- 1Department of Orthopaedics, Henan Provincial People’s Hospital, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou, Henan, China
- 2Department of Orthopaedics, Second Affiliated Hospital of Luohe Medical College, Luohe, Henan, China
- 3Department of Orthopaedics, Huaihe Hospital of Henan University, Kaifeng, Henan, China
- 4Department of Traditional Chinese Orthopedics, First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan, China
Background: While the value of blood coagulation markers, such as D-Dimer, Fibrinogen, platelet count/mean platelet volume ratio (PC/MPV), and Fibrin Degradation Product (FDP), in the diagnosis of periprosthetic joint infection (PJI) has been explored in recent years, the significance of synovial fluid coagulation markers in PJI diagnosis remains unclear. Therefore, this study aims to investigate the potential value of synovial fluid D-Dimer (sD-Dimer) and synovial fluid FDP (sFDP) in the diagnosis of PJI.
Materials and methods: In a prospective study, the levels of serum C-reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), sD-Dimer, and sFDP were measured and compared in 56 patients with PJI (Group A) and 40 patients with aseptic loosening (Group B) who presented at our department from March 1st, 2020, to December 31st, 2023. The diagnostic efficacy of these markers in PJI diagnosis was assessed using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve.
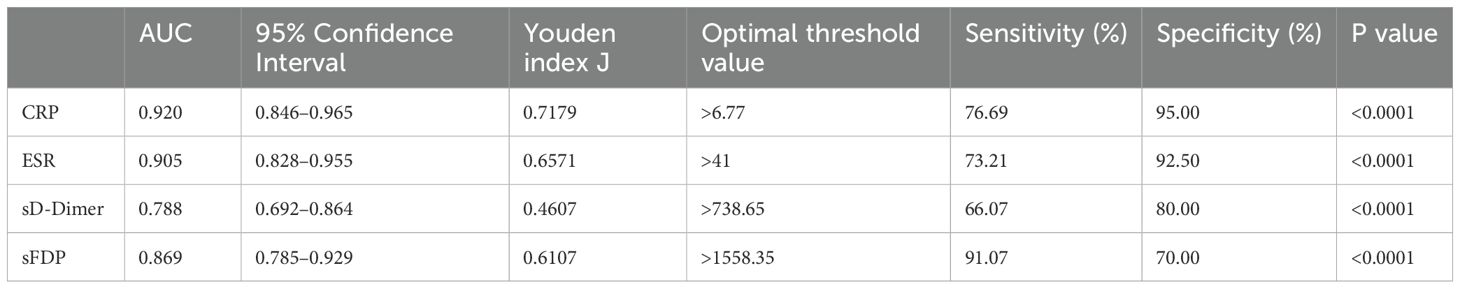
Results: The levels of CRP, ESR, sD-Dimer, and sFDP in Group A were significantly higher than the levels in Group B. The AUC values, optimal threshold values, sensitivity, and specificity for CRP, ESR, sD-Dimer, and sFDP in PJI diagnosis were as follows: CRP [0.920 (95% confidence interval (CI), 0.846–0.965), >6.77, 76.69%, 95.00%], ESR [0.905 (95% CI, 0.828–0.955), >41, 73.21%, 92.50%], sD-Dimer [0.788 (95% CI, 0.692–0.864), >738.65, 66.07%, 80.00%], and sFDP [0.869 (95% CI, 0.785–0.929), >1558.35, 91.07%, 70.00%]. Furthermore, sFDP demonstrated similar performance in PJI diagnosis to CRP and ESR, while sD-Dimer exhibited inferior performance in PJI diagnosis compared to CRP and ESR.
Conclusions: sFDP shows promise as a valuable new adjunctive diagnostic marker for PJI. Further investigations with larger sample sizes are warranted.
Introduction
Despite the publication of guidelines by the 2010 American Academy of Orthopedic Surgeons (Della Valle et al., 2011), 2013 Musculoskeletal Infection Society (MSIS) (Parvizi and Gehrke, 2014), 2018 International Consensus Meeting (ICM) (Parvizi et al., 2018), 2014 European Bone and Joint Infection Society (EBJIS) (Tande and Patel, 2014), and 2021 EBJIS criteria (McNally et al., 2021) for PJI diagnosis, the timely and accurate diagnosis of periprosthetic joint infection (PJI) remains challenging.
Synovial fluid, also referred to as joint fluid, is situated within the articular joint cavity. It undergoes significant alterations during joint pathology and is considered a crucial component of the diagnostic algorithm for confirming or excluding PJI (Vale et al., 2023). Traditionally, the analysis of synovial fluid has primarily focused on parameters such as synovial leukocyte count, synovial polymorphonuclear percentage (sPMN%), and bacterial culture (Squire et al., 2011). However, the optimal thresholds and diagnostic value of synovial leukocyte count and sPMN% in PJI diagnosis remain subjects of debate (Yi et al., 2015; Pagliaccetti et al., 2021; Abdelaziz et al., 2022). Furthermore, synovial culture has demonstrated only moderate accuracy, with the confirmation or exclusion of PJI often requiring several days of culture (Carli et al., 2019). Consequently, the evaluation of numerous novel synovial markers, including calprotectin (Peng et al., 2022), interleukin-6 (Mihalic et al., 2020), S100 calcium-binding protein A8 (S100A8), S100 calcium-binding protein A9 (S100A9) (Xu et al., 2023), lactate glucose ratio (Berthoud et al., 2020), D-lactate (Karbysheva et al., 2020), and α-Defensin (Zeng et al., 2021) for diagnosing PJI has been undertaken. While some of these emerging synovial markers show promising performance in PJI diagnosis, their widespread adoption in routine clinical practice is hindered by the requirement for specialized antibodies and equipment. Therefore, it is imperative to identify convenient and efficient synovial markers for the diagnosis of PJI.
Although coagulation markers have traditionally been utilized for detecting venous thromboembolism, recent studies have indicated that elevated blood coagulation markers, such as D-Dimer (Shahi et al., 2017; Huang et al., 2019), Fibrinogen (Huang et al., 2021), platelet count/mean platelet volume ratio (PC/MPV) (Paziuk et al., 2020; Sahin et al., 2021) and Fibrin Degradation Product (FDP) (Fujimoto et al., 2018; Xu et al., 2019) may serve as indicators of PJI. Furthermore, research has shown that sFDP and sD-Dimer are markedly expressed in the synovium during inflammatory conditions like rheumatoid arthritis (Anil et al., 2022), and the expression of sD-Dimer is notably elevated in foals with septic joints compared to those without infection (Ewald, 1989). Nevertheless, the applicability of sFDP and sD-Dimer in the diagnosis of PJI remains uncertain.
As C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are widely recommended as PJI diagnostic markers in various guidelines (Della Valle et al., 2011; Parvizi and Gehrke, 2014; Tande and Patel, 2014; Parvizi et al., 2018; McNally et al., 2021), the objective of this investigation is to assess the diagnostic utility of sD-Dimer and sFDP in the context of PJI compared with CRP and ESR. Our hypothesis posits that: (i) levels of sD-Dimer and sFDP in PJI patients will exhibit elevation compared to individuals with aseptic loosening; (ii) sD-Dimer and sFDP will demonstrate comparable diagnostic efficacy in PJI detection when compared with CRP and ESR.
Materials and methods
This study was conducted in strict adherence to the ethical guidelines outlined in the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects) and received approval from the Ethics Board of Henan Provincial People’s Hospital (Approval No. 202080). Prior to participation, informed consent was obtained from all participants or their legally authorized representatives, ensuring the protection of their rights and welfare throughout the research process.
Inclusion criteria
The inclusion criteria for this study encompassed patients who presented with either chronic PJI or aseptic loosening and subsequently underwent relevant treatments, including conservative management, debridement, antibiotics, and implant retention (DAIR) surgery, prosthesis removal with antibiotic bone cement spacer implantation surgery, or revision arthroplasty at our institution between March 1, 2020, and December 31, 2023.
Exclusion criteria
The exclusion criteria for this study comprised patients who met any of the following conditions: 1) a history of anticoagulant therapy within the preceding 2 weeks; 2) recent joint dislocation or trauma occurring within the past 2 weeks; 3) presence of systemic inflammatory conditions, such as rheumatoid arthritis, systemic lupus erythematosus (SLE), psoriasis, polymyalgia rheumatica, or inflammatory bowel disease (IBD); 4) history of hypercoagulable disorders; 5) synovial fluid samples contaminated with blood; 6) initial synovial sample concentrations of sD-Dimer and sFDP falling below the lower limits of detection of the analytical instrument; 7) post-dilution of synovial samples (10-fold, 20-fold, 40-fold and 80-fold dilution were achieved by mixing the sample with respective volume of saline solution.) resulting in sD-Dimer and sFDP concentrations surpassing the upper limits of detection of the instrument; and 8) presence of tumors.
Study Population
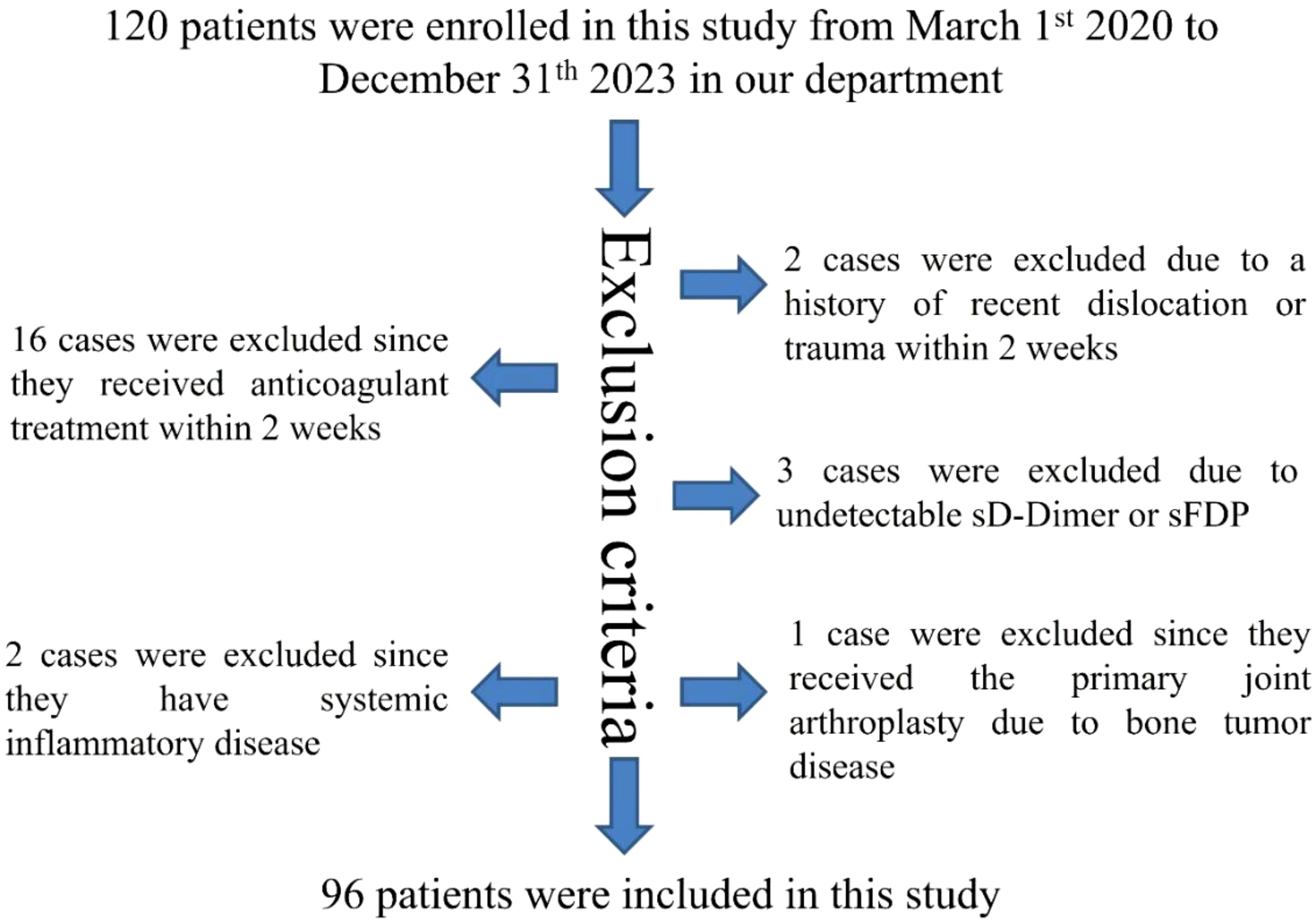
A total of 120 patients were recruited for this study between March 1, 2020, and December 31, 2023, at our department. Among them, 16 cases were excluded due to recent anticoagulant therapy within 2 weeks, 2 cases were excluded for recent joint dislocation or trauma within the same timeframe, 3 cases were excluded for undetectable levels of (sD-Dimer or sFDP, 2 cases were excluded for systemic inflammatory conditions, and 1 case was excluded for undergoing primary joint arthroplasty for bone tumor disease. Ultimately, a cohort of 96 patients met the study’s inclusion and exclusion criteria, as illustrated in Figure 1.
Definition of PJI and aseptic loosening
PJI was defined using the MSIS criteria (Della Valle et al., 2011). Aseptic loosening was defined using the criteria in our previous published paper (Ewald, 1989; Anil et al., 2022).
Measuring methods
Preoperative levels of serum CRP and ESR were assessed prior to surgery. Synovial fluid samples were obtained either before or during the surgical procedure. Following collection, the synovial fluid samples were placed in tubes containing 3.8% sodium citrate solution (at a ratio of 1:9 citrate to synovial fluid). Within the initial 2 hours post-collection, the samples underwent centrifugation at 1000× g for 10 minutes, after which the supernatants were carefully pipetted and stored at -80°C for subsequent analyses. The concentrations of sD-Dimer and sFDP were determined using an automated coagulation analyzer (Sysmex Europe, CS-5100) with commercially available reagents and controls as per the manufacturer’s instructions. In cases where the concentrations of sD-Dimer or sFDP in the original synovial sample exceeded the upper limits of the analyzer’s detection range, 10-fold, 20-fold, 40-fold and 80-fold dilution were achieved by mixing the sample with respective volume of saline solution. The reported results were adjusted by the corresponding dilution factor.
Statistical analysis
The normality of quantitative data was initially assessed using the Kolmogorov-Smirnov test. Data conforming to a normal distribution were presented as mean ± standard deviation, and between-group comparisons of quantitative data were conducted using the Independent-sample t test. Non-normally distributed data were expressed as Median (Interquartile Range), and non-parametric methods were employed for between-group quantitative comparisons. Categorical data were compared using the Chi-square test (χ2). All statistical analyses were performed using IBM SPSS Statistics (version 19, IBM SPSS Software).
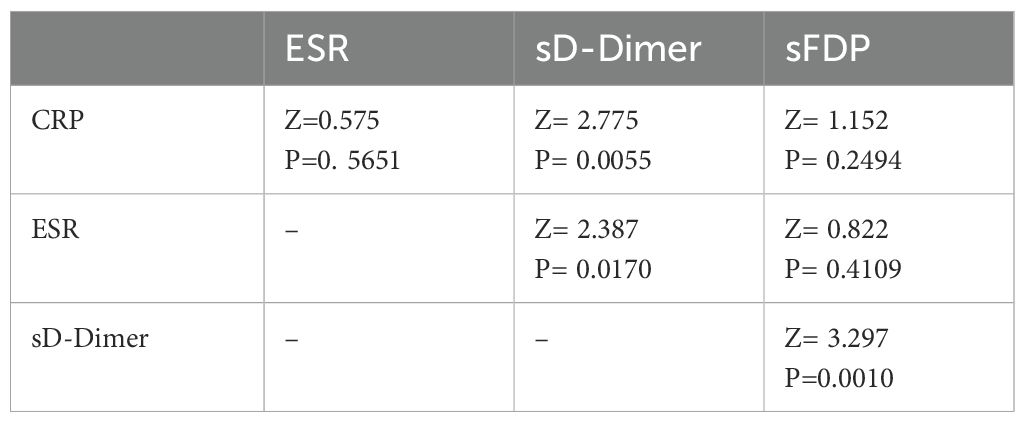
The diagnostic performance of CRP, ESR, sD-Dimer, and sFDP in PJI diagnosis was evaluated through receiver operating characteristic (ROC) analyses using MedCalc 19.0.4 (MedCalc Software, Ostend, Belgium). Sensitivity, specificity, and the area under the ROC curve (AUC) were key parameters assessed. DeLong’s test (DeLong et al., 1988) was utilized to compare the AUC values between CRP and ESR, CRP and sD-Dimer, CRP and sFDP, ESR and sD-Dimer, as well as ESR and sFDP. A significance level of p < 0.05 was considered indicative of a statistically significant difference.
Results
General information of participants
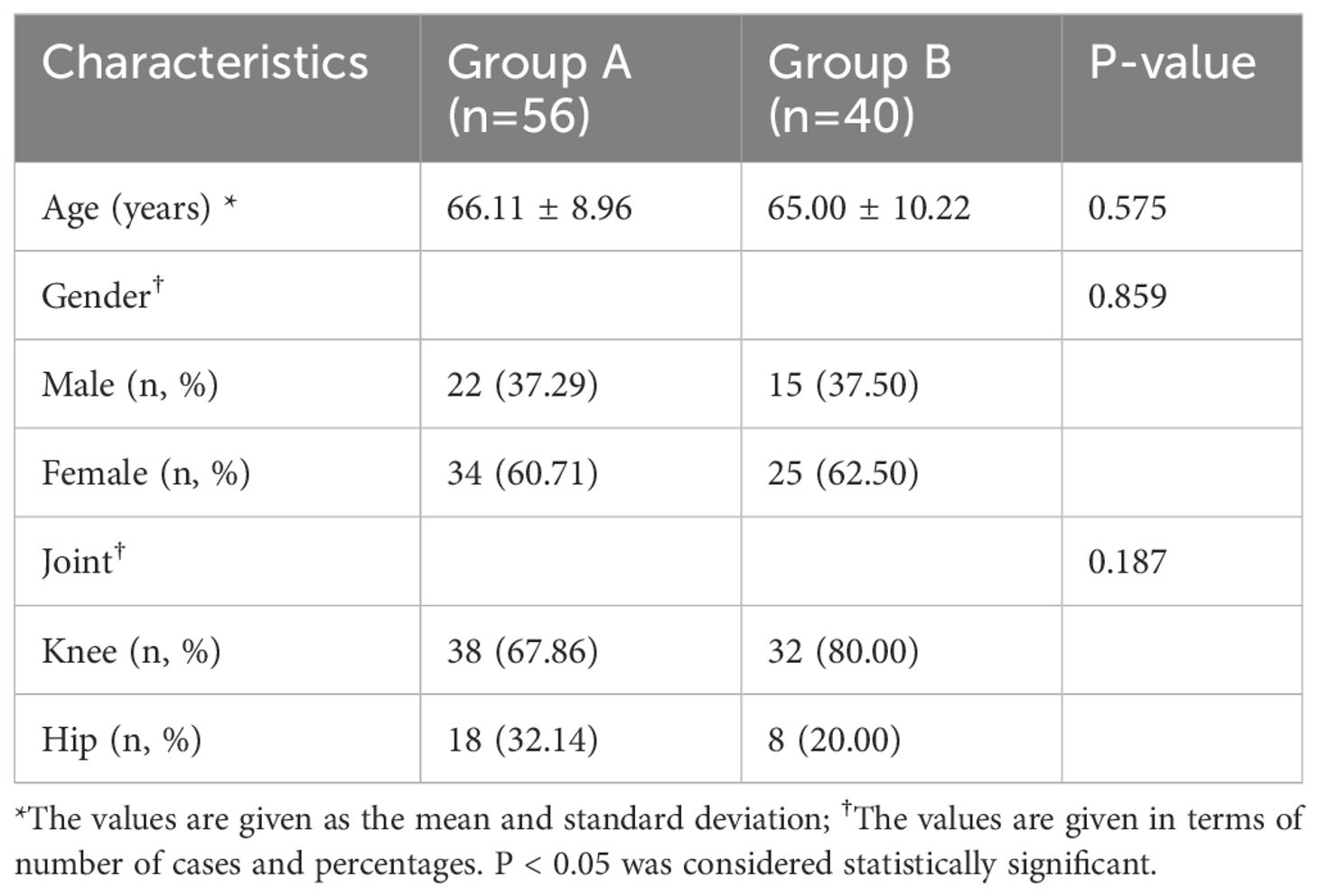
Patients were stratified into two groups: group A (comprising 56 patients with periprosthetic joint infection) and group B (comprising 40 patients with aseptic loosening). Detailed patient demographics are summarized in Table 1, showing no significant differences in baseline characteristics between the two groups.
Comparison of PJI diagnostic value of CRP, ESR, sD-Dimer and s-FDP
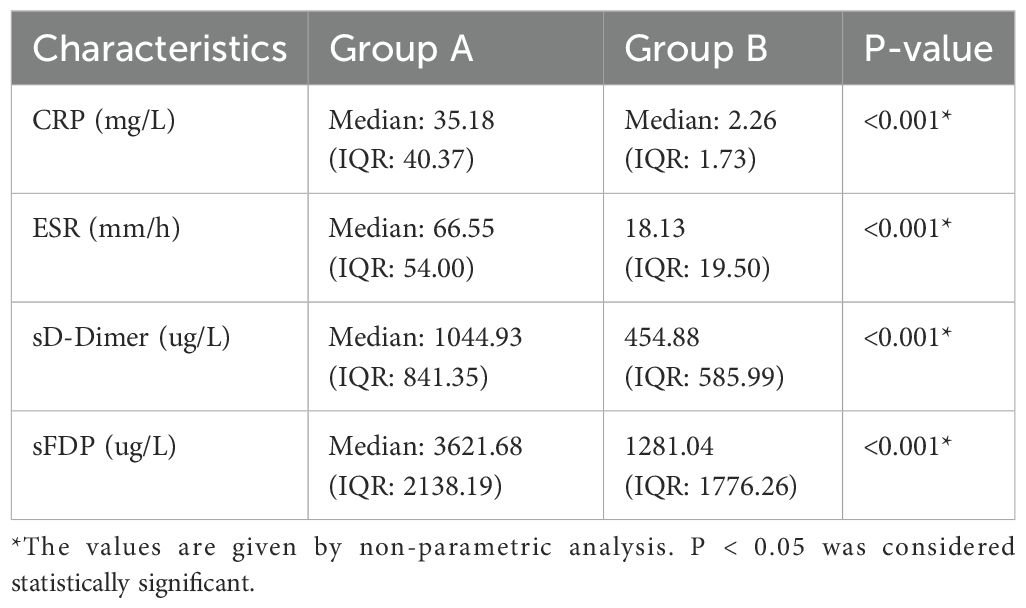
The levels of CRP, ESR, sD-Dimer, and sFDP in group A were significantly higher than the levels observed in Group B (Table 2). The receiver operating characteristic (ROC) curve analysis (Table 3) revealed that AUC of both sFDP and sD-Dimer were lower than CRP and ESR in PJI diagnosis. In order to know whether there was significant difference when compared the AUC among sFDP, sD-Dimer, CRP and ESR in PJI diagnosis, DeLong’s test (DeLong et al., 1988) was performed and DeLong’s test indicated that sFDP demonstrated comparable diagnostic performance in PJI diagnosis to CRP and ESR. However, sD-Dimer exhibited inferior diagnostic performance in PJI diagnosis compared to CRP and ESR (Table 4; Figure 2).
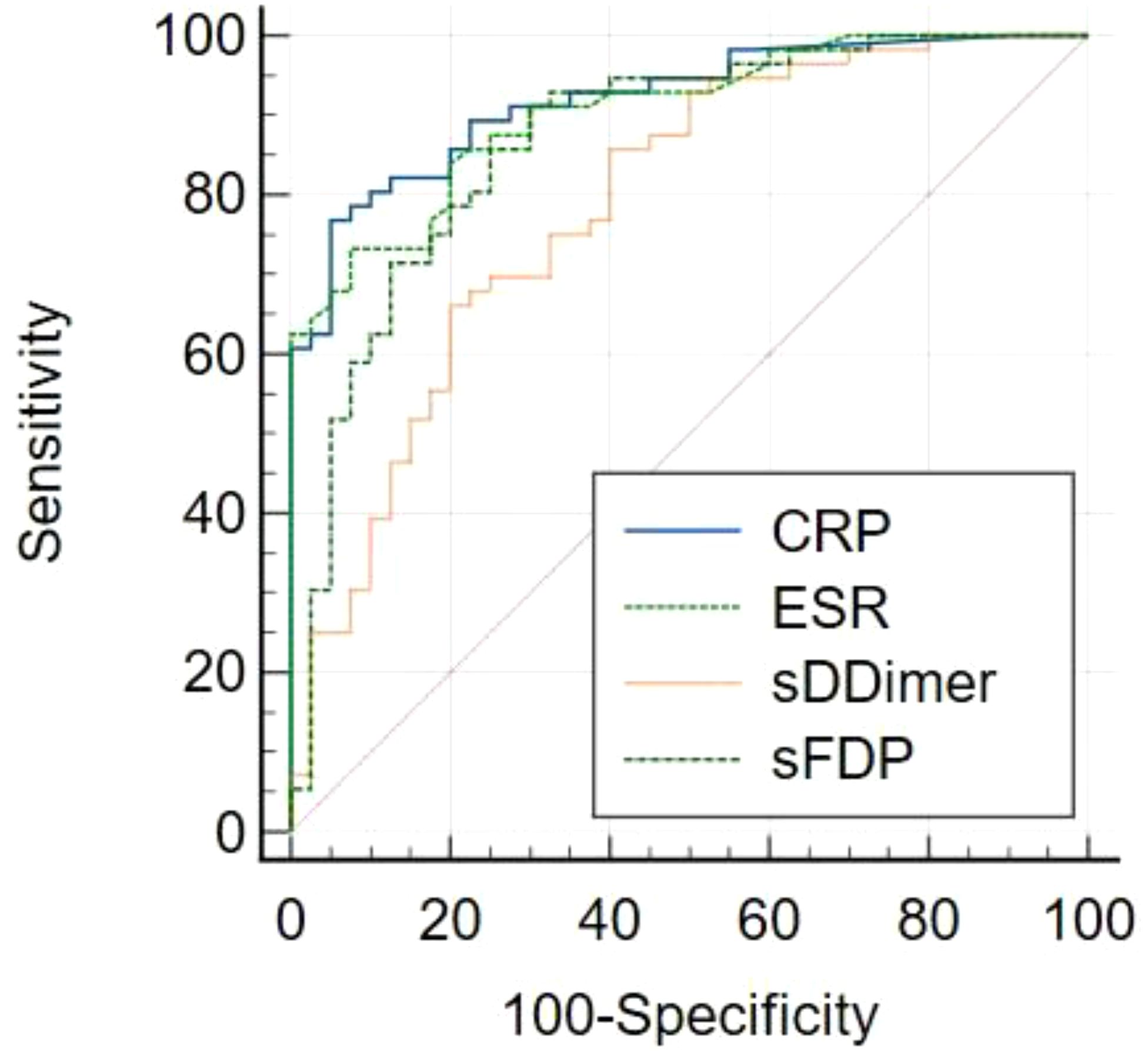
Figure 2. Receiver operating characteristic (ROC) curves of CRP, ESR, sD-Dimer, sFDP in PJI diagnosis.
Discussion
The precise diagnosis of PJI in patients lacking typical symptoms, such as sinus tract formation, redness, swelling, fever, or persistent pain, remains a significant challenge for orthopedic surgeons. The search for reliable biomarkers for PJI diagnosis continues to be a focus of research. To our knowledge, this study represents the first investigation into the diagnostic value of sD-Dimer and sFDP in PJI diagnosis. Our findings demonstrate that sFDP performs comparably to CRP and ESR in the diagnosis of PJI. With a calculated optimal threshold value of >1558.35, sFDP exhibits a sensitivity of 91.07% and a specificity of 70.00% in PJI diagnosis. Given the rapid analysis, ease of identification, and cost-effectiveness of FDP detection, our results introduce a novel adjunct marker for PJI diagnosis.
Numerous studies have explored various biomarkers and diagnostic tools for PJI, with a particular focus on synovial fluid markers due to their proximity to the site of infection. For instance, synovial calprotectin has shown high sensitivity and specificity in PJI diagnosis, with some studies reporting sensitivity and specificity values of up to 95% and 97%, respectively (Zhang et al., 2020; Peng et al., 2022; Warren et al., 2022). Karbysheva et al. demonstrated that synovial fluid D-Lactate had a sensitivity of 94.3% and specificity of 78.4% in PJI diagnosis when using Musculoskeletal Infection Society criteria (Karbysheva et al., 2020). Theil et al. showed that synovial fluid pH had a strong correlation with synovial leukocyte count and can potentially serve as a diagnostic marker for chronic PJI (Theil et al., 2022). Chen et al. found that synovial fluid leukocyte esterase can used as a rapid PJI diagnostic tool, with a pooled sensitivity of 87% and specificity of 96% (Chen et al., 2019). Although these studies suggested that synovial fluid biomarkers hold promise as new diagnostic indicators for PJI, most of these published synovial fluid biomarkers often require specialized antibodies and equipment for detection, limiting their widespread adoption in routine clinical practice. Different from most of these published synovial fluid biomarkers, FDP detection is already routinely performed in clinical settings, making sFDP a more accessible and practical option for PJI diagnosis.
It is important to note that while sFDP shows promise as a valuable new adjunctive diagnostic marker for PJI, its relatively modest specificity suggests that it should be used in combination with other biomarkers. The integration of multiple parameters for PJI diagnosis has been explored in other studies, with some reporting improved diagnostic accuracy when combining different biomarkers. Lee et al. found that combining synovial fluid calprotectin with CRP and IL-6 significantly improved PJI diagnostic performance (Lee et al., 2017). Diniz et al. found that combined synovial fluid alpha-2-microglobulin and synovial fluid CRP demonstrated improved PJI diagnostic accuracy (Diniz et al., 2023). Yu et al. found that combined platelet-to-albumin ratio with CRP and ESR had a high PJI diagnostic accuracy, with sensitivity and specificity values of 93.8% and 92.5%, respectively (Yu et al., 2025). All these findings highlighted the potential of combining multiple biomarkers to improve diagnostic accuracy. Therefore, further investigations are needed to explore the synergistic use of sFDP with other established biomarkers.
However, it is imperative to interpret the findings of this study within the context of several limitations. Firstly, our study included a modest cohort of 96 patients, underscoring the need for larger, higher-quality studies to comprehensively assess the diagnostic value of sD-Dimer and sFDP in PJI diagnosis. Secondly, we did not compare the AUC values of sD-Dimer and sFDP with synovial fluid leukocyte count and sPMN% in PJI diagnosis. Consequently, we are unable to definitively ascertain whether the diagnostic performance of sD-Dimer and sFDP in PJI diagnosis surpasses that of synovial fluid leukocyte count and sPMN%. Thirdly, our exclusion criteria encompassed patients with systemic inflammatory diseases, a history of anticoagulant therapy, or recent dislocations or trauma within a two-week period, constituting approximately 20% of patients in our department. This exclusion criterion may somewhat constrain the generalizability of our conclusions in the clinical evaluation of PJI. Fourthly, due to the viscosity of synovial fluid and the relatively elevated concentrations of sD-Dimer and sFDP in PJI patients, there were instances where we had to dilute the synovial fluid with an appropriate volume of saline solution to enhance detection success rates. However, this dilution process may introduce a degree of error in concentration detection. Lastly, since this study primarily focuses on preoperative diagnosis, it did not aim to establish a specific minimum follow-up period. Consequently, it is plausible that some patients who were not operated on in our hospital during the study period may have been diagnosed with PJI at a later stage.
Conclusion
In conclusion, sFDP emerges as a promising adjunctive parameter for PJI diagnosis. Nonetheless, given its relatively modest specificity, the integration of sFDP with other biomarkers is recommended. The synergistic utilization of multiple parameters warrants exploration in more extensive clinical trials and holds potential utility, especially in cases where diagnoses remain inconclusive. Nevertheless, further comprehensive studies are imperative to validate and refine this combined diagnostic approach.
Future directions
The findings in this study should be interpreted with caution due to the study’s limitations. Further comprehensive research is essential to confirm these results and refine the diagnostic approach for PJI using synovial fluid markers. The integration of multiple biomarkers, along with larger and more diverse patient populations, will be crucial in enhancing the reliability and clinical applicability of these diagnostic tools.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Board of Henan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JH: Funding acquisition, Writing – original draft. PC: Data curation, Software, Writing – review & editing. ZZ: Methodology, Software, Writing – review & editing. CC: Methodology, Writing – original draft. PP: Formal Analysis, Writing – original draft. YL: Project administration, Writing – original draft. DM: Data curation, Supervision, Validation, Writing – review & editing. TL: Methodology, Supervision, Conceptualization, Writing – review & editing. YJ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Project Sponsored by the Advanced Scientific Research Foundation for the Returned Overseas Chinese Scholars in Henan Province (2024HNSLXRY08); Henan Provincial and Ministry Co-construction Project (SBGJ202102031); National Natural Science Foundation of China (82002840); Key Scientific and Technological Projects in Henan Province (LHGJ20240046); Overseas Training Program for Medical Science and Technology Talents in Henan Province (H20240055).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRP, C-reactive Protein (CRP); ESR, Erythrocyte Sedimentation Rate; sD-Dimer, synovial fluid D-Dimer; sFDP, synovial fluid Fibrin Degradation Product; AUC, Area under the curve; ROC, Receiver operating characteristics; IQR, Interquartile range; PJI, Periprosthetic joint infection; PMN, Polymorph neutrophiles; THA, Total hip arthroplasty; TJA, Total joint arthroplasty; TKA, Total knee arthroplasty; ICM, International consensus meeting; MSIS, Musculoskeletal infection society.
References
Abdelaziz, H., Aljawabra, A., Rossmann, M., Tien, C. S., Citak, M., Klatte, T. O., et al. (2022). What is the impact of automated synovial cell counting on different aseptic causes and periprosthetic conditions associated with revision THA? Clin. Orthop Relat. Res. 480, 905–914. doi: 10.1097/CORR.0000000000002063
Anil, U., Singh, V., Schwarzkopf, R. (2022). Diagnosis and detection of subtle aseptic loosening in total hip arthroplasty. J. Arthroplasty 37, 1494–1500. doi: 10.1016/j.arth.2022.02.060
Berthoud, O., Coiffier, G., Albert, J. D., Gougeon-Jolivet, A., Goussault, C., Bendavid, C., et al. (2020). Performance of a new rapid diagnostic test the lactate/glucose ratio of synovial fluid for the diagnosis of septic arthritis. Joint Bone Spine 87, 343–350. doi: 10.1016/j.jbspin.2020.03.009
Carli, A. V., Abdelbary, H., Ahmadzai, N., Cheng, W., Shea, B., Hutton, B., et al. (2019). et al: diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: A systematic review. J. Bone Joint Surg. Am. 101, 635–649. doi: 10.2106/JBJS.18.00632
Chen, Y., Kang, X., Tao, J., Zhang, Y., Ying, C., Lin, W. (2019). Reliability of synovial fluid alpha-defensin and leukocyte esterase in diagnosing periprosthetic joint infection (PJI): a systematic review and meta-analysis. J. Orthop Surg. Res. 14, 453. doi: 10.1186/s13018-019-1395-3
Della Valle, C., Parvizi, J., Bauer, T. W., DiCesare, P. E., Evans, R. P., Segreti, J., et al. (2011). American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J. Bone Joint Surg. Am. 93, 1355–1357. doi: 10.2106/JBJS.9314ebo
Diniz, S. E., Ribau, A., Vinha, A., Oliveira, J. C., Abreu, M. A., Sousa, R. (2023). Simple and inexpensive synovial fluid biomarkers for the diagnosis of prosthetic joint infection according to the new EBJIS definition. J. Bone Jt Infect. 8, 109–118. doi: 10.5194/jbji-8-109-2023
Ewald, F. C. (1989). The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin. Orthop Relat. Res. 248, 9–12. doi: 10.1097/00003086-198911000-00003
Fujimoto, T., Kaneko, T., Sunakawa, T., Ikegami, H., Musha, Y. (2018). Elevation of fibrin degradation product (FDP) values prevents the negative conversion of serum CRP values after total knee arthroplasty. J. Orthop 15, 940–944. doi: 10.1016/j.jor.2018.08.005
Huang, J. C., Chen, X., Qiang, S., Zheng, W. D., Zheng, J., Jin, Y. (2021). Exciting performance of plasma fibrinogen in periprosthetic joint infection diagnosis. Orthop Surg. 13, 812–816. doi: 10.1111/os.12964
Huang, J., Zhang, Y., Wang, Z., Dong, Y., Zhao, Y., Zheng, J., et al. (2019). The serum level of D-Dimer is not suitable for distinguishing between prosthetic joint infection and aseptic loosening. J. Orthop Surg. Res. 14, 407. doi: 10.1186/s13018-019-1461-x
Karbysheva, S., Yermak, K., Grigoricheva, L., Renz, N., Perka, C., Trampuz, A. (2020). Synovial fluid d-lactate-A novel pathogen-specific biomarker for the diagnosis of periprosthetic joint infection. J. Arthroplasty 35, 2223–2229 e2222. doi: 10.1016/j.arth.2020.03.016
Lee, Y. S., Koo, K. H., Kim, H. J., Tian, S., Kim, T. Y., Maltenfort, M. G., et al. (2017). Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: A systematic review and meta-analysis. J. Bone Joint Surg. Am. 99, 2077–2084. doi: 10.2106/JBJS.17.00123
McNally, M., Sousa, R., Wouthuyzen-Bakker, M., Chen, A. F., Soriano, A., Vogely, H. C., et al. (2021). The EBJIS definition of periprosthetic joint infection. Bone Joint J. 103-B, 18–25. doi: 10.1302/0301-620X.103B1.BJJ-2020-1381.R1
Mihalic, R., Zdovc, J., Brumat, P., Trebse, R. (2020). Synovial fluid interleukin-6 is not superior to cell count and differential in the detection of periprosthetic joint infection. Bone Jt Open 1, 737–742. doi: 10.1302/2633-1462.112.BJO-2020-0166.R1
Pagliaccetti, J., Pannu, T. S., Villa, J. M., Piuzzi, N. S., Higuera, C. A. (2021). Variability and interpretation of synovial cell count and differential: A perspective in hip and knee arthroplasty. Orthopedics 44, e320–e325. doi: 10.3928/01477447-20210508-01
Parvizi, J., Gehrke, T. (2014). International Consensus Group on Periprosthetic Joint I: Definition of periprosthetic joint infection. J. Arthroplasty 29, 1331. doi: 10.1016/j.arth.2014.03.009
Parvizi, J., Tan, T. L., Goswami, K., Higuera, C., Della Valle, C., Chen, A. F., et al. (2018). The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J. Arthroplasty 33, 1309–1314 e1302. doi: 10.1016/j.arth.2018.02.078
Paziuk, T., Rondon, A. J., Goswami, K., Tan, T. L., Parvizi, J. (2020). A novel adjunct indicator of periprosthetic joint infection: platelet count and mean platelet volume. J. Arthroplasty 35, 836–839. doi: 10.1016/j.arth.2019.10.012
Peng, X., Zhang, H., Xin, P., Bai, G., Ge, Y., Cai, M., et al. (2022). Synovial calprotectin for the diagnosis of periprosthetic joint infection: a diagnostic meta-analysis. J. Orthop Surg. Res. 17, 2. doi: 10.1186/s13018-021-02746-2
Sahin, E., Karaismailoglu, B., Ozsahin, M. K., Guven, M. F., Kaynak, G. (2021). Low value of platelet count to mean platelet volume ratio to diagnose chronic PJI: A case control study. Orthop Traumatol Surg. Res. 107, 102899. doi: 10.1016/j.otsr.2021.102899
Shahi, A., Kheir, M. M., Tarabichi, M., Hosseinzadeh, H. R. S., Tan, T. L., Parvizi, J. (2017). Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J. Bone Joint Surg. Am. 99, 1419–1427. doi: 10.2106/JBJS.16.01395
Squire, M. W., Della Valle, C. J., Parvizi, J. (2011). Preoperative diagnosis of periprosthetic joint infection: role of aspiration. AJR Am. J. Roentgenol 196, 875–879. doi: 10.2214/AJR.10.5160
Tande, A. J., Patel, R. (2014). Prosthetic joint infection. Clin. Microbiol. Rev. 27, 302–345. doi: 10.1128/CMR.00111-13
Theil, C., Ackmann, T., Gosheger, G., Puetzler, J., Moellenbeck, B., Schwarze, J., et al. (2022). Synovial fluid pH is as specific as synovial leukocyte count but less sensitive for the diagnosis of chronic prosthetic joint infection. J. Orthop Traumatol 23, 52. doi: 10.1186/s10195-022-00672-5
Vale, J. S., Castelo, F. S., Barros, B. S., Ribau, A. C., Carvalho, A. D., Sousa, R. J. G. (2023). Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection-A systematic review and meta-analysis of their diagnostic accuracy according to different definitions. J. Arthroplasty 38, 2731–2738 e2733. doi: 10.1016/j.arth.2023.06.017
Warren, J. A., Klika, A. K., Bowers, K., Colon-Franco, J., Piuzzi, N. S., Higuera, C. A. (2022). Calprotectin lateral flow test: consistent across criteria for ruling out periprosthetic joint infection. J. Arthroplasty 37, 1153–1158. doi: 10.1016/j.arth.2022.01.082
Xu, Y., Ma, X., Guo, H., Tang, H., Liu, J., Wang, C., et al. (2023). Diagnostic value of synovial fluid biomarkers for periprosthetic joint infection: A prospective, double-blind trial. Med. Sci. Monit 29, e940842.
Xu, H., Xie, J., Huang, Q., Lei, Y., Zhang, S., Pei, F. (2019). Plasma fibrin degradation product and D-dimer are of limited value for diagnosing periprosthetic joint infection. J. Arthroplasty 34, 2454–2460. doi: 10.1016/j.arth.2019.05.009
Yi, P. H., Cross, M. B., Moric, M., Levine, B. R., Sporer, S. M., Paprosky, W. G., et al. (2015). Do serologic and synovial tests help diagnose infection in revision hip arthroplasty with metal-on-metal bearings or corrosion? Clin. Orthop Relat. Res. 473, 498–505.
Yu, Y., Wen, Y., Xia, J., Dong, G., Niu, Y. (2025). Blood cell ratio combinations for diagnosing periprosthetic joint infections: A preliminary study. Infect. Drug Resist. 18, 635–645. doi: 10.2147/IDR.S489201
Zeng, Y. Q., Deng, S., Zhu, X. Y., Sun, X. B., Feng, W. J., Zeng, J. C., et al. (2021). Diagnostic accuracy of the synovial fluid alpha-defensin lateral flow test in periprosthetic joint infection: A meta-analysis. Orthop Surg. 13, 708–718. doi: 10.1111/os.12966
Keywords: prosthetic joint infection, synovial fluid, CRP, ESR, D-dimer, fibrin degradation product, diagnosis
Citation: Huang J, Chen P, Zhang Z, Cheng C, Peng P, Li Y, Meng D, Liu T and Jin Y (2025) Synovial fluid fibrin degradation product can be used as a new auxiliary marker for periprosthetic joint infection diagnosis. Front. Cell. Infect. Microbiol. 15:1435970. doi: 10.3389/fcimb.2025.1435970
Received: 21 May 2024; Accepted: 19 March 2025;
Published: 02 May 2025.
Edited by:
Diana Manolescu, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Marie Louise Guadalupe Attwood, North Bristol NHS Trust, United KingdomEmil Robert Stoicescu, Victor Babes University of Medicine and Pharmacy, Romania
Copyright © 2025 Huang, Chen, Zhang, Cheng, Peng, Li, Meng, Liu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Jin, aG5qaW55aW1kQDE2My5jb20=
 Jincheng Huang
Jincheng Huang Peng Chen2
Peng Chen2 Puji Peng
Puji Peng