- 1Department of Emergency, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 2National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing, China
- 3Chongqing Key Laboratory of Child Rare Diseases in Infection and Immunity, Chongqing, China
- 4Department of Neonatology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 5Department of Clinical Laboratory, Children’s Hospital of Chongqing Medical University, Chongqing, China
Introduction: Sepsis remains a leading cause of mortality and morbidity worldwide. This study aimed to investigate the clinical characteristics of children with sepsis and septic shock, with emphasis to evaluate the predictive value of C-reactive protein (CRP), procalcitonin (PCT), and nucleated red blood cell (NRBC) count in pediatric sepsis and septic shock patients.
Methods: We included a total of 121 children, including 80 with sepsis and 41 with septic shock, who were admitted to the Children’s Hospital of Chongqing Medical University between January 2021 and June 2024.
Results: No significant differences in sex, age, weight, or basic diseases were observed between the sepsis and septic shock groups (P > 0.05). However, the laboratory findings showed significantly lower platelet counts and hemoglobin levels as well as higher CRP, PCT, NRBC, lactic acid, ALT, CK-MB, urea nitrogen, and APTT levels in the septic shock group (P < 0.05). Poorer outcomes were observed in the septic shock group, with higher rates of disease progression or death (63.4% vs. 31.2%, P < 0.05). ROC analysis showed that the combination of these three biomarkers achieved greater predictive accuracy (AUC = 0.956), outperforming CRP and PCT alone. Compared with those with sepsis, children with septic shock presented worse clinical and laboratory profiles, required more intensive treatments, and had poorer outcomes.
Conclussion: The inclusion of the NRBC count, combined with CRP and PCT, significantly increases the predictive efficacy of disease severity or progression, particularly indicative of septic shock, highlighting the potential of this combination for early diagnosis and management in pediatric patients.
Introduction
Sepsis represents a series of clinical syndromes that are caused by a dysregulated host response to infection, which leads to widespread inflammation, tissue damage, and potentially life-threatening multiple organ dysfunction (Singer et al., 2016; Schlapbach et al., 2024). Sepsis poses a significant global health challenge, with approximately 50 million cases reported annually, half of which occur in children under 19 years of age (Rudd et al., 2020). In 2017, the World Health Assembly formally recognized sepsis as a critical focus area for the next decade through a global resolution (Kissoon et al., 2017; Reinhart et al., 2017). Notably, a substantial number of sepsis-related deaths occur shortly after hospital admission, primarily due to severe septic shock and rapid organ failure (Härtel et al., 2020). Furthermore, children who survive sepsis often experience long-term complications, including organ dysfunction or neurodevelopmental impairments (Watson et al., 2024). Therefore, the rapid and accurate recognition of septic shock, followed by timely and effective interventions, is important.
Conventional blood cultures have long been considered the gold standard for diagnosing bacterial sepsis. However, their long processing times and susceptibility to contamination limit their clinical utility. As a result, researchers have used clinical symptoms and laboratory biomarkers to predict or diagnose sepsis and septic shock in pediatric patients. However, the clinical presentation of pediatric sepsis is often ambiguous, and current available laboratory markers lack sufficient sensitivity and specificity, presenting a significant challenge for timely diagnosis (Chiesa et al., 2004).
Current protocols for diagnosing pediatric sepsis and septic shock rely on clinical criteria and biomarkers, such as C-reactive protein (CRP), procalcitonin (PCT), and white blood cell (WBC) counts. While these markers have demonstrated utility in identifying inflammation and infection, their diagnostic accuracy remains limited, especially in pediatric patients. CRP, for instance, is a widely used acute-phase biomarker; however, its delayed elevation—requiring 24–48 hours to peak—reduces its sensitivity in detecting early infections.
Furthermore, CRP is non-specific and may be elevated in a variety of inflammatory conditions, limiting its diagnostic precision. Similarly, PCT, which is an immunologically active protein that is released during systemic inflammatory responses, plays a role in immune regulation and vascular contraction. Its levels increase within 3–4 hours of bacterial infection and peak at 24 hours, making it a more dynamic marker than CRP (Reinhart et al., 2000). However, PCT levels can also increase in noninfectious inflammatory conditions, such as severe trauma or burns, which reduces its specificity. In addition, the high cost and limited availability of PCT testing in primary hospitals and resource-limited settings restrict its routine application.
Recently, nucleated red blood cells (NRBCs) have emerged as a potential biomarker for pediatric sepsis. NRBCs are immature precursors of red blood cells that are not present in the circulation of healthy children (older than 28 days) or adults (healthy newborns have circulating NRBCs that rapidly disappear within a few weeks after birth) (Pedersen et al., 2022). NRBCs help diagnose hematological disorders related to erythropoiesis, anemia, or hemolysis. Recent studies have shown that this economical and easily accessible analysis has potential for the diagnosis and prognosis of critically ill adults and preterm infants (Christensen et al., 2011; Desai et al., 2012; Narcı et al., 2021). However, the value of NRBCs in predicting septic shock remains largely unknown.
Given the limitations of individual conventional biomarkers, combining multiple parameters may enhance diagnostic accuracy and prognostic evaluation. Therefore, this study aimed to analyze the predictive performance of individual biomarkers, including CRP, PCT, and NRBCs, in identifying septic shock. Furthermore, we aimed to compare the predictive performance of NRBCs with that of traditional biomarkers and evaluate the combined predictive utility of CRP, PCT, and NRBCs. By exploring the complementary roles of these biomarkers, we hope to provide a more reliable approach for the early diagnosis and management of pediatric sepsis.
Materials and methods
Study design and patients
This retrospective study was conducted at the Children’s Hospital of Chongqing Medical University (Chongqing, China). Pediatric patients who were diagnosed with sepsis at admission between January 2021 and June 2024 were included.
Patients were excluded if they met any of the following criteria: (1) age <28 days or adjusted gestational age <44 weeks; (2) diagnosed with blood disorders (e.g., leukemia, lymphoma, or anemia); (3) congenital or acquired immune deficiencies; or (4) hospitalization duration <72 hours with insufficient clinical documentation. Sepsis and septic shock were defined based on the criteria established by the International Consensus Conference on Pediatric Sepsis (Goldstein et al., 2005) as follows. A diagnosis of sepsis required (1) the presence of ≥2 systemic inflammatory response syndrome (SIRS) criteria; and (2) confirmed or suspected invasive infection. A diagnosis of septic shock required (1) the presence of age-specific SIRS criteria (≥2 items); (2) confirmed or suspected invasive infection; and (3) objective evidence of cardiovascular dysfunction. A diagnosis of cardiovascular dysfunction required documentation of (1) persistent hypotension (age-specific blood pressure values exceeding 2 standard deviations below normative means) following the administration of 40–60 mL/kg fluid resuscitation; (2) the requirement for vasopressor/inotropic support; and (3) objective evidence of hypoperfusion. The threshold of CRP and PCT is 8 mg/L and 0.05 ng/mL, respectively.
This study was approved by the Institutional Review Board of the Children’s Hospital of Chongqing Medical University (No. 133). This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Data collection
Clinical data were extracted from electronic medical records and the hospital’s centralized data platform. The collected variables included demographic characteristics, vasoactive medication use, length of hospitalization, and clinical outcomes. The laboratory parameters at admission included the following: (1) hematological indices: white blood cell count, platelet count, hemoglobin, NRBC; After blood was taken from patients, the number of NRBC was manually counted per high-power microscopic field under a microscope (×400). (2) biochemical markers: troponin, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), serum creatinine (Scr), potassium, sodium, calcium, and glucose, and lactate; (3) inflammatory markers: CRP and PCT; (4) coagulation profiles: international normalized ratio (INR) and activated partial thromboplastin time (APTT); and (5) microbiological data: pathogen culture results. The outcomes were stratified into two categories: (1) favorable: sepsis resolution with hospital discharge; (2) unfavorable: disease progression despite treatment, severe sequelae, treatment withdrawal, and sepsis-related mortality.
Statistical analysis
The data distribution for continuous measures are summarized using median values with interquartile ranges (IQRs). Comparative assessments of proportions across categorical parameters were conducted via χ2 or Fisher’s exact tests based on sample size adequacy. Between-group differences in non-normally distributed variables were evaluated through Wilcoxon rank-sum tests, whereas parametric comparisons utilized independent samples t tests. ROC curve analyses were performed for CRP, PCT, and NRBC individually and in combination, with optimal cutoffs derived by maximizing Youden’s index. All the computational workflows and visualizations were executed in SPSS 26.0 (IBM Corp., Armonk, NY, USA). P values less than 0.05 were predefined as statistically significant thresholds.
Results
Demographic characteristics
The present study enrolled 121 children, including 80 patients with sepsis and 41 patients with septic shock. The demographic characteristics of the study participants are presented in Table 1. No significant differences were observed between the sepsis and septic shock groups in terms of sex, age, weight, disease course or basic disease. A significantly greater proportion of patients in the septic shock group had a history of surgery compared to the sepsis group (73% vs. 35%, P < 0.05). The sources of infection varied between the two groups, with respiratory tract infections being more prevalent in the septic shock group than in the sepsis group (65.8% vs. 49%, P < 0.05), whereas bloodstream infections were less common (2.4% vs. 12.5%, P < 0.05).
Laboratory indicators
There were statistically significant differences in laboratory indicators between the sepsis and septic shock groups. In terms of blood parameters, platelet count and hemoglobin levels were significantly lower, whereas the CRP levels were significantly higher in the septic shock group than in the sepsis group (P < 0.05) (Figure 1). WBC counts were not significantly different between the two groups. In addition, positive blood culture rates were significantly higher in the septic shock group than in the sepsis group (36.5% vs. 12.5%) (P < 0.001) (Table 1). The most common etiology of blood cultures was Escherichia coli in both groups (Figure 2). For biochemical markers, PCT, NRBC, lactic acid, ALT, CK-MB, urea nitrogen, and APTT levels were significantly higher in the septic shock group than in the sepsis group (P < 0.05) (Figures 3, 4).
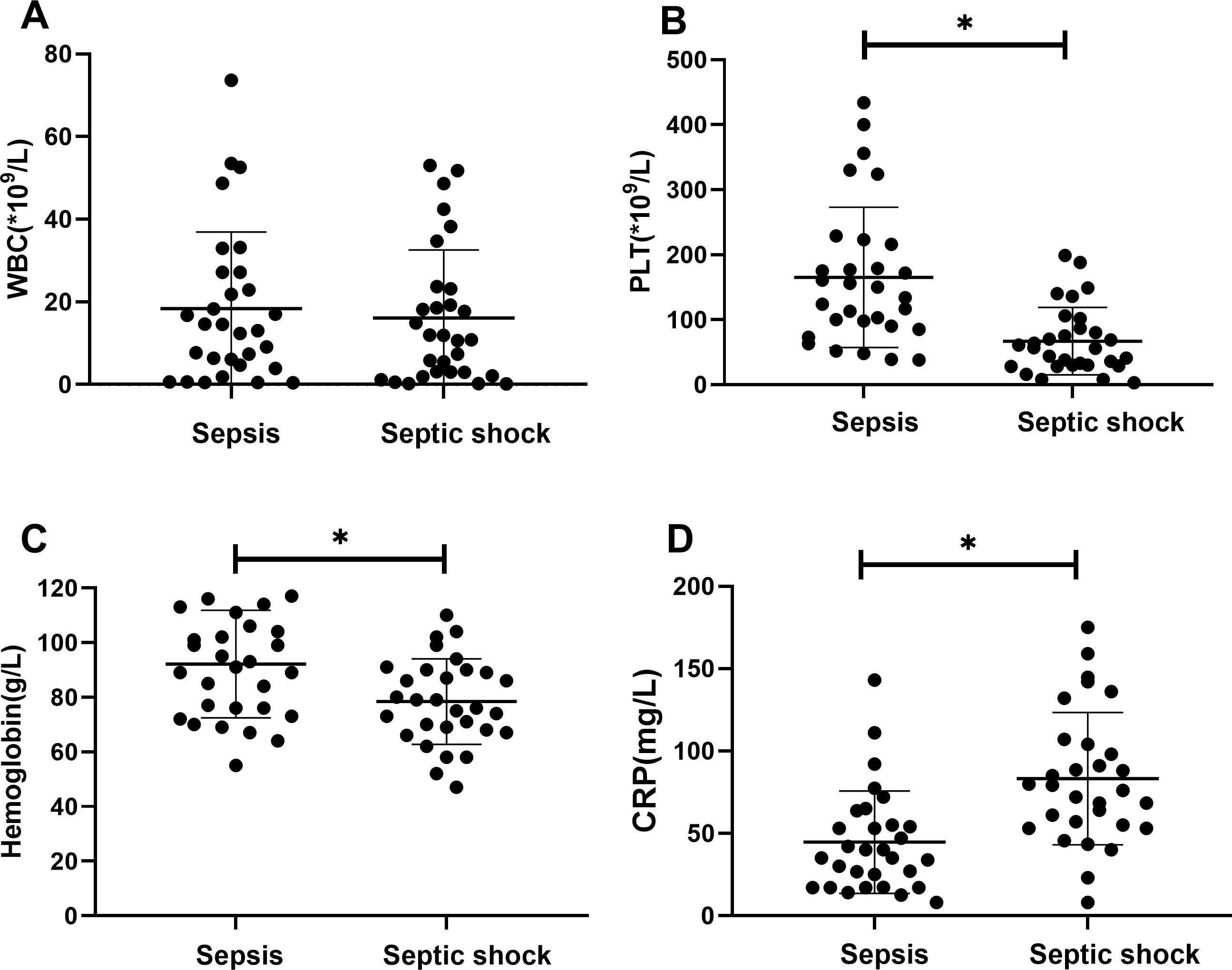
Figure 1. Blood index for septic children with and without nucleated red blood cells (NRBCs) before and after treatment. WBC (A), PLT (B), Hemoglobin (C) and CRP (D) levels present in the blood of the respective groups are shown. Asterisk denotes a statistically significant difference between sepsis group and septic shock group (P < 0.05). WBC, white blood cell; PLT, platelet; CRP, c-reactive protein.
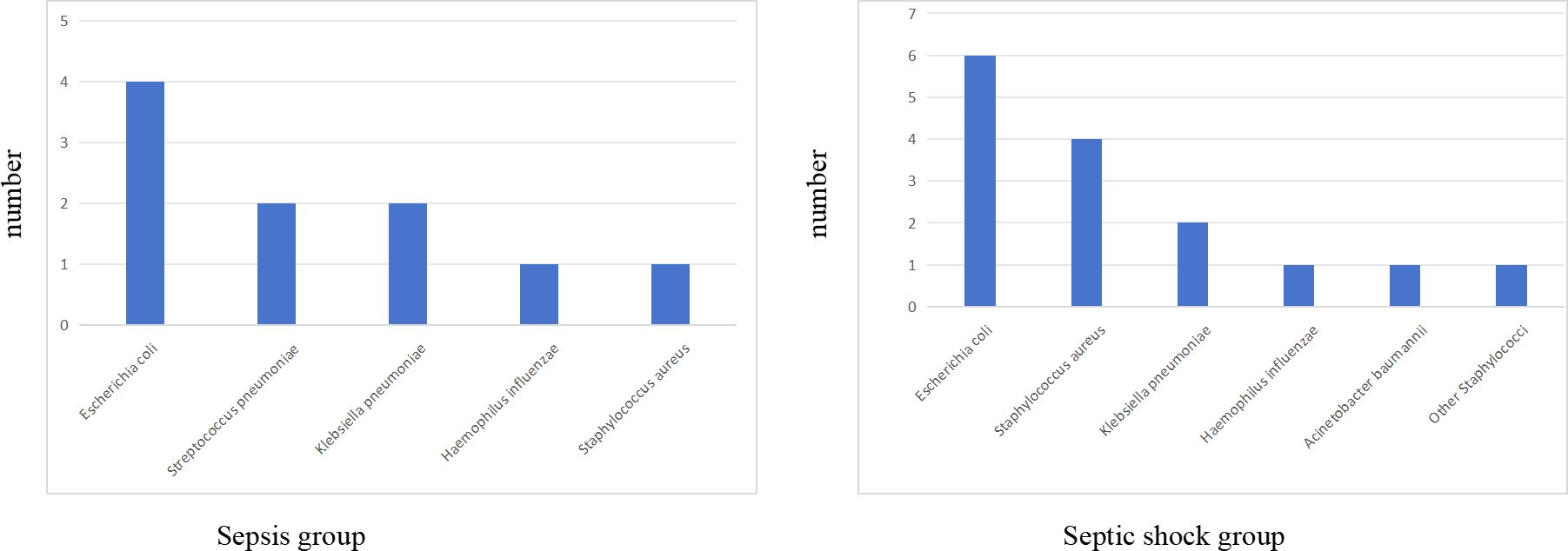
Figure 2. The bacterial species distribution of blood cultures in sepsis group and septic shock group.
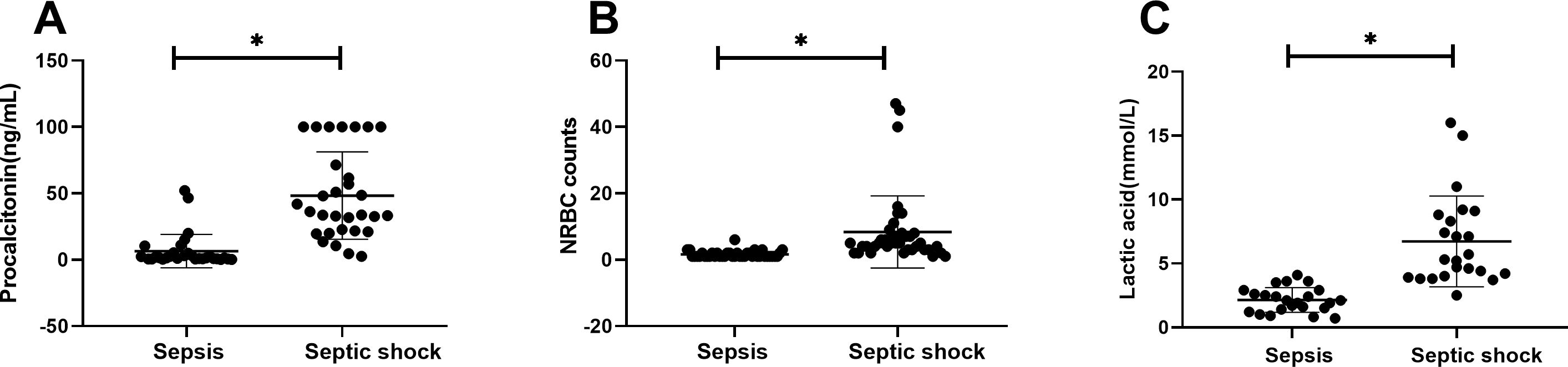
Figure 3. Laboratory values for children of sepsis group and septic shock group. Procalcitonin (A), NRBC counts (B) and lactic acid (C) levels present in the blood of the respective groups are shown. Asterisk denotes a statistically significant difference between sepsis group and septic shock group (P < 0.05). NRBC, nucleated red blood cell.
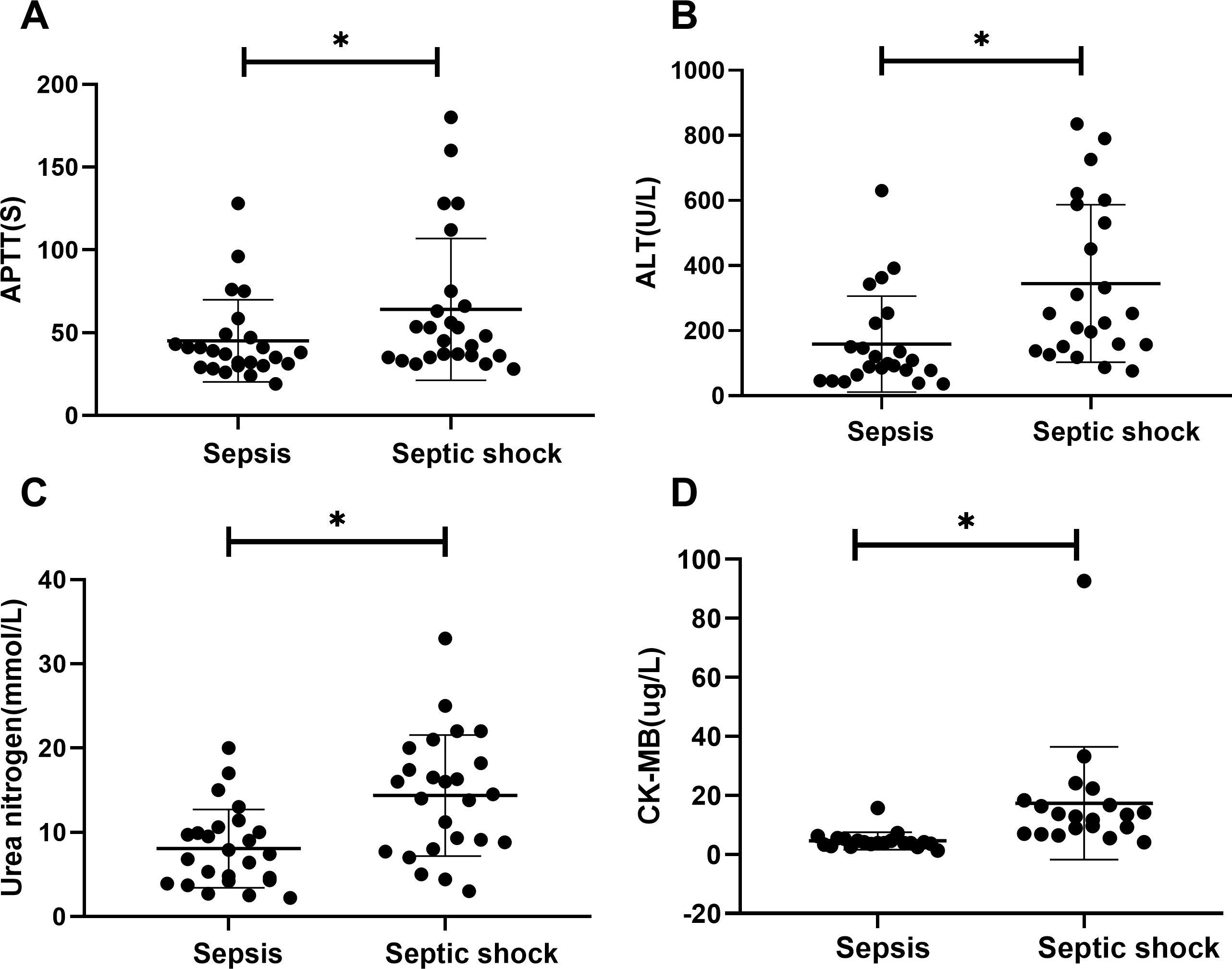
Figure 4. Laboratory values for children of sepsis group and septic shock group. APTT (A), ALT (B), urea nitrogen (C) and CK-MB (D) levels present in the blood of the respective groups are shown. Asterisk denotes a statistically significant difference between sepsis group and septic shock group (P < 0.05). APTT, activated partial thromboplastin time; ALT, alanine aminotransferase; CK-MB, creatine kinase muscle and brain isoenzyme.
Treatment and clinical outcomes in children with sepsis
The treatment of sepsis and septic shock followed the guidelines established by the International Consensus Conference on Pediatric Sepsis [14]. The proportion of patients requiring invasive mechanical ventilation was significantly higher in the septic shock group than in the sepsis group (100% vs. 27.5%, P < 0.001). Conversely, the proportion of patients requiring non-invasive or no respiratory support was significantly lower in the septic shock group than in the sepsis group (0% vs. 10%, P = 0.02; 0% vs. 62.5%, P < 0.001). Patients in the septic shock group were more likely to undergo blood transfusions (34/41, 83% vs. 45/80, 56%, P < 0.001) and hemopurification (28/41, 68.3% vs. 14/80, 17.5%, P < 0.001) (Table 2). The septic shock group had longer hospital stays than did the sepsis group (median 20.2 days vs. median 16.4 days, P < 0.05). Additionally, a greater proportion of patients in the sepsis group improved after treatment (68.8% vs. 36.6%, P < 0.05), whereas 63.4% of children in the septic shock group experienced disease progression or even death.
ROC curve analysis
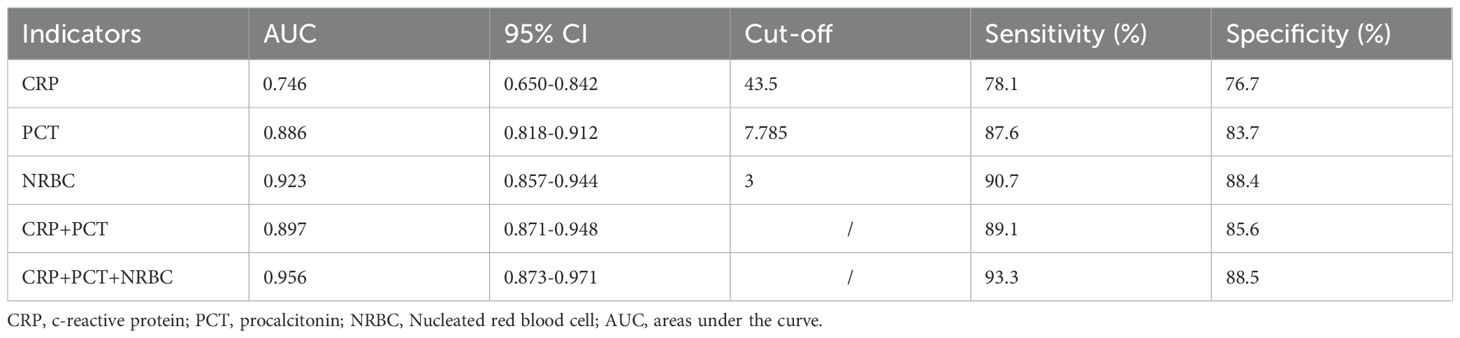
The diagnostic accuracy of CRP, PCT, and the NRBC for the prediction of septic shock in children was assessed. The areas under the curve (AUCs) of the receiver operating characteristic (ROC) curves of CRP, PCT and the NRBC count for the prediction of sepsis in children were 0.746 [95% confidence interval (CI) 0.650–0.842], 0.868 [95% CI 0.818–0.912] and 0.923 [95% CI 0.857–0.944], respectively. The AUC for the combination of CRP, PCT, and the NRBC count for the prediction of septic shock in children was 0.956 [95% CI 0.873–0.971] (Figure 5, Table 3). Compared to CRP or PCT alone, and combination of these two biomarkers, the combination of CRP, PCT and NRBC achieved a greater predictive accuracy.
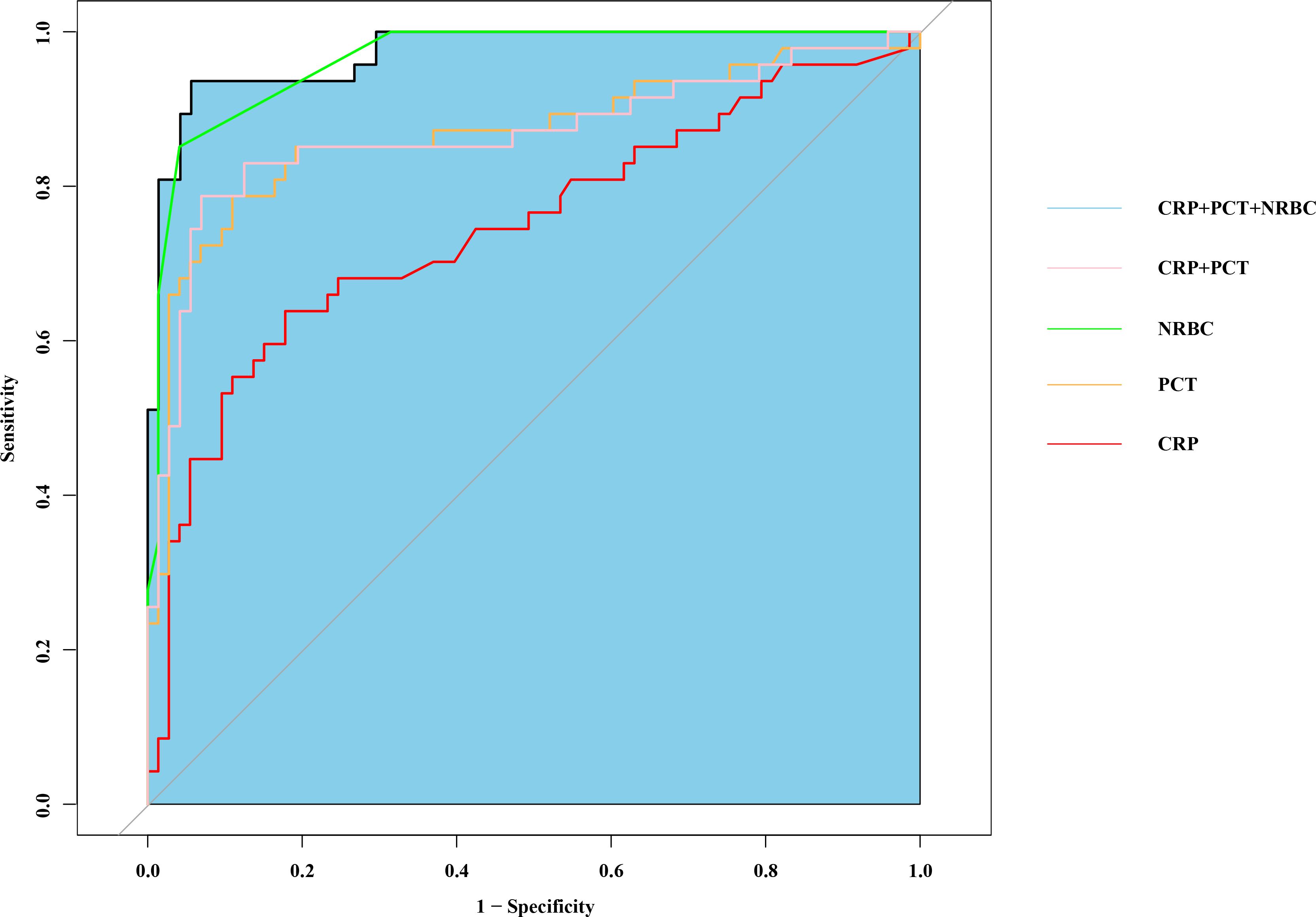
Figure 5. Combination of nucleated red blood cell, PCT and CRP to predict septic shock by receiver operating characteristic (ROC) curves. PCT, procalcitonin; CRP, c-reactive protein.
Discussion
This study evaluated the individual and combined diagnostic accuracies of CRP, PCT and NRBCs for predicting the occurrence and severity of sepsis in the pediatric population. Our findings revealed that NRBC counts, when analyzed alongside CRP and PCT levels, are valuable biomarkers for distinguishing between sepsis and septic shock. The combination of these three biomarkers demonstrated superior predictive accuracy compared with CRP or PCT alone, highlighting the enhanced diagnostic potential of this panel. These results emphasize the role of NRBCs as a complementary biomarker that not only improves diagnostic precision but also reflects the physiological stress and disease severity associated with sepsis.
Sepsis is a life-threatening condition that imposes a substantial burden worldwide. According to the Global Burden of Disease (GBD) study, approximately 25 million sepsis cases occur annually in pediatric and neonatal populations, resulting in approximately 3 million deaths (Watson et al., 2024). The absence of definitive biomarkers for the timely diagnosis of sepsis exacerbates the inappropriate use of antibiotics. Therefore, identifying a potential combination of biomarkers to improve the early diagnosis of sepsis is imperative.
In this study, the positive rates of blood culture in sepsis and septic shock patients were 12.5% and 36.5%, respectively. The most common bacterial isolate was Escherichia coli. These findings differ from those of a meta-analysis by Droz et al (Droz et al., 2019), which reported a pooled blood culture positivity rate of 28% (95% CI: 13.2–45.8%) among pediatric populations in low- and middle-income Asian countries. The lower positivity rate observed in our sepsis cohort may reflect differences in patient demographics, clinical settings, or regional pathogen prevalence. Although blood culture is considered the gold standard for diagnosing sepsis, its sensitivity is influenced by multiple factors. For example, delays in sample collection, previous antibiotic administration, technical limitations of detection methods, and insufficient blood sample volume all contribute to variability in positive rates (Lambregts et al., 2019; Huber et al., 2020; Whelan et al., 2024). These challenges are particularly pronounced in pediatric patients, where obtaining adequate blood volumes for culture is often more difficult than in adults.
Our analysis revealed significantly higher CRP concentrations in patients with septic shock than in those diagnosed with sepsis (P < 0.05). However, CRP has shown limited diagnostic reliability because of its gradual elevation during the early phases of infection and its frequent elevation in noninfectious conditions. In this study, the ROC curve analysis revealed that plasma CRP level exhibited the poorest diagnostic performance, with low specificity in the early diagnosis of sepsis in children. Current evidence suggests integrating CRP with other markers to optimize diagnostic accuracy rather than employing it as a standalone tool for sepsis screening (Morad et al., 2020).
Serum PCT is widely recognized as a biomarker of severe bacterial infections and the early diagnosis of sepsis, as well as for monitoring disease progression in infectious conditions. A meta-analysis by Menon et al. on severity-related variables in children demonstrated that PCT levels are positively correlated with mortality rates, which can serve as a predictive indicator for septic shock patients (Menon et al., 2022). In the present study, PCT levels were significantly higher in the septic shock group than in the sepsis group (P < 0.05). One study reported that a procalcitonin threshold of 19.1 ng/mL was associated with septic shock in children with sepsis (Rey et al., 2007). In this study, using a cutoff value of PCT ≥7.785 μg/L determined by the ROC curve, PCT demonstrated a sensitivity of 87.6% and specificity of 83.7% (Table 3). In pediatric septic shock patients, procalcitonin demonstrated superior predictive capability relative to CRP, a finding consistent with established clinical research (Rey et al., 2007; Wacker et al., 2013; Morad et al., 2020).
Early recognition and differentiation of sepsis and septic shock are critical for initiating timely and appropriate management. However, diagnostic challenges persist owing to nonspecific clinical presentations and the limitations of existing laboratory tests. These limitations highlight the urgent need for novel biomarkers that can increase diagnostic accuracy, allow for early risk stratification, and guide personalized treatment strategies. Our study addresses this need by demonstrating that NRBCs improve the diagnostic performance of traditional biomarkers.
NRBCs represent immature erythroid precursors that enter circulation during severe physiological stressors such as hypoxia, systemic inflammation, or bone marrow activation (Pedersen et al., 2022). Their presence in circulation beyond the neonatal period is considered pathological and has been associated with poor outcomes in critically ill patients. In this study, NRBC counts were significantly higher in children with septic shock than in those with sepsis (P < 0.05). Our findings confirm the prognostic utility of NRBC counts in pediatric septic shock. These results confirm the prognostic value of NRBC quantification for mortality risk stratification in pediatric patients with septic shock, confirming previous the results of previous studies (Pedersen et al., 2022; Noor et al., 2023; Kirsch et al., 2024).
Previous work from our group identified NRBC positivity as an independent risk factor for disease severity and mortality in pediatric sepsis (Li et al., 2023). In the adult population, Desai et al. observed that surgical sepsis patients with detectable NRBCs exhibited significantly elevated mortality risks in both ICU and general ward settings. Their threshold analysis revealed mortality rates exceeding 50% at NRBC peaks >500/μL, with survival rates dropping below 12% for counts >2000/μL (Desai et al., 2012). Narcı et al.’s retrospective analysis also established NRBC counts as independent predictors of mortality across trauma, sepsis, and critically ill populations (Narcı et al., 2021). Collectively, these findings substantiate NRBC elevation as a biomarker of pathophysiological dysregulation proportional to disease severity.
In this study, when a cutoff value of NRBC count ≥ 3 was used, as determined by ROC curve analysis, the NRBC count demonstrated a sensitivity of 90.7% and a specificity of 88.4% (Table 3). When combined with CRP and PCT, the NRBC count improved the risk prediction accuracy (AUC increased from 0.746 to 0.956), enabling more precise clinical assessment in pediatric sepsis management. This combined biomarker strategy facilitates earlier recognition of sepsis and severity stratification, thereby supporting time-sensitive clinical decisions regarding therapeutic escalation.
The pathophysiological mechanisms underlying elevated NRBC counts in pediatric sepsis and septic shock patients require further investigation. Under conditions of hypoxia, systemic inflammation, or tissue damage, signaling molecules such as erythropoietin and interleukin proteins (e.g., interleukin-3 and interleukin-6 [IL-6]) are activated (Stachon et al., 2005; Kirsch et al., 2024). Experimental evidence has identified IL-6 as a key regulator that promotes erythropoietin synthesis during systemic inflammatory response syndrome following septic shock in pediatric populations (Krafte-Jacobs and Bock, 1996). These biochemical mediators stimulate the maturation of immature red blood cells in the bone marrow, leading to the appearance of NRBCs in the circulation. Our findings suggest that this phenomenon is likely related to tissue hypoxia caused by systemic hypoperfusion.
In our study, children with septic shock required more mechanical ventilation and had higher lactate levels than those with sepsis alone did, supporting the role of oxygen deprivation in NRBC production. Additionally, elevated CRP and PCT levels in children with septic shock further indicate the involvement of inflammatory mediators in the synthesis and release of NRBCs. Further research is needed to elucidate the mechanisms underlying this association.
This study has several limitations. First, the study population was drawn from a single center, resulting in a relatively small sample size, which may limit the generalizability of our findings. In addition, the retrospective design of the study may have introduced bias in data recording and collection. Further research with larger sample sizes and multicenter designs is needed to validate these findings. At last, the future prospective studies are required to validate the mechanism of NRBC sources, due to the effects of anemia or hypoxia.
Conclusions
In conclusion, this study demonstrated that the NRBC count combined with the CRP and PCT levels have value for the diagnosis and prognosis of pediatric sepsis and septic shock. This biomarker combination improves the ability to reliably distinguish between sepsis and the progression of septic shock, representing a substantial advancement in sepsis care. By enabling earlier detection, more precise risk stratification, and more effective therapeutic interventions, the findings of the present study help address critical challenges in the management of pediatric sepsis. Given the global burden of pediatric sepsis and the limitations of existing diagnostic tools, incorporating NRBC measurements into clinical practice has the potential to transform sepsis management and improve outcomes for children worldwide. Future research should aim to validate these findings in larger, multicenter studies, explore their applicability across diverse populations, and investigate the broader implications of NRBC measurement for sepsis management and public health strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the Children’s Hospital of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
KP: Conceptualization, Investigation, Writing – original draft. CW: Writing – original draft. JC: Data curation, Formal analysis, Software, Writing – review & editing. DC: Data curation, Validation, Writing – review & editing. LT: Conceptualization, Funding acquisition, Resources, Writing – review & editing. JT: Conceptualization, Investigation, Validation, Visualization, Writing – review & editing. HL: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Chongqing (NO. CSTB2024NSCQ-MSX0326, CSTB2024TIAD-KPX0034), the Chongqing Science and Technology Bureau and Health Commission Joint Medical Project (NO.2024QNXM011), the Science and Technology Research Program of Chongqing Municipal Education Commission (No. KJQN202300459).
Acknowledgments
We are grateful to American Journal Experts for their valuable advice to the linguistic revision of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chiesa, C., Panero, A., Osborn, J. F., Simonetti, A. F., and Pacifico, L. (2004). Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin. Chem. 50, 279–287. doi: 10.1373/clinchem.2003.025171
Christensen, R. D., Henry, E., Andres, R. L., and Bennett, S. T. (2011). Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology 99, 289–294. doi: 10.1159/000320148
Desai, S., Jones, S. L., Turner, K. L., Hall, J., and Moore, L. J. (2012). Nucleated red blood cells are associated with a higher mortality rate in patients with surgical sepsis. Surg. Infect. (Larchmt.) 13, 360–365. doi: 10.1089/sur.2011.089
Droz, N., Hsia, Y., Ellis, S., Dramowski, A., Sharland, M., and Basmaci, R. (2019). Bacterial pathogens and resistance causing community acquired paediatric bloodstream infections in low- and middle-income countries: a systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 8, 207. doi: 10.1186/s13756-019-0673-5
Goldstein, B., Giroir, B., Randolph, A., and International Consensus Conference on Pediatric Sepsis (2005). International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6, 2–8. doi: 10.1097/01.PCC.0000149131.72248.E6
Härtel, C., Faust, K., Fortmann, I., Humberg, A., Pagel, J., Haug, C., et al. (2020). Sepsis related mortality of extremely low gestational age newborns after the introduction of colonization screening for multi-drug resistant organisms. Antimicrob. Resist. Infect. Control 9, 144. doi: 10.1186/s13756-020-00804-8
Huber, S., Hetzer, B., Crazzolara, R., and Orth-Höller, D. (2020). The correct blood volume for paediatric blood cultures: a conundrum. Clin. Microbiol. Infect. 26, 168–173. doi: 10.1016/j.cmi.2019.10.006
Kirsch, A., Niebhagen, F., Goldammer, M., Waske, S., Heubner, L., Petrick, P., et al. (2024). Nucleated red blood cells as a prognostic marker for mortality in patients with SARS-CoV-2-induced ARDS: an observational study. J. anesthesia analgesia Crit. Care 4, 38. doi: 10.1186/s44158-024-00174-2
Kissoon, N., Reinhart, K., Daniels, R., MaChado, M., Schachter, R. D., and Finfer, S. (2017). Sepsis in children: global implications of the world health assembly resolution on sepsis. Pediatr. Crit. Care Med. 18, e625–e627. doi: 10.1097/PCC.0000000000001340
Krafte-Jacobs, B. and Bock, G. H. (1996). Circulating erythropoietin and interleukin-6 concentrations increase in critically ill children with sepsis and septic shock. Crit. Care Med. 24, 1455–1459. doi: 10.1097/00003246-199609000-00005
Lambregts, M., Bernards, A. T., van der Beek, M. T., Visser, L. G., and de Boer, M. G. (2019). Time to positivity of blood cultures supports early re-evaluation of empiric broad-spectrum antimicrobial therapy. PLoS One 14, e0208819. doi: 10.1371/journal.pone.0208819
Li, H., Tu, Q., Feng, K., Cheng, J., Zou, Z., Li, S., et al. (2023). Nucleated red blood cells as a novel biomarker in the diagnosis and prediction of sepsis severity in children. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1264607
Menon, K., Schlapbach, L. J., Akech, S., Argent, A., Biban, P., Carrol, E. D., et al. (2022). Criteria for pediatric sepsis-A systematic review and meta-analysis by the pediatric sepsis definition taskforce. Crit. Care Med. 50, 21–36. doi: 10.1097/CCM.0000000000005294
Morad, E. A., Rabie, R. A., Almalky, M. A., and Gebriel, M. G. (2020). Evaluation of procalcitonin, C-reactive protein, and interleukin-6 as early markers for diagnosis of neonatal sepsis. Int. J. Microbiol. 2020, 8889086. doi: 10.1155/2020/8889086
Narcı, H., Oktay, M. M., Ayrık, C., and Çimen, M. (2021). Nucleated red blood cells as predictor of all-cause mortality in emergency department. Am. J. Emerg. Med. 46, 335–338. doi: 10.1016/j.ajem.2020.10.002
Noor, T., Imran, A., Raza, H., Sarwar, M., Umer, S., and Fatima, M. (2023). Frequency of nucleated red blood cells in the peripheral blood of ICU-admitted patients. Cureus 15, e33827. doi: 10.7759/cureus.33827
Pedersen, S., Chok, R., McKillop, S., Rojas-Vasquez, M., Duff, J. P., Szkotak, A., et al. (2022). Peripheral nucleated red blood cells and mortality in critically ill children. J. Pediatr. Hematol. Oncol. 44, 79–83. doi: 10.1097/MPH.0000000000002294
Reinhart, K., Daniels, R., Kissoon, N., MaChado, F. R., Schachter, R. D., and Finfer, S. (2017). Recognizing sepsis as a global health priority - A WHO resolution. N. Engl. J. Med. 377, 414–417. doi: 10.1056/NEJMp1707170
Reinhart, K., Karzai, W., and Meisner, M. (2000). Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 26, 1193–1200. doi: 10.1007/s001340000624
Rey, C., Los Arcos, M., Concha, A., Medina, A., Prieto, S., Martinez, P., et al. (2007). Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med. 33, 477–484. doi: 10.1007/s00134-006-0509-7
Rudd, K. E., Johnson, S. C., Agesa, K. M., Shackelford, K. A., Tsoi, D., Kievlan, D. R., et al. (2020). Global, regional, and national sepsis incidence and mortality 1990-2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211. doi: 10.1016/S0140-6736(19)32989-7
Schlapbach, L. J., Watson, R. S., Sorce, L. R., Argent, A. C., Menon, K., Hall, M. W., et al. (2024). International consensus criteria for pediatric sepsis and septic shock. JAMA 331, 665–674. doi: 10.1001/jama.2024.0179
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810. doi: 10.1001/jama.2016.0287
Stachon, A., Bolulu, O., Holland-Letz, T., and Krieg, M. (2005). Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock 24, 34–39. doi: 10.1097/01.shk.0000164693.11649.91
Wacker, C., Prkno, A., Brunkhorst, F. M., and Schlattmann, P. (2013). Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect. Dis. 13, 426–435. doi: 10.1016/S1473-3099(12)70323-7
Watson, R. S., Carrol, E. D., Carter, M. J., Kissoon, N., Ranjit, S., and Schlapbach, L. J. (2024). The burden and contemporary epidemiology of sepsis in children. Lancet Child Adolesc. Health 8, 670–681. doi: 10.1016/S2352-4642(24)00140-8
Keywords: C-reactive protein, procalcitonin, nucleated red blood cell, sepsis, septic shock
Citation: Pu K, Wang C, Cheng J, Chen D, Tan L, Tan J and Li H (2025) Combination of nucleated red blood cells and inflammatory biomarkers (PCT and CRP) for predicting sepsis and septic shock in children. Front. Cell. Infect. Microbiol. 15:1603216. doi: 10.3389/fcimb.2025.1603216
Received: 31 March 2025; Accepted: 28 July 2025;
Published: 14 August 2025.
Edited by:
Kuldeep Gupta, University of Arizona, United StatesReviewed by:
Isha Pandey, Rutgers University, Newark, United StatesDan Justin Kalenda Yombo, AstraZeneca Biopharmaceuticals, United States
Copyright © 2025 Pu, Wang, Cheng, Chen, Tan, Tan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jihong Tan, NDM0NDM3MTYxQHFxLmNvbQ==; Hongdong Li, NDEzNzQ1MzA4QHFxLmNvbQ==
†These authors have contributed equally to this work
 Kaibin Pu1,2,3
Kaibin Pu1,2,3 Chunyi Wang
Chunyi Wang Jie Cheng
Jie Cheng Liping Tan
Liping Tan Jihong Tan
Jihong Tan Hongdong Li
Hongdong Li