- 1Jiangxi Key Laboratory of Molecular Medicine, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2School of Medicine, Yichun University, Yichun, Jiangxi, China
- 3Rehabilitation Department, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
Background: Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can affect nearly every organ system in the human body and present with diverse clinical manifestations. However, its effects on cardiovascular outcomes remain discrepant.
Aim: The objective of this study was to determine whether blood inflammatory levels on admission were associated with in-hospital mortality risk in patients with congestive heart failure (CHF) and COVID-19.
Methods: We performed a retrospective analysis of 4,711 inpatients with confirmed SARS-CoV-2 infection from the Dryad database. Among these individuals, 541 CHF patients with COVID-19 were compared with hospitalized non-CHF patients (n = 4,170). Admission variables including demographic characteristics, vital signs, preexisting comorbidities, and laboratory indicators were obtained as potential confounders for in-hospital mortality risk.
Results: Univariate analysis with Kaplan–Meier curves suggested that higher inflammatory levels on admission—including white blood cell (WBC) count, interleukin-6 (IL-6), ferritin, procalcitonin, and C-reactive protein—were associated with a significantly higher risk of in-hospital mortality compared with lower levels. Consistently, multivariate Cox regression analysis showed that apart from ferritin [0.8 (0.7, 0.9), <0.001], the WBC [1.3 (1.1, 1.5), 0.013], IL-6 [1.4 (1.2, 1.7), <0.001], procalcitonin [1.2 (1.0, 1.3), 0.031] and C-reactive protein [1.2 (1.1, 1.4), 0.002] were independently associated with increased risk of in-hospital mortality in non-CHF patients in the adjusted II model. However, these independent relationships were not observed in CHF patients.
Conclusion: Elevated systemic inflammatory levels on admission were significantly associated with increased in-hospital mortality risk in non-CHF patients with COVID-19 but not in CHF patients. These findings may provide a clinical basis for risk stratification in CHF patients. Further studies are needed to support these results.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Stalder and Thiel, 2021; Jackson et al., 2022), has undergone continuous mutation, leading to varied clinical manifestations with high morbidity and mortality, particularly among elderly patients with chronic underlying conditions such as cardiovascular and respiratory diseases (Rey et al., 2020). SARS-CoV-2 can invade myocardial cells via the angiotensin-converting enzyme 2 (ACE2) receptor, resulting in acute cardiovascular events such as myocarditis and acute myocardial infarction (MI), which may subsequently lead to congestive heart failure (CHF), arrhythmia, and even cardiac arrest (Guzik et al., 2020; Liu et al., 2020). In addition, the virus can induce a severe inflammatory cytokine storm or immune response, causing further damage to myocardial tissue and coronary arteries (Guzik et al., 2020; Liu et al., 2020). Previous studies have demonstrated that patients with confirmed COVID-19 and heart failure (HF) face an increased risk of morbidity and mortality (Panhwar et al., 2019; Isath et al., 2023). A recent high-quality study also reported that COVID-19 infection in patients with acute HF is significantly associated with increased in-hospital mortality (Hashem et al., 2023). Inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) have been correlated with mortality risk in non-critically ill patients with COVID-19 (Guzik et al., 2020; Liu et al., 2020). However, other inflammatory markers—including interleukin-6 (IL-6), ferritin, C-reactive protein, and procalcitonin—have not been fully explored in these patients. Moreover, little evidence exists on the association between systemic inflammatory levels on admission and in-hospital mortality risk in CHF patients with confirmed COVID-19 (Huang et al., 2022; Xie et al., 2022).
Therefore, we analyzed a large sample of inpatients with confirmed SARS-CoV-2 infection (n = 4,711) from the Dryad database to assess whether blood levels of inflammatory biomarkers—white blood cell (WBC) count, IL-6, ferritin, C-reactive protein, and procalcitonin—on admission serve as independent predictors of in-hospital mortality risk among CHF and non-CHF patients.
Methods
Study population
Our study data were obtained from the Dryad database (https://doi.org/10.5061/dryad.7d7wm37sz) where a retrospective study including a total of 4,711 patients with confirmed SARS-CoV-2 infection who were admitted to four hospitals within the Montefiore Health System between March 1 and April 16, 2020 (Altschul, 2021; Eskandar et al., 2021). Clinical information, including demographic characteristics, preexisting comorbidities, admission vital signs, admission laboratory indicators, medications, and in-hospital mortality, was confirmed by the healthcare surveillance software package (Clinical Looking Glass; Streamline Health, Atlanta, GA) and by review of the primary medical records (Altschul et al., 2020).
Inclusion criteria: all patients with confirmed SARS-CoV-2 infection tested by real-time reverse transcriptase PCR assay were included.
Exclusion criteria: 1) patients who died before admission or were not admitted were excluded because they did not have a complete set of laboratory indicators; 2) only the last report was considered for analysis if patients had multiple admissions. For the purpose of this study, 4,711 patients were finally enrolled in the analysis. According to the regulations of the Dryad database, we registered and then obtained the dataset for free for further analysis without additional ethical consent.
In-hospital mortality and admission variables
To achieve the purpose of this study, in-hospital mortality was considered the primary outcome. Mortality data were collected by querying in-hospital deaths and deaths recorded in the National Death Registry. Only laboratory indicators obtained at admission were included for analysis. Comorbidities were identified by the International Classification of Diseases, 10th Revision (ICD-10), and the comorbidities chosen were those used in the Charlson Comorbidity Index (Sasson et al., 2017; Altschul et al., 2020; Eskandar et al., 2021). Each patient’s medical record was reviewed for diagnoses occurring within 5 years.
Statistical analysis
To evaluate the relevance of inflammatory indicators at admission with in-hospital mortality risk among CHF and non-CHF patients with COVID-19, all patients were divided into two subgroups (with CHF and without CHF). Mean ± standard deviation (SD) was used for the statistical description of continuous variables with a normal distribution and was compared by an independent t-test. Median (25–75 percentiles) was used for continuous variables with a non-normal distribution and was compared using the Mann–Whitney U test. The N (%) was used for categorical variables and was compared using Pearson’s chi-square test. Firstly, univariate Kaplan–Meier curve analysis was performed to evaluate in-hospital mortality risk in these patients. Then, to confirm independent associations of inflammatory indicators (WBC, IL-6, ferritin, C-reactive protein, and procalcitonin) with in-hospital mortality risk, multivariate logistic regression analysis was performed using odds ratios (ORs) with 95% confidence intervals (CIs). To further confirm independent predictors of in-hospital mortality risk, multivariate Cox regression analysis was conducted using these admission inflammatory indicators as exposure variables, associated with the primary outcome by hazard ratios (HRs) with 95% CIs. The confounding factors were as follows: 1) Adjusted I included age, Black, White, Asian, and Latino; 2) Adjusted II included age, Black, White, Asian, Latino, temperature, mean arterial pressure, and oxygen saturation.
We finally used sensitivity analysis to evaluate the predictive value of inflammatory indicators at admission for in-hospital mortality risk. The number of days from admission to in-hospital death was used as the time-to-event data. For all statistical analyses in this study, a p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Empower (version 4.1) or SPSS (version 24.0).
Results
Admission characteristics (baseline)
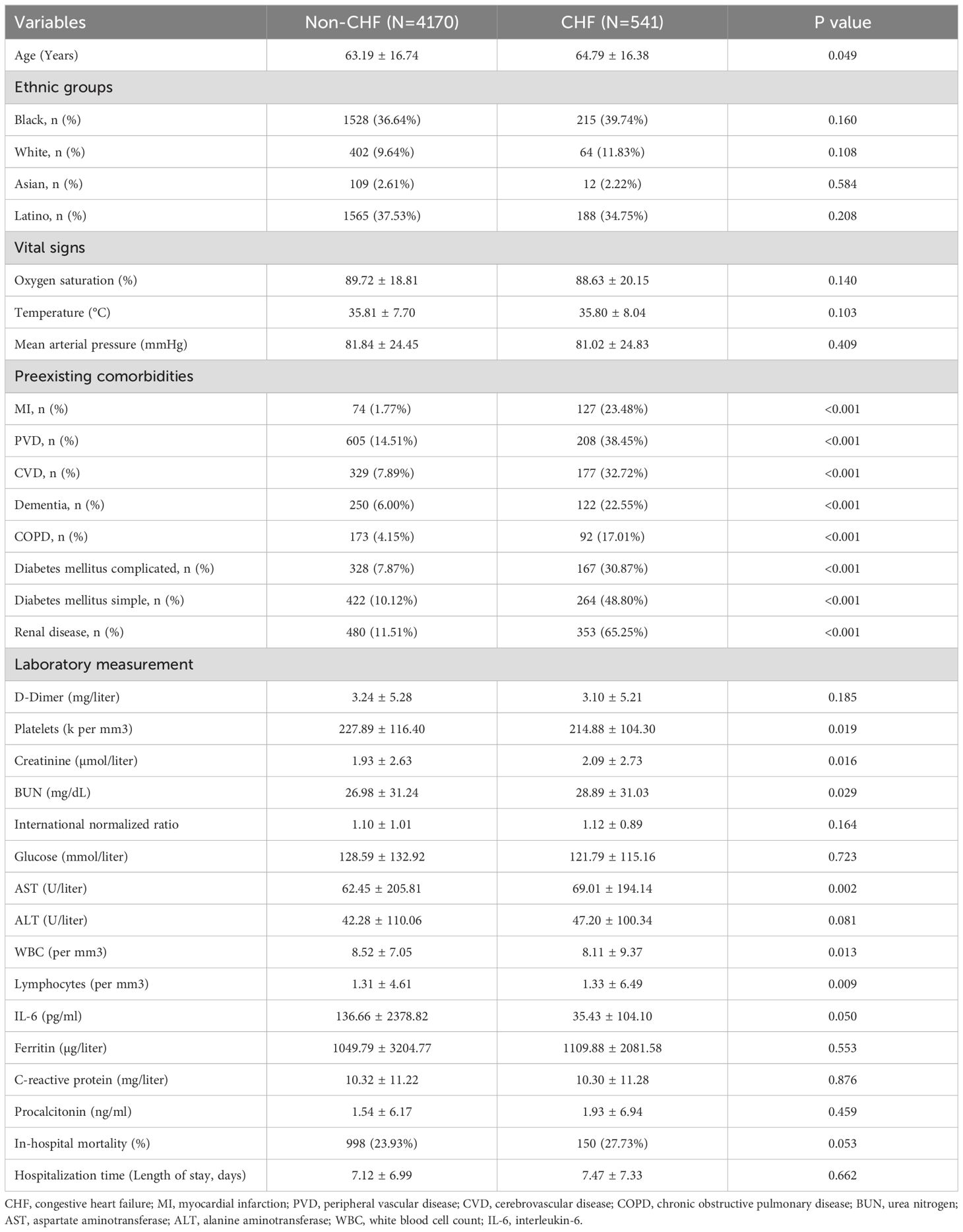
As shown in Table 1, among the 4,711 patients with confirmed COVID-19 infection who were recorded in the Montefiore Health System, 541 (11.5%) individuals were diagnosed with CHF. These CHF patients were older and had a higher prevalence of preexisting comorbidities, including MI, peripheral vascular disease (PVD), cerebrovascular disease (CVD), dementia, chronic obstructive pulmonary disease (COPD), complicated diabetes mellitus, uncomplicated diabetes mellitus, renal disease, and abnormal liver function [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], compared with those without CHF (n = 4,170). Interestingly, CHF patients did not have worse outcomes in admission vital signs (oxygen saturation, temperature, and mean arterial pressure), inflammatory indicators at admission [ferritin, C-reactive protein, and procalcitonin, but not WBC, lymphocytes, or IL-6], or in-hospital mortality compared with non-CHF patients (all p > 0.05). In addition, there was no significant difference in ethnic group distribution between these two subgroups (p > 0.05).
Multivariate logistic regression analysis
We first applied multivariate logistic regression analysis to evaluate the potential associations between inflammatory biomarkers at admission and in-hospital mortality risk after adjusting for sociodemographics and vital signs (Table 2). The adjusted I model showed that, in addition to WBC and IL-6, the inflammatory biomarkers ferritin, procalcitonin, and C-reactive protein were significantly and independently associated with a higher rate of in-hospital mortality after adjusting for age and ethnic group in both CHF and non-CHF patients. Consistently, after further adjusting for age, ethnic group, and vital signs, the adjusted II model suggested that, in addition to WBC and IL-6, ferritin, procalcitonin, and C-reactive protein remained associated with a higher rate of in-hospital mortality in the two subgroups.
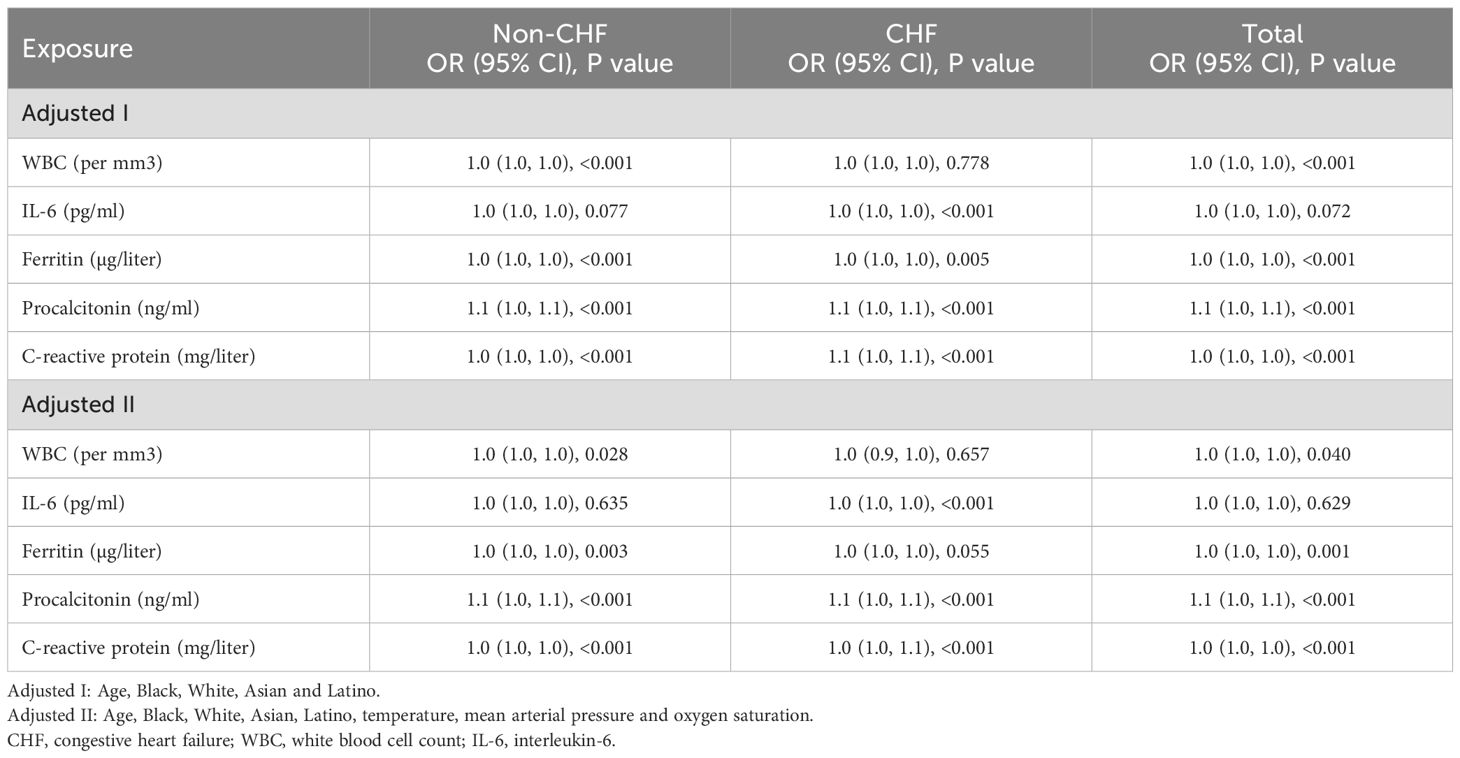
Table 2. Multivariate logistic regression analysis for the association of blood inflammatory markers with in-hospital mortality risk.
Furthermore, we divided these five biomarkers into binary variables to estimate in-hospital mortality risk (Table 3). We observed that elevated inflammatory levels—including WBC (<4,800 or >10,800 per mm³), IL-6 (>150 pg/ml), procalcitonin (>0.1 ng/ml), and C-reactive protein (>10 mg/l)—were significantly associated with a higher rate of in-hospital mortality after controlling for age, Black, White, Asian, Latino, temperature, mean arterial pressure, and oxygen saturation in all patients (CHF and non-CHF), as shown in the adjusted II model.
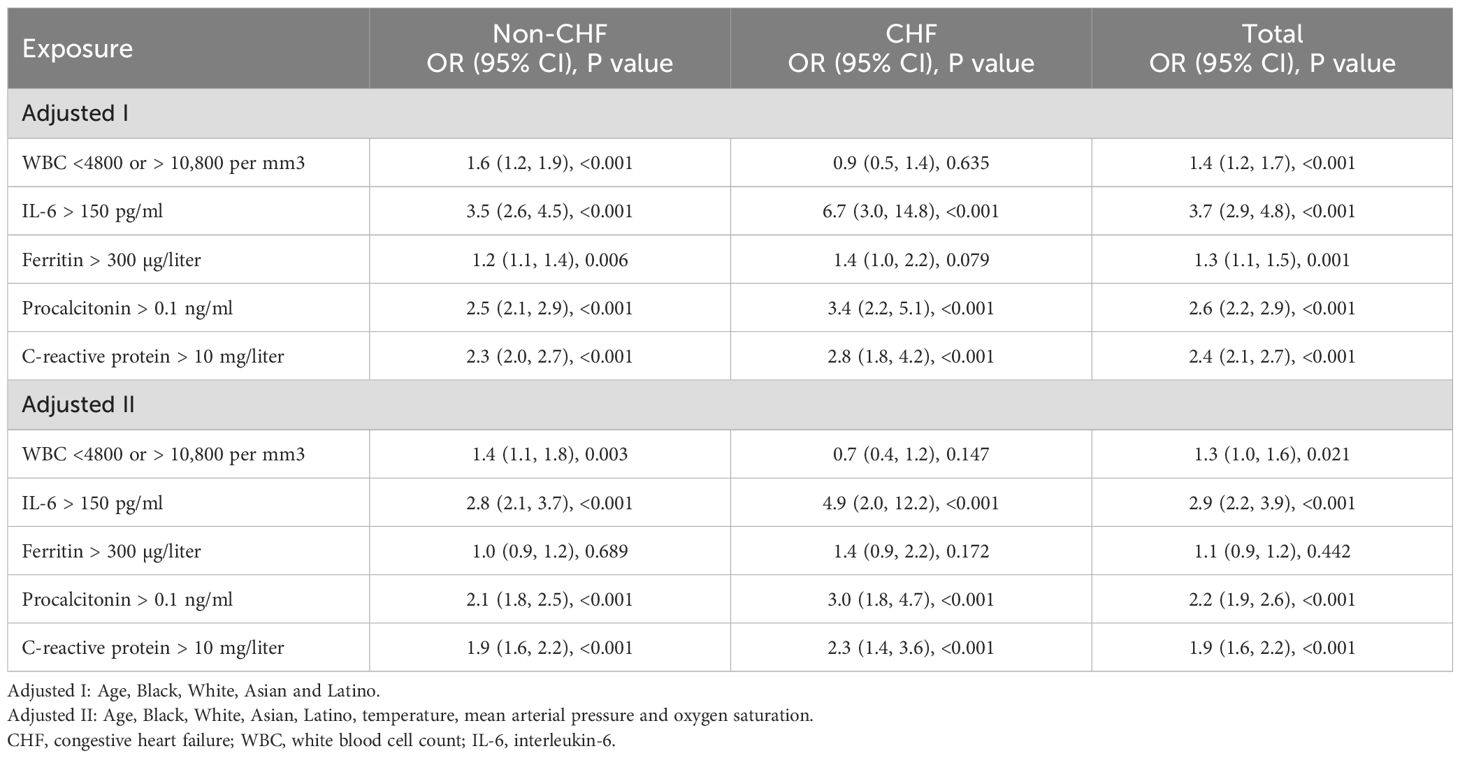
Table 3. Multivariate logistic regression analysis for the association of blood inflammatory markers with in-hospital mortality risk.
Multivariate Cox regression analysis
The univariate analysis of Kaplan–Meier curves suggested that higher inflammatory levels—including WBC (<4,800 or >10,800 per mm3), IL-6 (>150 pg/ml), ferritin (>300 µg/l), procalcitonin (>0.1 ng/ml), and C-reactive protein (>10 mg/l)—were associated with a significantly higher risk of in-hospital mortality compared with lower levels [WBC (>4800 and <10,800 per mm3), IL-6 (<150 pg/ml), ferritin (< 300 µg/liter), procalcitonin (<0.1 ng/ml) and C-reactive protein (<10 mg/l)], as shown in Figures 1A–E.
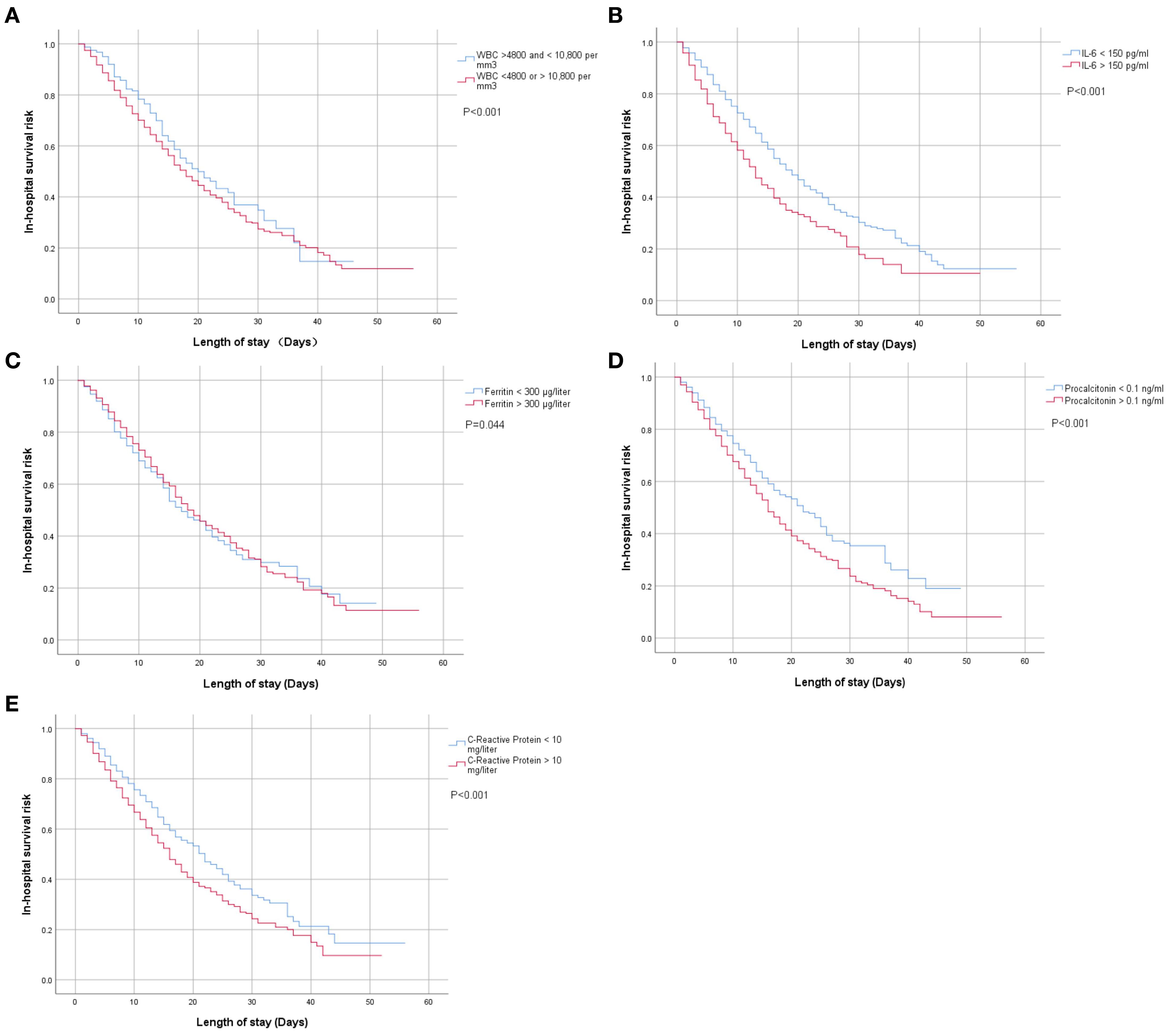
Figure 1. (A–E) Univariate Kaplan–Meier analysis of the associations between blood inflammatory biomarkers at admission and in-hospital mortality risk among patients with confirmed COVID-19 infection.
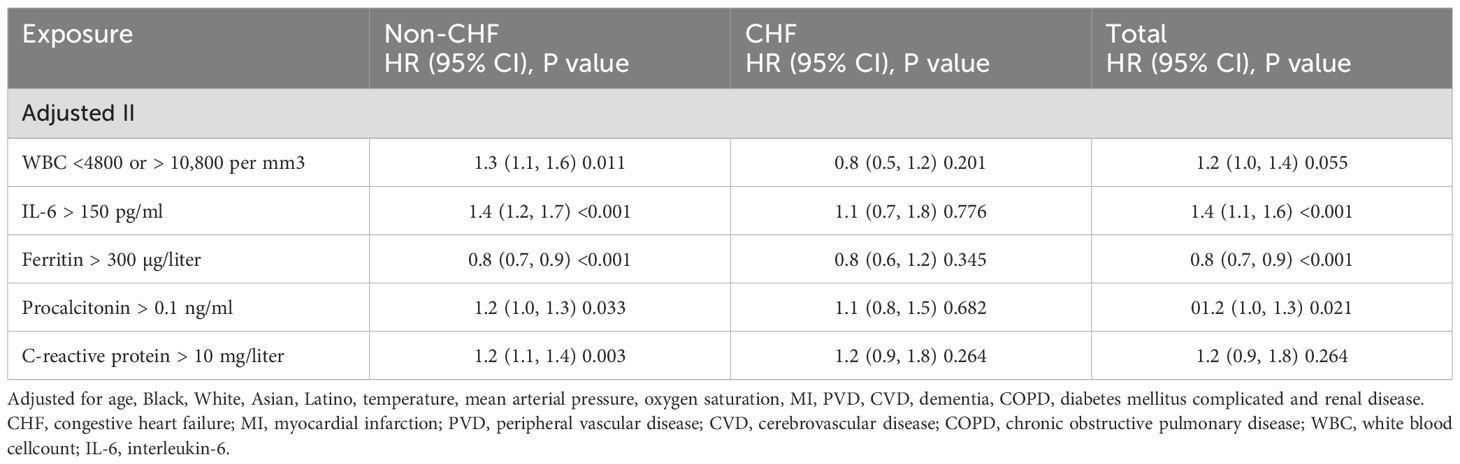
Findings were consistent with the above results. Multivariate Cox regression analysis in the adjusted II model demonstrated that, in addition to ferritin [0.8 (0.7, 0.9), <0.001], WBC [1.3 (1.1, 1.5), 0.013], IL-6 [1.4 (1.2, 1.7), <0.001], procalcitonin [1.2 (1.0, 1.3), 0.031], and C-reactive protein [1.2 (1.1, 1.4), 0.002] contributed to a higher risk of in-hospital mortality in non-CHF patients but not in CHF patients (Table 4).
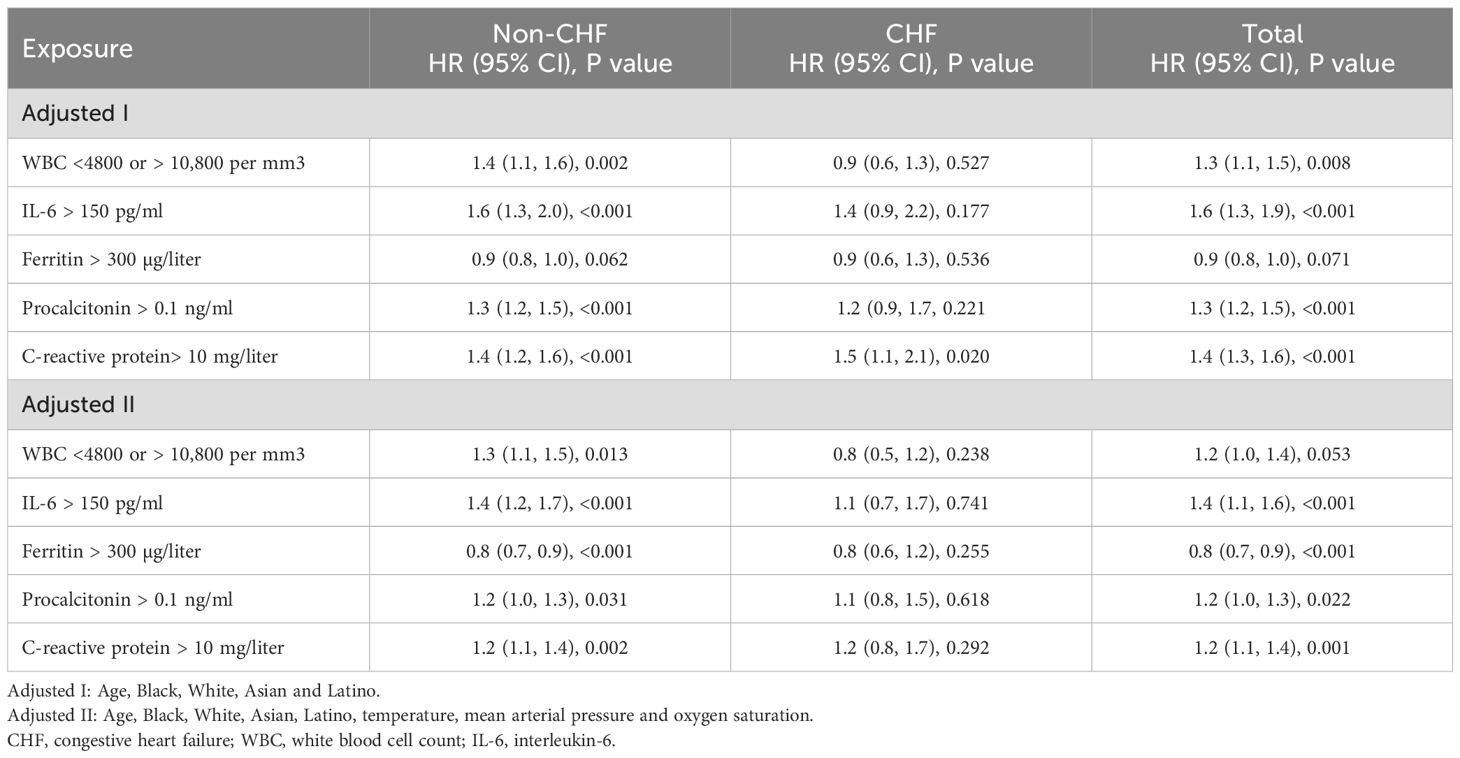
Table 4. Multivariate Cox regression analysis for the association of blood inflammatory markers with in-hospital mortality risk.
Sensitivity analysis
Further sensitivity analysis suggested that WBC (<4800 or >10,800 per mm3), IL-6 (>150 pg/ml), ferritin (>300 µg/liter), procalcitonin (>0.1 ng/ml) and C-reactive protein (>10 mg/liter) were still associated with in-hospital mortality risk in non-CHF patients but not in CHF patients by adjusted for age, Black, White, Asian, Latino, temperature, mean arterial pressure, oxygen saturation, MI, PVD, CVD, dementia, COPD, diabetes mellitus complicated and renal disease (Table 5).
Discussion
We analyzed a large inpatient cohort with confirmed COVID-19 infection, evaluating the predictive value of systemic inflammatory indicators at admission for in-hospital mortality risk. As expected, a significant increase in inflammatory levels contributed to a higher in-hospital mortality risk, but this relationship existed in non-CHF patients rather than in CHF patients.
The COVID-19 pandemic has caused significant morbidity and mortality (Wang et al., 2020; Wu et al., 2023), which can also be complicated by acute or chronic cardiovascular syndromes, in addition to severe respiratory complications (Chung et al., 2021; Patone et al., 2022). These syndromes can manifest as acute coronary syndrome, myocarditis, cardiac arrhythmias, or clinical HF, with or without hemodynamic instability (Driggin et al., 2020; Sala et al., 2020; Heidecker et al., 2022; Zuin et al., 2023a). Such early or late cardiovascular complications may occur at any point during hospitalization or even after discharge, once respiratory symptoms have improved (Bhatraju et al., 2020; Fried et al., 2020).
Previous evidence has indicated that COVID-19 infection may result in adverse cardiovascular events attributable to a severe inflammatory cytokine storm (Guzik et al., 2020; Liu et al., 2020). This is consistent with our findings, which showed that high levels of systemic inflammation significantly increased the risk of in-hospital mortality.
In fact, a number of preexisting comorbidities or risk factors have been associated with worse clinical outcomes in patients with COVID-19 infection. For example, previous studies confirmed that older patients with COVID-19 tended to have a higher risk of requiring mechanical ventilation, composite death, and/or ICU admission compared with younger patients (Guan et al., 2020). Evidence from Chinese populations has shown that the presence of preexisting cardiovascular comorbidities can increase the disease severity of hospital-treated COVID-19 (Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020). These studies concluded that among patients with COVID-19, the fatality rate was much higher in those with comorbidities than in those without (Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020). Reported mortality rates included 10.5% for patients with cardiovascular disease (CVD), 6.0% for hypertension, 7.3% for diabetes, 6.0% for cancer, and 6.3% for chronic respiratory disease (Epidemiology Working Group for NCIP Epidemic Response, 2020; Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020; Ruan, 2020; The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020). Clearly, the occurrence of CVD significantly increased the mortality rate to a greater extent than other preexisting comorbidities (Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020). These findings were also supported by a previous meta-analysis, which reported that CVD was present in 4.2% of the total population with COVID-19, and among these, 22.7% died (Epidemiology Working Group for NCIP Epidemic Response, 2020). However, there was large heterogeneity in reported death rates due to differences in study populations with various cardiovascular comorbidities. Another report from a Chinese population showed that HF was considered an adverse outcome in 23% of individuals with COVID-19; approximately 52% of non-survivors had HF, whereas only 12% of survivors had HF (Zhou et al., 2020). Similarly, in our study, CHF patients (27.73%) had a slightly higher in-hospital mortality rate than non-CHF patients (23.93%), but the statistical difference between the two groups was not significant (p = 0.053).
A previous meta-analysis involving patients who had recovered from COVID-19 (N = 21,463,173; mean age, 54.5 years; 58.7% male) was performed. This study concluded that the risk for incident HF may occur even in individuals at low cardiovascular risk, which was inconsistent with most previous conclusions (Zuin et al., 2023b). The authors explained that the relatively high heterogeneity might be due to differences in baseline population characteristics, preexisting CVD risk, vaccination status for COVID-19, or prior HF history. Our study also found that high systemic inflammatory levels at admission were significantly associated with an increased risk of in-hospital mortality in non-CHF patients but not in CHF patients. HF is well known as the terminal stage of heart disease. Many conditions—including diabetes, hypertension, coronary heart disease, valvular disease, and myocarditis—can lead to HF (Daugherty et al., 2021; Salah et al., 2022; Wang et al., 2022). The heterogeneity of these causes may partly explain the seemingly contradictory phenomena. Other possible sources of variation include differences in population selection by age and race, therapeutic drugs (β-blockers, ACE inhibitors, glucose-lowering, antihypertensive, and lipid-lowering agents), medical history, statistical methods, inclusion criteria, and other factors (Daugherty et al., 2021; Salah et al., 2022; Wang et al., 2022). For instance, previous studies, including meta-analyses, did not systematically analyze prior HF history, limiting the ability to further evaluate death risk (Daugherty et al., 2021; Salah et al., 2022; Wang et al., 2022). The pathological mechanism underlying the higher risk of death in CHF patients with COVID-19 has not yet been fully confirmed. Direct viral invasion causing myocardial damage or endothelial cell infection may partly explain the increased risk of worsening HF or death in most cases (Nishiga et al., 2020; Cowie et al., 2022). However, in our CHF patients, chronically elevated baseline inflammation was not significant, which may explain why inflammatory markers were not directly and significantly correlated with mortality in CHF patients in our analysis. More evidence from prospective cohort studies is needed to further validate our findings.
Undoubtedly, our study findings provide several implications for clinical practice. The risk of in-hospital mortality may be predicted by systemic inflammatory levels at admission during the acute phase of COVID-19 infection in non-CHF patients but not in CHF patients. Although controversies remain regarding mortality risk during hospitalization among CHF patients, our results support the conclusion that patients with preexisting CHF or a prior HF history may be at increased risk of in-hospital mortality. There is no doubt that the present study has several limitations. First, this was a retrospective study, and some variables—such as treatment during hospitalization (e.g., corticosteroids and antivirals), cardiac function, and cause of death—were missing, limiting further refinement of the study. Second, gender data were missing; given that previous studies have suggested that COVID-19 sequelae may differ by gender, this could introduce potential bias. Finally, although sensitivity analysis was performed in our study, additional subgroup analyses stratified by age, comorbidities, or biomarker levels should also be conducted to evaluate the robustness of these findings.
Conclusion
Our findings showed that elevated inflammatory levels at admission in CHF patients were not associated with an increased risk of in-hospital mortality. However, higher inflammatory levels were associated with a greater risk of in-hospital mortality among non-CHF patients. More evidence is needed in the future to support these findings.
Data availability statement
The datasets presented in this study can be found in online repositories. Our study data were obtained from the Dryad database (https://doi.org/10.5061/dryad.7d7wm37sz).
Ethics statement
Our study data were obtained from the Dryad database (https://doi.org/10.5061/dryad.7d7wm37sz). The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
RW: Writing – original draft, Data Curation, Validation. YH: Supervision, Writing – original draft, Writing – review & editing. ZT: Writing – original draft, Data Curation, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by Jiangxi Provincial Natural Science Foundation (20224BAB216019) and (20242BAB25434).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altschul, D. J., Unda, S. R., Benton, J., de la Garza Ramos, R., Cezayirli, P., Mehler, M., et al. (2020). A novel severity score to predict inpatient mortality in COVID-19 patients. Sci. Rep. 10, 16726. doi: 10.1038/s41598-020-73962-9, PMID: 33028914
Bhatraju, P. K., Ghassemieh, B. J., Nichols, M., Kim, R., Jerome, K. R., Nalla, A. K., et al. (2020). Covid-19 in critically ill patients in the seattle region - case series. N Engl. J. Med. 382, 2012–2022. doi: 10.1056/NEJMoa2004500, PMID: 32227758
Chung, M. K., Zidar, D. A., Bristow, M. R., Cameron, S. J., Chan, T., Harding, C. V., 3rd, et al. (2021). COVID-19 and cardiovascular disease: from bench to bedside. Circ. Res. 128, 1214–1236. doi: 10.1161/CIRCRESAHA.121.317997, PMID: 33856918
Cowie, M. R., Mourilhe-Rocha, R., Chang, H. Y., Volterrani, M., Ban, H. N., Campos de Albuquerque, D., et al. (2022). The impact of the COVID-19 pandemic on heart failure management: Global experience of the OPTIMIZE Heart Failure Care network. Int. J. Cardiol. 363, 240–246. doi: 10.1016/j.ijcard.2022.06.022, PMID: 35750302
Daugherty, S. E., Guo, Y., Heath, K., Dasmariñas, M. C., Jubilo, K. G., Samranvedhya, J., et al. (2021). Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 373, n1098. doi: 10.1136/bmj.n1098, PMID: 34011492
Driggin, E., Madhavan, M. V., Bikdeli, B., Chuich, T., Laracy, J., Biondi-Zoccai, G., et al. (2020). Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 75, 2352–2371. doi: 10.1016/j.jacc.2020.03.031, PMID: 32201335
Epidemiology Working Group for NCIP Epidemic Response (2020). The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin. J. Epidemiol. 41, 145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003, PMID: 32064853
Eskandar, E. N., Altschul, D. J., de la Garza Ramos, R., Cezayirli, P., Unda, S. R., Benton, J., et al. (2021). Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. 96, e1527–e1538. doi: 10.1212/WNL.0000000000011356, PMID: 33443111
Fried, J. A., Ramasubbu, K., Bhatt, R., Topkara, V. K., Clerkin, K. J., Horn, E., et al. (2020). The variety of cardiovascular presentations of COVID-19. Circulation. 141, 1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164, PMID: 32243205
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N Engl. J. Med. 382, 1708–1720. doi: 10.1056/NEJMoa2002032, PMID: 32109013
Guzik, T. J., Mohiddin, S. A., Dimarco, A., Patel, V., Savvatis, K., Marelli-Berg, F. M., et al. (2020). COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 116, 1666–1687. doi: 10.1093/cvr/cvaa106, PMID: 32352535
Hashem, A., Khalouf, A., Mohamed, M. S., Nayfeh, T., Elkhapery, A., Elbahnasawy, M., et al. (2023). COVID-19 infection is associated with increased in-hospital mortality and complications in patients with acute heart failure: insight from national inpatient sample (2020). J. Intensive Care Med. 38, 1068–1077. doi: 10.1177/08850666231182380, PMID: 37350092
Heidecker, B., Dagan, N., Balicer, R., Eriksson, U., Rosano, G., Coats, A., et al. (2022). Myocarditis following COVID-19 vaccine: incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 24, 2000–2018. doi: 10.1002/ejhf.2669, PMID: 36065751
Huang, L., Li, X., Gu, X., Zhang, H., Ren, L., Guo, L., et al. (2022). Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir. Med. 10, 863–876. doi: 10.1016/S2213-2600(22)00126-6, PMID: 35568052
Isath, A., Malik, A., Bandyopadhyay, D., Goel, A., Hajra, A., Dhand, A., et al. (2023). COVID-19, heart failure hospitalizations, and outcomes: A nationwide analysis. Curr. Probl Cardiol. 48, 101541. doi: 10.1016/j.cpcardiol.2022.101541, PMID: 36529234
Jackson, C. B., Farzan, M., Chen, B., and Choe, H. (2022). Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23, 3–20. doi: 10.1038/s41580-021-00418-x, PMID: 34611326
Liu, H., Gai, S., Wang, X., Zeng, J., Sun, C., Zhao, Y., et al. (2020). Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc. Res. 116, 1733–1741. doi: 10.1093/cvr/cvaa191, PMID: 32638018
Nishiga, M., Wang, D. W., Han, Y., Lewis, D. B., and Wu, J. C. (2020). COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 17, 543–558. doi: 10.1038/s41569-020-0413-9, PMID: 32690910
Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (2020). The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41, 145–151.
Panhwar, M. S., Kalra, A., Gupta, T., Kolte, D., Khera, S., Bhatt, D. L., et al. (2019). Effect of influenza on outcomes in patients with heart failure. JACC Heart Fail. 7, 112–117. doi: 10.1016/j.jchf.2018.10.011, PMID: 30611718
Patone, M., Mei, X. W., Handunnetthi, L., Dixon, S., Zaccardi, F., Shankar-Hari, M., et al. (2022). Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 28, 410–422. doi: 10.1038/s41591-021-01630-0, PMID: 34907393
Rey, J. R., Caro-Codón, J., Rosillo, S. O., Iniesta, Á. M., Castrejón-Castrejón, S., Marco-Clement, I., et al. (2020). Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur. J. Heart Fail. 22, 2205–2215. doi: 10.1002/ejhf.1990, PMID: 32833283
Ruan, S. (2020). Likelihood of survival of coronavirus disease 2019. Lancet Infect. Dis. 20, 630–631. doi: 10.1016/S1473-3099(20)30257-7, PMID: 32240633
Sala, S., Peretto, G., Gramegna, M., Palmisano, A., Villatore, A., Vignale, D., et al. (2020). Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 41, 1861–1862. doi: 10.1093/eurheartj/ehaa286, PMID: 32267502
Salah, H. M., Fudim, M., O’Neil, S. T., Manna, A., Chute, C. G., and Caughey, M. C. (2022). Post-recovery COVID-19 and incident heart failure in the National COVID Cohort Collaborative (N3C) study. Nat. Commun. 13, 4117. doi: 10.1038/s41467-022-31834-y, PMID: 35840623
Sasson, G., Bai, A. D., Showler, A., Burry, L., Steinberg, M., Ricciuto, D. R., et al. (2017). Staphylococcus aureus bacteremia in immunosuppressed patients: a multicenter, retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 36, 1231–1241. doi: 10.1007/s10096-017-2914-y, PMID: 28251359
Stalder, H. and Thiel, V. (2021). Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19, 155–170. doi: 10.1038/s41579-020-00468-6, PMID: 33116300
The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (2020). China CDC Wkly 2, 113–122.
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan. China. JAMA 323, 1061–1069. doi: 10.1001/jama.2020.1585, PMID: 32031570
Wang, W., Wang, C. Y., Wang, S. I., and Wei, J. C. (2022). Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. 53, 101619. doi: 10.1016/j.eclinm.2022.101619, PMID: 35971425
Wu, N., Joyal-Desmarais, K., Ribeiro, P. A. B., Vieira, A. M., Stojanovic, J., Sanuade, C., et al. (2023). Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 11, 439–452. doi: 10.1016/S2213-2600(23)00015-2, PMID: 36780914
Xie, Y., Xu, E., Bowe, B., and Al-Aly, Z. (2022). Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28, 583–590. doi: 10.1038/s41591-022-01689-3, PMID: 35132265
Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395, 1054–1062. doi: 10.1016/S0140-6736(20)30566-3, PMID: 32171076
Zuin, M., Rigatelli, G., Battisti, V., Costola, G., Roncon, L., and Bilato, C. (2023a). Increased risk of acute myocardial infarction after COVID-19 recovery: A systematic review and meta-analysis. Int. J. Cardiol. 372, 138–143. doi: 10.1016/j.ijcard.2022.12.032, PMID: 36535564
Keywords: COVID-19, in-hospital mortality, heart failure, inflammation, risk stratification
Citation: Wan R, Tan Z and Huang Y (2025) Infectious biomarkers upon admission predict in-hospital mortality in COVID-19 patients with and without chronic heart failure. Front. Cell. Infect. Microbiol. 15:1577214. doi: 10.3389/fcimb.2025.1577214
Received: 15 February 2025; Accepted: 15 September 2025;
Published: 24 October 2025.
Edited by:
Luciane Amorim Santos, Bahiana School of Medicine and Public Health, BrazilCopyright © 2025 Wan, Tan and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Huang, aHluYW5jaGFuZzg4ODhAMTYzLmNvbQ==
†The first author
 Rong Wan1†
Rong Wan1† Zhaochong Tan
Zhaochong Tan Ying Huang
Ying Huang