- 1School of Biomedical Engineering, Capital Medical University, Beijing, China
- 2Beijing Institute of Heart, Lung, and Blood Vessel Diseases, Beijing, China
- 3Emergency and Critical Care Center, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 4Peking University Shenzhen Graduate School, Shenzhen Bay Laboratory, Shenzhen, Guangdong, China
- 5Department of Critical Care Medicine, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, Heilongjiang, China
- 6Medical Image College, Mudanjiang Medical University, Mudanjiang, Heilongjiang, China
Aneurysm, as life-threatening vascular pathologies, are significantly influenced by hemodynamic factors in their development. The combine of numerical simulation and in vitro experiment have laid the foundation for high-precision hemodynamic analysis, while the integration of deep learning technologies has significantly enhanced computational efficiency. However, current researches still face challenges such as limitations in biomimetic materials, and incomplete understanding of mechano-biological coupling mechanisms. In this review, we systematize traditional and emerging methodologies characterizing hemodynamic perturbations across the pathophysiological continuum of aneurysmal expansion, rupture, and thrombosis progression. This review aims to (1) elucidate mechanistic underpinnings of aneurysm destabilization, (2) inspire people to establish standardized quantification protocols for hemodynamic analysis, and (3) pave the way for patient-specific risk stratification enabling data-driven clinical interventions.
1 Introduction
Aneurysms, as a potentially fatal vascular disease, have always been a focus of research in the field of cardiovascular and cerebrovascular diseases. An aneurysm is defined as a pathological bulging that occurs locally in the artery wall. It encompasses multiple types and is typically classified according to anatomical location. These include IAs (Ferns et al., 2011), carotid artery aneurysms (Feng et al., 2020; Welleweerd et al., 2015), CAA (Angelini, 2007), TAA (Senser et al., 2021), AAA (Anagnostakos and Lal, 2021), popliteal artery aneurysms (Jergovic et al., 2022), and other less common site-specific expansive lesions.
Although aneurysms occurring in various anatomical sites all involve structural changes in the arterial wall, they differ significantly in terms of incidence, pathogenesis, and prognosis (Camasão and Mantovani, 2021; Yang et al., 2020). IAs have an incidence rate of approximately 3%–5% in the general population. Such aneurysms typically arise due to abnormal WSS or genetic factors, such as mutations, leading to endothelial cell damage, disruption of the elastic membrane, and subsequent local inflammatory reactions, ultimately weakening the arterial wall (Chalouhi et al., 2013; Ferns et al., 2011; Miyata et al., 2022; Leemans et al., 2019; Sekhar and Heros, 1981). The most severe consequence of IAs is rupture, resulting in subarachnoid hemorrhage and poor clinical outcomes (CHMAYSSANI et al., 2011; Hadad et al., 2024; Hejazi and Phani, 2022). CAA are relatively rare, with an incidence rate between 0.3% and 4.9% among patients undergoing coronary angiography. Atherosclerosis is the most common cause of CAA, involving damage to the medial layer of the vessel wall and breakdown of elastic fibers (Serruys et al., 2023; Gutierrez et al., 2017; Iemura et al., 2000; Gellis et al., 2022). Such aneurysms may lead to severe cardiovascular events, including myocardial infarction and arrhythmia (Taskesen et al., 2021; Conrad et al., 2024). TAA have an annual incidence rate of approximately six per 100,000 individuals and are closely associated with genetic conditions such as Marfan syndrome and Loeys-Dietz syndrome (Senser et al., 2021). These genetic disorders accelerate degenerative changes in the aortic wall by affecting the stability of collagen and elastin proteins. TAA often remain undetected until symptomatic, and rupture results in high mortality. AAA are more common in elderly males over 65 years, with approximately 8% prevalence in this population (Hellmann et al., 2007; Bossone and Eagle, 2021; Heussel et al., 1997). Smoking, hypertension, and atherosclerosis are major risk factors (Tang et al., 2005; Wang et al., 2023). The mortality rate following AAA rupture is exceedingly high, making rupture prevention a primary focus in AAA management (Wang et al., 2024). The risk of rupture increases with aneurysm diameter, and surgical intervention is recommended particularly when the diameter exceeds 5.5 cm (Anagnostakos and Lal, 2021). Despite the differences in clinical manifestations and risk factors, aneurysms across various anatomical sites pose significant threats to patient health and survival. Understanding these diseases contributes to improving diagnostic accuracy and treatment efficiency, reducing patient mortality risks.
Aneurysms occurring at different anatomical locations, differ markedly not only in clinical manifestations but also in lesion structure, flow patterns, and surrounding tissue environments, presenting substantial challenges for precise diagnosis and individualized treatment. Throughout aneurysm formation, expansion, and potential rupture, mechanical factors play indispensable roles. The development of aneurysms is closely related to mechanical factors such as WSS, circumferential stress, and flow stagnation. These factors accelerate aneurysm expansion and rupture by damaging vascular endothelial cells, promoting collagen degradation, or increasing thrombosis risk. Abnormal variations in WSS can cause endothelial cell injury, increased circumferential stress may lead to collagen degradation and vessel wall remodeling, while flow stagnation and vortices frequently induce thrombosis and embolization (Frösen et al., 2019). In CAA, abnormal WSS can weaken the arterial wall, causing localized vessel dilation and elevating rupture risk. In IAs, studies indicate that abnormal hemodynamics, particularly elevated shear stress, are strongly associated with aneurysm formation and rupture. Areas of flow stagnation in IAs, especially at arterial bifurcations, are prone to thrombosis, and subsequent embolization can result in severe complications. Meanwhile, AAA typically involve expansion and weakening of the abdominal aortic wall, closely associated with local changes in circumferential and shear stress. In AAA, elevated circumferential stress can exceed the mechanical strength of the arterial wall, potentially leading to aneurysm rupture.
In summary, current mechanical studies on aneurysmal disease primarily focus on aneurysm expansion, rupture, and thrombosis deposition. Alterations in the hemodynamic environment significantly influence aneurysm expansion rate, rupture risk, and thrombosis formation, dictating disease progression. Thus, elucidating the mechanical mechanisms underlying aneurysm pathogenesis and progression to guide precise intervention strategies remains an urgent issue in cardiovascular research. Figure 1 illustrates the morphological classification of CAA, along with related adverse cardiovascular events and common treatment options. This classification is broadly similar to that of IAs and AAA, which also include dissecting and pseudoaneurysm types. Treatment options for AAA include EVAR, open surgery, and medication to control blood pressure and limit aneurysm growth.
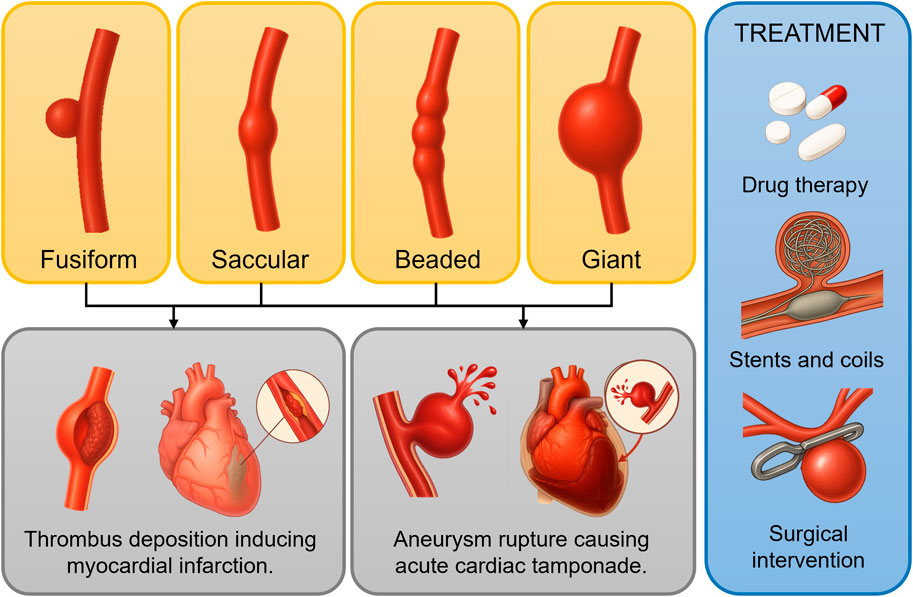
Figure 1. Morphological classification of coronary artery aneurysms (CAAs) and adverse events associated with aneurysm progression. The yellow box shows four morphological classifications of CAAs; the gray box illustrates potential cardiovascular adverse events resulting from thrombus deposition, growth, and rupture; the blue box presents commonly used treatment strategies.
Therefore, this review will focus on recent advances and challenges in aneurysm-related hemodynamic research, including numerical simulations, in vitro experimental measurements, and artificial intelligence-based mechanical analysis methods. Additionally, we will compare the mechanical heterogeneity among aneurysms at different anatomical locations, explore the potential and limitations of various research methods in clinical translation, and propose critical future research directions aimed at enhancing aneurysm diagnosis and treatment.
2 Mechanistic insights into aneurysm progression
2.1 Morphology and mechanics in aneurysm expansion
The coupling between morphological features of aneurysms and local hemodynamics is a critical driver for aneurysm initiation, progression, and rupture. According to anatomical location, aneurysms are classified into CAA, IAs, and AAA. Based on morphological features, they are categorized into fusiform and saccular aneurysms. Common geometric parameters in current studies include directly measured maximum aneurysm diameter (Dmax), length (L), volume (V), surface area (A), curvature (τ), torsion (κ), neck width, neck diameter (DNeck), neck angle, and tilt angle (θ). Three-dimensional vascular geometries are acquired through various imaging techniques such as CTA, MRA, and DSA. To enhance measurement accuracy, two-dimensional image measurement via multiplanar reconstruction from CT images is often cross-validated with three-dimensional geometry measurements. In addition to directly measured geometric parameters, researchers calculate ratio-based parameters like ASI, SR, aspect ratio, sphericity index, UI, bifurcation angle, CE, and LE. Generally, aneurysm morphologies vary across locations, and specific geometric features are more suitable for different aneurysm types. Researchers must select relevant parameters according to their specific research context.
Significant coupling relationships exist between aneurysm geometric parameters and local hemodynamics, and these couplings strongly influence aneurysm expansion. Taking CAA an example, larger aneurysm diameters and volumes typically correlate with reduced TAWSS, which has been linked to accelerated luminal dilatation. Increased aneurysm size also corresponds to higher OSI and RRT, thus aggravating disturbed flow that facilitates wall remodeling and continued enlargement (Zhang et al., 2024). Fusiform aneurysms, characterized by axial elongation, generally experience less flow disturbance yet may still undergo progressive enlargement under sustained circumferential wall stress. In contrast, saccular aneurysms exhibit pronounced flow separation and vortical flow fields due to their distinct neck structures, a pattern associated with localized wall weakening and outward bulging (Rafiei and Saidi, 2022; Zhang et al., 2024). For instance, studies on IAs show substantial reductions in intra-aneurysmal velocity and vortex formation, causing significant enlargement of low-WSS regions that precede measurable diameter growth (Hoi et al., 2004; Hadad et al., 2024). Similarly, in CAA, substantial expansion or neck constriction commonly leads to low-velocity flow areas and widespread low-WSS distribution, further accelerating mural remodeling and progressive dilatation (Singhal and Gupta, 2024; Wong et al., 2020; Hoi et al., 2004). Long-term follow-up of AAA cohorts has revealed that patients exposed to persistently low TAWSS (<0.4 Pa) exhibit mean growth rates exceeding 0.8 mm year-1, underscoring the clinical relevance of the hemodynamic–morphology coupling described above (Bappoo et al., 2021; Faisal et al., 2025). Table 1 summarizes current research on the relationships between morphological parameters and hemodynamic indicators. Figure 2 summarizes the current research progress on the geometryss–mechanicss–biology interplay in aneurysms, illustrating how different geometric features influence the development of aneurysmal disease.
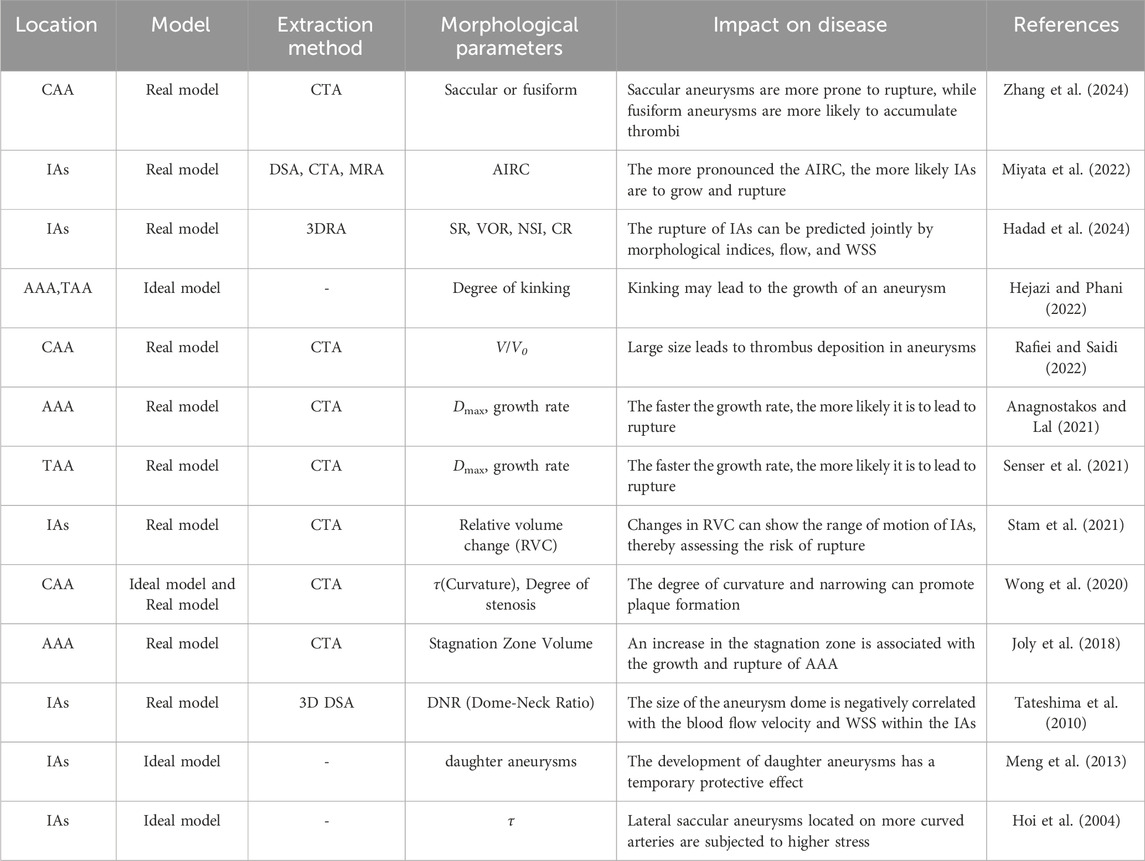
Table 1. Research progress on the relationship between morphological parameters and hemodynamic indicators.
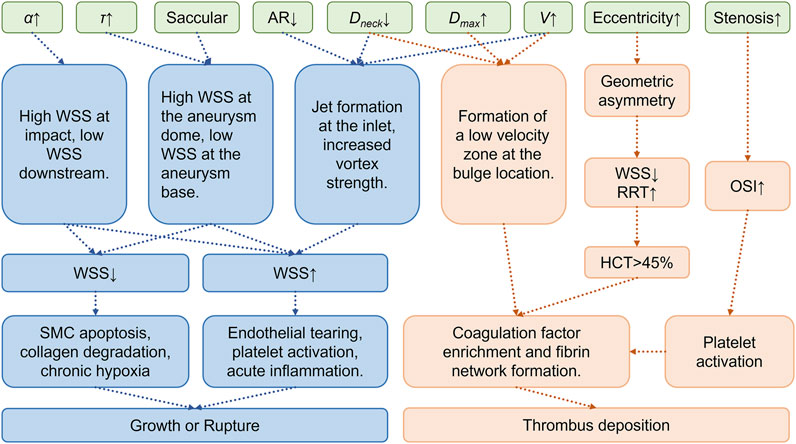
Figure 2. Morphological-mechanical-biological coupling mechanism of aneurysm growth, rupture, and thrombus deposition.Green indicates geometric parameters, blue indicates mechanisms of growth and rupture, and orange indicates mechanisms of thrombus deposition.α: Bifurcation angle, τ: Curvature, AR: Aspect Ratio, Dmax: Maximum aneurysm diameter, Dneck: Neck diameter, V: Aneurysm volume, HCT: Hematocrit.
Currently, CFD numerical simulations are the primary research method, quantitatively analyzing aneurysm hemodynamics through solving velocity, pressure, and WSS-related fields. Additional indices, such as helicity and cross-flow index (CFI), evaluate flow multidirectionality and turbulence’s effects on aneurysm walls, enriching morphology-dynamics coupling analyses. As research advances, multi-scale patient-specific modeling and precise CFD simulations become prevalent, enhancing morphological parameters’ value for clinical risk prediction and therapeutic strategy development.
2.2 Hemodynamic of aneurysm rupture
Aneurysm rupture is a complex process involving multiple mechanical and biological mechanisms. Endothelial dysfunction and abnormal local blood flow—characterized by pronounced flow deceleration, recirculation, and vortices—create hemodynamic environments that lower WSS and raise OSI, ILT formation and setting the stage for wall failure (Wang et al., 2024; Al-Jumaily et al., 2023). These abnormal flow patterns decrease WSS and increase OSI, promoting thrombus formation and reducing endothelial cell viability. Low-WSS areas undergo chronic hypoxia, triggering inflammatory cell aggregation, elevated MMP expression, and apoptosis, ultimately causing arterial wall structural degeneration, including elastin and collagen degradation, thereby critically weakening the wall and predisposing it to rupture (Arslan and Salman, 2023; Belkacemi et al., 2023; Philip et al., 2022). Enlarged aneurysm size is often accompanied by markedly reduced TAWSS and elevated OSI and RRT; these hemodynamic changes promote ILT deposition, intensify wall hypoxia, and therefore markedly elevate rupture risk, as demonstrated for CAA, IAs, and AAA (Zhang et al., 2024). In AAA, rupture usually occurs in weakened wall regions such as posterior or lateral walls (Singh et al., 2021; Golledge, 2019), whereas IAs commonly rupture at inflow impingement sites or arterial bifurcations (Hadad et al., 2024; Miyata et al., 2022). Saccular morphologies with narrow necks concentrate inflow jets, generating high-WSS impingement zones that can coexist with surrounding low-WSS regions, collectively accelerating structural degradation and precipitating rupture (Rafiei and Saidi, 2022). Conversely, fusiform aneurysms may rupture after prolonged periods of progressive dilatation when mural stress exceeds tensile strength despite relatively smoother core flow patterns. Peak wall stress (PWS) plays a critical role in rupture events, with high-PWS areas indicating mechanical stress concentration, increasing local degeneration and rupture likelihood (Singh et al., 2021).
The role of shear stress in aneurysm rupture remains controversial. The high WSS hypothesis suggests that high shear forces, particularly in IAs, directly damage endothelial cells, triggering acute inflammatory responses and increasing rupture risk (Zhang et al., 2019; Wang et al., 2018; Cho, 2023). Conversely, the low WSS hypothesis emphasizes chronic pathologies induced by low shear regions, particularly in AAA, where long-term hypoxia-driven matrix degradation and endothelial dysfunction are primary rupture triggers (Zhang et al., 2016; Miura et al., 2013; Boyd et al., 2016; Zhou et al., 2017). Recent studies indicate significantly increased rupture risks when WSS exceeds a threshold (12.3 dyne/cm2), especially in anterior communicating artery aneurysms (ACoA), with rupture risks multiplying per unit increase of WSS (Miura et al., 2013). High WSS regions involve concentrated flow impingement, mechanical endothelial damage, and significant inflammatory responses, accelerating MMP release and matrix degradation, ultimately compromising wall strength and structural integrity. Low WSS mechanisms first manifest as reduced and oscillatory blood flow (increased OSI), promoting ILT formation (Wang et al., 2018). Thrombus deposition exacerbates wall hypoxia, endothelial dysfunction, and chronic inflammation, including macrophage and neutrophil aggregation and MMP release, accelerating vascular matrix degradation and smooth muscle cell apoptosis (Zhang et al., 2019). Moreover, low WSS diminishes endothelial protective functions by reducing NO production, exacerbating inflammation and vessel wall fragility (Miura et al., 2013; Zhang et al., 2016). Numerical simulations and clinical evidence further suggest chronic low WSS environments induce inflammation and hypoxia, structurally damaging aneurysm walls, ultimately causing mechanical instability and rupture under repetitive blood pressure fluctuations (Boyd et al., 2016; Zhou et al., 2017). However, studies also highlight dynamic changes in relationships between WSS magnitude and aneurysm expansion and rupture. Early aneurysm expansion stages might exhibit high WSS, transitioning to low WSS dominance as geometry evolves, forming chronic hypoxia and inflammatory conditions, ultimately leading to rupture (Singh et al., 2021; Wang et al., 2018; Zhou et al., 2017; Cho, 2023). This shift from high to low WSS underscores WSS’s varying influence across aneurysm development stages.
Although CFD and FSI numerical simulations have significantly advanced our understanding of aneurysmal hemodynamics, how precisely high and low WSS differentially drive aneurysm expansion and rupture remains unclear, necessitating integrated research involving clinical imaging and pathology for further elucidation.
2.3 Thrombus deposition dynamics
The formation of ILT is a pathological process influenced by complex hemodynamic and biological mechanisms (Stark and Massberg, 2021; Hathcock, 2006; Mihalko and Brown, 2020). Currently, two distinct mechanical hypotheses, namely, the low-WSS hypothesis and the high-WSS hypothesis, explain aneurysm thrombosis formation, each with significant mechanistic differences and ongoing debates.
The low-WSS hypothesis emphasizes that regions of significantly reduced or stagnant blood flow within aneurysms often accompany vortex, recirculation, and swirl flow structures (Buck et al., 2018; Deplano and Siouffi, 1999). For example, Cao et al. performed numerical simulations on Kawasaki Disease (KD) coronary artery aneurysms, demonstrating that low TAWSS, high OSI, and high RRT significantly increased thrombotic risk, with thrombi frequently located at the proximal and myocardial sides of aneurysms (Cao et al., 2023). Additionally, another study by Cao et al. (2022) indicated that the combined presence of low TAWSS, high OSI, and high RRT notably enhanced thrombus formation risks in KD coronary aneurysms. Under low-WSS conditions, the blood velocity is extremely low, extending the residence time of RBC, platelets, and coagulation factors (Van der Waerden et al., 2025; Millon et al., 2015). Prolonged stagnation decreases local oxygen levels, triggering endothelial cell dysfunction characterized by significantly diminished secretion of antithrombotic substances like NO and prostacyclin (PGI2), thereby reducing normal anticoagulant function. Hypoxia and prolonged low shear stress enhance endothelial cell expression of adhesion molecules, intensifying platelet-endothelial adhesion (Millon et al., 2015). Stagnant blood flow results in the local accumulation of coagulation factors, triggering and amplifying the coagulation cascade reaction, eventually forming stable fibrin networks and progressively larger thrombotic structures (Zhou et al., 2023).
The high-WSS hypothesis emphasizes abnormally high shear forces at aneurysm entrances or local stenotic areas, leading to endothelial cell damage and rapid platelet adhesion and aggregation (Casa and Ku, 2017). According to the high-WSS theory, aneurysm entrances or localized narrow regions exhibit significantly elevated blood flow velocity and shear stress (Dolan et al., 2013). High shear conditions continuously expose endothelial cells to mechanical stimuli, resulting in mechanical damage or activation (Sho et al., 2002; Masuda et al., 1999; Sho et al., 2003). Under high shear stress, vWF molecules undergo mechanical stretching, increasing binding sites for platelet glycoprotein receptors (GP Ib), significantly enhancing rapid platelet adhesion and aggregation onto damaged endothelium, forming initial platelet-rich thrombotic cores (Tada et al., 2011; Tada et al., 2010). Furthermore, endothelial injury exposes procoagulant substances such as subendothelial collagen fibers, rapidly initiating thrombogenesis. Sustained mechanical stress and endothelial damage trigger local inflammatory responses, releasing inflammatory mediators (such as ADP) and tissue factors, further accelerating the coagulation cascade.
The low-WSS and high-WSS mechanisms differ in focus: low-WSS emphasizes gradual thrombus deposition due to blood stagnation, whereas high-WSS highlights rapid initial platelet aggregation due to mechanical injury. Although both mechanisms likely coexist clinically, detailed exploration of their respective mechanisms and clinical significance is crucial for comprehensively understanding aneurysm thrombosis and developing precise intervention strategies.
The mechanism of thrombus deposition also demonstrates significant anatomical specificity. For example, in intracranial aneurysms, dynamic thrombus deposition and shedding directly influence downstream embolization risk (Ridker and Rane, 2021; Malek et al., 1999; Deplano and Siouffi, 1999). Conversely, thrombus deposition in AAA temporarily alleviates mechanical stress on aneurysm walls but may eventually contribute to further wall weakening (Wang et al., 2023; Xu et al., 2023; Hayashi et al., 2006). Studies on abnormal flow parameters and thrombus formation in coronary artery aneurysms have gained considerable attention. For instance, Woźniak et al. (2024) confirmed significant associations between low WSS, high OSI, high RRT, and thrombus deposition in coronary aneurysms.
2.4 Risk stratification of aneurysms
Aneurysm risk stratification in contemporary clinical practice has evolved from a “one-size-fits-all” diameter paradigm toward nuanced, disease-specific schemes that integrate demographics, imaging surrogates and, increasingly, biologic read-outs of wall vulnerability.
For IAs, prospective outcome cohorts underpin three complementary tools. The PHASES score translates six readily available variables—population, hypertension, age, diameter, prior subarachnoid haemorrhage and site—into an absolute 5-year rupture probability that ranges from <0.5% (score 0–2) to ≈18% (score ≥13), and is now embedded in both European and North-American guidelines (Greving et al., 2014). Growth prediction is addressed by the ELAPSS score, in which history of rupture, aneurysm location, age, population, morphology and size yield a 5-year enlargement risk exceeding 10% once the sum reaches 12 points; this facilitates personalized surveillance intervals and early endovascular referral (Backes et al., 2017). When therapeutic equipoise persists, the multidisciplinary UIATS consensus model quantifies 29 patient-, aneurysm- and treatment-related factors on two opposing columns; a net difference of ±3 provides a reproducible threshold either for intervention or watchful waiting (Etminan et al., 2015; Backes et al., 2017). Beyond morphology, high-resolution vessel-wall MRI has introduced the aneurysm-to-pituitary-stalk contrast ratio; a value ≥0.5 denotes circumferential wall enhancement that correlates with inflammatory cell infiltration and identifies unstable lesions even when diameter is small (Wu et al., 2022).
For AAA, the maximal anteroposterior diameter remains the cornerstone: elective repair is advocated at ≥55 mm in men and ≥50 mm in women, or earlier when yearly growth surpasses 10 mm, as codified by the 2022 Society for Vascular Surgery guideline update (Chaikof et al., 2018). Yet diameter alone incompletely captures wall frailty. Finite-element modelling shows that a PWS >200 kPa—or, more sensitively, a peak wall rupture index (PWRI) elevated relative to patient-specific wall strength—distinguishes ruptured from size-matched intact aneurysms (Singh et al., 2021). Concomitantly, the burden and geometry of intraluminal thrombus have emerged as pivotal modifiers: posterior thrombus thickness >10 mm or a volumetric occupancy >40% accelerates hypoxic medial degeneration and triples rupture odds despite lowering computed wall stress, underscoring the dual biomechanical-biological nature of this substrate (Haller et al., 2018). Thoracic aortic disease follows a size-indexed logic tempered by genotype and growth rate. In patients with tricuspid aortic valves the current ACC/AHA statement recommends surgery once the ascending aorta reaches 55 mm, but lowers the threshold to 50 mm—or an aortic cross-sectional area/height ratio >10 cm2 m-1—for Marfan, Loeys-Dietz or familial forms, and mandates expedited repair when expansion exceeds 3 mm year-1 (Isselbacher et al., 2022).
Risk models for CAA secondary to KD rely on body-surface-normalised Z-scores: aneurysms with Z < 5 usually regress; those with Z 5–10 persist and entail chronic antiplatelet therapy; and “giant” lesions (Z ≥ 10 or absolute diameter ≥8 mm) carry a ≥20% 5-year thrombosis/myocardial-infarction risk, warranting lifelong anticoagulation and advanced imaging surveillance (Kato et al., 2023; McCrindle et al., 2017).
Collectively, these disease-specific stratification frameworks illustrate how clinical decision-making now weaves together absolute size, dynamic growth, composite risk scores, biomechanical metrics and imaging biomarkers to delineate a personalised trajectory from benign dilatation to catastrophic rupture or occlusion.
3 Current status of research methods on hemodynamic characteristics
3.1 Numerical simulations
CFD or FSI simulations are common tools to characterize hemodynamic properties within aneurysms. Recently, CFD methods have been widely applied in cardiovascular diseases such as coronary artery stenosis, aortic aneurysms, and cerebral aneurysms (Feng et al., 2020; Fan et al., 2019a; Feng et al., 2018). Figure 3 summarizes representative studies based on numerical simulation methods that investigate thrombus deposition, expansion, and rupture of aneurysms, including both idealized models and patient-specific vascular models.
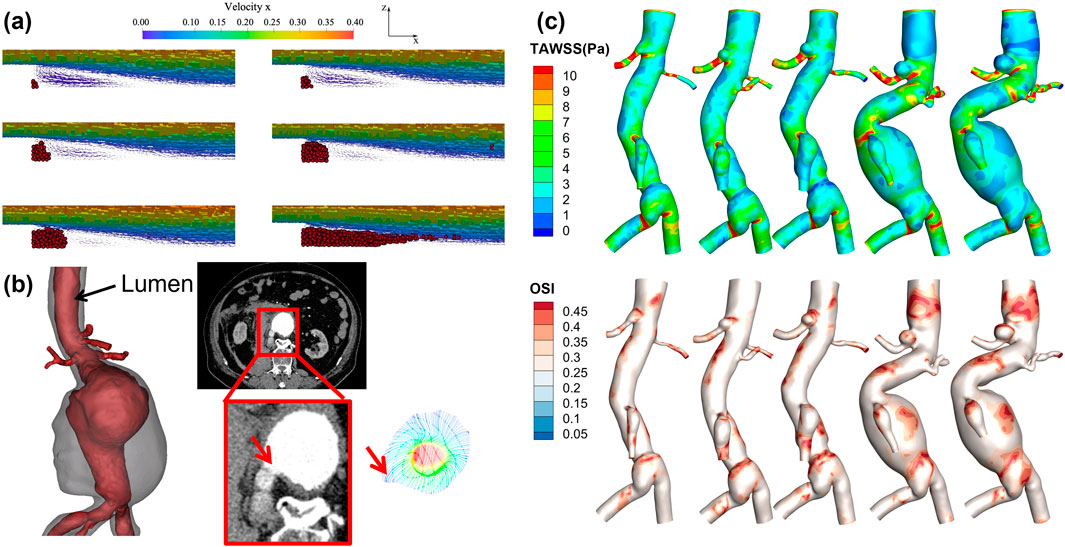
Figure 3. Numerical simulation-based study of thrombus formation and the progression and rupture of aneurysms. (a) is based on the Smoothed Particle Hydrodynamics method for simulating thrombus deposition processes in a backward step model (Monteleone et al., 2023); (b) Velocity field simulation at the rupture site of an abdominal aortic aneurysm, with the red arrow indicating the rupture point; (c) Aneurysm growth over five follow-ups in the same patient, along with contour plots of TAWSS and OSI.
The standard procedure for CFD simulations typically begins with the three-dimensional reconstruction of arterial geometry using medical imaging data (e.g., CTA, MRI, Micro-CT, or DSA) to obtain patient-specific anatomical models. The accuracy of vascular geometry reconstruction directly influences simulation outcomes. Multi-threshold segmentation techniques combined with manual corrections are generally employed to ensure geometric models are consistent with actual anatomical structures. During the meshing process, tetrahedral or polyhedral meshes are frequently used, supplemented by prism layer refinement to adequately capture wall shear layers and near-wall turbulent characteristics. The mesh element count typically ranges from hundreds of thousands to millions. However, current mesh sizes are often limited to scales of several hundred micrometers, which may fail to accurately capture small-scale flow features, particularly in regions sensitive to WSS, such as aneurysm cavities or vascular bifurcations, potentially introducing errors (Ren et al., 2022; Le et al., 2013; Kandangwa et al., 2022). In selecting algorithms for hemodynamic simulations, CFD usually employs the FVM to solve Navier–Stokes equations. Common spatial discretization schemes include the second-order upwind scheme and central difference method. Typical temporal integration methods include semi-implicit Euler schemes, BDF2, and other high-order implicit or semi-implicit methods to ensure simulation stability and accuracy. In complex flow regions such as AAA or intracranial aneurysms, LES methods are also utilized to capture richer turbulent structures (Vergara et al., 2017; Lancellotti et al., 2017).
Regarding the assumption of blood fluid properties, most studies still adopt the Newtonian fluid assumption, considering blood viscosity as a constant value (approximately 4–4.5 mPa·s) (Feng et al., 2020; Fan et al., 2019b). However, studies indicate that this simplification might introduce significant errors in low-flow regions or aneurysm vortex areas, failing to accurately simulate shear-thinning effects of blood, thereby affecting precise calculation of parameters like WSS and turbulence indices. Consequently, Non-Newtonian fluid models (such as Carreau or Carreau-Yasuda models) are gaining attention in research to more accurately represent blood rheological properties (Salman et al., 2019; Nørgaard et al., 2014; Miranda et al., 2021).
Currently, simulations commonly assume arterial walls as rigid structures, neglecting their actual deformable characteristics. This assumption is clearly limited, particularly given the significant histological differences between arterial regions. For example, coronary artery walls are relatively thin with notable tissue elasticity; cerebral artery walls, rich in elastic fibers, are relatively fragile; and aortic walls have a thicker elastic medial structure. Neglecting these tissue characteristics and structural differences can lead to errors in predicting hemodynamic parameters (e.g., pressure gradients, WSS, vortex stability). To enhance simulation accuracy, FSI methods have increasingly become a research trend. Unlike traditional CFD, which focuses solely on blood flow, FSI couples interactions between blood and arterial walls, thereby providing more realistic blood flow-wall deformation interaction models (Mendez et al., 2018; Lin et al., 2017). Typical FSI applications include stress redistribution after stent implantation, aortic valve opening and closing processes, and aneurysm wall rupture risk prediction (Pavlin-Premrl et al., 2021; Miranda et al., 2021; Pinho et al., 2019). For example, after coronary stent implantation, FSI can more accurately simulate stress distribution between the stent and vascular wall and the vessel remodeling process, offering more clinically valuable evaluations.
In determining boundary conditions, traditional methods commonly employ typical pressure/flow waveforms of the aorta or coronary arteries from clinical measurements or literature as inlet conditions (Huo et al., 2012; Cao et al., 2021; Tang et al., 2020; Salman et al., 2019). Outlet conditions generally adopt zero-pressure or simple linear resistance models. However, these simplified boundary conditions cannot fully reflect the physiological characteristics of the actual microcirculation or downstream vascular bed, possibly leading to over- or under-estimations of local hemodynamic characteristics. Some studies have begun incorporating more physiological Windkessel or other distributed parameter models to obtain more precise simulation results at outlet boundary conditions (Wang et al., 2023; Van der Horst et al., 2013; Sommer et al., 2022; Mei et al., 2020).
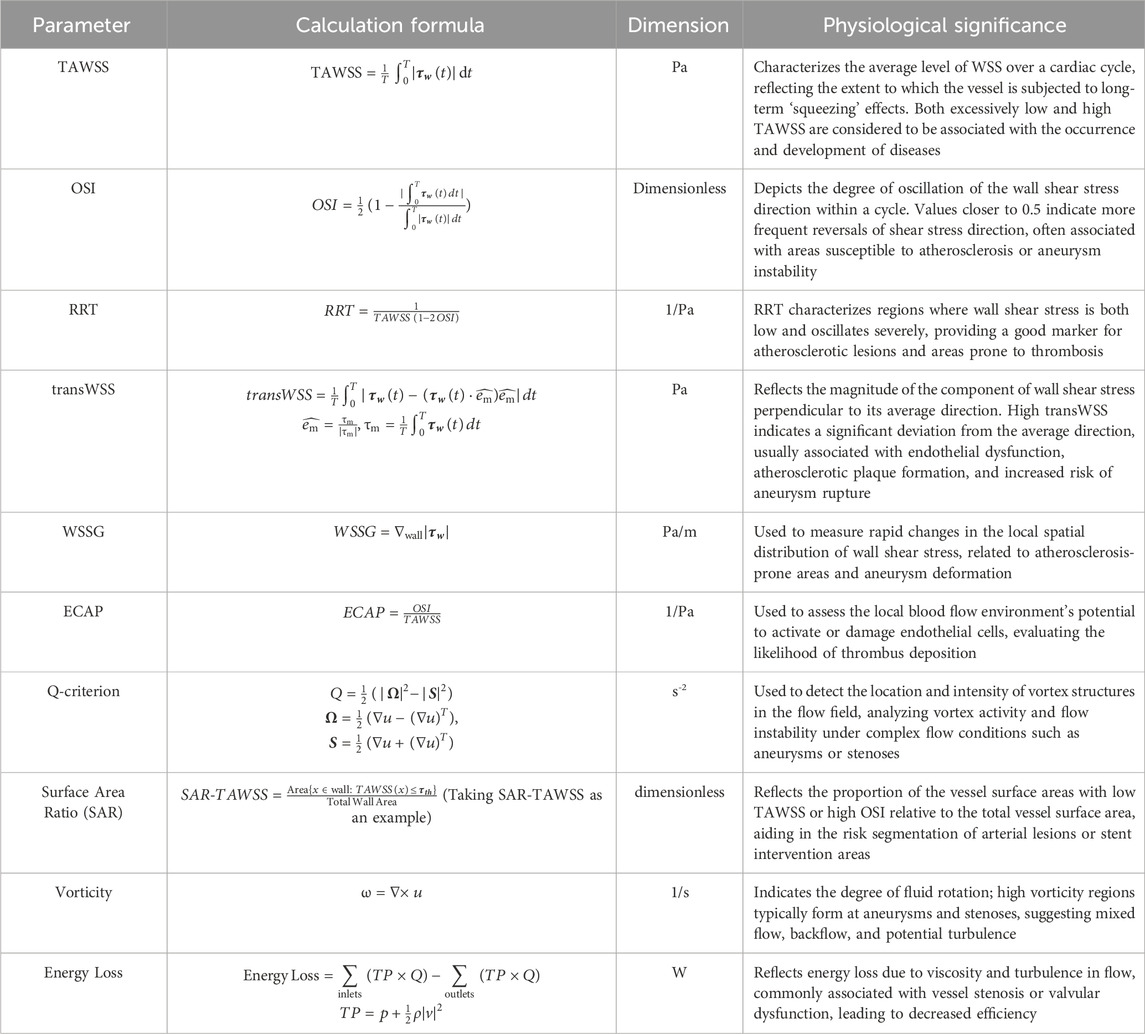
Concerning hemodynamic parameters, current studies mainly focus on WSS, TAWSS, OSI, RRT, vortex criteria (e.g., Q-criterion), ECAP, EL, among others. Accurate selection and prediction of these parameters have significant implications for clinical risk assessment. Table 2 summarizes commonly used hemodynamic indices, their formulas, and physiological significance. The accurate prediction of hemodynamic indices strongly depends on mesh precision, fluid model selection, wall boundary conditions, and outlet condition settings, and their reliability requires further validation. Present simulation method limitations mainly manifest in two areas: firstly, excessive dependence on boundary conditions, where uncertainties or simplifications in inlet and outlet conditions might skew results; secondly, insufficient clinical measurements to rigorously validate numerical simulation outcomes. Thus, future research should enhance coupling validation between experimental and numerical simulations, fully integrating medical imaging data, flow and pressure measurements, and physiological parameters to develop more refined FSI models and boundary conditions.
3.2 In Vitro experiment
Simulations based solely on CFD may easily lead to inaccurate results due to differences in vascular model processing methods or boundary condition settings by different researchers or clinicians. Consequently, verification with in vitro MCL systems becomes an essential auxiliary step, helping to confirm the fidelity of CFD results. Chen et al. (2022); Liang et al. (2022) study validated the accuracy of CFD numerical simulations using an in vitro modeling approach. The results showed that the average flow distribution ratio (FDR) difference between the CFD simulations and the standard data was 2.4% ± 1.70%. The comparison primarily employed paired t-tests to assess the statistical differences between the two methods (with a significance level set at p < 0.05), thereby confirming that, with proper selection of patient-specific geometries and physical properties (such as using a hyperelastic material model), the CFD simulation results regarding pressure waveforms and flow distribution are highly consistent with the in vitro experimental data.
The basic procedure of in vitro simulation experiments typically includes obtaining patient-specific vascular geometrical data through medical imaging (e.g., CT or MRI), followed by personalized 3D reconstruction using software such as Geomagic Wrap, and then manufacturing the vascular model via 3D printing. Silicone materials, such as Sylgard 184, are commonly used to construct vascular models, exhibiting an elastic modulus between two and 8 MPa, a Poisson’s ratio of approximately 0.49, and a density around 1,060 kg/m3 (Liang et al., 2022; Chen et al., 2022). Alternatively, transparent Plexiglass can be utilized for flow visualization, with an elastic modulus ranging from 2.5 to 3 GPa and a Poisson’s ratio of approximately 0.35 (Liang et al., 2022). Blood analog fluids typically comprise glycerin-water solutions (density: 1,050–1,060 kg/m3; viscosity: 3.5–4.1 mPa·s) to replicate the rheological properties of blood (Jamiolkowski et al., 2020; Espa et al., 2019).
The distal vascular resistance and compliance of the vascular system are usually modeled using a three-element Windkessel model (Diamond and Forrester, 1972; Shaw et al., 2002). The compliance chamber utilizes the compressibility of air to simulate vascular compliance, whereas the glycerin-water solution flow simulates the viscous resistance of blood flow (Bardi et al., 2024; Salman et al., 2019; Kolli et al., 2016; Liang et al., 2023). Adjusting the pressure and volume within the air chamber precisely controls system compliance, thus simulating vascular elasticity characteristics under various physiological or pathological conditions (Shaw et al., 2002; Alfonso et al., 1994). After establishing the MCL, experimental data such as velocity fields can be measured using PIV or Doppler ultrasound, and real-time pressure waveforms, WSS, and flow rate data can be recorded using pressure sensors (Jamiolkowski et al., 2020; Espa et al., 2019; Rotman et al., 2019). The experimental data can be processed by filtering techniques (e.g., Savitzky-Golay filter) to remove high-frequency noise originating from the experimental setup and environment, ensuring accurate comparisons between experimental and CFD simulation results (He et al., 2024; Liang et al., 2022; Zhao et al., 2021; Jamiolkowski et al., 2020). This approach provides precise evidence for subsequent aneurysm diagnosis and treatment. However, it is noteworthy that many researchers currently restrict their focus to local vascular regions for in vitro validation or CFD simulations. Since the experimental scope significantly impacts simulation and numerical results, such as WSS and velocity distributions, attention should be paid to incorporating broader vascular pathways rather than being limited solely to lesion sites during numerical and experimental analyses. Figure 4 illustrates the general procedure and results processing of in vitro modeling.
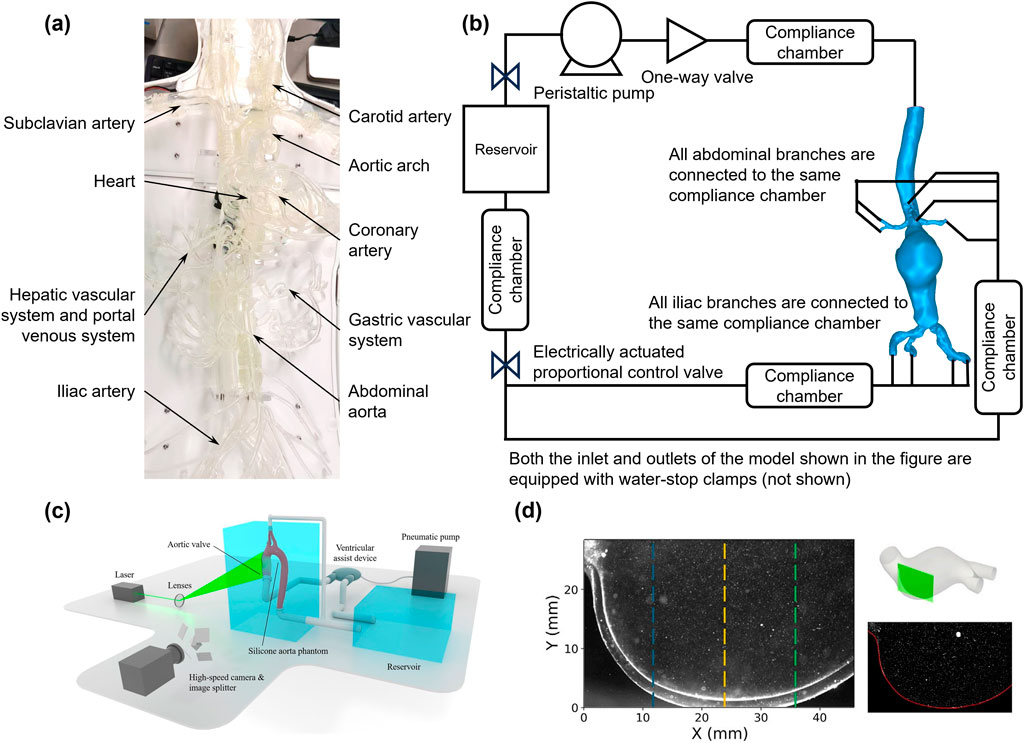
Figure 4. Experimental methods for hemodynamic analysis using physical models. (a) Setup of a physical in vitro vascular model; (b) Simulated circulation loop for an abdominal aortic aneurysm; (c) Schematic diagram of the 3D-PIV setup, consisting of a simulated circulation loop with electrical and optical components (Zeugin et al., 2024); (d) LED-PIV experimental results for two regions of interest in a patient’s actual AAA model (Bardi et al., 2024).
Nevertheless, in vitro simulations still present several limitations. Existing materials struggle to fully replicate the nonlinear elasticity, anisotropy, and biological responses of real blood vessels. Furthermore, accurately simulating the mechanical characteristics of vascular intima, media, and adventitia, as well as plaque and thrombus, remains challenging in vitro. Therefore, investigating more realistic in vitro simulation materials is a crucial area for future exploration.
3.3 4D-flow MRI
4D-flow MRI has emerged as a non-invasive imaging modality for hemodynamic evaluation, providing time-resolved, three-dimensional velocity fields across the cardiac cycle through the application of velocity-encoding gradients integrated into phase-contrast MR imaging (Bissell et al., 2023). This technique allows for comprehensive quantification of hemodynamic parameters, including velocity, WSS, OSI, RRT, and pulse wave velocity (PWV), making it highly suitable for detailed assessment of complex flow patterns encountered in intracranial aneurysms (Peng et al., 2025; Schnell et al., 2012; Liu et al., 2018).
Recent studies have validated the accuracy and reliability of 4D-flow MRI-derived hemodynamic parameters through comparison with numerical simulation, in vitro experiment, and standard clinical imaging protocols. For example, Liu et al. (2018) developed an accelerated 4D-flow MRI approach combining advanced undersampling (CIRCUS) with compressed sensing reconstruction, achieving high temporal resolution (below 30 m) within clinically feasible scan times (∼5 min). Their work demonstrated robust qualitative and quantitative agreement with conventional imaging methods in both healthy volunteers and intracranial aneurysm patients, highlighting its ability to accurately visualize complex flow structures such as intra-aneurysmal vortices and recirculating flow patterns (Liu et al., 2018). Furthermore, Ferdian et al. (2022) introduced WSSNet, a deep-learning model specifically designed to improve WSS estimation accuracy from clinical-resolution 4D-flow MRI data, which significantly correlated with CFD-derived results (r = 0.92), addressing key challenges related to spatial resolution limitations.
Clinical applications of 4D-flow MRI have expanded significantly, particularly within aneurysm 00research. Misaki et al. (2021) validated the clinical utility of intracranial 4D-flow MRI by demonstrating strong correlations between MRI-derived flow parameters and CFD-simulated results, particularly in predicting rupture risk associated with abnormal hemodynamic features in aneurysms. Similarly, Koizumi et al. (2024) introduced the aneurysm damping index (ADI), an innovative parameter derived from 4D-flow MRI data, quantifying flow-induced mechanical energy attenuation within aneurysms, thereby offering new insights into aneurysmal wall compliance and stiffness, important markers for aneurysm stability. Brindise et al. (2019) further confirmed the robustness of dimensionless hemodynamic indices such as OSI across various modalities, underscoring the potential of 4D-flow MRI to reliably capture clinically relevant hemodynamic metrics even under spatial resolution constraints.
Looking forward, advancements in acquisition and reconstruction techniques, including multi-VENC encoding, compressed sensing, and machine-learning-based image reconstruction, will likely further enhance the capability of 4D-flow MRI to detect subtle yet clinically meaningful flow disturbances (Bissell et al., 2023; Brindise et al., 2019; Gao et al., 2019; Liu et al., 2018). These developments, coupled with ongoing standardization efforts detailed in recent expert consensus documents, suggest a promising trajectory toward widespread clinical adoption. Consequently, 4D-flow MRI is anticipated to play a pivotal role in future intracranial aneurysm management by improving patient-specific risk stratification, facilitating targeted therapeutic decisions, and enabling more precise longitudinal follow-up of aneurysm progression.
3.4 New techniques for predicting flow fields: deep learning
As mentioned in the previous section, obtaining more accurate simulation results requires developing more comprehensive mechanical models and precise boundary conditions. However, this approach significantly increases computational cost and time consumption, severely limiting clinical applications. Balancing computational efficiency and accuracy of results remains a critical issue for researchers.
In recent years, the integration of DL and CFD has become an essential trend in hemodynamic research. Although traditional CFD simulations hold irreplaceable precision in biomedical engineering, the incorporation of DL methods, particularly frameworks such as PINN, CNN, and GCN, has enabled researchers to predict and analyze hemodynamics quickly and accurately. Figure 5 summarizes current studies on aneurysmal hemodynamic characteristics based on deep learning methods, including flow field generation using deep learning, automatic segmentation and detection of aneurysm morphology from medical images, and risk prediction models for aneurysms. In coronary artery simulation, Alzhanov et al. (2024a), Alzhanov et al. (2024b) developed a hybrid CFD-PINN framework. By embedding the incompressible Navier-Stokes equations directly into the neural network training process, their method ensures mass and momentum conservation during training. This approach not only delivers accurate individualized predictions but also significantly reduces computational costs, representing a promising non-invasive functional diagnostic tool. Meanwhile, Suk et al. (2024a), Suk et al. (2024b) proposed a novel method based on SE (3)-equivariant Graph Convolutional Networks (GEM-GCN). By explicitly considering the local geometry and connectivity of mesh surfaces and using group-equivariant convolutions, this approach effectively captures detailed flow characteristics on vascular surfaces, enabling rapid and precise estimation of coronary artery WSS. This method demonstrates robustness to complex topologies and arbitrary spatial transformations, achieving approximately 7.6% error, indicating strong potential for clinical real-time assessment. Additionally, a comprehensive image-to-flow processing pipeline has received increased attention. Yao et al. (Yao et al., 2024) developed the Image2Flow network, ingeniously combining 3D CNN and GCN to directly segment pulmonary arteries from cardiac MRI images and estimate corresponding CFD flow fields rapidly and automatically. The significant advantage lies in its extremely fast processing (only hundreds of milliseconds) while maintaining good balance between segmentation accuracy (Dice ≈0.9) and flow prediction error (∼10%), promising widespread application in cardiopulmonary disease diagnosis and treatment processes.
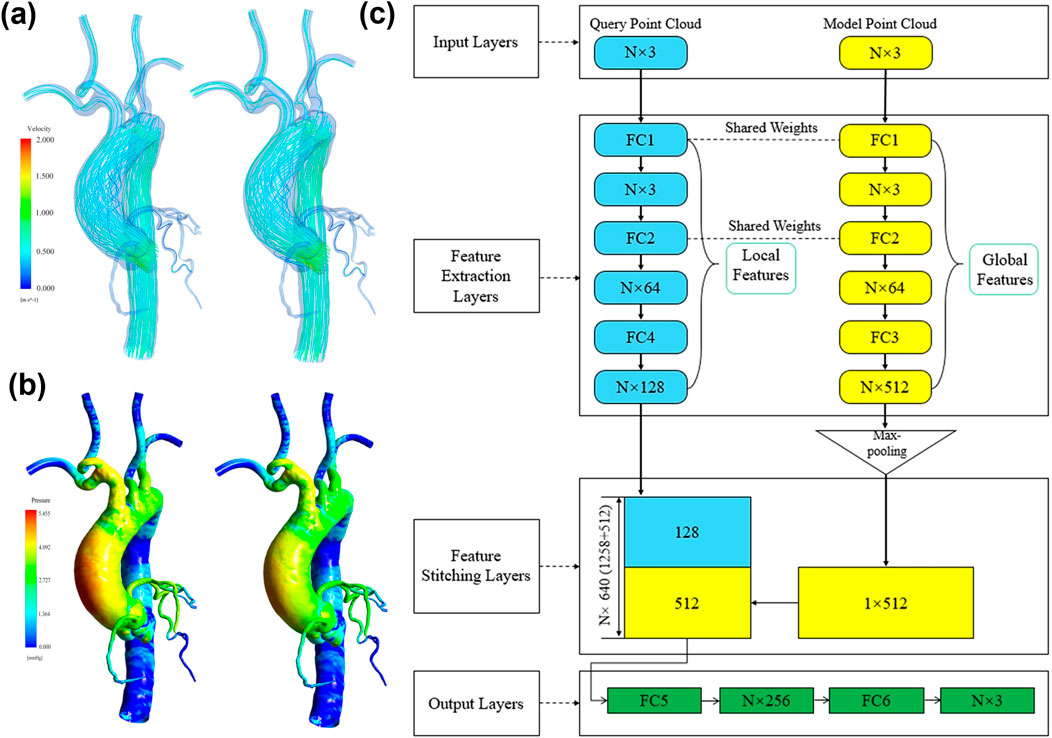
Figure 5. Deep learning-based reconstruction of coronary and aortic flow fields. (a) Velocity field reconstruction: CFD results on the left, deep learning results on the right; (b) Wall shear stress reconstruction: CFD results on the left, deep learning results on the right; (c) Deep learning network architecture for flow field reconstruction proposed by (Li et al., 2021).
Moreover, research efforts also focus on combining CFD simulations with structural analysis and machine learning to enhance clinical predictions of disease risk. Siogkas et al. (2024) integrated CFD-derived hemodynamic indices with structural simulation-derived local stresses to predict carotid plaque rupture risk using GBT, achieving diagnostic accuracy up to 88%. This model utilizes realistic pulsatile blood flow boundary conditions generated from 3D MRI data and ultrasound-measured flow waveforms, accurately simulating physiological environments, offering practical value for stroke risk prediction. Furthermore, considering the clinical importance of iFR, Liu J. et al. (2024), Liu X. et al. (2024) introduced a simplified model combining DL and physiological regulatory mechanisms to rapidly estimate non-invasive iFR. Trained on real clinical patient coronary CTA data and high-precision CFD simulations, their deep neural network predicts resistance due to coronary stenosis. Coupled with microcirculation auto-regulatory mechanisms, this method enables fast, non-invasive iFR (iFRCT) calculation with a diagnostic accuracy of up to 88.3%. Explicit consideration of physiological regulatory mechanisms enhances the physiological plausibility and clinical applicability of this model.
The studies mentioned above also highlight an increasing emphasis on interpretability. For example, the hybrid CNN-GCN model by Yao et al. (Yao et al., 2024) explicitly integrates spatial and vascular morphology information, providing a geometric perspective of interpretability, despite the absence of detailed feature attribution analysis. In contrast, Alamir et al. (2024) utilized integrated gradient methods to attribute neural network features, explicitly revealing the significance of vessel radius and inflow velocity in determining coronary artery WSS distributions. These approaches shift DL models from complete “black boxes” towards interpretability, supporting clinical decision-making more effectively. However, it is noteworthy that most researchers developing DL algorithms have not utilized high-fidelity simulations during dataset preparation, such as neglecting pulsatile flow, non-Newtonian blood properties, or realistic boundary conditions. For DL to achieve more precise blood flow simulations, high-fidelity simulation datasets or actual patient-measured data are necessary prerequisites. Additionally, many studies train models on local hemodynamic data while ignoring accuracy on large-scale models. Given physiological considerations, rapid flow field computation, lesion detection, and risk prediction for large-scale vessels (such as intracranial arteries, coronary arteries, or the entire aorta) deserve further attention.
To further mitigate the overfitting risk associated with small-sample training conditions and enhance model generalizability, current studies are increasingly incorporating hybrid strategies that combine data augmentation, transfer learning, and multi-task learning. For example, in the context of vascular flow simulation, Yao et al. (2024) employed self-supervised pretraining on synthetic flow data, followed by fine-tuning on patient-specific geometries, which significantly improved prediction accuracy with limited real-world samples. Additionally, methods like Bayesian Neural Networks (BNNs) and Monte Carlo Dropout (Gal and Ghahramani, 2015) have been adopted to quantify uncertainty in predictions and regularize model weights, thus preventing overfitting. From the interpretability perspective, techniques such as Integrated Gradients, SHAP (SHapley Additive exPlanations), and attention heatmaps are increasingly utilized to expose model decision processes, allowing clinicians to verify whether predictions align with known hemodynamic risk factors (e.g., low WSS, high OSI). Importantly, as suggested by Alamir et al. (2024), these interpretability tools not only identify which geometric or flow-related features contribute most to predictions but also support clinical trust-building by providing biologically meaningful rationales behind each diagnostic decision. Going forward, the integration of interpretable physics-constrained models, such as KANs and PINNs, with interactive visualization dashboards for clinical end-users, may offer an effective pathway toward regulatory acceptance and real-world adoption (Liu Z. et al., 2024; Liu J. et al., 2024; Ranasinghe et al., 2024).
In summary, recent studies applying DL to CFD-based hemodynamics exhibit three core trends: first, physics-informed or constrained DL (e.g., PINN) clearly shows advantages in accuracy and physical consistency; second, computational efficiency is significantly improved, greatly facilitating real-time or near-real-time applications and clinical feasibility; lastly, increased attention is being paid to model interpretability and decision-support capabilities, promoting model transparency and clinical acceptance. These innovations indicate that DL techniques are gradually moving hemodynamic simulations towards real-time, precise, and personalized clinical applications, potentially revolutionizing cardiovascular disease diagnostics and treatment paradigms in the future.
4 Conclusion
Significant advancements have been achieved in aneurysm hemodynamics research over the past few decades, uncovering the critical roles mechanical factors play in aneurysm initiation, progression, and rupture through multidisciplinary integration. This review systematically summarizes the heterogeneity observed across aneurysms located at various anatomical sites concerning morphological characteristics, mechanical environments, and mechanisms of thrombus formation. Particular emphasis has been placed on the complex associations between key hemodynamic parameters and aneurysm progression.
Integration of numerical simulations and in vitro experiments has provided essential tools for high-precision hemodynamic analyses, while the incorporation of DL techniques has substantially enhanced computational efficiency and clinical translation potential. Nonetheless, current research still encounters numerous challenges: rigid wall simplifications, uncertainties in boundary conditions within CFD simulations may introduce inaccuracies; DL models heavily rely on high-quality training datasets and suffer from insufficient interpretability; and biomimetic in vitro experimental materials often fail to replicate authentic vascular biomechanical properties. Furthermore, controversies surrounding the high/low WSS hypothesis and incomplete elucidation of mechanobiological coupling mechanisms necessitate integrated multi-scale and multi-omics studies.
Future research could benefit from further enhancing multidisciplinary collaboration, potentially integrating mechanobiology, multi-omics analyses, and artificial intelligence technologies to explore the dynamic mechanisms through which mechanical stimuli might influence aneurysm progression via endothelial cell signaling pathways. It may also be valuable to consider the development of patient-specific “digital twin” platforms that incorporate real-time imaging, blood flow simulations, and surgical planning, which could contribute to establishing a new paradigm for personalized aneurysm treatment.
Author contributions
TF: Funding acquisition, Project administration, Writing – original draft, Writing – review and editing, Methodology. JW: Formal Analysis, Investigation, Writing – original draft, Data curation. XW: Data curation, Validation, Writing – original draft. XC: Formal Analysis, Investigation, Writing – original draft. DZ: Funding acquisition, Project administration, Software, Writing – original draft, Writing – review and editing. FX: Funding acquisition, Project administration, Validation, Resources, Writing – original draft, Writing – review and editing. GC: Data curation, Resources, Conceptualization, Methodology, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is supported in part by National Natural Science Foundation of China (Grant No. 12202292, 12202017), the R&D Program of Beijing Municipal Education Commission (KM202310025021), Shenzhen Science and Technology Program (JCYJ20210324130401005, RCBS20231211090712024), and Shenzhen Medical Research Fund (SMRF A2303037), Heilongjiang Natural Science Foundation Project (SS 2024H001), National Key Clinical Specialty Capacity Building Project of the Department of Critical Care Medicine, Hongqi Hospital Affiliated to Mudanjiang Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alamir, S. H., Tufaro, V., Trilli, M., Kitslaar, P., Mathur, A., Baumbach, A., et al. (2024). Rapid prediction of wall shear stress in stenosed coronary arteries based on deep learning. Front. Bioeng. Biotechnol. 12, 1360330. doi:10.3389/fbioe.2024.1360330
Alfonso, F., Macaya, C., Goicolea, J., Hernandez, R., Segovia, J., Zamorano, J., et al. (1994). Determinants of coronary compliance in patients with coronary artery disease: an intravascular ultrasound study. J. Am. Coll. Cardiol. 23 (4), 879–884. doi:10.1016/0735-1097(94)90632-7
Al-Jumaily, A. M., Embong, A. H. B., Al-Rawi, M., Mahadevan, G., and Sugita, S. (2023). Aneurysm rupture prediction based on strain energy-CFD modelling. Bioengineering 10 (10), 1231. doi:10.3390/bioengineering10101231
Alzhanov, N., Ng, E. Y. K., and Zhao, Y. (2024a). Three-dimensional physics-informed neural network simulation in coronary artery Trees. Fluids 9 (7), 153. doi:10.3390/fluids9070153
Alzhanov, N., Ng, E. Y. K., and Zhao, Y. (2024b). Hybrid CFD PINN FSI simulation in coronary artery Trees. Fluids 9 (12), 280. doi:10.3390/fluids9120280
Anagnostakos, J., and Lal, B. K. (2021). Abdominal aortic aneurysms. Prog. Cardiovasc. Dis. 65, 34–43. doi:10.1016/j.pcad.2021.03.009
Angelini, P. (2007). Coronary artery anomalies. Circulation 115 (10), 1296–1305. doi:10.1161/CIRCULATIONAHA.106.618082
Arslan, A. C., and Salman, H. E. (2023). Effect of intraluminal thrombus burden on the risk of abdominal aortic aneurysm rupture. J. Cardiovasc. Dev. Dis. 10 (6), 233. doi:10.3390/jcdd10060233
Backes, D., Rinkel, G. J. E., Greving, J. P., Velthuis, B. K., Murayama, Y., Takao, H., et al. (2017). ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology 88 (17), 1600–1606. doi:10.1212/WNL.0000000000003865
Bappoo, N., Syed, M. B. J., Khinsoe, G., Kelsey, L. J., Forsythe, R. O., Powell, J. T., et al. (2021). Low shear stress at baseline predicts expansion and aneurysm-related events in patients with abdominal aortic aneurysm. Circ. Cardiovasc. Imaging 14 (12), 1112–1121. doi:10.1161/CIRCIMAGING.121.013160
Bardi, F., Gasparotti, E., Vignali, E., Antonuccio, M. N., Storto, E., Avril, S., et al. (2024). A hybrid mock circulatory loop integrated with a LED-PIV system for the investigation of AAA compliant phantoms. Front. Bioeng. Biotechnol. 12, 1452278. doi:10.3389/fbioe.2024.1452278
Belkacemi, D., Tahar Abbes, M., Al-Rawi, M., Al-Jumaily, A. M., Bachene, S., and Laribi, B. (2023). Intraluminal thrombus characteristics in AAA patients: non-invasive diagnosis using CFD. Bioengineering 10 (5), 540. doi:10.3390/bioengineering10050540
Bissell, M. M., Raimondi, F., Ait Ali, L., Allen, B. D., Barker, A. J., Bolger, A., et al. (2023). 4D Flow cardiovascular magnetic resonance consensus statement: 2023 update. J. Cardiov. Magn. Reson. 25 (1), 40. doi:10.1186/s12968-023-00942-z
Bossone, E., and Eagle, K. A. (2021). Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 18 (5), 331–348. doi:10.1038/s41569-020-00472-6
Boyd, A. J., Kuhn, D. C. S., Lozowy, R. J., and Kulbisky, G. P. (2016). Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J. Vasc. Surg. 63 (6), 1613–1619. doi:10.1016/j.jvs.2015.01.040
Brindise, M. C., Rothenberger, S., Dickerhoff, B., Schnell, S., Markl, M., Saloner, D., et al. (2019). Multi-modality cerebral aneurysm haemodynamic analysis: in vivo 4D flow MRI, in vitro volumetric particle velocimetry and in silico computational fluid dynamics. J. R. Soc. Interface 16 (158), 20190465. doi:10.1098/rsif.2019.0465
Buck, A. K. W., Groszek, J. J., Colvin, D. C., Keller, S. B., Kensinger, C., Forbes, R., et al. (2018). Combined in silico and in vitro approach predicts low wall shear stress regions in a hemofilter that correlate with thrombus formation in vivo. Asaio. J. 64 (2), 211–217. doi:10.1097/MAT.0000000000000649
Camasão, D. B., and Mantovani, D. (2021). The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater. Today Bio 10, 100106. doi:10.1016/j.mtbio.2021.100106
Cao, H., Li, Y., Zhao, Y., Xiong, T., Liu, Z., Zheng, T., et al. (2021). Hemodynamic characteristics of patients with suspected coronary heart disease at their initial visit. Front. Physiol. 12, 714438. doi:10.3389/fphys.2021.714438
Cao, H., Xiong, Z., Liu, Z., Li, Y., Pu, H., Liu, J., et al. (2023). Influence of morphology and hemodynamics on thrombosis in kawasaki disease patients. Med. Nov. Technol. Devices 18, 100225. doi:10.1016/j.medntd.2023.100225
Cao, H., Zheng, T., Li, D., Liu, J., Liu, Z., and Peng, L. (2022). Thrombotic risk stratification of coronary aneurysms in Kawasaki disease patients: the study of morphology and hemodynamics. Chin. Med. J. Engl. 135 (18), 2253–2255. doi:10.1097/CM9.0000000000001931
Casa, L. D. C., and Ku, D. N. (2017). Thrombus Formation at high shear rates. Annu. Rev. Biomed. Eng. 19 (1), 415–433. doi:10.1146/annurev-bioeng-071516-044539
Chaikof, E. L., Dalman, R. L., Eskandari, M. K., Jackson, B. M., Lee, W. A., Mansour, M. A., et al. (2018). The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 67 (1), 2–77.e2. doi:10.1016/j.jvs.2017.10.044
Chalouhi, N., Hoh, B. L., and Hasan, D. (2013). Review of cerebral aneurysm formation, growth, and rupture. Stroke 44 (12), 3613–3622. doi:10.1161/STROKEAHA.113.002390
Chen, D., Liang, S., Li, Z., Mei, Y., Dong, H., Ma, Y., et al. (2022). A mock circulation loop for in vitro hemodynamic evaluation of aorta: application in aortic dissection. J. Endovasc. Ther. 29 (1), 132–142. doi:10.1177/15266028211034863
Chmayssani, M., Rebeiz, J. G., Rebeiz, T. J., Batjer, H. H., and Bendok, B. R. (2011). Relationship of growth to aneurysm rupture in asymptomatic aneurysms ≤ 7 mm: a systematic analysis of the literature. Neurosurgery 68 (5), 1164–1171. doi:10.1227/neu.0b013e31820edbd3
Cho, K. (2023). The current limitations and advanced analysis of hemodynamic study of cerebral aneurysms. Neurointervention 18 (2), 107–113. doi:10.5469/neuroint.2023.00164
Conrad, N., Molenberghs, G., Verbeke, G., Zaccardi, F., Lawson, C., Friday, J. M., et al. (2024). Trends in cardiovascular disease incidence among 22 million people in the UK over 20 years: population based study. Bmj 385, e078523. doi:10.1136/bmj-2023-078523
De Nisco, G., Lodi Rizzini, M., Verardi, R., Chiastra, C., Candreva, A., De Ferrari, G., et al. (2023). Modelling blood flow in coronary arteries: Newtonian or shear-thinning non-Newtonian rheology? Comput. Methods. Programs. Biomed 242, 107823. doi:10.1016/j.cmpb.2023.107823
Deplano, V., and Siouffi, M. (1999). Experimental and numerical study of pulsatile flows through stenosis:. J. Biomech. 32 (10), 1081–1090. doi:10.1016/S0021-9290(99)00098-6
Diamond, G., and Forrester, J. S. (1972). Effect of coronary artery disease and acute myocardial infarction on left ventricular compliance in man. N. Y. N.Y. 45 (1), 11–19. doi:10.1161/01.CIR.45.1.11
Dolan, J. M., Kolega, J., and Meng, H. (2013). High wall shear stress and spatial gradients in vascular pathology: a review. Ann. Biomed. Eng. 41 (7), 1411–1427. doi:10.1007/s10439-012-0695-0
Espa, S., Moroni, M., and Boniforti, M. A. (2019). In-Vitro simulation of the blood flow in an axisymmetric abdominal aortic aneurysm. Appl. Sci. 9 (21), 4560. doi:10.3390/app9214560
Etminan, N., Brown, R. D. J., Beseoglu, K., Juvela, S., Raymond, J., Morita, A., et al. (2015). The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology 85 (10), 881–889. doi:10.1212/WNL.0000000000001891
Faisal, M. A. A., Mutlu, O., Mahmud, S., Tahir, A., Chowdhury, M. E. H., Bensaali, F., et al. (2025). Rapid wall shear stress prediction for aortic aneurysms using deep learning: a fast alternative to CFD. Med. Biol. Eng. Comput. doi:10.1007/s11517-025-03311-3
Fan, T., Zhou, Z., Fang, W., Wang, W., Xu, L., and Huo, Y. (2019a). Morphometry and hemodynamics of coronary artery aneurysms caused by atherosclerosis. Atherosclerosis 284, 187–193. doi:10.1016/j.atherosclerosis.2019.03.001
Fan, T., Zhou, Z., Fang, W., Wang, W., Xu, L., and Huo, Y. (2019b). Morphometric and hemodynamic parameter dataset for coronary artery aneurysms caused by atherosclerosis. Data Brief. 25, 104293. doi:10.1016/j.dib.2019.104293
Feng, Y., Liu, J., Fan, T., Zhang, W., Yin, X., E, Y., et al. (2020). Vertebral artery stenoses contribute to the development of diffuse plaques in the basilar artery. Front. Bioeng. Biotechnol. 8, 168. doi:10.3389/fbioe.2020.00168
Feng, Y., Wang, X., Fan, T., Li, L., Sun, X., Zhang, W., et al. (2018). Bifurcation asymmetry of small coronary arteries in juvenile and adult mice. Front. Physiol. 9, 519. doi:10.3389/fphys.2018.00519
Ferdian, E., Dubowitz, D. J., Mauger, C. A., Wang, A., and Young, A. A. (2022). WSSNet: aortic wall shear stress estimation using deep learning on 4D flow MRI. Front. Cardiovasc. Med. 8, 769927. doi:10.3389/fcvm.2021.769927
Ferns, S. P., Sprengers, M. E. S., van Rooij, W. J. J., van den Berg, R., Velthuis, B. K., de Kort, G. A. P., et al. (2011). De novo aneurysm formation and growth of untreated aneurysms. Stroke 42 (2), 313–318. doi:10.1161/STROKEAHA.110.591594
Frösen, J., Cebral, J., Robertson, A. M., and Aoki, T. (2019). Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg. Focus 47 (1), E21. doi:10.3171/2019.5.FOCUS19234
Gal, Y., and Ghahramani, Z. (2015). “Dropout as a bayesian approximation: representing model uncertainty,” in Deep learning.
Gao, Q., Liu, X., Wang, H., Li, F., Wu, P., Niu, Z., et al. (2019). Post-processing techniques of 4D flow MRI: velocity and wall shear stress.
Gellis, L., Castellanos, D. A., Oduor, R., Gauvreau, K., Dionne, A., Newburger, J., et al. (2022). Comparison of coronary artery measurements between echocardiograms and cardiac CT in Kawasaki disease patients with aneurysms. J. Cardiovasc. Comput. Tomogr. 16 (1), 43–50. doi:10.1016/j.jcct.2021.09.002
Golledge, J. (2019). Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 16 (4), 225–242. doi:10.1038/s41569-018-0114-9
Greving, J. P., Wermer, M. J. H., Brown, R. D. J., Morita, A., Juvela, S., Yonekura, M., et al. (2014). Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 13 (1), 59–66. doi:10.1016/S1474-4422(13)70263-1
Gutierrez, N. G. M., Shirinsky, O. M., Gagarina, N. M., Lyskina, G. M., Fukazawa, R. M., Ogawa, S. M., et al. (2017). Assessment of coronary artery aneurysms caused by kawasaki disease using transluminal attenuation gradient analysis of computerized Tomography angiograms. Am. J. Cardiol. 120 (4), 556–562. doi:10.1016/j.amjcard.2017.05.025
Hadad, S., Mut, F., Slawski, M., Robertson, A. M., and Cebral, J. R. (2024). Evaluation of predictive models of aneurysm focal growth and bleb development using machine learning techniques. J. Neurointerventional Surg. 16 (4), 392–397. doi:10.1136/jnis-2023-020241
Haller, S. J., Crawford, J. D., Courchaine, K. M., Bohannan, C. J., Landry, G. J., Moneta, G. L., et al. (2018). Intraluminal thrombus is associated with early rupture of abdominal aortic aneurysm. J. Vasc. Surg. 67 (4), 1051–1058.e1. doi:10.1016/j.jvs.2017.08.069
Hathcock, J. J. (2006). Flow effects on coagulation and thrombosis. Arteriosclerosis, Thrombosis, Vasc. Biol. 26 (8), 1729–1737. doi:10.1161/01.ATV.0000229658.76797.30
Hayashi, H., Hidaka, F., Kumazaki, T., and Ochi, M. (2006). Serial assessment of the development of inflammatory abdominal aortic aneurysm from ordinary atherosclerotic abdominal aortic aneurysm using multidetector-row computed tomographic angiography. Heart. Vessels. 21 (5), 334–337. doi:10.1007/s00380-005-0895-8
He, W., Jiao, M., Fang, X., Shen, Z., Cai, Q., and Zhang, L. (2024). In vitro study of flow characteristics in abdominal aortic aneurysm. Aip Adv. 14 (1). doi:10.1063/5.0184229
Hejazi, M., and Phani, A. S. (2022). On growth, buckling, and rupture of aneurysms: cylindrical tube analogy. J. Biomech. 144, 111313. doi:10.1016/j.jbiomech.2022.111313
Hellmann, D. B., Grand, D. J., and Freischlag, J. A. (2007). Inflammatory abdominal aortic aneurysm. Jama 297 (4), 395–400. doi:10.1001/jama.297.4.395
Heussel, C. P., Kauczor, H. U., Heussel, G., Mildenberger, P., and Dueber, C. (1997). Aneurysms complicating inflammatory diseases in immunocompromised hosts: value of contrast-enhanced CT. Eur. Radiol. 7 (3), 316–319. doi:10.1007/s003300050157
Hoi, Y., Meng, H., Woodward, S. H., Bendok, B. R., Hanel, R. A., Guterman, L. R., et al. (2004). Effects of arterial geometry on aneurysm growth: three-dimensional computational fluid dynamics study. J. Neurosurg. 101 (4), 676–681. doi:10.3171/jns.2004.101.4.0676
Huo, Y., Finet, G., Lefevre, T., Louvard, Y., Moussa, I., and Kassab, G. S. (2012). Which diameter and angle rule provides optimal flow patterns in a coronary bifurcation? J. Biomech. 45 (7), 1273–1279. doi:10.1016/j.jbiomech.2012.01.033
Iemura, M., Ishii, M., Sugimura, T., Akagi, T., and Kato, H. (2000). Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart 83 (3), 307–311. doi:10.1136/heart.83.3.307
Isselbacher, E. M., Preventza, O., Hamilton Black, J., Augoustides, J. G., Beck, A. W., Bolen, M. A., et al. (2022). 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation 146 (24), e334–e482. doi:10.1161/CIR.0000000000001106
Jamiolkowski, M. A., Hartung, M. C., Malinauskas, R. A., and Lu, Q. (2020). An in vitro blood flow loop system for evaluating the thrombogenicity of medical devices and biomaterials. Asaio. J. 66 (2), 183–189. doi:10.1097/MAT.0000000000000958
Jergovic, I., Cheesman, M. A., Siika, A., Khashram, M., Paris, S. M., Roy, J., et al. (2022). Natural history, growth rates, and treatment of popliteal artery aneurysms. J. Vasc. Surg. 75 (1), 205–212.e3. doi:10.1016/j.jvs.2021.07.243
Joly, F., Soulez, G., Garcia, D., Lessard, S., and Kauffmann, C. (2018). Flow stagnation volume and abdominal aortic aneurysm growth: insights from patient-specific computational flow dynamics of Lagrangian-coherent structures. Comput. Biol. Med. 92, 98–109. doi:10.1016/j.compbiomed.2017.10.033
Kandangwa, P., Torii, R., Gatehouse, P. D., Sherwin, S. J., and Weinberg, P. D. (2022). Influence of right coronary artery motion, flow pulsatility and non-Newtonian rheology on wall shear stress metrics. Front. Bioeng. Biotechnol. 10, 962687. doi:10.3389/fbioe.2022.962687
Kato, T., Miura, M., Kobayashi, T., Kaneko, T., Fukushima, N., Suda, K., et al. (2023). Analysis of coronary arterial aneurysm regression in patients with kawasaki disease by aneurysm severity: factors associated with regression. J. Am. Heart Assoc. 12 (3), e022417. doi:10.1161/JAHA.121.022417
Kawsara, A., Núñez Gil, I. J., Alqahtani, F., Moreland, J., Rihal, C. S., and Alkhouli, M. (2018). Management of coronary artery aneurysms. Jacc Cardiovasc. Interv. 11 (13), 1211–1223. doi:10.1016/j.jcin.2018.02.041
Koizumi, S., Kin, T., Sekine, T., Kiyofuji, S., Umekawa, M., and Saito, N. (2024). Intracranial aneurysm stiffness assessment using 4D Flow MRI. J. Neuroradiol. 51 (6), 101221. doi:10.1016/j.neurad.2024.101221
Kolli, K. K., Min, J. K., Ha, S., Soohoo, H., and Xiong, G. (2016). Effect of varying hemodynamic and vascular conditions on fractional flow reserve: an in vitro study. J. Am. Heart Assoc. 5 (7), e003634. doi:10.1161/JAHA.116.003634
Kung, E., Kahn, A. M., Burns, J. C., and Marsden, A. (2014). In vitro validation of patient-specific hemodynamic simulations in coronary aneurysms caused by kawasaki disease. Cardiovasc. Eng. Technol. 5 (2), 189–201. doi:10.1007/s13239-014-0184-8
Lancellotti, R. M., Vergara, C., Valdettaro, L., Bose, S., and Quarteroni, A. (2017). Large eddy simulations for blood dynamics in realistic stenotic carotids. Int. J. Numer. Meth. Biomed. 33 (11). doi:10.1002/cnm.2868
Le, T. B., Troolin, D. R., Amatya, D., Longmire, E. K., and Sotiropoulos, F. (2013). Vortex phenomena in sidewall aneurysm hemodynamics: experiment and numerical simulation. Ann. Biomed. Eng. 41 (10), 2157–2170. doi:10.1007/s10439-013-0811-9
Leemans, E. L., Cornelissen, B. M. W., Said, M., van den Berg, R., Slump, C. H., Marquering, H. A., et al. (2019). Intracranial aneurysm growth: consistency of morphological changes. Neurosurg. Focus 47 (1), E5. doi:10.3171/2019.4.FOCUS1987
Li, G., Wang, H., Zhang, M., Tupin, S., Qiao, A., Liu, Y., et al. (2021). Prediction of 3D Cardiovascular hemodynamics before and after coronary artery bypass surgery via deep learning. Commun. Biol. 4 (1), 99. doi:10.1038/s42003-020-01638-1
Liang, S., Jia, H., Zhang, X., Guo, W., Zhou, G., Li, S., et al. (2022). In-vitro and in-silico haemodynamic analyses of a novel embedded iliac branch device. Front. Cardiovasc. Med. 9, 828910. doi:10.3389/fcvm.2022.828910
Liang, X., Peng, F., Yao, Y., Yang, Y., Liu, A., and Chen, D. (2023). Aneurysm wall enhancement, hemodynamics, and morphology of intracranial fusiform aneurysms. Front. Aging Neurosci. 15, 1145542. doi:10.3389/fnagi.2023.1145542
Lin, S., Han, X., Bi, Y., Ju, S., and Gu, L. (2017). Fluid-structure interaction in abdominal aortic aneurysm: effect of modeling techniques. Biomed. Res. Int. 2017, 1–10. doi:10.1155/2017/7023078
Liu, J., Koskas, L., Faraji, F., Kao, E., Wang, Y., Haraldsson, H., et al. (2018). Highly accelerated intracranial 4D flow MRI: evaluation of healthy volunteers and patients with intracranial aneurysms. Biol. Med. 31 (2), 295–307. doi:10.1007/s10334-017-0646-8
Liu, J., Li, B., Yang, Y., Huang, S., Sun, H., Liu, J., et al. (2024a). A comprehensive approach to prediction of fractional flow reserve from deep-learning-augmented model. Comput. Biol. Med. 169, 107967. doi:10.1016/j.compbiomed.2024.107967
Liu, X., Xie, B., Zhang, D., Zhang, H., Gao, Z., and de Albuquerque, V. H. C. (2024b). Unsupervised physics-informed deep learning for assessing pulmonary artery hemodynamics. Expert Syst. Appl. 257, 125079. doi:10.1016/j.eswa.2024.125079
Liu, Z., Ma, P., Wang, Y., Matusik, W., and Tegmark, M. (2024c). Kan 2.0. Kolmogorov-Arnold Networks Meet Science.
Lyu, Z., Mu, N., Rezaeitaleshmahalleh, M., Zhang, X., Mcbane, R., and Jiang, J. (2024). Automatic segmentation of intraluminal thrombosis of abdominal aortic aneurysms from CT angiography using a mixed-scale-driven multiview perception network (M2Net) model. Comput. Biol. Med. 179, 108838. doi:10.1016/j.compbiomed.2024.108838
Malek, A. M., Alper, S. L., and Izumo, S. (1999). Hemodynamic shear stress and its role in atherosclerosis. Jama J. Am. Med. Assoc. 282 (21), 2035–2042. doi:10.1001/jama.282.21.2035
Masuda, H., Zhuang, Y., Singh, T. M., Kawamura, K., Murakami, M., Zarins, C. K., et al. (1999). Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arteriosclerosis, Thrombosis, Vasc. Biol. 19 (10), 2298–2307. doi:10.1161/01.atv.19.10.2298
Mccrindle, B. W., Rowley, A. H., Newburger, J. W., Burns, J. C., Bolger, A. F., Gewitz, M., et al. (2017). Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation 135 (17), e927–e999. doi:10.1161/CIR.0000000000000484
Mei, Y., Xu, H., Ma, W., Li, Z., Yang, R., Yuan, H., et al. (2020). Retrograde branched extension limb assembling stent of pararenal abdominal aortic aneurysm: a longitudinal hemodynamic analysis for stent graft migration. Int. J. Numer. Meth. Biomed. 36 (11), e3394. doi:10.1002/cnm.3394
Mendez, V., Di Giuseppe, M., and Pasta, S. (2018). Comparison of hemodynamic and structural indices of ascending thoracic aortic aneurysm as predicted by 2-way FSI, CFD rigid wall simulation and patient-specific displacement-based FEA. Comput. Biol. Med. 100, 221–229. doi:10.1016/j.compbiomed.2018.07.013
Meng, H., Feng, Y., Woodward, S. H., Bendok, B. R., Hanel, R. A., Guterman, L. R., et al. (2013). Mathematical model of the rupture mechanism of intracranial saccular aneurysms through daughter aneurysm formation and growth. Neurol. Res. 27 (5), 459–465. doi:10.1179/016164105X25171
Mihalko, E., and Brown, A. C. (2020). Clot structure and implications for bleeding and thrombosis. Semin. Thromb. Hemost. 46 (01), 096–104. doi:10.1055/s-0039-1696944
Millon, A., Sigovan, M., Boussel, L., Mathevet, J., Louzier, V., Paquet, C., et al. (2015). Low WSS induces intimal thickening, while large WSS variation and inflammation induce medial thinning, in an animal model of atherosclerosis. Plos One 10 (11), e0141880. doi:10.1371/journal.pone.0141880
Miranda, E., Sousa, L. C., Antonio, C. C., Castro, C. F., and Pinto, S. (2021). Role of the left coronary artery geometry configuration in atherosusceptibility: CFD simulations considering sPTT model for blood. Comput. Methods Biomech. Biomed. Eng. 24 (13), 1488–1503. doi:10.1080/10255842.2021.1894555
Misaki, K., Futami, K., Uno, T., Nambu, I., Yoshikawa, A., Kamide, T., et al. (2021). Inflow hemodynamics of intracranial aneurysms: a comparison of computational fluid dynamics and 4D flow magnetic resonance imaging. J. Stroke Cerebrovasc. Dis. 30 (5), 105685. doi:10.1016/j.jstrokecerebrovasdis.2021.105685
Miura, Y., Ishida, F., Umeda, Y., Tanemura, H., Suzuki, H., Matsushima, S., et al. (2013). Low wall shear stress is independently associated with the rupture status of middle cerebral artery aneurysms. Stroke 44 (2), 519–521. doi:10.1161/STROKEAHA.112.675306
Miyata, T., Kataoka, H., Shimizu, K., Okada, A., Yagi, T., Imamura, H., et al. (2022). Predicting the growth of middle cerebral artery bifurcation aneurysms using differences in the bifurcation angle and inflow coefficient. J. Neurosurg. 138, 1357–1365. doi:10.3171/2022.8.JNS22597
Monteleone, A., Viola, A., Napoli, E., and Burriesci, G. (2023). Modelling of thrombus formation using smoothed particle hydrodynamics method. Plos One 18 (2), e0281424. doi:10.1371/journal.pone.0281424
Nishi, H., Cancelliere, N. M., Rustici, A., Charbonnier, G., Chan, V., Spears, J., et al. (2024). Deep learning-based cerebral aneurysm segmentation and morphological analysis with three-dimensional rotational angiography. J. Neurointerventional Surg. 16 (2), 197–203. doi:10.1136/jnis-2023-020192
Nørgaard, B. L., Leipsic, J., Gaur, S., Seneviratne, S., Ko, B. S., Ito, H., et al. (2014). Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed Tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J. Am. Coll. Cardiol. 63 (12), 1145–1155. doi:10.1016/j.jacc.2013.11.043
Pavlin-Premrl, D., Boopathy, S. R., Nemes, A., Mohammadzadeh, M., Monajemi, S., Ko, B. S., et al. (2021). Computational fluid dynamics in intracranial atherosclerosis - lessons from cardiology: a review of CFD in intracranial atherosclerosis. J. Stroke Cerebrovasc. Dis. 30 (10), 106009. doi:10.1016/j.jstrokecerebrovasdis.2021.106009
Peng, F., Xia, J., Zhang, F., Lu, S., Wang, H., Li, J., et al. (2025). Intracranial aneurysm instability prediction model based on 4D-Flow MRI and HR-MRI. Neurotherapeutics 22 (1), e00505. doi:10.1016/j.neurot.2024.e00505
Philip, N. T., Patnaik, B. S. V., and Sudhir, B. J. (2022). Hemodynamic simulation of abdominal aortic aneurysm on idealised models: investigation of stress parameters during disease progression. Comput. Methods. Programs. Biomed. 213, 106508. doi:10.1016/j.cmpb.2021.106508
Pinho, N., Castro, C. F., António, C. C., Bettencourt, N., Sousa, L. C., and Pinto, S. I. S. (2019). Correlation between geometric parameters of the left coronary artery and hemodynamic descriptors of atherosclerosis: FSI and statistical study. Med. Biol. Eng. Comput. 57 (3), 715–729. doi:10.1007/s11517-018-1904-2
Qiu, Y., Wang, J., Zhao, J., Wang, T., Zheng, T., and Yuan, D. (2022). Association between blood flow pattern and rupture risk of abdominal aortic aneurysm based on computational fluid dynamics. Eur. J. Vasc. Endovasc. Surg. 64 (2-3), 155–164. doi:10.1016/j.ejvs.2022.05.027
Rafiei, A., and Saidi, M. (2022). Aneurysm geometric features effect on the hemodynamic characteristics of blood flow in coronary artery: CFD simulation on CT angiography-based model. Med. Biol. Eng. Comput. 60 (12), 3357–3375. doi:10.1007/s11517-022-02676-z
Raissi, M., Yazdani, A., and Karniadakis, G. E. (2020). Hidden fluid mechanics: learning velocity and pressure fields from flow visualizations. Science 367 (6481), 1026–1030. doi:10.1126/science.aaw4741
Ranasinghe, N., Xia, Y., Seneviratne, S., and Halgamuge, S. (2024). GINN-KAN: interpretability pipelining with applications. Phys. Inf. Neural Netw. doi:10.48550/arXiv.2408.14780
Ren, S., Liu, Q., Chen, Z., Deng, X., Sun, A., and Luan, J. (2022). Hemodynamic evaluation of endarterectomy and stenting treatments for carotid web. Front. Cardiovasc. Med. 9, 993037. doi:10.3389/fcvm.2022.993037
Ridker, P. M., and Rane, M. (2021). Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 128 (11), 1728–1746. doi:10.1161/CIRCRESAHA.121.319077
Rotman, O. M., Kovarovic, B., Chiu, W., Bianchi, M., Marom, G., Slepian, M. J., et al. (2019). Novel polymeric valve for transcatheter aortic valve replacement applications: in vitro hemodynamic study. Ann. Biomed. Eng. 47 (1), 113–125. doi:10.1007/s10439-018-02119-7
Salman, H. E., Ramazanli, B., Yavuz, M. M., and Yalcin, H. C. (2019). Biomechanical investigation of disturbed hemodynamics-induced tissue degeneration in abdominal aortic aneurysms using computational and experimental techniques. Front. Bioeng. Biotechnol. 7, 111. doi:10.3389/fbioe.2019.00111
Schnell, S., Ansari, S. A., Vakil, P., Hurley, M., Carr, J., Batjer, H., et al. (2012). Characterization of cerebral aneurysms using 4D FLOW MRI. J. Cardiov. Magn. Reson. 14 (S1), W2. doi:10.1186/1532-429X-14-S1-W2
Sekhar, L. N., and Heros, R. C. (1981). Origin, growth, and rupture of saccular aneurysms: a review. Neurosurgery 8 (2), 248–260. doi:10.1227/00006123-198102000-00020
Senser, E. M., Misra, S., and Henkin, S. (2021). Thoracic aortic aneurysm. Clin. 39 (4), 505–515. doi:10.1016/j.ccl.2021.06.003
Serruys, P. W., Kotoku, N., Nørgaard, B. L., Garg, S., Nieman, K., Dweck, M. R., et al. (2023). Computed tomographic angiography in coronary artery disease. Eurointervention 18 (16), e1307–e1327. doi:10.4244/EIJ-D-22-00776
Shaw, J. A., Walton, A. S., Kingwell, B. A., Cameron, J. D., and Dart, A. M. (2002). Determinants of coronary artery compliance in subjects with and without angiographic coronary artery disease. J. Am. Coll. Cardiol. 39, 268. doi:10.1016/S0735-1097(02)81203-7
Sho, E., Komatsu, M., Sho, M., Nanjo, H., Singh, T. M., Xu, C., et al. (2003). High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor. Exp. Mol. Pathol. 75 (1), 1–11. doi:10.1016/S0014-4800(03)00032-7
Sho, E., Sho, M., Singh, T. M., Nanjo, H., Komatsu, M., Xu, C., et al. (2002). Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp. Mol. Pathol. 73 (2), 142–153. doi:10.1006/exmp.2002.2457
Singh, T. P., Moxon, J. V., Iyer, V., Gasser, T. C., Jenkins, J., and Golledge, J. (2021). Comparison of peak wall stress and peak wall rupture index in ruptured and asymptomatic intact abdominal aortic aneurysms. Br. J. Surg. 108 (6), 652–658. doi:10.1002/bjs.11995
Singhal, M., and Gupta, R. (2024). Patient-specific modelling of coronary hemodynamics: state of the art. Sādhanā 49 (4), 255. doi:10.1007/s12046-024-02589-7
Siogkas, P. K., Pleouras, D., Pezoulas, V., Kigka, V., Tsakanikas, V., Fotiou, E., et al. (2024). Combining computational fluid dynamics, structural analysis, and machine learning to predict cerebrovascular events: a mild ml approach. Diagnostics 14 (19), 2204. doi:10.3390/diagnostics14192204
Sommer, K. N., Bhurwani, M. M. S., Iyer, V., and Ionita, C. N. (2022). Comparison of fluid dynamics changes due to physical activity in 3D printed patient specific coronary phantoms with the Windkessel equivalent model of coronary flow. Med 8 (1), 10. doi:10.1186/s41205-022-00138-8
Stam, L. B., Aquarius, R., de Jong, G. A., Slump, C. H., Meijer, F. J. A., and Boogaarts, H. D. (2021). A review on imaging techniques and quantitative measurements for dynamic imaging of cerebral aneurysm pulsations. Sci. Rep. 11 (1), 2175. doi:10.1038/s41598-021-81753-z
Stark, K., and Massberg, S. (2021). Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18 (9), 666–682. doi:10.1038/s41569-021-00552-1
Suk, J., de Haan, P., Lippe, P., Brune, C., and Wolterink, J. M. (2024a). Mesh neural networks for SE(3)-equivariant hemodynamics estimation on the artery wall. Comput. Biol. Med. 173, 108328. doi:10.1016/j.compbiomed.2024.108328
Suk, J., Nannini, G., Rygiel, P., Brune, C., Pontone, G., Redaelli, A., et al. (2024b). Deep vectorised operators for pulsatile hemodynamics estimation in coronary arteries from a steady-state prior. doi:10.48550/arxiv.2410.11920
Tada, Y., Kitazato, K. T., Yagi, K., Shimada, K., Matsushita, N., Kinouchi, T., et al. (2011). Statins promote the growth of experimentally induced cerebral aneurysms in estrogen-deficient rats. Stroke 42 (8), 2286–2293. doi:10.1161/STROKEAHA.110.608034
Tada, Y., Yagi, K., Kitazato, K. T., Tamura, T., Kinouchi, T., Shimada, K., et al. (2010). Reduction of endothelial tight junction proteins is related to cerebral aneurysm formation in rats. J. Hypertens. 28 (9), 1883–1891. doi:10.1097/HJH;0b013e32833c2273
Tang, C. X., Liu, C. Y., Lu, M. J., Schoepf, U. J., Tesche, C., Bayer, R. R., et al. (2020). CT FFR for ischemia-specific CAD with a new computational fluid dynamics algorithm. Jacc Cardiovasc. Imaging 13 (4), 980–990. doi:10.1016/j.jcmg.2019.06.018
Tang, T., Boyle, J. R., Dixon, A. K., and Varty, K. (2005). Inflammatory abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 29 (4), 353–362. doi:10.1016/j.ejvs.2004.12.009
Taskesen, T., Osei, K., Ugwu, J., Hamilton, R., Tannenbaum, M., and Ghali, M. (2021). Coronary artery aneurysm presenting as acute coronary syndrome: two case reports and a review of the literature. J. Thromb. Thrombolysis 52 (2), 683–688. doi:10.1007/s11239-021-02418-2
Tateshima, S., Chien, A., Sayre, J., Cebral, J., and Viñuela, F. (2010). The effect of aneurysm geometry on the intra-aneurysmal flow condition. Neuroradiology 52 (12), 1135–1141. doi:10.1007/s00234-010-0687-4
Van der Horst, A., Boogaard, F. L., Van'T Veer, M., Rutten, M. C. M., Pijls, N. H. J., and van de Vosse, F. N. (2013). Towards patient-specific modeling of coronary hemodynamics in healthy and diseased state. Comput. Math. Method Med. 2013, 1–15. doi:10.1155/2013/393792
Van der Waerden, R., Spendlove, J., Entwistle, J., Xu, X., Narracott, A., Gunn, J., et al. (2025). Analysis of blood stasis for stent thrombosis using an advection-diffusion lattice Boltzmann scheme. Mathematics 13 (3), 376. doi:10.3390/math13030376
Vergara, C., Le Van, D., Quadrio, M., Formaggia, L., and Domanin, M. (2017). Large eddy simulations of blood dynamics in abdominal aortic aneurysms. Med. Eng. Phys. 47, 38–46. doi:10.1016/j.medengphy.2017.06.030
Wang, J., Fan, T., Zhang, H., Ge, Y., Lu, W., Liu, F., et al. (2023). Aortic hemodynamic and morphological analysis before and after repair of thoracoabdominal aortic aneurysm using a G-Branch endograft. Front. Physiol. 14, 1234989. doi:10.3389/fphys.2023.1234989
Wang, L., Jiang, X., Zhang, K., Chen, K., Wu, P., and Li, X. (2024). A hemodynamic analysis of energy loss in abdominal aortic aneurysm using three-dimension idealized model. Front. Physiol. 15, 1330848. doi:10.3389/fphys.2024.1330848
Wang, Y., Leng, X., Zhou, X., Li, W., Siddiqui, A. H., and Xiang, J. (2018). Hemodynamics in a middle cerebral artery aneurysm before its growth and fatal rupture: case study and review of the literature. World Neurosurg. 119, e395–e402. doi:10.1016/j.wneu.2018.07.174
Welleweerd, J. C., den Ruijter, H. M., Nelissen, B. G. L., Bots, M. L., Kappelle, L. J., Rinkel, G. J. E., et al. (2015). Management of extracranial carotid artery aneurysm. Eur. J. Vasc. Endovasc. Surg. 50 (2), 141–147. doi:10.1016/j.ejvs.2015.05.002
Wong, K. K. L., Wu, J., Liu, G., Huang, W., and Ghista, D. N. (2020). Coronary arteries hemodynamics: effect of arterial geometry on hemodynamic parameters causing atherosclerosis. Med. Biol. Eng. Comput. 58 (8), 1831–1843. doi:10.1007/s11517-020-02185-x
Woźniak, P., Iwańczyk, S., Błaszyk, M., Stępień, K., Lesiak, M., Mularek-Kubzdela, T., et al. (2024). Coronary artery aneurysm or ectasia as a form of coronary artery remodeling: etiology, pathogenesis, diagnostics, complications, and treatment. Biomedicines 12 (9), 1984. doi:10.3390/biomedicines12091984
Wu, X. B., Zhong, J. L., Wang, S. W., Su, Y., Chen, P. S., Li, Z. J., et al. (2022). Circumferential wall enhancement with contrast ratio measurement in unruptured intracranial aneurysm for aneurysm instability. Brain Behav. 12 (5), e2568. doi:10.1002/brb3.2568
Xu, J., Bettendorf, B., D Oria, M., and Sharafuddin, M. J. (2023). Multidisciplinary diagnosis and management of inflammatory aortic aneurysms. J. Vasc. Surg. 78 (1), 231–242.e2. doi:10.1016/j.jvs.2022.12.024
Yang, S., Gong, X., Qi, Y., and Jiang, Z. (2020). Comparative study of variations in mechanical stress and strain of human blood vessels: mechanical reference for vascular cell mechano-biology. Biomech. Model. Mechanobiol. 19 (2), 519–531. doi:10.1007/s10237-019-01226-1
Yao, T., Pajaziti, E., Quail, M., Schievano, S., Steeden, J., and Muthurangu, V. (2024). Image2Flow: a proof-of-concept hybrid image and graph convolutional neural network for rapid patient-specific pulmonary artery segmentation and CFD flow field calculation from 3D cardiac MRI data. Plos Comput. Biol. 20 (6), e1012231. doi:10.1371/journal.pcbi.1012231
Zeugin, T., Coulter, F. B., Gülan, U., Studart, A. R., and Holzner, M. (2024). In vitro investigation of the blood flow downstream of a 3D-printed aortic valve. Sci. Rep. 14 (1), 1572. doi:10.1038/s41598-024-51676-6
Zhang, K., Song, P., Pei, Y., Liu, X., Dai, M., and Wen, J. (2024). Numerical investigation on the impact of different coronary aneurysms morphologies on thrombus formation and hemodynamics: a comparative study. Biomech. Model. Mechanobiol. 23 (5), 1631–1647. doi:10.1007/s10237-024-01859-x
Zhang, X., Karuna, T., Yao, Z., Duan, C., Wang, X., Jiang, S., et al. (2019). High wall shear stress beyond a certain range in the parent artery could predict the risk of anterior communicating artery aneurysm rupture at follow-up. J. Neurosurg. 131 (3), 868–875. doi:10.3171/2018.4.JNS173179
Zhang, Y., Jing, L., Zhang, Y., Liu, J., and Yang, X. (2016). Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: case report and literature review. Bmc Neurol. 16 (1), 231. doi:10.1186/s12883-016-0759-0
Zhao, Y., Li, T., Wu, M., Zeng, Z., Gao, M., Bao, X., et al. (2021). Simultaneous transcatheter treatment of ascending aortic aneurysm with aortic and mitral regurgitation: anin vitro study. Interact. Cardiovasc. Thorac. Surg. 33 (3), 474–482. doi:10.1093/icvts/ivab101
Zhou, G., Zhu, Y., Yin, Y., Su, M., and Li, M. (2017). Association of wall shear stress with intracranial aneurysm rupture: systematic review and meta-analysis. Sci. Rep. 7 (1), 5331. doi:10.1038/s41598-017-05886-w
Zhou, M., Yu, Y., Chen, R., Liu, X., Hu, Y., Ma, Z., et al. (2023). Wall shear stress and its role in atherosclerosis. Front. Cardiovasc. Med. 10, 1083547. doi:10.3389/fcvm.2023.1083547
Glossary
AAA Abdominal Aortic Aneurysm
ASI Aneurysm Shape Index
BDF2 Backward Differentiation Formula 2
CAA Coronary Artery Aneurysm
CE Cross-sectional Eccentricity
CFD Computational Fluid Dynamics
CNN Convolutional Neural Networks
CTA Computed Tomography Angiography
DL Deep Learning
DNR Dome-Neck Ratio
DSA Digital Subtraction Angiography
ECAP Endothelial Cell Activation Potential
FSI Fluid-Structure Interaction
FVM Finite Volume Method
GBT Gradient Boosting Trees
GCN Graph Convolutional Networks
IAs Intracranial Aneurysm
iFR Instantaneous wave-free Ratio
ZILT Intraluminal Thrombus
LE Longitudinal Eccentricity
LES Large Eddy Simulation
MCL Mock Circulation Loop
Micro-CT Micro Computed Tomography
MMP Matrix Metalloproteinase
MRA Magnetic Resonance Angiography
MRI Magnetic Resonance Imaging
NO Nitric Oxide
OSI Oscillatory Shear Index
PINN Physics-Informed Neural Networks
PIV Particle Image Velocimetry
RBC Red Blood Cell
RRT Relative Residence Time
SR SizZe Ratio
TAA Thoracic Aortic Aneurysm
TAWSS Time-Averaged Wall Shear Stress
UI Undulation Index
vWF von Willebrand Factor
WSS Wall Shear Stress
Keywords: aneurysm, hemodynamic, numerical simulation, deep learning, in vitro experiment
Citation: Fan T, Wang J, Wang X, Chen X, Zhao D, Xie F and Chen G (2025) Integrated multidisciplinary approach to aneurysm hemodynamic analysis: numerical simulation, in Vitro experiment, and deep learning. Front. Bioeng. Biotechnol. 13:1602190. doi: 10.3389/fbioe.2025.1602190
Received: 29 March 2025; Accepted: 25 April 2025;
Published: 03 June 2025.
Edited by:
Yonghui Qiao, Northwestern Polytechnical University, ChinaCopyright © 2025 Fan, Wang, Wang, Chen, Zhao, Xie and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Fan, ZnR0QGNjbXUuZWR1LmNu; Dongliang Zhao, emhhb2RvbmdsaWFuZ0BzemJsLmFjLmNu; Fengjie Xie, bWRqeGZqMTk3MUBzaW5hLmNu; Guangxin Chen, dnZ2Y2d4QDEyNi5jb20=
 Tingting Fan
Tingting Fan Jinhang Wang
Jinhang Wang Xu Wang
Xu Wang Xi Chen
Xi Chen Dongliang Zhao4*
Dongliang Zhao4*