- 1Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 2Department of Microbiology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 3Pediatric Congenital Hematologic Disorders Research Center, Mofid Children Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4HIV/AIDS Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Department of Bacteriology & Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Colorectal cancer (CRC) is known as the second leading cause of cancer-related deaths around the world. Rectal bleeding, changes in bowel movements, weight loss, and fatigue are the main clinical presentations of CRC. While the exact etiology of the disease is unknown, CRC is considered a complex and multifactorial disease resulted from an intricate interplay of genetic and environmental factors. Moreover, CRC is known as a chronic inflammation–associated tumor, and patients with inflammatory bowel disease (IBD) or Irritable bowel syndrome (IBS) are susceptible groups to CRC development. The gut microbiota and its metabolites play a crucial role in the development and progression of CRC. CRC can be created after anaerobic bacterial infections such as Enterotoxigenic Bacteroides fragilis (ETBF), Fusobacterium, Clostridium difficile, Clostridium perfringens, Clostridium septicum, Peptostreptococcus, Prevotella, Veillonella, etc. Activation of Wnt signaling, loss of tissue architecture, proinflammatory signaling, and genotoxic cellular DNA damage are the primary mechanisms by which anaerobic bacteria induce carcinogenesis in CRC. Besides, spore germination and toxin production are done in hypoxic and acidic conditions. Therefore, according to the presence of this condition (anaerobic glycolysis) in tumor tissue, the tumor environment is suitable for the formation of anaerobic infections. The current review‐based study aims to discuss the important aspects of these mechanisms and their possible roles in the initiation, development, and exacerbation of CRC.
Introduction
Colorectal cancer (CRC) is the third most common cancer around the world (Bray et al., 2024; Cao et al., 2024; Hong et al., 2024). Despite the existence of different treatment regimens, CRC ranks as the second leading cause of cancer-related deaths with an estimated 3.2 million new cases and 1.6 million deaths in 2024 (BGI Genomics, 2024). Rectal bleeding, changes in bowel movements, weight loss, and fatigue are the primary clinical presentations of CRC (Koliarakis et al., 2024). CRC is a multifactorial disease, and its etiology is not fully determined. It is presumed that genetic and environmental factors are linked to the initiation, progression, and exacerbation of CRC (Cheng et al., 2024). Indeed, CRC is known as a chronic inflammation–associated tumor, and patients with ulcerative colitis or Crohn’s disease (inflammatory bowel disease) are susceptible groups to CRC development (Eskandari-Malayeri et al., 2024).
Globally, previously published studies have stated that CRC can be triggered by anaerobic bacterial infections, including Enterotoxigenic Bacteroides fragilis (ETBF), Fusobacterium nucleatum, Clostridium difficile, C. perfringens, C. septicum, Peptostreptococcus, Prevotella, and Veillonella (Kajihara et al., 2023; Conde-Pérez et al., 2024; Hong et al., 2024). The gut microbiota and its metabolites play a crucial role in the development and progression of CRC (Cheng et al., 2024). Disruptions in microbiota composition are termed dysbiosis (Shariati et al., 2019a; Azimi et al., 2020). Microbiota-related carcinogenesis is primarily caused by dysbiosis-related inflammation and carcinogen formation (Azimi et al., 2018; Shariati et al., 2019b). Moreover, secondary bile acids (BAs) and short-chain fatty acids (SCFAs), which are two main bacterial metabolites in the colon, play either protective or harmful roles in the initiation and development of CRC (Cheng et al., 2024). Activation of Wnt signaling, loss of tissue architecture, proinflammatory signaling, and genotoxic cellular DNA damage are the primary mechanisms by which anaerobic bacteria induce carcinogenesis in CRC (Hong et al., 2024). The role and possible mechanisms of anaerobic pathogenic bacteria are summarized in Table 1. According to what was mentioned earlier and with respect to possible roles of anaerobic bacteria in cancer development and progression, the current review‐based study aims to discuss their potential roles and important aspects of mechanisms in the initiation, development, and exacerbation of CRC.
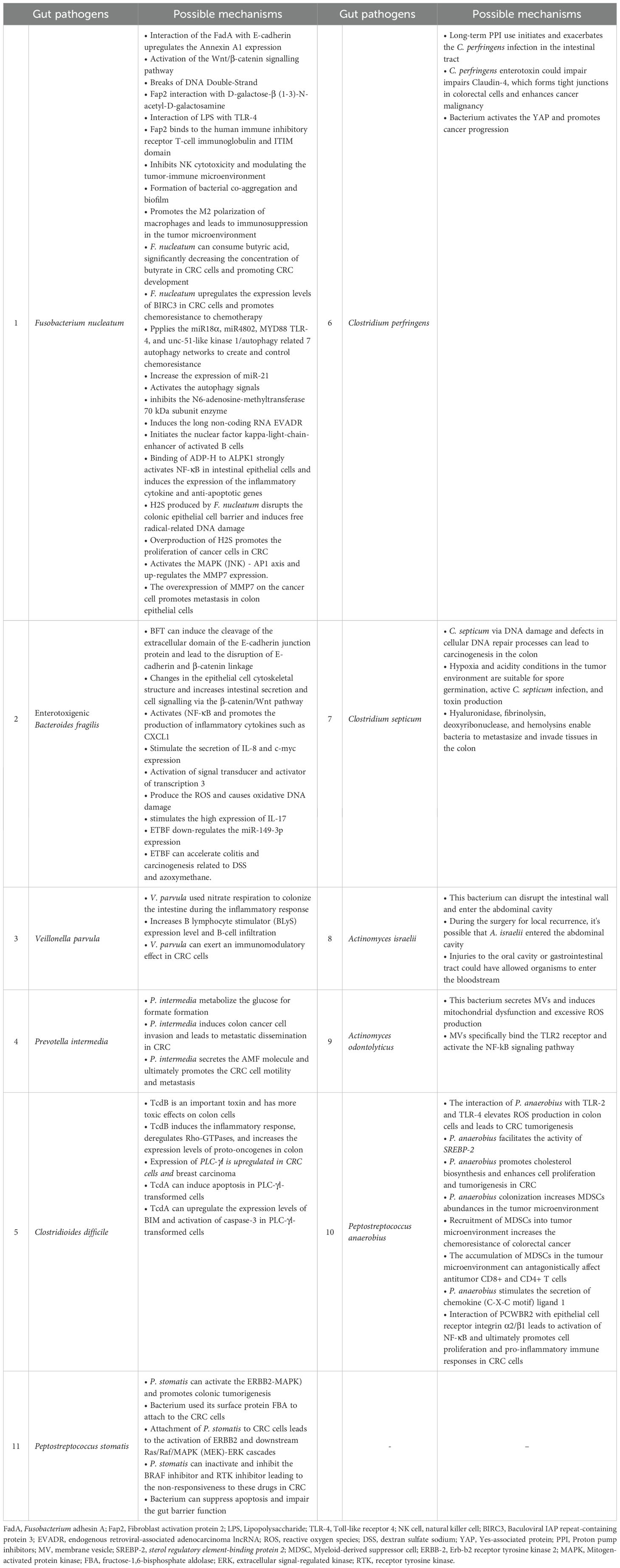
Table 1. The possible mechanisms of anaerobic gut bacteria implicated in the initiation, exacerbation, and development of human CRC.
Fusobacterium nucleatum
Fusobacterium nucleatum (F. nucleatum) is a spindle-shaped, non-spore-forming Gram-negative anaerobe bacterium. It is identified as a member of the human normal microbiota in oral cavity[12]. However, prior studies have indicated that F. nucleatum is an invasive, pro-inflammatory pathogen associated with various human disease conditions (Koliarakis et al., 2024).
F. nucleatum plays a key role in initiating and developing various cancers, such as liver, lung, and CRC (Liu et al., 2021). It has been discovered that different mechanisms of CRC are mediated by F. nucleatum. In most cases, the entrance of F. nucleatum into colorectal tissues has been facilitated in three main ways (Figure 1): 1) interaction of the effector Fusobacterium adhesin A (FadA) with E-cadherin, 2) fibroblast activation protein 2 (Fap2) interaction with D-galactose-β (1-3)-N-acetyl-D-galactosamine (Gal-GalNAc), and 3) interaction of Lipopolysaccharide (LPS) of F. nucleatum with Toll-like receptor 4 (TLR4) (Wang et al., 2023a; Martin-Gallausiaux et al., 2024).
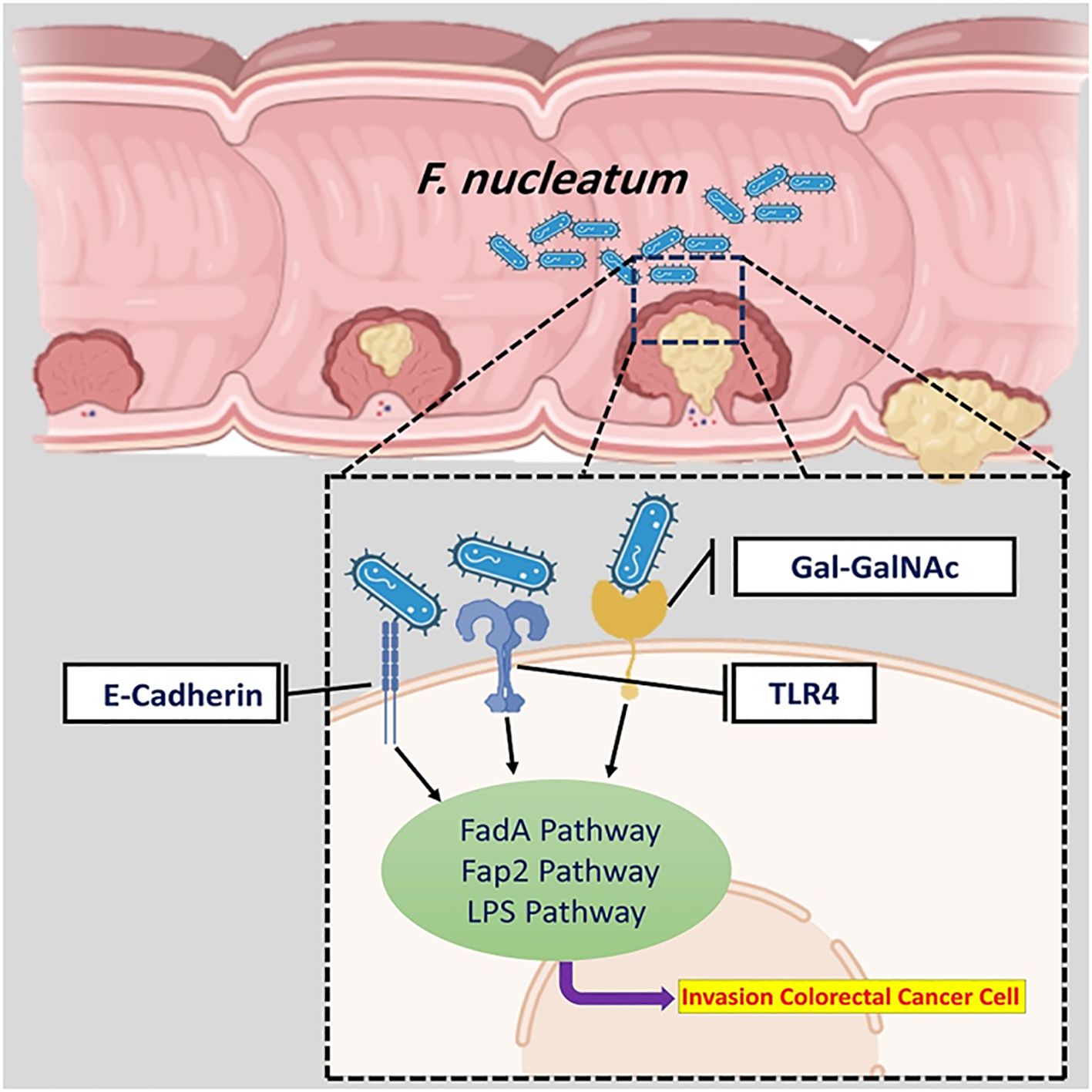
Figure 1. The overall mechanism of F. nucleatum in CRC. The entrance of F. nucleatum into colorectal tissues has been facilitated in three main ways.
Previously published studies have revealed overexpressed FadA gene levels in CRC patients’ faecal samples (Martin-Gallausiaux et al., 2024; Wang et al., 2024). Annexin A1 is a 35–40 kDa molecule and is considered as a main part of FadA protein. It is proposed that Annexin A1 can enable the FadA protein to influence different biological processes such as apoptosis, cell differentiation, cell proliferation, cell migration, and tumor growth (Figure 2) (Liu et al., 2024). The binding of the FadA protein to E-cadherin upregulates the Annexin A1 expression and can stimulate carcinogenesis (Cheng et al., 2024). Moreover, by activating the Wnt/β-catenin signalling pathway cyclin D1 (CycD1), it can induce and trigger oncogenic and inflammatory responses (Pignatelli et al., 2023). Interaction of Annexin A1 with epidermal growth factor receptor (EGFR) induces immune suppression in the tumor microenvironment (Araújo et al., 2021).
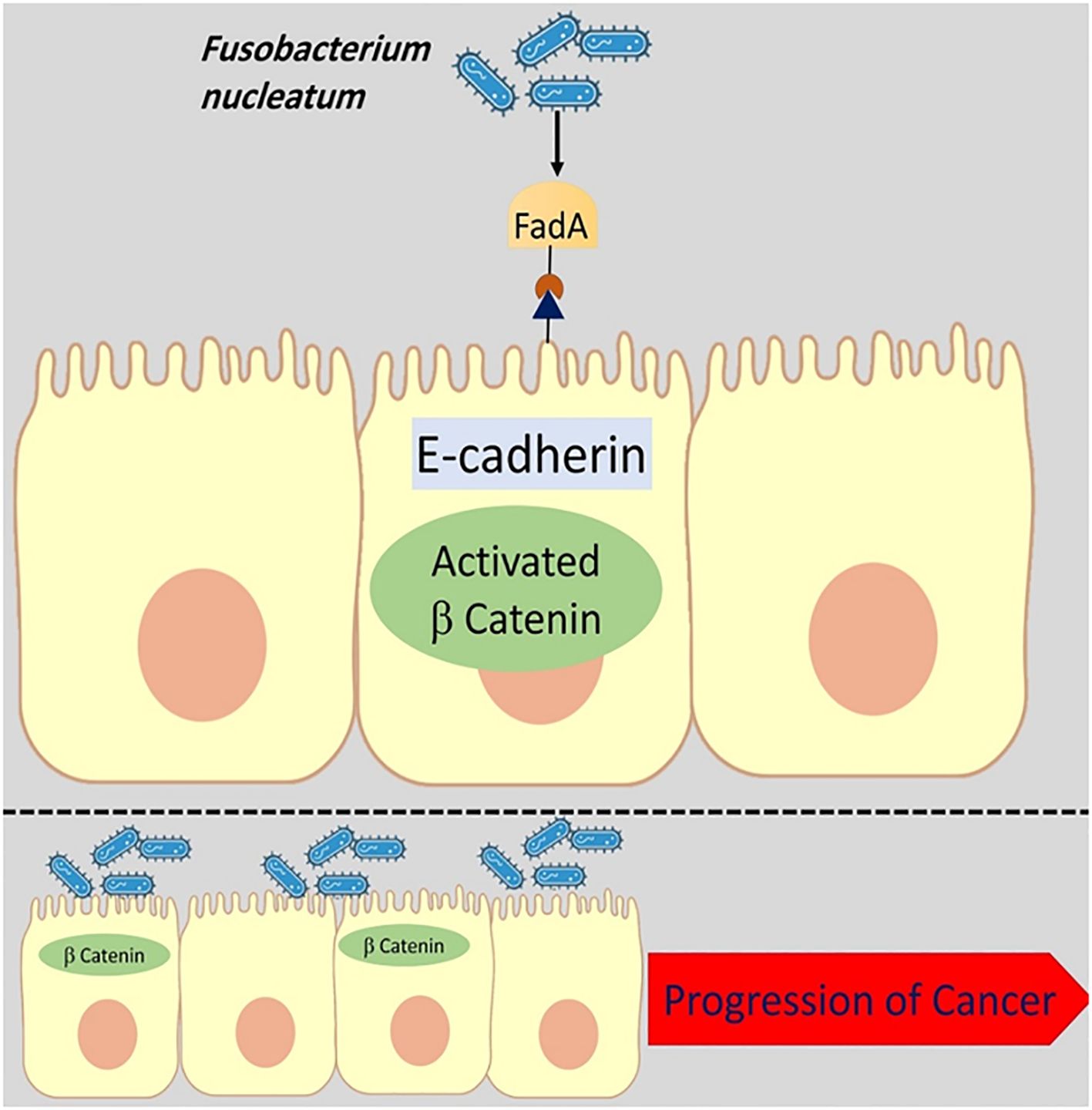
Figure 2. The binding of the FadA protein to E-cadherin upregulates the Annexin A1 expression and can stimulate carcinogenesis. The disruption of E-cadherin and β-catenin linkage leads to changes in the epithelial cell cytoskeletal structure and increases cancer progression.
In addition, the interaction of F. nucleatum FadA protein with E-cadherin leads to DNSA damage (DNA Double-Strand breaks (DSBs)) and has a key role in cancer development (Guo et al., 2020).
F. nucleatum Fap2 protein plays a significant role in attachment and instability of intestinal barriers. Fap2 can promote the entrance of F. nucleatum to intestinal epithelial cells (Luo et al., 2024). Furthermore, by interacting with tumor-specific sugar residue Gal-GalNAc, FadA and Fap2 proteins promote the attachment of F. nucleatum to biofilm and facilitate its localization and enrichment in CRC (Figure 3) (Zeddou, 2023). Fap2 binds to human immune inhibitory receptor T-cell immunoglobulin and ITIM domain, inhibiting natural killer cell (NK) cytotoxicity and modulating the tumor-immune microenvironment. Therefore, Fap2 can inhibit antitumor immunity in tumor microenvironment (Gur et al., 2015).
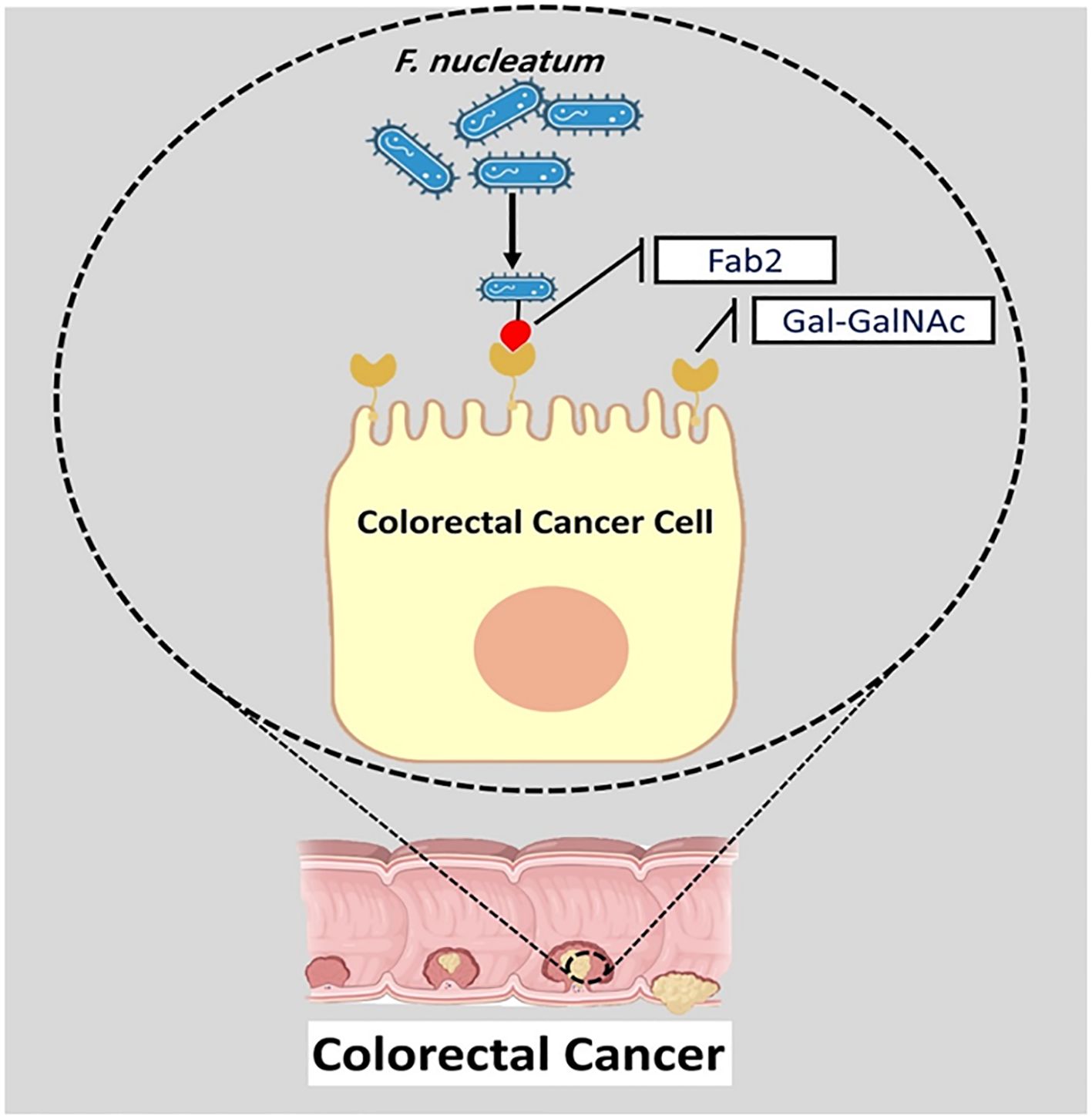
Figure 3. The interaction of Fap2 with tumor-specific sugar residue Gal-GalNAc promotes the attachment of F. nucleatum to biofilm and facilitates its localization and enrichment in CRC.
F. nucleatum has numerous adhesins on its surface, including RadD, Aid1, and FomA, that help in formation of bacterial co-aggregation and biofilm. In CRC, F. nucleatum dominant biofilms are a common finding, particularly when the cancer is on the right side of the colon (Liu et al., 2024).
M2 macrophages have a crucial role in tissue healing and inflammation. A previously published study stated that the interaction of F. nucleatum with a TLR4-dependent mechanism promotes the M2 polarization of macrophages. The promotion of M2 polarization of macrophages by F. nucleatum leads to immunosuppression in the tumor microenvironment (Chen et al., 2018; Mohammadi et al., 2022).
Butyrate is the main energy source for colonic cells and has anti-inflammatory and immunomodulatory effects. It inhibits tumor cell proliferation and migration, downregulates the Wnt signalling pathway, and limits tumor angiogenesis (Martin-Gallausiaux et al., 2024). Therefore, butyrate can inhibit tumor development in colonic cells. Wu et al. suggested that F. nucleatum can actively consume butyric acid, significantly reduce the concentration of butyrate in CRC cells, and promote CRC development. They presumed that F. nucleatum promotes the occurrence and development of CRC by reducing the population of butyrate-producing bacteria (Wu et al., 2024).
The combination of 5-Fluorouracil (5-Fu), irinotecan, or oxaliplatin is used as a standard treatment of advanced CRC (André et al., 2009). One of the primary causes of poor prognosis in CRC is chemoresistance. During the period of drug treatment, F. nucleatum upregulates the expression levels of Baculoviral IAP repeat-containing protein 3 (BIRC3) in CRC cells. An increase in expression levels of BIRC3 promotes chemoresistance to chemotherapy (Martin-Gallausiaux et al., 2024).
Moreover, F. nucleatum applies the miR18α, miR4802, MYD88 TLR-4, and unc-51-like kinase 1/autophagy related 7 autophagy networks to create and control chemoresistance in CRC cells (Figure 4) (Bostanghadiri et al., 2023).
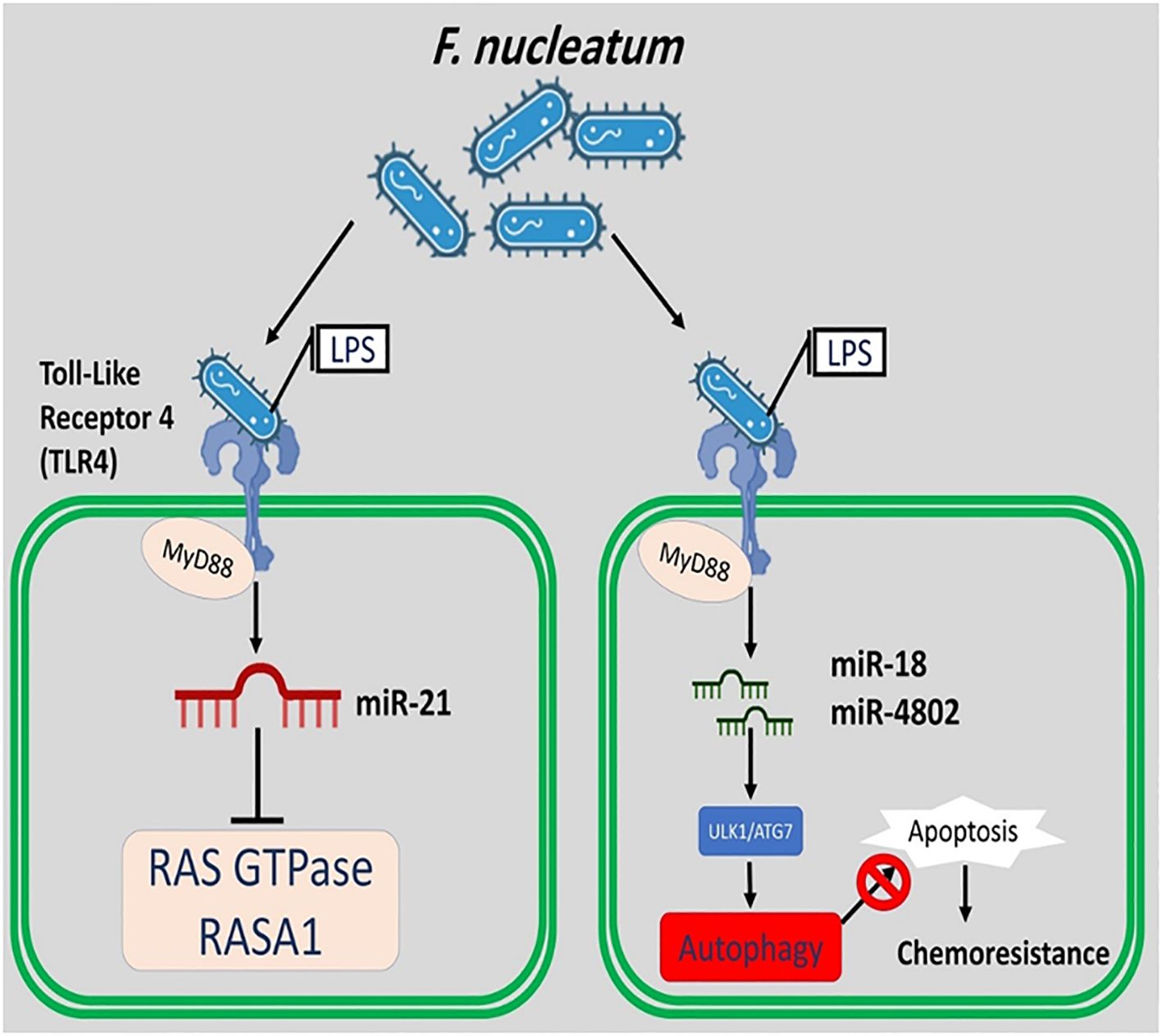
Figure 4. F. nucleatum applies the miR18, miR4802, MYD88, TLR-4, and unc-51-like kinase 1/autophagy related 7 autophagy networks to create and control chemoresistance in CRC cells. F. nucleatum induces the expression of miR-21 and suppresses the RAS GTPase and RASA1.
It is revealed that F. nucleatum infection can promote CRC cell metastasis by several mechanisms. This bacterium activates the autophagy signals, inhibits the N6-adenosine-methyltransferase 70 kDa subunit (METTL3) enzyme, induces the long non-coding RNA endogenous retroviral-associated adenocarcinoma lncRNA (EVADR), and initiates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Du et al., 2019; Chen et al., 2022; Liu et al., 2024).
ADP-heptose (ADP-H) is known as the pathogen-associated molecular pattern (PAMP) produced by F. nucleatum (Chen et al., 2022). Alpha kinase 1 (ALPK1) is the new pattern recognition receptor (PRR) that senses ADP-H. The binding of ADP-H to ALPK1 strongly activates nuclear factor-κB (NF-κB) in intestinal epithelial cells and induces the expression of inflammatory cytokines and anti-apoptotic genes (Milivojevic et al., 2017; Martin-Gallausiaux et al., 2022). It is revealed that the binding of ADP-H to ALPK1 increases the expression levels of IL-6, IL-8, IL-17, TNF-α, TLR-2, and TLR-4 genes in CRC cells. Moreover, this process reduces the chemosensitivity of CRC cells (Martin-Gallausiaux et al., 2024).
Previously published studies have identified a significant relationship between miR-21 expression and high levels of F. nucleatum in CRC cells. The expression of miR-21 is upregulated by hyperactive NF-kB binding to its promoter in CRC cells. This finding is partial support for the role of F. nucleatum in carcinogenesis by inducing miR-21. It is possible that F. nucleatum induces the expression of miR-21 and suppresses the Ras p21 protein activator 1 (RASA1) (a regulator of Ras GDP and GTP) (Bostanghadiri et al., 2023).
Both hydrogen sulfide (H2S) and sulfur-containing amino acids can initiate or promote the development of various cancers, such as CRC (Kim et al., 2016). F. nucleatum metabolizes sulfur-containing amino acids and produces H2S. Moreover, this bacterium can produce H2S from L-cysteine. H2S produced by F. nucleatum disrupts the colonic epithelial cell barrier and induces free radical-related DNA damage. It is revealed that the overproduction of H2S promotes the proliferation of cancer cells in CRC patients (Wang et al., 2023b).
Matrix metalloproteinase 7 (MMP7) is a significant member of the MMPs family and has a significant role in metastasis, tumor growth, and angiogenesis (Pezeshkian et al., 2021). MMP7 played a vital role in the activation of other MMPs, such as pro-MMP2 and pro-MMP9. It is revealed that F. nucleatum activates the MAPK (JNK) - AP1 axis (a transcription factor which targets MMP7) and upregulates the MMP7 expression. The overexpression of MMP7 in cancer cells promotes metastasis in colon epithelial cells (Ou et al., 2023).
Enterotoxigenic Bacteroides fragilis
Bacteroides fragilis (B. fragilis) is a gram-negative anaerobic bacterium that has been identified as a human colonic symbiont (Jo et al., 2023). B. fragilis constitutes 1% of the total intestinal microbiome, and 80% of children and adults are tested positive for this bacterium in the intestines (Dadgar-Zankbar et al., 2023). This bacterium is divided into two types: 1) enterotoxigenic B. fragilis (ETBF) and 2) non-enterotoxigenic types of B. fragilis. B. fragilis toxin (bft) is a 20 kDa zinc metalloprotease toxin with three isotypes, including 1) bft-1, 2) bft-2, and 3) bft-3 (Cheng et al., 2020). Published studies have shown that bft-2 is more carcinogenic than bft-1 (Dadgar-Zankbar et al., 2023). ETBF plays a main role in initiating and exacerbating gastrointestinal diseases such as CRC through multiple molecular processes (Qu et al., 2023).
E-cadherin is an important regulator of epithelial-mesenchymal transition (EMT) (Tabowei et al., 2022). Both in vitro and in vivo model studies have revealed that BFT can induce the cleavage of extracellular domain of the E-cadherin junction protein and lead to the disruption of E-cadherin and β-catenin linkage. The disruption of E-cadherin and β-catenin linkage leads to changes in epithelial cell cytoskeletal structure. It increases intestinal secretion and cell signalling via the β-catenin/Wnt pathway. Moreover, the cleavage of E-cadherin activates nuclear factor-κB (NF-κB) and promotes the production of inflammatory cytokines such as CXCL1 (C-X-C Motif Chemokine Ligand 1)(Figure 5) (Cheng et al., 2020; Khodaverdi et al., 2021). It is revealed that the disruption of E-cadherin and β-catenin linkage can stimulate the secretion of IL-8 and c-myc expression. C-myc is often persistently expressed in cancer cells. IL-8 can lead to activation of signal transducer and activator of transcription 3 (Stat3; regulators of various cell functions such as acute inflammation). Stat3 can act as an oncogene, constitutively activating it to promote chronic inflammation and tumorigenesis (Figure 6). Moreover, studies have stated that Stat3 can skew host immune responses towards tolerance in ETBF infections (Hwang et al., 2013; Purcell et al., 2022). BFT also produces reactive oxygen species (ROS) and causes oxidative DNA damage (Figure 5). Besides, ETBF stimulates the high expression of IL-17 and increases the risk of CRC (Khodaverdi et al., 2021). MicroRNAs (miRNAs) are noncoding and single-stranded RNAs with approximately 22 base pairs. Protein translational processes are regulated by miRNAs. Moreover, the molecular and cellular processes in cancer and inflammatory states are greatly influenced by miRNAs (Bartel, 2018; Slack and Chinnaiyan, 2019).
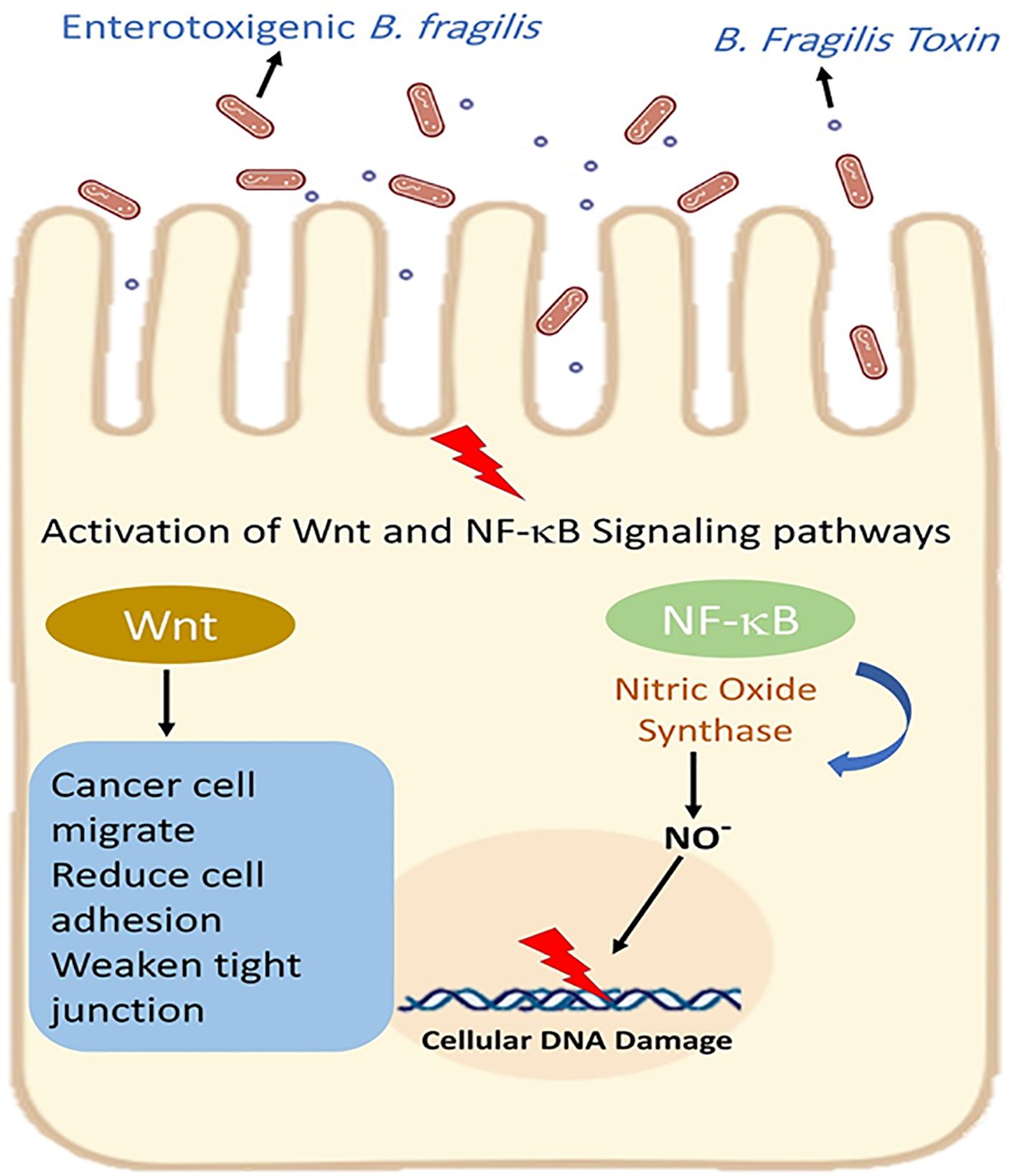
Figure 5. Enterotoxigenic B. fragilis increases intestinal secretion and cell signaling via the β-catenin/Wnt pathway. Moreover, BFT activates nuclear factor-κB (NF-κB) and promotes the production of inflammatory cytokines.
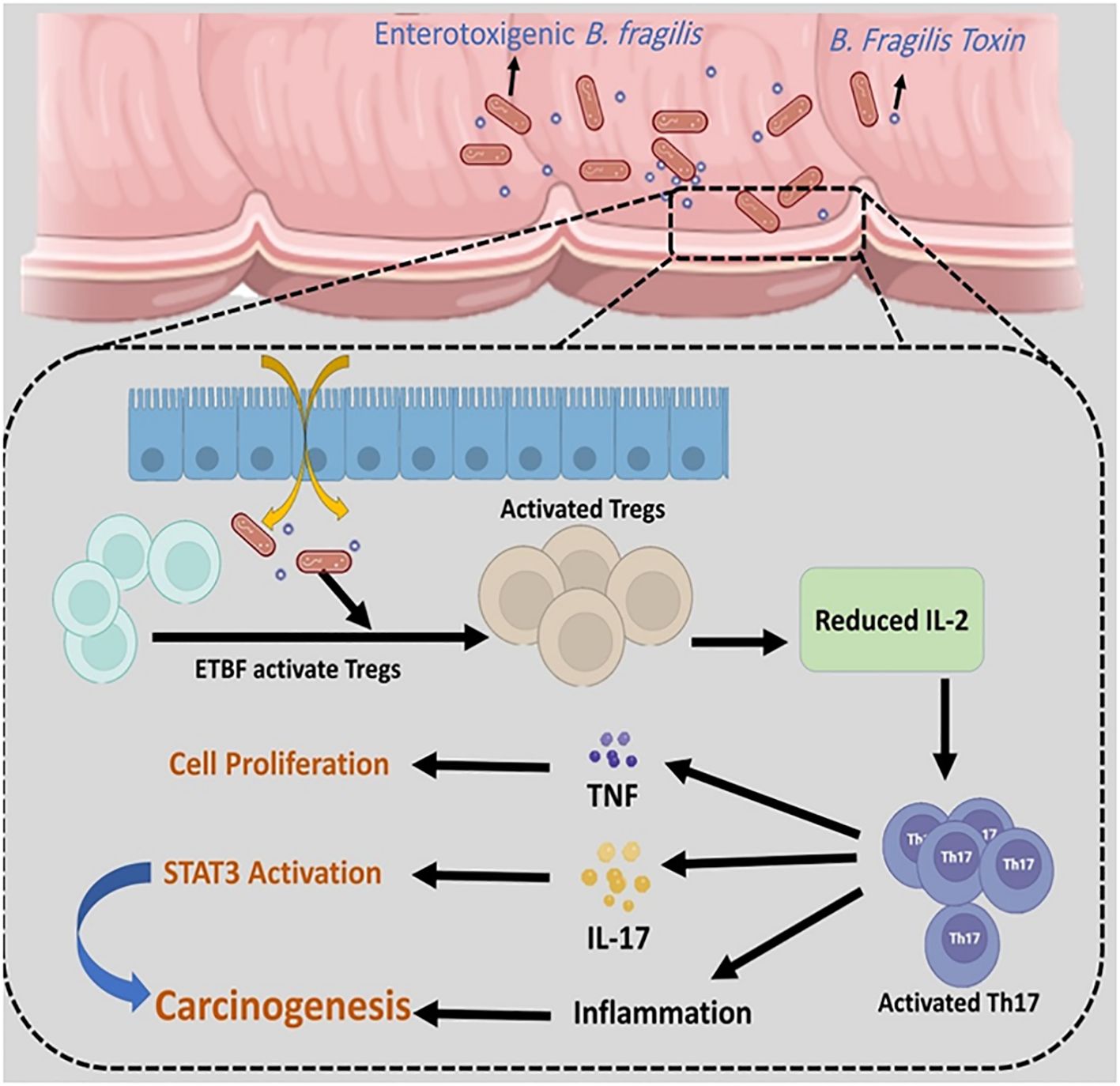
Figure 6. ETBF can activate regulatory T cells (Tregs) and stimulate the high expression of Th17. The overexpression of Th17 leads to an increase in TNF-α, IL-17, and inflammation. STAT3 can act as an oncogene, and constitutively activating it promotes chronic inflammation and tumorigenesis.
Another carcinogenic process related to ETBF is the downregulation of miR-149-3p. It is revealed that ETBF downregulates the miR-149-3p and promotes intestinal inflammation and malignancy in CRC cells (Cao et al., 2021). Moreover, animal model studies have shown that ETBF can accelerate colitis and carcinogenesis related to dextran sulfate sodium (DSS) and azoxymethane (AOM). However, the molecular mechanism of this process remains unclear (Tabowei et al., 2022).
Veillonella parvula
Veillonella parvula (V. parvula) is a strictly gram-negative anaerobic coccus-shaped bacterium belonging to the genus Veillonella. This bacterium is a member of normal microbiota in human intestinal, oral, and respiratory tract (Qian et al., 2023). V. parvula uses nitrate respiration to colonize the intestine during the inflammatory response. Therefore, V. parvula could play a significant role in different digestive diseases such as CRC (Rojas-Tapias et al., 2022). This bacterium increases B lymphocyte stimulator (BLyS) expression level and B-cell infiltration in CRC patients (Qian et al., 2023). Studies have indicated that an increase in BLyS expression level leads to an increase in the level of B cells in tumor tissues (Du et al., 2022; Qian et al., 2023). It is presumed that V. parvula can exert an immunomodulatory effect in CRC cells. CRC patients with high fecal V. parvula abundance are more likely to develop an advanced tumor stage, higher rates of lymph node metastasis, and a lower prognosis (Qian et al., 2023).
Prevotella
Colorectal adenomas (CRAs) are precancerous lesions present in 30–40% and <20% of individuals aged ≥ 70 years and ≤ 50 years, respectively[50]. Prevotella intermedia (P. intermedia) is an anaerobic bacterium that can transform CRAs into colorectal cancer (CRC). Previously published studies have revealed that this bacterium can increase cell migration and invasion in CRC cells[7, 50, 51]. The gut microbial metabolite formate plays a key role in activating aryl hydrocarbon receptor (AhR) signalling [52]. AhR is a ligand-activated transcription factor and a receptor for multiple physiological ligands[53]. Interestingly, P. intermedia utilizes several enzymes to produce formate. Furthermore, this bacterium metabolizes glucose for formate formation. The formate produced by P. intermedia induces colon cancer cell invasion and leads to metastatic dissemination in CRC[50, 54]. Autocrine motility factor (AMF), also known as glucose-6-phosphate isomerase (GPI), secreted by tumor cells, drives epithelial-mesenchymal transition, and stimulates invasion and metastasis in various cancers, including CRC[55]. It is supposed that P. intermedia secretes the AMF molecule and ultimately promotes CRC cell motility and metastasis [56, 57].
Clostridioides difficile
Clostridioides difficile (C. difficile) is an anaerobic gram-positive spore-forming bacterium. 5-15% of the adult population is tested positive for this bacterium as a normal gut microbiota (Tirelli et al., 2023). Most patients infected with this bacterium are asymptomatic or have limited to mild diarrhea. However, C. difficile can cause different forms of infectious diseases, ranging from infectious diarrhea (antibiotic-associated diarrhea), pseudomembranous colitis, and fulminant colitis (toxic megacolon). Pseudomembranous colitis and fulminant colitis are seen in 25% and 1-3% of patients, respectively (Magat et al., 2020; Tirelli et al., 2023). Several studies have reported that infection by toxin-producing C. difficile has a potential role in disruption of gut microbiome and leads to tissue damage and malignant transformation of cells in the colon (Jahani-Sherafat et al., 2019; Kaźmierczak-Siedlecka et al., 2020). This bacterium can produce three toxins, including TcdA (C. difficile Toxin A), TcdB (C. difficile Toxin A), and cdtA/B (C. difficile binary toxin A and B). In comparison to TcdA, TcdB is an important toxin and has more toxic effects on colon cells in C. difficile infection (CDI) (Dicks et al., 2019).
It is proven that TcdB toxin induces the inflammatory response, deregulates Rho-GTPases, and increases the expression levels of proto-oncogenes in colon (Jank et al., 2007). Briefly, TcdB binds to its specific receptors, and the toxin-receptor complex enters the host cells by endocytosis. At the next step, TcdB passes from the acidic endosomal membrane and is translocated into the cytosol. Finally, the active part of the TcdB toxin (glucosyltransferase) is released into the cytosol. Glucosyltransferase transfers glucose molecules into Rho proteases and prevents the normal binding of Guanosine-5’-triphosphate (GTP) to Guanosine diphosphate (GDP) bound form of Rho protein (Fettucciari et al., 2023). Therefore, the TcdB toxin can inactivate the Rho protein (Jena et al., 2013; Dicks et al., 2019). Inactivation of the Rho protein stimulates pro-inflammatory interleukin production, tumor necrosis factor (TNF) activation, increases vascular permeability, and finally can lead to the growth and proliferation of tumor cells in CRC cells (Dicks et al., 2019). Phospholipase C-γl (PLC-γl) is an important signalling molecule, and overexpression of PLC-γl is related to cellular transformation (Nam et al., 2012). It is shown that the expression of PLC-γl is upregulated in CRC cells and breast carcinoma. TcdA can induce apoptosis in PLC-γl-transformed cells. Moreover, TcdA can upregulate the expression levels of BIM (a novel member of the BCL-2 family that promotes apoptosis) and activation of caspase-3 in PLC-γl-transformed cells. The high upregulation of PLC-γl by TcdA suggests that this toxin may have good potential as an anticancer agent against different malignancies such as CRC (Warny et al., 2000; Nam et al., 2012).
Clostridium perfringens
Clostridium perfringens (C. perfringens) is a gram-positive, anaerobic spore-forming bacterium belonging to the Clostridiaceae family (Huang and Wang, 2020). This bacterium can be isolated from sewage, soil, human genital (reproductive) and urinary systems, and the intestinal tract (Kohya et al., 2022). C. perfringens is a causative agent of food poisoning after trauma and gas gangrene. This bacterium could lead to death in a short time in patients with cancer or immunocompromised individuals (Huang and Wang, 2020). C. perfringens has the highest frequency in patients with CRC, pancreatic cancer and gastric cancer, respectively (Kohya et al., 2022). First-line treatment for peptic gastrointestinal disorders involves proton pump inhibitors (PPIs). PPIs have been proven to inhibit colon cancer cell growth and carcinogenesis (Zeng et al., 2016; Sasaki et al., 2020). By suppressing the overexpressed protein kinase in T-LAK cells in various cancers, PPIs can inhibit the development and progression of cancer. Furthermore, membrane-bound ATP-binding cassette transporters are suppressed by PPIs, which can reduce drug resistance in cancer (Sasaki et al., 2020). It is revealed that long-term PPI use initiates and exacerbates the C. perfringens infection in intestinal tract. C. perfringens enterotoxin could impair Claudin-4 (CLDN4), which forms tight junctions in colorectal cells and enhances cancer malignancy (Sasaki et al., 2020). Moreover, this bacterium activates the Yes-associated protein (YAP) and promotes cancer progression (Fujiwara-Tani et al., 2018).
Clostridium septicum
Clostridium septicum (C. septicum) is an anaerobic spore-forming gram-positive rod-shaped bacterium belonging to the Clostridium family (Lintin et al., 2014). This bacterium is a highly virulent pathogen that produces different toxins and has been linked to hematological malignancy, colorectal malignancy, cyclical neutropenia, diabetes mellitus, and immunosuppression. (Nanjappa et al., 2015) Overall, 50 to 85% of C. septicum infections are associated with underlying malignancy (Kennedy et al., 2005). Anaerobic and spore-forming abilities allow C. septicum to flourish in the gut. It enters the bloodstream directly after passing through the mucosal layer (Lintin et al., 2014). Nevertheless, the pathophysiology behind how this bacterium promotes colonic malignancy is still unclear. A previously published study pointed out that C. septicum and UshA genotoxin in Escherichia coli may follow a similar pathogenesis. It is presumed that these bacteria, via DNA damage and defects in cellular DNA repair processes, can lead to carcinogenesis in the colon (Abraham and Padam, 2023). Spore germination, active C. septicum infection, and toxin production are done in hypoxic and acidic conditions. Therefore, according to the presence of this condition (anaerobic glycolysis) in tumor tissue, the tumor environment is suitable for the formation of active infection (Jessamy et al., 2016; Justesen et al., 2022). Furthermore, blood supply is relatively low in tumor tissue and this condition could prepare the environment for C. septicum proliferation (Justesen et al., 2022). C. septicum has several toxins and enzymes such as hyaluronidase, fibrinolysin, deoxyribonuclease, and hemolysins, which enable bacteria to metastasize and invade tissues in the colon (Bickerton et al., 2022).
Actinomyces
Actinomycetes are a heterogeneous group of gram-positive bacteria commonly found in the human digestive and genital tract, and oral cavity (Fukunaga et al., 2024). The bacteria belonging to the Actinomycetes can cause severe infections in the peritoneal cavity, abdominal wall, face, neck and chest (Acquaro et al., 2010; Valour et al., 2014). Actinomyces israelii (A. israelii) is a rod-shaped anaerobic gram-positive bacterium and is considered as the most common pathogen among Actinomycetes (Fukunaga et al., 2024). Actinomycosis is a chronic suppurative and granulomatous disease that is caused by A. israelii (Valour et al., 2014). A few studies have presumed that A. israelii could have a role in initiation or exacerbation of CRC (Fukunaga et al., 2024). There are three conceivable reasons for inflammation and perforation of gastrointestinal tract caused by this bacterium; 1) this bacterium can disrupt the intestinal wall and enter the abdominal cavity; 2) during the surgery for local recurrence, it’s possible that A. israelii enters the abdominal cavity; 3) injuries to the oral cavity or gastrointestinal tract could have allowed organisms to enter the bloodstream (Yang and Im, 2018; Fukunaga et al., 2024).
Actinomyces odontolyticus (A. odontolyticus) is another anaerobic bacterium in human oral cavity and gastrointestinal tract (Könönen and Wade, 2015). Previously published studies have reported that this bacterium is frequently isolated from stool samples of patients with early-stage CRC (Yachida et al., 2019; Breau, 2024). It is revealed that A. odontolyticus plays a main role in colorectal dysplasia. This bacterium secretes membrane vesicles (MVs) and induces mitochondrial dysfunction and excessive reactive oxygen species (ROS) production. ROS production can lead to DNA damage and cellular transformation in colonic epithelium (Miyakawa et al., 2024). Furthermore, MVs enriched for lipoteichoic acid (LTA) specifically binds with the TLR2 receptor and activates the NF-kB signaling pathway (Breau, 2024).
Peptostreptococcus
Peptostreptococcus anaerobius (P. anaerobius) is an anaerobic gram-positive coccus and is considered as a member of the human oral and gut normal microbiota (Gu et al., 2023). This bacterium can cause several infectious diseases, including endocarditis, periodontal disease, genitourinary and gastrointestinal tract infections (Tsoi et al., 2017). Results of studies have shown that this bacterium was significantly present in stool and mucosal samples of patients with CRC (Yu et al., 2017; Brennan and Garrett, 2019; Cheng et al., 2020). The interaction of P. anaerobius with TLR-2 and TLR-4 elevates ROS production in colon cells and leads to CRC tumorigenesis (Tsoi et al., 2017). The increases in the level of intracellular ROS enhance the biosynthesis of cholesterol (Tsoi et al., 2017). Briefly, P. anaerobius facilitates the activity of sterol regulatory element-binding protein 2 (SREBP-2). SREBP2 is a key regulator of cholesterol biosynthesis, and this molecule controls the expression levels of genes involved in the synthesis of cholesterol. Therefore, P. anaerobius promotes ROS production and cholesterol biosynthesis and enhances cell proliferation and tumorigenesis in CRC (Tsoi et al., 2017; Huang et al., 2024).
Myeloid-derived suppressor cells (MDSCs) are known as the main immune suppressant cells in tumor microenvironment and are linked to cancer progression (Liu et al., 2024). It is presumed that the intratumoral bacteria play a main role in accumulation of MDSCs in CRC cells (Gu et al., 2023). Results of a study performed on the mouse models revealed that P. anaerobius colonization increases MDSCs abundances in tumor microenvironment (Figure 7) (Liu et al., 2024). The presence of P. anaerobius in tumor microenvironment stimulates the secretion of chemokine (C-X-C motif) ligand 1 (CXCL1) (Long et al., 2019; Liu et al., 2024). CXCL1 is a main chemokine in the recruitment and activation of neutrophils for microbial killing. This chemokine plays a main role in host immune response. CXCL1 participates in the chemotaxis of CXCR2 (C-X-C Motif Chemokine Receptor 2) + MDSCs. The accumulation of MDSCs in tumor microenvironment can antagonistically affect antitumor CD8+ and CD4+ T cells (Long et al., 2019; Gu et al., 2023). Moreover, it is revealed that the recruitment of MDSCs into tumor microenvironment increases the chemoresistance of colorectal cancer to oxaliplatin and mediates anti-PD1 therapy resistance (Liu et al., 2024).
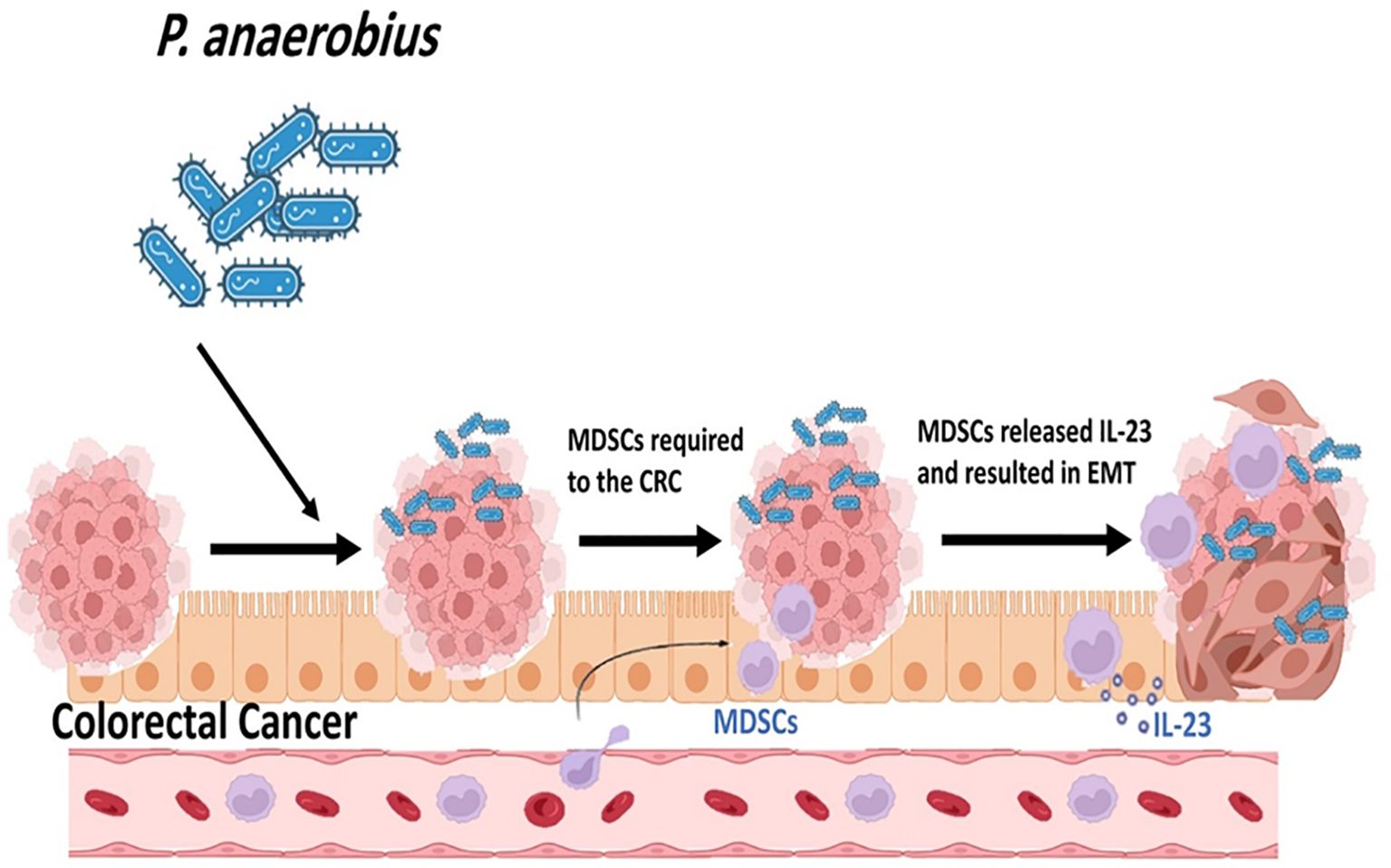
Figure 7. P. anaerobius colonization increases Myeloid-derived suppressor cells' (MDSCs) abundances in tumor microenvironment.
The putative cell wall binding repeat 2 (PCWBR2) is a surface protein of P. anaerobius. The direct interaction of PCWBR2 with epithelial cell receptor integrin α2/β1 leads to the activation of B cells (NF-κB) cascade and ultimately promotes cell proliferation and pro-inflammatory immune responses in CRC cells (Long et al., 2019).
Peptostreptococcus stomatis (P. stomatis) is another species of Peptostreptococcus spp. that is frequently isolated from patients with CRC (Osman et al., 2021). Results of a previously published animal model study indicated that P. stomatis can activate the erb-b2 receptor tyrosine kinase 2 (ERBB2)-mitogen-activated protein kinase (MAPK) and promote colonic tumorigenesis (Huang et al., 2024). This bacterium applies its surface protein fructose-1,6-bisphosphate aldolase (FBA) to become attached to CRC cells. The attachment of P. `stomatis to CRC cells leads to the activation of ERBB2 and downstream of Ras/Raf/MAPK (MEK)- extracellular signal-regulated kinase (ERK) cascades (Figure 8) (Shen et al., 2021). Moreover, it is revealed that P. stomatis can inactivate and inhibit the BRAF inhibitor (a member of RAF serine/threonine kinases) (vemurafenib) and receptor tyrosine kinase (RTK) inhibitor (cetuximab, erlotinib), leading to the non-responsiveness to these drugs in CRC. This bacterium can suppress apoptosis and impair the gut barrier function in mouse models of CRC (Osman et al., 2021; Huang et al., 2024).
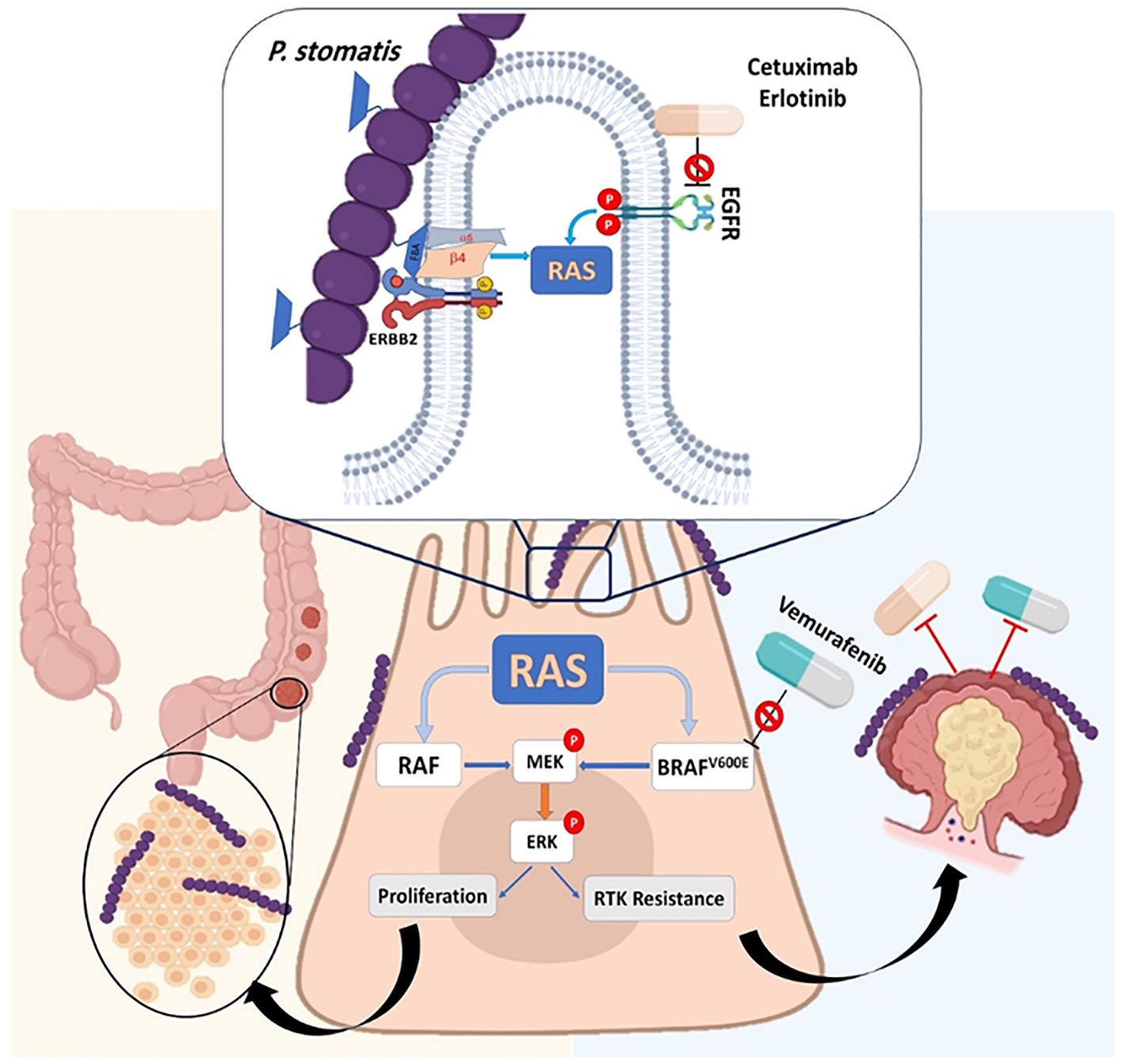
Figure 8. P. stomatis can activate the erb-b2 receptor tyrosine kinase 2 (ERBB2)-mitogen-activated protein kinase (MAPK) and promote colonic tumorigenesis. This bacterium applies its surface protein fructose-1,6-bisphosphate aldolase (FBA) to attachment on the CRC cells. The attachment of P. stomatis to CRC cells leads to the activation of ERBB2 and downstream Ras/Raf/MAPK (MEK)- extracellular signal-regulated kinase (ERK) cascades. P. stomatis can inactivate and inhibit the BRAF inhibitor (a member of RAF serine/threonine kinases) (vemurafenib) and receptor tyrosine kinase (RTK) inhibitor (cetuximab, erlotinib), leading to non-responsiveness to these drugs in CRC.
Conclusion
In general, the exact role of anaerobic gut bacteria in initiation, exacerbation and development of human CRC is not completely identified. In the present study, we searched various databases and gathered the results of previously published studies and clinical and epidemiological signs and the possible mechanisms of some anaerobic gut bacteria in CRC were shown. Different studies have revealed that many anaerobic gut bacteria including Fusobacterium nucleatum, C. difficile, Enterotoxigenic B. fragilis, P. anaerobius, P. stomatis, etc. could contribute to initiation, development, and exacerbation of CRC in animal models and human. However, more animal models and clinical trial studies are required for a precise conclusion regarding the role of anaerobic gut bacteria in CRC development.
Author contributions
SS: Conceptualization, Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. HS: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. HG: Data curation, Investigation, Software, Writing – original draft. MA: Conceptualization, Data curation, Software, Writing – original draft. TA: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This work is part of the PhD. thesis of Sahar Sabour, which was approved by the Infectious and Tropical Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Moreover, we would like to thank “The Department of Bacteriology & Virology, School of Medicine, Shiraz University of Medical Science, Shiraz, Iran” for their kind cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abraham, A. T. and Padam, S. (2023). Clostridium septicum bacteremia as the presenting sign of colon cancer. Cureus 15, e45343. doi: 10.7759/cureus.45343
Acquaro, P., Tagliabue, F., Confalonieri, G., Faccioli, P., and Costa, M. (2010). Abdominal wall actinomycosis simulating a Malignant neoplasm: Case report and review of the literature. World J. Gastrointest Surg. 2, 247–250. doi: 10.4240/wjgs.v2.i7.247
André, T., Boni, C., Navarro, M., Tabernero, J., Hickish, T., Topham, C., et al. (2009). Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 27, 3109–3116. doi: 10.1200/JCO.2008.20.6771
Araújo, T. G., Mota, S. T. S., Ferreira, H. S. V., Ribeiro, M. A., Goulart, L. R., and Vecchi, L. (2021). Annexin A1 as a regulator of immune response in cancer. Cells 10, 2245. doi: 10.3390/cells10092245
Azimi, T., Nasiri, M. J., Chirani, A. S., Pouriran, R., and Dabiri, H. (2018). The role of bacteria in the inflammatory bowel disease development: a narrative review. Apmis 126, 275–283. doi: 10.1111/apm.12814
Azimi, T., Nasser, A., Shariati, A., Shiadeh, S. M., Safari, H., Alizade-Sani, M., et al. (2020). The possible role of pathogenic and non-pathogenic bacteria in initiation and exacerbation of celiac disease; a comprehensive review. Curr. Pharm. Biotechnol. 21, 452–466. doi: 10.2174/1389201021666191219160729
BGI Genomics (2024). Global State of Colorectal Cancer Awareness Report (BGI Center). Available online at: https://www.bgi.com/global/news/bgi-genomics-2024-global-state-of-colorectal-cancer-awareness-report.
Bickerton, S., Awopetu, A., Abood, A., Lee, H., Lane, T., and Reid, A. (2022). Atraumatic Clostridium septicum myonecrosis presenting as upper limb ischaemia in a patient with undiagnosed bowel cancer. Ann. R Coll. Surg. Engl. 104, e95–e97. doi: 10.1308/rcsann.2021.0153
Bostanghadiri, N., Razavi, S., Shariati, A., Talebi, M., Mirkalantari, S., Emami Razavi, A., et al. (2023). Exploring the interplay between Fusobacterium nucleatum with the expression of microRNA, and inflammatory mediators in colorectal cancer. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1302719
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. doi: 10.3322/caac.21834
Breau, K. A. (2024). Actinomyces odontolyticus: from carries to colorectal cancer. Cell Mol. Gastroenterol. Hepatol. 17, 879–880. doi: 10.1016/j.jcmgh.2024.02.009
Brennan, C. A. and Garrett, W. S. (2019). Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166. doi: 10.1038/s41579-018-0129-6
Cao, W., Qin, K., Li, F., and Chen, W. (2024). Comparative study of cancer profiles between 2020 and 2022 using global cancer statistics (GLOBOCAN). J. Natl. Cancer Cent. 4, 128–134. doi: 10.1016/j.jncc.2024.05.001
Cao, Y., Wang, Z., Yan, Y., Ji, L., He, J., Xuan, B., et al. (2021). Enterotoxigenic Bacteroides fragilis promotes intestinal inflammation and Malignancy by inhibiting exosome-packaged miR-149-3p. Gastroenterology 161, 1552–1566.e12. doi: 10.1053/j.gastro.2021.08.003
Chen, T., Li, Q., Wu, J., Wu, Y., Peng, W., Li, H., et al. (2018). Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 67, 1635–1646. doi: 10.1007/s00262-018-2233-x
Chen, S., Zhang, L., Li, M., Zhang, Y., Sun, M., Wang, L., et al. (2022). Fusobacterium nucleatum reduces METTL3-mediated m6A modification and contributes to colorectal cancer metastasis. Nat. Commun. 13, 1248. doi: 10.1038/s41467-022-28913-5
Cheng, W. T., Kantilal, H. K., and Davamani, F. (2020). The mechanism of Bacteroides fragilis toxin contributes to colon cancer formation. Malays J. Med. Sci. 27, 9–21. doi: 10.21315/mjms2020.27.4.2
Cheng, W., Li, F., and Yang, R. (2024). The roles of gut microbiota metabolites in the occurrence and development of colorectal cancer: Multiple insights for potential clinical applications. Gastro Hep Adv. 3, 855–870. doi: 10.1016/j.gastha.2024.05.012
Cheng, Y., Ling, Z., and Li, L. (2020). The intestinal microbiota and colorectal cancer. Front. Immunol. 11. doi: 10.3389/fimmu.2020.615056
Conde-Pérez, K., Aja-Macaya, P., Buetas, E., Trigo-Tasende, N., Nasser-Ali, M., Rumbo-Feal, S., et al. (2024). The multispecies microbial cluster of Fusobacterium, Parvimonas, Bacteroides and Faecalibacterium as a precision biomarker for colorectal cancer diagnosis. Mol. Oncol. 18, 1093–1122. doi: 10.1002/1878-0261.13604
Dadgar-Zankbar, L., Shariati, A., Bostanghadiri, N., Elahi, Z., Mirkalantari, S., Razavi, S., et al. (2023). Evaluation of enterotoxigenic Bacteroides fragilis correlation with the expression of cellular signaling pathway genes in Iranian patients with colorectal cancer. Infect. Agent Cancer 18, 48. doi: 10.1186/s13027-023-00523-w
Dicks, L. M., Mikkelsen, L. S., Brandsborg, E., and Marcotte, H. (2019). Clostridium difficile, the difficult “Kloster” fuelled by antibiotics. Curr. Microbiol. 76, 774–782. doi: 10.1007/s00284-018-1543-8
Du, X., Li, Q., Tang, Z., Yan, L., Zhang, L., Zheng, Q., et al. (2022). Alterations of the gut microbiome and fecal metabolome in colorectal cancer: implication of intestinal metabolism for tumorigenesis. Front. Physiol. 13. doi: 10.3389/fphys.2022.854545
Du, F., Qiao, C., Li, X., Chen, Z., Wu, S., Hu, S., et al. (2019). Forkhead box K2 promotes human colorectal cancer metastasis by upregulating ZEB1 and EGFR. Theranostics 9, 3879–3902. doi: 10.7150/thno.31716
Eskandari-Malayeri, F., Rezeai, M., Narimani, T., Esmaeil, N., and Azizi, M. (2024). Investigating the effect of Fusobacterium nucleatum on the aggressive behavior of cancer-associated fibroblasts in colorectal cancer. Discov. Oncol. 15, 292. doi: 10.1007/s12672-024-01156-0
Fettucciari, K., Fruganti, A., Stracci, F., Spaterna, A., Marconi, P., and Bassotti, G. (2023). Clostridioides difficile toxin B induced senescence: A new pathologic player for colorectal cancer? Int. J. Mol. Sci. 24, 8155. doi: 10.3390/ijms24098155
Fujiwara-Tani, R., Sasaki, T., Luo, Y., Goto, K., Kawahara, I., Nishiguchi, Y., et al. (2018). Anti-claudin-4 extracellular domain antibody enhances the antitumoral effects of chemotherapeutic and antibody drugs in colorectal cancer. Oncotarget. 9, 37367–37378. doi: 10.18632/oncotarget26427:37367-37378
Fukunaga, Y., Maeda, H., Yamaguchi, S., Tsutsui, M., Okamoto, K., Tanaka, T., et al. (2024). An actinomycosis infection resembling peritoneal dissemination of rectal cancer: a case report. Surg. Case Rep. 10, 207. doi: 10.1186/s40792-024-02005-6
Gu, J., Lv, X., Li, W., Li, G., He, X., Zhang, Y., et al. (2023). Deciphering the mechanism of Peptostreptococcus anaerobius-induced chemoresistance in colorectal cancer: The important roles of MDSC recruitment and EMT activation. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1230681
Guo, P., Tian, Z., Kong, X., Yang, L., Shan, X., Dong, B., et al. (2020). FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J. Exp. Clin. Cancer Res. 39, 202. doi: 10.1186/s13046-020-01677-w
Gur, C., Ibrahim, Y., Isaacson, B., Yamin, R., Abed, J., Gamliel, M., et al. (2015). Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355. doi: 10.1016/j.immuni.2015.01.010
Hong, B.-Y., Chhaya, A., Robles, A., Cervantes, J., and Tiwari, S. (2024). The role of Fusobacterium nucleatum in the pathogenesis of colon cancer. J. Investig. Med. 72, 819–827. doi: 10.1177/10815589241277829
Huang, P., Ji, F., Cheung, A. H.-K., Fu, K., Zhou, Q., Ding, X., et al. (2024). Peptostreptococcus stomatis promotes colonic tumorigenesis and receptor tyrosine kinase inhibitor resistance by activating ERBB2-MAPK. Cell Host Microbe 32, 1365–1379.e10. doi: 10.1016/j.chom.2024.07.001
Huang, C.-Y. and Wang, M.-C. (2020). Clostridium perfringens bacteremia associated with colorectal cancer in an elderly woman. Turk J. Gastroenterol. 31, 960–961. doi: 10.5152/tjg.2020.19987
Hwang, S., Gwon, S.-Y., Kim, M. S., Lee, S., and Rhee, K.-J. (2013). Bacteroides fragilis toxin induces IL-8 secretion in HT29/C1 cells through disruption of E-cadherin junctions. Immune Netw. 13, 213–217. doi: 10.4110/in.2013.13.5.213
Jahani-Sherafat, S., Azimirad, M., Alebouyeh, M., Amoli, H. A., Hosseini, P., Ghasemian-Safaei, H., et al. (2019). The rate and importance of Clostridium difficile in colorectal cancer patients. Gastroenterol. Hepatol. Bed Bench. 12, 358–363.
Jank, T., Giesemann, T., and Aktories, K. (2007). Rho-glucosylating Clostridium difficile toxins A and B: new insights into structure and function. Glycobiology 17, 15R–22R. doi: 10.1093/glycob/cwm004
Jena, P. K., Trivedi, D., Chaudhary, H., Sahoo, T. K., and Seshadri, S. (2013). Bacteriocin PJ4 active against enteric pathogen produced by Lactobacillus helveticus PJ4 isolated from gut microflora of wistar rat (Rattus norvegicus): partial purification and characterization of bacteriocin. Appl. Biochem. Biotechnol. 169, 2088–2100. doi: 10.1007/s12010-012-0044-7
Jessamy, K., Ojevwe, F. O., Ubagharaji, E., Sharma, A., Anozie, O., Gilman, C. A., et al. (2016). Clostridium septicum: an unusual link to a lower gastrointestinal bleed. Case Rep. Gastroenterol. 10, 489–493. doi: 10.1159/000448881
Jo, M., Hwang, S., Lee, C.-G., Hong, J.-E., Kang, D.-H., Yoo, S.-H., et al. (2023). Promotion of colitis in B cell-deficient C57BL/6 mice infected with enterotoxigenic bacteroides fragilis. Int. J. Mol. Sci. 25, 364. doi: 10.3390/ijms25010364
Justesen, U. S., Nielsen, S. L., Jensen, T. G., Dessau, R. B., Møller, J. K., Coia, J. E., et al. (2022). Bacteremia with anaerobic bacteria and association with colorectal cancer: a population-based cohort study. Clin. Infect. Dis. 75, 1747–1753. doi: 10.1093/cid/ciac259
Kajihara, T., Yahara, K., Kitamura, N., Hirabayashi, A., Hosaka, Y., and Sugai, M. (2023). Distribution, trends, and antimicrobial susceptibility of Bacteroides, Clostridium, Fusobacterium, and Prevotella species causing bacteremia in Japan during 2011–2020: a retrospective observational study based on national surveillance data. Open Forum Infect. Dis. 10, ofad334. doi: 10.1093/ofid/ofad334
Kaźmierczak-Siedlecka, K., Daca, A., Fic, M., van de Wetering, T., Folwarski, M., and Makarewicz, W. (2020). Therapeutic methods of gut microbiota modification in colorectal cancer management–fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 11, 1518–1530. doi: 10.1080/19490976.2020.1764309
Kennedy, C. L., Krejany, E. O., Young, L. F., O’Connor, J. R., Awad, M. M., Boyd, R. L., et al. (2005). The α-toxin of Clostridium septicum is essential for virulence. Mol. Microbiol. 57, 1357–1366. doi: 10.1111/j.1365-2958.2005.04774.x
Khodaverdi, N., Zeighami, H., Jalilvand, A., Haghi, F., and Hesami, N. (2021). High frequency of enterotoxigenic Bacteroides fragilis and Enterococcus faecalis in the paraffin-embedded tissues of Iranian colorectal cancer patients. BMC Cancer 21, 1353. doi: 10.1186/s12885-021-09110-x
Kim, M.-J., Yoon, J.-H., and Ryu, J.-H. (2016). Mitophagy: a balance regulator of NLRP3 inflammasome activation. BMB Rep. 49, 529–535. doi: 10.5483/BMBRep.2016.49.10.115
Kohya, R., Murai, T., Taguchi, Y., Sawai, K., Takehara, M., Nagahama, M., et al. (2022). An autopsy case of rapidly aggravated clostridium perfringens septicemia with colorectal cancer. Case Rep. Infect. Dis. 2022, 1071582. doi: 10.1155/2022/1071582
Koliarakis, I., Lagkouvardos, I., Vogiatzoglou, K., Tsamandouras, I., Intze, E., Messaritakis, I., et al. (2024). Circulating bacterial DNA in colorectal cancer patients: the potential role of fusobacterium nucleatum. Int. J. Mol. Sci. 25, 9025. doi: 10.3390/ijms25169025
Könönen, E. and Wade, W. G. (2015). Actinomyces and related organisms in human infections. Clin. Microbiol. Rev. 28, 419–442. doi: 10.1128/CMR.00100-14
Lintin, L., Wheeler, R., Whiston, R., Gordon, A., Berry, D., and Torkington, J. (2014). Mycotic thoracic aortic arch aneurysm from haematogenous spread of Clostridium septicum due to metastatic colorectal cancer: a survival guide. J. Surg. Case Rep. 2014, rju117. doi: 10.1093/jscr/rju117
Liu, Y., Wong, C. C., Ding, Y., Gao, M., Wen, J., Lau, H. C.-H., et al. (2024). Peptostreptococcus anaerobius mediates anti-PD1 therapy resistance and exacerbates colorectal cancer via myeloid-derived suppressor cells in mice. Nat. Microbiol. 9, 1467–1482. doi: 10.1038/s41564-024-01695-w
Liu, K., Yang, X., Zeng, M., Yuan, Y., Sun, J., He, P., et al. (2021). The role of fecal Fusobacterium nucleatum and pks+ Escherichia coli as early diagnostic markers of colorectal cancer. Dis. Markers. 2021, 1171239. doi: 10.1155/2021/1171239
Liu, H., Yu, Y., Dong, A., Elsabahy, M., Yang, Y. W., and Gao, H. (2024). Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges. Explor. (Beijing). 4, 20230092. doi: 10.1002/EXP.20230092
Long, X., Wong, C., Tong, L., Chu, E., Ho Szeto, C., Go, M., et al. (2019). Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 4, 2319–2330. doi: 10.1038/s4156:4-019
Luo, M., Li, Q., Gu, Q., and Zhang, C. (2024). Fusobacterium nucleatum: a novel regulator of antitumor immune checkpoint blockade therapy in colorectal cancer. Am. J. Cancer Res. 14, 3962–3975. doi: 10.62347/MYZA2640
Magat, E. M., Balanag, G. A., CariÑo, A. M., Fellizar, A., Ortin, T. S., Guevarra, L., Jr., et al. (2020). Clostridioides difficile antibody response of colorectal cancer patients versus clinically healthy individuals. Biosci. Microbiota Food Health 39, 123–127. doi: 10.12938/bmfh.2020-010
Martin-Gallausiaux, C., Garcia-Weber, D., Lashermes, A., Larraufie, P., Marinelli, L., Teixeira, V., et al. (2022). Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway. Gut Microbes 14, 2110639. doi: 10.1080/19490976.2022.2110639
Martin-Gallausiaux, C., Salesse, L., Garcia-Weber, D., Marinelli, L., Beguet-Crespel, F., Brochard, V., et al. (2024). Fusobacterium nucleatum promotes inflammatory and anti-apoptotic responses in colorectal cancer cells via ADP-heptose release and ALPK1/TIFA axis activation. Gut Microbes 16, 2295384. doi: 10.1080/19490976.2023.2295384
Milivojevic, M., Dangeard, A.-S., Kasper, C. A., Tschon, T., Emmenlauer, M., Pique, C., et al. (2017). ALPK1 controls TIFA/TRAF6-dependent innate immunity against heptose-1, 7-bisphosphate of gram-negative bacteria. PloS Pathog. 13, e1006224. doi: 10.1371/journal.ppat.1006224
Miyakawa, Y., Otsuka, M., Shibata, C., Seimiya, T., Yamamoto, K., Ishibashi, R., et al. (2024). Gut bacteria-derived membrane vesicles induce colonic dysplasia by inducing DNA damage in colon epithelial cells. Cell Mol. Gastroenterol. Hepatol. 17, 745–767. doi: 10.1016/j.jcmgh.2024.01.010
Mohammadi, M., Mirzaei, H., and Motallebi, M. (2022). The role of anaerobic bacteria in the development and prevention of colorectal cancer: A review study. Anaerobe 73, 102501. doi: 10.1016/j.anaerobe
Nam, H.-J., Kang, J.-K., Chang, J.-S., Lee, M.-S., Nam, S.-T., Jung, H.-W., et al. (2012). Cells transformed by PLC-gamma 1 overexpression are highly sensitive to Clostridium difficile toxin A-induced apoptosis and mitotic inhibition. J. Microbiol. Biotechnol. 22, 50–57. doi: 10.4014/jmb.1107.07018
Nanjappa, S., Shah, S., and Pabbathi, S. (2015). Clostridium septicum gas gangrene in colon cancer: importance of early diagnosis. Case Rep. Infect. Dis. 2015, 694247. doi: 10.1155/2015/694247
Osman, M. A., Neoh, H.-m., Ab Mutalib, N.-S., Chin, S.-F., Mazlan, L., Raja Ali, R.A., et al. (2021). Parvimonas micra, Peptostreptococcus stomatis, Fusobacterium nucleatum and Akkermansia muciniphila as a four-bacteria biomarker panel of colorectal cancer. Sci. Rep. 11, 2925. doi: 10.1038/s41598-021-82465-0
Ou, S., Chen, H., Wang, H., Ye, J., Liu, H., Tao, Y., et al. (2023). Fusobacterium nucleatum upregulates MMP7 to promote metastasis-related characteristics of colorectal cancer cell via activating MAPK (JNK)-AP1 axis. J. Transl. Med. 21, 704. doi: 10.1186/s12967-023-04527-3
Pezeshkian, Z., Nobili, S., Peyravian, N., Shojaee, B., Nazari, H., Soleimani, H., et al. (2021). Insights into the role of matrix metalloproteinases in precancerous conditions and in colorectal cancer. Cancers (Basel) 13, 6226. doi: 10.3390/cancers13246226
Pignatelli, P., Nuccio, F., Piattelli, A., and Curia, M. C. (2023). The role of Fusobacterium nucleatum in oral and colorectal carcinogenesis. Microorganisms. 11, 2358. doi: 10.3390/microorganisms11092358
Purcell, R. V., Permain, J., and Keenan, J. I. (2022). Enterotoxigenic Bacteroides fragilis activates IL-8 expression through Stat3 in colorectal cancer cells. Gut Pathog. 14, 16. doi: 10.1186/s13099-022-00489-x
Qian, Y., Kang, Z., Zhao, L., Chen, H., Zhou, C., Gao, Q., et al. (2023). Berberine might block colorectal carcinogenesis by inhibiting the regulation of B-cell function by Veillonella parvula. Chin. Med. J. (Engl). 136, 2722–2731. doi: 10.1097/CM9.0000000000002752
Qu, R., Zhang, Y., Ma, Y., Zhou, X., Sun, L., Jiang, C., et al. (2023). Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv. Sci. (Weinh) 10, e2205563. doi: 10.1002/advs.202205563
Rojas-Tapias, D. F., Brown, E. M., Temple, E. R., Onyekaba, M. A., Mohamed, A. M., Duncan, K., et al. (2022). Inflammation-associated nitrate facilitates ectopic colonization of oral bacterium Veillonella parvula in the intestine. Nat. Microbiol. 7, 1673–1685. doi: 10.1038/s41564-022-01224-7
Sasaki, T., Mori, S., Kishi, S., Fujiwara-Tani, R., Ohmori, H., Nishiguchi, Y., et al. (2020). Effect of proton pump inhibitors on colorectal cancer. Int. J. Mol. Sci. 21, 3877. doi: 10.3390/ijms21113877
Shariati, A., Aslani, H. R., Shayesteh, M. R., Taghipour, A., Nasser, A., Safari, H., et al. (2019a). Are viruses and parasites linked to celiac disease? A question that still has no definite answer. Curr. Pharm. Biotechnol. 20, 1181–1193. doi: 10.2174/1389201020666190828124924
Shariati, A., Fallah, F., Pormohammad, A., Taghipour, A., Safari, H., Chirani, A. S., et al. (2019b). The possible role of bacteria, viruses, and parasites in initiation and exacerbation of irritable bowel syndrome. J. Cell Physiol. 234, 8550–8569. doi: 10.1002/jcp.27828
Shen, X., Li, J., Li, J., Zhang, Y., Li, X., Cui, Y., et al. (2021). Fecal enterotoxigenic Bacteroides fragilis–peptostreptococcus stomatis–parvimonas micra biomarker for noninvasive diagnosis and prognosis of colorectal laterally spreading tumor. Front. Oncol. 11. doi: 10.3389/fonc.2021.661048
Slack, F. J. and Chinnaiyan, A. M. (2019). The role of non-coding RNAs in oncology. Cell. 179, 1033–1055. doi: 10.1016/j.cell.2019.10.017
Tabowei, G., Gaddipati, G. N., Mukhtar, M., Alzubaidee, M. J., Dwarampudi, R. S., Mathew, S., et al. (2022). Microbiota Dysbiosis a cause of colorectal Cancer or not? A systematic review. Cureus 14, e30893. doi: 10.7759/cureus.30893
Tirelli, F., Lorenzon, L., Biondi, A., Langellotti, L., Santoro, G., Agnes, A., et al. (2023). Predictors of Clostridium difficile infection after stoma reversal following TaTME surgery. Updates Surg. 75, 1589–1596. doi: 10.1007/s13304-023-01614-4
Tsoi, H., Chu, E. S., Zhang, X., Sheng, J., Nakatsu, G., Ng, S. C., et al. (2017). Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology 152, 1419–1433.e5. doi: 10.1053/j.gastro.2017.01.009
Valour, F., Sénéchal, A., Dupieux, C., Karsenty, J., Lustig, S., Breton, P., et al. (2014). Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Resist. 7, 183–197. doi: 10.2147/IDR.S39601
Wang, M., Li, Y., Yang, X., Liu, Z., Wang, K., Gong, D., et al. (2023a). Effects of metronidazole on colorectal cancer occurrence and colorectal cancer liver metastases by regulating Fusobacterium nucleatum in mice. Immun. Inflammation Dis. 11, e1067. doi: 10.1002/iid3.1067
Wang, M., Wang, Z., Lessing, D. J., Guo, M., and Chu, W. (2023b). Fusobacterium nucleatum and its metabolite hydrogen sulfide alter gut microbiota composition and autophagy process and promote colorectal cancer progression. Microbiol. Spectr. 11, e0229223. doi: 10.1128/spectrum.02292-23
Wang, N., Zhang, L., Leng, X.-X., Xie, Y.-L., Kang, Z.-R., Zhao, L.-C., et al. (2024). Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the Hippo pathway. Gut Microbes 16, 2333790. doi: 10.1080/19490976.2024.2333790
Warny, M., Keates, A. C., Keates, S., Castagliuolo, I., Zacks, J. K., Aboudola, S., et al. (2000). p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Invest. 105, 1147–1156. doi: 10.1172/JCI7545
Wu, Q.-L., Fang, X.-T., Wan, X.-X., Ding, Q.-Y., Zhang, Y.-J., Ji, L., et al. (2024). Fusobacterium nucleatum-induced imbalance in microbiome-derived butyric acid levels promotes the occurrence and development of colorectal cancer. World J. Gastroenterol. 30, 2018–2037. doi: 10.3748/wjg.v30.i14.2018
Yachida, S., Mizutani, S., Shiroma, H., Shiba, S., Nakajima, T., Sakamoto, T., et al. (2019). Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976. doi: 10.1038/s41591-019-0458-7
Yang, S. S. and Im, Y. C. (2018). Severe abdominopelvic actinomycosis with colon perforation and hepatic involvement mimicking advanced sigmoid colon cancer with hepatic metastasis: a case study. BMC Surg. 18, 51. doi: 10.1186/s12893-018-0386-3
Yu, T., Guo, F., Yu, Y., Sun, T., Ma, D., Han, J., et al. (2017). Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 170, 548–563.e16. doi: 10.1016/j.cell.2017.07.008
Zeddou, M. (2023). Class i hla allele predicted restricted antigenic coverages for fap2 protein of Fusobacterium nucleatum are associated with colorectal cancer incidence. Asian Pac J. Cancer Prev. 24, 3629–3636. doi: 10.31557/APJCP.2023.24.10.3629
Keywords: colorectal cancer, CRC, anaerobic gut bacterial, gut bacterial, narrative review
Citation: Sabour S, Sadeghi Koupaei H, Ghasemi H, Amin M and Azimi T (2025) Anaerobic gut bacteria and their potential role in the initiation, exacerbation, and development of human colorectal cancer: a narrative review. Front. Cell. Infect. Microbiol. 15:1559001. doi: 10.3389/fcimb.2025.1559001
Received: 11 January 2025; Accepted: 30 June 2025;
Published: 25 July 2025.
Edited by:
Vibha Rani, Jaypee Institute of Information Technology, IndiaReviewed by:
Yuhang Wang, The University of Iowa, United StatesSaied Ghorbani, Iran University of Medical Sciences, Iran
Copyright © 2025 Sabour, Sadeghi Koupaei, Ghasemi, Amin and Azimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taher Azimi, VGFoZXJhemltaTVAZ21haWwuY29t
 Sahar Sabour
Sahar Sabour Hanieh Sadeghi Koupaei3
Hanieh Sadeghi Koupaei3 Hadi Ghasemi
Hadi Ghasemi Taher Azimi
Taher Azimi