- 1"Ion Ionescu de la Brad" Iasi University of Life Sciences, Iasi, Romania
- 2Department of Veterinary Medicine and Animal Productions, University of Naples Federico II, Naples, Italy
Feline oral squamous cell carcinoma (FOSCC) is the most common oral malignancy in cats, characterized by aggressive local invasion, high metastatic potential, and poor clinical outcomes. Its etiology is multifactorial, involving genetic mutations (notably TP53), viral infections (such as papillomavirus), environmental exposures to xenobiotics and chronic oral inflammation, though definitive causal relationships remain unclear due to limited studies. FOSCC primarily affects older, non-pedigree cats, with no clear sex or breed predisposition, and most frequently arises in the gingiva, sublingual region, and tongue. FOSCC presents with non-specific signs like weight loss, oral ulceration, and difficult eating, often leading to late diagnosis. FOSCC displays highly infiltrative growth with marked cellular pleomorphism and frequent bone invasion. Recent advances have identified various biomarkers, such as Ki-67, Cyclin D1, Bmi-1, and EMT-related proteins, that enhance diagnostic accuracy and prognostic assessment, while emerging research into tumor mutational burden and metabolic pathways offers promising therapeutic targets. Prognosis remains poor, with median survival times typically under 2 months and limited response to conventional treatments; however, surgical intervention and novel targeted therapies show potential for improved outcomes. This review synthesizes recent progress in understanding FOSCC etiology, pathology, and therapeutic strategies, and highlights ongoing challenges and future directions in the management of this devastating feline cancer.
1 Introduction
Cancer poses serious health challenges in domestic animals, with feline oral squamous cell carcinoma (FOSCC) being the most common and aggressive oral cancer in cats. FOSCC exhibits rapid local invasion and metastasis, driven by complex genetic, structural, environmental, and infectious factors. Advances in veterinary oncology have revealed key molecular mechanisms and biomarkers, enabling new targeted and immunotherapeutic strategies (1–8). Despite progress, gaps remain in understanding specific risk factors and treatment resistance (9–11). This review emphasizes recent advances in FOSCC research in terms of etiology, epidemiology, prognosis, pathology and explores future perspectives, including new therapeutic approaches and molecular diagnosis, that could further enhance understanding and treatment of this challenging feline cancer.
2 Etiology
2.1 Viral infection
Several viruses, including FcaPV (Felis catus papillomavirus), FIV (Feline immunodeficiency virus), FeLV (Feline leukemia virus), and EBV (Epstein–Barr virus) have been investigated for their role in FOSCC (10–17).
Feline papillomaviruses have been detected in tumor samples, particularly Felis catus papillomavirus type 2 (FcaPV-2) (9, 13–15, 17–25). The viral oncoproteins E6 and E7 contribute to oncogenesis by disrupting key tumor suppressor pathways, specifically p53 and pRb, thereby promoting cancer development (22, 26, 27). High viral DNA loads correlate with elevated E6 and E7 expression, indicating that FcaPV-2 can actively drive tumor growth in affected cases (28–31). The overexpression of p16, a surrogate biomarker linked to E7-mediated pRb disruption, may be involved in FcaPV-related FOSCC, although its exact role requires more investigation (13, 17, 25). Disruption of the viral E2 gene, which normally regulates viral transcription, leads to unchecked expression of these oncogenes (22). Notably, the occasional co-expression of L1 capsid protein alongside E6/E7 in tumors suggests ongoing viral replication, maintaining a persistent immune response that fosters a pro-tumorigenic inflammatory microenvironment through tissue damage and cytokine release (25, 28, 32). Interestingly, recent studies indicate that different FcaPV types are detectable in in situ carcinoma of the oral cavity, suggesting a viral-driven multi-step carcinogenesis and providing additional evidence of their role in FOSCC development (33). Due to pathological similarities with human head and neck squamous cell carcinoma (HNSCC) associated with high-risk human papillomavirus infection, FcaPV-positive FOSCC is proposed as a relevant animal model for HPV-driven HNSCC (34–37).
FIV, a lentivirus causing immunosuppression similarly to human HIV, infects the oral cavity, creating a viral reservoir that promotes chronic inflammation and immune dysfunction. This environment facilitates cellular dysregulation, increasing neoplastic risk and contributing to FOSCC development (16). The immunosuppressive effects of FIV promote repeated cellular turnover and damage, critical steps in carcinogenesis (38).
FeLV, a retrovirus known for causing lymphomas and sarcomas, may also contribute to FOSCC through insertional mutagenesis, impairing oncogenes and tumor suppressor genes and triggering malignant transformation (39, 40). Therefore, FeLV-induced immune dysregulation and chronic inflammation may further increase susceptibility to FOSCC.
Additionally, EBV has been detected in one FOSCC case, but further research is needed to understand better the possible role of this virus in the etiopathogenesis (12, 41).
Dated studies presented conflicting results regarding detection of viral infection in FOSCC. Variability in sample size and viral detection methods may justify this apparent inconsistency with most recent research. Techniques differ widely, from PCR-based viral DNA detection and immunohistochemistry to in situ hybridization, each with varying sensitivity and specificity. Standardization of methodological approaches represents a significant challenge to be addressed in future research in order to definitely clarify the role of viral infections in FOSCC etiology.
2.2 Environmental and lifestyle factors
Exposure to environmental tobacco smoke (ETS) has been investigated as a risk factor for FOSCC. However, studies have not found a statistically significant correlation, despite some suggesting a twofold increased risk in exposed cats (1–3, 42). Unlike in humans, no dose–response relationship was observed between exposure to cigarette smoke and cancer development (1–3, 42). Dietary factors have also been investigated, with wet food consumption, especially canned tuna, being associated with a 3.6-fold increased risk. This association may be due to nutrient differences in these foods or because high canned food intake leads to poor dental hygiene, promoting tartar buildup, bacterial toxins, oral inflammation, and potentially neoplastic transformation, though statistical significance was not established (1, 2).
The use of ectoparasite control methods, such as flea collars, has been linked to an increased risk (5.3-fold), where chronic exposure to chemical compounds in these products may induce cellular damage, oxidative stress, or immune disruption, potentially contributing to carcinogenesis, but evidence remains inconclusive (1, 2). Clumping clay cat litter and flea collar use were reported as significant risk factors (ORs 1.66 and 4.48, respectively), possibly related to carcinogenic substances such as crystalline silica in clay litters and tetrachlorvinphos in flea collars (43). These findings suggest environmental chemical exposures may play a role in FOSCC development and warrant further research.
2.3 Chronic inflammation and comorbidities
Chronic oral conditions such as periodontal disease (PD), feline chronic gingivostomatitis (FCGS), and other oral inflammatory conditions may contribute to carcinogenesis, though studies directly linking them to FOSCC are lacking (9, 44–47). In humans, chronic inflammation is known to induce genetic mutations and epigenetic alterations, leading to cancer (48). The involvement of inflammatory mediators like cytokines, prostaglandins, and metalloproteinases, which promote tumor progression, has been demonstrated in both human and feline SCC (5, 7, 48–51). Additionally, a case of Trichinella spp. infection was reported in an FOSCC sample, but its carcinogenic role remains unclear, further research is required (44, 52).
2.4 Genetic and molecular events
Genetic mutations, particularly in the TP53 gene, are commonly found in FOSCC and are thought to play a critical role in tumor development (2, 17, 25, 53, 54). Increased expression of the tumor suppressor protein p53 has been observed in some ETS-exposed cats, no direct link to tobacco exposure was confirmed (2, 3). The overexpression of p16, a biomarker for cell senescence, has been assessed in several studies, but no statistically significant correlation with papillomavirus infection was found, more studies are needed in this area (13, 17, 20, 25, 31, 55, 56). Other molecular pathways, such as cyclooxygenase (COX), signal transducer and activator of transcription 3 (STAT3), epidermal growth factor receptor (EGFR), and vascular endothelial growth factor (VEGF), have been implicated in tumor progression, suggesting a complex interplay of molecular alterations in FOSCC development (5, 7, 49, 51, 57).
2.5 Development and structural factors
It has been proposed that FOSCC may originate from dental structures, such as the dental lamina and enamel organ epithelium, similar to dentigerous cyst-associated SCC in humans (58, 59). While this hypothesis remains speculative, it aligns with observations in other species, suggesting a possible developmental contribution to the disease.
2.6 Microbial influences and oral flora
Human studies show that imbalances in oral bacteria can promote oral cancer by increasing inflammation (60). In cats, infection with FIV is associated with harmful shifts in oral bacteria, increasing the risk of oral squamous cell carcinoma (61). Differences in oral microbiota between healthy cats and those with periodontitis indicate that bacterial imbalance may also raise cancer risk in felines, paralleling findings in humans (62).
The influence of oral microbiota on the development of FOSCC is an area of ongoing research. Exploring the role of microbial profiles in oral health and disease could identify specific pathogens as potential risk factors for feline oral cancer.
Understanding the causes of FOSCC is limited due to few studies, many with small sample sizes or case series (1–3). Using owner-reported data for exposure to ETS may cause bias, as smoking households with affected cats might be underrepresented (1–3). The lack of standard methods for virus detection and missing information about the patient health status may also underestimate the role of infection (9–11, 63). Future research should involve larger, well-controlled studies to better clarify how these factors contribute to FOSCC development (Figure 1).
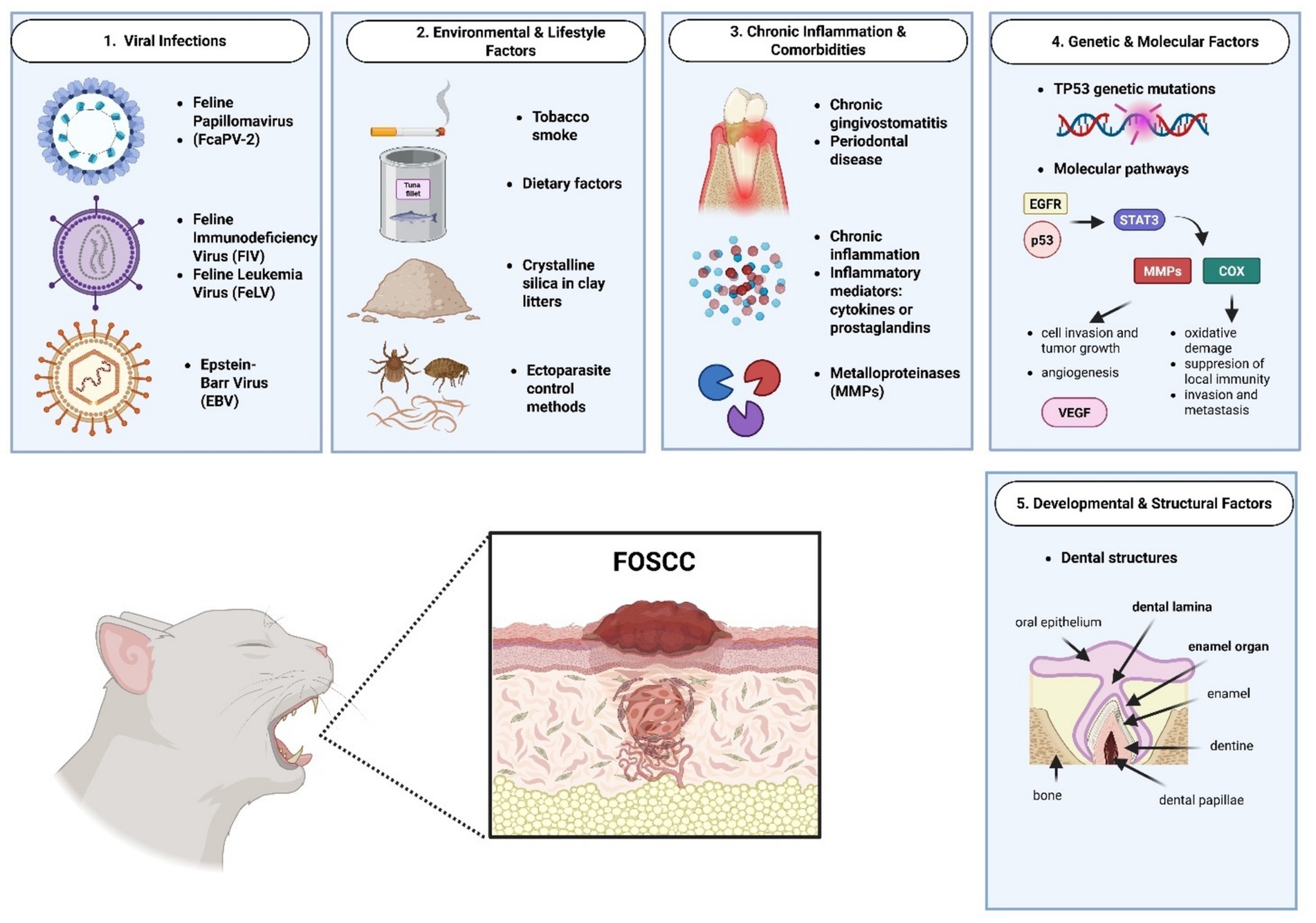
Figure 1. Schematic representation of feline oral squamous cell carcinoma etiologic factors. Created in BioRender. Tutu, P. (2025) https://BioRender.com/zpfsdkn.
3 Epidemiology
FOSCC is the most common malignant oral tumor in cats, accounting for 46–61.2% of all oral neoplasms in multiple surveys (64, 65). Epidemiological studies have been conducted worldwide, with data collected from the USA, UK, Italy, New Zealand, Slovenia, and Japan, encompassing hundreds of cases (38). Most studies are retrospective and based on histopathological review of biopsy samples, this limitation may influence the possibility in establishing a direct causal relationship between the neoplasia and the potential etiologic factors described in chapter 2 (17, 38, 66–68).
3.1 Breed and demographics
Non-pedigree (domestic) cats represent the majority of FOSCC cases, 84–96% in several studies, with domestic shorthair being the most common, purebred cats are less affected, but breeds such as Burmese, Maine Coon, Persian, Chartreux, and Siamese are known to be affected (38, 50, 66–68). The median age at diagnosis typically ranges from 11 to 13.5 years, with reported ranges spanning 1 to 21 years (17, 50, 64, 67, 68). Both sexes are affected, with studies reporting near-equal or slightly higher female representation (17, 50, 67–69).
3.2 Anatomical sites and tumor characteristics
FOSCC most frequently arises in the gingiva (mandibular and maxillary), sublingual region, and tongue (17, 68–70). The tongue is more commonly affected in younger cats (mean age 11.9 years), while gingival tumors occur in slightly older cats (mean age 13.6 years). Tumors are often invasive, with frequent bone involvement (osteolysis), especially in maxillary (48%) and mandibular (33%) cases (68, 70).
4 Pathology
4.1 Histopathology
Oral squamous cell carcinomas in domestic animals consist of invasive nests of cancerous epithelial cells penetrating the submucosa, with basaloid outer cells and larger eosinophilic central cells. These tumors show abnormal maturation, keratinization irregularities, and solid masses with intercellular bridges (71). They grow aggressively, often spreading single cells beyond the main tumor (Figure 2A), causing ulceration, inflammation, and sometimes bone invasion with surrounding fibrous tissue. Tumors vary in differentiation, influencing prognosis, and may contain abnormal nuclei, necrosis indicating aggressiveness, multinucleated cells, and lymphocyte infiltration, while stromal fibrosis is generally minimal (71–73).
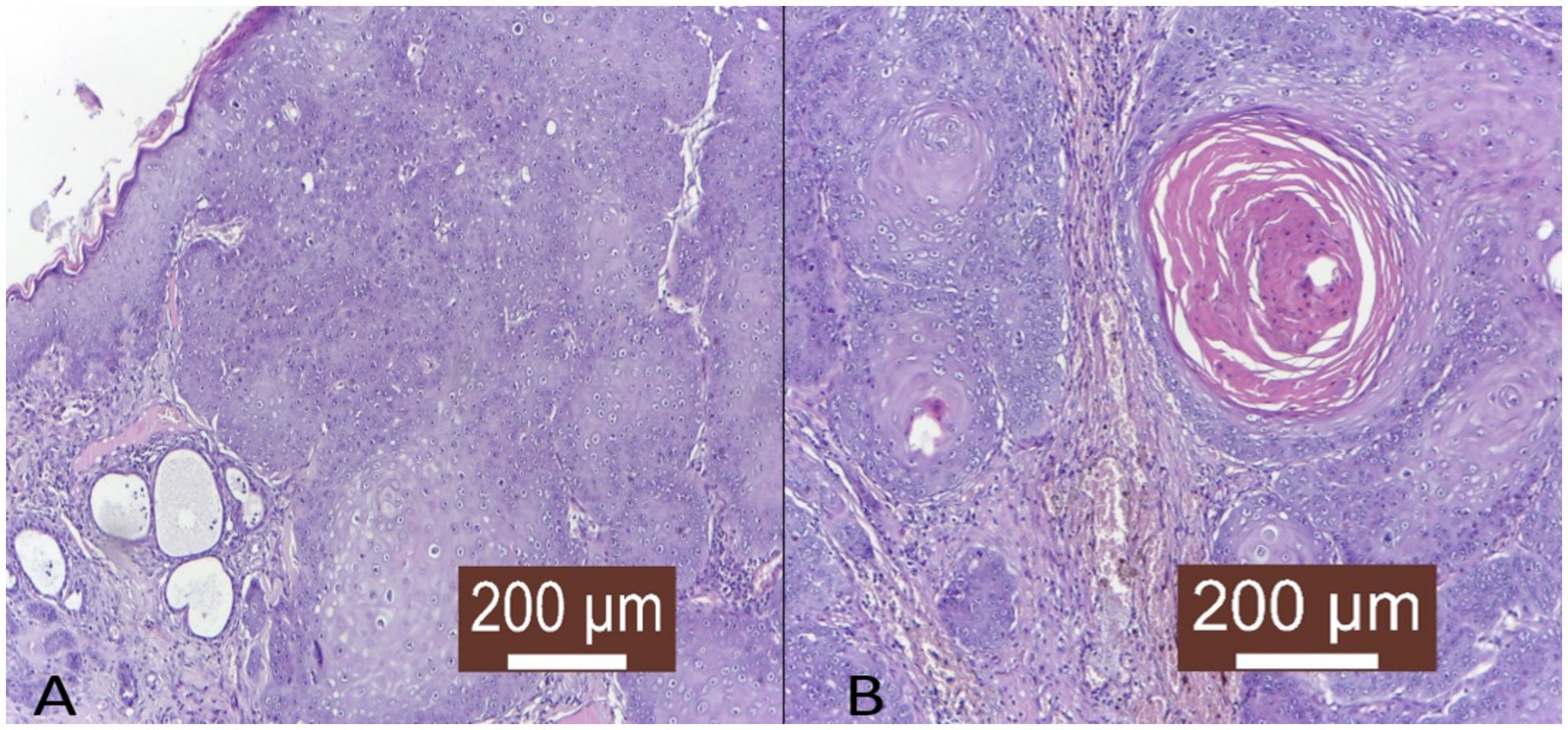
Figure 2. Histopathological representation of feline oral squamous cell carcinoma. (A) Irregular, columnar, diffuse tumor infiltration in the deep layers of the skin with the formation of keratin-rich tumor islands (HE X100). (B) Tumor islet with high keratin content in the profound layer of the skin (HE X100).
Several distinct histological subtypes can be identified in FOSCC based on criteria adapted from HNSCC classifications. These subtypes primarily include well-differentiated keratinizing squamous cell carcinoma, poorly differentiated non-keratinizing squamous cell carcinoma, and basaloid squamous cell carcinoma. The well-differentiated subtype is characterized by the presence of keratin pearls and an organized arrangement of squamous cells (Figure 2B), indicative of maintained differentiation. Poorly differentiated variants lack these organized features, often exhibiting a higher degree of pleomorphic cells, which can result in more aggressive clinical behavior (66, 74).
The basaloid variant, although less frequent, presents a distinct histological profile with high cellularity and minimal keratin formation. This subtype can exhibit a pattern similar to that of adenoid cystic carcinoma, which poses diagnostic challenges as it might be misidentified without careful histopathological evaluation (66). The presence of such variants in FOSCC suggests the need for meticulous histological examination and classification, as they correlate strongly with clinical outcomes and prognostic indicators (34, 75).
In both cats and humans, OSCC is the most common oral cancer, characterized by invasive malignant keratinocytes. A key difference is that keratin pearls, common in human OSCC, are rare in cats, reflecting faster progression in felines (74).
4.2 Clinical appearance
FOSCC is an aggressive neoplasm that can arise in various locations within the oral cavity, most commonly affecting the mandibular (Figure 3A), maxillary, and sublingual regions (50, 70, 76). Less frequently, tumors develop on the hard palate, soft palate, larynx, pharynx, or lips, although these sites account for less than 2% of cases (70). Regardless of location, SCC tend to be locally invasive and destructive, leading to severe clinical signs.
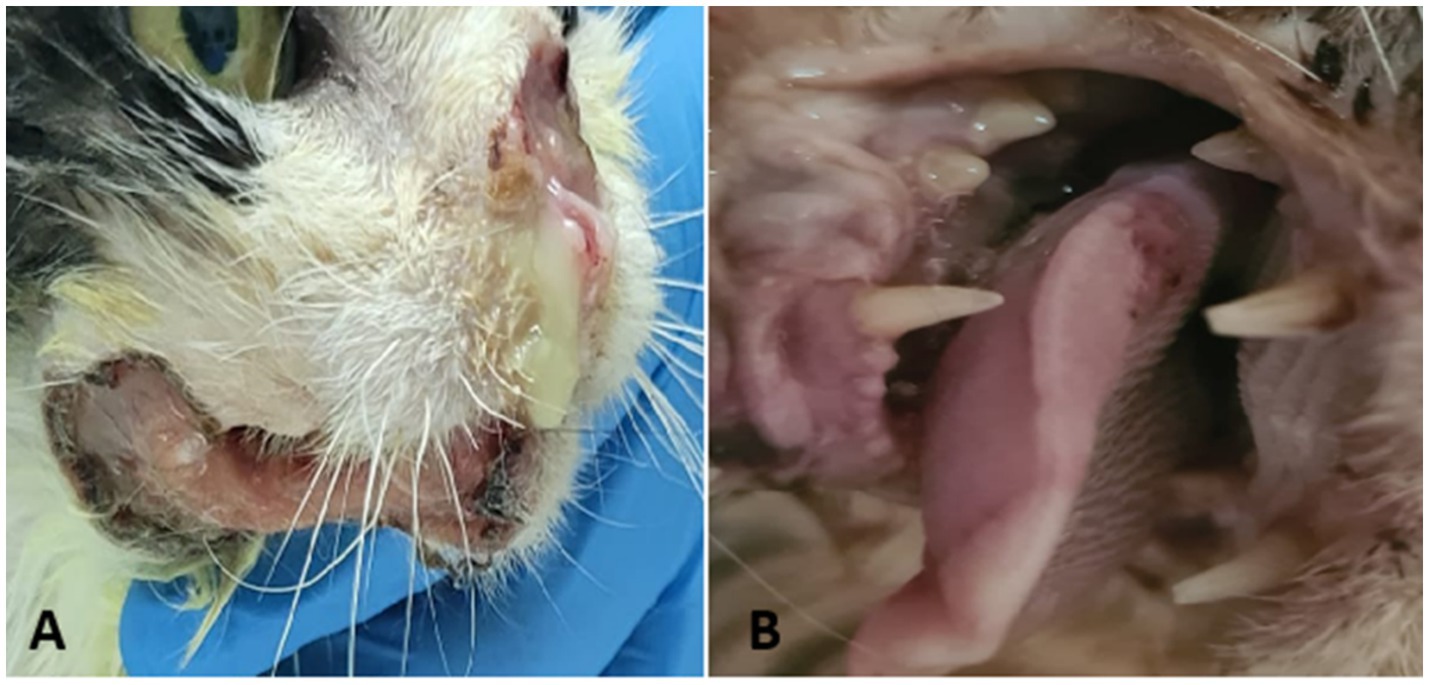
Figure 3. Feline oral squamous cell carcinoma—clinical presentation. (A) Ulcerated and infected nasal and mandibular lesion. (B) Ulcerated lesion at the base of the tongue.
In the initial stages, FOSCC may appear as a small, raised, fleshy mass or as an ulcerated lesion with little visible proliferation. Despite their relatively subtle appearance on physical examination, these tumors often invade surrounding tissues extensively. Affected cats commonly exhibit nonspecific signs such as reduced appetite, weight loss, lethargy, halitosis, excessive drooling, and decreased grooming. Owners frequently report difficulty in eating, and veterinarians may observe loose or mobile teeth in the affected area. While tooth extraction may temporarily improve appetite, the extraction site often fails to heal, forming a persistent ulcer. Tumors that develop in the sublingual and lingual areas (Figure 3B) can look like a foreign body invading the tongue aggressively. This leads to a firm thickening of the tongue, reduced movement, ulceration, and in severe cases necrosis due to poor blood supply. As these tumors grow, the tongue may stick out of the mouth, which can cause trauma, bleeding, and make eating difficult. Maxillary SCC are highly destructive, spreading into bone and causing bone loss with large lesions. Tumors located toward the back of the upper jaw may interfere with eye movement, while those at the front often cause teeth to become loose or fall out, even if the gums appear healthy (71).
Mandibular SCC show similar signs, including ulceration, loose teeth, and in some cases, new bone formation and bone loss even without visible ulceration. Occasionally, tumors originate within the jawbone itself, resembling intraosseous carcinoma (77).
4.3 Biomarkers
The potential role of biomarkers as immunohistochemical markers in feline tumors is a subject of current exploration. A number of significant biomarkers have been evaluated in FOSCC, demonstrating correlations with their counterparts in HNSCC, indicating shared molecular pathways and disease mechanisms.
These biomarkers are classified into several categories, including proliferation markers, epithelial-mesenchymal transition (EMT) factors, immune checkpoints, angiogenesis-related proteins, stromal remodeling elements, genetic alterations, and metabolic regulators. Among these, p53, p16, EGFR, VEGF and COX-2 have been the most studied (2–8, 17, 25, 54, 66, 78–89). However, there are some new markers, particularly Ki67, Bax, Bcl2, Caspase3, NQO1 and TERT, which offers new insights and new perspectives into the FOSCC molecular evolution and targetability (Figure 4; Table 1) (5, 79, 80, 90–92).
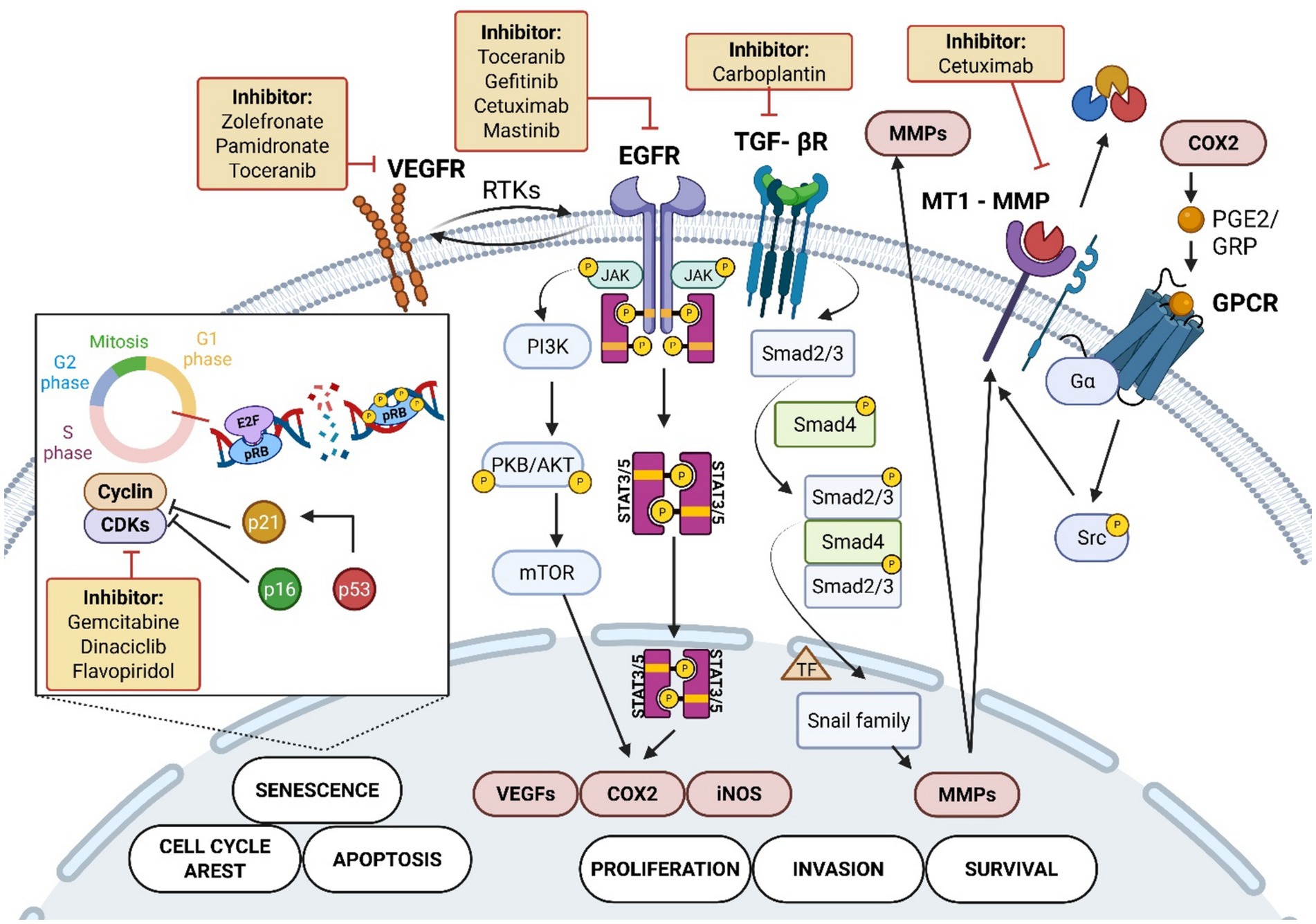
Figure 4. Key biomarkers and targeted treatments in feline oral squamous cell carcinoma. Carcinogens activate receptor tyrosine kinases (RTK) such as VEGFR and EGFR, triggering signaling cascades including JAK/STAT3/5, PI3K/AKT/mTOR, and TGF-β receptor/Smad pathways. These pathways regulate cellular processes such as proliferation (COX2, VEGF), survival (MMP), invasion (Snail), apoptosis, senescence, and cell cycle arrest (p53, p16, p21, CDK, Cyclins, E2F, pRB). COX2 activation involves GPCR and Src kinase signaling leading to MMP activity promoting the invasive activity of the tumor. The inset shows cell cycle control through pRB phosphorylation and CDK regulation (5, 8, 25, 102, 139, 144–146). The figure also depicts pharmacological inhibitors targeting these molecules, including Toceranib, Gefitinib, Cetuximab, Gemcitabine, Carboplatin, and others, used in the treatment of FOSCC (4, 46, 91, 96, 115, 116, 124, 125, 128, 135, 142). Created in BioRender. Tutu, P. (2025) https://BioRender.com/3hns4gp.
The analysis of biomarkers in FOSCC and their correlation with HNSCC presents the notion of using FOSCC as a valuable naturally occurring model for studying in human’s counterparts. The substantial similarity in biomarker profiles indicates the feasibility of translational research, whereby feline cancer studies could provide insights that inform human medicine, particularly in the context of exploring novel therapeutic strategies and preventive measures (5, 34, 83). Furthermore, given the similarity in the pathways of tumour evolution exhibited by both cancers, interventions targeting shared biomarkers may result in advancements in treatment outcomes across species.
5 Prognosis
FOSCC poses significant challenges in veterinary medicine due to its aggressive nature and poor prognosis. Understanding the prognostic factors associated with FOSCC is critical for enhancing treatment strategies and overall outcomes. Much like HNSCC, various biomarkers, clinical indicators and treatment modalities influence the clinical course and therapeutic response of FOSCC.
5.1 Treatment modalities
FOSCC carries a poor prognosis, with median survival times (MST) of 44 to 60 days and a one-year survival rate of 5–10% (50, 63, 78, 93). Surgical excision, especially mandibulectomy, can improve survival, with MST reported up to 420 days, though recurrence rates remain high at 38% (94, 95). Traditional radiation and chemotherapy are generally ineffective, accelerated radiation combined with carboplatin has extended MST to around 163 days (96). FOSCC is notably resistant to conventional therapies, with mechanisms of resistance still not well understood (94, 95, 97, 98).
5.2 Tumor location
Tumor site influences prognosis. Cats with maxillary SCC tend to have longer survival compared to other oral locations (63). Oropharyngeal SCCs show longer MSTs than sublingual or other sites, possibly linked to differences in cancer-associated fibroblasts (87). Bone invasion does not seem to affect prognosis significantly, reflecting the highly invasive nature of these tumors regardless of histology (17). Metastasis drastically worsens survival, with MST of 24 days for cats with multiple lymph node or distant metastases versus 90 days for non-metastatic cases (63, 93, 94).
5.3 Molecular markers
Several molecular markers have prognostic relevance. Diffuse cyclooxygenase-1 (COX-1) expression correlates with longer survival (50). The Ki67 proliferation index shows mixed results: some studies link high Ki67 to worse outcomes, but this is not confirmed in other studies (78, 79). EGFR expression generally does not correlate significantly with survival, though lower survival trends imply potential as a therapeutic target (78, 79). Tumor vascularization assessed by microvessel density (MVD) lacks strong survival correlation, despite higher MVD in tongue tumors (99, 100).
Immunohistochemical markers like p16 associate with longer survival independently of papillomavirus infection (101, 102), while p53 expression is unreliable for prognosis, suggesting diverse oncogenic pathways in FOSCC development (13, 17, 103, 104). Histologic differentiation and invasion patterns have not consistently predicted outcomes (105–107).
FOSCC remains a highly aggressive neoplasm with limited treatment success. The identification of molecular markers may enhance prognostic predictions and guide treatment decisions. Future studies should focus on refining prognostic markers and exploring targeted therapies to improve clinical outcomes for affected cats.
6 Therapy
FOSCC remains a challenging disease. Radiation therapy, chemotherapy, and surgery are standard treatments, often used together to control the disease and improve quality of life. Emerging approaches such as metabolic therapy, bisphosphonates, stem cell therapy, immunotherapy, tyrosine kinase inhibitors (TKI), analgesics, and gene therapy are being explored to enhance outcomes (Table 2). Traditional treatments focus on tumor control, while newer therapies attempt to target tumor growth, manage pain, and improve survival, aiming at the diversification and enrichment of the arsenal against this aggressive cancer.
6.1 Radiation therapy
Radiation therapy for FOSCC demonstrates clinical feasibility through various protocols that offer symptom relief and tumor control, but outcomes and tolerability vary considerably. Stereotactic Radiation Therapy (SRT) provides rapid symptom improvement with an overall response rate of 38.5% and median survival around 106 days. However, factors such as high tumor microvascular density and keratinization negatively impact survival, and complications like mandibular fractures can impair quality of life (80). Accelerated hypofractionated radiation (4.8 Gy × 10) shows promising tumor responses and extended progression-free and overall survival with generally manageable mucositis and some late effects, although long-term toxicity remains a concern (108).
Coarse Fractionation Radiotherapy (8 Gy × 3) offers limited palliation and shorter median survival (60 days) but poses substantial risks including mucositis, pain, and dysphagia, which can affect clinical tolerability (97). A similar coarse fractionated megavoltage protocol (24–40 Gy in 3–4 fractions) improves quality of life in a majority of cats, with median survival ranging near 92 days (109). The accelerated protocol (3.5 Gy × 14 over 9 days) is moderately tolerable and achieves a median survival of 86 days, while cats achieving complete response may survive substantially longer (110). Microbrachytherapy with holmium-166 microspheres shows encouraging local control rates (55%) and minimal side effects, allowing for less extensive surgery in some cases and improved survival in responders (111).
Despite initial radiosensitivity, FOSCC frequently develops radioresistance over time, driven by mechanisms such as enhanced DNA repair, cancer stem cell activation, EMT, and tumor microenvironment changes. These adaptations reduce long-term treatment efficacy and contribute to the overall poor prognosis (112–114). Current limitations include variable survival benefits, toxicities affecting quality of life, and the inevitability of radioresistance. Future directions emphasize the development of multimodal strategies that integrate radiation therapy with surgery, systemic treatments, or molecular targeted therapies to overcome resistance and improve clinical outcomes (112–114).
6.2 Chemotherapy
Chemotherapy shows clinical feasibility with several agents explored, though overall efficacy remains limited and survival benefits modest. Low-dose gemcitabine combined with palliative radiotherapy achieves partial or complete responses in some cats, with a median survival time of 111.5 days (115).
Carboplatin acts as a radiosensitizer in accelerated radiation protocols, particularly benefiting tonsillar SCC with manageable toxicity and modest antitumor activity (96, 116, 117). Ultrasound and microbubble-enhanced chemotherapy (USMB) using bleomycin is a clinically feasible and safe approach to improve drug delivery and tumor perfusion but has shown limited clinical efficacy in cats (118). The novel agent IB-DNQ, targeting NQO1 to generate cytotoxic reactive oxygen species, presents promising targeted therapeutic potential (92). Conversely, liposomal cisplatin (L-NDDP) proved ineffective, yielding no tumor responses and poor survival despite acceptable toxicity (119). Ethyl Cellulose-Ethanol Ablation (ECEA), which combines chemotherapy with localized electric pulses to retain ethanol intratumorally, produced transient tumor shrinkage but poor functional outcomes in lingual and sublingual SCC, limiting its applicability to these sites (120).
Limitations of chemotherapy include generally modest survival improvements, inconsistent tumor responses, and treatment-related toxicities that affect quality of life (118–120). Future directions should focus on refining multimodal protocols integrating chemotherapy with radiation, surgery, and novel targeted agents to enhance efficacy. Continued research into innovative drug delivery systems and molecularly targeted therapies is essential to overcome current therapeutic challenges and improve prognosis in FOSCC.
6.3 Surgical interventions
Surgical interventions include maxillectomy and mandibulectomy. These are aggressive procedures but offer a potential curative approach if the tumor is localized and able to extract (94, 121).
Maxillectomy is an effective treatment for FOSCC, achieving good local tumor control and extended survival times. The procedure includes various techniques, such as unilateral rostral, bilateral rostral, segmental, caudal, and total unilateral maxillectomy. While intraoperative complications occur in 16.7% of cases, postoperative complications are more common, with hyporexia and incisional dehiscence affecting 20% of cats. Despite these challenges, survival rates are promising, with a two-year survival rate of 83% for FOSCC cases. Poor prognostic factors include a high mitotic index, the need for adjuvant chemotherapy, and local recurrence, which significantly impact survival (121).
Radical mandibulectomy is another aggressive surgical approach for managing extensive FOSCC. The procedure involves removing 75 to 90% of the mandible, necessitating feeding tube placement in all cases. While some cats experience local recurrence, others achieve long-term survival, with some living beyond 1 year. The mean estimated survival time following mandibulectomy is 712 days. Importantly, six out of eight cats were able to resume independent food intake postoperatively. With appropriate perioperative supportive care, radical mandibulectomy is a viable option for treating extensive feline oral neoplasia and can result in prolonged survival for selected patients (94).
Future directions should focus on minimizing surgical morbidity, improving perioperative care, and integrating multimodal therapies to address local recurrence and metastatic disease. Investigating less invasive techniques or adjunct treatments to enhance surgical success and quality of life is warranted.
6.4 Metabolic therapy
Metabolic therapy represents a clinically feasible and innovative approach by targeting cancer-specific energy and nutrient metabolism. MD-1 therapy disrupts glycolytic and mitochondrial metabolism, particularly oxidative phosphorylation (OXPHOS), effectively killing FOSCC cell lines and reducing tumor growth in both subcutaneous and orthotopic models. These promising in vitro and in vivo findings position MD-1 as a potential novel treatment for FOSCC and possibly HNSCC (89).
Another metabolic strategy targets polyamine synthesis using 2-Difluoromethylornithine (DFMO), which lowers tumor polyamine levels essential for proliferation. While DFMO monotherapy can reduce tumors, notable toxicities such as ototoxicity and subclinical thrombocytopenia present limitations that require further optimization (122, 123). The combination of DFMO with MQT 1426 is feasible and safe, yielding modest clinical benefits like stable disease or tumor regression; however, dosing adjustments are necessary to reduce vestibular toxicity.
Although metabolic therapies show promise by exploiting tumor-specific metabolic vulnerabilities, challenges remain regarding toxicity and optimal dosing protocols. Future directions should involve refining such metabolic interventions, integrating them with conventional therapies, and expanding translational research to improve outcomes for FOSCC and related human cancers.
6.5 Bisphosphonates
Bisphosphonates are useful for managing bone-invasive FOSCC. They work by blocking osteoclastic bone resorption and angiogenesis, which helps reduce pain. Zoledronate can slow tumor growth and reduce bone damage. It lowers levels of serum VEGF and C-terminal telopeptide (CTx), markers linked to tumor activity. When given with meloxicam, zoledronate is well-tolerated. Meloxicam helps slow tumor growth, while zoledronate prevents bone breakdown (4, 124).
Pamidronate is another bisphosphonate that also blocks bone resorption and blood vessel growth. A small study in eight cats with bone-invasive cancers, including FOSCC, found pamidronate to be safe and feasible. It showed modest benefits, like stabilizing the disease in some cats. However, no direct tumor shrinkage was seen. Still, pamidronate’s ability to inhibit tumor cells in lab tests and ease bone-related symptoms supports further research (125).
Bisphosphonates mainly offer palliative benefits rather than curing the disease. More studies are needed to improve their use, possibly combining them with other treatments. This could help improve quality of life and clinical outcomes in cats with bone-invasive FOSCC.
6.6 Emerging therapies
Emerging therapies, such as TKI, stem cell therapy and gene therapy were used in FOSCC patience as alternative treatments.
Mastinib, a TKI, is effective at slowing cancer cell growth. This approach targets important pathways and works in both cats and dogs (126). Gene therapy using TBG-RNAi-fCK2αα’ is safe and shows some signs of shrinking tumors (127). Both treatments appear feasible and deserve further trials (126, 127). Toceranib, a TKI, offers modest efficacy, extending median survival to 123 days compared to 45 days in untreated cats, with a biological response rate of 56.5%. Improved outcomes are noted when combined with NSAIDs, yet long-term survival is still poor (128).
Stem cell therapy using feline umbilical cord MSCs can reduce symptoms for a short time (128). However, the benefits are temporary, and disease quickly worsens. Immunotherapy with lymphokine-activated killer (LAK) cells is safe even in older cats, but it has not been shown to extend survival or slow cancer (129).
All therapies have limited or inconsistent effects. Stem cell treatment only relieves symptoms briefly without improving survival (128). Immunotherapy is well tolerated but lacks proof of effectiveness (129). Gene therapy needs better dosing and more reliable results (127). The TKI data come from small studies and need stronger evidence from larger trials (126).
More research with larger, prospective studies is needed. Combining TKI with other treatments might improve results. Gene therapy should be fine-tuned for dosing and timing. Immunotherapy approaches must identify better targets and boost immune responses. Using multiple therapies together could offer better tumor control and longer survival. Developing biomarkers will help make treatments to individual cats for better outcomes.
7 Future perspectives
7.1 New areas of interest
Research in FOSCC is advancing by focusing on several promising areas. Tumor mutational burden (TMB), defined as the number of somatic mutations per megabase in tumor DNA, is emerging as a biomarker to predict response to immune checkpoint inhibitor therapy. FOSCC shows a high TMB (>5.0), similar to HNSCC, suggesting potential responsiveness to future immune checkpoint therapies (83, 130).
Genomic studies have identified polyamine-related signatures that influence tumor metabolism and the microenvironment, offering new therapeutic targets (131). Additionally, EMT-related genes such as SNAI1, TWIST1, ZEB1, ZEB2, and mesenchymal markers FN1, VIM, and CDH2 are enriched in FOSCC and contribute to metastasis (83). Although immune checkpoint inhibitors like PD-L1 and CTLA-4 are not yet available for FOSCC treatment, they remain promising candidates for immunotherapy trials (6, 66, 83–85). Metabolic targeting approaches, including dual MCT1/MCT4 inhibitors and pathways regulated by hypoxia-inducible factors such as HIF-1α, are being explored as potential therapies (89).
The focus on molecular and metabolic factors, such as high TMB and EMT gene expression, that highlight tumor vulnerabilities, appears to be effective in the FOSCC research. These findings support immune checkpoint inhibitors and metabolic targeting as promising therapeutic strategies because they address key mechanisms driving tumor growth and spread.
7.2 New therapeutic frontiers
FOSCC is an aggressive malignancy with poor prognosis and novel treatment approaches are challenging. Recent advances are related to innovative strategies enhancing therapeutic outcomes.
7.2.1 Electrochemotherapy (ECT)
Electrochemotherapy (ECT) has emerged as a promising localized treatment for FOSCC. Studies have demonstrated its efficacy, particularly in combination with bleomycin. One study reported an 81.8% complete response rate in superficial SCC lesions, with some responses lasting over 3 years, highlighting its durability and tolerability (132). Another study showed that ECT with bleomycin significantly outperformed bleomycin alone, achieving an 89% overall response rate and a median progression-free survival of 30.5 months, compared to 3.9 months in the control group (133). These findings underscore ECT’s potential for managing advanced SCC in critical areas like the head.
7.2.2 Targeted molecular therapies
Targeted molecular therapies for FOSCC include EGFR-targeted agents, telomerase inhibitors, nanobody-targeted photodynamic therapy, and bone-targeted treatments, each designed to interfere with specific pathways involved in tumor growth and progression.
EGFR is a key driver in epithelial cancers due to its frequent overexpression or mutation, which leads to persistent activation of signaling pathways that promote tumor cell proliferation, survival, invasion, and metastasis, making it a critical molecular target for therapies such as TKI and monoclonal antibodies (134–139).
Cetuximab, an anti-EGFR monoclonal antibody used as therapeutic agent against HNSCC, has demonstrated efficacy in FOSCC cell lines. It inhibits EGFR activation and downstream signaling pathways such as Akt, reducing proliferation, promoting apoptosis, and impairing invasion by downregulating matrix metalloproteinases (MMP-2/-9) and EMT markers (82, 135). These findings suggest that Cetuximab could be a valuable addition to feline cancer therapy.
Gefitinib, an EGFR TKI, has been shown to suppress cell proliferation and migration in FOSCC. Resistance to gefitinib can occur, not due to mutations in its kinase domain. RNA interference (RNAi) targeting EGFR has demonstrated potential in overcoming this resistance and exhibits an additive effect when combined with radiation therapy (140).
7.2.3 Immunotherapy
The potential of immunotherapy in FOSCC treatment is being explored, with particular focus on immune checkpoint inhibitors. Nivolumab, an anti-PD-1 antibody approved for recurrent or metastatic HNSCC in humans, has demonstrated significant survival benefits over standard therapies. While its application in FOSCC is under investigation, its success in human oncology suggests a promising translational opportunity (141).
7.2.4 Chemotherapeutics
Several chemotherapeutic agents used for other cancers have demonstrated efficacy against FOSCC. Methotrexate, actinomycin D, and CDK inhibitors such as dinaciclib and flavopiridol have shown strong anti-proliferative effects on FOSCC cell lines while sparing normal fibroblasts. These agents induce apoptosis and alter cell cycle progression, making them viable candidates for further clinical trials in feline oncology (91, 142). Additionally, methotrexate’s established efficacy in HNSCC supports its potential use in FOSCC (143). The utilization of these agents in the human oncology field, accompanied by their results in FOSCC cell lines, makes them promising targets in FOSCC future therapy.
8 Conclusion
FOSCC remains the most common and aggressive oral malignancy in cats, posing significant challenges for both diagnosis and treatment. Its multifactorial etiology highlights the complexity of this disease and the need for a holistic approach to both research and clinical management. Despite advances in our understanding of the molecular and cellular mechanisms of FOSCC, the prognosis for affected cats remains poor, with median survival times rarely exceeding few months.
Recent research has revealed some promising opportunities for improving the diagnosis, prognosis, and treatment of FOSCC. The identification of key biomarkers, such as Ki-67, Cyclin D1, Bmi-1, and EMT-related proteins, has improved our ability to diagnose and prognosticate FOSCC, while studies on genetic mutations and molecular pathways (including TP53, COX, STAT3, EGFR, and VEGF) have provided valuable insights into tumor behavior and potential therapeutic targets. New areas of interest, such as TMB and immune checkpoint molecules, suggest that immunotherapy and metabolic targeting may play a future role in treatment.
FOSCC is characterized by its rapid local invasion, and destructive nature, often leading to severe oral discomfort and a marked decline in quality of life. While surgical excision offers the best chance for prolonged survival, it is rarely feasible due to the tumor’s location and extent at diagnosis. Traditional therapies, including radiation and chemotherapy, have shown limited efficacy, though novel protocols and combination treatments show some promise.
Progress in the management of FOSCC will depend on early detection, larger and better epidemiological studies, and applying molecular discoveries into practical clinical tools. The integration of advanced diagnostics, personalized medicine, and innovative therapies holds the potential to improve both survival and quality of life for affected cats. Ongoing collaboration among researchers, clinicians, and pet owners will be essential to stimulate innovation and ensure that scientific advances benefit feline patients in tangible ways.
Author contributions
PT: Conceptualization, Writing – original draft, Writing – review & editing. FD: Supervision, Writing – original draft, Writing – review & editing. GA: Conceptualization, Writing – review & editing. MD: Writing – review & editing. LH: Writing – review & editing. OS: Writing – review & editing. OT: Writing – review & editing. GB: Conceptualization, Writing – review & editing. MM: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The financial support was received for the publication of this article from IULS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bertone, ER, Snyder, LA, and Moore, AS. Environmental and lifestyle risk factors for oral squamous cell carcinoma in domestic cats. J Vet Intern Med. (2003) 17:557–62. doi: 10.1111/J.1939-1676.2003.TB02478.X
2. Snyder, LA, Bertone, ER, Jakowski, RM, Dooner, MS, Jennings-Ritchie, J, and Moore, AS. p53 expression and environmental tobacco smoke exposure in feline oral squamous cell carcinoma. Vet Pathol. (2004) 41:209–14. doi: 10.1354/VP.41-3-209
3. Renzi, A, De Bonis, P, Morandi, L, Lenzi, J, Tinto, D, Rigillo, A, et al. Prevalence of p53 dysregulations in feline oral squamous cell carcinoma and non-neoplastic oral mucosa. PLoS One. (2019) 14:e0215621. doi: 10.1371/JOURNAL.PONE.0215621
4. Wypij, JM, Fan, TM, Fredrickson, RL, Barger, AM, De Lorimier, LP, and Charney, SC. In vivo and in vitro efficacy of zoledronate for treating oral squamous cell carcinoma in cats. J Vet Intern Med. (2008) 22:158–63. doi: 10.1111/J.1939-1676.2007.0010.X
5. Brown, ME, Bear, MD, Rosol, TJ, Premanandan, C, Kisseberth, WC, and London, CA. Characterization of STAT3 expression, signaling and inhibition in feline oral squamoufs cell carcinoma. BMC Vet Res. (2015) 11:505. doi: 10.1186/S12917-015-0505-7
6. Harris, K, Gelberg, HB, Kiupel, M, and Helfand, SC. Immunohistochemical features of epithelial-mesenchymal transition in feline Oral squamous cell carcinoma. Vet Pathol. (2019) 56:826–39. doi: 10.1177/0300985819859873
7. Kabak, YB, Sozmen, M, Devrim, AK, Sudagidan, M, Yildirim, F, Guvenc, T, et al. Expression levels of angiogenic growth factors in feline squamous cell carcinoma. Acta Vet Hung. (2020) 68:37–48. doi: 10.1556/004.2020.00005
8. Nasry, WHS, Rodriguez-Lecompte, JC, and Martin, CK. In vitro expression of genes encoding HIF1α, VEGFA, PGE2 synthases, and PGE2 receptors in feline oral squamous cell carcinoma. J Vet Diagn Invest. (2025) 37:223–33. doi: 10.1177/10406387251315677
9. Chu, S, Wylie, TN, Wylie, KM, Johnson, GC, Skidmore, ZL, Fleer, M, et al. A virome sequencing approach to feline oral squamous cell carcinoma to evaluate viral causative factors. Vet Microbiol. (2020) 240:108491. doi: 10.1016/j.vetmic.2019.108491
10. Little, S, Levy, J, Hartmann, K, Hofmann-Lehmann, R, Hosie, M, Olah, G, et al. 2020 AAFP feline retrovirus testing and management guidelines. J Feline Med Surg. (2020) 22:5–30. doi: 10.1177/1098612X19895940
11. Beatty, JA, and Hartmann, K. Advances in feline viruses and viral diseases. Viruses. (2021) 13:923. doi: 10.3390/V13050923
12. Milman, G, Smith, KC, and Erles, K. Serological detection of Epstein-Barr virus infection in dogs and cats. Vet Microbiol. (2011) 150:15–20. doi: 10.1016/J.VETMIC.2010.12.013
13. Munday, JS, Knight, CG, and French, AF. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Res Vet Sci. (2011) 90:280–3. doi: 10.1016/J.RVSC.2010.06.014
14. Dunowska, M, Munday, JS, Laurie, RE, and Hills, SFK. Genomic characterisation of Felis catus papillomavirus 4, a novel papillomavirus detected in the oral cavity of a domestic cat. Virus Genes. (2014) 48:111–9. doi: 10.1007/S11262-013-1002-3
15. Altamura, G, Cuccaro, B, Eleni, C, Strohmayer, C, Brandt, S, and Borzacchiello, G. Investigation of multiple Felis catus papillomavirus types (-1/-2/-3/-4/-5/-6) DNAs in feline oral squamous cell carcinoma: a multicentric study. J Vet Med Sci. (2022) 84:881–4. doi: 10.1292/JVMS.22-0060
16. Miller, C, Boegler, K, Carver, S, MacMillan, M, Bielefeldt-Ohmann, H, and VandeWoude, S. Pathogenesis of oral FIV infection. PLoS One. (2017) 12:138. doi: 10.1371/JOURNAL.PONE.0185138
17. Munday, JS, He, Y, Aberdein, D, and Klobukowska, HJ. Increased p16CDKN2A, but not p53, immunostaining is predictive of longer survival time in cats with oral squamous cell carcinomas. Vet J. (2019) 248:64–70. doi: 10.1016/J.TVJL.2019.04.007
18. Munday, JS, Kiupel, M, French, AF, and Howe, L. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet Dermatol. (2008) 19:259–63. doi: 10.1111/J.1365-3164.2008.00685.X
19. Egberink, H, Hartmann, K, Mueller, R, Pennisi, MG, Belák, S, Tasker, S, et al. Feline papillomatosis. Viruses. (2025) 17:59. doi: 10.3390/V17010059
20. Munday, JS, and French, AF. Felis catus papillomavirus types 1 and 4 are rarely present in neoplastic and inflammatory oral lesions of cats. Res Vet Sci. (2015) 100:220–2. doi: 10.1016/J.RVSC.2015.03.002
21. O’Neill, SH, Newkirk, KM, Anis, EA, Brahmbhatt, R, Frank, LA, and Kania, SA. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Vet Dermatol. (2011) 22:68–74. doi: 10.1111/J.1365-3164.2010.00912.X
22. Altamura, G, Corteggio, A, and Borzacchiello, G. Felis catus papillomavirus type 2 E6 oncogene enhances mitogen-activated protein kinases and Akt activation but not EGFR expression in an in vitro feline model of viral pathogenesis. Vet Microbiol. (2016) 195:96–100. doi: 10.1016/J.VETMIC.2016.09.013
23. Altamura, G, Jebara, G, Cardeti, G, and Borzacchiello, G. Felis catus papillomavirus type-2 but not type-1 is detectable and transcriptionally active in the blood of healthy cats. Transbound Emerg Dis. (2018) 65:497–503. doi: 10.1111/tbed.12732
24. Munday, JS, Howe, L, French, A, Squires, RA, and Sugiarto, H. Detection of papillomaviral DNA sequences in a feline oral squamous cell carcinoma. Res Vet Sci. (2009) 86:359–61. doi: 10.1016/J.RVSC.2008.07.005
25. Supsavhad, W, Dirksen, WP, Hildreth, BE, and Rosol, TJ. p16, pRb, and p53 in feline oral squamous cell carcinoma. Vet Sci. (2016) 3:18. doi: 10.3390/VETSCI3030018
26. Altamura, G, Power, K, Martano, M, degli Uberti, B, Galiero, G, De Luca, G, et al. Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation. Sci Rep. (2018) 8:17529. doi: 10.1038/S41598-018-35723-7
27. Altamura, G, Corteggio, A, Pacini, L, Conte, A, Pierantoni, GM, Tommasino, M, et al. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology. (2016) 496:1–8. doi: 10.1016/J.VIROL.2016.05.017
28. Thomson, NA, Munday, JS, and Dittmer, KE. Frequent detection of transcriptionally active felis catus papillomavirus 2 in feline cutaneous squamous cell carcinomas. J Gen Virol. (2016) 97:1189–97. doi: 10.1099/JGV.0.000416/CITE/REFWORKS
29. Mazzei, M, Forzan, M, Carlucci, V, Anfossi, AG, Alberti, A, Albanese, F, et al. A study of multiple Felis catus papillomavirus types (1, 2, 3, 4) in cat skin lesions in Italy by quantitative PCR. J Feline Med Surg. (2018) 20:772–9. doi: 10.1177/1098612X17732255
30. Vascellari, M, Mazzei, M, Zanardello, C, Melchiotti, E, Albanese, F, Forzan, M, et al. Felis catus papillomavirus types 1, 2, 3, 4, and 5 in feline Bowenoid in situ carcinoma: an in situ hybridization study. Vet Pathol. (2019) 56:818–25. doi: 10.1177/0300985819859874
31. Altamura, G, Cardeti, G, Cersini, A, Eleni, C, Cocumelli, C, Bartolomé del Pino, LE, et al. Detection of Felis catus papillomavirus type-2 DNA and viral gene expression suggest active infection in feline oral squamous cell carcinoma. Vet comp. Oncologia. (2020) 18:494–501. doi: 10.1111/vco.12569
32. Roperto, S, Russo, V, Ozkul, A, Corteggio, A, Sepici-Dincel, A, Catoi, C, et al. Productive infection of bovine papillomavirus type 2 in the urothelial cells of naturally occurring urinary bladder tumors in cattle and water buffaloes. PLoS One. (2013) 8:e62227. doi: 10.1371/JOURNAL.PONE.0062227
33. Munday, JS, Bell, CM, and Gulliver, EL. Feline oral in situ carcinoma associated with papillomavirus infection: a case series of 7 cats. Vet Pathol. [Online ahead of print.] (2025) 1–8. doi: 10.1177/03009858251352594
34. Wypij, JM. A naturally occurring feline model of head and neck squamous cell carcinoma. Pathol Res Int. (2013) 2013:1–7. doi: 10.1155/2013/502197
35. Supsavhad, W, Dirksen, WP, Martin, CK, and Rosol, TJ. Animal models of head and neck squamous cell carcinoma. Vet J. (2016) 210:7–16. doi: 10.1016/J.TVJL.2015.11.006
36. Tanaka, TI, and Alawi, F. Human papillomavirus and oropharyngeal Cancer. Dent Clin N Am. (2018) 62:111–20. doi: 10.1016/J.CDEN.2017.08.008
37. Altamura, G, and Borzacchiello, G. HPV related head and neck squamous cell carcinoma: new evidences for an emerging spontaneous animal model. Oral Oncol. (2019) 88:84. doi: 10.1016/j.oraloncology.2018.11.027
38. Sequeira, I, Pires, M d A, Leitão, J, Henriques, J, Viegas, C, and Requicha, J. Feline oral squamous cell carcinoma: a critical review of etiologic factors. Vet Sci. (2022) 9:558. doi: 10.3390/vetsci9100558
39. Lin, J, Kouznetsova, VL, and Tsigelny, IF. Molecular mechanisms of feline cancers. OBM Genet. (2021) 5:11. doi: 10.21926/OBM.GENET.2102131
40. Carneiro, CS, de Queiroz, GF, Pinto, ACBCF, Dagli, MLZ, and Matera, JM. Feline injection site sarcoma: immunohistochemical characteristics. J Feline Med Surg. (2019) 21:314–21. doi: 10.1177/1098612X18774709
41. Al-Thawadi, H, Gupta, I, Jabeen, A, Skenderi, F, Aboulkassim, T, Yasmeen, A, et al. Co-presence of human papillomaviruses and Epstein-Barr virus is linked with advanced tumor stage: a tissue microarray study in head and neck cancer patients. Cancer Cell Int. (2020) 20:361. doi: 10.1186/S12935-020-01348-Y
42. Jiang, X. Tobacco and oral squamous cell carcinoma: a review of carcinogenic pathways. Tob Induc Dis. (2019) 17:1–9. doi: 10.18332/TID/111652
43. Noall, L, Lee, S, Burton, JH, Marquardt, TM, Cermak, J, Thombs, LA, et al. A multi-institutional epidemiologic study evaluating environmental risk factors for feline oral squamous cell carcinoma. Vet Comp Oncol. (2023) 21:509–19. doi: 10.1111/VCO.12914
44. Moisan, PG, Lorenz, MD, Stromberg, PC, and Simmons, HA. Concurrent trichinosis and oral squamous cell carcinoma in a cat. J Vet Diagn Invest. (1998) 10:199–202. doi: 10.1177/104063879801000220
45. O’Neill, DG, Church, DB, McGreevy, PD, Thomson, PC, and Brodbelt, DC. Prevalence of disorders recorded in cats attending primary-care veterinary practices in England. Vet J. (2014) 202:286–91. doi: 10.1016/J.TVJL.2014.08.004
46. Olmsted, GA, Farrelly, J, Post, GS, and Smith, J. Tolerability of toceranib phosphate (Palladia) when used in conjunction with other therapies in 35 cats with feline oral squamous cell carcinoma: 2009–2013. J Feline Med Surg. (2017) 19:568–75. doi: 10.1177/1098612X16638118
47. Pavlin, D, Dolenšek, T, Švara, T, and Nemec, A. Solid type primary intraosseous squamous cell carcinoma in a cat. BMC Vet Res. (2018) 14:23. doi: 10.1186/S12917-018-1344-0
48. Gopinath, D, Menon, RK, Veettil, SK, Botelho, MG, and Johnson, NW. Periodontal diseases as putative risk factors for head and neck cancer: systematic review and meta-analysis. Cancers (Basel). (2020) 12:1–15. doi: 10.3390/CANCERS12071893
49. Looper, JS, Malarkey, DE, Ruslander, D, Proulx, D, and Thrall, DE. Epidermal growth factor receptor expression in feline oral squamous cell carcinomas. Vet Comp Oncol. (2006) 4:33–40. doi: 10.1111/J.1476-5810.2006.00091.X
50. Hayes, AM, Adams, VJ, Scase, TJ, and Murphy, S. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice: paper. J Small Anim Pract. (2007) 48:394–9. doi: 10.1111/J.1748-5827.2007.00393.X
51. Nasry, WHS, and Martin, CK. Intersecting mechanisms of hypoxia and prostaglandin E2-mediated inflammation in the comparative biology of oral squamous cell carcinoma. Front Oncol. (2021) 11:1–15. doi: 10.3389/FONC.2021.539361
52. Liao, C, Cheng, X, Liu, M, Wang, X, and Boireau, P. Trichinella spiralis and tumors: cause, coincidence or treatment? Anti Cancer Agents Med Chem. (2017) 18:1091–9. doi: 10.2174/1871520617666171121115847
53. Teifke, JP, and Lohr, CV. Immunohistochemical detection of P53 overexpression in paraffin wax-embedded squamous cell carcinomas of cattle, horses, cats and dogs. J Comp Pathol. (1996) 114:205–10. doi: 10.1016/S0021-9975(96)80010-7
54. Nasir, L, Krasner, H, Argyle, DJ, and Williams, A. Immunocytochemical analysis of the tumour suppressor protein (p53) in feline neoplasia. Cancer Lett. (2000) 155:1–7. doi: 10.1016/S0304-3835(00)00337-2
55. Yamashita-Kawanishi, N, Sawanobori, R, Matsumiya, K, Uema, A, Chambers, JK, Uchida, K, et al. Detection of felis catus papillomavirus type 3 and 4 DNA from squamous cell carcinoma cases of cats in Japan. J Vet Med Sci. (2018) 80:1236–40. doi: 10.1292/JVMS.18-0089
56. Muss, HB, Smitherman, A, Wood, WA, Nyrop, K, Tuchman, S, Randhawa, PK, et al. p16 a biomarker of aging and tolerance for cancer therapy. Transl Cancer Res. (2020) 9:5732–42. doi: 10.21037/TCR.2020.03.39
57. Hayes, A, Scase, T, Miller, J, Murphy, S, Sparkes, A, and Adams, V. COX-1 and COX-2 expression in feline oral squamous cell carcinoma. J Comp Pathol. (2006) 135:93–9. doi: 10.1016/J.JCPA.2006.06.001
58. Quigley, PJ, Leedale, A, and Dawson, IMP. Carcinoma of mandible of cat and dog simulating osteosarcoma. J Comp Pathol. (1972) 82:15–IN4. doi: 10.1016/0021-9975(72)90021-7
59. Yasuoka, T, Yonemoto, K, Kato, Y, and Tatematsu, N. Squamous cell carcinoma arising in a dentigerous cyst. J Oral Maxillofac Surg. (2000) 58:900–5. doi: 10.1053/JOMS.2000.8219
60. Zhang, L, Liu, Y, Zheng, HJ, and Zhang, CP. The Oral microbiota may have influence on Oral Cancer. Front Cell Infect Microbiol. (2020) 9:1–11. doi: 10.3389/FCIMB.2019.00476
61. Older, CE, Gomes, MDOS, Hoffmann, AR, Policano, MD, Dos Reis, CAC, Carregaro, AB, et al. Influence of the FIV status and chronic gingivitis on feline oral microbiota. Pathogens. (2020) 9:1–11. doi: 10.3390/PATHOGENS9050383
62. Rodrigues, MX, Bicalho, RC, Fiani, N, Lima, SF, and Peralta, S. The subgingival microbial community of feline periodontitis and gingivostomatitis: characterization and comparison between diseased and healthy cats. Sci Rep (2019) 9:1–10. doi: 10.1038/S41598-019-48852-4;SUBJMETA
63. Gendler, A, Lewis, JR, Reetz, JA, and Schwarz, T. Computed tomographic features of oral squamous cell carcinoma in cats: 18 cases (2002-2008). J Am Vet Med Assoc. (2010) 236:319–25. doi: 10.2460/JAVMA.236.3.319
64. Stebbins, KE, Morse, CC, and Goldschmidt, MH. Feline Oral neoplasia: a ten-year survey. Vet Pathol. (1989) 26:121–8. doi: 10.1177/030098588902600204
65. Manuali, E, Forte, C, Vichi, G, Genovese, DA, Mancini, D, De Leo, AAP, et al. Tumours in European shorthair cats: a retrospective study of 680 cases. J Feline Med Surg. (2020) 22:1095–102. doi: 10.1177/1098612X20905035
66. Sparger, EE, Murphy, BG, Kamal, FM, Arzi, B, Naydan, D, Skouritakis, CT, et al. Investigation of immune cell markers in feline oral squamous cell carcinoma. Vet Immunol Immunopathol. (2018) 202:52–62. doi: 10.1016/j.vetimm.2018.06.011
67. Wingo, K. Histopathologic diagnoses from biopsies of the Oral cavity in 403 dogs and 73 cats. J Vet Dent. (2018) 35:7–17. doi: 10.1177/0898756418759760
68. Zaccone, R, Renzi, A, Chalfon, C, Lenzi, J, Bellei, E, Marconato, L, et al. Environmental risk factors for the development of oral squamous cell carcinoma in cats. J Vet Intern Med. (2022) 36:1398–408. doi: 10.1111/JVIM.16372
69. Falcão, F, Faísca, P, Viegas, I, de Oliveira, JT, and Requicha, JF. Feline oral cavity lesions diagnosed by histopathology: a 6-year retrospective study in Portugal. J Feline Med Surg. (2020) 22:977–83. doi: 10.1177/1098612X19900033/FORMAT/EPUB
70. Martin, C, Tannehill-Gregg, S, Wolfe, TD, and Rosol, TJ. Bone-invasive oral squamous cell carcinoma in cats: pathology and expression of parathyroid hormone-related protein. Vet Pat. (2011) 48:302–12. doi: 10.1177/0300985810384414
71. Munday, JS, Löhr, CV, and Kiupel, M. Tumors of the alimentary tract. Hawaii Med J. (2017) 8:499–601. doi: 10.1002/9781119181200.CH13
72. Dan, H, Liu, S, Liu, J, Liu, D, Yin, F, Wei, Z, et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-κB pathway in oral squamous cell carcinoma. Mol Oncol. (2020) 14:795–807. doi: 10.1002/1878-0261.12644
73. Fu, TY, Tsai, MH, Wang, JS, and Ger, LP. Antioxidant enzymes in oral verrucous carcinoma. J Oral Pathol Med. (2017) 46:46–9. doi: 10.1111/JOP.12460
74. Bilgic, O, Duda, L, Sánchez, MD, and Lewis, JR. Feline oral squamous cell carcinoma: clinical manifestations and literature review. J Vet Dent. (2015) 32:30–40. doi: 10.1177/089875641503200104
75. Martin, CK, Werbeck, JL, Thudi, NK, Lanigan, LG, Wolfe, TD, Toribio, RE, et al. Zoledronic acid reduces bone loss and tumor growth in an orthotopic xenograft model of osteolytic oral squamous cell carcinoma. Cancer Res. (2010) 70:8607–16. doi: 10.1158/0008-5472.CAN-10-0850
77. McCaw, D, Pope, E, Payne, J, West, MK, Tompson, RV, and Tate, D. Treatment of canine oral squamous cell carcinomas with photodynamic therapy. Br J Cancer. (2000) 82:1297–9. doi: 10.1054/bjoc.1999.1094
78. Bergkvist, GT, Argyle, DJ, Morrison, L, Macintyre, N, Hayes, A, and Yool, DA. Expression of epidermal growth factor receptor (EGFR) and Ki67 in feline oral squamous cell carcinomas (FOSCC). Vet Comp Oncol. (2011) 9:106–17. doi: 10.1111/J.1476-5829.2010.00239.X
79. Yoshikawa Dr, H, Ehrhart, EJ, Charles, JB, Thamm, DH, and LaRue, SM. Immunohistochemical characterization of feline oral squamous cell carcinoma. Am J Vet Res. (2012) 73:1801–6. doi: 10.2460/AJVR.73.11.1801
80. Yoshikawa, H, Ehrhart, EJ, Charles, JB, Custis, JT, and Larue, SM. Assessment of predictive molecular variables in feline oral squamous cell carcinoma treated with stereotactic radiation therapy. Vet Comp Oncol. (2016) 14:39–57. doi: 10.1111/VCO.12050
81. Giuliano, A, Swift, R, Arthurs, C, Marote, G, Abramo, F, McKay, J, et al. Quantitative expression and co-localization of Wnt Signalling related proteins in feline squamous cell carcinoma. PLoS One. (2016) 11:e0161103. doi: 10.1371/JOURNAL.PONE.0161103
82. Altamura, G, Martano, M, Matrone, A, Corteggio, A, and Borzacchiello, G. Monoclonal antibody cetuximab impairs matrix metalloproteinases 2 and 9, epithelial–mesenchymal transition and cell migration in feline oral squamous cell carcinoma in vitro. Vet Comp Oncol. (2024) 22:149–55. doi: 10.1111/VCO.12943
83. Rodney, AR, Skidmore, ZL, Grenier, JK, Griffith, OL, Miller, AD, Chu, S, et al. Genomic landscape and gene expression profiles of feline oral squamous cell carcinoma. Front Vet Sci. (2023) 10:1079019. doi: 10.3389/FVETS.2023.1079019/BIBTEX
84. Brunetti, D, Bottani, E, and Giuliano, A. Companion animal model in translational oncology; feline oral squamous cell carcinoma and canine oral melanoma. Biology. (2021) 11:54. doi: 10.3390/BIOLOGY11010054
85. Maekawa, N, Konnai, S, Asano, Y, Otsuka, T, Aoki, E, Takeuchi, H, et al. Molecular characterization of feline immune checkpoint molecules and establishment of PD-L1 immunohistochemistry for feline tumors. PLoS One. (2023) 18:e0281143. doi: 10.1371/JOURNAL.PONE.0281143
86. Mineshige, T, Tanaka, Y, Watanabe, K, Tagawa, M, Tomihari, M, and Kobayashi, Y. Histologic, immunohistochemical, and in situ hybridization study of myxoid stroma in feline oral squamous cell carcinoma. J Vet Med Sci. (2024) 86:258–65. doi: 10.1292/JVMS.23-0356
87. Klobukowska, HJ, and Munday, JS. High numbers of stromal Cancer-associated fibroblasts are associated with a shorter survival time in cats with Oral squamous cell carcinoma. Vet Pathol. (2016) 53:1124–30. doi: 10.1177/0300985816629713
88. Tandon, S, Tudur-Smith, C, Riley, RD, Boyd, MT, and Jones, TM. A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol Biomarkers Prev. (2010) 19:574–87. doi: 10.1158/1055-9965.EPI-09-0981
89. Khammanivong, A, Saha, J, Spartz, AK, Sorenson, BS, Bush, AG, Korpela, DM, et al. A novel MCT1 and MCT4 dual inhibitor reduces mitochondrial metabolism and inhibits tumour growth of feline oral squamous cell carcinoma. Vet Comp Oncol. (2020) 18:324–41. doi: 10.1111/VCO.12551
90. Altamura, G, degli Uberti, B, Galiero, G, De Luca, G, Power, K, Licenziato, L, et al. The small molecule BIBR1532 exerts potential anti-cancer activities in preclinical models of feline oral squamous cell carcinoma through inhibition of telomerase activity and down-regulation of TERT. Front Vet Sci. (2021) 7:620776. doi: 10.3389/FVETS.2020.620776/BIBTEX
91. Piegols, HJ, Takada, M, Parys, M, Dexheimer, T, Yuzbasiyan-Gurkan, V, and Piegols, HJ. Investigation of novel chemotherapeutics for feline oral squamous cell carcinoma. Oncotarget. (2018) 9:33098–109. doi: 10.18632/ONCOTARGET.26006
92. Lundberg, AP, Boudreau, MW, Selting, KA, Chatkewitz, LE, Samuelson, J, Francis, JM, et al. Utilizing feline oral squamous cell carcinoma patients to develop NQO1-targeted therapy. Neoplasia. (2021) 23:811–22. doi: 10.1016/J.NEO.2021.06.008
93. Reeves, NP, Turrel, J, and Withrow, S. Oral squamous cell carcinoma in the cat Lakewood, Colorado, USA: Journal of the American Animal Hospital Association. (1993) 29, 438–441.
94. Northrup, NC, Selting, KA, Rassnick, KM, Kristal, O, O’Brien, MG, Dank, G, et al. Outcomes of cats with oral tumors treated with mandibulectomy: 42 cases. J Am Anim Hosp Assoc. (2006) 42:350–60. doi: 10.5326/0420350
95. Hutson, CA, Willauer, CC, Walder, EJ, Stone, JL, and Klein, MK. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987-1989). J Am Vet Med Assoc. (1989) 201:777–81.
96. Fidel, J, Lyons, J, Tripp, C, Houston, R, Wheeler, B, and Ruiz, A. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. J Vet Intern Med. (2011) 25:504–10. doi: 10.1111/J.1939-1676.2011.0721.X
97. Bregazzi, VS, LaRue, SM, Powers, BE, Fettman, MJ, Ogilvie, GK, and Withrow, SJ. Response of feline oral squamous cell carcinoma to palliative radiation therapy. Vet Radiol Ultrasound. (2001) 42:77–9. doi: 10.1111/J.1740-8261.2001.TB00907.X
98. Bostock, DE. Neoplasms of the skin and subcutaneous tissues in dogs and cats. Br Vet J. (1986) 142:1–19. doi: 10.1016/0007-1935(86)90002-3
99. Weidner, N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. (1995) 36:169–80. doi: 10.1007/BF00666038
100. Jain, RK. Molecular regulation of vessel maturation. Nat Med. (2003) 9:685–93. doi: 10.1038/nm0603-685
101. Sedghizadeh, PP, Billington, WD, Paxton, D, Ebeed, R, Mahabady, S, Clark, GT, et al. Is p16-positive oropharyngeal squamous cell carcinoma associated with favorable prognosis? A systematic review and meta-analysis. Oral Oncol. (2016) 54:15–27. doi: 10.1016/J.ORALONCOLOGY.2016.01.002
102. Parry, D, Bates, S, Mann, DJ, and Peters, G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. (1995) 14:503–11. doi: 10.1002/J.1460-2075.1995.TB07026.X
103. Karpathiou, G, Monaya, A, Forest, F, Froudarakis, M, Casteillo, F, Marc Dumollard, J, et al. p16 and p53 expression status in head and neck squamous cell carcinoma: a correlation with histological, histoprognostic and clinical parameters. Pathology. (2016) 48:341–8. doi: 10.1016/J.PATHOL.2016.01.005
104. Munday, JS, Dunowska, M, and De Grey, S. Detection of two different papillomaviruses within a feline cutaneous squamous cell carcinoma: case report and review of the literature. NZ Vet J. (2011) 57:248–51. doi: 10.1080/00480169.2009.36911
105. Almangush, A, Bello, IO, Keski-Säntti, H, Mäkinen, LK, Kauppila, JH, Pukkila, M, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. (2014) 36:811–8. doi: 10.1002/HED.23380
106. Bryne, M, Koppang, HS, Lilleng, R, and Kjærheim, Å. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. (1992) 166:375–81. doi: 10.1002/PATH.1711660409
107. Chang, YC, Nieh, S, Chen, SF, Jao, SW, Lin, YL, and Fu, E. Invasive pattern grading score designed as an independent prognostic indicator in oral squamous cell carcinoma. Histopathology. (2010) 57:295–303. doi: 10.1111/J.1365-2559.2010.03616.X
108. Poirier, VJ, Kaser-Hotz, B, Vail, DM, and Straw, RC. Efficacy and toxicity of an accelerated hypofractionated radiation therapy protocol in cats with oral squamous cell carcinoma. Vet Radiol Ultrasound. (2013) 54:81–8. doi: 10.1111/J.1740-8261.2012.01970.X
109. Sabhlok, A, and Ayl, R. Palliative radiation therapy outcomes for cats with oral squamous cell carcinoma (1999–2005). Vet Radiol Ultrasound. (2014) 55:565–70. doi: 10.1111/VRU.12157
110. Fidel, JL, Sellon, RK, Houston, RK, and Wheeler, BA. A nine-day accelerated radiation protocol for feline squamous cell carcinoma. Vet Radiol Ultrasound. (2007) 48:482–5. doi: 10.1111/J.1740-8261.2007.00283.X
111. van Nimwegen, SA, Bakker, RC, Kirpensteijn, J, van Es, RJJ, Koole, R, Lam, MGEH, et al. Intratumoral injection of radioactive holmium (166 ho) microspheres for treatment of oral squamous cell carcinoma in cats. Vet Comp Oncol. (2018) 16:114–24. doi: 10.1111/VCO.12319
112. Zhan, Y, and Fan, S. Multiple mechanisms involving in Radioresistance of nasopharyngeal carcinoma. J Cancer. (2020) 11:4193–204. doi: 10.7150/JCA.39354
113. Zhou, T, Zhang, LY, He, JZ, Miao, ZM, Li, YY, Zhang, YM, et al. Review: mechanisms and perspective treatment of radioresistance in non-small cell lung cancer. Front Immunol. (2023) 14:1133899. doi: 10.3389/FIMMU.2023.1133899/XML/NLM
114. Busato, F, Khouzai B El, M, and Mognato, M. Biological mechanisms to reduce radioresistance and increase the efficacy of radiotherapy: state of the art. Int J Mol Sci. (2022) 23:10211. doi: 10.3390/IJMS231810211
115. Jones, PD, de Lorimier, LP, Kitchell, BE, and Losonsky, JM. Gemcitabine as a radiosensitizer for nonresectable feline oral squamous cell carcinoma. J Am Anim Hosp Assoc. (2003) 39:463–7. doi: 10.5326/0390463
116. Kisseberth, WC, Vail, DM, Yaissle, J, Jeglum, KA, Couto, CG, Ward, H, et al. Phase I clinical evaluation of carboplatin in tumor-bearing cats: a veterinary cooperative oncology group study. J Vet Intern Med. (2008) 22:83–8. doi: 10.1111/J.1939-1676.2007.0017.X
117. Treggiari, E, Romanelli, G, Ferro, S, and Roccabianca, P. Long-term survival in a cat with tonsillar squamous cell carcinoma treated with surgery and chemotherapy. JFMS Open Rep. (2021) 7:1–6. doi: 10.1177/2055116920984387
118. de Maar, JS, Zandvliet, MMJM, Veraa, S, Tobón Restrepo, M, Moonen, CTW, and Deckers, R. Ultrasound and microbubbles mediated bleomycin delivery in feline Oral squamous cell carcinoma-an in vivo veterinary study. Pharmaceutics. (2023) 15:1–16. doi: 10.3390/PHARMACEUTICS15041166
119. Fox, LE, Rosenthal, RC, King, RR, Levine, PB, Vail, DM, Helfand, SC, et al. Use of cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum (II), a liposomal cisplatin analogue, in cats with oral squamous cell carcinoma. Am J Vet Res. (2000) 61:791–5. doi: 10.2460/AJVR.2000.61.791
120. Lai, YHE, Morhard, R, Ramanujam, N, and Nolan, MW. Minimally invasive ethyl cellulose ethanol ablation in domesticated cats with naturally occurring head and neck cancers: six cats. Vet Comp Oncol. (2021) 19:492–500. doi: 10.1111/VCO.12687
121. Liptak, JM, Thatcher, GP, Mestrinho, LA, Séguin, B, Vernier, T, Martano, M, et al. Outcomes of cats treated with maxillectomy: 60 cases. A veterinary Society of Surgical Oncology retrospective study. Vet Comp Oncol. (2021) 19:641–50. doi: 10.1111/VCO.12634
122. L, JR, O, TG, S, KA, K, EL, R, AM, J, MW, et al. Polyamine inhibitors for treatment of feline oral squamous cell carcinoma: a proof-of-concept study. J Vet Dent. (2013) 30:140–5. doi: 10.1177/089875641303000301
123. Skorupski, KA, O’Brien, TG, Guerrero, T, Rodriguez, CO, and Burns, MR. Phase I/II clinical trial of 2-difluoromethyl-ornithine (DFMO) and a novel polyamine transport inhibitor (MQT 1426) for feline oral squamous cell carcinoma. Vet Comp Oncol. (2011) 9:275–82. doi: 10.1111/J.1476-5829.2011.00264.X
124. Martin, CK, Dirksen, WP, Carlton, MM, Lanigan, LG, Pillai, SP, Werbeck, JL, et al. Combined zoledronic acid and meloxicam reduced bone loss and tumour growth in an orthotopic mouse model of bone-invasive oral squamous cell carcinoma. Vet Comp Oncol. (2015) 13:203–17. doi: 10.1111/VCO.12037
125. Wypij, JM, and Heller, DA. Pamidronate disodium for palliative therapy of feline bone-invasive tumors. Vet Med Int. (2014) 2014:1–8. doi: 10.1155/2014/675172
126. Rathore, K, Alexander, M, and Cekanova, M. Piroxicam inhibits Masitinib-induced cyclooxygenase 2 expression in oral squamous cell carcinoma cells in vitro. Transl Res. (2014) 164:158–68. doi: 10.1016/J.TRSL.2014.02.002
127. Cannon, CM, Trembley, JH, Kren, BT, Unger, GM, O’Sullivan, MG, Cornax, I, et al. Therapeutic targeting of protein kinase CK2 gene expression in feline oral squamous cell carcinoma: a naturally occurring large-animal model of head and neck cancer. Hum Gene Ther Clin Dev. (2017) 28:80–6. doi: 10.1089/HUMC.2017.008
128. Park, MK, and Song, KH. Case report: allogeneic feline umbilical cord-derived mesenchymal stem cell transplantation for feline oral squamous cell carcinoma. Front Vet Sci. (2024) 11:1443110. doi: 10.3389/FVETS.2024.1443110
129. Maeta, N, Tamura, K, Takemitsu, H, and Miyabe, M. Lymphokine-activated killer cell transplantation after anti-cancer treatment in two aged cats. Open Vet J. (2019) 9:147–50. doi: 10.4314/OVJ.V9I2.9
130. Merino, DM, McShane, LM, Fabrizio, D, Funari, V, Chen, SJ, White, JR, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the friends of Cancer research TMB harmonization project. J Immunother Cancer. (2020) 8:e000147. doi: 10.1136/JITC-2019-000147
131. Tang, J, Wu, X, Cheng, B, and Lu, Y. Identification of a polyamine-related signature and six novel prognostic biomarkers in oral squamous cell carcinoma. Front Mol Biosci. (2023) 10:1073770. doi: 10.3389/FMOLB.2023.1073770/BIBTEX
132. Tozon, N, Pavlin, D, Sersa, G, Dolinsek, T, and Cemazar, M. Electrochemotherapy with intravenous bleomycin injection: an observational study in superficial squamous cell carcinoma in cats. J Feline Med Surg. (2014) 16:291–9. doi: 10.1177/1098612X13507071
133. Spugnini, EP, Pizzuto, M, Filipponi, M, Romani, L, Vincenzi, B, Menicagli, F, et al. Electroporation enhances bleomycin efficacy in cats with periocular carcinoma and advanced squamous cell carcinoma of the head. J Vet Intern Med. (2015) 29:1368–75. doi: 10.1111/JVIM.13586
134. Londhe, P, Gutwillig, M, and London, C. Targeted therapies in veterinary oncology. Vet Clin N Am Small Anim Pract. (2019) 49:917–31. doi: 10.1016/j.cvsm.2019.04.005
135. Altamura, G, and Borzacchiello, G. Anti-EGFR monoclonal antibody Cetuximab displays potential anti-cancer activities in feline oral squamous cell carcinoma cell lines. Front Vet Sci. (2022) 9:1–9. doi: 10.3389/FVETS.2022.1040552
136. Beirão, BCB, Raposo, T, Jain, S, Hupp, T, and Argyle, DJ. Challenges and opportunities for monoclonal antibody therapy in veterinary oncology. Vet J. (2016) 218:40–50. doi: 10.1016/J.TVJL.2016.11.005
137. Singer, J, Weichselbaumer, M, Stockner, T, Mechtcheriakova, D, Sobanov, Y, Bajna, E, et al. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol. (2012) 50:200–9. doi: 10.1016/J.MOLIMM.2012.01.002
138. Concu, R, and Cordeiro, MNDS. Cetuximab and the head and neck squamous cell Cancer. Curr Top Med Chem. (2018) 18:192–8. doi: 10.2174/1568026618666180112162412
139. Levantini, E, Maroni, G, Del Re, M, and Tenen, DG. EGFR signaling pathway as therapeutic target in human cancers. Semin Cancer Biol. (2022) 85:253–75. doi: 10.1016/J.SEMCANCER.2022.04.002
140. Bergkvist, GT, Argyle, DJ, Pang, LY, Muirhead, R, and Yool, DA. Studies on the inhibition of feline EGFR in squamous cell carcinoma: enhancement of radiosensitivity and rescue of resistance to small molecule inhibitors. Cancer Biol Ther. (2011) 11:927–37. doi: 10.4161/CBT.11.11.15525
141. Ferris, RL, Blumenschein, G, Fayette, J, Guigay, J, Colevas, AD, Licitra, L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMOA1602252
142. Smolensky, D, Rathore, K, Bourn, J, and Cekanova, M. Inhibition of the PI3K/AKT pathway sensitizes Oral squamous cell carcinoma cells to anthracycline-based chemotherapy in vitro. J Cell Biochem. (2017) 118:2615–24. doi: 10.1002/JCB.25747
143. Singh Kushwaha, V, Gupta, S, Husain, N, Khan, H, Negi, M, Jamal, N, et al. Gefitinib, methotrexate and methotrexate plus 5-fluorouracil as palliative treatment in recurrent head and neck squamous cell carcinoma. Cancer Biol Ther. (2015) 16:346–51. doi: 10.4161/15384047.2014.961881
144. Takeuchi, K, and Ito, F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. (2010) 277:316–26. doi: 10.1111/J.1742-4658.2009.07450.X
145. Wojtkowska, A, Małek, A, Giziński, S, Sapierzyński, R, Rodo, A, Sokołowska, J, et al. Comparison of MMP-2, MMP-9, COX-2, and PGP expression in feline injection-site and feline noninjection-site sarcomas—pilot study. Animals. (2024) 14:2110. doi: 10.3390/ANI14142110/S1
146. Daraban Bocaneti, F, Altamura, G, Corteggio, A, Tanase, OI, Dascalu, MA, Pasca, SA, et al. Expression of matrix metalloproteinases (MMPs)-2/-7/-9/-14 and tissue inhibitors of MMPs (TIMPs)-1/-2 in bovine cutaneous fibropapillomas associated with BPV-2 infection. Front Vet Sci. (2022) 9:1063580. doi: 10.3389/fvets.2022.1063580
147. Jennings, RN, Miller, MA, and Ramos-Vara, JA. Comparison of CD34, CD31, and factor VIII-related antigen Immunohistochemical expression in feline vascular neoplasms and CD34 expression in feline nonvascular neoplasms. Vet Pathol. (2012) 49:532–7. doi: 10.1177/0300985811429312
148. Kummer, S, Klang, A, Strohmayer, C, Walter, I, Jindra, C, Kneissl, S, et al. Feline SCCs of the head and neck display partial epithelial-mesenchymal transition and harbor stem cell-like cancer cells. Pathogens. (2023) 12:1288. doi: 10.3390/PATHOGENS12111288/S1
149. Nasry, WHS, Wang, H, Jones, K, Dirksen, WP, Rosol, TJ, Rodriguez-Lecompte, JC, et al. CD147 and cyclooxygenase expression in feline oral squamous cell carcinoma. Vet Sci. (2018) 5, 1–15. doi: 10.3390/vetsci5030072
150. DiBernardi, L, Doré, M, Davis, JA, Owens, JG, Mohammed, SI, Guptill, CF, et al. Study of feline oral squamous cell carcinoma: potential target for cyclooxygenase inhibitor treatment. Prostaglandins Leukot Essent Fatty Acids. (2007) 76:245–50. doi: 10.1016/J.PLEFA.2007.01.006
151. Nishibori, S, Kaneko, MK, Nakagawa, T, Nishigaki, K, Kato, Y, Igase, M, et al. Development of anti-feline PD-1 antibody and its functional analysis. Sci Rep. (2023) 13:6420. doi: 10.1038/S41598-023-31543-6
152. Boston, SE, van Stee, LL, Bacon, NJ, Szentimrey, D, Kirby, BM, van Nimwegen, S, et al. Outcomes of eight cats with oral neoplasia treated with radical mandibulectomy. Vet Surg. (2020) 49:222–32. doi: 10.1111/VSU.13341
153. Cakiroglu, H, Deveci Ozkan, A, Erman, G, Fatih Bozkurt, M, Yanar, S, Kale Bakir, E, et al. Comparative analysis of IscM and Isc Qu in feline oral squamous cell carcinoma treatment: cytotoxic and apoptotic insights. Front Vet Sci. (2025) 12:1549550. doi: 10.3389/FVETS.2025.1549550
154. Holanda, AGA, Cesário, BC, Silva, VM, Francelino, LEC, Nascimento, BHM, Damasceno, KFA, et al. Use of cold atmospheric plasma in the treatment of squamous cell carcinoma: in vitro effects and clinical application in feline tumors: a pilot study. Top Companion Anim Med. (2023) 53-54:100773–54. doi: 10.1016/j.tcam.2023.100773
Keywords: biomarkers, FcaPV, feline, monoclonal antibody, neoplasia, FOSCC
Citation: Tutu P, Daraban Bocaneti F, Altamura G, Dascalu MA, Horodincu L, Soreanu OD, Tanase OI, Borzacchiello G and Mares M (2025) Feline oral squamous cell carcinoma: recent advances and future perspectives. Front. Vet. Sci. 12:1663990. doi: 10.3389/fvets.2025.1663990
Edited by:
Francisco José Pallarés, University of Cordoba, SpainReviewed by:
Genilson Queiroz, Federal University Rural Semi-Arid, BrazilDanial Khayatan, Columbia University Irving Medical Center, United States
Copyright © 2025 Tutu, Daraban Bocaneti, Altamura, Dascalu, Horodincu, Soreanu, Tanase, Borzacchiello and Mares. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florentina Daraban Bocaneti, ZmxvcmVudGluYWJvY2FuZXRpQHlhaG9vLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Paul Tutu
Paul Tutu Florentina Daraban Bocaneti
Florentina Daraban Bocaneti Gennaro Altamura
Gennaro Altamura Mihaela Anca Dascalu
Mihaela Anca Dascalu Loredana Horodincu
Loredana Horodincu Octavian Dumitru Soreanu
Octavian Dumitru Soreanu Oana Irina Tanase
Oana Irina Tanase Giuseppe Borzacchiello
Giuseppe Borzacchiello Mihai Mares
Mihai Mares