- 1Department of Respiratory and Critical Care Medicine, The Second Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Nuclear Medicine, The Second Hospital of Jilin University, Changchun, Jilin, China
Background: It can be difficult to distinguish lung disease caused by nontuberculous mycobacteria (NTM), Mycobacterium tuberculosis, and mixed infections (MIs) that include NTM. Metagenomic next generation sequencing (mNGS) is a highly sensitive method that can reliably identify lung pathogens. We retrospectively analyzed the records of patients who had MIs of the lungs that included NTM and received mNGS testing.
Methods: The records of 36 patients who were diagnosed with NTM infection of the lungs at the Second Hospital of Jilin University from Nov 2023 to Jun 2024 were analyzed. Initial empirical treatments were ineffective in all patients, leading to the application of mNGS of bronchoalveolar lavage fluid (BALF).
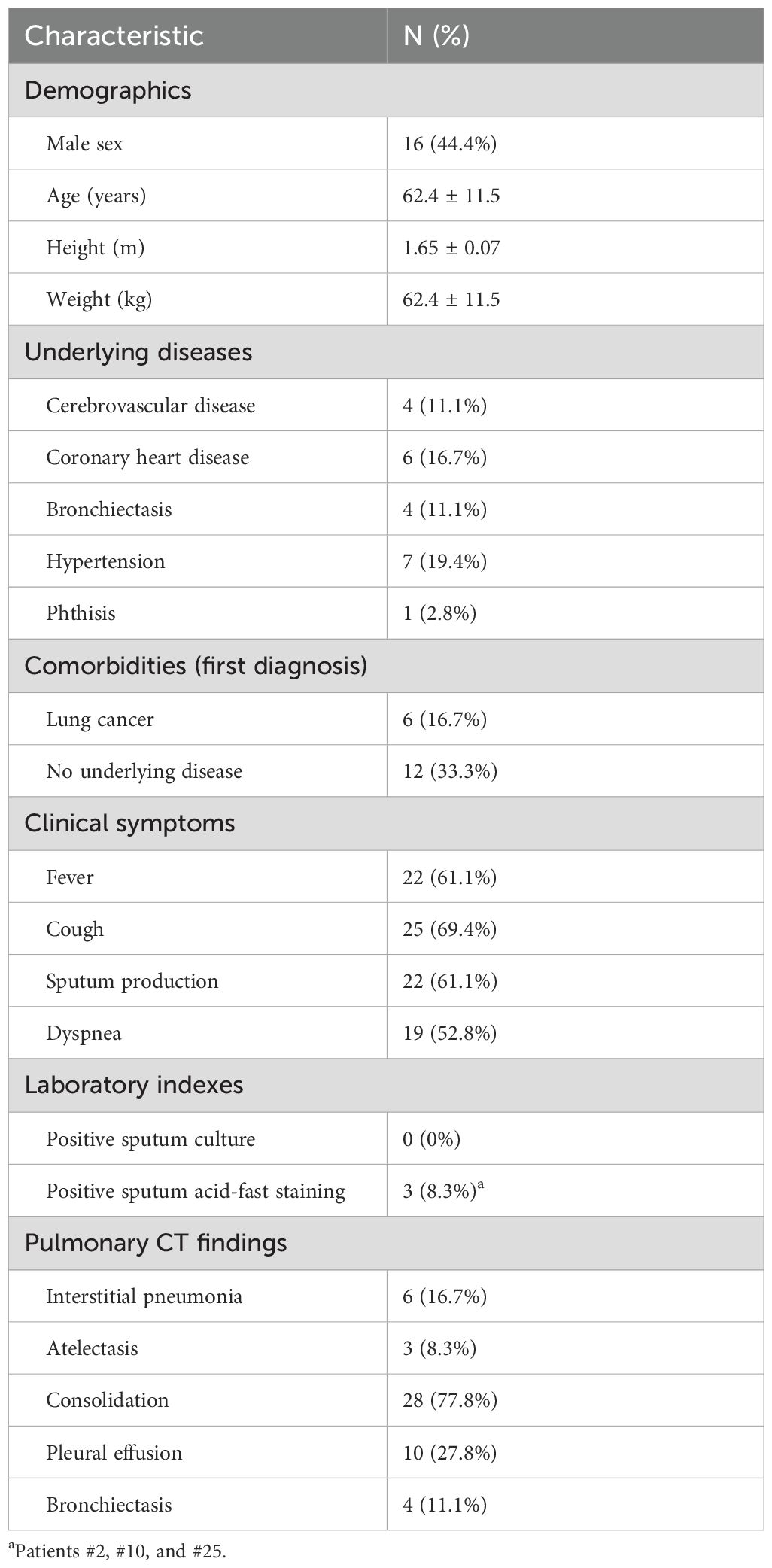
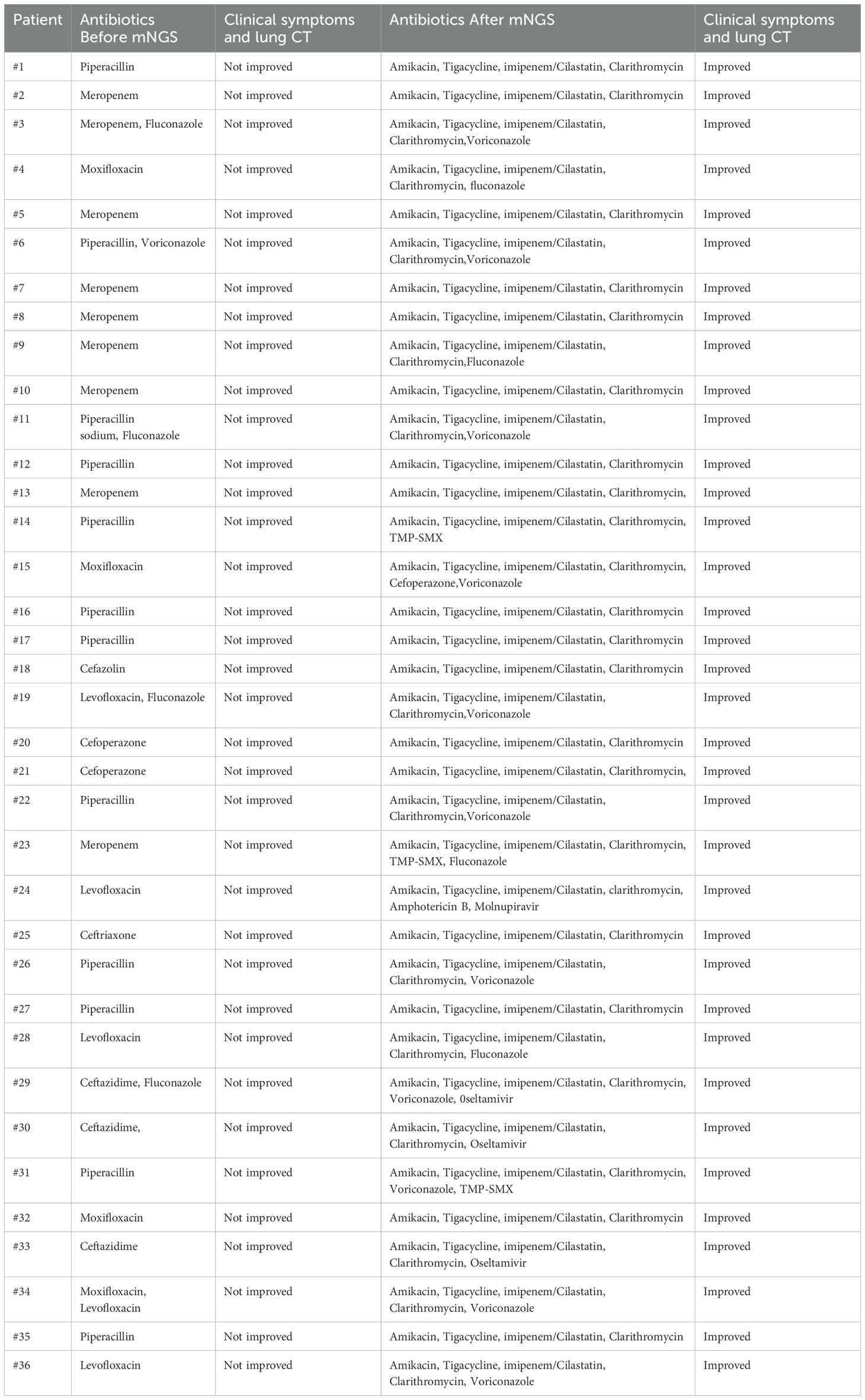
Results: The average patient age was 62.4 years, 22 patients had one or more underlying chronic disease, and all patients had at least one respiratory symptom (cough, sputum production, fever, or dyspnea). Chest CT examinations showed that patients had different degrees of pneumonia and pleural effusion. Among tested patients, there were elevated levels of erythrocyte sedimentation rate in 81.8% (18/22) and elevated C-reactive protein in 90.5% (19/21). There were variable results from acid-fast staining of bronchoalveolar lavage fluid (BALF; 3/36, 8.3%), and transbronchial lung biopsy (TBLB; 5/14, 35.7%). mNGS identified seven NTM species. Treatment based on the mNGS results led to the resolution of clinical symptoms and absorption of lung lesions in all patients.
Conclusions: Most of the 36 patients with MIs of the lungs that included NTM had underlying diseases. The results of traditional tests, including sputum or BALF culture and smear, acute phase markers, and TBLB pathological examination, were problematic. mNGS provides rapid and reliable diagnosis, allowing the rapid implementation of appropriate therapy in patients with MIs of the lungs that include NTM.
Introduction
Non-tuberculous mycobacteria (NTM) pulmonary diseases are systemic lung infections that can threaten human life and well-being, and are an increasingly serious global health problem. Recent changes in the global climate and the development of more sophisticated technologies for identification of pathogens have led to the increased detection of NTM and other pathogens (Porvaznik et al., 2017; Chin et al., 2020; Gopalaswamy et al., 2020; Sharma and Upadhyay, 2020).
Identification of the etiological pathogen is essential for the clinical diagnosis of NTM lung disease. However, the traditional culture methods have low rates of positive identification and are time-consuming, making them unsuitable for obtaining rapid and accurate clinical diagnoses. It is also difficult to distinguish between infections by NTM and by Mycobacterium tuberculosis, and these infections require different treatments (Kalaiarasan et al., 2020). For a patient who has a mixed infection (MI) with an NTM, the signs of the NTM infection may be masked by a strong pathogenic response to the more easily detected co-infecting pathogen, resulting in persistent symptoms, prolonged treatment time, increased use of medical resources, and poor prognosis (Cullen et al., 2000). The limitations of traditional methods are especially problematic for patients with atypical clinical symptoms and imaging results, and for patients who have multiple negative sputum smears and negative culture results for acid-fast bacilli. Reliance on the traditional methods for pathogen identification often leads to misdiagnosis and inappropriate treatment (Quan et al., 2017; Kalaiarasan et al., 2020; Morais et al., 2022; Khare and Brown-Elliott, 2023). Metagenomic next generation sequencing (mNGS) can identify and type all pathogens in a clinical sample by directly measuring DNA and RNA (Zheng et al., 2021; Hilt and Ferrieri, 2022). Thus, mNGS has great potential because it provides rapid and reliable identification of etiological pathogens, and allows clinicians to select the most appropriate treatment regimens for patients who have complex MIs.
We conducted a single-center retrospective study to investigate the value of mNGS for the diagnosis of NTM in patients with MIs. We described the details of this process for 36 patients with MIs who presented with atypical clinical symptoms and lung imaging features and who had poor resolution of symptoms following empirical treatment or treatment based on sputum culture results. We ultimately confirmed the presence of a MI with an NTM in each patient. This study aims to improve understanding of the value of mNGS for the diagnosis of NTM in patients with MIs.
Methods
Patients and sample collection
This study initially examined 36 patients who had complex MIs and received mNGS testing for detection of NTM at the Second Hospital of Jilin University (Changchun, China) between Nov 2023 and Jun 2024. Each included patient had poor outcome from empirical treatment before admission, agreed to undergo testing of bronchoalveolar lavage fluid with NGS, and had NGS results indicating MIs with NTM. Clinical data and basic information (age, sex, etc.) were collected from all patients. Data from laboratory tests and imaging, such as routine blood parameters and chemistry, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), and chest computed tomography (CT), were also collected. All patients underwent bronchofiberscopy for collection of BALF, and 8 patients received transbronchial lung biopsy (TBLB). The mNGS procedures, including nucleic acid extraction, library construction, and shotgun sequencing, were performed using the Illumination NextSeq 550DX. Bioinformatics analysis was carried out by WillingMed Technology Co, Ltd. (Beijing, China). A sample with 10 or more qualified reads was considered to indicate a pathogenic species.
NTM smear and culture
Acid-fast bacilli (AFB) were detected by Ziehl-Neelsen staining of smears prepared from either bronchoalveolar lavage fluid or concentrated 24-hour sputum samples. For NTM culture, we adopted the conventional Löwenstein-Jensen method.
Library preparation and metagenomic sequencing
DNA library construction was performed by automated nucleic acid extraction, enzymatic fragmentation, end-repair, terminal adenylation, and adaptor ligation, as described previously (Luan et al., 2021). Finished libraries were quantified by real-time polymerase chain reaction(PCR) (KAPA HiFi Kit, Roche) and pooled. Shotgun sequencing was performed using the Illumina Nextseq platform. Approximately 20 million 50 bp single-end reads were generated for each library. Bioinformatic analysis was conducted as described previously (Shen et al., 2021). Briefly, sequences of human origin were filtered (GRCh38.p13), and the remaining reads were aligned to a reference database (NCBI nt, GenBank, and an in-house curated genomic database) to identify microbial species and read counts. For each sequencing run, a negative control (culture medium with 104 Jurkat cells/mL) was included.
mNGS reporting criteria
Microbial reads identified from a library were reported if: (i) the sequencing data passed the quality control filters (library concentration > 50 pM, Q20 > 85%, Q30 > 80%) and (ii) the negative control (NC) in the same sequencing run did not contain the species or an excess of reads per million (RPM[sample]/RPM[NC] ≥ 5). This criterion was determined empirically in previous studies as a cutoff for discriminating true-positives from background contamination (Schlaberg et al., 2017; Wilson et al., 2019; Luan et al., 2021).
Ethics statement
This study was approved by the Ethics Committee of the Second Hospital of Jilin University (approval number: 2024-166). All patients gave written informed consent for the analysis of personal and clinical details, along with any identifying images, to be published in this study. All methods were performed in accordance with the relevant guidelines and regulations.
Statistical analyses
SPSS (version 21.0, USA) was used for all statistical analyses. Normally distributed variables are expressed as means ± standard deviations (SDs) and categorical variables as counts and percentages.
Results
Clinicopathological data
The 36 patients had an age range of 38 to 83 years (mean: 62.4 years). The Body Mass Index(BMI) range was 11.72 to 31.25 kg/m2 (mean: 23.11 kg/m2), and there were 13 patients with BMI greater than 25 kg/m2 and 3 patients with BMI below 18.5 kg/m2 (Table 1). Seven patients had hypertension, 6 had a pulmonary malignancy, 6 had coronary heart disease, 4 had bronchiectasis, 4 had cerebrovascular disease, 1 had phthisis, and the remaining 12 patients had good general health with no underlying diseases. All patients had at least one respiratory symptom, such as fever, cough, production of sputum, and dyspnea. Cough was the most common symptom (25 cases, 69.4%), followed by fever and sputum production (22 cases, 61.1%).
Laboratory data
Laboratory examinations showed that 19 patients had increased levels of CRP, and 6 had increased levels of the white blood cell count, neutrophil ratio and count, and procalcitonin. The ESR was increased in 81.8% (18/22) of tested patients. All 36 patients underwent fiberoptic bronchoscopy, but there were no abnormalities in the airways of 28 cases. There was no evidence of Mycobacterium tuberculosis or NTM in the sputum and BALF cultures of all 36 patients. Three patients tested positive in acid-fast staining of BALF (3/36, 8.3%).
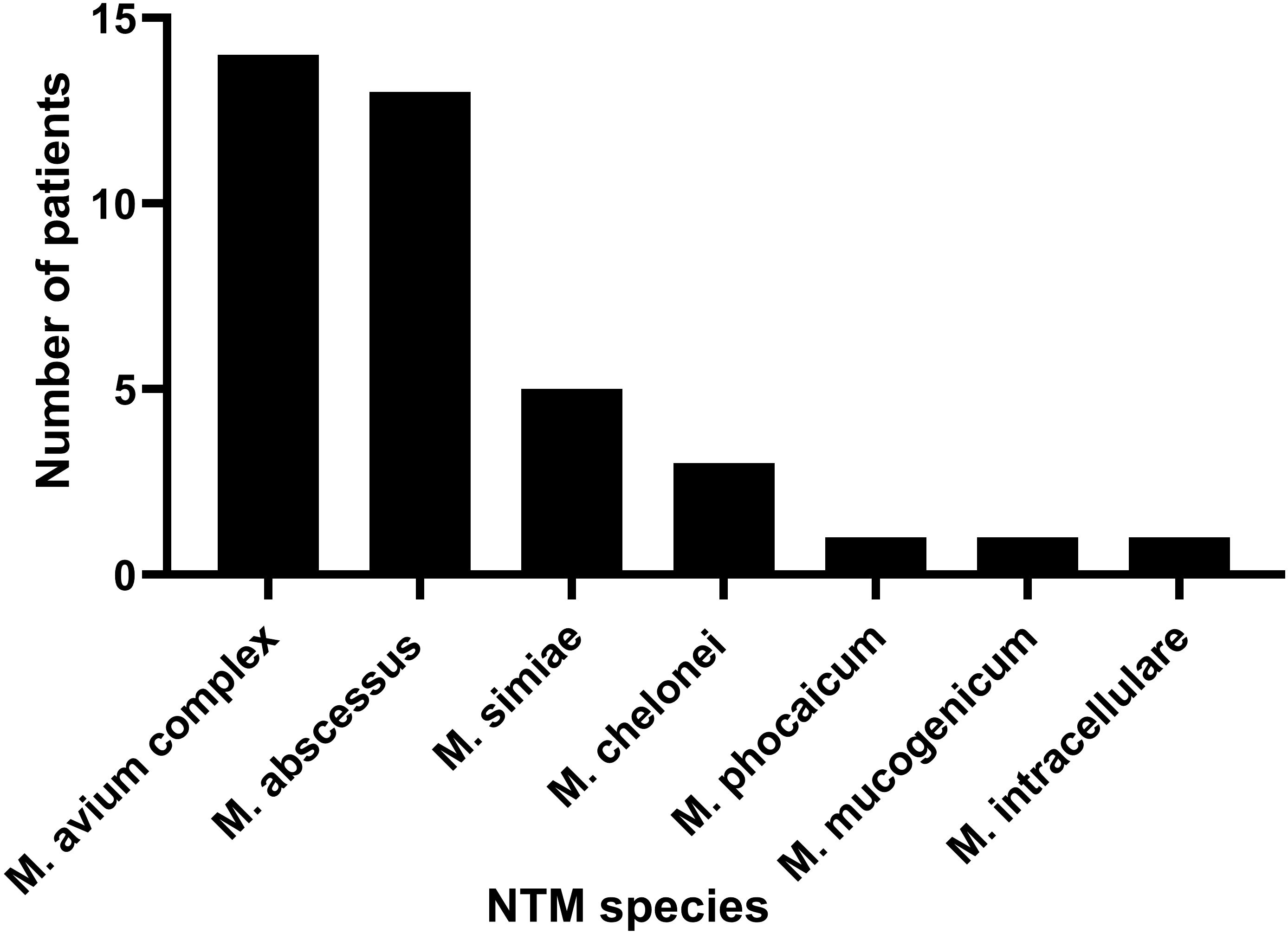
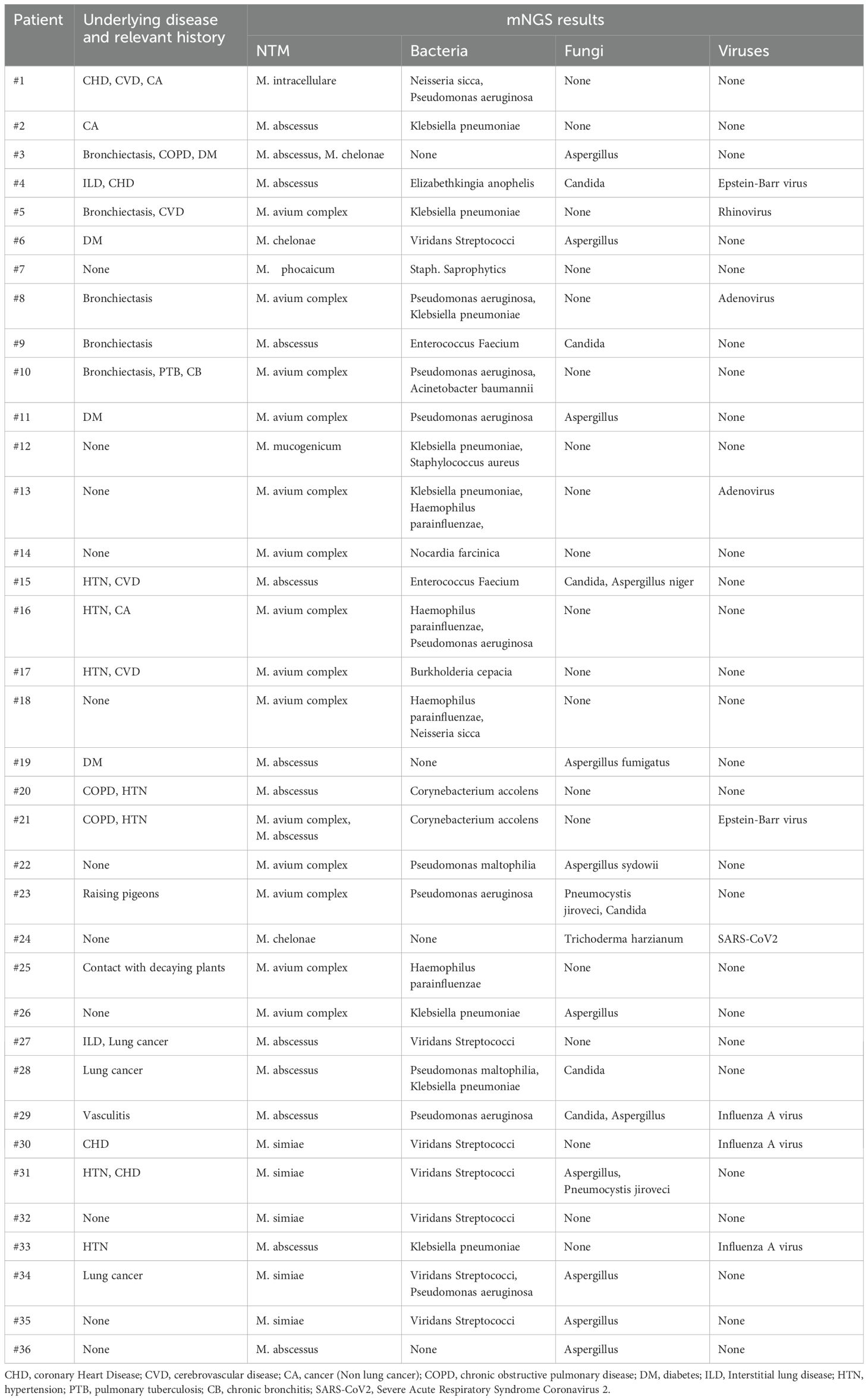
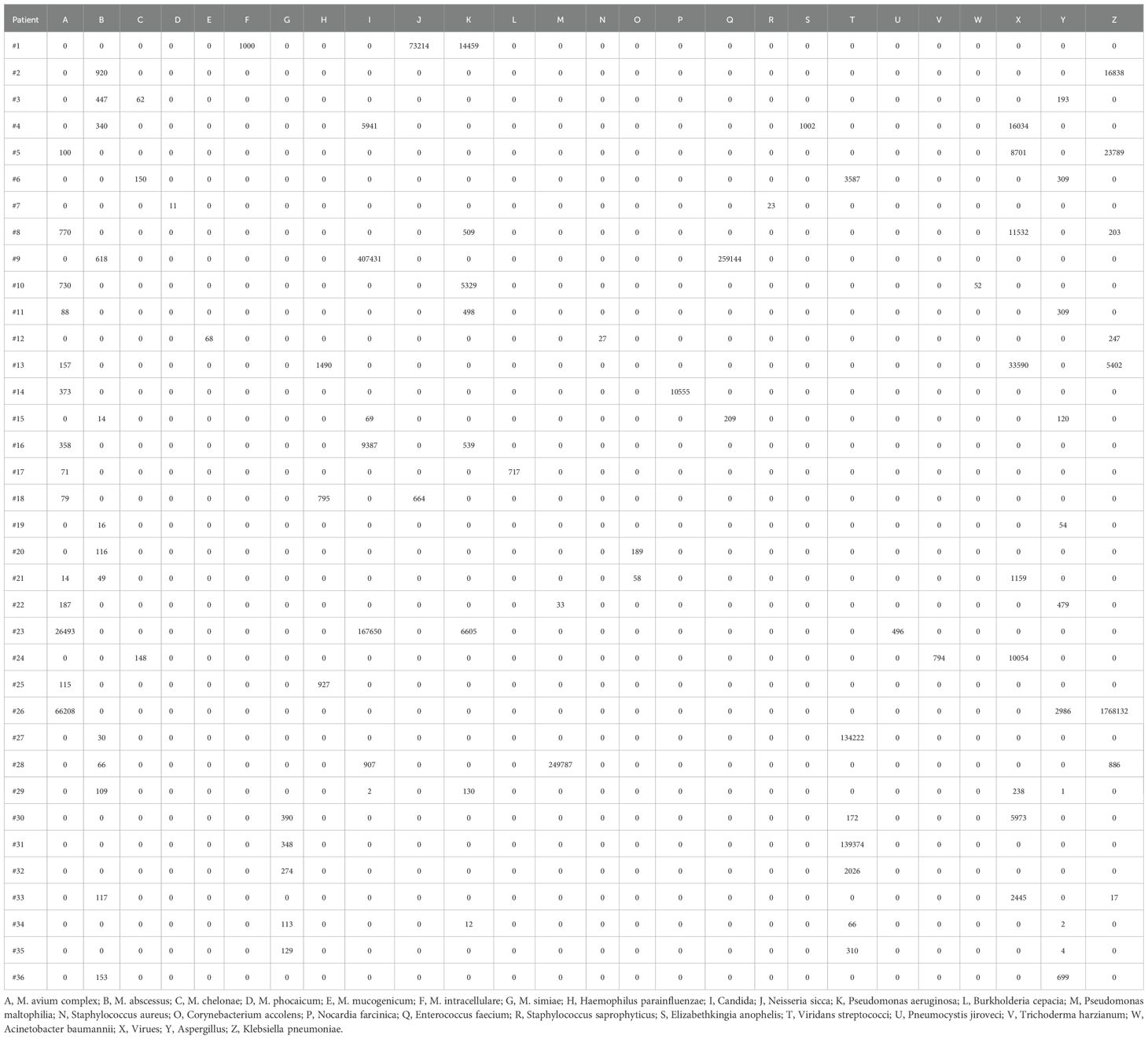
All patients had clear evidence pulmonary infections but poor response to empirical therapy, leading to the use of mNGS of BALF. The mNGS results identified NTM in all 36 patients (Figure 1; Table 2). These 7 NTM species were M. avium complex (14 patients), M. abscessus (13 patients), M. simiae (5 patients), M. chelonae (3 patients), M. phocaicum (1 patient), M. mucogenicum (1 patients), and M. intracellulare (1 patient). All patients were also complicated with infections by other bacteria, fungi, or viruses (Figure 2; Tables 2, 3). The most common co-occurring species were Pseudomonas aeruginosa (8, 22.2%), Klebsiella pneumoniae (8, 22.2%), and Aspergillus (11, 30.6%). The first mNGS result in Patient #7 was negative, but acid smear of the BALF was positive, and our re-examination using mNGS combined with qPCR confirmed M. phocaicum.
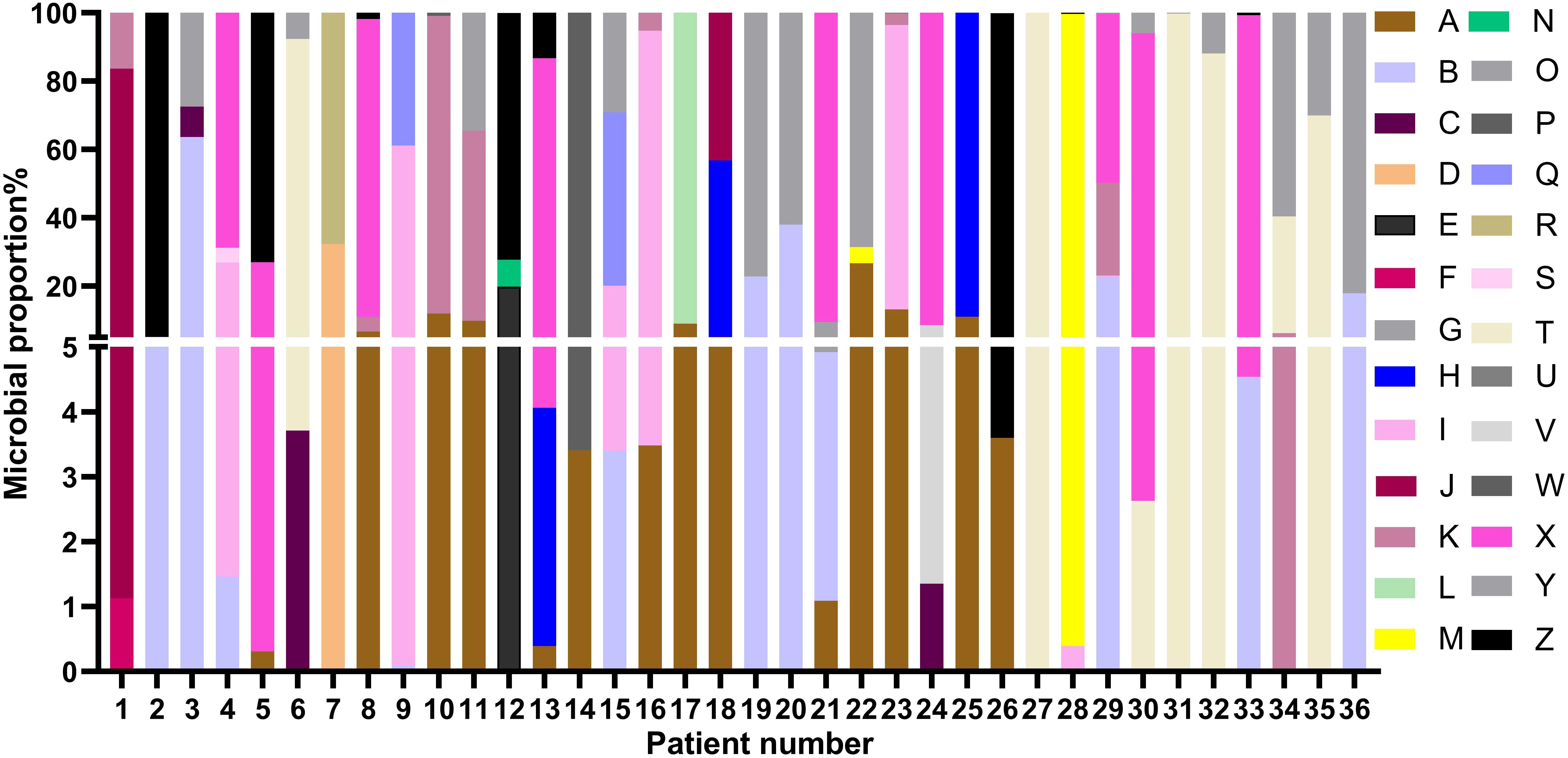
Figure 2. Relative abundances of NTM and non-NTM pathogens in each patient based on mNGS of BALF. The colors represent different pathogenic microorganisms (from A to Z) and length represents the percentage of pathogenic microorganisms. (A), M. avium complex; (B), M. abscessus; (C), M. chelonae; (D), M. phocaicum; (E), M. mucogenicum; (F), M. intracellulare; (G), M. simiae; (H), Haemophilus parainfluenzae; (I), Candida; (J), Neisseria sicca; (K), Pseudomonas aeruginosa; (L), Burkholderia cepacia; (M), Pseudomonas maltophilia; (N), Staphylococcus aureus; (O), Corynebacterium accolens; (P), Nocardia farcinica; (Q), Enterococcus faecium; (R), Staphylococcus saprophyticus; (S), Elizabethkingia anophelis; (T), Viridans streptococci; (U), Pneumocystis jiroveci; (V), Trichoderma harzianum; (W), Acinetobacter baumannii; (X), Virues; (Y), Aspergillus; (Z), Klebsiella pneumoniae.
Imaging results
On admission, all 36 patients received chest CT examinations. The imaging findings had a variety of pathological patterns, such as consolidation accompanied with ground-glass opacity, widened interlobular septa, diffuse interstitial changed in the lungs (Figures 3A–C), consolidation with associated traction bronchiectasis (Figure 3D), bronchovascular bundle thickened, with multiple nodular diffusely distributed in the lung fields and pleural effusion presented (Figure 3E), consolidation with cavitation, and accompanied by surrounding fibrous strands and patchy opacities (Figure 3F), cavitary lesion containing an intracavitary spherical opacity (Figure 3G), consolidation with bronchial cutoff sign and bronchiectasis (Figure 3H), consolidation with cavitation in the right upper lobe, accompanied by surrounding fibrotic strands (Figure 3I).
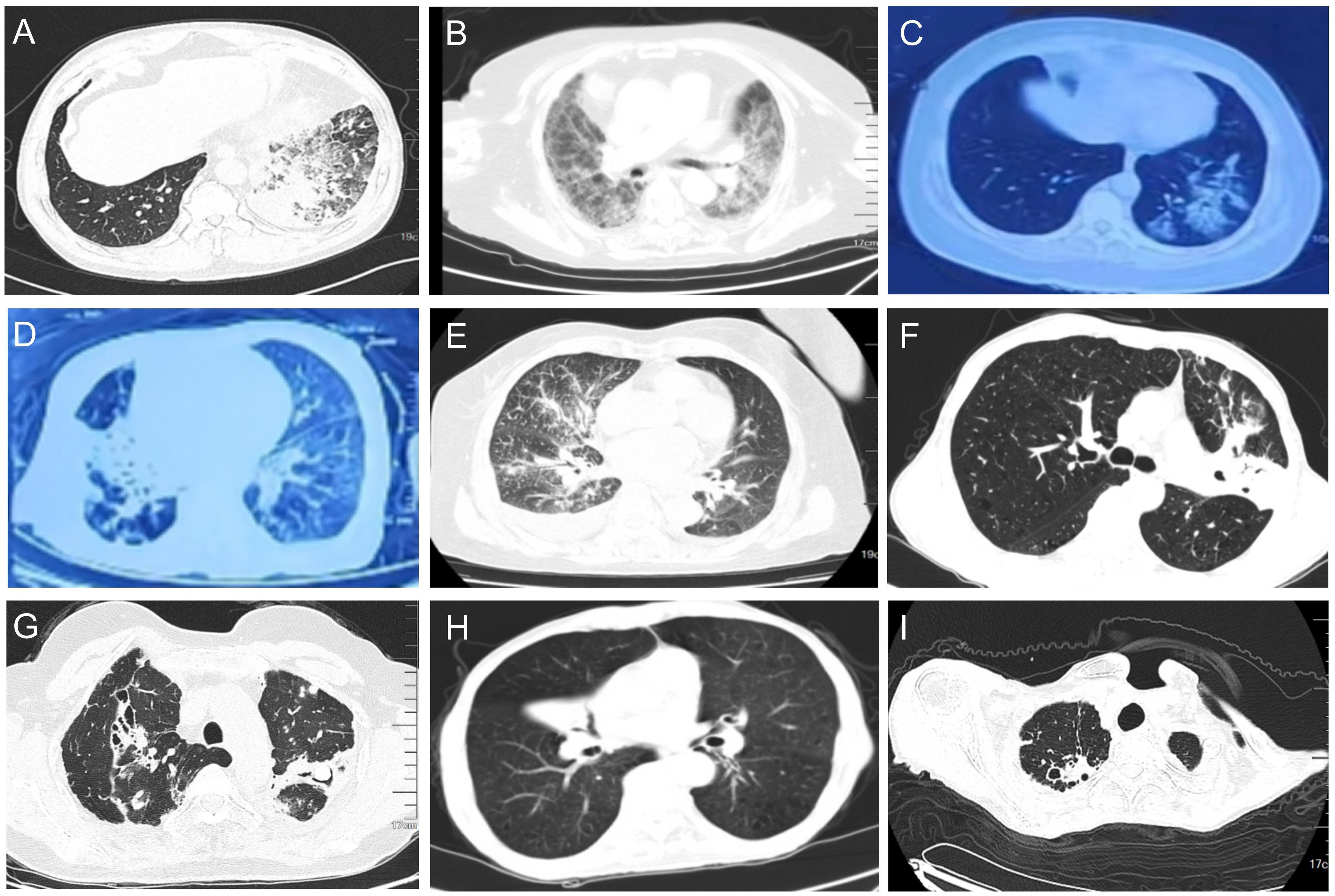
Figure 3. Pulmonary CT (lung window) representative findings of NTM patients at admission. (A) Consolidation accompanied with ground-glass opacity were observed in the lower lobe of the left lung. Widened interlobular septa were also observed. (B) Diffuse interstitial changed in the lungs. (C) Ground glass opacities in the lower lobe of the left lung. (D) Consolidation of the middle lobe of the right lung were observed, with associated traction bronchiectasis within the consolidated lesion. (E) The right bronchovascular bundle thickened, with multiple nodular diffusely distributed in the lung fields. A small amount of right pleural effusion presented. (F) Consolidation in the left upper lobe with cavitation, accompanied by surrounding fibrous strands and patchy opacities. (G) Consolidation in the upper lobes with traction bronchiectasis. A cavitary lesion is noted within the consolidation of the left lobe, containing an intracavitary spherical opacity. Scattered micronodules presented in the surrounding parenchyma. (H) Right middle lobe consolidation with bronchial cutoff sign and intralesional bronchiectasis. (I) Consolidation with cavitation in the right upper lobe, accompanied by surrounding fibrotic strands.
Co-infections
The co-infections were mainly by Gram-positive cocci and Gram-positive bacilli. Among all 36 patients, 32 (88.9%) had infections by bacteria, 17 (47.2%) had infections by fungi, and 24 (66.7%) had an underlying disease with co-infection. A mixed infection can increase the difficulty of treatment and prolong the treatment time.
Pathological outcome
14 patients received TBLB, and 5 of them had typical granulomas with necrosis (Figure 4) with positive acid-fast staining. Eight cases had chronic inflammatory cell infiltration.
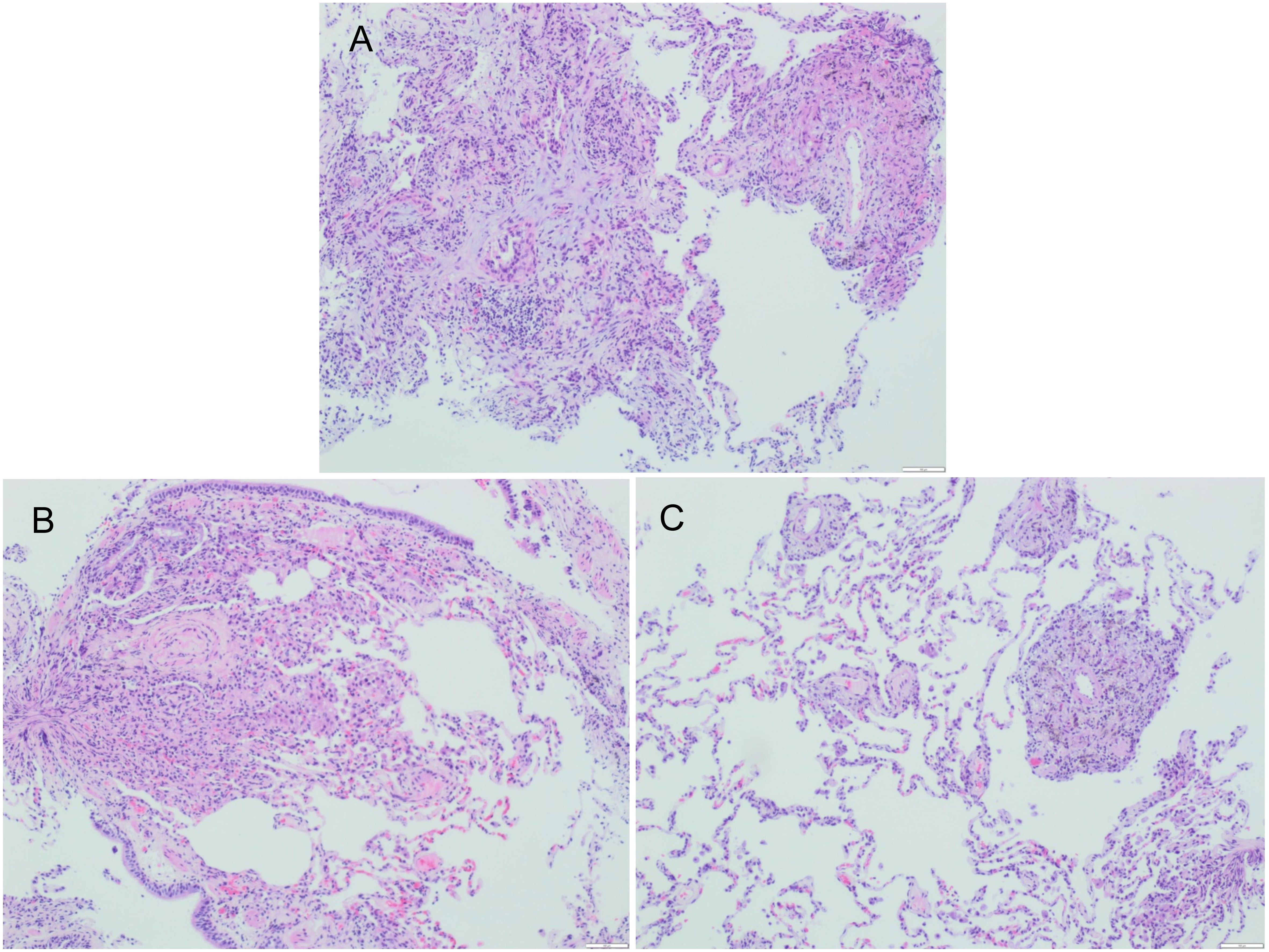
Figure 4. Fibrobronchoscopic lung biopsy specimen of Patient #2, (A–C) showing granuloma (H$E staining).
Treatments and outcome
Immediately after admission, all patients received empirical anti-infection therapy with a beta-lactamase inhibitor or carbapenem or quinolone, and some patients received anti-fungal treatment. However, these treatments did not lead to significant relief of clinical symptoms in any of the 36 patients, and the pulmonary CT lesions were not absorbed in re-examination. This led to the use of mNGS of BALF. The results from mNGS indicated that all patients had infections by NTM, along with other bacteria (Klebsiella pneumoniae, Staphylococcus saprophyticus, Enterococcus faecium, Burkholderia cenocepacia, Haemophilus parainfluenzae, Neisseria sicca), fungi (Candida, Aspergillus, Pneumocystis yersoni), or viruses (Adenovirus, Rhinovirus, Epstein-Barr virus, Severe Acute Respiratory Syndrome Coronavirus 2(SARS-CoV2), Influenza A virus). After the NTM diagnoses, the anti-bacteria regimens were adjusted by adding different appropriate agents (Rifamycin, Ethambutol, Roxithromycin) according to guidelines for diagnosis and treatment of nontuberculous mycobacteriosis (Table 4) (Chinese Medical Association Tuberculosis Branch, 2020). At the same time, the treatments with other anti-infective or antifungal drugs were also adjusted(oseltamivir, fluconazole, or voriconazole) based on the mNGS results. The clinical manifestations in all 36 patients gradually resolved after treatment with roxithromycin, rifampicin, ethambutol, which lasted for 12 months. Figure 5 shows the chest CT results of a typical patient before and after NTM treatment. Chest CT (Figures 5A–E, a-e) showed right upper lung consolidation with thickening of the bronchovascular bundle at the right lung. Scattered ground-glass opacities and nodulars were distributed throughout the pulmonary parenchyma. Right pleural effusion and enlarged right hilar lymph nodes were also observed. (Figures 5A’–E’, a ‘- e’) Follow-up imaging demonstrated significant resolution of the right lung abnormalities. The previously noted right pleural effusion has completely resolved, and the right hilar lymph nodes showed reduction in size.
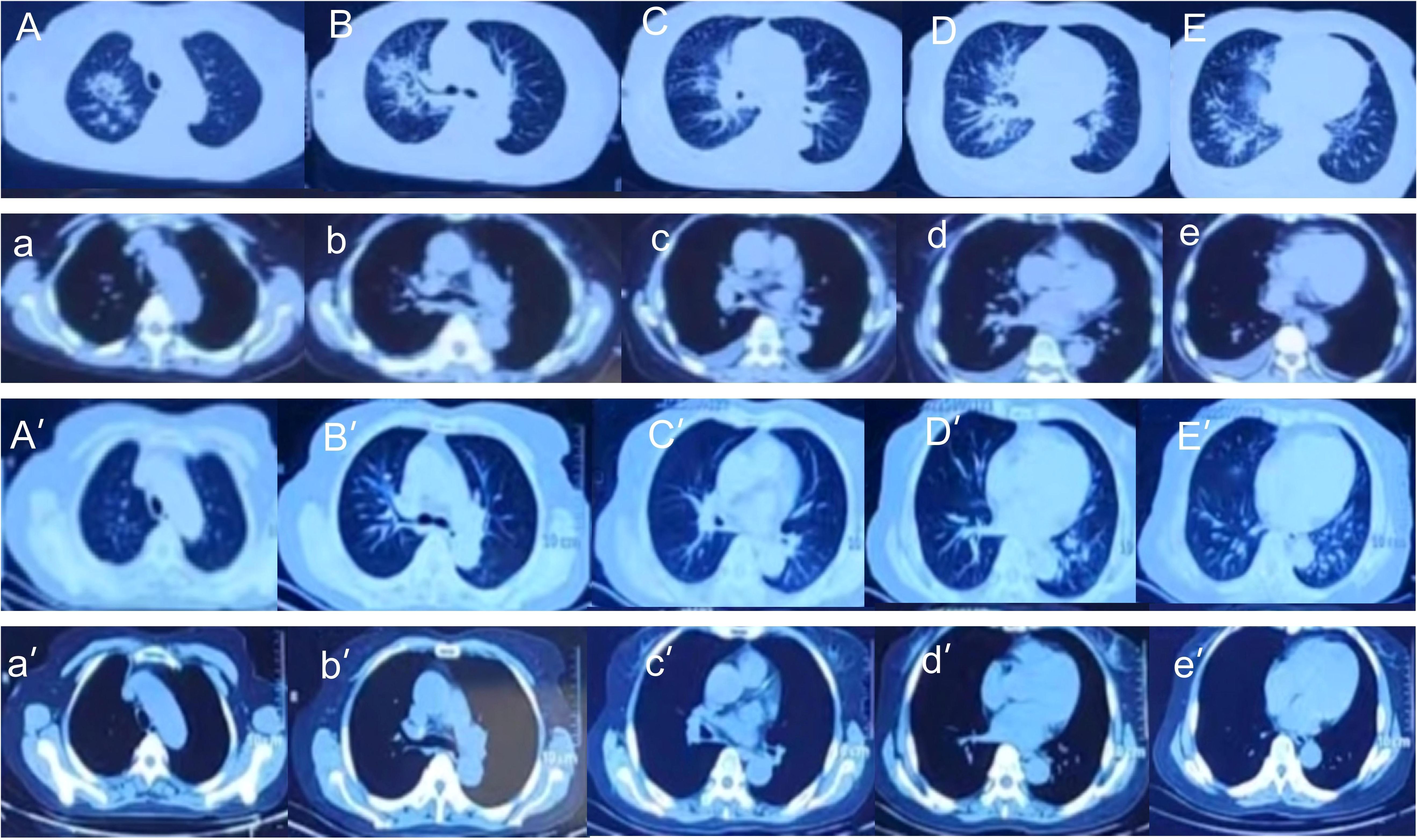
Figure 5. Chest CT imaging of Patient #1 before treatment (top 2 rows) and after treatment (bottom 2 rows). (A–E, a-e) Right upper lung consolidation with thickening of the bronchovascular bundle at the right lung. Scattered ground-glass opacities and nodulars were distributed throughout the pulmonary parenchyma. Right pleural effusion and enlarged right hilar lymph nodes were also observed. (A’–E’, a ‘- e’) Follow-up imaging demonstrated significant resolution of the right lung abnormalities. The previously noted right pleural effusion has completely resolved, and the right hilar lymph nodes showed reduction in size.
Discussion
The prevalence of NTM lung disease continues to increase globally, and it is now a widespread serious public health problem (Ahmed et al., 2020; Johansen et al., 2020; Varghese and Al-Hajoj, 2020). NTM pulmonary disease is a systemic disease caused by different species of NTM that mainly infect the lungs. The different species of NTM are common in natural environments, such as soil, dust, and water, and NTM infections are generally opportunistic and acquired from the environment. Although NTM species are generally less pathogenic to humans than M. tuberculosis, a human host with impaired local or systemic immune response can develop lung lesions. Most NTM lung diseases have slow onset, lack specific clinical manifestations, and are difficult to distinguish from other diseases (Cowman et al., 2019; Loret and Dumoutier, 2019; Chin et al., 2020; Sharma and Upadhyay, 2020; Nogueira et al., 2021). Clinicians may therefore ignore or overlook the existence of NTM lung disease, and patients are often misdiagnosed or remain undiagnosed.
Clinical characteristics and laboratory examinations of NTM mixed infection
The clinical symptoms of the 36 patients in our study were variable, and included fever, cough, sputum production, and dyspnea (Table 1). The lung CT findings were also variable, with evidence of consolidation, nodules, pleural effusion, interstitial changes, and other abnormalities (Table 1, Figure 3). Most of our patients received anti-infection treatments at different hospitals before admission to our hospital, and their clinical symptoms and lung CT results showed no changes following these initial treatments. Due to the receipt of anti-microbial treatment before admission, some patients also had atypical symptoms and laboratory results. After admission to our hospital, we provided empirical antibacterial treatment and some patients also received empirical antifungal treatment. Because the clinical symptoms and lung CT results of the 36 patients did not change after this initial treatment, we performed mNGS detection of BALF in all 36 patients, and TBLB examination for 14 patients.
It is important to improve the understanding of NTM pulmonary disease and to identify NTM species as soon as possible so that prompt and appropriate treatment can be used. The traditional diagnosis of NTM infection relies on clinical manifestations, histopathology, acid-fast smears, and isolation of NTM from culture (Hahn et al., 1987; Maboni et al., 2024). In recent years, the T-SPOT® test, qPCR, and other methods have also been used for detection and diagnosis of NTM infections (Ying et al., 2024). However, these methods can have low sensitivity, are time-consuming, and require special equipment, so many hospitals lack the capability to diagnose NTM pulmonary disease.
Our results are somewhat inconsistent with those from previous studies. Our sputum or BAL cultures were negative for NTM, and the positive rate from acid-fast staining was only 8.3% (3/36), significantly lower than that of mNGS. Moreover, positive acid-fast staining cannot distinguish tuberculosis(TB) from NTM, and use of these results alone could lead to misdiagnosis and inappropriate treatment (Xu et al., 2022). This may be because of the interference or dominance of other strains in the MIs, the small sample size, and possible sample bias. Our pathological positivity rate from TBLB was only 35.7%, possibly due to the small number of samples obtained from the TBLB. We also found that T-SPOT® test for NTM had low sensitivity and specificity, consistent with the literature (Xu et al., 2022).
Use of mNGS for the diagnosis of NTM mixed infections
mNGS can be used to confirm the presence of pathogenic microorganisms in the lower respiratory tract, and this method is also fast, highly accurate, low-cost, has wide coverage, and can be used to discover new pathogens (Zheng et al., 2021; Chen et al., 2022; Diao et al., 2022). mNGS can detect all nucleic acid fragments in a clinical sample, identify the species and types of different pathogens using bioinformatics analysis, and provide quantitative data (such as sequence number and coverage) of all pathogens. An advantage of mNGS is that it may lead to the administration of more appropriate antibiotics in patients with lower respiratory tract infections, and thereby decrease the risk of death (Yan et al., 2025). For example, mNGS can provide reliable diagnosis of invasive pulmonary aspergillosis (Yang et al., 2024) and detection of various pulmonary TB samples. A study of children with pneumonia showed that conventional microbial tests had a positivity rate of 68% relative to mNGS (100%) (Lu et al., 2024). mNGS is therefore an important tool for the etiological diagnosis of pulmonary TB and determining the microecological characteristics of the lungs (Zhou et al., 2024). A retrospective study that compared NTM detection rates from mNGS with the BACTEC Mycobacterial Growth Indicator Tube 960 (the current gold standard for diagnosis of NTM) showed that mNGS had greater sensitivity in analysis of BALF, lung needle biopsy specimens, and sputum (Wei et al., 2023). mNGS can detect NTM quickly and with high sensitivity in a variety of samples, and can also detect pathogens that occur as co-infections. In agreement with previous studies, we found that mNGS was reliable for the detection of mixed MIs and for identification of genes responsible for antibiotic resistance. An additional advantage of mNGS is that it can rapidly screen multiple drug resistance genes associated with the development of NTM disease, and thereby enable patients with multiple and extensively resistant bacterial infections to receive more effective and individualized treatment regimens (Liu et al., 2022; Xu et al., 2022; Matsumoto and Nakamura, 2023). Our retrospective analysis indicated that patients who had MIs with an NTM often had an underlying disease or a coexisting viral infection that contributed to immune dysfunction. The most common coexisting pathogens, including P. aeruginosa, Aspergillus, and several others, are associated with different coexisting underlying diseases, consistent with the traditional understanding of NTM as conditional pathogens (Hahn et al., 1987; Lopes et al., 2022).
Limitations of NGS detection
The advancement of sequencing technology led to significant decreases in the cost of generating sequence data, but the total cost of required reagents and sample processing remains high. The cost of operating and maintaining the bioinformatics analysis process is also relatively high. The significantly higher total cost of mNGS compared to many other clinical methods greatly limits its use in routine clinical applications. However, with the standardization, optimization, and increased automation in mNGS, and the continuing increases in the possible application of mNGS in different clinical scenarios, the total cost of mNGS continues to decrease and it is increasingly accepted by more clinicians and patients (Liu et al., 2025; Qin et al., 2025).
Importantly, we used established auditing standards for NGS testing, and this allowed screening out of nonpathogenic (background) bacteria. At the same time, we also considered the patient’s medical history, clinical symptoms, physical signs, and imaging results when interpreting the NGS findings. After treatment, we observed the patient’s symptoms, laboratory results, and imaging results, especially when the NGS indicated evidence of NTM, which have lower pathogenicity than M. tuberculosis. This cautious approach to diagnosis is necessary because it can be difficult to clearly distinguish background bacteria from pathogenic bacteria. For example, empirical antibiotic treatment is usually ineffective in patients with chronic obstructive pulmonary disease (COPD) who experience difficulty breathing, coughing, and sputum production. However, if a pulmonary CT shows consolidation with a cavity shadow in the right middle lobe of the lung and the NGS result indicates drug-resistant Klebsiella pneumoniae and NTM, we will consider drug-resistant Klebsiella pneumoniae and NTM as pathogenic, rather than background. In this case, we adjust the anti-infective treatment according to the patient’s condition and provide anti-TB treatment. We also observe changes in the patient’s symptoms and imaging results to determine whether further adjustment of treatment is needed. Therefore, we did not rely solely on NGS results. Although NGS has high sensitivity, we still need to make a comprehensive judgment based on the patient’s medical history and other factors. At the same time, we also recognize the possible need to adjust the medications and observe changes in the patient’s condition when interpreting the NGS results.
In short, patients with NTM pulmonary infections can have diverse clinical symptoms and imaging findings, especially those with MIs, and this can lead to misdiagnosis. Therefore, the rapid and accurate identification of NTM and of co-occurring pathogens by mNGS has many important advantages, such as high efficiency, high sensitivity, and wide coverage. mNGS is particularly useful for the accurate identification of NTM.
This study had several limitations. First, it was a single-center retrospective analysis, and most samples were from hospitalized patients with who responded poorly to initial empirical treatment. Therefore, the sample size was small, and the rate of NTM detection was significantly higher than the reported prevalence rate of NTM in the general population. These factors could have biased our detection of NTM mixed bacterial infections. Future large and multi-center studies that use an appropriate control group should be used to analyze the morbidities and co-infection of patients admitted for NTM infection.
Conclusions
As a new technology for etiological diagnosis, mNGS can provide improved sensitivity for the diagnosis of NTM pulmonary infections in patients who have MIs of the lungs. mNGS can also detect multiple bacteria, fungi, and viruses, and therefore reduce the incidence of misdiagnosis and delayed diagnosis and treatment. However, it is also necessary to consider diagnostic specificity and to use strict quality control measures when using mNGS for diagnosis of NTM pulmonary infections.
Data availability statement
The data presented in the study are deposited in the China National center for Bioinformation(CNCB) repository, accession number PRJCA041435.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from All patients gave written informed consent for personal and clinical details, along with any identifying images, to be published in this study. A copy of the consents form is available for review by the Editor of this journal. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JY: Data curation, Writing – original draft. XL: Formal analysis, Writing – original draft. QW: Data curation, Writing – original draft. MG: Data curation, Writing – original draft. WL: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant no. 81800080), Natural Science Foundation of Jilin Province (grant no. YDZJ202201ZYTS120).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NTM, non-tuberculous mycobacteria; MI, mixed infection; mNGS, next generation metagenomic sequencing; BALF, bronchoalveolar lavage fluid; TBLB, transbronchial lung biopsy; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PCT, procalcitonin; CT, chest computed tomography; AFB, Acid-fast bacilli; PCR, polymerase chain reaction; NC, negative control; SDs, standard deviations; BMI, Body Mass Index; SARS-CoV2, Severe Acute Respiratory Syndrome Coronavirus 2; TB, tuberculosis; COPD, chronic obstructive pulmonary disease.
References
Ahmed, I., Tiberi, S., Farooqi, J., Jabeen, K., Yeboah-Manu, D., Migliori, G. B., et al. (2020). Non-tuberculous mycobacterial infections-A neglected and emerging problem. Int. J. Infect. Dis. 92S, S46–S50. doi: 10.1016/j.ijid.2020.02.022
Chen, S., Kang, Y., Li, D., and Li, Z. (2022). Diagnostic performance of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in patients with pulmonary infections: Systematic review and meta-analysis. Int. J. Infect. Dis. 122, 867–873. doi: 10.1016/j.ijid.2022.07.054
Chin, K. L., Sarmiento, M. E., Alvarez-Cabrera, N., Norazmi, M. N., and Acosta, A. (2020). Pulmonary non-tuberculous mycobacterial infections: current state and future management. Eur. J. Clin. Microbiol. Infect. Dis. 39, 799–826. doi: 10.1007/s10096-019-03771-0
Chinese Medical Association Tuberculosis Branch (2020). Guidelines for the Diagnosis and Treatment of nontuberculous Mycobacteriosis (2020 edition). Chin. J. Tuberculosis Respir. Dis. 43, 918–946.
Cowman, S., van Ingen, J., Griffith, D. E., and Loebinger, M. R. (2019). Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J., 54. doi: 10.1183/13993003.00250-2019
Cullen, A. R., Cannon, C. L., Mark, E. J., and Colin, A. A. (2000). Mycobacterium abscessus infection in cystic fibrosis. Colonization or infection? Am. J. Respir. Crit. Care Med. 161, 641–645. doi: 10.1164/ajrccm.161.2.9903062
Diao, Z., Han, D., Zhang, R., and Li, J. (2022). Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J. Adv. Res. 38, 201–212. doi: 10.1016/j.jare.2021.09.012
Gopalaswamy, R., Shanmugam, S., Mondal, R., and Subbian, S. (2020). Of tuberculosis and non-tuberculous mycobacterial infections - a comparative analysis of epidemiology, diagnosis and treatment. J. BioMed. Sci. 27, 74. doi: 10.1186/s12929-020-00667-6
Hahn, Y. S., McLone, D. G., and Uden, D. (1987). Cervical intervertebral disc calcification in children. Childs Nerv Syst. 3, 274–277. doi: 10.1007/BF00271822
Hilt, E. E. and Ferrieri, P. (2022). Next generation and other sequencing technologies in diagnostic microbiology and infectious diseases. Genes (Basel) 13 (9), 1566. doi: 10.3390/genes13091566
Johansen, M. D., Herrmann, J. L., and Kremer, L. (2020). Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 18, 392–407. doi: 10.1038/s41579-020-0331-1
Kalaiarasan, E., Thangavelu, K., Krishnapriya, K., Muthuraj, M., Jose, M., and Joseph, N. M. (2020). Diagnostic performance of real time PCR and MALDI-TOF in the detection of nontuberculous mycobacteria from clinical isolates. Tuberculosis (Edinb) 125, 101988. doi: 10.1016/j.tube.2020.101988
Khare, R. and Brown-Elliott, B. A. (2023). Culture, identification, and antimicrobial susceptibility testing of pulmonary nontuberculous mycobacteria. Clin. Chest Med. 44, 743–755. doi: 10.1016/j.ccm.2023.06.001
Liu, B., Bao, Z., Chen, W., Xi, X., Ge, X., Zhou, J., et al. (2025). Targeted next-generation sequencing in pneumonia: applications in the detection of responsible pathogens, antimicrobial resistance, and virulence. Infect. Drug Resist. 18, 407–418. doi: 10.2147/IDR.S504392
Liu, Y., Ma, X., Chen, J., Wang, H., and Yu, Z. (2022). Nontuberculous mycobacteria by metagenomic next-generation sequencing: Three cases reports and literature review. Front. Public Health 10, 972280. doi: 10.3389/fpubh.2022.972280
Lopes, M., Batista, M., Garcia, T., Alves, H., Boaventura, L., Pontes, C., et al. (2022). Non-tuberculous mycobacteria: clinical and laboratory characterization (2009 and 2019). Epidemiol. Infect. 151, e8. doi: 10.1017/S0950268822000899
Loret, J. F. and Dumoutier, N. (2019). Non-tuberculous mycobacteria in drinking water systems: A review of prevalence data and control means. Int. J. Hyg Environ. Health 222, 628–634. doi: 10.1016/j.ijheh.2019.01.002
Lu, S., Sun, L., Cao, L., Zhao, M., Guo, Y., Li, M., et al. (2024). Analysis of lung microbiota in pediatric pneumonia patients using BALF metagenomic next-generation sequencing: A retrospective observational study. Med. (Baltimore) 103, e40860. doi: 10.1097/MD.0000000000040860
Luan, Y., Hu, H., Liu, C., Chen, B., Liu, X., Xu, Y., et al. (2021). et al: A proof-of-concept study of an automated solution for clinical metagenomic next-generation sequencing. J. Appl. Microbiol. 131, 1007–1016. doi: 10.1111/jam.v131.2
Maboni, G., Prakash, N., and Moreira, M. A. S. (2024). Review of methods for detection and characterization of non-tuberculous mycobacteria in aquatic organisms. J. Vet. Diagn. Invest. 36, 299–311. doi: 10.1177/10406387231194619
Matsumoto, Y. and Nakamura, S. (2023). Rapid and comprehensive identification of nontuberculous mycobacteria. Methods Mol. Biol. 2632, 247–255. doi: 10.1007/978-1-0716-2996-3_17
Morais, F. C. L., Bello, G. L., Costi, C., Schmid, K. B., Soares, T. D. S., Barcellos, R. B., et al. (2022). Detection of non-tuberculosis mycobacteria (NTMs) in lung samples using 16S rRNA. Mem Inst Oswaldo Cruz 117, e220031. doi: 10.1590/0074-02760220031
Nogueira, L. B., Garcia, C. N., Costa, M., Moraes, M. B., Kurizky, P. S., and Gomes, C. M. (2021). Non-tuberculous cutaneous mycobacterioses. Bras. Dermatol. 96, 527–538. doi: 10.1016/j.abd.2021.04.005
Porvaznik, I., Solovic, I., and Mokry, J. (2017). Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Adv. Exp. Med. Biol. 944, 19–25. doi: 10.1007/5584_2016_45
Qin, L., Liang, M., Song, J., Chen, P., Zhang, S., Zhou, Y., et al. (2025). Utilizing targeted next-generation sequencing for rapid, accurate, and cost-effective pathogen detection in lower respiratory tract infections. Infect. Drug Resist. 18, 329–340. doi: 10.2147/IDR.S494558
Quan, Z., Haiming, T., Xiaoyao, C., Weifeng, Y., Hong, J., and Hongfei, Z. (2017). Development of one-tube multiplex polymerase chain reaction (PCR) for detecting Mycobacterium bovis. J. Vet. Med. Sci. 78, 1873–1876. doi: 10.1292/jvms.15-0216
Schlaberg, R., Chiu, C. Y., Miller, S., Procop, G. W., Weinstock, G., Professional Practice, C., et al. (2017). Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch. Pathol. Lab. Med. 141, 776–786. doi: 10.5858/arpa.2016-0539-RA
Sharma, S. K. and Upadhyay, V. (2020). Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J. Med. Res. 152, 185–226. doi: 10.4103/ijmr.IJMR_902_20
Shen, H., Shen, D., Song, H., Wu, X., Xu, C., Su, G., et al. (2021). Clinical assessment of the utility of metagenomic next-generation sequencing in pediatric patients of hematology department. Int. J. Lab. Hematol. 43, 244–249. doi: 10.1111/ijlh.13370
Varghese, B. and Al-Hajoj, S. (2020). A global update on rare non-tuberculous mycobacteria in humans: epidemiology and emergence. Int. J. Tuberc Lung Dis. 24, 214–223. doi: 10.5588/ijtld.19.0194
Wei, W., Cao, J., Wu, X. C., Cheng, L. P., Shen, X. N., Sha, W., et al. (2023). Diagnostic performance of metagenomic next-generation sequencing in non-tuberculous mycobacterial pulmonary disease when applied to clinical practice. Infection 51, 397–405. doi: 10.1007/s15010-022-01890-z
Wilson, M. R., Sample, H. A., Zorn, K. C., Arevalo, S., Yu, G., Neuhaus, J., et al. (2019). Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl. J. Med. 380, 2327–2340. doi: 10.1056/NEJMoa1803396
Xu, P., Yang, K., Yang, L., Wang, Z., Jin, F., Wang, Y., et al. (2022). Next-generation metagenome sequencing shows superior diagnostic performance in acid-fast staining sputum smear-negative pulmonary tuberculosis and non-tuberculous mycobacterial pulmonary disease. Front. Microbiol. 13, 898195. doi: 10.3389/fmicb.2022.898195
Yan, M., Shang, L., Wang, Y., Wang, C., and Cao, B. (2025). Metagenomic next-generation sequencing on treatment strategies and prognosis of patients with lower respiratory tract infections: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 65, 107440. doi: 10.1016/j.ijantimicag.2024.107440
Yang, J., Wu, X., Zhang, Q., Lin, C., Yu, Y., Zhang, X., et al. (2024). Metagenomic next-generation sequencing and galactomannan testing for the diagnosis of invasive pulmonary aspergillosis. Sci. Rep. 14, 31389. doi: 10.1038/s41598-024-82806-9
Ying, C., Zhang, L., Jin, X., Zhu, D., and Wu, W. (2024). Advances in diagnosis and treatment of non-tuberculous mycobacterial lung disease. Diagn. Microbiol. Infect. Dis. 109, 116254. doi: 10.1016/j.diagmicrobio.2024.116254
Zheng, Y., Qiu, X., Wang, T., and Zhang, J. (2021). The diagnostic value of metagenomic next-generation sequencing in lower respiratory tract infection. Front. Cell Infect. Microbiol. 11, 694756. doi: 10.3389/fcimb.2021.694756
Zhou, H., Pei, Y., Xie, Q., Nie, W., Liu, X., Xia, H., et al. (2024). Diagnosis and insight into the unique lung microbiota of pediatric pulmonary tuberculosis patients by bronchoalveolar lavage using metagenomic next-generation sequencing. Front. Cell Infect. Microbiol. 14, 1492881. doi: 10.3389/fcimb.2024.1492881
Keywords: non-tuberculous mycobacteria (NTM), metagenomic next generation sequencing (mNGS), mixed infection (MI), bronchoalveolar lavage fluid (BALF), diagnosis, treatment
Citation: Yu J, Lv X, Wang Q, Gao M and Li W (2025) Metagenomic sequencing for identification of nontuberculous mycobacteria and other pathogens in patients with mixed infection of the lung. Front. Cell. Infect. Microbiol. 15:1592216. doi: 10.3389/fcimb.2025.1592216
Received: 12 March 2025; Accepted: 30 May 2025;
Published: 19 June 2025.
Edited by:
Lia Danelishvili, Oregon State University, United StatesReviewed by:
Arryn Craney, Petrified Bugs LLC, United StatesDeby Kusumaningrum, Airlangga University, Indonesia
Copyright © 2025 Yu, Lv, Wang, Gao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, bGl3ZWkxNDE3QGpsdS5lZHUuY24=
 Jinyan Yu
Jinyan Yu Xuejiao Lv
Xuejiao Lv Qi Wang1
Qi Wang1 Wei Li
Wei Li