- 1First Clinical Medical College, Heilongjiang University of Chinese Medicine, Harbin, China
- 2Department of Gynecology II, Second Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
Rising rates of infertility have stimulated interest in dietary supplements to improve oocyte quality through mitochondrial function, antioxidant activity, and epigenetically regulated metabolic pathways. Mitochondria provides adenosine triphosphate for oocyte maturation, with Coenzyme Q10 (CoQ10) demonstrating efficacy in animal models by alleviating oxidative damage and enhancing blastocyst formation. In aged mice, CoQ10 restored mitochondrial activity and reduced chromosomal abnormalities, while preliminary human studies noted improved embryo quality in poor responders, though randomized controlled trials (RCTs) remain inconclusive. Antioxidants like melatonin counter reactive oxygen species (ROS)-induced spindle defects and mitochondrial dysfunction, showing benefits in murine oocyte maturation and blastocyst development. Resveratrol enhanced bovine oocyte quality through metabolic modulation. Human trials on antioxidants show reduced granulosa cell stress but lack robust evidence. Epigenetically, folate supports DNA methylation critical for embryonic gene expression, with deficiencies linked to hyperhomocysteinemia and developmental defects in animal models. Human observational studies associate folate-rich diets with lower aneuploidy and better assisted reproductive technology outcomes, while omega-3 fatty acids aid chromatin remodeling via histone deacetylase regulation. Despite compelling preclinical data, human trials face inconsistencies due to variable designs and confounders. Standardized RCTs are urgently needed to translate mechanistic insights into clinical guidelines, addressing the disconnect between animal studies and human reproductive outcomes.
1 Introduction
Infertility is defined as the inability to conceive after 1 year of unprotected sexual intercourse. While male factors, such as low sperm count, poor sperm motility, or hormonal imbalances, contribute to this condition, reduced female reproductive capacity remains a critical determinant (Hosseini et al., 2024). Poor female fertility is frequently associated with clinical features such as elevated body mass index (BMI), advanced maternal age, and polycystic ovary syndrome (PCOS). For instance, PCOS is characterized by hormonal imbalances, including elevated anti-Müllerian hormone (AMH), luteinizing hormone (LH), and androgen levels, alongside reduced follicle-stimulating hormone (FSH), which impair ovulation and fertility (Zabieglo et al., 2025). Furthermore, age-related declines in ovarian reserve and BMI-related metabolic disturbances, such as increased free androgen index (FAI) and decreased sex hormone-binding globulin (SHBG), are also significant contributors to fertility challenges.
In vitro fertilization (IVF) has emerged as a leading assisted reproductive technology (ART) for overcoming infertility, offering hope to millions of couples worldwide. However, the high costs associated with IVF treatments, coupled with their emotional and psychological toll, pose significant challenges. Couples often spend an average of $61,377 out-of-pocket across 2.7 cycles to achieve a live birth, placing substantial financial and emotional strain on individuals and families (Peterson et al., 2025). This burden is further exacerbated by the uncertain outcomes of IVF procedures, which can lead to repeated cycles and additional costs. In light of these challenges, optimizing the efficacy of IVF protocols, including the use of dietary supplements, becomes even more critical to reduce the overall economic and psychological toll on patients.
Although pharmacological therapies retain their efficacy, dietary supplements, including coenzyme Q10 (CoQ10), myo-inositol, melatonin, and vitamins, have emerged as a promising adjunct in infertility management due to their potential to mitigate oxidative stress, improve hormonal balance, and enhance reproductive capacity (Vitagliano et al., 2021). However, despite accumulating evidence, inconsistencies in findings related to macromolecule and nutrient intake highlight the need for clearer mechanistic insights. For example, CoQ10 has demonstrated efficacy in improving oocyte quality and clinical pregnancy rates, particularly in the context of ovarian aging (Shang et al., 2024). Similarly, other supplements such as myo-inositol, melatonin, and vitamins have also show significant benefits in IVF outcomes. Myo-inositol, for example, enhances embryo quality and reduces unsuitable oocytes under optimal drug stimulation (Zheng et al., 2017; Wdowiak et al., 2025). Notably, myo-inositol is a key molecule in FSH signaling and oocyte maturation, and its supplementation has been shown to improve the metaphase II (MII) oocyte rate and fertilization rate, particularly in women with PCOS and non-obese PCOS (Zhang et al., 2025). Additionally, it reduces the amount of gonadotropins required for ovarian stimulation and shortens the stimulation length, especially in women with PCOS (Lagana et al., 2018). Emerging evidence also suggests that myo-inositol supplementation may improve ART outcomes in poor ovarian responders, evidenced by increased fertilization rates, elevated ovarian sensitivity index, and reduced gonadotropin demands (Mohammadi et al., 2021).
Furthermore, while serum mineral supplementation has been associated with improved oocyte quality, supports embryo development, mitigates oxidative stress, regulates hormonal balance, and optimizes IVF outcomes, while concurrently reducing miscarriage rates (Kapper et al., 2024). In patients with PCOS, vitamin D supplementation elevates ovulation and pregnancy rates, lowers androgen levels and miscarriage rates, and reduces FSH and LH concentrations, despite having no measurable impact on cleavage or fertilization rates (Yang et al., 2023). Astaxanthin, a potent antioxidant, enhances oocyte quality and reduces oxidative stress in ART procedures (Maleki-Hajiagha et al., 2024). Notably, astaxanthin improves total antioxidant capacity (TAC) in follicular fluid but exerts inconsistent effects on catalase (CAT), malondialdehyde (MDA), or superoxide dismutase (SOD) levels. While it moderately enhances oocyte and embryo quality, its impact on fertility rates remains statistically insignificant (Rodrigues et al., 2025). Oral melatonin administration during IVF cycles has been associated with increased mature oocytes yields and higher clinical pregnancy rates, although these improvements lack statistical significance (Mejlhede et al., 2021). However, melatonin supplementation during controlled ovarian stimulation, while linked to low rates of congenital abnormalities, shows no clinical benefits in terms of oocyte retrieval efficiency or miscarriage prevention (Seko et al., 2014). Mitochondrial supplementation, though safe, fails to improve oocyte competence, with studies reporting no significant changes in embryo development or fertilization rates (Ferreira et al., 2021).
Despite accumulating evidence, inconsistencies in findings related to macromolecule and nutrient intake highlight the need for clearer mechanistic insights. This review synthesizes current evidence on molecular pathways underlying infertility interventions and emphasizes priority directions for future research.
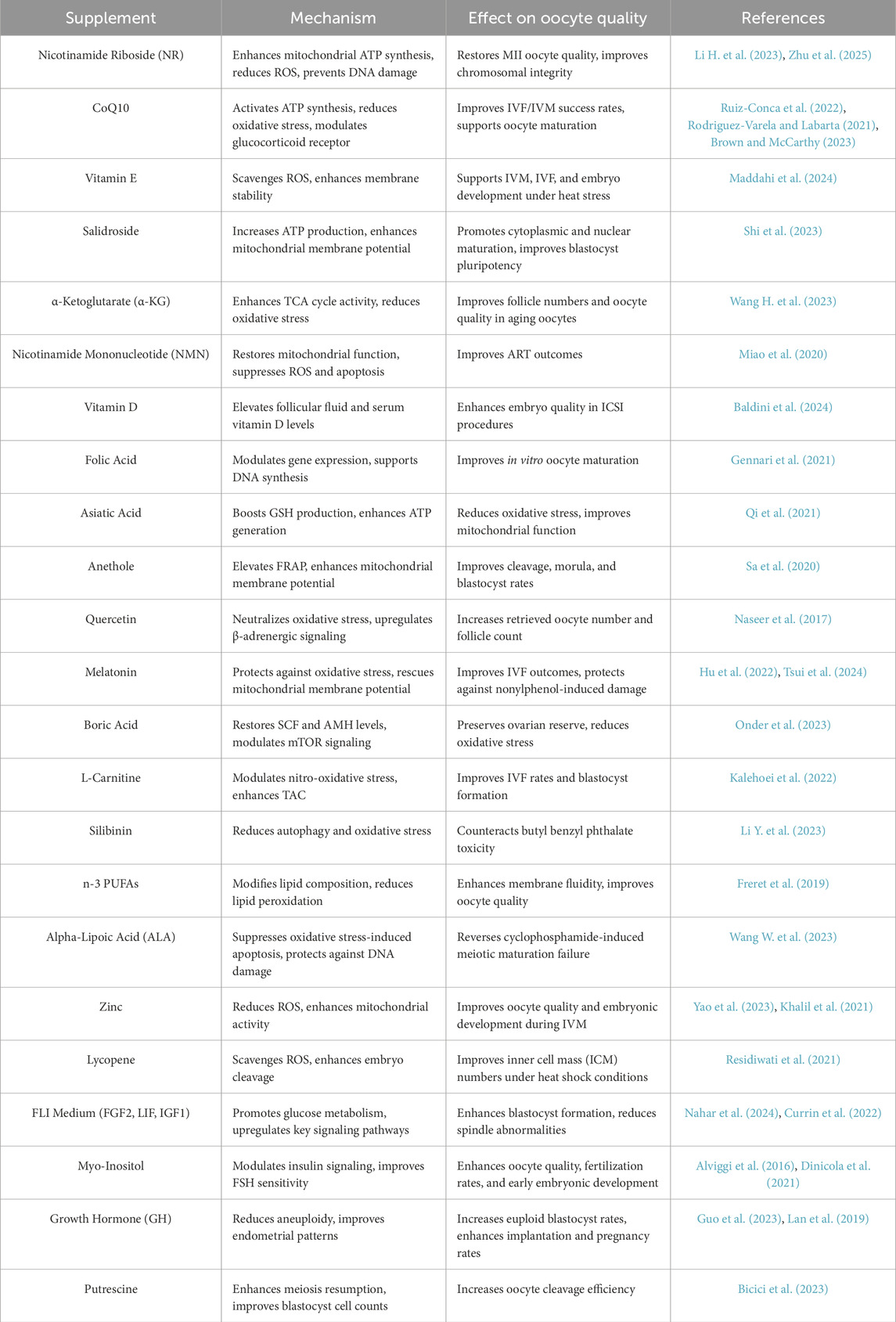
2 Mechanistic and biological pathways involving dietary supplements on oocytes
The rising rates of infertility have stimulated interest in dietary supplements to improve oocyte quality through mitochondrial function, antioxidant activity, and epigenetically regulated metabolic pathways. This section discusses the mechanisms and biological pathways of dietary supplements in improving oocyte quality, with a focus on their roles in different animal models and human studies.
2.1 Mitochondrial function
Mitochondrial dysfunction is a key factor in oocyte quality decline, and dietary supplements have shown promising effects in enhancing mitochondrial adenosine triphosphate (ATP) synthesis, reducing oxidative stress, and ultimately improving oocyte quality. Specifically, nicotinamide riboside (NR) supplementation during early embryonic development has been demonstrated to mitigate reactive oxygen species (ROS) accumulation, thereby preventing apoptosis and DNA damage in IVM mouse models. Although nicotinamide adenine dinucleotide (NAD+), a critical redox cofactor, declines in post-ovulatory oocytes, NR supplementation effectively prevents this loss, restoring metaphase II (MII) oocyte quality by maintaining chromosomal integrity and alleviating mitochondrial dysfunction, which may enhance ART success (Li H. et al., 2023). Notably, NAD + precursors also show therapeutic potential for ovarian infertility in a PCOS mouse model (Zhu et al., 2025). Furthermore, CoQ10 increases glucocorticoid receptor expression while reducing immunophilins (FK506-binding protein 5, FKBP5) and hydroxysteroid dehydrogenases (HSD11B1), thereby promoting oocyte maturation (Ruiz-Conca et al., 2022). In addition, CoQ10 activates ATP synthesis and mitigates mitochondrial ROS-induced oxidative damage (Rodriguez-Varela and Labarta, 2021). Particularly in older women, CoQ10 improves IVF/IVM success rates by restoring Krebs cycle activity, balancing ROS levels, and reducing DNA damage and oocyte apoptosis (Brown and McCarthy, 2023). Interestingly, in cattle, vitamin E has been shown to outperform CoQ10 and vitamin C in supporting IVM, IVF, and embryo development under heat stress, as evidenced by elevated trophectoderm, ICM, and blastocyst cell counts (Maddahi et al., 2024).
In addition to these supplements, salidroside has been found to reduce ROS levels and enhance intracellular glutathione (GSH) concentrations, promoting cytoplasmic maturation via increased ATP production, mitochondrial membrane potential, and mtDNA copy number. Moreover, salidroside activates MAPK phosphorylation, driving nuclear oocyte maturation and blastocyst pluripotency (Shi et al., 2023). Similarly, α-Ketoglutarate (α-KG), a TCA cycle metabolite, enhances follicle numbers and oocyte quality in aging oocytes (Wang H. et al., 2023). Nicotinamide mononucleotide (NMN) supplementation also restores mitochondrial function, suppresses ROS and apoptosis, and improves ART outcomes (Miao et al., 2020). Importantly, vitamin D supplementation has been shown to improve embryo quality in ICSI procedures by elevating follicular fluid and serum vitamin D levels (Baldini et al., 2024).
Finally, folic acid has been observed to influence in vitro oocyte maturation and gene expression patterns (Gennari et al., 2021), while asiatic acid reduces oxidative stress by boosting GSH production, ATP generation, and mitochondrial membrane potential (Qi et al., 2021). In a similar vein, anethole elevates ferric-reducing antioxidant power (FRAP), cleavage/morula/blastocyst rates, and mitochondrial membrane potential, all of which contribute to improved embryo development (Sa et al., 2020). These findings collectively underscore the significant role of dietary supplements in enhancing mitochondrial function and oocyte quality, thereby offering potential therapeutic strategies for improving ART outcomes.
2.2 Antioxidant activity
Oxidative stress, which is a major contributor to oocyte aging, is mitigated by antioxidant supplements that operate downstream of mitochondrial dysfunction by directly neutralizing oxidative damage through exogenous radical scavenging and endogenous defense potentiation, thereby enhancing cellular redox balance and preserving oocyte genomic integrity and developmental competence (Lucia Dos Santos Silva et al., 2023). For instance, in rabbit models, a quercetin-supplemented diet positively correlates with retrieved oocyte number, follicle count, and the proportion of A-grade oocytes; however, it does not significantly affect oocyte maturation (Naseer et al., 2017). Similarly, in mice, Euterpe oleracea exhibits variable effects on antioxidant pathways and cell growth by upregulating β-adrenergic signaling while downregulating apoptosis and proinflammatory signaling (Katz-Jaffe et al., 2020). Furthermore, melatonin plays a critical role in protecting against nonylphenol-induced oxidative stress and DNA damage in mice by rescuing mitochondrial membrane potential and correcting aberrant mitochondrial distribution via lysosomal regulation through Rab11 and lysosomal-associated membrane protein 2 (LAMP2) (Hu et al., 2022). Additionally, melatonin improves IVF outcomes by upregulating ATPase copper-transporting beta (ATP7B) and glutathione peroxidase 4 (GPX4) gene expression, thereby enhancing resilience against metal-induced toxicity and oxidative stress. In vitro studies using HGL5 cells, melatonin has been demonstrated to restore glycolysis, tricarboxylic acid (TCA) cycle activity, and redox balance, effectively protecting oocytes (Tsui et al., 2024).
In addition to melatonin, boric acid has been shown to preserve ovarian reserve by restoring stem cell factor (SCF) and AMH levels, downregulating sirtuin 1 (SIRT1), and upregulating mTOR signaling. Consequently, boric acid reduces CAT and SOD activity while suppressing MDA, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and neutrophil protease (NP) levels (Onder et al., 2023). Moreover, L-carnitine, when combined with bone marrow mesenchymal stem cell-conditioned medium, enhances IVF rates by modulating endometriosis-induced nitro-oxidative stress, increasing TAC, reducing NO levels, and correlating with improved blastocyst formation (Kalehoei et al., 2022). Silibinin also counteracts butyl benzyl phthalate toxicity by reducing autophagy and oxidative stress (Li Y. et al., 2023). Notably, zearalenone (ZEN) disrupts oocyte-cumulus cell interactions, delays cell cycle progression, and induces cytoskeletal abnormalities; however, these effects are mitigated by ZEN modification with hydrated sodium calcium aluminosilicate (Xu et al., 2021).
The beneficial effects of supplementing n-3 polyunsaturated fatty acids (PUFAs) on cow reproduction have been previously reported. Freret et al. confirmed that supplementation with n-3 PUFAs modify lipid composition in oocyte membranes, enhancing membrane fluidity and reducing susceptibility to lipid peroxidation (Freret et al., 2019). Alpha-lipoic acid (ALA) supplementation has been shown to reverse cyclophosphamide (CY)-induced meiotic maturation failure in oocytes. CY disrupts cytoskeletal assembly, organelle dynamics, and cortical granule/mitochondrial integrity—key indicators of cytoplasmic maturation. Remarkably, ALA suppresses oxidative stress-induced apoptosis and DNA damage, thereby protecting oocytes from CY-induced deterioration (Wang W. et al., 2023). In sheep models, zinc reduces ROS levels and enhances mitochondrial activity and GSH concentrations, thereby improving oocyte quality and embryonic development during in vitro maturation (IVM) (Yao et al., 2023). Similarly, zinc chloride (ZnCl2) and sodium selenite (Na2SeO3) promote oocyte progression to metaphase II during IVM, increasing TAC and reducing hydrogen peroxide (H2O2) and MDA levels, which correlate with upregulated antiapoptotic and antioxidative gene expression (Khalil et al., 2021). In contrast, lycopene scavenges oocyte ROS and enhances embryo cleavage under heat shock conditions, increasing inner cell mass (ICM) numbers without significantly affecting blastocyst development (Residiwati et al., 2021). These findings collectively highlight the diverse mechanisms by which antioxidants can mitigate oxidative stress and improve oocyte quality, offering promising therapeutic avenues for enhancing reproductive outcomes.
2.3 Epigenetically regulated metabolic pathways
Epigenetically regulated metabolic pathways play a pivotal role in enhancing oocyte quality and developmental competence. Notably, fibroblast growth factor 2 (FGF2), leukemia inhibitory factor (LIF), and insulin-like growth factor 1 (IGF1), collectively termed FLI medium, significantly improve mouse oocyte quality, as evidenced by enhanced blastocyst formation rates. Specifically, FLI medium promotes glucose metabolism through the pentose phosphate pathway, hexosamine biosynthesis, and glycolysis, while also upregulating transcripts of endothelial growth factor-like factors, reducing spindle abnormalities, and enhancing cumulus cell expansion. During IVM, FLI medium activates key signaling pathways, including the phosphorylation of protein kinase B (AKT), mitogen-activated protein kinase 1/3 (MAPK1/3), signal transducer and activator of transcription 3 (STAT3), and the mammalian target of rapamycin (mTOR) downstream target ribosomal protein S6 kinase B1 (RPS6KB1) (Nahar et al., 2024). Intriguingly, FLI medium, when combined with porcine follicular fluid, methionine, and cysteine supplementation, correlates with reduced polyspermic zygote formation and increased monospermic zygote formation (Currin et al., 2022). Additionally, L-carnitine modulates gene expression during embryo development (Carrillo-G et al., 2023), while alanine enhances embryonic competence by upregulating fibroblast growth factor receptor 2 (FGFR2) and POU class 5 homeobox 1 (POU5F1) mRNA levels, as well as increasing intra-oocyte GSH concentrations (Lee et al., 2019).
Other interventions further underscore the importance of metabolic regulation in oocyte development. For instance, the cathepsin B inhibitor E-64, when added to IVM medium, improves the developmental competence of ovum pick-up-derived immature oocytes (Balboula et al., 2022). Similarly, granulocyte colony-stimulating factor (G-CSF) enhances IVM of poor-quality cumulus-oocyte complexes (Cai et al., 2024). Interestingly, introducing mitochondrial DNA (mtDNA) into Sus scrofa oocytes alters DNA methylation profiles and gene expression, thereby modifying epigenetic programming during oogenesis (Okada et al., 2022). mtDNA supplementation studies reveal upregulated blastocyst-related genes, addressing mtDNA deficiency during pregnancy (Oka et al., 2023). Furthermore, myo-inositol plays a critical role in female endocrine and metabolic balance. Beyond its antioxidant activity, myo-inositol improves oocyte quality, enhances fertilization rates, and supports early embryonic development by modulating insulin signaling and FSH sensitivity (Alviggi et al., 2016). Recent evidence highlights that myo-inositol, administered with D-chiro-inositol in a physiological ratio of 40:1, which synergistically improves ovarian function and metabolic parameters in patients with PCOS (Dinicola et al., 2021). The efficacy of myo-inositol in improving IVF outcomes is further supported by clinical trials. For instance, supplementation with 1 g myo-inositol and 400 µg folic acid significantly increased mature oocytes in 133 PCOS women (Vartanyan et al., 2017), while 4 g myo-inositol and 400 µg folic acid reduced rFSH dose and cycle duration while increasing pregnancy rates in 98 infertile PCOS patients (Emekci Ozay et al., 2017).
Growth hormone (GH) supplementation has also been shown to increase euploid blastocyst rates, potentially reducing aneuploidy and improving pregnancy outcomes in cases of recurrent pregnancy loss (Guo et al., 2023). Additionally, GH lowers cycle cancellation rates, improves endometrial patterns, and enhances implantation and pregnancy rates (Lan et al., 2019). Similarly, putrescine increases meiosis resumption rates, oocyte cleavage efficiency, and blastocyst cell counts (Bicici et al., 2023). Propylene glycol alters gene expression and follicular fluid composition (Gamarra et al., 2018), while cytokine supplementation improves somatic cell nuclear transfer (SCNT) efficiency in IVF (Keim et al., 2023). Importantly, dietary interventions targeting oocyte quality are guided by follicular fluid fatty acid composition analyses, which reveal elevated levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Kermack et al., 2021). Probiotics and prebiotics modulate gut microbiota activity through amino acid metabolism, thereby mitigating metabolic syndrome and supporting female reproductive health (Dai et al., 2015). Collectively, these findings highlight the intricate interplay between epigenetic regulation, metabolic pathways, and oocyte quality, offering promising strategies to optimize reproductive outcomes Table 1.
3 Human clinical trials on dietary supplements and oocyte outcomes
In human clinical trials, dietary interventions with methyl donors during FSH stimulation in women with PCOS have demonstrated significant benefits. Specifically, these interventions reduce FSH requirements and improve implantation rates, while follicular homocysteine levels are negatively correlated with clinical pregnancy rates. These findings suggest that methyl donor-enriched diets may enhance outcomes in PCOS patients (Kucuk et al., 2023). Similarly, flaxseed oil supplementation in women with diminished ovarian reserve (DOR) has been shown to reduce recombinant human follicle-stimulating hormone (r-hFSH) dosage and stimulation duration, while increasing peak estradiol (E2), MII oocyte rate, fertilization, cleavage, and high-quality embryo rates. This indicates a positive correlation between flaxseed oil supplementation and improved MII oocyte rates in DOR patients (Chu et al., 2024). Furthermore, dehydroepiandrosterone (DHEA) supplementation in aging women has been found to increase day 3 embryo yield and top-quality day 3 embryos, as well as improve ongoing pregnancy rates and clinical pregnancy rates. These results suggest that DHEA enhances IVF outcomes in aging women, partially through the reprogramming of metabolic pathways (Li et al., 2021).
On the other hand, not all dietary interventions yield positive results. For instance, antioxidant supplementation in women with unexplained subfertility has not shown significant changes in age, BMI, basal FSH, mature MII oocyte count, or clinical pregnancy rate. This indicates that oral multivitamin/mineral antioxidants do not improve oocyte quality (Youssef et al., 2015). However, certain combination therapies have shown promise. Melatonin and myo-inositol combination therapy in patients with PCOS has demonstrated synergistic enhancement of oocyte quality and embryo quality, suggesting that this combined therapy is recommended for IVF protocols in PCOS (Pacchiarotti et al., 2016). Additionally, vitamin E and D3 co-supplementation in patients with PCOS has been found to increase clinical pregnancy and implantation rates while decreasing serum TAC. Although there is a weak association between vitamin D and implantation rate, these findings indicate that vitamins E/D3 improve IVF success via antioxidant mechanisms (Fatemi et al., 2017).
Moreover, n-3 PUFAs intervention in reproductive-age women has shown reduced omega-6/omega-3 ratio, FSH, and FSH response to GnRH, as well as decreased serum IL-1β/TNF-α in the obese subgroup. This suggests that omega-3 PUFA benefits women with diminished ovarian reserve (Al-Safi et al., 2016). In PCOS patients undergoing IVF, n-3 PUFA specifically improves oocyte maturation by inhibiting oxidative stress, clearing free radicals, and maintaining spindle/chromosome integrity (Ma et al., 2023). Furthermore, myo-inositol supplementation in poor responders undergoing ICSI has not shown differences in gonadotropin dose, oxidative stress index (OSI), or total retrieved and mature oocytes count. However, ovulation triggering improves fertilization rates and embryo quality (Nazari et al., 2020). Standardized multi-nutrient supplementation in women undergoing ICSI has been shown to improve pregnancy outcomes, indicating that multi-nutrient regimens enhance embryo developmental competence (Nouri et al., 2017). Multi-nutrient supplementation also confers positive effects on follicular output rate while reducing gonadotropin requirements (Gopinath et al., 2024). Additionally, early-onset estrogen supplementation improves the quality of retrieved immature oocytes, enhancing maturation rates during IVM cycles (Hatirnaz et al., 2021) Figure 1.
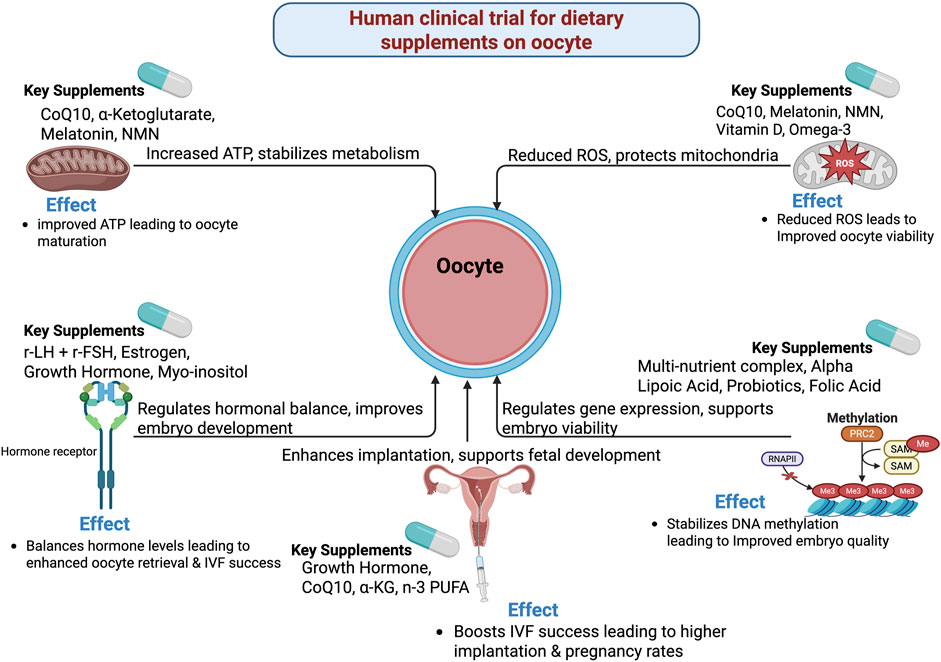
Figure 1. Mechanistic and biological pathways on human clinical trial for dietary supplements on oocyte.
4 Precision medicine in oocyte enhancement
The integration of precision medicine into reproductive health offers transformative potential for addressing female infertility, particularly through targeting the enhancement of oocyte quality. Precision medicine customizes interventions based on genetic, metabolic, and biomarker profiles, addressing the molecular heterogeneity that underlying infertility. Although the efficacy of dietary supplements remains debated, they have emerged as critical modulators of oocyte health, exerting their effects through antioxidant, mitochondrial, and hormonal pathways.
4.1 Genetic strategies
Genetic variability significantly influences ovarian reserve, oxidative stress responses, and mitochondrial function, thereby shaping individual responses to supplementation. For instance, polymorphisms in genes such as SOD or GPX4 can influence the efficacy of antioxidant therapies. Melatonin, which upregulates the expression of ATP7B and GPX4 in cumulus cells, may be particularly beneficial individuals with genetic variants that disrupt metal ion homeostasis or lipid peroxidation defenses (Tsui et al., 2024). Similarly, CoQ10’s ability to restore mitochondrial electron transport chain activity may be critical for carriers of PDSS2 or COQ6 mutations linked to age-related oocyte decline (Ben-Meir et al., 2015; Desbats et al., 2015).
In PCOS, INSR variants correlate with insulin resistance, guiding the use of myo-inositol to improve FSH signaling and glucose uptake (Unfer et al., 2017; Han et al., 2024). Genetic screening for such variants could stratify patients for personalized supplementation protocols, thereby enhancing ART outcomes.
4.2 Metabolic profiling
Metabolic dysregulation, including mitochondrial dysfunction or insulin resistance, significantly influences the efficacy of supplements. NAD + precursors such as NR can mitigate age-related NAD + depletion in oocytes, thereby restoring chromosomal integrity and mitochondrial membrane potential in murine models (Li H. et al., 2023). Women with metabolic syndrome or PCOS, often exhibiting mitochondrial inefficiency, may benefit from NMN to boost ATP production and reduce ROS (Miao et al., 2020). Lipidomic profiling of follicular fluid reveals elevated EPA and DHA correlating with improved oocyte maturation, particularly in PCOS patients (Freret et al., 2019; Ma et al., 2023). Precision lipid supplementation could thus target individuals with suboptimal fatty acid profiles. Similarly, α-KG, a TCA cycle intermediate, enhances follicle numbers in aging oocytes by restoring metabolic flux, suggesting utility in women with diminished ovarian reserve (Wang H. et al., 2023).
4.3 Biomarker-driven optimization
Biomarkers such as AMH, oxidative stress markers, and follicular fluid composition provide a means for real-time monitoring of interventions. CoQ10 supplementation has been shown to increase AMH-positive follicles and reduces oxidative DNA damage in cisplatin-treated rats, highlighting its role in preserving ovarian reserve (Ozcan et al., 2016). In humans, follicular fluid vitamin D levels correlate with embryo quality, supporting its use in vitamin D-deficient patients undergoing ICSI (Baldini et al., 2024).
Dynamic biomarkers, such as mitochondrial membrane potential or redox balance (GSH/GSSG ratio), could guide dose adjustments. For example, melatonin’s restoration of glycolysis and TCA cycle activity in HGL5 granulosa cells suggests that women with metabolic dysregulation in cumulus-oocyte complexes may require higher doses (Tsui et al., 2024). Similarly, elevated FDX1 levels linked to mitochondrial efficiency could identify candidates for CoQ10 or ubiquinol supplementation (Serrano et al., 2022).
4.4 Challenges and future directions
Although significant progress has been made, key challenges remain, including genetic heterogeneity such as MTHFR variants complicating folate dosing, limited clinical validation of oxidative stress biomarkers for infertility, and reliance on animal models, highlighting the need for human RCTs with stratified cohorts (Vitagliano et al., 2021; Woodward et al., 2022). Emerging tools such as multi-omics (genomics, metabolomics) and AI-driven predictive models offer solutions for instance, integrating genomic data with follicular fluid metabolomics to predict antioxidant responses (astaxanthin and vitamin E) (Maddahi et al., 2024; Tripathi et al., 2023). Precision medicine is reshaping oocyte enhancement by aligning interventions like melatonin and CoQ10 (targeting mitochondrial dysfunction and oxidative stress in age-related decline) or myo-inositol and omega-3 PUFAs (addressing PCOS-specific dysregulation) with individual genetic, metabolic, and biomarker profiles. To realize this potential, future research must prioritize human trials, biomarker validation, and integrative omics approaches, advancing fertility care from empirical practices to evidence-based precision therapeutics.
5 Limitations of current evidence and recommendations for future research
The following section discusses the key limitations of existing studies on dietary interventions and oocyte quality, as well as actionable recommendations to address these gaps and advance future research. While the cited articles provide valuable insights, their mechanistic and clinical heterogeneities highlight the need for a more standardized and pathway-centric approach in this field.
5.1 Limitations
5.1.1 Mechanistic gaps
One of the primary obstacles in optimizing dietary interventions for oocyte quality is the lack of clarity regarding nutrient-specific pathways. For instance, while quercetin has been linked to improved IVF oocyte retrieval outcomes, its apparent negative impact on embryo quality remains mechanistically unclear, which complicates its clinical application (Baltazar et al., 2018). Similarly, cysteamine’s dual role in upregulating molecular markers during cumulus-oocyte complex (COC) maturation while downregulating embryonic development-related genes highlights a knowledge gap in its gene regulatory networks (Doroftei et al., 2024). The role of α-KG in oocyte quality and stem cell dynamics despite its documented influence on differentiation and reprogramming lacks clarity, particularly in how these processes directly translate to reproductive performance (Karayiannis et al., 2018). Oxidative stress interventions also face mechanistic ambiguities: GSH only partially rescues ovarian follicle loss, suggesting incomplete understanding of redox balance in follicular survival (Hohos et al., 2020), while melatonin’s ability to ameliorate age-related meiotic defects via the SIRT2-dependent H4K16 deacetylation pathway requires deeper exploration to define its broader applicability (Wu et al., 2024). Metabolic interactions further exemplify mechanistic uncertainties. High-energy diets, though promoting follicular growth through elevated insulin, paradoxically impair oocyte quality, indicating unresolved links between metabolic signaling and oocyte competence (Li Z. et al., 2023). Similarly, fatty acid supplementation in bovine models fails to modify lipid metabolism or developmental capacity, underscoring a disconnect between fatty acid’s theoretical benefits and practical outcomes (Gardinal et al., 2018).
5.1.2 Clinical heterogeneity
Clinical translation is hindered by variability in diagnostic and dosing frameworks. The absence of standardized criteria for ovarian dysfunction and “poor responder” classification limits generalizability across studies, complicating the identification of target populations (Chantrasiri et al., 2025). Dosing inconsistencies further exacerbate reproducibility challenges: melatonin’s broad dosage range (2–18 mg/day) and unclear optimal thresholds for selenium in female reproductive health reflect a lack of precision in therapeutic protocols (Genario et al., 2019; Chowdhury et al., 2021). Variable supplement durations, as seen in bovine studies where prepartum whole raw soybean supplementation showed no effect on oocyte quality, highlight the need for standardized timelines (Tomita et al., 2023). Species-specific differences also limit translational relevance. For example, melatonin’s integration into bovine breeding management faces practical challenges despite its theoretical benefits, and rodent-derived data on high-fat diets or fatty acid supplementation poorly predict human responses, emphasizing the need for human-centric models (Piscopo et al., 2025). Even promising interventions, like sunflower seed supplementation or crude protein’s adverse effects on embryo development, lack consistency across species, underscoring the risks of extrapolating animal data to clinical practice (Plante-Dube et al., 2021; Lim et al., 2020).
5.2 Recommendations
5.2.1 Pathway-centric trials
Mitochondrial and Redox Pathways: Targeted interventions aimed at enhancing mitochondrial function and redox balance are critical for improving oocyte quality. The evaluation of compounds such as CoQ10, DHEA, and Cleo-20 T3 could potentially increase the levels of ferredoxin 1 (FDX1), a key regulator of mitochondrial TCA cycle activity and electron transport chain efficiency, thereby mitigating lipid peroxidation and apoptosis in oocytes (Serrano et al., 2022). FDX1’s dual role in energy metabolism and oxidative stress reduction positions it as a therapeutic target for age-related declines in oocyte competence, particularly in IVF settings where mitochondrial dysfunction is prevalent.
5.2.1.1 Kinase signaling
Evaluating kinase-driven pathways offers opportunities to address metabolic and maturation defects in oocytes. For instance, combining myo-inositol with folic acid in PCOS patients modulates extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation, reducing phosphorylated AKT levels and improving insulin sensitivity, a key factor in PCOS-associated infertility (Unfer et al., 2017). Similarly, FLI medium in follicular fluid enhances oocyte meiotic maturation by activating MAPK pathways, which regulate cytoskeletal dynamics and developmental competence (Thongkittidilok et al., 2022). Prioritizing trials on kinase signaling could refine therapies for conditions like PCOS and poor ovarian response.
5.2.1.2 Antioxidant synergy
Optimizing antioxidant combinations, such as chlorogenic acid, curcumin, and β-mercaptoethanol, may amplify protection against oxidative damage during embryo development. While individual antioxidants show partial efficacy, synergistic formulations could enhance blastocyst formation rates by targeting multiple redox pathways simultaneously (Anchordoquy et al., 2022). This approach is particularly relevant for patients with elevated oxidative stress, such as those with advanced maternal age or metabolic disorders.
5.2.2 Standardized dosing
5.2.2.1 Reproductive protocols
Defining optimal dosages for supplements like melatonin (2–18 mg/day) is essential to balance efficacy and safety, especially regarding gestational and neonatal outcomes (Chowdhury et al., 2021). Trace minerals (Cu, Se, Mn, Zn) during in vitro maturation (IVM) improve embryo quality, but standardized thresholds are needed to avoid toxicity and ensure reproducibility (Tabatabaie et al., 2022). Similarly, omega-3 PUFAs require dose-dependent studies to validate their role in restoring ovarian gene expression disrupted by high-fat diets (Li et al., 2020).
5.2.2.2 Dietary interventions
Standardizing components of the Mediterranean diet rich in antioxidants, monounsaturated fats, and polyphenols could improve IVF pregnancy rates by reducing inflammation and oxidative stress (Machado et al., 2020). Concurrently, validating peripartum oleic acid dosing is critical to support follicular and oocyte health during metabolic stressors like negative energy balance (Mintziori et al., 2020).
5.2.2.3 Toxicity thresholds
Establishing safe upper limits for supplements is vital. For example, crude protein over-supplementation may impair embryo development, necessitating guidelines to avoid unintended harm (Lim et al., 2020). Similarly, while niacin reduces oxidative stress, its optimal dosing for ovarian follicle rescue without adverse effects remains undefined (Almubarak et al., 2021). Prioritizing pathway-centric trials and standardized dosing frameworks will bridge mechanistic knowledge gaps and clinical inconsistencies, enabling precision-based strategies to enhance oocyte quality and fertility outcomes.
6 Conclusion
Dietary supplements and nutritional interventions influence oocyte quality through diverse mechanisms across species, targeting oxidative stress, mitochondrial function, and metabolic pathways. Key findings include the benefits of antioxidants in reducing ROS and improving IVF outcomes, the role of myo-inositol and n-3 PUFAs in enhancing oocyte quality in patients with PCOS, and the ability of CoQ10 and α-KG to counteract age-related oocyte decline. Preclinical studies highlight promising compounds such as quercetin, SDF-1, and lycopene, which improve oocyte retrieval, cytoplasmic maturation, and stress resilience in rodents and bovines. However, several limitations hinder translational progress, including inconsistent dosages, mixed outcomes, and a lack of standardized protocols. High-energy diets and crude protein supplementations may impair oocyte quality, while clinical heterogeneity and species-specific responses further complicate the application of preclinical findings. TO address these challenges, future research should prioritize human-centric studies, standardized dosing frameworks, and pathway-centric trials targeting mitochondrial function and antioxidant synergy. Establishing evidence-based protocols and focusing on clinical reproducibility will be essential to translate these insights into reliable therapies for infertility. In summary, while dietary supplements offer significant potential to improve oocyte quality, a more systematic and standardized approach is urgently needed to bridge the gap between preclinical discoveries and clinical applications.
Author contributions
HC: Writing – original draft. SW: Writing – original draft. MS: Writing – original draft. DY: Writing – review and editing. HL: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Figure was Created in https://BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almubarak, A. M., Kim, E., Yu, I. J., and Jeon, Y. (2021). Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 169, 36–46. doi:10.1016/j.theriogenology.2021.04.005
Al-Safi, Z. A., Liu, H., Carlson, N. E., Chosich, J., Harris, M., Bradford, A. P., et al. (2016). Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J. Clin. Endocrinol. Metab. 101 (1), 324–333. doi:10.1210/jc.2015-2913
Alviggi, C., Cariati, F., Conforti, A., De Rosa, P., Vallone, R., Strina, I., et al. (2016). The effect of FT500 Plus(®) on ovarian stimulation in PCOS women. Reprod. Toxicol. 59, 40–44. doi:10.1016/j.reprotox.2015.10.014
Anchordoquy, J. P., Balbi, M., Farnetano, N. A., Fabra, M. C., Carranza-Martin, A. C., Nikoloff, N., et al. (2022). Trace mineral mixture supplemented to in vitro maturation medium improves subsequent embryo development and embryo quality in cattle. Vet. Res. Commun. 46 (4), 1111–1119. doi:10.1007/s11259-022-09982-9
Balboula, A. Z., Aboelenain, M., Sakatani, M., Yamanaka, K. I., Bai, H., Shirozu, T., et al. (2022). Effect of E-64 supplementation during in vitro maturation on the developmental competence of bovine OPU-derived oocytes. Genes (Basel) 13 (2), 324. doi:10.3390/genes13020324
Baldini, G. M., Russo, M., Proietti, S., Forte, G., Baldini, D., and Trojano, G. (2024). Supplementation with vitamin D improves the embryo quality in in vitro fertilization (IVF) programs, independently of the patients' basal vitamin D status. Arch. Gynecol. Obstet. 309 (6), 2881–2890. doi:10.1007/s00404-024-07473-7
Baltazar, A. L., de Mattos, G. M., Ropelli, B. M., Firetti, S., Castilho, C., Pugliesi, G., et al. (2018). Supplementation with sunflower seeds in beef cattle did not impact on oocyte and in vitro embryo production. Reprod. Domest. Anim. 53 (3), 801–808. doi:10.1111/rda.13173
Ben-Meir, A., Burstein, E., Borrego-Alvarez, A., Chong, J., Wong, E., Yavorska, T., et al. (2015). Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 14 (5), 887–895. doi:10.1111/acel.12368
Bicici, E., Satilmis, F., Bodu, M., Demirel, M. A., Karakas Alkan, K., and Alkan, H. (2023). Effect of putrescine supplementation to in vitro maturation medium on embryo development and quality in cattle. Anim. Biotechnol. 34 (8), 3887–3896. doi:10.1080/10495398.2023.2236660
Brown, A. M., and McCarthy, H. E. (2023). The Effect of CoQ10 supplementation on ART treatment and oocyte quality in older women. Hum. Fertil. (Camb) 26 (6), 1544–1552. doi:10.1080/14647273.2023.2194554
Cai, L., Jeong, Y. W., Hwang, W. S., and Hyun, S. H. (2024). Optimization of human recombinant granulocyte-colony stimulating factor supplementation during in vitro production of porcine embryos to improve the efficiency of resource utilization of poor-quality cumulus-oocyte complexes. Theriogenology 216, 93–102. doi:10.1016/j.theriogenology.2023.12.017
Carrillo-Gonzalez, D. F., Hernandez-Herrera, D. Y., and Maldonado-Estrada, J. G. (2023). The role of L-carnitine in bovine embryo metabolism. A review of the effect of supplementation with a metabolic modulator on in vitro embryo production. Anim. Biotechnol. 34 (2), 413–423. doi:10.1080/10495398.2021.1938593
Chantrasiri, R., Somboonchai, P., Piromlertamorn, W., Pantasri, T., and Sanmee, U. (2025). Effects of dietary quercetin on retrieved mouse oocytes and in vitro fertilization outcomes. JBRA Assist. Reprod. 29 (1), 16–20. doi:10.5935/1518-0557.20240073
Chowdhury, M. M. R., Park, J., Afrin, F., Ko, Y. G., Kim, C. L., Lee, S. S., et al. (2021). Transcriptome profiling of in vitro-matured oocytes from a Korean native cow (hanwoo) after cysteamine supplementation. Anim. Biotechnol. 32 (4), 401–412. doi:10.1080/10495398.2019.1706545
Chu, Q., Yu, Y. X., Zhang, J. Z., Zhang, Y. T., and Yu, J. P. (2024). Effects of flaxseed oil supplementation on metaphase II oocyte rates in IVF cycles with decreased ovarian reserve: a randomized controlled trial. Front. Endocrinol. (Lausanne) 15, 1280760. doi:10.3389/fendo.2024.1280760
Currin, L., Glanzner, W. G., Gutierrez, K., de Macedo, M. P., Guay, V., Baldassarre, H., et al. (2022). Optimizing swine in vitro embryo production with growth factor and antioxidant supplementation during oocyte maturation. Theriogenology 194, 133–143. doi:10.1016/j.theriogenology.2022.10.005
Dai, Z., Wu, Z., Hang, S., Zhu, W., and Wu, G. (2015). Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 21 (5), 389–409. doi:10.1093/molehr/gav003
Desbats, M. A., Lunardi, G., Doimo, M., Trevisson, E., and Salviati, L. (2015). Genetic bases and clinical manifestations of coenzyme Q10 (CoQ10) deficiency. J. Inherit. Metabolic Dis. 38 (1), 145–156. doi:10.1007/s10545-014-9749-9
Dinicola, S., Unfer, V., Facchinetti, F., Soulage, C. O., Greene, N. D., Bizzarri, M., et al. (2021). Inositols: from established knowledge to novel approaches. Int. J. Mol. Sci. 22 (19), 10575. doi:10.3390/ijms221910575
Doroftei, B., Ilie, O. D., Timofeiov, S., Dabuleanu, A. M., Scripcariu, I. S., Micu, R., et al. (2024). A scoping review regarding reproductive capacity modulation based on alpha-ketoglutarate supplementation. Reproduction 168 (5), e240137. doi:10.1530/REP-24-0137
Emekci Ozay, O., Ozay, A. C., Cagliyan, E., Okyay, R. E., and Gulekli, B. (2017). Myo-inositol administration positively effects ovulation induction and intrauterine insemination in patients with polycystic ovary syndrome: a prospective, controlled, randomized trial. Gynecol. Endocrinol. 33 (7), 524–528. doi:10.1080/09513590.2017.1296127
Fatemi, F., Mohammadzadeh, A., Sadeghi, M. R., Akhondi, M. M., Mohammadmoradi, S., Kamali, K., et al. (2017). Role of vitamin E and D(3) supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: a double blinded randomized placebo-controlled trial. Clin. Nutr. ESPEN 18, 23–30. doi:10.1016/j.clnesp.2017.01.002
Ferreira, A. F., Soares, M., Almeida Reis, S., Ramalho-Santos, J., Sousa, A. P., and Almeida-Santos, T. (2021). Does supplementation with mitochondria improve oocyte competence? A systematic review. Reproduction 161 (3), 269–287. doi:10.1530/REP-20-0351
Freret, S., Oseikria, M., Bourhis, D. L., Desmarchais, A., Briant, E., Desnoes, O., et al. (2019). Effects of a n-3 polyunsaturated fatty acid-enriched diet on embryo production in dairy cows. Reproduction 158 (1), 71–83. doi:10.1530/REP-18-0644
Gamarra, G., Ponsart, C., Lacaze, S., Nuttinck, F., Cordova, A., Mermillod, P., et al. (2018). Oral propylene glycol modifies follicular fluid and gene expression profiles in cumulus-oocyte complexes and embryos in feed-restricted heifers. Reprod. Fertil. Dev. 30 (3), 417–429. doi:10.1071/RD17037
Gardinal, R., Calomeni, G. D., Zanferari, F., Vendramini, T. H. A., Takiya, C. S., Del Valle, T. A., et al. (2018). Different durations of whole raw soybean supplementation during the prepartum period: milk fatty acid profile and oocyte and embryo quality of early-lactating Holstein cows. J. Dairy Sci. 101 (1), 675–689. doi:10.3168/jds.2016-12504
Genario, R., Morello, E., Bueno, A. A., and Santos, H. O. (2019). The usefulness of melatonin in the field of obstetrics and gynecology. Pharmacol. Res. 147, 104337. doi:10.1016/j.phrs.2019.104337
Gennari, V. C., Credendio, E. M., Fernandes, A., Vila, R. A., Libardi Miranda Furtado, C., Silveira Ramos, E., et al. (2021). Folic acid supplementation during oocytes maturation influences in vitro production and gene expression of bovine embryos. Zygote 29 (5), 342–349. doi:10.1017/S0967199421000022
Gopinath, M., Khadijah, I. S., Ruhaima, R., Nuguelis, R., and Mukhri, H. (2024). The impact of oral multinutrient supplementation on in vitro fertilisation or intracytoplasmic sperm injection outcomes: a prospective controlled study. Med. J. Malays. 79 (6), 715–720.
Guo, Q., Liu, P., Zhou, W., Xia, M., Li, J., Lu, J., et al. (2023). Growth hormone supplementation ameliorates blastocyst euploidy rates and improves pregnancy outcomes in women undergoing preimplantation genetic testing for aneuploidy cycles. Front. Endocrinol. (Lausanne) 14, 1117706. doi:10.3389/fendo.2023.1117706
Han, Y., Hou, Y., Han, Q., Yuan, X., and Chen, L. (2024). Dietary supplements in polycystic ovary syndrome-current evidence. Front. Endocrinol. (Lausanne) 15, 1456571. doi:10.3389/fendo.2024.1456571
Hatirnaz, E., Hatirnaz, S., Kanat-Pektas, M., Dokuzeylul Gungor, N., Erol, O., Kalyoncu, S., et al. (2021). The impact of timing for estrogen supplementation in polycystic ovary syndrome patients undergoing primed in vitro maturation. J. Obstet. Gynaecol. Res. 47 (8), 2684–2691. doi:10.1111/jog.14858
Hohos, N. M., Elliott, E. M., Cho, K. J., Lin, I. S., Rudolph, M. C., and Skaznik-Wikiel, M. E. (2020). High-fat diet-induced dysregulation of ovarian gene expression is restored with chronic omega-3 fatty acid supplementation. Mol. Cell Endocrinol. 499, 110615. doi:10.1016/j.mce.2019.110615
Hosseini, M., Khalafiyan, A., Zare, M., Karimzadeh, H., Bahrami, B., Hammami, B., et al. (2024). Sperm epigenetics and male infertility: unraveling the molecular puzzle. Hum. Genomics 18 (1), 57. doi:10.1186/s40246-024-00626-4
Hu, L. L., Li, H. G., Li, X. M., Xu, Y., Pang, Y. Q., Wang, B., et al. (2022). Nonylphenol exposure-induced oocyte quality deterioration could be reversed by melatonin supplementation in mice. Environ. Pollut. 305, 119317. doi:10.1016/j.envpol.2022.119317
Kalehoei, E., Moradi, M., Azadbakht, M., Zhaleh, H., Parvini, M., Cheraghbaeigi, S., et al. (2022). In vitro maturation medium supplementation: utilization of repaglinide, L-carnitine, and mesenchymal stem cell-conditioned medium to improve developmental competence of oocytes derived from endometriosis mouse models. Braz J. Med. Biol. Res. 55, e11948. doi:10.1590/1414-431X2022e11948
Kapper, C., Stelzl, P., Oppelt, P., Ganhor, C., Gyunesh, A. A., Arbeithuber, B., et al. (2024). The impact of minerals on female fertility: a systematic review. Nutrients 16 (23), 4068. doi:10.3390/nu16234068
Karayiannis, D., Kontogianni, M. D., Mendorou, C., Mastrominas, M., and Yiannakouris, N. (2018). Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 33 (3), 494–502. doi:10.1093/humrep/dey003
Katz-Jaffe, M. G., Lane, S. L., Parks, J. C., McCallie, B. R., Makloski, R., and Schoolcraft, W. B. (2020). Antioxidant intervention attenuates aging-related changes in the murine ovary and oocyte. Life (Basel) 10 (11), 250. doi:10.3390/life10110250
Keim, J., Liu, Y., Regouski, M., Stott, R., Singina, G. N., White, K. L., et al. (2023). Cytokine supplemented maturation medium improved development to term following somatic cell nuclear transfer (SCNT) in cattle. Reprod. Fertil. Dev. 35 (11), 575–588. doi:10.1071/RD23011
Kermack, A. J., Wellstead, S. J., Fisk, H. L., Cheong, Y., Houghton, F. D., Macklon, N. S., et al. (2021). The fatty acid composition of human follicular fluid is altered by a 6-week dietary intervention that includes marine omega-3 fatty acids. Lipids 56 (2), 201–209. doi:10.1002/lipd.12288
Khalil, W. A., Yang, C. Y., El-Moghazy, M. M., El-Rais, M. S., Shang, J. H., and El-Sayed, A. (2021). Effect of zinc chloride and sodium selenite supplementation on in vitro maturation, oxidative biomarkers, and gene expression in buffalo (Bubalus bubalis) oocytes. Zygote 29 (5), 393–400. doi:10.1017/S0967199421000162
Kucuk, T., Horozal, P. E., Karakulak, A., Timucin, E., and Dattilo, M. (2023). Follicular homocysteine as a marker of oocyte quality in PCOS and the role of micronutrients. J. Assist. Reprod. Genet. 40 (8), 1933–1941. doi:10.1007/s10815-023-02847-3
Lagana, A. S., Vitagliano, A., Noventa, M., Ambrosini, G., and D'Anna, R. (2018). Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: a systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 298 (4), 675–684. doi:10.1007/s00404-018-4861-y
Lan, K. C., Lin, P. Y., Chang, Y. C., Chen, Y. J., Tsai, Y. R., Ismaeil Mohamed, I. S., et al. (2019). Growth hormone supplementation may improve the pregnancy rate and endometrial receptivity among women aged more than 40 years undergoing in vitro fertilization. Biomed. J. 42 (6), 411–416. doi:10.1016/j.bj.2019.05.003
Lee, Y., Shim, J., Ko, N., Kim, H. J., Park, J. K., Kwak, K., et al. (2019). Effect of alanine supplementation during in vitro maturation on oocyte maturation and embryonic development after parthenogenesis and somatic cell nuclear transfer in pigs. Theriogenology 127, 80–87. doi:10.1016/j.theriogenology.2019.01.001
Li, C., He, X., Huang, Z., Han, L., Wu, X., Li, L., et al. (2020). Melatonin ameliorates the advanced maternal age-associated meiotic defects in oocytes through the SIRT2-dependent H4K16 deacetylation pathway. Aging (Albany NY) 12 (2), 1610–1623. doi:10.18632/aging.102703
Li, C. J., Lin, L. T., and Tsui, K. H. (2021). Dehydroepiandrosterone shifts energy metabolism to increase mitochondrial biogenesis in female fertility with advancing age. Nutrients 13 (7), 2449. doi:10.3390/nu13072449
Li, H., Wang, H., Xu, J., Zeng, X., Sun, Y., and Yang, Q. (2023). Nicotinamide riboside supplementation ameliorated post-ovulatory oocyte quality decline. Reproduction 165 (1), 103–111. doi:10.1530/REP-22-0095
Li, Y., Xiong, B., Miao, Y., and Gao, Q. (2023). Silibinin supplementation ameliorates the toxic effects of butyl benzyl phthalate on porcine oocytes by eliminating oxidative stress and autophagy. Environ. Pollut. 329, 121734. doi:10.1016/j.envpol.2023.121734
Li, Z., Zhang, K., Zhou, Y., Zhao, J., Wang, J., and Lu, W. (2023). Role of melatonin in bovine reproductive biotechnology. Molecules 28 (13), 4940. doi:10.3390/molecules28134940
Lim, J., Ali, S., Liao, L. S., Nguyen, E. S., Ortiz, L., Reshel, S., et al. (2020). Antioxidant supplementation partially rescues accelerated ovarian follicle loss, but not oocyte quality, of glutathione-deficient mice†. Biol. Reprod. 102 (5), 1065–1079. doi:10.1093/biolre/ioaa009
Lucia Dos Santos Silva, R., de Sousa Barberino, R., and Tavares de Matos, M. H. (2023). Impact of antioxidant supplementation during in vitro culture of ovarian preantral follicles: a review. Theriogenology 207, 110–122. doi:10.1016/j.theriogenology.2023.05.027
Ma, R., Wang, S., Xue, M., Zhang, H., He, Z., Jueraitetibaike, K., et al. (2023). Effects of n-3 PUFA supplementation on oocyte in vitro maturation in mice with polycystic ovary syndrome. J. Ovarian Res. 16 (1), 87. doi:10.1186/s13048-023-01162-w
Machado, A. F., Guimaraes, S. E. F., Guimaraes, J. D., Santos, G. M., Silva, A. L., Silva, Y., et al. (2020). Effect of protein supplement level on the productive and reproductive parameters of replacement heifers managed in intensive grazing systems. PLoS One 15 (10), e0239786. doi:10.1371/journal.pone.0239786
Maddahi, A., Saberivand, A., Hamali, H., Jafarpour, F., and Saberivand, M. (2024). Exploring the impact of heat stress on oocyte maturation and embryo development in dairy cattle using a culture medium supplemented with vitamins E, C, and coenzyme Q10. J. Therm. Biol. 119, 103759. doi:10.1016/j.jtherbio.2023.103759
Maleki-Hajiagha, A., Shafie, A., Maajani, K., and Amidi, F. (2024). Effect of astaxanthin supplementation on female fertility and reproductive outcomes: a systematic review and meta-analysis of clinical and animal studies. J. Ovarian Res. 17 (1), 163. doi:10.1186/s13048-024-01472-7
Mejlhede, M. A. B., Jepsen, J. B., and Knudsen, U. B. (2021). Oral melatonin supplementation during in vitro fertilization treatment: a systematic PRISMA review and meta-analysis of randomized controlled trials. Gynecol. Endocrinol. 37 (12), 1079–1085. doi:10.1080/09513590.2021.1974378
Miao, Y., Cui, Z., Gao, Q., Rui, R., and Xiong, B. (2020). Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 32 (5), 107987. doi:10.1016/j.celrep.2020.107987
Mintziori, G., Mousiolis, A., Duntas, L. H., and Goulis, D. G. (2020). Evidence for a manifold role of selenium in infertility. Horm. (Athens) 19 (1), 55–59. doi:10.1007/s42000-019-00140-6
Mohammadi, S., Eini, F., Bazarganipour, F., Taghavi, S. A., and Kutenaee, M. A. (2021). The effect of Myo-inositol on fertility rates in poor ovarian responder in women undergoing assisted reproductive technique: a randomized clinical trial. Reprod. Biol. Endocrinol. 19 (1), 61. doi:10.1186/s12958-021-00741-0
Nahar, A., Becker, J., Pasquariello, R., Herrick, J., Rogers, H., Zhang, M., et al. (2024). FGF2, LIF, and IGF-1 supplementation improves mouse oocyte in vitro maturation via increased glucose metabolism†. Biol. Reprod. 110 (4), 672–683. doi:10.1093/biolre/ioae014
Naseer, Z., Ahmad, E., Epikmen, E. T., Ucan, U., Boyacioglu, M., Ipek, E., et al. (2017). Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 96, 136–141. doi:10.1016/j.theriogenology.2017.03.029
Nazari, L., Salehpour, S., Hosseini, S., Saharkhiz, N., Azizi, E., Hashemi, T., et al. (2020). Effect of myo-inositol supplementation on ICSI outcomes among poor ovarian responder patients: a randomized controlled trial. J. Gynecol. Obstet. Hum. Reprod. 49 (5), 101698. doi:10.1016/j.jogoh.2020.101698
Nouri, K., Walch, K., Weghofer, A., Imhof, M., Egarter, C., and Ott, J. (2017). The impact of a standardized oral multinutrient supplementation on embryo quality in in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized trial. Gynecol. Obstet. Invest 82 (1), 8–14. doi:10.1159/000452662
Okada, T., McIlfatrick, S., Hin, N., Aryamanesh, N., Breen, J., and St John, J. C. (2022). Mitochondrial supplementation of Sus scrofa metaphase II oocytes alters DNA methylation and gene expression profiles of blastocysts. Epigenetics Chromatin 15 (1), 12. doi:10.1186/s13072-022-00442-x
Okada, T., McIlfatrick, S., and St John, J. C. (2023). Mitochondrial DNA deficiency and supplementation in Sus scrofa oocytes influence transcriptome profiles in oocytes and blastocysts. Int. J. Mol. Sci. 24 (4), 3783. doi:10.3390/ijms24043783
Onder, G. O., Goktepe, O., Karaman, E., Karakas, E., Mat, O. C., Bolat, D., et al. (2023). Nonylphenol exposure-induced oocyte quality deterioration could be reversed by boric acid supplementation in rats. Biol. Trace Elem. Res. 201 (9), 4518–4529. doi:10.1007/s12011-023-03657-5
Ozcan, P., Ficicioglu, C., Kizilkale, O., Yesiladali, M., Tok, O. E., Ozkan, F., et al. (2016). Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J. Assist. Reprod. Genet. 33 (9), 1223–1230. doi:10.1007/s10815-016-0751-z
Pacchiarotti, A., Carlomagno, G., Antonini, G., and Pacchiarotti, A. (2016). Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 32 (1), 69–73. doi:10.3109/09513590.2015.1101444
Peterson, S. K., Jennings Mayo-Wilson, L., Spigel, L., Morgan, I., and Parker, A. (2025). Health care experiences of individuals accessing or undergoing in vitro fertilization (IVF) in the U.S.: a narrative review of qualitative studies. Front. Reprod. Health 7, 1490917. doi:10.3389/frph.2025.1490917
Piscopo, F., Gasparrini, B., van Halderen, R., Brouwers, J. F., van den Broek, J., van Tol, H. T. A., et al. (2025). Periparturient oleic acid-rich fat supplementation affects the lipid profile in blood and results in an increased oocyte yield in postpartum dairy cows. Theriogenology 236, 33–44. doi:10.1016/j.theriogenology.2025.01.018
Plante-Dube, M., Picard, C., Gilbert, I., Robert, C., Fievez, V., Vlaeminck, B., et al. (2021). Effects of a dietary supplement enriched in palmitoleic acid on fatty acid composition of follicular fluid, granulosa cell metabolism, and oocyte developmental capacity in early lactation dairy cows. J. Dairy Sci. 104 (3), 3693–3706. doi:10.3168/jds.2020-19191
Qi, J. J., Li, X. X., Zhang, Y., Diao, Y. F., Hu, W. Y., Wang, D. L., et al. (2021). Supplementation with asiatic acid during in vitro maturation improves porcine oocyte developmental competence by regulating oxidative stress. Theriogenology 172, 169–177. doi:10.1016/j.theriogenology.2021.06.013
Residiwati, G., Azari-Dolatabad, N., Tuska, H. S. A., Sidi, S., Van Damme, P., Benedetti, C., et al. (2021). Effect of lycopene supplementation to bovine oocytes exposed to heat shock during in vitro maturation. Theriogenology 173, 48–55. doi:10.1016/j.theriogenology.2021.07.014
Rodrigues, V. D., Boaro, B. L., Laurindo, L. F., Chagas, E. F. B., de Lima, E. P., Laurindo, L. F., et al. (2025). Exploring the benefits of astaxanthin as a functional food ingredient: its effects on oxidative stress and reproductive outcomes in women with PCOS - a systematic review and single-arm meta-analysis of randomized clinical trials. Naunyn Schmiedeb. Arch. Pharmacol. 398 (2), 1155–1169. doi:10.1007/s00210-024-03432-w
Rodriguez-Varela, C., and Labarta, E. (2021). Does coenzyme Q10 supplementation improve human oocyte quality? Int. J. Mol. Sci. 22 (17), 9541. doi:10.3390/ijms22179541
Ruiz-Conca, M., Gardela, J., Mogas, T., Lopez-Bejar, M., and Alvarez-Rodriguez, M. (2022). Apoptosis and glucocorticoid-related genes mRNA expression is modulated by coenzyme Q10 supplementation during in vitro maturation and vitrification of bovine oocytes and cumulus cells. Theriogenology 192, 62–72. doi:10.1016/j.theriogenology.2022.08.030
Sa, N. A. R., Vieira, L. A., Ferreira, A. C. A., Cadenas, J., Bruno, J. B., Maside, C., et al. (2020). Anethole supplementation during oocyte maturation improves in vitro production of bovine embryos. Reprod. Sci. 27 (8), 1602–1608. doi:10.1007/s43032-020-00190-x
Seko, L. M., Moroni, R. M., Leitao, V. M., Teixeira, D. M., Nastri, C. O., and Martins, W. P. (2014). Melatonin supplementation during controlled ovarian stimulation for women undergoing assisted reproductive technology: systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 101 (1), 154–161.e4. doi:10.1016/j.fertnstert.2013.09.036
Serrano, A. M., Silvestri, G., Kiazim, L. G., Vining, L. M., Zak, L. J., Walling, G. A., et al. (2022). Supplementation of porcine in vitro maturation medium with FGF2, LIF, and IGF1 enhances cytoplasmic maturation in prepubertal gilts oocytes and improves embryo quality. Zygote 30 (6), 801–808. doi:10.1017/S0967199422000284
Shang, Y., Song, N., He, R., and Wu, M. (2024). Antioxidants and fertility in women with ovarian aging: a systematic review and meta-analysis. Adv. Nutr. 15 (8), 100273. doi:10.1016/j.advnut.2024.100273
Shi, S., Geng, Z., Yu, X., Hu, B., Liu, L., Chi, Z., et al. (2023). Salidroside supplementation affects in vitro maturation and preimplantation embryonic development by promoting meiotic resumption. Genes (Basel) 14 (9), 1729. doi:10.3390/genes14091729
Tabatabaie, M., Amiri, S., Golestan Jahromi, M., Sene, A. A., Zandieh, Z., Mehdizadeh, M., et al. (2022). The effect of Myo-Inositol supplement on molecular regulation of folliculogenesis, steroidogenesis, and assisted reproductive technique outcomes in patients with polycystic ovarian syndrome. Mol. Biol. Rep. 49 (2), 875–884. doi:10.1007/s11033-021-06833-9
Thongkittidilok, C., Le, Q. A., Lin, Q., Takebayashi, K., Do, T. K. L., Namula, Z., et al. (2022). Effects of individual or in-combination antioxidant supplementation during in vitro maturation culture on the developmental competence and quality of porcine embryos. Reprod. Domest. Anim. 57 (3), 314–320. doi:10.1111/rda.14063
Tomita, K., Ishii, T., Endo, N., and Tanaka, T. (2023). Effects of short-term dietary supplementation on the number of ovarian follicles, quantity and quality of oocytes, and in vitro embryo production in Japanese Black cows. J. Reprod. Dev. 69 (2), 65–71. doi:10.1262/jrd.2022-103
Tripathi, S. K., Nandi, S., Gupta, P. S. P., and Mondal, S. (2023). Antioxidants supplementation improves the quality of in vitro produced ovine embryos with amendments in key development gene expressions. Theriogenology 201, 41–52. doi:10.1016/j.theriogenology.2022.11.048
Tsui, K. H., Li, C. J., and Lin, L. T. (2024). Melatonin supplementation attenuates cuproptosis and ferroptosis in aging cumulus and granulosa cells: potential for improving IVF outcomes in advanced maternal age. Reprod. Biol. Endocrinol. 22 (1), 138. doi:10.1186/s12958-024-01311-w
Unfer, V., Facchinetti, F., Orru, B., Giordani, B., and Nestler, J. (2017). Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr. Connect. 6 (8), 647–658. doi:10.1530/EC-17-0243
Vartanyan, E. V., Tsaturova, K. A., Devyatova, E. A., Mikhaylyukova, A. S., Levin, V. A., Petuhova, N. L., et al. (2017). Improvement in quality of oocytes in polycystic ovarian syndrome in programs of in vitro fertilization. Gynecol. Endocrinol. 33 (Suppl. 1), 8–11. doi:10.1080/09513590.2017.1399699
Vitagliano, A., Petre, G. C., Francini-Pesenti, F., De Toni, L., Di Nisio, A., Grande, G., et al. (2021). Dietary supplements for female infertility: a critical review of their composition. Nutrients 13 (10), 3552. doi:10.3390/nu13103552
Wang, H., Xu, J., Li, H., Chen, W., Zeng, X., Sun, Y., et al. (2023). Alpha-ketoglutarate supplementation ameliorates ovarian reserve and oocyte quality decline with aging in mice. Mol. Cell Endocrinol. 571, 111935. doi:10.1016/j.mce.2023.111935
Wang, W., Zhang, D., Sun, L., Zhang, Z., Zhang, Y., Zhang, Y., et al. (2023). Alpha-lipoic acid supplementation reverses the declining quality of oocytes exposed to cyclophosphamide. Food Chem. Toxicol. 181, 114090. doi:10.1016/j.fct.2023.114090
Wdowiak, A., Bakalczuk, S., Filip, M., Lagana, A. S., and Unfer, V. (2025). The clinical use of myo-inositol in IVF-et: a position statement from the experts group on inositol in basic and clinical research and on PCOS (egoi-PCOS), the polish society of andrology, and the international scientific association for the support and development of medical technologies. J. Clin. Med. 14 (2), 558. doi:10.3390/jcm14020558
Woodward, A. A., Urbanowicz, R. J., Naj, A. C., and Moore, J. H. (2022). Genetic heterogeneity: challenges, impacts, and methods through an associative lens. Genet. Epidemiol. 46 (8), 555–571. doi:10.1002/gepi.22497
Wu, C. C., Li, C. J., Lin, L. T., Wen, Z. H., Cheng, J. T., and Tsui, K. H. (2024). Examining the effects of nutrient supplementation on metabolic pathways via mitochondrial ferredoxin in aging ovaries. Nutrients 16 (10), 1470. doi:10.3390/nu16101470
Xu, Y., Sun, M. H., Li, X. H., Ju, J. Q., Chen, L. Y., Sun, Y. R., et al. (2021). Modified hydrated sodium calcium aluminosilicate-supplemented diet protects porcine oocyte quality from zearalenone toxicity. Environ. Mol. Mutagen 62 (2), 124–132. doi:10.1002/em.22399
Yang, M., Shen, X., Lu, D., Peng, J., Zhou, S., Xu, L., et al. (2023). Effects of vitamin D supplementation on ovulation and pregnancy in women with polycystic ovary syndrome: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 14, 1148556. doi:10.3389/fendo.2023.1148556
Yao, Y., Tang, Y., Qin, H., Meng, R., Zhang, C., Zhang, Y., et al. (2023). Zinc supplementation promotes oocyte maturation and subsequent embryonic development in sheep. Theriogenology 206, 161–169. doi:10.1016/j.theriogenology.2023.04.025
Youssef, M. A., Abdelmoty, H. I., Elashmwi, H. A., Abduljawad, E. M., Elghamary, N., Magdy, A., et al. (2015). Oral antioxidants supplementation for women with unexplained infertility undergoing ICSI/IVF: randomized controlled trial. Hum. Fertil. (Camb) 18 (1), 38–42. doi:10.3109/14647273.2014.927595
Zabieglo, E., Jach, R., and Pirog, M. (2025). Age and BMI-related changes in hormonal profile in women with polycystic ovary syndrome (PCOS): association with infertility. Int. J. Gynaecol. Obstet. doi:10.1002/ijgo.70160
Zhang, J., Zhang, H., Zhou, W., Jiang, M., and Lin, X. (2025). Effect of myo-inositol supplementation in mixed ovarian response IVF cohort: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 16, 1520362. doi:10.3389/fendo.2025.1520362
Zheng, X., Lin, D., Zhang, Y., Lin, Y., Song, J., Li, S., et al. (2017). Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Med. Baltim. 96 (49), e8842. doi:10.1097/MD.0000000000008842
Keywords: oocyte process, quality ovary, dietary supplements, lifestyle changes, clinical trials
Citation: Chen H, Wang S, Song M, Yang D and Li H (2025) Oocyte and dietary supplements: a mini review. Front. Cell Dev. Biol. 13:1619758. doi: 10.3389/fcell.2025.1619758
Received: 28 April 2025; Accepted: 13 June 2025;
Published: 26 June 2025.
Edited by:
Marcela Alejandra Michaut, CONICET Dr. Mario H. Burgos Institute of Histology and Embryology (IHEM), ArgentinaReviewed by:
Ousman Bajinka, University of the Gambia, GambiaMichele Russo, Lo. Li. Pharma s. r. l., Italy
Copyright © 2025 Chen, Wang, Song, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongxia Yang, eWR4eWFuZ2Rvbmd4aWEyMDI1QDEyNi5jb20=; Hongmei Li, bGhtbGlob25nbWVpMjAyNUAxMjYuY29t
 Hao Chen1
Hao Chen1 Hongmei Li
Hongmei Li