- 1Microbiology Laboratory, College of Veterinary Medicine, Konkuk University, Seoul, Republic of Korea
- 2VIP Animal Medical Center KR, Seoul, Republic of Korea
Porphyromonas spp. are oral anaerobes that play a key role in the pathogenesis of canine periodontal disease. Despite their clinical relevance in veterinary medicine, data on the antimicrobial susceptibility of canine Porphyromonas isolates remain limited. Therefore, we assessed the antimicrobial susceptibility of Porphyromonas spp. isolated from the subgingival plaque of dogs with periodontitis in South Korea. Fifty-eight dogs diagnosed with periodontal disease were screened for Porphyromonas spp., and species identification was confirmed using PCR and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Antimicrobial susceptibility testing was performed using the broth microdilution method with the Sensititre AN02B panel. Overall, 40 isolates were recovered from 30 of the 58 dogs sampled, comprising 15 Porphyromonas gulae, 11 Porphyromonas macacae, eight Porphyromonas gingivalis, five Porphyromonas gingivicanis, and one Porphyromonas crevioricanis. Resistance was detected in six isolates (15%) to penicillin, three (7.5%) to imipenem, three (7.5%) to meropenem, 15 (25%) to clindamycin, and seven (17.5%) to ampicillin. No resistance was observed to ampicillin/sulbactam, amoxicillin/clavulanic acid, cefotetan, cefoxitin, metronidazole, chloramphenicol, piperacillin, tetracycline, mezlocillin, or piperacillin/tazobactam. These findings provide crucial insights into the antimicrobial susceptibility patterns of canine oral Porphyromonas spp. and highlight the importance of judicious antimicrobial use in veterinary dentistry.
1 Introduction
Periodontal disease is a chronic inflammatory condition that leads to the progressive destruction of the supporting structures of the teeth, including the gingiva, alveolar bone, cementum, and periodontal ligament. It is primarily driven by complex bacterial communities that colonize dental plaque and calculus (1, 2). Among these microorganisms, species of the genus Porphyromonas are frequently implicated in both the initiation and progression of periodontal disease in dogs (1, 2).
Porphyromonas spp. are part of the commensal oral microbiota in dogs, being detectable in both healthy and diseased states. However, their prevalence is considerably higher in diseased populations, where they often act as keystone members contributing to disease progression (3, 4). This dual presence highlights their role as part of the normal commensal flora in health and as major pathogens in periodontal disease.
Porphyromonas spp., along with other periodontal pathogens, typically reside within biofilms, structured microbial communities encased in a self-produced extracellular matrix. Biofilm formation markedly diminishes the efficacy of antimicrobial agents through multiple mechanisms including the physical barrier imposed by the extracellular polymeric matrix, reduced metabolic activity of bacteria in deeper biofilm layers, and the presence of persister cells (5–9). Consequently, the primary treatment for periodontal disease has traditionally relied on mechanical debridement (10, 11). However, the frequent recurrence of periodontitis following mechanical therapy alone has raised concerns about the sufficiency of this approach (12). To enhance treatment outcomes, the use of antimicrobial agents as short-term adjuncts in cases of periodontitis has become increasingly common, particularly following procedures such as scaling or extractions (10, 13). A retrospective study indicated that approximately 16.4% of canine dental procedures involved the administration of systemic or local antimicrobials (13). Beyond the oral cavity, Porphyromonas spp. have been associated with extraoral infections such as aspiration pneumonia, wound infections, sepsis, and hepatic abscesses, conditions that necessitate systemic antimicrobial therapy (14–20). Despite their clinical relevance in veterinary medicine, data on the antimicrobial susceptibility of canine Porphyromonas isolates remain limited. The fastidious nature of these bacteria and the technical challenges associated with their cultivation hinder large-scale surveillance efforts. Moreover, empirical antimicrobial use in the absence of susceptibility data may disrupt commensal microbiota and promote the emergence of resistant strains (21, 22).
In this study, we aimed to address this knowledge gap by characterizing the antimicrobial susceptibility profiles of Porphyromonas spp. isolated from the subgingival plaque of dogs with periodontitis in South Korea. The findings will support improved periodontal management and promote antimicrobial stewardship in veterinary practice.
2 Materials and methods
2.1 Sample collection
Dental plaque samples were collected from 58 dogs diagnosed with periodontal disease at the VIP Animal Medical Center (Seoul, South Korea) between October 2019 and August 2020. Samples were obtained from the maxillary molar region and placed in a transport medium consisting of Brucella broth supplemented with 1% yeast extract, 5% defibrinated sheep blood, 0.5 g/L cysteine, 5 μg/mL hemin, 10 μg/mL vitamin K, and 0.3% agar. Samples were immediately transported under anaerobic conditions to the Microbiology Laboratory, College of Veterinary Medicine, Konkuk University. Detailed clinical metadata were not available because the samples were provided by the collaborating veterinary hospital during routine dental procedures.
2.2 Bacterial isolation and identification
Samples were homogenized in 0.5 mL distilled water and streaked onto Porphyromonas Blood Agar (Tryptic Soy Agar supplemented with 1% yeast extract, 5% defibrinated sheep blood, 0.5 g/L cysteine, 5 μg/mL hemin, and 10 μg/mL vitamin K). Plates were incubated anaerobically at 37 °C for up to 2 weeks. All black-pigmented colonies, characteristic of Porphyromonas spp., were subcultured until pure isolates were obtained. Each Porphyromonas isolate from a given sample was stored in brain heart infusion broth containing 10% skim milk at −80 °C.
Frozen stocks were revived by streaking onto Porphyromonas Blood Agar and incubating anaerobically at 37 °C. Colonies were then inoculated into supplemented Brucella broth and incubated anaerobically at 37 °C for 2 weeks. Following incubation, bacterial cultures were smeared onto a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) target plate. To each smear, 1.5 μL of 70% formic acid was applied and allowed to dry completely, followed by 1.5 μL of matrix solution. Once dry, the samples were analyzed using MALDI-TOF MS by NosVet Co. (South Korea).
For molecular confirmation, genomic DNA was extracted from cultured isolates using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The 16S rRNA gene was amplified by PCR using primers 8F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1429R (5′-GGT TAC CTT GTT ACG ACT T-3′) (23, 24), in accordance with the i-Taq DNA polymerase protocol (iNtRON Biotechnology, South Korea).
The PCR conditions included an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min and 40 s, with a final extension at 72 °C for 7 min.
PCR products were visualized by electrophoresis on a 2% agarose gel containing RedSafe (iNtRON Biotechnology, South Korea) and purified using the MEGAquick-spin Plus Total Fragment DNA Purification Kit (iNtRON Biotechnology, South Korea) according to the manufacturer’s protocol. Sanger sequencing was performed by BIONICS Co. (South Korea), and taxonomic identification was confirmed using BLAST searches against the NCBI nr/nt database.
2.3 Antimicrobial susceptibility testing
Three to five well-isolated colonies were suspended in 5 mL of cation-adjusted Mueller–Hinton broth (ThermoFisher Scientific) and adjusted to a 0.5 McFarland standard.
A 100-μL aliquot of the suspension was transferred to Brucella broth supplemented with 5% defibrinated sheep blood, 5 μg/mL hemin, and 10 μg/mL vitamin K, yielding a final bacterial concentration of 1 × 106 CFU/mL. The inoculum was dispensed into Sensititre AN02B trays (UniScience, South Korea) and incubated anaerobically at 37 °C until visible growth was observed in the positive control wells. The antimicrobials tested included ampicillin/sulbactam (SAM), amoxicillin/clavulanic acid (AMC), cefotetan (CTT), penicillin (PEN), imipenem (IPM), meropenem (MEM), clindamycin (CLI), cefoxitin (FOX), metronidazole (MTZ), chloramphenicol (CHL), ampicillin (AMP), piperacillin (PIP), tetracycline (TET), mezlocillin (MEZ), and piperacillin/tazobactam (TZP).
The minimum inhibitory concentration (MIC) was defined as the lowest concentration of an antimicrobial that completely inhibited visible bacterial growth. Bacteroides fragilis (ATCC 25285) was used as the quality control strain. All assays were performed in triplicate. MIC interpretation was based on the breakpoints established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for anaerobic bacteria.
3 Results
3.1 Resource identification initiative
Overall, 40 Porphyromonas spp. were isolated from 30 of the 58 dogs sampled. The species distribution comprised 15 Porphyromonas gulae, 11 Porphyromonas macacae, eight Porphyromonas gingivalis, five Porphyromonas gingivicanis, and one Porphyromonas crevioricanis. Species identification was consistent across methods, with BLAST identity scores above 98.3% (Supplementary Table S1).
3.1.1 Overall susceptibility patterns
No resistance was observed to SAM, AMC, CTT, FOX, MTZ, CHL, PIP, TET, MEZ, or TZP (Table 1).
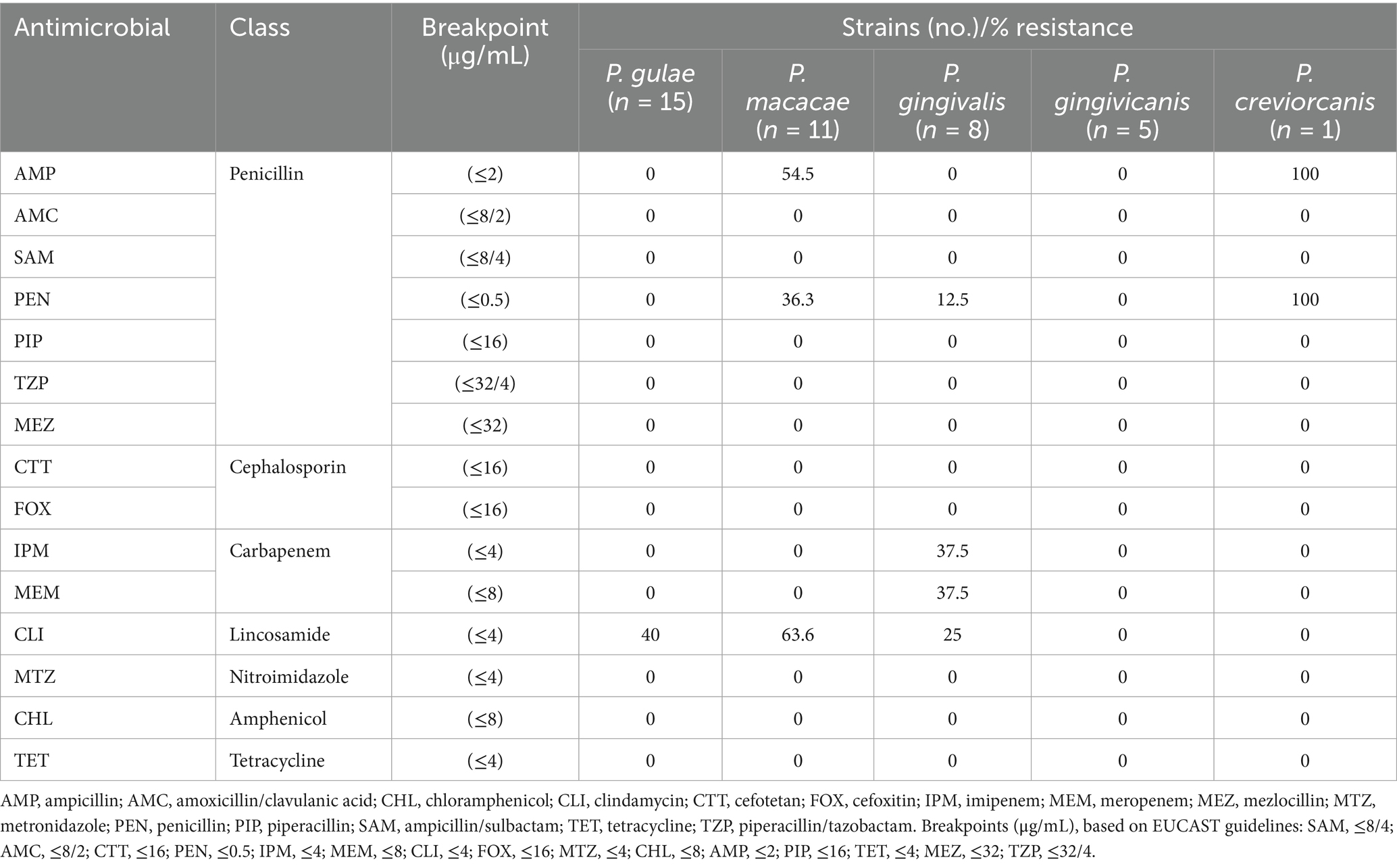
Table 1. Antimicrobial susceptibility of Porphyromonas isolates from the subgingival plaque of dogs with periodontitis.
3.1.2 Penicillin-class agents
Resistance to PEN was detected in six isolates (15%): four P. macacae (36.4%), one P. gingivalis (12.5%), and the single P. crevioricanis isolate (100%).
Resistance to AMP was observed in seven isolates (17.5%): six P. macacae (54.5%) and one P. crevioricanis (100%).
3.1.3 Carbapenems
Resistance to IPM and MEM was detected in three isolates each (7.5%), all belonging to P. gingivalis (37.5%).
3.1.4 Lincosamides
Resistance to CLI was identified in 15 isolates (25%): six P. gulae (40%), seven P. macacae (63.6%), and two P. gingivalis (25%).
Detailed species-specific resistance profiles are provided in Supplementary Tables S2–S6.
4 Discussion
In this study, we determined antimicrobial resistance profiles of 40 Porphyromonas isolates recovered from 30 of the 58 dogs with periodontal disease. To the best of our knowledge, this is the first report from South Korea to investigate the antimicrobial susceptibility of Porphyromonas spp. isolated from the canine dental plaque.
In this study, Porphyromonas spp. were isolated from only half of the sampled dogs. This observation likely reflects inter-individual variation in oral microbial communities, differences in disease stage, and host or environmental factors (4). Notably, studies utilizing 16S rRNA sequencing often report higher prevalence rates of Porphyromonas, as DNA-based approaches can detect non-cultivable or low-abundance populations (25). In contrast, the culture-based methods employed in the present study may underestimate prevalence due to the fastidious growth requirements of these organisms. This methodological distinction should be considered when interpreting the prevalence data presented here.
CLI is commonly prescribed for the management of bacterial periodontal infections and is frequently used to treat gingivitis, periodontitis, dental root abscesses, and as prophylaxis following oral surgery (26, 27). Among the antimicrobials tested, CLI exhibited the highest resistance rate, in contrast to a previous study by Senorinho et al. (28), which reported no CLI resistance among P. gulae and P. macacae isolates. Despite this, most Porphyromonas isolates in our study remained susceptible to a broad range of antimicrobials. This may reflect their localization within biofilms, which reduces antimicrobial exposure and the reliance on mechanical debridement (i.e., scaling) as the primary therapy, combined with generally judicious antimicrobial use in veterinary practice (5, 6, 9–11).
In human dentistry, resistance of Porphyromonas spp. to clindamycin has been documented in several studies (29, 30), particularly among P. gingivalis isolates from patients with periodontal disease, whereas data in companion animals remain scarce. Our study provides region-specific insights that contribute to bridging this gap.
Antimicrobial susceptibility testing is rarely conducted in clinical settings prior to treatment, often resulting in inappropriate or excessive use (31–33). Although antimicrobial susceptibility testing should ideally inform treatment decisions, empirical therapy is sometimes necessary. Porphyromonas spp. are fastidious anaerobes that may require up to 2 weeks of incubation to yield visible growth, presenting a challenge in time-sensitive conditions such as sepsis or aspiration pneumonia. In such cases, access to region-specific antimicrobial resistance data is essential to guide empirical therapy until isolate-specific results are available.
In addition to their role in periodontal disease, oral bacteria such as Porphyromonas spp. may enter the bloodstream during dental procedures and contribute to systemic conditions, including infective endocarditis (34, 35). Therefore, antimicrobial decisions in veterinary dentistry should consider not only the local oral microbiota but also the potential for systemic sequelae. This highlights the importance of prudent, case-specific antimicrobial use.
A limitation of this study is the absence of detailed clinical metadata for the sampled dogs. Information such as age, sex, breed, disease stage, and prior antimicrobial exposure was not available, as the samples were obtained during routine dental procedures at a collaborating veterinary hospital without access to the patients’ full medical histories. The lack of such data restricted our ability to evaluate potential associations between host characteristics, treatment history, and antimicrobial resistance patterns. Future studies incorporating comprehensive clinical information will be important to better understand risk factors for resistance in canine periodontal pathogens.
Despite these limitations, our findings highlight the clinical importance of considering antimicrobial resistance in periodontal pathogens. Accordingly, veterinarians should consider the use of clindamycin in empirical therapy for canine periodontal infections, whenever feasible, perform culture and susceptibility testing.
5 Conclusion
This study confirms the presence of clindamycin-resistant Porphyromonas spp. in dogs with periodontitis in South Korea. While resistance was limited, these findings provide region-specific data to guide empirical therapy and highlight the value of susceptibility testing in veterinary dentistry.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements. All clinical samples were obtained from client-owned dogs during routine veterinary dental treatment, with no additional procedures performed for research purposes. Written informed consent was not obtained from the owners for the participation of their animals in this study because Owner consent for the use of these samples in research was obtained verbally at the time of treatment, in accordance with institutional policy.
Author contributions
TK: Conceptualization, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Y-DC: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Investigation. WH: Conceptualization, Writing – original draft, Writing – review & editing, Investigation. S-WL: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. T-ML: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The authors verify and take full responsibility for the use of generative AI in the preparation of this manuscript. Generative AI was used solely for grammar correction and rephrasing sentences to align with academic writing style. All content, data interpretation, and conclusions are the original work of the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1684907/full#supplementary-material
References
1. Bi, R, Yang, Y, Liao, H, Ji, G, Ma, Y, Cai, L, et al. Porphyromonas gingivalis induces an inflammatory response via the cGAS-STING signaling pathway in a periodontitis mouse model. Front Microbiol. (2023) 14:1183415. doi: 10.3389/fmicb.2023.1183415
2. Murugaiyan, V, Utreja, S, Hovey, KM, Sun, Y, LaMonte, MJ, Wactawski‑Wende, J, et al. Defining Porphyromonas gingivalis strains associated with periodontal disease. Scient Rep. (2024) 14:6222. doi: 10.1038/s41598-024-56849-x
3. Kačírová, J, Sondorová, M, Maďari, A, Styková, E, Mucha, R, Nemcová, R, et al. Detection of periodontal pathogens from dental plaques of dogs with and without periodontal disease. Pathogens. (2022) 11:480. doi: 10.3390/pathogens11040480
4. Kwack, KH, Jang, E-Y, Kim, C, Choi, Y-S, Lee, J-H, and Moon, J-H. Porphyromonas gulae and canine periodontal disease: current understanding and future directions. Virulence. (2025) 16:2449019. doi: 10.1080/21505594.2024.2449019
5. Bessa, LJ, Botelho, J, Machado, V, Alves, R, and Mendes, JJ. Managing oral health in the context of antimicrobial resistance. Int J Environ Res Public Health. (2022) 19:16448. doi: 10.3390/ijerph192416448
6. Pai, L, Patil, S, Liu, S, and Wen, F. A growing battlefield in the war against biofilm-induced antimicrobial resistance: insights from reviews on antibiotic resistance. Front Cell Infect Microbiol. (2023) 13:1327069. doi: 10.3389/fcimb.2023.1327069
7. Singh, B, Dahiya, M, Kumar, V, Ayyagari, A, Chaudhari, DN, and Ahire, JJ. Biofilm and antimicrobial resistance: mechanisms, implications, and emerging solutions. Microbiol Res. (2025) 16:183. doi: 10.3390/microbiolres16080183
8. Stewart, PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. (2002) 292:107–113. doi: 10.1078/1438-4221-00196
9. Zhang, Q, Zhen, M, Wang, X, Zhao, FX, Dong, Y, Wang, X, et al. Antibiotic exposure enriches streptococci carrying resistance genes in periodontitis plaque biofilms. PeerJ. (2025) 13:e18835. doi: 10.7717/peerj.18835
10. Laforgia, A, Inchingolo, AD, Piras, F, Colonna, V, Giorgio, RV, Carone, C, et al. Therapeutic strategies and genetic implications for periodontal disease management: a systematic review. Int J Mol Sci. (2024) 25:7217. doi: 10.3390/ijms25137217
11. Schulz, S, Stein, JM, Schumacher, A, Kupietz, D, Yekta-Michael, SS, Schittenhelm, F, et al. Nonsurgical periodontal treatment options and their impact on subgingival microbiota. J Clin Med. (2022) 11:1187. doi: 10.3390/jcm11051187
12. Liss, A, Abrahamsson, KH, Welander, M, and Tomasi, C. Effectiveness of nonsurgical re‐instrumentation of residual pockets as step 3 of periodontal therapy: A field study. J Periodontol (2025). doi: 10.1002/jper.24-0532
13. Weese, JS, Battersby, I, Morrison, J, Spofford, N, and Soltero-Rivera, M. Antimicrobial use practices in canine and feline dental procedures performed in primary care veterinary practices in the United States. PLoS One. (2023) 18:e0295070. doi: 10.1371/journal.pone.0295070
14. Atagi, Y, Homma, Y, Yamashi, S, Kikuchi, K, and Nagashima, Y. Fatal renal abscess caused by Porphyromonas gingivalis and subcapsular hemorrhage, Japan. Emer Infect Dis. (2024) 30:2214–2217. doi: 10.3201/eid3010.240078
15. Jover-Díaz, F, Cuadrado, JM, Laveda, R, Andreu, L, and Merino, J. Porphyromonas asaccharolytica liver abscess. Anaerobe. (2003) 9:87–9. doi: 10.1016/S1075-9964(03)00065-9
16. Nie, S, Lin, D, and Li, X. Clinical characteristics and management of 106 patients with pyogenic liver abscess in a traditional Chinese hospital. Front Surg. (2023) 9:1041746. doi: 10.3389/fsurg.2022.1041746
17. Okabe, T, Kamiya, Y, Kikuchi, T, Goto, H, Umemura, M, Suzuki, Y, et al. Porphyromonas gingivalis components/secretions synergistically enhance pneumonia caused by Streptococcus pneumoniae in mice. Int J Mol Sci. (2021) 22:12704. doi: 10.3390/ijms222312704
18. Sha, J, Shao, J, Lu, S, Yao, W, Deng, Y, Chen, J, et al. Pyopneumothorax with bronchopleural fistula due to pulmonary infection caused by Porphyromonas gingivalis in a patient with periodontitis. Clin Respir J. (2023) 17:962–965. doi: 10.1111/crj.13684
19. Wang, X, Liu, L, Lu, B, Duan, X, Zhou, X, Huang, D, et al. The thoracoabdominal wall abscess and sepsis caused by Porphyromonas pogonae: case report and literature review. Heliyon. (2023) 9:e14860. doi: 10.1016/j.heliyon.2023.e14860
20. Yuanjun, Z, Yan, C, Qingyan, Z, and Feng, X. Porphyromonas gingivalis-induced hematogenous disseminated severe pneumonia: a case report. J Int Méd Res. (2024) 52:03000605231213760. doi: 10.1177/03000605231213760
21. Dietl, B, Boix-Palop, L, Gisbert, L, Mateu, A, Garreta, G, Xercavins, M, et al. Risk factors associated with inappropriate empirical antimicrobial treatment in bloodstream infections. A cohort study. Front Pharmacol. (2023) 14:1132530. doi: 10.3389/fphar.2023.1132530
22. Li, S, Liu, J, Zhang, X, Gu, Q, Wu, Y, Tao, X, et al. The potential impact of antibiotic exposure on the microbiome and human health. Microorganisms. (2025) 13:602. doi: 10.3390/microorganisms13030602
23. Eden, PA, Schmidt, TM, Blakemore, RP, and Pace, NR. Phylogenetic Analysis of Aquaspirillum magnetotacticum Using Polymerase Chain Reaction-Amplified 16S rRNA-Specific DNA. Int J Syst Evol Microbiol. (1991) 41:324–325. doi: 10.1099/00207713-41-2-324
24. Jiang, H, Dong, H, Zhang, G, Yu, B, Chapman, LR, and Fields, MW. Microbial Diversity in Water and Sediment of Lake Chaka, an Athalassohaline Lake in Northwestern China. Appl Environ Microbiol. (2006) 72:7430–7430. doi: 10.1128/aem.02230-06
25. Bai, Y, Song, P, Shen, Z, Shi, H, Jiang, Z, Lin, J, et al. Porphyromonas gulae infection in canines, pet owners and veterinarians in China: an epidemiological study and risk factor analysis. One Health Adv. (2023) 1:9. doi: 10.1186/s44280-023-00007-x
26. Abdullah, FM, Hatim, QY, Oraibi, AI, Alsafar, TH, Alsandook, TA, Lutfi, W, et al. Antimicrobial management of dental infections: updated review. Medicine. (2024) 103:e38630. doi: 10.1097/MD.0000000000038630
27. Luchian, I, Goriuc, A, Martu, MA, and Covasa, M. Clindamycin as an alternative option in optimizing periodontal therapy. Antibiotics. (2021) 10:814. doi: 10.3390/antibiotics10070814
28. Senhorinho, GNA, Nakano, V, Liu, C, Song, Y, Finegold, SM, and Avila-Campos, MJ. Occurrence and antimicrobial susceptibility of Porphyromonas spp. and Fusobacterium spp. in dogs with and without periodontitis. Anaerobe. (2012) 18:381–385. doi: 10.1016/j.anaerobe.2012.04.008
29. Ardila, CM, López, MA, and Guzmán, IC. High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas Gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Medicina Oral Patologia Oral Cirugia Bucal. (2010) 15:e947–51.
30. Conrads, G, Klomp, T, Deng, D, Wenzler, J-S, Braun, A, and Abdelbary, MMH. The antimicrobial susceptibility of Porphyromonas gingivalis: genetic repertoire, global phenotype, and review of the literature. Antibiotics. (2021) 10:1438. doi: 10.3390/antibiotics10121438
31. Muzny, CA, and Sobel, JD. The role of antimicrobial resistance in refractory and recurrent bacterial vaginosis and current recommendations for treatment. Antibiotics. (2022) 11:500. doi: 10.3390/antibiotics11040500
32. Reissier, S, Penven, M, Guérin, F, and Cattoir, V. Recent trends in antimicrobial resistance among anaerobic clinical isolates. Microorganisms. (2023) 11:1474. doi: 10.3390/microorganisms11061474
33. Shaker, HO, Naguib, M, Abdelaziz, BM, and Bourini, MMSEE. Clindamycin use evaluation retrospective observational study in critical care units in Alexandria main university hospital. Egypt J Int Med. (2024) 36:33. doi: 10.1186/s43162-024-00300-0
34. Bumm, CV, and Folwaczny, M. Infective endocarditis and oral health—a narrative review. Cardiovasc Diag Ther. (2021) 11:1403–15. doi: 10.21037/cdt-20-908
Keywords: antimicrobial susceptibility testing, Porphyromonas, periodontitis, clindamycin, minimum inhibitory concentration
Citation: Kim T, Choi Y-D, Hur W, Lee S-W and La T-M (2025) Antimicrobial susceptibility of Porphyromonas spp. isolated from dogs with periodontal disease in South Korea. Front. Vet. Sci. 12:1684907. doi: 10.3389/fvets.2025.1684907
Edited by:
Maria M. Soltero-Rivera, University of California, Davis, United StatesReviewed by:
Se Eun Kim, Chonnam National University, Republic of KoreaClaire A. Shaw, University of California, Davis, United States
Copyright © 2025 Kim, Choi, Hur, Lee and La. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae-Min La, Zmt4b2Fsc0Brb25rdWsuYWMua3I=
†These authors have contributed equally to this work
 Taesoo Kim
Taesoo Kim Yi-Don Choi2†
Yi-Don Choi2† Sang-Won Lee
Sang-Won Lee Tae-Min La
Tae-Min La