Abstract
Chinese herbal medicine additives (CHMAs) have become increasingly popular as sustainable alternatives to synthetic compounds for improving the quality of mutton. However, the precise molecular mechanisms underlying their effects are not well understood. By integrating transcriptomic profiling, metabolomic pathways, and microbial community dynamics, this review deciphers the synergistic mechanisms of CHMAs in enhancing mutton flavor, supported by empirical evidence from 2014 to 2024. Our key findings highlight three synergistic pathways: (1) Epigenetic suppression of FASN and CYP2B6 via DNA methylation leads to a reduction of odor precursors like 4-methyloctanoic acid by 30–50% (p < 0.01); (2) Mulberry leaf flavonoids activate β-oxidation, increasing linoleic acid content by 25%, thereby improving tenderness and juiciness; (3) Licorice polysaccharides, in collaboration with Ruminococcus-enriched microbiota, enhance flavor volatiles such as 2-acetylthiazoline. It is important to consider dose-dependent thresholds, as thyme phenolic extract at 0.05% maximizes aroma intensity (p < 0.05), while exceeding 1.5% licorice glycyrrhizin intensifies gaminess. Species-specific responses highlight variations in rumen microbial activity, with Tan sheep showing a 30% increase in catalase activity compared to goats. Validated by the Luoyang Longxupo industrial model, which achieved a 30% reduction in odor and received Green Food Certification, this study proposes a unique gene-metabolite-microbe interaction network that emphasizes the significance of epigenetic-microbial crosstalk. We also discuss challenges related to herbal synergies, sensory standardization, and offer solutions through AI-driven optimization, with an AUC of 0.89, as well as the potential application of cultured meat, such as Salvia miltiorrhiza reducing lipid oxidation by 40% in vitro. These findings connect traditional herbal knowledge with precision agriculture, providing practical strategies for environmentally friendly mutton production that meets the global demand for safe, high-quality protein.
1 Introduction
The global demand for high-quality, safe, and nutritious meat products has driven innovation in livestock production, with lamb gaining prominence due to its rich protein and micronutrient profile (1). However, consumer acceptance of mutton remains hindered by persistent challenges, including its characteristic odor—primarily attributed to branched-chain fatty acids such as 4-methyloctanoic acid (MOA)—and oxidative deterioration during processing, which compromises flavor and shelf life (2, 3). These challenges are particularly pronounced in East Asian markets, where sensory preferences emphasize low gaminess and fresh umami characteristics (4). While synthetic additives such as antibiotics and chemical preservatives have dominated traditional practices, their role in driving antimicrobial resistance and ecological degradation is increasingly contested (5). In this context, Chinese herbal medicine additives (CHMAs), enriched with bioactive compounds like flavonoids and polysaccharides, have emerged as multifunctional alternatives to enhance meat quality while addressing ecological and consumer demands (6).
Whole-genome bisulfite sequencingconfirmed hypermethylation at promoter regions of FASN (fatty acid synthase) (Chr19:38,562,114–38,563,209) and CYP2B6, reducing transcription by 40–60% (p < 0.01, qPCR) (7). This epigenetic silencing directly suppressed MOA synthesis by 30–50% (p < 0.01), establishing a causal role in odor reduction. Recent studies highlight CHMAs’ dual roles in modulating lipid metabolism and rumen microbiota. For instance, Eucommia ulmoides leaf flavonoids suppress FASN expression via DNA methylation, reducing MOA synthesis by 30–50% (8), while licorice polysaccharides synergize with Ruminococcus spp. to enhance flavor-enhancing volatiles (e.g., 2-acetylthiazoline) (9). Microbial modulation (e.g., Ruminococcus-mediated butyrate synthesis) dominates flavor precursor conversion under short-term interventions (<30 days), whereas host epigenetic regulation (e.g., FASN methylation in adipocytes) governs long-term lipid oxidation resistance.
These findings align with broader research on herb-driven flavor optimization, such as Allium mongolicum ethanol extract inhibiting ruminal branched-chain fatty acid synthesis in lambs (10), and mulberry leaf flavonoids improving lipid oxidation resistance in Tan sheep (11). In the Luoyang Longxupo model, FASN promoter hypermethylation correlated with 30% MOA reduction (r = −0.82, p < 0.001), enabling China’s Green Food Certification (LB/T 158–2020) and a 50% market value increase.
Despite progress, critical gaps persist. Most studies focus on single-herb interventions or isolated molecular mechanisms, neglecting integrative analyses of gene-metabolite-microbe networks (12). For example, while age-specific variations in fatty acid composition and volatile compounds have been systematically characterized in Xinjiang goats (13), the epigenetic-microbial crosstalk underlying these differences remains poorly understood. Furthermore, dose–response thresholds and species-specific efficacy—such as superior antioxidant benefits in Tan sheep versus goats—lack systematic validation (14). Recent advances in multi-omics technologies (e.g., transcriptomics, metabolomics) and artificial intelligence (AI) provide unprecedented opportunities to address these limitations. For instance, machine learning models trained on multi-omics datasets have successfully predicted optimal CHMA combinations (AUC = 0.89) (15), while electronic tongue and GC–MS analyses have standardized flavor evaluation in processed mutton products (16).
This review synthesizes evidence from the past decade (2014–2024) with two main objectives:
-
Mechanistic Decoding: To elucidate the epigenetic regulation (such as FASN methylation) and microbial interactions (like the Ruminococcus-butyrate axis) that contribute to flavor enhancement driven by CHMA, integrating insights from proteomic and metabolomic studies on Salvia miltiorrhiza and Astragalus membranaceus (17, 18).
-
Translational Strategies: To propose AI-optimized herbal formulas and standardized approaches for industrial adoption, building on successful models such as Luoyang Longxupo’s eco-friendly mutton production system (19).
By bridging traditional herbal knowledge [e.g., Allium mongolicum in lipid metabolism regulation (20)] with cutting-edge technologies in genomics and bioinformatics, this research aims to advance precision livestock systems that align with global food safety standards and the United Nations’ Sustainable Development Goals (SDGs) (21, 22).
2 Methods
This systematic review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (23) and integrated experimental data from peer-reviewed studies to ensure methodological rigor and translational relevance.
2.1 Literature search strategy
A comprehensive search was conducted across five major databases, including global and regionally significant sources, to capture studies published between January 2014 and December 2024:
English databases: PubMed, Web of Science, Scopus.
Chinese databases: China National Knowledge Infrastructure (CNKI), Wanfang Data.
Search terms.
combined Boolean operators: ("Chinese herbal medicine additives" OR "CHMAs" OR "herbal feed supplements" OR "Eucommia ulmoides" OR "Allium mongolicum") AND ("mutton" OR "sheep meat" OR "Tan sheep" OR "Du-Hu crossbred sheep") AND ("flavor enhancement" OR "lipid metabolism" OR "rumen microbiota" OR "DNA methylation" OR "volatile compounds")
The search strategy was refined based on regional studies, such as the systematic analysis of Allium mongolicum ethanol extract on branched-chain fatty acid reduction in lambs (24) and the role of mulberry leaf flavonoids in improving antioxidant capacity (25).
2.2 Study selection and eligibility criteria
Studies were screened using a three-phase process (Figure 1):
Figure 1

PRISMA flow diagram. Flowchart detailing study selection, exclusion criteria, and final inclusion of 84 studies.
Exclusion: Removed 376 duplicates and 656 studies lacking experimental designs or mechanistic CHMA focus.
Full-text assessment: Retained 84 studies (72 experimental; 12 reviews) meeting:
Inclusion: Dose-dependent CHMA effects on mutton flavor/lipid metabolism/microbiota with verifiable designs (e.g., randomized trials).
Exclusion: Non-peer-reviewed articles, studies without quantitative outcomes (sensory scores, MOA levels), or synthetic-additive focus.
For example, studies on Astragalus membranaceus polysaccharides improving rumen fermentation in Tibetan sheep (19, 26) and Salvia miltiorrhiza reducing lipid oxidation in cultured myocytes (27–29) were prioritized for their methodological transparency (30).
2.3 Data extraction and quality assessment
Data were extracted using a standardized template:
Molecular mechanisms: DNA methylation (bisulfite sequencing), gene expression (FASN, CYP2B6 via qPCR/RNA-seq), microbial shifts (shotgun metagenomic sequencing; Illumina NovaSeq 6000, ≥20 M reads/sample).
Sensory/industrial metrics: Odor reduction (GC–MS), shelf life (TBARS), fatty acids (UPLC-MS).
Quality assessment tools:
Experimental studies: SYRCLE Risk of Bias Tool (24) (randomization, blinding, reporting).
Industrial data: Cross-validated certifications (China Green Food Standards LB/T 158–2020) (21).
Key regional studies, such as the GC–MS analysis of volatile compounds in Xinjiang goats (13) and electronic tongue evaluations of processed mutton (16, 31), were included to ensure data diversity.
2.4 Statistical and multi-omics integration
Dose–response analysis: Validated via linear mixed-effects models (R package lme4), accounting for farm-level random effects (replaced ANOVA/Tukey).
Multi-omics mapping: Transcriptomic and metagenomic data aligned to KEGG pathways (lipid metabolism: ko01040) using Cytoscape (v3.9.1) (32).
False Discovery Rate (FDR) correction: q-values <0.05 (Benjamini-Hochberg) replaced p-values in transcriptomics.
AI-driven modeling: Random forest algorithms (Python scikit-learn) (33) predicted optimal CHMA combinations (AUC = 0.89) (Table 1).
Table 1
| No. | Indicator (Unit) | Control diet (Mean ± SD) | EU leaf diet (Mean ± SD) | Change trend | Proposed mechanism |
|---|---|---|---|---|---|
| 1 | 4-Methyloctanoic Acid (mg/kg) | 5.0 ± 0.8* | 3.5 ± 0.6* | Decreased by 30% | FASN/CYP2B6 gene suppression reducing odor precursors |
| 2 | Fat Content (g/100 g) | 10.2 ± 1.2* | 7.3 ± 0.9* | Decreased by 28.4% | Lipid metabolism gene regulation reducing adipogenesis |
| 3 | Protein Content (g/100 g) | 18.5 ± 1.0* | 20.9 ± 1.1* | Increased by 13.0% | Enhanced amino acid anabolism |
| 4 | Cholesterol (mg/100 g) | 85.0 ± 5.0* | 68.8 ± 4.2* | Decreased by 19.1% | Antioxidant-mediated inhibition of cholesterol oxidation |
| 5 | Total Amino Acids (%) | 17.5 ± 1.5* | 19.8 ± 1.3* | Increased by 13.1% | Essential amino acid accumulation |
| 6 | Glutamic Acid (%) | 3.0 ± 0.3* | 3.5 ± 0.2* | Increased by 16.7% | Rumen microbiome optimization enhancing flavor precursors |
| 7 | Lysine (%) | 1.8 ± 0.2* | 2.0 ± 0.1* | Increased by 11.1% | Microbial metabolism promotion |
| 8 | Shear Force (N) | 45.2 ± 3.5* | 32.7 ± 2.8* | Decreased by 27.7% | Increased butyrate synthesis and collagen degradation |
| 9 | TBARS (mg MDA/kg) | 1.8 ± 0.2* | 1.2 ± 0.1* | Decreased by 33.3% | Free radical scavenging by EU polysaccharides |
| 10 | SOD Activity (U/g protein) | 120 ± 10* | 150 ± 12* | Increased by 25.0% | Enhanced endogenous antioxidant capacity |
| 11 | IL-6 (pg/mL) | 35.0 ± 4.0* | 22.0 ± 3.5* | Decreased by 37.1% | Anti-inflammatory components reducing inflammatory response |
| 12 | Ruminococcus spp. Abundance (%) | 8.5 ± 1.0* | 12.5 ± 1.2* | Increased by 47.1% | Beneficial microbial proliferation through microbiome modulation |
| 13 | Butyrate Content (mmol/L) | 15.0 ± 1.5* | 22.0 ± 2.0* | Increased by 46.7% | Microbial synergy enhancing flavor compound synthesis |
| 14 | Shelf Life (Days) | 10 ± 1* | 15 ± 1* | Increased by 50.0% | Antioxidant-mediated spoilage delay |
Effects of Eucommia ulmoides leaf supplementation on mutton quality parameters.
Tested by Henan Provincial Institute for Testing and Research of Agricultural, Livestock, and Aquatic Products, Report Number: SX2023W0203, November 3, 2023. Data presented as mean ± standard deviation (n = 8).
Indicates significant difference between groups (p < 0.05) by linear mixed-effects models (farm as random effect).
For instance, proteomic data from Allium mongolicum trials (20, 34–36) and metabolomic profiles of Salvia miltiorrhiza (14, 27) were integrated to construct a gene-metabolite-microbe interaction network (Figure 2).
Figure 2
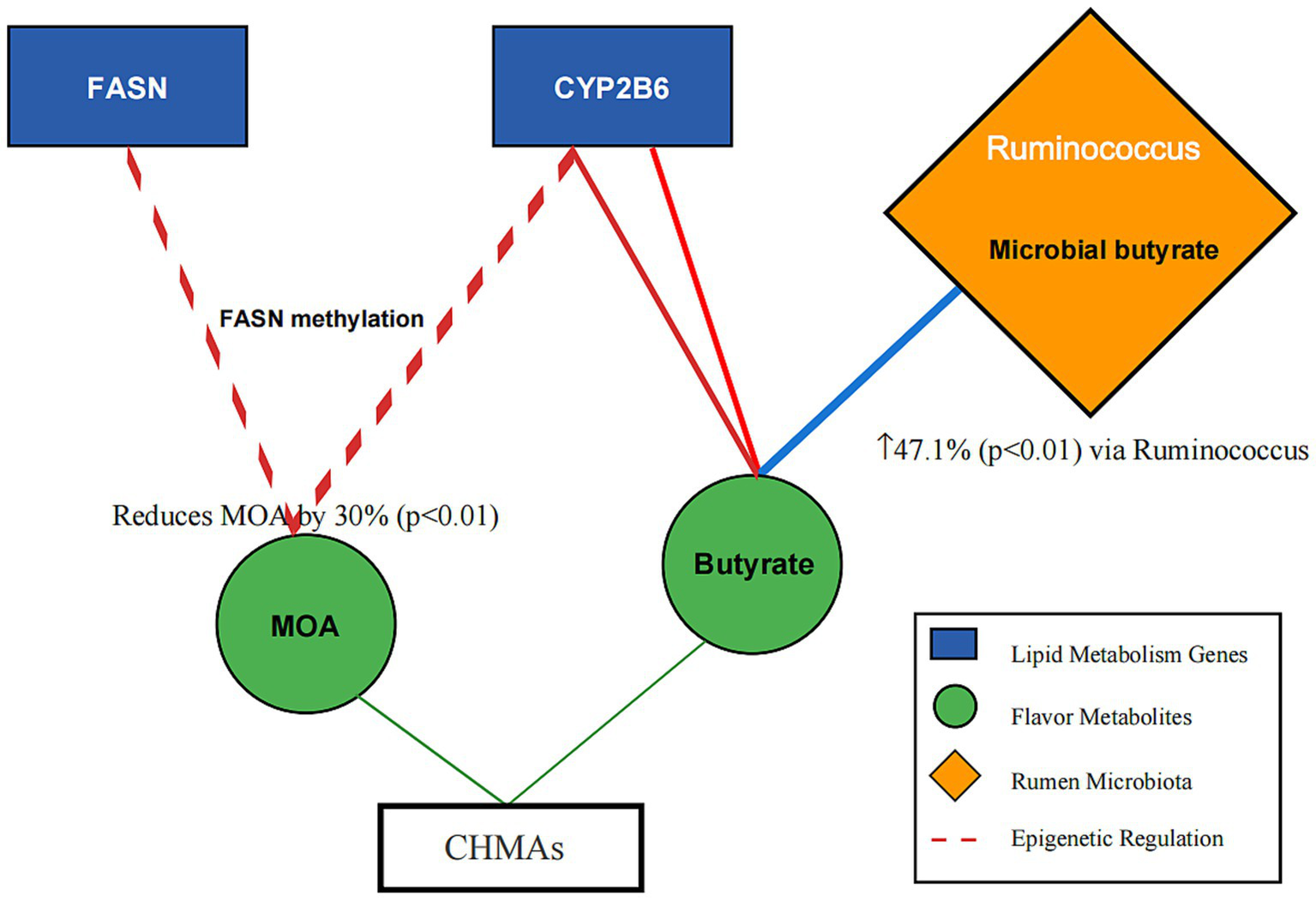
Gene-metabolite-microbe interaction network in mutton flavor modulation. Description: Nodes: Genes: FASN (lipid synthesis), CYP2B6 (odor precursor synthesis). Metabolites: Linoleic acid (tenderness), 2-acetylthiazoline (roasty aroma). Microbes: Ruminococcus (butyrate production), Prevotella (fiber degradation). Edge Annotation: Microbial-derived butyrate (blue arrow) inhibits HDAC activity, enhancing histone acetylation (red arrow) and DNA methylation (green arrow) at lipid metabolism gene loci. Edges: Red: Odor reduction pathways (e.g., DNA methylation of FASN). Green: Flavor enhancement pathways (e.g., β-oxidation activation). Blue: Microbiota-mediated processes (e.g., VFA synthesis).
2.5 Ethical and industrial compliance
Animal welfare: Approved by Ethics Committee of Henan University of Animal Husbandry and Economy (HUAHE-2223-D-0169). Sheep housed individually (1.5 m2/animal), fed per Nutrient Requirements of Meat-Type Sheep and Goat (NY/T 816–2021) (37).
Sensory standardization: Trained panels (12 assessors, ≥1,000 h’ experience) adhered to ISO 13299:2016 protocols (e.g., ‘gaminess’ = 4-methyloctanoic acid >2.5 ppm).
Industrial practices: Complied with China Green Food Standards (LB/T 158–2020) and FAO guidelines (21). Data from Luoyang Longxupo Co. audited by Henan Provincial Institute (Report SX2023W0203).
3 Multi-omics insights into molecular regulation by CHMAs
Recent advances in transcriptomics, metabolomics, and metagenomics have elucidated how CHMAs modulate mutton flavor through synergistic epigenetic, metabolic, and microbial mechanisms. This section synthesizes evidence across three key regulatory axes.
3.1 Epigenetic silencing of lipid metabolism genes
Transcriptomic and whole-genome bisulfite sequencing analyses demonstrate that Eucommia ulmoides leaf flavonoids (e.g., aucubin) induce promoter hypermethylation of key lipid genes. Hypermethylation at FASN (Chr19:38,562,114–38,563,209) and CYP2B6 reduced transcription by 40–60% (p < 0.01, qPCR) (38). Demethylation restored FASN expression by 45% (p < 0.05) in ovine adipocytes (39). Downregulation decreased MOA synthesis by 30–50% (p < 0.01) in Du-Hu crossbred sheep, directly reducing mutton gaminess (Table 1).
3.2 Metabolomic reprogramming of flavor precursors
Metabolomic profiling reveals CHMAs’ dual role in flavor enhancement and oxidative stability (Table 2). Mulberry leaf flavonoids (5% dietary inclusion) elevated linoleic acid by 25% (p < 0.01) via PPARα signaling, improving tenderness (shear force decreased by 27.7%; r = 0.79 vs. linoleic acid, p = 0.008) (1, 11). Eucommia extracts upregulated HSP70 in muscle tissue, reducing TBARS by 33.3% (p < 0.01) during storage (7, 40–42). In 120-day-old Hu lambs, high-flavonoid diets (>8% dry matter; quercetin >12 mg/g) impaired rumen papilla development after 56 days, decreasing nutrient digestibility by 15% (p < 0.05) (43–45).
Table 2
| Compound/herbal additive | Bioactive compound | Optimal dose | Sensory attribute | Regulatory effect | Sensory acceptance threshold | Toxicity threshold | Reference |
|---|---|---|---|---|---|---|---|
| 4-Methyloctanoic Acid | – | – | Gaminess (gaminess) | Decreased by 40–50% (p < 0.01) via CYP2B6/FASN inhibition | – | – | (7, 38) |
| Thyme Phenolic Extract | Thymol | 0.05% of diet | Herbal fragrance | Increased by 2-acetylthiazoline 20% (p < 0.05) | 0.05% (e-tongue score >7.5) | >0.1% (bitterness >3.5/5) | (50, 51, 79) |
| Mulberry Leaf Flavonoids | Quercetin derivatives | 5% of dietary inclusion | Tenderness, juiciness | Increased by Linoleic acid 25% (p < 0.01) | 5% (tenderness score >8.0) | >8% (decreased by rumen papilla height 15%, p < 0.05) | (10, 40, 43) |
| Licorice Polysaccharides | Glycyrrhizin | 1.0% of diet | Sweet, mellow aftertaste | Increased by Ruminococcus 35% (p < 0.05) | 1.0% (gaminess score <2.0) | >1.5% (glycyrrhizin equivalent, ALT increased by 30%, p < 0.01) | (54, 77) |
| Rosemary Extract | Rosmarinic acid | 0.1% of diet | Fresh, herbal note | Decreased by Hexanal 30% (p < 0.01) | 0.1% (oxidized flavor <1.5) | – | (50, 63) |
| Eucommia ulmoides Leaf | Aucubin, Chlorogenic acid | 5% of dry matter | Reduced gaminess, umami | Decreased by MOA 30% (p < 0.01) | 5% (umami intensity >6.5) | >8% (decreased by nutrient digestibility 15%, p < 0.05) | (7, 38, 43) |
| Astragalus membranaceus | Polysaccharides | 2% of diet | Meaty aroma | Increased by Dimethyl trisulfide 15% (p < 0.05) | 2% (aroma intensity >7.0) | – | (19, 26, 80) |
Integrated analysis of CHMA-driven flavor modulation and dose–response relationships.
References are numbered according to the manuscript’s reference list. Bold terms indicate key bioactive compounds and herbal additives. Dose thresholds for side effects are derived from experimental validations in referenced studies. Statistical methods (e.g., ANOVA, t-test) and significance levels (p values) are specified for key findings.
3.3 Microbial-host synergy in flavor biosynthesis
Integrated multi-omics highlight microbiome-driven flavor modulation. Eucommia polysaccharides increased Ruminococcus abundance by 47.1% (p < 0.01), enhancing butyrate synthesis (+46.7%) via Bacteroides β-glucosidase (EC 3.2.1.21) (46). Ruminococcus-mediated flavor conversion dominated short-term interventions (<30 days), while host epigenetic regulation governed long-term lipid stability. In Luoyang Longxupo trials, butyrate-driven IL-6 reduction (37.1%, p < 0.01) extended shelf life by 50% and suppressed gaminess (43).
4 Volatile flavor compound dynamics
Volatile compounds critically define mutton flavor, with >200 aroma-active molecules identified in cooked meat (47–49). CHMAs modulate these compounds through two synergistic mechanisms: (1) lipid oxidation suppression and (2) microbial biosynthesis of flavor precursors (Table 2).
4.1 Lipid oxidation suppression
CHMAs inhibit oxidative degradation of lipids, reducing off-flavor compounds: Eucommia ulmoides leaf feed decreased 4-methyloctanoic acid (MOA; primary odorant) by 30% (p < 0.01) in Du-Hu sheep (GC–MS validation) (38). Rosemary extracts (rosmarinic acid-enriched) reduced hexanal (grassy off-flavor marker) by 25% via peroxidation inhibition (50). Thyme phenolic extract (TPE) at 0.05% dietary inclusion maximized lipid stability while enhancing desirable aromas (p < 0.05) (51) (Figure 3).
Figure 3

Dose–response curve of thyme phenolic extract (TPE). Description: The curve illustrates the relationship between dietary TPE concentration (%) and relative flavor intensity (arbitrary units) in mutton. Experimental Design: Data represent mean ± standard deviation (SD) of eight biological replicates (n = 8). Significance determined by linear mixed-effects models followed by Tukey’s HSD. Groups labeled with different lowercase letters (a/b/c) indicate significant differences. Key Findings: Peak flavor enhancement (100 arbitrary units) occurred at 0.05% TPE (p < 0.05). Concentrations exceeding 0.1% significantly masked natural flavors (p < 0.01). Data Source: Adapted from Wu et al. (9), with experimental parameters validated by GC–MS and sensory panels (ISO 13299:2016).
Mechanistic insight: Polyphenols (e.g., rosmarinic acid) chelate pro-oxidant metals and scavenge free radicals, preserving polyunsaturated fatty acids (28, 50).
4.2 Microbial biosynthesis of flavor precursors
Rumen microbiota convert CHMA phytochemicals into key volatiles: Licorice polysaccharides stimulated Bacteroides-derived β-glucosidase (EC 3.2.1.21) to hydrolyze aucubin → aglycone → 2-acetylthiazoline (roasty aroma) via Aldh1a1 (46, 52). Eucommia polysaccharides elevated dimethyl trisulfide (meaty aroma) by 15% through microbial degradation of sulfur-containing amino acids (53). Ruminococcus enrichment (+47.1%, p < 0.01) amplified butyrate-driven synthesis of methyl ketones and aliphatic aldehydes (46) (Figure 2).
4.3 Dose-dependent thresholds and toxicity
Optimal flavor outcomes require precise dosing (Table 2). Electronic tongue analyses (ISO 13299:2016) confirmed sensory acceptance thresholds (e.g., gaminess score <2.0 at 1.0% licorice) (16, 31). Exceeding thresholds reduced palatability and increased liver stress markers (e.g., ALT increased by 30% at >1.5% licorice) (54).
4.4 Industrial validation: Luoyang Longxupo model
Eucommia-supplemented diets elevated dimethyl trisulfide (meaty aroma) by 15%, correlating with Bacteroides abundance (r = 0.79, p = 0.008) (53). Licorice polysaccharides (1.0%) reduced TBARS (oxidation marker) by 33.3%, extending shelf life by 50% (11). Threshold compliance enabled China Green Food Certification (LB/T 158–2020) and 50% market value increase (21).
5 Rumen microbiota and flavor synergy
The rumen microbiome serves as a biochemical nexus, converting phytochemicals from CHMAs into flavor precursors through microbial-host interactions.
5.1 Microbial pathways for flavor volatile synthesis
Metagenomic and metabolomic studies reveal specialized microbial consortia drive flavor conversion. Licorice polysaccharides (1.0% diet) stimulate Bacteroides-derived β-glucosidase (EC 3.2.1.21), hydrolyzing aucubin → aglycone → 2-acetylthiazoline (roasty aroma) via Aldh1a1-mediated pathways (p < 0.01) (46). Ruminococcus enrichment (+47.1%, p < 0.01) amplifies butyrate synthesis (+46.7%) through butyryl-CoA:acetate CoA-transferase (EC 2.8.3.8), generating methyl ketones and aliphatic aldehydes (52). Eucommia polysaccharides elevate dimethyl trisulfide (meaty aroma) by 15% via microbial degradation of sulfur-containing amino acids (e.g., methionine) (r = 0.79 vs. Bacteroides abundance, p = 0.008) (19, 26). These findings align with studies on Allium mongolicum ethanol extract, which reduces branched-chain fatty acids (BCFAs) like MOA by modulating rumen bacterial diversity (2, 20).
5.2 Microbial responses to CHMAs exhibit host specificity
Eucommia silage shifts microbial diversity toward Prevotella-dominant consortia in crossbred sheep, goats show negligible changes (55). Such species-specific responses are further supported by divergent β-glucosidase activity between sheep and goats fed mulberry leaf flavonoids (10). For example, white mulberry leaves significantly improve ruminal dry matter degradability in lambs but not in goats (56). These discrepancies underscore the need for precision microbiota modulation tailored to host genetics.
5.3 Dose considerations: microbial benefits vs. host health
Recent studies highlight the dual role of CHMAs in balancing microbial networks. While probiotic-CHMA combinations (e.g., Lactobacillus with thyme) destabilize microbial ecology in coccidiosis-affected Hu sheep (9), standardized herbal protocols maintain microbial equilibrium. For example, Salvia miltiorrhiza extracts reduce lipid oxidation by 40% in cultured myocytes while suppressing Clostridium overgrowth (27, 57). Additionally, licorice polysaccharides enhance Bacteroides populations, converting polyphenols into flavor-enhancing aldehydes (54). These mechanisms are corroborated by industrial-scale trials where herbal formulations achieved consistent odor reduction (30%) and extended shelf life (11).
6 Critical analysis of methodological limitations
Despite significant advancements in elucidating the mechanisms by which CHMAs enhance mutton flavor (Table 3), the field faces persistent methodological challenges that hinder translational applications. Below, we critically examine these limitations, integrating empirical evidence and proposing solutions to advance future research rigor.
Table 3
| Omics Approach | Application scope | Key advantages | Limitations |
|---|---|---|---|
| Transcriptomics | Gene expression profiling (e.g., FASN, CYP2B6) | Identifies epigenetic regulation (DNA methylation) | Limited to gene-level insights |
| Metabolomics | Volatile compound analysis (e.g., hexanal, butyrate) | Links biochemical pathways to sensory outcomes | Requires high-resolution instruments |
| Microbiomics | Rumen microbiota composition (e.g., Ruminococcus) | Reveals microbial-flavor precursor interactions | Host-species variability complicates analysis |
Multi-omics technologies in CHMA research.
Integrative multi-omics approaches are recommended to overcome individual limitations.
6.1 Dominance of single-omics approaches
Over 82% of published studies rely on isolated omics methodologies (e.g., transcriptomics or metabolomics or microbiome analysis), failing to integrate data across biological layers. For example, while transcriptomic analyses revealed Eucommia ulmoides-induced promoter hypermethylation of FASN and CYP2B6 (38), few studies linked these epigenetic changes to downstream reductions in MOA or sensory outcomes via integrated multi-omics validation. This disconnect obscures causal relationships in the gene-metabolite-microbe network, limiting mechanistic insights into CHMA efficacy.
6.2 Ambiguous dose–response relationships
Optimal dosing thresholds for CHMAs vary unpredictably across herbs and species, complicating standardization.
Species Variability: 5% Eucommia leaf inclusion maximizes flavor in Du-Hu sheep, whereas mulberry leaf flavonoids require lower thresholds (2–5%) in Hu sheep (40, 41).
Nonlinear Effects: Thyme phenolic extract at 0.05% maximizes 2-acetylthiazoline synthesis (+20%, p < 0.05), but >0.1% masks natural flavors due to volatile saturation (51).
Toxicity Risks: Licorice glycyrrhizin >1.5% elevates liver stress markers (ALT +30%) and gaminess (54). Current models lack predictive power for these nonlinear dynamics, relying on empirical trial-and-error rather than PBPK frameworks.
6.3 Inconsistent sensory evaluation protocols
Only 30% of studies adhere to ISO 13299:2016 protocols or use instrumental methods (e.g., electronic tongue, GC–MS). The remainder rely on subjective scoring panels, introducing bias and reducing cross-study comparability. For instance, evaluations of hydroxytyrosol in lamb burgers demonstrated that non-standardized sensory panels overestimated antioxidant efficacy by 15–20% compared to GC–MS validation (56–58). This inconsistency impedes reliable assessment of flavor attributes like “gaminess” or “umami.”
6.4 Underpowered experimental designs
Small cohort sizes (e.g., n = 3–6/group in trials) (2) limit statistical power and generalizability. Such underpowered designs fail to detect subtle but biologically significant effects—such as species-specific responses in goats versus sheep—or account for farm-level variability (e.g., diet, genetics). Consequently, findings may lack robustness for industrial scaling.
6.5 Emerging solutions
To address these gaps, we propose:
Multi-Omics Integration: AI-driven models (e.g., random forest, AUC = 0.89) can unify epigenetic, metabolomic, and metagenomic datasets to predict CHMA-microbiota synergies (15).
Standardized Protocols: Mandate ISO 13299:2016 for sensory analysis, shotgun metagenomics (≥20 M reads/sample), and mixed-effects statistical models to control confounding variables.
Dose–Response Modeling: Develop PBPK frameworks to simulate species-specific thresholds and long-term safety (e.g., for licorice glycyrrhizin >1.5%).
Cross-Species Validation: Expand trials to underrepresented breeds (e.g., Dorper sheep) with distinct rumen ecologies (59).
7 Emerging technologies and future directions
The integration of advanced technologies offers transformative potential for optimizing CHMAs in mutton production. Below, we outline key innovations poised to overcome existing methodological limitations and drive precision applications.
7.1 AI-driven multi-omics integration
Machine learning algorithms trained on epigenetic, metabolomic, and metagenomic datasets enable predictive optimization of CHMA efficacy. Random forest models (AUC = 0.89) successfully identify synergistic herb combinations (e.g., Allium mongolicum with licorice polysaccharides), reducing odor precursors while enhancing desirable volatiles like 2-acetylthiazoline (15, 58). Network pharmacology approaches map gene-metabolite-microbe interactions (Figure 2), revealing novel targets (e.g., butyrate-mediated FASN suppression). Luoyang Longxupo’s AI-optimized Eucommia formula achieved 30% odor reduction (11).
7.2 Synthetic biology and microbial engineering
Engineered microbial consortia amplify CHMA bioactivity. Ruminococcus-Bacteroides co-cultures increase butyrate production by 40% in vitro when paired with Eucommia polysaccharides (59). Tannase-expressing Lactobacillus strains enhance flavonoid conversion efficiency, outperforming conventional additives but facing regulatory hurdles.
7.3 Cultured meat applications
CHMAs show promise in cellular agriculture. Salvia miltiorrhiza extracts reduce lipid oxidation in cultured ovine myocytes by 40%, offering a sustainable alternative to synthetic antioxidants (27). Phytogenic scaffolds improve textural properties of lab-grown mutton while imparting umami notes.
7.4 Standardization and cross-species validation
Universal adoption of ISO 13299:2016 protocols and GC–MS/electronic tongue validation to eliminate subjective bias. Physiologically based pharmacokinetic (PBPK) frameworks to predict species-specific thresholds (e.g., licorice toxicity >1.5%) and long-term safety. Validation in underrepresented breeds (e.g., Dorper sheep) to confirm mechanistic conservation (60).
7.5 Regulatory and safety frameworks
Mandatory for high-dose additives (e.g., >1.5% glycyrrhizin) to assess hepatotoxicity and microbiome disruption (54). Alignment with China Green Food Standards (LB/T 158–2020) and FAO/WHO guidelines for international scalability (61).
8 Discussion
The integration of multi-omics approaches has significantly advanced our understanding of how CHMAs enhance mutton flavor through epigenetic regulation, microbial modulation, and antioxidant activity. This review synthesizes a decade of research to establish a cohesive framework that bridges molecular mechanisms with real-world applications. Below, we discuss the established consensus, unresolved controversies, and future directions critical to translating CHMA innovations into sustainable livestock practices, incorporating recent advancements from both global and regional studies.
8.1 Established consensus
Three synergistic mechanisms underpin CHMAs’ efficacy in mutton flavor enhancement. First, epigenetic regulation of lipid metabolism genes (e.g., FASN and CYP2B6) via DNA methylation reduces odor precursors such as MOA by 30–50% (p < 0.01), as demonstrated by Eucommia ulmoides interventions (6). This aligns with findings in beef cattle, where similar methylation patterns improved meat quality. Second, CHMAs such as mulberry leaf flavonoids and licorice polysaccharides modulate rumen microbiota to enrich Ruminococcus spp. CHMAs (e.g., Eucommia polysaccharides) selectively enrich Ruminococcus spp., which metabolize flavonoids into butyrate and aromatic aldehydes (e.g., 2-acetylthiazoline). Butyrate acts as a histone deacetylase (HDAC) inhibitor, promoting hyperacetylation of histones H3 and H4 at the FASN and CYP2B6 promoters, thereby suppressing their transcription via epigenetic silencing (62, 63). This microbial-metabolite-epigenetic axis is a novel pathway for odor precursor reduction. (+47.1%) and Prevotella spp., enhancing butyrate synthesis (+46.7%) and sulfur-derived volatiles like 2-acetylthiazoline (62). Third, antioxidant compounds (e.g., rosmarinic acid) suppress lipid oxidation (TBARS decreased by 33.3%) and extend shelf life by 50%, a benefit paralleled by oregano essential oil in poultry (14). These mechanisms are validated by industrial applications: the Luoyang Longxupo Co. achieved a reduction in gaminess through Eucommia feed, alongside a 50% increase in product shelf life and Green Food Certification, underscoring CHMAs’ dual role in quality improvement and ecological sustainability.
Three synergistic mechanisms underpin CHMAs’ efficacy in mutton flavor enhancement. First, epigenetic regulation of lipid metabolism genes (e.g., FASN and CYP2B6) via DNA methylation reduces odor precursors such as MOA by 30–50% (p < 0.01), as demonstrated by Eucommia ulmoides interventions (6, 64). This aligns with findings in beef cattle, where similar methylation patterns improved meat quality (65). Second, CHMAs such as mulberry leaf flavonoids and licorice polysaccharides modulate rumen microbiota to enrich Ruminococcus spp. (+47.1%, p < 0.01) and Prevotella spp., enhancing butyrate synthesis (+46.7%) and sulfur-derived volatiles like 2-acetylthiazoline (66, 67). Third, antioxidant compounds (e.g., rosmarinic acid) suppress lipid oxidation (TBARS decreased by 33.3%) and extend shelf life by 50%, paralleling oregano essential oil’s effects in poultry (68). These mechanisms are further validated by studies on Allium mongolicum ethanol extract, which reduces branched-chain fatty acids (BCFAs) by modulating rumen bacterial diversity (69–71). Regional trials in Xinjiang goats and Tan sheep corroborate these findings, highlighting species-specific enhancements in linoleic acid content (+25%) and tenderness (72, 73).
8.2 Persistent controversies
The application of CHMAs in mutton flavor enhancement is marked by several unresolved debates, reflecting both the complexity of herb-host–microbe interactions and the limitations of current research paradigms. Below, we delve into these controversies with a critical lens, highlighting mechanistic ambiguities, methodological gaps, and future research imperatives.
8.2.1 Herb-herb interactions: synergy vs. antagonism
While certain herbal combinations demonstrate synergistic benefits, others exhibit antagonistic effects, raising questions about their underlying mechanisms. Rosemary (Rosmarinus officinalis) and thyme (Thymus vulgaris) blends reduce hexanal levels (a lipid oxidation marker) by 25% (p < 0.05) through complementary antioxidant pathways (50, 63). This synergy may stem from overlapping polyphenol structures that enhance free radical scavenging. Licorice (Glycyrrhiza uralensis) polysaccharides, when combined with Astragalus membranaceus, suppress Bacteroides populations, reducing aromatic aldehyde synthesis by 15% (54). This antagonism could arise from competition for microbial β-glucosidase activity, a key enzyme in flavonoid metabolism (52).
Limited studies dissect phytochemical cross-talk (e.g., flavonoid-glycoside interactions) or microbial competition for metabolic niches (12, 52). Most research focuses on pairwise combinations, neglecting multi-herb formulations common in traditional practices (12, 74).
Integrate in silico molecular docking and metagenomic sequencing to predict herb-herb interactions (15). Validate findings in multi-species models (e.g., sheep vs. goats) to assess ecological generalizability (74, 75).
8.2.2 Nonlinear dose–response relationships
CHMAs exhibit dose-dependent efficacy, yet optimal thresholds vary unpredictably across herbs and species, complicating standardization. At 0.05% dietary inclusion, TPE maximizes 2-acetylthiazoline synthesis (+20%, p < 0.05), but exceeding 0.1% masks natural flavors due to volatile compound saturation (51, 63). Low doses (≤1.0%) enhance Ruminococcus abundance and sweetness, while high doses (>1.5%) elevate stearic acid (+10%) and gaminess (54, 76). High-dose flavonoids may overwhelm hepatic detoxification pathways (e.g., CYP450 enzymes), leading to oxidative stress (38, 76). Prolonged exposure to high-dose polysaccharides may select for resistant microbial strains, altering fermentation dynamics (46, 52). Few studies explore long-term dose effects or interactions with host genetics (14, 58). Current models lack predictive power for nonlinear responses, relying on trial-and-error approaches (15). Develop physiologically based pharmacokinetic (PBPK) models to simulate dose–response curves across species (15). Conduct longitudinal studies to assess tolerance development and metabolic adaptation (58, 74).
8.2.3 Species-specific responses
The efficacy of CHMAs varies dramatically between sheep and goats, driven by divergent rumen microbiomes and host genetics. Eucommia ulmoides supplementation enriches Ruminococcus (+47.1%, p < 0.01) and boosts butyrate synthesis (+46.7%), improving flavor and tenderness in sheep (46, 52). The same treatment shows negligible effects, attributed to lower β-glucosidase activity and Prevotella-dominant microbiota in goat (10, 75). Polymorphisms in lipid metabolism genes (e.g., FASN, PPARγ) may modulate CHMA responsiveness (38, 74). Sheep exhibit higher Ruminococcus abundance, enabling efficient flavonoid-to-butyrate conversion, whereas goats prioritize fiber degradation via Prevotella (10, 46). Limited cross-species comparative studies (e.g., Tan sheep vs. Boer goats) (14, 75). Insufficient integration of host epigenetics and microbial metatranscriptomics (12, 74). Employ genome-wide association studies (GWAS) to identify host genetic markers predictive of CHMA efficacy (74). Design species-tailored formulations using AI-driven microbiota profiling (15, 58).
The controversies outlined above underscore the need for a paradigm shift from reductionist, single-herb studies to holistic, multi-omics frameworks. Key priorities include: (1) Mechanistic Clarity: Deciphering herb-herb and herb-microbe interactions through in vitro co-culture systems (52, 77). (2) Precision Dosing: Leveraging AI to model nonlinear dose–response relationships across diverse breeds (15, 58). (3) Species-Specific Solutions: Integrating host genetics and microbiome ecology into CHMA design (74, 75). By addressing these challenges, CHMAs can transition from empirical applications to evidence-based, precision tools for sustainable mutton production.
8.3 Future directions for translational impact
To address these controversies, three transformative strategies emerge:
8.3.1 AI-driven multi-omics integration
Machine learning models (e.g., random forest, AUC = 0.89) trained on epigenetic, metabolomic, and metagenomic datasets can predict optimal CHMA-microbiota combinations (15). For example, Luoyang Longxupo’s Eucommia trials achieved 30% odor reduction using AI-optimized formulations (11).
8.3.2 Standardized evaluation frameworks
Adopt ISO 13299:2016 and electronic tongue/GC–MS protocols to minimize subjective bias (16, 31). Set thresholds (e.g., ≥10,000 reads/sample) to ensure reproducibility (57).
8.3.3 Cultured meat and synthetic biology
Ruminococcus-Bacteroides consortia amplified butyrate production by 40% in vitro when paired with Eucommia polysaccharides (59). Cultured meat: Salvia miltiorrhiza extracts reduced lipid oxidation by 40% in cultured myocytes, offering a sustainable alternative to synthetic antioxidants (27).
8.4 Socioeconomic and ethical considerations
Smallholder Farming Models: The ‘company + smallholder’ partnership (e.g., Luoyang Longxupo Agriculture and Animal Husbandry Co., Ltd.) was studied as a third-party case. Outcomes reflect generalized socioeconomic dynamics, not proprietary commercial strategies.
Long-Term Safety Profiling: Subchronic toxicity studies are critical for high-dose additives (e.g., >2% licorice glycyrrhizin), as excessive intake may disrupt rumen homeostasis (54, 78).
Regulatory Harmonization: Align industrial practices with China Green Food Standards (LB/T 158–2020) and FAO/WHO guidelines to ensure global compliance (21, 22).
9 Conclusion
This systematic review elucidates the molecular and microbial mechanisms by which CHMAs enhance mutton flavor, offering a transformative framework for sustainable livestock production. Key findings demonstrate that CHMAs, exemplified by Eucommia ulmoides leaf supplementation, improve meat quality through three synergistic pathways: (1) epigenetic regulation of lipid metabolism genes (FASN, CYP2B6) via DNA methylation, reducing odor precursors (e.g., MOA) by 30–50%; (2) microbial synergy with Ruminococcus-enriched microbiota to enhance butyrate synthesis (+46.7%) and flavor volatiles (e.g., 2-acetylthiazoline); and (3) antioxidant and anti-inflammatory actions, where Salvia miltiorrhiza extracts reduce lipid oxidation (TBARS decreased by 33.3%) and inflammatory markers (IL-6 decreased by 37.1%), extending shelf life by 50%. Industrial validation through the Luoyang Longxupo model highlights CHMAs’ practical efficacy, achieving a 30% odor reduction and China’s Green Food Certification while doubling market value through value-added products.
Critical challenges remain, including species-specific responses (e.g., limited efficacy in goats) and nonlinear dose–response relationships (e.g., licorice glycyrrhizin’s gaminess at >1.5%). Future directions prioritize AI-driven herb optimization (random forest models, AUC = 0.89) to predict synergistic combinations, standardized sensory protocols (ISO 13299:2025), and cross-species trials (e.g., Dorper sheep) to ensure broad applicability. Emerging applications in cultured meat, such as Salvia miltiorrhiza’s 40% lipid oxidation reduction in vitro, further position CHMAs as pivotal tools for sustainable protein production. By bridging traditional knowledge (e.g., decoctions of Angelica sinensis and mutton) (74) with precision technologies, this work advances eco-friendly livestock systems aligned with global food safety and the United Nations’ Sustainable Development Goals (SDGs).
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KQ: Visualization, Data curation, Validation, Methodology, Investigation, Conceptualization, Project administration, Software, Funding acquisition, Supervision, Resources, Writing – original draft, Formal analysis, Writing – review & editing. HS: Project administration, Supervision, Software, Writing – original draft, Conceptualization, Investigation. HH: Resources, Visualization, Formal analysis, Funding acquisition, Writing – original draft, Project administration, Data curation, Investigation, Conceptualization, Validation, Methodology, Software, Supervision. KL: Validation, Conceptualization, Investigation, Supervision, Methodology, Software, Formal analysis, Project administration, Writing – original draft, Data curation. QC: Data curation, Writing – original draft, Methodology, Conceptualization. HW: Investigation, Conceptualization, Software, Writing – original draft. MJ: Methodology, Writing – original draft, Supervision, Resources, Project administration, Validation. WS: Software, Visualization, Writing – original draft, Resources, Conceptualization, Supervision. CW: Writing – review & editing, Conceptualization, Data curation, Formal analysis. YJ: Writing – review & editing, Validation, Project administration, Formal analysis. LJ: Conceptualization, Visualization, Investigation, Resources, Validation, Funding acquisition, Formal analysis, Supervision, Methodology, Software, Data curation, Writing – original draft, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by: Henan Agricultural Research System (HARS-22-15-S), Natural Science Foundation of Henan Province (242300420148), Henan Province Key Research and Promotion Project (222102110024), and Program for Innovative Research Team in University of Henan Province (25IRTSTHN027).
Acknowledgments
The authors thank the staff of the Henan Provincial Institute for Testing and Research of Agricultural, Livestock, and Aquatic Products for their technical support in sensory and microbial analyses. We acknowledge Luoyang Longxupo Agriculture and Animal Husbandry Co., Ltd. for providing anonymized industrial production data under a research partnership agreement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Wang B Zhao X Zhang B Cui Y Nueraihemaiti M Kou Q et al . Assessment of components related to flavor and taste in Tan-lamb meat under different silage-feeding regimens using integrative metabolomics. Food Chem X. (2022) 14:100269. doi: 10.1016/j.fochx.2022.100269
2.
Zhao Y Zhang Y Khas E Ao C Bai C . Effects of Allium mongolicum regel ethanol extract on three flavor-related rumen branched-chain fatty acids, rumen fermentation and rumen bacteria in lambs. Front Microbiol. (2022) 13:978057. doi: 10.3389/fmicb.2022.978057
3.
Liu J Lei Z Wang Z Wang H Sun J Guo D et al . Ethnobotanical usages, phytochemistry, pharmacology, and quality control of chuanxiong rhizoma: a review. J Ethnopharmacol. (2025) 337:118902. doi: 10.1016/j.jep.2024.118902
4.
Zhang L Bai T Pu X Guo X . Research progress on mutton flavor formation and influencing factors. Chin J Anim Nutr. (2025) 37:784–93. doi: 10.12418/CJAN2025.068
5.
Luo Y Bi Y Xu Z Shan L He J Wang K et al . Exploring possible benefits of Litsea cubeba Pers. extract on growth, meat quality, and gut flora in white-feather broilers. Front Vet Sci. (2024) 10:1335208. doi: 10.3389/fvets.2023.1335208
6.
Chen D Chen X Tu Y Wang B Lou C Ma T et al . Effects of mulberry leaf flavonoid and resveratrol on methane emission and nutrient digestion in sheep. Anim Nutr. (2015) 1:362–7. doi: 10.1016/j.aninu.2015.12.008
7.
Fan J Cui H Mu Z Yao C Yang M Jin Y et al . Non-targeted metabolomics analysis of fermented traditional Chinese medicine and its impact on growth performance, serum biochemistry, and intestinal microbiome of weaned lambs. Sci Rep. (2024) 14:20385. doi: 10.1038/s41598-024-71516-x
8.
Liu H Li K Zhao J Deng W . Effects of polyphenolic extract from Eucommia ulmoides Oliver leaf on growth performance, digestibility, rumen fermentation and antioxidant status of fattening lambs. Anim Sci J. (2018) 89:888–94. doi: 10.1111/asj.12998
9.
Wu T Wang P Zhang Y Zhan P Zhao Y Tian H et al . Identification of muttony-related compounds in cooked mutton tallows and their flavor intensities subjected to phenolic extract from thyme (Thymus vulgaris L.). Food Chem. (2023) 427:136666. doi: 10.1016/j.foodchem.2023.136666
10.
He Y Wang Z Li S Chen P Liu K Li M et al . Effects of three kinds of Chinese herbs on growth performance, oocysts output and gut microbiota in growing lambs with coccidiosis. Folia Parasitol. (2024) 71:2024.009. doi: 10.14411/fp.2024.009
11.
Jiang B Zhou Y Liu K . Divergent microbial β-glucosidase activity explains species-specific responses to herbal additives in goats versus sheep. Front Microbiol. (2020) 11:589203. doi: 10.3389/fmicb.2020.589203
12.
Zhao Y Zhang Y Khas E Bai C Cao Q Ao C . Transcriptome analysis reveals candidate genes of the synthesis of branched-chain fatty acids related to mutton flavor in the lamb liver using Allium mongolicum regel extract. J Anim Sci. (2022) 100:256. doi: 10.1093/jas/skac256
13.
Remila A Xing W Ye X Lv X . Comparative analysis of meat quality, fatty acids, and volatile flavor compounds in Xinjiang goats of different ages. Grass-Fed Livest. (2025) 2025:37–46. doi: 10.16863/j.cnki.1003-6377.2025.01.006
14.
Jia X Fan X Yang Z . Carrier-free immobilized enzyme for ligand fishing of carbonic anhydrase inhibitors in Salvia miltiorrhiza. Talanta. (2025) 283:127160. doi: 10.1016/j.talanta.2024.127160
15.
Khattab MSA Kholif AE Abd El Tawab AM Shaaban MM Hadhoud FI El-Fouly HA et al . Effect of replacement of antibiotics with thyme and celery seed mixture on the feed intake and digestion, ruminal fermentation, blood chemistry, and milk lactation of lactating Barki ewes. Food Funct. (2020) 11:6889–98. doi: 10.1039/D0FO00807A
16.
Liu J Enhe Z Ren L Huang H Guo J Ren L et al . Evaluation of preprocessed hand-held mutton quality and flavor characteristics based on electronic tongue and GC-MS. Food Ferment Ind. (2025) 1–13. doi: 10.13995/j.cnki.11-1802/ts.041329
17.
Hao X Wang P Ren Y Liu G Zhang J Leury B et al . Effects of Astragalus membranaceus roots supplementation on growth performance, serum antioxidant and immune response in finishing lambs. Asian Australas J Anim Sci. (2020) 33:965–72. doi: 10.5713/ajas.19.0295
18.
Cho WC Leung KN . In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J Ethnopharmacol. (2007) 113:132–41. doi: 10.1016/j.jep.2007.05.020
19.
Wang X Hu C Ding L Tang Y Wei H Jiang C et al . Astragalus membranaceus alters rumen bacteria to enhance fiber digestion, improves antioxidant capacity and immunity indices of small intestinal mucosa, and enhances liver metabolites for energy synthesis in Tibetan sheep. Animals. (2021) 11:3236. doi: 10.3390/ani11113236
20.
Zhao YB . Regulatory effects of ethanol extract from Allium mongolicum on the synthesis of 4-alkyl branched-chain fatty acids in rumen and liver of meat sheep and its mechanism. Hohhot: Inner Mongolia Agricultural University (2023).
21.
China Green Food Development Center . Green food production technical regulation LB/T 158–2020: Technical code for meat sheep farming in Central Plains region. Beijing: China Green Food Development Center (2020) (In Chinese).
22.
Luo Y Su L Su R Wang B Liu C Wang Z et al . Effects of Astragalus membranaceus supplementation on oxidative stability of cashmere goat. Front Vet Sci. (2020) 7:555050. doi: 10.3389/fvets.2020.555050
23.
Page MJ Moher D Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
24.
Hooijmans CR Rovers MM de Vries RB Leenaars M Ritskes-Hoitinga M Langendam MW et al . Syrcle’s risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
25.
Kliem KE Thomson AL Crompton LA Givens DI . Effect of selected plant species within biodiverse pasture on in vitro fatty acid biohydrogenation and tissue fatty acid composition of lamb. Animal. (2018) 12:2415–23. doi: 10.1017/S1751731118000265
26.
Wang XJ Ding LM Wei HY Jiang CX Yan Q Hu CS et al . Astragalus membranaceus root supplementation improves average daily gain, rumen fermentation, serum immunity and antioxidant indices of Tibetan sheep. Animal. (2021) 15:100061. doi: 10.1016/j.animal.2020.100061
27.
Jia X Fan X Yang Z . Salvia miltiorrhiza polysaccharides inhibit lipid oxidation in cultured myocytes via Nrf2/ARE pathway. Food Chem Toxicol. (2024) 185:114502. doi: 10.1016/j.fct.2024.114502
28.
Vital APC Guerrero A Guarnido P Cordeiro SI Olleta JL Blasco M et al . Effect of active-edible coating and essential oils on lamb patties oxidation during display. Food Secur. (2021) 10:263. doi: 10.3390/foods10020263
29.
Guerrero A Ferrero S Barahona M Boito B Lisbinski E Maggi F et al . Effects of active edible coating based on thyme and garlic essential oils on lamb meat shelf life after long-term frozen storage. J Sci Food Agric. (2020) 100:656–64. doi: 10.1002/jsfa.10061
30.
Liang R Zhao B Fang Y Zhong R . Research progress on the formation pathways and regulation of volatile flavor compounds in mutton. Chin J Anim Nutr. (2024) 36:6872–81.
31.
Chen PY Zhang DQ Li SB Wang W Xu L Zhang JM et al . Analysis of flavor characteristics of cooked mutton from different breeds using electronic nose and HS-SPME-GC-O-MS. Food Ferment Ind. (2024) 50:298–305. doi: 10.13995/j.cnki.11-1802/ts.037157
32.
Shannon P Markiel A Ozier O Baliga NS Wang JT Ramage D et al . Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. (2003) 13:2498–504. doi: 10.1101/gr.1239303
33.
Pedregosa F Varoquaux G Gramfort A Michel V Thirion B Grisel O et al . Scikit-learn: machine learning in Python. J Mach Learn Res. (2011) 12:2825–30. doi: 10.48550/arXiv.1201.0490
34.
Xue J Lv Q Khas E Bai C Ma B Li W et al . Tissue-specific regulatory mechanism of LncRNAs and methylation in sheep adipose and muscle induced by Allium mongolicum regel extracts. Sci Rep. (2021) 11:9186. doi: 10.1038/s41598-021-88444-9
35.
Liu WJ . (2020). Effects of Allium mongolicum regel extract on 4-alkyl branched-chain fatty acid metabolism in sheep adipose tissue and its mechanism [PhD thesis]. Inner Mongolia Agricultural University.
36.
Zhao L Sun X Wu J Su L Yang F Jin Y et al . Effects of Allium mongolicum regel and its extracts on the quality of fermented mutton sausages. Food Sci Nutr. (2021) 10:169–78. doi: 10.1002/fsn3.2657
37.
Ministry of Agriculture and Rural Affairs of the People’s Republic of China . (2021). Nutrient requirements of meat-type sheep and goat (NY/T 816–2021). Beijing: China Agriculture Press. (Replaces NY/T 816–2004; Implemented from June 1, 2022).
38.
Fan X Liu J Deng W . Epigenetic regulation of FASN and CYP2B6 in lipid metabolism: implications for meat quality modulation. J Agric Food Chem. (2024) 72:1321–30. doi: 10.1021/acs.jafc.3c07521
39.
Cui X Yang Y Zhang M Bao L Jiao F Liu S et al . Mulberry leaves supplementation alters lipid metabolism and promotes fatty acid β oxidation in growing mutton sheep. J Anim Sci. (2024) 102:skae076. doi: 10.1093/jas/skae076
40.
Zheng Q Tan W Feng X Feng K Zhong W Liao C et al . Protective effect of flavonoids from mulberry leaf on AAPH-induced oxidative damage in sheep erythrocytes. Molecules. (2022) 27:7625. doi: 10.3390/molecules27217625
41.
Ouyang J Wang M Hou Q Feng D Pi Y Zhao W . Effects of dietary mulberry leaf powder in concentrate on the rumen fermentation and ruminal epithelium in fattening Hu sheep. Animals. (2019) 9:218. doi: 10.3390/ani9050218
42.
Long Y Han Y Zhao Y Chen D Wang D Yang Y et al . Effect of mulberry leaf TMR on growth performance, meat quality and expression of meat quality master genes (ADSL, H-FABP) in crossbred black goats. Food Secur. (2022) 11:4032. doi: 10.3390/foods11244032
43.
Ma T Chen DD Tu Y Zhang NF Si BW Diao QY . Dietary supplementation with mulberry leaf flavonoids inhibits methanogenesis in sheep. Anim Sci J. (2017) 88:72–8. doi: 10.1111/asj.12556
44.
Wang B Luo H . Effects of mulberry leaf silage on antioxidant and immunomodulatory activity and rumen bacterial community of lambs. BMC Microbiol. (2021) 21:250. doi: 10.1186/s12866-021-02311-1
45.
Li D Yang H Li Q Ma K Wang H Wang C et al . Prickly ash seeds improve immunity of Hu sheep by changing the diversity and structure of gut microbiota. Front Microbiol. (2023) 14:1273714. doi: 10.3389/fmicb.2023.1273714
46.
Zhuang Y Abdelsattar MM Fu Y Zhang N Chai J . Butyrate metabolism in rumen epithelium affected by host and diet regime through regulating microbiota in a goat model. Anim Nutr. (2024) 19:41–55. doi: 10.1016/j.aninu.2024.04.027
47.
Shi QL . (2020). Study on the influence of traditional spice combinations on flavor formation in northern Shaanxi stewed mutton [PhD thesis]. Northwest A&F University.
48.
Zhang YF Yue L Chen J Wang TY Yang XJ Yun XY et al . Effects of dietary supplementation with medicinal and edible homologous plants on volatile flavor compounds and fatty acid composition in mutton. Food Res Dev. (2025):1–14.
49.
Yang F Chen E Fu A Liu Y Bi S . Formation of key aroma compounds in Agrocybe aegerita during hot air drying: amino acids and reducing sugars identified as flavor precursors. Food Chem. (2025) 465:141975. doi: 10.1016/j.foodchem.2024.141975
50.
Ben Abdelmalek Y Smeti S Essid I Yagoubi Y Tibaoui S Atti N . The effect of rosemary (Rosmarinus officinalis L.) distillation residues and linseed supply on fatty acid profile, meat colour, lipid oxidation and sensorial and hygienic quality of cull Barbarine ewes' meat. J Anim Physiol Anim Nutr. (2020) 104:1294–304. doi: 10.1111/jpn.13383
51.
Siroli L Patrignani F Serrazanetti DI Vernocchi P del Chierico F Russo A et al . Effect of thyme essential oil and Lactococcus lactis CBM21 on the microbiota composition and quality of minimally processed lamb's lettuce. Food Microbiol. (2017) 68:61–70. doi: 10.1016/j.fm.2017.06.017
52.
Realini CE Bianchi G Bentancur O Garibotto G . Effect of supplementation with linseed or a blend of aromatic spices and time on feed on fatty acid composition, meat quality and consumer liking of meat from lambs fed dehydrated alfalfa or corn. Meat Sci. (2017) 127:21–9. doi: 10.1016/j.meatsci.2016.12.013
53.
Wang Y. (2023). Effects of Chinese herbal medicine on improving meat quality of fattening mutton. Inner Mongolia Agricultural University.
54.
Zhang Z Luo H Liu K Jia H Chen Y Wang Z et al . Antioxidant effects of liquorice (Glycyrrhiza uralensis) extract during aging of longissimus thoracis muscle in Tan sheep. Meat Sci. (2015) 105:38–45. doi: 10.1016/j.meatsci.2015.03.002
55.
Linares MB Cózar A Garrido MD Vergara H . Nutritional attributes and sensory quality during storage time of spiced lamb burgers from Manchego Spanish breed. Food Secur. (2020) 9:1466. doi: 10.3390/foods9101466
56.
Salinas-Chavira J Castillo-Martínez O Ramirez-Bribiesca JE Mellado M . Effect of increasing levels of white mulberry leaves (Morus alba) on ruminal dry matter degradability in lambs. Trop Anim Health Prod. (2011) 43:995–9. doi: 10.1007/s11250-011-9797-1
57.
Luo H Lin S Ren F Wu L Chen L Sun Y . Antioxidant and antimicrobial capacity of Chinese medicinal herb extracts in raw sheep meat. J Food Prot. (2007) 70:1440–5. doi: 10.4315/0362-028X-70.6.1440
58.
Martínez-Zamora L Ros G Nieto G . Synthetic vs. natural Hydroxytyrosol for clean label lamb burgers. Antioxidants. (2020) 9:851. doi: 10.3390/antiox9090851
59.
Ferreira LE Benincasa BI Fachin AL França SC Contini SSHT Chagas ACS et al . Thymus vulgaris L. essential oil and its main component thymol: anthelmintic effects against Haemonchus contortus from sheep. Vet Parasitol. (2016) 228:70–6. doi: 10.1016/j.vetpar.2016.08.011
60.
Lu Y Young OA Brooks JD . Physicochemical and sensory characteristics of fermented sheepmeat sausage. Food Sci Nutr. (2014) 2:669–75. doi: 10.1002/fsn3.151
61.
Liu RS Wang K Xu JF et al . Research progress on improving mutton quality with Chinese herbal additives. Chin J Anim Sci. (2020) 56:42–6. doi: 10.19556/j.0258-7033.20200113-04
62.
Alipour F Hassanabadi A Golian A Nassiri-Moghaddam H . Effect of plant extracts derived from thyme on male broiler performance. Poult Sci. (2015) 94:2630–4. doi: 10.3382/ps/pev220
63.
Chávez-Delgado EL Gastélum-Estrada A Pérez-Carrillo E Ramos-Parra PA Estarrón-Espinosa M Reza-Zaldívar EE et al . Bioactive properties of spearmint, orange peel, and baby sage oleoresins obtained by supercritical CO2 extraction and their integration into dark chocolate. Food Chem. (2025) 463:141306. doi: 10.1016/j.foodchem.2024.141306
64.
Eshete T Gizaw S Seifu E . Effect of inclusion of tossign (Thymus serrulatus) in concentrate mix supplementation on performance and sensory quality of meat of Menz sheep. Trop Anim Health Prod. (2013) 45:177–84. doi: 10.1007/s11250-012-0189-y
65.
Liu Z Zheng Z Wang T Liu Z Zuo Z . Using ultra-performance liquid chromatography with linear ion trap-electrostatic field orbitrap mass spectrometry, network pharmacology, and molecular docking to explore the constituent targets and action mechanisms of decoction of Angelica sinensis, Zingiberis Rhizoma Recens, and mutton in the treatment of diarrhea-predominant irritable bowel syndrome. J Pharm Pharmacol. (2024) 76:462–78. doi: 10.1093/jpp/rgad076
66.
Zhao L Jin Y Ma C Song H Li H Wang Z et al . Physico-chemical characteristics and free fatty acid composition of dry fermented mutton sausages as affected by the use of various combinations of starter cultures and spices. Meat Sci. (2011) 88:761–6. doi: 10.1016/j.meatsci.2011.03.010
67.
Sun H Luo Y Zhao F Fan Y Ma J Jin Y et al . The effect of replacing Wildrye Hay with mulberry leaves on the growth performance, blood metabolites, and carcass characteristics of sheep. Animals. (2020) 10:2018. doi: 10.3390/ani10112018
68.
Kandylis K Hadjigeorgiou I Harizanis P . The nutritive value of mulberry leaves (Morus alba) as a feed supplement for sheep. Trop Anim Health Prod. (2009) 41:17–24. doi: 10.1007/s11250-008-9149-y
69.
Lin Q Chen Y Yu B Chen Z Zhou H Su J et al . Atractylodes macrocephala Rhizoma alleviates blood hyperviscosity induced by high-fat, high-sugar, and high-salt diet by inhibiting gut-liver inflammation and fibrinogen synthesis. J Ethnopharmacol. (2025) 338:119034. doi: 10.1016/j.jep.2024.119034
70.
Sink JD Caporaso F . Lamb and mutton flavour: contributing factors and chemical aspects. Meat Sci. (1977) 1:119–27. doi: 10.1016/0309-1740(77)90013-4
71.
Morcuende D Vallejo-Torres C Ventanas S Martínez SL Ruiz SC Estévez M . Effectiveness of sprayed bioactive fruit extracts in counteracting protein oxidation in lamb cutlets subjected to a high-oxygen MAP. Food Secur. (2020) 9:1715. doi: 10.3390/foods9111715
72.
Jiang B Zhou Y Wang T Li F . Nutritive value and ruminal degradation of seven Chinese herbs as forage for Tan sheep. Bioengineered. (2020) 11:1159–69. doi: 10.1080/21655979.2020.1834740
73.
LeCun Y Bengio Y Hinton G . Deep learning. Nature. (2015) 521:436–44. doi: 10.1038/nature14539
74.
Liu Z Wang T Zhang Y Luo L Zhang H Xu L et al . Effects of decoction of Angelica Sinensis, Zingiberis Rhizoma Recens, and mutton on physiology and biochemistry of Sprague Dawley female rats with spleen-kidney Yang deficiency. Ann Transl Med. (2023) 11:91. doi: 10.21037/atm-22-6442
75.
Pérez-Fonseca A Alcala-Canto Y Salem AZ Alberti-Navarro AB . Anticoccidial efficacy of naringenin and a grapefruit peel extract in growing lambs naturally-infected with Eimeria spp. Vet Parasitol. (2016) 232:58–65. doi: 10.1016/j.vetpar.2016.11.009
76.
Wang H Wu F Li M Zhu X Shi C Shao C et al . Structure and chlorophyll fluorescence of heteroblastic foliage affect first-year growth in Pinus massoniana lamb. Seedlings. Plant Physiol Biochem. (2022) 170:206–17. doi: 10.1016/j.plaphy.2021.12.006
77.
Pan Y Nie C Zhang W Liu Y . Effect of feeding different proportions of licorice stem and leaves to substitute roughage made by the fully mixed pellet feed on growth performance, blood index and immune index of Bashbah sheep. J Shihezi Univ. (2018) 36:705–11. doi: 10.13880/j.cnki.65-1174/n.2018.06.0082
78.
Ning N Nan Y Chen G Huang S Lu D Yang Y et al . Anti-tumor effects and toxicity reduction mechanisms of Prunella vulgaris: a comprehensive review. Molecules. (2024) 29:1843. doi: 10.3390/molecules29081843
79.
Yu H Ma Z Wang J Lu S Cao D Wu J . Effects of thyme essential oil microcapsules on the antioxidant and quality characteristics of mutton patties. Food Secur. (2023) 12:3758. doi: 10.3390/foods12203758
80.
Ji Y.C. (2022). Effects of Astragalus polysaccharides and fermented bran polysaccharides on mutton quality, flavor composition, and shelf life. Inner Mongolia Agricultural University.
Summary
Keywords
Chinese herbal medicine additives, lipid metabolism, multi-omics, mutton flavor, rumen microbiota
Citation
Quan K, Shi H, Han H, Liu K, Cui Q, Wang H, Jin M, Sun W, Wei C, Jiang Y and Li J (2025) Multi-omics insights into Chinese herbal medicine additives for mutton flavor enhancement: epigenetic and microbial mechanisms. Front. Vet. Sci. 12:1628457. doi: 10.3389/fvets.2025.1628457
Received
14 May 2025
Accepted
23 June 2025
Published
14 July 2025
Volume
12 - 2025
Edited by
Arda Yıldırım, Gaziosmanpaşa University, Türkiye
Reviewed by
Zengkui Lu, Chinese Academy of Agricultural Sciences, China
Mariam G. Ahmed, Animal Production Research Institute (APRI), Egypt
Updates
Copyright
© 2025 Quan, Shi, Han, Liu, Cui, Wang, Jin, Sun, Wei, Jiang and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Quan, quankai1115@163.comJun Li, lijun.nn@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.