- 1Host-Pathogen Interface Laboratory, Department of Cell Biology, Institute of Biology, University of Brasília, Brasília, DF, Brazil
- 2Faculty of Ceilândia, University of Brasília, Brasília, DF, Brazil
1 Introduction
Vector-borne diseases contribute to over 17% of total infectious diseases (WHO, 2023). Mosquitoes, ticks, fleas, sand flies and triatomines are hematophagous arthropod vectors of debilitating pathogenic microorganisms that cause widespread human infectious diseases, such as malaria, zika, dengue fever, yellow fever, Japanese encephalitis, tick-borne encephalitis, Lyme disease, plague, rickettsiosis, leishmaniasis and Chagas disease (WHO, 2023). Scientists worldwide have been making significant contributions to the field of parasitic infections transmitted by arthropod vectors investigating their molecular biology. Arthropod proteases play essential roles in their blood-feeding behavior, egg development, and immunity (Santiago et al., 2017). Proteases are multifunctional enzymes that hydrolyze one or more peptide bonds in a protein or peptide. Their activity can result in modification/activation/inactivation of proteins, enzymes and peptides, protein targeting, and amino acids recycling (Rawlings and Salvesen, 2013). Due to their inherent involvement in many key physiological processes, inhibition or abnormal enzyme production or secretion can lead to various pathological conditions (López-Otín and Bond, 2008).
Otherwise, the remarkable substrate affinity and specificity of proteases are features that make enzyme therapy an important approach for treating/managing multiple ailments (Shankar et al., 2021). In fact, the accumulated knowledge on the catalytic and functional diversity of proteases has driven the development of therapeutic approaches for cardiovascular disease, inflammation, sepsis, digestive and retinal disorders, among others (Craik et al., 2011), and also their incorporation into dermatological products (Del Rosso, 2013). Several protease therapies have been approved by the U.S. Food and Drug Administration, and many are in clinical development (Badalamente and Hurst, 2007; Thomas and Bayat, 2010; Ranieri et al., 2012; Gelbard et al., 2013; Brunengraber et al., 2014; Lyden et al., 2019; Jadhav et al., 2020; Kaufman-Janette et al., 2021; Tamimi et al., 2021; Obed et al., 2022).
Our previous review covered contemporary advances in the proteases from hematophagous arthropod vectors up to 2016 (Santiago et al., 2017). In this opinion article, we summarize further research findings on vector proteases and emphasize their biotechnological potential for the development of innovative protease-based drugs with broad clinical applications. This potential arises from the considerable effort that has been made by high-throughput transcriptomic and proteomic approaches to catalog arthropod vector proteins, followed by structural biology and protease activity investigations.
2 Finding the molecule and its biological activity
The progress in biotechnology and the application of high-throughput sequencing technologies have unveiled a remarkable number of proteases in hematophagous arthropod vector tissues, giving rise to an emerging field of scientific exploration. Hematophagous consume blood from vertebrate hosts as a nutrient source. Their ability to locate the prey, their behavior, their mouthparts morphology, and their physiology are an interesting combination of tools very well adapted to obtain blood meals. Once the host is found at the right place and time, the hematophagous pursue their meal boldly. During the bite, the host suffers tissue and vascular injuries, which trigger a series of interrelated mechanisms, such as hemostasis, inflammation, and immune responses (Ribeiro, 1987; Ribeiro, 1995). Large-scale sialotranscriptomic (salivary glands transcriptome) and sialoproteomic (salivary glands proteome) analyses have been reported for various blood-feeding arthropods (Andersen et al., 2007; Arcà et al., 2007; Calvo et al., 2007; Assumpcao et al., 2008; Chmelar et al., 2008; Andersen et al., 2009; Alves-Silva et al., 2010; Schwarz et al., 2014; Santiago et al., 2016; Santiago et al., 2018; Praça et al., 2021) disclosing the saliva of hematophagous is indeed a potent pharmacologically active fluid capable of counteracting the hemostatic, inflammatory, and immune responses of the vertebrate host (Ribeiro, 1987; Ribeiro, 1995). The comprehensive mapping of already reported sialomes (salivary glands transcriptomic and proteomic analyses) revealed different protease families are produced by salivary glands cells (Andersen et al., 2007; Arcà et al., 2007; Calvo et al., 2007; Assumpcao et al., 2008; Chmelar et al., 2008; Andersen et al., 2009; Alves-Silva et al., 2010; Schwarz et al., 2014; Santiago et al., 2016; Santiago et al., 2018; Praça et al., 2021).
Metalloprotease and serine protease sequences have been disclosed in the salivary glands of ticks (Valenzuela et al., 2002; Harnnoi et al., 2007; Decrem et al., 2008a) and triatomines (Santiago et al., 2016; Santiago et al., 2018), and their functions are under investigation. Metalloprotease members are known to inhibit platelet aggregation and hydrolyze fibrinogen and fibronectin preventing blood clotting (Huang et al., 1993; Feitosa et al., 1998; da Silveira et al., 2007; Hsu et al., 2007; Hsu et al., 2008; Trevisan-Silva et al., 2010). Interestingly, metalloproteases are abundant in snake venoms showing important antithrombotic and hemorrhagic activities (Gutiérrez et al., 2005; Sajevic et al., 2011). In agreement, functional studies of Metis 1 and Metis 2, two metalloproteases found in the salivary glands of Ixodes ricinus ticks, have shown that these proteins play a significant role in regulating fibrinolysis (Decrem et al., 2008b). Although the exact role of these components in saliva remains unknown, it is important to explore their potential in producing peptides that can specifically target inflammation and coagulation cascades (Amino et al., 2001). This could lead to the development of more effective and precise protease-based drugs, which could be used to treat hemorrhagic and thrombotic disorders, as well as cardiovascular and cerebrovascular diseases by preventing thrombus formation.
During the digestion process of blood components in the gut of hematophagous organisms, proteases function within a network of multiple enzymes that break down hemoglobin. These multi-peptidase repertoires are mainly composed of serine proteases in mosquitoes and cysteine and aspartic proteases in ticks and triatomines (Santiago et al., 2017). Trypanosoma cruzi, the causative agent of Chagas disease, proliferates and develops inside the intestines of triatomine vectors. As part of the feeding process, while consuming blood, the vector releases T. cruzi contaminated feces onto the skin of the vertebrate host. The protozoan can infect the host through the bite injury or intact mucosae. In this context, it has been suggested that T. cruzi can modulate insect metabolism, increasing the activity levels of digestive enzymes (Borges et al., 2006; Buarque et al., 2013). The investigation of protease activity in Rhodnius prolixus triatomine unveiled distinct sequential patterns of protease expression within the insect digestive system, including cathepsin L-like and cathepsin D-like proteases (Table 1) (Henriques et al., 2020). It is important to conduct comprehensive studies to better understand the role of proteases in the trypanosome/vector interaction. We must consider whether T. cruzi modulation of digestive enzymes could enhance the infection process in humans. From this knowledge, strategies for direct intervention in the vector gut physiology or the modulation of interactions between the pathogen and digestive enzymes may be developed. This hypothesis is an attractive area of research, as the digestive enzymes produced by triatomines could potentially be used as a parasite control intervention strategy.
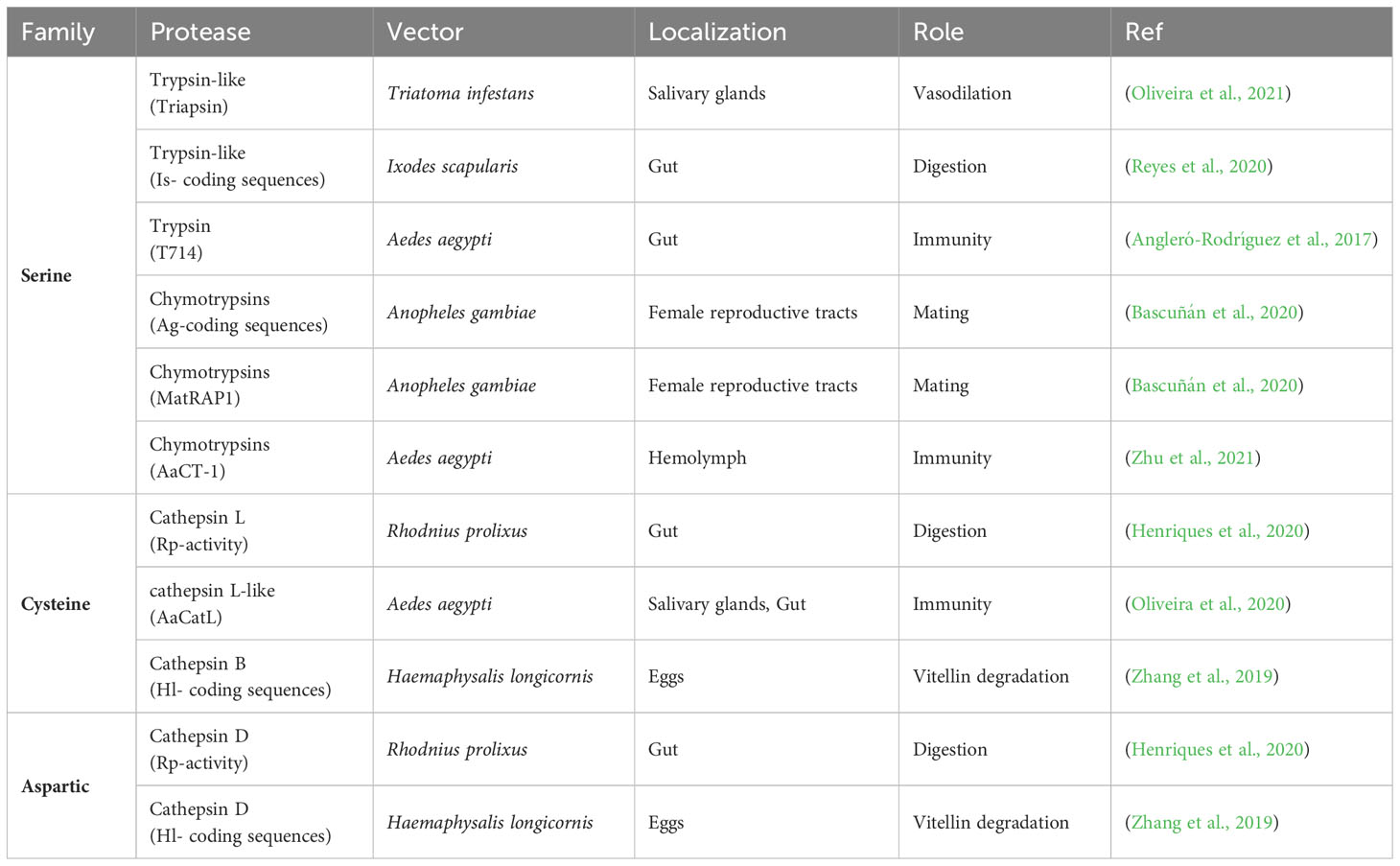
Table 1 Novel information on members from the serine, cysteine and aspartic protease families disclosed in triatomine, tick and mosquito tissues from 2017 to 2023.
Yet concerning triatomine proteases, triapsin (Table 1), a serine protease from the saliva of Triatoma infestans first described in 2001 (Amino et al., 2001) and still under investigation, is capable of inducing hydrolysis of protease-activated receptors (PARs), with a distinct preference for cleaving the PAR-2 peptide. Mass spectrometry analysis has corroborated the presence of a single cleavage site, corresponding to the activation site of the PAR-2 receptor. Moreover, nitric oxid (NO) levels measurements have demonstrated that triapsin induces a dose-dependent release of NO in cultured human umbilical vein endothelial cells. NO appears to play a role in the vasorelaxant activity of triapsin. Furthermore, observations of increased mouse ear venular diameter following triapsin exposure suggest a plausible link between triapsin activity mediated by PAR-2 and vasodilation induced by T. infestans saliva (Table 1) (Oliveira et al., 2021).
While consuming blood, ticks are capable of transmitting viral and bacterial diseases, such as tick-borne encephalitis caused by the tick-borne encephalitis virus, and Lyme disease caused by Borrelia burgdorferi spirochetes (Nuttall, 1999). Ticks have a digestive system that consists of a combination of cysteine-aspartic proteases, which operate together in hemoglobinolysis (Sojka et al., 2008). Previous studies have highlighted the significance of IrCD 1-3, which are three different isoforms of cathepsin, in playing various biological roles in Ixodes ricinus tick. These enzymes are expressed not only in gut cells but also in salivary glands and ovaries, and may generate antimicrobial peptides, that aid in immune responses against foreign invaders (Sojka et al., 2012; Sojka et al., 2016). Of interest, in ticks, trypsin-like serine proteases may participate in the liberation of dipeptides and free amino acids in the intracellular midgut vesicles and outside the digestive vesicles (Horn et al., 2009). In Ixodes scapularis, vector of Lyme disease, trypsin levels increase significantly after repletion. Knockdown of tick serine proteases was shown to lower hemoglobin degradation and negatively impacted levels of active trypsin in the midgut of I. scapularis, as well as blood feeding, survival, and fecundity in this species (Table 1) (Reyes et al., 2020). Another potential candidate for investigation is the vitellin degrading cysteine endopeptidase (VTDCE), found in Boophilus microplus tick eggs. This enzyme plays a role in both vitellogenesis and embryonic development (Seixas et al., 2003). VTDCE also possesses antimicrobial activity, particularly reported against Staphylococcus epidermidis (Oldiges et al., 2012). Proteases like IrCD and VTDCE hold significant biotechnological potential as therapeutic agents for pathogen control. More recently, it was also shown that vitellin degradation in Haemaphysalis longicornis eggs involves three enzymes: cathepsin B, cathepsin D, and acid phosphatase (Table 1) (Zhang et al., 2019).
In mosquitoes, it was demonstrated that the reproductive success of Anopheles gambiae, an important vector of the malaria parasite Plasmodium spp, relies on a single copulation event after which most females become permanently refractory to further mating. In females, two chymotrypsin‐like serine proteases regulated by the male‐synthetized steroid hormone 20‐hydroxyecdysone (20E) play an important role in modulating their susceptibility to mating. The depletion of the Mating Regulated Atrial Protease 1 (MatRAP1), one of these proteases, by RNA interference, reduced female refractoriness to further copulation, allowing a significant proportion of females mate again (Table 1) (Bascuñán et al., 2020). In Aedes aegypti, a cathepsin L-like peptidase (AaCatL) was cloned, expressed, purified, and biochemically characterized. Transcripts of AaCatL were detected in the salivary glands and midgut from Ae. aegypti and seem to be negatively correlated with DENV-2 virus titers, indicating AaCatL may have a role during mosquito-DENV interactions. Purified recombinant AaCatL has a typical cathepsin L-like substrate profile. Authors suggest AaCatL may inhibit the activation of caspases (Table 1) (Oliveira et al., 2020). In field-caught Ae. aegypti, the gut-associated fungus Talaromyces was shown to profoundly down-regulate digestive enzyme genes and trypsin activity in the mosquito and to render Ae. aegypti more permissive to DENV infection (Table 1) (Angleró-Rodríguez et al., 2017). Interestingly, it was reported that the expression of some chymotrypsins from Ae. aegypti and Aedes albopictus, such as AaCT-1 (Table 1) are suppressed by the human blood-derived microRNA hsa-miR- 150-5p, enhancing DENV and ZIKV loads in these mosquitoes (Table 1) (Zhu et al., 2021).
Table 1 presents novel information on eleven members from the serine, cysteine and aspartic protease families disclosed in triatomine, tick and mosquito tissues, from 2017 to 2023. For a list of proteases from hematophagous arthropod vectors disclosed before this period, we suggest the information that was written in our previous publication (Santiago et al., 2017).
3 Maxadilan, the vasodilator from sand flies
Turning to the clinical applications of proteins from hematophagous arthropod vectors, although not a protease, a potent vasodilator peptide, named maxadilan, from the salivary glands of Lutzomyia longipalpis, the sand fly that transmits Leishmania spp., selectively and potently activate the PAC1 receptor (Lerner et al., 2007), following intradermal injection (Marynissen et al., 2022). This receptor is activated in the pathophysiology of migraine. Maxadilan was proposed to be used as a novel pharmacodynamic biomarker for the early clinical development of PAC1 receptor antagonists (Marynissen et al., 2022), highlighting the potential clinical use of proteins from hematophagous arthropod vectors.
4 The ongoing challenge
Developing new therapies for infectious diseases transmitted by arthropod vectors, as well as for controlling disorders that affect platelet function and blood clotting is a considerable challenge. Understanding the functional role of proteins is a significant task of the post-genome research era. The journey from discovering a promising compound to progressing to human clinical trials and reaching the market spans many years and entails substantial financial investments. However, these paths begin with fundamental science. In this opinion article, we wanted to emphasize the biotechnological potential of hematophagous vector proteases. Realizing this potential requires a deeper research interest in this area and a commitment to developing effective methodologies for structural and functional studies.
Author contributions
CA: Conceptualization, Project administration, Writing – review & editing. PS: Conceptualization, Project administration, Writing – review & editing. GC: Writing – original draft. GS: Writing – original draft. RM: Writing – original draft. IB: Writing – review & editing. JS: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants awarded by the Fundação de Apoio à Pesquisa do Distrito Federal (FAP-DF, grants 00193-00000825/2021-19 and 00193-00002600/2022-70), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, grant 88881.711954/2022-0 CAPES-COFECUB), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, INCT-MCTI/CNPq/CAPES/FAPs 16/2014), Financiadora de Estudos e Projetos (Finep, grant CT-Infra 0439/11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alves-Silva, J., Ribeiro, J. M., Van Den Abbeele, J., Attardo, G., Hao, Z., Haines, L. R., et al. (2010). An insight into the sialome of Glossina morsitans morsitans. BMC Genomics 11, 213. doi: 10.1186/1471-2164-11-213
Amino, R., Tanaka, A. S., Schenkman, S. (2001). Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas' disease Triatoma infestans (Hemiptera: Reduviidae). Insect Biochem. Mol. Biol. 31 (4-5), 465–472. doi: 10.1016/s0965-1748(00)00151-x
Andersen, J. F., Hinnebusch, B. J., Lucas, D. A., Conrads, T. P., Veenstra, T. D., Pham, V. M., et al. (2007). An insight into the sialome of the oriental rat flea, Xenopsylla cheopis (Rots). BMC Genomics 8, 102. doi: 10.1186/1471-2164-8-102
Andersen, J. F., Pham, V. M., Meng, Z., Champagne, D. E., Ribeiro, J. M. (2009). Insight into the sialome of the Black Fly, Simulium vittatum. J. Proteome Res. 8 (3), 1474–1488. doi: 10.1021/pr8008429
Angleró-Rodríguez, Y. I., Talyuli, O. A., Blumberg, B. J., Kang, S., Demby, C., Shields, A., et al. (2017). An Aedes aEgypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. Elife 6. doi: 10.7554/eLife.28844
Arcà, B., Lombardo, F., Francischetti, I. M., Pham, V. M., Mestres-Simon, M., Andersen, J. F., et al. (2007). An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem. Mol. Biol. 37 (2), 107–127. doi: 10.1016/j.ibmb.2006.10.007
Assumpcao, T. C., Francischetti, I. M., Andersen, J. F., Schwarz, A., Santana, J. M., Ribeiro, J. M. (2008). An insight into the sialome of the blood-sucking bug Triatoma infestans, a vector of Chagas' disease. Insect Biochem. Mol. Biol. 38 (2), 213–232. doi: 10.1016/j.ibmb.2007.11.001. doi.org/S0965-1748(07)00253-6.
Badalamente, M. A., Hurst, L. C. (2007). Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren's contracture. J. Handb. Surg. Am. 32 (6), 767–774. doi: 10.1016/j.jhsa.2007.04.002
Bascuñán, P., Gabrieli, P., Mameli, E., Catteruccia, F. (2020). Mating-regulated atrial proteases control reinsemination rates in Anopheles Gambiae females. Sci. Rep. 10 (1), 21974. doi: 10.1038/s41598-020-78967-y
Borges, E. C., MaChado, E. M., Garcia, E. S., Azambuja, P. (2006). Trypanosoma cruzi: effects of infection on cathepsin D activity in the midgut of Rhodnius prolixus. Exp. Parasitol. 112 (2), 130–133. doi: 10.1016/j.exppara.2005.09.008
Brunengraber, L. N., Jayes, F. L., Leppert, P. C. (2014). Injectable Clostridium histolyticum collagenase as a potential treatment for uterine fibroids. Reprod. Sci. 21 (12), 1452–1459. doi: 10.1177/1933719114553449
Buarque, D. S., Braz, G. R., Martins, R. M., Tanaka-Azevedo, A. M., Gomes, C. M., Oliveira, F. A., et al. (2013). Differential expression profiles in the midgut of Triatoma infestans infected with Trypanosoma cruzi. PloS One 8 (5), e61203. doi: 10.1371/journal.pone.0061203
Calvo, E., Dao, A., Pham, V. M., Ribeiro, J. M. (2007). An insight into the sialome of Anopheles funestus reveals an emerging pattern in anopheline salivary protein families. Insect Biochem. Mol. Biol. 37 (2), 164–175. doi: 10.1016/j.ibmb.2006.11.005
Chmelar, J., Anderson, J. M., Mu, J., Jochim, R. C., Valenzuela, J. G., Kopecký, J. (2008). Insight into the sialome of the castor bean tick, Ixodes ricinus. BMC Genomics 9, 233. doi: 10.1186/1471-2164-9-233
Craik, C. S., Page, M. J., Madison, E. L. (2011). Proteases as therapeutics. Biochem. J. 435 (1), 1–16. doi: 10.1042/BJ20100965
da Silveira, R. B., Wille, A. C., Chaim, O. M., Appel, M. H., Silva, D. T., Franco, C. R., et al. (2007). Identification, cloning, expression and functional characterization of an astacin-like metalloprotease toxin from Loxosceles intermedia (brown spider) venom. Biochem. J. 406 (2), 355–363. doi: 10.1042/BJ20070363
Decrem, Y., Beaufays, J., Blasioli, V., Lahaye, K., Brossard, M., Vanhamme, L., et al. (2008a). A family of putative metalloproteases in the salivary glands of the tick Ixodes ricinus. FEBS J. 275 (7), 1485–1499. doi: 10.1111/j.1742-4658.2008.06308.x
Decrem, Y., Mariller, M., Lahaye, K., Blasioli, V., Beaufays, J., Zouaoui Boudjeltia, K., et al. (2008b). The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. Int. J. Parasitol. 38 (5), 549–560. doi: 10.1016/j.ijpara.2007.09.003
Del Rosso, J. Q. (2013). Application of protease technology in dermatology: rationale for incorporation into skin care with initial observations on formulations designed for skin cleansing, maintenance of hydration, and restoration of the epidermal permeability barrier. J. Clin. Aesthet Dermatol. 6 (6), 14–22.
Feitosa, L., Gremski, W., Veiga, S. S., Elias, M. C., Graner, E., Mangili, O. C., et al. (1998). Detection and characterization of metalloproteinases with gelatinolytic, fibronectinolytic and fibrinogenolytic activities in brown spider (Loxosceles intermedia) venom. Toxicon 36 (7), 1039–1051. doi: 10.1016/s0041-0101(97)00083-4
Gelbard, M., Goldstein, I., Hellstrom, W. J., McMahon, C. G., Smith, T., Tursi, J., et al. (2013). Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J. Urol 190 (1), 199–207. doi: 10.1016/j.juro.2013.01.087
Gutiérrez, J. M., Rucavado, A., Escalante, T., Díaz, C. (2005). Hemorrhage induced by snake venom metalloproteinases: biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 45 (8), 997–1011. doi: 10.1016/j.toxicon.2005.02.029
Harnnoi, T., Sakaguchi, T., Nishikawa, Y., Xuan, X., Fujisaki, K. (2007). Molecular characterization and comparative study of 6 salivary gland metalloproteases from the hard tick, Haemaphysalis longicornis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 147 (1), 93–101. doi: 10.1016/j.cbpb.2006.12.008
Henriques, B. S., Gomes, B., Oliveira, P. L., Garcia, E. S., Azambuja, P., Genta, F. A. (2020). Characterization of the temporal pattern of blood protein digestion in. Front. Physiol. 11. doi: 10.3389/fphys.2020.509310
Horn, M., Nussbaumerová, M., Sanda, M., Kovárová, Z., Srba, J., Franta, Z., et al. (2009). Hemoglobin digestion in blood-feeding ticks: mapping a multipeptidase pathway by functional proteomics. Chem. Biol. 16 (10), 1053–1063. doi: 10.1016/j.chembiol.2009.09.009
Hsu, C. C., Wu, W. B., Chang, Y. H., Kuo, H. L., Huang, T. F. (2007). Antithrombotic effect of a protein-type I class snake venom metalloproteinase, kistomin, is mediated by affecting glycoprotein Ib-von Willebrand factor interaction. Mol. Pharmacol. 72 (4), 984–992. doi: 10.1124/mol.107.038018
Hsu, C. C., Wu, W. B., Huang, T. F. (2008). A snake venom metalloproteinase, kistomin, cleaves platelet glycoprotein VI and impairs platelet functions. J. Thromb. Haemost. 6 (9), 1578–1585. doi: 10.1111/j.1538-7836.2008.03071.x
Huang, T. F., Chang, M. C., Teng, C. M. (1993). Antiplatelet protease, kistomin, selectively cleaves human platelet glycoprotein Ib. Biochim. Biophys. Acta 1158 (3), 293–299. doi: 10.1016/0304-4165(93)90028-7
Jadhav, S. B., Shah, N., Rathi, A., Rathi, V. (2020). Serratiopeptidase: Insights into the therapeutic applications. Biotechnol. Rep. (Amst) 28, e00544. doi: 10.1016/j.btre.2020.e00544
Kaufman-Janette, J., Joseph, J. H., Kaminer, M. S., Clark, J., Fabi, S. G., Gold, M. H., et al. (2021). Collagenase clostridium histolyticum-aaes for the treatment of cellulite in women: results from two phase 3 randomized, placebo-Controlled trials. Dermatol. Surg. 47 (5), 649–656. doi: 10.1097/DSS.0000000000002952
Lerner, E. A., Iuga, A. O., Reddy, V. B. (2007). Maxadilan, a PAC1 receptor agonist from sand flies. Peptides 28 (9), 1651–1654. doi: 10.1016/j.peptides.2007.06.021
López-Otín, C., Bond, J. S. (2008). Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 283 (45), 30433–30437. doi: 10.1074/jbc.R800035200
Lyden, P., Pryor, K. E., Coffey, C. S., Cudkowicz, M., Conwit, R., Jadhav, A., et al. (2019). Final results of the RHAPSODY trial: A multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, A recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann. Neurol. 85 (1), 125–136. doi: 10.1002/ana.25383
Marynissen, H., Buntinx, L., Bamps, D., Depre, M., Ampe, E., Van Hecken, A., et al. (2022). First-in-human development of a pharmacodynamic biomarker for PAC. Clin. Transl. Sci. 15 (8), 1968–1977. doi: 10.1111/cts.13309
Nuttall, P. A. (1999). Pathogen-tick-host interactions: Borrelia burgdorferi and TBE virus. Zentralbl Bakteriol 289 (5-7), 492–505. doi: 10.1016/s0934-8840(99)80002-4
Obed, D., Salim, M., Schlottmann, F., Bingoel, A. S., Panayi, A. C., Dastagir, K., et al. (2022). Short-term efficacy and adverse effects of collagenase clostridium histolyticum injections, percutaneous needle fasciotomy and limited fasciectomy in the treatment of Dupuytren's contracture: a network meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 23 (1), 939. doi: 10.1186/s12891-022-05894-6
Oldiges, D. P., Parizi, L. F., Zimmer, K. R., Lorenzini, D. M., Seixas, A., Masuda, A., et al. (2012). A Rhipicephalus (Boophilus) microplus cathepsin with dual peptidase and antimicrobial activity. Int. J. Parasitol. 42 (7), 635–645. doi: 10.1016/j.ijpara.2012.04.013
Oliveira, F. A. A., Buri, M. V., Rodriguez, B. L., Costa-da-Silva, A. L., Araújo, H. R. C., Capurro, M. L., et al. (2020). The first characterization of a cystatin and a cathepsin L-like peptidase from Aedes aEgypti and their possible role in DENV infection by the modulation of apoptosis. Int. J. Biol. Macromol 146, 141–149. doi: 10.1016/j.ijbiomac.2019.12.010
Oliveira, K. A., Torquato, R. J. S., Lustosa, D. C. G. G., Ribeiro, T., Nascimento, B. W. L., de Oliveira, L. C. G., et al. (2021). Proteolytic activity of Triatoma infestans saliva associated with PAR-2 activation and vasodilation. J. Venom Anim. Toxins Incl Trop. Dis. 27, e20200098. doi: 10.1590/1678-9199-JVATITD-2020-0098
Praça, Y. R., Santiago, P. B., Charneau, S., Mandacaru, S. C., Bastos, I. M. D., Bentes, K. L. D. S., et al. (2021). An integrative sialomic analysis reveals molecules from. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.798924
Ranieri, V. M., Thompson, B. T., Barie, P. S., Dhainaut, J. F., Douglas, I. S., Finfer, S., et al. (2012). Drotrecogin alfa (activated) in adults with septic shock. N Engl. J. Med. 366 (22), 2055–2064. doi: 10.1056/NEJMoa1202290
Rawlings, N. D., Salvesen, G. (2013). Handbook of Proteolytic Enzymes Vol. 3 (Academic Press, Oxford, United King: Academic Press). doi: 10.1016/B978-0-12-382219-2.00848-6
Reyes, J., Ayala-Chavez, C., Sharma, A., Pham, M., Nuss, A. B., Gulia-Nuss, M. (2020). Blood digestion by trypsin-like serine proteases in the replete lyme disease vector tick, Ixodes scapularis. Insects 11, 201 (3). doi: 10.3390/insects11030201
Ribeiro, J. (1987). Role of saliva in blood-feeding by arthropods. Annu. Rev. entomology 32 (1), 463–478. doi: 10.1146/annurev.en.32.010187.002335
Ribeiro, J. M. (1995). Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 4 (3), 143–152.
Sajevic, T., Leonardi, A., Križaj, I. (2011). Haemostatically active proteins in snake venoms. Toxicon 57 (5), 627–645. doi: 10.1016/j.toxicon.2011.01.006
Santiago, P. B., Assumpção, T. C., de Araújo, C. N., Bastos, I. M., Neves, D., da Silva, I. G., et al. (2016). A Deep Insight into the Sialome of Rhodnius neglectus, a Vector of Chagas Disease. PLoS Negl. Trop. Dis. 10 (4), e0004581. doi: 10.1371/journal.pntd.0004581
Santiago, P. B., de Araújo, C. N., Charneau, S., Bastos, I. M. D., Assumpção, T. C. F., Queiroz, R. M. L., et al. (2018). Exploring the molecular complexity of Triatoma dimidiata sialome. J. Proteomics 174, 47–60. doi: 10.1016/j.jprot.2017.12.016
Santiago, P. B., de Araújo, C. N., Motta, F. N., Praça, Y. R., Charneau, S., Bastos, I. M., et al. (2017). Proteases of haematophagous arthropod vectors are involved in blood-feeding, yolk formation and immunity - a review. Parasit Vectors 10 (1), 79. doi: 10.1186/s13071-017-2005-z
Schwarz, A., Medrano-Mercado, N., Schaub, G. A., Struchiner, C. J., Bargues, M. D., Levy, M. Z., et al. (2014). An updated insight into the Sialotranscriptome of Triatoma infestans: developmental stage and geographic variations. PLoS Negl. Trop. Dis. 8 (12), e3372. doi: 10.1371/journal.pntd.0003372
Seixas, A., Dos Santos, P. C., Velloso, F. F., Da Silva Vaz, I., Masuda, A., Horn, F., et al. (2003). A Boophilus microplus vitellin-degrading cysteine endopeptidase. Parasitology 126 (Pt 2), 155–163. doi: 10.1017/s0031182002002731
Shankar, R., Upadhyay, P. K., Kumar, M. (2021). Protease enzymes: highlights on potential of proteases as therapeutics agents. Int. J. Pept. Res. Ther. 27, 1281–1296. doi: 10.1007/s10989-021-10167-2
Sojka, D., Franta, Z., Frantová, H., Bartosová, P., Horn, M., Váchová, J., et al. (2012). Characterization of gut-associated cathepsin D hemoglobinase from tick Ixodes ricinus (IrCD1). J. Biol. Chem. 287 (25), 21152–21163. doi: 10.1074/jbc.M112.347922
Sojka, D., Franta, Z., Horn, M., Hajdusek, O., Caffrey, C. R., Mares, M., et al. (2008). Profiling of proteolytic enzymes in the gut of the tick Ixodes ricinus reveals an evolutionarily conserved network of aspartic and cysteine peptidases. Parasit Vectors 1 (1), 7. doi: 10.1186/1756-3305-1-7
Sojka, D., Hartmann, D., Bartošová-Sojková, P., Dvořák, J. (2016). Parasite cathepsin D-like peptidases and their relevance as therapeutic targets. Trends Parasitol. 32 (9), 708–723. doi: 10.1016/j.pt.2016.05.015
Tamimi, Z., Al Habashneh, R., Hamad, I., Al-Ghazawi, M., Roqa'a, A. A., Kharashgeh, H. (2021). Efficacy of serratiopeptidase after impacted third molar surgery: a randomized controlled clinical trial. BMC Oral. Health 21 (1), 91. doi: 10.1186/s12903-021-01451-0
Thomas, A., Bayat, A. (2010). The emerging role of Clostridium histolyticum collagenase in the treatment of Dupuytren disease. Ther. Clin. Risk Manag 6, 557–572. doi: 10.2147/TCRM.S8591
Trevisan-Silva, D., Gremski, L. H., Chaim, O. M., da Silveira, R. B., Meissner, G. O., Mangili, O. C., et al. (2010). Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles). Biochimie 92 (1), 21–32. doi: 10.1016/j.biochi.2009.10.003
Valenzuela, J. G., Francischetti, I. M., Pham, V. M., Garfield, M. K., Mather, T. N., Ribeiro, J. M. (2002). Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 205 (Pt 18), 2843–2864. doi: 10.1242/jeb.205.18.2843
WHO (2023). Vector-borne diseases. Available at: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
Zhang, T. T., Qiu, Z. X., Li, Y., Wang, W. Y., Li, M. M., Guo, P., et al. (2019). The mRNA expression and enzymatic activity of three enzymes during embryonic development of the hard tick Haemaphysalis longicornis. Parasit Vectors 12 (1), 96. doi: 10.1186/s13071-019-3360-8
Keywords: protease, arthropod vector, hematophagous vector, biothechnological potential, protease-based therapy
Citation: de Araújo CN, Santiago PB, Causin Vieira G, Silva GdS, Moura RP, Bastos IMD and Santana JMd (2023) The biotechnological potential of proteases from hematophagous arthropod vectors. Front. Cell. Infect. Microbiol. 13:1287492. doi: 10.3389/fcimb.2023.1287492
Received: 01 September 2023; Accepted: 09 October 2023;
Published: 26 October 2023.
Edited by:
Martina Paoletta, Instituto Nacional de Tecnología Agropecuaria, ArgentinaReviewed by:
Isaura Simões, University of Coimbra, PortugalCopyright © 2023 de Araújo, Santiago, Causin Vieira, Silva, Moura, Bastos and Santana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula Beatriz Santiago, cGF1bGEuYmVhdHJpekB1bmIuYnI=
 Carla Nunes de Araújo
Carla Nunes de Araújo Paula Beatriz Santiago
Paula Beatriz Santiago Giulia Causin Vieira
Giulia Causin Vieira Gabriel dos Santos Silva
Gabriel dos Santos Silva Renan Pereira Moura
Renan Pereira Moura Izabela Marques Dourado Bastos
Izabela Marques Dourado Bastos Jaime Martins de Santana
Jaime Martins de Santana