- 1Department of Gynecology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, China
- 2Department of Oncology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- 3Department of Pathology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, China
- 4Department of Epidemiology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, China
Background: Coexistent cervical intraepithelial neoplasia (CIN) and vaginal intraepithelial neoplasia (VaIN) is problematic, posing challenges for patient management. This study focused on the clinical characteristics of coexistent CIN 2/3 and VaIN (all degrees), evaluating the proclivity for disease recurrence/persistence at 6 months after treatment.
Methods: A retrospective case–control study of women treated for coexistent CIN 2/3 and VaIN (CE group) was undertaken between January 2018 and December 2020. During the same period, women with CIN 2/3 only were selected chronologically (1:2 ratio) for comparison (sCIN group). A loop electrosurgical excision procedure (LEEP) was the standard treatment for CIN 2/3, performing electrofulguration of VaIN in tandem. First follow-up visits at 6 months thereafter entailed testing for human papillomavirus (HPV). Univariate and multivariate analyses served to assess pertinent risk factors.
Results: There were 91 CE group members, each treated for coexistent CIN 2/3 and VaIN (VaIN 1, 35; VaIN 2/3, 56). Age ≥50 years (OR = 3.362, 95% CI: 1.421–7.954) emerged as an independent risk factor for coexistent disease. Positive margins and persistent high-risk HPV (HR-HPV) infection after treatment were more common in the CE (vs. sCIN) group (p = 0.012 and p < 0.001, respectively), as was recurrent/persistent high-grade disease (17.6% vs. 2.2%; p < 0.001). In the CE group, persistent HR-HPV infection 6 months after treatment (OR = 21.320, 95% CI: 2.509–181.188) was the sole independent risk factor for disease recurrence/persistence at 6 months.
Conclusions: Comprehensive vaginal wall examinations are warranted for women with CIN 2/3, particularly those >50 years old. Close follow-up by HPV test is also indicated if CIN 2/3 and VaIN coexist, given a heightened incidence of recurrent/persistent disease.
Introduction
Cervical cancer is the fourth most common female-related cancer worldwide and represents a significant health problem for women (Sung et al., 2021). Early identification and treatment of cervical intraepithelial neoplasia (CIN) is critical for cervical cancer prevention (Palumbo et al., 2023). High-risk human papillomavirus (HR-HPV) infections, which are linked to precancerous lesions or invasive cancers of the cervix (Bogani et al., 2017), may similarly develop in vaginal and vulvar locations (Bertoli et al., 2019). In recent years, the incidence of vaginal intraepithelial neoplasia (VaIN) has risen (Zhang et al., 2016), with low-grade (VaIN 1) or high-grade (VaIN 2/3) lesions more often detected in the setting of past hysterectomy for cervical cancer or high-grade CIN (CIN 2/3) (Preti et al., 2020). Because VaIN may accompany CIN 2/3 (Perkins et al., 2020), delineating associated clinical characteristics may help alert clinicians to its potential development.
To treat high-grade CIN, a loop electrosurgical excision procedure (LEEP) or cold-knife conization (CKC) is usually recommended (Perkins et al., 2020). However, even if lesions are effectively removed as above (virtually eradicating HPV infection), the risk of recurrence and progression to cervical cancer is higher for such women, compared with others in the general population (Ouh et al., 2020). A recent study suggested that post-treatment HPV vaccination with the nonavalent vaccine (Gardasil 9) may facilitate viral clearance in women treated for HPV-related cervical lesions (Palumbo et al., 2025). However, vaccination uptake remains low in China. In terms of VaIN, the incidence of progression to vaginal squamous cell carcinoma ranges from 2% to 7% (Ratnavelu et al., 2013). Although VaIN 1 is not a preinvasive threat, its rate of spontaneous regression is lower in the presence (vs. absence) of CIN 2/3 or vulvar intraepithelial neoplasia (VIN) 2/3 (67% vs. 91%) (Kesic et al., 2023), and once identified, the premalignant nature of VaIN 2/3 requires intervention. Consequently, all grades of VaIN should be addressed when treating CIN 2/3.
A variety of treatment options have been adopted for managing VaIN, including topical agents, ablation, excision, and radiotherapy (Gurumurthy et al., 2020). Commonly used ablation therapies include CO2 laser vaporization, photodynamic therapy, and electrofulguration, the latter being a good choice for VaIN treatment (Chen et al., 2016). The use of the same device for LEEP and electrofulguration is a suitable approach in patients with coexistent CIN 2/3 and VaIN. However, women with VaIN are at high risk of recurrence (Kim et al., 2018). Despite simultaneous treatment, the ramifications of coexistent CIN 2/3 remain unclear in this regard, meriting further research.
The present study was undertaken to investigate the clinical characteristics of coexistent CIN 2/3 and VaIN, analyzing risk factors for disease recurrence/persistence at 6 months after treatment.
Materials and methods
Study population
This retrospective, case–control study involved women with histologically confirmed CIN 2/3, each subjected to LEEP between January 2018 and December 2020 at Liaoning Cancer Hospital and Institute of China Medical University. Among them, 91 displayed both CIN 2/3 and VaIN 1 or VaIN 2/3 (CE group), undergoing electrofulguration of vaginal lesions concurrently. Another 182 diagnosed with CIN 2/3 exclusively comprised the single CIN (sCIN) group, having been selected at random according to order of colposcopy-guided biopsy. Exclusion criteria were the following: (1) postoperative pathology upgraded to microinvasive or invasive carcinoma; (2) lack of follow−up data; (3) second conization or total hysterectomy within 3 months after LEEP; (4) conditions likely impacting HPV susceptibility or CIN progression, including autoimmune disorders, genetic disorders, or organ transplantation; and (5) pregnancy. The Ethics Committee of Liaoning Cancer Hospital and Institute approved our study protocol (reference number: 20230162). All subjects were duly informed, granting signed consent before submitting to colposcopy and operative procedures.
Clinical data
We collected demographic and clinicopathologic data on each patient, recording age at the time of conization, transformation zone type, pre- and postoperative liquid-based cytology (LBC) results, pre- and postoperative HPV infection status, conization outcomes (grade of dysplasia and margins), and follow-up biopsy histopathology. Using ThinPrep technology (Hologic, Marlborough, MA, USA), reporting of LBC results followed the 2014 Bethesda System guidelines. The Digene Hybrid Capture 2 (HC2) test (Qiagen, Hilden, Germany) provided semiquantitative measures of HR-HPV DNA content, equating positivity with a >1 ratio of relative light units to positive control cutpoint (RLU/PC). HPV DNA genotyping was achieved using the HPV GenoArray test kit (Hybribio Ltd, Kowloon Bay, Hong Kong), combining DNA amplification and flow-through hybridization techniques (Liu et al., 2010). Each sample was screened for a total of 21 HPV genotypes (6, 11, 16, 18, 31, 33, 35, 39, 42–45, 51–53, 56, 58, 59, 66, 68, and CP8304).
Colposcopy and biopsy techniques
Colposcopy was routinely undertaken in our Cervical Disease Clinic, examining the cervix, vagina, and vulva comprehensively. Any suspicious areas were subjected to biopsy under colposcopic guidance, acquiring multiple samples on occasion in areas of patchy involvement or across particularly broad lesions to exclude occult invasion. Colposcopic examinations were deferred if needed, pending treatment of cervical/vaginal infections or postmenopausal dystrophy (i.e., estrogenic therapy). Within the sCIN group, we biopsied vaginal wall at points unstained by Lugol’s iodine where VaIN might reside. Histologic sections of routinely processed tissues were independently assessed by two expert pathologists.
Operative procedures
All LEEP and electrofulguration operations were performed under the guidance of colposcopy. In subsequent pathologic examinations of cone specimens (marked at 12 o’clock), positive margins were those harboring CIN 1–3 or cancer at one or both resected edges (ectocervical or endocervical). We set the power rating of electrofulguration output to 30 W, placing the head of the ball electrode close to its surface target. Treatment range was 3 mm beyond the outer edge of lesion, at a depth of 1.0–1.5 mm.
Follow-up monitoring
Study enrollees regularly returned for first follow-up visits 6 months post-treatment, at which time each supplied samples for HC2 HPV DNA testing and LBC. Detectable abnormalities called for colposcopy-directed biopsy of the cervix or endocervical curettage. Disease recurrence/persistence was signaled by histologically confirmed CIN 2/3, VaIN 2/3, or both on post-treatment biopsies at 6 months.
Statistical analysis
We expressed non-normally distributed quantitative variables as median values and interquartile ranges (IQRs), using the Mann–Whitney U test to compare differences. Categorical data were shown as counts and percentages. To compare groups, Pearson’s χ2 test was employed, applying Fisher’s exact test to small-sized samplings. Logistic regression served to generate odds ratios (ORs) and 95% confidence intervals (CIs). Independent risk factors were identified through multivariate logistic regression. All computations were driven by standard software (SPSS v22.0; IBM Corp, Armonk, NY, USA), with significance set at p < 0.05.
Results
Population characteristics
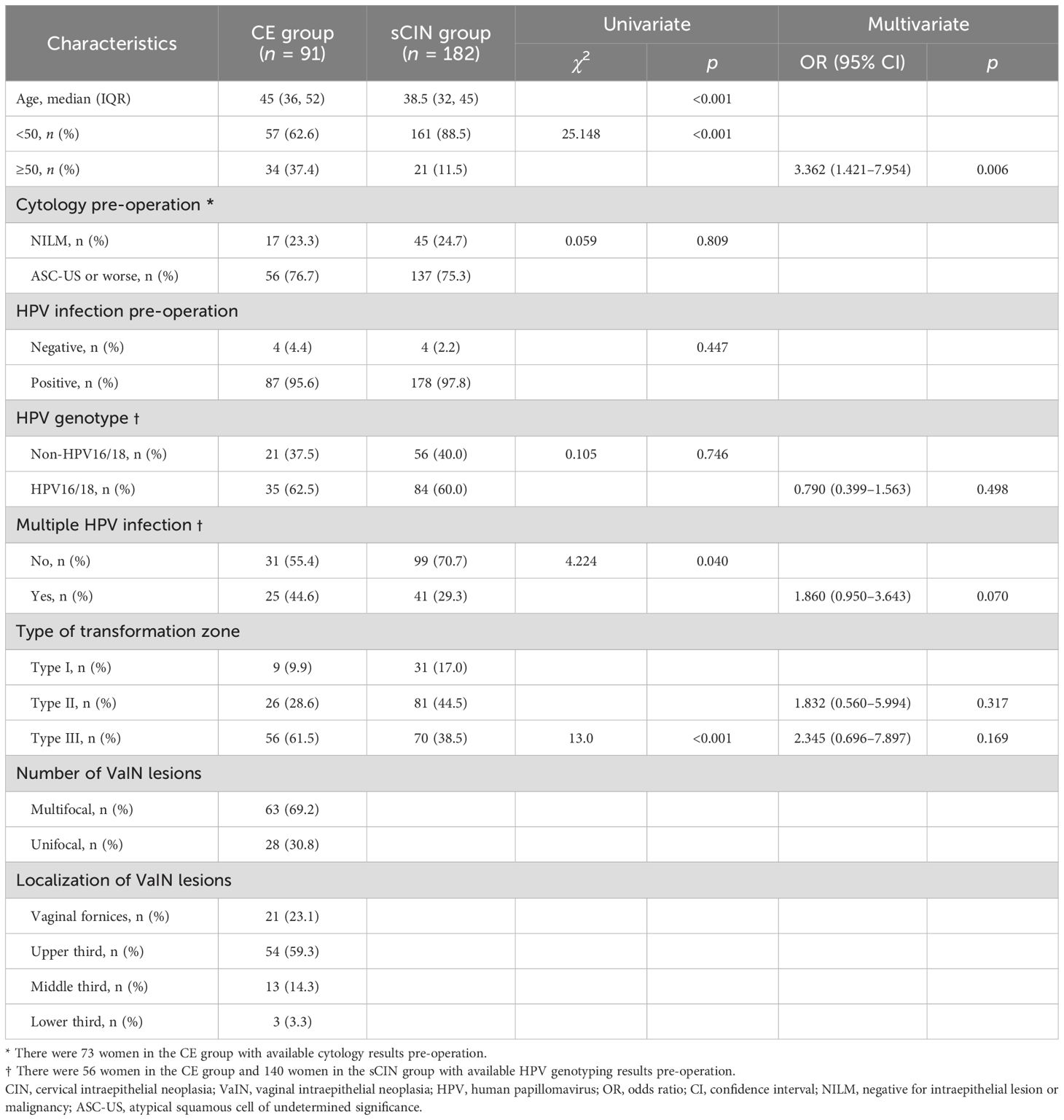
A total of 91 women qualified for the CE group, demonstrating coexistent CIN 2/3 and VaIN (VaIN 1, 35; VaIN 2/3, 56). In the sCIN group, there were 182 women with CIN 2/3 only. The clinical characteristics of the study population are shown by group in Table 1. Median age in the CE group (45 years, IQR: 36–52; range 21–68 years) significantly surpassed that determined for the sCIN group (38.5 years, IQR: 32–45; range, 22–62 years; p < 0.001), with significantly more women ≥50 years old by comparison (37.4% vs. 11.5%; p < 0.001). Results of HPV genotyping, which were available in part (CE group, 56; sCIN group, 140), showed similarity in terms of HPV16/18 infection prevalence (62.5% vs. 60.0%; p = 0.746). However, multiple HPV infections were significantly more common in the CE (vs. sCIN) group (44.6% vs. 29.3%; p = 0.04), with type III cervical transformation zones predominating (61.5% vs. 38.5%; p < 0.001). VaIN found in CE group members initially was largely multifocal (63/91, 69.2%), far less inclined to unifocal involvement (28/91, 30.8%). VaIN lesions were confined to vaginal fornices or the upper one-thirds of vaginal canals in a majority (75/91, 82.4%) of women.
Multivariate analysis succeeded in identifying age ≥50 years (OR = 3.362, 95% CI: 1.421–7.954) as the sole independent risk factor for coexistent CIN 2/3 and VaIN, failing to implicate HPV genotype, multiple HPV infections, or type III transformation zone.
Disease recurrence/persistence 6 months after treatment
Women of both groups presented for first follow-up visits 6 months after treatment. Clinicopathologic characteristics following therapy are listed by group in Table 2. The margin positivity rate was significantly higher for the CE (vs. sCIN) group (33.0% vs. 19.2%; p = 0.012). At this time, persistent HR-HPV infection was also significantly more common in CE (vs. sCIN) group members (48.4% vs. 23.1%; p < 0.001), mirrored by the corresponding rates of recurrent/persistent high-grade disease [17.6% (16/91) vs. 2.2% (4/182); p < 0.001]. However, there were no instances of progression to invasive cancer. In the CE group, 12 women experienced recurrent/persistent disease of both cervix and vagina, whereas vaginal lesions alone were noted in four others. In contrast, recurrent/persistent lesions of the cervix only presented in three women of the sCIN group, with one exhibiting both cervical and vaginal lesions.
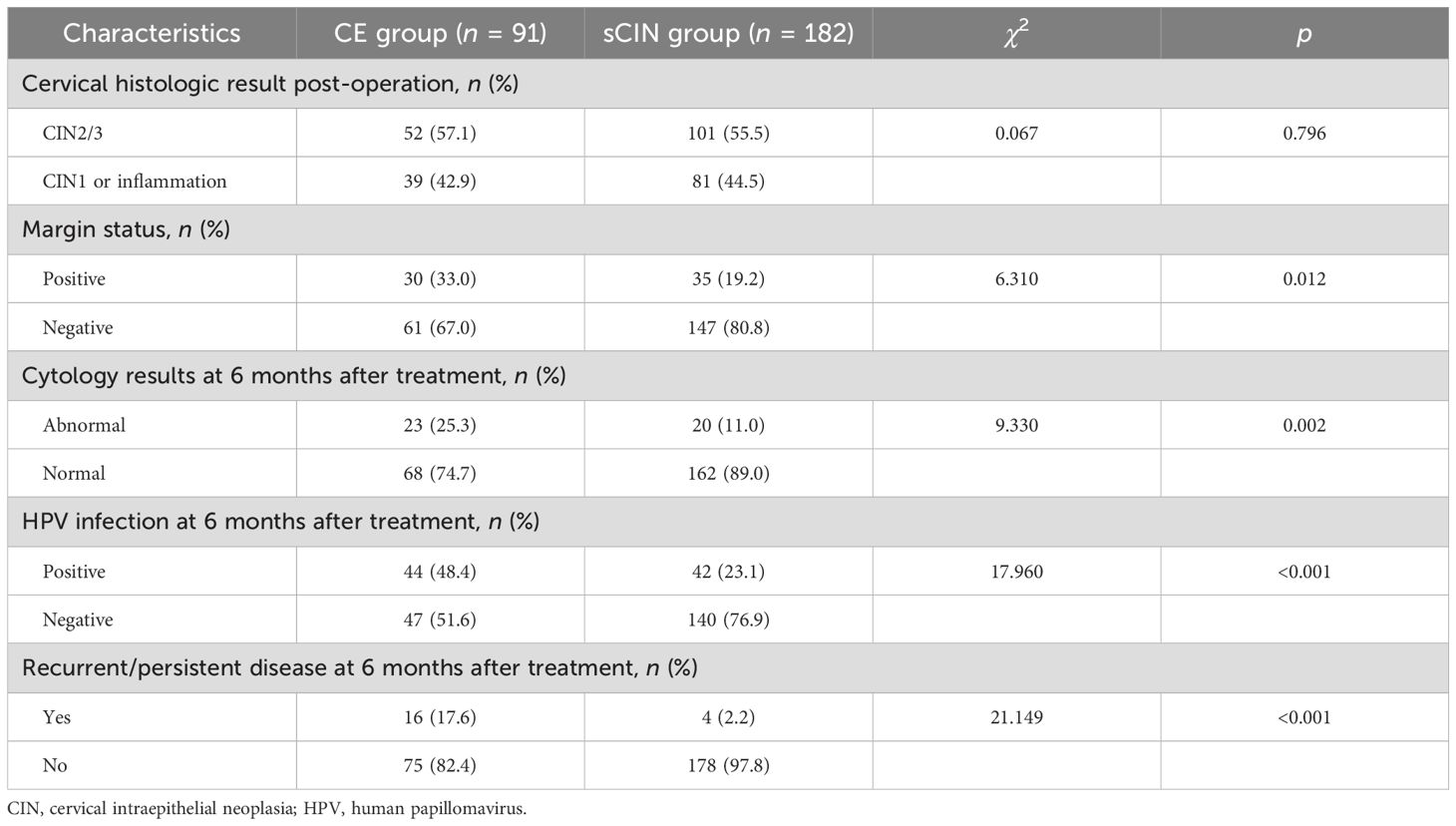
Table 2. Comparisons of the clinicopathological factors after treatment between the CE group and the sCIN group.
Risk factors for disease recurrence/persistence 6 months after treatment (CE group)
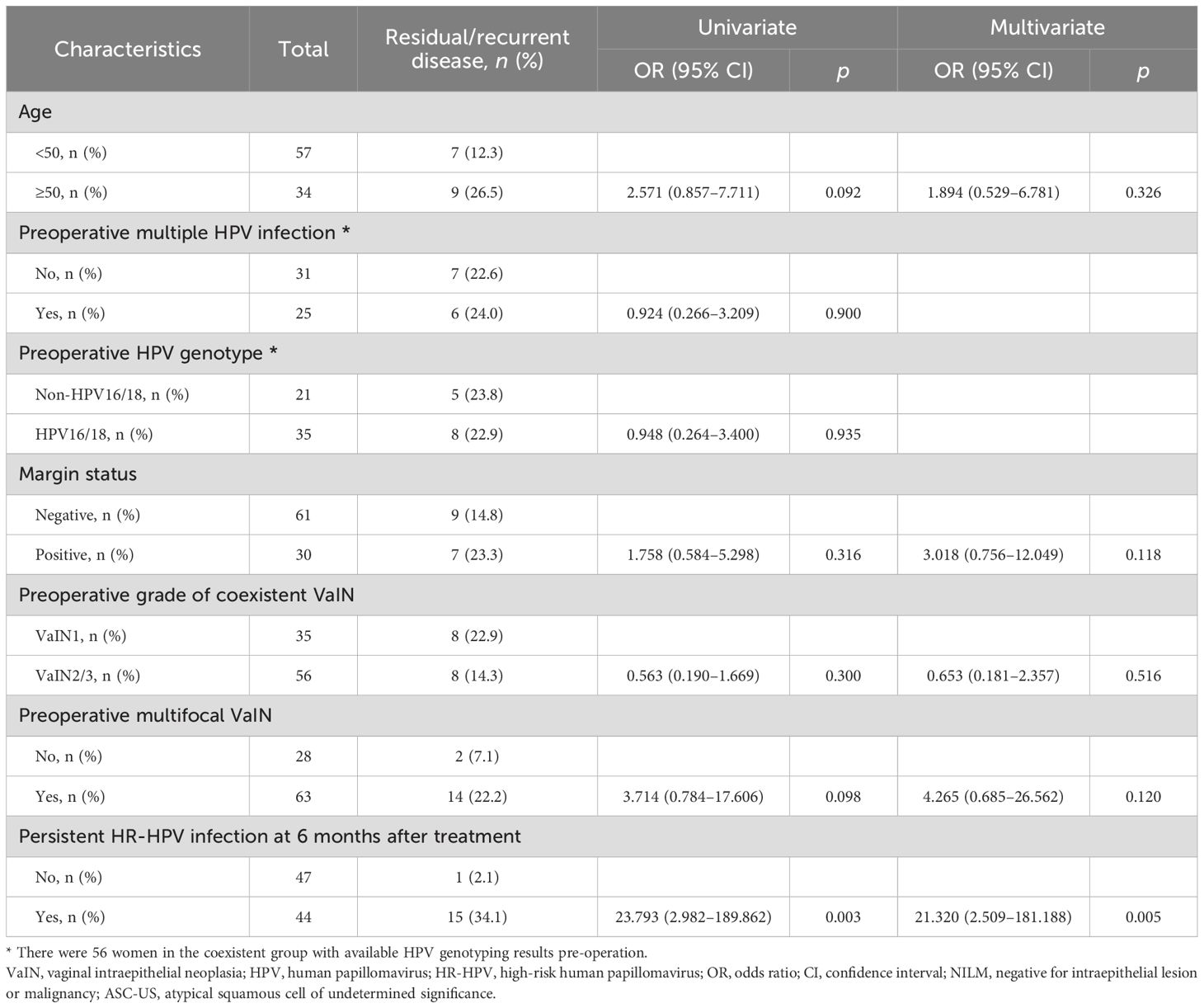
In univariate logistic regression analysis, persistent HR-HPV infection 6 months after treatment (OR = 23.793, 95% CI: 2.982–189.862) emerged as the sole variable associated with post-treatment disease recurrence/persistence (Table 3). Age, a multiplicity of preoperative HPV infections, HPV genotype, margin status, preoperative grade of coexistent VaIN, and preoperative multi- or unifocal VaIN were unrelated factors.
Multivariate analysis was carried out as well, adjusting for potential confounding effects (i.e., age, margin status, preoperative grade of coexistent VaIN, and preoperative multi- or unifocal VaIN). The only risk factor identified for post-treatment disease recurrence/persistence was HR-HPV infection 6 months after treatment (OR = 21.320, 95% CI: 2.509–181.188) (Table 3).
Discussion
Results of the present study confirm that the coexistence of CIN 2/3 and VaIN is a rather common event. The one related risk factor that emerged in the course of our analysis was age ≥50 years. During follow-up, a poorer prognosis was evident for the CE group by comparison, having shown an increased rate of margin positivity, greater persistence of HR-HPV infection, and higher risk of recurrent/persistent disease 6 months after treatment. In fact, persistent HR-HPV infection 6 months after treatment proved to be an independent risk factor for disease recurrence/persistence at 6 months in the CE group. Compared with the sCIN group, CE group members were more prone to recurrent/persistent vaginal wall lesions.
In the past, hysterectomy due to CIN 2/3 and stage I cervical cancer has been the chief issue driving the clinical awareness of VaIN. However, it is now apparent that CIN and VaIN often coexist, reported at rates of 2.2%–15.7% in previous studies (Zhang et al., 2016; Gonzalez-Bosquet et al., 2017) and increasingly raising concerns. Previous studies have linked coexisting CIN and VaIN to older age, multiple HPV infections, high HPV viral load, HPV16 infection, and immunosuppression (Gonzalez-Bosquet et al., 2017; Zhang et al., 2022; Cho et al., 2021). Consistent with these findings, our study identified age ≥50 years and multiple HPV infections as risk factors; however, only age ≥50 years remained an independent predictor. This association may reflect reduced estrogen levels and age-related immunologic decline. Thinning of the vaginal epithelium in older women may increase vulnerability to microtrauma, thereby facilitating HPV infection and impairing viral clearance (Zhang et al., 2024).
Vaginal colposcopy poses greater challenges than cervical colposcopy due to the nonspecific and variable appearance of VaIN on colposcopic examination (Sopracordevole et al., 2018). Notably, occult invasive carcinoma has been detected in 2.6%–5.5% of cases (Chai et al., 2020; Sopracordevole et al., 2020), underscoring the importance of obtaining multiple biopsies, particularly from areas with the most suspicious colposcopic features.
The management of VaIN remains challenging due to the proximity of adjacent organs such as the bladder and bowel and the complexity of treating multifocal or extensive lesions (Gurumurthy et al., 2020). While surgical excision may be preferred, its utility is limited by the often patchy and multifocal nature of VaIN. In our cohort, multifocal VaIN was present in 69.2% of women with coexistent disease. Alternatively, ablation is considered effective for VaIN 2/3, especially for multifocal lesions, and is associated with low complication rates (Cho et al., 2021; Sopracordevole et al., 2020). Both ablative and excisional approaches have demonstrated comparable efficacy (Bogani et al., 2018). Furthermore, the equipment used for LEEP can also be applied to electrofulguration, which has proven to be a safe and effective treatment with favorable cure rates and minimal complications (Kesic et al., 2023).
Ahead of ablative procedures, lesions should be fully visible and adequately biopsied to exclude invasion. VaIN 1 in the presence (vs. absence) of CIN 2/3 carries a lower spontaneous regression rate due to the different biologic behavior (Kesic et al., 2023). In our study population, we treated multiple foci of VaIN 1 and CIN 2/3 concurrently to achieve full eradication and reduce the subsequent recurrent/persistent disease rate in CE group members. A prospective study is needed going forward to better assess the benefit of this approach.
Even if lesions are effectively removed by various procedures, such as resection or ablation, some risk of persistent HR-HPV infection remains post-treatment in affected patients. Several earlier studies examining HR-HPV status following LEEP for CIN 2/3 have cited rates of persistent HPV at 6 months in the range of 14.3%–33.0% (Kim et al., 2010; Pirtea et al., 2016). The testing we required during first follow-up visits (6 months post-treatment) confirmed a significantly higher rate of persistent HR-HPV infection in the CE (vs. sCIN) group (48.4% vs. 23.1%; p < 0.001). This outcome perhaps reflects a pathogenesis inherent in multicentric lesions of lower genital tract that is marked by divergent cervical and vaginal proclivities for specific HPV genotypic infections (Zhang et al., 2021). Currently, overall rates reported in the literature for CIN 2+ recurrences after LEEP range from 5% to 25% (Ouh et al., 2020; Fernández-Montolí et al., 2020). Recurrences of VaIN are also quite frequent (10% to 42%), despite standard treatment (Field et al., 2020; Dodge et al., 2001), and recurrence rates for multicentric lesions tend to exceed those observed for isolated CIN or VaIN involvement (Ait Menguellet et al., 2007). In our hands, the rate of high-grade lesion recurrence/persistence in CE group members was 17.6% during follow-up, readily surpassing that of the sCIN group (2.2%; p < 0.001). Hence, it is our view that women with coexistent CIN 2/3 and VaIN should be closely monitored during post-treatment periods.
Predicting recurrence expeditiously after surgery is vital. Recurrence rates are ostensibly influenced by menopause, lesion size, smoking habit, CIN detected at margins, HIV positivity, and persistent HR-HPV infection (Bogani et al., 2018; Wu et al., 2016; Jing et al., 2018; Ricci et al., 2025). Although margin status is a convenient predictor of recurrence and a customary risk marker, it is not an acknowledged independent risk factor (Massad et al., 2013). HR-HPV testing has instead assumed a more critical role in postoperative follow-up (Ryu et al., 2012; Bruhn et al., 2022). The 2019 guidelines of the American Society of Colposcopy and Cervical Pathology (ASCCP) recommended HPV-based testing at first follow-up (preferably 6 months after treatment of high-grade CIN), regardless of margin status (Perkins et al., 2020). There is mounting evidence that HR-HPV persistence is pivotal in predicting recurrence after conization for CIN 2/3 (Bogani et al., 2018; Gosvig et al., 2015; Du et al., 2013). Unfortunately, no data are available as yet to support this premise in the context here. Our findings with respect to the CE group seem to corroborate HR-HPV persistence as an independent risk factor for disease recurrence/persistence. Multifocality is a notable characteristic of VaIN. A recent study identified multifocality as an independent risk factor for recurrence or persistence of VaIN following laser vaporization (Boonlikit and Tangterdchanakit, 2024). However, in the present study, multifocality was not significantly associated with recurrence or persistence (OR = 3.714, 95% CI: 0.784–17.606). Further research into broader populations is needed for validation.
Ultimately, we have demonstrated that women with coexistent CIN 2/3 and VaIN are more apt to experience vaginal wall recurrence/persistence. In the sCIN group, a solitary instance of post-treatment coexistent disease was apparent. It is feasible that this specific virus was latent within the vaginal wall, underscoring the need for full examinations during follow-up colposcopy.
The main strengths of this study include its large roster of enrollees and the substantial volume of comprehensive data, relative to other published reports. Moreover, this study is among the few to examine the clinical characteristics of coexisting CIN 2/3 and VaIN, thereby highlighting the need for increased clinical attention to women presenting with both lesions.
Nevertheless, several limitations must be acknowledged. First, the retrospective and single-center design necessitates cautious interpretation of the findings. Second, because of the lack of a prospective cohort, some confounding variables such as smoking status, immunosuppression, and HPV vaccination status could not be assessed, limiting causal inferences. Third, although HC2 assays are commonly used during follow-up to detect persistent HR-HPV infections, they do not allow genotypic confirmation of viral persistence. Additionally, we were unable to distinguish between cervical and vaginal HPV infections, precluding the assessment of concordance between sites. The short follow-up period, while useful for early observations, limits the ability to draw conclusions regarding long-term recurrence. Notably, HR-HPV infection at 6 months post-treatment (OR = 21.320; 95% CI: 2.509–181.188) was the only identified risk factor for post-treatment disease recurrence/persistence. However, the wide CI reflects substantial uncertainty, likely due to the small number of recurrence/persistence events. Future studies with prospective larger cohorts and extended follow-up are needed to enhance the precision of risk estimates and validate these findings.
Conclusions
In conclusion, more attention must be devoted to coexistent CIN and VaIN, because an undetected vaginal reservoir may impact HPV clearance rates after treatment. Compared with the sCIN group, the CE group exhibited a significantly higher rate of disease recurrence or persistence. Notably, persistent HR-HPV infection at 6 months post-treatment was identified as an independent risk factor for recurrence/persistence in the CE group. These findings underscore the importance of close post-treatment monitoring using HPV-based testing after LEEP and electrofulguration for coexistent CIN 2/3 and VaIN. Surveillance colposcopy should entail full assessment of both cervix and vagina. Further prospective studies are needed in this specific realm, collecting data long-term to better evaluate the clinical implications of the above risk factors for disease recurrence/persistence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethical committee of Liaoning Cancer Hospital and Institute. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. LW: Conceptualization, Data curation, Methodology, Writing – review & editing. YZ: Data curation, Writing – review & editing. GL: Methodology, Writing – review & editing. DW: Conceptualization, Funding acquisition, Methodology, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program (2017YFC0114204).
Acknowledgments
The authors would like to thank Miss Yumei Xiu, Miss Dan Sun, and Miss Xiaopan Hu for their excellent assistance in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ait Menguellet, S., Collinet, P., Houfflin Debarge, V., Nayama, M., Vinatier, D., and Leroy, J. L. (2007). Management of multicentric lesions of the lower genital tract. Eur. J. Obstet Gynecol Reprod. Biol. 132, 116–120. doi: 10.1016/j.ejogrb.2006.04.011
Bertoli, H. K., Rasmussen, C. L., Sand, F. L., Albieri, V., Norrild, B., Verdoodt, F., et al. (2019). Human papillomavirus and p16 in squamous cell carcinoma and intraepithelial neoplasia of the vagina. Int. J. Cancer 145, 78–86. doi: 10.1002/ijc.32078
Bogani, G., Ditto, A., Martinelli, F., Mosca, L., Chiappa, V., Rossetti, D., et al. (2018). LASER treatment for women with high-grade vaginal intraepithelial neoplasia: A propensity-matched analysis on the efficacy of ablative versus excisional procedures. Lasers Surg. Med. 50, 933–939. doi: 10.1002/lsm.22941
Bogani, G., Taverna, F., Lombardo, C., Borghi, C., Martinelli, F., Signorelli, M., et al. (2017). Retrospective study of the influence of HPV persistence on outcomes among women with high-risk HPV infections and negative cytology. Int. J. Gynaecol Obstet 138, 62–68. doi: 10.1002/ijgo.12170
Boonlikit, S. and Tangterdchanakit, P. (2024). Multicentricity and the risk of recurrence/persistence after laser vaporization for high-grade vulvar and vaginal intraepithelial neoplasia. World J. Oncol. 15, 90–99. doi: 10.14740/wjon1743
Bruhn, L. V., Hyldig, N., and Schledermann, D. (2022). HPV test as test of cure after conization for CIN2+: A nationwide register-based cohort study. J. Low Genit Tract Dis. 26, 287–292. doi: 10.1097/LGT.0000000000000693
Chai, Y. K., Cheung, S. Y. C., and Chan, K. K. L. (2020). Outcome of vaginal stripping for vaginal intraepithelial neoplasia: A 20-year observational study. J. Obstet Gynaecol Res. 46, 2511–2517. doi: 10.1111/jog.14482
Chen, L., Hu, D., Xu, S., Wang, X., Chen, Y., Lv, W., et al. (2016). Clinical features, treatment and outcomes of vaginal intraepithelial neoplasia in a Chinese tertiary centre. Ir J. Med. Sci. 185, 111–114. doi: 10.1007/s11845-014-1231-z
Cho, H. W., Hong, J. H., and Lee, J. K. (2021). Detection of high-risk human papillomavirus infection and treatment of high-grade vaginal intraepithelial neoplasia: A single-institution study. Int. J. Gynaecol Obstet 154, 227–232. doi: 10.1002/ijgo.13583
Dodge, J. A., Eltabbakh, G. H., Mount, S. L., Walker, R. P., and Morgan, A. (2001). Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 83, 363–369. doi: 10.1006/gyno.2001.6401
Du, R., Meng, W., Chen, Z. F., Zhang, Y., Chen, S. Y., and Ding, Y. (2013). Post-treatment human papillomavirus status and recurrence rates in patients treated with loop electrosurgical excision procedure conization for cervical intraepithelial neoplasia. Eur. J. Gynaecol Oncol. 34, 548–551.
Fernández-Montolí, M. E., Tous, S., Medina, G., Castellarnau, M., García-Tejedor, A., and de Sanjosé, S. (2020). Long-term predictors of residual or recurrent cervical intraepithelial neoplasia 2–3 after treatment with a large loop excision of the transformation zone: a retrospective study. BJOG 127, 377–387. doi: 10.1111/1471-0528.15996
Field, A., Bhagat, N., Clark, S., Speed, T., and Razvi, K. (2020). Vaginal intraepithelial neoplasia: A retrospective study of treatment and outcomes among a cohort of UK women. J. Low Genit Tract Dis. 24, 43–47. doi: 10.1097/LGT.0000000000000502
Gonzalez-Bosquet, E., Mazarico, E., Lorente, N., and Gomez-Roig, M. D. (2017). Risk factors to develop multicentric lesions of the lower genital tract. Eur. J. Gynaecol Oncol. 38, 10–13.
Gosvig, C. F., Huusom, L. D., Deltour, I., Andersen, K. K., Duun-Henriksen, A. K., Madsen, E. M., et al. (2015). Role of human papillomavirus testing and cytology in follow-up after conization. Acta Obstet Gynecol Scand. 94, 405–411. doi: 10.1111/aogs.12601
Gurumurthy, M., Leeson, S., Tidy, J., and Cruickshank, M. E. (2020). UK national survey of the management of vaginal intraepithelial neoplasia. J. Obstet Gynaecol 40, 694–698. doi: 10.1080/01443615.2019.1652887
Jing, L., Dan, W., Zhunan, L., Ying, X., and Yi, C. (2018). Residual lesions in uterine specimens after loop electrosurgical excision procedure in patients with CIN. Arch. Gynecol Obstet 298, 805–812. doi: 10.1007/s00404-018-4881-7
Kesic, V., Carcopino, X., Preti, M., Vieira-Baptista, P., Bevilacqua, F., Bornstein, J., et al. (2023). The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD), and the European Federation for Colposcopy (EFC) consensus statement on the management of vaginal intraepithelial neoplasia. Int. J. Gynecol Cancer 33, 446–461. doi: 10.1136/ijgc-2022-004213
Kim, M. K., Lee, I. H., and Lee, K. H. (2018). Clinical outcomes and risk of recurrence among patients with vaginal intraepithelial neoplasia: a comprehensive analysis of 576 cases. J. Gynecol Oncol. 29, e6. doi: 10.3802/jgo.2018.29.e6
Kim, Y. T., Lee, J. M., Hur, S. Y., Cho, C. H., Kim, Y. T., Kim, S. C., et al. (2010). Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int. J. Cancer 126, 1903–1909. doi: 10.1002/ijc.24794
Liu, S. S., Leung, R. C., Chan, K. K., Cheung, A. N., and Ngan, H. Y. (2010). Evaluation of a newly developed GenoArray human papillomavirus (HPV) genotyping assay and comparison with the Roche Linear Array HPV genotyping assay. J. Clin. Microbiol. 48, 758–764. doi: 10.1128/JCM.00989-09
Massad, L. S., Einstein, M. H., Huh, W. K., Katki, H. A., Kinney, W. K., Schiffman, M., et al. (2013). 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 121, 829–846. doi: 10.1097/AOG.0b013e3182883a34
Ouh, Y. T., Cho, H. W., Kim, S. M., Min, K. J., Lee, S. H., Song, J. Y., et al. (2020). Risk factors for type-specific persistence of high-risk human papillomavirus and residual/recurrent cervical intraepithelial neoplasia after surgical treatment. Obstet Gynecol Sci. 63, 631–642. doi: 10.5468/ogs.20049
Palumbo, M., Della Corte, L., Ronsini, C., Guerra, S., Giampaolino, P., and Bifulco, G. (2023). Surgical treatment for early cervical cancer in the HPV era: state of the art. Healthcare (Basel) 11, 2942. doi: 10.3390/healthcare11222942
Palumbo, M., Lavitola, G., Di Filippo, C., Foreste., V., Granata, M., Imperatore, O., et al. (2025). Impact of Human papillomavirus 9-valent vaccine on viral clearance after surgical treatment: A single-center retrospective observational study. Eur. J. Obstet Gynecol Reprod. Biol. 310, 113994. doi: 10.1016/j.ejogrb.2025.113994
Perkins, R. B., Guido, R. S., Castle, P. E., Chelmow, D., Einstein, M. H., Garcia., F., et al. (2020). 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J. Low Genit Tract Dis. 24, 102–131. doi: 10.1097/LGT.0000000000000525
Pirtea, L., Grigoraş, D., Matusz, P., Pirtea, M., Moleriu, L., Tudor, A., et al. (2016). Human papilloma virus persistence after cone excision in women with cervical high grade squamous intraepithelial lesion: A prospective study. Can. J. Infect. Dis. Med. Microbiol. 2016, 3076380. doi: 10.1155/2016/3076380
Preti, M., Rosso, S., Micheletti, L., Libero, C., Sobrato, I., Giordano, L., et al. (2020). Risk of HPV-related extra-cervical cancers in women treated for cervical intraepithelial neoplasia. BMC Cancer 20, 972. doi: 10.1186/s12885-020-07452-6
Ratnavelu, N., Patel, A., Fisher, A. D., Galaal, K., Cross, P., and Naik, R. (2013). High-grade vaginal intraepithelial neoplasia: can we be selective about who we treat? BJOG 120, 887–893. doi: 10.1111/1471-0528.12223
Ricci, C., Di Pumpo, M., Nicolotti, N., Capelli, G., Zannoni, G. F., Evangelista, M. T., et al. (2025). Major predictive factors for recurrence of CIN after treatment: an exploratory analysis towards a predictive model. Eur. Rev. Med. Pharmacol. Sci. 29, 23–29. doi: 10.26355/eurrev_202501_37056
Ryu, A., Nam, K., Kwak, J., Kim, J., and Jeon, S. (2012). Early human papillomavirus testing predicts residual/recurrence after LEEP. J. Gynecol Oncol. 23, 217–225. doi: 10.3802/jgo.2012.23.4.217
Sopracordevole, F., Barbero, M., Clemente, N., Fallani, M. G., Cattani, P., Agarossi, A., et al. (2018). Colposcopic patterns of vaginal intraepithelial neoplasia: a study from the Italian Society of Colposcopy and Cervico-Vaginal Pathology. Eur. J. Cancer Prev. 27, 152–157. doi: 10.1097/CEJ.0000000000000287
Sopracordevole, F., Clemente, N., Di, Giuseppe, J., Barbero, M., Fallani, M. G., Cattani, P., et al. (2020). Clinical characteristics and long-term follow-up of patients treated for high-grade vaginal intraepithelial neoplasia: results from a 20-year survey in Italy. J. Low Genit Tract Dis. 24, 381–386. doi: 10.1097/LGT.0000000000000567
Sung, H., Ferlay, J., Siegel., R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Wu, J., Jia, Y., Luo, M., and Duan, Z. (2016). Analysis of residual/recurrence and its risk factors after loop electrosurgical excision procedure for high-grade cervical intraepithelial neoplasia. Gynecol Obstet Invest 81, 296–301. doi: 10.1159/000437423
Zhang, J., Chang, X., Qi, Y., Zhang, Y., and Zhang, S. (2016). A retrospective study of 152 women with vaginal intraepithelial neoplasia. Int. J. Gynaecol Obstet 133, 80–83. doi: 10.1016/j.ijgo.2015.08.014
Zhang, J., Liu, G., Cui, X., Yu, H., and Wang, D. (2021). Human papillomavirus genotypes and the risk factors associated with multicentric intraepithelial lesions of the lower genital tract: a retrospective study. BMC Infect. Dis. 21, 554. doi: 10.1186/s12879-021-06234-0
Zhang, Y. Y., Xia, R., Chen, D., and Zhang, X. (2022). Analysis of related factors of cervical intraepithelial neoplasia complicated with vaginal intraepithelial neoplasia. Clin. Transl. Oncol. 24, 902–908. doi: 10.1007/s12094-021-02739-x
Keywords: high-grade cervical intraepithelial neoplasia, vaginal intraepithelial neoplasia, persistent high-risk human papillomavirus infection, disease recurrence, loop electrosurgical excision procedure
Citation: Zhang J, Wu L, Zhu Y, Liu G and Wang D (2025) Human papillomavirus infection and disease recurrence/persistence after treatment for women of high-grade cervical intraepithelial neoplasia with coexisting vaginal intraepithelial neoplasia. Front. Cell. Infect. Microbiol. 15:1602216. doi: 10.3389/fcimb.2025.1602216
Received: 29 March 2025; Accepted: 17 June 2025;
Published: 09 July 2025.
Edited by:
Maria Isaguliants, Riga Stradiņš University, LatviaReviewed by:
Mario Palumbo, Federico II University Hospital, ItalyAnna Stasulane, Riga Stradiņš University, Latvia
Copyright © 2025 Zhang, Wu, Zhu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danbo Wang, d2FuZ2RhbmJvQGNhbmNlcmhvc3AtbG4tY211LmNvbQ==
†These authors share first authorship
 Jing Zhang
Jing Zhang Lina Wu2†
Lina Wu2† Yanmei Zhu
Yanmei Zhu Danbo Wang
Danbo Wang