- 1Department of Endocrinology and Metabolism, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, Jiangsu, China
- 2Department of Geriatrics, Shanghai Health and Medical Center, Wuxi, Jiangsu, China
- 3Department of Surgery, Shanghai Health and Medical Center, Wuxi, Jiangsu, China
Background: Helicobacter pylori (HP) infection is one of the most common chronic infections worldwide, closely related to various gastrointestinal diseases and metabolic disorders. In recent years, the relationship between HP infection and abnormal glucose and lipid metabolism has received significant attention, although its specific mechanisms remain unclear. This study aims to explore the association between HP infection and lipid metabolism abnormalities, particularly the role of the apolipoprotein B/A1 (ApoB/ApoA1) ratio.
Methods: This cross-sectional study retrospectively analyzed data from 9,218 patients who underwent physical examinations at Shanghai Health and Medical Center in 2022. HP infection status was determined using the carbon-13 breath test, and clinical data, biochemical indicators, and lipid metabolism-related data were collected. Multiple regression analysis was employed to investigate the relationship between HP infection and the ApoB/ApoA1 ratio.
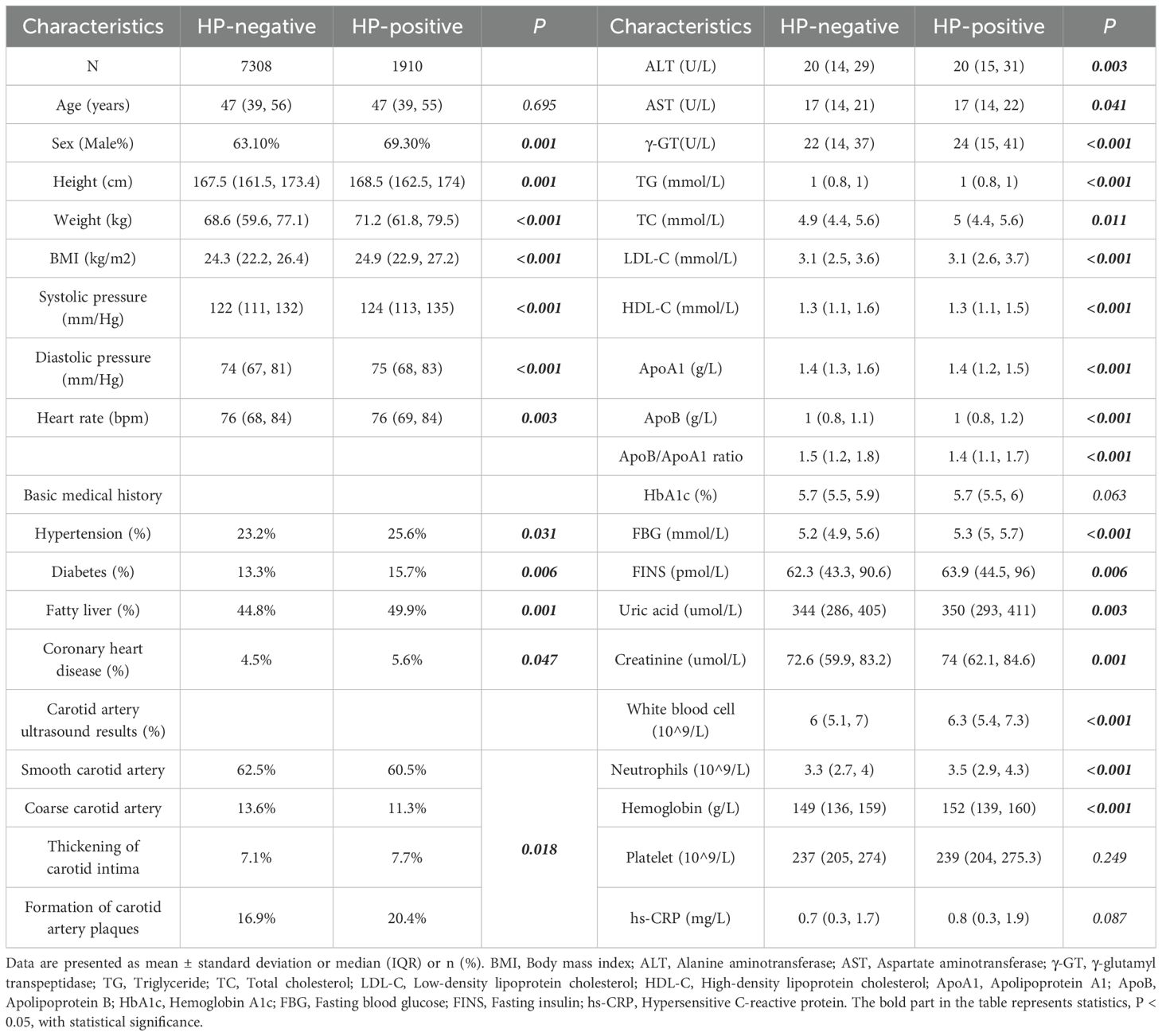
Results: Patients in the HP-positive group were older and had a higher proportion of males. Their body mass index (BMI), blood pressure, γ-glutamyl transpeptidase (γ-GT), total cholesterol (TC), fasting blood glucose (FBG), Creatinine and White blood Cell were significantly higher than those in the HP-negative group. The HP-positive group exhibited a higher prevalence of underlying diseases (e.g., hypertension, diabetes, coronary heart disease) and significant abnormalities in glucose and lipid metabolism, uric acid, high-sensitivity C-reactive protein (hs-CRP), and other indicators. The ApoB/ApoA1 ratio was significantly elevated in the HP-positive group and was not influenced by gender. Multiple regression analysis revealed that the ApoB/ApoA1 ratio is an independent risk factor for HP infection.
Conclusion: HP infection is closely associated with abnormal lipid metabolism, and the ApoB/ApoA1 ratio is an independent risk factor for HP infection, demonstrating significant advantages over other lipid indicators. This large-scale study highlights a significant association between HP infection and an elevated ApoB/ApoA1 ratio. The findings suggest that HP may contribute to cardiovascular risk via dyslipidemia, with the ApoB/ApoA1 ratio serving as a potential biomarker. Further research should explore whether HP eradication could mitigate these metabolic disturbances.
1 Introduction
Helicobacter pylori (HP) is a microaerophilic, spiral-shaped, Gram-negative bacterium that colonizes the stomach. HP infection is one of the most common chronic infections globally, affecting over 50% of the world’s population, with approximately 4.4 billion individuals infected (Li et al., 2017). HP infection is a well-established independent risk factor for gastrointestinal diseases such as peptic ulcers, chronic gastritis, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma. Recently, attention has shifted to the correlation between HP infection and metabolic disorders. Studies have shown that HP infection is closely associated with impaired glucose and lipid metabolism, including diabetes, obesity, dyslipidemia, atherosclerosis, and nonalcoholic fatty liver disease (Bravo et al., 2018).
Several studies have reported that patients with HP infection exhibit adverse lipid profiles that promote atherosclerosis (Moretti et al., 2014; Zhao et al., 2019; Abdu et al., 2020; Martín-Núñez et al., 2020; Lu et al., 2022). Following treatment, blood lipid levels have been shown to improve after HP eradication with oral antibiotics (Ando et al., 2006). However, the relationship between HP infection and lipid metabolism remains unclear. For instance, Negussie et al. found that total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) were elevated in HP-infected patients (Sarbecha et al., 2024), while Sun et al. reported no significant differences in TC and LDL-C levels between HP-infected and non-infected individuals, with HP infection only showing a significant correlation with high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG) (Sun et al., 2016). Xiao et al. observed that TC, TG, and LDL-C levels were higher in the HP-infected group, while HDL-C levels were lower compared to the control group (Yuliandari and Mayura, 2024). Although these findings suggest that HP-infected patients exhibit adverse lipid profiles, the correlation between HP infection and abnormal lipid metabolism varies across studies. Whether other lipid indicators offer potential advantages warrants further investigation.
Apolipoproteins, synthesized in the liver, play a crucial role in lipid transport and redistribution. Apolipoproteins primarily consist of apolipoprotein B (ApoB) and apolipoprotein A1 (ApoA1). The measurement of ApoB and ApoA1 does not require fasting samples and is standardized, making the ApoB/ApoA1 ratio a more balanced, comprehensive, and stable indicator of lipid metabolism and a predictor of cardiovascular disease and diabetes (Jing et al., 2014; Nurtazina et al., 2020). Apolipoproteins also play a significant role in immune regulation and inflammatory responses (Zhong et al., 2022). However, no studies have yet reported a correlation between HP infection and apolipoproteins.
In this study, we conducted a cross-sectional investigation to explore the differences in glucose and lipid metabolism disorders between HP-positive and HP-negative patients, the correlation between HP infection and the ApoB/ApoA1 ratio, and to further elucidate the potential relationship between them. This large-scale study highlights a significant association between HP infection and an elevated ApoB/ApoA1 ratio. The findings suggest that HP may contribute to cardiovascular risk via dyslipidemia, with the ApoB/ApoA1 ratio serving as a potential biomarker. Further research should explore whether HP eradication could mitigate these metabolic disturbances.
2 Subjects and methods
2.1 Research design
This cross-sectional study was conducted at the Shanghai Health and Medical Center in Wuxi, China. The primary objective was to evaluate the correlation between carbon-13 breath test results and ApoA1, ApoB, and ApoB/ApoA1 ratio.
We collected data from subjects who underwent physical examinations at the Shanghai Health Care Center in 2022. Subjects were screened based on inclusion and exclusion criteria, and general information (e.g., age, gender, height, weight, blood pressure, heart rate), medical history (e.g., diabetes, hypertension, coronary heart disease, vascular plaque), routine biochemical examinations (e.g., white blood cell count, neutrophil count, hemoglobin, high-sensitivity C-reactive protein (hs-CRP), fasting blood glucose (FBG), Hemoglobin A1c, creatinine (HbA1c), alanine aminotransferase (ALT),aspartate aminotransferase (AST),γ-glutamyl transpeptidase (γ-GT), TG, cholesterol, apolipoproteins), and C13 breath test results were collected. Patients were grouped based on C13 breath test results, and intergroup differences were evaluated. Further subgroup analysis by gender was conducted to assess potential influencing factors. Finally, the correlation between C13 breath test results and ApoA1, ApoB, and ApoB/ApoA1 ratio was evaluated.
The confirmation of fatty liver comes from abdominal color Doppler ultrasound. The main diagnostic features include enhanced echo in the front field of the liver (“bright liver”), attenuated echo in the far field, and unclear display of the pipeline structure in the liver to diagnose fatty liver.
The confirmation and classification of carotid atherosclerosis comes from cervical vascular color Doppler ultrasound. Smooth carotid artery: Smooth carotid intima, uniform thickness, IMT less than 1.0mm, and enlargement less than 1.2mm. Coarse carotid artery: The intima of the carotid artery is not smooth, with intermittent echoes or localized thickening of the intima, and an IMT greater than 1.0mm. Thickening of carotid intima: The IMT is between 1.0-1.5mm, and the vascular wall is thickened, but no plaque is formed. Formation of carotid artery plaque: When the IMT is greater than or equal to 1.5mm, there may be plaques protruding into the lumen on the local vascular wall. The plaques can be hypoechoic, mixed echoic, or calcified echoic, and may be accompanied by ulcers or irregular shapes.
2.2 Inclusion and exclusion criteria
Inclusion criteria:
1. Age ≥ 18 years.
2. Male or female.
Exclusion criteria:
1. History of gastrointestinal surgery.
2. Antibiotics were used in the last 4 weeks before the examination, regardless of the type and duration of use.
3. Long-term antibiotic use.
4. Alanine aminotransferase or aspartate aminotransferase ≥ 3 times the upper limit of normal.
5. No clear C13 breath test results.
6. Patients with mental, speech, or behavioral disorders.
2.3 Study population
This study retrospectively analyzed data from 36,845 patients who underwent physical examinations at Shanghai Health and Medical Center in 2022. Among them, 19,592 patients who did not undergo the C13 breath test were excluded, along with 286 patients with a history of gastrointestinal surgery, malignant tumors, or long-term antibiotic use, 112 patients with elevated liver enzymes, and 7,637 patients with missing data. A total of 9,218 patients were included in the study.
2.4 Statistical analysis
Continuous variables with normal distribution are presented as mean ± standard deviation, while non-normally distributed variables are presented as median and interquartile range. Categorical data are presented as frequencies and percentages. One-way analysis of variance (ANOVA) was used to compare clinical features between groups, the Bonferroni-corrected Mann-Whitney U test was used for non-normally distributed variables, and the chi-square test was used for categorical variables. Stepwise multiple regression analysis was conducted to identify independent risk factors, including major clinical risk factors and variables with p-values ≤ 0.1 in univariate analysis. Regression results are expressed as odds ratios (OR) with 95% confidence intervals (CI).
3 Results
3.1 Baseline characteristics of HP-positive and HP-negative groups
Compared to the HP-negative group, the HP-positive group was older and had a higher proportion of males. Additionally, the HP-positive group had significantly higher BMI, blood pressure, and other indicators. The prevalence of underlying diseases (e.g., hypertension, diabetes, coronary heart disease, vascular plaque) was higher in the HP-positive group, with statistically significant differences. Liver and kidney function, harmful lipid metabolism, glucose metabolism indicators, uric acid, sh-CRP, and white blood cell were also higher in the HP-positive group. Significant differences were observed in ApoB and the ApoB/ApoA1 ratio (p < 0.001 for both). HDL-C and Apo A1 levels were lower in the HP-positive group (p < 0.001 for both), with statistically significant differences (Table 1).
3.2 Clinical characteristics differences between HP-positive and HP-negative in different gender groups
In the male group, BMI, blood pressure, diabetes history and fatty liver disease history differed significantly between HP-positive and HP-negative patients. In the female group, only BMI showed a significant difference (Table 2A). Regardless of gender, LDL-C, HDL-C, ApoA1, ApoB, ApoB/ApoA1 ratio, and white blood cells differed significantly between HP-positive and HP-negative patients. In the male group, γ-GT, TG, FBG, and HbA1c levels also differed significantly (p<0.05), while these differences were not observed in the female group (Table 2B).
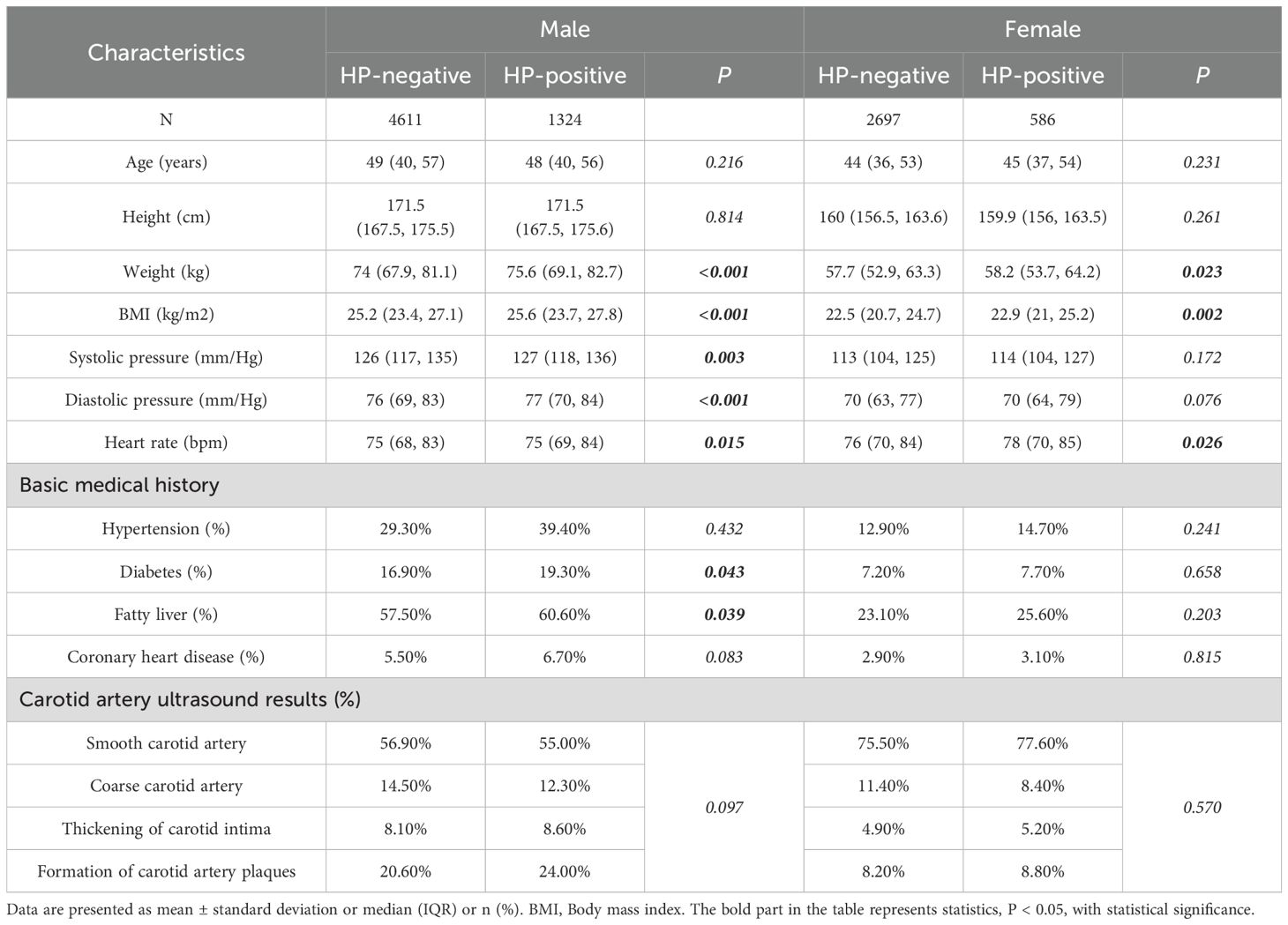
Table 2A. Clinical characteristics differences between HP-positive and HP-negative in different gender groups.
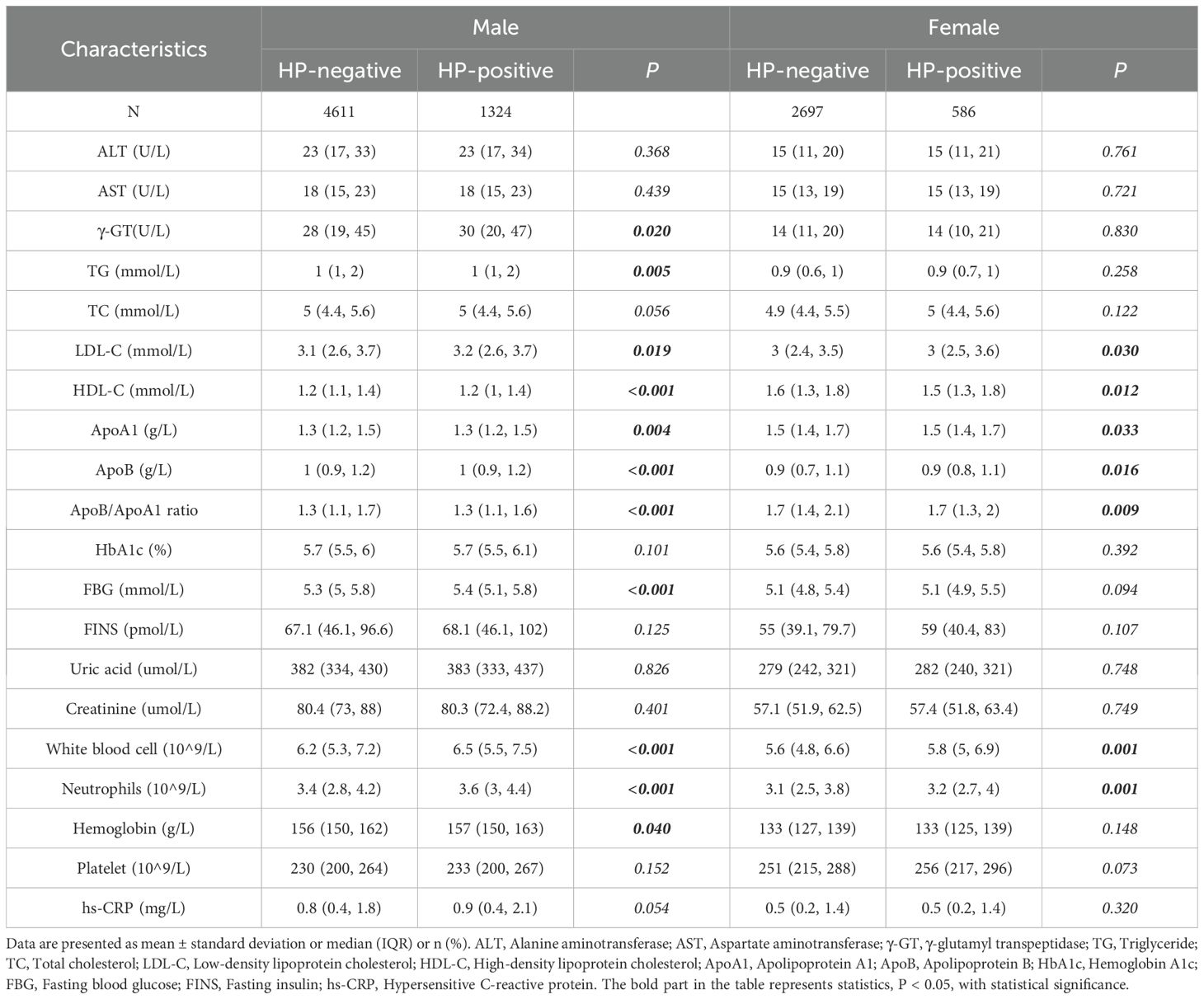
Table 2B. Clinical characteristics differences between HP-positive and HP-negative in different gender groups.
3.3 Characteristics of HP-positive and HP-negative in different groups
After grouping by gender, there are still statistical differences in BMI, LDL-C, HDL-C, ApoB/ApoA 1 ratio, white blood cell and neutrophils between HP negative and positive patients (Figure 1). The differences in ALT, AST, TC, Uric acid and Creatinine between HP negative and HP positive patients before grouping by gender disappeared after grouping by gender (Figure 1).
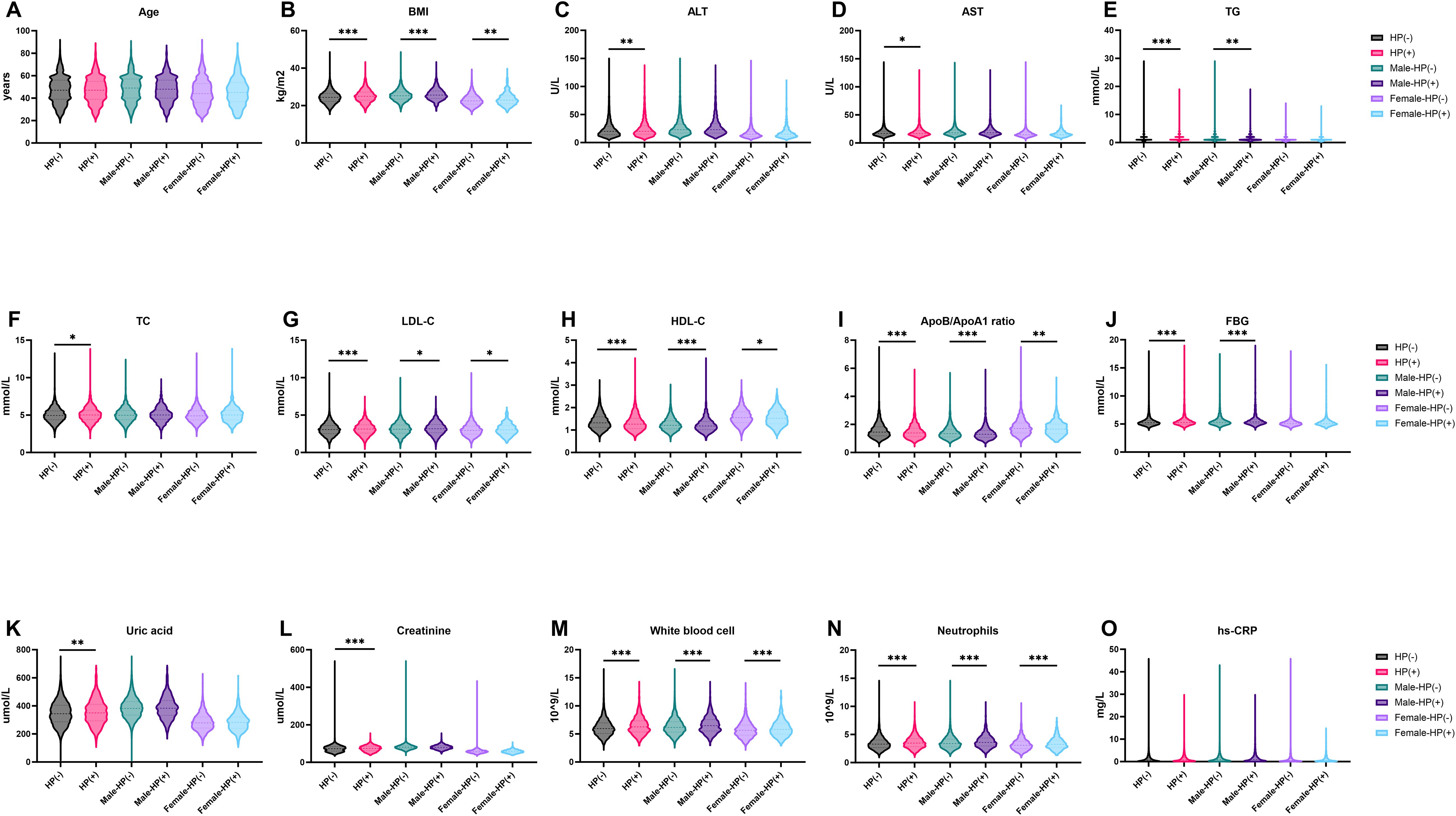
Figure 1. Characteristics of HP-Positive and HP-Negative in different groups. (A, B) show the distribution characteristics of Age and BMI in different groups; (C–I) show the distribution characteristics of ALT, AST, TG, TC, LDL-C, HDL-C and ApoB/ApoA1ratio in different groups; (J-O) show the distribution characteristics of FBG, Uric acid, Creatine, White blood cell, Neutrophils and hs-CRP in different groups. *P < 0.05, **P < 0.01, ***P < 0.001. BMI, Bodymass index; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; TG, Triglyceride; TC, Total cholesterol; LDL-C, Low-density lipoprotein cholesterol; HDL-C, High-density lipoprotein cholesterol; ApoA1, ApolipoproteinA1; ApoB, ApolipoproteinB; FBG, Fastingbloodglucose; hs-CRP, HypersensitiveC-reactiveprotein.
3.4 Single factor logistic regression analysis of HP infection
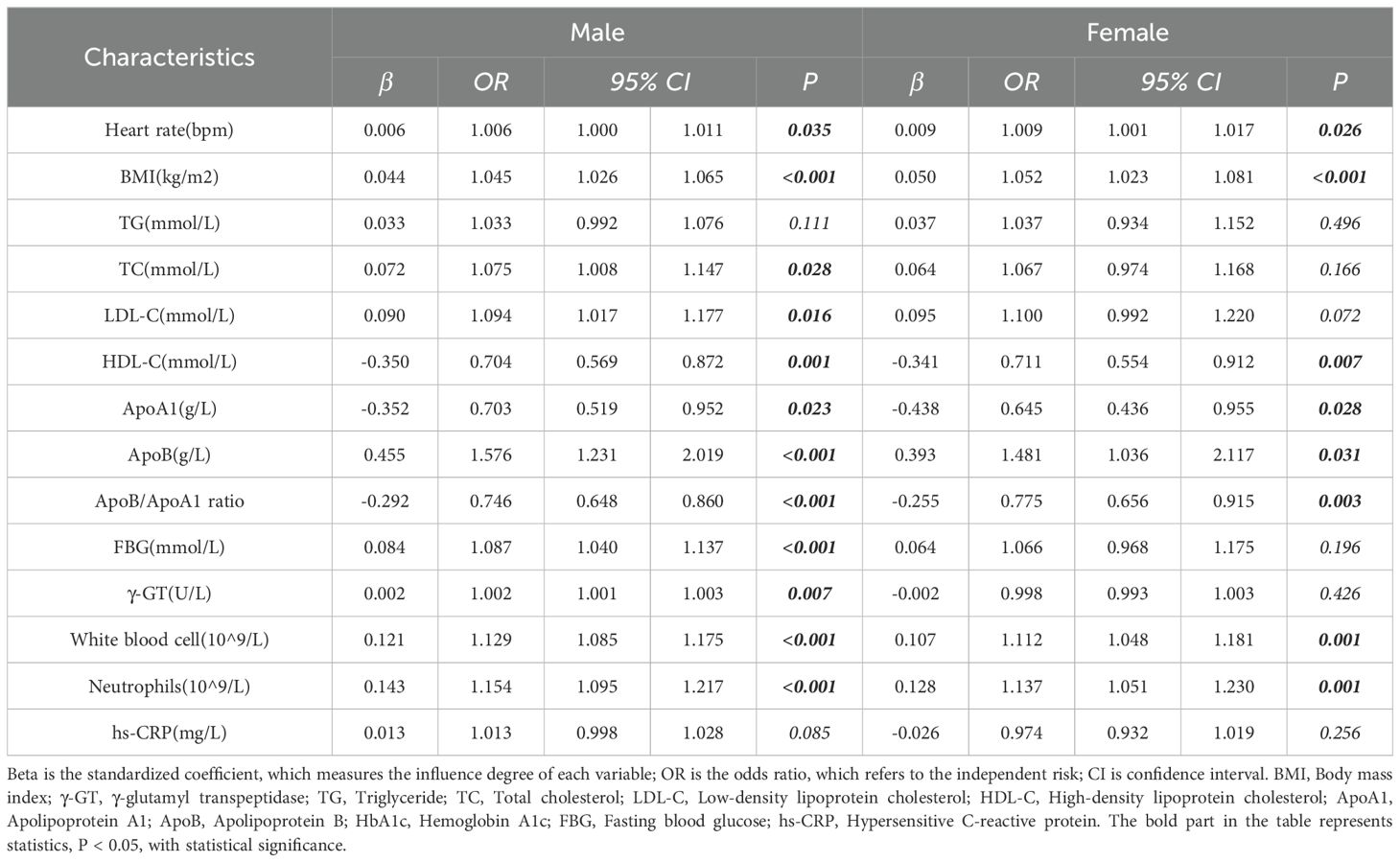
In the male group, BMI, heart rate, LDL-C, HDL-C, ApoB, ApoB/ApoA1 ratio, FBG, γ-GT, white blood cells, neutrophils, and hs-CRP were significant risk factors for HP positivity (p < 0.05) (Table 3). In the female group, BMI, heart rate, HDL-C, ApoB, ApoB/ApoA1 ratio, white blood cells, and neutrophils were significant risk factors for HP positivity (p < 0.05) (Table 3).
3.5 HP positive ratio in different lipids after quartile division
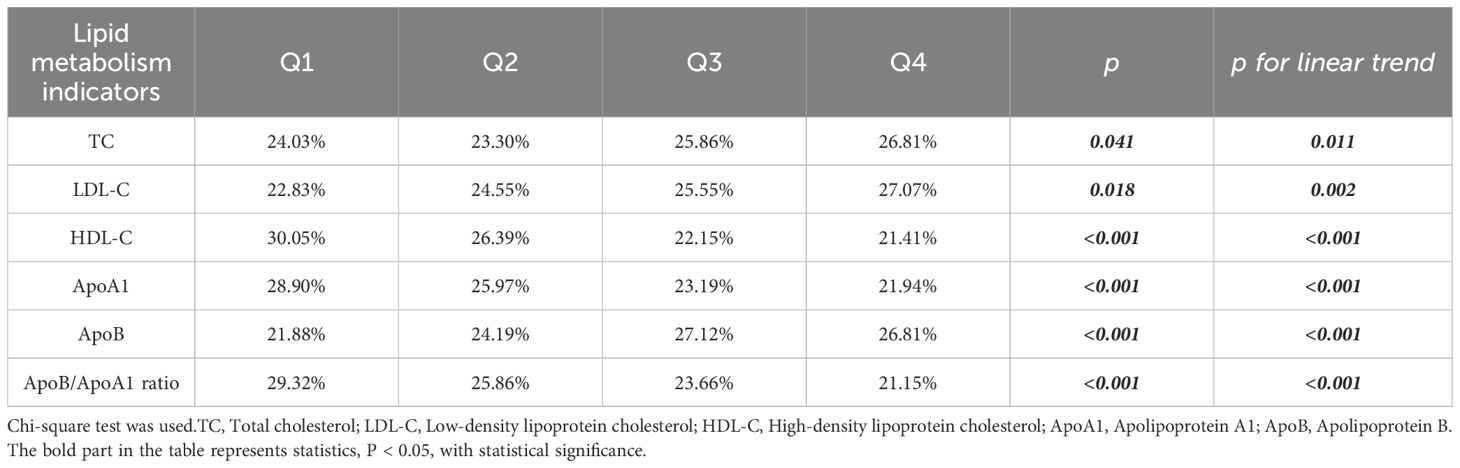
After dividing TC, LDL-C, HDL-C, ApoA 1, ApoB and ApoB/ApoA 1 ratio into quartiles, the results showed that the positive ratio of Helicobacter pylori patients was different, with a linear trend, which was statistically significant (Table 4). With the gradual increase of TC, LDL-C and ApoB from Q1 to Q4, the positive rate of patients with Helicobacter pylori gradually increased, with statistical significance (p<0.05). With the increase of HDL-C, ApoA 1 and ApoB/ApoA 1 ratio, the positive rate of patients with Helicobacter pylori gradually decreased, with statistical significance (p<0.05).
3.6 Multivariate logistic regression analysis of HP infection
In male patients, FBG, white blood cells, ApoB/ApoA1 ratio, and BMI were independent risk factors for HP positivity (p < 0.05), with OR values of 1.065, 1.106, 0.84, and 1.029, respectively (Table 5). In female patients, white blood cells, ApoB/ApoA1 ratio, BMI, and hs-CRP were independent risk factors for HP positivity (p < 0.05), with OR values of 1.113, 0.818, 1.047, and 0.918, respectively (Table 5).
4 Discussion
This study found that HP infection is closely associated with multiple clinical parameters. The prevalence of underlying diseases, such as hypertension, diabetes, coronary heart disease, and vascular plaque, was higher in the HP-positive group. Significant differences were observed in glucose and lipid metabolism-related indicators, uric acid, hs-CRP, and white blood cells between the HP-positive and HP-negative groups, consistent with previous studies (Bravo et al., 2018; Park et al., 2021; Feng et al., 2024; Yang et al., 2024). These findings suggest that HP-positive individuals may experience systemic glucose and lipid metabolism disorders and chronic inflammation, with HP infection closely linked to cardiovascular system damage.
Multiple pathways connect HP infection and lipid metabolism disorders. The chronic low-grade inflammation induced by HP infection leads to the accumulation of pro-inflammatory cytokines, such as C-reactive protein, interleukin-6, and interleukin-8, exacerbating oxidative stress and lipid metabolism disturbances (Chen et al., 2023). Inflammatory signals activate nuclear factor kappa B (NF-κB), downregulating lipid metabolism-related genes and enhancing pro-inflammatory gene expression (Shi et al., 2022; Wang et al., 2022; Chen et al., 2024). These factors significantly impact host lipid metabolism and atherosclerosis progression, increasing cardiovascular risk (Robinson et al., 2015; Krupa et al., 2021). Additionally, HP infection modulates host energy metabolism by upregulating mTORC1 expression and altering branched-chain amino acid metabolism (Kim et al., 2018; Feng et al., 2020). HP infection also affects metabolic hormone secretion (e.g., ghrelin, leptin, GLP-1) and induces host inflammatory responses by altering gut microbiota composition, impacting host metabolism and energy homeostasis (Cuomo et al., 2020). Conversely, lipid metabolism disorders can affect gastric blood circulation and mucosal barrier function, creating favorable conditions for the colonization and reproduction of Hp. They can also lead to changes in the lipid composition of gastric mucosa, affecting mucosal integrity and permeability, making it easier for Hp to invade the submucosal layer and aggravating the degree of infection (Feng et al., 2020). In addition, lipid metabolism disorders can lead to an imbalance in immune regulation, resulting in a decrease in the body’s immune defense against HP.
Our study also revealed gender differences in clinical characteristics. Male HP-positive patients exhibited significant differences in age, medical history, liver function, lipid metabolism, glucose metabolism, uric acid, and hs-CRP compared to HP-negative males. In contrast, female HP-positive patients showed differences in only a few indicators, which seems to suggest that women themselves have an inhibitory effect on HP induced glucose and lipid metabolism disorders. Limited research exists on gender differences in HP infection.
Previous studies have found a decrease in Hp serum positivity among individuals using contraceptive pills, suggesting a potential protective effect of oral contraceptives against Helicobacter pylori infection (Fong and Wang, 2021). Previous epidemiological studies and animal models have shown that female hormones, especially estrogen, have a protective effect on Hp induced gastric cancer, and this study further reveals that Helicobacter pylori cells adsorbed with estrogen may be prevented from adhering to gastric epithelial cells, thereby exerting a protective effect (Hosoda et al., 2009). Animal studies have demonstrated that estrogen supplementation alleviates gastric damage in HP-infected male mice by enhancing IL-10 function and reducing IFN-γ and IL-1β responses (Ohtani et al., 2011). Research has found that female mice undergoing ovariectomy develop Hp-induced gastric cancer, while female mice undergoing ovariectomy can inhibit the occurrence of gastric cancer by supplementing with estrogen. Estrogen therapy can alleviate gastric lesions by increasing the expression of Foxp3+ and interleukin-10 (IL-10), reducing the expression of IFN-e and IL-1e (Sheh et al., 2011). At the same time, studies have found that G protein coupled estrogen receptors prevent the activation of NF-κB promoter by Hp cytotoxin related gene A in gastric cancer cells, and inhibit the expression of tumor necrosis factor alpha (TNF-T), IL-6, and IL-1 (Kang et al., 2021; Okamoto et al., 2023),. These studies all suggest that female estrogen has the ability to inhibit inflammation and downstream pathway activation caused by Helicobacter pylori. However, there is no direct research report on whether estrogen is related to glucose and lipid metabolism disorders in Hp, which also deserves further investigation.
Our study further identified through multiple regression analysis that, regardless of gender, BMI, ApoB/ApoA1 ratio, and white blood cells are independent risk factors for HP infection. The ApoB/ApoA1 ratio was significantly elevated in HP-positive individuals and demonstrated superiority over other lipid indicators, a finding not previously reported.
Apolipoproteins play a crucial role in immune regulation and inflammatory responses. During acute infection, ApoA1 inhibits neutrophil phagocytosis and reactive oxygen species production. It also affects the inflammatory response and apoptosis of neutrophils through various pathways, such as activating the AMPK pathway and regulating NF-κF signaling pathways (Meng et al., 2024). While ApoB is a strong inflammatory marker positively correlated with IL-6 and CRP (Zhong et al., 2022). Apolipoproteins, in addition to serving as structural components of lipoproteins, also act as ligands for cell surface receptors and cofactors for enzymes, playing a crucial role in microbial invasion of host cells. The interaction between serum lipids and host cell membrane lipid rafts may alter HP-host cell interactions, leading to chronic inflammation and increased cardiovascular risk (Oakley et al., 2009; Majkova et al., 2010; Kumar et al., 2015; Chyuan et al., 2018). Therefore, the increase of ApoB/ApoA1 is related to chronic inflammation in vivo. It is speculated that Hp infection may lead to the increase of ApoB/ApoA1 by causing the occurrence and development of inflammation in the host, and the increase of ApoB/ApoA1 is involved in the interaction between Hp and host cells, and mediates the further progress of inflammation, ultimately leading to atherosclerosis and increased risk of cardiovascular and cerebrovascular diseases. However, the mechanisms underlying the interaction between HP infection and apolipoproteins remain unclear and warrant further investigation.
4.1 Limitations
This study has several limitations. As a cross-sectional study, it lacks control variables and long-term follow-up, potentially introducing recall bias, detection bias, and information bias. As well, the ratio of the HP-positive and HP-negative groups is 1: 3 to 1: 4, which may affect the testing efficiency to some extent. Additionally, the study did not collect data on smoking and alcohol consumption, limiting the ability to analyze their potential roles in HP infection or metabolic abnormalities.
5 Conclusion
In summary, our study demonstrates that HP infection is closely associated with lipid metabolism abnormalities. The ApoB/ApoA1 ratio is an independent risk factor for HP infection, unaffected by gender, and offers significant advantages over other lipid indicators.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Shanghai Health and Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CL: Conceptualization, Data curation, Formal Analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. XPZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JLP: Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. ZQZ: Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Formal Analysis, Funding acquisition, Resources, Writing – original draft, Writing – review & editing. XWZ: Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the 11th Hospital level Project of Shanghai Health and Medical Center (2024016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdu, A., Cheneke, W., Adem, M., Belete, R., and Getachew, A. (2020). Dyslipidemia and associated factors among patients suspected to have helicobacter pylori infection at jimma university medical center, jimma, Ethiopia. Int. J. Gen. Med. 13, 311–321. doi: 10.2147/IJGM.S243848
Ando, T., Minami, M., Ishiguro, K., Maeda, O., Watanabe, O., Mizuno, T., et al. (2006). Changes in biochemical parameters related to atherosclerosis after Helicobacter pylori eradication. Aliment Pharmacol. Ther. 24 SUPPL.4, 58–64. doi: 10.1111/j.1365-2036.2006.00026.x
Bravo, D., Hoare, A., Soto, C., Valenzuela, M. A., and Quest, A. F. (2018). Helicobacter pylori in human health and disease: Mechanisms for local gastric and systemic effects. World J. Gastroenterol. 24, 3071–3089. doi: 10.3748/wjg.v24.i28.3071
Chen, X., Peng, R., Peng, D., Liu, D., and Li, R. (2024). Helicobacter pylori infection exacerbates metabolic dysfunction-associated steatotic liver disease through lipid metabolic pathways: a transcriptomic study. J. Transl. Med. 22, 1–13. doi: 10.1186/s12967-024-05506-y
Chen, Y., You, N., Shen, C., Wu, J., and Zhang, J. (2023). Helicobacter pylori infection increases the risk of nonalcoholic fatty liver disease in diabetic population. Front. Nutr. 10 February. doi: 10.3389/fnut.2023.1076579
Chyuan, I. T., Tsai, H. F., Wu, C. S., Sung, C. C., and Hsu, P. N. (2018). TRAIL-mediated suppression of T cell receptor signaling inhibits T cell activation and inflammation in experimental autoimmune encephalomyelitis. Front. Immunol. 9 JAN, 1–14. doi: 10.3389/fimmu.2018.00015
Cuomo, P., Papaianni, M., Sansone, C., Iannelli, A., Iannelli, D., Medaglia, C., et al. (2020). An in vitro model to investigate the role of helicobacter pylori in type 2 diabetes, obesity, alzheimers disease and cardiometabolic disease. Int. J. Mol. Sci. 21, 1–12. doi: 10.3390/ijms21218369
Feng, G., Chen, Y., and Li, K. (2020). Helicobacter pylori promote inflammation and host defense through the cagA -dependent activation of mTORC1. J. Cell Physiol. 235, 10094–10108. doi: 10.1002/jcp.29826
Feng, Z., Chen, L., Wu, Q., Xu, F., Tong, Q., and Wang, G. (2024). Acute Helicobacter pylori infection prevalence and association with metabolic abnormality in general Chinese population: A retrospective study. Med. (Baltimore). 103, e37117. doi: 10.1097/MD.0000000000037117
Fong, P. and Wang, Q. T. (2021). Protective effect of oral contraceptive against Helicobacter pylori infection in US adult females: NHANES 1999–2000. Epidemiol. Infect. 149, e120. doi: 10.1017/S0950268821000923
Hosoda, K., Shimomura, H., Hayashi, S., Yokota, K., Oguma, K., and Hirai, Y. (2009). Anabolic utilization of steroid hormones in Helicobacter pylori. FEMS Microbiol. Lett. 297, 173–179. doi: 10.1111/j.1574-6968.2009.01685.x
Jing, F., Mao, Y., Guo, J., Zhang, Z., Li, Y., Ye, Z., et al. (2014). The value of Apolipoprotein B/Apolipoprotein A1 ratio for metabolic syndrome diagnosis in a Chinese population: a cross-sectional study. Lipids Health Dis. 13, 81. doi: 10.1186/1476-511X-13-81
Kang, M.-H., Eyun, S., and Park, Y.-Y. (2021). Estrogen-related receptor-gamma influences Helicobacter pylori infection by regulating TFF1 in gastric cancer. Biochem. Biophys. Res. Commun. 563, 15–22. doi: 10.1016/j.bbrc.2021.05.076
Kim, I.-J., Lee, J., Oh, S. J., Yoon, M.-S., Jang, S.-S., Holland, R. L., et al. (2018). Helicobacter pylori Infection Modulates Host Cell Metabolism through VacA-Dependent Inhibition of mTORC1. Cell Host Microbe 23, 583–593.e8. doi: 10.1016/j.chom.2018.04.006
Krupa, A., Gonciarz, W., Rusek-Wala, P., Rechciński, T., Gajewski, A., Samsel, Z., et al. (2021). Helicobacter pylori infection acts synergistically with a high-fat diet in the development of a proinflammatory and potentially proatherogenic endothelial cell environment in an experimental model. Int. J. Mol. Sci. 22, 3394. doi: 10.3390/ijms22073394
Kumar, B., Kowluru, A., and Kowluru, R. A. (2015). Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 56, 2985–2992. doi: 10.1167/iovs.15-16466
Li, J-Z., Li, J-Y., Wu, T-F., Xu, J-H., Huang, C-Z., Cheng, D., et al. (2017). Helicobacter pylori infection is associated with type 2 diabetes, not type 1 diabetes: An updated meta-analysis. Gastroenterol. Res. Pract. 2017:1–15. doi: 10.1155/2017/5715403
Lu, J., Van Hoang, D., Hayashi, Y., Hashimoto, M., Kubo, S., Kajio, H., et al. (2022). Negative-high titer of helicobacter pylori antibody and lipid profiles. BioMed. Res. Int. 2022, 1. doi: 10.1155/2022/9984255
Majkova, Z., Toborek, M., and Hennig, B. (2010). The role of caveolae in endothelial cell dysfunction with a focus on nutrition and environmental toxicants. J. Cell Mol. Med. 14, 2359–2370. doi: 10.1111/j.1582-4934.2010.01064.x
Martín-Núñez, G. M., Cornejo-Pareja, I., Roca-Rodríguez M del, M., Clemente-Postigo, M., Cardona, F., Fernández-García, J. C., et al. (2020). H. pylori eradication treatment causes alterations in the gut microbiota and blood lipid levels. Front. Med. 7, 3–9. doi: 10.3389/fmed.2020.00417
Meng, J., Yang, W., Chen, Z., Pei, C., Peng, X., Li, C., et al. (2024). ApoA1, ApoB, ApoA1/B for pathogenic prediction of chronic obstructive pulmonary disease complicated by acute lower respiratory tract infection: A cross-sectional study. Int. J. Chron Obstruct Pulmon Dis. 19 January, 309–317. doi: 10.2147/COPD.S441503
Moretti, E., Gonnelli, S., Campagna, M., Nuti, R., Collodel, G., and Figura, N. (2014). Influence of Helicobacter pylori infection on metabolic parameters and body composition of dyslipidemic patients. Intern. Emerg. Med. 9, 767–772. doi: 10.1007/s11739-013-1043-6
Nurtazina, A., Kozhakhmetova, D., Dautov, D., Shakhanova, A., and Chattu, V. K. (2020). Apolipoprotein B/A1 ratio as a diagnostic alternative to triglycerides and HDL-cholesterol for the prediction of metabolic syndrome among hypertensives in Kazakhstan. Diagnostics 10, 510. doi: 10.3390/diagnostics10080510
Oakley, F. O., Smith, R. L., and Engelhardt, J. F. (2009). Lipid rafts and caveolin-1 coordinate interleukin-1β (IL-1β)-dependent activation of NFκB by controlling endocytosis of Nox2 and IL-1β receptor 1 from the plasma membrane. J. Biol. Chem. 284, 33255–33264. doi: 10.1074/jbc.M109.042127
Ohtani, M., Ge, Z., Garcia, A., Rogers, A. B., Muthupalani, S., Taylor, N. S., et al. (2011). 17 -Estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis 32, 1244–1250. doi: 10.1093/carcin/bgr072
Okamoto, M., Miura, A., Ito, R., Kamada, T., Mizukami, Y., and Kawamoto, K. (2023). G-protein-coupled estrogen receptor prevents nuclear factor-kappa B promoter activation by Helicobacter pylori cytotoxin-associated gene A in gastric cancer cells. J. Vet. Med. Sci. 85, 23–0054. doi: 10.1292/jvms.23-0054
Park, Y., Kim, T. J., Lee, H., Yoo, H., Sohn, I., Min, Y. W., et al. (2021). Eradication of Helicobacter pylori infection decreases risk for dyslipidemia: A cohort study. Helicobacter 26, 1–9. doi: 10.1111/hel.12783
Robinson, K., White, J., and Winter, J. (2015). Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes. J. Inflammation Res. 8, 137. doi: 10.2147/JIR.S64888
Sarbecha, N., Fikade, M., Wondimnew, T., Kene, K., Kebede, N., Gebresillasie, H., et al. (2024). Comparison of hematologic parameters, serum electrolytes, and lipid profiles among dyspeptic patients with and without Helicobacter pylori infection attending Jimma Medical Center, Jimma, South West Ethiopia. PloS One 19, e0310047. doi: 10.1371/journal.pone.0310047
Sheh, A., Ge, Z., Parry, N. M. A., Muthupalani, S., Rager, J. E., Raczynski, A. R., et al. (2011). 17β-estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in helicobacter pylori –infected INS-GAS male mice. Cancer Prev. Res. 4, 1426–1435. doi: 10.1158/1940-6207.CAPR-11-0219
Shi, Y., Ning, J., Norbu, K., Hou, X., Zheng, H., Zhang, H., et al. (2022). The tibetan medicine Zuozhu-Daxi can prevent Helicobacter pylori induced-gastric mucosa inflammation by inhibiting lipid metabolism. Chin. Med. (United Kingdom). 17, 1–15. doi: 10.1186/s13020-022-00682-9
Sun, Y., Fu, D., Wang, Y. K., Liu, M., and Liu, X. D. Prevalence of Helicobacter pylori infection and its association with lipid profiles. Bratislava Med. J. (2016) 117, 521–524. doi: 10.4149/BLL_2016_103
Wang, Z., Wang, W., Gong, R., Yao, H., Fan, M., Zeng, J., et al. (2022). Eradication of Helicobacter pylori alleviates lipid metabolism deterioration: a large-cohort propensity score-matched analysis. Lipids Health Dis. 21, 1–10. doi: 10.1186/s12944-022-01639-5
Yang, C., You, N., Chen, Y., and Zhang, J. (2024). Helicobacter pylori infection increases the risk of dyslipidemia in Chinese diabetic Population: a retrospective cross-sectional study. BMC Infect. Dis. 24, 1–8. doi: 10.1186/s12879-024-09597-2
Yuliandari, P. and Mayura, I. P. B. (2024). Response to article “Effect of helicobacter pylori infection on glucose metabolism, lipid metabolism and inflammatory cytokines in nonalcoholic fatty liver disease patients” [Letter. J. Multidiscip Healthc 17 March, 1413–1414. doi: 10.2147/JMDH.S469382
Zhao, M.-M., Krebs, J., Cao, X., Cui, J., Chen, D.-N., Li, Y., et al. (2019). Helicobacter pylori infection as a risk factor for serum bilirubin change and less favourable lipid profiles: a hospital-based health examination survey. BMC Infect. Dis. 19, 157. doi: 10.1186/s12879-019-3787-8
Keywords: Helicobacter pylori, lipid metabolism abnormalities, ApoB/ApoA1 ratio, cross-sectional studies, cardiovascular disease
Citation: Liu C, Zhu X, Pu J, Zou Z, Zhou L and Zhu X (2025) Helicobacter pylori infection and apolipoprotein B/apolipoprotein A1 ratio: a cross-sectional study. Front. Cell. Infect. Microbiol. 15:1582843. doi: 10.3389/fcimb.2025.1582843
Received: 27 February 2025; Accepted: 23 April 2025;
Published: 20 May 2025.
Edited by:
Hui Han, University of Liège, BelgiumReviewed by:
Sinem Oktem Okullu, Acibadem Mehmet Ali Aydinlar University, TürkiyeWaleed Samy, Professor of InterMedicine, Egypt
Li Li, University of California, San Francisco, United States
Copyright © 2025 Liu, Zhu, Pu, Zou, Zhou and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowei Zhu, emh1eHdAbmptdS5lZHUuY24=; Lu Zhou, NTUyNzU1OThAcXEuY29t
†These authors have contributed equally to this work
 Chao Liu1†
Chao Liu1† Xuping Zhu
Xuping Zhu Zhuoqun Zou
Zhuoqun Zou