Abstract
More than two decades after cloning the cystic fibrosis transmembrane regulator (CFTR) gene, the defective gene in cystic fibrosis (CF), we still do not understand how dysfunction of this ion channel causes lung disease and the tremendous neutrophil burden which persists within the airways; nor why chronic colonization by Pseudomonas aeruginosa develops in CF patients who are thought to be immunocompetent. It appears that the microenvironment within the lung of CF patients provides favorable conditions for both P. aeruginosa colonization and neutrophil survival. In this context, the ability of bacteria to induce hypoxia, which in turn affects neutrophil survival is an additional level of complexity that needs to be accounted for when controlling neutrophil fate in CF. Recent studies have underscored the importance of neutrophils in innate immunity and their functions appear to extend far beyond their well-described role in antibacterial defense. Perhaps a disturbance in neutrophil reprogramming during the course of an infection severely modulates the inflammatory response in CF. Furthermore there is an emerging concept that the CFTR itself may be an immune modulator and stimulating CFTR function in CF patients could promote neutrophil and macrophages antimicrobial function. Fostering the resolution of inflammation by favoring neutrophil apoptosis could preserve their microbicidal activities but decrease their proinflammatory potential. In this context, triggering neutrophil apoptosis with roscovitine may be a potential therapeutic option and this is currently being evaluated in CF patients. In the present review we discuss how neutrophils functions are disturbed in CF and how this may relate to chronic infection with P. aeuginosa and we propose novel research directions aimed at modulating neutrophil survival, dampening lung inflammation and ultimately leading to an amelioration of the lung disease.
Chronic infection and inflammation in cystic fibrosis are indicative of a defect in the immune response
Cystic fibrosis (CF) is the most frequent hereditary genetic disease in the Caucasian population, originating from mutations within the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. These mutations result in an often-lethal respiratory disease affecting approximately 1 in 4,500 births in Europe and North America. Median predicted survival at birth ranges from 40 to 50 years in developed countries and longevity continues to improve (MacKenzie et al., 2014), with a predicted increase in the number of CF patients (Burgel et al., 2015). Mutation of the CFTR leads to the secretion of a viscous and abundant mucus in the lung that is conducive to bacterial infections (Martin et al., 2014; Figure 1). In fact, almost all patients suffer from pulmonary infections caused by various pathogens, which can be isolated from sputum or bronchoalveolar lavages. Infections are treated by administrating antibiotics and in the long term this generates bacterial multi-resistance. Pulmonary bacterial infections are the major cause of death (Martin et al., 2016a).
Figure 1
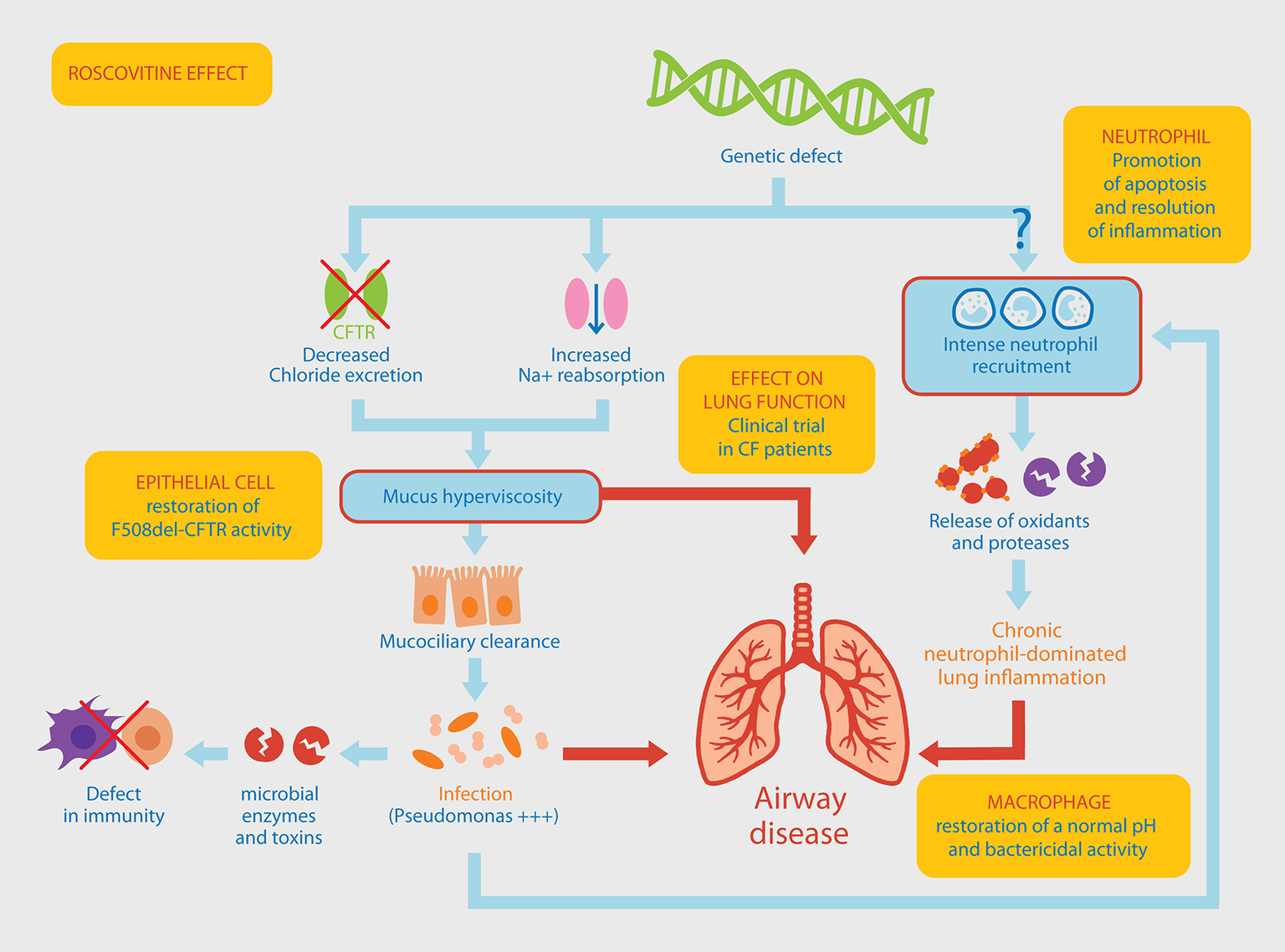
Pathophysiological mechanisms involved in Pseudomonas aeruginosa chronic lung infection associated with neutrophil-dominated inflammation. The original genetic defect in CF mutations within the CFTR gene leading to a defective chloride ions excretion and an increased sodium reabsorption, resulting in the production of a thick mucus in the airways. Altered mucociliary clearance favors an increased susceptibility to infections, a chronic colonization of the lung by P. aeruginosa, a massive neutrophil recruitment within the airways and a persistent airway inflammation. An alternative pathway to this classical view of the CF could be a “constitutive defect in innate immunity” linking the genetic defect to the intense recruitment of neutrophils. This would result in an increased activation state (release of oxidants and proteases) and a delayed apoptosis explaining their persistence at the site of inflammation. Yellow boxes indicate the different effects of roscovitine (see main text for details) which is currently tested in a CF clinical trial.
Neutrophils are the first and the most abundant leukocyte that migrate to the site of infection followed by monocytes, which differentiate locally into macrophages. In the acute phase of infections or inflammatory diseases, neutrophils can engulf and kill invading microorganisms (Witko-Sarsat et al., 2000). In CF, several studies have shown abnormalities in innate immunity where neutrophils are major instigators (Bals et al., 1999; Cohen and Prince, 2012; Bonfield, 2015). Neutrophils can no longer migrate through viscous mucus, thereby reducing the capture and destruction of bacteria (Matsui et al., 2005). Chronic pulmonary neutrophil-dominated inflammation occurs very early in CF patient and it represents a key element in disease severity (Cantin, 1995; Khan et al., 1995). The underlying mechanisms contributing to the disease remain largely unknown, raising the question of a relationship between genetic deficit, chronic pulmonary inflammation and specificity of the infection by P. aeruginosa (Hayes et al., 2011; Figure 1).
CF airways contain an abundant amount of nuclear material that is believed to originate from neutrophils. This pathophysiological characteristic is linked to the ability of neutrophils to release DNA through neutrophil extracellular traps (NETs), something which has been described in CF (Manzenreiter et al., 2012; Dwyer et al., 2014). Targeting nuclear material with DNase I improved lung function, putatively through NETs degradation (Gray et al., 2015). NETs can kill P. aeruginosa, although acquired resistances have been described among clinical isolates (Young et al., 2011). Oxidative burst and NADPH activity are essential to the process of NET formation with myeloperoxidase (MPO) and neutrophil elastase (NE), which stand as essential co-factors (Papayannopoulos et al., 2010). The main issue in the characterization of NETs in CF is to decipher the extent to which the abundant nuclear material accumulation in CF airways originate from dead cells or if, as proposed, it is the result of an active and coordinated process of release through NETs. Further investigations will be required to address this controversial question.
Pseudomonas aeruginosa: a well-adapted bacteria to the CF lung
While there is not usually any pathogenic microbe in respiratory secretion in CF patients at birth, systematic bronchoalveolar lavage of young children with CF reveals that bacterial infection usually occurs within the first months of life and is associated with lung function impairment and airway structural damage (Ramsey et al., 2014). The nature of the microbes infecting CF lungs depend on the patient and varies with age: the predominant bacteria during childhood are usually Staphylococcus aureus and Haemophilus influenzae, whereas P. aeruginosa is the major pathogen cultured from CF airways in adolescents and adults (Vaincre la Mucoviscidose et Ined)1. One of the first microbes identified in the CF airways is S. aureus, a gram positive bacterium. It has been suggested that early S. aureus infection, which contributes to structural damage of the airways, as well as antibiotic treatments, promote the implantation of P. aeruginosa (Govan and Nelson, 1993; Ratjen et al., 2001). The first positive culture containing P. aeruginosa can occur either within the first few months of life or are delayed by several decades (Pernet et al., 2014). For example, P. aeruginosa infection is less prevalent in patients with CFTR mutations associated with residual CFTR function (Burgel et al., 2010) and variants in dynactin 4 have been associated with late occurrence of the first P. aeruginosa culture in CF patients (Emond et al., 2012; Viel et al., 2016).
Chronic P. aeruginosa infection is responsible for increased morbidity and mortality (Emerson et al., 2002). Recent studies have shown that the predominance of P. aeruginosa in older CF patients was not due solely to a resistance to antibiotics. Indeed, the bacterium itself may manipulate host immune responses by stimulating airway epithelium cells to produce phospholipase A2 type-IIA (sPLA2-IIA) which is capable of killing Gram positive bacteria such as S. aureus (Pernet et al., 2014).
Hypoxic conditions in CF and the impact on P. aeruginosa virulence and immune response efficiency
Lungs, along with the skin, are the human organs exposed to the highest oxygen levels, due to the direct contact between the epithelium and atmospheric air. The alveolar partial pressure of oxygen (pO2) is 100–110 mmHg (vs. 160 mmHg in atmospheric air; Pittman, 2013). Oxygen diffuses through a thin mucus layer (2–10 μm in trachea), mainly composed of Muc5ac and Muc5b gel-forming mucins (Burgel et al., 2007). It has been reported that hypoxia is induced in the airways of CF patients (Worlitzsch et al., 2002). Similar observations were made during other pathogen infections including Shigella flexneri (Arena et al., 2017) or Mycobacterium tuberculosis (Tsai et al., 2006; Rustad et al., 2009) and in sterile inflammations (Karhausen et al., 2004; Campbell et al., 2014). More specifically, it has been shown that induction of hypoxia is associated with the establishment of an oxygen gradient within a thicker mucus layer in CF patients where P. aeruginosa was preferentially found within the hypoxic niches (Worlitzsch et al., 2002). Although reactive oxygen species (ROS) production has been shown in vitro to increase mucin expression (Muc5ac; Yan et al., 2008), no clear link has been defined between the induction of hypoxia and mucin production in CF patients. However, the increased production of mucins in CF lung disease is anticipated to exacerbate hypoxia.
P. aeruginosa is a Gram-negative, rod-shaped facultative anaerobe, which has the ability to colonize a wide range of microenvironments. P. aeruginosa aerobic respiration relies on the reduction of O2 which is mediated by Cytochrome cbb3 oxidase, Cytochrome aa3 oxidase and Cytochrome bo3 oxidase. Alternatively, in the absence of available oxygen, P. aeruginosa energy production is mediated by the anaerobic respiratory chain allowing for the reduction of nitrate (nitrate reductase) or nitrite (nitrite reductase) (Cook et al., 2014). This dual respiratory capacity appears to be essential to allow P. aeruginosa to colonize lung mucus and to persist within the hypoxic environment. The metabolic shift associated to P. aeruginosa adaptation under hypoxic conditions was recently studied: a set of genes specifically expressed under hypoxic conditions were identified (azu, cbb3-1, cbb3-2, ccpR, icd, idh, oprF, himD, and nuoA) and these genes were proposed to stand as markers for hypoxic adaptation of P. aeruginosa within the CF lung environment (Eichner et al., 2014).
Two hypotheses have been proposed to explain hypoxia induction within CF mucus layer. Either P. aeruginosa is responsible for oxygen depletion or activated neutrophils consume dioxygen for reactive oxygen species production catalyzed by NADPH oxidase, as reported during sterile inflammation (Campbell et al., 2014). Neutrophil NADPH oxidase function has been shown to play an essential role in P. aeruginosa killing (Mizgerd and Brain, 1995). The impact of hypoxia on the propagation of infection is currently unclear: does it promote P. aeruginosa colonization capacity or does it foster the immune response efficiency? To date this question remains unanswered.
The impact of hypoxia on CF is not yet fully understood and has been controversial to some extent. It has been shown that hypoxia protects epithelial cells from P. aeruginosa internalization in vitro, suggesting that mimicking hypoxia responses in vivo with hydroxylase inhibitor dimethyloxallyl glycine (DMOG) could represent a potential therapeutic approach (Schaible et al., 2012, 2013). Conversely, it has been reported that oxygen-limiting conditions increase antibiotic tolerance, biofilm formation, and alginate synthesis, promoting P. aeruginosa persistence (Schobert and Tielen, 2010). Induction of hypoxia would promote neutrophils survival, as these cells are mostly glycolytic, producing ATP mainly through glycolysis not respiration (Maianski et al., 2004). Neutrophils are well adapted to hypoxia and this is mostly attributed to the transcriptional regulator HIFs (1 and 2; Walmsley et al., 2005; Monceaux et al., 2016).
NETs have been observed in CF airways and characterized as the most efficient neutrophil antimicrobial function. However, their antimicrobial function together with the associated cell-death process (named NETosis) have been challenged by several authors (Simon et al., 2013; Malachowa et al., 2016; Nauseef and Kubes, 2016). Here, we focus on the relevance of NETs formation and antimicrobial function in the context of hypoxic microenvironments. Dioxygen is a unique substrate of the NADPH oxidase, which catalyze singlet oxygen production (O2•−). In hypoxic environments, NADPH oxidase activity and production are likely limited. NADPH oxidase activity has been shown to be essential for NETs formation (Kirchner et al., 2012; Palmer et al., 2012), similarly to ROS, including hydrogen peroxide (H2O2) (Fuchs et al., 2007) or singlet oxygen (Nishinaka et al., 2011). Under hypoxic conditions, NETs production may be decreased, promoting P. aeruginosa persistence in these microenvironments. Further investigations will be required to address this important question, as most studies were performed in vitro under atmospheric conditions, which do not reflect pathophysiological conditions.
Hypoxia induction should be seriously considered to fully comprehend CF causes and clinical outcomes and also in the development of novel antimicrobial molecules targeting P. aeruginosa. It was recently reported that hypoxia promoted P. aeruginosa antibiotic resistance by modulating multidrug efflux pumps composition and subunits stoichiometry (Eichner et al., 2014). Deciphering the extent to which hypoxia modulates P. aeruginosa virulence mechanisms and impacts on its adaptation within infection foci requires further investigations in CF animal models.
Neutrophil functions in CF: still under investigation
Dissecting effector functions in CF neutrophils, already detailed in extensive reviews (Downey et al., 2009; Hayes et al., 2011; Laval et al., 2016), has not revealed a major deficiency in neutrophil functions. However, it has been described that airway neutrophils exhibit profound functional (Laval et al., 2013) and signaling changes mediated by the cytoskeleton-associated kinase that regulate granule exocytosis (Tirouvanziam et al., 2008). Likewise, microarray analysis was used to compare the expression of more than 1,000 genes in neutrophils isolated from blood of CF patients and healthy donors and it revealed the upregulation of 62 genes including those encoding chemokines, and downregulation of 27 genes suggesting a specific disturbance in the mechanisms regulating inflammation (Adib-Conquy et al., 2008).
Although the presence of CFTR in neutrophils was controversial for years, it has been detected in membranes (Pohl et al., 2014) and its localization in both secretory vesicles and phagolysosomes has been reported (Painter et al., 2006). Upon neutrophil activation, NADPH assembly combined with myeloperoxidase chlorinating activity leads to the generation of oxidants essential for bacterial killing (Nauseef, 2007). CF neutrophils exhibit a defective intraphagolysosomal HOCl production, although a normal extracellular production of MPO-derived HOCl is observed (Painter et al., 2006). Chloride is essential for P. aeruginosa killing by neutrophils. CF neutrophils have a reduced bactericidal capacity compared non-CF neutrophils in the presence of chloride, strongly suggesting that a defective CFTR might compromise the ability of CF neutrophils to clear P. aeruginosa (Painter et al., 2008). These results are consistent with previous reports regarding the role of CFTR in the acidification of macrophages' phagolysosome (Di et al., 2006). Accordingly, CFTR activation represents an appealing therapeutic strategy and this is currently in development (Son et al., 2017).
Defect in neutrophil apoptosis in CF leading to failure in the resolution of inflammation
Neutrophil apoptosis, a process of programmed cell death that prevents the release of neutrophil histotoxic contents including oxidants and proteinases, is tightly regulated and limits the destruction of surrounding tissue (Geering and Simon, 2011; Witko-Sarsat et al., 2011). The subsequent recognition and phagocytosis of apoptotic neutrophils by macrophages is central to the successful resolution of an inflammatory response (Kennedy and DeLeo, 2009) and to avoid autoimmunity (Thieblemont et al., 2016). Dying neutrophils exert an anti-inflammatory effect through modulation of macrophage inflammatory cytokine release (Bratton and Henson, 2011). Neutrophil apoptosis may be delayed, induced or enhanced by micro-organisms depending on their immune evasion strategies and the health of the host they encounter (McCracken and Allen, 2014).
Several abnormalities have been described in macrophages from CF patients but so far no defect in the phagocytosis of apoptotic cells has been reported (Bruscia and Bonfield, 2016). This was illustrated in macrophages from CFTR−/− mice infected with P. aeruginosa showing an enhanced cytokine production and secretion suggesting that the macrophage response may be an important therapeutic target for decreasing the morbidity of CF lung disease (Bruscia et al., 2009). At the site of infection, neutrophils represent more than 95% of the cells from the bronchoalveolar lavage and increased neutrophil lifespan is critical for effective host defense but delays in apoptosis can lead to persistent tissue damage. In neutrophils, analysis of genes expression during inflammation clearly showed a modulation of genes involved in apoptosis (Kobayashi et al., 2003).
The short-lived pro-survival Bcl-2 family protein, Mcl-1 (myeloid cell leukemia-1), is instrumental in controlling apoptosis in response to inflammatory stimuli (Moulding et al., 2001; Milot and Filep, 2011). Notably, neutrophils from CF patients chronically infected with P. aeruginosa have a prolonged survival (Dibbert et al., 1999; McKeon et al., 2008). An increased survival was also found in neutrophils isolated from the parents of CF patients, suggesting that at least in part, the defect in neutrophils may be genetically determined (Moriceau et al., 2010). In the latter study, the delayed apoptosis observed in CF patients was not reversed by inhibition of CFTR functions strongly suggesting that some CFTR functions in neutrophils may be independent of its chloride channel and rather due to its ability to associate with other proteins regulating cell functions.
Promoting resolution of inflammation by targeting neutrophils: the case of roscovitine
Today, treatment of inflammatory diseases with non-steroidal anti-inflammatories is based on inhibiting the synthesis or action of inflammatory mediators that drive the host response to injury (Perretti et al., 2015). An alternative approach for the development of novel therapeutics is now based on endogenous mechanisms that switch off acute inflammation and bring about its resolution (Serhan et al., 2007). To date, conventional anti-inflammatory therapies in CF, using glucocorticoids or non-steroid anti-inflammatory drugs, such as ibuprofen, have shown beneficial, albeit marginal, effects by slowing down CF disease progression (Eigen et al., 1995). These modest results may be attributed to the fact that none of these treatments specifically targeted neutrophils, which represent the main cellular actors in inflammation associated with lung disease in CF. In addition, serious side effect of prednisone on growth in children have precluded their therapeutic use in CF (Lai et al., 2000). Inhibition of neutrophil elastase has been tested in order to decrease lung inflammation and is still under investigation (Kelly et al., 2008). A strategy based on interfering with neutrophil recruitment using BIIL 284, an LTB4 receptor (Konstan et al., 2014) or SB 656933, a CXCR2 (Moss et al., 2013) antagonist have been investigated in CF. Unfortunately, these compounds appear to enhance inflammation and have proven to be detrimental to the clinical status of the CF patients. Consequently, novel approaches need to regulate rather than inhibit neutrophils in CF.
In line with this, neutrophil apoptosis represents an important mechanism in the resolution of inflammation and could be considered as a good target to dampen inflammation in CF (Jones et al., 2016). Roscovitine is a low molecular weight pharmacological inhibitor of cyclin-dependent kinases (CDKs) discovered over 20 years ago during studies on the regulation of cell division in starfish oocytes (Meijer et al., 1997; Meijer and Raymond, 2003). This molecule has been used as a pharmacological tool to investigate cell cycle control, apoptosis, neuronal functions, etc. Furthermore roscovitine has been evaluated as a drug candidate in numerous diseases ranging from cancers, especially neuroblastoma (Bettayeb et al., 2010; Delehouze et al., 2014), viral infections, neurodegeneration, rheumatoid arthritis, glaucoma to polycystic kidney disease. Roscovitine has already been administrated to over 500 patients. While roscovitine was originally believed to exert its effects mainly on proliferating cells, it was reported that roscovitine also affected neutrophils which are deficient in proliferative capacities (Savio et al., 2006; Leitch et al., 2009). In neutrophils, roscovitine triggers apoptosis thereby favoring their phagocytosis by macrophages to promote the resolution of inflammation (Rossi et al., 2006). Notably, this activity was due to the inhibition of CDK7 and CDK9 involved in the regulation of RNA transcription (Leitch et al., 2012). Roscovitine has proven beneficial in enhancing neutrophil apoptosis in a model of meningitis (Koedel et al., 2009). However, the modulation of innate and adaptive immunity of roscovitine extends beyond its effect on neutrophils (Meijer et al., 2016). Roscovitine can act on CF alveolar macrophages to rescue acidification in phagolysosomes, which show abnormally high pH (Di et al., 2006). As a result, roscovitine restores their bactericidal activity (Riazanski et al., 2015). Whether roscovitine can modulate the microbicidal activities of neutrophils from healthy subjects or from CF patients is yet to be tested and should be addressed. In addition, roscovitine can correct the CFTR defect, as it partially protects F508del-CFTR from proteolytic degradation and favors its trafficking to the plasma membrane (Norez et al., 2014). Altogether, roscovitine has multiple activities resulting in a strong therapeutic potential in CF and this is currently being evaluated in a first clinical trial with P. aeruginosa infected CF patients (Meijer et al., 2016; Figure 1).
However, the question remains whether enhancing neutrophil clearance could represent a potential danger of decreasing the antibacterial defense provided by neutrophils. Importantly, CF patients are not prone to neutropenia (the definition of which is an absolute blood count of 500/mm3 in non-CF individuals, Bodey et al., 1966) and the only cases reported in CF so far were drug-induced.
PCNA scaffold as a novel key to favor neutrophil apoptosis: regulatory role of p21/waf1 in lung inflammation during persistent P. aeruginosa infection
Neutrophils are terminally differentiated cells deprived of proliferating capacities, and are committed to death. Despite their lack of proliferation, we have discovered that mature neutrophils express high levels of proliferating cell nuclear antigen (PCNA), which was exclusively localized in the cytosol (Witko-Sarsat et al., 2010). This was unexpected because PCNA was known a nuclear factor involved in DNA replication and repair of proliferating cells (Moldovan et al., 2007), Notably, in mature neutrophils PCNA plays a pivotal role in neutrophil survival and changes in parallel with neutrophil apoptosis (Witko-Sarsat and Ohayon, 2016). In the neutrophil cytosol, PCNA was associated with procaspase-8 and procaspase-9 to prevent their activation. In accordance with our hypothesis that PCNA promotes neutrophil survival, we previously showed by immunocytochemistry that PCNA is highly expressed in inflammatory neutrophils within CF lung explants (Chiara et al., 2012). In keeping with these latter results, PCNA is more abundant in the cytosol of CF neutrophils (Western blot analysis) compared to non-CF neutrophils (Martin et al., 2016b). We have provided evidence that treatment with the p21 peptide (Warbrick, 2000), capable of binding the PCNA interdomain connecting loop, reversed the delay in apoptosis observed in neutrophils from CF patients and restored apoptosis levels to that of healthy controls (Martin et al., 2016b). Hence, the pro-apoptotic effect of the p21 peptide is a proof-of-concept that p21/waf1 interfers with cytoplasmic PCNA to trigger neutrophil apoptosis (Chiara et al., 2012). However, p21 protein is hardly expressed in neutrophils and it is unlikely that endogenous p21 regulates neutrophil apoptosis under basal state. In sharp contrast, p21/waf1 mRNA was strongly induced in human neutrophils following LPS challenge suggesting that p21/waf 1 was involved in the regulation of neutrophil activation under inflammatory conditions (Martin et al., 2016a).
To understand the potential role of p21/waf1 in P. aeruginosa infection in the lung, we used a model of persistent lung infection triggered by the instillation of agarose beads-coated P. aeruginosa in mice which results in accumulation of neutrophils in peribronchial area and in alveolar consolidation (Martin et al., 2016b). After 7 days of lung infection with P. aeruginosa, inflammation was more intense in p21−/− mice compared to WT as evidenced by morphologic analysis of the lung. Since no intrinsic defect in the phagocytosis of apoptotic neutrophils by macrophages is found in p21−/− mice, the accumulation of neutrophils at the site of inflammation in these mice could be attributable to a defect in neutrophil apoptosis rather than impaired clearance by macrophages. Accordingly, in vitro, neutrophils isolated from p21−/− mice displayed enhanced survival in response to TNF-α and G-CSF. In keeping with these data obtained in vivo in murine models, an induction of p21 mRNA was observed in responses to both cytokines in human neutrophils (Martin et al., 2016b). Our study provides clear evidence that p21/waf1 expression is a key regulator of neutrophil fate in vivo, especially during P. aeruginosa infection. In conclusion, similarly to roscovitine, targeting PCNA in neutrophils using the p21 competing peptide could accelerate the resolution of inflammation in an infectious context and could be considered as a potential therapeutic strategy in CF.
Remaining open questions: the dilemma of targeting neutrophil survival in CF
We urgently need to identify the molecular mechanisms underlying neutrophil dysfunction in CF, how it relates to CFTR and how it promotes infection with P. aeruginosa. Given the importance and the renewed interest in neutrophils as instrumental actors in immune deregulation associated with lung disease in CF, promoting CFTR-dependent antimicrobial function (Son et al., 2017) or targeting neutrophils to promote their apoptosis (Martin et al., 2016b) is a timely issue that should be addressed. The persistence of neutrophils in CF airways relies on multiple parameters and this enigma will be solved by taking into account the complexity of neutrophil plasticity in response to the hypoxic inflammatory microenvironment and the influence of P. aeruginosa on neutrophil survival mechanisms.
Statements
Author contributions
All authors have written, discussed and approved the final manuscript. VW is an expert in neutrophil-Pseudomonas aeruginosa interaction expert in CF. BM is an expert in bacteria infection and hypoxia. PB is a medical doctor involved in CF patient care. LM is an expert in roscovitine treatment.
Acknowledgments
The work was supported by the Association “Vaincre la Mucoviscidose” (LM and VW), the association ABCF Mucoviscidose (VW), the Fondation Laurette Fugain (BM) and institutional fundings (VW): Investissements d'Avenir programme ANR-11-IDEX-0005-02, Sorbonne Paris Cite, Labex INFLAMEX, the Chancellerie des Universités de Paris (Legs Poix VW).
Conflict of interest
Laurent MEIJER is CEO & CSO of ManRos Therapeutics. The other authors have no conflict of interest to declare.
Footnotes
1.^ Vaincre la Mucoviscidose et Ined Registre Français de la Mucoviscidose–Bilan des données 2015 [Online]. Paris. Available: (Accessed January 28th 2017).
References
1
Adib-Conquy M. Pedron T. Petit-Bertron A. F. Tabary O. Corvol H. Jacquot J. et al . (2008). Neutrophils in cystic fibrosis display a distinct gene expression pattern. Mol. Med.14, 36–44. 10.2119/2007-00081.Adib-Conquy
2
Arena E. T. Tinevez J. Y. Nigro G. Sansonetti P. J. Marteyn B. S. (2017). The infectious hypoxia: occurrence and causes during Shigella infection. Microb. Infect.19, 157–165. 10.1016/j.micinf.2016.10.011
3
Bals R. Weiner D. J. Wilson J. M. (1999). The innate immune system in cystic fibrosis lung disease. J. Clin. Invest.103, 303–307.
4
Bettayeb K. Baunbaek D. Delehouze C. Loaec N. Hole A. J. Baumli S. et al . (2010). CDK Inhibitors Roscovitine and CR8 Trigger Mcl-1 Down-Regulation and Apoptotic Cell Death in Neuroblastoma Cells. Genes Cancer1, 369–380. 10.1177/1947601910369817
5
Bodey G. P. Buckley M. Sathe Y. S. Freireich E. J. (1966). Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med.64, 328–340.
6
Bonfield T. L. (2015). Macrophage Dysfunction in Cystic Fibrosis: a therapeutic target to enhance self-immunity. Am. J. Respir. Crit. Care Med.192, 1406–1407. 10.1164/rccm.201509-1811ED
7
Bratton D. L. Henson P. M. (2011). Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol.32, 350–357. 10.1016/j.it.2011.04.009
8
Bruscia E. M. Bonfield T. L. (2016). Cystic Fibrosis Lung Immunity: the role of the macrophage. J. Innate Immun.8, 550–563. 10.1159/000446825
9
Bruscia E. M. Zhang P. X. Ferreira E. Caputo C. Emerson J. W. Tuck D. et al . (2009). Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am. J. Respir. Cell Mol. Biol.40, 295–304. 10.1165/rcmb.2008-0170OC
10
Burgel P. R. Bellis G. Olesen H. V. Viviani L. Zolin A. Blasi F. et al . (2015). Future trends in cystic fibrosis demography in 34 European countries. Eur. Respir. J.46, 133–141. 10.1183/09031936.00196314
11
Burgel P. R. Fajac I. Hubert D. Grenet D. Stremler N. Roussey M. et al . (2010). Non-classic cystic fibrosis associated with D1152H CFTR mutation. Clin. Genet.77, 355–364. 10.1111/j.1399-0004.2009.01294.x
12
Burgel P. R. Montani D. Danel C. Dusser D. J. Nadel J. A. (2007). A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax62, 153–161. 10.1136/thx.2006.062190
13
Campbell E. L. Bruyninckx W. J. Kelly C. J. Glover L. E. McNamee E. N. Bowers B. E. (2014). Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity40, 66–77. 10.1016/j.immuni.2013.11.020
14
Cantin A. (1995). Cystic fibrosis lung inflammation: early, sustained, and severe. Am. J. Respir. Crit. Care Med.151, 939–941.
15
Chiara A. D. Pederzoli-Ribeil M. Burgel P. R. Danel C. Witko-Sarsat V. (2012). Targeting cytosolic proliferating cell nuclear antigen in neutrophil-dominated inflammation. Front. Immunol.3:311. 10.3389/fimmu.2012.00311
16
Cohen T. S. Prince A. (2012). Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat. Med.18, 509–519. 10.1038/nm.2715
17
Cook G. M. Greening C. Hards K. Berney M. (2014). Energetics of pathogenic bacteria and opportunities for drug development. Adv. Microb. Physiol.65, 1–62. 10.1016/bs.ampbs.2014.08.001
18
Delehouze C. Godl K. Loaec N. Bruyere C. Desban N. Oumata N. et al . (2014). CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells. Oncogene33, 5675–5687. 10.1038/onc.2013.513
19
Di A. Brown M. E. Deriy L. V. Li C. Szeto F. L. Chen Y. et al . (2006). CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol.8, 933–944. 10.1038/ncb1456
20
Dibbert B. Weber M. Nikolaizik W. H. Vogt P. Schoni M. H. Blaser K. et al . (1999). Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc. Natl. Acad. Sci. U.S.A.96, 13330–13335.
21
Downey D. G. Bell S. C. Elborn J. S. (2009). Neutrophils in cystic fibrosis. Thorax64, 81–88. 10.1136/thx.2007.082388
22
Dwyer M. Shan Q. D'Ortona S. Maurer R. Mitchell R. Olesen H. et al . (2014). Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J. Innate Immun.6, 765–779. 10.1159/000363242
23
Eichner A. Gunther N. Arnold M. Schobert M. Heesemann J. Hogardt M. (2014). Marker genes for the metabolic adaptation of Pseudomonas aeruginosa to the hypoxic cystic fibrosis lung environment. Int. J. Med. Microbiol.304, 1050–1061. 10.1016/j.ijmm.2014.07.014
24
Eigen H. Rosenstein B. J. FitzSimmons S. Schidlow D. V. (1995). A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic fibrosis foundation prednisone trial group. J. Pediatr.126, 515–523. 10.1016/S0022-3476(95)70343-8
25
Emerson J. Rosenfeld M. McNamara S. Ramsey B. Gibson R. L. (2002). Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol.34, 91–100. 10.1002/ppul.10127
26
Emond M. J. Louie T. Emerson J. Zhao W. Mathias R. A. Knowles M. R. et al . (2012). Exome sequencing of extreme phenotypes identifies DCTN4 as a modifier of chronic Pseudomonas aeruginosa infection in cystic fibrosis. Nat. Genet.44, 886–889. 10.1038/ng.2344
27
Fuchs T. A. Abed U. Goosmann C. Hurwitz R. Schulze I. Wahn V. et al . (2007). Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol.176, 231–241. 10.1083/jcb.200606027
28
Geering B. Simon H. U. (2011). Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ.18, 1457–1469. 10.1038/cdd.2011.75
29
Govan J. R. Nelson J. W. (1993). Microbiology of cystic fibrosis lung infections: themes and issues. J. R. Soc. Med.86(Suppl. 20), 11–18.
30
Gray R. D. McCullagh B. N. McCray P. B. (2015). NETs and CF lung disease: current status and future prospects. Antibiotics (Basel), 4, 62–75. 10.3390/antibiotics4010062
31
Hayes E. Pohl K. McElvaney N. G. Reeves E. P. (2011). The cystic fibrosis neutrophil: a specialized yet potentially defective cell. Arch. Immunol. Ther. Exp. (Warsz).59, 97–112. 10.1007/s00005-011-0113-6
32
Jones H. R. Robb C. T. Perretti M. Rossi A. G. (2016). The role of neutrophils in inflammation resolution. Seminars Immunol.28, 137–145. 10.1016/j.smim.2016.03.007
33
Karhausen J. Furuta G. T. Tomaszewski J. E. Johnson R. S. Colgan S. P. Haase V. H. (2004). Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest.114, 1098–1106. 10.1172/JCI200421086
34
Kelly E. Greene C. M. McElvaney N. G. (2008). Targeting neutrophil elastase in cystic fibrosis. Expert Opin. Ther. Targets12, 145–157. 10.1517/14728222.12.2.145
35
Kennedy A. D. DeLeo F. R. (2009). Neutrophil apoptosis and the resolution of infection. Immunol. Res.43, 25–61. 10.1007/s12026-008-8049-6
36
Khan T. Z. Wagener J. S. Bost T. Martinez J. Accurso F. J. Riches D. W. (1995). Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Resp. Crit. Care Med.151, 1075–1082. 10.1164/ajrccm.151.4.7697234
37
Kirchner T. Möller S. Klinger M. Solbach W. Laskay T. Behnen M. (2012). The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat. Inflamm.2012:849136. 10.1155/2012/849136
38
Kobayashi S. D. Braughton K. R. Whitney A. R. Voyich J. M. Schwan T. G. Musser J. M. et al . (2003). Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. U.S.A.100, 10948–10953. 10.1073/pnas.1833375100
39
Koedel U. Frankenberg T. Kirschnek S. Obermaier B. Häcker H. Paul R. et al . (2009). Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog.5:e1000461. 10.1371/journal.ppat.1000461
40
Konstan M. W. Döring G. Heltshe S. L. Lands L. C. Hilliard K. A. Koker P. et al . (2014). A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros.13, 148–155. 10.1016/j.jcf.2013.12.009
41
Lai H.-C. FitzSimmons S. C. Allen D. B. Kosorok M. R. Rosenstein B. J. Campbell P. W. et al . (2000). Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N. Engl. J. Med.342, 851–859. 10.1056/NEJM200003233421204
42
Laval J. Ralhan A. Hartl D. (2016). Neutrophils in cystic fibrosis. Biol. Chem.397, 485–496. 10.1515/hsz-2015-0271
43
Laval J. Touhami J. Herzenberg L. A. Conrad C. Taylor N. Battini J. L. et al . (2013). Metabolic adaptation of neutrophils in cystic fibrosis airways involves distinct shifts in nutrient transporter expression. J. Immunol.190, 6043–6050. 10.4049/jimmunol.1201755
44
Leitch A. E. Haslett C. Rossi A. G. (2009). Cyclin-dependent kinase inhibitor drugs as potential novel anti-inflammatory and pro-resolution agents. Br. J. Pharmacol.158, 1004–1016. 10.1111/j.1476-5381.2009.00402.x
45
Leitch A. E. Lucas C. D. Marwick J. A. Duffin R. Haslett C. Rossi A. G. (2012). Cyclin-dependent kinases 7 and 9 specifically regulate neutrophil transcription and their inhibition drives apoptosis to promote resolution of inflammation. Cell Death Diff.19, 1950–1961. 10.1038/cdd.2012.80
46
MacKenzie T. Gifford A. H. Sabadosa K. A. Quinton H. B. Knapp E. A. Goss C. H. et al . (2014). Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the cystic fibrosis foundation patient registry. Ann. Intern. Med.161, 233–241. 10.7326/M13-0636
47
Maianski N. A. Geissler J. Srinivasula S. M. Alnemri E. S. Roos D. Kuijpers T. W. (2004). Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ.11, 143–153. 10.1038/sj.cdd.4401320
48
Malachowa N. Kobayashi S. D. Quinn M. T. DeLeo1 F. R. (2016). NET confusion. Front. Immunol.7:259. 10.3389/fimmu.2016.00259
49
Manzenreiter R. Kienberger F. Marcos V. Schilcher K. Krautgartner W. D. Obermayer A. et al . (2012). Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cyst. Fibros.11, 84–92. 10.1016/j.jcf.2011.09.008
50
Martin C. Frija-Masson J. Burgel P. R. (2014). Targeting mucus hypersecretion: new therapeutic opportunities for COPD?Drugs74, 1073–1089. 10.1007/s40265-014-0235-3
51
Martin C. Hamard C. Kanaan R. Boussaud V. Grenet D. Abely M. et al . (2016a). Causes of death in French cystic fibrosis patients: the need for improvement in transplantation referral strategies!J. Cyst. Fibros.15, 204–212. 10.1016/j.jcf.2015.09.002
52
Martin C. Ohayon D. Alkan M. Mocek J. Pederzoli-Ribeil M. Candalh C. et al . (2016b). Neutrophil-expressed p21/waf1 favors inflammation resolution in Pseudomonas aeruginosa infection. Am. J. Resp. Cell Mol. Biol.54, 740–750. 10.1165/rcmb.2015-0047OC
53
Matsui H. Verghese M. W. Kesimer M. Schwab U. E. Randell S. H. Sheehan J. K. et al . (2005). Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J. Immunol.175, 1090–1099. 10.4049/jimmunol.175.2.1090
54
McCracken J. M. Allen L. A. (2014). Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death7, 15–23. 10.4137/JCD.S11038
55
McKeon D. J. Condliffe A. M. Cowburn A. S. Cadwallader K. C. Farahi N. Bilton D. et al . (2008). Prolonged survival of neutrophils from patients with Delta F508 CFTR mutations. Thorax63, 660–661. 10.1136/thx.2008.096834
56
Meijer L. Borgne A. Mulner O. Chong J. P. Blow J. J. Inagaki N. et al . (1997). Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem.243, 527–536.
57
Meijer L. Nelson D. J. Riazanski V. Gabdoulkhakova A. G. Hery-Arnaud G. Le Berre R. et al . (2016). Modulating Innate and Adaptive Immunity by (R)-Roscovitine: potential Therapeutic Opportunity in Cystic Fibrosis. J. Innate Immun.8, 330–349. 10.1159/000444256
58
Meijer L. Raymond E. (2003). Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc Chem. Res.36, 417–425. 10.1021/ar0201198
59
Milot E. Filep J. G. (2011). Regulation of neutrophil survival/apoptosis by Mcl-1. ScientificWorldJournal11, 1948–1962. 10.1100/2011/131539
60
Mizgerd J. P. Brain J. D. (1995). Reactive oxygen species in the killing of Pseudomonas aeruginosa by human leukocytes. Curr. Microbiol.31, 124–128.
61
Moldovan G. L. Pfander B. Jentsch S. (2007). PCNA, the maestro of the replication fork. Cell129, 665–679. 10.1016/j.cell.2007.05.003
62
Monceaux V. Chiche-Lapierre C. Chaput C. Witko-Sarsat V. Prevost M. C. Taylor C. T. et al . (2016). Anoxia and glucose supplementation preserve neutrophil viability and function. Blood128, 993–1002. 10.1182/blood-2015-11-680918
63
Moriceau S. Lenoir G. Witko-Sarsat V. (2010). In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: evidence for an innate neutrophil disturbanceJ. Innate Immun.2, 260–266. 10.1159/000295791
64
Moss R. B. Mistry S. J. Konstan M. W. Pilewski J. M. Kerem E. Tal-Singer R. et al . (2013). Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J. Cyst. Fibros.12, 241–248. 10.1016/j.jcf.2012.08.016
65
Moulding D. A. Akgul C. Derouet M. White M. R. Edwards S. W. (2001). BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J. Leukoc. Biol.70, 783–792.
66
Nauseef W. M. (2007). How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev.219, 88–102. 10.1111/j.1600-065X.2007.00550.x
67
Nauseef W. M. Kubes P. (2016). Pondering neutrophil extracellular traps with healthy skepticism. Cell. Microbiol.18, 1349–1357. 10.1111/cmi.12652
68
Nishinaka Y. Arai T. Adachi S. Takaori-Kondo A. Yamashita K. (2011). Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem. Biophys. Res. Commun.413, 75–79. 10.1016/j.bbrc.2011.08.052
69
Norez C. Vandebrouck C. Bertrand J. Noel S. Durieu E. Oumata N. et al . (2014). Roscovitine is a proteostasis regulator that corrects the trafficking defect of F508del-CFTR by a CDK-independent mechanism. Br. J. Pharmacol.171, 4831–4849. 10.1111/bph.12859
70
Painter R. G. Bonvillain R. W. Valentine V. G. Lombard G. A. LaPlace S. G. Nauseef W. M. et al . (2008). The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol.83, 1345–1353. 10.1189/jlb.0907658
71
Painter R. G. Valentine V. G. Lanson N. A. Jr. Leidal K. Zhang Q. Lombard G. et al . (2006). CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry45, 10260–10269. 10.1021/bi060490t
72
Palmer L. J. Cooper P. R. Ling M. R. Wright H. J. Huissoon A. Chapple I. L. (2012). Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol.167, 261–268. 10.1111/j.1365-2249.2011.04518.x
73
Papayannopoulos V. Metzler K. D. Hakkim A. Zychlinsky A. (2010). Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol.191, 677–691. 10.1083/jcb.201006052
74
Pernet E. Guillemot L. Burgel P. R. Martin C. Lambeau G. Sermet-Gaudelus I. et al . (2014). Pseudomonas aeruginosa eradicates Staphylococcus aureus by manipulating the host immunity. Nat. Commun.5:5105. 10.1038/ncomms6105
75
Perretti M. Leroy X. Bland E. J. Montero-Melendez T. (2015). Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci.36, 737–755. 10.1016/j.tips.2015.07.007
76
Pittman R. N. (2013). Oxygen transport in the microcirculation and its regulation. Microcirculation20, 117–137. 10.1111/micc.12017
77
Pohl K. Hayes E. Keenan J. Henry M. Meleady P. Molloy K. et al . (2014). A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood124, 999–1009. 10.1182/blood-2014-02-555268
78
Ramsey K. A. Ranganathan S. Park J. Skoric B. Adams A. M. Simpson S. J. et al . (2014). Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am. J. Respir. Crit. Care Med.190, 1111–1116. 10.1164/rccm.201407-1277OC
79
Ratjen F. Comes G. Paul K. Posselt H. G. Wagner T. O. Harms K. et al . (2001). Effect of continuous antistaphylococcal therapy on the rate of P. aeruginosa acquisition in patients with cystic fibrosis. Pediatr. Pulmonol.31, 13–16. 10.1002/1099-0496(200101)31:1<13::AID-PPUL1001>3.0.CO;2-N
80
Riazanski V. Gabdoulkhakova A. G. Boynton L. S. Eguchi R. R. Deriy L. V. Hogarth D. K. et al . (2015). TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc. Natl. Acad. Sci. U.S.A.112, E6486–E6495. 10.1073/pnas.1518966112
81
Rossi A. G. Sawatzky D. A. Walker A. Ward C. Sheldrake T. A. Riley N. A. et al . (2006). Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med.12, 1056–1064. 10.1038/nm1468
82
Rustad T. R. Sherrid A. M. Minch K. J. Sherman D. R. (2009). Hypoxia: a window into Mycobacterium tuberculosis latency. Cell. Microbiol.11, 1151–1159. 10.1111/j.1462-5822.2009.01325.x
83
Savio M. Cerri M. Cazzalini O. Perucca P. Stivala L. A. Pichierri P. et al . (2006). Replication-dependent DNA damage response triggered by roscovitine induces an uncoupling of DNA replication proteins. Cell Cycle5, 2153–2159. 10.4161/cc.5.18.3235
84
Schaible B. McClean S. Selfridge A. Broquet A. Asehnoune K. Taylor C. T. et al . (2013). Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE8:e56491. 10.1371/journal.pone.0056491
85
Schaible B. Taylor C. T. Schaffer K. (2012). Hypoxia increases antibiotic resistance in Pseudomonas aeruginosa through altering the composition of multidrug efflux pumps. Antimicrob. Agents Chemother.56, 2114–2118. 10.1128/AAC.05574-11
86
Schobert M. Tielen P. (2010). Contribution of oxygen-limiting conditions to persistent infection of Pseudomonas aeruginosa. Future Microbiol.5, 603–621. 10.2217/fmb.10.16
87
Serhan C. N. Brain S. D. Buckley C. D. Gilroy D. W. Haslett C. O'Neill L. A. et al . (2007). Resolution of inflammation: state of the art, definitions and terms. FASEB J.21, 325–332. 10.1096/fj.06-7227rev
88
Simon D. Simon H.-U. Yousefi S. (2013). Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy68, 409–416. 10.1111/all.12111
89
Son J.-H. Zhu J. S. Phuan P. W. Cil O. Teuthorn A. P. Ku C. K. et al . (2017). High-Potency Phenylquinoxalinone Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Activators. J. Med. Chem.60, 2401–2410. 10.1021/acs.jmedchem.6b01759
90
Thieblemont N. Wright H. L. Edwards S. W. Witko-Sarsat V. (2016). Human neutrophils in auto-immunity. Seminars Immunol.28, 159–173. 10.1016/j.smim.2016.03.004
91
Tirouvanziam R. Gernez Y. Conrad C. K. Moss R. B. Schrijver I. Dunn C. E. et al . (2008). Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc. Natl. Acad. Sci. U.S.A.105, 4335–4339. 10.1073/pnas.0712386105
92
Tsai M. C. Chakravarty S. Zhu G. Xu J. Tanaka K. Koch C. et al . (2006). Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell. Microbiol.8, 218–232. 10.1111/j.1462-5822.2005.00612.x
93
Viel M. Hubert D. Burgel P.-R. Génin E. Honoré I. Martinez B. et al . (2016). DCTN4 as a modifier of chronic Pseudomonas aeruginosa infection in cystic fibrosis. Clin. Respir. J.10, 777–783. 10.1111/crj.12288
94
Walmsley S. R. Print C. Farahi N. Peyssonnaux C. Johnson R. S. Cramer T. et al . (2005). Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med.201, 105–115. 10.1084/jem.20040624
95
Warbrick E. (2000). The puzzle of PCNA's many partners. Bioessays22, 997–1006. 10.1002/1521-1878(200011)22:11<997::AID-BIES6>3.0.CO;2-#
96
Witko-Sarsat V. Mocek J. Bouayad D. Tamassia N. Ribeil J. A. Candalh C. et al . (2010). Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival. J. Exp. Med.207, 2631–2645. 10.1084/jem.20092241
97
Witko-Sarsat V. Ohayon D. (2016). Proliferating cell nuclear antigen in neutrophil fate. Immunol. Rev.273, 344–356. 10.1111/imr.12449
98
Witko-Sarsat V. Pederzoli-Ribeil M. Hirsh E. Sozzani S. Cassatella M. A. (2011). Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 32, 117–124. 10.1016/j.it.2011.01.001
99
Witko-Sarsat V. Rieu P. Descamps-Latscha B. Lesavre P. Halbwachs-Mecarelli L. (2000). Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest.80, 617–653. 10.1038/labinvest.3780067
100
Worlitzsch D. Tarran R. Ulrich M. Schwab U. Cekici A. Meyer K. C. et al . (2002). Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest.109, 317–325. 10.1172/JCI13870
101
Yan F. Li W. Jono H. Li Q. Zhang S. Li J. D. et al . (2008). Reactive oxygen species regulate Pseudomonas aeruginosa lipopolysaccharide-induced MUC5AC mucin expression via PKC-NADPH oxidase-ROS-TGF-alpha signaling pathways in human airway epithelial cells. Biochem. Biophys. Res. Commun.366, 513–519. 10.1016/j.bbrc.2007.11.17211
102
Young R. L. Malcolm K. C. Kret J. E. Caceres S. M. Poch K. R. Nichols D. P. et al . (2011). Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS ONE6:e23637. 10.1371/journal.pone.0023637
Summary
Keywords
inflammation, cystic fibrosis, Pseudomonas, PCNA, apoptosis, hypoxia, roscovitine
Citation
Marteyn BS, Burgel P-R, Meijer L and Witko-Sarsat V (2017) Harnessing Neutrophil Survival Mechanisms during Chronic Infection by Pseudomonas aeruginosa: Novel Therapeutic Targets to Dampen Inflammation in Cystic Fibrosis. Front. Cell. Infect. Microbiol. 7:243. doi: 10.3389/fcimb.2017.00243
Received
30 March 2017
Accepted
26 May 2017
Published
30 June 2017
Volume
7 - 2017
Edited by
Lee-Ann H. Allen, University of Iowa, United States
Reviewed by
Paul Proost, KU Leuven, Belgium; Tracey L. Bonfield, Case Western Reserve University, United States
Updates
Copyright
© 2017 Marteyn, Burgel, Meijer and Witko-Sarsat.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Véronique Witko-Sarsat veronique.witko@inserm.fr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.