- 1Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 2Universal Scientific Education and Research Network (USERN) Office, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 3Department of Microbiology, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 4Department of Microbiology, Faculty of Medicine, Shahed University, Tehran, Iran
- 5Department of Biology, School of Natural Sciences, University of Tabriz, Tabriz, Iran
- 6Pharmaceutical Sciences Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
The human microbiome refers to the genomic content of microorganisms inhabiting the human body, including the lungs, oral cavity, intestinal tract, esophagus, and other areas. The human oral microbiota is a diverse and complex ecosystem that includes bacteria, microeukaryotes, archaea, and viruses. These communities have a highly structured biogeography resulting from the various microenvironments in the oral cavity, shaping local metabolic exchange. Dietary nitrate (NO3-) is an ion naturally present in vegetables, especially leafy greens. When consumed, it leads to the production of nitric oxide (NO). This bioactive molecule benefits bodily functions like host defense and neuronal communication and improves vascular and metabolic health. Dietary NO3- is reduced to NO via the nitrate-nitrite-NO pathway, facilitated by nitrate-reducing bacteria inside the oral cavity. NO has a leading role in different types of diseases, including cancer, cardiovascular disease, and diabetes. The bioavailability of NO is greatly enhanced by the activity of bacteria residing in the mouth, which reduces NO3-to NO2- and increases the concentration of circulating NO2-. NO is the key to causing different malignancies, including gastrointestinal cancers. NO can cause cell death by inducing DNA damage and anti-apoptotic signaling pathways. Low to moderate levels of NO derived from tumors can activate angiogenesis and promote an invasive phenotype, while high levels of NO may have an anti-tumor effect in protecting against cancer. In this review, we intend to discuss the human microbiome, dietary NO3-consumption, the vital role of NO in the human body, types of cancers, and treatments based on it.
1 Introduction
The human microbiome refers to the diverse microbial communities and their collective genetic material that inhabit various regions of the human body (Morrison et al., 2023). These microbial residents, encompassing bacteria, fungi, viruses, and archaea, inhabit a variety of anatomical niches, including the lungs, oral cavity, intestinal tract, esophagus, and skin. Each locale nurtures its distinct microbial community, meticulously sculpted by environmental factors such as pH, oxygen levels, and nutrient availability. Far from being passive occupants, these microorganisms are active participants in our physiology, playing critical roles in digestion, immune regulation, and defense against pathogens (Kozak and Pawlik, 2023; Morrison et al., 2023).
The human microbiome occupies specific anatomical niches and significantly impacts essential physiological processes, such as immune regulation, metabolic function, and homeostasis maintenance. The gut microbiota interacts with the host immune system, promoting the development and maturation of immune responses and affecting susceptibility to inflammatory and autoimmune diseases (Belkaid and Hand, 2014). Microbiota-driven metabolism plays a crucial role in nutrient digestion, energy balance, and metabolic signaling pathways, influencing metabolic health and contributing to conditions like obesity and type 2 diabetes (Fan and Pedersen, 2021). Microbiota-derived signals are crucial for maintaining physiological homeostasis, as demonstrated by their role in regulating barrier integrity and local immune tolerance at mucosal surfaces (Thaiss et al., 2016). The functional impact of the microbiome significantly surpasses its compositional diversity across various body sites, highlighting its importance in both health and disease.
The tongue, teeth, gums, and salivary glands each harbor unique microbial populations, engaged in complex metabolic interactions reflective of their specific microenvironments (Arweiler and Netuschil, 2016)., This dynamic ecosystem is shaped by an array of influences, including diet, oral hygiene practices, age, genetics, and overall health status. Beyond its role as a sentinel of oral well-being, the oral microbiome exerts systemic effects, with recent studies implicating it in conditions extending well beyond the mouth, including oncological processes (Schwartz et al., 2021; Wade, 2021).
A remarkable nexus between the oral microbiome and systemic health emerges through the metabolism of dietary nitrates (NO3-), naturally occurring compounds plentiful in vegetables like spinach, beets, and leafy greens. Upon ingestion, these NO3- initiate an elegant biochemical cascade that culminates in the production of NO, a molecule of profound physiological importance. NO serves as a multifaceted signaling agent, renowned for its contributions to vasodilation, neurotransmission, and immune response. Yet, in the realm of cancer, NO exhibits a striking duality: it may either foster tumor progression or suppress malignancy, a behavior contingent upon its concentration, exposure duration, and the surrounding biological context (Hezel and Weitzberg, 2015; Bedale et al., 2016; Bryan et al., 2022; Morou-Bermúdez et al., 2022; Moran et al., 2024; Olas, 2024; Luetic et al., 2025).
Importantly, this microbiome-mediated conversion of dietary NO3- to NO2- and NO has significant implications for cancer pathogenesis, particularly in the gastrointestinal tract. NO2-, produced by oral bacteria such as Veillonella and Rothia, can lead to the formation of carcinogenic nitrosamines in the acidic stomach environment, increasing the risk of gastric cancer (Figure 1). Similarly, in esophageal cancer, the reflux of NO2-rich gastric contents contributes to nitrosative stress and DNA damage in the esophageal epithelium (Lundberg et al., 2008a; Hyde et al., 2014). In colorectal cancer (CRC), NO2- from swallowed saliva, along with translocated oral bacteria like Fusobacterium nucleatum, can influence the colonic microenvironment, potentially modulating tumor progression through NO-mediated signaling. Even in cancers outside the gastrointestinal tract, such as pancreatic cancer and glioblastoma, systemic NO levels, partly derived from microbiome-mediated NO3-metabolism, may play a role (Hyde et al., 2014). Thus, the oral microbiome serves as a critical link between dietary NO3-intake, NO production, and cancer risk, with dysbiosis, a condition known as the repercussions can be significant, potentially altering the abundance or activity of NO2-producing bacteria and affecting tumorigenesis (Hezel and Weitzberg, 2015; Blekkenhorst et al., 2018; Khan et al., 2020).
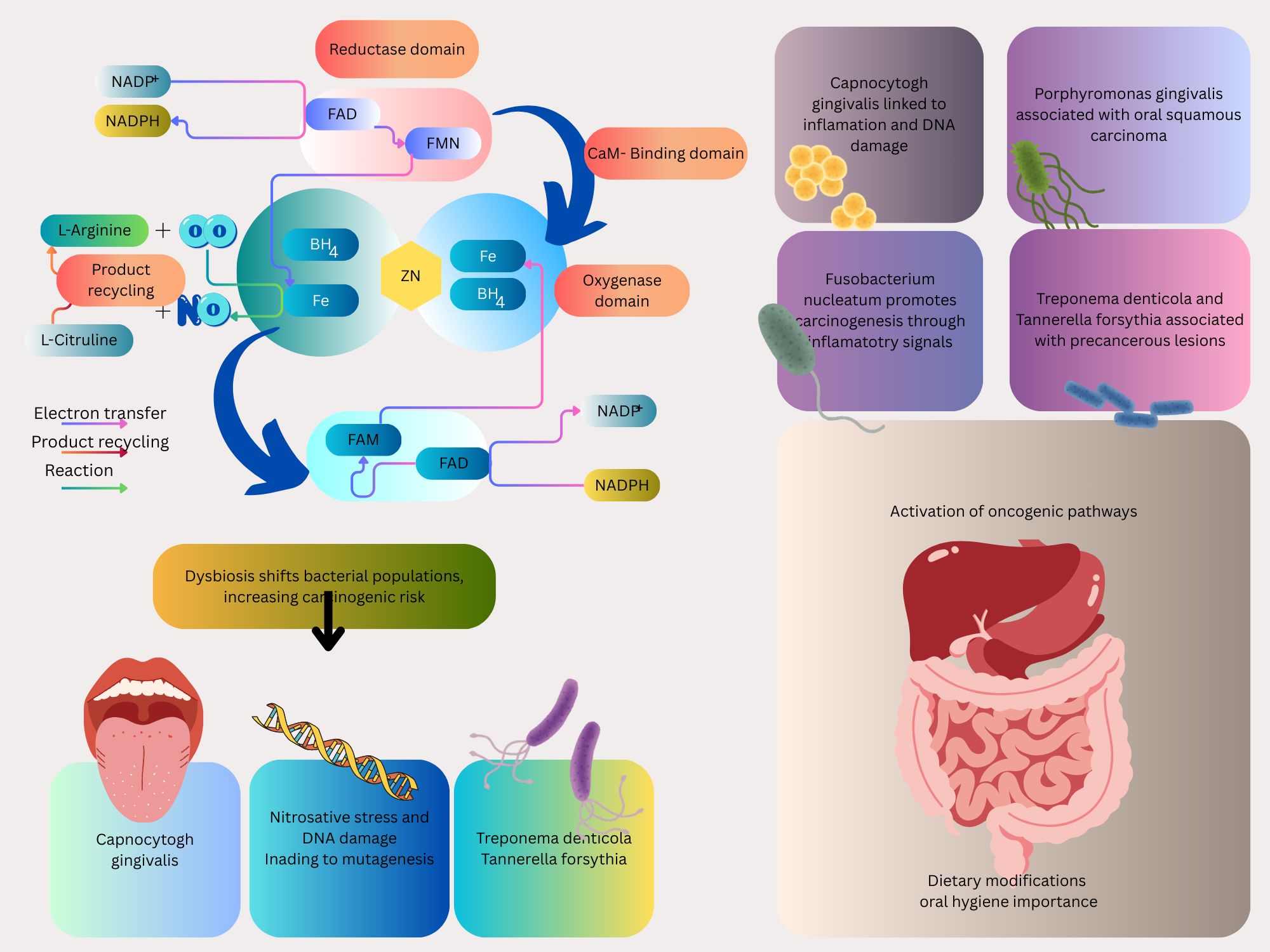
Figure 1. Schematic illustrating the role of dietary NO3- in nitrogen cycle and oral microbiota in metabolizing dietary NO3- into NO2- and NO, highlighting specific bacterial species involved.
NO’s dualistic nature, capable of either promoting or inhibiting tumorigenesis, renders it a focal point in oncological research (Korde Choudhari et al., 2013; Kandalai et al., 2023). At modest concentrations, NO may drive carcinogenesis by enhancing angiogenesis, suppressing programmed cell death (apoptosis), and fostering an invasive tumor phenotype. At elevated levels, NO can inflict DNA damage, exert cytotoxic effects, and mobilize immune-mediated tumor suppression. This behavior positions the oral microbiome, via its modulation of NO synthesis, as a potential influencer of cancer risk and progression, particularly in gastrointestinal malignancies where microbially derived NO exerts its most immediate effects (Kamm et al., 2019; Król and Kepinska, 2020; Mintz et al., 2021). In this exploration, we will undertake a thorough examination of the human microbiome, with a special emphasis on the oral cavity and its pivotal role in mediating the effects of dietary NO3- on NO production. We will probe the multifaceted roles of NO in health and disease, with a keen focus on its implications for cancer pathogenesis, particularly within the gastrointestinal tract. Additionally, assess emerging NO-based therapeutic modalities, ranging from NO donors to immunomodulatory agents, and their promise in augmenting existing cancer therapies. By illuminating these intricate relationships, aim to deepen our grasp of cancer biology and catalyze the development of transformative approaches to patient care.
2 Dietary nitrate consumption
NO3- and NO2- are ubiquitous chemical compounds naturally present in a wide array of dietary sources, including vegetables, fruits, processed foods, drinking water, and also serve as food additives in certain products. Vegetables constitute the primary source of dietary NO3-, accounting for approximately 80–90% of intake in most populations (Hord et al., 2009). Among these, leafy greens such as spinach, arugula, lettuce, Swiss chard, and celery are notably rich, frequently containing NO3-concentrations exceeding 2500 mg kg-¹ fresh weight (Luetic et al., 2025). Root vegetables, including beetroot, and cruciferous vegetables like cabbage and broccoli also contribute substantial amounts, with levels influenced by soil composition, irrigation, and agricultural practices, such as the application of nitrogen-based fertilizers.
Fruits, while less nitrate-dense than vegetables, are noteworthy contributors to dietary intake. Apples, pears, grapes, berries, bananas, melons, and stone fruits typically contain NO3- in the range of 10–100 mg kg (Colla et al., 2018; Olas, 2024). Additional sources include grains, pod vegetables, mushrooms, onions, and garlic, which harbor lower but detectable NO3-levels, which are integral to a balanced diet, and are valued not only for their NO3-content but also for their provision of essential vitamins, minerals, and antioxidants (Bedale et al., 2016; Ferysiuk and Wójciak, 2020).
NO2-s, by contrast, are less prevalent in fresh produce but are commonly introduced through processed foods, particularly cured meats such as bacon, sausages, and ham. In these products, sodium or potassium NO2- is employed as a preservative to enhance color, flavor, and microbial stability (Ferysiuk and Wójciak, 2020). Certain vegetables, including spinach and celery, also contain naturally occurring NO2-s, though in smaller quantities (Luetic et al., 2023). Drinking water represents another source of both NO3- and NO2-s, with concentrations varying based on geological factors and agricultural runoff, though regulatory standards aim to limit exposure (Ward et al., 2018).
The dietary intake of NO3- and NO2- reflects regional and cultural dietary patterns. In Western diets, adults typically consume 50–150 mg of NO3- daily, predominantly from vegetables, while NO2- intake is lower, ranging from 0.1–10 mg, largely from processed meats and water (Song et al., 2015; Srour et al., 2023). Preparation methods further influence NO3-content: boiling, for instance, can reduce vegetable NO3-levels by 20–50%, whereas fresh or minimally processed produce retains higher concentrations (Tiso and Schechter, 2015).
Moreover, additional biological and dietary aspects could influence how NO3-, NO2-s, and their associated chemicals affect the body. Some oral bacteria strains have been found to convert NO3-present in meals to NO2- (Tiso and Schechter, 2015). For example, certain species of Veillonella or Rothia, located on the back of the tongue, use saliva NO3-as an energy source and convert it into NO2- (Lundberg et al., 2008b).
Researchers discovered that eating foods high in NO3- was linked to a lower risk of gastric cancer and that eating foods high in NO2- and NDMA was linked to an increased risk of cancer (Song et al., 2015).
When NO2- comes into contact with the stomach’s highly acidic secretions, they are transformed into nitrous acid, which interacts with amines to create nitrosamines (Kobayashi et al., 2015). Generally speaking, dietary NO2-, the primary nitrosating agent acquired from meals and reduction of salivary NO3-, is catalyzed in the acidic stomach to form NO-related compounds, such as S-nitroso, N-nitroso, O-nitroso compounds, and NO (Gago et al., 2008; Pinheiro et al., 2015). According to reports, nitrosamines can cause esophageal, gastric, and colon cancers (Park et al., 2015; Bedale et al., 2016; Alaei et al., 2021).
Eating foods rich in NO3- and NO2-s, particularly animal sources, has been linked to an increased risk of cancer, including breast, gastric, renal cell, adult glioma, colorectal, esophageal, and thyroid cancers. Such foods contain high levels of amines and amides, as well as heme iron, which may promote the production of endogenous N-nitroso compounds, thus contributing to cancer risk (Karwowska and Kononiuk, 2020). Also, some data point to a possible connection between CRC and NO3- in drinking water (Schullehner et al., 2018; Temkin et al., 2019). This is due to the conversion of NO3- into carcinogenic N-nitroso compounds (Schullehner et al., 2018). Thus, eating vegetables(consumption of NO3-) is seen as a component of a healthy diet for people, whereas increased consumption of NO2- and NDMA seems to be a risk factor for cancer (Song et al., 2015).
3 Nitric oxide production pathway
NO production begins with the substrate L-arginine, which is converted to NO and L-citrulline by the enzyme NO synthase (NOS). This reaction occurs within different cells, such as endothelial cells, neurons, and macrophages (Figure 1). The enzyme NOS exists in three isoforms: endothelial (eNOS), neuronal (nNOS), and inducible (iNOS). Each of these isoforms is involved in a physiological process, including vascular homeostasis, neurotransmission, and immunological defense mechanisms.
Further, the NO produced via this pathway exerts its effects by stimulating guanylate cyclase, leading to increased levels of cyclic guanosine monophosphate (cGMP). This molecule acts as a secondary messenger, resulting in various physiological responses such as vasodilation and inhibition of platelet aggregation. Thus, the L-arginine-NO pathway plays a vital role in maintaining physiological homeostasis (Gonzalez et al., 2025). Endothelial cells, which are critical for NO production through endothelial eNOS, do not play a direct role in the oxidation of NO2- to NO3-under physiological conditions. eNOS catalyzes the conversion of L-arginine to NO and L-citrulline, and the NO produced can be oxidized to NO2- in plasma or further to NO3-by oxyhemoglobin (HbO2) in erythrocytes (Ignarro, 1990). However, there is no evidence that endothelial cells themselves possess the enzymatic machinery to oxidize NO2- to NO3-. Instead, endothelial cells may contribute indirectly to NO2- dynamics by generating NO, which is then metabolized in the bloodstream to form NO2- and NO3-.
In certain contexts, such as hypoxia, endothelial cells can reduce NO2- to NO, mediated by enzymes like xanthine oxidoreductase or eNOS acting as a NO2- reductase, enhancing local NO bioavailability (Su et al., 2020). Under inflammatory conditions, endothelial cells may express enzymes like myeloperoxidase, which could theoretically oxidize NO2-, but this is not a primary function and occurs mainly in pathological states (Pacher et al., 2007). Thus, while endothelial cells are integral to NO synthesis and NO2- reduction, their role in NO2- oxidation is negligible, with HbO2 in the blood serving as the dominant mediator.
This distinction is relevant to cancer biology, as the balance between NO2- and NO3-levels influences the availability of substrates for the NO3-NO2-NO pathway, potentially affecting tumor microenvironments. For instance, excessive NO2- accumulation due to dysregulated metabolism could contribute to nitrosative stress, promoting DNA damage in gastrointestinal cancers (Lundberg et al., 2008a).
3.1 Nitric oxide generation from dietary nitrate
The generation of NO from dietary NO3- represents a critical metabolic pathway mediated by the oral microbiome and gastrointestinal environment. Dietary NO3-, from vegetables, is ingested and absorbed into the bloodstream (Keller et al., 2020; Liu et al., 2020), where they are concentrated in saliva by the salivary glands (Figure 1). In the oral cavity, facultative anaerobic bacteria, notably Veillonella and Rothia species, reduce NO3- to NO2- through enzymatic activity. These bacteria, residing predominantly on the tongue, utilize NO3-as an electron acceptor, producing NO2- as a metabolic byproduct, as NO, and other reactive nitrogen oxides. NO2- can also be converted into NO and other bioactive nitrogen oxides in the blood and tissues by enzymatic and nonenzymatic mechanisms. Interestingly, these mechanisms are accelerated under hypoxic and acidic conditions when oxygen-dependent NOS enzymes may not function properly. Therefore, the NO3-NO2-NO pathway is considered a backup system that ensures NO bioactivity when there is low NOS output (Lundberg and Weitzberg, 2022).
Following its production, NO is a highly reactive molecule that undergoes rapid metabolism in the body, with one of its primary fates being oxidation to NO3-. This process is predominantly mediated by oxyheme-containing proteins, such as (HbO2 in red blood cells and oxymyoglobin in muscle tissues. These proteins, which contain heme groups with iron in the ferrous (Fe²+) state bound to oxygen, facilitate the oxidation of NO to NO3-through a series of biochemical reactions (Ignarro, 1990).
In the bloodstream, HbO2, is the primary mediator of NO oxidation. NO reacts with oxyhemoglobin to form methemoglobin (where the heme iron is oxidized to Fe³+) and NO3-, as described by the reaction:
This reaction occurs rapidly in the vascular compartment, particularly in erythrocytes, ensuring that NO is efficiently converted to NO3-, which is then excreted in urine or recycled through the NO3-NO2-NO pathway. The high concentration of HbO2 in blood (approximately 10 mM heme) makes this a dominant pathway for NO clearance in the systemic circulation, preventing excessive NO accumulation that could lead to cytotoxicity or vasodilation (Ignarro, 1990).
In tissues, particularly skeletal and cardiac muscle, HbO2 plays a similar role. NO reacts with HbO2 to produce NO3-and metmyoglobin, effectively regulating NO levels in these oxygen-rich environments. This process is especially relevant in tissues with high metabolic activity, where NO produced by endothelial or inducible iNOS must be tightly controlled to maintain homeostasis (Shiva, 2013).
Additionally, NO can be oxidized to NO3- in other oxygen-rich environments, such as the lungs, where inhaled oxygen or Reactive oxygen species (ROS) may contribute to non-enzymatic oxidation. However, the oxyheme-mediated pathway remains the primary mechanism due to its efficiency and prevalence (Lundberg et al., 2008a). The resulting NO3-is either excreted by the kidneys or concentrated in saliva by the salivary glands, re-entering the NO3-NO2-NO pathway, thus completing a cyclical process that integrates dietary NO3- metabolism with endogenous NO turnover.
This oxidation process has implications for cancer biology, as NO3- levels in the body, whether derived from dietary sources or NO metabolism, can influence the availability of substrates for the NO3-NO2-NO pathway, potentially modulating tumor microenvironment dynamics. For instance, excessive NO3-production from NO oxidation in dysbiotic conditions could amplify nitrosative stress, contributing to DNA damage and carcinogenesis, particularly in gastrointestinal cancers (Khan et al., 2020).
4 Characteristics of the oral microbiota in carcinogenesis
The oral microbiota, a complex community of over 700 microbial species including bacteria, fungi, viruses, and protozoa, plays a critical role in metabolizing dietary NO3- into NO, as outlined in the preceding section (Caselli et al., 2020; Tuominen and Rautava, 2021), is shaped by various factors, such as dietary habits, oral hygiene practices, the host’s immune response, systemic health conditions, and the use of medications. The composition of microbial communities is significantly influenced by diet, which affects nutrient availability and pH levels (Wade, 2013). The immune system, especially innate immunity and mucosal defenses, regulates microbial balance and prevents the overgrowth of pathogenic species (Belkaid and Hand, 2014). Systemic diseases, including diabetes and autoimmune disorders, can disturb microbial balance and lead to oral dysbiosis (Lamont and Hajishengallis, 2015). Furthermore, antimicrobial drug treatments, particularly broad-spectrum antibiotics, may diminish microbial diversity and facilitate the growth of opportunistic pathogens (Bhalodi et al., 2019). Age and hormonal changes influence the composition of oral microbiota over time (Huttenhower et al., 2012; Lloyd-Price et al., 2016). Beyond its contribution to NO production via the NO3-NO2-NO pathway, the oral microbiota is increasingly recognized for its role in carcinogenesis, particularly in oral, esophageal, gastric, and colorectal cancers, where dysbiosis may amplify NO-related oncogenic processes (Figure 2) (Lundberg et al., 2008a; Pennisi et al., 2017; Zhang et al., 2018; Cali et al., 2019; Sun et al., 2020). Dysbiosis of the oral microbiota can significantly affect the host’s metabolism and immune system and lead to local and systemic disorders such as systemic lupus erythematosus and cancers such as squamous cell carcinoma (SCC) which, in this situation, excessive NO production can lead to nitrosative stress, causing DNA damage and tumor initiation. This mechanism is particularly relevant in esophageal cancer, which affects 604,100 individuals annually (Lamont and Hajishengallis, 2015; Helmink et al., 2019; Li et al., 2020; Sarkar et al., 2021). For instance, one of the risk factors for oral squamous cell carcinomas (OSCC), a subgroup of head and neck (SCC) (Tandon et al., 2017; Bray et al., 2018) is homeostasis dysregulation. Due to the possibility that bacteria may encourage inflammation, cell proliferation, and the production of some oncogenic substances, several species, including Capnocytophaga gingivalis, Fusobacterium sp, Streptococcus sp, Peptostreptococcus sp, Porphyromonas gingivalis, and Prevotella sp, have been strongly correlated with oral carcinoma (Karpiński, 2019). Porphyromonas gingivalis, Fusobacterium nucleatum, Treponema denticola, and Streptococcus anginosus, four common oral cavity residents, have been identified as probable causative agents of OSCC (Zhang et al., 2019).

Figure 2. Various cancers-associated bacteria are depicted, which intricate interplay between these microorganisms and NO signaling pathways may affect cancer development and progression.
The findings show that six families (Prevotellaceae, Fusobacteriaceae, Flavobacteriaceae, Lachnospiraceae, Peptostreptococcaceae, and Campylobacteraceae) and 13 genera, including Porphyromonas, Alloprevotella, and Fusobacterium, are enriched in cancer tissues. Also, Fusobacterium nucleatum, Prevotella intermedia, Aggregatibacter segnis, Capnocytophaga leadbetteri, Peptostreptococcus stomatis, and five additional species have significantly higher abundances at the species level, indicating a probable link between these bacteria and OSCC (Zhang et al., 2019).
Esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are the two primary histological forms of esophageal cancer (Figure 2) (Kelly, 2019) (Uhlenhopp et al., 2020; Sung et al., 2021).
Fusobacterium nucleatum has been demonstrated to promote CRC in experimental tests by producing chemokines and activating the E-cadherin/β-catenin signaling pathway via its FadA adhesin (Figure 2) (Rubinstein et al., 2013; Franzosa et al., 2014; Ferlay et al., 2015; Derakhshankhah et al., 2017; Douaiher et al., 2017; Siegel et al., 2020; Uchino et al., 2021) (Pignatelli et al., 2021) (Sun et al., 2017). IL-8 and growth-related oncogene (GRO), two CXC-chemokine genes, are expressed in cultured epithelial cells by viable Streptococcus anginosus isolated from esophageal cancer tissues (GRO) (Narikiyo et al., 2004). Also, the presence of Streptococcus anginosus has been documented in tissues from esophageal cancer, gastric cancer, dysplasia of the esophagus, and head and neck squamous cell carcinomas. These indicate that Streptococcus anginosus is involved in the carcinogenic process (Sasaki et al., 1998; Tateda et al., 2000).
Besides oral cancer correlated with oral cavity microbiome, the fourth most prevalent cancer in the world, and the fifth most frequently diagnosed malignancy in gastric cancer, which is associated with oral cavity microbiome, has been correlated with poor oral health (Ndegwa et al., 2018; Sung et al., 2021). The development of gastric cancer and the oral microbiome have been linked. Streptococcus salivarius was the first bacterium found in gastric tumors (Ijyuuin and Umehara, 2012). Based on the studies conducted about the oral microbiome and gastric cancer, it has been found that the microbial abundance of Tenericutes, M. orale, E. yurii, and Cutibacterium decreases. In contrast, the abundance of Betaproteobacteria, Neisseriales, Neisseriaceae, N. mucosa, and P. pleuritidis increases (Yang et al., 2022). In a recent study conducted in the United States, the prevalence of periodontal pathogens was compared in samples of dental plaque and saliva from 35 patients with precancerous gastric lesions and 70 controls. They discovered precancerous gastric lesions were more likely to develop when Tannerella forsythia, Treponema denticola, and Actinobacillus actinomycetemcomitans loads were present (Sun et al., 2017).
Maintaining oral microbial balance through rigorous hygiene practices, such as regular brushing and tongue cleaning, may reduce pathogenic bacterial loads and modulate NO production, potentially lowering cancer risk (Tanda et al., 2019). The oral microbiota’s dual role in NO generation and dysbiosis underscores its significance in carcinogenesis, particularly in the context of dietary NO3-metabolism, and highlights the need for further research into its mechanistic contributions to cancer development.
5 Resilience and composition of oral microbiome
In the context of oral cancer and NO production, resilience refers to the microbiome’s capacity to maintain or restore its compositional and functional stability, particularly its role in metabolizing dietary NO3- into NO despite perturbations such as dysbiosis, cancer development, or therapeutic intervention (Krasse, 2001; Trombelli et al., 2004; Izadi et al., 2016; Kilian et al., 2016; Rosier et al., 2018; Zhang et al., 2018; Dashper et al., 2019; Caselli et al., 2020; Shouval et al., 2020; Welch et al., 2020; Ingham et al., 2021; Wade, 2021).
5.1 Factors influencing resilience
Resilience is challenged by both cancer development and its treatments. Oral hygiene practices (e.g., brushing, tongue cleaning) and dietary NO3-intake (e.g., from spinach, beetroot) promote resilience by supporting beneficial bacteria (Blekkenhorst et al., 2018). Conversely, poor hygiene or low NO3-intake may exacerbate dysbiosis, increasing cancer risk through altered NO dynamics (Rosier et al., 2018). The oral microbiome’s resilience is crucial for maintaining balanced NO production, influencing cancer risk through NO3-metabolism. Dysbiosis, treatment-induced shifts, and lifestyle factors can disrupt this balance, highlighting the need for strategies like targeted hygiene or dietary interventions to enhance resilience and mitigate oncogenic NO effects.
Chemotherapy and radiotherapy, common in OSCC management, induce significant microbial shifts, reducing beneficial nitrate-reducing bacteria and increasing opportunistic pathogens like Candida species (Alnuaimi et al., 2015). These treatments disrupt microbial biofilms, impair salivary function, and exacerbate inflammation, further compromising microbiome resilience (Gaetti-Jardim et al., 2009). For instance, post-treatment reductions in Veillonella may diminish NO production from dietary NO3-, while dysbiotic enrichment of Fusobacterium nucleatum may enhance inflammation-driven carcinogenesis via NO-mediated pathways (Gaetti-Jardim et al., 2009). Despite these perturbations, the oral microbiome often demonstrates partial recovery, with resilient taxa like Streptococcus and Actinomyces recolonizing within months post-treatment, potentially restoring NO3-metabolism (He et al., 2015).
The interplay between microbiome resilience, oral cancer, and NO production underscores the importance of maintaining microbial balance. Strategies to enhance resilience, such as targeted oral hygiene and dietary interventions, may mitigate dysbiosis and modulate NO-related oncogenic processes, offering potential avenues for cancer prevention and treatment optimization.
6 Exploring the role of NO in cancers
NO can lead to mutations primarily through the induction of nitrosative stress. Under conditions where ROS are present, NO rapidly reacts with superoxide anions (O2·−) to generate PeroxyNO2- (ONOO−).
ONOO− is a highly reactive oxidant that can modify critical cellular components, including DNA (Radi, 2013). ONOO− and other RNS can cause direct modifications to DNA bases, and nitration and nitrosation reactions may transform guanine into 8-nitroguanine or other altered bases (Pacher et al., 2007; Semenikhina et al., 2022). These modifications can lead to mispairing during DNA replication and eventually result in point mutations. The oxidative and nitrosative stress generated by ONOO− can induce both single-strand and double-strand breaks in DNA. Such strand breaks compromise the integrity of the genome and increase the likelihood of mutagenesis if not adequately repaired (Semenikhina et al., 2022).
Also, NO and its derivatives can impair the function of key DNA repair enzymes. This interference can diminish the cell’s ability to rectify errors arising during DNA replication, allowing mutations to accumulate over time.
Another function in which NO can also contribute to the formation of nitrosamines, especially in acidic environments, which are known to be mutagenic. Although this mechanism is more prominent in the context of dietary NO2- and processed meats, it further supports the link between NO-related biochemical reactions and DNA mutation processes (Pacher et al., 2007; Semenikhina et al., 2022).
At low to moderate levels, NO produced by the oral microbiome supports cellular homeostasis and immune surveillance. For instance, in the oral cavity, NO derived from nitrate-reducing bacteria enhances antimicrobial defenses, potentially inhibiting early tumor initiation (Wink et al., 2011; Khan et al., 2020). However, in the context of microbial dysbiosis, excessive NO production can drive oncogenic processes. Dysbiosis, characterized by enrichment of pathogenic bacteria like Porphyromonas gingivalis and Fusobacterium nucleatum in OSCC, disrupts the balance of nitrate-reducing bacteria, leading to elevated NO levels that induce nitrosative stress (Karpiński, 2019; Sun et al., 2020). This stress causes DNA damage, protein nitrosylation, and activation of oncogenic signaling pathways, promoting tumor initiation and progression in OSCC (Bray et al., 2018).
Similarly, ROS, such as hydrogen peroxide (H2O2), produced by dysbiotic oral bacteria via enzymes like NADPH oxidase, acts as a second messenger in signaling pathways, promoting cell proliferation at low concentrations or inducing cell death at higher levels. The interplay between NO and ROS amplifies oxidative and nitrosative stress, exacerbating carcinogenesis in OSCC (McBee et al., 2017; Kandalai et al., 2023).
This interplay between the oral microbiome, NO production, and cancer underscores the need for targeted interventions. Strategies to modulate microbial composition, such as probiotics or nitrate-rich diets, may optimize NO levels to favor antitumor effects while minimizing oncogenic nitrosative stress. These insights highlight the oral microbiome’s critical role in shaping NO’s impact on cancer, particularly in the context of dietary NO3-metabolism. Further illustrates the oral-gut microbiome axis. Oral bacteria such as Fusobacterium nucleatum and Peptostreptococcus stomatis translocate to the gut via saliva, where they promote NO-driven inflammation and ROS production, enhancing tumor growth (Uchino et al., 2021). Fusobacterium nucleatum activates β-catenin signaling, while gut bacteria produce ROS/RNS via NADPH oxidase, contributing to oncogenic signaling in early CRC and potentially tumor-suppressive effects in advanced stages (Ndegwa et al., 2018). The dual role of NO and ROS/RNS highlights their context-dependent effects, with microbiome-driven production shaping cancer outcomes (Table 1).
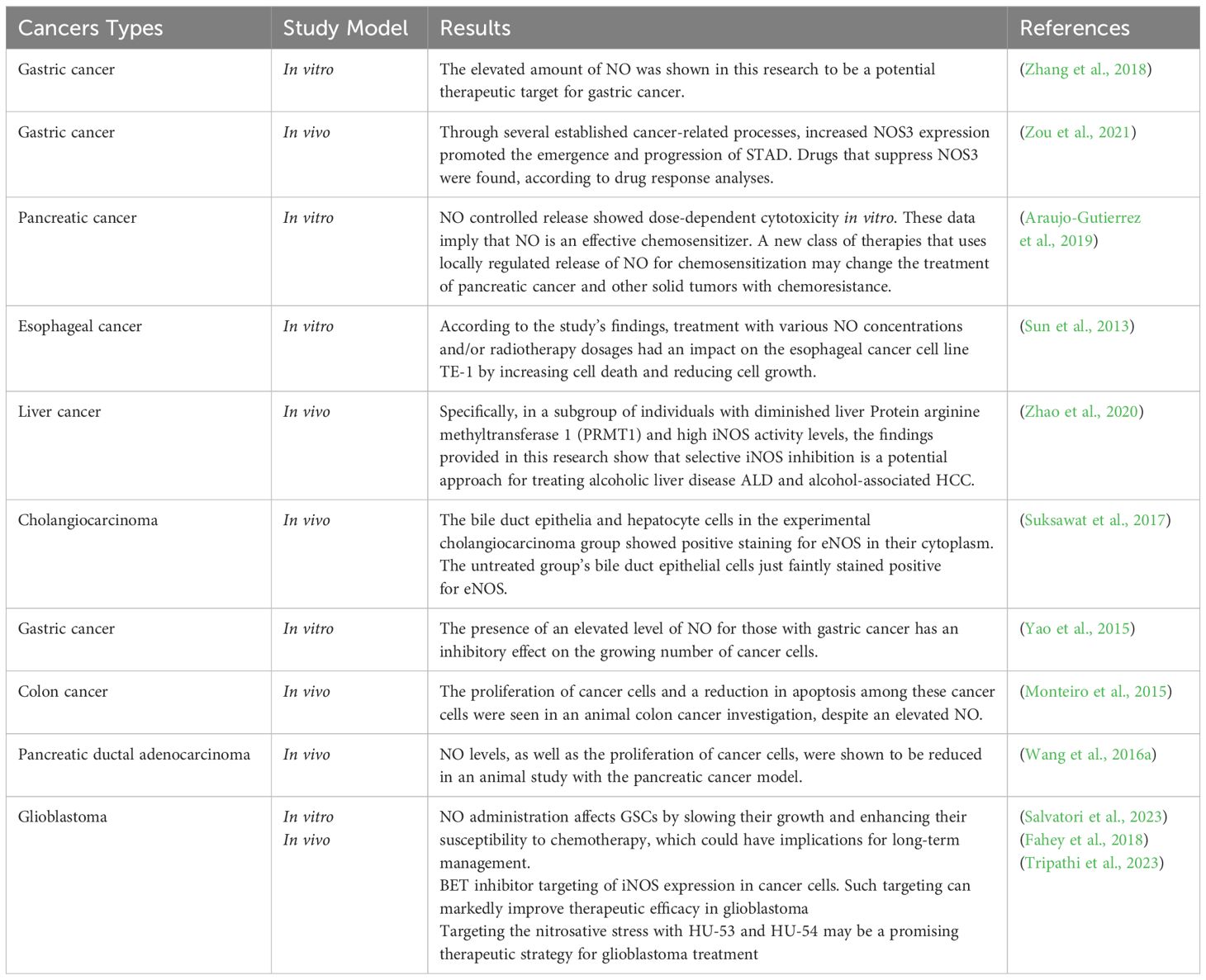
Table 1. The results of preclinical studies regarding the different effects of nitric oxide in various types of cancer.
6.1 NO’s role in promoting tumorigenesis
Angiogenesis is a primary pro-tumorigenic effect, as NO stimulates vascular endothelial growth factor (VEGF) expression, promoting blood vessel formation to supply nutrients and oxygen to tumors (Korde Choudhari et al., 2013). This is evident in colorectal and gastric cancers, where NO enhances tumor vascularization, supporting growth and metastasis. Inhibition of apoptosis is another key mechanism, with NO suppressing caspase activity and upregulating anti-apoptotic proteins like Bcl-2, allowing tumor cells to evade programmed cell death (Khan et al., 2020). Additionally, NO facilitates tumor invasion by activating matrix metalloproteinases (MMPs), which degrade extracellular matrix, enabling cancer cell migration, as seen in pancreatic and esophageal cancers (Monteiro et al., 2019). These effects are amplified in dysbiotic conditions, where oral bacteria like Fusobacterium nucleatum increase NO production, contributing to tumor progression (Sun et al., 2020). Collectively, these mechanisms highlight NO’s role in fostering an oncogenic environment at low concentrations.
6.2 NO’s anti-cancer properties
At high concentrations, NO exhibits anti-cancer properties, counteracting tumor growth through cytotoxic and immune-mediated mechanisms. DNA damage is a primary effect, as elevated NO levels, often from iNOS, induce nitrosative stress, causing single- and double-strand DNA breaks that trigger tumor cell apoptosis (Pacher et al., 2007). This is observed in glioblastoma, where high NO levels sensitize tumor cells to chemotherapy (Tripathi et al., 2023). Immune activation is another critical mechanism, with NO enhancing cytotoxic T-cell and macrophage activity, promoting tumor cell killing. In the oral cavity, NO from nitrate-reducing bacteria supports immune surveillance, potentially inhibiting early tumor initiation. Additionally, high NO levels disrupt mitochondrial function, inducing cytotoxicity and apoptosis in tumor cells, as seen in pancreatic and hepatocellular carcinomas. These anti-tumorigenic effects underscore NO’s potential as a therapeutic agent, particularly when modulated to achieve high local concentrations (Mintz et al., 2021).
6.3 Gastrointestinal cancer
GI cancers represent more than one-fourth of all cancer incidence and one-third of cancer-related mortality (Huang et al., 2023). The positive expression of P53, iNOS, and vascular endothelial growth factor is significantly increased in gastric cancer. NOS can induce DNA mutations in tumor suppressor genes, leading to the occurrence of gastric cancer. In gastric cancer, for example, NO can inhibit the growth and proliferation of cancer cells and promote apoptosis in cancer cells. Additionally, NO can also help to regulate inflammation and oxidative stress in the stomach, which can contribute to the development of gastric cancer (Sang et al., 2011; de Oliveira et al., 2017).
iNOS was reported to be involved in the occurrence, development, invasiveness, and metastasis of esophageal carcinoma. Also, high iNOS, eNOS, and NOS mRNA expression has been found in human colorectal cancer tissues. The accumulation and phosphorylation of p53 caused by NO leads to cell cycle arrest, and the levels of iNOS and p53-p-Ser15 positively correlate with the degree of inflammation (Hu et al., 2020).
In esophageal cancer, chronic exposure to NO can lead to the formation of nitrosamines, which are potent carcinogens that can contribute to the development of cancer. However, NO can also help to protect against the development of esophageal cancer by inhibiting the growth and proliferation of cancer cells (Hofseth et al., 2003).
In CRC, NO can have both pro- and anti-cancer effects. In some cases, NO can inhibit the growth and proliferation of cancer cells in the intestines, while in other cases, NO can promote the survival and growth of cancer cells (Wang et al., 2020).
Additionally, NO can also modulate the immune response in the intestines, which can impact the development and progression of cancer (Mokry et al., 2020).
6.4 Colorectal cancer
Some microbiomes, including E. Coli and Species belonging to genera Bacteroides and Prevotella, have also been noted as being significantly more abundant in CRC patients. The commensal bacteria Fusobacterium nucleatum was also found to increase intestinal tumorigenesis without aggravating colitis or inflammatory pathways (Kandalai et al., 2023).
ROS plays an essential role in our bodies as a moderate level of ROS leads to cell damage and promotes cancer; however, an excessively high level of ROS induces cancer cell death, acting as an anti-cancer. ROS are usually higher in CRC cells and induce cell death in cancer cells while not affecting normal cells (Lin et al., 2018). Different NOS3 and NOS1 polymorphisms described in colorectal tissue are suspiciously associated with the development of colon cancer. NOS2 and NOS3 promote colon cancer’s migration and invasive capacity by activating soluble guanylate cyclase (Monteiro et al., 2019).
NO produced by NOS2 exacerbates colon cancer at inflammatory sites. NOS2 is expressed in 50–60% of colon cancer patients, and those with high NOS2 expression have the prognosis. In healthy individuals, NOS2 expression is absent in colon epithelial cells, and it is low-to-intermediate in patients with chronic colitis and is high in patients with colon cancer. In the primary stages of colon cancer, low NOS2 expression is found (Monteiro et al., 2019).
In the case of CRC, Veillonella and Rothia facilitate the reduction of dietary NO3- into NO2-, which can form carcinogenic nitrosamines in the stomach’s acidic environment, elevating gastric cancer risk. In esophageal cancer, particularly adenocarcinoma, NO2- rich saliva from oral microbial activity can reflux into the esophagus, contributing to nitrosative stress and DNA damage (Table 2) (Hyde et al., 2014; Camañes-Gonzalvo et al., 2024).
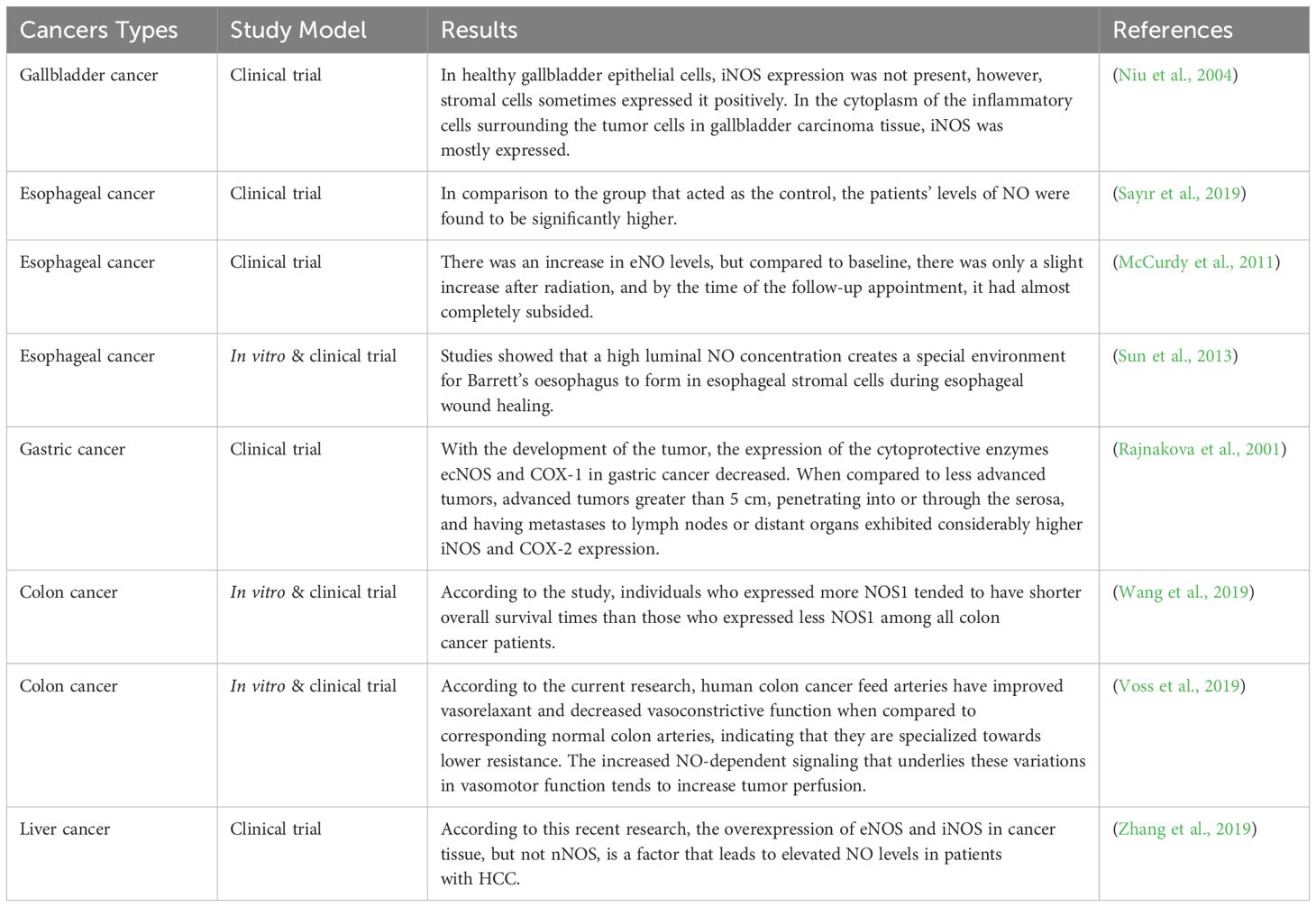
Table 2. The results of clinical studies regarding the different effects of nitric oxide in different types of cancer.
6.5 Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related mortality worldwide. NOS expression and autophagy inhibition have been linked to cancer cell death (Figure 3) (Al-Shahari et al., 2022). Lei Zhou et al., in a 2012 study, indicated that decreased levels of NO/NOS-2 in liver tissues may be relevant to the development and metastasis of HCC. It was also found that the level of NO was higher in HCC tissues without metastasis than in those with metastasis (Zhou et al., 2012). In a 2016 study, Lei Zhou et al. found that NO inhibits the proliferation of HepG2 cells, with more significant suppression when using increasing concentrations of sodium nitroprusside (SNP), and NO stopped HepG2 cells in the G1 phase. The results indicated that cell apoptosis depends on NO concentration. NO suppresses proliferation, migration, and invasion, stops the cell cycle, and induces apoptosis in HepG2 cells; however, the detailed mechanism is still unclear, and further investigation is required (Zhou et al., 2016).
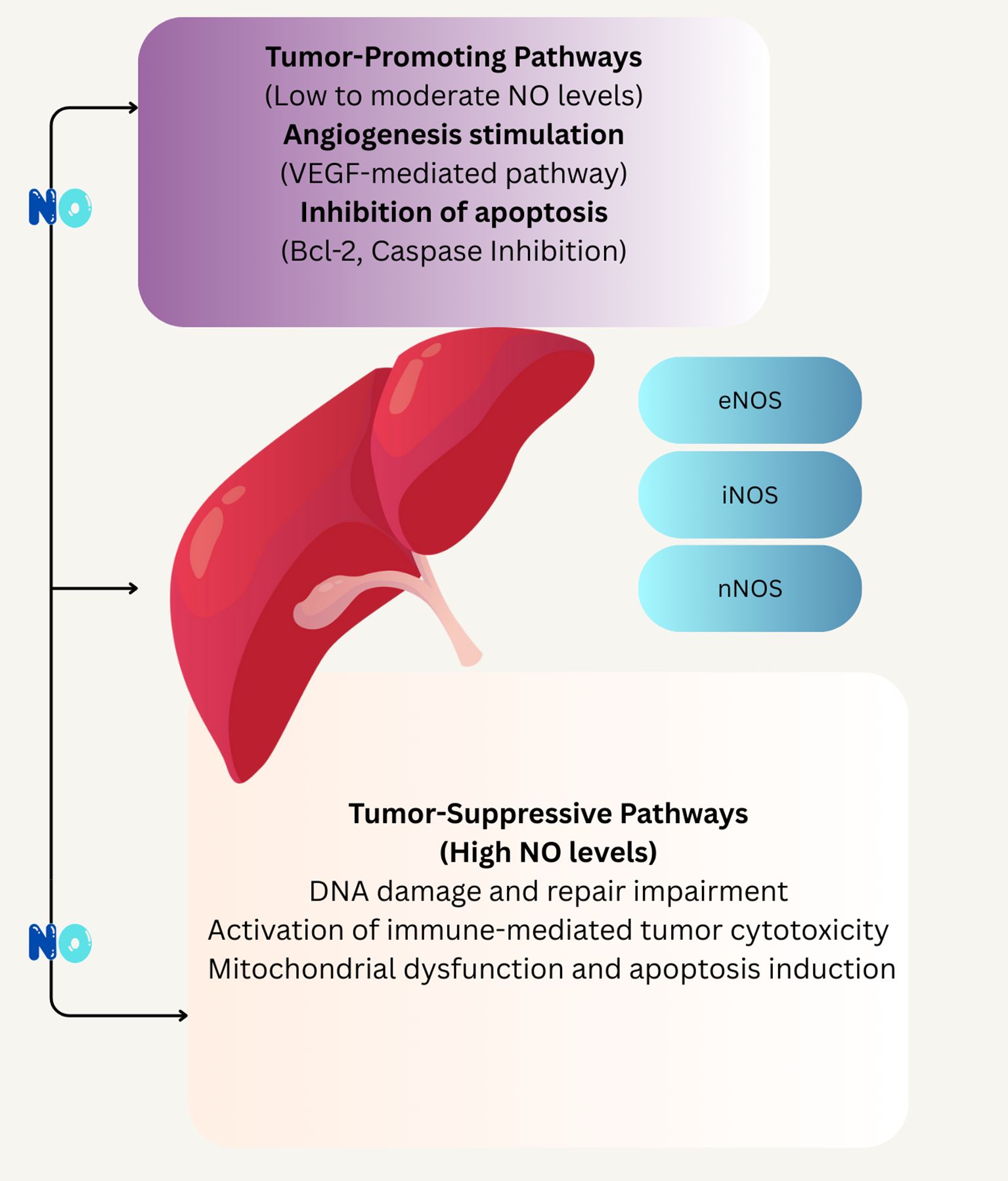
Figure 3. Schematic illustrates of the concentration-dependent effects of NO on tumor progression and suppression. At low to moderate NO levels, tumor-promoting pathways are activated, including VEGF-mediated angiogenesis and inhibition of apoptosis through Bcl-2 and caspase pathways. In contrast, high NO levels trigger tumor-suppressive mechanisms such as DNA damage, impaired repair, immune-mediated cytotoxicity, and mitochondrial dysfunction leading to apoptosis. The involvement of different NOS isoforms eNOS, iNOS, and nNOS s highlighted in mediating these divergent outcomes.
6.6 Gastric cancer
H. pylori is a Gram-negative bacterium colonizing the human gastric mucosa (Kim et al., 2011). H. pylori is challenged by a toxic environment that produces reactive oxygen species, such as hydrogen peroxide and superoxide anions, and reactive nitrogen species, most notably NO (Allen et al., 2023). This bacterium is mainly responsible for persistent oxidative stress in the stomach and induction of chronic immune responses, resulting in DNA damage and gastric cancer (also called stomach cancer). Oxidative stress results from the excessive release of ROS/RNS by activated neutrophils, whereas bacteria also produce ROS in host cells (Jain et al., 2021). Elevated NO is caused by up-regulated iNOS following inflammation or tissue damage in gastric epithelial cells. Increased iNOS expression in H. pylori-associated chronic gastritis diminishes and returns to baseline once it is eradicated (Figure 3) (Saaed et al., 2021). Gobert et al. reported that H. pylori expresses the gene rocF that encodes arginase, effectively dampening NO production by iNOS in macrophages (Gobert et al., 2001). Also, NOS2 expression is seen to increase in gastric adenocarcinoma. In a 2017 study, Oliveira et al. recognized 12 miRNAs associated with NOS2 gene expression in gastric carcinoma (Table 2) (de Oliveira et al., 2017).
6.7 Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest epithelial malignancies in the world (Connor and Gallinger, 2022). Excessive NO production and the expression of iNOS are closely related to PDAC, promoting the occurrence of cancer and angiogenesis (Xiong et al., 2021). A study conducted in 2003 on PDAC patients found that the expression rate of iNOS was 62.7%, which was related to lymph node metastasis (Liu et al., 2003). In another study in 2016, Jian Wang et al. suggested that PDAC expresses a high level of NOS2, leading to sustained NO production. Also, enhanced NOS2 expression in tumors was significantly associated with poor survival in PDAC patients (Wang et al., 2016a). Although less directly exposed to oral microbial products, pancreatic cancer may be influenced by systemic NO derived from dietary NO3-metabolism, which contributes to the body’s NO pool. This systemic NO could reach the pancreas via circulation or ductal connections with the duodenum, potentially affecting tumor angiogenesis and progression. While the connection is less pronounced than in gastrointestinal cancers, dysbiosis altering NO2- production may still play a subtle role in pancreatic ductal adenocarcinoma (Wang et al., 2016b).
6.8 Bile duct cancers
Biliary tract cancers (BTC), including intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), and gallbladder carcinoma (GBC), account for approximately 3% of gastrointestinal malignancies (Fava et al., 2007). NO and inducible iNOS play significant roles in BTC pathogenesis, particularly through their regulation of Notch signaling, a critical pathway in bile duct development and carcinogenesis.
In cholangiocytes, inflammatory cytokines (e.g., TNF-α, IL-1β) induce iNOS expression, leading to elevated NO production, which promotes malignant transformation (Fava et al., 2007). NO regulates Notch signaling through multiple mechanisms. First, NO mediates S-nitrosylation of Notch receptors or ligands, stabilizing the Notch intracellular domain (NICD) and enhancing its nuclear translocation to activate oncogenic target genes like HES1 and HEY1 (Charles et al., 2010). Second, NO upregulates Notch-1 expression by activating transcription factors such as NF-κB, which is heightened in the inflammatory tumor microenvironment of cholangiocarcinoma (Ma et al., 2013). Third, NO-induced nitrosative stress impairs DNA repair mechanisms, leading to epigenetic changes (e.g., histone modifications) that further amplify Notch signaling, promoting tumor progression (Khan et al., 2020).
Notch-1 is hyper-expressed in cholangiocarcinoma cells, correlating with tumor growth and invasion, and its dependency on NO generation positions NO as a key regulator of BTC pathogenesis (Fava et al., 2007). The oral microbiome may indirectly contribute to this process, as NO derived from dietary NO3-metabolism by bacteria like Veillonella and Rothia increases systemic NO levels, potentially enhancing Notch activation in tumors (Hyde et al., 2014). Targeting NO-Notch interactions, such as with iNOS or Notch inhibitors, holds therapeutic promise for BTC, warranting further clinical exploration.
6.9 Esophageal cancer
Esophageal cancer is one of the most fatal malignancies in the world. Esophageal SCC and esophageal adenocarcinoma (EAC) are the two main types of esophageal cancer (Shi and Chen, 2022). Esophageal adenocarcinoma involves chronic exposure of the distal epithelium to stomach and bile acids, causing inflammation and intestinal metaplasia, which is also known as Barrett’s esophagus (BE). The esophageal injury can be caused by reflux directly or by the generation of ROS indirectly. Other risk factors for EAC include obesity and gastroesophageal reflux disease (GERD). The gram-negative, dysbiotic microbiota in the EAC may stimulate iNOS, leading to lower esophageal sphincter relaxation and inducing GERD. NO, and iNOS induce apoptosis, angiogenesis, and DNA damage during tumorigenesis in esophageal adenocarcinoma. Studies have also shown high iNOS expression in BE patients (Sharma et al., 2022).
NOS2 expression is upregulated in esophageal cancer. Mutations in the NOS2 gene or its promoter are related to NOS induction and increased rates of different cancers, including esophageal cancer. Also, mutations of the p53 gene are seen in this malignancy (de Oliveira et al., 2017).
Esophagus (SCC) is characterized by increased expression of genes encoding glucose transporter 1 (GLUT1), iNOS, and ornithine decarboxylase (ODC) of the L-arginine/NO/polyamine pathway (Bednarz-Misa et al., 2020). Increased esophageal (SCC) rates are also associated with specific changes in the salivary microbiota (Chen et al., 2015). An increase in harmful bacteria shifts the balance towards a disease state. An imbalanced microbiota environment, also known as dysbiosis, leads to immune activation and chronic inflammation of the esophageal mucosa (Table 2) (Chiang et al., 2023).
6.10 Glioblastoma
Gliomas represent the most prevalent form of brain tumors, among which glioblastomas are the most malignant subtype. Despite advances in comprehending their biology and treatment strategies, median survival remains disappointingly low. Inflammatory processes involving NO critically contribute to glioma formation. The inducible isoform iNOS is highly overexpressed in gliomas and has been linked to resistance against temozolomide (TMZ) treatment, neoplastic transformation, and modulation of immune response. At low levels, NO enhances tumor growth, while high levels may induce cytotoxicity, reflecting its dual nature
While both in vitro and in vivo studies showed the potential of iNOS inhibitors as effective treatments for gliomas, no clinical trials on gliomas have been published (Figure 4) (Mazurek and Rola, 2021; Tripathi et al., 2023).
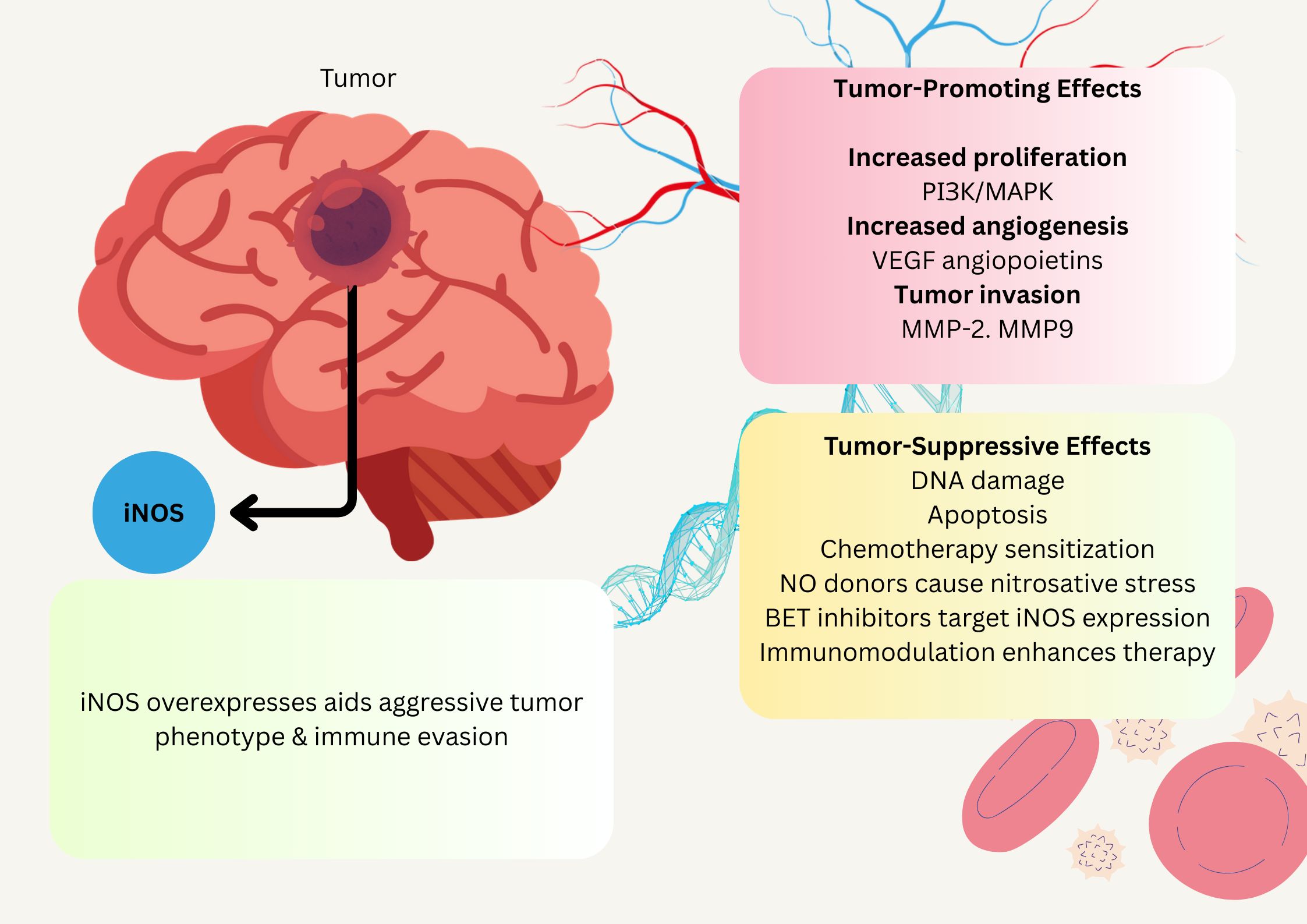
Figure 4. Schematic illustration of NO association effect on glioblastoma. NO administration affects glioblastoma by slowing their growth and enhancing their susceptibility to chemotherapy. This has potential implications for long-term management. Additionally, BET inhibitors targeting iNOS expression in cancer cells can significantly improve therapeutic efficacy in glioblastoma.
Merenzone et al.’s systematic review demonstrated that inhibitors exhibit substantial potential as treatment options for oncologic lesions, and they have demonstrated a safe toxicity profile in humans for other pathological conditions. Their research endeavors should be focused on investigating their potential effects on brain tumors (Merenzon et al., 2023). While glioblastoma’s connection to the oral microbiome is less direct, systemic NO levels partly derived from dietary NO3-metabolism may still be relevant. As circulating NO could influence the tumor microenvironment or immune responses in the brain. Although endogenous NO production via iNOS dominates in glioblastoma, microbiome-derived NO might subtly modulate systemic inflammation, warranting further exploration of this indirect link (Fahey et al., 2018).
6.11 Triple-negative breast cancer
This type of breast is an aggressive subtype occurring more in younger woman, characterized by rapid metastasis, a poor prognosis, absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor expression (Obidiro et al., 2023). NOS2 plays a significant role in TNBC progression as high levels of NOS2 expression correlates with poor prognosis, increased metastasis, and cancer recurrence (Figure 5) (Nafea et al., 2021).

Figure 5. Illustration highlights the aggressive nature of TNBC, characterized by the absence of ER, and PR. High NOS2 expression promotes tumor progression, metastasis, and poor prognosis. The diagram depicts immune modulation through NOS2 depletion and COX2 inhibition, enhancing CD8+ T-cell infiltration and anti-tumor response. It also shows the therapeutic potential of NO donor–aurovertin B hybrids in inducing apoptosis and suppressing tumor growth.
One study suggests that reducing NOS2 expression alters the spatial orientation and density of lymphoid cells, particularly CD8 T cells within the tumor microenvironment and when NOS2 is depleted and COX2 is inhibited, tumor growth slows and the immune response enhances (Somasundaram et al., 2022).
Another study focuses on NO donors and shows that NO donor-aurovertin hybrids can be used to treat TNBC by inducing apoptosis. These hybrids combine aurovertin B (AVB), a natural polyketide with anti-proliferative effects, with NO donors to enhance therapeutic efficacy (Figure 5) (Ma et al., 2024).
6.12 Nitric oxide and NOS2 as prognostic markers in cancer
High levels of NO and iNOS have been identified as markers of poor prognosis in several cancers, reflecting their role in promoting tumor progression, angiogenesis, and treatment resistance.
In PDAC, elevated NOS2 expression is strongly associated with poor survival. A study of PDAC patients found that 62.7% of tumors expressed high NOS2 levels, correlating with lymph node metastasis and reduced overall survival (Wang et al., 2016b). This suggests that NOS2-driven NO production enhances tumor aggressiveness, making it a robust prognostic marker. For CRC, NOS2 expression is observed in 50–60% of cases, with high levels linked to worse prognosis, particularly in advanced stages. Patients with elevated NOS2 expression exhibit increased tumor invasiveness and reduced survival, likely due to NO-mediated inflammation and angiogenesis (Monteiro et al., 2019). This positions NOS2 as a marker of poor outcomes in CRC. Also, HCC, overexpression of iNOS and endothelial eNOS contributes to elevated NO levels, associated with tumor progression and poorer prognosis. Clinical studies show that high iNOS expression correlates with increased tumor size, vascular invasion, and reduced survival in HCC patients (Zhang et al., 2019). NO’s role in promoting angiogenesis and inhibiting apoptosis underscores its prognostic relevance.
Glioblastoma also demonstrates high iNOS expression as a marker of poor prognosis. Elevated iNOS levels drive resistance to temozolomide and enhance tumor growth, correlating with shorter survival times in preclinical and clinical (Merenzon et al., 2023).
In gastric cancer, the prognostic role of NOS2 is less consistent but notable in advanced cases. High iNOS expression is associated with larger tumors (>5 cm), serosal invasion, and lymph node metastases, linked to reduced survival (Rajnakova et al., 2001). However, some studies report variability, suggesting context-dependent effects. In other cancers, such as esophageal cancer and bile duct cancers, evidence is less conclusive. While iNOS expression is elevated in esophageal (SCC) and cholangiocarcinoma, its direct correlation with poor prognosis is not consistently reported, warranting further investigation (Suksawat et al., 2017; Bednarz-Misa et al., 2020).
In summary, high NO/NOS2 levels serve as markers of poor prognosis in pancreatic, colorectal, hepatocellular, glioblastoma, and, to a lesser extent, gastric cancers. These associations reflect NO’s role in enhancing tumor aggressiveness and resistance to therapy. Future studies should validate these markers in larger cohorts and explore their integration into clinical prognostic models, potentially guiding NO-targeted therapies.
7 Nitric oxide-based anti-cancer therapies
Four major categories of NO-based anti-cancer therapies—NO donors, phosphodiesterase inhibitors (PDE-i), soluble guanylyl cyclase (sGC) activators, and immunomodulators NO to target cancer cells, offering promising avenues for treatment (Mintz et al., 2021). NO donors, such as glyceryl trinitrate, release NO directly into the tumor microenvironment, inducing cytotoxicity by disrupting mitochondrial function and sensitizing cancer cells to chemotherapy and radiotherapy (Huang et al., 2017). Phosphodiesterase inhibitors, like sulindac sulfone, block cGMP degradation, enhancing NO-cGMP signaling to inhibit tumor cell proliferation and promote apoptosis (Mehta and Patel, 2019). Soluble guanylyl cyclase activators, such as riociguat, stimulate cGMP production, amplifying NO signaling to suppress tumor growth by inhibiting proliferation and inducing apoptosis, particularly in resistant cancers (Schenk et al., 2021). Immunomodulators, including TLR7 agonists combined with iNOS inhibitors, modulate NO levels to enhance cytotoxic T-cell and macrophage activity, boosting immune-mediated tumor cell killing (PeÑarando et al., 2019). These mechanisms, detailed in the following subsections, highlight NO’s therapeutic potential, potentially modulated by oral microbiome-derived NO from dietary NO3- (Morou-Bermúdez et al., 2022).
7.1 NO donors
NO donors are compounds capable of generating NO, including Glyceryl triNO3-(GTN) (Huang et al., 2017). They function by elevating NO or NO isoforms without endogenous production (Mintz et al., 2021). NO donors work through different mechanisms, i.e., increased NO concentration within tissue beds (Alimoradi et al., 2019). In cancer therapy, NO donors can work alone, combined with other therapeutics, such as chemo–, radio–, or immunotherapy, and hybridization of NO donors. Recently, organic NO3- has joined the clinical field of cancer treatment and affects tumor vessels by improving tumor oxygenation (Huang et al., 2017). In a 2015 phase I study, Illum et al. used the combination of 5-FU, topical GTN, and radiation therapy against advanced operable rectal cancer to evaluate the maximum tolerable dose of NO donor (Illum et al., 2015). Planchette et al.’s study shows that NO can potentially bypass chemotherapy resistance mechanisms associated with platinum-based drugs. Research has focused on combining platinum chemotherapeutic drugs with NO donors. These combination regimens have demonstrated antitumor benefits, particularly in non-small cell lung cancer (Plenchette et al., 2017). Phosphodiesterase is a group of iso-enzymes that hydrolyze cyclic nucleotides and lowering intracellular cAMP and cGMP, leading to tumorigenic effects. Many tumors show a decreased level of intracellular cAMP due to high PDE expression. For example, Sulindac sulfone (exisulind) inhibits PDE2 and PDE5 isoforms and induces apoptosis in colon cancer cells (Mehta and Patel, 2019). Soluble guanylate cyclase (sGC) is a protein essential for sensing NO and is upregulated in disease progression, leading to chemoresistance. Genetic reduction of sGC levels or pharmacological inhibition of sGC activity with an NOS inhibitor re-sensitized a progression in treatment. In a 2021 study, Schenk et al. concluded that the sGC signaling pathway can be the reason for relapsing small cell lung cancer (SCLC) (Schenk et al., 2021).
7.2 Immunomodulators
Immunomodulators represent a promising class of NO-based anti-cancer therapies, leveraging NO’s role in modulating the immunosuppressive tumor microenvironment to enhance immune responses. This subsection explores how NO-targeted immunomodulators can improve cancer immunotherapy, with potential links to microbiome-mediated NO production.
Activated macrophages generate NO or have cytotoxic antitumor activity, indicating NO’s pivotal role in the immune system. NO plays a vital role in the immunosuppressive tumor environment; therefore, therapies against NO have been considered in different types of tumors to increase the efficacy of immunotherapy (PeÑarando et al., 2019). For example, administering the Toll-like receptor 7 (TLR7) agonist imiquimod requires the simultaneous inhibition of iNOS activity to promote tumor antigen-specific Th1 responses and suppress tumor growth in vivo (Ito et al., 2015). Arginine is an amino acid used in protein, cell division, and wound healing biosynthesis that can be obtained from daily food intake or synthesized in the body. Arginine-dependent migration requires arginine to be metabolized by the NO synthase enzyme (NOS) (Al-Koussa et al., 2020). Feng et al.’s study on glioblastoma, based on mRNA sequencing data of 560 IDH-wildtype glioblastoma patients from three public cohorts, aimed to construct an arginine metabolism-related genes signature (ArMRS) based on four essential arginine metabolism-related genes (ArMGs). Analyses of tumor immune microenvironment revealed that higher ArMRS was correlated with more immune infiltration and a relatively “hot” immunological phenotype. Also, demonstrated that ArMRS was positively correlated with the expression of multiple immunotherapy targets, including PD1 and B7-H3. Additionally, the glioblastomas in the ArMRS high-risk group would present with more cytotoxic T cells (CTLs) infiltration and better-predicted response to immune checkpoint inhibitors (ICIs) (Feng et al., 2023).
The oral microbiome may indirectly influence these therapies by contributing to systemic NO levels through dietary NO3-metabolism. Nitrate-reducing bacteria (Veillonella, Rothia) produce NO2-, which can increase circulating NO, potentially modulating immune responses in tumors (Hyde et al., 2014). Future research should explore how microbiome-targeted interventions could optimize NO levels for immunotherapy.
In summary, NO-based immunomodulators offer a novel approach to overcome tumor immunosuppression, with potential synergies with microbiome-derived NO. Clinical trials are needed to validate these strategies and integrate them with existing immunotherapies.
8 Future prospects
As research into the human microbiome and its influence on NO production advances, the complex relationship between dietary NO3-, the oral microbiome, and cancer risk emerges as a pivotal area for future investigation. Dietary NO3-, primarily derived from vegetables and processed meats, exhibits a dual role in cancer biology, as discussed, they can either protect against or promote carcinogenesis, depending on their source and the physiological context. This duality underscores the need for a nuanced understanding to inform cancer prevention and therapeutic strategies. Evidence suggests that NO3- from vegetables, which are accompanied by antioxidants such as vitamin C, generally exert protective effects against gastrointestinal cancers, including gastric and CRC, by inhibiting the formation of carcinogenic NOCs (Song et al., 2015). Conversely, NO2- from processed meats, which lack these protective compounds and are rich in amines, facilitates NOC formation, elevating cancer risk (Said Abasse et al., 2022). However, the tipping point at which dietary NO3- transitions from beneficial to harmful remains poorly defined (Li et al., 2013). Conditions like acidic gastric pH, H. pylori infection, or atrophic gastritis can enhance NOC synthesis from NO2-s (Bryan et al., 2012). Genetic variations affecting inflammation or DNA repair may further amplify susceptibility to NOCs (Mubayi et al., 2011).
While a broad consensus supports the protective role of vegetable-derived NO3- and the risks posed by processed meat NO2-s, inconsistencies persist. Some studies find no clear link between NO3-intake and cancer risk, particularly for NO3- from drinking water, suggesting a need for improved dietary assessment methods and control of confounding variables (Xie et al., 2016). Additionally, the safe thresholds for NO3- and NO2- consumption remain contentious, necessitating further research to establish evidence-based limits. To address these uncertainties, future studies should prioritize the following: mixed genomics, transcriptomics, proteomics, and metabolomics to get the full picture of NO in cancer. That could uncover new targets and show us how NO teams up with things like reactive oxygen species or cytokines maybe even spark some clever combo treatments. Further steps could include modeling blend NO data with a patient’s tumor stage, microbial mix, and genetics. Which could fine-tune NO donor doses or pair them with immune checkpoint inhibitors think glioblastoma, where arginine and immunotherapy overlap. That’s personalized medicine in action.
These steps feel like the natural next move. They build on what we’ve learned and push us closer to making NO a real tool against cancer. The way it ties into the microbiome and therapy, it’s a wide-open field.
9 Conclusion
The intricate relationship between NO, the human microbiome, and cancer pathogenesis, as elucidated in this review, underscores NO’s remarkable dual nature as both a promoter and suppressor of tumor development. It’s a molecule that wears two hats one minute it’s nudging tumors to grow, the next it’s putting the brakes on them. We’ve dug into how NO gets made, whether through the L-arginine-NO synthase NOS pathway or the NO3-NO2-NO pathway route powered by the oral microbiome. That link between what we eat, the bacteria in our mouths, and cancer risk? It’s honestly pretty fascinating. What stands out is how NO plays out differently depending on the situation. In cancers like gastric, esophageal, colorectal, pancreatic, and even glioblastoma, it’s a bit of a wildcard. At lower doses, it’s like a cheerleader for tumor growth, helping blood vessels form and making cancer cells bigger. But crank up the levels, and NO flips the script, triggering cell death and hinting at a built-in defense against tumors. The oral microbiome is a big piece of this puzzle, turning dietary NO3- into NO and tying our daily habits straight to cancer outcomes. Ideas like NO donors, phosphodiesterase inhibitors, soluble guanylyl cyclase activators, and immunomodulators could shake up current treatments, maybe even help patients beat drug resistance or live longer, as some clinical studies backing this up, showing how NOS levels and NO activity shift in cancer tissues and track with how the disease progresses. Then there’s the tumor microenvironment, where NO interacts with immune cells, stromal bits, and oxidative stress in ways that are, frankly, a little mind-boggling. It’s this complexity that makes NO so intriguing, it could either fuel cancer or give us a way to stop it, depending on how we approach it. The clinical patterns talked about, like how NOS ties to prognosis, make me think we’re onto something big, like personalized treatments tailored to each patient’s unique setup.
This review shines a light on NO’s double-edged role in cancer and gets us thinking about how to turn that into real-world solutions. It’s a field brimming with potential, and I’d say we’ve laid a solid foundation for what’s next.
Author contributions
ZI: Funding acquisition, Supervision, Validation, Writing – review & editing. SJ: Investigation, Project administration, Writing – original draft. HF: Investigation, Methodology, Writing – original draft. MA: Investigation, Writing – original draft. MB: Resources, Writing – original draft. MF: Data curation, Methodology, Writing – original draft. RA: Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Kermanshah University of Medical Sciences. All authors report no biomedical financial interests or potential conflicts of interest. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI-based tools were used for language polishing and formatting assistance during manuscript preparation, but all scientific content and analyses were developed by the authors.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alaei, L., Izadi, Z., Jafari, S., Jahanshahi, F., Jaymand, M., Mohammadi, P., et al. (2021). Irreversible thermal inactivation and conformational lock of alpha glucosidase. J. Biomolecular Structure Dynamics. 39, 3256–3262. doi: 10.1080/07391102.2020.1762742
Alimoradi, H., Greish, K., Gamble, A. B., and Giles, G. I. (2019). Controlled delivery of nitric oxide for cancer therapy. Pharm. Nanotechnology. 7, 279–303. doi: 10.2174/2211738507666190429111306
Al-Koussa, H., El Mais, N., Maalouf, H., Abi-Habib, R., and El-Sibai, M. (2020). Arginine deprivation: A potential therapeutic for cancer cell metastasis? A review. Cancer Cell Int. 20, 1–7. doi: 10.1186/s12935-020-01232-9
Allen, M. G., Bate, M. Y., Tramonte, L. M., Avalos, E. Y., Loh, J., Cover, T. L., et al. (2023). Regulation of helicobacter pylori urease and acetone carboxylase genes by nitric oxide and the crdRS two-component system. Microbiol. Spectr. 11 (1), e04633–e04622. doi: 10.1128/spectrum.04633-22
Alnuaimi, A. D., Wiesenfeld, D., O’Brien-Simpson, N. M., Reynolds, E. C., and McCullough, M. J. (2015). Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oral. Oncol. 51, 139–145. doi: 10.1016/j.oraloncology.2014.11.008
Al-Shahari, E. A., El Barky, A. R., Mohamed, T. M., Abdelaal, K., and Alm-Eldeen, A. A. (2022). L-arginine is potential than doxorubicin or their combination in improving hepatocellular carcinoma-induced liver nitric oxide overproduction and arginase expression downregulation. Fresenius Environ. Bulletin. 31, 4572–4580.
Araujo-Gutierrez, R., Van Eps, J., Kirui, D., Bryan, N., Kang, Y., Fleming, J., et al. (2019). Enhancement of gemcitabine cytotoxicity in pancreatic adenocarcinoma through controlled release of nitric oxide. Biomed. Microdevices. 21, 1–8. doi: 10.1007/s10544-019-0375-z
Arweiler, N. B. and Netuschil, L. (2016). “The oral microbiota,” in Microbiota of the human body: implications in health and disease, 45–60. (Switzerland: Springer, Cham).
Bedale, W., Sindelar, J. J., and Milkowski, A. L. (2016). Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 120, 85–92. doi: 10.1016/j.meatsci.2016.03.009
Bednarz-Misa, I., Fortuna, P., Fleszar, M. G., Lewandowski, Ł., Diakowska, D., Rosińczuk, J., et al. (2020). Esophageal squamous cell carcinoma is accompanied by local and systemic changes in L-arginine/NO pathway. Int. J. Mol. Sci. 21, 6282. doi: 10.3390/ijms21176282
Belkaid, Y. and Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell. 157, 121–141. doi: 10.1016/j.cell.2014.03.011
Bhalodi, A. A., van Engelen, T. S. R., Virk, H. S., and Wiersinga, W. J. (2019). Impact of antimicrobial therapy on the gut microbiome. J. Antimicrobial Chemotherapy 74, i6–i15. doi: 10.1093/jac/dky530
Blekkenhorst, L. C., Bondonno, N. P., Liu, A. H., Ward, N. C., Prince, R. L., Lewis, J. R., et al. (2018). Nitrate, the oral microbiome, and cardiovascular health: a systematic literature review of human and animal studies. Am. J. Clin. Nutr. 107, 504–522. doi: 10.1093/ajcn/nqx046
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Bryan, N. S., Alexander, D. D., Coughlin, J. R., Milkowski, A. L., and Boffetta, P. (2012). Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food Chem. Toxicology. 50, 3646–3665. doi: 10.1016/j.fct.2012.07.062
Bryan, N. S., Burleigh, M. C., and Easton, C. (2022). The oral microbiome, nitric oxide and exercise performance. Nitric. Oxide 125, 23–30. doi: 10.1016/j.niox.2022.05.004
Cali, F., Cantone, M., Cosentino, F. I. I., Lanza, G., Ruggeri, G., Chiavetta, V., et al. (2019). Interpreting genetic variants: hints from a family cluster of parkinson’s disease. J. Parkinsons Dis. 9, 203–206. doi: 10.3233/JPD-171292
Camañes-Gonzalvo, S., Montiel-Company, J. M., Lobo-de-Mena, M., Safont-Aguilera, M. J., Fernández-Diaz, A., López-Roldán, A., et al. (2024). Relationship between oral microbiota and colorectal cancer: A systematic review. J. Periodontal Res. 59, 1071–1082. doi: 10.1111/jre.13289
Caselli, E., Fabbri, C., D’Accolti, M., Soffritti, I., Bassi, C., Mazzacane, S., et al. (2020). Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. 20, 1–19. doi: 10.1186/s12866-020-01801-y
Charles, N., Ozawa, T., Squatrito, M., Bleau, A. M., Brennan, C. W., Hambardzumyan, D., et al. (2010). Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 6, 141–152. doi: 10.1016/j.stem.2010.01.001
Chen, X., Winckler, B., Lu, M., Cheng, H., Yuan, Z., Yang, Y., et al. (2015). Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PloS One 10, e0143603. doi: 10.1371/journal.pone.0143603
Chiang, H. C., Hughes, M., and Chang, W. L. (2023). The role of microbiota in esophageal squamous cell carcinoma: A review of the literature. Thorac. Cancer. 14, 2821–2829. doi: 10.1111/1759-7714.15096
Colla, G., Kim, H.-J., Kyriacou, M. C., and Rouphael, Y. (2018). Nitrate in fruits and vegetab les. Scientia Horticulturae. 237, 221–238. doi: 10.1016/j.scienta.2018.04.016
Connor, A. A. and Gallinger, S. (2022). Pancreatic cancer evolution and heterogeneity: integrating omics and clinical data. Nat. Rev. Cancer. 22, 131–142. doi: 10.1038/s41568-021-00418-1
Dashper, S., Mitchell, H., Lê Cao, K.-A., Carpenter, L., Gussy, M., Calache, H., et al. (2019). Temporal development of the oral microbiome and prediction of early childhood caries. Sci. Rep. 9, 19732. doi: 10.1038/s41598-019-56233-0
de Oliveira, G. A., Cheng, R. Y., Ridnour, L. A., Basudhar, D., Somasundaram, V., McVicar, D. W., et al. (2017). Inducible nitric oxide synthase in the carcinogenesis of gastrointestinal cancers. Antioxidants Redox Signaling 26, 1059–1077. doi: 10.1089/ars.2016.6850
Derakhshankhah, H., Izadi, Z., Alaei, L., Lotfabadi, A., Saboury, A. A., Dinarvand, R., et al. (2017). Colon cancer and specific ways to deliver drugs to the large intestine. Anti-Cancer Agents Medicinal Chem. (Formerly Curr. Medicinal Chemistry-Anti-Cancer Agents). 17, 1317–1327. doi: 10.2174/1871520617666170213142030
Douaiher, J., Ravipati, A., Grams, B., Chowdhury, S., Alatise, O., and Are, C. (2017). Colorectal cancer-global burden, trends, and geographical variations. J. Surg. Oncol. 115, 619–630. doi: 10.1002/jso.v115.5
Fahey, J. M., Stancill, J. S., Smith, B. C., and Girotti, A. W. (2018). Nitric oxide antagonism to glioblastoma photodynamic therapy and mitigation thereof by BET bromodomain inhibitor JQ1. J. Biol. Chem. 293, 5345–5359. doi: 10.1074/jbc.RA117.000443
Fan, Y. and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Fava, G., Marzioni, M., Benedetti, A., Glaser, S., DeMorrow, S., Francis, H., et al. (2007). Molecular pathology of biliary tract cancers. Cancer Letters. 250, 155–167. doi: 10.1016/j.canlet.2006.09.011
Feng, W., Zuo, M., Li, W., Chen, S., Wang, Z., Yuan, Y., et al. (2023). A novel score system based on arginine metabolism-related genes to predict prognosis, characterize immune microenvironment, and forecast response to immunotherapy in IDH-wildtype glioblastoma. Front. Pharmacol. 14. doi: 10.3389/fphar.2023.1145828
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. cancer. 136, E359–EE86. doi: 10.1002/ijc.v136.5
Ferysiuk, K. and Wójciak, K. M. (2020). Reduction of nitrite in meat products through the application of various plant-based ingredients. Antioxidants. 9, 711. doi: 10.3390/antiox9080711
Franzosa, E. A., Morgan, X. C., Segata, N., Waldron, L., Reyes, J., Earl, A. M., et al. (2014). Relating the metatranscriptome and metagenome of the human gut. Proc. Natl. Acad. Sci. U S A. 111, E2329–E2338. doi: 10.1073/pnas.1319284111
Gaetti-Jardim, E., Marcelino, S. L., Feitosa, A. C. R., Romito, G. A., and Avila-Campos, M. J. (2009). Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J. Med. Microbiol. 58, 1568–1575. doi: 10.1099/jmm.0.013383-0
Gago, B., Nyström, T., Cavaleiro, C., Rocha, B. S., Barbosa, R. M., Laranjinha, J., et al. (2008). The potent vasodilator ethyl nitrite is formed upon reaction of nitrite and ethanol under gastric conditions. Free Radical Biol. Med. 45, 404–412. doi: 10.1016/j.freeradbiomed.2008.04.027
Gobert, A. P., McGee, D. J., Akhtar, M., Mendz, G. L., Newton, J. C., Cheng, Y., et al. (2001). Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: A strategy for bacterial survival. Proc. Natl. Acad. Sci. 98, 13844–13849. doi: 10.1073/pnas.241443798
Gonzalez, M., Clayton, S., Wauson, E., Christian, D., and Tran, Q. K. (2025). Promotion of nitric oxide production: mechanisms, strategies, and possibilities. Front. Physiol. 16, 1545044. doi: 10.3389/fphys.2025.1545044
He, J., Li, Y., Cao, Y., Xue, J., and Zhou, X. (2015). The oral microbiome diversity and its relation to human diseases. Folia Microbiol. (Praha). 60, 69–80. doi: 10.1007/s12223-014-0342-2
Helmink, B. A., Khan, M. W., Hermann, A., Gopalakrishnan, V., and Wargo, J. A. (2019). The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388. doi: 10.1038/s41591-019-0377-7
Hezel, M. and Weitzberg, E. (2015). The oral microbiome and nitric oxide homoeostasis. Oral. diseases. 21, 7–16. doi: 10.1111/odi.2014.21.issue-1
Hofseth, L. J., Hussain, S. P., Wogan, G. N., and Harris, C. C. (2003). Nitric oxide in cancer and chemoprevention. Free Radical Biol. Med. 34, 955–968. doi: 10.1016/S0891-5849(02)01363-1
Hord, N. G., Tang, Y., and Bryan, N. S. (2009). Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am. J. Clin. Nutr. 90, 1–10. doi: 10.3945/ajcn.2008.27131
Hu, Y., Xiang, J., Su, L., and Tang, X. (2020). The regulation of nitric oxide in tumor progression and therapy. J. Int. Med. Res. 48, 0300060520905985. doi: 10.1177/0300060520905985
Huang, Z., Fu, J., and Zhang, Y. (2017). Nitric oxide donor-based cancer therapy: advances and prospects. J. Med. Chem. 60, 7617–7635. doi: 10.1021/acs.jmedchem.6b01672
Huang, J., Lucero-Prisno, D. E., III, Zhang, L., Xu, W., Wong, S. H., Ng, S. C., et al. (2023). Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 20, 271–287. doi: 10.1038/s41575-022-00726-3
Huttenhower, C., Gevers, D., Knight, R., Abubucker, S., Badger, J. H., Chinwalla, A. T., et al. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214.
Hyde, E. R., Andrade, F., Vaksman, Z., Parthasarathy, K., Jiang, H., Parthasarathy, D. K., et al. (2014). Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PloS One 9, e88645. doi: 10.1371/journal.pone.0088645
Ignarro, L. J. (1990). Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 30, 535–560. doi: 10.1146/annurev.pa.30.040190.002535
Ijyuuin, T. and Umehara, F. (2012). Case of Streptococcus salivarius bacteremia/meningoencephalitis leading to discovery of early gastric cancer. Rinsho Shinkeigaku= Clin. Neurology. 52, 360–363. doi: 10.5692/clinicalneurol.52.360
Illum, H., Wang, D. H., Dowell, J. E., Hittson, W. J., Torrisi, J. R., Meyer, J., et al. (2015). Phase I dose escalation trial of nitroglycerin in addition to 5-fluorouracil and radiation therapy for neoadjuvant treatment of operable rectal cancer. Surgery. 158, 460–465. doi: 10.1016/j.surg.2015.04.007
Ingham, A. C., Kielsen, K., Mordhorst, H., Ifversen, M., Aarestrup, F. M., Müller, K. G., et al. (2021). Microbiota long-term dynamics and prediction of acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Microbiome. 9, 1–28. doi: 10.1186/s40168-021-01100-2
Ito, H., Ando, T., Ogiso, H., Arioka, Y., and Seishima, M. (2015). Inhibition of induced nitric oxide synthase enhances the anti-tumor effects on cancer immunotherapy using TLR7 agonist in mice. Cancer Immunology Immunother. 64, 429–436. doi: 10.1007/s00262-014-1644-6
Izadi, Z., Divsalar, A., Saboury, A. A., and Sawyer, L. (2016). β-lactoglobulin–pectin nanoparticle-based oral drug delivery system for potential treatment of colon cancer. Chem. Biol. Drug design. 88, 209–216. doi: 10.1111/cbdd.2016.88.issue-2
Jain, U., Saxena, K., and Chauhan, N. (2021). Helicobacter pylori induced reactive oxygen Species: A new and developing platform for detection. Helicobacter. 26, e12796. doi: 10.1111/hel.12796
Kamm, A., Przychodzen, P., Kuban-Jankowska, A., Jacewicz, D., Dabrowska, A. M., Nussberger, S., et al. (2019). Nitric oxide and its derivatives in the cancer battlefield. Nitric. Oxide 93, 102–114. doi: 10.1016/j.niox.2019.09.005
Kandalai, S., Li, H., Zhang, N., Peng, H., and Zheng, Q. (2023). The human microbiome and cancer: a diagnostic and therapeutic perspective. Cancer Biol. Ther. 24, 2240084. doi: 10.1080/15384047.2023.2240084
Karpiński, T. M. (2019). Role of oral microbiota in cancer development. Microorganisms. 7, 20. doi: 10.3390/microorganisms7010020
Karwowska, M. and Kononiuk, A. (2020). Nitrates/nitrites in food—Risk for nitrosative stress and benefits. Antioxidants. 9, 241. doi: 10.3390/antiox9030241
Keller, R. M., Beaver, L., Prater, M. C., and Hord, N. G. (2020). Dietary nitrate and nitrite concentrations in food patterns and dietary supplements. Nutr. Today 55, 218–226. doi: 10.1097/NT.0000000000000253
Kelly, R. J. (2019). Emerging multimodality approaches to treat localized esophageal cancer. J. Natl. Compr. Canc Netw. 17, 1009–1014. doi: 10.6004/jnccn.2019.7337
Khan, F. H., Dervan, E., Bhattacharyya, D. D., McAuliffe, J. D., Miranda, K. M., and Glynn, S. A. (2020). The role of nitric oxide in cancer: master regulator or not? Int. J. Mol. Sci. 21, 9393. doi: 10.3390/ijms21249393
Kilian, M., Chapple, I. L., Hannig, M., Marsh, P. D., Meuric, V., Pedersen, A. M., et al. (2016). The oral microbiome - an update for oral healthcare professionals. Br. Dent. J. 221, 657–666. doi: 10.1038/sj.bdj.2016.865
Kim, S. S., Ruiz, V. E., Carroll, J. D., and Moss, S. F. (2011). Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Letters. 305, 228–238. doi: 10.1016/j.canlet.2010.07.014
Kobayashi, J., Ohtake, K., and Uchida, H. (2015). NO-rich diet for lifestyle-related diseases. Nutrients. 7, 4911–4937. doi: 10.3390/nu7064911
Korde Choudhari, S., Chaudhary, M., Bagde, S., Gadbail, A. R., and Joshi, V. (2013). Nitric oxide and cancer: a review. World J. Surg. Oncol. 11, 1–11. doi: 10.1186/1477-7819-11-118
Kozak, M. and Pawlik, A. (2023). The role of the oral microbiome in the development of diseases. Int. J. Mol. Sci. 24, 5231. doi: 10.3390/ijms24065231
Krasse, B. (2001). The Vipeholm Dental Caries Study: recollections and reflections 50 years later. J. Dental Res. 80, 1785–1788. doi: 10.1177/00220345010800090201
Król, M. and Kepinska, M. (2020). Human nitric oxide Synthase—Its functions, polymorphisms, and inhibitors in the context of inflammation, diabetes and cardiovascular diseases. Int. J. Mol. Sci. 22, 56. doi: 10.3390/ijms22010056
Lamont, R. J. and Hajishengallis, G. (2015). Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 21, 172–183. doi: 10.1016/j.molmed.2014.11.004
Li, Q., Lan, Q., Zhang, Y., Bassig, B. A., Holford, T. R., Leaderer, B., et al. (2013). Role of one-carbon metabolizing pathway genes and gene-nutrient interaction in the risk of non-Hodgkin lymphoma. Cancer Causes Control. 24, 1875–1884. doi: 10.1007/s10552-013-0264-3
Li, B.-Z., Zhou, H.-Y., Guo, B., Chen, W.-J., Tao, J.-H., Cao, N.-W., et al. (2020). Dysbiosis of oral microbiota is associated with systemic lupus erythematosus. Arch. Oral. Biol. 113, 104708. doi: 10.1016/j.archoralbio.2020.104708
Lin, S., Li, Y., Zamyatnin, A. A., Jr., Werner, J., and Bazhin, A. V. (2018). Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 233, 5119–5132. doi: 10.1002/jcp.v233.7
Liu, Y., Croft, K. D., Hodgson, J. M., Mori, T., and Ward, N. C. (2020). Mechanisms of the protective effects of nitrate and nitrite in cardiovascular and metabolic diseases. Nitric. Oxide 96, 35–43. doi: 10.1016/j.niox.2020.01.006
Liu, J., Li, K., and Dou, K. (2003). The Expression of iNOS and COX-2 in Pancreatic Cancer and its Clinical Significance. Cancer Res. Prev. Treat. 30, 361. doi: 10.3971/j.issn.1000-8578.1426
Lloyd-Price, J., Abu-Ali, G., and Huttenhower, C. (2016). The healthy human microbiome. Genome Med. 8, 51. doi: 10.1186/s13073-016-0307-y
Luetic, S., Knezovic, Z., Jurcic, K., Majic, Z., Tripkovic, K., and Sutlovic, D. (2023). Leafy vegetable nitrite and nitrate content: potential health effects. Foods. 12, 45–62. doi: 10.3390/foods12081655
Luetic, S., Knezovic, Z., Jurcic, K., Perasovic, M. L., and Sutlovic, D. (2025). Nitrates and nitrites in leafy vegeta bles: the influence of culinary processing on concentration levels and possible impact on health. Int. J. Mol. Sci. 26, 3018. doi: 10.3390/ijms26073018
Lundberg, J. O. and Weitzberg, E. (2022). Nitric oxide signaling in health and disease. Cell. 185, 2853–2878. doi: 10.1016/j.cell.2022.06.010
Lundberg, J. O., Weitzberg, E., and Gladwin, M. T. (2008a). The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167. doi: 10.1038/nrd2466
Lundberg, J. O., Weitzberg, E., and Gladwin, M. T. (2008b). The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug discovery. 7, 156–167. doi: 10.1038/nrd2466
Ma, J., Xia, J., Miele, L., Sarkar, F. H., and Wang, Z. (2013). Notch signaling pathway in pancreatic cancer progression. Pancreat Disord. Ther. 3, 101–115. doi: 10.4172/2165-7092.1000114
Ma, L. F., Xu, L. L., Yuan, L. J., Yang, X., Wu, R., Bao, S. M., et al. (2024). Discovery of NO donor-aurovertin hybrids as dual ferroptosis and apoptosis inducers for treating triple negative breast cancer. J. Med. Chem. 67, 13089–13105. doi: 10.1021/acs.jmedchem.4c01070
Mazurek, M. and Rola, R. (2021). The implications of nitric oxide metabolism in the treatment of glial tumors. Neurochemistry Int. 150, 105172. doi: 10.1016/j.neuint.2021.105172
McBee, M. E., Chionh, Y. H., Sharaf, M. L., Ho, P., Cai, M. W. L., and Dedon, P. C. (2017). Production of superoxide in bacteria is stress- and cell state-dependent: A gating-optimized flow cytometry method that minimizes ROS measurement artifacts with fluorescent dyes. Front. Microbiol. 4592017 doi: 10.3389/fmicb.2017.00459
McCurdy, M. R., Wazni, M. W., Martinez, J., McAleer, M. F., and Guerrero, T. (2011). Exhaled nitric oxide predicts radiation pneumonitis in esophageal and lung cancer patients receiving thoracic radiation. Radiotherapy Oncol. 101, 443–448. doi: 10.1016/j.radonc.2011.08.035
Mehta, A. and Patel, B. M. (2019). Therapeutic opportunities in colon cancer: Focus on phosphodiesterase inhibitors. Life Sci. 230, 150–161. doi: 10.1016/j.lfs.2019.05.043
Merenzon, M. A., Hincapie Arias, E., Bhatia, S., Shah, A. H., Higgins, D. M. O., Villaverde, M., et al. (2023). Nitric oxide synthase inhibitors as potential therapeutic agents for gliomas: A systematic review. Nitric. Oxide 138-139, 10–16. doi: 10.1016/j.niox.2023.06.002
Mintz, J., Vedenko, A., Rosete, O., Shah, K., Goldstein, G., Hare, J. M., et al. (2021). Current advances of nitric oxide in cancer and anticancer therapeutics. Vaccines. 9, 94. doi: 10.3390/vaccines9020094
Mokry, R. L., Schumacher, M. L., Hogg, N., and Terhune, S. S. (2020). Nitric oxide circumvents virus-mediated metabolic regulation during human cytomegalovirus infection. Mbio. 11, 230–245. doi: 10.1128/mbio.02630-20
Monteiro, H., Costa, P., Reis, A., and Stern, A. (2015). Nitric oxide: protein tyrosine phosphorylation and protein S-nitrosylation in cancer. Biomed. J. 38, 88–95. doi: 10.4103/2319-4170.158624
Monteiro, H. P., Rodrigues, E. G., Amorim Reis, A. K. C., Longo, L. S., Jr., Ogata, F. T., Moretti, A. I. S., et al. (2019). Nitric oxide and interactions with reactive oxygen species in the development of melanoma, breast, and colon cancer: A redox signaling perspective. Nitric. Oxide 89, 1–13. doi: 10.1016/j.niox.2019.04.009
Moran, S. P., Rosier, B. T., Henriquez, F. L., and Burleigh, M. C. (2024). The effects of nitrate on the oral microbiome: a systematic review investigating prebiotic potential. J. Oral. Microbiol. 16, 2322228. doi: 10.1080/20002297.2024.2322228
Morou-Bermúdez, E., Torres-Colón, J. E., Bermúdez, N. S., Patel, R. P., and Joshipura, K. J. (2022). Pathways linking oral bacteria, nitric oxide metabolism, and health. J. Dental Res. 101, 623–631. doi: 10.1177/00220345211064571
Morrison, A. G., Sarkar, S., Umar, S., Lee, S. T., and Thomas, S. M. (2023). The contribution of the human oral microbiome to oral disease: A review. Microorganisms. 11, 318. doi: 10.3390/microorganisms11020318
Mubayi, A., Greenwood, P., Wang, X., Castillo-Chávez, C., Gorman, D. M., Gruenewald, P., et al. (2011). Types of drinkers and drinking settings: an application of a mathematical model. Addiction. 106, 749–758. doi: 10.1111/j.1360-0443.2010.03254.x
Nafea, H., Youness, R. A., Abou-Aisha, K., and Gad, M. Z. (2021). LncRNA HEIH/miR-939-5p interplay modulates triple-negative breast cancer progression through NOS2-induced nitric oxide production. J. Cell Physiol. 236, 5362–5372. doi: 10.1002/jcp.v236.7
Narikiyo, M., Tanabe, C., Yamada, Y., Igaki, H., Tachimori, Y., Kato, H., et al. (2004). Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer science. 95, 569–574. doi: 10.1111/j.1349-7006.2004.tb02488.x
Ndegwa, N., Ploner, A., Liu, Z., Roosaar, A., Axéll, T., and Ye, W. (2018). Association between poor oral health and gastric cancer: a prospective cohort study. Int. J. Cancer. 143, 2281–2288. doi: 10.1002/ijc.v143.9
Niu, X.-J., Wang, Z.-R., Wu, S.-L., Geng, Z.-M., Zhang, Y.-F., and Qing, X.-L. (2004). Relationship between inducible nitric oxide synthase expression and angiogenesis in primary gallbladder carcinoma tissue. World J. gastroenterology. 10, 725. doi: 10.3748/wjg.v10.i5.725
Obidiro, O., Battogtokh, G., and Akala, E. O. (2023). Triple negative breast cancer treatment options and limitations: future outlook. Pharmaceutics. 15, 312–328. doi: 10.3390/pharmaceutics15071796
Olas, B. (2024). The cardioprotective role of nitrate-rich vegetab les. Foods. 13, 691. doi: 10.3390/foods13050691
Pacher, P., Beckman, J. S., and Liaudet, L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424. doi: 10.1152/physrev.00029.2006
Park, J.-E., Seo, J.-E., Lee, J.-Y., and Kwon, H. (2015). Distribution of seven N-nitrosamines in food. Toxicological Res. 31, 279–288. doi: 10.5487/TR.2015.31.3.279
PeÑarando, J., Aranda, E., and RodrÍguez-Ariza, A. (2019). Immunomodulatory roles of nitric oxide in cancer: tumor microenvironment says “NO” to antitumor immune response. Transl. Res. 210, 99–108. doi: 10.1016/j.trsl.2019.03.003
Pennisi, M., Lanza, G., Cantone, M., Schepis, C., Ferri, R., Barone, R., et al. (2017). Unusual neurological presentation of nevoid basal cell carcinoma syndrome (Gorlin-Goltz syndrome). J. Clin. Neurology. 13, 439–441. doi: 10.3988/jcn.2017.13.4.439
Pignatelli, P., Iezzi, L., Pennese, M., Raimondi, P., Cichella, A., Bondi, D., et al. (2021). The potential of colonic tumor tissue fusobacterium nucleatum to predict staging and its interplay with oral abundance in colon cancer patients. Cancers (Basel) 13, 175–190. doi: 10.3390/cancers13051032
Pinheiro, L. C., Amaral, J. H., Ferreira, G. C., Portella, R. L., Ceron, C. S., Montenegro, M. F., et al. (2015). Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radical Biol. Med. 87, 252–262. doi: 10.1016/j.freeradbiomed.2015.06.038
Plenchette, S., Paul, C., and Bettaieb, A. (2017). “Chapter 6 - nitric oxide and platinum-derivative-based regimens for cancer treatment: from preclinical studies to clinical trials,” in Nitric oxide (Donor/induced) in chemosensitizing. 1. Ed. Bonavida, B. (Elsevier, Amsterdam, Netherlands: Academic Press), 91–103.
Radi, R. (2013). Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 288, 26464–26472. doi: 10.1074/jbc.R113.472936
Rajnakova, A., Moochhala, S., Goh, P. M., and Ngoi, S.-S. (2001). Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer letters. 172, 177–185. doi: 10.1016/S0304-3835(01)00645-0
Rosier, B. T., Marsh, P. D., and Mira, A. (2018). Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dent. Res. 97, 371–380. doi: 10.1177/0022034517742139
Rubinstein, M. R., Wang, X., Liu, W., Hao, Y., Cai, G., and Han, Y. W. (2013). Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206. doi: 10.1016/j.chom.2013.07.012
Saaed, H. K., Chiggiato, L., Webb, D.-L., Rehnberg, A.-S., Rubio, C. A., Befrits, R., et al. (2021). Elevated gaseous luminal nitric oxide and circulating IL-8 as features of Helicobacter pylori-induced gastric inflammation. Upsala J. Med. Sci. 126, 50–67. doi: 10.48101/ujms.v126.8116
Said Abasse, K., Essien, E. E., Abbas, M., Yu, X., Xie, W., Sun, J., et al. (2022). Association between dietary nitrate, nitrite intake, and site-specific cancer risk: A systematic review and meta-analysis. Nutrients 14, 120–135. doi: 10.3390/nu14030666
Salvatori, L., Malatesta, S., Illi, B., Somma, M. P., Fionda, C., Stabile, H., et al. (2023). Nitric oxide prevents glioblastoma stem cells’ Expansion and induces temozolomide sensitization. Int. J. Mol. Sci. 24, 11286. doi: 10.3390/ijms241411286
Sang, J., Chen, Y., and Tao, Y. (2011). Nitric oxide inhibits gastric cancer cell growth through the modulation of the Akt pathway. Mol. Med. Rep. 4, 1163–1167. doi: 10.3892/mmr.2011.535
Sarkar, P., Malik, S., Laha, S., Das, S., Bunk, S., Ray, J. G., et al. (2021). Dysbiosis of oral microbiota during oral squamous cell carcinoma development. Front. Oncol. 11, 614448. doi: 10.3389/fonc.2021.614448
Sasaki, H., Ishizuka, T., Muto, M., Nezu, M., Nakanishi, Y., Inagaki, Y., et al. (1998). Presence of Streptococcus anginosus DNA in esophageal cancer, dysplasia of esophagus, and gastric cancer. Cancer Res. 58, 2991–2995.
Sayır, F., Şehitoğulları, A., Demir, H., Aslan, M., Çobanoğlu, U., and Bilgin, C. (2019). Serum prolidase activity, total oxidant/antioxidant, and nitric oxide levels in patients with esophageal squamous cell carcinoma. Turkish J. Thorac. Cardiovasc. Surgery. 27, 206. doi: 10.5606/tgkdc.dergisi.2019.16888
Schenk, M. W., Humphrey, S., Hossain, A., Revill, M., Pearsall, S., Lallo, A., et al. (2021). Soluble guanylate cyclase signalling mediates etoposide resistance in progressing small cell lung cancer. Nat. Commun. 12, 6652. doi: 10.1038/s41467-021-26823-6
Schullehner, J., Hansen, B., Thygesen, M., Pedersen, C. B., and Sigsgaard, T. (2018). Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. cancer. 143, 73–79. doi: 10.1002/ijc.v143.1
Schwartz, M., Canon, F., Feron, G., Neiers, F., and Gamero, A. (2021). Impact of oral microbiota on flavor perception: From food processing to in-mouth metabolization. Foods. 10, 2006. doi: 10.3390/foods10092006
Semenikhina, M., Stefanenko, M., Spires, D. R., Ilatovskaya, D. V., and Palygin, O. (2022). Nitric-oxide-mediated signaling in podocyte pathophysiology. Biomolecules. 12, 200–216. doi: 10.3390/biom12060745
Sharma, T., Gupta, A., Chauhan, R., Bhat, A. A., Nisar, S., Hashem, S., et al. (2022). Cross-talk between the microbiome and chronic inflammation in esophageal cancer: potential driver of oncogenesis. Cancer Metastasis Rev. 41, 281–299. doi: 10.1007/s10555-022-10026-6
Shi, N. and Chen, T. (2022). Chemopreventive properties of black raspberries and strawberries in esophageal cancer review. Antioxidants. 11, 1815. doi: 10.3390/antiox11091815
Shiva, S. (2013). Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 1, 40–44. doi: 10.1016/j.redox.2012.11.005
Shouval, R., Eshel, A., Dubovski, B., Kuperman, A. A., Danylesko, I., Fein, J. A., et al. (2020). Patterns of salivary microbiota injury and oral mucositis in recipients of allogeneic hematopoietic stem cell transplantation. Blood advances. 4, 2912–2917. doi: 10.1182/bloodadvances.2020001827
Siegel, R. L., Miller, K. D., Goding Sauer, A., Fedewa, S. A., Butterly, L. F., Anderson, J. C., et al. (2020). Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164. doi: 10.3322/caac.21601
Somasundaram, V., Ridnour, L. A., Cheng, R. Y. S., Walke, A. J., Kedei, N., Bhattacharyya, D. D., et al. (2022). Systemic Nos2 Depletion and Cox inhibition limits TNBC disease progression and alters lymphoid cell spatial orientation and density. Redox Biol. 58, 102529. doi: 10.1016/j.redox.2022.102529
Song, P., Wu, L., and Guan, W. (2015). Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients. 7, 9872–9895. doi: 10.3390/nu7125505
Srour, B., Chazelas, E., Druesne-Pecollo, N., Esseddik, Y., de Edelenyi, F. S., Agaësse, C., et al. (2023). Dietary exposure to nitrites and nitrates in association with type 2 diabetes risk: Results from the NutriNet-Santé population-based cohort study. PloS Med. 20, e1004149. doi: 10.1371/journal.pmed.1004149
Su, H., Liu, X., Du, J., Deng, X., and Fan, Y. (2020). The role of hemoglobin in nitric oxide transport in vascular system. Med. Novel Technol. Devices. 5, 100034. doi: 10.1016/j.medntd.2020.100034
Suksawat, M., Techasen, A., Namwat, N., Yongvanit, P., Khuntikeo, N., Titapun, A., et al. (2017). Upregulation of endothelial nitric oxide synthase (eNOS) and its upstream regulators in Opisthorchis viverrini associated cholangiocarcinoma and its clinical significance. Parasitol. Int. 66, 486–493. doi: 10.1016/j.parint.2016.04.008
Sun, G.-G., Hu, W.-N., Zhang, J., Li, C.-L., and Yang, C.-R. (2013). Effect of nitric oxide on esophageal cancer cell line TE-1. Chin. Med. Sci. J. 28, 44–49. doi: 10.1016/S1001-9294(13)60018-8
Sun, J., Tang, Q., Yu, S., Xie, M., Xie, Y., Chen, G., et al. (2020). Role of the oral microbiota in cancer evolution and progression. Cancer Med. 9, 6306–6321. doi: 10.1002/cam4.v9.17
Sun, J., Zhou, M., Salazar, C. R., Hays, R., Bedi, S., Chen, Y., et al. (2017). Chronic periodontal disease, periodontal pathogen colonization, and increased risk of precancerous gastric lesions. J. Periodontol. 88, 1124–1134. doi: 10.1902/jop.2017.160829
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Tanda, N., Washio, J., Kamei, T., Akazawa, K., Takahashi, N., and Koseki, T. (2019). Professional oral care reduces carcinogenic acetaldehyde levels in mouth air of perioperative esophageal cancer patients: A prospective comparative study. Tohoku J. Exp. Med. 249, 75–83. doi: 10.1620/tjem.249.75
Tandon, P., Dadhich, A., Saluja, H., Bawane, S., and Sachdeva, S. (2017). The prevalence of squamous cell carcinoma in different sites of oral cavity at our Rural Health Care Centre in Loni, Maharashtra–a retrospective 10-year study. Contemp. Oncology/Współczesna Onkologia. 21, 178–183. doi: 10.5114/wo.2017.68628
Tateda, M., Shiga, K., Saijo, S., Sone, M., Hori, T., Yokoyama, J., et al. (2000). Streptococcus anginosus in head and neck squamous cell carcinoma: implication in carcinogenesis. Int. J. Mol. Med. 6, 699–1402. doi: 10.3892/ijmm.6.6.699
Temkin, A., Evans, S., Manidis, T., Campbell, C., and Naidenko, O. V. (2019). Exposure-based assessment and economic valuation of adverse birth outcomes and cancer risk due to nitrate in United States drinking water. Environ. Res. 176, 108442. doi: 10.1016/j.envres.2019.04.009
Thaiss, C. A., Zmora, N., Levy, M., and Elinav, E. (2016). The microbiome and innate immunity. Nature. 535, 65–74. doi: 10.1038/nature18847
Tiso, M. and Schechter, A. N. (2015). Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PloS One 10, e0119712. doi: 10.1371/journal.pone.0119712
Tripathi, M. K., Ojha, S. K., and Amal, H. (2023). 532P Nitric oxide is a target by a combo-drug for glioblastoma treatment. Ann. Oncol. 34, S403. doi: 10.1016/j.annonc.2023.09.1726
Trombelli, L., Tatakis, D. N., Scapoli, C., Bottega, S., Orlandini, E., and Tosi, M. (2004). Modulation of clinical expression of plaque-induced gingivitis: II. Identification of “high-responder” and “low-responder” subjects. J. Clin. periodontology. 31, 239–252. doi: 10.1111/j.1600-051x.2004.00478.x
Tuominen, H. and Rautava, J. (2021). Oral microbiota and cancer development. Pathobiology. 88, 116–126. doi: 10.1159/000510979
Uchino, Y., Goto, Y., Konishi, Y., Tanabe, K., Toda, H., Wada, M., et al. (2021). Colorectal cancer patients have four specific bacterial species in oral and gut microbiota in common-A metagenomic comparison with healthy subjects. Cancers (Basel) 13, 300–315. doi: 10.3390/cancers13133332
Uhlenhopp, D. J., Then, E. O., Sunkara, T., and Gaduputi, V. (2020). Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 13, 1010–1021. doi: 10.1007/s12328-020-01237-x
Voss, N. C., Kold-Petersen, H., and Boedtkjer, E. (2019). Enhanced nitric oxide signaling amplifies vasorelaxation of human colon cancer feed arteries. Am. J. Physiology-Heart Circulatory Physiol. 316, H245–HH54. doi: 10.1152/ajpheart.00368.2018
Wade, W. G. (2013). The oral microbiome in health and disease. Pharmacol. Res. 69, 137–143. doi: 10.1016/j.phrs.2012.11.006
Wade, W. G. (2021). Resilience of the oral microbiome. Periodontology 2000. 86, 113–122. doi: 10.1111/prd.12365
Wang, J., He, P., Gaida, M., Yang, S., Schetter, A. J., Gaedcke, J., et al. (2016a). Inducible nitric oxide synthase enhances disease aggressiveness in pancreatic cancer. Oncotarget. 7, 52993. doi: 10.18632/oncotarget.10323
Wang, J., He, P., Gaida, M., Yang, S., Schetter, A. J., Gaedcke, J., et al. (2016b). Inducible nitric oxide synthase enhances disease aggressiveness in pancreatic cancer. Oncotarget. 7, 52993–53004. doi: 10.18632/oncotarget.10323
Wang, H., Wang, L., Xie, Z., Zhou, S., Li, Y., Zhou, Y., et al. (2020). Nitric oxide (NO) and NO synthases (NOS)-based targeted therapy for colon cancer. Cancers. 12, 1881. doi: 10.3390/cancers12071881
Wang, Q., Ye, S., Chen, X., Xu, P., Li, K., Zeng, S., et al. (2019). Mitochondrial NOS1 suppresses apoptosis in colon cancer cells through increasing SIRT3 activity. Biochem. Biophys. Res. Commun. 515, 517–523. doi: 10.1016/j.bbrc.2019.05.114
Ward, M. H., Jones, R. R., Brender, J. D., de Kok, T. M., Weyer, P. J., Nolan, B. T., et al. (2018). Drinking water nitrate and human health: an updated review. Int. J. Environ. Res. Public Health 15, 80–95. doi: 10.3390/ijerph15071557
Welch, J. L. M., Ramírez-Puebla, S. T., and Borisy, G. G. (2020). Oral microbiome geography: micron-scale habitat and niche. Cell Host Microbe 28, 160–168. doi: 10.1016/j.chom.2020.07.009
Wink, D. A., Hines, H. B., Cheng, R. Y., Switzer, C. H., Flores-Santana, W., Vitek, M. P., et al. (2011). Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 89, 873–891. doi: 10.1189/jlb.1010550
Xie, L., Mo, M., Jia, H.-X., Liang, F., Yuan, J., and Zhu, J. (2016). Association between dietary nitrate and nitrite intake and site-specific cancer risk: evidence from observational studies. Oncotarget. 7, 150–165. doi: 10.18632/oncotarget.10917
Xiong, H., Wang, R., and Zhang, C. (2021). A review on the research progress of inducible nitric oxide synthase in the pathogenesis of pancreatic Malignancy. J. Clin. Nurs. Res. 5, 151–153. doi: 10.26689/jcnr.v5i4.2281
Yang, Y., Long, J., Wang, C., Blot, W. J., Pei, Z., Shu, X., et al. (2022). Prospective study of oral microbiome and gastric cancer risk among Asian, African American and European American populations. Int. J. Cancer. 150, 916–927. doi: 10.1002/ijc.v150.6
Yao, X., Wu, Y., Zhu, M., Qian, H., and Chen, Y. (2015). Nitric oxide/cyclic guanosine monophosphate inducers sodium nitroprusside and L-arginine inhibit the proliferation of gastric cancer cells via the activation of type II cyclic guanosine monophosphate-dependent protein kinase. Oncol. Letters. 10, 479–484. doi: 10.3892/ol.2015.3229
Zhang, X., Jin, L., Tian, Z., Wang, J., Yang, Y., Liu, J., et al. (2019). Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. Cancer science. 110, 1054–1063. doi: 10.1111/cas.2019.110.issue-3
Zhang, L., Liu, Y., Zheng, H. J., and Zhang, C. P. (2019). The oral microbiota may have influence on oral cancer. Front. Cell Infect. Microbiol. 9, 476. doi: 10.3389/fcimb.2019.00476
Zhang, Y., Wang, X., Li, H., Ni, C., Du, Z., and Yan, F. (2018). Human oral microbiota and its modulation for oral health. Biomedicine Pharmacotherapy. 99, 883–893. doi: 10.1016/j.biopha.2018.01.146
Zhang, W.-L., Wang, S.-S., Wang, H.-F., Tang, Y.-J., Tang, Y.-L., and Liang, X.-H. (2019). Who is who in oral cancer? Exp. Cell Res. 384, 111634. doi: 10.1016/j.yexcr.2019.111634
Zhang, Y. Z., Wang, C. F., and Zhang, L. F. (2018). Cucurbitacin D impedes gastric cancer cell survival via activation of the iNOS/NO and inhibition of the Akt signalling pathway. Oncol. Rep. 39, 2595–2603. doi: 10.3892/or.2018.6361
Zhao, J., O’Neil, M., Schonfeld, M., Komatz, A., Weinman, S. A., and Tikhanovich, I. (2020). Hepatocellular protein arginine methyltransferase 1 suppresses alcohol-induced hepatocellular carcinoma formation by inhibition of inducible nitric oxide synthase. Hepatol. Commun. 4, 790–808. doi: 10.1002/hep4.1488
Zhou, L., Wang, Y., Tian, D.-A., Yang, J., and Yang, Y.-Z. (2012). Decreased levels of nitric oxide production and nitric oxide synthase-2 expression are associated with the development and metastasis of hepatocellular carcinoma. Mol. Med. Rep. 6, 1261–1266. doi: 10.3892/mmr.2012.1096
Zhou, L., Zhang, H., and Wu, J. (2016). Effects of nitric oxide on the biological behavior of HepG2 human hepatocellular carcinoma cells. Exp. Ther. Med. 11, 1875–1880. doi: 10.3892/etm.2016.3128
Keywords: nitric oxide, dietary nitrate, nitrite (NONO2-), nitrate (NO3-), oral microbiome, microbiota, gastrointestinal cancers, iNOS
Citation: Jasemi SK, Faridafshar H, Amin MN, Babamohamadi M, Falahati M, Amirian R and Izadi Z (2025) NO: a key player in microbiome dynamics and cancer pathogenesis. Front. Cell. Infect. Microbiol. 15:1532255. doi: 10.3389/fcimb.2025.1532255
Received: 21 November 2024; Accepted: 29 May 2025;
Published: 26 June 2025.
Edited by:
Xin-Ru Wang, Upstate Medical University, United StatesReviewed by:
Erika M Palmieri, National Cancer Institute at Frederick (NIH), United StatesNikhilesh Joardar, Washington University in St. Louis, United States
Pratibha Gaur, International Centre for Genetic Engineering and Biotechnology, India
Copyright © 2025 Jasemi, Faridafshar, Amin, Babamohamadi, Falahati, Amirian and Izadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhila Izadi, emFkaV96aEByYXppLnR1bXMuYWMuaXI=; aXphZGkuemhpbGFAeWFob28uY29t; Roshanak Amirian, cm9hbWlyaWFuQGdtYWlsLmNvbQ==
 Seyedeh Kimia Jasemi1,2
Seyedeh Kimia Jasemi1,2 Mohammed Namiq Amin
Mohammed Namiq Amin Mehregan Babamohamadi
Mehregan Babamohamadi Roshanak Amirian
Roshanak Amirian Zhila Izadi
Zhila Izadi