Abstract
Objectives:
Inappropriate dosing of direct oral anticoagulants (DOACs) may increase the risk of thromboembolism or bleeding in patients with atrial fibrillation (AF). The inappropriate use of these medications presents a significant clinical challenge. Our study aimed to analyze the current utilization of rivaroxaban and edoxaban among Chinese patients with AF, as well as the factors influencing the use of nonstandard doses.
Methods:
This study evaluated patients diagnosed with AF between January 2017 and December 2023. Descriptive analyses were performed to summarize the characteristics of the study population. Inappropriate dosing was identified based on the guidelines. Multivariate analysis was performed to identify factors associated with inappropriate dosing in these patients.
Results:
A total of 1,066 patients diagnosed with AF, comprising 852 individuals treated with rivaroxaban and 214 individuals treated with edoxaban, were included. Their median age was 69 years, and 58.7% of them were males. Among them, 573 patients (53.8%) received inappropriate dosages. Among the patients prescribed rivaroxaban, 503 (59.0%) were underdosed and eight (0.9%) were overdosed. Among the patients prescribed edoxaban, 49 patients (22.9%) were underdosed and 13 patients (6.1%) were overdosed. Multivariate analysis identified independent factors associated with inappropriate medication dosing, including advanced age [adjusted odds ratio (OR) 1.031, 95% confidence interval (CI) 1.010–1.052], combined use of antiplatelet drugs (adjusted OR 1.649, 95% CI 1.111–2.447), and reduced use of dronedarone (adjusted OR 0.332, 95% CI 0.126–0.877).
Conclusions:
The incidence of inappropriate DOAC dosing in Chinese patients with AF was high. Advanced age, the concurrent use of antiplatelet medications, and the nonuse of dronedarone have been identified as independent factors associated with inappropriate dosing.
1 Introduction
Atrial fibrillation (AF) is the most prevalent type of sustained cardiac arrhythmia (1). Approximately 30% of individuals with AF are hospitalized at least once per year, with 10% requiring more than two admissions (2–4). Furthermore, AF is associated with a fivefold increase in the risk of ischemic stroke. Guidelines recommended antithrombotic therapy with an oral anticoagulant (OAC) as the primary regimen for thrombotic prevention in patients with AF, effectively decreasing the incidence of cardioembolic events and stroke among high-risk individuals (1, 5, 6).
Warfarin is a classic anticoagulant typically used to prevent thrombotic events in patients with AF and remains one of the most widely prescribed anticoagulants globally. Warfarin has been shown to significantly reduce the risk of stroke in patients with non-valvular AF, with reported efficacy rates reaching up to 64% (7). Despite being a well-established anticoagulant, warfarin also has several limitations that restrict its utilization, such as the slow onset of action, the necessity for regular international normalized ratio monitoring, and a narrow therapeutic range. The optimal dosage of anticoagulant can vary significantly among individuals. In addition to dietary choices, medications, and comorbidities that may impact warfarin metabolism, genetic factors also contribute to individual variability in the required warfarin dosing (8).
The emergence of direct oral anticoagulants (DOACs) has offered new treatment approaches for preventing thromboembolism in patients with AF and has gradually become a prevailing trend in the treatment of AF. These DOACs include direct anti-Xa inhibitors such as apixaban, rivaroxaban, and edoxaban, along with the direct thrombin inhibitor dabigatran, which offers significant advantages over traditional potions like warfarin. A meta-analysis has revealed that compared with warfarin, DOACs are associated with a 19% reduction in the risk of stroke and systemic embolic events, a 10% decrease in all-cause mortality, and a 52% lower risk of hemorrhagic stroke, but a 25% increased risk of gastrointestinal bleeding risk (9). Currently, DOACs are recognized as the first-line treatment for stroke prevention in patients with AF (1, 5, 6). Contrary to warfarin, DOACs provide a more predictable therapeutic effect with a fixed-dose regimen due to their predictable pharmacokinetic and pharmacodynamic profiles. In addition, they do not require routine monitoring and are associated with fewer drug–drug and drug–food interactions (10).
A study conducted in China (2013–2014; 2015–2016) demonstrated a gradual increase in OAC prescription rates for AF patients, increasing from 21.0% to 41.0% over time (11). A systematic review indicated that suboptimal utilization of anticoagulants remains a persisting challenge, despite the availability of DOACs (12). To reduce the risk of bleeding and other adverse effects associated with DOACs, physicians may prescribe low-dose DOACs to patients. However, underdosing of DOAC can be linked to an elevated risk of thromboembolic stroke (13, 14).
To the best of our knowledge, this study is the first to evaluate the factors influencing inappropriate DOAC doses in Chinese hospitalized patients with AF. The study findings are expected to provide valuable insights and recommendations for enhancing the appropriate use of DOAC drugs.
2 Patients and methods
2.1 Study design
This retrospective, single-center study was conducted at the Beijing Tongren Hospital, a tertiary hospital with 1,700 beds in China. Ethical approval for this study was obtained from the Ethics Committee of Beijing Tongren Hospital (approval no.: TREC2024-KY152). Given its retrospective nature, the necessity for obtaining written informed consent from patients was waived. This study included individuals who were consecutively admitted to the hospital from January 2017 to December 2023 with a diagnosis of AF. A total of 7,456 patients diagnosed with AF were identified, excluding 162 who had a mechanical heart valve or were suffering from moderate-to-severe mitral stenosis, 136 who were admitted with venous thromboembolism, 5,764 who were discharged without receiving rivaroxaban or edoxaban, 118 who had incomplete clinical data (no serum creatinine or weight available), 210 who had multiple admissions (only the first record was retained), and 1,066 patients were ultimately included (Figure 1).
Figure 1
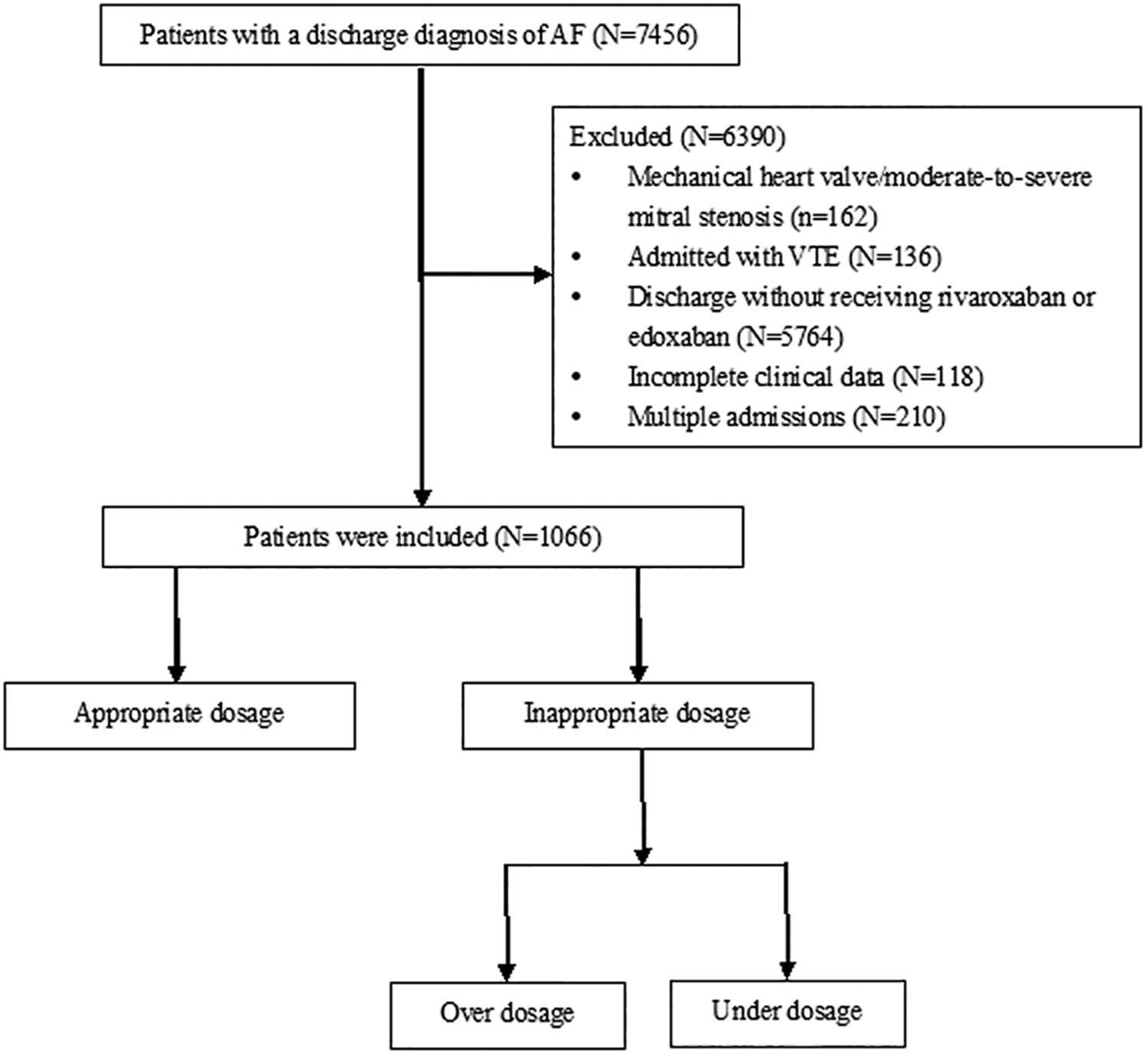
Flow chart of study population.
2.2 Data collection
Trained personnel collected patient clinical data through the electronic health record. Basic demographic information included department, age, gender, weight, history of bleeding, length of hospital stay, and diagnosis. Laboratory data were collected at the end of hospitalization, including serum creatinine, hemoglobin, and alanine aminotransferase (ALT). Systolic blood pressure and medications were prescribed at discharge. The Cockcroft–Gault formula was used to calculate the creatinine clearance. CHA2DS2-VA scores were calculated at discharge (1).
2.3 Evaluation criteria
The appropriate dosage standards for medications are based on the ESC Atrial Fibrillation Guidelines and the related drug package inserts (Table 1) (1, 15). Underdosing refers to prescribing a medication at a lower dose when the patient dose not fulfill the criteria for dose reduction. Overdosing is characterized by a prescription dose that remains unchanged despite the patient meeting the criteria for dose reduction, or when the total daily dose exceeds the limits recommended in the dosing guidelines.
Table 1
| Category | Rivaroxaban | Edoxaban |
|---|---|---|
| Standard dose | 20 mg qd | 60 mg qd |
| Reduced dose | 15 mg qd | 30 mg qd |
| Dose-reduction criteria | CrCl 15∼49 mL/min | - creatinine clearance 15–50 mL/min - body weight ≤ 60kg - concomitant use of ciclosporin, dronedarone, erythromycin, or ketoconazole |
| Contraindicated | CrCl <15 mL/min | CrCl <15 mL/min |
Dose selection criteria for NOACs.
2.4 Statistical analysis
The Kolmogorov–Smirnov test was utilized to assess the normality of continuous variables. Normally distributed variables were presented as means ± standard deviations, whereas nonnormally distributed variables were expressed as medians and interquartile ranges (IQR). For categorical variables, frequencies and percentages were utilized for description, with either the chi-square test or Fisher's exact test applied for comparisons. To determine the risk factors associated with the inappropriate dose of DOACs, binary logistic regression analysis was performed. Statistically significant variables (p < 0.05) in the univariable analysis were subsequently included in the multivariable regression model. All statistical analyses were performed using the Statistical Package for Social Sciences version 22.0, adhering to a significance threshold of α = 0.05.
3 Results
3.1 Study population characteristics
This study included a total of 1,066 patients diagnosed with AF, comprising 852 treated with rivaroxaban and 214 who received edoxaban. The cohort's median age was 69 (IQR, 62–78) years, and 58.7% of them were males. The median length of hospital stay was 9 (IQR, 6–12) days. Catheter ablation was performed in 34.4% of patients. The most common comorbidities were hypertension (71.5%), hyperlipidemia (60.8%), and diabetes (35.0%). The median number of medications prescribed at discharge was 7 (IQR, 5–10). The median CHA2DS2-VA scores were 3 (IQR, 2–5) (Table 2).
Table 2
| Characteristic | Total (N = 1,066) | Appropriate dose (N = 493) | Inappropriate dose (N = 573) | p-value |
|---|---|---|---|---|
| Age, years, median (IQR) | 69 (62,78) | 67 (60,75) | 71 (65,79) | <0.001 |
| Gender, Male, n (%) | 626 (58.7) | 297 (60.2) | 329 (57.4) | 0.350 |
| Length of stay, days, median (IQR) | 9 (6, 12) | 8 (6, 11) | 9 (6, 13) | 0.094 |
| Catheter ablation, n (%) | 367 (34.4) | 205 (41.6) | 162 (28.3) | <0.001 |
| Number of medications at discharge, median (IQR) | 7 (5,10) | 7 (5,9) | 8 (5,10) | <0.001 |
| Comorbidities | ||||
| Heart failure, n (%) | 346 (32.5) | 152 (30.8) | 194 (33.9) | 0.293 |
| Hypertension, n (%) | 762 (71.5) | 328 (66.5) | 434 (75.7) | <0.001 |
| Diabetes, n (%) | 373 (35.0) | 157 (31.8) | 216 (37.7) | 0.046 |
| Coronary heart disease, n (%) | 347 (32.6) | 142 (28.8) | 205 (35.8) | 0.015 |
| Hyperlipidemia, n (%) | 648 (60.8) | 297 (60.2) | 351 (61.3) | 0.736 |
| Stroke, n (%) | 234 (22.0) | 82 (16.6) | 152 (26.5) | <0.001 |
| Hyperuricemia, n (%) | 250 (23.5) | 129 (26.2) | 121 (21.1) | 0.052 |
| Infectious disease, n (%) | 240 (22.5) | 102 (20.7) | 138 (24.1) | 0.186 |
| Hypoalbuminemia, n (%) | 64 (6.0) | 27 (5.5) | 37 (6.5) | 0.502 |
| Anemia, n (%) | 135 (12.7) | 62 (12.6) | 73 (12.7) | 0.936 |
| COPD, n (%) | 30 (2.8) | 10 (2.0) | 20 (3.5) | 0.150 |
| Malignancy, n (%) | 55 (5.2) | 25 (5.1) | 30 (5.2) | 0.904 |
| Bleeding history, n (%) | 78 (7.3) | 25 (5.1) | 53 (9.2) | 0.009 |
| SBP at discharge, mmHg, median (IQR) | 124 (115, 132) | 123 (115, 130) | 124 (116, 132) | 0.05 |
| ALT at dischargea, median (IQR) | 19 (14, 29) | 20 (14, 30) | 19 (13, 27) | 0.047 |
| Hemoglobin count at dischargeb, median (IQR) | 134 (121, 146) | 135 (122, 148) | 133 (120, 145) | 0.045 |
| CrCl, mL/min, median (IQR) | 75.4 (56.6, 94.6) | 80.1 (55.3, 102.8) | 72.0 (57.6, 88.8) | <0.001 |
| CHA2DS2-VA, median (IQR) | 3 (2, 5) | 3 (1, 4) | 4 (2, 5) | <0.001 |
| Medications at discharge | ||||
| Antiplatelet drugs, n (%) | 169 (15.9) | 57 (11.6) | 112 (19.5) | <0.001 |
| Amiodarone, n (%) | 167 (15.7) | 94 (19.1) | 73 (12.7) | 0.005 |
| Dronedarone, n (%) | 26 (2.4) | 19 (3.9) | 7 (1.2) | 0.005 |
| Propafenone, n (%) | 26 (2.4) | 12 (2.4) | 14 (2.4) | 0.992 |
| β-blocker, n (%) | 645 (60.5) | 302 (61.3) | 343 (59.9) | 0.642 |
| Digoxin, n (%) | 72 (6.8) | 34 (6.9) | 38 (6.6) | 0.864 |
| Diltiazem, n (%) | 12 (1.1) | 5 (1.0) | 7 (1.2) | 0.749 |
| Statins, n (%) | 786 (73.7) | 348 (70.6) | 438 (76.4) | 0.030 |
| ACEI/ARB/ARNI, n (%) | 536 (50.3) | 240 (48.7) | 296 (51.7) | 0.333 |
| NSAID, n (%) | 7 (0.7) | 1 (0.2) | 6 (1.0) | 0.099 |
| MRA, n (%) | 171 (16.0) | 74 (15.0) | 97 (16.9) | 0.395 |
| Diuretics, n (%) | 310 (29.1) | 135 (27.4) | 175 (30.5) | 0.258 |
| Steroids, n (%) | 12 (1.1) | 4 (0.8) | 8 (1.4) | 0.367 |
| PPI, n (%) | 558 (52.3) | 257 (52.1) | 301 (52.5) | 0.896 |
| Anti-infection drug, n (%) | 68 (6.4) | 26 (5.3) | 42 (7.3) | 0.171 |
Factors contributing to inappropriate prescriptions (N = 1,066).
Data are shown as mean ± standard deviation, medians (first to third quartiles) or n (%).
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine transaminase; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; SBP, systolic blood pressure.
Full data not available: Total n = 1,055, Appropriate dose n = 487, Inappropriate dose n = 568.
Full data not available: Total n = 1,050, Appropriate dose n = 484, Inappropriate dose n = 566.
3.2 Appropriateness of the prescribed rivaroxaban or edoxaban dose levels
According to the criteria of the guidelines, 493 patients (46.2%) received the appropriate dose, whereas 573 patients (53.8%) were administered inappropriate doses. Among those prescribed rivaroxaban, 503 (59.0%) were underdosed and eight (0.9%) were overdosed. For those prescribed edoxaban, 49 patients (22.9%) were underdosed and 13 (6.1%) were overdosed. The proportion of appropriate prescriptions was higher for edoxaban (71.0%) than for rivaroxaban (40.0%) (Figure 2).
Figure 2
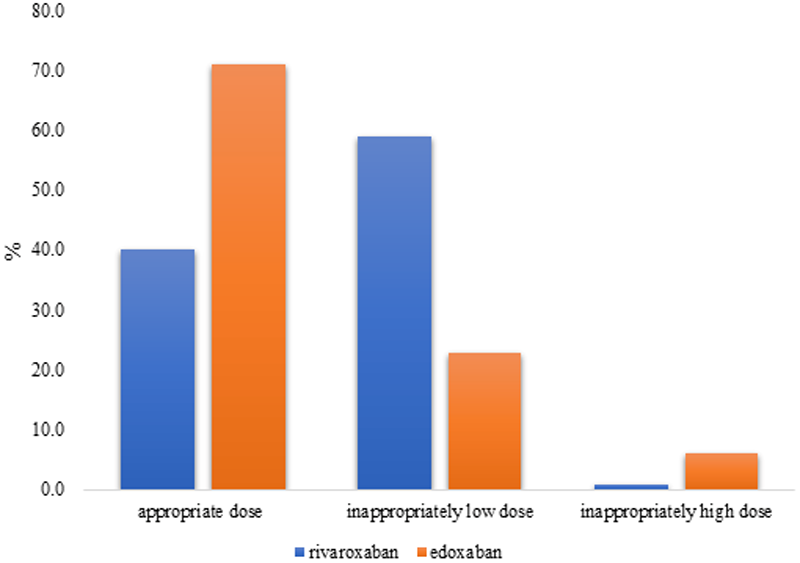
Frequency of appropriate vs. inappropriate dosing.
3.3 Factors associated with inappropriate prescriptions
Univariate analysis identified several factors significantly associated with inappropriate prescriptions, including advanced age, a higher total number of medications at discharge, and the presence of comorbidities such as hypertension, diabetes, coronary heart disease, stroke, and bleeding history. In addition, lower ALT, hemoglobin, and creatinine clearance levels, as well as higher CHA2DS2-VA scores and concomitant prescription of antiplatelet drugs and statins, were associated with inappropriate prescribing. The proportion of patients who underwent catheter ablation, as well as those prescribed amiodarone and dronedarone, was lower in patients prescribed inappropriate medication dosage compared with those who were treated with appropriate regimens (Table 2). Multivariate analysis identified independent factors, including advanced age [adjusted odds ratio (OR) 1.031, 95% confidence interval (CI) 1.010–1.052], the combined use of antiplatelet drugs (adjusted OR 1.649, 95% CI 1.111–2.447), and reduced use of dronedarone (adjusted OR 0.332, 95% CI 0.126–0.877), were associated with inappropriate medication (Table 3).
Table 3
| Variable | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Age | 1.031 (1.010–1.052) | 0.004 |
| Catheter ablation | 0.991 (0.710–1.382) | 0.956 |
| Number of medications | 0.989 (0.943–1.037) | 0.647 |
| Hypertension | 1.212 (0.863–1.702) | 0.267 |
| Diabetes | 0.985 (0.706–1.374) | 0.930 |
| Coronary heart disease | 0.881 (0.621–1.248) | 0.474 |
| Stroke | 1.293 (0.836–2.002) | 0.248 |
| Bleeding history | 1.322 (0.786–2.223) | 0.293 |
| ALT | 1.000 (0.993–1.006) | 0.883 |
| Hemoglobin count | 1.003 (0.995–1.010) | 0.481 |
| CrCl | 1.001 (0.996–1.007) | 0.624 |
| CHA2DS2-VA | 1.092 (0.920–1.298) | 0.314 |
| Antiplatelet drugs | 1.649 (1.111–2.447) | 0.013 |
| Amiodarone | 0.696 (0.471–1.028) | 0.069 |
| Dronedarone | 0.332 (0.126–0.877) | 0.026 |
| Statins | 0.942 (0.689–1.289) | 0.710 |
Multivariable logistic regression analysis of factors associated with inappropriate of DOACs.
ALT, alanine transaminase; CrCl, creatinine clearance.
Univariate analysis identified the following factors significantly associated with rivaroxaban underdosing: older age, the higher total number of medications at discharge, hypertension, stroke, bleeding history, lower hemoglobin levels, and creatinine clearance, higher CHA2DS2-VA scores, and concurrent prescription of antiplatelet drugs and nonsteroidal anti-inflammatory drugs (NSAIDs). Among those receiving inappropriate medications, a lower percentage of patients underwent catheter ablation or were prescribed amiodarone compared with those receiving appropriate medication (Table 4). In the multivariate analysis, older age (adjusted OR 1.053, 95% CI 1.029–1.078) and the combined use of antiplatelet drugs (adjusted OR 1.949, 95% CI 1.238–3.069) were independent factors found to be associated with rivaroxaban underdosing (Table 5).
Table 4
| Characteristic | Total (N = 844) | Appropriate dose (N = 341) | Inappropriate low dose (N = 503) | p-value |
|---|---|---|---|---|
| Age, years, median (IQR) | 69 (62, 77) | 65 (57, 71) | 71 (65, 79) | <0.001 |
| Gender, Male, n (%) | 512 (60.7) | 216 (63.3) | 296 (58.8) | 0.189 |
| Length of stay, days, median (IQR) | 9 (6,12) | 9 (6,12) | 9 (6,12) | 0.499 |
| Catheter ablation, n (%) | 288 (34.1) | 150 (44.0) | 138 (27.4) | <0.001 |
| Number of medications at discharge, median (IQR) | 7 (5,10) | 6 (5,9) | 7 (5,10) | <0.001 |
| Comorbidities | ||||
| Heart failure, n (%) | 268 (31.8) | 100 (29.3) | 168 (33.4) | 0.212 |
| Hypertension, n (%) | 609 (72.2) | 231 (67.7) | 378 (75.1) | 0.018 |
| Diabetes, n (%) | 291 (34.5) | 107 (31.4) | 184 (36.6) | 0.119 |
| Coronary heart disease, n (%) | 282 (33.4) | 104 (30.5) | 178 (35.4) | 0.140 |
| Hyperlipidemia, n (%) | 517 (61.3) | 208 (61.0) | 309 (61.4) | 0.899 |
| Stroke, n (%) | 186 (22.0) | 56 (16.4) | 130 (25.8) | 0.001 |
| Hyperuricemia, n (%) | 201 (23.8) | 95 (27.9) | 106 (21.1) | 0.023 |
| Infectious disease, n (%) | 177 (21.0) | 62 (18.2) | 115 (22.9) | 0.101 |
| Hypoalbuminemia, n (%) | 46 (5.5) | 17 (5.0) | 29 (5.8) | 0.624 |
| Anemia, n (%) | 88 (10.4) | 30 (8.8) | 58 (11.5) | 0.202 |
| COPD, n (%) | 23 (2.7) | 5 (1.5) | 18 (3.6) | 0.064 |
| Malignancy, n (%) | 48 (5.7) | 21 (6.1) | 27 (5.4) | 0.627 |
| Bleeding history, n (%) | 61 (7.2) | 16 (4.7) | 45 (8.9) | 0.019 |
| SBP at discharge, mmHg, median (IQR) | 124 (116, 132) | 123 (116, 130) | 125 (116, 133) | 0.096 |
| ALT at dischargea, median (IQR) | 19 (14, 29) | 20 (14, 31) | 19 (13, 27) | 0.067 |
| Hemoglobin count at dischargeb, median (IQR) | 135 (122, 147) | 137 (124, 150) | 133 (121, 146) | 0.006 |
| CrCl, mL/min, median (IQR) | 76.64 (58.4, 96.0) | 85.77 (60.0, 108.9) | 72.28 (58.0, 89.2) | <0.001 |
| CHA2DS2-VA, median (IQR) | 3 (2, 5) | 2 (1, 4) | 4 (2, 5) | <0.001 |
| Medications at discharge | ||||
| Antiplatelet drugs, n (%) | 139 (16.5) | 36 (10.6) | 103 (20.5) | <0.001 |
| Amiodarone, n (%) | 142 (16.8) | 73 (21.4) | 69 (13.7) | 0.003 |
| Dronedarone, n (%) | 3 (0.4) | 1 (0.3) | 2 (0.4) | 0.803 |
| Propafenone, n (%) | 17 (2.0) | 7 (2.1) | 10 (2.0) | 0.948 |
| β-blocker, n (%) | 514 (60.9) | 206 (60.4) | 308 (61.2) | 0.810 |
| Digoxin, n (%) | 55 (6.5) | 22 (6.5) | 33 (6.6) | 0.950 |
| Diltiazem, n (%) | 10 (1.2) | 3 (0.9) | 7 (1.4) | 0.500 |
| Statins, n (%) | 626 (74.2) | 242 (71.0) | 384 (76.3) | 0.080 |
| ACEI/ARB/ARNI, n (%) | 420 (49.8) | 160 (46.9) | 260 (51.7) | 0.174 |
| NSAID, n (%) | 6 (0.7) | 0 (0.0) | 6 (1.2) | 0.043 |
| MRA, n (%) | 138 (16.4) | 52 (15.2) | 86 (17.1) | 0.476 |
| Diuretics, n (%) | 241 (28.6) | 88 (25.8) | 153 (30.4) | 0.146 |
| Steroids, n (%) | 8 (0.9) | 3 (0.9) | 5 (1.0) | 0.866 |
| PPI, n (%) | 440 (52.1) | 182 (53.4) | 258 (51.3) | 0.553 |
| Anti-infection drug, n (%) | 46 (5.5) | 14 (4.1) | 32 (6.4) | 0.157 |
Factors influencing inadequate low dose of rivaroxaban (N = 844).
Data are shown as mean ± standard deviation, medians (first to third quartiles) or n(%).
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine transaminase; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; SBP, systolic blood pressure.
Full data not available: Total n = 839, Appropriate dose n = 339, Inappropriate low dose n = 500.
Full data not available: Total n = 831, Appropriate dose n = 335, Inappropriate low dose n = 496.
Table 5
| Variable | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Age | 1.053 (1.029–1.078) | <0.001 |
| Catheter ablation | 0.857 (0.587–1.252) | 0.429 |
| Number of medications | 1.000 (0.946–1.057) | 0.996 |
| Hypertension | 1.087 (0.745–1.587) | 0.665 |
| Stroke | 1.392 (0.871–2.226) | 0.167 |
| Hyperuricemia | 0.718 (0.502–1.026) | 0.069 |
| Bleeding history | 1.153 (0.615–2.161) | 0.657 |
| Hemoglobin count | 1.002 (0.994–1.010) | 0.631 |
| CrCl | 1.001 (0.994–1.008) | 0.744 |
| CHA2DS2-VA | 1.017 (0.868–1.192) | 0.834 |
| Antiplatelet drugs | 1.949 (1.238–3.069) | 0.004 |
| Amiodarone | 0.750 (0.484–1.163) | 0.199 |
Multivariable logistic regression analysis of factors associated with inappropriate low dose of rivaroxaban.
CrCl, creatinine clearance; NSAID, non-steroidal anti-inflammatory drug.
The variable “NSAID use” was excluded from the multivariable logistic regression model due to complete/quasi-complete separation in the data, which precluded stable estimation of its effect and led to unreliable odds ratios and confidence intervals.
Factors that were significantly associated with edoxaban underdosing in the univariate analysis included longer hospitalization; higher total number of medications at discharge; hypertension, coronary heart disease, and stroke comorbidities; higher CHA2DS2-VA scores; and combined use of anti-infection drugs. The proportion of patients who underwent catheter ablation and were prescribed dronedarone or β-blockers was lower among those receiving inappropriate medication compared with those receiving appropriate medication (Table 6). In the multivariate analysis, the presence of hypertension was independently associated with edoxaban underdosing (adjusted OR 3.529, 95% CI 1.304–9.548). β-blocker use was associated with a lower risk of inappropriate dosing (adjusted OR 0.427, 95% CI 0.203–0.897) (Table 7).
Table 6
| Characteristic | Total (N = 201) | Appropriate dose (N = 152) | Inappropriate low dose (N = 49) | p-value |
|---|---|---|---|---|
| Age, years, median (IQR) | 73 (65.5, 82.0) | 72 (64.0, 80.8) | 74 (68.5, 82.5) | 0.125 |
| Gender, Male, n (%) | 107 (53.2) | 81 (53.3) | 26 (53.1) | 0.978 |
| Length of stay, days, median (IQR) | 8 (6, 11) | 8 (6, 10) | 10 (7, 14) | 0.011 |
| Catheter ablation, n (%) | 66 (32.8) | 55 (36.2) | 11 (22.4) | 0.075 |
| Number of medications at discharge, median (IQR) | 8 (6, 10) | 7 (5, 10) | 9 (7, 12) | 0.001 |
| Comorbidities | ||||
| Heart failure, n (%) | 74 (36.8) | 52 (34.2) | 22 (44.9) | 0.177 |
| Hypertension, n (%) | 139 (69.2) | 97 (63.8) | 42 (85.7) | 0.004 |
| Diabetes, n (%) | 73 (36.3) | 50 (32.9) | 23 (46.9) | 0.075 |
| Coronary heart disease, n (%) | 60 (29.9) | 38 (25.0) | 22 (44.9) | 0.008 |
| Hyperlipidemia, n (%) | 118 (58.7) | 89 (58.6) | 29 (59.2) | 0.938 |
| Stroke, n (%) | 43 (21.4) | 26 (17.1) | 17 (34.7) | 0.009 |
| Hyperuricemia, n (%) | 46 (22.9) | 34 (22.4) | 12 (24.5) | 0.759 |
| Infectious disease, n (%) | 60 (29.9) | 40 (26.3) | 20 (40.8) | 0.054 |
| Hypoalbuminemia, n (%) | 17 (8.5) | 10 (6.6) | 7 (14.3) | 0.092 |
| Anemia, n (%) | 45 (22.4) | 32 (21.1) | 13 (26.5) | 0.424 |
| COPD, n (%) | 6 (3.0) | 5 (3.3) | 1 (2.0) | 0.655 |
| Malignancy, n (%) | 7 (3.5) | 4 (2.6) | 3 (6.1) | 0.246 |
| Bleeding history, n (%) | 14 (7.0) | 9 (5.9) | 5 (10.2) | 0.306 |
| SBP at discharge, mmHg, median (IQR) | 123 (114.5, 131.0) | 122 (113.0, 130.0) | 123 (119.0, 132.0) | 0.145 |
| ALT at dischargea, median (IQR) | 19 (14, 27) | 19.5 (15, 27) | 19 (12, 26) | 0.536 |
| Hemoglobin count at dischargeb, mean ± standard | 129.5 ± 20.3 | 130.2 ± 19.4 | 127.2 ± 23.0 | 0.369 |
| CrCl, mL/min, median (IQR) | 72.3 (50.0, 88.4) | 71.5 (48.6, 88.4) | 74.1 (60.1, 90.0) | 0.457 |
| CHA2DS2-VA, median (IQR) | 3 (2, 5) | 3 (2, 4) | 5 (3, 6) | <0.001 |
| Medications at discharge | ||||
| Antiplatelet drugs, n (%) | 29 (14.4) | 21 (13.8) | 8 (16.3) | 0.664 |
| Amiodarone, n (%) | 23 (11.4) | 21 (13.8) | 2 (4.1) | 0.063 |
| Dronedarone, n (%) | 18 (9.0) | 18 (11.8) | 0 (0.0) | 0.012 |
| Propafenone, n (%) | 7 (3.5) | 5 (3.3) | 2 (4.1) | 0.793 |
| β-blocker, n (%) | 118 (58.7) | 96 (63.2) | 22 (44.9) | 0.024 |
| Digoxin, n (%) | 17 (8.5) | 12 (7.9) | 5 (10.2) | 0.613 |
| Diltiazem, n (%) | 2 (1.0) | 2 (1.3) | 0 (0.0) | >0.999 |
| Statins, n (%) | 147 (73.1) | 106 (69.7) | 41 (83.7) | 0.056 |
| ACEI/ARB/ARNI, n (%) | 106 (52.7) | 80 (52.6) | 26 (53.1) | 0.958 |
| NSAID, n (%) | 1 (0.5) | 1 (0.7) | 0 (0.0) | >0.999 |
| MRA, n (%) | 31 (15.4) | 22 (14.5) | 9 (18.4) | 0.512 |
| Diuretics, n (%) | 66 (32.8) | 47 (30.9) | 19 (38.8) | 0.309 |
| Steroids, n (%) | 3 (1.5) | 1 (0.7) | 2 (4.1) | 0.148 |
| PPI, n (%) | 104 (51.7) | 75 (49.3) | 29 (59.2) | 0.231 |
| Anti-infection drug, n (%) | 22 (10.9) | 12 (7.9) | 10 (20.4) | 0.015 |
Factors influencing inadequate low dose of edoxaban (N = 201).
Data are shown as mean ± standard deviation, medians (first to third quartiles) or n (%).
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine transaminase; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; SBP, systolic blood pressure.
Full data not available: Total n = 195, Appropriate dose n = 148, Inappropriate low dose n = 47.
Full data not available: Total n = 198, Appropriate dose n = 149, Inappropriate low dose n = 49.
Table 7
| Variable | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Length of stay | 1.045 (0.961–1.135) | 0.304 |
| Number of medications | 1.050 (0.931–1.184) | 0.426 |
| Hypertension | 3.529 (1.304–9.548) | 0.013 |
| Coronary heart disease | 1.783 (0.690–4.605) | 0.232 |
| Stroke | 1.963 (0.665–5.797) | 0.222 |
| CHA2DS2-VA | 0.924 (0.647–1.319) | 0.663 |
| Dronedarone | 0.000 (0.000-) | 0.998 |
| β-blocker | 0.427 (0.203–0.897) | 0.025 |
| Anti-infection drug | 2.194 (0.754–6.379) | 0.149 |
Multivariable logistic regression analysis of factors associated with inappropriate low dose of edoxaban.
4 Discussion
Our findings provide real-world evidence that may facilitate optimal treatment choice. The key results of this study are summarized as follows: (1) About 53.8% of patients with AF were given inappropriate dosing, with 51.8% treated with underdoses and 2.0% with overdoses. Among those prescribed rivaroxaban, 59.0% were underdosed, and 0.9% were overdosed. Of the patients prescribed edoxaban, 22.9% were underdosed and 6.1% were overdosed. (2) Several independent factors were found to be associated with inappropriate medication dosing, including advanced age, the concurrent use of antiplatelet drugs, and reduced usage of dronedarone. (3) Older age and the concurrent usage of antiplatelet agents were factors independently associated with rivaroxaban underdosing. For edoxaban underdosing, the presence of hypertension was identified as a significant factor. Additionally, β-blocker administration was found to correlate with a lower risk of underdosing.
Rivaroxaban received its first clinical approved in China in 2009, while edoxaban was approved for clinical use in 2018. This discrepancy might explain the lower prescription rates of edoxaban observed in our study. A cross-sectional study conducted in Belgium indicated that inappropriate prescribing occurred in 16.9% of patients, with underdosing (9.7%) being more prevalent than overdosing (6.9%) (16). An Australia study revealed that 28.9% of patients with AF were prescribed inappropriate doses. Among them, underdosing was the predominant issue, with 22.9% for apixaban, 7.1% for dabigatran, and 25.1% for rivaroxaban (17). Similarly, the ANATOLIA-AF study reported that 24.9% of participants did not receive the appropriate DOAC dosage (18). A French registration study also found that 46% of patients were treated with inappropriate underdosing, with a higher prevalence observed among those reveiving apixaban (19). A retrospective registry revealed that 57.3% of patients were administered off-label doses of DOACs, including rivaroxaban and dabigatran (20). A study utilizing medical data from a multicenter health care system in Taiwan revealed that approximately 27% and 5% of AF patients were treated with underdosing and overdosing of NOACs, respectively (21). The variation in the prevalence of inappropriate DOAC dosing observed in real-world studies may be attributed to several factors, including the types of NOACs assessed, differences in the criteria of appropriate doses, ethnic differences in the bleeding risk associated with DOACs, availability of targeted anticoagulation reversal agents, and level of physicians' knowledge (22, 23).
Previous studies have indicated that patients receiving off-label DOAC doses do not receive the full benefit of anticoagulation (24). The GARFIELD-AF (Global Anticoagulant Registry in the FIELD-AF) study, conducted between 2013 and 2016, revealed that 72.9% received the recommended dosing, whereas 23.2% were underdosed and 3.8% were overdosed. Non-recommended dosing was associated with a higher risk of all-cause mortality when compared with the recommended dosing (14). A retrospective registry study found that DOACs administered at off-label underdosed were linked to a notably lower risk of major bleeding and all-cause mortality compared with on-label doses. However, no significant differences were observed in the risks of thrombotic events or minor bleeding (20). A systematic review assessed the clinical efficacy and safety of low-dose DOAC treatment vs. on-label dosing in Asian patients with AF. The outcomes of the low-dose treatment did not display significant differences when compared to the standard-dose treatment (25). A study conducted in a tertiary medical center in Taiwan revealed that off-label low-dosing of rivaroxaban should be avoided due to the higher risk of ischemic stroke, without a corresponding reduction in the risk of intracranial hemorrhage when compared to on-label dosing (26).
The reported clinical factors associated with inappropriate dosing vary between studies. Our results indicate that advanced age and the concurrent use of antiplatelet drugs are independent factors influencing inappropriate medication dosing and the underdosing of rivaroxaban.
Previous studies have established that advanced age is an independent risk factor for inappropriate medication use (18, 19, 27). Our present study observed results consistent with these findings because of the increased occurrence of bleeding events in elderly patients, who frequently present with multiple comorbidities, polypharmacy, low weight, frailty, and falls. Our study found that older age was also an independent risk factor associated with rivaroxaban underdosing; however, it was not recognized as a risk factor for edoxaban underdosing. In addition, the incidence of edoxaban underdosing in this study was lower than that of rivaroxaban, which may be associated with edoxaban showing a significantly lower risk of bleeding (28, 29). Another study indicated that the net clinical benefit is more pronounced in the elderly population receiving standard DOAC doses compared to those treated with lower doses (30).
Previous studies have demonstrated that the risk of bleeding increases with the concomitant use of DOACs and antiplatelet agents, and they are well-known in clinical practice (31). Concerns regarding the risk of bleeding may lead clinicians to prescribe low-dose DOACs. Previous guidelines recommended that clinicians utilize the HAS-BLED scores to assess the risk of bleeding in patients with AF. The HAS-BLED score awards 1 point for the use of an antiplatelet drug (15). However, the use of bleeding risk scores was not recommended by the new guidelines; instead, they suggest that modifiable bleeding risk factors should be managed to improve safety (1).
This study revealed that patients using amiodarone and dronedarone were more likely to be prescribed standard DOAC doses, a finding that may differ from previous studies (18). Amiodarone and dronedarone are known potential inhibitors of P-glycoprotein (P-gp) and CYP3A4, which might be associated with an increased risk of bleeding when used with DOACs (32). The results may be attributed to closer monitoring or stricter medication management during hospitalization for patients who underwent catheter ablation. It is important to note that NOACs do not require frequent INR monitoring which benefits patients, but also imposes a risk of clinical consequences due to unrecognized interactions.
Hypertension is a risk factor for inappropriate medication dosing and rivaroxaban underdosing. It has also been identified as an independent risk factor for edoxaban underdosing. Among patients diagnosed with AF, hypertension has been associated with an increased risk of stroke, heart failure, and major bleeding (33, 34). In this population, hypertension frequently exists with other risk factors, such as coronary heart disease and stroke. Additionally, factors such as lower hemoglobin, reduced creatinine clearance, a bleeding history, and a higher CHA2DS2-VASc score are also important risk factors for inappropriate medication dosing, as reported in previous studies (19, 35, 36). In our study, these factors were identified as univariate variables associated with inappropriate medication dosing; however, the multivariate analysis did not reveal any statistically significant differences.
The unexpected observation regarding edoxaban was that the use of β-blockers was associated with appropriate prescriptions. We hypothesize that this finding may be attributed to the absence of drug interactions with edoxaban, which alleviates concerns about bleeding risks. Conversely, previous studies have reported NSAIDs as factors that could increase the risks associated with low-dose NOAC use due to their potential to increase bleeding risk (18, 37). However, this study reported that NSAIDs exhibited a significant difference in the univariate analysis, but no statistical difference was observed in the multivariate analysis, which may be due to the small number of patients using NSAIDs.
Despite their numerous advantages, NOACs present significant safety challenges, particularly regarding potential drug interactions in patients undergoing polypharmacy and the absence of specific monitoring indicators. While NOACs have fewer drug-drug interactions than warfarin—a clear benefit—this advantage may paradoxically lead clinicians to underestimate the importance of screening for NOAC interactions. Furthermore, since routine therapeutic monitoring is not required for dose adjustment with NOACs, deviations from recommended prescribing information make it difficult to assess the level of anticoagulation. To mitigate inappropriate prescribing, several strategies can be implemented: (1) enhancing pharmacist-led medication reviews to optimize anticoagulant dosing; (2) establishing a multidisciplinary “anticoagulation team” focused on defining optimal management strategies and reducing potentially inappropriate medications, particularly in polypharmacy patients; and (3) implementing computerized decision-support systems (CDSS) to manage anticoagulation and minimize inappropriate anticoagulant prescribing.
This study is, to our knowledge, the first to explore factors associated with inappropriate DOAC dosing in AF patients in China. However, several limitations should be acknowledged. First, this retrospective, single-center study was conducted at a tertiary hospital in Beijing, a major metropolitan area, which may limit generalizability to other regions, countries, or hospital levels and introduces potential selection bias. Second, dabigatran was excluded due to limited availability of only a single dose formulation at our institution, while apixaban was excluded because it was not available at all, both of which constrain the comprehensiveness of our findings. Third, the use of inpatient rather than outpatient data—chosen for superior feature coverage—may limit extrapolation to outpatient settings and real-world practice. Finally, we did not examine the relationship between clinical outcomes and inappropriate DOAC dosing, which warrants investigation in future research.
5 Conclusions
This study reveals a high incidence of inappropriate dosing of DOACs in patients with AF in China, with underdosing occurring more frequently, particularly among those prescribed rivaroxaban. Advanced age, concurrent use of antiplatelet medications, and the nonuse of dronedarone were identified as independent factors associated with inappropriate dosing. Currently, patient undertreatment and non-adherence to guidelines remain significant issues, and thereby, the standardization of medication use should be improved.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Tongren Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YB: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. JW: Data curation, Investigation, Supervision, Writing – review & editing. GL: Investigation, Writing – review & editing. ZZ: Formal analysis, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
We sincerely thank and are grateful to all the authors who participated in this study.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Van Gelder IC Rienstra M Bunting KV Casado-Arroyo R Caso V Crijns HJGM et al 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2024) 45:3314–414. 10.1093/eurheartj/ehae176
2.
Kirchhof P Schmalowsky J Pittrow D Rosin L Kirch W Wegscheider K et al Management of patients with atrial fibrillation by primary-care physicians in Germany: 1-year results of the ATRIUM registry. Clin Cardiol. (2014) 37:277–84. 10.1002/clc.22272
3.
Meyre P Blum S Berger S Aeschbacher S Schoepfer H Briel M et al Risk of hospital admissions in patients with atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. (2019) 35:1332–43. 10.1016/j.cjca.2019.05.024
4.
Steinberg BA Kim S Fonarow GC Thomas L Kowey AJ R P et al Drivers of hospitalization for patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J. (2014) 167:735–42.e2. 10.1016/j.ahj.2014.02.003
5.
Joglar JA Chung MK Armbruster AL Benjamin EJ Chyou JY Cronin EM et al 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2024) 149:e1–e156. 10.1161/CIR.0000000000001193
6.
Andrade JG Aguilar M Atzema C Bell A Cairns JA Cheung CC et al The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. (2020) 36:1847–948. 10.1016/j.cjca.2020.09.001
7.
Hart RG Pearce LA Aguilar MI . Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. 10.7326/0003-4819-146-12-200706190-00007
8.
Johnson JA Caudle KE Gong L Whirl-Carrillo M Stein CM Scott SA et al Clinical pharmacogenetics implementation consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. (2017) 102:397–404. 10.1002/cpt.668
9.
Ruff CT Giugliano RP Braunwald E Hoffman EB Deenadayalu N Ezekowitz MD et al Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. 10.1016/S0140-6736(13)62343-0
10.
Samama MM Contant G Spiro TE Perzborn E Le Flem L Guinet C et al Laboratory assessment of rivaroxaban: a review. Thromb J. (2013) 11:11. 10.1186/1477-9560-11-11
11.
Liu X Feng G Marler SV Huisman MV Lip GYH Ma C . Real world time trends in antithrombotic treatment for newly diagnosed atrial fibrillation in China: reports from the GLORIA-AF phase III registry: trends in antithrombotic therapy use in China. Thromb J. (2023) 21:83. 10.1186/s12959-023-00527-x
12.
Alamneh EA Chalmers L Bereznicki LR . Suboptimal use of oral anticoagulants in atrial fibrillation: has the Introduction of direct oral anticoagulants improved prescribing practices?Am J Cardiovasc Drugs. (2016) 16:183–200. 10.1007/s40256-016-0161-8
13.
Kubota K Ooba N . Effectiveness and safety of reduced and standard daily doses of direct oral anticoagulants in patients with nonvalvular atrial fibrillation: a cohort study using national database representing the Japanese population. Clin Epidemiol. (2022) 14:623–39. 10.2147/CLEP.S358277
14.
Camm AJ Cools F Virdone S Bassand JP Fitzmaurice DA Arthur Fox KA et al Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol. (2020) 76:1425–36. 10.1016/j.jacc.2020.07.045
15.
Hindricks G Potpara T Dagres N Arbelo E Bax JJ Blomström-Lundqvist C et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehaa612
16.
Moudallel S Cornu P Dupont A Steurbaut S . Determinants for under- and overdosing of direct oral anticoagulants and physicians’ implementation of clinical pharmacists’ recommendations. Br J Clin Pharmacol. (2022) 88:753–63. 10.1111/bcp.15017
17.
Li RJ Caughey GE Shakib S . Appropriateness of inpatient dosing of direct oral anticoagulants for atrial fibrillation. J Thromb Thrombolysis. (2022) 53:425–35. 10.1007/s11239-021-02528-x
18.
Kocabaş U Ergin I Yavuz V Murat S Özdemir I Genç Ö et al Prevalence and associated factors of inappropriate dosing of direct oral anticoagulants in patients with atrial fibrillation: the ANATOLIA-AF study. Cardiovasc Drugs Ther. (2024) 38:581–99. 10.1007/s10557-022-07409-w
19.
Guenoun M Cohen S Villaceque M Sharareh A Schwartz J Hoffman O et al Characteristics of patients with atrial fibrillation treated with direct oral anticoagulants and new insights into inappropriate dosing: results from the French national prospective registry: PAFF. Europace. (2023) 25:euad302. 10.1093/europace/euad302
20.
Xu W Lv M Wu T Huang N Zhang W Su J et al Off-label dose direct oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation: a new evidence of Asian dose. Int J Cardiol. (2023) 371:184–90. 10.1016/j.ijcard.2022.09.073
21.
Chan YH Chao TF Chen SW Lee HF Yeh YH Huang YC et al Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm. (2020) 17(12):2102–10. 10.1016/j.hrthm.2020.07.022
22.
Chao TF Chen SA Ruff CT Hamershock RA Mercuri MF Antman EM et al Clinical outcomes, edoxaban concentration, and anti-factor Xa activity of Asian patients with atrial fibrillation compared with non-Asians in the ENGAGE AF-TIMI 48 trial. Eur Heart J. (2019) 40:1518–27. 10.1093/eurheartj/ehy807
23.
Pollack CV Jr Reilly PA van Ryn J Eikelboom JW Glund S Bernstein RA et al Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. (2017) 377: 431–41. 10.1056/NEJMoa1707278
24.
Santos J António N Rocha M Fortuna A . Impact of direct oral anticoagulant off-label doses on clinical outcomes of atrial fibrillation patients: a systematic review. Br J Clin Pharmacol. (2020) 86:533–47. 10.1111/bcp.14127
25.
Choi J No JE Lee JY Choi SA Chung WY Ah YM et al Efficacy and safety of clinically driven low-dose treatment with direct oral anticoagulants in Asians with atrial fibrillation: a systematic review and meta-analysis. Cardiovasc Drugs Ther. (2022) 36:333–45. 10.1007/s10557-021-07171-5
26.
Cheng WH Chao TF Lin YJ Chang SL Lo LW Hu YF et al Low-dose rivaroxaban and risks of adverse events in patients with atrial fibrillation. Stroke. (2019) 50(9):2574–7. 10.1161/STROKEAHA.119.025623
27.
Steinberg BA Shrader P Pieper K Thomas L Allen LA Ansell J et al Frequency and outcomes of reduced dose non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (the outcomes registry for better informed treatment of atrial fibrillation II). J Am Heart Assoc. (2018) 7:e007633. 10.1161/JAHA.117.007633
28.
Kim SM Jeon ET Jung JM Lee JS . Real-world oral anticoagulants for Asian patients with non-valvular atrial fibrillation: a PRISMA-compliant article. Medicine (Baltimore). (2021) 100:e26883. 10.1097/MD.0000000000026883
29.
Bonanad C Formiga F Anguita M Petidier R Gullón A . Oral anticoagulant use and appropriateness in elderly patients with atrial fibrillation in complex clinical conditions: aCONVENIENCE study. J Clin Med. (2022) 11:7423. 10.3390/jcm11247423
30.
Alnsasra H Haim M Senderey AB Reges O Leventer-Roberts M Arnson Y et al Net clinical benefit of anticoagulant treatments in elderly patients with nonvalvular atrial fibrillation: experience from the real world. Heart Rhythm. (2019) 16:31–7. 10.1016/j.hrthm.2018.08.016
31.
Hijazi Z Oldgren J Lindbäck J Alexander JH Alings M De Caterina R et al Evaluation of the age, biomarkers, and clinical history-bleeding risk score in patients with atrial fibrillation with combined aspirin and anticoagulation therapy enrolled in the ARISTOTLE and RE-LY trials. JAMA Netw Open. (2020) 3:e2015943. 10.1001/jamanetworkopen.2020.15943
32.
Gronich N Stein N Muszkat M . Association between use of pharmacokinetic-interacting drugs and effectiveness and safety of direct acting oral anticoagulants: nested case-control study. Clin Pharmacol Ther. (2021) 110:1526–36. 10.1002/cpt.2369
33.
Friberg L Rosenqvist M Lip GY . Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J. (2012) 33:1500–10. 10.1093/eurheartj/ehr488
34.
Potpara TS Polovina MM Licina MM Marinkovic JM Lip GY . Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: the Belgrade atrial fibrillation study. Eur J Heart Fail. (2013) 15:415–24. 10.1093/eurjhf/hft004
35.
Anouassi Z Atallah B Alsoud LO El Nekidy W Al Mahmeed W AlJaabari M et al Appropriateness of the direct oral anticoagulants dosing in the Middle East gulf region. J Cardiovasc Pharmacol. (2021) 77:182–8. 10.1097/FJC.0000000000000913
36.
Rymer JA Chiswell K Young L Chiu A Liu L Webb L et al Analysis of oral anticoagulant dosing and adherence to therapy among patients with nonvalvular atrial fibrillation. JAMA Netw Open. (2023) 6:e2317156. 10.1001/jamanetworkopen
37.
Olsen AS McGettigan P Gerds TA Fosbøl EL Olesen JB Sindet-Pedersen C et al Risk of gastrointestinal bleeding associated with oral anticoagulation and non-steroidal anti-inflammatory drugs in patients with atrial fibrillation: a nationwide study. Eur Heart J Cardiovasc Pharmacother. (2020) 6:292–300. 10.1093/ehjcvp/pvz069
Summary
Keywords
atrial fibrillation, direct oral anticoagulant (DOAC), inappropriate dosing, medication review, risk factors
Citation
Bai Y, Wang J, Li G and Zhou Z (2026) Factors influencing the inappropriate dosing of rivaroxaban and edoxaban in Chinese hospitalized patients with atrial fibrillation. Front. Cardiovasc. Med. 12:1694976. doi: 10.3389/fcvm.2025.1694976
Received
29 August 2025
Revised
05 December 2025
Accepted
15 December 2025
Published
13 January 2026
Volume
12 - 2025
Edited by
dimitrios vrachatis, National and Kapodistrian University of Athens, Greece
Reviewed by
Catalina Lionte, Grigore T. Popa University of Medicine and Pharmacy, Romania
Ehsan Habeeb, Taibah University, Saudi Arabia
Updates
Copyright
© 2026 Bai, Wang, Li and Zhou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Ying Bai felisha_bai@hotmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.