- 1Parasitology Laboratory, Veterinary College, Xinjiang Agricultural University, Urumqi, Xinjiang, China
- 2National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido, Japan
- 3Laboratory of Sustainable Animal Environment, Graduate School of Agriculture Science, Tohoku University, Sendai, Japan
- 4Department of Internal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
- 5Department of Biochemistry and Chemistry of Nutrition, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
- 6Research Center for Asian Infectious Diseases, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
Babesia and Theileria are microscopic parasites that infect livestock, leading to substantial economic losses. Current treatments are often limited by challenges such as drug resistance and incomplete parasite eradication. This study investigates potential of Etoposide (EP), a well-known anticancer drug that disrupts DNA Topoisomerase II, as a treatment for these parasitic infections. The research focused on EP’s ability to inhibit Babesia bovis, Babesia caballi, and Theileria equi, evaluating its impact on parasite viability, structural changes, and protective role for host red blood cells. Parasites were exposed to various concentrations of EP (ranging from 0.50 × to 4 × IC50), and their viability and morphology were assessed through bioassays and Giemsa-stained slides analysis. In vivo experiments were conducted using a mouse model infected with Babesia microti, to examine changes in parasite burden, red blood cell counts, and fluorescence-based signals. The results demonstrated that EP inhibited parasite growth in a dose-dependent manner, with IC50 values of 11.23 ± 2.82 μM for B. bovis, 0.037 ± 0.039 μM for B. caballi, and 0.68 ± 0.39 μM for T. equi. Importantly, parasites treated with EP did not recover when returned to untreated culture conditions. Morphological changes included distinct spots in B. bovis and B. caballi, along with abnormal structures in T. equi. These findings suggest that EP has potential as a complementary therapy, enhancing the effectiveness of existing treatments for Babesia and Theileria infections. Further research is warranted to refine its application and investigate its role in combination therapy strategies.
1 Introduction
Babesia spp. are globally distributed, tick-borne protozoan parasites that cause significant diseases in livestock, such as bovine babesiosis in cattle and equine piroplasmosis in horses, resulting in substantial economic losses (1). In cattle, Babesia bovis and Babesia bigemina are the primary causative agents leading to symptoms that include high fever, hemolytic anemia, hemoglobinuria, and, in severe cases, death (2). In horses, equine piroplasmosis is caused by Babesia caballi and Theileria equi, which induce similar clinical signs (3). Although significant progress has been made in developing drugs targeting Babesia, further research and innovation remain essential (4).
DNA topoisomerase II (TopoII) is indispensable for chromosome dynamics in rapidly dividing protozoan parasites, resolving topological stress through transient double-stranded DNA break formation and religation (5). Critically, structural and functional distinctions exist between parasitic TopoII isoforms and human topoisomerases (hTop) (6), enabling selective targeting for antiprotozoal drug development. This therapeutic potential is evidenced by: (i) Stage-specific expression of Plasmodium PfTopoVIB and PfSpo11 during late schizogony, coinciding with mitochondrial genome segregation (7); (ii) Dual-stage antiplasmodial activity of (ii) Dual-stage antiplasmodial activity of Aloe marlothii constituents, with aloesaponarin I and aloesaponol IV computationally identified as β-hemoglobin and TopoII inhibitors, respectively (8); (iii) Lethal DNA damage in parasites induced by etoposide (EP)-mediated stabilization of TopoII-DNA cleavage complexes (9).
Recent studies have expanded the scope of drug research to include anti-inflammatory, antibacterial, anti-tumor, and immunomodulatory effects. Novel hybrid compounds, such as 7-chloro-4-aminoquinoline-benzimidazole (10) and monoanionic gold bis (dithiolene) complexes (11) demonstrate this paradigm shift. However, application to protozoal diseases beyond malaria, toxoplasmosis, and trypanosomiasis—prioritized for their zoonotic significance application to protozoal diseases beyond malaria, toxoplasmosis, and trypanosomiasis—prioritized for their zoonotic significance (12). Effective babesiosis control is clinically imperative (13) particularly with emerging pathogens like Babesia duncani (14) alongside established agents B. microti and B. divergens. This study provides mechanistic evidence supporting antitumor drug repurposing against Babesia species. Collectively, these insights advance understanding of blood-protozoan pathogenesis and potential hematological implications.
2 Materials and methods
Babesia bovis (Texas strain), B. caballi (USDA strain), and T. equi (USDA strain) were cultured using purified bovine and horse red blood cells (RBCs) in GIT and RPMI 1640 media. Cultures were maintained in 24-well plates in a 37 °C in an incubator with a gas mixture comprising 5% CO2 and 5% O2. The culture medium was refreshed every 24 h (2). Concurrently, B. microti (Munich strain) was administered to 7-week-old female BALB/c mice (CLEA Japan Inc., Tokyo, Japan). The SYBR Green I (SGI) nucleic acid stain (Lonza, USA; 10,000 ×) and lysis buffer were prepared following the procedure described by Li et al. (2), solutions were stored at −30 °C and 4 °C, respectively. Stock solutions of diminazene aceturate (DA, Novartis, Japan), Etoposide (EP, Sigma-Aldrich, Japan) were prepared at a concentration of 100 mM and stored at −30 °C until use. Cell toxicity was evaluated using the Cell Counting Kit-8 (CCK-8, Japan). IC50 values for B. bovis, B. caballi, and T. equi were calculated for concentrations ranging from 0.10 to 250 μM using non-linear regression analysis (curve fit) in GraphPad Prism 7 (GraphPad Software Inc., USA). Non-infected RBCs and 0.5% dimethyl sulfoxide (DMSO) were used as blank and negative controls, respectively. Each compound concentration was tested in triplicate, and the experiment was repeated three times.
The viability of B. bovis treated with drugs was assessed using the protocol (2). In brief, 10 μL of 1% infected RBCs (iRBCs) and 90 μL of medium containing varying drug concentrations were dispensed into a 96-well plate. The plate was incubated under the previously established conditions, and the medium was replaced every 24 h for 4 days. Fresh medium containing the respective concentrations of EP (0.50×, 1×, 2×, and 4 × the IC₅₀) was added during each medium change. On the fifth day, 3 μL of RBCs from the treated wells were mixed with 7 μL of fresh RBCs in a new 96-well plate without the drug, and the medium was refreshed daily for the next 5 days. Thin blood smears were prepared and stained with Giemsa (GBS) on the fifth day. Each experiment was performed in triplicate and repeated three times. To evaluate cell viability in response to drug treatment, the Cell Counting Kit-8 (CCK-8, Japan) assay was utilized. In summary, Madin-Darby Bovine Kidney (MDBK) cells were seeded at a density of 5 × 104 cells/mL in 96-well plates, with 100 μL of cell suspension per well, and incubated for 24 h. Next, 100 μL of drug solutions at various concentrations (5–1,000 μM) were added in triplicate to the wells. After a 24-h incubation period, 10 μL of CCK-8 reagent was added to each well followed by an additional 4-h incubation. Absorbance at 450 nm was then measured using an MTP-500 microplate reader (Corona Electric, Japan). Wells containing only culture medium served as blanks, while wells with cells in medium with 0.5% DMSO were used as controls (2).
The therapeutic efficacy of selected compounds was assessed using a mouse model infected with B. microti, following the protocol outlined by Rizk et al. (15). Thirty-five 7-week-old female BALB/c mice (From CLEA Japan Inc., Tokyo, Japan) were randomly divided into four groups. The first group received EP treatment at a dose of 1 mg/kg, while the second group was administered the standard drug DA at 25 mg/kg. The third and fourth groups were positive (Infected and untreated) and negative (Uninfected and untreated) controls, respectively. Treatments were delivered via intraperitoneal injection (IP) for five consecutive days (From days 4–8) after parasitemia levels reached 1%. Following previously study (2), a B. microti-positive mouse model was established by retrieving B. microti from the cell bank and I njecting into a mouse. Parasitemia was determined every other day by examining GBS under a 100 × oil immersion Eclipse E200 microscope (Nikon, Tokyo, Japan). The percentage of parasitized erythrocytes was calculated by counting 103 erythrocytes. When parasitemia reached 30%, blood was collected, diluted in 1 × PBS to 2 × 107 infected red blood cells (iRBCs)/mL, and 0.5 mL of this suspension was injected intraperitoneally into all groups, except the negative control. The final concentration of injected iRBCs was 1 × 107/mL. Parasitemia was monitored bi-daily for 32 days, or until parasitemia was cleared using GBS as described earlier. Tail blood (2.5 μL) was collected every 2 days and analyzed in 96-well plates using RPMI 1640 medium, lysis buffer, and 2 × SGI stain, followed by fluorescence measurement after 1 h of incubation. Hematological analyses were performed every 4 days using a hemocytometer (Celltac α MEK-6450, NihonKohden, Japan). All experiments were carried out in duplicate.
The IC50 values for EP and DA were calculated using non-linear regression analysis with GraphPad Prism 6.0 (GraphPad Software Inc., USA). The half-maximal inhibitory concentration (IC₅₀) values for EP and DA were determined by non-linear regression analysis using GraphPad Prism 6.0 (GraphPad Software Inc., USA). Specifically, a four-parameter logistic model (4PL) was fitted to the dose–response data obtained from at least three independent experiments, each performed in technical triplicate. The model provides the IC₅₀ as the inflection point of the sigmoidal curve. All reported IC₅₀ values are presented as the mean ± standard error of the mean (SEM) derived from these independent replicates. This analysis included independent Student’s t-tests and one-way ANOVA. A p-value of less than 0.05 was considered statistically significant, with values below 0.01 indicated a highly significant difference. The study protocol received approval from the Committee on the Ethics of Animal Experiments at Obihiro University of Agriculture and Veterinary Medicine, Japan (Permit numbers: animal experiment, 230,244; DNA experiment, AP0001299621; Pathogen, AP0001299622; Ethical approval numbers, 22–23 and 23–17).
3 Results
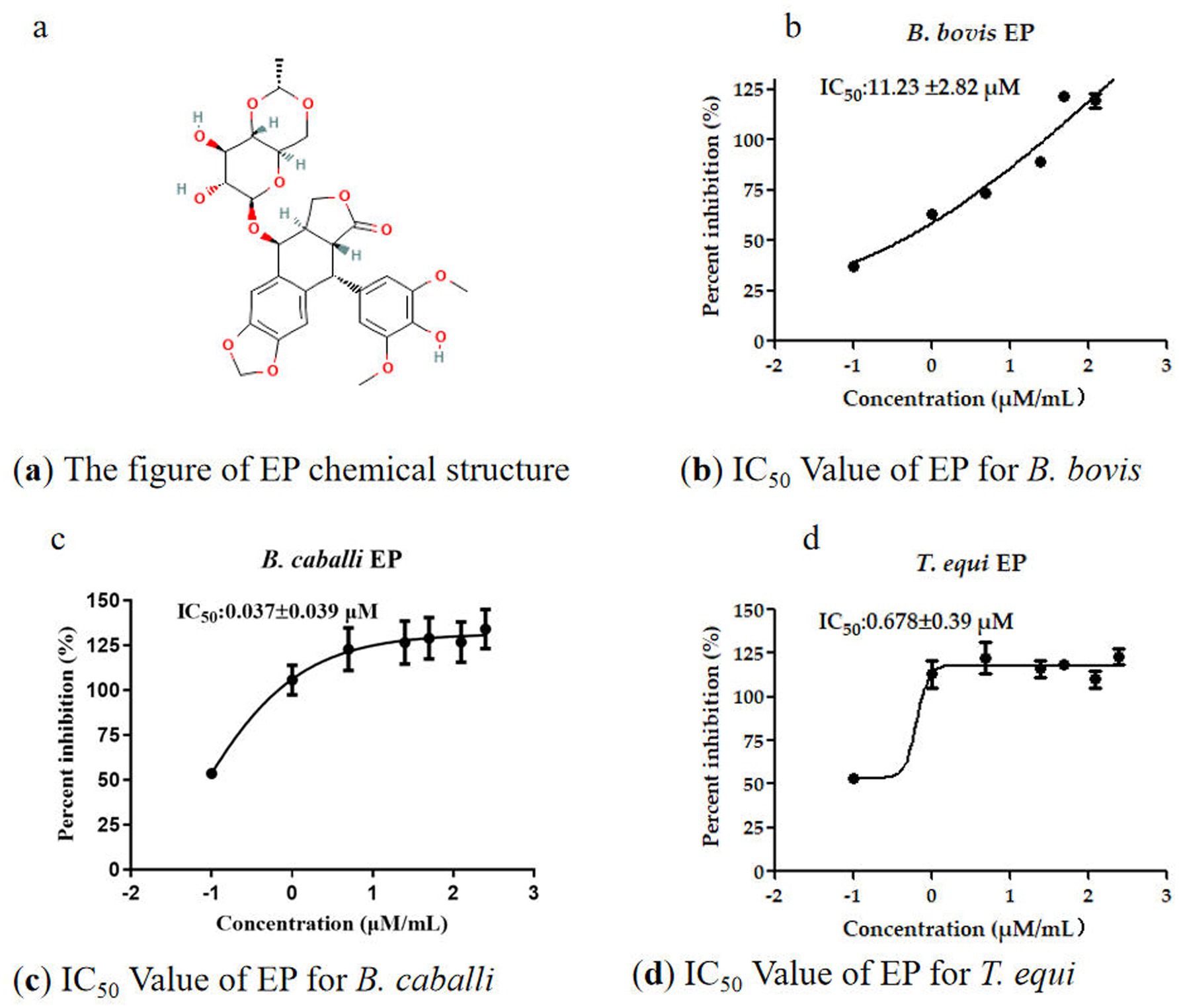
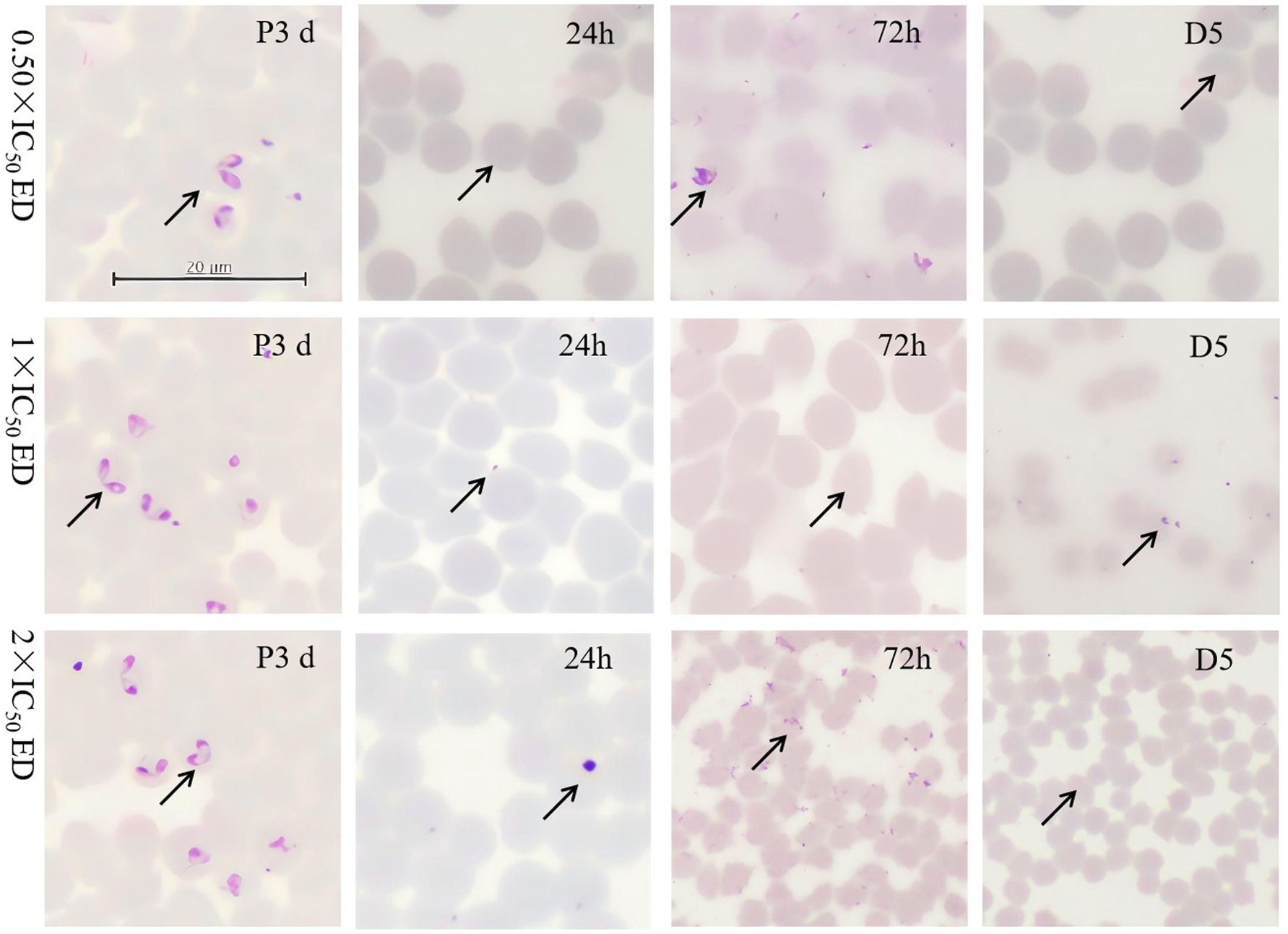
The EP demonstrated potent inhibitory effects on B. bovis, B. caballi and T. equi and the IC50 values for EP against these parasites were 11.23 ± 2.82 μM, 0.037 ± 0.039 μM, and 0.68 ± 0.39 μM, respectively (Figures 1a–d). To further validate the effectiveness of the identified EP as inhibitor of B. bovis, B. caballi, and T. equi, both viability assays and morphological assessments of treated cultures were performed. Following treatment with varying concentrations of EP (IC50 ranging from 0.50 × to 4×), no recovery of B. bovis, B. caballi, or T. equi was observed when the cultures were returned to standard medium conditions. GBS were used to examine parasitemia at 24 h, 72 h, and 5 days. All quantitative data are presented as the mean ± standard error of the mean (SEM) from at least three independent experiments, each performed in technical triplicate. Statistical analyses were conducted using one-way ANOVA followed by Tukey’s multiple-comparison test or Student’s t-test where appropriate. Statistical significance was annotated directly on the graphs as p < 0.05, p < 0.01, and p < 0.001. Error bars in all figures represent the SEM. Where no bars are visible, the errors are smaller than the symbols. All analyses were performed using GraphPad Prism 7.0 (GraphPad Software Inc., USA).
Morphological analysis revealed small spots within RBCs in B. bovis and B. caballi treated with 0.50 × IC50 of EP on day 5, compared to the positive control (P3d). In T. equi, suspected parasite bodies appeared at 72 h and on day 5 following treatment with 0.50 × and 2 × IC50. Notably, no regrowth of B. bovis, B. caballi, or T. equi was observed at EP concentrations of 11.23, 22.46, and 44.92 μM; 0.074, 0.148, and 0.296 μM; and 1.36, 2.72, and 5.44 μM, respectively (Figure 2, Supplementary Figures S1, S2).
Treatment with EP in vitro showed no cytotoxic effects on MDBK cells at concentrations up to 500 μM. Notably, EP, identified as a potent natural compound, exhibited a low IC50 value for MDBK cells while demonstrating a very high selectivity index (SI). The cytotoxicity assay results were used to calculate the selectivity index (SI = IC₅₀ for MDBK cells ÷ IC₅₀ for parasites), providing a comparative measure of safety. The SI values for EP were 44.5 for B. bovis, 13,514 for B. caballi, and 735 for T. equi, indicating a favorable safety margin across all tested species. For comparison, the standard anti-babesial drug diminazene aceturate (DA) exhibits reported SI values in the range of 250–500 under similar assay conditions (2). Thus, while EP’s antiparasitic potency is modest relative to DA, its markedly higher SI—especially against B. caballi and T. equi—suggests low mammalian cytotoxicity and potential utility as a lead compound for safer combination therapy development.
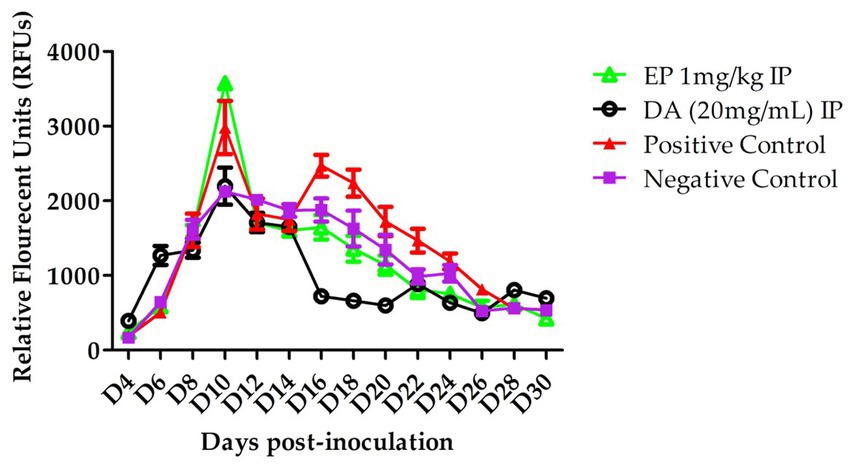
The in vivo efficacy of EP against B. microti was evaluated. In the EP treated group, a slower increase in parasitemia was observed following intraperitoneal (IP) injections on days 14, 16, and 18. However, statistical analysis revealed no significant difference compared to the control group (p > 0.05). On day 16, relative fluorescence units (RFUs) values for the treatedgroup were recorded at 1,800 for 1 mg/kg EP and 960 for 20 mg/kg DA, whereas the control group exhibited a peak RFU of 2,500 (Figure 3).
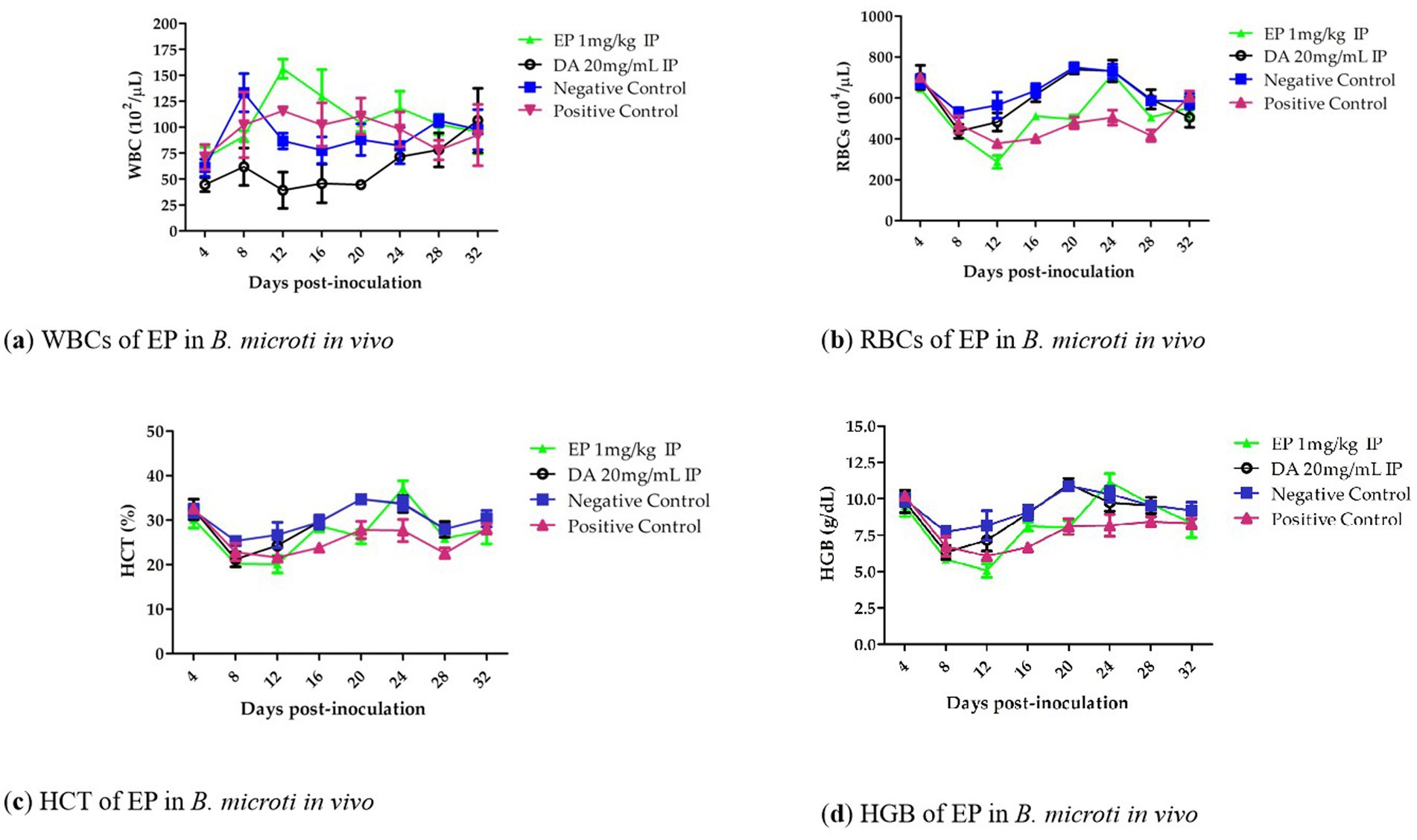
Notably, hematological parameters including RBC count (Figure 4a), hemoglobin (HGB) concentration (Figure 4b), hematocrit (HCT) percentage (Figure 4c), and HGB count (Figure 4d), showed an increasing trend from days 12 to 20 in the EP-treated groups when compared to the untreated infected group. However, no statistically significant reduction (p > 0.05) in RBC count, HGB concentration, or HCT percentage was observed in the EP-treated groups relative to the DA-treated group.
4 Discussion
Tick-borne diseases (TBDs) in animals are increasingly prevalent in subtropical and tropical regions; however, research focusing on Asian countries remains limited (16). Babesiosis, one of the TBDs, is primarily transmitted by Ixodid ticks (17, 18). As a region adjacent to Xinjiang, Pakistan continues to report cases of bovine babesiosis and theileriosis. For instance, Kebzai et al. (19) detected Theileria annulata in cattle blood samples from Loralai and Zhob districts, with infection rates of 12.75 and 12.25% via PCR. Notably, the prevalence among young cattle reached 85.82% (19). Additionally, T. annulata has been found to infect camels in Pakistan, showing an overall infection rate of 13.5% (20).
In recent years, several new therapeutic approaches have been developed for bovine theileriosis and babesiosis, including Juglone, Buparvaquone (21), imidocarb dipropionate, and the MCD protocol (metronidazole, clindamycin, and doxycycline) (19, 22). Following treatments such as Juglone and Buparvaquone, a decrease in total leukocyte and lymphocyte counts was observed in recovering cattle (21). In T. equi infections, affected horses exhibited reduced levels of total and free testosterone, as well as tri-iodothyronine (T3), alongside elevated thyroxine (T4) and cortisol levels compared to healthy animals (23). Interestingly, Ozkan et al. (24) reported an unusual case of Babesia bigemina infection in eight dogs—a pathogen typically associated with cattle.
Etoposide (EP), a topoisomerase II (Top2) inhibitor that induces DNA damage, is clinically employed against small cell lung cancer (25). EP exerts its antitumor effects by preventing DNA re-ligation, thereby inducing critical errors during DNA synthesis in the premitotic phase and promoting cancer cell apoptosis. Its activity is cell cycle-dependent, with predominant effects during the S and G2 phases, mediated specifically through the inhibition of the topoisomerase II alpha isoform (26). However, the clinical utility of etoposide is constrained by its low aqueous solubility, short half-life, and nonspecific toxicities, which collectively limit its bioavailability and therapeutic efficacy. Notably, within the etoposide and cisplatin (EP) regimen, a dose of 500 mg/m2 represents a critical threshold beyond which adverse effects—such as alopecia, gastrointestinal toxicity, and acute hypersensitivity reactions—become frequent. For in vivo contexts, a previous study reported a murine intraperitoneal LD50 of 64 mg/kg (27). The concentration of 500 μM etoposide used in our experiments translates to approximately 125–150 mg/kg in vivo, which is considered appropriate for evaluating cytotoxicity in the present study. The limited antiparasitic efficacy of etoposide phosphate (EP) observed in the B. microti mouse model, characterized by a non-significant reduction in parasitemia compared to control groups, may be attributed to several factors. Firstly, the dosage used in this study may have been insufficient, as the maximum tolerated dose (MTD) for EP in Balb/C mice was previously established at 75 mg/kg, a regimen associated with distinct weight loss profiles (28). Consequently, higher or optimized dosing regimens might yield stronger antbabesial effects in future investigations. Furthermore, the pharmacokinetic profile of EP likely influenced the weak in vivo outcome. Etoposide is known to exhibit low aqueous solubility, a short half-life, and nonspecific toxicities, which collectively limit its bioavailability and efficacy. This is supported by findings from Garg et al. (29), who reported altered etoposide pharmacokinetics in malnourished animals, including a ~ 60% increase in the area under the concentration-time curve (AUC) and decreases in clearance, volume of distribution, and half-life of ~37%, 53%, and 24%, respectively. Additionally, the systemic exposure of etoposide, which is partly metabolized by cytochrome P450 enzyme 3A4 (CYP3A4), can be increased by concomitant administration of CYP3A4 inhibitors such as aprepitant (30).
Protozoan topoisomerases exhibit structural distinctions from mammalian isoforms, presenting potential antiparasitic targets (6). While cellular mechanisms resolving EP-induced Top2-DNA covalent complexes remain incompletely characterized, Plasmodium falciparum studies demonstrate EP binding at PfTopoII’s Tyr-829 active site—shared by edotecarin, namitecan, AR-67, and belotecan—inhibiting DNA relaxation by stabilizing the cleavage complex and preventing religation (25, 31). Blood smear analyses suggest EP similarly inhibits religation in B. bovis, B. caballi, and T. equi, inducing irreversible DNA double-strand breaks that trigger apoptosis—consistent with EP’s antitumor mechanism where transient damage becomes permanent (31, 32). Teniposide (VM-26), an EP derivative with analogous activity FDA-approved 32 years ago, remains utilized in pediatric acute lymphoblastic leukemia and gliomas (25).
DNA replication machinery represents a validated target for apicomplexan parasites given its essential role in proliferation (33). Topoisomerase inhibitors are clinically deployed against bacterial infections and malignancies (34), with emerging evidence supporting their utility against Babesia/Theileria species. Ciprofloxacin derivatives, nitidine chloride, and camptothecin exhibit efficacy against these parasites [Tayebwa et al., 2018; (47)], while Plasmodium topoisomerase homologs exist in Theileria annulata (34). Notably, apicoplast-localized DNA gyrase (absent in humans) is essential for plastid function; P. falciparum GyrA-knockout parasites (ΔPfGyrA) require isopentenyl pyrophosphate (IPP) supplementation and show reduced ciprofloxacin sensitivity but retain EP insensitivity (35). Current babesiosis therapies rely on atovaquone-azithromycin or clindamycin-quinine combinations (36). In this study, EP induced irreversible viability loss in B. bovis, B. caballi, and T. equi—contrasting with transient growth suppression by conventional agents. Species-dependent IC₅₀ variation (highest in B. bovis) may reflect differential Top2 expression or structure (3). Morphological alterations including intraerythrocytic spots in Babesia spp. and residual structures in T. equi align with DNA-damage phenotypes and resemble changes induced by mitochondrial disruptors, hemozoin inhibitors, or dual-mechanism agents like pyronaridine (Top2/hemozoin suppression) and isocoumarins (DNA gyrase/hemozoin inhibition) (37–39). EP (VP-16), as a topoisomerase II inhibitor, exhibits a distinct mechanism of action compared to current anti-Babesial agents such as diminazene (DA), atovaquone combinations (15, 40), or histone deacetylase inhibitors (e.g., panobinostat). While DA demonstrates direct inhibitory effects against B. bovis (0.16 ± 0.02 μg/mL), T. equi (0.28 ± 0.01 μg/mL), and B. caballi (0.012 ± 0.003 μg/mL) (40), EP’s efficacy against apicomplexan parasites remains quantitatively inferior and non-selective. In vitro studies indicate that EP’s half-maximal inhibitory concentration (IC₅₀) against Babesia spp. exceeds 10 μM (approximately 5.87 μg/mL), which is significantly higher than DA’s potency (0.012–0.28 μg/mL) (15). This contrasts sharply with panobinostat, which shows promising anti-B. duncani activity (EC₅₀ 0.0084 μM) and a wide therapeutic window (TC₅₀ 130 μM, SI > 15,000) (41). The primary limitation of EP lies in its mechanism: it stabilizes topoisomerase II-DNA cleavage complexes in rapidly dividing cells, but apicomplexan parasites possess divergent topoisomerase II isoforms with low structural homology to human enzymes.
In terms of safety, the calculated selectivity indices (SI) further support EP’s favorable toxicity profile in mammalian cells. Although EP demonstrated comparatively weaker antiparasitic potency than diminazene aceturate, its higher SI values (ranging from 44 to over 13,000, depending on the species tested) indicate low cytotoxicity to host cells. These results align with the known pharmacological behavior of etoposide, which primarily targets rapidly dividing cells with high Topoisomerase II activity while sparing quiescent cells. Such differential selectivity may allow EP to serve as a scaffold for structure-activity optimization toward parasite-selective analogues. Future work should evaluate EP derivatives or formulation approaches that enhance antiparasitic potency while preserving this advantageous safety profile.
Although T. annulata and B. bovis encode Topoisomerase IB (6), Top2 remains unconfirmed in Babesia/Theileria, necessitating mechanistic studies on EP-parasite interactions. EP’s irreversible effects prevent recrudescence post-withdrawal, suggesting utility against drug-resistant strains via combination therapies. While nitidine chloride outperformed diminazene aceturate against B. microti (47), EP’s lower efficacy here may reflect suboptimal dosing (1 mg/kg vs. 20 mg/kg in prior studies). Prodrug strategies to enhance bioavailability (42) could optimize natural inhibitors like quercetin, which targets protozoan topoisomerases (43). Intriguingly, despite limited parasitological efficacy against B. microti, EP significantly improved hematological parameters (RBC, HGB, HCT)—potentially via erythropoietic stimulation during myelosuppression (44) warranting further mechanistic investigation. EP’s differential activity against piroplasms (B. bovis, B. caballi, T. equi) versus B. microti may stem from variations in Top2cc repair pathways between protozoan species. Investigating EP’s interaction with parasite DNA repair mechanisms—particularly the presence or absence of homologous recombination components—could reveal novel therapeutic targets. Prodrug strategies (42) may enhance delivery of topoisomerase-targeting compounds like quercetin (43), potentially optimizing EP’s dual role: direct antiparasitic action against susceptible species and hematoprotection in babesiosis.
Although EP showed limited standalone efficacy in this study, its mechanism of targeting topoisomerase II presents potential applicability to other protozoan diseases. Its ability to disrupt the parasite life cycle without regrowth indicates promise in eliminating cryptic parasite stages, a critical factor in preventing chronic or latent infections. Exploring EP’s effectiveness in Plasmodium infections, where DNA topoisomerase is a validated target, might provide broader insights into its therapeutic potential across parasitic diseases. Consequently, EP lacks specificity for parasitic targets, leading to off-target toxicity in host cells. Additionally, EP’s immunosuppressive properties could exacerbate Babesiosis-related complications by impairing host defenses, whereas DA or atovaquone-based therapies directly target parasitic metabolic pathways (e.g., mitochondrial electron transport) with higher selectivity. Thus, EP may not a best candidate for Babesiosis treatment, and future efforts should focus on parasite-specific topoisomerase II inhibitors or combination therapies to enhance selectivity and overcome resistance. Therefore, developing inhibitors that specifically target the structural differences between host and parasite topoisomerase II could minimize off-target effects (45). Additionally, leveraging high-throughput screening and structure-based drug design may accelerate the discovery of novel compounds with improved efficacy and safety profiles. Combining such agents with existing antiparasitic drugs might also reduce the likelihood of resistance development, offering a more robust therapeutic strategy against Babesia infections.
The markedly different sensitivities we observed between species (e.g., relatively high IC₅₀ for B. bovis versus low IC₅₀ for B. caballi and modest in vivo activity against B. microti) likely reflect multiple, non–mutually exclusive biological and pharmacological factors (46). First, structural and sequence variation in parasite topoisomerase(s) could alter drug binding and stabilization of the TopoII–DNA cleavage complex; even single amino-acid differences at the drug-binding pocket can change affinity and cytotoxic outcome. Second, stage- and species-specific expression levels of TopoII (or alternative topoisomerase isoforms) will modify the fraction of parasites that are vulnerable during S/G₂-like replication phases—species with a higher proportion of actively replicating parasites in culture will appear more sensitive to a cell cycle–dependent poison such as etoposide. Third, differences in DNA damage response and repair capacity (for example, efficiency of homologous recombination, activity of tyrosyl-DNA phosphodiesterases, or other Top2cc resolution pathways) will determine whether TopoII-mediated double-strand breaks are converted into lethal lesions or efficiently repaired. Fourth, variable drug uptake, efflux or intracellular sequestration (including erythrocyte versus parasite membrane permeability, and presence of parasite transporters) and species-specific metabolism could alter intracellular etoposide concentrations. Finally, the in vivo pharmacokinetic context (distribution, protein binding, metabolism by host CYPs, and intrared blood cell bioavailability) differs from in vitro exposure and can reduce effective concentration at the parasite. Collectively, these mechanisms provide plausible explanations for why etoposide produced irreversible in vitro effects in some piroplasms yet only modest in vivo efficacy against B. microti. Future mechanistic studies—including comparative TopoII sequencing and structural modelling, recombinant enzyme inhibition assays, expression profiling of topoisomerases and DNA-repair genes, and measurements of intra-erythrocytic drug accumulation-will be required to pinpoint the dominant determinants of species-specific sensitivity and to inform rational design of parasite-selective TopoII inhibitors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Obihiro University of Agriculture and Veterinary Medicine. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YL: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ZC: Data curation, Investigation, Writing – review & editing. JL: Data curation, Validation, Writing – review & editing. YZ: Validation, Writing – review & editing. IZ: Writing – review & editing. MR: Writing – review & editing. HL: Resources, Writing – review & editing. SE-S: Writing – review & editing. NY: Resources, Writing – review & editing. QG: Funding acquisition, Project administration, Resources, Writing – review & editing. XX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. BC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the following projects Special Projects of the Autonomous Region Natural Science Foundation Youth Science Fund (No. 2022D01B83), Central Government in Guidance of Local Science and Technology Development (No. ZYYD2023C03–2), Innovative research team on biological vectors and transmission of vector-borne zoonotic diseases in the Xinjiang Uygur Autonomous Region (No. 2023TSYCTD0008), Autonomous Region Major Science and Technology Special Project (No. 2023A02007-2), Postdoctoral program of Xinjiang Agricultural University, Xinjiang Uygur Autonomous Region, the National Natural Science Foundation of China (Grant No. 12561093), the AMED project (Grant No. JP23WM02250317) and the Strategic International Collaborative Research Project (JP008837) promoted by the Ministry of Agriculture, Forestry, and Fisheries of Japan, Local Government-Sponsored Overseas Study Program of Xinjiang Uyghur Autonomous Region, China. This work was partly supported by Local Government-Sponsored Overseas Study Program of Xinjiang Uyghur Autonomous Region, China. This work was partly supported by Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University. The research resources (facility) and technical advices were kindly provided by the Joint Usage/Research Program of Cancer Research Institute, Kanazawa University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1689833/full#supplementary-material
References
1. Renard, I, and Ben Mamoun, C. Treatment of human Babesiosis: then and now. Pathogens. (2021) 10:1120. doi: 10.3390/pathogens10091120
2. Li, Y, Rizk, MA, Galon, EM, Liu, M, Li, J, Ringo, AE, et al. Discovering the potent inhibitors against Babesia bovis in vitro and Babesia microti in vivo by repurposing the natural product compounds. Front Vet Sci. (2021) 8:762107. doi: 10.3389/fvets.2021.762107
3. Chen, Z, Vallega, KA, Wang, D, Quan, Z, Fan, S, Wang, Q, et al. DNA topoisomerase II inhibition potentiates osimertinib's therapeutic efficacy in EGFR-mutant non-small cell lung cancer models. J Clin Invest. (2024) 134:e172716. doi: 10.1172/JCI172716
4. Ma, Y, Jian, Y, Wang, G, Li, X, Wang, G, Hu, Y, et al. Molecular identification of Babesia and Theileria infections in livestock in the Qinghai-Tibetan plateau area, China. Animals. (2024) 14:476. doi: 10.3390/ani14030476
5. Lopes, SB, Aleixo, RV, Silva, JM, Cruz, G, Ferreira, E, and Rabadão, E. Recurrent mucosal leishmaniasis of the epiglottis in an immunosuppressed patient. ID Cases. (2023) 33:e01860. doi: 10.1016/j.idcr.2023.e01860
6. Lamba, S, and Roy, A. DNA topoisomerases in the unicellular protozoan parasites: unwinding the mystery. Biochem Pharmacol. (2022) 203:115158. doi: 10.1016/j.bcp.2022.115158
7. Singh, P, Tabassum, W, Fangaria, N, Dey, S, Padhi, S, Bhattacharyya, MK, et al. Plasmodium topoisomerase VIB and Spo11 constitute functional type IIB topoisomerase in malaria parasite: its possible role in mitochondrial DNA segregation. Microbiol Spectr. (2023) 11:e0498022. doi: 10.1128/spectrum.04980-22
8. Mianda, SM, Invernizzi, L, van der Watt, ME, Reader, J, Moyo, P, Birkholtz, LM, et al. In vitro dual activity of Aloe marlothii roots and its chemical constituents against plasmodium falciparum asexual and sexual stage parasites. J Ethnopharmacol. (2022) 297:115551. doi: 10.1016/j.jep.2022.115551
9. Le, TT, Wu, M, Lee, JH, Bhatt, N, Inman, JT, Berger, JM, et al. Etoposide promotes DNA loop trapping and barrier formation by topoisomerase II. Nat Chem Biol. (2023) 19:641–50. doi: 10.1038/s41589-022-01235-9
10. Krstulović, L, Rastija, V, Pessanha de Carvalho, L, Held, J, Rajić, Z, Živković, Z, et al. Design, synthesis, antitumor, and antiplasmodial evaluation of new 7-Chloroquinoline-Benzimidazole hybrids. Molecules. (2024) 29:2997. doi: 10.3390/molecules29132997
11. Vitré, C, Le Gal, Y, Vacher, A, Roisnel, T, Lorcy, D, Santana, S, et al. Structure-activity relationship of anticancer and antiplasmodial gold bis(dithiolene) complexes. Dalton Trans. (2024) 53:11903–13. doi: 10.1039/d4dt01458h
12. Vargas Rigo, G, Gomes Cardoso, F, Bongiorni Galego, G, da Rosa, DF, Dos Santos, ALS, and Tasca, T. Metallopeptidases as key virulence attributes of clinically relevant protozoa: new discoveries, perspectives, and frontiers of knowledge. Curr Protein Pept Sci. (2023) 24:307–28. doi: 10.2174/1389203724666230306153001
13. Yin, F, Mu, D, Tian, Z, Li, D, Ma, X, Wang, J, et al. Molecular detection of Babesia gibsoni in cats in China. Animals. (2022) 12:3066. doi: 10.3390/ani12223066
14. Li, F, Zhao, P, Wang, S, Luo, W, Xia, Y, Li, D, et al. Babesia duncani pyruvate kinase inhibitor screening and identification of key active amino acid residues. Microorganisms. (2024) 12:1141. doi: 10.3390/microorganisms12061141
15. Rizk, MA, El-Sayed, SAE, Sayed-Ahmed, MZ, Almoshari, Y, Alqahtani, SS, Ahmad, S, et al. Evaluation of the inhibitory effect of Moringa oleifera leaves Methanolic extract against in vitro growth of several Babesia species and Theileria equi and the in vivo growth of Babesia microti. J Trop Med. (2023) 2023:4285042. doi: 10.1155/2023/4285042
16. Nawab, Y, Ijaz, M, Ayyub, RM, Ahmed, A, Muzammil, I, Ghumman, NZ, et al. Molecular detection of Theileria annulata infection: an emerging disease of pet dogs in Pakistan. Kafkas Univ Vet Fak Derg. (2023) 29:41–8. doi: 10.9775/kvfd.2022.28455
17. Babiker, EM, Elshafie, EI, Salih, DA, Saaid, AA, and Elmalik, KH. Diverse techniques in detecting the tropical theileriosis among cattle in Blue Nile state, Sudan. Int J Vet Sci. (2024) 13:127–31. doi: 10.47278/journal.ijvs/2023.078
18. Rafique, MN, Akram, W, Aslam, MA, Ather, AB, Rehman, M, Zahid, A, et al. Uncovering strategies for the detection of Babesia species. Trends Anim Plant Sci. (2024) 4:1–7. doi: 10.62324/TAPS/2024.041
19. Kebzai, F, Ashraf, K, Rehman, MU, Akbar, H, and Avais, M. Molecular analysis and associated risk factors of Theileria annulata in cattle from various zones of Balochistan, Pakistan. Kafkas Univ Vet Fak Derg. (2024) 30:15–21. doi: 10.9775/kvfd.2023.30151
20. Aslam, F, Rehman, M, Saleem, G, Ashraf, K, Hafeez, MA, and Saqib, M. Identification and molecular characterization of Theileria annulata with associated risk factors in naturally infected camels from selected districts in Punjab, Pakistan. Pak Vet J. (2023) 1:4. doi: 10.29261/pakvetj/2022.084
21. Atif, FA, Nazir, MU, Roheen, T, Mehnaz, S, and Hussain, I. Antitheilerial efficacy of juglone, buparvaquone and oxytetracycline against tropical theileriosis in naturally infected crossbred cattle. Pak Vet J. (2024) 44:129–34. doi: 10.29261/pakvetj/2023.116
22. Milošević, S, Bozovic, AI, Magas, V, and Sukara, R. First clinical evidence with one-year monitoring of Babesia gibsoni monoinfection in two dogs from Serbia. Pak Vet J. (2024) 44:1315–21. doi: 10.29261/pakvetj/2024.286
23. Thabet, NF, Kandil, OM, and Fararh, KM. Impact of Theileria equi infection on Arabian stallion fertility: serological and hormonal perspectives. Int J Vet Sci. (2024) 13:1002–8. doi: 10.47278/journal.ijvs/2024.196
24. Ozkan, O, Banucicek,, Yucesan, B, Yilmaz, Y, and Ocal, Z. Detection of Babesia bigemina cases in dogs in rural areas/Türkiye by molecular methods. Kafkas Univ Vet Fak Derg. (2024) 30:41–5. doi: 10.9775/kvfd.2023.30356
25. Buzun, K, Bielawska, A, Bielawski, K, and Gornowicz, A. DNA topoisomerases as molecular targets for anticancer drugs. J Enzyme Inhib Med Chem. (2020) 35:1781–99. doi: 10.1080/14756366.2020.1821676
26. Chen, M, Suzuki, A, Thakkar, S, Yu, K, Hu, C, and Tong, W. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today. (2016) 21:648–53. doi: 10.1016/j.drudis.2016.02.015
27. Herrmann, SM, Abudayyeh, A, Gupta, S, Gudsoorkar, P, Klomjit, N, Motwani, SS, et al. Diagnosis and management of immune checkpoint inhibitor-associated nephrotoxicity: a position statement from the American Society of Onco-nephrology. Kidney Int. (2025) 107:21–32. doi: 10.1016/j.kint.2024.09.017
28. Aston, WJ, Hope, DE, Nowak, AK, Robinson, BW, Lake, RA, and Lesterhuis, WJ. A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice. BMC Cancer. (2017) 17:684. doi: 10.1186/s12885-017-3677-7
29. Garg, M, Gandhi, K, Jadhav, SM, Gurjar, M, and Gota, V. Effect of moderate malnutrition on the pharmacokinetics of etoposide and vincristine in freshly weaned rats. Eur J Drug Metab Pharmacokinet. (2023) 48:657–63. doi: 10.1007/s13318-023-00851-7
30. Strik, J, de Jong, LAW, Sijm, J, Desar, IME, and van Erp, NP. Effect of Aprepitant on etoposide pharmacokinetics in patients with testicular Cancer: a pharmacokinetic study to determine the absence of a clinically relevant interaction. Clin Pharmacol Ther. (2024) 115:135–8. doi: 10.1002/cpt.3081
31. Dar, A, Godara, P, Prusty, D, and Bashir, M. Plasmodium falciparum topoisomerases: emerging targets for anti-malarial therapy. Eur J Med Chem. (2024) 265:116056. doi: 10.1016/j.ejmech.2023.116056
32. Kuruppu, AI, Paranagama, P, and Goonasekara, CL. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm J. (2019) 27:565–73. doi: 10.1016/j.jsps.2019.02.004
33. Musabyimana, JP, Musa, S, Manti, J, Distler, U, Tenzer, S, Ngwa, CJ, et al. The plasmodium falciparum histone methyltransferase SET10 participates in a chromatin modulation network crucial for intraerythrocytic development. mSphere. (2024) 9:e0049524. doi: 10.1128/msphere.00495-24
34. Singh, P, Rani, K, Gotmare, A, and Bhattacharyya, S. A tale of topoisomerases and the knotty genetic material in the backdrop of plasmodium biology. Biosci Rep. (2022) 42:BSR20212847. doi: 10.1042/BSR20212847
35. Tan, S, Mudeppa, DG, Kokkonda, S, White, J, Patrapuvich, R, and Rathod, PK. Properties of plasmodium falciparum with a deleted apicoplast DNA gyrase. Antimicrob Agents Chemother. (2021) 65:e0058621. doi: 10.1128/AAC.00586-21
36. Vannier, E, Hunfeld, KP, Smith, RP, and Krause, PJ. Management of human babesiosis -approaches and perspectives. Expert Rev Anti-Infect Ther. (2025) 23:739–52. doi: 10.1080/14787210.2025.2526843
37. Bailly, C. Pyronaridine: an update of its pharmacological activities and mechanisms of action. Biopolymers. (2021) 112:e23398. doi: 10.1002/bip.23398
38. Coronado, L, Zhang, XQ, Dorta, D, Escala, N, Pineda, LM, Ng, MG, et al. Semisynthesis, Antiplasmodial activity, and mechanism of action studies of Isocoumarin derivatives. J Nat Prod. (2021) 84:1434–41. doi: 10.1021/acs.jnatprod.0c01032
39. Soliman, TN, Keifenheim, D, Parker, PJ, and Clarke, DJ. Cell cycle responses to topoisomerase II inhibition: molecular mechanisms and clinical implications. J Cell Biol. (2023) 222:e202209125. doi: 10.1083/jcb.202209125
40. Scarpa, AB, Salvador, VF, Leal, LLLL, de Morais, IML, Couto, LFM, Heller, LM, et al. Evaluation of different supportive treatments during packed cell volume replacement in calves naturally infected with tick fever agents. Vet Res Commun. (2024) 48:2441–55. doi: 10.1007/s11259-024-10423-y
41. Asquith, M, Prior, S, and Brüning-Richardson, A. Human babesiosis: the past, present and future. Expert Rev Mol Med. (2025) 27:e30. doi: 10.1017/erm.2025.10016
42. Sundararaman, SA, Miller, JJ, Daley, EC, O'Brien, KA, Kasak, P, Daniels, AM, et al. Prodrug activation in malaria parasites mediated by an imported erythrocyte esterase, acylpeptide hydrolase (APEH). Proc Natl Acad Sci USA. (2025) 122:e2417682122. doi: 10.1073/pnas.2417682122
43. Memariani, H, Memariani, M, and Ghasemian, A. Quercetin as a promising Antiprotozoan phytochemical: current knowledge and future research avenues. Biomed Res Int. (2024) 2024:1–37. doi: 10.1155/2024/7632408
44. Liu, WM, Bamford, C, Slevin, M, and Joel, SP. Effects of haemopoietic growth factors in combination with etoposide on sister chromatid exchange frequencies in peripheral blood mononuclear cells. Cancer Chemother Pharmacol. (1998) 41:343–6. doi: 10.1007/s002800050749
45. Dews, EA, Teixeira, JE, Huston, CD, Meyers, MJ, and Hyson, PR. A propidium iodide-based in vitro screen of the "bug box" against Babesia duncani reveals potent inhibitors. Antimicrob Agents Chemother. (2025) 69:e0003525. doi: 10.1128/aac.00035-25
46. Kozhabaev, M, Kuzerbaeva, A, Baizhanov, K, Tulemetova, S, and Nukhodzhaev, N. Study of the dynamics of distribution, seasonality, and degree of infection with bovine theileriosis in the territory of Turkestan region. Int J Vet Sci. (2023) 12:382–8. doi: 10.47278/journal.ijvs/2022.203
Keywords: bovine babesiosis, equine piroplasmosis, etoposide, in vitro, in vivo, inhibitory effects
Citation: Li Y, Cui Z, Li J, Zhang Y, Zafar I, Rizk MA, Li H, El-Sayed SAE-S, Yokoyama N, Guo Q, Xuan X and Chahan B (2025) Leveraging topoisomerase II-mediated DNA damage: repurposing etoposide as a lead compound for apicomplexan parasite control. Front. Vet. Sci. 12:1689833. doi: 10.3389/fvets.2025.1689833
Edited by:
Vikrant Sudan, Guru Angad Dev Veterinary and Animal Sciences University, IndiaReviewed by:
Qingxia Wu, Tibet Agricultural and Animal Husbandry University, ChinaKhalid Mehmood, Islamia University of Bahawalpur, Pakistan
Copyright © 2025 Li, Cui, Li, Zhang, Zafar, Rizk, Li, El-Sayed, Yokoyama, Guo, Xuan and Chahan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongchang Li, bHljXzg3NjIwMTdAMTYzLmNvbQ==; Bayin Chahan, MjUxNDA2Mjg4MUBxcS5jb20=; Xuenan Xuan, Z2dlbkBnLmVjYy51LXRva3lvLmFjLmpw
†These authors have contributed equally to this work and share first authorship
 Yongchang Li
Yongchang Li Zeyun Cui1†
Zeyun Cui1† Iqra Zafar
Iqra Zafar Mohamed Abdo Rizk
Mohamed Abdo Rizk Hang Li
Hang Li Shimaa Abd El-Salam El-Sayed
Shimaa Abd El-Salam El-Sayed