- 1Department of Biology, University of Rome “Tor Vergata”, Rome, Italy
- 2Faculty of Engineering, University of Porto (FEUP), Porto, Portugal
- 3School of Medicine and Biomedical Sciences (ICBAS), Porto, Portugal
- 4PhD Program in Cellular and Molecular Biology, University of Rome “Tor Vergata”, Rome, Italy
- 5PhD Program in Space Science and Technology, University of Trento, Trento, Italy
- 6Department of Industrial Engineering, University of Rome “Tor Vergata”, Rome, Italy
- 7Space sustainability Center (SSC), University of Rome “Tor Vergata”, Rome, Italy
Background: Long-duration crewed missions on the Moon and Mars rely on support technologies based on locally available resources. Rock-weathering cyanobacteria are key enablers to transform minerals, carbon dioxide and urine (from crew waste) into biomass to be used to feed heterotrophic bacteria for downstream production of consumables. However, cyanobacterial cultivation in media based on water-released minerals is hindered by reduced light penetration due to the medium turbidity. The biomass production from two desert isolates of Chroococcidiopsis, a strain capable of Far-red Light Photoacclimation (FaRLiP) and a non-FaRLiP strain, was compared to investigate if the former better faced regolith shading.
Methods: The FaRLiP strain CCMEE 010 and non-FaRLiP CCMEE 029 were cultivated for 21 days under VL in Martian water-released minerals with 10 mM urea and 2.4 mM perchlorate and in BG-11 control medium. A comparison was made of cell morphology, photosynthetic pigment emission spectrum and presence of urea transport and catabolism genes.
Results: No morphological changes occurred among the two strains, but the FaRLiP strain exhibited adaptation to regolith shadowing as shown by an emission peak related to FaRLiP early phase. The absence of pigment bleaching suggested the tolerance towards prolonged cultivation with Mars-relevant perchlorate and urea. The latter was used as a nitrogen source enabled by genes for urea transport and catabolism. Biomass lysates from both strains supported the growth of heterotrophic bacteria, although the FaRLiP-positive strain cultivated in both Martian water-released minerals and BG-11 medium accumulated more biomass and thus promoted greater bacterial growth.
Conclusion: The cultivation under VL with Martian water-released minerals (with perchlorate and urea) showed that the FaRLiP strain suffered less growth detriment in the turbid medium, though the potential role of this process in Bio-ISRU remains unclear.
Introduction
As humanity moves towards a new era of deep space exploration, envisioning sustainable outposts on the Moon and Mars requires surpassing numerous technological limitations as defined by the International Space Exploration Coordination Group and NASA’s Moon to Mars Strategies Objectives (NASA, 2023). While the Environmental Control and Life Support System (ECLSS) on the International Space Station (ISS) provides most of the water and breathable air (Williamson et al., 2023; Vega, 2021), astronauts still rely on the delivery of supplies from Earth, such as food and medicine.
One of the most critical components for human exploration of deep space is reducing reliance on Earth-based resupplies. This requires the development of artificial ecosystems, known as Bioregenerative Life Support Systems (BLSS) to recycle wastes and provide essential resources such as oxygen, water and food (De Micco et al., 2023; Liu H. et al., 2021). In addition, the development of in-situ resource utilization (ISRU) technologies relying on physical and chemical methods (Zhang et al., 2023), should be enhanced. These approaches can be complemented by leveraging the capabilities of living organisms, particularly microorganisms, to grow using resources available on site and perform biochemical processes to produce a wide range of consumable, a concept known as biological in-situ resource utilization (Bio-ISRU) (Averesch et al., 2023).
Besides being able to use resources available on site, an ideal microorganism for space applications requires a unique blend of robustness, efficiency, and versatility. It must withstand space constraints including altered gravity and ionizing radiation; be transported at initial low mass and volume; offer high versatility for biotechnological applications and require minimal attenuation of superficial conditions at the extraterrestrial destination (Cockell, 2022). In this context the identification of suitable strains among cyanobacteria and algae is gaining an increasing interest, as they perform oxygenic photosynthesis and carbon dioxide fixation (Mapstone, et al., 2022; Santomartino et al., 2023). Such a capability is harnessed in the European Space Agency’s Micro-Ecological Life Support System Alternative (MELiSSA), a pilot project which employs Limnospira indica, previously called Arthrospira, which is an edible cyanobacterium with high protein content largely used on Earth (Lafarga et al., 2020). Beyond human consumption, rock-weathering cyanobacteria are promising candidates for Bio-ISRU as they can use local resources and the yielded biomass can be further used as feedstock for bacteria relevant to bioprocesses, which likely cannot use raw extraterrestrial soils, poor in organics, fixed nitrogen and readily available mineral nutrients (Rothschild, 2016; Verseux et al., 2016). Moreover, cyanobacterial biomass can provide biofertilizers and/or biostimulants for space farming, thus offering potential benefits for crop production in challenging extraterrestrial environments (Renaud et al., 2023).
When applied to Bio-ISRU on Mars the cultivation of cyanobacteria will depend on regolith and light availability as a source of minerals and energy and additionally, it will be affected by the presence of perchlorates in the soil (Rzymski et al., 2024). When flooding the Martian regolith simulant MGS-1 with water, initial high values of turbidity have been reported, although they decrease over time, resulting in increased transparency and availability of photosynthetically active radiation (Rzymski et al., 2023). However, cyanobacterial cultivations benefit from agitation and aeration; hence to bypass regolith shading a photobioreactor has been proposed in which the cyanobacterium Anabaena sp. PCC 7938 and the Martian regolith simulant are physically separated in compartments connected through dialysis membranes to allow small molecule exchange (Ramalho et al., 2022). It was also shown that cyanobacterial productivity can be augmented by increasing regolith concentrations up to a perchlorate content that is toxic to the cyanobacterium (Ramalho et al., 2024). Indeed, it was anticipated that the most promising microorganisms for Bio-ISRU are those that tolerate perchlorate ions in the 2.2–2.5 mM range (Rzymski et al., 2024).
Harnessing for Bio-ISRU rock-inhabitant cyanobacteria of the Chroococcidiopsis genus is interesting considering their desiccation- and radiation-resistance along with tolerance towards 100 mM perchlorate ions (Billi et al., 2021). Notably, strain CCMEE 029 has been successfully cultivated with lunar and Martian regolith simulants supplemented with synthetic human urine and, when relevant, with 2.4 mM perchlorate ions (Fernandez et al., 2023). Planktonic cultures and biofilms were obtained either when the cyanobacterium was cultivated in direct contact with regolith gains or in water-released minerals (Fernandez et al., 2023). To overcome the regolith shading, planktonic cultures were shaken occasionally, to promote cyanobacterial growth primarily on the top of sedimented grains (Figure 4; Verseux et al., 2016). In contrast, when using a medium based on water-released minerals from the Martian regolith simulant JSC Mars-1, planktonic cultures faced a certain degree of turbulence due to leached iron and uplift of fine grains not removed by low-speed centrifugation of water/regolith mixture (Figures 1, 3 this work). JSC Mars-1A is a first-generation simulant based on altered volcanic ash, specifically plagioclase with minor Ti magnetite, Ca-rich pyroxene, olivine, glassy, ferric oxide particles, and traces of crystalline clay minerals or phyllosilicates (Allen et al., 1998). The simulant’s reflectance spectrum shows a significant absorption of light in the visible range, a key spectral characteristic of ferric iron, and a weak absorption across near-infrared wavelengths (Allen et al., 1998).

Figure 1. Photographs of water-released minerals obtained from the Martian regolith simulant JSC Mars-1A. (A) Medium obtained after shaking at 6 r.p.m. for 8 hs at RT and collecting the supernatant aftre centrifugation at 100 g for 10 min. (B) Medium based on water-released minerals after shaking. Medium supplemented with perchlorate and urea.
The present work aimed to compare the bacterial feedstock production from strain CCMEE 029 with that from Chroococcidiopsis sp. CCMEE 010, a desert strain capable of performing photosynthesis in the far-red light (FRL) region of the electromagnetic spectrum (Billi et al., 2022). This acclimation process is known as Far-red Light Photoacclimation (FaRLiP) and occurs in cyanobacteria, like those found in deserts, caves, or beach rocks, adapted to environments depleted in VL and enriched in FRL due to physical processes or above presence of photoautotrophs (Jung et al., 2023; Sanfilippo et al., 2019). FaRLiP consists in a remodelling of the photosynthetic apparatus and synthesis of far-red absorbing chlorophylls, namely, Chl f and d, that enable the absorption and utilization of FRL (Gan and Bryant, 2015).
The FaRLiP acclimation is considered advantageous for biotechnological applications since at the bottom of bioreactors cyanobacteria experience FRL-enriched conditions (Chen and Blankenship, 2011; Liu D. et al., 2021). This adaptation might also be advantageous for Bio-ISRU based on cyanobacteria given that, compared to Earth, the light reaching the Martian surface has less intensity and is shifted towards longer wavelengths (Thomas et al., 1999).
Hence the suitability of Chroococcidiopsis sp. CCMEE 010 for Bio-ISRU was investigated in comparison with the non-FaRLiP strain Chroococcidiopsis CCMEE 029 by verifying if: i) its growth can be supported by water-released minerals from JSC Mars-1 supplemented with perchlorate ions and urea; ii) FaRLiP acclimation occurs under VL in the presence of regolith shading; iii) the yielded biomass can be used as bacterial feedstock. Finally, the capability of strains CCMEE 010 and CCMEE 029 of using urea as a nitrogen source, was investigated by performing a bioinformatic analysis to search for genes involved in the urea transport and catabolism.
Materials and methods
Cyanobacterial and bacterial strains
The two Chroococcidiopsis strains used in this study are part of the Culture Collection of Microorganisms from Extreme Environments (CCMEE) established by E. Imre Friedmann and Roseli Ocampo-Friedmann and maintained at the Department of Biology, University of Rome Tor Vergata (Table 1). Both strains were grown in liquid Blue Green-11 Medium (BG-11), at 25 °C and under a photon flux density of 20 μmol/m2s provided by white tubular led lights (OSRAM LEDs). For comparison reason the FaRLiP strain CCMEE 010 was cultured under far-red light using a photon flux density of 5 μmol/m2s provided by far-red tubular LED lights (OSRAM LEDs). Escherichia coli W (ATCC 9637), a fast-growing strain that utilizes sucrose as a carbon source (Archer et al., 2011), was purchased from the American Type Culture Collection (Manassas, VA, United States) and grown in Luria-Bertani (LB) broth at 37 °C with orbital shaking.
Medium based on water-released minerals
The Martian regolith simulant JSC Mars-1A (Allen et al., 1998), provided by Orbital Technologies Corporation (Madison, WI, United States), was used to prepare water-released minerals for cyanobacterial growth. The autoclaved simulant was resuspended in double-distilled water (ddH2O) at a concentration of 0.2 g/mL and subjected to shaking at 6 r.p.m. for 8 hs, at room temperature (RT). Afterwards, the mixture was centrifuged at 100 g for 10 min, the supernatant was recovered and supplemented with 10 mM urea and 2.4 mM perchlorate ions (60% calcium perchlorate and 40% magnesium perchlorate), as previously reported (Fernandez et al., 2023). Both were previously filtered with a 0.22 µm filter, to ensure sterility. The water-released medium showed a brownish colour with a certain degree of turbulence (Figure 1A) likely due to iron in the solution and fine grains that were not removed by low-speed centrifugation and that uplifted after shaking (Figure 1B).
Cyanobacterial growth with water-released minerals
The two cyanobacterial strains were grown in BG-11 liquid medium until a stationary phase was achieved. Pellets of about 1 × 108 cells (determined using a Bürker chamber) were washed twice with ddH2O and resuspended in: (i) 10 mL of water-released minerals supplemented with 10 mM urea and 2.4 mM perchlorate (experimental), (ii) 10 mL of BG-11 medium (positive control), or (iii) 10 mL of ddH2O (negative control). Cultures were incubated for 21 days at 25 °C at 25 °C and under a photon flux density of 20 μmol/m2s provided by white tubular led lights (OSRAM LEDs). All conditions were performed in triplicate.
Preparation of cyanobacterial lysates
After 21 days of growth, each 10-mL cyanobacterial culture was centrifuged at 5000 g for 5 min, each pellet was collected in a 1.5 mL Eppendorf and dried overnight in a laminar flow hood. Cyanobacterial biomass was evaluated by determining the chlorophyll a to dry biomass ratio as previously described (Fernandez et al., 2023). Dry pellets from cultures grown in BG-11 medium were normalized to 1 mg/mL ddH2O aliquots, the same dilution was used to normalize pellets from cultures grown in water-released medium. Cell lysis was performed by modifying a previously developed method (Fernandez et al., 2023) by replacing liquid nitrogen with a homogenizer (GeneReady Hangzhou Lifereal Biotechnology Co., Ltd., China), as shown in Figure 2. Briefly, one volume of glass beads was added to pellets resuspended in 500 µL ddH2O, then the cells/water/bead mixture was subjected to three cycles of 2-min homogenization, 1-min heating at 60 °C and 1-min vortexing. After centrifugation at 6000 g for 5 min at room temperature the supernatant was collected, and the lysis procedure was repeated by resuspending the pellet with 500 µL ddH2O. Finally, the two supernatant fractions were collected in an Eppendorf and used as feedstock for bacteria.
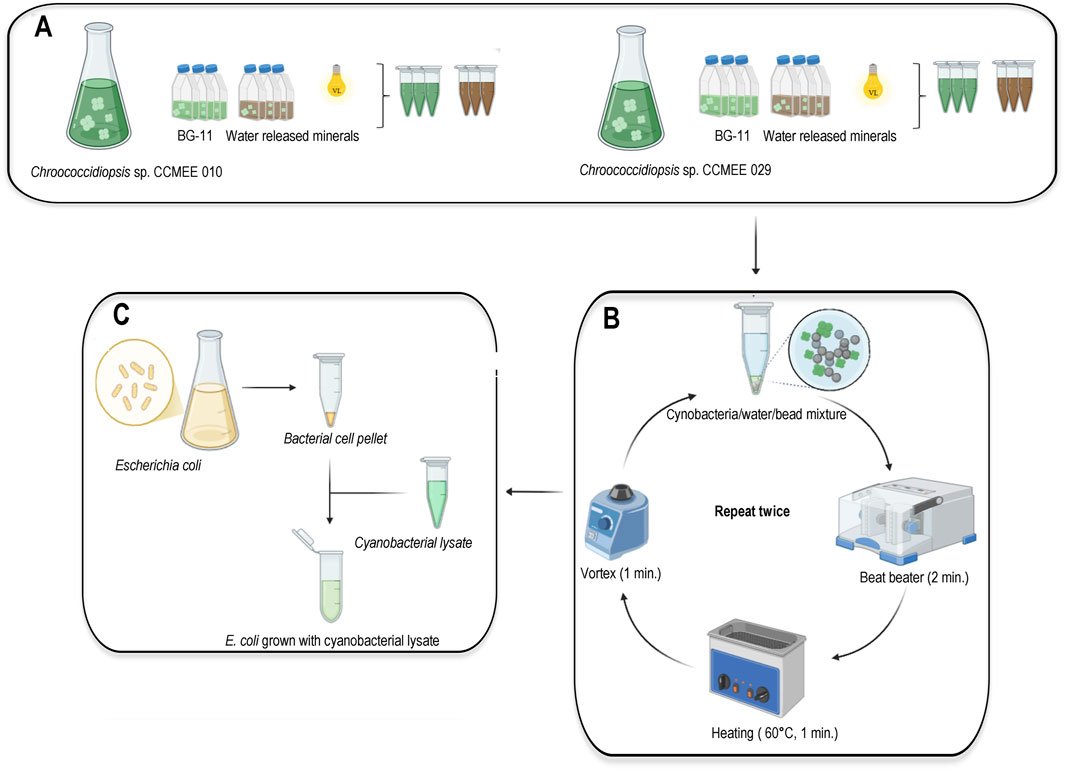
Figure 2. Workflow of bacterial feedstock preparation from cyanobacterial biomass. (A) cyanobacteria were cultivated for 21 days in water-released minerals and BG-11 medium under visible light. (B) Each cyanobacteria/water/bead mixture was subjected to three cycles of 2-min homogenization, 1-min at 60 °C and 1-min vortexing; after centrifugation each supernatant was collected, the pellet was resuspended in water and subjected to a second cycle of mechanical lysis. (C) for each sample the two supernatant fractions were collected and used as feedstock to grow bacteria. Created with BioRender.com.
Bacterial growth with cyanobacterial lysates
After overnight growth in LB medium, cultures of E. coli W were washed twice with Phosphate-Buffered Saline (PBS) and pellets of about 1 × 107 cells were resuspended in 1 mL of cyanobacterial lysate, 1 mL of LB medium and 1 mL of ddH2O. Cultures were incubated in 2 mL Eppendorf tubes at 37 °C with orbital shaking. Bacterial growth was assessed by measurement of the optical density at 600 nm at 24 h of incubation. All conditions were performed in triplicate.
Confocal analysis of cyanobacteria
Cyanobacteria grown in water-released minerals and BG-11 were examined with a Confocal Laser Scanning Microscopy System (CLSM; Olympus Fluoview 1000). Cells were immobilized on top of microscope slides with BG-11 containing 1.5% (w/v) agarose. CLSM lambda scans were performed with a 488-nm excitation laser and emission was detected from 550 nm to 790 nm. Plots of the emission spectra were constructed with GraphPad Prism 8.0.1 (GraphPad Software, San Diego, CA, United States), using the mean fluorescence intensity and upper standard deviation. To enable direct comparison between experimental groups, values were normalized as percentages of the largest value in each data set.
Bioinformatics analysis
A comparative genomic analysis was conducted to identify urea metabolism genes in Chroococcidiopsis sp. CCMEE 010 and CCMEE 029 by using Synechocystis sp. PCC 6803 as reference genome for urea-related sequences. Putative genes were identified using BLASTp searches against the target genome, and hits with a default expected threshold and sequence identity ≥30% were considered for further analysis. Gene prediction was conducted using Prokka (Seemann, 2014). Then InterProScan and eggNOG-mapper tool (Huerta-Cepas et al., 2019) were employed to validate the retrieved gene sequences.
Statistical tests
Data were analysed using GraphPad Prism 8.0.1 (GraphPad Software, San Diego, CA, United States) for Windows.
Results
Unaltered morphology of cyanobacteria in Martian water-released mineral medium
Cultures of the two Chroococcidiopsis strains, namely, the FaRLiP strain CCMEE 010 and non-FaRLiP strain CCMEE 029, grown for 21 days under VL-conditions with Martian water-released mineral medium (supplemented with 10 mM urea and 2.4 mM perchlorate ions), showed a brownish colour (Figures 3A,B). This colour was due to the iron in the solution and uplift of fine simulant grains that were not removed duirng the medium preparation (shaking at 6 r.p.m. for 8 hs followed by low-speed centrifugation) and presence of iron in the solution (see Figure 1 in Materials and Methods). No growth occurred when the two strains were inoculated in ddH2O (not shown).
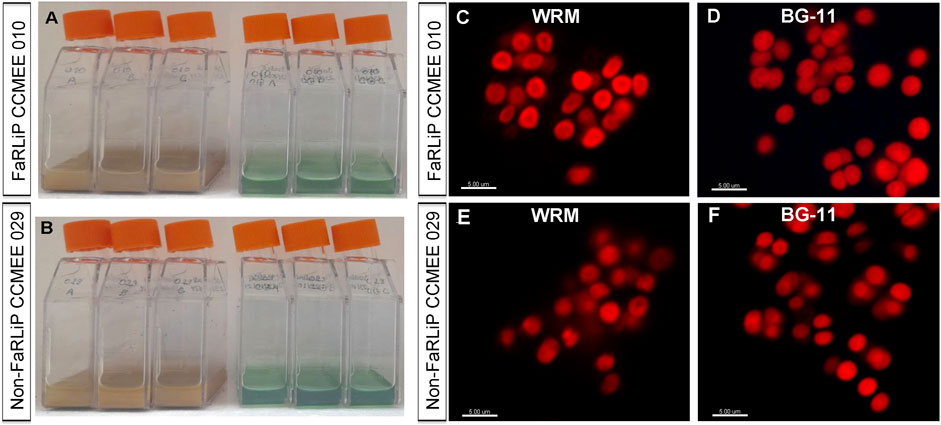
Figure 3. Chroococcidiopsis strains after 21 days of growth under VL. (A) Cultures of the FaRLiP strain CCMEE 010 and (B) non-FaRLiP strain CCMEE 029 grown in Martian water-released minerals supplemented with 10 mM urea and 2.4 mM (WRM) and in control BG-11 medium. CLSM imaging of the FaRLiP strain CCMEE 010 grown in WRM (C) and in BG-11 medium (D), non-FaRLiP strain CCMEE 029 grown in WRM (E) and in BG-11 medium (F). Bar scale = 5 μm.
The analysis at the CLSM with a 635-nm excitation laser revealed no morphological changes in the FaRLiP strain CCMEE 010 after 21 days of cultivation under VL condition in water-released mineral medium (Figure 3C) when compared to cells grown in BG-11 medium (Figure 3D). Moreover, in both cultivation media, cells exhibited an intense autofluorescence of the photosynthetic pigments (Figures 3C,D). Similarly, a comparable morphology and autofluorescence of the photosynthetic pigments was observed in the non-FaRLiP strain CCMEE 029 when cultivated in the Martian water-released mineral medium (Figure 3E) or in BG-11 (Figure 3F).
Acclimation of FaRLiP cyanobacterial strain in Martian water-released mineral medium
After for 21 days of cultivation under VL condition in Martian water-released mineral medium (supplemented with 10 mM urea and 2.4 mM perchlorate ions), the spectral feature of the photosynthetic pigments of the Chroococcidiopsis FaRLiP strain CCMEE 010 and the non-FaRLiP strain CCMEE 029 were investigated at the single-cell level by CLSM-λscan (Figure 4). When cultivated in water-released mineral medium the FaRLiP strain CCMEE 010 exhibited an emission spectrum with a peak in the 650–660 nm range attributed to phycobiliproteins and Chl a along with an additional peak at about 670 nm (Figure 3A). The 670-nm peak is typical of the emission spectrum of strain CCMEE 010 after 21-day exposure to FRL in BG-11 medium, along with a peak in the 715–727 nm range due to Chl f (Figure 4A). The non-FaRLiP strain CCMEE 029 grown in Martian water-released minerals and BG-11 medium exhibited a similar emission spectrum with a peak in the 650–660 nm range due to phycobiliproteins and Chl a (Figure 4B). This peak was comparable in shape and intensity to that shown by the FaRLiP strain CCMEE 010 incubated under the same growth conditions (Figure 4B).
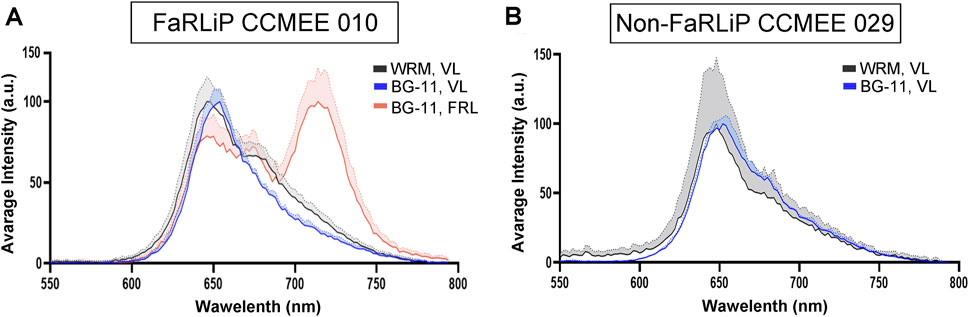
Figure 4. CSLM λscan of Chroococcidiopsis strains grown for 21 days (A) FaRLiP strain CCMEE 010 grown under VL in Martian water-released minerals (with 2.4 mM perchlorates and 10 mM urea; WRM, VL) and in BG-11 (BG-11, VL); FaRLiP strain CCMEE 010 grown in BG-11 medium under FLR (BG-11, FRL). (B) Non-FaRLiP strain CCMEE 029 grown for 21 days under VL in Martian water-released minerals (with 2.4 mM perchlorates and 10 mM urea; WRM, VL) and in BG-11 medium (BG-11, VL).
Cyanobacteria grown in Martian water-released mineral medium as feedstock for heterotrophic producers
The biomass production of the Chroococcidiopsis FaRLiP strain CCMEE 010 after 21 days of cultivation under VL condition in Martian water-released minerals (with perchlorates and urea) is shown in Figure 5. The non-FaRLiP strain CCMEE 029 produced a biomass corresponding to about the 27% of when cultivated in BG-11 medium, as previously reported (Fernandez et al., 2023), while the FaRLiP strain CCMEE 010 yielded a biomass corresponding to about 58% of the biomass from cultures in BG-11 medium.
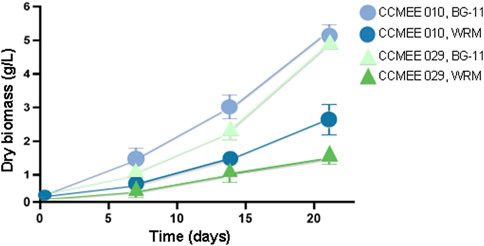
Figure 5. Biomass production from Chroococcidiopsis strains grown for 21 days under VL condition. FaRLiP strain CCMEE 010 and non-FaRLiP strain CCMEE 029 cultivated in Martian water-released minerals supplemented with 10 mM urea and 2.4 mM (WRM) and in BG-11 medium (BG-11).
Each biomass obtained from cultures in Martian water-released minerals was subjected to cell lysis and the yielded lysates used to feed E. coli W. When the lysate from the FaRLiP strain CCMEE 010 was inoculated with 1 × 107 bacterial cells after 24 hs of incubation under optimal growth conditions, the cell density increased to 5.9 × 107 cells/mL (Figure 6). While the lysate from the non-FaRLiP strain CMME 029 supported an increase to 2.5 × 107 cells/mL (Figure 6).
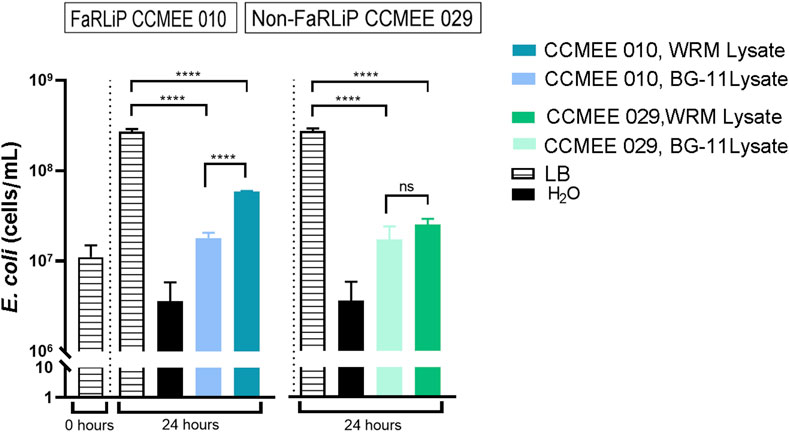
Figure 6. Growth of E. coli with lysates from Chroococcidiopsis strains grown for 21 days under VL condition. Lysate from FaRLiP strain CCMEE 010 and non-FaRLiP strain CCMEE 029 grown in Martian water-released minerals with 2.4 mM perchlorates and 10 mM urea (WRM Lysate) and in BG-11 (BG-11 Lysate). Controls: E. coli inoculated into LB medium and into ddH20.
To allow the comparison with previuos results reported for strain CCMEE 029 (Fernandez et al., 2023) the lysates from cells grown in BG-11 were normalized to 1 mg/mL dry wieght and used as growth medium for 1 × 107 cells of E. coli W. After 24-h of incubation under optimal growth conditions a comparable increase of the bacterial cell densities was obtained when using either the lysate from the FaRLiP strain CCMEE 010 and the non-FaRLiP strain (Figure 6). The increases of the bacterial cell density increases were lower than those occurring when using LB medium.
Analysis of the urea transport and catabolism genomic region
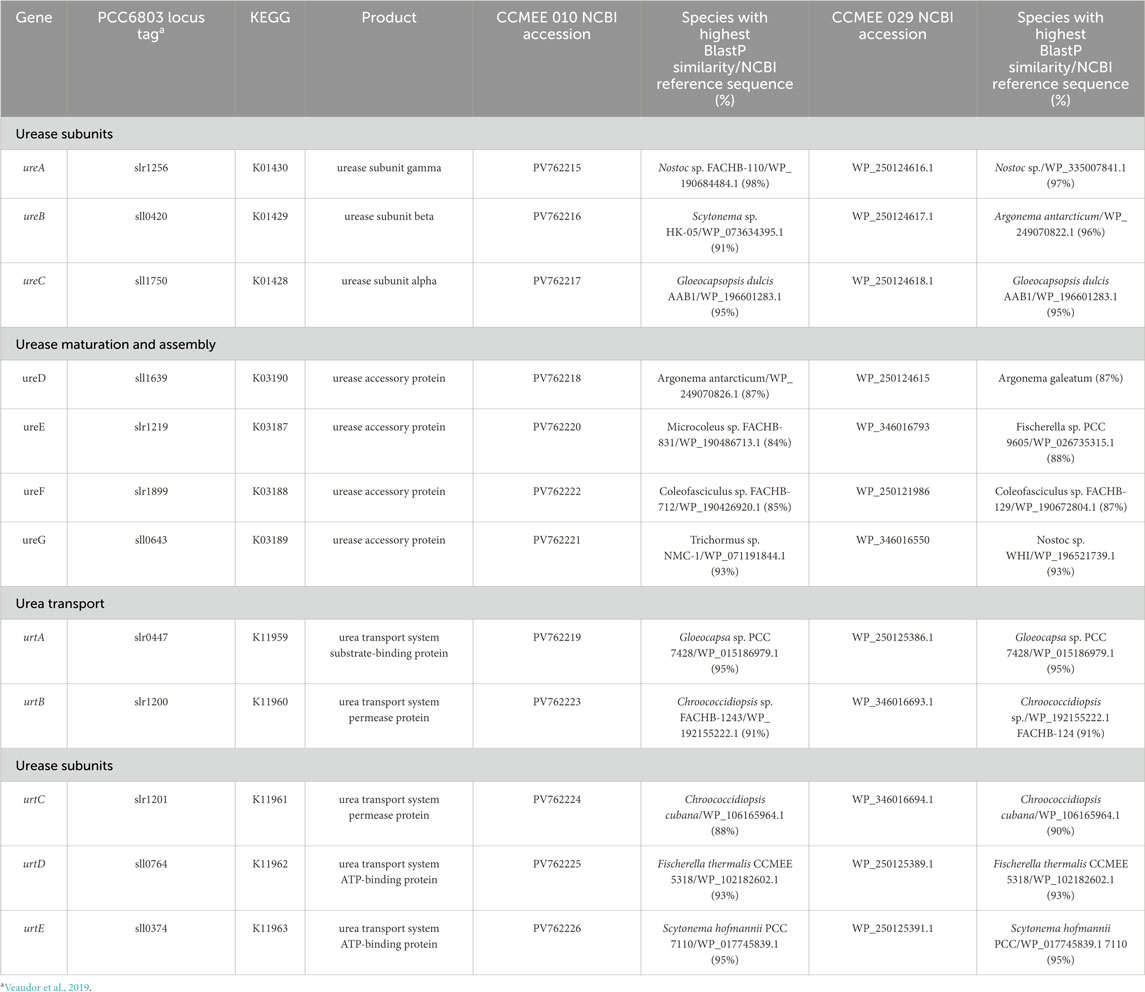
The genomic regions of Chroococcidiopsis sp. CCMEE 010 and CCMEE 029 associated with urea metabolism were identified by using Synechocystis sp. PCC 6803 as reference (Veaudor et al., 2019). The Blast analysis identified the presence in both genomes of protein orthologs to the urease subunits UreABC, maturation and assembly of urease components UreDEFG and urea transporters UrtABCDE (Table 2). Beside the fact that BLAST results in which the highest similarities of strain CCMEE 010 were shared with strain CCMEE 029 were omitted, the proteins of both cyanobacteria shared the highest similarity with orthologs of cyanobacteria isolated from extreme environments such as Scytonema sp. HK-05 from a semiarid crust in Japan, Argonema antarcticum from Western Antarctica, Chroococcidiopsis cubana form a dried pool in Cuba or Gloeocapsopsis dulcis from the Atacama Desert, Chile (Table 2).
The comparative structure of the genomic region for urea transport and catabolism in Chroococcidiopsis sp. CCMEE 010 and CCMEE 029 showed the presence of the three main functional modules–urease catalytic subunits, accessory proteins, and urea transporters–with moderate to high levels of protein similarity with Synechocystis sp. PCC 6803, ranging from 39.5% to 85% (Figure 7). Moreover, compared to PCC 6803 the three main functional modules were mote continuously linked with the urea transport genes (urtABCDE) in one cluster and the urease (ureABCDEFG) located in three positions: One comprising the cluster ureABCD, one the cluster ureEF and the other one the ureG gene (Figure 7).
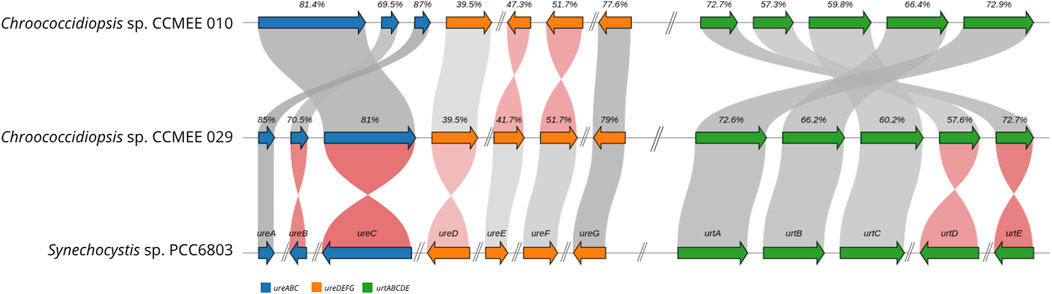
Figure 7. Genomic region associated with urea acquisition and catabolism in Chroococcidiopsis sp. CCMEE 010 and CCMEE 029. Annotations are based on Synechocystis sp. PCC6803. Genes not continuously linked are indicated by hash marks. Arrows represent the transcriptional orientation of the genes coloured according to functional category: urease subunits UreABC (blue), urease maturation and assembly UreDEFG (orange), urea transporters UrtABCDE (green); protein similarity relative to Synechocystis sp. PCC 6803 is also shown. See Table 2 for details.
Discussion
With the upcoming Artemis Lunar exploration program and the Lunar Gateway Station serving as a staging point for future human missions to Mars (Crusan et al., 2019; Smith et al., 2020) the development of life support technologies is fundamental. Rock-weathering cyanobacteria might be key enablers to transform locally available resources - such as atmospheric carbon dioxide, regolith-derived minerals, and urea from crew’s urine - into biomass to feed heterotrophic bacteria for downstream production of consumables.
The present study investigated if the constraints imposed by the regolith shading might be overcome by FaRLiP cyanobacteria, capable of photosynthesis beyond the VL range, such as a few desert strains of the Chroococcidiopsis genus (Antonaru et al., 2023). Overall, after 21 days of cultivation under visible light and Martian water-released minerals (supplemented with 10 mM urea and 2.4 mM perchlorate ions) the FaRLiP strain CCMEE 010 yielded an increased biomass compared to the non-FaRLiP strain CCMEE 029, that allowed an enhanced growth of E. coli W.
During the cultivation, acclimation of the FaRLiP strain CCMEE 010 was indicated by the appearance of a 670-nm peak in the emission spectrum of the photosynthetic pigments. Notably this peak occurred during the early phase (3 days) of the FRL acclimation of strain CCMEE 010 along with a peak at 715–727 nm due to FRL-shifted chlorophylls that appears after 7 days of FRL acclimation (Di Stefano et al., 2024). Hence the regolith shielding - due to the uplift of the finest grains that were not removed after low-speed centrifugation of the water/regolith mixture - might have influenced the spectral quality of the light and triggered a slower acclimation process under suboptimal conditions. Indeed, the FaRLiP response requires extended periods of fully establish FRL condition (Ho et al., 2017). Concordantly, FRL-shifted chlorophylls were detected in strain CCMEE 010 only after 7 days of FRL condition (Di Stefano et al., 2024).
However extended periods of cultivation under VL and Martian water-released minerals (supplemented with 10 mM urea and 2.4 mM perchlorate ions) were not investigated, making impossible to establish whether strain CCMEE 010 did experience a slower FaRLiP process due to VL attenuation caused by regolith shading. It has been reported that when cultured at high cell density under VL condition, the FaRLiP cyanobacterium Chlorogloeopsis fritschii produced FRL-shifted chlorophylls, an effect attributed to VL-depletion and FRL-enrichment caused by self-shading (Airs et al., 2014). Hence, the potential role of FaRLiP in the better performance of strain CCMEE 010 when cultivated in the Martian water-released minerals remains to be unravelled, for example, by investigating the growth capability of the FaRLiP and non-FaRLiP strain in BG-11 medium containing inert particles in order not to change its nutrition properties.
In the present work, the absence in the emission spectrum of any peaks related to FRL-shifted chlorophylls, in the FaRLiP strain CCMEE 010 grown for 21 days in BG-11 medium, suggested that the cell density was not enough to establish FRL-enriched microenvironments. In addition, exception made for the 670-nm peak, the comparable intensity of the fluorescence spectra of strains CCMEE 010 and CCMEE 029 when cultivated in both Martian water-released minerals and BG-11 medium, indicated that 2.4 mM perchlorate and 10 mM urea did not cause pigment bleaching. These results suggest that the FaRLiP strain CCMEE 010 can tolerate a prolonged cultivation in water-released minerals - supplemented with perchlorate and urea, - as previously reported for the non-FaRLiP strain CCMEE 029 (Billi et al., 2021; Fernandez et al., 2023). Such a tolerance is particularly relevant since Synechocystis sp. PCC 6803 has been shown to undergo chlorosis after 14 days of growth in 5 mM urea, with higher urea concentrations further reducing the period of healthy growth and biomass production (Veaudor et al., 2018). Perchlorate tolerance is shared by several Chroococcidiopsis strains (Rzymski et al., 2022), and the capability of utilizing urea has also been reported for Chroococcidiopsis thermalis CCALA 050 (Fais et al., 2024).
The ability of Chroococcidiopsis strains CCMEE 010 and CCMEE 029 to utilize urea as a nitrogen source was further investigated through a bioinformatic analysis. Both strains endowed single copies of genes for urease activity (ureABCDEFG) and urea transport (urtABCDE). The selection against gene redundancy has been ascribed to the fact that high urea transport and catabolism are toxic under high-urea concentration (≥10 mM) or prolonged cultivation period (Veaudor et al., 2019). The bioinformatic analysis revealed that the genomic context of urea transport and catabolism in the strains CCMEE 010 and CCMEE 029 differed from Synechocystis sp. PCC 6803 in which these genes are scattered through the genome. On the contrary, in strains CCMEE 010 and CCMEE 029, the urea transport and catabolism genes are mapped in four clusters, similarly to Nostoc sp. PCC 7120 (Veaudor et al., 2019) and share a higher similarity with orthologs of cyanobacteria from extreme environments rather than Synechocystis sp. PCC 6803.
The biomass lysates of strains CCMEE 010 and CCMEE 029 cultivated in Martian water-released minerals were used to feed E. coli W, a model organism for biotechnological applications (Yang et al., 2021). The Martian water-released minerals did not provide optimal growth conditions for cyanobacteria, resulting in a lower biomass production compared to BG-11 medium. Nevertheless, the biomass lysates supported bacterial growth. Notably, the lysate from the FaRLiP strain CCMEE 010 cultivated in water-released minerals yielded a greater increase of the bacterial cell density than the non-FaRLiP strain, due to an enhanced biomass production. While the normalized lysates of two strains grown in BG-11 yielded comparable bacterial cell densities. However, these values were slightly lower than those previously reported when using the biomass lysates of strain CCMEE 029 (Fernandez et al., 2023). This might be attributed to the modifications introduced to the former protocol, to make it less complex and more reproducible beyond Earth laboratories. Cyanobacterial lysis was achieved through homogenization rather than using liquid nitrogen, which might have reduced lysis efficiency. Moreover, filtration steps of the water-released minerals were avoided to minimize the process complexity, hence some turbulence remained in the medium due to residual fine particles not removed after low-speed centrifugation. Such experimental growth condition was better faced by the FaRLiP strain CCMEE 010 strain as suggested by the higher efficiency of its lysate as feedstock. These findings support strain CCMEE 010 as a suitable candidate for Bio-ISRU. Notably, the proven capability of this strain to adapt to altered VL light conditions could be relevant in a scenario where light exposure is limited or spectrally shifted, as is the case of the Martian surface (Kuhn and Atreya, 1979).
Conclusion
This work demonstrated the advantages of cultivating FaRLiP cyanobacteria using Martian water-released minerals to produce biomass to support biotechnologically relevant bacteria, while simultaneously contributing to crew-waste recycle. When cultivated under VL in Martian water-released minerals Chroococcidiopsis strain CCMEE 010 adapted to the shadowing caused by the uplift of the fine grains as shown by the emission spectrum of the photosynthetic pigments thus suggesting a slower FaRLiP process. Hence the improvement consists in using a cyanobacterium capable of driving photosynthesis with wavelengths available in the visible or near-infrared range.
Compared to the non-FaRLiP strain CCMEE 029, an enhanced E. coli growth was supported by strain CCMEE 010 due to a greater cyanobacterial biomass production. Indeed, compared to CCMEE 029, the FaRLiP strain accumulated more biomass also in BG-11 medium, but it suffered less the growth impairment in the turbid medium based on Martian water-released minerals. The supplementation of the water-released minerals with perchlorate and urea - which could be sourced from astronaut urine -, further demonstrates the cyanobacterial potential for recycling of crew-generated waste in limited-resource conditions and relevance for Bio-ISRU. The possibility of using the biomass of a FaRLiP cyanobacterium as feedstock further underscored their value as a nutrient source for heterotrophic bacteria, regardless of their cultivation in a perchlorate-rich medium and reduced VL condition. Finally, results suggest expanding into FRL the photosynthetically active radiation of space-relevant cyanobacteria not capable of FaRLiP, but suitable to engineering biology approaches.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
IS: Formal Analysis, Writing – original draft, Methodology, Writing – review and editing. GD: Methodology, Writing – original draft, Writing – review and editing, Investigation, Data curation. AD: Writing – review and editing, Methodology. CM: Writing – review and editing, Methodology. AC: Formal Analysis, Software, Writing – review and editing. GR: Formal Analysis, Writing – review and editing, Software. LS: Writing – review and editing. DB: Funding acquisition, Supervision, Writing – review and editing, Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was carried out within the Space It Up project funded by the Italian Space Agency, ASI, and the Ministry of University and Research, MUR, under contract n. 2024-5-E.0 - CUP n. I53D24000060005.
Acknowledgments
The authors would like to thank Elena Romano from the Centre of Advanced Microscopy, Department of Biology, University of Rome Tor Vergata, for her skillful assistance in the use of the facility.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Airs, R. L., Temperton, B., Sambles, C., Farnham, G., Skill, S. C., and Llewellyn, C. A. (2014). Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation. FEBS Lett. 588, 3770–3777. doi:10.1016/j.febslet.2014.08.026
Allen, C. C., Jager, K. M., Morris, R. V., Lindstrom, D. J., Lindstrom, M. M., and Lockwood, J. P. (1998). Martian soil simulant available for scientific, educational study. Eos Trans. Am. Geophys. Union 79, 405–409. doi:10.1029/98EO00309
Antonaru, L. A., Selinger, V. M., Jung, P., Di Stefano, G., Sanderson, N. D., Barker, L., et al. (2023). Common loss of far-red light photoacclimation in cyanobacteria from hot and cold deserts: a case study in the Chroococcidiopsidales. ISME Commun. 3, 113. doi:10.1038/s43705-023-00319-4
Archer, C. T., Kim, J. F., Jeong, H., Park, J. H., Vickers, C. E., Lee, S. Y., et al. (2011). The genome sequence of E. coli W (ATCC 9637): comparative genome analysis and an improved genome-scale reconstruction of E. coli. BMC Genomics 12, 9. doi:10.1186/1471-2164-12-9
Averesch, N. J. H., Berliner, A. J., Nangle, S. N., Zezulka, S., Vengerova, G. L., Ho, D., et al. (2023). Microbial biomanufacturing for space-exploration—what to take and when to make. Nat. Commun. 14, 2311. doi:10.1038/s41467-023-37910-1
Billi, D., Gallego Fernandez, B., Fagliarone, C., Chiavarini, S., and Rothschild, L. J. (2021). Exploiting a perchlorate-tolerant desert cyanobacterium to support bacterial growth for in situ resource utilization on Mars. Int. J. Astrobiol. 20, 29–35. doi:10.1017/S1473550420000300
Billi, D., Napoli, A., Mosca, C., Fagliarone, C., de Carolis, R., Balbi, A., et al. (2022). Identification of far-red light acclimation in an endolithic Chroococcidiopsis strain and associated genomic features: implications for oxygenic photosynthesis on exoplanets. Front. Microbiol. 13, 933404. doi:10.3389/fmicb.2022.933404
Chen, M., and Blankenship, R. E. (2011). Expanding the solar spectrum used by photosynthesis. Trends Plant. Sci. 16, 427–431. doi:10.1016/j.tplants.2011.03.011
Cockell, C. S. (2022). Bridging the gap between microbial limits and extremes in space: space microbial biotechnology in the next 15 years. Microb. Biotechnol. 15, 29–41. doi:10.1111/1751-7915.13927
Crusan, J., Bleacher, J., Caram, J., Craig, D., Goodliff, K., Herrmann, N., et al. (2019). “NASA’s Gateway: an update on progress and plans for extending human presence to cislunar space,” in IEEE aerospace conference Proceedings, (big sky, MT, USA), 1–19. doi:10.1109/AERO.2019.8741561
De Micco, V., Amitrano, C., Mastroleo, F., Aronne, G., Battistelli, A., Carnero-Diaz, E., et al. (2023). Plant and microbial science and technology as cornerstones to bioregenerative life support Systems in space. NPJ Microgravity 9, 69. doi:10.1038/s41526-023-00317-9
Di Stefano, G., Battistuzzi, M., La Rocca, N., Selinger, V. M., Nürnberg, D. J., and Billi, D. (2024). Far-red light photoacclimation in a desert Chroococcidiopsis strain with a reduced FaRLiP gene cluster and expression of its chlorophyll f synthase in space-resistant isolates. Front. Microbiol. 15, 1450575. doi:10.3389/fmicb.2024.1450575
Fais, G., Casula, M., Sidorowicz, A., Manca, A., Margarita, V., Fiori, P. L., et al. (2024). Cultivation of Chroococcidiopsis thermalis using available in situ resources to sustain life on Mars. Life 14, 251. doi:10.3390/life14020251
Fernandez, B. G., Rothschild, L. J., Fagliarone, C., Chiavarini, S., and Billi, D. (2023). Feasibility as feedstock of the cyanobacterium Chroococcidiopsis sp. 029 cultivated with urine-supplemented moon and mars regolith simulants. Algal Res. 71, 103044. doi:10.1016/j.algal.2023.103044
Gan, F., and Bryant, D. A. (2015). Adaptive and acclimative responses of cyanobacteria to far-red light. Environ. Microbiol. 17, 3450–3465. doi:10.1111/1462-2920.12992
Ho, M. Y., Soulier, N. T., Canniffe, D. P., Shen, G., and Bryant, D. A. (2017). Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr. Opin. Plant Biol. 37, 24–33. doi:10.1016/j.pbi.2017.03.006
Huerta-Cepas, J., Szklarczyk, D., Heller, D., Hernández-Plaza, A., Forslund, S. K., Cook, H., et al. (2019). EggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47 (D1), D309–D314. doi:10.1093/nar/gky1085
Jung, P., Harion, F., Wu, S., Nürnberg, D. J., Bellamoli, F., Guillen, A., et al. (2023). Dark blue-green: cave-inhabiting cyanobacteria as a model for astrobiology. Front. Astron. Space Sci. 10, 1107371. doi:10.3389/fspas.2023.1107371
Kuhn, W. R., and Atreya, S. K. (1979). Solar radiation incident on the Martian surface. J. Mol. Evol. 14, 57–64. doi:10.1007/BF01732367
Lafarga, T., Fernández-Sevilla, J. M., González-López, C., and Acién-Fernández, F. G. (2020). Spirulina for the food and functional food industries. Food Res. Int. 137, 109356. doi:10.1016/j.foodres.2020.109356
Liu, H., Yao, Z., Fu, Y., and Feng, J. (2021). Review of research into bioregenerative life support system(s) which can support humans living in space. Life Sci. Space Res. (Amst) 31, 113–120. doi:10.1016/j.lssr.2021.09.003
Liu, D., Liberton, M., Hendry, J. I., Aminian-Dehkordi, J., Maranas, C. D., and Pakrasi, H. B. (2021). Engineering biology approaches for food and nutrient production by cyanobacteria. Curr. Opin. Biotechnol. 67, 1–6. doi:10.1016/j.copbio.2020.09.011
Mapstone, L. J., Leite, M. N., Purton, S., Crawford, I. A., and Dartnell, L. (2022). Cyanobacteria and microalgae in supporting human habitation on Mars. Biotechnol. Adv. 59, 107946. doi:10.1016/j.biotechadv.2022.107946
NASA (2023). NASA’S Moon to Mars Strategy and Objectives Development - a blueprint for sustained human presence and exploration throughout the Solar system. Available online at: https://www.nasa.gov/wp-content/uploads/2023/04/m2m_strategy_and_objectives_development.pdf (Accessed June 15, 2025).
Ramalho, T. P., Chopin, G., Salman, L., Baumgartner, V., Heinicke, C., and Verseux, C. (2022). On the growth dynamics of the cyanobacterium Anabaena sp. PCC 7938 Martian regolith. NPJ Microgravity 8, 43. doi:10.1038/s41526-022-00240-5
Ramalho, T. P., Baumgartner, V., Kunst, N., Rodrigues, D., Bohuon, E., Leroy, B., et al. (2024). Resource-efficiency of cyanobacterium production on Mars: assessment and paths forward. Algal Res. 84, 103801. doi:10.1016/j.algal.2024.103801
Renaud, C., Leys, N., and Wattiez, R. (2023). Photosynthetic microorganisms, an overview of their biostimulant effects on plants and perspectives for space agriculture. J. Plant Interact. 18, 2242697. doi:10.1080/17429145.2023.2242697
Rothschild, L. J. (2016). Synthetic biology meets bioprinting: enabling technologies for humans on Mars (and Earth). Biochem. Soc. Trans. 44, 1158–1164. doi:10.1042/BST20160067
Rzymski, P., Poniedziałek, B., Hippmann, N., and Kaczmarek, Ł. (2022). Screening the survival of cyanobacteria under perchlorate stress. Potential implications for Mars in situ resource utilization. Astrobiology 22, 672–684. doi:10.1089/ast.2021.0100
Rzymski, P., Klimaszyk, P., Kasianchuk, N., Jakubiak, P., Proch, J., and Niedzielski, P. (2023). Blue on red: chemical conditions of liquid water emerging on simulated Martian regolith. Icarus 389, 115263. doi:10.1016/j.icarus.2022.115263
Rzymski, P., Losiak, A., Heinz, J., Szukalska, M., Florek, E., Poniedziałek, B., et al. (2024). Perchlorates on mars: occurrence and implications for putative life on the red planet. Icarus 421, 116246. doi:10.1016/j.icarus.2024.116246
Sanfilippo, J. E., Garczarek, L., Partensky, F., and Kehoe, D. M. (2019). Chromatic acclimation in cyanobacteria: a diverse and widespread process for optimizing photosynthesis. Annu. Rev. Microbiol. 73, 407–433. doi:10.1146/annurev-micro-020518-115738
Santomartino, R., Averesch, N. J. H., Bhuiyan, M., Cockell, C. S., Colangelo, J., Gumulya, Y., et al. (2023). Toward sustainable space exploration: a roadmap for harnessing the power of microorganisms. Nat. Commun. 14, 1391. doi:10.1038/s41467-023-37070-2
Seemann, T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. doi:10.1093/bioinformatics/btu153
Smith, M., Craig, D., Herrmann, N., Mahoney, E., Krezel, J., McIntyre, N., et al. (2020). “The Artemis Program: an Overview of NASA’s activities to return humans to the Moon,” in IEEE aerospace Conference proceedings, 1–10. doi:10.1109/AERO47225.2020.9172323
Thomas, N., Markiewicz, W. J., Sablotny, R. M., Wuttke, M. W., Keller, H. U., Johnson, J. R., et al. (1999). The color of the Martian sky and its influence on the illumination of the Martian surface. J. Geophys. Res. Planets 104, 8795–8808. doi:10.1029/98JE02556
Veaudor, T., Ortega-Ramos, M., Jittawuttipoka, T., Bottin, H., Cassier-Chauvat, C., and Chauvat, F. (2018). Overproduction of the cyanobacterial hydrogenase and selection of a mutant thriving on urea, as a possible step towards the future production of hydrogen coupled with water treatment. PLoS One 13, e0198836. doi:10.1371/journal.pone.0198836
Veaudor, T., Cassier-Chauvat, C., and Chauvat, F. (2019). Genomics of urea transport and catabolism in cyanobacteria: biotechnological implications. Front. Microbiol. 10, 2052. doi:10.3389/fmicb.2019.02052
Vega, L. (2021). Environmental control and life support (ECLS) systems. In Handbook of bioastronautics L. R. Young, and J. P. Sutton 69–82. doi:10.1007/978-3-319-12191-8_132
Verseux, C., Baqué, M., Lehto, K., De Vera, J.-P., Rothschild, L. J., and Billi, D. (2016). Sustainable life support on Mars - the potential roles of cyanobacteria. Int. J. Astrobiol. 15, 65–92. doi:10.1017/S147355041500021X
Williamson, J., Wilson, J. P., Robinson, K., and Luong, H. (2023). “Status of ISS water management and recovery,” in 52nd International Conference on Environmental Systems 12-16 July 2023 (Calgary, Canada).
Yang, D., Prabowo, C. P. S., Eun, H., Park, S. Y., Cho, I. J., Jiao, S., et al. (2021). Escherichia coli as a platform microbial host for systems metabolic engineering. Essays Biochem. 65, 225–246. doi:10.1042/EBC20200172
Keywords: bioregenerative life support systems, desert cyanobacteria, FaRLiP, in-situ resource utilization, space sustainability
Citation: Santos de Sousa I, Di Stefano G, D’Agostino A, Martella CM, Chirico A, Rigano G, Santo L and Billi D (2025) The potential of far-red light-acclimating cyanobacteria to support sustainable outposts on Mars. Front. Astron. Space Sci. 12:1658632. doi: 10.3389/fspas.2025.1658632
Received: 02 July 2025; Accepted: 20 August 2025;
Published: 18 September 2025.
Edited by:
Josep M. Trigo-Rodríguez, Spanish National Research Council (CSIC), SpainCopyright © 2025 Santos de Sousa, Di Stefano, D’Agostino, Martella, Chirico, Rigano, Santo and Billi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Billi, YmlsbGlAdW5pcm9tYTIuaXQ=
†These authors have contributed equally to this work and share first authorship
 Isabel Santos de Sousa
Isabel Santos de Sousa Giorgia Di Stefano
Giorgia Di Stefano Andrea D’Agostino1,4
Andrea D’Agostino1,4 Antonio Chirico
Antonio Chirico Daniela Billi
Daniela Billi