1
Department of Experimental Neurophysiology, Medical Faculty, Ruhr University Bochum, Bochum, Germany
2
International Graduate School of Neuroscience, Ruhr University Bochum, Bochum, Germany
In the hippocampus in vivo, both synaptic plasticity and network activity are closely interdependent. We have found that immediately after an attempt to induce long-term potentiation (LTP), changes in theta (5–10 Hz) and gamma (30–100 Hz) activity correlate tightly with the occurrence of LTP, suggesting that tetanisation-driven activation of sensory inputs synchronises the activity of granule cells and interneurons, and thus, facilitates the encoding of acquired stimuli. This results in increase of theta and gamma power, and elevates the probability that afferent stimuli both coincide with the peak of theta cycle and reach their post-synaptic target within the gamma time-window (of 10–30 ms). Both these mechanisms can effectively shift the direction, of tetanisation-induced changes in synaptic weight, towards potentiation and induction of LTP. Here, we discuss our findings in the context of possible mechanisms that link theta and gamma oscillations with LTP induction, as well as their role in information processing and formation of memories.
The ability to analyse and learn new information and, if necessary, modify ongoing behaviour, based on a combination of previous and recent memories, is an important factor for the survival of organisms in an ever-changing world. Creating and managing these memory traces, comprise core functions of the central nervous system, which rely on its ability to engage in synaptic plasticity. They can be monitored at different levels, ranging from the subcellular to the behavioural.
Since its discovery in the late 1960s (Andersen and Lømo, 1967
; Bliss and Lømo, 1973
; see for review: Lømo, 2003
), long-term potentiation (LTP) has been the subject of intense study, using a plethora of induction protocols, species, and various preparations both in vivo and in vitro. Nowadays, LTP, along with long-term depression (LTD), is widely used as a model of synaptic information storage and are believed to represent the processes of learning and memory in neuronal networks (Abraham, 2003
; Bear, 1996
; Bliss and Collingridge, 1993
; Kemp and Manahan-Vaughan, 2007
; Lynch, 2004
; Whitlock et al., 2006
).
Synaptic plasticity in the form of LTP, typically results in both functional and structural reorganisation of the synapse, that evolves over time and comprises different phases, namely LTP-induction (post-tetanic potentiation), short-term potentiation, LTP expression (early LTP) and maintenance (late LTP) (Abraham, 2003
; Bliss and Collingridge, 1993
; Frey et al., 1993
; Huang et al., 1996
; Nguyen et al., 1994
). Furthermore, each of these phases is characterised by the different involvement of neurotransmitter systems acting via both ionotropic and metabotropic receptors. For the induction phase, several lines of evidence underlie the important role of the activation of N-methyl-D-aspartate (NMDA) receptors, at least for the hippocampal CA1 region and dentate gyrus (Abraham and Mason, 1988
; Collingridge et al., 1983
; Errington et al., 1987
; Fox et al., 2006
; Frey et al., 1996
; Manahan-Vaughan, 1997
; Niewoehner et al., 2007
). For early LTP, activation of the metabotropic glutamate receptors (mGluRs), particularly group I mGluRs, plays a critical role (Aiba et al., 1994
; Anwyl, 1999
; Manahan-Vaughan, 1997
; Manahan-Vaughan and Braunewell, 2005
; Manahan-Vaughan and Reymann, 1996
; Manahan-Vaughan et al., 1998
; Richter-Levin et al., 1994
). Late-LTP (maintenance phase) requires de novo protein synthesis (Frey et al., 1996
; Krug et al., 1984
; Otani and Abraham, 1989
) and activation of several immediate-early genes and late-response genes (Abraham et al., 1991
; Brakeman et al., 1997
; Cole et al., 1989
; Link et al., 1995
; Messaoudi et al., 2007
; Wisden et al., 1990
; Yin et al., 2002
). Additionally, various neuromodulators can either increase or decrease the probability, magnitude and/or longevity of changes in synaptic transmission, hence affecting the outcomes of patterned synaptic activation (Granado et al., 2008
; Kemp and Manahan-Vaughan, 2005
, 2008
; Kulla and Manahan-Vaughan, 2002
; Lemon and Manahan-Vaughan, 2006
; Li et al., 2003
, 2007
; Manahan-Vaughan and Kulla, 2003
; Swanson-Park et al., 1999
).
Besides the different relative involvement of neurotransmitter systems, the induction and/or expression of LTP, as a reflection of central synapses to acquire, process and subsequently store new information, depends upon several other factors, including environmental enrichment, various forms of stress, etc. (Artola et al., 2006
; Avital et al., 2006
; Bruel-Jungerman et al., 2005
; Duffy et al., 2001
; Foster et al., 2000
; Kopp et al., 2006
; Seidenbecher et al., 1997
; van Praag et al., 1999
; Xu et al., 1998
), which couple the sensory input to a current behavioural state and provide the contextual frame for processing of sensory stimuli. Thus, taking into account the plethora of mechanisms underlying the phenomenon of LTP, it is not surprising that various factors and/or experimental manipulations can markedly affect its different phases. However, for most forms of LTP, and particularly for electrically induced LTP, the state of the animal (synaptic and behavioural) during a short period of time, both during and immediately after tetanisation, appears decisive in determining the persistence of potentiation (Abraham, 2003
).
In our study (Bikbaev and Manahan-Vaughan, 2007
), we applied high-frequency tetanisation (HFT) in freely behaving adult rats and monitored field excitatory synaptic potentials (fEPSPs) in parallel with the intrahippocampal electroencephalogram over a period of 24 h. Despite the fact that an identical stimulation protocol was employed for all animals, substantial variation in the outcomes of tetanisation was found. In the first group of animals, a robust LTP occurred that was stable for at least 24 h (LTP group), in the second group short-term potentiation that endured for up to 3 h was induced (STP group), whereas in the third group a failure of potentiation occurred, i.e. no persistent increase in synaptic transmission was seen (failure group). Given this range of tetanisation results, we analysed them in the context of the changes in network activity that occurred during and within first 300 s after tetanisation.
Oscillatory activity is an integral part of functional neuronal networks, and significant variability in patterns of oscillatory activity can be registered under certain conditions both in vivo and in vitro. Modification of synaptic weights occurs very fast during the activated state of the hippocampus (Buzsáki, 1996
), with entorhinal–hippocampal network oscillations at theta frequency playing a crucial role in this process (Kamondi et al., 1998
). Particularly in the hippocampus, theta oscillations (theta rhythm, rhythmic slow activity) have been proposed to play a role in a wide variety of hippocampal functions (see for review: Buzsáki, 2005
). In rodents, periods of prominent theta oscillations occur during exploratory behaviour and phases of rapid eye movement REM (sleep); conditions which are generally termed theta-associated behaviour (Bland, 1986
; Buzsáki, 2002
, 2005
; Vanderwolf, 1969
). These behavioural states, and particularly exploratory behaviour, are characterised by neocortico-hippocampal transfer of new spatial information when acquisition and/or encoding of sensory information should be facilitated, whereas transfer of the information from the hippocampus to neocortex is associated with other patterns, such as sharp waves and ripples (Bragin et al., 1995
; Buzsáki, 1996
; Buzsáki et al., 2002
; Chrobak et al., 2000
). Gamma oscillatory activity represents another pattern of network activity, which is characteristic for cortical locations, and is proposed to be involved in various cognitive functions (Buzsáki and Chrobak, 1995
; Fell et al., 2001
; Fries et al., 2001
; Gray and Singer, 1989
; Hermann et al., 2004
; Hopfield, 1995
; Lisman, 1999
; Lisman and Idiart, 1995
; Montgomery and Buzsáki, 2007
; Singer, 1993
). In the hippocampus of freely moving animals, the amplitude of gamma oscillations is higher in the dentate gyrus than in other hippocampal regions expressing gamma oscillations (Bragin et al., 1995
), and varies as a function of the theta cycle (Csicsvari et al., 2003
; Penttonen et al., 1998
). Hence, hippocampal theta and gamma oscillations comprise functionally-associated patterns of network activity that emerge as a consequence of the combination of intrinsic oscillatory properties of principal cells and interneurons, the rhythmic activation of which is driven by intra- and extrahippocampal connections (Bartos et al., 2007
; Bragin et al., 1995
; Csicsvari et al., 2003
; Klausberger et al., 2003
; Penttonen et al., 1998
; Ylinen et al., 1995
).
The results of our study demonstrated that the successful potentiation of synaptic transmission (LTP and STP groups) in freely moving rats, was preceded by a higher relative theta power in the period of 300 s after HFT, when compared with the pre-tetanisation period (Figure 1
). In contrast, a gradual recovery of theta power to pre-tetanisation values, but not an increase, was found in the LTP-failure group. In other words, successful induction of potentiation (either in the form of STP or LTP), resulted in an enhancement of theta oscillatory activity after tetanisation, whereas no such enhancement occurred in rats that showed failure of LTP. The mean amplitude of a single theta cycle was also significantly higher within the post-tetanisation period in the LTP and STP groups, when compared to the group of failed synaptic potentiation. Taken together, these data suggest that a transient increase of theta power is necessary for the proper handling of sensory input activation (mimicked here by HFT). This, in turn, may play a permissive role in LTP induction, and, in general, in the formation of memory trace(s) of newly acquired information.
Figure 1. Transient enhancement of theta and gamma power in the post-tetanisation period correlates with potentiation of synaptic transmission in the dentate gyrus of freely moving rats. (A) Examples of EEG epochs, which comprise 100 s long periods of tetanisation (200 Hz, 10 trains: first train is marked by arrow) and following 300 s, recorded in rats that showed either LTP (left panel), STP (middle panel) or failure of potentiation (right panel), respectively. Asterisk denotes the period immediately after HFT, when the amplitude of network oscillations is higher in LTP and STP cases, in comparison with failed potentiation. Scale bar: 20 s. (B) Successful potentiation (LTP and STP) of synaptic transmission is associated with a prominent increase of the relative theta and gamma power particularly in the period encompassing 100 s after tetanisation. The results were pooled for LTP, STP and failure groups and presented as Mean ± S.E.M. (modified from Bikbaev and Manahan-Vaughan, 2007
).
However, the differences between LTP, STP and failed potentiation groups were not limited in our study by theta oscillations. We found that within the first 100 s after HFT the increase in theta power, and the mean amplitude of a single theta cycle, was accompanied by higher gamma power in the LTP group, and higher amplitude of gamma oscillations within a single theta cycle in both the LTP and STP groups, when compared with cases of failed potentiation (Figure 1
B). This indicates that not the increase of theta power per se, but a correlated increase of both theta and gamma power is important for enabling or mediating the plastic changes in synaptic weights. Furthermore, our results show that these enhancements of oscillatory activity should take place within a relatively short period of time for synaptic plasticity to occur. Taking into account that the amplitude of gamma oscillations in the dentate gyrus, but not in the CA3 or CA1 regions, is significantly higher in the presence of theta oscillations than in non-theta states (Csicsvari et al., 2003
), these findings suggest that the tetanisation-induced facilitation of theta activity can play a causal role for the enhancement of gamma oscillations.
In the dentate gyrus of the intact brain, the power of both theta and gamma activity is driven by and strongly depends upon entorhinal input, and is higher during exploratory behaviour (Bragin et al., 1995
; Csicsvari et al., 2003
). Furthermore, behavioural data indicating enhanced eyeblink conditioning in rabbits that received pairings of stimuli during epochs of prominent theta activity, when compared to those stimulated during non-theta periods (Seager et al., 2002
), provide additional support for a facilitatory role of theta oscillations in learning. However, one should emphasise that the increase of theta power occurred in our study in the period after tetanisation. Thus, the differences in theta power that we observed, did not comprise an endogenous pre-condition for synaptic plasticity, but rather occurred as a consequence of perforant path stimulation. Accordingly, if natural theta rhythm is necessary for the acquisition or processing of sensory stimuli, one can presume that the artificial activation of sensory inputs (in the form of HFT) would require and/or induce an enhancement of oscillatory activity in the theta frequency range, in order to temporally organise neuronal activity and favour synaptic plasticity. The depolarisation of dendritic compartments of dentate gyrus granule cells via activation of NMDA receptors and mGluRs, after strong activation of the glutamatergic perforant path, is likely to contribute to such an enhancement. Perforant path stimulation of the dentate gyrus, in conjunction with subsequent firing of mossy fiber collaterals, will also activate parvalbumin-expressing basket cells (Kneisler and Dingledine, 1995
), which via ionotropic γ-aminobutyric acid (GABAA) receptors provide rhythmic inhibition of somata and perisomatic region of principal cells and play a pivotal role in the generation of both theta (Buzsáki, 2002
; Klausberger et al., 2003
; Sik et al., 1997
; Ylinen et al., 1995
) and gamma (Bartos et al., 2001
, 2007
; Penttonen et al., 1998
; Vida et al., 2006
) oscillations. Additionally, activation of GABAA receptors has been reported to be critical also for the generation of transient tetanically induced gamma oscillations in vitro (Traub et al., 2004
; Whittington et al., 1997
). Hence, the combination of NMDAR-induced dendritic excitation and GABAAR-mediated somatic inhibition results in current flow through distal dendrites, which is important for the generation and maintenance of extracellular theta currents (Buzsáki, 2002
). This could also effectively trigger and/or enhance theta oscillations in post-HFT period. In turn, theta oscillations may dynamically modulate the probability of NMDAR activation, which is highest on the peak of the theta cycle and the lowest on the trough (Vertes, 2005
). The increase of gamma power in the post-tetanisation period may rely on the activation of group I mGluRs, which was reported to induce GABAAR-dependent gamma oscillations in vitro (Whittington et al., 1995
), and increase gamma power in vivo (Martin, 2001
). We have found recently that prolonged inhibition of mGluR5 results in a significant impairment of LTP associated with a marked suppression of gamma oscillations in the dentate gyrus of freely moving rats (Bikbaev et al., 2008
). Taken together, correlated activation of granule cells and interneurons, via fast and slow glutamatergic excitation, not only can contribute to long-term synaptic potentiation, but, complemented with fast rhythmic GABAergic inhibition, can affect network oscillations on both short- and long-term time-scales (Figure 2
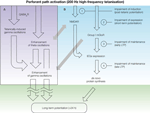
). In this respect, pharmacological modulation of selected neurotransmitter systems, including the glutamatergic, GABAergic and cholinergic systems, coupled with correlation analysis of network activity and long-term changes in synaptic transmission, would be necessary for a more conclusive support of this suggestion. For instance, such analysis after activation of the cholinergic system, which can facilitate oscillatory activity in the theta frequency band, and simultaneous inhibition of GABAAR- and/or mGluR-mediated signalling, which affects hippocampal gamma activity, could help to dissect the roles of theta and gamma oscillations in the shaping of synaptic plasticity.
Figure 2. A schematic representation of the consequences of high-frequency tetanisation that occur on the network and cellular levels, and lead towards long-term potentiation of synaptic transmission. (A) Strong afferent stimulation results in an enhancement of both theta and gamma oscillations, which occurs within 5 min interval after tetanisation and can be mediated via GABARs, NMDARs and mGluRs. (B) Tetanisation-triggered activation of both ionotropic and metabotropic glutamate receptors is followed by the expression of plasticity-related immediate-early genes (IEGs) and protein synthesis, which can underlie structural synaptic reorganisation and long-term increase in synaptic efficacy. Experimental procedures that inhibit the induction, expression or maintenance of LTP can result in impairment of post-tetanic potentiation, STP, early LTP or late LTP (1–4, respectively). Grey arrows denote possible links between NMDAR and mGluR activation and facilitation of network activity in theta and gamma frequency bands
.
The apparent relationship between theta activity and certain types of behaviour, and the relative regularity and stability of this oscillatory pattern suggests that theta rhythm may serve as an internal timing mechanism for individual elements of spatially distributed neuronal ensembles (Buzsáki, 2005
). In other words, enhanced theta oscillations may provide an appropriate time-window for the firing of individual neurons within currently activated ensemble(s) and, therefore, optimise the encoding of the acquired signal. Indeed, the firing rate of both principal cells and several classes of interneurons in the hippocampus has been found to be theta phase-locked, i.e. to depend upon the phase of the ongoing theta cycle (Csicsvari et al., 1999
; Klausberger et al., 2003
; O’Keefe and Recce, 1993
). An important link between hippocampal theta oscillations and the probability of potentiation of synaptic transmission is provided by observations with regard to increases or decreases of synaptic efficacy, which were caused by trains of high-frequency stimulation delivered either at the peak or the trough of theta cycle, and which resulted in LTP or LTD respectively (Huerta and Lisman, 1993
, 1995
; McCartney et al., 2004
; Pavlides et al., 1988
). During naturally occurring, locomotion-induced theta oscillations, LTP, that persists for at least 48 h, is preferentially induced by stimuli delivered near the local theta peak in behaving animals (Hyman et al., 2003
; Orr et al., 2001
), whereas in anesthetised animals a long-lasting enhancement of fEPSP is elicited, if hippocampal afferents are stimulated synchronously on the peak of theta oscillations (Hölscher et al., 1997
; Pavlides et al., 1988
). This demonstrates that not only the parameters of the stimulation protocol, but also the timing of tetanisation relative to the phase of theta cycle, can change the direction of synaptic plasticity (LTD vs. LTP).
Thus, tetanisation-driven increases of theta and gamma oscillations, such as those observed in our study (Bikbaev and Manahan-Vaughan, 2007
), seem to be intrinsically interconnected, and play complementary roles in synaptic potentiation. The heightened theta power in the period after tetanisation is likely to temporally organise the firing of principal cells within theta time-windows, hence supporting the increase of the amplitude of gamma oscillations. Bearing in mind that the higher amplitude of gamma oscillations reflects a higher degree of synchronisation in the network (see for review: Axmacher et al., 2006
), the increase of gamma power in the period immediately after tetanisation may indicate that, during the facilitated theta activity, the firing of principal cells and interneurons is more synchronised (or precise) and/or recruits more elements into the network activity. Accordingly, enhanced gamma oscillations synchronise neuronal firing within narrower gamma time windows and elevate the probability for afferent stimuli to coincide with the peak of theta cycle, where the gamma amplitude is highest, hence resulting in the strengthening of the activated synaptic pathway. The outcomes of studies of spike-timing dependent plasticity (see for review: Dan and Poo, 2004
) demonstrate that such synchronisation of neuronal spiking, within a 10–30 ms window: which precisely matches to a single gamma cycle, can dramatically increase the probability of potentiation of activated synaptic connections. Moreover, in in vitro recordings from the primary visual cortex, where theta activity is generally not so prominent as in the hippocampus, pairing the stimuli with either the peak or trough of oscillations in the beta and gamma frequency ranges has been found to effectively drive synaptic plasticity towards potentiation or depression, respectively (Wespatat et al., 2004
).
In this context, one can conclude that the transient enhancement of theta and gamma oscillations in the post-tetanisation period reflects the engagement of the system in encoding of acquired information. This can result in a subsequent “reconfiguration” of the network, coupled with the strengthening of currently activated connections, and the weakening of the others. Furthermore, facilitated theta activity can support a tighter timing control of the firing of principal cells and interneurons in the network, which serves to optimise information processing. Tetanisation-triggered enhancement of gamma oscillations is associated, on the other hand, with higher synchronisation and reflects the coordinated ensemble activity aimed to encode/retrieve relevant memories. Accordingly, the absence or inconsistency of such changes in network activity can be related to the lack, or insufficiency, of activation of required neurotransmitter systems, sub-optimal conditions for the processing of sensory information and, therefore, a lower probability for LTP.
For several decades, theta rhythm was regarded in rodents as an electrographic hallmark of exploratory behaviour, that occurs when the hippocampus is engaged in the acquisition and processing of sensory stimuli, which can lead in turn to experience-based modification of ongoing behaviour. Gamma oscillations were also proposed to play a role in various cognitive processes both in human and animals. At the neuronal level, the acquisition and formation of memory traces are believed to rely on activity-related changes in synaptic efficacy, and LTP of synaptic transmission after patterned afferent stimulation is considered as an adequate experimental model of these processes. However, despite the existence of extensive experimental data showing the involvement of hippocampal theta and gamma oscillations in cognitive processes, a relationship between the coordinated enhancement of both theta and gamma power immediately after tetanisation and the consequences of induction of synaptic plasticity in freely moving rats was not, to our knowledge, described earlier. We found that changes in theta and gamma oscillatory activity precede electrically induced potentiation of synaptic transmission. Importantly, the correlated tetanisation-driven enhancement of both theta and gamma oscillations is associated with successful short- and LTP, but not with its failure. These findings link LTP with the oscillatory activity of rather large assemblies of hippocampal principal cells and interneurons, and provide additional support for the role of hippocampal theta and gamma oscillations in information processing and the formation of new memories.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by a Deutsche Forschungsgemeinschaft grant to D. Manahan-Vaughan (Ma1843).
Niewoehner, B., Single, F. N., Hvalby, Ø., Jensen, V., Borgloh, S. M., Seeburg, P. H., Rawlins, J. N., Sprengel, R., and Bannerman, D. M. (2007). Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur. J. Neurosci. 25, 837–846.
Swanson-Park, J. L., Coussens, C. M., Mason-Parker, S. E., Raymond, C. R., Hargreaves, E. L., Dragunow, M., Cohen, A. S., and Abraham, W. C. (1999). A double dissociation within the hippocampus of dopamine D1/D5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience 92, 485–497.