- 1College of Life and Health Science, Northeastern University, Shenyang, Liaoning, China
- 2Department of Laboratory Animal Center, General Hospital of Northern Theater Command, Shenyang, Liaoning, China
Cancer remains one of the most significant causes of mortality across the world. Despite remarkable advancements made in early detection, therapeutic strategies, and the advent of immunotherapy in recent years, numerous challenges continue to hinder optimal outcomes. The development and progression of cancer are driven not only by genetic and epigenetic alterations within tumor cells but also by dynamic interactions occurring with the surrounding tumor microenvironment (TME). It is a highly complex milieu composed of tumor cells, non-tumor stromal cells, extracellular matrix components, immune cells, blood vessels, and diverse signaling molecules. Emerging evidence underscores the pivotal role of fungi in influencing cancer biology, including initiation, progression, immune evasion, and the modulation of TME. Fungi, which are omnipresent microorganisms, have traditionally been considered opportunistic pathogens. However, recent research highlights their broader impact on host immunity and their potential contributions to cancer pathogenesis. For instance, in patients with cancer, fungal infections not only exacerbate clinical complications but also create conditions conducive to tumor growth, metastasis, and immune escape by altering the immune microenvironment. In addition, fungal-derived metabolites and their interactions with host immune pathways can significantly modulate the efficacy of immunotherapies. These findings have spurred interest in exploring antifungal strategies as adjunctive approaches in cancer management, positioning antifungal therapy as a burgeoning area of oncological research. This review provides an in-depth exploration of the complex interplay between fungi and cancer. It examines the multifaceted role of fungal infections in tumor biology, the mechanisms through which fungi reshape the TME through immune modulation and their influence on immune-evasion strategies and therapeutic resistance. Furthermore, the potential for integrating antifungal therapies into comprehensive cancer treatment regimens has been highlighted, offering insights into novel avenues for improving patient outcomes.
1 Introduction
Cancer is one of the most significant causes of mortality globally (Mullard, 2020). According to the World Health Organization, nearly 10 million people succumbed to cancer in 2020 alone (Mullard, 2020) (Liu et al., 2023a). In the relentless effort to combat this disease, research and advancements in cancer treatment have continued unabated (Dolgin, 2021; Gilbertson, 2011; Kroemer and Pouyssegur, 2008). Cancer management is a multifaceted and highly individualized process, influenced by factors such as the cancer type, stage, patient health, and the molecular characteristics of the tumor (Duggan et al., 2017; Hui and Bruera, 2020; Jost and Roila, 2009; Samuel et al., 2014). Traditional treatment modalities, including surgery, radiotherapy, and chemotherapy, remain foundational (Allen et al., 2017) (Figure 1). However, the emergence of cancer immunotherapy in recent years represents a transformative breakthrough in oncology (Borgers et al., 2021; Derynck et al., 2021; Morrison et al., 2018; Szeto and Finley, 2019).
Figure 1. This illustration summarizes the primary therapeutic strategies employed in cancer management, including surgery, chemotherapy, radiotherapy, immunotherapy, and chimeric antigen receptor T-cell (CAR-T) therapy. Each modality targets the tumor from a distinct angle, aiming to remove, destroy, or modulate malignant cells and the tumor microenvironment. These approaches are often used in combination to enhance efficacy and reduce the risk of recurrence.
Immunotherapy functions by enhancing or modulating the patient’s immune system to recognize and destroy cancer cells (Brandenburg et al., 2024). A prominent example of this is immune checkpoint inhibitors (Abril-Rodriguez and Ribas, 2017), which block inhibitory signals that suppress immune responses, thereby activating the immune system to target malignant cells (Abril-Rodriguez and Ribas, 2017; Curry and Lim, 2015). This approach has demonstrated remarkable efficacy in treating various types of cancers, including melanoma and non-small-cell lung cancer (Arbour and Riely, 2019; Derosa et al., 2018; McGranahan et al., 2016).
Another innovative therapy is chimeric antigen receptor T-cell therapy, wherein patient’s T-cells are genetically engineered to identify and attack cancer cells (Gumber and Wang, 2022; Liu et al., 2022b). While chimeric antigen receptor T-therapy has demonstrated significant success in hematological malignancies such as leukemia and lymphoma (Uscanga-Palomeque et al., 2023), its application to solid tumors presents substantial challenges that remain the focus of ongoing research (Chohan et al., 2023; Ma et al., 2019; Uscanga-Palomeque et al., 2023; Zhang et al., 2023, 2022).
Cancer vaccines represent a preventative approach, with notable examples including the human papillomavirus vaccine and the hepatitis B vaccine (Liu et al., 2023c; Saxena et al., 2021), both of which effectively prevent certain virus-associated cancers (Liu et al., 2024e). However, the development of therapeutic cancer vaccines is still in its early stages (Liu et al., 2024e; Lopes et al., 2019).
Targeted therapy is another promising strategy in cancer treatment (Chang et al., 2021). By aiming at specific genetic mutations or molecular markers unique to cancer cells (Chang et al., 2021), it disrupts tumor growth with greater precision relative to that of the traditional chemotherapy (Crooke et al., 2018; Liu and Wang, 2024), thereby sparing normal cells and minimizing the inevitable collateral damage (Crooke et al., 2018). Several targeted agents have been successfully integrated into clinical practice (Liu and Wang, 2024; Mancarella et al., 2023; Wang et al., 2020), such as small-molecule epidermal growth factor receptor inhibitors (e.g., gefitinib and erlotinib) (Damaraju et al., 2014; Kohsaka et al., 2017; Yang et al., 2017) and human epidermal growth factor receptor 2-targeted drugs (e.g., trastuzumab) (Cameron et al., 2017; Hurvitz et al., 2023). Monoclonal antibodies, including trastuzumab and bevacizumab (Limousin et al., 2023; Pierga et al., 2012; Wahid et al., 2016), bind to surface antigens on cancer cells, leading to their destruction or inhibiting their proliferation, yielding significant clinical benefits (Singh et al., 2024).
The introduction of immune checkpoint inhibitors has revolutionized cancer therapy, shifting scientific attention to the critical role of immune evasion within the tumor microenvironment (TME) (Galassi et al., 2024). Therefore, understanding and manipulating the TME to overcome immunosuppressive mechanisms and enhance immune-mediated tumor destruction is now a key research priority, driving innovation in the next-generation immunotherapies (Barkley et al., 2022; Galassi et al., 2024; Tang et al., 2021; Zhang et al., 2024).
2 TME and microbial relationships
The relationship between the TME and the microbiota has emerged as a dynamic and growing field in the cancer research domain (Lam et al., 2021; Lu et al., 2022). Historically, cancer studies have primarily focused on tumor cells, the immune system, and genetic mutations, often overlooking the role of microbiota (Goenka et al., 2023; Liu et al., 2024b). However, advancements in microbiomics and immunology have increasingly highlighted the profound influence of the microbiota (Liu et al., 2024b), particularly bacteria, within the tumor environment on processes such as tumor initiation, progression, metastasis, immune evasion, and therapeutic response (Guillot et al., 2023; Jiang et al., 2023; Liu et al., 2024b; You et al., 2024).
While bacteria have garnered substantial attention over the past few decades for their role in cancer (Souza et al., 2023), fungi—a significant component of the microbiota—have been comparatively understudied (Gilbertson, 2011; Kroemer and Pouyssegur, 2008). Fungi encompass a diverse range of organisms, including clinically relevant pathogens such as Aspergillus, Candida, Cryptococcus, and Pneumocystis (Strickland and Shi, 2021). In nature, fungi have become indispensable for ecological balance (Dean et al., 2012; Strickland and Shi, 2021), contributing to organic matter decomposition, nutrient cycling, and forming symbiotic relationships with other organisms (Biedermann and Vega, 2020; Jia and Chen, 2025).
Despite their larger size compared to bacteria, fungi account for a minor proportion of the gut microbiota in terms of abundance but may have disproportionate effects on host immunity and metabolism, fungi represent only approximately 0.6% of total microbial DNA (Chen et al., 2017; Takeuchi et al., 2024), which has led to the underestimation of their potential pathogenicity. Fungal infections account for more than 1.5 million deaths annually and have significant implications on the host immune system as well as the overall microbiota composition. Recent research has confirmed that fungi play a critical role in shaping the TME, thereby influencing cancer development and progression (Wang et al., 2024c).
Although fungi comprise only 0.01–2% of the gut microbiota, their functional impact on health and disease far exceeds this proportion (Cheng et al., 2024; Dart, 2019; Galloway-Peña et al., 2024; Saftien et al., 2023). Their interactions with bacteria and the host immune system are highly complex (Belkaid and Hand, 2014; Mazmanian et al., 2005; Sepich-Poore et al., 2021). In addition, owing to their larger cellular size, the total biomass of symbiotic fungi possibly surpassed that of bacteria (How et al., 2022; Saftien et al., 2023). Traditionally, fungi have been studied primarily in the context of infectious diseases (Fernandes and Carter, 2020; Strickland and Shi, 2021; Wang et al., 2024a), while their role in symbiosis remains underexplored (Dohlman et al., 2022; Narunsky-Haziza et al., 2022). Moreover, certain symbiotic fungi exhibit opportunistic behavior, adding complexity to their functional roles (Dohlman et al., 2022; Narunsky-Haziza et al., 2022). Research efforts are further complicated by the high individual and temporal variability in microbiota composition, which often exceeds that observed in bacterial communities (Lin et al., 2022; Liu et al., 2022a).
Most studies have indicated that the Ascomycota and Basidiomycota phyla dominate fungal populations across various body sites (Angelova et al., 2023; Guarro et al., 1999; Sandargo et al., 2019), with the gut representing the most extensively studied ecological niche owing to its dense microbial ecosystem (Saftien et al., 2023).
Fungi exist in diverse forms, including yeasts and hyphae (Witchley et al., 2019). The ability of yeasts to convert into hyphae is a key pathogenic trait of fungi like Candida albicans (Honorato et al., 2022; Kiss et al., 2019; Witchley et al., 2019). Yeast cells are generally more resistant to macrophage killing and immune responses when compared to their hyphal forms (Witchley et al., 2019). The transition from the yeast form to the hyphae form is typically influenced by environmental factors such as pH, CO2 levels, anaerobic conditions, and temperature (Honorato et al., 2022; Lu et al., 2014; Sudbery, 2011). Fungi inhabit multiple ecological niches in the human body, including the gastrointestinal tract and the surfaces of other mucosal membranes (Lohse et al., 2018; Soll, 2024). The relationship between fungi and human health is deeply interconnected (Lohse et al., 2018).
Fungal infections can be classified into two categories: superficial and systemic (Brown et al., 2012).
Superficial infections: These include infections of the skin, nails, and mucous membranes (Mayer et al., 2013; Otašević and Hay, 2023). The most common examples are Candida infections (e.g., oral thrush and vaginal candidiasis) and dermatophyte infections (e.g., tinea capitis, tinea corporis, and tinea cruris) (Mayer et al., 2013).
Systemic infections: These infections occur when the immune system is compromised, allowing fungal infections to spread to the internal organs (Bing et al., 2024). Common examples include pulmonary infections (e.g., aspergillosis and cryptococcosis) and bloodstream infections (e.g., Candida bloodstream infections) (Esher Righi et al., 2023). These infections tend to be more severe and are especially threatening to immunocompromised patients (Esher Righi et al., 2023; Morales-López et al., 2017; Park et al., 2024).
In recent years, the incidence of fungal infections has steadily increased owing to the widespread use of antibiotics and immunosuppressive drugs (Parsons and Diekema, 2023; Spallone and Schwartz, 2021). This rise is particularly noticeable among cancer patients, organ transplant recipients, and individuals with HIV/AIDS, in whom fungal infections pose a serious complication (Benitez and Carver, 2019; Brüggemann et al., 2022; Gøtzsche and Johansen, 2014; Pagano and Caira, 2014). Some of the most commonly used antifungal drugs are as follows:
Fluconazole and itraconazole (broad-spectrum antifungal agents mainly used for treating systemic fungal infections).
Amphotericin B (a broad-spectrum antifungal drug that is often used for treating severe fungal infections).
Voriconazole (primarily used for treating invasive fungal infections) (Arendrup et al., 2020; Chen and Sorrell, 2007).
However, the increasing use of antifungal treatments has seen a parallel rise in the corresponding antifungal resistance (Kozubowski and Berman, 2025; Lockhart et al., 2023), especially with Candida species and Aspergillus species, which are developing resistance to standard antifungal medications (Carolus et al., 2021; Gregor et al., 2023; Lee et al., 2021). This aspect is driven by research efforts to develop new antifungal drugs (Nicola et al., 2019).
In the early 20th century, scientists began recording a co-occurrence between certain fungal infections and cancer in patients (Liu et al., 2023a). However, most of these studies were descriptive and did not explore the potential role of fungi in cancer development (Wu et al., 2024a). In recent years, the rapid advancement of omics research has facilitated the unraveling of the relationship between fungi and cancer, revealing a deeper and more complex connection.
3 Literature search and inclusion criteria
To ensure a comprehensive overview of the topic, we conducted a literature search using PubMed, Scopus, and Web of Science databases up to February 2025. The keywords included “fungi and cancer,” “mycobiome and tumor,” “fungal metabolites and carcinogenesis,” “antifungal therapy and oncology,” among others. We included English-language peer-reviewed articles focusing on experimental models, clinical studies, or mechanistic insights related to fungi and cancer. Articles were screened based on relevance, and duplicates were removed.
3.1 Fungi as diagnostic biomarkers
Research on fungi as tumor biomarkers has emerged as a new field in recent years. The conventional tumor biomarkers are mainly based on tumor cells or their metabolic products (Dohlman et al., 2022). However, past studies have suggested that certain fungi and their metabolites display distinct changes in cancer patients, implicating their potential applications in the early diagnosis, prediction, and treatment of tumors (Dohlman et al., 2022; Lin et al., 2022; Liu et al., 2022a; Su et al., 2024) (Figure 2).
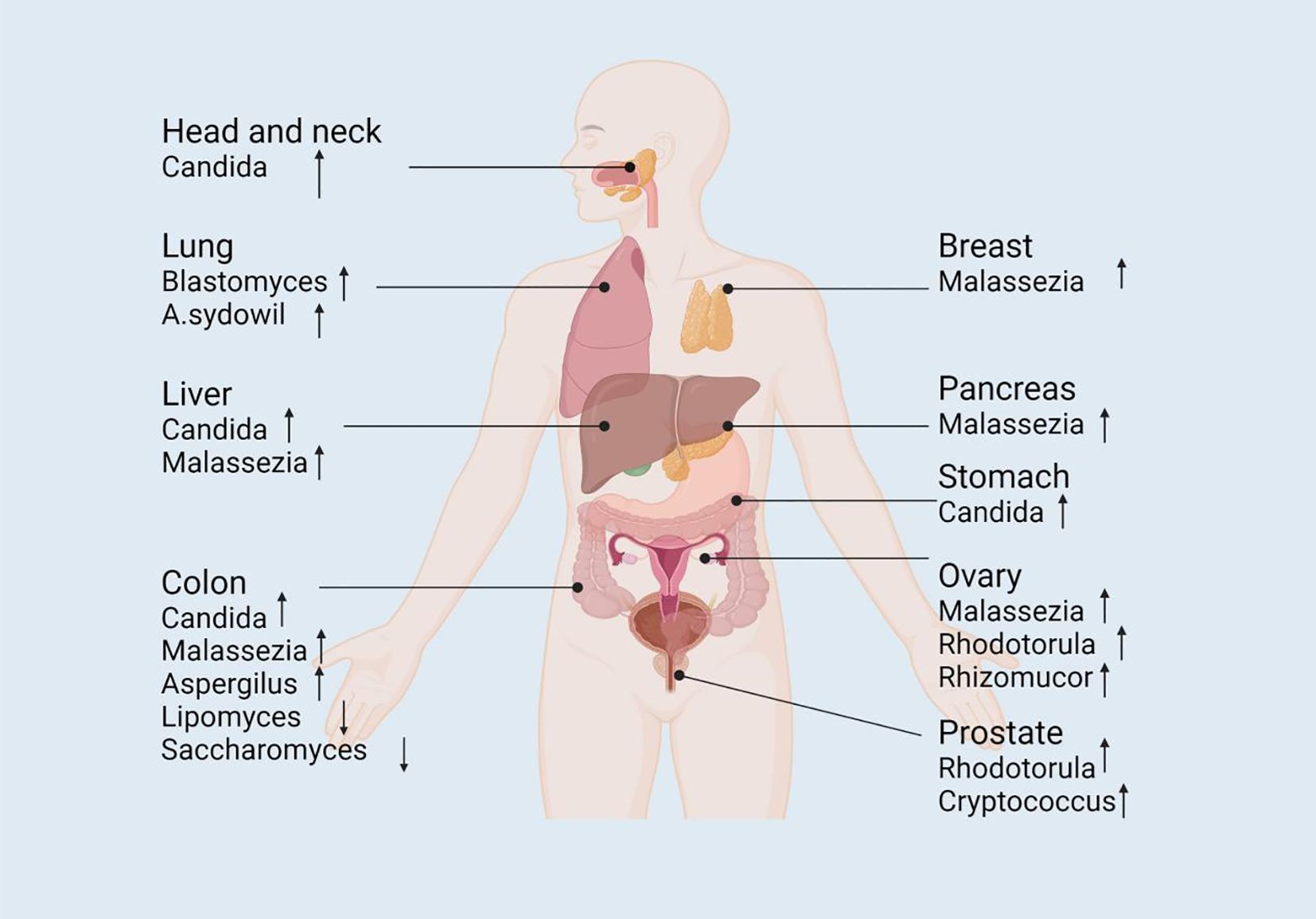
Figure 2. The alteration of mycobiome in abundance across different tumor sites. The composition of fungal mycobiome is altered in different body sites (e.g., colorectum, pancreas, stomach, liver, head and neck, lung, and breast) that are associated with tumorigenesis, serving as potential diagnostic or prognostic biomarkers to promote the study of the complicated mechanistic investigation of fungal involvement in carcinogenesis. ↓decrease; ↑increase. Pathways with dashed arrows represent hypothetical interactions yet to be validated in clinical studies (Dohlman et al., 2022; Su et al., 2024).
3.1.1 Diagnostic potential of fungi as biomarkers in gastrointestinal tumors
The gastrointestinal tract is the area with the highest prevalence of fungi in the human body (Dohlman et al., 2022; Wu et al., 2024a). Researchers have identified the presence of fungi in gastrointestinal tumors as well as discovered a close association between fungi and cancer development (Dohlman et al., 2022; Lin et al., 2022; Liu et al., 2022a; Su et al., 2024).
Globally, colorectal cancer (CRC) is one of the most common causes of death, with continuously rising incidence rates, accounting for approximately 900,000 deaths annually (Dekker et al., 2019). Past studies have reported that the occurrence of CRC correlates with fungal abundance relative to that in healthy controls (Bai et al., 2022; Schürch et al., 2020). These alterations include an enrichment of the Basidiomycota/Ascomycota ratio (Sokol et al., 2017), an abundance of Malasseziomycetes, and a depletion of Saccharomycetes and Pneumocystidomycetes proportion (Coker et al., 2019). Several studies have observed an increased Basidiomycota/Ascomycota ratio in colorectal cancer patients; however, most of these studies involve small cohorts and observational designs, and thus the predictive value remains speculative and requires further validation in larger, well-controlled studies. Furthermore, the population of specific fungal species such as Lipomyces starkeyi and Saccharomyces cerevisiae are reduced, while those of others like Malassezia globosa and Aspergillus flavus are enriched in CRC patients (Coker et al., 2019). Moreover, past research has revealed that a combination of fungal and bacterial biomarkers was more accurate in distinguishing CRC patients from healthy individuals when compared to using only bacterial species (Lin et al., 2022; Liu et al., 2022a).
In patients with liver and gastric cancers (GC), a decrease in alpha diversity and an increase in the number of opportunistic fungi (such as Malassezia and Candida) have been detected (Zhong et al., 2021). For instance, Dohlman reported that C. albicans mediates GC by reducing the diversity and richness of gastric fungi, thereby promoting the pathogenesis of GC (Dohlman et al., 2022). A similar phenomenon was observed in adenomas, wherein fungal diversity was reduced in comparison to healthy tissues (Luan et al., 2015). Furthermore, Aykut et al. reported microbial dysbiosis in the tumors of pancreatic and oral cancer patients in both mouse and human studies (Aykut et al., 2019).
3.1.2 Diagnostic potential in non-gastrointestinal cancers
A similar phenomenon has been observed in non-gastrointestinal cancers, demonstrating the significant potential of fungi as tumor biomarkers.
Through the Cancer Genome Atlas cohort, detected an enrichment of Blastomyces dermitidis/gilchristii in cancer patients. Notably, in the Weizmann cohort, smokers displayed a higher abundance of Aspergillus and Agaricus in their tumors compared to non-smokers with lung cancer (Narunsky-Haziza et al., 2022). In breast cancer, Malassezia was found to be significantly enriched, while Aspergillus and Malassezia were found to form a hub for fungal-bacterial co-occurrence (Dohlman et al., 2022) (Narunsky-Haziza et al., 2022). When compared to patients with cirrhosis, those with hepatocellular carcinoma (HCC) exhibited significantly reduced gut microbiome diversity, but an increase in C. albicans abundance (Liu et al., 2022c).
In 2022, a collaborative study between the Weizmann Institute of Science (Israel) and the University of California, San Diego (USA) comprehensively characterized cancer microbiota in 17,401 patients across four independent cohorts with 35 cancer types. The study reported a low abundance of fungal DNA and cells in several major human cancers when compared with fungal communities with matching bacterial communities and immune profiles; this study also explored the role of fungi in prognosis and diagnosis. The results of this past study provided new insights into cancer detection and treatment (Narunsky-Haziza et al., 2022).
The research on fungi as tumor biomarkers is progressing rapidly, especially in the areas of specific metabolic products (Shuai et al., 2022; Vitali et al., 2022), DNA/RNA detection, and microbiome structure analysis (Liao et al., 2023). Although multi-omics studies offer integrative insights, most suffer from small sample sizes, lack of replication, and inconsistent bioinformatics pipelines, limiting their generalizability. In the future, by integrating multi-omics technologies and efficient detection methods, fungal biomarkers are expected to play a crucial role in early cancer diagnosis, treatment monitoring, and prognosis assessment (Galloway-Peña et al., 2024; Liu et al., 2022a; Saftien et al., 2023). In addition, investigating the interactions and regulatory mechanisms between fungi and the TME is expected to further promote the development of precision medicine in this are (Hiam-Galvez et al., 2021; Ruffin et al., 2023).
3.2 Fungi Affecting TME through immune modulation
The impact of fungi on the TME is a complex and increasingly focused area of research (Jiang et al., 2022b). Fungi not only play important roles in human health but also influence the occurrence, development, and response to treatments through various mechanisms (Saftien et al., 2023). Fungi can alter the TME both directly and indirectly, thereby affecting processes such as tumor cell proliferation, immune evasion, and metastasis (Blake et al., 2024; Cao et al., 2024; Saftien et al., 2023; Wang et al., 2024c) (Figure 3).
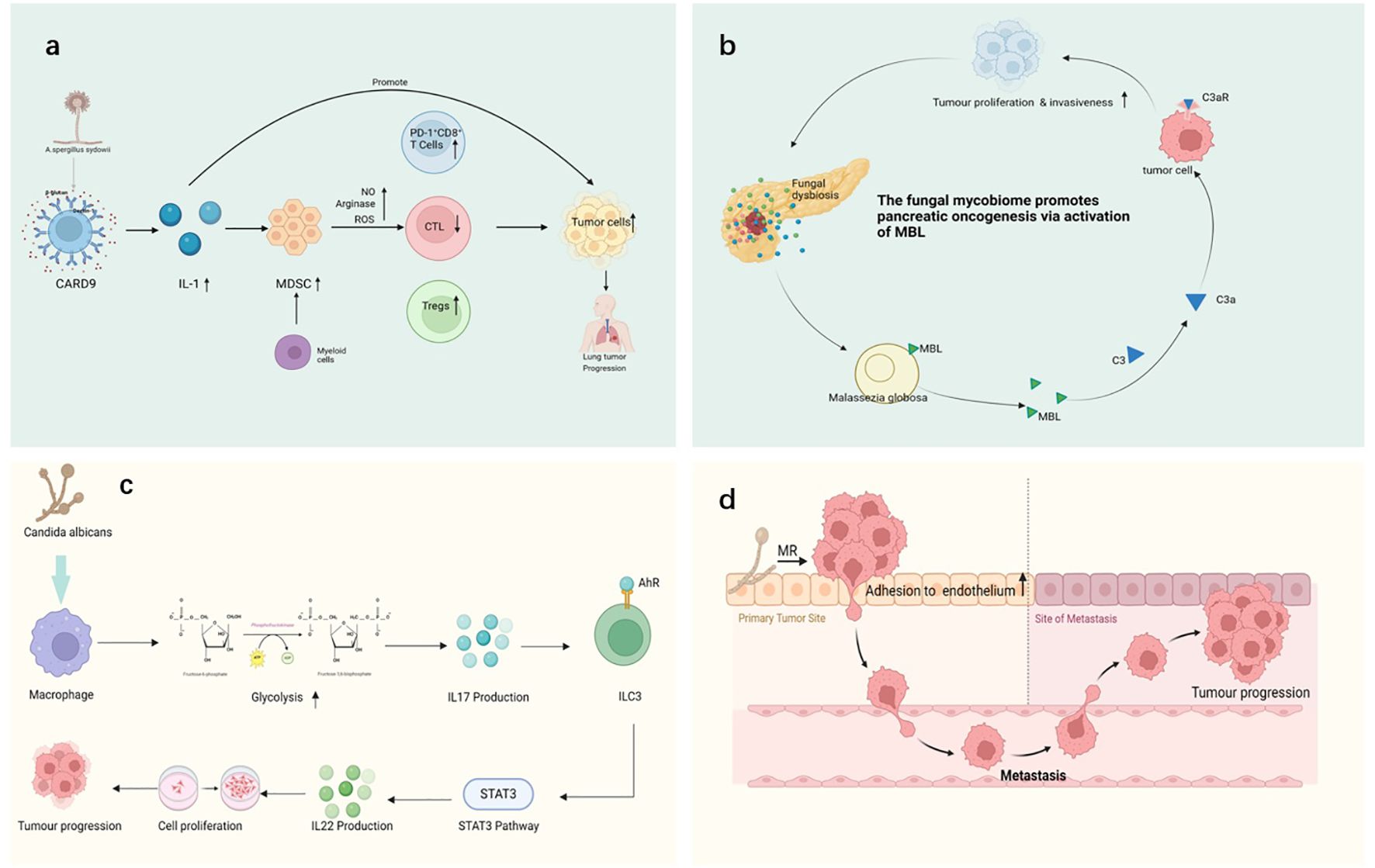
Figure 3. Various mechanisms through which fungi interact with cancer, including: (a) A sydowii activates immune responses through the CARD9 pathway, promoting the upregulation of IL-1 and myeloid-derived suppressor cells (MDSCs). By inducing the production of nitric oxide (NO), arginase, and reactive oxygen species (ROS), it impairs the effect of cytotoxic T-lymphocytes (CTLs) and increases the proportion of PD-1+ CD8+ T-cells. These immune responses may contribute to tumor progression, causing the proliferation of lung cancer cells. (b) The activation of Malassezia globosa triggers the mannose-binding lectin (MBL) pathway, the MBL (mannose-binding lectin) pathway initiates complement activation via MASP-1 and MASP-2, which cleave C4 and C2 to generate C3 convertase, leading to downstream immune signaling, promoting the development of pancreatic cancer. This process involves tumor proliferation, invasiveness, and immune modulation. (c) In murine models, C albicans infection has been shown to enhance IL-17 production via macrophage glycolytic reprogramming, subsequently activating ILC3 cells and promoting IL-22 secretion through the VEGF 3 pathway. While this cascade has been linked to increased tumor proliferation in experimental systems, its clinical relevance remains debated, and contradictory data suggest that IL-17 may have dual roles depending on cancer type and immune context (Aggor et al., 2020; X. Wang et al., 2023b). (d) Fungi enhance tumor cell adhesion to endothelial cells through interactions with tumor cell surface mannose receptors (MR), thereby facilitating the metastasis of cancer cells. This mechanism allows tumor cells to migrate from the primary site to metastatic sites, driving cancer progression. Pathways with dashed arrows represent hypothetical interactions yet to be validated in clinical studies (Heung et al., 2023; Riquelme and McAllister, 2021; Sheng et al., 2024; Soerens et al., 2023).
Fungi can significantly alter the TME by regulating immune cell functions, thereby influencing tumor growth, immune evasion, and response to treatment (Blake et al., 2024; Saftien et al., 2023; Wang et al., 2024c) (Figures 3A, B). Fungal infections not only directly affect the host immune system through their pathogenicity but also regulate immune cell functions via a range of mechanisms, which results in immune suppression, immune evasion, and a chronic inflammatory environment that favors tumor growth and metastasis (Heung et al., 2023; Riquelme and McAllister, 2021; Sheng et al., 2024; Soerens et al., 2023). Several of the key pathways through which fungi modulate immune cell functions and alter the TME are discussed below:
3.2.1 Fungi modulating dendritic cells to alter the TME
DCs serve as a bridge between the innate and adaptive immune systems, initiating T-cell immune responses by phagocytosing and presenting antigens (Palucka and Banchereau, 2012). Fungi interact with DCs through their cell wall components (such as β-glucans and chitin) and other molecules (e.g., lipids and carbohydrates), binding to pattern-recognition receptors (such as Dectin-1 and TLR2) and affecting the function of DCs (Chamilos et al., 2010; Karnam et al., 2021; Koning and Mebius, 2016).
3.2.1.1 Induction of immune tolerance
After activation, DCs secrete pro-inflammatory or anti-inflammatory cytokines, which determines the type of immune response (Nelson et al., 2020; Ramirez-Ortiz and Means, 2012). However, fungal infections, particularly chronic infections, often result in alterations of the DC function, converting them into immune-suppressive types (Anderson et al., 2021; Gringhuis et al., 2022). Chronic fungal infections may induce immune tolerance in DCs when initiating adaptive immune responses by secreting immunosuppressive factors such as IL-10 and TGF-β (Iberg et al., 2017). This aspect suppresses the activity of effector T-cells, leading to immune evasion in the TME(Y. Guo et al., 2020; Iberg et al., 2017).
3.2.1.2 Polarization of DCs
For instance, fungi such as C. albicans can activate the Th17 response of DCs via β-glucans (Li et al., 2022b) (Figure 3C). This response leads to the secretion of large amounts of IL-17, which promotes chronic inflammation and increases the accumulation of immunosuppressive cells (e.g., Tregs), thereby altering the immune characteristics of the TME (Ramirez-Garcia et al., 2016).
3.2.2 Macrophage polarization and its impact on the TME
Macrophages are important effector cells of the immune system, capable of regulating immune responses through pathogen phagocytosis and cytokine secretion (Varol et al., 2015; Wynn and Vannella, 2016). Fungal infections activate different functional states of macrophages through their surface molecules (such as β-glucans), which, especially, affect their polarization (Casadevall, 2022; Gilbert et al., 2014).
3.2.2.1 Macrophages and immune suppression
M1 macrophages are pro-inflammatory and can enhance anti-tumor immune responses by secreting cytokines (such as TNF-α and IL-12) (Li et al., 2023b; Pu and Ji, 2022). Fungal infections may initially activate M1 macrophages, but, as the infection progresses, macrophages tend to polarize into M2 macrophages (Chen et al., 2024a, 2023a; Kaplanov et al., 2019). M2 macrophages secrete immunosuppressive factors (such as IL-10 and TGF-β), promoting tumor immune evasion and angiogenesis, thereby providing a favorable environment for tumor cell growth and metastasis (Chen et al., 2023a; Wang et al., 2023b; Zhou et al., 2019).
3.2.2.2 Impact of fungal infection on macrophage polarization
For example, Aspergillus infections can lead to M2 macrophage polarization(J. J. Chen et al., 2020; Guibo et al., 2024), thereby inhibiting effector immune responses and enhancing the immunosuppressive environment in the TME (Casadevall, 2022; Sprenger et al., 2018), which, in turn, supports tumor growth and metastasis (Ma et al., 2024; Matusiak et al., 2024; Narunsky-Haziza et al., 2022) (Figure 3D).
3.2.3 Fungi modulating T-cell function to alter the TME
T-cells play a central role in the tumor immune responses, and fungi can modulate T-cell functions through direct or indirect mechanisms, thereby influencing the TME (Chapman et al., 2020; Song et al., 2023; Zhou et al., 2024a).
3.2.3.1 Treg cell accumulation and immune suppression
Regulatory T-cells (Tregs) play a critical role in immune tolerance and evasion (Savage et al., 2020; Wing et al., 2019). Fungi activate DCs and macrophages to promote the proliferation and accumulation of Tregs (Atarashi et al., 2011; Sui et al., 2020), which secrete immunosuppressive factors such as IL-10 and TGF-β, inhibiting effector T-cell function and leading to immune evasion (Arpaia et al., 2013; Atarashi et al., 2013; Furusawa et al., 2013). For instance, Aspergillus infection triggers an increase in the number of Tregs (Yan et al., 2021), thereby enhancing the tumor’s immune evasion mechanisms and inhibiting anti-tumor immune responses (Hezaveh et al., 2022; Kumagai et al., 2024; Moreno Ayala et al., 2023).
3.2.3.2 Role of Th17 cells and IL-17
Fungal infections often promote the activation of Th17 cells, which increases the production of IL-17 (Cifaldi et al., 2022; Mills, 2023). IL-17 promotes chronic inflammation in the TME and, in some cases, enhances immune suppression (Seif et al., 2023; Sun et al., 2022). IL-17 not only activates immune cells but also induces local immune tolerance, thereby increasing the accumulation of Tregs and further inhibiting anti-tumor immune responses (Fidelle et al., 2023; Lee et al., 2023).
3.2.4 Accumulation of immunosuppressive cells to alter the TME
Fungi alter the immune status of the TME by affecting the function of immunosuppressive cells (such as M2 macrophages, Tregs, and myeloid-derived suppressor cells [MDSCs]), which promotes tumor growth and metastasis (Lyu et al., 2022; Xu et al., 2018; Zhao et al., 2018).
3.2.4.1 Role of MDSCs
MDSCs are immune-suppressive cell populations in cancer and chronic infection (Nakamura and Smyth, 2020; Schneider et al., 2022; Yang et al., 2022b). They suppress the function of effector T-cells by secreting immunosuppressive factors (such as TGF-β and IL-10), which, in turn, promotes tumor immune evasion (Cervantes-Villagrana et al., 2020; Kuang et al., 2023; Yi et al., 2023). Fungal infections (e.g., Cryptococcus infection) can enhance the generation and function of MDSCs (Li et al., 2022c), leading to the generation of an immunosuppressive environment in the TME and the reduction in the effectiveness of anti-tumor immune responses (Li et al., 2022c).
3.2.4.2 Immune evasion and immune tolerance
Fungi activate immunosuppressive cells such as Tregs, M2 macrophages, and MDSCs, which facilitate the immune evasion of tumors (Marcos et al., 2016; Pais et al., 2016). For example, chronic Candida infection can secrete immunosuppressive factors such as IL-10 (Candon et al., 2020), thereby inhibiting the activity of effector immune cells and supporting tumor cell growth and immune evasion (Candon et al., 2020; MaChado and Torres, 2018; Szabo et al., 2023).
3.2.5 Fungi’s impact on TME via immune evasion mechanisms
Fungal infections can induce immune evasion through various mechanisms, thereby supporting tumors (Cheng et al., 2024). The key mechanisms of immune evasion are discussed below (Cheng et al., 2024; Pulendran and Davis, 2020):
3.2.5.1 Establishment of immune tolerance
Fungi contribute to immune tolerance within the TME by suppressing effector T-cell activity, thereby enabling tumor cells to evade immune surveillance (Arner and Rathmell, 2023; Harris et al., 2024; Hiam-Galvez et al., 2021). Through the modulation of DCs, macrophages, and T-cells, fungi create an immunosuppressive environment that fosters tumor growth (Bilal et al., 2025; Galloway-Peña et al., 2024).
3.2.5.2 Promotion of chronic inflammation
Chronic inflammation driven by fungal infections plays a multifaceted role in TME (Denk and Greten, 2022; Greten and Grivennikov, 2019). Fungi induce the secretion of pro-inflammatory cytokines, including IL-17, IL-6, and TNF-α, which activate immune-suppressive mechanisms that, paradoxically, support tumor survival and progression (Elinav et al., 2013; Lochhead et al., 2021).
3.3 Fungi regulate TME through metabolites
The regulatory role of fungal metabolites within TME is a complex and rapidly evolving area of research (Hanus et al., 2021; Xiao et al., 2024). Emerging evidence suggests that fungi influence tumor dynamics not only as pathogenic agents but also by directly or indirectly modulating the TME through their metabolites (Cheng et al., 2024; Saftien et al., 2023). These metabolites contribute to key processes such as tumor initiation, progression, immune evasion, and metastasis (Eniafe and Jiang, 2021; Li et al., 2024a).
3.3.1 Mechanisms of fungal metabolite regulation in the TME
Fungal metabolites, including mycotoxins, volatile organic compounds (VOCs), and small molecular metabolites, modulate immune responses within the TME (Kozieł et al., 2021; Rushing and Selim, 2019). These compounds can alter immune cell activation, differentiation, proliferation, and responsiveness to tumor cells, thereby influencing immune surveillance and tumor immune evasion (Mafe and Büsselberg, 2024; Pitt and Miller, 2017) (Figure 4).
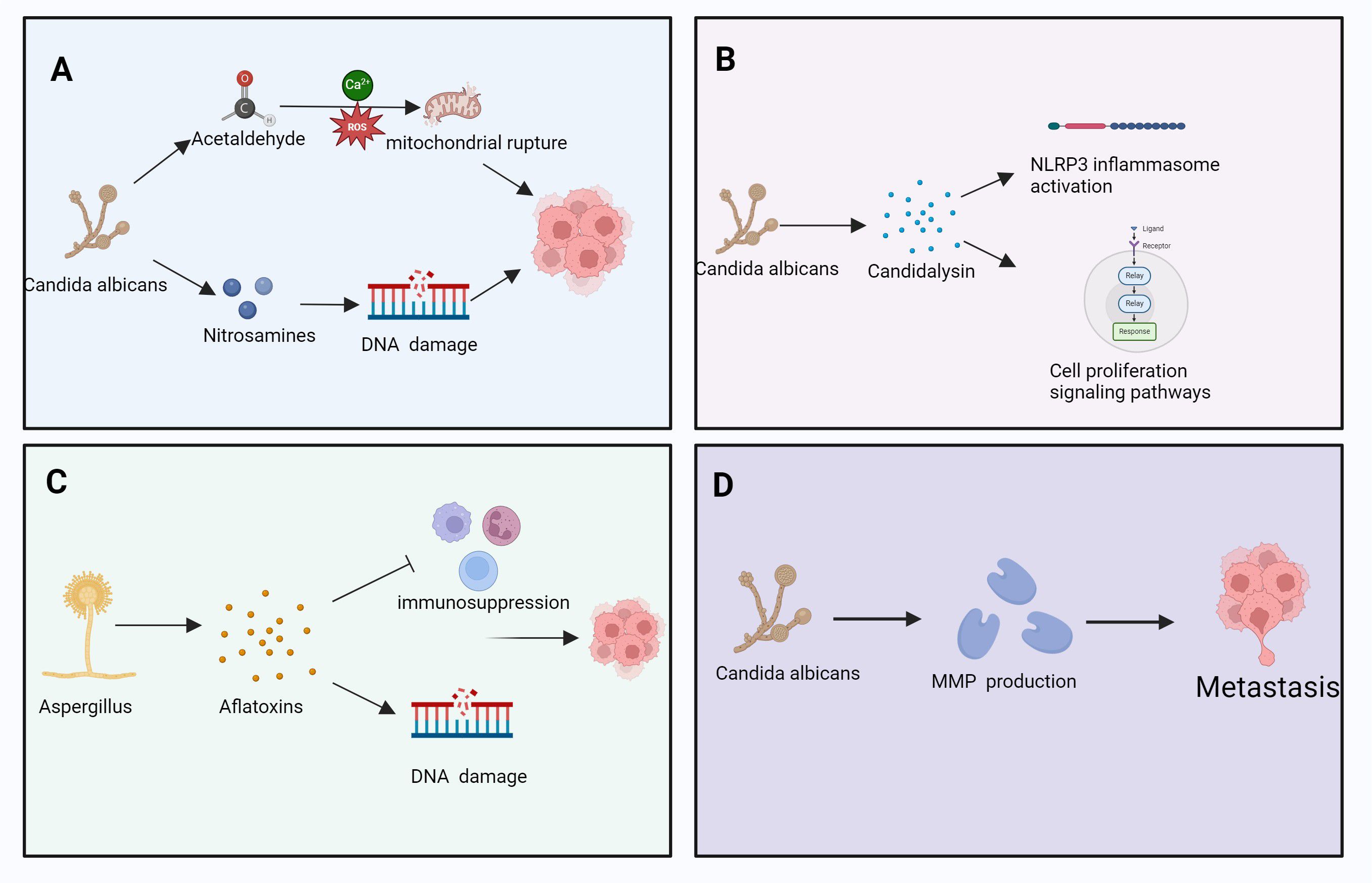
Figure 4. The role of various fungal metabolites in cancer development, involving processes such as DNA damage, immune suppression, cell proliferation, and metastasis. Specifically, it includes: (A) C albicans induces DNA damage through its metabolites acetaldehyde and nitrosamines. Acetaldehyde generates reactive oxygen species (ROS) mediated by calcium ions (Ca²+), leading to mitochondrial rupture, which further disrupts cell function and promotes cancer progression. (B) C albicans secretes candidalysin, which activates the NLRP3 inflammasome. This process regulates cell proliferation-signaling pathways, promoting tumor cell proliferation and advancing cancer progression. (C) Aspergillus secretes aflatoxins, leading to immune suppression and DNA damage. The immunosuppressive effect of aflatoxins creates a favorable environment for tumor cell proliferation and survival. (D) C albicans promotes the production of matrix metalloproteinases (MMPs) through its metabolites, thereby facilitating the metastasis of tumor cells. This process helps tumor cells traverse the basement membrane and spread to other tissues. Pathways with dashed arrows represent hypothetical interactions yet to be validated in clinical studies (Kozieł et al., 2021; Rushing and Selim, 2019).
3.3.1.1 Immunosuppressive effects of mycotoxins
Mycotoxins, such as aflatoxin and muscarine, Aflatoxins are produced primarily by Aspergillus flavus and A. parasiticus, while muscarine is associated with Inocybe and Clitocybe species,inhibit immune cell functions, including those of T-cells and macrophages, thereby weakening the host’s antitumor response (Claeys et al., 2020; Marchese et al., 2018; Unicsovics et al., 2024). By altering the immune cell composition within the TME, mycotoxins contribute to a suppressed antitumor immune landscape, enhancing the tumor’s ability to evade immune detection and destruction (Kraft et al., 2021) (Figure 4A).
3.3.1.2 Regulation of immune cell metabolism
Fungal metabolites significantly affect immune cell metabolism within the TME, Notable fungal metabolites involved include candidalysin, gliotoxin, and patulin, which modulate immune signaling and epithelial integrity, thereby influencing their functional capacity (Cheng et al., 2024; Wang et al., 2024c; Zhou et al., 2024e). For instance, these metabolites may suppress T-cell effector function by modulating key metabolic pathways, including glycolysis and fatty id oxidation (He et al., 2021; Jia et al., 2024a). In addition, fungal metabolites may promote the accumulation and activation of immunosuppressive cells, such as Tregs and tumor-associated macrophages, further enhancing immune evasion (Campbell et al., 2020; Ma et al., 2017) (Figures 4B, C).
3.3.2 Metabolic reprogramming and tumor growth
Fungal metabolites play a role in tumor cell metabolic reprogramming (Baixauli et al., 2022). Cancer cells frequently exhibit the “Warburg effect,” favoring anaerobic glycolysis over oxidative phosphorylation, even in oxygen-rich environments, metabolites such as ethanol, acetaldehyde, and farnesol have been shown to promote glycolysis in tumor and immune cells, so as to meet their energy demands (Takeuchi et al., 2024). So as to meet their energy demands (Takeuchi et al., 2024). Certain fungal metabolites, such as organic acids, ketones, and fatty acids, can influence these metabolic pathways, thereby affecting tumor cell growth and proliferation (Takeuchi et al., 2024). For example, some fungal metabolites enhance glycolysis in tumor cells, thereby providing additional energy to support the rapid tumor expansion (Bacigalupa et al., 2024; Zhou et al., 2024d) (Figure 4D).
3.3.3 Regulation of autophagy and apoptosis in tumor cells
Some fungal metabolites influence key cellular processes, including autophagy and apoptosis, within tumor cells. Autophagy is a critical survival mechanism that enables tumor cells to adapt to nutrient deprivation and cellular stress (Bacigalupa et al., 2024). Certain fungal metabolites regulate autophagy, allowing tumor cells to survive unfavorable conditions by modulating pathways such as the mechanistic target of rapamycin signaling (Bacigalupa et al., 2024; Ngwa et al., 2019; Zhou et al., 2024c). Secondary metabolites of fungi may enhance tumor cell survival by interacting with these pathways, thereby contributing to tumor progression and resistance to therapeutic interventions (Lee et al., 2024) (Claeys et al., 2020).
3.3.4 Fungal metabolites and immune cell interactions in the TME
Fungal metabolites significantly affect interactions between immune cells and tumor cells within the TME, reshaping its immune landscape (Takeuchi et al., 2024; Wu et al., 2020). These metabolites can enhance or inhibit the recruitment and infiltration of specific immune cell populations, thereby altering immune cell composition and functionality (Bachem et al., 2019; Chen et al., 2021; Takeuchi et al., 2024). Such changes influence immune evasion mechanisms and tumor growth dynamics (Wu et al., 2024b). Examples include gliotoxin, which suppresses NF-κB activation, and indole-3-lactic acid, which modulates host inflammation through AhR signaling. For example, certain metabolites may increase the infiltration of immunosuppressive cells or decrease the presence of cytotoxic immune cells, tipping the balance in favor of tumor survival (Bacigalupa et al., 2024; Staudt et al., 2023; Uribe-Herranz et al., 2020; Wu et al., 2024b).
3.3.5 Promotion of an immunosuppressive microenvironment
Fungal metabolites play a crucial role in the polarization of tumor-associated macrophages within TME (Rangel Rivera et al., 2021). They facilitate the shift from the pro-inflammatory M1 phenotype, which exerts antitumor effects, to the immunosuppressive M2 phenotype (Wu et al., 2020). This transition enhances immune suppression and creates a microenvironment conducive to tumor growth and immune evasion, allowing cancer cells to proliferate unchecked (Arpaia et al., 2013).
3.3.6 Regulation of cytokine and chemokine expression
The expression of cytokines and chemokines within the TME is intricately regulated by fungal metabolites (Bhat et al., 2022; Propper and Balkwill, 2022). These metabolites modulate immune cell activity by upregulating or downregulating cytokine and chemokine levels (Gupta et al., 2022). For instance, certain fungal compounds may induce tumor cells to secrete immunosuppressive cytokines, such as IL-10 and TGF-β (Gupta et al., 2022; Singha et al., 2015). These cytokines suppress immune responses, reduce the activity of cytotoxic T-cells, and promote Treg function, collectively facilitating tumor immune evasion (Cheng et al., 2016; Drouillard et al., 2023).
3.3.7 Fungal interactions with microbial communities in the TME
The TME encompasses a diverse array of microbial communities, including both bacteria and fungi (Jiang et al., 2024). Fungal metabolites examples include gliotoxin, which suppresses NF-κB activation, and indole-3-lactic acidcan interact with bacterial populations.Fungal metabolites can interact with bacterial populations, influencing the overall microbiota composition and the activity within TME (Jiang et al., 2022a, 2024; Liu et al., 2024a). These interactions may alter immune responses and contribute to tumor progression (Qiu et al., 2020). Recent studies have highlighted the dynamic interplay between fungi and bacteria in modulating tumor growth, immune suppression, and the overall immune milieu (Bi et al., 2024; Qian et al., 2024; Qiu et al., 2020).
3.3.8 Synergistic effects of gut microbiota and immunotherapy
The gut microbiota plays a pivotal role in determining the effectiveness of cancer immunotherapy (Kim and Lee, 2021). Emerging research suggests that fungal communities within the gut microbiota significantly impact immune modulation and therapeutic outcomes (Kim and Lee, 2021; Masheghati et al., 2024; Yao et al., 2024). Certain fungal metabolites influence the composition and functionality of the gut microbiota, thereby affecting the host’s systemic immune responses (Dong et al., 2021; Gao et al., 2021). By shaping the gut microbiota, fungi indirectly modulate the immune characteristics of the TME, potentially enhancing or diminishing the efficacy of immunotherapeutic strategies (Dong et al., 2021; Jamal et al., 2023). These findings underscore the importance of integrating microbiome studies into cancer treatment paradigms so as to optimize therapeutic responses (Zhao et al., 2024).
In 2015, Thomas and his colleagues first noticed that there were correlations between gut microbiota and ICI immunotherapy. They used mice which were harbored with different commensal microbiota, then compared the melanoma growth of these mice. They also found that different microbiota might relate to different spontaneous antitumor immunity. Of which, they found that Bifidobacterium could facilitate antitumor effect of PD-L1 blockade (Sivan et al., 2015).
3.3.9 Typical fungal metabolites and their effects on TME
3.3.9.1 Mycotoxins
Aflatoxin, a potent carcinogenic mycotoxin produced by A. flavus, directly interacts with DNA, thereby inducing tumor formation (Marchese et al., 2018). In addition to its genotoxic effects, aflatoxin modulates immune system function, promoting tumor cell growth and metastasis by impairing immune surveillance mechanisms and facilitating immune evasion (Dong et al., 2022; Zhu et al., 2021).
3.3.9.2 Ochratoxin
Ochratoxin, secreted by Aspergillus ochraceus, exerts significant immunosuppressive effects within the TME (Llobregat et al., 2022; Parussolo et al., 2019). It alters immune cell infiltration and functionality, creating an immune-suppressive milieu that supports tumor progression (Liu et al., 2024d; Więckowska et al., 2024). This mycotoxin disrupts the balance of immune responses, further promoting tumor proliferation and metastatic potential (Liu et al., 2024d; Więckowska et al., 2024).
3.3.9.3 VOCs
Fungal VOCs are small molecules with diverse biological activities, including the regulation of plant growth and modulation of tumor cell behavior (Gouzerh et al., 2022; Zhou et al., 2024b). These VOCs can influence the TME by altering immune cell functions, by either promoting or suppressing immune responses (Gouzerh et al., 2022; Stone, 2022; Zhou et al., 2024b). Through such mechanisms, fungal VOCs indirectly affect immune cell activation, differentiation, and cytokine production, thereby modulating the immune status of the TME and contributing to tumor progression (Gouzerh et al., 2022; Wekking et al., 2024).
3.3.9.4 Secondary metabolites of fungi
Fungal secondary metabolites, such as mycophenolic acid, tacrolimus (cyclosporine), and polyamide compounds, are critical mediators in the TME (Liu et al., 2024b; Nesic et al., 2014). These metabolites can influence tumor immune evasion and cell proliferation (Domingos et al., 2022; Duizer and de Zoete, 2023; Lin et al., 2021; Staszczak, 2021) (He et al., 2020). For instance, tacrolimus, an immunosuppressant commonly used in clinical settings, inhibits T-cell function and contributes to immune suppression, which facilitates tumor cell survival and growth (He et al., 2020) (Julianti et al., 2022).
Fungal interactions within the TME extend beyond direct effects on immune and tumor cells. Metabolites influence microbial communities, regulate immune cell activity, and alter tumor cell metabolism, collectively contributing to immune evasion, proliferation, metastasis, and therapeutic resistance. This complex interplay highlights fungal metabolites as promising targets for innovative antitumor strategies and reinforces the importance of this emerging research area in the field of cancer biology.
3.4 Fungi influence TME through angiogenesis and tumor metastasis
3.4.1 Promotion of angiogenesis
Angiogenesis is essential for tumor growth and metastasis, providing tumor cells with oxygen and nutrients while offering a pathway for dissemination (Dudley and Griffioen, 2023b; Wang et al., 2015). Fungal metabolites and structural components, such as β-glucan, play critical roles in promoting angiogenesis by stimulating the release of pro-angiogenic factors, including vascular endothelial growth factor (VEGF) (Choi et al., 2022; Xu et al., 2024). Proposed pathway based on limited experimental evidence; quantitative validation is needed (Xu et al., 2024).
Several fungal species, such as Aspergillus and C. albicans, induce localized inflammatory responses that enhance angiogenesis through the secretion of metabolites like lipids and toxins (Wang et al., 2024b, 2015). These factors influence vascular remodeling and blood vessel formation within the TME, enabling tumor expansion and metastatic spread (Cao et al., 2023b; Dudley and Griffioen, 2023a, 2023b).
3.4.2 Secretion of angiogenesis factors
Fungal infections activate host immune cells, including macrophages and DCs, prompting the secretion of VEGF and basic fibroblast growth factor (Kuo et al., 2018; Onyishi et al., 2023). These angiogenesis factors drive the formation of new vasculature, supporting tumor growth and facilitating tumor cell migration into the circulatory system (Liu et al., 2023d; Patel et al., 2023).
For example, Aspergillus species promote angiogenesis by inducing VEGF production, enhancing nutrient delivery to tumor cells while providing a conduit for metastasis (Ben-Ami et al., 2013; Ito, 2013; Park et al., 2019). Similarly, C. albicans and Cryptococcus leverage cell wall polysaccharides, such as β-glucan, to activate local immune responses (Iyer et al., 2021). This activation leads to upregulated angiogenesis factor secretion, which further contributes to vascular proliferation and metastatic progression within the TME (Bhat et al., 2021; Pérez-Tomás and Pérez-Guillén, 2020).
3.4.3 Tumor cell metastasis
Fungi contribute to tumor metastasis by altering immune cell composition in the TME, modulating immune evasion mechanisms, and regulating pro-inflammatory cytokines (Arner and Rathmell, 2023; Liu et al., 2024b). Gut microbiota dysbiosis, including fungal imbalances, is closely linked to metastatic progression (Li et al., 2019; Liu et al., 2024b).
Tumor metastasis refers to the dissemination of tumor cells from their primary site to distant tissues—a process facilitated by angiogenesis, immune evasion, and enhanced tumor cell invasiveness (Li et al., 2019; Liu et al., 2024b). Fungi influence metastasis through multiple mechanisms, which significantly alters the TME (Fu et al., 2023).
3.4.4 Regulation of Treg cells and immune evasion
Fungi can induce the accumulation of Tregs, which suppress antitumor immunity (Liu et al., 2024b). Tregs secrete immunosuppressive cytokines such as IL-10 and TGF-β, inhibiting effector T-cell activity and enabling tumor cells to escape immune surveillance (Fu et al., 2023; Liu et al., 2024b). This immune-suppressive environment promotes metastasis, particularly within newly established tumor sites, where conditions favor tumor cell proliferation and spread (Hoshino et al., 2015; Wong and Yu, 2024).
3.4.5 Relationship between angiogenesis and tumor metastasis
Angiogenesis supplies tumor cells with oxygen and nutrients while providing direct routes for tumor cells to enter the circulatory and lymphatic systems(C. Jiang et al., 2021; Singhal and Augustin, 2020). Fungi contribute to angiogenesis through metabolites that enhance endothelial cell proliferation and vascular permeability, thereby increasing tumor cell invasiveness and metastatic potential (Mehrian-Shai et al., 2019; Ribatti, 2024).
3.4.6 Fungal metabolites and the expression of metastasis-related proteins
Fungal metabolites activate signaling pathways associated with metastasis in tumor cells (Sharma-Walia et al., 2012). For example, C. albicans infection stimulates the MAPK/ERK pathway, enhancing tumor cell invasiveness and migratory capacity (Ho et al., 2019; Song et al., 2022). This signaling cascade promotes the dissemination of tumor cells to secondary tissues, facilitating metastasis (Sharma-Walia et al., 2012).
3.4.7 Pro-inflammatory factors and tumor metastasis
Chronic inflammation induced by fungal infections promotes the release of pro-inflammatory cytokines, which then contribute to tumor cell migration and metastatic progression (Yaniv et al., 2024; Zhu et al., 2024). Pro-inflammatory mediators such as TNF-α, IL-1β, and IL-6 not only stimulate angiogenesis but also alter tumor cell adhesion and infiltration, thereby enhancing metastatic behavior (Cruceriu et al., 2020; Kobelt et al., 2020; Zhang et al., 2021).
3.4.7.1 Fungi and IL-17 regulation in metastasis
Fungi, including C. albicans, drive IL-17 production, a cytokine associated with chronic inflammation and immune suppression (Aggor et al., 2020; Shao et al., 2019). IL-17 fosters an immune-suppressive environment while upregulating pro-inflammatory mediators, thereby increasing tumor cell invasiveness and metastatic potential (Guo et al., 2022; Li et al., 2024b). This dual role makes IL-17 a pivotal link between chronic fungal infections and tumor metastasis (Chen et al., 2023b; Liu et al., 2024c).
3.5 Interactions between fungi and symbiotic microbiota in the TME
The complex interplay between fungi and other symbiotic microorganisms within the TME has emerged as a significant area of research (Takeuchi et al., 2024). Fungal metabolites influence the TME directly and indirectly by interacting with bacterial, viral, and other microbial populations (Takeuchi et al., 2024). The collective activity of these microbial communities, particularly the gut microbiota, plays a critical role in tumor initiation, progression, immune evasion, and response to therapy (Gagnière et al., 2016; Pickard et al., 2017). As the key members of the microbiota, fungi contribute to tumorigenesis by modulating the composition and functionality of these microbial networks, thereby reshaping the TME to support tumor growth and metastasis (Heidari et al., 2024; Wang et al., 2024c) (Figure 5).
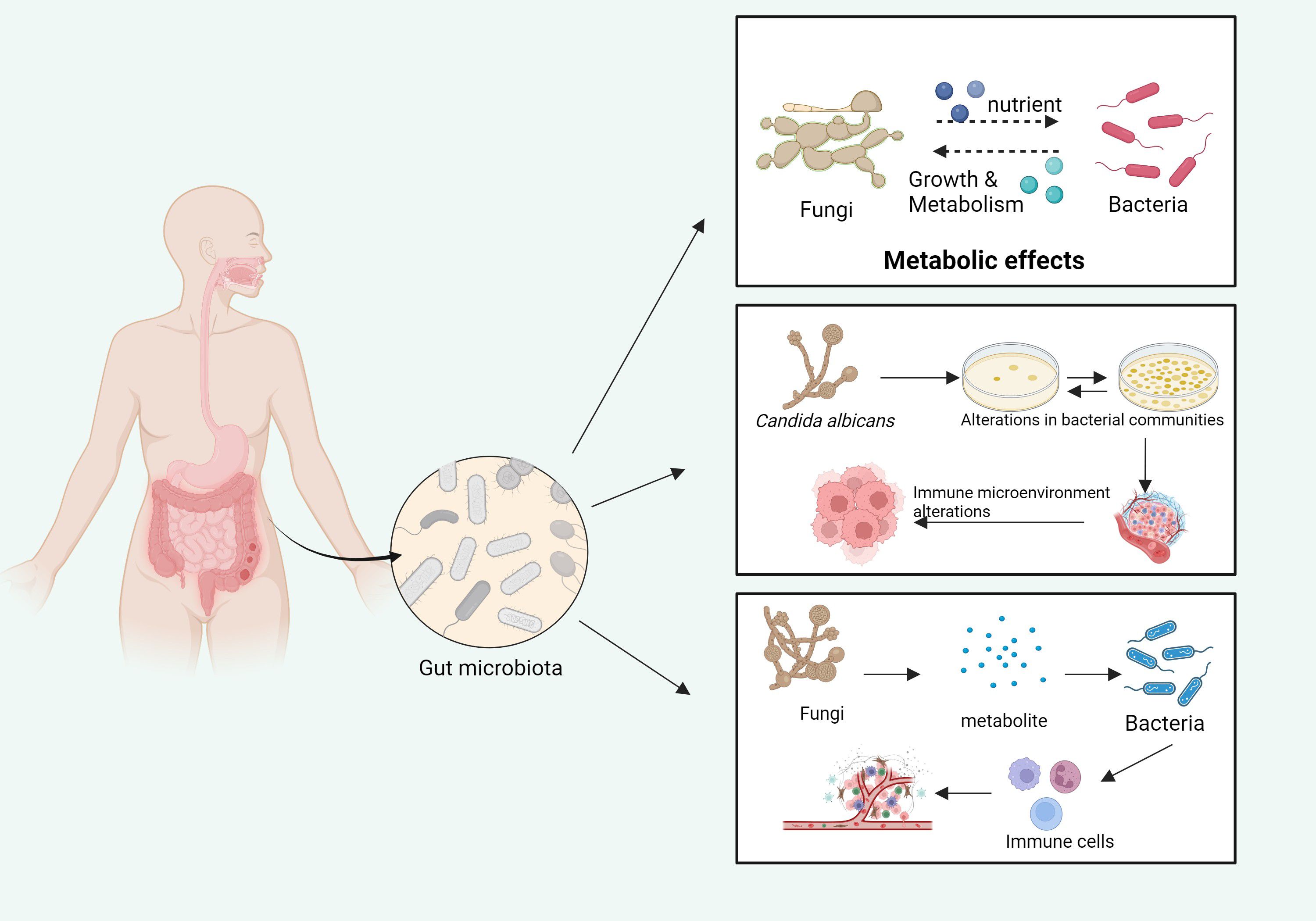
Figure 5. The influence of fungi and bacteria on each other’s growth and metabolism in the gut microbiota. Fungi, by metabolizing various nutrients, impact bacterial growth and metabolic processes. In turn, the metabolites produced by bacteria influence fungal growth and activity. These interactions cause alterations in the gut microbiome, which, in turn, modulate the local immune microenvironment, potentially affecting systemic immune responses (Top panel): fungi, through their metabolic consumption of nutrients, directly influence the growth and metabolic activities of bacterial communities within the gut. This metabolic interaction contributes to the formation of distinct microbial community structures. (Middle panel): Specifically, C. albicans can alter the composition of bacterial populations. The resulting disruption in the microbiome leads to significant changes in the immune microenvironment, affecting the host’s immune responses, including the modulation of inflammatory and anti-inflammatory pathways. (Bottom panel): The metabolites produced by fungi interact with bacterial metabolites to influence immune cell activation. These interactions not only alter the gut microenvironment but can also affect systemic immune functions, potentially influencing host susceptibility to infections and disease progression. Pathways with dashed arrows represent hypothetical interactions yet to be validated in clinical studies (Takeuchi et al., 2024).
3.5.1 Synergistic effects of fungi and symbiotic microbiota in the TME
The TME comprises not only tumor and immune cells but also diverse microbial communities, including bacteria, fungi, and viruses (Brennan and Garrett, 2019; Schwabe and Jobin, 2013). These microbial populations exert significant influence on tumor biology through intricate interactions (Schwabe and Jobin, 2013). As integral members of these communities, fungi interact with symbiotic microbiota in ways that shape the TME (Chen et al., 2017).
3.5.1.1 Interactions among microbial communities
Fungi in the TME do not exist in isolation; they interact dynamically with bacteria, viruses, and other microorganisms (Chen et al., 2017; Wang et al., 2024c). For instance, certain fungal metabolites may serve as nutrients for bacteria or modify microbial metabolic outputs, notably, Bacteroides, Lactobacillus, and Prevotella species appear to benefit from fungal metabolic interactions, thereby influencing the overall microbiota composition (Wang et al., 2024c). Conversely, bacterial metabolic by-products, such as short-chain fatty acids and lactic acid, can impact fungal growth and metabolism, thereby creating bidirectional regulatory networks that affect TME (Koliarakis et al., 2019; Lu et al., 2022).
3.5.1.2 Impact of gut microbiota on immunotherapy
A growing body of research has highlighted the critical role of gut microbiota composition in determining the success of cancer immunotherapy (Galloway-Peña et al., 2024; Wong and Yu, 2023). Certain gut bacteria enhance antitumor immune responses, and fungi may act as modulators in this process (Dohlman et al., 2021; Tong et al., 2021). Fungal metabolites can influence bacterial populations within the gut, alter immune responses, and subsequently affect tumor immune evasion and therapeutic outcomes (Hanus et al., 2021; Li et al., 2023a; Wang et al., 2024c).
3.5.2 Fungi and gut microbiota interactions
The gut microbiota, comprising bacteria, fungi, viruses, and other microorganisms, plays a pivotal role in host health, immune regulation, and disease progression (Li et al., 2023a, 2024a; Papon et al., 2021; Wang et al., 2024c). Dysbiosis within this complex ecosystem has been closely linked to cancer development, with fungi contributing to gut microbiota alterations and tumor progression through several mechanisms (Li et al., 2023b; Wang et al., 2024c).
3.5.2.1 Fungi’s role in maintaining gut microbial balance
Fungal species such as C. albicans and Aspergillus are the normal components of the gut microbiota, where they help maintain microbial homeostasis (Li et al., 2023b; Wang et al., 2024c). However, conditions such as immunosuppression or antibiotic use can disrupt this balance, leading to fungal overgrowth and dysbiosis (Gou et al., 2024; Shi et al., 2020). Fungal dysbiosis can destabilize the gut microbiota equilibrium, fostering an environment conducive to tumor initiation and progression (Bi et al., 2024; Chen et al., 2024a; Malik et al., 2018). Furthermore, fungal metabolites can reshape the bacterial community, activating immune cells and modulating antitumor immune responses, either enhancing or suppressing immune activity (Bi et al., 2024).
3.5.2.2 Relationship between gut microbiota and immune response
The gut microbiota profoundly influences local and systemic immune responses, thereby directly impacting tumor immune evasion (Guo et al., 2021; Schürch et al., 2020; Zhou et al., 2021). Bacterial metabolic products, such as short-chain fatty acids, enhance local immune activity and suppress tumor growth (Chen et al., 2022; Jiang et al., 2023). Fungi indirectly affect immune regulation by modifying the synthesis of these metabolites (Chen et al., 2022; Jiang et al., 2023; Ngwa et al., 2019; Wang et al., 2023a). For example, C. albicans interacts with gut bacteria to either stimulate or suppress immune responses, thereby playing a pivotal role in tumor initiation and progression through its influence on immune modulation and microbiota composition (d’Enfert et al., 2021; Pierre et al., 2023).
3.5.2.3 Immunotherapy and microbiota interactions
Increasing evidence suggests that the composition of the gut microbiota significantly influences the effectiveness of immunotherapy (Routy et al., 2018; Trompette et al., 2014). For example, the efficacy of immune checkpoint inhibitors, such as PD-1/PD-L1 inhibitors, is closely linked to the gut microbiota (Routy et al., 2018). The fungal communities within the gut may play a role in this response (Trompette et al., 2014). Certain fungal metabolites, through interactions with the gut bacteria, can either enhance or suppress the effectiveness of these inhibitors (Matson et al., 2021). Therefore, modulating the gut microbiota, particularly by balancing the relationship between fungi and bacteria, may offer a novel strategy to improve immunotherapy outcomes (Chrysostomou et al., 2023; Matson et al., 2021; Routy et al., 2018; Zhou et al., 2021).
While fungal modulation of the gut microbiota may impact immunotherapeutic efficacy, findings remain inconsistent across studies. Some reports failed to demonstrate a significant correlation between fungal diversity and immune checkpoint response, potentially due to confounding factors such as antibiotic exposure, diet, tumor type, and baseline immune heterogeneity. Therefore, a clearer understanding of how fungi interact with host immunity—and under what circumstances they enhance or suppress therapy—is still required (Chrysostomou et al., 2023).
3.5.3 Fungi and interactions with other microbial communities
Beyond the gut microbiota, fungi also interact with other microbial populations within the TME, such as the skin and oral microbiota, thereby influencing tumor initiation and progression (Flowers and Grice, 2020; Hong et al., 2019).
3.5.3.1 Oral microbiota and tumors
Fungi in the oral cavity, especially Candida, are strongly associated with the development of oral and esophageal cancers (Alnuaimi et al., 2015; Deshpande et al., 2018). Candida interacts with bacterial communities in the oral cavity through its metabolites, altering the local immune environment and promoting tumor growth (Huo et al., 2022; Shuai et al., 2022). Certain fungi may directly stimulate tumor cells or modulate local immune responses, thereby contributing to tumor progression and metastasis (Wang et al., 2024c).
3.5.3.2 Skin microbiota and tumors
Fungi on the skin, such as Candida and Malassezia, are vital for maintaining the skin microbiota balance (Hau et al., 2015). The fungal community on the skin is linked to the development of skin cancers, including melanoma (Hanes et al., 2021; Shiao et al., 2021). Fungi may promote tumor development by influencing the local immune response or by interacting with skin bacteria, thereby further contributing to tumor initiation and progression (Byrd et al., 2018; Li et al., 2023c).
3.5.4 Therapeutic potential of modulating the TME
Modulating fungal communities or balancing their metabolites within the TME may provide new avenues for cancer treatment (Narunsky-Haziza et al., 2022). Adjusting the fungal populations in the gut or other microbiota may enhance tumor immune responses and improve the efficacy of antitumor immunotherapy (Narunsky-Haziza et al., 2022). In addition, natural products derived from fungi, such as antifungal drugs (e.g., voriconazole) or their metabolites, may serve as adjunctive agents in cancer therapy (Bilal et al., 2025; Dickson, 2019).
Fungi interact with symbiotic microbiota within the TME, including gut, oral, and skin microbiota, influencing tumor initiation, progression, immune evasion, and treatment response. Through their metabolites—such as mycotoxins and VOCs—fungi modulate immune systems, metabolic pathways, and tumor cell behavior. Investigating the mechanisms of fungal interactions with the microbiota in the TME offers valuable insights for tumor immunotherapy and microbiota modulation, potentially leading to breakthroughs in future cancer treatments.
3.6 Antifungal therapy and cancer treatment
The integration of antifungal therapy with cancer treatment is an emerging and promising area of research (Bilal et al., 2025; Weng et al., 2023). While much of the current literature focuses on the interactions between fungal infections and the immune systems of patients with cancer, an increasing body of evidence suggests that antifungal therapy can play a more significant role than merely addressing infections (Liu et al., 2023b; Neoh et al., 2024; Weng et al., 2023). In fact, antifungal therapy may have the potential to regulate the TME and improve cancer treatment outcomes (Jia et al., 2024b; Yang et al., 2022a) (Figure 6).
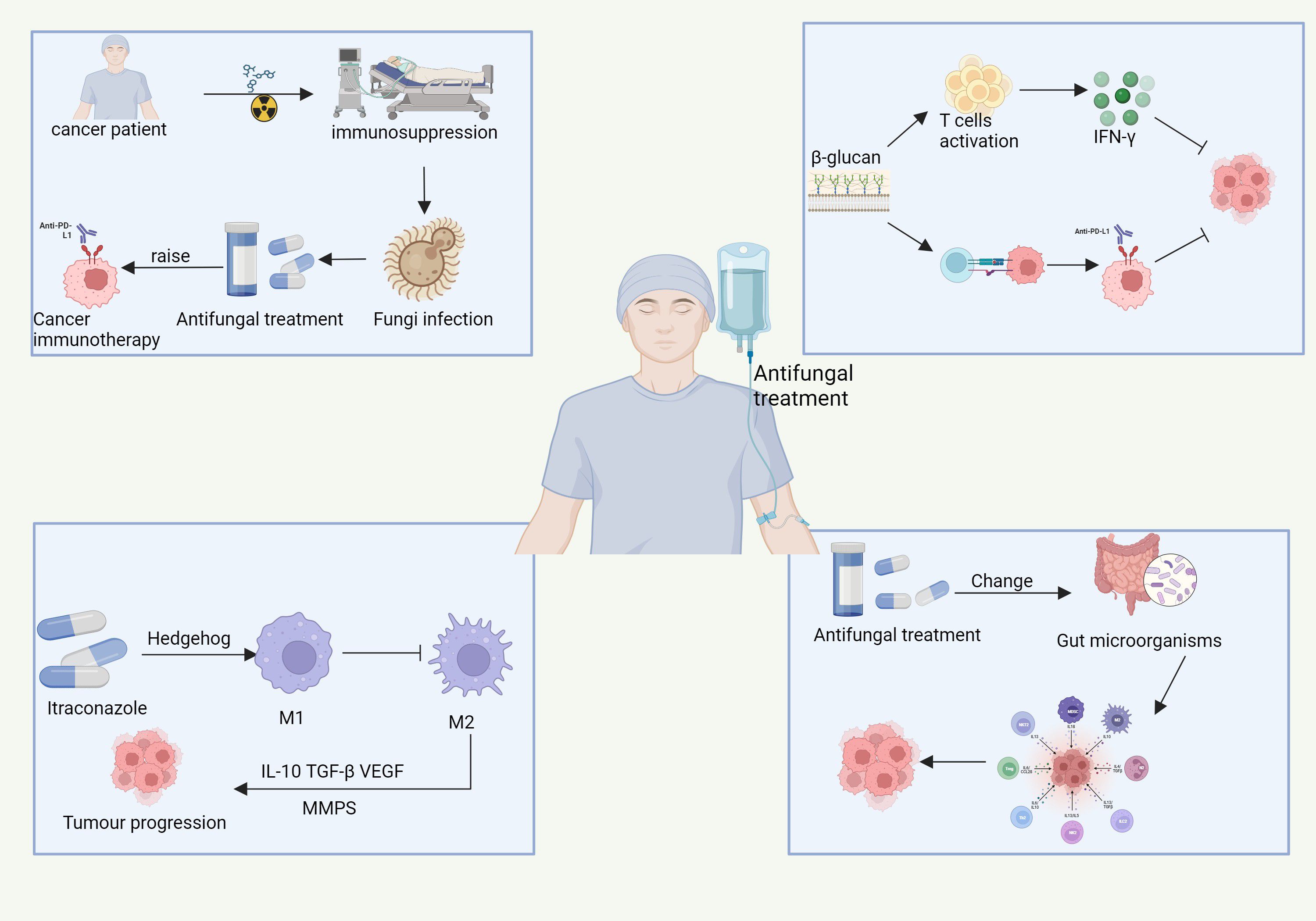
Figure 6. The complex interactions between antifungal treatment, cancer immunotherapy, and tumor progression in immunocompromised cancer patients. Cancer therapies, such as chemotherapy and immunotherapy, induce immune suppression, increasing vulnerability to fungal infections. These infections can complicate cancer treatment. Antifungal treatments, like β-glucan, not only help combat fungal infections but also activate immune responses, stimulating T-cells and promoting IFN-γ production. This immune activation can enhance the effectiveness of cancer immunotherapy, particularly anti-PD-L1 therapy. Furthermore, antifungal treatments such as itraconazole influence tumor progression by modulating immune pathways, including the Hedgehog-signaling pathway, which shifts macrophage polarization from the immune-activating M1 phenotype to the immunosuppressive M2 phenotype, supporting tumor growth. Moreover, antifungal treatment can alter the gut microbiota, indirectly influencing systemic immunity and affecting cancer progression. Pathways with dashed arrows represent hypothetical interactions yet to be validated in clinical studies (Jia et al., 2024b; Yang et al., 2022a).
3.6.1 Impact of antifungal therapy on the immune system
Cancer therapies such as chemotherapy, radiotherapy, and immunotherapy often induce immunosuppression, rendering patients with cancer more susceptible to fungal infections (e.g., Candida and Aspergillus) (K. Li et al., 2021). Under these circumstances, apart from eliminating infections, antifungal therapy may have important effects on immune system modulation (Lionakis et al., 2023; Pathakumari et al., 2020).
3.6.1.1 Restoration of immune function
Immunosuppression is a significant driver of tumor immune evasion(X. Cao et al., 2023a; Chen et al., 2023). By clearing fungal infections, antifungal drugs can help restore immune function, alleviate immune system stress, and promote more robust antitumor immune responses (Fisher et al., 2022; Iyer et al., 2021). For example, antifungal drugs can inhibit the growth of immunosuppressive fungi, such as Candida, thereby preventing them from dampening immune responses and potentially enhancing the efficacy of cancer immunotherapy (Lionakis et al., 2023; H. Lu et al., 2023).
3.6.1.2 Modulation of the immune microenvironment
Certain antifungal drugs—such as voriconazole and itraconazole—may modulate the immune cell landscape within the TME (Jang et al., 2023; Jia et al., 2024b). These drugs can influence immune cell polarization, leading to enhanced antitumor immune responses (Jia et al., 2024b; Yang et al., 2022a). For instance, antifungal therapy stimulates DCs, which play a pivotal role in antigen presentation. Enhanced DC activity leads to improved T-cell activation, which can intensify the body’s immune response against the tumor (Fites et al., 2018; Wang et al., 2021).
3.6.2 Direct effects of antifungal drugs on tumor cells
In addition to their immune-modulating effects, some antifungal drugs have demonstrated direct antitumor activity (Shanholtzer et al., 2022; Wei et al., 2017). These drugs can influence tumor cell proliferation, migration, and resistance to treatment through a variety of mechanisms (Dembitsky et al., 2021; Yamaguchi et al., 1993).
3.6.2.1 Inhibition of tumor cell proliferation
Antifungal agents such as voriconazole and itraconazole have been identified as having antitumor properties, including the ability to inhibit tumor cell proliferation (Benitez and Carver, 2019; D’Arcy et al., 2020). These drugs exert their effects by disrupting the key metabolic pathways involved in tumor growth, such as fatty acid synthesis, or by targeting signaling pathways such as the mechanistic target of the rapamycin pathway that is critical for tumor cell survival and proliferation (Liu et al., 2023b; Weng et al., 2023).
3.6.2.2 Enhancing drug sensitivity
Antifungal drugs may enhance the sensitivity of tumor cells to chemotherapy or immunotherapy, thereby improving the overall efficacy of these treatments (Weng et al., 2023). For instance, several studies have demonstrated that certain antifungal drugs can potentiate the effects of chemotherapy by inhibiting multidrug resistance proteins (MDR) in tumor cells, thereby reducing tumor cell resistance to chemotherapy agents (Bilal et al., 2025; Weng et al., 2023).
3.6.2.3 Induction of tumor cell apoptosis
Certain antifungal drugs induce apoptosis in tumor cells (Chen et al., 2019). Fungal cell wall components and metabolites can bind to receptors on the surface of tumor cells, triggering apoptotic signaling pathways and leading to programmed cell death (Chakrabarti and Ray, 2016; Yang et al., 2023). This ability to induce apoptosis can further enhance the therapeutic effects of antifungal agents when combined with other cancer treatments (Forma and Bryś, 2021).
3.6.3 Synergistic effects of antifungal therapy and immune checkpoint inhibitors
Immune checkpoint inhibitors (such as PD-1/PD-L1 inhibitors and CTLA-4 inhibitors) have emerged as a significant advancement in cancer immunotherapy (Naimi et al., 2022). Moreover, antifungal therapy may have a synergistic effect when used in conjunction with immune checkpoint inhibitors, potentially improving the outcomes of these treatments (Butterfield and Najjar, 2024; Jia et al., 2024a).
While several studies suggest that gut fungi may enhance immune checkpoint blockade efficacy, other analyses have reported inconsistent associations, possibly due to antibiotic use, diet, or inter-individual variability. Thus, the role of the mycobiome in immunotherapy response remains complex and warrants further investigation.
3.6.3.1 Regulation of microbiota
The gut microbiota plays a crucial role in determining the efficacy of immune checkpoint inhibitors (Li et al., 2022a). Dysbiosis in the gut microbiota can negatively affect the effectiveness of these inhibitors (Derosa et al., 2024). Antifungal drugs, by regulating the fungal populations within the gut microbiota, may help restore microbial balance, which could, in turn, enhance the efficacy of immune checkpoint inhibitors (Wurster et al., 2022). This modulation of the microbiota highlights a novel mechanism through which antifungal therapy can augment the effectiveness of immunotherapy (Mercer and O’Neil, 2020).
3.6.3.2 Modulation of the immune microenvironment
Antifungal therapy may influence the immune cell composition within the TME (Yang et al., 2022a). By modulating the activity of immune cells such as macrophages, DCs, and T-cells, antifungal drugs can promote more efficient tumor antigen presentation and activate immune responses (Jang et al., 2023; Zhuo et al., 2022). For example, certain antifungal drugs, such as voriconazole and itraconazole, may activate specific immune pathways that boost antitumor immune responses (Benitez and Carver, 2019). This effect, in turn, enhances the therapeutic effects of immune checkpoint inhibitors, contributing to improved outcomes in cancer immunotherapy (Jang et al., 2023; Jia et al., 2024b).
3.6.4 Effects of antifungal drugs in the TME
The fungal communities, immune cells, and fibroblasts within the TME interact to influence tumor progression (Aykut et al., 2019). Antifungal therapy not only clears infections and directly affects TME but may also regulate TME indirectly through the following mechanisms:
3.6.4.1 Impact on immune cells in the TME
Interactions between fungi and various immune cells in the TME, such as macrophages and DCs, may contribute to tumor immune evasion (Harris et al., 2024). Antifungal therapy has the potential to restore normal immune cell function and enhance the immune microenvironment within the TME (Jia et al., 2024b; Yang et al., 2022a). This improvement can lead to enhanced antitumor immune responses, thereby supporting the body’s ability to fight the tumor more effectively (Yang et al., 2022a).
3.6.4.2 Alleviating the immunosuppressive microenvironment
Certain fungi, such as Candida, can interact with the immune system to create an immunosuppressive environment that promotes tumor growth and metastasis (Lin et al., 2023). By clearing these fungi, antifungal therapy may reduce immune tolerance, thereby strengthening the immune system’s capacity to target and destroy tumor cells (Daley et al., 2017). This process not only improves immune surveillance, but may also inhibit tumor progression.
3.6.5 Effects of antifungal therapy on the gut microbiota
The growing recognition of the role of gut microbiota in tumor immunotherapy has highlighted the potential influence of antifungal drugs on the composition of the gut microbiota (Fernandes et al., 2022; Park et al., 2022; Wong and Yu, 2023). Past studies have suggested that antifungal therapy can alter the structure of the gut microbiota, which, in turn, could modify the host’s immune responses and metabolic state, thereby indirectly impacting tumor growth and immune surveillance (d’Enfert et al., 2021; Renga et al., 2024). For example, certain antifungal drugs, such as amphotericin B, directly target the gut fungal community, which potentially disrupts its balance and subsequently affects both immune responses and antitumor activity (Demir et al., 2022; Wheeler et al., 2016).
3.6.6 Combination of antifungal therapy with radiotherapy/chemotherapy
Antifungal drugs can be integrated into combination therapies with radiotherapy or chemotherapy to improve the treatment outcomes (Souza et al., 2020). The following are the key mechanisms of how antifungal therapy possibly augments these conventional treatments:
3.6.6.1 Enhancing chemotherapy effects
Chemotherapy often leads to immunosuppression, which increases the risk of fungal infections (Wojciechowski and Wiseman, 2021). Antifungal drugs can mitigate this risk, while also potentially enhancing chemotherapy’s antitumor effects by modulating the immune microenvironment (Wojciechowski and Wiseman, 2021). Furthermore, some antifungal drugs possess direct antitumor properties and may synergize with chemotherapy drugs, enhancing their efficacy (Saeedi et al., 2019).
3.6.6.2 Improving radiotherapy effects
Certain antifungal drugs, such as voriconazole, can improve the effects of radiotherapy (Cucchetto et al., 2015). These drugs may help modulate immune cells and tumor cell responses within the TME, thereby enhancing immune cell function and increasing tumor cell sensitivity to radiation (Dandachi et al., 2018). By serving as potential adjuncts to radiotherapy, antifungal drugs can offer an additional means to improve treatment outcomes (Dandachi et al., 2018).
3.6.7 Side Effects and challenges of antifungal therapy
Despite the potential benefits of antifungal drugs in cancer treatment, their use presents several challenges, particularly in terms of the side effects induced during prolonged treatment.
3.6.7.1 Drug Toxicity
Some antifungal drugs may lead to toxicity in the organs such as the liver and kidneys, especially in immunosuppressed patients with cancer (Assress et al., 2020; Silva et al., 2023). This effect necessitates careful monitoring of the organ function throughout antifungal therapy (Silva et al., 2023). Dose adjustments may be required to minimize toxicity and prevent harm, which underscores the importance of a personalized approach to antifungal treatment in patients with cancer (Silva et al., 2023).
3.6.7.2 Drug resistance
Fungi can develop resistance to antifungal drugs, particularly with prolonged use, particularly with prolonged use of specific agents (Fisher et al., 2018). This resistance poses a significant challenge to effective treatment, necessitating the continuous development of new antifungal agents (Perlin et al., 2017). Ongoing research should therefore explore novel antifungal compounds, alternative therapeutic strategies, and approaches to mitigate the emergence of such resistance cases (Perlin et al., 2017).
The combination of antifungal therapy with cancer treatment offers valuable new insights into cancer care. By regulating the TME, restoring immune system function, enhancing immunotherapy efficacy, and directly inhibiting tumor cell proliferation, antifungal drugs could serve as an important adjunct in cancer therapy. Future research is likely to reveal the intricate interactions between fungi and the TME, thereby uncovering the full potential of antifungal drugs in cancer treatment. Meanwhile, ensuring the rational use of antifungal agents and mitigating their potential side effects are deemed crucial in refining future treatment strategies. long-term toxicities of antifungal agents, such as hepatotoxicity or nephrotoxicity, require further investigation in cancer patients (Table 1).
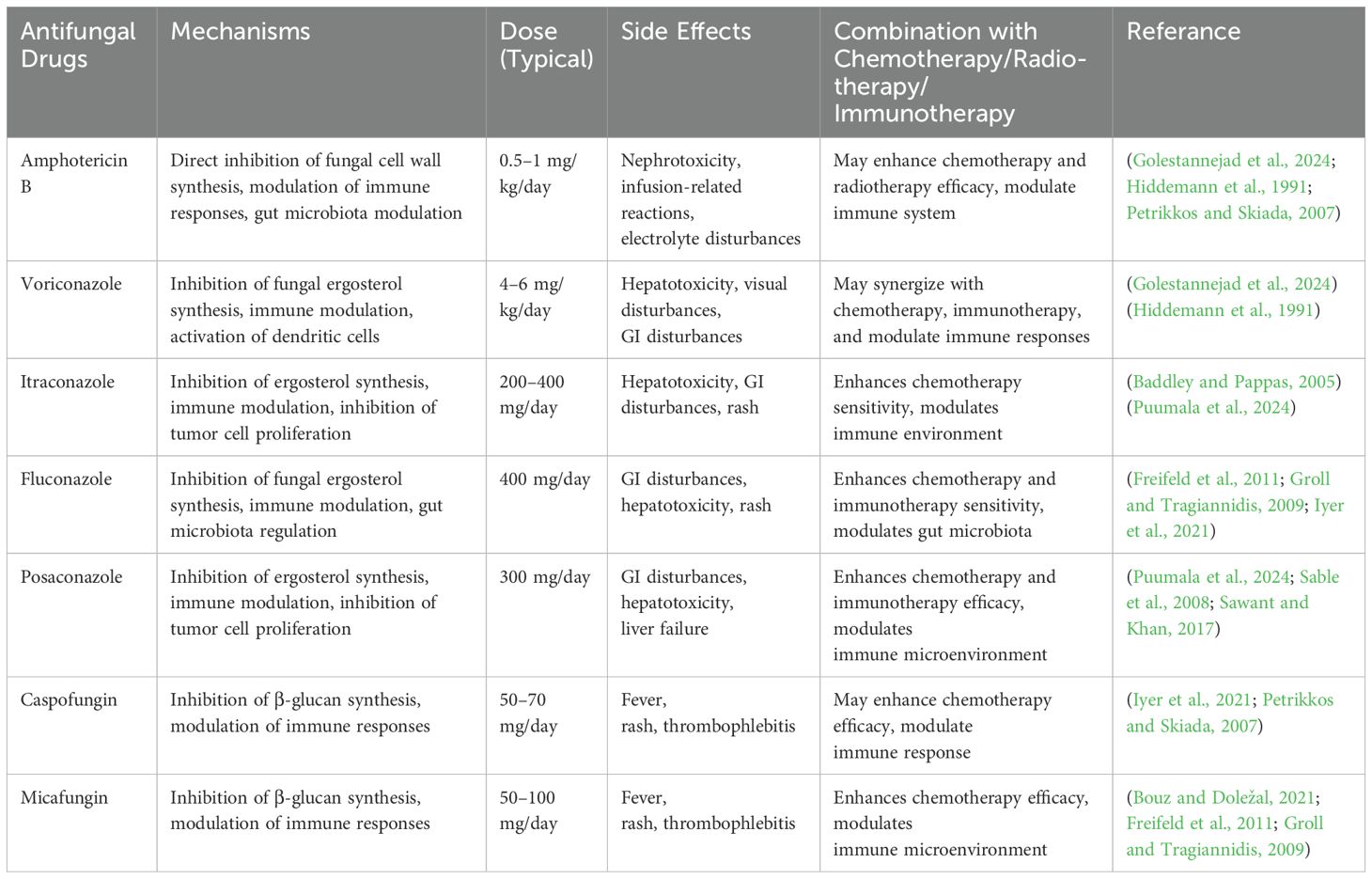
Table 1. summarizes key antifungal agents with relevance to both fungal control and potential tumor modulation mechanisms.
4 Summary
The research on the role of fungi in cancer is still in its early stages, with several challenges hindering its progress. One of the main obstacles is the difficulty in detecting fungi within the TME. While fungal detection technologies, such as FungiQuant, have made considerable strides, issues such as sampling difficulties, genomic contamination, operational complexity, and challenges in clinical application remain unresolved. Despite these challenges, the recognition of fungi’s potential role in cancer initiation and progression is growing. However, the mechanisms through which fungi influence cancer remain complex and multifaceted, warranting further investigation. To bridge the gap between basic and clinical research, future studies should focus on deepening our current understanding of the relationship between fungi and cancer, while simultaneously developing more effective strategies for diagnosis, treatment, and prevention.
The relationship between fungi and cancer is a multidimensional research area with considerable promise. Future advancements in this field may include the following: 1. exploration of the impact of fungal infections on cancer initiation and progression, 2. Investigation of the anticancer potential of fungal metabolites and their therapeutic implications, 3. Promotion of fungal-mediated immune modulation in clinical settings, and 4. Identification of novel fungal-related biomarkers for early detection and tailored treatment strategies. Furthermore, the integration of novel antifungal drugs with immunotherapy presents an exciting frontier for future cancer treatment research. As advancements in molecular biology, genetic engineering, immunology, and other related fields continue, the application of fungal resources in cancer care is believed to lead to innovative strategies and groundbreaking approaches for cancer treatment.
5 Future directions and priority areas
Moving forward, key priorities include: (1) establishing standardized protocols for mycobiome profiling, (2) validating fungal biomarkers in large multi-cohort studies, (3) dissecting causal versus correlative fungal-tumor interactions using functional models, and (4) exploring the pharmacodynamics and clinical integration of antifungal therapy in oncology. Addressing these gaps will be essential for translating mycobiome insights into clinical applications.
Author contributions
CS: Funding acquisition, Resources, Writing – original draft, Writing – review & editing. WZ: Conceptualization, Data curation, Formal analysis, Investigation, Software, Supervision, Validation, Writing – original draft. YG: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Software, Supervision, Validation, Writing – original draft. JL: Conceptualization, Data curation, Supervision, Writing – original draft. HZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Liaoning Province Science and Technology Plan Joint Project (2023JH2/101700140 to CS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abril-Rodriguez, G. and Ribas, A. (2017). SnapShot: immune checkpoint inhibitors. Cancer Cell 31, 848–848.e841. doi: 10.1016/j.ccell.2017.05.010
Aggor, F. E. Y., Break, T. J., Trevejo-Nuñez, G., Whibley, N., Coleman, B. M., Bailey, R. D., et al. (2020). Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci. Immunol. 5. doi: 10.1126/sciimmunol.aba0570
Allen, C., Her, S., and Jaffray, D. A. (2017). Radiotherapy for cancer: present and future. Adv. Drug Delivery Rev. 109, 1–2. doi: 10.1016/j.addr.2017.01.004
Alnuaimi, A. D., Wiesenfeld, D., O’Brien-Simpson, N. M., Reynolds, E. C., and McCullough, M. J. (2015). Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oncol. 51, 139–145. doi: 10.1016/j.oraloncology.2014.11.008
Anderson, D. A., 3rd, Dutertre, C. A., Ginhoux, F., and Murphy, K. M. (2021). Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 21, 101–115. doi: 10.1038/s41577-020-00413-x
Angelova, G., Stefanova, P., Brazkova, M., and Krastanov, A. (2023). Molecular and morphological characterization of Xylaria karsticola (Ascomycota) isolated from the fruiting body of Macrolepiota procera (Basidiomycota) from Bulgaria. PloS One 18, e0287679. doi: 10.1371/journal.pone.0287679
Arbour, K. C. and Riely, G. J. (2019). Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. Jama 322, 764–774. doi: 10.1001/jama.2019.11058
Arendrup, M. C., Friberg, N., Mares, M., Kahlmeter, G., Meletiadis, J., and Guinea, J. (2020). How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol Infect. 26, 1464–1472. doi: 10.1016/j.cmi.2020.06.007
Arner, E. N. and Rathmell, J. C. (2023). Metabolic programming and immune suppression in the tumor microenvironment. Cancer Cell 41, 421–433. doi: 10.1016/j.ccell.2023.01.009
Arpaia, N., Campbell, C., Fan, X., Dikiy, S., van der Veeken, J., deRoos, P., et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. doi: 10.1038/nature12726
Assress, H. A., Nyoni, H., Mamba, B. B., and Msagati, T. A. M. (2020). Occurrence and risk assessment of azole antifungal drugs in water and wastewater. Ecotoxicol Environ. Saf. 187, 109868. doi: 10.1016/j.ecoenv.2019.109868
Atarashi, K., Tanoue, T., Oshima, K., Suda, W., Nagano, Y., Nishikawa, H., et al. (2013). Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236. doi: 10.1038/nature12331
Atarashi, K., Tanoue, T., Shima, T., Imaoka, A., Kuwahara, T., Momose, Y., et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. doi: 10.1126/science.1198469
Aykut, B., Pushalkar, S., Chen, R., Li, Q., Abengozar, R., Kim, J. I., et al. (2019). The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267. doi: 10.1038/s41586-019-1608-2
Bachem, A., Makhlouf, C., Binger, K. J., de Souza, D. P., Tull, D., Hochheiser, K., et al. (2019). Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity 51, 285–297.e285. doi: 10.1016/j.immuni.2019.06.002
Bacigalupa, Z. A., Landis, M. D., and Rathmell, J. C. (2024). Nutrient inputs and social metabolic control of T cell fate. Cell Metab. 36, 10–20. doi: 10.1016/j.cmet.2023.12.009
Baddley, J. W. and Pappas, P. G. (2005). Antifungal combination therapy: clinical potential. Drugs 65, 1461–1480. doi: 10.2165/00003495-200565110-00002
Bai, X., Wei, H., Liu, W., Coker, O. O., Gou, H., Liu, C., et al. (2022). Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 71, 2439–2450. doi: 10.1136/gutjnl-2021-325021
Baixauli, F., Piletic, K., Puleston, D. J., Villa, M., Field, C. S., Flachsmann, L. J., et al. (2022). An LKB1-mitochondria axis controls T(H)17 effector function. Nature 610, 555–561. doi: 10.1038/s41586-022-05264-1
Barkley, D., Moncada, R., Pour, M., Liberman, D. A., Dryg, I., Werba, G., et al. (2022). Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet. 54, 1192–1201. doi: 10.1038/s41588-022-01141-9
Belkaid, Y. and Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. doi: 10.1016/j.cell.2014.03.011
Ben-Ami, R., Albert, N. D., Lewis, R. E., and Kontoyiannis, D. P. (2013). Proangiogenic growth factors potentiate in situ angiogenesis and enhance antifungal drug activity in murine invasive aspergillosis. J. Infect. Dis. 207, 1066–1074. doi: 10.1093/infdis/jis940
Benitez, L. L. and Carver, P. L. (2019). Adverse effects associated with long-term administration of azole antifungal agents. Drugs 79, 833–853. doi: 10.1007/s40265-019-01127-8
Bhat, A. A., Nisar, S., Singh, M., Ashraf, B., Masoodi, T., Prasad, C. P., et al. (2022). Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun. (Lond) 42, 689–715. doi: 10.1002/cac2.12295
Bhat, A. A., Yousuf, P., Wani, N. A., Rizwan, A., Chauhan, S. S., Siddiqi, M. A., et al. (2021). Tumor microenvironment: an evil nexus promoting aggressive head and neck squamous cell carcinoma and avenue for targeted therapy. Signal Transduct Target Ther. 6, 12. doi: 10.1038/s41392-020-00419-w
Bi, X., Wang, J., and Liu, C. (2024). Intratumoral microbiota: metabolic influences and biomarker potential in gastrointestinal cancer. Biomolecules 14. doi: 10.3390/biom14080917
Biedermann, P. H. W. and Vega, F. E. (2020). Ecology and evolution of insect-fungus mutualisms. Annu. Rev. Entomol 65, 431–455. doi: 10.1146/annurev-ento-011019-024910
Bilal, H., Khan, M. N., Khan, S., Shafiq, M., Fang, W., Zeng, Y., et al. (2025). Fungal influences on cancer initiation, progression, and response to treatment. Cancer Res. 85, 413–423. doi: 10.1158/0008-5472.Can-24-1609
Bing, J., Guan, Z., Zheng, T., Ennis, C. L., Nobile, C. J., Chen, C., et al. (2024). Rapid evolution of an adaptive multicellular morphology of Candida auris during systemic infection. Nat. Commun. 15, 2381. doi: 10.1038/s41467-024-46786-8
Blake, S. J., Wolf, Y., Boursi, B., and Lynn, D. J. (2024). Role of the microbiota in response to and recovery from cancer therapy. Nat. Rev. Immunol. 24, 308–325. doi: 10.1038/s41577-023-00951-0
Borgers, J. S. W., Heimovaara, J. H., Cardonick, E., Dierickx, D., Lambertini, M., Haanen, J., et al. (2021). Immunotherapy for cancer treatment during pregnancy. Lancet Oncol. 22, e550–e561. doi: 10.1016/s1470-2045(21)00525-8
Bouz, G. and Doležal, M. (2021). Advances in antifungal drug development: an up-to-date mini review. Pharmaceuticals (Basel) 14. doi: 10.3390/ph14121312
Brandenburg, A., Heine, A., and Brossart, P. (2024). Next-generation cancer vaccines and emerging immunotherapy combinations. Trends Cancer 10, 749–769. doi: 10.1016/j.trecan.2024.06.003
Brennan, C. A. and Garrett, W. S. (2019). Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol 17, 156–166. doi: 10.1038/s41579-018-0129-6
Brown, G. D., Denning, D. W., and Levitz, S. M. (2012). Tackling human fungal infections. Science 336, 647. doi: 10.1126/science.1222236
Brüggemann, R. J., Verheggen, R., Boerrigter, E., Stanzani, M., Verweij, P. E., Blijlevens, N. M. A., et al. (2022). Management of drug-drug interactions of targeted therapies for haematological Malignancies and triazole antifungal drugs. Lancet Haematol 9, e58–e72. doi: 10.1016/s2352-3026(21)00232-5
Butterfield, L. H. and Najjar, Y. G. (2024). Immunotherapy combination approaches: mechanisms, biomarkers and clinical observations. Nat. Rev. Immunol. 24, 399–416. doi: 10.1038/s41577-023-00973-8
Byrd, A. L., Belkaid, Y., and Segre, J. A. (2018). The human skin microbiome. Nat. Rev. Microbiol 16, 143–155. doi: 10.1038/nrmicro.2017.157
Cameron, D., Piccart-Gebhart, M. J., Gelber, R. D., Procter, M., Goldhirsch, A., de Azambuja, E., et al. (2017). 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389, 1195–1205. doi: 10.1016/s0140-6736(16)32616-2
Campbell, C., McKenney, P. T., Konstantinovsky, D., Isaeva, O. I., Schizas, M., Verter, J., et al. (2020). Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479. doi: 10.1038/s41586-020-2193-0
Candon, S., Rammaert, B., Foray, A. P., Moreira, B., Gallego Hernanz, M. P., Chatenoud, L., et al. (2020). Chronic disseminated candidiasis during hematological Malignancies: an immune reconstitution inflammatory syndrome with expansion of pathogen-specific T helper type 1 cells. J. Infect. Dis. 221, 1907–1916. doi: 10.1093/infdis/jiz688
Cao, X., Geng, Q., Fan, D., Wang, Q., Wang, X., Zhang, M., et al. (2023a). m(6)A methylation: a process reshaping the tumour immune microenvironment and regulating immune evasion. Mol. Cancer 22, 42. doi: 10.1186/s12943-022-01704-8
Cao, Y., Langer, R., and Ferrara, N. (2023b). Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug Discov. 22, 476–495. doi: 10.1038/s41573-023-00671-z
Cao, Y., Xia, H., Tan, X., Shi, C., Ma, Y., Meng, D., et al. (2024). Intratumoural microbiota: a new frontier in cancer development and therapy. Signal Transduct Target Ther. 9, 15. doi: 10.1038/s41392-023-01693-0
Carolus, H., Pierson, S., Muñoz, J. F., Subotić, A., Cruz, R. B., Cuomo, C. A., et al. (2021). Genome-wide analysis of experimentally evolved candida auris reveals multiple novel mechanisms of multidrug resistance. mBio 12. doi: 10.1128/mBio.03333-20
Casadevall, A. (2022). Immunity to invasive fungal diseases. Annu. Rev. Immunol. 40, 121–141. doi: 10.1146/annurev-immunol-101220-034306
Cervantes-Villagrana, R. D., Albores-García, D., Cervantes-Villagrana, A. R., and García-Acevez, S. J. (2020). Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct Target Ther. 5, 99. doi: 10.1038/s41392-020-0205-z
Chakrabarti, M. and Ray, S. K. (2016). Anti-tumor activities of luteolin and silibinin in glioblastoma cells: overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 21, 312–328. doi: 10.1007/s10495-015-1198-x
Chamilos, G., Ganguly, D., Lande, R., Gregorio, J., Meller, S., Goldman, W. E., et al. (2010). Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PloS One 5, e12955. doi: 10.1371/journal.pone.0012955
Chang, L., Ruiz, P., Ito, T., and Sellers, W. R. (2021). Targeting pan-essential genes in cancer: Challenges and opportunities. Cancer Cell 39, 466–479. doi: 10.1016/j.ccell.2020.12.008
Chapman, N. M., Boothby, M. R., and Chi, H. (2020). Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70. doi: 10.1038/s41577-019-0203-y
Chen, Y., Chen, H. N., Wang, K., Zhang, L., Huang, Z., Liu, J., et al. (2019). Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J. Hepatol 70, 66–77. doi: 10.1016/j.jhep.2018.09.022
Chen, J., Domingue, J. C., and Sears, C. L. (2017). Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin. Immunol. 32, 25–34. doi: 10.1016/j.smim.2017.08.001
Chen, J. J., He, Y. S., Zhong, X. J., Cai, Z. L., Lyu, Y. S., Zhao, Z. F., et al. (2020). Ribonuclease T2 from Aspergillus fumigatus promotes T helper type 2 responses through M2 polarization of macrophages. Int. J. Mol. Med. 46, 718–728. doi: 10.3892/ijmm.2020.4613
Chen, M. L., Huang, X., Wang, H., Hegner, C., Liu, Y., Shang, J., et al. (2021). CAR directs T cell adaptation to bile acids in the small intestine. Nature 593, 147–151. doi: 10.1038/s41586-021-03421-6
Chen, Z., Qiao, S., Yang, L., Sun, M., Li, B., Lu, A., et al. (2023b). Mechanistic insights into the roles of the IL-17/IL-17R families in pancreatic cancer. Int. J. Mol. Sci. 24. doi: 10.3390/ijms241713539
Chen, G., Ren, Q., Zhong, Z., Li, Q., Huang, Z., Zhang, C., et al. (2024b). Exploring the gut microbiome’s role in colorectal cancer: diagnostic and prognostic implications. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1431747
Chen, S., Saeed, A., Liu, Q., Jiang, Q., Xu, H., Xiao, G. G., et al. (2023a). Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 8, 207. doi: 10.1038/s41392-023-01452-1
Chen, S. C. and Sorrell, T. C. (2007). Antifungal agents. Med. J. Aust. 187, 404–409. doi: 10.5694/j.1326-5377.2007.tb01313.x
Chen, C., Wang, Z., Ding, Y., and Qin, Y. (2023). Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1133308
Chen, F. W., Wu, Y. L., Cheng, C. C., Hsiao, Y. W., Chi, J. Y., Hung, L. Y., et al. (2024a). Inactivation of pentraxin 3 suppresses M2-like macrophage activity and immunosuppression in colon cancer. J. BioMed. Sci. 31, 10. doi: 10.1186/s12929-023-00991-7
Chen, L., Zhang, G., Li, G., Wang, W., Ge, Z., Yang, Y., et al. (2022). Ifnar gene variants influence gut microbial production of palmitoleic acid and host immune responses to tuberculosis. Nat. Metab. 4, 359–373. doi: 10.1038/s42255-022-00547-3
Cheng, W., Li, F., Gao, Y., and Yang, R. (2024). Fungi and tumors: The role of fungi in tumorigenesis (Review). Int. J. Oncol. 64. doi: 10.3892/ijo.2024.5640
Cheng, Z. H., Shi, Y. X., Yuan, M., Xiong, D., Zheng, J. H., and Zhang, Z. Y. (2016). Chemokines and their receptors in lung cancer progression and metastasis. J. Zhejiang Univ Sci. B 17, 342–351. doi: 10.1631/jzus.B1500258
Chohan, K. L., Siegler, E. L., and Kenderian, S. S. (2023). CAR-T cell therapy: the efficacy and toxicity balance. Curr. Hematol. Malig Rep. 18, 9–18. doi: 10.1007/s11899-023-00687-7
Choi, M., Lee, S. M., Lee, J. W., Kim, I., Pack, C. G., and Ha, C. H. (2022). Yeast beta-glucan mediates histone deacetylase 5-induced angiogenesis in vascular endothelial cells. Int. J. Biol. Macromol 211, 556–567. doi: 10.1016/j.ijbiomac.2022.05.057
Chrysostomou, D., Roberts, L. A., Marchesi, J. R., and Kinross, J. M. (2023). Gut microbiota modulation of efficacy and toxicity of cancer chemotherapy and immunotherapy. Gastroenterology 164, 198–213. doi: 10.1053/j.gastro.2022.10.018
Cifaldi, C., Ursu, G. M., D’Alba, I., Paccoud, O., Danion, F., Lanternier, F., et al. (2022). Main human inborn errors of immunity leading to fungal infections. Clin. Microbiol Infect. 28, 1435–1440. doi: 10.1016/j.cmi.2022.06.031
Claeys, L., Romano, C., De Ruyck, K., Wilson, H., Fervers, B., Korenjak, M., et al. (2020). Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr Rev. Food Sci. Food Saf. 19, 1449–1464. doi: 10.1111/1541-4337.12567
Coker, O. O., Nakatsu, G., Dai, R. Z., Wu, W. K. K., Wong, S. H., Ng, S. C., et al. (2019). Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 68, 654–662. doi: 10.1136/gutjnl-2018-317178
Crooke, S. T., Witztum, J. L., Bennett, C. F., and Baker, B. F. (2018). RNA-targeted therapeutics. Cell Metab. 27, 714–739. doi: 10.1016/j.cmet.2018.03.004
Cruceriu, D., Baldasici, O., Balacescu, O., and Berindan-Neagoe, I. (2020). The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol. (Dordr) 43, 1–18. doi: 10.1007/s13402-019-00489-1
Cucchetto, G., Cazzadori, A., Conti, M., Cascio, G. L., Braggio, P., and Concia, E. (2015). Treatment of chronic pulmonary aspergillosis with voriconazole: review of a case series. Infection 43, 277–286. doi: 10.1007/s15010-014-0711-4
Curry, W. T. and Lim, M. (2015). Immunomodulation: checkpoint blockade etc. Neuro Oncol. 17 Suppl 7, vii26–vii31. doi: 10.1093/neuonc/nov174
D’Arcy, M. E., Pfeiffer, R. M., Rivera, D. R., Hess, G. P., Cahoon, E. K., Arron, S. T., et al. (2020). Voriconazole and the risk of keratinocyte carcinomas among lung transplant recipients in the United States. JAMA Dermatol. 156, 772–779. doi: 10.1001/jamadermatol.2020.1141
d’Enfert, C., Kaune, A. K., Alaban, L. R., Chakraborty, S., Cole, N., Delavy, M., et al. (2021). The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev. 45. doi: 10.1093/femsre/fuaa060
Daley, D., Mani, V. R., Mohan, N., Akkad, N., Ochi, A., Heindel, D. W., et al. (2017). Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 23, 556–567. doi: 10.1038/nm.4314
Damaraju, V. L., Scriver, T., Mowles, D., Kuzma, M., Ryan, A. J., Cass, C. E., et al. (2014). Erlotinib, gefitinib, and vandetanib inhibit human nucleoside transporters and protect cancer cells from gemcitabine cytotoxicity. Clin. Cancer Res. 20, 176–186. doi: 10.1158/1078-0432.Ccr-13-2293
Dandachi, D., Wilson Dib, R., Fernández-Cruz, A., Jiang, Y., Chaftari, A. M., Hachem, R., et al. (2018). Invasive pulmonary aspergillosis in patients with solid tumours: risk factors and predictors of clinical outcomes. Ann. Med. 50, 713–720. doi: 10.1080/07853890.2018.1518581
Dean, R., Van Kan, J. A., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Spanu, P. D., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M., and Wallace, M. B. (2019). Colorectal cancer. Lancet 394, 1467–1480. doi: 10.1016/s0140-6736(19)32319-0
Dembitsky, V. M., Ermolenko, E., Savidov, N., Gloriozova, T. A., and Poroikov, V. V. (2021). Antiprotozoal and antitumor activity of natural polycyclic endoperoxides: origin, structures and biological activity. Molecules 26. doi: 10.3390/molecules26030686
Demir, M., Lang, S., Hartmann, P., Duan, Y., Martin, A., Miyamoto, Y., et al. (2022). The fecal mycobiome in non-alcoholic fatty liver disease. J. Hepatol 76, 788–799. doi: 10.1016/j.jhep.2021.11.029
Denk, D. and Greten, F. R. (2022). Inflammation: the incubator of the tumor microenvironment. Trends Cancer 8, 901–914. doi: 10.1016/j.trecan.2022.07.002
Derosa, L., Hellmann, M. D., Spaziano, M., Halpenny, D., Fidelle, M., Rizvi, H., et al. (2018). Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 29, 1437–1444. doi: 10.1093/annonc/mdy103
Derosa, L., Iebba, V., Silva, C. A. C., Piccinno, G., Wu, G., Lordello, L., et al. (2024). Custom scoring based on ecological topology of gut microbiota associated with cancer immunotherapy outcome. Cell 187, 3373–3389.e3316. doi: 10.1016/j.cell.2024.05.029
Derynck, R., Turley, S. J., and Akhurst, R. J. (2021). TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34. doi: 10.1038/s41571-020-0403-1
Deshpande, N. P., Riordan, S. M., Castaño-Rodríguez, N., Wilkins, M. R., and Kaakoush, N. O. (2018). Signatures within the esophageal microbiome are associated with host genetics, age, and disease. Microbiome 6, 227. doi: 10.1186/s40168-018-0611-4
Dickson, I. (2019). Fungal dysbiosis associated with colorectal cancer. Nat. Rev. Gastroenterol. Hepatol 16, 76. doi: 10.1038/s41575-019-0105-2
Dohlman, A. B., Arguijo Mendoza, D., Ding, S., Gao, M., Dressman, H., Iliev, I. D., et al. (2021). The cancer microbiome atlas: a pan-cancer comparative analysis to distinguish tissue-resident microbiota from contaminants. Cell Host Microbe 29, 281–298.e285. doi: 10.1016/j.chom.2020.12.001
Dohlman, A. B., Klug, J., Mesko, M., Gao, I. H., Lipkin, S. M., Shen, X., et al. (2022). A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 185, 3807–3822.e3812. doi: 10.1016/j.cell.2022.09.015
Domingos, L. T. S., Martins, R. D. S., Lima, L. M., Ghizelini, A. M., Ferreira-Pereira, A., and Cotinguiba, F. (2022). Secondary metabolites diversity of aspergillus unguis and their bioactivities: A potential target to be explored. Biomolecules 12. doi: 10.3390/biom12121820
Dong, J., Gao, H. L., Wang, W. Q., Yu, X. J., and Liu, L. (2021). Bidirectional and dynamic interaction between the microbiota and therapeutic resistance in pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 1875, 188484. doi: 10.1016/j.bbcan.2020.188484
Dong, L., Lu, D., Chen, R., Lin, Y., Zhu, H., Zhang, Z., et al. (2022). Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell 40, 70–87.e15. doi: 10.1016/j.ccell.2021.12.006
Drouillard, D., Craig, B. T., and Dwinell, M. B. (2023). Physiology of chemokines in the cancer microenvironment. Am. J. Physiol. Cell Physiol. 324, C167–c182. doi: 10.1152/ajpcell.00151.2022
Dudley, A. C. and Griffioen, A. W. (2023a). The modes of angiogenesis: an updated perspective. Angiogenesis 26, 477–480. doi: 10.1007/s10456-023-09895-4
Dudley, A. C. and Griffioen, A. W. (2023b). Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis 26, 313–347. doi: 10.1007/s10456-023-09876-7
Duggan, C., Dvaladze, A. L., Tsu, V., Jeronimo, J., Constant, T. K. H., Romanoff, A., et al. (2017). Resource-stratified implementation of a community-based breast cancer management programme in Peru. Lancet Oncol. 18, e607–e617. doi: 10.1016/s1470-2045(17)30592-2
Duizer, C. and de Zoete, M. R. (2023). The role of microbiota-derived metabolites in colorectal cancer. Int. J. Mol. Sci. 24. doi: 10.3390/ijms24098024
Elinav, E., Nowarski, R., Thaiss, C. A., Hu, B., Jin, C., and Flavell, R. A. (2013). Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771. doi: 10.1038/nrc3611
Eniafe, J. and Jiang, S. (2021). The functional roles of TCA cycle metabolites in cancer. Oncogene 40, 3351–3363. doi: 10.1038/s41388-020-01639-8
Esher Righi, S., Harriett, A. J., Lilly, E. A., Fidel, P. L., Jr., and Noverr, M. C. (2023). Candida-induced granulocytic myeloid-derived suppressor cells are protective against polymicrobial sepsis. mBio 14, e0144623. doi: 10.1128/mbio.01446-23
Fernandes, M. R., Aggarwal, P., Costa, R. G. F., Cole, A. M., and Trinchieri, G. (2022). Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer 22, 703–722. doi: 10.1038/s41568-022-00513-x
Fernandes, K. E. and Carter, D. A. (2020). Cellular plasticity of pathogenic fungi during infection. PloS Pathog 16, e1008571. doi: 10.1371/journal.ppat.1008571
Fidelle, M., Rauber, C., Alves Costa Silva, C., Tian, A. L., Lahmar, I., de la Varende, A. M., et al. (2023). A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296. doi: 10.1126/science.abo2296
Fisher, M. C., Alastruey-Izquierdo, A., Berman, J., Bicanic, T., Bignell, E. M., Bowyer, P., et al. (2022). Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol 20, 557–571. doi: 10.1038/s41579-022-00720-1
Fisher, M. C., Hawkins, N. J., Sanglard, D., and Gurr, S. J. (2018). Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360, 739–742. doi: 10.1126/science.aap7999
Fites, J. S., Gui, M., Kernien, J. F., Negoro, P., Dagher, Z., Sykes, D. B., et al. (2018). An unappreciated role for neutrophil-DC hybrids in immunity to invasive fungal infections. PloS Pathog 14, e1007073. doi: 10.1371/journal.ppat.1007073
Flowers, L. and Grice, E. A. (2020). The skin microbiota: balancing risk and reward. Cell Host Microbe 28, 190–200. doi: 10.1016/j.chom.2020.06.017
Forma, E. and Bryś, M. (2021). Anticancer activity of propolis and its compounds. Nutrients 13. doi: 10.3390/nu13082594
Freifeld, A. G., Bow, E. J., Sepkowitz, K. A., Boeckh, M. J., Ito, J. I., Mullen, C. A., et al. (2011). Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 52, e56–e93. doi: 10.1093/cid/cir073
Fu, A., Yao, B., Dong, T., and Cai, S. (2023). Emerging roles of intratumor microbiota in cancer metastasis. Trends Cell Biol. 33, 583–593. doi: 10.1016/j.tcb.2022.11.007
Furusawa, Y., Obata, Y., Fukuda, S., Endo, T. A., Nakato, G., Takahashi, D., et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. doi: 10.1038/nature12721
Gagnière, J., Raisch, J., Veziant, J., Barnich, N., Bonnet, R., Buc, E., et al. (2016). Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 22, 501–518. doi: 10.3748/wjg.v22.i2.501
Galassi, C., Chan, T. A., Vitale, I., and Galluzzi, L. (2024). The hallmarks of cancer immune evasion. Cancer Cell 42, 1825–1863. doi: 10.1016/j.ccell.2024.09.010
Galloway-Peña, J., Iliev, I. D., and McAllister, F. (2024). Fungi in cancer. Nat. Rev. Cancer 24, 295–298. doi: 10.1038/s41568-024-00665-y
Gao, G., Ma, T., Zhang, T., Jin, H., Li, Y., Kwok, L. Y., et al. (2021). Adjunctive Probiotic Lactobacillus rhamnosus Probio-M9 Administration Enhances the Effect of Anti-PD-1 Antitumor Therapy via Restoring Antibiotic-Disrupted Gut Microbiota. Front. Immunol. 12. doi: 10.3389/fimmu.2021.772532
Gilbert, A. S., Wheeler, R. T., and May, R. C. (2014). Fungal pathogens: survival and replication within macrophages. Cold Spring Harb Perspect. Med. 5, a019661. doi: 10.1101/cshperspect.a019661
Goenka, A., Khan, F., Verma, B., Sinha, P., Dmello, C. C., Jogalekar, M. P., et al. (2023). Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun. (Lond) 43, 525–561. doi: 10.1002/cac2.12416
Golestannejad, Z., Saberi, Z., Jamshidi, M., Dehghan, P., Khozeimeh, F., Faghihian, E., et al. (2024). Evaluation of antifungal effect of amphotericin B in comparison with nystatin on Candida species derived from patients undergoing head-and-neck radiotherapy. Dent. Res. J. (Isfahan) 21, 66. doi: 10.4103/drj.drj_352_23
Gøtzsche, P. C. and Johansen, H. K. (2014). Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database Syst. Rev. 2014, Cd000026. doi: 10.1002/14651858.CD000026.pub2
Gou, H., Zeng, R., Lau, H. C. H., and Yu, J. (2024). Gut microbial metabolites: Shaping future diagnosis and treatment against gastrointestinal cancer. Pharmacol. Res. 208, 107373. doi: 10.1016/j.phrs.2024.107373
Gouzerh, F., Bessière, J. M., Ujvari, B., Thomas, F., Dujon, A. M., and Dormont, L. (2022). Odors and cancer: Current status and future directions. Biochim. Biophys. Acta Rev. Cancer 1877, 188644. doi: 10.1016/j.bbcan.2021.188644
Gregor, J. B., Gutierrez-Schultz, V. A., Hoda, S., Baker, K. M., Saha, D., Burghaze, M. G., et al. (2023). An expanded toolkit of drug resistance cassettes for Candida glabrata, Candida auris, and Candida albicans leads to new insights into the ergosterol pathway. mSphere 8, e0031123. doi: 10.1128/msphere.00311-23
Greten, F. R. and Grivennikov, S. I. (2019). Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41. doi: 10.1016/j.immuni.2019.06.025
Gringhuis, S. I., Kaptein, T. M., Remmerswaal, E. B. M., Drewniak, A., Wevers, B. A., Theelen, B., et al. (2022). Fungal sensing by dectin-1 directs the non-pathogenic polarization of T(H)17 cells through balanced type I IFN responses in human DCs. Nat. Immunol. 23, 1735–1748. doi: 10.1038/s41590-022-01348-2
Groll, A. H. and Tragiannidis, A. (2009). Recent advances in antifungal prevention and treatment. Semin. Hematol. 46, 212–229. doi: 10.1053/j.seminhematol.2009.03.003
Guarro, J., Gené, J., and Stchigel, A. M. (1999). Developments in fungal taxonomy. Clin. Microbiol Rev. 12, 454–500. doi: 10.1128/cmr.12.3.454
Guibo, L., Chunxu, D., Biao, C., Zhaolei, H., Wenwen, L., Xiangnan, J., et al. (2024). Dectin-1 participates in the immune-inflammatory response to mouse Aspergillus fumigatus keratitis by modulating macrophage polarization. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1431633
Guillot, N., Roméo, B., Manesh, S. S., Milano, G., Brest, P., Zitvogel, L., et al. (2023). Manipulating the gut and tumor microbiota for immune checkpoint inhibitor therapy: from dream to reality. Trends Mol. Med. 29, 897–911. doi: 10.1016/j.molmed.2023.08.004
Gumber, D. and Wang, L. D. (2022). Improving CAR-T immunotherapy: Overcoming the challenges of T cell exhaustion. EBioMedicine 77, 103941. doi: 10.1016/j.ebiom.2022.103941
Guo, C., Guo, D., Fang, L., Sang, T., Wu, J., Guo, C., et al. (2021). Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr Polym 267, 118231. doi: 10.1016/j.carbpol.2021.118231
Guo, Y., Kasahara, S., Jhingran, A., Tosini, N. L., Zhai, B., Aufiero, M. A., et al. (2020). During aspergillus infection, monocyte-derived DCs, neutrophils, and plasmacytoid DCs enhance innate immune defense through CXCR3-dependent crosstalk. Cell Host Microbe 28, 104–116.e104. doi: 10.1016/j.chom.2020.05.002
Guo, B., Zuo, Z., Di, X., Huang, Y., Gong, G., Xu, B., et al. (2022). Salidroside attenuates HALI via IL-17A-mediated ferroptosis of alveolar epithelial cells by regulating Act1-TRAF6-p38 MAPK pathway. Cell Commun. Signal 20, 183. doi: 10.1186/s12964-022-00994-1
Gupta, M., Chandan, K., and Sarwat, M. (2022). Natural products and their derivatives as immune check point inhibitors: Targeting cytokine/chemokine signalling in cancer. Semin. Cancer Biol. 86, 214–232. doi: 10.1016/j.semcancer.2022.06.009
Hanes, M. R., Giacomantonio, C. A., and Marshall, J. S. (2021). Mast cells and skin and breast cancers: A complicated and microenvironment-dependent role. Cells 10. doi: 10.3390/cells10050986
Hanus, M., Parada-Venegas, D., Landskron, G., Wielandt, A. M., Hurtado, C., Alvarez, K., et al. (2021). Immune system, microbiota, and microbial metabolites: the unresolved triad in colorectal cancer microenvironment. Front. Immunol. 12. doi: 10.3389/fimmu.2021.612826
Harris, M. A., Savas, P., Virassamy, B., O’Malley, M. M. R., Kay, J., Mueller, S. N., et al. (2024). Towards targeting the breast cancer immune microenvironment. Nat. Rev. Cancer 24, 554–577. doi: 10.1038/s41568-024-00714-6
Hau, C. S., Tada, Y., Kanda, N., and Watanabe, S. (2015). Immunoresponses in dermatomycoses. J. Dermatol. 42, 236–244. doi: 10.1111/1346-8138.12718
He, Y., Fu, L., Li, Y., Wang, W., Gong, M., Zhang, J., et al. (2021). Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 33, 988–1000.e1007. doi: 10.1016/j.cmet.2021.03.002
He, Q., Zeng, Q., Shao, Y., Zhou, H., Li, T., Song, F., et al. (2020). Anti-cervical cancer activity of secondary metabolites of endophytic fungi from Ginkgo biloba. Cancer Biomark 28, 371–379. doi: 10.3233/cbm-190462
Heidari, M., Maleki Vareki, S., Yaghobi, R., and Karimi, M. H. (2024). Microbiota activation and regulation of adaptive immunity. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1429436
Heung, L. J., Wiesner, D. L., Wang, K., Rivera, A., and Hohl, T. M. (2023). Immunity to fungi in the lung. Semin. Immunol. 66, 101728. doi: 10.1016/j.smim.2023.101728
Hezaveh, K., Shinde, R. S., Klötgen, A., Halaby, M. J., Lamorte, S., Ciudad, M. T., et al. (2022). Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340.e328. doi: 10.1016/j.immuni.2022.01.006
Hiam-Galvez, K. J., Allen, B. M., and Spitzer, M. H. (2021). Systemic immunity in cancer. Nat. Rev. Cancer 21, 345–359. doi: 10.1038/s41568-021-00347-z
Hiddemann, W., Essink, M. E., Fegeler, W., Zühlsdorf, M., Sauerland, C., and Büchner, T. (1991). Antifungal treatment by amphotericin B and 5-fluorocytosine delays the recovery of normal hematopoietic cells after intensive cytostatic therapy for acute myeloid leukemia. Cancer 68, 9–14. doi: 10.1002/1097-0142(19910701)68:1<9::aid-cncr2820680103>3.0.co;2-u
Ho, J., Yang, X., Nikou, S. A., Kichik, N., Donkin, A., Ponde, N. O., et al. (2019). Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat. Commun. 10, 2297. doi: 10.1038/s41467-019-09915-2
Hong, B. Y., Sobue, T., Choquette, L., Dupuy, A. K., Thompson, A., Burleson, J. A., et al. (2019). Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 7, 66. doi: 10.1186/s40168-019-0679-5
Honorato, L., de Araujo, J. F. D., Ellis, C. C., Piffer, A. C., Pereira, Y., Frases, S., et al. (2022). Extracellular vesicles regulate biofilm formation and yeast-to-hypha differentiation in candida albicans. mBio 13, e0030122. doi: 10.1128/mbio.00301-22
Hoshino, A., Costa-Silva, B., Shen, T. L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. doi: 10.1038/nature15756
How, C. W., Ong, Y. S., Low, S. S., Pandey, A., Show, P. L., and Foo, J. B. (2022). How far have we explored fungi to fight cancer? Semin. Cancer Biol. 86, 976–989. doi: 10.1016/j.semcancer.2021.03.009
Hui, D. and Bruera, E. (2020). Models of palliative care delivery for patients with cancer. J. Clin. Oncol. 38, 852–865. doi: 10.1200/jco.18.02123
Huo, X., Li, D., Wu, F., Li, S., Qiao, Y., Wang, C., et al. (2022). Cultivated human intestinal fungus Candida metapsilosis M2006B attenuates colitis by secreting acyclic sesquiterpenoids as FXR agonists. Gut 71, 2205–2217. doi: 10.1136/gutjnl-2021-325413
Hurvitz, S. A., Hegg, R., Chung, W. P., Im, S. A., Jacot, W., Ganju, V., et al. (2023). Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401, 105–117. doi: 10.1016/s0140-6736(22)02420-5
Iberg, C. A., Jones, A., and Hawiger, D. (2017). Dendritic cells as inducers of peripheral tolerance. Trends Immunol. 38, 793–804. doi: 10.1016/j.it.2017.07.007
Ito, J. I. (2013). Enhancing angiogenesis in invasive aspergillosis: a novel therapeutic approach. J. Infect. Dis. 207, 1031–1033. doi: 10.1093/infdis/jis944
Iyer, K. R., Revie, N. M., Fu, C., Robbins, N., and Cowen, L. E. (2021). Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat. Rev. Microbiol 19, 454–466. doi: 10.1038/s41579-021-00511-0
Jamal, R., Messaoudene, M., de Figuieredo, M., and Routy, B. (2023). Future indications and clinical management for fecal microbiota transplantation (FMT) in immuno-oncology. Semin. Immunol. 67, 101754. doi: 10.1016/j.smim.2023.101754
Jang, H., Ojha, U., Jeong, J. H., Park, K. G., Lee, S. Y., and Lee, Y. M. (2023). Myriocin suppresses tumor growth by modulating macrophage polarization and function through the PI3K/Akt/mTOR pathway. Arch. Pharm. Res. 46, 629–645. doi: 10.1007/s12272-023-01454-1
Jia, D. and Chen, S. (2025). Commensal fungi, a force to be reckoned with. Cell Host Microbe 33, 6–8. doi: 10.1016/j.chom.2024.12.012
Jia, D., Wang, Q., Qi, Y., Jiang, Y., He, J., Lin, Y., et al. (2024a). Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 187, 1651–1665.e1621. doi: 10.1016/j.cell.2024.02.022
Jia, M., Yuan, Z., Yu, H., Feng, S., Tan, X., Long, Z., et al. (2024b). Rapamycin circumvents anti PD-1 therapy resistance in colorectal cancer by reducing PD-L1 expression and optimizing the tumor microenvironment. BioMed. Pharmacother. 176, 116883. doi: 10.1016/j.biopha.2024.116883
Jiang, S., Ma, W., Ma, C., Zhang, Z., Zhang, W., and Zhang, J. (2024). An emerging strategy: probiotics enhance the effectiveness of tumor immunotherapy via mediating the gut microbiome. Gut Microbes 16, 2341717. doi: 10.1080/19490976.2024.2341717
Jiang, J., Mei, J., Yi, S., Feng, C., Ma, Y., Liu, Y., et al. (2022a). Tumor associated macrophage and microbe: The potential targets of tumor vaccine delivery. Adv. Drug Delivery Rev. 180, 114046. doi: 10.1016/j.addr.2021.114046
Jiang, S. S., Xie, Y. L., Xiao, X. Y., Kang, Z. R., Lin, X. L., Zhang, L., et al. (2023). Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 31, 781–797.e789. doi: 10.1016/j.chom.2023.04.010
Jiang, T., Yang, T., Chen, Y., Miao, Y., Xu, Y., Jiang, H., et al. (2022b). Emulating interactions between microorganisms and tumor microenvironment to develop cancer theranostics. Theranostics 12, 2833–2859. doi: 10.7150/thno.70719
Jiang, C., Zhang, N., Hu, X., and Wang, H. (2021). Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer 20, 117. doi: 10.1186/s12943-021-01411-w
Jost, L. and Roila, F. (2009). Management of cancer pain: ESMO clinical recommendations. Ann. Oncol. 20 Suppl 4, 170–173. doi: 10.1093/annonc/mdp164
Julianti, E., Abrian, I. A., Wibowo, M. S., Azhari, M., Tsurayya, N., Izzati, F., et al. (2022). Secondary metabolites from marine-derived fungi and actinobacteria as potential sources of novel colorectal cancer drugs. Mar Drugs 20. doi: 10.3390/md20010067
Kaplanov, I., Carmi, Y., Kornetsky, R., Shemesh, A., Shurin, G. V., Shurin, M. R., et al. (2019). Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. U S A 116, 1361–1369. doi: 10.1073/pnas.1812266115
Karnam, A., Bonam, S. R., Rambabu, N., Wong, S. S. W., Aimanianda, V., and Bayry, J. (2021). Wnt-β-catenin signaling in human dendritic cells mediates regulatory T-cell responses to fungi via the PD-L1 pathway. mBio 12, e0282421. doi: 10.1128/mBio.02824-21
Kim, J. and Lee, H. K. (2021). Potential role of the gut microbiome in colorectal cancer progression. Front. Immunol. 12. doi: 10.3389/fimmu.2021.807648
Kiss, E., Hegedüs, B., Virágh, M., Varga, T., Merényi, Z., Kószó, T., et al. (2019). Comparative genomics reveals the origin of fungal hyphae and multicellularity. Nat. Commun. 10, 4080. doi: 10.1038/s41467-019-12085-w
Kobelt, D., Zhang, C., Clayton-Lucey, I. A., Glauben, R., Voss, C., Siegmund, B., et al. (2020). Pro-inflammatory TNF-α and IFN-γ Promote tumor growth and metastasis via induction of MACC1. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00980
Kohsaka, S., Nagano, M., Ueno, T., Suehara, Y., Hayashi, T., Shimada, N., et al. (2017). A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci. Transl. Med. 9. doi: 10.1126/scitranslmed.aan6566
Koliarakis, I., Messaritakis, I., Nikolouzakis, T. K., Hamilos, G., Souglakos, J., and Tsiaoussis, J. (2019). Oral bacteria and intestinal dysbiosis in colorectal cancer. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20174146
Koning, J. J. and Mebius, R. E. (2016). Fungi take control of lymphocyte recirculation. Immunity 44, 211–213. doi: 10.1016/j.immuni.2016.01.017
Kozieł, M. J., Ziaja, M., and Piastowska-Ciesielska, A. W. (2021). Intestinal barrier, claudins and mycotoxins. Toxins (Basel) 13. doi: 10.3390/toxins13110758
Kozubowski, L. and Berman, J. (2025). The impact of phenotypic heterogeneity on fungal pathogenicity and drug resistance. FEMS Microbiol Rev. 49. doi: 10.1093/femsre/fuaf001
Kraft, S., Buchenauer, L., and Polte, T. (2021). Mold, mycotoxins and a dysregulated immune system: A combination of concern? Int. J. Mol. Sci. 22. doi: 10.3390/ijms222212269
Kroemer, G. and Pouyssegur, J. (2008). Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13, 472–482. doi: 10.1016/j.ccr.2008.05.005
Kuang, Z., Liu, X., Zhang, N., Dong, J., Sun, C., Yin, M., et al. (2023). USP2 promotes tumor immune evasion via deubiquitination and stabilization of PD-L1. Cell Death Differ 30, 2249–2264. doi: 10.1038/s41418-023-01219-9
Kumagai, S., Itahashi, K., and Nishikawa, H. (2024). Regulatory T cell-mediated immunosuppression orchestrated by cancer: towards an immuno-genomic paradigm for precision medicine. Nat. Rev. Clin. Oncol. 21, 337–353. doi: 10.1038/s41571-024-00870-6
Kuo, C. J., Hansen, M., and Troemel, E. (2018). Autophagy and innate immunity: Insights from invertebrate model organisms. Autophagy 14, 233–242. doi: 10.1080/15548627.2017.1389824
Lam, K. C., Araya, R. E., Huang, A., Chen, Q., Di Modica, M., Rodrigues, R. R., et al. (2021). Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184, 5338–5356.e5321. doi: 10.1016/j.cell.2021.09.019
Lee, Y. H., Chuah, S., Nguyen, P. H. D., Lim, C. J., Lai, H. L. H., Wasser, M., et al. (2023). IFNγ(-)IL-17(+) CD8 T cells contribute to immunosuppression and tumor progression in human hepatocellular carcinoma. Cancer Lett. 552, 215977. doi: 10.1016/j.canlet.2022.215977
Lee, P. J., Hung, C. M., Yang, A. J., Hou, C. Y., Chou, H. W., Chang, Y. C., et al. (2024). MS-20 enhances the gut microbiota-associated antitumor effects of anti-PD1 antibody. Gut Microbes 16, 2380061. doi: 10.1080/19490976.2024.2380061
Lee, Y., Puumala, E., Robbins, N., and Cowen, L. E. (2021). Antifungal drug resistance: molecular mechanisms in candida albicans and beyond. Chem. Rev. 121, 3390–3411. doi: 10.1021/acs.chemrev.0c00199
Li, F., Gao, Y., Cheng, W., Su, X., and Yang, R. (2023a). Gut fungal mycobiome: A significant factor of tumor occurrence and development. Cancer Lett. 569, 216302. doi: 10.1016/j.canlet.2023.216302
Li, L., Huang, X., and Chen, H. (2024a). Unveiling the hidden players: exploring the role of gut mycobiome in cancer development and treatment dynamics. Gut Microbes 16, 2328868. doi: 10.1080/19490976.2024.2328868
Li, W., Huang, X., Han, X., Zhang, J., Gao, L., and Chen, H. (2024b). IL-17A in gastric carcinogenesis: good or bad? Front. Immunol. 15. doi: 10.3389/fimmu.2024.1501293
Li, Z., Ju, Y., Xia, J., Zhang, Z., Zhen, H., Tong, X., et al. (2023c). Integrated human skin bacteria genome catalog reveals extensive unexplored habitat-specific microbiome diversity and function. Adv. Sci. (Weinh) 10, e2300050. doi: 10.1002/advs.202300050
Li, X. V., Leonardi, I., Putzel, G. G., Semon, A., Fiers, W. D., Kusakabe, T., et al. (2022a). Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603, 672–678. doi: 10.1038/s41586-022-04502-w
Li, K., Shi, H., Zhang, B., Ou, X., Ma, Q., Chen, Y., et al. (2021). Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 6, 362. doi: 10.1038/s41392-021-00670-9
Li, J., Sun, J., Zeng, Z., Liu, Z., Ma, M., Zheng, Z., et al. (2023b). Tumour-associated macrophages in gastric cancer: From function and mechanism to application. Clin. Transl. Med. 13, e1386. doi: 10.1002/ctm2.1386
Li, Y. N., Wang, Z. W., Li, F., Zhou, L. H., Jiang, Y. S., Yu, Y., et al. (2022c). Inhibition of myeloid-derived suppressor cell arginase-1 production enhances T-cell-based immunotherapy against Cryptococcus neoformans infection. Nat. Commun. 13, 4074. doi: 10.1038/s41467-022-31723-4
Li, X., Zhang, S., Guo, G., Han, J., and Yu, J. (2022a). Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine 82, 104163. doi: 10.1016/j.ebiom.2022.104163
Li, R., Zhou, R., Wang, H., Li, W., Pan, M., Yao, X., et al. (2019). Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ 26, 2447–2463. doi: 10.1038/s41418-019-0312-y
Liao, X., Deng, R., Warriner, K., and Ding, T. (2023). Antibiotic resistance mechanism and diagnosis of common foodborne pathogens based on genotypic and phenotypic biomarkers. Compr Rev. Food Sci. Food Saf. 22, 3212–3253. doi: 10.1111/1541-4337.13181
Limousin, W., Laurent-Puig, P., Ziol, M., Ganne-Carrié, N., Nahon, P., Ait-Omar, A., et al. (2023). Molecular-based targeted therapies in patients with hepatocellular carcinoma and hepato-cholangiocarcinoma refractory to atezolizumab/bevacizumab. J. Hepatol 79, 1450–1458. doi: 10.1016/j.jhep.2023.08.017
Lin, Y., Lau, H. C., Liu, Y., Kang, X., Wang, Y., Ting, N. L., et al. (2022). Altered mycobiota signatures and enriched pathogenic aspergillus rambellii are associated with colorectal cancer based on multicohort fecal metagenomic analyses. Gastroenterology 163, 908–921. doi: 10.1053/j.gastro.2022.06.038
Lin, S., Li, Y., Wang, D., Huang, C., Marino, D., Bollt, O., et al. (2021). Fascin promotes lung cancer growth and metastasis by enhancing glycolysis and PFKFB3 expression. Cancer Lett. 518, 230–242. doi: 10.1016/j.canlet.2021.07.025
Lin, L., Wang, M., Zeng, J., Mao, Y., Qin, R., Deng, J., et al. (2023). Sequence Variation of Candida albicans Sap2 Enhances Fungal Pathogenicity via Complement Evasion and Macrophage M2-Like Phenotype Induction. Adv. Sci. (Weinh) 10, e2206713. doi: 10.1002/advs.202206713
Lionakis, M. S., Drummond, R. A., and Hohl, T. M. (2023). Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 23, 433–452. doi: 10.1038/s41577-022-00826-w
Liu, Y., An, L., Huang, R., Xiong, J., Yang, H., Wang, X., et al. (2022b). Strategies to enhance CAR-T persistence. Biomark Res. 10, 86. doi: 10.1186/s40364-022-00434-9
Liu, Z. L., Chen, H. H., Zheng, L. L., Sun, L. P., and Shi, L. (2023d). Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 8, 198. doi: 10.1038/s41392-023-01460-1
Liu, C., Fu, L., Wang, Y., and Yang, W. (2024a). Influence of the gut microbiota on immune cell interactions and cancer treatment. J. Transl. Med. 22, 939. doi: 10.1186/s12967-024-05709-3
Liu, X., Huang, P., Yang, R., and Deng, H. (2023c). mRNA cancer vaccines: construction and boosting strategies. ACS Nano 17, 19550–19580. doi: 10.1021/acsnano.3c05635
Liu, N. N., Jiao, N., Tan, J. C., Wang, Z., Wu, D., Wang, A. J., et al. (2022a). Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol 7, 238–250. doi: 10.1038/s41564-021-01030-7
Liu, Z., Li, Y., Li, C., Lei, G., Zhou, L., Chen, X., et al. (2022c). Intestinal candida albicans promotes hepatocarcinogenesis by up-regulating NLRP6. Front. Microbiol 13. doi: 10.3389/fmicb.2022.812771
Liu, N., Tu, J., Huang, Y., Yang, W., Wang, Q., Li, Z., et al. (2023b). Target- and prodrug-based design for fungal diseases and cancer-associated fungal infections. Adv. Drug Delivery Rev. 197, 114819. doi: 10.1016/j.addr.2023.114819
Liu, Y. and Wang, H. (2024). Biomarkers and targeted therapy for cancer stem cells. Trends Pharmacol. Sci. 45, 56–66. doi: 10.1016/j.tips.2023.11.006
Liu, W., Wang, X., and Wu, W. (2024c). Role and functional mechanisms of IL−17/IL−17R signaling in pancreatic cancer (Review). Oncol. Rep. 52. doi: 10.3892/or.2024.8803
Liu, J. L., Xu, X., Rixiati, Y., Wang, C. Y., Ni, H. L., Chen, W. S., et al. (2024b). Dysfunctional circadian clock accelerates cancer metastasis by intestinal microbiota triggering accumulation of myeloid-derived suppressor cells. Cell Metab. 36, 1320–1334.e1329. doi: 10.1016/j.cmet.2024.04.019
Liu, X., Yan, C., Chang, C., Meng, F., Shen, W., Wang, S., et al. (2024d). Ochratoxin A promotes chronic enteritis and early colorectal cancer progression by targeting Rinck signaling. Phytomedicine 122, 155095. doi: 10.1016/j.phymed.2023.155095
Liu, Y., Yan, Q., Zeng, Z., Fan, C., and Xiong, W. (2024e). Advances and prospects of mRNA vaccines in cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer 1879, 189068. doi: 10.1016/j.bbcan.2023.189068
Liu, N.-N., Yi, C.-X., Wei, L.-Q., Zhou, J.-A., Jiang, T., Hu, C.-C., et al. (2023a). The intratumor mycobiome promotes lung cancer progression via myeloid-derived suppressor cells. Cancer Cell 41, 1927–1944.e1929. doi: 10.1016/j.ccell.2023.08.012
Llobregat, B., González-Candelas, L., and Ballester, A. R. (2022). Ochratoxin A defective aspergillus carbonarius mutants as potential biocontrol agents. Toxins (Basel) 14. doi: 10.3390/toxins14110745
Lochhead, R. B., Strle, K., Arvikar, S. L., Weis, J. J., and Steere, A. C. (2021). Lyme arthritis: linking infection, inflammation and autoimmunity. Nat. Rev. Rheumatol 17, 449–461. doi: 10.1038/s41584-021-00648-5
Lockhart, S. R., Chowdhary, A., and Gold, J. A. W. (2023). The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol 21, 818–832. doi: 10.1038/s41579-023-00960-9
Lohse, M. B., Gulati, M., Johnson, A. D., and Nobile, C. J. (2018). Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol 16, 19–31. doi: 10.1038/nrmicro.2017.107
Lopes, A., Vandermeulen, G., and Préat, V. (2019). Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 38, 146. doi: 10.1186/s13046-019-1154-7
Lu, H., Hong, T., Jiang, Y., Whiteway, M., and Zhang, S. (2023). Candidiasis: From cutaneous to systemic, new perspectives of potential targets and therapeutic strategies. Adv. Drug Delivery Rev. 199, 114960. doi: 10.1016/j.addr.2023.114960
Lu, Y., Su, C., and Liu, H. (2014). Candida albicans hyphal initiation and elongation. Trends Microbiol 22, 707–714. doi: 10.1016/j.tim.2014.09.001
Lu, H., Xu, X., Fu, D., Gu, Y., Fan, R., Yi, H., et al. (2022). Butyrate-producing Eubacterium rectale suppresses lymphomagenesis by alleviating the TNF-induced TLR4/MyD88/NF-κB axis. Cell Host Microbe 30, 1139–1150.e1137. doi: 10.1016/j.chom.2022.07.003
Lu, Y., Yuan, X., Wang, M., He, Z., Li, H., Wang, J., et al. (2022). Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J. Hematol. Oncol. 15, 47. doi: 10.1186/s13045-022-01273-9
Luan, C., Xie, L., Yang, X., Miao, H., Lv, N., Zhang, R., et al. (2015). Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci. Rep. 5, 7980. doi: 10.1038/srep07980
Lyu, M., Suzuki, H., Kang, L., Gaspal, F., Zhou, W., Goc, J., et al. (2022). ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 610, 744–751. doi: 10.1038/s41586-022-05141-x
Ma, E. H., Bantug, G., Griss, T., Condotta, S., Johnson, R. M., Samborska, B., et al. (2017). Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357. doi: 10.1016/j.cmet.2016.12.011
Ma, Y., Chen, H., Li, H., Zheng, M., Zuo, X., Wang, W., et al. (2024). Intratumor microbiome-derived butyrate promotes lung cancer metastasis. Cell Rep. Med. 5, 101488. doi: 10.1016/j.xcrm.2024.101488
Ma, S., Li, X., Wang, X., Cheng, L., Li, Z., Zhang, C., et al. (2019). Current progress in CAR-T cell therapy for solid tumors. Int. J. Biol. Sci. 15, 2548–2560. doi: 10.7150/ijbs.34213
MaChado, Á. and Torres, T. (2018). Guselkumab for the treatment of psoriasis. BioDrugs 32, 119–128. doi: 10.1007/s40259-018-0265-6
Mafe, A. N. and Büsselberg, D. (2024). Mycotoxins in food: cancer risks and strategies for control. Foods 13. doi: 10.3390/foods13213502
Malik, A., Sharma, D., Malireddi, R. K. S., Guy, C. S., Chang, T. C., Olsen, S. R., et al. (2018). SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity 49, 515–530.e515. doi: 10.1016/j.immuni.2018.08.024
Mancarella, C., Morrione, A., and Scotlandi, K. (2023). PROTAC-based protein degradation as a promising strategy for targeted therapy in sarcomas. Int. J. Mol. Sci. 24. doi: 10.3390/ijms242216346
Marchese, S., Polo, A., Ariano, A., Velotto, S., Costantini, S., and Severino, L. (2018). Aflatoxin B1 and M1: biological properties and their involvement in cancer development. Toxins (Basel) 10. doi: 10.3390/toxins10060214
Marcos, C. M., de Oliveira, H. C., de Melo, W. C., da Silva, J. F., Assato, P. A., Scorzoni, L., et al. (2016). Anti-immune strategies of pathogenic fungi. Front. Cell Infect. Microbiol 6. doi: 10.3389/fcimb.2016.00142
Masheghati, F., Asgharzadeh, M. R., Jafari, A., Masoudi, N., and Maleki-Kakelar, H. (2024). The role of gut microbiota and probiotics in preventing, treating, and boosting the immune system in colorectal cancer. Life Sci. 344, 122529. doi: 10.1016/j.lfs.2024.122529
Matson, V., Chervin, C. S., and Gajewski, T. F. (2021). Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology 160, 600–613. doi: 10.1053/j.gastro.2020.11.041
Matusiak, M., Hickey, J. W., van, I. D. G. P., Lu, G., Kidziński, L., Zhu, S., et al. (2024). Spatially segregated macrophage populations predict distinct outcomes in colon cancer. Cancer Discov. 14, 1418–1439. doi: 10.1158/2159-8290.Cd-23-1300
Mayer, F. L., Wilson, D., and Hube, B. (2013). Candida albicans pathogenicity mechanisms. Virulence 4, 119–128. doi: 10.4161/viru.22913
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O., and Kasper, D. L. (2005). An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118. doi: 10.1016/j.cell.2005.05.007
McGranahan, N., Furness, A. J., Rosenthal, R., Ramskov, S., Lyngaa, R., Saini, S. K., et al. (2016). Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469. doi: 10.1126/science.aaf1490
Mehrian-Shai, R., Reichardt, J. K. V., Harris, C. C., and Toren, A. (2019). The gut-brain axis, paving the way to brain cancer. Trends Cancer 5, 200–207. doi: 10.1016/j.trecan.2019.02.008
Mercer, D. K. and O’Neil, D. A. (2020). Innate inspiration: antifungal peptides and other immunotherapeutics from the host immune response. Front. Immunol. 11. doi: 10.3389/fimmu.2020.02177
Mills, K. H. G. (2023). IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 23, 38–54. doi: 10.1038/s41577-022-00746-9
Morales-López, S. E., Parra-Giraldo, C. M., Ceballos-Garzón, A., Martínez, H. P., Rodríguez, G. J., Álvarez-Moreno, C. A., et al. (2017). Invasive infections with multidrug-resistant yeast candida auris, Colombia. Emerg Infect. Dis. 23, 162–164. doi: 10.3201/eid2301.161497
Moreno Ayala, M. A., Campbell, T. F., Zhang, C., Dahan, N., Bockman, A., Prakash, V., et al. (2023). CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8(+) T cell antitumor immunity. Immunity 56, 1613–1630.e1615. doi: 10.1016/j.immuni.2023.06.003
Morrison, A. H., Byrne, K. T., and Vonderheide, R. H. (2018). Immunotherapy and prevention of pancreatic cancer. Trends Cancer 4, 418–428. doi: 10.1016/j.trecan.2018.04.001
Mullard, A. (2020). Addressing cancer’s grand challenges. Nat. Rev. Drug Discov. 19, 825–826. doi: 10.1038/d41573-020-00202-0
Naimi, A., Mohammed, R. N., Raji, A., Chupradit, S., Yumashev, A. V., Suksatan, W., et al. (2022). Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal 20, 44. doi: 10.1186/s12964-022-00854-y
Nakamura, K. and Smyth, M. J. (2020). Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol. Immunol. 17, 1–12. doi: 10.1038/s41423-019-0306-1
Narunsky-Haziza, L., Sepich-Poore, G. D., Livyatan, I., Asraf, O., Martino, C., Nejman, D., et al. (2022). Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 185, 3789–3806.e3717. doi: 10.1016/j.cell.2022.09.005
Nelson, B. N., Hawkins, A. N., and Wozniak, K. L. (2020). Pulmonary macrophage and dendritic cell responses to cryptococcus neoformans. Front. Cell Infect. Microbiol 10. doi: 10.3389/fcimb.2020.00037
Neoh, C. F., Jeong, W., Kong, D. C. M., Beardsley, J., Kwok, P. C. L., Slavin, M. A., et al. (2024). New and emerging roles for inhalational and direct antifungal drug delivery approaches for treatment of invasive fungal infections. Expert Rev. Anti Infect. Ther. 22, 1085–1098. doi: 10.1080/14787210.2024.2409408
Nesic, K., Ivanovic, S., and Nesic, V. (2014). Fusarial toxins: secondary metabolites of Fusarium fungi. Rev. Environ. Contam Toxicol. 228, 101–120. doi: 10.1007/978-3-319-01619-1_5
Ngwa, V. M., Edwards, D. N., Philip, M., and Chen, J. (2019). Microenvironmental metabolism regulates antitumor immunity. Cancer Res. 79, 4003–4008. doi: 10.1158/0008-5472.Can-19-0617
Nicola, A. M., Albuquerque, P., Paes, H. C., Fernandes, L., Costa, F. F., Kioshima, E. S., et al. (2019). Antifungal drugs: New insights in research & development. Pharmacol. Ther. 195, 21–38. doi: 10.1016/j.pharmthera.2018.10.008
Onyishi, C. U., Desanti, G. E., Wilkinson, A. L., Lara-Reyna, S., Frickel, E. M., Fejer, G., et al. (2023). Toll-like receptor 4 and macrophage scavenger receptor 1 crosstalk regulates phagocytosis of a fungal pathogen. Nat. Commun. 14, 4895. doi: 10.1038/s41467-023-40635-w
Otašević, S. and Hay, R. (2023). Editorial: Superficial fungal infections. Front. Cell Infect. Microbiol 13. doi: 10.3389/fcimb.2023.1285771
Pagano, L. and Caira, M. (2014). The role of primary antifungal prophylaxis in patients with haematological Malignancies. Clin. Microbiol Infect. 20 Suppl 6, 19–26. doi: 10.1111/1469-0691.12464
Pais, P., Costa, C., Cavalheiro, M., Romão, D., and Teixeira, M. C. (2016). Transcriptional control of drug resistance, virulence and immune system evasion in pathogenic fungi: A cross-species comparison. Front. Cell Infect. Microbiol 6. doi: 10.3389/fcimb.2016.00131
Palucka, K. and Banchereau, J. (2012). Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277. doi: 10.1038/nrc3258
Papon, N., Hohl, T. M., and Zhai, B. (2021). Mycobiota dysbiosis and gastric tumorigenesis. Theranostics 11, 7488–7490. doi: 10.7150/thno.61480
Park, E. M., Chelvanambi, M., Bhutiani, N., Kroemer, G., Zitvogel, L., and Wargo, J. A. (2022). Targeting the gut and tumor microbiota in cancer. Nat. Med. 28, 690–703. doi: 10.1038/s41591-022-01779-2
Park, J. Y., Ji, Y. S., Zhu, H., Zhang, Y., Park, D. H., Kim, Y. J., et al. (2019). Anti-angiogenic effect of asperchalasine A via attenuation of VEGF signaling. Biomolecules 9. doi: 10.3390/biom9080358
Park, G., Munley, J. A., Kelly, L. S., Kannan, K. B., Mankowski, R. T., Sharma, A., et al. (2024). Gut mycobiome dysbiosis after sepsis and trauma. Crit. Care 28, 18. doi: 10.1186/s13054-023-04780-4
Parsons, M. G. and Diekema, D. J. (2023). What is new in fungal infections? Mod Pathol. 36, 100187. doi: 10.1016/j.modpat.2023.100187
Parussolo, G., Oliveira, M. S., Garcia, M. V., Bernardi, A. O., Lemos, J. G., Stefanello, A., et al. (2019). Ochratoxin A production by Aspergillus westerdijkiae in Italian-type salami. Food Microbiol 83, 134–140. doi: 10.1016/j.fm.2019.05.007
Patel, S. A., Nilsson, M. B., Le, X., Cascone, T., Jain, R. K., and Heymach, J. V. (2023). Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin. Cancer Res. 29, 30–39. doi: 10.1158/1078-0432.Ccr-22-1366
Pathakumari, B., Liang, G., and Liu, W. (2020). Immune defence to invasive fungal infections: A comprehensive review. BioMed. Pharmacother. 130, 110550. doi: 10.1016/j.biopha.2020.110550
Pérez-Tomás, R. and Pérez-Guillén, I. (2020). Lactate in the tumor microenvironment: an essential molecule in cancer progression and treatment. Cancers (Basel) 12. doi: 10.3390/cancers12113244
Perlin, D. S., Rautemaa-Richardson, R., and Alastruey-Izquierdo, A. (2017). The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect. Dis. 17, e383–e392. doi: 10.1016/s1473-3099(17)30316-x
Petrikkos, G. and Skiada, A. (2007). Recent advances in antifungal chemotherapy. Int. J. Antimicrob Agents 30, 108–117. doi: 10.1016/j.ijantimicag.2007.03.009
Pickard, J. M., Zeng, M. Y., Caruso, R., and Núñez, G. (2017). Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 279, 70–89. doi: 10.1111/imr.12567
Pierga, J. Y., Petit, T., Delozier, T., Ferrero, J. M., Campone, M., Gligorov, J., et al. (2012). Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol. 13, 375–384. doi: 10.1016/s1470-2045(12)70049-9
Pierre, J. F., Peters, B. M., La Torre, D., Sidebottom, A. M., Tao, Y., Zhu, X., et al. (2023). Peptide YY: A Paneth cell antimicrobial peptide that maintains Candida gut commensalism. Science 381, 502–508. doi: 10.1126/science.abq3178
Pitt, J. I. and Miller, J. D. (2017). A concise history of mycotoxin research. J. Agric. Food Chem. 65, 7021–7033. doi: 10.1021/acs.jafc.6b04494
Propper, D. J. and Balkwill, F. R. (2022). Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 19, 237–253. doi: 10.1038/s41571-021-00588-9
Pu, Y. and Ji, Q. (2022). Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front. Immunol. 13. doi: 10.3389/fimmu.2022.874589
Pulendran, B. and Davis, M. M. (2020). The science and medicine of human immunology. Science 369. doi: 10.1126/science.aay4014
Puumala, E., Fallah, S., Robbins, N., and Cowen, L. E. (2024). Advancements and challenges in antifungal therapeutic development. Clin. Microbiol Rev. 37, e0014223. doi: 10.1128/cmr.00142-23
Qian, C., Hui, J., Peng, Z., Sun, X., and Zhang, J. (2024). Mucosal microbiota characterization in gastric cancer identifies immune-activated-related transcripts relevant gastric microbiome signatures. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1435334
Qiu, Q., Lin, Y., Ma, Y., Li, X., Liang, J., Chen, Z., et al. (2020). Exploring the emerging role of the gut microbiota and tumor microenvironment in cancer immunotherapy. Front. Immunol. 11. doi: 10.3389/fimmu.2020.612202
Ramirez-Garcia, A., Rementeria, A., Aguirre-Urizar, J. M., Moragues, M. D., Antoran, A., Pellon, A., et al. (2016). Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol 42, 181–193. doi: 10.3109/1040841x.2014.913004
Ramirez-Ortiz, Z. G. and Means, T. K. (2012). The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans). Virulence 3, 635–646. doi: 10.4161/viru.22295
Rangel Rivera, G. O., Knochelmann, H. M., Dwyer, C. J., Smith, A. S., Wyatt, M. M., Rivera-Reyes, A. M., et al. (2021). Fundamentals of T cell metabolism and strategies to enhance cancer immunotherapy. Front. Immunol. 12. doi: 10.3389/fimmu.2021.645242
Renga, G., Nunzi, E., Stincardini, C., Pariano, M., Puccetti, M., Pieraccini, G., et al. (2024). CPX-351 exploits the gut microbiota to promote mucosal barrier function, colonization resistance, and immune homeostasis. Blood 143, 1628–1645. doi: 10.1182/blood.2023021380
Ribatti, D. (2024). Microbiota and angiogenesis in the intestinal vasculature. Tissue Cell 89, 102466. doi: 10.1016/j.tice.2024.102466
Riquelme, E. and McAllister, F. (2021). Bacteria and fungi: The counteracting modulators of immune responses to radiation therapy in cancer. Cancer Cell 39, 1173–1175. doi: 10.1016/j.ccell.2021.08.004
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillère, R., et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97. doi: 10.1126/science.aan3706
Ruffin, A. T., Li, H., Vujanovic, L., Zandberg, D. P., Ferris, R. L., and Bruno, T. C. (2023). Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat. Rev. Cancer 23, 173–188. doi: 10.1038/s41568-022-00531-9
Rushing, B. R. and Selim, M. I. (2019). Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 124, 81–100. doi: 10.1016/j.fct.2018.11.047
Sable, C. A., Strohmaier, K. M., and Chodakewitz, J. A. (2008). Advances in antifungal therapy. Annu. Rev. Med. 59, 361–379. doi: 10.1146/annurev.med.59.062906.071602
Saeedi, M., Eslamifar, M., and Khezri, K. (2019). Kojic acid applications in cosmetic and pharmaceutical preparations. BioMed. Pharmacother. 110, 582–593. doi: 10.1016/j.biopha.2018.12.006
Saftien, A., Puschhof, J., and Elinav, E. (2023). Fungi and cancer. Gut 72, 1410–1425. doi: 10.1136/gutjnl-2022-327952
Samuel, N., Villani, A., Fernandez, C. V., and Malkin, D. (2014). Management of familial cancer: sequencing, surveillance and society. Nat. Rev. Clin. Oncol. 11, 723–731. doi: 10.1038/nrclinonc.2014.169
Sandargo, B., Chepkirui, C., Cheng, T., Chaverra-Muñoz, L., Thongbai, B., Stadler, M., et al. (2019). Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 37, 107344. doi: 10.1016/j.bioteChadv.2019.01.011
Savage, P. A., Klawon, D. E. J., and Miller, C. H. (2020). Regulatory T cell development. Annu. Rev. Immunol. 38, 421–453. doi: 10.1146/annurev-immunol-100219-020937
Sawant, B. and Khan, T. (2017). Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. BioMed. Pharmacother. 96, 1478–1490. doi: 10.1016/j.biopha.2017.11.127
Saxena, M., van der Burg, S. H., Melief, C. J. M., and Bhardwaj, N. (2021). Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378. doi: 10.1038/s41568-021-00346-0
Schneider, K. M., Mohs, A., Gui, W., Galvez, E. J. C., Candels, L. S., and Hoenicke, L. (2022). Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat. Commun. 13, 3964. doi: 10.1038/s41467-022-31312-5
Schürch, C. M., Bhate, S. S., Barlow, G. L., Phillips, D. J., Noti, L., and Zlobec, I. (2020). Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182, 1341–1359.e1319. doi: 10.1016/j.cell.2020.07.005
Schwabe, R. F. and Jobin, C. (2013). The microbiome and cancer. Nat. Rev. Cancer 13, 800–812. doi: 10.1038/nrc3610
Seif, F., Torki, Z., Zalpoor, H., Habibi, M., and Pornour, M. (2023). Breast cancer tumor microenvironment affects Treg/IL-17-producing Treg/Th17 cell axis: Molecular and therapeutic perspectives. Mol. Ther. Oncolytics 28, 132–157. doi: 10.1016/j.omto.2023.01.001
Sepich-Poore, G. D., Zitvogel, L., Straussman, R., Hasty, J., Wargo, J. A., and Knight, R. (2021). The microbiome and human cancer. Science 371. doi: 10.1126/science.abc4552
Shanholtzer, C. N., Rice, C., Watson, K., Carreon, H., and Long, T. E. (2022). Effect of copper on the antifungal activity of disulfiram (Antabuse®) in fluconazole-resistant Candida strains. Med. Mycol 60. doi: 10.1093/mmy/myac016
Shao, T. Y., Ang, W. X. G., Jiang, T. T., Huang, F. S., Andersen, H., Kinder, J. M., et al. (2019). Commensal candida albicans positively calibrates systemic th17 immunological responses. Cell Host Microbe 25, 404–417.e406. doi: 10.1016/j.chom.2019.02.004
Sharma-Walia, N., Patel, K., Chandran, K., Marginean, A., Bottero, V., Kerur, N., et al. (2012). COX-2/PGE2: molecular ambassadors of Kaposi’s sarcoma-associated herpes virus oncoprotein-v-FLIP. Oncogenesis 1, e5. doi: 10.1038/oncsis.2012.5
Sheng, D., Jin, C., Yue, K., Yue, M., Liang, Y., Xue, X., et al. (2024). Pan-cancer atlas of tumor-resident microbiome, immunity and prognosis. Cancer Lett. 598, 217077. doi: 10.1016/j.canlet.2024.217077
Shi, Y., Zheng, W., Yang, K., Harris, K. G., Ni, K., Xue, L., et al. (2020). Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J. Exp. Med. 217. doi: 10.1084/jem.20192282
Shiao, S. L., Kershaw, K. M., Limon, J. J., You, S., Yoon, J., Ko, E. Y., et al. (2021). Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 39, 1202–1213.e1206. doi: 10.1016/j.ccell.2021.07.002
Shuai, M., Fu, Y., Zhong, H. L., Gou, W., Jiang, Z., Liang, Y., et al. (2022). Mapping the human gut mycobiome in middle-aged and elderly adults: multiomics insights and implications for host metabolic health. Gut 71, 1812–1820. doi: 10.1136/gutjnl-2021-326298
Silva, J. T., Husain, S., and Aguado, J. M. (2023). Isavuconazole for treating invasive mould disease in solid organ transplant recipients. Transpl Int. 36. doi: 10.3389/ti.2023.11845
Singh, H., Lowder, K. E., Kapner, K., Kelly, R. J., Zheng, H., McCleary, N. J., et al. (2024). Clinical outcomes and ctDNA correlates for CAPOX BETR: a phase II trial of capecitabine, oxaliplatin, bevacizumab, trastuzumab in previously untreated advanced HER2+ gastroesophageal adenocarcinoma. Nat. Commun. 15, 6833. doi: 10.1038/s41467-024-51271-3
Singha, B., Gatla, H. R., and Vancurova, I. (2015). Transcriptional regulation of chemokine expression in ovarian cancer. Biomolecules 5, 223–243. doi: 10.3390/biom5010223
Singhal, M. and Augustin, H. G. (2020). Beyond angiogenesis: exploiting angiocrine factors to restrict tumor progression and metastasis. Cancer Res. 80, 659–662. doi: 10.1158/0008-5472.Can-19-3351
Sivan, A., Corrales, L., Hubert, N., Williams, J. B., Aquino-Michaels, K., Earley, Z. M., et al. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089. doi: 10.1126/science.aac4255
Soerens, A. G., Künzli, M., Quarnstrom, C. F., Scott, M. C., Swanson, L., Locquiao, J. J., et al. (2023). Functional T cells are capable of supernumerary cell division and longevity. Nature 614, 762–766. doi: 10.1038/s41586-022-05626-9
Sokol, H., Leducq, V., Aschard, H., Pham, H. P., Jegou, S., Landman, C., et al. (2017). Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048. doi: 10.1136/gutjnl-2015-310746
Soll, D. R. (2024). White-opaque switching in Candida albicans: cell biology, regulation, and function. Microbiol Mol. Biol. Rev. 88, e0004322. doi: 10.1128/mmbr.00043-22
Song, P., Peng, G., Yue, H., Ogawa, T., Ikeda, S., Okumura, K., et al. (2022). Candidalysin, a virulence factor of candida albicans, stimulates mast cells by mediating cross-talk between signaling pathways activated by the dectin-1 receptor and MAPKs. J. Clin. Immunol. 42, 1009–1025. doi: 10.1007/s10875-022-01267-9
Song, X., Zhang, H., Zhang, Y., Goh, B., Bao, B., Mello, S. S., et al. (2023). Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature 619, 837–843. doi: 10.1038/s41586-023-06265-4
Souza, V. G. P., Forder, A., Pewarchuk, M. E., Telkar, N., de Araujo, R. P., Stewart, G. L., et al. (2023). The complex role of the microbiome in non-small cell lung cancer development and progression. Cells 12. doi: 10.3390/cells12242801
Souza, E. S. V. C., Oliveira, V. C., Sousa Á, F. L., Bim, F. L., Macedo, A. P., Andrade, D., et al. (2020). Prevalence and susceptibility profile of Candida spp. isolated from patients in cancer therapy. Arch. Biol. 119, 104906. doi: 10.1016/j.archoralbio.2020.104906
Spallone, A. and Schwartz, I. S. (2021). Emerging fungal infections. Infect. Dis. Clin. North Am. 35, 261–277. doi: 10.1016/j.idc.2021.03.014
Sprenger, M., Kasper, L., Hensel, M., and Hube, B. (2018). Metabolic adaptation of intracellular bacteria and fungi to macrophages. Int. J. Med. Microbiol 308, 215–227. doi: 10.1016/j.ijmm.2017.11.001
Staszczak, M. (2021). Fungal secondary metabolites as inhibitors of the ubiquitin-proteasome system. Int. J. Mol. Sci. 22. doi: 10.3390/ijms222413309
Staudt, S., Ziegler-Martin, K., Visekruna, A., Slingerland, J., Shouval, R., Hudecek, M., et al. (2023). Learning from the microbes: exploiting the microbiome to enforce T cell immunotherapy. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1269015
Stone, L. (2022). Urinary VOCs as bladder cancer biomarkers. Nat. Rev. Urol 19, 256. doi: 10.1038/s41585-022-00595-0
Strickland, A. B. and Shi, M. (2021). Mechanisms of fungal dissemination. Cell Mol. Life Sci. 78, 3219–3238. doi: 10.1007/s00018-020-03736-z
Su, Q., Wong, O. W. H., Lu, W., Wan, Y., Zhang, L., Xu, W., et al. (2024). Multikingdom and functional gut microbiota markers for autism spectrum disorder. Nat. Microbiol 9, 2344–2355. doi: 10.1038/s41564-024-01739-1
Sudbery, P. E. (2011). Growth of Candida albicans hyphae. Nat. Rev. Microbiol 9, 737–748. doi: 10.1038/nrmicro2636
Sui, H., Zhang, L., Gu, K., Chai, N., Ji, Q., Zhou, L., et al. (2020). YYFZBJS ameliorates colorectal cancer progression in Apc(Min/+) mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun. Signal 18, 113. doi: 10.1186/s12964-020-00596-9
Sun, K., Xu, R., Ma, F., Yang, N., Li, Y., Sun, X., et al. (2022). scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat. Commun. 13, 4943. doi: 10.1038/s41467-022-32627-z
Szabo, I., Badii, M., Gaál, I. O., Szabo, R., Popp, R. A., Joosten, L. A. B., et al. (2023). Enhanced innate and acquired immune responses in systemic sclerosis primary peripheral blood mononuclear cells (PBMCs). Int. J. Mol. Sci. 24. doi: 10.3390/ijms241914438
Szeto, G. L. and Finley, S. D. (2019). Integrative approaches to cancer immunotherapy. Trends Cancer 5, 400–410. doi: 10.1016/j.trecan.2019.05.010
Takeuchi, T., Nakanishi, Y., and Ohno, H. (2024). Microbial metabolites and gut immunology. Annu. Rev. Immunol. 42, 153–178. doi: 10.1146/annurev-immunol-090222-102035
Tang, T., Huang, X., Zhang, G., Hong, Z., Bai, X., and Liang, T. (2021). Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. 6, 72. doi: 10.1038/s41392-020-00449-4
Tong, Y., Gao, H., Qi, Q., Liu, X., Li, J., Gao, J., et al. (2021). High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 11, 5889–5910. doi: 10.7150/thno.56157
Trompette, A., Gollwitzer, E. S., Yadava, K., Sichelstiel, A. K., Sprenger, N., Ngom-Bru, C., et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166. doi: 10.1038/nm.3444
Unicsovics, M., Molnár, Z., Mézes, M., Posta, K., Nagyéri, G., Várbíró, S., et al. (2024). The possible role of mycotoxins in the pathogenesis of endometrial cancer. Toxins (Basel) 16. doi: 10.3390/toxins16060236
Uribe-Herranz, M., Rafail, S., Beghi, S., Gil-de-Gómez, L., Verginadis, I., Bittinger, K., et al. (2020). Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Invest. 130, 466–479. doi: 10.1172/jci124332
Uscanga-Palomeque, A. C., Chávez-Escamilla, A. K., Alvizo-Báez, C. A., Saavedra-Alonso, S., Terrazas-Armendáriz, L. D., Tamez-Guerra, R. S., et al. (2023). CAR-T cell therapy: from the shop to cancer therapy. Int. J. Mol. Sci. 24. doi: 10.3390/ijms242115688
Varol, C., Mildner, A., and Jung, S. (2015). Macrophages: development and tissue specialization. Annu. Rev. Immunol. 33, 643–675. doi: 10.1146/annurev-immunol-032414-112220
Vitali, F., Colucci, R., Di Paola, M., Pindo, M., De Filippo, C., Moretti, S., et al. (2022). Early melanoma invasivity correlates with gut fungal and bacterial profiles. Br. J. Dermatol. 186, 106–116. doi: 10.1111/bjd.20626
Wahid, M., Mandal, R. K., Dar, S. A., Jawed, A., Lohani, M., Areeshi, M. Y., et al. (2016). Therapeutic potential and critical analysis of trastuzumab and bevacizumab in combination with different chemotherapeutic agents against metastatic breast/colorectal cancer affecting various endpoints. Crit. Rev. Oncol. Hematol. 104, 124–130. doi: 10.1016/j.critrevonc.2016.06.009
Wang, Y., Bai, M., Peng, Q., Li, L., Tian, F., Guo, Y., et al. (2024b). Angiogenesis, a key point in the association of gut microbiota and its metabolites with disease. Eur. J. Med. Res. 29, 614. doi: 10.1186/s40001-024-02224-5
Wang, L., Cao, Y., Lou, E., Zhao, X., and Chen, X. (2024a). The role of gut fungi in Clostridioides difficile infection. BioMed. J. 47, 100686. doi: 10.1016/j.bj.2023.100686
Wang, Z., Dabrosin, C., Yin, X., Fuster, M. M., Arreola, A., Rathmell, W. K., et al. (2015). Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 35 Suppl, S224–s243. doi: 10.1016/j.semcancer.2015.01.001
Wang, L., Qin, W., Huo, Y. J., Li, X., Shi, Q., Rasko, J. E. J., et al. (2020). Advances in targeted therapy for Malignant lymphoma. Signal Transduct Target Ther. 5, 15. doi: 10.1038/s41392-020-0113-2
Wang, J. B., Qiu, Q. Z., Zheng, Q. L., Zhao, Y. J., Xu, Y., Zhang, T., et al. (2023a). Tumor immunophenotyping-derived signature identifies prognosis and neoadjuvant immunotherapeutic responsiveness in gastric cancer. Adv. Sci. (Weinh) 10, e2207417. doi: 10.1002/advs.202207417
Wang, Y., Wang, Y., Zhou, Y., Feng, Y., Sun, T., and Xu, J. (2024c). Tumor-related fungi and crosstalk with gut fungi in the tumor microenvironment. Cancer Biol. Med. 21, 977–994. doi: 10.20892/j.issn.2095-3941.2024.0240
Wang, X., Wu, S., Wu, W., Zhang, W., Li, L., Liu, Q., et al. (2023b). Candida albicans Promotes Oral Cancer via IL-17A/IL-17RA-Macrophage Axis. mBio 14, e0044723. doi: 10.1128/mbio.00447-23
Wang, Z., Xu, F., Hu, J., Zhang, H., Cui, L., Lu, W., et al. (2021). Modulation of lactate-lysosome axis in dendritic cells by clotrimazole potentiates antitumor immunity. J. Immunother Cancer 9. doi: 10.1136/jitc-2020-002155
Wei, S., Li, L., Shu, Y., Zhao, K., and Ji, Z. (2017). Synthesis, antifungal and antitumor activity of two new types of imidazolin-2-ones. Bioorg Med. Chem. 25, 6501–6510. doi: 10.1016/j.bmc.2017.10.033
Wekking, D., Senevirathne, T. H., Pearce, J. L., Aiello, M., Scartozzi, M., Lambertini, M., et al. (2024). The impact of COVID-19 on cancer patients. Cytokine Growth Factor Rev. 75, 110–118. doi: 10.1016/j.cytogfr.2023.11.004
Weng, N., Zhang, Z., Tan, Y., Zhang, X., Wei, X., and Zhu, Q. (2023). Repurposing antifungal drugs for cancer therapy. J. Adv. Res. 48, 259–273. doi: 10.1016/j.jare.2022.08.018
Wheeler, M. L., Limon, J. J., Bar, A. S., Leal, C. A., Gargus, M., Tang, J., et al. (2016). Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19, 865–873. doi: 10.1016/j.chom.2016.05.003
Więckowska, M., Cichon, N., Szelenberger, R., Gorniak, L., and Bijak, M. (2024). Ochratoxin A and its role in cancer development: A comprehensive review. Cancers (Basel) 16. doi: 10.3390/cancers16203473
Wing, J. B., Tanaka, A., and Sakaguchi, S. (2019). Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 50, 302–316. doi: 10.1016/j.immuni.2019.01.020
Witchley, J. N., Penumetcha, P., Abon, N. V., Woolford, C. A., Mitchell, A. P., and Noble, S. M. (2019). Candida albicans morphogenesis programs control the balance between gut commensalism and invasive infection. Cell Host Microbe 25, 432–443.e436. doi: 10.1016/j.chom.2019.02.008
Wojciechowski, D. and Wiseman, A. (2021). Long-term immunosuppression management: opportunities and uncertainties. Clin. J. Am. Soc. Nephrol. 16, 1264–1271. doi: 10.2215/cjn.15040920
Wong, C. C. and Yu, J. (2023). Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 20, 429–452. doi: 10.1038/s41571-023-00766-x
Wong, C. C. and Yu, J. (2024). Mapping the pancancer metastasis tumor microbiome. Cell 187, 2126–2128. doi: 10.1016/j.cell.2024.03.040
Wu, D., Guan, Y. X., Li, C. H., Zheng, Q., Yin, Z. J., Wang, H., et al. (2024a). Nutrient-fungi-host” tripartite interaction in cancer progression. Imeta 3, e170. doi: 10.1002/imt2.170
Wu, Z., Lamao, Q., Gu, M., Jin, X., Liu, Y., Tian, F., et al. (2024b). Unsynchronized butyrophilin molecules dictate cancer cell evasion of Vγ9Vδ2 T-cell killing. Cell Mol. Immunol. 21, 362–373. doi: 10.1038/s41423-024-01135-z
Wu, K., Yuan, Y., Yu, H., Dai, X., Wang, S., Sun, Z., et al. (2020). The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 136, 501–515. doi: 10.1182/blood.2019003990
Wurster, S., Watowich, S. S., and Kontoyiannis, D. P. (2022). Checkpoint inhibitors as immunotherapy for fungal infections: Promises, challenges, and unanswered questions. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1018202
Wynn, T. A. and Vannella, K. M. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462. doi: 10.1016/j.immuni.2016.02.015
Xiao, J., Wang, S., Chen, L., Ding, X., Dang, Y., Han, M., et al. (2024). 25-Hydroxycholesterol regulates lysosome AMP kinase activation and metabolic reprogramming to educate immunosuppressive macrophages. Immunity 57, 1087–1104.e1087. doi: 10.1016/j.immuni.2024.03.021
Xu, M., Pokrovskii, M., Ding, Y., Yi, R., Au, C., Harrison, O. J., et al. (2018). c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377. doi: 10.1038/nature25500
Xu, C., Wang, F., Guan, S., and Wang, L. (2024). β-Glucans obtained from fungus for wound healing: A review. Carbohydr Polym 327, 121662. doi: 10.1016/j.carbpol.2023.121662
Yamaguchi, H., Abe, S., and Tokuda, Y. (1993). Immunomodulating activity of antifungal drugs. Ann. N Y Acad. Sci. 685, 447–457. doi: 10.1111/j.1749-6632.1993.tb35905.x
Yan, W., Zhao, Y. S., Xie, K., Xing, Y., and Xu, F. (2021). Aspergillus fumigatus Influences Gasdermin-D-Dependent Pyroptosis of the Lung via Regulating Toll-Like Receptor 2-Mediated Regulatory T Cell Differentiation. J. Immunol. Res. 2021, 5538612. doi: 10.1155/2021/5538612
Yang, H., Berezowska, S., Dorn, P., Zens, P., Chen, P., Peng, R. W., et al. (2022a). Tumor-infiltrating lymphocytes are functionally inactivated by CD90+ stromal cells and reactivated by combined Ibrutinib and Rapamycin in human pleural mesothelioma. Theranostics 12, 167–185. doi: 10.7150/thno.61209
Yang, Z., Hackshaw, A., Feng, Q., Fu, X., Zhang, Y., Mao, C., et al. (2017). Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: A meta-analysis. Int. J. Cancer 140, 2805–2819. doi: 10.1002/ijc.30691
Yang, Z., Huo, Y., Zhou, S., Guo, J., Ma, X., Li, T., et al. (2022b). Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 34, 2018–2035.e2018. doi: 10.1016/j.cmet.2022.10.010
Yang, Y., Jin, Y., Yin, L., Liu, P., Zhu, L., and Gao, H. (2023). Sertaconazole nitrate targets IDO1 and regulates the MAPK signaling pathway to induce autophagy and apoptosis in CRC cells. Eur. J. Pharmacol. 942, 175515. doi: 10.1016/j.ejphar.2023.175515
Yaniv, D., Mattson, B., Talbot, S., Gleber-Netto, F. O., and Amit, M. (2024). Targeting the peripheral neural-tumour microenvironment for cancer therapy. Nat. Rev. Drug Discov. 23, 780–796. doi: 10.1038/s41573-024-01017-z
Yao, H., Ma, S., Huang, J., Si, X., Yang, M., Song, W., et al. (2024). Trojan-horse strategy targeting the gut-liver axis modulates gut microbiome and reshapes microenvironment for orthotopic hepatocellular carcinoma therapy. Adv. Sci. (Weinh) 11, e2310002. doi: 10.1002/advs.202310002
Yi, M., Li, T., Niu, M., Mei, Q., Zhao, B., Chu, Q., et al. (2023). Exploiting innate immunity for cancer immunotherapy. Mol. Cancer 22, 187. doi: 10.1186/s12943-023-01885-w
You, S., Li, S., Zeng, L., Song, J., Li, Z., Li, W., et al. (2024). Lymphatic-localized Treg-mregDC crosstalk limits antigen trafficking and restrains anti-tumor immunity. Cancer Cell 42, 1415–1433.e1412. doi: 10.1016/j.ccell.2024.06.014
Zhang, Y., Liu, Z., Yang, X., Lu, W., Chen, Y., Lin, Y., et al. (2021). H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics 11, 1473–1492. doi: 10.7150/thno.51245
Zhang, L., Xu, J., Zhou, S., Yao, F., Zhang, R., You, W., et al. (2024). Endothelial DGKG promotes tumor angiogenesis and immune evasion in hepatocellular carcinoma. J. Hepatol 80, 82–98. doi: 10.1016/j.jhep.2023.10.006
Zhang, X., Zhang, H., Lan, H., Wu, J., and Xiao, Y. (2023). CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1101495
Zhang, X., Zhu, L., Zhang, H., Chen, S., and Xiao, Y. (2022). CAR-T cell therapy in hematological Malignancies: current opportunities and challenges. Front. Immunol. 13. doi: 10.3389/fimmu.2022.927153
Zhao, L., He, R., Long, H., Guo, B., Jia, Q., Qin, D., et al. (2018). Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat. Med. 24, 1536–1544. doi: 10.1038/s41591-018-0205-5
Zhao, X., Liu, C., Peng, L., and Wang, H. (2024). Metformin facilitates anti-PD-L1 efficacy through the regulation of intestinal microbiota. Genes Immun. 25, 7–13. doi: 10.1038/s41435-023-00234-7
Zhong, M., Xiong, Y., Zhao, J., Gao, Z., Ma, J., Wu, Z., et al. (2021). Candida albicans disorder is associated with gastric carcinogenesis. Theranostics 11, 4945–4956. doi: 10.7150/thno.55209
Zhou, X., Chen, Z., Yu, Y., Li, M., Cao, Y., Prochownik, E. V., et al. (2024c). Increases in 4-acetaminobutyric acid generated by phosphomevalonate kinase suppress CD8(+) T cell activation and allow tumor immune escape. Adv. Sci. (Weinh) 11, e2403629. doi: 10.1002/advs.202403629
Zhou, Y., Han, W., Feng, Y., Wang, Y., Sun, T., and Xu, J. (2024e). Microbial metabolites affect tumor progression, immunity and therapy prediction by reshaping the tumor microenvironment (Review). Int. J. Oncol. 65. doi: 10.3892/ijo.2024.5661
Zhou, X., Li, W., Wang, S., Zhang, P., Wang, Q., Xiao, J., et al. (2019). YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 27, 1176–1189.e1175. doi: 10.1016/j.celrep.2019.03.028
Zhou, J., Liu, J., Wang, B., Li, N., Liu, J., Han, Y., et al. (2024a). Eosinophils promote CD8(+) T cell memory generation to potentiate anti-bacterial immunity. Signal Transduct Target Ther. 9, 43. doi: 10.1038/s41392-024-01752-0
Zhou, M., Wang, Q., Lu, X., Zhang, P., Yang, R., Chen, Y., et al. (2024b). Exhaled breath and urinary volatile organic compounds (VOCs) for cancer diagnoses, and microbial-related VOC metabolic pathway analysis: a systematic review and meta-analysis. Int. J. Surg. 110, 1755–1769. doi: 10.1097/js9.0000000000000999
Zhou, X., Wang, G., Tian, C., Du, L., Prochownik, E. V., and Li, Y. (2024d). Inhibition of DUSP18 impairs cholesterol biosynthesis and promotes anti-tumor immunity in colorectal cancer. Nat. Commun. 15, 5851. doi: 10.1038/s41467-024-50138-x
Zhou, C. B., Zhou, Y. L., and Fang, J. Y. (2021). Gut microbiota in cancer immune response and immunotherapy. Trends Cancer 7, 647–660. doi: 10.1016/j.trecan.2021.01.010
Zhu, Q., Ma, Y., Liang, J., Wei, Z., Li, M., Zhang, Y., et al. (2021). AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct Target Ther. 6, 299. doi: 10.1038/s41392-021-00713-1
Zhu, Q., Zhang, K., Cao, Y., and Hu, Y. (2024). Adipose stem cell exosomes, stimulated by pro-inflammatory factors, enhance immune evasion in triple-negative breast cancer by modulating the HDAC6/STAT3/PD-L1 pathway through the transporter UCHL1. Cancer Cell Int. 24, 385. doi: 10.1186/s12935-024-03557-1
Zhuo, Y., Li, S., Hu, W., Zhang, Y., Shi, Y., Zhang, F., et al. (2022). Targeting SNORA38B attenuates tumorigenesis and sensitizes immune checkpoint blockade in non-small cell lung cancer by remodeling the tumor microenvironment via regulation of GAB2/AKT/mTOR signaling pathway. J. Immunother Cancer 10. doi: 10.1136/jitc-2021-004113
Keywords: cancer, tumor microenvironment, immunity, fungal derived metabolites, antifungal therapy fungi, fungal-derived metabolites, antifungal therapy
Citation: Zhang W, Zhang H, Gao Y, Lei J and Suo C (2025) Fungi and cancer: unveiling the complex role of fungal infections in tumor biology and therapeutic resistance. Front. Cell. Infect. Microbiol. 15:1596688. doi: 10.3389/fcimb.2025.1596688
Received: 20 March 2025; Accepted: 05 May 2025;
Published: 10 June 2025.
Edited by:
Yuanwei Zhang, Nanjing Normal University, ChinaReviewed by:
José Ascención Martínez-Álvarez, University of Guanajuato, MexicoAmr A Mohamed, Cairo University, Egypt
Copyright © 2025 Zhang, Zhang, Gao, Lei and Suo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenhao Suo, c2NoNzE1M0AxNjMuY29t
†These authors have contributed equally to this work
 Wanli Zhang1†
Wanli Zhang1† He Zhang
He Zhang Yiru Gao
Yiru Gao Chenhao Suo
Chenhao Suo