Abstract
In the early stages of mammalian embryonic development, the bipotential gonads can differentiate into the testes or ovaries. These organs are essential for gamete production, transmitting genetic information to offspring via sperm or oocytes. Testis differentiation is triggered by the Y chromosome sex-determining region (SRY) genetic program, with male reproductive health largely established during the early stages of testis development. However, SRY is only transiently activated in precursor Sertoli cells, initiating their differentiation. At later stages, differentiated Sertoli cells are crucial for male sex determining in other cell lineages, including germ cells, Leydig cells involved in steroid hormone synthesis, and the establishment of vascular patterns. Clearly, Sertoli cells play an essential role in testis development and function, and are indispensable for male reproduction. In this review, we examine the composition and functional dynamics of the testis, highlighting how single-cell transcriptomics has redefined our understanding of testicular cellular architecture and functional diversity. We focus on the pivotal regulatory roles of Sertoli cells in orchestrating the development and functional coordination of germ cells, Leydig cells, peritubular myoid cells, and testicular macrophages. Furthermore, we discuss the pathological consequences of Sertoli cell dysfunction and its mechanistic contributions to male reproductive disorders, providing molecular insights into spermatogenic failure and androgen dysregulation.
1 Introduction
The testis is the primary organ of male sexual development and reproduction, secreting androgens to promote body masculinization and producing sperm for reproduction (Jiménez et al., 2021; Luo et al., 2022). Sertoli cells (SCs), as essential somatic cells in the testis, play a central role in testis formation during fetal development, as well as in the initiation and maintenance of spermatogenesis during puberty and adulthood. SCs also establish an immunoprivileged environment for germ cells (GCs), preventing autoimmune responses triggered by neoantigens during spermatogenesis (Luaces et al., 2023). Furthermore, SCs are key regulators of cell lineage differentiation within the testis, which promote male gonadal development (Rotgers et al., 2018), and specifies Leydig cells (LCs) and peritubular myoid cells (PMCs) fate (Li et al., 2016; Zhao et al., 2021). Postnatally, SCs also play a significant role in androgen production by LCs, development of PMCs, and regulation of the vasculature. Disruption of SCs function can lead to male reproductive disorders (see Figure 1). In this review, we systematically describe the central role of SCs in the testis, explore the mechanisms underlying their pathological dysfunction, and highlight how SCs serve as key factors in testicular development and functional maintenance. We hope this review offers insights into testis function and male reproductive health, encouraging further detailed investigations.
FIGURE 1
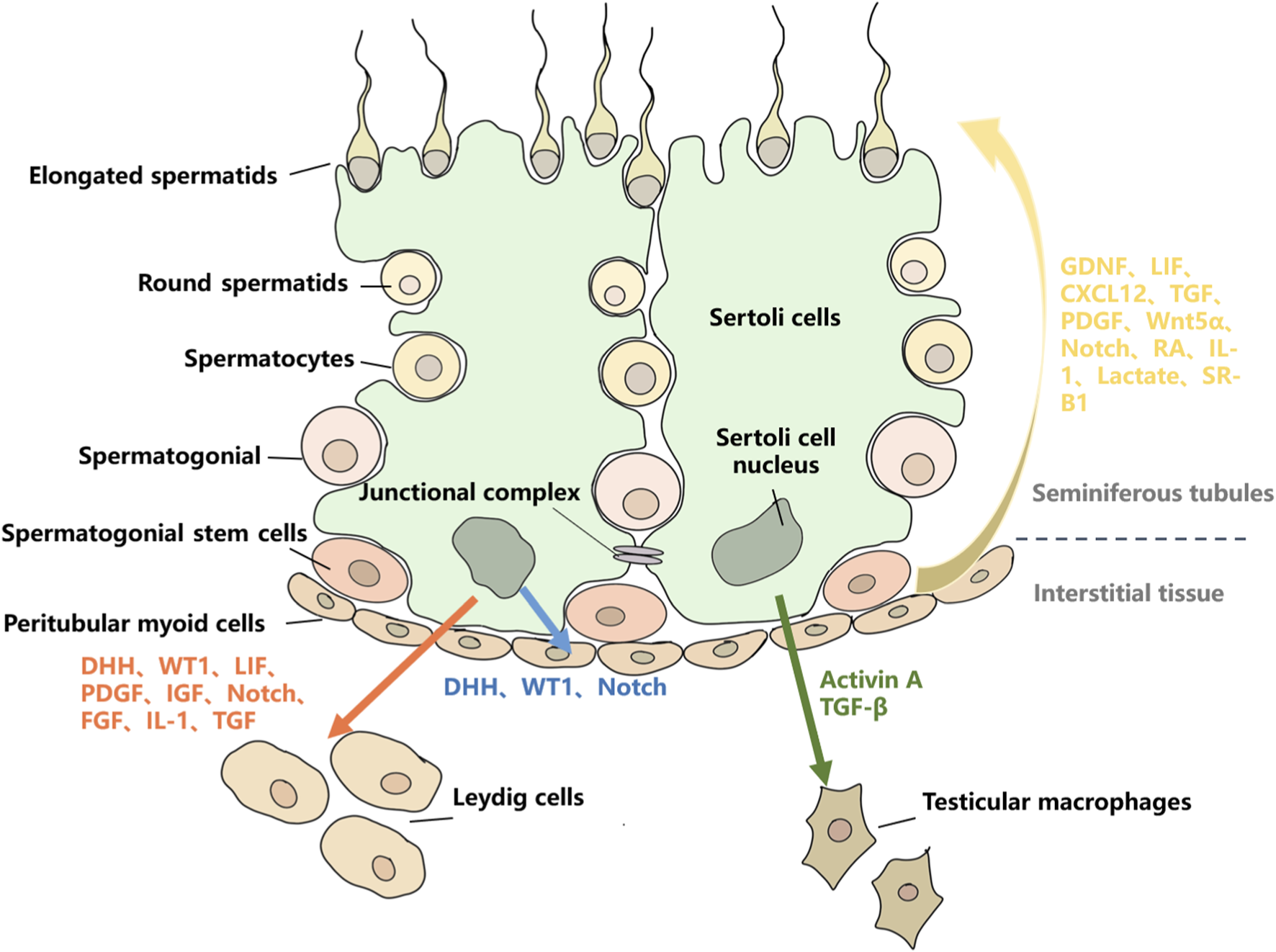
The regulatory roles of SCs on other testicular cell types. Red arrows represent the regulatory effects of SCs on LCs, mainly through the secretion of DHH, WT1, LIF, PDGF, IGF, Notch, FGF, IL-1, and TGF. Blue arrows represent the regulatory effects of SCs on PMCs, mainly through the secretion of DHH, WT1, and Notch. Green arrows represent the regulatory effects of SCs on TMs, mainly through the secretion of Activin-A and TGF-β. Yellow arrows represent the regulatory effects of SCs on GCs, mainly through the secretion of GDNF, LIF, CXCL12, TGF, PDGF, Wnt5α, Notch, RA, IL-1, Lactate, and SR-B1.
2 Composition and function of testis
The testis comprises various types of cells, including GCs, SCs, LCs, PMCs, and testicular macrophages (TMs) (Mäkelä et al., 2019). Its tissue structure is primarily divided into two regions: the seminiferous tubules and the interstitial tissue (Oatley and Brinster, 2012). In the seminiferous tubules, SCs and PMCs form the basal lamina. GCs are located on the basal membrane and are classified into three types: spermatogonial stem cells (SSCs, undifferentiated spermatogonia), spermatogonia (SSCs that undergo active mitosis), and preleptotene spermatocytes (differentiated spermatogonia) (Wanjari and Gopalakrishnan, 2024). SCs support normal spermatogenesis by providing a cellular matrix and secreting specific growth factors (Chojnacka et al., 2016; Luca et al., 2018a). During spermatogenesis, SCs exhibit significant dynamic changes and are capable of supporting the simultaneous development of various types of GCs (Sawaied et al., 2025). The types and numbers of GCs supported by SCs vary at different stages of the spermatogenic cycle, typically including one to two types of spermatogonia, one to two types of spermatocytes, and one to two types of haploid spermatids. The number of GCs supported by SCs is regulated by various factors, primarily including species differences, the developmental status of SCs before puberty, SCs density within the seminiferous tubules, and the endocrine environment. In the mouse, spermatogenesis is divided into 12 stages, forming a complete spermatogenic cycle (O’Donnell et al., 2022a). In rodents such as mice, the spermatogenic cycle occurs sequentially along the length of the tubules, while in humans and primates, multiple spermatogenic cycles are interwoven, forming a more complex spermatogenic structure (O’Donnell et al., 2022b). The interstitial space contains LCs, mesenchymal cells, and immune cells (Oatley and Brinster, 2012). LCs produce androgens and cytokines, which may act directly or indirectly to regulate SSCs self-renewal (Zaker et al., 2022). GCs, Somatic cells, and various cytokines synthesized and secreted within the testis together form the testicular microenvironment (see Figure 2) (Zhou et al., 2019). Studies have shown that within the microenvironment, different types of cells communicate through cytokines to promote spermatogenesis and testosterone synthesis (Arora et al., 2022; Li et al., 2023). These interactions are essential for the normal functioning of the testis, including cell proliferation, differentiation, metabolism, and protein transport (Arora et al., 2022). SCs are the most critical somatic cells in testis, closely associated with GCs, supporting and guiding their development into mature spermatozoa during spermatogenesis (Griswold, 2018). Similarly, SCs play a vital role in maintaining the stability of SSCs pool (Ibtisham et al., 2020).
FIGURE 2
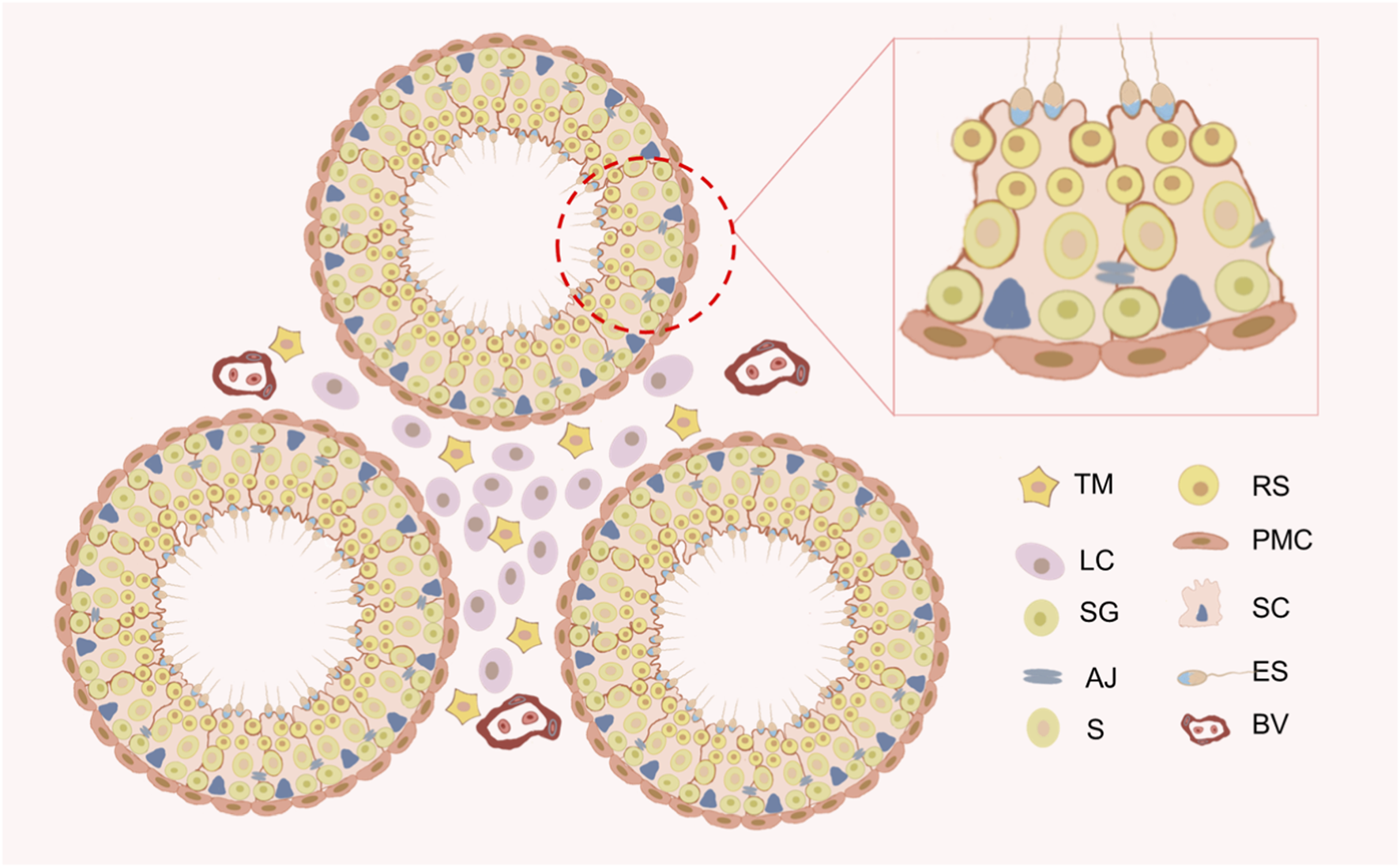
Schematic diagram of the testicular microenvironment of the mouse. The testis comprises two main components: the seminiferous tubules and the interstitial spaces. Peritubular myoid cells (PMCs) and Sertoli cells (SCs) together form the basal lamina of the seminiferous tubules. Germ cells develop in the seminiferous epithelium formed by SCs. Germ cells include spermatogonium (SG), spermatocytes (S), round spermatids (RS), and elongated spermatids (ES). The interstitial spaces primarily consist of Leydig cells (LCs) and testicular macrophages (TMs). Blood vessels (BV) are located within the interstitial spaces.
2.1 SCs
SCs, also known as “nanny cells”, are among the most intricate cells in the testis (França et al., 2016) and play crucial roles in protecting spermatogenic cells, providing nutrients and physical support for spermatogenesis and testis development (Griswold, 2018). SCs are classified based on stages into two types: immature Sertoli cells (ISCs, from fetal to pre-pubertal) and adult Sertoli cells (ASCs, from pubertal and post-pubertal) (You et al., 2021). ISCs participate in fetal sexual differentiation and pre-pubertal testicular development (Sekido and Lovell-Badge, 2013). Studies have shown that at E10-11 of mouse embryonic development, coelomic epithelial cells differentiate into gonadal somatic cells, which are bipotential can develop into either an ovary or a testis (Poulsen et al., 2019). The transition from the ovarian to the testis pathway is driven by the SRY gene expressed in pre-SCs during the narrow window of embryonic development (E10.5) (Hiramatsu et al., 2009; Bunce et al., 2023). By E12.5 of embryonic development, SCs form seminiferous cords around gonocytes (Yokonishi and Capel, 2021). Fetal Sertoli cells (FSCs) proliferation is essential for the lengthening and expansion of testicular seminiferous cords (Archambeault and Yao, 2010). After birth, SCs continue to proliferate until puberty, at which point they become non-proliferative and terminally differentiated (Meroni et al., 2019). ASCs maintain spermatogenesis during puberty and adulthood (Griswold, 2018) and provide immune protection for spermatogenic cells (Kaur et al., 2014). Normal SCs function is essential for GCs survival and fertility, and SCs dysfunction can lead to testicular pathology and male sterility (Murphy and Richburg, 2014; Ni et al., 2020). SCs exhibit strong secretory function, releasing various of substances, such as growth factors (including GDNF (Garcia et al., 2017), TGFs (Fang et al., 2023), NFG (Niu et al., 2020), SCF (Chui et al., 2011), ILs (Fadlalla, 2019), IGFs (Cannarella et al., 2019), FGFs (Tian et al., 2019), Activin (O’Donnell et al., 2022a)), transport protein (such as transferrin (Yefimova et al., 2008), androgen-binding proteins (Yefimova et al., 2008), sulfated glycoprotein (Hartmann et al., 2016), and ceruloplasmin-like protein proteins (Skinner and Griswold, 1983)) and regulatory proteins (AMH (Rodriguez et al., 2022), endothelin (Chan et al., 2008), and inhibin (Lucas-Herald and Mitchell, 2022)), these substances regulate the self-renewal and differentiation of spermatogenic cells, as well as the growth and development of the male reproductive system.
2.2 LCs
LCs are located in the interstitial spaces of the seminiferous tubules and are mainly responsible for androgen synthesis and secretion, essential for spermatogenesis (Fang et al., 2021; Medici et al., 2024). In mammals, at least two types of LCs exist: fetal Leydig cells (FLCs) and adult Leydig cells (ALCs). FLCs synthesize androgen in the testis before birth, while ALCs perform this function after birth (Teerds and Huhtaniemi, 2015; Shima, 2019). FLCs produce androstenedione, which requires converted to testosterone in the presence of FSCs (Shima et al., 2013). During embryonic development, FLCs and FSCs jointly produce high androgen levels, promoting male reproductive organ differentiation and brain masculinization (Lee et al., 2024). Postnatally, FLCs decrease in number, resulting in reduced androgen production, which eventually reaches a minimum. With the development of ALCs from testicular stem cells, androgen levels gradually increase (Chauvigné et al., 2012; Zirkin and Papadopoulos, 2018). Testosterone regulates mammalian spermatogenesis, and its deficiency in adults leads to reduced fertility, fat accumulation, impaired cognitive function, weakened immune response, and fatigue (Chen H et al., 2017). Rodent LCs development proceeds through four main stages: stem Leydig cells (SLCs), progenitor Leydig cells (PLCs), immature Leydig cells (ILCs), and ALCs, as illustrated in Figure 3A (Chen M et al., 2017). However, another type of LCs exists in the primate and human testis, short-lived during the neonatal stage, thought their origin remains unclear (Nistal et al., 1986; Prince, 1992; Prince, 2001). The hypothalamic-pituitary-testicular axis (HPTA) is transiently activated during the first 6 months after birth, promoting neonatal LCs development and increasing androgen levels. After this period, the HPTA returns to inactivity, and neonatal LCs either involute or dedifferentiate (as shown in Figure 3B) (Prince, 1990). During this brief period, the function of nascent LCs is largely unstudied and remains unknown. It is hypothesized that testosterone synthesis by neonatal LCs is linked to the central nervous system and contributes to brain development (Martin, 2016).
FIGURE 3
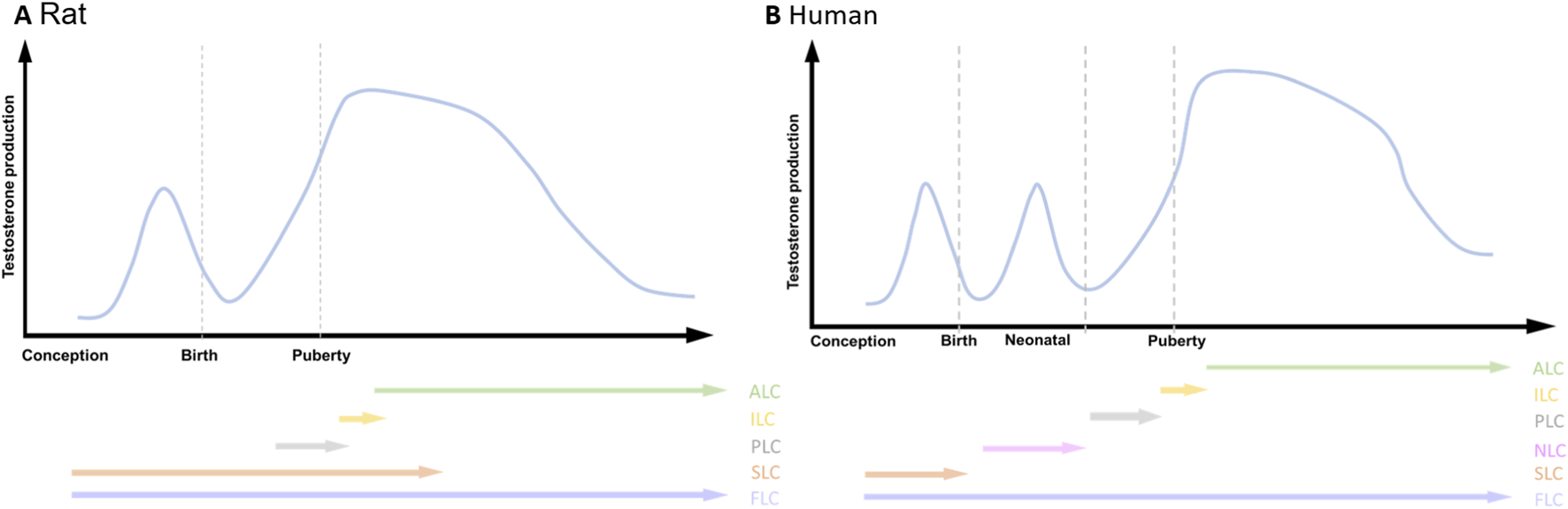
Testosterone production and the emergence of LCs during the life span. (A) Rat; (B) Human. adult Leydig cells (ALC), immature Leydig cells (ILC), progenitor Leydig cells (PLC), neonatal Leydig cells (NLC), stem Leydig cells (SLC), fetal Leydig cells (FLC). Two androgenic peaks are present in Rat, while three androgenic peaks are present in human.
2.3 TMs
TMs represent the largest reservoir of immune cells in the mammalian testis, accounting for over 20% of interstitial cells (Hume et al., 1984; Masone, 2023). TMs preserve testicular immune privilege by modulating the innate immune response (Bhushan et al., 2015; Bhushan and Meinhardt, 2017), thus attenuating pro-inflammatory responses triggered by external stimuli (Meinhardt et al., 2018). TMs are categorized into two types: interstitial macrophages and peritubular macrophages, with the former originating from embryo yolk sac cells (DeFalco et al., 2014). Fetal TMs play a crucial role in testicular morphological changes during early gonadal development and regulate vascular remodeling (Li Q et al., 2021). After birth, bone marrow progenitor cells differentiated into peritubular macrophages, which colonize empty niches as specialized tissue-resident macrophages (Mossadegh-Keller et al., 2017). Cytokines secreted by TMs promote LCs proliferation and differentiation (Gaytan et al., 1994), and stimulate steroid hormone synthesis (Hales, 2002; Hutson, 2006). These factors also contribute to the maintenance and differentiation of SSCs (DeFalco et al., 2015; Mossadegh-Keller et al., 2017), which are essential for testicular development and spermatogenesis.
2.4 PMCs
PMCs are a major component of the seminiferous tubule wall, characterized by smooth muscle-like features and playing a key role in sperm transport (Wang et al., 2018a). Evidence suggests that PMCs are closely related to spermatogenesis. During embryonic development, PMCs and SCs together form the basal lamina of the seminiferous epithelium, creating a unique microenvironment for SSCs self-renew, which maintain the number of spermatogonia and spermatocytes in the testis (Richardson et al., 1995; Uchida et al., 2020; Min et al., 2022). PMCs secrete leucine-rich repeat-containing G protein-coupled receptor 4 (LGR4), colony-stimulating factor-1 (CSF-1), and glial cell-derived neurotrophic factor (GDNF), all of which regulate spermatogenesis and testicular function (Maekawa et al., 1996; Fernandez et al., 2008; Qian et al., 2013; Chen and Liu, 2016). However, when spermatogenesis is disrupted, the structure and function of PMCs undergo changes (Mayerhofer, 2013). Additionally, other cells in the TME secrete paracrine factors that can act on PMCs, promoting cell contraction, relaxation, and seminiferous tubule peristalsis (Fleck et al., 2021; Kawabe et al., 2022). Loss of PMCs contractile function results in sterility (Wang et al., 2018b).
2.5 GCs
In contrast to somatic cells in the testis, GCs play a crucial role in reproduction by accurately transmitting genetic information from one generation to the next (Yamashita, 2020). GCs comprise SSCs, spermatogonia, early spermatocytes, spermatids, and elongated spermatozoa (Hancock et al., 2021; Ren et al., 2022). In mammals, including mice and humans, male GCs development occurs in three main stages (see Figure 4): specification of primordial germ cells (PGCs), male sex differentiation, and spermatogenesis (Saitou and Miyauchi, 2016). PGCs are induced following the differentiation of trophectoderm and primitive endoderm (Saitou and Yamaji, 2012; Sasaki et al., 2016). PGCs, the earliest GCs in mice (appearing at E6.25), are detected around E7.25 (Richardson and Lehmann, 2010; Saitou and Yamaji, 2010). PGCs begin migrating at E7.5 and colonize the genital ridge at E10.5 (Seki et al., 2007; Nicholls and Page, 2021). Upon reaching the genital ridge, PGCs undergo mitotic arrest, remaining in the G0/G1 phase and becoming quiescent (Western et al., 2008). The first spermatogonia typically appear shortly after birth, and then the spermatogonia migrate from the center of the seminiferous tubules to the periphery, where they continue to proliferate (De Rooij and Russell, 2000; Nagano et al., 2000). In many male species (such as rats, mice, cats, sheep, donkeys, cattle), spermatogenesis occurs continuously, forming meiotic spermatocytes, spermatids, and ultimately elongated sperm. However, in humans and other primates, there is a long interval between the appearance of spermatogonia and the completion of spermatogenesis, which typically begins at puberty. Additionally, in seasonally breeding mammals, the first wave of spermatogenesis must occur at the start of each breeding season because a complete set of germ cells must be formed from spermatogonia (Jones et al., 2021). It has been reported that spermatogenesis begins on postnatal day 5 in mice, and a portion of the PGCs are recruited as SSCs, which migrate to the basement membrane of the seminiferous tubules and gradually move toward the lumen as their differentiation progresses (Oatley and Brinster, 2012; Yoshida, 2020). SSCs Self-renewal and differentiation maintain the stem cell pool, forming the foundation for sperm production (Walker, 2010). In rodents, Asingle spermatogonium are considered SSCs. Through mitosis, Asingle spermatogonium form two Apaired spermatogonia, if no intercellular bridges are present, they undergo self-renewal, producing two new Asingle spermatogonium; if intercellular bridges form, they differentiate into Aaligned-4, Aaligned-8, and Aaligned-16 (Dym et al., 2009). Aaligned-16 differentiate into A1 spermatogonia, which give rise to A2, A3, A4, intermediate, and B spermatogonia through successive mitotic divisions, eventually forming primary spermatocytes (Wakayama et al., 2015). Primary spermatocytes undergo two meiotic divisions to form secondary spermatocytes and round spermatids, which then undergo spermatid metamorphosis to become elongated spermatozoa (Zhang L. et al., 2022). In mice, Asingle, Apair, and Aaligned are considered undifferentiated spermatogonia, with Asingle accounting for 10% of this cell population (Nakagawa et al., 2007; Fayomi and Orwig, 2018). However, in primates, the classification of spermatogonia differs from that in mice. The spermatogonia of primates are classified into three types: Adark, Apale, and B. Adark spermatogonia are considered reserve-type, while Apale spermatogonia are proliferative-type, and B spermatogonia correspond to the differentiated cell population in mice (Lord and Oatley, 2017; Potter and DeFalco, 2017). Research indicates that Adark spermatogonia are a form of quiescent SSCs that typically only proliferate during puberty or when a large number of spermatogonia are lost due to certain factors, in order to restore the self-renewal capacity of SSCs. Apale spermatogonia constitute the majority of SSCs and undergo self-renewal and proliferation in a regulated manner according to the spermatogenic epithelial cycle (Kubota and Brinster, 2018). However, there is some debate surrounding this concept.
FIGURE 4
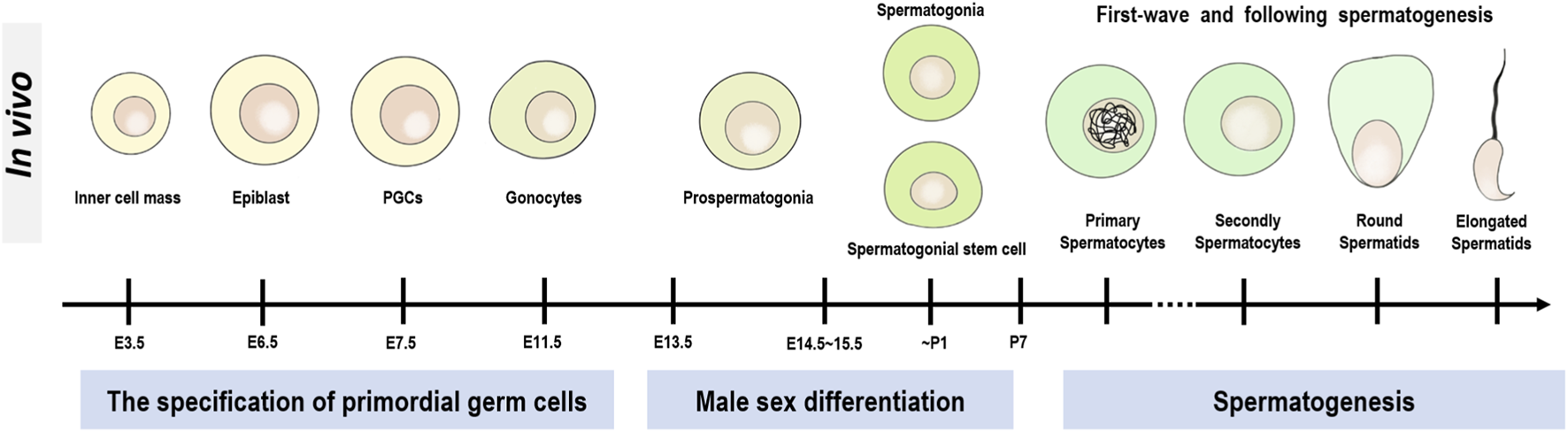
Development of GCs in male mice. The entire development of male mice GCs is divided into three main stages, the first being the specification of primordial germ cells (PGCs), the second being the stage of male sex differentiation, and the third being the stage of spermatogenesis.
2.6 Single-cell transcriptomics redefines testicular cellular architecture and functional diversity
Spermatogenesis, the cornerstone of male reproduction, involves the proliferation and differentiation of diploid germ cells into haploid flagellated spermatozoa. This tightly orchestrated process relies on intricate interactions between testicular somatic cells (e.g., SCs and LCs) and GCs. However, its pronounced cellular heterogeneity has posed long-standing challenges in resolving stage-specific cellular populations. While the roles of somatic cells in spermatogenesis are relatively well-defined, the molecular mechanisms governing their developmental maturation remain incompletely elucidated.
The application of single-cell RNA sequencing (scRNA-seq) has systematically characterized the heterogeneity of mammalian testicular cells and redefined their cellular atlases. In yaks, pigs, humans, and other species, scRNA-seq has identified GCs subpopulations (spermatogonial stem cells, spermatocytes, and spermatids) and somatic subtypes (SCs, LCs, PMCs) with distinct markers (e.g., UCHL1, SOX9, PRND) (Wang et al., 2023a; Yan et al., 2024). Pseudotime trajectory analyses further indicate a shared progenitor origin for LCs and PMCs (Yan et al., 2024), and functional enrichment demonstrated synergistic regulation between somatic signaling pathways (PI3K-AKT, Wnt) and germline modules (spermatogenesis, DNA binding) (Yan et al., 2024). Cross-species comparisons show that these cell types exhibit both conserved functions and species-specific heterogeneity in pigs, humans, and mice (Zhang W. et al., 2022; Wang et al., 2024).
Compared with other testicular somatic cell types, the developmental trajectory of SCs has been extensively characterized by scRNA-seq. Most studies classify SCs into three stages: immature (during fetal/perinatal phase/neonatal), maturing (during juvenile/prepubertal life), and mature (at puberty and adulthood) (Guo et al., 2020; Tan et al., 2020; Zhao et al., 2020; Su et al., 2025). In human testes, SCs undergo three consecutive stages during normal maturation: Stage_a, b, and c, each with a distinct gene expression profile. Stage_a is characterized by high expression of EGR3, JUN, and NR4A1; Stage_b by S100A13, ENO1, and BEX1; and Stage_c cells primarily express HOPX, DEFB119, and CST9L. This trajectory shows marked age dependence. Stage_a cells are mainly SCs from 2 years old. The numbers of Stage_a and Stage_b cells decrease with age, reaching a minimum at 11 years. Thereafter, functionally mature Stage_c cells emerge and become predominant in late adolescence and adulthood. Functional enrichment analysis further supports the transition between developmental stages. Stage_a cells are significantly enriched for biological processes such as “stem cell differentiation” and “cell number maintenance”, indicating that SCs at this stage primarily exhibit characteristics of stem or progenitor cells. In contrast, Stage_c cells are enriched for processes including “compound rescue in cellular metabolism”, “protein transmembrane transport”, and “phagosome maturation”, indicating that mature SCs primarily function to phagocytose GCs and their metabolic byproducts (Zhao et al., 2020). Guo et al. also identified two immature SCs stages in human testes, designated Immature #1 and Immature #2, which ultimately merged into mature SCs at 11 years of age (Guo et al., 2020). Comparing the two studies, we observe that Immature one resembles Stage_a, while Immature two is closer to Stage_b. In testicular disease research, transcriptomic analyses comparing normal and Klinefelter syndrome (KS) testes indicate that SCs are the most severely affected somatic cell type (Winge et al., 2020). KS SCs are largely arrested at Stage_b and display altered energy metabolism features, such as upregulation of genes associated with oxidative phosphorylation and glycolysis, while triglyceride metabolism levels are suppressed (Zhao et al., 2020). They also show broad transcriptional dysregulation, including upregulation of immune-related genes (B2M and MIF (del Campo et al., 2014; Sumaiya et al., 2022)) and reduced expression of sex hormone-regulated gene GNRH1 (Zhao et al., 2020). Notably, X-linked genes are overexpressed in KS SCs. Mahyari et al. provide a key explanation for this phenomenon: they identified a distinct subset of KS SCs lacking XIST expression, this mechanistic defect likely contributes to the upregulation of X-linked genes within KS SCs (Mahyari et al., 2021).
However, scRNA-seq’s reliance on tissue dissociation limits spatial insights into microenvironmental interactions. Recent advances in spatial transcriptomics now enable the characterization of molecular features and intercellular dynamics within native tissue contexts. These technologies map spatially variable genes, delineate cellular neighborhoods, reconstruct communication networks, and detect molecular alterations under pathological conditions (Xu and Chen, 2025). For instance, Slide-seq preserves spatial information to systematically unveil germ-somatic interaction networks (e.g., spermatogonial stem cells adjacent to SCs) within seminiferous tubules (Rajachandran et al., 2023). They also identify conserved (mouse-human) and disease-specific spatial gene modules (e.g., inflammatory signaling in diabetic models), providing a transformative framework for understanding testicular pathologies and therapeutic development (Chen et al., 2021).
3 Influence of SCs on gonadal development
3.1 SCs coordinate gonadal sex differentiation
Gonadal development is a complex process initiated by the migration of PGCs (Yildirim et al., 2020). During embryonic development, PGCs migrate alongside other cell types to the gonadal ridge, where they are induced to differentiate into ovaries or testes in response to different factors (Poulsen et al., 2019). In the early formation of the mouse genital ridge, coelomic epithelial cells express transcription factors WT1, SF1, GATA4, and LHX9, all of which are essential for the gonad formation (Birk et al., 2000; Wilhelm and Englert, 2002; Klattig et al., 2007; Chen H et al., 2017). The supporting cells are the first differentiated somatic cells, playing a pivotal role in orchestrating gonadal sex determination and promoting gametogenesis (Rotgers et al., 2018). These cells differentiate into SCs in the testis and granulosa cells in the ovary (Carré and Greenfield, 2016). This review focuses on the relationship between SCs and male gonadal development.
In mice, sex determination occurs shortly after the coelomic epithelium migrates into the gonadal ridge, simultaneously, the tunica albuginea forms at the junction between the gonad and the coelomic epithelium, preventing cell entry (Karl and Capel, 1998). Compared to other somatic lineages, SCs are almost entirely XY in chromosome composition of the XX-XY mouse chimera (Palmer and Burgoyne, 1991), that is: the SRY acts cell-autonomously in pre-SCs to trigger their differentiation, with further somatic masculinization resulting from SCs signaling. Karl and his colleagues’ research confirms this view, in the testis, SCs differentiate from the first wave of cells entering the gonads, while subsequent progenitor cells differentiate into LCs, PMCs, and TMs (Karl and Capel, 1998). Before differentiation, coelomic epithelial progenitor pools express both SF1 and WT1 (Liu et al., 2016). After differentiation, SCs retain WT1 expression, whereas LCs maintain higher levels of SF1 (Schmahl et al., 2000; Miyabayashi et al., 2013; McClelland et al., 2015). When the WT1 gene is deleted in the gonadal primordium before sex determination, progenitor cells fail to activate the development programs for XX and XY gonads, leading instead to their differentiation into SF1-expressing steroidogenic cells (Chen M et al., 2017). One study indicates that WT1 influences progenitor cell fate towards SCs differentiation by reducing Nr5a1 transcription (Bale and Epperson, 2015).
Testis differentiation is regulated by the sex-determining gene SRY on the Y chromosome, which is expressed only in pre-SCs (Okashita and Tachibana, 2021). At the onset of sex determination, SRY genes are transiently upregulated in SCs (Del Valle et al., 2017). After sex determination, the SRY gene is silenced in mice, while in humans, there are lower levels of SRY gene expression persist even in mature testes (Hanley et al., 2000; Bullejos and Koopman, 2001). SRY gene expression is initiated by transcription factors, including SF1, WT1, GATA4, FOG2 and CBX2, in null mutant of these factors, the gonads exhibit reduced or missing SRY expression, resulting in XY sex reversal (Barbara et al., 2001; Hossain and Saunders, 2001; Tevosian et al., 2002; Miyamoto et al., 2008; Katoh-Fukui et al., 2012; Warr et al., 2012). As well, histone modifications, DNA methylation, and chromatin repression also regulate SRY expression (Kuroki and Tachibana, 2018). The SRY protein binds DNA, activating genes involved in testis development and repressing genes associated with ovary differentiation (Rotgers et al., 2018). The primary target gene of SRY is SOX9. SRY expression activates SOX9, which in turn activates FGF9, promoting the differentiation of pre-SCs (Sekido and Lovell-Badge, 2008; Zhao and Koopman, 2012). Once SCs are formed, they induce FLCs development through hedgehog signaling (Yao et al., 2002), promoting the production of testosterone and insulin-like 3 (INSL3) (Barsoum et al., 2009). This process further induces testicular descent and the masculinization of external genitalia (Hutson, 2013; Roth et al., 2021). In parallel, SOX9 recruits other proteins (PADI, AMH, and PDGS) to facilitate testis development (Otake and Park, 2016; Tsuji-Hosokawa et al., 2018; Lucas-Herald and Mitchell, 2022). However, the absence of SRY expression in pre-SCs leads to the differentiation of the gonad into ovaries (Albrecht and Eicher, 2001). Similarly, abnormal SRY expression causes the genital ridge to develop into ovaries (Larney et al., 2014). Therefore, SRY expressed by pre-SCs is essential for directing gonadal development towards masculinization.
3.2 SCs induce testis cords formation
After the gonadal primordium is established and the fate of the supporting cells is determined, the gonad develops into either ovaries or testes (Poulsen et al., 2019). Once specified for testis development, pre-SCs polarize, aggregate, and assemble around pre-GCs to form SC-GC clusters, then endothelial cells migrate and guide to form testis-specific tubular structures, known as testis cords, which later develop into seminiferous tubules in the adult testis (Rossi et al., 2000; Combes et al., 2009; Svingen and Koopmam, 2013). The maintenance of fetal testis cords is normal testis development (Archambeault and Yao, 2010; Chen et al., 2010; Cool et al., 2012; Zheng et al., 2015). The dramatic changes in testis cords morphology are driven by the rapid proliferation of FSCs, alterations in FSCs number influence the cord structures development, potentially leading to involution and the formation of blind-ending tubules (Bagheri-Fam et al., 2011). The initiation of cord formation requires a large number of FSCs to separate and enclose the GCs within the lumen (Brennan and Capel, 2004; Svingen and Koopman, 2013; Chen et al., 2016). Growing evidence suggests that TGFβ is associated with testis cord formation, though its effects may be indirect. TGFβ plays an important role in regulating and SCs proliferation. The specific deletion of Smad4 (a core component of TGFβ signaling) in SCs leads to defective proliferation and dysplastic testis cords (Archambeault and Yao, 2014). DNA damage binding protein 1 (DDB1) promotes SCs proliferation by activation of the TGFβ pathway, deletion of DDB1 in the FSCs impairs their proliferation and disrupts the coiling of testis cords, leading to smaller testes and reduced sperm production (Zheng et al., 2019). In addition, activin A, produced by FSCs, is a unique regulator of SCs proliferation and cords expansion (Archambeault and Yao, 2010). Taken together, FSCs play a significant role in promoting the elongation and expansion of cord structures, laying the foundation for postnatal testis development.
4 SCs promote spermatogenesis
4.1 SCs maintain the SSCs pools homeostasis
The SSCs niche consists of GCs, SCs, interstitial cells, PMCs, and growth factors from the vascular network (Kanatsu-Shinohara et al., 2007; Oatley and Brinster, 2012). A stable niche facilitates the self-renewal of SSCs and maintains the stem cell pool (Ibtisham et al., 2020). SSCs are located on the basement membrane of the seminiferous tubule, in close contact with SCs (Oatley and Brinster, 2012; Guo et al., 2020). In the presence of SCs, SSCs resume proliferation and undergo several mitotic divisions to form spermatocytes, which give rise to haploid spermatids by meiosis (Zhang et al., 2012). Studies have shown that SCs are activated to produce GDNF in response to FSH, it is a key factor in SSCs self-renewal (Garcia et al., 2017). Ablation of GDNF or its receptors Ret and Gfra1 in SSCs leads to the loss of stem cells and their progeny (Naughton et al., 2006). However, excessive GDNF inhibits differentiation, leading to more undifferentiated SSCs (Hofmann, 2008; Zhang et al., 2012). Furthermore, NOTCH signaling in SCs regulates GDNF expression through HES1 and HEY1 transcription factors, and undifferentiated GCs expressing the NOTCH ligand JAG1 participate in GDNF modulation within the stem cell niche via negative feedback regulation (Garcia et al., 2017). SCs stabilize the SSCs niche by secreting leukemia inhibitory factor (LIF) and chemokines CXCL12 (Oatley et al., 2011; Oatley and Brinster, 2012; Yang et al., 2021). SCs also secrete fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and Wnt5a, which contribute to SSCs self-renewal (Yeh et al., 2011; Hasegawa and Saga, 2014; Ren et al., 2020). These factors are regulated by FSH signaling, which activates the FSH receptor (FSHR) on SCs and targets functional and transcription factors via the cAMP/PKA pathway (Lim and Hwang, 1995; Sharpe et al., 2003; Wang et al., 2022). Interestingly, exogenous increases SCs leads to a corresponding increase in the number of niches (Oatley et al., 2011). However, excessive self-renewal or differentiation of SSCs can disrupt spermatogenesis, ultimately leading to male infertility (Li S et al., 2021).
4.2 SCs create a unique metabolic microenvironment for spermatogenesis
One important event in spermatogenesis is the metabolic cooperation between SCs and developing GCs. After birth, the initial entry into the spermatogenic cycle is triggered by a pubertal upsurge in retinoic acid (RA) (Teletin et al., 2019). RA is an intermediate metabolite of vitamin A (Raverdeau et al., 2012). Within SCs, retinol is oxidized to retinal by RDH10 and then to RA by RALDH1a1 (Theodosiou et al., 2010). RDH10 is essential for the biosynthesis of RA and its absence in SCs results in defective spermatogonia differentiation (Sandell et al., 2012; Tong et al., 2013). RA interacts with heterodimers of the RA receptors (RARs) and the retinoid X receptors (RXRs), binding to RA response elements (RAREs) in target genes, and recruiting co-activators or co-repressors to either induce or inhibit transcription (Mark et al., 2015). Studies have shown that RA deficiency gradually reduces the number of GCs in rats, leaving only SCs and undifferentiated spermatogonia in the seminiferous tubules (Li et al., 2019). Besides, RARα is an important RA member expressed in SCs, when lack RARα, sperm release fails (Vernet et al., 2008). The spermatogenic cycle is supported by subsequent RA pulses (Hogarth et al., 2015; Hofmann and McBeath, 2022). These pulses were observed to influence spermiation at stages VIII–IX of the seminiferous epithelium cycle and were linked to the stage-specific localization of mRNAs associated with retinoid storage, RA synthesis, and degradation (van Pelt and de Rooij, 1990; Vernet et al., 2006). This was confirmed by the discovery of the RA-responsive gene Stra8, which is primarily expressed at stages VIII–IX of the seminiferous cycle (Endo et al., 2015).
The testis is a highly specialized organ characterized by low and unevenly distributed oxygen levels. In the testis, SCs exhibit a high glycolytic flux to produce the elevated lactate levels necessary for GCs development (Qian et al., 2023). Glucose is absorbed from the extracellular space via glucose transporter proteins, particularly GLUT1 and GLUT3, and then converted to lactate, in this process, the lactate dehydrogenase system plays a key role by reversibly catalyzing the conversion of pyruvate to lactate (Custódio et al., 2021). Lactate is then transported across the plasma membrane by monocarboxylate transporters (MCT) to the GCs as an energy source (Bernardino et al., 2019). Studies have shown that during spermatogenesis, increased expression of mitochondrial respiratory-related genes in differentiated GCs leads to pyruvate and lactate replacing glucose as the fuel for mitochondrial respiration in spermatogonia and spermatocytes (Mita and Hall, 1982; Nakamura et al., 1984; Ren et al., 2022). Furthermore, intratesticular injection of lactate significantly improved spermatogenesis in the testes of adult cryptorchid rats (Courtens and Plöen, 1999). In this metabolic environment, SCs are co-regulated by both external and internal factors, such as insulin, sex hormones, cytokines, and AMP-activated kinases (Boussouar and Benahmed, 2004). Research suggests that a lack of insulin decreases the expression of LDHA, which in turn reduces the conversion of pyruvate to lactate and MCT4 (Oliveira et al., 2012). These findings align with another study, which demonstrated that insulin and FSH increase lactate production in ISCs, suggesting a positive correlation between insulin levels and lactate production (Oonk et al., 1985).
4.3 The blood-testis barrier (BTB) established by SCs is involved in spermatogenesis
The BTB comprises multiple protein complexes between adjacent SCs, including tight junctions (TJ), ectoplasmic specialization (ES, specific adhesion junctions in the testis), gap junctions (GJ) and desmosome junctions (DJ) (Mao et al., 2020). TJ forms barriers dividing the seminiferous epithelium into basal and apical compartments, preventing the diffusion of soluble substances and macromolecules into the apical compartment and limiting the translocation of proteins and lipids between these compartments (Mruk and Cheng, 2015). SCs express occludin and claudin-11, the major regulators of TJ (McCabe et al., 2016). The basal compartment contains SSCs, spermatogonia, and preleptotene spermatocytes (Wanjari and Gopalakrishnan, 2024). Preleptotene spermatocytes enter the apical compartment through the transient opening of the BTB junction complex, where they undergo two meiotic divisions to form spermatocytes, eventually becoming elongated spermatozoa, which enter the lumen of the seminiferous tubules (Cheng and Mruk, 2012). To prevent the self-antigens of spermatocytes and spermatozoa in the apical compartment from triggering an immune response, the BTB isolates many GCs specific antigens, acting as an immune barrier for spermatogenesis (Luaces et al., 2023). ES is mainly composed of cadherin-catenin complexes and can be categorized into two types based on their location: one is basal ES between the SCs (Qian et al., 2014). The other is the apical ES between SCs and spermatids, they interact with the acrosomal region of the spermatid and promote rapid elongation and maturation by mechanically grasping the head of the spermatids (Mruk and Cheng, 2004; Berruti and Paiardi, 2014; Qian et al., 2014). SCs participate in spermatogenesis by providing nutrients (sugars, amino acids) and secreting cytokines (Chojnacka et al., 2016; Luca et al., 2018b). GJ-mediated intercellular substance transport realizes this function in SCs, and GJ can selectively release substances according to those required by GCs at different times (Haverfield et al., 2014; Ni et al., 2019), this regulation of bioactive substance concentration provides a suitable microenvironment for spermatogenesis (O’Donnell et al., 2011). In addition, GJ expresses abundant connexin43 (Pointis et al., 2010), which interacts with Plakophilin2, β-catenin, N-cadherin, and c-Src to mediate signaling during the reconstruction of the seminiferous epithelium in spermatogenesis and regulate the migration of preleptotene spermatocyte (Carette et al., 2010; Gerber et al., 2016).
The BTB is a highly dynamic ultrastructure that undergoes cyclic reorganization in response to various factors, facilitating the completion of meiosis in leptotene spermatocytes (Mruk and Cheng, 2015; Liu et al., 2024). It has been shown that TGF-β and TNF-α regulate BTB reorganization by activating the MAPK signaling pathway, promoting protein endocytosis and ectosome degradation, which interfere with TJ barrier function (Xia et al., 2009; Liu J et al., 2018). Besides, spermatocytes induce SCs to produce and highly IL-1 during stage VIII of the spermatogenic epithelial cycle, regulating the BTB reorganization and spermatozoa release (Sarkar et al., 2008). Androgens promote not only the completion of meiosis and development of spermatocytes (Christin-Maitre and Young, 2022) but also the production of TJ proteins, which maintain the SCs’ barrier function and BTB integrity (McCabe et al., 2012; Kabbesh et al., 2022). In the testis, where androgen concentration fluctuates markedly, the BTB isolates seminiferous tubules from the interstitial tissue, allowing SCs to express androgen-binding protein (ABP) (Walker, 2010; Chen T et al., 2022; Washburn and Dufour, 2023). Androgen binds to ABP, alleviating fluctuations and enabling stable release (Ma et al., 2015). Androgens regulate GCs adhesion and sperm release through the activation of a non-classical testosterone pathway, binding to DNA regulatory elements via androgen receptors (AR) on SCs (Holdcraft and Braun, 2004; Cheng et al., 2007; Deng et al., 2017). Transferrin, located on SCs, transfers iron ions from the blood to cell membranes and transmit them to GCs at different stages, promoting the development, differentiation, and maturation of GCs (Yefimova et al., 2008). Additionally, the BTB confers polarity to the spermatogenic epithelium and guides GCs migration, ensuring proper directional movement of spermatogenic cells (Liu Y et al., 2018). In summary, the BTB established by SCs contributes to spermatogenesis. when damaged, it leads to abnormalities in the testicular microenvironment, including disorganization and loss of polarity of SCs and GCs, disrupting normal spermatogenesis in the testis.
4.4 Sperm production and output are directed by SCs
Normal spermatogenesis depends on specific signals from the hypothalamic-pituitary-gonadal axis (HPGA). The hypothalamus produces of gonadotropin-releasing hormone (GnRH), which stimulates the anterior pituitary to synthesize and secrete FSH and LH through activation of its receptor (GnRHR) (Lenzi et al., 2009; Smith and Walker, 2014). However, GCs do not express FSH receptors (FSHR) and must signal through SCs to produce cytokines and metabolites that promote GCs maturation (Meachem et al., 2005; Wu et al., 2015). Specifically, binding of FSH to FSHR induces GTP-binding protein in SCs to recruit other molecules, stimulate Gαs or inhibit Gαi, and regulate the cAMP synthesis, subsequently, cAMP activates the downstream transcription factor CREB, maintaining the supportive role of SCs in spermatogenesis (Scobey et al., 2001). LH acts on receptors (LHR) on LCs to promote testosterone synthesis by inducing proliferation, differentiation and maturation of LCs (Smith and Walker, 2014). Testosterone induces spermatogonia to complete meiosis via the AR on SCs, ensuring that GCs acquire the ability to divide (De Gendt et al., 2004; Larose et al., 2020). Testosterone deficiency alters the expression of proteins involved in RNA processing, modification, apoptosis, oxidative stress, and meiosis thereby impeding spermatogenesis (Stanton et al., 2012). Damage to FSH or AR on SCs leads to GCs apoptosis and reduced spermatogenesis efficiency (Ruwanpura et al., 2010; Willems et al., 2010; De Gendt et al., 2014). One study demonstrated that LHR knockout mice lacked testosterone production and exhibited impaired spermatogenesis (Lei et al., 2001), whereas exogenous testosterone administration restored spermatogenesis (Oduwole et al., 2014). It is suggested that SCs play a critical role in transducing of FSH and androgen signaling to coordinate spermatogenesis.
During spermatogenesis, SCs continuously alter their function, cytoskeleton, specialized junctions, gene and protein expression to provide nutrients and physical support to GCs at various stages (Dunleavy et al., 2019). As sperm mature, they need to detach from SCs and enter the seminiferous tubules, eventually being transported to the epididymis for further processing (O’Donnell, 2014). During sperm release, SCs also act as “quality inspectors” by screening and phagocytosing abnormal spermatozoa (O’Donnell et al., 2011). This process dependent on the binding of the scavenger receptor class B type I (SR-B1) in SCs to the membrane phospholipid phosphatidylserine on the target cell (Nakagawa et al., 2005; Zohar-Fux et al., 2022). Phosphatidylserine is normally confined to the inner leaflet of the bilayer membrane, but is transferred to the outer leaflet and exposesd on the cell surface during apoptosis (Park and Kim, 2019). In the process of GCs deformation, a large amount of waste is produced, and SCs recycle this waste for their own energy production (Regueira et al., 2018). Sperm are highly sensitive to microenvironmental changes, and disruptions in SC signaling pathways or drastic hormonal changes can lead to sperm release failure (Kumar et al., 2018). The GCs undergo spermatogenesis to form species-specific elongated spermatids, which are eventually released from the seminiferous epithelium (Du et al., 2021). In the mouse testis, a complete spermatogenic cycle occurs along the seminiferous tubules, while in humans, multiple cycles are intertwined (Johnston et al., 2008; O’Donnell et al., 2022a), meaning that human SCs undergo a more complex process during spermatogenesis.
5 SCs determine the fate of LCs
5.1 SCs promote LCs differentiation and maturation
At least two types of LCs are present in mammalian embryonic and postnatal testis: FLCs and ALCs (Heinrich and DeFalco, 2020). During early embryonic development, pre-SCs promote the formation of fetal PLCs (Rotgers et al., 2018), once FLCs are appointed, their dependence on FSCs diminishes (Fouchécourt et al., 2016; Mäkelä and Hobbs, 2019). Cytokines secreted by FSCs are involved in the proliferation and survival of FLCs, including KIT ligand (Tsikolia et al., 2009), Desert Hedgehog (DHH) (Yao et al., 2002), and PDGF-A (Brennan et al., 2003). Studies have shown that knocking down DHH in mice reduces the number of FLCs (Yao et al., 2002), while activation of DHH induces the expression of SMO and the downstream transcription factors GLI-1, promoting the proliferation of FLCs (Barsoum et al., 2013). FLCs express PDGFRα, which promotes their differentiation in response to PDGF-A secreted by FSCs (Basciani et al., 2002; Brennan et al., 2003). In contrast, the absence of PDGF-A leads to developmental defects in ALCs but does not affect the number of FLCs (Gnessi et al., 2000). Male gonadal differentiation is facilitated by the interaction of FLCs and FSCs (Svingen and Koopman, 2013; Shima, 2019). Although both FLCs and ALCs are capable of producing androgens (Val et al., 2003), testosterone during the fetal period requires conversion by the 17β-HSD enzyme in FSCs, as FLCs only synthesize androstenedione (O’Shaughnessy et al., 2002). Androgens produced by FLCs and FSCs contribute to the development of the epididymis, seminal vesicles, and vas deferens (O’Donnell et al., 2022b). During the fetal period, genital tissues produce 5α-reductase, which converts testosterone produced by FSCs to dihydrotestosterone, inducing the development of the male urethra, prostate, and penis (Kim et al., 2002; Markouli and Michala, 2023).
After birth, ALCs gradually replace FLCs and begin synthesizing and secreting androgens at puberty (Shima, 2019). The genealogical relationship between FLCs and ALCs has not been fully elucidated. Some scholars believe that ALCs originate from stem cells and are independent of FLCs (Habert, 1993; Lo et al., 2004; Ge et al., 2006; Griswold and Behringer, 2009). Others suggest that a subset of the ALCs arises from a subpopulation of de-differentiated FLCs that remain after birth (Kilcoyne et al., 2014; Kaftanovskaya et al., 2015; Shima et al., 2015; Shima et al., 2018), which is regulated by WT1 in SCs (Wen et al., 2014). WT1 is highly expressed in SCs and induces LCs proliferation and differentiation by regulating DHH in SCs, promoting DHH binding to the Ptch1 receptor on LCs (Chen et al., 2014; Min et al., 2022). WT1 knockout in mice leads to reduced numbers of ALCs, downregulation of steroidogenic enzyme expression, and decreased testosterone levels (Chen et al., 2014). Additionally, Rebourcet’s study found that the number of SCs during development predicts the number of LCs in the adult testis (Rebourcet et al., 2017). Complete ablation of SCs in neonates restricts the differentiation and development of the LCs population (Rebourcet et al., 2014a).
5.2 SCs contribute to LCs steroid hormone synthesis
At the onset of spermatogenesis, SCs play a crucial role in providing continuous support to LCs, ensuring their optimal functionality. Studies have shown that the removal of ASCs leads to the apoptosis of ALCs and a significant reduction in their cell number (Rebourcet et al., 2014b). This suggests a dependency between the number of SCs and LCs. SCs secrete various cytokines, such as LIF, PDGF, IGF1, FGF2, TGF, Kit ligand, and Notch ligand, which regulate LCs development and steroidogenesis (Hu et al., 2010; Martin, 2016; Chen M et al., 2017). Among them, IGF1, FGF2 and TGF increased the expression of StAR, promoting the transfer of cholesterol from the outer to the inner mitochondrial membrane to form pregnenolone (Manna et al., 2006). Additionally, SCs can convert testosterone to estrogen via aromatase, which negatively regulates steroidogenesis (Zhang Z. et al., 2022). In co-cultured studies, a reciprocal relationship between SCs and LCs was observed, suggesting that SCs might influence LCs function. In the co-culture system, LCs exhibit a more developed smooth endoplasmic reticulum and higher testosterone content (Saez et al., 1989), while medium collected from SCs increased LCs steroid hormone synthesis (Khan and Rai, 2005). In addition, reducing estrogen levels in SCs promotes testosterone synthesis in co-cultured LCs (Deng et al., 2018). LCs can also influence the metabolic activity of SCs by secreting cytokines (Khan and Rai, 2004). SCs release numerous exosomes, which can cross the BTB to act on LCs functions, providing new evidence for exosomal communication between SCs and LCs. Ma et al. demonstrated that exosomes secreted by SCs promote the survival of LCs by activating the CCL20-AKT pathway (Ma et al., 2022). Exposure to PFOS significantly increases the expression level of miR-9-3p in both SCs and their exosomes, and this miRNA can be transferred to LCs, where it targets and suppresses StAR expression, thereby inhibiting the testosterone synthesis and secretion (Huang et al., 2022). Furthermore, Liang found that miR-145-5p can be transported to LCs via SCs-derived exosomes and exert its function by targeting the SF-1 gene, which in turn downregulated the expression of steroidogenesis-related genes, ultimately inhibited testosterone production (Liang et al., 2021).
In summary, SCs play a crucial role in regulating the development and function of both FLCs and ALCs. The interactions between these 2 cell types are essential for the spermatogenesis, and their crosstalk may underlie various male reproductive dysfunctions. Further research is needed to elucidate the specific mechanisms of these interactions.
6 Effects of SCs on other somatic cells in the testis
6.1 Influence of SCs on PMCs
PMCs are the least studied cells in the testis, and their origin remains controversial. Most researchers believe that PMCs and LCs originate from a common progenitor, a mesenchymal stem cell (Ademi et al., 2022; Aksel et al., 2023; Robinson et al., 2023; Tao et al., 2023), This was also confirmed by Guo useing single-cell transcriptome sequencing, who found that in human testis, LCs and PMCs share a common progenitor, and these progenitor cells co-express LCs and PMCs markers (Guo et al., 2020). SCs are essential for the proliferation and differentiation of PMCs. It has been shown that DHH secreted by SCs promotes the proliferation and differentiation of PMCs, and knocked out of the DHH gene in SCs results in impaired PMCs during development in the testis (Pierucci-Alves et al., 2001). DHH signaling confer polarity to PMCs in organoid culture studies and promotes reorganization of spermatogenic tubules in vitro (Min et al., 2022). SCs also produce WT1, and early reports indicated that specific knockdown of WT1 on SCs using Amh-Cre mice disrupts fetal testicular cords formation (Gao et al., 2006). Wen and colleagues discovered that WT1 regulates the development of FLCs and interstitial progenitor cell lineages through Notch signaling and plays a role in PMCs development, and that the absence of WT1 in SCs leads to reduced differentiation of PMCs and impedes fetal testis compartmentalization (Wen et al., 2016). During testicular cord assembly, PMCs secrete collagen I, collagen IV, and fibronectin, while SCs produce and deposit collagen IV and small amounts of laminin, forming the basal lamina of the seminiferous tubules, which is important for maintaining the ecological niche stability of SSCs after birth (Creemers et al., 2002; Kanatsu-Shinohara et al., 2003; Zhou et al., 2019). The stable number of neonatal SCs facilitates the maintenance of PMCs polarity. When SCs are ablated, PMCs depolarization accelerates, leading to disorganized seminiferous tubules morphology (Rebourcet et al., 2014a; Tao et al., 2023). In the adult testis, SCs ablation does not reduce the number of PMCs but diminishes their activity, which subsequently induces the loss of ALCs (Rebourcet et al., 2014b). It is evident that SCs are crucial for PMCs differentiation and contribute to the maintenance of normal PMCs function in adulthood.
6.2 SCs impact on TMs
The testis is a unique immune microenvironment where dendritic cells, mast cells, lymphocytes, and TMs play important roles (Pérez et al., 2013; Bhushan et al., 2015; Bhushan et al., 2020). In the testis, immune responses are generally suppressed, which protects GCs from the immune system and sustaining fertility (Fijak et al., 2011; Li et al., 2012). SCs secrete various immunomodulatory factors that are essential for the function of interstitial immune cells (Luca et al., 2018a). However, SCs derived immunomodulators require androgens support, and when AR are absent in SCs, TJ in SCs are damaged, leading to changes in interstitial immune cells and loss of immune privilege (Meng et al., 2011). TMs constitute a significant portion of the testicular immune microenvironment and are key contributors to its immune function (Bhushan et al., 2020). TMs are present in early undifferentiated fetal gonads (DeFalco et al., 2014; Wang et al., 2021) and are recruited by FSCs to promote organ-specific developmental functions during a narrow time window of embryonic development (Gu et al., 2023). Activin A, a member of the TGF-β superfamily, is primarily secreted by SCs and exerts both pro-inflammatory and anti-inflammatory effects (Hedger and de Krester, 2013; Wijayarathna and de Kretser, 2016), promoting the production of various inflammatory mediators by TMs (de Kretser et al., 2012; Morianos et al., 2019). Activin A also plays a crucial role in regulating macrophage development and function (Hedger and de Kretser, 2013) and helps maintain TMs numbers and testicular immune privilege (Biniwale et al., 2022). It has been long believed that SCs protect GCs from immune attacks by forming the BTB (Kaur et al., 2014; França et al., 2016). However, recent studies suggest that SCs can produce GCs-specific proteins and release them into the interstitial space, potentially promoting peripheral immune tolerance (Tung et al., 2017; O’Donnell et al., 2021). These findings reveal the complex mechanisms by which SCs maintain testicular immune privilege.
Previous research has focused primarily on the interactions between SCs and GCs. However, the specific mechanisms and pathways by which SCs regulate other somatic cells in the testis remain unclear and warrant further investigation. SCs secrete various specific mRNAs and proteins that affect only neighboring somatic and GCs but may also regulate overall male reproductive health. Therefore, an in-depth study of SC-specific gene expression will help elucidate the molecular mechanisms by which SCs modulate the function of somatic cells within the testicular microenvironment.
7 Dysfunction of SCs affects male reproduction
SCs are the “custodians” of the spermatogenic microenvironment, the integrity of their structure and function directly determines the efficiency of spermatogenesis and male fertility. The principal drivers affecting SC function fall into three categories: genetic factors, environmental exposures, and lifestyle/dietary patterns. These factors can disrupt BTB, perturb the HPG axis and immune homeostasis, trigger oxidative stress and mitochondrial damage, and remodel metabolic and paracrine signaling, thereby weakening nutritional and protective support for GCs and ultimately leading to impaired spermatogenesis.
7.1 Key genes regulation
In most mammals (including humans and mice), early gonadal development is non-dimorphic, generating a primordial, bipotential gonad. Its fate is determined by expression of the sex-determining gene SRY on the Y chromosome (Chen R et al., 2022). SRY cooperates with NR5A1/SF1 to bind the testicular enhancer core (TESCO) sequence, rapidly initiating SOX9 expression and thereby establishing the SC lineage (Sekido and Lovell-Badge, 2008; Gonen et al., 2017). Once activated, SOX9 forms a positive feedback loop through prostaglandin D2 (PGD2) and FGF9, and directly inhibits the RSPO1/WNT4/β-catenin pathway that drives ovarian differentiation, ensuring testis cord formation and AMH expression (Rotgers et al., 2018; Chen and Gao, 2022). During this process, FGF9/FGFR2 signaling is critical for promoting proliferation of precursor cells and for inhibiting WNT4 to stabilize the male developmental trajectory (Bagheri-Fam et al., 2017). Studies also show that loss of RSPO1 can cause abnormal expansion of androgen-producing cells (Rotgers et al., 2018). The upstream regulator WT1 functions as a hub with multiple roles, it guides somatic cells of the genital ridge toward differentiation into SCs or FLCs during early development; after sex determination, it maintains cell polarity and BTB function by activating non-canonical Wnt pathways (Wnt-PCP and Wnt/Ca2+), while continuously inhibiting β-catenin signaling. Experiments confirm that absence of Wt1 during the fetal or prepubertal period severely disrupts testicular architecture and LCs development, resulting in male infertility (Rotgers et al., 2018; Liang et al., 2019). Notably, WT1 exerts time-dependent bidirectional control over SF1 expression (enhancing it before sex determination but antagonizing it in SCs thereafter), highlighting WT1’s central role in maintaining lineage stability and the testicular microenvironment (Wilhelm and Englert, 2002; Chen et al., 2012).
In the late differentiation phase and after birth, DMRT1 becomes essential for preserving SCs identity. From E12.5 onward, DMRT1 consolidates male fate by continuously repressing ovarian genes such as Wnt4 and Foxl2, while upregulating Ptgds, Fgfr2, and Gdnf (Matson et al., 2011; Xu et al., 2020). Postnatal loss of DMRT1 leads to transdifferentiation of SCs into granulosa-like cells and can even trigger reversal of gonadal fate (Matson et al., 2011). Furthermore, absence of Raptor, a core component of the mTORC1 pathway, induces dedifferentiation of ASCs, suggesting that nutrient sensing and epigenetic programs jointly establish a metabolic gate that secures mature cellular homeostasis (Xiong et al., 2018). At the hormone regulation level, NR5A1/SF1 directly activates the AMH promoter, and its specific deletion in SCs also downregulates AR expression and delays cellular maturation (Kato et al., 2012; Anamthathmakula et al., 2019), providing a molecular explanation for phenotypes observed in certain pathogenic NR5A1/SF1 variants. X-linked loss-of-function mutations in AR are the predominant cause of androgen insensitivity syndrome (AIS), a prototypical SC-related disorder/difference of sex development (DSD). In complete AIS, AR variants are detectable in more than 95% of cases, underscoring the indispensability of this pathway for terminal SCs maturation (Delli Paoli et al., 2023; Karseladze et al., 2024). Importantly, the molecular underpinnings of 46,XY DSD are highly heterogeneous. More than 60 genes have been implicated, and approximately 60% of cases can be attributed to mutations in AR, SRD5A2, or NR5A1/SF1 (Delot and Vilain, 2021; Zheng et al., 2023). A recent study further identified DHX37, MYRF, and PPP2R3C as 46,XY DSD-associated genes. DHX37 encodes a DEAD-box RNA helicase; hotspot mutations within its RecA-like domains (e.g., p.R308Q, p.R674W) are strongly associated with gonadal dysgenesis, cryptorchidism, and micropenis, suggesting that ribosome biogenesis, cell-cycle control, and the NF-κB and Wnt signaling pathways may collectively mediate the impact of DHX37 on the SCs lineage (McElreavey et al., 2020; Zhang et al., 2022; Zhang et al., 2025).
7.2 Etiologies of sertoli cell-only syndrome
Sertoli cell-only syndrome (SCOS) is the most common and severe subtype of nonobstructive azoospermia (NOA), with a detection rate of 26.3%–69.6% among NOA patients, thereby posing a serious threat to male reproductive health (Chen T et al., 2022). The etiology of SCOS is complex and involves genetic, hormonal, and environmental factors, including Y-chromosome microdeletions, chromosomal abnormalities, cryptorchidism, varicocele, radiotherapy, and harmful occupational exposures (Ghanami Gashti et al., 2021; Hamoda et al., 2025). Studies indicate that karyotypic abnormalities (e.g., Klinefelter syndrome) and microdeletions in the Y-chromosome azoospermia factor (AZF) regions are key markers for SCOS subtyping. The AZF loci are located on the long arm of the Y chromosome (Yq11). There are five fragile sites on Yq11, and their disruption can lead to recurrent deletions of large DNA segments ranging from 0.8 Mb to 7.7 Mb (Wang et al., 2023b; Krausz et al., 2024). Histologically, seminiferous tubules in SCOS contain only SCs, with a complete absence of GCs, and are often accompanied by tubular atrophy and basement membrane thickening (Ghanami Gashti et al., 2021; Wang et al., 2023a). In a minority of cases, focal “residual spermatogenesis” may be present, offering the possibility of biological fatherhood via testicular sperm extraction (TESE) combined with intracytoplasmic sperm injection (ICSI), although this also carries genetic risks for the offspring (Ghanami Gashti et al., 2021; Krausz et al., 2024). Patients with SCOS typically exhibit elevated FSH with largely normal LH and testosterone levels, suggesting compensatory pituitary feedback for spermatogenic failure and relatively preserved LC function (Ghanami Gashti et al., 2021; Wang et al., 2023a). SCOS can be classified as primary or secondary: the former originates from defects in the directed migration or differentiation of PGCs during embryogenesis and most commonly presents as complete SCOS (cSCOS). The latter relates to abnormalities in the maintenance, self-renewal, or differentiation of SSCs after birth, or to dysfunction of the somatic niche, typically presenting early as incomplete SCOS (iSCOS) and potentially progressing over time to cSCOS. The evolution of iSCOS into cSCOS has been demonstrated in multiple genetically engineered models (Wang et al., 2023b), indicating that “intrinsic SSC defects” and “niche failure” are two key mechanisms that converge on a common terminal phenotype. In addition to the classic AZF deletion, aberrant expression of genes such as NANOS1, SYCP3, TEX11, and HSF has been implicated in SCOS (Chen T et al., 2022; Wang et al., 2023a). Transcriptomic analyses further show that downregulated genes in SCOS are enriched in cell-cycle and reproduction-related pathways, whereas upregulated genes are associated with inflammation and immune responses. Protein–protein interaction network analysis identifies hub genes centered on CCNB1, AURKA, and CDC20, these all belong to suppressed cell-cycle modules, suggesting that spermatogenic arrest is accompanied by marked cell-cycle dysregulation (Chen R et al., 2022). Additionally, immune cell infiltration is increased in SCOS samples, and CD56bright NK cells are significantly associated with multiple hub genes, indicating that “cell-cycle arrest” and “activation of the immune microenvironment” together constitute the dual molecular underpinnings of SCOS. In addition, noncoding RNAs participate in niche imbalance and the regulation of SSC fate by modulating signaling pathways such as Notch, EGFR, and LIF/IL-6, thereby providing new insights for a comprehensive elucidation of SCOS pathogenesis (Hofmann and McBeath, 2022; Wang et al., 2023b).
7.3 Sertoli cell tumor
Sertoli cell tumor (SCT) is the second most common testicular sex cord–stromal tumor in males and accounts for approximately 1% of all testicular neoplasms. Based on histopathological features, SCTs are categorized into two types: not otherwise specified (NOS) and large cell calcifying SCT (LCCSCT) (Patrikidou et al., 2023). LCCSCT occurs in prepubertal and postpubertal patients, and some of them are associated with cancer-predisposition syndrome and germline variations (Al-Obaidy et al., 2022). SCT may arise from system-level imbalances of SCs in the multilayered development and homeostatic regulatory network and involves three interrelated pathological modules. First, the non-classical Wnt/PCP pathway regulated by WT1 maintains cell polarity and lineage stability, whereas the classical Wnt/β-catenin pathway is physiologically restrained to preserve the resting state and the BTB. When this regulatory axis is perturbed, it drives aberrant proliferation and dedifferentiation of SCs (Wang et al., 2013; Lobo et al., 2023). Evidence indicates that miR-202-3p targets LRP6 and cyclin D1 within the Wnt/β-catenin cascade, thereby restraining cell-cycle progression (Yang et al., 2019), suggesting that “enhancement of Wnt signaling” together with “cell-cycle propulsion” constitutes a key pro-proliferative circuit in SCs whose molecular features closely align with tumorigenesis. In addition to abnormalities in the Wnt pathway, loss of the homeostatic functions of developmental transcription factors further accelerates malignant transformation; for example, SF1/NR5A1 regulates the survival and ordered proliferation of embryonic SCs through the MDM2/TP53 signaling pathway (Anamthathmakula et al., 2019), and imbalance of this pathway may attenuate TP53-dependent checkpoint control, permitting expansion of aberrant clones. Finally, epigenetic disequilibrium amplifies these processes. Pervasive histone acetylation abnormalities have been observed in testes with SCOS (Ghanami Gashti et al., 2021), indicating systemic alterations in the SC microenvironment and chromatin openness; this provides an epigenetic basis for sustained activation of pro-proliferative transcriptional programs, including the Wnt–cyclin D axis. Notably, some SCOS pathological specimens show loss of epithelial characteristics in SCs accompanied by disruption of seminiferous tubule architecture (Peirouvi et al., 2021). This deterioration of the tissue microenvironment confers spatial growth advantages and immune-evasion conditions to abnormal clones, providing a plausible explanation for the clinically observed low-grade malignant tendency and risk of local recurrence in SCT.
7.4 Systemic aging
SCs are the most age-sensitive cell type in the testis; compared to young individuals, the number of age-related differentially expressed genes increases in elderly testes (Huang et al., 2023). Systemic factors associated with aging, along with changes in the local microenvironment, reduce the number of SCs and impair their function, further affecting spermatogenesis (Rebourcet et al., 2017; Xiao et al., 2024). At the ultrastructural level, aging leads to changes in SCs, including abnormal nuclear morphology, periodic loss of organelles, relaxation of the endoplasmic reticulum, and enlarged lysosomes, resulting in a decline in their function (Santiago et al., 2019).
Specifically, aging leads to lipid metabolism disorders in SCs. Studies have shown that genes involved in sterol biosynthesis and transport (such as Msmo1, Sqle, Tspo, Hmgcr, Elovl5, Fads1, and Dhcr24) are upregulated in aged SCs (Nie et al., 2022), indicating the accumulation of lipid inclusions within SCs (Paniagua et al., 1987; Santiago et al., 2019). Sulfogalactosylglycerolipid (SGG) is essential for spermatogenesis (Tanphaichitr et al., 2018), and arylsulfatase A (ARSA) produced by SCs can degrade SGG in apoptotic GCs and their remnants (Kongmanas et al., 2021). When SCs fail to degrade SGG promptly, lipid droplet accumulation occurs. However, whether reduced ARSA activity in senescent SCs leads to SGG accumulation, accompanied by higher ROS levels, thereby affecting spermatogenesis, requires further investigation. Moreover, SCs secrete various signaling molecules, and their secretory capacity gradually declines with aging. For example, GDNF secreted by SCs regulates the self-renewal of SSCs (Parekh et al., 2019), but its expression levels in SCs decrease with advancing age (Ryu et al., 2006). This may be one of the key reasons for the reduced number of SSCs in aged individuals. Additionally, periodic RA pulses serve as key regulators of spermatogenesis (Endo et al., 2017; Saracino et al., 2020). The RA receptor signaling pathway is significantly decreased in aged SCs (Huang et al., 2023). This suggests that aged SCs have a reduced capacity to guide spermatocyte differentiation, resulting in slower GCs entry into meiosis. scRNA-seq analysis further revealed that key transcription regulators such as WT1, GATA4, and AR exhibited significantly reduced expression levels in aged SCs (Huang et al., 2023). This will affect the expression levels of FYN, a gene closely associated with GCs’ survival and differentiation (Luo et al., 2012; Yang et al., 2022). In addition to secretory functions, SCs also have prominent phagocytic functions (Tysoe, 2024). During spermatogenesis, SCs are responsible for clearing metabolic waste and residual cytoplasm generated throughout the process (Yefimova et al., 2018; Tysoe, 2024). However, in aged animals, the seminiferous tubules harbor substantial accumulations of residual bodies and arrested round spermatids that have not been cleared. This buildup may disrupt the microenvironmental homeostasis of the seminiferous tubules and impede spermatogenesis (Dong et al., 2016). Additionally, increased expression of inflammation-related genes (such as Il1a, Il6st, Ifi27, Ccl2, and Mif) was observed in aged SCs (Nie et al., 2022). Given the nutritional and supportive role of SCs in the GCs lineage, these altered transcriptional levels indicate a decline in overall metabolic function and heightened inflammation in aged SCs, thereby impairing their capacity to support GCs development.
7.5 Endocrine disrupting chemicals
Endocrine disrupting chemicals (EDCs) are pollutants widely present in the environment, originating largely from the pharmaceutical, cosmetics, and packaging industries. These substances can enter the human body, interfere with normal endocrine activity, and adversely affect male reproductive health (Feijo et al., 2025). In the male reproductive system, EDCs impair the function of SCs by inhibiting proliferation, disrupting the cytoskeleton and BTB integrity, and interfering with adhesion junctions between SCs and GCs, ultimately leading to testicular dysfunction and infertility (Mitra et al., 2017; Stukenborg et al., 2021; Adegoke et al., 2023). Common EDCs that may adversely affect male reproductive health include cadmium, phthalates, bisphenol A (BPA), perfluorinated compounds, pesticides, among others (Gao et al., 2025).
As industrial development continues, heavy metals are increasingly released into the environment, either intentionally or unintentionally, harming human and animal health through inhalation or consumption of contaminated water sources. Cadmium (Cd), a prevalent environmental pollutant from industrial processes and cigarette smoking, has been shown to accumulate in the body, particularly in the testes, despite a daily intake as low as 1.06 μg/kg body weight (Wan et al., 2013). This accumulation can disrupt various bodily functions. SCs are a primary target of Cd, pregnant rats on GD12 received low-dose intraperitoneal injections of Cd, which led to decreased expression DHH and FSHR genes in SCs, although it did not significantly affect SCs number, it may have impacted the development and function of other testicular cells (Li et al., 2018). Cd exposure in lactating rats led to vacuolization of SCs and loss of spermatogenic epithelial cells in adult rats (Bekheet, 2011). Additionally, Cd induces excessive autophagy in SCs, leading to apoptosis and an increase in reactive ROS (Chen et al., 2024). SCs support spermatogenesis by forming the BTB (Luaces et al., 2023). However, in rats, Cd disrupts BTB structure by upregulating TGF-β3, which activates p38MAPK signaling (Lui et al., 2003; Wong et al., 2004). Focal adhesion kinase (FAK), a non-receptor protein tyrosine kinase, regulates the TJ proteins (such as occludin and ZO-1) in the BTB, and Cd downregulates FAK expression, damaging the BTB and further inhibiting spermatogenesis (Venditti et al., 2021; Li et al., 2024).
BPA, a prototypical environmental estrogen, is a significant EDC that negatively affects male reproductive health. Most human exposure to BPA occurs through household items, medical devices, and food packaging made from polycarbonate plastics (Corpuz-Hilsabeck and Culty, 2023). BPA can induce metabolic disorders and abnormal spermatogenesis through mechanisms including mimicking estrogenic activity, interfering with DNA methylation, and altering enzyme activities (Barbagallo et al., 2020; Deng et al., 2024). Numerous studies indicate that the reproductive toxicity of BPA primarily arises from impairment of SCs function (Corpuz-Hilsabeck and Culty, 2023). During fetal development, the effects of BPA are complex. One study reported that intrauterine BPA exposure downregulates the expression of AMH, a marker of male fetal SCs, and reduces testosterone levels, suggesting impaired function of both SCs and LCs (Lv et al., 2019). However, an organ culture study observed that BPA exposure increased numbers and markers of FSCs in mice, while GCs and FLCs were significantly reduced, the authors therefore hypothesized that BPA may disrupt the normal proportions and cellular communication among various testicular cell types, and that this imbalance in cellular niche serving as a key mechanism of reproductive dysfunction (Park et al., 2021). Rossi et al. examined primary SCs isolated from 7-day-old postnatal mice and found that exposure to 0.5 μM BPA enhanced endocannabinoid (eCB) signaling, a lipid-based signaling system that acts on cannabinoid receptors and is known to regulate spermatogenesis and sperm fertilization capacity. Because FSH regulates eCB signaling in ISCs, the authors propose that BPA may disrupt FSH signaling (Rossi et al., 2020). In a TM4 cell model, BPA showed a biphasic effect: micromolar levels of BPA inhibited cell proliferation, whereas nanomolar levels stimulated energy metabolism, evidenced by elevated ATP levels and increased mitochondrial activity (Ge et al., 2014). Additionally, BPA induces apoptosis in adolescent rat SCs (PND 18–22) via activating the Fas/FasL and p38/JNK pathways (Qi et al., 2014). Co-exposure to BPA and phthalates has been reported to synergistically disrupt Sertoli–germ cell tight and gap junctions, markedly reducing AR and junctional protein expression and thereby compromising BTB integrity (de Freitas et al., 2016).
7.6 Lifestyle/dietary patterns
In recent years, rapid socio-economic development, combined with unhealthy eating habits and poor lifestyles, has led to a steady increase in global obesity rates (Chooi et al., 2019). Obesity is a major risk factor for non-communicable diseases and is strongly associated with diabetes, fatty liver disease, cardiovascular disease, and cancer (Lauby-Secretan et al., 2016). Obesity has been shown to cause reproductive dysfunction in men, and excess body fat affecting sperm production and quality, making men more susceptible to oligozoospermia and azoospermia (Ma et al., 2019). Previous studies have found that obesity impairs spermatogenesis by disrupting the BTB (Zhang et al., 2014). Recent studies suggest that male obesity cause hormonal imbalances in the HPGA, leading to secondary hypogonadism (Fernandez et al., 2019). Specifically, white adipose tissue produces high levels of cytochrome P450 aromatase, which converts androgens to estrogens, and estrogens then act on the hypothalamus through negative feedback, inhibiting the production and secretion of FSH and LH, thereby impairing testosterone synthesis and spermatogenesis (Barbagallo et al., 2021). The inflammasome NLRP3 is a sensor of cellular damage, once activated, it recruits ASCs to form a caspase-1 activation platform, inducing interleukin secretion and creating an inflammatory microenvironment (Karki and Kanneganti, 2019). Researchers found that decreased AMPKα expression and increased NADPH oxidase activity activated NLRP3 expression in the testes of obese mice, further exacerbating obesity induced SCs dysfunction and male infertility (Mu et al., 2022). Leptin, produced by adipocytes, regulates the body’s energy balance, however, plasma leptin concentrations are high in most obese individuals and are proportional to systemic adiposity. Evidence suggests that leptin promotes the phosphorylation of IRS family members in the hypothalamus, and that phosphorylation of IRS inhibitory sites can impair interactions between FSH and IGF1 in SCs, potentially affecting spermatogenesis (Duan et al., 2004). The impairment of spermatogenesis caused by obesity is regulated by multiple factors, and the specific mechanisms remain to be further investigated.
8 Conclusion and outlook
Male infertility is a major public health issue affecting approximately 10% of couples of reproductive age worldwide, with 50% of cases attributable to male factors (Agarwal et al., 2021). In recent years, male reproductive health in industrialized countries has significantly deteriorated, as evidenced by reduced sperm quality (Lv et al., 2021), lower serum testosterone levels (Travison et al., 2009), and an increased incidence of testicular cancer (Skakkebæk et al., 2022). Consequently, elucidating the mechanisms that regulate testicular function is especially important. In this context, SCs play a central role. They participate in testicular development and gonadal differentiation, by secreting various cytokines, act together with GCs, LCs, and PMCs to form a complex signaling network that maintains homeostasis of the testicular microenvironment. Existing studies indicate that functional impairment of SCs is closely associated with endocrine disruptors, oxidative stress, inflammation, and other environmental factors. However, most current research on SCs relies on two-dimensional culture models that struggle to accurately recapitulate the complex in vivo environment. Moreover, although multi-omics technologies (including proteomics, epigenomics, transcriptomics, and metabolomics) can identify key molecules in SCs, major challenges remain in systematically integrating multi-omics datasets, building reliable biomarker networks, and validating them effectively in vivo. To address these gaps, future research should focus on developing organoids and three-dimensional culture systems to construct testicular microenvironment models with greater physiological relevance. By integrating multi-omics data and fostering interdisciplinary collaboration, a systematic dissection of SC regulatory networks under physiological and pathological conditions may provide stronger theoretical foundations and new therapeutic strategies for the mechanistic study and clinical intervention of male infertility.
Statements
Author contributions
XF: Conceptualization, Writing – original draft. JT: Writing – review and editing. SH: Conceptualization, Writing – original draft. FW: Conceptualization, Writing – review and editing. YL: Writing – original draft. SZ: Writing – original draft. LO: Writing – original draft. ZH: Writing – original draft. XC: Conceptualization, Writing – original draft, Writing – review and editing. JH: Conceptualization, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. National Key Research and Development Program of China, Grant/Award Number: 2022YFD1300200; Shaanxi Province Agricultural Key Core Technology Research Project, Grant/Award Number: 2024NYGG005.
Acknowledgments
We are grateful to the participants for their contribution in accomplishing this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adegoke E. O. Rahman M. S. Amjad S. Pang W. K. Ryu D. Y. Park Y. J. et al (2023). Environmentally relevant doses of endocrine disrupting chemicals affect Male fertility by interfering with sertoli cell glucose metabolism in mice. Chemosphere337, 139277. 10.1016/j.chemosphere.2023.139277
2
Ademi H. Djari C. Mayère C. Neirijnck Y. Sararols P. Rands C. M. et al (2022). Deciphering the origins and fates of steroidogenic lineages in the mouse testis. Cell Rep.39 (11), 110935. 10.1016/j.celrep.2022.110935
3
Agarwal A. Baskaran S. Parekh N. Cho C.-L. Henkel R. Vij S. et al (2021). Male infertility. Lancet397 (10271), 319–333. 10.1016/S0140-6736(20)32667-2
4
Aksel S. Cao M. Derpinghaus A. Baskin L. S. Cunha G. R. (2023). Ontogeny of mouse Sertoli, Leydig and peritubular myoid cells from embryonic day 10 to adulthood. Differentiation129, 96–108. 10.1016/j.diff.2022.02.006
5
Al-Obaidy K. I. Idrees M. T. Abdulfatah E. Kunju L. P. Wu A. Ulbright T. M. (2022). Large cell calcifying sertoli cell tumor: a clinicopathologic Study of 18 cases with comprehensive review of the literature and reappraisal of Prognostic features. Am. J. Surg. Pathol.46 (5), 688–700. 10.1097/PAS.0000000000001849
6
Albrecht K. H. Eicher E. M. (2001). Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol.240 (1), 92–107. 10.1006/dbio.2001.0438
7
Anamthathmakula P. Miryala C. S. J. Moreci R. S. Kyathanahalli C. Hassan S. S. Condon J. C. et al (2019). Steroidogenic factor 1 (Nr5a1) is required for Sertoli cell survival post sex determination. Sci. Rep.9 (1), 4452. 10.1038/s41598-019-41051-1
8
Archambeault D. R. Yao H. H. (2010). Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc. Natl. Acad. Sci. U. S. A.107 (23), 10526–10531. 10.1073/pnas.1000318107
9
Archambeault D. R. Yao H. H. (2014). Loss of smad4 in Sertoli and Leydig cells leads to testicular dysgenesis and hemorrhagic tumor formation in mice. Biol. Reprod.90 (3), 62. 10.1095/biolreprod.113.111393
10
Arora H. Qureshi R. Khodamoradi K. Seetharam D. Parmar M. Van Booven D. J. et al (2022). Leptin secreted from testicular microenvironment modulates hedgehog signaling to augment the endogenous function of Leydig cells. Cell Death and Dis.13 (3), 208. 10.1038/s41419-022-04658-3
11
Bagheri-Fam S. Argentaro A. Svingen T. Combes A. N. Sinclair A. H. Koopman P. et al (2011). Defective survival of proliferating Sertoli cells and androgen receptor function in a mouse model of the ATR-X syndrome. Hum. Mol. Genet.20 (17), 3535. 10.1093/hmg/ddr267
12
Bagheri-Fam S. Bird A. D. Zhao L. Ryan J. M. Yong M. Wilhelm D. et al (2017). Testis determination requires a specific FGFR2 Isoform to repress FOXL2. Endocrinology158 (11), 3832–3843. 10.1210/en.2017-00674
13
Bale T. L. Epperson C. N. (2015). Sex differences and stress across the lifespan. Nat. Neurosci.18 (10), 1413–1420. 10.1038/nn.4112
14
Barbagallo F. Condorelli R. A. Mongioi L. M. Cannarella R. Aversa A. Calogero A. E. et al (2020). Effects of bisphenols on testicular steroidogenesis. Front. Endocrinol.11, 373. 10.3389/fendo.2020.00373
15
Barbagallo F. Condorelli R. A. Mongioi L. M. Cannarella R. Cimino L. Magagnini M. C. et al (2021). Molecular mechanisms underlying the relationship between obesity and Male infertility. Metabolites11 (12), 840. 10.3390/metabo11120840
16
Barbara P. D. Méjean C. Moniot B. Malclès M. H. Berta P. Boizet-Bonhoure B. (2001). Steroidogenic factor-1 contributes to the cyclic-adenosine monophosphate down-regulation of human SRY gene expression. Biol. Reprod.64 (3), 775–783. 10.1095/biolreprod64.3.775
17
Barsoum I. B. Bingham N. C. Parker K. L. Jorgensen J. S. Yao H. H. C. (2009). Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev. Biol.329 (1), 96–103. 10.1016/j.ydbio.2009.02.025
18
Barsoum I. B. Kaur J. Ge R. S. Cooke P. S. Yao H. H. C. (2013). Dynamic changes in fetal Leydig cell populations influence adult Leydig cell populations in mice. Faseb J.27 (7), 2657–2666. 10.1096/fj.12-225060
19
Basciani S. Mariani S. Arizzi M. Ulisse S. Rucci N. Jannini E. A. et al (2002). Expression of platelet-derived growth factor-A (PDGF-A), PDGF-B, and PDGF receptor-alpha and -beta during human testicular development and disease. J. Clin. Endocrinol. Metab.87 (5), 2310–2319. 10.1210/jcem.87.5.8476
20
Bekheet S. H. M. (2011). Comparative effects of repeated administration of cadmium chloride during pregnancy and lactation and selenium protection against cadmium toxicity on some organs in immature rats' offsprings. Biol. Trace Elem. Res.144 (1-3), 1008–1023. 10.1007/s12011-011-9084-z
21
Bernardino R. L. D'Souza W. N. Rato L. Rothstein J. L. Dias T. R. Chui D. et al (2019). Knockout of MCT1 results in total absence of spermatozoa, sex hormones dysregulation, and morphological alterations in the testicular tissue. Cell Tissue Res.378 (2), 333–339. 10.1007/s00441-019-03028-4
22
Berruti G. Paiardi C. (2014). The dynamic of the apical ectoplasmic specialization between spermatids and Sertoli cells: the case of the small GTPase Rap1. Biomed. Res. Int.2014, 635979. 10.1155/2014/635979
23
Bhushan S. Meinhardt A. (2017). The macrophages in testis function. J. Reprod. Immunol.119, 107–112. 10.1016/j.jri.2016.06.008
24
Bhushan S. Tchatalbachev S. Lu Y. N. Fröhlich S. Fijak M. Vijayan V. et al (2015). Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J. Immunol.194 (11), 5455–5464. 10.4049/jimmunol.1401132
25
Bhushan S. Theas M. S. Guazzone V. A. Jacobo P. Wang M. Fijak M. et al (2020). Immune cell subtypes and their function in the testis. Front. Immunol.11, 583304. 10.3389/fimmu.2020.583304
26
Biniwale S. Wijayarathna R. Pleuger C. Bhushan S. Loveland K. L. Meinhardt A. et al (2022). Regulation of macrophage number and gene transcript levels by activin A and its binding protein, follistatin, in the testes of adult mice. J. Reprod. Immunol.151, 103618. 10.1016/j.jri.2022.103618
27
Birk O. S. Casiano D. E. Wassif C. A. Cogliati T. Zhao L. Zhao Y. et al (2000). The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature403 (6772), 909–913. 10.1038/35002622
28
Boussouar F. Benahmed M. (2004). Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab.15 (7), 345–350. 10.1016/j.tem.2004.07.003
29
Brennan J. Capel B. (2004). One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat. Rev. Genet.5 (7), 509–521. 10.1038/nrg1381
30
Brennan J. Tilmann C. Capel B. (2003). Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes and Dev.17 (6), 800–810. 10.1101/gad.1052503
31
Bullejos M. Koopman P. (2001). Spatially dynamic expression of Sry in mouse genital ridges. Dev. Dyn.221 (2), 201–205. 10.1002/dvdy.1134
32
Bunce C. Barske L. Zhang G. R. Capel B. (2023). Biased precursor ingression underlies the center-to-pole pattern of male sex determination in mouse. Development150 (5), dev201060. 10.1242/dev.201060
33
Cannarella R. Arato I. Condorelli R. A. Luca G. Barbagallo F. Alamo A. et al (2019). The IGF1 receptor is involved in follicle-stimulating hormone signaling in porcine Neonatal Sertoli cells. J. Clin. Med.8 (5), 577. 10.3390/jcm8050577
34
Carette D. Weider K. Gilleron J. Giese S. Dompierre J. Bergmann M. et al (2010). Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev. Biol.346 (1), 54–67. 10.1016/j.ydbio.2010.07.014
35
Carré G. A. Greenfield A. (2016). The gonadal supporting cell lineage and Mammalian sex determination: the differentiation of Sertoli and granulosa cells. Results Probl. Cell Differ.58, 47–66. 10.1007/978-3-319-31973-5_3
36
Chan Y. F. Tang F. S. O W. (2008). Adrenomedullin in the rat testis. II: its production, actions on inhibin secretion, regulation by follicle-stimulating hormone, and its interaction with endothelin 1 in the Sertoli cell. Biol. Reprod.78 (4), 780–785. 10.1095/biolreprod.107.060863
37
Chauvigné F. Verdura S. Mazón M. J. Duncan N. Zanuy S. Gómez A. et al (2012). Follicle-Stimulating hormone and luteinizing hormone mediate the androgenic pathway in Leydig cells of an evolutionary advanced teleost. Biol. Reprod.87 (2), 35. 10.1095/biolreprod.112.100784
38
Chen M. Gao F. (2022). The regulation of gonadal somatic cell differentiation in humans. Genomics Proteomics Bioinforma.20 (2), 219–222. 10.1016/j.gpb.2022.04.003
39
Chen S. R. Liu Y. X. (2016). Testis cord maintenance in Mouse embryos: genes and signaling. Biol. Reprod.94 (2), 42. 10.1095/biolreprod.115.137117
40
Chen G. C. Yang L. Q. Begum S. Xu L. (2010). GPR56 is essential for Testis development and Male fertility in mice. Dev. Dyn.239 (12), 3358–3367. 10.1002/dvdy.22468
41
Chen H. Palmer J. S. Thiagarajan R. D. Dinger M. E. Lesieur E. Chiu H. et al (2012). Identification of novel markers of mouse fetal ovary development. PLoS One7 (7), e41683. 10.1371/journal.pone.0041683
42
Chen M. Wang X. Wang Y. Zhang L. Xu B. Lv L. et al (2014). Wt1 is involved in leydig cell steroid hormone biosynthesis by regulating paracrine factor expression in mice. Biol. Reprod.90 (4), 71. 10.1095/biolreprod.113.114702
43
Chen L. Y. Willis W. D. Eddy E. M. (2016). Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc. Natl. Acad. Sci. U. S. A.113 (7), 1829–1834. 10.1073/pnas.1517994113
44
Chen H. Murray E. Sinha A. Laumas A. Li J. Lesman D. et al (2021). Dissecting mammalian spermatogenesis using spatial transcriptomics. Cell Rep.37 (5), 109915. 10.1016/j.celrep.2021.109915
45
Chen N. Wan X. Y. Cheng S. Tang G. J. Xia D. Xu Y. L. et al (2024). Defective autophagic flux aggravates cadmium-induced Sertoli cell apoptosis. Ecotoxicol. Environ. Saf.273, 116095. 10.1016/j.ecoenv.2024.116095
46
Chen H. Wang Y. Y. Ge R. S. Zirkin B. R. (2017). Leydig cell stem cells: identification, proliferation and differentiation. Mol. Cell. Endocrinol.445 (C), 65–73. 10.1016/j.mce.2016.10.010
47
Chen M M. Zhang L. Cui X. Lin X. Li Y. Wang Y. et al (2017). Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression. Development144 (1), 44–53. 10.1242/dev.144105
48
Chen R. Wang F. Chen Y. Han D. (2022). Immune homeostasis and disorder in the testis - roles of Sertoli cells. J. Reprod. Immunol.152, 103625. 10.1016/j.jri.2022.103625
49
Chen T. Wang Y. Tian L. Guo X. Xia J. Wang Z. et al (2022). Aberrant gene expression profiling in men with Sertoli cell-only syndrome. Front. Immunol.13, 821010. 10.3389/fimmu.2022.821010
50
Cheng C. Y. Mruk D. D. (2012). The blood-testis barrier and its implications for Male contraception. Pharmacol. Rev.64 (1), 16–64. 10.1124/pr.110.002790
51
Cheng J. Watkins S. C. Walker W. H. (2007). Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology148 (5), 2066–2074. 10.1210/en.2006-1465
52
Chojnacka K. Zarzycka M. Mruk D. D. (2016). Biology of the sertoli cell in the fetal, pubertal, and adult Mammalian testis. Results Probl. Cell Differ.58, 225–251. 10.1007/978-3-319-31973-5_9
53
Chooi Y. C. Ding C. Magkos F. (2019). The epidemiology of obesity. Metabolism-Clinical Exp.92, 6–10. 10.1016/j.metabol.2018.09.005
54
Christin-Maitre S. Young J. (2022). Androgens and spermatogenesis. Ann. D. Endocrinol.83 (3), 155–158. 10.1016/j.ando.2022.04.010
55
Chui K. Trivedi A. Cheng C. Y. Cherbavaz D. B. Dazin P. F. Huynh A. L. et al (2011). Characterization and functionality of proliferative human Sertoli cells. Cell Transpl.20 (5), 619–635. 10.3727/096368910x536563
56
Combes A. N. Wilhelm D. Davidson T. Dejana E. Harley V. Sinclair A. et al (2009). Endothelial cell migration directs testis cord formation. Dev. Biol.326 (1), 112–120. 10.1016/j.ydbio.2008.10.040
57
Cool J. DeFalco T. Capel B. (2012). Testis formation in the fetal mouse: dynamic and complex de novo tubulogenesis. Wiley Interdiscip. Reviews-Developmental Biol.1 (6), 847–859. 10.1002/wdev.62
58
Corpuz-Hilsabeck M. Culty M. (2023). Impact of endocrine disrupting chemicals and pharmaceuticals on Sertoli cell development and functions. Front. Endocrinol. (Lausanne)14, 1095894. 10.3389/fendo.2023.1095894
59
Courtens J. L. Plöen L. (1999). Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol. Reprod.61 (1), 154–161. 10.1095/biolreprod61.1.154
60
Creemers L. B. Meng X. den Ouden K. van Pelt A. M. M. Izadyar F. Santoro M. et al (2002). Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol. Reprod.66(6): 1579–1584. 10.1095/biolreprod66.6.1579
61
Custódio T. F. Paulsen P. A. Frain K. M. Pedersen B. P. (2021). Structural comparison of GLUT1 to GLUT3 reveal transport regulation mechanism in sugar porter family. Life Sci. Alliance4 (4), e202000858. 10.26508/lsa.202000858
62
de Freitas A. Ribeiro M. A. Pinho C. F. Peixoto A. R. Domeniconi R. F. Scarano W. R. (2016). Regulatory and junctional proteins of the blood-testis barrier in human Sertoli cells are modified by monobutyl phthalate (MBP) and bisphenol A (BPA) exposure. Toxicol. Vitro34, 1–7. 10.1016/j.tiv.2016.02.017
63
De Gendt K. Swinnen J. V. Saunders P. T. K. Schoonjans L. Dewerchin M. Devos A. et al (2004). A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. U. S. A.101 (5), 1327–1332. 10.1073/pnas.0308114100
64
De Gendt K. Verhoeven G. Amieux P. S. Wilkinson M. F. (2014). Research resource: Genome-Wide identification of AR-Regulated genes translated in Sertoli cells in vivo using the RiboTag approach. Mol. Endocrinol.28 (4), 575–591. 10.1210/me.2013-1391
65
de Kretser D. M. O'Hehir R. E. Hardy C. L. Hedger M. P. (2012). The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol. Cell. Endocrinol.359 (1-2), 101–106. 10.1016/j.mce.2011.10.009
66
De Rooij D. G. Russell L. D. (2000). All you wanted to know about spermatogonia but were afraid to ask. J. Androl.21 (6), 776–798. 10.1002/j.1939-4640.2000.tb03408.x
67
DeFalco T. Bhattacharya I. Williams A. V. Sams D. M. Capel B. (2014). Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc. Natl. Acad. Sci. U. S. A.111 (23), E2384–E2393. 10.1073/pnas.1400057111
68
DeFalco T. Potter S. J. Williams A. V. Waller B. Kan M. J. Capel B. (2015). Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep.12 (7), 1107–1119. 10.1016/j.celrep.2015.07.015
69
del Campo A. B. Kyte J. A. Carretero J. Zinchencko S. Mendez R. Gonzalez-Aseguinolaza G. et al (2014). Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int. J. Cancer134 (1), 102–113. 10.1002/ijc.28338
70
Del Valle I. Buonocore F. Duncan A. J. Lin L. Barenco M. Parnaik R. et al (2017). A genomic atlas of human adrenal and gonad development. Wellcome Open Res.2, 25. 10.12688/wellcomeopenres.11253.2
71
Delli Paoli E. Di Chiano S. Paoli D. Lenzi A. Lombardo F. Pallotti F. (2023). Androgen insensitivity syndrome: a review. J. Endocrinol. Invest46 (11), 2237–2245. 10.1007/s40618-023-02127-y
72
Delot E. C. Vilain E. (2021). Towards improved genetic diagnosis of human differences of sex development. Nat. Rev. Genet.22 (9), 588–602. 10.1038/s41576-021-00365-5
73
Deng Q. Wu Y. Zhang Z. Wang Y. Li M. H. Liang H. et al (2017). Androgen receptor localizes to plasma membrane by binding to Caveolin-1 in mouse Sertoli cells. Int. J. Endocrinol.2017, 3985916. 10.1155/2017/3985916
74
Deng S. L. Wang Z. P. Jin C. Kang X. L. Batool A. Zhang Y. et al (2018). Melatonin promotes sheep Leydig cell testosterone secretion in a co-culture with Sertoli cells. Theriogenology106, 170–177. 10.1016/j.theriogenology.2017.10.025
75
Deng X. Liang S. Tang Y. Li Y. Xu R. Luo L. et al (2024). Adverse effects of bisphenol A and its analogues on male fertility: an epigenetic perspective. Environ. Pollut.345, 123393. 10.1016/j.envpol.2024.123393
76
Dong Y. S. Hou W. G. Li Y. Liu D. B. Hao G. Z. Zhang H. F. et al (2016). Unexpected requirement for a binding partner of the syntaxin family in phagocytosis by murine testicular Sertoli cells. Cell Death Differ.23 (5), 787–800. 10.1038/cdd.2015.139
77
Du L. Chen W. Cheng Z. X. Wu S. He J. Han L. et al (2021). Novel gene regulation in normal and abnormal spermatogenesis. Cells10 (3), 666. 10.3390/cells10030666
78
Duan C. J. Li M. H. Rui L. Y. (2004). SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J. Biol. Chem.279 (42), 43684–43691. 10.1074/jbc.M408495200
79
Dunleavy J. E. M. O'Bryan M. K. Stanton P. G. O'Donnell L. (2019). The cytoskeleton in spermatogenesis. Reproduction157 (2), R53–R72. 10.1530/Rep-18-0457
80
Dym M. Kokkinaki M. He Z. (2009). Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res. C Embryo Today87 (1), 27–34. 10.1002/bdrc.20141
81
Endo T. Romer K. A. Anderson E. L. Baltus A. E. de Rooij D. G. Page D. C. (2015). Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. U. S. A.112 (18), E2347–E2356. 10.1073/pnas.1505683112
82
Endo T. Freinkman E. de Rooij D. G. Page D. C. (2017). Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc. Natl. Acad. Sci. U. S. A.114 (47), E10132–E10141. 10.1073/pnas.1710837114
83
Fadlalla K. (2019). 99 temporal and spatial characterization of interleukin-6 protein in the caprine testis. J. Anim. Sci.97, 83. 10.1093/jas/skz258.171
84
Fang Y. W. Su Y. F. Xu J. Hu Z. Y. Zhao K. Liu C. Y. et al (2021). Varicocele-Mediated Male infertility: from the perspective of testicular immunity and inflammation. Front. Immunol.12, 729539. 10.3389/fimmu.2021.729539
85
Fang X. Nie L. Putluri S. Ni N. Bartholin L. Li Q. (2023). Sertoli cell-specific activation of transforming growth factor beta receptor 1 leads to testicular granulosa cell tumor Formation. Cells12 (23), 2717. 10.3390/cells12232717
86
Fayomi A. P. Orwig K. E. (2018). Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res.29, 207–214. 10.1016/j.scr.2018.04.009
87
Feijo M. Carvalho T. M. A. Fonseca L. R. S. Vaz C. V. Pereira B. J. Cavaco J. E. B. et al (2025). Endocrine-disrupting chemicals as prostate carcinogens. Nat. Rev. Urol.22 (9), 609–631. 10.1038/s41585-025-01031-9
88
Fernandez D. Bertoldi M. V. Gomez L. Morales A. Callegari E. Lopez L. A. (2008). Identification and characterization of Myosin from rat testicular peritubular myoid cells. Biol. Reprod.79 (6), 1210–1218. 10.1095/biolreprod.107.066472
89
Fernandez C. J. Chacko E. C. Pappachan J. M. (2019). Male obesity-related secondary hypogonadism - pathophysiology, clinical implications and management. Eur. Endocrinol.15 (2), 83–90. 10.17925/ee.2019.15.2.83
90
Fijak M. Bhushan S. Meinhardt A. (2011). Immunoprivileged sites: the testis. Methods Mol. Biol.677, 459–470. 10.1007/978-1-60761-869-0_29
91
Fleck D. Kenzler L. Mundt N. Strauch M. Uesaka N. Moosmann R. et al (2021). ATP activation of peritubular cells drives testicular sperm transport. Elife10, e62885. 10.7554/eLife.62885
92
Fouchécourt S. Livera G. Messiaen S. Fumel B. Parent A. S. Marine J. C. et al (2016). Apoptosis of Sertoli cells after conditional ablation of murine double minute 2 (Mdm2) gene is p53-dependent and results in male sterility. Cell Death Differ.23 (3), 521–530. 10.1038/cdd.2015.120
93
França L. R. Hess R. A. Dufour J. M. Hofmann M. C. Griswold M. D. (2016). The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology4 (2), 189–212. 10.1111/andr.12165
94
Gao F. Maiti S. Alam N. Zhang Z. Deng J. M. Behringer R. R. et al (2006). The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc. Natl. Acad. Sci. U. S. A.103 (32), 11987–11992. 10.1073/pnas.0600994103
95
Gao S. Fang X. Guo L. (2025). Construction of testicular organoids and their applications in the field of toxicology. Arch. Toxicol.99 (12), 4737–4755. 10.1007/s00204-025-04177-y
96
Garcia T. X. Parekh P. Gandhi P. Sinha K. Hofmann M. C. (2017). The NOTCH ligand JAG1 regulates GDNF expression in Sertoli cells. Stem Cells Dev.26 (8), 585–598. 10.1089/scd.2016.0318
97
Gaytan F. Bellido C. Aguilar E. Vanrooijen N. (1994). Requirement for testicular macrophages in leydig-cell proliferation and differentiation during prepubertal development in rats. J. Reprod. Fertil.102 (2), 393–399. 10.1530/jrf.0.1020393
98
Ge R. S. Dong Q. A. Sottas C. M. Papadopoulos V. Zirkin B. R. Hardy M. P. (2006). In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. U. S. A.103 (8), 2719–2724. 10.1073/pnas.0507692103
99
Ge L. C. Chen Z. J. Liu H. Zhang K. S. Su Q. Ma X. Y. et al (2014). Signaling related with biphasic effects of bisphenol A (BPA) on Sertoli cell proliferation: a comparative proteomic analysis. Biochim. Biophys. Acta1840 (9), 2663–2673. 10.1016/j.bbagen.2014.05.018
100
Gerber J. Heinrich J. Brehm R. (2016). Blood-testis barrier and Sertoli cell function: lessons from SCCx43KO mice. Reproduction151 (2), R15–R27. 10.1530/Rep-15-0366
101
Ghanami Gashti N. Sadighi Gilani M. A. Abbasi M. (2021). Sertoli cell-only syndrome: etiology and clinical management. J. Assist. Reprod. Genet.38 (3), 559–572. 10.1007/s10815-021-02063-x
102
Gnessi L. Basciani S. Mariani S. Arizzi M. Spera G. Wang C. et al (2000). Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A-deficient mice. J. Cell Biol.149 (5), 1019–1026. 10.1083/jcb.149.5.1019
103
Gonen N. Quinn A. O'Neill H. C. Koopman P. Lovell-Badge R. (2017). Correction: normal levels of Sox9 expression in the developing mouse Testis depend on the TES/TESCO enhancer, but this does not Act alone. PLoS Genet.13 (2), e1006584. 10.1371/journal.pgen.1006584
104
Griswold M. D. (2018). 50 years of spermatogenesis: sertoli cells and their interactions with germ cells. Biol. Reprod.99 (1), 87–100. 10.1093/biolre/ioy027
105
Griswold S. L. Behringer R. R. (2009). Fetal Leydig cell origin and development. Sex. Dev.3 (1), 1–15. 10.1159/000200077
106
Gu X. W. Heinrich A. Li S. Y. DeFalco T. (2023). Testicular macrophages are recruited during a narrow fetal time window and promote organ-specific developmental functions. Nat. Commun.14 (1), 1439. 10.1038/s41467-023-37199-0
107
Guo J. Nie X. Giebler M. Mlcochova H. Wang Y. Grow E. J. et al (2020). The dynamic transcriptional cell Atlas of testis development during human puberty. Cell Stem Cell26 (2), 262–276 e264. 10.1016/j.stem.2019.12.005
108
Habert R. (1993). In vivo acute testicular testosterone response to injection of luteinizing hormone in the rat fetus. Acta Endocrinol. (Copenh)128 (3), 268–273. 10.1530/acta.0.1280268
109
Hales D. B. (2002). Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol.57 (1-2), 3–18. 10.1016/S0165-0378(02)00020-7
110
Hamoda T. Shah R. Mostafa T. Pinggera G. M. Atmoko W. Rambhatla A. et al (2025). Global Andrology Forum (GAF) Clinical Guidelines on the management of non-obstructive azoospermia: bridging the gap between controversy and consensus. World J. Mens. Health43, e26. 10.5534/wjmh.250037
111
Hancock G. V. Wamaitha S. E. Peretz L. Clark A. T. (2021). Mammalian primordial germ cell specification. Development148 (6), dev189217. 10.1242/dev.189217
112
Hanley N. A. Hagan D. M. Clement-Jones M. Ball S. G. Strachan T. Salas-Cortés L. et al (2000). SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech. Dev.91 (1-2), 403–407. 10.1016/S0925-4773(99)00307-X
113
Hartmann K. Bennien J. Wapelhorst B. Bakhaus K. Schumacher V. Kliesch S. et al (2016). Current insights into the sulfatase pathway in human testis and cultured Sertoli cells. Histochem Cell Biol.146 (6), 737–748. 10.1007/s00418-016-1503-y
114
Hasegawa K. Saga Y. (2014). FGF8-FGFR1 signaling acts as a niche factor for maintaining undifferentiated spermatogonia in the mouse. Biol. Reprod.91 (6), 145. 10.1095/biolreprod.114.121012
115
Haverfield J. T. Meachem S. J. Nicholls P. K. Rainczuk K. E. Simpson E. R. Stanton P. G. (2014). Differential permeability of the blood-testis barrier during reinitiation of spermatogenesis in adult Male rats. Endocrinology155 (3), 1131–1144. 10.1210/en.2013-1878
116
Hedger M. P. de Kretser D. M. (2013). The activins and their binding protein, follistatin-diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine and Growth Factor Rev.24 (3), 285–295. 10.1016/j.cytogfr.2013.03.003
117
Heinrich A. DeFalco T. (2020). Essential roles of interstitial cells in testicular development and function. Andrology8 (4), 903–914. 10.1111/andr.12703
118
Hiramatsu R. Matoba S. Kanai-Azuma M. Tsunekawa N. Katoh-Fukui Y. Kurohmaru M. et al (2009). A critical time window of Sry action in gonadal sex determination in mice. Development136 (1), 129–138. 10.1242/dev.029587
119
Hofmann M. C. (2008). Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol. Cell. Endocrinol.288 (1-2), 95–103. 10.1016/j.mce.2008.04.012
120
Hofmann M. C. McBeath E. (2022). Sertoli cell-germ cell interactions within the niche: paracrine and Juxtacrine molecular communications. Front. Endocrinol.13, 897062. 10.3389/fendo.2022.897062
121
Hogarth C. A. Arnold S. Kent T. Mitchell D. Isoherranen N. Griswold M. D. (2015). Processive pulses of retinoic acid Propel asynchronous and continuous murine sperm production. Biol. Reprod.92 (2), 37. 10.1095/biolreprod.114.126326
122
Holdcraft R. W. Braun R. E. (2004). Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development131 (2), 459–467. 10.1242/dev.00957
123
Hossain A. Saunders G. F. (2001). The human sex-determining gene SRY is a direct target of WT1. J. Biol. Chem.276 (20), 16817–16823. 10.1074/jbc.M009056200
124
Hu G. X. Lin H. Chen G. R. Chen B. B. Lian Q. Q. Hardy D. O. et al (2010). Deletion of the Igf1 gene: suppressive effects on adult Leydig cell development. J. Androl.31 (4), 379–387. 10.2164/jandrol.109.008680
125
Huang J. Ren H. Chen A. Li T. Wang H. Jiang L. et al (2022). Perfluorooctane sulfonate induces suppression of testosterone biosynthesis via Sertoli cell-derived exosomal/miR-9-3p downregulating StAR expression in Leydig cells. Environ. Pollut.301, 118960. 10.1016/j.envpol.2022.118960
126
Huang D. Zuo Y. Zhang C. Sun G. Jing Y. Lei J. et al (2023). A single-nucleus transcriptomic atlas of primate testicular aging reveals exhaustion of the spermatogonial stem cell reservoir and loss of Sertoli cell homeostasis. Protein Cell14 (12), 888–907. 10.1093/procel/pwac057
127
Hume D. A. Halpin D. Charlton H. Gordon S. (1984). The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of endocrine organs. Proc. Natl. Acad. Sci. U. S. A.81 (13), 4174–4177. 10.1073/pnas.81.13.4174
128
Hutson J. C. (2006). Physiologic interactions between macrophages and Leydig cells. Exp. Biol. Med.231 (1), 1–7. 10.1177/153537020623100101
129
Hutson J. M. (2013). Journal of Pediatric Surgery-Sponsored Fred McLoed Lecture. Undescended testis: the underlying mechanisms and the effects on germ cells that cause infertility and cancer. J. Pediatr. Surg.48 (5), 903–908. 10.1016/j.jpedsurg.2013.02.001
130
Ibtisham F. Awang-Junaidi A. H. Honaramooz A. (2020). The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res.380 (2), 393–414. 10.1007/s00441-020-03212-x
131
Jiménez R. Burgos M. Barrionuevo F. J. (2021). Sex maintenance in mammals. Genes (Basel)12 (7), 999. 10.3390/genes12070999
132
Johnston D. S. Wright W. W. DiCandeloro P. Wilson E. Kopf G. S. Jelinsky S. A. (2008). Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc. Natl. Acad. Sci. U. S. A.105 (24), 8315–8320. 10.1073/pnas.0709854105
133
Jones S. L. Styles J. Geyer C. B. (2021). A new translation and reader's guide to the first detailed description of the first wave of spermatogenesis in the mouse. Mol. Reprod. Dev.88 (7), 473–478. 10.1002/mrd.23519
134
Kabbesh H. Bulldan A. Konrad L. Scheiner-Bobis G. (2022). The role of ZIP9 and androgen receptor in the establishment of tight junctions between adult rat Sertoli cells. Biology-Basel11 (5), 668. 10.3390/biology11050668
135
Kaftanovskaya E. M. Lopez C. Ferguson L. Myhr C. Agoulnik A. I. (2015). Genetic ablation of androgen receptor signaling in fetal Leydig cell lineage affects Leydig cell functions in adult testis. Faseb J.29 (6), 2327–2337. 10.1096/fj.14-263632
136
Kanatsu-Shinohara M. Ogonuki N. Inoue K. Miki H. Ogura A. Toyokuni S. et al (2003). Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod.69 (2), 612–616. 10.1095/biolreprod.103.017012
137
Kanatsu-Shinohara M. Inoue K. Ogonuki N. Miki H. Yoshida S. Toyokuni S. et al (2007). Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture. Biol. Reprod.76 (1), 55–62. 10.1095/biolreprod.106.055863
138
Karki R. Kanneganti T. D. (2019). Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer19 (4), 197–214. 10.1038/s41568-019-0123-y
139
Karl J. Capel B. (1998). Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev. Biol.203 (2), 323–333. 10.1006/dbio.1998.9068
140
Karseladze A. I. Asaturova A. V. Kiseleva I. A. Badlaeva A. S. Tregubova A. V. Zaretsky A. R. et al (2024). Androgen insensitivity syndrome with bilateral gonadal sertoli cell lesions, Sertoli-Leydig cell tumor, and paratesticular leiomyoma: a case report and first systematic literature review. J. Clin. Med.13 (4), 929. 10.3390/jcm13040929
141
Kato T. Esaki M. Matsuzawa A. Ikeda Y. (2012). NR5A1 is required for functional maturation of Sertoli cells during postnatal development. Reproduction143 (5), 663–672. 10.1530/REP-11-0365
142
Katoh-Fukui Y. Miyabayashi K. Komatsu T. Owaki A. Baba T. Shima Y. et al (2012). Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology153 (2), 913–924. 10.1210/en.2011-1055
143
Kaur G. Thompson L. A. Dufour J. M. (2014). Sertoli cells - immunological sentinels of spermatogenesis. Seminars Cell and Dev. Biol.30, 36–44. 10.1016/j.semcdb.2014.02.011
144
Kawabe Y. Numabe T. Tanemura K. Hara K. (2022). Characteristics of alpha smooth muscle actin-positive peritubular cells in prepubertal bovine testes. Biochem. Biophys. Res. Commun.609, 48–53. 10.1016/j.bbrc.2022.03.149
145
Khan U. W. Rai U. (2004). In vitro effect of FSH and testosterone on Sertoli cell nursing function in wall lizard Hemidactylus flaviviridis (Rüppell) ruppell. General Comp. Endocrinol.136 (2), 225–231. 10.1016/j.ygcen.2003.12.015
146
Khan U. W. Rai U. (2005). Endocrine and paracrine control of Leydig cell steroidogenesis and proliferation in the wall lizard: an in vitro study. General Comp. Endocrinol.140 (2), 109–115. 10.1016/j.ygcen.2004.10.009
147
Kilcoyne K. R. Smith L. B. Atanassova N. Macpherson S. McKinnell C. van den Driesche S. et al (2014). Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc. Natl. Acad. Sci. U. S. A.111 (18), E1924–E1932. 10.1073/pnas.1320735111
148
Kim K. S. Liu W. Cunha G. R. Russell D. W. Huang H. Shapiro E. et al (2002). Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res.307 (2), 145–153. 10.1007/s004410100464
149
Klattig J. Sierig R. Kruspe D. Makki M. S. Englert C. (2007). WT1-mediated gene regulation in early urogenital ridge development. Sex. Dev.1 (4), 238–254. 10.1159/000104774
150
Kongmanas K. Saewu A. Kiattiburut W. Baker M. A. Faull K. F. Burger D. et al (2021). Accumulation of seminolipid in Sertoli cells is associated with increased levels of reactive oxygen species and Male subfertility: studies in aging Arsa null Male mice. Antioxidants (Basel)10 (6), 912. 10.3390/antiox10060912
151
Krausz C. Navarro-Costa P. Wilke M. Tuttelmann F. (2024). EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state of the art 2023. Andrology12 (3), 487–504. 10.1111/andr.13514
152
Kubota H. Brinster R. L. (2018). Spermatogonial stem cells. Biol. Reprod.99 (1), 52–74. 10.1093/biolre/ioy077
153
Kumar A. Raut S. Balasinor N. H. (2018). Endocrine regulation of sperm release. Reprod. Fertil. Dev.30 (12), 1595–1603. 10.1071/Rd18057
154
Kuroki S. Tachibana M. (2018). Epigenetic regulation of mammalian sex determination. Mol. Cell. Endocrinol.468, 31–38. 10.1016/j.mce.2017.12.006
155
Larney C. Bailey T. L. Koopman P. (2014). Switching on sex: transcriptional regulation of the testis-determining gene Sry. Development141 (11), 2195–2205. 10.1242/dev.107052
156
Larose H. Kent T. Ma Q. Y. Shami A. N. Harerimana N. Li J. Z. et al (2020). Regulation of meiotic progression by Sertoli-cell androgen signaling. Mol. Biol. Cell31 (25), 2841–2862. 10.1091/mbc.E20-05-0334
157
Lauby-Secretan B. Scoccianti C. Loomis D. Grosse Y. Bianchini F. Straif K. et al (2016). Body fatness and cancer - viewpoint of the IARC Working Group. N. Engl. J. Med.375 (8), 794–798. 10.1056/NEJMsr1606602
158
Lee R. Lee W. Y. Park H. J. (2024). Diuron-induced fetal Leydig cell dysfunction in in vitro organ cultured fetal testes. Reprod. Toxicol.123, 108497. 10.1016/j.reprotox.2023.108497
159
Lei Z. M. Mishra S. Zou W. Xu B. Foltz M. Li X. et al (2001). Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol. Endocrinol.15 (1), 184–200. 10.1210/me.15.1.184
160
Lenzi A. Balercia G. Bellastella A. Colao A. Fabbri A. Foresta C. et al (2009). Epidemiology, diagnosis, and treatment of male hypogonadotropic hypogonadism. J. Endocrinol. Investig.32 (11), 934–938. 10.1007/Bf03345775
161
Li N. Wang T. Han D. S. (2012). Structural, cellular and molecular aspects of immune privilege in the testis. Front. Immunol.3, 152. 10.3389/fimmu.2012.00152
162
Li X. Wang Z. Jiang Z. Guo J. Zhang Y. Li C. et al (2016). Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc. Natl. Acad. Sci. U. S. A.113 (10), 2666–2671. 10.1073/pnas.1519395113
163
Li X. J. Liu J. P. Wu S. W. Zheng W. W. Li H. T. Bao S. H. et al (2018). In utero single low-dose exposure of cadmium induces rat fetal Leydig cell dysfunction. Chemosphere194, 57–66. 10.1016/j.chemosphere.2017.11.159
164
Li X. Long X. Y. Xie Y. J. Zeng X. Chen X. Mo Z. C. (2019). The roles of retinoic acid in the differentiation of spermatogonia and spermatogenic disorders. Clin. Chim. Acta497, 54–60. 10.1016/j.cca.2019.07.013
165
Li Y. X. Chen Y. G. Wu W. P. Li N. Hua J. L. (2023). MMPs, ADAMs and ADAMTSs are associated with mammalian sperm fate. Theriogenology200, 147–154. 10.1016/j.theriogenology.2023.02.013
166
Li X.-W. Li S. Yang Y. Talukder M. Xu X.-W. Li C.-X. et al (2024). The FAK/occludin/ZO-1 complex is critical for cadmium-induced testicular damage by disruption of the integrity of the blood-testis barrier in chickens. J. Hazard. Mater.470, 134126. 10.1016/j.jhazmat.2024.134126
167
Li Q. Li H. H. Liang J. L. Mei J. X. Cao Z. Zhang L. et al (2021). Sertoli cell-derived exosomal MicroRNA-486-5p regulates differentiation of spermatogonial stem cell through PTEN in mice. J. Cell. Mol. Med.25 (8), 3950–3962. 10.1111/jcmm.16347
168
Li S Y. Gu X. W. Heinrich A. Hurley E. G. Capel B. DeFalco T. (2021). Loss of Mafb and Maf distorts myeloid cell ratios and disrupts fetal mouse testis vascularization and organogenesis†. Biol. Reprod.105 (4), 958–975. 10.1093/biolre/ioab098
169
Liang J. Wang N. He J. Du J. Guo Y. Li L. et al (2019). Induction of Sertoli-like cells from human fibroblasts by NR5A1 and GATA4. Elife8, e48767. 10.7554/eLife.48767
170
Liang J. L. Li H. H. Mei J. X. Cao Z. Tang Y. Huang R. F. et al (2021). Sertoli cell-derived exosome-mediated transfer of miR-145-5p inhibits Leydig cell steroidogenesis by targeting steroidogenic factor 1. Faseb J.35 (6), e21660. 10.1096/fj.202002589RRRR
171
Lim K. Hwang B. D. (1995). Follicle-Stimulating-Hormone transiently induces expression of protooncogene C-Myc in primary sertoli-cell cultures of early pubertal and prepubertal rat. Mol. Cell. Endocrinol.111 (1), 51–56. 10.1016/0303-7207(95)03543-G
172
Liu C. Rodriguez K. Yao H. H. C. (2016). Mapping lineage progression of somatic progenitor cells in the mouse fetal testis. Development143 (20), 3700–3710. 10.1242/dev.135756
173
Liu M. R. He Q. Yuan Z. H. Chen N. N. Ren S. Du Q. et al (2024). HDAC3 promotes Sertoli cell maturation and maintains the blood-testis barrier dynamics. Faseb J.38 (5), e23526. 10.1096/fj.202301349RR
174
Liu J. Ren L. H. Wei J. L. Zhang J. Zhu Y. P. Li X. Y. et al (2018). Fine particle matter disrupts the blood-testis barrier by activating TGF-β3/p38 MAPK pathway and decreasing testosterone secretion in rat. Environ. Toxicol.33 (7), 711–719. 10.1002/tox.22556
175
Liu Y. Hu Y. Wang L. Xu C. (2018). Expression of transcriptional factor EB (TFEB) in differentiating spermatogonia potentially promotes cell migration in mouse seminiferous epithelium. Reprod. Biol. Endocrinol.16 (1), 105. 10.1186/s12958-018-0427-x
176
Lo K. C. Lei Z. M. Rao C. V. Beck J. Lamb D. J. (2004). De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of leydig stem cells. Endocrinology145 (9), 4011–4015. 10.1210/en.2003-1729
177
Lobo J. Acosta A. M. Netto G. J. (2023). Molecular biomarkers with potential clinical application in testicular cancer. Mod. Pathol.36 (10), 100307. 10.1016/j.modpat.2023.100307
178
Lord T. Oatley J. M. (2017). A revised Asingle model to explain stem cell dynamics in the mouse male germline. Reproduction154 (2), R55–R64. 10.1530/Rep-17-0034
179
Luaces J. P. Toro-Urrego N. Otero-Losada M. Capani F. (2023). What do we know about blood-testis barrier? Current understanding of its structure and physiology. Front. Cell Dev. Biol.11, 1114769. 10.3389/fcell.2023.1114769
180
Luca G. Arato I. Sorci G. Cameron D. F. Hansen B. C. Baroni T. et al (2018a). Sertoli cells for cell transplantation: pre-clinical studies and future perspectives. Andrology6 (3), 385–395. 10.1111/andr.12484
181
Luca G. Baroni T. Arato I. Hansen B. C. Cameron D. F. Calafiore R. (2018b). Role of Sertoli cell proteins in immunomodulation. Protein Pept. Lett.25 (5), 440–445. 10.2174/0929866525666180412163151
182
Lucas-Herald A. K. Mitchell R. T. (2022). Testicular sertoli cell hormones in differences in sex development. Front. Endocrinol.13, 919670. 10.3389/fendo.2022.919670
183
Lui W. Y. Wong C. H. Mruk D. D. Cheng C. Y. (2003). TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology144 (4), 1139–1142. 10.1210/en.2002-0211
184
Luo J. Gupta V. Kern B. Tash J. S. Sanchez G. Blanco G. et al (2012). Role of FYN kinase in spermatogenesis: defects characteristic of Fyn-null sperm in mice. Biol. Reprod.86 (1), 1–8. 10.1095/biolreprod.111.093864
185
Luo D. He Z. Yu C. Guan Q. (2022). Role of p38 MAPK signalling in Testis development and Male fertility. Oxid. Med. Cell Longev.2022, 6891897. 10.1155/2022/6891897
186
Lv Y. Li L. Fang Y. Chen P. Wu S. Chen X. et al (2019). In utero exposure to bisphenol A disrupts fetal testis development in rats. Environ. Pollut.246, 217–224. 10.1016/j.envpol.2018.12.006
187
Lv M. Q. Ge P. Zhang J. Yang Y. Q. Zhou L. Zhou D. X. (2021). Temporal trends in semen concentration and count among 327 373 Chinese healthy men from 1981 to 2019: a systematic review. Hum. Reprod.36 (7), 1751–1775. 10.1093/humrep/deab124
188
Ma Y. Yang H. Z. Xu L. M. Huang Y. R. Dai H. L. Kang X. N. (2015). Testosterone regulates the autophagic clearance of androgen binding protein in rat Sertoli cells. Sci. Rep.5, 8894. 10.1038/srep08894
189
Ma J. X. Wu L. Zhou Y. Zhang H. Xiong C. L. Peng Z. et al (2019). Association between BMI and semen quality: an observational study of 3966 sperm donors. Hum. Reprod.34 (1), 155–162. 10.1093/humrep/dey328
190
Ma Y. Zhou Y. Zou S. S. Sun Y. Chen X. F. (2022). Exosomes released from Sertoli cells contribute to the survival of Leydig cells through CCL20 in rats. Mol. Hum. Reprod.28 (2), gaac002. 10.1093/molehr/gaac002
191
Maekawa M. Kamimura K. Nagano T. (1996). Peritubular myoid cells in the testis: their structure and function. Arch. Histol. Cytol.59 (1), 1–13. 10.1679/aohc.59.1
192
Mahyari E. Guo J. Lima A. C. Lewinsohn D. P. Stendahl A. M. Vigh-Conrad K. A. et al (2021). Comparative single-cell analysis of biopsies clarifies pathogenic mechanisms in Klinefelter syndrome. Am. J. Hum. Genet.108 (10), 1924–1945. 10.1016/j.ajhg.2021.09.001
193
Mäkelä J. A. Hobbs R. M. (2019). Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction158 (5), R169–R187. 10.1530/Rep-18-0476
194
Mäkelä J. A. Koskenniemi J. J. Virtanen H. E. Toppari J. (2019). Testis development. Endocr. Rev.40 (4), 857–905. 10.1210/er.2018-00140
195
Manna P. R. Chandrala S. P. Jo Y. Stocco D. M. (2006). cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J. Mol. Endocrinol.37 (1), 81–95. 10.1677/jme.1.02065
196
Mao B. P. Bu T. Mruk D. Li C. Sun F. Cheng C. Y. (2020). Modulating the blood-testis barrier towards increasing drug delivery. Trends Pharmacol. Sci.41 (10), 690–700. 10.1016/j.tips.2020.07.002
197
Mark M. Teletin M. Vernet N. Ghyselinck N. B. (2015). Role of retinoic acid receptor (RAR) signaling in post-natal male germ cell differentiation. Biochim. Biophys. Acta1849 (2), 84–93. 10.1016/j.bbagrm.2014.05.019
198
Markouli M. Michala L. (2023). Fertility potential in 5α-reductase type 2 deficient males. J. Pediatr. Urol.19 (1), 108–114. 10.1016/j.jpurol.2022.09.002
199
Martin L. J. (2016). Cell interactions and genetic regulation that contribute to testicular Leydig cell development and differentiation. Mol. Reprod. Dev.83 (6), 470–487. 10.1002/mrd.22648
200
Masone M. C. (2023). Embryonal origin of adult testicular macrophages. Nat. Rev. Urol.20 (5), 262. 10.1038/s41585-023-00770-x
201
Matson C. K. Murphy M. W. Sarver A. L. Griswold M. D. Bardwell V. J. Zarkower D. (2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature476 (7358), 101–104. 10.1038/nature10239
202
Mayerhofer A. (2013). Human testicular peritubular cells: more than meets the eye. Reproduction145 (5), R107–R116. 10.1530/Rep-12-0497
203
McCabe M. J. Allan C. M. Foo C. F. H. Nicholls P. K. McTavish K. J. Stanton P. G. (2012). Androgen initiates Sertoli cell tight junction formation in the hypogonadal (hpg) mouse. Biol. Reprod.87 (2), 38. 10.1095/biolreprod.111.094318
204
McCabe M. J. Foo C. F. Dinger M. E. Smooker P. M. Stanton P. G. (2016). Claudin-11 and occludin are major contributors to Sertoli cell tight junction function, in vitro. Asian J. Androl.18 (4), 620–626. 10.4103/1008-682x.163189
205
McClelland K. S. Bell K. Larney C. Harley V. R. Sinclair A. H. Oshlack A. et al (2015). Purification and transcriptomic analysis of Mouse Fetal Leydig cells reveals candidate genes for specification of gonadal steroidogenic cells. Biol. Reprod.92 (6), 145. 10.1095/biolreprod.115.128918
206
McElreavey K. Jorgensen A. Eozenou C. Merel T. Bignon-Topalovic J. Tan D. S. et al (2020). Pathogenic variants in the DEAH-box RNA helicase DHX37 are a frequent cause of 46,XY gonadal dysgenesis and 46,XY testicular regression syndrome. Genet. Med.22 (1), 150–159. 10.1038/s41436-019-0606-y
207
Meachem S. J. Ruwanpura S. M. Ziolkowski J. Ague J. M. Skinner M. K. Loveland K. L. (2005). Developmentally distinct in vivo effects of FSH on proliferation and apoptosis during testis maturation. J. Endocrinol.186 (3), 429–446. 10.1677/joe.1.06121
208
Medici C. Jorgensen N. Juul A. Albrethsen J. Kreiberg M. Lauritsen J. et al (2024). Insulin-like factor 3, basal and human chorionic gonadotropin-stimulated testosterone as biomarkers to predict the effect of testosterone replacement in testicular cancer survivors with mild Leydig cell insufficiency. Clin. Genitourin. Cancer22 (1), e106–e112. 10.1016/j.clgc.2023.08.005
209
Meinhardt A. Wang M. Schulz C. Bhushan S. (2018). Microenvironmental signals govern the cellular identity of testicular macrophages. J. Leukoc. Biol.104 (4), 757–766. 10.1002/Jlb.3mr0318-086rr
210
Meng J. Greenlee A. R. Taub C. J. Braun R. E. (2011). Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. Biol. Reprod.85 (2), 254–260. 10.1095/biolreprod.110.090621
211
Meroni S. B. Galardo M. N. Rindone G. Gorga A. Riera M. F. Cigorraga S. B. (2019). Molecular mechanisms and signaling pathways involved in Sertoli cell proliferation. Front. Endocrinol.10, 224. 10.3389/fendo.2019.00224
212
Min M. Song T. Sun M. D. Wang T. T. Tan J. Zhang J. D. (2022). Dhh signaling pathway regulates reconstruction of seminiferous tubule-like structure. Reprod. Biol.22 (4), 100684. 10.1016/j.repbio.2022.100684
213
Mita M. Hall P. F. (1982). Metabolism of round spermatids from rats: lactate as the preferred substrate. Biol. Reprod.26 (3), 445–455. 10.1095/biolreprod26.3.445
214
Mitra S. Srivastava A. Khandelwal S. (2017). Long term impact of the endocrine disruptor tributyltin on male fertility following a single acute exposure. Environ. Toxicol.32 (10), 2295–2304. 10.1002/tox.22446
215
Miyabayashi K. Katoh-Fukui Y. Ogawa H. Baba T. Shima Y. Sugiyama N. et al (2013). Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. Plos One8 (6), e68050. 10.1371/journal.pone.0068050
216
Miyamoto Y. Taniguchi H. Hamel F. Silversides D. W. Viger R. S. (2008). A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. Bmc Mol. Biol.9, 44. 10.1186/1471-2199-9-44
217
Morianos I. Papadopoulou G. Semitekolou M. Xanthou G. (2019). Activin-A in the regulation of immunity in health and disease. J. Autoimmun.104, 102314. 10.1016/j.jaut.2019.102314
218
Mossadegh-Keller N. Gentek R. Gimenez G. Bigot S. Mailfert S. Sieweke M. H. (2017). Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med.214 (10), 2829–2841. 10.1084/jem.20170829
219
Mruk D. D. Cheng C. Y. (2004). Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol. Metab.15 (9), 439–447. 10.1016/j.tem.2004.09.009
220
Mruk D. D. Cheng C. Y. (2015). The Mammalian blood-testis barrier: its biology and regulation. Endocr. Rev.36 (5), 564–591. 10.1210/er.2014-1101
221
Mu Y. Yin T. L. Zhang Y. Yang J. Wu Y. T. (2022). Diet-induced obesity impairs spermatogenesis: the critical role of NLRP3 in Sertoli cells. Inflamm. Regen.42 (1), 24. 10.1186/s41232-022-00203-z
222
Murphy C. J. Richburg J. H. (2014). Implications of Sertoli cell induced germ cell apoptosis to testicular pathology. Spermatogenesis4 (2), e979110. 10.4161/21565562.2014.979110
223
Nagano R. Tabata S. Nakanishi Y. Ohsako S. Kurohmaru M. Hayashi Y. (2000). Reproliferation and relocation of mouse male germ cells (gonocytes) during prespermatogenesis. Anat. Rec.258 (2), 210–220. 10.1002/(SICI)1097-0185(20000201)258:2<210::AID-AR10>3.0.CO;2-X
224
Nakagawa A. Shiratsuchi A. Tsuda K. Nakanishi Y. (2005). In vivo analysis of phagocytosis of apoptotic cells by testicular Sertoli cells. Mol. Reprod. Dev.71 (2), 166–177. 10.1002/mrd.20278
225
Nakagawa T. Nabeshima Y. I. Yoshida S. (2007). Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell12 (2), 195–206. 10.1016/j.devcel.2007.01.002
226
Nakamura M. Okinaga S. Arai K. (1984). Metabolism of pachytene primary spermatocytes from rat testes: pyruvate maintenance of adenosine triphosphate level. Biol. Reprod.30 (5), 1187–1197. 10.1095/biolreprod30.5.1187
227
Naughton C. K. Jain S. Strickland A. M. Gupta A. Milbrandt J. (2006). Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate.(vol 74, pg 314, 2006). Biol. Reprod.75 (4), 660. 10.1095/biolreprod.106.052027
228
Ni F. D. Hao S. L. Yang W. X. (2019). Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis. Cell Death Dis.10 (8), 541. 10.1038/s41419-019-1782-z
229
Ni F. D. Hao S. L. Yang W. X. (2020). Molecular insights into hormone regulation via signaling pathways in Sertoli cells: with discussion on infertility and testicular tumor. Gene753, 144812. 10.1016/j.gene.2020.144812
230
Nicholls P. K. Page D. C. (2021). Germ cell determination and the developmental origin of germ cell tumors. Development148 (8), dev198150. 10.1242/dev.198150
231
Nie X. Munyoki S. K. Sukhwani M. Schmid N. Missel A. Emery B. R. et al (2022). Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev. Cell57 (9), 1160–1176.e1165. 10.1016/j.devcel.2022.04.004
232
Nistal M. Paniagua R. Regadera J. Santamarìa L. Amat P. (1986). A quantitative morphological study of human Leydig cells from birth to adulthood. Cell Tissue Res.246 (2), 229–236. 10.1007/bf00215884
233
Niu Q. Cao M. Yuan C. Huang Y. Zhao Z. Zhang B. et al (2020). Effect of nerve growth factor on the proliferation in newborn bovine testicular Sertoli cells. Reproduction160 (3), 405–415. 10.1530/rep-19-0601
234
O'Donnell L. (2014). Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis4 (2), e979623. 10.4161/21565562.2014.979623
235
O'Donnell L. Nicholls P. K. O'Bryan M. K. McLachlan R. I. Stanton P. G. (2011). Spermiation: the process of sperm release. Spermatogenesis1 (1), 14–35. 10.4161/spmg.1.1.14525
236
O'Donnell L. Rebourcet D. Dagley L. F. Sgaier R. Infusini G. O'Shaughnessy P. J. et al (2021). Sperm proteins and cancer-testis antigens are released by the seminiferous tubules in mice and men. Faseb J.35 (3), e21397. 10.1096/fj.202002484R
237
O'Donnell L. Smith L. B. Rebourcet D. (2022a). Sertoli cells as key drivers of testis function. Seminars Cell and Dev. Biol.121, 2–9. 10.1016/j.semcdb.2021.06.016
238
O'Donnell L. Whiley P. A. F. Loveland K. L. (2022b). Activin A and sertoli cells: key to fetal testis steroidogenesis. Front. Endocrinol. (Lausanne)13, 898876. 10.3389/fendo.2022.898876
239
O'Shaughnessy P. J. Johnston H. Willerton L. Baker P. J. (2002). Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J. Cell Sci.115 (17), 3491–3496. 10.1242/jcs.115.17.3491
240
Oatley J. M. Brinster R. L. (2012). The germline stem cell niche unit in Mammalian testes. Physiol. Rev.92 (2), 577–595. 10.1152/physrev.00025.2011
241
Oatley M. J. Racicot K. E. Oatley J. M. (2011). Sertoli cells dictate spermatogonial stem cell niches in the mouse testis. Biol. Reprod.84 (4), 639–645. 10.1095/biolreprod.110.087320
242
Oduwole O. O. Vydra N. Wood N. E. M. Samanta L. Owen L. Keevil B. et al (2014). Overlapping dose responses of spermatogenic and extragonadal testosterone actions jeopardize the principle of hormonal male contraception. Faseb J.28 (6), 2566–2576. 10.1096/fj.13-249219
243
Okashita N. Tachibana M. (2021). Transcriptional regulation of the Y-Linked Mammalian testis-determining gene SRY. Sex. Dev.15 (5-6), 351–359. 10.1159/000519217
244
Oliveira P. F. Alves M. G. Rato L. Laurentino S. Silva J. Sá R. et al (2012). Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim. Biophys. Acta-General Subj.1820 (2), 84–89. 10.1016/j.bbagen.2011.11.006
245
Oonk R. B. Grootegoed J. A. van der Molen H. J. (1985). Comparison of the effects of insulin and follitropin on glucose metabolism by Sertoli cells from immature rats. Mol. Cell Endocrinol.42 (1), 39–48. 10.1016/0303-7207(85)90005-x
246
Otake S. Park M. K. (2016). Expressional changes of AMH signaling system in the quail testis induced by photoperiod. Reproduction152 (5), 575–589. 10.1530/Rep-16-0175
247
Palmer S. J. Burgoyne P. S. (1991). In situ analysis of fetal, prepuberal and adult XX----XY chimaeric mouse testes: sertoli cells are predominantly, but not exclusively, XY. Development112 (1), 265–268. 10.1242/dev.112.1.265
248
Paniagua R. Nistal M. Amat P. Rodriguez M. C. Martin A. (1987). Seminiferous tubule involution in elderly men. Biol. Reprod.36 (4), 939–947. 10.1095/biolreprod36.4.939
249
Parekh P. A. Garcia T. X. Hofmann M. C. (2019). Regulation of GDNF expression in Sertoli cells. Reproduction157 (3), R95–R107. 10.1530/REP-18-0239
250
Park S. Y. Kim I. S. (2019). Stabilin receptors: role as phosphatidylserine receptors. Biomolecules9 (8), 387. 10.3390/biom9080387
251
Park H. J. Lee W. Y. Do J. T. Park C. Song H. (2021). Evaluation of testicular toxicity upon fetal exposure to bisphenol A using an organ culture method. Chemosphere270, 129445. 10.1016/j.chemosphere.2020.129445
252
Patrikidou A. Cazzaniga W. Berney D. Boormans J. de Angst I. Di Nardo D. et al (2023). European Association of Urology Guidelines on testicular cancer: 2023 update. Eur. Urol.84 (3), 289–301. 10.1016/j.eururo.2023.04.010
253
Peirouvi T. Aliaghaei A. Eslami Farsani B. Ziaeipour S. Ebrahimi V. Forozesh M. et al (2021). COVID-19 disrupts the blood-testis barrier through the induction of inflammatory cytokines and disruption of junctional proteins. Inflamm. Res.70 (10-12), 1165–1175. 10.1007/s00011-021-01497-4
254
Pérez C. V. Theas M. S. Jacobo P. V. Jarazo-Dietrich S. Guazzone V. A. Lustig L. (2013). Dual role of immune cells in the testis: protective or pathogenic for germ cells?Spermatogenesis3 (1), e23870. 10.4161/spmg.23870
255
Pierucci-Alves F. Clark A. M. Russell L. D. (2001). A developmental study of the Desert hedgehog-null mouse testis. Biol. Reprod.65 (5), 1392–1402. 10.1095/biolreprod65.5.1392
256
Pointis G. Gilleron J. Carette D. Segretain D. (2010). Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos. Trans. R. Soc. B-Biological Sci.365 (1546), 1607–1620. 10.1098/rstb.2009.0114
257
Potter S. J. DeFalco T. (2017). Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction153 (4), R151–R162. 10.1530/REP-16-0588
258
Poulsen K. H. Nielsen J. E. Frederiksen H. Melau C. Hare K. J. Thuesen L. L. et al (2019). Dysregulation of FGFR signalling by a selective inhibitor reduces germ cell survival in human fetal gonads of both sexes and alters the somatic niche in fetal testes. Hum. Reprod.34 (11), 2228–2243. 10.1093/humrep/dez191
259
Prince F. P. (1990). Ultrastructural evidence of mature Leydig cells and Leydig cell regression in the neonatal human testis. Anat. Rec.228 (4), 405–417. 10.1002/ar.1092280406
260
Prince F. P. (1992). Ultrastructural evidence of indirect and direct autonomic innervation of human Leydig-Cells - comparison of neonatal, childhood and pubertal ages. Cell Tissue Res.269 (3), 383–390. 10.1007/Bf00353893
261
Prince F. P. (2001). The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J. Endocrinol.168 (2), 213–216. 10.1677/joe.0.1680213
262
Qi S. Fu W. Wang C. Liu C. Quan C. Kourouma A. et al (2014). BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod. Toxicol.50, 108–116. 10.1016/j.reprotox.2014.10.013
263
Qian Y. Liu S. J. Guan Y. T. Pan H. J. Guan X. Qiu Z. W. et al (2013). Lgr4-mediated Wnt/β-catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development140 (8), 1751–1761. 10.1242/dev.093641
264
Qian X. J. Mruk D. D. Cheng Y. H. Tang E. I. Han D. S. Lee W. M. et al (2014). Actin binding proteins, spermatid transport and spermiation. Seminars Cell and Dev. Biol.30, 75–85. 10.1016/j.semcdb.2014.04.018
265
Qian G. Q. Wang X. C. Zhang X. Shen B. Liu Q. (2023). Pyruvate kinase M in germ cells is essential for sperm motility and male fertility but not spermatogenesis. Asian J. Androl.26 (2), 212–219. 10.4103/aja202350
266
Rajachandran S. Zhang X. Cao Q. Caldeira-Brant A. L. Zhang X. Song Y. et al (2023). Dissecting the spermatogonial stem cell niche using spatial transcriptomics. Cell Rep.42 (7), 112737. 10.1016/j.celrep.2023.112737
267
Raverdeau M. Gely-Pernot A. Féret B. Dennefeld C. Benoit G. Davidson I. et al (2012). Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl. Acad. Sci. U. S. A.109 (41), 16582–16587. 10.1073/pnas.1214936109
268
Rebourcet D. O'Shaughnessy P. J. Monteiro A. Milne L. Cruickshanks L. Jeffrey N. et al (2014a). Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. Plos One9 (8), e105687. 10.1371/journal.pone.0105687
269
Rebourcet D. O'Shaughnessy P. J. Pitetti J. L. Monteiro A. O'Hara L. Milne L. et al (2014b). Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development141 (10), 2139–2149. 10.1242/dev.107029
270
Rebourcet D. Darbey A. Monteiro A. Soffientini U. Tsai Y. T. Handel I. et al (2017). Sertoli cell number defines and predicts germ and Leydig cell population sizes in the adult mouse testis. Endocrinology158 (9), 2955–2969. 10.1210/en.2017-00196
271
Regueira M. Gorga A. Rindone G. M. Pellizzari E. H. Cigorraga S. B. Galardo M. N. et al (2018). Apoptotic germ cells regulate Sertoli cell lipid storage and fatty acid oxidation. Reproduction156 (6), 515–525. 10.1530/rep-18-0181
272
Ren F. Fang Q. Xi H. M. Feng T. Y. Wang L. Q. Hu J. H. (2020). Platelet-derived growth factor-BB and epidermal growth factor promote dairy goat spermatogonial stem cells proliferation via Ras/ERK1/2 signaling pathway. Theriogenology155, 205–212. 10.1016/j.theriogenology.2020.06.012
273
Ren M. D. Xu Y. Phoon C. K. L. Erdjument-Bromage H. Neubert T. A. Rajan S. et al (2022). Condensed mitochondria assemble into the acrosomal matrix during spermiogenesis. Front. Cell Dev. Biol.10, 867175. 10.3389/fcell.2022.867175
274
Richardson B. E. Lehmann R. (2010). Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat. Rev. Mol. Cell Biol.11 (1), 37–49. 10.1038/nrm2815
275
Richardson L. L. Kleinman H. K. Dym M. (1995). Basement-Membrane gene-expression by Sertoli and peritubular myoid cells in-vitro in the rat. Biol. Reprod.52 (2), 320–330. 10.1095/biolreprod52.2.320
276
Robinson M. Haegert A. Li Y. Y. Morova T. Zhang A. Y. Y. Witherspoon L. et al (2023). Differentiation of peritubular myoid-like cells from human induced pluripotent stem cells. Adv. Biol.7 (7), e2200322. 10.1002/adbi.202200322
277
Rodriguez K. F. Brown P. R. Amato C. M. Nicol B. Liu C. F. Xu X. et al (2022). Somatic cell fate maintenance in mouse fetal testes via autocrine/paracrine action of AMH and activin B. Nat. Commun.13 (1), 4130. 10.1038/s41467-022-31486-y
278
Rossi F. Guerrini L. Pasimeni G. Markouizou A. Fabbrini A. Santiemma V. (2000). Testicular peritubular myoid cells are a target for adrenomedullin. Arch. Androl.44 (2), 103–107. 10.1080/014850100262263
279
Rossi G. Dufrusine B. Lizzi A. R. Luzi C. Piccoli A. Fezza F. et al (2020). Bisphenol A deranges the endocannabinoid System of primary sertoli cells with an impact on inhibin B production. Int. J. Mol. Sci.21 (23), 8986. 10.3390/ijms21238986
280
Rotgers E. Jorgensen A. Yao H. H. (2018). At the crossroads of fate-somatic cell lineage specification in the fetal gonad. Endocr. Rev.39 (5), 739–759. 10.1210/er.2018-00010
281
Roth D. M. Bayona F. Baddam P. Graf D. (2021). Craniofacial development: neural crest in molecular embryology. Head. Neck Pathol.15 (1), 1–15. 10.1007/s12105-021-01301-z
282
Ruwanpura S. M. McLachlan R. I. Meachem S. J. (2010). Hormonal regulation of male germ cell development. J. Endocrinol.205 (2), 117–131. 10.1677/Joe-10-0025
283
Ryu B. Y. Orwig K. E. Oatley J. M. Avarbock M. R. Brinster R. L. (2006). Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells24 (6), 1505–1511. 10.1634/stemcells.2005-0580
284
Saez J. M. Avallet O. Naville D. Perrard-Sapori M. H. Chatelain P. G. (1989). Sertoli-Leydig cell communications. Ann. N. Y. Acad. Sci.564, 210–231. 10.1111/j.1749-6632.1989.tb25899.x
285
Saitou M. Miyauchi H. (2016). Gametogenesis from pluripotent stem cells. Cell Stem Cell18 (6), 721–735. 10.1016/j.stem.2016.05.001
286
Saitou M. Yamaji M. (2010). Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences. Reproduction139 (6), 931–942. 10.1530/Rep-10-0043
287
Saitou M. Yamaji M. (2012). Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol.4 (11), a008375. 10.1101/cshperspect.a008375
288
Sandell L. L. Lynn M. L. Inman K. E. McDowell W. Trainor P. A. (2012). RDH10 oxidation of vitamin A is a critical control step in synthesis of retinoic acid during Mouse embryogenesis. Plos One7 (2), e30698. 10.1371/journal.pone.0030698
289
Santiago J. Silva J. V. Alves M. G. Oliveira P. F. Fardilha M. (2019). Testicular aging: an overview of ultrastructural, cellular, and molecular alterations. J. Gerontol. A Biol. Sci. Med. Sci.74 (6), 860–871. 10.1093/gerona/gly082
290
Saracino R. Capponi C. Di Persio S. Boitani C. Masciarelli S. Fazi F. et al (2020). Regulation of Gdnf expression by retinoic acid in Sertoli cells. Mol. Reprod. Dev.87 (4), 419–429. 10.1002/mrd.23323
291
Sarkar O. Mathur P. P. Cheng C. Y. Mruk D. D. (2008). Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol. Reprod.78 (3), 445–454. 10.1095/biolreprod.107.064501
292
Sasaki K. Nakamura T. Okamoto K. Yabuta Y. Iwatani C. Tsuchiya H. et al (2016). The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev. Cell39 (2), 169–185. 10.1016/j.devcel.2016.09.007
293
Sawaied A. Levy B. E. Arazi E. Lunenfeld E. Shi Q. Huleihel M. (2025). Follicle-Stimulating hormone and testosterone play a role in the regulation of Sertoli cell functions following germ cell depletion in vitro. Int. J. Mol. Sci.26 (6), 2702. 10.3390/ijms26062702
294
Schmahl J. Eicher E. M. Washburn L. L. Capel B. (2000). Sry induces cell proliferation in the mouse gonad. Development127 (1), 65–73. 10.1242/dev.127.1.65
295
Scobey M. Bertera S. Somers J. Watkins S. Zeleznik A. Walker W. (2001). Delivery of a cyclic adenosine 3',5'-monophosphate response element-binding protein (creb) mutant to seminiferous tubules results in impaired spermatogenesis. Endocrinology142 (2), 948–954. 10.1210/endo.142.2.7948
296
Seki Y. Yamaji M. Yabuta Y. Sano M. Shigeta M. Matsui Y. et al (2007). Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development134 (14), 2627–2638. 10.1242/dev.005611
297
Sekido R. Lovell-Badge R. (2008). Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature453 (7197), 930–934. 10.1038/nature06944
298
Sekido R. Lovell-Badge R. (2013). Genetic control of Testis development. Sex. Dev.7 (1-3), 21–32. 10.1159/000342221
299
Sharpe R. M. McKinnell C. Kivlin C. Fisher J. S. (2003). Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction125 (6), 769–784. 10.1530/rep.0.1250769
300
Shima Y. (2019). Development of fetal and adult Leydig cells. Reprod. Med. Biol.18 (4), 323–330. 10.1002/rmb2.12287
301
Shima Y. Miyabayashi K. Haraguchi S. Arakawa T. Otake H. Baba T. et al (2013). Contribution of Leydig and Sertoli cells to testosterone production in Mouse fetal testes. Mol. Endocrinol.27 (1), 63–73. 10.1210/me.2012-1256
302
Shima Y. Matsuzaki S. Miyabayashi K. Otake H. Baba T. Kato S. et al (2015). Fetal Leydig cells persist as an androgen-independent subpopulation in the postnatal testis. Mol. Endocrinol.29 (11), 1581–1593. 10.1210/me.2015-1200
303
Shima Y. Miyabayashi K. Sato T. Suyama M. Ohkawa Y. Doi M. et al (2018). Fetal Leydig cells dedifferentiate and serve as adult Leydig stem cells. Development145 (23), dev169136. 10.1242/dev.169136
304
Skakkebæk N. E. Lindahl-Jacobsen R. Levine H. Andersson A. M. Jorgensen N. Main K. M. et al (2022). Environmental factors in declining human fertility. Nat. Rev. Endocrinol.18 (3), 139–157. 10.1038/s41574-021-00598-8
305
Skinner M. K. Griswold M. D. (1983). Sertoli cells synthesize and secrete a ceruloplasmin-like protein. Biol. Reprod.28 (5), 1225–1229. 10.1095/biolreprod28.5.1225
306
Smith L. B. Walker W. H. (2014). The regulation of spermatogenesis by androgens. Seminars Cell and Dev. Biol.30, 2–13. 10.1016/j.semcdb.2014.02.012
307
Stanton P. G. Sluka P. Foo C. F. H. Stephens A. N. Smith A. I. McLachlan R. I. et al (2012). Proteomic changes in rat spermatogenesis in response to in vivo androgen manipulation; impact on meiotic cells. Plos One7 (7), e41718. 10.1371/journal.pone.0041718
308
Stukenborg J. B. Mitchell R. T. Soder O. (2021). Endocrine disruptors and the male reproductive system. Best. Pract. Res. Clin. Endocrinol. Metab.35 (5), 101567. 10.1016/j.beem.2021.101567
309
Su J. Yang Y. Wang D. Su H. Zhao F. Zhang C. et al (2025). A dynamic transcriptional cell atlas of testes development after birth in Hu sheep. BMC Biol.23 (1), 78. 10.1186/s12915-025-02186-y
310
Sumaiya K. Langford D. Natarajaseenivasan K. Shanmughapriya S. (2022). Macrophage migration inhibitory factor (MIF): a multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol. Ther.233, 108024. 10.1016/j.pharmthera.2021.108024
311
Svingen T. Koopman P. (2013). Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes and Dev.27 (22), 2409–2426. 10.1101/gad.228080.113
312
Tan K. Song H. W. Wilkinson M. F. (2020). Single-cell RNAseq analysis of testicular germ and somatic cell development during the perinatal period. Development147 (3), dev183251. 10.1242/dev.183251
313
Tanphaichitr N. Kongmanas K. Faull K. F. Whitelegge J. Compostella F. Goto-Inoue N. et al (2018). Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction. Prog. Lipid Res.72, 18–41. 10.1016/j.plipres.2018.08.002
314
Tao H. P. Lu T. F. Li S. Jia G. X. Zhang X. N. Yang Q. E. et al (2023). Pancreatic lipase-related protein 2 is selectively expressed by peritubular myoid cells in the murine testis and sustains long-term spermatogenesis. Cell Mol. Life Sci.80 (8), 217. 10.1007/s00018-023-04872-y
315
Teerds K. J. Huhtaniemi I. T. (2015). Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum. Reprod. Update21 (3), 310–328. 10.1093/humupd/dmv008
316
Teletin M. Vernet N. Yu J. S. Klopfenstein M. Jones J. W. Féret B. et al (2019). Two functionally redundant sources of retinoic acid secure spermatogonia differentiation in the seminiferous epithelium. Development146 (1), dev170225. 10.1242/dev.170225
317
Tevosian S. G. Albrecht K. H. Crispino J. D. Fujiwara Y. Eicher E. M. Orkin S. H. (2002). Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development129 (19), 4627–4634. 10.1242/dev.129.19.4627
318
Theodosiou M. Laudet V. Schubert M. (2010). From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci.67 (9), 1423–1445. 10.1007/s00018-010-0268-z
319
Tian R. Yao C. Yang C. Zhu Z. Li C. Zhi E. et al (2019). Fibroblast growth factor-5 promotes spermatogonial stem cell proliferation via ERK and AKT activation. Stem Cell Res. Ther.10 (1), 40. 10.1186/s13287-019-1139-7
320
Tong M. H. Yang Q. E. Davis J. C. Griswold M. D. (2013). Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc. Natl. Acad. Sci. U. S. A.110 (2), 543–548. 10.1073/pnas.1214883110
321
Travison T. G. Araujo A. B. Hall S. A. McKinlay J. B. (2009). Temporal trends in testosterone levels and treatment in older men. Curr. Opin. Endocrinol. Diabetes Obes.16 (3), 211–217. 10.1097/MED.0b013e32832b6348
322
Tsikolia N. Merkwitz C. Sass K. Sakurai M. Spanel-Borowski K. Ricken A. M. (2009). Characterization of bovine fetal Leydig cells by KIT expression. Histochem. Cell Biol.132 (6), 623–632. 10.1007/s00418-009-0640-y
323
Tsuji-Hosokawa A. Kashimada K. Kato T. Ogawa Y. Nomura R. Takasawa K. et al (2018). Peptidyl arginine deiminase 2 (Padi2) is expressed in Sertoli cells in a specific manner and regulated by SOX9 during testicular development. Sci. Rep.8, 13263. 10.1038/s41598-018-31376-8
324
Tung K. S. Harakal J. Qiao H. Rival C. Li J. C. Paul A. G. et al (2017). Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Invest127 (3), 1046–1060. 10.1172/jci89927
325
Tysoe O. (2024). Sertoli cell lysosomal dysfunction drives age-related testicular degeneration. Nat. Rev. Endocrinol.20 (7), 386. 10.1038/s41574-024-01001-y
326
Uchida A. Sakib S. Labit E. Abbasi S. Scott R. W. Underhill T. M. et al (2020). Development and function of smooth muscle cells is modulated by Hic1 in mouse testis. Development147 (13), dev185884. 10.1242/dev.185884
327
Val P. Lefrançois-Martinez A. M. Veyssière G. Martinez A. (2003). SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl. Recept1 (1), 8. 10.1186/1478-1336-1-8
328
van Pelt A. M. de Rooij D. G. (1990). Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol. Reprod.43 (3), 363–367. 10.1095/biolreprod43.3.363
329
Venditti M. Ben Rhouma M. Romano M. Z. Messaoudi I. Reiter R. J. Minucci S. (2021). Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis. Ecotoxicol. Environ. Saf.226, 112878. 10.1016/j.ecoenv.2021.112878
330
Vernet N. Dennefeld C. Guillou F. Chambon P. Ghyselinck N. B. Mark M. (2006). Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. Embo J.25 (24), 5816–5825. 10.1038/sj.emboj.7601447
331
Vernet N. Dennefeld C. Klopfenstein M. Ruiz A. Bok D. Ghyselinck N. B. et al (2008). Retinoid X receptor beta (RXRB) expression in Sertoli cells controls cholesterol homeostasis and spermiation. Reproduction136 (5), 619–626. 10.1530/Rep-08-0235
332
Wakayama T. Nakata H. Kumchantuek T. Gewaily M. S. Iseki S. (2015). Identification of 5-bromo-2'-deoxyuridine-labeled cells during mouse spermatogenesis by heat-induced antigen retrieval in lectin staining and immunohistochemistry. J. Histochem Cytochem63 (3), 190–205. 10.1369/0022155414564870
333
Walker W. H. (2010). Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. B-Biological Sci.365 (1546), 1557–1569. 10.1098/rstb.2009.0258
334
Wan H. T. Mruk D. D. Wong C. K. C. Cheng C. Y. (2013). The apical ES-BTB-BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol. Med.19 (7), 396–405. 10.1016/j.molmed.2013.03.006
335
Wang X. N. Li Z. S. Ren Y. Jiang T. Wang Y. Q. Chen M. et al (2013). The Wilms tumor gene, Wt1, is critical for mouse spermatogenesis via regulation of sertoli cell polarity and is associated with non-obstructive azoospermia in humans. PLoS Genet.9 (8), e1003645. 10.1371/journal.pgen.1003645
336
Wang Y. Q. Batool A. Chen S. R. Liu Y. X. (2018a). GATA4 is a negative regulator of contractility in mouse testicular peritubular myoid cells. Reproduction156 (4), 343–351–351. 10.1530/Rep-18-0148
337
Wang Y. Q. Chen S. R. Liu Y. X. (2018b). Selective deletion of WLS in peritubular myoid cells does not affect spermatogenesis or fertility in mice. Mol. Reprod. Dev.85 (7), 559–561. 10.1002/mrd.22988
338
Wang M. Yang Y. L. Cansever D. Wang Y. M. Kantores C. Messiaen S. et al (2021). Two populations of self-maintaining monocyte-independent macrophages exist in adult epididymis and testis. Proc. Natl. Acad. Sci. U. S. A.118 (1), e2013686117. 10.1073/pnas.2013686117
339
Wang J. M. Li Z. F. Yang W. X. Tan F. Q. (2022). Follicle-stimulating hormone signaling in Sertoli cells: a licence to the early stages of spermatogenesis. Reprod. Biol. Endocrinol.20 (1), 97. 10.1186/s12958-022-00971-w
340
Wang X. Liu X. Qu M. Li H. (2023a). Sertoli cell-only syndrome: advances, challenges, and perspectives in genetics and mechanisms. Cell Mol. Life Sci.80 (3), 67. 10.1007/s00018-023-04723-w
341
Wang X. Pei J. Xiong L. Guo S. Cao M. Kang Y. et al (2023b). Single-Cell RNA sequencing reveals Atlas of yak testis cells. Int. J. Mol. Sci.24 (9), 7982. 10.3390/ijms24097982
342
Wang X. Wang Y. Wang Y. Guo Y. Zong R. Hu S. et al (2024). Single-cell transcriptomic and cross-species comparison analyses reveal distinct molecular changes of porcine testes during puberty. Commun. Biol.7 (1), 1478. 10.1038/s42003-024-07163-9
343
Wanjari U. R. Gopalakrishnan A. V. (2024). Blood-testis barrier: a review on regulators in maintaining cell junction integrity between Sertoli cells. Cell Tissue Res.396 (2), 157–175. 10.1007/s00441-024-03894-7
344
Warr N. Carre G. A. Siggers P. Faleato J. V. Brixey R. Pope M. et al (2012). Gadd45γ and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev. Cell23 (5), 1020–1031. 10.1016/j.devcel.2012.09.016
345
Washburn R. L. Dufour J. M. (2023). Complementing testicular immune regulation: the relationship between Sertoli cells, complement, and the immune response. Int. J. Mol. Sci.24 (4), 3371. 10.3390/ijms24043371
346
Wen Q. Zheng Q. S. Li X. X. Hu Z. Y. Gao F. Cheng C. Y. et al (2014). Wt1 dictates the fate of fetal and adult Leydig cells during development in the mouse testis. Am. J. Physiology-Endocrinology Metab.307 (12), E1131–E1143. 10.1152/ajpendo.00425.2014
347
Wen Q. Wang Y. Tang J. Cheng C. Y. Liu Y. X. (2016). Sertoli cell Wt1 regulates peritubular myoid cell and fetal Leydig cell differentiation during fetal Testis development. PLoS One11 (12), e0167920. 10.1371/journal.pone.0167920
348
Western P. S. Miles D. C. van den Bergen J. A. Burton M. Sinclair A. H. (2008). Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells26 (2), 339–347. 10.1634/stemcells.2007-0622
349
Wijayarathna R. de Kretser D. M. (2016). Activins in reproductive biology and beyond. Hum. Reprod. Update22 (3), 342–357. 10.1093/humupd/dmv058
350
Wilhelm D. Englert C. (2002). The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev.16 (14), 1839–1851. 10.1101/gad.220102
351
Willems A. De Gendt K. Allemeersch J. Smith L. B. Welsh M. Swinnen J. V. et al (2010). Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int. J. Androl.33 (3), 507–517. 10.1111/j.1365-2605.2009.00964.x
352
Winge S. B. Soraggi S. Schierup M. H. Rajpert-De Meyts E. Almstrup K. (2020). Integration and reanalysis of transcriptomics and methylomics data derived from blood and testis tissue of men with 47,XXY Klinefelter syndrome indicates the primary involvement of Sertoli cells in the testicular pathogenesis. Am. J. Med. Genet. C Semin. Med. Genet.184 (2), 239–255. 10.1002/ajmg.c.31793
353
Wong C. H. Mruk D. D. Lui W. Y. Cheng C. Y. (2004). Regulation of blood-testis barrier dynamics: an in vivo study. J. Cell Sci.117 (5), 783–798. 10.1242/jcs.00900
354
Wu Q. Y. Shui Y. C. Xia X. Y. Huang Y. F. (2015). FSH and FSHR gene polymorphisms and male infertility: an update. Zhonghua Nan Ke Xue21 (11), 1031–1034. 10.13263/j.cnki.nja.2015.11.015
355
Xia W. L. Wong E. W. P. Mruk D. D. Cheng C. Y. (2009). TGF-β3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev. Biol.327 (1), 48–61. 10.1016/j.ydbio.2008.11.028
356
Xiao Z. Liang J. Huang R. Chen D. Mei J. Deng J. et al (2024). Inhibition of miR-143-3p restores blood-testis barrier function and ameliorates sertoli cell senescence. Cells13 (4), 313. 10.3390/cells13040313
357
Xiong Z. Wang C. Wang Z. Dai H. Song Q. Zou Z. et al (2018). Raptor directs Sertoli cell cytoskeletal organization and polarity in the mouse testis. Biol. Reprod.99 (6), 1289–1302. 10.1093/biolre/ioy144
358
Xu Q. Chen H. (2025). Applications of spatial transcriptomics in studying spermatogenesis. Andrology13, 1181–1189. 10.1111/andr.70043
359
Xu C. Mohsin A. Luo Y. Xie L. Peng Y. Wang Q. et al (2020). Inducing non-genetically modified induced embryonic sertoli cells derived from embryonic stem cells with recombinant protein factors. Front. Cell Dev. Biol.8, 533543. 10.3389/fcell.2020.533543
360
Yamashita Y. M. (2020). When the family treasure is a doormat. Dev. Cell52 (1), 3–4. 10.1016/j.devcel.2019.12.013
361
Yan Z. Wang P. Yang Q. Gun S. (2024). Single-Cell RNA sequencing reveals an Atlas of Hezuo pig Testis cells. Int. J. Mol. Sci.25 (18), 9786. 10.3390/ijms25189786
362
Yang C. Yao C. Tian R. Zhu Z. Zhao L. Li P. et al (2019). miR-202-3p regulates sertoli cell proliferation, synthesis function, and apoptosis by targeting LRP6 and Cyclin D1 of Wnt/β-Catenin signaling. Mol. Ther. Nucleic Acids14, 1–19. 10.1016/j.omtn.2018.10.012
363
Yang F. Whelan E. C. Guan X. B. Deng B. Q. Wang S. Sun J. C. et al (2021). FGF9 promotes mouse spermatogonial stem cell proliferation mediated by p38 MAPK signalling. Cell Prolif.54 (1), e12933. 10.1111/cpr.12933
364
Yang Y. Yao M. Zeng J. Zheng D. Li Q. Ni Y. et al (2022). FYN regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization via its effect on Arp3 in the mouse testis. Front. Immunol.13, 915274. 10.3389/fimmu.2022.915274
365
Yao H. H. C. Whoriskey W. Capel B. (2002). Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes and Dev.16 (11), 1433–1440. 10.1101/gad.981202
366
Yefimova M. G. Sow A. Fontaine I. Guilleminot V. Martinat N. Crepieux P. et al (2008). Dimeric transferrin inhibits phagocytosis of residual bodies by testicular rat Sertoli cells. Biol. Reprod.78 (4), 697–704. 10.1095/biolreprod.107.063107
367
Yefimova M. G. Messaddeq N. Meunier A. C. Cantereau A. Jegou B. Bourmeyster N. (2018). Phagocytosis by sertoli cells: analysis of main phagocytosis steps by confocal and Electron Microscopy. Methods Mol. Biol.1748, 85–101. 10.1007/978-1-4939-7698-0_8
368
Yeh J. R. Zhang X. F. Nagano M. C. (2011). Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J. Cell Sci.124 (14), 2357–2366. 10.1242/jcs.080903
369
Yildirim E. Aksoy S. Onel T. Yaba A. (2020). Gonadal development and sex determination in mouse. Reprod. Biol.20 (2), 115–126. 10.1016/j.repbio.2020.01.007
370
Yokonishi T. Capel B. (2021). Differentiation of fetal sertoli cells in the adult testis. Reproduction162 (2), 141–147. 10.1530/Rep-21-0106
371
Yoshida S. (2020). Mouse spermatogenesis reflects the Unity and diversity of tissue stem cell niche systems. Cold Spring Harb. Perspect. Med.10 (11), a036186. 10.1101/cshperspect.a036186
372
You X. Chen Q. Yuan D. Zhang C. C. Zhao H. X. (2021). Common markers of testicular Sertoli cells. Expert Rev. Mol. Diagn.21 (6), 613–626. 10.1080/14737159.2021.1924060
373
Zaker H. Razi M. Mahmoudian A. Soltanalinejad F. (2022). Boosting effect of testosterone on GDNF expression in Sertoli cell line (TM4); comparison between TM3 cells-produced and exogenous testosterone. Gene812, 146112. 10.1016/j.gene.2021.146112
374
Zhang Y. Wang S. Wang X. X. Liao S. Y. Wu Y. J. Han C. S. (2012). Endogenously produced FGF2 is essential for the survival and proliferation of cultured mouse spermatogonial stem cells. Cell Res.22 (4), 773–776. 10.1038/cr.2012.17
375
Zhang H. J. Yin Y. M. Wang G. S. Liu Z. M. Liu L. Sun F. (2014). Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep.4, 4260. 10.1038/srep04260
376
Zhang H. L. Chen P. Y. Liu Y. X. Xie W. Q. Fan S. J. Yao Y. C. et al (2022). Estrogen signaling regulates seasonal changes of the prostate in wild ground squirrels (Spermophilus dauricus). J. Steroid Biochem. Mol. Biol.218, 106058. 10.1016/j.jsbmb.2022.106058
377
Zhang L. Guo M. Liu Z. Liu R. Zheng Y. Yu T. et al (2022a). Single-cell RNA-seq analysis of testicular somatic cell development in pigs. J. Genet. Genomics49 (11), 1016–1028. 10.1016/j.jgg.2022.03.014
378
Zhang W. Mao J. Wang X. Sun B. Zhao Z. Zhang X. et al (2022b). Case report: novel compound heterozygotic variants in PPP2R3C gene causing syndromic 46, XY gonadal dysgenesis and literature review. Front. Genet.13, 871328. 10.3389/fgene.2022.871328
379
Zhang Z. Miao J. Wang Y. (2022c). Mitochondrial regulation in spermatogenesis. Reproduction163 (4), R55–r69. 10.1530/rep-21-0431
380
Zhang W. Wang X. Mao J. Cao Y. Zhang X. Nie M. et al (2025). MYRF variants in patients with 46,XY Differences/Disorders of sex development and literature review. Am. J. Med. Genet. A197 (6), e64002. 10.1002/ajmg.a.64002
381
Zhao L. Koopman P. (2012). SRY protein function in sex determination: thinking outside the box. Chromosome Res.20 (1), 153–162. 10.1007/s10577-011-9256-x
382
Zhao L. Yao C. Xing X. Jing T. Li P. Zhu Z. et al (2020). Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat. Commun.11 (1), 5683. 10.1038/s41467-020-19414-4
383
Zhao X. Wen X. Ji M. Guan X. Chen P. Hao X. et al (2021). Differentiation of seminiferous tubule-associated stem cells into leydig cell and myoid cell lineages. Mol. Cell Endocrinol.525, 111179. 10.1016/j.mce.2021.111179
384
Zheng B. Zhao D. Zhang P. Shen C. Guo Y. S. Zhou T. et al (2015). Quantitative proteomics reveals the essential roles of stromal interaction molecule 1 (STIM1) in the testicular cord Formation in mouse testis. Mol. and Cell. Proteomics14 (10), 2682–2691. 10.1074/mcp.M115.049569
385
Zheng W. Nazish J. Wahab F. Khan R. Jiang X. H. Shi Q. H. (2019). DDB1 regulates sertoli cell proliferation and Testis cord remodeling by TGFβ pathway. Genes10 (12), 974. 10.3390/genes10120974
386
Zheng G. Y. Chu G. M. Li P. P. He R. (2023). Phenotype and genetic characteristics in 20 Chinese patients with 46,XY disorders of sex development. J. Endocrinol. Invest46 (8), 1613–1622. 10.1007/s40618-023-02020-8
387
Zhou R. Wu J. R. Z. Liu B. Jiang Y. Q. Chen W. Li J. et al (2019). The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci.76 (14), 2681–2695. 10.1007/s00018-019-03101-9
388
Zirkin B. R. Papadopoulos V. (2018). Leydig cells: formation, function, and regulation. Biol. Reprod.99 (1), 101–111. 10.1093/biolre/ioy059
389
Zohar-Fux M. Ben-Hamo-Arad A. Arad T. Volin M. Shklyar B. Hakim-Mishnaevski K. et al (2022). The phagocytic cyst cells in Drosophila testis eliminate germ cell progenitors via phagoptosis. Sci. Adv.8 (24), eabm4937. 10.1126/sciadv.abm4937
Summary
Keywords
testis, Sertoli cells, gonadal development, spermatogenesis, testosterone synthesis, testis immunity
Citation
Feng X, Tian J, Han S, Wen F, Li Y, Zhang S, Ouyang L, Hu Z, Chen X and Hu J (2025) Sertoli cells as a hub in testicular development and male reproductive. Front. Cell Dev. Biol. 13:1693878. doi: 10.3389/fcell.2025.1693878
Received
27 August 2025
Revised
04 November 2025
Accepted
24 November 2025
Published
10 December 2025
Volume
13 - 2025
Edited by
Charles H. Muller, University of Washington, United States
Reviewed by
Zumrut Dogan, Adiyaman University, Türkiye
Najmeh Salehi, University of Tehran, Iran
Updates
Copyright
© 2025 Feng, Tian, Han, Wen, Li, Zhang, Ouyang, Hu, Chen and Hu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhong Hu, hjh19732008@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.