- 1Department of Orthopaedics, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 2National Engineering Research Center of Immunological Products, Third Military Medical University (Army Medical University), Chongqing, China
- 3Institute of Cancer, Xinqiao Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 4Department of Orthopaedics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Bone infections, specifically chronic osteomyelitis, are characterized by recurrent episodes. They are considered intractable clinical diseases as they require protracted and difficult-to-cure courses. Staphylococcus aureus (S. aureus) is the most common pathogen responsible for bone infections and has high destruction rates. Previous literature has indicated that during S. aureus osteomyelitis, immune evasion mainly involves three mechanisms: biofilm formation, intracellular infection, and abscess formation. However, recently, it was observed that S. aureus can enter and persist for a long time in the Osteocyte lacuno-canalicular network (OLCN), a bone microstructure. Furthermore, it has been found to successfully evade the host’s immune system via natural physical barriers, chemical properties, and bone microstructure’s immune escape mechanisms. Therefore, S. aureus bone infections are more difficult to cure than soft-tissue infections. Currently, there are only a few studies on OLCN invasion by S. aureus, and the clinical evidence is not sufficient. Therefore, this review aimed to combine relevant published literature on the OLCN-mediated immune escape of S. aureus to elaborate on the pathological mechanisms associated with protracted and difficult-to-cure bone infections. The findings will provide a scientific basis and theoretical foundation for future comprehensive analysis of how S. aureus invades OLCN and novel treatment strategies for bone infections.
1 Introduction
Chronic osteomyelitis (COM) is a chronic infection of bone primarily caused by pathogenic microorganisms disseminating via hematogenous or exogenous routes. COM is manifested in bones and surrounding soft tissues, usually after months to years of continuous infection. Furthermore, it is mostly diagnosed secondary to neglected or incompletely treated hematogenous osteomyelitis and is a common complication observed after open fractures and orthopedic-related surgeries. COM is associated with local bone tissue necrosis and abscess formation; therefore, it is characterized by long-course complex infections with a high recurrence rate. In severe cases, it can cause bone defects and even become a lifelong disease. Reports have indicated that COM can relapse 80 years after the primary onset (Kremers et al., 2015). The clinical treatment of COM is difficult and costly, which significantly impacts patients’ quality of life and the healthcare system and has become a major challenge for orthopedic surgeons (Wu et al., 2019; Yousaf et al., 2021).
Chronic osteomyelitis predominantly occurs after fractures and internal fixation implantation surgeries. However, in rare cases, it is also caused by lower-limb ischemic ulcers due to diabetes, sickle-cell disease, and malnutrition (Jiang et al., 2024). The subtype classification of COM includes implant-related osteomyelitis [including periprosthetic joint infection (PJI) and instrumented spinal infection], fracture-related infection, acute hematogenous osteomyelitis, diabetic foot infection, septic arthritis, congenital spinal osteomyelitis, etc. (Masters et al., 2022). Its primary characteristics include the persistent presence of microorganisms, low-grade inflammation, sequestra (bone fragments), and sinus tract formation, which differentiates it from acute osteomyelitis (Caputo et al., 1994). Recurrence at the same site accompanied by fever is an obvious sign of COM. Moreover, persistent clinical symptoms for > 10 days have been linked with the formation of sequestra and the development of COM (Norden et al., 1992).
Various microorganisms have been associated with bone infections. Some common microorganisms causing chronic bone infections include Staphylococcus aureus (S. aureus), coagulase-negative staphylococci (CoNS) (such as Staphylococcus epidermidis), Streptococcus spp., Enterococcus spp., Diphtheroids, Gram-negative bacteria, Pseudomonas spp., and Enterobacteriaceae spp. Of these, S. aureus is the most common, prevalent, and destructive pathogen related to bone infections (Masters et al., 2022). This Gram-positive bacterium can infect about every type of human tissue and cause asymptomatic skin colonization to life-threatening diseases. Furthermore, S. aureus is specifically pathogenic in bone infections because it can invade, colonize, and grow within bones (Masters et al., 2022). Moreover, its virulence factors can disrupt the host’s immune defenses, thereby causing bone destruction. For instance, S. aureus protein A (SpA), an extracellular and cell-binding protein, can induce severe inflammatory responses (Kumar et al., 2007), inhibit osteogenesis and promote osteoclastogenesis (Jin et al., 2013). Similarly, > 50% of bone infections are caused by methicillin-resistant S. aureus (MRSA), which is difficult to treat (Gallarate et al., 2021).
Chronic bone infections are primarily controlled using the following 6 measures: thorough debridement, local stabilization, dead space obliteration, adequate drainage, effective coverage, as well as applying local and systemic sensitive antibiotics. However, these clinical treatment strategies face 2 challenges. First, antibiotics have a low bone tissue diffusion rate, and although administered intravenously, the required plasma concentration and tissue penetration are difficult to achieve (Fantoni et al., 2019). Furthermore, microorganisms have evolved various mechanisms to effectively evade the host’s innate and adaptive immune attacks and continuously develop antibiotic resistance for persistent colonization in the host (Qin et al., 2024), which increases the challenges of COM treatment. Secondly, thorough surgical debridement of inflammatory tissues, sinus tracts, scar tissues, infected granulation tissues, medullary cavity abscesses, sclerotic bone, sequestra, etc., is crucial for COM treatment. Most current studies suggest that it is necessary to expand the debridement region, and tissue that is infected or might be infected, as well as the surrounding soft tissues, should be removed as much as possible (Simpson et al., 2001; Hevroni and Koplewitz, 2007; Hogan et al., 2013). However, the accurate identification of the infection boundary is difficult to delineate during surgery for thorough debridement. Therefore, even after antibiotic treatment and surgical debridement, the treatment failure rate is 20% (Conterno and Turchi, 2013), and the postsurgical reinfection rate is 33% (Azzam et al., 2009; Rosas et al., 2017).
Several studies have indicated that the following mechanisms are responsible for immune evasion and S. aureus persistence during COM: Biofilm formation (Ricciardi et al., 2018; Masters et al., 2019), Intracellular infection (Ellington et al., 2003; Bosse et al., 2005; Sendi et al., 2006; Josse et al., 2015; Yang et al., 2018; Krauss et al., 2019; Alder et al., 2020; Roper et al., 2020), Staphylococcal abscess communities (SACs) (Carek et al., 2001; Cheng et al., 2011; Kobayashi et al., 2015; Malachowa et al., 2016; Kavanagh et al., 2018). These mechanisms have been comprehensively studied and, therefore, will not be discussed in this paper. The S. aureus invasion of the Osteocyte lacuno-canalicular network (OLCN) via a novel pathway for bacterial persistence and immune evasion can explain the long-term bacterial persistence and treatment failure in COM (de Mesy Bentley et al., 2017; de Mesy Bentley et al., 2018) (Figure 1A). However, this pathway has not been systematically investigated, and comprehensive research is needed to determine better COM treatment. Therefore, this paper reviewed this pathway to provide the theoretical foundation for exploring the mechanism of S. aureus invading the OLCN and developing targeted treatment strategies in the future.
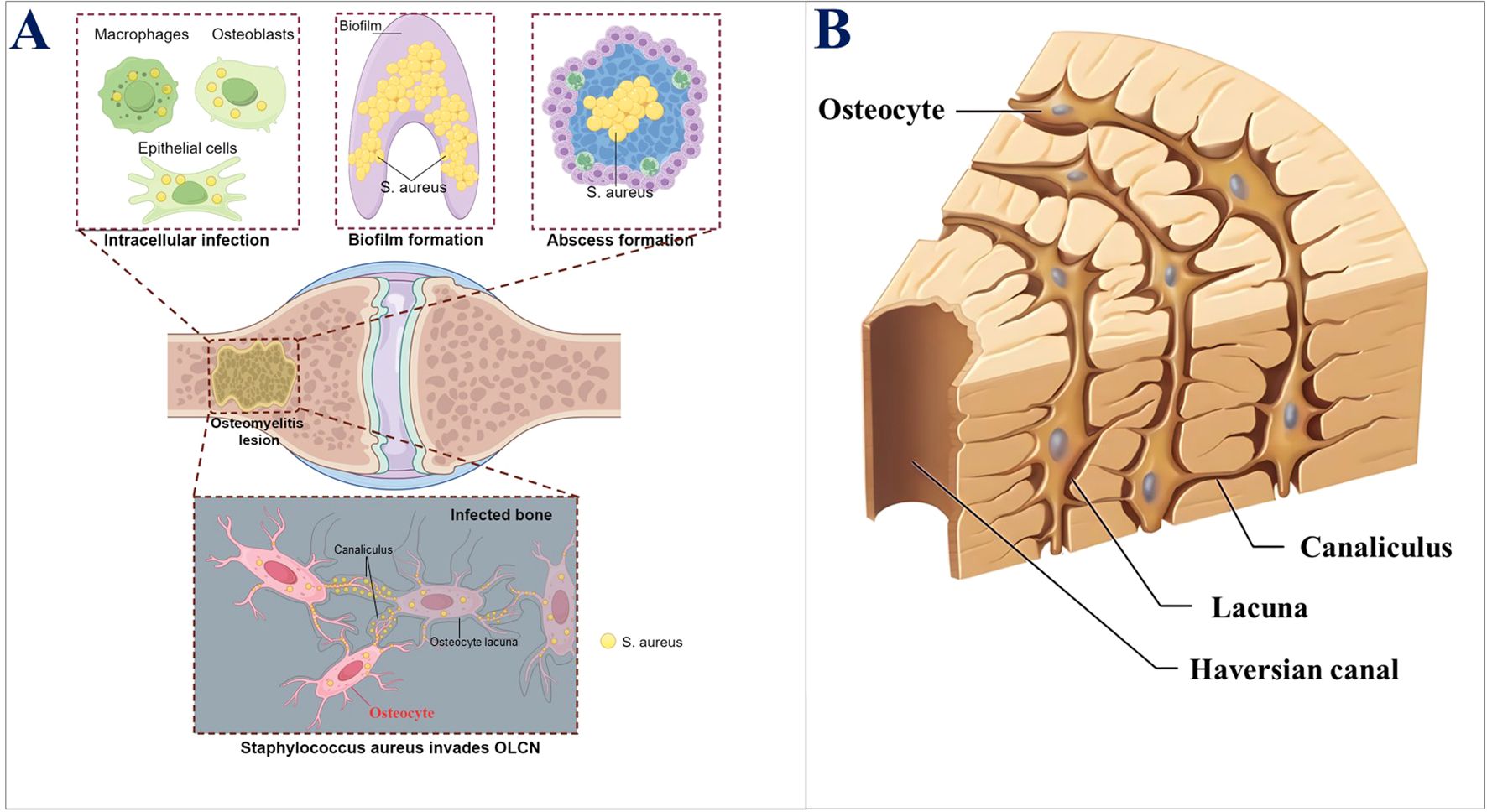
Figure 1. (A) Key mechanisms of the persistence of S. aureus in bone infections. (B) Schematic diagram of the three-dimensional structure of OLCN.
2 Osteocyte lacuno-canalicular network
Osteocytes are the primary cells in the bone matrix (Parfitt, 1977). Furthermore, they are the most mature and terminally differentiated cells within the osteoblast lineage. Moreover, they are found embedded in the lacunae (or bone lacunae) of the hard extracellular matrix (ECM). Lacunae are the spaces that contain a single osteocyte. The osteocyte has a flat and oval-shaped cell body, a slightly darker colored nucleus, and basophilic cytoplasm, with observable mitochondria and Golgi apparatus. Moreover, glycogen granules and lipid droplets can be revealed by special staining. Osteocytes have numerous slender processes, which extend into the small canals (called bone canaliculi) around the bone lacunae. That is, the bone canaliculi connect adjacent bone lacunae, which are channels containing the cytoplasmic processes of osteocytes (Yu et al., 2020). The most prominent feature of osteocytes is their cell processes, which extend within the bone canaliculi and connect with adjacent osteocytes. These structures allow osteocytes in the deep part to connect and communicate with the cell processes and bone canaliculi near the bone surface. These cell processes are connected via gap junctions (Doty, 1981; Shapiro, 1997). This huge three-dimensional network structure is called an OLCN (Burger and Klein-Nulend, 1999) (Figure 1B). The osteocyte OLCN has various crucial functions, such as mechanical sensing, bone remodeling balance, and homeostasis of body mineral metabolism (Repp et al., 2017). The bone canaliculi are transport channels for blood and nutrients within the bone. They supply nutrients and oxygen to the bone and are the only route for the bone tissue to communicate with the outside. Osteocytes in the bone matrix maintain the ecological balance within the bone by transporting nutrients and metabolic products via bone canaliculi and also provide a signal transmission pathway within the bone. Therefore, it was inferred that OLCN provides a suitable living environment, such as sufficient nutrients, oxygen, and an appropriate pH, for the invading S. aureus, enabling it to survive persistently in the OLCN and evade the immune system, which might explain the protracted course of chronic bone infections. The relationship between the OLCN environment and S. aureus survival warrants further research.
3 Clinical evidence of S. aureus invading the OLCN
It has been determined that most COM cases involve S. aureus infections (either single-microbe or multi-microbe). Despite aggressive surgical debridement and antibiotic treatment, recurrence remains common. The 2023 International Consensus Conference on Musculoskeletal Infections emphasized that eradicating residual bacteria is crucial for treating implant-related osteomyelitis (Jennings et al., 2024). As mentioned above, the invasion of S. aureus into the OLCN may render it a challenging pathogen in bone infections. This newly discovered unique immune evasion phenomenon of S. aureus can be initially traced back to a clinical case reported in 2018. This report described a patient with an infected diabetic foot ulcer complicated by COM due to S. aureus. Gram-staining confirmed the presence of Gram-positive bacteria in a fan-shaped pattern in the bone tissue adjacent to the bone marrow. Transmission electron microscopy (TEM) was used to describe that in the sub-micron OLCN of the amputated bone tissue, S. aureus transformed from spherical cocci into rod-shaped bacteria. This was the first evidence of the transformation of S. aureus and its invasion into the OLCN in human bones, supporting a new mechanism for the persistent existence of S. aureus in the pathogenesis of COM (de Mesy Bentley et al., 2018).
Subsequently, Louise Kruse Jensen et al. also studied two patients with COM (one with diabetic foot osteomyelitis and one with fracture-related infection). The microbiological test results of all patients were positive for Staphylococcus. Meanwhile, the clinical relevance of bacterial invasion into the sub-micron OLCN in bone tissue was confirmed through testing. Based on immunohistochemistry and electron microscopy, S. aureus was identified in the OLCN of all patients. These findings solidified that bacterial OLCN invasion is a clinically relevant part of the disease biology of osteomyelitis (Jensen et al., 2023), especially regarding osteomyelitis recurrence. The literature mentioned above represents the only conclusive clinical evidence of S. aureus invading OLCN. It provides new insights into immune evasion and persistence during S. aureus osteomyelitis and indicates a new direction for future research, thus being of great value. However, they merely point out the real-world phenomenon of S. aureus invading OLCN during osteomyelitis without further exploring the reasons for its invasion, the specific biological processes, and mechanisms in combination with clinical cases. Many subsequent studies mainly rely on in-vitro platforms and animal models, which may differ from clinical cases and cannot be fully used to guide future clinical practice. Therefore, continued in-depth research in combination with cases of chronic bone infection caused by S. aureus may also be one of the most promising research directions in the future.
4 The OLCN invading mechanism of S. aureus
For chronic bone infection, the extensive and thorough debridement, irrigation, removal of all implants in traditional revision surgeries, and systemic antibiotic therapy are effective against planktonic bacteria, SACs, and surface biofilms; however, these measures remain ineffective against the bacteria in OLCN (Ren et al., 2022; Ren et al., 2023). S. aureus can invade OLCN by various mechanisms, making chronic bone infections more difficult to treat. Firstly, the OLCN is located deep within the bone cortex in the bone mineral matrix, an environment that restricts immune-cell-mediated immune surveillance, making it completely immune to attacks from immune cells. Further, osteocytes have a long life and can survive in the body for decades (Prideaux et al., 2016). These factors make OLCN a perfect site for the persistent survival of S. aureus. Secondly, bacteria may survive for years by dissolving the surrounding bone mineral matrix as a source of nutrients. Lastly, the depth to which S. aureus invades the OLCN remains unclear; however, it might be a primary factor responsible for the failure of surgical removal of bone infection foci. In summary, theoretically, amputation may be the only treatment approach to eradicate S. aureus in the OLCN of living bone tissue (Masters et al., 2020). Therefore, a comprehensive investigation of the mechanisms by which S. aureus invades the OLCN is required for the development of novel treatment strategies and for reducing the recurrence and disability rate associated with chronic bone infections.
Bone canaliculi have about 0.5 μm diameter and connect osteocyte lacunae (You et al., 2004); however, the diameter of most clinically relevant bacteria is much larger (for example, S. aureus is 1 μm in diameter). Therefore, it is generally believed that bacteria cannot enter the OLCN, and indeed, the OLCN invasion by S. aureus is challenged by morphological variation. However, the literature suggests that for OLCN invasion, S. aureus transforms from cocci to rod-shaped bacteria with a diameter less than half its original size (de Mesy Bentley et al., 2017; Yu et al., 2020). Furthermore, since S. aureus has no flagella, its invasion of OLCN is also contrary to the consensus that S. aureus is a non-motile coccus (Masters et al., 2022). These findings have attracted many scholars to study the mechanism by which S. aureus enters the OLCN after morphological variation.
De Mesy Bentley et al. performed transmission electron microscopy (TEM) and revealed that S. aureus (UAMS-1, USA300 LAC) exists as a single cocci and sub-micron rod-shaped bacteria in the canals of the living cortical bone of mice and forms biofilms in the osteocyte compartments. They also found that the deformed bacteria can enter the canals via asymmetric binary fission, proliferate along the osteocyte lacunae edges, and migrate into the lacunae’s interior (de Mesy Bentley et al., 2017). Another study suggests that S. aureus invades OLCN by stimulating its cell-wall synthesis mechanism and surface adhesins under the guidance of durotaxis (Raimon et al., 2016) and haptotaxis (Hsu et al., 2005), respectively. To identify the virulence genes responsible for haptotaxis and durotaxis, an in-vitro platform model, the microfluidic silicon membrane-canal array (μSiM-CA), was developed (de Mesy Bentley et al., 2017; Elysia et al., 2019). This model mimics canaliculi’s physiological dimensions and determines the genes required for S. aureus to invade the canaliculi. It has been observed that the Agr quorum-sensing system may not be necessary for the in-vitro transmission of S. aureus (UAMS-1) through 0.5-micrometer nanopores (Elysia et al., 2019). Furthermore, in a murine implant-related osteomyelitis model, Agr gene knockdown S. aureus [UAMS-1 agr-null strain (UAMS-1Δagr.:tetM)] invaded the sub-micron canal network of bone (Gowrishankar et al., 2019). Penicillin-binding proteins 3 and 4 (PBP3 and PBP4) genes encode the non-essential cell-wall transpeptidases, PBP3 and PBP4, respectively, which function in the final stage of cell-wall synthesis (Costa et al., 2018). Elysia A Masters et al. employed the μSiM-CA platform model to screen the S. aureus transposon-insertion mutant library (Elysia et al., 2019; Masters et al., 2020) and indicated that PBP4 gene deletion significantly inhibited the in-vitro transmission of S. aureus (USA300) through nanopores, inhibited S. aureus invasion of OLCN, and reduced the degree of bone loss at the infection site in a murine model of implant-related osteomyelitis. Moreover, they investigated the in-vitro nanopore transmission and in-vivo osteomyelitis pathogenesis of selected S. aureus cell wall synthesis mutants. The results of the in-vitro study showed that the deletion of cell-wall synthesis mutants [PBP3, autolysin (Atl)] and surface adhesin mutants [clumping factor A (ClfA) and SasC] inhibited S. aureus transmission through nanopores. Furthermore, in a murine implant-related osteomyelitis model, PBP3 and Atl deletion [USA300 pbp3-null (Δpbp3) and atl null (Δatl)] reduced the loosening of infected implants and the formation of S. aureus abscesses in the bone marrow cavity, while the deletion of surface adhesins had no significant difference. TEM imaging revealed that PBP3 was the only mutant gene associated with reduced in vivo sub-micron canal invasion (Elysia et al., 2021). These in-vitro and in-vivo analyses confirmed that PBP3 and PBP4 are key genes for S. aureus’ sub-micron transmission and OLCN invasion (Masters et al., 2020). Therefore, inhibiting PBP3 and/or PBP4 may prevent S. aureus invasion of OLCN, and their inhibitors can be used as adjuvants in the antibacterial treatment of S. aureus osteomyelitis (Masters et al., 2022). Overall, the cell wall synthases of S. aureus are crucial for in vivo OLCN invasion and osteomyelitis pathogenesis.
The survival of bacteria requires cell wall protection, and the main structural unit of the bacterial cell wall is peptidoglycan (also called mucopetide), a unique component that is composed of sugars and amino acids. Peptidoglycan is a derivative of heteropolysaccharide and is considered “bricks,” which are crucial for maintaining cell morphology, integrity, mechanical strength, and the survival of bacteria (Vollmer et al., 2008a; Silhavy et al., 2010; Turner et al., 2014). However, peptidoglycan alone is insufficient, and to build a wall using these bricks, penicillin-binding proteins (PBPs) are required, which act as “cement.” The PBPs can cross-link peptidoglycan into a complete cell wall and are involved in the final assembly stage of peptidoglycan into the bacterial cell wall. The repair of S. aureus cell wall has four endogenous PBPs (PBP1, PBP2, PBP3, PBP4). Of these, only PBP1 and PBP2 are required for peptidoglycan synthesis, as they can perform all the transpeptidase (side-chain-linking) functions required for cell growth and division (Pinho et al., 2001; Pereira et al., 2007; Eric et al., 2008; Katarzyna et al., 2022). PBP3 and PBP4 are non-essential, monofunctional PBPs with only transpeptidase activity (Pinho et al., 2000; Scheffers and Pinho, 2005). PBP3 is a class B high-molecular-weight PBP, and its role in the S. aureus cell cycle remains elusive (Yoshida et al., 2012; Kylväjä et al., 2016). PBP4 also has a low molecular weight and is the only PBP responsible for the extensive peptidoglycan cross-linking in the S. aureus cell wall (Navratna et al., 2010). Peptidoglycan hydrolases balance the function of PBPs by degrading the cell wall during growth and division. Atl is the major peptidoglycan hydrolase of S. aureus, which consists of two subunits: amidase (Amd) and glucosaminidase (Gmd) (Vollmer et al., 2008b). It participates in cell wall hydrolysis during cell division (Clarke and Foster, 2006; Vollmer et al., 2008b) and also binds ECM ligands as a surface adhesin. Therefore, it was proposed that Atl may be involved in haptotaxis and durotaxis functions (Clarke and Foster, 2006; Bose et al., 2012; Hirschhausen et al., 2012; Schlesier et al., 2020), which may be related to the invasion of OLCN by S. aureus (Elysia et al., 2021).
Theoretically, S. aureus must deform and divide into smaller-size/diameter cells to invade OLCN. Bacterial cell division (mainly binary fission) is a relatively complex process, where during DNA molecule separation, the cell membrane and wall grow inward to form a septum, dividing the cytoplasm into two halves. Other than peptidoglycan (the main structural component of the septum) and PBPs, the cell wall of S. aureus contains other crucial components, including tyrosine, β-glucosidase, lactic acid, and lactate dehydrogenase. These components coordinate with each other to form special cell wall structures and perform various functions, such as cell protection, cell shape maintenance, and drug resistance. Furthermore, they also modulate the formation of the septum and cell division in terms of space and time. An in-depth evaluation of the S. aureus cell wall components will help understand their biological characteristics and application value.
Currently, the research on the mechanism of S. aureus invading OLCN is mostly based on in-vitro simulation platforms and murine osteomyelitis model experiments, and the OLCN invading mechanism of S. aureus in clinical chronic bone infection patients remains undetermined. Therefore, it is crucial to research clinical cases to comprehensively understand the underlying mechanism and better prevent the occurrence and development of chronic bone infections.
5 Treatments for S. aureus invasion of OLCN
Due to the lack of research on the colonization of S. aureus in the OLCN and the evidence of clinical efficacy, the use of local antibiotics to treat bone infections is being questioned. Researchers have searched PubMed using “osteocyte lacuno-canalicular network” and/or “OLCN” as keywords, to investigate the treatment of S. aureus invasion of the OLCN, which revealed the following three aspects (Figure 2).
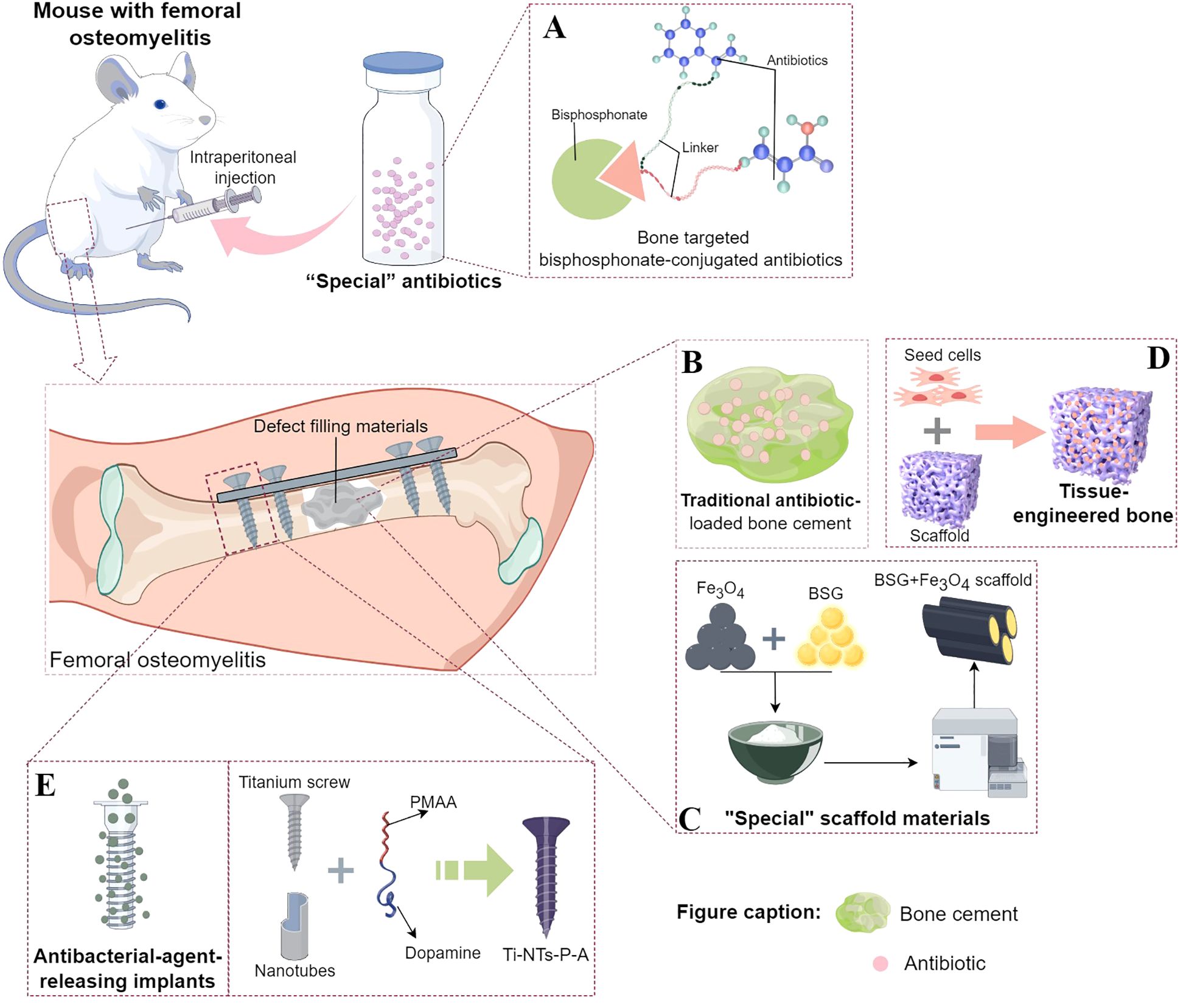
Figure 2. Existing treatment strategies targeting the immune escape mechanism of Staphylococcus aureus invasion into the OLCN. (A) The composition of “special” antibiotic, bisphosphonate-conjugated antibiotics. Part of the design was cited from Reference 74 and redrawn. (B) The basic structure of traditional antibiotic-loaded bone cement. (C) A schematic diagram taking the “special” scaffold material composed of borosilicate bioactive glass (BSG) + Fe3O4 magnetic nanoparticles as an example. Part of the design was cited from Reference 77 and redrawn. (D) The composition of tissue - engineered bone, including seed cells and scaffolds. (E) A schematic diagram of the implant material capable of sustained - release of antibacterial agents, taking Ti - NTs - P - A as an example. Part of the design was cited from Reference 90 and redrawn.
5.1 Development of antibiotics with “special functions.”
Since the systemic use of antibiotics generally fails to completely eradicate bacteria within the OLCN (Theuretzbacher et al., 2020), researchers have evaluated the local bactericidal concentration of “special” antibiotics in the bone infection area. Adjei-Sowah, E. et al. used fluorescent bisphosphonate probes in a murine S. aureus (UAMS-1 and USA300LAC)-infected tibia model and indicated labeling of the bone surface near the bacteria. They proposed that bisphosphonate-conjugated antibiotics (BCA) application and a “targeting and releasing” can better deliver antibiotics to the site of bone infection. Bisphosphonic acid and hydroxy-bisphosphonate-conjugated antibiotics of sitafloxacin and tedizolid were synthesized using hydroxyphenyl and aminophenyl carbamates, respectively. These conjugates indicated significant serum stability. Furthermore, Sitafloxacin O-phenyl carbamate BCA successfully eradicated static biofilms, whereas the less-stable tedizolid N-phenyl carbamate BCA had limited efficacy against Methicillin-Sensitive S. aureus (MSSA) and MRSA. These results prove that BCA can efficiently eradicate S. aureus biofilms on the OLCN bone surface and support the in-vivo drug development of sitafloxacin BCA (Adjei-Sowah et al., 2021) (Figure 2A). Ren Y et al. also employed the “targeting and releasing” kinetic approach to propose the design of two bone-targeting bisphosphonate-conjugated antibiotics, bisphosphonate-conjugated sitafloxacin (BCS) and hydroxy-bisphosphonate-conjugated sitafloxacin (HBCS). They evaluated the in-vivo efficacy of BCS and HBCS relative to bisphosphonate, sitafloxacin, and vancomycin in a murine implant-related osteomyelitis model. The vancomycin, sitafloxacin, and placebo groups indicated the presence of autolytic bacteria of colonized S. aureus (USA300LAC) in the OLCN of infected tibias of mice, whereas the abundance of most bacteria in the OLCN of infected tibias of BCA-treated mice was relatively low. Compared with the placebo and free-antibiotic controls, BCA also significantly increased the OLCN diameter. These findings support the bone-targeting strategy of BCA to overcome the bi-distribution limitation of standardized antibiotic treatment, which has difficulty accessing the OLCN (Ren et al., 2022).
5.2 Development of ideal bone defect filling and repair materials
Chronic bone infections are often accompanied by extensive bone defects. Therefore, it is critical to develop bone defect-filling materials that possess an appropriate elastic modulus to eliminate dead space, exhibit good antibacterial and osteogenic activities, and are degradable. Since the 1960s, antibiotic-loaded bone cement has been the primary choice for the clinical treatment of infected bone defects and was once widely regarded as a standard strategy (Jiranek, 2005; Schwarz et al., 2021) (Figure 2B). However, there are several limitations to antibiotic-loaded bone cement, such as incompletely eradicating biofilm-associated bacteria, not repairing bone defects, and being non-biodegradable, necessitating a second-stage surgery. Biomaterials for treating bone infection defects mainly fall into the following categories: bioactive glass (with intrinsic antibacterial, osteoconductive, and angiogenic properties), antibiotic-impregnated calcium-based bone substitutes and calcium phosphates (whose chemical and crystal structures are similar to the inorganic components of bone and have a good infection control rate, yet attention should be paid to the degradation rates of different materials), and polymers (natural polymers such as collagen, chitosan, and gelatin are hydrophobic and highly biocompatible, with the main drawback of being prone to trigger immune responses. Synthetic polymers have a longer shelf life, uniform microstructure, and good mechanical strength, but they can be divided into degradable and non-degradable types) (Jiang et al., 2024). For example, a BSG + Fe3O4 magnetic scaffold was developed based on the bactericidal properties of magnetic Fe3O4 nanoparticles in an alternating magnetic field, combined with the excellent osteogenic induction and immunomodulatory properties of borosilicate bioactive glass (BSG), (Figure 2C). The antibacterial and osteogenic properties of the BSG + Fe3O4 magnetic scaffold against S. aureus (ATCC29213) bone infections were evaluated in vitro and in vivo. The results showed that the BSG + 5% Fe3O4 magnetic scaffold enhanced the osteogenesis of mesenchymal stem cells (MSCs) and promoted the polarization of macrophages to the M2 type in vitro. The rabbit implant-related S. aureus bone infection model also confirmed that this magnetic scaffold had improved antibacterial effects at the implantation site, effectively controlling the Staphylococcus abscess communities and S. aureus within the OLCN. Furthermore, it also promotes new bone formation around the primary infected site, thereby effectively addressing the treatment challenges in the infected bone defects. Moreover, the degradation rate of this bioactive scaffold can effectively match the bone formation rate (Jin et al., 2024).
Other than antibiotic-loaded bone cement, autologous or allogeneic bone is also the optimal source for repairing infected bone defects because of its good osteogenic activity. However, the clinical application of autologous or allogeneic bone is often restricted by several factors, such as donor shortage, discomfort at the bone-harvesting site, infection, and bleeding (Mitra et al., 2017; McNeill et al., 2020). Furthermore, it cannot achieve local anti-infection simultaneously. Therefore, the development of novel bone-repair materials with excellent mechanical properties, osteogenic activity, and local anti-infection capabilities has become a research hotspot. With the rapid advancements in cell technology, biomimetic materials, and microsurgical techniques, the development of tissue-engineered bone (TEB) has significantly progressed and can also be employed as a bone-repair material to treat infected bone defects (Qin et al., 2024). The TEB comprises scaffold materials, seed cells, and cytokines (Figure 2D). The scaffold material is the core component of TEB because all other components are loaded onto the scaffold to function. Based on the basic mechanical support requirements, an ideal scaffold system for bone infection repair should provide infection treatment with bone defect regeneration. The composite scaffold systems for infected bone defect repair are categorized into hydrogel scaffolds and solid bone tissue substitutes. The hydrogel scaffolds are loaded with stem cells, growth factors, and nanoparticles, whereas the solid bone tissue substitutes mainly include growth factors, nanoparticles, exosomes, and stem cells (Qin et al., 2024). In the treatment of bone infections, hydrogel scaffolds can load bioactive molecules such as antibiotics and growth factors. These molecules can exert their biological effects through diffusion and osmosis, thus effectively inhibiting the growth and reproduction of bacteria. At the same time, they can promote the repair and regeneration of bone tissue and alleviate bone infections. For example, studies have shown that hydrogel scaffolds loaded with rat bone marrow mesenchymal stem cells (rBMSCs) (Bastami et al., 2024) and adipose-derived stem cells (ASCs) (Tang et al., 2020) have excellent adhesion, proliferation, and differentiation abilities, and significant new bone regeneration ability. Hydrogel scaffolds loaded with growth factors such as bone morphogenetic protein (BMP) and stromal cell-derived factor-1 (SDF-1) enhance bone regeneration (Ratanavaraporn et al., 2011). Hydrogel scaffolds loaded with nanoparticles can achieve a gradual and sustained release of antibiotics by incorporating antibiotics into the hydrogel and then embedding the hydrogel in the nanoparticles. They have good injectability and antibacterial activity, thus achieving the purpose of preventing bone infections (Posadowska et al., 2016; Loebel et al., 2017; Wassif et al., 2024). Solid scaffolds, on the other hand, have advantages such as stable shape, complex structure, and diverse functions. Most importantly, they can provide support and elastic modulus that match the mechanical properties of bone tissue through material optimization. Therefore, in the strategy for treating infectious bone defects, it is also a very good choice to use functional-integrated solid scaffolds for filling and treatment. Most solid implants usually require modification of their surface itself to address issues such as bacterial infections in bone infections (avoiding bacterial adhesion, killing bacteria, and reducing biofilm formation), inflammatory responses, and bone regeneration (Epstein et al., 2011; Vlamakis et al., 2013; Ghosh et al., 2019). However, currently, there are no studies on specifically eradicating S. aureus in bone microstructures using TEB methods, and such future research on bone infection treatment via TEB technology could focus on loading targeted antibiotics onto matrix materials with different properties and simultaneously choosing appropriate scaffold preparation methods. The treatment goal is to clear bacteria in biofilms and OLCN to promote infection eradication and bone regeneration. This approach can overcome the bottleneck of traditional treatment methods for bone infection and defects, which often require multiple surgeries.
5.3 Development of internal fixation materials with anti-infection functions
In the surgical treatment of infected bone defects, after thorough debridement, internal fixators are usually required to firmly fix the bone stumps (or the bone defect area) for good mechanical stability, which is conducive to bone repair. However, the internal fixator’s surface is a prone site for bacterial biofilm formation (Zimmerli et al., 2004; Yousif et al., 2015), which acts as a barrier, creating a stable environment for bacterial growth. The biofilm protects bacterial cells from extreme conditions such as high temperatures, nutrient deficiency, pH changes, and antibiotics. Furthermore, systemic antibiotics often fail to completely eradicate bacteria within the biofilms and OLCN (Theuretzbacher et al., 2020). Therefore, it is essential to elucidate methods that allow internal fixators to locally release sufficient concentrations of antibiotics in the infected area to effectively combat bacteria while minimizing systemic toxicity (Birk et al., 2021; Sayed et al., 2022; Wang et al., 2022). Therefore, various local antibacterial release systems have been developed to treat bone infections, specifically in titanium alloy implants widely used in orthopedics (Wu et al., 2021; Zhang et al., 2023; Zhou et al., 2023). Previous literature suggests that the acidic environment in a bacterial infection can be employed to develop a pH-responsive antibacterial surface on implants (Chen et al., 2020; Zhang et al., 2023; Zhou M. et al., 2024). Some scholars have successfully synthesized pH-responsive polymethacrylic acid (PMAA)-gated TiO2 nanotubes on titanium plates. The PMAA molecules provide an on-demand release of antibacterial peptides through the “swelling-collapse” transition (Chen et al., 2020). Moreover, to improve the nanotube’s stability, a new type of screw was developed by preparing enhanced TiO2 nanotubes on titanium screws; it immobilizes PMAA and loads the antibacterial peptide HHC36 (Figure 2E). In an acidic infected environment, this novel screw indicated significant pH-responsiveness and enhanced antibacterial effect by the on-demand HHC36 release. The simulated clinical implantation process has indicated that this novel screw can maintain excellent pH-responsive antibacterial performance under mechanical stress (Zhou H. et al., 2024). Further, the research also revealed that the antibacterial peptide HHC36 completely eradicates the residual bacteria in SACs and OLCN (Zhou H. et al., 2024). Altogether, the development of internal fixators with controlled and on-demand sustained release of antibiotics can effectively eradicate bacteria that have invaded the OLCN, providing a new direction for the prevention and treatment of recurrent chronic bone infections.
6 Discussion and foresight
The invasion of bacteria into the OLCN of bone tissue is the basis of COM pathology and is crucial for clinical treatment, especially for recurrent osteomyelitis. The three-dimensional structure of OLCN indicates that it is an ideal “refuge” for S. aureus. Firstly, osteocytes, the main OLCN components, are enclosed in the bone canaliculus network and send out cellular processes that extend into the canaliculi to connect adjacent osteocytes. In vitro studies have indicated that S. aureus can invade and survive within osteocytes without causing cell death (Yang et al., 2018). Furthermore, during osteocyte infection, S. aureus adapts to the environment by adopting a survival mode similar to that of small-colony variants (SCVs), thereby supporting persistent or occult infections. Clinical case studies have also confirmed significant intracellular colonization of S. aureus in osteoblasts and osteocytes in chronically infected bone tissues (Bosse et al., 2005; Sendi et al., 2006) than other cell types, such as macrophages (Garzoni and Kelley, 2011), epithelial cells (Dziewanowska et al., 1999), keratinocytes (Kintarak et al., 2004), and endothelial cells (Edwards et al., 2010). S. aureus infection of osteocytes is particularly pathogenic. S. aureus intracellularly infects osteoblasts, which, upon differentiation or maturation into osteocytes, serve as a reservoir for long-term bacterial colonization in bone tissue and easily evade the immune system (Alder et al., 2020). Furthermore, S. aureus can induce the secretion of osteoclast-related cytokines, which promote pathological bone loss. Moreover, large-sized bacteria like S. aureus (1 μm diameter) can change their shape and reduce their volume by nearly half to penetrate the sub-micron-level (0.5 μm diameter) interstitial channel network in the dense bone structure (You et al., 2004; Cai et al., 2022). This allows them to survive for an extended period and evade the surveillance of immune cells. Although bromodeoxyuridine labeling studies on mice have revealed that orally administered small molecules can reach S. aureus colonized within the OLCN (Sultan et al., 2019), other studies suggest that the combined use of high-dose local and systemic antibiotics fails to eradicate OLCN invasion by MSSA (Veis and Cassat, 2021) and MRSA (Masters et al., 2020). This might be because their adaptive responses are associated with persistent intracellular survival and SCV formation (Schwarz et al., 2021). Lastly, the small-sized S. aureus SCVs that invade and survive within osteocytes may also be secreted into the osteocyte lacunae and canaliculi. The survival of S. aureus in such bone microstructures makes them a continuous source of occult infection. Therefore, new treatment methods targeting the morphological changes of S. aureus (such as transformation into SCVs) during its invasion of the OLCN should be developed for effective COM treatment.
6.1 OLCN is a potential “special” niche for the persistent S. aureus SCV survival
S. aureus SCVs are a special bacterial phenotype that grows slowly and forms tiny colonies on agar plates. Compared to the normal S. aureus colonies, their diameter is significantly smaller (the colony size is only approximately one-tenth of wild-type bacterial colonies) (Proctor et al., 2006; Nguyen et al., 2009; Garcia et al., 2013). They were first reported approximately 100 years ago and were described as naturally occurring populations, typically the “G” type or “dwarf” colonies of many bacterial species, including S. aureus (Eiff et al., 2006; Proctor et al., 2006). Due to the special S. aureus SCVs morphology and significantly reduced metabolic rate, they have markedly reduced sensitivity to antibiotics, making their detection and identification difficult in routine testing and culturing conditions. As a result, they are associated with persistent and recurrent infections, which have been extensively studied (Proctor et al., 2006).
The identification of factors that induce the formation of S. aureus SCVs is significant for preventing chronic S. aureus bone infections, specifically for reducing the entry of S. aureus into the OLCN and its survival as SCVs. Some early studies considered SCVs as gonidial mutants or “G” forms that develop within specific mother cells under adverse conditions. They are extremely small and may represent the primitive stage of the bacterial life cycle (Swingle, 1935; Wise and Spink, 1954). Recent reports isolated “dwarf” colonies from animals and humans after antibiotic treatment (Goudie and Goudie, 1955; Sompolinsky et al., 1974). Previous literature on SCVs of different bacterial species has indicated that SCV formation is a natural survival mechanism for many bacteria and is mostly generated under selective conditions such as antibiotic treatment, cold stress, disinfectant exposure, or within eukaryotic cells (Atalla et al., 2011). Therefore, it is essential to further explore the inducing factors and underlying mechanisms of this bacterial phenotype. The small volume of SCVs may help S. aureus enter bone lacunae and canaliculi from osteocytes. The identification of the relationship between the formation of S. aureus SCVs and OLCN invasion may help in the development of targeted treatment strategies for the SCV phenotype. This mechanism can reduce the bacterial load in the OLCN, thereby decreasing the incidence of persistent infections.
6.2 Sub-minimum inhibitory concentration antibiotics may play a crucial role in SCV formation and OLCN invasion
6.2.1 Clinically, the antibiotics for treating COM caused by S. aureus are primarily administered locally or systemically
The commonly used systemic antibiotics include β-lactams, clindamycin, and fluoroquinolones (Tuchscherr et al., 2016). The minimum inhibitory concentration (MIC) is defined as the lowest concentration of an antibiotic that can inhibit the pathogenic bacteria growth in the culture medium after 18 to 24 hours of in vitro bacterial culture. MIC measures the antibacterial activity of antimicrobial agents. Theoretically, when the drug concentration reaches the MIC, bacterial growth and reproduction will be inhibited. In vivo, if the drug concentration at the infection site is continuously maintained at or above the MIC, and the bacteria becomes sensitive to the drug, thereby gradually relieving the patient’s symptoms, such as fever, redness, swelling, and pain. Furthermore, inflammatory indicators, including white blood cell (WBC) count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT), also gradually decrease to the normal range. Moreover, the body’s immune system slowly eliminates the inhibited bacteria, thus achieving the goal of the treatment. However, clinically, the blood and local drug concentrations at the infection site cannot reach the MIC due to various reasons, thus forming sub-minimum inhibitory concentration (sub-MIC). Antibiotics at sub-MIC have no inhibitory effect on bacterial growth and induce metabolic and morphological changes in the bacteria. To cope with the drug pressure, bacteria produce some proteins or enzymes to resist the drug’s action and maintain their survival, which may lead to drug resistance. The literature points out that the action of antibiotics at sub-MIC can promote three main types of morphological changes in S. aureus, including cell morphological deformation, cell wall component alteration, and cell wall rupture (Braga et al., 1997; Mette et al., 2013; Guanghui et al., 2014). In 2021, Juan Chen et al. reviewed the impact of sub-MIC antibiotics on S. aureus morphology (Juan et al., 2021). They evaluated the impact of antibiotics, including dicloxacillin, cefodizime, cefotaxime, ceftriaxone, cefodizime, ciprofloxacin (CFX), berberine, tetracycline, thioridazine, and ceftobiprole at sub-MIC on the morphological variations of S. aureus. They revealed varying degrees of morphological alteration, such as increased volume, irregular deformation, damaged and ruptured cell walls, increased cell membrane permeability, and decreased adhesiveness. Moreover, they showed that antibiotics at different sub-MIC may have different effects on S. aureus’s cell morphology (Juan et al., 2021). Furthermore, certain sub-MIC antibiotics (such as methicillin and cefoxitin) can weaken the cell wall of S. aureus by binding to PBPs (Shang et al., 2019). Therefore, it was speculated that S. aureus morphological variations are caused by sub-MIC antibiotics, and their binding with PBPs may be associated with the invasion of S. aureus into the OLCN. However, further exploration and verification are warranted.
6.2.2 The sub-MIC antibiotics also affect S. aureus adhesion and invasion
Research has shown that the pathogenicity of S. aureus is markedly linked with its ability to adhere to host cells or the ECM (Josse et al., 2017). Similarly, S. aureus’s entrance into osteocytes and canaliculi is important for its adhesion and colonization in the OLCN. The adhesion molecules responsible for S. aureus adhesion mainly include clumping factors A and B, staphylococcal fibrinogen-binding proteins A and B, serine-aspartate repeat-containing protein D, etc. (Zheng et al., 2020). Juan Chen et al. revealed that the effects of sub-MIC antibiotics on the adhesion and invasion of S. aureus may vary depending on the bacterial strains and host cell models used (Juan et al., 2021). Furthermore, several studies have indicated that the effects of sub-MIC antibiotics on the adhesion of different S. aureus strains also vary. Some strains indicate an enhanced adhesion effect, while others may show decreased adhesion ability. Further in-depth investigation of the effects of sub-MIC antibiotics on the adhesion and invasion of S. aureus is crucial for the proper application of antibiotics and the reduction of the bacterial load in the OLCN.
6.2.3 Local application of antibiotics in the bone infection site is also a critical means and classic strategy for treating COM caused by S. aureus
The advent of antibiotic-loaded bone cement is advantageous for repairing infected bone defects by eliminating dead spaces and promoting antibacterial effects locally. Therefore, it is widely used for the clinical treatment of infected bone defects. Antibacterial drugs in bone cement should have thermal stability, water-solubility, a broad antibacterial spectrum, high antibacterial efficacy, few naturally resistant bacteria, less binding to proteins, low allergenicity, no systemic toxic reactions, and minimal impact on the mechanical strength of the bone cement. Currently, the antibiotics employed in antibiotic-loaded bone cement depend on the types of infected microorganisms and primarily include gentamicin, tobramycin, and vancomycin powder (Saeed et al., 2019). However, there are certain disadvantages of using antibiotic-loaded bone cement such as when the local antibiotic concentration fails to reach the MICs, it promotes the formation of SCVs. L. Tuchscherr et al. showed that in vitro, rifampicin can almost clear infected osteoblasts in acute and chronic phases. Whereas low-concentration of gentamicin, moxifloxacin, and clindamycin can induce SCV formation. Moreover, gentamicin, fosfomycin, and clindamycin promote the rapid formation of SCVs within osteoblasts, which may result in chronic infections. The acute and chronic mouse osteomyelitis model revealed that during the acute phase, only rifampicin significantly reduced the bacterial load in bone tissue, while cefuroxime and gentamicin showed poor effects. Moreover, gentamicin induces the formation of SCVs (Tuchscherr et al., 2016), thus confirming that gentamicin is a potent inducer of SCVs.
Based on the above three aspects, about 70% of patients receiving long-term antibiotic treatment may develop S. aureus SCVs infections (Melter and Radojevič, 2010). Therefore, the role of sub-MIC antibiotics (especially those like gentamicin) in promoting the formation of S. aureus SCVs should not be ignored, as it significantly impacts the promotion of persistent and recurrent infections in clinical practice. Furthermore, the SCVs-inducing activity of antibiotics such as gentamicin should be considered specifically during the treatment of infected bone defects. Since antibiotic-loaded bone cement is often applied locally as a surface coating for internal fixators or a filler for defect areas, antibiotics like gentamicin should be used in higher concentrations to achieve a bactericidal effect. Therefore, since sub-MIC gentamicin can induce SCV formation, its doses in host cells, bone defect areas, or bone tissues should be monitored. The rapidly formed SCVs promote prolonged bacterial survival in the host. The persistent survival, adhesion, and colonization of S. aureus in osteocytes and canaliculi are the main causes of COM. In the future, the relationship and mechanism of action between S. aureus SCVs induced by sub-MIC antibiotics and OLCN invasion should be comprehensively investigated. Developing a bone-targeted antibiotic with a local concentration that can exceed the MIC is significant for the treatment of deep bone infections.
6.3 OLCN may provide a suitable environment for the survival of S. aureus
The relatively closed OLCN microenvironment provides stable metabolic support and suitable survival conditions for pathogenic bacteria (Moriishi and Komori, 2022; Evans et al., 2024). The pH of OLCN plays a crucial role in the osteocyte’s function and also provides favorable conditions for bacteria invasion. Recently, several researchers from the bone biology field have investigated the pH of the OLCN microenvironment. The literature suggests that under normal physiological conditions, the OLCN’s pH remains relatively stable and slightly alkaline, around 7.4 - 7.6. The metabolic activities of osteocytes and the composition of the surrounding tissue fluid jointly maintain the pH in the OLCN. During metabolic processes such as aerobic respiration, osteocytes produce carbon dioxide, which combines with water to form carbonic acid, which in turn dissociates to produce hydrogen and bicarbonate ions. The hydrogen ion concentration is finely regulated by the intracellular buffer system and ion transport mechanisms, keeping the OLCN’s pH within an appropriate range. For example, carbonic anhydrase in osteocytes can catalyze the reaction between carbon dioxide and water, whereas the cell membrane transport proteins and ion channels transport bicarbonate and hydrogen ions across the transmembrane, thereby maintaining the intra- and inter-cellular acid-base balance. In infectious diseases such as implant-related S. aureus osteomyelitis, the metabolic products of bacteria and the inflammatory response may decrease OLCN’s pH. S. aureus produces acidic substances, such as lactic acid and acetic acid, during metabolism. The local accumulation of these acidic metabolic products can reduce OLCN’s pH to 6.5 or even lower. Furthermore, inflammatory mediators such as TNF-α and IL-1 released by inflammatory cells can stimulate osteoclasts and promote bone resorption. Osteoclasts secrete acidic substances, including hydrogen ions, during bone resorption, which further exacerbates the acidic environment in the OLCN (Zhou H. et al., 2024). The pH changes in OLCN’s environment affect the normal functions of osteocytes. It may promote bacterial survival and reproduction, forming a vicious cycle and exacerbating the infection. In an acidic environment, osteocyte’s metabolic activities are inhibited, their ability to synthesize and secrete bone matrix reduces, and their survival is threatened. For bacteria, although a lower pH environment can inhibit their growth to a certain extent, some bacteria, such as S. aureus, have acid tolerance and can not only survive but also cause persistent infections in the acidic OLCN. For instance, the acidic environment (pH 3-5) of bone resorption lacunae may inhibit the functions of complement and antimicrobial peptides. It has been observed that in pH < 6, the synthesis of complement C3 and C5 convertases is inhibited, which weakens the complement cascade reaction, including C3b deposition and C5a generation (Hany Ibrahim et al., 2015). Antimicrobial peptides, defensins, rely on their cationic properties to bind to the bacterial membrane and form pores. An acidic environment alters the cationic characteristics of these peptides and weakens their bacterial cell membrane binding ability, thus reducing their bactericidal effect (Mahmoud H et al., 2014). Similarly, the acidic environment may enhance pathogenic bacteria’s tolerance to acid stress by inducing the outer-membrane proteins of pathogenic bacteria (such as ClfA in S. aureus) or lipid-metabolic pathways. The hypoxic environment in the bone microstructure may induce a dormant state in pathogenic bacteria, which reduces energy consumption and enhances their tolerance to the immune system to adapt to the hypoxic environment. The literature also revealed that the acidic OLCN environment reduces the body’s immune function, decreases the bactericidal effect of antimicrobial peptides, and provides a favorable survival environment for pathogenic bacteria such as S. aureus. There is research on the association between the pH environment of the OLCN and S. aureus; however, several factors require further investigation. In future research, the whole-genome expression profiles and proteomic changes of S. aureus under different pH OLCN environments should be analyzed to comprehensively understand its survival-adaptation and pathogenic mechanisms. Further, more precise and efficient pH-regulation technologies and combination therapies, as well as pre-clinical and clinical trials, are required to translate the research results into clinical applications and provide better treatment options for bone infection patients.
7 Conclusion
In conclusion, both clinical case reports and some animal model studies have indicated that the invasion of S. aureus into OLCN is one of the important mechanisms that cannot be ignored for its immune escape and the persistence of bone infections, which are difficult to heal. Existing studies have shown that various mechanisms mainly involving the components of the cell wall of S. aureus are involved in its morphological variation and promote its invasion into OLCN. In view of this special mechanism of S. aureus entering OLCN, innovative strategies and techniques for treating bone infections have been proposed from three aspects, namely, improving antibiotics, developing new bone defect repair materials, and implant materials. Meanwhile, the interaction among osteocytes, antibiotics at sub-MIC, and the formation of SCVs may play an important role in the invasion of the bone microstructure by S. aureus. This not only provides a new perspective for understanding chronic bone infections but also opens up new avenues for their treatment, and promotes the research in this field to develop in a more precise direction. Through this comprehensive perspective, we earnestly expect to achieve significant breakthroughs in the treatment of bone infections in the future, thereby effectively improving the treatment outcomes and quality of life of patients.
Author contributions
ZR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. XC: Data curation, Investigation, Writing – original draft. LQ: Conceptualization, Data curation, Software, Writing – original draft. XW: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft. FL: Conceptualization, Methodology, Resources, Validation, Writing – original draft. QZ: Conceptualization, Supervision, Visualization, Writing – review & editing. HZ: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by grants from the National Natural Science Foundation of China (No. 32270989).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adjei-Sowah, E., Peng, Y., Weeks, J., Jonason, J. H., Bentley, K., Masters, E., et al. (2021). Development of Bisphosphonate-Conjugated Antibiotics to Overcome Pharmacodynamic Limitations of Local Therapy: Initial Results with Carbamate Linked Sitafloxacin and Tedizolid. Antibiotics. 10 (6). doi: 10.3390/antibiotics10060732
Alder, K. D., Lee, I., Munger, A. M., Kwon, H. K., Morris, M. T., Cahill, S. V., et al. (2020). Intracellular Staphylococcus aureus in bone and joint infections: A mechanism of disease recurrence, inflammation, and bone and cartilage destruction. Bone 141, 115568. doi: 10.1016/j.bone.2020.115568
Atalla, H., Gyles, C., and Mallard, B. (2011). Staphylococcus aureus small colony variants (SCVs) and their role in disease. Anim. Health Res. Rev. 12, 33–45. doi: 10.1017/S1466252311000065
Azzam, K., McHale, K., Austin, M., Purtill, J. J., and Parvizi, J. (2009). Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin. Orthopaedics Related Res. 467, 1706–1714. doi: 10.1007/s11999-009-0739-4
Bastami, F., Safavi, S. M., Seifi, S., Nadjmi, N., and Khojasteh, A. (2024). Addition of bone-marrow mesenchymal stem cells to 3D-printed alginate/gelatin hydrogel containing freeze-dried bone nanoparticles accelerates regeneration of critical size bone defects. Macromol. Biosci. 24, e2300065. doi: 10.1002/mabi.202300065
Birk, S., Boisen, A., and Nielsen, L. (2021). Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 174, 30–52. doi: 10.1016/j.addr.2021.04.005
Bose, J., Lehman, M., Fey, P., and Bayles, K. (2012). Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One 7, e42244. doi: 10.1371/journal.pone.0042244
Bosse, M. J., Gruber, H. E., and Ramp, W. K. (2005). Internalization of bacteria by osteoblasts in a patient with recurrent, long-term osteomyelitis. A case report. J. Bone Joint Surg. Am. 87, 1343–1347. doi: 10.2106/00004623-200506000-00022
Braga, P. C., Dal Sasso, M., and Maci, S. (1997). Cefodizime: effects of sub-inhibitory concentrations on adhesiveness and bacterial morphology of Staphylococcus aureus and Escherichia coli: comparison with cefotaxime and ceftriaxone. J. Antimicrob. Chemother. 39 (1), 79–84. doi: 10.1093/jac/39.1.79
Burger, E. H. and Klein-Nulend, J. (1999). Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J. 13, S101–S112. doi: 10.1096/fasebj.13.9001.s101
Cai, Y., Huang, C., Chen, X., Chen, Y., Huang, Z., Zhang, C., et al. (2022). Staphylococcus aureusThe role of small colony variants in intraosseous invasion and colonization in periprosthetic joint infection. Bone Joint Res. 11, 843–853. doi: 10.1302/2046-3758.1112.BJR-2021-0590.R1
Caputo, G. M., Cavanagh, P. R., Ulbrecht, J. S., Gibbons, G. W., and Karchmer, A. W. (1994). Assessment and management of foot disease in patients with diabetes. N. Engl. J. Med. 331, 854–860. doi: 10.1056/NEJM199409293311307
Carek, P. J., Dickerson, L. M., and Sack, J. L. (2001). Diagnosis and management of osteomyelitis. Am. Fam. Phys. 63, 2413–2420.
Chen, J., Shi, X., Zhu, Y., Chen, Y., Gao, M., Gao, H., et al. (2020). On-demand storage and release of antimicrobial peptides using Pandora’s box-like nanotubes gated with a bacterial infection-responsive polymer. Ranostics 10, 109–122. doi: 10.7150/thno.38388
Cheng, A. G., DeDent, A. C., Schneewind, O., and Missiakas, D. (2011). A play in four acts: Staphylococcus aureus abscess formation. Trends Microb. 19, 225–232. doi: 10.1016/j.tim.2011.01.007
Clarke, S. and Foster, S. (2006). Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51, 187–224. doi: 10.1016/S0065-2911(06)51004-5
Conterno, L. O. and Turchi, M. D. (2013). Antibiotics for treating chronic osteomyelitis in adults. Cochr. Database Syst. Rev. 2013, CD004439. doi: 10.1002/14651858.CD004439.pub3
Costa, T., Oliveira, C., Chambers, H. F., and Chatterjee, S. S. (2018). PBP4: A new perspective on staphylococcus aureus β-lactam resistance. Microorganisms 6, 57. doi: 10.3390/microorganisms6030057
de Mesy Bentley, K. L., MacDonald, A., Schwarz, E. M., and Oh, I. (2018). Chronic osteomyelitis with staphylococcus aureus deformation in submicron canaliculi of osteocytes: A case report. JBJS Case Connect 8, e8. doi: 10.2106/JBJS.CC.17.00154
de Mesy Bentley, K. L., Trombetta, R., Nishitani, K., Bello-Irizarry, S. N., Ninomiya, M., Zhang, L., et al. (2017). Awad HA et al: Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J. Bone Miner Res. 32, 985–990. doi: 10.1002/jbmr.3055
Doty, S. B. (2001). Morphological evidence of gap junctions between bone cells. J Orthop Res. 19 (5), 985–9. doi: 10.1007/BF02409482
Dziewanowska, K., Patti, J., Deobald, C., Bayles, K., Trumble, W., and Bohach, G. (1999). Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67, 4673–4678. doi: 10.1128/IAI.67.9.4673-4678.1999
Edwards, A., Potts, J., Josefsson, E., and Massey, R. (2010). Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS Pathog. 6, e1000964. doi: 10.1371/journal.ppat.1000964
Eiff, C. V., Peters, G., and Becker, K. (2006). The small colony variant (SCV) concept – the role of staphylococcal SCVs in persistent infections. Injury S26–33. doi: 10.1016/j.injury.2006.04.006
Ellington, J. K., Harris, M., Webb, L., Smith, B., Smith, T., Tan, K., et al. (2003). Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J. Bone Joint Surg. Br. 85, 918–921. doi: 10.1302/0301-620X.85B6.13509
Elysia, A. M., Alec, T. S., Stefano, B., Emma, N. L., Sydney, C. B., Clyde, T. O., et al. (2019). An in vitro platform for elucidating the molecular genetics of S. aureus invasion of the osteocyte lacuno-canalicular network during chronic osteomyelitis. Nanomedicine 21, 102039. doi: 10.1016/j.nano.2019.102039
Elysia, A. M., Gowrishankar, M., Lananh, H., Ann Lindley, G., Karen L, d., Chad, A. G., et al. (2021). Staphylococcus aureus cell wall biosynthesis modulates bone invasion and osteomyelitis pathogenesis. Front. Microb. 12. doi: 10.3389/fmicb.2021.723498
Epstein, A. K., Pokroy, B., Seminara, A., and Aizenberg, J. (2011). Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc. Natl. Acad. Sci. U. S. A. 108, 995–1000. doi: 10.1073/pnas.1011033108
Eric, S., Frédéric, K., Mohammed, T., Juan, A. A., and Paulette, C. (2008). The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microb. Rev. 32 (2), 234–58. doi: 10.1111/j.1574-6976.2008.00105.x
Evans, H., Andrews, R., Abedi, F., Sprules, A., Trend, J., Lovric, G., et al. (2024). Evidence for peri-lacunar remodeling and altered osteocyte lacuno-canalicular network in mouse models of myeloma-induced bone disease. JBMR Plus 8, ziae093. doi: 10.1093/jbmrpl/ziae093
Fantoni, M., Taccari, F., and Giovannenze, F. (2019). Systemic antibiotic treatment of chronic osteomyelitis in adults. Eur. Rev. Med. Pharmacol. Sci. 23, 258–270. doi: 10.26355/eurrev_201904_17500
Gallarate, M., Chirio, D., Chindamo, G., Peira, E., and Sapino, S. (2021). Osteomyelitis: focus on conventional treatments and innovative drug delivery systems. Curr. Drug Deliv. 18, 532–545. doi: 10.2174/1567201817666200915093224
Garcia, L., Lemaire, S., Kahl, B., Becker, K., Proctor, R., Denis, O., et al. (2013). Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 68, 1455–1464. doi: 10.1093/jac/dkt072
Garzoni, C. and Kelley, W. (2011). Return of the Trojan horse: intracellular phenotype switching and immune evasion by Staphylococcus aureus. EMBO Mol. Med. 3, 115–117. doi: 10.1002/emmm.201100123
Ghosh, C., Sarkar, P., Issa, R., and Haldar, J. (2019). Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microb. 27, 323–338. doi: 10.1016/j.tim.2018.12.010
Goudie, J. G. and Goudie, R. B. (1955). Recurrent infections by a stable dwarf-colony variant of Staphylococcus aureus. J. Clin. Pathol. 8 (4), 284–7. doi: 10.1136/jcp.8.4.284
Gowrishankar, M., Elysia, A. M., John, L. D., and Edward M, S. (2019). Mechanisms of immune evasion and bone tissue colonization that make staphylococcus aureus the primary pathogen in osteomyelitis. Curr. Osteoporos Rep. 17 (6), 395–404. doi: 10.1007/s11914-019-00548-4
Guanghui, L., Mingyu, Q., Yan, G., Xin, W., Yunfeng, X., and Xiaodong, X. (2014). Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog. Dis. 11 (9), 677–83. doi: 10.1089/fpd.2013.1731
Hany Ibrahim, K., Ismet, B., and Alan, B. (2015). Complement-coagulation cross-talk: A potential mediator of the physiological activation of complement by low pH. Front. Immunol. 6. doi: 10.3389/fimmu.2015.00215
Hevroni, A. and Koplewitz, B. Z. (2007). Images in clinical medicine. Bone within bone–chronic osteomyelitis. N. Engl. J. Med. 356, e7. doi: 10.1056/NEJMicm055247
Hirschhausen, N., Schlesier, T., Peters, G., and Heilmann, C. (2012). Characterization of the modular design of the autolysin/adhesin Aaa from Staphylococcus aureus. PLoS One 7, e40353. doi: 10.1371/journal.pone.0040353
Hogan, A., Heppert, V. G., and Suda, A. J. (2013). Osteomyelitis. Arch. Orthop. Trauma Surg. 133, 1183–1196. doi: 10.1007/s00402-013-1785-7
Hsu, S., Thakar, R., Liepmann, D., and Li, S. (2005). Effects of shear stress on endothelial cell haptotaxis on micropatterned surfaces. Biochem. Biophys. Res. Commun. 337 (1), 401–9. doi: 10.1016/j.bbrc.2005.08.272
Jennings, J. A., Arts, J. J., Abuhussein, E., Alt, V., Ashton, N., Baertl, S., et al. (2024). Ducheyne P et al: 2023 International Consensus Meeting on musculoskeletal infection: Summary from the treatment workgroup and consensus on treatment in preclinical models. J. Orthop. Res. 42, 500–511. doi: 10.1002/jor.25765
Jensen, L. K., Birch, J. M., Jensen, H. E., Kirketerp-Møller, K., and Gottlieb, H. (2023). Bacterial invasion of the submicron osteocyte lacuna-canaliculi network (OLCN): a part of osteomyelitis disease biology. Apmis 131, 325–332. doi: 10.1111/apm.v131.7
Jiang, C., Zhu, G., and Liu, Q. (2024). Current application and future perspectives of antimicrobial degradable bone substitutes for chronic osteomyelitis. Front. Bioeng. Biotechnol. 12, 1375266. doi: 10.3389/fbioe.2024.1375266
Jin, Y., Liu, H., Chu, L., Yang, J., Li, X., Zhou, H., et al. (2024). Initial therapeutic evidence of a borosilicate bioactive glass (BSG) and Fe3O4 magnetic nanoparticle scaffold on implant-associated Staphylococcal aureus bone infection. Bioact. Mater. 40, 148–167. doi: 10.1016/j.bioactmat.2024.05.040
Jin, T., Zhu, Y. L., Li, J., Shi, J., He, X. Q., Ding, J., et al. (2013). Staphylococcal protein A, Panton-Valentine leukocidin and coagulase aggravate the bone loss and bone destruction in osteomyelitis. Cell Physiol. Biochem. 32, 322–333. doi: 10.1159/000354440
Jiranek, W. (2005). Antibiotic-loaded cement in total hip replacement: current indications, efficacy, and complications. Orthopedics 28, s873–7. doi: 10.3928/0147-7447-20050802-14
Josse, J., Laurent, F., and Diot, A. (2017). Staphylococcal adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front. Microbiol. 8, 2433. doi: 10.3389/fmicb.2017.02433
Josse, J., Velard, F., and Gangloff, S. C. (2015). Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis. Front. Cell Infect. Microb. 5, 85. doi: 10.3389/fcimb.2015.00085
Juan, C., Huyue, Z., Jingbin, H., Rong, Z., and Xiancai, R. (2021). Virulence alterations in staphylococcus aureus upon treatment with the sub-inhibitory concentrations of antibiotics. J. Adv. Res. 31, 165–175. doi: 10.1016/j.jare.2021.01.008
Katarzyna, W., Vincenzo, A. R., Xinyue, C., Lucia, L., Manuel, P., Simon, B., et al. (2022). Penicillin-binding protein 1 (PBP1) of staphylococcus aureus has multiple essential functions in cell division. mBio 13 (4), e0066922. doi: 10.1128/mbio.00669-22
Kavanagh, N., Ryan, E. J., Widaa, A., Sexton, G., Fennell, J., O’Rourke, S., et al. (2018). Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin. Microb. Rev. 31 (2). doi: 10.1128/CMR.00084-17
Kintarak, S., Whawell, S., Speight, P., Packer, S., and Nair, S. (2004). Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 72, 5668–5675. doi: 10.1128/IAI.72.10.5668-5675.2004
Kobayashi, S. D., Malachowa, N., and DeLeo, F. R. (2015). Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 185, 1518–1527. doi: 10.1016/j.ajpath.2014.11.030
Krauss, J. L., Roper, P. M., Ballard, A., Shih, C. C., Fitzpatrick, J. A. J., Cassat, J. E., et al. (2019). Staphylococcus aureus infects osteoclasts and replicates intracellularly. mBio 10 (5). doi: 10.1128/mBio.02447-19
Kremers, H. M., Nwojo, M. E., Ransom, J. E., Wood-Wentz, C. M., Melton, L. J., 3rd, and Huddleston, P. M., 3rd (2015). Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J. Bone Joint Surg. Am. 97, 837–845. doi: 10.2106/JBJS.N.01350
Kumar, A., Tassopoulos, A. M., Li, Q., and Yu, F. S. (2007). Staphylococcus aureus protein A induced inflammatory response in human corneal epithelial cells. Biochem. Biophys. Res. Commun. 354, 955–961. doi: 10.1016/j.bbrc.2007.01.072
Kylväjä, R., Ojalehto, T., Kainulainen, V., Virkola, R., and Westerlund-Wikström, B. (2016). Penicillin binding protein 3 of Staphylococcus aureus NCTC 8325–4 binds and activates human plasminogen. BMC Res. Notes 9, 389. doi: 10.1186/s13104-016-2190-4
Loebel, C., Rodell, C. B., Chen, M. H., and Burdick, J. A. (2017). Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 12, 1521–1541. doi: 10.1038/nprot.2017.053
Mahmoud H, A. A., Leah, R. R., Nicholas, D. G., Kelsey, A. S., Alexander, R. H., David, A. S., et al. (2014). pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. U S A 111 (52), 18703–8. doi: 10.1073/pnas.1422091112
Malachowa, N., Kobayashi, S. D., Porter, A. R., Braughton, K. R., Scott, D. P., Gardner, D. J., et al. (2016). Contribution of staphylococcus aureus coagulases and clumping factor A to abscess formation in a rabbit model of skin and soft tissue infection. PLoS One 11, e0158293. doi: 10.1371/journal.pone.0158293
Masters, E. A., Bentley, K., Gill, A. L., Hao, S. P., Galloway, C. A., Salminen, A. T., et al. (2020). Identification of Penicillin Binding Protein 4 (PBP4) as a critical factor for Staphylococcus aureus bone invasion during osteomyelitis in mice. PLoS Pathog. 16 (10), e1008988. doi: 10.1371/journal.ppat.1008988
Masters, E. A., Ricciardi, B. F., Bentley, K. L. M., Moriarty, T. F., Schwarz, E. M., and Muthukrishnan, G. (2022). Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat. Rev. Microb. 20, 385–400. doi: 10.1038/s41579-022-00686-0
Masters, E. A., Trombetta, R. P., de Mesy Bentley, K. L., Boyce, B. F., Gill, A. L., Gill, S. R., et al. (2019). Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy. Bone Res. 7, 20. doi: 10.1038/s41413-019-0061-z
McNeill, E. P., Zeitouni, S., Pan, S., Haskell, A., Cesarek, M., Tahan, D., et al. (2020). Garcia M et al: Characterization of a pluripotent stem cell-derived matrix with powerful osteoregenerative capabilities. Nat. Commun. 11 (1), 3025. doi: 10.1038/s41467-020-16646-2
Melter, O. and Radojevič, B. (2010). Small colony variants of Staphylococcus aureus–review. Folia Microb. 55, 548–558. doi: 10.1007/s12223-010-0089-3
Mette, T., Janne, K. K., Magda, L. A., Marianne, N. S., Hans Jørn, K., Sérgio, R. F., et al. (2013). Thioridazine induces major changes in global gene expression and cell wall composition in methicillin-resistant Staphylococcus aureus USA300. PLoS One 8 (5), e64518. doi: 10.1371/journal.pone.0064518
Mitra, D., Whitehead, J., Yasui, O. W., and Leach, J. K. (2017). Bioreactor culture duration of engineered constructs influences bone formation by mesenchymal stem cells. Biomaterials 146, 29–39. doi: 10.1016/j.biomaterials.2017.08.044
Moriishi, T. and Komori, T. (2022). Osteocytes: their lacunocanalicular structure and mechanoresponses. Int. J. Mol. Sci. 23 (8). doi: 10.3390/ijms23084373
Navratna, V., Nadig, S., Sood, V., Prasad, K., Arakere, G., and Gopal, B. (2010). Molecular basis for the role of Staphylococcus aureus penicillin binding protein 4 in antimicrobial resistance. J. Bacteriol. 192, 134–144. doi: 10.1128/JB.00822-09
Nguyen, H. A., Denis, O., Vergison, A., Theunis, A., Tulkens, P. M., Struelens, M. J., et al. (2009). Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus small-colony variant strain isolated from a cystic fibrosis patient: pharmacodynamic evaluation and comparison with isogenic normal-phenotype and revertant strains. Antimicrob. Agents Chemother. 53, 1434–1442. doi: 10.1128/AAC.01145-08
Norden, C., Nelson, J. D., Mader, J. T., and Calandra, G. B. (1992). Evaluation of new anti-infective drugs for the treatment of infections of prosthetic hip joints. Infectious Diseases Society of America and the Food and Drug Administration. Clin. Infect. Dis. 15, S177–S181. doi: 10.1093/clind/15.Supplement_1.S177
Parfitt, A. M. (1977). The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption–bone flow theory. Clin. Orthop. Relat. Res. (127), 236–247. doi: 10.1097/00003086-197709000-00036
Pereira, S. F. F., Henriques, A. O., Pinho, M. G., Lencastre, H. D., and Tomasz, A. (2007). Role of PBP1 in cell division of Staphylococcus aureus. J. Bacteriol. 189 (9), 3525–31. doi: 10.1128/JB.00044-07
Pinho, M., de Lencastre, H., and Tomasz, A. (2000). Cloning, characterization, and inactivation of the gene pbpC, encoding penicillin-binding protein 3 of Staphylococcus aureus. J. Bacteriol. 182, 1074–1079. doi: 10.1128/JB.182.4.1074-1079.2000
Pinho, M. G., Filipe, S. R., de Lencastre, H., and Tomasz, A. (2001). Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 183 (22), 6525–31. doi: 10.1128/JB.183.22.6525-6531.2001
Posadowska, U., Brzychczy-Wloch, M., Drozdz, A., Krok-Borkowicz, M., Wlodarczyk-Biegun, M., Dobrzynski, P., et al. (2016). Injectable hybrid delivery system composed of gellan gum, nanoparticles and gentamicin for the localized treatment of bone infections. Expert Opin. Drug Deliv. 13, 613–620. doi: 10.1517/17425247.2016.1146673
Prideaux, M., Findlay, D. M., and Atkins, G. J. (2016). Osteocytes: The master cells in bone remodelling. Curr. Opin. Pharmacol. 28, 24–30. doi: 10.1016/j.coph.2016.02.003
Proctor, R., von Eiff, C., Kahl, B., Becker, K., McNamara, P., Herrmann, M., et al. (2006). Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4, 295–305. doi: 10.1038/nrmicro1384
Qin, L., Yang, S., Zhao, C., Yang, J., Li, F., Xu, Z., et al. (2024). Xiong C et al: Prospects and challenges for the application of tissue engineering technologies in the treatment of bone infections. Bone Res. 12, 28. doi: 10.1038/s41413-024-00332-w
Raimon, S., Vito, C., Jorge, E., Alberto, E.-A., Anna, L., Léo, V., et al. (2016). Collective cell durotaxis emerges from long-range intercellular force transmission. Science 353 (6304), 1157–61. doi: 10.1126/science.aaf7119
Ratanavaraporn, J., Furuya, H., Kohara, H., and Tabata, Y. (2011). Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials 32, 2797–2811. doi: 10.1016/j.biomaterials.2010.12.052
Ren, Y., Weeks, J., Xue, T., Rainbolt, J., de Mesy Bentley, K., Shu, Y., et al. (2023). Evidence of bisphosphonate-conjugated sitafloxacin eradication of established methicillin-resistant S. aureus infection with osseointegration in murine models of implant-associated osteomyelitis. Bone Res. 11, 51. doi: 10.1038/s41413-023-00287-4
Ren, Y., Xue, T., Rainbolt, J., Bentley, K., Galloway, C. A., Liu, Y., et al. (2022). Ebetino FH et al: Efficacy of Bisphosphonate-Conjugated Sitafloxacin in a Murine Model of S. aureus Osteomyelitis: Evidence of “Target & Release” Kinetics and Killing of Bacteria Within Canaliculi. Front. Cell. Infect. Microbiol. 12, 910970. doi: 10.3389/fcimb.2022.910970
Repp, F., Kollmannsberger, P., Roschger, A., Berzlanovich, A., Gruber, G. M., Roschger, P., et al. (2017). Coalignment of osteocyte canaliculi and collagen fibers in human osteonal bone. J. Struct. Biol. 199, 177–186. doi: 10.1016/j.jsb.2017.07.004
Ricciardi, B. F., Muthukrishnan, G., Masters, E., Ninomiya, M., Lee, C. C., and Schwarz, E. M. (2018). Staphylococcus aureus evasion of host immunity in the setting of prosthetic joint infection: biofilm and beyond. Curr. Rev. Musculoskelet Med. 11, 389–400. doi: 10.1007/s12178-018-9501-4
Roper, P. M., Shao, C., and Veis, D. J. (2020). Multitasking by the OC lineage during bone infection: bone resorption, immune modulation, and microbial niche. Cells 9 (10). doi: 10.3390/cells9102157
Rosas, S., Ong, A. C., Buller, L. T., Sabeh, K. G., Law, T. Y., Roche, M. W., et al. (2017). Season of the year influences infection rates following total hip arthroplasty. World J. Orthoped. 8, 895–901. doi: 10.5312/wjo.v8.i12.895
Saeed, K., McLaren, A., Schwarz, E., Antoci, V., Arnold, W., Chen, A., et al. (2019). 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J. Orthopaed. Res. 37, 1007–1017. doi: 10.1002/jorr.v37.5
Sayed, F. A.-Z., Eissa, N. G., Shen, Y., Hunstad, D. A., Wooley, K. L., and Elsabahy, M. (2022). Morphologic design of nanostructures for enhanced antimicrobial activity. J. Nanobiotechnol. 20 (1), 536. doi: 10.1186/s12951-022-01733-x
Scheffers, D. and Pinho, M. (2005). Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 69, 585–607. doi: 10.1128/MMBR.69.4.585-607.2005
Schlesier, T., Siegmund, A., Rescher, U., and Heilmann, C. (2020). Characterization of the Atl-mediated staphylococcal internalization mechanism. Int. J. Med. Microbiol. 310, 151463. doi: 10.1016/j.ijmm.2020.151463
Schwarz, E. M., McLaren, A. C., Sculco, T. P., Brause, B., Bostrom, M., Kates, S. L., et al. (2021). Adjuvant antibiotic-loaded bone cement: Concerns with current use and research to make it work. J. Orthopaed. Res. 39 (2), 227–239. doi: 10.1002/jor.24616
Sendi, P., Rohrbach, M., Graber, P., Frei, R., Ochsner, P. E., and Zimmerli, W. (2006). Staphylococcus aureus small colony variants in prosthetic joint infection. Clin. Infect. Dis. 43, 961–967. doi: 10.1086/cid.2006.43.issue-8
Shang, W., Rao, Y., Zheng, Y., Yang, Y., Hu, Q., Hu, Z., et al. (2019). Tan L et al: β-Lactam Antibiotics Enhance the Pathogenicity of Methicillin-Resistant Staphylococcus aureus via SarA-Controlled Lipoprotein-Like Cluster Expression. mBio 10 (3). doi: 10.1128/mBio.00880-19
Shapiro, F. (1997). Variable conformation of GAP junctions linking bone cells: a transmission electron microscopic study of linear, stacked linear, curvilinear, oval, and annular junctions. Calcified Tissue Int. 61 (4), 285–93. doi: 10.1007/s002239900337
Silhavy, T., Kahne, D., and Walker, S. (2010). The bacterial cell envelope. Cold Spring Harbor Perspect. Biol. 2, a000414. doi: 10.1101/cshperspect.a000414
Simpson, A. H., Deakin, M., and Latham, J. M. (2001). Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J. Bone Joint Surg. Br. 83, 403–407. doi: 10.1302/0301-620X.83B3.0830403
Sompolinsky, D., Cohen, M., and Ziv, G. (1974). Epidemiological and biochemical studies on thiamine-less dwarf-colony variants of Staphylococcus aureus as etiological agents of bovine mastitis. Infect. Immun. 9 (2), 217–28. doi: 10.1128/iai.9.2.217-228.1974
Sultan, A., Samuel, L., Umpierrez, E., Swiergosz, A., Rabin, J., Mahmood, B., et al. (2019). Routine use of commercial antibiotic-loaded bone cement in primary total joint arthroplasty: a critical analysis of the current evidence. Ann. Trans. Med. 7, 73. doi: 10.21037/atm.2018.11.50
Swingle, E. L. (1935). Studies on small colony variants of staphylococcus aureus. J. Bacteriol. 29 (5), 467–89. doi: 10.1128/jb.29.5.467-489.1935
Tang, Y., Tong, X., Conrad, B., and Yang, F. (2020). Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo. Theranostics 10, 6035–6047. doi: 10.7150/thno.41096
Theuretzbacher, U., Outterson, K., Engel, A., and Karlén, A. (2020). The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 18, 275–285. doi: 10.1038/s41579-019-0288-0
Tuchscherr, L., Kreis, C., Hoerr, V., Flint, L., Hachmeister, M., Geraci, J., et al. (2016). Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J. Antimicrob Chemother. 71, 438–448. doi: 10.1093/jac/dkv371
Turner, R., Vollmer, W., and Foster, S. (2014). Different walls for rods and balls: the diversity of peptidoglycan. Mol. Microbiol. 91, 862–874. doi: 10.1111/mmi.2014.91.issue-5
Veis, D. and Cassat, J. (2021). Infectious osteomyelitis: marrying bone biology and microbiology to shed new light on a persistent clinical challenge. J. Bone Min. Res. 36, 636–643. doi: 10.1002/jbmr.4279
Vlamakis, H., Chai, Y., Beauregard, P., Losick, R., and Kolter, R. (2013). Sticking together: building a biofilm the Bacillus subtilis way. Nat. Rev. Microb. 11, 157–168. doi: 10.1038/nrmicro2960
Vollmer, W., Blanot, D., and de Pedro, M. (2008a). Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167. doi: 10.1111/j.1574-6976.2007.00094.x
Vollmer, W., Joris, B., Charlier, P., and Foster, S. (2008b). Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 32, 259–286. doi: 10.1111/j.1574-6976.2007.00099.x
Wang, Y., Chang, R. Y. K., Britton, W. J., and Chan, H.-K. (2022). Advances in the development of antimicrobial peptides and proteins for inhaled therapy. Adv. Drug Deliv. Rev. 180, 114066. doi: 10.1016/j.addr.2021.114066
Wassif, R. K., Elkheshen, S. A., Shamma, R. N., Amer, M. S., Elhelw, R., and El-Kayal, M. (2024). Injectable systems of chitosan in situ forming composite gel incorporating linezolid-loaded biodegradable nanoparticles for long-term treatment of bone infections. Drug Deliv. Transl. Res. 14, 80–102. doi: 10.1007/s13346-023-01384-x
Wise, R. I. and Spink, W. W. (1954). The influence of antibiotics on the origin of small colonies (G variants) of Micrococcus pyogenes var. aureus. J. Clin. Invest. 33 (12), 1611–22. doi: 10.1172/JCI103041
Wu, S., Xu, J., Zou, L., Luo, S., Yao, R., Zheng, B., et al. (2021). Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 12 (1), 3303. doi: 10.1038/s41467-021-23069-0
Wu, Z. Q., Zeng, D. L., Yao, J. L., Bian, Y. Y., Gu, Y. T., Meng, Z. L., et al. (2019). Research progress on diagnosis and treatment of chronic osteomyelitis. Chin. Med. Sci. J. 34, 211–220. doi: 10.24920/003493
Yang, D., Wijenayaka, A. R., Solomon, L. B., Pederson, S. M., Findlay, D. M., Kidd, S. P., et al. (2018). Novel Insights into Staphylococcus aureus Deep Bone Infections: the Involvement of Osteocytes. mBio 9 (2). doi: 10.1128/mBio.00415-18
Yoshida, H., Kawai, F., Obayashi, E., Akashi, S., Roper, D., Tame, J., et al. (2012). Crystal structures of penicillin-binding protein 3 (PBP3) from methicillin-resistant Staphylococcus aureus in the apo and cefotaxime-bound forms. J. Mol. Biol. 423, 351–364. doi: 10.1016/j.jmb.2012.07.012
You, L.-D., Weinbaum, S., Cowin, S. C., and Schaffler, M. B. (2004). Ultrastructure of the osteocyte process and its pericellular matrix. Anatom. Record. Part A. Discov. Mol. Cell. Evol. Biol. 278 (2), 505–13. doi: 10.1002/ar.a.v278a:2
Yousaf, A., Muhammad, S., Zoghoul, S. B. M., Alam, S. I., and Elsyaed, A. M. (2021). Chronic recurrent multifocal osteomyelitis and its management. Cureus 13, e18872. doi: 10.7759/cureus.18872
Yousif, A., Jamal, M. A., and Raad, I. (2015). Biofilm-based central line-associated bloodstream infections. Adv. Exp. Med. Biol. 830, 157–179. doi: 10.1007/978-3-319-11038-7_10
Yu, B., Pacureanu, A., Olivier, C., Cloetens, P., and Peyrin, F. (2020). Assessment of the human bone lacuno-canalicular network at the nanoscale and impact of spatial resolution. Sci. Rep. 10, 4567. doi: 10.1038/s41598-020-61269-8
Zhang, J., Zhuang, Y., Sheng, R., Tomás, H., Rodrigues, J., Yuan, G., et al. (2023). Smart stimuli-responsive strategies for titanium implant functionalization in bone regeneration and therapeutics. Mater. Horizons. 11 (1), 12–36. doi: 10.1039/d3mh01260c
Zheng, J., Tu, H., Sun, X., Xu, G., Chen, J., Deng, Q., et al. (2020). In vitro activities of telithromycin against biofilms compared with azithromycin, clindamycin, vancomycin and daptomycin. J. Med. Microbiol. 69, 120–131. doi: 10.1099/jmm.0.001122
Zhou, M., Li, G., Yu, J., Zhou, Q., Wang, K., Kang, J., et al. (2024). Interfacial delivery of carbon monoxide via smart titanium implant coating for enhanced soft tissue integration with switchable antibacterial and immunomodulatory properties. Bioact. Mater. 40, 318–333. doi: 10.1016/j.bioactmat.2024.06.010
Zhou, H., Ren, Y., Zou, K., Jin, Y., Liu, H., Jiang, H., et al. (2024). Efficacy of pH-Responsive Surface Functionalized Titanium Screws in Treating Implant-associated S. aureus Osteomyelitis with Biofilms Formation. Adv. Healthc. Mater. 14 (3), e2403261. doi: 10.1002/adhm.202403261
Zhou, H., Ye, S., Xu, M., Hao, L., Chen, J., Fang, Z., et al. (2023). Dynamic surface adapts to multiple service stages by orchestrating responsive polymers and functional peptides. Biomaterials 301, 122200. doi: 10.1016/j.biomaterials.2023.122200
Keywords: Staphylococcus aureus, osteocyte lacuno-canalicular network, immune escape, bone infection, chronic osteomyelitis
Citation: Rong Z, Chen X, Qin L, Wang X, Luo F, Zou Q and Zeng H (2025) Immune escape of Staphylococcus aureus mediated by osteocyte lacuna-canalicular network leads to persistent and uncured bone infection. Front. Cell. Infect. Microbiol. 15:1592086. doi: 10.3389/fcimb.2025.1592086
Received: 12 March 2025; Accepted: 30 April 2025;
Published: 02 June 2025.
Edited by:
Percy Schröttner, Technische Universität Dresden, GermanyReviewed by:
Elena Marcello, Polytechnic University of Turin, ItalyNirmal Muthukumarasamy, Sanford USD Medical Center, United States
Copyright © 2025 Rong, Chen, Qin, Wang, Luo, Zou and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Zeng, emVuZzExMDlAMTYzLmNvbQ==; Quanming Zou, cW16b3UyMDA3QDE2My5jb20=
†These authors share first authorship
 Zhigang Rong
Zhigang Rong Xiaozhen Chen3†
Xiaozhen Chen3† Quanming Zou
Quanming Zou Hao Zeng
Hao Zeng