- 1School of Health Sciences, University of Tasmania, Launceston, TAS, Australia
- 2Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia
- 3Child and Maternal Health Division, Menzies School of Health Research, Darwin, NT, Australia
Nontypeable Haemophilus influenzae (NTHi) is a major respiratory pathogen that imposes a substantial disease burden, globally. Further amplifying the burden of NTHi-associated infections is the rapidly expanding spectrum and prevalence of antibiotic resistance, and the lack of an effective vaccination strategy. In 2017, the World Health Organization list of “priority pathogens”, highlighted the urgent need for new therapeutic agents against NTHi. Consequently, alternative preventative or treatment approaches that do not rely on antibiotic susceptibility or stable vaccine targets are becoming more attractive. The nutritional dependency for haem/iron at all stages of NTHi pathogenesis exposes a vulnerability that may be exploited for the development of such therapies. This review explores the role of haem/iron in all facets of NTHi pathogenesis, the host-bacterial competition for this vital nutrient, and the therapeutic potential of strategies that interfere with its acquisition.
1 NTHi is a major pathogen in the respiratory tract
Members of the Haemophilus genus are Gram-negative coccobacilli belonging to the Pasteurellaceae family. Most infections associated with this genus are caused by H. influenzae; other species such as H. aegyptius, H. parainfluenzae and H. ducreyi are rarely isolated from clinical samples but have been documented to cause a variety of mild respiratory or genitourinary tract infections (Nørskov-Lauritsen, 2014; Van Eldere et al., 2014). H. influenzae can be subtyped into encapsulated strains, which express different serotypes of capsular polysaccharide (designated types a–f), and nonencapsulated strains, which are designated nontypeable H. influenzae or NTHi. Prior to widespread implementation of the Haemophilus influenzae type b (Hib)-conjugate vaccination programs in the 1990s, Hib was the most common cause of bacterial meningitis in children under the age of five (Heath et al., 2001; Maguire et al., 2018; Soeters et al., 2018). Concurrent with the decline in Hib disease, the prevalence of NTHi in human carriage and disease has increased such that NTHi is now the most common phenotype isolated from clinical infection sites (Van Eldere et al., 2014).
NTHi must first colonise the nasopharynx before migrating to other anatomical sites where it can cause a wide spectrum of disease; however, the exact mechanisms that influence the behavioural shift of NTHi from coloniser to pathogen are not understood (Van Eldere et al., 2014). Asymptomatic nasopharyngeal NTHi colonisation is common among adults (20-30%) and children under the age of five (52-84%) (Mackenzie et al., 2010; Bajanca-Lavado et al., 2022). Colonisation typically begins within two years of birth and follows a dynamic and diverse course of rapid genotype turnover and simultaneous carriage of multiple strains (Faden et al., 1995; Van Kempen et al., 2001; Mukundan et al., 2007; Puig et al., 2014). NTHi is a common cause of opportunistic mucosal infections including sinusitis, paediatric conjunctivitis and community-acquired pneumonia (Van Eldere et al., 2014; Cerquetti and Giufrè, 2016). The post-Hib vaccine era has also seen a steady global increase in the incidence of invasive infections among young children and the elderly (from 10-17% in 1989 to 84-90% in 2009-2015) caused by NTHi (Ladhani et al., 2010; Cheong et al., 2015; Collins et al., 2015; Whittaker et al., 2017; Giufrè et al., 2018; Soeters et al., 2018). The highest NTHi morbidity is seen in two clinical settings: otitis media (middle ear infection) in children, and individuals with chronic lung disease (Van Eldere et al., 2014; Cerquetti and Giufrè, 2016).
1.1 Otitis media
OM is an important disease in early childhood globally - it is one of the most common reasons for health care visits and antibiotic prescription, and the leading cause of conductive hearing loss in children (Monasta et al., 2012). OM incidence varies geographically with recent global epidemiological estimates reporting ~391–709 million cases of OM each year (Monasta et al., 2012; Huang et al., 2025), with approximately 83% of children experiencing at least one episode by the age of three (Teele et al., 1989; Sierra et al., 2011). When taking into account recurrent episodes and seasonal variation, NTHi accounts for approximately 60% of cases (Haggard, 2008; Barkai et al., 2009). OM occurs when bacteria within the nasopharynx ascend the eustachian tube and gain access to the middle ear space (Kaur et al., 2011). Compared to other otopathogens, NTHi-associated OM is associated with higher disease severity, treatment failure, recurrence, persistence and need for repeat surgery (Barkai et al., 2009; Seppanen et al., 2020). Recurrent episodes occur in 26-54% of cases (Teele et al., 1989; Leibovitz, 2008; Arguedas et al., 2010) and greatly increase a child’s risk of developing chronic suppurative otitis media (CSOM); a complication characterised by chronic inflammation of the middle ear cavity with recurrent discharge through tympanic perforation (Welp and Bomberger, 2020). Hearing loss and consequential impairment of childhood psychosocial and cognitive development are common complications of CSOM in children (Monasta et al., 2012) and although rare, neurological sequelae account for 21,000 deaths annually (Monasta et al., 2012; Penido et al., 2016).
1.2 Chronic lung disease
NTHi is the leading bacterial cause of infectious exacerbations in adults with chronic obstructive pulmonary disease (COPD) and the second leading cause of infections in paediatric cystic fibrosis (Finney et al., 2014; Marshall et al., 2022). Due to a combination of host and microbial factors, NTHi persists in the lower airways of these individuals which is associated with an increased frequency of exacerbations, worsening symptoms, inflammation causing tissue damage, compositional changes to the lung microbiome and overall worse clinical prognosis (Sethi and Murphy, 2001; Sriram et al., 2017; Welp and Bomberger, 2020; Dicker et al., 2021). Similarly, children with protracted bacterial bronchitis (a disease caused by chronic infection of the conducting airways) who are positive for NTHi have reduced lung function (Craven and Everard, 2013) and are 7-fold more likely to progress to bronchiectasis within 2 years (Wurzel et al., 2016). Children with bronchiectasis experience significant morbidity and, if untreated, have poorer clinical outcomes in later life compared to patients with adult-onset bronchiectasis (King et al., 2009). As such, NTHi is an important contributor to the functional decline, disease progression, morbidity and mortality in paediatric, and adult chronic airway diseases (Sethi and Murphy, 2001).
2 Current management of NTHi infections necessitates alternative therapeutic strategies
2.1 Antibiotic therapy
NTHi-associated lower respiratory infections are typically treated with moderate spectrum β-lactam (typically amoxicillin or amoxicillin-clavulanic acid) antibiotics (Marchant et al., 2024). In COPD patients where an infection is suspected, empiric amoxicillin and doxycycline (in addition to oral corticosteroids depending on disease severity) are recommended (Yang IA et al., 2024); however, antibiotic selection may be based on local susceptibility patterns (Wedzicha et al., 2017). Although macrolides (e.g. azithromycin) have good efficacy against common respiratory pathogens and desirable anti-inflammatory properties, their use is cautioned due to potential significant adverse effects (Ni et al., 2015; Maddi et al., 2017; Yang IA et al., 2024). Amoxicillin treatment is strongly recommended for persistent OM (with or without effusion) among high-risk children (Leach et al., 2021), with amoxicillin-clavulanic acid or 2nd-3rd generation cephalosporins (e.g. cefuroxime, ceftriaxone, cefpodoxime) being recommended where initial treatment fails (Siddiq and Grainger, 2015). Best practice guidelines for Australian First Nations children recommend CSOM is treated with ear cleaning plus topical ciprofloxacin 2–3 times per day until the ear has no discharge for 3 consecutive days (Leach, 2020). Azithromycin is recommended for acute OM cases where adherence is difficult or there is no access to refrigeration (Leach et al., 2021), and cefdinir or co-trimoxazole have been recommended for children allergic to penicillin (Siddiq and Grainger, 2015; Leach et al., 2021).
There is a growing frequency of antibiotic treatment failure among patients with NTHi-associated infections, largely owing to a rapidly evolving resistance profile (Sriram et al., 2017). In 2017, NTHi resistance was highlighted in the World Health Organisation’s list of “priority pathogens” for which new therapeutic/preventative agents are urgently needed (Organization WH, 2017). However, there have since been no advances in NTHi-targeted therapeutics and the bacterium remains on this list as of 2024 (Organization WH, 2024). The prevalence of amoxicillin/ampicillin resistance varies markedly between regions and has grown substantially over the past decade from 20-44% between 1997-2010 (Ito et al., 2010; Dabernat and Delmas, 2012; Resman et al., 2012) to 19-74% in 2016-2021 (Wang et al., 2019; Li et al., 2020; Zhou et al., 2022; El Nouwar et al., 2025). More recently, studies have reported the emergence of treatment failure with amoxicillin-clavulanic acid and cephalosporins used to treat NTHi-associated OM and community-acquired pneumonia (Patel et al., 1995; Dagan et al., 1997; Puig et al., 2015). Although global isolation of these strains is currently low (12-17%) (Dabernat and Delmas, 2012; Heliodoro et al., 2020; Li et al., 2020), their prevalence is increasing, particularly in regions such as Japan and Taiwan (Skaare et al., 2014; Honda et al., 2018; Yamada et al., 2020; Abavisani et al., 2024). Additionally, mechanisms of acquired resistance to macrolides and fluoroquinolones have also been reported, and multidrug-resistant NTHi isolates have been recovered from blood, middle ear, sputum, and nasopharyngeal specimens (Pérez-Vázquez et al., 2004; Phaff et al., 2006; Pfeifer et al., 2013; Ni et al., 2015; Puig et al., 2015; Su et al., 2020; Abavisani et al., 2024). Although currently uncommon, these resistance mechanisms have the potential to spread owing to the ability of NTHi to transfer resistant genes on mobile genetic elements and by chromosomal recombination (Hegstad et al., 2020).
The growing prevalence and spectrum of NTHi antibiotic resistance necessitates changes to antibiotic treatment guidelines that carefully balance intended clinical outcomes with the risk of further promoting antibiotic resistance. Extremely high antibiotic prescription rates are associated with the management of OM and exacerbations of COPD, a high proportion of which are considered unnecessary (Turnidge and Meleady, 2017). Antibiotic therapy may reduce middle ear damage in some patients with acute OM (Venekamp et al) but in 60% of cases, the infection spontaneously clears without antibiotic intervention and without complications (Venekamp et al]; Haggard, 2008). Similarly, antibiotic management reduces exacerbation frequency in COPD patients (Roede et al., 2009; Rothberg et al., 2010) but has no overall impact on airway destruction and disease progression (Dixit et al., 2016). In addition, NTHi frequently evades antibiotic clearance through the formation of biofilm communities or invasion of host cells (García-Cobos et al., 2014; Duell et al., 2016). For these reasons, antibiotic treatment is often insufficient to prevent relapse caused by persistent bacterial communities and does not offer long-term protection against reinfection with different strains, particularly in COPD airways (Leibovitz, 2008; García-Cobos et al., 2014; Miravitlles and Anzueto, 2015; Sriram et al., 2017).
2.2 Preventative strategies
In addition to the aforementioned treatment challenges, single courses of antibiotics cannot prevent reinfection and there is currently no effective NTHi vaccine. Despite the enormous success of the Hib-conjugate vaccine in preventing invasive infections caused by capsular type b H.influenzae, it has no effect on NTHi because these strains lacks the type b capsule (the vaccine target) (Hariadi et al., 2015). Although other vaccines comprising different antigen targets have been developed, none have proven effective in preventing infections caused by NTHi (Smith-Vaughan et al., 2014). Challenges in developing an effective vaccine arise from the enormous genetic heterogeneity among NTHi strains and the high rates of phase-variable expression of many putative vaccine targets (Murphy, 2015; Jalalvand and Riesbeck, 2018; Novotny et al., 2019). The only vaccine that has shown any potential protection against NTHi disease is the ten-valent pneumococcal conjugate vaccine containing protein D (NTHi antigen) as a carrier protein (PHiD-CV; Synflorix™, GSK Vaccines) licensed in 2008 by a number of countries for active immunisation against acute OM caused by NTHi (Clarke et al., 2017). However, randomised controlled trials of PHiD-CV among infants in Finland and Australian First Nations populations found no significant impact on NTHi carriage rates or development of OM (Vesikari et al., 2016; Beissbarth et al., 2021).
3 Host-NTHi competition for haem-iron
Iron is an essential micronutrient for pathogen and host alike, with critical roles in many vital cellular processes, both as an inorganic ion and through incorporation into haem, which is a molecule composed of a protoporphyrin IX (PPIX) ring and a single central iron atom (Parrow et al., 2013; Barber and Elde, 2015). Biochemical (White and Granick, 1963) and genomic studies (Cavallaro et al., 2008) indicate that NTHi lack multiple enzymes required for biosynthesis of PPIX. NTHi possesses a ferrochelatase (encoded by hemH), allowing it to catalyse the insertion of iron into PPIX (Schlör et al., 2000); however, there is no significant source of free PPIX in host tissues and hemH is not required for host colonisation or infection (Cavallaro et al., 2008), hence it appears that the growth requirement for haem must be fulfilled by acquisition of intact haem from the host. The redox potential of iron underpins both its catalytic utility in biological reactions, and production of cell-damaging free radicals. The host must therefore maintain tight regulation of systemic and cellular iron homeostasis to simultaneously meet the body’s iron demand, prevent cellular toxicity, and withhold nutrients from invading pathogens (Cassat and Skaar, 2013).
3.1 Haem-iron regulation in the respiratory tract
Sequestration of free iron is achieved by iron-binding proteins (such as ferritin, transferrin and lactoferrin) and by capturing haem within haemoproteins (Cassat and Skaar, 2013; Barber and Elde, 2015) (Figure 1). The majority of total iron in the body is found complexed as haemoglobin within erythrocytes (~1.5–2 g iron), whereas the major store of inorganic iron is ferritin, found within hepatocytes (~1 g) and macrophages (~600 mg) (Szelestey et al., 2013; Hariadi et al., 2015). Plasma iron concentrations are maintained at a low level (as complexes with transferrin), and this decreases substantially during the acute phase response to infection or other inflammatory stimuli (Ganz, 2018; Saini et al., 2024). The intracellular sequestration and the paucity of free extracellular haem-iron ensures that there is scarce availability of this essential nutrient for pathogens (Cassat and Skaar, 2013).
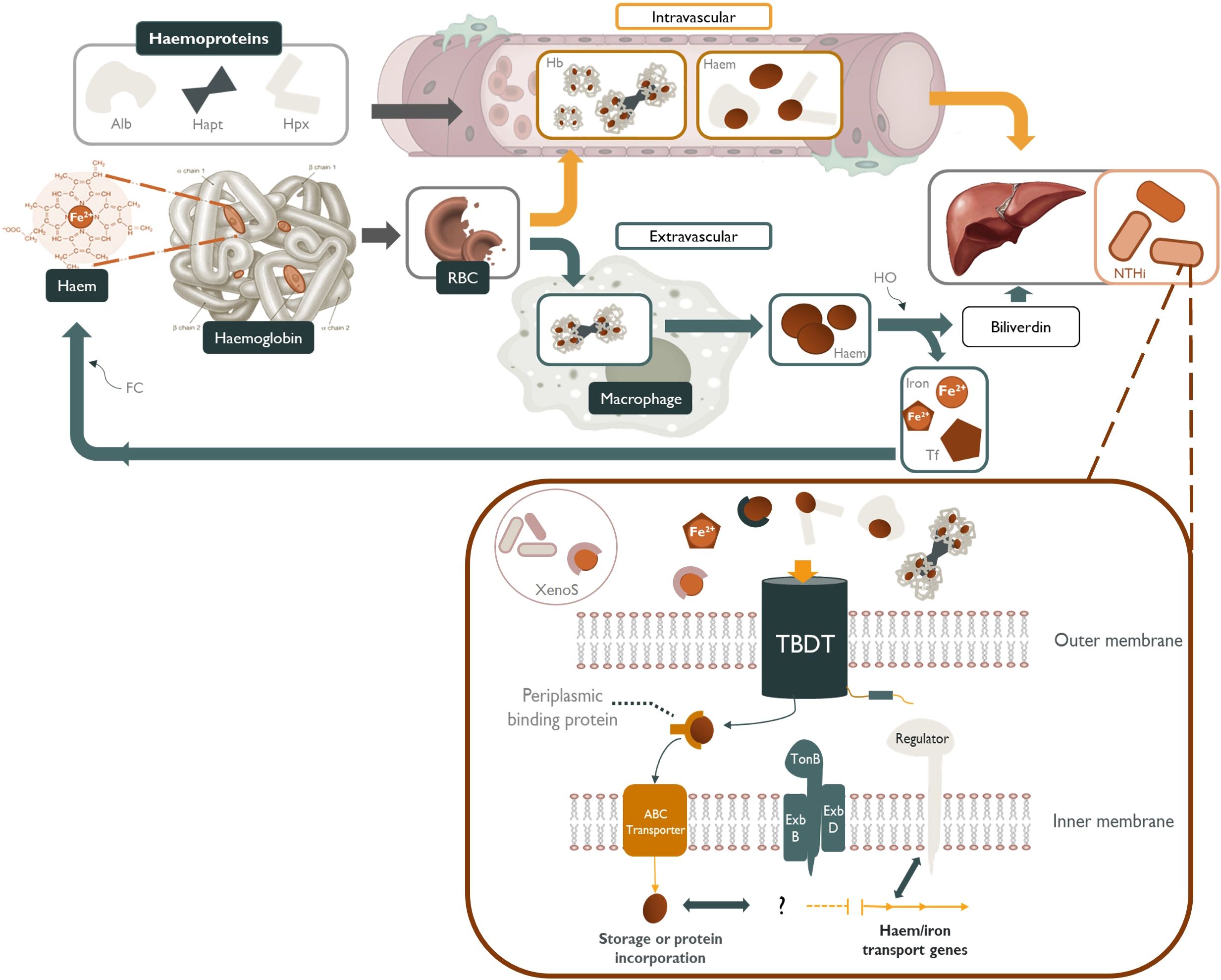
Figure 1. NTHi sequestration of host-derived sources of haem and other iron-containing moieties. Haem-iron released into the plasma by erythrocyte (RBC) senescence is quickly scavenged by haemoproteins such as, albumin (Alb) and hemopexin (Hpx). Any haemoglobin (Hb) released into the serum is tightly bound by haptoglobin (Hapt) and subsequently cleared by tissue macrophages (Szelestey et al., 2013; Hariadi et al., 2015). Free-haem, free-iron, transferrin-bound (Tf) iron, xenosiderophore-bound (XenoS) and haem-containing haemoproteins are readily available to NTHi as sources of nutritional haem/iron. These iron-containing molecules are transported across the periplasmic space by specific TonB-dependent transporters (TBDT) powered by a cytoplasmic transmembrane protein complex (TonB, ExbB and ExbD), followed by transport across the inner membrane by an ATP-binding cassette transporter (ABC transporter). Haem/iron acquisition in NTHi is regulated in accordance with environmental cues by iron-repressive regulators such as fur.
Iron levels are suspected to be low in the upper respiratory tract, with an estimated daily exposure of approximately 10-25 μg or around 1/1000th of that encountered by the gastrointestinal tract (Ali et al., 2017; Cloonan et al., 2017). Iron may be sourced from the pulmonary vasculature in free-, or transferrin/lactoferrin-bound forms, or as a component of haemoprotein-bound haemoglobin/haem. The high abundance of transferrin present in bronchoalveolar lavage fluid indicates that iron regulation in the lung may be primarily controlled by transferrin (Ali et al., 2017). Both alveolar macrophages and the bronchial/alveolar epithelia are able to sequester iron by various mechanisms including receptor-mediated uptake of ferric haem-iron (followed by storage within ferritin) and endocytosis of haemoglobin-haptoglobin complexes (Smith and McCulloh, 2015; Cloonan et al., 2017). The upper respiratory tract may also be exposed to inhaled atmospheric iron and iron-containing particulate matter; however, little is known about the bioavailability of this source to the host or upper respiratory microbiota (Cloonan et al., 2017).
3.2 NTHi acquisition of host-derived haem-iron sources
Despite the inherently low availability of haem-iron in the respiratory tract, NTHi is adept at scavenging a variety of host-derived haem-iron sources from intracellular and extracellular reservoirs, using a highly complex and redundant repertoire of haem acquisition systems (Table 1). These mechanisms capture iron/haem molecules that are free in solution or complexed with host proteins and shuttle the iron/haem moiety across the cell wall and both bacterial membranes in a series of protein-ligand interactions (Figure 1) (Cassat and Skaar, 2013). NTHi is also capable of storing excess haem which creates an intracellular surplus that can be donated to starved bacterial cells in haem-iron deplete conditions; however, the mechanisms are currently unknown (Rodríguez-Arce et al., 2019).
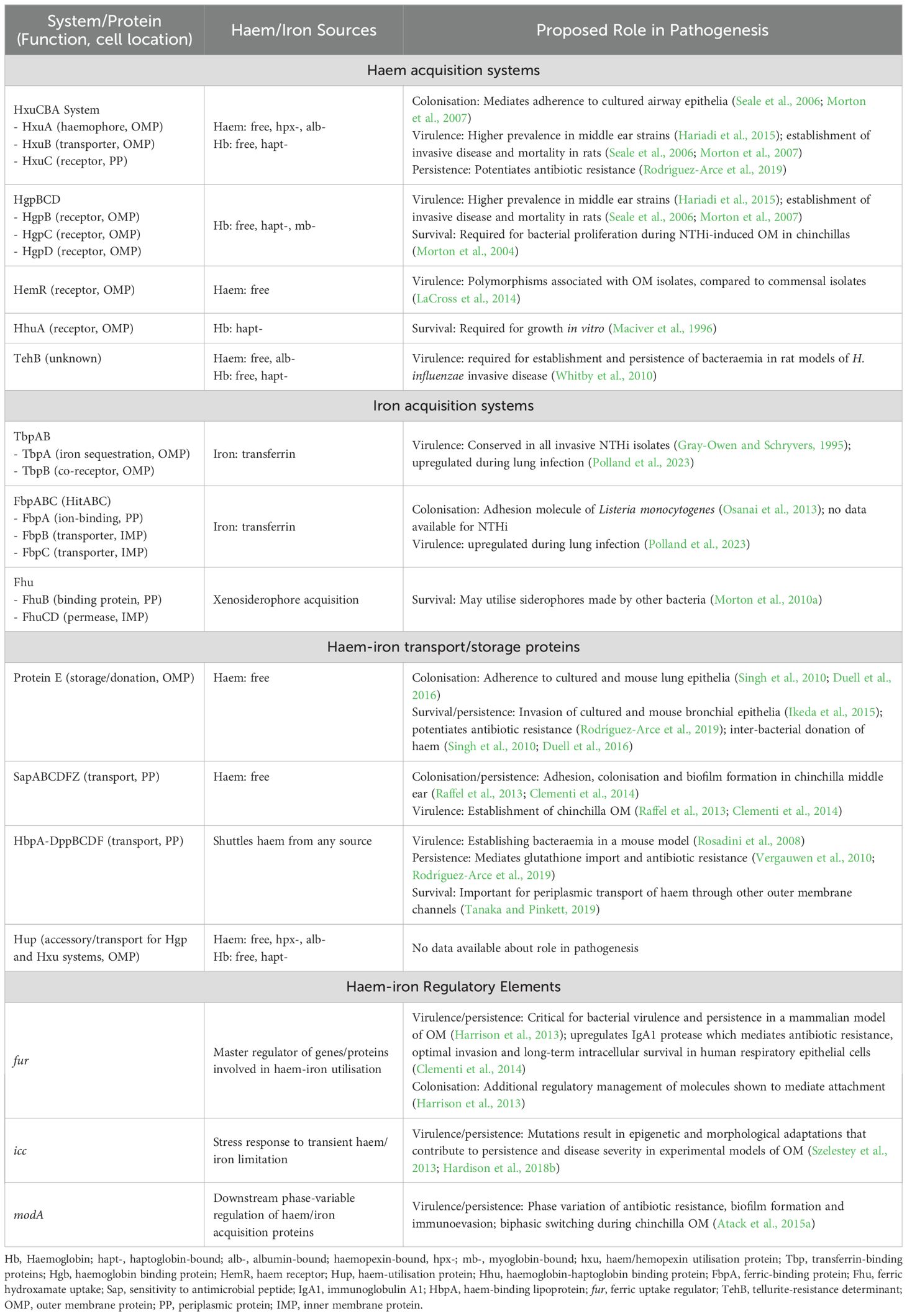
Table 1. NTHi proteins and regulatory elements involved in the acquisition of host-derived haem-iron and non-haem iron sources in vivo, and evidence for their potential roles in pathogenesis.
Haemoprotein-mediated sequestration (as opposed to acquisition of free iron) from the extracellular environment is the most common mode of iron acquisition used by NTHi. Target-specific surface-anchored proteins function by taking up free haem or extracting it from haemoproteins in the extracellular medium and delivering it to a TonB-dependent outer membrane receptor (Hariadi et al., 2015). Quantitatively, haemoglobin and haemoglobin-haptoglobin complexes are likely to be the most significant extracellular sources of haem in vivo (Seale et al., 2006). This is reflected by the sheer number of mechanisms possessed by NTHi for uptake of these molecules and the attenuated pathogenesis of strains unable to utilise them (Morton et al., 2004). Although less common, NTHi (approximately 3% of isolates) also express receptors that directly recognise and extract iron from transferrin and/or lactoferrin (Barber and Elde, 2015). In other bacterial species, the most common strategy for obtaining iron from these molecules involves the secretion of siderophores; small-molecule ferric chelators which sequester iron from host transferrin (Bullen et al., 1999). Unlike most bacteria, NTHi does not possess genes encoding proteins for siderophore synthesis; however, an iron-repressible siderophore utilisation locus was discovered in several NTHi strains that may enable utilisation of xenosiderophores produced by other microorganisms in vivo (Morton et al., 2010a). Such a tactic would further expand the pool of iron sources available to NTHi, without the energy burden associated with siderophore synthesis (Hariadi et al., 2015).
Once complexed by haem-targeting proteins or xenosiderophores, a set of TonB-dependent transporters (TBDTs) are required to transport the haem/iron into the periplasmic space, followed by transport across the inner membrane by ATP-binding cassette (ABC) transporters (Sgheiza et al., 2017). A cytoplasmic transmembrane protein complex composed of three proteins, TonB, ExbB and ExbD, spans the periplasm and interacts with specific TBDTs. This TonB complex transduces the proton motive force of the cytoplasmic membrane to energise transport of substrates through a specific TBDT (Noinaj et al., 2010). Once inside the cell, the expectation is that haem-bound iron can be liberated through enzymatic cleavage of the porphyrin ring by haem oxygenase enzymes that are present in many bacterial species (Richard et al., 2019). Haem-iron acquisition in NTHi is regulated in accordance with environmental iron-bioavailability by iron-repressive transcriptional regulators such as fur, with the result that iron uptake is coordinated with storage and efflux to ensure iron homeostasis (Cassat and Skaar, 2013). Tight regulation of haem-iron uptake is critical for balancing the metabolic requirement with the potentially toxic consequences of excess iron (Harrison et al., 2013).
3.3 Dysregulation of host haem-iron homeostasis
High iron availability has been shown to increase the pathogenic potential of many bacteria in tissue and animal infection models by enhancing growth, cellular adhesion, invasion and epithelial damage (Reid et al., 2009; Kortman et al., 2012; Nairz et al., 2017). Thus, disorders that interfere with iron-restricting host responses may predispose individuals to NTHi infections. High susceptibility to infection with a variety of bacterial genera, including Haemophilus, has been described in several iron overload disorders, such as hereditary haemochromatosis, β-thalassemia, sideroblastic anaemia, transfusion-dependence, or chronic liver disease (Weinberg, 2000; Rahav et al., 2006; Kontoghiorghes et al., 2010; Quenee et al., 2012). In these conditions, the iron-binding capacity of serum transferrin is exceeded, resulting in low affinity complexes of iron with other plasma components (Cassat and Skaar, 2013), thus providing an easily accessible iron source for microbes. Iron overload is also associated with defective chemotaxis and phagocytosis by neutrophils and macrophages, as well as decreased bactericidal activity that contributes to decreased immune function (Porto and De Sousa, 2007; Cherayil, 2010). Thus, a combination of impaired immune function and increased iron availability may contribute to the heightened susceptibility to infection in conditions of iron-overload.
High levels of iron and/or ferritin have been detected in the airways of individuals with COPD and cystic fibrosis, and in heavy smokers, suggesting dysregulation of iron homeostasis in these conditions (Stites et al., 1999; Ali et al., 2017). In these conditions, excess iron appears to reside intracellularly in alveolar macrophages and, to a lesser extent, within the epithelial lining fluid of the lung (Mateos et al., 1998). While these findings suggest a role of excess iron in the characteristically high predisposition to NTHi infections observed in these patient groups (Fenker et al., 2018), correlative studies are required to determine if this is the case. Similarly, geogenic particles from remote regions of Australia (communities with a disproportionate burden of respiratory disease and infection) with high iron oxide levels were shown to increase NTHi invasion of bronchial epithelial cells in vitro compared to low iron oxide particulate matter from other regions (Williams et al., 2023). However, further studies are required to determine whether there is a direct correlation between NTHi disease prevalence and local geogenic iron oxide particulate levels among the broader population.
4 Haem-iron is an important mediator of NTHi pathogenesis and persistence
The success of NTHi as a pathogen is reliant on its ability to perform interactions with the host, many of which are regulated directly or indirectly by the bioavailability of haem or other iron-containing moieties (Harrison et al., 2013). Haem-iron acquisition is coordinated by the ferric uptake regulator fur; a master regulator of genes involved in the uptake of iron and iron-containing moieties in many bacterial species (Hassan and Troxell, 2013). Additional regulatory management of NTHi-host cell interactions by fur have been reported (Hassan and Troxell, 2013). Haem and iron acquisition systems have also demonstrated a role in sensing host environmental cues to mediate interactions with host epithelial cells. As such, the regulatory feedback between haem/iron sequestration and interactions with the host plays an important role in all facets if NTHi pathogenesis (Harrison et al., 2013; Rodríguez-Arce et al., 2019). Haem/iron acquisition systems and evidence for their role in NTHi pathogenesis are summarised in Table 1, Figure 2.
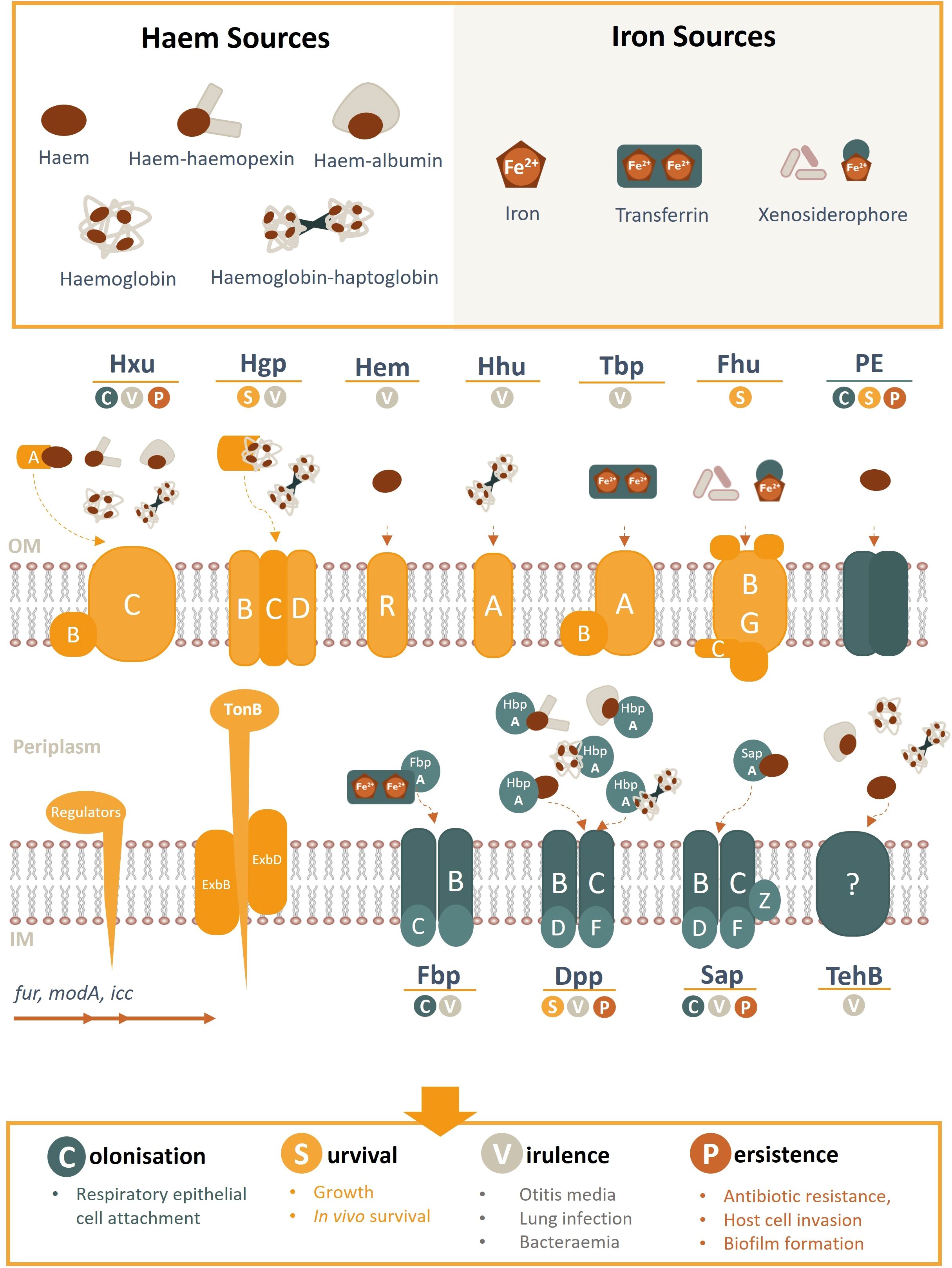
Figure 2. Schematic overview of NTHi haem/iron uptake systems and transporters, their cognate haem/iron sources, and role in pathogenesis. Known haem acquisition systems include HxuABC, HgpBCD, HemR and HhuA. The Hxu system involves the HxuAB two-partner secretion system whereby HxuA is exposed at the cell surface and leads to haem release and its capture by the HxuC receptor. The Hgp system involves haem sequestration by a secreted haemophore which is transported across the outer membrane by the HgpBCD receptor. Outer membrane receptors HemR and HhuA bind directly to host haem or haemoproteins. Iron uptake (free- or transferrin-bound) is coordinated by TbpAB, Fhu (xenosiderophores) and FbpABC (HitABC). Once within the periplasm, haem/iron are transported across the inner membrane by ABC transporters (e.g. Fbp, DppBCDF, SapBCDFZ and TehB) by their periplasmic proteins (e.g. SapA, HbpA, FbpA). PE is an outer membrane protein that binds haem as a dimer. Regulatory gene elements are indicated and systems highlighted in yellow are TonB-dependant (opposed to blue TonB independent receptors). OM, outer membrane; IM, inner membrane.
4.1 Host attachment and colonisation
In all disease contexts, nasopharyngeal colonisation by NTHi is a critical antecedent of subsequent ear and lower airway infections (Hare et al., 2010; Duell et al., 2016). Additionally, a higher strain turnover and density of NTHi colonisation has been linked with an in increased risk of developing OM (Harabuchi et al., 1994; Smith-Vaughan et al., 2006; Kirkham et al., 2010; Smith-Vaughan et al., 2013), acute exacerbations in COPD (Van Kempen et al., 2001; Sethi et al., 2002) and lower airway infections in children with bronchiectasis (Hare et al., 2010). For this reason, populations with high NTHi carriage rates experience the highest OM disease burden (Sun et al., 2012; Coleman et al., 2018). Likewise, among patients with COPD, nasopharyngeal acquisition of a new NTHi strain is frequently associated with the onset of an acute exacerbation (Sethi et al., 2002). Furthermore, elevated nasopharyngeal NTHi bacterial load can result in a clinically significant increase in COPD symptoms, even in the absence of a clinical exacerbation (Pettigrew et al., 2012).
Successful host colonisation is achieved by a suite of outer membrane proteins that mediate attachment to the nasopharyngeal epithelium (Atto et al., 2019). Upregulation of proteins involved in host-cell adhesion has been demonstrated in iron depleted media designed to replicate respiratory tract conditions (Pidcock et al., 1988; Qu et al., 2010), suggesting that iron bioavailability influences adhesin expression. Transcriptomic and proteomic analysis of fur has revealed additional regulatory management of several molecules that can mediate attachment and colonisation, such as the high-molecular weight proteins (HMW) (Qu et al., 2010; Harrison et al., 2013). HMW1 and HMW2 are major NTHi adhesins present in 75-80% of isolates (Buscher et al., 2004). These glycopeptides bind to integrin-receptors on epithelial cell surfaces and are vital to the bacterium’s ability to adhere to cultured human respiratory epithelial cells and to colonise the upper respiratory tract of rhesus macaques in vivo (Rempe et al., 2016).
Interestingly, some proteins involved in host-cell colonisation have demonstrated moonlighting functions directly involved in haem or iron acquisition. The outer membrane adhesin, protein E (PE), is ubiquitous among NTHi clinical isolates and hijacks host vitronectin-integrin binding to promote cell adhesion (Ikeda et al., 2015). The requirement for PE in the optimal adherence and persistence of NTHi within the airways has been demonstrated in mice immunised with anti-PE antibodies (Ronander et al., 2009). More recently, PE was shown to form high affinity interactions with haem and influence the ability of H. influenzae to acquire haem in vitro. PE-bound haem was also donated to haem-starved populations during co-culture, suggesting a secondary role for PE as a haem storage site that could be later distributed to nearby starved cells and promote survivability under conditions of fluctuating haem availability (Al Jubair et al., 2014; Rodríguez-Arce et al., 2019). The Sap (sensitivity to antimicrobial peptide) inner membrane ATP-binding-cassette (ABC) transport complex also appears to be important in the ability of NTHi to acquire haem-iron and colonise the host (Mason et al., 2011). Deletion of the Sap structural ATPase protein, SapF, simultaneously inhibits recovery of depleted internal haem-iron stores and reduces the ability of NTHi to colonise the nasopharynx or cause acute infection in a chinchilla model of acute OM (Vogel et al., 2012). The periplasmic binding protein, SapA was also found to be essential for haem transport and utilisation by haem-starved NTHi (Mason et al., 2011) and adherence to polarised epithelial cells through host environmental cues (Raffel et al., 2013). The shared host-cell adhesion and haem-binding functionality of many NTHi proteins suggests that bacterial haem-utilisation plays an important role in its interplay with host epithelial cells and may provide an adaptive advantage for colonisation in environments with low haem-iron availability (Ghio, 2009).
4.2 Survivability and virulence in the respiratory tract
Continued NTHi survival in the respiratory tract requires a secure source of host-derived haem; accordingly, strains with a reduced capacity to acquire haem were shown to have a substantially shortened lifespan in broth culture and in chinchilla airways (Maciver et al., 1996; Morton et al., 2004). The higher prevalence of multiple haem-acquisition genes in disease-associated isolates compared to throat colonising strains or the non-pathogenic relative H. haemolyticus is consistent with the notion that the ability to acquire diverse haem sources provides an adaptive advantage in pathogenic isolates (Qu et al., 2010; Hariadi et al., 2015). A conserved genetic island unique to disease-associated NTHi features an overrepresentation of genes associated with haem acquisition and transport (Zhang et al., 2012). Inactivation of multiple genes associated with haem-utilisation was found to attenuate NTHi virulence-determinants and disease severity/duration in animal models of acute OM and lung infection (Morton et al., 2004; Morton et al., 2009; Szelestey et al., 2013; Rodríguez-Arce et al., 2019). Similarly, an isogenic mutant of two haem-acquisition pathways was unable to sustain bacteraemia or produce meningitis in a rat model of invasive disease (Seale et al., 2006). Collectively, these data indicate that iron-dependent fur-regulated genes contribute to longer and more severe infections by regulating iron within the cell (Ahearn et al., 2017).
The ability to acquire specific haem sources may also be linked to the pathogenic potential of NTHi isolates. Capacity to utilise iron from transferrin or from haptoglobin-bound haemoglobin has been associated with invasive Haemophilus spp. isolates (Hardie et al., 1993; Zhang et al., 2012) and is not an ability shared among non-pathogenic members of the Haemophilus genus (Williams et al., 1990). Additionally, NTHi strains deficient in the ability to acquire haemoglobin-haptoglobin have an attenuated ability to cause infection (Seale et al., 2006). Individual NTHi strains are capable of producing up to four different haemoglobin/haemoglobin-haptoglobin binding proteins (Hgp) that collectively display affinity for all known human haptoglobin phenotypes (Morton et al., 2006). The presence of multiple hgp genes may allow for selective expression of the Hgp with greatest affinity for the predominant host haemoglobin-haptoglobin phenotype, or alternatively as a strategy to evade the host immune response mounted against a particular Hgp-expressing population (Langlois and Delanghe, 1996). There are 2 major haptoglobin alleles (hp1 and hp2), with each allele coding for a subunit capable of binding one haemoglobin αβ dimer. The Hp1/2 proteins form different oligomers, such that a dimeric Hp1–1 molecule can bind two haemoglobin αβ dimers, while the polymeric Hp2–1 and Hp 2–2 molecules have the capacity to bind greater numbers of haemoglobin αβ dimers (Morton et al., 2006). The prevalence of expressed haptoglobin phenotypes varies between populations, with the Hp2–2 phenotype predominating in Indian and Australian First Nations populations (Langlois and Delanghe, 1996). A growth preference for the Hp 1–1 phenotype has been demonstrated by H. influenzae in vitro (Morton et al., 2006); however, the correlation with susceptibility to infection in vivo has not been investigated. This also highlights the need for consideration of haem source in in vitro models, where free haem is typically added as the sole source in culture media: a condition which may not reflect predominant sources in the respiratory and middle ear niches. For example, the iron-containing moiety lactotransferrin was more than 10-fold more abundant in middle ear effusions collected from children with chronic OM than haemoglobin (Val et al., 2016).
There is also evidence to suggest that haem-iron accessibility may influence adaptive transitions between commensal and pathogenic states. For many opportunistic pathogens, iron starvation not only triggers activation of genes involved in its uptake, but also those involved in virulence determinants (Chen et al., 2011; Hassan and Troxell, 2013; Jung and Do, 2013). In Pseudomonas aeruginosa, positive selection for promotor mutations that increase expression of the bacterial uptake system phu (pyoverdine siderophore) occurs exclusively among isolates from patients with cystic fibrosis that show enhanced bacterial growth (Marvig et al., 2014). NTHi haem-iron acquisition proteins (along with proteins associated with biofilm formation, antibiotic resistance and immune evasion) are subject to changes in gene expression, which are differentially regulated during experimental OM (Atack et al., 2015a) and in isolates recovered from COPD airways (Rao et al., 1999; Ren et al., 1999; Poole et al., 2013) by the modA phase-variable regulon (phasevarion). Switching of modA expression from OFF to ON state within the middle ear produces a phenotypic switch in several genes during infection that increases disease severity and permits bacterial persistence by increasing cell adhesion, invasion and robust biofilm formation (Atack et al., 2015a; Brockman et al., 2016; Brockman et al., 2017; Brockman et al., 2018). Specifically, in a ModA16 ON state, NTHi upregulates multiple iron-acquisition factors including an iron ABC transporter substrate-binding protein, and haem/haemopexin-binding proteins HxuC and HxuB (Tram et al., 2024). Independent phase-variable expression has also been observed in genes involved in haemoglobin-haptoglobin acquisition (Phillips et al., 2022) which is hypothesised to provide an adaptive response to fluctuations in haem-iron availability/source influenced by the stage or site of infection (Atack et al., 2015b). Additionally, strand slippage may provide a mechanism to avoid the host immunological response by expressing proteins that are functionally similar, but antigenically distinct (Rao et al., 1999; Ren et al., 1999).
4.3 Persistence in the respiratory tract
NTHi persistence within the respiratory tract not only enables the potential for disease recurrence/relapse but also induces persistent airway inflammation and promotes disease progression in COPD lungs (Murphy et al., 2004; Finney et al., 2014; Ahearn et al., 2017). To persist within the airways, NTHi must subvert or evade clearance by antibiotics or the innate host immune response. This is achieved by a variety of mechanisms that include specific neutralisation of antimicrobial agents, resistance to nutritional immunity, invasion of host epithelial cells and formation of protective biofilms (Morton et al., 2004; King, 2012; Vogel et al., 2012). Haem-iron bioavailability has been shown to influence many NTHi behaviours associated with persistence (Szelestey et al., 2013).
An important first-line defence by the host against microbial growth is to further reduce circulating iron concentrations to limit microbial survival; a process known as nutritional immunity. Siderocalins contribute to this antimicrobial defence by sequestration of microbial siderophores (Clifton et al., 2009). NTHi uses siderophore-independent mechanisms, such as haem-uptake, to acquire iron and so siderocalins are ineffective and this may provide an adaptive advantage over competing bacteria in the respiratory tract that rely on siderophore-mediated acquisition of iron for survival (Nelson et al., 2005; Holden and Bachman, 2015). The initial immune response against bacterial infection of mucosal surfaces in the respiratory tract also involves recruitment of immune cells and abundant production of antimicrobial peptides (AMPs) (Geitani et al., 2020). IgA1 is the principal immunoglobulin subclass produced by respiratory mucosal tissues and plays a major role in host defence by inhibiting microbial adherence, inactivating bacterial toxins, and promoting humoral immunity (Geitani et al., 2020). NTHi counteracts this response by producing an IgA1 protease, an extracellular endopeptidase which specifically cleaves IgA1 (Rao et al., 1999). IgA1 protease production appears to be exclusive to pathogenic Haemophilus species and is upregulated by fur in response to iron-restricted conditions (Rao et al., 1999).
Coordination between bacterial immune evasion and haem homeostasis has been observed for several NTHi proteins involved in the acquisition/utilisation of haem. HxuCBA, SapA, and PE have been shown to confer resistance to AMP LL-37 and a homologue of human β-defensin in a chinchilla model of acute OM (Mason et al., 2005; Vogel et al., 2012; Rodríguez-Arce et al., 2019). The consequence of inhibiting AMP resistance was observed in a sapA mutant which had an attenuated ability to survive in both the nasopharynx and the chinchilla middle ear, compared to the parent strain (Mason et al., 2005; Shelton et al., 2011). HbpA, the substrate binding protein that transports haem within the periplasm, is responsible for transport of glutathione, a thiol-containing tripeptide that confers protection against oxidative, xenobiotic, and metal iron stresses (Vergauwen et al., 2010; Rodríguez-Arce et al., 2019). NTHi cannot synthesise glutathione and absence of glutathione from the growth medium has been shown to reduce survival of H. influenzae Rd in culture (Vergauwen et al., 2003). Thus, NTHi may take advantage of the same import systems to both obtain haem and confer resistance to the innate mucosal immune response and oxidative stress (Mason et al., 2006; Rodríguez-Arce et al., 2019). This may contribute to a predisposition to NTHi infections in CF and COPD patients who demonstrate compromised lower airway AMP activity despite mounting an inflammatory response (Persson et al., 2017; Geitani et al., 2020).
NTHi invasion of host epithelial cells may not only provide a means of evading host immune pressures, but also provide an alternative reservoir for nutrient acquisition when bacteria are exposed to nutrient-limiting conditions (Harrison et al., 2013). Chinchilla middle ears challenged with sapA mutants deficient in haem-iron uptake demonstrated a tendency towards a more persistent phenotype, favouring intracellular survival and a dampened cytokine response compared to the parent strain (Raffel et al., 2013). Similarly, expression of fur-regulated IgA1 protease (Harrison et al., 2013) was required for optimal invasion and long-term intracellular survival in bronchial epithelial cells (Clementi et al., 2014). While these observations suggest environments restrictive of iron promote NTHi invasion and persistence, other studies have demonstrated a more nuanced relationship between haem/iron availability and NTHi phenotype. NTHi mutants lacking hxuCBA, hbpA, hpe or sapA haem-acquisition systems had an impaired ability to invade type II pneumocytes (Rodríguez-Arce et al., 2019). Similarly, mutants lacking the conserved iron-regulon fur also exhibited reduced persistence in middle ears of chinchillas (Harrison et al., 2013). Two studies (Szelestey et al., 2013; Hardison et al., 2018a) have attempted to understand this discrepancy through transient haem-iron restriction of NTHi. Exposure to excess haem-iron was only found to promote a persistent phenotype in experimental OM models for NTHi strains previously starved of haem-iron, compared to those continuously exposed to haem-replete conditions (Szelestey et al., 2013; Hardison et al., 2018b). This response was attributed to microevolutions through mutations in icc which result in epigenetic and morphological adaptations that contribute to persistence and disease severity (Hardison et al., 2018a). Recurrent episodes in a separate pre-clinical OM model also demonstrated continuous microevolution of a haemoglobin-binding gene that resulted in a highly invasive NTHi phenotype that persisted for at least one month following clinical resolution of the infection (Harrison et al., 2020).
The ability to form sedentary communities or biofilms in anatomical sites, such as the middle ear, has also been shown to contribute to the in vivo persistence of NTHi (García-Cobos et al., 2014). In addition to protection from immune- or antibiotic-mediated killing, biofilm formation may also act as a mucosal reservoir for NTHi following resolution of clinical disease, thus promoting bacterial persistence and re-infection within the airways (Jung and Do, 2013; Raffel et al., 2013). Biofilm-resident bacteria exhibit a reduced metabolism and an altered proteome compared to their planktonic counterparts, features that contribute to their reduced susceptibility to immune effectors and commonly used antibiotic treatments (Novotny et al., 2019). This mechanism is hypothesised to contribute to the chronic and recurrent nature of NTHi-associated infections, including bronchitis, acute exacerbations of COPD, conjunctivitis, sinusitis, and OM (Kaya et al., 2013; Gu et al., 2014; Novotny et al., 2019; Welp and Bomberger, 2020). Haem-iron restriction or loss of the utilisation protein SapF results in morphological plasticity and enhanced community development and biofilm architecture (Vogel et al., 2012; Szelestey et al., 2013). Conversely, excess haem-iron availability was shown to increase peak height and architectural complexity of NTHi biofilms following a period of haem-iron restriction (Szelestey et al., 2013).
5 Disruption of haem-iron assimilation: an effective therapeutic strategy against NTHi infection?
The diminishing effectiveness of current antibiotic treatments and the challenges associated with vaccine development have encouraged exploration of novel therapeutic strategies to prevent and/or treat NTHi infections. The dependence for haem-iron at all stages of NTHi pathogenesis exposes a vulnerability that provides promising targets for the development of new therapies that may disrupt iron uptake (Stites et al., 1999; Ahearn et al., 2017). In animal models, extracellular iron restriction was shown to be effective in preventing respiratory infection and dissemination of pneumonia caused by a variety of Gram-negative bacteria, including H. influenzae (Minandri et al., 2016; Michels et al., 2017). Similarly, disruption of haem or iron acquisition mechanisms significantly affects the ability of NTHi to cause disease in animal models (Ahearn et al., 2017). Currently, multiple host- and bacterial-targeted approaches in development aim to disrupt haem and/or iron assimilation by a range of pathogens and may have utility against NTHi (Table 2).
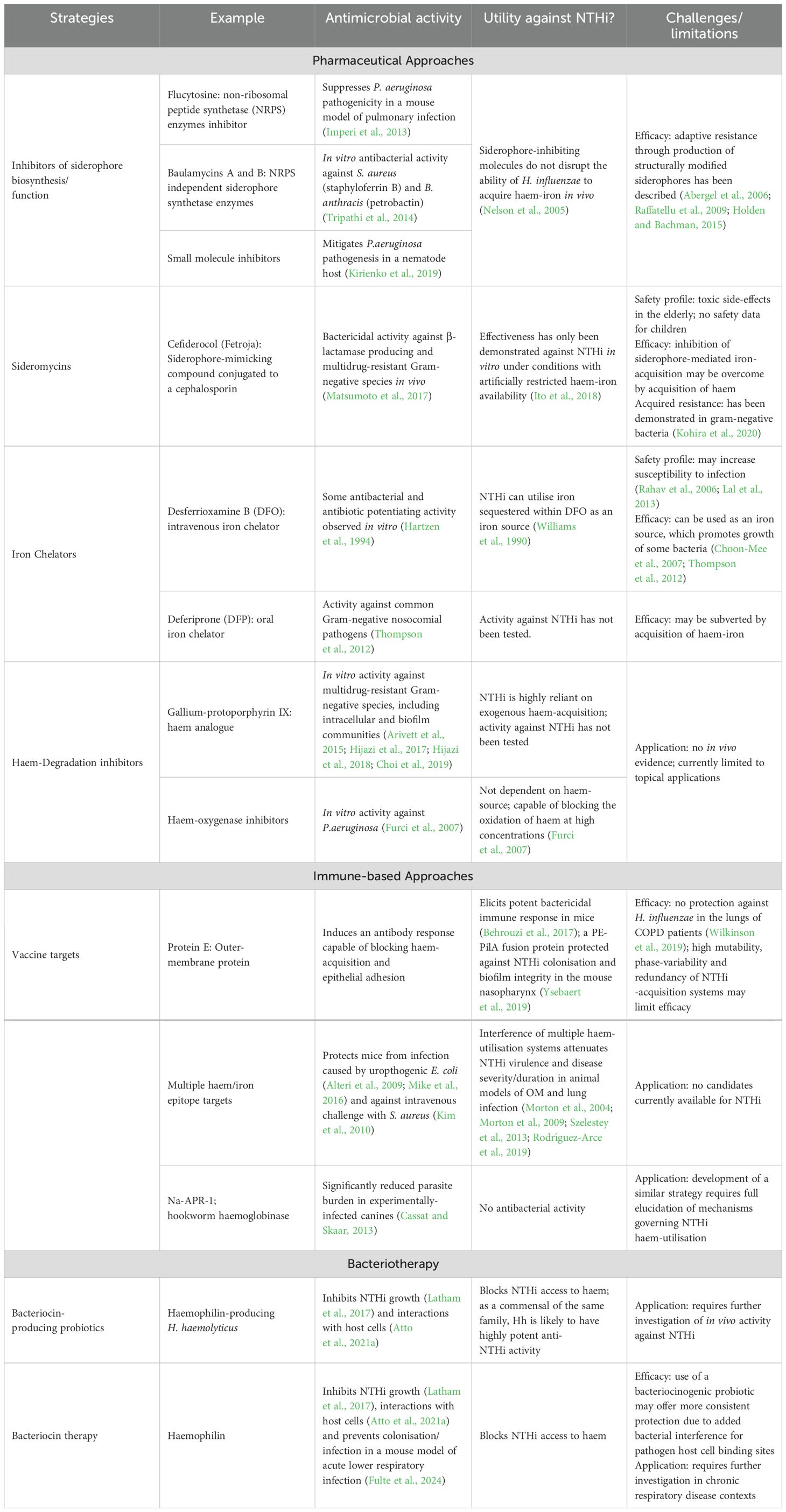
Table 2. Strategies that target bacterial haem-iron assimilation and their potential application for the prevention or treatment of NTHi infections.
5.1 Antimicrobials that prevent haem/iron assimilation
Several pharmaceutical agents in development target microbial haem-iron acquisition. These agents can be broadly categorised by their intended approach; either those that inhibit haem-iron availability or bacterial acquisition pathways, or toxic haem/iron-mimicking compounds that gain entry to bacterial cells through existing uptake systems (Cassat and Skaar, 2013).
5.1.1 Siderophore synthesis inhibitors
One of the earliest antimicrobials used for tuberculosis treatment, para-aminosalicylic acid, inhibits synthesis of the bacterial siderophore, mycobactin (Brown and Ratledge, 1975). Subsequently, a large number of siderophore biosynthesis inhibitors have been developed that have capacity to prevent bacterial growth under iron-limiting conditions (Ribeiro and Simões, 2019). Flucytosine, a synthetic fluorinated pyrimidine used to treat fungal respiratory tract infections, has also been shown to inhibit expression of the pyoverdine siderophore-biosynthesis gene in Pseudomonas aeruginosa, and subsequently suppress pathogenicity in a mouse model of pulmonary infection (Imperi et al., 2013). Similarly, disruption of the function of the pyoverdine protein also mitigated P. aeruginosa pathogenesis in a nematode host (Kirienko et al., 2019). Although NTHi possess the capacity to utilise siderophores, genes associated with siderophore biosynthesis have not been identified (Holden and Bachman, 2015). Therefore, the utility of these compounds in restricting NTHi access to iron is limited and may only disrupt a minor iron source by proxy of decreased xenosiderophore production by local microbial communities. Additionally, local microbiota may have the potential to develop adaptive resistance through production of structurally modified siderophores (Abergel et al., 2006; Raffatellu et al., 2009; Holden and Bachman, 2015).
5.1.2 Sideromycins
An alternative approach is the use of xenosiderophores covalently linked to antibiotics, known as sideromycins, that encourage antibiotic uptake through existing iron uptake systems. Sideromycins have demonstrated bactericidal activity against β-lactamase producing Enterobacterales, and other antibiotic-resistant strains of P. aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii, including producers of the class B metallo-β-lactamases and class C serine-β-lactamases (Page et al., 2010; Brown et al., 2013; Murphy-Benenato et al., 2015). Sideromycins have also been used in vivo to successfully treat P. aeruginosa infections and prevent systemic infection with S. pneumoniae and Y. enterocolitica in mouse models (Pramanik et al., 2007; McPherson et al., 2012). However, translation into human use has historically faced diminished clinical utility due to compound instability in vivo, emergence of adaptive resistance during exposure, or side-effects accompanying treatment (Ji and Miller, 2012; Page, 2013). As a result, only the novel catechol siderophore-conjugated cephalosporin antibiotic, cefiderocol has progressed beyond the first phase of human safety trials, owing to a unique combination of structural features derived from cefepime and ceftazidime that overcomes that stability problems associated with earlier iterations (Page et al., 2010).
Cefiderocol has recently been approved as a last-line treatment for complicated urinary tract infections and pneumonia caused by antibiotic resistant Gram-negative bacteria, such as carbapenem-resistant P. aeruginosa, A. baumannii, and K. pneumoniae (Matsumoto et al., 2017; Ito et al., 2018). Recently, cefiderocol was used to successfully treat ventilator-associated pneumonia caused by Stenotrophomonas maltophilia in a preterm neonate (Koirala et al., 2023). However, clinical studies have reported a higher all-cause mortality among patients treated with cefiderocol for hospital-acquired pneumonia, and thus treatment regimens require close monitoring to avoid toxic side-effects, particularly among elderly patients (El-Lababidi and Rizk, 2020). The uncertain safety profile among elderly patients and a lack of data from children lends poorly to routine treatment of NTHi infections which primarily affects these age groups. Additionally, NTHi is adept at scavenging iron from a variety of sources in vivo and siderophore-mediated iron acquisition is a minor contributor to total iron acquisition in NTHi (Morton et al., 2010b). This characteristic has been implicated in the intrinsic resistance of NTHi to siderophore-targeting compounds observed in vivo. In a nasal colonisation model, production of lipocalin, an acute-phase inhibitor of siderophore-mediated iron uptake, was upregulated but did not affect the ability of H. influenzae to acquire host-derived sources of haem-iron (Nelson et al., 2005; Cassat and Skaar, 2013; Leal et al., 2013). Additionally, NTHi cannot utilise catechol-containing siderophores (Williams et al., 1990; Page, 2019) and so is unlikely to possess the necessary machinery to uptake cefiderocol. Thus, it is unlikely that the activity of catechol sideromycins against NTHi will be greater than that of the antibiotic alone. Additionally, production of multiple β-lactamases has been shown to contribute to the emergence of cefiderocol non-susceptibility in several Gram-negative isolates (Kohira et al., 2020). Thus, sideromycins may be vulnerable to the same antibiotic resistance mechanisms faced by traditional antibiotic therapies.
5.1.3 Chelating agents
An alternative approach that mitigates the potential for adaptive resistance involves targeting multiple iron sources through chelating agents (Kontoghiorghes et al., 2010). Chelation therapy prevents accumulation of excess iron and reduces its availability to invading pathogens (Mobarra et al., 2016). This approach has been effective in protecting mice against K. pneumoniae pneumonia and dissemination (Michels et al., 2017). However, as is the case with xenosiderophores, iron-containing chelating molecules such as desferrioxamine B (DFO), used in the treatment of iron-loading disorders, can be utilised as an iron source by bacteria that harbour the necessary receptor, including NTHi (Williams et al., 1990; Choon-Mee et al., 2007; Arifin et al., 2014). Chelation therapy with DFO may therefore increase iron availability and increase the risk of infection. More severe infections, and higher liver and kidney bacterial burdens have been demonstrated in DFO-treated mice following intravenous challenge with S. aureus (Arifin et al., 2014). A positive correlation between DFO use and higher rates of infection with S. aureus and other opportunistic bacteria in patients suffering from thalassemia-associated iron overload has also been reported (Rahav et al., 2006; Lal et al., 2013). Research has therefore moved towards synthetic iron chelators that can be taken orally, such as Deferiprone (DFP) which does not promote bacterial growth (Kim and Shin, 2009). DFP has been found to not only reduce the growth of some strains of common Gram-negative nosocomial pathogens, but also reduce the minimum inhibitory concentrations of antibiotics (Thompson et al., 2012). However, the activity of these compounds against NTHi have not been tested and their effectiveness may be subverted by acquisition of haem-iron.
The majority of NTHi iron requirement is fulfilled by acquisition of haem therefore compounds that restrict haem-assimilation may have higher therapeutic value than those solely targeting iron acquisition. Haem assimilation was first targeted with the analogue gallium-protoporphyrin IX (GaPP), a bactericidal metalloporphyrin that uses existing haem uptake machinery to gain entry to the bacterial cell (Stojiljkovic et al., 1999). Incorporation of Ga(III) in place of iron disrupts the iron-dependent redox process as Ga(III) cannot be reduced to Ga(II) under physiological conditions, and thus cannot be liberated from porphyrins by haem oxygenase (Choi et al., 2019). GaPP has demonstrated inhibitory effects against A. baumanii and P. aeruginosa in model respiratory cell lines, and other multidrug-resistant Gram-positive and Gram-negative species in vitro (Arivett et al., 2015; Hijazi et al., 2017; Hijazi et al., 2018). This compound is also effective against biofilm and intracellular communities (Choi et al., 2019). The activity of GaPP is enhanced when combined with DFP which has been demonstrated by a topical hydrogel with anti-biofilm and antibiotic-potentiating properties against S. aureus in an artificial wound model (Richter et al., 2017a; Richter et al., 2017b). Although GaPP has recently been shown to inhibit the growth and intracellular viability of COPD NTHi isolates in limited haemin conditions (Baker et al., 2022), it is limited to topical applications in its current form and cannot be used to prevent future infections. Beyond GaPP, exploration of haem-targeting compounds is scarce. Additional haem-oxygenase inhibitors against P. aeruginosa and Neisseria meningitidis have only been identified through virtual screening and an in vitro growth assay, which were capable of blocking the oxidation of haem at concentrations in excess of that available to pathogens in the respiratory tract (Furci et al., 2007).
5.2 Immunotherapies
Recently, vaccine strategies have exploited the haem-dependant NTHi pathogenic mechanisms by incorporating the surface haemoprotein receptor, PE as a vaccine antigen. As previously discussed, PE is an adhesin of NTHi with functions involved in haem binding, storage and inter-bacterial donation (Duell et al., 2016). PE is expressed by 98% of NTHi strains, the epithelial cell-binding region of which is highly conserved among strains (Singh et al., 2010). Serum from mice immunised with recombinant truncated PE demonstrated a strong bactericidal effect against NTHi (Behrouzi et al., 2017). Incorporation of PE in a fusion protein with PilA enhanced immunogenicity and protected against NTHi colonisation and disrupted biofilm integrity in the mouse nasopharynx (Ysebaert et al., 2019). More recently, the PE-PilA fusion protein, combined with Protein D, has completed phase 2 clinical trials, demonstrating an acceptable reactogenicity and safety profile in adults with moderate/severe COPD (Leroux-Roels et al., 2016). Despite these promising results, the isolation of H. influenzae from sputum samples did not differ between the vaccine and the placebo group (Wilkinson et al., 2019).
The effectiveness of PE-based approaches may be undermined by the same genetic heterogeneity and phase-variable expression of other potential NTHi surface antigens (Murphy, 2015; Jalalvand and Riesbeck, 2018; Novotny et al., 2019). A high degree of polymorphisms within the gene encoding an NTHi haemoglobin-binding protein has been reported, which alters the protein affinity for iron capture/usage (Duell et al., 2016). This limitation is exacerbated by the high level of redundancy and multi-functionality of NTHi proteins, particularly those involved in the acquisition of haem-iron (Whitby et al., 2009). Therefore, only an antibody response capable of blocking a variety of epitopes may cause sufficient malnutrition to inhibit survival and host-pathogen interactions (Hare, 2017). This approach has been used against uropathogenic E. coli strains, where mucosal immunisation with six outer membrane iron receptors or siderophores protected against urinary tract infection in mice (Alteri et al., 2009; Mike et al., 2016). Antibodies targeting the IsdA and IsdB haem-acquisition systems of S. aureus protected mice against intravenous challenge (Kim et al., 2010). In addition to targeting iron acquisition proteins, some efficacy has been achieved with vaccines targeting iron homeostasis in pathogens. The Na-APR-1 protease from human hookworm, Necator americanus, is essential for enzymatic activity to support blood feeding. Vaccination with a mutated form of Na-APR-1 significantly reduced parasite burden in experimentally-infected canines (Cassat and Skaar, 2013). A similar strategy that targets multiple haem-iron acquisition systems, or their regulation may offer similar protection against NTHi infection. Indeed, interference of multiple NTHi haem-utilisation systems or disruption of the master haem-regulon fur has proven effective in attenuating NTHi disease severity/duration in animal models of OM and lung infection (Morton et al., 2004; Morton et al., 2009; Harrison et al., 2013; Szelestey et al., 2013; Rodríguez-Arce et al., 2019).
5.3 Bacteriotherapies
The issues inherent to pharmacological- and immunological-based approaches has necessitated the exploration of alternative therapies against NTHi infections. The vital role of haem-iron in the survival of NTHi and other bacteria in the upper respiratory tract raises the stakes for evolutionary conflicts to arise in the struggle for this limiting nutrient (Barber and Elde, 2015). Thus, a commensal bacterium that can outcompete NTHi for haem-iron may have potential as a probiotic therapy by making the environment inhospitable for NTHi growth (De Boeck et al., 2021). A probiotic is defined as a live microorganism that, when administered in adequate amounts, confers a health benefit to the host (Martín and Langella, 2019). Probiotics that outcompete pathogens for iron have demonstrated high levels of protection against infection in the gastrointestinal tract. The Nissle 1917 strain of E. coli has been applied as a probiotic treatment that supresses gastroenteritis by outcompeting for the siderophore-mediated iron assimilation of Salmonella enterica serovar Typhimurium (Deriu et al., 2013; Holden and Bachman, 2015). This inter-bacterial relationship exposes the protective potential of beneficial microbes to combat pathogens through iron sequestration.
Probiotics administered directly to the upper respiratory tract have close proximity to pathobionts and may therefore interfere with colonisation and development of disease by competing for host cell binding sites and nutrients (e.g. iron) (Falagas et al., 2008). This competitive inhibition appears to be highly effective in formulations where the antagonising commensal bacterium belongs to the same family and occupies the same niche as the pathogenic species. Nasal and oral probiotic sprays containing α-haemolytic streptococcal strains are effective in treating (Manti et al., 2020) and preventing episodes of acute pharyngotonsillitis caused by β-haemolytic group A streptococci, pneumococcal OM in children (Marchisio et al., 2015; Andaloro et al., 2019) and pneumococcal pneumonia in mice (Shekhar et al., 2019). Similarly, intranasal delivery of the closely related commensal Neisseria lactamica prevented meningococcal meningitis in mice (Li et al., 2006) and nasal delivery of a commensal Pasteurellaceae species was able to delay onset of OM in mice (Granland et al., 2020). Recently, a nasopharyngeal H. haemolyticus isolate was discovered that has potent inhibitory activity against NTHi and Hib isolates by outcompeting them for haem (Latham et al., 2017). This ability was attributed to the discovery of a novel haemophore-like protein (now named haemophilin; Hpl) produced by H. haemolyticus (Latham et al., 2020). Hpl-producing H. haemolyticus strains were shown to outcompete NTHi in a broth co-culture system (Atto et al., 2020) and treatments containing Hpl-producing H. haemolyticus (or Hpl alone) protected model respiratory cell lines from NTHi attachment and invasion (Atto et al., 2021a). Furthermore, a small-scale epidemiological study of 257 healthy adults in Australia found a strong correlation between pharyngeal carriage of H. haemolyticus strains containing hpl and a reduced likelihood and density of NTHi co-colonisation, compared to participants colonised with H. haemolyticus strains incapable of producing Hpl (Atto et al., 2021b). Recently, intranasal administration of Hpl or Hpl-producing H. haemolyticus reduced respiratory tract colonisation and infection with NTHi in a mouse model of acute lower airway infection (Fulte et al., 2024). Hpl may therefore have potent clinical utility in preventing NTHi infections; however, further studies are needed to assess efficacy in chronic respiratory disease contexts. Probiotic approaches (such as Hpl) that exploit NTHi haem-iron dependency deliver several benefits over standard antibiotic therapy which make them an asset to dampening emergent resistance. For example, their narrow spectrum of activity does not provoke collateral effects on the whole microbiota or promote enrichment of resistant clones/strains (Rea et al., 2010; Hols et al., 2019). Additionally, the presence of a competitive probiotic in the nasopharynx may also provide additional inhibition of pathogen colonisation, or prevent strain replacement through interference for host cell binding sites (Drutz et al., 1966).
6 Conclusion
The growing prevalence of resistance to first- and second-line antibiotics, in the absence of an effective preventative strategy necessitates the need for alternative therapies that can reduce the enormous disease burden associated with NTHi infections. Strategies that target haem-iron assimilation of NTHi have a potentially high impact on the ability of NTHi to survive and cause disease within host airways. Unlike pharmacological- and immunological-based approaches, bacteriotherapy may provide an effective strategy that both treats, and prevents NTHi infections, which is not compromised by antigenic heterogeneity or bacterial resistance mechanisms. However, targeting the haem-iron assimilation of NTHi requires careful consideration and improved modelling of haem-iron sources and fluctuations present in respiratory niches during health and disease.
Author contributions
BA: Conceptualization, Writing – original draft, Writing – review & editing. DG: Writing – review & editing. RM: Writing – review & editing. ST: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abavisani, M., Keikha, M., and Karbalaei, M. (2024). First global report about the prevalence of multi-drug resistant Haemophilus influenzae: a systematic review and meta-analysis. BMC Infect. Diseases. 24, 90. doi: 10.1186/s12879-023-08930-5
Abergel, R. J., Wilson, M. K., Arceneaux, J. E., Hoette, T. M., Strong, R. K., Byers, B. R., et al. (2006). Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc. Natl. Acad. Sci. 103, 18499–18503. doi: 10.1073/pnas.0607055103
Ahearn, C. P., Gallo, M. C., and Murphy, T. F. (2017). Insights on persistent airway infection by non-typeable Haemophilus influenzae in chronic obstructive pulmonary disease. Pathog. Dis. 75. doi: 10.1093/femspd/ftx042
Ali, M. K., Kim, R. Y., Karim, R., Mayall, J. R., Martin, K. L., Shahandeh, A., et al. (2017). Role of iron in the pathogenesis of respiratory disease. Int. J. Biochem. Cell Biol. 88, 181–195. doi: 10.1016/j.biocel.2017.05.003
Al Jubair, T., Singh, B., Fleury, C., Blom, A. M., Mörgelin, M., Thunnissen, M. M., et al. (2014). Haemophilus influenzae stores and distributes hemin by using protein E. Int. J. Med. Microbiol. 304, 662–668. doi: 10.1016/j.ijmm.2014.04.015
Alteri, C. J., Hagan, E. C., Sivick, K. E., Smith, S. N., and Mobley, H. L. (2009). Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 5, e1000586. doi: 10.1371/journal.ppat.1000586
Andaloro, C., Santagati, M., Stefani, S., and La Mantia, I. (2019). Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a oral spray for children with recurrent streptococcal pharyngotonsillitis: a randomized placebo-controlled clinical study. Eur. Arch. Oto-Rhino-Laryngology. 276, 879–887. doi: 10.1007/s00405-019-05346-3
Arguedas, A., Kvaerner, K., Liese, J., Schilder, A., and Pelton, S. (2010). Otitis media across nine countries: disease burden and management. Int. J. Pediatr. otorhinolaryngology. 74, 1419–1424. doi: 10.1016/j.ijporl.2010.09.022
Arifin, A. J., Hannauer, M., Welch, I., and Heinrichs, D. E. (2014). Deferoxamine mesylate enhances virulence of community-associated methicillin resistant Staphylococcus aureus. Microbes infection 16, 967–972. doi: 10.1016/j.micinf.2014.09.003
Arivett, B. A., Fiester, S. E., Ohneck, E. J., Penwell, W. F., Kaufman, C. M., Relich, R. F., et al. (2015). Antimicrobial activity of gallium protoporphyrin IX against Acinetobacter baumannii strains displaying different antibiotic resistance phenotypes. Antimicrobial Agents chemotherapy. 59, 7657–7665. doi: 10.1128/AAC.01472-15
Atack, J. M., Srikhanta, Y. N., Fox, K. L., Jurcisek, J. A., Brockman, K. L., Clark, T. A., et al. (2015a). A biphasic epigenetic switch controls immunoevasion, virulence and niche adaptation in non-typeable Haemophilus influenzae. Nat. Commun. 6, 1–12. doi: 10.1038/ncomms8828
Atack, J. M., Winter, L. E., Jurcisek, J. A., Bakaletz, L. O., Barenkamp, S. J., and Jennings, M. P. (2015b). Selection and counterselection of Hia expression reveals a key role for phase-variable expression of Hia in infection caused by nontypeable Haemophilus influenzae. J. Infect. Dis. 212, 645–653. doi: 10.1093/infdis/jiv103
Atto, B., Eapen, M. S., Sharma, P., Frey, U., Ammit, A. J., Markos, J., et al. (2019). New therapeutic targets for the prevention of infectious acute exacerbations of COPD: role of epithelial adhesion molecules and inflammatory pathways. Clin. Science. 133, 1663–1703. doi: 10.1042/CS20181009
Atto, B., Kunde, D., Gell, D. A., and Tristram, S. (2021a). Haemophilin-producing strains of Haemophilus haemolyticus protect respiratory epithelia from NTHi colonisation and internalisation. Pathogens 10, 29. doi: 10.3390/pathogens10010029
Atto, B., Kunde, D., Gell, D. A., and Tristram, S. (2021b). Oropharyngeal carriage of hpl-containing Haemophilus haemolyticus predicts lower prevalence and density of NTHi colonisation in healthy adults. Pathogens 10, 577. doi: 10.3390/pathogens10050577
Atto, B., Latham, R., Kunde, D., Gell, D. A., and Tristram, S. (2020). In vitro anti-NTHi activity of haemophilin-producing strains of Haemophilus haemolyticus. Pathogens 9, 243. doi: 10.3390/pathogens9040243
Bajanca-Lavado, M. P., Cavaco, L., Fernandes, M., Touret, T., Candeias, C., Simões, A. S., et al. (2022). Haemophilus influenzae carriage among healthy children in Portugal, 2015–2019. Microorganisms 10, 1964. doi: 10.3390/microorganisms10101964
Baker, J. M., Baba-Dikwa, A., Shah, R., Lea, S., and Singh, D. (2022). Gallium protoporphyrin as an antimicrobial for non-typeable Haemophilus influenzae in COPD patients. Life Sci. 305, 120794. doi: 10.1016/j.lfs.2022.120794
Barber, M. F. and Elde, N. C. (2015). Buried treasure: evolutionary perspectives on microbial iron piracy. Trends Genet. 31, 627–636. doi: 10.1016/j.tig.2015.09.001
Barkai, G., Leibovitz, E., Givon-Lavi, N., and Dagan, R. (2009). Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatr. Infect. Dis. J. 28, 466–471. doi: 10.1097/INF.0b013e3181950c74
Behrouzi, A., Bouzari, S., Vaziri, F., Fateh, A., Afrough, P., Motlagh, A. D. V., et al. (2017). Recombinant truncated E protein as a new vaccine candidate against nontypeable H. influenzae: its expression and immunogenic evaluation. Microbial pathogenesis. 110, 431–438. doi: 10.1016/j.micpath.2017.07.025
Beissbarth, J., Wilson, N., Arrowsmith, B., Binks, M. J., Oguoma, V., Lawrence, K., et al. (2021). Nasopharyngeal carriage of otitis media pathogens in infants receiving 10-valent non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10), 13-valent pneumococcal conjugate vaccine (PCV13) or a mixed primary schedule of both vaccines: A randomised controlled trial. Vaccine 39, 2264–2273. doi: 10.1016/j.vaccine.2021.03.032
Brockman, K. L., Azzari, P. N., Branstool, M. T., Atack, J. M., Schulz, B. L., Jen, F. E.-C., et al. (2018). Epigenetic regulation alters biofilm architecture and composition in multiple clinical isolates of nontypeable Haemophilus influenzae. MBio 9. doi: 10.1128/mBio.01682-18
Brockman, K. L., Branstool, M. T., Atack, J. M., Robledo-Avila, F., Partida-Sanchez, S., Jennings, M. P., et al. (2017). The ModA2 phasevarion of nontypeable Haemophilus influenzae regulates resistance to oxidative stress and killing by human neutrophils. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-03552-9
Brockman, K. L., Jurcisek, J. A., Atack, J. M., Srikhanta, Y. N., Jennings, M. P., and Bakaletz, L. O. (2016). ModA2 phasevarion switching in nontypeable Haemophilus influenzae increases the severity of experimental otitis media. J. Infect. diseases. 214, 817–824. doi: 10.1093/infdis/jiw243
Brown, M. F., Mitton-Fry, M. J., Arcari, J. T., Barham, R., Casavant, J., Gerstenberger, B. S., et al. (2013). Pyridone-conjugated monobactam antibiotics with gram-negative activity. J. medicinal Chem. 56, 5541–5552. doi: 10.1021/jm400560z
Brown, K. A. and Ratledge, C. (1975). The effect of p-aminosalicyclic acid on iron transport and assimilation in mycobacteria. Biochim. Biophys. Acta (BBA)-General Subjects. 385, 207–220. doi: 10.1016/0304-4165(75)90349-9
Bullen, J., Griffiths, E., and Edmiston, C. E. (1999). Iron and infection: molecular, physiological and clinical aspects (Chichester: LWW).
Buscher, A. Z., Burmeister, K., Barenkamp, S. J., and St. Geme, J. W., III (2004). Evolutionary and functional relationships among the nontypeable Haemophilus influenzae HMW family of adhesins. J. bacteriology. 186, 4209–4217. doi: 10.1128/JB.186.13.4209-4217.2004
Cassat, J. E. and Skaar, E. P. (2013). Iron in infection and immunity. Cell Host Microbe 13, 509–519. doi: 10.1016/j.chom.2013.04.010
Cavallaro, G., Decaria, L., and Rosato, A. (2008). Genome-based analysis of heme biosynthesis and uptake in prokaryotic systems. J. Proteome Res. 7, 4946–4954. doi: 10.1021/pr8004309
Cerquetti, M. and Giufrè, M. (2016). Why we need a vaccine for non-typeable Haemophilus influenzae. Hum. Vaccines immunotherapeutics 12, 2357–2361. doi: 10.1080/21645515.2016.1174354
Chen, C., Pande, K., French, S. D., Tuch, B. B., and Noble, S. M. (2011). An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 10, 118–135. doi: 10.1016/j.chom.2011.07.005
Cheong, J. W. S., Smith, H., Heney, C., Robson, J., Schlebusch, S., Fu, J., et al. (2015). Trends in the epidemiology of invasive Haemophilus influenzae disease in Queensland, Australia from 2000 to 2013: what is the impact of an increase in invasive non-typable H. influenzae (NTHi)? Epidemiol. Infection. 143, 2993–3000.
Cherayil, B. J. (2010). Iron and immunity: immunological consequences of iron deficiency and overload. Archivum immunologiae therapiae experimentalis. 58, 407–415. doi: 10.1007/s00005-010-0095-9
Choi, S.-r., Britigan, B. E., and Narayanasamy, P. (2019). Iron/heme metabolism-targeted gallium (III) nanoparticles are active against extracellular and intracellular Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrobial Agents chemotherapy 63. doi: 10.1128/AAC.02643-18
Clarke, C., Bakaletz, L. O., Ruiz-Guiñazú, J., Borys, D., and Mrkvan, T. (2017). Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage. Expert Rev. Vaccines 16, 751–764. doi: 10.1080/14760584.2017.1333905
Clementi, C. F., Håkansson, A. P., and Murphy, T. F. (2014). Internalization and trafficking of nontypeable Haemophilus influenzae in human respiratory epithelial cells and roles of IgA1 proteases for optimal invasion and persistence. Infection immunity. 82, 433–444. doi: 10.1128/IAI.00864-13
Clifton, M. C., Corrent, C., and Strong, R. K. (2009). Siderocalins: siderophore-binding proteins of the innate immune system. Biometals 22, 557–564. doi: 10.1007/s10534-009-9207-6
Cloonan, S. M., Mumby, S., Adcock, I. M., Choi, A. M., Chung, K. F., and Quinlan, G. J. (2017). The “iron”-y of iron overload and iron deficiency in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 196, 1103–1112. doi: 10.1164/rccm.201702-0311PP
Coleman, A., Wood, A., Bialasiewicz, S., Ware, R. S., Marsh, R. L., and Cervin, A. (2018). The unsolved problem of otitis media in indigenous populations: a systematic review of upper respiratory and middle ear microbiology in indigenous children with otitis media. Microbiome 6, 1–15. doi: 10.1186/s40168-018-0577-2
Collins, S., Litt, D. J., Flynn, S., Ramsay, M. E., Slack, M. P., and Ladhani, S. N. (2015). Neonatal invasive Haemophilus influenzae disease in England and Wales: epidemiology, clinical characteristics, and outcome. Clin. Infect. Diseases. 60, 1786–1792. doi: 10.1093/cid/civ194
Craven, V. and Everard, M. L. (2013). Protracted bacterial bronchitis: reinventing an old disease. Arch. Dis. childhood. 98, 72–76. doi: 10.1136/archdischild-2012-302760
Dabernat, H. and Delmas, C. (2012). Epidemiology and evolution of antibiotic resistance of Haemophilus influenzae in children 5 years of age or less in France, 2001–2008: a retrospective database analysis. Eur. J. Clin. Microbiol. Infect. diseases. 31, 2745–2753. doi: 10.1007/s10096-012-1623-9
Dagan, R., Abramson, O., Leibovitz, E., Greenberg, D., Lang, R., Goshen, S., et al. (1997). Bacteriologic response to oral cephalosporins: are established susceptibility breakpoints appropriate in the case of acute otitis media? J. Infect. Dis. 176, 1253–1259. doi: 10.1086/jid.1997.176.issue-5
De Boeck, I., Spacova, I., Vanderveken, O. M., and Lebeer, S. (2021). Lactic acid bacteria as probiotics for the nose? Microbial Biotechnol. 3), 859–869. doi: 10.1111/1751-7915.13759
Deriu, E., Liu, J. Z., Pezeshki, M., Edwards, R. A., Ochoa, R. J., Contreras, H., et al. (2013). Probiotic bacteria reduce Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe 14, 26–37. doi: 10.1016/j.chom.2013.06.007
Dicker, A. J., Huang, J. T., Lonergan, M., Keir, H. R., Fong, C. J., Tan, B., et al. (2021). The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 147, 158–167. doi: 10.1016/j.jaci.2020.02.040
Dixit, D., Bridgeman, M. B., Madduri, R. P., Kumar, S. T., and Cawley, M. J. (2016). Pharmacological management and prevention of exacerbations of chronic obstructive pulmonary disease in hospitalized patients. Pharm. Ther. 41, 703.
Drutz, D. J., Van Way, M. H., Schaffner, W., and Koenig, M. G. (1966). Bacterial interference in the therapy of recurrent staphylococcal infections: multiple abscesses due to the implantation of the 502A strain of Staphylococcus. New Engl. J. Med. 275, 1161–1165. doi: 10.1056/NEJM196611242752104
Duell, B. L., Su, Y. C., and Riesbeck, K. (2016). Host–pathogen interactions of nontypeable Haemophilus influenzae: from commensal to pathogen. FEBS letters. 590, 3840–3853. doi: 10.1002/feb2.2016.590.issue-21
El-Lababidi, R. M. and Rizk, J. G. (2020). Cefiderocol: A siderophore cephalosporin. Ann. Pharmacotherapy. 54, 1215–1231. doi: 10.1177/1060028020929988
El Nouwar, R., Prevost, B., Wautier, M., Yin, N., Hites, M., and Martiny, D. (2025). Epidemiology of invasive Haemophilus influenzae infections in Belgium: 2018–2022. Eur. J. Clin. Microbiol. Infect. Dis. 44 (4), 855–865. doi: 10.1007/s10096-025-05040-9
Faden, H., Duffy, L., Williams, A., Pediatrics, T. W., Krystofik, D. A., and Wolf, J. (1995). Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 years of life. J. Infect. Diseases. 172, 132–135. doi: 10.1093/infdis/172.1.132
Falagas, M. E., Rafailidis, P. I., and Makris, G. C. (2008). Bacterial interference for the prevention and treatment of infections. Int. J. antimicrobial agents. 31, 518–522. doi: 10.1016/j.ijantimicag.2008.01.024
Fenker, D. E., McDaniel, C. T., Panmanee, W., Panos, R. J., Sorscher, E. J., Sabusap, C., et al. (2018). Comparison between two pathophysiologically different yet microbiologically similar lung diseases: cystic fibrosis and chronic obstructive pulmonary disease. Int. J. Respir. Pulm. Med. 5 (2), 098. doi: 10.23937/2378-3516/1410098
Finney, L. J., Ritchie, A., Pollard, E., Johnston, S. L., and Mallia, P. (2014). Lower airway colonization and inflammatory response in COPD: a focus on Haemophilus influenzae. Int J Chron Obstruct Pulmon Dis 9, 1119–32. doi: 10.2147/COPD.S54477
Fulte, S, Atto, B, McCarty, A, Horn, KJ, Redzic, JS, Eisenmesser, E, et al. (2024). Heme sequestration by hemophilin from Haemophilus haemolyticus reduces respiratory tract colonization and infection with non-typeable Haemophilus influenzae. Msphere 9 (3), e0000624. doi: 10.1128/msphere.00006-24
Furci, L. M., Lopes, P., Eakanunkul, S., Zhong, S., MacKerell, A. D., and Wilks, A. (2007). Inhibition of the bacterial heme oxygenases from Pseudomonas aeruginosa and Neisseria meningitidis: novel antimicrobial targets. J. medicinal Chem. 50, 3804–3813. doi: 10.1021/jm0700969
García-Cobos, S., Moscoso, M., Pumarola, F., Arroyo, M., Lara, N., Pérez-Vázquez, M., et al. (2014). Frequent carriage of resistance mechanisms to β-lactams and biofilm formation in Haemophilus influenzae causing treatment failure and recurrent otitis media in young children. J. Antimicrobial Chemotherapy. 69, 2394–2399. doi: 10.1093/jac/dku158
Geitani, R., Moubareck, C. A., Xu, Z., Karam Sarkis, D., and Touqui, L. (2020). Expression and roles of antimicrobial peptides in innate defense of airway mucosa: potential implication in cystic fibrosis. Front. Immunol. 11, 1198. doi: 10.3389/fimmu.2020.01198
Ghio, A. J. (2009). Disruption of iron homeostasis and lung disease. Biochim. Biophys. Acta (BBA)-General Subjects. 1790, 731–739. doi: 10.1016/j.bbagen.2008.11.004
Giufrè, M., Fabiani, M., Cardines, R., Riccardo, F., Caporali, M. G., D’Ancona, F., et al. (2018). Increasing trend in invasive non-typeable Haemophilus influenzae disease and molecular characterization of the isolates, Italy, 2012–2016. Vaccine 36 (45), 6615–6622. doi: 10.1016/j.vaccine.2018.09.060
Granland, C. M., Scott, N. M., Lauzon-Joset, J.-F., Langereis, J. D., De Gier, C., Sutherland, K. M., et al. (2020). Nasal delivery of a commensal Pasteurellaceae species inhibits nontypeable Haemophilus influenzae colonization and delays onset of otitis media in mice. Infection immunity. 88, e00685–e00619. doi: 10.1128/IAI.00685-19
Gray-Owen, S. D. and Schryvers, A. B. (1995). Characterization of transferrin binding proteins 1 and 2 in invasive type b and nontypeable strains of Haemophilus influenzae. Infection immunity. 63, 3809–3815. doi: 10.1128/iai.63.10.3809-3815.1995
Gu, X., Keyoumu, Y., Long, L., and Zhang, H. (2014). Detection of bacterial biofilms in different types of chronic otitis media. Eur. Arch. Oto-Rhino-Laryngology. 271, 2877–2883. doi: 10.1007/s00405-013-2766-8
Haggard, M. (2008). Otitis media: prospects for prevention. Vaccine 26, G20–GG4. doi: 10.1016/j.vaccine.2008.11.009
Harabuchi, Y., Faden, H., Yamanaka, N., Duffy, L., Wolf, J., Krystofik, D., et al. (1994). Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. J. Infect. diseases. 170, 862–866. doi: 10.1093/infdis/170.4.862
Hardie, K., Adams, R. A., and Towner, K. (1993). Transferrin-binding ability of invasive and commensal isolates of Haemophilus spp. J. Med. Microbiol. 39, 218–224. doi: 10.1099/00222615-39-3-218
Hardison, R. L., Harrison, A., Wallace, R. M., Heimlich, D. R., O’Bryan, M. E., Sebra, R. P., et al. (2018a). Microevolution in response to transient heme-iron restriction enhances intracellular bacterial community development and persistence. PloS pathogens. 14, e1007355. doi: 10.1371/journal.ppat.1007355
Hardison, R. L., Heimlich, D. R., Harrison, A., Beatty, W. L., Rains, S., Moseley, M. A., et al. (2018b). Transient nutrient deprivation promotes macropinocytosis-dependent intracellular bacterial community development. MSphere 3. doi: 10.1128/mSphere.00562-18
Hare, S. A. (2017). Diverse structural approaches to haem appropriation by pathogenic bacteria. Biochim. Biophys. Acta (BBA)-Proteins Proteomics. 1865, 422–433. doi: 10.1016/j.bbapap.2017.01.006
Hare, K. M., Grimwood, K., Leach, A. J., Smith-Vaughan, H., Torzillo, P. J., Morris, P. S., et al. (2010). Respiratory bacterial pathogens in the nasopharynx and lower airways of Australian indigenous children with bronchiectasis. J. pediatrics. 157, 1001–1005. doi: 10.1016/j.jpeds.2010.06.002
Hariadi, N. I., Zhang, L., Patel, M., Sandstedt, S. A., Davis, G. S., Marrs, C. F., et al. (2015). Comparative profile of heme acquisition genes in disease-causing and colonizing nontypeable Haemophilus influenzae and Haemophilus haemolyticus. J. Clin. Microbiol. 53, 2132–2137. doi: 10.1128/JCM.00345-15
Harrison, A., Hardison, R. L., Fullen, A. R., Wallace, R. M., Gordon, D. M., White, P., et al. (2020). Continuous microevolution accelerates disease progression during sequential episodes of infection. Cell Rep. 30, 2978–88.e3. doi: 10.1016/j.celrep.2020.02.019
Harrison, A., Santana, E. A., Szelestey, B. R., Newsom, D. E., White, P., and Mason, K. M. (2013). Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae. Infection Immun. 81, 1221–1233. doi: 10.1128/IAI.01227-12
Hartzen, S. H., Frimodt-MØLler, N., and Thomsen, V. F. (1994). The antibacterial activity of a siderophore: 3. The activity of deferoxamine in vitro and its influence on the effect of antibiotics against Escherichia coli, Proteus mirabilis and coagulase-negative staphylococci. APMIS 102, 219–226. doi: 10.1111/j.1699-0463.1994.tb04868.x
Heath, P. T., Booy, R., Azzopardi, H. J., Slack, M. P., Fogarty, J., Moloney, A. C., et al. (2001). Non-type b Haemophilus influenzae disease: clinical and epidemiologic characteristics in the Haemophilus influenzae type b vaccine era. Pediatr. Infect. Dis. J. 20, 300–305. doi: 10.1097/00006454-200103000-00016
Hegstad, K., Mylvaganam, H., Janice, J., Josefsen, E., Sivertsen, A., and Skaare, D. (2020). Role of horizontal gene transfer in the development of multidrug resistance in Haemophilus influenzae. Msphere 5. doi: 10.1128/mSphere.00969-19
Heliodoro, C. I. M., Bettencourt, C. R., and Bajanca-Lavado, M. P. (2015). Portuguese Group for the Study of Haemophilus influenzae invasive infection. Molecular epidemiology of invasive Haemophilus influenzae disease in Portugal: an update of the post-vaccine period, 2011-2018. Eur. J. Clin. Microbiol Infect Dis. 39 (8), 1471–1480. doi: 10.1007/s10096-020-03865-0
Hijazi, S., Visaggio, D., Pirolo, M., Frangipani, E., Bernstein, L., and Visca, P. (2018). Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front. Cell. infection Microbiol. 8, 316. doi: 10.3389/fcimb.2018.00316
Hijazi, S., Visca, P., and Frangipani, E. (2017). Gallium-protoporphyrin IX inhibits Pseudomonas aeruginosa growth by targeting cytochromes. Front. Cell. infection Microbiol. 7, 12. doi: 10.3389/fcimb.2017.00012
Holden, V. I. and Bachman, M. A. (2015). Diverging roles of bacterial siderophores during infection. Metallomics 7, 986–995. doi: 10.1039/C4MT00333K
Hols, P., Ledesma-García, L., Gabant, P., and Mignolet, J. (2019). Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends Microbiol. 27, 690–702. doi: 10.1016/j.tim.2019.03.007
Honda, H., Sato, T., Shinagawa, M., Fukushima, Y., Nakajima, C., Suzuki, Y., et al. (2018). Multiclonal expansion and high prevalence of β-lactamase-negative Haemophilus influenzae with high-level ampicillin resistance in Japan and susceptibility to quinolones. Antimicrobial Agents chemotherapy. 62. doi: 10.1128/AAC.00851-18
Huang, G.-J., Lin, B.-R., Li, P.-S., Tang, N., Fan, Z.-J., and Lu, B.-Q. (2025). The global burden of otitis media in 204 countries and territories from 1992 to 2021: a systematic analysis for the Global Burden of Disease study 2021. Front. Public Health 12, 1519623. doi: 10.3389/fpubh.2024.1519623
Ikeda, M., Enomoto, N., Hashimoto, D., Fujisawa, T., Inui, N., Nakamura, Y., et al. (2015). Nontypeable Haemophilus influenzae exploits the interaction between protein-E and vitronectin for the adherence and invasion to bronchial epithelial cells. BMC Microbiol. 15, 1–11. doi: 10.1186/s12866-015-0600-8
Imperi, F., Massai, F., Facchini, M., Frangipani, E., Visaggio, D., Leoni, L., et al. (2013). Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc. Natl. Acad. Sci. 110, 7458–7463. doi: 10.1073/pnas.1222706110
Ito, M., Hotomi, M., Maruyama, Y., Hatano, M., Sugimoto, H., Yoshizaki, T., et al. (2010). Clonal spread of β-lactamase-producing amoxicillin–clavulanate-resistant (BLPACR) strains of non-typeable Haemophilus influenzae among young children attending a day care in Japan. Int. J. Pediatr. otorhinolaryngology. 74, 901–906. doi: 10.1016/j.ijporl.2010.05.008
Ito, A., Sato, T., Ota, M., Takemura, M., Nishikawa, T., Toba, S., et al. (2018). In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrobial Agents chemotherapy 62. doi: 10.1128/AAC.01454-17
Jalalvand, F. and Riesbeck, K. (2018). Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert Rev. Vaccines 17, 503–512. doi: 10.1080/14760584.2018.1484286
Ji, C. and Miller, M. J. (2012). Chemical syntheses and in vitro antibacterial activity of two desferrioxamine B-ciprofloxacin conjugates with potential esterase and phosphatase triggered drug release linkers. Bioorganic medicinal Chem. 20, 3828–3836. doi: 10.1016/j.bmc.2012.04.034
Jung, W. H. and Do, E. (2013). Iron acquisition in the human fungal pathogen Cryptococcus neoformans. Curr. Opin. Microbiol. 16, 686–691. doi: 10.1016/j.mib.2013.07.008
Kaur, R., Chang, A., Xu, Q., Casey, J. R., and Pichichero, M. E. (2011). Phylogenetic relatedness and diversity of non-typable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J. Med. Microbiol. 60, 1841. doi: 10.1099/jmm.0.034041-0
Kaya, E., Dag, I., Incesulu, A., Gurbuz, M. K., Acar, M., and Birdane, L. (2013). Investigation of the presence of biofilms in chronic suppurative otitis media, nonsuppurative otitis media, and chronic otitis media with cholesteatoma by scanning electron microscopy. Sci. World J. 2013, 638715. doi: 10.1155/2013/638715
Kim, CM, Park, YJ, and Shin, SH (2007). A widespread deferoxamine-mediated iron-uptake system in Vibrio vulnificus. J. Infect. Dis. 196, 1537–1545. doi: 10.1086/523108
Kim, H. K., DeDent, A., Cheng, A. G., McAdow, M., Bagnoli, F., Missiakas, D. M., et al. (2010). IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 28, 6382–6392. doi: 10.1016/j.vaccine.2010.02.097
Kim, C.-M. and Shin, S.-H. (2009). Effect of iron-chelator deferiprone on the in vitro growth of staphylococci. J. Korean Med. science. 24, 289. doi: 10.3346/jkms.2009.24.2.289
King, P. (2012). Haemophilus influenzae and the lung (Haemophilus and the lung). Clin. Trans. Med. 1, 10. doi: 10.1186/2001-1326-1-10
King, P. T., Holdsworth, S. R., Farmer, M., Freezer, N., Villanueva, E., and Holmes, P. W. (2009). Phenotypes of adult bronchiectasis: onset of productive cough in childhood and adulthood. COPD: J. Chronic Obstructive Pulmonary Disease. 6, 130–136. doi: 10.1080/15412550902766934
Kirienko, D. R., Kang, D., and Kirienko, N. V. (2019). Novel pyoverdine inhibitors mitigate Pseudomonas aeruginosa pathogenesis. Front. Microbiol. 9, 3317. doi: 10.3389/fmicb.2018.03317
Kirkham, L.-A. S., Wiertsema, S. P., Mowe, E. N., Bowman, J. M., Riley, T. V., and Richmond, P. C. (2010). Nasopharyngeal carriage of Haemophilus haemolyticus in otitis-prone and healthy children. J. Clin. Microbiol. 48, 2557–2559. doi: 10.1128/JCM.00069-10
Kohira, N., Hackel, M. A., Ishioka, Y., Kuroiwa, M., Sahm, D. F., Sato, T., et al. (2020). Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J. Global Antimicrobial Resistance. 22, 738–741. doi: 10.1016/j.jgar.2020.07.009
Koirala, A., Krishnappa, B., Banh, C., Brandenburg, U., Findlay, M., and Williams, P. C. (2023). Successful use of cefiderocol to treat a multidrug-resistant Stenotrophomonas maltophilia ventilator-associated pneumonia in an extremely preterm neonate. Pediatr. Infect. Dis. J. 42, 1012–1016. doi: 10.1097/INF.0000000000004051
Kontoghiorghes, G. J., Kolnagou, A., Skiada, A., and Petrikkos, G. (2010). The role of iron and chelators on infections in iron overload and non iron loaded conditions: prospects for the design of new antimicrobial therapies. Hemoglobin 34, 227–239. doi: 10.3109/03630269.2010.483662
Kortman, G. A., Boleij, A., Swinkels, D. W., and Tjalsma, H. (2012). Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS One 7, e29968. doi: 10.1371/journal.pone.0029968
LaCross, N. C., Marrs, C. F., and Gilsdorf, J. R. (2014). Otitis media associated polymorphisms in the hemin receptor HemR of nontypeable Haemophilus influenzae. Infection Genet. Evol. 26, 47–57. doi: 10.1016/j.meegid.2014.05.002
Ladhani, S., Slack, M. P., Heath, P. T., Von Gottberg, A., Chandra, M., Ramsay, M. E., et al. (2010). Invasive Haemophilus influenzae disease, Europe, 1996–2006. Emerging Infect. diseases. 16, 455. doi: 10.3201/eid1603.090290
Lal, A., Porter, J., Sweeters, N., Ng, V., Evans, P., Neumayr, L., et al. (2013). Combined chelation therapy with deferasirox and deferoxamine in thalassemia. Blood Cells Molecules Diseases. 50, 99–104. doi: 10.1016/j.bcmd.2012.10.006
Langlois, M. R. and Delanghe, J. R. (1996). Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 42, 1589–1600. doi: 10.1093/clinchem/42.10.1589
Latham, R. D., Gell, D. A., Fairbairn, R. L., Lyons, A. B., Shukla, S. D., Cho, K. Y., et al. (2017). An isolate of Haemophilus haemolyticus produces a bacteriocin-like substance that inhibits the growth of nontypeable Haemophilus influenzae. Int. J. Antimicrobial Agents 49, 503–506. doi: 10.1016/j.ijantimicag.2016.12.010
Latham, R. D., Torrado, M., Atto, B., Walshe, J. L., Wilson, R., Guss, J. M., et al. (2020). A heme-binding protein produced by Haemophilus haemolyticus inhibits non-typeable Haemophilus influenzae. Mol. Microbiol. 113, 381–398. doi: 10.1111/mmi.v113.2
Leach, A. J., Morris, P. S., Coates, H. L., Nelson, S., O'Leary, S. J., Richmond, P. C., et al (2021). Otitis media guidelines for Australian Aboriginal and Torres Strait Islander children: summary of recommendations. Med. J. Aust. 214 (5), 228–233. doi: 10.5694/mja2.50953
Leach, A. J., Morris, P. S., Coates, H. L., Nelson, S., O’Leary, S. J., Richmond, P. C., et al. (2021). Otitis media guidelines for Australian Aboriginal and Torres Strait Islander children: summary of recommendations. Med. J. Australia. 214, 228. doi: 10.5694/mja2.v214.5
Leal, S. M., Jr., Roy, S., Vareechon, C., Carrion, Sd, Clark, H., Lopez-Berges, M. S., et al. (2013). Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog. 9, e1003436. doi: 10.1371/journal.ppat.1003436
Leibovitz, E. (2008). Complicated otitis media and its implications. Vaccine 26, G16–GG9. doi: 10.1016/j.vaccine.2008.11.008
Leroux-Roels, G., Van Damme, P., Haazen, W., Shakib, S., Caubet, M., Aris, E., et al. (2016). randomized, observer-blind, placebo-controlled studies to evaluate the safety, reactogenicity and immunogenicity of an investigational non-typeable Haemophilus influenzae (NTHi) protein vaccine in adults. Vaccine 34, 3156–3163. doi: 10.1016/j.vaccine.2016.04.051
Li, X.-X., Xiao, S.-Z., Gu, F.-F., He, W.-P., Ni, Y.-X., and Han, L.-Z. (2020). Molecular epidemiology and antimicrobial resistance of haemophilus influenzae in adult patients in Shanghai, China. Front. Public Health 8, 95. doi: 10.3389/fpubh.2020.00095
Li, Y., Zhang, Q., Winterbotham, M., Mowe, E., Gorringe, A., and Tang, C. M. (2006). Immunization with live Neisseria lactamica protects mice against meningococcal challenge and can elicit serum bactericidal antibodies. Infection immunity. 74, 6348–6355. doi: 10.1128/IAI.01062-06
Maciver, I., Latimer, J. L., Liem, H., Muller-Eberhard, U., Hrkal, Z., and Hansen, E. J. (1996). Identification of an outer membrane protein involved in utilization of hemoglobin-haptoglobin complexes by nontypeable Haemophilus influenzae. Infection Immun. 64, 3703–3712. doi: 10.1128/iai.64.9.3703-3712.1996
Mackenzie, G. A., Leach, A. J., Carapetis, J. R., Fisher, J., and Morris, P. S. (2010). Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect. diseases. 10, 1–10. doi: 10.1186/1471-2334-10-304
Maddi, S., Kolsum, U., Jackson, S., Barraclough, R., Maschera, B., Simpson, K. D., et al. (2017). Ampicillin resistance in Haemophilus influenzae from COPD patients in the UK. Int. J. chronic obstructive pulmonary disease. 12, 1507–1518. doi: 10.2147/COPD.S135338
Maguire, J. E., Beard, F., Méder, K., Dey, A., Macartney, K., and McIntyre, P. (2018). Australian vaccine preventable disease epidemiological review series: invasive Haemophilus influenzae type b disease, 2000-2017. Communicable Dis. Intell. . 2020, 44. doi: 10.33321/cdi.2020.44.11
Manti, S., Parisi, G. F., Papale, M., Licari, A., Salpietro, C., Miraglia del Giudice, M., et al. (2020). Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for treatment of upper respiratory tract infections in children: a pilot study on short-term efficacy. Ital. J. pediatrics. 46, 1–7. doi: 10.1186/s13052-020-0798-4
Marchant, J. M., Chang, A. B., Kennedy, E., King, D., Perret, J. L., Schultz, A., et al. (2024). Cough in Children and Adults: Diagnosis, Assessment and Management (CICADA). Summary of an updated position statement on chronic cough in Australia. Med. J. Australia. 220, 35–45. doi: 10.5694/mja2.v220.1
Marchisio, P., Santagati, M., Scillato, M., Baggi, E., Fattizzo, M., Rosazza, C., et al. (2015). Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur. J. Clin. Microbiol. Infect. Diseases. 34, 2377–2383. doi: 10.1007/s10096-015-2491-x
Marshall, B., Faro, A., and Brown, W. (2022). Cystic Fibrosis Foundation patient registry: 2020 annual data report, 2021.
Martín, R. and Langella, P. (2019). Emerging health concepts in the probiotics field: streamlining the definitions. Front. Microbiol. 10, 1047. doi: 10.3389/fmicb.2019.01047
Marvig, R. L., Damkiær, S., Khademi, S. H., Markussen, T. M., Molin, S., and Jelsbak, L. (2014). Within-host evolution of Pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. MBio 5. doi: 10.1128/mBio.00966-14
Mason, K. M., Bruggeman, M. E., Munson, R. S., and Bakaletz, L. O. (2006). The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol. Microbiol. 62, 1357–1372. doi: 10.1111/j.1365-2958.2006.05460.x
Mason, K. M., Munson, R. S., and Bakaletz, L. O. (2005). A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infection immunity. 73, 599–608. doi: 10.1128/IAI.73.1.599-608.2005
Mason, K. M., Raffel, F. K., Ray, W. C., and Bakaletz, L. O. (2011). Heme utilization by nontypeable Haemophilus influenzae is essential and dependent on Sap transporter function. J. bacteriology. 193, 2527–2535. doi: 10.1128/JB.01313-10
Mateos, F., Brock, J. H., and Pérez-Arellano, J. L. (1998). Iron metabolism in the lower respiratory tract. Thorax 53, 594–600. doi: 10.1136/thx.53.7.594
Matsumoto, S., Singley, C. M., Hoover, J., Nakamura, R., Echols, R., Rittenhouse, S., et al. (2017). Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrobial Agents chemotherapy 61. doi: 10.1128/AAC.00700-17
McPherson, C. J., Aschenbrenner, L. M., Lacey, B. M., Fahnoe, K. C., Lemmon, M. M., Finegan, S. M., et al. (2012). Clinically relevant Gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrobial Agents chemotherapy. 56, 6334–6342. doi: 10.1128/AAC.01345-12
Michels, K. R., Zhang, Z., Bettina, A. M., Cagnina, R. E., Stefanova, D., Burdick, M. D., et al. (2017). Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2. doi: 10.1172/jci.insight.92002
Mike, L. A., Smith, S. N., Sumner, C. A., Eaton, K. A., and Mobley, H. L. (2016). Siderophore vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. Proc. Natl. Acad. Sci. 113, 13468–13473. doi: 10.1073/pnas.1606324113
Minandri, F., Imperi, F., Frangipani, E., Bonchi, C., Visaggio, D., Facchini, M., et al. (2016). Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infection immunity. 84, 2324–2335. doi: 10.1128/IAI.00098-16
Miravitlles, M. and Anzueto, A. (2015). Antibiotic prophylaxis in COPD: Why, when, and for whom? Pulmonary Pharmacol. Ther. 32, 119–123. doi: 10.1016/j.pupt.2014.05.002
Mobarra, N., Shanaki, M., Ehteram, H., Nasiri, H., Sahmani, M., Saeidi, M., et al. (2016). A review on iron chelators in treatment of iron overload syndromes. Int. J. hematology-oncology Stem Cell Res. 10, 239.
Monasta, L., Ronfani, L., Marchetti, F., Montico, M., Brumatti, L. V., Bavcar, A., et al. (2012). Burden of disease caused by otitis media: systematic review and global estimates. PloS One 7, e36226. doi: 10.1371/journal.pone.0036226
Morton, D. J., Bakaletz, L. O., Jurcisek, J. A., VanWagoner, T. M., Seale, T. W., Whitby, P. W., et al. (2004). Reduced severity of middle ear infection caused by nontypeable Haemophilus influenzae lacking the hemoglobin/hemoglobin–haptoglobin binding proteins (Hgp) in a chinchilla model of otitis media. Microbial pathogenesis. 36, 25–33. doi: 10.1016/j.micpath.2003.08.007
Morton, D. J., Seale, T. W., Bakaletz, L. O., Jurcisek, J. A., Smith, A., VanWagoner, T. M., et al. (2009). The heme-binding protein (HbpA) of Haemophilus influenzae as a virulence determinant. Int. J. Med. Microbiol. 299, 479–488. doi: 10.1016/j.ijmm.2009.03.004
Morton, D. J., Seale, T. W., Madore, L. L., VanWagoner, T. M., Whitby, P. W., and Stull, T. L. (2007). The haem–haemopexin utilization gene cluster (hxuCBA) as a virulence factor of Haemophilus influenzae. Microbiology 153, 215–224. doi: 10.1099/mic.0.2006/000190-0
Morton, D. J., Turman, E. J., Hensley, P. D., VanWagoner, T. M., Seale, T. W., Whitby, P. W., et al. (2010a). Identification of a siderophore utilization locus in nontypeable Haemophilus influenzae. BMC Microbiol. 10, 1–12. doi: 10.1186/1471-2180-10-113
Morton, D. J., Turman, E. J., Hensley, P. D., Whitby, P. W., Stull, T. L., Seale, T. W., et al. (2010b). Identification of a siderophore utilization locus in nontypeable Haemophilus influenzae. BMC Microbiol. 10, 113. doi: 10.1186/1471-2180-10-113
Morton, D. J., VanWagoner, T. M., Seale, T. W., Whitby, P. W., and Stull, T. L. (2006). Differential utilization by Haemophilus influenzae of haemoglobin complexed to the three human haptoglobin phenotypes. FEMS Immunol. Med. Microbiol. 46, 426–432. doi: 10.1111/j.1574-695X.2006.00052.x
Mukundan, D., Ecevit, Z., Patel, M., Marrs, C. F., and Gilsdorf, J. R. (2007). Pharyngeal colonization dynamics of Haemophilus influenzae and Haemophilus haemolyticus in healthy adult carriers. J. Clin. Microbiol. 45, 3207–3217. doi: 10.1128/JCM.00492-07
Murphy, T. F. (2015). Vaccines for nontypeable Haemophilus influenzae: the future is now. Clin. Vaccine Immunol. 22, 459–466. doi: 10.1128/CVI.00089-15
Murphy, T. F., Brauer, A. L., Schiffmacher, A. T., and Sethi, S. (2004). Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 170, 266–272. doi: 10.1164/rccm.200403-354OC
Murphy-Benenato, K. E., Bhagunde, P. R., Chen, A., Davis, H. E., Durand-Reville, T. F., Ehmann, D. E., et al. (2015). Discovery of efficacious Pseudomonas aeruginosa-targeted siderophore-conjugated monocarbams by application of a semi-mechanistic pharmacokinetic/pharmacodynamic model. J. medicinal Chem. 58, 2195–2205. doi: 10.1021/jm501506f
Nairz, M., Schroll, A., Haschka, D., Dichtl, S., Tymoszuk, P., Demetz, E., et al. (2017). Genetic and dietary iron overload differentially affect the course of Salmonella typhimurium infection. Front. Cell. infection Microbiol. 7, 110. doi: 10.3389/fcimb.2017.00110
Nelson, A. L., Barasch, J. M., Bunte, R. M., and Weiser, J. N. (2005). Bacterial colonization of nasal mucosa induces expression of siderocalin, an iron-sequestering component of innate immunity. Cell. Microbiol. 7, 1404–1417. doi: 10.1111/j.1462-5822.2005.00566.x
Ni, W., Shao, X., Cai, X., Wei, C., Cui, J., Wang, R., et al. (2015). Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One 10, e0121257. doi: 10.1371/journal.pone.0121257
Noinaj, N., Guillier, M., Barnard, T. J., and Buchanan, S. K. (2010). TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64, 43–60. doi: 10.1146/annurev.micro.112408.134247
Nørskov-Lauritsen, N. (2014). Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin. Microbiol. Rev. 27, 214–240. doi: 10.1128/CMR.00103-13
Novotny, L. A., Brockman, K. L., Mokrzan, E. M., Jurcisek, J. A., and Bakaletz, L. O. (2019). Biofilm biology and vaccine strategies for otitis media due to nontypeable Haemophilus influenzae. J. Pediatr. Infect. Dis. 14, 69. doi: 10.1055/s-0038-1660818
Organization WH (2017). Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis (New York : John Wiley: World Health Organization).
Organization WH (2024). WHO bacterial priority pathogens list, 2024: bacterial pathogens of public health importance, to guide research, development, and strategies to prevent and control antimicrobial resistance (New York : John Wiley: World Health Organization).
Osanai, A., Li, S. J., Asano, K., Sashinami, H., Hu, D. L., and Nakane, A. (2013). Fibronectin-binding protein, FbpA, is the adhesin responsible for pathogenesis of Listeria monocytogenes infection. Microbiol. Immunol. 57, 253–262. doi: 10.1111/1348-0421.12030
Page, M. G. (2013). Siderophore conjugates. Ann. New York Acad. Sci. 1277, 115–126. doi: 10.1111/nyas.2013.1277.issue-1
Page, M. G. (2019). The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin. Infect. Dis. 69, S529–SS37. doi: 10.1093/cid/ciz825
Page, M. G., Dantier, C., and Desarbre, E. (2010). In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant gram-negative bacilli. Antimicrobial Agents chemotherapy. 54, 2291–2302. doi: 10.1128/AAC.01525-09
Parrow, N. L., Fleming, R. E., and Minnick, M. F. (2013). Sequestration and scavenging of iron in infection. Infection immunity. 81, 3503–3514. doi: 10.1128/IAI.00602-13
Patel, J. A., Reisner, B., Vizirinia, N., Owen, M., Chonmaitree, T., and Howie, V. (1995). Bacteriologic failure of amoxicillin-clavulanate in treatment of acute otitis media caused by nontypeable Haemophilus influenzae. J. pediatrics. 126, 799–806. doi: 10.1016/S0022-3476(95)70415-9
Penido, N., Chandrasekhar, S. S., Borin, A., Maranhão, A., and Testa, J. R. G. (2016). Complications of otitis media-a potentially lethal problem still present. Braz. J. otorhinolaryngology. 82, 253–262. doi: 10.1016/j.bjorl.2015.04.007
Pérez-Vázquez, M., Román, F., Aracil, B., Cantón, R., and Campos, J. (2004). Laboratory detection of Haemophilus influenzae with decreased susceptibility to nalidixic acid, ciprofloxacin, levofloxacin, and moxifloxacin due to GyrA and ParC mutations. J. Clin. Microbiol. 42, 1185–1191. doi: 10.1128/JCM.42.3.1185-1191.2004
Persson, L. J., Aanerud, M., Hardie, J. A., Nilsen, R. M., Bakke, P. S., Eagan, T. M., et al. (2017). Antimicrobial peptide levels are linked to airway inflammation, bacterial colonisation and exacerbations in chronic obstructive pulmonary disease. Eur. Respir. J. 49. doi: 10.1183/13993003.01328-2016
Pettigrew, M. M., Laufer, A. S., Gent, J. F., Kong, Y., Fennie, K. P., and Metlay, J. P. (2012). Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl. Environ. Microbiol. 78, 6262–6270. doi: 10.1128/AEM.01051-12
Pfeifer, Y., Meisinger, I., Brechtel, K., and Gröbner, S. (2013). Emergence of a multidrug-resistant Haemophilus influenzae strain causing chronic pneumonia in a patient with common variable immunodeficiency. Microbial Drug Resistance. 19, 1–5. doi: 10.1089/mdr.2012.0060
Phaff, S. J., Tiddens, H. A., Verbrugh, H. A., and Ott, A. (2006). Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J. Antimicrobial Chemotherapy. 57, 741–746. doi: 10.1093/jac/dkl014
Phillips, Z. N., Jennison, A. V., Whitby, P. W., Stull, T. L., Staples, M., and Atack, J. M. (2022). Examination of phase-variable haemoglobin–haptoglobin binding proteins in non-typeable Haemophilus influenzae reveals a diverse distribution of multiple variants. FEMS Microbiol. Lett. 369, fnac064. doi: 10.1093/femsle/fnac064
Pidcock, K. A., Wooten, J. A., Daley, B. A., and Stull, T. L. (1988). Iron acquisition by Haemophilus influenzae. Infection Immun. 56, 721–725. doi: 10.1128/iai.56.4.721-725.1988
Polland, L., Rydén, H., Su, Y., and Paulsson, M. (2023). In vivo gene expression profile of Haemophilus influenzae during human pneumonia. Microbiol. spectrum. 11, e01639–e01623. doi: 10.1128/spectrum.01639-23
Poole, J., Foster, E., Chaloner, K., Hunt, J., Jennings, M. P., Bair, T., et al. (2013). Analysis of nontypeable Haemophilus influenzae phase-variable genes during experimental human nasopharyngeal colonization. J. Infect. diseases. 208, 720–727. doi: 10.1093/infdis/jit240
Porto, G. and De Sousa, M. (2007). Iron overload and immunity. World J. gastroenterology: WJG. 13, 4707. doi: 10.3748/wjg.v13.i35.4707
Pramanik, A., Stroeher, U. H., Krejci, J., Standish, A. J., Bohn, E., Paton, J. C., et al. (2007). Albomycin is an effective antibiotic, as exemplified with Yersinia enterocolitica and Streptococcus pneumoniae. Int. J. Med. Microbiol. 297, 459–469. doi: 10.1016/j.ijmm.2007.03.002
Puig, C., Marti, S., Fleites, A., Trabazo, R., Calatayud, L., Linares, J., et al. (2014). Oropharyngeal colonization by nontypeable Haemophilus influenzae among healthy children attending day care centers. Microbial Drug Resistance. 20, 450–455. doi: 10.1089/mdr.2013.0186
Puig, C., Tirado-Vélez, J. M., Calatayud, L., Tubau, F., Garmendia, J., Ardanuy, C., et al. (2015). Molecular characterization of fluoroquinolone resistance in nontypeable Haemophilus influenzae clinical isolates. Antimicrobial Agents chemotherapy. 59, 461–466. doi: 10.1128/AAC.04005-14
Qu, J., Lesse, A. J., Brauer, A. L., Cao, J., Gill, S. R., and Murphy, T. F. (2010). Proteomic expression profiling of Haemophilus influenzae grown in pooled human sputum from adults with chronic obstructive pulmonary disease reveal antioxidant and stress responses. BMC Microbiol. 10, 1–12. doi: 10.1186/1471-2180-10-162
Quenee, L. E., Hermanas, T. M., Ciletti, N., Louvel, H., Miller, N. C., Elli, D., et al. (2012). Hereditary hemochromatosis restores the virulence of plague vaccine strains. J. Infect. diseases. 206, 1050–1058. doi: 10.1093/infdis/jis433
Raffatellu, M., George, M. D., Akiyama, Y., Hornsby, M. J., Nuccio, S.-P., Paixao, T. A., et al. (2009). Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5, 476–486. doi: 10.1016/j.chom.2009.03.011
Raffel, F. K., Szelestey, B. R., Beatty, W. L., and Mason, K. M. (2013). The Haemophilus influenzae Sap transporter mediates bacterium-epithelial cell homeostasis. Infection immunity. 81, 43–54. doi: 10.1128/IAI.00942-12
Rahav, G., Volach, V., Shapiro, M., Rund, D., Rachmilewitz, E. A., and Goldfarb, A. (2006). Severe infections in thalassaemic patients: prevalence and predisposing factors. Br. J. haematology. 133, 667–674. doi: 10.1111/j.1365-2141.2006.06082.x
Rao, V. K., Krasan, G. P., Hendrixson, D. R., Dawid, S., and St. Geme, J. W., III (1999). Molecular determinants of the pathogenesis of disease due to non-typable Haemophilus influenzae. FEMS Microbiol. Rev. 23, 99–129. doi: 10.1111/j.1574-6976.1999.tb00393.x
Rea, M. C., Sit, C. S., Clayton, E., O’Connor, P. M., Whittal, R. M., Zheng, J., et al. (2010). a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. 107, 9352–9357. doi: 10.1073/pnas.0913554107
Reid, D. W., Anderson, G. J., and Lamont, I. L. (2009). Role of lung iron in determining the bacterial and host struggle in cystic fibrosis. Am. J. Physiology-Lung Cell. Mol. Physiol. 297, L795–L802. doi: 10.1152/ajplung.00132.2009
Rempe, K. A., Porsch, E. A., Wilson, J. M., and Geme, J. W. S. (2016). The HMW1 and HMW2 adhesins enhance the ability of nontypeable Haemophilus influenzae to colonize the upper respiratory tract of rhesus macaques. Infection immunity. 84, 2771–2778. doi: 10.1128/IAI.00153-16
Ren, Z., Jin, H., Whitby, P. W., Morton, D. J., and Stull, T. L. (1999). Role of CCAA nucleotide repeats in regulation of hemoglobin and hemoglobin-haptoglobin binding protein genes of Haemophilus influenzae. J. bacteriology 181, 5865–5870. doi: 10.1128/JB.181.18.5865-5870.1999
Resman, F., Ristovski, M., Forsgren, A., Kaijser, B., Kronvall, G., Medstrand, P., et al. (2012). Increase of β-lactam-resistant invasive Haemophilus influenzae in Sweden, 1997 to 2010. Antimicrobial Agents chemotherapy. 56, 4408–4415. doi: 10.1128/AAC.00415-12
Ribeiro, M. and Simões, M. (2019). Advances in the antimicrobial and therapeutic potential of siderophores. Environ. Chem. Letters. 17, 1485–1494. doi: 10.1007/s10311-019-00887-9
Richard, K. L., Kelley, B. R., and Johnson, J. G. (2019). Heme uptake and utilization by gram-negative bacterial pathogens. Front. Cell. infection Microbiol. 9, 81. doi: 10.3389/fcimb.2019.00081
Richter, K., Thomas, N., Claeys, J., McGuane, J., Prestidge, C. A., Coenye, T., et al. (2017a). A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity. Antimicrobial Agents chemotherapy 61. doi: 10.1128/AAC.00481-17
Richter, K., Thomas, N., Zhang, G., Prestidge, C. A., Coenye, T., Wormald, P.-J., et al. (2017b). Deferiprone and gallium-protoporphyrin have the capacity to potentiate the activity of antibiotics in Staphylococcus aureus small colony variants. Front. Cell. infection Microbiol. 7, 280. doi: 10.3389/fcimb.2017.00280
Rodríguez-Arce, I., Al-Jubair, T., Euba, B., Fernández-Calvet, A., Gil-Campillo, C., Martí, S., et al. (2019). Moonlighting of Haemophilus influenzae heme acquisition systems contributes to the host airway-pathogen interplay in a coordinated manner. Virulence 10, 315–333. doi: 10.1080/21505594.2019.1596506
Roede, B. M., Bresser, P., Prins, J. M., Schellevis, F., Verheij, T. J., and Bindels, P. J. (2009). Reduced risk of next exacerbation and mortality associated with antibiotic use in COPD. Eur. Respir. J. 33, 282–288. doi: 10.1183/09031936.00088108
Ronander, E., Brant, M., Eriksson, E., Mörgelin, M., Hallgren, O., Westergren-Thorsson, G., et al. (2009). Nontypeable Haemophilus influenzae adhesin protein E: characterization and biological activity. J. Infect. diseases. 199, 522–531. doi: 10.1086/596211
Rosadini, C. V., Wong, S. M., and Akerley, B. J. (2008). The periplasmic disulfide oxidoreductase DsbA contributes to Haemophilus influenzae pathogenesis. Infection immunity. 76, 1498–1508. doi: 10.1128/IAI.01378-07
Rothberg, M. B., Pekow, P. S., Lahti, M., Brody, O., Skiest, D. J., and Lindenauer, P. K. (2010). Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA 303, 2035–2042. doi: 10.1001/jama.2010.672
Saini, M., Trehan, K., Thakur, S., Modi, A., and Jain, S. K. (2024). Advances in iron deficiency anaemia management: exploring novel drug delivery systems and future perspectives. Curr Drug Deliv. doi: 10.2174/0115672018300804240426070552
Schlör, S., Herbert, M., Rodenburg, M., Blass, J., and Reidl, J. (2000). Characterization of ferrochelatase (hemH) mutations in Haemophilus influenzae. Infection Immun. 68, 3007–3009. doi: 10.1128/IAI.68.5.3007-3009.2000
Seale, T. W., Morton, D. J., Whitby, P. W., Wolf, R., Kosanke, S. D., VanWagoner, T. M., et al. (2006). Complex role of hemoglobin and hemoglobin-haptoglobin binding proteins in Haemophilus influenzae virulence in the infant rat model of invasive infection. Infection immunity. 74, 6213–6225. doi: 10.1128/IAI.00744-06
Seppanen, E. J., Thornton, R. B., North, H. J., Corscadden, K. J., Wiertsema, S. P., Vijayasekaran, S., et al. (2020). Bacterial reservoirs in the middle ear of otitis-prone children are associated with repeat ventilation tube insertion. Pediatr. Infect. Dis. J. 39, 91–96. doi: 10.1097/INF.0000000000002541
Sethi, S., Evans, N., Grant, B. J., and Murphy, T. F. (2002). New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. New Engl. J. Med. 347, 465–471. doi: 10.1056/NEJMoa012561
Sethi, S. and Murphy, T. F. (2001). Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14, 336–363. doi: 10.1128/CMR.14.2.336-363.2001
Sgheiza, V., Novick, B., Stanton, S., Pierce, J., Kalmeta, B., Holmquist, M. F., et al. (2017). Covalent bonding of heme to protein prevents heme capture by nontypeable Haemophilus influenzae. FEBS Open Bio 7, 1778–1783. doi: 10.1002/feb4.2017.7.issue-11
Shekhar, S., Khan, R., Schenck, K., and Petersen, F. C. (2019). Intranasal immunization with the commensal Streptococcus mitis confers protective immunity against pneumococcal lung infection. Appl. Environ. Microbiol. 85. doi: 10.1128/AEM.02235-18
Shelton, C. L., Raffel, F. K., Beatty, W. L., Johnson, S. M., and Mason, K. M. (2011). Sap transporter mediated import and subsequent degradation of antimicrobial peptides in Haemophilus. PloS Pathog. 7, e1002360. doi: 10.1371/journal.ppat.1002360
Siddiq, S. and Grainger, J. (2015). The diagnosis and management of acute otitis media: American Academy of Pediatrics Guidelines 2013. Arch. Dis. Childhood-Education Practice. 100, 193–197. doi: 10.1136/archdischild-2013-305550
Sierra, A., Lopez, P., Zapata, M. A., Vanegas, B., Castrejon, M. M., DeAntonio, R., et al. (2011). Non-typeable Haemophilus influenzae and Streptococcus pneumoniae as primary causes of acute otitis media in Colombian children: a prospective study. BMC Infect. diseases. 11, 4. doi: 10.1186/1471-2334-11-4
Singh, B., Brant, M., Kilian, M., Hallström, B., and Riesbeck, K. (2010). Protein E of Haemophilus influenzae is a ubiquitous highly conserved adhesin. J. Infect. diseases. 201, 414–419. doi: 10.1086/649782
Skaare, D., Anthonisen, I. L., Kahlmeter, G., Matuschek, E., Natås, O. B., Steinbakk, M., et al. (2014). Emergence of clonally related multidrug resistant Haemophilus influenzae with penicillin-binding protein 3-mediated resistance to extended-spectrum cephalosporins, Norway, 2006 to 2013. Eurosurveillance 19, 20986. doi: 10.2807/1560-7917.ES2014.19.49.20986
Smith, A. and McCulloh, R. J. (2015). Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front. Physiol. 6, 187. doi: 10.3389/fphys.2015.00187
Smith-Vaughan, H. C., Binks, M. J., Marsh, R. L., Kaestli, M., Ward, L., Hare, K. M., et al. (2013). Dominance of Haemophilus influenzae in ear discharge from Indigenous Australian children with acute otitis media with tympanic membrane perforation. BMC Ear Nose Throat Disord. 13, 1–9. doi: 10.1186/1472-6815-13-12
Smith-Vaughan, H., Byun, R., Nadkarni, M., Jacques, N. A., Hunter, N., Halpin, S., et al. (2006). Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 6, 10. doi: 10.1186/1472-6815-6-10
Smith-Vaughan, H. C., Chang, A. B., Sarovich, D. S., Marsh, R. L., Grimwood, K., Leach, A. J., et al. (2014). Absence of an important vaccine and diagnostic target in carriage-and disease-related nontypeable Haemophilus influenzae. Clin. Vaccine Immunol. 21, 250–252. doi: 10.1128/CVI.00632-13
Soeters, H. M., Blain, A., Pondo, T., Doman, B., Farley, M. M., Harrison, L. H., et al. (2018). Current epidemiology and trends in invasive Haemophilus influenzae disease—United States, 2009–2015. Clin. Infect. Diseases. 67, 881–889. doi: 10.1093/cid/ciy187
Sriram, K. B., Cox, A. J., Clancy, R. L., Slack, M. P., and Cripps, A. W. (2018). Nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease: a review for clinicians. Crit. Rev. Microbiol. 44 (2), 125–142. doi: 10.1080/1040841X.2017.1329274
Stites, S. W., Plautz, M. W., Bailey, K., O’Brien-Ladner, A. R., and Wesselius, L. J. (1999). Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am. J. Respir. Crit. Care Med. 160, 796–801. doi: 10.1164/ajrccm.160.3.9811018
Stojiljkovic, I., Kumar, V., and Srinivasan, N. (1999). Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol. Microbiol. 31, 429–442. doi: 10.1046/j.1365-2958.1999.01175.x
Su, P.-Y., Huang, A.-H., Lai, C.-H., Lin, H.-F., Lin, T.-M., and Ho, C.-H. (2020). Extensively drug-resistant Haemophilus influenzae–emergence, epidemiology, risk factors, and regimen. BMC Microbiol. 20, 1–10. doi: 10.1186/s12866-020-01785-9
Sun, W., Jacoby, P., Riley, T. V., Bowman, J., Leach, A. J., Coates, H., et al. (2012). Association between early bacterial carriage and otitis media in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia: a cohort study. BMC Infect. diseases. 12, 1–9. doi: 10.1186/1471-2334-12-366
Szelestey, B. R., Heimlich, D. R., Raffel, F. K., Justice, S. S., and Mason, K. M. (2013). Haemophilus responses to nutritional immunity: epigenetic and morphological contribution to biofilm architecture, invasion, persistence and disease severity. PloS pathogens. 9, e1003709. doi: 10.1371/journal.ppat.1003709
Tanaka, K. J. and Pinkett, H. W. (2019). Oligopeptide-binding protein from nontypeable Haemophilus influenzae has ligand-specific sites to accommodate peptides and heme in the binding pocket. J. Biol. Chem. 294, 1070–1082. doi: 10.1074/jbc.RA118.004479
Teele, D. W., Klein, J. O., Rosner, B., and Group GBOMS (1989). Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. diseases. 160, 83–94. doi: 10.1093/infdis/160.1.83
Thompson, M. G., Corey, B. W., Si, Y., Craft, D. W., and Zurawski, D. V. (2012). Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrobial Agents chemotherapy. 56, 5419–5421. doi: 10.1128/AAC.01197-12
Tram, G., Jen, F. E.-C., Phillips, Z. N., Lancashire, J. F., Timms, J., Poole, J., et al. (2024). Phasevarions in Haemophilus influenzae biogroup aEgyptius control expression of multiple proteins. Microbiol. Spectrum. 12, e02601–e02623. doi: 10.1128/spectrum.02601-23
Tripathi, A., Schofield, M. M., Chlipala, G. E., Schultz, P. J., Yim, I., Newmister, S. A., et al. (2014). broad-spectrum antibiotics identified as inhibitors of siderophore biosynthesis in Staphylococcus aureus and Bacillus anthracis. J. Am. Chem. Society. 136, 1579–1586. doi: 10.1021/ja4115924
Troxell, B. and Hassan, H. M. (2013). Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front. Cell. infection Microbiol. 3, 59. doi: 10.3389/fcimb.2013.00059
Turnidge, J. D. and Meleady, K. T. (2017). Antimicrobial Use and Resistance in Australia (AURA) surveillance system: coordinating national data on antimicrobial use and resistance for Australia. Aust. Health Review. 42, 272–276. doi: 10.1071/AH16238
Val, S., Poley, M., Brown, K., Choi, R., Jeong, S., Colberg-Poley, A., et al. (2016). Proteomic characterization of middle ear fluid confirms neutrophil extracellular traps as a predominant innate immune response in chronic otitis media. PloS One 11, e0152865. doi: 10.1371/journal.pone.0152865
Van Eldere, J., Slack, M. P., Ladhani, S., and Cripps, A. W. (2014). Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect. diseases. 14, 1281–1292. doi: 10.1016/S1473-3099(14)70734-0
Van Kempen, M., Dhooge, I., Vaneechoutte, M., and Claeys, G. (2001). Turnover of Haemophilus influenzae isolates in otitis-prone children. Acta oto-rhino-laryngologica belgica. 55, 83–86.
Venekamp, R. P., Sanders, S. L., Glasziou, P. P., Del Mar, C. B., and Rovers, M. M. (2015). Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2015 (6), CD000219. doi: 10.1002/14651858.CD000219.pub4
Vergauwen, B., Elegheert, J., Dansercoer, A., Devreese, B., and Savvides, S. N. (2010). Glutathione import in Haemophilus influenzae Rd is primed by the periplasmic heme-binding protein HbpA. Proc. Natl. Acad. Sci. 107, 13270–13275. doi: 10.1073/pnas.1005198107
Vergauwen, B., Pauwels, F., and Van Beeumen, J. J. (2003). Glutathione and catalase provide overlapping defenses for protection against respiration-generated hydrogen peroxide in Haemophilus influenzae. J. bacteriology 185, 5555–5562. doi: 10.1128/JB.185.18.5555-5562.2003
Vesikari, T., Forsten, A., Seppä, I., Kaijalainen, T., Puumalainen, T., Soininen, A., et al. (2016). Effectiveness of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein d–conjugated vaccine (PHiD-CV) against carriage and acute otitis media—a double-blind randomized clinical trial in Finland. J. Pediatr. Infect. Dis. Society. 5, 237–248. doi: 10.1093/jpids/piw010
Vogel, A. R., Szelestey, B. R., Raffel, F. K., Sharpe, S. W., Gearinger, R. L., Justice, S. S., et al. (2012). SapF-mediated heme-iron utilization enhances persistence and coordinates biofilm architecture of Haemophilus. Front. Cell. infection Microbiol. 2, 42. doi: 10.3389/fcimb.2012.00042
Wang, H.-J., Wang, C.-Q., Hua, C.-Z., Yu, H., Zhang, T., Zhang, H., et al. (2019). Antibiotic resistance profiles of Haemophilus influenzae isolates from children in 2016: a Multicenter study in China. Can. J. Infect. Dis. Med. Microbiol. 2019, 6456321. doi: 10.1155/2019/6456321
Wedzicha, J. A., Miravitlles, M., Hurst, J. R., Calverley, P. M., Albert, R. K., Anzueto, A., et al. (2017). Management of COPD exacerbations: a European respiratory society/American thoracic society guideline. Eur. Respir. J. 49. doi: 10.1183/13993003.00791-2016
Weinberg, E. (2000). Microbial pathogens with impaired ability to acquire host iron. Biometals 13, 85–89. doi: 10.1023/A:1009293500209
Welp, A. L. and Bomberger, J. M. (2020). Bacterial community interactions during chronic respiratory disease. Front. Cell. Infection Microbiol. 10, 213. doi: 10.3389/fcimb.2020.00213
Whitby, P. W., Seale, T. W., Morton, D. J., VanWagoner, T. M., and Stull, T. L. (2010). Characterization of the Haemophilus influenzae tehB gene and its role in virulence. Microbiology 156, 1188. doi: 10.1099/mic.0.036400-0
Whitby, P. W., Seale, T. W., VanWagoner, T. M., Morton, D. J., and Stull, T. L. (2009). The iron/heme regulated genes of Haemophilus influenzae: comparative transcriptional profiling as a tool to define the species core modulon. BMC Genomics 10, 1–19. doi: 10.1186/1471-2164-10-6
White, D. C. and Granick, S. (1963). Hemin biosynthesis in Haemophilus. J. bacteriology. 85, 842–850. doi: 10.1128/jb.85.4.842-850.1963
Whittaker, R., Economopoulou, A., Dias, J. G., Bancroft, E., Ramliden, M., and Celentano, L. P. (2017). Epidemiology of invasive Haemophilus influenzae disease, Europe, 2007–2014. Emerging Infect. diseases. 23, 396. doi: 10.3201/eid2303.161552
Wilkinson, T. M., Schembri, S., Brightling, C., Bakerly, N. D., Lewis, K., MacNee, W., et al. (2019). Non-typeable Haemophilus influenzae protein vaccine in adults with COPD: a phase 2 clinical trial. Vaccine 37, 6102–6111. doi: 10.1016/j.vaccine.2019.07.100
Williams, P., Morton, D. J., Towner, K. J., Stevenson, P., and Griffiths, E. (1990). Utilization of enterobactin and other exogenous iron sources by Haemophilus influenzae, H. parainfluenzae and H. paraphrophilus. Microbiology 136, 2343–2350. doi: 10.1099/00221287-136-12-2343
Williams, L. J., Tristram, S. G., and Zosky, G. R. (2023). Geogenic particles induce bronchial susceptibility to non-typeable Haemophilus influenzae. Environ. Res. 236, 116868. doi: 10.1016/j.envres.2023.116868
Wurzel, D. F., Marchant, J. M., Yerkovich, S. T., Upham, J. W., Petsky, H. L., Smith-Vaughan, H., et al. (2016). Protracted bacterial bronchitis in children: natural history and risk factors for bronchiectasis. Chest 150, 1101–1108. doi: 10.1016/j.chest.2016.06.030
Yamada, S., Seyama, S., Wajima, T., Yuzawa, Y., Saito, M., Tanaka, E., et al. (2020). β-Lactamase-non-producing ampicillin-resistant Haemophilus influenzae is acquiring multidrug resistance. J. infection Public Health 13, 497–501. doi: 10.1016/j.jiph.2019.11.003
Yang, I. A., George, J., McDonald, C. F., Disler, R., Ordman, R., Goodwin, A., et al. (2024). The COPD-X Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease 2024. Version 2.76, September 2024. Published online 16 November 2024. https://copdx.org.au/copd-x-plan/.
Ysebaert, C., Denoël, P., Weynants, V., Bakaletz, L. O., Novotny, L. A., Godfroid, F., et al. (2019). A protein E-PilA fusion protein shows vaccine potential against nontypeable Haemophilus influenzae in mice and chinchillas. Infection Immun. 87. doi: 10.1128/IAI.00345-19
Zhang, L., Xie, J., Patel, M., Bakhtyar, A., Ehrlich, G. D., Ahmed, A., et al. (2012). Nontypeable Haemophilus influenzae genetic islands associated with chronic pulmonary infection. PLoS One 7, e44730. doi: 10.1371/journal.pone.0044730
Keywords: haem acquisition, new therapeutic agents, nontypeable H. influenzae, respiratory infections, iron, host-pathogen interactions, heme
Citation: Atto B, Gell DA, Marsh R and Tristram S (2025) Exploiting haem-iron dependence of nontypeable Haemophilus influenzae: an avenue for future therapeutic development. Front. Cell. Infect. Microbiol. 15:1548048. doi: 10.3389/fcimb.2025.1548048
Received: 19 December 2024; Accepted: 22 April 2025;
Published: 15 May 2025.
Edited by:
Maria Paula Bajanca-Lavado, National Health Institute Doutor Ricardo Jorge (INSA), PortugalReviewed by:
Kenneth L. Brockman, Medical College of Wisconsin, United StatesHao-Chieh Chiu, National Taiwan University, Taiwan
Copyright © 2025 Atto, Gell, Marsh and Tristram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brianna Atto, YnJpYW5uYS5hdHRvQHV0YXMuZWR1LmF1
 Brianna Atto
Brianna Atto David A. Gell2
David A. Gell2 Robyn Marsh
Robyn Marsh Stephen Tristram
Stephen Tristram