Efremov, EE, Novikov, BV, Balyshev, VM, Tsibanov, SZ, Kalinovsky, T, et al. Vaccination with viral protein-mimicking peptides postpones mortality in domestic pigs infected by African swine fever virus. Mol Med Rep. (2011) 4:395–401. doi: 10.3892/mmr.2011.454
75. Hua, RH, Liu, J, Zhang, SJ, Liu, RQ, Zhang, XF, He, XJ, et al. Mammalian cell-line-expressed CD2v protein of African swine fever virus provides partial protection against the HLJ/18 strain in the early infection stage. Viruses. (2023) 15:1467. doi: 10.3390/v15071467
76. Argilaguet, JM, Pérez-Martín, E, Nofrarías, M, Gallardo, C, Accensi, F, Lacasta, A, et al. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS One. (2012) 7:e40942. doi: 10.1371/journal.pone.0040942
77. Lacasta, A, Ballester, M, Monteagudo, PL, Rodríguez, JM, Salas, ML, Accensi, F, et al. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J Virol. (2014) 88:13322–32. doi: 10.1128/JVI.01893-14
78. Imatdinov Almaz, R, Kazakova, AS, Šekler, M, Morozova, DY, and Lyska, VM. Immunization of pigs with recombinant plasmids containing genes of ubiquitinated p30, p54 and CD2V proteins of African swine fever virus. Acta Vet Brno. (2020) 70:92–109. doi: 10.2478/acve-2020-0007
79. Sunwoo, SY, Pérez-Núñez, D, Morozov, I, Sánchez, EG, Gaudreault, NN, Trujillo, JD, et al. DNA-protein vaccination strategy does not protect from challenge with African swine fever virus Armenia 2007 strain. Vaccines (Basel). (2019) 7:12. doi: 10.3390/vaccines7010012
80. Jancovich, JK, Chapman, D, Hansen, DT, Robida, MD, Loskutov, A, Craciunescu, F, et al. Immunization of pigs by DNA prime and recombinant vaccinia virus boost to identify and rank African swine fever virus immunogenic and protective proteins. J Virol. (2018) 92:17. doi: 10.1128/JVI.02219-17
81. Bosch-Camós, L, López, E, Collado, J, Navas, MJ, Blanco-Fuertes, M, Pina-Pedrero, S, et al. M448R and MGF505-7R: two African swine fever virus antigens commonly recognized by ASFV-specific T-cells and with protective potential. Vaccines (Basel). (2021) 9:508. doi: 10.3390/vaccines9050508
82. Luong, HQ, HTL, L, Truong, LQ, Nguyen, TN, Vu, HD, Nguyen, HT, et al. Comparative analysis of swine antibody responses following vaccination with live-attenuated and killed African swine fever virus vaccines. Vaccine. (2023) 11:1687. doi: 10.3390/vaccines11111687
83. Munang’andu, HM, Mutoloki, S, and Evensen, Ø. Non‐replicating vaccines. Fish Vaccin. (2014) 1:22–32. doi: 10.1002/9781118806913.ch3
84. Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. (2007) 18:546–56. doi: 10.1016/j.copbio.2007.10.010
85. Vanaparthy, R, Mohan, G, Vasireddy, D, and Atluri, P. Review of COVID-19 viral vector-based vaccines and COVID-19 variants. Infez Med. (2021) 29:328–38. doi: 10.53854/liim-2903-3
86. Zhu, JJ. African swine fever vaccinology: the biological challenges from immunological perspectives. Viruses. (2022) 14:2021. doi: 10.3390/v14092021
87. Fan, YC, Chiu, HC, Chen, LK, Chang, GJJ, and Chiou, SS. Formalin inactivation of Japanese encephalitis virus vaccine alters the antigenicity and immunogenicity of a neutralization epitope in envelope protein domain III. PLoS Negl Trop Dis. (2015) 9:e0004167. doi: 10.1371/journal.pntd.0004167
88. Gaidamakova, EK, Myles, IA, McDaniel, DP, Fowler, CJ, Valdez, PA, Naik, S, et al. Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio- protective Mn2+-peptide complex from Deinococcus. Cell Host Microbe. (2012) 12:117–24. doi: 10.1016/j.chom.2012.05.011
Keywords: African swine fever virus, inactivated, subunit, vaccine efficacy, meta-analysis
Citation: Ntakiyisumba E, Tanveer M, Hirwa F and Won G (2025) Quantitative evaluation of the efficacy of non-replicating vaccines for controlling African swine fever in domestic pigs: a systematic review and meta-analysis. Front. Vet. Sci. 12:1614479. doi: 10.3389/fvets.2025.1614479
Edited by:
Suresh Kumar Tikoo, University of Saskatchewan, CanadaReviewed by:
Tiangang Xu, China Animal Health and Epidemiology Center, ChinaSonu S. Nair, Indian Council of Agricultural Research (ICAR), India
Copyright © 2025 Ntakiyisumba, Tanveer, Hirwa and Won. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gayeon Won, Z3l3b25AamJudS5hYy5rcg==
 Eurade Ntakiyisumba
Eurade Ntakiyisumba Maryum Tanveer
Maryum Tanveer Fabrice Hirwa
Fabrice Hirwa Gayeon Won
Gayeon Won