- 1Department of Microbiology, JSS Medical College and Hospital, JSS Academy of Higher Education and Research, Mysuru, India
- 2Department of Microbiology, Mysore Medical College and Research Institute, Mysuru, India
- 3Quorum Sensing Laboratory, School of Chemical and Biotechnology, Sastra Deemed to be University, Thanjavur, India
Introduction: Escherichia coli (E. coli) causes most cases of the urinary tract infections (UTIs) via virulence factors like biofilms. This study identifies key phenotypic and genotypic virulence attributes of Uropathogenic Escherichia coli.
Methodology: A total of 180 uropathogenic E. coli (UPEC) isolated from patients with different categories (cystitis, pyelonephritis, recurrent UTI, catheter-associated UTI, and asymptomatic bacteriuria) of UTI and 30 commensal E. coli isolated from healthy individuals were evaluated for biofilm production by phenotypic methods using tissue culture plate, tube adherence, and Congo red method, and RT-PCR was used to genetically characterize them.
Results: This study analyzed 1,600 urine samples from UTI patients, with 498 showing significant bacterial growth and 180 identifying E. coli as the pathogen. The female-to-male ratio of UTI cases was 0.74. Antibiotic susceptibility testing revealed 100% sensitivity to tigecycline and fosfomycin as well as 89.44%, 86.11%, 81.66%, and 72.22% sensitivity to nitrofurantoin, amikacin, imipenem, and meropenem, respectively. Only 64.44% were sensitive to ciprofloxacin, with 10% being multidrug-resistant (MDR). Moreover, 18.33% of the UPEC isolates produced mettalo-beta-lactamases (MBL), and 13.33% produced AmpC beta-lactamases. Biofilm production was observed in 72.22% of UPEC isolates compared to 16.66% in commensal isolates. The biofilm-forming UPEC, compared to commensal E. coli, has significantly higher antibiotic resistance, with a 128-fold reduction in ciprofloxacin susceptibility. Additionally, the fimH gene was detected in 98.33% of the UPEC isolates.
Conclusion: This study shows that UPEC strains produce specific virulence determinants like adhesion to uroepithelial cells. Screening for virulence factors should be integrated into microbiology laboratories. Specific virulence genes linked to UPEC may serve as potential targets for prophylactic strategies to prevent recurrent infections and improve management.
1 Introduction
Urinary tract infection (UTI) results in the inflammation of the urinary tract due to the growth of a significant number (>105 CFU/ml) of uropathogens. UTIs can be classified depending on the severity, such as urosepsis, pyelonephritis, and cystitis. Clinically, UTIs can be classified as uncomplicated and complicated UTIs. UTI is the most common reason for visiting a healthcare facility, with approximately 150 million people developing UTIs annually. Women are more prone to develop UTI, and it is estimated that 40% of women develop UTI at least once during their lifetime, and about 11% of women above the age of 18 years develop an episode of UTI per year. Though UTIs can be treated effectively by antibiotics, recurrence is widespread. Recurrent UTIs may be due to bacterial virulence factors and host deficiencies (Foxman and Brown, 2003; Micali et al., 2014; Flores-Mireles et al., 2015).
Uropathogenic Escherichia coli (UPEC) is responsible for approximately 90% of community-acquired and 50% of nosocomial UTIs. This may be due to a multitude of virulence factors (VFs), which facilitate them to survive, grow, and persist in the adverse settings of the urinary tract (Epp and Larochelle, 2010; Soto et al., 2011; Jhang and Kuo, 2017). These virulence factors are coded by large regions of mobile genomic material called genomic islands (GI). The genomic islands containing more than one virulence gene are called pathogenicity islands (PAIs). VFs in UPEC can be classified as cell surface factors, including type 1 fimbriae (fimH), P fimbriae (pap), S fimbriae (sfa), F1C fimbriae, afimbrial adhesion I (afaI), thin aggregative fimbriae (also called curli), flagella, capsule, outer membrane proteins, and lipopolysaccharides. Additionally, there are exported virulence factors, which include alpha-haemolysin (hlyA), cytotoxic necrotizing factor 1, cytolethal distending toxin, secreted autotransporter toxin, cytolysin A, and siderophores like enterobactin, aerobactin (aer), and yersiniabactin (Hooton and Stamm, 1997; Oelschlaeger et al., 2002; Vila et al., 2002; Yan and Polk, 2004; Wiles et al., 2008).
The cell surface factors enable UPEC to form multicellular communities called biofilms. Biofilm formation allows UPEC to persist in the urinary tract by providing several survival advantages, including antibiotic resistance, expression of various virulence factors through quorum sensing, and resistance to host defense mechanisms such as phagocytosis. Infections caused by biofilm-producing UPEC strains are challenging to treat due to their high levels of resistance to antibiotics (Mobley and Warren, 1996; Hooton and Stamm, 1997; Oelschlaeger et al., 2002; Wiles et al., 2008). These infections have been linked to recurrent infections and prolonged hospital admissions, resulting in increased healthcare costs and a greater risk of acquiring additional nosocomial infections. Consequently, there is an elevated likelihood of increased morbidity and mortality. Therefore, it is crucial to determine the phenotypic and genotypic virulence attributes of UPEC, especially its biofilm-producing capability. This approach aids in improving the management and prognosis of UTIs, reducing their economic burden, enhancing treatment plans, assessing patient risks, improving infection control procedures, and allocating resources to reduce antimicrobial resistance. Thus, the present study aimed to determine the biofilm-forming capacity of UPEC.
2 Methodology
2.1 Sample collection/study design
This laboratory-based prospective study was conducted at a tertiary care hospital in Mysuru, Karnataka. The present study evaluated and compared the biofilm-forming capacity of UPEC and intestinal commensal E. coli. Urine samples sent for culture and sensitivity from clinically suspected cases of UTI were received in the laboratory and processed according to the standard protocol.
A total of 180 urine samples with significant pyuria (≥5 inflammatory cells) and significant growth of E. coli (≥105 log colony-forming units) were included in the study. Gram-positive and Gram-negative organisms other than E. coli isolated from the urine specimens, E. coli isolated in insignificant numbers (<105), and repeat isolates from the same patient were excluded from the study. Relevant patient demographic and clinical information details were collected from the hospital information system and medical records. Stool samples from healthy adults were collected after informed consent and cultured on MacConkey agar. The lactose-fermenting colonies identified as E. coli were included in the study as intestinal commensal E. coli. A total of 30 intestinal commensal E. coli were included in the study.
2.2 Sample processing
Microscopic examination: Uncentrifuged urine samples were examined microscopically using the wet mount technique under a 40× objective lens to screen for inflammatory cells, red blood cells, and organisms. A finding of ≥5 inflammatory cells per high-power field (HPF) was considered significant pyuria (Baron and Finegold, 2007).
Semiquantitative culture: Semiquantitative culture was carried out using a sterile calibrated nichrome wire loop delivering 0.001 mL of urine (measuring 2 mm in internal diameter) on Urichrome agar (UCA). The inoculated UCA plates were incubated aerobically at 37°C for 18 to 24 h. After incubation, violet-colored E. coli colonies on UCA were counted using a magnifying lens. The number of colonies counted was multiplied by 1,000 to calculate the colony-forming unit (CFU) per milliliter of urine. The presence of 100,000 or more colonies was considered a significant bacteriuria. The violet-colored E. coli colonies were confirmed using an automated identification system (Vitek-2 compact system by bioMérieux, France).
Antimicrobial susceptibility testing (AST): Antibiotic sensitivity testing was carried out using an automated Vitek-2 compact system by bioMérieux, France. The results were interpreted according to the CLSI guidelines (M-100, 31st edition) (Clinical and Laboratory Standards Institute, 2020). Additionally, the antimicrobial susceptibility testing for fosfomycin was carried out using an Epsilometer strip (E-strip) on cation-adjusted Muller Hinton Agar (MHA) (HiMedia Laboratories, Mumbai, India).
2.3 Phenotypic methods for the detection of biofilm formation among UPEC
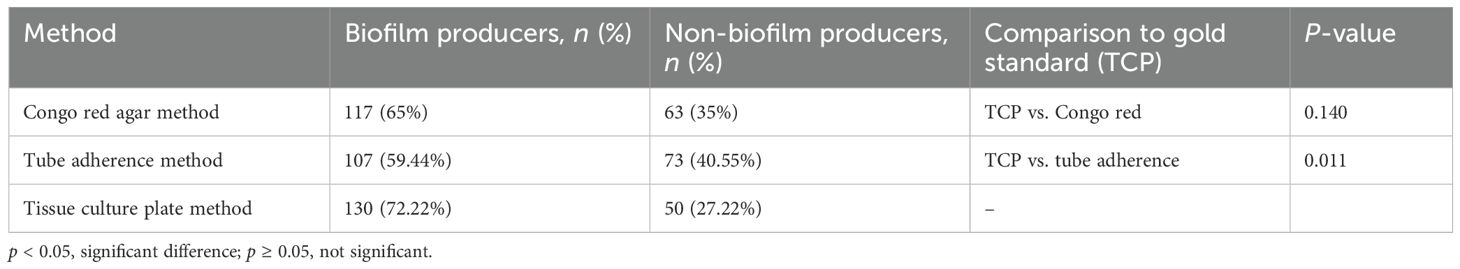
This study used three phenotypic methods to detect biofilm formation among UPEC and intestinal commensal E. coli. Among the three methods, the tissue culture plate method was considered the gold standard for biofilm detection, but it is labor-intensive and technically demanding. Hence, we evaluated the efficacy of simpler alternatives, the Congo red agar method (CRA) and tube adherence methods, to determine their reliability as screening tools.
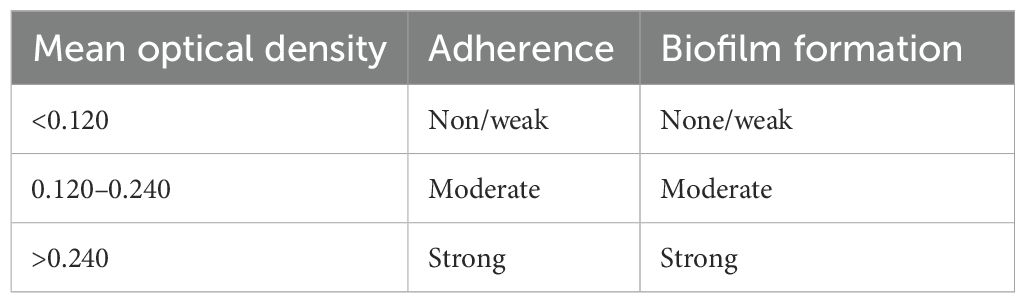
Tissue culture plate method (TCM): The organisms from fresh agar plates were inoculated in trypticase soy broth with 2% glucose and incubated for 24 h at 37°C in stationary conditions. The broth was diluted 1:100 with fresh medium. Individual sterile polystyrene wells of a 96-well flat-bottomed tissue culture plate (TCP) were filled with 200 µL of diluted cultures. Only the medium in the well served as a control to check the sterility of the media and the nonspecific binding of the media to the well. The TCP was incubated for 24 h at 37°C. After incubation, the contents of each well were gently removed by tapping the plates. The wells were washed four times with 0.2 mL of phosphate-buffered saline (PBS, pH 7.2) to remove free-floating planktonic bacteria. Biofilms formed by adherent “sessile” organisms in a plate were fixed with sodium acetate (2%) and stained with crystal violet (0.1% w/v) for 1 min. Excess stain was rinsed off by washing with deionized water, and the plates were kept for drying. The biofilms formed and stained uniformly with crystal violet. The optical density (OD) of stained adherent bacteria was determined with a micro-ELISA auto reader (model 680, Bio-Rad) at a wavelength of 570 nm (OD570). This OD value was considered as an index of bacterial adherence/biofilm (Christensen et al., 1985).
The experiment was performed in triplicate; the data generated was averaged, and the standard deviation was calculated. To compensate for background absorbance, OD readings from sterile medium, fixation, and dye were averaged and subtracted from all test values. S. epidermidis ATCC 35984 was used as a positive control for biofilm formation, and S. epidermidis ATCC 12228 was used as a negative control.
Classification of bacterial adherence was based on the OD values obtained for individual strains, categorized as strongly positive, moderately positive, weakly positive, and non-biofilm forming, as indicated in Table 1 (Park et al., 2009).
Tube adherence method (TAM): A loopful of the test organisms was inoculated into a test tube containing 10 mL of sterile brain heart infusion broth. The tube was incubated aerobically at 36°C ± 1°C for 24 h. The tube content was discarded, and the tube was washed with 9 mL phosphate buffer saline at ph 7.2. The biofilm formed was fixed by adding 10 mL of freshly prepared sodium acetate (2%) into each tube and leaving it for 10 min. The contents of the tube were discarded, and 10 mL of crystal violet (0.1%) was added to each tube and left at room temperature for 30 min. The stain was discarded, and the washing step was repeated. The tubes were allowed to dry in an inverted position at room temperature. Interpretation: Biofilm formation was detected by the presence of visible film on the wall and bottom of the tube. The biofilm was graded visually as absent, moderate, and strong biofilm formation (Christensen et al., 1982).
Congo red agar method (CRA): Congo red agar was prepared by mixing brain heart infusion agar (37 g) and sucrose (50 g) in 800 mL of distilled water and autoclaving the mixture. Congo red stain (200 mL) was added when the agar cooled to 55°C. The test organisms were plated onto the CRA plate and incubated aerobically at 37°C for 24 h. Interpretation: A black-colored colony was considered a biofilm producer (Freeman et al., 1989).
2.4 Genotypic attributes of biofilm among E. coli isolates
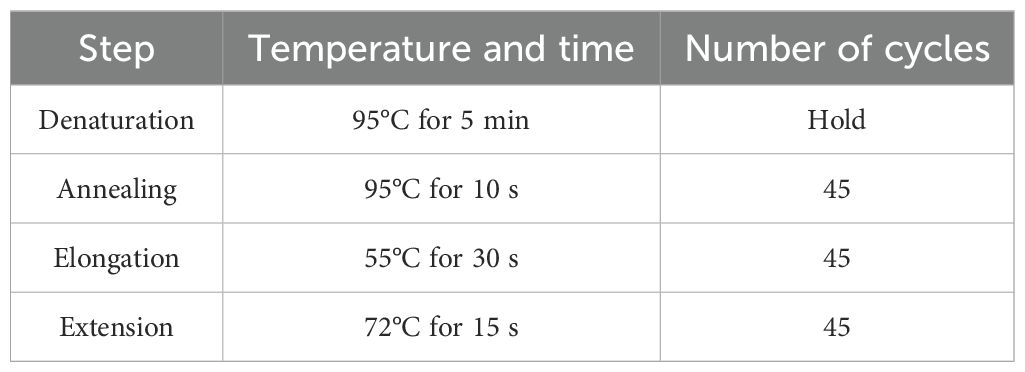
Deoxyribonucleic acid (DNA) was extracted from fresh 24-h cultures of the test isolates grown on nutrient agar using a commercial Spinster total DNA extraction kit (ADT Biotech, Kuala Lumpur, Malaysia) following the manufacturer’s protocol. The extracted nucleic acid was subjected to real-time multiplex polymerase chain reaction (PCR) for the detection of six adhesion genes. Type-it HRM master mix (purchased from Qiagen company from Germany) was used according to the manufacturer’s instructions. Multiplex PCR was employed to identify six adhesion genes, namely, fimH (465 bp), papC (203 bp), papGII (190 bp), papGIII (258 bp), afa/draBC (593 bp), and sfa/focDE (408 bp). The details of the primers are given in Table 2. Based on the melting curve of gene amplicons, a multiplex PCR was designed. The temperature profile for real-time PCR is detailed in Table 3. The melting curve analysis was done at 70°C to 90°C.
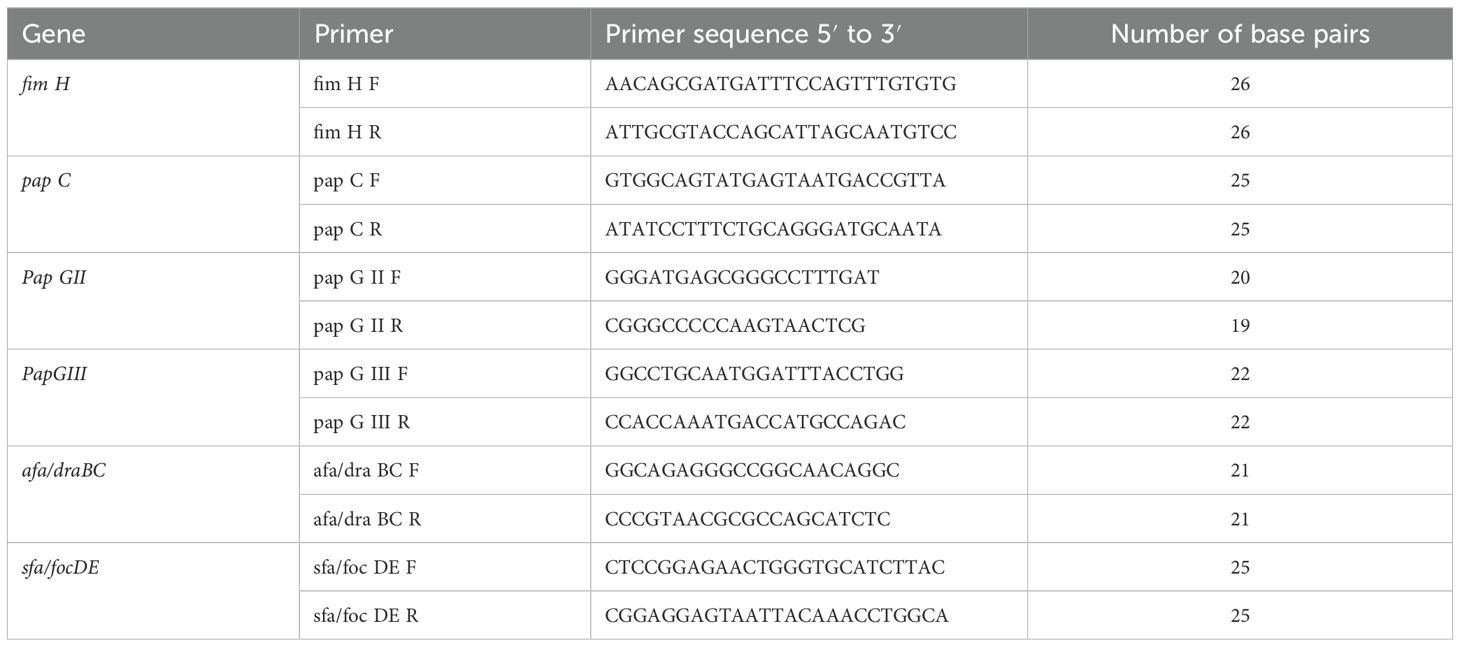
Table 2. The details of the primers’ reference (Yamamoto et al., 1995; Johnson and Stell, 2000; Usein et al., 2001; Wang et al., 2002; Mapes et al., 2007; Park et al., 2009; Yun et al., 2014; Munkhdelger et al., 2017; Rashki et al., 2017).
Statistical analysis: Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 17.0 software package. We applied descriptive statistics such as percentage, mean, and standard deviation. Chi-square test was applied. The difference and association were interpreted as statistically significant when p was less than 0.05.
3 Results
3.1 Patients’ demographic and clinical characteristics
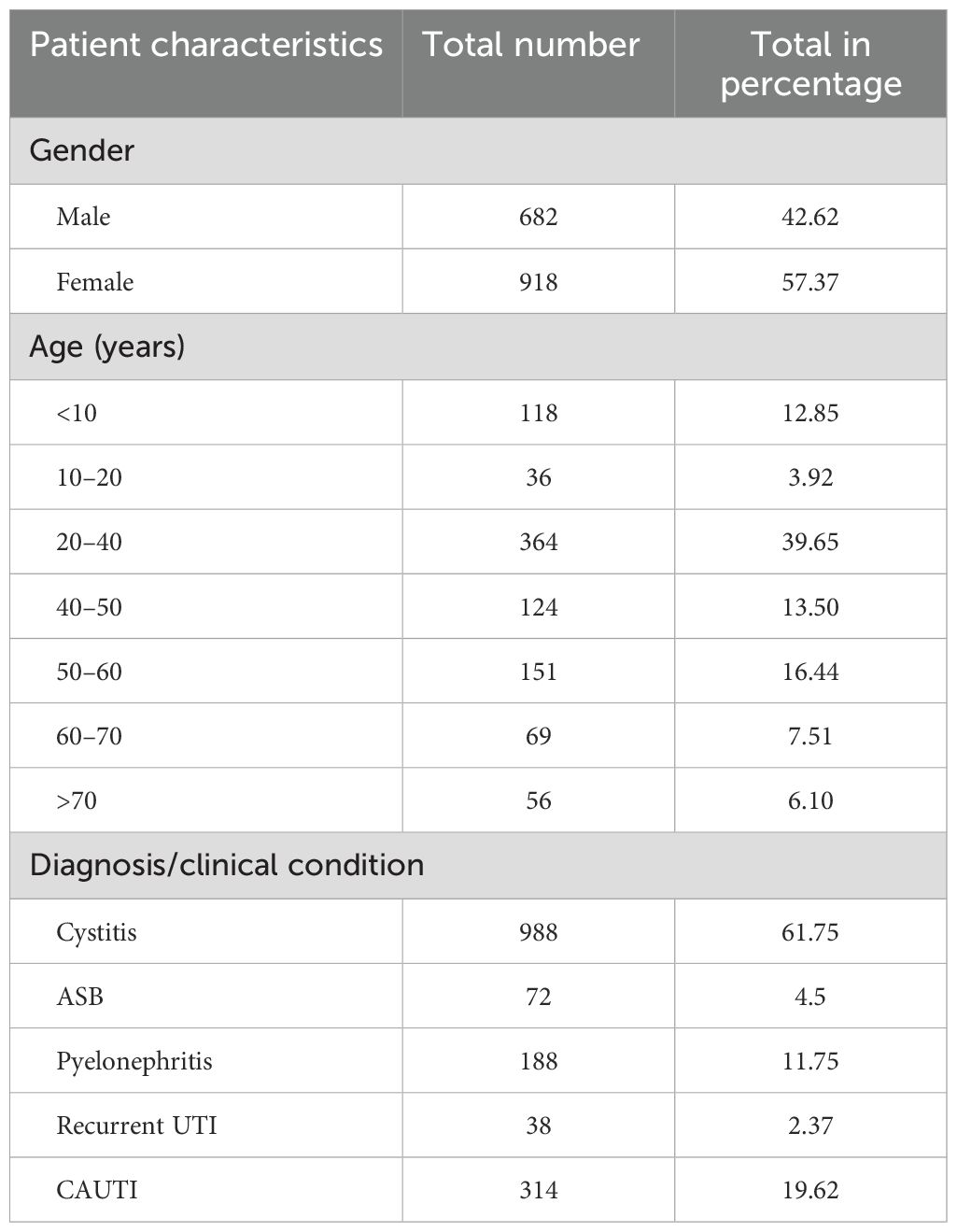
In this study, 1,600 urine samples were received from patients with suspected UTIs. Out of these, 918 (57.37%) samples were from female patients and 682 (42.62%) samples were from male patients, with a female-to-male ratio of 0.74. The majority of the patients belonged to the age group of 20–40 years (n = 618, 38.62%), followed by 50–60 years (n = 266, 16.62%). Table 4 shows the demographic and clinical profile of the study samples. Of the 1,600 urine samples received, 988 (61.75%) samples were from patients with cystitis, 188 (11.75%) from pyelonephritis, 314 (19.62%) from catheterized patients, 72 (4.5%) from asymptomatic bacteriuria (ABU), and 38 (2.37%) from patients with recurrent UTIs.
3.2 Semiquantitative culture results
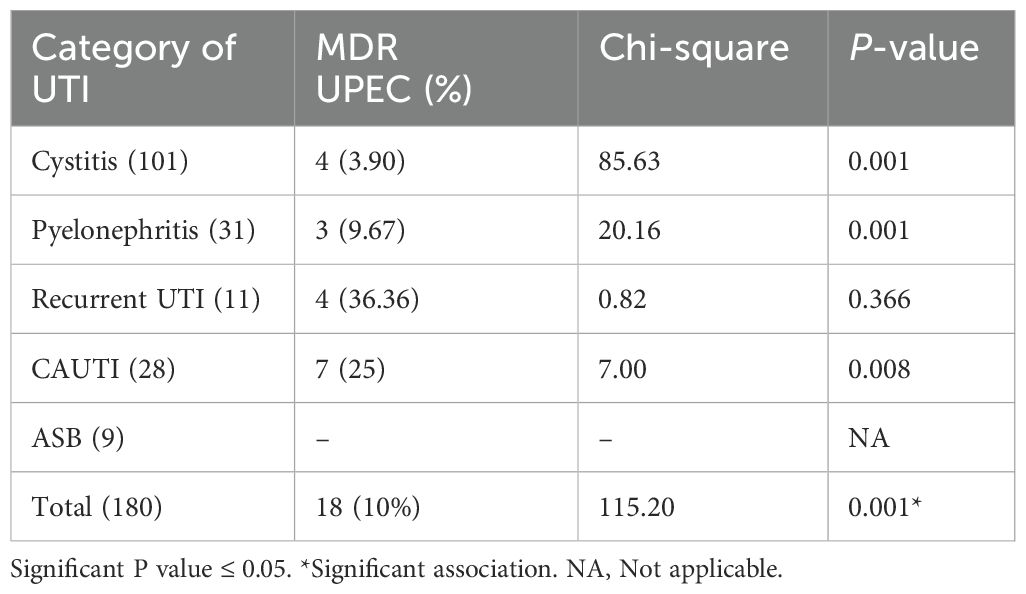
Of the 180 UPEC isolates included in the study, 101 (56.11%) were from patients with cystitis, 31 (17.22%) were from patients with pyelonephritis, 28 (15.55%) were from catheterized patients, 11 (6.11%) were from patients with recurrent UTI, and nine (5%) were from patients with asymptomatic bacteriuria.
3.3 Antibiotic susceptibility pattern of UPEC and commensal Escherichia coli
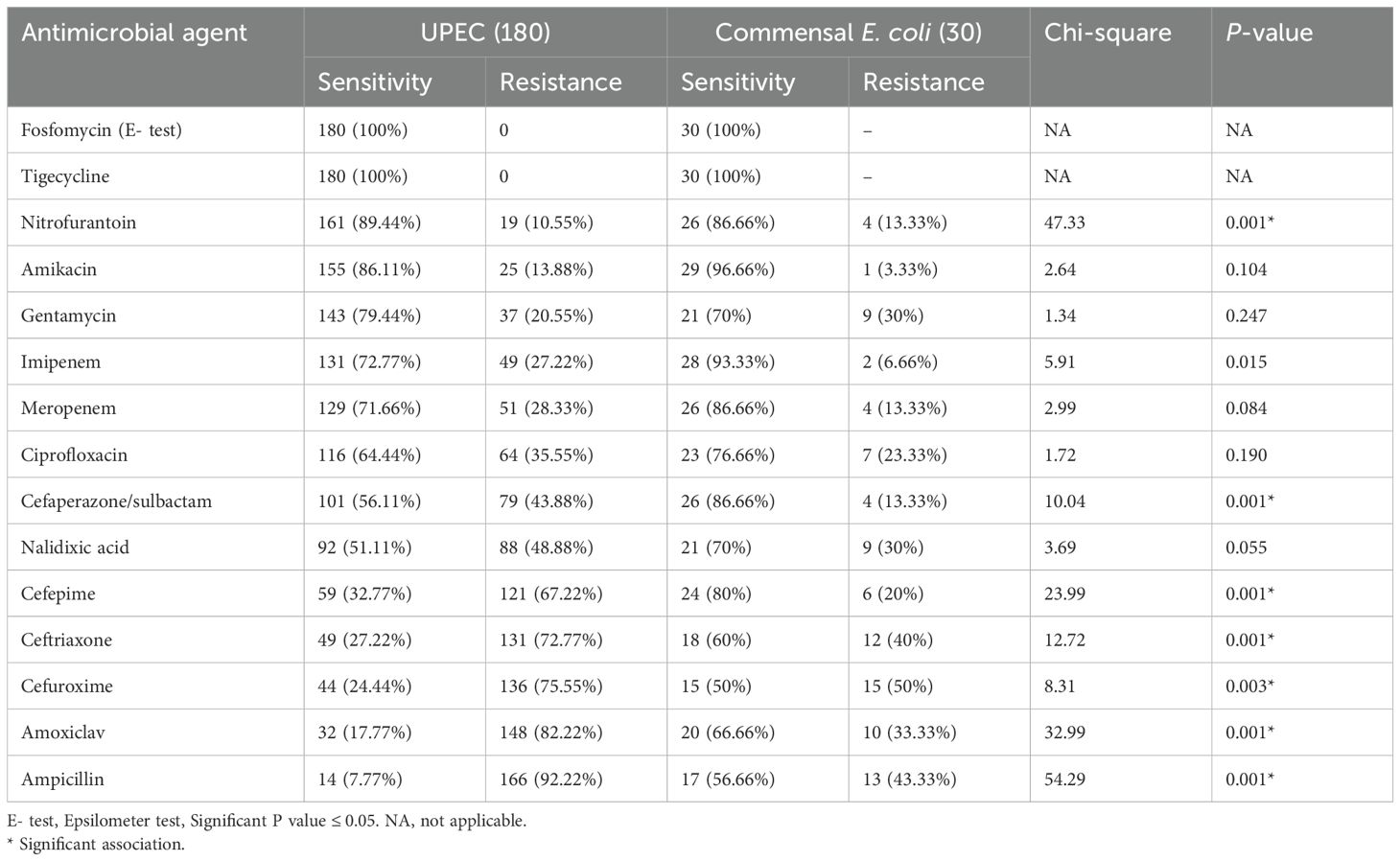
As given in Table 5, the antibiotic susceptibility profile for UPEC isolates in this study revealed high sensitivity to nitrofurantoin (89.44%), followed by amikacin (86.11%), imipenem (72.77%), meropenem (71.66%), and cefoparazone/sulbactam (56.11%). Notably, all UPEC isolates (100%) were sensitive to fosfomycin and tigecycline. None of the commensal E. coli were multidrug-resistant; however, three isolates showed resistance to both carbapenems tested. None of the commensal isolates were MBL or AmpC producers. Antimicrobial resistance was compared between UPEC and fecal E. coli isolates. The UPEC isolates showed a higher degree of resistance when compared to the fecal isolates.
Among the 180 UPEC isolates, 18 were identified as multidrug-resistant (MDR), showing resistance to at least one agent in three or more antibiotic classes. As given in Table 6, among the 18 MDR isolates, seven (38.88%) isolates were from catheterized patients, four (22.22%) were from patients with recurrent UTI, three (16.66%) were from patients with pyelonephritis, and four (22.22%) were from patients with cystitis. This also accounts for 25% of the CAUTI isolates, 36.36% of recurrent UTI isolates, 3.90% of cystitis isolates, and 9.67% of pyelonephritis isolates as MDR. None of the asymptomatic bacteriuria isolates were MDR (Table 6). Additionally, among the 180 UPEC, 33 (18.33%) were metallo-beta-lactamase (MBL) producers and 24 (13.33%) were Amp C producers; among these, 18 and 11 isolates were also MDR, respectively.
3.4 Biofilm formation among UPEC and commensal E. coli
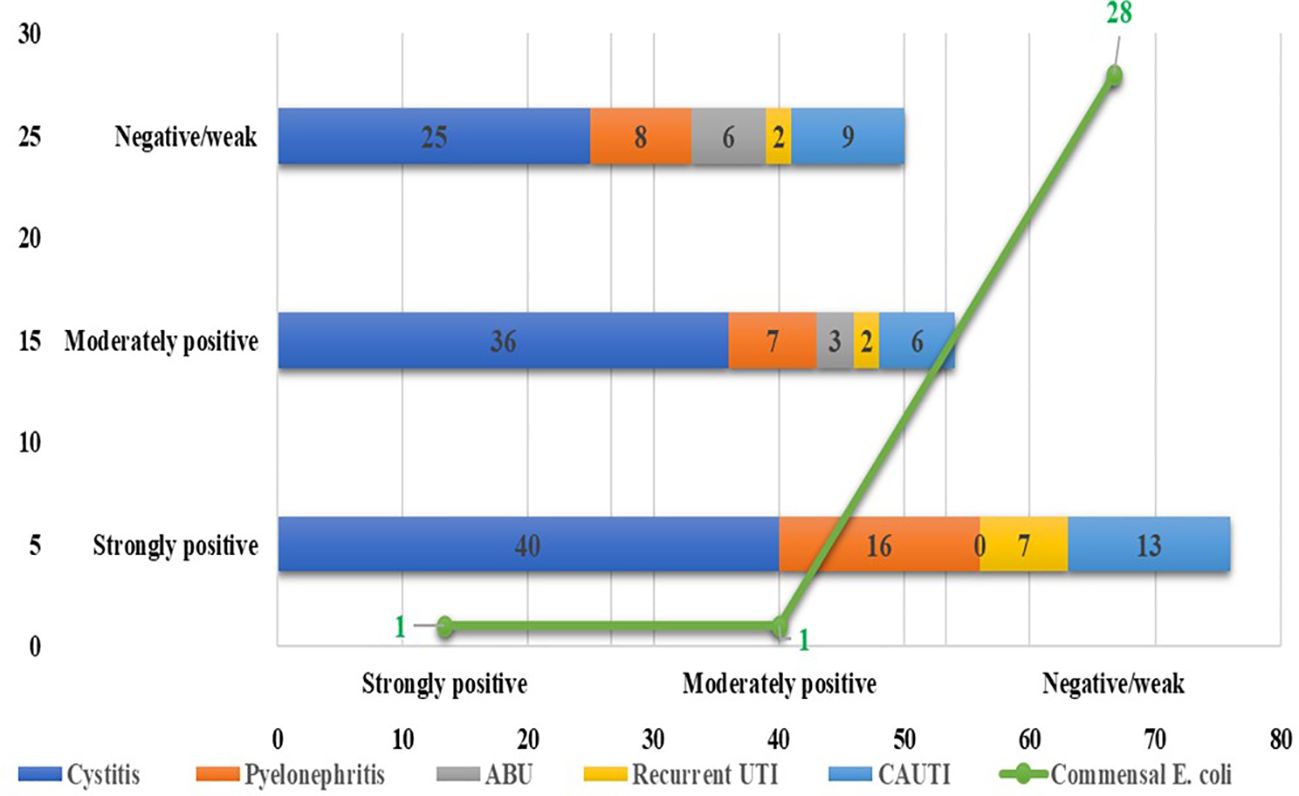
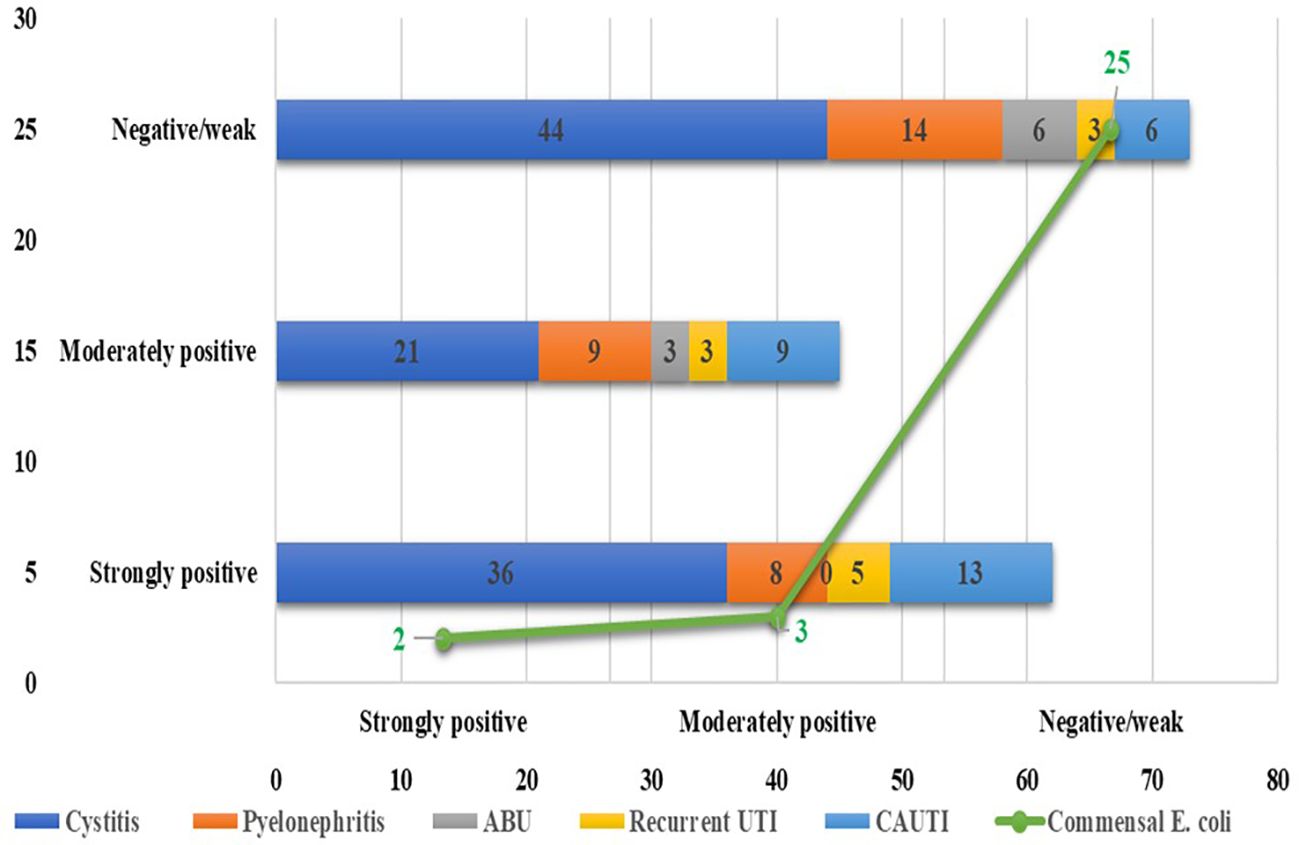
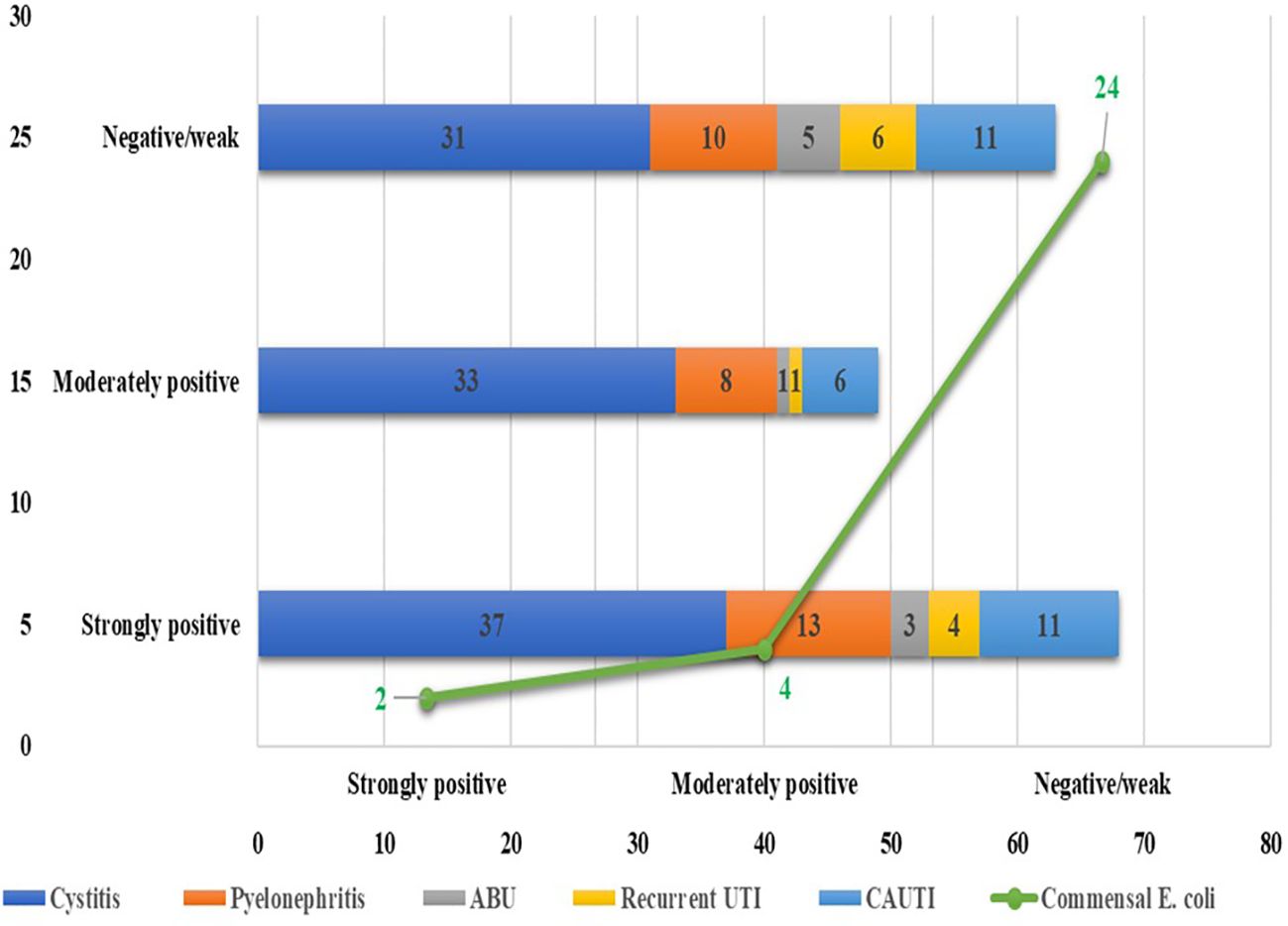
Among the 180 UPEC isolates, biofilm production was detected in 130 (72.22%) isolates by the tissue culture plate method (Figure 1), 107 (59.44%) by the tube adherence method (Figure 2), and 117 (65%) by the CRA method (Figure 3). In comparison, among the 30 commensal E. coli isolates, biofilm formation was observed in only two (6.66%) isolates by the tissue culture plate method, five (16.66%) by the tube adherence method, and six (20%) by the CRA method. The comparison of biofilm detection by different phenotypic methods is provided in Table 7.
The tissue culture plate method, considered the gold standard for identifying biofilm producers, identified 130 biofilm producers (72.22%) and 50 non-biofilm producers (27.78%). In comparison, the Congo red agar method identified 117 biofilm producers (65.0%) and 63 non-biofilm producers (35.0%), with no significant difference observed compared to TCP (p = 0.140), indicating a comparable performance. However, the tube adherence method identified 107 biofilm producers (59.44%) and 73 non-biofilm producers (40.56%) and showed a significant difference compared to TCP (p = 0.011), suggesting that it may underperform relative to the gold standard.
3.5 Prevalence of biofilm-forming genes among UPEC and commensal E. coli
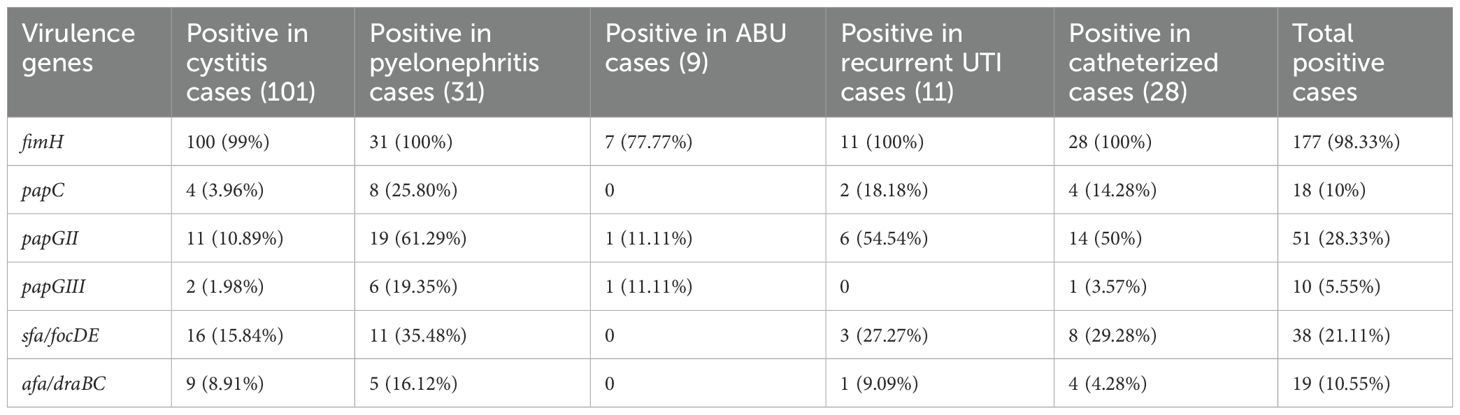
Real-time multiplex PCR identified the fimH gene, which codes for type I fimbriae, in 98.33% of the UPEC isolates. The gene was present in 100% of the pyelonephritis isolates and recurrent UTI and CAUTI isolates, 99% of cystitis isolates, and 77.77% of asymptomatic bacteriuria (ASB) isolates. The papC gene was detected in 79 (43.88%) UPEC isolates, with a higher frequency among recurrent UTI, pyelonephritis, and CAUTI isolates. The sfa/focED gene, coding for S fimbriae, was found in 21.11% of the UPEC isolates, predominantly in those associated with pyelonephritis and CAUTI. Afimbrial adhesins encoded by afa/draBC were observed in only 10.55% of the isolates, mostly among pyelonephritis and cystitis isolates, as detailed in Table 8. Additionally, a comparative analysis of the genotypic results of UPEC and commensal E. coli is given in Table 9.
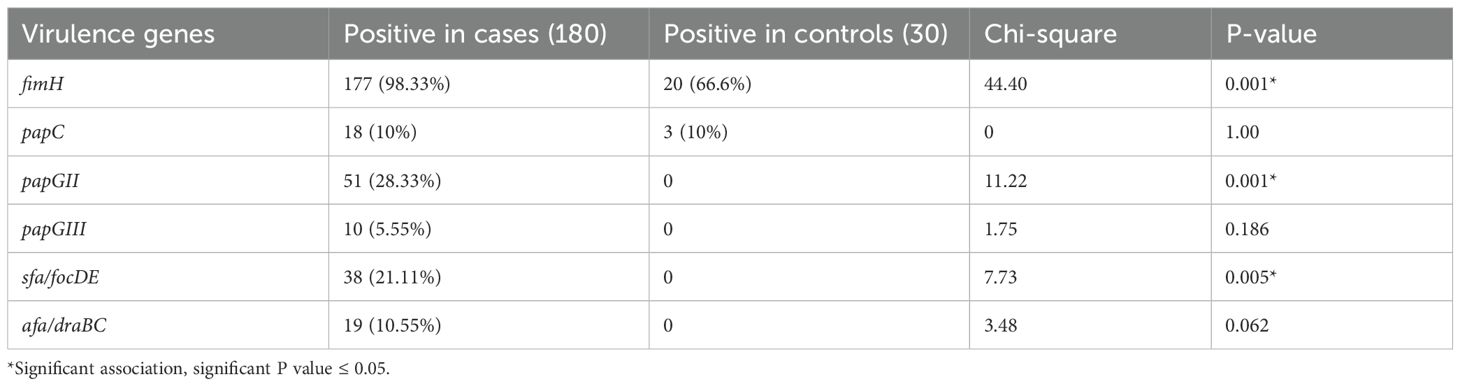
Table 9. Comparison of the presence of various biofilm genes among UPEC and intestinal commensal E. coli.
As shown in Table 9, among the 30 intestinal commensal E. coli isolates, fimH gene was identified in 20 (66.6%) isolates, while the papC gene was detected in three (10%) isolates. However, none of the commensal isolates harbored papGII, papGIII, sfa/focDE, and afa/draBC genes.
3.6 Antimicrobial susceptibility pattern of biofilm-producing and non-biofilm-producing UPEC
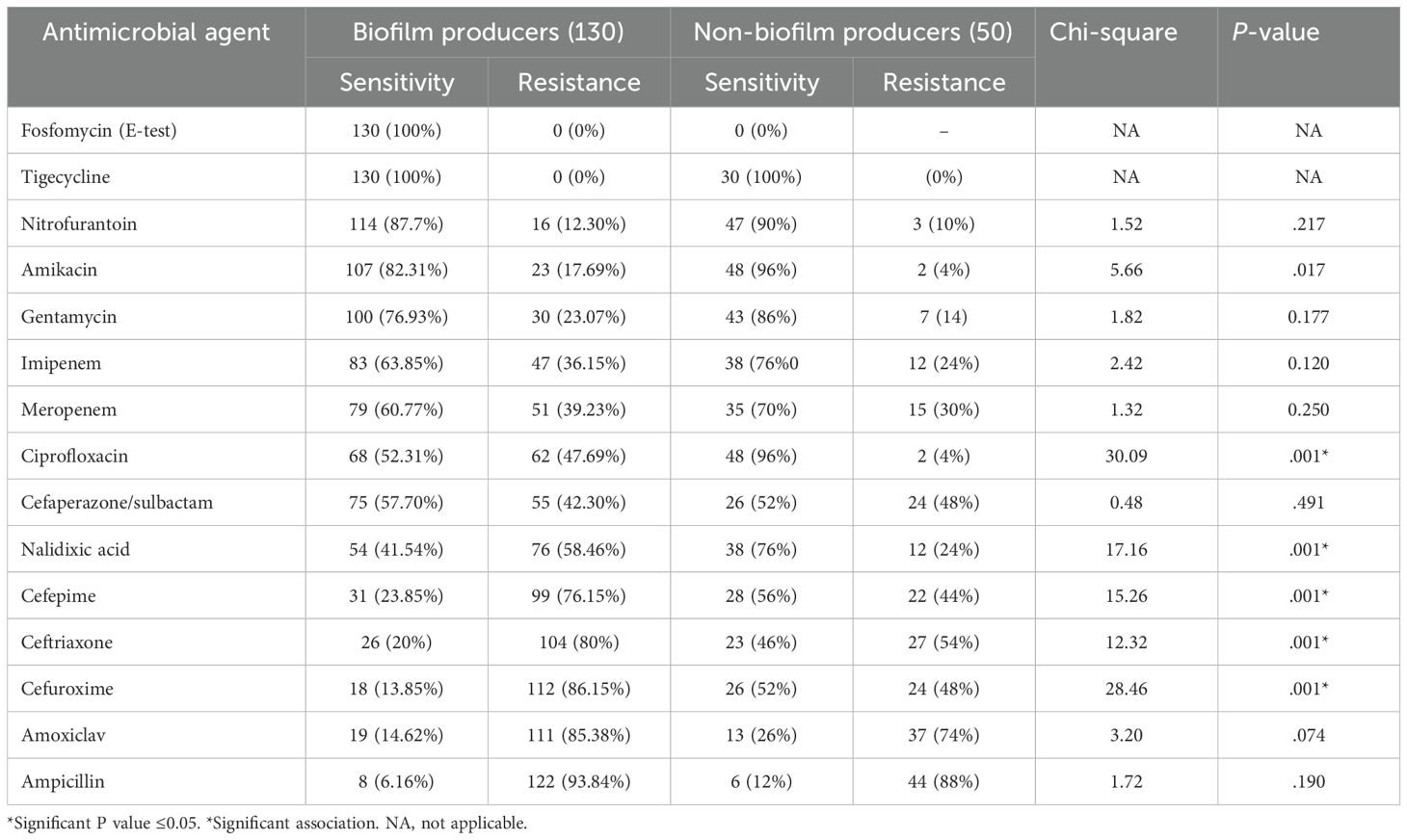
Antimicrobial resistance was compared, and it was observed that the biofilm-producing strains were more resistant to the commonly used antimicrobials than the non-biofilm-producing strains. All of the biofilm-forming isolates were sensitive to fosfomycin and tigecycline, as shown in Table 10.
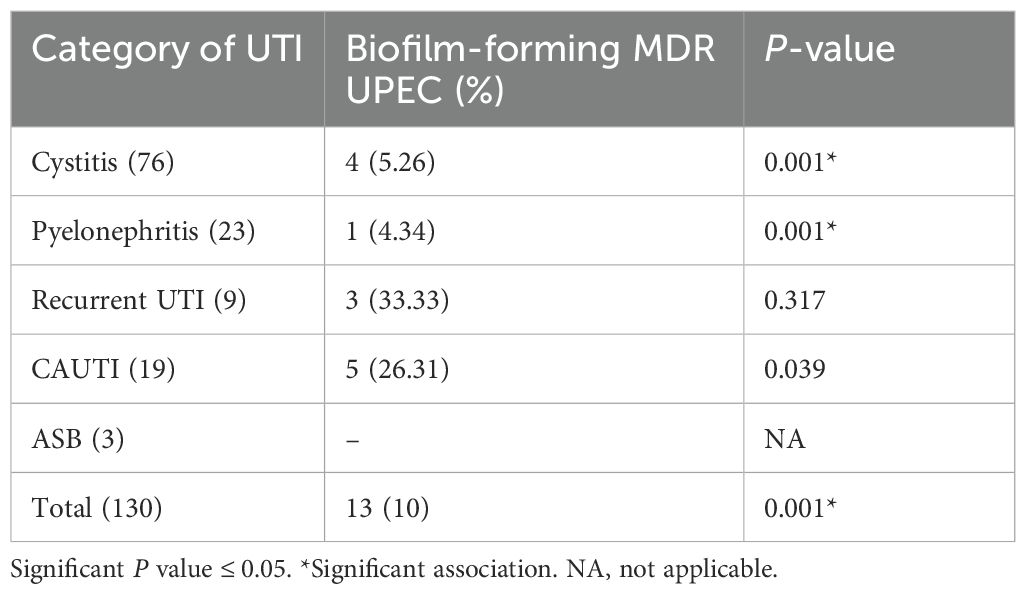
As shown in Table 11, 18 of the 180 UPEC isolates were MDR, of which 13 were biofilm-forming, accounting for 72.22% (13/18) of the MDR isolates to be biofilm-forming. Of the 130 biofilm-forming UPEC, 13 (10%) were MDR isolates, 33.33% of the biofilm-forming recurrent UTI isolates were MDR, and 26.31% of the biofilm-forming catheter isolates were MDR. Of the 130 biofilm-forming isolates, 25 (19.23%) were MBL producers, and among these 25, 13 were also MDR isolates. Among 130 biofilms producing UPEC, 17 (13.07%) were AmpC producers, and among the 17, 10 were MDR isolates.
4 Discussion
UTIs are among the most common bacterial infections in humans. UTIs are a common cause of morbidity and affect persons of all age groups, including young women, children, and the elderly. E. coli accounts for more than 80%–90% of all UTIs (Desai et al., 2013). The ability of UPEC to cause UTI is associated with the expression of a variety of virulence factors (Klemm and Schembri, 2000; Roos et al., 2006). The severity of the infection is dependent on both the virulence of UPEC and also on the susceptibility of the host (Santo et al., 2006). One of the important virulence factors of UPEC is biofilm formation; understanding the rate of biofilm formation will aid in the proper management and initiation of appropriate antibiotics, which will help in the prevention of antimicrobial resistance.
It was observed in our study that among the 180 E. coli isolates, 101 (56.11%) were from patients with cystitis, 31 (17.22%) were from patients with pyelonephritis, 28 (15.55%) were from catheterized patients, 11 (6.11%) were from patients with recurrent UTI, and nine (5%) were from patients with asymptomatic bacteriuria. The main treatment modality for UTIs is the use of antibiotics such as β-lactams, trimethoprim, nitrofurantoin, and quinolone, but due to the widespread misuse of these antibiotics, strains of the isolates have developed, thus making the sensitivity reports essential for the selection of appropriate antibiotics. In this study, it is observed that UPEC exhibit a high degree of resistance to commonly used antibiotics like ampicillin, cefepime, cefuroxime, and ceftriaxone. A maximum number of isolates were sensitive to nitrofurantoin, amikacin, and carbapenems. Furthermore, 100% sensitivity was observed from tigecycline and fosfomycin; these observations correlated with the observations made by Manjula A Vagarali et al. (Annapurna et al., 2014a), M. Eshwarappa et al. (Eshwarappa et al., 2011), and Arindam Chakraborty et al. (Chakraborty et al., 2017). The antibiotic resistance pattern of UPEC was compared with fecal E. coli isolated from healthy individuals. We found that fecal E. coli was more sensitive to the antibiotics tested than the UPEC isolates.
UPEC is one of the most common uropathogens associated with UTI (Raksha et al., 2003; Davis and Flood, 2011; Foxman, 2014). To initiate an infective process in the urinary tract, UPEC has to survive the host defense mechanisms like exfoliation of uroepithelial cells, micturition, and endogenous antimicrobial agents. Given this, UPEC possesses many virulence and fitness factors (Dhakal et al., 2008). Genes located on the pathogenicity island code for the various VFs in UPEC (Rao, 1994). The severity of UTI depends on the number of virulence factors expressed by the uropathogen and also on host susceptibility (Fowler and Stamey, 1977).
In our study, biofilm production was observed in 72.22% of UPEC and was found to be one of the most common virulence factors among UPEC. A similar observation was made by Lalith Meshram et al. (Mesharam et al., 2012), who reported that 76% of UPEC have biofilm-forming capacity. Shah et al. (2019) reported in their study that 62% of UPEC were biofilm producers, and Annapurna et al. (2014b) have reported 73.30% of the isolates to be biofilm producers (Annapurna et al., 2014a). E. Suman et al. (Suman et al., 2007), in their study, also reported a very high rate (92%) of biofilm-forming UPEC, and our results are in contrast to the results obtained by Pramodhini and Niveditha (2012) who reported only 39.60% of E. coli isolates to be biofilm producers. Biofilm formation is an important virulence factor which gives several survival advantages to the isolate. It allows them to persist in the urinary tract by protecting them from host defense mechanisms, and it also renders the isolates resistant to antimicrobial agents, which interferes with their eradication (Jadhav et al., 2011; Fattahi et al., 2015; Vijayalakshmi et al., 2015).
In this study, 6.6% of the commensal E. coli were biofilm producers; this finding is statistically significant. A similar observation was made by Fattahi et al., Karam et al., and Soto et al., who have reported a higher prevalence of biofilm formation among UPEC than in controls (Jadhav et al., 2011; Fattahi et al., 2015; Vijayalakshmi et al., 2015; Karam et al., 2018). However, 6% of our control strains were biofilm producers. This could be because it is the usual living condition of bacteria in natural environments. Biofilm formation was most commonly associated with UPEC isolated from CAUTI (92.85%), followed by isolates from recurrent UTI (81.81%) and acute cystitis, and only 11.1% of ABU isolates were biofilm producers. These findings are in agreement with the findings of Karigoudar et al. (2019) who have reported 89.5% of CAUTI isolates to be biofilm producers. Tabasi et al. (2015) have reported a high incidence of biofilm production among recurrent UTI isolates, which is in correlation with our study.
In the present study, biofilm-forming UPEC were resistant to ampicillin (93.84%), followed by amoxiclav (85.38%), cefuroxime (86.15%), ceftriaxone (80%), and cefepime (76.15%). Most of the biofilm-producing UPEC were sensitive to aminoglycosides and carbapenems, and all of the strains were sensitive to nitrofurantoin, tigecycline, and fosfomycin. A similar high resistance to various antibiotics was noted by Tajbakhsh et al., R. karigowder et al., Poovendran et al., Karam et al., Tadepalli et al., Tabasi et al., and Sevanan et al., all of whom have also reported a higher frequency of resistance to antibiotics among biofilm producers (Sevanan et al., 2011; Poovendran and Ramanathan, 2014; Tabasi et al., 2015; Tadepalli et al., 2016; Tajbakhsh et al., 2016; Karam et al., 2018; Karigoudar et al., 2019). In the present study, a comparative increase in the resistance among biofilm producers to all the tested antibiotics was noted; however, a statistically significant correlation was observed with nalidixic acid, ciprofloxacin, cefuroxime, ceftriaxone, and cefepime.
In our study, 18 out of the 180 UPEC were found to be MDR, of which 13 were biofilm producers; this accounted for 72.22% (13/18) of the MDR isolates to be biofilm producers. Of the 130 biofilms forming UPEC, 13 (10%) were MDR isolates. In addition, 33.33% and 26.31% of the biofilm-forming UPEC from recurrent UTI and CAUTI were MDR. A statistically significant association between biofilm production and MDR was observed. This is in contrast to the study by Shrestha et al. (Shrestha et al., 2019). The higher rate of resistance among biofilm producers is attributed to insufficient antimicrobial concentration within the biofilm matrix, delayed penetration of the antibiotic into the deeper layers of the biofilms, and the relatively inactive state of the isolate within the biofilm (Fattahi et al., 2015). We report a higher rate of MDR UPEC, and most of the MDR isolates were from CAUTI. These isolates were MDR as they were nosocomial strains and, secondly, because of selective pressure due to the over-the-counter use of broad-spectrum antibiotics.
It is well known that one of the characteristic features of biofilm is its ability to tolerate antibiotics (Bjarnsholt et al., 2005). It is reported that bacteria in biofilms tolerate 100–1,000 times higher concentrations of antibiotics than planktonic cells (Mah and O’Toole, 2001; Donlan and Costerton, 2002; Alhede et al., 2011). Thus, there may be treatment failure when the choice of antibiotics is done based on the MIC report, as MIC determines the minimum inhibitory concentration against the planktonic state and does not quantify the concentration required to inhibit bacteria in biofilm. Thus, determination of MBEC will be more useful to adjust the dosage of the antibiotic required to eradicate bacteria in the biofilm mode of life (Ghanwate, 2012). The MBEC assay was developed by Ceri et al. (Sepandj et al., 2004). The MBEC assay was done to evaluate the change in the susceptibility pattern of one of the common antibiotics used to treat UTI—ciprofloxacin—and one less commonly used antibiotic in our setting—fosfomycin.
In this study, genes coding for adhesins like fimH, papC, papGII, papGIII, Sfa/focDE, and afa/draBC were studied. The fimH gene codes for type I fimbriae, and it was present in 98.33% of the UPEC isolates. The gene was present in 100% of the pyelonephritis, recurrent UTI, and CAUTI isolates and was found in 99% of the cystitis isolates and 77.77% of the ABU isolates. The fimH gene was seen in 98.33% of UPEC and 66/6% of fecal E. coli. This result is comparable to the studies conducted by Arindam Chakraborty et al. (189) (90%), Kudinha et al., and Mora et al., who have also demonstrated a high prevalence of fimH genes among the UPEC isolates (Martinez et al., 2000; Mora et al., 2009; Chakraborty et al., 2017). Yun et al. (2014) have reported fimH to be present in 100% of pyelonephritis isolates and 96% of ABU isolates, which is under our study. Type 1 fimbria was commonly associated with cystitis and was found to help in the development of IBCs in a mouse model of UTI (Connell et al., 1996; Johnson et al., 2001; Gunther NW et al., 2002). Type 1 fimbriae facilitate bacteria to adhere to each other, resulting in biofilm-like communities in the urinary tract.
P fimbriae, the principal mannose-resistant adherence organelle of UPEC, contributes to pathogenesis as it is involved in bacterial colonization and stimulates an injurious host inflammatory response (Tiba et al., 2008). Within the Pap operon, there are genes which code for the outer membrane protein (papC), a minor structural subunit of the fimbriae (papE/F) and papG adhesins (papGI/papGII/papGIII) (Daigle et al., 1994). The Pap G II adhesin is associated with the strains causing pyelonephritis and bacteremia, while the Pap G III adhesin is associated with the strains causing cystitis (Féria et al., 2001; Ghazvini et al., 2019), and PapGII adhesin is prevalent among fecal isolates.
In the present study, the pap gene was present in 79 (43.88%) of the UPEC isolates. Similar observations were made by Arindam Chakraborty et al. who reported 49% and Ki Wook Yun et al. who reported 45.3% of their isolates to be positive for the pap gene (Yun et al., 2014). In our study, it was observed that the occurrence of various Pap genes (papC, papGI, and papGII was 25%, 51%, and 19% respectively) was more frequent in pyelonephritis strains, which is in concordance with the studies conducted by Ghazvini et al. (36%). Mabbett et al. have reported a slightly higher frequency of this gene among pyelonephritis strains than in our study (Lane and Mobley, 2007; Mabbett et al., 2009). In the present study, it was also found that the papGII gene occurred more frequently in pyelonephritis isolates. This is in agreement with the findings of Monique et al. However, papGIII was not commonly associated with cystitis strains in our study (Daigle et al., 1994).
P fimbriae are most commonly expressed by isolates causing pyelonephritis, recurrent UTI, and CAUTI (Herias et al., 1995); however, these fimbriae are also required by the commensal E. coli for colonization and persistence in the gut. At times, these fimbriae help fecal E. coli to spread to extra-intestinal sites like the urinary tract (Wold et al., 1992; Malagolini et al., 2000). Moreover, 10% of the control strains in this study harbored the papC gene. The sfa/foc ED gene codes for S fimbriae, which recognizes and adheres to the sialic acid expressed on the receptors of epithelial cells in the kidney and vascular endothelial cells. Sialic acid residues are also found in uroplakin proteins present on the bladder luminal surface and, thus, may have a role in the pathogenesis of cystitis (Wold et al., 1992).
In our study, Sfa/foc ED was seen in 21.11% of the UPEC isolates and was more commonly associated with pyelonephritis and CAUTI isolates. This finding is in concordance with the findings of Monique et al. (27.8%) (Malagolini et al., 2000). Ki Wook Yun et al. (Goluszko et al., 1997 have reported a slightly lower prevalence of this gene (15.6%). The afa/Dr family consists of both fimbrial adhesive organelles and the afimbrial adhesins. The afa/Dr-associated proteins are invasins, which help in the internalization of bacteria by the host cells (Behzadi, 2017). Afimbrial adhesins coded by afa/draBC were seen in only 10.55% of the isolates and were more commonly observed in pyelonephritis and cystitis isolates. Shahin et al. have also reported a low prevalence of this gene among UPEC. However, the literature review has reported that up to 65% of UPEC cause cystitis, 26% cause pyelonephritis, and 6% cause asymptomatic bacteriuria to harbor this gene (Wood, 2009). None of the control strains harbored this gene.
Limitations of the study: The molecular detection of AmpC and MBL could not be done because of financial constraints, and antibiotic susceptibility testing was interpreted using guidelines representing the serum concentration of the antibiotics.
5 Conclusion
E. coli is a major pathogen causing UTIs. Many VFs in UPEC have been studied, and it is observed that no single VF could be established as a marker of urovirulence, thus suggesting the role of multiple virulence factors in uropathogenesis. However, biofilm formation is consistently seen in recurrent UTI, CAUTI, and pyelonephritis cases and thus could be used as a marker for such complications, thereby helping in choosing the appropriate dosage for treatment. A genotypic study helps in understanding the molecular pathogenesis, which, in turn, will help in developing clinical strategies for the prevention and management of UTIs. It was observed that the majority of the UPEC were biofilm-forming, and there was a significant difference in the antibiotic susceptibility pattern of biofilm producers and non-biofilm producers. Treatment of UTI is by selecting an antibiotic that the isolate is sensitive to, but the sensitivity reports are generated against planktonic bacteria and may not be effective against the organisms within the biofilms, resulting in recurrent infections and treatment failures. Thus, to conclude, it is important to incorporate routine biofilm detection and MBEC detection methods in a diagnostic laboratory, which will help in the appropriate selection of the dose of the antibiotic, which, in turn, will help overcome treatment failures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Ethical Committee, JSS Medical College and Hospital, JSS AHER, Mysuru, India. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Ethical clearance number: JSS/MC/IEC/09/967/2019-20.
Author contributions
RM: Data curation, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. AK: Project administration, Supervision, Writing – review & editing. AP: Project administration, Supervision, Writing – review & editing. YM: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. MS: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study did not receive any funding to conduct the study, but publication charges will be provided by JSS Academy of Higher Education and Research, Mysuru, India.
Acknowledgments
The authors would like to acknowledge the management of JSS AHER for permitting us to conduct this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhede, M., Kragh, K. N., Qvortrup, K., Allesen-Holm, M., van Gennip, M., Christensen, L. D., et al. (2011). Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface-attached biofilm. PLoS One 6, e27943. doi: 10.1371/journal.pone.0027943
Annapurna, Y. V. S., Swapna Reddy, B., and Lakshmi, V. V. (2014a). Multidrug resistance and virulence phenotypes among uropathogenic Escherichia coli. International Journal of current Microbiology and Applied Science 3, 222–229.
Annapurna, Y. V. S., Swapna Reddy, B., and Lakshmi, V. V. (2014b). MultidrugvResistance and virulence phenotypes among uropathogenic Escherichiacoli 3, 222–229.
Baron, E. J. and Finegold, S. M. (2007). “Baily and Scott’s,” in Diagnostic Microbiology-Ed, 12th ed (CV Mosby Company, Philadelphia).
Behzadi, P. (2017). Uropathogenic Escherichia coli and Fimbrial Adhesins. Urinary tract infection: the result of the strength of the pathogen, or the weakness of the host, 65.
Bjarnsholt, T., Jensen, P. O., Burmolle, M., Hentzer, M., Haagensen, J. A. J., Hougen, H. P, et al. (2005). Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151, 373–383. doi: 10.1099/mic.0.27463-0
Chakraborty, A., Adhikari, P., Shenoy, S., and Saralaya, V. (2017). Molecular characterisation of uropathogenic Escherichia coli isolates at a tertiary care hospital in South India. Indian Journal of Medical Microbiology 35, 305–331. doi: 10.4103/ijmm.IJMM_14_291
Christensen, G. D., Simpson, W. A., Bisno, A. L., and Beachey, E. H. (1982). Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37, 318–326. doi: 10.1128/iai.37.1.318-326.1982
Christensen, G. D., Simpson, W. A., Younger, J. J., Baddour, L. M., Barrett, F. F., Melton, D. M., et al. (1985). Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22, 996–1006. doi: 10.1128/jcm.22.6.996-1006.1985
Clinical and Laboratory Standards Institute. (2020). *M100: Performance standards for antimicrobial susceptibility testing* (30th ed.). Available at: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf.
Connell, I., Agace, W., Klemm, P., Schembri, M., Mărild, S., and Svanborg, C. (1996). Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. U.S.A. 93, 9827–9832. doi: 10.1073/pnas.93.18.9827
Daigle, F., Harel, J., Fairbrother, J. M., and Lebel, P. (1994). Expression and detection of pap, sfa, and afa- encoded fimbrial adhesin systems among uropathogenic Escherichia coli. Clin. J. Microbiol. 40, 286–291. doi: 10.1139/m94-046
Davis, F. and Flood, H. D. (2011). “The pathogenesis of urinary tract infections,” in Clinical management of complicated urinary tract infection (Croatia: InTech), 101–120.
Desai, S., Rajput, A., Kagal, A., and Bharadwaj, R. (2013). Virulence factors in uropathogenic E. coli causing urinary tract infections. Indian J. Basic Appl. Med. Res. 2, 886–896.
Dhakal, B., Kulesus, R., and Mulvey, M. (2008). Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur. J. Clin. Invest. 38, 2–11. doi: 10.1111/j.1365-2362.2008.01986.x
Donlan, R. M. and Costerton, J. W. (2002). Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193. doi: 10.1128/CMR.15.2.167-193.2002
Epp, A. and Larochelle, A. (2010). Recurrent urinary tract infection. Urogynaecology committee, family physician advisory committee. J. Obstet Gynaecol Can. 32, 1082–1090. doi: 10.1016/S1701-2163(16)34717-X
Eshwarappa, M., Dosegowda, R., Aprameya, V., Khan, M. W., Shiva Kumar, P., and Kempegowda, P. (2011). Clinico-microbiological profile of urinary tract infection in south India. Indian J. Nephrol. 21, 30–36. doi: 10.4103/0971-4065.75226
Fattahi, S., Kafil, H. S., Nahai, M. R., Asgharzadeh, M., Nori, R., and Aghazadeh, M. (2015). Relationship of biofilm formation and different virulence genes in uropathogenic Escherichia coli isolates from Northwest Iran. GMS hygiene infection control, 1-7. doi: 10.3205/dgkh000254
Féria, C., MaChado, J., Duarte-Correia, J., Gonçalves, J., and Gaastra, W. (2001). Distribution of papG alleles among uropathogenic Escherichia coli isolated from different species. FEMS Microbiol. Lett. 202, 205–208. doi: 10.1016/S0378-1097(01)00310-X
Flores-Mireles, A. L., Walker, J. N., Caparon, M., and Hultgren, S. J. (2015). Urinary tract infections: epidemiology, mechanism of infection and treatment options. NatRev Microbiol. 13, 269–284. doi: 10.1038/nrmicro3432
Fowler, J. E., Jr and Stamey, T. A. (1977). Studies of intraorbital colonization in women with recurrent urinary infections. VII. The role of bacterial adherence. J. Urol. 117, 472–476. doi: 10.1016/S0022-5347(17)58501-8
Foxman, B. (2014). Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N Am. 28, 1–13. doi: 10.1016/j.idc.2013.09.003
Foxman, B. and Brown, P. (2003). Epidemiology of Urinary tract infections: transmission and risk factors, incidence and costsinfect. Dis. Clin. North Am. 17, 227–241. doi: 10.1016/S0891-5520(03)00005-9
Freeman, D. J., Falkiner, F. R., and Keane, C. T. (1989). New method for detecting slime production by coagulase-negative staphylococci. J. Clin. Pathol. 42, 872 4. doi: 10.1136/jcp.42.8.872
Ghanwate, N. A. (2012) Biofilm eradication studies on uropathogenic E. coli using ciprofloxacin and nitrofurantoin. Int. J. Pharm. Biomed. Res. 3(2):127–31.
Ghazvini, H., Taheri, K., Edalati, E., Sedighi, M., and Mirkalantari, S. (2019). Virulence factors and antimicrobial resistance in uropathogenic Escherichiacoli strains isolated from cystitis and pyelonephritis. Turk J. Med. Sci. 49, 361–367. doi: 10.3906/sag-1805-100
Goluszko, P., Popov, V., Selvarangan, R., Nowicki, S., Pham, T., and Nowicki, B. J. (1997). Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J. Infect. Dis. 176, 158–167. doi: 10.1086/jid.1997.176.issue-1
Gunther NW, I. V., Snyder, J. A., Lockatell, V., Blomfield, I., Johnson, D. E., and Mobley, H. L. (2002). Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect. Immun. 70, 3344–3354. doi: 10.1128/IAI.70.7.3344-3354.2002
Herias, M. V., Midvedt, T., Hanson, L. A., and Wold, A. E. (1995). Role of Escherichia coli P fimbriae in intestinal colonization in gnotobiotic rats. Infect. Immun. 63, 4781–4789. doi: 10.1128/iai.63.12.4781-4789.1995
Hooton, T. M. and Stamm, W. E. (1997). Diagnosis and treatment of uncomplicated urinary tract infections. Infect. Dis. Clinics North America 11, 551–581. doi: 10.1016/S0891-5520(05)70373-1
Jadhav, S., Hussain, A., Devi, S., Kumar, A., Parveen, S., Gandham, N., et al. (2011). Virulence characteristics and genetic affinities of multiple drug resistant uropathogenic Escherichia coli from a semi-urban locality in India. PLoS One 6, e18063. doi: 10.1371/journal.pone.0018063
Jhang, J.-F. and Kuo, H.-C. (2017). Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. TZU CHI Med. J. 29, 131–137. doi: 10.4103/tcmj.tcmj_53_17
Johnson, J. R., O’Bryan, T. T., Kuskowski, M., and Maslow, J. N. (2001). Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect. Immun. 69, 5363–5374. doi: 10.1128/IAI.69.9.5363-5374.2001
Johnson, J. R. and Stell, A. L. (2000). Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181, 261–272. doi: 10.1086/jid.2000.181.issue-1
Karam, M. R. A., Habibi, M., and Bouzari, S. (2018). Relationships between virulence factors and antimicrobial resistance among Escherichia coli isolated from urinary tract infections and commensal isolates in Tehran, Iran. Osong Public Health Res. perspectives. 9, 217–224. doi: 10.24171/j.phrp.2018.9.5.02
Karigoudar, R. M., Karigoudar, M. H., Wavare, S. M., and Mangalgi, S. S. (2019). Detection of biofilm among uropathogenic Escherichia coli and its correlation with antibiotic resistance pattern. J. Lab. Physicians. 11, 17–22. doi: 10.4103/JLP.JLP_98_18
Klemm, P. and Schembri, M. (2000). Bacterial adhesins: function and structureBacterial adhesins: function and structure. Int. J. Med. Microbiol. 290, 27–35. doi: 10.1016/S1438-4221(00)80102-2
Lane, M. C. and Mobley, H. L. (2007). Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 72, 19–125. doi: 10.1038/sj.ki.5002230
Mabbett, A. N., Ulett, G. C., Watts, R. E., Tree, J. J., Totsika, M., Cheryl-Lynn, Y. O., et al. (2009). Virulence properties of asymptomatic bacteriuria Escherichia coli. Int. J. Med. Microbiol. 299, 53–63. doi: 10.1016/j.ijmm.2008.06.003
Mah, T. F. and O’Toole, G. A. (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39. doi: 10.1016/S0966-842X(00)01913-2
Malagolini, N., Cavallone, D., Wu, X. R., and Serafín-Cessi, F. (2000). Terminal glycosylation of bovine uroplakin III, one of the major integral-membrane glycoproteins of the mammalian bladder. Biochim. Biophys. Acta 1475, 231–237. doi: 10.1016/S0304-4165(00)00073-8
Mapes, S., Rhodes, D. M., Wilson, W. D., Leutenegger, C. M., and Pusterla, N. (2007). Comparison of five real-time PCR assays for detecting virulence genes in isolates of Escherichia coli from septicaemic neonatal foals. Veterinary Rec. 161, 716–718. doi: 10.1136/vr.161.21.716
Martinez, J. J., Mulvey, M. A., Schilling, J. D., Pinkner, J. S., and Hultgren, S. J. (2000). Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19, 2803–2812. doi: 10.1093/emboj/19.12.2803
Mesharam, L., Patidar, R. K., khare, M., Bagde, S., Sahare, K. N., and singh, v. (2012). Comparative analysis b/w Biofilm formation of commensal and pathogenic E. coli isolates. Asiatic J. @ Biotechnol. Resour. 03, 1441–1446.
Micali, S., Isgro, G., Miceli, N., Calapai, G., and Navarra, M. (2014). Cranberry and recurrent cystitis: more than thinking? Crit. Rev. Food Sci. Nutr. 54, 1063–1073. doi: 10.1080/10408398.2011.625574
Mobley, H. L. T. and Warren, J. W. (1996). Urinary tract infections: Molecular pathogenesis and clinical management (Washington, DC, USA: ASM Press).
Mora, A., López, C., Dabhi, G., Blanco, M., Blanco, J. E., Alonso, M. P., et al. (2009). Extraintestinal pathogenic Escherichia coli O1:K1:H7/NM from human and avian origin: Detection of clonal groups B2 ST95 and D ST59 with different host distribution. BMC Microbiol. 9, 132. doi: 10.1186/1471-2180-9-132
Munkhdelger, Y., Gunregjav, N., Dorjpurev, A., Juniichiro, N., and Sarantuya, J. (2017). Detection of virulence genes, phylogenetic group and antibiotic resistance of uropathogenic Escherichia coli in Mongolia. J. Infect. Dev. Ctries 11, 51–57. doi: 10.3855/jidc.7903
Oelschlaeger, T. A., Dobrindt, U., and Hacker, J. (2002). Pathogenicity islands of uropathogenic E. coli and the evolution of virulence. Int. J. antimicrobial Agents 19, 517–521. doi: 10.1016/S0924-8579(02)00092-4
Park, H.-K., Jung, Y.-J., Chae, H.-C., Shin, Y.-J., Woo, S.-Y., Park, H.-S., et al. (2009). Comparison of Escherichia coli uropathogenic genes (kps, usp and ireA) and enteroaggregative genes (aggR and aap) via multiplex polymerase chain reaction from suprapubic urine specimens of young children with fever. Scandinavian J. Urol. Nephrol. 43, 51–57. doi: 10.1080/00365590802299338
Poovendran, P. and Ramanathan, N. (2014). In vitro study on antibiotic susceptibility pattern of biofilm-producing uropathogenic Escherichia coli isolates and their molecular characterization. Asian J. Pharm. Cl Res. 7, 181–185.
Pramodhini, S. and Niveditha, S. (2012). Antibiotic resistance pattern of bioflim forming uropathogens isolated from catheterized patients in Pondicherry, India. AMJ. Australas. Med. J. 5, 344—348. doi: 10.4066/AMJ.2012.1193
Raksha, R., Srinivasa, H., and Macaden, R. S. (2003). Occurrence and characterisation of uropathogenic E. coli in urinary tract infections. Indian J. Med. Microbiol. 21, 102–107. doi: 10.1016/S0255-0857(21)03130-3
Rao, J. P. (1994). Study of virulence factors of uropathogenic E. coli Vol. 47 (Hyderabad: Gandhi Medical College).
Rashki, A., Abdi, H. A., and Shookohi, M. (2017). Prevalence of genes encoding outer membrane virulence factors among fecal Escherichia coli isolates. Int. J. Basic Sci. Med. 2, 52–57. doi: 10.15171/ijbsm.2017.11
Roos, V., Ulett, G., Schembri, M., and Klemm, P. (2006). The asymptomatic bacteriuria Escherichia coli strain 83972 outcompetes uropathogenic E. coli strains in human urine. Infect. Immun. 74, 615–624. doi: 10.1128/iai.74.1.615-624.2006
Santo, E., Macedo, C., and Marin, J. M. (2006). Virulence factors of uropathogenic Escherichia coli from a university hospital in Ribeirao Preto, Sao Paulo, Brazil. Rev. Inst. Med. Trop. S. Paulo 48, 185–188. doi: 10.1590/S0036-46652006000400002
Sepandj, F., Ceri, H., Gibb, A., Read, R., and Olson, M. (2004). Minimum inhibitory concentration (MIC) versus minimum biofilm eliminating concentration (MBEC) in the evaluation of antibiotic sensitivity of gram-negative bacilli causing peritonitis. Perit Dial Int. 24, 65–67. doi: 10.1177/089686080402400107
Sevanan, M., Pongiya, U., and Peedikavil, N. J. (2011). Antimicrobial susceptibility pattern of biofilm-producing Escherichia coli of urinary tract infections. Curr. Res. Bacteriol. 4, 73–80. doi: 10.3923/crb.2011.73.80
Shah, C., Baral, R., Bartaula, B., and Shrestha, L. B. (2019). Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 19, 204. doi: 10.1186/s12866-019-1587-3
Shrestha, R., Khanal, S., Poudel, P., et al. (2019). Extended-spectrum β-lactamase producing uropathogenic Escherichia coli and the correlation of biofilm with antibiotics resistance in Nepal. Ann. Clin. Microbiol. Antimicrob. 18, 42. doi: 10.1186/s12941-019-0340-y
Soto, S. M., Marco, F., Guiral, E., and Vila, J. (2011). Biofilm formation in uropathogenic Escherichia coli strains: relationship with neurovirulence factors and antimicrobial resistance, 159-170. doi: 10.5772/24626
Suman, E., Jose, J., Varghese, S., and Kotian, M. S. (2007). Study of biofilm production in Escherichia coli causing urinary tract infection. Indian J. Med. Microbiol. 25, 305–306. doi: 10.1016/S0255-0857(21)02135-6
Tabasi, M., Asadi Karam, M. R., Habibi, M., Yekaninejad, M. S., and Bouzari, S. (2015). Phenotypic assays to determine virulence factors of Uropathogenic Escherichia coli (UPEC) isolates and their correlation with antibiotic resistance pattern. Osong Public Health Res. perspectives. 6, 261–268. doi: 10.1016/j.phrp.2015.08.002
Tadepalli, S., Prudhivi, S., Babu Myneni, R., and Rao, S. (2016). Biofilm formation in uropathogenic Escherichia coli isolates and its association with extended-spectrum beta-lactamase production and drug resistance. Saudi J. Pathol. Microbiol. 1, 60–64.
Tajbakhsh, E., Ahmadi, P., Abedpour-Dehkordi, E., Arbab-Soleimani, N., and Khamesipour, F. (2016). Biofilm formation, antimicrobial susceptibility, serogroups and virulence genes of uropathogenic E. coli isolated from clinical samples in Iran. Antimicrob. Resist. Infect. Control 5, 11. doi: 10.1186/s13756-016-0109-4
Tiba, M. R., Yano, T., and da Silva Leite, D. (2008). Genotypic characterization of virulence factors in Escherichia coli strains from patients with cystitis. Rev. Inst. Med. Trop. S. Paulo 50, 255-260. doi: 10.1590/S0036-46652008000500001
Usein, C.-R., Damian, M., Tatu-Chitoiu, D., Capusa, C., Fagaras, R., Tudorache, D., et al. (2001). Prevalence of virulence genes in Escherichia coli strains isolated from Romanian adult urinary tract infection cases. J. Cell. Mol. Med. 5, 303–310. doi: 10.1111/j.1582-4934.2001.tb00164.x
Vijayalakshmi, J., Hymavathi, R., Renuka Devi, A., Ramanamma, M. V., Swarnalatha, G., Surekha, A., et al. (2015). Study of virulence factors in uropathogenic Escherichia coli Journal of the evolution of medical and dental sciences. Journal of Evolution of Medical and Dental Sciences 4, 1297–1305. doi: 10.14260/jemds/2015/182
Vila, J., Simon, K., Ruiz, Horcajada, J. P., Velasco, M., Barranco, M., et al. (2002). Are quinolone-resistant Escherichia coli less virulent? J. Infect. Dis. 86 (7), 0022–1899.doi: 10.1086/342955
Wang, G., Clark, C. G., and Rodgers, F. G. (2002). Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J. Clin. Microbiol., 3613–3619. doi: 10.1128/JCM.40.10.3613-3619.2002
Wiles, T. J., Kulesus, R. R., and Mulvey, M. A. (2008). Origins and virulence mechanisms of uropathogenic E. coli. Exp. Mol. Pathol. 85, 11–19. doi: 10.1016/j.yexmp.2008.03.007
Wold, E. A., Caugant, D. A., Lidin-Janson, G., De Man, P., and Svanborg, C. (1992). Resident colonic Escherichia coli strains frequently display uropathogenic characteristics. J. Infect. Dis. 165, 46–52. doi: 10.1093/infdis/165.1.46
Wood, T. K. (2009). Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ. Microbiol. 11, 1–15. doi: 10.1111/j.1462-2920.2008.01768.x
Yamamoto, S., Terai, A., Yuri, K., Kurazono, H., Takeda, Y., and Yoshida, O. (1995). Detection of urovirulence factors in Escherichia coZi by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 12, 85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x
Yan, F. and Polk, D. B. (2004). “Commensal bacteria in the gut: learning who our friends are,” in Current Opinion in Gastroenterology (USA, Current Opinion in Gastroenterology), vol. 20, 565–571.
Keywords: biofilm production, uropathogenic Escherichia coli, multiplex PCR, urinary tract infection, Congo-red method, tissue culture plate method
Citation: Mahale RP, K A, Princy A, Maheshwarappa YD and Sumana MN (2025) Comparative evaluation of biofilm-forming capacity in uropathogenic and commensal Escherichia coli. Front. Cell. Infect. Microbiol. 15:1570422. doi: 10.3389/fcimb.2025.1570422
Received: 19 February 2025; Accepted: 23 May 2025;
Published: 31 July 2025.
Edited by:
Ariadnna Cruz-Córdova, Federico Gómez Children’s Hospital, MexicoReviewed by:
Verónica Iranzú Martínez-Santos, Autonomous University of Guerrero, MexicoPrabin Dawadi, University of Mississippi, United States
Copyright © 2025 Mahale, K, Princy, Maheshwarappa and Sumana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahadevaiah Neelambike Sumana, bW5zdW1hbmFAanNzdW5pLmVkdS5pbg==
†These authors have contributed equally to this work
 Rashmi P. Mahale
Rashmi P. Mahale Anuradha K2†
Anuradha K2† Adeline Princy
Adeline Princy Yogeesh D. Maheshwarappa
Yogeesh D. Maheshwarappa Mahadevaiah Neelambike Sumana
Mahadevaiah Neelambike Sumana