- 1Departments of Gastroenterology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 2Departments of Otolaryngology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 3Home Ward, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 4Health Management Center, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 5Taizhou Hospital of Zhejiang Province, Shaoxing University, Taizhou, China
Background: Obesity has become a global health concern, particularly in relation to abdominal fat which is associated with various diseases. The connection between Helicobacter pylori (H. pylori) and obesity is still debated, with limited research on the link between H. pylori and visceral fat. This study aimed to examine the correlation between H. pylori infection and visceral fat area (VFA) among participants.
Methods: A total of 18,076 individuals participated in this study, undergoing assessments of VFA, physical parameter measurements, and serum examinations. Bioelectrical impedance was used to analyze VFA, with the triglyceride-glucose (TyG) index indicating levels of insulin resistance (IR) in the population. Multiple linear regression analysis was utilized to determine the factors influencing VFA, while a generalized additive model was employed to assess potential non-linear associations between the TyG index and VFA.
Results: H. pylori was recognized as a contributing factor to VFA exclusively within diabetic populations. In these populations, a significant nonlinear relationship existed between the TyG index and VFA. Furthermore, mediation analysis indicated that the TyG index acted as a mediator in the relationship between H. pylori and VFA.
Conclusion: H. pylori is closely associated with VFA in individuals with diabetes, with IR serving as a mediating factor in the relationship between H. pylori and VFA.
Introduction
Obesity is a widespread problem that is on the rise globally, resulting in substantial financial costs (Finkelstein et al., 2009; Afshin et al., 2017). The causes of obesity are multifaceted, encompassing sedentary lifestyle, excessive caloric intake, and additional factors such as psychosocial influences (McAllister et al., 2009). Obesity contributes to higher rates of illness and death due to the link between surplus body weight and elevated chances of developing a range of health issues such as heart diseases, strokes, diabetes, and non-alcoholic fatty liver disease (NAFLD) (Marchesini et al., 2003; Nguyen et al., 2011; Lin and Li, 2021). However, not all excess fat poses the same disease risks (Vilalta et al., 2022). Studies have shown that visceral fat poses a greater danger than subcutaneous fat due to the proteins released by visceral fat cells that can trigger inflammation, atherosclerosis, dyslipidemia, and hypertension (Vilalta et al., 2022; Mao et al., 2024). Moreover, new evidence suggests a connection between visceral obesity and insulin resistance (IR) (Neeland et al., 2019). Despite being the gold standard for assessing IR, the hyperinsulinemic-euglycemic clamp test is not feasible in clinical settings due to practical, ethical, and financial constraints (Du et al., 2014). The triglyceride-glucose (TyG) index, a new clinical surrogate for IR, is gaining research interest for its ease of use, availability, and consistency (Xue et al., 2022; Zhang et al., 2023).
Body Mass Index (BMI), a widely used anthropometric measure, serves as an indicator for fat mass and obesity. (Goossens, 2017). While BMI is valuable for tracking population weight trends and identifying significant potential health risks in individuals with high BMI, it has serious limitations in assessing individual over fatness and fat distribution within the body (Bray, 2023). Bioelectrical Impedance Analysis (BIA) is gaining popularity in large-scale studies for its ease, quickness, and cost-effectiveness as a method for analyzing body composition (Ricciardi and Talbot, 2007; Popiolek-Kalisz and Szczygiel, 2023). BIA offers more precise body composition measurements compared to BMI, establishing it as a more dependable approach for evaluating obesity (Brener et al., 2021).
Recent studies have shown the important impact of gut bacteria on obesity development (Kumari and Kozyrskyj, 2017; Cornejo-Pareja et al., 2019; Scheithauer et al., 2020). Helicobacter pylori (H. pylori) infection is a prevalent gastrointestinal infection that is widely spread worldwide (Zamani et al., 2018). H. pylori strains residing in the gastric mucosa have the capability to secrete vacuolating cytotoxin, urease, and cytotoxins, even when most infected individuals remain asymptomatic (Kusters et al., 2006). Reports suggest that apart from causing gastrointestinal disorders like chronic gastritis, peptic ulcers, and gastric cancer, H. pylori infection can also lead to extra-gastric conditions including metabolic syndrome, cardiovascular diseases, and liver diseases (Kuipers, 1997; Franceschi et al., 2019; Liu et al., 2023).
Diabetes and obesity are both manifestations of metabolic syndrome, with mutual influence, in which IR plays a significant role (Lemieux and Després, 2020). Nevertheless, the connection between H. pylori infection and obesity is still uncertain. Moreover, no research has yet confirmed the correlation between H. pylori infection and visceral fat area (VFA). Hence, this study aims to explore the relationship between H. pylori and VFA in the population, as well as the mediating role of IR.
Methods
Research participants
Individuals who received health screenings at Taizhou Hospital from January 2017 to April 2023 were the subjects of this study. To meet the inclusion criteria, participants had to be at least 18 years old, provide full personal details, blood test results, and have completed both a urea enzyme test and bioelectrical impedance analysis. Exclusion criteria included being pregnant, lacking clinical data, having a past of stomach surgery, serious heart conditions (such as individuals with heart pacemakers), and having concurrent cancerous growths. Individuals previously diagnosed with diabetes, who have received antihyperglycemic medication treatment, or whose fasting blood glucose (FBG) levels were ≥7.0 mmol/L or glycated hemoglobin A1c (HbA1c) levels were ≥6.5% at the time of the examination, were classified as the diabetic population. A total of 18,076 individuals participated in this study, including 2,155 diagnosed with diabetes.
Detection of H. pylori
H. pylori status was assessed through 13C or 14C urea breath tests during routine health examinations. During the 13C breath test, participants rinsed their mouths after fasting, exhaled normally for breath sample collection, swallowed a 13C urea capsule, waited for 30 minutes, exhaled for another sample collection, and analyzed both samples using the instrument. In the 14C breath test, participants swallowed a 14C urea capsule, sat quietly for 15 minutes, exhaled into a gas collection card through a mouthpiece, and underwent testing.
Detection of visceral fat area
We conducted BIA to measure the participants’ VFA. Participants were required to empty their bladder and bowels in a fasted state before the measurement and remove any metal accessories from their bodies. They stood still for 5 minutes pre-measurement to allow for stable water distribution in the body and adjust to the position for testing. Wearing lightweight clothing, participants stood barefoot on the footpads, grasped the electrodes with both hands aligning their heels with the electrodes at the back of their feet, kept their arms straight without touching the sides of their bodies, and ensured their thighs did not touch.
Blood biochemical results and parameters
Licensed nurses collected age, gender, medical history, systolic blood pressure (SBP), and diastolic blood pressure (DBP). A fasting blood sample was collected from each participant to measure FBG, HbA1c, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), total protein (TP), albumin (Alb) levels, and blood creatinine (Cr). The TyG index was calculated using the formula: ln [TG (mg/dL) × FBG (mg/dL)/2] (Guerrero-Romero et al., 2010).
Statistical analysis
Continuous variables underwent analysis through T-tests and were presented as mean ± standard deviation. Categorical variables were evaluated using chi-square tests and reported as counts and percentages. Multiple linear regression analyses were conducted to further identify factors influencing VFA, with adjustments made for confounding variables on multiple occasions. Generalized additive models (GAM) were applied to investigate any nonlinear associations between the TyG index and VFA. Subsequently, a mediation analysis was conducted to explore the mediating role of the TyG index in the relationship between H. pylori and VFA. The statistical analysis was conducted using R 4.1.3 software. The GAM was implemented through the “mgcv” package, while mediation analysis was executed using the “mediation” package within the R environment.
Results
Baseline characteristics
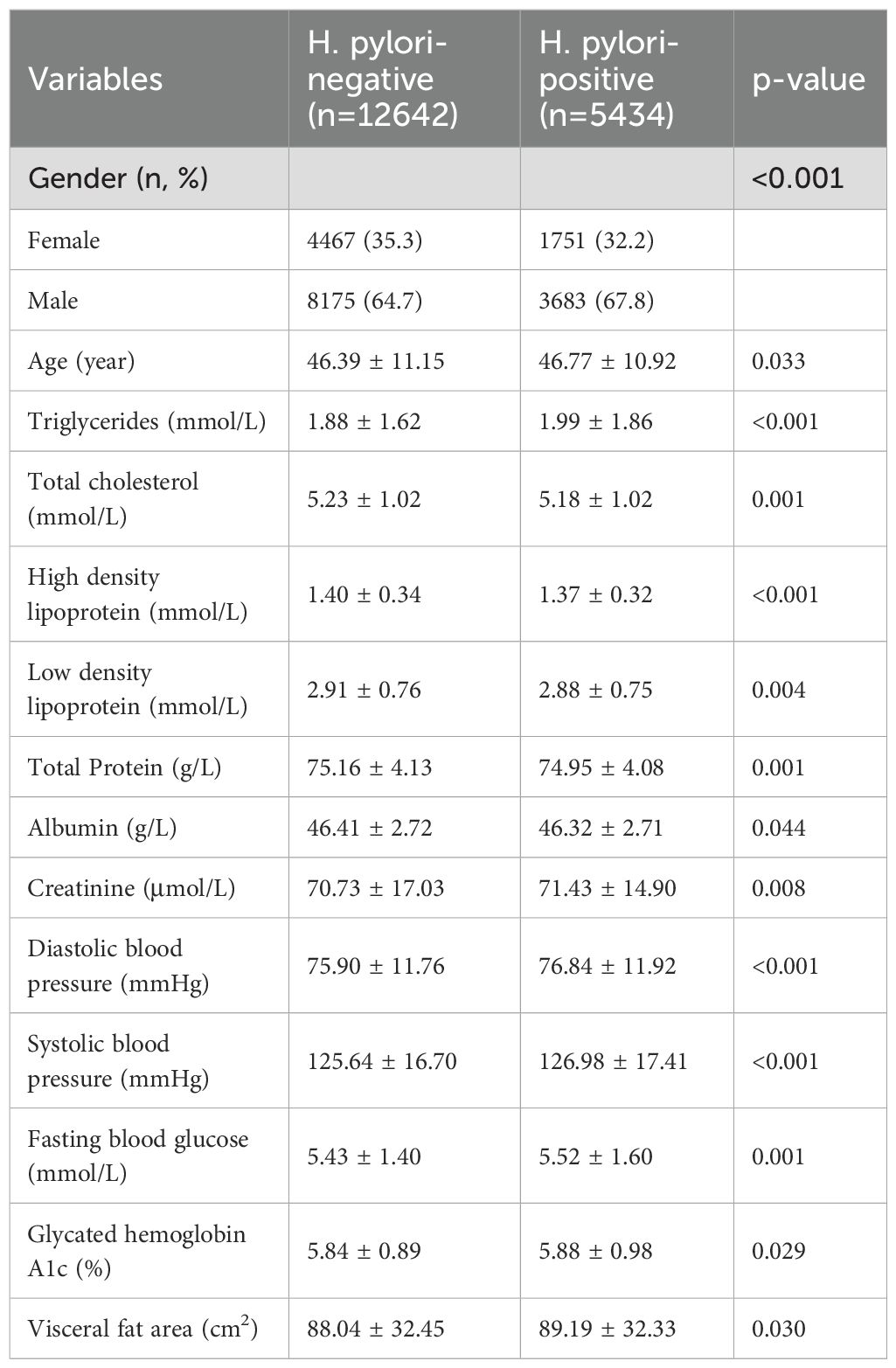
This study involved a total of 18,076 individuals, with participant characteristics detailed in Table 1. The H. pylori positive group, as opposed to the H. pylori negative group, exhibited a higher proportion of males, with 67.8% compared to 64.7%. In addition, the H. pylori positive group showed higher levels of TG, Cr, blood pressure, blood glucose, and VFA, while presenting lower levels of TC, HDL, LDL, and Alb.
Differences in VFA in different populations
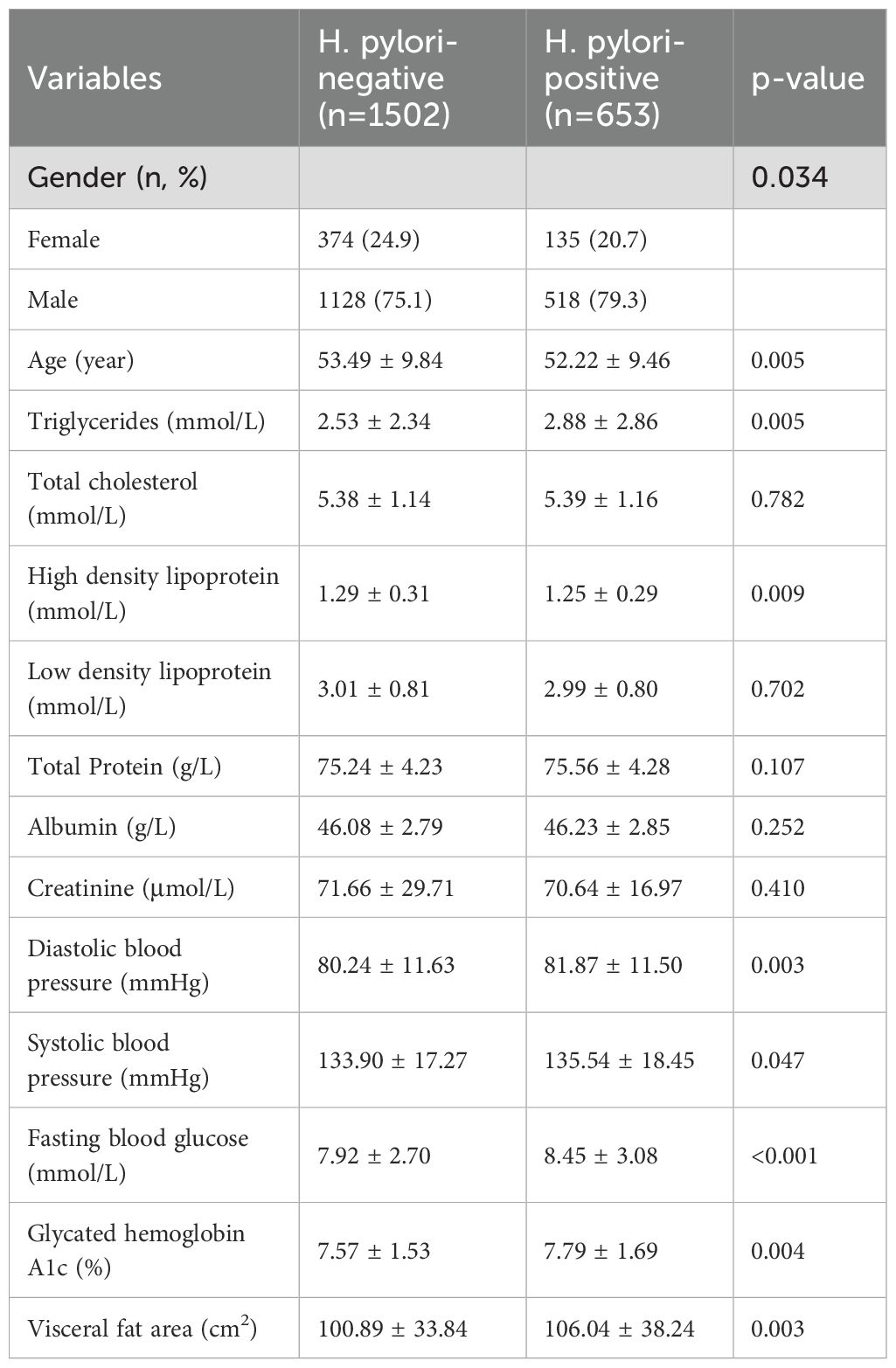
Given the inconsistent impact of H. pylori infection on diabetic and non-diabetic populations, we conducted a stratified analysis on VFA among these diverse groups. Among individuals without diabetes, the existence of H. pylori infection did not demonstrate a notable variation in VFA levels (Supplementary Table 1). Conversely, in the diabetic cohort, individuals in the H. pylori positive group exhibited a notable increase in VFA (P=0.003), as demonstrated in Table 2. Similar to the overall population, the H. pylori positive group displayed higher levels of TG, blood pressure, and blood glucose, along with lower HDL levels.
The impact of H. pylori on VFA
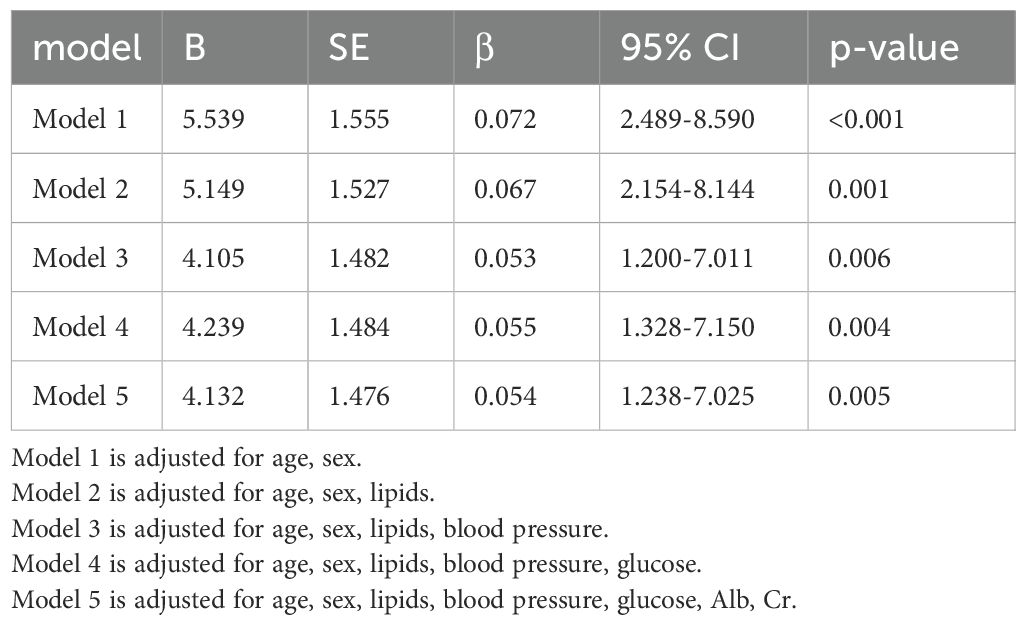
To investigate the impact of H. pylori on VFA, we employed multivariable linear regression. After adjusting for lipid profile, blood pressure, blood glucose, Alb, Cr, as well as gender and age, H. pylori infection persisted as a risk factor for VFA in the diabetic population, as shown in Table 3. The correlation among variables was illustrated in Figure 1.
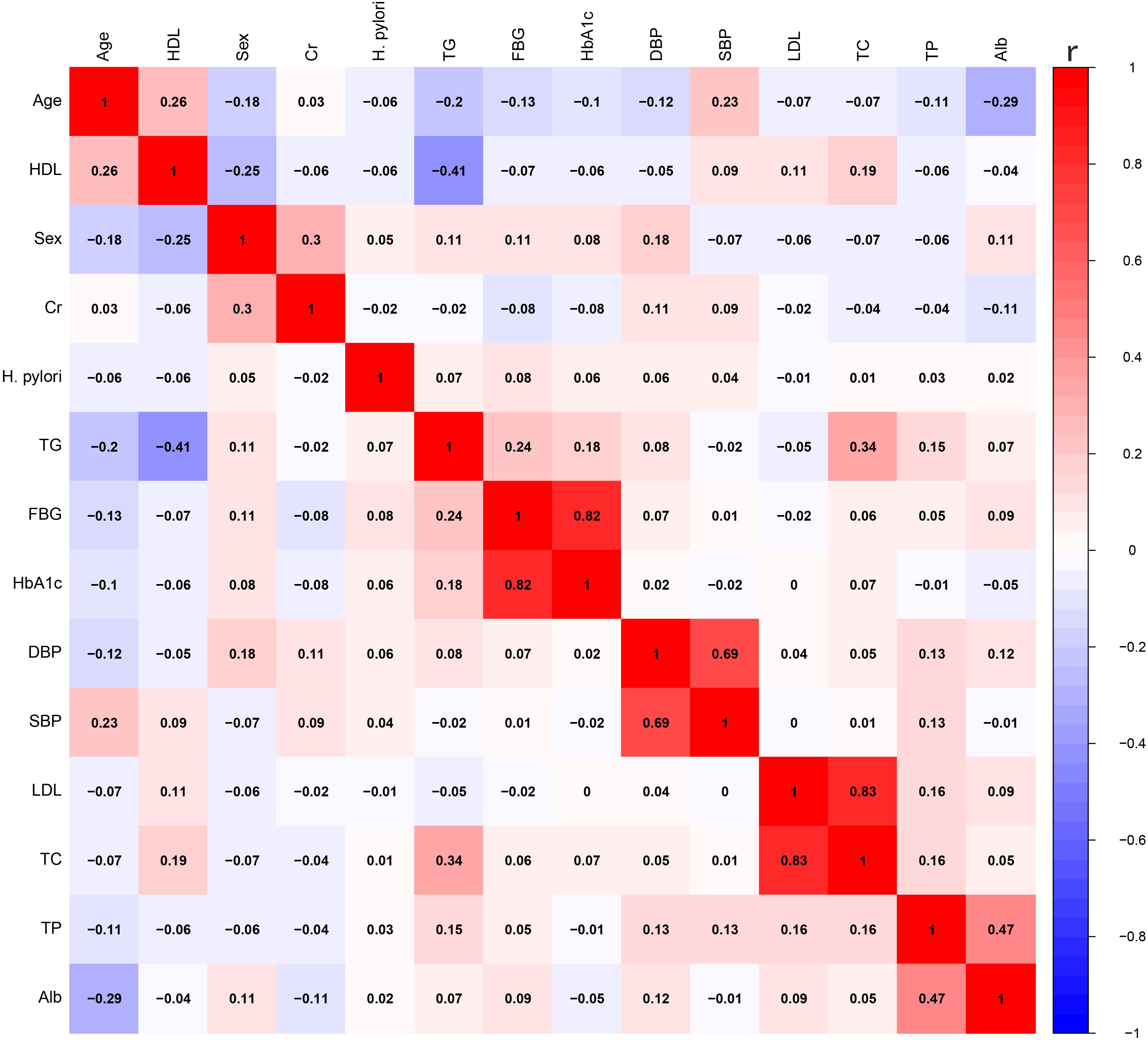
Figure 1. The correlation between various variables. HDL, high-density lipoprotein; Cr, creatinine; TG, triglycerides; FBG, fasting blood glucose; HbA1c, glycated hemoglobin A1c; DBP, diastolic blood pressure; SBP, systolic blood pressure; LDL, low-density lipoprotein; TC, total cholesterol; TP, total protein; Alb, albumin.
Relationship between TyG index and VFA
Based on the GAM curve, there was a nonlinear relationship between TyG index and VFA. When the TyG index was below 9.07, an increase in the TyG index was linked to an elevation in VFA (P < 0.001). Conversely, if the TyG index exceeded 9.07, no significant correlation existed between the TyG index and VFA (P = 0.617) as illustrated in Figure 2.
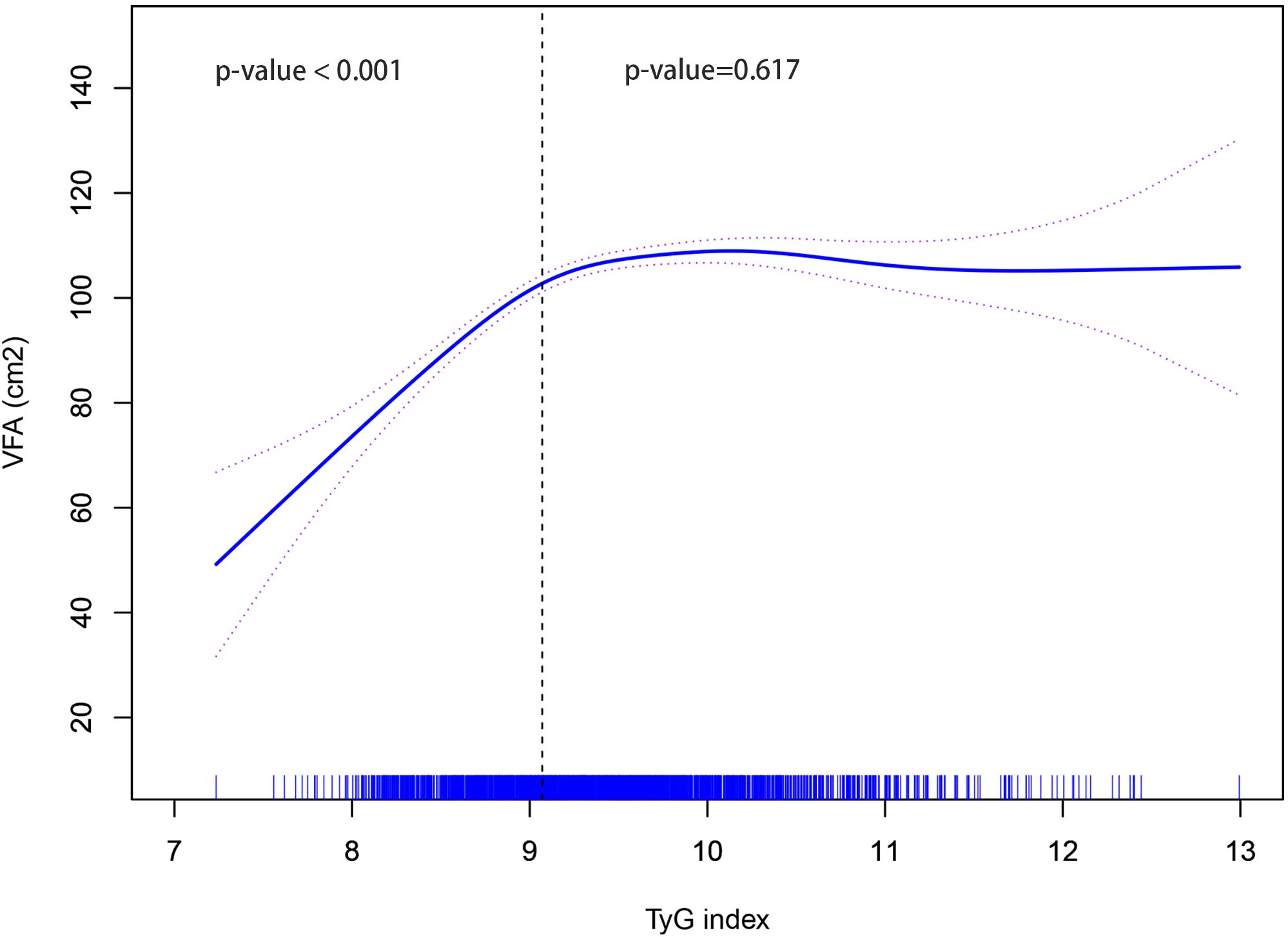
Figure 2. Non-linear relationship between TyG index and VFA. TyG, triglyceride-glucose; VFA, visceral fat area.
Mediation effect analysis
To further investigate the role of the TyG index in the relationship between H. pylori and VFA, we conducted a mediation analysis. The result indicated that the TyG index partially mediated the relationship between H. pylori and VFA, with a mediation effect size of 0.821 (P < 0.001), a direct effect of 4.330 (P = 0.010), and a mediation ratio of 15.9% (P = 0.006), as illustrated in Figure 3.

Figure 3. Mediating role of TyG index in the relationship between H. pylori and VFA. TyG, triglyceride-glucose; VFA, visceral fat area. Proportion mediated = mediation effect/(mediation effect + direct effect).
Discussion
Opinions on the link between H. pylori infection and obesity are divided. In China, a cross-sectional study found a significant correlation between obesity and H. pylori infection (Zhang et al., 2015). Furthermore, H. pylori infection was positively correlated with higher BMI in several studies involving large samples (Suki et al., 2018; Zhang et al., 2020). However, conflicting conclusions have been reported. An analysis of Mendelian randomization found no association between H. pylori infection and obesity (den Hollander et al., 2017). Additionally, in some developed countries, H. pylori infection was negatively correlated with obesity rates (Lender et al., 2014). Nevertheless, there is a lack of research exploring the association between H. pylori and VFA, stratified by diabetes status.
In our research, we discovered that H. pylori infection did not exhibit a significant impact on VFA in the non-diabetic cohort. Yet, individuals with diabetes showed a correlation between H. pylori infection and a rise in VFA. Previous research indicates that when VFA exceeds 100 cm², the risk of obesity-related diseases markedly increases (New criteria for ‘obesity disease’ in Japan, 2002; Kim et al., 2022). Although women typically have less visceral fat than men, they remain at an elevated risk for developing diabetes due to increases in visceral fat (Kim et al., 2022). Our further analysis using multiple linear regression confirmed that H. pylori infection is a significant risk factor for elevated VFA. Infection with H. pylori disturbs the equilibrium of beneficial bacterial species in the stomach lining, causing changes in the human gut microbiome, which is essential for the body’s immune response and metabolic balance (Iino et al., 2018; Lopetuso et al., 2018). In fact, gut microbiota imbalance is closely associated with obesity, diabetes, and metabolic syndrome (Cornejo-Pareja et al., 2019; Dabke et al., 2019; Wu et al., 2023). The progression of diabetes involves chronic low-grade metabolic inflammation, closely intertwined with disturbances in glucose metabolism pathways (Scheithauer et al., 2020). Adipose tissue not only serves as an important energy storage organ but also is significantly associated with inflammation and IR (Gregor and Hotamisligil, 2011; Wensveen et al., 2015; Lu et al., 2019). Previous studies have demonstrated that IR can promote the accumulation of visceral fat and exacerbate the progression of diabetes through various inflammatory factors and metabolic pathways (de Mutsert et al., 2018; Yaribeygi et al., 2019; Lee et al., 2022). This study established a significant correlation between IR and VFA in diabetic populations. The relationship between H. pylori and IR has been reported in earlier studies, with some suggesting that H. pylori infection may contribute to the development of IR by activating chronic inflammatory responses, disrupting insulin signaling pathways, increasing oxidative stress levels, and altering gastrointestinal hormone secretion (Aslan et al., 2006; Azami et al., 2021). Our results confirmed that IR plays a significant mediating role in the relationship between H. pylori and VFA, indicating that IR may be one mechanism by which H. pylori affects visceral fat deposition. Notably, diabetic populations typically exhibit higher levels of IR, and H. pylori may exert its effects on visceral obesity specifically under this pathological state. Consequently, early intervention targeting H. pylori infection, particularly eradication of H. pylori, may hold significant clinical implications for reducing visceral fat accumulation in diabetic populations. Although H. pylori secretes various virulence factors, the specific factors affecting VFA remain unidentified. VFA is closely linked to a range of chronic diseases, and if not addressed, its continued accumulation may have serious health consequences. Therefore, future large-scale longitudinal studies and investigations into the biological mechanisms are needed to further elucidate the precise pathways through which H. pylori infection contributes to metabolic regulation in diabetic individuals and to explore the potential benefits of H. pylori eradication therapy in diabetic patients with visceral obesity.
Our study validated the influence of H. pylori infection on VFA in people with diabetes. However, this study still has shortcomings. Firstly, being single-centered, whether the research findings can be generalized to other regions necessitates further validation through multicenter studies. Besides, the study lacks examination of factors such as lifestyle, psychosocial influences, and socioeconomic status, all of which are crucial influences on visceral obesity. Moreover, the specific mechanism by which H. pylori infection solely affects the VFA in individuals with diabetes remains unclear, demanding prospective studies and additional biological experiments for verification.
Conclusion
In diabetic populations, there is a significant association between H. pylori infection and VFA, with IR serving as a mediating factor in the association between H. pylori and VFA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Taizhou Hospital (approval number: K20220790). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YiC: Writing – original draft, Formal analysis, Methodology, Conceptualization. BN: Investigation, Writing – original draft, Data curation. NY: Conceptualization, Investigation, Writing – original draft. YaC: Conceptualization, Visualization, Writing – original draft, Data curation. JZ: Validation, Formal analysis, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the hospital staff for their contribution to the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1598223/full#supplementary-material
References
Afshin, A., Forouzanfar, M. H., Reitsma, M. B., Sur, P., Estep, K., Lee, A., et al. (2017). Health effects of overweight and obesity in 195 countries over 25 years. New Engl. J. medicine. 377, 13–27. doi: 10.1056/NEJMoa1614362
Aslan, M., Horoz, M., Nazligul, Y., Bolukbas, C., Bolukbas, F. F., Selek, S., et al. (2006). Insulin resistance in H pylori infection and its association with oxidative stress. World J. gastroenterology. 12, 6865–6868. doi: 10.3748/wjg.v12.i42.6865
Azami, M., Baradaran, H. R., Dehghanbanadaki, H., Kohnepoushi, P., Saed, L., Moradkhani, A., et al. (2021). Association of Helicobacter pylori infection with the risk of metabolic syndrome and insulin resistance: an updated systematic review and meta-analysis. Diabetol. Metab. syndrome. 13, 145. doi: 10.1186/s13098-021-00765-x
Brener, A., Peleg, I., Rosenfeld, T., Kern, S., Uretzky, A., Elkon-Tamir, E., et al. (2021). Beyond body mass index - body composition assessment by bioimpedance in routine endocrine practice. Endocrine Pract. 27, 419–425. doi: 10.1016/j.eprac.2020.10.013
Cornejo-Pareja, I., Muñoz-Garach, A., Clemente-Postigo, M., and Tinahones, F. J. (2019). Importance of gut microbiota in obesity. Eur. J. Clin. nutrition. 72, 26–37. doi: 10.1038/s41430-018-0306-8
Dabke, K., Hendrick, G., and Devkota, S. (2019). The gut microbiome and metabolic syndrome. J. Clin. investigation. 129, 4050–4057. doi: 10.1172/JCI129194
de Mutsert, R., Gast, K., Widya, R., de Koning, E., Jazet, I., Lamb, H., et al. (2018). Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: the Netherlands epidemiology of obesity study. Metab. syndrome related Disord. 16, 54–63. doi: 10.1089/met.2017.0128
den Hollander, W. J., Broer, L., Schurmann, C., Meyre, D., den Hoed, C. M., Mayerle, J., et al. (2017). Helicobacter pylori colonization and obesity - a Mendelian randomization study. Sci. reports. 7, 14467. doi: 10.1038/s41598-017-14106-4
Du, T., Yuan, G., Zhang, M., Zhou, X., Sun, X., and Yu, X. (2014). Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. diabetology. 13, 146. doi: 10.1186/s12933-014-0146-3
Examination Committee of Criteria for ‘Obesity Disease’ in Japan and Japan Society for the Study of Obesity. (2002). New criteria for ‘obesity disease’ in Japan. Circ. J. 66, 987–992. doi: 10.1253/circj.66.987
Finkelstein, E. A., Trogdon, J. G., Cohen, J. W., and Dietz, W. (2009). Annual medical spending attributable to obesity: payer-and service-specific estimates. Health affairs (Project Hope) 28, w822–w831. doi: 10.1377/hlthaff.28.5.w822
Franceschi, F., Covino, M., and Roubaud Baudron, C. (2019). Review: Helicobacter pylori and extragastric diseases. Helicobacter. 24 Suppl 1, e12636. doi: 10.1111/hel.2019.24.issue-S1
Goossens, G. H. (2017). The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obesity facts. 10, 207–215. doi: 10.1159/000471488
Gregor, M. F. and Hotamisligil, G. S. (2011). Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445. doi: 10.1146/annurev-immunol-031210-101322
Guerrero-Romero, F., Simental-Mendía, L. E., González-Ortiz, M., Martínez-Abundis, E., Ramos-Zavala, M. G., Hernández-González, S. O., et al. (2010). The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351. doi: 10.1210/jc.2010-0288
Iino, C., Shimoyama, T., Chinda, D., Arai, T., Chiba, D., Nakaji, S., et al. (2018). Infection of helicobacter pylori and atrophic gastritis influence lactobacillus in gut microbiota in a Japanese population. Front. Immunol. 9, 712. doi: 10.3389/fimmu.2018.00712
Kim, E. H., Kim, H. K., Lee, M. J., Bae, S. J., Choe, J., Jung, C. H., et al. (2022). Sex differences of visceral fat area and visceral-to-subcutaneous fat ratio for the risk of incident type 2 diabetes mellitus. Diabetes Metab. J. 46, 486–498. doi: 10.4093/dmj.2021.0095
Kuipers, E. J. (1997). Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Alimentary Pharmacol. Ther. 11 Suppl 1, 71–88. doi: 10.1046/j.1365-2036.11.s1.5.x
Kumari, M. and Kozyrskyj, A. L. (2017). Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obesity Rev. 18, 18–31. doi: 10.1111/obr.12484
Kusters, J. G., van Vliet, A. H., and Kuipers, E. J. (2006). Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 19, 449–490. doi: 10.1128/CMR.00054-05
Lee, S. H., Park, S. Y., and Choi, C. S. (2022). Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab. J. 46, 15–37. doi: 10.4093/dmj.2021.0280
Lemieux, I. and Després, J. P. (2020). Metabolic syndrome: past, present and future. Nutrients. 12. doi: 10.3390/nu12113501
Lender, N., Talley, N. J., Enck, P., Haag, S., Zipfel, S., Morrison, M., et al. (2014). Review article: Associations between Helicobacter pylori and obesity–an ecological study. Alimentary Pharmacol. Ther. 40, 24–31. doi: 10.1111/apt.2014.40.issue-1
Lin, X. and Li, H. (2021). Obesity: epidemiology, pathophysiology, and therapeutics. Front. endocrinology. 12, 706978. doi: 10.3389/fendo.2021.706978
Liu, Y., Shuai, P., Chen, W., Liu, Y., and Li, D. (2023). Association between Helicobacter pylori infection and metabolic syndrome and its components. Front. endocrinology. 14, 1188487. doi: 10.3389/fendo.2023.1188487
Lopetuso, L. R., Napoli, M., Rizzatti, G., Scaldaferri, F., Franceschi, F., and Gasbarrini, A. (2018). Considering gut microbiota disturbance in the management of Helicobacter pylori infection. Expert Rev. gastroenterology hepatology. 12, 899–906. doi: 10.1080/17474124.2018.1503946
Lu, J., Zhao, J., Meng, H., and Zhang, X. (2019). Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front. Immunol. 10, 1173. doi: 10.3389/fimmu.2019.01173
Mao, J., Gan, S., Gong, S., Zhou, Q., Yu, F., Zhou, H., et al. (2024). Visceral fat area is more strongly associated with arterial stiffness than abdominal subcutaneous fat area in Chinese patients with type 2 diabetes. Diabetol. Metab. syndrome. 16, 123. doi: 10.1186/s13098-024-01356-2
Marchesini, G., Bugianesi, E., Forlani, G., Cerrelli, F., Lenzi, M., Manini, R., et al. (2003). Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology (Baltimore Md). 37, 917–923. doi: 10.1053/jhep.2003.50161
McAllister, E. J., Dhurandhar, N. V., Keith, S. W., Aronne, L. J., Barger, J., Baskin, M., et al. (2009). Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. nutrition. 49, 868–913. doi: 10.1080/10408390903372599
Neeland, I. J., Ross, R., Després, J. P., Matsuzawa, Y., Yamashita, S., Shai, I., et al. (2019). Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes endocrinology. 7, 715–725. doi: 10.1016/S2213-8587(19)30084-1
Nguyen, N. T., Nguyen, X. M., Lane, J., and Wang, P. (2011). Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999-2006. Obesity surgery. 21, 351–355. doi: 10.1007/s11695-010-0335-4
Popiolek-Kalisz, J. and Szczygiel, K. (2023). Bioelectrical impedance analysis and body composition in cardiovascular diseases. Curr. problems Cardiol. 48, 101911. doi: 10.1016/j.cpcardiol.2023.101911
Ricciardi, R. and Talbot, L. A. (2007). Use of bioelectrical impedance analysis in the evaluation, treatment, and prevention of overweight and obesity. J. Am. Acad. Nurse Practitioners. 19, 235–241. doi: 10.1111/j.1745-7599.2007.00220.x
Scheithauer, T. P. M., Rampanelli, E., Nieuwdorp, M., Vallance, B. A., Verchere, C. B., van Raalte, D. H., et al. (2020). Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 11, 571731. doi: 10.3389/fimmu.2020.571731
Suki, M., Leibovici Weissman, Y., Boltin, D., Itskoviz, D., Tsadok Perets, T., Comaneshter, D., et al. (2018). Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders: a cohort study. Eur. J. gastroenterology hepatology. 30, 143–148. doi: 10.1097/MEG.0000000000001014
Vilalta, A., Gutiérrez, J. A., Chaves, S., Hernández, M., Urbina, S., and Hompesch, M. (2022). Adipose tissue measurement in clinical research for obesity, type 2 diabetes and NAFLD/NASH. Endocrinology Diabetes Metab. 5, e00335. doi: 10.1002/edm2.v5.3
Wensveen, F. M., Jelenčić, V., Valentić, S., Šestan, M., Wensveen, T. T., Theurich, S., et al. (2015). NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 16, 376–385. doi: 10.1038/ni.3120
Wu, J., Yang, K., Fan, H., Wei, M., and Xiong, Q. (2023). Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. endocrinology. 14, 1114424. doi: 10.3389/fendo.2023.1114424
Xue, Y., Xu, J., Li, M., and Gao, Y. (2022). Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index-related parameters. Front. endocrinology. 13, 951689. doi: 10.3389/fendo.2022.951689
Yaribeygi, H., Farrokhi, F. R., Butler, A. E., and Sahebkar, A. (2019). Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 234, 8152–8161. doi: 10.1002/jcp.v234.6
Zamani, M., Ebrahimtabar, F., Zamani, V., Miller, W. H., Alizadeh-Navaei, R., Shokri-Shirvani, J., et al. (2018). Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Alimentary Pharmacol. Ther. 47, 868–876. doi: 10.1111/apt.2018.47.issue-7
Zhang, J., Chen, Y., Chen, W., Xu, H., Wang, H., Chen, L., et al. (2020). Persistent infection of Helicobacter pylori affects weight loss in obese population compared with persistent negative: A case-control study based on healthy Chinese. Helicobacter. 25, e12697. doi: 10.1111/hel.12697
Zhang, Y., Du, T., Chen, X., Yu, X., Tu, L., and Zhang, C. (2015). Association between Helicobacter pylori infection and overweight or obesity in a Chinese population. J. infection developing countries. 9, 945–953. doi: 10.3855/jidc.6035
Keywords: Helicobacter pylori, visceral fat area, triglyceride-glucose index, mediation analysis, diabetes
Citation: Chen Y, Ni B, You N, Chen Y and Zhang J (2025) Relationship between Helicobacter pylori infection and visceral fat area in diabetic populations: the mediating role of insulin resistance. Front. Cell. Infect. Microbiol. 15:1598223. doi: 10.3389/fcimb.2025.1598223
Received: 22 March 2025; Accepted: 28 April 2025;
Published: 16 May 2025.
Edited by:
Rashu Barua, New York University, United StatesReviewed by:
Mohammad Rashedul Islam, University of Chittagong, BangladeshMd Saqline Mostaq, University of Louisiana at Monroe, United States
Copyright © 2025 Chen, Ni, You, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yahong Chen, Y2hlbnloQGVuemVtZWQuY29t; Jinshun Zhang, emhhbmdqaW5zaHVuQGVuemVtZWQuY29t
†These authors have contributed equally to this work
 Yi Chen
Yi Chen Bingqian Ni2†
Bingqian Ni2† Jinshun Zhang
Jinshun Zhang