Abstract
Introduction:
While the deterioration in the general health of patients with long COVID (LC) is well documented, no studies have assessed changes in medication use and their relationships with vascular health. This study aimed to evaluate the increase in the use of various drug classes in LC and its relationship with vascular structure and function.
Methods:
Each participant in the sample of 305 subjects diagnosed with LC completed a questionnaire on medication use, verified in medical records. Pre-pandemic and current drug use were recorded. Arterial stiffness was measured with the VaSera device, which estimates the cardio-ankle vascular index and brachial-ankle pulse wave velocity (ba-PWV); carotid-femoral pulse wave velocity was determined using the Sphygmocor device. Vascular structure was assessed by carotid intima-media thickness (c-IMT), measured with a Sonosite Micromax ultrasound. This analysis focuses exclusively on macrovascular parameters. Statistical analyses were performed with SPSS software.
Results:
Use of all classes of medication increased. Patients with a greater rise in drug use after an LC diagnosis showed higher vascular parameters. Greater cardiovascular drug use was positively associated with ba-PWV, an indicator of arterial stiffness (β = 0.301, 95%CI: 0.024–0.577). Increased anti-inflammatory/analgesic drug use was positively associated with c-IMT, a marker of vascular wall thickness (β = 0.012, 95%CI: 0.001–0.023).
Conclusions:
Medication use rose from 2019 to the time of inclusion in the study. The increase in cardiovascular and anti-inflammatory/analgesic drug use was positively associated with ba-PWV and c-IMT, respectively, suggesting a link between greater drug use and impaired vascular health in LC.
Highlights
-
•
Long COVID is associated with impaired vascular structure and function in a well-defined post-COVID cohort.
-
•
Vascular alterations persist despite pharmacological treatment, highlighting unmet therapeutic needs.
-
•
Increased drug use reflects the physical and mental health burden in individuals with Long COVID.
-
•
This is the first study to investigate the relationship between drug consumption and vascular parameters in Long COVID.
1 Introduction
The health landscape has been transformed by the COVID-19 pandemic, not just because of the severity of the acute infection, but also because of the long-term symptoms, a condition known as long COVID (LC) (1). The pathophysiology of LC is multifactorial and unknown, but chronic inflammation and endotheliopathy stand out (2). These mechanisms boost the progression of pre-existing chronic diseases and the emergence of new pathologies, affecting both cardiovascular (3) and mental health (4). Specifically, LC has been linked to an increase in cardiovascular risk factors such as hypertension (5),dyslipidemia (6),diabetes mellitus (DM) (7) or increased risk of thrombotic events (8). In addition, a higher incidence of psychiatric pathologies such as depression or anxiety has been reported in patients with LC (9). The growth in these pathologies and the need to control the persistent symptoms of the disease [more than 200 symptoms have been described (10)] gives rise to greater use of medications and health resources among LC patients. This increased drug use is more pronounced than in healthy individuals (11). Different therapy options have been proposed for the different effects of LC (12). To combat chronic inflammation, patients with LC use more anti-inflammatory and immunomodulatory drugs (13). The presence of anxious-depressive symptoms, dysautonomia or chronic fatigue generate increased use of antihypertensive drugs (14), antihyperlipidaemics (15), antidepressants and anxiolytics (16). Polypharmacy in patients with LC reflects symptom variability, the multifactorial nature of the disease, and the limited knowledge about effective treatments, all of which increases the risk of overtreatment and iatrogenesis (17).
Vascular alteration (18) and cardiovascular risk factors (19) are key determinants in the development of LC. Controlling cardiovascular risk factors in the acute phase of COVID-19 has been linked to a lower probability of LC developing or less severe symptoms if it does occur (19, 20). Vascular health analysis (vascular structure and function) is a predictor of cardiovascular risk (21). Vascular health can be assessed noninvasively by the following measures: carotid intima-media thickness (c-IMT), which assesses vascular structure (22); carotid-femoral pulse wave velocity (cf-PWV) for central arterial stiffness; brachial-ankle pulse wave velocity (ba-PWV) for peripheral arterial stiffness; and the cardio-ankle vascular index (CAVI), which estimates both central and peripheral arterial stiffness (23). The present study focuses exclusively on macrovascular parameters, assessed through validated non-invasive techniques.
Vascular damage (especially that produced in LC) is triggered by alterations in the renin-angiotensin-aldosterone system and inflammatory mediators (24). These signalling pathways are therapeutic targets for numerous medication groups. COVID-19 interacts with angiotensin-converting enzyme 2 (ACE2), leading to increased angiotensin II levels and promoting endothelial dysfunction, inflammation (25), and elevated blood pressure (26). The relationship between dyslipidemia and SARS-CoV-2 infection is bidirectional: elevated cholesterol may enhance viral entry through increased ACE2 expression (27), while the infection itself raises cholesterol and triglyceride levels and promotes atheromatous plaque formation through oxidative stress (15). Lipid-lowering therapy can counteract these effects by reducing vascular inflammation through the endothelial nitric oxide synthase/ nitric oxide (eNOS/NO) and Yes-associated protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ) pathways, stabilizing atherosclerotic plaque, and activating longevity genes such as Klotho (28). In DM, SARS-CoV-2 can cause direct β-cell injury and reduced insulin production (29), while insulin resistance and chronic inflammation favour the persistence of LC (30). Beyond glucose control, agents such as metformin modulate immune and oxidative stress responses and may even inhibit viral replication (31, 32). Persistent endothelial injury and microclot formation, together with complement dysregulation and an increased von Willebrand factor/ a disintegrin and metalloproteinase with thrombospondin type 1 motif member 13 (ADAMTS13) ratio, underlie the prothrombotic state characteristic of LC, which could be mitigated by antiplatelet or anticoagulant therapy (33, 34).
While the action of certain antihypertensives (35, 36), antihyperlipidaemics and hypoglycaemics on vascular health is well known (28)), other drugs, such as antidepressants and anxiolytics, also have off-target vascular effects (37). Treatment with fluvoxamine, in addition to its effects on anxiety and depressive symptoms, has shown favourable outcomes for LC, reducing viral load and the concentration of pro-inflammatory cytokines (38). The use of selective serotonin reuptake inhibitors (SSRIs) during acute infection has also been associated with a lower risk of developing LC (39). The “vascular depression” hypothesis proposes that microvascular disease, endothelial dysfunction, and vascular inflammation contribute to depressive symptoms, mechanisms that may overlap with the neuropsychiatric manifestations of LC (40, 41). Systemic inflammation and immune dysregulation are likewise central to LC pathophysiology, and early exposure to anti-inflammatory drugs during acute infection has been linked to a greater risk of persistent symptoms, highlighting the complex and bidirectional relationship between inflammation, drug use, and vascular health (42). Figure 1 shows the interrelationships between drug use, vascular health, and LC.
Figure 1
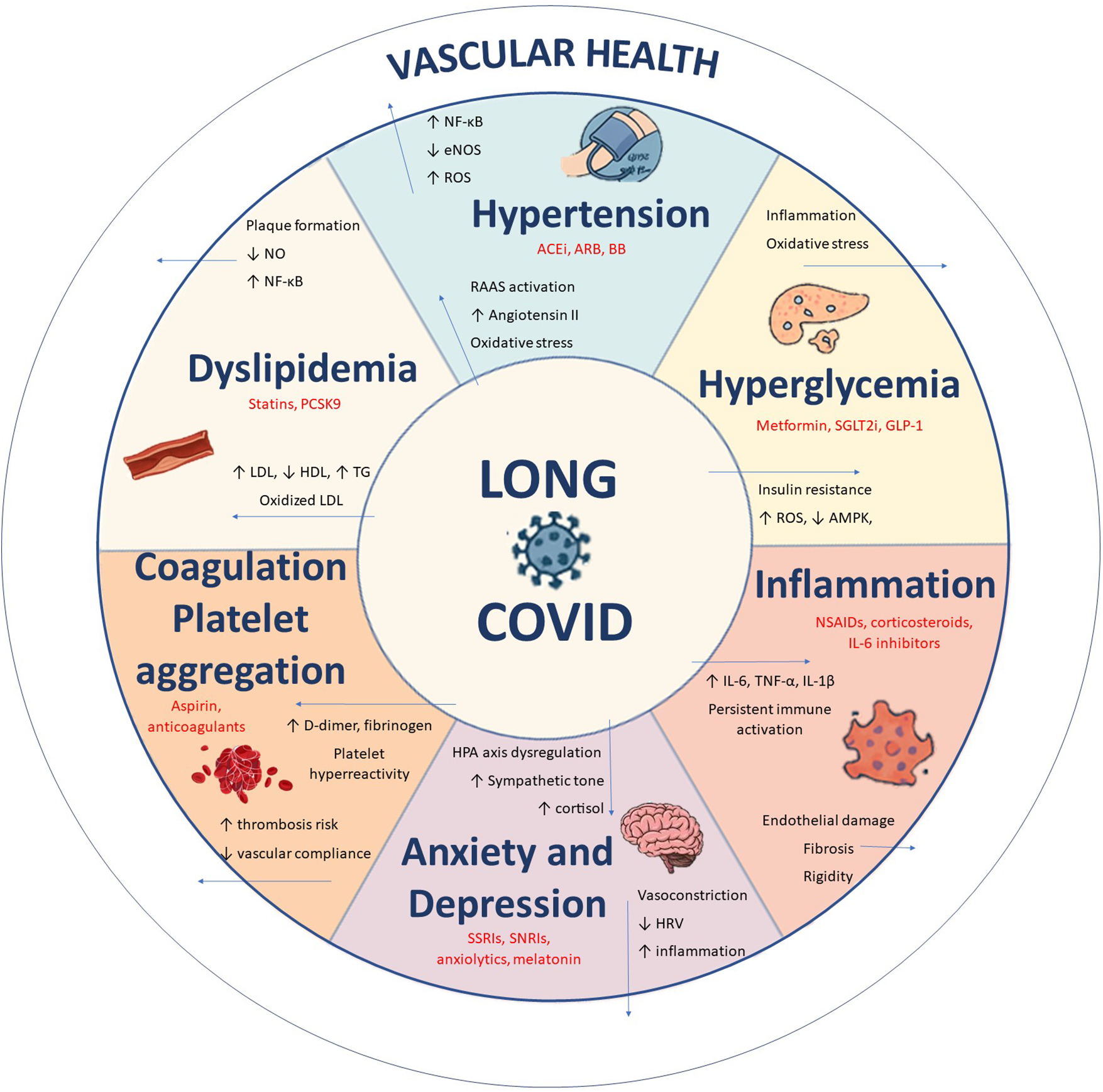
Pathophysiological pathways linking LC to multisystem alterations and vascular dysfunction. Source: own elaboration.
COVID-19 infection (43) and lifestyle choices (44) have been shown to influence vascular health in patients with LC, yet the relationship between vascular health and drug use in LC has not been evaluated. Further research is needed to clarify how medication use influences vascular outcomes in these patients.
2 Materials and methods
2.1 Objectives
The aims of this study were to analyse the increase in the use of cardiovascular, psychotropic and anti-inflammatory/analgesic drugs, its relationship with parameters of vascular structure and function in subjects diagnosed with LC, and the differences by sex.
2.2 Study design
This work is part of the BioICOPER study, a descriptive cross-sectional study conducted at the Salamanca Primary Care Research Unit. The study protocol has been previously published (45). The study was registered on ClinicalTrials.gov in April 2023 (registration number NCT05819840).
2.3 Study population
Using consecutive sampling, 305 participants diagnosed with LC were recruited throughout 2023 from the primary care registries and the LC clinic of Salamanca University Hospital's internal medicine department.
The inclusion criteria were:
- 1.
A confirmed diagnosis of LC according to the World Health Organization (WHO) (46): symptoms that starts within three months of the acute phase of COVID-19, last for at least two months, cannot be explained by another diagnosis, and may persist, recur after initial recovery, or fluctuate over time (46).
- 2.
Age ≥ 18 years.
- 3.
Ability to complete the clinical assessments and questionnaires.
Exclusion criteria were:
- 1.
Subjects excluded were those in a terminal state.
- 2.
Established cardiovascular disease (ischemic heart disease or cerebrovascular event).
- 3.
Severe chronic kidney disease (glomerular filtration rate below 30 mL/min/1.73 m2).
- 4.
Inability to travel to the health centre for the evaluations.
2.4 Variables and measurement instruments
Four trained healthcare professionals administered the necessary examinations and questionnaires following a standardised protocol. The questionnaires included and assessment of previous and current drug consumption. An independent researcher was responsible for data quality control.
2.4.1 Sociodemographic variables
Participants' age and sex were recorded at the moment of inclusion in the study.
2.4.2 Drug use
The use of medication was assessed with a standardised questionnaire administered to each participant and subsequently verified in medical records, both prior to the illness (2019) and at the time of inclusion in the study (current). The increase in drug use was determined by evaluating those participants currently taking the medication group in question but who did not use it in 2019; that is, participants who started taking the drug after developing LC. The use of the following medications was recorded: cardiovascular drugs (antihypertensives, antihyperlipidaemics, hypoglycaemics, anticoagulants and platelet aggregation inhibitors), antidepressants and anxiolytics (benzodiazepines and hypnotics), and anti-inflammatory and analgesic drugs (non-steroidal anti-inflammatory drugs, oral corticosteroids, paracetamol, and other analgesics).
2.4.3 Vascular structure and arterial stiffness
2.4.3.1 Vascular structure
c-IMT was measured to assess vascular structure (47). c-IMT was determined with a Sonosite Micromaxx ultrasound system (FUJIFILM Sonosite, USA) with a high-resolution linear probe and Sonocal software. Automatic measurements were performed in accordance with a previously published protocol (48). A 10 mm section of the common carotid artery was selected, 1 cm from the bifurcation. Measurements of the proximal and distal walls of the carotid artery were taken from this section in anterior, lateral and posterior projections. Ultrasound images were obtained with the subject in the supine position, with the head hyperextended and tilted toward the contralateral shoulder. The staff responsible for the imaging were previously trained.
2.4.3.2 Arterial stiffness
Arterial stiffness was assessed using cf-PWV, ba-PWV and CAVI. Cf-PWV was measured using the SphygmoCor device (AtCor Medical Pty Ltd, Head Office, Australia), with the participant in the supine position. The distance between the sternal jugulum and the sensor location on the carotid and radial arteries or carotid and femoral arteries was determined (49), and the time measurement was based on the delay of the carotid, radial and femoral artery pulse waves relative to the R wave of the electrocardiogram.
Ba-PWV and CAVI were analysed using the VaSera VS-2000 device (Fukuda Denshi Co., Ltd., Japan), with the patient silent and motionless. Electrodes were attached to the arms and ankles, and a heart sound microphone was placed in the second intercostal space. Ba-PWV was estimated using the following equation: ba-PWV = (0.5934 × height in cm + 14.4724)/tba, where tba refers to the interval between the detection of brachial waves and ankle waves (50). CAVI was estimated with the equation: stiffness parameter β = 2ρ × 1/(SBP-DBP) × ln(SBP/DBP) × PWV, where ρ is the blood density, SBP is the systolic blood pressure, DBP is the diastolic blood pressure and PWV is the pulse wave velocity determined between the aortic valve and the ankle. Measurements obtained after three consecutive heartbeats were considered valid (51).
2.4.4 Cardiovascular risk factors
2.4.4.1 Blood pressure measurement
SBP and DBP were measured in accordance with the European Society of Hypertension recommendations (52). After remaining seated for at least 5 min, three measurements were taken on the participant's dominant arm using an OMRON M10-IT sphygmomanometer (Omron Healthcare, Japan), with the average of the last two measurements being recorded. “Hypertension” was considered if the participants had blood pressure ≥ 140/90 mmHg or if they consumed antihypertensive drugs.
2.4.4.2 Analytical parameters
We determined the following analytical parameters: total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and fasting plasma glucose (FPG). On the second visit, venous blood was drawn between 8:00 and 9:00 am, with subjects fasting, without having drunk alcohol or caffeine, or smoked in the previous 12 h. “Dyslipidaemia” was diagnosed if participants had total cholesterol levels ≥240 mg/dL, LDL ≥160 mg/dL, HDL <40 mg/dL in men and <50 mg/dL in women, or 153 triglycerides ≥150 mg/dL; or if they were taking lipid-lowering drugs. Subjects with FPC ≥126 mg/dL or those taking hypoglycaemic medication were classified as having “DM”.
2.4.4.3. Anthropometric measurements
Participants were weighed (in kg) using the InBody 230 monitor (InBody Co., Ltd., South Korea), after fasting for 2 h, without shoes and wearing light clothing. Their height (cm) was measured standing barefoot on a stadiometer (Seca 222, Medical Scale and Measurement Systems, United Kingdom) and body mass index (BMI) was calculated by dividing weight in kg by height in metres squared (m2). “Obesity” was diagnosed if BMI was ≥ 30 kg/m2.
2.4.4.4. Smoking status
To assess smoking status, we used a four-item questionnaire adapted from the WHO MONICA project (53). Participants were classified as active smokers or non-smokers.
2.5 Statistical analysis
The means of two-category quantitative variables were compared using Student's t-test, while the chi-square test was used to compare two categories of categorical variables. Spearman's rho correlation coefficient was used to correlate the relationship between measures of vascular structure and function and the increase in the use of different medications. Multiple regression models were applied to identify the association between increased drug use and vascular parameters. The vascular parameters used as dependent variables were c-IMT measured in mm, cf-PWV and ba-PWV measured in m/s, and CAVI. The increases in the use of cardiovascular medications, antidepressants/anxiolytics and anti-inflammatory/analgesic medications were taken as independent variables. Age in years and sex were considered as control variables. A p value <0.05 was regarded as statistically significant. Data were analysed using SPSS for Windows, v28.0 (IBM Corp., Armonk, USA).
2.6 Ethical principles
The study was approved by the Ethics Committee for Drug Research of the Salamanca Health Area on June 27, 2022 (CEIm reference code: Ref. PI 2022 06 1048), and the guidelines of the Declaration of Helsinki (54) and the WHO were followed throughout. Participant confidentiality was guaranteed at all times in accordance with Organic Law 3/2018, European Regulation 2016/679, and Council Regulation (EC) No. 27/04/2016 on Data Protection. All participants signed informed consent before being included in the study and after receiving information about the procedures involved.
3 Results
3.1 Participant characteristics
The mean time from infection to inclusion in the study was 38.7 ± 10.0 months overall, with no differences between men (38.5 ± 10.0 months) and women (38.7 ± 9.4 months), p = 0.10.
Table 1 shows the use of medication by participants prior to the pandemic (2019), overall and by sex. Before COVID-19 infection, men used significantly more cardiovascular drugs than women including antihypertensives (23.7% vs. 12.0%, p = 0.009) and antihyperlipidaemics (24.7% vs. 7.2%, p < 0.001). Conversely, women took more antidepressants and anxiolytics than men (antidepressants: 13% vs. 5.2%, p = 0.038; anxiolytics: 11.1% vs. 4.1%, p = 0.047). No differences were found in the use of hypoglycaemic agents, platelet aggregation inhibitors, anticoagulants, anti-inflammatory drugs or analgesics (all p > 0.05).
Table 1
| Drugs | Overall (n° = 305) |
Women (n° = 208) |
Men (n° = 97) |
P |
|---|---|---|---|---|
| Cardiovascular drugs, mean ± SD | 0.42 ± 0.84 | 0.29 ± 0.69 | 0.70 ± 1.05 | <0.001 |
| Cardiovascular drugs, n (%) | 79 (25.9%) | 40 (19.2%) | 39 (40.2%) | <0.001 |
| Antihypertensives, mean ± SD | 0.20 ± 0.52 | 0.15 ± 0.44 | 0.32 ± 0.65 | 0.011 |
| Antihypertensives, n (%) | 48 (15.7%) | 25 (12.0%) | 23 (23.7%) | 0.009 |
| Antihyperlipidaemics, mean ± SD | 0.13 ± 0.35 | 0.08 ± 0.29 | 0.24 ± 0.45 | 0.001 |
| Antihyperlipidaemics, n (%) | 39 (12.8%) | 15 (7.2%) | 24 (24.7%) | <0.001 |
| Hypoglycaemics, mean ± SD | 0.07 ± 0.25 | 0.05 ± 0.21 | 0.10 ± 0.31 | 0.056 |
| Hypoglycaemics, n (%) | 20 (6.6%) | 10 (4.8%) | 10 (10.3%) | 0.071 |
| Platelet aggregation inhibitors/Anticoagulants, mean ± SD | 0.03 ± 0.16 | 0.02 ± 0.14 | 0.04 ± 0.20 | 0.164 |
| Platelet aggregation inhibitors/Anticoagulants, n (%) | 8 (2.6%) | 4 (1.9%) | 4 (4.1%) | 0.263 |
| Antidepressants/Anxiolytics, mean ± SD | 0.20 ± 0.52 | 0.25 ± 0.58 | 0.10 ± 0.37 | 0.004 |
| Antidepressants/Anxiolytics, n (%) | 46 (15.1%) | 38 (18.3%) | 8 (8.2%) | 0.023 |
| Antidepressants, mean ± SD | 0.11 ± 0.31 | 0.13 ± 0.34 | 0.05 ± 0.22 | 0.008 |
| Antidepressants n (%) | 32 (10.5%) | 27 (13.0%) | 5 (5.2%) | 0.038 |
| Anxiolytics mean ± SD | 0.10 ± 0.33 | 0.12 ± 0.35 | 0.05 ± 0.27 | 0.030 |
| Anxiolytics n (%) | 27 (8.9%) | 23 (11.1%) | 4 (4.1%) | 0.047 |
| Anti-inflammatory agents/Analgesics, mean ± SD | 0.18 ± 0.50 | 0.20 ± 0.51 | 0.12 ± 0.46 | 0.092 |
| Anti-inflammatory agents/Analgesics, n (%) | 41 (13.4%) | 33 (15.9%) | 8 (8.2%) | 0.069 |
| Anti-inflammatory agents, mean ± SD | 0.11 ± 0.31 | 0.12 ± 0.33 | 0.07 ± 0.26 | 0.084 |
| Anti-inflammatory agents, n (%) | 32 (10.5%) | 25 (12.0%) | 7 (7.2%) | 0.202 |
| Analgesics, mean ± SD | 0.07 ± 0.34 | 0.08 ± 0.35 | 0.05 ± 0.30 | 0.233 |
| Analgesics, n (%) | 15 (4.9%) | 12 (5.8%) | 3 (3.1%) | 0.314 |
Drug use in subjects with long COVID before the pandemic, overall and by sex.
Values are means and standard deviations for continuous data, and number and proportions for categorical data. SD, standard deviations.
p value: differences between men and women.
Table 2 shows the participants' general characteristics, comorbidities and their use of medication at the time of inclusion in the study. The mean age of the sample was 52.7 ± 11.9 years. Men showed a higher burden of cardiovascular risk factors, including SBP, DBP, triglycerides, FPG, body weight, BMI (all p < 0.001). Hypertension was present in 35.2% of the sample, dyslipidaemia in 66.3%, diabetes mellitus in 12.2%, obesity in 32.5%, and 5.7% of participants were active smokers. These comorbidities are specified to provide context for their potential influence on vascular measurements. Women showed higher levels of total cholesterol (p = 0.029) and HDL cholesterol (p < 0.001).
Table 2
| Variable | Overall (n° = 305) |
Women (n° = 208) |
Men (n° = 97) |
P |
|---|---|---|---|---|
| Age, (years) | 52.71 ± 11.94 | 51.32 ± 11.54 | 55.70 ± 12.28 | 0.001 |
| Cardiovascular Risk Factors | ||||
| SBP, (mmHg) | 119.95 ± 16.75 | 115.52 ± 15.94 | 129.45 ± 14.37 | <0.001 |
| DBP, (mmHg) | 76.85 ± 11.11 | 74.30 ± 10.20 | 82.34 ± 11.04 | <0.001 |
| Hypertension, n (%) | 109 (35.2) | 57 (27.5) | 52 (53.6) | <0.001 |
| Total cholesterol, (mg/dL) | 187.45 ± 34.30 | 189.95 ± 34.71 | 182.11 ± 32.94 | 0.029 |
| LDL cholesterol, (mg/dL) | 113.03 ± 31.76 | 112.77 ± 31.67 | 113.59 ± 32.12 | 0.417 |
| HDL cholesterol, (mg/dL) | 56.92 ± 13.58 | 60.73 ± 13.06 | 48.78 ± 10.86 | <0.001 |
| Triglycerides, (mg/dL) | 102.23 ± 50.81 | 95.09 ± 47.52 | 117.47 ± 54.39 | <0.001 |
| Dyslipidaemia, n (%) | 201 (66.3) | 130 (63.1) | 71 (73.2) | 0.053 |
| FPG, (mg/dL) | 87.88 ± 17.67 | 84.84 ± 15.74 | 94.37 ± 19.78 | <0.001 |
| Diabetes Mellitus, n (%) | 37 (12.2) | 15 (7.3) | 22 (22.7) | <0.001 |
| Weight, kg | 75.95 ± 17.39 | 70.29 ± 15.46 | 88.09 ± 14.95 | <0.001 |
| Height, cm | 164.50 ± 8.71 | 160.77 ± 6.52 | 172.51 ± 7.35 | <0.001 |
| BMI, (kg/m2) | 27.97 ± 5.55 | 27.21 ± 5.78 | 29.60 ± 4.64 | <0.001 |
| Obesity, n (%) | 99 (32.5) | 55 (26.4) | 44 (45.4) | <0.001 |
| Active smoker, n (%) | 17 (5.7) | 9 (4.5) | 8 (8.4) | 0.065 |
| Vascular structure and function | ||||
| c-IMT, (mm) | 0.64 ± 0.09 | 0.62 ± 0.07 | 0.68 ± 0.12 | <0.001 |
| cf-PWV, (m/s) | 7.79 ± 2.55 | 7.30 ± 2.06 | 8.85 ± 3.13 | <0.001 |
| ba-PWV, (m/s) | 12.79 ± 2.38 | 12.39 ± 2.26 | 13.63 ± 2.40 | <0.001 |
| CAVI | 7.50 ± 1.27 | 7.32 ± 1.18 | 7.90 ± 1.36 | <0.001 |
| Drugs | ||||
| Cardiovascular drugs, mean ± SD | 0.80 ± 1.19 | 0.61 ± 1.04 | 1.23 ± 1.38 | <0.001 |
| Cardiovascular drugs, n (%) | 132 (43.3%) | 70 (33.7%) | 62 (63.9%) | <0.001 |
| Antihypertensives, mean ± SD | 0.36 ± 0.70 | 0.30 ± 0.66 | 0.49 ± 0.78 | 0.024 |
| Antihypertensives, n (%) | 79 (25.9%) | 45 (21.6%) | 34 (35.1%) | 0.013 |
| Antihyperlipidaemics, mean ± SD | 0.29 ± 0.56 | 0.20 ± 0.48 | 0.47 ± 0.66 | <0.001 |
| Antihyperlipidaemics, n (%) | 75 (24.6%) | 35 (16.8%) | 40 (41.2%) | <0.001 |
| Hypoglycaemics, mean ± SD | 0.11 ± 0.31 | 0.07 ± 0.25 | 0.19 ± 0.39 | 0.004 |
| Hypoglycaemics, n (%) | 32 (10.5%) | 14 (6.7%) | 18 (18.6%) | 0.002 |
| Platelet aggregation inh/Anticoagulants, mean ± SD | 0.05 ± 0.22 | 0.04 ± 0.19 | 0.08 ± 0.28 | 0.080 |
| Platelet aggregation inh/Anticoagulants, n (%) | 16 (5.2%) | 8 (3.8%) | 8 (8.2%) | 0.108 |
| Antidepressants/Anxiolytics mean ± SD | 0.49 ± 0.74 | 0.57 ± 0.79 | 0.30 ± 0.58 | <0.001 |
| Antidepressants/Anxiolytics n (%) | 106 (34.8%) | 83 (39.9%) | 23 (23.7%) | 0.006 |
| Antidepressants, mean ± SD | 0.25 ± 0.43 | 0.30 ± 0.46 | 0.14 ± 0.35 | 0.001 |
| Antidepressants n (%) | 76 (24.9%) | 62 (29.8%) | 14 (14.4%) | 0.004 |
| Anxiolytics mean ± SD | 0.24 ± 0.46 | 0.27 ± 0.49 | 0.16 ± 0.39 | 0.012 |
| Anxiolytics n (%) | 67 (22.0%) | 53 (25.5%) | 14 (14.4%) | 0.030 |
| Anti-inflammatory agents/Analgesics, mean ± SD | 0.49 ± 0.77 | 0.55 ± 0.80 | 0.34 ± 0.68 | 0.017 |
| Anti-inflammatory agents/Analgesics, n (%) | 104 (34.1%) | 80 (38.5%) | 24 (24.7%) | 0.019 |
| Anti-inflammatory agents, mean ± SD | 0.26 ± 0.47 | 0.31 ± 0.50 | 0.16 ± 0.39 | 0.001 |
| Anti-inflammatory agents, n (%) | 76 (24.9%) | 62 (29.8%) | 14 (14.4%) | 0.004 |
| Analgesics, mean ± SD | 0.22 ± 0.50 | 0.24 ± 0.52 | 0.19 ± 0.46 | 0.188 |
| Analgesics, n (%) | 56 (18.4%) | 41 (19.7%) | 15 (15.5%) | 0.372 |
Characteristics of the participants at the time of inclusion in the study, overall and by sex.
Values are means and standard deviations for continuous data, and number and proportions for categorical data. SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high–density lipoprotein cholesterol; BMI, body mass index; c-IMT, intima–media thickness of common carotid; cf-PWV, carotid-femoral pulse wave velocity; ba-PWV, brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular index.
p value: differences between men and women.
Men presented significantly worse vascular parameters: higher cf-PWV (men: 8.85 ± 3.13 vs. women: 7.30 ± 2.06), ba-PWV (men: 13.63 ± 2.40 vs. women: 12.39 ± 2.26), and CAVI values (men: 7.90 ± 1.36 vs. women: 7.32 ± 1.18) (all p < 0.001), as well as slightly higher c-IMT (women 0.62 ± 0.07 mm vs. men 0.68 ± 0.12 mm, p < 0.001) (Table 2).
At the time of inclusion, men continued to use more cardiovascular drugs than women (antihypertensives p = 0.024; antihyperlipidaemics p < 0.001; hypoglycaemics p = 0.004). Women had a greater use of antidepressants (p = 0.001), anxiolytics (p = 0.012), and anti-inflammatory agents (p = 0.001). No sex differences were found in antiplatelet/anticoagulant drug use (p > 0.05) or analgesic use (p > 0.05) (Table 2).
3.2 Increase in drug use
Table 3 shows the increase in the use of medications from the pre-pandemic period to the time subjects were included in the study, both overall and by sex. All drug groups saw increased use. While men had a greater increase in the use of cardiovascular drugs: antihyperlipidaemics and hypoglycaemics (an increase of 21.6% and 8.2%, respectively, p < 0.05)), the increase in the use of anxiolytics was more pronounced in women (an increase of 24.7% p = 0.038). These findings highlight a global rise in medication consumption after LC onset, with sex-specific patterns depending on therapeutic class.
Table 3
| Drugs | Global | Women | Men | P |
|---|---|---|---|---|
| Cardiovascular drugs, mean ± SD | 0.38 ± 0.77 | 0.31 ± 0.67 | 0.53 ± 0.94 | 0.023 |
| Cardiovascular drugs, n (%) | 88 (28.9%) | 50 (24.0%) | 38 (39.2%) | 0.007 |
| Antihypertensives, mean ± SD | 0.16 ± 0.48 | 0.15 ± 0.45 | 0.17 ± 0.55 | 0.426 |
| Antihypertensives, n (%) | 48 (15.7%) | 30 (14.4%) | 18 (18.6%) | 0.356 |
| Antihyperlipidaemics, mean ± SD | 0.16 ± 0.46 | 0.12 ± 0.41 | 0.24 ± 0.56 | 0.033 |
| Antihyperlipidaemics, n (%) | 44 (14.4%) | 23 (11.1%) | 21 (21.6%) | 0.014 |
| Hypoglycaemics, mean ± SD | 0.04 ± 0.20 | 0.02 ± 0.14 | 0.08 ± 0.28 | 0.018 |
| Hypoglycaemics, n (%) | 12 (3.9%) | 4 (1.9%) | 8 (8.2%) | 0.008 |
| Platelet aggregation inhibitors/Anticoagulants, mean ± SD | 0.03 ± 0.21 | 0.02 ± 0.20 | 0.04 ± 0.25 | 0.201 |
| Platelet aggregation inhibitors/Anticoagulants, n (%) | 11 (3.6%) | 6 (2.9%) | 5 (5.2%) | 0.322 |
| Antidepressants/Anxiolytics mean ± SD | 0.28 ± 0.60 | 0.32 ± 0.65 | 0.20 ± 0.47 | 0.028 |
| Antidepressants/Anxiolytics n (%) | 73 (24.3%) | 57 (27.9%) | 16 (16.5%) | 0.030 |
| Antidepressants, mean ± SD | 0.14 ± 0.40 | 0.17 ± 0.42 | 0.09 ± 0.33 | 0.044 |
| Antidepressants n (%) | 49 (16.1%) | 39 (18.8%) | 10 (10.3%) | 0.062 |
| Anxiolytics mean ± SD | 0.14 ± 0.36 | 0.15 ± 0.38 | 0.10 ± 0.31 | 0.106 |
| Anxiolytics n (%) | 73 (23.9%) | 57 (27.4%) | 16 (16.5%) | 0.038 |
| Anti-inflammatory agents/Analgesics, mean ± SD | 0.31 ± 0.72 | 0.35 ± 0.74 | 0.22 ± 0.68 | 0.060 |
| Anti-inflammatory agents/Analgesics, n (%) | 80 (26.2%) | 60 (28.8%) | 20 (20.6%) | 0.128 |
| Anti-inflammatory agents, mean ± SD | 0.16 ± 0.45 | 0.19 ± 0.47 | 0.08 ± 0.40 | 0.018 |
| Anti-inflammatory agents, n (%) | 80 (26.2%) | 60 (28.8%) | 20 (20.6%) | 0.128 |
| Analgesics, mean ± SD | 0.15 ± 0.46 | 0.16 ± 0.47 | 0.13 ± 0.45 | 0.333 |
| Analgesics, n (%) | 45 (14.8%) | 32 (15.4%) | 13 (13.4%) | 0.649 |
Increase in drug use between the two measurements, overall and by sex (current-2019).
Values are means and standard deviations for continuous data, and number and proportions for categorical data.
p value: differences between men and women.
3.3 Vascular structure and function in relation to increased drug use
As shown in Table 4, participants with increased use of cardiovascular drugs presented significantly higher vascular structure and function parameters. In this group, c-IMT (0.66 ± 0.07 vs. 0.63 ± 0.10 mm; p = 0.003), cf-PWV (8.68 ± 3.02 vs. 7.43 ± 2.24 m/s; p < 0.001), ba-PWV (13.76 ± 2.37 vs. 12.40 ± 2.27 m/s; p < 0.001) and CAVI (7.89 ± 1.19 vs. 7.34 ± 1.26; p < 0.001) were all significantly elevated.
Table 4
| c-IMT (mm) | Increase | No increase | P |
|---|---|---|---|
| Cardiovascular Drugs, mean ± SD | 0.66 ± 0.07 | 0.63 ± 0.10 | 0.003 |
| Antihypertensives, mean ± SD | 0.66 ± 0.08 | 0.63 ± 0.10 | 0.020 |
| Antihyperlipidaemics, mean ± SD | 0.67 ± 0.07 | 0.63 ± 0.09 | 0.006 |
| Hypoglycaemics, mean ± SD | 0.67 ± 0.07 | 0.64 ± 0.09 | 0.108 |
| Platelet aggregation inhibitors/Anticoagulants, mean ± SD | 0.64 ± 0.07 | 0.64 ± 0.09 | 0.470 |
| Antidepressants/Anxiolytics mean ± SD | 0.63 ± 0.07 | 0.64 ± 0.10 | 0.195 |
| Antidepressants, mean ± SD | 0.63 ± 0.08 | 0.64 ± 0.10 | 0.159 |
| Anxiolytics mean ± SD | 0.63 ± 0.07 | 0.64 ± 0.10 | 0.223 |
| Anti-inflammatory agents/Analgesics, mean ± SD | 0.65 ± 0.12 | 0.63 ± 0.08 | 0.068 |
| Anti-inflammatory agents, mean ± SD | 0.65 ± 0.12 | 0.63 ± 0.08 | 0.068 |
| Analgesics, mean ± SD | 0.66 ± 0.11 | 0.64 ± 0.09 | 0.063 |
| cf-PWV (m/s) | |||
| Cardiovascular drugs, mean ± SD | 8.68 ± 3.02 | 7.43 ± 2.24 | <0.001 |
| Antihypertensives, mean ± SD | 8.73 ± 3.18 | 7.62 ± 2.38 | 0.013 |
| Antihyperlipidaemics, mean ± SD | 8.59 ± 2.58 | 7.65 ± 2.52 | 0.012 |
| Hypoglycaemics, mean ± SD | 9.88 ± 3.02 | 7.70 ± 2.50 | 0.002 |
| Platelet aggregation inhibitors/Anticoagulants, mean ± SD | 7.01 ± 2.13 | 7.82 ± 2.56 | 0.151 |
| Antidepressants/Anxiolytics mean ± SD | 7.91 ± 2.74 | 7.69 ± 2.47 | 0.275 |
| Antidepressants, mean ± SD | 7.98 ± 3.01 | 7.75 ± 2.44 | 0.282 |
| Anxiolytics mean ± SD | 8.03 ± 2.38 | 7.75 ± 2.58 | 0.250 |
| Anti-inflammatory agents/Analgesics, mean ± SD | 7.83 ± 2.45 | 7.77 ± 2.59 | 0.430 |
| Anti-inflammatory agents, mean ± SD | 7.83 ± 2.45 | 7.77 ± 2.59 | 0.430 |
| Analgesics, mean ± SD | 7.99 ± 2.44 | 7.77 ± 2.62 | 0.312 |
| ba-PWV (m/s) | |||
| Cardiovascular drugs, mean ± SD | 13.76 ± 2.37 | 12.40 ± 2.27 | <0.001 |
| Antihypertensives, mean ± SD | 13.63 ± 2.44 | 12.63 ± 2.34 | 0.004 |
| Antihyperlipidaemics, mean ± SD | 13.95 ± 2.06 | 12.59 ± 2.38 | <0.001 |
| Hypoglycaemics, mean ± SD | 14.02 ± 1.50 | 12.74 ± 2.40 | 0.034 |
| Platelet aggregation inhibitors/Anticoagulants, mean ± SD | 14.23 ± 2.76 | 12.73 ± 2.35 | 0.020 |
| Antidepressants/Anxiolytics mean ± SD | 12.42 ± 2.12 | 12.89 ± 2.40 | 0.093 |
| Antidepressants, mean ± SD | 12.55 ± 2.49 | 12.83 ± 2.37 | 0.231 |
| Anxiolytics mean ± SD | 12.48 ± 2.25 | 12.84 ± 2.40 | 0.181 |
| Anti-inflammatory agents/Analgesics, mean ± SD | 12.51 ± 1.93 | 12.88 ± 2.51 | 0.089 |
| Anti-inflammatory agents, mean ± SD | 12.51 ± 1.93 | 12.88 ± 2.51 | 0.089 |
| Analgesics, mean ± SD | 12.91 ± 1.81 | 12.79 ± 2.43 | 0.386 |
| CAVI | |||
| Cardiovascular drugs, mean ± SD | 7.89 ± 1.19 | 7.34 ± 1.26 | <0.001 |
| Antihypertensives, mean ± SD | 7.74 ± 1.17 | 7.46 ± 1.28 | 0.077 |
| Antihyperlipidaemics, mean ± SD | 7.97 ± 1.18 | 7.42 ± 1.26 | 0.004 |
| Hypoglycaemics, mean ± SD | 8.13 ± 0.83 | 7.48 ± 1.27 | 0.041 |
| Platelet aggregation inhibitors/Anticoagulants, mean ± SD | 8.24 ± 0.76 | 7.47 ± 1.27 | 0.004 |
| Antidepressants/Anxiolytics mean ± SD | 7.23 ± 1.09 | 7.57 ± 1.30 | 0.036 |
| Antidepressant drugs, mean ± SD | 7.36 ± 1.14 | 7.53 ± 1.29 | 0.188 |
| Anxiolytics drugs mean ± SD | 7.29 ± 1.21 | 7.54 ± 1.27 | 0.116 |
| Anti-inflammatory agents/Analgesics, mean ± SD | 7.32 ± 1.17 | 7.57 ± 1.29 | 0.070 |
| Anti-inflammatory drugs, mean ± SD | 7.32 ± 1.17 | 7.57 ± 1.29 | 0.070 |
| Analgesic drugs, mean ± SD | 7.61 ± 1.22 | 7.51 ± 1.28 | 0.322 |
Vascular parameter values in subjects with and without increased drug use.
Values are means and standard deviations for continuous data, and number and proportions for categorical data. c-IMT, intima–media thickness of common carotid; cf-PWV, carotid-femoral pulse wave velocity; ba-PWV, brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular index.
p value: differences between increase and no increase.
For antidepressants/anxiolytics, only CAVI was higher in participants without increased use (with increase: 7.23 ± 1.09 vs. 7.57 ± 1.30, p = 0.036). The anti-inflammatory/analgesic group showed no significant differences overall (p > 0.05).
In summary, the greatest vascular alterations were observed in participants with increased cardiovascular drug use.
3.4 Relationship between increased drug use and vascular parameters
Figures 2–5 and Supplementary Table S1 show the overall and sex-specific correlations between vascular structure and function measures and increased drug use. Greater use of cardiovascular, antihypertensive and antihyperlipidaemics was positively correlated with c-IMT (r = 0.222, r = 0.151 and r = 0.171, respectively). Taking more cardiovascular, antihypertensive, antihyperlipidaemic and hypoglycaemic drugs was positively correlated with cf-PWV (r = 0.196, r = 0.117, r = 0.153 and r = 0.171, respectively) and ba-PWV (r = 0.256, r = 0.113, r = 0.231, r = 0.146, repectively), and using more cardiovascular and antihyperlipidaemics had a positive correlation with CAVI (r = 0.200). All p values were <0.005. No significant correlations were found between increased use of antidepressants/anxiolytics or anti-inflammatories/analgesics and vascular parameters.
Figure 2
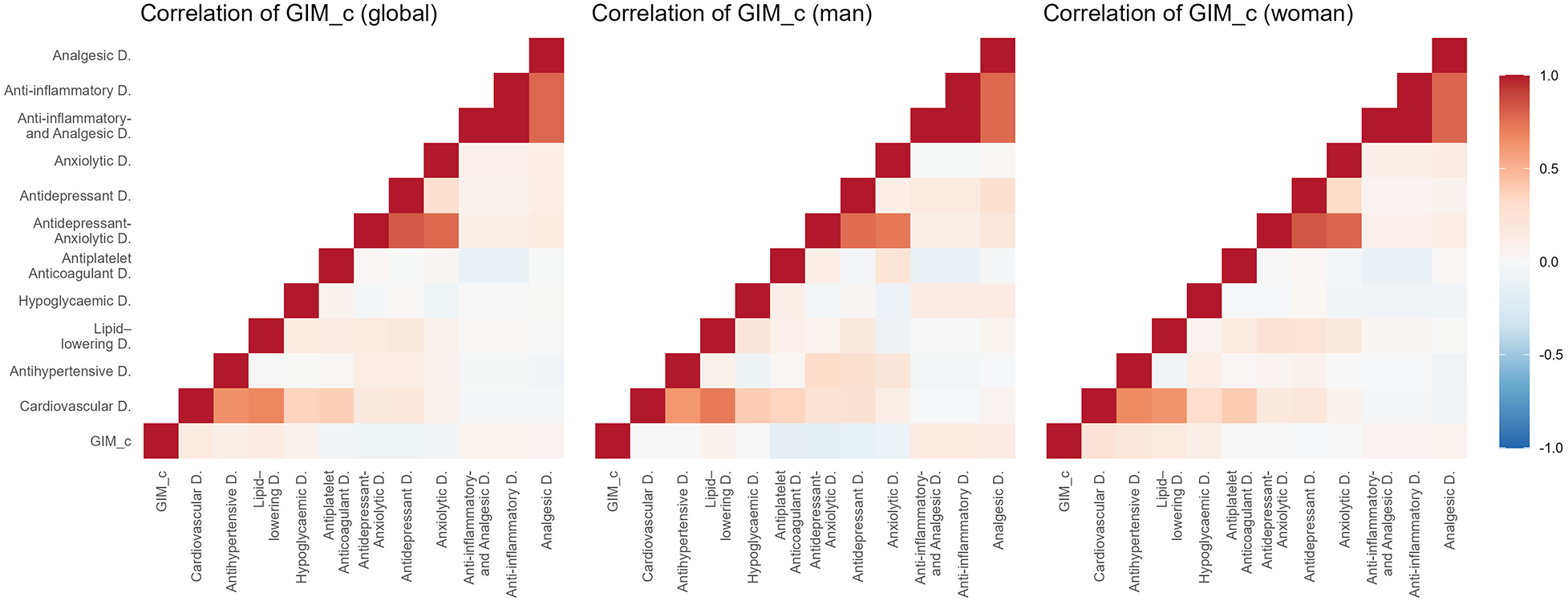
Heatmap of correlation between c-IMT and increased drug use, overall and by sex. GIM_c: c-IMT: intima–media thickness of common carotid.
Figure 3
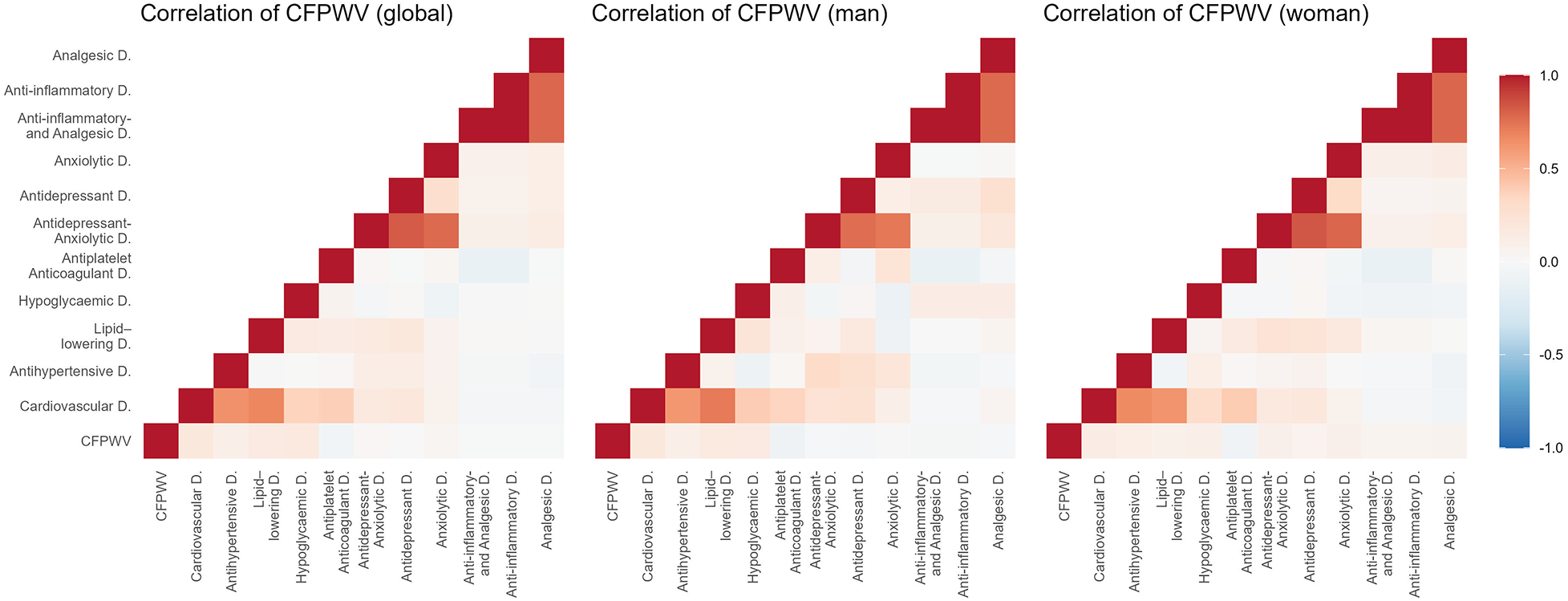
Heatmap of correlation between cf-PWV and increased drug use, overall and by sex. Cf-PWV: carotid-femoral pulse wave velocity.
Figure 4
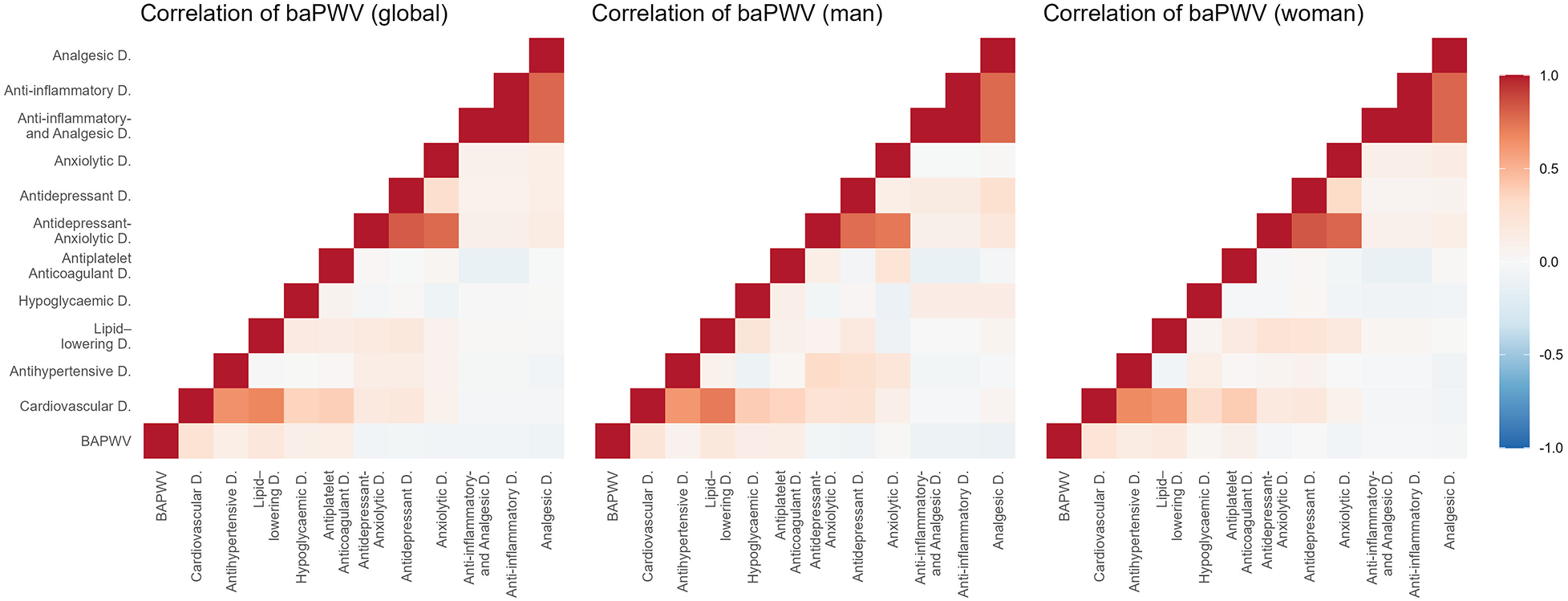
Heatmap of correlation between ba-PWV and increased drug use, overall and by sex. Ba-PWV, brachial-ankle pulse wave velocity.
Figure 5
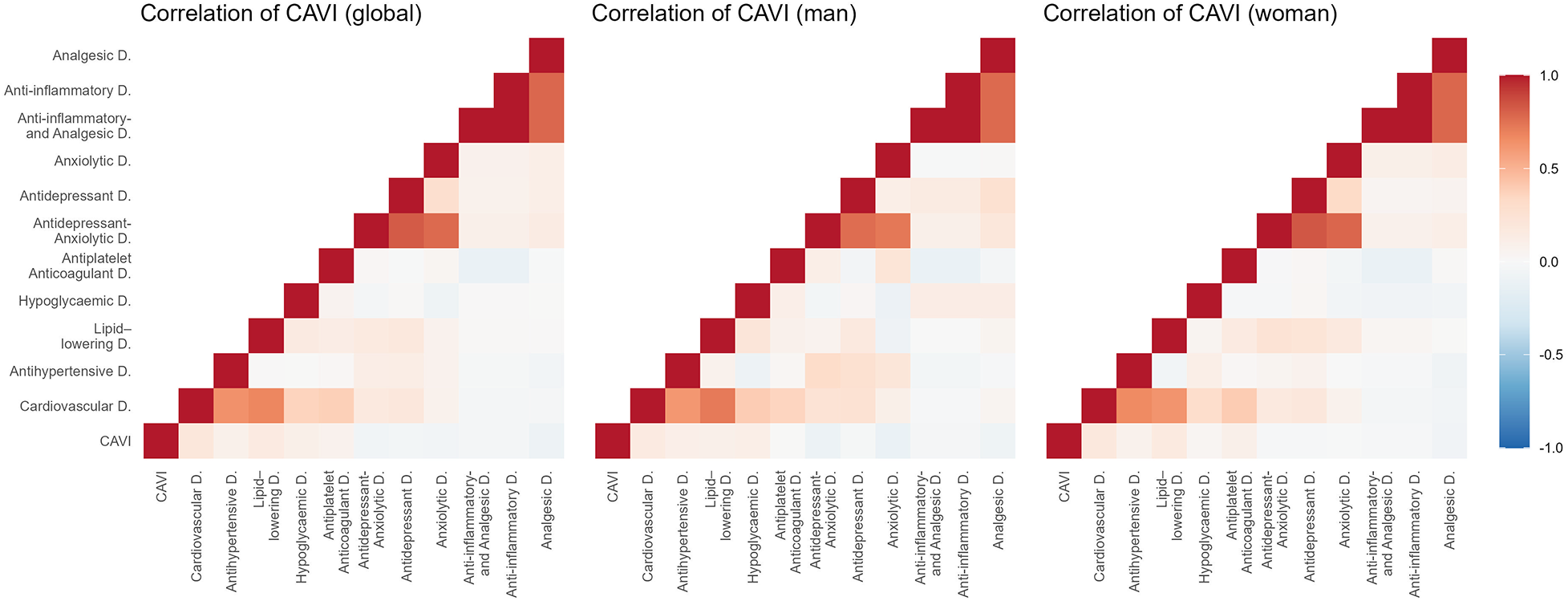
Heatmap of correlation between CAVI and increased drug use, overall and by sex. CAVI, cardio-ankle vascular index.
The results of the multiple regression analysis, adjusted for age and sex, are shown in Figure 6 and Supplementary Table S2. Increased use of cardiovascular medications was positively associated with ba-PWV (β = 0.301, 95% CI: 0.024–0.577). Increased use of anti-inflammatory/analgesic medications was positively associated with c-IMT (β = 0.012, 95% CI: 0.001–0.023). No other associations reached statistical significance.
Figure 6
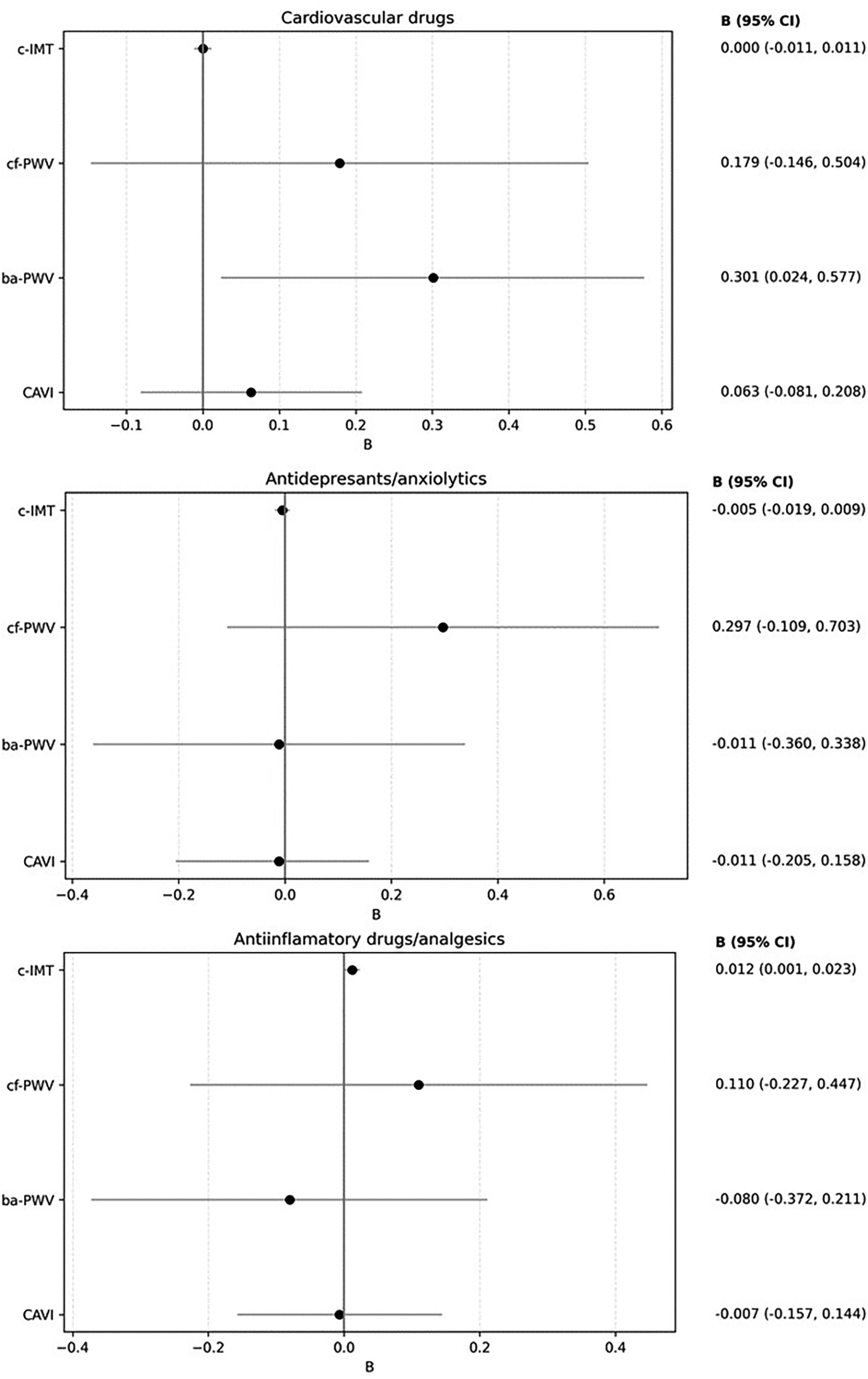
Forest plot: association between increased drug use and vascular parameters. Multiple regression analysis. Dependent variable: “c-IMT”, “cf-PWV”, “ba-PWV” and “CAVI”. Independent variables: increased drug use (cardiovascular drugs, antidepressants/anxiolytics and Anti-inflammatory agents/analgesics). Adjustment variables: age and sex. c-IMT, Intima–media thickness of common carotid; cf-PWV, carotid-femoral pulse wave velocity; ba-PWV, Brachial-ankle pulse wave velocity; CAVI: Cardio-ankle vascular index.
A summary figure illustrating the associations between the increase in drug use and arterial stiffness parameters during LC is provided in Figure 7.
Figure 7
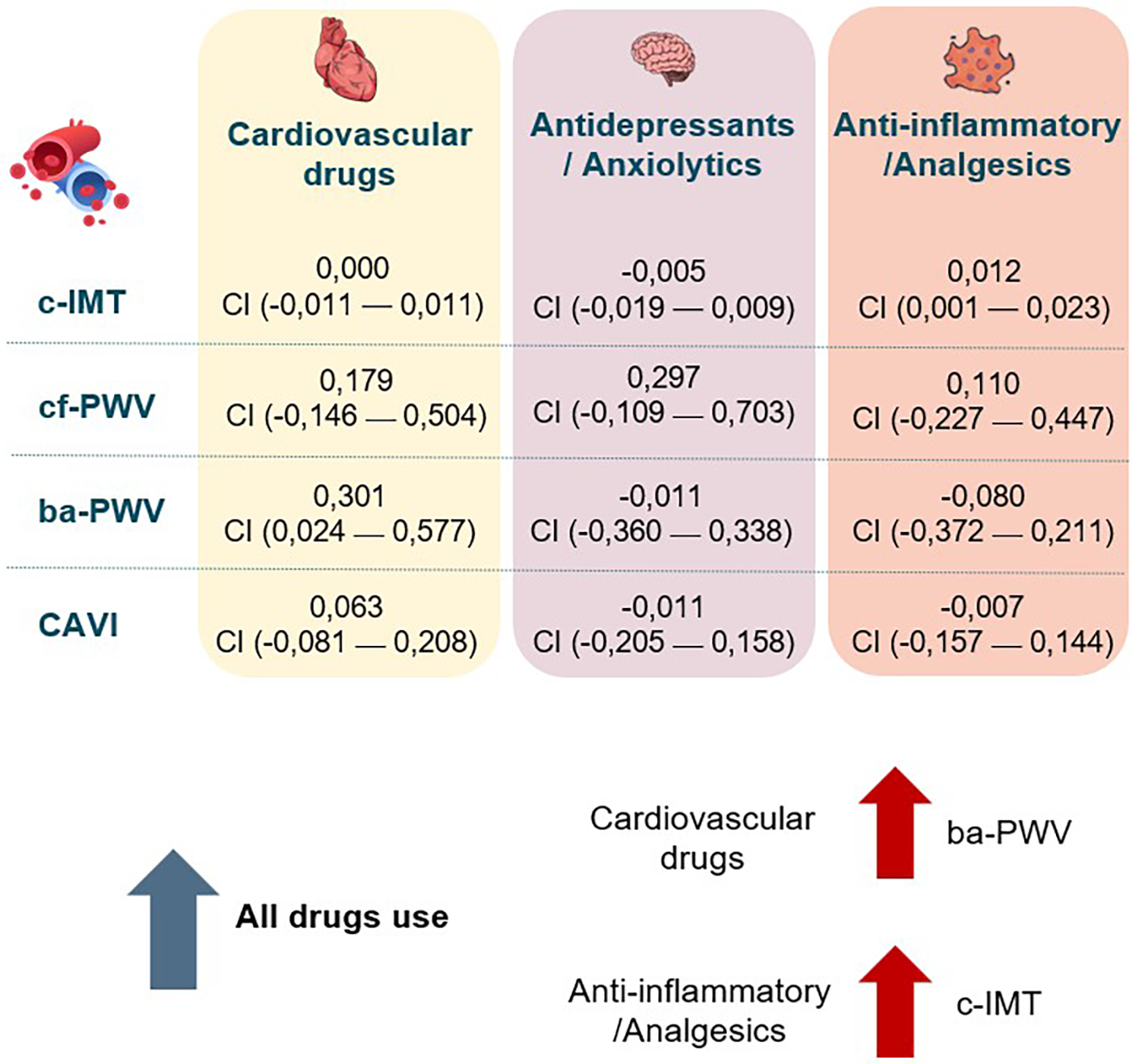
Summary of associations between increased drug use and vascular parameters. c-IMT, Intima–media thickness of common carotid; cf-PWV, carotid-femoral pulse wave velocity; ba-PWV, Brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular index.
4 Discussion
Although several studies have described increased use of medication among patients with LC, there are no detailed analyses of this use and its relationship to vascular health. This study addresses a previously unexplored aspect of increased drug use following the onset of LC and its relationship with vascular structure parameters (determined by c-IMT) and arterial stiffness (by cf-PWV, ba-PWV, and CAVI). While the main findings suggest an increase in the use of all drug classes, positive associations were only found between the increase in cardiovascular drugs and ba-PWV, and between anti-inflammatory/analgesic drugs and c-IMT, after adjusting for age and sex.
4.1 Relationship between cardiovascular drug use and vascular parameters
As Figure 1 shows, the bidirectional relationship between LC and cardiovascular risk factors has been reported in numerous studies (55). For example, the study by Tudoran et al. (56) reinforced this association, highlighting that persistent inflammation and comorbidities contribute to diastolic dysfunction in LC. It is thus hardly surprising to observe greater use of cardiovascular medications in this population. Arterial stiffness is an established cardiovascular risk factor, and several studies have identified an association between SARS-CoV-2 infection and elevated cf-PWV (57).
4.1.1 Antihypertensives
In line with previous studies, we found that use of antihypertensive drugs increased after LC symptoms started (12). This can be directly attributed to the appearance of new-onset hypertension after SARS-CoV-2 infection, dysautonomia (58) and the widespread use (especially of beta-blockers) in the treatment of postural orthostatic tachycardia syndrome (POTS) (59). A 65% increase in the risk of new-onset hypertension has been reported after infection compared to non-infected individuals (60). While hypertension produces structural and functional changes in the blood vessels, promoting an increase in arterial stiffness (24), antihypertensive treatment may reverse this effect (36). Several studies have described lower arterial stiffness (independent of the decrease in blood pressure) produced by taking antihypertensives (61), although no such association was found in the present study. This discrepancy could be due to the influence of the different medications: drugs that interact with the renin-angiotensin-aldosterone system and calcium channel blockers have been associated with lower PWV, mediated by vascular smooth muscle relaxation and a higher elastin-to-collagen ratio. Thiazide diuretics have shown a negative relationship with CAVI, but a positive relationship with PWV, mediated by decreased plasma volume and the resulting peripheral vasoconstriction. Beta-blockers on the other hand, widely used by LC patients (as a treatment for POTS and autonomic dysfunction), lack vasodilatory properties and have no effect on vascular health (28, 35). This study did not analyse the differences between each type of antihypertensive drug, which may partly explain these findings.
4.1.2 Antihyperlipidaemics
Patients with LC start using antihyperlipidaemics in connection with the development of dyslipidemia and cardiovascular complications during the course of the disease (62). In line with our results, research in Romania on patients with LC found a doubling in the number of participants with dyslipidemia in the post-pandemic period compared to the pre-pandemic period (63), as well as an increase in the prescription of antihyperlipidaemics (64). Apart from the alterations produced in the lipid profile, the need for secondary treatment of cardiovascular complications present in LC, such as cardiovascular events, has also increased the use of antihyperlipidaemics (13).
Both dyslipidemia and LC can alter vascular structure. A recent study has shown an increase in c-IMT in patients with LC (65). As with antihypertensive drugs, the level of vascular protection of antihyperlipidaemics varies across groups and doses. High-dose statins and, especially, the use of ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors (iPCSK9) were associated with lower PWV and c-IMT, while fibrates showed no correlation (66, 67). The differences may reflect the lack of subgroup analysis. In addition, we analysed the start of antihyperlipidaemics use from the onset of LC, so the prevalence of high-dose statin, ezetimibe or iPCSK9 use, is lower than in cases of long-established hypertension.
4.1.3 Hypoglycaemic agents
Consistent with this study, an increase in DM has been observed in patients with LC (7). This increase in turn has led to many patients starting hypoglycaemic therapy. The presence of DM-induced microvascular disease is related to the severity of the infection (68) and to the alteration of the vascular structure and arterial stiffness (69).
All groups of hypoglycaemics were linked to a reduction in PWV through the activation of eNOS (28). The influence on arterial stiffness is greater in the new antidiabetics [sodium-glucose cotransporter-2 inhibitors (iSGLT-2), dipeptidyl peptidase-4 inhibitors (iDPP-4) or glucagon-like peptide-1 (GLP-1) receptor agonists], which could explain their effects on cardiovascular level (70). The positive correlation between increased hypoglycaemics use and vascular parameters in the present study demonstrates the multifactorial nature of vascular damage in patients with LC. In line with our findings, we have not found any research linking use of antihyperlipidaemics or DM with vascular structure (c-IMT).
4.1.4 Antiplatelet and anticoagulant agents
Starting antiplatelet and anticoagulant drug use in patients with LC is a response to the persistent prothrombotic state (71). Nevertheless, antithrombotic treatment in LC is not yet well established (72). A reduction in peripheral arterial stiffness has been demonstrated with the intake of acetylsalicylic acid (73), purinergic receptor P2Y12 inhibitors (74) and anticoagulants (75).
In short, in contrast to previous studies reporting a reduction in arterial stiffness with the use of cardiovascular drugs, this study shows a positive association between the use of these drugs and arterial stiffness. In addition to the individual characteristics of each medication group, these discrepancies can be explained by the relationship between active and passive arterial stiffness. Active arterial stiffness is mediated by vascular smooth muscle making it dynamic and reversible, while passive arterial stiffness is mediated by collagen and vascular calcification and is therefore permanent (76). Cardiovascular drugs can reverse active arterial stiffness, but not passive stiffness (77). In the case of LC chronic inflammation produces irreversible changes in the vascular wall, promoting collagen deposition. This means that arterial stiffness is not reversible with drug use, which explains why this study shows higher levels of arterial stiffness with increased drug use: the damage caused by LC exceeds the capacity for pharmacological reversibility. This highlights the multifactorial and potentially irreversible nature of vascular damage in LC. In addition, LC-related vascular injury also involves microvascular dysfunction (particularly endothelial glycocalyx degradation and microclot formation) which recent studies have identified as key pathophysiological mechanisms (78). Although the present analysis focuses on macrovascular parameters, these microvascular alterations are complementary and help contextualize the vascular impairment observed in our cohort.
4.2 Relationship between the use of antidepressants and anxiolytics and vascular parameters
The psychological impact of the pandemic and the presence of persistent symptoms have contributed to worsening mental health in patients with LC. In line with this research, numerous studies have found an increase in the use of antidepressants and anxiolytics after the pandemic (16, 79). However, there is a lack of studies in patients with LC.
There are studies that have demonstrated an increase in c-IMT and arterial stiffness in depressive or anxiety disorders (80, 81). Consistent with our data (negative association, albeit not significant), several studies have demonstrated the beneficial effect of SSRI on arterial stiffness and carotid atherosclerosis (82), mediated by a reduction in proinflammatory cytokines (37). However, dual serotonin and norepinephrine reuptake inhibitor increase arterial stiffness through adrenergic activation (83). We have not found any studies that assess this relationship with anxiolytic drugs.
4.3 Relationship between the use of anti-inflammatory and analgesic drugs and vascular parameters
The increasing use of analgesics in LC development reflects the lack of specificity of the symptoms. Several studies highlight an increase in the use of analgesic drugs to mitigate persistent pain (84).
Although the anti-inflammatory drugs are generally associated with reduced arterial stiffness (28), this effect was not observed for analgesics. The present study found no differences in arterial stiffness, which could be due to the unification of both pharmacological groups into one. Furthermore, in contrast to the data from this study, a decrease in c-IMT was found with anti-inflammatory treatment in patients with rheumatoid arthritis (85). Nevertheless, one study supports the data we obtained, showing worse vascular health in patients with opioid use (86).
4.4 Limitations and strengths
This study has several limitations: 1. As a cross-sectional study, causation cannot be inferred; 2. The analysis by sex may be misleading, as the sexes are not represented equally; 3. Change in drug use was analysed by drug group, not individually. The study also has strengths: 1. It offers a unique and detailed perspective on the increase in drug use in patients with LC; 2. The updated WHO definition of LC was applied; 3. The analysis of vascular health was performed by trained and validated researchers following a standardised protocol; 4. The results are adjusted for age and sex, which avoids confounding factors; 5. We consider that the results have important clinical implications and lay the foundation for further research.
5 Conclusion
Drug use increased from 2019 to the time of inclusion in the study. While the increase in cardiovascular drugs had a positive association with ba-PWV, increased anti-inflammatory/analgesic drug use was positively linked with c-IMT in subjects diagnosed with LC.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found on ZENODO under the DOI: 10.5281/zenodo.14282873 (https://zenodo.org/records/14282873).
Ethics statement
The studies involving humans were approved by the Ethics Committee for Drug Research of the Salamanca Health Area on June 27, 2022 (Reference code: PI 2022 06 1048). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SA-R: Conceptualization, Writing – original draft, Investigation, Writing – review & editing, Resources, Formal analysis, Methodology, Data curation. LG-S: Supervision, Writing – review & editing, Writing – original draft. NS-M: Conceptualization, Investigation, Formal analysis, Methodology, Writing – review & editing, Data curation. AN-C: Writing – review & editing, Investigation, Methodology, Data curation, Formal analysis, Conceptualization. AD-M: Methodology, Data curation, Investigation, Formal analysis, Conceptualization, Writing – review & editing. CL-S: Methodology, Software, Formal analysis, Investigation, Conceptualization, Funding acquisition, Project administration, Writing – review & editing, Data curation. SG-S: Conceptualization, Software, Funding acquisition, Resources, Writing – review & editing, Validation, Project administration, Data curation, Formal analysis. AS-M: Visualization, Formal analysis, Writing – review & editing. ER-S: Conceptualization, Visualization, Formal analysis, Data curation, Supervision, Writing – review & editing. LG-O: Data curation, Software, Visualization, Conceptualization, Resources, Validation, Writing – review & editing, Supervision, Project administration, Funding acquisition. EN-M: Conceptualization, Funding acquisition, Writing – review & editing, Validation, Supervision, Formal analysis, Investigation, Software, Data curation, Methodology, Project administration, Visualization, Resources. MG-M: Data curation, Methodology, Conceptualization, Software, Validation, Investigation, Writing – review & editing, Supervision, Resources, Visualization, Formal analysis, Project administration, Funding acquisition.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study has been funded by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (ISCIII). RD21/0016/0010 (Network for Research on Chronicity, Primary Care, and Health Promotion (RICAPPS), Project PI21/00454, funded by the Carlos III Health Institute (ISCIII) and co-funded by the European Union. The government of Castilla y León also collaborated with the funding of this study through the research projects (GRS 2501/B/22). The CIBER CB22/06/00035 from the area of Respiratory Diseases.
Acknowledgments
We would like to thank all members of the BIOICOPER. We would also like to thank the patient advisers who collaborated with the study investigators.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1691153/full#supplementary-material
Abbreviations
ACE 2, angiotensin-converting enzyme 2; ADAMST13, a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13; ba-PWV, brachial-ankle pulse wave velocity; BMI, body mass index; CAVI, cardio-ankle vascular index; cf-PWV, carotid-femoral pulse wave velocity; CI, confidence interval; c-IMT, carotid intima-media thickness; DBP, diastolic blood pressure; DM, diabetes mellitus; eNOS, endothelial nitric oxide synthase; FPG, fasting plasma glucose; GLP-1, inhibitors, glucagon-like peptide-1; HDL-c, high-density lipoprotein cholesterol; iDPP-4, dipeptidyl peptidase-4 inhibitors; iPCSK9, proprotein convertase subtilisin/kexin type 9 inhibitors; iSGLT-2, sodium-glucose cotransporter-2 inhibitors; LC, Long COVID; LDL-c, low-density lipoprotein cholesterol; NO, nitric oxide; POTS, postural orthostatic tachycardia syndrome; PWV, pulse wave velocity; SBP, systolic blood pressure; SD, standard deviation; SSRI, selective serotonin reuptake inhibitors; Tba, time between brachial and ankle; WHO, World Health Organization; YAP/TAZ, Yes-associated protein/transcriptional coactivator with PDZ-binding motif.
References
1.
World Health Organization (WHO). Post COVID-19 condition (long COVID) (n.d.). Available online at:https://www.who.int/news-room/fact-sheets/detail/post-covid-19-condition-(long-covid) (Accessed April 19, 2025).
2.
Castanares-Zapatero D Chalon P Kohn L Dauvrin M Detollenaere J Maertens de Noordhout C et al Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. (2022) 54:1473–87. 10.1080/07853890.2022.2076901
3.
Tudoran C Tudoran M Lazureanu VE Marinescu AR Cut TG Oancea C et al Factors influencing the evolution of pulmonary hypertension in previously healthy subjects recovering from a SARS-CoV-2 infection. J Clin Med. (2021) 10:5272. 10.3390/jcm10225272
4.
Zhang T Li Z Mei Q Walline JH Zhang Z Liu Y et al Cardiovascular outcomes in long COVID-19: a systematic review and meta-analysis. Front Cardiovasc Med. (2025) 12:1450470. 10.3389/fcvm.2025.1450470
5.
Tadic M Saeed S Grassi G Taddei S Mancia G Cuspidi C . Hypertension and COVID-19: ongoing controversies. Front Cardiovasc Med. (2021) 8:639222. 10.3389/fcvm.2021.639222
6.
Huang L-W Li H-M He B Wang X-B Zhang Q-Z Peng W-X . Prevalence of cardiovascular symptoms in post-acute COVID-19 syndrome: a meta-analysis. BMC Med. (2025) 23:70. 10.1186/s12916-025-03908-3
7.
Doğan EE Rasulova N Bayramova F Hacisahinoğulları H Yalın GY Selçukbiricik ÖS et al The effect of COVID-19 pandemic on new-onset adult diabetes and its one-year follow-up. Prim Care Diabetes. (2025) 19:74–81. 10.1016/j.pcd.2024.11.004
8.
Xie Y Choi T Al-Aly Z . Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect Dis. (2024) 24:239–55. 10.1016/S1473-3099(23)00684-9
9.
Chai Y Lam ICH Man KKC Hayes JF Wan EYF Li X et al Psychiatric and neuropsychiatric sequelae of COVID-19 within 2 years: a multinational cohort study. BMC Med. (2025) 23:144. 10.1186/s12916-025-03952-z
10.
Fernández-de-Las-Peñas C . One year later: prevalence of long-COVID symptoms. Eur J Intern Med. (2023) 115:37–8. 10.1016/j.ejim.2023.07.001
11.
DeVoss R Carlton EJ Jolley SE Perraillon MC . Healthcare utilization patterns before and after a long COVID diagnosis: a case-control study. BMC Public Health. (2025) 25:514. 10.1186/s12889-025-21393-4
12.
Peluso MJ Deeks SG . Mechanisms of long COVID and the path toward therapeutics. Cell. (2024) 187:5500–29. 10.1016/j.cell.2024.07.054
13.
Golzardi M Hromić-Jahjefendić A Šutković J Aydin O Ünal-Aydın P Bećirević T et al The aftermath of COVID-19: exploring the long-term effects on organ systems. Biomedicines. (2024) 12:913. 10.3390/biomedicines12040913
14.
Gopinath G Suryavanshi Chinmay A Pallavi L . Long-term cognitive and autonomic effects of COVID-19 in young adults: a cross-sectional study at 28 months. Ann Med. (2025) 57:2453082. 10.1080/07853890.2025.2453082
15.
Li J Zhou Y Ma J Zhang Q Shao J Liang S et al The long-term health outcomes, pathophysiological mechanisms and multidisciplinary management of long COVID. Sig Transduct Target Ther. (2023) 8:1–19. 10.1038/s41392-023-01640-z
16.
Tiger M Castelpietra G Wesselhoeft R Lundberg J Reutfors J . Utilization of antidepressants, anxiolytics, and hypnotics during the COVID-19 pandemic. Transl Psychiatry. (2024) 14:1–6. 10.1038/s41398-024-02894-z
17.
Michael HU Brouillette M-J Fellows LK Mayo NE . Medication utilization patterns in patients with post-COVID syndrome (PCS): implications for polypharmacy and drug–drug interactions. J Am Pharm Assoc (2003). (2024) 64:102083. 10.1016/j.japh.2024.102083
18.
Ambrosino P Sanduzzi Zamparelli S Mosella M Formisano R Molino A Spedicato GA et al Clinical assessment of endothelial function in convalescent COVID-19 patients: a meta-analysis with meta-regressions. Ann Med. (2022) 54:3233–48. 10.1080/07853890.2022.2136403
19.
Raad-Sarabia M Aroca-Martínez G Miranda CC Ramos-Clason E Otálvaro AT Arnedo RD et al Factores de riesgo cardiovasculares y metabólicos asociados con la aparición de síndrome poscovid: un estudio de cohorte retrospectivo. Rev Colombiana Endocrinol Diabetes Metab. (2024) 11:350–65. 10.53853/encr.11.3.878
20.
Rach S Kühne L Zeeb H Ahrens W Haug U Pohlabeln H . Mild COVID-19 infection associated with post-COVID-19 condition after 3 months – a questionnaire survey. Ann Med. (2023) 55:2226907. 10.1080/07853890.2023.2226907
21.
Mazzolai L Teixido-Tura G Lanzi S Boc V Bossone E Brodmann M et al 2024 ESC guidelines for the management of peripheral arterial and aortic diseases. Eur Heart J. (2024) 45:3538–700. 10.1093/eurheartj/ehae179
22.
Yu B Wu Y Li W Zhou L Lin Y Wang W et al Predictive effect of different blood lipid parameters combined with carotid intima-media thickness on coronary artery disease. Front Cardiovasc Med. (2022) 9:1105413. 10.3389/fcvm.2022.1105413
23.
Claessens PJ Peeters R Claessens L Claessens C Claessens J Claessens PM . Pulse wave analysis measurements: important, underestimated and undervalued parameters in cardiovascular health problems. Front Cardiovasc Med. (2023) 10:1266258. 10.3389/fcvm.2023.1266258
24.
Quarti-Trevano F Cuspidi C Dell’Oro R Ambrosino P Grassi G . Association between arterial stiffness, high blood pressure, and hypertensive phenotypes: insights from the PAMELA study. J Clin Med. (2025) 14:2230. 10.3390/jcm14072230
25.
Chen G Li X Gong Z Xia H Wang Y Wang X et al Hypertension as a sequela in patients of SARS-CoV-2 infection. PLoS One. (2021) 16:e0250815. 10.1371/journal.pone.0250815
26.
Ostrowska A Wojciechowska W Rajzer M Weber T Bursztyn M Persu A et al The impact of the COVID-19 pandemic on hypertension phenotypes (ESH ABPM COVID-19 study). Eur J Intern Med. (2025) 131:58–64. 10.1016/j.ejim.2024.08.027
27.
Shoemark DK Colenso CK Toelzer C Gupta K Sessions RB Davidson AD et al Molecular simulations suggest vitamins, retinoids and steroids as ligands of the free fatty acid pocket of the SARS-CoV-2 spike protein. Angew Chem, Int Ed. (2021) 60:7098–110. 10.1002/anie.202015639
28.
Roth L Dogan S Tuna BG Aranyi T Benitez S Borrell-Pages M et al Pharmacological modulation of vascular ageing: a review from VascAgeNet. Ageing Res Rev. (2023) 92:102122. 10.1016/j.arr.2023.102122
29.
Al-kuraishy HM Al-Gareeb AI Alblihed M Guerreiro SG Cruz-Martins N Batiha GE-S . COVID-19 in relation to hyperglycemia and diabetes Mellitus. Front Cardiovasc Med. (2021) 8:644095. 10.3389/fcvm.2021.644095
30.
Ashique S Mishra N Garg A Garg S Farid A Rai S et al A critical review on the long-term COVID-19 impacts on patients with diabetes. Am J Med. (2025) 138:308–29. 10.1016/j.amjmed.2024.02.029
31.
He Y Zheng Q Zhifang Z Xiaofeng N Shenggen W Xue M et al When COVID-19 meets diabetes: a bibliometric analysis. Diabetes Res Clin Pract. (2025) 223:112118. 10.1016/j.diabres.2025.112118
32.
Bramante CT Beckman KB Mehta T Karger AB Odde DJ Tignanelli CJ et al Favorable antiviral effect of metformin on SARS-CoV-2 viral load in a randomized, placebo-controlled clinical trial of COVID-19. Clin Infect Dis. (2024) 79:354–63. 10.1093/cid/ciae159
33.
Alfaro E Díaz-García E García-Tovar S Galera R Casitas R Torres-Vargas M et al Endothelial dysfunction and persistent inflammation in severe post-COVID-19 patients: implications for gas exchange. BMC Med. (2024) 22:242. 10.1186/s12916-024-03461-5
34.
Cervia-Hasler C Brüningk SC Hoch T Fan B Muzio G Thompson RC et al Persistent complement dysregulation with signs of thromboinflammation in active long COVID. Science. (2024) 383:eadg7942. 10.1126/science.adg7942
35.
Cavero-Redondo I Martinez-Rodrigo A Saz-Lara A Moreno-Herraiz N Casado-Vicente V Gomez-Sanchez L et al Antihypertensive drug recommendations for reducing arterial stiffness in patients with hypertension: machine learning–based multicohort (RIGIPREV) study. J Med Internet Res. (2024) 26:e54357. 10.2196/54357
36.
Schettini IVG Barreto SM Brant LCC Ribeiro ALP Mill JG Rios DRA et al Use of antihypertensive drugs and arterial stiffness in the longitudinal study of adult health (ELSA-brasil). Cardiovasc Drugs Ther. (2025) 39:287–96. 10.1007/s10557-023-07529-x
37.
Dimoula A Fotellis D Aivalioti E Delialis D Polissidis A Patras R et al Off-target effects of antidepressants on vascular function and structure. Biomedicines. (2021) 10:56. 10.3390/biomedicines10010056
38.
Bonnet U Juckel G . Die bedeutung von antidepressiva bei COVID-19 und long-COVID – ein scoping-review update. Fortschr Neurol Psychiatr. (2024):2374–2218. 10.1055/a-2374-2218
39.
Butzin-Dozier Z Ji Y Deshpande S Hurwitz E Anzalone AJ Coyle J et al SSRI Use during acute COVID-19 and risk of long COVID among patients with depression. BMC Med. (2024) 22:445. 10.1186/s12916-024-03655-x
40.
Hage Z Madeira MM Koliatsis D Tsirka SE . Convergence of endothelial dysfunction, inflammation and glucocorticoid resistance in depression-related cardiovascular diseases. BMC Immunol. (2024) 25:61. 10.1186/s12865-024-00653-9
41.
Zhou S Wei T Liu X Liu Y Song W Que X et al Causal effects of COVID-19 on structural changes in specific brain regions: a Mendelian randomization study. BMC Med. (2023) 21:261. 10.1186/s12916-023-02952-1
42.
Lee Y-S Kim H Kwon S Kim T-H . Association between long COVID and nonsteroidal anti-inflammatory drug use by patients with acute-phase COVID-19: a nationwide Korea national health insurance service cohort study. PLoS One. (2024) 19:e0312530. 10.1371/journal.pone.0312530
43.
Theresa C Katebe B Shibao CA Kirabo A . Arterial stiffness in adults with long COVID in sub-saharan Africa. Physiol Rep. (2024) 12:e70029. 10.14814/phy2.70029
44.
Arroyo-Romero S Gómez-Sánchez L Suárez-Moreno N Navarro-Cáceres A Domínguez-Martín A Lugones-Sánchez C et al Relationship between alcohol consumption and vascular structure and arterial stiffness in adults diagnosed with persistent COVID: bioICOPER study. Nutrients. (2025) 17:703. 10.3390/nu17040703
45.
Gómez-Sánchez L Tamayo-Morales O Suárez-Moreno N Bermejo-Martín JF Domínguez-Martín A Martín-Oterino JA et al Relationship between the structure, function and endothelial damage, and vascular ageing and the biopsychological situation in adults diagnosed with persistent COVID (BioICOPER study). A research protocol of a cross-sectional study. Front Physiol. (2023) 14:1236430. 10.3389/fphys.2023.1236430
46.
Soriano JB Murthy S Marshall JC Relan P Diaz JV . WHO Clinical case definition working group on post-COVID-19 condition. A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infect Dis. (2022) 22:e102–7. 10.1016/S1473-3099(21)00703-9
47.
Ray A Tamsma JT Hovens MMC Roodt J Huisman MV . Accuracy of carotid plaque detection and intima–media thickness measurement with ultrasonography in routine clinical practice. Eur J Intern Med. (2010) 21:35–9. 10.1016/j.ejim.2009.10.001
48.
Gómez-Marcos MA Recio-Rodríguez JI Patino-Alonso MC Agudo-Conde C Gómez-Sanchez L Gómez-Sanchez M et al Protocol for measuring carotid intima-media thickness that best correlates with cardiovascular risk and target organ damage. Am J Hypertens. (2012) 25:955–61. 10.1038/ajh.2012.72
49.
Fortier C Agharazii M . Arterial stiffness gradient. Pulse (Basel). (2016) 3:159–66. 10.1159/000438852
50.
Yamashina A Tomiyama H Takeda K Tsuda H Arai T Hirose K et al Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. (2002) 25:359–64. 10.1291/hypres.25.359
51.
Shirai K Hiruta N Song M Kurosu T Suzuki J Tomaru T et al Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. (2011) 18:924–38. 10.5551/jat.7716
52.
Kreutz R Brunström M Burnier M Grassi G Januszewicz A Muiesan ML et al 2024 European society of hypertension clinical practice guidelines for the management of arterial hypertension. Eur J Intern Med. (2024) 126:1–15. 10.1016/j.ejim.2024.05.033
53.
WHO MONICA Project Principal Investigators. The world health organization MONICA project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA project principal investigators. J Clin Epidemiol. (1988) 41:105–14. 10.1016/0895-4356(88)90084-4
54.
World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. 10.1001/jama.2013.281053
55.
Liu Y Lou X . The bidirectional association between metabolic syndrome and long-COVID-19. Diabetes Metab Syndr Obes. (2024) 17:3697–710. 10.2147/DMSO.S484733
56.
Tudoran C Tudoran M Cut TG Lazureanu VE Bende F Fofiu R et al The impact of metabolic syndrome and obesity on the evolution of diastolic dysfunction in apparently healthy patients suffering from post-COVID-19 syndrome. Biomedicines. (2022) 10:1519. 10.3390/biomedicines10071519
57.
Jannasz I Pruc M Rahnama-Hezavah M Targowski T Olszewski R Feduniw S et al The impact of COVID-19 on carotid–femoral pulse wave velocity: a systematic review and meta-analysis. J Clin Med. (2023) 12:5747. 10.3390/jcm12175747
58.
Marques KC Quaresma JAS Falcão LFM . Cardiovascular autonomic dysfunction in “long COVID”: pathophysiology, heart rate variability, and inflammatory markers. Front Cardiovasc Med. (2023) 10:1256512. 10.3389/fcvm.2023.1256512
59.
Chadda KR Blakey EE Huang CL-H Jeevaratnam K . Long COVID-19 and postural orthostatic tachycardia syndrome- is dysautonomia to be blamed?Front Cardiovasc Med. (2022) 9:860198. 10.3389/fcvm.2022.860198
60.
Angeli F Zappa M Verdecchia P . Global burden of new-onset hypertension associated with severe acute respiratory syndrome coronavirus 2 infection. Eur J Intern Med. (2024) 119:31–3. 10.1016/j.ejim.2023.10.016
61.
Cavero-Redondo I Saz-Lara A Lugones-Sánchez C Pozuelo-Carrascosa DP Gómez-Sánchez L López-Gil JF et al Gómez-Marcos MÁ. Comparative effect of antihypertensive drugs in improving arterial stiffness in adults with hypertension (RIGIPREV study). A network meta-analysis. Front Pharmacol. (2023) 14:1225795. 10.3389/fphar.2023.1225795
62.
Wrona M Skrypnik D . New-onset diabetes Mellitus, hypertension, dyslipidaemia as sequelae of COVID-19 infection-systematic review. Int J Environ Res Public Health. (2022) 19:13280. 10.3390/ijerph192013280
63.
Abdulan IM Feller V Oancea A Maștaleru A Alexa AI Negru R et al Evolution of cardiovascular risk factors in post-COVID patients. J Clin Med. (2023) 12:6538. 10.3390/jcm12206538
64.
Kurdi A Millar M Nnabuko U McTaggart S Mueller T Proud E et al A population-based study of incident prescribing for hypercholesterolaemia and hypertension in Scotland: is the healthcare system recovering from the impact of COVID-19? Curr Med Res Opin. (2025) 41:447–53. 10.1080/03007995.2025.2482674
65.
Cozma A Briciu V Sitar-Tăut AV Leucuța D Sporiș N-D Lazar A-L Mălinescu T-V Ganea A-M Vlad CV Lupșe M et al Cardiac dysfunction and subclinical atherosclerosis in post-COVID-19 patients. Cardiac Failure Review. (2024) 11:e09. 10.15420/cfr.2024.21
66.
Reklou A Katsiki N Karagiannis A Athyros V . Effects of lipid lowering drugs on arterial stiffness: one more way to reduce cardiovascular risk?Curr Vasc Pharmacol. (2020) 18:38–42. 10.2174/1570161117666190121102323
67.
Alidadi M Montecucco F Jamialahmadi T Al-Rasadi K Johnston TP Sahebkar A . Beneficial effect of statin therapy on arterial stiffness. Biomed Res Int. (2021) 2021:5548310. 10.1155/2021/5548310
68.
Tochel C Engelmann J Giarratano Y Dhillon B Megaw R Bernabeu MO . Microvascular disease and severe COVID-19 outcomes in UKBiobank participants with diabetes. Acta Diabetol. (2025) 62:293–301. 10.1007/s00592-024-02420-z
69.
Reis M Teixeira A Cardoso J Borges T Afonso AC Correia-Costa L . Association between proinflammatory cytokines and arterial stiffness in type 1 diabetic adolescents. J Pediatr Endocrinol Metab. (2024) 37:405–12. 10.1515/jpem-2023-0530
70.
Neutel CHG Wesley CD Van Praet M Civati C Roth L De Meyer GRY et al Empagliflozin decreases ageing-associated arterial stiffening and vascular fibrosis under normoglycemic conditions. Vascul Pharmacol. (2023) 152:107212. 10.1016/j.vph.2023.107212
71.
Turner S Khan MA Putrino D Woodcock A Kell DB Pretorius E . Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab. (2023) 34:321–44. 10.1016/j.tem.2023.03.002
72.
Violi F Harenberg J Pignatelli P Cammisotto V . COVID-19 and long-COVID thrombosis: from clinical and basic science to therapeutics. Thromb Haemost. (2024) 124:286–96. 10.1055/s-0043-1776713
73.
Roth L Rombouts M Schrijvers DM Emini Veseli B Martinet W De Meyer GRY . Acetylsalicylic acid reduces passive aortic wall stiffness and cardiovascular remodelling in a mouse model of advanced atherosclerosis. Int J Mol Sci. (2021) 23:404. 10.3390/ijms23010404
74.
Vlachopoulos C Georgakopoulos C Pietri P Ioakeimidis N Koutouzis M Vaina S et al Effect of ticagrelor versus clopidogrel on aortic stiffness in patients with coronary artery disease. J Am Heart Assoc. (2019) 8:e012521. 10.1161/JAHA.119.012521
75.
Junejo RT Gupta D Snowdon RL Lip GYH Fisher JP . Relationship of warfarin and apixaban with vascular function in patients with atrial fibrillation. J Vasc Res. (2024) 61:59–67. 10.1159/000535618
76.
Wagner HP Humphrey JD . Differential passive and active biaxial mechanical behaviors of muscular and elastic arteries: basilar versus common carotid. J Biomech Eng. (2011) 133:051009-1–10. 10.1115/1.4003873
77.
Pewowaruk RJ Hein AJ Carlsson CM Korcarz CE Gepner AD . Effects of nitroglycerin-induced vasodilation on elastic and muscular artery stiffness in older veterans. Hypertens Res. (2022) 45:1997–2007. 10.1038/s41440-022-00981-6
78.
Tricarico G Travagli V . SARS-CoV-2 infections and long COVID as drivers of accelerated biological aging in endothelial cells related to oxidative stress. Coronaviruses. (2025) 7:1–5. 10.2174/0126667975354302250126132207
79.
García Diez S De Nicolás Valdés M Diéguez Varela C Fernández Martínez P Suárez Gil P Navarro Rodríguez Y . Impacto del confinamiento por COVID-19 en la prescripción de benzodiacepinas. Aten Primaria. (2023) 55:102552. 10.1016/j.aprim.2022.102552
80.
Barhwal KK Parida B Pattnaik J Rowlo P Mahakud S Patra S et al Reduced reward responsiveness in treatment resistant depression of middle-aged adults: association with carotid artery stiffness and tetrahydrobiopterin. PLoS One. (2023) 18:e0290784. 10.1371/journal.pone.0290784
81.
Eriksson MD Eriksson J Kautiainen H Salonen MK Mikkola TM Kajantie E et al Higher carotid-radial pulse wave velocity is associated with non-melancholic depressive symptoms in men – findings from Helsinki birth cohort study. Ann Med. (2021) 53:531–40. 10.1080/07853890.2021.1904277
82.
Delialis D Mavraganis G Dimoula A Patras R Dimopoulou A-M Sianis A et al A systematic review and meta-analysis on the effect of selective serotonin reuptake inhibitors on endothelial function. J Affect Disord. (2022) 316:71–5. 10.1016/j.jad.2022.08.007
83.
Mäki-Petäjä KM Barrett SML Evans SV Cheriyan J McEniery CM Wilkinson IB . The role of the autonomic nervous system in the regulation of aortic stiffness. Hypertension. (2016) 68:1290–7. 10.1161/HYPERTENSIONAHA.116.08035
84.
Carrasco-Garrido P Palacios-Ceña D Hernández-Barrera V Jiménez-Trujillo I Gallardo-Pino C Fernández-de-las-Peñas C . Patterns of opioid and non-opioid analgesic consumption in patients with post-COVID-19 conditions. J Clin Med. (2023) 12:6586. 10.3390/jcm12206586
85.
Kim H-J Kim M-J Lee C-K Hong Y-H . Effects of methotrexate on carotid intima-media thickness in patients with rheumatoid arthritis. J Korean Med Sci. (2015) 30:1589–96. 10.3346/jkms.2015.30.11.1589
86.
Reece AS Hulse GK . Impact of lifetime opioid exposure on arterial stiffness and vascular age: cross-sectional and longitudinal studies in men and women. BMJ Open. (2014) 4:e004521. 10.1136/bmjopen-2013-004521
Summary
Keywords
drug use, long COVID, arterial stiffness, vascular structure, cardiovascular risk
Citation
Arroyo-Romero S, Gómez-Sánchez L, Suárez-Moreno N, Navarro-Cáceres A, Domínguez-Martín A, Lugones-Sánchez C, González-Sánchez S, Sánchez-Moreno A, Rodríguez-Sánchez E, García-Ortiz L, Navarro-Matias E and Gómez-Marcos MA (2026) Association between cardiovascular, psychotropic and anti-inflammatory/analgesic drug use and vascular dysfunction in individuals with long COVID. BioICOPER study. Front. Cardiovasc. Med. 12:1691153. doi: 10.3389/fcvm.2025.1691153
Received
22 August 2025
Revised
22 November 2025
Accepted
23 December 2025
Published
12 January 2026
Volume
12 - 2025
Edited by
Yun Fang, The University of Chicago, Chicago, United States
Reviewed by
Cristina Tudoran, Victor Babes University of Medicine and Pharmacy, Romania
Valter Travagli, University of Siena, Italy
Updates
Copyright
© 2026 Arroyo-Romero, Gómez-Sánchez, Suárez-Moreno, Navarro-Cáceres, Domínguez-Martín, Lugones-Sánchez, González-Sánchez, Sánchez-Moreno, Rodríguez-Sánchez, García-Ortiz, Navarro-Matias and Gómez-Marcos.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Silvia Arroyo-Romero silvia_ar@usal.es
†These authors share first authorship
‡These authors share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.