Abstract
Background:
Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) have emerged as a promising cell-free therapeutic strategy for various diseases due to their anti-inflammatory, anti-apoptotic, and regenerative properties. Numerous meta-analyses have evaluated MSC-EV efficacy in preclinical animal models, but a comprehensive synthesis across diverse conditions is lacking.
Objective:
This umbrella review aims to systematically evaluate the therapeutic efficacy, mechanisms, and methodological quality of MSC-EVs in preclinical models across multiple diseases.
Methods:
A systematic search of Scopus and Web of Science was conducted to identify meta-analyses published up to July 2025, focusing on MSC-EV interventions in preclinical animal models. Data were extracted on study characteristics, exosome sources, animal models, outcomes, and risk of bias. The AMSTAR 2 tool assessed meta-analysis quality, while SYRCLE and CAMARADES tools evaluated primary study bias. Narrative and quantitative syntheses summarized efficacy, heterogeneity, and publication bias.
Results:
Forty-seven meta-analyses covering 27 diseases were included, spanning neurological, renal, wound healing, liver, musculoskeletal, respiratory, and reproductive disorders. MSC-EVs demonstrated high efficacy, significantly improving functional scores, reducing inflammation, and promoting regeneration. Bone marrow-, adipose-, and umbilical cord-derived EVs were most effective, with modified EVs showing enhanced outcomes. Methodological quality was moderate (AMSTAR 2), with high heterogeneity (I2 > 70%) and frequent risk of bias due to poor randomization and blinding. Publication bias was noted but often robust after adjustments.
Conclusion:
MSC-EVs exhibit robust therapeutic potential across diverse preclinical models, supporting their development as a versatile regenerative therapy. Standardization of EV protocols, improved study quality, and mechanistic insights are critical for clinical translation. This review provides a comprehensive framework for advancing MSC-EV research and application.
1 Introduction
Mesenchymal stem cells (MSCs) have garnered significant attention in regenerative medicine due to their multipotent differentiation capacity, immunomodulatory properties, and ability to promote tissue repair (Song et al., 2020). Derived from various sources such as bone marrow, adipose tissue, and umbilical cord, MSCs have shown therapeutic promise in preclinical and clinical studies across a wide range of conditions, including neurological, cardiovascular, renal, and musculoskeletal disorders (Zhidu et al., 2024). However, challenges such as immune rejection, variable efficacy, and potential tumorigenicity (Zhou et al., 2021) have prompted exploration of cell-free alternatives, particularly MSC-derived extracellular vesicles (MSC-EVs).
MSC-EVs, including exosomes and microvesicles, are nano-sized membrane-bound structures that carry bioactive molecules such as microRNAs, proteins, and lipids (Dabrowska et al., 2020). These vesicles mediate intercellular communication and recapitulate many of the therapeutic effects of their parent cells, including anti-inflammatory, anti-apoptotic, and regenerative actions (Kou et al., 2022). Unlike whole-cell therapies, MSC-EVs offer advantages such as lower immunogenicity, enhanced stability, and the ability to cross biological barriers, making them a promising platform for next-generation therapeutics (Kou et al., 2022). Preclinical studies in animal models have demonstrated MSC-EV efficacy in diverse conditions, from ischemic stroke (Zhao et al., 2023) and spinal cord injury (SCI) (Yi and Wang, 2021) to diabetic wounds (Soltani et al., 2024) and liver fibrosis (Zhou et al., 2024), highlighting their broad therapeutic potential.
Despite this promise, the field faces challenges, including variability in EV sources, isolation methods, and dosing regimens, as well as inconsistencies in preclinical study design and reporting (Dai et al., 2025). Numerous meta-analyses have synthesized evidence on MSC-EV efficacy for specific diseases, but a comprehensive overview integrating these findings across conditions is lacking. Umbrella reviews, which systematically synthesize meta-analyses, provide a high-level perspective to assess the consistency, quality, and generalizability of evidence, guiding future research and clinical translation.
This umbrella review aims to evaluate the therapeutic efficacy of MSC-EVs in preclinical animal models across diverse diseases. By analyzing outcomes, exosome sources, mechanisms of action, and methodological quality, we seek to provide a robust synthesis of the current evidence, identify gaps, and propose directions for advancing MSC-EV-based therapies. This work addresses the critical need for a unified understanding of MSC-EV potential, paving the way for standardized protocols and clinical applications.
2 Materials and methods
This umbrella review was conducted to systematically synthesize evidence from meta-analyses evaluating the therapeutic efficacy of MSC-EVs in preclinical animal models across diverse diseases and conditions. The methodology followed established guidelines for systematic reviews, including the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Joanna Briggs Institute (JBI) framework for umbrella reviews. Below, we detail the materials and methods used, organized into subsections for clarity.
2.1 Study design
This study is an umbrella review, defined as a systematic review of systematic reviews and meta-analyses. The objective was to aggregate and evaluate the therapeutic potential, mechanisms, and methodological quality of MSC-EV interventions in preclinical animal models. The review focused on meta-analyses to provide a high-level synthesis of evidence, capturing a broad range of diseases, exosome sources, and outcomes. The protocol was developed a priori and registered with PROSPERO to ensure transparency and reproducibility.
2.2 Search strategy
A comprehensive and systematic literature search was conducted to identify relevant meta-analyses. The search strategy was designed to capture studies evaluating MSC-EV therapeutic efficacy in preclinical models, with specific queries tailored to extracellular vesicles, mesenchymal stem cells, and meta-analyses (Table 1). The search was executed across multiple electronic databases, and the strategy was adapted from Table 1 of the provided article. The Scopus and Web of Science databases were searched from inception to July 2025 by two independent reviewers (N.M.M. and K.R.Z.) using standardized search protocols. Search results were exported to EndNote 20 for deduplication, and duplicates were removed using both automated and manual checks (Figure 1). The search strategy was validated by a medical librarian to ensure comprehensiveness and accuracy.
TABLE 1
| Code | Queries |
|---|---|
| #1 | “Extracellular Vesicles” OR “Exosomes” OR “Extracellular Vesicle” OR “Vesicle, Extracellular” OR “Vesicles, Extracellular” OR “Exovesicles” OR “Exovesicle” |
| #2 | “Mesenchymal Stem Cells” OR “Stem Cell, Mesenchymal” OR “Mesenchymal Stem Cell” OR “Stem Cells, Mesenchymal” OR “Mesenchymal Stromal Cells” OR “Mesenchymal Stromal Cell” OR “Stromal Cell, Mesenchymal” OR “Stromal Cells, Mesenchymal” OR “Wharton Jelly Cells” OR “Wharton’s Jelly Cells” OR “Wharton’s Jelly Cell” OR “Whartons Jelly Cells” OR “Bone Marrow Stromal Cells” OR “Bone Marrow Stromal Cell” OR “Bone Marrow Stromal Cells, Multipotent” OR “Multipotent Bone Marrow Stromal Cell” OR “Multipotent Bone Marrow Stromal Cells” OR “Bone Marrow Stromal Stem Cells” OR “Mesenchymal Progenitor Cell” OR “Mesenchymal Progenitor Cells” OR “Progenitor Cell, Mesenchymal” OR “Progenitor Cells, Mesenchymal” OR “Multipotent Mesenchymal Stromal Cells” OR “Mesenchymal Stromal Cells, Multipotent” OR “Multipotent Mesenchymal Stromal Cell” OR “Bone Marrow Mesenchymal Stem Cells” OR “Bone Marrow Mesenchymal Stem Cell” OR “Adipose-Derived Mesenchymal Stem Cells” OR “Adipose Derived Mesenchymal Stem Cells” OR “Adipose-Derived Mesenchymal Stromal Cells” OR “Adipose Derived Mesenchymal Stromal Cells” OR “Mesenchymal Stem Cells, Adipose-Derived” OR “Mesenchymal Stem Cells, Adipose Derived” OR “Adipose Tissue-Derived Mesenchymal Stromal Cell” OR “Adipose Tissue Derived Mesenchymal Stromal Cell” OR “Adipose Tissue-Derived Mesenchymal Stromal Cells” OR “Adipose Tissue Derived Mesenchymal Stromal Cells” OR “Adipose Tissue-Derived Mesenchymal Stem Cell” OR “Adipose Tissue Derived Mesenchymal Stem Cell” OR “Adipose Tissue-Derived Mesenchymal Stem Cells” OR “Adipose Tissue Derived Mesenchymal Stem Cells” OR “Adipose-Derived Mesenchymal Stem Cell” OR “Adipose Derived Mesenchymal Stem Cell” |
| #3 | “meta-analysis” or “meta analysis” |
| #4 | #1 AND #2 AND #3 (Filter: language restriction (English), Date limitation: up to 31 July 2025) |
Systematic search strategy for screening of meta-analysis articles evaluating mesenchymal stromal/stem cells-derived extracellular vesicles.
FIGURE 1
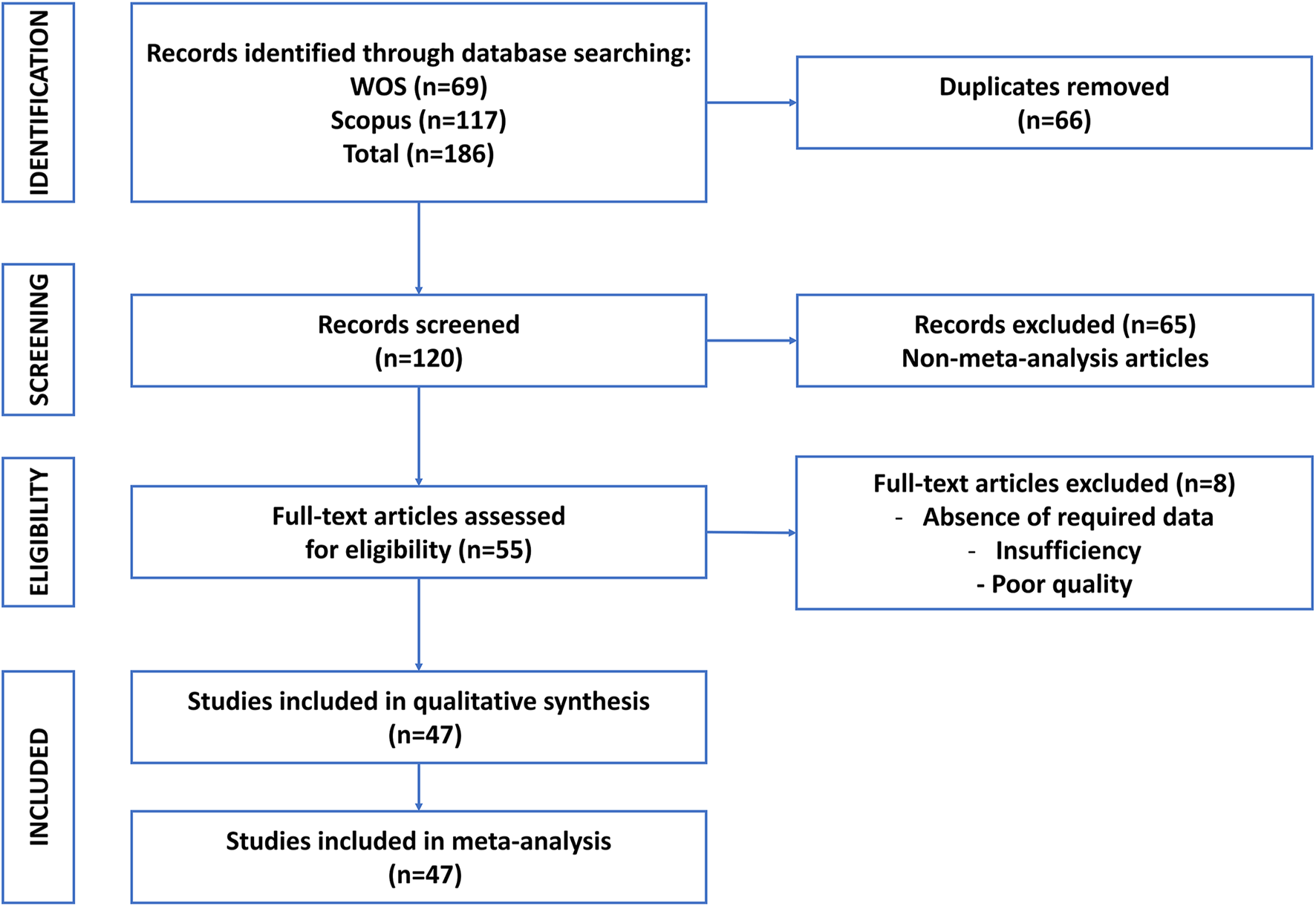
Flowchart of literature search and screening process for umbrella review of meta-analysis articles of mesenchymal stem cell-derived extracellular vesicles in preclinical models.
2.3 Eligibility criteria
For inclusion in this umbrella review, studies were selected based on predefined inclusion and exclusion criteria to ensure both relevance and methodological quality. Eligible studies were systematic reviews that included meta-analyses of preclinical studies, specifically those investigating MSC-EVs—including exosomes, microvesicles, or other EV subtypes—as the primary therapeutic intervention. Studies combining MSC-EVs with other therapies, such as scaffolds or pharmacological agents, were included provided that MSC-EVs remained the central focus. The target population comprised preclinical animal models used to study a broad range of diseases or conditions. Included studies had to report quantitative outcomes relevant to therapeutic efficacy, such as functional assessments, histological evaluations, molecular biomarkers, or survival rates. Only English-language, peer-reviewed journal articles were considered.
Studies were excluded if they were narrative reviews, systematic reviews without meta-analyses, or primary research articles. Additional exclusion criteria included studies that focused on EVs not derived from MSCs, unless MSC-EVs constituted a major component of the analysis. Clinical trials or studies involving human subjects were excluded, as were meta-analyses limited solely to in vitro data. Non-English publications, conference abstracts, grey literature, preprints, and other non-peer-reviewed materials were also excluded from this review.
2.4 Study selection
The study selection process was conducted in two distinct stages to ensure methodological rigor and transparency. In the first stage, titles and abstracts were independently screened by two reviewers (A.B. and M.A.K.). This initial screening was performed against the predefined eligibility criteria. Any discrepancies between the reviewers were resolved through discussion or, if necessary, by consulting a third reviewer (A.T.). In the second stage, the full texts of studies deemed potentially eligible were retrieved and independently evaluated by two additional reviewers (A.B. and M.A.K.) to determine their final inclusion. At this stage, specific reasons for exclusion were carefully documented. To provide a clear overview of the selection process, a PRISMA flow diagram was generated, outlining the number of records identified, screened, included, and excluded at each phase of the review (Figure 1).
2.5 Data extraction
Data extraction was carried out independently by two reviewers (N.M.M. and K.R.Z.) using a standardized form developed in Microsoft Excel. This form was piloted on five studies to ensure consistency, clarity, and completeness in data capture. After extraction, data were cross-verified for accuracy by the reviewers. Any inconsistencies were resolved through consensus or, when necessary, by consulting a senior author (A.T.).
The data extraction encompassed several key elements. For study characteristics, information was collected on the authors, year of publication, journal name, and reference number, along with the total number of studies included in each meta-analysis and the specific disease or condition being investigated. Intervention details included the type of MSC-EVs, the origin of the MSCs, and the method of delivery.
Regarding animal models, data were gathered on the species used, the specific strains, and the experimental disease models employed. Outcomes extracted included both primary outcomes and secondary outcomes. Where available, effect sizes such as standardized mean differences (SMD), weighted mean differences (WMD), hazard ratios (HR), or odds ratios (OR) were recorded, along with their corresponding 95% confidence intervals. Measures of heterogeneity, such as the I2 statistic, were also documented.
In terms of methodological quality, each study’s risk of bias was assessed using established tools like SYRCLE or CAMARADES. The overall risk of bias was categorized as low, moderate, high, or unclear. Evaluation of publication bias included methods such as Egger’s test and visual inspection of funnel plots. Furthermore, the AMSTAR 2 tool was used to appraise the methodological quality of the included systematic reviews and meta-analyses, with ratings categorized as high, moderate, low, or critically low, and critical flaws explicitly noted. Data were extracted from main texts, tables, and Supplementary Material. When numerical data was missing, attempts were made to contact the original authors for clarification. In cases where no response was obtained, data were estimated from graphical figures.
2.6 Quality assessment
To evaluate the methodological rigor of the included meta-analyses and the risk of bias in the primary studies they synthesized, two complementary assessment tools were employed. The AMSTAR 2 was used to appraise the overall quality of the included meta-analyses. Two independent reviewers (A.B. and G.A.T.) applied the 16-item checklist, with particular attention to critical domains such as protocol registration (item 2), comprehensiveness of the literature search strategy (item 4), justification for excluded studies (item 7), risk of bias assessment of included studies (item 9), appropriateness of the meta-analytic methods (item 11), and consideration of publication bias (item 15). Based on the number and severity of critical flaws identified, each meta-analysis was rated as having high, moderate, low, or critically low confidence in its findings. Any disagreements between reviewers were resolved through discussion and consensus. AMSTAR-2 ratings were assigned according to the number of critical domains rated ‘No.’ Reviews with ≥1 critical flaw were downgraded to low or critically low confidence.
The risk of bias in the primary studies included within each meta-analysis was assessed using the tools employed by the original meta-analyses themselves. The most commonly used instruments were the SYRCLE risk of bias tool and the CAMARADES checklist. These tools evaluated key domains of bias, including selection bias, performance bias, detection bias, attrition bias, and reporting bias. The overall risk of bias for each meta-analysis—categorized as low, moderate, high, or unclear—was recorded as reported in the studies. If a meta-analysis utilized a custom or non-standard assessment tool, its specific criteria were documented accordingly.
To improve clarity, we distinguished the use of the SYRCLE and CAMARADES tools based on the model type and reporting structure of the original meta-analyses. Specifically, the SYRCLE tool was applied when the included meta-analysis assessed basic animal studies with heterogeneous outcomes such as behavioral scores, histological findings, or inflammatory markers. In contrast, the CAMARADES checklist was used when analyzing more structured preclinical models—particularly in neurological and cardiovascular studies—where endpoints such as infarct volume, mNSS, or neurobehavioral scores were commonly and consistently reported. In instances where both tools were used or a modified version was employed, we recorded that distinction accordingly in Table 4.
2.7 Data synthesis
Data were synthesized both narratively and quantitatively to comprehensively evaluate the therapeutic efficacy of MSC-EVs across various diseases, exosome sources, and outcome measures. The synthesis was structured to align with the objectives of the umbrella review, with a particular focus on therapeutic effectiveness, underlying mechanisms of action, and the methodological quality of the included meta-analyses.
A narrative synthesis was performed to describe the diversity of conditions addressed in the included studies, the types and tissue sources of MSC-EVs used, the animal models employed, and the administration routes applied. This synthesis also outlined the primary outcomes assessed, their consistency across studies, and the proposed mechanisms of action, such as anti-inflammatory, anti-apoptotic, and regenerative effects. Findings were organized into comprehensive tables and illustrative figures to facilitate interpretation and comparison. For instance, Table 3 presents a detailed summary of exosome-based therapies across different diseases and conditions, while visual aids such as bar graphs and merged heatmaps were used to depict data trends and outcome distributions.
In the quantitative synthesis, effect sizes, heterogeneity measures, and statistical significance were summarized based on the results reported in the included meta-analyses. Key metrics included SMD, WMD, HR, and OR, all accompanied by 95% confidence intervals. These metrics were typically reported for primary outcomes such as functional recovery scores, wound healing rates, or infarct volume reduction. Heterogeneity across studies was assessed using the I2 statistic, with values greater than 50% considered indicative of substantial variability. Where available, subgroup analyses or sensitivity analyses were reported to explore sources of heterogeneity. Publication bias was evaluated based on the original meta-analyses.
No additional meta-analyses were conducted within this umbrella review, as the aim was to synthesize and evaluate existing meta-analytic evidence rather than generate new pooled estimates. However, reported effect sizes were qualitatively summarized to identify therapeutic trends—for example, MSC-EVs demonstrated high efficacy in preclinical models of stroke and moderate effects in kidney transplantation models.
Because umbrella reviews synthesize findings from published meta-analyses without re-analyzing primary studies, we did not exclude individual studies on the basis of heterogeneity. Instead, we applied a rule-based classification: outcomes were labeled as High effectiveness only when SMD >1.5, p < 0.01, and I2 < 70% in ≥2 independent meta-analyses. Outcomes with I2 ≥ 70% were reclassified as Promising but heterogeneous and interpreted with caution. Sensitivity summaries were added to indicate whether conclusions remained robust after considering only meta-analyses with I2 < 70% and without AMSTAR-2 critical flaws.
Because this is an umbrella review, we did not exclude meta-analyses solely on the basis of high heterogeneity. Instead, we applied a rule-based classification: outcomes were labeled as High effectiveness only when SMD >1.5, p < 0.01, and I2 < 70% in ≥2 independent reviews. Outcomes with I2 ≥ 70% were reclassified as Promising but heterogeneous and interpreted with caution.
2.8 Subgroup and sensitivity analyses
Subgroup analyses reported within the included meta-analyses were extracted to identify factors that may influence the therapeutic efficacy of MSC-EVs. These analyses explored variations based on the source of exosomes—such as bone marrow-derived MSCs (BM-MSCs), adipose-derived MSCs (AD-MSCs), and human umbilical cord-derived MSCs (hUC-MSCs)—as well as animal model characteristics, including species and specific strains used in the experiments. Differences in disease models were also considered, such as contusion versus compression injury models for SCI, to evaluate how pathophysiological variations affect outcomes.
Additional subgroup variables included the route of MSC-EV administration and the timing and dosage of EV delivery. These factors were examined to determine their potential role in modulating therapeutic effectiveness across studies.
Sensitivity analyses conducted within the original meta-analyses were also summarized. These included procedures such as excluding studies with a high risk of bias to test the stability of the main findings, as well as statistical methods like trim-and-fill adjustments to evaluate the impact of publication bias. Together, these subgroup and sensitivity analyses provided important insights into the robustness and generalizability of MSC-EV therapy outcomes across different experimental conditions.
2.9 Ethical considerations
As this study involved no primary data collection or animal experimentation, ethical approval was not required. However, the review considered the ethical conduct of included studies, noting compliance with animal welfare regulations as reported by the meta-analyses.
2.10 Statistical software and tools
Several tools were employed to facilitate data management and ensure methodological consistency throughout the review process. EndNote 20 was used for reference management and to identify and remove duplicate records prior to screening. For data extraction and the creation of summary tables, Microsoft Excel was utilized, offering a structured format to capture and organize information efficiently. Additionally, RStudio was employed to generate heatmap graphs, enabling visual representation of data patterns and relationships derived from the synthesized findings.
No new statistical analyses were performed in this umbrella review, as its primary goal was to synthesize and interpret results from existing meta-analyses. However, statistical metrics reported in the included studies were carefully reviewed and verified for accuracy to ensure the reliability of the synthesized findings.
3 Results
This umbrella review synthesizes findings from 47 meta-analyses evaluating the therapeutic efficacy of MSC-EVs in preclinical animal models across a wide range of diseases and conditions (Table 2). The systematic search identified studies published between 2016 and 2025, covering diverse therapeutic applications, exosome sources, animal models, and outcome measures. The results are organized into subsections to provide a detailed overview of MSC-EV efficacy, mechanisms, sources, and methodological considerations.
TABLE 2
| Authors, reference | Year | Journal | Number of studies | Disease/ Condition |
Intervention | Exosome source | Animal model | Outcomes | Main findings | Risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Aghayan et al. (2024) | 2024 | Stem Cell Rev Rep | 30 | Sepsis | MSC-EVs | BM, UC, AT, placenta | Mice, rats, sheep | Survival, organ function, cytokines | Improved survival (HR 0.33, 95% CI 0.27–0.41), reduced organ damage, modulated inflammation. BM-EVs most effective | High certainty (GRADE); low risk (SYRCLE, novel tool) |
| Bailey et al. (2022) | 2022 | Stem Cell Rev Rep | 10 | Diabetic wounds | MSC-EVs | BM, UC, AT, synovia, others | Mice, rats | Wound closure, angiogenesis, inflammation | Enhanced closure (SMD 5.48, 95% CI 3.55–8.13), angiogenesis, reduced inflammation. RNA-enriched EVs more effective | Unclear risk (SYRCLE); unclear blinding, randomization |
| Bernardi et al. (2025) | 2025 | Mol Neurobiol | 35 | Ischemic stroke | MSC-EVs | BM, UC, AT | Rodents, monkeys, ewes | Microglia, cytokines | Reduced inflammation, Iba1+, TNF-α | Moderate-high risk (SYRCLE); 67% moderate, 33% high |
| Chen et al. (2024) | 2024 | Heliyon | 55 | Traumatic brain injury | MSC-EVs | BM | Mice, rats | Neurological scores, lesion volume | Improved mNSS, MWM; reduced lesion volume | Moderate risk (CAMARADES, SYRCLE); mean score 5.75 |
| Chen et al. (2023) | 2023 | Heliyon | 7 | Intrauterine adhesion | Stem cells EVs | UC, BM, AT, others | Rats, rabbits | Fibrosis, endometrial repair | Reduced fibrosis, increased embryo number | High risk (SYRCLE); publication bias (Egger’s p = 0.005) |
| Dai et al. (2025) | 2025 | Lipids Health Dis | 14 | NAFLD, NASH | MSC-EVs | UC, AT, BM | Mice, rats | Liver markers, cytokines | Reduced AST, ALT, TG, NAS, oxidative stress | High risk (SYRCLE); poor methodology |
| Fang et al. (2023) | 2023 | J Pers Med | 39 | Liver diseases | MSC-EVs | BM, UC, AT, ESCs | Mice, rats | Liver function, histology, cytokines | Reduced damage, inflammation | Moderate risk (CAMARADES); high heterogeneity |
| Fang et al. (2022) | 2022 | Transplant Rev | 7 | Kidney transplantation | MSC-EVs, immune cell-EVs | BM, AT | Mice, rats | Graft survival, SCr, BUN | Immune-EVs more effective than MSC-EVs | Moderate-high risk (SYRCLE) |
| Firouzabadi et al. (2024a) | 2024 | Stem Cell Rev Rep | 19 | Asthma | MSC-EVs | BM, UC, AT, iPSC | Mice, rats | Inflammation, airway responsiveness | Reduced inflammation, hyper-responsiveness. Dose/timing critical | Unclear risk (SYRCLE); publication bias (Egger’s p < 0.05) |
| Firouzabadi et al. (2024b) | 2024 | J Ovarian Res | 29 | Primary ovarian insufficiency | MSC-EVs | UC, BM, AT, others | Mice, rats | Follicle count, hormones, pregnancy | Improved follicle number, hormones, pregnancy rate | Low quality (SYRCLE); high heterogeneity, bias |
| Gunjan et al. (2024) | 2024 | Mol Cell Probes | 8 | Diabetic wound healing | MSC-EVs | BM, UC, AT | Mice, rats | Wound closure, angiogenesis | Enhanced closure, angiogenesis | High risk (SYRCLE); high heterogeneity, bias |
| He et al. (2022) | 2022 | Stem Cell Res Ther | 9 | Subarachnoid hemorrhage | MSC-EVs, MSCs | BM, UC | Mice, rats | Neurobehavior, brain edema | EVs outperformed MSCs in neuroprotection | High risk (CAMARADES); high heterogeneity |
| He et al. (2023) | 2023 | Stem Cell Res Ther | 11 | Osteoporosis | MSC-EVs, ESC-EVs | UC, BM, AT, ESCs | Mice, rats | Bone mass, structure | Increased BMD, microarchitecture | High risk (SYRCLE); low randomization, blinding |
| Hickson et al. (2021) | 2021 | Stem Cells Transl Med | 40 | Diabetic kidney disease | MSC-EVs, MSCs | BM, UC, AT | Mice, rats, shrews | Renal function, inflammation | Reduced creatinine, fibrosis, inflammation | Low risk (SYRCLE); strong reporting |
| Himanshu et al. (2025) | 2025 | Biochem Biophys Rep | 15 | Chronic kidney disease | MSC-EVs | BM, UC, AT | Mice, rats | SCr, BUN, renal damage | Reduced SCr, BUN, inflammation | Moderate risk (SYRCLE); moderate heterogeneity |
| Jabermoradi et al. (2025) | 2024 | Arch Acad Emerg Med | 65 | SCI | MSC-EVs | BM, UC, AT | Mice, rats | Locomotion, neural markers | Improved motor recovery, reduced apoptosis | Moderate risk (SYRCLE); low bias for most outcomes |
| Kirkham et al. (2022) | 2022 | Stem Cell Rev Rep | 13 | Bone injury | MSC-EVs | BM, UC, AT, dental | Mice, rats | BV/TV, bone formation | Improved BV/TV, bone formation. Modified EVs no added benefit | Unclear risk (SYRCLE); no publication bias |
| Liu et al. (2020a) | 2020 | Stem Cell Res Ther | 31 | Acute kidney injury | MSC-EVs | BM, UC, AT, others | Mice, rats | SCr, BUN, inflammation | Improved SCr, BUN, reduced inflammation | Unclear risk (CAMARADES); publication bias adjusted |
| Liu et al. (2024) | 2024 | Front Cell Dev Biol | 25 | Osteosarcoma | MSC-EVs | BM, unspecified | Mice | Tumor volume, weight | Engineered EVs more effective; macrophage EVs promoted growth | Unclear risk (SYRCLE); possible bias |
| Lou et al. (2025) | 2025 | Stem Cell Res Ther | 20 | Erectile dysfunction | MSC-EVs | MSC, AT, UC | Rats | ICP/MAP, nNOS, eNOS | Improved function, no source/model difference | Moderate-high quality (SYRCLE); publication bias |
| Lv et al. (2025) | 2025 | Front Pharmacol | 14 | Kidney fibrosis | MSC-EVs | UC | Rats, mice | SCr, BUN, fibrosis | Reduced fibrosis, inflammation; increased E-Cadherin | Low-moderate risk (SYRCLE); no bias |
| Mou et al. (2025) | 2025 | Front Neurol | 21 | Acute SCI | MSC-EVs | BM, UC, AT, placenta | Rats | BBB scores | Improved locomotor recovery, reduced inflammation | Low risk (SYRCLE); slight publication bias |
| Nowak et al. (2022) | 2022 | Stem Cell Rev Rep | 35 | Chronic kidney disease | MSC-EVs | BM, UC, AT | Mice, rats | SCr, GFR, fibrosis | Improved renal outcomes, miRNA-mediated | Unclear risk (CAMARADES); bias adjusted |
| Shang et al. (2024) | 2024 | Cytotherapy | 40 | Traumatic SCI | Stem cell-EVs | BM, AT, UC, NSCs | Rats | BBB scores | Improved motor function (WMD 1.58–4.54); NSCs-EVs most effective | Unclear risk (SYRCLE); publication bias |
| Soltani et al. (2024) | 2024 | Stem Cell Rev Rep | 20 | Diabetic wounds | ADSC-EVs | AT | Mice, rats | Wound closure, angiogenesis | Enhanced closure (SMD 4.22, 95% CI 3.07–5.36), angiogenesis | Unclear risk (SYRCLE); unclear bias details |
| Tieu et al. (2021) | 2021 | J Extracell Vesicles | 11 | Respiratory diseases | MSC-EVs | BM, UC, AT | Preclinical models | Inflammation, fibrosis | Improved acute/chronic respiratory outcomes | Not specified |
| Wang et al. (2024) | 2024 | Stem Cells Int | 12 | Hemorrhagic stroke | Stem cell-EVs | MSC, AT | Mice, rats | Neurobehavioral scores | Improved SAH, chronic ICH outcomes (SMD -3.49, 2.38) | High quality (CAMARADES); publication bias |
| Wang et al. (2020) | 2020 | Respir Res | 17 | Acute lung injury/ARDS | MSC-EVs | BM, UC, AT, neural | Mice, rats, pigs | Lung injury, survival | Reduced injury (SMD -4.02), improved survival (OR 6.45) | Moderate-high heterogeneity; bias not detailed |
| Wang et al. (2025) | 2025 | Front Pharmacol | 28 | Knee osteoarthritis | MSC-EVs | BM, UC, AT, others | Rats | Cartilage repair, inflammation | Improved repair (OARSI SMD -2.97), reduced inflammation | Moderate quality (SYRCLE); unclear bias reporting |
| Wendt et al. (2018) | 2018 | Sci Rep | 43 | Cardiovascular diseases | EVs | MSC, cardiac cells | Rodents, pigs | Cardiac function, inflammation | Reduced injury, improved function, angiogenesis | Low reporting bias; unclear EV characterization |
| Xu et al. (2024) | 2024 | Front Pharmacol | 38 | Ischemic stroke | Stem cell-EVs | BM, UC, AT, others | Mice, rats | Infarct volume, mNSS | BMSC-EVs most effective; engineered EVs enhanced efficacy | Moderate risk (SYRCLE); publication bias |
| Xun et al. (2022) | 2022 | Front Immunol | 12 | Multiple sclerosis | MSC-EVs | BM, UC, AT, dental | Mice, rats | Clinical score | Improved symptoms (SMD -2.17); PDLSCs most effective | Unclear risk (SYRCLE); insufficient reporting |
| Yang et al. (2023a) | 2023 | Neural Regen Res | 49 | SCI, TBI | MSC-EVs | BM, UC, AT, placenta | Mice, rats | BBB, mNSS, Foot Fault | Improved SCI (SMD 4.46), TBI outcomes; reduced inflammation | Moderate risk (SYRCLE); publication bias |
| Yang et al. (2022) | 2022 | Front Neurosci | 13 | Spinal cord injury | MSC-EVs, miRNA-EVs | BM, UC, AT | Rats | BBB scores | miRNA-EVs improved motor function; contusion models better | Low risk (SYRCLE); publication bias |
| Yang et al. (2023b) | 2023 | Front Neurosci | 20 | Traumatic brain injury | EVs | MSC, astrocytes, NSCs | Mice, rats | mNSS, MWM | Astrocyte-EVs most effective; improved mNSS, MWM. | Uneven quality (SYRCLE); no bias for mNSS. |
| Ye et al. (2024) | 2024 | Front Mol Neurosci | 30 | SCI | BMSC-EVs | BM | Rats | BBB, inflammation, apoptosis | Improved outcomes; dose-response correlation | High risk (SYRCLE); unclear randomization, blinding |
| Yi and Wang (2021) | 2021 | Open Med | 35 | Acute SCI | EVs | MSC, HUVECs, PC12 | Mice, rats | BBB, BMS | Improved locomotor recovery; intrathecal better | Low risk (SYRCLE); publication bias in BBB. |
| Yue et al. (2024) | 2024 | Front Endocrinol | 21 | Type II diabetic wounds | MSC-EVs | AT, BM, UC, others | Mice, rats | Wound closure, inflammation | Improved healing (SMD >3), reduced inflammation | Unclear risk (SYRCLE); publication bias |
| Zhang et al. (2016a) | 2016 | Exp Ther Med | 13 | Acute kidney injury | MSC-EVs | BM, UC, AT | Rodents | SCr | EVs more effective than CM; early administration better | High heterogeneity; no publication bias |
| Zhang et al. (2016b) | 2016 | Stem Cells Int | 6 | Myocardial I/R injury | MSC-EVs | ESC-MSC, others | Mice, pigs | Cardiac function | Improved EF, FS; EVs better than CM. | High heterogeneity; no bias analysis |
| Zhang et al. (2022) | 2022 | Neural Plast | 24 | Cerebral I/R injury | MSC-EVs | BM, UC, AT | Mice, rats | Infarct volume, neuro score | Reduced infarct, improved neurology | Moderate quality (SYRCLE); publication bias |
| Zhang et al. (2025) | 2025 | Brain Res Bull | 73 | Ischemic stroke | MSC-EVs | BM, UC, AT | Mice, rats | Infarct volume, mNSS | Reduced infarct, improved function (P < 0.01) | High quality (CAMARADES); median score 8/10 |
| Zhou et al. (2023a) | 2023 | BMC Oral Health | 11 | Periodontitis | MSC-EVs | BM, dental | Mice, rats | BV/TV, CEJ-ABC | Improved BV/TV, reduced CEJ-ABC. | Unclear risk (SYRCLE); no bias in key metrics |
| Zhou et al. (2025) | 2025 | J Orthop Surg Res | 17 | Periodontitis | MSC-EVs | Dental, BM, UC | Mice, rats, beagles | BV/TV, BMD, CEJ-ABC | Improved BV/TV (SMD 13.99), BMD; no Tb.Th effect | Unclear risk (SYRCLE); high heterogeneity |
| Zhou et al. (2024) | 2024 | Front Pharmacol | 18 | Liver fibrosis | MSC-EVs | BM, UC, AT | Mice, rats | Liver function, fibrosis | Improved function, fibrosis; EVs + drugs better | Mixed risk (Cochrane); high heterogeneity |
| Zhou et al. (2023b) | 2023 | Stem Cell Rev Rep | 28 | POI, IUA | MSC-EVs | BM, UC, menstrual | Mice, rats | AMH, endometrial thickness | Improved AMH, endometrium; better with scaffolds | Unclear risk (SYRCLE); bias for AMH in POI. |
| Zhu et al. (2025) | 2025 | J Transl Med | 83 | Skin regeneration | MSC-EVs | BM, UC, AT, others | Mice, rats | Wound closure, collagen | Improved closure, collagen; ApoSEVs best | Low quality (MISEV2023); high heterogeneity |
Descriptive summary of meta-analyses evaluating mesenchymal stem cell-derived extracellular vesicles in preclinical studies.
Abbreviations: MSC-EVs, Mesenchymal stem cell-derived extracellular vesicles; BM, bone marrow; UC, umbilical cord; AT, adipose tissue; SCr, Serum creatinine; BUN, blood urea nitrogen; BBB, basso, Beattie, Bresnahan; mNSS, modified neurological severity score; MWM, morris water maze; SMD, standardized mean difference; HR, hazard ratio; CI, confidence interval; BV/TV, Bone volume/total volume; CEJ-ABC, Cementoenamel junction-alveolar bone crest; NAFLD, Non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; IUA, intrauterine adhesion; POI, primary ovarian insufficiency; TBI, traumatic brain injury; SCI, spinal cord injury.
3.1 Therapeutic efficacy across diseases
MSC-EVs demonstrated high therapeutic efficacy across most evaluated diseases, with consistent improvements in functional, histological, and molecular outcomes (Figure 2). The following summarizes key findings by disease category (Figure 3; Supplementary Table S1). MSC-EVs consistently reduced inflammation and apoptosis, while enhancing functional scores and histological repair. Effectiveness was high across most conditions, with bone marrow-derived MSC-EVs (BMSC-EVs) and preconditioned EVs showing superior results, though heterogeneity was moderate to high and risk of bias varied. The classification of therapeutic effectiveness into “high” and “moderate” was based on reported meta-analytic metrics. “High” effectiveness was assigned to outcomes with standardized mean difference (SMD) > 1.5, p < 0.01, and low-to-moderate heterogeneity (I2 < 70%) observed in at least two independent meta-analyses. “Moderate” effectiveness was applied to outcomes with SMD values between 0.8 and 1.5 or when heterogeneity exceeded 70%.
FIGURE 2
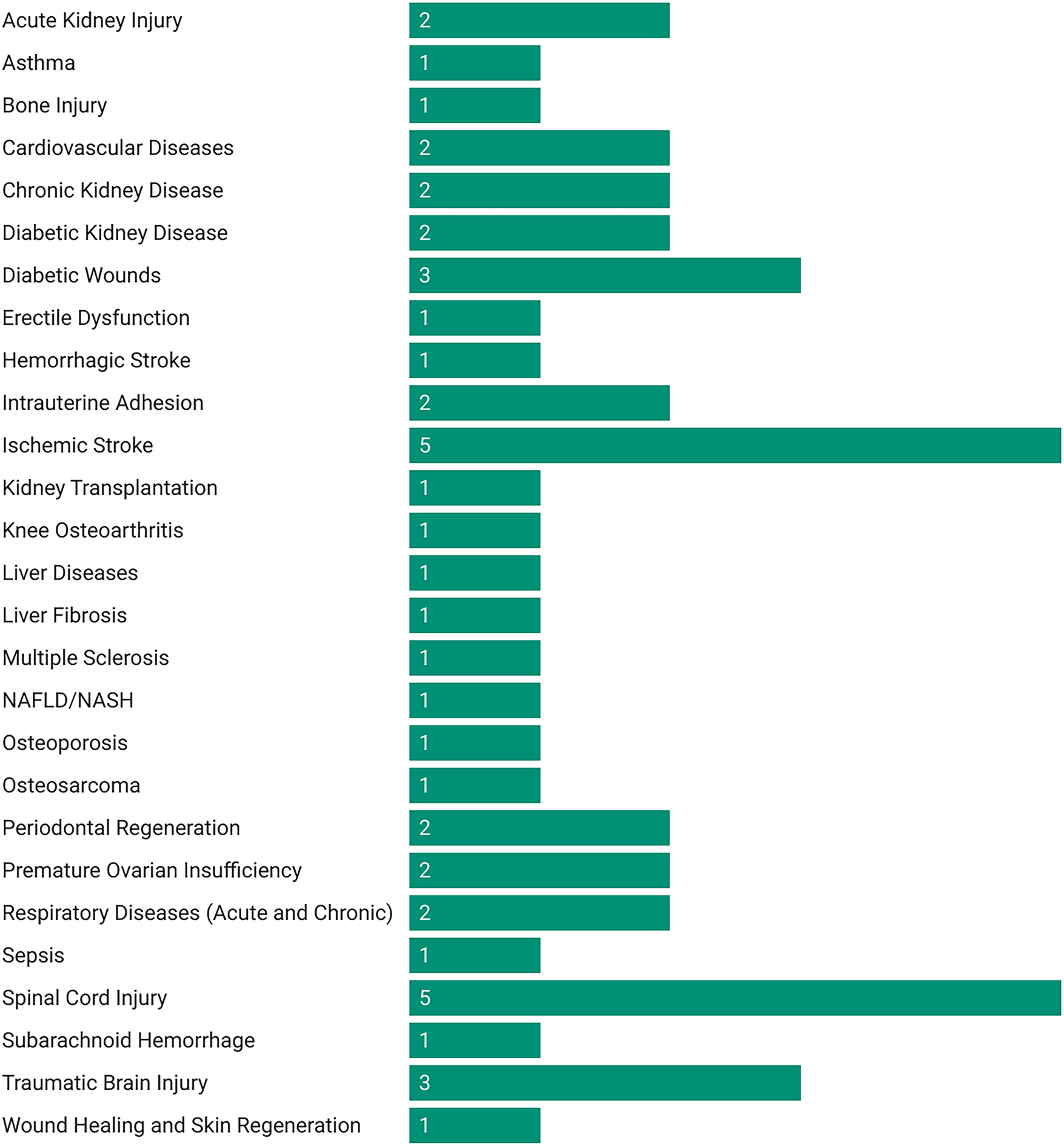
Number of meta-analyses evaluating MSC-EV therapies in preclinical models by disease category.
FIGURE 3
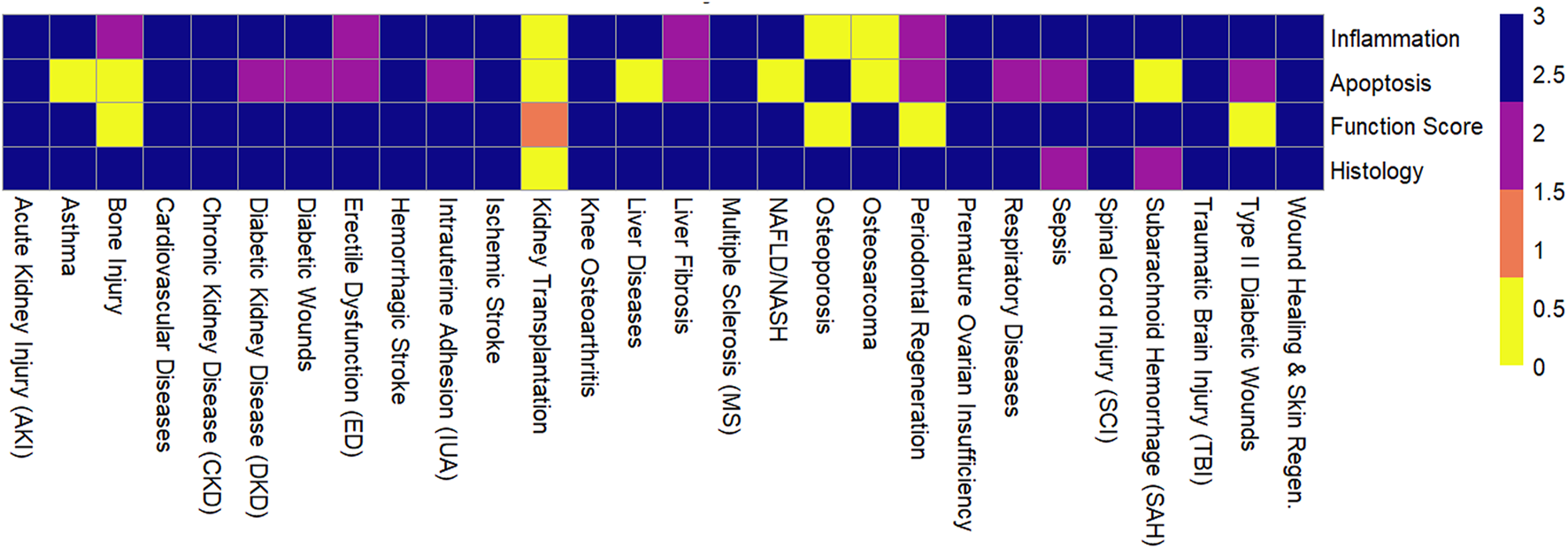
Effectiveness of mesenchymal stem cell-derived extracellular vesicles across outcomes for various diseases.
MSC-EVs exert their therapeutic effects through a range of interconnected biological mechanisms. These mechanisms contribute to the regenerative and protective roles of MSC-EVs in various pathological conditions.
One of the most prominent mechanisms is the anti-inflammatory effect. MSC-EVs were consistently shown to downregulate proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), while simultaneously upregulating anti-inflammatory mediators including interleukin-10 (IL-10) and transforming growth factor-beta 1 (TGF-β1). These immunomodulatory effects were observed across multiple disease models, particularly in stroke, SCI (SCI), acute kidney injury (AKI), and asthma.
Anti-apoptotic effects were also widely reported. MSC-EVs reduced markers of apoptosis, such as caspase-3 and Bax, in neurological, renal, and cardiovascular models. By inhibiting apoptotic pathways, MSC-EVs helped preserve tissue integrity and cell viability in damaged organs.
Functional improvements were another key therapeutic outcome, with enhanced performance in disease-specific scoring systems such as the Basso, Beattie, Bresnahan (BBB) score for SCI, the modified Neurological Severity Score (mNSS) for stroke, and the Osteoarthritis Research Society International (OARSI) score for joint degeneration. These improvements were largely attributed to mechanisms such as neuroregeneration, angiogenesis, and overall tissue repair facilitated by MSC-EVs.
Finally, histological improvements supported the regenerative potential of MSC-EVs. Across studies, MSC-EVs were shown to stimulate collagen deposition, promote angiogenesis and neurogenesis, and reduce fibrosis, lesion size, and tissue damage. These histological changes were particularly evident in models of wound healing, liver fibrosis, and kidney disease, underscoring the broad-spectrum therapeutic action of MSC-EVs across organ systems.
Across conditions such as ischemic stroke, diabetic wounds, SCI, and acute kidney injury, MSC-EVs significantly reduced inflammation, apoptosis, and tissue damage while enhancing functional recovery and histological repair (Table 3). BMSC-EVs, adipose-derived MSC-EVs (ADSC-EVs), and preconditioned EVs showed superior efficacy in conditions like ischemic stroke, diabetic wounds, and multiple sclerosis, with notable improvements in neurovascular repair, wound closure, and clinical scores. However, effectiveness was low in kidney transplantation, where MSC-EVs showed no significant benefit. Consistency across studies was moderate (I2 = 23–95%) for most conditions, with high heterogeneity in bone injury (I2 = 97–98%) and acute kidney injury (I2 = 96%), likely due to variability in animal models, exosome sources, and administration methods. For disease areas where heterogeneity was very high (I2 ≥ 70%), such as bone injury and acute kidney injury, the results were reclassified as Promising but heterogeneous. While these conditions showed large effect sizes, the variability across studies limits certainty in the pooled estimates. For such disease areas with I2 ≥ 70%, outcomes were downgraded to Promising but heterogeneous. While effect sizes were large, the variability across studies limits the certainty of pooled estimates.
TABLE 3
| Disease/Condition | Number of reviews | Animal models | Exosome sourcea | Main outcomes | Effectivenessb | Consistency (I2) |
|---|---|---|---|---|---|---|
| Acute Kidney Injury | 1 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, others | Reduced SCr (MD 0.93, 95% CI 0.67–1.20), BUN, TNF-α; increased IL-10; improved renal function | Promising but heterogeneous (EVs > CM) | Low (96%) |
| Asthma | 1 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, iPSC-MSCs | Reduced IL-4, eosinophils, collagen, AHR; increased IL-10 | Promising but heterogeneous | Moderate (72%–93%) |
| Bone Injury | 1 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, dental MSCs | Increased BV/TV (22.2%), NBF (26.1%), mTOR/AKT, BMP2 activation | Promising but heterogeneous | Low (97%–98%) |
| Cardiovascular Diseases | 2 | Mice, rats, pigs | BM-MSCs, UC-MSCs, AD-MSCs, CPCs, ESCs | Reduced infarct size (SMD -5.87, 95% CI -7.07 to −4.67), apoptosis; improved EF (SMD 1.57, 95% CI 0.86–1.26), angiogenesis | Promising but heterogeneous | Moderate (86%–94%) |
| Chronic Kidney Disease | 2 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs | Reduced fibrosis, inflammation; improved GFR, renal function | Promising but heterogeneous | Moderate (67%–95%) |
| Diabetic Kidney Disease | 2 | Mice, rats, shrews | BM-MSCs, UC-MSCs, AD-MSCs, others | Reduced SCr, BUN, fibrosis; increased IL-10; improved histology | Promising but heterogeneous | Moderate (60%–94%) |
| Diabetic Wounds | 2 | Mice, rats | AD-MSCs, BM-MSCs, UC-MSCs, others | Enhanced closure (SMD 4.22, 95% CI 3.07–5.36), angiogenesis (SMD 9.27, 95% CI 4.70–13.83), collagen | Promising but heterogeneous (ADSC-EVs, ApoSEVs best) | Moderate-High (39%–88%) |
| Erectile Dysfunction | 1 | Rats | MSCs, AD-MSCs, UC-MSCs | Improved ICP/MAP, NOS, smooth muscle ratio | Promising but heterogeneous | Moderate (74%–86%) |
| Hemorrhagic Stroke | 1 | Mice, rats | BM-MSCs, AD-MSCs, UC-MSCs | Improved neurobehavior in SAH (SMD -3.49, 95% CI -4.23 to −2.75), chronic ICH; reduced apoptosis, inflammation | Promising but heterogeneous (SAH, chronic ICH) | Moderate (23%–92%) |
| Intrauterine Adhesion | 1 | Rats, rabbits | UC-MSCs, BM-MSCs, AD-MSCs, others | Increased endometrial thickness (WMD 132.36, 95% CI 118.99–145.74), glands; reduced fibrosis | Promising but heterogeneous (HA/collagen enhanced) | Moderate (54%–95%) |
| Ischemic Stroke | 4 | Mice, rats, monkeys, ewes | BM-MSCs, UC-MSCs, AD-MSCs, NSCs, others | Reduced infarct volume (SMD -3.76, 95% CI -4.22 to −3.29), mNSS; enhanced neurovascular repair | Promising but heterogeneous (BMSC-EVs best) | Moderate (43%–92%) |
| Kidney Transplantation | 1 | Mice, rats | BM-MSCs, AD-MSCs | Prolonged graft survival; MSC-EVs not significant | Low (MSC-EVs) | Low (91%–94%) |
| Knee Osteoarthritis | 1 | Rats | BM-MSCs, UC-MSCs, AD-MSCs, others | Improved OARSI score (SMD -2.97, 95% CI -3.62 to −2.31), collagen II; reduced IL-1β, TNF-α | Promising but heterogeneous (UMSC-EVs best) | Moderate (0%–81%) |
| Liver Diseases | 1 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, others | Improved liver enzymes, reduced fibrosis, inflammation | Promising but heterogeneous | Moderate-High (0%–80%) |
| Liver Fibrosis | 1 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs | Reduced collagen (SMD -2.92, 95% CI -4.76 to −1.08), α-SMA; improved ALT, AST | Promising but heterogeneous (ADSC-EVs, EV + drugs best) | Moderate (70%–91%) |
| Multiple Sclerosis | 1 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, PDLSCs | Improved clinical score (SMD -2.17, 95% CI -3.99 to −0.34); reduced inflammation | Promising but heterogeneous (PDLSCs best) | Moderate (84%) |
| NAFLD/NASH | 1 | Mice, rats | UC-MSCs, AD-MSCs, BM-MSCs | Reduced liver fat, inflammation; increased SOD | Promising but heterogeneous | Not reported |
| Osteoporosis | 1 | Mice, rats | UC-MSCs, BM-MSCs, AD-MSCs | Improved BMD, bone microstructure | Promising but heterogeneous | Low-Moderate (71%–87%) |
| Osteosarcoma | 1 | Mice | BM-MSCs, AD-MSCs, macrophages | Reduced tumor volume; macrophage-EVs most effective | Promising but heterogeneous | Moderate (40%–70%) |
| Periodontal Regeneration | 2 | Mice, rats, beagles | BM-MSCs, UC-MSCs, dental MSCs | Increased BV/TV (WMD 14.07, 95% CI 6.73–21.41), BMD; reduced CEJ-ABC | Promising but heterogeneous (preconditioned EVs best) | Moderate (36%–99%) |
| Premature Ovarian Insufficiency | 1 | Mice | BM-MSCs, UC-MSCs, AD-MSCs, others | Improved AMH (SMD 5.39, 95% CI 3.43–7.36), E2; reduced FSH | Promising but heterogeneous | Moderate (76%–95%) |
| Respiratory Diseases | 2 | Mice, rats, pigs | BM-MSCs, UC-MSCs, AD-MSCs | Reduced lung injury (SMD -4.02, 95% CI -5.28 to −2.23); improved survival (OR 6.45, 95% CI 2.78–14.97) | Promising but heterogeneous | Moderate (67%–95%) |
| Sepsis | 1 | Mice, rats, sheep | BM-MSCs, UC-MSCs, AD-MSCs | Improved survival, organ function; reduced TNF-α, IL-6 | Promising but heterogeneous | Moderate (Not reported) |
| Spinal Cord Injury | 4 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, NSCs | Improved BBB score (WMD 3.47, 95% CI 3.31–3.63); reduced inflammation, apoptosis | Promising but heterogeneous (BMSC-EVs, NSC-EVs best) | Moderate (75%–81%) |
| Subarachnoid Hemorrhage | 1 | Mice, rats | BM-MSCs, UC-MSCs | Improved neurobehavior; reduced brain edema | Promising but heterogeneous | Moderate (58%–89%) |
| Traumatic Brain Injury | 2 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, astrocytes | Improved mNSS (SMD -4.48), MWM; reduced inflammation, lesion volume | Promising but heterogeneous (AEVs best early) | Moderate (76%–94%) |
| Wound Healing/Skin Regeneration | 1 | Mice, rats | BM-MSCs, UC-MSCs, AD-MSCs, others | Improved closure (SMD 3.60, 95% CI 3.23–3.96), angiogenesis, collagen | Promising but heterogeneous (ApoSEVs, ADSC-EVs best) | Moderate (82%–85%) |
Comprehensive summary of mesenchymal stem cell-derived extracellular vesicles-based therapies across diseases and conditions.
Abbreviations: MSC-EVs, Mesenchymal stem cell-derived extracellular vesicles; BM-MSCs, Bone marrow MSCs; UC-MSCs, Umbilical cord MSCs; AD-MSCs, Adipose tissue MSCs; SCr, Serum creatinine; BUN, blood urea nitrogen; BBB, basso, Beattie, Bresnahan; mNSS, modified neurological severity score; MWM, morris water maze; SMD, standardized mean difference; WMD, weighted mean difference; CI, confidence interval; BV/TV, Bone volume/total volume; CEJ-ABC, Cementoenamel junction-alveolar bone crest; AHR, Airway hyper-responsiveness; AMH, Anti-Müllerian hormone; NAFLD, Non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
Administration routes are summarized by disease model; CNS, models frequently employed intrathecal or intranasal delivery, whereas local injection/hydrogel strategies were common in wound and periodontal models.
High effectiveness required SMD >1.5, p < 0.01, and I2 < 70% in ≥2 independent meta-analyses. Outcomes with I2 ≥ 70% were reclassified as Promising but heterogeneous.
Administration routes varied substantially across conditions. Intravenous delivery was the predominant method in most disease models, including renal and hepatic injury. For CNS models such as spinal cord injury and ischemic stroke, intrathecal, intranasal, or intracerebroventricular administration was frequently used and, in some cases, demonstrated greater efficacy by enabling direct delivery across the blood–brain barrier. For local diseases such as diabetic wounds and periodontal regeneration, local injections or hydrogel/scaffold-based delivery systems were commonly applied, supporting tissue retention and enhancing therapeutic benefit. These findings, summarized in Table 4, indicate that administration route is an important factor influencing MSC-EV efficacy and should be tailored to the target organ and disease.
TABLE 4
| Author(s) (Year) (references) | MSC source | EV dose | Administration route | Dose unit | Dose-response studied |
|---|---|---|---|---|---|
| Aghayan et al. (2024) | BM-MSCs UC-MSCs AD-MSCs |
2 μg–300 µg 1 × 108 to 1 × 1011 particles |
IV IP IT |
µg Particle number |
Not |
| Bailey et al. (2022) | Various tissues | 10–200 µg 1.83 × 1010–5.22 × 1010 particles |
Hydrogel Intradermal SC Direct injection |
µg Particle number |
Not |
| Bernardi et al. (2025) | BM-MSCs UC-MSCs AD-MSCs |
Single or multiple bolus various time points (0–168 h post-ischemia) |
IV IC IN Intraarterial Others |
Not uniformly reported (µg or particle number) | Yes |
| Chen et al. (2023) | BM-MSCs | 20–100 μg EV protein (injected daily for 3–7 days) 1.6–4.2 × 108 particles |
IV IN Local injection |
µg Particle number |
Partially |
| Chen et al. (2024) | BM-MSCs UC-MSCs AD-MSCs uMSCs MenSCs |
25–100 µg (mass) 0.25–0.5 mL (volume) 2.13 × 107· particles |
Intrauterine IV |
µg mL Particle number |
Not |
| Dai et al. (2025) | BM-MSCs UC-MSCs AD-MSCs |
30–200 µg or ∼1 × 109 particles | IV | µg Particle number |
Yes |
| Fang et al. (2022) | BM-MSCs AD-MSCs |
9.6–11.7 µg 1–1.4 × 109 particles 10 µg |
IV Intrasplenic |
µg Particle number |
Not |
| Fang et al. (2023) | BM-MSCs UC-MSCs AD-MSCs ESC-MSCs |
100–500 µg per injection | IV Local injection |
µg | Not |
| Firouzabadi et al. (2024a) | BM-MSCs UC-MSCs AD-MSCs iPSC-MSCs |
20–100 µg 1 × 109 to 5 × 105 particles mostly single or 2-dose regimens |
IV IN |
µg Particle number |
Not |
| Firouzabadi et al. (2024b) | BM-MSCs UC-MSCs AD-MSCs iPSC-MSCs AF-MSC |
10 μg–400 µg total dose ranged from 10 to 1200 µg |
IV Intra-ovarian IP |
µg Particle number |
Not |
| Gunjan et al. (2024) | BM-MSCs UC-MSCs AD-MSCs |
Varied from 30 to 150 µg ∼1.8 × 1010 to 5.2 × 1010 particles |
Local SC Hydrogel |
µg Particle number |
Not |
| He et al. (2022) | BM-MSCs UC-MSCs |
100 µg 200–400 µg 2 × 105 MSCs |
IV ICV IN |
µg Particle number |
Not |
| He et al. (2023) | BM-MSCs UC-MSCs AD-MSCs ESC-MSCs |
20–200 µg ∼1–5 × 109 |
IV Local injection |
µg Particle number |
Yes |
| Hickson et al. (2021) | BM-MSCs UC-MSCs AD-MSCs |
40–200 µg protein per dose 1 × 109–1 × 1011 particles |
IV IP |
µg Particle number |
Not |
| Himanshu et al. (2025) | BM-MSCs UC-MSCs AD-MSCs PSC-MSCs |
20–250 µg protein 1 × 105–1 × 1011 particles |
IV IM IP IC |
µg Particle number |
Not |
| Jabermoradi et al. (2025) | BM-MSCs UC-MSCs AD-MSCs |
20–150 µg 5 × 109 × 1010 particles |
IV IT Direct spinal cord |
µg Particle number |
Yes |
| Kirkham et al. (2022) | BM-MSCs AD-MSCs UC-MSCs Dental MSCs |
1–200 µg or 1–1000 × 108 particles | Local implantation (hydrogel/scaffold) Local injection IV |
µg Particle number |
Not |
| Liu et al. (2020a) | BM-MSCs UC-MSCs WJ-MSCs AD-MSCs UVECs |
100 µg (20–200 µg) 2–5 × 1010 particles |
IV Renal capsule |
µg Particle number |
Yes |
| Liu et al. (2024) | BM-MSCs UC-MSCs AD-MSCs |
30–150 µg 1 × 109 × 1011 particles per dose |
IV IN ICV |
µg Particle number |
Not |
| Lou et al. (2025) | BM-MSCs UC-MSCs AD-MSCs |
25–100 µg per dose occasional studies used 1 × 1010 particles |
Corpus cavernosum IV |
µg Particle number |
Not |
| Lv et al. (2025) | UCB-MSCs | 1 × 104 to 1 × 106 | IV IP |
Particle number | Yes |
| Mou et al. (2025) | BM-MSCs UC-MSCs AD-MSCs Placenta- MSCs |
20–200 µg 1 × 109 to 2 × 1010 particles per dose |
IV IT Local injection |
µg Particle number |
Not |
| Nowak et al. (2022) | BM-MSCs UC-MSCs AD-MSCs |
30–200 µg 1 × 109–2 × 1010 particles per dose |
IV Renal capsule |
µg Particle number |
Not |
| Shang et al. (2024) | BM-MSCs AD-MSCs UC-MSCs NSCs |
40–200 µg per dose | IV | µg | Not |
| Soltani et al. (2024) | AD-MSCs | 10–200 µg per dose 1 × 109–2 × 1010 particles; mostly single dose |
SC Hydrogel/dressing delivery |
µg Particle number |
Yes |
| Tieu et al. (2021) | BM-MSCs AD-MSCs UC-MSCs |
50–250 µg 1 × 109 × 1011 particles |
SC Topical IV |
µg Particle number |
Partially |
| Wang et al. (2020) | BM-MSCs UC-MSCs AD-MSCs WJ-MSCs |
10–100 µg protein 1 × 105–108 particles |
IV IT Intratracheal |
µg Particle number |
Not |
| Wang et al. (2024) | BM-MSCs UC-MSCs AD-MSCs |
20–400 µg protein 3 × 106 cells equivalent |
IV Intraventricular |
µg Cell-equivalent |
Yes |
| Wang et al. (2025) | UC-MSCs BM-MSCs |
30–200 µg 1 × 109–1 × 1010 particles per injection |
IV IN |
µg Particle number |
Not |
| Wendt et al. (2018) | BM-MSCs | 30–100 µg per injection | IV Local injection |
µg | Not |
| Xu et al. (2024) | BM-MSCs UC-MSCs AD-MSCs iPSC-MSCs |
10–300 µg 2 × 106–3 × 1011 particles 10–200 μg/kg 800 ng-100 µg |
IV IN Intracerebral |
µg µg/kg Particle number |
Yes |
| Xun et al. (2022) | UC-MSCs AD-MSCs |
100–300 µg 1 × 109 to 2 × 1010 particles |
IV IN |
µg Particle number |
Partially |
| Yang et al. (2022) | BM-MSCs AD-MSCs |
100–700 µg | IV Intrathecal |
µg | Yes |
| Yang et al. (2023a) | BM-MSCs UC-MSCs AD-MSCs NSCs |
3–200 µg 3 × 1010 particles 1.5 × 106 cells |
IV Intraventricular Retroorbital |
µg Particle number Cell-based equivalent |
Yes |
| Yang et al. (2023b) | BM-MSCs UC-MSCs AD-MSCs Placenta-MSCs |
100 µg 20–400 µg 1 × 109–3 × 1011 particles |
IV IT IN Retroorbital ICV |
µg Particle number |
Yes |
| Ye et al. (2024) | BM-MSCs | 100 µg per injection 100–500 µg |
IV IT |
µg | Yes |
| Yi and Wang (2021) | BM-MSCs UC-MSCs AD-MSCs NSCs EF-MSCs |
10–700 µg per injection 200 μg/mL 5 × 1010 particles |
IV IT IN Intracerebral Retroorbital |
µg µg/mL Particle number |
Yes |
| Yue et al. (2024) | BM-MSCs UC-MSCs AD-MSCs |
10–200 µg per injection 1 × 109 to 2 × 1010 particles |
SC Intradermal Hydrogel-assisted topical delivery |
µg Particle number |
Not |
| Zhang et al. (2016a) | BM-MSCs UC-MSCs AD-MSCs |
10–200 µg per dose | IV | µg | Not |
| Zhang et al. (2016b) | ESC-MSCs BM-MSCs |
100–200 µg | IV | µg CM-equivalent |
Not |
| Zhang et al. (2022) | BM-MSCs UC-MSCs AD-MSCs |
50–200 µg 1 × 109–2 × 1010 particles |
IV IN |
µg Particle number |
Yes |
| Zhang et al. (2025) | BM-MSCs UC-MSCs AD-MSCs |
100 µg Up to 300 µg |
IV Local cerebral injection |
µg | Yes |
| Zhou et al. (2023a) | BM-MSCs Dental MSCs |
100–300 µg 1 × 109–2 × 1010 particles |
Local gingival injection IV Scaffold implantation |
µg Particle number |
Not |
| Zhou et al. (2023b) | BM-MSCs Dental MSCs |
100 µg 1.5 × 109 particles |
Local injection | µg Particle number |
Not |
| Zhou et al. (2024) | BM-MSCs UC-MSCs AD-MSCs TMSC AMSCs |
40–400 µg 100–250 µg |
IV IP Liver lobe injection |
µg | Yes |
| Zhou et al. (2025) | BM-MSCs UC-MSCs MenSCs |
10–100 µg per injection | Intrauterine IV |
µg | Not |
| Zhidu et al. (2024) | PDLSCs DPSCs SCAPs SHEDs |
50–300 µg 1 × 109–2 × 1010 particles |
Bone defect implantation Local injection |
µg Particle number |
Not |
| Zhu et al. (2025) | BM-MSCs UC-MSCs AD-MSCs |
30–200 µg 1 × 109–2 × 1010 particles |
Topical hydrogel SC |
µg Particle number |
Yes |
Overview of mesenchymal stem cell-derived extracellular vesicle (MSC-EV) dosing strategies, sources, administration routes, dose units, and evaluation of dose-response effects in preclinical meta-analyses.
Abbreviations: MSC, mesenchymal stem cell; BM-MSCs, Bone Marrow-Derived Mesenchymal Stem Cells; UC-MSCs, Umbilical Cord-Derived Mesenchymal Stem Cells; AD-MSCs, Adipose Tissue-Derived Mesenchymal Stem Cells; WJ-MSCs, Wharton’s Jelly-Derived Mesenchymal Stem Cells; UCB-MSCs, Umbilical Cord Blood-Derived Mesenchymal Stem Cells; AF-MSCs, Amniotic Fluid-Derived Mesenchymal Stem Cells; ESC-MSCs, Embryonic Stem Cell-Derived Mesenchymal Stem Cells; IV, intravenous; IP, intraperitoneal; IT, intrathecal; IC, intracardiac; IN, intranasal; ICV, intracerebroventricular; IM, intramuscular; iPSC-MSCs, Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells; PSC-MSCs, Pluripotent Stem Cell-Derived Mesenchymal Stem Cells; NSCs, Neural Stem Cells; EF-MSCs, Endometrial Fibroblast-Derived Mesenchymal Stem Cells; Dental MSCs, Dental Tissue-Derived Mesenchymal Stem Cells; DPSCs, Dental Pulp Stem Cells; SHEDs, Stem Cells from Human Exfoliated Deciduous Teeth; SCAPs, Stem Cells from Apical Papilla; PDLSCs, Periodontal Ligament Stem Cells; TMSCs, Tonsil-Derived Mesenchymal Stem Cells; AMSCs, Amniotic Membrane-Derived Mesenchymal Stem Cells; MenSCs, Menstrual Blood-Derived Mesenchymal Stem Cells; uMSCs, Uterine-Derived Mesenchymal Stem Cells; Placenta-MSCs, Placenta-Derived Mesenchymal Stem Cells; SC, subcutaneous.
Across the included meta-analyses, MSC-EV doses varied widely depending on disease model, administration route, and MSC source. The reported doses ranged from as low as 2 μg to as high as 700 μg of EV protein per injection, or from 1 × 105 to 1 × 1011 particles per dose. Most studies administered EVs intravenously, although intranasal, intrathecal, subcutaneous, intrauterine, and local delivery via hydrogels or scaffolds were also frequently reported. A new supplementary table (Table 4) was created to summarize these dosing parameters, including dose units, routes, and whether dose-response relationships were investigated. Among the reviewed studies, approximately one-third conducted some form of dose-response assessment, with 100 μg per injection emerging as a commonly effective dose across multiple conditions, including spinal cord injury, ischemic stroke, and diabetic wound healing.
To integrate the evidence across sources and disease categories, we created a Bubble chart (Figure 4) mapping MSC-EV sources against disease models. This visualization includes only meta-analyses with AMSTAR-2 high or moderate confidence and I2 < 70%. Cells indicate the number of supporting meta-analyses, with darker shading representing stronger evidence. Hollow dots mark disease–source pairs where evidence exists but heterogeneity was high (I2 ≥ 70%). This figure highlights consistent support for BM-MSC-EVs in neurological diseases (stroke, SCI), AD-MSC-EVs in diabetic wound healing, and UC-MSC-EVs in musculoskeletal and periodontal regeneration.
FIGURE 4
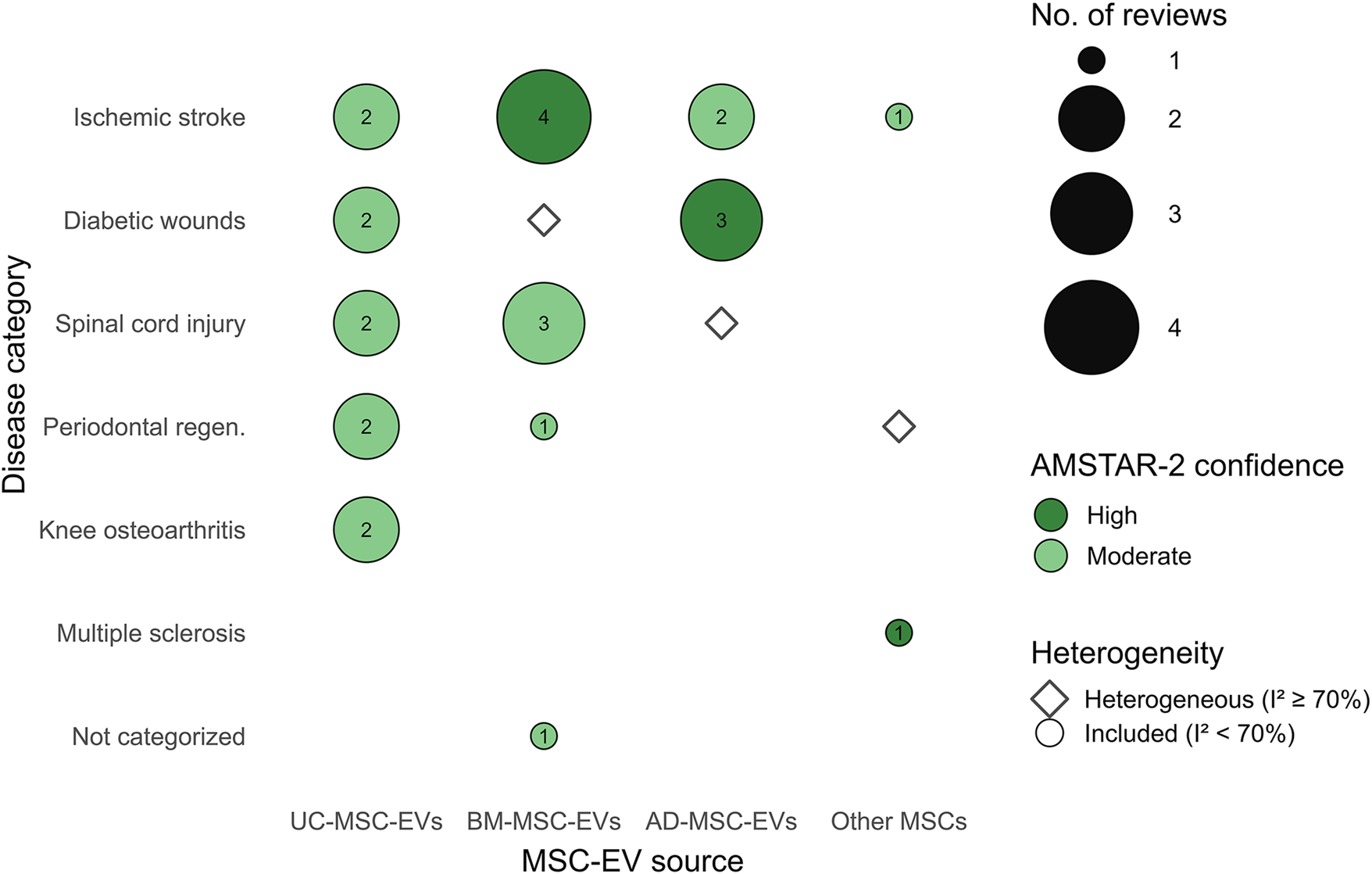
Bubble map of MSC-EV sources across disease categories, summarizing higher-quality meta-analyses. Filled bubbles indicate pairs supported by reviews with AMSTAR-2 High/Moderate confidence and I2 < 70%; bubble size encodes the number of such reviews. Hollow diamonds mark pairs reported with I2 ≥ 70% (promising but heterogeneous). Other MSCs aggregates less-frequent sources. Abbreviations: BM-MSC-EVs, bone marrow–derived; AD-MSC-EVs, adipose-derived; UC-MSC-EVs, umbilical cord–derived.
3.2 Exosome source and therapeutic efficacy
The therapeutic efficacy of MSC-EVs varied notably depending on their cellular source (Table 5). Among the sources, BM-MSCs were the most extensively studied, with approximately 308 studies. These EVs demonstrated high effectiveness across multiple conditions, including ischemic stroke, SCI, acute kidney injury, and cardiovascular diseases. BMSC-EVs were particularly effective in reducing infarct size, improving neurological function scores, and promoting neuroregeneration.
TABLE 5
| Stem cell source | Number of studies | Common disease targets | Key outcomes | Reported efficacy |
|---|---|---|---|---|
| Bone Marrow (BM-MSC) | 308 | Ischemic Stroke, IUA, TBI, Diabetic Wounds, Kidney Transplantation, Liver Diseases, NAFLD/NASH, SAH, Osteoporosis, DKD, SCI, Bone Injury, Osteosarcoma, AKI, CKD, POI, Asthma, Hemorrhagic Stroke, ALI/ARDS, Knee OA, MS, Cardiovascular Diseases, Periodontal Regeneration, Wound Healing, Liver Fibrosis | Reduced cerebral infarct volume (SMD -3.76), mNSS (SMD -2.11), SCr (MD -0.93 mg/dL), inflammation (TNF-α, IL-6, IL-1β; SMD -3.12), apoptosis (SMD -4.52), fibrosis, ALT, AST; improved AMH (SMD 5.39), BV/TV (WMD 14.07%), BBB score (WMD 3.47), wound closure (SMD 3.60), angiogenesis (SMD 4.64), EF (SMD 1.57) | High (less effective for kidney transplantation, acute/subacute ICH; best for revascularization) |
| Adipose Tissue (AD-MSC) | 154 | Ischemic Stroke, IUA, Diabetic Wounds, Sepsis, Kidney Transplantation, Liver Diseases, NAFLD/NASH, Osteoporosis, DKD, SCI, Bone Injury, Osteosarcoma, AKI, ED, CKD, Asthma, Hemorrhagic Stroke, ALI/ARDS, Knee OA, MS, Cardiovascular Diseases, TBI, POI, Wound Healing, Liver Fibrosis | Reduced inflammation (IL-6, TNF-α; SMD -2.30), cerebral infarct volume (SMD -3.76), SCr (MD -0.93 mg/dL), fibrosis, ALT, AST; improved wound closure (SMD 4.22), angiogenesis (SMD 4.64), AMH (SMD 5.39), BBB score (SMD -3.29), GFR | High (most effective for angiogenesis, wound closure; less effective for SCI, acute/subacute ICH) |
| Umbilical Cord (hUC-MSC) | 119 | Ischemic Stroke, IUA, Diabetic Wounds, Sepsis, Liver Diseases, NAFLD/NASH, SAH, Osteoporosis, DKD, SCI, Bone Injury, AKI, CKD, POI, Asthma, Hemorrhagic Stroke, ALI/ARDS, Knee OA, MS, Cardiovascular Diseases, TBI, Periodontal Regeneration, Wound Healing, Liver Fibrosis | Reduced inflammation (IL-6, TNF-α; SMD -2.30), cerebral infarct volume (SMD -3.76), SCr (MD -0.93 mg/dL), fibrosis, ALT, AST; improved AMH (SMD 5.39), wound closure (SMD 3.60), angiogenesis (SMD 4.64), BBB score (SMD -3.29), EF (SMD 1.57) | High (most effective for knee OA, periodontal regeneration) |
| Menstrual Blood (MenSC) | 6 | IUA, Diabetic Wounds, Liver Diseases, POI, Wound Healing | Reduced fibrosis, inflammation, ALT, AST; improved wound closure (SMD 3.60), angiogenesis, AMH, E2, pregnancy odds | High |
| Uterus (uMSC) | 1 | IUA | Reduced fibrosis; increased gland number | High |
| Synovial (SMSC) | 6 | Diabetic Wounds, Knee OA, Wound Healing | Reduced IL-1β, TNF-α, MMP-13; improved wound closure (SMD 3.60), angiogenesis, OARSI score, type II collagen, aggrecan, IL-10 | High (superior for knee OA) |
| Decidua MSCs | 2 | Diabetic Wounds, Wound Healing | Reduced inflammation (IL-6; SMD -2.30); improved wound closure (SMD 3.16), angiogenesis (SMD 4.64), re-epithelialization (SMD 4.68) | High |
| Gingival MSCs | 3 | Diabetic Wounds, Type II Diabetic Wounds, Periodontal Regeneration | Reduced inflammation (IL-6; SMD -2.30); improved wound closure (SMD 3.16), angiogenesis (SMD 4.64), BV/TV (WMD 14.07%), CEJ-ABC (WMD -0.12 mm) | High |
| Amniotic (AMSC) | 4 | Liver Diseases, Wound Healing | Reduced ALT, AST, fibrosis; improved wound closure (SMD 3.60), angiogenesis, collagen deposition | High |
| Tonsil (TSC) | 1 | Liver Diseases | Reduced ALT, AST, fibrosis | High |
| Placental (hPMSC) | 6 | NAFLD/NASH, SCI, Asthma, Wound Healing | Reduced AST, ALT, inflammation, BALF IL-4; improved locomotion (BBB), neuro-regeneration, wound closure (SMD 3.60), angiogenesis | High |
| Urine-Derived (USC) | 7 | Osteoporosis, DKD, ED, CKD | Reduced SCr, BUN, inflammation; improved BMD, BV/TV, ICP/MAP, nNOS, eNOS, GFR | High |
| Wharton’s Jelly (hWJMSC) | 2 | SCI, AKI | Reduced inflammation, SCr, BUN, TNF-α; improved locomotion (BBB), neuro-regeneration, IL-10 | High |
| Dental Pulp (DPSC) | 5 | SCI, Knee OA, Ischemic Stroke, Periodontal Regeneration | Reduced IL-1β, TNF-α, cerebral infarct volume; improved locomotion (BBB), OARSI score, BV/TV (WMD 14.07%), type II collagen, IL-10 | High |
| Mouse Umbilical Cord (mUCMSC) | 1 | SCI | Reduced inflammation, GFAP; improved locomotion, neuro-regeneration | High |
| Kidney-Derived (KMSC) | 2 | AKI | Reduced SCr, BUN, TNF-α, apoptosis; increased IL-10 | High |
| Human Liver Stem Cell (HLSC) | 3 | AKI, CKD | Reduced SCr, BUN, TNF-α, apoptosis; increased IL-10, GFR | High |
| Human Umbilical Cord Blood (hUCB-MSC) | 8 | DKD, CKD, ED | Reduced SCr, BUN, inflammation, fibrosis; improved IL-10, E-Cadherin, ICP/MAP, nNOS, eNOS, GFR | High |
| Muscle-Derived Stem Cells (MDSC) | 1 | ED | Improved ICP/MAP, nNOS, eNOS, smooth muscle/collagen ratio | High |
| Amniotic Fluid (AF-MSC) | 11 | CKD, POI, Knee OA | Reduced IL-1β, TNF-α, SCr, BUN; improved OARSI score, type II collagen, GFR, AMH, E2, pregnancy odds | High |
| Induced Pluripotent Stem Cell (iPSC-MSC) | 6 | POI, Asthma, Wound Healing | Reduced BALF IL-4; improved follicle count, AMH, E2, pregnancy odds, wound closure (SMD 3.60), angiogenesis | High |
| Clonal MSC (H-cMSC) | 1 | POI | Improved follicle count, AMH, E2, pregnancy odds | High |
| Periodontal Ligament (PDLSC) | 9 | MS, Periodontal Regeneration | Reduced inflammation (IL-17, IFN-γ, IL-1β), microglial activation; improved clinical score (SMD -2.17), BV/TV (WMD 14.07%), remyelination, Tregs | High (most effective for MS) |
| Neural Stem Cell (NSCEVs) | 12 | TBI, SCI | Reduced inflammation; improved mNSS (MD -2.0), BBB score (SMD 0.91), neuro-regeneration | High (early effect in SCI) |
| Dental Follicle Stem Cells (DFSCs) | 2 | Periodontal Regeneration | Improved BV/TV (WMD 14.07%), BMD (SMD 0.29); reduced CEJ-ABC (WMD -0.12 mm), Tb.Sp (SMD -0.08) | High (effective for bone regeneration) |
| Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs) | 2 | Periodontal Regeneration | Improved BV/TV (WMD 14.07%), BMD (SMD 0.29); reduced CEJ-ABC (WMD -0.12 mm), Tb.Sp (SMD -0.08) | High (effective for bone regeneration) |
| Apical Papilla Stem Cells (SCAPs) | 1 | Periodontal Regeneration | Improved BV/TV (SMD 13.99), BMD (SMD 0.29); reduced CEJ-ABC (SMD -0.22), Tb.Sp (SMD -0.08) | High (effective for bone regeneration) |
| Hair Follicle MSCs | 1 | Wound Healing | Improved wound closure (SMD 3.60), angiogenesis, collagen deposition | High (superior for wound closure in diabetic models) |
| Oral Mucosa Lamina MSCs | 3 | Wound Healing | Improved wound closure (SMD 3.60), angiogenesis, collagen deposition | High |
| Orbicularis Oculi Muscle MSCs | 1 | Wound Healing | Improved wound closure (SMD 3.60), angiogenesis, collagen deposition | High |
Comprehensive analysis of mesenchymal stem cell-derived extracellular vesicles sources and their therapeutic efficacy across diseases.
AD-MSCs, represented in about 154 studies, showed the highest efficacy in the treatment of diabetic wounds. These EVs promoted angiogenesis and accelerated wound closure, and also demonstrated consistent therapeutic benefits in models of liver fibrosis and chronic kidney disease.
hUC-MSCs, reported in around 119 studies, were most effective in models of knee osteoarthritis, periodontal tissue regeneration, and skin wound healing. hUC-MSC-EVs consistently reduced inflammation and improved functional outcomes across various disease models.
EVs derived from other MSC sources, such as menstrual blood, synovial tissue, and dental pulp, were less frequently studied but showed high therapeutic potential in specific conditions. For example, EVs from menstrual blood and synovial MSCs were effective in intrauterine adhesion and osteoarthritis, respectively, while periodontal ligament-derived EVs showed strong efficacy in models of multiple sclerosis and periodontal regeneration.
Notably, modified or engineered EVs—such as those loaded with specific microRNAs or preconditioned under hypoxic conditions—often outperformed their native counterparts. These engineered vesicles showed enhanced efficacy in models of stroke, SCI, and diabetic wounds. The method of EV delivery also influenced outcomes to some extent; while hydrogels and scaffold-based approaches were used in several studies, no delivery method demonstrated consistent superiority over direct injection.
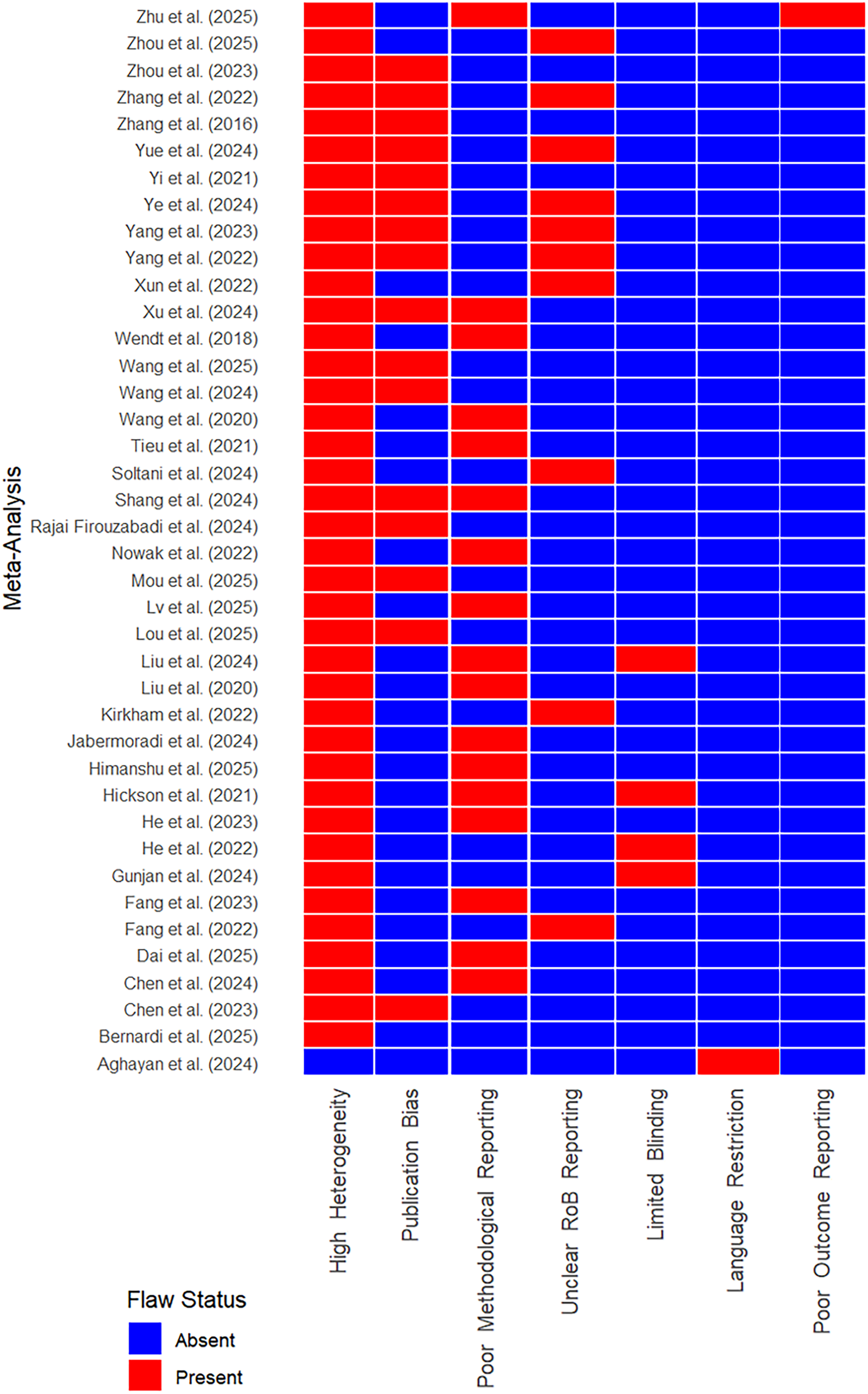
3.3 Methodological quality and risk of bias
The methodological rigor of the included meta-analyses and their underlying primary studies revealed several key challenges (Table 6). Most reviews reported a moderate to high risk of bias, assessed using tools such as SYRCLE and CAMARADES. Common methodological shortcomings included unclear random sequence generation, lack of blinding of personnel and outcome assessors, and insufficient details regarding allocation concealment. Furthermore, publication bias was detected in several high-interest disease models—including stroke, SCI, and diabetic wounds—although many findings remained robust after trim-and-fill adjustments. Across the included reviews, the most frequent biases were inadequate or unclear random sequence generation, lack of blinding of investigators and outcome assessors, and insufficient allocation concealment. These issues were consistently reported in the majority of meta-analyses and represent systemic weaknesses in preclinical MSC-EV research.
TABLE 6
| Authors, reference | Tool used | Overall RoB rating | Most common biasesa |
|---|---|---|---|
| Aghayan et al. (2024) | Novel Tool | Unclear | Methodological heterogeneity, data extraction limitations |
| Bailey et al. (2022) | SYRCLE | Unclear | Unclear randomization, allocation concealment, blinding |
| Bernardi et al. (2025) | SYRCLE | Moderate-High | Allocation, blinding, random housing |
| Chen et al. (2024) | CAMARADES | Moderate | Sample size calculation, allocation concealment, blinding |
| Chen et al. (2023) | SYRCLE | High | Allocation concealment, performance bias, detection bias |
| Dai et al. (2025) | SYRCLE | Moderate | Allocation sequence, blinding, baseline similarity |
| Fang et al. (2023) | CAMARADES | Moderate | Sample size calculation, blinding, random outcome assessment |
| Fang et al. (2022) | SYRCLE | High | Selection bias (random allocation), attrition bias |
| Firouzabadi et al. (2024a) | SYRCLE | Moderate | Blinding, allocation concealment, random outcome assessment |
| Firouzabadi et al. (2024b) | SYRCLE | Low | Sequence generation, allocation concealment, blinding |
| Gunjan et al. (2024) | SYRCLE | Moderate | Blinding, allocation concealment |
| He et al. (2022) | CAMARADES | Moderate | Sample size calculation, blinded SAH induction |
| He et al. (2023) | SYRCLE | Moderate | Allocation concealment, blinding, random outcome assessment |
| Hickson et al. (2021) | SYRCLE | Moderate | Allocation concealment, blinding, random housing, outcome assessment |
| Himanshu et al. (2025) | SYRCLE | Moderate | Blinding, allocation concealment, random housing |
| Jabermoradi et al. (2025) | SYRCLE | Moderate | Allocation concealment, blinding, random housing, outcome assessment |
| Kirkham et al. (2022) | SYRCLE | Unclear | Blinding, allocation concealment, selective reporting, randomization |
| Liu et al. (2020a) | CAMARADES | Moderate | Sample size calculation, blinded model induction, blinded outcome assessment |
| Liu et al. (2024) | SYRCLE | Unclear | Blinding, random outcome assessment, allocation concealment |
| Lou et al. (2025) | Custom (9 criteria) | High-Moderate | Blinding, sample size calculation, follow-up duration |
| Lv et al. (2025) | SYRCLE | Moderate | Allocation concealment, blinding, randomization |
| Mou et al. (2025) | SYRCLE | Low | Minor issues in randomization, blinding |
| Nowak et al. (2022) | CAMARADES | Moderate | Randomization, blinded outcome assessment, conflict of interest statement |
| Shang et al. (2024) | SYRCLE | High | Unclear randomization, allocation concealment, limited blinding (25/40 studies) |
| Soltani et al. (2024) | SYRCLE | Unclear | Lack of randomization details, unclear allocation concealment, no blinding |
| Tieu et al. (2021) | SYRCLE | Moderate | Unclear randomization, allocation concealment, partial blinding of outcome assessors |
| Wang et al. (2024) | CAMARADES | High | Lack of blinding, no sample size calculation, unclear random housing |
| Wang et al. (2020) | SYRCLE | Moderate | Unclear randomization, allocation concealment, lack of blinding, variable assessment |
| Wang et al. (2025) | SYRCLE | Moderate | Unclear randomization (24/28 studies), allocation concealment, limited blinding |
| Wendt et al. (2018) | SYRCLE | Moderate | Unclear randomization, allocation concealment, limited blinding, variable EV reporting |
| Xu et al. (2024) | SYRCLE | Moderate | Unclear randomization (32/38 studies), allocation concealment, limited blinding |
| Xun et al. (2022) | SYRCLE | Unclear | Unclear randomization, allocation concealment, blinding, incomplete outcome reporting |
| Yang et al. (2023a) | SYRCLE | Moderate | Unclear randomization, allocation concealment, limited blinding, uneven study quality |
| Yang et al. (2022) | SYRCLE | Unclear | Unclear attrition bias, selective reporting (92% unclear), publication bias |
| Yang et al. (2023b) | SYRCLE | Moderate | Unclear randomization, allocation concealment, high heterogeneity (I2 = 94% for mNSS) |
| Ye et al. (2024) | SYRCLE | Unclear | Unclear randomization (29/30 studies), blinding, allocation concealment, publication bias |
| Yi and Wang (2021) | SYRCLE | Unclear | Unclear randomization, blinding, publication bias for BBB scores (Egger’s p = 0.00) |
| Yue et al. (2024) | SYRCLE | Unclear | Unclear randomization, allocation concealment, blinding, publication bias (Egger’s p = 0.000) |
| Zhang et al. (2016a) | SYRCLE | Unclear | Unclear randomization, allocation concealment, blinding, no publication bias |
| Zhang et al. (2016b) | SYRCLE | Unclear | Unclear randomization, allocation concealment, blinding, potential publication bias |
| Zhang et al. (2022) | SYRCLE | Moderate | Unclear randomization (22/24 studies), no allocation concealment, publication bias |
| Zhang et al. (2025) | CAMARADES | Moderate | Sample size calculation, unclear randomization, blinding |
| Zhou et al. (2023a) | SYRCLE, NIH | Unclear | Unclear randomization, limited blinding, publication bias for AMH |
| Zhou et al. (2025) | Cochrane | Unclear | Unclear randomization, allocation concealment, blinding, incomplete outcome data |
| Zhou et al. (2024) | SYRCLE | Unclear | Unclear randomization, allocation concealment, lack of blinding |
| Zhou et al. (2023b) | SYRCLE | Unclear | Unclear allocation concealment, blinding, high risk for random housing |
| Zhu et al. (2025) | SYRCLE | Unclear | Unclear randomization, allocation concealment, blinding, poor dose reporting |
Comprehensive summary of risk of bias assessments in meta-analysis of mesenchymal stem cell-derived extracellular vesicles-based studies.
Common recurring issues across studies were unclear randomization procedures, lack of blinding, and poor allocation concealment.
In terms of methodological quality, all included meta-analyses received a moderate AMSTAR 2 rating (Figure 5; Supplementary Table S2). This was primarily due to high heterogeneity (with I2 values ranging from 35% to 99%) and limited reporting of essential methodological components such as randomization procedures and blinding. Important methodological shortcomings were identified in several studies, particularly incomplete or unclear risk of bias assessments and lack of consideration for publication bias. Where AMSTAR-2 critical domains were rated ‘No,’ these reviews were classified as low or critically low confidence.
FIGURE 5
Distribution of critical flaws across meta-analysis of mesenchymal stem cell-derived extracellular vesicles-based studies in AMSTAR 2 assessments.
Heterogeneity was a significant concern across the dataset, with I2 values often exceeding 70%. This variability was largely attributed to differences in animal models, MSC sources, EV dosages, and delivery routes. Despite this, sensitivity and subgroup analyses frequently confirmed the robustness of results, suggesting that the therapeutic effects of MSC-EVs were consistent across different experimental conditions.
4 Discussion
4.1 Therapeutic efficacy and clinical implications
The review suggests that MSC-EVs exhibit high efficacy across multiple disease categories, including neurological, renal, wound healing, liver, musculoskeletal, respiratory, and reproductive disorders. Notably, MSC-EVs consistently reduced inflammation and apoptosis while promoting tissue regeneration, angiogenesis, and functional recovery. For instance, in ischemic stroke, MSC-EVs reduced cerebral infarct volume (SMD -3.76) and improved neurological scores (mNSS; SMD -2.11), with BMSC-EVs showing superior efficacy (Zhao et al., 2023). Similarly, in diabetic wounds, adipose-derived EVs (ADSC-EVs) accelerated wound closure (SMD 4.22) and enhanced angiogenesis (SMD 9.27), highlighting their potential in regenerative medicine (Soltani et al., 2024).
These findings align with the broader literature on MSC-EVs, which emphasizes their role as bioactive mediators carrying microRNAs, proteins, and lipids that modulate cellular processes. The high efficacy observed in conditions like SCI and traumatic brain injury, where MSC-EVs improved locomotor scores (BBB; WMD 3.47) and cognitive outcomes (mNSS; SMD -4.48), underscores their neuroprotective and regenerative capabilities (Chen et al., 2024; Ye et al., 2024). The ability of MSC-EVs to outperform conditioned medium in acute kidney injury (Liu C. et al., 2020; Zhang G. et al., 2016) and to match or exceed MSC-based therapies in subarachnoid hemorrhage (He et al., 2022) further supports their therapeutic advantage, likely due to their stability, low immunogenicity, and ability to cross biological barriers.
The clinical implications are significant. MSC-EVs offer a cell-free therapeutic approach that circumvents challenges associated with MSC transplantation, such as immune rejection and tumorigenic risks. Their efficacy in diverse preclinical models suggests potential for broad clinical applications, particularly in conditions with high unmet needs, such as stroke, SCI, and diabetic complications. However, the variability in efficacy across diseases highlights the need for disease-specific optimization of EV sources, dosing, and delivery methods.
4.2 Exosome source and optimization
The review reveals that exosome source significantly influences therapeutic outcomes. AD-MSC-EVs excelled in wound healing, particularly diabetic wounds, where they promoted angiogenesis and collagen deposition, while BM-MSC-EVs demonstrated superior effects in neurological models. hUC-MSCs showed superior efficacy in knee osteoarthritis and periodontal regeneration, possibly due to their high proliferative capacity and immunomodulatory properties.
Emerging sources, such as periodontal ligament (PDLSCs) for multiple sclerosis (Xun et al., 2022) and menstrual blood (MenSCs) for intrauterine adhesion (Chen et al., 2023), demonstrated high efficacy despite fewer studies, suggesting untapped potential. Modified EVs, such as miRNA-loaded or hypoxia-pretreated EVs, consistently outperformed native EVs, as seen in SCI (Hu et al., 2021; Liu W. et al., 2020; Yang et al., 2024) and stroke (Li et al., 2023; Song et al., 2024), where engineered EVs enhanced functional recovery by targeting specific pathways. These findings align with recent studies emphasizing the role of EV cargo engineering in enhancing therapeutic specificity.
Delivery methods also influenced outcomes. Intravenous and intrathecal routes were most common, with intrathecal administration showing superior efficacy in SCI. Hydrogels and scaffolds improved outcomes in some contexts, but their benefit was not universal, as seen in diabetic wounds where non-hydrogel methods were equally effective (Bailey et al., 2022; Chen et al., 2025). These observations underscore the need for tailored delivery strategies based on disease pathophysiology and target tissue.
The administration route is another determinant of therapeutic outcomes. While intravenous delivery remains the most frequently used method, it may not be optimal for all disease contexts. For CNS conditions, intrathecal and intranasal delivery were more effective in bypassing the blood–brain barrier and enhancing neuroprotective outcomes. For local pathologies, such as wounds and periodontal disease, local injection and hydrogel-mediated delivery improved retention and tissue-specific effects. These observations underscore the need for future preclinical and clinical studies to systematically evaluate route-dependent biodistribution and efficacy of MSC-EVs.
This crosswalk illustrates the concentration of higher-quality evidence, showing clear clusters of BM-MSC-EVs with neurological models, AD-MSC-EVs with wound healing, and UC-MSC-EVs with musculoskeletal and periodontal regeneration. These patterns emphasize the importance of tailoring MSC-EV source selection to disease context.
4.3 Considerations on MSC-EV dose optimization
One critical but under-addressed variable in MSC-EV therapy is dosing strategy. Our umbrella review found substantial variability in reported doses, with most studies using a fixed dose (often 100 μg) without justification or titration. While several studies—such as those on SCI, stroke, and reproductive models—performed subgroup or network meta-analyses to examine dose-response relationships, the overall evidence remains fragmented and underpowered. In some cases, 100–200 μg was reported as optimal for neuroprotection or tissue regeneration, yet other studies used much higher doses (up to 700 μg) or particle-based quantifications (1 × 109 to 1011 particles).
The lack of standardized dosing metrics (mass vs. particle count), inconsistent reporting of EV characterization, and variable injection regimens further complicate cross-study comparisons. Notably, some studies administered EVs via specialized delivery systems, which could enhance local bioavailability and reduce systemic loss. However, head-to-head comparisons across these delivery platforms remain limited.
To support clinical translation, future preclinical trials should incorporate formal dose-response analyses, adopt standardized reporting in line with MISEV2023 guidelines, and evaluate pharmacokinetics and tissue distribution in parallel with efficacy outcomes (Su et al., 2025).
4.4 Mechanisms of action
The therapeutic effects of MSC-EVs are mediated through multiple mechanisms, including anti-inflammatory, anti-apoptotic, and regenerative pathways (Liao et al., 2022). The consistent reduction in proinflammatory cytokines and upregulation of IL-10 across diseases like asthma, sepsis, and liver fibrosis highlight their immunomodulatory role. In neurological disorders, MSC-EVs reduced neuronal apoptosis and promoted neurogenesis and axonal regeneration, contributing to functional recovery (Dabrowska et al., 2020). In wound healing, enhanced angiogenesis and collagen deposition were driven by EV-mediated delivery of growth factors and microRNAs (Pulido-Escribano et al., 2023).
These mechanisms are consistent with the literature, which attributes MSC-EV efficacy to their cargo of bioactive molecules, including miRNAs, proteins, and lipids. The ability of MSC-EVs to modulate multiple pathways simultaneously explains their broad efficacy but also complicates efforts to pinpoint specific mechanisms for each disease (Tsuji et al., 2020). Future studies should leverage omics technologies to elucidate disease-specific EV cargos and their targets, facilitating precision medicine approaches.
4.5 Methodological quality and limitations
A major limitation across the evidence base is the prevalence of randomization bias, lack of blinding, and inadequate allocation concealment, as summarized in Table 6. These issues undermine internal validity and may inflate reported effect sizes. The review identified significant methodological challenges that temper the interpretation of findings. Most meta-analyses reported moderate to high risk of bias, primarily due to unclear randomization, lack of blinding, and inadequate allocation concealment in primary studies. The SYRCLE and CAMARADES tools highlighted these issues, with only a few studies achieving low risk across all domains. High heterogeneity (I2 often >70%) was another concern, driven by variations in animal models, EV sources, doses, and administration protocols. While sensitivity analyses and trim-and-fill adjustments often confirmed robust findings, publication bias was evident in conditions like stroke and SCI, suggesting a potential overestimation of effect sizes. Although some outcomes showed very large effect sizes, they were accompanied by high heterogeneity (I2 ≥ 70%). In this umbrella review, we did not exclude these results but reclassified them as Promising but heterogeneous to preserve comprehensiveness while reflecting their limited certainty.
The AMSTAR 2 assessments rated all meta-analyses as moderate quality, reflecting limitations in reporting randomization, blinding, and publication bias assessments. The lack of standardized EV characterization further complicates comparisons across studies. These methodological issues align with broader challenges in preclinical research, where poor reporting and experimental design can undermine reproducibility (Simon-Tillaux et al., 2022).
The umbrella review itself has limitations. The restriction to English-language studies may have excluded relevant non-English meta-analyses (Wang et al., 2015). The reliance on reported data from included meta-analyses meant that incomplete or inconsistent reporting could affect our synthesis. Additionally, the diversity of diseases and outcomes precluded a formal meta-analysis of effect sizes, limiting our ability to quantify overall efficacy.
4.6 Limitations and considerations
Because umbrella reviews rely on published meta-analyses, we cannot exclude or re-pool individual primary studies. Instead, we downgraded evidence strength for outcomes with I2 ≥ 70% to Promising but heterogeneous. This ensures transparency while retaining the comprehensive scope of the umbrella review.
Several limitations must be considered when interpreting the findings of this umbrella review. Study quality was a notable concern, as poor reporting of critical methodological aspects such as randomization, blinding, and allocation concealment limited the reliability of some conclusions. Many primary studies scored between 3 and 7 on the SYRCLE scale, reflecting low to moderate methodological quality.
Several included reviews were of low or critically low confidence according to AMSTAR-2, and while retained for completeness, sensitivity summaries excluding these reviews are presented to indicate robustness of conclusions.
Future preclinical MSC-EV studies should implement rigorous randomization and blinding, with transparent allocation concealment, in line with ARRIVE reporting standards, to improve the reliability of pooled evidence.
Publication bias was evident in numerous conditions, including stroke, SCI, and post-operative ileus, as indicated by asymmetrical funnel plots and significant Egger’s or Begg’s test results. However, subsequent trim-and-fill analyses often confirmed the stability of the observed effects, lending credibility to the synthesized outcomes.
Another issue was the variability in exosome characterization. Some studies did not include essential quality control data, such as electron microscopy images or expression analysis of EV surface markers, which may affect the comparability and reproducibility of MSC-EV therapies.
Lastly, translational challenges remain. While MSC-EVs demonstrated high efficacy across a range of preclinical disease models, differences in dosing regimens, timing of administration, and delivery strategies must be standardized to advance these findings toward clinical application.
4.7 Future directions
Several key priorities have emerged to guide future research on MSC-EVs, with the goal of enhancing scientific rigor and accelerating clinical translation. First and foremost, there is a critical need for standardization. Uniform protocols for EV isolation, characterization, and dosing must be developed and widely adopted to ensure reproducibility and comparability across studies. In this context, strict adherence to the MISEV2023 (Minimal Information for Studies of Extracellular Vesicles) guidelines should be considered essential (Welsh et al., 2024).
In addition, mechanistic studies should be expanded using advanced omics technologies—such as proteomics, transcriptomics, and metabolomics—alongside bioinformatics tools, to elucidate disease-specific EV cargos and their molecular targets. Such insights will support the development of more tailored and effective therapeutic strategies. Optimization of MSC-EV therapies is another important area of focus. This includes exploring novel and less-studied EV sources, such as PDLSCs and MenSCs, as well as employing bioengineering strategies like microRNA loading or surface modification to enhance therapeutic potency.
Across the included meta-analyses, the most commonly reported methods were hypoxic preconditioning, miRNA-engineering, cytokine/growth factor priming, and scaffold-based conditioning. These preconditioning approaches were consistently associated with improved therapeutic efficacy, including enhanced angiogenesis, neuroprotection, and anti-inflammatory effects. For example, hypoxia-enhanced EVs showed superior functional outcomes in spinal cord injury models, while miRNA-modified EVs demonstrated targeted regulation of inflammatory and regenerative pathways (Jiang et al., 2025). Scaffold incorporation also supported sustained EV release and localized tissue repair (Leung et al., 2022). These findings suggest that preconditioning may be a key determinant of EV potency, and future research should prioritize standardized evaluation of these strategies (Liu et al., 2025).
For clinical translation, the field must now progress toward conducting early-phase clinical trials (Phase I/II) to assess the safety, tolerability, and efficacy of MSC-EVs in human subjects. Priority should be given to high-impact conditions where preclinical data already show strong therapeutic potential, such as ischemic stroke and diabetic wounds. Alongside these translational efforts, improving methodological rigor in preclinical studies is crucial. This involves proper implementation of randomization, blinding, and allocation concealment, with transparent reporting practices aligned with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Finally, addressing publication bias remains a vital consideration. The use of prospective study registration and open-access data platforms can help ensure that both positive and negative results are reported, thereby strengthening the integrity of the evidence base. By tackling these research priorities, the field can move toward more reliable, effective, and clinically applicable MSC-EV therapies.
5 Conclusion
MSC-EVs demonstrate remarkable therapeutic potential across diverse preclinical models, with high efficacy in reducing inflammation, apoptosis, and tissue damage while promoting regeneration and functional recovery. BM-, adipose-, and umbilical cord-derived EVs are particularly promising, with modified EVs offering enhanced benefits. Despite methodological limitations, the consistency of positive outcomes supports MSC-EVs as a viable therapeutic strategy. However, current studies are limited by small sample sizes, heterogeneous isolation and characterization methods, and variable outcome measures, which hinder comparability and reproducibility. Future studies should prioritize standardized protocols, robust mechanistic investigations, and rigorous experimental design to address these shortcomings. Addressing standardization, mechanistic understanding, and study quality will be critical to translating these findings into clinical practice, potentially revolutionizing treatment for a wide range of diseases.
Statements
Author contributions
NM: Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft. KZ: Investigation, Methodology, Software, Writing – original draft. AB: Formal Analysis, Investigation, Validation, Writing – review and editing. MK: Formal Analysis, Investigation, Validation, Writing – review and editing. AT: Conceptualization, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the program-targeted financing on scientific programs of the Ministry of Healthcare of the Republic of Kazakhstan « Development of an exosome isolation kit from umbilical cord mesenchymal stem cell culture for therapeutic and research application» (2024–2026) (IRN BR25593457).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1655623/full#supplementary-material
References
1
Aghayan A. H. Mirazimi Y. Fateh K. Keshtkar A. Rafiee M. Atashi A. (2024). Therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in sepsis: a systematic review and meta-analysis of preclinical studies. Stem Cell Rev. Rep.20 (6), 1480–1500. 10.1007/s12015-024-10741-3
2
Bailey A. J. M. Li H. Kirkham A. M. Tieu A. Maganti H. B. Shorr R. et al (2022). MSC-Derived extracellular vesicles to heal diabetic wounds: a systematic review and meta-analysis of preclinical animal studies. Stem Cell Rev. Rep.18 (3), 968–979. 10.1007/s12015-021-10164-4
3
Bernardi L. P. Hugentobler Schlickmann T. Carello-Collar G. De Bastiani M. A. Rigon Zimmer E. Braganhol E. et al (2025). Microglial responses to MSC-EVs treatment in animal and cellular models of ischemic stroke: a systematic review with meta-analysis. Mol. Neurobiol.10.1007/s12035-025-05025-x
4
Chen W. H. Chen S. R. Hu X. X. Huang Q. Y. Chen J. M. Lin S. et al (2023). Effects of treatment with stem cell-derived extracellular vesicles in preclinical rodent models of intrauterine adhesion: a meta-analysis. Heliyon9 (12), e22902. 10.1016/j.heliyon.2023.e22902
5
Chen C. Peng C. Hu Z. Ge L. (2024). Effects of bone marrow mesenchymal stromal cells-derived therapies for experimental traumatic brain injury: a meta-analysis. Heliyon10 (3), e25050. 10.1016/j.heliyon.2024.e25050
6
Chen L. Liu J. He Y. Zeng C. Liao W. Luo C. (2025). A systematic review and meta-analysis to investigate the effectiveness of exosome for diabetic wounds. J. Tissue Viability34 (3), 100917. 10.1016/j.jtv.2025.100917
7
Dabrowska S. Andrzejewska A. Janowski M. Lukomska B. (2020). Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front. Immunol.11, 591065. 10.3389/fimmu.2020.591065
8
Dai Q. Zhu D. Du X. Tan H. Chen Q. (2025). Therapeutic potential of mesenchymal stem cell-derived extracellular vesicle in nonalcoholic fatty liver disease: a systematic review and meta-analysis of preclinical evidence. Lipids Health Dis.24 (1), 217. 10.1186/s12944-025-02635-1
9
Fang Y. Bouari S. Hoogduijn M. J. Ijzermans J. N. M. de Bruin R. W. F. Minnee R. C. (2022). Therapeutic efficacy of extracellular vesicles to suppress allograft rejection in preclinical kidney transplantation models: a systematic review and meta-analysis. Transpl. Rev.36 (4), 100714. 10.1016/j.trre.2022.100714
10
Fang X. Gao F. Yao Q. Xu H. Yu J. Cao H. et al (2023). Pooled analysis of mesenchymal stromal cell-derived extracellular vesicle therapy for liver disease in preclinical models. J. Pers. Med.13 (3), 441. 10.3390/jpm13030441
11
Firouzabadi S. R. Mohammadi I. Ghafourian K. Kiani A. Hashemi S. M. (2024a). Mesenchymal stem cell-derived extracellular vesicle therapy for asthma in murine models: a systematic review and meta-analysis. Stem Cell Rev. Rep.20 (5), 1162–1183. 10.1007/s12015-024-10704-8
12
Firouzabadi S. R. Mohammadi I. Ghafourian K. Mofidi S. A. Firouzabadi S. R. Hashemi S. M. et al (2024b). Mesenchymal stem cell-derived extracellular vesicles therapy for primary ovarian insufficiency: a systematic review and meta-analysis of pre-clinical studies. J. Ovarian Res.17 (1), 200. 10.1186/s13048-024-01513-1
13
Gunjan H. Pandey R. P. Mukherjee R. Chang C. M. (2024). Advanced meta-analysis on therapeutic strategies of mesenchymal derived exosome for diabetic chronic wound healing and tissue remodeling. Mol. Cell Probes77, 101974. 10.1016/j.mcp.2024.101974
14
He J. Liu J. Huang Y. Lan Z. Tang X. Hu Z. (2022). Mesenchymal stem cells-derived therapies for subarachnoid hemorrhage in preclinical rodent models: a meta-analysis. Stem Cell Res. Ther.13 (1), 42. 10.1186/s13287-022-02725-2
15
He X. Wang Y. Liu Z. Weng Y. Chen S. Pan Q. et al (2023). Osteoporosis treatment using stem cell-derived exosomes: a systematic review and meta-analysis of preclinical studies. Stem Cell Res. Ther.14 (1), 72. 10.1186/s13287-023-03317-4
16
Hickson L. J. Abedalqader T. Ben-Bernard G. Mondy J. M. Bian X. Conley S. M. et al (2021). A systematic review and meta-analysis of cell-based interventions in experimental diabetic kidney disease. Stem Cells Transl. Med.10 (9), 1304–1319. 10.1002/sctm.19-0419
17
Himanshu G. Pandey R. P. Mukherjee R. Chang C. M. (2025). Meta-analysis study of the therapeutic impact of Mesenchymal stem cells derived exosomes for chronic kidney diseases. Biochem. Biophys. Rep.43, 102072. 10.1016/j.bbrep.2025.102072
18
Hu M. Cao Z. Jiang D. (2021). The effect of miRNA-Modified exosomes in animal models of spinal cord injury: a meta-analysis. Front. Bioeng. Biotechnol.9, 819651. 10.3389/fbioe.2021.819651
19
Jabermoradi S. Paridari P. Ramawad H. A. Gharin P. Roshdi S. Toloui A. et al (2025). Stem cell-derived exosomes as a therapeutic option for spinal cord injuries; a systematic review and meta-analysis. Arch. Acad. Emerg. Med.13 (1), e2. 10.22037/aaem.v12i1.2261
20
Jiang J. Wang Z. Bao Q. Chen S. Xu W. Jiang J. (2025). Extracellular vesicles as emerging therapeutic strategies in spinal cord injury: ready to Go. Biomedicines13 (5), 1262. 10.3390/biomedicines13051262
21
Kirkham A. M. Bailey A. J. M. Tieu A. Maganti H. B. Montroy J. Shorr R. et al (2022). MSC-Derived extracellular vesicles in preclinical animal models of bone injury: a systematic review and meta-analysis. Stem Cell Rev. Rep.18 (3), 1054–1066. 10.1007/s12015-021-10208-9
22
Kou M. Huang L. Yang J. Chiang Z. Chen S. Liu J. et al (2022). Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool?Cell Death Dis.13 (7), 580. 10.1038/s41419-022-05034-x
23
Leung K. S. Shirazi S. Cooper L. F. Ravindran S. (2022). Biomaterials and extracellular vesicle delivery: current status, applications and challenges. Cells11 (18), 2851. 10.3390/cells11182851
24
Li P. Yin R. Chen Y. Chang J. Yang L. Liu X. et al (2023). Engineered extracellular vesicles for ischemic stroke: a systematic review and meta-analysis of preclinical studies. J. Nanobiotechnology21 (1), 396. 10.1186/s12951-023-02114-8
25
Liao C. Chen G. Yang Q. Liu Y. Zhou T. (2022). Potential therapeutic effect and mechanisms of mesenchymal stem cells-extracellular vesicles in renal fibrosis. Front. Cell Dev. Biol.10, 824752. 10.3389/fcell.2022.824752
26
Liu C. Wang J. Hu J. Fu B. Mao Z. Zhang H. et al (2020a). Extracellular vesicles for acute kidney injury in preclinical rodent models: a meta-analysis. Stem Cell Res. Ther.11 (1), 11. 10.1186/s13287-019-1530-4
27
Liu W. Rong Y. Wang J. Zhou Z. Ge X. Ji C. et al (2020b). Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflammation17 (1), 47. 10.1186/s12974-020-1726-7
28
Liu X. Ye J. Guo W. Wang J. (2024). Significance of exosomes in osteosarcoma research: a systematic review and meta-analysis of a singular clinical investigation. Front. Cell Dev. Biol.12, 1473044. 10.3389/fcell.2024.1473044
29
Liu X. Li Z. Fu J. Wang R. He J. Yao J. et al (2025). Tailored extracellular vesicles from dental stem cells: advances in specific modifications for enhanced therapeutic applications. Int. J. Nanomedicine20, 8327–8341. 10.2147/ijn.S528190
30
Lou K. Hu J. Tong J. Wang Z. (2025). Nanoscale therapeutics for erectile dysfunction: a meta-analysis of stem cell-derived extracellular vesicles as natural nanoparticles in diabetic rat models. Stem Cell Res. Ther.16 (1), 278. 10.1186/s13287-025-04389-0
31
Lv Y. Hua Z. Lu X. (2025). Protective effects and possible mechanisms of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles against kidney fibrosis in animal models: a systematic review and meta-analysis. Front. Pharmacol.15, 1511525. 10.3389/fphar.2024.1511525
32
Mou C. Xia Z. Wang X. Dai X. Wang J. Zhang C. et al (2025). Stem cell-derived exosome treatment for acute spinal cord injury: a systematic review and meta-analysis based on preclinical evidence. Front. Neurol.16, 1447414. 10.3389/fneur.2025.1447414
33
Nowak N. Yamanouchi M. Satake E. (2022). The nephroprotective properties of extracellular vesicles in experimental models of chronic kidney disease: a systematic review. Stem Cell Rev. Rep.18 (3), 902–932. 10.1007/s12015-021-10189-9
34
Pulido-Escribano V. Torrecillas-Baena B. Dorado G. Gálvez-Moreno M. Á. Camacho-Cardenosa M. Casado-Díaz A. (2023). Combination of biomaterials and extracellular vesicles from mesenchymal Stem-Cells: new therapeutic strategies for skin-wound healing. Appl. Sci.13 (4), 2702. 10.3390/app13042702
35
Shang Z. Wanyan P. Wang M. Zhang B. Cui X. Wang X. (2024). Stem cell-derived exosomes for traumatic spinal cord injury: a systematic review and network meta-analysis based on a rat model. Cytotherapy26 (1), 1–10. 10.1016/j.jcyt.2023.09.002
36
Simon-Tillaux N. Gerard A. L. Rajendrabose D. Tubach F. Dechartres A. (2022). A methodological review with meta-epidemiological analysis of preclinical systematic reviews with meta-analyses. Sci. Rep.12 (1), 20066. 10.1038/s41598-022-24447-4
37
Soltani S. Zahedi A. Vergara A. J. S. Noli M. Soltysik F. M. Pociot F. et al (2024). Preclinical therapeutic efficacy of extracellular vesicles derived from adipose-derived mesenchymal Stromal/Stem cells in diabetic wounds: a systematic review and meta-analysis. Stem Cell Rev. Rep.20 (8), 2016–2031. 10.1007/s12015-024-10753-z
38
Song N. Scholtemeijer M. Shah K. (2020). Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol. Sci.41 (9), 653–664. 10.1016/j.tips.2020.06.009
39
Song J. Zhou D. Cui L. Wu C. Jia L. Wang M. et al (2024). Advancing stroke therapy: innovative approaches with stem cell-derived extracellular vesicles. Cell Commun. Signal22 (1), 369. 10.1186/s12964-024-01752-1
40
Su X. Wang H. Li Q. Chen Z. (2025). Extracellular vesicles: a review of their therapeutic potentials, sources, biodistribution, and administration routes. Int. J. Nanomedicine20, 3175–3199. 10.2147/ijn.S502591
41
Tieu A. Hu K. Gnyra C. Montroy J. Fergusson D. A. Allan D. S. et al (2021). Mesenchymal stromal cell extracellular vesicles as therapy for acute and chronic respiratory diseases: a meta-analysis. J. Extracell. Vesicles10 (12), e12141. 10.1002/jev2.12141
42
Tsuji K. Kitamura S. Wada J. (2020). Immunomodulatory and regenerative effects of mesenchymal stem cell-derived extracellular vesicles in renal diseases. Int. J. Mol. Sci.21 (3), 756. 10.3390/ijms21030756
43
Wang Z. Brito J. P. Tsapas A. Griebeler M. L. Alahdab F. Murad M. H. (2015). Systematic reviews with language restrictions and no author contact have lower overall credibility: a methodology study. Clin. Epidemiol.7, 243–247. 10.2147/CLEP.S78879
44
Wang F. Fang B. Qiang X. Shao J. Zhou L. (2020). The efficacy of mesenchymal stromal cell-derived therapies for acute respiratory distress syndrome-a meta-analysis of preclinical trials. Respir. Res.21 (1), 307. 10.1186/s12931-020-01574-y
45
Wang C. Yan B. Liao P. Chen F. Lei P. (2024). Meta-Analysis of the therapeutic effects of stem cell-derived extracellular vesicles in rodent models of Hemorrhagic stroke. Stem Cells Int.2024, 3390446. 10.1155/2024/3390446
46
Wang Z. Hu Z. Niu L. Xu Y. Qi Y. (2025). Mesenchymal stem cell-derived exosomes for the treatment of knee osteoarthritis: a systematic review and meta-analysis based on rat model. Front. Pharmacol.16, 1588841. 10.3389/fphar.2025.1588841
47
Welsh J. A. Goberdhan D. C. I. O'Driscoll L. Buzas E. I. Blenkiron C. Bussolati B. et al (2024). Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles13 (2), e12404. 10.1002/jev2.12404
48
Wendt S. Goetzenich A. Goettsch C. Stoppe C. Bleilevens C. Kraemer S. et al (2018). Evaluation of the cardioprotective potential of extracellular vesicles - a systematic review and meta-analysis. Sci. Rep.8 (1), 15702. 10.1038/s41598-018-33862-5
49
Xu K. Zhao X. He Y. Guo H. Zhang Y. (2024). Stem cell-derived exosomes for ischemic stroke: a conventional and network meta-analysis based on animal models. Front. Pharmacol.15, 1481617. 10.3389/fphar.2024.1481617
50
Xun C. Deng H. Zhao J. Ge L. Hu Z. (2022). Mesenchymal stromal cell extracellular vesicles for multiple sclerosis in preclinical rodent models: a meta-analysis. Front. Immunol.13, 972247. 10.3389/fimmu.2022.972247
51
Yang Z. Rao J. Liang Z. Xu X. Lin F. Lin Y. et al (2022). Efficacy of miRNA-modified mesenchymal stem cell extracellular vesicles in spinal cord injury: a systematic review of the literature and network meta-analysis. Front. Neurosci.16, 989295. 10.3389/fnins.2022.989295
52
Yang Z. Liang Z. Rao J. Lin F. Lin Y. Xu X. et al (2023a). Mesenchymal stem cell-derived extracellular vesicles therapy in traumatic central nervous system diseases: a systematic review and meta-analysis. Neural Regen. Res.18 (11), 2406–2412. 10.4103/1673-5374.371376
53
Yang Z. L. Liang Z. Y. Lin Y. K. Lin F. B. Rao J. Xu X. J. et al (2023b). Efficacy of extracellular vesicles of different cell origins in traumatic brain injury: a systematic review and network meta-analysis. Front. Neurosci.17, 1147194. 10.3389/fnins.2023.1147194
54
Yang Z. Liang Z. Rao J. Xie H. Zhou M. Xu X. et al (2024). Hypoxic-preconditioned mesenchymal stem cell-derived small extracellular vesicles promote the recovery of spinal cord injury by affecting the phenotype of astrocytes through the miR-21/JAK2/STAT3 pathway. CNS Neurosci. Ther.30 (3), e14428. 10.1111/cns.14428
55
Ye Z. Zheng Y. Li N. Zhang H. Li Q. Wang X. (2024). Repair of spinal cord injury by bone marrow mesenchymal stem cell-derived exosomes: a systematic review and meta-analysis based on rat models. Front. Mol. Neurosci.17, 1448777. 10.3389/fnmol.2024.1448777
56
Yi H. Wang Y. (2021). A meta-analysis of exosome in the treatment of spinal cord injury. Open Med. (Wars)16 (1), 1043–1060. 10.1515/med-2021-0304
57
Yue G. Li Y. Liu Z. Yu S. Cao Y. Wang X. (2024). Efficacy of MSC-derived small extracellular vesicles in treating type II diabetic cutaneous wounds: a systematic review and meta-analysis of animal models. Front. Endocrinol.15, 1375632. 10.3389/fendo.2024.1375632
58
Zhang G. Wang D. Miao S. Zou X. Liu G. Zhu Y. (2016a). Extracellular vesicles derived from mesenchymal stromal cells may possess increased therapeutic potential for acute kidney injury compared with conditioned medium in rodent models: a meta-analysis. Exp. Ther. Med.11 (4), 1519–1525. 10.3892/etm.2016.3076
59
Zhang H. Xiang M. Meng D. Sun N. Chen S. (2016b). Inhibition of myocardial Ischemia/Reperfusion Injury by exosomes secreted from mesenchymal stem cells. Stem Cells Int.2016, 4328362. 10.1155/2016/4328362
60
Zhang L. Pei C. Hou D. Yang G. Yu D. (2022). Inhibition of cerebral Ischemia/Reperfusion injury by MSCs-Derived small extracellular vesicles in rodent models: a systematic review and meta-analysis. Neural Plast.2022, 3933252. 10.1155/2022/3933252
61
Zhang X. Guo Y. Fang K. Huang X. Lan D. Wang M. et al (2025). Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles in ischemic stroke: a meta-analysis of preclinical studies. Brain Res. Bull.221, 111219. 10.1016/j.brainresbull.2025.111219
62
Zhao J. Deng H. Xun C. Chen C. Hu Z. Ge L. et al (2023). Therapeutic potential of stem cell extracellular vesicles for ischemic stroke in preclinical rodent models: a meta-analysis. Stem Cell Res. Ther.14 (1), 62. 10.1186/s13287-023-03270-2
63
Zhidu S. Ying T. Rui J. Chao Z. (2024). Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. Stem Cell Res. Ther.15 (1), 266. 10.1186/s13287-024-03885-z
64
Zhou T. Yuan Z. Weng J. Pei D. Du X. He C. et al (2021). Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol.14 (1), 24. 10.1186/s13045-021-01037-x
65
Zhou H. Qi Y. X. Zhu C. H. Li A. Pei D. D. (2023a). Mesenchymal stem cell-derived extracellular vesicles for treatment of bone loss within periodontitis in pre-clinical animal models: a meta-analysis. BMC Oral Health23 (1), 701. 10.1186/s12903-023-03398-w
66
Zhou Y. Li Q. You S. Jiang H. Jiang L. He F. et al (2023b). Efficacy of mesenchymal stem cell-derived extracellular vesicles in the animal model of female reproductive diseases: a meta-analysis. Stem Cell Rev. Rep.19 (7), 2299–2310. 10.1007/s12015-023-10576-4
67
Zhou X. Xu Y. Wang X. Lu W. Tang X. Jin Y. et al (2024). Single and combined strategies for mesenchymal stem cell exosomes alleviate liver fibrosis: a systematic review and meta-analysis of preclinical animal models. Front. Pharmacol.15, 1432683. 10.3389/fphar.2024.1432683
68
Zhou L. Cai W. Zhang Y. Zhong W. He P. Ren J. et al (2025). Therapeutic effect of mesenchymal stem cell-derived exosome therapy for periodontal regeneration: a systematic review and meta-analysis of preclinical trials. J. Orthop. Surg. Res.20 (1), 27. 10.1186/s13018-024-05403-6
69
Zhu Y. Yang H. Xue Z. Tang H. Chen X. Liao Y. (2025). Mesenchymal stem cells-derived small extracellular vesicles and apoptotic extracellular vesicles for wound healing and skin regeneration: a systematic review and meta-analysis of preclinical studies. J. Transl. Med.23 (1), 364. 10.1186/s12967-024-05744-0
Summary
Keywords
mesenchymal stem cells, extracellular vesicles, exosomes, preclinical models, umbrella review, regenerative medicine
Citation
Mussin NM, Zhilisbayeva KR, Baspakova A, Kurmanalina MA and Tamadon A (2025) Umbrella review of mesenchymal stem cell-derived extracellular vesicles in preclinical models: therapeutic efficacy across diverse conditions. Front. Cell Dev. Biol. 13:1655623. doi: 10.3389/fcell.2025.1655623
Received
28 June 2025
Accepted
29 September 2025
Published
13 October 2025
Volume
13 - 2025
Edited by
Francesco De Francesco, Azienda Ospedaliero Universitaria delle Marche, Italy
Reviewed by
Hakan Darici, University of Istinye, Türkiye
Vitali Maldonado, University of Arkansas, United States
Updates
Copyright
© 2025 Mussin, Zhilisbayeva, Baspakova, Kurmanalina and Tamadon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amin Tamadon, amintamaddon@yahoo.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.