- 1School of Nursing, Jinan University, Guangzhou, China
- 2Department of Metabolic and Bariatric Surgery, The First Affiliated Hospital, Jinan University, Guangzhou, China
- 3Centre of Health Management, The First Affiliated Hospital, Jinan University, Guangzhou, China
- 4School of Medicine, Jinan University, Guangzhou, China
- 5Health Science Center, Jinan University, Guangzhou, China
Background: Spleen deficiency syndrome (SDS) is one of the primary Traditional Chinese Medicine (TCM) syndromes in Non-alcoholic fatty liver disease (NAFLD). Diet influences NAFLD and SDS through the intestinal microbiota. The current study aimed to investigate the interrelationships of intestinal bacteria, fungi and dietary nutrient intake in NAFLD patients with SDS.
Methods: The NAFLD TCM Patient Reported Outcome (PRO) Scale was administered to evaluate the TCM clinical symptoms of NAFLD patients. The Spleen Deficiency PRO Scale and Food Frequency Questionnaire (FFQ) were employed to respectively diagnose spleen deficiency syndrome and assess dietary nutrient intake, energy-adjusted dietary inflammatory index (E-DII), and dietary diversity scores (DDS) in NAFLD patients. Subsequently, stool samples were collected for 16S rRNA gene and ITS2 region sequencing to analyze the interrelationships among target intestinal bacteria, fungi, and dietary nutrient intake.
Results: The NAFLD TCM PRO Scale indicated that the average score for symptoms related to SDS in NAFLD patients was 4.13 ± 0.40. Compared with NAFLD patients without SDS, those with SDS had insufficient dietary nutrient intake of diet-derived antioxidants such as carotene and folic acid, stronger pro-inflammatory effects of food, and reduced dietary diversity (P < 0.05). Additionally, sufficient dietary diversity was identified as a protective factor against SDS in NAFLD (OR: 0.424; 95% CI: 0.309, 0.583; P < 0.001). 16S rRNA gene and ITS2 region sequencing results showed that Collinsella (LDA = 3.947, P = 0.046) and Rhizopus (LDA = 3.196, P = 0.01) were enriched in NAFLD patients with SDS, whereas Intestinimonas was markedly increased in NAFLD patients without SDS (LDA = 2.015, P = 0.02). Correlation analysis demonstrated that Gemmiger and Rhizopus were significantly positively correlated (r = 0.778, P < 0.001), as were Candida and Segatella (r = 0.569, P < 0.001). Intestinimonas was positively correlated with the intake of antioxidant and anti-inflammatory nutrients such as dietary fiber, vitamin C, and iron (0.2 < r < 0.5, P < 0.05), while niacin intake was negatively correlated with Rhizopus abundance (r = -0.39, P = 0.025).
Conclusion: Symptoms related to SDS are common in patients with NAFLD. The independent and interactive effects of intestinal bacteria and fungi might have collectively influenced the immune function and inflammation levels in NAFLD patients with SDS. These processes were likely associated with the intake of antioxidant and anti-inflammatory nutrients, as well as niacin.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease worldwide, characterized by excessive hepatic fat accumulation, affecting approximately 38% of the global population (Wong et al., 2023). Approximately 29.6% of the Chinese population is affected by NAFLD (Fan et al., 2024). NAFLD is also associated with a variety of extrahepatic diseases, such as cardiovascular disease, chronic kidney disease, and multiple extrahepatic cancers (Miao et al., 2024a), leading to substantial healthcare expenditures, economic losses, and decreased health-related quality of life (Lazarus et al., 2022). Traditional Chinese medicine (TCM), as a widely recognized form of complementary and alternative therapy, provides novel insights for the prevention and treatment of NAFLD, owing to its distinctive therapeutic approach (Zhang et al., 2024b). Our preclinical study confirmed that the TCM formula Shenling Baizhu San, known for its spleen-tonifying effects, significantly ameliorated NAFLD (Chen et al., 2024). Spleen deficiency syndrome (SDS) is one of the primary TCM syndromes in NAFLD (Dai et al., 2022). NAFLD patients with SDS often present with symptoms such as decreased appetite, loose stool, insomnia, and increased susceptibility to infections (Dai et al., 2022; Zheng et al., 2020). Therefore, early identification of symptoms and biomarkers in NAFLD patients with SDS may effectively reduce the economic burden and improve the quality of life for these patients.
Intestinal microbiota influences the progression of both NAFLD and SDS. Comprising intestinal bacteria, fungi, and viruses, intestinal microbiota exerts critical functions in maintaining intestinal homeostasis, immune responses, and microbial balance through bacterial-fungal interactions (Li et al., 2024a). Intestinal microbiota dysbiosis increases intestinal permeability, potentially exposing the liver to harmful substances, thereby promoting hepatic fat accumulation and liver fibrosis (Hu et al., 2020). Current evidence indicates that the pathogenesis of SDS is closely related to intestinal microbiota dysbiosis, with significant intestinal bacterial disturbances observed in both SDS mouse models and patients (Peng et al., 2014, 2020; Yu et al., 2024). Dietary nutrient intake directly modulates intestinal microbiota composition, which manifests through increased intestinal permeability and elevated inflammatory mediators, thereby accelerating liver disease progression (Jiménez-González et al., 2025). Understanding the impact of diet on the interaction between the intestinal microbiota and the host immune system may provide valuable insights for developing nutritional strategies to maintain intestinal health in NAFLD patients with SDS (Zhang, 2022).
Currently, most research focuses on the relationship between NAFLD and intestinal microbiota, while the role of intestinal microbiota in NAFLD patients with SDS remains unclear, particularly regarding the role of intestinal fungi. Therefore, we collected stool samples from NAFLD patients with and without SDS and performed 16S rRNA gene and ITS2 region sequencing to investigate the characteristics of intestinal bacteria and fungi. Additionally, we explored the interactions between dietary nutrient intake and intestinal microbiota in NAFLD patients with SDS, ultimately providing novel insights into the prevention, treatment, and dietary management of NAFLD patients with SDS.
2 Materials and methods
2.1 Diagnostic criteria
The diagnosis of NAFLD was established according to the Asia-Pacific Working Party on NAFLD guidelines, incorporating three essential criteria: 1) B-ultrasound examination confirmed hepatic steatosis; 2) absence or minimal alcohol consumption (defined as < 70 g/week for women and < 140 g/week for men); and (3) exclusion of alternative etiologies of hepatic steatosis, including but not limited to autoimmune liver diseases and viral hepatitis (Wong et al., 2018). The diagnostic criterion for SDS was based on the Spleen Deficiency PRO scale developed by the Institute of Longhua Hospital, Shanghai University of Traditional Chinese Medicine (SR1362577) (Dai et al., 2022). Patients with a total score ≥ 20 were identified as NAFLD patients with spleen deficiency syndrome (NAFLD_P), whereas those with a score < 20 were categorized as NAFLD patients without spleen deficiency syndrome (NAFLD_NP).
2.2 Patient recruitment
This study included patient from patients visiting the First Affiliated Hospital of Jinan University from January to October 2024. Detailed inclusion criteria: 1) Meet the diagnostic criterion; 2) Voluntary participation in the study with signed informed consent; 3) Age range 18 to 70 years; 4) According to the requirements of the FFQ, participants had resided in Guangzhou for at least 6 months and had an energy intake within the normal range. The general energy intake range is 500–3500 kcal/day for females and 800–4000 kcal/day for males; 5) Participants from whom stool samples were collected must not have used antibiotics, probiotics, prebiotics, hormones, immunosuppressants, or Chinese herbs in the past 3 months (Miao et al., 2024b). Participants with any of the following conditions were excluded from the study: Individuals with severe systemic or infectious diseases, such as malignancies, severe cardiopulmonary disorders, neurological diseases, HIV infection, etc.
This study was approved by the Medical Ethics Review Board of Jinan University (Approval No. JUNKY-2023-0130) and was supervised by the Ethics Committee throughout the research process. Written informed consent was obtained from all participants prior to their enrollment in the study.
2.3 Research tools
2.3.1 The NAFLD traditional Chinese medicine reported outcome scale (The NAFLD TCM PRO)
We assessed the TCM clinical symptoms in NAFLD patients using the NAFLD TCM PRO scale. This self-assessment tool enables participants to evaluate their condition based on subjective feelings. Comprising three domains and nine subdomains, the scale has undergone multiple validations and demonstrates robust reliability and validity, with a total Cronbach’s α coefficient exceeding 0.8. Utilizing a 5-point Likert rating system, higher scores on the scale indicate better health status and quality of life (Shen, 2017).
2.3.2 The spleen deficiency reported outcome scale
Patients were asked to score the severity of ten symptoms based on their own experience, and the final symptom score was calculated by multiplying the individual symptom scores by their corresponding weights. This questionnaire has been validated for reliability among the study population, demonstrating good reliability and validity with a Cronbach’s α coefficient of 0.773, which indicates acceptable internal consistency (Dai et al., 2022).
2.3.3 The food frequency questionnaire
The FFQ used in this study encompasses the range of foods commonly consumed by individuals in the Guangzhou area. The survey examines the participants’ dietary habits over the past six months, with a focus on three primary aspects: food types, frequency of consumption, and the average intake. The questionnaire includes 7 major food categories and 82 individual food items. Dietary data were collected through one-on-one, face-to-face interviews during patient clinic visits, and food portion reference charts were provided to aid participants in accurately estimating their portion sizes (Zhang and Ho, 2009).
2.4 Research methods
2.4.1 Dietary nutrient intake
Dietary nutrient intake was computed from the FFQ data. Nutrient intake was energy-adjusted using the nutrient density method, whereby participants’ original daily dietary nutrient intakes were converted to intake amounts per 1000 kcal (Mccullough and Byrd, 2023).
2.4.2 Energy-adjusted dietary inflammatory index
The calculation of the Dietary Inflammatory Index (DII) was based on the dietary data collected through the FFQ. For each food item, actual intake values were standardized using the global dietary standard library’s average intake and standard deviation. Z-scores were computed and then converted into percentile values. These values were subsequently doubled and subtracted by 1, resulting in a zero-centered, symmetrical distribution. The DII score for each food item was obtained by multiplying the percentile value by the corresponding inflammatory effect score. The total DII score was calculated by summing the individual DII scores of all food items. E-DII employed in this study followed a similar calculation method, but prior to Z-score conversion, the raw dietary nutrient intake values were adjusted to correspond to a 1000 kcal energy intake. Similarly, the average intake and standard deviation in the global dietary standard library were also adjusted for a 1000 kcal energy intake (Shivappa et al., 2014; Chen et al., 2022).
2.4.3 Dietary diversity scores
The food items collected through the FFQ were categorized into nine groups based on the Chinese Dietary Guidelines (2022): cereal, vegetables, fruits, Soybeans and their products, eggs, meat, fish, milk and dairy products, and oil. The frequency of food intake was classified into five levels: “almost every day”, “not every day, but once a week at least”, “not every week, but once a month at least”, “not every month, but sometimes”, “seldom or never”. In this study, participants were assigned 1 point for consuming a food item “almost every day” or “once a week at least”, and 0 points for all other frequencies. Each food category was scored only once. A higher score reflects a higher dietary diversity level, with the maximum possible score being 9 (Zhu et al., 2024).
2.5 Stool sample collection
The researcher prepared disposable sterile 5 ml stool collection tubes (Manufacturer: Beijing BIORIDA Technology Co., Ltd., Catalog No. BA-0206) in advance and instructed the patients on stool collection procedures. Approximately 2–3 g of stool sample for each patient was collected from the inside (not the surface) using clean stool collection paper and a sterile spoon to avoid contamination by urine or water. After collection, the sample must be immediately placed in a portable ice box and promptly transferred to a -80°C refrigerator for storage until further processing (Kyriazopoulou et al., 2025).
2.6 Gut microbiota detection
HiPure Stool DNA was extracted using the HiPure Stool DNA Extraction Kit (Magen, Guangzhou, China). PCR amplification was conducted for the V3-V4 hypervariable region of the 16S rRNA gene and the ITS2 region (16S rRNA primer sequences: 5’-3’, 341F: CCTACGGGNGGCWGCAG, 806R: GGACTACHVGGGTATCTAAT; ITS2 primer sequence: 5’-3’, ITS3_KYO2: GATGAAGAACGYAGYRAA, ITS4: TCCTCCGCTTATTGATATGC). Subsequently, Illumina sequencing was performed, and 2% agarose gel electrophoresis was used to preliminarily assess the quality of the amplification products. The PCR products were purified using AMPure XP Beads (Beckman, CA, USA). The purified products were quantified using Qubit 3.0, and sequencing libraries were constructed using the Illumina DNA Prep Kit (Illumina, CA, USA). The quality of the libraries was assessed using the ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, USA). Qualified libraries were sequenced and analyzed on the NovaSeq 6000 platform using the PE250 mode. All quality control and sequencing procedures were conducted by GENEDENOVO Biotechnology Company (Guangzhou, China). The obtained data were quality-controlled, clustered, and de-chimerized to obtain operational taxonomic units (OTUs). Species annotation was performed based on OTU sequences to obtain species abundance information at each level. Subsequently, bioinformatics analysis were performed on species composition (Krona, version 2.6), indicator species (R VennDiagram package, version 1.6.16), Alpha diversity (QIIME, version 1.9.1), Beta diversity (Muscle, version 3.8.31), functional prediction (PICRUSt, version 2.1.4), and environmental factor association (R Vegan Package, version 2.5.3) (Caporaso et al., 2010; Chen and Boutros, 2011).
2.7 Statistical methods
Continuous variables that followed a normal distribution were expressed as mean ± standard deviation. When the assumption of equal variances was met, group comparisons were performed using Student’s t-test; otherwise, Welch’s t-test was used. Non-normally distributed variables were expressed as medians (interquartile range), with group comparisons conducted using the Mann-Whitney U test. Categorical variables were presented as counts (percentages) and analyzed using the chi-square test. Pearson, mantel test, and procrustes analysis were employed to assess the relationships between variables. Binary logistic regression was used to identify the influencing factors of NAFLD patients with SDS. A P-value of < 0.05 was considered statistically significant.
3 Results
3.1 The NAFLD TCM PRO scale scores
In this study, a total of 388 NAFLD patients completed the NAFLD TCM PRO scale. The general information of the patients and the laboratory examination results were detailed in the Supplementary Material (Supplementary Table S1-2). The scores of NAFLD patients in various domains were presented in Table 1-1, with the lowest average item score observed in the treatment domain (3.75 ± 0.66) points. The detailed scores for each aspect of the physiological domain in NAFLD patients were presented in Table 1-2, with the total score for symptoms related to SDS being (20.70 ± 2.14) points, a mean item score of (4.13 ± 0.40) points, ranking third. Table 1–3 specifically displayed the symptoms related to SDS item scores, among which the item “Do you experience loose or unformed stools?” showed the lowest mean score of (3.82 ± 0.87) points.
3.2 Participant characteristics
3.2.1 Baseline characteristics
In total, 245 NAFLD patients were recruited, comprising 106 cases of NAFLD_NP (43.27%) and 139 cases of NAFLD_P (56.73%). The spleen deficiency score in NAFLD_P patients was significantly higher than that in NAFLD_NP patients (12.03 ± 4.67 vs. 35.31 ± 11.59, P < 0.001). Compared to NAFLD_NP patients, NAFLD_P patients exhibited significant differences in personal income (P = 0.018), weekly exercise time (P = 0.002), daily sleep duration (P = 0.03), and family history of liver disease (P = 0.037), as detailed in Table 2-1. Laboratory examination results revealed that total protein (TP, P = 0.009) and albumin (ALB, P = 0.035) levels in NAFLD_P were lower than in NAFLD_NP, while total cholesterol (TC, P = 0.008) and low-density lipoprotein (LDL, P = 0.039) levels were significantly high in NAFLD_P compared to NAFLD_NP, with further details provided in Table 2-2.
3.2.2 Dietary nutrient intake
The comparative analysis of dietary nutrient intake (per 1000 kcal), as shown in Table 2-3, revealed that NAFLD_P patients had insufficient dietary nutrient intake of vitamin A (P = 0.009), carotenoids (P = 0.013), iron (P = 0.035), betaine (P = 0.018), and folate compared (P = 0.024) to NAFLD_NP patients. Although food had a pro-inflammatory effect in both NAFLD_NP and NAFLD_P patients, the E-Dll score of NAFLD_P patients was higher than that of NAFLD_NP patients, indicating that food had a stronger pro-inflammatory effect in NAFLD_P patients (0.02 ± 1.20 vs. 0.35 ± 1.18, P = 0.028, Table 2-4). The dietary diversity score of NAFLD_P was lower than that of NAFLD_NP (7.88 ± 0.91 vs. 7.09 ± 1.08, P < 0.001, Table 2-5). Notably, the number of NAFLD_NP patients who consumed soy and soy products (P < 0.001), fruits (P = 0.001), fish (P = 0.026), milk (P = 0.03), and dairy products on a weekly basis was markedly higher than that of NAFLD_P patients.
3.2.3 Logistic regression analysis of factors influencing spleen deficiency in NAFLD patients
Indicators with statistically significant differences in dietary analysis between NAFLD_NP and NAFLD_P patients were included in logistic regression analysis. The results (Table 2-6) indicated that only sufficient dietary diversity is a protective factor against spleen deficiency in NAFLD patients (OR:0.424; 95% CI:0.309, 0.583; P < 0.001). A one-unit increase in dietary diversity score was associated with a 0.426-fold decrease in the risk of spleen deficiency in NAFLD patients.
3.3 Characteristics of intestinal microbiota
3.3.1 Characteristics of intestinal bacteria
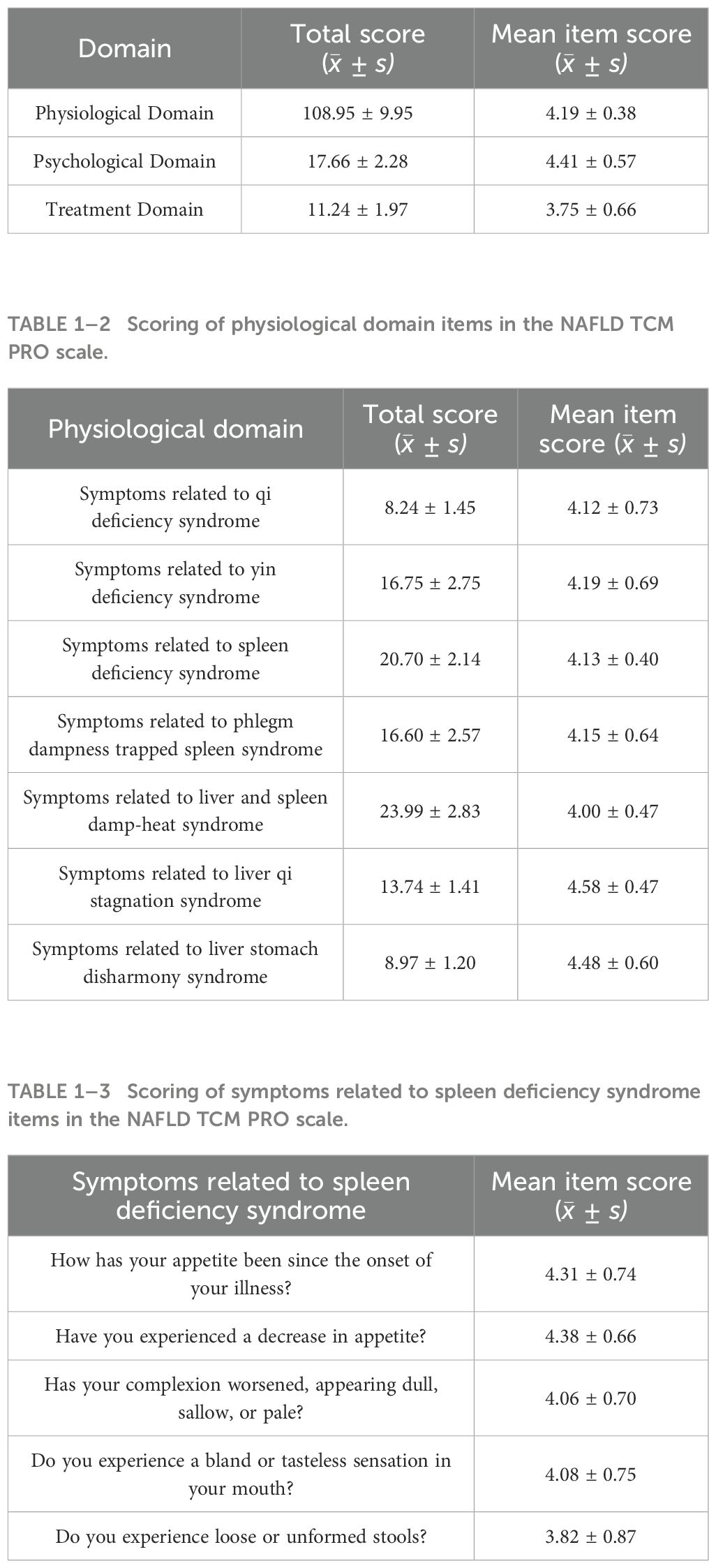
To further investigate the potential role of intestinal bacteria in NAFLD with SDS, a total of 76 stool samples (30 for NAFLD_P, 46 for NAFLD_NP). The sequencing results revealed that there were 1,847 OTUs common to both groups, with 341 OTUs unique to the NAFLD_P patients and 374 OTUs unique to the NAFLD_NP patients (Figure 1A). The alpha diversity analysis showed that there was no statistically significant differences in both the Sob and Shannon index between the NAFLD_P and NAFLD_NP patients (P = 0.737 for Sob index, P = 0.727 for Shannon index, Welch’s t-test, Figures 1B, C). Beta diversity was evaluated using Bray-Curtis distances via principal component analysis (PCoA) combined with the Adonis (PERMANOVA) test, indicating no significant structural differences between the two groups (P = 0.379, Figures 1D, E). The relative abundance of species in the top 10 at the phylum and genus levels for the two groups is shown in Figures 1F, G, respectively. The relative abundances of Actinomycetota (P < 0.001) and Verrucomicrobiota (P = 0.036) at the phylum level, and Akkermansia (P = 0.036), Bifidobacterium (P = 0.004), and Collinsella (P = 0.023) at the genus level, were higher in NAFLD_P patients than in NAFLD_NP patients (Figures 1H, I).
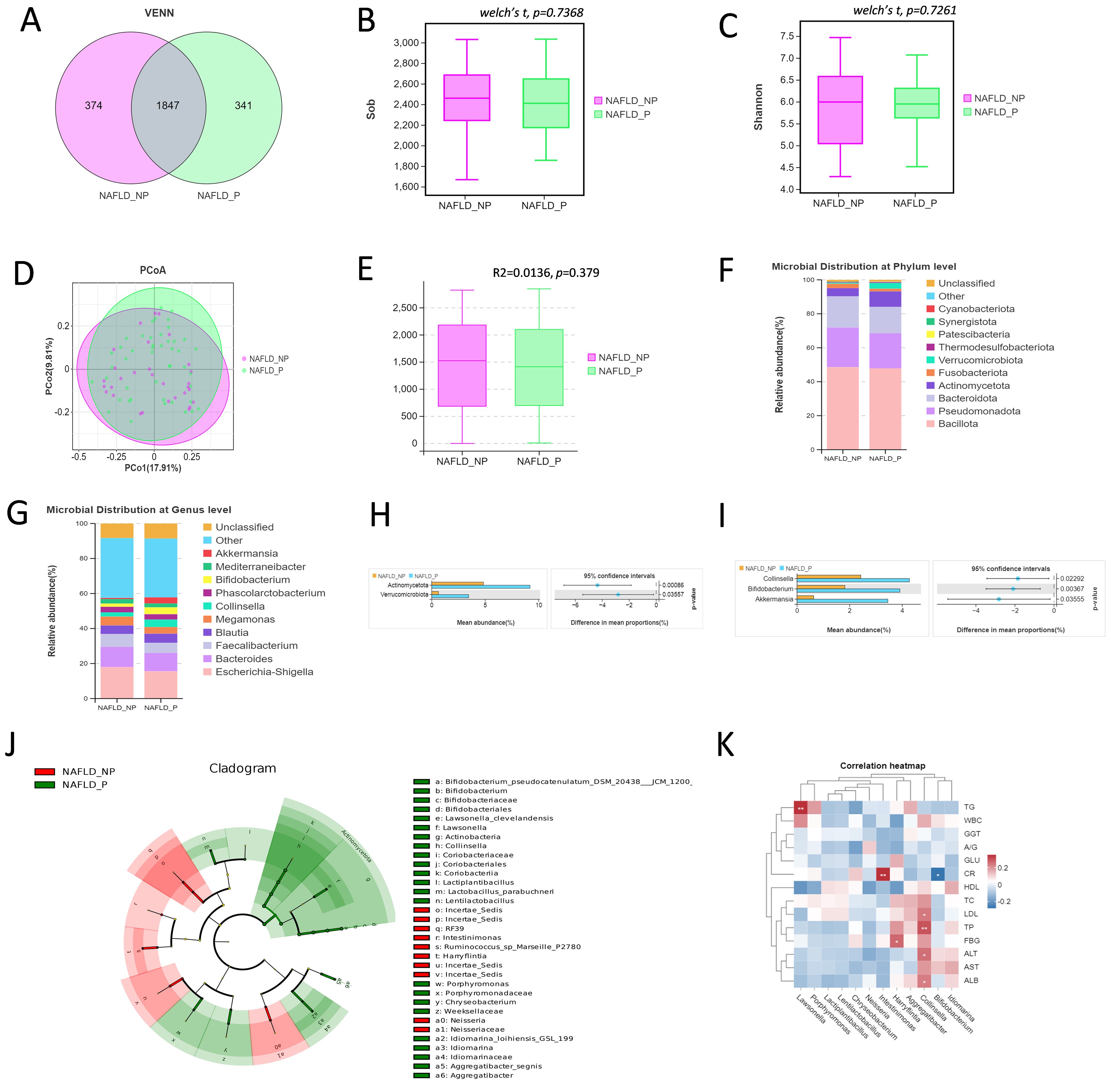
Figure 1. Intestinal bacteria in the NAFLD_NP and NAFLD_P patients. (A) Comparison of intestinal bacteria OTUs between NAFLD_NP and NAFLD_P patients. (B, C) Alpha diversity is indicated by Sob index and Shannon diversity index. (D) Principal coordinate analysis (PCoA) based on bray. (E) Adonis analysis of similarities, R2 = 0.0136, P = 0.379. (F) Top 10 most relatively abundant intestinal bacteria at the phylum level. (G) Top 10 most relatively abundant intestinal bacteria at the genus level. (H) Significant differences in intestinal bacteria at the phylum level (top 10). (I) Significant differences in intestinal bacteria at the genus level (top 10). (J) Cladogram of 57 differential intestinal bacteria with LEfSe analysis (LDA > 2, P < 0.05). (K) Pearson correlation analysis of the relative abundance of differential potential biomarker intestinal bacteria at the genus level with biochemical indices. *P < 0.05 or **P < 0.01.
LEfSE analysis was used to determine the contribution of intestinal bacteria in distinguishing between NAFLD_P and NAFLD_NP. As shown in Figure 1J, the results revealed that 18 intestinal bacteria were significantly enriched in the NAFLD_P group, while 7 intestinal bacteria were enriched in the NAFLD_NP group with LDA > 2 and P < 0.05. It is noteworthy that the relative abundance of Collinsella is significantly high in the NAFLD_P patients (LDA=3.947, P = 0.046), while Intestinimonas is significantly high in the NAFLD_NP patients (LDA= 2.015, P = 0.02). Further association of differential intestinal bacteria at the genus level with biochemical indices revealed that Collinsella was positively correlated with Alanine Aminotransferase (ALT, r = 0.262, P = 0.022), LDL (r = 0.246, P = 0.032), TP (r = 0.302, P = 0.008), and ALB (r = 0.24, P = 0.037), while Intestinimonas is positively correlated with CR (r = 0.353, P = 0.002, Figure 1K).
3.3.2 Characteristics of intestinal fungi
33 stool samples were subjected to ITS2 region sequencing analysis to characterize the intestinal fungi in NAFLD patients (21 for NAFLD_P, 12 for NAFLD_NP). A total of 73 OTUs were shared between the two groups, with 72 OTUs unique to the NAFLD_NP group and 91 OTUs unique to the NAFLD_P group (Figure 2A). The Shannon and Simpson indices were used to reflect the abundance and evenness of the intestinal fungi. The results showed that there was no significant differences between the two groups (P = 0.808 for Sob index, P = 0.778 for Simpson index, Welch’s t-test, Figures 2B, C). Beta diversity was analyzed via Non-metric Multidimensional Scaling (NMDS) and Analysis of Similarities (ANOSIM). Although the NMDS plot showed some overlap between groups (Figure 2D), significant differences in fungal composition were observed, with inter-group differences being greater than intra-group differences (R = 0.1442, P = 0.033, Figure 2E). The composition of the top ten intestinal fungi at both the phylum and genus levels in the NAFLD_NP and NAFLD_P patients was shown in Figures 2F ,G. The result of LEfSe analysis indicated that 9 and 10 intestinal fungi were respectively enriched in NAFLD_P and NAFLD_NP patients with LDA > 2, P <0.05 (Figure 2H). Notably, the Rhizopus was significantly enriched in the NAFLD_P patients and is classified as an opportunistic pathogen capable of causing mucormycosis (LDA = 3.196, P = 0.01) (Petrikkos et al., 2012). Further correlation analysis between differential intestinal fungi at the genus level and biochemical indices revealed that Rhizopus was negatively correlated with ALB (r = -0.392, P = 0.024, Figure 2I).
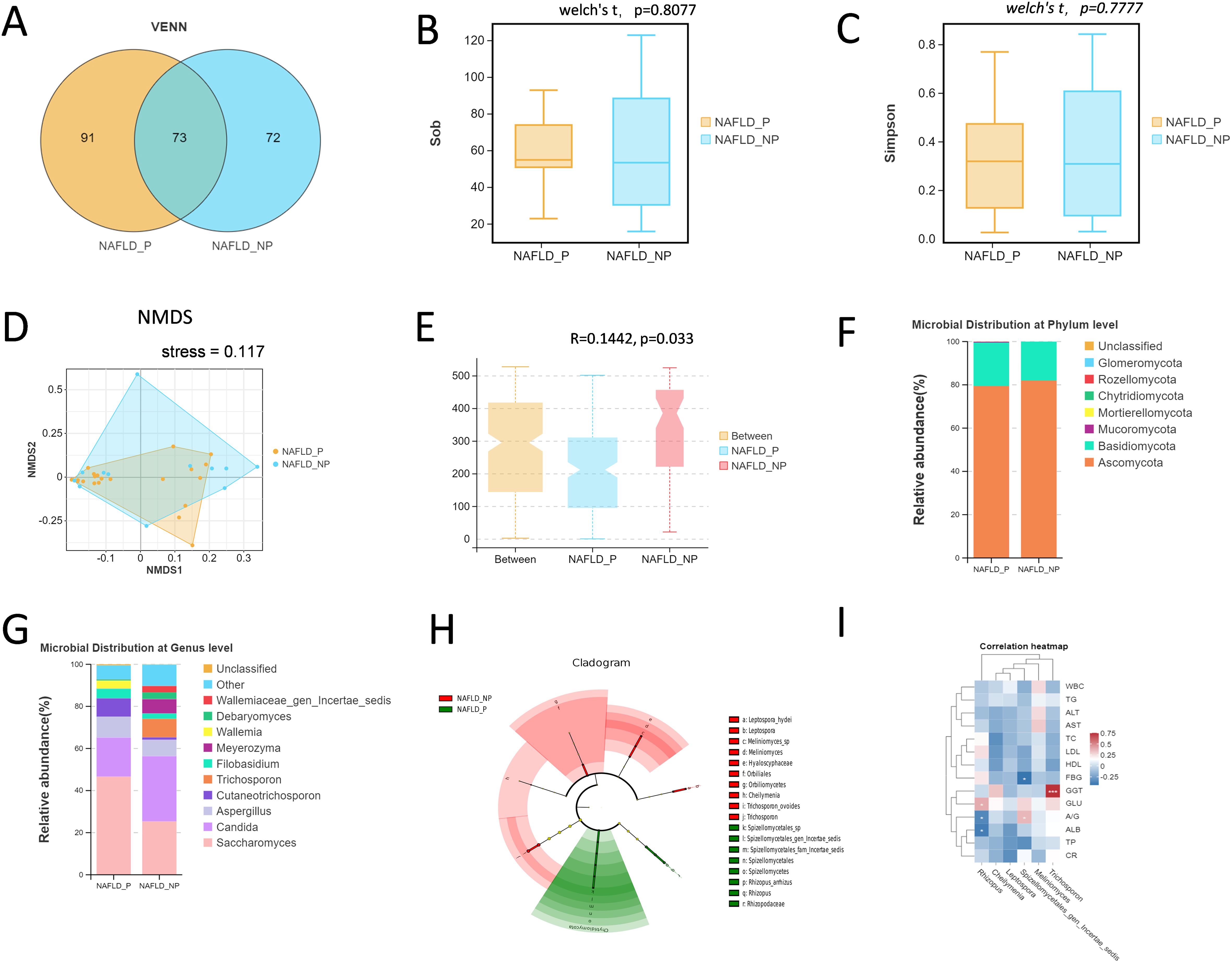
Figure 2. Intestinal fungi in the NAFLD_NP and NAFLD_P patients. (A) Comparison of intestinal fungi OTUs between NAFLD_NP and NAFLD_P patients. (B, C) Alpha diversity is indicated by Sob index and Simpson diversity index. (D) Non-metric multidimensional scaling (NMDS) based on bray. (E) NMDS Analysis of similarities (ANOSIM), If 1 > R > 0, it suggests that the inter-group differences exceed the intra-group variations, R = 0.1442, P = 0.033. (F) Top 10 intestinal fungi at phylum level. (G) Top 10 intestinal fungi at the genus level. (H) Cladogram of 19 differential intestinal fungi with LEfSe analysis (LDA > 2, P < 0.05). (I) Pearson correlation analysis of the relative abundance of differential potential biomarker intestinal fungi at the genus level with biochemical indices. *P < 0.05 or ***P < 0.001.
3.3.3 Correlation analysis between intestinal bacteria and fungi
In the Procrustes analysis based on Bray distance at the OTU level, we found that the community structures of intestinal bacteria and fungi did not differ significantly between NAFLD_P and NAFLD_NP patients. Additionally, samples were widely dispersed in the principal coordinate space, reflecting low similarity of community structures (M²= 0.917, P = 0.118, Figure 3A). Consequently, we conducted a network analysis of intestinal bacteria and fungi at the genus level in both NAFLD_P and NAFLD_NP patient groups and identified several potentially meaningful associations. The results demonstrated that bacteria-fungi associations were stronger in NAFLD_NP patients compared to NAFLD_P patients(Figures 3B, C). Nevertheless, meaningful associations were still observed in NAFLD_P patients, including a strong positive correlation between intestinal bacteria Gemmiger and fungi Rhizopus (r = 0.778, P < 0.001), as well as between intestinal bacteria Segatella and fungi Candida (r = 0.569, P < 0.001).
Figure 3. The association between intestinal bacteria and fungi in NAFLD_NP and NAFLD_P patients. (A) Procrustes analysis of intestinal bacteria and fungal community structures between NAFLD_P and NAFLD_NP patients based on Bray-Curtis distance at the OTU level. (B) Pearson correlation network of bacteria and fungi at the genus level in the NAFLD_NP patients. (C) Pearson correlation network of bacteria and fungi at the genus level in the NAFLD_P patients. The networks are composed of nodes and edges, where nodes represent species and edges represent correlations, circles are used to denote bacteria, and triangles denote fungi, the size of the nodes indicates the degree of connectivity, solid red lines represent positive correlations, while dashed blue lines represent negative correlations. P value: 0 to 0.05, correlation index: -1 to -0.5 or 0.5 to 1.
3.4 Correlation analysis between the intestinal microbiota and dietary nutrient intake
The Mantel test results showed a significant weak correlation between the OTU distance matrix of intestinal bacteria and dietary nutrient intake distance matrix (r = 0.167, P < 0.001, Figure 4A), while no significant association was found between the OTU distance matrix of intestinal fungi and dietary nutrient intake distance matrix (r = 0.044, P = 0.259, Figure 4B). Pearson correlation analysis was further employed to explore the relationship between the relative abundances of genus-level differential potential biomarkers in the intestinal microbiota and dietary nutrient intakes. The results revealed significant positive correlations between the relative abundance of Intestinimonas and dietary fiber, carbohydrate (r = 0.364, P < 0.001), vitamin A (r = 0.397, P < 0.001), vitamin B1 (r = 0.331, P = 0.004), vitamin B6 (r = 0.371, P < 0.001), vitamin C (r = 0.376, P < 0.001), iron (r = 0.236, P = 0.04), potassium (r = 0.284, P = 0.013), and magnesium (r = 0.262, P = 0.022) (Figure 4C). Additionally, a significant negative correlation was found between the relative abundance of Rhizopus and Niacin (r = -0.39, P = 0.025, Figure 4D). Furthermore, the relative abundance of intestinal fungi Trichosporon exhibited significant positive correlations with vitamin B2 (r = 0.351, P = 0.045), vitamin B12 (r = 0.567, P < 0.001), sodium (r = 0.444, P = 0.01), and calcium (r = 0.437, P = 0.011).
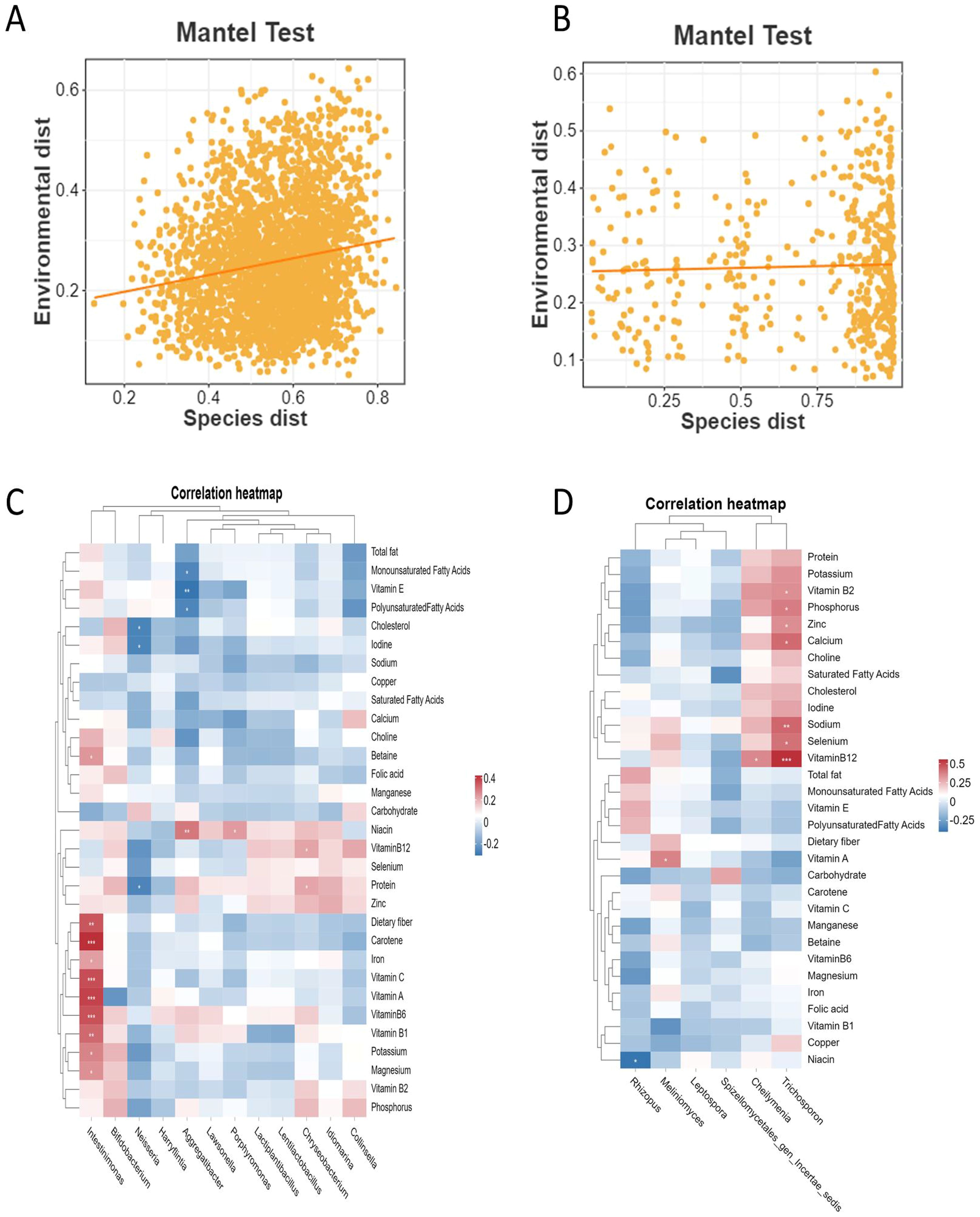
Figure 4. The association analysis of dietary nutrient intake and intestinal microbes in NAFLD_NP and NAFLD_P patients. (A) Mantel test result for the correlation between the OTU distance matrix of intestinal bacteria and dietary nutrient intake distance matrix. (B) Mantel test result for the correlation between the OTU distance matrix of intestinal fungi and dietary nutrient intake distance matrix. (C) A heatmap illustrating the correlation between the relative abundance of differential potential intestinal bacteria and dietary nutrient intake. (D) A heatmap illustrating the correlation between the relative abundance of differential potential intestinal fungi and dietary nutrient intake. Pearson correlation analysis was conducted to assess the association between differential potential biomarker intestinal microbes at the genus level and dietary nutrient intake in both the NAFLD_NP and NAFLD_P patients. *P < 0.05, **P < 0.01, ***P < 0.001.
4 Discussion
Patient self-management played a crucial role in the progression of NAFLD (Kwon et al., 2023). This study revealed that NAFLD patients scored lowest in the treatment domain, reflecting poorer therapeutic adherence, which might result from insufficient disease knowledge, complex treatment protocols, and inadequate self-management strategies. Notably, we also found that symptoms associated with SDS were relatively common among NAFLD patients, with loose or unformed stools being the most frequent symptom. Typical symptoms and signs could effectively reflect the TCM syndromes of patients. The weakened spleen’s impaired transport function and the deficient stomach’s inability to digest led to food-fluid retention, which disrupts spleen yang ascent and ultimately caused diarrhea (Fan et al., 2023). Consequently, SDS might play a critical role in the occurrence and development of NAFLD, and this study will further explored the mechanism of SDS in NAFLD.
Engaging in at least 150–240 minutes of moderate aerobic exercise per week or sleeping 6–8 hours daily can effectively reduced hepatic steatosis in patients (Keating et al., 2023; Mikolasevic et al., 2021). In this study, fewer NAFLD patients with SDS engaged in 6–8 hours of daily sleep duration and 150 minutes of moderate-intensity exercise per week compared to those without SDS. Additionally, lack of physical activity weakened spleen transport and transformation functions, led to insufficient qi and blood production, which in turn affected sleep quality due to impaired nourishment of the heart and mind (Chen, 2019). Spleen deficiency might also lead to metabolic disorders such as lipid metabolism, amino acid metabolism, and energy metabolism (Li et al., 2023; Sun et al., 2024; Zhan et al., 2024), as evidenced by our results showed that NAFLD patients with SDS exhibited abnormalities in TP, ALB, LDL, and total cholesterol (TG) levels. In accordance with TCM, a deficiency in the spleen and stomach’s ability to transform food into essential nutrients resulted in inadequate production of qi and blood, caused a deficiency in the five zang-fu organs, diminished resistance to pathogenic factors, immune system imbalances, and ultimately inflammation (Qiu et al., 2024). Diet-derived antioxidants served as ideal supplements for NAFLD prevention, effectively inhibiting inflammation. In accordance with TCM, the insufficiency of spleen and stomach to convert food into essential nutrients might weaken the body’s resistance to disease factors, disrupt the immune system, and ultimately lead to inflammation (Chen et al., 2023). In the current study, evaluation of nutrient intake and the E-DII scores revealed significantly low daily levels of vitamin A, carotene, iron, betaine, and folic acid in NAFLD patients with SDS compared to those without. Carotene, iron, betaine, and folic acid were confirmed to have the function of inhibiting hepatic lipid accumulation and ameliorating hepatic oxidative stress (Hu et al., 2024; Liu et al., 2024b; Ma et al., 2025; Tyczynska et al., 2024). Despite the acknowledged antioxidant properties of vitamin A, the association between dietary vitamin A intake and the risk of NAFLD remained inconclusive (Liu et al., 2024a). Current research suggested that supplementation with specific vitamins and minerals might alleviate NAFLD symptoms, but the optimal dosage range remained unclear (Tyczynska et al., 2024). Sufficient dietary diversity was a protective factor for NAFLD patients with SDS. We also assessed the DDS of NAFLD patients, finding that patients with SDS had lower DDS scores than those without. Sufficient dietary diversity offered a richer selection of foods, and increased the intake of beneficial substances such as micronutrients and dietary fiber, thereby reducing NAFLD risk (Luo et al., 2022).
In the NAFLD population, Collinsella is notably high in patients with liver depression and spleen deficiency compared to those with damp-heat depression syndrome (Ma, 2022). Besides, Collinsella, as a pro-inflammatory genus, is also significantly enriched in NASH (Astbury et al., 2020). Previous research showed that Administration of jujube with spleen-tonifying properties to spleen deficiency rats for ten days significantly improved the relative abundance of Collinsella (Li, 2017). Current results showed that Collinsella is significantly enriched in NAFLD patients with SDS. Collinsella has been reported to influence host lipid metabolism via specific pathways (Astbury et al., 2020), and our findings further demonstrated that there was significant positive correlation between Collinsella and LDL, ALT, as well as TP levels. This correlation suggests that Collinsella may be implicated in metabolic dysregulation by modulating both lipid metabolic and protein synthetic processes. Additionally, study have indicated that, in a spleen deficiency rat model, the abundance of Collinsella and Intestinimonas was significantly increased and decreased, respectively (Sun, 2020). Furthermore, Butyrate, a common short-chain fatty acid, played a crucial role in reducing inflammation, maintaining colon health, and supporting the intestinal barrier (Cai et al., 2020). As a beneficial bacterium with anti-inflammatory and anti-obesity properties (Cai et al., 2020), accumulating evidences indicated that Intestinimonas was capable of fermenting fiber to produce butyrate and converting fructoselysine, an advanced glycation end-product, into butyrate (Niu et al., 2024; Kahleova et al., 2023). Similarly, Intestinimonas is significantly enriched in NAFLD patients without SDS in our study. Further analysis also revealed a positive correlation between the relative abundance of Intestinimonas and Cr, and low Cr levels has been reported as a predictor of NASH (Wu et al., 2021). It is speculated that NAFLD patients without SDS have a low risk of progressing to hepatitis compared to NAFLD patients with SDS.
Concurrently, previous studies indicated that Akkermansia and Bifidobacterium, recognized as beneficial bacteria, may increase in response to enhanced dietary carbohydrate bioavailability and can rise compensatory under disease conditions to restore microbial balance (Cox et al., 2021; Fava et al., 2013; Hizo and Rampelotto, 2023). Notably, excessive increases in microbial abundance may compromise gut microbiota stability and diversity, leading to metabolic dysfunction. Akkermansia, a mucin-degrading bacterium, may impair the intestinal barrier and promote LPS translocation when overabundant, thereby triggering low-grade inflammation (Qu et al., 2023). In our results, the significant increase in Akkermansia and Bifidobacterium relative abundance in NAFLD_P patients may be attributed to the factors mentioned above. Future studies should further investigate specific roles of these bacterium in NAFLD patients with SDS and evaluate the potential as therapeutic targets or biomarkers.
Rhizopus is a distinguishing fungi in metabolic-associated fatty liver disease, differentiating it from alcohol-related liver disease (Zhang et al., 2024a). Mucor sp. (a common opportunistic pathogen) and Rhizopus belong to the same order, Mucorales, and Mucor sp. has been shown to be positively correlated with liver inflammation and fibrosis (Demir et al., 2022). Besides, activation of innate immunity, as the first line of defense, plays a crucial role in liver inflammation in NAFLD (Arrese et al., 2016). Prior study showed that Rhizopus was the most common genus responsible for fatal mucormycosis infections in immunocompromised patients (Petrikkos et al., 2012). Correspondingly, a significant enrichment of Rhizopus also existed in NAFLD patients with SDS. Moreover, ALB has anti-inflammatory and antioxidant functions (Yang et al., 2021), while NAFLD patients exhibited lower ALB levels due to hepatic lipid accumulation, which might exacerbate the progression of liver inflammation (Peiseler et al., 2022; Li et al., 2024b). In our study, the relative abundance of Rhizopus was negatively correlated with ALB. Compared to NAFLD patients without SDS, those with SDS might have even low levels of ALB due to the pro-inflammatory effects of Rhizopus. Therefore, we hypothesize that the enrichment of pro-inflammatory bacteria and fungi in NAFLD patients with SDS is associated with elevated inflammation levels and impaired immunity.
Intestinal bacteria and fungi exhibit complex interrelationships in health and disease. In this study, though no significant association was detected between the community structures of intestinal bacteria and fungi, we identified potential meaningful interactions in NAFLD patients with SDS. In these patients, Candida was positively correlated with Segatella. Previous studies have shown that fungi could directly or indirectly reduce the levels of Sutterella (Scanu et al., 2024). Sutterella, a pathogenic intestinal microorganism linked to intestinal inflammation and gastrointestinal disorders, degrades IgA to impair intestinal immune function, thereby facilitating the invasion of pathogenic commensal bacteria (Singh et al., 2024; Kaakoush, 2020). Additionally, previous study demonstrated that the relative abundance of Candida significantly increased in mice fed high-fat and high-fructose diets, and was positively correlated with the NAFLD activity score (Zheng et al., 2024). Candida albicans, a common Candida species frequently found in NAFLD patients, was not only able to activate host Toll-like receptors(TLR), leading to the release of pro-inflammatory cytokines (Li et al., 2024a; Netea et al., 2006, 2002), but also to induce epigenetic reprogramming of innate immune cells (Netea et al., 2015). In addition, we also observed a positive correlation between Rhizopus and Gemmiger. In NAFLD patients with SDS and compromised immune function, Rhizopus promoted inflammation, while Gemmiger induced inflammation and related symptoms by downregulating TLR1 expression (Zhou et al., 2023). The limited biodiversity of bacteria and fungi in these patients may drive novel cross-kingdom interactions, potentially affecting host immune and inflammatory regulation (Yadav et al., 2022; Sokol et al., 2017). Therefore, we hypothesize that the interactions between Rhizopus and Gemmiger, as well as between Candida and Segatella, in NAFLD patients with SDS may collectively modulate the host’s immune and inflammatory responses.
Intestinal bacteria are not only associated with long-term diet but also influenced by factors such as exercise, pharmacologic factors, and host genetic factors (Chang and Kao, 2019; Zhang et al., 2025; Hoffmann et al., 2013). In the present study, long-term dietary intake was characterized using the food frequency questionnaire, and the results revealed a weak correlation between the OTU distance matrix of intestinal bacteria and dietary nutrient intake distance matrix. This suggests that, in addition to dietary influences, other complex factors may be involved in regulating the changes in intestinal bacteria in NAFLD patients with SDS. However, dietary intervention could still serve as a potential regulatory strategy. In contrast, no significant association was observed between the OTU distance matrix of intestinal fungi and dietary nutrient intake distance matrix, which may be attributed to the low abundance of fungi in the gut and their more rapid response to short-term dietary changes (Hoffmann et al., 2013). It is worth noting that Intestinimonas is an anti-inflammatory beneficial bacterium that ferments fiber to produce butyrate (Kahleova et al., 2023). In this study, we found that the relative abundance of Intestinimonas was found to be positively correlated not only with dietary fiber intake but also with carbohydrates, vitamins A, B1, B6, and C intake, as well as with iron potassium, and magnesium intake. Consequently, NAFLD patients without SDS showed Intestinimonas enrichment, potentially associated with high intake of antioxidant and anti-inflammatory foods compared to SDS patients. Furthermore, niacin intake was negatively correlated with the relative abundance of Rhizopus. Niacin intake in NAFLD patients was lower than in healthy controls, particularly among those with SDS. Although the relationship between niacin and NAFLD is not fully understood, niacin could exert beneficial effects by reducing triglyceride synthesis and improving lipid profiles, and to mitigated free radical damage and reduce inflammation, which was crucial to alleviate NAFLD (Antentas et al., 2024; Zhou and Han, 2024). In our study, the relative abundance of Rhizopus was negatively correlated with the albumin-to-globulin (A/G) ratio, which was a potential biomarker of liver damage to some extent. Therefore, we speculate that dietary niacin may contribute to liver tissue damage through its interaction with Rhizopus. In the future, the optimal intake of niacin will still require further validation studies.
There are two main limitations in this study. First, the observational design without intervention precluded causal inference between intestinal microbiota and NAFLD patients with SDS. Second, the absence of metabolomic analyses prevented comprehensive characterization of microbiota-metabolome interactions. In future, studies should integrate longitudinal interventions with multi-omics approaches.
5 Conclusions
In this study, symptoms related to SDS were more common in NAFLD patients. The intake of dietary nutrients in NAFLD patients with SDS might have exhibited a pro-inflammatory effect, potentially due to insufficient intake of antioxidant nutrients. The independent and interactive effects of intestinal bacteria and fungi might have collectively influenced the immune function and inflammation levels in NAFLD patients with SDS. These processes were likely associated with the intake of antioxidant and anti-inflammatory nutrients, as well as niacin. These findings provide a theoretical basis for the clinical treatment and dietary management of NAFLD patients with SDS.
Data availability statement
The raw gut microbiome sequencing data from this study have been deposited in the NCBI Sequence Read Archive (SRA) with BioProject accession number PRJNA1299057.
Ethics statement
The studies involving humans were approved by the Jinan University Ethics Committee (Ethical approval number: JUNKY-2023-0130). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GL: Formal analysis, Investigation, Methodology, Writing – original draft. WO: Data curation, Investigation, Methodology, Writing – original draft. JY: Data curation, Formal analysis, Validation, Visualization, Writing – original draft. DC: Data curation, Methodology, Resources, Writing – original draft. YW: Project administration, Resources, Writing – original draft. AW: Investigation, Project administration, Writing – original draft. LG: Project administration, Resources, Writing – original draft. WQ: Project administration, Resources, Writing – original draft. CL: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing. YL: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (No. 82174256), Program of China Scholarships Council (No. 202206785007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1586212/full#supplementary-material
References
Antentas, M., Rojo-López, M. I., Vendrell, P., Granado-Casas, M., Genua, I., Fernandez-Camins, B., et al. (2024). Impact of dietary niacin on metabolic dysfunction-associated steatotic liver disease in mediterranean subjects: A population-based study. Nutrients 16, 4178. doi: 10.3390/nu16234178, PMID: 39683571
Arrese, M., Cabrera, D., Kalergis, A. M., and Feldstein, A. E. (2016). Innate immunity and inflammation in NAFLD/NASH. Digestive Dis. Sci. 61, 1294–1303. doi: 10.1007/s10620-016-4049-x, PMID: 26841783
Astbury, S., Atallah, E., Vijay, A., Aithal, G. P., Grove, J. I., and Valdes, A. M. (2020). Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes 11, 569–580. doi: 10.1080/19490976.2019.1681861, PMID: 31696774
Cai, W., Xu, J. X., Li, G., Liu, T., Guo, X. L., Wang, H. J., et al. (2020). Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 130. doi: 10.1016/j.foodres.2019.108939, PMID: 32156386
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303, PMID: 20383131
Chang, C. S. and Kao, C. Y. (2019). Current understanding of the gut microbiota shaping mechanisms. J. BioMed. Sci. 26, 59. doi: 10.1186/s12929-019-0554-5, PMID: 31434568
Chen, G. J. (2019). The Clinical Study of Tianrendi Sancai Moxibustion in TreatingInsomnia of Deficiency of the Heart and Spleen. Shandong University of Traditional Chinese Medicine, Jinan(Shandong.
Chen, H. and Boutros, P. C. (2011). VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinf. 12, 35. doi: 10.1186/1471-2105-12-35, PMID: 21269502
Chen, L. L., Fan, Z. Q., Sun, X. D., Qiu, W., Mu, W. T., Chai, K. Y., et al. (2023). Diet-derived antioxidants and nonalcoholic fatty liver disease: a Mendelian randomization study. Hepatol. Int. 17, 326–338. doi: 10.1007/s12072-022-10443-3, PMID: 36352064
Chen, Y., Maitiniyazi, G., Li, Z., Li, T., Liu, Y., Zhang, R., et al. (2022). TNF-α Mediates the association between dietary inflammatory index and depressive symptoms in breast cancer. Nutrients 15. doi: 10.3390/nu15010084, PMID: 36615742
Chen, D. L., Wang, Y. F., Yang, J. M., Ou, W. Y., Lin, G. R., Zeng, Z., et al. (2024). Shenling Baizhu San ameliorates non-alcoholic fatty liver disease in mice by modulating gut microbiota and metabolites. Front. Pharmacol. 15. doi: 10.3389/fphar.2024.1343755, PMID: 38720776
Chinese Nutrition Society. (2022). Revision and Analysis of the Chinese Food Guide Pagoda. Available online at: http://dg.cnsoc.org/article/04/RMAbPdrjQ6CGWTwmo62hQg.html (Accessed July 26, 2025).
Cox, L. M., Maghzi, A. H., Liu, S., Tankou, S. K., Dhang, F. H., Willocq, V., et al. (2021). Gut microbiome in progressive multiple sclerosis. Ann. Neurol. 89, 1195–1211. doi: 10.1002/ana.26084, PMID: 33876477
Dai, L., Xu, J. J., Zhou, W. J., Lü, A. P., and Ji, G. (2022). Appraisal of treatment outcomes in integrative medicine using metabonomics: Taking non-alcoholic fatty liver disease with spleen deficiency syndrome as an example. J. Integr. Medicine-Jim 20, 524–533. doi: 10.1016/j.joim.2022.08.002, PMID: 36031542
Demir, M., Lang, S., Hartmann, P., Duan, Y., Martin, A., Miyamoto, Y., et al. (2022). The fecal mycobiome in non-alcoholic fatty liver disease. J. Hepatol. 76, 788–799. doi: 10.1016/j.jhep.2021.11.029, PMID: 34896404
Fan, J. G., Xu, X. Y., Yang, R. X., Nan, Y. M., Wei, L., Jia, J. D., et al. (2024). Guideline for the prevention and treatment of metabolic dysfunction-associated fatty liver disease (Version 2024). J. Clin. Trans. Hepatol. 12, 955–974. doi: 10.14218/jcth.2024.00311, PMID: 39544247
Fan, Y., Zhao, Q., Wei, Y., Wang, H., Ga, Y., Zhang, Y., et al. (2023). Pingwei san ameliorates spleen deficiency-induced diarrhea through intestinal barrier protection and gut microbiota modulation. Antioxidants (Basel) 12, 1122. doi: 10.3390/antiox12051122, PMID: 37237988
Fava, F., Gitau, R., Griffin, B. A., Gibson, G. R., Tuohy, K. M., and Lovegrove, J. A. (2013). The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome 'at-risk' population. Int. J. Obes. (Lond) 37, 216–223. doi: 10.1038/ijo.2012.33, PMID: 22410962
Hizo, G. H. and Rampelotto, P. H. (2023). The role of bifidobacterium in liver diseases: A systematic review of next-generation sequencing studies. Microorganisms 11, 2999. doi: 10.3390/microorganisms11122999, PMID: 38138143
Hoffmann, C., Dollive, S., Grunberg, S., Chen, J., Li, H., Wu, G. D., et al. (2013). Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PloS One 8, e66019. doi: 10.1371/journal.pone.0066019, PMID: 23799070
Hu, H. M., Lin, A. Z., Kong, M. W., Yao, X. W., Yin, M. Z., Xia, H., et al. (2020). Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J. Gastroenterol. 55, 142–158. doi: 10.1007/s00535-019-01649-8, PMID: 31845054
Hu, B. H., Sui, J., Wang, Y., Li, L. H., Gong, D. C., Zhu, Z. X., et al. (2024). A systematic review of dietary and circulating carotenoids and liver disease. Food Funct. 15, 9813–9832. doi: 10.1039/d4fo03082f, PMID: 39229651
Jiménez-González, C., Alonso-Peña, M., Argos Vélez, P., Crespo, J., and Iruzubieta, P. (2025). Unraveling MASLD: the role of gut microbiota, dietary modulation, and AI-driven lifestyle interventions. Nutrients 17, 1580. doi: 10.3390/nu17091580, PMID: 40362889
Kaakoush, N. O. (2020). Sutterella species, igA-degrading bacteria in ulcerative colitis. Trends Microbiol. 28, 519–522. doi: 10.1016/j.tim.2020.02.018, PMID: 32544438
Kahleova, H., Holtz, D. N., Strom, N., La Reau, A., Kolipaka, S., Schmidt, N., et al. (2023). A dietary intervention for postmenopausal hot flashes: A potential role of gut microbiome. An exploratory analysis. Complementary Therapies Med. 79. doi: 10.1016/j.ctim.2023.103002, PMID: 37949415
Keating, S. E., Sabag, A., Hallsworth, K., Hickman, I. J., Macdonald, G. A., Stine, J. G., et al. (2023). Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: A position statement from exercise and sport science Australia. Sports Med. 53, 2347–2371. doi: 10.1007/s40279-023-01918-w, PMID: 37695493
Kwon, O. Y., Kim, S. U., Ahn, S. H., and Jang, Y. (2023). Self-management and associated factors among patients with non-alcoholic fatty liver disease: A cross-sectional study. Int. J. Environ. Res. Public Health 20, 667. doi: 10.3390/ijerph20010667, PMID: 36612985
Kyriazopoulou, E., Stylianakis, E., Damoraki, G., Koufargyris, P., Kollias, I., Katrini, K., et al. (2025). Procalcitonin-guided early cessation of antibiotics prevents gut inflammation and preserves gut microbiome: Data from the PROGRESS controlled trial. Int. J. Antimicrob. Agents 66, 107507. doi: 10.1016/j.ijantimicag.2025.107507, PMID: 40216091
Lazarus, J. V., Mark, H. E., Anstee, Q. M., Arab, J. P., Batterham, R. L., Castera, L., et al. (2022). Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78. doi: 10.1038/s41575-021-00523-4, PMID: 34707258
Li, Y. (2017). Studies on the Bioactive Components and Action Mechanism of Jujubae Fructus Reinforcing Spleen and Stomach Effects. Nanjing University of Chinese Medicine, Nanjing(Jiangsu.
Li, L. W., Cai, F. Q., Guo, C., Liu, Z., Qin, J. M., and Huang, J. A. (2024a). Gut microbiome and NAFLD: impact and therapeutic potential. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1500453, PMID: 39664063
Li, X. M., Liu, S. L., He, Y. J., and Shu, J. C. (2024b). Using new indices to predict metabolism dysfunction-associated fatty liver disease (MAFLD): analysis of the national health and nutrition examination survey database. BMC Gastroenterol. 24, 109. doi: 10.1186/s12876-024-03190-2, PMID: 38491451
Li, Y., Zhang, Y., Cao, R., Niu, J., Bian, T., Ma, D., et al. (2023). Identifications of metabolic differences between Hedysari Radix Praeparata Cum Melle and Astragali Radix Praeparata Cum Melle for spleen-qi deficiency rats: A comparative study. J. Pharm. BioMed. Anal. 236, 115689. doi: 10.1016/j.jpba.2023.115689, PMID: 37677887
Liu, J. Q., Liu, Y. X., Chen, Y. S., Liu, Y. H., Huang, C. Q., Luo, Y. J., et al. (2024b). Betaine alleviates nonalcoholic fatty liver disease (NAFLD) via a manner involving BHMT/FTO/m6A/ PGC1α signaling. J. Nutr. Biochem. 134. doi: 10.1016/j.jnutbio.2024.109738, PMID: 39154792
Liu, C., Sun, X. A., Peng, J., Yu, H. Q., Lu, J., and Feng, Y. H. (2024a). Association between dietary vitamin A intake from different sources and non-alcoholic fatty liver disease among adults. Sci. Rep. 14, 1851. doi: 10.1038/s41598-024-52077-5, PMID: 38253816
Luo, X. F., Li, Y., Zhou, Y., Zhang, C., Li, L. J., Luo, Y. T., et al. (2022). Association of non-alcoholic fatty liver disease with salt intake and dietary diversity in chinese medical examination adults aged 18–59 years: A cross-sectional study. Front. Nutr. 9. doi: 10.3389/fnut.2022.930316, PMID: 35903450
Ma, Y. D. (2022). Microbiota of non-alcoholic fatty liver disease complicated with abnormal glucose metabolism syndrome in patients with Liver depression and spleen deficiency. Guangdong Pharmaceutical University, Guangzhou(Guangdong.
Ma, H., Liu, H., Yang, Y. T., Han, M., and Jiang, C. M. (2025). The effect of folate deficiency and different doses of folic acid supplementation on liver diseases. Br. J. Nutr. 133, 37–47. doi: 10.1017/s000711452400285x, PMID: 39534991
Mccullough, L. E. and Byrd, D. A. (2023). Total energy intake: implications for epidemiologic analyses. Am. J. Epidemiol. 192, 1801–1805. doi: 10.1093/aje/kwac071, PMID: 35419586
Miao, L., Targher, G., Byrne, C. D., Cao, Y. Y., and Zheng, M. H. (2024a). Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 35, 697–707. doi: 10.1016/j.tem.2024.02.007, PMID: 38429161
Miao, T., Zhang, X., Zhang, C., Wu, J., Zhu, Y., Xiao, M., et al. (2024b). Type 3 resistant starch from Canna edulis reduce lipid levels in patients with mild hyperlipidemia through altering gut microbiome: A double- blind randomized controlled trial. Pharmacol. Res. 205, 107232. doi: 10.1016/j.phrs.2024.107232, PMID: 38825157
Mikolasevic, I., Domislovic, V., Kanizaj, T. F., Radic-Kristo, D., Krznaric, Z., Milovanovic, T., et al. (2021). Relationship between coffee consumption, sleep duration and smoking status with elastographic parameters of liver steatosis and fibrosis; controlled attenuation parameter and liver stiffness measurements. Int. J. Clin. Pract. 75. doi: 10.1111/ijcp.13770, PMID: 33070425
Netea, M. G., Gow, N. A., Munro, C. A., Bates, S., Collins, C., Ferwerda, G., et al. (2006). Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116, 1642–1650. doi: 10.1172/jci27114, PMID: 16710478
Netea, M. G., Joosten, L. A. B., van der Meer, J. W. M., Kullberg, B. J., and Van De Veerdonk, F. L. (2015). Immune defence against Candida fungal infections. Nat. Rev. Immunol. 15, 630–642. doi: 10.1038/nri3897, PMID: 26388329
Netea, M. G., van der Graaf, C. A., Vonk, A. G., Verschueren, I., van der Meer, J. W., and Kullberg, B. J. (2002). The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 185, 1483–1489. doi: 10.1086/340511, PMID: 11992285
Niu, Y. L., Zhao, T. T., Liu, Z. J., Li, D. T., Wen, D. X., Li, B., et al. (2024). Brassica rapa L. crude polysaccharide meditated synbiotic fermented whey beverage ameliorates hypobaric hypoxia induced intestinal damage. Food Funct. 15, 11975–11989. doi: 10.1039/d4fo04667f, PMID: 39555987
Peiseler, M., Schwabe, R. F., Hampe, J., Kubes, P., Heikenwaelder, M., and Tacke, F. (2022). Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J. Hepatol. 77, 1136–1160. doi: 10.1016/j.jhep.2022.06.012, PMID: 35750137
Peng, Y., Wu, C., Yang, J., and Li, X. (2014). Gut microbial diversity in rat model induced by rhubarb. Exp. Anim. 63, 415–422. doi: 10.1538/expanim.63.415, PMID: 25048267
Peng, Y., Zhang, S., Liu, Z., Ji, J., Wu, C., Yang, J., et al. (2020). Gut microbiota and Chinese medicine syndrome: altered fecal microbiotas in spleen (Pi)-deficient patients. J. Tradit Chin. Med. 40, 137–143., PMID: 32227775
Petrikkos, G., Skiada, A., Lortholary, O., Roilides, E., Walsh, T. J., and Kontoyiannis, D. P. (2012). Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 54, S23–S34. doi: 10.1093/cid/cir866, PMID: 22247442
Qiu, Z. Y., Wen, Y., and Gao, T. S. (2024). Effect of Astragalus polysaccharide on serum inflammatory factors and thyroid tissue of auto-immune thyroiditis NOD. 2h4 mice impact. Lishizhen Med. Materia Med. Res. 35, 2362–2368. doi: 10.3969/j.issn.1008-0805.2024.10.09
Qu, S., Zheng, Y., Huang, Y., Feng, Y., Xu, K., Zhang, W., et al. (2023). Excessive consumption of mucin by over-colonized Akkermansia muciniphila promotes intestinal barrier damage during Malignant intestinal environment. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1111911, PMID: 36937258
Scanu, M., Toto, F., Petito, V., Masi, L., Fidaleo, M., Puca, P., et al. (2024). An integrative multi-omic analysis defines gut microbiota, mycobiota, and metabolic fingerprints in ulcerative colitis patients. Front. Cell. Infection Microbiol. 14. doi: 10.3389/fcimb.2024.1366192, PMID: 38779566
Shen, Q. Y. (2017). Development of a Traditional Chinese Medicine PRO Scale for Non-alcoholic Fatty Liver Disease. Beijing University of Chinese Medicine, Beijing (China).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R., and Hébert, J. R. (2014). Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17, 1689–1696. doi: 10.1017/s1368980013002115, PMID: 23941862
Singh, V., Mahra, K., Jung, D., and Shin, J. H. (2024). Gut microbes in polycystic ovary syndrome and associated comorbidities; type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD), and the potential of microbial therapeutics. Probiotics Antimicrob. Proteins 16, 1744–1761. doi: 10.1007/s12602-024-10262-y, PMID: 38647957
Sokol, H., Leducq, V., Aschard, H., Pham, H. P., Jegou, S., Landman, C., et al. (2017). Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048. doi: 10.1136/gutjnl-2015-310746, PMID: 26843508
Sun, S. D. (2020). Study on the mechanism of Qiweibaizhu decoction on intest inal flora-short chain fatty acids and mucus barrier of diarrhea rats with spleen deficiency syndrome. Guangzhou University of Chinese Medicine, Guangzhou(Guangdong.
Sun, S., Guo, H., Shang, E., Guo, Q., Ju, A., Li, Y., et al. (2024). Lipidomics study of Liujunzi decoction in hyperlipidemia rats with spleen deficiency based on UPLC-Q-TOF/MS. Heliyon 10, e31710. doi: 10.1016/j.heliyon.2024.e31710, PMID: 38882295
Tyczynska, M., Hunek, G., Szczasny, M., Brachet, A., Januszewski, J., Forma, A., et al. (2024). Supplementation of micro- and macronutrients-A role of nutritional status in non-alcoholic fatty liver disease. Int. J. Mol. Sci. 25, 4916. doi: 10.3390/ijms25094916, PMID: 38732128
Wong, V. W., Chan, W. K., Chitturi, S., Chawla, Y., Dan, Y. Y., Duseja, A., et al. (2018). Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J. Gastroenterol. Hepatol. 33, 70–85. doi: 10.1111/jgh.13857, PMID: 28670712
Wong, V. W. S., Ekstedt, M., Wong, G. L. H., and Hagström, H. (2023). Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 79, 842–852. doi: 10.1016/j.jhep.2023.04.036, PMID: 37169151
Wu, X. X., Zheng, K., Boursier, J., Chan, W. K., Yilmaz, Y., Romero-Gómez, M., et al. (2021). acNASH index to diagnose nonalcoholic steatohepatitis: a prospective derivation and global validation study. Eclinicalmedicine 41. doi: 10.1016/j.eclinm.2021.101145, PMID: 34646997
Yadav, M., Ali, S., Shrode, R. L., Shahi, S. K., Jensen, S. N., Hoang, J., et al. (2022). Multiple sclerosis patients have an altered gut mycobiome and increased fungal to bacterial richness. PloS One 17. doi: 10.1371/journal.pone.0264556, PMID: 35472144
Yang, X. W., Mao, Z. M., Huang, Y. R., Yan, H. Z., Yan, Q. J., Hong, J. R., et al. (2021). Reductively modified albumin attenuates DSS-Induced mouse colitis through rebalancing systemic redox state. Redox Biol. 41. doi: 10.1016/j.redox.2021.101881, PMID: 33601276
Yu, H. C., Meng, Y. Y., Wang, E. K., Yuan, J. Y., Peng, Y., and Li, X. B. (2024). Buzhong Yiqi Decoction ameliorates spleen deficiency syndrome by regulating gut microbiota. Zhongguo Zhong Yao Za Zhi 49, 1028–1043. doi: 10.19540/j.cnki.cjcmm.20231013.701, PMID: 38621910
Zhan, X., Xiao, Y., Jian, Q., Dong, Y., Ke, C., Zhou, Z., et al. (2024). Integrated analysis of metabolomic and transcriptomic profiling reveals the effect of Atractylodes oil on Spleen Yang Deficiency Syndrome in rats. J. Ethnopharmacol 319, 117205. doi: 10.1016/j.jep.2023.117205, PMID: 37741473
Zhang, P. (2022). Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 23, 4916. doi: 10.3390/ijms23179588, PMID: 36076980
Zhang, C. X. and Ho, S. C. (2009). Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac J. Clin. Nutr. 18, 240–250., PMID: 19713184
Zhang, L., Liu, R., Song, Z., and Zhang, X. (2025). Exercise, diet, and brain health: from the perspective of gut microbiota regulation. Nutrients 17, 1686. doi: 10.3390/nu17101686, PMID: 40431427
Zhang, D. Y., Wang, Q., Li, D., Chen, C., Lv, Y. T., Huang, S. M., et al. (2024a). Different fungal signatures in ALD and MAFLD. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1510507, PMID: 39669777
Zhang, W. Y., Wang, M. H., and Xie, C. (2024b). Potential of traditional Chinese medicine in the treatment of nonalcoholic fatty liver disease: A promising future. World J. Gastroenterol. 30, 4597–4601. doi: 10.3748/wjg.v30.i43.4597, PMID: 39575403
Zheng, R. Y., Xiang, X. W., Shi, Y., Xie, J. Y., Xing, L., Zhang, T., et al. (2024). Gut microbiota and mycobiota change with feeding duration in mice on a high-fat and high-fructose diet. BMC Microbiol. 24, 504. doi: 10.1186/s12866-024-03663-0, PMID: 39609794
Zheng, Y. Y., Zeng, X., Chen, P., Chen, T. T., Peng, W., and Su, W. W. (2020). Integrating pharmacology and gut microbiota analysis to explore the mechanism of citri reticulatae pericarpium against reserpine-induced spleen deficiency in rats. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.586350, PMID: 33192528
Zhou, J. and Han, J. (2024). Association of niacin intake and metabolic dysfunction-associated steatotic liver disease: findings from National Health and Nutrition Examination Survey. BMC Public Health 24, 2742. doi: 10.1186/s12889-024-20161-0, PMID: 39379884
Zhou, J., Qiu, X. M., Chen, X. J., Ma, S. H., Chen, Z. Y., Wang, R. Z., et al. (2023). Comprehensive analysis of gut microbiota alteration in the patients and animal models with polycystic ovary syndrome. J. Microbiol. 61, 821–836. doi: 10.1007/s12275-023-00079-9, PMID: 37824034
Keywords: non-alcoholic fatty liver disease, spleen deficiency syndrome, intestinal bacteria, intestinal fungi, dietary nutrient intake
Citation: Lin G, Ou W, Yang J, Chen D, Wang Y, Wu A, Gao L, Qu W, Lin C and Liang Y (2025) Correlation of intestinal bacteria, fungi and dietary nutrient intake in NAFLD patients with spleen deficiency syndrome. Front. Cell. Infect. Microbiol. 15:1586212. doi: 10.3389/fcimb.2025.1586212
Received: 02 March 2025; Accepted: 15 July 2025;
Published: 11 August 2025.
Edited by:
Ehsaneh Taheri, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Abbas Yadegar, Shahid Beheshti University of Medical Sciences, IranHuanzhuo Mai, Capital Medical University, China
Copyright © 2025 Lin, Ou, Yang, Chen, Wang, Wu, Gao, Qu, Lin and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenli Lin, dGNoZW5saWxpbkBqbnUuZWR1LmNu; Yinji Liang, dGx5akBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Guiru Lin
Guiru Lin Wanyi Ou1†
Wanyi Ou1† Yinji Liang
Yinji Liang