- Seijo Kobayashi Veterinary Clinic, Tokyo, Japan
Aging is characterized by chronic systemic inflammation accompanied by cellular senescence, immunosenescence, organ dysfunction, and age-related diseases. A chronic low-grade pro-inflammatory state known as “inflammaging” accelerates age-related diseases such as obesity, diabetes, vascular diseases, and certain types of cancer. Senescent cells drive age-related tissue dysfunction partially by inducing a chronic senescence-associated secretory phenotype (SASP) associated with various diseases. Obesity and insulin resistance change with advancing age and are linked to low-grade chronic inflammation, leading to age-related diseases. Obesity results in significant changes in the adipokine profile, such as reduced levels of anti-inflammatory adipokines, e.g., adiponectin. Cats are more prone to obesity than dogs owing to the unique characteristics of their glucose and lipid metabolism. Severely obese cats show excessive visceral fat accumulation, significantly increased triglyceride, free fatty acids, and TNF-α plasma concentrations as pro-inflammatory markers, and a significant decrease in adiponectin. Aged obese cats with excessive visceral fat exhibit fatty liver and enlarged adipocytes with macrophage infiltration. A healthy lifestyle is recognized as the most effective way to maintain health and fight aging. Aging is inevitable in animals; however, delaying the onset of age-related disease through adequate interventions at the early stages of SASP induction is possible. Adequate nutrition, moderate exercise, and a good mental state can effectively prevent age-related obesity in cats.
1 Introduction
As aging progresses, the ability of the animal body to resolve inflammation is reduced significantly, resulting in an imbalance between proinflammation and anti-inflammation. This results in a chronic low-grade pro-inflammatory state known as “inflammaging,” which accelerates age-related diseases like obesity, diabetes, vascular diseases, and certain types of cancer (1). Inflammaging occurs in senescent tissues and is involved in the development of age-related diseases (2). Oxidative stress (OS) is associated with various age-related conditions, including sarcopenia and frailty (3), and OS-induced aging and associated disorders cause soft tissue deterioration and homeostatic imbalances (4, 5). Moreover, stressed senescent cells exhibit an altered secretome, referred to as the senescence-associated secretory phenotype (SASP), which results in the secretion of pro-inflammatory cytokines (6).
The increase in the prevalence of overweight and obesity represents a worldwide phenomenon that is associated with various chronic diseases such as type-2 diabetes (T2D), cancer, rheumatoid arthritis and osteoarthritis (OA), cognitive impairment and dementia, and those affecting the cardiovascular (CV) system (7). Obesity superimposed on aging (age-related obesity) represents an additional risk factor for the older age group in which the prevalence of chronic diseases, as well as the occurrence of complications, increases (8, 9). In cats, whose glucose and lipid metabolism differ from that in dogs, obesity and its associated diseases increase significantly with age (10, 11). In this review, we outline chronic inflammation as the basic pathophysiology of age-related diseases and discuss the relationship between age-related obesity and inflammaging in cats.
2 Senescence-associated secretory phenotype and inflammaging
Aging is characterized by chronic systemic inflammation accompanied by cellular senescence, immunosenescence, organ dysfunction, and age-related diseases (12). Cellular senescence is a state of permanent cell proliferation arrest induced by persistent DNA damage and other stress-induced signals. Cellular senescence has since been reported not only in cultured cells but also in vivo in cells of various organisms, ranging from yeast to mammals (13). In vivo, cellular senescence is induced by DNA damage-associated stress. The role and mechanism underlying senescence-associated secretory phenotypes (SASP) have been increasingly recognized, as they are suspected to be associated with various diseases (14). Many senescent cells secrete a wide spectrum of bioactive factors, including inflammatory cytokines, chemokines, growth factors, matrix metalloproteases, lipids, nucleotides, extracellular vesicles, and soluble factors, termed SASP (15). The combination of these molecules forms the SASP, which determines various processes in the body associated with regeneration (16), tissue remodeling (17), inflammation (14), and carcinogenesis (18). The SASP is a dynamic process that can be divided into several phases. The first phase starts immediately after DNA damage, followed by an early SASP phase characterized by increased synthesis spanning several days. Within 4–10 days, the secretion of most SASP factors increases through autocrine exposure, leading up to the mature phase of the SASP (19). SASP regulation occurs at both the transcriptional and post-transcriptional levels. Nuclear factor kappa B (NF-κB) plays a key role in regulating the expression of genes that are the main components of the SASP (20). Senescent cell accumulation and long-term SASP secretion may result in disrupted tissue function, accelerated aging, and the development of age-related pathologies (21). Mitochondrial dysfunction is an often-unappreciated hallmark of cellular senescence which plays important roles not only in the senescence growth arrest but also in the development of the SASP and resistance to cell-death (22).
The term “inflammaging,” first used by Franceschi et al. in 2000, is associated with chronic sub-clinical inflammatory processes and biological aging (23). The SASP phenotype has been proposed as the underlying cause of inflammaging and comprises various soluble factors, including pro-inflammatory mediators (e.g., IL-6 and IL-8) and matrix-degrading molecules characterized by the release of pro-inflammatory cytokines (24). Senescent cells exhibit molecular (e.g., senescence marker expression) and morphological features (e.g., enlarged or flattened cells) (25).
3 Inflammaging and age-related diseases
Aging is the strongest risk factor for most chronic diseases, including obesity. Central obesity and inflammation have consistently been found to be strongly associated with the severity and future risk of severe multimorbidity. The pro-inflammatory state of aging has been suggested to be a proxy biomarker of the pace of aging. Strong epidemiological evidence suggests that elevated levels of pro-inflammatory markers in older animals are associated with the risk of developing most diseases typical of aging (26). The systemic consequences of aging on the development of aging phenotypes can be roughly summarized into four major domains: (1) changes in body composition, (2) an imbalance between energy availability and demand, (3) dysregulation of signaling networks that maintain homeostasis, and (4) neurodegeneration with impaired neuroplasticity (26). Age-related changes in body composition and physical fitness are among the most apparent and unavoidable effects of aging, and cause metabolic dysfunction (Table 1). Visceral fat, which is responsible for many obesity-related pathologies and an independent risk factor for coronary artery disease, stroke, and death, continuously accumulates and is reflected in an increase in waist circumference throughout life (27). Evidently, all organs experience some changes in tissue composition throughout life, and the related changes are directly associated with sub-clinical and clinical pathology, including neurodegeneration (28), physical frailty, increase in fibro-connective build-up in muscles, and demineralization and loss of bone strength (29).
The balance between energy availability and demand is tightly regulated, and ATP is constantly resynthesized because its storage is sufficient for only a few seconds (30). In muscle cells and neurons, this stability is co-adjuvanted by the phosphocreatine buffering system, which accumulates chemical energy to be promptly used when demand suddenly increases. Most energy muscles use is generated through aerobic metabolism; hence, energy consumption can be estimated indirectly from oxygen consumption. Older individuals with multiple comorbidities have less available energy and require more energy at rest and during physical activity. The amount of energy used at rest decreases with age largely because of a loss of lean body mass but declines less in those with multiple chronic conditions (physical inactivation) (31). Hence, sick older individuals use most of their available energy to perform activities essential to daily living.
A mild pro-inflammatory state develops in most aging individuals, reflected by high levels of pro-inflammatory markers, such as IL-6 and C-reactive protein (CRP) (32). These hormones, inflammatory biomarkers, and antioxidants are part of complex signaling networks that control homeostasis, and individual biomarker levels may reflect adaptations within homeostatic feedback loops rather than causative factors. The number of neurons also declines throughout life, as neurons generally stop reproducing shortly after birth (31). With aging, microglia acquire a predisposition to reactive inflammation, and brain tissue from older individuals exhibits higher levels of pro-inflammatory cytokines and lower levels of anti-inflammatory cytokines than those in the brain tissue of younger individuals. Higher inflammation has been associated with lower cognition and reduced neuronal plasticity, which are expressed as reduced capacities for adaptation and compensation (33). Clinically, inflammaging is characterized by increased blood levels of several inflammatory biomarkers, including CRP, IL-6, IL-8, and TNF-α (34). Furthermore, serum IL-6 levels also predict incident disability and frailty (35).
Obesity and insulin resistance are altered with advancing age and are linked to low-grade chronic inflammation, leading to age-related systemic metabolic dysfunction, physical limitation, and frailty (36). Mitochondrial hormesis may also play a role in aging, and mild mitochondrial toxicity may trigger beneficial compensatory responses that improve cellular fitness (37). Resveratrol and metformin, which inhibit cellular energy metabolism by increasing AMP levels, activating AMP-activated protein kinase (AMPK), and decreasing oxygen uptake, are possible examples of this (38). Obesity has become a prominent health problem globally and is closely associated with many chronic diseases, such as diabetes mellitus, cardiovascular diseases, and certain types of cancer (39). Obesity develops when energy intake exceeds energy expenditure and is characterized by excessive adipose tissue (AT) accumulation. When AT reaches its maximum capacity for energy storage, it releases free fatty acids (FFA), causing ectopic lipid deposition in other tissues, such as the liver, skeletal muscle, and vasculature. Adipose tissue shows increased macrophage infiltration during the development of obesity (40, 41). Consequently, these AT macrophages secrete high levels of pro-inflammatory cytokines, resulting in obesity-associated chronic low-grade inflammation and impaired insulin signaling (42).
Obesity results in significant changes in the adipokine profile, creating a shift toward elevated levels of pro-inflammatory adipokines, such as leptin and resistin, and reduced levels of anti-inflammatory adipokines, such as adiponectin (43). Obesity is also associated with increased perivascular fat, expressed as pro-inflammatory markers, including serum amyloid A (SAA) (44). SAA subtypes 1–3 are well-described acute-phase reactants that are elevated in acute inflammatory conditions such as infection, tissue injury, and trauma. SAA subtypes have also been implicated in chronic metabolic diseases, including obesity, diabetes, and cardiovascular disease, and, passively, in autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease (45). Circulating SAA levels are positively associated with visceral adiposity (46), suggesting that visceral fat is a potential source of SAA. These changes in circulating levels of adipokines are exactly SASP.
4 Obesity in cats
Obesity is the most common age-related disease in cats. Similar to that in humans, an increased incidence of obesity in cats has been observed to accompany aging in recent years (11, 47), and its prevalence is assumed to be 30–40% (47, 48). In cats, obesity is associated with the development of insulin resistance (49) and T2D (50, 51) and is considered a good model of human metabolic syndrome (52). Cats are more prone to obesity than dogs owing to their unique glucose and lipid metabolism characteristics (53, 54). In feline livers, glucokinase, the rate-limiting enzyme in glycolysis, is lacking (53), and gluconeogenic enzyme activity is higher than that in canine livers (55). Additionally, the expression levels of mRNA associated with the insulin signaling pathway, including insulin receptor substrate (IRS)-1, IRS-2, phosphatidylinositol 3-kinase (PI3K) P-85α, are significantly lower in cats than those in dogs (54), and expression levels of IRS-2 and PI3K mRNA significantly decreased in liver and skeletal muscle of obese cats (56). Furthermore, adiponectin, an adipokine that improves insulin sensitivity, is lower in cats in the normal state (54) and with weight gain (56). Thus, adiponectin appears to play an important role in the development of obesity-related metabolic disturbances in cats. Collectively, this evidence suggests that, similar to humans, cats have an inherently lower ability to process glucose and are predisposed to obesity and insulin resistance, as well as visceral obesity-induced lipid metabolism abnormalities.
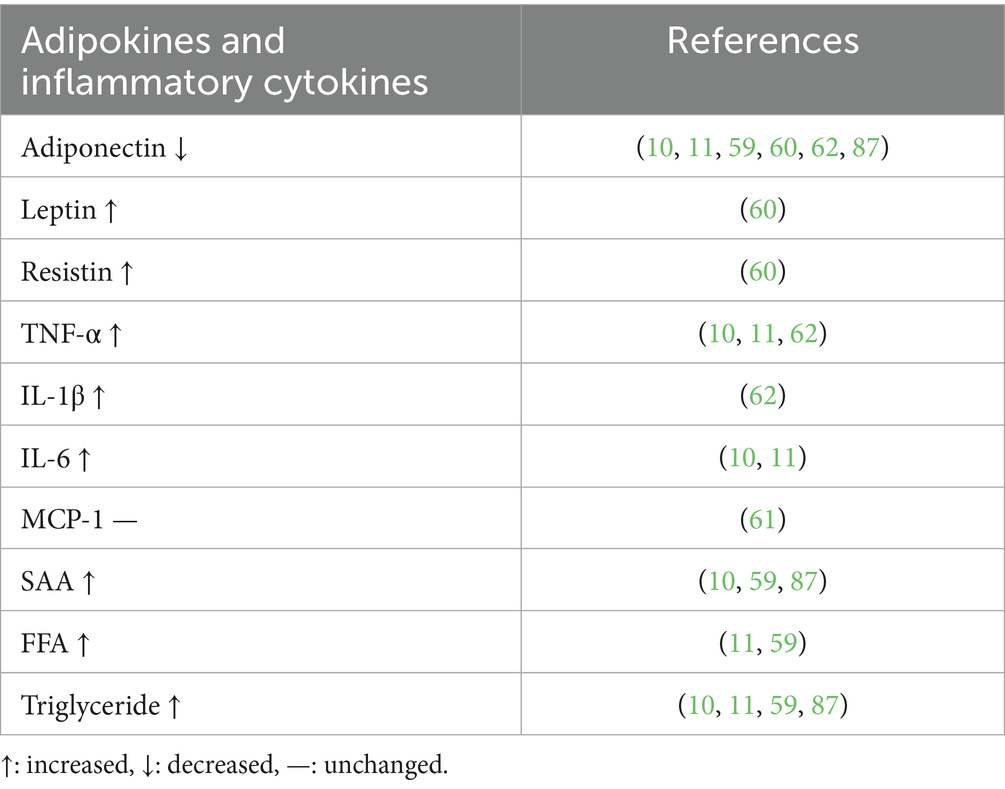
Much like in human medicine, consensus on objective biochemical and mechanical parameters such as body mass index and its reference values for classifying weight status is lacking in veterinary medicine. Body condition score (BCS) is a commonly accepted semi-quantitative method for evaluating weight status. It involves subjective visual observation and palpation made by an observer, using a scale from 1 to 9, where 1 indicates emaciation, 5 is ideal, and 9 is extremely fat (57). Severely obese cats with a BCS of 9 showed excessive amounts of visceral fat accumulation and a significant increase in plasma concentrations of triglyceride, FFA, and TNF-α as pro-inflammatory markers and a significant decrease in adiponectin concentrations (10, 11). Aged obese cats with excessive visceral fat show enlarged adipocytes with macrophage infiltration (10, 11). mRNA expression levels of FAS and SREBP-1 in abdominal AT and livers of obese cats were significantly increased (58). In the aged obese cats, ectopic lipid accumulation was accelerated, and fatty liver is observed frequently (10, 11, 59). Changes in circulating levels of adipokines (adiponectin, leptin, and resistin), inflammatory cytokines (TNF-α, IL-1β, IL-6, MCP-1, and SAA) and lipids (FFA and triglyceride) in obese cats are summarized in Table 2 (59–62).
On the other hand, adipose tissue adiponectin mRNA and circulating adiponectin do not exhibit a correlation (63). Feline and human studies have shown that adiponectin gene expression is adipose depot-dependent (52), indicating that circulating adiponectin levels are dependent on other factors in addition to adiposity and fat depot location. Remodeling adipocytes are senescent cells and cannot produce adiponectin. This SASP is one of the characteristics of feline obesity and is considered to induce insulin resistance, followed by severe metabolic disorders such as obesity, diabetes, and vascular dysfunction, among others.
5 Intervention strategies for age-related obesity in cats
A healthy lifestyle has long been recognized as the most effective way to maintain health and prevent the deleterious effects of aging (64, 65). Adequate nutrition, moderate exercise, and a good mental state can effectively delay aging (66, 67). Balanced and adequate nutritional intake positively affects aging. Exercise is an efficient strategy for delaying aging due to various mechanisms, including DNA damage (68) and OS (69). Calorie restriction in animals is associated with a substantial reduction in pro-inflammatory markers in blood (70). Weight loss combined with exercise improves functional status, reduces some features of frailty in obese individuals, improves the cardiovascular risk profile, and reduces the risk of some types of cancer (71, 72). As aging progresses, living organisms experience a series of progressive degenerative changes and become more sensitive to internal and external stimuli, leading to OS aggravation, increased inflammation, apoptosis, and structural and functional cell and organ damage, resulting in a SASP followed by age-related diseases (73, 74). The SASP has been proposed as the underlying cause of inflammation and consists of various soluble factors, such as pro-inflammatory mediators (e.g., IL-6 and IL-8) and matrix-degrading molecules characterized by the release of pro-inflammatory cytokines (23). Alleviation of inflammaging will help prevent age-related diseases. The transcription factor NF-κB represents a promising target for SASP control. Incidentally, several NF-κB-dependent pro-inflammatory SASP factors are downregulated. Moreover, NF-κB is a key upstream regulator of the SASP and is, simultaneously, a transcriptional target of NF-κB (75). Metformin, an antidiabetic drug with pleiotropic effects, also targets senescent cells (76), negatively affecting NF-κB without affecting other inflammatory pathways such as p38 and JNK. Metformin-mediated inhibition of the SASP may contribute to the anti-aging effects observed after metformin treatment (77). Metformin activates AMPK and activated AMPK phosphorylates the acetyl-CoA carboxylase, inhibiting fat synthesis and promoting fat oxidation instead, thus reducing hepatic lipid stores and enhancing hepatic insulin sensitivity (78).
Various phytochemicals have been developed as senolytic drugs (12). Resveratrol, a natural polyphenol found in plants such as peanuts, grapes, and strawberries (79), modulates the expression of pro- and anti-apoptotic factors, neutralizes free radical species, affects mitochondrial function, chelates redox-active transition-metal ions, and prevents protein aggregation (80). Resveratrol inhibits the SASP through the SIRT1/NF-κB signaling pathway and delayed aging (81, 82) (Supplementary Figure 1). Quercetin (12) and curcumin (83) have shown anti-SASP and anti-inflammatory activities similar to those of resveratrol. For alleviation of inflammaging in age-related obesity cats, resveratrol supplementation (59), and quercetin supplementation (84) were effective. Metformin, which enhances peripheral insulin sensitivity and reduces hepatic glucose output, is used as anti-diabetic drug, however studies on metformin in age-related obesity cats are currently in progress (85).
Early diagnosis of the SASP by detecting various pro-inflammatory cytokines and inflammatory markers is possible (45, 86). In age-related obesity cats, SAA can be good diagnostic marker at early stage of inflammaging (87). Aging is inevitable in animals; however, delaying the onset of age-related diseases through adequate interventions in the early stage of the SASP is possible. Adequate nutrition, moderate exercise, and a good mental state can effectively prevent age-related diseases, including obesity, in cats.
6 Conclusion
Aging is characterized by chronic systemic inflammation, which is accompanied by cellular senescence, immunosenescence, organ dysfunction, and age-related diseases such as obesity, diabetes, vascular diseases, and even certain types of cancer. Senescent cells partially drive age-related tissue dysfunction by inducing a chronic SASP associated with various diseases. Obesity results in significant changes in the adipokine profile, creating a shift toward elevated levels of pro-inflammatory adipokines, such as leptin and resistin, and reduced levels of anti-inflammatory adipokines, such as adiponectin. Cats are more prone to obesity than dogs owing to their unique glucose and lipid metabolism characteristics. Severely obese cats show excessive visceral fat accumulation, a significant increase in plasma triglyceride, FFA, and TNF-α concentrations as pro-inflammatory markers, and a significant decrease in adiponectin concentrations. A healthy lifestyle is recognized as the most effective way to maintain health and fight the effects of aging. Adequate nutrition, moderate exercise, and a good mental state can effectively prevent age-related obesity in cats.
Author contributions
MiK: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Writing – review & editing. MoK: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1639055/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Resveratrol activates AMPK in a SIRT1-dependent manner through deacetylation of LKB1. High dose of resveratrol activates AMPK directly and low dose of resveratrol activate AMPK via SIRT1, and stimulate mitochondrial biogenesis that results in improvement of lipid metabolism in tissues (81). LKB1, liver kinase B1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator1-alpha; NRF, nuclear respiratory factor; TFAM, transcription factor A, mitochondrial; NAD, nicotinamide adenine dinucleotide.
Abbreviations
AMP, Adenosine 5′-monophosphate; AMPK, AMP-activated protein kinase; AT, Adipose tissue; BCS, Body condition score; CRP, C-reactive protein; FAS, Fatty acid synthase; FFA, Free fatty acid; IL-6, Interleukin-6; IRS, Insulin receptor substrate; JNK, c-Jun N-terminal kinase; MCP-1, Monocyte chemoattractant protein-1; NAD, Nicotinamide adenine dinucleotide; NFκB, Nuclear factor kappa B; OS, Oxidative stress; PI3K, Phosphatidylinositol 3-kinase; SAA, Serum amyloid A; SASP, Senescence-associated secretory phenotype; SREBP-1, Sterol regulatory element binding protein-1; TNF-α, Tumor necrosis factor-α.
References
1. Xia, S, Zhang, X, Zheng, S, Khanabdali, R, Kalionis, B, Wu, J, et al. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. (2016) 2016:8426874. doi: 10.1155/2016/8426874
2. Li, T, Huang, Y, Cai, W, Chen, X, Men, X, Lu, T, et al. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis. (2020) 11:932. doi: 10.1038/s41419-020-03137-x
3. Bonomini, F, Rodella, LF, and Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. (2015) 6:109–20. doi: 10.14336/AD.2014.0305
4. Biala, AK, Dhingra, R, and Kirshenbaum, LA. Mitochondrial dynamics: orchestrating the journey to advance age. J Mol Cell Cardiol. (2015) 83:37–43. doi: 10.1016/j.yjmcc.2015.04.015
5. Bratic, A, and Larsson, NG. The role of mitochondria in aging. J Clin Invest. (2013) 123:951–7. doi: 10.1172/JCI64125
6. Campisi, J. Aging, cellular senescence, and cancer. Annu Rev Physiol. (2013) 75:685–705. doi: 10.1146/annurev-physiol-030212-183653
7. Frasca, D, Blomberg, BB, and Paganelli, R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. (2017) 8:1745. doi: 10.3389/fimmu.2017.01745
8. Ottaviani, S, Allanore, Y, Tubach, F, Forien, M, Gardette, A, Pasquert, B, et al. Body mass index influence to inflixmab in ankylosing spondylitis. Arthritis Res Ther. (2012) 14:R115. doi: 10.1186/ar3841
9. Sandberg, ME, Bengtsson, V, Kallberg, H, Wesley, A, Klareskog, L, Alfredsson, L, et al. Overweight decreases the chance of achieving good response and low disease activity in early rhumetoid arthrrithis. Ann Rheum Dis. (2014) 73:2029–33. doi: 10.1136/1nnrheumdis-2013-205094
10. Okada, Y, Kobayashi, M, Sawamura, M, and Arai, T. Comparison of visceral fat accumulation and metabolome markers among cats of varying BCS and novel classification of feline obesity and metabolic syndrome. Front Vet Sci. (2017) 4:17. doi: 10.3389/fvets.2017.00017
11. Okada, Y, Ueno, H, Mizorogi, T, Ohara, K, Kawasumi, K, and Arai, T. Diagnostic criteria for obesity disease in cats. Front Vet Sci. (2019) 6:284. doi: 10.3389/fvets.2019.00284
12. Li, X, Li, C, Zhang, W, Wang, Y, Qian, P, and Huang, H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. (2023) 8:239. doi: 10.1038/s41392-023-01502-8
13. Lohr, JN, Galimov, ER, and Gems, D. Does senescence promote fitness in Caenorhabditis elegans by causing death? Ageing Res Rev. (2019) 50:58–71. doi: 10.1016/j.arr.2019.01.008
14. Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): can it be controlled by senolysis? Inflam Regen. (2022) 42:11. doi: 10.1186/s41232-022-00197-8
15. Zhang, L, Pitcher, LE, Yousefzadeh, MJ, Niedernhofer, LJ, Robbins, PD, and Zhu, Y. Cellular senescence: a key therapeutic target in aging and disease. J Clin Invest. (2022) 132:e158450. doi: 10.1172/JCI158450
16. Ritschka, B, Storer, M, Mas, A, Heinzmann, F, Ortells, MC, Morton, JP, et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. (2017) 31:172–83. doi: 10.1101/gad.290635.116
17. Birch, J, and Gil, J. Senescence and the SASP: many therapeutic avenues. Genes Dev. (2020) 34:1565–76. doi: 10.1101/gad.343129.120
18. Rao, SG, and Jackson, JG. SASP: tumor suppressor or promoter? Yes! Trends Cancer. (2016) 2:676–87. doi: 10.1016/j.trecan.2016.10.001
19. Prašnikar, E, Borišek, J, and Perdih, A. Senescent cells as promising targets to tackle age-related diseases. Ageing Res Rev. (2021) 66:101251. doi: 10.1016/j.arr.2020.101251
20. Salminen, A, Kauppinen, A, and Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. (2012) 24:835–45. doi: 10.1016/j.cellsig.2011.12.006
21. Khavinson, V, Linkova, N, Dyatlova, A, Kantemirova, R, and Kozlov, K. Senescence-associated secretory phenotype of cardiovascular system cells and inflammaging: perspectives of peptide regulation. Cells. (2022) 12:106. doi: 10.3390/cells12010106
22. Martini, H, and Passos, JF. Cellular senescence: all roads lead to mitochondria. FEBS J. (2023) 290:1186–202. doi: 10.1111/febs.16361
23. Franceschi, C, Bonafè, M, Valensin, S, Olivieri, F, De De, LM, Ottaviani, E, et al. Inflammaging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. (2000) 908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x
24. Robbins, PD. Extracellular vesicles and aging. Stem Cell Investig. (2017) 4:98. doi: 10.21037/sci.2017.12.03
25. Sharpless, NE, and Sherr, CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. (2015) 15:397–408. doi: 10.1038/nrc3960
26. Bektas, A, Schurman, SH, Sen, R, and Ferrucci, L. Aging, inflammation and the environment. Exp Gerontol. (2018) 105:10–8. doi: 10.1016/j.exger.2017.12.015
27. Folsom, AR, Kaye, SA, Sellers, TA, Hong, CP, Cerhan, JR, Potter, JD, et al. Body fat distribution and 5-year risk of death in older women. J Am Med Assoc. (1993) 269:483–7. doi: 10.1001/jama.1993.03500040049035
28. Camandola, S, and Mattson, MP. Brain metabolism in health, aging, and neurodegeneration. EMBO J. (2017) 36:1474–92. doi: 10.15252/embj.201695810
29. Ferrucci, L, Baroni, M, Ranchelli, A, Lauretani, F, Maggio, M, Mecocci, P, et al. Interaction between bone and muscle in older persons with mobility limitations. Curr Pharm Des. (2014) 20:3178–97. doi: 10.2174/13816128113196660690
30. Casey, A, and Greenhaff, PL. Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am J Clin Nutr. (2000) 72:607S–17S. doi: 10.1093/ajcn/72.2.607S
31. Ferrucci, L, Hesdorffer, C, Bandinelli, S, and Simonsick, EM. Frailty as a nexus between the biology of aging, environmental conditions and clinical geriatrics. Public Health Rev. (2010) 32:475–88. doi: 10.1007/BF03391612
32. Ferrucci, L, Corsi, A, Lauretani, F, Bandinelli, S, Bartali, B, Taub, DD, et al. The origins of age-related proinflammatory state. Blood. (2005) 105:2294–9. doi: 10.1182/blood-2004-07-2599
33. Norden, DM, and Godbout, JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. (2013) 39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x
34. Scheller, J, Chalaris, A, Schmidt-Arras, D, and Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. (2011) 1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034
35. Soysal, P, Stubbs, B, Lucato, P, Luchini, C, Solmi, M, Peluso, R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. (2016) 31:1–8. doi: 10.1016/j.arr.2016.08.006
36. Stout, MB, Justice, JN, Nicklas, BJ, and Kirkland, JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda). (2017) 32:9–19. doi: 10.1152/physiol.00012.2016
37. López-Otín, C, Blasco, MA, Partridge, L, Serrano, M, and Kroemer, G. The hallmarks of aging. Cell. (2013) 153:1194–217. doi: 10.1016/j.cell.2013.05.039
38. Hawley, SA, Ross, FA, Chevtzoff, C, Green, KA, Evans, A, Fogarty, S, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. (2010) 11:554–65. doi: 10.1016/j.cmet.2010.04.001
39. Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. (2017) 960:1–17. doi: 10.1007/978-3-319-48382-5_1
40. Xu, H, Barnes, GT, Yang, Q, Tan, G, Yang, D, Chou, CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. (2003) 112:1821–30. doi: 10.1172/JCI200319451
41. Castoldi, A, Naffah de Souza, C, Câmara, NO, and Moraes-Vieira, PM. The macrophage switch in obesity development. Front Immunol. (2015) 6:637. doi: 10.3389/fimmu.2015.00637
42. Gao, D, Madi, M, Ding, C, Fok, M, Steele, T, Ford, C, et al. Interleukin-1beta mediates macrophage- induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab. (2014) 307:E289–304. doi: 10.1152/ajpendo.00430.2013
43. Chung, HS, and Choi, KM. Organokines in disease. Adv Clin Chem. (2020) 94:261–321. doi: 10.1016/bs.acc.2019.07.012
44. Shridas, P, Ji, A, Trumbauer, AC, Noffsinger, VP, Leung, SW, Dugan, AJ, et al. Adipocyte-derived serum amyloid a promotes angiotensin II-induced abdominal aortic aneurysms in obese C57BL/6J mice. Arterioscler Thromb Vasc Biol. (2022) 42:632–43. doi: 10.1161/ATVBAHA.121.317225
45. Den Hartigh, LJ, May, KS, Zhang, XS, Chait, A, and Blaser, MJ. Serum amyloid a and metabolic disease: evidence for a critical role in chronic inflammatory conditions. Front Cardiovasc Med. (2023) 10:1197432. doi: 10.3389/fcvm.2023.1197432
46. Lee, CG, Carr, MC, Murdoch, SJ, Mitchell, E, Woods, NF, Chen, MH, et al. Adipokines, inflammation and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab. (2009) 94:1104–10. doi: 10.1210/jc.2008-0701
47. Chandler, M, Cunningham, S, Lund, EM, Khanna, C, Naramore, R, Patel, A, et al. Obesity and associated comorbidities in people and companion animals: a one health perspective. J Comp Pathol. (2017) 156:296–309. doi: 10.1016/j.jcpa.2017.03.006
48. Mori, N, Okada, Y, Tsuchida, N, Hatano, Y, Habara, M, Ishikawa, S, et al. Preliminary analysis of modified low-density lipoproteins in the serum of healthy and obese dogs and cats. Front Vet Sci. (2015) 2:34. doi: 10.3389/fvets.2015.00034
49. Brennan, CL, Hoenig, M, and Ferguson, DC. GLUT4 but not GLUT1 expression decreases early in the development of feline obesity. Domest Anim Endocrinol. (2004) 26:291–301. doi: 10.1016/j.domaniend.2003.11.003
50. Laflamme, DP. Companion animals symposium: obesity in dogs and cats: what is wrong with being fat? J Anim Sci. (2012) 90:1653–62. doi: 10.2527/jas.2011-4571
51. Osto, M, and Lutz, TA. Translational value of animal models of obesity–focus on dogs and cats. Eur J Pharmacol. (2015) 759:240–52. doi: 10.1016/j.ejphar.2015.03.036
52. Van de Velde, H, Janssens, GPJ, de Rooster, H, Polis, I, Peters, I, Ducatelle, R, et al. The cat as a model for human obesity: insights into depot-specific inflammation associated with feline obesity. Br J Nutr. (2013) 110:1326–35. doi: 10.1017/S0007114513000226
53. Tanaka, A, Inoue, A, Takeguchi, A, Washizu, T, Bonkobara, M, and Arai, T. Comparison of expression of glucokinase gene and activities of enzymes related to glucose metabolism in livers between dog and cat. Vet Res Commun. (2005) 29:477–85. doi: 10.1007/s11259-005-1868-1
54. Mori, A, Lee, P, Takemitsu, H, Sako, T, and Arai, T. Comparison of insulin signaling gene expression in insulin sensitive tissues between cats and dogs. Vet Res Commun. (2009) 33:211–26. doi: 10.1007/s11259-008-9168-1
55. Washizu, T, Tanaka, A, Sako, T, Washizu, M, and Arai, T. Comparison of the activities of enzymes related to glycolysis and gluconeogenesis in the liver of dogs and cats. Res Vet Sci. (1999) 67:205–6. doi: 10.1053/rvsc.1998.0305
56. Mori, A, Lee, P, Takemitsu, H, Iwasaki, E, Kimura, N, Yagishita, M, et al. Decresed gene expression of insulin signaling genes in insulin sensitive tissues of obese cats. Vet Res Commun. (2009) 33:315–29. doi: 10.1007/s11259-008-9179-y
57. Verkest, KR, Rand, JS, Fleeman, LM, Morton, JM, Richards, AA, Rose, FJ, et al. Distinct adiponectin profiles might contribute to differences in susceptibility to type 2 diabetes in dogs and humans. Domest Anim Endocrinol. (2011) 41:67–73. doi: 10.1016/j.domaniend.2011.03.003
58. Lee, P, Mori, A, Takemitsu, H, Yamamoto, I, and Arai, T. Lipogenic gene expression in abdominal adipose and liver tissue of diet-induced overweight cats. Vet J. (2011) 190:e150–3. doi: 10.1016/j.tvjl.2011.04.003
59. Yun, JE, Kang, SR, Kim, JY, Kim, HY, Kobayashi, M, and Arai, T. Effect of resveratrol supplementation on lipid metabolism in healthy and obese cats. Front Vet Sci. (2025) 12:1565367. doi: 10.3389/fvets.2025.1565367
60. Takashima, S, Nishi, N, Kobatake, Y, Kiyosue, M, Kimura, S, and Kitagawa, H. Concentrations of leptin, adiponectin, and resistin in the serum of obese cats during weight loss. J Vet Med Sci. (2019) 81:1294–300. doi: 10.1292/jvms.19-0091
61. Sternberg, K, Gensby, L, Cremer, SE, Nielsen, MM, and Reinhard, BC. Analytical performance of a canine ELISA monocyte chemoattractant protein-1 assay for use in cats and evaluation of circulating levels in normal weight and obese cats. Acta Vet Scand. (2022) 64:22. doi: 10.1186/s13028-022-00640-3
62. Araujo, SL, Martins, PL, de Souza Pereira, TH, Sampaio, TL, Paula, RR, de Menzes, PB, et al. Evidence of obesity-induced inflammatory changes in client-owned cats. Vet World. (2024) 17:1685–92. doi: 10.14202/vetworld.2024.1685-1692
63. Stenberg, K, Novotny, GW, Lutz, TA, Mandrup-Poulsen, T, and Reinhard, BC. Obesity-induced changes in gene expression in feline adipose and skeletal muscle tissue. J Anim Physiol Anim Nutr. (2023) 107:1262–78. doi: 10.1111/jpn.13802
64. Campisi, J, Kapahi, P, Lithgow, GJ, Melov, S, Newman, JC, and Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. (2019) 571:183–92. doi: 10.1038/s41586-019-1365-2
65. Sharma, R, Diwan, B, Sharma, A, and Witkowski, JM. Emerging cellular senescence-centric understanding of immunological aging and its potential modulation through dietary bioactive components. Biogerontology. (2022) 23:699–729. doi: 10.1007/s10522-022-09995-6
66. Dasgupta, M, Sharkey, JR, and Wu, G. Inadequate intakes of indispensable amino acids among homebound older adults. J Nutr Elder. (2005) 24:85–99. doi: 10.1300/J052v24n03_07
67. Li, Y, Pan, A, Wang, DD, Liu, X, Dhana, K, Franco, OH, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. (2018) 138:345–55. doi: 10.1161/CIRCULATIONAHA.117.032047
68. Radák, Z, Naito, H, Kaneko, T, Tahara, S, Nakamoto, H, Takahashi, R, et al. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. (2002) 445:273–8. doi: 10.1007/s00424-002-0918-6
69. Gomez-Cabrera, MC, Domenech, E, and Viña, J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. (2008) 44:126–31. doi: 10.1016/j.freeradbiomed.2007.02.001
70. Meydani, SN, Das, SK, Pieper, CF, Lewis, MR, Klein, S, Dixit, VD, et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging. (2016) 8:1416–31. doi: 10.18632/aging.100994
71. Ma, C, Avenell, A, Bolland, M, Hudson, J, Stewart, F, Robertson, C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. (2017) 359:j4849. doi: 10.1136/bmj.j4849
72. Ligibel, JA, Basen-Engquist, K, and Bea, JW. Weight management and physical activity for breast cancer prevention and control. Am Soc Clin Oncol Educ Book. (2019) 39:e22–33. doi: 10.1200/EDBK_237423
73. Pyo, IS, Yun, S, Yoon, YE, Choi, JW, and Lee, SJ. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules. (2020) 25:4649. doi: 10.3390/molecules25204649
74. Zhou, DD, Luo, M, Huang, SY, Saimaiti, A, Shang, A, Gan, RY, et al. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxidative Med Cell Longev. (2021) 2021:9932218. doi: 10.1155/2021/9932218
75. Orjalo, AV, Bhaumik, D, Gengler, BK, Scott, GK, and Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL6/IL-8 cytokine network. Proc Natl Acad Sci USA. (2009) 106:17031–6. doi: 10.1073/pnas.0905299106
76. Algire, C, Moiseeva, O, Deschênes-Simard, X, Amrein, L, Petruccelli, L, Birman, E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila). (2012) 5:536–43. doi: 10.1158/1940-6207.CAPR-11-0536
77. Anisimov, VN, Berstein, LM, Popovich, IG, Zabezhinski, MA, Egormin, PA, Piskunova, TS, et al. If stated early life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging. (2011) 3:148–57. doi: 10.18632/aging.100273
78. Rena, G, Hardie, DG, and Pearson, ER. The mechanisms of action of metformin. Diabetologia. (2017) 60:1577–85. doi: 10.1007/s00125-017-4342-z
79. Zhang, D, Zhang, J, Zeng, J, Li, Z, Zuo, H, Huang, C, et al. Nano-gold loaded with resveratrol enhance the anti-hepatoma effect of resveratrol in vitro and in vivo. J Biomed Nanotechnol. (2019) 15:288–300. doi: 10.1166/jbn.2019.2682
80. Yessenkyzy, A, Saliev, T, Zhanaliyeva, M, Masoud, AR, Umbayev, B, Sergazy, S, et al. Polyphenols as caloric-restriction mimetics and autophagy inducers in aging research. Nutrients. (2020) 12:1344. doi: 10.3390/nu12051344
81. Price, NL, Gomes, AP, Ling, AJY, Duarte, FV, Martin-Montalvo, A, North, BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondria function. Cell Metab. (2012) 15:675–90. doi: 10.1016/j.cmet.2012.04.003
82. Zeng, XL, Yang, XN, and Liu, XJ. Resveratrol attenuates cigarette smoke extract induced cellular senescence in human airway epithelial cells by regulating the miR-34a/SIRT1/NF-κB pathway. Medicine (Baltimore). (2022) 101:e31944. doi: 10.1097/MD.0000000000031944
83. Matacchione, G, Gurău, F, Silvestrini, A, Tiboni, M, Mancini, L, Valli, D, et al. Anti-SASP and anti-nflammatory activity of resveratrol, curcumin and β-caryophyllene association on human endothelial and monocytic cells. Biogerontology. (2021) 22:297–313. doi: 10.1007/s10522-021-09915-0
84. Kobayashi, M, Okada, Y, Ueno, H, Mizorogi, T, Ohara, K, Kawasumi, K, et al. Effects of supplementation with anti-inflammatory compound extracted from herbs in healthy and obese cats. Vet Med (Auckl). (2020) 11:39–44. doi: 10.2147/VMRR.S240516
85. Martin, G, and Rand, J. Current understanding of feline diabetes: part 2, treatment. J Feline Med Surg. (2000) 2:3–17. doi: 10.1053/jfms.2000.0057
86. Zhang, L, Pitcher, LE, Prahalad, V, Niedernhofer, LJ, and Robbins, PD. Recent advances in the discovery of senolytics. Mech Ageing Dev. (2021) 200:111587. doi: 10.1016/j.mad.2021.111587
Keywords: aging, inflammaging, cats, obesity, senescence-associated secretory phenotype
Citation: Kobayashi M and Kobayashi M (2025) Age-related obesity and inflammaging in cats. Front. Vet. Sci. 12:1639055. doi: 10.3389/fvets.2025.1639055
Edited by:
Anna Maria Giudetti, University of Salento, ItalyReviewed by:
Adriano Carrasco, State University of Midwest Paraná, BrazilSteffi Araujo, State University of Ceará, Brazil
Copyright © 2025 Kobayashi and Kobayashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Motoo Kobayashi, bW9zdWtlYW1tZ0BtZS5jb20=
 Miki Kobayashi
Miki Kobayashi Motoo Kobayashi
Motoo Kobayashi