- 1Division of Basic & Translational Research, Department of Surgery, University of Minnesota, Minneapolis, MN, United States
- 2BioTechnology Institute, University of Minnesota, Saint Paul, MN, United States
Fecal microbiota transplantation (FMT) has been established as a highly restorative therapeutic approach for treating recurrent Clostridioides difficile infection (rCDI). Recently, the use of capsule-based fecal microbiota transplantation (cFMT) has been shown to be a clinically effective approach to restore intestinal microbiota composition. This convenient, oral delivery provides an easy route of administration and a newfound flexibility for clinicians and patients. In this review, we discuss the development of cFMT, paying particular attention to lyophilized cFMT products. We review the available published clinical studies comparing cFMT with lower endoscopic FMT (eFMT) or placebo. We further discuss the pharmacokinetics of FMT, which should be understood in a framework of microbial ecology that considers the complex and dynamic interactions of gut microbiota with host factors and other microorganisms. Promisingly, the results of multiple trials investigating cFMT vs. eFMT in rCDI show cFMT to be as effective as eFMT at preventing rCDI. However, its efficacy in non-rCDI conditions, including obesity and metabolic syndrome, inflammatory bowel disease, HIV, and neurologic conditions, is less clear and more research is needed in these areas. Standardization of formulation, dose, and timing of administration to ensure optimal microbiota engraftment and clinical response is also a challenge to be addressed. Overall, cFMT is a practical method for fecal microbiota transplantation, with similar efficacy to eFMT in the resolution of rCDI, that holds therapeutic potential in a variety of other diseases.
Background
The intestinal (gut) microbiome is defined as the complete collection of microorganisms, including bacteria, viruses, protozoa, and fungi, in addition to their collective genetic material that is present in the gastrointestinal tract (Shreiner et al., 2015). The trillions of gut bacterial cells can be further classified into thousands of different species including more than 5,000 bacterial strains. The gut microbiota is crucial in maintaining a healthy gut; this biological system produces the essential vitamins B12 and K and digests and metabolizes nutrients from ingested substances such as complex polysaccharides (e.g., fiber) and medications (Shreiner et al., 2015). Importantly, the commensal microbiota also provides protection from pathogen colonization through Toll-like receptor-mediated immune activation (Brandl et al., 2007), modulation of host metabolites (Nagao-Kitamoto et al., 2020), and production of bactericidal compounds (Coyne et al., 2019). Indirectly, the competitive exclusion of pathogens via niche specialization and efficient consumption of available nutrients also occurs. Given these and other roles extending beyond the gastrointestinal tract, the microbiota is now recognized as a critical component of health and disease (Lozupone et al., 2012; Marchesi et al., 2016).
Factors such as environment, diet, host genetics, and medications contribute to a person’s unique microbial composition (Lozupone et al., 2012; Lloyd-Price et al., 2017). Medications, especially antibiotics, can have an adverse effect on the gut microbiome, and the extensive use of antibiotics in medicine over the last few decades has contributed to the depletion of the gut microbiota and led to the increased development of antibiotic-resistant pathogens (Fischbach and Walsh, 2009; Modi et al., 2014). Furthermore, the rapid, diminishing diversity of the gut microbiota following antibiotic exposure directly causes a loss of function and structure of the microbial community (Dethlefsen et al., 2008; Dethlefsen and Relman, 2011). Both antibiotic-resistant pathogens as well as the decreased diversity are gaining recognition as prominent health concerns related to the use and overuse of antibiotics (Khoruts and Sadowsky, 2016).
Fecal Microbiota Transplantation
In response to issues associated with antibiotic use described above, fecal microbiota transplantation (FMT) has reemerged as a restorative therapeutic approach and is especially recognized for treating recurrent Clostridioides difficile infection (rCDI) once antibiotics have proven ineffective (Hamilton et al., 2012; Borody et al., 2013; van Nood et al., 2013). This procedure involves the transplantation of the gut microbiota from a healthy donor to a patient to restore normal diversity and function. FMT results in donor-like normalization of the gut microbial community structure and functionality without causing dysbiosis associated with antibiotic treatments, a predominant causal risk factor for rCDI in most patients (Weingarden et al., 2015; Hui et al., 2019). Use of FMT has shown a high rate of efficacy (~90%) when treating rCDI (Drekonja et al., 2015).
FMT has evolved over the last decade toward the use of increasingly regulated and standardized products that are more easily integrated into mainstream clinical practice (Hamilton et al., 2012; Khoruts et al., 2021). Traditionally, the United States has performed FMT via a lower endoscopic route of administration (eFMT), which has the advantage of direct application to the colon (Hamilton et al., 2012). In Europe, administration through a nasogastric or nasoduodenal tube (NGT/NDT) is more commonly done (van Nood et al., 2013). In a study that aimed at comparing the clinical efficacy of both routes, it was found that the rCDI cure rates did not significantly differ between methods (Postigo and Kim, 2012; Youngster et al., 2014a), although more recent accounts suggest the superiority of colonoscopic delivery (Ramai et al., 2020). Despite the procedural differences present in the routes of administration, FMT via NGT or colonoscopy appears to be safe and highly effective for the resolution and management of CDI (Postigo and Kim, 2012; Ramai et al., 2020).
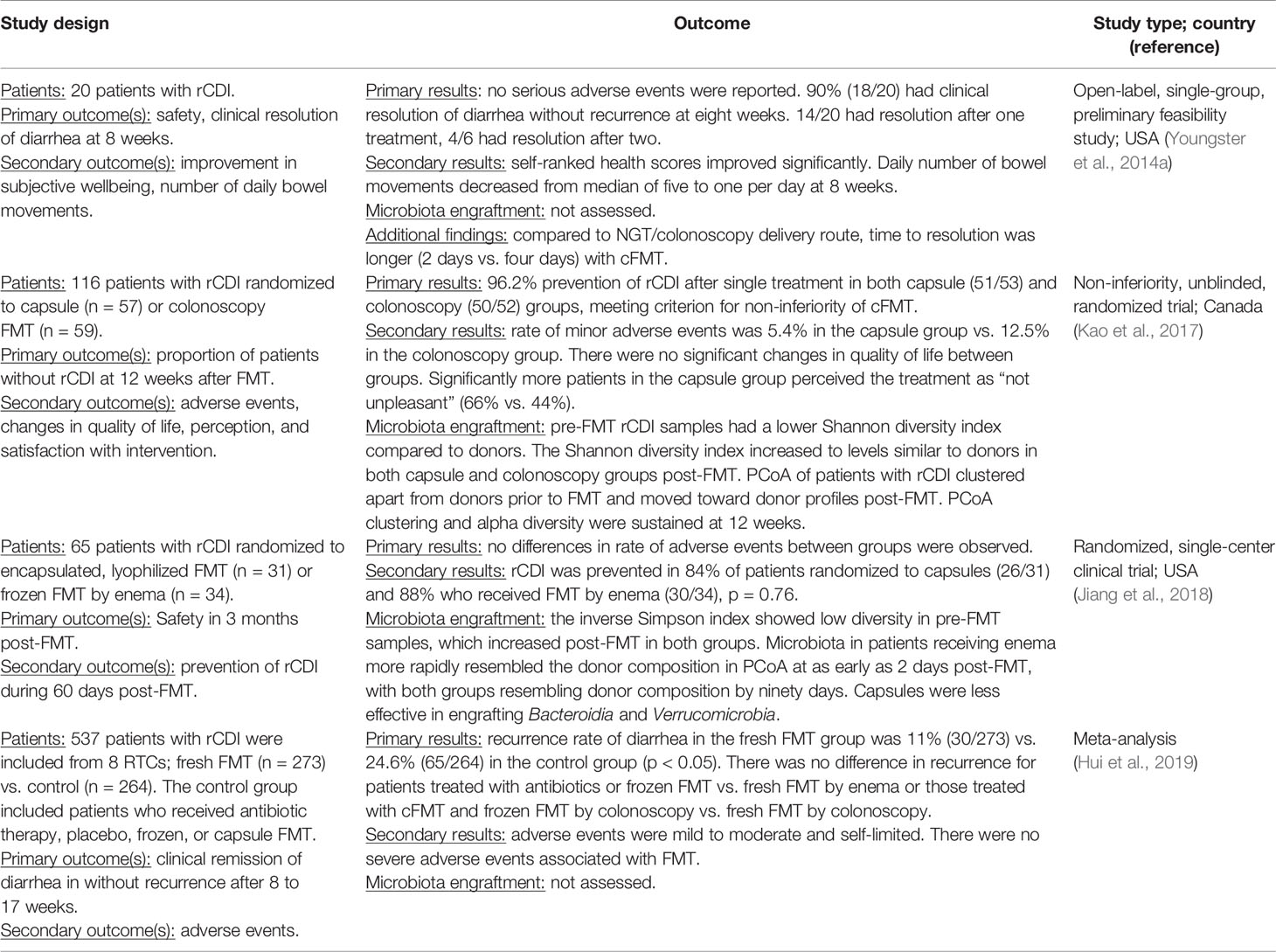
Clinical Efficacy of Capsule-Based FMT
The push toward clinical practicality and flexibility has recently resulted in the development of an oral FMT administration route using encapsulated frozen or freeze-dried material (cFMT) (Youngster et al., 2014a; Staley et al., 2017a). This oral preparation is preferable for many patients and providers due to its greater ease of administration and less invasive nature (Kao et al., 2017). The efficacy of cFMT is an area of ongoing study, and in the setting of rCDI, multiple recent trials (Youngster et al., 2014b; Kao et al., 2017; Jiang et al., 2018; Ramai et al., 2020) have demonstrated comparable clinical results of cFMT to eFMT (Table 1). Results from these trials showed the non-inferiority of cFMT compared to eFMT with 84%-96% clinical resolution of rCDI in patients treated with capsules and no significant difference in the rate of adverse events. In a recent meta-analysis of 26 studies, including 16 that administered FMT via colonoscopy and four that used capsules, both routes of administration achieved equivalent response rates of 94.8% (CI 92.4–96.8%) and 92.1% (CI 88.6–95.0%), respectively (Ramai et al., 2020). Based on these findings, cFMT represents a less cumbersome, more practical, and more flexible approach for patients to restore gut microbiota diversity with the advantage that it can be administered in the outpatient setting. More standardized, oral products, such as SER-109, composed of purified Firmicutes spores (McGovern et al., 2021), are also showing promising results in preventing recurrence of C. difficile infection (Feuerstadt et al., 2022).
While cFMT has gained attention for its use in treating rCDI, it is also now being applied to a wide range of microbially associated diseases that may benefit from repopulation of the gut with a healthy microbiota (Sadowsky and Khoruts, 2016). cFMT is actively being studied in a wide range of conditions including inflammatory bowel disease (IBD), obesity/metabolic syndrome, and neurologic disorders, among others. In this review, we will discuss the development of the FMT capsule product, its clinical efficacy, and its pharmacokinetics in comparison to eFMT, as well as future directions for this restorative therapy.
Development of the Capsule Product
Despite medical literature dating back to the fourth century, FMT research is still a developing procedure, specifically in regard to donor selection (Stripling and Rodriguez, 2018). When FMT first emerged in contemporary medicine, it was thought that individuals closely related to the patient would provide the optimal, fresh donor fecal microbiota for restorative repopulation (Bibbò et al., 2020). Patients were often tasked with finding a suitable donor; however, this presented a logistical difficulty and added to patient burden in addition to their illness. Moreover, no benefit was observed using fresh vs. frozen fecal preparations (Lee et al., 2016). In a practical effort to make donor fecal material readily available, the use of prescreened “universal” donors whose stool could be banked became a common practice internationally (Hamilton et al., 2012; Cammarota et al., 2019). However, recent concerns related to extended-spectrum beta-lactamase (ESBL) Escherichia coli and other multidrug-resistant species remain a concern when using banked stool and have resulted in patient death following FMT (DeFilipp et al., 2019). Therefore, it is critical for patient safety that donors and biobanked specimens are rigorously screened and checked regularly to ensure product safety.
Throughout its modern usage, though, the selection of the optimal donor in addition to screening protocols for safety has actively evolved and there is still a lack of clear resolution regarding the features of the optimal donor (Woodworth et al., 2017; Bibbò et al., 2020). Several small-scale studies have proposed the use of FMT super-donors, as FMT success has shown to be dependent on the composition and microbial diversity of the stool donor (Wilson et al., 2021). While this hypothesis is tempting, the absence of large, randomized clinical trials of FMT for the treatment of rCDI or other conditions suggests that the existence of FMT super-donors is yet to be supported by concrete empirical evidence. Nevertheless, an international consensus regarding the use of banked frozen donor material and consistently emerging recommendations may help standardize this practice (Cammarota et al., 2019).
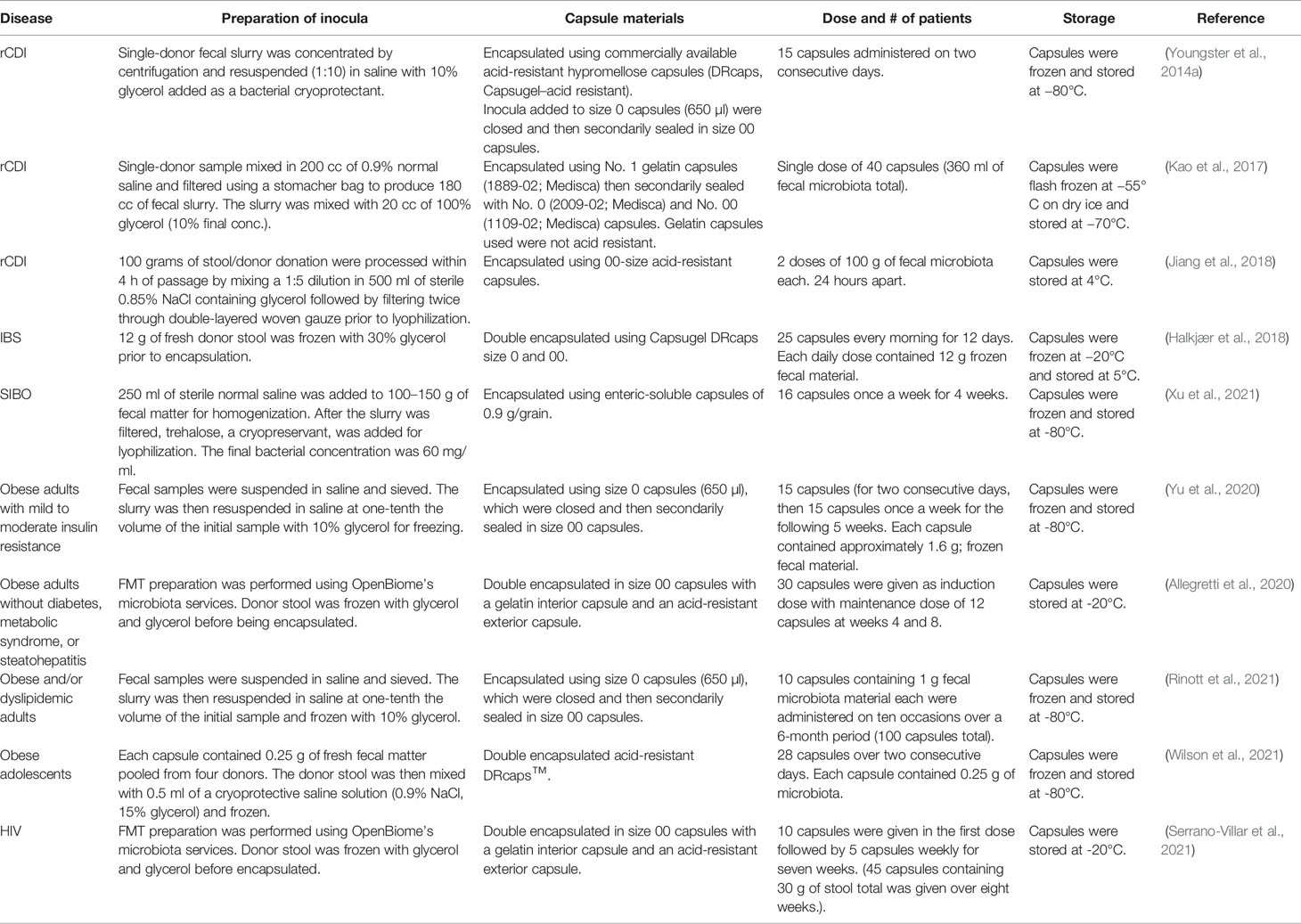
The encapsulation of donor material for oral delivery represented the next practical step in improving both the aesthetics of FMT and its safety and availability. Similar to endoscopic and nasoduodenal approaches to administration, the first encapsulated FMT products were made from frozen donor preparations (Youngster et al., 2014a). These preparations were amended with 10% glycerol for ultralow-temperature storage, but uniformity of preparations and storage remained practical barriers to high-throughput usage. To address these concerns, our group pioneered the development of a freeze-dried preparation that would preserve the viability and diversity of the microbiota while at the same time reducing cumbersome mechanical barriers to capsule production (Staley et al., 2017a). These capsules were manufactured under Good Manufacturing Practice (GMP) standards, and use of the lyophilized powder did not significantly reduce the membrane integrity of the microbiota relative to fresh stool (Staley et al., 2017a). Due to a lack of standardized methods, emerging encapsulated FMT products vary somewhat in their formulation and to a greater degree in their mechanical properties, dosage, and delivery regimen, with the majority of capsules still made using frozen donor material (Table 2).
Donor Material Preparation
Homogenization of the donor material is a ubiquitous first step to capsule preparation and is typically carried out in normal saline with dilution factors ranging from 5× to 10× (Table 2). Among frozen preparations, the donor slurry is typically amended with 10% glycerol. However, the use of freeze-dried preparations necessitated the use of different lyoprotectants to reach a practical viscosity for encapsulation (Staley et al., 2017a). Various lyoprotectants including sucrose, trehalose, mannitol, and skim milk, alone or in combination, at concentrations ranging from 2.5% to 10% were tested to identify which products provided an optimal consistency while maintaining bacterial viability. Milk was not suitable for pharmaceutical preparations due to potential allergic reactions and batch variability. Both trehalose and mannitol were found to produce preparations that were easily ground into powder and encapsulated. Trehalose was found superior in preserving bacterial viability, with membrane integrity tests comparable to frozen products (Staley et al., 2017a), and it appears to work consistently among other groups pursuing lyophilized encapsulated microbiota (Xu et al., 2021). While the use of trehalose has been suggested to enhance the virulence of some strains of C. difficile (Collins et al., 2018), there are several studies that show trehalose supplementation does not interfere with the clinical efficacy of cFMT (Saund et al., 2020; Buckley et al., 2021).
Capsule Construction
The size and types of capsules used have also varied among different groups based on availability and the intended location of release (Table 2). However, the majority of studies have used size 00 capsules or smaller as a manageable size for patient use. When choosing the capsule material itself, the choice is dependent on the need for a specific location of release and engraftment. In order for the capsule to maintain its integrity as well as the bacterial viability, the use of acid-resistant encapsulation is crucial in its ability to provide protective properties against the harsh nature of the oral route of administration (Table 2). While most of the studies utilized an acid-resistant capsule and/or double encapsulation, this choice has varied considerably. In a study done to test the capsules’ ability to withstand the acidic environment present during the capsule transit, it was found that acid-resistant capsules were able to maintain membrane stability at a pH of 3 or less at 37°C before they began to leak (Varga et al., 2021).
Similar to the different choices of capsule material and size, the storage temperature of the encapsulated product varies among the studies (Table 2). The majority of the studies reported storage of their capsule products at -80°C for long-term storage, and -20°C along with 4°C for short-term storage. While it was known that frozen FMT material was required to be stored at ultralow freezing temperatures, the encapsulated product thermal stability has allowed some flexibility in storage. Lyophilized, encapsulated microbiota was tested in storage for 96 h at 20°C, 4°C, -20°C, and -80°C, with no significant differences in the microbiota viability as determined by membrane integrity (Staley et al., 2017a). This suggests that, at least among lyophilized preparations, short-term storage in patients’ homes may allow greater flexibility in FMT administration.
Capsule Administration
Capsule administration provides a more flexible, less invasive, and more palatable option for FMT delivery without a reduction in clinical efficacy (Kao et al., 2017; Ramai et al., 2020). Colonoscopic administration benefits from the ability to visualize the colon and directly deliver the microbiota to the targeted area, and the ability to deliver larger quantities of microbiota; however, there is a risk due to the use of anesthesia as well as that of bowel perforation (Ramai et al., 2019). In comparison, upper gastrointestinal administration uses less stool but also has a greater risk of adverse events including aspiration, hemorrhage, and perforation (Wang et al., 2016; Ramai et al., 2020). Thus, in addition to increasing the flexibility of administration while reducing the unpleasantness associated with the procedure, cFMT may also represent a safer route of administration.
Capsule dosage and administration regimens have varied widely among studies (Table 2). The average total dose present in the studies ranged from 100 to 400 mg; however, the timing of administration and quantity of the capsules varied considerably based on study timelines and objectives. In our experience, doses ranging from 2.1 × 1011 to 2.5 × 1012 bacteria did not significantly affect clinical efficacy or the extent of microbiota engraftment (Staley et al., 2017a), nor did a prior bowel cleansing using polyethylene glycol (tested in four patients). However, several groups have also taken into account other medications that may interfere with microbiota engraftment, primarily proton pump inhibitors (PPIs) that may impair capsule opening (Kao et al., 2017). PPIs are commonly administered to lessen the symptoms of acid reflux and severe heartburn (Freston, 2004). Fortunately, multiple studies found no difference in clinical efficacy or engraftment among patients taking PPIs vs. patients not taking them (Youngster et al., 2014a; Staley et al., 2017a; Hong et al., 2020). The current weight of evidence suggests that, despite a number of potential confounding elements, oral administration of FMT is a relatively flexible and durable approach for microbiota restoration.
Clinical Applications of cFMT
The gut microbiota has been linked to various other diseases, including inflammatory bowel diseases (IBD), neurologic disorders, and obesity, and there has been much enthusiasm around using FMT to target potential dysbiosis that may contribute to these conditions. While the clinical efficacy, non-inferior outcomes (>90% cure rate), and advantageous delivery method of cFMT in treating rCDI have been well established, the use of FMT in treating non-rCDI diseases is still a burgeoning area of interest. A PubMed search of clinical trials investigating cFMT revealed seven published studies after excluding those focused on rCDI (Table 3). These were all small, pilot studies and included irritable bowel syndrome (IBS), small intestinal bacterial overgrowth (SIBO), obesity, and HIV. A search of all currently registered cFMT clinical trials on Clinicaltrials.gov yielded over 100 results where cFMT is being evaluated in the treatment of a wide range of pathologies including rCDI, obesity and metabolic syndrome, IBD, IBS, cancers, HIV, the gut–brain–microbiota axis (in conditions such as depression and autism spectrum disorder), atopy and allergies, non-alcoholic fatty liver disease, hypertension, and graft-vs.-host disease, among others (Table 4). The efficacy of FMT in treating these diseases is less straightforward than in cases of rCDI, owing, in part, to increased complexity of diseases including multifactorial etiologies and lack of clear, infectious targets. Further studies of cFMT in these contexts may help expound on the pathologies of these diseases while also improving patient outcomes.
Inflammatory Bowel Disease
Alterations in host microbiota are thought to contribute to the multifactorial pathogenesis of IBD. Studies have shown that patients with ulcerative colitis (UC) and Crohn’s disease (CD) have reduced microbial diversity and decreased abundances of predominant phyla, specifically Firmicutes and Bacteroidetes (Vaughn et al., 2016; Levy and Allegretti, 2019). It is hypothesized that dysbiosis contributes to intestinal inflammation resulting in aberrant host immune responses and that normalizing the microbiota of IBD patients using FMT may improve symptoms and induce remission. Overall, the clinical efficacy of FMT in IBD patients has demonstrated equivocal outcomes (Table 5).
There are five randomized clinical trials (RCTs) that investigated FMT in UC (Moayyedi et al., 2015; Rossen et al., 2015; Paramsothy et al., 2017a; Costello et al., 2019; Crothers et al., 2021). One small pilot RCT has been conducted evaluating FMT in CD (Sokol et al., 2020), but most data come from open-label cohort trials. In all IBD studies, FMT was delivered by enema, colonoscopy, or NDT; only one used cFMT, but this was in conjunction with an initial dose delivered by colonoscopy (Crothers et al., 2021). A meta-analysis found remission rates of 36% (201/555) in UC, 50.5% (42/83) in CD, and 21.5% (5/23) in pouchitis (Paramsothy et al., 2017b). When only RCTs in UC were analyzed, a significant benefit from FMT was found with odds ratio (OR) of 2.89 (p = 0.006). In subanalyses, greater rates of remission were associated with delivery via lower endoscopy and greater number of eFMT infusions received. Although no studies have been published yet regarding the efficacy of cFMT in IBD, there are currently 18 registered trials investigating this subject (Table 4). Evidence thus far suggests that FMT may be an efficacious treatment for UC. It is more difficult to determine its effect in CD and pouchitis given the lack of RCTs. More data are needed about FMT in the IBD population, in general, before any conclusions can be drawn about its role in future clinical practice.
Neurological and Psychiatric Disorders
The gut–brain–microbiome axis refers to the bidirectional communication of the central nervous system and the gut microbiota via metabolites, hormones, and immunomodulators (Martin et al., 2018). Perturbations in this circuit are thought to contribute to a host of diseases, such as obesity, IBS, anxiety, depression, Parkinson’s disease (PD), and autism spectrum disorders (ASD). Use of FMT to restore a more “normal” brain–gut axis and alleviate symptoms of neurologic and psychiatric disorders is under investigation.
Preliminary clinical studies in patients with ASD have found promising results related to improvement of GI symptoms after FMT. Adults and children with ASD often have GI symptoms, including constipation and/or diarrhea, abdominal pain, and indigestion concomitant with behavioral symptoms (social skill and communication deficits, irritability, hyperactivity, repetitive behaviors, etc.), and these have been found to correlate in severity (Kang et al., 2017). Children with ASD have altered gut microbiota compared to children without ASD, leading to the hypothesis that the dysbiotic gut microbiota, potentially due to increased antibiotic use in early childhood, leads to changes in GI function and alteration in metabolites produced by microbiota, impacting neurobiological pathways (Kang et al., 2017). In a non-randomized, open-label clinical trial performed on 18 children and adolescents with ASD who underwent a 10-week course of FMT delivered via rectal administration or an oral powder mixed with chocolate milk, GI symptoms were significantly reduced by 80%, and gradually over the course of the study period, ASD-related behaviors also significantly improved. These improvements were sustained over the follow-up period of 8 weeks (Kang et al., 2017). Engraftment, measured by UniFrac distance (Lozupone and Knight, 2005), increased community diversity, which was lower at baseline in ASD subjects compared to non-ASD controls, and increased abundances of Bifidobacterium and Prevotella were observed. In a 2-year follow-up study of this cohort of patients, GI symptoms remained significantly improved and ASD-related behaviors continued to improve after the end of the treatment period (Kang et al., 2019). Analysis of plasma and fecal metabolites demonstrated significant changes in plasma metabolites from baseline after FMT, including increased nicotinamide riboside and IMP and decreased caprylate and heptanoate, suggesting a potential biochemical cause for symptoms of ASD (Kang et al., 2020).
The contribution of the gut–brain–microbiome axis to psychiatric diseases is another area of active study, and the role of FMT in major depressive disorders and anxiety disorders is being explored. A recent review article identified eight clinical trials assessing the effect of FMT on depression and anxiety symptoms (Chinna Meyyappan et al., 2020). While three of these studies were case reports and six primarily examined other disorders (i.e., IBS) with depression or anxiety symptom relief as secondary outcomes, these trials demonstrated significant improvements in short-term depression and/or anxiety, but variable long-term results (Chinna Meyyappan et al., 2020). Further study is warranted to clarify the role of FMT in psychiatric illnesses given the high prevalence of these diseases, stigmatism and morbidity associated with them, and need for effective treatment options.
Parkinson’s disease is a neurodegenerative disorder characterized by motor (tremor, bradykinesia, rigidity, shuffling gait) and non-motor symptoms, in particular constipation, in up to 80% of cases. Dysbiosis leading to alterations of the gut–brain axis has been implicated in the development of PD symptoms, and recent preliminary clinical studies have demonstrated that FMT can ameliorate constipation and improve motor symptoms (Huang et al., 2019; Xue et al., 2020; Kuai et al., 2021).
While there are no published cFMT trials in the context of neurologic and psychiatric diseases, a number are ongoing (Table 4). An encapsulated formulation is pertinent to the needs of this specific population of patients for whom invasive medical therapies may be especially taxing due to behavioral or mobility issues, frailty, anxiety, and agoraphobia.
Obesity/Metabolic Syndrome
The basis of obesity, thought to be due to a complex interplay of environmental and genetic factors, is still not fully understood. Obesity is increasingly common and has been linked to disease states associated with the metabolic syndrome including type II diabetes and non-alcoholic fatty liver disease. Abnormalities in the gut microbiota have been linked to obesity: lower baseline species alpha diversity is often observed in obese individuals as well has a higher ratio of Firmicutes to Bacteroidetes compared to lean individuals (Napolitano and Covasa, 2020). Obesity-associated microbiota may contribute to increased caloric absorption and energy production, chronic inflammation and immune responses leading to insulin resistance, and dysregulated fatty acid metabolism (Napolitano and Covasa, 2020). While FMT studies conducted in obese and lean mice have robustly demonstrated the transferability of the obese phenotype, definitive clinical results have not been borne out in human trials. In a 2012 study, FMT delivered by NDT from lean donors to obese males with impaired fasting glucose resulted in significantly improved peripheral insulin sensitivity compared to obese recipients of autologous FMT (Vrieze et al., 2012). However, there was no change in weight, body mass index (BMI), glycated hemoglobin, resting energy expenditure, or glucoregulatory hormones between recipients of lean donor FMT and controls. A subsequent follow-up study in obese males who received either lean donor or autologous FMT through NDT infusion revealed significant improvement in peripheral insulin sensitivity among lean-donor recipients and marginal improvements in glycated hemoglobin at 6 weeks post-FMT (Kootte et al., 2017). Again, there were no changes in weight, BMI, resting energy expenditure, or enteroendocrine hormones between the two groups. Obese individuals who received lean-donor FMT had a significant increase in the fecal short-chain fatty acid (SCFA) acetate, which is inversely correlated with insulin resistance. Additionally, lean-donor recipients demonstrated an increase in the acetate-producing species Bifidobacterium pseudolongum within the duodenum, pointing to the potential role of microbiota-mediated SCFA production on insulin sensitivity in patients with metabolic syndrome. An increase in plasma amino acid γ-aminobutyric acid, a metabolite that promotes insulin sensitivity in rodent models, was also noted among recipients of lean-donor FMT. Lastly, the authors of this study noted that lower baseline fecal microbiota diversity predicted clinical response to FMT treatment.
Four trials investigating obesity and metabolic parameters using cFMT have been published to date (Table 3). Two recent studies showed no significant improvements in BMI or metabolic parameters after intervention (Allegretti et al., 2020; Yu et al., 2020). One study, which examined maintenance of weight loss after autologous cFMT within patients who underwent different dietary interventions, showed mixed results with significantly decreased weight regain after autologous cFMT in only one of the dietary intervention groups (Rinott et al., 2021). The most recent study (Wilson et al., 2021) examined engraftment after cFMT in obese adolescents but did not interrogate clinical or biochemical metabolic parameters. Although there was no clinical improvement in these trials, there was evidence of microbiota engraftment in all four studies. It is likely that the lack of clinical improvement in obesity and metabolic syndrome after cFMT is not due to a failure of the capsule technology itself, but rather that the efficacy of FMT in general is less well-established in obesity, as demonstrated in the equivocal results of the human FMT trials (Vrieze et al., 2012; Kootte et al., 2017). While dysbiosis exacerbates the development of obesity through various established pathways (i.e., microbial energy harvest, SCFA production, inflammation), obesity is a multifactorial disease; more data are needed to determine who will most likely benefit from FMT and what aspects of obesity-associated metabolic syndrome can be improved by manipulation of the microbiota.
Other Conditions
cFMT is under investigation in a multitude of other non-rCDI diseases, including IBS, drug-resistant organisms, hematologic disorders, graft-versus-host disease, malignancies, and allergy (Table 3). Only a few studies have been published at this point. One completed study investigating cFMT in IBS (Halkjær et al., 2018) showed no improvement in clinical parameters; however, it did demonstrate microbiota engraftment. Another published study investigated HIV patients (Serrano-Villar et al., 2021) and found that cFMT did not lead to improved CD4 counts or inflammatory markers but did resolve dysbiosis typically associated with HIV and led to a decrease in biomarkers of intestinal injury. Many of the cFMT studies in non-rCDI diseases were small-scale, pilot studies limited in their statistical power (Table 3). In the future, RCTs with larger cohorts are needed to better evaluate the efficacy of cFMT in these various disease states. Another challenge that needs to be simultaneously addressed is to optimize and standardize capsule formulation and dose for more rigorous inter-study evaluations.
Pharmacokinetics of cFMT
The pharmacology of FMT, an active biological community, is an emerging discipline with distinct principles from that of conventional drugs. The pharmacology of FMT must be understood in a framework of microbial ecology that considers the complex and dynamic interactions of gut microbiota with host factors such as diet, medications, and lifestyle, and relationships with other microorganisms (Khoruts et al., 2021). The standard concepts of pharmacokinetics (absorption, distribution, metabolism, and excretion) are poorly applicable to FMT therapies given that the “drug” is a metabolically active, complex consortium. Thus, FMT pharmacokinetics could be thought of largely in terms of microbiota engraftment. As previously discussed, engraftment is affected by many steps along the way in cFMT product formulation, from donor selection to the choice of preservation method to encapsulation strategy. In this section, rather than focusing on traditional pharmacological principles [reviewed recently (Khoruts et al., 2021)], we will discuss the pharmacokinetics of FMT as the kinetics of microbiota engraftment.
The assessment of microbial engraftment is an often overlooked or oversimplified metric in studies of FMT. Early studies relied on detection of taxonomic units, often as broadly as phyla, in post-FMT patient samples that were also present in the donor sample (Hamilton et al., 2013; van Nood et al., 2013; Seekatz et al., 2014; Kelly et al., 2016). Engraftment was established as a return of alpha diversity (richness and evenness) and a taxonomic distribution of bacteria dominated by members of the Firmicutes and Bacteroidetes phyla. However, high-resolution taxonomic compositions (i.e., species and strains) were not considered, partially due to limitations of the 16S rRNA gene amplicon sequencing read length, as well as potential non-detects based on limits of detection. Methods were refined to assess correlations between donor and recipient fecal communities (Weingarden et al., 2015; Jalanka et al., 2016), which provided a statistical evaluation of engraftment while still suffering from similar technical limitations. More recently, our group and others have utilized Bayesian approaches to infer levels of engraftment and invasion by specific taxa following FMT or animal cohousing (Ridaura et al., 2013; Khanna et al., 2017; Staley et al., 2017b; Le Bastard et al., 2018; Sokol et al., 2020; Haifer et al., 2021). Packages like SourceTracker (Knights et al., 2011) that employ this approach have been shown to be able to differentiate individual donor samples (Staley et al., 2018), determine taxa associated with engraftment (Ridaura et al., 2013; Staley et al., 2017b), and may be applied to emerging metagenomics datasets for use with high resolution taxonomic data. Below, we will discuss the current state of encapsulated microbial engraftment kinetics assessed using robust computational methods for quantitative assessment, with a focus on human studies of rCDI and clinical response to therapy.
Kinetics of Engraftment in cFMT vs. eFMT
One of the striking differences in microbial engraftment between cFMT and more traditional eFMT is a delay in engraftment following cFMT relative to eFMT, despite similar clinical efficacy (Staley et al., 2017b; Jiang et al., 2018). Administration of frozen donor fecal material via colonoscopy has been shown to result in donor-like normalization of the patient microbiota within 1 week following administration by either colonoscopy or NDT (Hamilton et al., 2013; van Nood et al., 2013; Weingarden et al., 2015; Jalanka et al., 2016), and complete engraftment may occur within the first 48 h following FMT (Weingarden et al., 2015; Jiang et al., 2018). In contrast, cFMT using lyophilized preparations of donor microbiota resulted in slower, punctuated engraftment, and engraftment levels similar to those seen with eFMT were not observed until 2–4 weeks following administration (Staley et al., 2017b; Jiang et al., 2018). This delay was reflected in slower expansion of the Bacteroidetes, which corresponded with a shift from predominantly primary to secondary fecal bile acids (Staley et al., 2017b), and may reflect a need for a greater concentration of bacteria using this method. To address the incongruence between clinical efficacy and engraftment kinetics following cFMT, we investigated whether early signatures of engraftment were predictive of clinical outcomes and found that relative abundances of members of predominant families Bacteroidaceae, Ruminococcaceae, and Lachnospiraceae were highly predictive of clinical response (Staley et al., 2018), suggesting that methods to improve engraftment and expansion of these groups may be a promising clinical target.
The reasons for the delay in engraftment between cFMT and eFMT have become an active area of research with primary foci on improving microbiota formulation and determining an optimal method for delivery. Notably, in a multicenter, unblinded, RCT, differences in microbiota engraftment, determined by qualitative taxonomic comparison, did not appear to be prominent when frozen microbiota were delivered by colonoscopy or capsule (Kao et al., 2017). These results suggest that a feature of the lyophilization process may impair the early resuscitation of certain members of the microbial community, e.g., members of phylum Bacteroidetes. However, a recent study by our group using identical preparations of lyophilized microbiota delivered colonoscopically or orally indicated that the route of administration was also a significant variable, with greater levels of donor similarity observed following the former delivery method within the first 2 weeks (Staley et al., 2021). Given discrepancies in capsule preparation and data analysis, definitive conclusions regarding the reasons underlying delayed engraftment of lyophilized microbiota following cFMT remain to be determined. Nevertheless, results of current studies suggest a definite possibility to improve formulation methods to overcome limitations caused by both lyophilization and oral delivery.
Durability of Engraftment Is Comparable in cFMT
The reconstitution of the donor microbiota following colonoscopic FMT has been investigated in a small subset of studies and was reported as generally stable over the period of 1 year (Weingarden et al., 2015; Jalanka et al., 2016). While fluctuations in the microbiota composition were observed, these were similar to those observed in healthy donors (Weingarden et al., 2015). However, even in cases of eFMT, donor engraftment is not always immediate, with one patient showing increased relative abundances of Firmicutes for 7 months following treatment before microbiota composition began to resemble that of the donor up to 4.5 years post-FMT, despite durable therapeutic success (Broecker et al., 2016). Following cFMT using lyophilized microbiota, donor engraftment of bacteria was recently reported to remain high for 6 months following FMT, although no evidence of fungal engraftment was observed (Haifer et al., 2021). Similarly, work by our group indicated that cFMT responders who received lyophilized microbiota maintained similarity to their donor for up to 1 year and patients receiving different cFMTs from differing donor lots could be significantly differentiated (Staley et al., 2019). We also noted three predominant patterns in engraftment—among 18 patients who responded to cFMT, 61% showed high (>50% donor similarity) and sustained engraftment, 22% showed high engraftment during the first month that later declined, and 17% showed very slow engraftment, reaching a maximum of <40% similarity by 1 year. We noted that abundances of Bacteroides and Parabacteroides were correlated with engraftment rate but typically reflected engraftment of only 2–4 strains, determined by oligotype analysis (Staley et al., 2019). Long-term engraftment following cFMT using frozen microbiota remains to be investigated, but early evidence suggests that cFMT results in durable long-term engraftment, similar to eFMT, in the absence of antibiotic and other provocations in the majority of patients.
Discussion
Encapsulated FMT is becoming a mainstream therapeutic option to treat rCDI, with applications to a variety of other conditions in which FMT has been recently explored or thought to be of benefit (Sadowsky and Khoruts, 2016). While there is still a paucity of data reflecting the use of cFMT in conditions other than rCDI, the ease of administration and potential for storage outside of ultra-low-temperature conditions make it a promising avenue to expand the therapeutic reach of FMT. Similar to existing concerns regarding the standardization of FMT materials (Cammarota et al., 2019), methods of capsule preparation remain highly variable among research groups, reflective predominantly of practical concerns. Systematic investigations of parameters such as the role of cyro-protectants, capsule coating, and mechanical features to accommodate gastric transit and control capsule opening are necessary to determine critical aspects of capsule formulation to improve clinical efficacy and standardize production. In addition to efficacy, understanding and optimizing microbiota engraftment and expansion of critical taxa will be necessary, especially in the majority of conditions in which antibiotic exposure is not a first-line treatment. Abiotic parameters such as metabolite (e.g., short-chain fatty acids or bile acids) concentrations may also contribute to the success and kinetics of bacterial engraftment (Staley et al., 2017b), in addition to physical parameters of the capsule, and represent further areas of study to improve encapsulate microbiota therapeutics.
Early experience with cFMT in rCDI has highlighted a paucity of data regarding the role of microbial ecology in the clinical success of FMT. In relation to standard pharmacology, the application of a metabolically active, complex consortium as a therapeutic raises similar concerns about off-target effects related to the interaction of the microbiota with other drugs, diet, and probiotics (Khoruts et al., 2021). As the use of FMT is expanded as a potential treatment in other conditions, evaluating these interactions will be increasingly important both to improve clinical efficacy and over deleterious off-target effects. Donor screening is now increasingly considered with a hypothesis that a specific microbiota consortium may prove more beneficial in correcting dysbiosis underlying a specific disease; e.g., bacterial sulfur metabolism related to IBD (Bryant et al., 2021). In addition, understanding the competitive dynamics associated with engraftment and invasion will be necessary to optimize formulation, dosing strategies, and adjunctive therapies to improve outcomes following FMT.
Author Contributions
HH and SB drafted the manuscript. CS provided the critical commentary and feedback. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allegretti, J. R., Kassam, Z., Mullish, B. H., Chiang, A., Carrellas, M., Hurtado, J., et al. (2020). Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. 18, 855–863.e2. doi: 10.1016/j.cgh.2019.07.006
Bibbò, S., Settanni, C. R., Porcari, S., Bocchino, E., Ianiro, G., Cammarota, G., et al. (2020). Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J. Clin. Med. 9, 1757. doi: 10.3390/jcm9061757
Borody, T. J., Brandt, L. J., Paramsothy, S., Agrawal, G. (2013). Fecal Microbiota Transplantation: A New Standard Treatment Option for Clostridium Difficile Infection. Expert Rev. Anti Infect. Ther. 11, 447–449. doi: 10.1586/eri.13.26
Brandl, K., Plitas, G., Schnabl, B., DeMatteo, R. P., Pamer, E. G. (2007). MyD88-Mediated Signals Induce the Bactericidal Lectin RegIII Gamma and Protect Mice Against Intestinal Listeria Monocytogenes Infection. J. Exp. Med. 204, 1891–1900. doi: 10.1084/JEM.20070563
Broecker, F., Klumpp, J., Schuppler, M., Russo, G., Biedermann, L., Hombach, M., et al. (2016). Long-Term Changes of Bacterial and Viral Compositions in the Intestine of a Recovered Clostridium Difficile Patient After Fecal Microbiota Transplantation. Cold Spring Harb Mol. Case Stud. a000448. doi: 10.1101/mcs.a000448
Bryant, R. V., Day, A. S., McGrath, K. C., Telfer, K., Yao, C. K., Costello, S. P. (2021). Fecal Microbiota Transplantation Augmented by a Sulfide-Reducing Diet for Refractory Ulcerative Colitis: A Case Report With Functional Metagenomic Analysis. JGH Open 5, 1099–1102. doi: 10.1002/JGH3.12623
Buckley, A. M., Moura, I. B., Arai, N., Spittal, W., Clark, E., Nishida, Y., et al. (2021). Trehalose-Induced Remodelling of the Human Microbiota Affects Clostridioides Difficile Infection Outcome in an In Vitro Colonic Model: A Pilot Study. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/FCIMB.2021.670935/FULL
Cammarota, G., Ianiro, G., Kelly, C. R., Mullish, B. H., Allegretti, J. R., Kassam, Z., et al. (2019). International Consensus Conference on Stool Banking for Faecal Microbiota Transplantation in Clinical Practice. Gut 68, 2111–2121. doi: 10.1136/GUTJNL-2019-319548
Chinna Meyyappan, A., Forth, E., Wallace, C. J. K., Milev, R. (2020). Effect of Fecal Microbiota Transplant on Symptoms of Psychiatric Disorders: A Systematic Review. BMC Psychiatry 20. doi: 10.1186/s12888-020-02654-5
Collins, J., Robinson, C., Danhof, H., Knetsch, C. W., Van Leeuwen, H. C., Lawley, T. D., et al. (2018). Dietary Trehalose Enhances Virulence of Epidemic Clostridium Difficile. Nature 553, 291–294. doi: 10.1038/nature25178
Costello, S. P., Hughes, P. A., Waters, O., Bryant, R. V., Vincent, A. D., Blatchford, P., et al. (2019). Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA - J. Am. Med. Assoc. (Am Med. Assoc) 156–164. doi: 10.1001/jama.2018.20046
Coyne, M. J., Béchon, N., Matano, L. M., McEneany, V. L., Chatzidaki-Livanis, M., Comstock, L. E. (2019). A Family of Anti-Bacteroidales Peptide Toxins Wide-Spread in the Human Gut Microbiota. Nat. Commun. 10. doi: 10.1038/S41467-019-11494-1
Crothers, J. W., Chu, N. D., Nguyen, L. T. T., Phillips, M., Collins, C., Fortner, K., et al. (2021). Daily, Oral FMT for Long-Term Maintenance Therapy in Ulcerative Colitis: Results of a Single-Center, Prospective, Randomized Pilot Study. BMC Gastroenterol. 21. doi: 10.1186/s12876-021-01856-9
Cui, B., Feng, Q., Wang, H., Wang, M., Peng, Z., Li, P., et al. (2015). Fecal Microbiota Transplantation Through Mid-Gut for Refractory Crohn’s Disease: Safety, Feasibility, and Efficacy Trial Results. J. Gastroenterol. Hepatol. 30, 51–58. doi: 10.1111/jgh.12727
DeFilipp, Z., Bloom, P. P., Torres Soto, M., Mansour, M. K., Sater, M. R. A., Huntley, M. H., et al. (2019). Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 381, 2043–2050. doi: 10.1056/NEJMOA1910437/SUPPL_FILE/NEJMOA1910437_DISCLOSURES.PDF
Dethlefsen, L., Huse, S., Sogin, M. L., Relman, D. A. (2008). The Pervasive Effects of an Antibiotic on the Human Gut Microbiota, as Revealed by Deep 16S rRNA Sequencing. PloS Biol. 6, e280. doi: 10.1371/journal.pbio.0060280
Dethlefsen, L., Relman, D. A. (2011). Incomplete Recovery and Individualized Responses of the Human Distal Gut Microbiota to Repeated Antibiotic Perturbation. Proc. Natl. Acad. Sci. U. S. A. 108, 4554–4561. doi: 10.1073/pnas.1000087107
Drekonja, D., Reich, J., Gezahegn, S., Greer, N., Shaukat, A., MacDonald, R., et al. (2015). Fecal Microbiota Transplantation for Clostridium Difficile Infection a Systematic Review. Ann. Intern. Med. 162, 630–638. doi: 10.7326/M14-2693
Feuerstadt, P., Louie, T. J., Lashner, B., Wang, E. E. L., Diao, L., Bryant, J. A., et al. (2022). SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides Difficile Infection. N. Engl. J. Med. 386, 220–229. doi: 10.1056/NEJMOA2106516/SUPPL_FILE/NEJMOA2106516_DATA-SHARING.PDF
Fischbach, M. A., Walsh, C. T. (2009). Antibiotics for Emerging Pathogens. Science 325, 1089–1093. doi: 10.1126/science.1176667
Freston, J. W. (2004). Therapeutic Choices in Reflux Disease: Defining the Criteria for Selecting a Proton Pump Inhibitor. Am. J. Med. 117. doi: 10.1016/j.amjmed.2004.07.020
Goyal, A., Yeh, A., Bush, B. R., Firek, B. A., Siebold, L. M., Rogers, M. B., et al. (2018). Safety, Clinical Response, and Microbiome Findings Following Fecal Microbiota Transplant in Children With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 24, 410–421. doi: 10.1093/ibd/izx035
Gutin, L., Piceno, Y., Fadrosh, D., Lynch, K., Zydek, M., Kassam, Z., et al. (2019). Fecal Microbiota Transplant for Crohn Disease: A Study Evaluating Safety, Efficacy, and Microbiome Profile. United Eur. Gastroenterol. J. 7, 807–814. doi: 10.1177/2050640619845986
Haifer, C., Paramsothy, S., Borody, T. J., Clancy, A., Leong, R. W., Kaakoush, N. O. (2021). Long-Term Bacterial and Fungal Dynamics Following Oral Lyophilized Fecal Microbiota Transplantation in Clostridioides Difficile Infection. mSystems 6, e00905–e00920. doi: 10.1128/MSYSTEMS.00905-20/SUPPL_FILE/MSYSTEMS.00905-20-SF009.TIF
Halkjær, S. I., Christensen, A. H., Lo, B. Z. S., Browne, P. D., Günther, S., Hansen, L. H., et al. (2018). Faecal Microbiota Transplantation Alters Gut Microbiota in Patients With Irritable Bowel Syndrome: Results From a Randomised, Double-Blind Placebo-Controlled Study. Gut 67, 2107–2115. doi: 10.1136/gutjnl-2018-316434
Hamilton, M. J., Weingarden, A. R., Sadowsky, M. J., Khoruts, A. (2012). Standardized Frozen Preparation for Transplantation of Fecal Microbiota for Recurrent Clostridium Difficile Infection. Am. J. Gastroenterol. 107, 761–767. doi: 10.1038/ajg.2011.482
Hamilton, M. J., Weingarden, A. R., Unno, T., Khoruts, A., Sadowsky, M. J. (2013). High-Throughput DNA Sequence Analysis Reveals Stable Engraftment of Gut Microbiota Following Transplantation of Previously Frozen Fecal Bacteria. Gut Microbes 4, 125–135. doi: 10.4161/gmic.23571
Hong, A. S., Yu, W. Y., Hong, J. M., Cross, C. L., Azab, M., Ohning, G., et al. (2020). Proton Pump Inhibitor in Upper Gastrointestinal Fecal Microbiota Transplant: A Systematic Review and Analysis. J. Gastroenterol. Hepatol. 35, 932–940. doi: 10.1111/jgh.14958
Huang, H., Xu, H., Luo, Q., He, J., Li, M., Chen, H., et al. (2019). Fecal Microbiota Transplantation to Treat Parkinson’s Disease With Constipation: A Case Report. Med. (United States) 98. doi: 10.1097/MD.0000000000016163
Hui, W., Li, T., Liu, W., Zhou, C., Gao, F. (2019). Fecal Microbiota Transplantation for Treatment of Recurrent C. Difficile Infection: An Updated Randomized Controlled Trial Meta-Analysis. PloS One 14. doi: 10.1371/journal.pone.0210016
Jalanka, J., Mattila, E., Jouhten, H., Hartman, J., de Vos, W. M., Arkkila, P., et al. (2016). Long-Term Effects on Luminal and Mucosal Microbiota and Commonly Acquired Taxa in Faecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection. BMC Med. 14, 155. doi: 10.1186/s12916-016-0698-z
Jiang, Z.-D., Jenq, R. R., Ajami, N. J., Petrosino, J. F., Alexander, A. A., Ke, S., et al. (2018). Safety and Preliminary Efficacy of Orally Administered Lyophilized Fecal Microbiota Product Compared With Frozen Product Given by Enema for Recurrent Clostridium Difficile Infection: A Randomized Clinical Trial. PloS One 13, e0205064. doi: 10.1371/journal.pone.0205064
Kang, D. W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., McDonough-Means, S., et al. (2019). Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 9. doi: 10.1038/s41598-019-42183-0
Kang, D.-W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., et al. (2017). Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 5, 10. doi: 10.1186/s40168-016-0225-7
Kang, D.-W., Adams, J. B., Vargason, T., Santiago, M., Hahn, J., Krajmalnik-Brown, R. (2020). Distinct Fecal and Plasma Metabolites in Children With Autism Spectrum Disorders and Their Modulation After Microbiota Transfer Therapy. mSphere 5. doi: 10.1128/msphere.00314-20
Kao, D., Roach, B., Silva, M., Beck, P., Rioux, K., Kaplan, G. G., et al. (2017). Effect of Oral Capsule– vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium Difficile Infection: A Randomized Clinical Trial. JAMA 318, 1985–1993. doi: 10.1001/jama.2017.17077
Kelly, C., Khoruts, A., Staley, C., Sadowsky, M., Abd, M., Alani, M., et al. (2016). Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium Difficile Infection: A Randomized Trial. Ann. Intern. Med. 165, 609–616. doi: 10.7326/M16-0271
Khanna, S., Vazquez-Baeza, Y., González, A., Weiss, S., Schmidt, B., Muñiz-Pedrogo, D. A., et al. (2017). Changes in Microbial Ecology After Fecal Microbiota Transplantation for Recurrent C. Difficile Infection Affected by Underlying Inflammatory Bowel Disease. Microbiome 5, 55. doi: 10.1186/S40168-017-0269-3/FIGURES/2
Khoruts, A., Sadowsky, M. J. (2016). Understanding the Mechanisms of Faecal Microbiota Transplantation. Nat. Rev. Gastroenterol. Hepatol. 13, 508–516. doi: 10.1038/nrgastro.2016.98
Khoruts, A., Staley, C., Sadowsky, M. J. (2021). Faecal Microbiota Transplantation for Clostridioides Difficile: Mechanisms and Pharmacology. Nat. Rev. Gastroenterol. Hepatol. 18, 67–80. doi: 10.1038/s41575-020-0350-4
Knights, D., Kuczynski, J., Charlson, E. S., Zaneveld, J., Mozer, M. C., Collman, R. G., et al. (2011). Bayesian Community-Wide Culture-Independent Microbial Source Tracking. Nat. Methods 8, 761–U107. doi: 10.1038/nmeth.1650
Kootte, R. S., Levin, E., Salojärvi, J., Smits, L. P., Hartstra, A. V., Udayappan, S. D., et al. (2017). Improvement of Insulin Sensitivity After Lean Donor Feces in Metabolic Syndrome is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 26, 611–619.e6. doi: 10.1016/j.cmet.2017.09.008
Kuai, X., Yao, X., Xu, L., Zhou, Y., Zhang, L., Liu, Y., et al. (2021). Evaluation of Fecal Microbiota Transplantation in Parkinson’s Disease Patients With Constipation. Microb. Cell Fact 20, 98. doi: 10.1186/s12934-021-01589-0
Le Bastard, Q., Ward, T., Sidiropoulos, D., Hillmann, B. M., Chun, C. L., Sadowsky, M. J., et al. (2018). Fecal Microbiota Transplantation Reverses Antibiotic and Chemotherapy-Induced Gut Dysbiosis in Mice. Sci. Rep. 8, 6219. doi: 10.1038/s41598-018-24342-x
Lee, C. H., Steiner, T., Petrof, E. O., Smieja, M., Roscoe, D., Nematallah, A., et al. (2016). Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium Difficile Infection: A Randomized Clinical Trial. JAMA 315, 142–149. doi: 10.1001/jama.2015.18098
Levy, A. N., Allegretti, J. R. (2019). Insights Into the Role of Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Therap Adv. Gastroenterol. 12, 1756284819836893. doi: 10.1177/1756284819836893
Lloyd-Price, J., Mahurkar, A., Rahnavard, G., Crabtree, J., Orvis, J., Hall, A. B., et al. (2017). Strains, Functions and Dynamics in the Expanded Human Microbiome Project. Nature 550, 61–66. doi: 10.1038/nature23889
Lozupone, C., Knight, R. (2005). UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 71, 8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., Knight, R. (2012). Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 489, 220–230. doi: 10.1038/nature11550
Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D. A., Hirschfield, G. M., Hold, G., et al. (2016). The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 65, 330–339. doi: 10.1136/gutjnl-2015-309990
Martin, C. R., Osadchiy, V., Kalani, A., Mayer, E. A. (2018). The Brain-Gut-Microbiome Axis. CMGH 6, 133–148. doi: 10.1016/j.jcmgh.2018.04.003
McGovern, B. H., Ford, C. B., Henn, M. R., Pardi, D. S., Khanna, S., Hohmann, E. L., et al. (2021). SER-109, an Investigational Microbiome Drug to Reduce Recurrence After Clostridioides Difficile Infection: Lessons Learned From a Phase 2 Trial. Clin. Infect. Dis. 72, 2132–2140. doi: 10.1093/CID/CIAA387
Moayyedi, P., Surette, M. G., Kim, P. T., Libertucci, J., Wolfe, M., Onischi, C., et al. (2015). Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 149, 102–109. doi: 10.1053/j.gastro.2015.04.001
Modi, S. R., Collins, J. J., Relman, D. A. (2014). Antibiotics and the Gut Microbiota. J. Clin. Invest 124, 4212–4218. doi: 10.1172/JCI72333
Nagao-Kitamoto, H., Leslie, J. L., Kitamoto, S., Jin, C., Thomsson, K. A., Gillilland, M. G., et al. (2020). Interleukin-22-Mediated Host Glycosylation Prevents Clostridioides Difficile Infection by Modulating the Metabolic Activity of the Gut Microbiota. Nat. Med. 26, 608–617. doi: 10.1038/S41591-020-0764-0
Napolitano, M., Covasa, M. (2020). Microbiota Transplant in the Treatment of Obesity and Diabetes: Current and Future Perspectives. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.590370
Paramsothy, S., Kamm, M. A., Kaakoush, N. O., Walsh, A. J., van den Bogaerde, J., Samuel, D., et al. (2017a). Multidonor Intensive Faecal Microbiota Transplantation for Active Ulcerative Colitis: A Randomised Placebo-Controlled Trial. Lancet 6736, 1–11. doi: 10.1016/S0140-6736(17)30182-4
Paramsothy, S., Paramsothy, R., Rubin, D. T., Kamm, M. A., Kaakoush, N. O., Mitchell, H. M., et al. (2017b). Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 11, 1180–1199. doi: 10.1093/ecco-jcc/jjx063
Postigo, R., Kim, J. H. (2012). Colonoscopic Versus Nasogastric Fecal Transplantation for the Treatment of Clostridium Difficile Infection: A Review and Pooled Analysis. Infection 40, 643–648. doi: 10.1007/s15010-012-0307-9
Ramai, D., Zakhia, K., Fields, P. J., Ofosu, A., Patel, G., Shahnazarian, V., et al. (2020). Fecal Microbiota Transplantation (FMT) With Colonoscopy is Superior to Enema and Nasogastric Tube While Comparable to Capsule for the Treatment of Recurrent Clostridioides Difficile Infection: A Systematic Review and Meta-Analysis. Dig Dis. Sci. 66, 369–380. doi: 10.1007/s10620-020-06185-7
Ramai, D., Zakhia, K., Ofosu, A., Ofori, E., Reddy, M. (2019). Fecal Microbiota Transplantation: Donor Relation, Fresh or Frozen, Delivery Methods, Cost-Effectiveness. Ann. Gastroenterol. 32, 30. doi: 10.20524/AOG.2018.0328
Ridaura, V. K., Faith, J. J., Rey, F. E., Cheng, J., Duncan, A. E., Kau, A. L., et al. (2013). Gut Microbiota From Twins Discordant for Obesity Modulate Metabolism in Mice. Science 341, 1241214. doi: 10.1126/science.1241214
Rinott, E., Youngster, I., Yaskolka Meir, A., Tsaban, G., Zelicha, H., Kaplan, A., et al. (2021). Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 160, 158–173.e10. doi: 10.1053/j.gastro.2020.08.041
Rossen, N. G., Fuentes, S., van der Spek, M. J., Tijssen, J. G., Hartman, J. H. A., Duflou, A., et al. (2015). Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 149, 110–118.e4. doi: 10.1053/j.gastro.2015.03.045
Sadowsky, M. J., Khoruts, A. (2016). Faecal Microbiota Transplantation is Promising But Not a Panacea. Nat. Microbiol. 1, 16015. doi: 10.1038/nmicrobiol.2016.15
Saund, K., Rao, K., Young, V. B., Snitkin, E. S. (2020). Genetic Determinants of Trehalose Utilization are Not Associated With Severe Clostridium Difficile Infection Outcome. Open Forum Infect. Dis. 7, ofz548. doi: 10.1093/OFID/OFZ548
Seekatz, A. M., Aas, J., Gessert, C. E. (2014). Recovery of the Gut Microbiome Following Fecal Microbiota. MBio 5, 1–9. doi: 10.1128/mBio.00893-14.Editor
Serrano-Villar, S., Talavera-Rodríguez, A., Gosalbes, M. J., Madrid, N., Pérez-Molina, J. A., Elliott, R. J., et al. (2021). Fecal Microbiota Transplantation in HIV: A Pilot Placebo-Controlled Study. Nat. Commun. 12, 1139. doi: 10.1038/s41467-021-21472-1
Shreiner, A. B., Kao, J. Y., Young, V. B. (2015). The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 31, 69–75. doi: 10.1097/MOG.0000000000000139
Sokol, H., Landman, C., Seksik, P., Berard, L., Montil, M., Nion-Larmurier, I., et al. (2020). Fecal Microbiota Transplantation to Maintain Remission in Crohn’s Disease: A Pilot Randomized Controlled Study. Microbiome 8, 12. doi: 10.1186/s40168-020-0792-5
Staley, C., Halaweish, H., Graiziger, C., Hamilton, M., Kabage, A., Galdys, A., et al. (2021). Lower Endoscopic Delivery of Freeze-Dried Intestinal Microbiota Results in More Rapid and Efficient Engraftment Than Oral Administration. Sci. Rep. 11, 4519. doi: 10.1038/S41598-021-84152-6
Staley, C., Hamilton, M. J., Vaughn, B. P., Graiziger, C. T., Newman, K. M., Kabage, A. J., et al. (2017a). Successful Resolution of Recurrent Clostridium Difficile Infection Using Freeze-Dried, Encapsulated Microbiota; Pragmatic Cohort Study. Am. J. Gastroenterol. 112, 940–947. doi: 10.1038/ajg.2017.6
Staley, C., Kaiser, T., Vaugh, B. P., Graiziger, C. T., Hamilton, M. J., Khoruts, A., et al. (2018). Predicting Recurrence of Clostridium Difficile Infection Following Encapsulated Fecal Microbiota Transplantation. Microbiome 6, 166. doi: 10.1186/s40168-018-0549-6
Staley, C., Kaiser, T., Vaughn, B. P., Graiziger, C., Hamilton, M. J., Kabage, A. J., et al. (2019). Durable Long-Term Bacterial Engraftment Following Encapsulated Fecal Microbiota Transplantation to Treat Clostridium Difficile Infection. MBio 10, e01586–e01519. doi: 10.1128/mBio.01586-19
Staley, C., Vaughn, B. P., Graiziger, C. T., Singroy, S., Hamilton, M. J., Yao, D., et al. (2017b). Community Dynamics Drive Punctuated Engraftment of the Fecal Microbiome Following Transplantation Using Freeze-Dried, Encapsulated Fecal Microbiota. Gut Microbes 8, 276–288. doi: 10.1080/19490976.2017.1299310
Stripling, J., Rodriguez, M. (2018). Current Evidence in Delivery and Therapeutic Uses of Fecal Nicrobiota Transplantation in Human Diseases —Clostridium Difficile Disease and Beyond. Am. J. Med. Sci. 356, 424–432. doi: 10.1016/J.AMJMS.2018.08.010
Suskind, D. L., Brittnacher, M. J., Wahbeh, G., Shaffer, M. L., Hayden, H. S., Qin, X., et al. (2015). Fecal Microbial Transplant Effect on Clinical Outcomes and Fecal Microbiome in Active Crohn’s Disease. Inflamm. Bowel Dis. 21, 556–563. doi: 10.1097/MIB.0000000000000307
van Nood, E., Vrieze, A., Nieuwdorp, M., Fuentes, S., Zoetendal, E. G., de Vos, W. M., et al. (2013). Duodenal Infusion of Donor Feces for Recurrent Clostridium Difficile. N. Engl. J. Med. 368, 407–415. doi: 10.1056/NEJMoa1205037
Varga, A., Kocsis, B., Sipos, D., Kása, P., Vigvári, S., Pál, S., et al. (2021). How to Apply FMT More Effectively, Conveniently and Flexible – A Comparison of FMT Methods. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.657320
Vaughn, B. P., Vatanen, T., Allegretti, J. R., Bai, A., Xavier, R. J., Korzenik, J., et al. (2016). Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn’s Disease. Inflamm. Bowel Dis. 22, 2182–2190. doi: 10.1097/MIB.0000000000000893
Vermeire, S., Joossens, M., Verbeke, K., Wang, J., Machiels, K., Sabino, J., et al. (2016). Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J. Crohn’s Colitis 10, 387–394. doi: 10.1093/ecco-jcc/jjv203
Vrieze, A., Van Nood, E., Holleman, F., Salojärvi, J., Kootte, R. S., Bartelsman, J. F. W. M., et al. (2012). Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 143, 913–6.e7. doi: 10.1053/j.gastro.2012.06.031
Wang, S., Xu, M., Wang, W., Cao, X., Piao, M., Khan, S., et al. (2016). Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PloS One 11, e0161174. doi: 10.1371/JOURNAL.PONE.0161174
Weingarden, A., González, A., Vázquez-Baeza, Y., Weiss, S., Humphry, G., Berg-Lyons, D., et al. (2015). Dynamic Changes in Short- and Long-Term Bacterial Composition Following Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection. Microbiome 3, 10. doi: 10.1186/s40168-015-0070-0
Wei, Y., Zhu, W., Gong, J., Guo, D., Gu, L., Li, N., et al. (2015). Fecal Microbiota Transplantation Improves the Quality of Life in Patients With Inflammatory Bowel Disease. Gastroenterol. Res. Pr 2015, 517597. doi: 10.1155/2015/517597
Wilson, B. C., Vatanen, T., Jayasinghe, T. N., Leong, K. S. W., Derraik, J. G. B., Albert, B. B., et al. (2021). Strain Engraftment Competition and Functional Augmentation in a Multi-Donor Fecal Microbiota Transplantation Trial for Obesity. Microbiome 9, 107. doi: 10.1186/s40168-021-01060-7
Woodworth, M. H., Carpentieri, C., Sitchenko, K. L., Kraft, C. S. (2017). Challenges in Fecal Donor Selection and Screening for Fecal Microbiota Transplantation: A Review. Gut Microbes 8, 225–237. doi: 10.1080/19490976.2017.1286006
Xiang, L., Ding, X., Li, Q., Wu, X., Dai, M., Long, C., et al. (2020). Efficacy of Faecal Microbiota Transplantation in Crohn’s Disease: A New Target Treatment? Microb. Biotechnol. 13, 760–769. doi: 10.1111/1751-7915.13536
Xue, L. J., Yang, X. Z., Tong, Q., Shen, P., Ma, S. J., Wu, S. N., et al. (2020). Fecal Microbiota Transplantation Therapy for Parkinson’s Disease: A Preliminary Study. Med. (Baltimore) 99, e22035. doi: 10.1097/MD.0000000000022035
Xu, F., Li, N., Wang, C., Xing, H., Chen, D., Wei, Y. (2021). Clinical Efficacy of Fecal Microbiota Transplantation for Patients With Small Intestinal Bacterial Overgrowth: A Randomized, Placebo-Controlled Clinic Study. BMC Gastroenterol. 21, 54. doi: 10.1186/s12876-021-01630-x
Youngster, I., Russell, G. H., Pindar, C., Ziv-Baran, T., Sauk, J., Hohmann, E. L. (2014a). Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium Difficile Infection. JAMA 312, 1772–1778. doi: 10.1001/jama.2014.13875
Youngster, I., Sauk, J., Pindar, C., Wilson, R. G., Kaplan, J. L., Smith, M. B., et al. (2014b). Fecal Microbiota Transplant for Relapsing Clostridium Difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin. Infect. Dis. 58, 1515–1522. doi: 10.1093/cid/ciu135
Keywords: gut microbiota, fecal microbiota transplant (FMT), microbial ecology, pharmacology, pharmacokinetics
Citation: Halaweish HF, Boatman S and Staley C (2022) Encapsulated Fecal Microbiota Transplantation: Development, Efficacy, and Clinical Application. Front. Cell. Infect. Microbiol. 12:826114. doi: 10.3389/fcimb.2022.826114
Received: 30 November 2021; Accepted: 21 February 2022;
Published: 17 March 2022.
Edited by:
Xingmin Sun, University of South Florida, United StatesReviewed by:
Yeshi Yin, Hunan University of Science and Engineering, ChinaMichael H. Woodworth, Emory University, United States
Srishti Saha, Mayo Clinic, United States
Copyright © 2022 Halaweish, Boatman and Staley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Staley, Y21zdGFsZXlAdW1uLmVkdQ==
†These authors share first authorship
 Hossam F. Halaweish
Hossam F. Halaweish Sonja Boatman
Sonja Boatman Christopher Staley
Christopher Staley