- 1Vision X Medical Technology Co., Ltd., Shanghai, China
- 2National Clinical Research Center for Ocular Diseases, Eye Hospital, Wenzhou Medical University, Wenzhou, China
- 3Shanghai E-Vision Eye Clinic, Shanghai, China
Objective: To develop and validate machine learning (ML) models for predicting cycloplegic spherical equivalent refraction (SER) using non-cycloplegic parameters, addressing challenges in pediatric ophthalmic assessments.
Methods: A prospective cohort of 2,274 Chinese children (4,548 eyes) aged 3∼16 years was stratified into development (n = 1819) and validation (n = 455) datasets. Six ML models (linear regression, random forest, extreme gradient boosting, multilayer perceptron, support vector machine, and light gradient boosting machine) were trained on demographics, non-cycloplegic refractive error, and ocular biometrics. Model performance was evaluated using R2, mean error (ME), mean absolute error (MAE), and clinical accuracy (proportions within ±0.50 D/±1.00 D).
Results: In the validation dataset, ML models predicted cycloplegic SER with high R2 (0.920∼0.934), low ME (−0.004∼0.015 D) and MAE (0.385∼0.413 D). The multilayer perceptron model achieved the highest accuracy (R2 = 0.934, MAE = 0.385 D), with 73.08% and 94.29% of predictions within ±0.50 D and ±1.00 D, respectively. Performance was optimal in children aged 7∼10 years (77.17∼79.70% within ±0.50 D) and those with low myopia (−3.00 to −0.50 D; 83.09∼83.56% within ±0.50 D). Non-cycloplegic measurements systematically overestimated myopia (mean difference: −0.39 ± 0.71 D, P < 0.001), particularly in younger children and hyperopic eyes.
Conclusion: ML models provide accurate estimates of cycloplegic SER using non-cycloplegic parameters, offering a practical alternative for pediatric refractive assessments when cycloplegia is infeasible.
1 Introduction
Accurate refractive error assessment in pediatric populations necessitates cycloplegic refraction to eliminate accommodation-induced measurement errors, establishing it as the clinical gold standard for both diagnosis and epidemiological studies (Morgan et al., 2015; Doherty et al., 2019; Flitcroft et al., 2019). However, application of cycloplegia faces practical challenges in large-scale pediatric research, including time constraints, resource limitations, and potential contraindications, often precluding its routine use in population-based screenings. To address the conflict between the necessity of cycloplegic refractive examination and the difficulty of performing cycloplegia in practice, emerging studies have explored statistical models to predict cycloplegic refractive error using non-cycloplegic parameters. These models exhibit inconsistent performance (R2 ranging from 0.26 to 0.935) (Ip et al., 2007; Kimura et al., 2007; He et al., 2015; Foo et al., 2016; Sankaridurg et al., 2017; Magome et al., 2021; Wang et al., 2022; Du et al., 2023; He et al., 2023; Ying et al., 2024), likely attributable to methodological heterogeneity, variable predictor selection, and population-specific biases.
Machine learning (ML), a branch of artificial intelligence, offers unparalleled capabilities in identifying complex patterns within high-dimensional datasets. Unlike conventional regression models constrained by linear assumptions, advanced ML algorithms (e.g., random forest [RF], support vector machine [SVM] and gradient boosting models) are capable of modeling intricate and non-linear relationships between predictors and outcomes, and have demonstrated great potential for application in ophthalmology and vision research (Wu et al., 2021; Cao et al., 2022; Wu et al., 2022; Chandra and Ying, 2023; 2024). Recent advancements highlight ML’s ability to predict key refractive parameters such as spherical equivalent refraction (SER) and axial length (AL) in pediatric cohorts. For instance, Zhu et al. (2023) demonstrated that ML models could predict SER and AL changes in children with high accuracy (R2 up to 0.90), leveraging longitudinal data to inform myopia progression. These studies collectively emphasize ML’s capacity to address both diagnostic and prognostic challenges in pediatric ophthalmology, particularly when traditional methods are constrained.
This study aimed to develop and validate ML models for predicting cycloplegic refractive error in Chinese children aged 3–16 years old using readily obtainable non-cycloplegic parameters, encompassing demographics, non-cycloplegic refractive error, and ocular biometric measurements. By leveraging the predictive power of ML, this work seeks to provide a clinically practical solution for determining true refractive status in pediatric populations where cycloplegic examinations are impractical.
2 Materials and methods
2.1 Participants
A prospective cohort of 2,274 children (4,548 eyes) aged 3–16 years was recruited from the Eye Hospital of Wenzhou Medical University between October 2021 and March 2024. Inclusion criteria included: (1) parental consent for cycloplegic procedures; (2) availability of pre-/post-cycloplegic clinical data. Exclusion criteria comprised ocular organic diseases, strabismus, amblyopia, prior ocular surgery, or hypersensitivity to 1% cyclopentolate hydrochloride. Participants were stratified by age and refractive status and randomly allocated into development (80%, n = 1819 children) and validation (20%, n = 455 children) datasets to ensure proportional representation.
The study protocol was approved by the Institutional Ethics Committee of the Eye Hospital of Wenzhou Medical University (approval no. 2021-233-K-203-03) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants’ parents or guardians.
2.2 Data collection
Demographic information (age, gender, and height) and ocular parameters were collected under non-cycloplegic conditions. Comprehensive ophthalmic examinations included: distance visual acuity test using retro-illuminated logMAR charts with tumbling-E optotypes, slit-lamp examination of the anterior segment, fundus examination with ophthalmoscopy, intraocular pressure measurement and the assessment of ocular biometrics under non-cycloplegic conditions (e.g., AL, corneal curvature [CR], central corneal thickness [CCT], aqueous depth [AD], lens thickness [LT], and corneal astigmatism) using the AB-800 optical biometer (Hangzhou Weixiao Medical Technology Co., Ltd., Hangzhou, China). AL measurements were retained if intra-test variability was ≤0.02 mm.
Cycloplegia was induced by administering two drops of 1% cyclopentolate (Alcon, Fortworth, TX, America) at 5-min intervals. Autorefraction (KR 800, Topcon, Tokyo, Japan) was performed pre-cycloplegia and 30 min after application of cyclopentolate (following confirmation of adequate pupillary dilation [>6 mm] and loss of light reflex). For both conditions, three consecutive autorefraction measurements were averaged, with repeat assessments if any spherical or cylindrical component differed by > 0.50 D between readings. All data underwent dual-entry verification (EpiData 3.1, Odense, Denmark) with source document reconciliation for discrepancies.
2.3 Machine learning models
The cycloplegic SER was calculated as sphere +0.5*cylinder. Six ML models were evaluated for cycloplegic SER prediction: (1) linear regression: identifies linear relationships through coefficient optimization, offering interpretability but constrained to additive patterns (Ludwig Fahrmeir and Lang, 2009). (2) RF: combines predictions from an ensemble of decision trees to mitigate overfitting (Breiman, 2001). (3) extreme gradient boosting (XGBoost): utilizes gradient-boosted trees and trained additively to enhance predictive accuracy (Chen and Guestrin, 2016). (4) multilayer perceptron (MLP): a feedforward neural network employing weighted linear summations and nonlinear activation functions for hierarchical feature transformation (Abu-Mostafa and Hsuan-Tien Lin, 2012). The MLP architecture comprised three fully connected layers: an input layer (11 neurons), a hidden layer (32 neurons), and an output layer (1 neuron). (5) SVM: predicts a continuous outcome with high generalization ability by transforming data to high-dimensional spaces (Cortes and Vapnik, 1995). (6) light gradient boosting machine (LightGBM): accelerates gradient boosting via histogram-based tree construction and leaf-wise growth, prioritizing efficiency for large datasets (Ke et al., 2017).
The predictors for the ML models were: demographics (age, gender, height), non-cycloplegic SER, biometric parameters (AL, CR, AL/CR ratio, CCT, AD, LT, and corneal astigmatic value). Hyperparameters were tuned for each of the models through 5-fold cross-validation on the development dataset to reduce overfitting risks and optimize R2 (the coefficient of determination). Final models were trained on the entire development dataset using optimized hyperparameters. The independent validation dataset was strictly reserved for final performance evaluation, thereby ensuring an unbiased assessment of the models’ generalizability.
2.4 Statistical analysis
Myopia was defined as cycloplegic SER -0.50 D or worse. Emmetropia was defined between −0.50 D and +0.50 D and hyperopia was defined as greater than +0.50 D. Continuous variables were expressed as mean ± standard deviation (SD). Paired t-test was performed to compare the differences between non-cycloplegic and cycloplegic SER across the entire cohort and in subgroups based on age, refractive error, axial length. Model performance was evaluated using R2, correlation coefficient r, mean error (ME), and mean absolute error (MAE) of differences between predicted and measured cycloplegic SER (calculated as predicted–measured), as well as the clinical accuracy proportions (predictions within ±0.50 D/±1.00 D). The predicted and measured cycloplegic SER were also statistically compared using paired t-test. For the best-performing model (selected based on the performance in the validation dataset), subgroup analyses were conducted by age and refractive error magnitude.
ML models implementation utilized Python 3.8 (scikit-learn version 1.3.0), while the statistical analysis was performed with IBM SPSS Statistics software version 27 (IBM, Armonk, NY). A P value of <0.05 was considered statistically significant.
3 Results
3.1 Participant characteristics
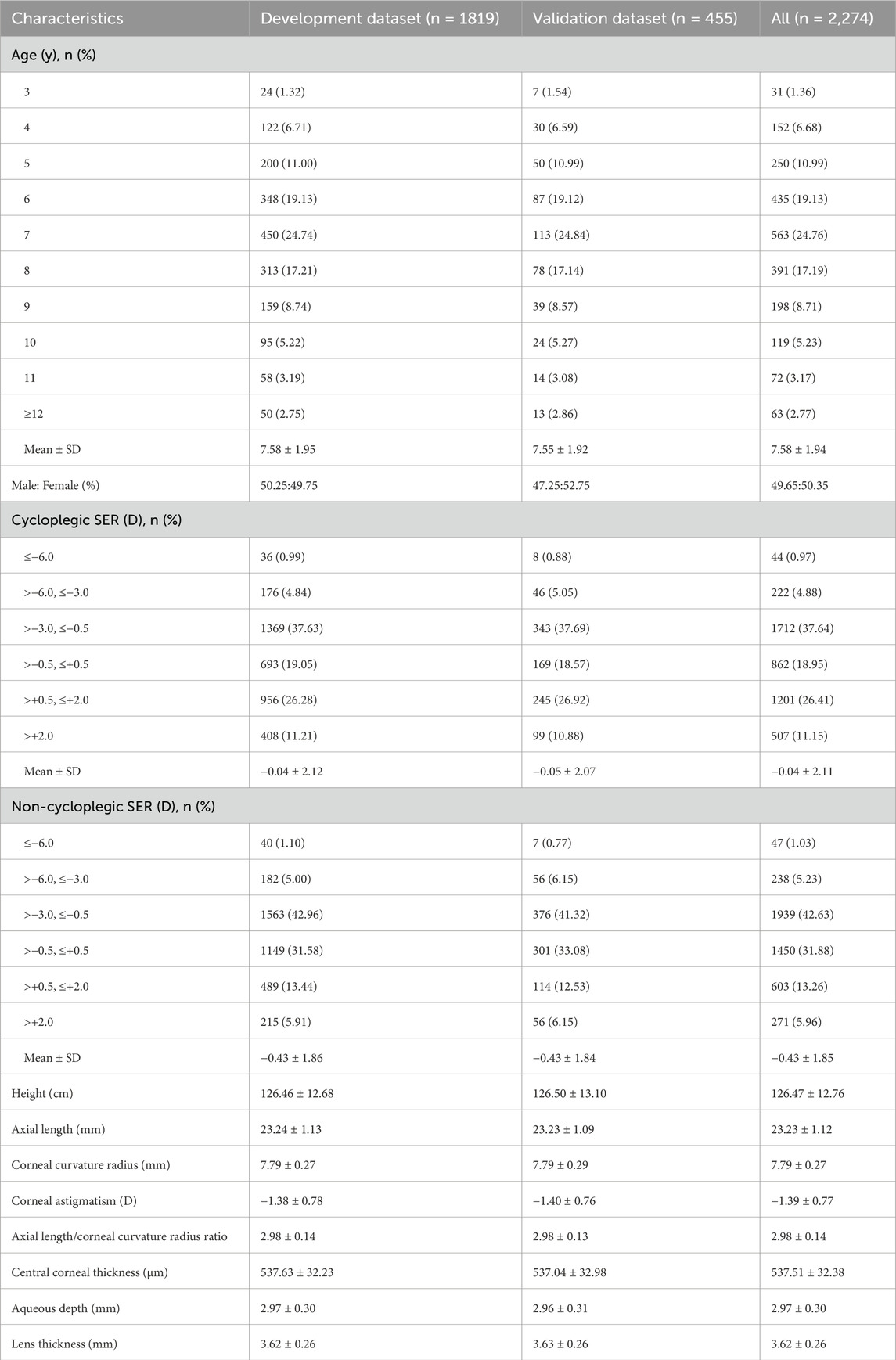
Data from 2,274 children (4,548 eyes) were included in the analysis, with a mean age of 7.58 ± 1.94 years (range: 3∼16 years). Females accounted for 50.35% of the study population. Participants were stratified into development (n = 1819 children, 3,638 eyes; mean age: 7.58 ± 1.95 years) and validation (n = 455 children, 910 eyes; mean age: 7.55 ± 1.92 years) datasets. The two datasets exhibited comparable distributions in age, gender, height, and ocular biometric parameters (all P values > 0.05). Demographic and ocular characteristics of participants in the development and validation datasets were summarized in Table 1.
3.2 Mean difference between non-cycloplegic and cycloplegic SER
Overall, there was a mean paired difference of −0.39 ± 0.71 D (95% confidence intervals: −0.41 to −0.37D, P < 0.001) between non-cycloplegic and cycloplegic SER, with the non-cycloplegic SER resulting in a more myopic (or less hyperopic) refractive error. Figure 1 illustrated the mean paired differences in non-cycloplegic and cycloplegic SER by age, cycloplegic refractive error, axial length. The bias was greatest with younger age, hyperopic refractive error and shorter axial length. Differences approached zero when cycloplegic SER measures were myopic, and became increasingly negative with emmetropia and hyperopia (all P < 0.05, except for the high myopia subgroup, P = 0.491).

Figure 1. Differences between non-cycloplegic versus cycloplegic refraction based on age (A), refractive error (B), axial length (C).
3.3 Machine learning model performance
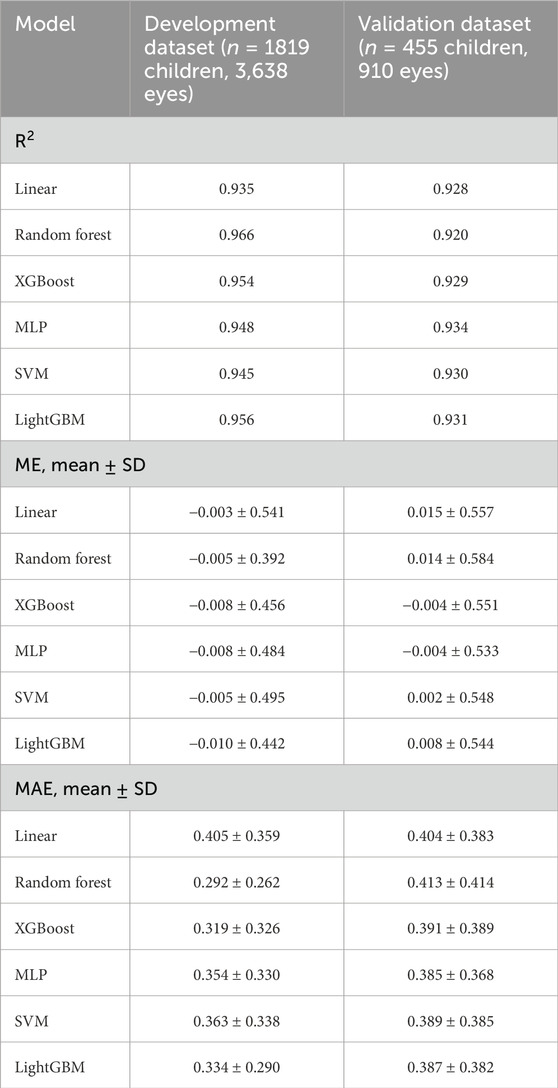
With the clinical parameter values obtained from the development dataset, we developed and evaluated 6 ML models for predicting cycloplegic SER. In the development dataset, all 6 ML models predicted cycloplegic SER well with R2 ranging from 0.935 (linear) to 0.966 (random forest), the correlation coefficient r of 0.952 (linear) to 0.973 (random forest), ME of −0.010 D (LightGBM) to −0.003 D (linear), and MAE of 0.292 D (random forest) to 0.405 D (linear) (Table 2). The random forest model performed best (R2 = 0.966, MAE = 0.292 D), followed by LightGBM (R2 = 0.956, MAE = 0.334 D). In the validation dataset, the MLP model outperformed others (R2 = 0.934, r = 0.956, MAE = 0.385 D), with LightGBM model ranking second (R2 = 0.931, r = 0.955, MAE = 0.387 D), and the random forest model performed worst (R2 = 0.920, r = 0.951, MAE = 0.413 D) (Table 2). Paired t-tests confirmed no significant differences between predicted and measured SER across all models (P = 0.430 for linear, P = 0.927 for RF, P = 0.652 for XGBoost, P = 0.459 for MLP, P = 0.836 for SVM, P = 0.830 for LightGBM).
3.4 Subgroup analysis of the best-performing model
We further evaluated the performance of the best-performing ML model in the validation dataset (MLP) by assessing its performance across subsets stratified by age and cycloplegic SER magnitude. The importance of features in MLP model was shown in Supplementary Figure 1. Non-cycloplegic SER, AL/CR ratio, and AL were the top three predictors for predicting cycloplegic SER. The scatter plot (Figure 2) illustrated a highly positive correlation between predicted cycloplegic SER by MLP model and measured cycloplegic SER values, with R2 of 0.948 in the development dataset and 0.934 in the validation dataset.
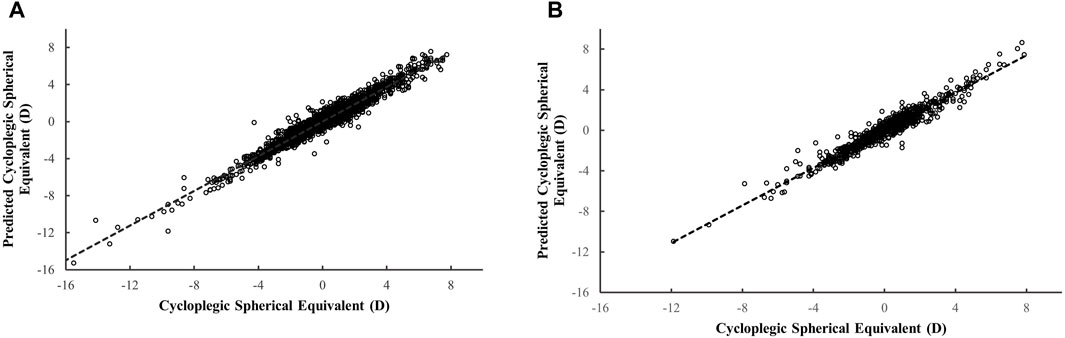
Figure 2. The scatter plot for the predicted versus measured cycloplegic spherical equivalent based on the MLP model. The scatter plot in the development dataset ((A) 3,638 eyes from 1819 children) and in the validation dataset ((B) 910 eyes from 455 children).
The overall ME between predicted and measured cycloplegic SER was −0.008 ± 0.484 D and −0.004 ± 0.533 D in two datasets (Table 3). In the development dataset, the ME was within 0.037 D and the MAE within 0.399 D across all age subgroups. When evaluating differences between predicted and measured based on magnitude of cycloplegic SER, the model exhibited the lowest MAE (≤0.328 D) for low myopia and emmetropia, and the highest MAE for high myopia and hyperopia > +2.00 D. Similar trends were observed in the validation dataset (Table 3).
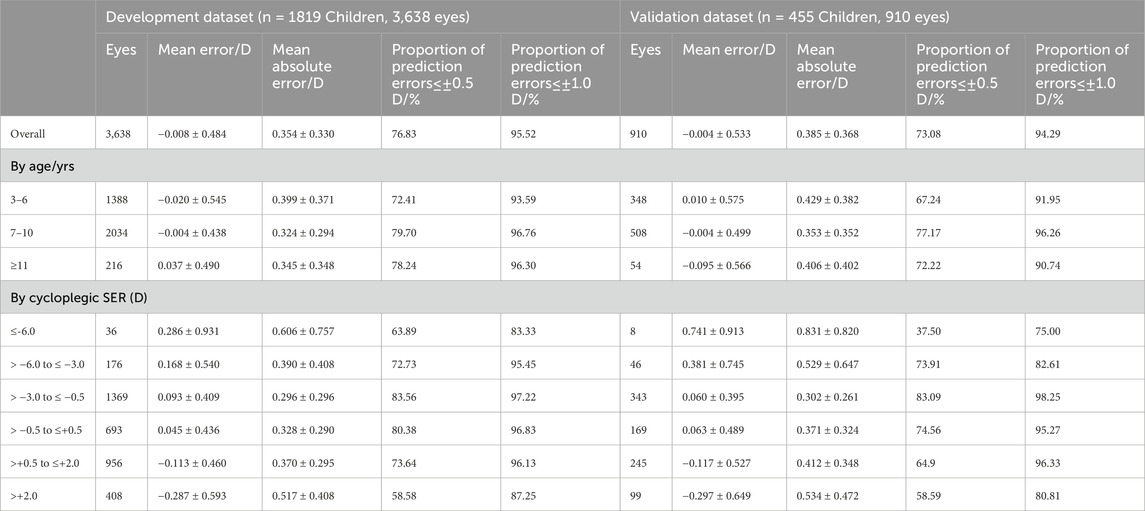
Table 3. Subgroup analysis of the best-performing model MLP in the development dataset and validation dataset.
To assess the clinical value of the ML model predictions of the refractive error, we evaluated the proportions of prediction errors within ±0.50 D and ±1.00 D compared with cycloplegic SER. The MLP model achieved clinical accuracy rates of 76.83% (±0.50 D) and 95.52% (±1.00 D) in the development dataset, and 73.08% (±0.50 D) and 94.29% (±1.00 D) in the validation dataset. Peak performance was observed in the subgroup of children aged 7–10 years (77.17∼79.70% within ±0.50 D) and for eyes with cycloplegic SER ranging from −3.00 to −0.50 D (83.09∼83.56% within ±0.50 D).
4 Discussion
This study developed and validated 6 ML models to predict cycloplegic refractive error using non-cycloplegic parameters in a large cohort of Chinese children. The results showed successful predictions of ML models in both the development and validation datasets, demonstrating the promising potential and practical value of ML-based tools to circumvent the challenges of cycloplegia in pediatric refractive assessments while maintaining diagnostic precision.
All the ML models performed well in predicting cycloplegic SER in the validation datasets (R2 ranging from 0.920 to 0.934), with the MLP model performed best. Several studies have employed statistical prediction models to estimate cycloplegic refractive error in pediatric populations, utilizing diverse predictors such as demographics, ocular biometric parameters (Magome et al., 2021), non-cycloplegic refractive error (Sankaridurg et al., 2017; Wang et al., 2022), and uncorrected visual acuity [UCVA](Sankaridurg et al., 2017), alongside modeling methodologies ranging from traditional regression to advanced ML techniques. However, the heterogeneity in study designs and populations has resulted in widely variable predictive performance (R2: 0.26∼0.935) (Ip et al., 2007; Kimura et al., 2007; He et al., 2015; Foo et al., 2016; Sankaridurg et al., 2017; Magome et al., 2021; Wang et al., 2022; Du et al., 2023; He et al., 2023; Ying et al., 2024). In particular, Du et al. (2023) developed 4 ML models (Support Vector Regression, Random Forest Regression, AdaBoost Regression, and Deep Neural Network) in 2,467 Chinese children aged 6–18 years, achieving R2 of 0.899∼0.927 and MAE of 0.372∼0.436 D. Among them, AdaBoost regression model exhibited the best overall prediction performance, with R2 = 0.927 and MAE = 0.372, slightly inferior to the optimal MLP-based model in our study. However, some predictors used in their models (e.g., the accommodation lag data) may not be easily available in clinical practice, which would limit the models’ applicability. Ying et al. (2024) also developed and validated 6 ML models based on 3,414 Chinese children ages 5–18 years by using age, gender, non-cycloplegic refractive error, biometric measures (AL, CR, AL/CR ratio, CCT, and anterior chamber depth), UCVA, intraocular pressure, and glasses-wearing status. The models predicted cycloplegic SER with R2 of 0.913∼ 0.935, and MAE of 0.393∼0.480 D, with top performance comparable to ours. Notably, while the ML methods used in our study were largely similar to those employed by Ying et al., the optimal model diverged (MLP vs. XGBoost). This discrepancy may be attributed to cohort differences: their population comprised older children (5∼18 years), with nearly 50% was low hyperopia (+0.50∼+3.00 D); whereas ours encompassed younger ages (3∼16 years old), dominated by low myopia (−3.00∼-0.50 D) and low hyperopia (+0.50∼+2.00 D). In comparison to prior work, our study prioritizes clinical practicality by exclusively incorporating parameters easily obtainable without cycloplegia, while maintaining robust accuracy (MLP model: R2 = 0.934). This balance between simplicity and precision positions our framework as a scalable solution for population-based studies, particularly in resource-limited settings where cycloplegic examinations are challenging. Furthermore, the included younger children (3–16 years old) with varied refractive status (from −16.13 to +7.88 D) enhances the model’s clinical relevance for early myopia risk stratification.
Substantial evidence demonstrates clinically significant discrepancies between cycloplegic and non-cycloplegic measurements, with non-cycloplegic SER systematically overestimated myopia (Zhao et al., 2004; Fotedar et al., 2007; Hu et al., 2015; Sankaridurg et al., 2017; Gu et al., 2022). Consistent with previous studies, such bias was also observed in our study: the mean SER changed from −0.04 ± 2.11 D to −0.43 ± 1.85 D under the non-cycloplegic condition with a mean difference of 0.39 ± 0.71 D toward myopia. Our systematic analysis of paired differences between cycloplegic and non-cycloplegic refraction across ages 3–16 years and all types of refractive error revealed that the overestimation of myopia or underestimated hyperopia with non-cycloplegia is greater in younger individuals, emmetropic or hyperopic eyes, and shorter AL. Accommodation significantly impacts the accurate measurement of refraction data in children. Age-related norms for accommodative responses in eyes without significant refractive error suggest that accommodative responses are quite variable under high accommodative demands, especially in younger individuals (McClelland and Saunders, 2004). Consequently, predicting refractive error without cycloplegia is challenging in young children. The inclusion of children under 5 years of age in this study provides a basis for addressing the difficulty of predicting cycloplegic refractive error in this age group.
The MLP model demonstrated robust clinical accuracy, with 73.08% and 94.29% of predictions falling within ±0.50 D and ±1.00 D of measured cycloplegic SER, respectively. This performance outperformed the traditional regression-based approach by He et al. (2023), which achieved an accuracy of 47% within ±0.50 D and 79% within ±1.00 D, using limited predictors (age, gender, AL and AL/CR ratio). While Du et al. (2023) reported marginally higher precision (75.2% within ±0.50 D) in their best-performing model, our framework maintains comparable efficacy while prioritizing clinically accessible parameters. Notably, the accuracy of MLP model peaked in children aged 7∼10 years (77.17∼79.70% within ±0.50 D) and those with low myopia (83.09∼83.56% within ±0.50 D). These subgroups represent pivotal stages for myopia surveillance and early intervention to mitigate progression to high myopia. These results position the ML model as a pragmatic tool for large-scale screenings in resource-limited settings.
There were several limitations in the present study. First, this study used the AB-800 optical biometer in a cohort of Chinese children, which may restrict the generalizability of the findings to other types of biometers or children of different races or ethnicities. External validation across diverse populations and devices is warranted. Second, the reduced accuracy in high myopia (≤−6.0 D) and moderate/high hyperopia (>+2.0 D) limits utility for these subgroups. Expanding data with extreme refractive errors and retraining models on enriched cohorts could improve performance and enhance the model’s applicability. Finally, the current ML models predict the cycloplegic refractive error at a single timepoint. Future longitudinal studies can evaluate the models’ capacity to track refractive progression over time.
5 Conclusion
This study developed and validated 6 ML models for predicting cycloplegic refractive error using readily available demographics and non-cycloplegic biometric parameters in a large cohort of Chinese children (3∼16 years). Among the evaluated algorithms, the MLP model demonstrated superior performance (R2 = 0.934), and exhibited enhanced accuracy in children aged 7–10 years and those with low myopia—key populations for myopia surveillance and intervention. These ML models address practical challenges in pediatric refractive assessments, offering a scalable tool for clinical practice and large-scale epidemiological research where administering cycloplegia is not feasible.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of the Eye Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BC: Data curation, Writing – original draft, Formal Analysis, Visualization. LT: Writing – original draft, Data curation, Investigation. FT: Writing – original draft, Investigation, Data curation. QY: Investigation, Writing – original draft, Data curation. YR: Writing – original draft, Data curation. YL: Formal Analysis, Writing – original draft. MC: Data curation, Writing – original draft. CW: Writing – original draft, Data curation. MY: Writing – original draft, Data curation. SX: Conceptualization, Writing – review and editing, Methodology, Supervision, Validation. RD: Writing – review and editing, Supervision, Conceptualization, Methodology, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Authors BC, YL were employed by Vision X Medical Technology Co., Ltd. and RD serves as a medical consultant for the same entity.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1608494/full#supplementary-material
References
Abu-Mostafa, Y. S., and Hsuan-Tien Lin, M.M.-I. (2012). Neural networks. Nairobi, Kenya: AML Publishers.
Cao, K., Verspoor, K., Sahebjada, S., and Baird, P. N. (2022). Accuracy of machine learning assisted detection of keratoconus: a systematic review and meta-analysis. J. Clin. Med. 11 (3), 478. doi:10.3390/jcm11030478
Chandra, R. S., and Ying, G. S. (2023). Evaluation of multiple machine learning models for predicting number of anti-VEGF injections in the comparison of AMD treatment trials (CATT). Transl. Vis. Sci. Technol. 12 (1), 18. doi:10.1167/tvst.12.1.18
Chandra, R. S., and Ying, G. S. (2024). Predicting visual acuity responses to anti-VEGF treatment in the comparison of age-related macular degeneration treatments trials using machine learning. Ophthalmol. Retina 8 (5), 419–430. doi:10.1016/j.oret.2023.11.010
Chen, T., and Guestrin, C. (2016). “XGBoost: a scalable tree boosting system,” in Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining (San Francisco, California, USA: Association for Computing Machinery).
Cortes, C., and Vapnik, V. (1995). Support-vector networks. Mach. Learn. 20 (3), 273–297. doi:10.1007/BF00994018
Doherty, S. E., Doyle, L. A., McCullough, S. J., and Saunders, K. J. (2019). Comparison of retinoscopy results with and without 1% cyclopentolate in school-aged children. Ophthalmic Physiol. Opt. 39 (4), 272–281. doi:10.1111/opo.12629
Du, B., Wang, Q., Luo, Y., Jin, N., Rong, H., Wang, X., et al. (2023). Prediction of spherical equivalent difference before and after cycloplegia in school-age children with machine learning algorithms. Front. Public Health 11, 1096330. doi:10.3389/fpubh.2023.1096330
Flitcroft, D. I., He, M., Jonas, J. B., Jong, M., Naidoo, K., Ohno-Matsui, K., et al. (2019). IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol. Vis. Sci. 60 (3), M20–m30. doi:10.1167/iovs.18-25957
Foo, V. H., Verkicharla, P. K., Ikram, M. K., Chua, S. Y., Cai, S., Tan, C. S., et al. (2016). Axial length/corneal radius of curvature ratio and myopia in 3-year-old children. Transl. Vis. Sci. Technol. 5 (1), 5. doi:10.1167/tvst.5.1.5
Fotedar, R., Rochtchina, E., Morgan, I., Wang, J. J., Mitchell, P., and Rose, K. A. (2007). Necessity of cycloplegia for assessing refractive error in 12-year-old children: a population-based study. Am. J. Ophthalmol. 144 (2), 307–309. doi:10.1016/j.ajo.2007.03.041
Gu, F., Gao, H. M., Zheng, X., Gu, L., Huang, J., Meng, J., et al. (2022). Effect of cycloplegia on refractive error measure in Chinese school students. Ophthalmic Epidemiol. 29 (6), 629–639. doi:10.1080/09286586.2021.1999986
He, X., Sankaridurg, P., Naduvilath, T., Wang, J., Xiong, S., Weng, R., et al. (2023). Normative data and percentile curves for axial length and axial length/corneal curvature in Chinese children and adolescents aged 4-18 years. Br. J. Ophthalmol. 107 (2), 167–175. doi:10.1136/bjophthalmol-2021-319431
He, X., Zou, H., Lu, L., Zhao, R., Zhao, H., Li, Q., et al. (2015). Axial length/corneal radius ratio: association with refractive state and role on myopia detection combined with visual acuity in Chinese schoolchildren. PLoS One 10 (2), e0111766. doi:10.1371/journal.pone.0111766
Hu, Y. Y., Wu, J. F., Lu, T. L., Wu, H., Sun, W., Wang, X. R., et al. (2015). Effect of cycloplegia on the refractive status of children: the Shandong children eye study. PLoS One 10 (2), e0117482. doi:10.1371/journal.pone.0117482
Ip, J. M., Huynh, S. C., Kifley, A., Rose, K. A., Morgan, I. G., Varma, R., et al. (2007). Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest Ophthalmol. Vis. Sci. 48 (10), 4846–4853. doi:10.1167/iovs.07-0101
Ke, G., Meng, Q., Finely, T., Wang, T., Chen, W., Ma, W., et al. (2017). “LightGBM: a highly efficient gradient boosting decision tree,” in Advances in neural information processing systems, 30.
Kimura, S., Hasebe, S., Miyata, M., Hamasaki, I., and Ohtsuki, H. (2007). Axial length measurement using partial coherence interferometry in myopic children: repeatability of the measurement and comparison with refractive components. Jpn. J. Ophthalmol. 51 (2), 105–110. doi:10.1007/s10384-006-0410-5
Ludwig Fahrmeir, T. K., and Lang, S. (2009). Regression - Modelle, Methoden und Anwendungen. Berlin, Heidelberg: Springer.
Magome, K., Morishige, N., Ueno, A., Matsui, T. A., and Uchio, E. (2021). Prediction of cycloplegic refraction for noninvasive screening of children for refractive error. PLoS One 16 (3), e0248494. doi:10.1371/journal.pone.0248494
McClelland, J. F., and Saunders, K. J. (2004). Accommodative lag using dynamic retinoscopy: age norms for school-age children. Optom. Vis. Sci. 81 (12), 929–933. doi:10.1097/01.OPX.0000147681.94971.28
Morgan, I. G., Iribarren, R., Fotouhi, A., and Grzybowski, A. (2015). Cycloplegic refraction is the gold standard for epidemiological studies. Acta Ophthalmol. 93 (6), 581–585. doi:10.1111/aos.12642
Sankaridurg, P., He, X., Naduvilath, T., Lv, M., Ho, A., Smith, E., et al. (2017). Comparison of noncycloplegic and cycloplegic autorefraction in categorizing refractive error data in children. Acta Ophthalmol. 95 (7), e633–e640. doi:10.1111/aos.13569
Wang, J., Wang, X., Gao, H. M., Zhang, H., Yang, Y., Gu, F., et al. (2022). Prediction for cycloplegic refractive error in Chinese school students: model development and validation. Transl. Vis. Sci. Technol. 11 (1), 15. doi:10.1167/tvst.11.1.15
Wu, J. H., Liu, T. Y. A., Hsu, W. T., Ho, J. H., and Lee, C. C. (2021). Performance and limitation of machine learning algorithms for diabetic retinopathy screening: meta-analysis. J. Med. Internet Res. 23 (7), e23863. doi:10.2196/23863
Wu, J. H., Nishida, T., Weinreb, R. N., and Lin, J. W. (2022). Performances of machine learning in detecting glaucoma using fundus and retinal optical coherence tomography images: a meta-analysis. Am. J. Ophthalmol. 237, 1–12. doi:10.1016/j.ajo.2021.12.008
Ying, B., Chandra, R. S., Wang, J., Cui, H., and Oatts, J. T. (2024). Machine learning models for predicting cycloplegic refractive error and myopia status based on non-cycloplegic data in Chinese students. Transl. Vis. Sci. Technol. 13 (8), 16. doi:10.1167/tvst.13.8.16
Zhao, J., Mao, J., Luo, R., Li, F., Pokharel, G. P., and Ellwein, L. B. (2004). Accuracy of noncycloplegic autorefraction in school-age children in China. Optom. Vis. Sci. 81 (1), 49–55. doi:10.1097/00006324-200401000-00010
Keywords: refractive error, prediction, machine learning, cycloplegic refraction, myopia
Citation: Chen B, Tian L, Tian F, Yang Q, Ruan Y, Li Y, Cao M, Wu C, Yang M, Xu S and Deng R (2025) Machine learning-driven prediction of cycloplegic refractive error in Chinese children. Front. Cell Dev. Biol. 13:1608494. doi: 10.3389/fcell.2025.1608494
Received: 09 April 2025; Accepted: 09 May 2025;
Published: 22 May 2025.
Edited by:
Weihua Yang, Southern Medical University, ChinaReviewed by:
Lin Wang, Bosch (China) Investment Ltd., ChinaNan Chen, Eye Hospital of Nanjing Medical University, China
Minghui Zhao, Shanghai Municipal Hospital of Traditional Chinese Medicine, China
Copyright © 2025 Chen, Tian, Tian, Yang, Ruan, Li, Cao, Wu, Yang, Xu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruzhi Deng, ZHJ6QGV5ZS5hYy5jbg==; Suzhong Xu, eHVzdXpob25nQG1haWwuZXllLmFjLmNu
 Bichi Chen1
Bichi Chen1 Ruzhi Deng
Ruzhi Deng