- Sorbonne Université, Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Institut de la Vision, Paris, France
The establishment of functional neuronal circuits critically relies on the ability of developing neurons to accurately sense and integrate a variety of guidance signals from their surrounding environment. Such signals are indeed crucial during key steps of neuronal circuit wiring, including neuronal migration and axon guidance, to guide developing neurons or extending axons towards their target destination in the developing brain. The growth cone, located at the tip of developing neurons, is a key subcellular structure in this process, that concentrates many different guidance receptors and signalling molecules and specialises in the probing and integration of extracellular signals into various guidance behaviours. Interestingly, the small primary cilium, long considered as a vestigial organelle, has progressively emerged as a cellular antenna specialised in cell signalling, and has been reported, just like the growth cone, to harbour a variety of guidance receptors. How primary cilium-elicited signals are then transduced into specific cellular processes to guide developing neurons and axons remains however obscure. In this review, we will summarise our emerging understanding of the role of primary cilium-elicited signalling pathways on neuronal guidance processes, by focusing on neuronal migration and axon guidance. We will highlight the primary cilium molecular diversity, and how it shapes the primary cilium functional versatility, allowing the ciliary compartment to instruct various guidance behaviours through the regulation of different cellular processes. We will moreover discuss current and future avenues of research, to unravel the different molecular effectors activated downstream of specific ciliary signals, and clues to be gained from studies performed in non-neuronal cells. Rising challenges of the field will also be addressed, such as the technical challenge induced by the dual subcellular localisation (i.e., ciliary and extra-ciliary) of many ciliary guidance receptors, and the importance of the development of new genetic/chemo-genetic/optogenetic tools. Finally, we will highlight the insight such studies will bring for our understanding of the aetiology of different disorders, including ciliopathies, neurodevelopmental and neurodegenerative disorders, but also cancer cell migration/invasion, which are associated with defective primary cilium formation and function.
1 Introduction
Neuronal guidance signalling encompasses all the signalling processes that ensure precise neuronal positioning and wiring (Yuasa-Kawada et al., 2022). Neuronal migration and axon pathfinding are two major steps of this guidance process. Newly generated neurons indeed migrate from their birthplace to their final destination in the developing brain and extend their growing axons towards the right synaptic targets. The neuron’s environment is a key ally in this developmental journey, as it provides different spatiotemporally-controlled guidance signals that enable developing neurons to ultimately integrate functional neuronal circuits. Depending on the neuronal subtype and/or the developmental stage, migration and axon navigation can occur either sequentially or concomitantly. Adding to this complexity, a same guidance signal can steer different populations of neurons and/or elicit different types of guidance behaviours (e.g., neuronal migration or axon guidance), highlighting the importance for developing neurons to accurately sense and integrate multiple extracellular signals in order for accurate neural circuit wiring to occur.
Extracellular guidance cues are sensed by receptors/channels expressed at the surface of developing neurons and come in many different flavours. They can be chemical, including diffusible extracellular or cell-bound ligands (proteins, lipids, small molecules …), but also mechanical, or even electrical (Gangatharan et al., 2018; Medvedeva and Pierani, 2020; Dorskind and Kolodkin, 2021). The growth cone, that is formed at the tip of extending axons and migrating neurons alike, is known to express many guidance receptors and is extensively studied as a key structure specialised in the probing and integration of the extracellular environment (Lowery and Van Vactor, 2009; Stoeckli, 2018; Nakajima et al., 2024). Interestingly, developing neurons–as almost all vertebrate cells–possess another key subcellular compartment, the primary cilium (PC), that has progressively emerged as a cell antenna specialised in collecting signals from the environment. Indeed, mutations affecting the PC structure and/or function have been found to induce a group of developmental disorders termed ciliopathies. While the clinical manifestations of ciliopathies are multisystemic, and include retinopathy, obesity, diabetes, skeletal malformations, and hepatic disease, ciliopathies are also characterised by a wide range of neurodevelopmental defects, such as in the Joubert (JBTS), Meckel-Grüber (also called Meckel syndrome, MKS) or Bardet–Biedl syndromes (Reiter and Leroux, 2017; Andreu-Cervera et al., 2021; Karalis et al., 2022). These defects include brain malformations, ataxia, epilepsy, mental disability and highlight the importance of primary cilia in neuronal circuit wiring and function. Accordingly, recent studies have located several receptors/effectors of major guidance signalling pathways to the ciliary compartment (Higginbotham et al., 2012; Loukil et al., 2023). However, the precise signalling events elicited in response to guidance signals within the PC and transduced to downstream intracellular effectors in order to regulate neuronal guidance behaviours remain poorly understood.
In this review, we will summarise our current understanding of the role of PC-elicited signalling pathways on neuronal guidance processes, focusing on neuronal migration and axon guidance. We will highlight the importance of the molecular diversity of the ciliary compartment, and how it determines the functional versatility of PC signalling during neuronal guidance, regulating: (i) different guidance processes (i.e., neuronal migration and axon navigation) sequentially or concomitantly, and (ii) different molecular mechanisms converging on a same guidance process (e.g., neuronal migration). It is indeed important to bear in mind that the generic PC does not exist, and that ciliary composition is highly versatile, at different levels. First, (i) the PC protein composition varies throughout the lifespan of the cell: for example, the expression of the ciliary marker, adenylate cyclase 3 (AC3; i.e., enzyme responsible for the cAMP cyclic nucleotide synthesis) is low in the embryonic brain, but increases during the first postnatal weeks, before decreasing again at later stages (Arellano et al., 2012). Ciliary protein composition is moreover (ii) highly dependent on the cell type, and depending on the cell type, (iii) a same ciliary protein can show different sub-ciliary localisation patterns (Hansen et al., 2022). We will moreover discuss current and future research avenues to unravel the many ramifications of molecular effectors activated downstream of specific PC-elicited guidance signals, and clues to be gained from studies performed in non-neuronal cells. Finally, we will highlight the insight such studies will bring for our understanding of ciliopathies, but also neurodevelopmental and neurodegenerative disorders or cancer cell migration, associated with defective PC formation and function.
2 The neuronal primary cilium: a signalling hub sensing environmental guidance cues
2.1 The primary cilium subcellular compartment
Primary cilia are small, microtubule-based structures that are contiguous with the plasma membrane and bud from the surface of almost all vertebrate cells. Observed as early as 1898 (Zimmermann, 1898), technical limitations have long relegated the PC to a vestigial organelle, until the development of transmission electron microscopy and the association made between primary cilia and ciliopathies gradually boosted our interest for this tiny organelle. Since then, ciliopathies have been reported one after the other, with the discovery of more and more ciliopathy-associated genes (Reiter and Leroux, 2017), the study of which has contributed to considerably increase our knowledge of the PC structure and function.
2.1.1 The primary cilium structure and composition
The architecture of the PC has been extensively studied. The PC is organised by a modified mother centriole, called the basal body, from which the ciliary microtubule core, called the axoneme (comprising nine microtubule doublets), extends, surrounded by the ciliary membrane (Figure 1). In mammalian neurons, the PC extends 2 to 12 μm from the cell surface, with a diameter ∼ 200–500 nm (DeMars et al., 2023; Macarelli et al., 2023). Two main ciliogenesis pathways have been described: the extracellular pathway, and the intracellular one, that is the most studied (Wang and Dynlacht, 2018; Hoffman and Prekeris, 2022; Zhao et al., 2023). While extracellular ciliogenesis occurs in most polarised epithelial cells, the intracellular pathway appears to be favoured by most other cell types (Sorokin, 1962; 1968; Molla-Herman et al., 2010; Labat-de-Hoz et al., 2021). In the intracellular pathway, ciliogenesis starts in the cytoplasm with the docking of the basal body to a large ciliary vesicle. The axoneme assembles from the basal body beneath this vesicle. As the axoneme extends, the ciliary vesicle expands to encapsulate the axoneme in a double membrane layer, with the ciliary membrane facing the axoneme and the ciliary sheat facing the cytoplasm. PC budding at the cell surface is then enabled by fusion of the ciliary sheat with the plasma membrane. Conversely, extracellular ciliogenesis is initiated by the docking of the basal body to the plasma membrane. As the axoneme extends from the basal body, the ciliary membrane is gradually formed from the plasma membrane. Whether in the extracellular or intracellular pathway, extension of the PC, in which translation does not occur, relies on a ciliary transport system, the intraflagellar transport (IFT), that uses the axoneme scaffold to provide all the building material required for membrane and axoneme extension, as well as for protein delivery and exit to and from the PC. IFT (Taschner and Lorentzen, 2016) is powered by the kinesin-II and dynein microtubule-based molecular motors for anterograde and retrograde transport along the axoneme, respectively. Trains of IFT particles, each composed of IFTA and IFTB subcomplexes, are assembled at the ciliary base and couple the molecular motors to the cargoes for ciliary trafficking to and from the PC tip.
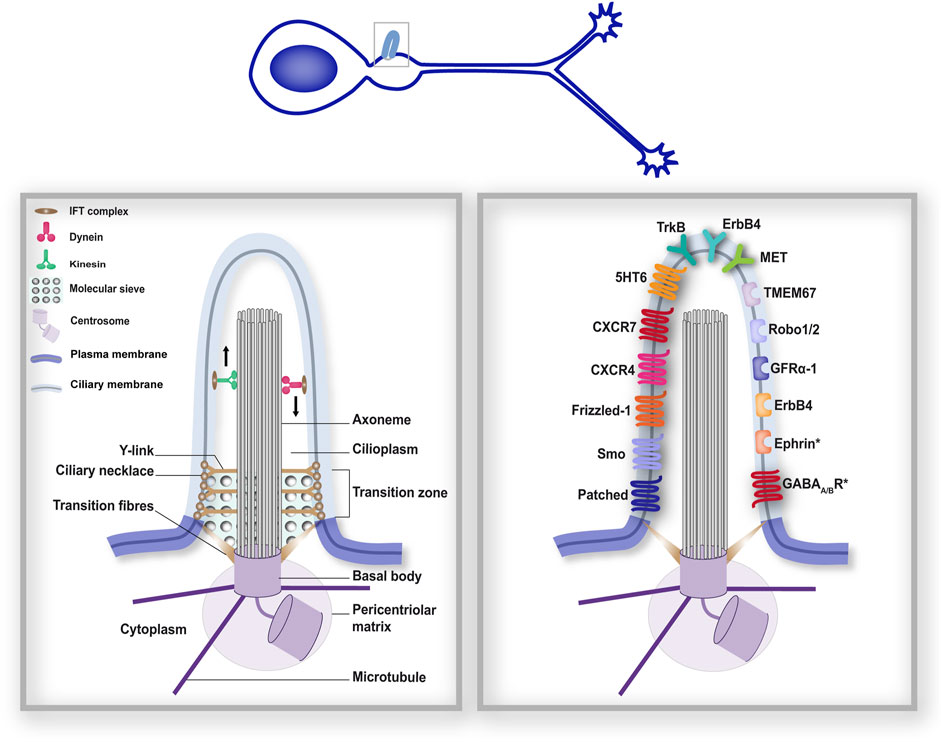
Figure 1. The primary cilium forms a distinct subcellular compartment that functions as a signalling hub. The structural organisation of the PC (left-hand boxed region) comprises different gating mechanisms that ensure a distinct protein composition of the ciliary compartment, in addition to its distinct lipidic composition. As a consequence, many membrane receptors have been reported at the surface of the PC. The right-hand boxed region depicts neuronal guidance-related membrane receptors reported at the surface of neuronal primary cilia during development (Rodriguez Gil and Greer, 2008; Williams et al., 2010; Petralia et al., 2011; Higginbotham et al., 2012; Toro-Tapia and Das, 2020). Receptors marked with an (*) were found in neuronal cilia postnatally (Loukil et al., 2023). Left- and right-hand boxed regions correspond to a higher magnification of the PC of the developing neuron depicted above.
2.1.2 The primary cilium: a signalling hub
This IFT system is important not only for ciliogenesis, but also for PC function. Indeed, the wide range of ciliopathy-associated phenotypes and target organs–ranging from skeletal, heart, kidney, renal or retinal malfunction to brain malformations and cognitive defects–highlights the crucial involvement of the highly conserved PC in the regulation of cell signalling and function. The PC is indeed now well established as a signalling hub at the crossroads between various signalling pathways (Christensen et al., 2012; Hilgendorf et al., 2016; Pala et al., 2017; Wheway et al., 2018; Anvarian et al., 2019; Nishimura et al., 2019; Mill et al., 2023). The IFT transport machinery plays an important part in the concentration and trafficking into and out of the tiny ciliary volume of many membrane receptors (e.g., G-protein coupled receptors, ion channels, extracellular matrix receptors, purinergic receptors …) and signalling molecules (e.g., second messengers, soluble proteins …). Of note, the precise molecular mechanisms involved in these various trafficking events remain to be clarified, and an IFT-independent lateral diffusion of certain ciliary membrane receptors along the axoneme has also been proposed (Milenkovic et al., 2009; Ye et al., 2013). Proteomic studies performed in non-neuronal systems have nevertheless contributed to confirm the diversity of proteins concentrated within the ciliary volume and hint at the wide variety of processes in which the PC signalling hub is involved (Ishikawa et al., 2012; Mick et al., 2015; Hansen et al., 2024; Liu et al., 2024).
This dense and diverse protein composition is a key feature of the PC compartment, along with its lipidic composition, that is distinct from that of the plasma membrane (Nakatsu, 2015; Conduit and Vanhaesebroeck, 2020). Different gating mechanisms, based on evolutionarily-conserved domains located at the base of the PC, act in concert with the IFT to strictly restrict the exchanges between the cytoplasm and the cilioplasm (Jensen and Leroux, 2017; Park and Leroux, 2022; Moran et al., 2024).
At the very base of the PC, the distal appendages (or transition fibres, see Figure 1) of the cell body connect the basal body to the ciliary membrane. IFT particles dock onto transition fibres before cargo trafficking to the ciliary compartment (Deane et al., 2001; Wei et al., 2013). Distal to the transition fibres, the transition zone is composed of Y-links that connect the axoneme to the ciliary membrane, and the ciliary necklace, comprising rows of membrane particles that encircle the base of the ciliary shaft. The transition zone appears to apply different gating mechanisms to safeguard the functional specificity of the ciliary compartment. Consistently, many ciliopathy-associated gene mutations affect transition zone proteins (Gonçalves and Pelletier, 2017). First, the transition zone appears to constitute a membrane diffusion barrier, with a ciliary zone of exclusion that prevents non-ciliary membrane proteins from entering the PC, but also maintains ciliary membrane proteins within the PC compartment (Williams et al., 2011; Cevik et al., 2013; Jensen and Leroux, 2017). Additionally, the transition fibres and transition zone appear to establish a soluble diffusion gate, in the way of a molecular sieve. Indeed, studies using a permeabilised system for ciliary trafficking in mammalian cells have reported that proteins of increasing size fused to GFP do not enter the PC with the same dynamics: while proteins below 4.8 nm enter the PC, entry is decreased for proteins between 4.8 and 8.6 nm, and is no longer detectable for larger proteins (Breslow et al., 2013). Similarly, diffusion of fluorescent proteins established a ciliary sieve-like barrier allowing the entry of soluble proteins with a Stokes radius as large as 7.9 nm (Lin et al., 2013). The precise molecular mechanisms involved in this sieve remain however elusive. A similarity with the nuclear pore complex (NPC) has been proposed, with studies revealing the implication of the nuclear transport machinery in ciliary trafficking (Dishinger et al., 2010; Fan et al., 2011; Kee et al., 2012), although some diffusion events may occur independently (Breslow et al., 2013).
This membrane and soluble diffusion barrier at the base of the PC allows the separation between the cytoplasm and the cilioplasm, and is essential for the functional specialisation of the ciliary antenna as an extracellular signal sensor. Consistently, studies have challenged the view that small second messenger signals (e.g., cAMP and cGMP cyclic nucleotides, calcium), locally produced within the PC compartment in response to the activation of ciliary membrane receptors, can freely diffuse between the cytoplasm and cilioplasm (Delling et al., 2016; Jiang et al., 2019), and argue in favour of a ciliary compartmentalisation of second messenger signals, that signal and function independently from the cytoplasmic pool. Indeed, in FRET experiments, Moore and colleagues reported that in inner medullary collecting duct cells (IMCD3), primary cilia have a high basal cAMP concentration with regards to the cytoplasm (∼5 times higher; Moore et al., 2016). In another study, pharmacological inhibition of the ciliary-localised vasopressin receptor type-2 in kidney epithelial cells induced increased cilioplasmic, but not cytoplasmic, cAMP levels. Conversely, fluid-shear stress decreased cilioplasmic cAMP levels, without affecting the cytoplasmic pool (Sherpa et al., 2019). In the case of cGMP, studies in Caenorhabditis elegans olfactory sensory neurons expressing a genetically encoded cGMP indicator show that, following odour exposure, ciliary cGMP levels transiently decreased, while cGMP levels in dendrites and soma gradually increased (Shidara et al., 2017). Similar observations have also been reported for calcium (Nauli et al., 2008; Delling et al., 2013; Jin et al., 2014; Sanchez et al., 2023; Shim et al., 2023). At the functional level, ciliary versus extra-ciliary second messenger signals have been reported to regulate different signalling pathways and mechanisms. For example, optogenetic increase of ciliary cAMP levels in zebrafish developing somites was shown to inhibit Hedgehog signalling, while cytoplasmic cAMP levels did not (Truong et al., 2021). Similarly, in developing zebrafish embryos, ciliary PKA, by contrast to cytosolic PKA, was found to specifically regulate the Hedgehog pathway (Zhang et al., 2024). In line with these observations, Hansen and colleagues unravelled a ciliary cAMP signalosome that is functionally distinct from the cytoplasm and drives kidney cyst formation (Hansen et al., 2022). Moreover, during cortical interneuron migration, ciliary cAMP and cGMP signals were found to antagonise each other to regulate cell polarity, while centrosome-located cAMP and cGMP acted in synergy to control another aspect of migration, which is nucleokinesis (Atkins et al., 2023b). Similar reports have been made concerning calcium, unravelling the PC as a calcium-mediated mechanosensory compartment that is necessary and sufficient to instruct left-right asymmetry during zebrafish development (Djenoune et al., 2023).
2.2 The primary cilium: a key signalling platform for neuronal guidance signalling pathways
Among the variety of signalling pathways and cell functions regulated by the PC signalling hub, receptors for some of the major signalling pathways that are involved in neuronal guidance processes have been found.
The first major evidence establishing the PC as a key signalling compartment in neuronal development arose in 2003 from a forward genetic screen conducted by Huangfu and colleagues in mouse embryos. They discovered that genes encoding intraflagellar transport machinery proteins are essential for embryonic ventral patterning through the signalling of Sonic hedgehog (Shh; Huangfu et al., 2003), one of the most important morphogens involved in neuronal development (Douceau et al., 2023). Since this pioneer study, the ciliary transduction of the Shh pathway–most commonly referred to as the canonical pathway (Teperino et al., 2014) – has been described (Rohatgi et al., 2007), and its role in neuronal development extensively reviewed (Bangs and Anderson, 2017). Since then, several components of the Shh transduction machinery have been localised to neuronal primary cilia (Figure 1, right-hand), such as the Patched receptor for Shh and the Smoothened (Smo) GPCR (a key signal transducer of the Shh pathway) in the PC of rat hippocampal neurons, or GPR161, which is a negative regulator of Shh canonical signalling (Mukhopadhyay et al., 2013), in the PC of dI1 commissural neurons (Petralia et al., 2011; Toro-Tapia and Das, 2020). Notably, Shh signalling at the PC has been involved in several neuronal guidance processes, including neuronal migration (Baudoin et al., 2012; Pedraza et al., 2024) and axon pathfinding (Dumoulin et al., 2024).
But the role of the PC in neuronal guidance processes is not limited to the transduction of the Shh signalling pathway. Another major guidance molecule, Wnt, primarily identified as a guidance molecule for navigating commissural axons in the mammalian spinal cord (Lyuksyutova et al., 2003) and subsequently involved in neuronal migration (Boitard et al., 2015; Bocchi et al., 2017), has been linked to the PC. The Wnt signalling pathway comprises a network of various signalling molecules, with Wnt ligands often activating frizzled receptors together with an array of different co-receptors. Two main branches of the pathway are classically distinguished: the canonical Wnt/β-catenin pathway and the non-canonical Wnt/PCP pathway. Signalling molecules of the Wnt transduction machinery have been found to localise to the PC of non-neuronal cells (e.g., Dishevelled, β-catenin, LRP5/6). Among these, some have been reported in the primary cilia of neurons. Such is the case, for example, of Frizzled-1, expressed in the PC of developing olfactory sensory neurons (Rodriguez Gil and Greer, 2008). The transmembrane Frizzled-like receptor Tmem67/MKS-3, a transition zone protein that functionally binds Wnt5a (Abdelhamed et al., 2015) and whose mutations are responsible for the MKS and JBTS ciliopathies, has moreover been located to the PC base of the C. elegans ciliated sensory neurons (Williams et al., 2010). It has further been shown to regulate canonical Wnt/β-catenin signalling in the developing cerebellum (Abdelhamed et al., 2019). However, the relationship between PC and Wnt signalling is complex. While Wnt signalling can regulate ciliogenesis, the PC can regulate Wnt signalling. Moreover, the question of whether the PC structure is required for the activation and transduction of the Wnt/β-catenin signalling pathways is controversial (Anvarian et al., 2019; Vuong and Mlodzik, 2023; Niehrs et al., 2025). Of note, several ciliary signalling components of the Wnt pathway are not exclusively localised to the PC. Such is the case of Frizzled-1, which has also been found in dendrites and axons of developing olfactory sensory neurons (Rodriguez Gil and Greer, 2008), highlighting the need for further studies to distinguish ciliary from extra ciliary regulations of Wnt-associated processes.
In addition to the Shh and Wnt pathways, extensively studied for their ciliary transduction, key molecular players in neuronal guidance pathways classically studied for their role in growth cones, have also been linked to the PC compartment. Immunohistochemistry experiments performed in migrating cortical interneurons have indeed identified several guidance receptors at the ciliary surface, namely, the TrkB receptor for BDNF (Brain-derived neurotrophic factor), the GFRα-1 receptor for GDNF (glial cell line-derived neurotrophic factor), CXCR4 and CXCR7 receptors for the CXCL12 chemokine, the ErbB4 receptor for Neuregulin1 (NRG-1), serotonin receptor 6 (5HT6), receptors Robo1 and 2 for Slit, and the MET receptor for HGF/SF (hepatocyte growth factor/scatter factor; Higginbotham et al., 2012). In addition to these receptors, an in vivo BioID (iBioID) proteomic screen has recently revealed in the PC of adult neurons (Loukil et al., 2023) the presence of Ephrin (involved both in neuronal migration and axon guidance processes) and GABA-A and GABA-B receptors, involved in synaptogenesis (Fiorentino et al., 2009; Sui et al., 2024) and neuronal migration (Heck et al., 2007). Finally, the receptor tyrosine kinase PDGFR-α (Clement et al., 2013), the CD44 hyaluronan receptor (Jones et al., 2012; Lee et al., 2020) and neuropilin 1 (Pinskey et al., 2017), all involved in different neuronal guidance processes (see sections below), have also been localised to the PC of non-neuronal cells. Future studies will be crucial to unravel how this multitude of ciliary signalling receptors regulate specific steps of neuronal guidance, in a cell type and cell stage specific manner.
Together, these studies pinpoint the neuronal PC as a key subcellular signalling compartment in neuronal guidance, integrating a variety of extracellular cues at the crossroads between different guidance processes. The downstream signalling effectors activated by ciliary guidance receptors, and how they regulate guidance processes, remain however obscure. This is mostly due to the technological challenge that represents the dissection of the ciliary-specific functions of guidance signalling receptors/effectors, with dual subcellular localisation (i.e., ciliary and extra-ciliary). Yet, during the past decade, some labs have developed innovative strategies to tackle this issue and provided important new insights into the molecular mechanisms underlying the PC-elicited regulation of neuronal guidance pathways. In the following sections, we will review our current knowledge of PC function in neuronal migration and axon guidance, and discuss future avenues to be explored.
3 Primary cilium signalling in neuronal migration
3.1 The primary cilium compartment in neuronal migration
A role for the PC in the acquisition of cell polarity and directed cell migration has long been established in various non-neuronal systems (Christensen et al., 2013; Veland et al., 2014). In fibroblasts, for example, the PC–together with the centrosome–re-orients prior to the initiation of migration (Katsumoto et al., 1994) and is then oriented parallel to the direction of the movement (Albrecht-Buehler, 1977). Furthermore, the PC genetic ablation abrogates chemical or electrical stimuli-evoked directed cell migration in fibroblasts or mesenchymal stem cells (Schneider et al., 2005; 2010; Pruski et al., 2016; 2019; Lee et al., 2020; Nakazato et al., 2023). Mutation of a ciliopathy-associated gene was also found to induce neural crest cell migration defects in the zebrafish model (Tobin et al., 2008). Despite such evidence, a role for primary cilia in neuronal migration has remained vaguer and more controversial, with some data reporting PC formation in the neocortex only after neuroblast migration has occurred, and no PC involvement in the establishment of neuronal polarity, neuronal migration or cortical laminar organisation (Arellano et al., 2012). By contrast, other groups have reported a role for primary cilia in the apico-basal polarity of radial glial cells (Higginbotham et al., 2013), in the tangential migration of cortical interneurons (Baudoin et al., 2012; Higginbotham et al., 2012), as well as in neuroblasts migrating postnatally through the rostral migratory stream towards the olfactory bulb (Matsumoto et al., 2019; Stoufflet et al., 2020). Strengthening the decisive role of the PC in neuronal migration, several gene mutations responsible for neurodevelopmental disorders–including ciliopathies or focal malformations of cortical development–and affecting ciliogenesis have been reported to impair radial or tangential neuronal migration in the developing cortex (Guo et al., 2015; Park et al., 2018).
3.2 Guidance cue-evoked primary cilium molecular pathways in neuronal migration
Neuronal migration is a well-documented cyclic saltatory process (Bellion et al., 2005; Schaar and McConnell, 2005; Tsai and Gleeson, 2005). In the first step of the cycle, migrating neurons probe their surroundings by extending and stabilising a leading process in an attractive or permissive environment. The centrosome then moves forwards to a proximal region within this process, called the dilatation or swelling compartment, before the nucleus dynamically translocates towards the centrosome in a process termed nucleokinesis. In 2012, Baudoin and colleagues showed that the PC genetic ablation altered the ability of interneurons migrating ex vivo in brain organotypic slices to exit their tangential migration stream and invade their target destination (i.e., the developing cortical plate), in a way that mimics Shh pathway inhibition, suggesting a role for Shh-initiated PC signalling in neuronal migration (Baudoin et al., 2012). The same year, Higginbotham and colleagues identified by immunohistochemistry experiments many guidance cue receptors in the PC of migrating cells (i.e., TrkB, GFRα-1, CXCR4, CXCR7, ErbB4, 5HT6, Robo1 and 2, MET). Using a microfluidic device, they moreover cultured cortical interneurons and dorsal cortical cells in two opposite chambers linked by microlanes, allowing to expose the cortical interneurons of one chamber to a gradient of migration-regulating cues secreted by the dorsal cortical cells of the other chamber. Using this setup, the authors further revealed that PC-ablated cortical interneurons (i.e., interneurons carrying a null-mutation for the small regulatory GTPase Arl13b) exhibit defective migration towards the source of the gradient, compared to wild-type interneurons (Higginbotham et al., 2012). These two pioneer studies have opened the exciting and complex question of how the activation of guidance receptors at the PC may regulate the different steps of neuronal migration: what are the specific downstream signalling events and cellular processes regulated by these PC-dependant guidance signals?
Very few studies have started to tackle this question. In a study performed in tangentially-migrating mouse neurons in the postnatal rostral migratory stream, genetic ablation of the PC led to altered nucleokinesis of migrating neurons, in a mechanism dependent on a centrosome-located cAMP hotspot, thereby linking the PC regulation of migration to a downstream centrosomal component (Stoufflet et al., 2020). Recently, the same group proposed a ciliary pathway involving GPR161 mechanosensitivity as the upstream trigger regulating the centrosomal cAMP hotspot and the organisation of the nuclear cage of microtubules, required for proper nucleokinesis to occur (Paillard et al., 2025). Given the wide range of guidance receptors expressed at the ciliary surface, linking specific PC-elicited guidance signals to specific downstream effectors and migratory behaviours remains however challenging. The fact that many ciliary membrane receptors are also expressed at the extra-ciliary plasma membrane further complexifies the situation, highlighting the need to develop new tools to bypass loss of function approaches and alter PC-elicited signals specifically at the ciliary compartment. Using newly developed genetically encoded molecular tools targeted to the PC to selectively modulate (i.e., increase or buffer) PC-elicited second messenger signals, combined with live cell imaging and pharmacological/genetic approaches, Atkins and colleagues recently added some pieces to the puzzle. They showed that the CXCL12 chemokine controls the cell polarity and branching behaviour of migrating cortical interneurons by decreasing the ciliary cAMP/cGMP ratio upon binding to its CXCR4 receptor (Atkins et al., 2023b; Figure 2, top). Such technological development paves the way towards the dissection of the specific role on migratory behaviours of other guidance receptors present at the ciliary surface, and to the identification of their specific downstream molecular effectors.
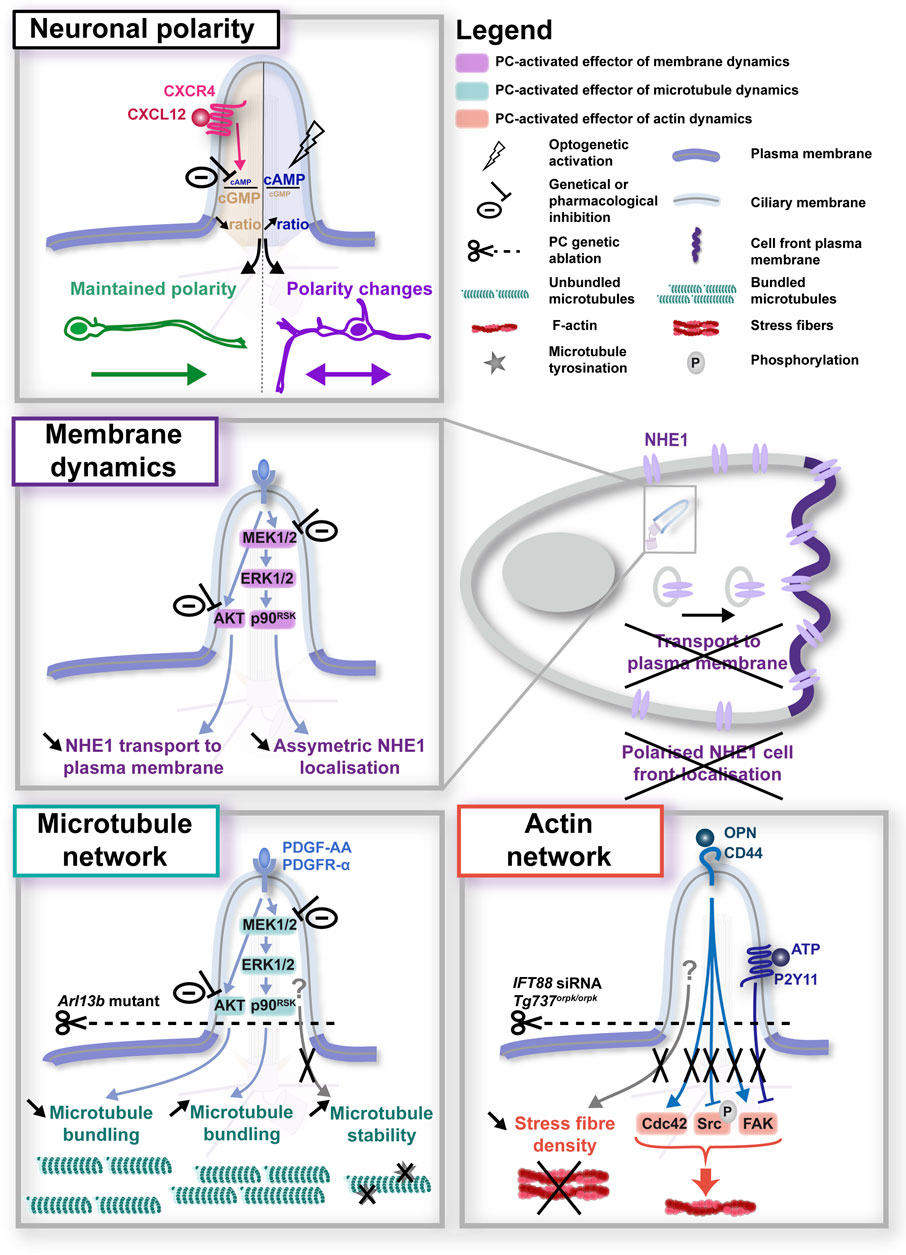
Figure 2. Primary cilium-elicited signalling pathways in neuronal migration. Top: in neurons, regulation of the ciliary cAMP/cGMP ratio downstream of CXCL12/CXCR4 activation at the PC surface was found to regulate the cell polarity and direction of migrating cells (top), although the downstream effectors activated in the cytoplasm remain to be identified (Atkins et al., 2023b). Middle and bottom summarise the research on downstream cytoplasmic effectors performed in migrating non-neuronal cells, that converge on the regulation of membrane dynamics (middle; Clement et al., 2013) or the microtubule (left-hand bottom; Clement et al., 2013; Pruski et al., 2016) and actin network (right-hand bottom; Jones et al., 2012; Mansini et al., 2019; Lee et al., 2020). In all panels, experimental manipulations (genetic, optogenetic or pharmacological) performed to alter ciliary signals, together with their phenotypic consequences, are colour-coded in black.
Precious clues may be gained from studies already linking ciliary molecular mechanisms to cell migration in non-neuronal cells. Interestingly, in such systems, PC-elicited signals have been reported to impact cell migration through the regulation of various mechanisms.
3.2.1 The primary cilium and the regulation of membrane dynamics
One of those mechanisms concerns the regulation of membrane dynamics (Figure 2, middle). In a study conducted by the Christensen lab in fibroblast cells, the Platelet-Derived Growth Factor AA (PDGF-AA) protein activated the PI3K-AKT and MEK1/2-ERK1/2-p90RSK pathways at the PC, and inhibiting these pathways counteracted the ability of PDGF-AA to stimulate migration in scratch-assay experiments (Clement et al., 2013), corroborating previous studies from the group (Schneider et al., 2005; 2010). Moreover, Clement et al. found that PDGF-AA signalling at the PC activates the Na+/H+ exchanger NHE1 and is critical for directed migration. More precisely, they show that while AKT inhibition impedes NHE1 vesicles from reaching the plasma membrane, inhibition of MEK1/2 abolishes the preferential localisation of NHE1 to the plasma membrane of the cell front, with cells displaying a broader NHE1 membrane distribution in multiple membrane locations (Clement et al., 2013). This study builds upon a previous study from the group involving NHE1 in directed cell migration downstream of ciliary PDGF-AA signalling (Schneider et al., 2009), and is in agreement with other studies establishing a role for NHE1 in cell migration and invasion (Cardone et al., 2005; Stock and Schwab, 2006; Stock and Pedersen, 2017), through various mechanisms, such as the regulation of cell polarity by anchoring actin filaments to the cell front plasma membrane (Denker and Barber, 2002). Of note, this role for ciliary MEK1/2 activation in NHE1 asymmetric membrane localisation is highly coherent with the well-established role of the PC in cell polarity and directed migration, also reported in migrating neurons (Atkins et al., 2023b). Together, these data open the possibility of a role for the PC in the regulation of the cell front behaviour through the control of membrane dynamics and/or the targeting of specific receptors to the plasma membrane (Figure 2). Interestingly, the PDGFR-α receptor for PDGF-AA has been found expressed in migrating neurons of the external germinal layer (EGL) of the cerebellum (Andrae et al., 2001). However, although it has been involved in the migration of astrocytes (Itoh et al., 2011), its role in neuronal migration remains uncharacterised. On the other hand, NHE1 has been involved in the migration and invasive behaviour of cancer cells in glioblastoma (Cong et al., 2014), as well as in early neurite outgrowth during neuronal development (Sin et al., 2009; 2020). To our knowledge, its regulation of neuronal migration has so far not been described, let alone downstream of neuronal PC activation. Thus, while they appear as attractive candidate players in PC-dependant cell migration, future studies will be required to determine whether PC-elicited guidance pathways, PDGFR-α-NHE1-related or -independent, may regulate membrane dynamics to control cell polarity or plasma membrane composition in a context of neuronal migration.
3.2.2 The primary cilium and the regulation of cytoskeletal dynamics
Another key cell process reported in non-neuronal migrating cells downstream of PC-elicited pathways is the regulation of cytoskeletal dynamics (Figure 2, bottom). Very few studies have analysed the effect of PC signalling on microtubule dynamics. The Christensen lab has nevertheless reported defects in extra-ciliary microtubule bundling downstream of PDGF-AA signalling at the PC (Clement et al., 2013), in addition to an effect of an Arl13b null mutation on microtubule detyrosination (i.e., a post-translationnal modification that correlates with a more stable state of microtubules) reported by Pruski and colleagues in mouse embryonic fibroblast cells (Pruski et al., 2016). By contrast, more studies have addressed the question of a role for the PC on actin dynamics during cell migration, with the identification of different F-actin regulators activated by PC signalling during cell migration. First, genetic ablation of the PC by siRNA-mediated knockdown of the intraflgellar transport 88 (IFT88) protein was found to abolish the phosphorylation of focal adhesion kinase (FAK, a tyrosine kinase that functions as a signalling scaffold for the assembly and maturation of the focal contacts regulating cell adhesion), that occurs in response to osteopontin (OPN) signalling at the PC in wild type migrating mesenchymal stem cells (Lee et al., 2020). A similar decrease in FAK phosphorylation following PC genetic ablation (deletion of intraflagellar transport protein Tg737: Tg737orpk/orpk) was observed in endothelial cells, in association with a decreased directionality of migrating cells (Jones et al., 2012). Moreover, in migrating cholangiocytes, ATP stimulation of the ciliary purinergic receptor P2Y11 induced a rapid degradation of FAK in ciliated cells, which was abolished in de-ciliated cells (Mansini et al., 2019). Another F-actin regulator targeted by PC signalling is the Src kinase, whose phosphorylation dynamics are disrupted in migrating cells upon PC genetic ablation compared to controls, whether in basal conditions or following OPN signalling (Lee et al., 2020). Of note, the same study reported an increased expression of the Cdc42 Rho GTPase in IFT88-silenced cells. Finally, and in addition to these different actin regulators, the PC has been suggested to regulate the stress fibre network of migrating endothelial cells, which regulates several functions in migrating cells, such as the generation of traction forces, the maturation of integrin-based adhesions, the establishment of cell polarity (Vicente-Manzanares et al., 2009). Intriguingly, studies report a reduction of the actin stress fibres observed in mutated endothelial cells displaying impaired PC assembly (Tg737orpk/orpk), compared to controls (Jones et al., 2012). To our knowledge, the P2Y11 purinergic receptor and the CD44 surface hyaluronan receptor (for OPN) have not been localised to neuronal primary cilia. However, independently of the PC, CD44 has been involved in the migration of neural precursor cells (Deboux et al., 2013). Similarly, purinergic receptors have been involved in neuronal migration or axon guidance (Rodrigues et al., 2019), although the P2Y11 receptor has not been reported so far in such processes.
Importantly, microtubule and F-actin remodelling are well established as key driving forces of neuronal migration (Schaar and McConnell, 2005; Shan et al., 2021) and axon guidance (Sánchez-Huertas and Herrera, 2021; Atkins et al., 2023a). Consistently, several guidance receptors found by the Anton lab in the PC of migrating cortical interneurons (Higginbotham et al., 2012; see Figure 1) are known to regulate membrane or cytoskeletal dynamics in a PC-independent context. These data highlight the need to dissect whether and how guidance signals elicited in neuronal primary cilia regulate cytoskeletal remodelling and/or membrane/receptor trafficking to drive specific migratory or axon steering behaviours.
4 Primary cilium signalling in axon guidance
4.1 The primary cilium compartment in axon guidance
Evidence of a role for the PC in axon navigation processes came from axonal tract defects observed in patients. Indeed, several ciliopathies (i.e., Joubert, Meckel Gruber, Acrocallosal and Orofacial Digital Syndromes) have been associated with a defective development of the corpus callosum (CC; Salonen, 1984; Odent et al., 1998; Holub et al., 2005; Takanashi et al., 2009; Poretti et al., 2011; Putoux et al., 2011), which consists in the largest axonal tract of the brain, formed by millions of axons that connect homologous cortical areas of the two brain cerebral hemispheres. Consistently, in Joubert Syndrome, defects of other major axonal tracts, displaying failure to cross the midline, have also been reported, such as the corticospinal tract (CST; Poretti et al., 2007; Théoret et al., 2013) and the superior cerebellar peduncle (SCP) tract (Spampinato et al., 2008). Of note, the molar tooth sign, characterised by thickened and elongated SCPs that fail to cross the midline, is one of the hallmarks of Joubert Syndrome and related disorders (Maria et al., 1999; Sattar and Gleeson, 2011; Romani et al., 2013). Defective decussation, fasciculation and/or branching of axonal tracts–including the SCP, CST, CC tracts and developing sensory corneal nerves–has also been reported in mouse models of Joubert syndrome and related disorders (Guo et al., 2019) or following the conditional knockout of the ciliopathy-associated IFT88 gene (Portal et al., 2019). Additionally, abnormal projection of thalamocortical axons towards the amygdala was reported in two ciliary mouse mutants (Magnani et al., 2015). Similarly, RNAi silencing of the Joubert Syndrome gene C5orf42 in chick embryos led to pathfinding defects of the commissural dI1 axons (Asadollahi et al., 2018). Corroborating these studies, in a genetic screen based on the in utero electroporation of a library of 30 shRNA targeting ciliopathy-linked genes in the cortex of E14,5 mouse embryos, Guo and colleagues identified aberrant axonal trajectory and fasciculation of neurons depleted for BBS5, BBS7, BBS9, BBS11, BBS12 and TMEM216 (Guo et al., 2015). Of note, changes in the adhesion properties of a developing neuron are likely to modify the way its axon will interact with other axons and/or cells from the surrounding environment, in a complex manner that can lead to axon guidance defects. Consistently, in the case of BBS5 and BBS7 knockdown, the authors moreover report defective axonal midline crossing towards the contralateral cortex, with miss-directed axons that, instead of crossing, project aberrantly towards subcortical targets once they have reached the midline.
4.2 Guidance cue-evoked primary cilium molecular pathways in axon guidance
While some of these axonal tract defects have been shown to occur in a non-cell autonomous manner, as a result of the defective distribution of glial and neuronal guide post cells (Benadiba et al., 2012; Laclef et al., 2015; Putoux et al., 2019), studies have also identified a cell autonomous role for the ciliary compartment in the regulation of axon pathfinding, involving different PC-elicited signalling pathways. In a study performed by the Anton lab, the conditional knockdown of the Joubert Syndrome-associated gene Arl13b in cultured deep cerebellar nuclei (DCN) neurons led to reduced dynamic axonal branching, aberrant growth cone morphology with altered filopodia-lamellipodia balance (i.e., numerous longer filopodial protrusions), as well as impaired axon-axon adhesion associated with reduced recruitment of the protocadherin-17 (Pcdh17) to axon-axon contacts (Guo et al., 2019; Figure 3, bottom and middle). Interestingly, these axonal and growth cone morphological defects were associated to an increase in the ciliary levels of the PIP3 second messenger. Using elegant tools based on the CIBN/CRY2 dimerization optogenetic system, Guo and colleagues showed that recruiting PIP3 or AKT to the PC of DCN neurons is sufficient to alter growth cone morphology and dynamics by inducing filopodial protrusions. They further use DREAAD chemo-genetic tools to show that modulating the activity of ciliary G-protein coupled receptors GPCRs (that are known to converge onto PIP3) recapitulates the PIP3-AKT-linked growth cone morphological defects (Figure 3, top). Together, these data highlight PIP3-AKT as a PC-elicited signalling pathway involved in growth cone remodelling and behaviour.
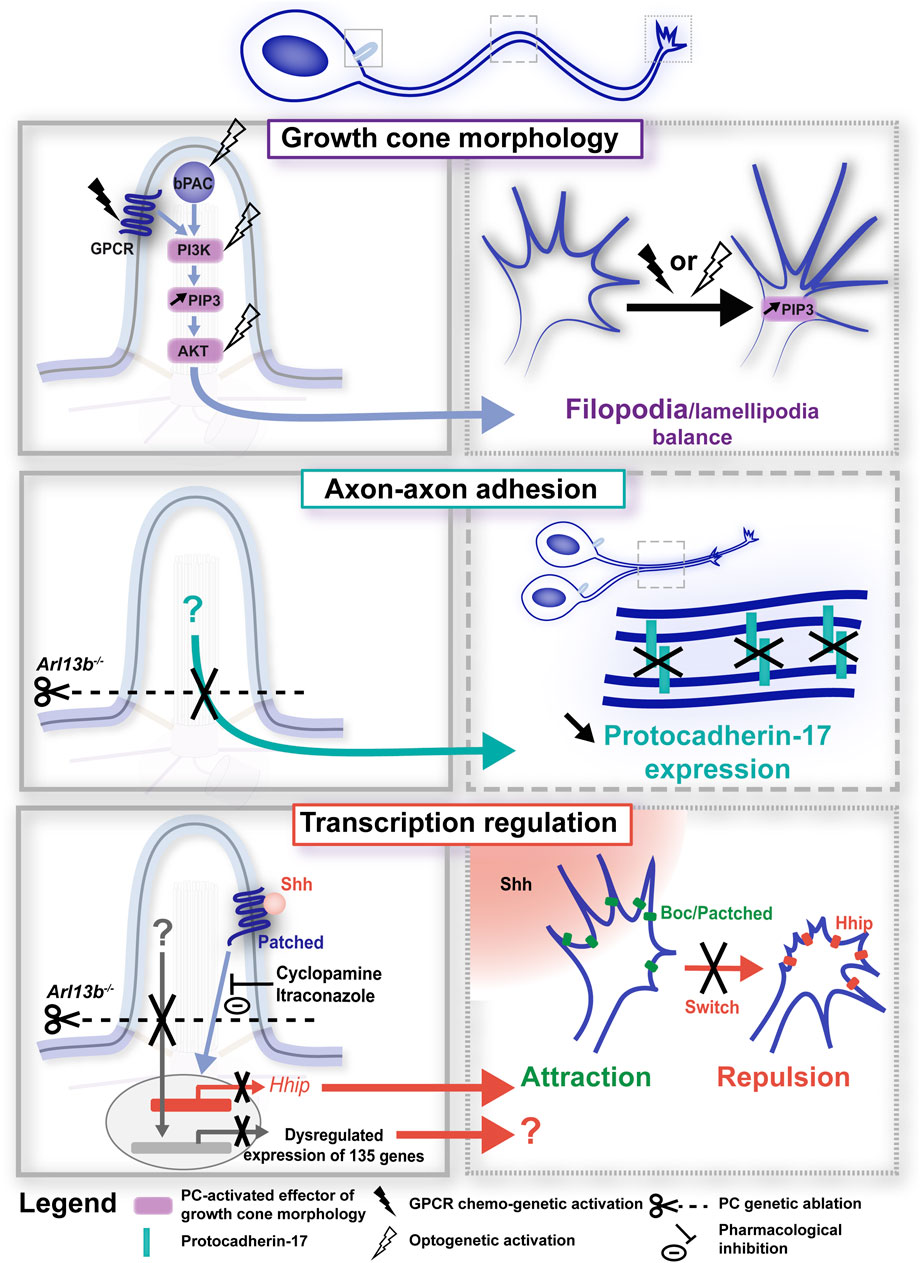
Figure 3. Primary cilium-elicited signalling pathways in axon pathfinding. Signalling pathways elicited at the PC (left-hand boxed regions, full line) induce phenotypic changes at the axonal and/or growth cone compartments (right-hand boxed region, large and small dotted lines, respectively). PC-elicited signalling pathways have been found to regulate axon pathfinding dynamics through the regulation of growth cone morphology (top; Guo et al., 2019), axon-axon adhesion (middle; Guo et al., 2019) and transcription (bottom; Guo et al., 2019; Dumoulin et al., 2024). In all panels, experimental manipulations (genetic, chemo-genetic, optogenetic or pharmacological) performed to alter ciliary signals, together with their phenotypic consequences, are colour-coded in black. Left- and right-hand boxed regions correspond to a higher magnification of the PC (full line), axon (large dotted line) or growth cone (small dotted line) compartments of the developing neuron depicted above. GPCR, G-protein coupled receptor; bPAC, bacterial (Beggiatoa) photoactivated adenylyl cyclase; Shh, Sonic hedgehog.
Given that the PC, that is organised by the centrosome, is located near the cell soma and consequently at a distance from the axonal growth cone, such results raise the question of the ciliary downstream molecular effectors and mechanisms that propagate the signals down the axon to the exploring growth cone. Interestingly, Guo and colleagues observed a gradual increase in PIP3 activity at the growth cone of DCN neurons following ciliary PIP3 activation (Guo et al., 2019; Figure 3, top), and propose that positive feedback networks involving kinase-dependent cascades may rapidly spread locally-induced PC signalling over long distances. Following RNA-seq analyses in E12.5 Arl13b−⁄− and control embryos, they further propose PC-induced regulation of transcriptional programs as an additional mechanism to regulate axon navigation processes (Figure 3, bottom). Their identification in ciliary mutants of differentially expressed genes involved (among other processes) in cell adhesion opens the possibility that the defective Pcdh17-mediated axon-axon adhesion observed in Arl13b conditional knockout neurons may be due to altered gene transcription. In agreement, the Stoeckli lab has recently identified a role for the PC of developing chick commissural axons in mediating a transcriptional switch of Shh receptors, required to elicit the well-documented behavioural switch (from attraction to repulsion) of commissural axons crossing the midline (Dumoulin et al., 2024). In chick dI1 neurons, the authors indeed showed that IFT88 silencing impaired dI1 axon midline crossing in a cell autonomous manner. IFT88 silencing was moreover associated in in situ hybridisation experiments with a reduced expression of the Hhip (hedgehog-interacting protein) receptor, which is required for the repulsive response to Shh and the rostral turn of post-crossing commissural axons (Bourikas et al., 2005; Wilson and Stoeckli, 2013). Importantly, preventing Smo entry in the PC in response to Shh activation, pharmacologically or genetically (using a hSmoCLD construct that prevents Smo ciliary localisation after endogenous Smo silencing), led to misprojecting commissural dI1 axons or reduced Hhip expression, respectively, supporting the requirement of Shh signalling at the PC for the induction of Hhip transcription and correct dI1 axon guidance (Dumoulin et al., 2024; Figure 3, bottom). This elegant study further opens the question of whether additional mechanisms required for axon guidance may be regulated by the PC, such as the axonal transport or exocytosis of Hhip at the growth cone membrane. In addition to a role for canonical Shh signalling in mediating gene transcription required for axon guidance, a non-canonical Shh pathway (i.e., that is transcription independent) that relies on the PC has been reported in the axonogenesis of chick postmitotic neurons (Toro-Tapia and Das, 2020). In developing chick embryos, neuroepithelial cells undergoing proliferation have been reported to delaminate from the neuroepithelium as they exit the cell cycle. Postmitotic neurons then initiate axon outgrowth and navigation for the formation of functional neuronal circuits. In this study, authors showed that as neuroepithelial cells delaminate, the PC is disassembled through apical abscission, followed by a PC re-assembly at the onset of axonogenesis. Preventing ciliary re-assembly by chromophore-assisted light inactivation impaired the axonogenesis of newborn neurons by inducing axonal collapse. Using a Gli reporter construct, authors further observed that canonical Shh signalling (i.e., Gli activity-dependent) in the PC is lost upon delamination, and is no longer observed in the newly assembled PC. Although this newly-assembled PC gradually displayed Smo accumulation (suggestive of Shh signalling), immunostaining revealed the presence of the GPR161 negative regulator of canonical Shh signalling. Finally, pharmacological inhibition of the Src family kinases, which mediate the cytoskeletal rearrangements downstream of non-canonical Shh signalling, induced axon collapse, supporting a model in which the re-assembled PC is required for axonogenesis by mediating non-canonical Shh signalling. Whether the non-canonical Shh signalling is also required during growth cone turning events, in addition to axon extension, remains to be uncovered.
Taken together, these studies show to what extent guidance signalling pathways initiated in the ciliary compartment close to the soma influence the axon and growth cone behaviours required for accurate axon navigation. It is interesting to note that a long-distance influence of ciliary signals was also reported to regulate the branching behaviour of the leading process in the case of neuronal migration (Atkins et al., 2023b). Further studies will be required to precisely unravel the molecular effectors linking ciliary signals to axonal and growth cone behavioural remodelling.
5 Conclusion: Insights to be gained from ciliary guidance pathways for our understanding of the aetiology of neurodevelopmental disorders
The increasing interest for the once-neglected ciliary compartment initially arose from the discovery of its involvement in a wide range of disorders. Indeed, in addition to ciliopathies, a dysfunction of the PC has now been involved in different neurodevelopmental (e.g., schizophrenia, autism spectrum disorder, bipolar disorder, intellectual disability …) and neurodegenerative disorders (Valente et al., 2014; Kaliszewski et al., 2015; Youn and Han, 2018; Park et al., 2019; Hasenpusch-Theil and Theil, 2021; Karalis et al., 2022; Ma et al., 2022; Volos et al., 2025), as well as in cancer cell migration/invasion (Eguether and Hahne, 2000; Higgins et al., 2019), including glioblastoma (Álvarez-Satta and Matheu, 2018). Conversely, studying the PC-elicited signalling pathways and molecular mechanisms regulating guidance processes in physiological conditions now appears as a key step to better understand the aetiology of such disorders. Interestingly, our increasing knowledge of PC-elicited guidance signalling and its functional and molecular versatility, both refines and complexifies our understanding of the role of this tiny organelle in pathology, at multiple levels.
First, the PC can regulate multiple aspects of a same neuronal guidance process. For example, during cell migration, the PC controls membrane dynamics, cytoskeletal dynamics but also focal adhesion dynamics. This occurs either through the activation of different ciliary membrane receptors (e.g., PDGFR-α, P2Y11, CXCR4), or through the activation of a same ciliary receptor (e.g., PDGFR-α) that can regulate multiple cellular mechanisms (e.g., membrane and microtubule dynamics, see Figure 2), sequentially or concomitantly through the activation of several parallel downstream pathways.
Second, a same ciliary signalling molecule can be involved in different stages of neuronal guidance. For example, in the genetic screen performed by Guo and colleagues, silencing of the Bardet-Biedl Syndrome-associated BBS7 gene led to a disrupted apical-basal polarity of radial glial cells, but also to a defective multipolar to bipolar transition of migrating principal neurons, and to altered axonal trajectory and fasciculation of cortical neurons (Guo et al., 2015). Likewise, while Shh appears to regulate the migration of developing cortical interneurons (Baudoin et al., 2012), it is also involved in the extension and navigation of developing axons, either through the transcriptional regulation of key guidance receptors (Dumoulin et al., 2024), or through a non-canonical pathway involving Src kinase activation (Toro-Tapia and Das, 2020).
Third, the presence of multiple guidance receptors both at the PC and growth cone surface highlights the importance of understanding the specific function of guidance receptor activation at each subcellular compartment. For example, guidance receptors such as Robo1/2 and ErBb4 have been linked to neurodevelopmental disorders, such as Autism Spectrum Disorder for Robo1/2 (Anitha et al., 2008) and schizophrenia and bipolar disorder for ErbB4 (Iwakura and Nawa, 2013; Mei and Nave, 2014). Identifying the specific contribution of the compartmentalised ciliary signalling of these receptors appears crucial in this context to better apprehend the complexity of such disorders and gain new insigths into their aetiology and treatment. Likewise, in cancer cell migration, while CXCL12 (Guo et al., 2016; Luo et al., 2019; Hayasaka et al., 2022) and Ephrin (Campbell et al., 2006; Wang, 2011; Cho et al., 2018) signalling have been linked to metastasis, the specific role on invasive behaviour of their local signalling at the ciliary compartment remains poorly characterised. Unravelling PC-elicited signalling pathways and downstream molecular effectors may therefore provide precious clues for future translational studies aiming to identify new therapeutic targets specific to PC signalling in order to selectively correct specific cell behaviours (i.e., invasion).
Understanding the specific role of the identified ciliary guidance receptors (see Figure 1) in different steps of neuronal guidance is a crucial step of this complex process. The complexity of the task lies in the diversity of ciliary receptors, that are not always exclusive to the ciliary compartment. Rising to this challenge will critically rely on the use and development of new tools to selectively manipulate (i.e., block/activate) specific membrane receptors located exclusively at the ciliary surface (without affecting the other ciliary receptors through PC genetic ablation, for example,) or their downstream second messenger signals. Such genetic, chemo-genetic and optogenetic tools are already starting to emerge to selectively buffer endogenous ciliary second messenger signals or trigger specific second messenger signalling within the ciliary compartment (Guo et al., 2019; Hansen et al., 2022; Atkins et al., 2023b).
Author contributions
MA: Writing – review and editing, Writing – original draft. CF: Writing – review and editing. XN: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We are grateful to Christine Métin for insightful comments and discussions. MA has received funding from the Brain and Behaviour Research Foundation (Young Investigator Grant) and from the Jérôme Lejeune and Sisley-d’Ornano postdoctoral fellowship program. The research of the XN and CF lab was supported by the Agence Nationale de la Recherche (ANR-20-CE16-0019), an IHU FOReSIGHT (ANR-18-IAHU-0001), the Fondation pour la Recherche Médicale (EQU202003010158), and by the DIM C-BRAINS, funded by the Conseil Régional d’Ile-de-France.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhamed, Z. A., Abdelmottaleb, D. I., El-Asrag, M. E., Natarajan, S., Wheway, G., Inglehearn, C. F., et al. (2019). The ciliary Frizzled-like receptor Tmem67 regulates canonical Wnt/β-catenin signalling in the developing cerebellum via Hoxb5. Sci. Rep. 9, 5446. doi:10.1038/s41598-019-41940-5
Abdelhamed, Z. A., Natarajan, S., Wheway, G., Inglehearn, C. F., Toomes, C., Johnson, C. A., et al. (2015). The Meckel-Gruber syndrome protein TMEM67 controls basal body positioning and epithelial branching morphogenesis in mice via the non-canonical Wnt pathway. Dis. Models Mech. 8, 527–541. doi:10.1242/dmm.019083
Albrecht-Buehler, G. (1977). Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell 12, 333–339. doi:10.1016/0092-8674(77)90109-x
Álvarez-Satta, M., and Matheu, A. (2018). Primary cilium and glioblastoma. Ther. Adv. Med. Oncol. 10, 1758835918801169. doi:10.1177/1758835918801169
Andrae, J., Hansson, I., Afink, G. B., and Nistér, M. (2001). Platelet-derived growth factor receptor-alpha in ventricular zone cells and in developing neurons. Mol. Cell Neurosci. 17, 1001–1013. doi:10.1006/mcne.2001.0989
Andreu-Cervera, A., Catala, M., and Schneider-Maunoury, S. (2021). Cilia, ciliopathies and hedgehog-related forebrain developmental disorders. Neurobiol. Dis. 150, 105236. doi:10.1016/j.nbd.2020.105236
Anitha, A., Nakamura, K., Yamada, K., Suda, S., Thanseem, I., Tsujii, M., et al. (2008). Genetic analyses of roundabout (ROBO) axon guidance receptors in autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 1019–1027. doi:10.1002/ajmg.b.30697
Anvarian, Z., Mykytyn, K., Mukhopadhyay, S., Pedersen, L. B., and Christensen, S. T. (2019). Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 15, 199–219. doi:10.1038/s41581-019-0116-9
Arellano, J. I., Guadiana, S. M., Breunig, J. J., Rakic, P., and Sarkisian, M. R. (2012). Development and distribution of neuronal cilia in mouse neocortex. J. Comp. Neurol. 520, 848–873. doi:10.1002/cne.22793
Asadollahi, R., Strauss, J. E., Zenker, M., Beuing, O., Edvardson, S., Elpeleg, O., et al. (2018). Clinical and experimental evidence suggest a link between KIF7 and C5orf42-related ciliopathies through Sonic Hedgehog signaling. Eur. J. Hum. Genet. 26, 197–209. doi:10.1038/s41431-017-0019-9
Atkins, M., Nicol, X., and Fassier, C. (2023a). Microtubule remodelling as a driving force of axon guidance and pruning. Semin. Cell Dev. Biol. 140, 35–53. doi:10.1016/j.semcdb.2022.05.030
Atkins, M., Wurmser, M., Darmon, M., Roche, F., Nicol, X., and Métin, C. (2023b). CXCL12 targets the primary cilium cAMP/cGMP ratio to regulate cell polarity during migration. Nat. Commun. 14, 8003. doi:10.1038/s41467-023-43645-w
Bangs, F., and Anderson, K. V. (2017). Primary cilia and mammalian hedgehog signaling. Cold Spring Harb. Perspect. Biol. 9, a028175. doi:10.1101/cshperspect.a028175
Baudoin, J.-P., Viou, L., Launay, P.-S., Luccardini, C., Espeso Gil, S., Kiyasova, V., et al. (2012). Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron 76, 1108–1122. doi:10.1016/j.neuron.2012.10.027
Bellion, A., Baudoin, J.-P., Alvarez, C., Bornens, M., and Métin, C. (2005). Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25, 5691–5699. doi:10.1523/JNEUROSCI.1030-05.2005
Benadiba, C., Magnani, D., Niquille, M., Morlé, L., Valloton, D., Nawabi, H., et al. (2012). The ciliogenic transcription factor RFX3 regulates early midline distribution of guidepost neurons required for corpus callosum development. PLoS Genet. 8, e1002606. doi:10.1371/journal.pgen.1002606
Bocchi, R., Egervari, K., Carol-Perdiguer, L., Viale, B., Quairiaux, C., De Roo, M., et al. (2017). Perturbed Wnt signaling leads to neuronal migration delay, altered interhemispheric connections and impaired social behavior. Nat. Commun. 8, 1158. doi:10.1038/s41467-017-01046-w
Boitard, M., Bocchi, R., Egervari, K., Petrenko, V., Viale, B., Gremaud, S., et al. (2015). Wnt signaling regulates multipolar-to-bipolar transition of migrating neurons in the cerebral cortex. Cell Rep. 10, 1349–1361. doi:10.1016/j.celrep.2015.01.061
Bourikas, D., Pekarik, V., Baeriswyl, T., Grunditz, A., Sadhu, R., Nardó, M., et al. (2005). Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat. Neurosci. 8, 297–304. doi:10.1038/nn1396
Breslow, D. K., Koslover, E. F., Seydel, F., Spakowitz, A. J., and Nachury, M. V. (2013). An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J. Cell Biol. 203, 129–147. doi:10.1083/jcb.201212024
Campbell, T. N., Attwell, S., Arcellana-Panlilio, M., and Robbins, S. M. (2006). Ephrin A5 expression promotes invasion and transformation of murine fibroblasts. Biochem. Biophysical Res. Commun. 350, 623–628. doi:10.1016/j.bbrc.2006.09.085
Cardone, R. A., Casavola, V., and Reshkin, S. J. (2005). The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795. doi:10.1038/nrc1713
Cevik, S., Sanders, A. A. W. M., Van Wijk, E., Boldt, K., Clarke, L., van Reeuwijk, J., et al. (2013). Active transport and diffusion barriers restrict Joubert Syndrome-associated ARL13B/ARL-13 to an Inv-like ciliary membrane subdomain. PLoS Genet. 9, e1003977. doi:10.1371/journal.pgen.1003977
Cho, H. J., Hwang, Y.-S., Yoon, J., Lee, M., Lee, H. G., and Daar, I. O. (2018). EphrinB1 promotes cancer cell migration and invasion through the interaction with RhoGDI1. Oncogene 37, 861–872. doi:10.1038/onc.2017.386
Christensen, S. T., Clement, C. A., Satir, P., and Pedersen, L. B. (2012). Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J. Pathol. 226, 172–184. doi:10.1002/path.3004
Christensen, S. T., Veland, I. R., Schwab, A., Cammer, M., and Satir, P. (2013). Analysis of primary cilia in directional cell migration in fibroblasts. Methods Enzymol. 525, 45–58. doi:10.1016/B978-0-12-397944-5.00003-1
Clement, D. L., Mally, S., Stock, C., Lethan, M., Satir, P., Schwab, A., et al. (2013). PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2–ERK1/2–p90RSK and AKT signaling pathways. J. Cell Sci. 126, 953–965. doi:10.1242/jcs.116426
Conduit, S. E., and Vanhaesebroeck, B. (2020). Phosphoinositide lipids in primary cilia biology. Biochem. J. 477, 3541–3565. doi:10.1042/BCJ20200277
Cong, D., Zhu, W., Shi, Y., Pointer, K. B., Clark, P. A., Shen, H., et al. (2014). Upregulation of NHE1 protein expression enables glioblastoma cells to escape TMZ-mediated toxicity via increased H⁺ extrusion, cell migration and survival. Carcinogenesis 35, 2014–2024. doi:10.1093/carcin/bgu089
Deane, J. A., Cole, D. G., Seeley, E. S., Diener, D. R., and Rosenbaum, J. L. (2001). Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11, 1586–1590. doi:10.1016/s0960-9822(01)00484-5
Deboux, C., Ladraa, S., Cazaubon, S., Ghribi-Mallah, S., Weiss, N., Chaverot, N., et al. (2013). Overexpression of CD44 in neural precursor cells improves trans-endothelial migration and facilitates their invasion of perivascular tissues in vivo. PLoS One 8, e57430. doi:10.1371/journal.pone.0057430
Delling, M., DeCaen, P. G., Doerner, J. F., Febvay, S., and Clapham, D. E. (2013). Primary cilia are specialized calcium signalling organelles. Nature 504, 311–314. doi:10.1038/nature12833
Delling, M., Indzhykulian, A. A., Liu, X., Li, Y., Xie, T., Corey, D. P., et al. (2016). Primary cilia are not calcium-responsive mechanosensors. Nature 531, 656–660. doi:10.1038/nature17426
DeMars, K. M., Ross, M. R., Starr, A., and McIntyre, J. C. (2023). Neuronal primary cilia integrate peripheral signals with metabolic drives. Front. Physiol. 14, 1150232. doi:10.3389/fphys.2023.1150232
Denker, S. P., and Barber, D. L. (2002). Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 159, 1087–1096. doi:10.1083/jcb.200208050
Dishinger, J. F., Kee, H. L., Jenkins, P. M., Fan, S., Hurd, T. W., Hammond, J. W., et al. (2010). Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat. Cell Biol. 12, 703–710. doi:10.1038/ncb2073
Djenoune, L., Mahamdeh, M., Truong, T. V., Nguyen, C. T., Fraser, S. E., Brueckner, M., et al. (2023). Cilia function as calcium-mediated mechanosensors that instruct left-right asymmetry. Science 379, 71–78. doi:10.1126/science.abq7317
Dorskind, J. M., and Kolodkin, A. L. (2021). Revisiting and refining roles of neural guidance cues in circuit assembly. Curr. Opin. Neurobiol. 66, 10–21. doi:10.1016/j.conb.2020.07.005
Douceau, S., Deutsch Guerrero, T., and Ferent, J. (2023). Establishing hedgehog gradients during neural development. Cells 12, 225. doi:10.3390/cells12020225
Dumoulin, A., Wilson, N. H., Tucker, K. L., and Stoeckli, E. T. (2024). A cell-autonomous role for primary cilium-mediated signaling in long-range commissural axon guidance. Development 151, dev202788. doi:10.1242/dev.202788
Eguether, T., and Hahne, M. (2018). Mixed signals from the cell’s antennae: primary cilia in cancer. EMBO reports 19, e46589. doi:10.15252/embr.201846589
Fan, S., Whiteman, E. L., Hurd, T. W., McIntyre, J. C., Dishinger, J. F., Liu, C. J., et al. (2011). Induction of Ran GTP drives ciliogenesis. Mol. Biol. Cell 22, 4539–4548. doi:10.1091/mbc.E11-03-0267
Fiorentino, H., Kuczewski, N., Diabira, D., Ferrand, N., Pangalos, M. N., Porcher, C., et al. (2009). GABAB receptor activation triggers BDNF release and promotes the maturation of GABAergic synapses. J. Neurosci. 29, 11650–11661. doi:10.1523/JNEUROSCI.3587-09.2009
Gangatharan, G., Schneider-Maunoury, S., and Breau, M. A. (2018). Role of mechanical cues in shaping neuronal morphology and connectivity. Biol. Cell 110, 125–136. doi:10.1111/boc.201800003
Gonçalves, J., and Pelletier, L. (2017). The ciliary transition zone: finding the pieces and assembling the gate. Mol. Cells 40, 243–253. doi:10.14348/molcells.2017.0054
Guo, F., Wang, Y., Liu, J., Mok, S. C., Xue, F., and Zhang, W. (2016). CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 35, 816–826. doi:10.1038/onc.2015.139
Guo, J., Higginbotham, H., Li, J., Nichols, J., Hirt, J., Ghukasyan, V., et al. (2015). Developmental disruptions underlying brain abnormalities in ciliopathies. Nat. Commun. 6, 7857. doi:10.1038/ncomms8857
Guo, J., Otis, J., Suciu, S. K., Catalano, C., Xing, L., Constable, S., et al. (2019). Primary cilia signaling promotes axonal tract development and is disrupted in Joubert syndrome-related disorders models. Dev. Cell 51, 759–774.e5. doi:10.1016/j.devcel.2019.11.005
Higgins, M., Obaidi, I., and McMorrow, T. (2019). Primary cilia and their role in cancer. Oncol Lett 17, 3041–3047. doi:10.3892/ol.2019.9942
Hansen, J. N., Kaiser, F., Leyendecker, P., Stüven, B., Krause, J., Derakhshandeh, F., et al. (2022). A cAMP signalosome in primary cilia drives gene expression and kidney cyst formation. EMBO Rep. 23, e54315. doi:10.15252/embr.202154315
Hansen, J. N., Sun, H., Kahnert, K., Westenius, E., Johannesson, A., Tzavlaki, K., et al. (2024). Intrinsic diversity in primary cilia revealed through spatial proteomics. 619273. doi:10.1101/2024.10.20.619273
Hasenpusch-Theil, K., and Theil, T. (2021). The multifaceted roles of primary cilia in the development of the cerebral cortex. Front. Cell Dev. Biol. 9, 630161. doi:10.3389/fcell.2021.630161
Hayasaka, H., Yoshida, J., Kuroda, Y., Nishiguchi, A., Matsusaki, M., Kishimoto, K., et al. (2022). CXCL12 promotes CCR7 ligand-mediated breast cancer cell invasion and migration toward lymphatic vessels. Cancer Sci. 113, 1338–1351. doi:10.1111/cas.15293
Heck, N., Kilb, W., Reiprich, P., Kubota, H., Furukawa, T., Fukuda, A., et al. (2007). GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb. Cortex 17, 138–148. doi:10.1093/cercor/bhj135
Higginbotham, H., Eom, T.-Y., Mariani, L. E., Bachleda, A., Hirt, J., Gukassyan, V., et al. (2012). Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev. Cell 23, 925–938. doi:10.1016/j.devcel.2012.09.019
Higginbotham, H., Guo, J., Yokota, Y., Umberger, N. L., Su, C.-Y., Li, J., et al. (2013). Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat. Neurosci. 16, 1000–1007. doi:10.1038/nn.3451
Hilgendorf, K. I., Johnson, C. T., and Jackson, P. K. (2016). The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr. Opin. Cell Biol. 39, 84–92. doi:10.1016/j.ceb.2016.02.008
Hoffman, H. K., and Prekeris, R. (2022). Roles of the actin cytoskeleton in ciliogenesis. J. Cell Sci. 135, jcs259030. doi:10.1242/jcs.259030
Holub, M., Potocki, L., and Bodamer, O. A. (2005). Central nervous system malformations in oral-facial-digital syndrome, type 1. Am. J. Med. Genet. A 136, 218. doi:10.1002/ajmg.a.30751
Huangfu, D., Liu, A., Rakeman, A. S., Murcia, N. S., Niswander, L., and Anderson, K. V. (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87. doi:10.1038/nature02061
Ishikawa, H., Thompson, J., Yates, J. R., and Marshall, W. F. (2012). Proteomic analysis of mammalian primary cilia. Curr. Biol. 22, 414–419. doi:10.1016/j.cub.2012.01.031
Itoh, Y., Toriumi, H., Yamada, S., Hoshino, H., and Suzuki, N. (2011). Astrocytes and pericytes cooperatively maintain a capillary-like structure composed of endothelial cells on gel matrix. Brain Res. 1406, 74–83. doi:10.1016/j.brainres.2011.06.039
Iwakura, Y., and Nawa, H. (2013). ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: pathological implications in schizophrenia and Parkinson’s disease. Front. Cell Neurosci. 7, 4. doi:10.3389/fncel.2013.00004
Jensen, V. L., and Leroux, M. R. (2017). Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr. Opin. Cell Biol. 47, 83–91. doi:10.1016/j.ceb.2017.03.012
Jiang, J. Y., Falcone, J. L., Curci, S., and Hofer, A. M. (2019). Direct visualization of cAMP signaling in primary cilia reveals up-regulation of ciliary GPCR activity following Hedgehog activation. Proc. Natl. Acad. Sci. U. S. A. 116, 12066–12071. doi:10.1073/pnas.1819730116
Jin, X., Mohieldin, A. M., Muntean, B. S., Green, J. A., Shah, J. V., Mykytyn, K., et al. (2014). Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol. Life Sci. 71, 2165–2178. doi:10.1007/s00018-013-1483-1
Jones, T. J., Adapala, R. K., Geldenhuys, W. J., Bursley, C., AbouAlaiwi, W. A., Nauli, S. M., et al. (2012). Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of Hsp27 dependent actin cytoskeletal organization. J. Cell. Physiol. 227, 70–76. doi:10.1002/jcp.22704
Kaliszewski, M., Knott, A. B., and Bossy-Wetzel, E. (2015). Primary cilia and autophagic dysfunction in Huntington’s disease. Cell Death Differ. 22, 1413–1424. doi:10.1038/cdd.2015.80
Karalis, V., Donovan, K. E., and Sahin, M. (2022). Primary cilia dysfunction in neurodevelopmental disorders beyond ciliopathies. J. Dev. Biol. 10, 54. doi:10.3390/jdb10040054
Katsumoto, T., Higaki, K., Ohno, K., and Onodera, K. (1994). The orientation of primary cilia during the wound response in 3Y1 cells. Biol. Cell 81, 17–21. doi:10.1016/0248-4900(94)90050-7
Kee, H. L., Dishinger, J. F., Blasius, T. L., Liu, C.-J., Margolis, B., and Verhey, K. J. (2012). A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 14, 431–437. doi:10.1038/ncb2450
Labat-de-Hoz, L., Rubio-Ramos, A., Casares-Arias, J., Bernabé-Rubio, M., Correas, I., and Alonso, M. A. (2021). A model for primary cilium biogenesis by polarized epithelial cells: role of the midbody remnant and associated specialized membranes. Front. Cell Dev. Biol. 8, 622918. doi:10.3389/fcell.2020.622918
Laclef, C., Anselme, I., Besse, L., Catala, M., Palmyre, A., Baas, D., et al. (2015). The role of primary cilia in corpus callosum formation is mediated by production of the Gli3 repressor. Hum. Mol. Genet. 24, 4997–5014. doi:10.1093/hmg/ddv221
Lee, M. N., Song, J. H., Oh, S.-H., Tham, N. T., Kim, J.-W., Yang, J.-W., et al. (2020). The primary cilium directs osteopontin-induced migration of mesenchymal stem cells by regulating CD44 signaling and Cdc42 activation. Stem Cell Res. 45, 101799. doi:10.1016/j.scr.2020.101799
Lin, Y.-C., Niewiadomski, P., Lin, B., Nakamura, H., Phua, S. C., Jiao, J., et al. (2013). Chemically inducible diffusion trap at cilia reveals molecular sieve-like barrier. Nat. Chem. Biol. 9, 437–443. doi:10.1038/nchembio.1252
Liu, X., Yam, P. T., Schlienger, S., Cai, E., Zhang, J., Chen, W.-J., et al. (2024). Numb positively regulates Hedgehog signaling at the ciliary pocket. Nat. Commun. 15, 3365. doi:10.1038/s41467-024-47244-1
Loukil, A., Ebright, E., Uezu, A., Gao, Y., Soderling, S. H., and Goetz, S. C. (2023). Identification of new ciliary signaling pathways in the brain and insights into neurological disorders. bioRxiv., 2023.12.20.572700. doi:10.1101/2023.12.20.572700
Lowery, L. A., and Van Vactor, D. (2009). The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10, 332–343. doi:10.1038/nrm2679
Luo, N., Chen, D., Liu, L., Li, L., and Cheng, Z. (2019). CXCL12 promotes human ovarian cancer cell invasion through suppressing ARHGAP10 expression. Biochem. Biophysical Res. Commun. 518, 416–422. doi:10.1016/j.bbrc.2019.07.098
Lyuksyutova, A. I., Lu, C.-C., Milanesio, N., King, L. A., Guo, N., Wang, Y., et al. (2003). Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984–1988. doi:10.1126/science.1089610
Ma, R., Kutchy, N. A., Chen, L., Meigs, D. D., and Hu, G. (2022). Primary cilia and ciliary signaling pathways in aging and age-related brain disorders. Neurobiol. Dis. 163, 105607. doi:10.1016/j.nbd.2021.105607
Macarelli, V., Leventea, E., and Merkle, F. T. (2023). Regulation of the length of neuronal primary cilia and its potential effects on signalling. Trends Cell Biol. S0962-8924 (23), 979–990. doi:10.1016/j.tcb.2023.05.005
Magnani, D., Morlé, L., Hasenpusch-Theil, K., Paschaki, M., Jacoby, M., Schurmans, S., et al. (2015). The ciliogenic transcription factor Rfx3 is required for the formation of the thalamocortical tract by regulating the patterning of prethalamus and ventral telencephalon. Hum. Mol. Genet. 24, 2578–2593. doi:10.1093/hmg/ddv021
Mansini, A. P., Peixoto, E., Jin, S., Richard, S., and Gradilone, S. A. (2019). The chemosensory function of primary cilia regulates cholangiocyte migration, invasion and tumor growth. Hepatology 69, 1582–1598. doi:10.1002/hep.30308
Maria, B. L., Quisling, R. G., Rosainz, L. C., Yachnis, A. T., Gitten, J., Dede, D., et al. (1999). Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J. Child. Neurol. 14, 368–376. doi:10.1177/088307389901400605
Matsumoto, M., Sawada, M., García-González, D., Herranz-Pérez, V., Ogino, T., Nguyen, H. B., et al. (2019). Dynamic changes in ultrastructure of the primary cilium in migrating neuroblasts in the postnatal brain. J. Neurosci. 39, 9967–9988. doi:10.1523/JNEUROSCI.1503-19.2019
Medvedeva, V. P., and Pierani, A. (2020). How do electric fields coordinate neuronal migration and maturation in the developing cortex? Front. Cell Dev. Biol. 8, 580657. doi:10.3389/fcell.2020.580657
Mei, L., and Nave, K.-A. (2014). Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 83, 27–49. doi:10.1016/j.neuron.2014.06.007
Mick, D. U., Rodrigues, R. B., Leib, R. D., Adams, C. M., Chien, A. S., Gygi, S. P., et al. (2015). Proteomics of primary cilia by proximity labeling. Dev. Cell 35, 497–512. doi:10.1016/j.devcel.2015.10.015
Milenkovic, L., Scott, M. P., and Rohatgi, R. (2009). Lateral transport of smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 187, 365–374. doi:10.1083/jcb.200907126
Mill, P., Christensen, S. T., and Pedersen, L. B. (2023). Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat. Rev. Genet. 24, 421–441. doi:10.1038/s41576-023-00587-9
Molla-Herman, A., Ghossoub, R., Blisnick, T., Meunier, A., Serres, C., Silbermann, F., et al. (2010). The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 123, 1785–1795. doi:10.1242/jcs.059519
Moore, B. S., Stepanchick, A. N., Tewson, P. H., Hartle, C. M., Zhang, J., Quinn, A. M., et al. (2016). Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc. Natl. Acad. Sci. U. S. A. 113, 13069–13074. doi:10.1073/pnas.1602393113
Moran, A. L., Louzao-Martinez, L., Norris, D. P., Peters, D. J. M., and Blacque, O. E. (2024). Transport and barrier mechanisms that regulate ciliary compartmentalization and ciliopathies. Nat. Rev. Nephrol. 20, 83–100. doi:10.1038/s41581-023-00773-2
Mukhopadhyay, S., Wen, X., Ratti, N., Loktev, A., Rangell, L., Scales, S. J., et al. (2013). The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 152, 210–223. doi:10.1016/j.cell.2012.12.026
Nakajima, C., Sawada, M., Umeda, E., Takagi, Y., Nakashima, N., Kuboyama, K., et al. (2024). Identification of the growth cone as a probe and driver of neuronal migration in the injured brain. Nat. Commun. 15, 1877. doi:10.1038/s41467-024-45825-8
Nakatsu, F. (2015). A phosphoinositide code for primary cilia. Dev. Cell 34, 379–380. doi:10.1016/j.devcel.2015.08.008
Nakazato, R., Matsuda, Y., Ijaz, F., and Ikegami, K. (2023). Circadian oscillation in primary cilium length by clock genes regulates fibroblast cell migration. EMBO Rep. 24, e56870. doi:10.15252/embr.202356870
Nauli, S. M., Kawanabe, Y., Kaminski, J. J., Pearce, W. J., Ingber, D. E., and Zhou, J. (2008). Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117, 1161–1171. doi:10.1161/CIRCULATIONAHA.107.710111
Niehrs, C., Silva, F. D., and Seidl, C. (2025). Cilia as Wnt signaling organelles. Trends Cell Biol. 35, 24–32. doi:10.1016/j.tcb.2024.04.001
Nishimura, Y., Kasahara, K., Shiromizu, T., Watanabe, M., and Inagaki, M. (2019). Primary cilia as signaling hubs in health and disease. Adv. Sci. 6, 1801138. doi:10.1002/advs.201801138
Odent, S., Le Marec, B., Toutain, A., David, A., Vigneron, J., Tréguier, C., et al. (1998). Central nervous system malformations and early end-stage renal disease in oro-facio-digital syndrome type I: a review. Am. J. Med. Genet. 75, 389–394. doi:10.1002/(sici)1096-8628(19980203)75:4<389::aid-ajmg8>3.0.co;2-l
Paillard, T., Allam, A., Doulazmi, M., Hautefeuille, M., Fouquet, C., Sarde, L., et al. (2025). GPR161 mechanosensitivity at the primary cilium drives neuronal saltatory migration. doi:10.1101/2025.03.05.641658
Pala, R., Alomari, N., and Nauli, S. M. (2017). Primary cilium-dependent signaling mechanisms. Int. J. Mol. Sci. 18, 2272. doi:10.3390/ijms18112272
Park, K., and Leroux, M. R. (2022). Composition, organization and mechanisms of the transition zone, a gate for the cilium. EMBO Rep. 23, e55420. doi:10.15252/embr.202255420
Park, S. M., Lim, J. S., Ramakrishina, S., Kim, S. H., Kim, W. K., Lee, J., et al. (2018). Brain somatic mutations in MTOR disrupt neuronal ciliogenesis, leading to focal cortical dyslamination. Neuron 99, 83–97.e7. doi:10.1016/j.neuron.2018.05.039
Park, S. M., Jang, H. J., and Lee, J. H. (2019). Roles of primary cilia in the developing brain. Front. Cell. Neurosci. 13, 218. doi:10.3389/fncel.2019.00218
Pedraza, M., Grampa, V., Scotto-Lomassese, S., Puech, J., Muzerelle, A., Mohammad, A., et al. (2024). The ciliary kinesin KIF7 controls the development of the cerebral cortex by acting differentially on SHH-signaling in dorsal and ventral forebrain. eLife 13. 586159. doi:10.7554/eLife.100328.1
Petralia, R. S., Schwartz, C. M., Wang, Y.-X., Mattson, M. P., and Yao, P. J. (2011). Subcellular localization of patched and smoothened, the receptors for sonic hedgehog signaling, in the hippocampal neuron. J. Comp. Neurol. 519, 3684–3699. doi:10.1002/cne.22681
Pinskey, J. M., Franks, N. E., McMellen, A. N., Giger, R. J., and Allen, B. L. (2017). Neuropilin-1 promotes Hedgehog signaling through a novel cytoplasmic motif. J. Biol. Chem. 292, 15192–15204. doi:10.1074/jbc.M117.783845
Poretti, A., Boltshauser, E., Loenneker, T., Valente, E. M., Brancati, F., Il’yasov, K., et al. (2007). Diffusion tensor imaging in Joubert syndrome. Am. J. Neuroradiol. 28, 1929–1933. doi:10.3174/ajnr.A0703
Poretti, A., Huisman, T. a. G. M., Scheer, I., and Boltshauser, E. (2011). Joubert syndrome and related disorders: spectrum of neuroimaging findings in 75 patients. Am. J. Neuroradiol. 32, 1459–1463. doi:10.3174/ajnr.A2517
Portal, C., Rompolas, P., Lwigale, P., and Iomini, C. (2019). Primary cilia deficiency in neural crest cells models anterior segment dysgenesis in mouse. eLife 8, e52423. doi:10.7554/eLife.52423
Pruski, M., Hu, L., Yang, C., Wang, Y., Zhang, J.-B., Zhang, L., et al. (2019). Roles for IFT172 and primary cilia in cell migration, cell division, and neocortex development. Front. Cell Dev. Biol. 7, 287. doi:10.3389/fcell.2019.00287
Pruski, M., Rajnicek, A., Yang, Z., Clancy, H., Ding, Y.-Q., McCaig, C. D., et al. (2016). The ciliary GTPase Arl13b regulates cell migration and cell cycle progression. Cell Adh Migr. 10, 393–405. doi:10.1080/19336918.2016.1159380
Putoux, A., Baas, D., Paschaki, M., Morlé, L., Maire, C., Attié-Bitach, T., et al. (2019). Altered GLI3 and FGF8 signaling underlies acrocallosal syndrome phenotypes in Kif7 depleted mice. Hum. Mol. Genet. 28, 877–887. doi:10.1093/hmg/ddy392
Putoux, A., Thomas, S., Coene, K. L. M., Davis, E. E., Alanay, Y., Ogur, G., et al. (2011). KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nat. Genet. 43, 601–606. doi:10.1038/ng.826
Reiter, J. F., and Leroux, M. R. (2017). Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547. doi:10.1038/nrm.2017.60
Rodrigues, R. J., Marques, J. M., and Cunha, R. A. (2019). Purinergic signalling and brain development. Seminars Cell Dev. Biol. 95, 34–41. doi:10.1016/j.semcdb.2018.12.001
Rodriguez Gil, D. J., and Greer, C. A. (2008). Wnt/frizzled family members mediate olfactory sensory neuron axon extension. J. Comp. Neurol. 511, 301–317. doi:10.1002/cne.21834
Rohatgi, R., Milenkovic, L., and Scott, M. P. (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372–376. doi:10.1126/science.1139740
Romani, M., Micalizzi, A., and Valente, E. M. (2013). Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet Neurol. 12, 894–905. doi:10.1016/S1474-4422(13)70136-4
Salonen, R. (1984). The Meckel syndrome: clinicopathological findings in 67 patients. Am. J. Med. Genet. 18, 671–689. doi:10.1002/ajmg.1320180414
Sanchez, G. M., Incedal, T. C., Prada, J., O’Callaghan, P., Dyachok, O., Echeverry, S., et al. (2023). The β-cell primary cilium is an autonomous Ca2+ compartment for paracrine GABA signaling. J. Cell Biol. 222, e202108101. doi:10.1083/jcb.202108101
Sánchez-Huertas, C., and Herrera, E. (2021). With the permission of microtubules: an updated overview on microtubule function during axon pathfinding. Front. Mol. Neurosci. 14, 759404. doi:10.3389/fnmol.2021.759404
Sattar, S., and Gleeson, J. G. (2011). The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Dev. Med. Child. Neurol. 53, 793–798. doi:10.1111/j.1469-8749.2011.04021.x
Schaar, B. T., and McConnell, S. K. (2005). Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. U. S. A. 102, 13652–13657. doi:10.1073/pnas.0506008102
Schneider, L., Cammer, M., Lehman, J., Nielsen, S. K., Guerra, C. F., Veland, I. R., et al. (2010). Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol. Biochem. 25, 279–292. doi:10.1159/000276562
Schneider, L., Clement, C. A., Teilmann, S. C., Pazour, G. J., Hoffmann, E. K., Satir, P., et al. (2005). PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861–1866. doi:10.1016/j.cub.2005.09.012
Schneider, L., Stock, C.-M., Dieterich, P., Jensen, B. H., Pedersen, L. B., Satir, P., et al. (2009). The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFR-α in the primary cilium. J. Cell Biol. 185, 163–176. doi:10.1083/jcb.200806019
Shan, Y., Farmer, S. M., and Wray, S. (2021). Drebrin regulates cytoskeleton dynamics in migrating neurons through interaction with CXCR4. Proc. Natl. Acad. Sci. U. S. A. 118, e2009493118. doi:10.1073/pnas.2009493118
Sherpa, R. T., Mohieldin, A. M., Pala, R., Wachten, D., Ostrom, R. S., and Nauli, S. M. (2019). Sensory primary cilium is a responsive cAMP microdomain in renal epithelia. Sci. Rep. 9, 6523. doi:10.1038/s41598-019-43002-2
Shidara, H., Hotta, K., and Oka, K. (2017). Compartmentalized cGMP responses of olfactory sensory neurons in Caenorhabditis elegans. J. Neurosci. 37, 3753–3763. doi:10.1523/JNEUROSCI.2628-16.2017
Shim, S., Goyal, R., Panoutsopoulos, A. A., Balashova, O. A., Lee, D., and Borodinsky, L. N. (2023). Calcium dynamics at the neural cell primary cilium regulate Hedgehog signaling–dependent neurogenesis in the embryonic neural tube. Proc. Natl. Acad. Sci. U. S. A. 120, e2220037120. doi:10.1073/pnas.2220037120
Sin, W.-C., Moniz, D. M., Ozog, M. A., Tyler, J. E., Numata, M., and Church, J. (2009). Regulation of early neurite morphogenesis by the Na+/H+ exchanger NHE1. J. Neurosci. 29, 8946–8959. doi:10.1523/JNEUROSCI.2030-09.2009
Sin, W. C., Tam, N., Moniz, D., Lee, C., and Church, J. (2020). Na/H exchanger NHE1 acts upstream of rho GTPases to promote neurite outgrowth. J. Cell Commun. Signal 14, 325–333. doi:10.1007/s12079-020-00556-5
Sorokin, S. (1962). Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363–377. doi:10.1083/jcb.15.2.363
Sorokin, S. P. (1968). Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3, 207–230. doi:10.1242/jcs.3.2.207
Spampinato, M. V., Kraas, J., Maria, B. L., Walton, Z. J., and Rumboldt, Z. (2008). Absence of decussation of the superior cerebellar peduncles in patients with Joubert syndrome. Am. J. Med. Genet. A 146A, 1389–1394. doi:10.1002/ajmg.a.32282
Stock, C., and Pedersen, S. F. (2017). Roles of pH and the Na+/H+ exchanger NHE1 in cancer: from cell biology and animal models to an emerging translational perspective? Seminars Cancer Biol. 43, 5–16. doi:10.1016/j.semcancer.2016.12.001
Stock, C., and Schwab, A. (2006). Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol. (Oxf) 187, 149–157. doi:10.1111/j.1748-1716.2006.01543.x
Stoeckli, E. T. (2018). Understanding axon guidance: are we nearly there yet? Development 145, dev151415. doi:10.1242/dev.151415
Stoufflet, J., Chaulet, M., Doulazmi, M., Fouquet, C., Dubacq, C., Métin, C., et al. (2020). Primary cilium-dependent cAMP/PKA signaling at the centrosome regulates neuronal migration. Sci. Adv. 6, eaba3992. doi:10.1126/sciadv.aba3992
Sui, Y., Mortensen, M., Yuan, B., Nicholson, M. W., Smart, T. G., and Jovanovic, J. N. (2024). GABAA receptors and neuroligin 2 synergize to promote synaptic adhesion and inhibitory synaptogenesis. Front. Cell Neurosci. 18, 1423471. doi:10.3389/fncel.2024.1423471
Takanashi, J., Tada, H., Ozaki, H., and Barkovich, A. J. (2009). Malformations of cerebral cortical development in oral-facial-digital syndrome type VI. Am. J. Neuroradiol. 30, E22–E23. doi:10.3174/ajnr.A1287
Taschner, M., and Lorentzen, E. (2016). The intraflagellar transport machinery. Cold Spring Harb. Perspect. Biol. 8, a028092. doi:10.1101/cshperspect.a028092
Teperino, R., Aberger, F., Esterbauer, H., Riobo, N., and Pospisilik, J. A. (2014). Canonical and non-canonical Hedgehog signalling and the control of metabolism. Seminars Cell Dev. Biol. 33, 81–92. doi:10.1016/j.semcdb.2014.05.007
Théoret, H., Gleeson, J., and Pascual-Leone, A. (2013). Neurophysiologic characterization of motor and sensory projections in Joubert syndrome. Clin. Neurophysiol. 124, 2283–2284. doi:10.1016/j.clinph.2013.06.006
Tobin, J. L., Di Franco, M., Eichers, E., May-Simera, H., Garcia, M., Yan, J., et al. (2008). Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome. Proc. Natl. Acad. Sci. U. S. A. 105, 6714–6719. doi:10.1073/pnas.0707057105
Toro-Tapia, G., and Das, R. M. (2020). Primary cilium remodeling mediates a cell signaling switch in differentiating neurons. Sci. Adv. 6, eabb0601. doi:10.1126/sciadv.abb0601
Truong, M. E., Bilekova, S., Choksi, S. P., Li, W., Bugaj, L. J., Xu, K., et al. (2021). Vertebrate cells differentially interpret ciliary and extraciliary cAMP. Cell 184, 2911–2926.e18. doi:10.1016/j.cell.2021.04.002
Tsai, L.-H., and Gleeson, J. G. (2005). Nucleokinesis in neuronal migration. Neuron 46, 383–388. doi:10.1016/j.neuron.2005.04.013
Valente, E. M., Rosti, R. O., Gibbs, E., and Gleeson, J. G. (2014). Primary cilia in neurodevelopmental disorders. Nat. Rev. Neurol. 10, 27–36. doi:10.1038/nrneurol.2013.247
Veland, I. R., Lindbæk, L., and Christensen, S. T. (2014). Linking the primary cilium to cell migration in tissue repair and brain development. Bioscience 64, 1115–1125. doi:10.1093/biosci/biu179
Vicente-Manzanares, M., Ma, X., Adelstein, R. S., and Horwitz, A. R. (2009). Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790. doi:10.1038/nrm2786
Volos, P., Fujise, K., and Rafiq, N. M. (2025). Roles for primary cilia in synapses and neurological disorders. Trends Cell Biol. 35, 6–10. doi:10.1016/j.tcb.2024.10.014
Vuong, L. T., and Mlodzik, M. (2023). The complex relationship of Wnt-signaling pathways and cilia. Curr. Top. Dev. Biol. 155, 95–125. doi:10.1016/bs.ctdb.2023.09.002
Wang, B. (2011). Cancer cells exploit the eph-ephrin system to promote invasion and metastasis: tales of unwitting partners. Sci. Signal. 4, pe28. doi:10.1126/scisignal.2002153
Wang, L., and Dynlacht, B. D. (2018). The regulation of cilium assembly and disassembly in development and disease. Development 145, dev151407. doi:10.1242/dev.151407
Wei, Q., Xu, Q., Zhang, Y., Li, Y., Zhang, Q., Hu, Z., et al. (2013). Transition fibre protein FBF1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nat. Commun. 4, 2750. doi:10.1038/ncomms3750
Wheway, G., Nazlamova, L., and Hancock, J. T. (2018). Signaling through the primary cilium. Front. Cell Dev. Biol. 6, 8. doi:10.3389/fcell.2018.00008
Williams, C. L., Li, C., Kida, K., Inglis, P. N., Mohan, S., Semenec, L., et al. (2011). MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192, 1023–1041. doi:10.1083/jcb.201012116
Williams, C. L., Masyukova, S. V., and Yoder, B. K. (2010). Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4. J. Am. Soc. Nephrol. 21, 782–793. doi:10.1681/ASN.2009060597
Wilson, N. H., and Stoeckli, E. T. (2013). Sonic hedgehog regulates its own receptor on postcrossing commissural axons in a glypican1-dependent manner. Neuron 79, 478–491. doi:10.1016/j.neuron.2013.05.025
Ye, F., Breslow, D. K., Koslover, E. F., Spakowitz, A. J., Nelson, W. J., and Nachury, M. V. (2013). Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife 2, e00654. doi:10.7554/eLife.00654
Youn, Y. H., and Han, Y.-G. (2018). Primary cilia in brain development and diseases. Am. J. Pathol. 188, 11–22. doi:10.1016/j.ajpath.2017.08.031
Yuasa-Kawada, J., Kinoshita-Kawada, M., Tsuboi, Y., and Wu, J. Y. (2022). Neuronal guidance genes in health and diseases. Protein Cell 14, 238–261. doi:10.1093/procel/pwac030
Zhang, H., Huang, Z., Chen, S., Chen, G., Ben, J., Ingham, P., et al. (2024). The basal ciliary but not cytosol PKA specifically regulates HH pathway downstream of smoothened. doi:10.1101/2024.12.30.630707
Zhao, H., Khan, Z., and Westlake, C. J. (2023). Ciliogenesis membrane dynamics and organization. Seminars Cell and Dev. Biol. 133, 20–31. doi:10.1016/j.semcdb.2022.03.021
Keywords: neuronal guidance, primary cilium, neuronal migration, axon guidance, signalling pathways
Citation: Atkins M, Fassier C and Nicol X (2025) Neuronal guidance behaviours: the primary cilium perspective. Front. Cell Dev. Biol. 13:1612555. doi: 10.3389/fcell.2025.1612555
Received: 15 April 2025; Accepted: 11 June 2025;
Published: 30 June 2025.
Edited by:
Junichi Yuasa-Kawada, Juntendo University, JapanReviewed by:
Fabienne E. Poulain, University of South Carolina, United StatesAlondra Schweizer Burguete, Columbia University, United States
Copyright © 2025 Atkins, Fassier and Nicol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melody Atkins, bWVsb2R5LmF0a2luc0BpbnNlcm0uZnI=
†These authors have contributed equally to this work and share last authorship
 Melody Atkins
Melody Atkins Coralie Fassier
Coralie Fassier Xavier Nicol†
Xavier Nicol†