- 1The Affiliated Changsha Central Hospital, Department of Cardiology, Hengyang Medical School, University of South China, Changsha, Hunan, China
- 2The Affiliated Changsha Central Hospital, Department of Laboratory Medicine, Hengyang Medical School, University of South China, Changsha, Hunan, China
- 3The Affiliated Changsha Central Hospital, Center of Tuberculosis Diagnosis and Treatment, Hengyang Medical School, University of South China, Changsha, Hunan, China
- 4The Affiliated Changsha Central Hospital, Technology Demonstration Base for Tuberculosis Diagnosis and Treatment in Hunan Province, University of South China, Changsha, Changsha, Hunan, China
- 5The Affiliated Changsha Central Hospital, Department of Science and Education, Hengyang Medical School, University of South China, Changsha, Hunan, China
- 6The Affiliated Changsha Central Hospital, Changsha Tuberculosis Research Institute, Hengyang Medical School, University of South China, Changsha, Hunan, China
- 7The Affiliated Changsha Central Hospital, Changsha Technology Innovation Center for Tuberculosis Diagnosis and Treatment, University of South China, Changsha, Hunan, China
Objective: To investigate the risk factors for all-cause mortality of previously untreated pulmonary tuberculosis patients complicated by hypertension and construct a predictive model.
Methods: We retrospectively analyzed the clinical data of inpatients with previously untreated pulmonary tuberculosis complicated by hypertension from 2019 to 2021 in Changsha Central Hospital. Patients’ survival status and cardiovascular events were collected through telephone follow-up. LASSO regression was utilized to screen predictive variables, and binary logistic regression identified mortality risk factors. A predictive nomogram model was developed using R software, and its precision and reliability were verified.
Results: Among the 1,014 patients, there were 100 (9.86%) deaths and 82 (8.09%) cardiovascular events. LASSO regression screened out 13 predictive variables. Multivariate logistic regression analysis revealed that smoking history, sputum bacteriology, pleural effusion, coronary heart disease, and chronic kidney disease were independent risk factors. Based on the training set data, a nomogram prognostic model was developed, showing an AUC of 0.712 (95% CI: 0.777-0.847), with 50.0% sensitivity and 84.3% specificity. The model’s fit was confirmed through internal and external validations.
Conclusion: The prediction model constructed in this study has high predictive ability and satisfactory clinical efficacy, and can provide an effective individualized prediction tool for assessing all-cause mortality risk in patients with previously untreated pulmonary tuberculosis complicated by hypertension.
Introduction
Tuberculosis (TB) is a long-term infectious disease caused by Mycobacterium tuberculosis (MTB), mainly spread via the respiratory tract. According to The World Health Organization’s (WHO) “Global Tuberculosis Report 2024” (World Health Organization, 2024), in 2023, there were about 10.8 million new TB cases globally, with an incidence rate of 134/100,000 and about 1.25 million fatal cases. Compared to 2015, the incidence and mortality rates of tuberculosis in 2023 have decreased by 8.3% and 23% respectively, which is far from reaching the second milestone goal set in the End TB Strategy, which aims to reduce the incidence by 50% and mortality by 75% by 2025. Identifying the risk factors for mortality in TB patients is crucial to reducing the burden and achieving the “End TB” goals.
The lung is the predominant site of tuberculosis infection, and pulmonary tuberculosis (PTB) is the most lethal variant of the disease. Meanwhile, previously untreated tuberculosis accounts for 80%-90% of the total number of newly diagnosed tuberculosis patients, and its drug resistance is relatively simple. In this context, some studies have analyzed the mortality causes of PTB patients and shown that the overall disease burden caused by TB can be attributed to post-TB sequelae (Menzies et al., 2021) or non-communicable diseases, such as respiratory diseases and cardiovascular diseases (Briggs et al., 2012). The latest meta-analysis evidence indicates that the all-cause mortality in patients treated for tuberculosis is largely attributed to cardiovascular diseases (20% [95% CI 15–26]; I²=92%) (Romanowski et al., 2019). A history of tuberculosis increases the risk of myocardial infarction, ischemic stroke, and peripheral arterial disease (Wang et al., 2017; Salindri et al., 2019). Recent literature reports that Mycobacterium bovis infection (BCG) can enhance atherosclerosis development (Huaman et al., 2020). A retrospective cohort study based on a health insurance research database in Taiwan, China (Chung et al., 2014) found that the proportion of hypertension in the pulmonary tuberculosis population was 38.7%, which was significantly higher than that in non-TB populations. Patients with PTB complicated by hypertension often have worse prognosis. The study by (Seegert et al., 2021). included 1,544 initial-treated PTB patients, in whom the proportion of those with hypertension was 12.8%. Compared with normotensive PTB patients, hypertensive PTB patients had a lower likelihood of successful treatment (odds ratio [OR] 0.76, 95% confidence interval [CI] 0.52-1.10) and a higher mortality rate during the two-year follow-up (hazard ratio [HR] 1.64, 95% CI 1.15-2.34). There is a relatively common comorbidity between pulmonary tuberculosis and hypertension, and the two diseases can interact with each other, increasing the death burden of each other.
In recent years, nomograms have been considered a feasible and effective predictive tool for disease diagnosis and prognosis outcome assessment (Jennings, 2015; Seegert et al., 2021). However, there has been no research on the prognosis of patients with a special condition of pulmonary tuberculosis combined with hypertension. The mycobacterial resistance in patients with primary-treated pulmonary tuberculosis is relatively simple, facilitating the evaluation of new therapeutic methods and enhancing the understanding of the disease’s natural course. This study was designed to delve into the risk factors influencing all-cause mortality among patients diagnosed with previously untreated pulmonary tuberculosis and hypertension, to create a nomogram prediction model applicable in clinical settings. The calibration and discrimination of the model were assessed to determine its effectiveness, which has good clinical value for evaluating clinical prognosis in the target population.
Methods
Study subject selection
Adult inpatients with previously untreated PTB complicated by hypertension in the Changsha Central Hospital Affiliated with the University of South China were enrolled as our primary cohort from 1st January, 2019 to 31st December, 2021. Patients in China receive treatment at designated facilities and obtain relevant insurance subsidies (Liu et al., 2020a). The Tuberculosis Department of Changsha Central Hospital, a specialized unit in Hunan Province, is among the facilities that admit and treat the highest volume of TB patients in the region. Consequently, the TB cases in Changsha Central Hospital provide a vital insight into the epidemiological trends and disease features prevalent in Hunan.
The research subjects were chosen according to the following criteria: 1) Initially treated PTB patients (Wu et al., 2024), which includes: (a) individuals who have never received anti-TB medications for PTB, (b) individuals who have not completed the full course of standard chemotherapy, and (c) individuals who have undergone irregular chemotherapy for less than one month; 2) Patients with essential hypertension; 3) individuals aged 18 years or older; 4) Patients who are not pregnant or lactating. 5) Patients without diseases that affect immune function, such as AIDS, malignant tumors, hematological diseases, systemic lupus erythematosus, rheumatoid arthritis, kidney transplantation, etc. 6) Patients had not used hormones and immunosuppressants within 4 months. 7) The clinical baseline data of patients were complete.
The study was conducted in accordance with the Declaration of Helsinki. It was a retrospective study that utilized patient data from the case management system, and ethical approval was obtained with waived informed consent. This ethical approval was granted by the Medical Ethics Sub-committee of Changsha Central Hospital (Ethics Approval Number: 2023-049).
Data collection
We conducted a retrospective data collection from 1,014 hospitalised patients with PTB combined with hypertension using the medical record system of Changsha Central Hospital. The extracted data included: 1) General clinical data such as age, gender, time of admission, type of medical insurance, educational background, and the presence of comorbidities like diabetes, chronic kidney disease, coronary heart disease, heart failure, COPD, as well as smoking history, drinking history, blood pressure and heart rate measurements at admission, the grading of hypertension, the duration of PTB, sputum bacteriological findings (If either AFB smear or sputum culture is positive, the case is considered sputum bacteriologically positive), drug resistance, and medication usage patterns for antihypertensives and lipid-lowering drugs. 2) Laboratory test results comprised neutrophil, platelet (PLT), lymphocytes, hemoglobin (Hb), albumin, transaminase, creatinine, blood lipid, erythrocyte sedimentation rates (ESR), and C-reactive protein (CRP). 3) Imaging findings involved the presence of cavities, pleural effusion, and the number of lung lobes affected by the lesion. 4) Follow-up data included the course of anti-tuberculosis treatment and adherence to medication regimens.
Statistical analysis
Statistical analyses were conducted using the R statistical package 4.3.2 (http://www.R-Project). Continuous measurements that followed a normal distribution were reported as the mean and standard deviation (SD), while those that did not conform to a normal distribution were presented as the median (IQR). Comparisons between the two groups were conducted using an independent samples t-test or the Mann-Whitney U test. Categorical variables were expressed as frequency percentages (%) and analyzed using the χ2 test. LASSO logistic regression analysis was employed to identify candidate predictors with substantial regression coefficients. Backward stepwise logistic regression was utilized for multivariate analysis to identify statistically significant prognostic risk factors in patients with previously untreated PTB complicated by hypertension. The model’s predictive performance was assessed through the receiver operating characteristic curve (ROC), calibration curve, and decision curve analysis (DCA). A confidence interval (CI) of 95% was established, and a p-value of <0.05 (bilateral) was deemed statistically significant.
Results
Traits of research subjects
The research included 1014 hospitalized patients with previously untreated PTB complicated by hypertension who were screened, as presented in Figure 1. The data was randomly partitioned into training (n=710) and validation(n=304) cohorts at a 7:3 ratio (random seed number: 20220326), with patients classified into death or survival groups based on all-cause mortality.
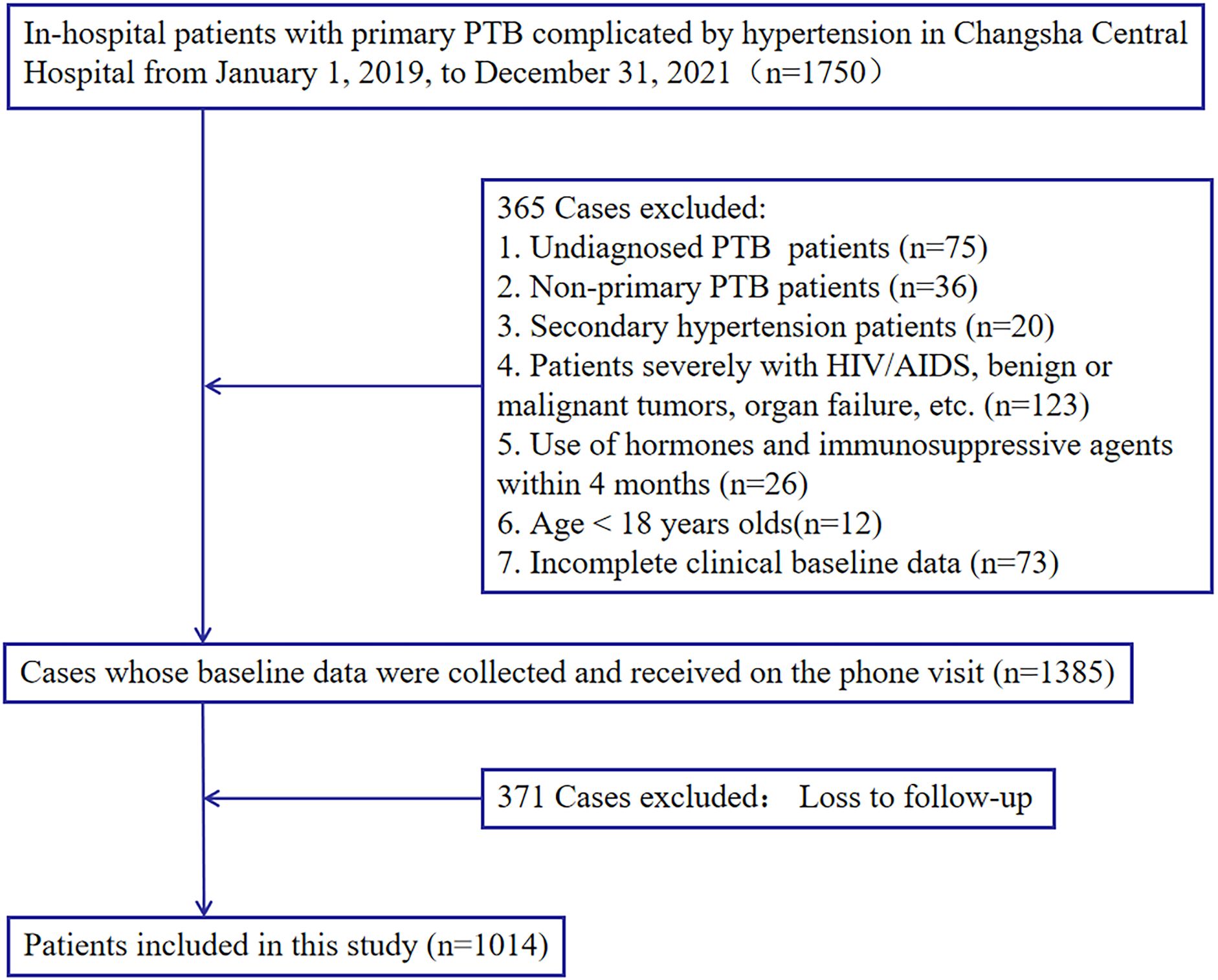
Figure 1. Study design. A total of 1,014 in-hospital patients with previously untreated PTB complicated by hypertension with complete relevant data were enrolled in this study.
Among the 1,014 subjects, 100 patients died, with a mortality rate of 9.86%, and 82 patients (8.09%) had cardiovascular events. The study included patients with an average age of 67. The proportion of male patients (70.32%) exceeded that of female patients (20.68%). Patients with junior high school education or below accounted for 81.46%. Regarding insurance, 53.96% had urban coverage, while 41.22% had rural coverage. Smokers (47.44%) and drinkers (26.73%) accounted for relative proportions. The patients (51.68%) were positive for sputum bacteriology, 44.38% had extrapulmonary tuberculosis, 28.60% had cavitary manifestations on chest imaging, and 27.31% had pleural effusion manifestations on imaging. Patients with comorbidities accounted for 39.30% diabetes, 8.38% heart failure, 19.92% hyperlipidemia, 14.69%stroke, 27.91%coronary heart disease, 10.26% chronic kidney disease (CKD), and 10.95% chronic obstructive pulmonary disease (COPD). Patients (40.4%) had adverse drug reactions during the treatment of PTB. Patients (73.08%) took antihypertensive drugs, while 23.67% took lipid-lowering drugs. Supplementary baseline data is available in Table 1.
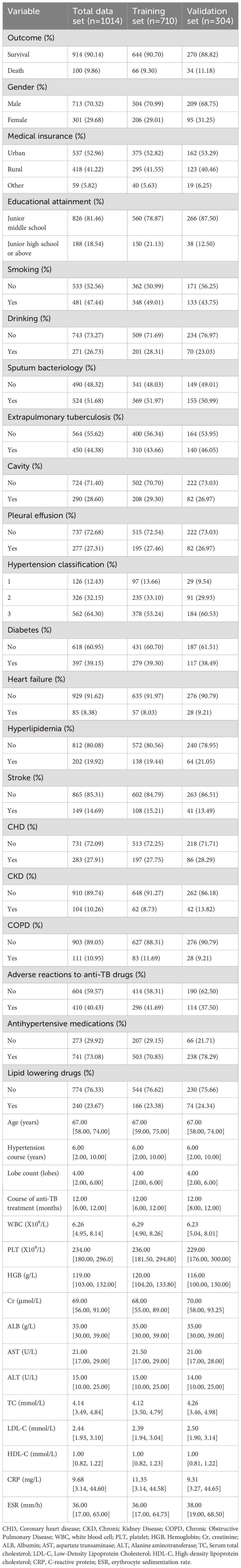
Table 1. Baseline characteristics of included in-hospital patients with previously untreated PTB complicated by hypertension.
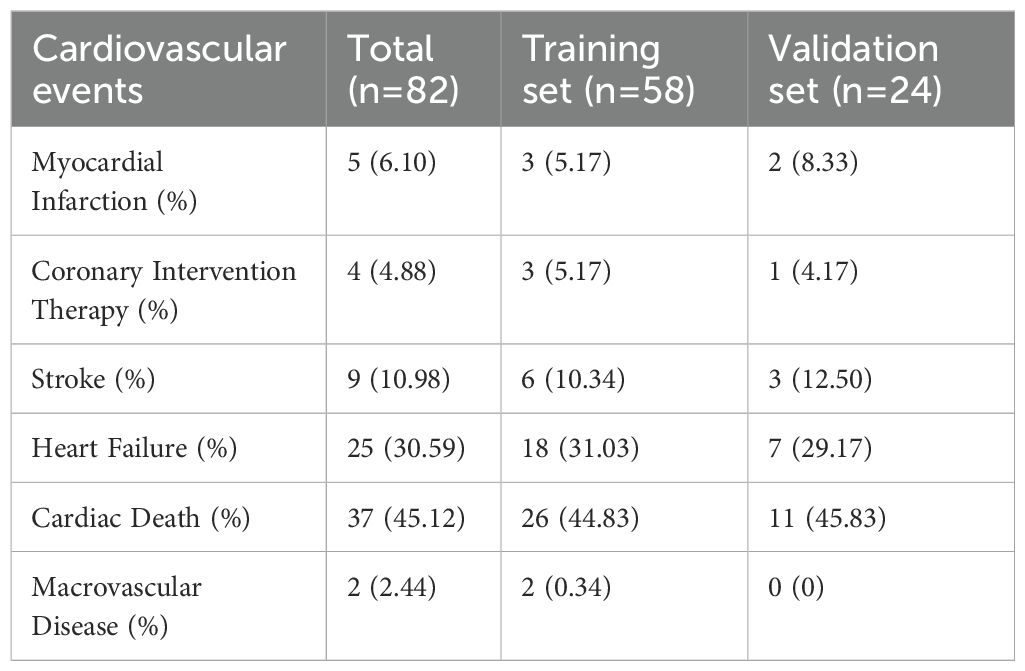
Among 1,014 research subjects, 82 patients had cardiovascular events, including 5 cases (6.10%) of myocardial infarction, 4 cases (4.88%) undergoing coronary interventional therapy, 9 cases (10.98%) of stroke, 25 cases (45.12%) of heart failure, 37 cases (45.12%) of cardiogenic death, and 2 cases (2.44%) of macrovascular lesions. (Table 2).
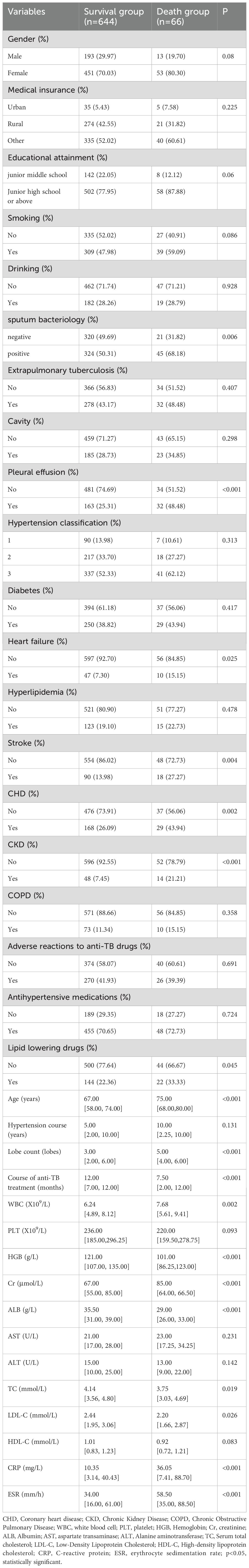
The 710 patients in the training set were divided into death and survival groups. The findings indicated statistically significant differences between the two groups regarding age, sputum bacteriology, pleural effusion, heart failure, stroke, CHD, CKD, number of lung lobes, anti-tuberculosis treatment, WBC, Cr, ESR, CRP, Hb, Alb, total cholesterol, and LDL (P < 0.05), as illustrated in Table 3.
Risk predictors for in-hospital patients initially diagnosed with primary PTB complicated by hypertension
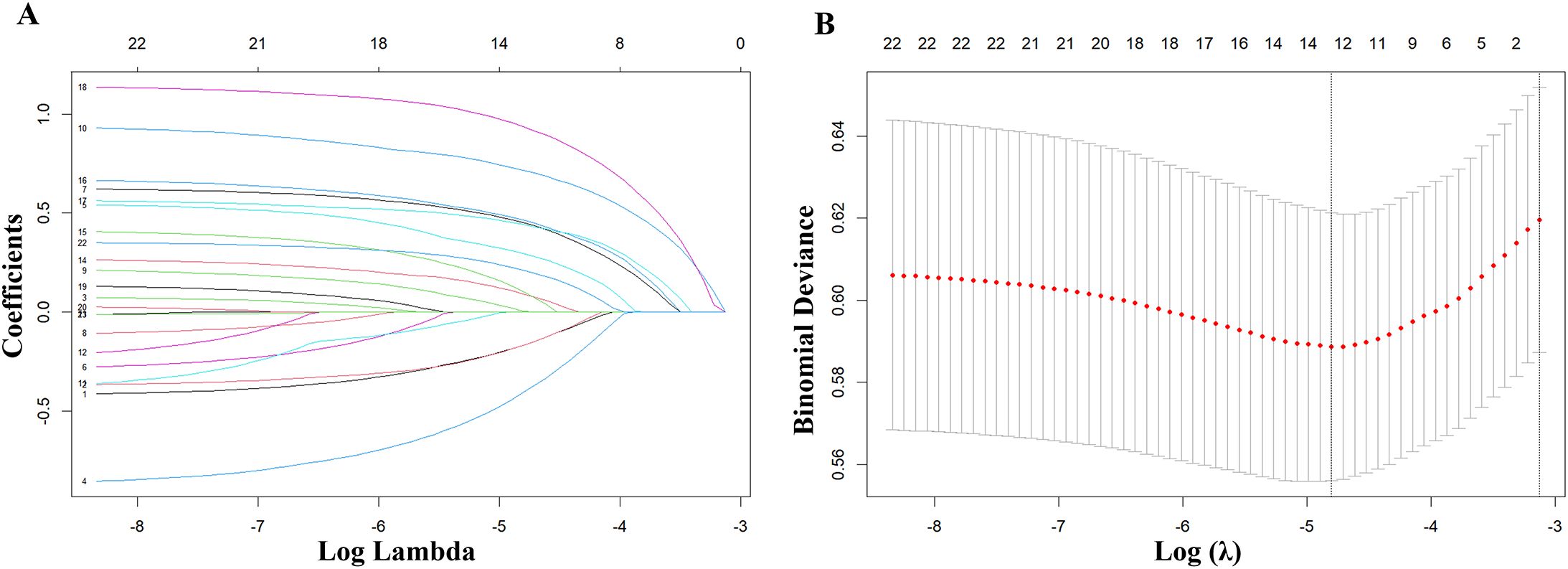
The LASSO regression analysis was used to screen out 13 predictive variables with non-zero coefficients, including gender, medical insurance, educational attainment, smoking history, sputum bacteriology, cavity, pleural effusion, heart failure, hyperlipidemia, stroke, coronary heart disease, chronic kidney disease, and taking lipid-lowering drugs. The LASSO regression analysis’s lasso coefficient profile diagram (Figure 2A) and cross-validation diagram (Figure 2B) were drawn.
Predictors demonstrating statistical significance (p<0.05) in preliminary analyses were subsequently included in the multivariate logistic regression model construction. The data presented in Table 4 and Figure 3 showed that smoking history (OR = 1.95; 95% CI: 1.13 - 3.41; p= 0.018), sputum bacteriology (OR = 2.00; 95% CI: 1.15 - 3.59; p = 0.017), pleural effusion (OR = 2.45; 95% CI: 1.42 - 4.22; p = 0.01), coronary heart disease (OR = 1.84; 95% CI: 1.04 - 3.22; p = 0.034), and chronic kidney disease (OR = 3.33; 95% CI: 1.59 - 6.67; p < 0.01) were identified as independent risk factors for mortality in patients with previously untreated PTB complicated by hypertension. (p < 0.05).
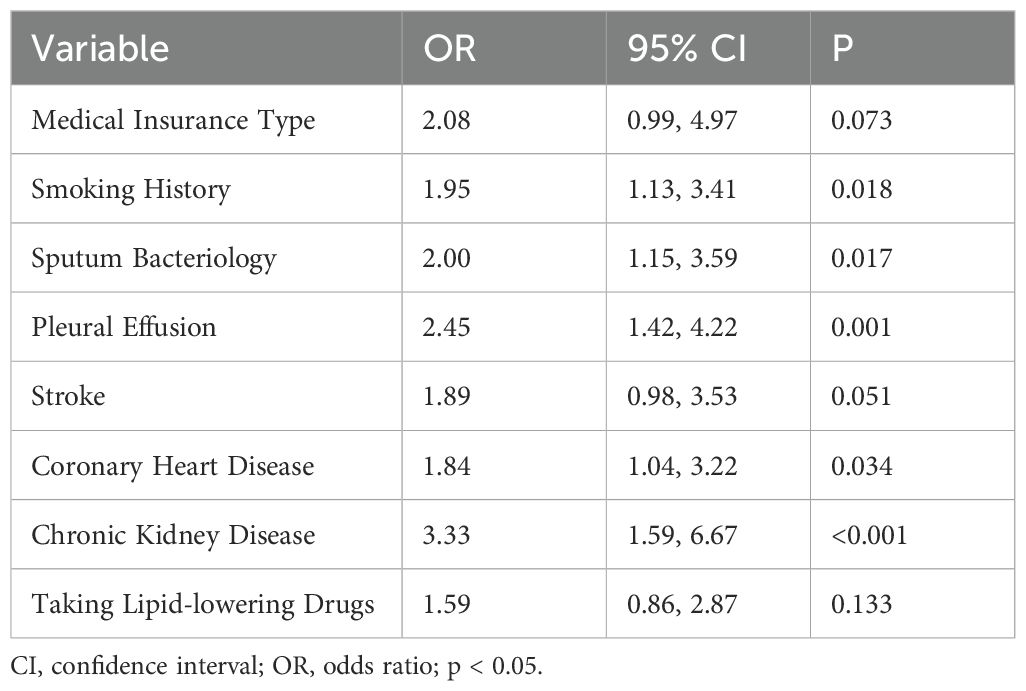
Table 4. Multivariate logistic regression analysis in the training set of in-hospital patients with previously untreated PTB complicated by hypertension.
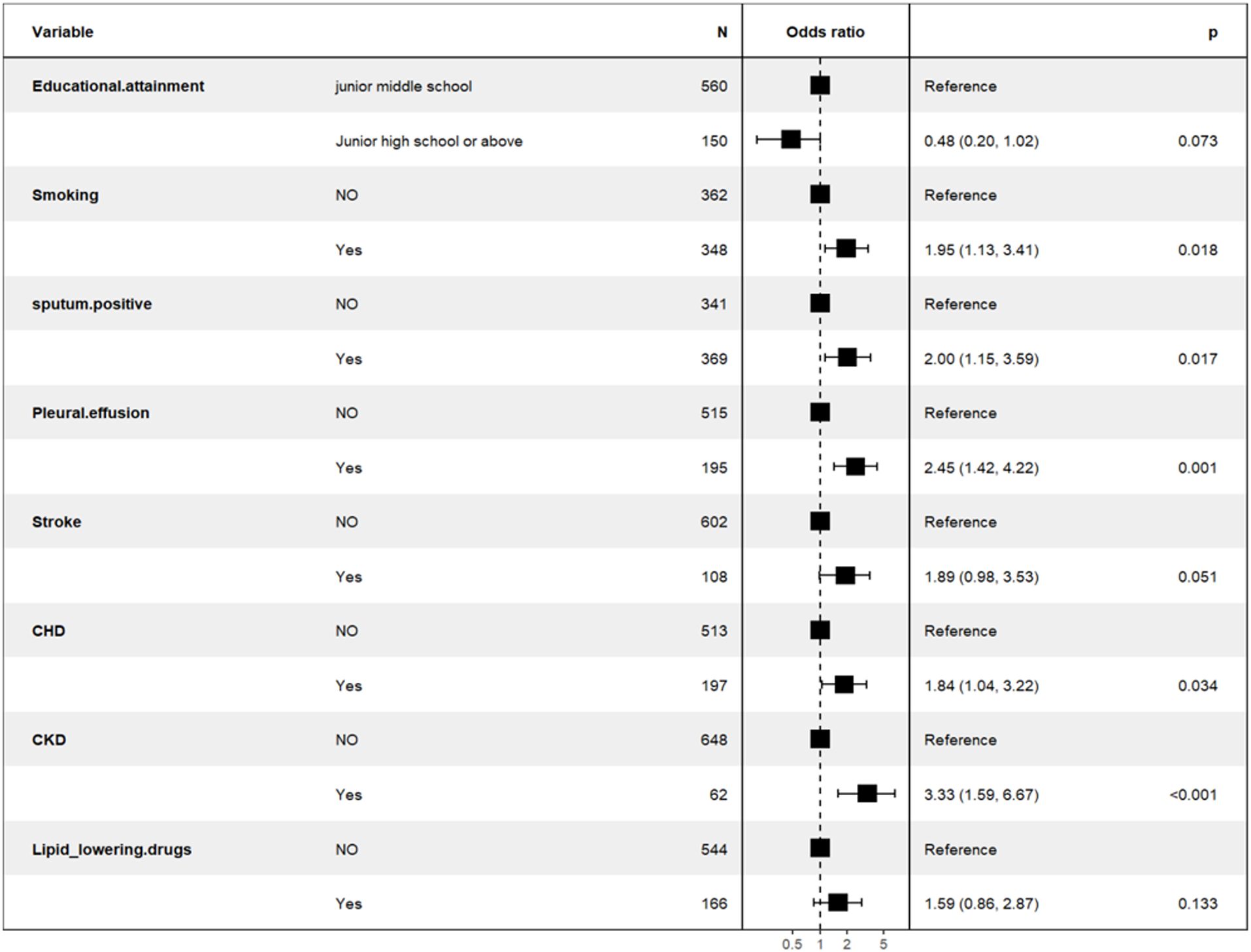
Figure 3. Backward stepwise logistic regression analysis of the training set of the population of in-hospital patients with previously untreated PTB complicated by hypertension.
A nomogram prognostic model was developed utilizing R software, incorporating five independent risk factors: smoking history, sputum bacteriology, pleural effusion, coronary heart disease, and chronic kidney disease, as shown in Figure 4.
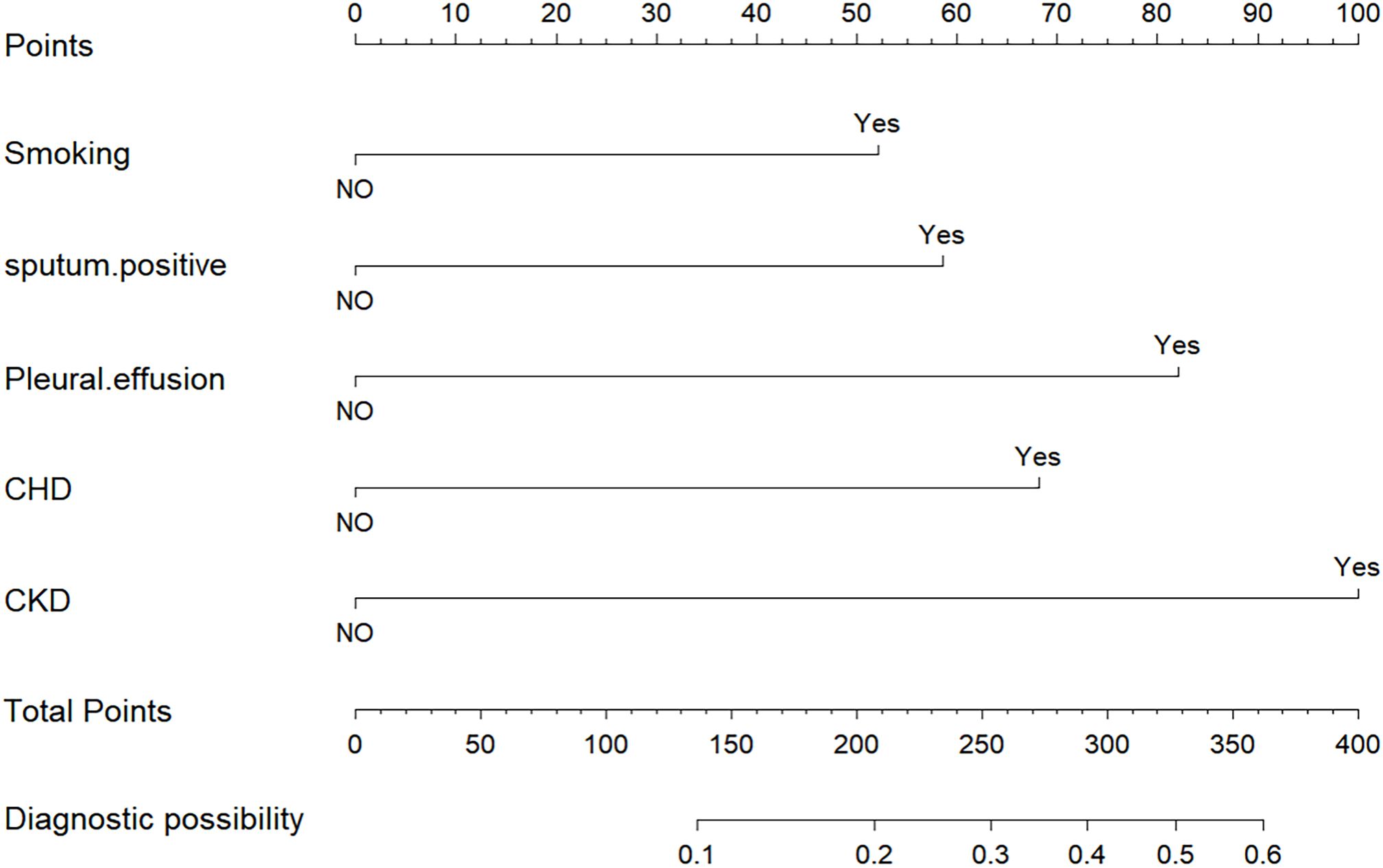
Figure 4. Nomogram to predict the outcomes of patients with previously untreated PTB complicated by hypertension.
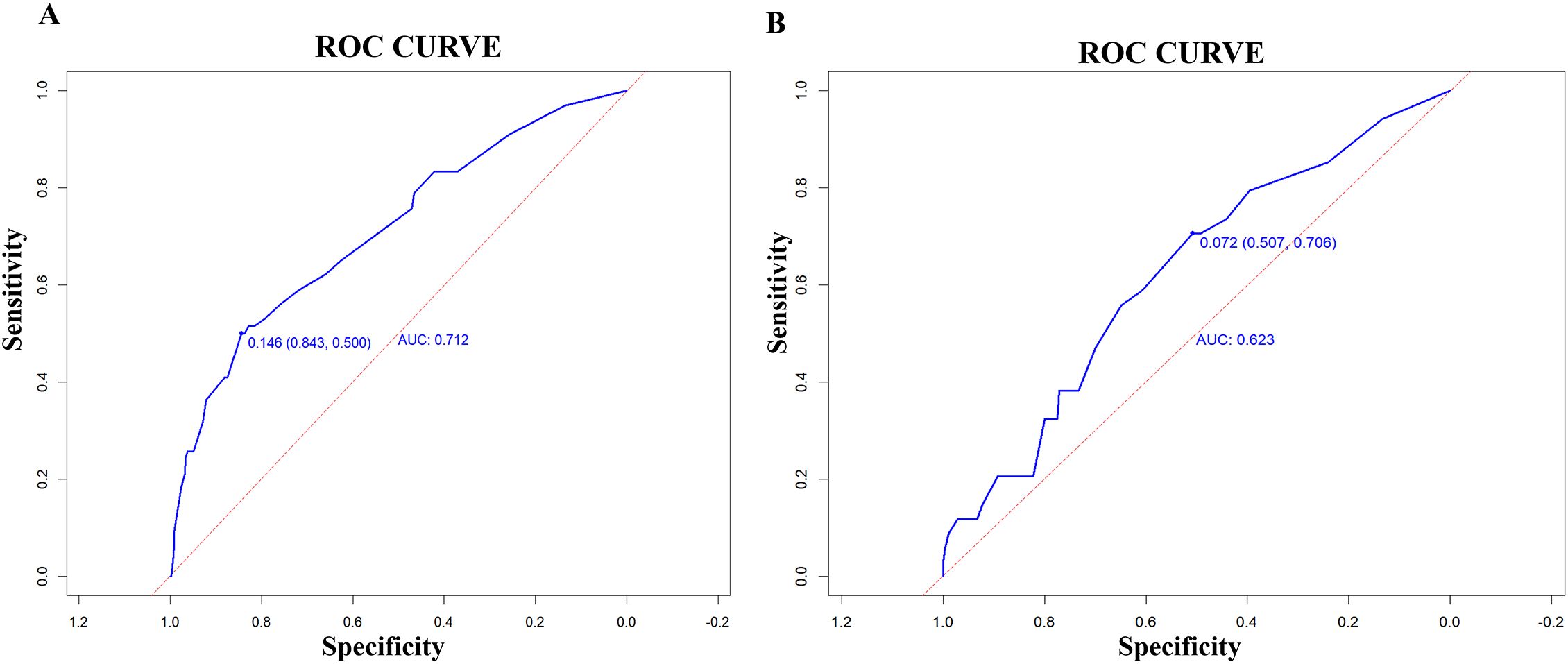
The model’s discriminatory performance was quantified using ROC curve analysis, yielding an AUC of 0.712 in the training cohort with corresponding sensitivity and specificity values of 50.0% and 84.3%, respectively (Figure 5A). External validation results showed moderately reduced performance, with an AUC of 0.620 accompanied by increased sensitivity (70.6%) but decreased specificity (50.7%) in the independent validation cohort (Figure 5B). To evaluate the model’s predictive accuracy, calibration plots were created using R software for both the training (Figure 6A) and validation (Figure 6B) cohorts. These plots feature a horizontal axis representing the predicted mortality risk for hospitalized patients with previously untreated PTB complicated by hypertension, while the vertical axis displays the observed mortality rates within this patient population. The Brier score (the mean squared error between anticipated and actual values) of the calibration curve of the training set was 0.078, indicating a relatively good calibration effect. The S:p value was 0.974, indicating that the fitted line closely aligned with the standard reference line and possessed a rather good predictive value. The P-value of the Hosmer-Lemeshow test was 0.89, indicating that there was no significant difference between the predicted probabilities and the actual probabilities. In addition, R software was used to draw the clinical decision curves (DCA curves) of the training set (Figure 7A) and the validation set (Figure 7B). In the training set, when the threshold probability was between 5% and 40%, the decision curve was located above the “None” line and the “All” line, indicating the model’s clinical applicability within this interval. and the model had clinical practicability within this range. Similarly, in the validation set, when the threshold probability was between 5% and 20%, the model had clinical practicability.
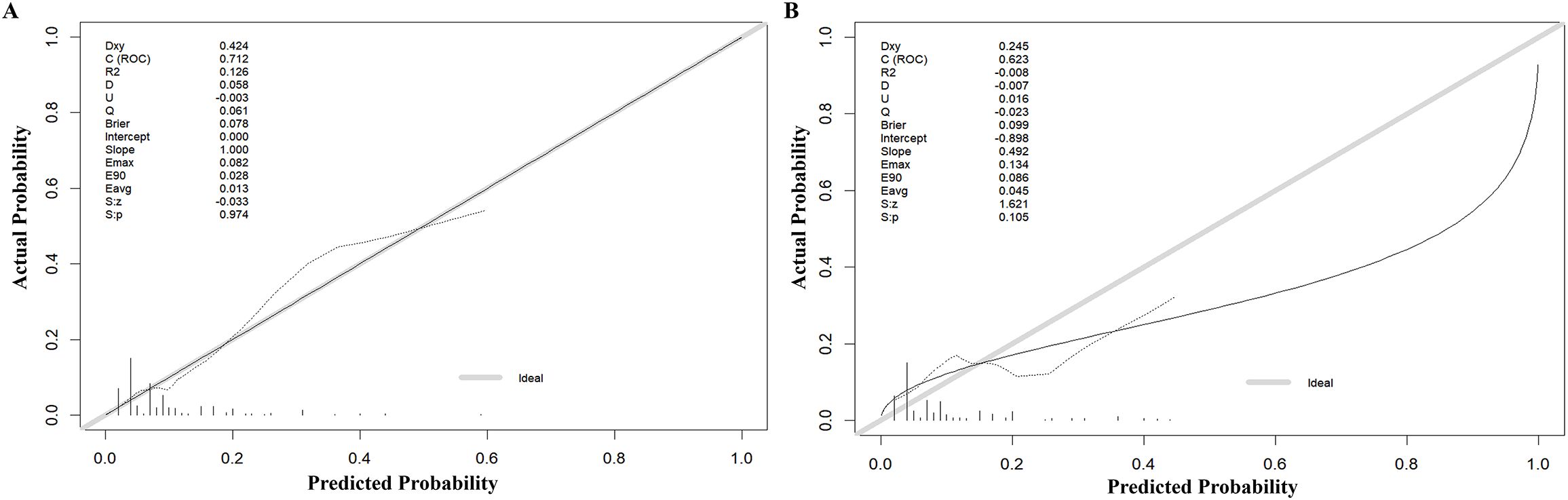
Figure 6. Calibration of the nomogram to predict the death in the training set (A) and validation set (B).
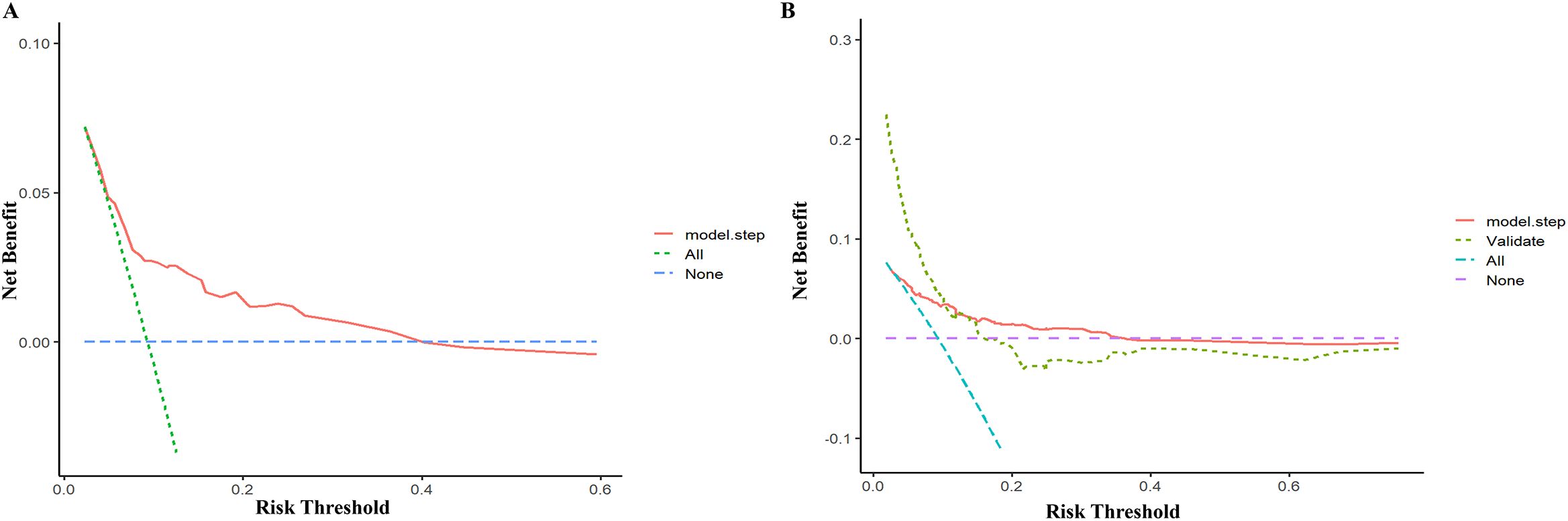
Figure 7. Decision cure analysis (DCA) for the nomogram in the training set (A) and validation set (B).
Discussion
China, as a nation with a significant tuberculosis burden, has experienced a gradual decrease in the mortality rate of the disease, however, the burden of death remains heavy (World Health Organization, 2024). As social and economic conditions improve and the population ages, the number of PTB patients with chronic non-communicable diseases like hypertension is increasing, further raising PTB mortality. Therefore, constructing a predictive model for the all-cause mortality risk in patients with previously untreated PTB complicated by hypertension is of great significance for improving patient prognosis.
The study’s target population comprised 1,014 patients with previously untreated PTB complicated by hypertension. The mortality rate was 9.86%, which is higher than the 4.9% mortality rate of patients with previously untreated PTB by Dan Li et al (Li et al., 2023), indicating that the complication of hypertension elevated the mortality risk for individuals with initially treated PTB to a significant degree. In another retrospective cohort study (Seegert et al., 2021), the treatment outcomes and prognoses of 1,544 individuals with previously untreated pulmonary tuberculosis (PTB) were followed up. The mortality rate of patients with previously untreated PTB complicated by hypertension within two years was significantly higher than those with normal blood pressure, further explaining this difference.
PTB and hypertension interact, increasing each other’s risk and severity, leading to a poorer prognosis. PTB can cause hypertension and cardiovascular diseases through chronic inflammation, immune responses (Caillon and Schiffrin, 2016; Rodriguez-Iturbe et al., 2017), and autoimmune reactions triggered by heat shock proteins (HSP) (Hazra et al., 2023). M. tuberculosis may exacerbate hypertension through lung tissue destruction and vascular inflammation (Kalla et al., 2020). Antituberculosis drugs like rifampicin (Liu et al., 2020b) may reduce the effectiveness of antihypertensive drugs. Hyperuricemia, a side effect of pyrazinamide (Yanai et al., 2021; Tian et al., 2023), increases cardiovascular disease risk. Hypertension promotes PTB progression through inflammatory factors and oxidative stress (Caillon and Schiffrin, 2016). Angiotensin II-induced hypertension may relate to TB severity (Cho et al., 2021). It is crucial to predict the mortality risk of patients with PTB complicated by hypertension in advance and take effective treatments promptly to improve the quality of life of patients.
This study created and validated a predictive nomogram model to assess the mortality risk of patients with previously untreated PTB complicated by hypertension. Eventually, five independent risk factors, namely smoking history, sputum bacteriology, pleural effusion, coronary heart disease, and chronic kidney disease, were screened out. Previous studies have found that age, male gender, smoking (Smith et al., 2015), sputum bacteriology (Wu et al., 2014; Liu et al., 2022), pleural effusion (Yen et al., 2017), combined chronic kidney disease, and malignant tumors are risk factors for the mortality of patients with PTB, which are consistent with the results of our study. In addition, we have newly discovered that combined coronary heart disease is an independent risk factor for them. A large amount of evidence-based medical evidence (Poole-Wilson et al., 2004; Jennings, 2015) has already shown that coronary heart disease increases the mortality risk.
Tuberculosis is linked to a heightened risk of developing atherosclerotic cardiovascular disease. Mycobacterial infection has been shown to aggravate atherosclerosis progression in mouse models lacking the low-density lipoprotein receptor (Ldlr-/-) (Huaman et al., 2020). In addition, hypertension accelerates the occurrence and development of coronary heart disease by inducing damage to vascular endothelial cells (Evans et al., 2021), promoting lipid deposition and platelet aggregation (D'Agostino et al., 2021), and increasing cardiac afterload (Hamada et al., 2022). When tuberculosis infection and hypertension coexist, the risk of coronary heart disease in patients increases sharply.
The AUC of the model was 0.712, indicating moderate predictive performance, with diagnostic sensitivity and specificity rates of 50.0% and 84.3%, respectively. A calibration curve assesses a model’s calibration performance by graphically comparing the predicted probabilities of outcomes against the empirically observed event frequencies. In this study, the predicted probabilities of the model were highly consistent with the actual probabilities, indicating that the model exhibits high accuracy. DCA evaluates the clinical utility of a model by quantifying its net benefit across varying threshold probabilities. In this study, DCA showed that the model provided clinical net benefit in the training set at threshold probabilities of 5%–40% and in the validation set at 5%–20%, indicating its clinical applicability within these ranges.
Study limitations
This study is not without its limitations. To begin with, being a retrospective study, it is susceptible to residual confounding factors and selection bias. Moreover, the sample size for positive outcome events was small, and the external validation cohort was sourced from a single hospital, failing to adequately represent the diverse characteristics of the entire patient population, potentially leading to inaccurate predictions when applied in different settings. Additionally, certain variables had to be excluded from the study due to missing data. Many cases were lost to follow - up owing to telephone follow - up, potentially compromising the model’s accuracy. Forthermore, the relatively low sensitivity in this study may pose a risk of misjudging high-risk patients. Finally, the clinically relevant data and experimental parameters were merely a portion of the patient’s clinical data and may have omitted other significant data. Future research should optimize the model by integrating imaging/genetic markers to enhance accuracy, conduct multi-center external validation with diverse patient cohorts from different regions to assess generalizability and enhance representativeness, use standardized questionnaires (demographics, lifestyle, comorbidities) for comprehensive bias control, and incorporate TB-cardiovascular comorbidity biomarkers to refine predictive performance. This approach will enable rigorous assessment of the model’s generalizability and clinical utility across populations.
Conclusions
Patients with previously untreated PTB complicated by hypertension have a relatively high mortality risk. The constructed nomogram prognostic model in this study may provide a feasible early assessment approach for the prognosis of patients with previously untreated PTB complicated by hypertension in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Sub-committee of Changsha Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study was a retrospective study that utilized patient data from the case management system.
Author contributions
AC: Data curation, Investigation, Methodology, Visualization, Writing – original draft. YN: Data curation, Investigation, Methodology, Visualization, Writing – original draft. ZZ: Writing – review & editing. YP: Writing – review & editing, Conceptualization, Funding acquisition. PD: Writing – review & editing, Conceptualization, Funding acquisition, Resources, Supervision. HX: Writing – review & editing, Data curation, Supervision, Validation. YL: Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported in part by the National Science Foundation of China (NSFC) Projects 82170475 (YL), Hunan Provincial Natural Science Foundation of China 2023JJ30065 (PD), The science and technology innovation Program of Hunan Province 2021SK53408 (YL), Scientific Research Project of Hunan Provincial Health Commission B202303016916 (YL), Hunan Province Degree and Graduate Education Reform Research Project 2022JGYB171 (PD), National Key Clinical Specialty Scientific Research Project (grant number: Z2023045) (YP), The science and technology innovation Program of Hunan Province (2023SK4071) (YP), Changsha Natural Science Foundation (kq2208438) (YP).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1574824/full#supplementary-material
References
Briggs, A. H., Weinstein, M. C., Fenwick, E. A., Karnon, J., Sculpher, M. J., Paltiel, A. D., et al. (2012). Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med. Decis Making 32, 722–732. doi: 10.1177/0272989X12458348
Caillon, A. and Schiffrin, E. L. (2016). Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr. Hypertens. Rep. 18, 21. doi: 10.1007/s11906-016-0628-7
Cho, S. N., Choi, J. A., Lee, J., Son, S. H., Lee, S. A., Nguyen, T. D., et al. (2021). Ang II-induced hypertension exacerbates the pathogenesis of tuberculosis. Cells 10 (9), 2478. doi: 10.3390/cells10092478
Chung, W. S., Lin, C. L., Hung, C. T., Chu, Y. H., Sung, F. C., Kao, C. H., et al. (2014). Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int. J. Tuberc Lung Dis. 18, 79–83. doi: 10.5588/ijtld.13.0288
D'Agostino, I., Tacconelli, S., Bruno, A., Contursi, A., Mucci, L., Hu, X., et al. (2021). Low-dose Aspirin prevents hypertension and cardiac fibrosis when thromboxane A(2) is unrestrained. Pharmacol. Res. 170, 105744. doi: 10.1016/j.phrs.2021.105744
Evans, C. E., Cober, N. D., Dai, Z., Stewart, D. J., and Zhao, Y. Y. (2021). Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur. Respir. J. 58 (3), 2003957. doi: 10.1183/13993003.03957-2020
Hamada, M., Ogimoto, A., Otani, T., Ohshima, K., Kono, T., Watanabe, Y., et al. (2022). Changes of left ventricular remodeling due to increased afterload in patients with essential hypertension. Int. J. Cardiol. 367, 74–80. doi: 10.1016/j.ijcard.2022.08.050
Hazra, J., Vijayakumar, A., and Mahapatra, N. R. (2023). Emerging role of heat shock proteins in cardiovascular diseases. Adv. Protein Chem. Struct. Biol. 134, 271–306. doi: 10.1016/bs.apcsb.2022.10.008
Huaman, M. A., Qualls, J. E., Jose, S., Schmidt, S. M., Moussa, A., Kuhel, D. G., et al. (2020). Mycobacterium bovis bacille-calmette-guerin infection aggravates atherosclerosis. Front. Immunol. 11. doi: 10.3389/fimmu.2020.607957
Jennings, G. L. (2015). A new guideline on treatment of hypertension in those with coronary artery disease: scientific statement from the american heart association, american college of cardiology, and american society of hypertension about treatment of hypertension in patients with coronary artery disease. Heart Lung Circ. 24, 1037–1040. doi: 10.1016/j.hlc.2015.05.022
Kalla, I. S., Miri, A., and Seedat, F. (2020). Occult pulmonary arterial hypertension in patients with previous pulmonary tuberculosis. Afr J. Thorac. Crit. Care Med. 26 (4), 110. doi: 10.7196/AJTCCM.2020.v26i4.110
Li, D., Tang, S. Y., Lei, S., Xie, H. B., and Li, L. Q. (2023). A nomogram for predicting mortality of patients initially diagnosed with primary pulmonary tuberculosis in Hunan province, China: a retrospective study. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1179369
Liu, K., Ai, L., Pan, J., Fei, F., Chen, S., Zhang, Y., et al. (2022). Survival analysis and associated factors for pulmonary tuberculosis death: evidence from the information system of tuberculosis disease and mortality surveillance in China. Risk Manag Healthc Policy 15, 1167–1178. doi: 10.2147/RMHP.S368593
Liu, K., Li, T., Vongpradith, A., Wang, F., Peng, Y., Wang, W., et al. (2020a). Identification and prediction of tuberculosis in eastern China: analyses from 10-year population-based notification data in Zhejiang Province, China. Sci. Rep. 10, 7425. doi: 10.1038/s41598-020-64387-5
Liu, W., Yan, T., Chen, K., Yang, L., Benet, L. Z., and Zhai, S. (2020b). Predicting interactions between rifampin and antihypertensive drugs using the biopharmaceutics drug disposition classification system. Pharmacotherapy 40, 274–290. doi: 10.1002/phar.2380
Menzies, N. A., Quaife, M., Allwood, B. W., Byrne, A. L., Coussens, A. K., Harries, A. D., et al. (2021). Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health 9, e1679–e1687. doi: 10.1016/S2214-109X(21)00367-3
Poole-Wilson, P. A., Lubsen, J., Kirwan, B. A., van Dalen, F. J., Wagener, G., Danchin, N., et al. (2004). Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 364, 849–857. doi: 10.1016/S0140-6736(04)16980-8
Rodriguez-Iturbe, B., Pons, H., and Johnson, R. J. (2017). Role of the immune system in hypertension. Physiol. Rev. 97, 1127–1164. doi: 10.1152/physrev.00031.2016
Romanowski, K., Baumann, B., Basham, C. A., Ahmad Khan, F., Fox, G. J., and Johnston, J. C. (2019). Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 19, 1129–1137. doi: 10.1016/S1473-3099(19)30309-3
Salindri, A. D., Wang, J. Y., Lin, H. H., and Magee, M. J. (2019). Post-tuberculosis incidence of diabetes, myocardial infarction, and stroke: Retrospective cohort analysis of patients formerly treated for tuberculosis in Taiwan 2002-2013. Int. J. Infect. Dis. 84, 127–130. doi: 10.1016/j.ijid.2019.05.015
Seegert, A. B., Patsche, C. B., Sifna, A., Gomes, V. F., Wejse, C., Storgaard, M., et al. (2021). Hypertension is associated with increased mortality in patients with tuberculosis in Guinea-Bissau. Int. J. Infect. Dis. 109, 123–128. doi: 10.1016/j.ijid.2021.06.062
Smith, G. S., Van Den Eeden, S. K., Baxter, R., Shan, J., Van Rie, A., Herring, A. H., et al. (2015). Cigarette smoking and pulmonary tuberculosis in northern California. J. Epidemiol. Community Health 69, 568–573. doi: 10.1136/jech-2014-204292
Tian, X., Chen, S., Wang, P., Xu, Q., Zhang, Y., Zhang, X., et al. (2023). Temporal relationship between hyperuricemia and hypertension and its impact on future risk of cardiovascular disease. Eur. J. Intern. Med. 111, 82–89. doi: 10.1016/j.ejim.2023.02.023
Wang, S. H., Chien, W. C., Chung, C. H., Lin, F. H., Peng, C. K., Chian, C. F., et al. (2017). Tuberculosis increases the risk of peripheral arterial disease: A nationwide population-based study. Respirology 22, 1670–1676. doi: 10.1111/resp.13117
World Health Organization (2024). Global tuberculosis report 2024. Available online at: https://iris.who.int/handle/10665/379339 (Accessed February 10, 2025).
Wu, L., Cai, X., and Jiang, X. (2024). Risk factors for multidrug-resistant tuberculosis: a predictive model study. Front. Med. (Lausanne) 11. doi: 10.3389/fmed.2024.1410690
Wu, Y. C., Lo, H. Y., Yang, S. L., and Chou, P. (2014). Factors correlated with tuberculosis reported after death. Int. J. Tuberc Lung Dis. 18, 1485–1490. doi: 10.5588/ijtld.14.0244
Yanai, H., Adachi, H., Hakoshima, M., and Katsuyama, H. (2021). Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. 22 (17), 9221. doi: 10.3390/ijms22179221
Keywords: previously untreated pulmonary tuberculosis, hypertension, mortality rate, risk factors, prediction model
Citation: Cao A, Nie Y, Zhong Z, Pei Y, Deng P, Xie H and Leng Y (2025) Predictive analysis of all-cause mortality of previously untreated pulmonary tuberculosis patients complicated by hypertension. Front. Cell. Infect. Microbiol. 15:1574824. doi: 10.3389/fcimb.2025.1574824
Received: 11 February 2025; Accepted: 24 July 2025;
Published: 11 August 2025.
Edited by:
Stephen Henry Gillespie, University of St Andrews, United KingdomReviewed by:
Madhur Sachan, Harvard Medical School, United StatesYunhuan Liu, Tongji University, China
Muge Cevik, University of St Andrews, United Kingdom
Copyright © 2025 Cao, Nie, Zhong, Pei, Deng, Xie and Leng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiping Leng, bHlwMDYyNkBhbGl5dW4uY29t; Hebin Xie, MjAxODA1MDY0MkB1c2MuZWR1LmNu; Ping Deng, cGFtZWxhZDIxMTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Anhua Cao1†
Anhua Cao1† You Nie
You Nie Hebin Xie
Hebin Xie Yiping Leng
Yiping Leng