- 1Department of Medical Care Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2School of Information and Engineering, Wenzhou Medical University, Wenzhou, China
- 3Oujiang Laboratory (Zhejiang Lab for Regenerative Medicine, Vision and Brain Health), School of Pharmaceutical Science, Wenzhou Medical University, Wenzhou, China
- 4Department of General Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 5Rehabilitation Medicine Center, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
Background: Metabolic dysfunction-associated fatty liver disease (MAFLD) has recently replaced nonalcoholic fatty liver disease (NAFLD) as a term that more accurately describes its pathogenesis. Helicobacter pylori (H. pylori), a bacterium that infects over half the world’s population, has been increasingly linked to various extragastric diseases. However, the impact of H. pylori on MAFLD in the Chinese population remains unexplored.
Methods: A retrospective cross-sectional study was conducted, encompassing 5619 participants from the First Affiliated Hospital of Wenzhou Medical University, spanning from April 2016 to August 2017. Detection of H. pylori was achieved through the 13C urea breath test or gastric biopsies with histochemical staining. Fatty liver was primarily diagnosed via ultrasound and assessed alongside metabolic indicators to confirm MAFLD. Logistic regression was utilized to evaluate the association between H. pylori and MAFLD.
Results: No significant correlation between H. pylori infection and MAFLD was found in the overall population through either univariate (OR=1.136, 95%CI 0.995-1.297, p=0.059) or multivariate logistic regression analysis (OR=1.036, 95%CI 0.877-1.224, p=0.675). However, subgroup analysis revealed a significant association in overweight individuals (BMI ≥ 23 kg/m2) within the MAFLD group (51.2% vs. 35.5%, p=0.002), a pattern not observed in the non-MAFLD group (47.7% vs. 45.4%, p=0.126). This association persisted after adjusting for confounders (OR=1.957, 95%CI 1.176-3.256, p=0.010).
Conclusion: Overweight individuals with MAFLD have a higher prevalence of H. pylori infection than their lean counterparts. This suggests a detrimental cycle between overweight status and H. pylori infection in MAFLD patients, potentially exacerbating metabolic deterioration. Therefore, eradication of H. pylori infection may have positive implications for reducing the incidence rate of overweight MAFLD.
Introduction
Metabolic-dysfunction-associated fatty liver disease (MAFLD), proposed in 2020 as an alternative to non-alcoholic fatty liver disease (NAFLD), is a prevalent chronic hepatic disease (Eslam et al., 2020a). MAFLD is perceived as a standalone disease related to known metabolic dysfunction and has a specific positive diagnosis. It represents a spectrum of liver disorders, ranging from steatosis and steatohepatitis to fibrosis, cirrhosis, and hepatocellular carcinoma. Globally, MAFLD affects approximately 13.48% to 31.79% of adults (Younossi et al., 2016; Yang et al., 2017; Qu et al., 2021; Lazarus et al., 2022), with its prevalence in China rising from 23.8% in 2001 to 32.9% in 2018 (Zhou et al., 2020). In morbidly obese individuals, prevalence may reach up to 90% (Doulberis et al., 2017a; Polyzos and Kountouras, 2019). MAFLD patients often exhibit higher rates of hypertriglyceridemia, diabetes, and hypertension, and are at greater risk for significant fibrosis (Lim et al., 2023). A large longitudinal cohort study in China showed that the definition of MAFLD is more effective in identifying individuals with poor clinical features and poor prognosis than NAFLD (Xu et al., 2023). Especially for individuals with normal weight, the presence of steatosis with at least two metabolic risk abnormalities can also be diagnosed as criteria for MAFLD in non-overweight/obese subjects. Hence, the proposed standards would cover the full spectrum of phenotypes from metabolically unhealthy normal weight to metabolically unhealthy obesity.
Helicobacter pylori (H. pylori) is a Gram-negative spiral-shaped pathogen discovered by Marshall and Warren in 1983 from gastric mucosa (Marshall and Warren, 1984). It is estimated that approximately half of the global population is infected with H. pylori (Cover and Blaser, 2009), with rates in developing countries reaching up to 70% (Adenote et al., 2021). Chronic infection by H. pylori is recognized for its association with several gastrointestinal diseases, including chronic gastritis, peptic ulcers, gastric mucosa-associated lymphomas, and gastric cancer (Malfertheiner et al., 2012; Matsuhisa and Aftab, 2012). Recent studies have also demonstrated a growing correlation between H. pylori and various extragastric disorders, such as metabolic syndrome, insulin resistance (IR), type 2 diabetes mellitus (T2DM), cardiovascular diseases, and neurodegenerative diseases (Suzuki et al., 2006; Doulberis et al., 2017b; Gravina et al., 2020).
The correlation between H. pylori infection and liver disease has prompted extensive discussion, particularly after Cindomk et al. (Cindoruk et al., 2008) isolated H. pylori DNA from nonalcoholic steatohepatitis (NASH) liver tissue in 2008. Several high-quality meta-analyses suggest a positive correlation between NAFLD and H. pylori infection (Mantovani et al., 2019; Ning et al., 2019; Zhou et al., 2019; Heydari et al., 2022), potentially influencing metabolic risk factors, the gut microbiome, the inflammatory state, and other metabolically active hormones. However, studies in Chinese and European populations suggest that H. pylori may not be a risk factor for NAFLD (Cai et al., 2018; Fan et al., 2018; Wang et al., 2022).
Given the emerging concept of metabolic-dysfunction-associated fatty liver disease (MAFLD) and the significant role of overweight and obesity, as determined by body mass index (BMI), in diagnosing MAFLD, research has indicated an association between H. pylori infection and increased BMI and obesity risk (Chen et al., 2018). Following the eradication of H. pylori, patients have reported weight loss (Martin-Nunez et al., 2021). Therefore, we conducted a cross-sectional and cohort survey to investigate the relationship between H. pylori infection, MAFLD, and overweight.
Subjects and methods
Study population
This retrospective cohort study included individuals who underwent routine health check-ups, including liver imaging and either 13C-urea breath tests or gastroscopy biopsies, at the First Affiliated Hospital of Wenzhou Medical University between April 2016 and August 2017. Subjects were excluded if they were under 18 years old, had a history of cancer, major cerebrovascular accident, end-stage renal disease, gastrectomy, or chronic liver disease such as viral hepatitis or alcohol-induced liver disease, had used medications that could interfere with the accuracy of H. pylori testing within the specified time frames, such as proton pump inhibitors within two weeks or antibiotics, sucralfate, bismuth within one month, or had incomplete clinical information regarding H. pylori and fatty liver disease (Malfertheiner et al., 2017). The final analysis included 5325 participants: 4219 with MAFLD and 1106 healthy controls. The study received approval from the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Collection of clinical indicators
After obtaining informed consent, a structured questionnaire was administered to collect data on age, BMI, gender, medical history, medication use, smoking, and alcohol consumption. Participants were categorized as lean (BMI < 23 kg/m2) or overweight (BMI ≥ 23 kg/m2) (Eslam et al., 2020b). Smoking status was classified as current, former (quit for more than six months), or never, with the last two categorized as non-current smoking status. Alcohol consumption was classified as heavy (intake > 210 g per week for males and > 140 g per week for females) or non-heavy. Systolic and diastolic blood pressures were measured using an automated sphygmomanometer while seated. Blood biochemical parameters including fasting plasma glucose (FBG), hemoglobin A1c (HbAlc), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT), album, triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid, creatinine, serum calcium, serum phosphorus, 25 hydroxyvitamin D, high-sensitivity C-reactive protein (hs-CRP), red blood cell (RBC), hemoglobin and white blood cell (WBC) were assessed using an automatic biochemical analyzer.
Diagnosis of H. pylori infection
H. pylori status was determined using a 13C urea breath test or gastric biopsies with histochemical staining (Malfertheiner et al., 2017). The 13C breath test involved: (a) collecting a baseline breath sample after a minimum of three hours of fasting; (b) administering 13C urea orally with warm water; (c) collecting a second breath sample 30 minutes later. A H. pylori infection was diagnosed if the Delta Over Baseline (DOB) value was ≥ 4. For gastric biopsies, the procedure included: (a) collecting mucosal specimens under endoscopic guidance by experienced physicians; (b) processing the specimens by fixing, dehydrating, embedding, slicing, and staining; (c) diagnosing via urease testing or histopathological examination.
Definition of MAFLD
MAFLD is diagnosed by the presence of hepatic steatosis and one or more of the following conditions: (1) overweight or obese (BMI ≥ 23 kg/m2) (2) diabetes mellitus, or (3) at least 2 metabolic risk abnormalities. These abnormalities include: (a) waist circumference (WC) ≥ 90 cm for men and ≥ 80 cm for women, (b) blood pressure ≥ 130/85 mmHg or specific drug treatment, (3) fasting plasma triglycerides ≥ 150 mg/dl or on specific drug treatment, (4) plasma HDL-C < 40 mg/dl for men and < 50 mg/dl for women or specific drug treatment, (5) prediabetes (fasting glucose 100–125 mg/dl or hemoglobin A1c 5.7–6.4%, (6) homeostasis model assessment of insulin resistance score ≥ 2.5, (7) plasma hs-CRP level > 2 mg/L(1). lean MAFLD is defined as hepatic steatosis with a BMI <25 kg/m2 (or <23 kg/m2 in Asians) (Eslam et al., 2020b).
Statistical analysis
Statistical analyses were conducted using SPSS 25.0 (SPSS Inc., Chicago, IL, USA). A P-value ≤ 0.05 was deemed statistically significant. Continuous variables were presented as means ± SD (for normally distributed data) or medians with interquartile ranges (for non-normally distribution date). Normality was assessed using the Kolmogorov-Smirnov test and normal Q-Q plots. The T-test was applied to normally distributed data, and the Mann–Whitney U-test was used otherwise. Categorical variables were expressed as counts and percentages and analyzed using the chi-square test or Fisher's exact test. Participants were stratified by the presence of MAFLD and/or H. pylori infection, as well as BMI. Multivariate logistic regression, adjusted for confounders, was employed to explore the relationship between groups, yielding odds ratios (OR) and 95% confidence intervals (CI).
Result
Baseline characteristics
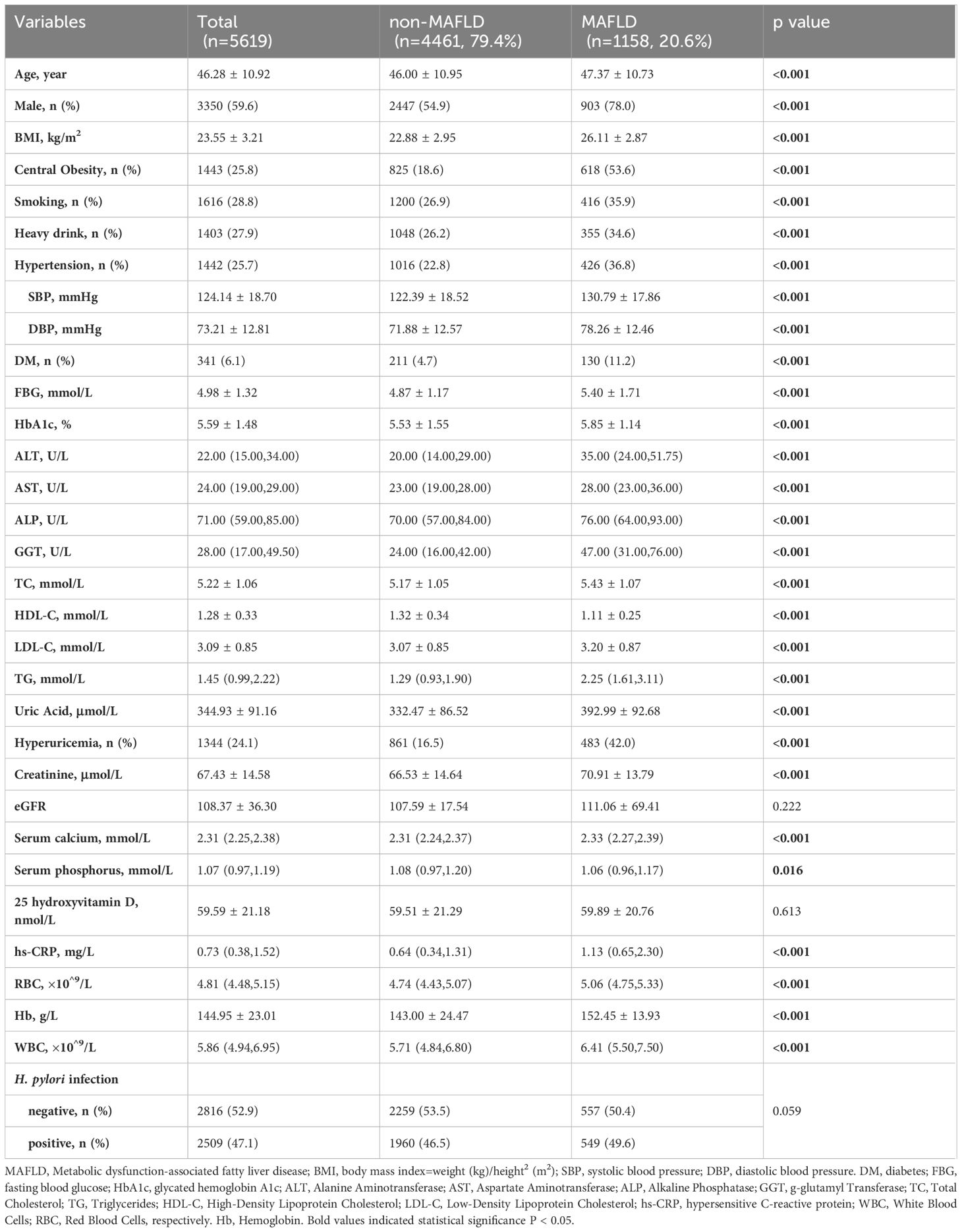
This study included 5619 participants: 4461 (79.4%) non-MAFLD and 1158 (20.6%) MAFLD. Among these, 5325 underwent routine health screening for H. pylori; 2816 (52.89%) tested negative, and 2509 (47.12%) positive. Demographically, the MAFLD group was older (46.00 ± 10.95 vs. 47.37 ± 10.73, p<0.001), had a higher proportion of males (78% vs. 54.9%, p<0.001), and exhibited more obesity (BMI 26.11 ± 2.87 vs. 22.88 ± 2.95, p<0.001). The MAFLD group also had higher incidences of smoking, heavy drinking, and metabolic abnormalities such as hypertension, diabetes mellitus, hyperuricemia, and elevated liver enzymes (ALT, AST, ALP, GGT), as well as worse lipid profiles (higher triglycerides and lower HDL-C) (Table 1) (all p<0.001). However, the distribution of MAFLD relative to H. pylori infection showed no significant difference (49.6% vs. 46.5%, p=0.059).
Logistic regression analyses for different markers and MAFLD
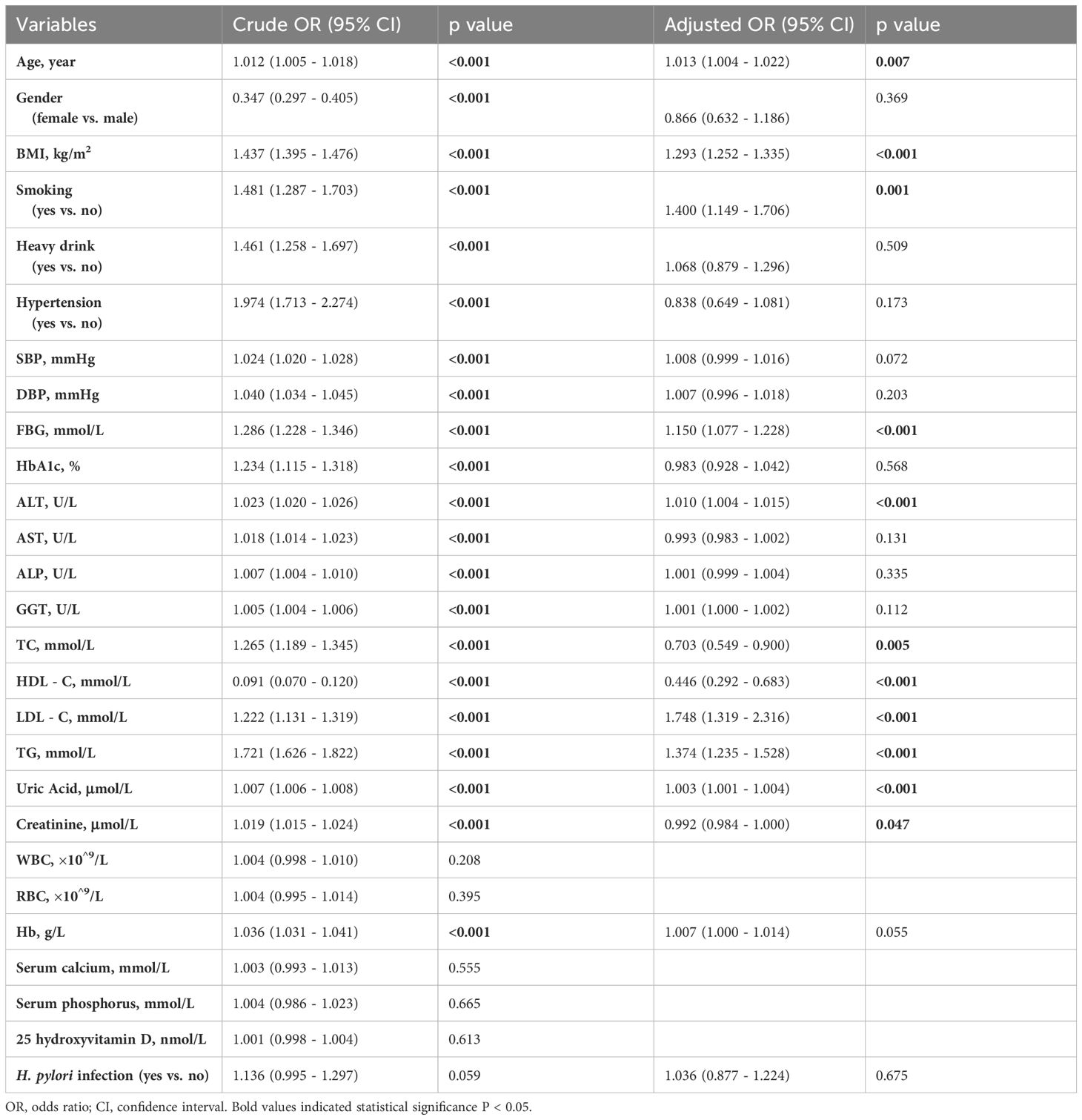
As displayed in Table 2, both univariate and multivariate logistic regression analyses were conducted to identify risk factors. Age, gender, BMI, smoking, heavy drinking, metabolic abnormalities, and hemoglobin were significantly associated with outcomes in the crude analysis (p <0.05). After adjustment, Age, BMI, smoking, FBG, ALT, HDL-C, LDL-C, TG, Uric Acid, and creatinine remained statistically significant (p<0.05). Gender, smoking, heavy drinking, hemoglobin A1c (HbA1c), and certain liver enzyme markers (AST, ALP, GGT) were no longer significantly correlated in the adjusted analysis. However, no significant relationship was found between H. pylori infection and MAFLD in the univariate analysis (p=0.059), nor after adjusting for confounding factors (p=0.675).
Tendency of H. pylori infection rate in different populations
Based on body mass index, participants were categorized into two groups: lean (BMI<23 kg/m2) and overweight (BMI ≥ 23 kg/m2). Subgroup analyses of general, non-MAFLD, and MAFLD populations were performed using the chi-square test to further investigate H. pylori infection status across different weight categories and MAFLD statuses (Figure 1). Overall, the infection rate was higher among overweight individuals (48.9% vs. 44.9%, p=0.0004). This trend was more pronounced in MAFLD populations (51.2% vs. 35.5%, p=0.002) but was not significant in non-MAFLD populations (47.7% vs. 45.4%, p=0.126).
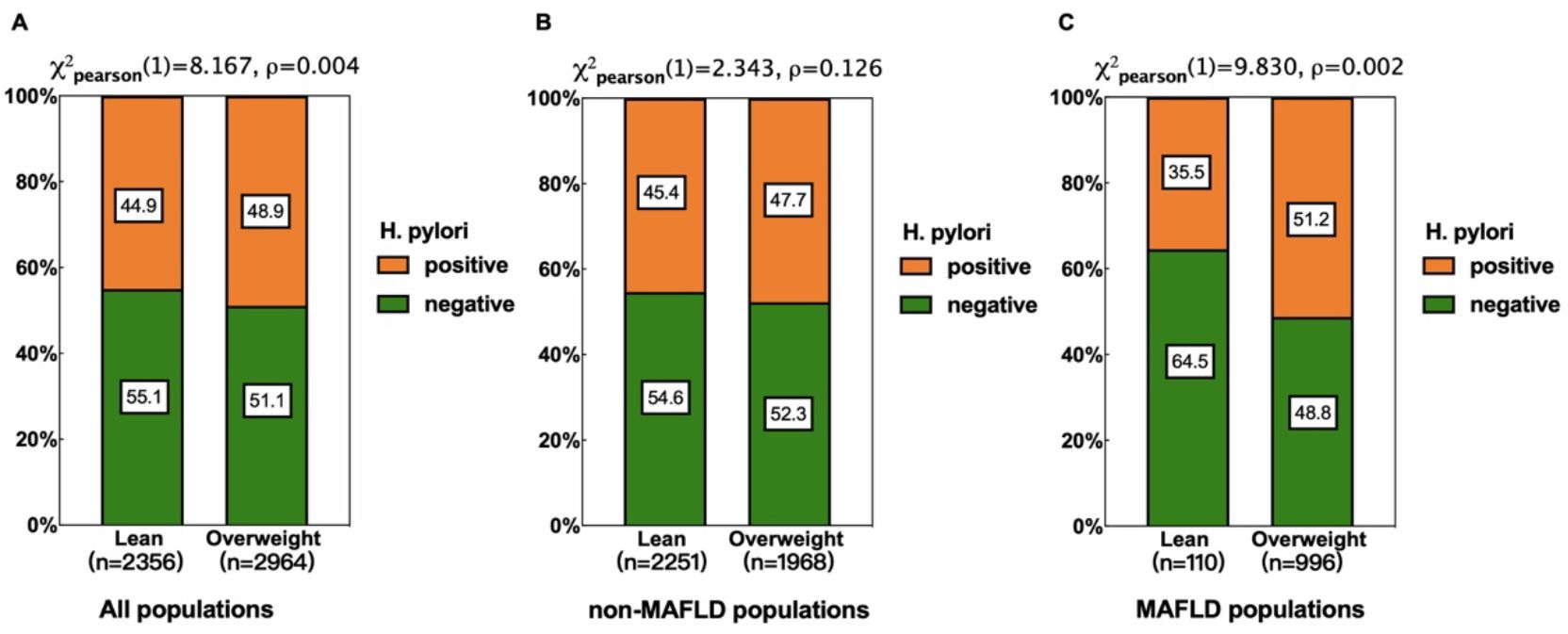
Figure 1. Relationship between H pylori infection and different weight in different populations. (A) All populations. (B) non-MAFLD populations. (C) MAFLD populations. Subgroup analyses revealed a higher H. pylori infection rate in overweight individuals in all populations (A), with a more pronounced difference in the MAFLD subgroup (B), but the differences were not significant in non-MAFLD populations (C).
Risk factors for MAFLD between lean and overweight groups
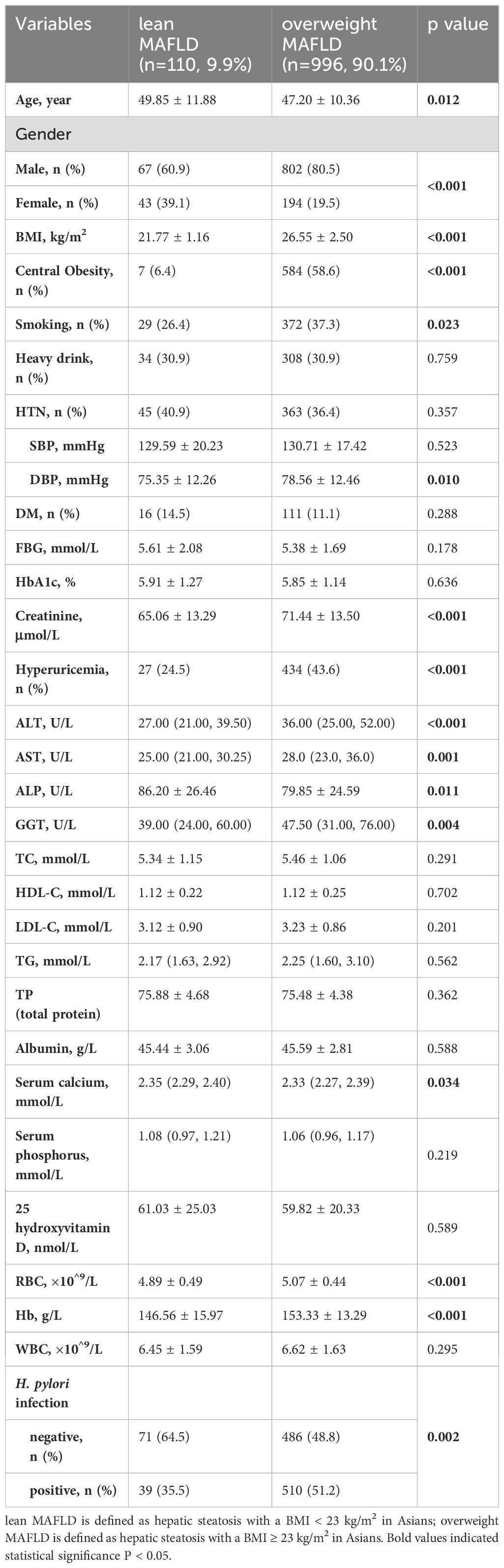
The baseline characteristics of the MAFLD population were shown in Table 3. The overweight MAFLD group had a significantly higher BMI (26.55 ± 2.50 vs. 21.77 ± 1.16, p<0.001) and more cases of central obesity (58.6% vs. 6.4%, p<0.001). Compared to the lean MAFLD group, the overweight group was younger, predominantly male, and included more smokers, had lower serum calcium levels and ALP, but exhibited higher diastolic blood pressure (DBP), creatinine, ALT, AST, GGT, and rates of hyperuricemia, RBD, Hb, as well as H. pylori infection rates (51.2% vs. 35.5%, p=0.002).
Effect of H. pylori infection on overweight MAFLD
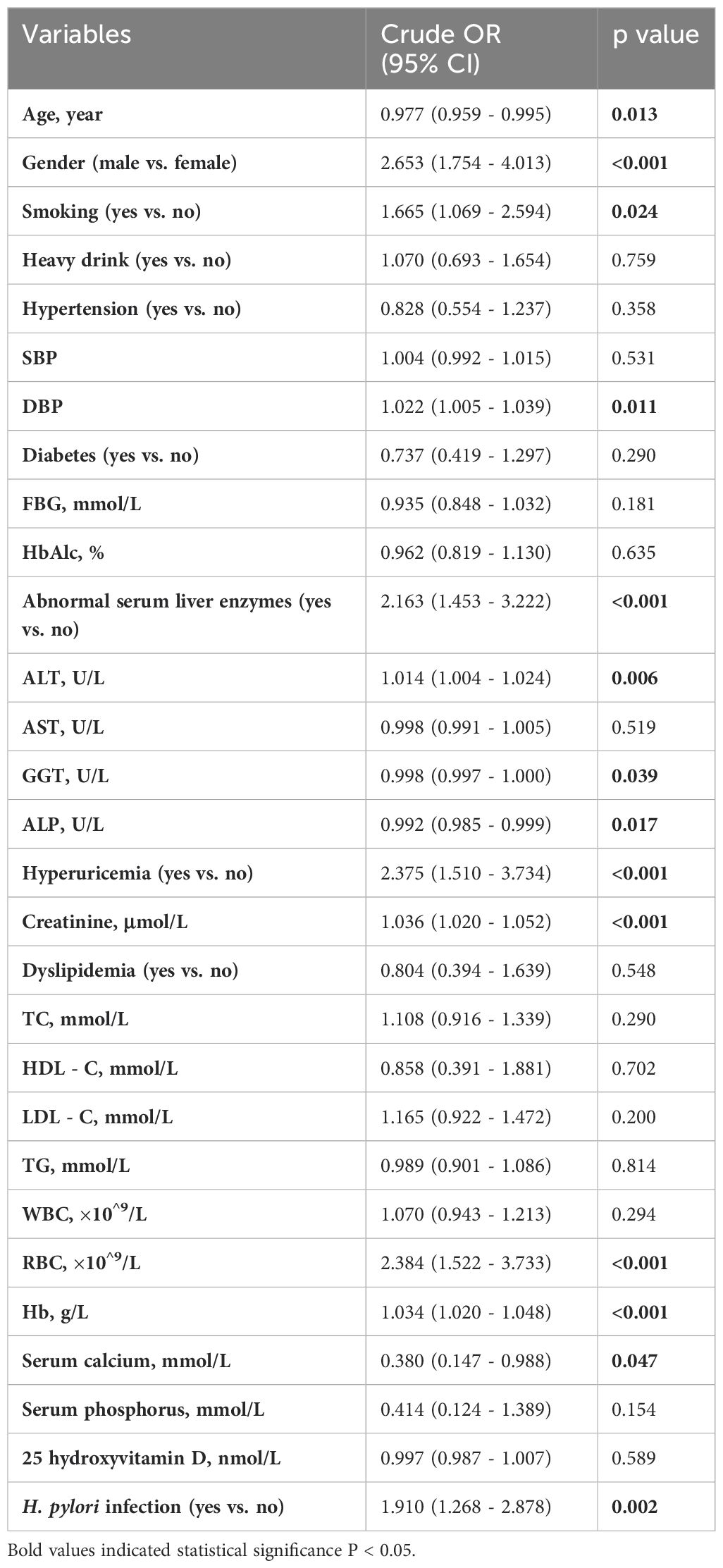
As shown in Table 4, univariate analysis identified several risk factors for overweight MAFLD classification by BMI, including age, male gender, smoking, DBP, ALT, GGT, ALP, hyperuricemia, creatinine, RBC, Hb, serum calcium, and H. pylori infection (p<0.05).
To control for confounding factors, adjustments were made in addition to age, gender, smoking (Model 0), DBP (Model 1), ALT, ALP, and GGT (Model 2), hyperuricemia and creatinine (Model 3), and RBC, Hb, and serum calcium (Model 4) respectively. The association between incident overweight MAFLD and H. pylori infection remained statistically significant (Table 5). To visualize this correlation, a forest plot was constructed based on Model 4 (Figure 2).
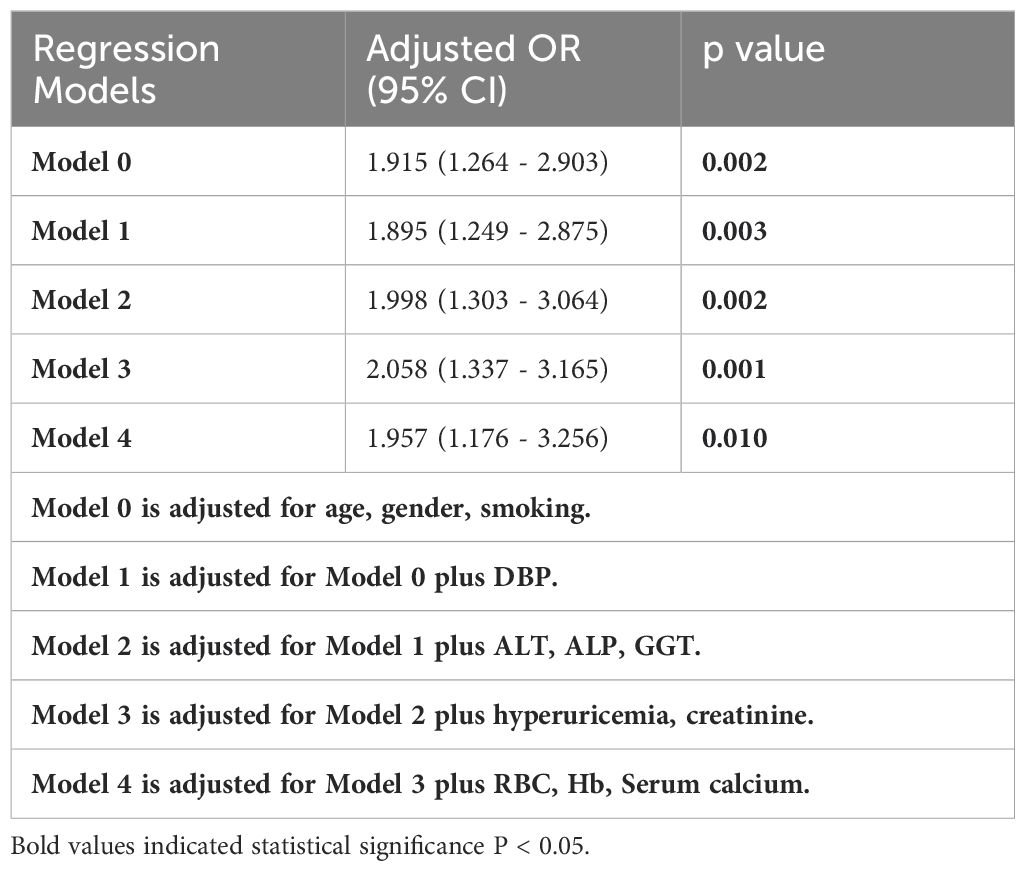
Table 5. Relationship between H. pylori infection and overweight MAFLD in different regression models.
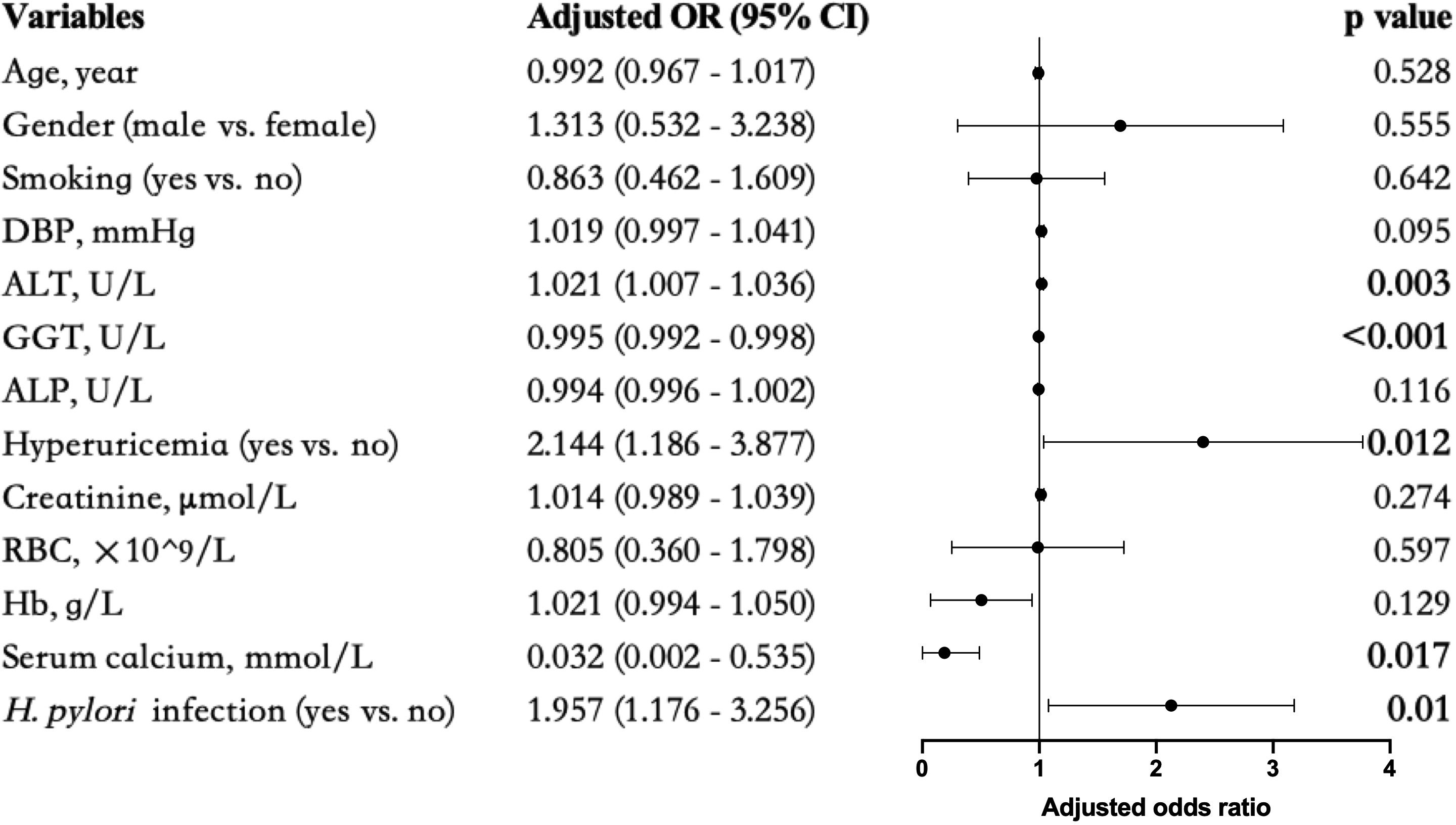
Figure 2. Forest plots of multivariate analysis for the association between different markers and overweight MAFLD. Bold values indicated statistical significance P < 0.05.
Discussion
The concept of MAFLD, introduced in 2020 as a replacement for Non-alcoholic Fatty Liver Disease (NAFLD), broadens the scope of fatty liver disease to include metabolic dysfunction as a central feature and has emerged as a significant global health concern (Eslam et al., 2020a). MAFLD increases the risk of end-stage liver disease, hepatocellular carcinoma, death, liver transplantation, and has extrahepatic consequences including cardiometabolic diseases and various cancers (Choi et al., 2022; Liu et al., 2022; Yuan et al., 2023; Crane et al., 2024; Fukunaga et al., 2024; Nakane et al., 2024). Its pathogenesis involves complex interactions among insulin resistance, adiposity, and lipid metabolism (Doulberis et al., 2017a; Polyzos and Kountouras, 2019; Lim et al., 2023; Xu et al., 2023).
In our study, we identified significant risk factors for MAFLD including older age, higher BMI, smoking, FBG, ALT, dyslipidemia (high TC, LDL-C, TG, and low HDL-C), uric acid, and creatinine. Further analysis revealed that lean MAFLD patients were older, had a higher percentage of females and hyperuricemia, and a lower proportion of smoking and drinking, lower diastolic blood pressure (DBP), and lower liver enzyme indicators (ALT, AST, ALP, and GGT) but higher serum calcium than overweight MAFLD patients. These results are consistent with previous studies on metabolic index changes and provide further insights into the risk factors for MAFLD (Eslam et al., 2020a; Chan et al., 2022; Yuan et al., 2022) and lean MAFLD (Buzova et al., 2020; Shah et al., 2020; Cheng et al., 2021; Eslam et al., 2022). Notably, we found a close correlation between high creatinine levels and MAFLD, persisting in further comparisons between overweight and lean MAFLD subgroups. A 2020 study (Sun et al., 2021) confirmed that the prevalence of CKD in MAFLD was higher than in non-metabolic dysfunction-associated NAFLD, and the severity of MAFLD was associated with a 1.34-fold higher risk of prevalent CKD. However, no significant differences were found in heavy drinking and hypertension between MAFLD and non-MAFLD. This discrepancy may necessitate further sample size expansion, and it is noted that fatty liver disease in Asia significantly differs from that in the West, where there are significantly higher rates of alcoholic liver disease (Younossi et al., 2022). Furthermore, when comparing the hypertension status of individuals aged 45 and above, those with MAFLD had a higher incidence of hypertension compared to those without MAFLD (44.9% vs. 32.4%, p<0.001). These findings suggest that individuals in the MAFLD group are more likely to possess metabolic risk factors and may require closer monitoring and management of their health conditions.
The scientific community has shown a growing interest in the relationship between MAFLD/NAFLD and H. pylori. However, it remains controversial whether H. pylori infection contributes to the increased risk of MAFLD. Several high-quality meta-analyses have demonstrated an increased risk of NAFLD in H. pylori -positive patients compared to H. pylori-negative patients, with odds ratios (ORs) ranging from 1.19 to 1.38 (Mantovani et al., 2019; Ning et al., 2019; Zhou et al., 2019; Heydari et al., 2022). Moreover, the eradication of H. pylori may reduce the risk of NAFLD (Polyzos et al., 2014). Although the majority of data supports a link between H. pylori infection and fatty liver disease, some studies dispute this association. Han et al. (2021) conducted a retrospective observational cohort study that showed no association between H. pylori seropositivity and NAFLD. This result is limited by the nature of H. pylori serology, which includes patients with past or present infection, and cannot accurately reflect active infection. Therefore, only active H. pylori infection should be considered when evaluating its relationship with NAFLD. Another study from southwestern China also found no positive correlation between H. pylori and NAFLD, but noted that H. pylori infection was more prevalent in patients with liver stiffness measurement (LSM) >7.4 kPa, suggesting an association with fibrosis (Liu et al., 2021).
Although no correlation between H. pylori infection and MAFLD was found in the overall population, a statistically significant correlation was observed between H. pylori infection and overweight in the MAFLD population, but not in the non-MAFLD population. Even after adjusting for multiple confounding factors in multivariate logistic regression, H. pylori infection remained an independent risk factor for overweight MAFLD patients compared to lean MAFLD. This suggests that H. pylori infection may synergistically promote the progression of overweight MAFLD. In other words, metabolic health is a dynamic state throughout the life cycle, and H. pylori infection may be a determining factor during the progression of overweight MAFLD phenotype. Previous research suggests that the pathophysiological mechanisms may involve H. pylori infection being associated with elevated levels of serum fetuin-A, which impairs insulin signaling via inhibition of insulin receptor tyrosine kinase activity and promotes inflammation through TLR4 activation (Pal et al., 2012; Goustin et al., 2013; Kahraman et al., 2013; von Loeffelholz et al., 2016). Additionally, H. pylori infection may affect gut barrier permeability, leading to the translocation of PAMPs to the liver via the gut-liver axis, thereby initiating inflammation and fibrosis (Quaresma et al., 2006; Dash et al., 2019). It is also linked to reduced serum levels of high-density lipoprotein (HDL) cholesterol, which promotes dyslipidemia (Upala et al., 2016). Moreover, there is an association between H. pylori infection and obesity. The infection induces inflammatory responses in gastric cells responsible for leptin and ghrelin production (Roper et al., 2008). Due to the anorexigenic effect of leptin, H. pylori infection may stimulate overeating and contribute to obesity mechanisms (Schwartz et al., 1996; Shintani et al., 2001). Obesity is typically associated with impaired immune function, and immune deterioration correlates with the degree of obesity (Marti et al., 2001). Studies have indicated that the maturation of monocytes into macrophages is diminished, and the bactericidal capacity of PMN cells is reduced in obese individuals (Palmblad et al., 1980; Krishnan et al., 1982), suggesting that the compromised immune state in obesity diminishes the ability to resist H. pylori infection.
For the first time, our study discovered that H. pylori was more prevalent in overweight MAFLD patients than lean individuals. However, the study has certain limitations that merit attention. Firstly, this is a single-center retrospective study, which may limit the generalizability of the findings. Multicenter, longitudinal studies are needed to provide more robust evidence. Secondly, because MAFLD is mainly diagnosed by ultrasound, it is not possible to determine the severity of MAFLD-associated hepatitis, and mild steatosis may go undetected. Nevertheless, the high sensitivity and specificity of ultrasound diagnosis for fatty hepatitis have led to its widespread use in clinical practice. Thirdly, we defined metabolic dysregulation as the presence of at least two of five metabolic risk abnormalities due to limited data on insulin resistance and high-sensitivity C-reactive protein, which may reduce the detection rate of MAFLD. Finally, although methods are being used to adjust for confounding factors, there may still be potential influences from other factors.
Conclusion
This study suggests a clear relationship between H. pylori infection and overweight MAFLD, indicating a vicious cycle between the two in MAFLD patients, leading to a worsening of metabolic status. Therefore, addressing the weight issues and H. pylori infection status of MAFLD patients may be beneficial for disease assessment. Moreover, controlling H. pylori infection may be a modifiable risk factor for preventing or treating overweight MAFLD. Further studies are needed to determine whether eradicating H. pylori and controlling body weight can improve metabolic associated fatty liver and prevent further liver disease.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Review of Ethics Committee in Clinical Research (ECCR) of the First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XC: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. JF: Conceptualization, Data curation, Formal Analysis, Writing – original draft. KJ: Data curation, Formal Analysis, Writing – original draft. ZY: Data curation, Formal Analysis, Writing – original draft. YQ: Data curation, Validation, Writing – original draft. KM: Investigation, Project administration, Writing – original draft. YW: Investigation, Project administration, Writing – original draft. JM: Software, Supervision, Writing – original draft. YD: Software, Supervision, Writing – original draft. ZZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. SL: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Key Research and Development Program of China grants 2016YFC1304000 (C Chen); The National Natural Scientific Foundation of China 82170017,82370085(C Chen); Zhejiang Provincial Key Research and Development Program 2020C03067 (C Chen); Wenzhou Scientific and Technology Program (Y2020174).
Acknowledgments
We thank all participants for the time dedicated to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adenote, A., Dumic, I., Madrid, C., Barusya, C., Nordstrom, C. W., and Rueda Prada, L. (2021). NAFLD and infection, a nuanced relationship. Can. J. Gastroenterol. Hepatol. 2021, 5556354. doi: 10.1155/2021/5556354
Buzova, D., Maugeri, A., Liguori, A., Napodano, C., Lo Re, O., Oben, J., et al. (2020). Circulating histone signature of human lean metabolic-associated fatty liver disease (MAFLD). Clin. Epigen. 12, 126. doi: 10.1186/s13148-020-00917-2
Cai, O., Huang, Z., Li, M., Zhang, C., Xi, F., and Tan, S. (2018). Association between helicobacter pylori infection and nonalcoholic fatty liver disease: A single-center clinical study. Gastroenterol. Res. Pract. 2018, 8040262. doi: 10.1155/2018/8040262
Chan, K. E., Koh, T. J. L., Tang, A. S. P., Quek, J., Yong, J. N., Tay, P., et al. (2022). Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: A meta-analysis and systematic review of 10 739–607 individuals. J. Clin. Endocrinol. Metab. 107, 2691–2700. doi: 10.1210/clinem/dgac321
Chen, L. W., Kuo, S. F., Chen, C. H., Chien, C. H., Lin, C. L., and Chien, R. N. (2018). A community-based study on the association between Helicobacter pylori Infection and obesity. Sci. Rep. 8, 10746. doi: 10.1038/s41598-018-28792-1
Cheng, Y. M., Kao, J. H., and Wang, C. C. (2021). The metabolic profiles and body composition of lean metabolic associated fatty liver disease. Hepatol. Int. 15, 405–412. doi: 10.1007/s12072-021-10147-0
Choi, J. M., Park, H. E., Han, Y. M., Lee, J., Lee, H., Chung, S. J., et al. (2022). Non-alcoholic/metabolic-associated fatty liver disease and helicobacter pylori additively increase the risk of arterial stiffness. Front. Med. (Lausanne). 9, 844954. doi: 10.3389/fmed.2022.844954
Cindoruk, M., Cirak, M. Y., Unal, S., Karakan, T., Erkan, G., Engin, D., et al. (2008). Identification of Helicobacter species by 16S rDNA PCR and sequence analysis in human liver samples from patients with various etiologies of benign liver diseases. Eur. J. Gastroenterol. Hepatol. 20, 33–36. doi: 10.1097/MEG.0b013e3282efa4f2
Cover, T. L. and Blaser, M. J. (2009). Helicobacter pylori in health and disease. Gastroenterology 136, 1863–1873. doi: 10.1053/j.gastro.2009.01.073
Crane, H., Eslick, G. D., Gofton, C., Shaikh, A., Cholankeril, G., Cheah, M., et al. (2024). Global prevalence of metabolic dysfunction-associated fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis. Clin. Mol. Hepatol. 30, 436–448. doi: 10.3350/cmh.2024.0109
Dash, N. R., Khoder, G., Nada, A. M., and Al Bataineh, M. T. (2019). Exploring the impact of Helicobacter pylori on gut microbiome composition. PloS One 14, e0218274. doi: 10.1371/journal.pone.0218274
Doulberis, M., Kotronis, G., Gialamprinou, D., Kountouras, J., and Katsinelos, P. (2017a). Non-alcoholic fatty liver disease: An update with special focus on the role of gut microbiota. Metabolism 71, 182–197. doi: 10.1016/j.metabol.2017.03.013
Doulberis, M., Kotronis, G., Thomann, R., Polyzos, S. A., Boziki, M., Gialamprinou, D., et al. (2017b). Review: Impact of Helicobacter pylori on Alzheimer’s disease: What do we know so far? Helicobacter 23 (1). doi: 10.1111/hel.12454
Eslam, M., El-Serag, H. B., Francque, S., Sarin, S. K., Wei, L., Bugianesi, E., et al. (2022). Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat. Rev. Gastroenterol. Hepatol. 19, 638–651. doi: 10.1038/s41575-022-00635-5
Eslam, M., Newsome, P. N., Sarin, S. K., Anstee, Q. M., Targher, G., Romero-Gomez, M., et al. (2020a). A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209. doi: 10.1016/j.jhep.2020.03.039
Eslam, M., Sanyal, A. J., George, J., and International Consensus, P. (2020b). MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158, 1999–2014 e1. doi: 10.1053/j.gastro.2019.11.312
Fan, N., Peng, L., Xia, Z., Zhang, L., Wang, Y., and Peng, Y. (2018). Helicobacter pylori infection is not associated with non-alcoholic fatty liver disease: A cross-sectional study in China. Front. Microbiol. 9, 73. doi: 10.3389/fmicb.2018.00073
Fukunaga, S., Mukasa, M., Nakane, T., Nakano, D., Tsutsumi, T., Chou, T., et al. (2024). Impact of non-obese metabolic dysfunction-associated fatty liver disease on risk factors for the recurrence of esophageal squamous cell carcinoma treated with endoscopic submucosal dissection: A multicenter study. Hepatol. Res. 54, 201–212. doi: 10.1111/hepr.13973
Goustin, A. S., Derar, N., and Abou-Samra, A. B. (2013). Ahsg-fetuin blocks the metabolic arm of insulin action through its interaction with the 95-kD beta-subunit of the insulin receptor. Cell Signal. 25, 981–988. doi: 10.1016/j.cellsig.2012.12.011
Gravina, A. G., Priadko, K., Ciamarra, P., Granata, L., Facchiano, A., Miranda, A., et al. (2020). Extra-gastric manifestations of helicobacter pylori infection. J. Clin. Med. 9 (12), 3887. doi: 10.3390/jcm9123887
Han, Y. M., Lee, J., Choi, J. M., Kwak, M. S., Yang, J. I., Chung, S. J., et al. (2021). The association between Helicobacter pylori with nonalcoholic fatty liver disease assessed by controlled attenuation parameter and other metabolic factors. PloS One 16, e0260994. doi: 10.1371/journal.pone.0260994
Heydari, K., Yousefi, M., Alizadeh-Navaei, R., Lotfi, P., Sheydaee, F., Raei, M., et al. (2022). Helicobacter pylori infection and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Turk. J. Gastroenterol. 33, 171–181. doi: 10.5152/tjg.2022.21467
Kahraman, A., Sowa, J. P., Schlattjan, M., Sydor, S., Pronadl, M., Wree, A., et al. (2013). Fetuin-A mRNA expression is elevated in NASH compared with NAFL patients. Clin. Sci. (Lond). 125, 391–400. doi: 10.1042/CS20120542
Krishnan, E. C., Trost, L., Aarons, S., and Jewell, W. R. (1982). Study of function and maturation of monocytes in morbidly obese individuals. J. Surg. Res. 33, 89–97. doi: 10.1016/0022-4804(82)90012-9
Lazarus, J. V., Mark, H. E., Anstee, Q. M., Arab, J. P., Batterham, R. L., Castera, L., et al. (2022). Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78. doi: 10.1038/s41575-021-00523-4
Lim, G. E. H., Tang, A., Ng, C. H., Chin, Y. H., Lim, W. H., Tan, D. J. H., et al. (2023). An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin. Gastroenterol. Hepatol. 21, 619–29 e7. doi: 10.1016/j.cgh.2021.11.038
Liu, Y., Li, D., Liu, Y., and Shuai, P. (2021). Association between helicobacter pylori infection and non-alcoholic fatty liver disease, hepatic adipose deposition and stiffness in Southwest China. Front. Med. (Lausanne). 8, 764472. doi: 10.3389/fmed.2021.764472
Liu, Z., Lin, C., Suo, C., Zhao, R., Jin, L., Zhang, T., et al. (2022). Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metabolism 127, 154955. doi: 10.1016/j.metabol.2021.154955
Malfertheiner, P., Megraud, F., O’Morain, C. A., Atherton, J., Axon, A. T., Bazzoli, F., et al. (2012). Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut 61, 646–664. doi: 10.1136/gutjnl-2012-302084
Malfertheiner, P., Megraud, F., O’Morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66 (1), 6–30. doi: 10.1136/gutjnl-2016-312288
Mantovani, A., Turino, T., Altomari, A., Lonardo, A., Zoppini, G., Valenti, L., et al. (2019). Association between Helicobacter pylori infection and risk of nonalcoholic fatty liver disease: An updated meta-analysis. Metabolism 96, 56–65. doi: 10.1016/j.metabol.2019.04.012
Marshall, B. J. and Warren, J. R. (1984). Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315. doi: 10.1016/S0140-6736(84)91816-6
Marti, A., Marcos, A., and Martinez, J. A. (2001). Obesity and immune function relationships. Obes. Rev. 2, 131–140. doi: 10.1046/j.1467-789x.2001.00025.x
Martin-Nunez, G. M., Cornejo-Pareja, I., Clemente-Postigo, M., and Tinahones, F. J. (2021). Gut microbiota: the missing link between helicobacter pylori infection and metabolic disorders? Front. Endocrinol. (Lausanne) 12, 639856. doi: 10.3389/fendo.2021.639856
Matsuhisa, T. and Aftab, H. (2012). Observation of gastric mucosa in Bangladesh, the country with the lowest incidence of gastric cancer, and Japan, the country with the highest incidence. Helicobacter 17, 396–401. doi: 10.1111/j.1523-5378.2012.00967.x
Nakane, T., Fukunaga, S., Nakano, D., Tsutsumi, T., Tanaka, H., Chou, T., et al. (2024). Impact of metabolic dysfunction-associated fatty liver disease on the incidence of Helicobacter pylori-negative gastric cancer. Hepatol. Res. 54, 540–550. doi: 10.1111/hepr.14010
Ning, L., Liu, R., Lou, X., Du, H., Chen, W., Zhang, F., et al. (2019). Association between Helicobacter pylori infection and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 31, 735–742. doi: 10.1097/MEG.0000000000001398
Pal, D., Dasgupta, S., Kundu, R., Maitra, S., Das, G., Mukhopadhyay, S., et al. (2012). Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285. doi: 10.1038/nm.2851
Palmblad, J., Hallberg, D., and Engstedt, L. (1980). Polymorphonuclear (PMN) function after small intestinal shunt operation for morbid obesity. Br. J. Haematol. 44, 101–108. doi: 10.1111/j.1365-2141.1980.tb01188.x
Polyzos, S. A. and Kountouras, J. (2019). Helicobacter pylori infection and nonalcoholic fatty liver disease: Time for large clinical trials evaluating eradication therapy. Helicobacter 24, e12588. doi: 10.1111/hel.2019.24.issue-3
Polyzos, S. A., Nikolopoulos, P., Stogianni, A., Romiopoulos, I., Katsinelos, P., and Kountouras, J. (2014). Effect of Helicobacter pylori eradication on hepatic steatosis, NAFLD fibrosis score and HSENSI in patients with nonalcoholic steatohepatitis: a MR imaging-based pilot open-label study. Arq. Gastroenterol. 51, 261–268. doi: 10.1590/S0004-28032014000300017
Qu, Y., Song, Y. Y., Chen, C. W., Fu, Q. C., Shi, J. P., Xu, Y., et al. (2021). Diagnostic performance of fibroTouch ultrasound attenuation parameter and liver stiffness measurement in assessing hepatic steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Clin. Transl. Gastroenterol. 12, e00323. doi: 10.14309/ctg.0000000000000323
Quaresma, J. A., Barros, V. L., Pagliari, C., Fernandes, E. R., Guedes, F., Takakura, C. F., et al. (2006). Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology 345, 22–30. doi: 10.1016/j.virol.2005.09.058
Roper, J., Francois, F., Shue, P. L., Mourad, M. S., Pei, Z., Olivares de Perez, A. Z., et al. (2008). Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J. Clin. Endocrinol. Metab. 93, 2350–2357. doi: 10.1210/jc.2007-2057
Schwartz, M. W., Seeley, R. J., Campfield, L. A., Burn, P., and Baskin, D. G. (1996). Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 98, 1101–1106. doi: 10.1172/JCI118891
Shah, P., Rathi, P., Mandot, A., Pal, A., and Ahire, D. (2020). Study and comparison of metabolic profile of lean and obese subjects with non alcoholic fatty liver disease. J. Assoc. Phys. India. 68 (8), 51–54.
Shintani, M., Ogawa, Y., Ebihara, K., Aizawa-Abe, M., Miyanaga, F., Takaya, K., et al. (2001). Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 50, 227–232. doi: 10.2337/diabetes.50.2.227
Sun, D. Q., Jin, Y., Wang, T. Y., Zheng, K. I., Rios, R. S., Zhang, H. Y., et al. (2021). MAFLD and risk of CKD. Metabolism 115, 154433. doi: 10.1016/j.metabol.2020.154433
Suzuki, H., Marshall, B. J., and Hibi, T. (2006). Overview: Helicobacter pylori and extragastric disease. Int. J. Hematol. 84, 291–300. doi: 10.1532/IJH97.06180
Upala, S., Jaruvongvanich, V., Riangwiwat, T., Jaruvongvanich, S., and Sanguankeo, A. (2016). Association between Helicobacter pylori infection and metabolic syndrome: a systematic review and meta-analysis. J. Dig. Dis. 17, 433–440. doi: 10.1111/cdd.2016.17.issue-7
von Loeffelholz, C., Horn, P., Birkenfeld, A. L., Claus, R. A., Metzing, B. U., Docke, S., et al. (2016). Fetuin A is a predictor of liver fat in preoperative patients with nonalcoholic fatty liver disease. J. Invest. Surg. 29, 266–274. doi: 10.3109/08941939.2016.1149640
Wang, W., Fan, M., Gong, R., Zhang, Y., Zeng, J., Xu, S., et al. (2022). Helicobacter pylori infection is not an independent risk factor of non-alcoholic fatty liver disease in China. BMC Gastroenterol. 22, 81. doi: 10.1186/s12876-022-02148-6
Xu, X., Zhou, X., Tian, T., Ding, Y., Yu, C., Zhao, W., et al. (2023). Comparison of clinical characteristics and outcomes of MAFLD and NAFLD in Chinese health examination populations. J. Clin. Transl. Hepatol. 11, 777–786. doi: 10.14218/JCTH.2022.00154
Yang, C., Yang, S., Xu, W., Zhang, J., Fu, W., and Feng, C. (2017). Association between the hyperuricemia and nonalcoholic fatty liver disease risk in a Chinese population: A retrospective cohort study. PloS One 12 (5), e0177249. doi: 10.1371/journal.pone.0177249
Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., and Wymer, M. (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84. doi: 10.1002/hep.28431
Younossi, Z. M., Paik, J. M., Al Shabeeb, R., Golabi, P., Younossi, I., and Henry, L. (2022). Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology 76, 1423–1437. doi: 10.1002/hep.32499
Yuan, Q., Wang, H., Gao, P., Chen, W., Lv, M., Bai, S., et al. (2022). Prevalence and risk factors of metabolic-associated fatty liver disease among 73,566 individuals in Beijing, China. Int. J. Environ. Res. Public Health 19 (4), 2096. doi: 10.3390/ijerph19042096
Yuan, X., Wang, X., Wu, S., Chen, S., Wang, Y., Wang, J., et al. (2023). Associations between metabolic dysfunction-associated fatty liver disease and extrahepatic cancers: a cohort in China. Hepatobil. Surg. Nutr. 12, 671–681. doi: 10.21037/hbsn-21-546
Zhou, B. G., Yang, H. J., Xu, W., Wang, K., Guo, P., and Ai, Y. W. (2019). Association between Helicobacter pylori infection and nonalcoholic fatty liver disease: A systematic review and meta-analysis of observational studies. Helicobacter 24, e12576. doi: 10.1111/hel.2019.24.issue-3
Keywords: metabolic dysfunction-associated fatty liver disease (MAFLD), Helicobacter pylori (H. pylori), body mass index (BMI), overweight, lean
Citation: Chen X, Fu J, Jin K, Yang Z, Qian Y, Mei K, Wang Y, Min J, Du Y, Zhu Z and Li S (2025) Overweight and Helicobacter pylori infection: a correlation in metabolic dysfunction-associated fatty liver disease. Front. Cell. Infect. Microbiol. 15:1565298. doi: 10.3389/fcimb.2025.1565298
Received: 22 January 2025; Accepted: 26 May 2025;
Published: 18 June 2025.
Edited by:
Anna Katrina Walduck, Charles Sturt University, AustraliaReviewed by:
Sasikala Muthusamy, Brigham and Women's Hospital and Harvard Medical School, United StatesWaleed Samy, Professor of InterMedicine, Egypt
Copyright © 2025 Chen, Fu, Jin, Yang, Qian, Mei, Wang, Min, Du, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaisheng Zhu, emh1emFpc2hlbmdAd211LmVkdS5jbg==; Shengcun Li, bGlzaGVuZ2N1bkB3bXUuZWR1LmNu
†These authors have contributed equally to this work
 Xu Chen
Xu Chen Jiayue Fu
Jiayue Fu Kejia Jin
Kejia Jin Zixuan Yang3,4
Zixuan Yang3,4 Yidan Qian
Yidan Qian Kehan Mei
Kehan Mei Zaisheng Zhu
Zaisheng Zhu Shengcun Li
Shengcun Li