- 1Department of Experimental Pathology, Immunology and Microbiology, Faculty of Medicine, American University of Beirut, Beirut, Lebanon
- 2Center for Infectious Diseases Research, American University of Beirut, Beirut, Lebanon
- 3World Health Organization (WHO) Collaborating Center for Reference and Research on Bacterial Pathogens, Beirut, Lebanon
Background: Helicobacter pylori is a globally prevalent bacterium associated with several gastrointestinal diseases, including peptic ulcers and gastric cancer. Growing interest has emerged in understanding how H. pylori affects gut microbiota and whether eradication therapies impact microbial balance, potentially influencing disease outcomes, including cancer progression.
Methods: A systematic review was conducted across PubMed, Scopus, and Web of Science databases using predefined keywords and Medical Subject Headings (MeSH) terms. Quality assessment was performed using the MINORS and Jadad scales.
Results: A total of 45 studies met the inclusion criteria, which evaluated microbial changes in H. pylori -infected individuals before and after eradication therapies. H. pylori infection resulted in significant alterations in gut and gastric microbiota, with a notable increase in inflammation-associated bacteria, such as Proteobacteria and Streptococcus. In gastric cancer patients, microbial diversity was reduced, with decreased levels of Bifidobacterium and Actinobacteria, and increased levels of Prevotella and Dialister, both associated with pro-inflammatory environments. Eradication therapies generally worsened dysbiosis initially, but probiotic supplementation promoted faster recovery of beneficial bacteria, improving microbial balance and reducing cancer-related dysbiosis.
Conclusion: H. pylori infection disrupts the gut microbiota, with eradication therapies further altering microbial composition. The restoration of microbial diversity is improved by probiotic supplementation. Understanding the long-term impacts of these therapies on gut health is essential for refining treatment strategies, particularly in preventing H. pylori -associated diseases like gastric cancer.
1 Introduction
Helicobacter pylori (H. pylori) is a small, spiral-shaped Gram-negative bacterium that measures between 0.5 to 1 μm in width and 2 to 4 μm in length (Öztekin et al., 2021). Its discovery in the 1980s transformed the understanding of gastrointestinal diseases, particularly peptic ulcers, which were previously attributed to stress and diet (Bornschein and Pritchard, 2022). The unique ability of H. pylori to survive in the acidic environment of the human stomach allows it to colonize over 50% of the global population, though many individuals remain asymptomatic (Frost et al., 2019; Fong, 2020; Charitos et al., 2021). In others, however, the infection can lead to gastritis, peptic ulcer disease, or even gastric cancer (Duan and Xu, 2025).
The bacterium survives the acidity of the stomach by producing urease, an enzyme that neutralizes stomach acid by converting urea into ammonia (Cunha et al., 2021). While this adaptation aids in its colonization, it also damages the stomach lining, contributing to ulcer formation in some people (Ali and AlHussaini, 2024). Testing for H. pylori is essential in patients with peptic ulcer symptoms or a family history of gastric cancer, though routine screening is not advised unless there is a clinical need (Garman et al., 2024). Beyond urease, other virulence factors, such as vacuolating cytotoxin A (vacA) and cytotoxin-associated gene A (cagA protein), are linked to varying disease outcomes (Baj et al., 2020). Notably, the cagA-positive strain of H. pylori is associated with a higher risk of gastric cancer (Peng et al., 2024).However, the reasons why some individuals develop severe disease while others remain asymptomatic remain unclear (Alexander et al., 2021).
Recent research has focused on the gut microbiota in patients with H. pylori infections, particularly in light of growing evidence linking gut microbiota dysbiosis with various health conditions (Yadav and Chauhan, 2024). Dysbiosis, or the imbalance in gut microbial communities, has been associated with gastrointestinal diseases and systemic conditions such as metabolic syndrome and cardiovascular disease (Yu et al., 2021). Importantly, dysbiosis is also implicated in the development of gastric cancer, which is the third leading cause of cancer-related deaths globally (Huang et al., 2023). H. pylori is believed to contribute to dysbiosis by inducing chronic inflammation, altering immune responses, and disrupting the natural microbial balance of the gut (Bakhti and Latifi-Navid, 2021). This disruption weakens the defenses of the stomach and facilitates the development of pre-cancerous lesions, which may promote the growth of harmful bacteria that accelerate cancer progression (Bakhti and Latifi-Navid, 2021).
This systematic review explores the relationship between gut microbiota and H. pylori, focusing on the gastrointestinal changes observed with and without therapeutic intervention. Additionally, it examines the impact of H. pylori on gut microbiota in patients with gastric cancer, highlighting the potential role of these microbial shifts in cancer progression.
2 Methodology
2.1 Search method and databases
A comprehensive search strategy was performed by multiple reviewers across PubMed, Scopus, and Web of Science using three keyword and MeSH term lists. These keywords were linked with the OR function, and lists were combined with the AND function, while the NOT function excluded review articles (Supplementary Data 1). No restrictions were placed on publication dates. Duplicates were removed using Rayyan AI and verified manually (Supplementary Data 2). The screening process included two stages: an initial independent screening of titles and abstracts, followed by a full-text review to confirm inclusion criteria. Data extraction involved details such as title, publication year, patient numbers, country, age, sample type, treatment regimen, microbial changes in H. pylori-positive patients, and consensus was achieved through discussion.
2.2 Inclusion criteria
The systematic review included original articles (Randomized Controlled Trials, Cohort Studies, Case-Control Studies, Cross-Sectional Studies, and Longitudinal Studies). Studies examining the impact of H. pylori infection without treatment on the composition of the gut and gastric microbiota, the effects of various H. pylori eradication antibiotic regimens on gut and gastric microbiome composition, and research reporting microbiota composition analyses in H. pylori-positive patients with gastric cancer were also considered.
2.3 Exclusion criteria
Studies that are not original articles (Reviews, Meta-analyses, Editorials, Chapters, Reports), involving animal models, or involving patients with recent antibiotic use were excluded.
2.4 Quality assessment
The quality of the included studies was assessed using the MINORS and Jadad scales. The MINORS tool, commonly used in systematic reviews and meta-analyses, was applied for non-randomized studies, distinguishing between comparative and non-comparative designs (Slim et al., 2003). Comparative studies were scored out of 24, while non-comparative studies were scored out of 16, with studies categorized into quartiles based on their scores. The Jadad scale was used for randomized controlled trials (RCTs), evaluating randomization, blinding, and withdrawals/dropouts, with scores ranging from 0 to 5, where higher scores indicated better quality (De Cassai et al., 2023).
3 Results
3.1 Search strategy outcomes and quality evaluation
3.1.1 Databases search outcomes
Two search queries identified relevant studies. Query 1 initially retrieved 1,640 articles, reduced to 808 after removing duplicates, while Query 2 retrieved 371, reduced to 208. From these, 307 articles from Query 1 and 192 from Query 2 were excluded as they were primarily reviews or animal studies. Further, 460 articles from Query 1 were removed, and 9 from Query 2 for being off topic based on title-abstract review. Ultimately, after retrieving full texts one study from Query 1 was excluded due to recent antibiotic use, and two from Query 2 were excluded for addressing gastric diseases unrelated to gastric cancer, resulting in 45 studies included in the systematic review. A PRISMA diagram summarizes this process (Supplementary Data 3).
3.1.2 Quality assessment
All 37 non-randomized articles were evaluated using the MINORS scale, which is suitable given the observational design of the included studies. The overall average MINORS score is 16.13± 3.47, with scores ranging from 9 to 21. Comparative studies have an average score of 17.82± 1.31 (range 16-21), while non-comparative studies have an average of 10 ± 0.53 (range 9-11). Among the comparative studies, 23 are in the third quartile and 6 in the fourth, while the 8 non-comparative studies are in the third quartile (Supplementary Table S1). For the randomized studies, all 8 studies were evaluated using the Jadad scale. The overall average Jadad score is 3.5± 0.74, with scores ranging from 3-5 (Supplementary Table S2).
3.2 Findings of studies evaluating the gut microbiota of Hp+ patients without treatment
In this section (Supplementary Table S3), 14 studies were analyzed. In total, gastric microbiota signatures were evaluated in 207 children (Age range: <14 years), 159 adolescents (Age range: 14–17 years), and 1,148 adults (Age range: >18). Various sample types were analyzed, including gastric biopsies, gastric fluid, and stool samples. The methods employed for taxonomic analysis included: 16S rRNA gene sequencing, shotgun metagenomic sequencing, and whole-genome sequencing.
In studies focusing on children and adolescents, alpha diversity was reported to increase in three studies (two stool samples, one gastric fluid) and decrease in two studies (gastric biopsies). Beta diversity remained unchanged in one study (gastric fluid), decreased in one (gastric biopsy), and distinguished between H. pylori-positive and -negative groups in two studies (gastric biopsy, stool). In gastric biopsy samples, Actinobacteria, Bacteroidetes, and Firmicutes levels decreased, while Proteobacteria levels increased in two studies. In gastric fluid samples, Actinobacteria and Lactobacillus levels decreased, whereas Streptococcus levels increased. In stool samples, Actinobacteria, Bacteroidetes, Clostridium, Eubacterium (in two studies), Firmicutes, Lactobacillus, Prevotella (in two studies), and Proteobacteria levels increased. Bifidobacterium levels showed mixed results, decreasing in one study and increasing in another, while Streptococcus levels increased in one study and remained unchanged in another (Supplementary Table S3).
In adult populations, alpha diversity increased in four studies (three stool, one gastric biopsy), decreased in three (two gastric biopsy, one stool), and showed no significant change in one (stool). Beta diversity differed between Hp+ and Hp- groups in five studies (three stool, two gastric biopsy). Lactobacillus levels decreased in one study (gastric biopsy) and showed no significant change in another (stool). Proteobacteria increased in two studies (stool, gastric biopsy) and decreased in one (gastric biopsy). Prevotella levels increased in three studies (two stool, one gastric biopsy), while Bifidobacterium decreased in one study (gastric biopsy) with no significant change in another (stool). Haemophilus increased in two studies (gastric biopsy, stool), and Verrucomicrobia phyla decreased in two studies (stool, gastric biopsy), with one study also reporting a decrease in Akkermansia muciniphila at the species level (stool), although this specie showed no significant change in another study (stool). The Acinetobacter genus decreased in one study, while the species Acinetobacter baumannii increased in another (both gastric biopsy). Bacteroides decreased in three studies (two stool, one gastric biopsy), and E. coli decreased in two studies (gastric biopsy, stool). One study found that the overall composition of the gastric microbiota in H. pylori-infected adults was like that of non-infected adults (gastric fluid) (Supplementary Table S3).
3.3 Findings of the studies evaluating the gut microbiota of Hp+ patients after different treatments
The population characteristics in the studies mainly focus on adult subjects, with sample sizes ranging from 11 to 1,214 participants (Supplementary Table S4). The age range of participants is typically between 3 and 80 years. The studies primarily utilize stool samples to analyze gut microbiota changes, after various therapeutic regimens for H. pylori. The methods used for taxonomic analysis are as follows: 81% of the studies employed 16S rRNA gene sequencing, 11% applied quantitative bacteriological culture techniques, 7% utilized shotgun metagenomic sequencing, and 3% used whole genome sequencing.
A total of 18 studies evaluated eradication therapies without probiotic supplementation, 9 of which employed triple therapy. Within 1–2 weeks of treatment initiation, these studies demonstrated variable effects on microbial diversity. Among those using triple therapy, 33.33% reported a decrease in alpha diversity, 22.22% showed significant changes in beta diversity, and 11.11% reported no significant change in beta diversity.
7 studies investigated the impact of bismuth quadruple therapy. At 1–2 weeks post-treatment, alpha diversity was reduced in 71.4% of studies, and beta diversity changes were significant in 57.1%. 3 studies assessed the effects of standard (non-bismuth) quadruple therapy. Within 1–2 weeks, alpha diversity decreased in 33.33% of studies, increased in another 33.33%, and beta diversity changes were significant in 66.66%. Levofloxacin-based quadruple therapy was evaluated in one study, which reported a reduction in alpha diversity and significant beta diversity alterations at week 2. High-dose dual therapy (HDDT) was investigated in a single study, where both alpha diversity was reduced and beta diversity significantly altered at week 2. Another study assessed vonoprazan–amoxicillin dual therapy (VA-dual therapy), which showed no significant changes in either alpha or beta diversity at 1 week. Polaprezinc-containing therapy was reported in one study, with no significant changes observed in alpha diversity at week 4.
At 2–3 months post-treatment, alpha diversity decreased in 11.1% of studies, showed no significant change in 16.6%, and had mixed outcomes in 27.7%, with reductions noted in C10, BQT, and EAML therapies at the genus level and VACT therapies. No significant change was seen with T14, VA dual therapy, HDDT, EAML at the species level, and PQT. Additionally, 5.5% of studies reported incomplete recovery, and another 5.5% showed restoration. Beta diversity showed significant changes in 5.5% of studies, recovery in 5.5%, no significant change in 11.1%, and mixed results in 22.2%. Restoration of beta diversity was observed with T14 therapy and EAML therapy at the species level, while significant changes were reported with C10, BQT, EAML at the genus level, and VAC TT therapies. In contrast, VA Dual therapy showed no significant beta diversity change.
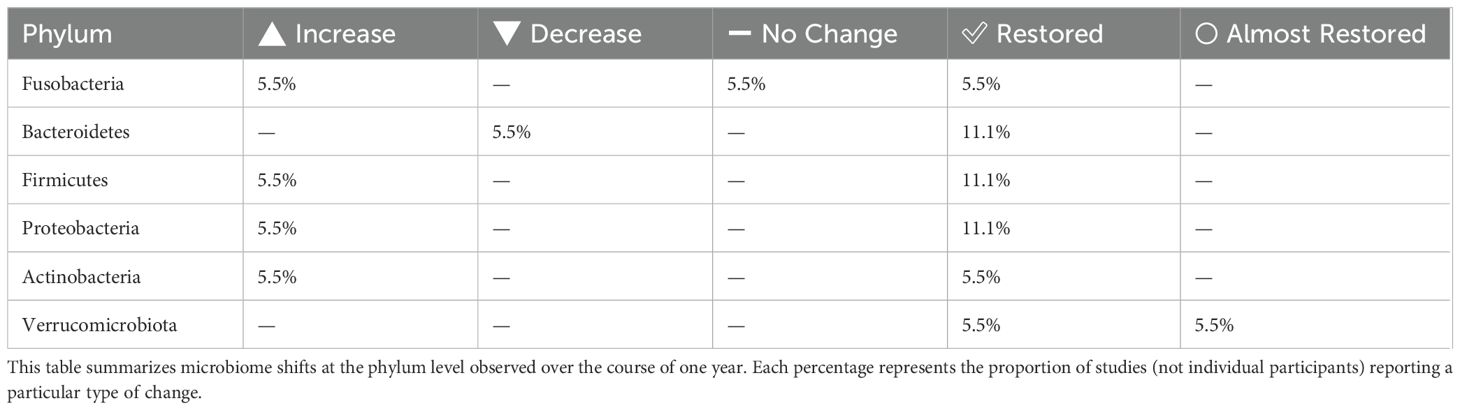
To facilitate clearer comparison and summary of the findings across different time points, the results are organized into the following tables. The impact of H. pylori eradication therapies on gut microbiota composition was assessed at both mid-term (2–3 months) and long-term (1 year) follow-ups. At 2–3 months, notable changes were observed across several phyla, with frequent decreases in Actinobacteria and Firmicutes, and mixed responses in Bacteroidetes and Proteobacteria (Table 1). One year post-treatment, alpha and beta diversity showed partial restoration in some studies, though changes persisted in others (Table 2). Phylum-level analysis at the 1-year mark revealed improved recovery trends in Firmicutes, Bacteroidetes, and Proteobacteria, while Verrucomicrobiota and Actinobacteria remained variably affected (Table 3). The impact of H. pylori eradication therapies on gut microbiota composition was assessed at both mid-term (2–3 months) and long-term (1 year) follow-ups. At 2–3 months, notable changes were observed across several phyla, with frequent decreases in Actinobacteria and Firmicutes, and mixed responses in Bacteroidetes and Proteobacteria (Table 1). One year post-treatment, alpha and beta diversity showed partial restoration in some studies, though changes persisted in others (Table 2). Phylum-level analysis at the 1-year mark revealed improved recovery trends in Firmicutes, Bacteroidetes, and Proteobacteria, while Verrucomicrobiota and Actinobacteria remained variably affected (Table 3).
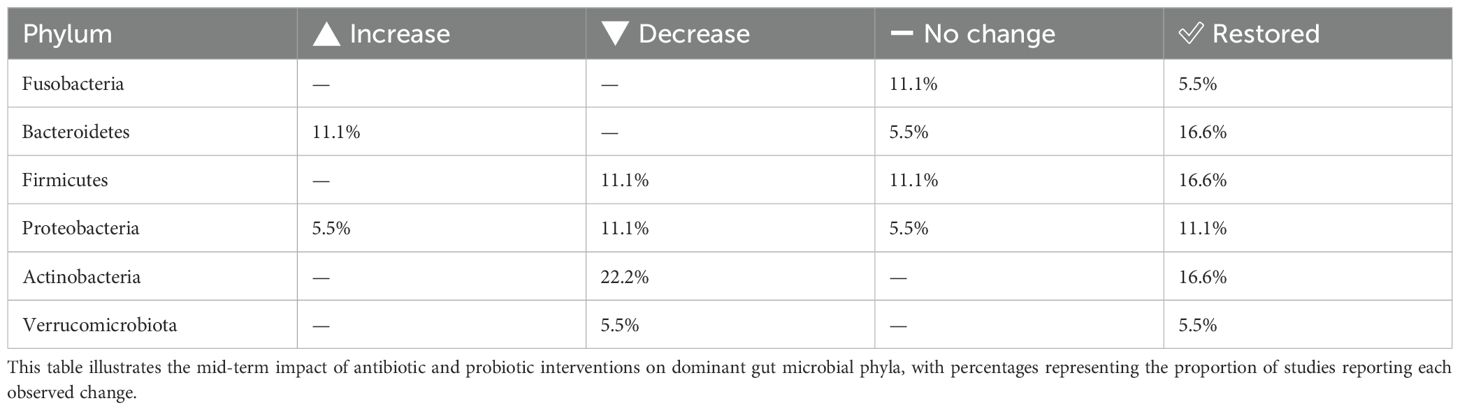
Table 1. Phylum-level gut microbiota changes observed 2–3 months after H. pylori eradication therapy.
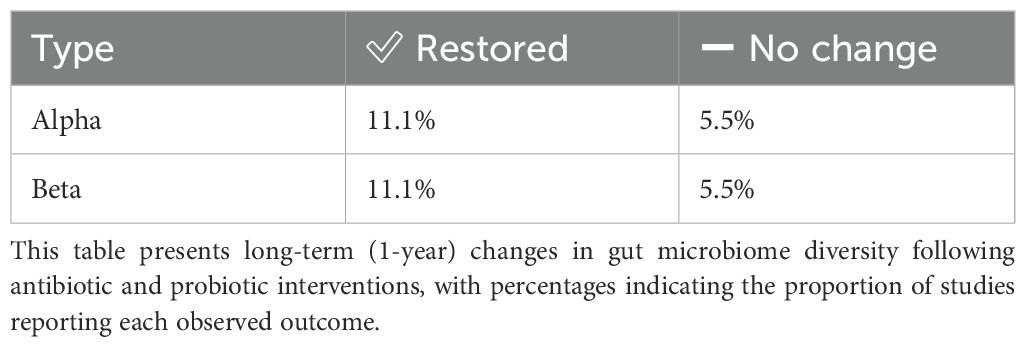
Table 2. Long-term (1-Year) changes in alpha and beta diversity following H. pylori eradication therapy.
Among the 18 included studies, 5 conducted comparative analyses between different H. pylori eradication therapies. These studies consistently showed that bismuth-based quadruple therapies (BQ10 and BQT) were associated with greater reductions in alpha diversity compared to other regimens such as T14, EAML14, and PQT. Beta diversity significantly distinguished treatment groups in all five studies, indicating distinct shifts in microbial community structure. In particular, BQT was linked to slower microbial recovery and greater dysbiosis compared to HDDT and PQT. Taxonomic analyses revealed increased Proteobacteria and decreased Firmicutes and Bacteroidetes shortly after treatment, with partial restoration over time. Species-level changes, such as elevated K.pneumoniae and Enterococcus spp., were more pronounced in the BQT group. Additionally, certain taxa like Parasutterella, Ruminococcus, and Anaerostipes were differentially abundant across treatment arms, highlighting therapy-specific microbial impacts.
It is worth noting that while all the included studies assessed the impact of H. pylori eradication therapies on gut microbiota composition, only two studies focused on pediatric populations, one involving children aged 3 to 14 and the other adolescents aged 15 to 16.
Among the studies evaluating H. pylori eradication regimens supplemented with probiotics, a range of strains were employed, including Clostridium butyricum (MIYA-BM®), Bacillus subtilis, Enterococcus faecium, and Saccharomyces boulardii. These were administered in various forms, tablets, enteric capsules, and powders, at differing doses and durations. Despite this heterogeneity, most studies reported favorable outcomes such as reduced dysbiosis, improved gastrointestinal symptoms, and enhanced eradication rates. Only one study involving pediatric participants aged 7 to 8 years also observed symptom relief and microbial stabilization with probiotic use.
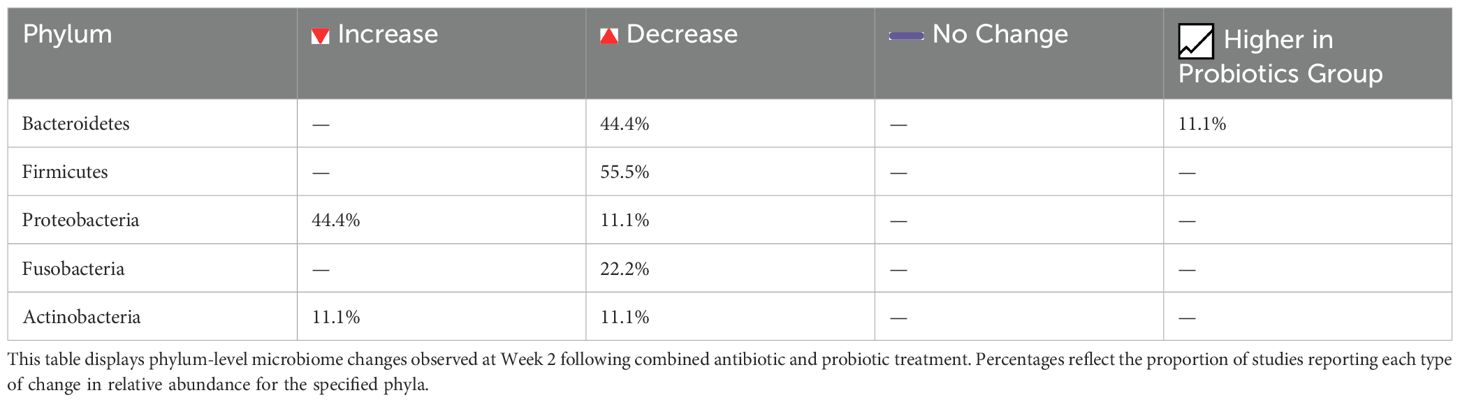
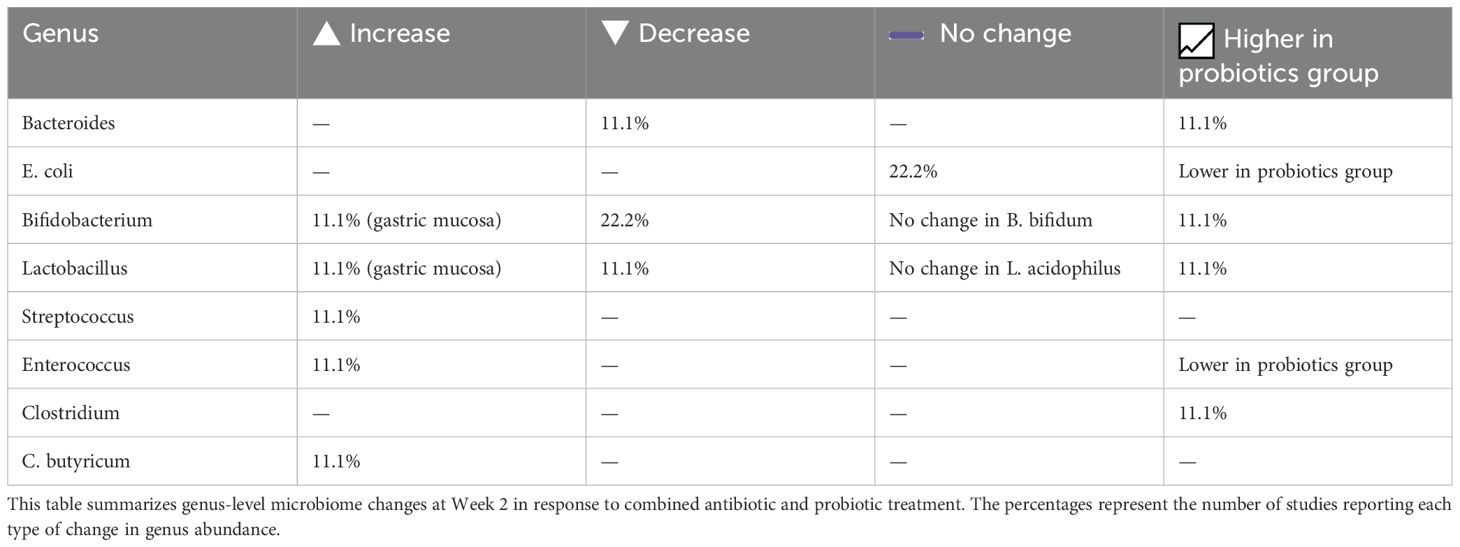
To complement the narrative description above, the following tables provide a structured summary of microbiome changes observed at Week 2 and during long-term follow-up. At Week 2, alpha diversity was reported to decrease in nearly half of the studies, while beta diversity showed significant compositional shifts in a subset of cases, reflecting the early impact of combined antibiotic and probiotic therapy (Table 4). Phylum-level changes at this time point highlighted marked reductions in Firmicutes and Bacteroidetes, alongside increased levels of Proteobacteria and Fusobacteria, with certain taxa found to be more abundant in the probiotic-treated groups (Table 5). At the genus level, notable changes included decreases in Bacteroides, E. coli, and Enterococcus, while beneficial genera such as Bifidobacterium, Lactobacillus, and Clostridium butyricum were more frequently preserved or increased in probiotic groups (Table 6). Long-term follow-up (1, 2, and 12 months) showed that most bacterial groups gradually returned to baseline, with transient fluctuations, such as temporary elevations in Enterococcus and Bacillus, resolving by Week 6 in almost all studies (Table 7).

Table 4. Microbiome diversity changes at week 2 following combined antibiotic and probiotic therapy.
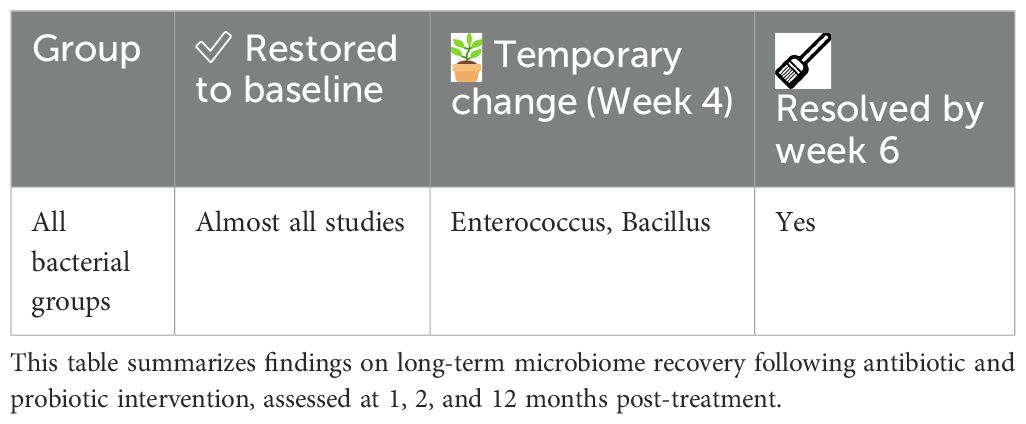
Table 7. Long-term recovery of gut microbiota at 1, 2, and 12 months after antibiotic and probiotic therapy.
100% of the studies that assessed different H. pylori eradication therapies in combination with probiotics reported that probiotic supplementation modulated gut microbiota composition in a treatment-specific manner. Across these studies, probiotics consistently attenuated the decline in alpha diversity and contributed to distinct beta diversity profiles between treatment groups. Several regimens, particularly those involving bismuth-based quadruple therapy, initially reduced beneficial taxa such as Bifidobacterium and Lactobacillus, while promoting opportunistic pathogens like Klebsiella, Enterobacter, and Shigella; however, the addition of probiotics helped suppress these shifts and facilitated microbial recovery. Notably, co-administration of Clostridium butyricum (CBM588) led to a dose-dependent decrease in C. difficile toxin A detection. Probiotic-treated groups also showed higher levels of beneficial genera (Faecalibacterium, Bacteroides) and more stable microbial communities, with several studies reporting near-complete restoration of baseline microbiota by week 22. Collectively, these findings underscore the consistent role of probiotics in mitigating dysbiosis and promoting microbiome resilience across all 9 studies evaluating combination therapies.
3.4 Findings of studies evaluating the gut microbiota alterations in H. pylori positive patients with gastric cancer
Five studies on microbiota changes in H. pylori-positive gastric cancer patients included participants aged 45 to 71, with one study focusing on patients under 18 (Supplementary Table S5). All studies used 16S rRNA gene sequencing for taxonomic analysis, while one also employed whole genome metagenomic analysis for a detailed view of microbial communities. Sixty percent of the studies utilized gastric biopsies, providing insights into bacterial populations in the stomach lining, while 40% analyzed stool samples. One study included both stool samples and gastric juice to enhance understanding of gut microbiota alterations in H. pylori-positive gastric cancer patients.
In terms of microbial findings, alpha diversity was reported to decrease in one study in stool samples and increase in two (both gastric biopsy). Beta diversity showed significant differences between H. pylori-positive and H. pylori-negative groups in one study involving stool samples. For specific bacterial groups, Actinobacteria levels decreased in two studies (gastric biopsy, gastric juice+stool) and increase in one (stool). Bifidobacterium levels also decreased in two studies (gastric biopsy, gastric juice+stool). Conversely, Proteobacteria levels in gastric juice and stool samples, as well as Streptococcus in gastric juice, biopsy, and stool samples, increased in two studies each. The Bacteroides genus showed mixed results, with an increase in one study (stool) and a decrease in another (gastric juice+stool). Firmicutes were dominant in early gastric cancer (EGC) in one study (gastric biopsy) but decreased in another (gastric juice+stool). Haemophilus was more prevalent in EGC compared to intestinal metaplasia (IM) in one gastric biopsy study, but its levels decreased in EGC in another biopsy study. Additionally, Enterococcus was uniquely found in EGC in one gastric biopsy study and increased in stool samples in another study.
4 Discussion
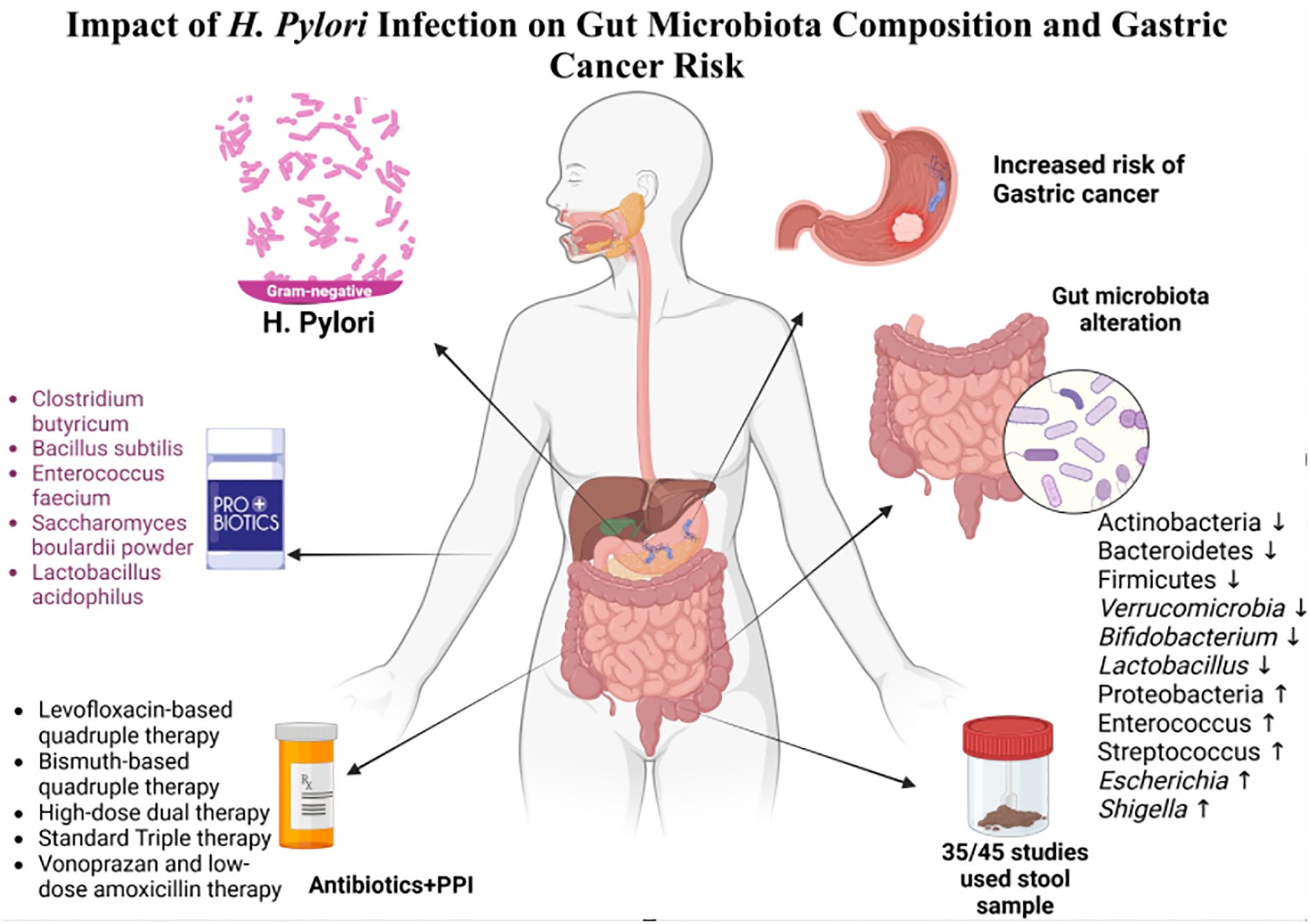
This systematic review explores the relationship between gut microbiota composition and H. pylori, focusing on gastrointestinal changes with and without treatment. It also examines how H. pylori alter the gut microbiota in gastric cancer patients, highlighting its potential role in cancer progression (Figure 1).
As a component of the gastrointestinal ecosystem, H. pylori infection and its effect on gastric acid secretion can influence the GI microbiome and overall health of the host (Chen et al., 2021).
Results concerning the key measures of microbial richness and composition, alpha and beta diversity respectively (Miao et al., 2020), were mixed across all age groups. The mixed results concerning alpha diversity suggest that microbial richness may be sample-type dependent (Nearing et al., 2021). On the other hand, the findings of the beta diversity were much more consistent. Almost all studies reported that the beta diversity had distinguished between Hp+ and Hp- individuals, with only one study reporting that the beta diversity remained unchanged. As mentioned in this study that a significance might be detected with a different analytical method or larger sample size (Dewayani et al., 2023).
On the phyla level, notable changes in the microbial community were observed in the young population. Gastric samples showed a decrease in the levels of Actinobacteria, Firmicutes, and Bacteroidetes but an increase in stool samples, highlighting a compartmentalized shift in microbial populations which suggests that H. pylori may potentially disrupt the gastric microbiota while also providing protection against external intestinal pathogens that lead to diarrhea (Kakiuchi et al., 2021; Martin-Nuñez et al., 2021). The observed decrease in gastric Firmicutes and Bacteroidetes could also be tied to changes in lipid and carbohydrate metabolism pathways, as these phyla play a crucial role in regulating lipid metabolism and maintaining energy homeostasis (Jian et al., 2022; Xiong, 2025). These metabolic and hormonal interactions may contribute to the unclear and controversial impact of H. pylori on growth and height in children as showed in 2 studies (Dharan and Wozny, 2022; Hong et al., 2022).
A consistent increase in Proteobacteria, a phylum often associated with inflammation and dysbiosis (Ibrahim and Belheouane, 2018), was observed across multiple studies in children and adolescents, indicating a potential link between H. pylori infection and the overgrowth of opportunistic pathogens (Sitkin et al., 2022). The increase in this phylum was also observed in adults; however, one study reported a decrease, indicating variability in the microbial response. This variability may be attributed to differences in ethnicity, genetic diversity, or diet (Dwiyanto et al., 2021).
In adults, Bacteroidetes showed a more consistent decrease, particularly in three studies. In contrast, Verrucomicrobia, a less common phylum, decreased in two studies, with a corresponding reduction in the genus Akkermansia, known for its role in maintaining gut mucosal integrity (Hajimohammad et al., 2024). This suggests that H. pylori infection may compromise the mucosal health of adults by depleting these beneficial bacteria. These findings underline an age-related microbial response to H. pylori, with greater consistency in adult studies regarding the depletion of certain beneficial bacterial groups (Walrath et al., 2021).
H. pylori infection elicits complex gut microbiota responses, differing by age. In children, probiotic genera like Bifidobacterium and Lactobacillus fluctuate, with some studies noting increases while others report declines. These bacteria support gut health by maturing the intestinal epithelium, maintaining its integrity, and regulating pH, alongside antimicrobial effects. Variations may be partly explained by regional hygiene practices (Gupta et al., 2020; Meliț et al., 2022). Eubacterium, a major butyrate producer, generally rises, particularly in children, benefiting gut health through anti-inflammatory short-chain fatty acid production (Du et al., 2024).
Prevotella levels consistently rise in infected individuals, suggesting that H. pylori promotes its carbohydrate-metabolizing capabilities across age groups (Prasoodanan et al., 2021). This elevation is consistent with other studies (Kakiuchi, 2023). Conversely, Bacteroides levels drop in adults, indicating disrupted gut balance (Zhang et al., 2023).
These shifts highlight a complex relationship between microbial changes and symptoms, with no single microbial group consistently predicting symptoms due to interspecies interactions (Simrén et al., 2012; Yu et al., 2023). Elevated levels of Sphingomonas in asymptomatic individuals suggest its potential as a distinguishing marker (Yu et al., 2023). This reduced levels of the bacterium in patients with persistent inflammation post-H. pylori eradication underscore its clinical relevance (Sung et al., 2020; Niu et al., 2021). Host immunity and environmental factors also shape microbial shifts (Woodhams et al., 2020). Adults show consistent microbial disruptions, particularly in beneficial bacteria, while children may tolerate H. pylori better, potentially activating protective responses against inflammation (Reyes, 2022; La Placa et al., 2025).
It has been shown that the colonization of the stomach and intestines by H. pylori leads to microbial alterations, and the treatment protocols used for its eradication also have a considerable impact on the microbiota in both the gastric and gut environments (Tohumcu et al., 2024).
Following H. pylori treatment without probiotic supplementation, alpha diversity generally declined, except in one study, likely due to variations in microbiome disruption caused by different treatments (Ramirez et al., 2020). In contrast, beta diversity findings were consistent, with nearly all studies reporting significant changes. At the phylum level, most studies observed a decrease in Actinobacteria, Firmicutes, and Bacteroidetes immediately after treatment (1–2 weeks). However, two studies found no change in Firmicutes, with Actinobacteria and Bacteroidetes levels even increasing, potentially due to treatment duration differences (7–14 days) (Lekang et al., 2022). Proteobacteria notably increased post-treatment, indicating gut dysbiosis (Gao et al., 2024).
At the genus level, Lactobacilli, Bifidobacterium, and Prevotella results varied, likely due to differing treatment regimens, while Verrucomicrobia consistently declined, suggesting compromised gut mucosal health (Fujio-Vejar et al., 2017; Mo et al., 2024). Most bacterial communities returned to baseline within 1–2 months, though some required 6–12 months. Findings suggest antibiotics rapidly decrease diversity and alter species abundance, leading to dysbiosis, with recovery often incomplete post-treatment, this underscores the importance of cautious antibiotic use to avoid lasting impacts on gut flora (Elvers et al., 2020).
When probiotics were co-administered during H. pylori eradication therapy, studies consistently reported beneficial outcomes across diverse probiotic regimens suggests a general protective effect of co-administration during H. pylori eradication therapy. These benefits likely stem from a combination of mechanisms, including modulation of host immune responses, suppression of opportunistic pathogens, and support for beneficial commensals (Raheem et al., 2021). However, substantial variability in the strains used, dosing schedules, and formulations complicates efforts to identify the most effective approach (Yang et al., 2024). Additionally, none of the studies employed head-to-head comparisons of different probiotic products, limiting the ability to draw conclusions about strain-specific efficacy. Standardization in future clinical protocols, including controlled comparisons of probiotic types and clearer reporting of microbiome outcomes, is essential to establish evidence-based recommendations for probiotic use in H. pylori treatment regimens (Wang et al., 2023).
Although current evidence supports the beneficial effects of probiotic supplementation during H. pylori eradication therapy, the lack of standardization across studies limits clinical applicability (Musazadeh et al., 2023). Nonetheless, several consistent findings allow for provisional recommendations. Strains such as Lactobacillus rhamnosus GG, Saccharomyces boulardii, Clostridium butyricum (particularly CBM588), and Bifidobacterium lactis have demonstrated efficacy in enhancing microbial recovery, reducing gastrointestinal side effects, and improving eradication rates (Imase et al., 2008). Most effective formulations delivered daily doses ranging from 1 × 109 to 1 × 1011 CFU, with higher doses offering greater microbiota protection, especially in patients receiving bismuth-based quadruple therapy (Yao et al., 2023). Probiotic administration was most beneficial when initiated concurrently with antibiotic treatment and continued for at least 2 to 4 weeks post-therapy to support microbiome stabilization and reduce dysbiosis-related complications (Mullish et al., 2024). Enteric-coated capsules and multi-strain powder formulations were associated with improved gastrointestinal survival and colonization efficiency (Govaert et al., 2024). In light of these findings, probiotic co-administration, particularly with microbiota-disruptive regimens, should be considered a supportive adjunct to eradication therapy (di Vito et al., 2022). Future clinical trials should prioritize head-to-head comparisons of probiotic strains, define optimal dosing schedules, and assess long-term microbiota recovery to inform standardized probiotic protocols in H. pylori management.
At week 2 following antibiotic and probiotic intervention, a decrease in alpha diversity was observed in 44.4% of the studies. This reduction in microbial diversity underscores the early disruptions to the gut microbiome caused by antibiotic therapy, even with probiotic supplementation. This is likely because probiotics can be inhibited by antibiotics, leading to a temporary collapse in microbial diversity (Yang et al., 2023).
Significant shifts in beta diversity were noted in almost all studies. These changes reflect alterations in community composition and inter-individual variability post-treatment, suggesting that probiotics may promote divergent microbiome recovery pathways in certain individuals (Chandrasekaran et al., 2024).
Probiotic supplementation during treatment is associated with an enrichment of beneficial bacterial genera, including Lactobacillus, Bifidobacterium, Bacteroidetes, and Clostridium, while pathogenic bacteria such as Enterococcus are depleted (Stojanov et al., 2020; Yan and Polk, 2020; Sabit et al., 2023). These findings suggest that probiotics play a crucial role in restoring and maintaining a healthier gut microbiota, potentially mitigating antibiotic-induced disruptions (Dahiya and Nigam, 2023). Furthermore, multiple studies demonstrate that treatment regimens supplemented with probiotics yield higher H. pylori eradication rates compared to standard therapies, while also effectively reducing gastrointestinal side effects (Elghannam et al., 2024).
These findings underscore the significant impact that H. pylori eradication regimens can have on gut microbiota, especially in the absence of probiotic supplementation. The observed persistent dysbiosis following Bismuth-based Quadruple Therapy (BQT) aligns with prior studies demonstrating its broad-spectrum antimicrobial effect, which tends to disrupt not only H. pylori but also commensal bacteria essential for maintaining gut homeostasis (Hsu et al., 2018). The reduced alpha diversity and altered beta diversity, along with increased prevalence of opportunistic pathogens such as Klebsiella pneumoniae and Enterococcus spp., raise concerns about secondary infections and long-term gut health following BQT, especially in vulnerable populations (Zhou et al., 2020).
In contrast, the relatively minimal microbiota disruption seen with Vonoprazan–Amoxicillin dual therapy (VA-dual therapy) supports its growing reputation as a safer first-line treatment. The stability in both alpha and beta diversity metrics during and after therapy indicates a more targeted antimicrobial action and a lesser ecological disturbance to the gut environment (Peng et al., 2023).
Importantly, the consistent protective effect of probiotics across all nine studies reinforces their utility as adjunctive agents in H. pylori treatment protocols. Supplementation was associated with the preservation of beneficial genera such as Bifidobacterium, Lactobacillus, and Faecalibacterium, which are known to support gut barrier integrity and modulate immune responses (Yao et al., 2023). This effect not only enhanced microbiota recovery but also attenuated the bloom of opportunistic and potentially pathogenic taxa. These observations suggest that probiotic co-administration is particularly critical when using regimens known for high microbial impact, such as BQT (Yao et al., 2023).
Taken together, the data suggests a paradigm shift in H. pylori treatment strategy may be warranted, prioritizing regimens like VA-dual therapy for their microbiota-sparing properties, and integrating probiotics as a standard co-treatment to mitigate the collateral damage of antibiotics. Moreover, future research should explore personalized approaches that balance eradication efficacy with preservation of microbiome integrity (Keikha and Karbalaei, 2021).
While all included studies examined the impact of H. pylori eradication therapies, either with or without probiotic supplementation, on gut microbiota composition, only three specifically focused on pediatric populations: one involving children aged 3 to 14 years, another adolescents aged 15 to 16 years, and a third with participants aged 7 to 8 years. The remainder of the studies enrolled adult participants, thereby limiting the ability to draw definitive conclusions about age-related differences in post-treatment dysbiosis (Kang et al., 2025). The broad age range across studies (3 to 80 years) further highlights a significant limitation in assessing age-specific susceptibility to microbiota disruption induced by antimicrobials or probiotics (Kang et al., 2025). Evidence from the broader microbiome literature suggests that children may demonstrate greater microbial resilience and more rapid recovery due to developmental plasticity and distinct immune regulatory profiles (Schoultz et al., 2025). However, the underrepresentation of pediatric cohorts in the current dataset prevented a thorough investigation of these age-dependent effects (Mohammadkhah et al., 2018). Moreover, most studies failed to stratify outcomes by age or control for key confounding variables such as diet, immune status, comorbidities, and microbiome developmental stage, all of which are known to influence the risk and severity of dysbiosis (Vujkovic-Cvijin et al., 2020). These limitations underscore the need for future studies to explicitly consider age as a biological variable and to systematically evaluate its role in shaping microbiota responses to H. pylori eradication regimens. While this review acknowledges the limited availability of pediatric data, this gap warrants stronger emphasis given the potential for age-specific differences in microbiota composition, immune maturation, and treatment response (Budzinski et al., 2024). Without age-specific data, clinical recommendations risk being disproportionately informed by adult-centric findings, which may not generalize to younger cohorts (Budzinski et al., 2024). Therefore, there is an urgent need for future studies to systematically incorporate pediatric populations and conduct age-stratified analyses (Gschwendtner et al., 2019). Such efforts are essential not only to optimize eradication protocols for younger individuals but also to understand how early-life microbiota disruptions might influence long-term gastrointestinal and metabolic health (Gschwendtner et al., 2019).
The studies collectively highlight the pivotal role of the gut microbiome, particularly in H. pylori-positive patients, in the development and progression of gastric cancer. Several studies demonstrate notable shifts in microbial diversity and composition, closely associated with the severity of gastric conditions and the presence of cancer.
For instance, two studies report a significant decline in Bifidobacterium levels, especially in patients with severe gastric cancer. These bacteria play a key role in anti-inflammatory responses, and their depletion may contribute to worsening gastric health (Virk et al., 2024). Simultaneously, elevated levels of inflammation-associated genera like Dialister and Prevotella suggest a pro-inflammatory environment, potentially accelerating tissue damage and carcinogenesis (Smet et al., 2022; Anthamatten et al., 2024). Similarly, early gastric cancer patients exhibited increased levels of pathogenic bacteria, such as Proteobacteria, Enterococcus, Streptococcus, and Escherichia-Shigella, further fostering a pro-inflammatory state that could promote cancer progression (Wang et al., 2020).
Interestingly, a 2023 study from Japan observed a reduction in Haemophilus in early gastric cancer (EGC) patients who had undergone successful H. pylori eradication. In contrast, a 2021 study from Portugal found higher levels of Haemophilus in EGC patients. This discrepancy may be attributed to the successful eradication of H. pylori in the former study, leading to a significant shift in the gastric microbiota, while in the latter study, the presence of early-stage cancer without prior eradication therapy could explain the higher Haemophilus levels (Xu et al., 2020).
5 Limitations of the studies
This systematic review offers a comprehensive overview of gut microbiota diversity and alterations in patients with H. pylori infection. However, the study has several limitations.
Firstly, the lack of detailed dietary information across most included studies is a significant limitation, as diet is crucial in shaping gut microbiota composition and diversity. This absence may have influenced the reported outcomes, introducing confounding factors related to participants’ varying dietary habits.
Secondly, considerable heterogeneity exists among the studies, particularly regarding the timing of taxonomic analysis after H. pylori treatment cessation, which may affect result comparability since gut microbiota can fluctuate over time post-treatment. Beyond timing, this heterogeneity stems from multiple methodological and contextual variables. These include differences in sequencing techniques (e.g., 16S rRNA gene sequencing versus shotgun metagenomics), which affect taxonomic depth and functional profiling; variation in sample types (gastric biopsies, gastric fluid, or stool); and disparities in patient demographics, such as age, geography, diet, and disease stage. Additionally, treatment regimens varied widely in antibiotic combinations, durations, and probiotic use, while follow-up intervals were inconsistent across studies. The absence of standardized protocols and analytical approaches further compounds this variability. Together, these factors limit the comparability of findings and the strength of generalizable conclusions. Future research should aim to minimize heterogeneity through harmonized methodologies and incorporate stratified analyses to account for population and protocol differences.
Thirdly, there is a scarcity of studies focusing on pediatric populations, with only two examining the impact of H. pylori treatment on children and adolescents. This limits the generalizability of findings to younger age groups, as gut microbiota responses to treatment may differ due to developmental variations in the microbiome and immune system.
Fourth, most of the studies used 16S rRNA sequencing as the primary method for analysing the gut microbiota. Although this technique is commonly employed, it has limitations in terms of taxonomic resolution and does not provide detailed functional or species-level information as effectively as other methods like shotgun metagenomics (Muhamad Rizal et al., 2020). As a result, this approach may lead to an incomplete understanding of the role of the microbiome in the progression of gastric cancer.
Finally, many studies assessing H. pylori treatment effects on gut microbiota lacked control groups of H. pylori-negative healthy individuals or untreated H. pylori-positive individuals. The absence of these controls hampers the ability to accurately evaluate the specific effects of eradication therapy on gut microbial composition, making it challenging to attribute observed changes to treatment rather than natural variations in the gut microbiome or H. pylori infection progression. This significantly limits the strength of the conclusions drawn from these studies.
6 Conclusion
The systematic review indicates that H. pylori significantly modify gut microbiota, demonstrating distinct patterns between age groups. Adults experience consistent microbial disruptions, whereas children exhibit variability and potential protective benefits. Antibiotic treatment temporarily decreases microbial diversity, leading to an increase in pathogenic Proteobacteria. Probiotic administration facilitates the restoration of beneficial bacteria. In gastric cancer patients infected with H. pylori, microbial shifts suggest a possible role of dysbiosis in cancer progression, highlighting the necessity for further research to elucidate these interactions and enhance therapeutic strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AA: Methodology, Validation, Formal analysis, Project administration, Supervision, Data curation, Conceptualization, Software, Investigation, Funding acquisition, Writing – original draft, Visualization, Resources, Writing – review & editing. FY: Data curation, Validation, Writing – review & editing. HA: Validation, Writing – review & editing. AH: Validation, Writing – review & editing. ES: Writing – original draft, Visualization. MB: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT was used for English linguistics corrections.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1592977/full#supplementary-material
References
Alexander, S. M., Retnakumar, R. J., Chouhan, D., Devi, T. N. B., Dharmaseelan, S., Devadas, K., et al. (2021). Helicobacter pylori in human stomach: the inconsistencies in clinical outcomes and the probable causes. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.713955
Ali, A. and AlHussaini, K. I. (2024). Helicobacter pylori: A contemporary perspective on pathogenesis, diagnosis and treatment strategies. Microorganisms. 12, 222. doi: 10.3390/microorganisms12010222
Anthamatten, L., Rogalla von Bieberstein, P., Menzi, C., Zünd, J. N., Lacroix, C., de Wouters, T., et al. (2024). Stratification of human gut microbiomes by succinotype is associated with inflammatory bowel disease status. Microbiome. 12, 186. doi: 10.1186/s40168-024-01897-8
Baj, J., Forma, A., Sitarz, M., Portincasa, P., Garruti, G., Krasowska, D., et al. (2020). Helicobacter pylori virulence factors—mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells. 10, 27. doi: 10.3390/cells10010027
Bakhti, S. Z. and Latifi-Navid, S. (2021). Interplay and cooperation of Helicobacter pylori and gut microbiota in gastric carcinogenesis. BMC Microbiol. 21, 258. doi: 10.1186/s12866-021-02315-x
Bornschein, J. and Pritchard, D. M. (2022). Myths and misconceptions in the management of Helicobacter pylori infection. Frontline Gastroenterol. 13, 245–253. doi: 10.1136/flgastro-2021-101826
Budzinski, L., Sempert, T., Lietz, L., Maier, R., Kang, G. U., von Stuckrad, A. S. L., et al. (2024). Age-stratification reveals age-specific intestinal microbiota signatures in juvenile idiopathic arthritis. Mol. Cell Pediatr. 11, 12. doi: 10.1186/s40348-024-00186-6
Chandrasekaran, P., Weiskirchen, S., and Weiskirchen, R. (2024). Effects of probiotics on gut microbiota: An overview. Int. J. Mol. Sci. 25, 6022. doi: 10.3390/ijms25116022
Charitos, I. A., D’Agostino, D., Topi, S., and Bottalico, L. (2021). 40 years of Helicobacter pylori: a revolution in biomedical thought. Gastroenterol. Insights 12, 111–135. doi: 10.3390/gastroent12020011
Chen, C. C., Liou, J. M., Lee, Y. C., Hong, T. C., El-Omar, E. M., and Wu, M. S. (2021). The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 13, 1–22. doi: 10.1080/19490976.2021.1909459
Cunha, E. S., Chen, X., Sanz-Gaitero, M., Mills, D. J., and Luecke, H. (2021). Cryo-EM structure of Helicobacter pylori urease with an inhibitor in the active site at 2.0 Å resolution. Nat. Commun. 12, 230. doi: 10.1038/s41467-020-20485-6
Dahiya, D. and Nigam, P. S. (2023). Antibiotic-therapy-induced gut dysbiosis affecting gut microbiota-brain axis and cognition: Restoration by intake of probiotics and synbiotics. Int. J. Mol. Sci. 24, 3074. doi: 10.3390/ijms24043074
De Cassai, A., Boscolo, A., Zarantonello, F., Pettenuzzo, T., Sella, N., Geraldini, F., et al. (2023). Enhancing study quality assessment: an in-depth review of risk of bias tools for meta-analysis—a comprehensive guide for anesthesiologists. J. Anesth. Analg Crit. Care 3, 44. doi: 10.1186/s44158-023-00129-z
Dewayani, A., Afrida Fauzia, K., Alfaray, R. I., Waskito, L. A., Doohan, D., Rejeki, P. S., et al. (2023). Gastric microbiome changes in relation with Helicobacter pylori resistance. PloS One 18, e0284958. doi: 10.1371/journal.pone.0284958
Dharan, M. and Wozny, D. (2022). Helicobacter pylori infection and small intestinal bacterial overgrowth: more than what meets the eye. World J. Clin. Cases. 10, 7209–7214. doi: 10.12998/wjcc.v10.i21.7209
di Vito, R., Conte, C., and Traina, G. (2022). A multi-strain probiotic formulation improves intestinal barrier function by the modulation of tight and adherent junction proteins. Cells. 11, 2617. doi: 10.3390/cells11162617
Du, Y., He, C., An, Y., Huang, Y., Zhang, H, Fu, W., et al. (2024). The role of short chain fatty acids in inflammation and body health. Int. J. Mol. Sci. 25, 7379. doi: 10.3390/ijms25137379
Duan, Y. and Xu, Y. (2025). Dou, Y. et al. Helicobacter pylori and gastric cancer: mechanisms and new perspectives. J. Hematol. Oncol. 18, 10. doi: 10.1186/s13045-024-01654-2
Dwiyanto, J., Hussain, M. H., Reidpath, D., Ong, K. S., Qasim, A., Lee, S. W. H., et al. (2021). Ethnicity influences the gut microbiota of individuals sharing a geographical location: a cross-sectional study from a middle-income country. Sci. Rep. 11, 2618. doi: 10.1038/s41598-021-82311-3
Elghannam, M. T., Hassanien, M. H., Ameen, Y. A., Turky, E. A., Elattar, G. M., ElRay, A. A., et al. (2024). Helicobacter pylori and oral-gut microbiome: Clinical implications. Infection. 52, 289–300. doi: 10.1007/s15010-023-02115-7
Elvers, K. T., Wilson, V. J., Hammond, A., Duncan, L., Huntley, A. L., Hay, A. D., et al. (2020). Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open 10. doi: 10.1136/bmjopen-2019-035677
Fong, I. W. (2020). “Helicobacter pylori infection: when should it be treated?,” in Current trends and concerns in infectious diseases. Emerging infectious diseases of the 21st century (Springer, Cham), 49–61. doi: 10.1007/978-3-030-36966-8_4
Frost, F., Kacprowski, T., Rühlemann, M., Bang, C., Franke, A., Zimmermann, K., et al. (2019). Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci. Rep. 9, 20100. doi: 10.1038/s41598-019-56631-4
Fujio-Vejar, S., Vasquez, Y., Morales, P., Magne, F., Vera-Wolf, P., Ugalde, J. A., et al. (2017). The gut microbiota of healthy Chilean subjects reveals a high abundance of the phylum verrucomicrobia. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01221
Gao, W., Liu, X., Zhang, S., Wang, J., Qiu, B., Shao, J., et al. (2024). Alterations in gut microbiota and inflammatory cytokines after administration of antibiotics in mice. Microbiol. Spectr. 12, e0309523. doi: 10.1128/spectrum.03095-23
Garman, K. S., Brown, H., Alagesan, P., McCall, S. J., Patierno, S., Wang, Q., et al. (2024). Helicobacter pylori testing prior to or at gastric cancer diagnosis and survival in a diverse US patient population. Gastric Cancer. 27, 28–35. doi: 10.1007/s10120-023-01448-4
Govaert, M., Rotsaert, C., Vannieuwenhuyse, C., Duysburgh, C., Medlin, S., Marzorati, M., et al. (2024). Survival of probiotic bacterial cells in the upper gastrointestinal tract and the effect of the surviving population on the colonic microbial community activity and composition. Nutrients. 16, 2791. doi: 10.3390/nu16162791
Gschwendtner, S., Kang, H., Thiering, E., Kublik, S., Fösel, B., Schulz, H., et al. (2019). Early life determinants induce sustainable changes in the gut microbiome of six-year-old children. Sci. Rep. 9, 12675. doi: 10.1038/s41598-019-49160-7
Gupta, V., Kumar, R., Sood, U., and Singhvi, N. (2020). Reconciling hygiene and cleanliness: a new perspective from human microbiome. Indian J. Microbiol. 60, 37–44. doi: 10.1007/s12088-019-00839-5
Hajimohammad, A., Fattahi, E., Ardebili, A., Kaboosi, H., and Ghaemi, E. A. (2024). The frequency of verrucomicrobia in the intestinal mucus of patients with cancer and polyps compared to healthy individuals. Jundishapur J. Microbiol. 17, e146159. doi: 10.5812/jjm-146159
Hong, T., Jiang, X., Zou, J., Yang, J., Zhang, H., Mai, H., et al. (2022). Hepatoprotective effect of curcumin against bisphenol A-induced hepatic steatosis via modulating gut microbiota dysbiosis. J. Nutr. Biochem. 109, 109103. doi: 10.1016/j.jnutbio.2022.109103
Hsu, P. I., Pan, C. Y., Kao, J. Y., Tsay, F. W., Peng, N. J., Kao, S. S., et al. (2018). Helicobacter pylori eradication with bismuth quadruple therapy leads to dysbiosis of gut microbiota with an increased relative abundance of Proteobacteria and decreased relative abundances of Bacteroidetes and Actinobacteria. Helicobacter. 23, e12498. doi: 10.1111/hel.12498
Huang, H., Zhong, W., Wang, X., Yang, Y., Wu, T., Chen, R., et al. (2023). The role of gastric microecological dysbiosis in gastric carcinogenesis. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1218395
Ibrahim, S. and Belheouane, M. (2018). “Methods for microbiota analysis: sample collection and laboratory methods,” in The Microbiome in Rheumatic Diseases and infection. Eds. Ragab, G., Atkinson, T., and Stoll, M. (Springer, Cham). doi: 10.1007/978-3-319-79026-8_2
Imase, K., Takahashi, M., Tanaka, A., Tokunaga, K., Sugano, H., Tanaka, M., et al. (2008). Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiol. Immunol. 52, 156–161. doi: 10.1111/j.1348-0421.2008.00026.x
Jian, Z., Zeng, L., Xu, T., Sun, S., Yan, S., Zhao, S., et al. (2022). The intestinal microbiome associated with lipid metabolism and obesity in humans and animals. J. Appl. Microbiol. 133, 2915–2930. doi: 10.1111/jam.15740
Kakiuchi, T. (2023). Commentary: Association between Helicobacter pylori infection and metabolic syndrome. Front. Endocrinol. (Lausanne). 14. doi: 10.3389/fendo.2023.1270855
Kakiuchi, T., Tanaka, Y., Ohno, H., Matsuo, M., and Fujimoto, K. (2021). Helicobacter pylori infection-induced changes in the intestinal microbiota of 14-year-old or 15-year-old Japanese adolescents: a cross-sectional study. BMJ Open 11, e047941. doi: 10.1136/bmjopen-2020-047941
Kang, D. W., Lee, J. W., Park, M. Y., Kim, S. H., Um, Y. H., Wang, S. M., et al. (2025). Impact of Helicobacter pylori eradication on age-specific risk of incident dementia in patients with peptic ulcer disease: a nationwide population-based cohort study. Geroscience. 47, 1161–1174. doi: 10.1007/s11357-024-01284-z
Keikha, M. and Karbalaei, M. (2021). Probiotics as the live microscopic fighters against Helicobacter pylori gastric infections. BMC Gastroenterol. 21, 388. doi: 10.1186/s12876-021-01977-1
La Placa, G., Covino, M., Candelli, M., Gasbarrini, A., Franceschi, F., and Merra, G. (2025). Relationship between human microbiome and helicobacter pylori. Microbiol. Res. 16, 24. doi: 10.3390/microbiolres16010024
Lekang, K., Shekhar, S., Berild, D., Petersen, F. C., and Winther-Larsen, H. C. (2022). Effects of different amoxicillin treatment durations on microbiome diversity and composition in the gut. PloS One 17. doi: 10.1371/journal.pone.0275737
Martin-Nuñez, G. M., Cornejo-Pareja, I., Clemente-Postigo, M., and Tinahones, F. J. (2021). Gut microbiota: the missing link between helicobacter pylori infection and metabolic disorders? Front. Endocrinol. (Lausanne) 12. doi: 10.3389/fendo.2021.639856
Meliț, L. E., Mărginean, C. O., and Săsăran, M. O. (2022). The challenges of eradicating pediatric helicobacter pylori infection in the era of probiotics. Children (Basel). 9, 795. doi: 10.3390/children9060795
Miao, R., Wan, C., and Wang, Z. (2020). The relationship of gastric microbiota and Helicobacter pylori infection in pediatrics population. Helicobacter. 25. doi: 10.1111/hel.12676
Mo, C., Lou, X., Xue, J., Shi, Z., Zhao, Y., Wang, F., et al. (2024). The influence of Akkermansia muciniphila on intestinal barrier function. Gut Pathog. 16, 41. doi: 10.1186/s13099-024-00635-7
Mohammadkhah, A. I., Simpson, E. B., Patterson, S. G., and Ferguson, J. F. (2018). Development of the gut microbiome in children, and lifetime implications for obesity and cardiometabolic disease. Children (Basel). 5, 160. doi: 10.3390/children5120160
Muhamad Rizal, N. S., Neoh, H.-m., Ramli, R., Periyasamy, P. R. A./K., Hanafiah, A., Abdul Samat, M. N., et al. (2020). Advantages and limitations of 16S rRNA next-generation sequencing for pathogen identification in the diagnostic microbiology laboratory: Perspectives from a middle-income country. Diagnostics (Basel). 10, 816. doi: 10.3390/diagnostics10100816
Mullish, B. H., Michael, D. R., Dabcheva, M., Webberley, T. S., Coates, N., John, D. A., et al. (2024). A double-blind, randomized, placebo-controlled study assessing the impact of probiotic supplementation on the symptoms of irritable bowel syndrome in females. Neurogastroenterol Motil. 36, e14751. doi: 10.1111/nmo.14751
Musazadeh, V., Nazari, A., Faghfouri, A. H., Emami, M., Kavyani, Z., Zokaei, M., et al. (2023). The effectiveness of treatment with probiotics in Helicobacter pylori eradication: results from an umbrella meta-analysis on meta-analyses of randomized controlled trials. Food Funct. 14, 7654–7662. doi: 10.1039/d3fo00300k
Nearing, J. T., Comeau, A. M., and Langille, M. G. I. (2021). Identifying biases and their potential solutions in human microbiome studies. Microbiome. 9, 113. doi: 10.1186/s40168-021-01059-0
Niu, Z. Y., Li, S. Z., Shi, Y. Y., and Xue, Y. (2021). Effect of gastric microbiota on quadruple Helicobacter pylori eradication therapy containing bismuth. World J. Gastroenterol. 27, 3913–3924. doi: 10.3748/wjg.v27.i25.3913
Öztekin, M., Yılmaz, B., Ağagündüz, D., and Capasso, R. (2021). Overview of Helicobacter pylori infection: clinical features, treatment, and nutritional aspects. Diseases. 9, 66. doi: 10.3390/diseases9040066
Peng, Y., Lei, X., Yang, Q., Zhang, G., He, S., Wang, M., et al. (2024). Helicobacter pylori CagA-mediated ether lipid biosynthesis promotes ferroptosis susceptibility in gastric cancer. Exp. Mol. Med. 56, 441–452. doi: 10.1038/s12276-024-01167-5
Peng, R., Zhang, Z., Qu, Y., and Chen, W. (2023). The impact of Helicobacter pylori eradication with vonoprazan-amoxicillin dual therapy combined with probiotics on oral microbiota: a randomized double-blind placebo-controlled trial. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1273709
Prasoodanan, P. K., Sharma, A. K., Mahajan, S., Dhakan, D. B., Maji, A., Scaria, J., et al. (2021). Western and non-western gut microbiomes reveal new roles of Prevotella in carbohydrate metabolism and mouth-gut axis. NPJ Biofilms Microbiomes. 7, 77. doi: 10.1038/s41522-021-00248-x
Raheem, A., Liang, L., Zhang, G., and Cui, S. (2021). Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 12. doi: 10.3389/fimmu.2021.616713
Ramirez, J., Guarner, F., Bustos Fernandez, L., Maruy, A., Sdepanian, V. L., and Cohen, H.. (2020). Antibiotics as major disruptors of gut microbiota. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.572912
Reyes, V. E. (2022). Helicobacter pylori immune response in children versus adults. Med. Res. Arch. 10, 3370. doi: 10.18103/mra.v10i12.3370
Sabit, H., Kassab, A., Alaa, D., Mohamed, S., Abdel-Ghany, S., Mansy, M., et al. (2023). The effect of probiotic supplementation on the gut-brain axis in psychiatric patients. Curr. Issues Mol. Biol. 45, 4080–4099. doi: 10.3390/cimb45050260
Schoultz, I., Claesson, M. J., Dominguez-Bello, M. G., Fåk Hållenius, F., Konturek, P., Korpela, K., et al. (2025). Gut microbiota development across the lifespan: Disease links and health-promoting interventions. J. Intern. Med. doi: 10.1111/joim.20089
Simrén, M., Barbara, G., Flint, H. J., Spiegel, B. M. R., Spiller, R. C., Vanner, S., et al. (2012). Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 62, 159–176. doi: 10.1136/gutjnl-2012-302167
Sitkin, S., Lazebnik, L., Avalueva, E., Kononova, S., and Vakhitov, T.. (2022). Gastrointestinal microbiome and Helicobacter pylori: eradicate, leave it as it is, or take a personalized approach? World J. Gastroenterol. 28, 766–774. doi: 10.3748/wjg.v28.i7.766
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., and Chipponi, J. (2003). Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J. Surg. 73, 712–716. doi: 10.1046/j.1445-2197.2003.02748.x
Smet, A., Kupcinskas, J., Link, A., Hold, G. L., and Bornschein, J. (2022). The role of microbiota in gastrointestinal cancer and cancer treatment: chance or curse? Cell Mol. Gastroenterol. Hepatol. 13, 857–874. doi: 10.1016/j.jcmgh.2021.08.013
Stojanov, S., Berlec, A., and Štrukelj, B. (2020). The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 8, 1715. doi: 10.3390/microorganisms8111715
Sung, J., Coker, O. O., Chu, E., Szeto, C. H., Luk, S. S., Lau, H. C. H., et al. (2020). Gastric microbes associated with gastric inflammation, atrophy, and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut. 69, 1572–1580. doi: 10.1136/gutjnl-2019-319826
Tohumcu, E., Kaitsas, F., Bricca, L., Ruggeri, A., Gasbarrini, A., Cammarota, G., et al. (2024). Helicobacter pylori and the human gastrointestinal microbiota: A multifaceted relationship. Antibiotics (Basel). 13, 584. doi: 10.3390/antibiotics13070584
Virk, M. S., Virk, M. A., He, Y., Tufail, T., Gul, M., Qayum, A., et al. (2024). The anti-inflammatory and curative exponent of probiotics: A comprehensive and authentic ingredient for the sustained functioning of major human organs. Nutrients. 16, 546. doi: 10.3390/nu16040546
Vujkovic-Cvijin, I., Sklar, J., Jiang, L., Natarajan, L., Knight, R., and Belkaid, Y. (2020). Host variables confound gut microbiota studies of human disease. Nature. 587, 448–454. doi: 10.1038/s41586-020-2881-9
Walrath, T., Dyamenahalli, K. U., Hulsebus, H. J., McCullough, R. L., Idrovo, J.-P., Boe, D. M., et al. (2021). Age-related changes in intestinal immunity and the microbiome. J. Leukoc. Biol. 109, 1045–1061. doi: 10.1002/JLB.3RI0620-405RR
Wang, J., Chen, W. D., and Wang, Y. D. (2020). The relationship between gut microbiota and inflammatory diseases: The role of macrophages. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.01065
Wang, Y., Wang, X., Cao, X. Y., Zhu, H. L., and Miao, L. (2023). Comparative effectiveness of different probiotics supplements for triple helicobacter pylori eradication: a network meta-analysis. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1120789
Woodhams, D. C., Bletz, M. C., Becker, C. G., Bender, H. A., Buitrago Rosas, D., Diebboll, H., et al. (2020). Host-associated microbiomes are predicted by immune system complexity and climate. Genome Biol. 21, 23. doi: 10.1186/s13059-020-01955-y
Xiong, S. (2025). Gut-microbiota-driven lipid metabolism: mechanisms and applications in swine production. Metabolites. 15, 248. doi: 10.3390/metabo15040248
Xu, L., Surathu, A., Raplee, I., Chockalingam, A., Stewart, S., Walker, L., et al. (2020). The effect of antibiotics on the gut microbiome: A metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genomics 21, 263. doi: 10.1186/s12864-020-6665-2
Yadav, M. and Chauhan, N. S. (2024). Role of gut-microbiota in disease severity and clinical outcomes. Brief Funct. Genomics 23, 24–37. doi: 10.1093/bfgp/elac037
Yan, F. and Polk, D. B. (2020). Probiotics and probiotic-derived functional factors—mechanistic insights into applications for intestinal homeostasis. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01428
Yang, Z., Zhou, Y., Han, Z., He, K., Zhang, Y., Wu, D., et al. (2024). The effects of probiotics supplementation on Helicobacter pylori standard treatment: an umbrella review of systematic reviews with meta-analyses. Sci. Rep. 14, 10069. doi: 10.1038/s41598-024-59399-4
Yang, L., Zhu, X., Zhao, W., and Wang, J. (2023). Effect of combined probiotics on the intestinal ecosystem during triple therapy for eradication of Helicobacter pylori. J. Biol. Regul. Homeost Agents. 37, 1075–1080. doi: 10.23812/j.biol.regul.homeost.agents.20233702.109
Yao, G., Fan, X., and Lu, D. (2023). Efficacy and safety of probiotic-supplemented bismuth quadruple therapy for the treatment of Helicobacter pylori infection: a systematic review and meta-analysis. J. Int. Med. Res. 51, 3000605231203841. doi: 10.1177/03000605231203841
Yu, T., Lu, T., Deng, W., Yao, D., He, C., Luo, P., et al. (2023). Microbiome and function alterations in the gastric mucosa of asymptomatic patients with Helicobacter pylori infection. Helicobacter. doi: 10.1111/hel.12965
Yu, D., Meng, X., de Vos, W. M., Wu, H., Fang, X., and Maiti, A. K. (2021). Implications of gut microbiota in complex human diseases. Int. J. Mol. Sci. 22, 12661. doi: 10.3390/ijms222312661
Zhang, L., Zhao, M., and Fu, X. (2023). Gastric microbiota dysbiosis and Helicobacter pylori infection. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1153269
Keywords: H. pylori, eradication therapies, gut microbiota, gastric cancer, probiotics
Citation: Albush A, Yassine F, Abbas H, Hanna A, Saba E and Bilen M (2025) The impact of Helicobacter pylori infection and eradication therapies on gut microbiota: a systematic review of microbial dysbiosis and its implications in gastric carcinogenesis. Front. Cell. Infect. Microbiol. 15:1592977. doi: 10.3389/fcimb.2025.1592977
Received: 13 March 2025; Accepted: 05 June 2025;
Published: 07 July 2025.
Edited by:
Ernesto Calderon Martinez, University of Texas Health Science Center at Houston, United StatesReviewed by:
Andrés González, University of Zaragoza, SpainChen Chen, Shenzhen Maternity and Child Healthcare Hospital, China
Xi Chen, Sichuan University, China
Copyright © 2025 Albush, Yassine, Abbas, Hanna, Saba and Bilen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melhem Bilen, bWIyMjhAYXViLmVkdS5sYg==
 Alia Albush
Alia Albush Fayez Yassine
Fayez Yassine Hassan Abbas
Hassan Abbas Aya Hanna
Aya Hanna Esber Saba
Esber Saba Melhem Bilen
Melhem Bilen