- 1Department of Urology, The First Hospital of Jilin University, Changchun, China
- 2Department of Cardiology, Affiliated Hospital of Changchun University of Traditional Chinese Medicine, Changchun, China
- 3Department of Pulmonology, Affiliated Hospital of Changchun University of Traditional Chinese Medicine, Changchun, China
- 4Lithotriptic Center, The First Hospital of Jilin University, Changchun, China
High mobility group box-1 (HMGB1) is a protein released from stressed or damaged cells that triggers immune activation and chronic inflammation. The NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) is a central component of the inflammasome, which activates caspase-1 and releases pro-inflammatory cytokines, including IL-1β and IL-18. The HMGB1/NLRP3 axis plays a critical role in regulating inflammation and immune responses, driving systemic inflammation and disease progression. Targeting this pathway offers promising therapeutic strategies for conditions such as autoimmune disorders, trauma, and chronic inflammatory diseases. In particular, inhibiting HMGB1 or NLRP3 can mitigate the exaggerated inflammatory response, reduce tissue damage, and slow disease progression. This review explores the bidirectional interactions between HMGB1 and NLRP3 and discusses current and emerging therapeutic approaches targeting this axis to modulate inflammation and improve clinical outcomes.
1 Introduction
The high mobility group box-1 (HMGB1) protein, a non-histone nuclear protein widely expressed in eukaryotic cells, plays a central role in DNA modulation and transcriptional regulation. Over the past few decades, HMGB1 has gained increasing recognition for its pivotal involvement in inflammation and the pathogenesis of systemic diseases (Goodwin et al., 1973; Rauvala et al., 2007; Chi et al., 2015). Upon cellular stress or injury, HMGB1 is released, either actively or passively, as a damage-associated molecular pattern (DAMP). It interacts with receptors such as toll-like receptors (TLRs) and the receptor for advanced glycation end products (RAGE), amplifying signaling pathways that drive inflammatory and immune responses (Yang et al., 2015; Harris et al., 2012). The NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome is another key player in inflammatory diseases. Composed of the sensor protein NLRP3, the adaptor protein ASC, and pro-caspase-1, it recognizes cellular damage and infections, triggering the release of pro-inflammatory cytokines like IL-1β and IL-18, and inducing pyroptosis (Bauernfeind et al., 2009; Yang et al., 2019). The relationship between HMGB1 and NLRP3 forms a critical feedback loop: HMGB1 activates NLRP3 through NF-κB signaling and ROS/ATP pathways, while NLRP3 activation further promotes the release of HMGB1, exacerbating inflammation (Yang et al., 2020a). This bidirectional interaction sustains chronic inflammation, contributing to the progression of various systemic diseases.
The HMGB1/NLRP3 axis plays a critical role in mediating the transition from localized inflammation to systemic responses, acting as a molecular bridge in chronic inflammation and immune dysregulation. Understanding the role of the HMGB1/NLRP3 signaling pathway is especially pertinent for systemic diseases such as autoimmune disorders, cardiovascular conditions, and neurodegenerative diseases. Inflammation driven by the HMGB1/NLRP3 axis contributes to disease progression and tissue damage in these contexts. This review provides a comprehensive analysis of the mechanistic roles of HMGB1 and NLRP3 in systemic inflammation and explores their potential as therapeutic targets for systemic diseases.
2 Structure and function of HMGB1 and NLRP3
2.1 HMGB1
HMGB1 is a multifunctional protein that plays a central role in regulating inflammation and immune responses. It consists of 215 amino acids and has a molecular weight of approximately 30 kDa. Its structure features two highly conserved DNA-binding domains, known as the A box (amino acids 1–79) and B box (amino acids 89–162), along with a C-terminal acidic tail (amino acids 186–215) (Figure 1A). These domains are arranged in an “L” shape, facilitating their interaction with DNA. The A and B boxes are positively charged, enabling them to bind to the minor groove of DNA, thereby inducing DNA bending and subsequently altering nucleosome stability and chromatin conformation (Figure 1B). The B box functions as the primary inflammatory region, while the A box acts as its antagonistic counterpart. The functions of these domains are influenced by the redox state of three key cysteines (Cys23, Cys45, and Cys106) (Kang et al., 2014; Frank et al., 2015a; Wang et al., 2019). The C-terminal acidic tail of HMGB1, rich in glutamic and aspartic acids, carries a negative charge and plays a role in its functions, while a short N-terminal region of lysine residues contributes to its interactions and biological activity (Chen et al., 2022; Bianchi et al., 1992). HMGB1 contains two nuclear localization signals (NLS1 and NLS2) that mediate its nuclear translocation. Structurally, the protein contains specific binding domains that facilitate interactions with pattern recognition receptors (PRRs), particularly TLR and RAGE, thereby promoting its diverse biological functions through these receptor-mediated pathways (Taverna et al., 2022) (Figure 1A).
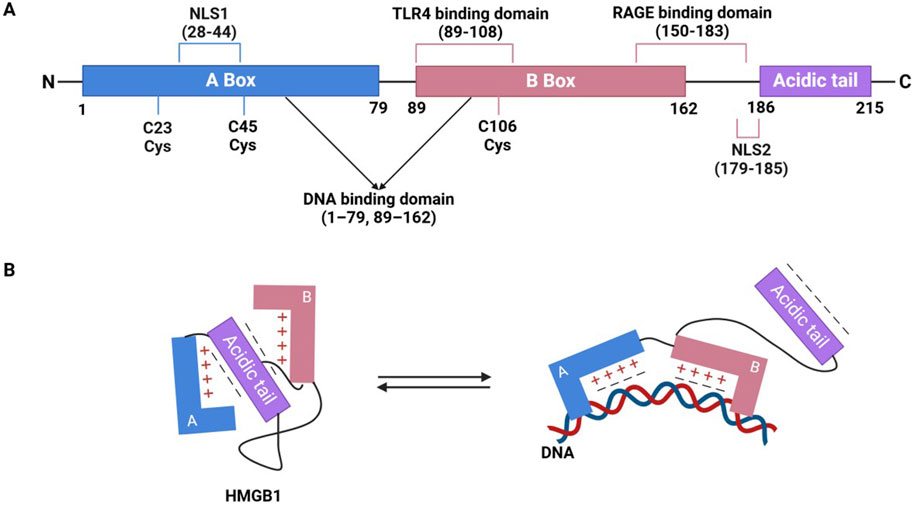
Figure 1. The structure of HMGB1 and its interaction with DNA. (A) Domain and functional sites of HMGB1. (B) Conformational transition of HMGB1 and its DNA binding mode.
Normally, HMGB1 is located in the nucleus, where it acts as a non-histone chromatin protein. By binding to DNA, it regulates chromatin structure and influences key processes such as gene expression, DNA repair, and replication (Bianchi and Agresti, 2005). However, under stress conditions such as infection, injury, or inflammation, HMGB1 can be released into the extracellular space, where it acts as DAMP. This release can occur through intrinsic immune activation or passive release following cellular damage, involving various cell types like immune cells, fibroblasts, and epithelial cells (Chen et al., 2022; Pisetsky, 2014; Bonaldi et al., 2003). Once outside the cell, HMGB1 binds to various receptors, thereby activating immune cells, amplifying inflammatory responses, and driving disease progression (Rapoport et al., 2020). Post-translational modifications, such as acetylation, phosphorylation, and oxidation, regulate its shuttling between the nucleus and cytoplasm, influencing processes like apoptosis, autophagy, and cell death, which ultimately determine (Tang et al., 2007; Wu et al., 2013; Ge et al., 2014; Cui et al., 2019; Kang et al., 2011).
The functional activity of HMGB1 is predominantly modulated by its redox state. In its unoxidized (reduced) form, HMGB1 primarily functions as a chemokine, recruiting immune cells to sites of injury or inflammation without triggering a significant inflammatory response. However, when oxidized, HMGB1 exhibits pro-inflammatory properties, activating signaling pathways such as NF-κB and MAPK, which amplify the inflammatory response (Park et al., 2003; Dumitriu et al., 2005). In addition, evidence indicates that redox status alone does not fully explain HMGB1’s pleiotropic functions. Its extracellular activity is also critically shaped by the type, expression level, and activation state of cell surface receptors within the local microenvironment. These receptors mainly include classical PRRs such as TLR4 and RAGE, as well as non-canonical receptors like TIM-3 (Tang and Lotze, 2012) and CXCR4 (Pirani et al., 2024), which are differentially expressed across immune cell subsets and pathological states, thereby contributing to distinct immunological outcomes in processes such as inflammation, tumor progression, and tissue repair. This dual regulation by redox state and receptor context adds a layer of complexity to HMGB1’s immunomodulatory functions. At lower concentrations, HMGB1 contributes to antimicrobial activity and transient inflammation. However, during infection, necrosis, or apoptosis, HMGB1 levels rise significantly, leading to severe inflammation, epithelial barrier disruption, organ dysfunction, and even death. Active HMGB1 also plays a role in various pathological conditions, including fever, anorexia, acute-phase reactions, and vascular leakage syndrome (Andersson and Tracey, 2011).
2.2 NLRP3
As a key sensor of the intrinsic immune system, NLRP3 sensitively detects endogenous cellular damage and exogenous pathogenic invasion. Structurally, NLRP3 contains three main functional domains: the Pyrin domain (PYD) for protein stabilization, the leucine-rich repeat (LRR) domain for protein-protein interactions, and the NACHT domain, responsible for nucleotide binding and ATP hydrolysis (Sharif et al., 2019; Swanson et al., 2019). In addition, the NACHT domain includes several subdomains: the Fish-specific NACHT-associated domain (FISNA), Nucleotide-binding domain (NBD), helical domains (HD1, HD2), and a winged helical domain (WHD), essential for NLRP3’s function (Fu and Wu, 2023; Zhang et al., 2015; Dekker et al., 2021) (Figure 2A).
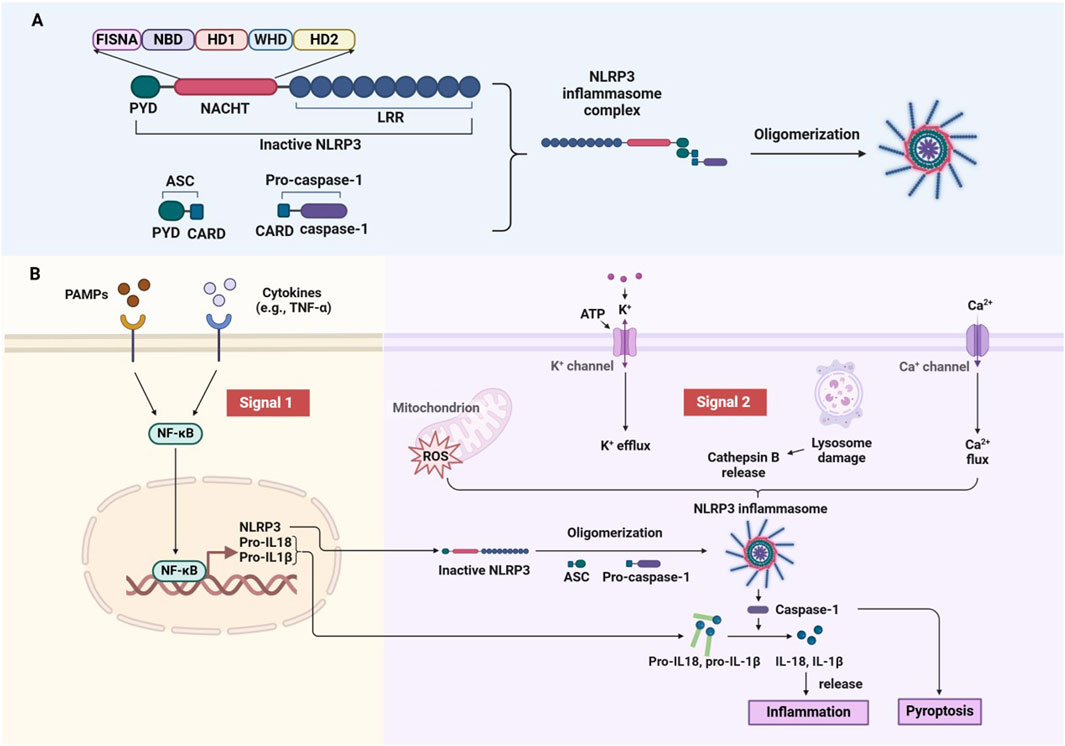
Figure 2. Structural domains and activation mechanism of the NLRP3 inflammasome. (A) Domain of NLRP3 and assembly of the Inflammasome. (B) Two-Signal model for NLRP3 inflammasome activation.
NLRP3 activation occurs in response to a wide range of endogenous and exogenous stimuli, including ATP, microbial agonists, particulate matter, and pore-forming toxins. The activation process is triggered by two signals. The initial signal is provided by PAMPs or cytokines (e.g., TNF-α), which activate the NF-κB pathway to induce NLRP3 and pro-IL-1β expression. The second signal is triggered by microbial or danger signals, leading to K+ efflux (Muñoz-Planillo et al., 2013), Ca2+ influx (Lee et al., 2012), cathepsin B leakage from lysosomes (Hornung et al., 2008), ROS production (Zhou et al., 2010), translocation to mitochondria (Zhou et al., 2011), and mitochondrial dysfunction (Iyer et al., 2013). When activated, NLRP3 forms a complex with apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase-1 (Agost et al., 2004). This complex drives the maturation and release of inflammatory cytokines such as IL-1β and IL-18, which promote further immune activation and recruit additional immune cells to the site of injury or infection (Schroder and Tschopp, 2010). Additionally, NLRP3 activation can lead to a form of programmed cell death called pyroptosis, which exacerbates inflammation and tissue damage (Boucher et al., 2018) (Figure 2B). While essential for pathogen defense, dysregulated NLRP3 activity is associated with various inflammatory and autoimmune diseases, such as arthritis, cardiovascular disease, and neurodegenerative disorders. Therefore, understanding how NLRP3 is activated and regulated is crucial for developing therapies aimed at modulating its activity in disease contexts.
3 Inflammatory mechanisms of the HMGB1/NLRP3 axis
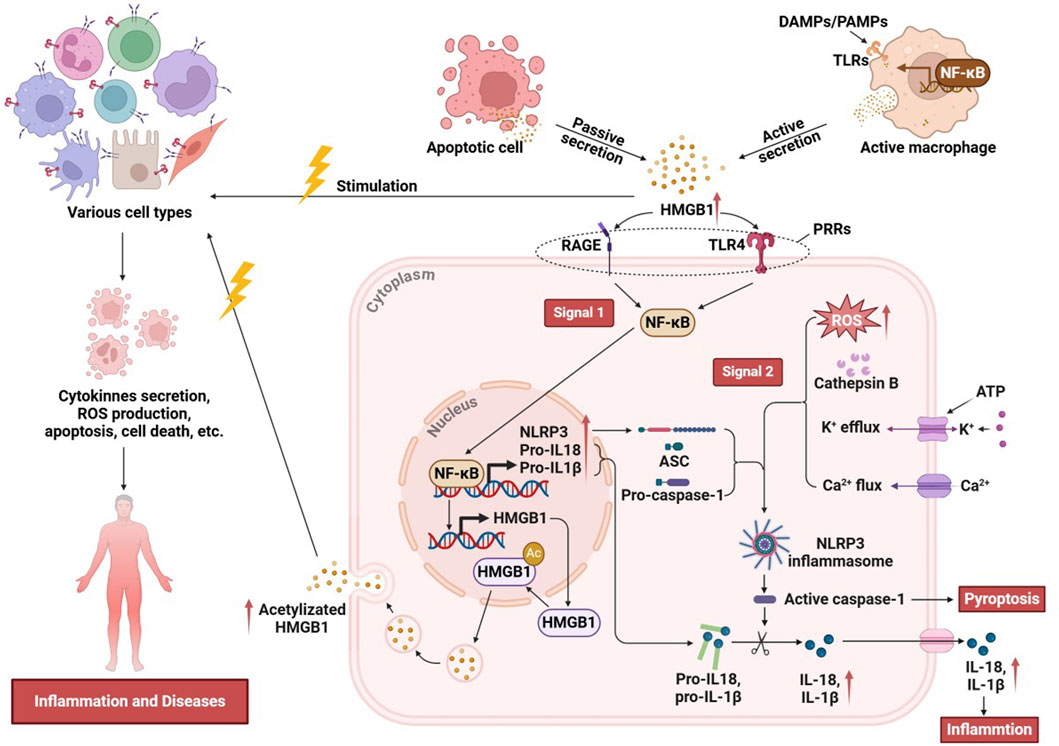
The interaction between HMGB1 and the NLRP3 inflammasome has emerged as a critical regulator of inflammatory responses across various pathological contexts. HMGB1, acting as a DAMP, interacts with immune receptors to initiate and amplify inflammation, particularly by engaging the NLRP3 pathway. The HMGB1/NLRP3 axis orchestrates a series of pro-inflammatory events that drive acute and chronic inflammatory states, making it a central focus in understanding the mechanisms of immune activation, tissue injury, and disease progression. HMGB1 can be actively secreted by cells exposed to inflammatory mediators, DAMPs, or PAMPs, or it can be transiently and passively released from apoptotic cells or from monocytes activated by exposure to apoptotic cells (Andersson and Tracey, 2011; Gauley and Pisetsky, 2009; Qin et al., 2006). Extracellularly secreted HMGB1 functions as a pro-inflammatory mediator, eliciting rapid responses from various cell types, including monocytes and macrophages (Li et al., 2003), dendritic cells (Yang et al., 2007), neutrophils (Fan et al., 2007), T-cells (Dumitriu et al., 2005), B-cells (Tian et al., 2007), epithelial cells (Yang, 2006), and smooth muscle cells (Porto et al., 2006) and so on. Extracellular HMGB1 binds to PRRs, through signaling pathways such as NF-κB, and activates NLRP3 inflammasomes, thereby delivering the “signal 1” required for NLRP3 activation (Wang et al., 2018; Vogel et al., 2018; Lamkanfi and Dixit, 2014). The relationship between HMGB1 and NLRP3 is not only that the former upregulates NLRP3 expression. In addition, HMGB1 may, under specific pathological conditions, enhance intracellular stress responses, such as ROS production, K+ efflux, and mitochondrial dysfunction, to facilitate the delivery of signal 2 required for NLRP3 inflammasome activation. Although these events are well-established canonical activation pathways, HMGB1 can act in concert with other stimuli to amplify and modulate the inflammasome activation process (Jiao et al., 2021; Lu et al., 2012). Once the NLRP3 inflammasome is assembled, caspase-1 is activated to cleave pro-IL-1β and pro-IL-18, generating their active forms, IL-1β and IL-18. Activation of the HMGB1/NLRP3 axis enhances the secretion of these proinflammatory cytokines, thereby amplifying both local and systemic inflammatory responses (Wang et al., 2020). Activated caspase-1 induces cellular pyroptosis, during which HMGB1 is further released as a DAMP that diffuses into surrounding tissues and activates the NLRP3 inflammasome in neighboring cells (Figure 3). Pyroptosis is also accompanied by the release of other DAMPs, amplifying the positive feedback loop of the HMGB1/NLRP3 axis and perpetuating a vicious cycle of inflammation (Wang et al., 2020). In summary, the HMGB1/NLRP3 axis plays a central role in amplifying both local and systemic inflammatory responses by activating the inflammasome and driving the secretion of pro-inflammatory cytokines. This pathway, through its dual-signal mechanism involving ROS generation and ionic flux alterations, mediates acute inflammation and contributes to chronic inflammatory states observed in various pathologies. Elucidating the intricacies of the HMGB1/NLRP3 axis underscores its potential as a therapeutic target for controlling inflammation-related diseases and enhancing immune regulation.
4 Role of HMGB1/NLRP3 axis in systemic diseases
4.1 Nervous system
4.1.1 Neuroinflammation
Neuroinflammation is a critical mediator in the initiation and progression of various brain disorders. Microglia, the resident immune cells of the central nervous system, are essential for sensing pathological insults and maintaining neural homeostasis. However, dysregulated microglial activity can lead to sustained inflammation, thereby accelerating neurodegenerative processes (Kwon and Koh, 2020; Niraula et al., 2017). For instance, Liu et al. demonstrated that exposure to PM2.5 particles in the atmosphere activates mouse microglia and induces neuroinflammation through the HMGB1/NLRP3 signaling pathway. Silencing HMGB1 in this context downregulated the NLRP3 inflammasome and the subsequent NF-κB/MAPK pathways, reduced pyroptotic cell death, mitigated hippocampal neuron damage, and improved synaptic function in neurons (Liu et al., 2022a). Similarly, Liao et al. found that inhibition of the HMGB1/NLRP3 axis in a rat stroke model reduced pro-inflammatory factors (IL-6, IL-1β, and TNF-α) in the brain, promoted the release of anti-inflammatory factors (IL-10, TGF-β, and brain-derived neurotrophic factors), and facilitated the shift of microglia from the M1 to M2 phenotype. This resulted in the preservation of blood-brain barrier integrity and improved the prognosis of ischemic stroke-related brain injury (Liao et al., 2024). Furthermore, Ye et al. (2019) emphasized the pivotal role of the TLR4/NF-κB pathway in mediating the interaction between HMGB1 and NLRP3 during microglial polarization, thus further driving neuroinflammation. As previously discussed, the redox state of HMGB1 significantly impacts its activity. This was corroborated by Frank et al. (2015b), who demonstrated that the disulfide form of HMGB1 exhibited pro-inflammatory effects both in vivo and in vitro. This form of HMGB1 directly induced microglial polarization toward an immune phenotype via TLR4 signaling, with NLRP3 playing a pivotal role in the downstream neuroinflammatory responses. Prolonged exposure to such inflammatory stimuli may further exacerbate the neuroinflammatory process.
4.1.2 Depression
Depression is a leading mental health disorder, with increasing global prevalence (Monroe and Harkness, 2022). Inflammatory pathways, including the HMGB1/NLRP3 axis, contribute to the pathophysiology of depression. Studies suggest that targeting this axis may offer therapeutic benefits. In depressed rats, overactivation of the hypothalamic-pituitary axis and elevated levels of HMGB1, RAGE, and NLRP3 were observed in the hippocampus. HMGB1 amplifies inflammation by activating RAGE and TLR4, activating the NF-κB signaling pathway, leading to the synthesis and release of pro-inflammatory cytokines. Additionally, caspase-1-specific inhibitor ameliorated depressive behaviors by inhibiting the pyroptosis of oligodendrocytes in the hippocampus during depression (Yang et al., 2020b). Recent studies have demonstrated that downregulation of HMGB1 and NLRP3 protein expression has been linked to alleviating depressive symptoms, making the HMGB1/NLRP3 axis a potential target for antidepressant therapies (Xie et al., 2021; Hendawy et al., 2023). These studies provide compelling evidence for the involvement of HMGB1 and the NLRP3 inflammasome in depression-associated neuroinflammatory pathways, where elevated activation levels correlate with both acute and chronic depression. By amplifying inflammation, the HMGB1/NLRP3 axis contributes to both the onset and persistence of depression, positioning it as a potential therapeutic target for inflammation-related depression subtypes.
4.1.3 Brain injury and cognitive dysfunction
Traumatic brain injury (TBI) is closely associated with cognitive dysfunction and neuroinflammation, with activation of the HMGB1/NLRP3 pathway playing a key role in these processes. Following TBI, increased Tau protein levels are observed in the dentate gyrus and thalamus, which correspond with elevated HMGB1 and NLRP3 activation, contributing to cognitive deficits. Inhibition of this axis has been found to improve spatial short-term memory, hippocampal spatial working memory, and restore nesting activity in mice (Zhao et al., 2021a). Moreover, a study by Shan et al. demonstrated that prenatal exposure to sevoflurane impairs learning and memory in rat offspring through HMGB1-induced activation of NLRP3/ASC inflammasome (Shan et al., 2023). Notably, the HMGB1/NLRP3 axis is also implicated in cognitive dysfunction associated with sepsis (Xiong et al., 2022; Ma et al., 2024), diabetes (Liu et al., 2022b), and Alzheimer’s disease (Elariny et al., 2024). Targeting this axis may attenuate these pathologies, though further clinical validation is needed. Additionally, the neuronal NLRP3 inflammasome serves as critical mediator of cortical neuroinflammation and trigeminal vascular activation. Microglia activation may further promote neuronal NLRP3 inflammasome-mediated cortical neuroinflammation through the HMGB1–TLR2/4 pathway, thereby contributing to migraine development (Chen et al., 2023a). In a study of 48 COVID-19 patients with headache symptoms, those with headaches exhibited a stronger inflammatory response compared to those without headaches. Elevated levels of HMGB1 and NLRP3 were implicated in the induction of the trigeminal system, with NLRP3 levels showed a moderate positive correlation with headache severity, length of hospitalization, and headache duration, while HMGB1 levels were positively correlated with headache severity (Bolay et al., 2021).
Ischemic brain injury, a critical factor affecting prognosis, further underscores the role of the HMGB1/NLRP3 axis. In a study by Zhang et al. (2024a), neutral polysaccharide from Gastrodia elata was able to attenuate the expression levels of HMGB1 and NLRP3 after cerebral ischemia/reperfusion injury. This alleviated neuroinflammation mediated by ferroptosis through the NRF2/HO-1 signaling pathway, although the precise relationship between anti-ferroptosis and anti-inflammatory effects remains unclear. Both Stress and cerebral ischemia synergistically increase HMGB1 levels in the serum and hippocampus, with activated microglia being the main source of its release. This activation further triggers the NLRP3 inflammasome pathway, impairing autophagic function in microglia and aggravating injury (Espinosa-Garcia et al., 2020). Notably, HMGB1 is released from cells during cerebral hemorrhage and binds to TLR4 on the surface of immune cells. Activation of the NLRP3 inflammasome promotes pyroptosis following cerebral hemorrhage (Lei et al., 2024). A case-control study investigating the correlation between NLRP3 expression and HMGB1 levels in the serum of children with febrile epilepsy revealed that serum levels of HMGB1, NLRP3, caspase-1, IL-1β, IL-6, and TNF-α were significantly higher in children with epilepsy compared to controls (Ye et al., 2024). Although they did not further explore how HMGB1 and NLRP3 interact to influence epileptogenesis, it focused on clinical cases and provided a valuable direction for future research. Additionally, the HMGB1/NLRP3 axis plays a significant role in both acute and chronic inflammation, contributing to neurological tumorigenesis and progression. In gliomas, HMGB1 has been shown to promote M1-like polarization of macrophages activate the NF-κB pathway by binding to RAGE, and enhance the release of the NLRP3 inflammasome. Bioinformatics analysis further revealed that HMGB1 levels were higher in glioma tissues than in non-tumor tissues, and elevated intracellular HMGB1 expression correlated with poor prognosis (Li et al., 2022).
4.1.4 Retinal damage and glaucoma
The HMGB1/NLRP3 axis is crucial in the progression of ocular diseases, particularly in retinal damage and glaucoma. For instance, infection with Staphylococcus pseudintermedius activates the NLRP3/HMGB1/ROS/GSDMD signaling axis in corneal epithelial cells, exacerbating apoptosis and leading to the formation of characteristic focal holes, numerous extracellular bubbles, and other localized phenomena (Wang et al., 2024a; Wang et al., 2024b). The increased release of HMGB1 regulates NLRP3 activation through an NF-κB-dependent mechanism, contributing to retinal damage and potentially progressing to glaucoma. This poses a serious threat to human vision and may result in irreversible blindness. Interestingly, inhibition of the HMGB1/NLRP3 axis has been shown to reduce inflammation and mitigate retinal injury, offering a potential strategy to protect vision and prevent irreversible blindness (Chi et al., 2015; Zhao et al., 2024a).
4.1.5 Other neurological disorders
Spinal cord injury (SCI) is a significant public health issue that imposes a substantial burden on both patients and society. Primary SCI causes cell death and vascular destruction, which subsequently trigger secondary processes, including inflammation, ischemia, and oxidative stress, that exacerbate the injury. The activation of inflammasomes plays a critical role in driving the tissue inflammatory process (Alizadeh et al., 2019). Recent research have shown that activating autophagy can inhibit the HMGB1/NF-κB/NLRP3 pathway, thereby improving motor function and reducing tissue damage caused by inflammatory responses following SCI (Gholaminejhad et al., 2022). Müller et al. demonstrated that lipocalin 2 increased the expression of HMGB1 and NLRP3 in SCI, and knocking down lipocalin 2 reduced gliosis and NLRP3 activation in astrocytes (Müller et al., 2023). Heatstroke, which causes vascular endothelial injury and multi-organ damage, also involves HMGB1 in the activation of NLRP3 inflammasomes. Regulating the HMGB1/NLRP3 axis could mitigate heatstroke-induced damage, suggesting a potential therapeutic strategy for these conditions (Zhang et al., 2024b; Pei et al., 2023). Additionally, Yin et al. (2022) delved into the thrombocytopenia that occurs after heatstroke from another direction, and they found that after heatstroke, HMGB1 induces high levels of ROS production via TLR4 and RAGE, which further activates NLRP3 inflammasomes involved in platelet activation and reduction in vivo.
4.2 Respiratory system
4.2.1 Acute lung injury (ALI)
Acute lung injury (ALI) involves damage to lung tissue, leading to dysfunction of alveoli, capillaries, and bronchioles. This inflammatory syndrome, which can progress to acute respiratory distress syndrome, is marked by the involvement of various inflammatory factors (Burkard et al., 2023; Matthay and Zemans, 2011). The HMGB1/NLRP3 axis plays a central role in this process. Activation of NLRP3 in ALI models induces expression of triggering receptor expressed on myeloid cells-1, which activates ROS–NF-κB signaling via HMGB1 and IL-18 secretion, creating a positive feedback loop that amplifies the inflammatory response (Zhong et al., 2020). Furthermore, inhibition of the HMGB1/NLRP3/Caspase-1 or HMGB1/RAGE/NF-κB signaling pathways in the lung attenuates inflammatory infiltration and cellular pyroptosis, thereby reducing lung injury (Ding et al., 2023; Chen et al., 2021; Wu et al., 2024). Targeting the expression and activation of these pathways may represent a novel strategy for ALI.
4.2.2 Pulmonary fibrosis (PF)
If left untreated, ALI can progress to pulmonary fibrosis (PF). Huang’s team found that bleomycin-induced acute lung injury in rats led to patchy fibrosis, atelectasis, hyperinflation, thickened alveolar septa, and significant inflammatory cell infiltration in lung tissues, accompanied by increased NLRP3 expression. Inhibition of HMGB1 expression promoted the nuclear translocation of nuclear factor erythroid 2-related factor 2 (NRF2) and enhanced HO-1 levels, thereby reducing NLRP3 expression and partially reversing lung fibrosis (Huang et al., 2023). The NLRP3 inflammasome has also been shown to regulate epithelial-mesenchymal transition in the development of pulmonary fibrosis, while HMGB1 promotes fibroblast proliferation and collagen accumulation. Furthermore, inhibition of the HMGB1/TLR4 axis and NLRP3 inflammasome/NF-κB signaling pathways attenuates both inflammation and fibrosis (Aydin et al., 2023; Elkhoely et al., 2023; Yang et al., 2024a). Targeting these pathways may offer new therapeutic options for PF, though clinical validation is needed.
4.2.3 Asthma
Asthma is a heterogeneous chronic inflammatory disease of the respiratory system that causes airflow obstruction and affects approximately 10% of adults. Among these, up to 6% have severe asthma, which is associated with a reduced quality of life and an increased risk of exacerbations, hospitalization, and death (Settipane et al., 2019; Varricchi et al., 2022). The HMGB1/NLRP3 axis plays a pivotal role in asthma initiation and progression. HMGB1 activates the NF-κB signaling pathway through TLR4 binding, which subsequently activates and releases the NLRP3 inflammasome. Additionally, HMGB1 induces autophagy in bronchial epithelial cells, further promoting NLRP3 inflammasome activation (Meng et al., 2023). Similarly, pre-treatment with ROS scavengers and NF-κB inhibitors in human bronchial epithelial cells downregulates NLRP3 inflammasome proteins, reduces IL-1β and IL-18 levels, and improves mitochondrial membrane potential (Jiao et al., 2021). Li et al. (2018) found that, in a mouse model of ovalbumin-induced asthma, the ATP/P2X7 axis activated NLRP3 inflammasomes, inducing the expression and release of HMGB1 in dendritic cells. HMGB1 was shown to regulate allergic airway inflammation, mucus production, and Th2 and Th17 polarization in asthmatic mice.
4.2.4 Other respiratory conditions
Inflammation and immunity play critical roles in the pathogenesis of pulmonary hypertension, with HMGB1 acting as a key DAMP released by ferroptotic cells to activate the NLRP3 inflammasome via binding to TLR4. Inhibition of ferroptosis in pulmonary artery endothelial cells attenuates pulmonary vascular remodeling and protects the right ventricle in pulmonary hypertensive rats (Xie et al., 2022). Additionally, HMGB1’s interaction with RAGE and NF-κB exacerbates inflammation through a positive feedback loop in the lung tissues of patients with drug-associated pulmonary toxicity (Abd Elmaaboud et al., 2024). Notably, although hyperactivation of the HMGB1/NLRP3 axis often plays a detrimental role in humans, Gao and Huang (2024) observed that is oflurane treatment in lung cancer cells activated HMGB1 and RAGE, upregulated NLRP3 expression, and promoted pyroptosis in tumor cells without affecting the viability of normal human bronchial epithelial cells. This finding suggests that isoflurane may be a more suitable anesthetic option for lung cancer surgery patients.
4.3 Digestive system
4.3.1 Liver injury
The importance of HMGB1 and the NLRP3 inflammasome in liver diseases has garnered increasing attention. In acute liver injury, macrophages release extracellular vesicles containing HMGB1, which are taken up by hepatocytes through transferrin-mediated endocytosis via binding to RAGE or TLR4. This process activates the NLRP3 inflammasome, triggering hepatocyte pyroptosis and injury. These findings suggest that HMGB1 not only serves as a marker of liver cell injury but also actively participates in regulating inflammatory responses (Wang et al., 2021; Chen et al., 2024a). Autophagy is essential for maintaining liver homeostasis. He et al. found that inducing autophagic flux reduced HMGB1 secretion in hepatocytes, which subsequently inhibited macrophage NLRP3 inflammasome activation, offering a potential therapeutic strategy for liver fibrosis (He et al., 2023). Conversely, autophagy-deficient hepatocytes actively release HMGB1, promoting cholangiocyte proliferation and tumorigenesis. This is driven by increased transcription of Caspase-11, triggered by sustained NRF2 activation, which activates inflammasomes and enhances HMGB1 release (Khambu et al., 2023). In alcoholic liver disease, HMGB1 translocates from the nucleus to the cytoplasm in hepatocytes, increasing apoptosis signal-regulating kinase 1-positive hepatocytes, passive HMGB1 release, and NLRP3 inflammasome activation, promoting inflammation and hepatic fibrosis (Adjei-Mosi et al., 2023). Inhibiting the NLRP3/HMGB1 signaling pathway reduces the feedback loop between inflammation and oxidative stress, protecting hepatocytes (Cao et al., 2022; Yao et al., 2024). Studies on hepatic ischemia-reperfusion injury (IRI) have demonstrated that hepatic IRI upregulates the expression of inflammation-related proteins, including HMGB1, TLR4, and NLRP3, which triggers inflammatory responses and hepatocyte injury (Du et al., 2021; Tong et al., 2023; El-Sisi et al., 2021). Xie et al. (2020) found that HBV protein X induced an increase in mitochondrial ROS under oxidative stress, activating the NLRP3 inflammasome. This activation led to hepatocyte death and the release of inflammatory factors, including IL-1β, IL-18, and HMGB1. Furthermore, the expression of inflammasome components, such as NLRP3 and IL-1β, was positively correlated with the HBV DNA load, suggesting the involvement of the NLRP3 inflammasome pathway in the progression of HBV-related hepatitis.
4.3.2 Gastrointestinal disorders
The release of HMGB1 is closely linked to the development of various diseases, including acute pancreatitis and gastrointestinal injury (Wu et al., 2021; Arab et al., 2022). In acute pancreatitis, HMGB1 upregulation promotes the activation of the NLRP3 inflammasome, leading to the release of pro-inflammatory cytokines, including IL-1β, which further exacerbates pancreatic injury (Wu et al., 2021). HMGB1 plays a critical role in ethanol-induced gastric ulcers by binding to TLR4 and RAGE, activating NF-κB, and inducing the activation of the NLRP3 inflammasome, which delays ulcer healing. Conversely, inhibiting HMGB1 expression accelerates the healing process (Alzokaky et al., 2020). In bile reflux gastritis, pyroptosis mediated by the NLRP3 inflammasome results in the release of HMGB1, which contributes to the inflammatory response. Additionally, ubiquitin-specific protease 50 interacts with ASC to deubiquitinate and activate the NLRP3 inflammasome, promoting the release of HMGB1. This release subsequently drives gastric tumorigenesis through the PI3K/AKT and MAPK/ERK pathways, revealing a novel mechanism in bile reflux-associated gastric carcinogenesis (Zhao et al., 2024b). Activation of the NLRP3/HMGB1 pathway disrupts intestinal tight junction proteins and promotes intestinal epithelial cell death, thereby compromising the integrity of the intestinal barrier (Li et al., 2024). Ulcerative colitis is a chronic inflammatory bowel disease affecting the colon and rectum, significantly impairing patients’ quality of life and causing long-term, burdensome complications (Le Berre et al., 2023). Bioinformatics analysis revealed high expression levels of several inflammation-associated genes, including TNF-α, HMGB1, and NLRP3, in the colonic tissues of patients with ulcerative colitis (Gao et al., 2024). Experiments have demonstrated that the anti-inflammatory effects achieved by inhibiting HMGB1 binding to TLR4 and suppressing the activation of the NF-κB/NLRP3 inflammasome pathway can ameliorate colonic mucosal barrier disruption and neutrophil recruitment in ulcerative colitis (Chen et al., 2023b; Sun et al., 2024). A prospective observational study revealed elevated serum levels of NLRP3 and HMGB1 in patients with ulcerative colitis, which were positively correlated with disease severity (Chen et al., 2020). Although the sample size of this study was small, it provides clinical evidence supporting the role of NLRP3 and HMGB1 in ulcerative colitis and suggests their potential as novel diagnostic markers for the disease.
4.4 Urinary system
In acute kidney injury, HMGB1 mediates cellular pyroptosis and renal tissue injury by binding to and activating TLR4 and RAGE receptors, triggering the NF-κB signaling pathway, and promoting downstream activation of NLRP3 and proinflammatory mediators (Hassan et al., 2024; Yang et al., 2024b; Henedak et al., 2024). Inhibition of HMGB1/NF-κB/NLRP3 pathway-related protein expression in HK-2 cells exerts anti-oxidative stress and anti-apoptotic effects. Treatment with glycyrrhizin analogs to inhibit HMGB1 levels accelerated the excretion of urea nitrogen and serum creatinine in septic mice, attenuated renal tissue injury, and preserved the integrity of the brush border (Qiang et al., 2023). Zhang et al. (2023) showed that DAMP accumulated after ferroptosis in renal cells recruits macrophages, activates HMGB1/RAGE/TLR4/MyD88 signaling, activates the NF - κB signaling pathway, and induces NLRP3 accumulation and cellular pyroptosis. Hypertension and diabetes mellitus, two major contributors to the global burden of chronic diseases, often lead to renal fibrosis during the progression of chronic kidney disease. HMGB1 and the NLRP3 inflammasome serve as key mediators of renal fibrosis and may even contribute to renal failure in hypertensive nephropathy and diabetic nephropathy (Awa et al., 2024; Zhang et al., 2020).
4.5 Genital system
Recurrent spontaneous abortion (URSA) is a common pregnancy complication with a complex etiology. In approximately 50% of cases, the underlying mechanism remains unknown. However, inflammation and abnormal immune tolerance are thought to be important pathogenic factors. Zhu et al. (2021) demonstrated that HMGB1, GSDMD, NLRP3, and caspase-1 proteins were elevated in the decidua tissues of URSA mice and patients. These results suggest that HMGB1, actively secreted by macrophages, may induce cellular pyroptosis via activation of the NF-κB signaling pathway, leading to aseptic inflammation, disruption of the maternal-fetal interface, and ultimately triggering URSA. Research indicates that HMGB1 and NLRP3 also contribute to pro-inflammatory processes in endometriosis and pre-eclampsia (Nunes et al., 2022; Huang et al., 2022). The HMGB1/RAGE/NLRP3 axis plays a pivotal role in cervical epithelial pyroptosis, promoting tumor cell proliferation and inflammation (You et al., 2021).
4.6 Circulatory system
4.6.1 Cardiac injury
Studies have shown that HMGB1 is upregulated in macrophages after cardiac injury and contributes to myocardial inflammation by promoting NLRP3 inflammasome activation under hypoxic conditions (Hu et al., 2022). Fan et al. found that elevated plasma levels of NLRP3 and HMGB1 were associated with poor prognosis in congenital heart disease, suggesting their potential as prognostic biomarkers for the condition (Fan et al., 2020). Furthermore, the HMGB1/NLRP3/caspase-1 pathway plays a role in hypoxia-reoxygenation-induced cellular pyroptosis in cardiomyocytes. Targeted inhibition of HMGB1 levels mitigates cytotoxicity and reduces the release of inflammatory factors (Fei et al., 2022). Song et al. demonstrated that Lipocalin-2 induces NLRP3 inflammasome activation through the HMGB1/TLR4 signaling pathway in mice with pressure overload-induced cardiac dysfunction, thereby impairing cardiac function (Song et al., 2017). Inhibiting HMGB1-dependent NLRP3 inflammasome activation reduces inflammation and apoptosis, enhances cellular antioxidant defenses, and shows potential for treating septic myocardial dysfunction and myocardial infarction (Zhao et al., 2021b; Wei et al., 2024).
4.6.2 Hematological disorders
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and can be either de novo or secondary to other processes (Bhansali et al., 2023). Using a mouse model of AML, Liu et al. (2021a) found that chronic restraint stress caused weight loss and reduced survival in AML mice. The proposed mechanism suggests that stress-induced HMGB1 secretion promotes AML progression by activating NLRP3 inflammasomes. The upregulation of the platelet NLRP3 inflammasome in patients with sickle cell disease is dependent on HMGB1/TLR4. This upregulation may regulate platelet responses and contribute to platelet aggregation, thrombosis, and vascular leakage through an autocrine or paracrine IL-1 receptor-mediated feed-forward loop (Vogel et al., 2018). Inhibiting the HMGB1/NLRP3 signaling pathway could serve as a potential therapeutic target for treating inflammation-induced thrombosis and vascular remodeling diseases in the future (Wei et al., 2022; Kim et al., 2018; Zhang et al., 2024c). During Burkitt lymphoma development, lysate cells express elevated levels of HMGB1, which activate the NLRP3 inflammasome to sustain ZEBRA expression. ZEBRA initiates the transition of the virus from the latent to the lytic state in lysate cells, thereby maintaining the lytic signal. This process facilitates viral replication and dissemination within host cells and may contribute to lymphoma progression (Reinhart et al., 2022). In sepsis, lipopolysaccharide, a cell wall component of Gram-negative bacteria, stimulates macrophages, leading to the release of HMGB1, an important inflammatory mediator. HMGB1 provides an inflammatory microenvironment conducive to the activation of NLRP3 inflammasomes. In turn, inflammatory factors released by activated NLRP3 inflammasomes further amplify HMGB1 release and inflammatory signaling. Together, these processes escalate the inflammatory response in sepsis, resulting in severe tissue injury, organ dysfunction, and ultimately poor patient prognosis (Xie et al., 2016). Studies have shown that the expression levels of NLRP3 and HMGB1 are markedly elevated in patients with myelodysplastic syndromes and myelofibrosis, suggesting their potential involvement in disease pathogenesis (Basiorka et al., 2016; Apodaca-Chávez et al., 2022; De Luca et al., 2024; Parciante et al., 2023). Although the direct mechanistic linkage between the two molecules remains to be fully elucidated, their concurrent upregulation under analogous pathological conditions suggests that the HMGB1/NLRP3 axis may play a pivotal role in driving disease progression and could serve as a promising target for therapeutic intervention.
4.7 Motor system
Following tendon injury, macrophages undergo cellular pyroptosis, and HMGB1 translocates from the nucleus to the cytoplasm, where it is subsequently packaged into extracellular vesicles and released into the extracellular space. The HMGB1/TLR axis activates the NF-κB signaling pathway in tendon-derived stem cells, working synergistically with NLRP3 inflammasomes to induce tendon-derived stem cell senescence and aberrant osteogenic differentiation, ultimately contributing to traumatic ectopic ossification (Li et al., 2023). HMGB1 triggers the assembly and activation of the NLRP3 inflammasome, leading to extracellular matrix disorganization, inflammation, and impaired healing after tendon injury (Thankam et al., 2018). Macrophages play a crucial role in maintaining tissue homeostasis and immune regulation, with M1-polarized macrophages promoting the production of inflammatory factors and influencing disc degeneration. While attenuating M1-polarized macrophage-mediated nucleus pulposus cell injury through inhibition of the HMGB1-MyD88-NF-κB pathway and NLRP3 inflammasome (Zhao et al., 2021c). The levels of HMGB1, NF-κB, NLRP3, IL-1β, and TNF-α proteins were significantly elevated in bone marrow mesenchymal stem cells following SCI, while inhibiting the HMGB1/NF-κB/NLRP3 inflammatory pathway, attenuating spinal cord tissue destruction, and improving the motor function of rats with SCI (Gholaminejhad et al., 2022).
4.8 Immune system
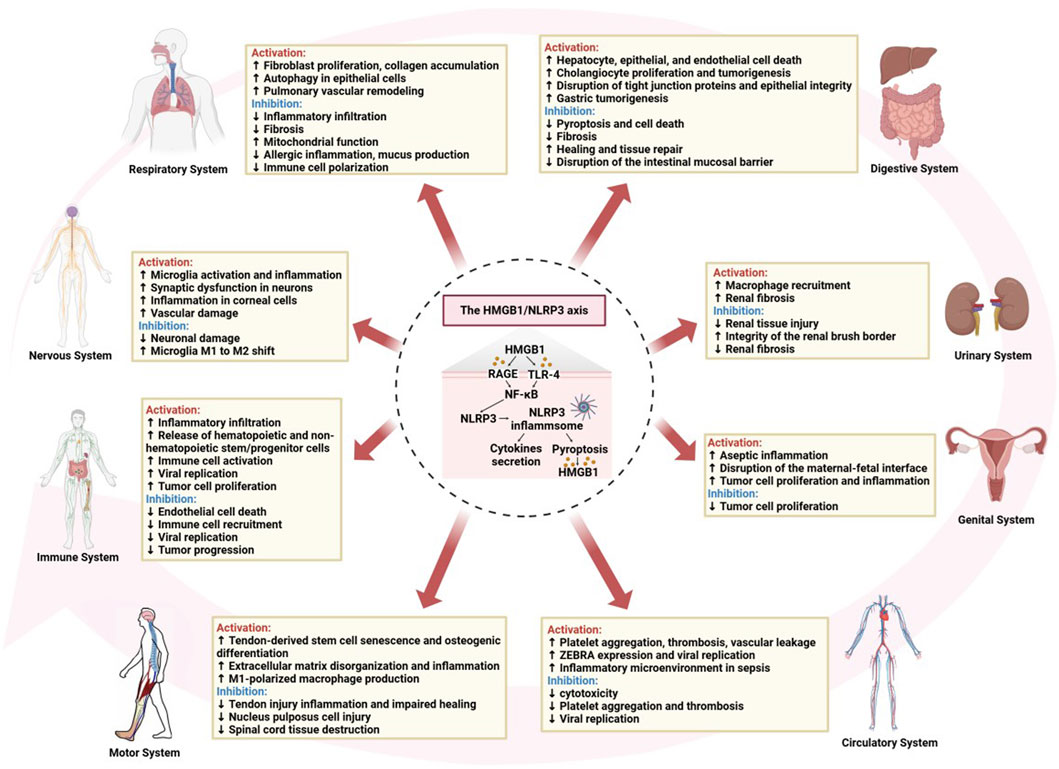
Macrophage exposure to inflammasome agonists induces the autophosphorylation of double-stranded RNA-dependent protein kinase, which broadly regulates inflammasome activation through physical interactions with various inflammasome components, including NLRP3, and is critical for caspase-1 activation, IL-1β cleavage, and HMGB1 release (Lu et al., 2012; Yu et al., 2019). HMGB1 also plays a crucial role in aseptic inflammation by activating the NLRP3 inflammasome, inducing IL-1β secretion and sepsis, and recruiting neutrophils and eosinophils, resulting in inflammatory infiltration and tissue damage (Jessop and Holian, 2015; Noguchi et al., 2021). Activation of the HMGB1/MyD88/NF-κB/NLRP3 signaling pathway plays a central role in inflammation in endothelial cells, which are classical antigen-presenting cells (Liu et al., 2021b; Yu et al., 2022). Interestingly, diurnal changes in ATP levels in peripheral blood regulate the diurnal release of hematopoietic and non-hematopoietic stem/progenitor cells from the bone marrow to peripheral blood through the activation of NLRP3/HMGB1 (Adamiak et al., 2020). In atopic dermatitis, HMGB1 activates the secretion of the NLRP3 inflammasome and inflammatory factors in epidermal keratinocytes by interacting with the RAGE receptor, as well as with IFN-γ and TNF-α, either individually or synergistically, leading to erythema and inflammation in the skin (Chang et al., 2023). Binsaleh et al. (2024) identified a strong association with the skin inflammatory response, influencing disease severity and patient depression, suggesting a potential link between this axis in skin inflammation and associated psychological states. Kawasaki disease is an immune-mediated vasculitis in which the body’s immune system is abnormally activated, with immune cells releasing large amounts of inflammatory mediators, resulting in vascular endothelial cell damage and an inflammatory response (McCrindle et al., 2017). Studies have shown that endothelial cellular pyroptosis is a key pathophysiological event in Kawasaki disease, triggered by high levels of HMGB1, leading to elevated RAGE expression and increased histone B activity, ultimately resulting in NLRP3 inflammasome-dependent, caspase-1-mediated endothelial cellular pyroptotic death. Circulating HMGB1 may serve as a sensitive predictor of endothelial injury in Kawasaki disease, and the inhibition of pyroptosis activation may represent a potential therapeutic target (Jia et al., 2019). Moreover, in addition to promoting viral replication and the inflammatory response during viral infection (Zhang et al., 2024d), HMGB1/NLRP3 is also involved in various biological processes in tumor cells, including cell proliferation, apoptosis, and immune regulation, thus offering new targets and strategies for tumor therapy (Chen et al., 2024b; Yang et al., 2024c) (Figure 4).
5 Potential therapeutic targets of the HMGB1/NLRP3 axis
Given the central role of the HMGB1/NLRP3 axis in various systemic diseases, therapeutic strategies targeting HMGB1 and NLRP3 have progressively emerged as a prominent area of research. By modulating the activation of this axis, it is possible to reduce the inflammatory response, block inflammation-induced tissue damage, and improve disease outcomes. Current therapeutic strategies focus on two primary approaches: first, inhibiting the release of HMGB1 or blocking its interaction with TLR/RAGE, and second, targeting NLRP3 to prevent inflammasome activation and subsequent pro-inflammatory cytokine release.
Recent advancements have highlighted a range of promising therapeutic agents targeting HMGB1 and NLRP3. Anti-HMGB1 monoclonal antibodies have shown efficacy in preclinical models of inflammatory diseases, such as sepsis and acute liver injury, by neutralizing HMGB1 and reducing the inflammatory response. Recombinant HMGB1 box A proteins, which specifically bind to the receptor-binding domain of HMGB1, have been shown to attenuate tissue damage in models of traumatic injury and chronic inflammation (Yang et al., 2020a; Andersson et al., 2018; Wang et al., 1999). Additionally, endogenous molecules such as immunoglobulins, antithrombin, thrombomodulin, vasoactive intestinal peptide, and growth hormone-releasing peptide have demonstrated potential in reducing HMGB1-mediated inflammation in various conditions, including infections and chemical toxicity (Yang et al., 2020a; Lu et al., 2014). Among natural compounds, niacin and mung bean extracts have been found to effectively inhibit HMGB1 release, offering novel therapeutic options for inflammatory diseases. Exogenous herbal ingredients are particularly promising due to their low toxicity and broad therapeutic potential, but their clinical development still faces hurdles, such as standardization of dosages and efficacy validation (Lu et al., 2014). In the field of NLRP3 inhibition, numerous compounds are currently under extensive investigation. MCC950, a potent small-molecule inhibitor of NLRP3, has demonstrated significant efficacy in attenuating inflammatory responses across both preclinical studies and early-phase clinical trials, showing therapeutic potential for a range of diseases, including Alzheimer’s disease, type 2 diabetes mellitus, and cardiovascular disorders (Li et al., 2022). Andrographolide, a bioactive natural compound isolated from Andrographis paniculata, has also been reported to effectively suppress NLRP3 inflammasome activation and ameliorate inflammatory pathologies in conditions such as asthma and rheumatoid arthritis (Agrawal and Nair, 2022). Additionally, synthetic small molecules, such as JC121, which exhibit high selectivity for NLRP3 targeting, have emerged as promising candidates for the treatment of inflammasome-mediated diseases (Blevins et al., 2022). Recent research efforts have facilitated the development of clinically promising NLRP3-targeted compounds. Dapansutrile Klück et al. (2020) and Inzomelid (NCT04015076), as next-generation NLRP3 inhibitors, have successfully entered clinical trials and demonstrated favorable safety profiles. These advances represent a critical step forward in the clinical translation of NLRP3-targeted therapies. HMGB1 and the NLRP3 inflammasome engage in a reciprocal activation process, establishing a positive feedback loop that amplifies inflammatory responses. Inhibiting either HMGB1 or the NLRP3 inflammasome alone reduces the activation of the other to some extent, attenuating the pro-inflammatory effects of the HMGB1/NLRP3 axis.
While these therapeutic strategies have shown potential, challenges remain in translating preclinical findings into clinical success. One of the primary concerns is the selectivity of inhibitors. Many compounds targeting NLRP3 also affect other immune signaling pathways, which can lead to unintended side effects. HMGB1 and NLRP3 are critically involved in multiple immune regulatory and inflammatory processes, maintaining a delicate and dynamic balance between inflammatory homeostasis and pathological responses. Although pharmacological inhibition of these key mediators holds promising therapeutic potential, it may also lead to immune dysregulation, aberrant inflammatory responses, and even tumor progression in certain pathological contexts. Furthermore, due to the structural diversity and functional complexity of the targets themselves, as well as the limited specificity and in vivo stability of current inhibitors, off-target toxicity remains a significant challenge. For instance, MCC950 was evaluated in a Phase II clinical trial for rheumatoid arthritis but was subsequently discontinued due to elevated serum liver enzyme levels observed in patients, Although the precise mechanism of its hepatotoxicity is not fully understood, it may be related to both its molecular structure and the high dosage used in clinical settings (Mangan et al., 2018). Therefore, bioavailability and the long-term safety of these agents require further validation in large-scale clinical trials.
6 Conclusion
The HMGB1/NLRP3 pathways serve as critical modulators in the development and progression of systemic inflammatory disorders. As pivotal drivers of innate immune responses, these pathways bridge inflammation and disease pathogenesis by orchestrating cellular stress signals, inflammasome activation, and the subsequent release of pro-inflammatory cytokines. Evidence increasingly underscores the centrality of HMGB1 and NLRP3 in promoting chronic inflammation and multi-organ damage across a spectrum of diseases, including autoimmune disorders, trauma, and stress. Notably, although the synergistic activation between HMGB1 and NLRP3 may form a pro-inflammatory positive feedback loop that accelerates disease progression, their regulatory mechanisms exhibit distinct variability and complexity across different tissue environments and pathological conditions. In particular, the functional roles and interactions of HMGB1 and NLRP3 may vary significantly depending on the cell type and stimulation context, and the ensuing activation of the associated inflammatory signaling cascades also displays diverse characteristics. In this review, we primarily describe the role of HMGB1/NLRP3 in systemic diseases and do not explore the related targeted therapeutic mechanisms in detail. Future studies should aim to unravel the precise molecular mechanisms underlying their interplay, enabling the development of highly specific, pathway-targeted interventions. Such approaches hold promise for improving outcomes in patients suffering from inflammation-driven systemic diseases. In summary, advancing our understanding of the HMGB1/NLRP3 axis will not only elucidate the molecular underpinnings of systemic inflammation but also pave the way for innovative therapeutic strategies to address the unmet medical needs in chronic inflammatory conditions.
Author contributions
LY: Conceptualization, Writing – original draft. XL: Data curation, Writing – original draft. XZ: Writing – original draft. FG: Writing – review and editing. YW: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We very much thank all of the participants who took part in the studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd Elmaaboud, M. A., Kabel, A. M., Borg, H. M., Magdy, A. A., Kabel, S. M., Arafa, E. S. A., et al. (2024). Omarigliptin/rosinidin combination ameliorates cyclophosphamide-induced lung toxicity in rats: the interaction between Glucagon-like Peptide-1, TXNIP/NLRP3 inflammasome signaling, and PI3K/Akt/FoxO1 axis. Biomed. & Pharmacother. 177, 117026. doi:10.1016/j.biopha.2024.117026
Adamiak, M., Ciechanowicz, A., Skoda, M., Cymer, M., Tracz, M., Xu, B., et al. (2020). Novel evidence that purinergic signaling - Nlrp3 inflammasome axis regulates circadian rhythm of hematopoietic stem/progenitor cells circulation in peripheral blood. Stem Cell Rev. Rep. 16, 335–343. doi:10.1007/s12015-020-09953-0
Adjei-Mosi, J., Sun, Q., Smithson, S. B., Shealy, G. L., Amerineni, K. D., Liang, Z., et al. (2023). Age-dependent loss of hepatic SIRT1 enhances NLRP3 inflammasome signaling and impairs capacity for liver fibrosis resolution. Aging Cell 22, e13811. doi:10.1111/acel.13811
Agrawal, P., and Nair, M. S. (2022). An insight into the pharmacological and analytical potential of andrographolide. Fundam. Clin. Pharmacol. 36, 586–600. doi:10.1111/fcp.12757
Agostini, L., Martinon, F., Burns, K., McDermott, M. F., Hawkins, P. N., and Tschopp, J. (2004). NALP3 forms an IL-1beta-Processing inflammasome with increased activity in muckle-wells autoinflammatory disorder. Immunity 20, 319–325. doi:10.1016/s1074-7613(04)00046-9
Alizadeh, A., Dyck, S. M., and Karimi-Abdolrezaee, S. (2019). Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 10, 282. doi:10.3389/fneur.2019.00282
Alzokaky, A.A.-M., Abdelkader, E. M., El-Dessouki, A. M., Khaleel, S. A., and Raslan, N. A. (2020). C-Phycocyanin protects against ethanol-induced gastric ulcers in rats: role of HMGB1/NLRP3/NF-κB pathway. Basic Clin. Pharmacol. Toxicol. 127, 265–277. doi:10.1111/bcpt.13415
Andersson, U., and Tracey, K. J. (2011). HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162. doi:10.1146/annurev-immunol-030409-101323
Andersson, U., Yang, H., and Harris, H. (2018). Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin. Ther. Targets 22, 263–277. doi:10.1080/14728222.2018.1439924
Apodaca-Chávez, E., Demichelis-Gómez, R., Rosas-López, A., Mejía-Domínguez, N. R., Galvan-López, I., Addorosio, M., et al. (2022). Circulating HMGB1 is increased in myelodysplastic syndrome but not in other bone marrow failure syndromes: proof-of-concept cross-sectional study. Ther. Adv. Hematol. 13, 20406207221125990. doi:10.1177/20406207221125990
Arab, H. H., Eid, A. H., El-Sheikh, A. A. K., Arafa, E. S. A., and Ashour, A. M. (2022). Irbesartan reprofiling for the amelioration of ethanol-induced gastric mucosal injury in rats: role of inflammation, apoptosis, and autophagy. Life Sci. 308, 120939. doi:10.1016/j.lfs.2022.120939
Awad, A. M., Elshaer, S. L., Gangaraju, R., Abdelaziz, R. R., and Nader, M. A. (2024). Ameliorative effect of montelukast against STZ induced diabetic nephropathy: Targeting HMGB1, TLR4, NF-κB, NLRP3 inflammasome, and autophagy pathways. Inflammopharmacology 32, 495–508. doi:10.1007/s10787-023-01301-1
Aydin, P., Aksakalli-Magden, Z. B., Civelek, M. S., Karabulut-Uzuncakmak, S., Mokhtare, B., Ozkaraca, M., et al. (2023). The melatonin agonist ramelteon attenuates bleomycin-induced lung fibrosis by suppressing the NLRP3/TGF-Β1/HMGB1 signaling pathway. Adv. Med. Sci. 68, 322–331. doi:10.1016/j.advms.2023.09.004
Basiorka, A. A., McGraw, K. L., Eksioglu, E. A., Chen, X., Johnson, J., Zhang, L., et al. (2016). The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 128, 2960–2975. doi:10.1182/blood-2016-07-730556
Bauernfeind, F. G., Horvath, G., Stutz, A., Alnemri, E. S., MacDonald, K., Speert, D., et al. (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791. doi:10.4049/jimmunol.0901363
Bhansali, R. S., Pratz, K. W., and Lai, C. (2023). Recent advances in targeted therapies in acute myeloid leukemia. J. Hematol. Oncol. 16, 29. doi:10.1186/s13045-023-01424-6
Bianchi, M. E., and Agresti, A. (2005). HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. & Dev. 15, 496–506. doi:10.1016/j.gde.2005.08.007
Bianchi, M. E., Falciola, L., Ferrari, S., and Lilley, D. M. (1992). The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. EMBO J. 11, 1055–1063. doi:10.1002/j.1460-2075.1992.tb05144.x
Binsaleh, A. Y., Ali, L. S., Bahaa, M. M., Elmasry, T. A., Negm, W. A., Hamouda, A. O., et al. (2024). Correlation between depression scores and serum NF-ĸB/NLRP3 axis, biotinidase, and HMGB1 after treatment with isotretinoin in patients with acne vulgaris. J. Cosmet. Dermatol 23, 3409–3417. doi:10.1111/jocd.16433
Blevins, H. M., Xu, Y., Biby, S., and Zhang, S. (2022). The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 14, 879021. doi:10.3389/fnagi.2022.879021
Bolay, H., Karadas, Ö., Oztürk, B., Sonkaya, R., Tasdelen, B., Bulut, T. D. S., et al. (2021). HMGB1, NLRP3, IL-6 and ACE2 levels are elevated in COVID-19 with headache: a window to the infection-related headache mechanism. J. Headache Pain 22, 94. doi:10.1186/s10194-021-01306-7
Bonaldi, T., Talamo, F., Scaffidi, P., Ferrera, D., Porto, A., Bachi, A., et al. (2003). Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 22, 5551–5560. doi:10.1093/emboj/cdg516
Boucher, D., Monteleone, M., Coll, R. C., Chen, K. W., Ross, C. M., Teo, J. L., et al. (2018). Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 215, 827–840. doi:10.1084/jem.20172222
Burkard, P., Schonhart, C., Vögtle, T., Köhler, D., Tang, L., Johnson, D., et al. (2023). A key role for platelet GPVI in neutrophil recruitment, migration, and NETosis in the early stages of acute lung injury. Blood 142, 1463–1477. doi:10.1182/blood.2023019940
Cao, Z., Yang, F., Lin, Y., Shan, J., Cao, H., Zhang, C., et al. (2022). Selenium antagonizes cadmium-induced inflammation and oxidative stress via suppressing the interplay between NLRP3 inflammasome and HMGB1/NF-κB pathway in duck hepatocytes. Int. J. Mol. Sci. 23, 6252. doi:10.3390/ijms23116252
Chang, Q.-X., Lyu, J.-L., Wu, P.-Y., Wen, K. C., Chang, C. C., and Chiang, H. M. (2023). Coffea arabica extract attenuates atopic dermatitis-like skin lesions by regulating NLRP3 inflammasome expression and skin barrier functions. Int. J. Mol. Sci. 24, 12367. doi:10.3390/ijms241512367
Chen, Y., Wu, D., and Sun, L. (2020). Clinical significance of high-mobility group box 1 protein (HMGB1) and nod-like receptor protein 3 (NLRP3) in patients with ulcerative colitis. Med. Sci. Monit. 26, e919530. doi:10.12659/MSM.919530
Chen, G., Hou, Y., Li, X., Pan, R., and Zhao, D. (2021). Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-κB pathway and NLRP3 inflammasome. Int. Immunopharmacol. 96, 107623. doi:10.1016/j.intimp.2021.107623
Chen, R., Kang, R., and Tang, D. (2022). The mechanism of HMGB1 secretion and release. Exp. & Mol. Med. 54, 91–102. doi:10.1038/s12276-022-00736-w
Chen, P.-Y., Yen, J.-C., Liu, T.-T., Chen, S. T., Wang, S. J., and Chen, S. P. (2023a). Neuronal NLRP3 inflammasome mediates spreading depolarization-evoked trigeminovascular activation. Brain 146, 2989–3002. doi:10.1093/brain/awad045
Chen, J., Lu, P., Liu, J., Yang, L., Li, Y., Chen, Y., et al. (2023b). 20(S)- protopanaxadiol saponins isolated from Panax notoginseng target the binding of HMGB1 to TLR4 against inflammation in experimental ulcerative colitis. Phytother. Res. 37, 4690–4705. doi:10.1002/ptr.7938
Chen, H., Wang, S., Chen, Q., Yu, W., Nie, H., Liu, L., et al. (2024a). Aloperine ameliorates acetaminophen-induced acute liver injury through HMGB1/TLR4/NF-κB and NLRP3/Inflammasome pathway. Mediat. Inflamm. 2024, 3938136. doi:10.1155/2024/3938136
Chen, S.-Q., Lu, X.-Y., Zhu, L.-Y., Zhu, H., Li, R. T., and Ye, R. R. (2024b). Design, synthesis, and antitumor mechanism investigation of Iridium(III) complexes conjugated with ibuprofen. J. Inorg. Biochem. 257, 112596. doi:10.1016/j.jinorgbio.2024.112596
Chi, W., Chen, H., Li, F., Zhu, Y., and Zhuo, Y. (2015). HMGB1 promotes the activation of NLRP3 and Caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J. Neuroinflammation 12, 137. doi:10.1186/s12974-015-0360-2
Cui, T., Zhang, W., Li, S., Chen, X., Chang, Y., Yi, X., et al. (2019). Oxidative stress-induced HMGB1 release from melanocytes: a paracrine mechanism underlying the cutaneous inflammation in vitiligo. J. Invest Dermatol 139, 2174–2184. doi:10.1016/j.jid.2019.03.1148
De Luca, G., Goette, N. P., Lev, P. R., Baroni Pietto, M. C., Marin Oyarzún, C. P., Castro Ríos, M. A., et al. (2024). Elevated levels of damage-associated molecular patterns HMGB1 and S100A8/A9 coupled with toll-like receptor-triggered monocyte activation are associated with inflammation in patients with myelofibrosis. Front. Immunol. 15, 1365015. doi:10.3389/fimmu.2024.1365015
Dekker, C., Mattes, H., Wright, M., Boettcher, A., Hinniger, A., Hughes, N., et al. (2021). Crystal structure of NLRP3 NACHT domain with an inhibitor defines mechanism of inflammasome inhibition. J. Mol. Biol. 433, 167309. doi:10.1016/j.jmb.2021.167309
Ding, P., Yang, R., Li, C., Fu, H. L., Ren, G. L., Wang, P., et al. (2023). Fibroblast growth factor 21 attenuates ventilator-induced lung injury by inhibiting the NLRP3/Caspase-1/GSDMD pyroptotic pathway. Crit. Care 27, 196. doi:10.1186/s13054-023-04488-5
Du, Y., Zhong, F., Cheng, H., Li, T., Chen, Y., Tan, P., et al. (2021). The dietary supplement γ-Oryzanol attenuates hepatic ischemia reperfusion injury via inhibiting endoplasmic reticulum stress and HMGB1/NLRP3 inflammasome. Oxid. Med. Cell Longev. 2021, 4628050. doi:10.1155/2021/4628050
Dumitriu, I. E., Baruah, P., Valentinis, B., Voll, R. E., Herrmann, M., Nawroth, P. P., et al. (2005). Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 174, 7506–7515. doi:10.4049/jimmunol.174.12.7506
Elariny, H. A., Kabel, A. M., Selim, H. M. R. M., Helal, A. I., Abdelrahman, D., Borg, H. M., et al. (2024). Repositioning canagliflozin for mitigation of aluminium chloride-induced Alzheimer’s disease: involvement of TXNIP/NLRP3 inflammasome axis, mitochondrial dysfunction, and SIRT1/HMGB1 signalling. Med. Kaunas. 60, 1805. doi:10.3390/medicina60111805
Elkhoely, A., Estfanous, R. S., Alrobaian, M., Borg, H. M., and Kabel, A. M. (2023). Repositioning itraconazole for amelioration of bleomycin-induced pulmonary fibrosis: targeting HMGB1/TLR4 axis, NLRP3 Inflammasome/NF-κB signaling, and autophagy. Life Sci. 313, 121288. doi:10.1016/j.lfs.2022.121288
El-Sisi, A. E.-D. E.-S., Sokar, S. S., Shebl, A. M., Mohamed, D. Z., and Abu-Risha, S. E. S. (2021). Octreotide and melatonin alleviate inflammasome-induced pyroptosis through inhibition of TLR4-NF-κB-NLRP3 pathway in hepatic ischemia/reperfusion injury. Toxicol. Appl. Pharmacol. 410, 115340. doi:10.1016/j.taap.2020.115340
Espinosa-Garcia, C., Atif, F., Yousuf, S., Sayeed, I., Neigh, G. N., and Stein, D. G. (2020). Progesterone attenuates stress-induced NLRP3 inflammasome activation and enhances autophagy following ischemic brain injury. Int. J. Mol. Sci. 21, 3740. doi:10.3390/ijms21113740
Fan, J., Li, Y., Levy, R. M., Fan, J. J., Hackam, D. J., Vodovotz, Y., et al. (2007). Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J. Immunol. 178, 6573–6580. doi:10.4049/jimmunol.178.10.6573
Fan, J., Qiu, Y., Zheng, Z., Yu, L., Shi, S., and Wu, X. (2020). NOD-like receptor protein 3 and high mobility group Box-1 are associated with prognosis of patients with congenital heart disease. J. Int. Med. Res. 48, 300060519884500. doi:10.1177/0300060519884500
Fei, L., Zhang, N., and Zhang, J. (2022). Mechanism of miR-126 in hypoxia-reoxygenation-induced cardiomyocyte pyroptosis by regulating HMGB1 and NLRP3 inflammasome. Immunopharmacol. Immunotoxicol. 44, 500–509. doi:10.1080/08923973.2022.2054819
Frank, M. G., Weber, M. D., Watkins, L. R., and Maier, S. F. (2015a). Stress sounds the alarmin: the role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav. Immun. 48, 1–7. doi:10.1016/j.bbi.2015.03.010
Frank, M. G., Weber, M. D., Fonken, L. K., Hershman, S. A., Watkins, L. R., and Maier, S. F. (2015b). The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: a role for the NLRP3 inflammasome. Brain, Behav. Immun. 55, 215–224. doi:10.1016/j.bbi.2015.10.009
Fu, J., and Wu, H. (2023). Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 41, 301–316. doi:10.1146/annurev-immunol-081022-021207
Gao, T., and Huang, Z. (2024). Effects of isoflurane on the cell pyroptosis in the lung cancer through the HMGB1/RAGE pathway. Appl. Biochem. Biotechnol. 196, 3786–3799. doi:10.1007/s12010-023-04739-9
Gao, B. B., Wang, L., Li, L. Z., Fei, Z. Q., Wang, Y. Y., Zou, X. M., et al. (2024). Beneficial effects of oxymatrine from Sophora flavescens on alleviating ulcerative colitis by improving inflammation and ferroptosis. J. Ethnopharmacol. 332, 118385. doi:10.1016/j.jep.2024.118385
Gauley, J., and Pisetsky, D. S. (2009). The translocation of HMGB1 during cell activation and cell death. Autoimmunity 42, 299–301. doi:10.1080/08916930902831522
Ge, X., Antoine, D. J., Lu, Y., Arriazu, E., Leung, T. M., Klepper, A. L., et al. (2014). High mobility group Box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J. Biol. Chem. 289, 22672–22691. doi:10.1074/jbc.M114.552141
Gholaminejhad, M., Jameie, S. B., Abdi, M., Abolhassani, F., Mohammed, I., and Hassanzadeh, G. (2022). All-trans retinoic acid–preconditioned mesenchymal stem cells improve motor function and alleviate tissue damage after spinal cord injury by inhibition of HMGB1/NF-κB/NLRP3 pathway through autophagy activation. J. Mol. Neurosci. 72, 947–962. doi:10.1007/s12031-022-01977-0
Goodwin, G. H., Sanders, C., and Johns, E. W. (1973). A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 38, 14–19. doi:10.1111/j.1432-1033.1973.tb03026.x
Harris, H. E., Andersson, U., and Pisetsky, D. S. (2012). HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 8, 195–202. doi:10.1038/nrrheum.2011.222
Hassan, N. F., El-Ansary, M. R., Selim, H. M. R. M., Ousman, M. S., Khattab, M. S., El-Ansary, M. R. M., et al. (2024). Alirocumab boosts antioxidant status and halts inflammation in rat model of sepsis-induced nephrotoxicity via modulation of Nrf2/HO-1, PCSK9/HMGB1/NF-ᴋB/NLRP3 and Fractalkine/CX3CR1 hubs. Biomed. & Pharmacother. 177, 116929. doi:10.1016/j.biopha.2024.116929
He, M., Chu, T., Wang, Z., Feng, Y., Shi, R., He, M., et al. (2023). Inhibition of macrophages inflammasome activation via autophagic degradation of HMGB1 by EGCG ameliorates HBV-induced liver injury and fibrosis. Front. Immunol. 14, 1147379. doi:10.3389/fimmu.2023.1147379
Hendawy, N., Salaheldin, T. H., and Abuelezz, S. A. (2023). PCSK9 inhibition reduces depressive like behavior in CUMS-exposed rats: highlights on HMGB1/RAGE/TLR4 pathway, NLRP3 inflammasome complex and IDO-1. J. Neuroimmune Pharmacol. 18, 195–207. doi:10.1007/s11481-023-10060-3
Henedak, N. T., El-Abhar, H. S., Abdallah, D. M., Ahmed, K. A., and Soubh, A. A. (2024). Demotion of Canonical/non-canonical inflammasome and pyroptosis alleviates Ischemia/reperfusion-induced acute kidney injury: novel role of the D2/D3 receptor agonist ropinirole. Eur. J. Pharmacol. 969, 176460. doi:10.1016/j.ejphar.2024.176460
Hornung, V., Bauernfeind, F., Halle, A., Samstad, E. O., Kono, H., Rock, K. L., et al. (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856. doi:10.1038/ni.1631
Hu, J., Liu, X., and Tang, Y. (2022). HMGB1/Foxp1 regulates hypoxia-induced inflammatory response in macrophages. Cell Biol. Int. 46, 265–277. doi:10.1002/cbin.11728
Huang, Y., Li, R., Hu, R., Yao, J., and Yang, Y. (2022). PEG2-Induced pyroptosis regulates the expression of HMGB1 and promotes hEM15A migration in endometriosis. Int. J. Mol. Sci. 23, 11707. doi:10.3390/ijms231911707
Huang, Y., Wang, A., Jin, S., Liu, F., and Xu, F. (2023). Activation of the NLRP3 inflammasome by HMGB1 through inhibition of the Nrf2/HO-1 pathway promotes bleomycin-induced pulmonary fibrosis after acute lung injury in rats. Allergol. Immunopathol. Madr. 51, 56–67. doi:10.15586/aei.v51i3.668
Iyer, S. S., He, Q., Janczy, J. R., Elliott, E. I., Zhong, Z., Olivier, A. K., et al. (2013). Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323. doi:10.1016/j.immuni.2013.08.001
Jessop, F., and Holian, A. (2015). Extracellular HMGB1 regulates multi-walled carbon nanotube-induced inflammation in vivo. Nanotoxicology 9, 365–372. doi:10.3109/17435390.2014.933904
Jia, C., Zhang, J., Chen, H., Zhuge, Y., Chen, H., Qian, F., et al. (2019). Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/Cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 10, 778. doi:10.1038/s41419-019-2021-3
Jiao, B., Guo, S., Yang, X., Sun, L., Sai, L., Yu, G., et al. (2021). The role of HMGB1 on TDI-induced NLPR3 inflammasome activation via ROS/NF-κB pathway in HBE cells. Int. Immunopharmacol. 98, 107859. doi:10.1016/j.intimp.2021.107859
Kang, R., Zeh, H. J., Lotze, M. T., and Tang, D. (2011). The beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18, 571–580. doi:10.1038/cdd.2010.191
Kang, R., Chen, R., Zhang, Q., Hou, W., Wu, S., Cao, L., et al. (2014). HMGB1 in health and disease. Mol. Asp. Med. 40, 1–116. doi:10.1016/j.mam.2014.05.001
Khambu, B., Cai, G., Liu, G., Bailey, N. T., Mercer, A. A., Baral, K., et al. (2023). NRF2 transcriptionally regulates Caspase-11 expression to activate HMGB1 release by autophagy-deficient hepatocytes. Cell Death Discov. 9, 270. doi:10.1038/s41420-023-01495-x
Kim, E. J., Park, S. Y., Baek, S. E., Jang, M. A., Lee, W. S., Bae, S. S., et al. (2018). HMGB1 increases IL-1β production in vascular smooth muscle cells via NLRP3 inflammasome. Front. Physiol. 9, 313. doi:10.3389/fphys.2018.00313
Klück, V., Jansen, T. L. T. A., Janssen, M., Comarniceanu, A., Efdé, M., Tengesdal, I. W., et al. (2020). Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, Proof-of-Concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280. doi:10.1016/s2665-9913(20)30065-5
Kwon, H. S., and Koh, S.-H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 9, 42. doi:10.1186/s40035-020-00221-2
Lamkanfi, M., and Dixit, V. M. (2014). Mechanisms and functions of inflammasomes. Cell 157, 1013–1022. doi:10.1016/j.cell.2014.04.007
Le Berre, C., Honap, S., and Peyrin-Biroulet, L. (2023). Ulcerative colitis. Lancet 402, 571–584. doi:10.1016/S0140-6736(23)00966-2
Lee, G.-S., Subramanian, N., Kim, A. I., Aksentijevich, I., Goldbach-Mansky, R., Sacks, D. B., et al. (2012). The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127. doi:10.1038/nature11588
Lei, C., Chen, K., Gu, Y., Li, Y., Wang, L., Zhu, X., et al. (2024). HMGB1/TLR4 axis promotes pyroptosis after ICH by activating the NLRP3 inflammasome. J. Neuroimmunol. 393, 578401. doi:10.1016/j.jneuroim.2024.578401
Li, J., Kokkola, R., Tabibzadeh, S., Yang, R., Ochani, M., Qiang, X., et al. (2003). Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 9, 37–45. doi:10.1007/bf03402105
Li, R., Wang, J., Li, R., Zhu, F., Xu, W., Zha, G., et al. (2018). ATP/P2X7-NLRP3 axis of dendritic cells participates in the regulation of airway inflammation and hyper-responsiveness in asthma by mediating HMGB1 expression and secretion. Exp. Cell Res. 366, 1–15. doi:10.1016/j.yexcr.2018.03.002
Li, Z., Fu, W.-J., Chen, X.-Q., Wang, S., Deng, R. S., Tang, X. P., et al. (2022). Autophagy-based unconventional secretion of HMGB1 in glioblastoma promotes chemosensitivity to temozolomide through macrophage M1-like polarization. J. Exp. Clin. Cancer Res. 41, 74. doi:10.1186/s13046-022-02291-8
Li, H., Guan, Y., Liang, B., Ding, P., Hou, X., Wei, W., et al. (2022). Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur. J. Pharmacol. 928, 175091. doi:10.1016/j.ejphar.2022.175091
Li, J., Wang, X., Yao, Z., Yuan, F., Liu, H., Sun, Z., et al. (2023). NLRP3-Dependent crosstalk between pyroptotic macrophage and senescent cell orchestrates trauma-induced heterotopic ossification during aberrant wound healing. Adv. Sci. (Weinh) 10, e2207383. doi:10.1002/advs.202207383
Li, Y., Ling, P., Li, Y., Wang, Y., Li, G., Qiu, C., et al. (2024). miR-138-5p ameliorates intestinal barrier disruption caused by acute superior mesenteric vein thrombosis injury by inhibiting the NLRP3/HMGB1 axis. PeerJ 12, e16692. doi:10.7717/peerj.16692
Liao, Y., Hu, J., Guo, C., Wen, A., Wen, L., Hou, Q., et al. (2024). Acteoside alleviates blood–brain barrier damage induced by ischemic stroke through inhibiting microglia HMGB1/TLR4/NLRP3 signaling. Biochem. Pharmacol. 220, 115968. doi:10.1016/j.bcp.2023.115968
Liu, N., Wu, Y., Wen, X., Li, P., Lu, F., and Shang, H. (2021a). Chronic stress promotes acute myeloid leukemia progression through HMGB1/NLRP3/IL-1β signaling pathway. J. Mol. Med. Berl. 99, 403–414. doi:10.1007/s00109-020-02011-9
Liu, X., Lu, B., Fu, J., Zhu, X., Song, E., and Song, Y. (2021b). Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-Kb signaling pathway in HUVEC cells. J. Hazard Mater 404, 124050. doi:10.1016/j.jhazmat.2020.124050
Liu, C., She, Y., Huang, J., Liu, Y., Li, W., Zhang, C., et al. (2022a). HMGB1-NLRP3-P2X7R pathway participates in PM2.5-Induced hippocampal neuron impairment by regulating microglia activation. Ecotoxicol. Environ. Saf. 239, 113664. doi:10.1016/j.ecoenv.2022.113664
Liu, L., Wang, N., Kalionis, B., Xia, S., and He, Q. (2022b). HMGB1 plays an important role in pyroptosis induced blood brain barrier breakdown in diabetes-associated cognitive decline. J. Neuroimmunol. 362, 577763. doi:10.1016/j.jneuroim.2021.577763
Lu, B., Nakamura, T., Inouye, K., Li, J., Tang, Y., Lundbäck, P., et al. (2012). Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674. doi:10.1038/nature11290
Lu, B., Wang, C., Wang, M., Li, W., Chen, F., Tracey, K. J., et al. (2014). Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Rev. Clin. Immunol. 10, 713–727. doi:10.1586/1744666X.2014.909730
Ma, Y., She, X., Liu, Y., and Qin, X. (2024). MSC-derived exosomal miR-140-3p improves cognitive dysfunction in sepsis-associated encephalopathy by HMGB1 and S-Lactoylglutathione metabolism. Commun. Biol. 7, 562. doi:10.1038/s42003-024-06236-z
Mangan, M. S. J., Olhava, E. J., Roush, W. R., Seidel, H. M., Glick, G. D., and Latz, E. (2018). Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17, 588–606. doi:10.1038/nrd.2018.97
Matthay, M. A., and Zemans, R. L. (2011). The acute respiratory distress syndrome: pathogenesis and treatment. Annu. Rev. Pathol. Mech. Dis. 6, 147–163. doi:10.1146/annurev-pathol-011110-130158
McCrindle, B. W., Rowley, A. H., Newburger, J. W., Burns, J. C., Bolger, A. F., Gewitz, M., et al. (2017). Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation 135, e927–e999. doi:10.1161/CIR.0000000000000484
Meng, X., Guo, S., Zhang, X., Jiao, B., Yang, X., Li, M., et al. (2023). HMGB1 inhibition reduces TDI-induced occupational asthma through ROS/AMPK/Autophagy pathway. Ecotoxicol. Environ. Saf. 266, 115575. doi:10.1016/j.ecoenv.2023.115575
Monroe, S. M., and Harkness, K. L. (2022). Major depression and its recurrences: life course matters. Annu. Rev. Clin. Psychol. 18, 329–357. doi:10.1146/annurev-clinpsy-072220-021440
Müller, N., Scheld, M., Voelz, C., Gasterich, N., Zhao, W., Behrens, V., et al. (2023). Lipocalin-2 deficiency diminishes canonical NLRP3 inflammasome formation and IL-1β production in the subacute phase of spinal cord injury. Int. J. Mol. Sci. 24, 8689. doi:10.3390/ijms24108689
Muñoz-Planillo, R., Kuffa, P., Martínez-Colón, G., Smith, B. L., Rajendiran, T. M., and Núñez, G. (2013). K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153. doi:10.1016/j.immuni.2013.05.016
Niraula, A., Sheridan, J. F., and Godbout, J. P. (2017). Microglia priming with aging and stress. Neuropsychopharmacology 42, 318–333. doi:10.1038/npp.2016.185
Noguchi, T., Sekiguchi, Y., Kudoh, Y., Naganuma, R., Kagi, T., Nishidate, A., et al. (2021). Gefitinib initiates sterile inflammation by promoting IL-1β and HMGB1 release via two distinct mechanisms. Cell Death Dis. 12, 49. doi:10.1038/s41419-020-03335-7
Nunes, P. R., Romao-Veiga, M., Matias, M. L., Ribeiro, V. R., de Oliveira, L., Peracoli, J. C., et al. (2022). Vitamin D decreases expression of NLRP1 and NLRP3 inflammasomes in placental explants from women with preeclampsia cultured with hydrogen peroxide. Hum. Immunol. 83, 74–80. doi:10.1016/j.humimm.2021.10.002
Parciante, E., Cumbo, C., Anelli, L., Zagaria, A., Redavid, I., Minervini, A., et al. (2023). The role of NLRP3, a star of excellence in myeloproliferative neoplasms. Int. J. Mol. Sci. 24, 4860. doi:10.3390/ijms24054860
Park, J. S., Arcaroli, J., Yum, H.-K., Yang, H., Wang, H., Yang, K. Y., et al. (2003). Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Cell Physiol. 284, C870–C879. doi:10.1152/ajpcell.00322.2002
Pei, Y., Ma, W., Wang, H., Chen, F., Xiao, W., Fan, M., et al. (2023). Mesenchymal stem cell-derived exosomal miR-548x-3p inhibits pyroptosis of vascular endothelial cells through HMGB1 in heat stroke. Genomics 115, 110719. doi:10.1016/j.ygeno.2023.110719
Pirani, E., Paparoditis, P., Pecoraro, M., Danelon, G., Thelen, M., Cecchinato, V., et al. (2024). Tumor cells express and maintain HMGB1 in the reduced isoform to enhance CXCR4-Mediated migration. Front. Immunol. 15, 1358800. doi:10.3389/fimmu.2024.1358800
Pisetsky, D. S. (2014). The expression of HMGB1 on microparticles released during cell activation and cell death in vitro and in vivo. Mol. Med. 20, 158–163. doi:10.2119/molmed.2014.00014
Porto, A., Palumbo, R., Pieroni, M., Aprigliano, G., Chiesa, R., Sanvito, F., et al. (2006). Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 20, 2565–2566. doi:10.1096/fj.06-5867fje
Qiang, X., Peng, Y., Wang, Z., Fu, W., Li, W., Zhao, Q., et al. (2023). Synthesis of glycyrrhizin analogues as HMGB1 inhibitors and their activity against sepsis in acute kidney injury. Eur. J. Med. Chem. 259, 115696. doi:10.1016/j.ejmech.2023.115696
Qin, S., Wang, H., Yuan, R., Li, H., Ochani, M., Ochani, K., et al. (2006). Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 203, 1637–1642. doi:10.1084/jem.20052203
Rapoport, B. L., Steel, H. C., Theron, A. J., Heyman, L., Smit, T., Ramdas, Y., et al. (2020). High mobility group box 1 in human cancer. Cells 9, 1664. doi:10.3390/cells9071664
Rauvala, H., and Rouhiainen, A. (2007). RAGE as a receptor of HMGB1 (amphoterin): roles in health and disease. Curr. Mol. Med. 7, 725–734. doi:10.2174/156652407783220750
Reinhart, N. M., Akinyemi, I. A., Frey, T. R., Xu, H., Agudelo, C., Brathwaite, J., et al. (2022). The danger molecule HMGB1 cooperates with the NLRP3 inflammasome to sustain expression of the EBV lytic switch protein in burkitt lymphoma cells. Virology 566, 136–142. doi:10.1016/j.virol.2021.12.002
Schroder, K., and Tschopp, J. (2010). The inflammasomes. Cell 140, 821–832. doi:10.1016/j.cell.2010.01.040
Settipane, R. A., Kreindler, J. L., Chung, Y., and Tkacz, J. (2019). Evaluating direct costs and productivity losses of patients with asthma receiving GINA 4/5 therapy in the United States. Ann. Allergy Asthma Immunol. 123, 564–572. doi:10.1016/j.anai.2019.08.462
Shan, Y., Liu, P., Zhou, Y., Ding, X., Liu, H., and Yang, J. (2023). Prenatal sevoflurane exposure impairs the learning and memory of rat offspring via HMGB1-Induced NLRP3/ASC inflammasome activation. ACS Chem. Neurosci. 14, 699–708. doi:10.1021/acschemneuro.2c00620
Sharif, H., Wang, L., Wang, W. L., Magupalli, V. G., Andreeva, L., Qiao, Q., et al. (2019). Structural mechanism for NEK7-Licensed activation of NLRP3 inflammasome. Nature 570, 338–343. doi:10.1038/s41586-019-1295-z
Song, E., Jahng, J. W., Chong, L. P., Sung, H. K., Han, M., Luo, C., et al. (2017). Lipocalin-2 induces NLRP3 inflammasome activation via HMGB1 induced TLR4 signaling in heart tissue of mice under pressure overload challenge. Am. J. Transl. Res. 9, 2723–2735.
Sun, K., Lin, W., Hong, Q., Chen, S., Li, J., and Qiu, S. (2024). Matrine: a promising treatment for ulcerative colitis by targeting the HMGB1/NLRP3/Caspase-1 pathway. Comb. Chem. High. Throughput Screen 28, 654–663. doi:10.2174/0113862073292384240209095838
Swanson, K. V., Deng, M., and Ting, J.P.-Y. (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489. doi:10.1038/s41577-019-0165-0
Tang, D., and Lotze, M. T. (2012). Tumor immunity times out: TIM-3 and HMGB1. Nat. Immunol. 13, 808–810. doi:10.1038/ni.2396
Tang, D., Shi, Y., Kang, R., Li, T., Xiao, W., Wang, H., et al. (2007). Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 81, 741–747. doi:10.1189/jlb.0806540
Taverna, S., Tonacci, A., Ferraro, M., Cammarata, G., Cuttitta, G., Bucchieri, S., et al. (2022). High mobility group box 1: biological functions and relevance in oxidative stress related chronic diseases. Cells 11, 849. doi:10.3390/cells11050849
Thankam, F. G., Roesch, Z. K., Dilisio, M. F., Radwan, M. M., Kovilam, A., Gross, R. M., et al. (2018). Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci. Rep. 8, 8918. doi:10.1038/s41598-018-27250-2
Tian, J., Avalos, A. M., Mao, S.-Y., Chen, B., Senthil, K., Wu, H., et al. (2007). Toll-like receptor 9-Dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8, 487–496. doi:10.1038/ni1457
Tong, L., Liu, R., Yang, Y., Zhao, J., Ye, S., Wang, X., et al. (2023). Ghrelin protects against Ischemia/reperfusion-induced hepatic injury via inhibiting Caspase-11-Mediated noncanonical pyroptosis. Transpl. Immunol. 80, 101888. doi:10.1016/j.trim.2023.101888
Varricchi, G., Ferri, S., Pepys, J., Poto, R., Spadaro, G., Nappi, E., et al. (2022). Biologics and airway remodeling in severe asthma. Allergy 77, 3538–3552. doi:10.1111/all.15473
Vogel, S., Arora, T., Wang, X., Mendelsohn, L., Nichols, J., Allen, D., et al. (2018). The platelet NLRP3 inflammasome is upregulated in sickle cell disease via HMGB1/TLR4 and bruton tyrosine kinase. Blood Adv. 2, 2672–2680. doi:10.1182/bloodadvances.2018021709
Wang, H., Bloom, O., Zhang, M., Vishnubhakat, J. M., Ombrellino, M., Che, J., et al. (1999). HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251. doi:10.1126/science.285.5425.248
Wang, R., Wu, W., Li, W., Huang, S., Li, Z., Liu, R., et al. (2018). Activation of NLRP3 inflammasome promotes foam cell formation in vascular smooth muscle cells and atherogenesis Via HMGB1. J. Am. Heart Assoc. 7, e008596. doi:10.1161/JAHA.118.008596
Wang, B., Lian, Y.-J., Su, W.-J., Liu, L. L., Li, J. M., Jiang, C. L., et al. (2019). Fr-HMGB1 and Ds-HMGB1 activate the kynurenine pathway via different mechanisms in association with depressive-like behavior. Mol. Med. Rep. 20, 359–367. doi:10.3892/mmr.2019.10225
Wang, J., Li, R., Peng, Z., Hu, B., and Rao, X. (2020). HMGB1 participates in LPS-Induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int. J. Mol. Med. 45, 61–80. doi:10.3892/ijmm.2019.4402
Wang, G., Jin, S., Huang, W., Li, Y., Wang, J., Ling, X., et al. (2021). LPS-induced macrophage HMGB1-Loaded extracellular vesicles trigger hepatocyte pyroptosis by activating the NLRP3 inflammasome. Cell Death Discov. 7, 337. doi:10.1038/s41420-021-00729-0
Wang, Z., Guo, L., Yuan, C., Zhu, C., Li, J., Zhong, H., et al. (2024a). Staphylococcus pseudintermedius induces pyroptosis of canine corneal epithelial cells by activating the ROS–NLRP3 signalling pathway. Virulence 15, 2333271. doi:10.1080/21505594.2024.2333271
Wang, Z., Guo, L., Zhu, C., Li, J., Guo, J., Zhu, X., et al. (2024b). NLRP3 targets HMGB1 to exacerbate the pyroptosis of canine corneal epithelial cells infected with Staphylococcus pseudintermedius. Exp. Eye Res. 248, 110096. doi:10.1016/j.exer.2024.110096
Wei, X., Zhang, B., Wei, F., Ding, M., Luo, Z., Han, X., et al. (2022). Gegen Qinlian Pills Alleviate Carrageenan-Induced Thrombosis in Mice Model by Regulating the HMGB1/NF-κB/NLRP3 Signaling. Phytomedicine 100, 154083. doi:10.1016/j.phymed.2022.154083
Wei, J., Leng, L., Sui, Y., Song, S., Owusu, F. B., Li, X., et al. (2024). Phenolic acids from Prunella vulgaris alleviate cardiac remodeling following myocardial infarction partially by suppressing NLRP3 activation. Phytother. Res. 38, 384–399. doi:10.1002/ptr.8024
Wu, F., Zhao, Z.-H., Ding, S.-T., Wu, H. H., and Lu, J. J. (2013). High mobility group box 1 protein is methylated and transported to cytoplasm in clear cell renal cell carcinoma. Asian Pac J. Cancer Prev. 14, 5789–5795. doi:10.7314/apjcp.2013.14.10.5789
Wu, X., Yang, Z., Wang, H., Zhao, Y., Gao, X., and Zang, B. (2021). High-mobility group box Protein-1 induces acute pancreatitis through activation of neutrophil extracellular trap and subsequent production of IL-1β. Life Sci. 286, 119231. doi:10.1016/j.lfs.2021.119231
Wu, Q., Zhou, M., Chen, Y., Zhu, B., Zhou, F., Ye, X., et al. (2024). Bletilla striata polysaccharides protect against ARDS by modulating the NLRP3/Caspase1/GSDMD and HMGB1/TLR4 signaling pathways to improve pulmonary alveolar macrophage pyroptosis. J. Ethnopharmacol. 319, 117361. doi:10.1016/j.jep.2023.117361
Xie, M., Yu, Y., Kang, R., Zhu, S., Yang, L., Zeng, L., et al. (2016). PKM2-Dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 7, 13280. doi:10.1038/ncomms13280
Xie, W., Ding, J., Xie, X., Yang, X. H., Wu, X. F., Chen, Z. X., et al. (2020). Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm. Res. 69, 683–696. doi:10.1007/s00011-020-01351-z
Xie, J., Bi, B., Qin, Y., Dong, W., Zhong, J., Li, M., et al. (2021). Inhibition of Phosphodiesterase-4 suppresses HMGB1/RAGE signaling pathway and NLRP3 inflammasome activation in mice exposed to chronic unpredictable mild stress. Brain, Behav. Immun. 92, 67–77. doi:10.1016/j.bbi.2020.11.029
Xie, S.-S., Deng, Y., Guo, S., Li, J. Q., Zhou, Y. C., Liao, J., et al. (2022). Endothelial cell ferroptosis mediates monocrotaline-induced pulmonary hypertension in rats by modulating NLRP3 inflammasome activation. Sci. Rep. 12, 3056. doi:10.1038/s41598-022-06848-7
Xiong, Y., Yang, J., Tong, H., Zhu, C., and Pang, Y. (2022). HMGB1 augments cognitive impairment in sepsis-associated encephalopathy by binding to MD-2 and promoting NLRP3-Induced neuroinflammation. Psychogeriatrics 22, 167–179. doi:10.1111/psyg.12794
Yang, D., Chen, Q., Yang, H., Tracey, K. J., Bustin, M., and Oppenheim, J. J. (2007). High mobility group Box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 81, 59–66. doi:10.1189/jlb.0306180
Yang, H., Wang, H., Chavan, S. S., and Andersson, U. (2015). High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol. Med. 21, S12. doi:10.2119/molmed.2015.00087
Yang, Y., Wang, H., Kouadir, M., Song, H., and Shi, F. (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10, 128–11. doi:10.1038/s41419-019-1413-8
Yang, H., Wang, H., and Andersson, U. (2020a). Targeting inflammation driven by HMGB1. Front. Immunol. 11, 484. doi:10.3389/fimmu.2020.00484
Yang, F., Zhu, W., Cai, X., Zhang, W., Yu, Z., Li, X., et al. (2020b). Minocycline alleviates NLRP3 inflammasome-dependent pyroptosis in monosodium glutamate-induced depressive rats. Biochem. Biophysical Res. Commun. 526, 553–559. doi:10.1016/j.bbrc.2020.02.149
Yang, S., Sun, Y., Luo, Y., Liu, Y., Jiang, M., Li, J., et al. (2024a). Hypermethylation of PPARG-encoding gene promoter mediates fine particulate matter-induced pulmonary fibrosis by regulating the HMGB1/NLRP3 axis. Ecotoxicol. Environ. Saf. 272, 116068. doi:10.1016/j.ecoenv.2024.116068
Yang, Y., Jiang, S., Mu, Y., Liu, C., Han, Y., Jiang, J., et al. (2024b). Berberine alleviated contrast-induced acute kidney injury by mitophagy-mediated NLRP3 inflammasome inactivation in a mice model. Toxicol. Appl. Pharmacol. 486, 116952. doi:10.1016/j.taap.2024.116952
Yang, M., Liu, H., Lou, J., Zhang, J., Zuo, C., Zhu, M., et al. (2024c). Alpha-emitter Radium-223 induces STING-dependent pyroptosis to trigger robust antitumor immunity. Small 20, e2307448. doi:10.1002/smll.202307448
Yang, R. (2006). Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 105–114. doi:10.2119/2006-00010
Yao, Y., Zuo, X., Shao, F., Yu, K., and Liang, Q. (2024). Jaceosidin attenuates the progression of hepatic fibrosis by inhibiting the VGLL3/HMGB1/TLR4 signaling pathway. Phytomedicine 128, 155502. doi:10.1016/j.phymed.2024.155502
Ye, Y., Jin, T., Zhang, X., Zeng, Z., Ye, B., Wang, J., et al. (2019). Meisoindigo protects against focal cerebral ischemia-reperfusion injury by inhibiting NLRP3 inflammasome activation and regulating microglia/macrophage polarization via TLR4/NF-κB signaling pathway. Front. Cell. Neurosci. 13, 553. doi:10.3389/fncel.2019.00553
Ye, X.-G., She, F.-Z., Yu, D.-N., Wu, L. Q., Tang, Y., Wu, B. Z., et al. (2024). Increased expression of NLRP3 associated with elevated levels of HMGB1 in children with febrile seizures: a case-control study. BMC Pediatr. 24, 44. doi:10.1186/s12887-024-04533-4
Yin, H., Wu, M., Lu, Y., Wu, X., Yu, B., Chen, R., et al. (2022). HMGB1-Activatied NLRP3 inflammasome induces thrombocytopenia in heatstroke rat. PeerJ 10, e13799. doi:10.7717/peerj.13799
You, L., Cui, H., Zhao, F., Sun, H., Zhong, H., Zhou, G., et al. (2021). Inhibition of HMGB1/RAGE axis suppressed the lipopolysaccharide (LPS)-induced vicious transformation of cervical epithelial cells. Bioengineered 12, 4995–5003. doi:10.1080/21655979.2021.1957750
Yu, S., Wang, D., Huang, L., Zhang, Y., Luo, R., Adah, D., et al. (2019). The complement receptor C5aR2 promotes protein kinase R expression and contributes to NLRP3 inflammasome activation and HMGB1 release from macrophages. J. Biol. Chem. 294, 8384–8394. doi:10.1074/jbc.RA118.006508
Yu, J., Jiang, Q., Liu, N., Fan, D., Wang, M., and Zhao, Y. (2022). Apigenin and Apigenin-7, 4′-O-Dioctanoate protect against acrolein-aggravated inflammation via inhibiting the activation of NLRP3 inflammasome and HMGB1/MYD88/NF-κB signaling pathway in human umbilical vein endothelial cells (HUVEC). Food Chem. Toxicol. 168, 113400. doi:10.1016/j.fct.2022.113400
Zhang, L., Chen, S., Ruan, J., Wu, J., Tong, A. B., Yin, Q., et al. (2015). Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409. doi:10.1126/science.aac5789
Zhang, K., Fan, C., Cai, D., Zhang, Y., Zuo, R., Zhu, L., et al. (2020). Contribution of TGF-Beta-Mediated NLRP3-HMGB1 activation to tubulointerstitial fibrosis in rat with angiotensin II-Induced chronic kidney disease. Front. Cell Dev. Biol. 8, 1. doi:10.3389/fcell.2020.00001
Zhang, D., Wu, C., Ba, D., Wang, N., Wang, Y., Li, X., et al. (2023). Ferroptosis contribute to neonicotinoid imidacloprid-evoked pyroptosis by activating the HMGB1-RAGE/TLR4-NF-κB signaling pathway. Ecotoxicol. Environ. Saf. 253, 114655. doi:10.1016/j.ecoenv.2023.114655
Zhang, Y., Ye, P., Zhu, H., Gu, L., Li, Y., Feng, S., et al. (2024a). Neutral polysaccharide from Gastrodia elata alleviates cerebral ischemia-reperfusion injury by inhibiting ferroptosis-mediated neuroinflammation via the NRF2/HO-1 signaling pathway. CNS Neurosci. Ther. 30, e14456. doi:10.1111/cns.14456
Zhang, Z., Wu, X., Zou, Z., Shen, M., Liu, Q., Zhangsun, Z., et al. (2024b). Heat stroke: pathogenesis, diagnosis, and current treatment. Ageing Res. Rev. 100, 102409. doi:10.1016/j.arr.2024.102409
Zhang, Y., Zhang, J.-X., Xiao, L.-X., Zheng, J. T., Qu, X. T., Liu, Y., et al. (2024c). The Synergistic Effect of Huangqi Gegen Decoction on Thrombosis Relates to the Astragalus Polysaccharide-Improved Oral Delivery of Puerarin. J. Ethnopharmacol. 335, 118622. doi:10.1016/j.jep.2024.118622
Zhang, Y., Li, J., Deng, H., Wan, H., Xu, P., Wang, J., et al. (2024d). High mobility group box 1 knockdown inhibits EV71 replication and attenuates cell pyroptosis through TLR4/NF-κB/NLRP3 axis. J. Biochem. Mol. Toxicol. 38, e23620. doi:10.1002/jbt.23620
Zhao, Y., Tan, S.-W., Huang, Z.-Z., Shan, F. B., Li, P., Ning, Y. L., et al. (2021a). NLRP3 inflammasome-dependent increases in high mobility group box 1 involved in the cognitive dysfunction caused by tau-overexpression. Front. Aging Neurosci. 13, 721474. doi:10.3389/fnagi.2021.721474
Zhao, H., Gu, Y., and Chen, H. (2021b). Propofol ameliorates endotoxin-induced myocardial cell injury by inhibiting inflammation and apoptosis via the PPARγ/HMGB1/NLRP3 axis. Mol. Med. Rep. 23, 176. doi:10.3892/mmr.2020.11815
Zhao, F., Guo, Z., Hou, F., Fan, W., Wu, B., and Qian, Z. (2021c). Magnoflorine alleviates “M1” polarized macrophage-induced intervertebral disc degeneration through repressing the HMGB1/Myd88/NF-κB pathway and NLRP3 inflammasome. Front. Pharmacol. 12, 701087. doi:10.3389/fphar.2021.701087
Zhao, Y.-Z., Zhang, X.-N., Yin, Y., Xiao, P. L., Gao, M., Zhang, L. M., et al. (2024a). N-Acetylserotonin alleviates retinal ischemia-reperfusion injury via HMGB1/RAGE/NF-κB pathway in rats. Int. J. Ophthalmol. 17, 228–238. doi:10.18240/ijo.2024.02.02
Zhao, C., Mu, M., Li, X., Dong, Z., Wang, J., Yao, C., et al. (2024b). USP50 regulates NLRP3 inflammasome activation in duodenogastric reflux-induced gastric tumorigenesis. Front. Immunol. 15, 1326137. doi:10.3389/fimmu.2024.1326137
Zhong, W.-J., Duan, J.-X., Liu, T., Yang, H. H., Guan, X. X., Zhang, C. Y., et al. (2020). Activation of NLRP3 inflammasome Up-Regulates TREM-1 expression in murine macrophages via HMGB1 and IL-18. Int. Immunopharmacol. 89, 107045. doi:10.1016/j.intimp.2020.107045
Zhou, R., Tardivel, A., Thorens, B., Choi, I., and Tschopp, J. (2010). Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140. doi:10.1038/ni.1831
Zhou, R., Yazdi, A. S., Menu, P., and Tschopp, J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. doi:10.1038/nature09663
Keywords: HMGB1, NLRP3, inflammation, systemic disorders, therapeutic targets
Citation: Yang L, Li X, Zhu X, Ge F and Wang Y (2025) Involvement of role of HMGB1-NLRP3 pathway in systemic disorders. Front. Cell Dev. Biol. 13:1600596. doi: 10.3389/fcell.2025.1600596
Received: 26 March 2025; Accepted: 20 June 2025;
Published: 04 July 2025.
Edited by:
John Hancock, University of the West of England, United KingdomReviewed by:
Geraldine De Luca, University of Buenos Aires, ArgentinaAbhinava Mishra, University of California, Santa Barbara, United States
Copyright © 2025 Yang, Li, Zhu, Ge and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Futao Ge, Z2UxZnUydGFvM0BqbHUuZWR1LmNu; Yuantao Wang, d2FuZ3l1YW50YW9ic0BqbHUuZWR1LmNu
†These authors have contributed equally to this work
 Lei Yang1
Lei Yang1 Yuantao Wang
Yuantao Wang