- 1The College of Veterinary Medicine, Southwest University, Chongqing, China
- 2Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Changchun, Jilin, China
- 3State Key Laboratory of Veterinary Etiological Biology, College of Veterinary Medicine, Lanzhou University, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, Gansu, China
- 4The Immunology Research Center, Medical Research Institute, Southwest University, Chongqing, China
Globally, brucellosis is a widespread zoonotic disease. It is prevalent in more than 170 countries and regions. It mostly damages an animal’s reproductive system and causes extreme economic losses to the animal husbandry industry. Once inside cells, Brucella resides in a vacuole, designated the BCV, which interacts with components of the endocytic and secretory pathways to ensure bacterial survival. Numerous studies conducted recently have revealed that Brucella’s ability to cause a chronic infection depends on how it interacts with the host. This paper describes the immune system, apoptosis, and metabolic control of host cells as part of the mechanism of Brucella survival in host cells. Brucella contributes to both the body’s non-specific and specific immunity during chronic infection, and it can aid in its survival by causing the body’s immune system to become suppressed. In addition, Brucella regulates apoptosis to avoid being detected by the host immune system. The BvrR/BvrS, VjbR, BlxR, and BPE123 proteins enable Brucella to fine-tune its metabolism while also ensuring its survival and replication and improving its ability to adapt to the intracellular environment.
1 Introduction
Brucellosis is a worldwide zoonotic disease that has brought great harm to both biosecurity and economic development. Some species of the genus Brucella can cause brucellosis, whose virulence is mainly reflected in its entry into cells and in its ability to survive and replicate (Gorvel and Moreno, 2002). Erythritol has been considered as an important factor in the pathogenesis of Brucella abortus 2308 and its ability to cause abortion in ruminants. Macrophages are immune cells, and their main function is to cause immune reactions and produce immune responses. Macrophage is one of the main target cells of Brucella infection. The survival and replication of Brucella in macrophages represent one of the strategies for Brucella to evade the host’s immune response, and it is also the reason for the failure of some patients to use anti-Brucella treatment. In addition, the prolonged existence of Brucella in macrophages will affect the signal pathway of the cell, and trigger a complex host response, so that it can adapt to the intracellular environment and reproduce widely in the host cell without destroying the basic cell function (Chaves-Olarte et al., 2002). Compared with acute diseases, the pathogens of chronic diseases have more virulence genes to ensure the persistence of infection (Hong et al., 2000). The intracellular environment allows Brucella to coordinate gene expression during infection. The host body specific internal resistance induced expression of genes is usually also an important virulence factor (Boschiroli et al., 2001).
2 Chronic intracellular infection with Brucella spp.
Brucella is a gram-negative facultatively intracellular bacteria that can invade and persist within the host cells and lead to chronic infections. Domestic animals, wild animals, and humans are susceptible to Brucella. There are six classical species: Brucella abortus (B. abortus), Brucella melitensis (B. melitensis), Brucella suis (B. suis), Brucella canis (B. canis), Brucella ovis (B. ovis) and Brucella neotomae (B. neotomae) (Erkyihun et al., 2022; Kurmanov et al., 2022). Among them, B. suis, B. abortus, and B. melitensis are the most harmful to humans (Olsen and Palmer, 2014). Brucella expresses atypical virulence factors, including lipopolysaccharide (LPS), virulence regulatory proteins, and phosphatidylcholine, but lacks classical virulence factors, such as invasive proteases, toxins, or virulence plasmids (Roop et al., 2003). Brucella is classified into two types based on differences in the structure of pathogenic bacteria LPS: smooth Brucella and rough Brucella (Stranahan and Arenas-Gamboa, 2021). Smooth LPS (S-LPS) consists of a polysaccharide O-chain, core, and lipid A, whereas rough strains lacked the O-side chain. The importance of the O-chain for the virulence of naturally occurring smooth Brucella strains is well documented. In general, rough (R) type Brucella shows reduced virulence, except for B. ovis and B. canis (Lopez-Santiago et al., 2019).
Brucellosis is typically divided into three distinct phases: the incubation phase before clinical symptoms are evident (within 2 days after infection), the acute phase during which time the pathogen invades and disseminates in host tissue (within 2 days to 3 weeks after infection), and the chronic phase that can eventually result in severe organ damage and death of the host organism (6 months to 1 year or more). (Grillo et al., 2012). It is generally believed that innate immunity is not highly activated during the incubation period. This allows Brucella to spread throughout the reticuloendothelial system and establish a replication mechanism within the phagocytes. There are no typical endotoxin symptoms (mainly the lipid A of LPS) during this process, which is different from other gram-negative infections. This clinical observation is consistent with brucellosis (Martirosyan et al., 2011). In the early stage of Brucella infection, Brucella barely activates the complement system and induces minimal levels of cytokines, the recruitment of pro-inflammatory cells at the infected site is poor (Barquero-Calvo et al., 2007). Since Brucella hardly activates the complement system through the classical pathway or activates granulocytes, it causes very little tissue damage and does not cause obvious blood changes, such as leukocytosis, increased neutrophilia, and decreased platelets, coagulation lesions rarely occur during the incubation period (Barquero-Calvo et al., 2007). After the incubation period, strong adaptive immunity begins to appear, and obvious clinical symptoms are observed, such as abortion and infertility in animals and high, undulating fever in humans. In the acute phase, Brucella begins to replicate actively in macrophages and dendritic cells (DC). One of the distinguishing features of Brucella is that the levels of chemokines and cytokines produced by infected macrophages and DC are low and prolonged, the production of proinflammatory cytokines released by polymorphonuclear (PMN) cells is low, and the activation and demand of natural killer cells (NK) are low (Barquero-Calvo et al., 2007). Although nonspecific immunity is used to control the proliferation of Brucella in the acute phase in mice, effective specific immunity is required in the later stage (Grillo et al., 2012).
The first line of defense against Brucella includes the phagocytosis action of PMN, macrophages, dendritic cells, NK cells, chemokines, pattern recognition receptors (PRR), and the complement system (Diacovich and Gorvel, 2010; Jiao et al., 2021). The specific immune response caused by Brucella infection has three main mechanisms. The first is the secretion of interferon by CD4+ T cell, CD8+ T cell, γδ T cell, which activates the bactericidal function of macrophages and prevents Brucella intracellular survival; the second is the cytotoxic effect of CD8+ T cells, which can kill infected macrophages; and the third is Th1 antibody subtypes, such as IgG2a and IgG3, which promote phagocytosis (Martirosyan et al., 2011; Grillo et al., 2012). Furthermore, cytokines such as interleukin-12 (IL-12), interferon-γ (IFN-γ), and tumor necrosis factor (TNF) are important in initiating both specific and non-specific immune responses. Brucella can participate in the regulation of innate immune mechanisms and the maintenance of intracellular replication by inhibiting Toll-like receptors (TLR) signaling pathways, the complement system, phagocytes, and apoptosis. Several immunomodulatory molecules, for example, proline racemase protein A (prpA) and the TIR domain-containing protein (TcpB) can influence the Th1 immune response by inhibiting the secretion of IFN-γ and promoting the secretion of interleukin-10 (IL-10) (Alaidarous et al., 2014; Spera et al., 2014). Due to an increase in CD4+ and CD25+ T cells in the spleen, chronic Brucella infection causes immunosuppression in the body (Bahador et al., 2014). The reduced recruitment of macrophages and DCs after Brucella infection leads to a decrease in CD8+ T lymphocyte activation, thus forming immunosuppression, which is conducive to immunosuppression being beneficial to the replication and chronic infection of Brucella (Pasquali et al., 2010).
In the initial stage of infection, macrophages, trophoblastic cell (TE), and other phagocytic cells are the main targets of Brucella infection (Xiao et al., 2022). For Brucella, they are not only the sites for both survival and replication but also a vehicle for transmission to other organ systems. Macrophages play a key role in the clearance and control of intracellular pathogens. Macrophages can not only kill pathogens and carry out non-specific immunity but also participate in antigen uptake and processing steps to initiate specific immunity. Meanwhile, its secreted cytokines, such as IL-1, IL-6, and IFN-γ, regulate the immune response and activate more lymphocytes and macrophages, playing a role in regulating the immune response and inflammatory immunity (Weiss and Schaible, 2015). Macrophages can eliminate most of the Brucella invading the body. However, there is still a small part of Brucella that can evade the immune system and use macrophages, as the host to invade, survive, and reproduce.
In its long evolutionary history, Brucella has interfered with both specific and nonspecific immune responses to establish a persistent infection, making it difficult to remove thoroughly. However, the specific and comprehensive mechanism of Brucella intracellular survival remains unknown.
3 Brucella mediates autophagy
More and more evidence suggest that nonspecific host immunity is important for Brucella intracellular infection. Autophagy is a non-specific immune process based on lysosomes, which can decompose non-essential cells or invading pathogens into cellular components to promote cell survival and provide more energy sources for cells. Some pathogens have evolved strategies to in turn use autophagy to survive inside cells, and Brucella can use the cell autophagy mechanism for intracellular replication to establish a good living environment. The specific processes include: quickly escaping from the phagocytic corpuscle to enter the cytoplasm; delaying the maturation of the phagocytic corpuscle at different stages before fusion with lysosomes; surviving and replicating in the degraded phagocytic environment; and completely or partially avoiding the endocytic pathway. All of these processes require microbiological agents to interfere with the function of macrophages (Celli, 2006).
First, Brucella relies on specific lipid rafts to enter macrophages. The outer membrane of Brucella consists of phospholipids, guanylic acid, lipoproteins, and nonstandard LPS, which replace the long aliphatic hydrocarbon chains. The content of negatively charged sugars in Brucella lipid A and core oligosaccharides (BR-LPS) is low; moreover, O-chains and associated polysaccharides are composed of homopolymers of non-reducing N-formyl peroxide sugars. These features help to reduce the negative charge on the surface of the bacteria. This special cell membrane structure prevents Brucella from combining with complement (both the classical and the MBL-mediated pathways), bactericidal defensin, bacitracin, or any other cationic bactericidal molecule and is effective against most bactericidal substances in lysosomal extracts, lysozyme, phospholipase, and lactoferrin(Fernandez-Prada et al., 2001; Cardoso et al., 2006). Specifically, the LPS O-chain modifies the fusion properties of BCV membranes or interacts with specific receptors located in lipid rafts to determine the entry of permissive cells. The type A scavenger receptor (SR-A) is considered as the receptor that binds to Brucella LPS, respectively (Watarai et al., 2003; Kim et al., 2004). The LPS O-chain also promotes Brucella survival by delaying fusion with the lysosome. This process is further enhanced by the disruptive effect of cyclic β-1,2-glucan on BCV lipid rafts (Celli, 2006). Cyclic β-1,2-glucan is secreted by intracellular Brucella, which disrupts cholesterol-rich lipid rafts located on the membrane of BCV and interferes with BCV maturation, thus preventing lysosomal fusion. A deletion mutant of Cgs, the gene encoding the synthetase of cyclic β-1,2-glucan, is unable to avoid fusion with the lysosome, suggesting that the production of cyclic β-1,2-glucan is necessary for Brucella intracellular cycle (Bohin, 2000; Arellano-Reynoso et al., 2005).
Upon entry into host cells, the Brucella reside in acidified phagosomal compartments known as endosomal Brucella-containing vacuoles (eBCVs). The eBCV stage is a necessary step in the intracellular circulation of Brucella. With the early and late interaction within the stage, most of the contents of BCV are subjected to enzymatic degradation, and 90% of internal Brucella are hydrolyzed and killed. However, the remaining 10% escaped from the host’s killing mechanisms through unknown mechanisms (Ke et al., 2015). This entire maturation process is in line with the complete maturation process, but after maturation, BCV still avoids fusion with the terminally degraded lysosomes, thus ensuring the intracellular survival of bacteria. This vacuole in this process is called eBCV (Figure 1). The sustained avoidance of macrophage degradation also requires VirB type IV secretion system (T4SS) and the conversion of eBCV into an endoplasmic reticulum-derived replicating compartment (rBCV). eBCV can provide the conditions necessary to induce the expression of the VirB operon that encodes a type IV secretion system (T4SS) and its transformation into rBCV, including lysosomal pH. In addition, eBCV can trigger intracellular bacterial growth before rBCV is formed (Porte et al., 1999; Celli, 2019).
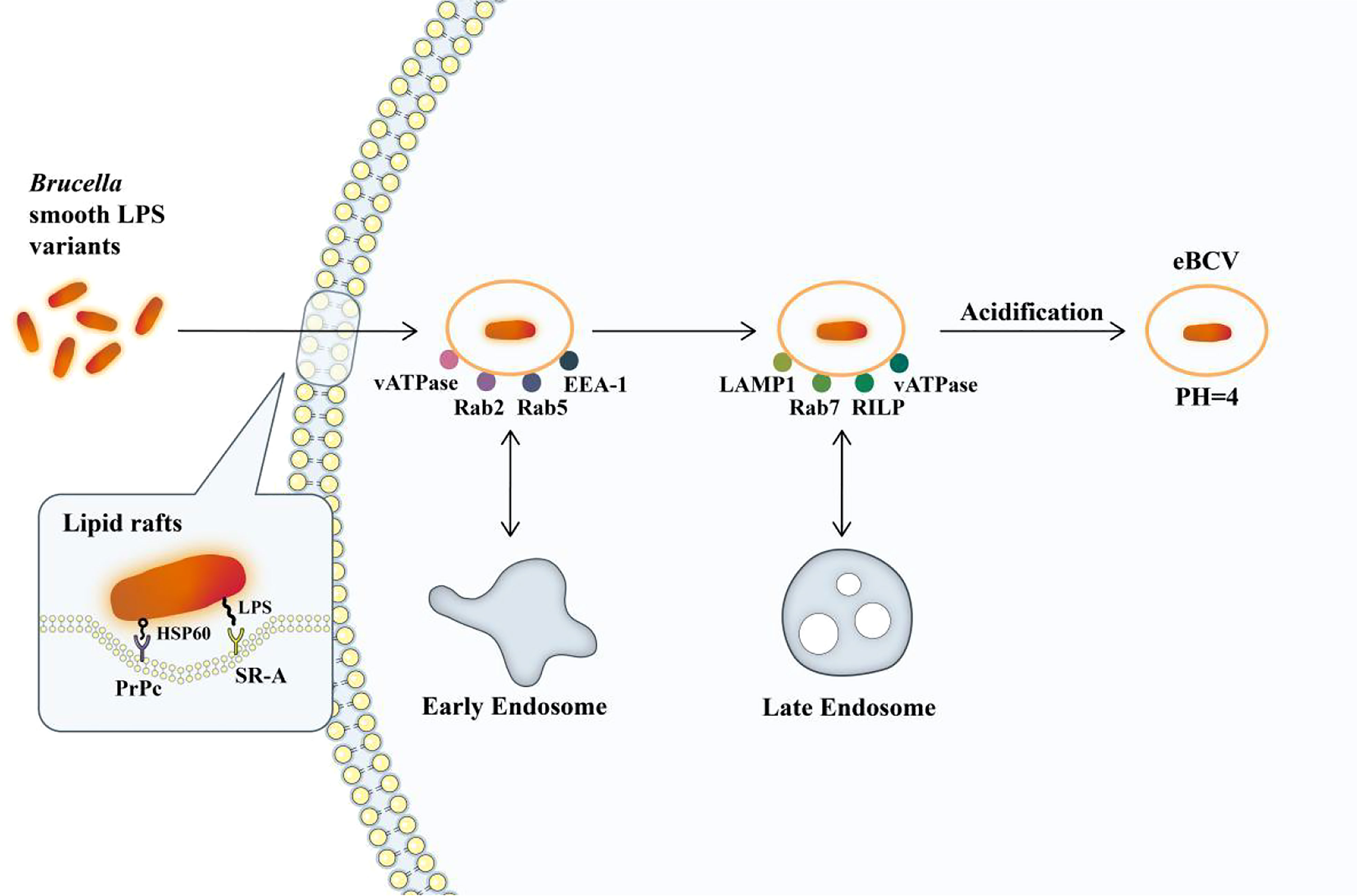
Figure 1 The formation of eBCV. Brucella enters macrophages via lipid rafts. Hsp60 and Brucella LPS bind to PRPC and SR-A receptors on lipid rafts. Brucella enters the cell and remains in the membrane envelope cavity, forming BCV containing Brucella. It interacts with early endosomes to obtain small GTPase Rab5 and early endosomal antigen (EEA-1) and subsequently obtains markers of late endosomes, such as membrane proteins recombinant lysosomal associated membrane protein 1 (LAMP1) and small GTPase Rab7. It will then be acidified, and the pH will reach 4, which is essential for the survival of Brucella and for the intracellular expression of the VirB T4SS (Porte et al., 1999; Boschiroli et al., 2002). The single arrows represent the flow of Brucella intracellular processes, and the double arrows represent interactions.
eBCV will gradually lose its endosomal marker and begin to continuously interact with the endoplasmic reticulum (ER) structure after interaction with the endosome. The site of action is the ER export site covered with coated vesicles II (COPII), and the interaction depends on the VirB IV secretion system, which controls its organization and function through COPII, and its activity is controlled by the small GTPase Sar1. The newly formed vesicles and tubules are fused with the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) or VTC, and the coated vesicles I (COPI), controlled by Arf1 GTPase, are transported to the Golgi apparatus or ER to play a role. Vacuoles containing VirB-deficient Brucella cannot sustain interactions and fuse with the ER. They eventually fuse with lysosomes. Studies have shown that eBCV interacts with the COPII coating structure of the proven functional endoplasmic reticulum exit sites (ERES) but doesn’t interact with the COPI coating structure (Celli et al., 2005; Xiong et al., 2021). Finally, eBCV obtains markers related to the ER membrane, such as calcium-binding protein, Sec61, and Pdi, indicating the turnover of the eBCV membrane and the accumulation of ER-derived membrane. At the same time, these vacuoles also acquire the structure and functional characteristics of ER, which further indicates that eBCV is gradually derived from ER (Pizarro-Cerda et al., 1998; Comerci et al., 2001; Celli et al., 2003). These changes in structure and function are associated with the initiation of bacterial replication. The vacuoles are then called rBCV, where the bacteria replicate.
The effectors of T4SS, BspA, BspB, and BspF inhibit the secretion of host proteins and promote bacterial replication (Myeni et al., 2013). Although it is not yet clear how BspA and BspF work, how BspB works has been revealed. The production of rBCV and the replication of bacteria are inseparable from BspB. BspB is delivered to the Golgi apparatus of host cells and interacts with the conserved oligomeric Golgi (COG) complex. As a result, the function of COG is changed, and the reverse Golgi vesicles that depend on COG are transported to BCV, thereby obtaining the Golgi apparatus source membrane. It is also known that the T4SS effector, RicA, is involved in controlling the formation of rBCV (Miller et al., 2017). At the same time, the unfolded protein response (UPR) transmembrane sensor, inositol-requiring enzyme-1 (IRE1), is also necessary for bacterial replication (Pandey et al., 2018). YPT-interacting protein 1A (Yip1A) is produced when IRE1 is activated, and it is then phosphorylated when combined with IRE1, which triggers XBP-1-dependent transcription (Taguchi et al., 2015). This activation also acts on the upregulation of COPII-coat complex subunits (Taguchi et al., 2015). The COPII-coat complex is essential for the formation of rBCV and is a crucial part of ERES and early secretory transport (Celli et al., 2005).
After extensive bacterial replication, autophagy BCV (aBCV) is produced (Figure 2). The production of aBCV requires typical autophagy nucleation but does not require the extended complex. Therefore, the lack of Beclin-1, ULK1, and Atg14 will prevent its formation, but the lack of Atg5, Atg7, Atg4, or Atg16L will not affect it. This is also the performance of Brucella using autophagy to complete its intracellular circulation. aBCV has the characteristics of advanced ribosomes, which are consistent with the mature autophagosome without ER markers, so its function is different from that of rBCV, but it is closely related to the release of bacteria and the spread between cells (Figure 2) (Boschiroli et al., 2002). The bacteria are released from the pores of the cell membrane into the intercellular substance, causing cell lysis. During the cell-to-cell propagation of Brucella, smooth (S) type Brucella may dissociate and become an R type. This dissociation is also enhanced in an acidic environment, facilitating the spread of Brucella from acidic phagocytosis. It is important to note that after dissociating into R type Brucella, S type Brucella can revert to S type. Therefore, S type Brucella may become R type when it needs to spread and then revert to S type when it escapes to resist intracellular killing. Then, Brucella will infect more macrophages and begin a new cycle of replication and dissociation. Rough mutants may be killed by complement or another cationic peptide-mediated cleavage (Pei et al., 2014).
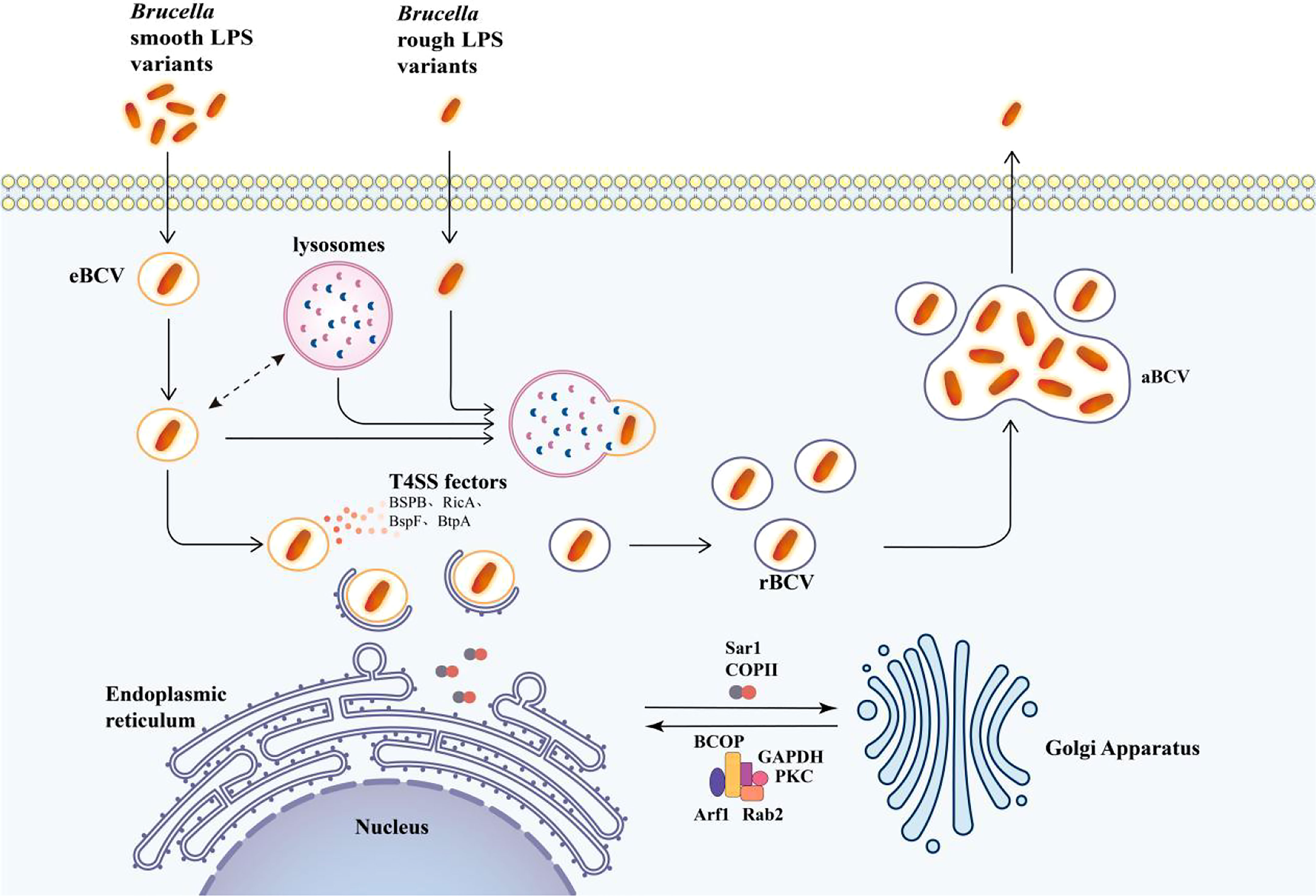
Figure 2 Model of intracellular transport of Brucella in macrophages. The lysosome interacts with eBCV and activates the transcription of T4SS effector proteins. T4SS effectors facilitate the arrival of eBCV at the endoplasmic reticulum exit site, and eBCV interacts with the surface coating of ERES to obtain the structural and functional characteristics of ER to form rBCV. The autophagy initiation proteins ULK1, ATG14L, and Beclin-1 play an important role in the formation of aBCV and finally release the pathogen from the cell. The mechanism of entry of Brucella rough LPS variants into cells is not clear, and after entering the cell, Brucella is degraded by lysosomal phagocytosis upon entry. The single arrows represent the flow of Brucella intracellular processes, and the double arrows represent interactions.
4 Brucella regulates metabolism
The ability to regulate its metabolism is also one of the keys to the successful adaptation of Brucella in vivo. Metabolic systems adapted to intracellular survival can make better use of nutrients at all stages of the infectious cycle (Brown et al., 2008; Lamontagne et al., 2009). When Brucella enters a cell and persists, its metabolic system adapts. The regulation of its metabolic system enables Brucella to take advantage of the metabolic pathways and intermediates provided by the host to adapt to various environmental conditions in the host cell. To adapt to different environments at different stages of the infection cycle, a comprehensive fine-tuning of gene expression is required to alter the corresponding functions of the bacteria. B. abortus is considered to have a slow metabolism during the period from entering the cell to starting to replicate (De Bolle et al., 2015). The protein obtained through sugar absorption, pentose phosphate pathway (PP), and tricarboxylic acid cycle (TCA) pathways, as well as the biosynthesis of amino acids, purines, and pyrimidines, is reduced. Brucella, on the other hand, does not store glycogen or poly-β-hydroxybutyrate, so it can maintain basic metabolism via protein and amino acid catabolism (Chain et al., 2005). It has also been suggested that B. abortus can even use macromolecules such as ribosomes at this stage. After entering the replication niche, metabolism began to strengthen (Lamontagne et al., 2009). Different strains of Brucella have different carbon sources. B. suis biovars 1 and 5, B. microti, and B. neotomae use C5 sugars such as xylose, arabinose, and ribose as the sole carbon source; some strains of B. abortus, B. melitensis, and B. suis use galactose as the sole carbon source when tested on the vitamin and mineral based medium; at the same time, some B. melitensis and B. suis strains grew on fructose and mannose as carbon sources (McCullough and Beal, 1951). Brucella breaks down hexose through the PP pathway and the incomplete Embden-Meyerhof-Parnas glycolytic pathway (EMP), and then further metabolites through TCA. But for the three most representative species of Brucella: B. abortus, B. melitensis, and B. suis, hexose is not the carbon source of choice. Instead, they preferentially utilize a four-carbon sugar alcohol (erythritol) and catabolize it to produce a trisaccharide phosphate. In addition, other studies have shown that some strains of Brucella could use polyols such as lactic acid and glycerol as peripheral carbon sources (Figure 3) (Barbier et al., 2018). The metabolic pathways of Brucella mainly include active PP and TCA cycles, potentially active Entner-Doudoroff (ED) and glyoxylate pathways, and incomplete EMP. Among them, the PP pathway plays an important role in the production of biological precursors and the degradation of sugars (Barbier et al., 2011).
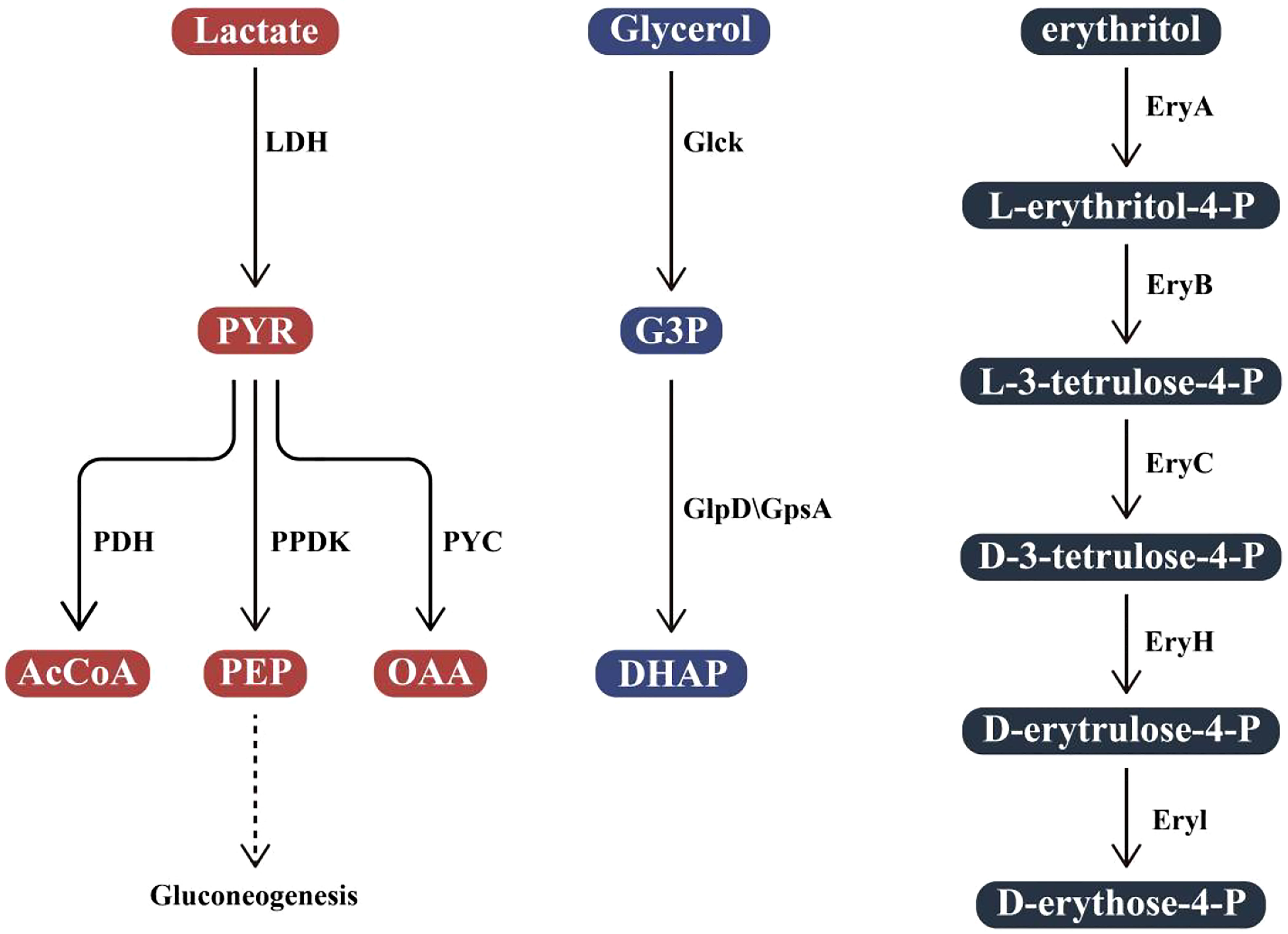
Figure 3 Utilization of polyols In Brucella. Lactate is converted into pyruvate (PYR) by L-lactate dehydrogenase (LDH). PYR is then phosphorylated by pyruvate phosphodikinase (PPDK) to produce phosphoenolpyruvate (PEP) and enter the gluconeogenesis pathway, converted to acetyl coenzyme A (AcCoA) by pyruvate dehydrogenase (PDH) or to oxaloacetate (OAA) by pyruvate carboxylase (PYC); Glycerol is converted to glyceraldehyde 3-phosphate (G3P) by glycerol kinase (GlcK). G3P is further activated by G3P dehydrogenase (GlpD) or G3P dehydrogenase (GpsA) to produce 3,4-dihydroxy acetophenone (DHAP). In B abortus, erythritol is first phosphorylated by EryA to L-erythritol-4-P, then L-erythritol-4-P is oxidized by EryB to L-3-tetramethysaccharide-4-P and then transformed into D-erythrocyte 4-P, catalyzed by EryC, EryH, and EryI in turn.
The two-component regulation system of Brucella, BvrR/BvrS, is the most distinctive two-component sensory regulation system of Brucella so far. BvrS is a membrane-bound homologous dimer protein belonging to the histidine protein kinase superfamily. It has three conserved regions: an amino-terminal periplasmic sensing domain with transmembrane segments, a cytoplasmic dimerization domain with a specific His residue, and the carboxy-terminal ATP-binding kinase domain. BvrR is a cytoplasmic protein that is highly similar to the reaction-regulating protein OmpR/PhoB subfamily, and its specific Asp residues are located in the conserved regulatory domain and have the effect domain of DNA binding activity (Lopez-Goni et al., 2002). It was originally discovered that BvrR/BvrS regulated the homeostasis and structure of several proteins in B. abortus cell membranes. However, with further research, it has been found that it also has functions related to metabolic function, thus contributing to the adaptation of B. abortus to an intracellular lifestyle (Lamontagne et al., 2009). Upon exposure to specific environmental stimuli, BvrS autophosphorylated on conserved histidine residues and mediated phosphate transfer to conserved aspartic acid on BvrR. The latter regulates cell expression through the differential expression of target genes. BvrS/BvrR is important for the virulence of bacteria, and translocation inactivation leads to defects in attachment, invasion, and intracellular replication (Sola-Landa et al., 1998). A recent transcriptional analysis shows that the BvrR mutation had significant effects on the expression of genes associated with carbohydrate, amino acid, fatty acid, and nitrogen metabolism (Viadas et al., 2010).
Quorum sensing (QS) is a regulatory system that can regulate gene expression at the population level according to the density of local bacteria. Recent transcriptional and proteomic analyses have shown that the inactivation of the two QS regulators, VjbR and BabR, has a strong effect on the genes involved in metabolism, especially the genes encoding the TCA cycle and glycolysis (Uzureau et al., 2010; Weeks et al., 2010). Since B. abortus’s intracellular expansion is limited until the replication site is reached, in response, B. abortus uses VjbR to slow down the metabolism of Brucella until it reaches endoplasmic reticulum derived rBCV. Subsequently, the BabR regulator acts on the reactivation of basal metabolism. BvrR can also activate the transcription of VjbR, so the two regulatory systems seem to be related (Martinez-Nunez et al., 2010; Viadas et al., 2010). To sum up, BvrS/BvrR, TCS, and QS systems are helpful to the regulation of B. abortus metabolism in the intracellular inventory.
The phosphoenolated pyruvate phosphotransferase system (PTS) provides an integrated system for bacteria to ensure optimal utilization of carbohydrates in complex environments, a feature that also plays an important role in host-bacterial interactions. The three genes, hprK, ptsM, and ptsO, are found downstream of the conserved two-component system genes (BvrS/BvrR, Exos/ChvI) associated with infection or symbiosis in all pathogenic or symbiotic -proteobacteria (Sola-Landa et al., 1998; Belanger et al., 2009). This genome structure shows a functional link between PTS and BvrS/BvrR (Boel et al., 2003; Barabote and Saier, 2005). Both BvrR and ptsP seem to modulate the expression of the QS modifier VjbR. VjbR, in turn, regulates virulence and metabolic determinants.
BPE123 is a T4SS effector protein of Brucella, indicating that the bacteria may manipulate the host carbohydrates synthesis or decomposition pathways through T4SS effector molecules. BPE123 interacts with the key glycolysis/gluconeogenesis host enzyme α-enolase, allowing it to bind to BCV and induce structural or functional changes that result in α-enolase activation. When this enzyme is depleted by RNA interference, the intracellular replication of B. abortus in HeLa cells is impaired, thus confirming the role of this protein in the infection process (Marchesini et al., 2016). Current data indicates that T4SS and its effector proteins could regulate the metabolic pathways of host cells and contribute to the intracellular survival of bacteria. However, how to control the secretion and action of effector proteins is still unclear (Hayek et al., 2019).
5 Brucella regulates apoptosis
Regulating the macrophages’ apoptosis is also one of the strategies used by Brucella to achieve intracellular persistence. By regulating the apoptosis of these host cells, especially macrophages, Brucella can reduce the bactericidal ability of immune cells. Brucella can promote or inhibit apoptosis in different conditions. Studies have shown that S type Brucella inhibited macrophage apoptosis, while attenuated R type Brucella induced macrophage apoptosis (Im et al., 2016). This may be related to the dissociation of the bacteria when they are released from the target cell. Gross et al. demonstrated that B. suis could disrupt the TNF-α apoptosis pathway by triggering cell signal transduction, by blocking the core steps of cell apoptosis (Gross et al., 2000). Galdeiro et al. found that the apoptosis of cells challenged with the B. abortus S19 strain was delayed when compared with lymphocytes and monocytes from healthy controls (Galdiero et al., 2000). These results suggest that Brucella is involved in the induction apoptosis, indicating that the immune system of the host in turn adapts them to infection.
Brucella infection induced the expression of zinc finger protein A20 in macrophages. A20, also known as tumor necrosis factor alpha-induced protein 3 (TNFAIP 3), is a dual inhibitor of macrophage activation and apoptosis, A20 has important physiological functions (Priem et al., 2020). On the one hand, activation of nuclear factor-κB (NF-κB) can inhibit apoptosis induced by tumor necrosis factor receptor 1 (TNFR1), and A20 can terminate the activity of NF-κB, so A20 can promote cell apoptosis (Vallabhapurapu and Karin, 2009; Vereecke et al., 2009). B. melitensis infection, on the other hand, significantly increases the expression of the TNF-α gene in macrophages, and A20, as one of the genes induced by TNF-α, also increases significantly (Wang et al., 2011). TNF-α induces macrophage apoptosis by signaling through complex I, Tradd-TRAF2-RIP (Micheau and Tschopp, 2003). A20 can ubiquitinate these proteins for degradation (He and Ting, 2002; Wertz et al., 2004). Therefore, A20 also has an anti-apoptotic function in macrophages. The results of Wei et al. revealed that A20 is involved in the inhibition of macrophage apoptosis in the process of B. abortus infection. The lack of A20 will inhibit the growth of B. abortus in macrophages, but it is not enough to trigger the apoptosis of macrophages. B. abortus induces A20 to promote B. abortus intracellular growth by inhibiting macrophage activation and apoptosis. This study provides a new explanation for the ability of B. abortus to grow and replicate in macrophages in the early stages of infection (Wei et al., 2015).
Reactive oxygen species (ROS) are the second messenger of apoptosis (Carrasco et al., 2016). When cells receive apoptosis signals, ROS levels increase, which may lead to increased Ca2+ influx, upregulation of Bax, the opening of the mitochondrial permeability transition pore (MPTP), activation of trypsin, and eventual cell death (Sun et al., 2016). Different levels of ROS determine apoptosis, necrosis, or the transformation from apoptosis to necrosis. Excessive ROS can change the activity of specific enzymes through redox reactions and participate in the regulation of autophagy and programmed cell death, thus adversely affecting the body. Therefore, ROS increased after apoptosis, which in turn promoted apoptosis. The anti-apoptotic protein BCL-2 inhibits ROS-induced lipid peroxidation by inhibiting ROS production. B. melitensis 16M can regulate the effects of the AIR domain on inflammatory factors, autophagy, and apoptosis in mouse macrophages through the ROS signaling pathway. The ability of B. melitensis 16M to promote apoptosis increased with infection time. AIR can also influence B. melitensis 16M-induced apoptosis via the ROS pathway (Li et al., 2016).
Calcium-activated cysteine protease 2 (Calpain-2) regulates macrophage apoptosis and necrosis under a variety of pathological conditions. Normally, an increase in intracellular calcium leads to the activation of Calpain-2, which induces macrophage apoptosis. On the other hand, B. abortus infection inhibits macrophage apoptosis by increasing intracellular calcium content. Nedd4 participates in apoptosis by ubiquitination and degradation of its substrates such as PTEN and caspase-9 (Ahn et al., 2008; Fombonne et al., 2012). Caspase-3 is one of the most important executors of apoptosis, and it plays a major role in the process of apoptosis (Cryns and Yuan, 1998). Current studies have shown that B. abortus infection triggers the degradation of Calpain-2 by activating Nedd4, and prevents the activation of the apoptotic effector caspase-3, thereby, inhibiting macrophage apoptosis. Calpain-2 is ubiquitinated by Nedd4, after infection with B. abortus, and degraded as intracellular calcium increases. These results indicate that after infection, B. abortus inhibits macrophage apoptosis through Nedd4-dependent Calpain-2 degradation (Cui et al., 2014).
JAK2/STAT3 signal transduction pathway is an important intracellular signal transduction pathway, as well as a common pathway for many cytokines and growth factors. It plays an essential role in cell proliferation, differentiation, apoptosis, and immune regulation. B. melitensis M5-90 infection regulates the apoptosis and proinflammatory response of RAW264.7 cells by activating the JAK2/STAT3 signaling pathway. AG490 is an inhibitor that inhibits JAK2 activity in macrophages of mice infected with B. melitensis M5-90 (Luo and Laaja, 2004). AG490 at various concentrations modulated the activation of the JAK2/STAT3 pathway to varying degrees but essentially inhibited TNF-α expression. TNF-α is involved in the induction of several distinct immune responses to intracellular infections (Aggarwal, 2003). Furthermore, TNF-α-mediated apoptosis is involved in the pathology of chronic inflammation and autoimmune diseases (Wang et al., 2009). TNF-α activates TNF-α receptor-1 and induces apoptosis by activating caspase in the death receptor pathway (Ashkenazi, 2002), implying that TNF-α can trigger macrophage apoptosis via the JAK2/STAT3 pathway. At the same time, TNF-α can also activate caspase-8 and caspase-3 to cause apoptosis (Dbaibo et al., 1997), and BCL-2 can regulate this effect. BCL-2 is an anti-apoptotic gene. Bax is a member of the BCL-2 family and can promote apoptosis (Karabay et al., 2014). The results show that the expression of caspase-3 and Bax decreased in RAW264.7 cells infected with B. melitensis M5-90 treated by AG490, whereas the expression of BCL-2 shows the opposite effect. In summary, B. melitensis M5-90 activates the JAK2/STAT3 signaling pathway and regulates TNF-α-induced apoptosis. Inhibition of the JAK2/STAT3 pathway can inhibit the Th1 immune response, inhibit apoptosis, and contribute to the intracellular survival of B. abortus (Yi et al., 2018).
Other studies have shown that BR-LPS O chain polysaccharide is also involved in the prevention of macrophage apoptosis. Zhang et al. found that Brucella outer membrane protein Omp31 inhibited TNF-α-mediated apoptosis during Brucella infection of macrophages (Zhang et al., 2016). The study by Liu also proved that the Omp31 protein could inhibit the apoptosis of microglia (Liu and Ma, 2017).
6 Conclusions
The interaction mechanism between pathogen and host is very complex, involving many biological factors and pathways. It is known that the adaptive regulation of immune response, metabolism, and apoptosis caused by Brucella after infection is necessary for its intracellular persistence and replication, and some of these biological factors and pathways have multiple roles. Although there have been a lot of related reports, there are still many issues worthy of further study.
Author contributions
Writing-original draft preparation, XG, HZ, ML, YX, and GG; writing-review and editing, ZS, XS, and JG; visualization, QH; supervision, BZ, and YC; project administration, YC and HJ; funding acquisition, YC and H.J. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the Fundamental Research Funds for the Central Universities (SWU-KT22013), the Key Talents Program of Gansu Province (2021RCXM047), the Natural Science Foundation of Chongqing (2022NSCQ-MSX2392, cstc2020jcyj-msxmX0446) and the National Science Foundation for Young Scientists of China (31802215).
Acknowledgments
I am very grateful to Jake J Wen from the department of cardiology, MD Anderson cancer center for helpful discussions during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
Aggarwal, B. B. (2003). Signaling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745. doi: 10.1038/nri1184
Ahn, Y., Hwang, C. Y., Lee, S. R., Kwon, K. S., Lee, C. (2008). The tumour suppressor PTEN mediates a negative regulation of the E3 ubiquitin-protein ligase Nedd4. Biochem. J. 412, 331. doi: 10.1042/BJ20071403
Alaidarous, M., Ve, T., Casey, L. W., Valkov, E., Ericsson, D. J., Ullah, M. O., et al. (2014). Mechanism of bacterial interference with TLR4 signaling by brucella toll/interleukin-1 receptor domain-containing protein TcpB. J. Biol. Chem. 289, 654. doi: 10.1074/jbc.M113.523274
Arellano-Reynoso, B., Lapaque, N., Salcedo, S., Briones, G., Ciocchini, A. E., Ugalde, R., et al. (2005). Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat. Immunol. 6, 618. doi: 10.1038/ni1202
Ashkenazi, A. (2002). Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2, 420. doi: 10.1038/nrc821
Bahador, A., Hadjati, J., Hassannejad, N., Ghazanfari, H., Maracy, M., Jafari, S., et al. (2014). Frequencies of CD4+ T regulatory cells and their CD25(high) and FoxP3(high) subsets augment in peripheral blood of patients with acute and chronic brucellosis. Osong Public Health Res. Perspect. 5, 161. doi: 10.1016/j.phrp.2014.04.008
Barabote, R. D., Saier, M. H., Jr. (2005). Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 69, 608. doi: 10.1128/MMBR.69.4.608-634.2005
Barbier, T., Nicolas, C., Letesson, J. J. (2011). Brucella adaptation and survival at the crossroad of metabolism and virulence. FEBS Lett. 585, 2929. doi: 10.1016/j.febslet.2011.08.011
Barbier, T., Zuniga-Ripa, A., Moussa, S., Plovier, H., Sternon, J. F., Lazaro-Anton, L., et al. (2018). Brucella central carbon metabolism: an update. Crit. Rev. Microbiol. 44, 182. doi: 10.1080/1040841X.2017.1332002
Barquero-Calvo, E., Chaves-Olarte, E., Weiss, D. S., Guzmán-Verri, C., Chacón-Díaz, C., Rucavado, A., et al. (2007). Brucella abortus Uses a Stealthy Strategy to Avoid Activation of the Innate Immune System during the Onset of Infection. PLoS ONE 2 (7), e631. doi: 10.1371/journal.pone.0000631
Belanger, L., Dimmick, K. A., Fleming, J. S., Charles, T. C. (2009). Null mutations in sinorhizobium meliloti exoS and chvI demonstrate the importance of this two-component regulatory system for symbiosis. Mol. Microbiol. 74, 1223. doi: 10.1111/j.1365-2958.2009.06931.x
Boel, G., Mijakovic, I., Maze, A., Poncet, S., Taha, M. K., Larribe, M., et al. (2003). Transcription regulators potentially controlled by HPr kinase/phosphorylase in gram-negative bacteria. J. Mol. Microbiol. Biotechnol. 5, 206. doi: 10.1159/000071072
Bohin, J. P. (2000). Osmoregulated periplasmic glucans in proteobacteria. FEMS Microbiol. Lett. 186, 11. doi: 10.1111/j.1574-6968.2000.tb09075.x
Boschiroli, M. L., Foulongne, V., O'Callaghan, D. (2001). Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4, 58. doi: 10.1016/s1369-5274(00)00165-x
Boschiroli, M. L., Ouahrani-Bettache, S., Foulongne, V., Michaux-Charachon, S., Bourg, G., Allardet-Servent, A., et al. (2002). The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. U.S.A. 99, 1544. doi: 10.1073/pnas.032514299
Brown, S. A., Palmer, K. L., Whiteley, M. (2008). Revisiting the host as a growth medium. Nat. Rev. Microbiol. 6, 657. doi: 10.1038/nrmicro1955
Cardoso, P. G., Macedo, G. C., Azevedo, V., Oliveira, S. C. (2006). Brucella spp noncanonical LPS: structure, biosynthesis, and interaction with host immune system. Microb. Cell Fact 5, 13. doi: 10.1186/1475-2859-5-13
Carrasco, E., Blazquez-Castro, A., Calvo, M. I., Juarranz, A., Espada, J. (2016). Switching on a transient endogenous ROS production in mammalian cells and tissues. Methods 109, 180. doi: 10.1016/j.ymeth.2016.08.013
Celli, J. (2006). Surviving inside a macrophage: the many ways of Brucella. Res. Microbiol. 157, 93. doi: 10.1016/j.resmic.2005.10.002
Celli, J. (2019). The intracellular life cycle of brucella spp. Microbiol. Spectr. 7, 10.1128. doi: 10.1128/microbiolspec.BAI-0006-2019
Celli, J., de Chastellier, C., Franchini, D. M., Pizarro-Cerda, J., Moreno, E., Gorvel, J. P. (2003). Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198, 545. doi: 10.1084/jem.20030088
Celli, J., Salcedo, S. P., Gorvel, J. P. (2005). Brucella coopts the small GTPase Sar1 for intracellular replication. Proc. Natl. Acad. Sci. U.S.A. 102, 1673. doi: 10.1073/pnas.0406873102
Chain, P. S., Comerci, D. J., Tolmasky, M. E., Larimer, F. W., Malfatti, S. A., Vergez, L. M., et al. (2005). Whole-genome analyses of speciation events in pathogenic Brucella. Infect. Immun. 73, 8353. doi: 10.1128/IAI.73.12.8353-8361.2005
Chaves-Olarte, E., Guzman-Verri, C., Meresse, S., Desjardins, M., Pizarro-Cerda, J., Badilla, J., et al. (2002). Activation of rho and rab GTPases dissociates Brucella abortus internalization from intracellular trafficking. Cell Microbiol. 4, 663. doi: 10.1046/j.1462-5822.2002.00221.x
Comerci, D. J., Martinez-Lorenzo, M. J., Sieira, R., Gorvel, J. P., Ugalde, R. A. (2001). Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol. 3, 159. doi: 10.1046/j.1462-5822.2001.00102.x
Cui, G., Wei, P., Zhao, Y., Guan, Z., Yang, L., Sun, W., et al. (2014). Brucella infection inhibits macrophages apoptosis via Nedd4-dependent degradation of calpain-2. Vet. Microbiol. 174, 195. doi: 10.1016/j.vetmic.2014.08.033
Dbaibo, G. S., Perry, D. K., Gamard, C. J., Platt, R., Poirier, G. G., Obeid, L. M., et al. (1997). Cytokine response modifier a (CrmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-alpha: CrmA and bcl-2 target distinct components in the apoptotic pathway. J. Exp. Med. 185, 481. doi: 10.1084/jem.185.3.481
De Bolle, X., Crosson, S., Matroule, J. Y., Letesson, J. J. (2015). Brucella abortus cell cycle and infection are coordinated. Trends Microbiol. 23, 812. doi: 10.1016/j.tim.2015.09.007
Diacovich, L., Gorvel, J. P. (2010). Bacterial manipulation of innate immunity to promote infection. Nat. Rev. Microbiol. 8, 117. doi: 10.1038/nrmicro2295
Erkyihun, G. A., Gari, F. R., Kassa, G. M. (2022). Bovine brucellosis and its public health significance in Ethiopia. Zoonoses 2 (1), 15. doi: 10.15212/ZOONOSES-2022-0005
Fernandez-Prada, C. M., Nikolich, M., Vemulapalli, R., Sriranganathan, N., Boyle, S. M., Schurig, G. G., et al. (2001). Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect. Immun. 69, 4407. doi: 10.1128/IAI.69.7.4407-4416.2001
Fombonne, J., Bissey, P. A., Guix, C., Sadoul, R., Thibert, C., Mehlen, P. (2012). Patched dependence receptor triggers apoptosis through ubiquitination of caspase-9. Proc. Natl. Acad. Sci. U.S.A. 109, 10510. doi: 10.1073/pnas.1200094109
Galdiero, E., Romano Carratelli, C., Vitiello, M., Nuzzo, I., Del Vecchio, E., Bentivoglio, C., et al. (2000). HSP and apoptosis in leukocytes from infected or vaccinated animals by Brucella abortus. New Microbiol. 23, 271.
Gorvel, J. P., Moreno, E. (2002). Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90, 281. doi: 10.1016/s0378-1135(02)00214-6
Grillo, M. J., Blasco, J. M., Gorvel, J. P., Moriyon, I., Moreno, E. (2012). What have we learned from brucellosis in the mouse model? Vet. Res. 43, 29. doi: 10.1186/1297-9716-43-29
Gross, A., Terraza, A., Ouahrani-Bettache, S., Liautard, J. P., Dornand, J. (2000). In vitro brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68, 342. doi: 10.1128/IAI.68.1.342-351.2000
Hayek, I., Berens, C., Luhrmann, A. (2019). Modulation of host cell metabolism by T4SS-encoding intracellular pathogens. Curr. Opin. Microbiol. 47, 59. doi: 10.1016/j.mib.2018.11.010
He, K. L., Ting, A. T. (2002). A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in jurkat T cells. Mol. Cell Biol. 22, 6034. doi: 10.1128/MCB.22.17.6034-6045.2002
Hong, P. C., Tsolis, R. M., Ficht, T. A. (2000). Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 68, 4102. doi: 10.1128/IAI.68.7.4102-4107.2000
Im, Y. B., Jung, M., Shin, M. K., Kim, S., Yoo, H. S. (2016). Expression of cytokine and apoptosis-related genes in bovine peripheral blood mononuclear cells stimulated with Brucella abortus recombinant proteins. Vet. Res. 47, 30. doi: 10.1186/s13567-016-0311-7
Jiao, H., Zhou, Z., Li, B., Xiao, Y., Li, M., Zeng, H., et al. (2021). The mechanism of facultative intracellular parasitism of Brucella. Int. J. Mol. Sci. 22, 3673. doi: 10.3390/ijms22073673
Karabay, A. Z., Aktan, F., Sunguroglu, A., Buyukbingol, Z. (2014). Methylsulfonylmethane modulates apoptosis of LPS/IFN-gamma-activated RAW 264.7 macrophage-like cells by targeting p53, bax, bcl-2, cytochrome c and PARP proteins. Immunopharmacol Immunotoxicol 36, 379. doi: 10.3109/08923973.2014.956752
Ke, Y., Wang, Y., W.andChen, Z. (2015). Type IV secretion system of Brucella spp. and its effectors. Front. Cell Infect. Microbiol. 5. doi: 10.3389/fcimb.2015.00072
Kim, S., Watarai, M., Suzuki, H., Makino, S., Kodama, T., Shirahata, T. (2004). Lipid raft microdomains mediate class a scavenger receptor-dependent infection of Brucella abortus. Microb. Pathog. 37, 11. doi: 10.1016/j.micpath.2004.04.002
Kurmanov, B., Zincke, D., Su, W., Hadfield, T. L., Aikimbayev, A., Karibayev, T., et al. (2022). Assays for identification and differentiation of Brucella species: a review. Microorganisms 10, 1584. doi: 10.3390/microorganisms10081584
Lamontagne, J., Forest, A., Marazzo, E., Denis, F., Butler, H., Michaud, J. F., et al. (2009). Intracellular adaptation of Brucella abortus. J. Proteome Res. 8, 1594. doi: 10.1021/pr800978p
Li, T., Xu, Y., Liu, L., Huang, M., Wang, Z., Tong, Z., et al. (2016). Brucella melitensis 16M regulates the effect of AIR domain on inflammatory factors, autophagy, and apoptosis in mouse macrophage through the ROS signaling pathway. PloS One 11, e0167486. doi: 10.1371/journal.pone.0167486
Liu, A. C. L., Ma, Q. L. (2017). Cloning and prokaryotic expression of protein-omp31 of brucellosis sheep and its effect on microglial cell apoptosis. J. Ningxia Med. Univ. 39, 667.
Lopez-Goni, I., Guzman-Verri, C., Manterola, L., Sola-Landa, A., Moriyon, I., Moreno, E. (2002). Regulation of Brucella virulence by the two-component system BvrR/BvrS. Vet. Microbiol. 90, 329. doi: 10.1016/s0378-1135(02)00218-3
Lopez-Santiago, R., Sanchez-Argaez, A. B., De Alba-Nunez, L. G., Baltierra-Uribe, S. L., Moreno-Lafont, M. C. (2019). Immune response to mucosal Brucella infection. Front. Immunol. 10. doi: 10.3389/fimmu.2019.01759
Luo, C., Laaja, P. (2004). Inhibitors of JAKs/STATs and the kinases: a possible new cluster of drugs. Drug Discovery Today 9, 268. doi: 10.1016/S1359-6446(03)03014-9
Marchesini, M. I., Morrone Seijo, S. M., Guaimas, F. F., Comerci, D. J. (2016). A T4SS effector targets host cell alpha-enolase contributing to Brucella abortus intracellular lifestyle. Front. Cell Infect. Microbiol. 6. doi: 10.3389/fcimb.2016.00153
Martinez-Nunez, C., Altamirano-Silva, P., Alvarado-Guillen, F., Moreno, E., Guzman-Verri, C., Chaves-Olarte, E. (2010). The two-component system BvrR/BvrS regulates the expression of the type IV secretion system VirB in Brucella abortus. J. Bacteriol 192, 5603. doi: 10.1128/JB.00567-10
Martirosyan, A., Moreno, E., Gorvel, J. P. (2011). An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 240, 211. doi: 10.1111/j.1600-065X.2010.00982.x
McCullough, N. B., Beal, G. A. (1951). Growth and manometric studies on carbohydrate utilization of Brucella. J. Infect. Dis. 89, 266. doi: 10.1093/infdis/89.3.266
Micheau, O., Tschopp, J. (2003). Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181. doi: 10.1016/s0092-8674(03)00521-x
Miller, C. N., Smith, E. P., Cundiff, J. A., Knodler, L. A., Bailey Blackburn, J., Lupashin, V., et al. (2017). A Brucella type IV effector targets the COG tethering complex to remodel host secretory traffic and promote intracellular replication. Cell Host Microbe 22, 317. doi: 10.1016/j.chom.2017.07.017
Myeni, S., Child, R., Ng, T. W., Kupko, J. J., 3rd, Wehrly, T. D., Porcella, S. F., et al. (2013). Brucella modulates secretory trafficking via multiple type IV secretion effector proteins. PloS Pathog. 9, e1003556. doi: 10.1371/journal.ppat.1003556
Olsen, S. C., Palmer, M. V. (2014). Advancement of knowledge of Brucella over the past 50 years. Vet. Pathol. 51, 1076. doi: 10.1177/0300985814540545
Pandey, A., Lin, F., Cabello, A. L., da Costa, L. F., Feng, X., Feng, H. Q., et al. (2018). Activation of host IRE1alpha-dependent signaling axis contributes the intracellular parasitism of Brucella melitensis. Front. Cell Infect. Microbiol. 8. doi: 10.3389/fcimb.2018.00103
Pasquali, P., Thornton, A. M., Vendetti, S., Pistoia, C., Petrucci, P., Tarantino, M., et al. (2010). CD4+CD25+ T regulatory cells limit effector T cells and favor the progression of brucellosis in BALB/c mice. Microbes Infect. 12, 3. doi: 10.1016/j.micinf.2009.09.005
Pei, J., Kahl-McDonagh, M., Ficht, T. A. (2014). Brucella dissociation is essential for macrophage egress and bacterial dissemination. Front. Cell Infect. Microbiol. 4. doi: 10.3389/fcimb.2014.00023
Pizarro-Cerda, J., Meresse, S., Parton, R. G., van der Goot, G., Sola-Landa, A., Lopez-Goni, I., et al. (1998). Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66, 5711. doi: 10.1128/IAI.66.12.5711-5724.1998
Porte, F., Liautard, J. P., Kohler, S. (1999). Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67, 4041. doi: 10.1128/IAI.67.8.4041-4047.1999
Priem, D., van Loo, G., Bertrand, M. J. M. (2020). A20 and cell death-driven inflammation. Trends Immunol. 41, 421. doi: 10.1016/j.it.2020.03.001
Roop, R. M., 2nd, Gee, J. M., Robertson, G. T., Richardson, J. M., Ng, W. L., Winkler, M. E. (2003). Brucella stationary-phase gene expression and virulence. Annu. Rev. Microbiol. 57, 57. doi: 10.1146/annurev.micro.57.030502.090803
Sola-Landa, A., Pizarro-Cerda, J., Grillo, M. J., Moreno, E., Moriyon, I., Blasco, J. M., et al. (1998). A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29, 125. doi: 10.1046/j.1365-2958.1998.00913.x
Spera, J. M., Comerci, D. J., Ugalde, J. E. (2014). Brucella alters the immune response in a prpA-dependent manner. Microb. Pathog. 67-68, 8. doi: 10.1016/j.micpath.2014.01.003
Stranahan, L. W., Arenas-Gamboa, A. M. (2021). When the going gets rough: the significance of Brucella lipopolysaccharide phenotype in host-pathogen interactions. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.713157
Sun, X. L., Chen, B. Y., Zhao, H. K., Cheng, Y. Y., Zheng, M. H., Duan, L., et al. (2016). Gas1 up-regulation is inducible and contributes to cell apoptosis in reactive astrocytes in the substantia nigra of LPS and MPTP models. J. Neuroinflamm. 13, 180. doi: 10.1186/s12974-016-0643-2
Taguchi, Y., Imaoka, K., Kataoka, M., Uda, A., Nakatsu, D., Horii-Okazaki, S., et al. (2015). Yip1A, a novel host factor for the activation of the IRE1 pathway of the unfolded protein response during Brucella infection. PloS Pathog. 11, e1004747. doi: 10.1371/journal.ppat.1004747
Uzureau, S., Lemaire, J., Delaive, E., Dieu, M., Gaigneaux, A., Raes, M., et al. (2010). Global analysis of quorum sensing targets in the intracellular pathogen Brucella melitensis 16 m. J. Proteome Res. 9, 3200. doi: 10.1021/pr100068p
Vallabhapurapu, S., Karin, M. (2009). Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693. doi: 10.1146/annurev.immunol.021908.132641
Vereecke, L., Beyaert, R., van Loo, G. (2009). The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 30, 383. doi: 10.1016/j.it.2009.05.007
Viadas, C., Rodriguez, M. C., Sangari, F. J., Gorvel, J. P., Garcia-Lobo, J. M., Lopez-Goni, I. (2010). Transcriptome analysis of the Brucella abortus BvrR/BvrS two-component regulatory system. PloS One 5, e10216. doi: 10.1371/journal.pone.0010216
Wang, F., Hu, S., Liu, W., Qiao, Z., Gao, Y., Bu, Z. (2011). Deep-sequencing analysis of the mouse transcriptome response to infection with Brucella melitensis strains of differing virulence. PloS One 6, e28485. doi: 10.1371/journal.pone.0028485
Wang, P., Qiu, W., Dudgeon, C., Liu, H., Huang, C., Zambetti, G. P., et al. (2009). PUMA is directly activated by NF-κB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ 16, 1192. doi: 10.1038/cdd.2009.51
Watarai, M., Kim, S., Erdenebaatar, J., Makino, S., Horiuchi, M., Shirahata, T., et al. (2003). Cellular prion protein promotes Brucella infection into macrophages. J. Exp. Med. 198, 5. doi: 10.1084/jem.20021980
Weeks, J. N., Galindo, C. L., Drake, K. L., Adams, G. L., Garner, H. R., Ficht, T. A. (2010). Brucella melitensis VjbR and C12-HSL regulons: contributions of the n-dodecanoyl homoserine lactone signaling molecule and LuxR homologue VjbR to gene expression. BMC Microbiol. 10, 167. doi: 10.1186/1471-2180-10-167
Wei, P., Cui, G., Lu, Q., Yang, L., Guan, Z., Sun, W., et al. (2015). A20 promotes Brucella intracellular growth via inhibition of macrophage cell death and activation. Vet. Microbiol. 175, 50. doi: 10.1016/j.vetmic.2014.11.006
Weiss, G., Schaible, U. E. (2015). Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 264, 182. doi: 10.1111/imr.12266
Wertz, I. E., O'Rourke, K. M., Zhou, H., Eby, M., Aravind, L., Seshagiri, S., et al. (2004). De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694. doi: 10.1038/nature02794
Xiong, X., Li, B., Zhou, Z., Gu, G., Li, M., Liu, J., et al. (2021). The VirB system plays a crucial role in Brucella intracellular infection. Int. J. Mol. Sci. 22, 13637. doi: 10.3390/ijms222413637
Yi, J., Wang, Y., Deng, X., Shao, Z., Zhang, H., Wang, B., et al. (2018). The role of JAK2/STAT3 signaling pathway regulation in macrophage apoptosis during Brucella M5-90 infection. Kafkas. Univ. Vet. Fak. Derg 24, 563. Available at: http://vetdergikafkas.org/uploads/pdf.
Xiao, Y, Li, M, Guo, X, Zeng, H, Shuai, X, Guo, J, et al. (2022). Inflammatory mechanism of Brucella infection in placental trophoblast cells. Int. J. Mol. Sci. 23, 13417. doi: 10.3390/ijms232113417
Keywords: Brucella, chronic infections, autophagy, metabolism, apoptosis
Citation: Guo X, Zeng H, Li M, Xiao Y, Gu G, Song Z, Shuai X, Guo J, Huang Q, Zhou B, Chu Y and Jiao H (2023) The mechanism of chronic intracellular infection with Brucella spp.. Front. Cell. Infect. Microbiol. 13:1129172. doi: 10.3389/fcimb.2023.1129172
Received: 21 December 2022; Accepted: 31 March 2023;
Published: 18 April 2023.
Edited by:
Alejandro Martin-Quiros, University Hospital La Paz, SpainReviewed by:
Tian Luo, University of Texas Medical Branch at Galveston, United StatesMałgorzata Gieryńska, Warsaw University of Life Sciences, Poland
Copyright © 2023 Guo, Zeng, Li, Xiao, Gu, Song, Shuai, Guo, Huang, Zhou, Chu and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhou, aG90dGFuazMyMTBAMTI2LmNvbQ==; Yuefeng Chu, Y2h1eXVlZmVuZ0BjYWFzLmNu; Hanwei Jiao, amlhb2hhbndlaUBzd3UuZWR1LmNu
†These authors have contributed equally to this work
 Xiaoyi Guo1
Xiaoyi Guo1 Yu Xiao
Yu Xiao Yuefeng Chu
Yuefeng Chu Hanwei Jiao
Hanwei Jiao