- 1Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, China
- 2Department of Oncology, Liuzhou Workers’ Hospital, Liuzhou, Guangxi, China
Objective: This study aimed to investigate hepatitis B virus (HBV) reactivation and its impact on postoperative survival in patients with HBV-related hepatocellular carcinoma (HCC) who underwent conversion therapy. The therapeutic regimen consisted of interventional procedures (hepatic artery infusion chemotherapy [HAIC] and/or transarterial chemoembolization [TACE]) combined with tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs).
Methods: A retrospective analysis was performed at a single institution involving 91 patients who had initially unresectable HCC linked to the hepatitis B virus. These patients achieved resectability following conversion therapy and subsequently underwent surgical tumor removal. Logistic regression identified risk factors for HBV reactivation (HBVr). Kaplan-Meier survival analysis and log-rank tests assessed survival differences. Cox proportional hazards regression was used to identify independent predictors of progression-free survival (PFS) and overall survival (OS).
Results: In our cohort, HBVr occurred in 17 patients (18.7%), all of whom received antiviral therapy. The incidence of HBVr was 16.7% (14/84) in patients with detectable baseline HBV DNA and 42.9% (3/7) in those with undetectable levels. Baseline HBV DNA ≥2000 IU/ml was identified as an independent protective factor against HBVr (OR 0.090, 95% CI 0.015–0.532; P = 0.008). The median PFS was significantly shorter in the reactivation group than in the non-reactivation group (12.1 months [95% CI 5.5–18.7] vs. 29.2 months [95% CI 23.6–34.7]; P < 0.001). However, no significant difference was observed in median OS between the two groups (not reached vs. 45.6 months [95% CI 41.7–49.5]; P = 0.117).
Conclusion: HBVr represents a potential complication in subjects receiving hepatectomy for hepatitis B virus associated HCC following conversion therapy involving interventional therapies combined with TKIs and ICIs. Patients experiencing HBVr exhibited significantly shorter progression-free survival compared to those without reactivation. Therefore, prophylactic antiviral therapy and meticulous HBV DNA monitoring are warranted during both conversion therapy and the perioperative period.
1 Introduction
Hepatocellular carcinoma (HCC) is a prevalent malignancy worldwide, with approximately 70% of new cases occurring in Asia (Sung et al., 2021). Projections estimate that there will be over one million new HCC cases and related deaths annually by 2040 (Rumgay et al., 2022). In regions with high HCC incidence, hepatitis B virus (HBV) infection is the primary etiological factor (Mysore and Leung, 2018). In recent years, systemic therapy has emerged as the mainstream treatment for advanced HCC. Specifically, the combination of tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) has achieved objective response rates (ORRs) of 20–30% in advanced or unresectable HCC, as demonstrated in landmark trials such as IMbrave150, ORIENT-32, HIMALAYA, and CARES-310. Furthermore, combining these systemic agents with locoregional therapies, such as transarterial chemoembolization (TACE) or hepatic arterial infusion chemotherapy (HAIC), has yielded even better outcomes (Ju et al., 2021; Cai et al., 2022; Fu et al., 2023; Zhu et al., 2023). Multiple studies have shown that surgical resection following successful conversion therapy offers superior long-term survival benefits compared to palliative treatments alone (Kulik et al., 2006; Lewandowski et al., 2009; Shindoh et al., 2021). Therefore, for patients with initially unresectable HCC, the selection of optimal treatment strategies and timing, alongside the effective management of complications, is of paramount importance for improving prognosis.
Among these complications, hepatitis B virus reactivation (HBVr) is a well-recognized challenge during HCC treatment (Voican et al., 2016). While HBVr is more frequent in patients positive for hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc), it can also manifest in individuals with resolved HBV infection (Hoofnagle, 2009). Existing antiviral agents are unable to completely eradicate covalently closed circular DNA (cccDNA), the viral reservoir in patients with chronic hepatitis B. Consequently, when cccDNA persists in the context of immunosuppression, control over HBV replication is compromised, leading to reactivation (Shi and Zheng, 2020). HBVr can trigger a spectrum of clinical events, ranging from mild hepatitis to fulminant liver failure and even death (Papatheodoridis et al., 2022). Moreover, HBVr can necessitate the interruption of anti-tumor therapy and adversely affect overall survival (Yang et al., 2024).
Previous research has reported an elevated risk of HBVr following surgical resection for HCC, which detrimentally affects patient prognosis (Huang et al., 2012; Dan et al., 2013; Xie et al., 2015). HBVr has also been observed during and after various anti-tumor regimens for intermediate-to-advanced HCC, including interventional therapies, TKIs, and ICIs, often leading to severe complications and negatively impacting long-term survival (Shen et al., 2023; Yang et al., 2024). Conversion therapy, the process of transforming an initially unresectable HCC into a resectable state, aims to enhance surgical eligibility and prognosis. Presently, a growing number of patients with unresectable HCC are undergoing triple therapy (interventional therapy plus TKIs and ICIs), which subsequently allows them to receive surgical treatment. However, for this specific population, the incidence of HBVr and its impact on prognosis remain unclear. This retrospective study, therefore, aims to investigate the occurrence of HBVr in HBV-related HCC patients who underwent surgical resection after conversion therapy with interventional treatment plus TKIs and ICIs, and to evaluate its influence on their prognosis.
2 Materials and methods
2.1 Patient recruitment and study design
This retrospective study enrolled patients with HCC who underwent tumor resection following conversion therapy with HAIC or TACE combined with TKIs and ICIs at the Guangxi Medical University Cancer Hospital from January 2021 to April 2024.The inclusion criteria were as follows: (1) age between 18 and 85 years; (2) histologically confirmed HCC; (3) Barcelona Clinic Liver Cancer (BCLC) stage A–C; (4) chronic or resolved HBV infection (defined as HBsAg-positive, or HBsAg-negative and anti-HBc-positive); (5) initiation of TACE/HAIC and TKIs within two weeks before or after the first dose of ICI; (6) receipt of at least one cycle of TACE/HAIC combined with at least one dose of a TKI and an ICI preoperatively; (7) concurrent receipt of prophylactic anti-HBV therapy during anti-tumor treatment; (8) Child-Pugh class A or B liver function; and (9) an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2.The exclusion criteria included: (1) presence of any other primary malignancy or extrahepatic metastases; (2) any prior anti-HCC treatment; (3) co-infection with other hepatotropic viruses or human immunodeficiency virus (HIV); (4) survival time of less than 3 months; (5) lack of HBV serological markers, HBV DNA monitoring, or imaging data during treatment; (6) history of organ or allogeneic bone marrow transplantation; (7) pregnancy or lactation; and (8) severe heart failure, uncontrolled diabetes, active infection, or other severe comorbidities. This study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital. The requirement for informed consent was waived due to the retrospective nature of the study. A total of 91 patients were ultimately included in the final analysis.
2.2 Conversion therapy
The conversion therapy regimen was tailored for each patient by a multidisciplinary team (MDT) based on their tumor status and liver function. The regimen consisted of transarterial interventional therapy (including TACE and HAIC) combined with a tyrosine kinase inhibitor (TKI) and a programmed cell death protein 1 (PD-1) inhibitor. Vascular interventional procedures (TACE and HAIC) were performed by interventional radiologists at our institution. Treatment with TKIs and PD-1 inhibitors was initiated within one week following the TACE or HAIC procedure, contingent upon the patient’s liver function recovery. The TKIs used in this study, consistent with the first-line treatment recommendations for advanced HCC in Chinese guidelines, included lenvatinib (8 mg daily for body weight <60 kg or 12 mg daily for body weight ≥60 kg), donafenib (0.2 g twice daily), sorafenib (400 mg twice daily), and apatinib (250 mg once daily). Bevacizumab was administered at 15 mg/kg every three weeks. The PD-1 inhibitors used were camrelizumab (200 mg intravenously IV every 2 weeks), tislelizumab (200 mg IV every 3 weeks), and sintilimab (200 mg IV every 3 weeks). The choice of specific agents was determined by the attending physician’s clinical judgment, the patient’s economic status, and personal preference. The dosage and frequency of all TKIs and PD-1 inhibitors were administered according to their respective package inserts.
2.3 Antiviral therapy
All patients were routinely screened for HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc, and serum HBV DNA levels upon their initial admission. HBV DNA was quantified using a real-time quantitative polymerase chain reaction (qPCR) assay with a lower limit of detection of 20 IU/ml. Antiviral therapy was immediately initiated for patients with HBsAg-positive status or those who were HBsAg-negative but had detectable HBV DNA. The antiviral agents included entecavir (ETV, 0.5 mg/day), tenofovir disoproxil fumarate (TDF, 300 mg/day), and tenofovir alafenamide (TAF, 25 mg/day). Patients were allowed to make an informed choice regarding the specific drug based on their socioeconomic status and personal preference. For patients already receiving antiviral treatment prior to admission, their existing regimen was continued. Lifelong antiviral therapy was recommended for all patients with HBV-related HCC. To monitor for HBVr and ensure medication adherence, HBV DNA levels were measured every 6 weeks during conversion therapy, and medication intake was documented. Antiviral therapy was continued throughout the perioperative period, with HBV DNA levels checked on postoperative day 7. For patients who developed HBVr during treatment, their antiviral regimen was switched, although drug resistance testing was not performed.
2.4 Postoperative management and follow-up
Following surgery, patients were followed up every 2–3 months for the first two years and every 6 months thereafter. Monitoring included serum tumor markers (e.g., alpha-fetoprotein [AFP], protein induced by vitamin K absence-II [PIVKA-II]), HBV DNA, HBV serological markers, abdominal ultrasound, and contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI).
2.5 Clinical and laboratory variables
Patient demographic characteristics and treatment histories were extracted from the electronic medical record system. Data on complete blood counts, blood biochemistry, AFP, HBV DNA, HBV serological markers, imaging studies, and tumor pathology were collected before and during anti-tumor treatment.
2.6 Outcome assessments
The primary endpoint was the incidence of HBVr, defined according to the Asian-Pacific Association for the Study of the Liver (APASL) clinical practice guidelines as one of the following: for patients with chronic HBV infection (HBsAg-positive), either (1) a ≥2 log10 IU/mL increase in HBV DNA level from baseline, or (2) an HBV DNA level >100 IU/mL in patients with previously undetectable baseline HBV DNA; for patients with resolved HBV infection (HBsAg-negative and anti-HBc-positive), either (1) HBsAg seroreversion (a change from HBsAg-negative to HBsAg-positive), or (2) a change from undetectable to detectable HBV DNA (Lau et al., 2021). Meeting any of these criteria signified an HBVr event. Secondary outcomes were overall survival (OS), progression-free survival (PFS), and loss to follow-up. OS was defined as the time from the initiation of the first treatment to cancer-related death or the last follow-up. PFS was defined as the time from surgical resection to disease progression, death from any cause, or the last follow-up. Tumor response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) and the HCC-specific modified RECIST (mRECIST) (Eisenhauer et al., 2009; Llovet and Lencioni, 2020). Tumor response was independently assessed by two radiologists who were blinded to the patients’ HBVr status.
2.7 Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) for normally distributed data and as median with interquartile range (IQR) for non-normally distributed data. Differences between groups were compared using the Student’s t-test or the Mann-Whitney U test, as appropriate. Categorical variables were described as numbers (n) and percentages (%) and were compared using the Chi-square (χ²) test or Fisher’s exact test. Univariate and multivariate logistic regression analyses were performed to identify risk factors for HBVr. Survival curves for PFS and OS were generated using the Kaplan-Meier method and compared with the log-rank test. The proportional hazards assumption for the Cox model was verified using the Schoenfeld residuals test. To identify independent prognostic factors for PFS and OS, univariate and multivariate analyses were conducted using the Cox proportional hazards model. A two-sided P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 25.0), and figures were generated with R software (version 4.4.2).
3 Results
3.1 Patient characteristics
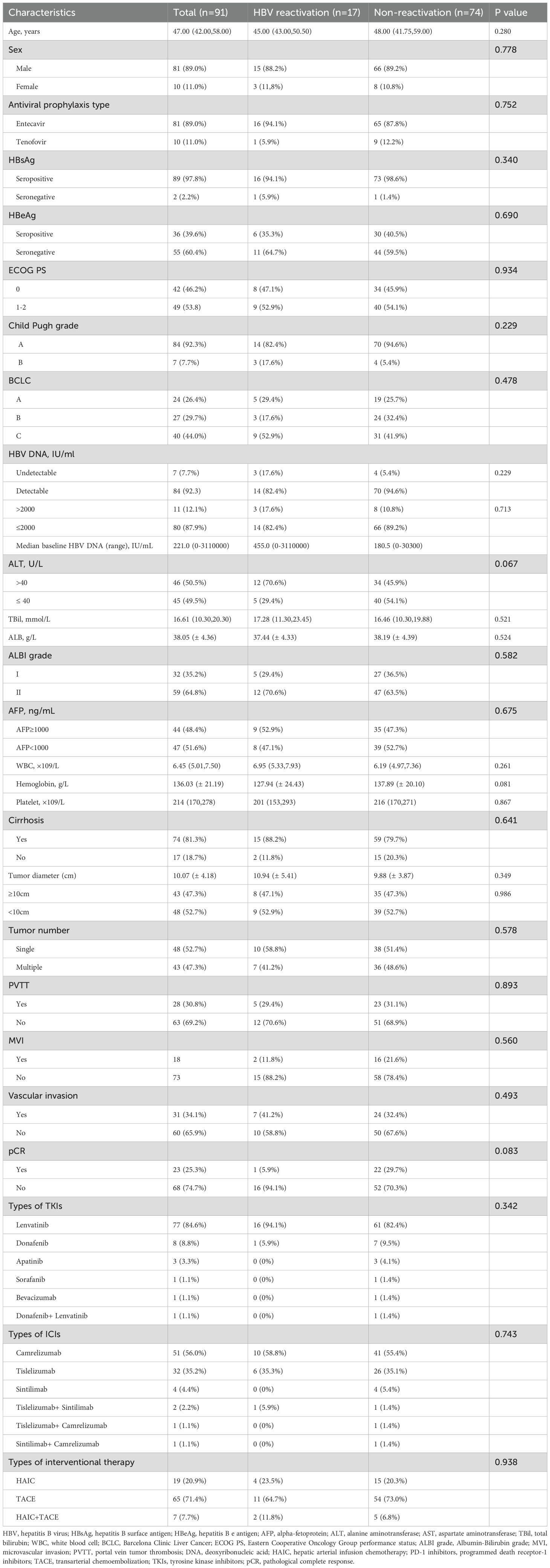
From January 2021 to April 2024, a total of 123 patients with HCC who underwent tumor resection following conversion therapy with TACE and/or HAIC plus TKIs and ICIs were initially screened. Of these, 32 patients were excluded: 3 were anti-HBc negative, and 29 had missing baseline or follow-up data. Ultimately, 91 patients were eligible and included in the final analysis (Figure 1). The detailed baseline characteristics of the enrolled patients are summarized in Table 1. The ICIs administered included sintilimab, camrelizumab, or tislelizumab. The TKIs included sorafenib, lenvatinib, bevacizumab, donafenib, or apatinib. The patient age ranged from 27 to 72 years (median, 47 years), with a predominance of male patients (n=81, 89.0%). At baseline, 90 patients (98.9%) were HBsAg-positive, while one patient had occult HBV infection (HBsAg-negative, anti-HBc-positive, and HBV DNA-positive). A detectable baseline HBV DNA level (median, 221 IU/mL; range, 20–1,270,000 IU/mL) was present in 84 patients (92.3%), and 11 of these patients had a serum HBV DNA level >2000 IU/mL. All patients received antiviral therapy during conversion treatment, with agents including entecavir, tenofovir disoproxil fumarate, or tenofovir alafenamide. The cohort comprised 84 patients (92.3%) with Child-Pugh class A liver function and 7 (7.7%) with class B. According to the BCLC staging system, 24 patients (26.4%) were stage A, 27 (29.7%) were stage B, and 40 (44.0%) were stage C. Postoperative pathology revealed that 48 patients had a solitary tumor, and 43 had multiple tumors. The mean tumor size was 10.07 ± 4.18 cm. Portal vein tumor thrombus (PVTT) was present in 28 patients (30.8%), and microvascular invasion (MVI) was observed in 31 patients (34.1%). A pathological complete response (pCR) was achieved in 23 patients (25.3%).
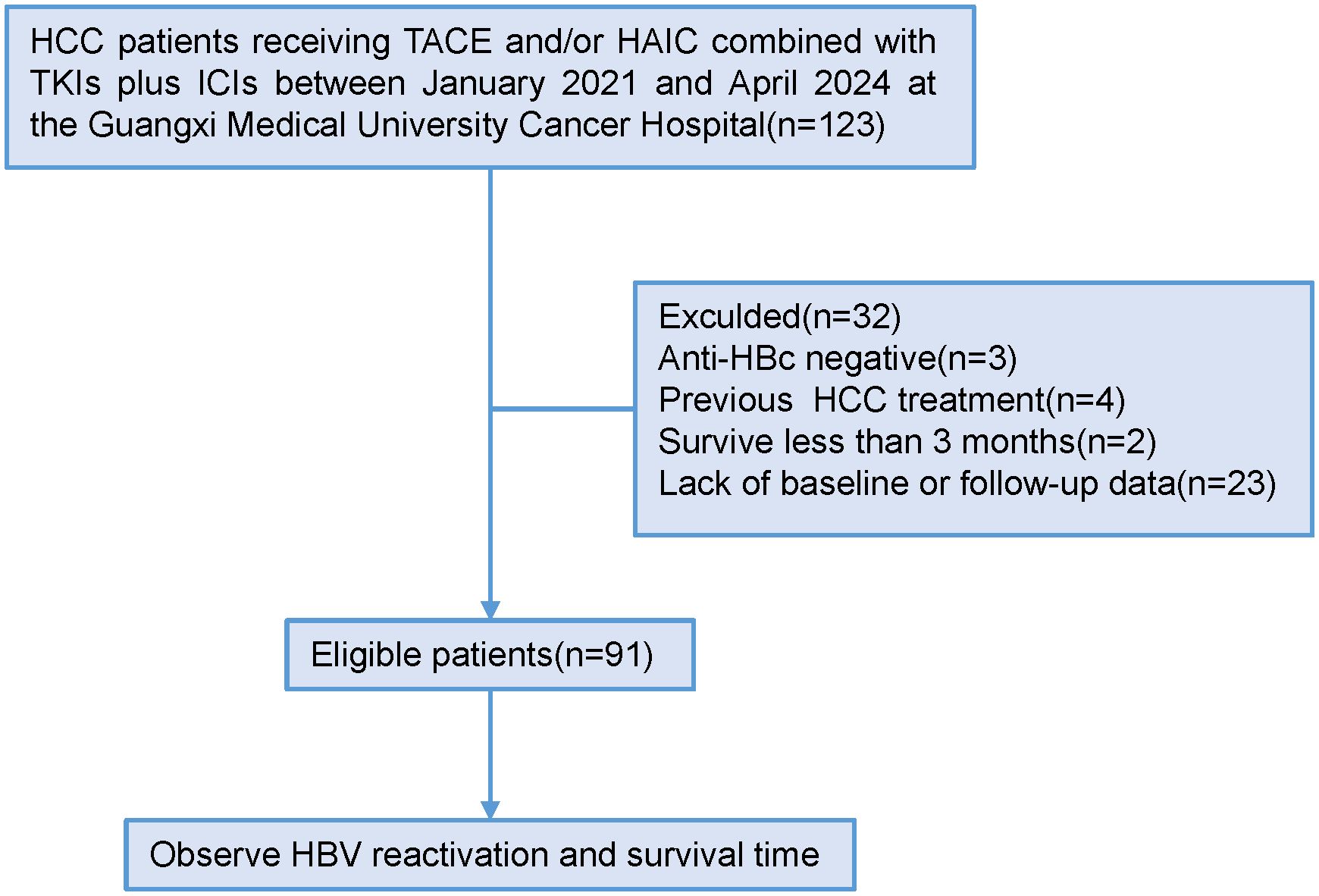
Figure 1. Patient enrollment and study flow. (Anti-HBc, antibody to hepatitis B core antigen; HAIC, hepatic artery infusion chemotherapy; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ICIs, immune checkpoint inhibitors; TACE, transarterial chemoembolization; TKIs, tyrosine kinase inhibitors).
3.2 HBV reactivation
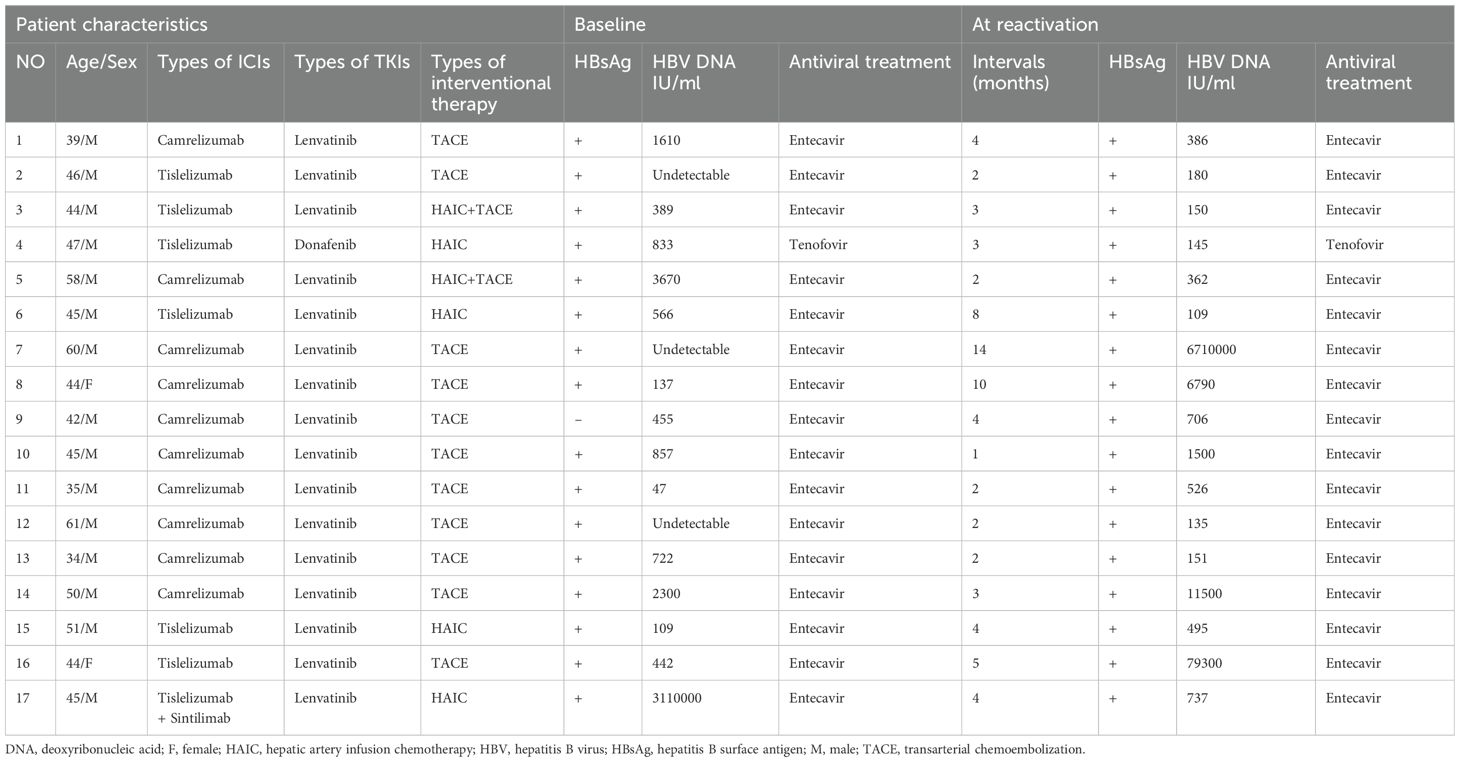
Among the 91 enrolled patients, HBVr occurred in a total of 17 patients (18.7%), with a median time to reactivation of 3 months (range, 1–10 months). Additionally, 19 patients experienced a certain degree of increase in viral load that did not meet the criteria for reactivation. Detailed characteristics of the 17 patients with HBVr are presented in Table 2 and Figure 2. Of these 17 patients, 15 were male. Three patients had undetectable HBV DNA at baseline. Among those with detectable baseline DNA, 12 achieved virological suppression during preoperative antiviral therapy. One patient was HBsAg-negative at baseline. At the onset of HBVr, the median HBV DNA level was 495 IU/mL (range, 109–6,710,000 IU/mL). All 17 patients with reactivation had received antiviral therapy since their initial diagnosis of hepatitis B, with 16 of them taking entecavir. The incidence of HBVr was 16.7% (14/84) in patients with detectable baseline HBV DNA and 42.9% (3/7) in those with undetectable baseline HBV DNA.
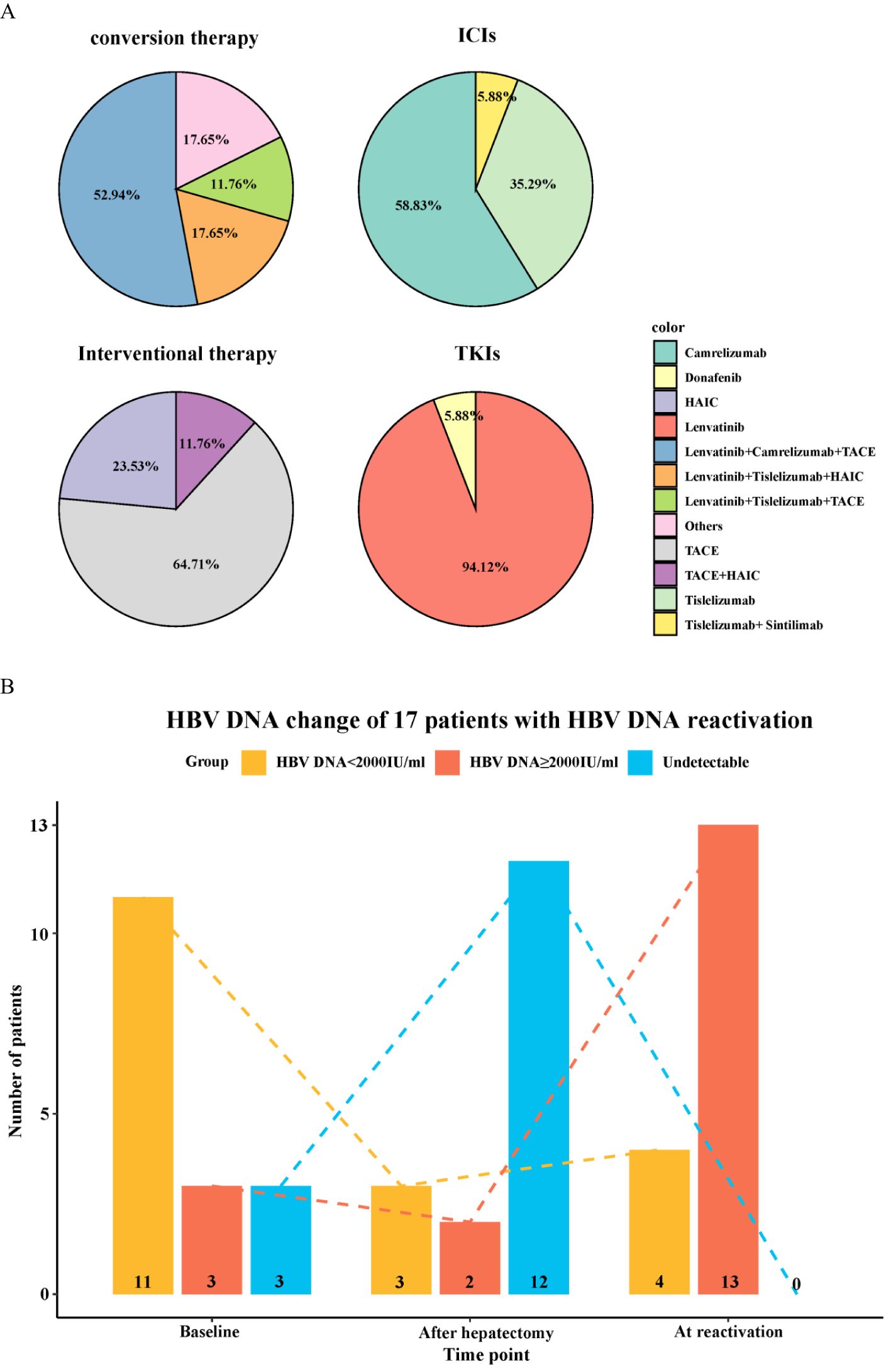
Figure 2. Characteristics of patients with hepatitis B virus reactivation. (A) Baseline demographics and clinical characteristics of the 17 patients who experienced HBVr. (B) Changes in HBV DNA levels over time in the 17 patients with HBVr.
3.3 Patterns of HBV reactivation
Among the 17 patients who experienced HBVr, a notable pattern was observed in 12 individuals who had initially achieved virological suppression (from detectable to undetectable) during preoperative antiviral therapy but subsequently showed detectable HBV DNA postoperatively. Furthermore, one HBsAg-negative patient experienced HBsAg seroreversion after surgery. All these patients were receiving ETV during the treatment period and remained HBsAg-positive post-reactivation, except for the single case of seroreversion.
3.4 Univariate and multivariable analyses for HBV reactivation
The results of the univariate and multivariable logistic regression analyses for HBVr are shown in Table 3. Both analyses consistently identified baseline HBV DNA ≥2000 IU/mL as the sole independent risk factor for HBVr (OR 3.939, 95% CI 1.169–13.272; P = 0.027).
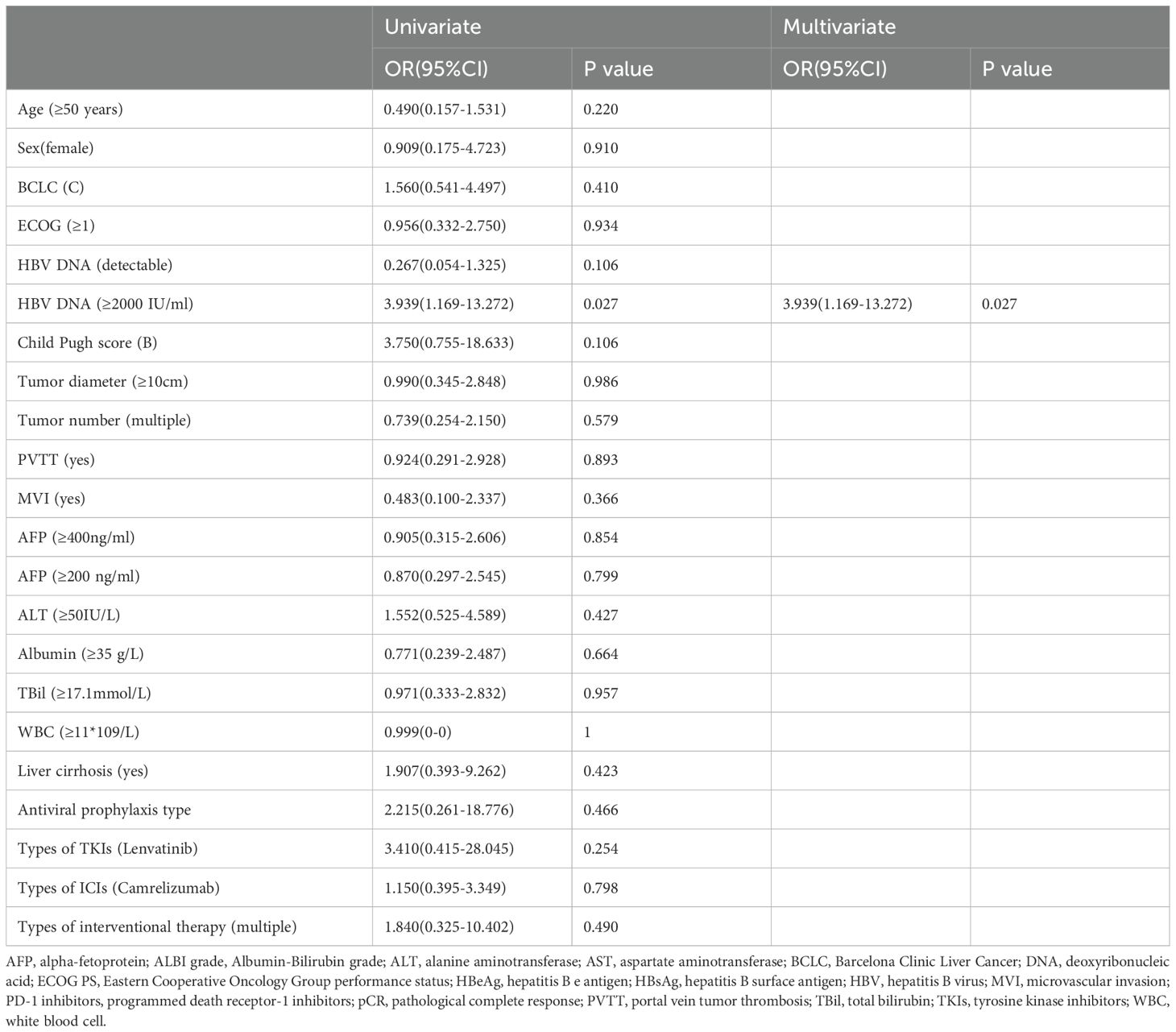
Table 3. Univariate and multivariate logistic regression analysis of risk factors for hepatitis B virus reactivation.
3.5 Patient prognosis
The median OS and PFS for the entire cohort of 91 patients were 47.0 months and 23.6 months, respectively (Supplementary Figure S1). The median follow-up time was 28.8 months in the HBVr group and 20.6 months in the non-reactivation group. No deaths were observed in the HBVr group during the follow-up period. The median OS was not reached in the HBVr group, compared to 45.6 months (95% CI 41.7–49.5) in the non-reactivation group (P = 0.117) (Figure 3A). However, the median PFS was significantly shorter in the HBVr group than in the non-reactivation group (12.1 months [95% CI 5.5–18.7] vs. 29.2 months [95% CI 23.6–34.7]; P < 0.001) (Figure 3B). These findings suggest that patients in the HBVr group had a higher risk of disease recurrence.
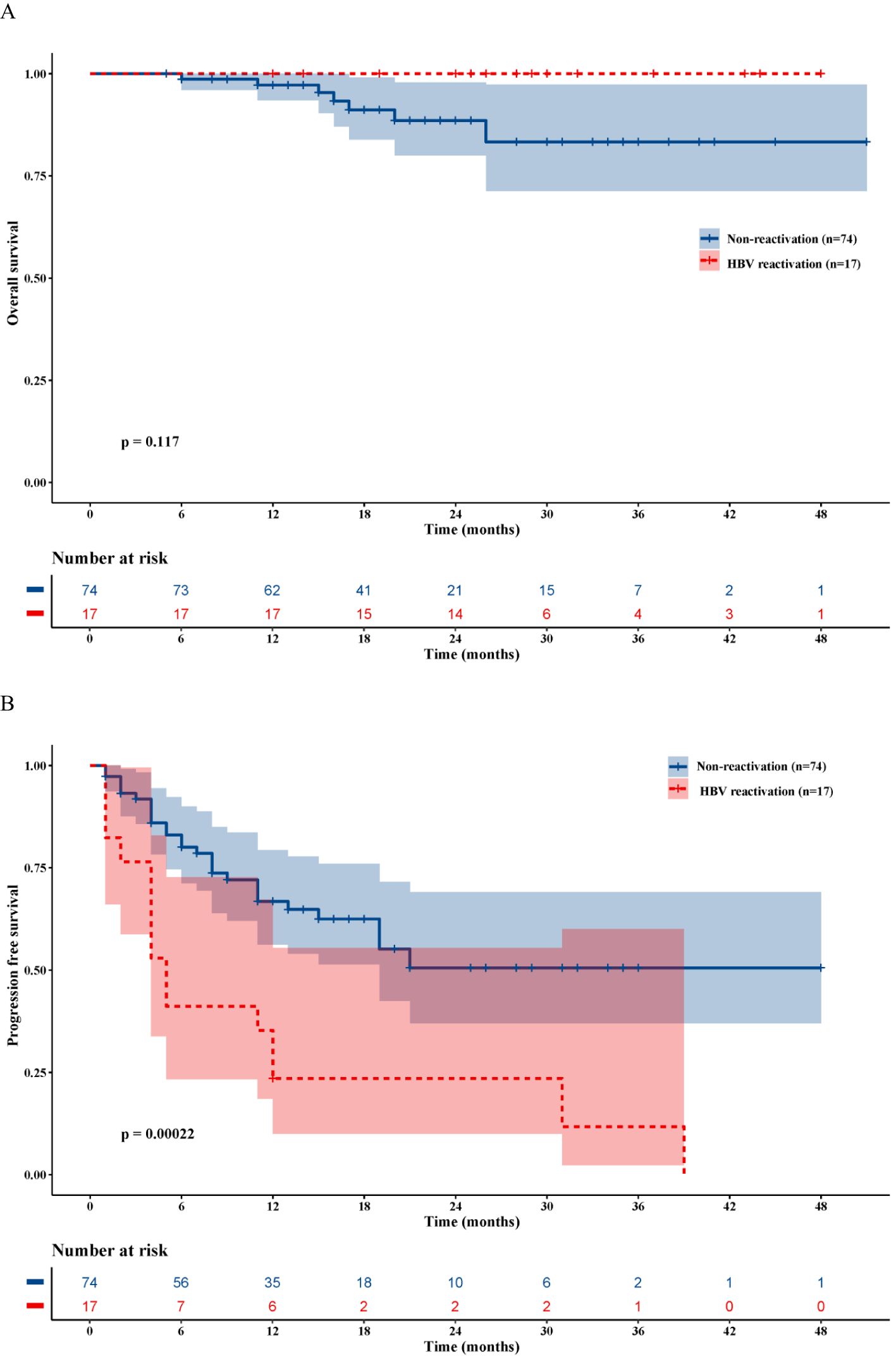
Figure 3. Kaplan-Meier survival analysis. (A) Overall survival curves for patients with and without HBVr. (B) Progression-free survival curves for patients with and without HBVr.
3.6 Univariate and multivariable analyses for PFS and OS
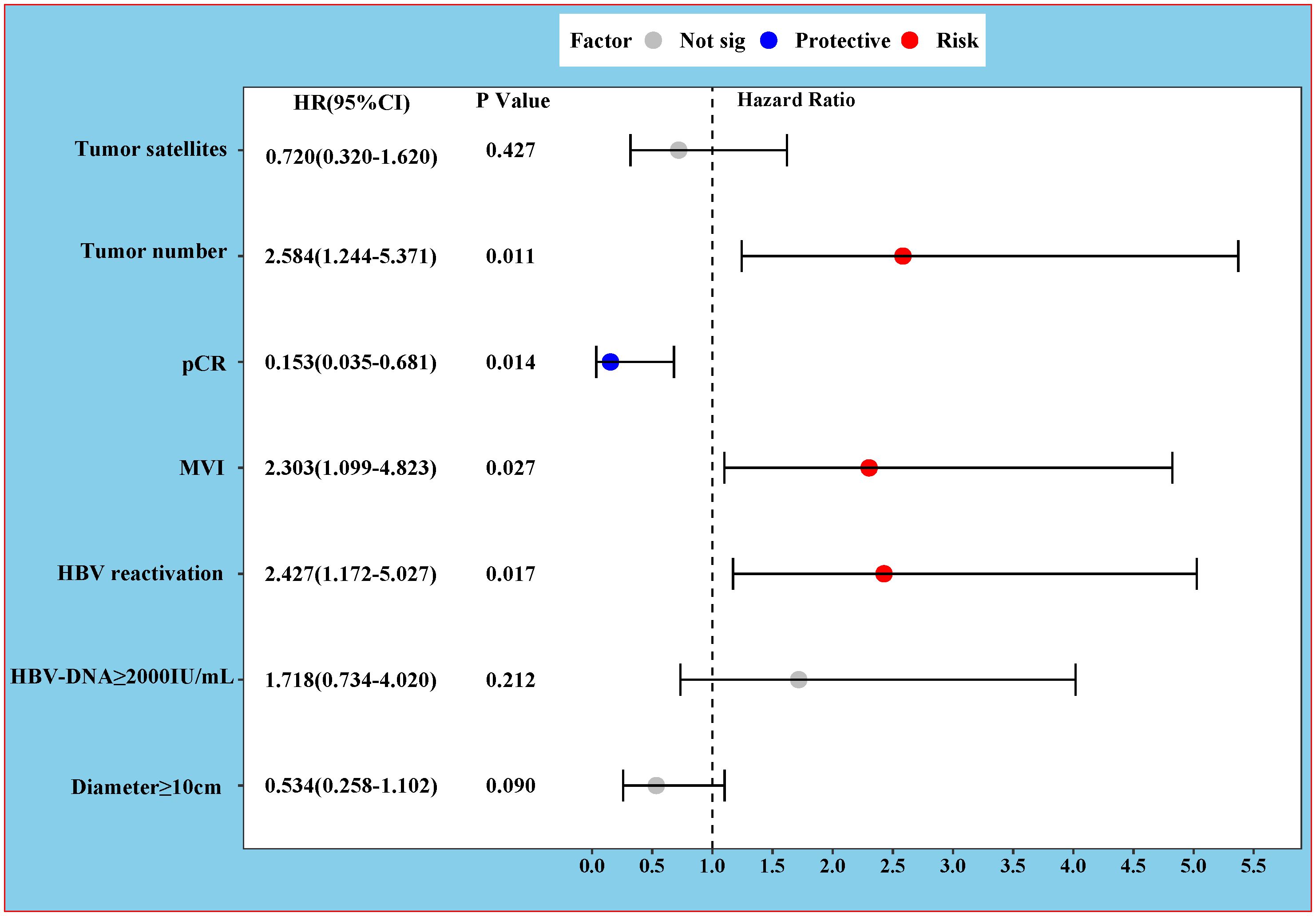
The results of the univariate and multivariable Cox regression analyses for PFS and OS are presented in Table 4. For PFS, univariate analysis identified several significant risk factors: multiple tumors (HR 2.418, 95% CI 1.283–4.557; P = 0.006), tumor diameter ≥10 cm (HR 2.433, 95% CI 1.256–4.714; P = 0.009), baseline HBV DNA ≥2000 IU/mL (HR 2.385, 95% CI 1.227–4.636; P = 0.010), HBVr (HR 3.085, 95% CI 1.623–5.863; P = 0.001), presence of satellite nodules (HR 2.117, 95% CI 1.058–4.236; P = 0.034), and MVI (HR 4.804, 95% CI 2.506–9.210; P < 0.001). pCR was a significant protective factor (HR 0.103, 95% CI 0.025–0.428; P = 0.002). In the multivariable analysis for PFS, multiple tumors (HR 2.584, 95% CI 1.244–5.371; P = 0.011), HBVr (HR 2.427, 95% CI 1.172–5.027; P = 0.017), and MVI (HR 2.303, 95% CI 1.099–4.823; P = 0.027) remained independent risk factors. pCR remained an independent protective factor (HR 0.153, 95% CI 0.035–0.681; P = 0.014) (Figure 4). For OS, both univariate and multivariable analyses identified baseline HBV DNA ≥2000 IU/mL as the sole independent risk factor (HR 6.549, 95% CI 1.458–29.408; P = 0.014). The proportional hazards assumption was met for all Cox models, as verified by Schoenfeld residual tests (P > 0.05 for all variables).
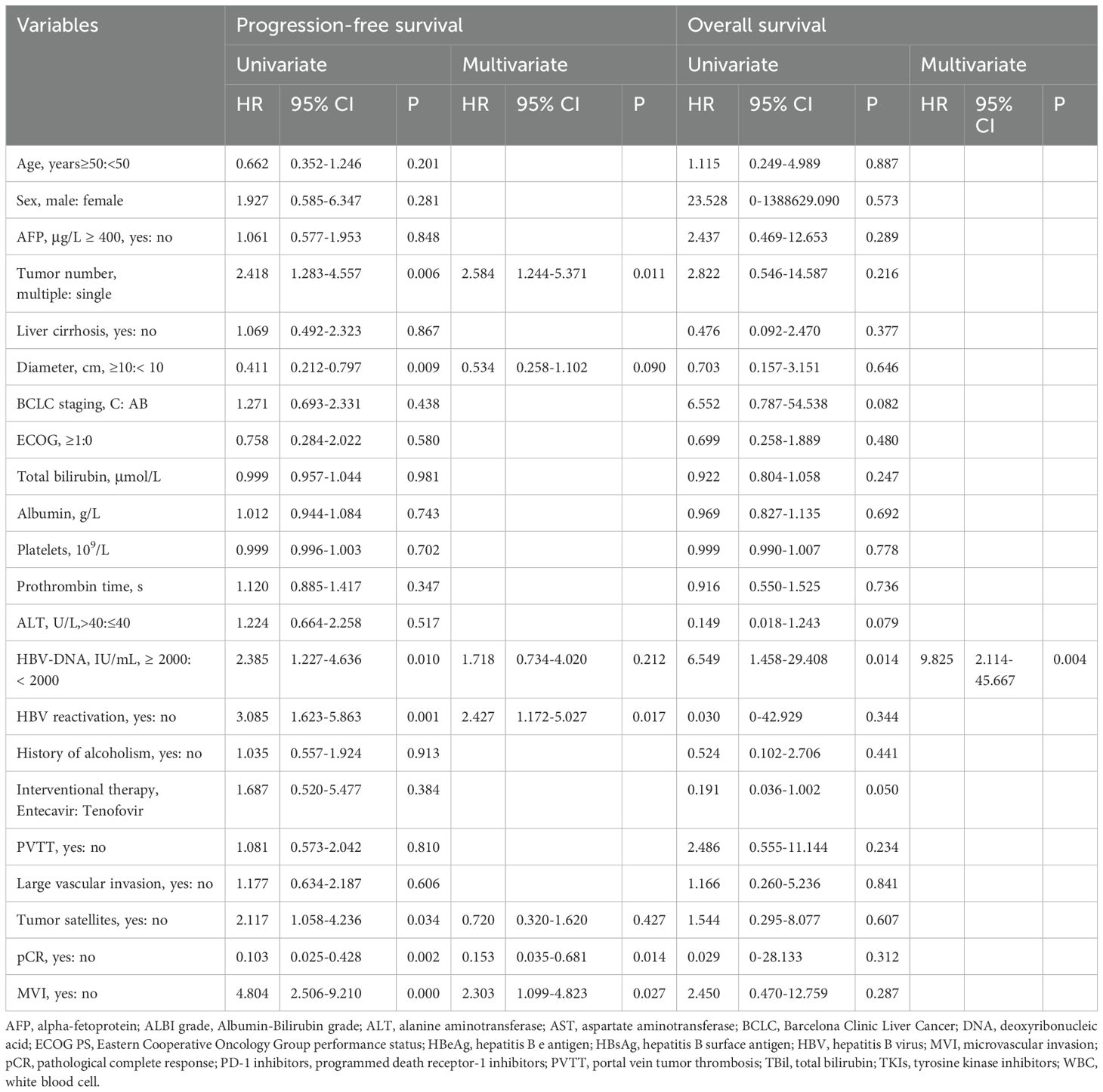
Table 4. Univariate and multivariate Cox regression analysis of independent predictors for progression-free survival and overall survival.
4 Discussion
This retrospective study is the first to elucidate the incidence of HBVr and evaluate its prognostic impact in patients with HBV-related HCC who underwent surgical resection following conversion therapy with interventional treatment, TKIs, and ICIs. We found that 17 (18.7%) patients experienced HBVr. Compared to the non-reactivation group, the HBVr group had a significantly shorter PFS, although no significant difference in OS was observed. The lack of a statistically significant OS difference may be attributable to the relatively small sample size, rendering the analysis underpowered. Furthermore, we identified a baseline HBV DNA level ≥2000 IU/mL as an independent risk factor for HBVr. For prognosis, multiple tumors, MVI, and HBVr were independent risk factors for tumor recurrence, whereas pCR was an independent protective factor. A baseline HBV DNA level ≥2000 IU/mL was the sole independent predictor of mortality.
Anti-tumor therapies, including surgery, TACE, HAIC, TKIs, and ICIs, have all been associated with HBVr. In our cohort, HBVr was observed in 18.7% of patients. This incidence is notably higher than that reported in studies of patients receiving combination therapies without subsequent surgery. For instance, the reported HBVr rate in HCC patients undergoing surgical resection with prophylactic antiviral therapy is typically between 1% and 5% (Papatheodoridi et al., 2022). In patients treated with TACE plus targeted and immune therapies, the HBVr rate was 10.1% (Shen et al., 2023), while for those on HAIC plus targeted and immune therapies, it was 7.5% (Yang et al., 2024). The primary cause of HBVr is an imbalance between the host’s immune response and viral replication. Surgical resection itself is a known risk factor for HBVr in HBsAg-positive patients, largely due to the surgical stress response, which can impair the host’s immune status, particularly in cases of concurrent infection or decompensated liver function (Papatheodoridi et al., 2022). The metabolic and immunological stress induced by hepatectomy, along with the acute release of stress hormones and cytokines, creates a transient window of immunosuppression, rendering patients susceptible to HBVr Burpee (Burpee et al., 2002). Moreover, partial hepatectomy can enhance viral replication due to immunosuppression from blood transfusions and ischemia-reperfusion injury (Huang et al., 2012). It is plausible that the combination of immunosuppression from conversion therapy and the subsequent surgical stress synergistically exacerbates immune dysfunction, leading to a higher HBVr rate than either treatment modality alone (Liu et al., 2021). Combination therapy is associated with an increased risk of HBVr. Indeed, several recent studies have identified combination therapy as an independent risk factor for this event (Lei et al., 2023; Wang et al., 2024). However, the underlying mechanisms for the elevated risk of HBV reactivation in patients undergoing surgical resection after conversion therapy remain to be fully elucidated. We speculate that this may be attributed to the incomplete recovery of host immune function following conversion therapy. This pre-existing immune compromise, when compounded by surgical stress, could lead to further immunosuppression, thereby resulting in a higher incidence of HBV reactivation.
In our study, HBVr occurred in 27.3% (3/11) of patients with baseline HBV DNA ≥2000 IU/mL and 17.5% (14/80) of those with levels <2000 IU/mL. Furthermore, multivariate analysis identified a baseline HBV DNA level of ≥2000 IU/mL as an independent risk factor for HBV reactivation. These findings are consistent with those of several previous reports. For instance, a study on HBV reactivation after radiofrequency ablation in patients with HCC reported that an HBV DNA level ≥2000 IU/mL was a significant risk factor (Liu et al., 2023). The observation that patients with higher HBV DNA levels are more prone to reactivation than those with lower levels has been well-documented in multiple studies (Cholongitas et al., 2018; Wang et al., 2021; Shen et al., 2023). However, some studies have reported no significant association between baseline HBV DNA levels and HBV reactivation in the context of combination therapy (He et al., 2021; Yang et al., 2024). This discrepancy may be attributable to the subsequent surgical intervention following conversion therapy, which could further alter both local and systemic immune statuses. This suggests that different treatment modalities may confer varying risks of HBV reactivation. Despite all patients receiving antiviral prophylaxis, HBVr still occurred. One possible explanation is the development of antiviral resistance resulting from prior treatments (Tenney et al., 2009; Guo et al., 2018). Another potential reason could be the disruption of antiviral therapy due to poor patient adherence, where patients fail to take their medication regularly. This phenomenon is not uncommon and has been documented in numerous studies (Jang, 2014; Shen et al., 2023; Yang et al., 2024). Our results showed no definitive link between the choice of specific interventional, targeted, or immune agents and HBVr risk. This suggests that the profound immunological insult from surgery may overshadow the differential effects of various conversion regimens. Therefore, for patients with high baseline HBV DNA levels, adopting a more potent antiviral strategy perioperatively may be warranted.
Histopathological features of the tumor were strongly associated with PFS. Our analysis confirmed that multiple tumors and MVI are independent risk factors for postoperative recurrence, while pCR is a strong protective factor. These findings align with established literature, where tumor size, multifocality, satellite nodules, and MVI have been consistently identified as predictors of a higher recurrence risk (Imamura et al., 2003; Sala et al., 2004; Ishizawa et al., 2008; Schiffman et al., 2010; Fuks et al., 2012; Li et al., 2013). Interestingly, we did not find a significant association between tumor size or satellite nodules and recurrence, which might be due to the larger tumor burden in our cohort compared to previous studies, or perhaps the preoperative conversion therapy altered the biological characteristics of the tumors.
Crucially, our study identified HBVr as an independent risk factor for postoperative tumor recurrence, corroborating findings from other recent studies (Lei et al., 2023; Yang et al., 2024). This association likely reflects a vicious cycle between the virus and the tumor. On one hand, HBVr involves a surge in viral replication and antigen release, triggering a robust inflammatory response. This chronic inflammation, often involving the activation of NF-κB and MAPK signaling pathways, creates a microenvironment conducive to hepatocellular mutagenesis and epigenetic alterations, thereby promoting HCC progression (Feitelson et al., 2022; Sivasudhan et al., 2022). This inflammatory state can also foster an immunosuppressive milieu by recruiting regulatory T cells (Tregs) and promoting anti-inflammatory cytokines, which impair immune surveillance (Chekol Abebe et al., 2021). On the other hand, the immunosuppressive environment created by tumor progression can facilitate HBVr. Tumors can upregulate immunosuppressive molecules like TGF-β and PD-L1 and pro-angiogenic factors like VEGF, which collectively inhibit T cell and NK cell function and promote the accumulation of Tregs and myeloid-derived suppressor cells (MDSCs) (Wang et al., 2011; Kalluri, 2016; Yang et al., 2018). Given this interplay, we propose that a more aggressive antiviral strategy should be considered during the perioperative period to minimize the risk of HBVr and, consequently, reduce the likelihood of tumor recurrence. However, the optimal timing to de-escalate back to a standard antiviral regimen postoperatively requires further investigation.
A high HBV DNA load is known to correlate with poor prognosis in HCC patients. In our study, a baseline HBV DNA level ≥2000 IU/mL was the sole independent risk factor for OS, although the wide confidence interval (HR 6.549, 95% CI 1.458–29.408) suggests that this finding may be limited by the sample size. This association has been repeatedly documented in the literature (Yu and Kim, 2014; Sun et al., 2021; Yang et al., 2024). We also observed a high HBVr rate (42.9%) among patients with undetectable baseline HBV DNA. This underscores the persistence of cccDNA in hepatocytes, which serves as a template for reactivation even when serum DNA is suppressed by nucleos(t)ide analogues (NAs) (Xia and Guo, 2020). Theoretically, even a single copy of cccDNA can lead to viral rebound and trigger chronic inflammation, perpetuating the malignant cycle of HBV and HCC (Shi and Zheng, 2020). Contrary to some previous reports, we did not find an association between tumor pathology or HBVr and OS. This could be due to the heterogeneity of post-recurrence treatments received by patients in our cohort, which would significantly influence survival outcomes. The impact of post-recurrence therapies in this specific patient population warrants further investigation.
This study has several limitations. First, as a single-center retrospective study with a relatively small sample size, selection bias cannot be ruled out. Second, we did not perform mechanistic studies to elucidate the biological links between HBVr and the combined treatment modality. Basic research is needed to explore these mechanisms. Finally, the screening intervals for HBV DNA and serological markers were not standardized, which may have led to delays in detecting some endpoint events. Therefore, large-scale, prospective, multicenter, randomized controlled trials are warranted to validate our conclusions.
5 Conclusion
This study indicates that in patients with HBV-related HCC undergoing surgery after conversion therapy, a high baseline HBV DNA level may lead to HBV reactivation and adversely affect long-term survival. Patients who experience HBV reactivation have a higher risk of recurrence than those who do not. Therefore, antiviral therapy and HBV DNA monitoring should be administered to patients with HBV-related HCC during conversion therapy and throughout the perioperative period.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Guangxi Medical University Cancer Hospital Science and Technology Ethical Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SX: Software, Conceptualization, Writing – original draft, Investigation, Formal analysis, Project administration, Data curation, Methodology. QP: Methodology, Investigation, Data curation, Writing – original draft, Software. MW: Methodology, Data curation, Writing – review & editing, Funding acquisition. DL: Project administration, Validation, Writing – review & editing. DY: Project administration, Data curation, Writing – original draft, Investigation. TB: Investigation, Conceptualization, Validation, Writing – review & editing. XW: Writing – review & editing, Validation, Supervision. ZT: Writing – review & editing, Project administration, Funding acquisition, Investigation, Validation, Conceptualization. FW: Conceptualization, Resources, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (82360537), Guangxi Natural Science Foundation Project (2025GXNSFBA069386), Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (GKE-ZZ202309), Youth Fund Project of Guangxi Medical University Cancer Hospital (2024-02) and Guangxi Science and Technology Program under Grant No.AD25069077.
Acknowledgments
The authors acknowledge and express their deepest gratitude to the participants of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1598193/full#supplementary-material
References
Burpee, S. E., Kurian, M., Murakame, Y., Benevides, S., and Gagner, M. (2002). The metabolic and immune response to laparoscopic versus open liver resection. Surg. Endosc 16, 899–904. doi: 10.1007/s00464-001-8122-x
Cai, M., Huang, W., Huang, J., Shi, W., Guo, Y., Liang, L., et al. (2022). Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front. Immunol. 13. doi: 10.3389/fimmu.2022.848387
Chekol Abebe, E., Asmamaw Dejenie, T., Mengie Ayele, T., Dagnew Baye, N., Agegnehu Teshome, A., and Tilahun Muche, Z. (2021). The role of regulatory B cells in health and diseases: A systemic review. J. Inflammation Res. 14, 75–84. doi: 10.2147/jir.S286426
Cholongitas, E., Haidich, A. B., Apostolidou-Kiouti, F., Chalevas, P., and Papatheodoridis, G. V. (2018). Hepatitis B virus reactivation in HBsAg-negative, anti-HBc-positive patients receiving immunosuppressive therapy: a systematic review. Ann. Gastroenterol. 31, 480–490. doi: 10.20524/aog.2018.0266
Dan, J. Q., Zhang, Y. J., Huang, J. T., Chen, M. S., Gao, H. J., Peng, Z. W., et al. (2013). Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur. J. Surg. Oncol. 39, 865–872. doi: 10.1016/j.ejso.2013.03.020
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. doi: 10.1016/j.ejca.2008.10.026
Feitelson, M. A., Arzumanyan, A., Spector, I., and Medhat, A. (2022). Hepatitis B x (HBx) as a component of a functional cure for chronic hepatitis B. Biomedicines 10. doi: 10.3390/biomedicines10092210
Fu, Y., Peng, W., Zhang, W., Yang, Z., Hu, Z., Pang, Y., et al. (2023). Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J. Gastroenterol. 58, 413–424. doi: 10.1007/s00535-023-01976-x
Fuks, D., Dokmak, S., Paradis, V., Diouf, M., Durand, F., and Belghiti, J. (2012). Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 55, 132–140. doi: 10.1002/hep.24680
Guo, X., Wu, J., Wei, F., Ouyang, Y., Li, Q., Liu, K., et al. (2018). Trends in hepatitis B virus resistance to nucleoside/nucleotide analogues in North China from 2009-2016: A retrospective study. Int. J. Antimicrob. Agents 52, 201–209. doi: 10.1016/j.ijantimicag.2018.04.002
He, M. K., Peng, C., Zhao, Y., Liang, R. B., Lai, Z. C., Kan, A., et al. (2021). Comparison of HBV reactivation between patients with high HBV-DNA and low HBV-DNA loads undergoing PD-1 inhibitor and concurrent antiviral prophylaxis. Cancer Immunol. Immunother. 70, 3207–3216. doi: 10.1007/s00262-021-02911-w
Hoofnagle, J. H. (2009). Reactivation of hepatitis B. Hepatology 49, S156–S165. doi: 10.1002/hep.22945
Huang, L., Li, J., Lau, W. Y., Yan, J., Zhou, F., Liu, C., et al. (2012). Perioperative reactivation of hepatitis B virus replication in patients undergoing partial hepatectomy for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 27, 158–164. doi: 10.1111/j.1440-1746.2011.06888.x
Imamura, H., Matsuyama, Y., Tanaka, E., Ohkubo, T., Hasegawa, K., Miyagawa, S., et al. (2003). Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 38, 200–207. doi: 10.1016/s0168-8278(02)00360-4
Ishizawa, T., Hasegawa, K., Aoki, T., Takahashi, M., Inoue, Y., Sano, K., et al. (2008). Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 134, 1908–1916. doi: 10.1053/j.gastro.2008.02.091
Jang, J. W. (2014). Hepatitis B virus reactivation in patients with hepatocellular carcinoma undergoing anti-cancer therapy. World J. Gastroenterol. 20, 7675–7685. doi: 10.3748/wjg.v20.i24.7675
Ju, S., Zhou, C., Yang, C., Wang, C., Liu, J., Wang, Y., et al. (2021). Apatinib plus camrelizumab with/without chemoembolization for hepatocellular carcinoma: A real-world experience of a single center. Front. Oncol. 11. doi: 10.3389/fonc.2021.835889
Kalluri, R. (2016). The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598. doi: 10.1038/nrc.2016.73
Kulik, L. M., Atassi, B., van Holsbeeck, L., Souman, T., Lewandowski, R. J., Mulcahy, M. F., et al. (2006). Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J. Surg. Oncol. 94, 572–586. doi: 10.1002/jso.20609
Lau, G., Yu, M. L., Wong, G., Thompson, A., Ghazinian, H., Hou, J. L., et al. (2021). APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol. Int. 15, 1031–1048. doi: 10.1007/s12072-021-10239-x
Lei, J., Yan, T., Zhang, L., Chen, B., Cheng, J., Gao, X., et al. (2023). Comparison of hepatitis B virus reactivation in hepatocellular carcinoma patients who received tyrosine kinase inhibitor alone or together with programmed cell death protein-1 inhibitors. Hepatol. Int. 17, 281–290. doi: 10.1007/s12072-022-10450-4
Lewandowski, R. J., Kulik, L. M., Riaz, A., Senthilnathan, S., Mulcahy, M. F., Ryu, R. K., et al. (2009). A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am. J. Transplant. 9, 1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x
Li, S. H., Wei, W., Guo, R. P., Shi, M., Guo, Z. X., Chen, Z. Y., et al. (2013). Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med. Oncol. 30, 696. doi: 10.1007/s12032-013-0696-3
Liu, K. X., Hong, J. G., Wu, R., Dong, Z. R., Yang, Y. F., Yan, Y. C., et al. (2021). Clinical benefit of antiviral agents for hepatocellular carcinoma patients with low preoperative HBV-DNA loads undergoing curative resection: A meta-analysis. Front. Oncol. 11. doi: 10.3389/fonc.2021.605648
Liu, J., Shen, H., Huang, S., Lin, J., Yan, Z., Qian, G., et al. (2023). Antiviral therapy inhibited HBV-reactivation and improved long-term outcomes in patients who underwent radiofrequency ablation for HBV-related hepatocellular carcinoma. World J. Surg. Oncol. 21, 42. doi: 10.1186/s12957-023-02921-1
Llovet, J. M. and Lencioni, R. (2020). mRECIST for HCC: Performance and novel refinements. J. Hepatol. 72, 288–306. doi: 10.1016/j.jhep.2019.09.026
Mysore, K. R. and Leung, D. H. (2018). Hepatitis B and C. Clin. Liver Dis. 22, 703–722. doi: 10.1016/j.cld.2018.06.002
Papatheodoridi, M., Tampaki, M., Lok, A. S., and Papatheodoridis, G. V. (2022). Risk of HBV reactivation during therapies for HCC: A systematic review. Hepatology 75, 1257–1274. doi: 10.1002/hep.32241
Papatheodoridis, G. V., Lekakis, V., Voulgaris, T., Lampertico, P., Berg, T., Chan, H. L. Y., et al. (2022). Hepatitis B virus reactivation associated with new classes of immunosuppressants and immunomodulators: A systematic review, meta-analysis, and expert opinion. J. Hepatol. 77, 1670–1689. doi: 10.1016/j.jhep.2022.07.003
Rumgay, H., Arnold, M., Ferlay, J., Lesi, O., Cabasag, C. J., Vignat, J., et al. (2022). Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77, 1598–1606. doi: 10.1016/j.jhep.2022.08.021
Sala, M., Fuster, J., Llovet, J. M., Navasa, M., Solé, M., Varela, M., et al. (2004). High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl 10, 1294–1300. doi: 10.1002/lt.20202
Schiffman, S. C., Woodall, C. E., Kooby, D. A., Martin, R. C., Staley, C. A., Egnatashvili, V., et al. (2010). Factors associated with recurrence and survival following hepatectomy for large hepatocellular carcinoma: a multicenter analysis. J. Surg. Oncol. 101, 105–110. doi: 10.1002/jso.21461
Shen, J., Wang, X., Wang, N., Wen, S., Yang, G., Li, L., et al. (2023). HBV reactivation and its effect on survival in HBV-related hepatocarcinoma patients undergoing transarterial chemoembolization combined with tyrosine kinase inhibitors plus immune checkpoint inhibitors. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1179689
Shi, Y. and Zheng, M. (2020). Hepatitis B virus persistence and reactivation. Bmj 370, m2200. doi: 10.1136/bmj.m2200
Shindoh, J., Kawamura, Y., Kobayashi, Y., Kobayashi, M., Akuta, N., Okubo, S., et al. (2021). Prognostic impact of surgical intervention after lenvatinib treatment for advanced hepatocellular carcinoma. Ann. Surg. Oncol. 28, 7663–7672. doi: 10.1245/s10434-021-09974-0
Sivasudhan, E., Blake, N., Lu, Z., Meng, J., and Rong, R. (2022). Hepatitis B viral protein HBx and the molecular mechanisms modulating the hallmarks of hepatocellular carcinoma: A comprehensive review. Cells 11. doi: 10.3390/cells11040741
Sun, F., Liu, Z., and Wang, B. (2021). Correlation between low-level viremia and hepatitis B-related hepatocellular carcinoma and recurrence: a retrospective study. BMC Cancer 21, 1103. doi: 10.1186/s12885-021-08483-3
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Tenney, D. J., Rose, R. E., Baldick, C. J., Pokornowski, K. A., Eggers, B. J., Fang, J., et al. (2009). Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 49, 1503–1514. doi: 10.1002/hep.22841
Voican, C. S., Mir, O., Loulergue, P., Dhooge, M., Brezault, C., Dréanic, J., et al. (2016). Hepatitis B virus reactivation in patients with solid tumors receiving systemic anticancer treatment. Ann. Oncol. 27, 2172–2184. doi: 10.1093/annonc/mdw414
Wang, B. J., Bao, J. J., Wang, J. Z., Wang, Y., Jiang, M., Xing, M. Y., et al. (2011). Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J. Gastroenterol. 17, 3322–3329. doi: 10.3748/wjg.v17.i28.3322
Wang, R., Tan, G., Lei, D., Li, Y., Gong, J., Tang, Y., et al. (2024). Risk of HBV reactivation in HCC patients undergoing combination therapy of PD-1 inhibitors and angiogenesis inhibitors in the antiviral era. J. Cancer Res. Clin. Oncol. 150, 158. doi: 10.1007/s00432-024-05677-7
Wang, X., Yang, X., Chen, F., Wu, S., Song, Z., and Fei, J. (2021). Hepatitis B virus reactivation potential risk factors in hepatocellular carcinoma via transcatheter arterial chemoembolization: A retrospective research. Can. J. Gastroenterol. Hepatol. 2021, 8864655. doi: 10.1155/2021/8864655
Xia, Y. and Guo, H. (2020). Hepatitis B virus cccDNA: Formation, regulation and therapeutic potential. Antiviral Res. 180, 104824. doi: 10.1016/j.antiviral.2020.104824
Xie, Z. B., Zhu, S. L., Peng, Y. C., Chen, J., Wang, X. B., Ma, L., et al. (2015). Postoperative hepatitis B virus reactivation and surgery-induced immunosuppression in patients with hepatitis B-related hepatocellular carcinoma. J. Surg. Oncol. 112, 634–642. doi: 10.1002/jso.24044
Yang, Z., Guan, R., Fu, Y., Hu, D., Zhou, Z., Chen, M., et al. (2024). Risk of hepatitis B virus reactivation and its effect on survival in advanced hepatocellular carcinoma patients treated with hepatic arterial infusion chemotherapy and lenvatinib plus programmed death receptor-1 inhibitors. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1336619
Yang, J., Yan, J., and Liu, B. (2018). Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 9. doi: 10.3389/fimmu.2018.00978
Yu, S. J. and Kim, Y. J. (2014). Hepatitis B viral load affects prognosis of hepatocellular carcinoma. World J. Gastroenterol. 20, 12039–12044. doi: 10.3748/wjg.v20.i34.12039
Keywords: hepatocellular carcinoma, conversion therapy, surgery, HBV reactivation, survival
Citation: Xu S, Pang Q, Wei M, Liu D, Yuan D, Bai T, Wang X, Tang Z and Wu F (2025) Postoperative hepatitis B virus reactivation and its impact on survival in HBV-related hepatocellular carcinoma patients undergoing conversion therapy with interventional therapy combined with tyrosine kinase inhibitors and immune checkpoint inhibitors. Front. Cell. Infect. Microbiol. 15:1598193. doi: 10.3389/fcimb.2025.1598193
Received: 22 March 2025; Accepted: 24 June 2025;
Published: 17 July 2025.
Edited by:
Ashraf A. Tabll, National Research Centre, EgyptReviewed by:
Jian Chen, Fudan University, ChinaSherif El-Kafrawy, King Abdulaziz University, Saudi Arabia
Copyright © 2025 Xu, Pang, Wei, Liu, Yuan, Bai, Wang, Tang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feixiang Wu, d3VmZWl4aWFuZ0BneG11LmVkdS5jbg==; Zhihong Tang, dGFuZ3poaWhvbmdAZ3htdS5lZHUuY24=
†These authors share first authorship
 Shaowei Xu
Shaowei Xu Qingqing Pang2†
Qingqing Pang2† Tao Bai
Tao Bai Feixiang Wu
Feixiang Wu